Abstract
In the last years, the systematic use of ultrasound mapping of the upper limb vascular network before the arteriovenous fistula (AVF) implantation, access maturation, and clinical management of late complications is widespread and expanding. Therefore, a good knowledge of theoretical outlines, instrumentation, and operative settings is undoubtedly required for a thorough examination. In this review, the essential Doppler parameters, B-Mode setting, and Doppler applications are considered. Basic concepts on the Doppler shift equation, angle correction, settings on pulse repetition frequency, operative Doppler frequency, gain are reported to ensure adequate and correct sampling of blood flow velocity. A brief analysis of the Doppler inherent artefacts (as random noise, blooming, aliasing, and motion artefacts) and the adjustment setting to minimize or eliminate the confounding artefacts are also considered. Doppler aliasing occurs when the pulse repetition frequency is set too low. This artefact is particularly frequent in vascular access sampling due to the high velocities range registered in the fistula’s different segments. Aliasing should be recognized because its correction is crucial to analyse the Doppler signals correctly. Recent advances in instrumentation are also considered about a potential purchase of a portable ultrasound machine or a top-of-line, high-end, or mid-range ultrasound system. Last, the pulse wave Doppler setting for vascular access B-Mode and Doppler assessment is summarized.
Keywords: B-mode ultrasound, pulse wave Doppler, arterio-venous fistula, Doppler parameters, Doppler/B-mode setting
Introduction
In the last years, the routine use of ultrasound in the mapping of upper limb vascular network before the arteriovenous fistula (AVF) implantation, access maturation, and clinical management of late complications is widespread and growing. Therefore, a good knowledge of theoretical outlines, instrumentation, and operative settings is undoubtedly required for a thorough examination.
Ultrasound, similar to MRI, is a “multiparametric” imaging technique because it analyses and represents native echo-signals originating from tissues and vessels in different ways. 1 B-Mode represents structural echoes as brightness dots and generates a grey scale image of the tissue. Doppler-mode imaging analyses in a region of interest (ROI) the backscattering from the red blood cells (RBCs) moving in the vessels. (a) Color Doppler represents the blood flow-velocity changes as a dynamic mapping where the flows move towards or away from the transducer and are respectively depicted with red and blue hues. (b) Spectral Doppler analyses in a small volume sample the velocity changes over time (cardiac cycle), generating a spectrum with positive and negative inflections.2–6 Contrast-enhanced ultrasound (CEUS) analyses vascular scattering enhancement after intravenous infusion of a low mechanical index amplifier.7,8 Indications of this technique in vascular access management are not specific. Last, ultrasound elastography represents the degree of tissue elasticity or stiffness. It does so by analyzing either tissue strain induced by transducer pressure or shear waves produced in the tissue by an acoustic radiation force impulse. 9 Through ultrafast-imaging algorithms, ultrasound elastography should be able to evaluate arterial wall elasticity and pulse wave velocity.
B-Mode and Color Doppler are the first-choice imaging in the morphological and functional assessment of vascular access (VA) and its early and late complications; it can compete with phlebography reliability. The availability of high-frequency small-parts probes (up to 20 MHz) with high spatial resolution and the remarkable improvement of Doppler performance and sensitivity are the reasons for this. The Doppler exam’s reliability is closely related to the operator’s clinical knowledge and skills, even more so if he is not daily dedicated to ultrasound diagnostics.
This review aims to present the basic knowledge necessary for a neophyte to approach ultrasound to perform an adequate and well-made examination.
Ultrasound imaging
The basic principles of US imaging are simple. A piezoelectric transducer excited by short bursts of electrical energy “vibrates” at its resonance frequency and sends a variable frequency pulse into the soft tissue. The echoes received are converted into a radiofrequency signal, analyzed, and represented on the video screen (Figure 1).
Figure 1.
Piezoelectric effect. A piezoelectric transducer generates a pulsed ultrasound beam that is launched into the soft tissues (a). Echo signals received by the probe are converted into a continuous radiofrequency signal analyzed by the receiver and displayed on the screen in different image modalities (B-Mode, color, and spectral Doppler) (b).
B-mode imaging
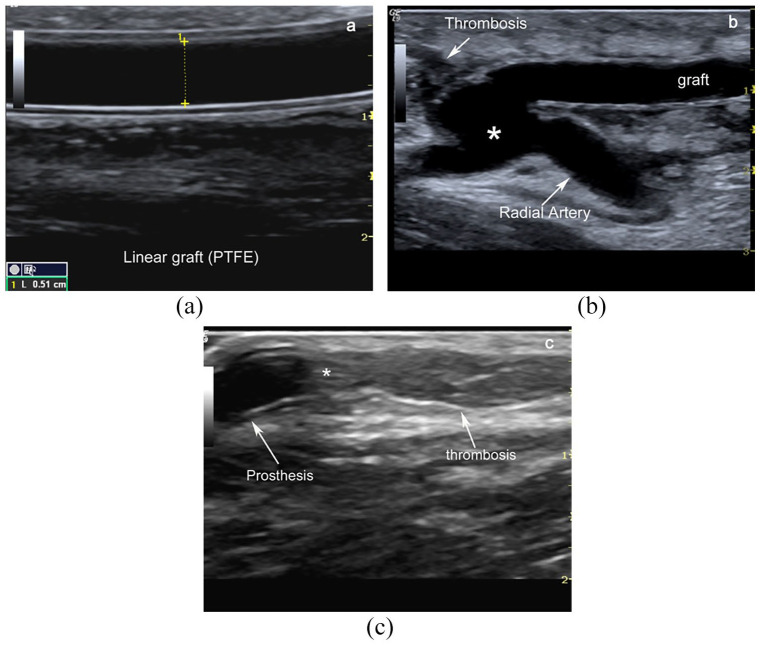
B-mode imaging is a brightness-modulated image in which depth is along the z-axis, and azimuth is along the x-axis. It is also known as “B-scan” or “2D mode.” The position of the echo is determined by its acoustic transit time and depth in the propagation axis. B-Mode represents two types of echo signals. The first type originates from specular and diffuse interfaces with a thickness greater than the pulse wavelength, such as vessel wall, muscle fasciae, renal collecting system, diaphragm, and liver capsule. The second one derives from the reflectors, which are smaller than the pulse wavelength, and from the Rayleigh scatterers that are very thin in size compared to pulse wavelength. 10 The RBCs (about 7 μm in diameter) flowing in the vessel as cell-clusters due to their rheological properties are Rayleigh scatterers. The B-Mode image represents the tissues and structures in a planar image. The grey shade of each pixel is related to the intensity of the native echo-signal and varies from black (anechoic or hypoechoic) to white (hyperechoic). Vessel features as relationship, site, diameter, course, intima-media wall thickness, plaques, calcifications, narrows, thrombosis are diagnosed with the grey scale ultrasound. Much morphological information can be obtained using B-Mode image in the native fistula (Figure 2) or prosthetic graft (Figure 3).
Figure 2.
B-Mode image of native arteriovenous fistula. Side-to-end well-functioning radiocephalic fistula at the wrist. (*) Anastomosis (a). Side-to-end radiocephalic AVF in a diabetic patient. Maturation of vascular access as well as the remodeling and dilation of the radial artery (Ra), anastomotic chamber (*), and draining vein are insufficient (b). Atherosclerotic and calcified radial artery in elderly diabetic patient with inflow stenosis. Brachial artery Qa in this patient was 180 ml/min (c). Coarse wall calcifications with shadowing (white arrows) into the anastomosis (*) of a well-functioning long-term radiocephalic fistula (d).
Figure 3.
B-Mode image of an arteriovenous graft. Typical grey scale image of a linear radial-cephalic antecubital linear graft (double-track sign) (a). Loop graft are usually placed within the forearm and anastomosed to the brachial artery and the basilic or brachial vein. Linear bridge in PTFE between antecubital radial artery and basilic vein after the thrombosis of vein draining proximal radio-cephalic fistula (*anastomotic chamber) (b). Intraluminal thrombotic material downstream of the venous anastomosis between prosthesis (*) and native cephalic vein (c).
Doppler effect
Backscattering from RBCs flowing in the vessels can be analyzed using the Doppler equation and represented through either a colorimetric map (color and power Doppler) or spectral analysis. Color-flow-imaging (CFI) overlaps the B-mode image with a color box representing a spatial map of RBCs mean velocity in each pixel. It encodes flow direction in colors (usually blue away from the transducer and red toward it), the amplitude of mean speed by brightness, and turbulence or variance by a third color (often green). Power Doppler is a color-coded image based on Doppler-shift intensity. Pulsed wave Doppler (PWD) uses discontinuous pulses to measure Doppler-shift or blood flow velocity in a volume sample. Both color-power Doppler and pulsed wave Doppler analyse the scattering information originating from RBCs in a region of interest. The resulting sonogram is superimposed/associated with the B-Mode image.
Doppler effect defines the variation of frequency observed when a US pulse from a fixed source strikes RBCs moving in the vessels. 11 The Doppler equation states that:
where ΔF is the Doppler shift, F0 is the pulse frequency, V is the velocity of blood flow towards, or away from the transducer, cos θ is the cosine function of the angle of incidence between the pulse wave and vectorial direction of the stream, and c is the ultrasound speed in soft tissue (1540 cm/s). In other words, ΔF is directly proportional to the transmitted frequency, blood flow velocity, and cosine function of the angle between the ultrasound beam and blood flow direction (Figure 4). At the same time, it is inversely proportional to the pulse velocity propagation through biological tissues. Doppler-Mode software solves this equation for the velocity-factor because it measures the Doppler shift and knows both ultrasound speed in the soft tissue and operative frequency.
Figure 4.
Doppler effect defines the variation of frequency observed when a US beam from a fixed source strikes red blood cells (RBCs) moving in the vessels towards or away from the transducer. Doppler shift (ΔF) is proportional to operative frequency (F0), blood flow velocity and cosine function (Θ) of the angle between ultrasound beam and blood flow direction. Thus it is inversely proportional to the pulse velocity propagation through biological tissues. Doppler-Mode software can solve and represent this equation for the velocity-factor.
Velocity versus time flow or V/t curve represents RBCs velocity assuming - by default - that the Doppler angle is 0°. The operator must correct the insonation angle in post-processing after the image freezing. The cosine function for a grade of 0° is 1, while the cosine function for an angle of 90° is 0 (Figure 4). Thus, Doppler-shift is maximum when the incident angle is near 0° or out of phase at 180°. Then, Doppler-shift has a positive or negative value depending on the flow direction. It is positive when the flow is toward the probe (0°–89°) and negative when the flow is away from the transducer (91°–180°). When the Doppler angle is 0°, the blood flow moves towards the transducer, while the blood flow moves away from the probe when the Doppler angle is at 180°. If the Doppler angle is at 90°, blood flow is perpendicular to the ultrasound beam, and no Doppler shift is detected.
The Doppler angle must be adequately set in the PWD that converts Doppler shift in a velocity spectrum of RBCs using the Fast Fourier Transform (FFT) algorithm.10–13 Any operator needs to learn the pivotal importance of the “Doppler angle” in the velocity calculation (Figure 5). A good compromise to obtain an adequate sample of superficial vessels is a 60°angle. In this way, the error in the speed calculation (about 20%–25%) is tolerable and not significant. This policy reduces the intra- and interobserver variability measuring the spectral curve’s quantitative parameters.
Figure 5.
Spectral representation of flow-velocities. Placing a volume sample in the vessel lumen as in the tributary artery of a radio cephalic AVF (a) or brachial artery (b) we can record a spectral Doppler trace. Velocities of RBCs, crossing the gate, are plotted versus time and represented as a velocity over time curve. The “y axis” represents velocity (m/sec) and “x axis” the time. Spectral curve provides a measurement of maximal and mean velocity enveloping the curve and integrating the velocities below the curve. Above the “zero line” the velocities are towards the transducer while below the “zero line” are away from the transducer.
Pulse wave Doppler
PWD analyses the Doppler shift in a volume sample placed along a sound field line; the sampling volume sample is freely located in the vessel lumen. The sonographer can vary the acoustic amplitude, Doppler operating frequency, and pulse repetition period (PRP) to improve the PWD sensitivity. However, this setting increases acoustics exposure and reduces spatial resolution.
The pulse repetition period (PRP) is the time interval between two successive pulses. During this period, the probe receives the echo signals from each point of the sound field to generate the B-mode image and analyse Doppler signals. The PRP varies according to reflector depth and distance travelled by US pulse in the round trip. It is usually represented in the monitor with its inverse value, that is, pulse repetition frequency (PRF), so 1/PRP = PRF (Figure 6).
Figure 6.
Pulse-echo pulsing. Pulsing characteristics for piezoelectric transducer are similar in all pulse-echo systems. The pulse duration (PD) is the fraction of time when the transducer actively transmits ultrasound. The PD is usually less than 1 μs to generate a broadband pulse and enhance spatial resolution. On the other hand, pulse repetition period (PRP) is the transducer’s receiving time and it is much greater than PD. Pulse repetition frequency (PRF) defines the frequency of acoustic pulses transmitted per second (KHz/s) and it is equal to inverse value of PRP (1/PRP).
The primary artefact of PWD is aliasing, that is, a spectral ambiguity, which arises when a continuous signal is discretely sampled at a rate inadequate to capture its changes. The artefact is inherent with the technical characteristics of the pulse transducers that generate the US discontinuously. The risk of aliasing increases when the PRF is low, because the time interval separating two successive pulses corresponding to receiving time of the transducer is not long enough to allow the return of the signals deriving from the deeper reflectors. The PRF is inversely proportional to the sample volume’s depth. The higher the sample depth in the sound field, the slower the PRF must be (as the pulse wave takes a more significant time to travel, one needs longer intervals to observe the returning wave). Therefore, the maximum velocity detectable decreases when the reflector depth in the sound field increases. The B-mode and Doppler PRF set-up are independent in the digital platform, so the risk of aliasing is considerably reduced.
PWD analyses the vessel backscattering for a short time interval, named gate or volume sample, along a single scan-line overlapped to the B-Mode image.12–14 The volume sample varies from 2 to 15 mm and allows the operator to restrict or extend the gate sampling. Doppler signals fall within the range of audible frequencies (20–18,000 Hz). So, they are simultaneously converted in a characteristic audio signal.10–12
Color Doppler
The CD data set is obtained in a region of interest (color box) superimposed to the B-Mode field (Figure 7). Echoes deriving from stationary reflectors in this region are represented as brightness points. Instead, the echoes from moving scatterers are analyzed by an autocorrelation detector to evaluate the mean flow-velocity changes, and they are represented as color pixels. The blue and red hues are conventionally assigned to display positive and negative Doppler shifts, respectively. Different shades (saturation) of red and blue are used to display velocity grading; lighter shades of color (light blue or yellow) are attributed to higher speeds.10,13–15 Ultrasound scanners are usually provided with several color maps to allow the operator to invert color assignments and alternate the chromatic map. Hue, saturation, and luminance of red and blue are the three characteristics of color used to indicate Doppler-shifted echoes’ nature, that is, their mean velocities, phase, amplitude, and variance. Variance in color flow imaging measures flow disturbance at the level of stenosis and corresponds to spectral broadening and fluttering in the PWD waveform distal to the stenosis. When using a chromatic scale that depicts flow toward the transducer with the red hue and flows away from the probe with a blue hue, the image sampled with a convex or curvilinear transducer appears the Figure 8.10,13–15
Figure 7.
Color Doppler imaging. Color Doppler imaging depicts inreal-time the flow-velocity changes in the vessels. Red and blue hues represent flow moving towards or away from the transducer. Desaturation of red or blue is an indirect measure of the velocity variations related to Doppler angle varying from 90° to 0° and 90° to 180°. Higher flow rates appear whiter than lower flow rates.
Figure 8.

Color Doppler imaging. CD data are evaluated in a region of interest superimposed to the B-Mode field (color-box or color field). Stationary echoes in the color box are represented as brightness points as in B-mode field.
The Doppler angle regulation is essential when linear array transducers are used to sample vessels running parallel to the skin surface. Doppler sampling, in this case, is obtained perpendicularly to the flow direction. The pulses and scan lines intersect the RBCs at a 90° angle, so no Doppler signal is registered. To solve this problem, 20° electronic steering, away from the transducer, is applied to pulses by the beamformer, regulating the pulse-delay sequences of active row excitation. In this way, color pulses and scan lines are steered at the same angle, and Doppler signals are represented on display. After steering is activated, sampling a vessel on the long axis using a linear probe can make it challenging to define the flow direction. A simple trick to understand the flow direction is to perform an orthogonal scan on the short axis and subsequently tilting the probe in a centripetal or centrifugal direction. In the first case, the color signals collected at 90° are confusing; in the second case, it will be red and, in the third case, naturally blue. For example, this trick helps to understand if, in an end-to-side radiocephalic fistula, there is or there is no theft of blood from the ulnar artery.
Since color Doppler is based on the same principles as PWD, it is also subject to aliasing. When the Doppler shift or blood flow velocity exceeds the Nyquist limit (0.4–0.7 m/s), aliasing appears as an abrupt inversion of the chromatic scale (mosaic pattern of colors), so the brightest shades of red and blue lie adjacent. Color scale aliasing helps the operator to detect high-velocity jets.
CDI mode is semiquantitative, angle-dependent imaging that provides an overall view of the regional vascular network and allows the operator to define the blood flow direction, exclude vessel’s thrombosis, and distinguish a vessel from a tubular structure, for example, a vein from a collection. CDI’s significant limitations are the poor spatial and temporal resolution, aliasing, and artefacts caused by the noise, blooming, and bruit.
Power Doppler imaging
Power Doppler imaging (PDI) is an alternative algorithm that estimates the Doppler shift’s total strength within each point in the color box. This processing is known as power or energy mode. It is angle-independent and sensitive to slow flows and does not vary significantly with flow direction.15,16 The PDI increases image persistence creating a pseudo-angiographic effect. The sensitivity makes PDI an excellent test for blood flow imaging of superficial vessels. The clutter due to arteries pulsatility and probe sliding is the principal limit to PDI. In the most recent platform, spatial-temporal clutter filtering has significantly improved CDI-PDI sensitivity in microvascular blood flow analysis without the infusion of a contrast agent amplifier. 17
Spectral tracing parameters
The spectral trace obtained by placing the sample in the lumen of an artery or vein represents the RBCs’ velocities crossing the gate in the time. A Velocity-over-time curve (V/t) is created using an FFT analyzer to convert a function of time (Doppler shift) in a mixture of frequencies represented in a Cartesian axis system. The inflections of the V/t curve can be positive or negative and represent the flow phases moving towards or away from the probe.
Several quantitative and semiquantitative parameters may be derived from the V/t curve.10,12–14 These are partly the same as those used in the analysis of the continuous wave Doppler curve.
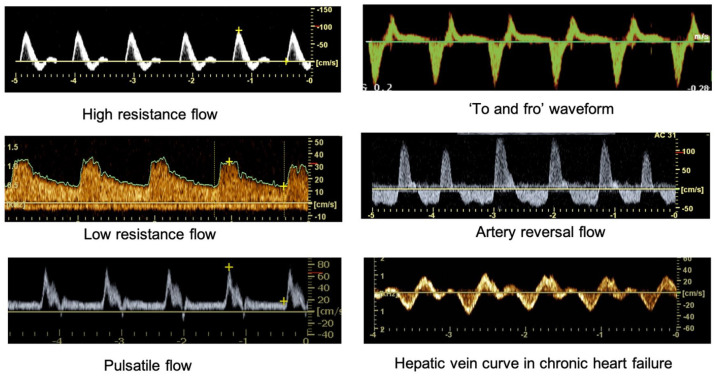
Direction and flow characteristics. A monophasic pattern with a significant diastolic flow reveals in an artery a low-resistance flow; a triphasic discontinuous curve characterizes a high-resistance arterial flow. On the contrary, a monophasic, slow centripetal velocity curve depicts the vein blood flow. Figure 9 illustrates how waveforms can be described based on the shape, number, and deflections phase.
Systolic velocity peak (SVP) and diastolic velocity (DV). Two main hemodynamic factors regulate these parameters: the cardiac output and total/regional resistance due to vessel diameter, length, blood viscosity, and wall elasticity. Thus, any typical waveforms indicate both these two physiological parameters. The SVP and DV are quantitative measurements of the RBCs velocity and characterize the flow variations due to critical stenosis. The “Venturi effect” occurs when the blood flow is pushed through the stenosis (Figure 10). For the principle of flow continuity and the principle of conservation of mechanical energy, in the stenosis point, the flow velocity significantly increases, whereas static pressure decreases.10,12–14
Regional resistance or impedance. Two semiquantitative indices evaluate the impedance: the resistance index (RI) and the pulsatility index (PI). The equation to calculate RI is IR = (SVP-DV)/SVP. The RI average value in a high-resistance vascular district as the arm is ≈ 1, whereas the regional RI in the upper limb after AVF implantation becomes <0.60. Unlike SVP and DV, the RI is not an angle-dependent parameter because it represents a ratio between two different velocities.10,12–14
Systolic/diastolic velocities ratio (S/D). This parameter is another index of peripheral resistance similar to RI.
Spectral broadening. It represents the dispersion of RBC velocities in the stenosis segment. When the blood flow velocity is very high, the laminar flow converts into a turbulent flow in the vessel branching or stenosis.
Time average maximum velocity (TAMV) and time-averaged mean velocity (TAVm). TAMV envelops the spectrum’s maximum speeds, while the TAVm defines the spectrum’s mean velocities during the cardiac cycle. In the feeding artery of AVF or graft, the blood flow rate of access (Qa) is quantified by the following equation: Qa = S·Vm, where S is the vessel’s cross-sectional area Vm, is TAMV or TAVm of the spectral trace. According to this equation, if the flow is constant, the cross-sectional area and the TAV are inversely proportional. Therefore, to calculate the Qa of an AVF or AVG, it is necessary to measure the cross-sectional area (diameter) of the feeding artery and TAVm (Figure 11).10,12–14
Figure 9.
Spectral inflections in V/t curve. Diagrams illustrate how normal waveforms can be characterized based on the number and phase inflections. The first is a triphasic trace with a reversal flow. It represents the high resistance flow rate of peripheral arteries at rest. The second is a low resistance monophasic trace such as in kidney, liver, brain; the third is a monophasic pulsatile flow such as in superior mesenteric artery. The diagram called “to-and-from” flow can be registered at the neck of a pseudoaneurysm. Artery reversal flow is the typical trace of the renal artery in a transplant with renal vein thrombosis; the last one is the hepatic veins trace in chronic heart failure.
Figure 10.

Doppler criteria of critical stenosis. Vein stenosis in a graft. The draining vein is reduced in diameter at the anastomosis level and shows an increase of PSV (>450 cm/s), spectral dispersion, aliasing and oversaturation at CD sampling. The Doppler angle and color box alignment are perfect.
Figure 11.
Brachial flow rate calculation in AVF. (a) High-flow rate in brachial-cephalic fistula. Qb = 1770 ml/min; brachial artery diameter 6.2 mm; TAVm 96.6 cm/s; RI 0.29; S/D ratio is 1.4. (b) High-flow rate in proximal brachial-cephalic fistula. Qb = 2224 ml/min; brachial artery diameter 6.3 mm; TAVm 118 cm/s; S/D ratio 2.1. (c) Normal flow-rate in distal brachial-cephalic fistula with multiple no significant draining vein stenoses. Qb = 511 ml/min; brachial artery diameter 5.0 mm; TAVm 43,1 cm/s; TAMV 68,9 cm/s; RI 0.59; S/D ratio is 2.4. (d) Low flow rate indistalbrachial-cephalic fistula with stenosis of outflow. Qb = 259 ml/min; brachial artery diameter 4.5 mm; TAVm 24.9 cm/s; RI 0.72; S/D ratio is 3.5.
Pulse-wave Doppler artefacts
Aliasing is a significant artefact of pulse-wave ultrasound systems; it is inherent with the pulse transducers’ technical characteristics that generate the ultrasound beam discontinuously. Aliasing occurs when transducer PRF is less than twice the frequency of the signal to be sampled. For example, to sample a Doppler shift of 1000 Hz, the PRF setting must be at least 2000 Hz so that the Doppler shift is sampled at least two times for each cycle. When the PRF is less than twice the maximum Doppler shift, the aliasing occurs. The PRF value equal to two times the Doppler shift is known as the Nyquist sampling rate. Figure 12 represents the sampling of two sine waves. The upper curve is sampled at discrete times indicated by arrows. The dotted lines on the bottom are the resultant sample signal. Sampling is good on the right side because the sampling rate frequency is over than Nyquist limit.10,13,14 On the left side, the sampling rate frequency is below this limit, and the resultant curve is an alias of the continuous signal. When sampling-rate is outside the Nyquist limit, aliasing appears in the spectral curve; it seems like an abrupt cut/inversion of waveform peak, while in CDI, it appears as an inappropriate progression of the chromatic scale (15-17) (Figure 13).
Figure 12.
Aliasing. Aliasing is the major Doppler artifact. It occurs when transducer PRF is less than twice the frequency of Doppler shift. In other words, the PRF setting must be great enough to sample at least two times for each cycle the Doppler signal. If the PRF is less than twice the maximum Doppler shift, the aliasing will occur. PRF value equal to 2fD is known as the Nyquist limit on the basis of the Shannon theorem. In the upper part of figure, a sampling of two sine waves is represented. The black curve is sampled at discrete times indicated by arrows. The dotted red lines on the bottom are the resultant sample signal. The sampling is adequate on the right side because the sampling rate is over the Nyquist limit. On the left side, the sampling rate is below this limit and the frequency of the resultant is an alias of the real signal. CD image represents critical stenosis of the cephalic arch at the level of infra-clavicular Mohrenheim’s fossa. Aliasing of spectral analysis appears as an abrupt cut and inversion of waveform peak. The table lists the useful procedures to correct the aliasing.
Figure 13.
Color Doppler aliasing. When sampling-rate is outside Nyquist limit, color Doppler aliasing appears as an altered progression of chromatic scale.
Blooming or color bleed artefacts appear as a color overflowing beyond the far wall of a superficial vessel. Sonographers can minimize them by decreasing the color gain. Color-bruit is an oversaturation artefact that appears in the tissues surrounding a vessel’s stenotic segment. Similar to the vascular bruit, it is determined by blood acceleration and vibrations transmitted in the tissues.
Advances in instrumentation
The history of sonography began in the 1960s by introducing the first real-time ultrasound scanners using mechanical scanning. Since then, a series of technical innovations have rapidly improved the image quality and the equipment’s reliability. In the 70s, the industry develops multi-channel technology with the electronic control of the transducer. The next two decades saw PWD development, real-time compound techniques, and harmonic tissue imaging, significantly improving image quality. In those same years, the first echo amplifier was marketed. All these developments derive from the availability and maturity of technical devices experienced in the laboratory. The microprocessor’s availability allowed the processing of the tissue’s echo signals and weak scattering deriving from RBCs, favoring the development of the real-time sonography and Doppler applications. The availability of low-cost analog/digital converters supported the full digitalization of the transmitting and receiving systems. Finally, the availability of broadband transducers and new piezoelectric materials has allowed the development of harmonic tissue imaging, and contrast-enhanced sonography, and 3D-ultrasound on the most recent platforms.
The first decade of this century saw technology’s miniaturization and the introduction to low-cost, small, portable, and high-performing equipment. These advancements have favored the organization of point of care of ultrasound (POCUS) in medical departments. The recent introduction of capacitive micromachined ultrasonic transducers (CMUT) is a breakthrough technology in ultrasound and the manufacture of multifrequency ultrasound probes, inexpensive compared to piezoelectric probes. CMUT is micro-electro-mechanical-systems-based and can be used to generate and receive acoustic signals in the ultrasonic range. By using CMUT technology ultra-high-resolution, wide-bandwidth, and highly miniaturized ultrasound transmitters and receivers, a new generation of pocket-sized, whole-body imaging ultrasound systems can be manufactured and marketed. Reducing costs is the primary barrier to the universal adoption of this operator-dependent “stethoscope of the future.”
The need and demand for ultrasonography training have grown in parallel to the expanded use of portable instruments. Hence, an affordable training solution to selective use of bedside ultrasound is essential. Bedside imaging is neither meant to replace a physical examination of vascular access nor other imaging techniques. Still, it is time to add insonation as the fifth component of advanced physical examination. POCUS for primary care for nephrologists and dialysis nurses in the next future should increase the surveillance and the performance of ultrasound-guided maneuvers useful for the correct management of vascular access. 18
Sonography is surely the first-line imaging for vascular access surveillance due to its simplicity, safety, and reliability, even if it is an operator-dependent technique. However, this disadvantage partly depends on different clinical experiences and the operator’s skill, partly due to the lack of familiarity with real-time ultrasound imaging and somewhat dependent upon the performance of vascular ultrasound machines. Last, the theoretical knowledge of many topics, such as the physic of ultrasound, instrumentation and transducer properties, signal processing, and operative setting, is undoubtedly necessary to adequately interact with the ultrasound platform to improve image quality and diagnostic performance.
The B-mode imaging highlights vascular morphology to obtain measurements as diameter, intimal-media thickness, atheromatous plaques, calcifications, and venous fibrosis. The PWD evaluates the blood flow rate and defines the quantitative/semiquantitative Doppler parameters as SVP, DV, RI TAMV, and Qb. The better the software characteristics, the more accurate the diagnosis and the number of exams carried out in each session. The software improves image quality and accuracy of diagnosis and depends upon platform characteristics and budget and the brand and the model chosen within that budget.
What to expect from a top-of-the-line or midrange vascular ultrasound systems category?
A broadband width linear transducer with a high signal-to-noise ratio and spatial resolution (7.5 to 20 MHz) obtains clear margin definition and detailed resolution. POCUS in Nephrology Unit should cover all vascular applications. At least one vascular probe should have a headlong below 25 mm for the central vein’s ultrasound-venepuncture or difficult vascular access.
PWD facilities are useful for CD and PWD regulation. The advanced vascular tools, such as automatic intimal-media thickness measurements and B flow, image optimization, and auto measurements, save time and decrease operator fatigue. The availability of automated scanning protocol, smart exam tools, or programmable protocol increases efficiency and improves exam speed.
Embedded battery for portability without shutting down the system. Dedicated measurement package and flexible report format for all applications.
An economy vascular ultrasound machine should have the same facilities and a good Doppler sensitivity for essential vascular exams like vascular access, superficial and deep veins, central veins, and carotid. Undoubtedly, lower features and hardware sets can hamper the usability and speed of the scanner. Therefore, these systems are more suitable for performing an emergency ultrasound examination (bedside, emergency room, or critical care). However, the miniaturization and cost containment are accompanied by an improvement in quality. 2D and 3D-4D images, ergonomics, and portable devices’ adaptability in the various clinical scenarios (touch-screens, pocket-system, and intelligent diagnostic systems) are almost spectacular.
B-mode setting
Ultrasound must be carried out in a well-tempered, quiet, and not very bright laboratory. The operator must use the most suitable probe and pre-processing configuration. When skin wounds are evident, the transducer should be coated with sterile cellophane or a condom; the contact between the transducer and the skin is guaranteed with a generous dose of antiseptic ultrasound gel.
Scan planes
In the short axis, the vessels appear as round-shaped structures. In contrast, in the long axis, in a lateral–medial or sagittal plane, they appear as tubuliform structure—rotating transducer to 90°, the short-axis view changes into a long-axis view, and vice versa. The vessel’s oblique view can be obtained by probe rotation between the short- and long-axis view. In a transversal or axial scan plane, the probe is placed transversely with the directional marker oriented at the right. The image faithfully repeats on the display the arrangement of the structures located at the vessel’s left and right sides. The operator’s view is specular, so he sees the right side’s anatomical structures to the left and those found on the left to the right, even if he can invert this setting. The directional probe marker must be oriented towards the centripetal direction in the sagittal scan plane. The screen image reproduces the most peripheral structures towards the top and the most caudal structures downwards. 10
The exam protocol for the upper limb vessels differs between the operators.19–22 The exam protocol for the upper limb vessels varies between different operators.19–22 In our laboratory, the CD exam is carried out with the patient sitting in front of the operator and arm extended. The superficial veins can be sampled with the arm along the trunk and the patient sitting on the hospital bed to take advantage of vein replenishment due to gravity. The cephalic vein along the deltoid sulcus, the cephalic arc, the axillary vein, and the subclavian vein must be sampled in the supine decubitus arm abducted at 90° from the trunk. The subclavian and axillary veins can be sampled behind the patient’s head, depending on the operator’s working habits.
The brachial artery is the most immediate anatomic landmark in the arm. Transverse and longitudinal scans must be performed along the median bicipital sulcus in the middle third of the arm. These scans allow identifying the artery or any abnormalities such as an early bifurcation in about 20% of cases. The brachial artery is easily distinguishable from the two satellite veins for its calibre, incompressibility, and spectral tracing. The comitantes veins are more compressible and have a spiral course around the artery. Once the brachial artery has been identified, the operator has to follow its path to the antecubital fossa, where the artery divides into its terminal branches. Then, radial and ulnar arteries are highlighted up to the carpal region.
A correct exploration of the arterial axis makes it easier to localize the fistula location (distal or proximal) and anastomosis type (end-to-end, side-to-side, end-to-side). In addition, this method allows for identifying the draining veins, despite not having anamnestic data on the AVF type. Transverse scans should always be used to define the course of the brachial and radial arteries and their anatomical relationships with the comitans veins and muscle structures. Long axis scans are essential for color mapping and spectral analysis, but they require more manual skills since arm vessels are very thin and easy to lose, even with the probe’s small sliding. In practice, this technique can be used to study the vascular tree, design AVF, and evaluate a normal or failing AVF.23,24
Doppler setting
The operator should obtain Doppler sampling of vascular access using high-frequency linear transducers with a wide bandwidth (from 7.5 to 20 MHz). The operative frequency of CD and spectral Doppler does not coincide with the B-Mode transmitted frequency in current platforms, so the operator can separately regulate Doppler and B-Mode operative frequency. A careful B-Mode setting (operative frequency, total and sectorial gain, focusing) and examination should precede the CD and PWD sampling to define the anatomy, relationship, and vessel’s features and establish the most suitable angle. Of course, since both the color box and the spectral analysis are superimposed to B-Mode, the image quality tends to decrease against excellent Doppler signals in the cheapest scanners. The best B-Mode image is obtained using an orthogonal incidence. 10
Color Doppler setting
The CDI mode activation superimposes on the B-Mode image a color-box. To adequately sample the Doppler signal in this region, the sonographer must adjust box dimension and depth, operative frequency, PRF, Doppler gain, wall filter and regulate the steering to avoid US orthogonal incidence. The steering function controls the phase-delays with which the digital beamformer excites the piezoelectric crystals. The maximum steering angle is 20–30° degrees. Beyond this value, the transducers lose sensitivity, and this function becomes not useful.10–15
Technical solutions adopted by manufacturers for multigate sampling vary, but the basic principle is similar. Each scan line of the color-box is examined with multiple sample volumes. Doppler signals are collected for short time-fractions to each sample size and at a fixed interval time concerning the number of samples.10–15 Return echoes along every line contain signals originating from either stationary or moving reflectors. An autocorrelation algorithm named phase-shift autocorrelation is used to quickly obtain Doppler information and compare the received with the signal transmitted.
In summary, echo signals deriving from moving reflectors and the different phases are temporarily stored and multiplied with the subsequent echo-signals. If the mirrors are stationary, the product of this multiplication is constant. On the contrary, if the reflector moves, like the RBCs in the vessels, the outcome varies over time. The differences are integrated and used to represent RBCs’ average speed. The autocorrelation process is repeated as often as packages of pulse color, number, and density of the color box’s scan lines. Color pixels are usually coarse due to the low spatial resolution of color Doppler. In this way, the vessel will appear blue or red-colored, depending on the flow direction. The chromatic scale does not provide any quantitative data but only qualitative information. It indicates the presence/absence of blood flow, describes the vessel course and allows the volume sample regulation to obtain a spectral curve. The primary colors’ desaturation (red to yellow, blue to light blue) represents the progressive increase of velocity as Doppler angle cosine. Higher flow rates appear whiter than lower flow rates.
PDI is an alternative CDI that does not estimate the RBCs velocities but the Doppler-shift amplitude within each point composing the color box. This image processing is known as power or energy mode. It does not vary significantly with the direction of the blood flow, so the PDI, unlike the CDI, is less dependent upon the insonation angle.
Spectral Doppler setting
PWD analyses the distribution of the RBCs velocities in a small gate placed into the vessel lumen. The sampling gate corresponds to a time interval, even though it is improperly called sample volume. The velocity (cm) or Doppler-shift (Hz) is represented in the y-axis, while in the x-axis is reported the time(s).12–15 The V/t curve inflections can be positive or negative (above or below the zero-crossing line) and represent the positive or negative Doppler shifts. The Doppler signal is simultaneously amplified and proposed as an audio signal that allows the operator to listen to “the sound” of the blood flow speed variations in the arteries and veins during systole and diastole. A high-frequency sound corresponds to a high-frequency waveform, and a low pitch sound corresponds to a low-frequency waveform.10–15 The best Doppler signals are obtained with angles close to 0°. The compromise is to use a 60° Doppler angle to standardize the error of velocity measurement. This method is not an absolute rule because <60° degree angles are certainly more accurate and precise. It is to remember that the percentage error in velocity calculation caused by a 5, 10, 15, and 20° in the Doppler angle is negligible or moderate at shallow angles, but it exponentially increases at higher grades.
Conclusion
Knowledge of theoretical outlines of sonography and instrumentation is fundamental to carry out an accurate B-Mode and Doppler examination of upper arm vessels and vascular access. In this review, the basics of physic, B-Mode, and Doppler applications are summarized to facilitate the ultrasound examination of vascular access and increase operators’ confidence and skills.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mario Meola  https://orcid.org/0000-0002-1330-2820
https://orcid.org/0000-0002-1330-2820
Jose Ibeas  https://orcid.org/0000-0002-1292-7271
https://orcid.org/0000-0002-1292-7271
Ilaria Petrucci  https://orcid.org/0000-0003-1931-1581
https://orcid.org/0000-0003-1931-1581
References
- 1. Mannaerts CK, Wildeboer RR, Remmers S, et al. Multiparametric ultrasound for prostate cancer detection and localization: correlation of B-mode, shear wave elastography and contrast enhanced ultrasound with radical prostatectomy specimens. J Urol 2019; 202: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 2. Harvey CJ, Pilcher JM, Eckersley RJ, et al. Advances in ultrasound. Clin Radiol 2002; 57: 157–177. [DOI] [PubMed] [Google Scholar]
- 3. Claudon M, Tranquart F, Evans DH, et al. Advances in ultrasound. Eur Radiol 2002; 12: 7–18. [DOI] [PubMed] [Google Scholar]
- 4. Lawrence JP. Physics and instrumentation of ultrasound. Crit Care Med 2007; 35: S314–S322. [DOI] [PubMed] [Google Scholar]
- 5. Meola M, PetruccI I. Ultrasound and color Doppler in nephrology. Physical and technical principles. G Ital Nefrol. 2012; 29: 81–91. [PubMed] [Google Scholar]
- 6. Meola M, Petrucci I, Bortolotto C, et al. Ultrasound and color Doppler in nephrology. Technology and applications. G Ital Nefrol 2012; 29: 210–223. [PubMed] [Google Scholar]
- 7. Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology 2010; 257: 24–39. [DOI] [PubMed] [Google Scholar]
- 8. Piscaglia F, Nolsøe C, Dietrich F, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012; 33: 33–59.62. [DOI] [PubMed] [Google Scholar]
- 9. Garra BS. Elastography: history, principles, and technique comparison. Abdom Imaging 2015; 40: 680–697. [DOI] [PubMed] [Google Scholar]
- 10. Meola M. Ecografia clinica in Nefrologia. Vol. I. Eureka Editore Lucca, 2015, pp.3–145. [Google Scholar]
- 11. White DN. Johan Christian Doppler and his effect - a brief history. Ultrasound Med Biol 1982; 8: 583–591. [DOI] [PubMed] [Google Scholar]
- 12. Hedrick WR, Hykes DL, Starchman DE. Chapter 7. Doppler spectral analysis. In: Hedrick WR, Hykes DL, Starchman DE. (eds) Ultrasound physics and instrumentation. 4th ed. St Louis, MO: Elsevier-Mosby, 2005, pp.221–237. [Google Scholar]
- 13. Kremkau FW. Chapter 4. Imaging instruments. In: FW Kremkau Diagnostic ultrasound – Principles and instruments. 7th ed. St Louis, MO: Saunders-Elseviers, 2006, pp.85–118. [Google Scholar]
- 14. Dauzat M. Chapter 1. Notions théoriques et thechnologiquesélémentaires.Bases de l’interprétation des signaux Doppler. In: Dauzat M, Laroche JP, De Bray JM, et al. (eds) Ultrasonographie vasculaire diagnostique. Theorie et pratique. 1st ed. Paris: Vigot, 1991, pp.3–35. [Google Scholar]
- 15. Powis RL. Color flow imaging. Radiographics 1994; 14: 415–428 [DOI] [PubMed] [Google Scholar]
- 16. Rubin JM. Power doppler. Section III: new imaging techniques. Technol Eur Radiol 1999; 9(Suppl. 3): S318–S322. [DOI] [PubMed] [Google Scholar]
- 17. Demené C, Deffieux C, Pernot M, et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and ultrasound sensitivity. IEEE Trans Med Imaging 34(11): 2271–2285. [DOI] [PubMed] [Google Scholar]
- 18. Narula J, Chandrashekhar Y, Braunwald E. Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and insonation. JAMA 2018; 3: 346–350. [DOI] [PubMed] [Google Scholar]
- 19. Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 2002; 225: 59–64. [DOI] [PubMed] [Google Scholar]
- 20. Wiese P, Nonnast-Daniel B. Colour Doppler ultrasound in dialysis access. Nephrol Dial Transplant 2004; 19: 1956–1963. [DOI] [PubMed] [Google Scholar]
- 21. Lomonte C, Casucci F, Antonelli M, et al. Is there a place for duplex screening of the brachial artery in the maturation of arteriovenous fistulas? Semin Dial 2005; 18: 243–246. [DOI] [PubMed] [Google Scholar]
- 22. Schberle W. Chapter 1. Fundamental principles. In: Schberle W. (ed.) Ultrasonography in vascular diagnosis. A therapy-oriented textbook and atlas. Berlino: Springer-Verlag, 2004, pp.5–30. [Google Scholar]
- 23. Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 2002; 62: 1109–1124. [DOI] [PubMed] [Google Scholar]
- 24. van Hooland S, Malik J. Hemodialysis vascular access ultrasonography: tips, tricks, pitfalls and a quiz. J Vasc Access 2010; 11: 255–262. [DOI] [PubMed] [Google Scholar]