Abstract
Arteriovenous fistula (AVF) complications are classified based on fistula outcomes. This review aims to update colour Doppler (CD) and pulse wave Doppler (PWD) roles in managing early and late complications of the native and prosthetic AVF. Vascular access (VA) failure occurs because inflow or outflow stenosis activates Wirchow’s triad inducing thrombosis. Therefore, the diagnosis of the tributary artery and outgoing vein stenosis will be the first topic considered. Post-implantation complications occur from the inability to achieve AVF maturation and dialysis suitability due to inflow/outflow stenosis. Late stenosis is usually a sequence of early defects repaired to maintain patency. Less frequently, in the mature AVF or graft, complications are acquired ‘de novo’. They derive either from incorrect management of vascular access (haematoma, pseudoaneurysm, prosthesis infection) or wall pathologies (aneurysm, myxoid valve degeneration, kinking, coiling, abnormal dilation from defects of elastic structures). High-resolution transducers (10–20 MHz) allow the characterization of the wall damage, haemodynamic dysfunctions, early and late complications even if phlebography remains the gold standard for the diagnosis for its sensitivity and specificity.
Keywords: AVF complications, inflow/outflow stenosis, fistula aneurysm, pseudoaneurysm, vascular access thrombosis, doppler imaging
Introduction
Based on the outcomes, AVF complications can be classified as early and late events. Primary patency is the most favourable clinical outcome in which the AVF provides an adequate flow for haemodialysis without any intervention.1,2 Assisted primary patency is the time interval between VA creation and the first occlusion (thrombosis-free VA survival) or patency measurement, including operative/endovascular interventions to maintain the VA. Secondary patency is the access survival until abandonment, that is, the time interval from access placement and abandonment, including all interventions to re-establish the haemodialysis suitability. Immediate failure of AVF occurs when the blood flow rate in the fistula circuit is inadequate due to poor condition of the vessels or surgical errors. Primary failure is the permanent AVF failure before it is suitable for haemodialysis. It occurs within 3 months of AVF implantation due to insufficient inflow or inappropriate remodelling of the incoming artery or outgoing vein. Secondary failure is the permanent failure and subsequent abandonment of an AVF that has reached dialysis suitability criteria.3,4
Early failure derives both from pre-existing or acquired anatomical anomalies and haemodynamic dysfunction after AVF implantation. Inflow defects are related to arterial wall quality (thinness, age-related pathologies such as atherosclerosis, diabetes, chronic kidney disease).5,6 Outflow defects are mainly related to pre-existing venous anomalies (thin or sclerotic veins, thrombosis due to phlebotomies or infusions, PICC position, incorrect use of accessory collateral veins for the AVF creation) and swing-segment lesions.7–11 Instead, iuxtaanastomotic and venous graft stenosis is due to vessel remodelling caused by the variation of wall shear stress (WSS) after access placement. The endothelium dysfunction controls inward and outward vessel remodelling and adaptative neointimal hyperplasia.12–14
Placement of an artificial arterio-venous anastomosis induces a sudden flow/pressure by-pass from high pressure and resistive vascular bed (tributary artery) to capacitance system at low pressure/resistance (draining vein). Furthermore, there is often a significant heterogeneity in the three-dimensional geometry of the swing segment. Arterial nonplanarity and curvature accelerate the iuxtaanastomotic hyperplasia. 15 A complex cascade of adaptive responses is activated by the endothelium and smooth muscle to obtain the vessel remodelling (artery and vein dilation, vein arterialization) and recover the basal WSS values. Active pathways inducing the neointimal hyperplasia are oxidative stress, activation of peroxynitrite and matrix metalloproteinases (MMP-2 and MMP-9), and the release of inflammatory cytokines (monocyte chemoattractant protein – MCP-1).16,17
In the last years, the attempt of Fistula First Breakthrough Initiative (FFBI) to create more native AFVs coincided with a substantial increase in the early failure rate from 10% to 25% in ‘70-the ‘80s to values of 20%–60% in the most recent series18,19 (Table 1). These data suggest that native AVF is not necessarily a ‘panacea’. It is the more appropriate procedure but imposes the knowledge of pre-existing or postoperative risk factors that prevent maturation and worsen vascular access’s primary outcomes.12–16
Late failure occurs after the VA has met dialysis suitability. This event recognizes the same primary causes of early failure, that is, reducing vein outflow or arterial inflow favouring stasis and thrombosis.
This paper focuses on the role of ultrasound and Doppler modalities in diagnosing VA complications. Early and late VA complications will be considered based on the conceptual model of inflow and outflow stenosis.
Stenosis
The term stenosis derives from the Greek ‘στένωσις’, which means ‘narrowing’. In haemodynamic, this term defines vessel lumen narrowing resulting from a parietal atherosclerotic plaque, or neointimal hyperplasia or obstructive thrombus. The stenosis percentage is measured either as a reduction in the linear diameter or a reduction in the cross-sectional area (Figure 1). The stenosis becomes critical or severe when vessel narrowing results in a significant drop in the downstream flow volume. Critical stenosis may or may not reduce resting blood flow depending on the organ’s autoregulation, volemia and collaterals’ development, reducing the smaller vessels’ overall resistance.
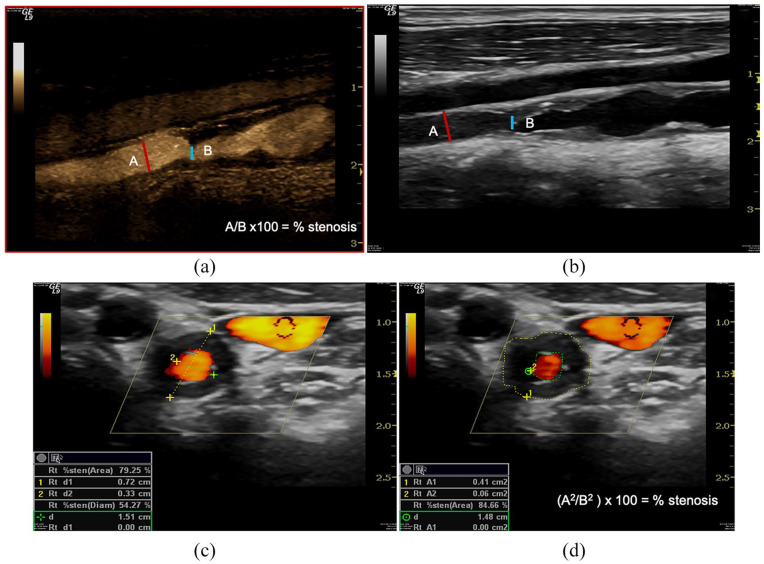
Figure 1.
Geometric stenosis. B-Mode evaluates reduction of the vessel lumen for a atheromatous plaque either as a percentage reduction in the linear diameter (A/B × 100 = % stenosis) (a, b), or as a percentage reduction in the cross-sectional area ((A2/B2) × 100 = % stenosis)) calculated from the diameter values (c) or cross-sectional areas (d). Angiography defines ‘critical’ as a reduction in diameter >50%.
Three different types of venous stenosis are described in the native AFVs.
Iuxtaanastomotic stenosis affects the first 2 cm of draining vein downstream of the anastomosis and represents 80% of all venous stenosis. This stenosis due to reactive neointimal hyperplasia leads to early failure. In mature AVF, it represents a progression of initial stenosis. Narrowing in this variant is tenacious, so long-term percutaneous transluminal angioplasty results (PTA) are not encouraging; even using drug-eluting balloon angioplasty and repair often requires a surgical revision (Figure 2).
Anastomotic breach stenosis is caused either by reactive neointimal hyperplasia or surgical errors. It is the most common cause of inflow stenosis, defect of maturation and AVF early failure. In native AFVs, 40% of accesses show inflow stenosis and coexisting lesion on the venous side (Figure 3).6,22
Stenosis of the venepuncture segment is a typical complication of a mature fistula. It can be either short in length or long/multiple. Short stenosis represents a late complication of pre-implantation phlebotomies and is localized between the two-venepuncture points. Vice versa, the multiple or extended stenosis represents the fibrotic response of the vein to repeated cannulation. Angioplasty is the first-choice treatment for this stenosis. However, the vessel’s elastic recoil may require surgical correction with patches or by-pass. 21
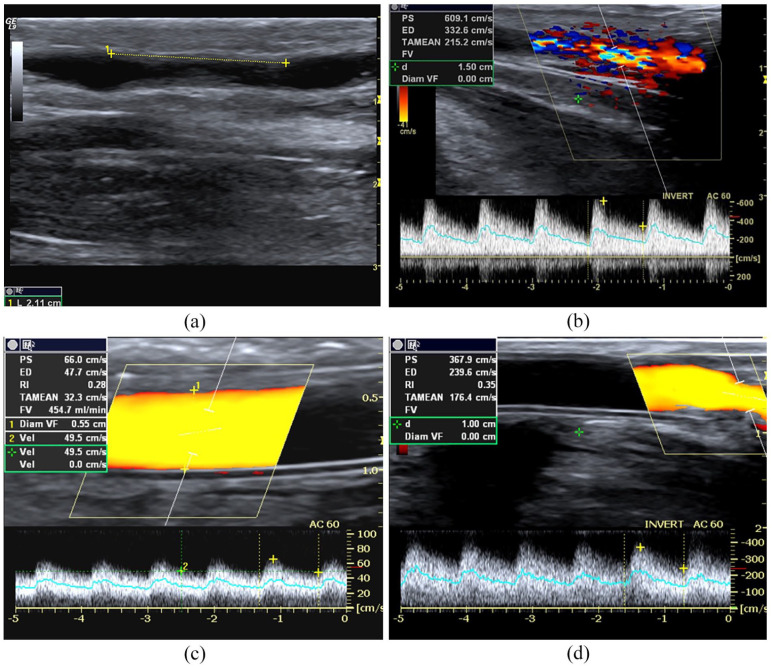
Figure 2.
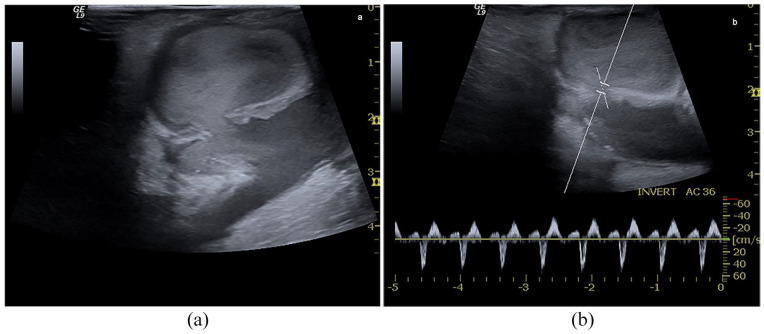
Outflow stenosis. (a) B-Mode image of an iuxta-anastomotic tract of a mature distal radio-cephalic fistula. Stenosis is 2.12 mm in length and derives from reactive neointimal hyperplasia is represented in the image by the mid-level parietal echoes, (b) PWD sampling of the same fistula. Aliasing and bruit colour are evident at CD, while the spectral curve shows PSV> 350 cm/s, DV >180 cm/s, spectral broadening and aliasing, (c) Power Doppler sampling of a brachio-cephalic AVG. The graft flow rate is reduced (450 mL/min) because stenosis is located at the venous anastomosis level (PSV 370 cm/s, DV 240 cm/s) (d).
Figure 3.
Inflow stenosis. (a) anastomotic breach CD sampling of a 1-month native side-to-end radio-cephalic fistula, (b) brachial flow rate calculation = 284 mL/min, and (c) spectral analysis at the level of breach anastomosis reveals severe stenosis: PSV > 400 cm/s, EDV 280 cm/s, spectral broadening, wall-thump, bruit colour and aliasing (Doppler angle = 28°).
In prosthetic grafts, inflow stenosis occurs in about 30% of cases. This complication often coexists with stenosis on the venous side that is more frequent and severe (Figure 2). Inflow stenosis as anastomosis stenosis is due to reactive neointimal hyperplasia induced by surgery, endothelial damage, different compliance between the prosthesis and native vein, and WSS variation.
Tributary arterial stenosis is rare and occurs in elderly patients when atherosclerotic degeneration of the wall prevents dilation and remodelling of the arterial lumen transforming non-critical defect into critical stenosis of inflow (Figure 4).6,20,21
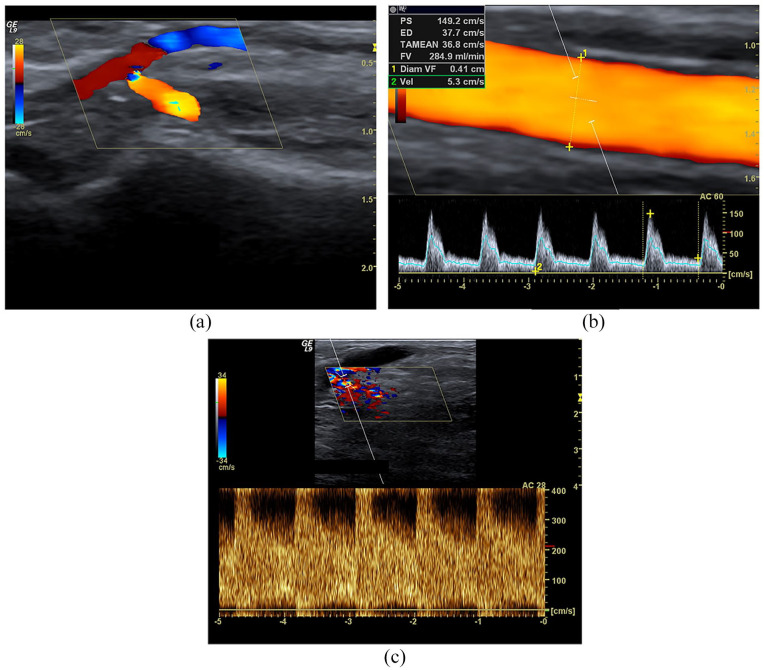
Figure 4.
Tributary artery stenosis. Male, 78 years old with maturation defect of distal radio-cephalic fistula 24 days after implantation: (a) Spectral Doppler highlights segmental stenosis of the tributary artery. Colour Doppler sampling shows aliasing and colour bruit; spectral analysis shows an explosive increase of PSV (>300 cm/s), and EDV at the level of stenosis (Doppler angle 36°), spectral broadening and aliasing; (b) downstream PSV is 130 cm/s and the peak-velocity ratio is 2 compared to the stenosis. (c) Upstream, the peak-velocity ratio is >2.5, but the spectral amplitude is elevated. Note that the Doppler angle is at 36°, so not perfectly aligned with the vessel’s longitudinal axis; this technical mistake underestimated the PSV.
Many factors influence the severity of mature AFVs and grafts stenosis. The stenotic segment’s length is essential because Poisieulle’s law states that the flow is inversely proportional to the vessel’s length. Also, the sites and diameter of stenosis, vein plasticity, inflow/outflow coexisting defects and haemodynamic factors such as cardiac output, blood pressure, aneurysms and geometric anomalies can influence a long time the AVF functioning. 20
Angiography defines as ‘critical’ a reduction in luminal diameter >50% judged by comparison with either adjacent vessel or graft. Morphological stenosis must be accompanied by haemodynamic or clinical abnormalities such as elevated BUN values recirculation (>10% when measured with the two-needle urea-based method, elevated values of static and dynamic venous pressure obtained at the last dialysis session, increase of negative arterial pre-pump pressures, and blood flow rate reduction and significant limb oedema.6,21,22
Ultrasound criteria of critical stenosis
High-resolution sonography defines the severity of the stenosis based on different criteria.
(1) Morphological or geometric stenosis. Artery stenosis is ‘critical’ when narrowing causes a significant reduction of downstream flow rate. It happens when a 60%–75% reduction of the artery diameter occurs. Vein stenosis is critical if the diameter in the narrowing point is <2–3 mm both on the short and long-axis, or the lumen shows a significant (⩾50%) reduction in transverse diameter compared to the mean value of the lumen measured downstream at the point where the vessel shows a uniform calibre.21–23
There are two main limitations of geometric criteria. The first one is the unreliability of measurements in a mature AVF when the draining vein shows a sequence of narrowing and dilation due to continuous cannulation. The second arises from the transducer’s spatial resolution. Generally, the low-frequency transducers (7.5 MHz) cannot highlight neointimal hyperplasia or valve apparatus in B-Mode.
(2) Haemodynamic criteria. These parameters derive from CD and PWD sampling. At CD, artefacts such as aliasing, bruit colour and colour variance are indirect signs of stenosis. At PWD, the peak systolic velocity (PSV) values are direct signs of stenosis when they are >200/250 cm/s in the tributary artery or >300–400 cm/s in the outgoing vein or graft.
- PSV-ratio between stenosis and normal vein. The ratio between the stenosis peak velocity and upstream peak velocity is an indirect parameter of trans-stenostic gradient pressure. In carotid or renal artery stenosis, a PSV ratio >4 or higher defines critical stenosis with an accuracy of 90%.21,22 In the graft, many authors consider a correct PSV ratio >2. 23
- Resistive indices or peripheral resistance indices. The Resistive index (RI) is calculated as (PSV) – Diastolic velocity (DV)/PSV and its normal range varies between 0.50 and 0.70; the systole-diastolic ratio (S/D) is the ratio between the maximum and minimum peak velocity. The increase of these parameters suggests a significant increase of resistance due to a stenosis inducing a significant reduction of the diastolic flow rate.
Direct as indirect haemodynamic criteria are reliable24–27 but not accurate if changes in the brachial artery’s flow rate are not considered.
4) Brachial artery flow rate. The typical values of a mature and well-functioning fistula are >600 mL/min. The absolute reduction of volume flow rate <500 mL/min or a reduction of >25% in the last month (if the blood flow rate is <1000 mL/min) is a pivotal sign of stenosis.25,27
Arterial stenosis diagnosis is simple because it is based on the encoded criteria (PSV >250 cm/s, spectral dispersion, doubling PSV compared to upstream). Instead, the stenosis diagnosis of outgoing vein must necessarily combine morphologic and functional criteria. 27 For example, a segmental increase of PSV 300–400 cm/s does not surely mean significant stenosis if it is not associated with a flow rate reduction (Qb < 600 mL/min, or a decrease >25% from previous measurements). Both criteria are necessary to distinguish true stenosis from borderline stenosis. If the brachial artery’s flow rate is normal, the increase in PSV aims to support the continuity of blood flow rate. PSV becomes indicative of critical stenosis when the flow rate drops. From a haemodynamically point of view, this event means that the stenosis is becoming critical. 27
The diagnostic accuracy of CD-PWD compared with angiography for detection of >50% diameter-reducing stenosis is approximately 80%13,28 with a pooled estimated sensitivity of 91% and a positive predictive value of 98%.6,29
Haemodynamic of a stenosis
Arterial or vein stenosis is a resistance in series along with the inflow or outflow of AVF. It may or may not cause a fall of downstream flow based on its haemodynamic severity. In any case, the spectral analysis registers acceleration at the level of narrowing point. For the law of flow continuity, this acceleration can have different haemodynamic significance. If the stenosis is not critical, the increase in PSV is not accompanied by a significant fall in the flow rate.
On the other hand, if systole-diastolic acceleration is associated with a fall in the flow rate, this means the stenosis is critical and, therefore, a repair is necessary.
The need for an intervention is even more definite if haemodynamic or clinical abnormalities are present. Therefore, the diagnosis of ‘critical’ stenosis requires the use of more criteria (vessel morphology, spectral velocity, flow rate, clinical abnormalities). These criteria have been partly validated with conventional or subtraction angiography. Still, the data evaluable refers to a limited number of controlled studies, generally with small sizes.26–31 Randomized and controlled trials are necessary to validate US/PWD diagnostic parameters and allow this test’s systematic use as surveillance for the native and prosthetic AFVs.
Maturation defects and early failure
K-DOQI guidelines establish the criteria of dialysis suitability (six rule) of VA: vein diameter ⩾ 6 mm, blood flow rate ⩾600 mL/min, vessel depth <0.6 cm and segment length for venepuncture >6 cm.32,33 In the FFBI trial, a flow rate variable from 300 to 800 mL/min measured on the outgoing vein’s first linear tract is considered normal. 34 The Haemodialysis Fistula Maturation Study Group defines haemodialysis’s suitability as a condition where the vein’s minimum diameter is ⩾4 mm and/or the blood flow rate is ⩾500 mL/min. When both these criteria are satisfied, the probability of good suitability is >95% of cases. When none of these criteria are reached, the dialysis suitability is only 33%. 34
In primary failure, CD/PWD sampling reveals an obstructive thrombosis of the anastomosis and efferent vein without perfusional signal, whereas the tributary artery keeps a high resistance triphasic spectral pattern.
The radial artery stenosis can become evident during the maturation period of a distal radio cephalic AVF when blood flow demand increases for the resistances’ drop. Thus, the stress tests (reactive hyperaemia, Allen test) must accurately evaluate the radial artery’s flow rate variations before AVF creation.
An explosive acceleration (PSV > 2.5–3.5 m/s) in the peri-anastomotic segment during the maturation period reveals an inflow or outflow stenosis if the brachial artery flow rate does not increase. Collateral vein development in this phase and an inadequate outgoing vein dilatation reveals outflow stenosis.
Late failure occurs after considerable time from the first cannulation but often derives from no-critical stenosis already evident during the maturation period and reducing the outflow progressively.
Early and late thrombosis
Early thrombosis is a postoperative complication and occurs in the first 24 h or the first week after fistula implantation. Technical mistakes, hypotension, hypercoagulability, kinking and torsion of the vein, compression due to a postoperative haematoma, prolonged vascular spasm and involuntary compression of the anastomosis during the night are the most common causes. Thrombosis concerns the anastomosis chamber and the draining vein. The artery is rarely involved unless atherosclerotic, iatrogenic or inflammatory parietal damage favours an inflow defect (Figure 5).
Figure 5.
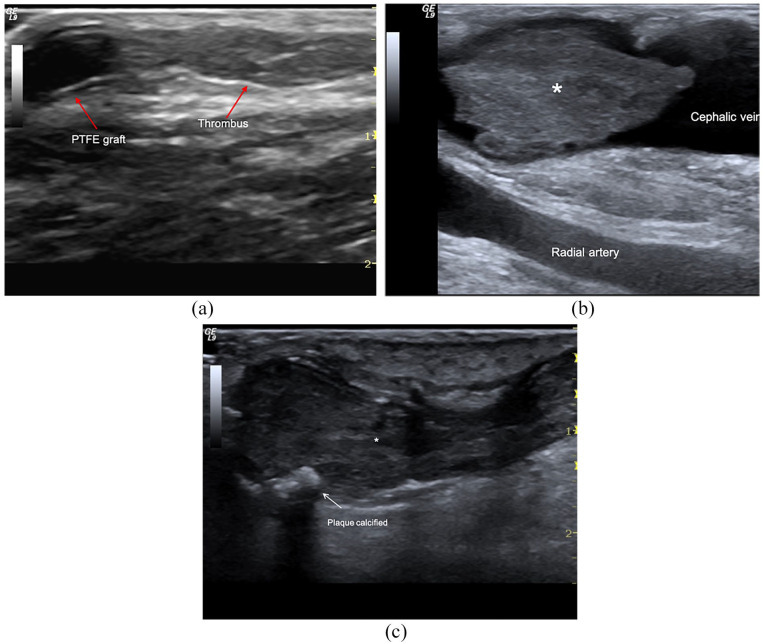
AVF thrombosis: (a) Brachio-cephalic graft. Occlusive clot (*) in the cephalic vein, (b) distal radio-cephalic fistula. Acute non-occlusive thrombosis of anastomosis and outgoing vein, and (c) acute obstructive thrombosis (<24 h) (*) of cephalic vein in mature AVF. Wall calcifications (arrows) are also evident.
The vein or the prosthesis appears obstructed by a hypo-hyperechoic material. The thrombus may be occlusive if the clot obstructs the blood flow completely or non-occlusive if around the clot is still evident a flow at CD. Acute and occlusive thrombosis is revealed by acute pain and the disappearance of thrill. It is an emergency and requires immediate percutaneous declotting in order not to lose vascular access.
Chronic thrombosis is an old clot that is over 1–2 months. The clot becomes harder and scars the vein. As a result of this process, the vein becomes much smaller and does not allow blood to flow freely through the vessel. The spectral curve is absent in the obstructed vein. When the obstruction is complete, the tributary artery’s spectral trace shows low diastolic velocities, up to take a high resistance triphasic pattern. The Doppler signs are crucial for referring the patient to immediate thrombolysis and remove the obstruction (Figure 6).
Thrombosis is the most common cause of vascular access failure in mature AVF. In 75% of cases, it is associated with critical stenosis on the venous side. Many factors contribute to determining the thrombosis, as the excessive compression of the AVF after the dialysis or during sleep, persistent hypotension, transient hypovolemia or subcritical arterial stenosis. The 9% of patients with recurrent thrombosis episodes show congenital thrombophilia (homo- or heterozygosis for factor V Leiden, levels of ATIII below 60% of normal values, proteins C and S deficiency).
In 77% of cases, anomalies in coagulation, such as high levels of factor VIII, fibrinogen, D-dimer, homocysteine, reduction in thrombin clotting time and presence of antiphospholipid antibodies, are due to chronic kidney disease. 35 About 50% of prosthetic fistulae with venous stenosis develop thrombosis. The leading causes of abnormal coagulation in prosthetic AFVs are antiphospholipid antibodies and abnormal levels of protein C, S and ATIII. 35
The CD/PWD is the most accurate method to diagnose thrombosis in the native or prosthetic access (sensitivity around 100%). Direct and indirect signs reveal the diagnosis. The vein appears full of solid material with low/medium-level echogenicity. Transducer compression does not cause collapsing, and the vein does not respond to dynamic manoeuvres (breathing, Valsalva manoeuvre) with flow variations. A typical indirect sign of obstructive thrombosis is a persistent high resistance triphasic trace in the fistula’s tributary artery.
CD helps establish thrombus extension because, in acute thrombosis, the clot is hypo- or anechoic like the blood. In long-term thrombosis, the clot becomes hyperechoic and calcified, and it is easily detected.
Aneurysms, haematomas, pseudoaneurysms, seroma
The outgoing vein’s wall injuries due to repeated cannulation can cause an aneurysm, haematoma and pseudoaneurysm formation. The aneurysm is a fusiform or saccular dilation of the vessel involving all three layers of its wall. Haematoma and pseudoaneurysm are instead post-traumatic blood collections. The haematoma is located outside the vessel and does not have any continuity with the lumen. Pseudo-aneurysm or false aneurysm is a perivascular collection; unlike the haematoma, it keeps continuity with the vessel lumen through a breach or collar, and unlike the aneurysm, it has no own wall. Still, it is delimited by reactive fibrous tissue covered by endothelium. 36 The risk of rupture is higher than that of a true aneurysm of comparable size due to inadequate support of the saccular wall. Thus, false aneurysms generally require treatment. The aneurysm, haematoma and pseudoaneurysm can involve the draining vein, prosthesis and, rarely, tributary artery. The favouring causes are anticoagulant drugs, repeated buttonhole punctures (particularly in prostheses), blind venepuncture, and venous hypertension due to venous stenosis. 36
Seroma is an unusual complication of prosthetic arteriovenous graft. It occurs in 2%–4% of cases within the first month after the graft’s placement for the transudation of sterile and clear serum from connective tissue surrounding the prosthesis. It remains confined as a collection by a non-secretory fibrous pseudo-membrane. Seroma is typically located close to arterial anastomosis. 37
Aneurysm and pseudoaneurysm appear as pulsatile mass at the physical examination, whereas haematoma and seroma are non-pulsatile. CD sampling allows for the immediate differential diagnosis between aneurysm and pseudoaneurysm versus the haematoma and seroma. When the mass progressively grows and the diagnosis is late, the skin covering the mass appears thin, translucent, reddened and can ulcerate. Rarely the pseudoaneurysm excludes itself from the circulation because of a spontaneous thrombosis. More often, like the aneurysm, it increases due to Laplace’s law and tends to rupture; it rarely irritates the median nerve or vein thrombosis by extrinsic compression. 37
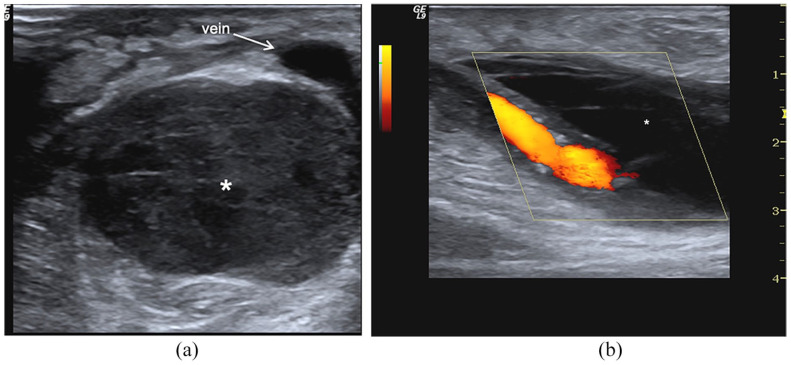
The CD/PWD with high-resolution transducers (7.5–20 MHz) is the first-choice imaging technique and allows the immediate differential diagnosis. Perivascular haematomas appear as a heterogeneous collection where debris and fibrin branches surround hypoechoic and corpuscular lacunae. Haematoma edges are usually undefined. The collection extends among the muscle bundles and subcutaneous tissue as a diffuse suffusion (Figure 7).
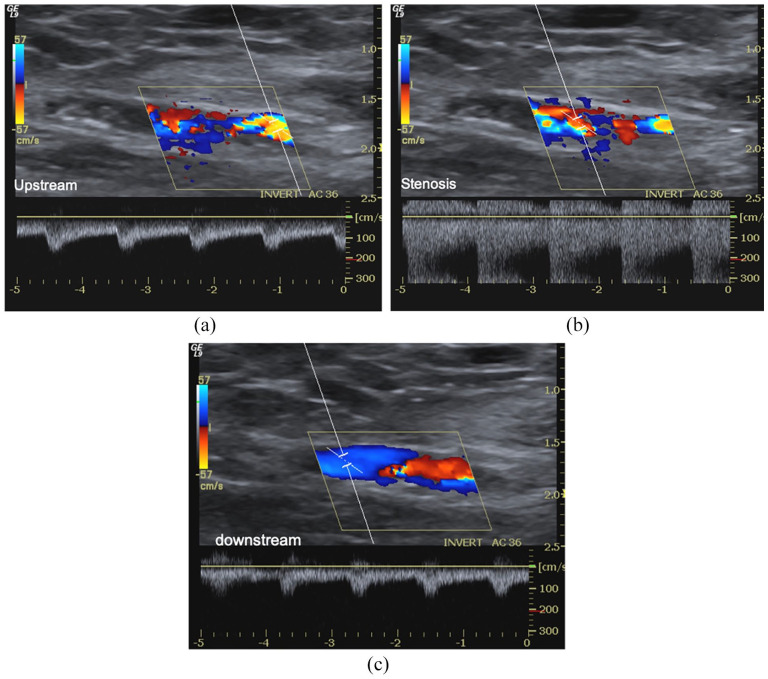
Figure 6.
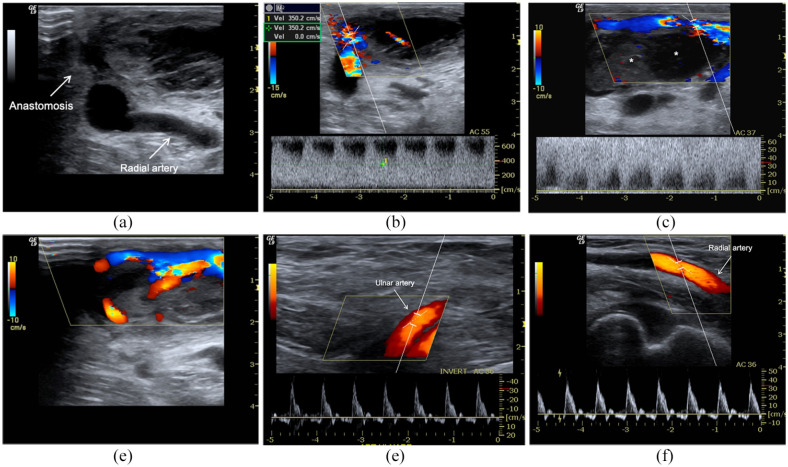
Early failure of radiocephalic AVF for inflow stenosis and thrombosis. AVF malfunction appears in the first HD session. (a) B-Mode, CD and PWD sampling, (b) highlight a sub-occlusive thrombosis (*) of the venous effluent favoured by severe inflow stenosis (aliasing, PSV > 600 cm/s, Doppler angle 55°). There is a thin jet between the thrombus and vein. The spectral trace of radial, (d) and ulnar artery reveals a high resistance velocity/time curve due to complete obstruction of anastomosis. The patient carried out percutaneous declotting even if the stenosis was not critical because the brachial artery flow rate was still >650 mL/min. (d) Distal mature AVF. Acute and obstructive thrombosis (<24 h) (*) of the cephalic vein. At B-Mode, there are also wall calcifications (arrows). Spectral trace of ulnar (e) radial (f) arteries reveals a high resistance velocity/time curve due to complete obstruction of anastomosis.
Figure 7.
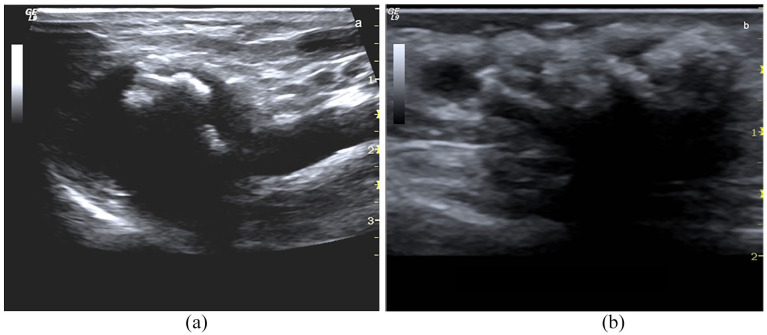
Haematoma: (a) At B-Mode, perivascular haematoma appears as a complex hypoechoic and corpuscolated fluid collection with debris and fibrin striae (*), (b) at CD sampling, it is not perfused (*) and surrounds the draining vein.
At the B-Mode, seroma appears as a simple anechoic collection, close to the arterial anastomosis, without debris or necrotic materials and colour signals. Ultrasound-guided fine-needle aspiration is necessary to clarify the diagnosis.
Consecutive aspirations are useful for the treatment, but they favour infection, skin necrosis, graft thrombosis and vascular access loss. Surgery draining and wrapping of graft by using microfibrillar collagen needs rarely. 36
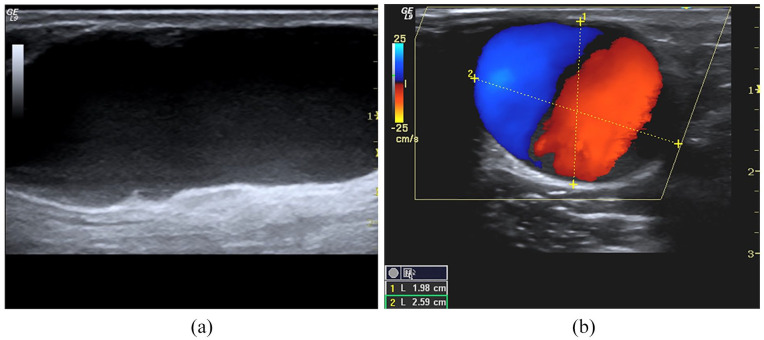
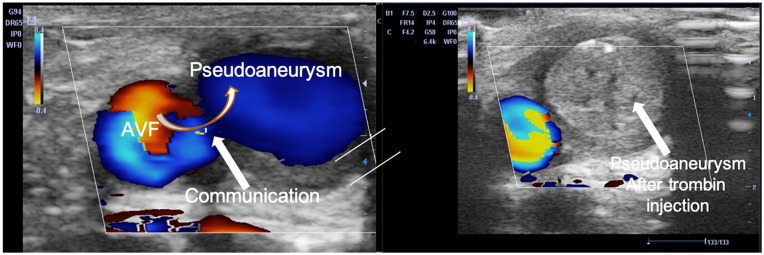
Usually, pseudoaneurysm appears as saccular dilatation close to the draining vein wall, while true aneurysm appears as a fusiform or saccular dilation of the vessel. Blood flow in the aneurysm cavity describes a circular vortex similar to a cloud of cigarette smoke, well evident in the B-Mode image due to the slowing of the blood flow. A giant red-blue vortex, named the ‘Korean flag’ sign, is evident at CD/PD sampling. In the pseudoaneurysm, a circular swirling may be seen on colour flow (yin-yang sign) (Figure 8). While a ‘to and fro’ spectral pattern may be registered with spectral Doppler in the collar’s correspondence. The blood enters the cavity and returns to the circulatory stream through the breach generating a large swirling, which draws a sizeable red-blue vortex in the colour sonogram. At the collar level, the flow generates aliasing. The artefact persists, even increasing the PRF values. It draws a characteristic iso-diphasic spectral trace (‘to and fro’ pattern) due to back-and-forth flow (Figure 9). Suffusion towards subcutaneous tissue as thickness reduction of superficial tissue is risk-signs of rupture. CT angiography and angiography are only needed to plan surgery or perform an endovascular procedure 37 . In many cases, the surgery can be planned without CT angiography if the CD/PWD is done comprehensively for the surgeon, and the surgeon has trust in the expertise of the operator.
Figure 8.
Aneurysm: (a) B-Mode long-axis scans of the cephalic vein. The true aneurysm appears as a fusiform or saccular vein dilation, (b) short axis of the vein. A large red-blue vortex similar to the ‘Korean flag’ is evident at CD/PD sampling in the aneurysmal sac.
Figure 9.
Arterial pseudoaneurysm: (a) B-Mode scans. A circular swirling in a brachial artery pseudoaneurysm is evident. The spontaneous blood echogenicity is due to the slowdown of blood in the pseudoaneurysm. At CD, the recirculation of blood draws the ‘yin-yang’ sign, (b) spectral Doppler in correspondence of the collar, shows a ‘to and fro’ pattern (isodiphasic deflection).
Aneurysm and pseudoaneurysm treatment vary according to the location, size and nature of the vessel (vein/artery) at the time of diagnosis. In particular, the effluent vein’s small pseudoaneurysms may not be treated and undergo clinical surveillance if they are stable in dimensions. Conversely, recent and not large pseudoaneurysm can be treated with the breach’s compression for 30–45 min using the ultrasound probe. Percutaneous injection of bovine thrombin (100–1500 IU) could be useful to promote a rapid thrombosis and closure of the pseudo-aneurysmatic cavity (Figure 10). 38 Thrombosis of the main vessel and allergic reactions are possible complications of the procedure. Regardless of volume, arterial pseudoaneurysms tend to increase due to the pressure levels and must always be treated surgically. The rapidity of growth increases the risk of rupture, the onset of infections and skin lesions.
Figure 10.
Venous pseudoaneurysm. A fresh and not very large pseudo-aneurysm can be treated with compression of the breach using the transducer. Ultrasound-guided percutaneous injection of bovine thrombin (100–1500 IU) promotes a rapid thrombosis of the pseudo-aneurysm.
Vascular surgery is the elective approach for treating the aneurysms and pseudoaneurysms of the tributary artery, prosthetic graft, or anastomosis, particularly if skin complications are present. 38 The vascular segment involved is replaced with a quick-use prosthetic bridge to avoid CVC implantation 35 . The stent-graft temporary placement should only be considered when it is evident an ongoing bleeding or a high risk of rupture pending surgical correction.
Infection
Since clinical diagnostic criteria of infection in the prosthesis are clear, ultrasound has little or no importance in this case. Therefore, it is mainly used to verify that the prosthesis is still open and there is no thrombosis. In graft, infection determines the appearance of complex and non-homogeneous material around the prosthesis, similar to haematoma. The differential diagnosis between a haematoma and a collection of pus is based on the clinical context and the physical evidence of tissue infection, that is, redness, pain and fever. This occurrence generally means that the AVF needs to be revised and the prosthesis removed and replaced completely. 39
Rare complications
In long-lasting AFVs, shear stress and high-flow rate favour vein wall fibrosis, myxoid degeneration of the valve systems causing ectasia’s appearance, kinking and coiling of the efferent vessel. These complications are particularly severe in patients with marfanoid habitus.
Persistent mechanical stress also promotes atherosclerotic calcifications at the level of the anastomotic chamber and venous effluent (Figure 11). When calcifications are severe and associated with an aneurysm or vessel kinking, they impose a surgical revision of the AVF.
Figure 11.
Calcifications: (a) Well-functioning long-term AVF with coarse calcifications of the anastomotic chamber, (b) chronic calcified thrombus in a non-functioning fistula.
Cephalic arch stenosis is a rare complication of radiocephalic fistula, but it is a common cause of brachiocephalic fistulas failure for the difficulty to treat with conventional angioplasty. A large part of brachiocephalic fistulas remodels into giant aneurysmatic fistulas due to high inflow pressure, causing circuit remodelling. The sharp turn of the cephalic arch, wall thickening and intimal hyperplasia due to abnormal wall shear stress induced by the high-flow regimen compared to the normal condition can partly explain the high occurrence of cephalic arch critical stenosis.40,41 Contributing factors are the myxoid degeneration of the venous valve apparatus and the vessel’s impingement in the clavipectoral fascia, and the deltopectoral groove’s structures. This syndrome’s clinical findings are venous hypertension, and development of collateral veins in the shoulder, prolonged bleeding, inadequate dialysis and rarely compartment syndrome. Angioplasty alone is the primary treatment option, but its effectiveness is suboptimal and brief.40,41 Recently, bare-metal stent placement and stent-graft have been proposed with variable results. 40
All these complications can be easily diagnosed by physical examination. The role of the US/PWD imaging is, as usual, to integrate clinical diagnosis before performing angiography.
Conclusions
In conclusion, US/PWD is the first-line imaging technique in diagnosing and working up early and late AFVs complications. Although angiography is the gold standard for vascular access complications, US/PWD reliability is very high, using a high-resolution transducer and high-sensitivity PWD. It provides useful information on the morphology and the function of vascular access. Furthermore, it can be used in the nephrology point of care of ultrasound and bedside whenever imaging is needed to integrate the physical examination or validate a complication’s clinical suspicion.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mario Meola  https://orcid.org/0000-0002-1330-2820
https://orcid.org/0000-0002-1330-2820
Antonio Marciello  https://orcid.org/0000-0003-2805-6842
https://orcid.org/0000-0003-2805-6842
Ilaria Petrucci  https://orcid.org/0000-0003-1931-1581
https://orcid.org/0000-0003-1931-1581
References
- 1. Gallieni M, Hollenbeck M, Inston N, et al. Clinical practice guideline on peri- and postoperative care of arteriovenous fistulas and grafts for haemodialysis in adults. Nephrol Dial Transplant 2019; 34(Supplement 2): ii1–ii42. [DOI] [PubMed] [Google Scholar]
- 2. Schmidli J, Widmer MK, Basile C, et al. Editor’s choice - vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 757–818. [DOI] [PubMed] [Google Scholar]
- 3. Schinstock CA, Albright RC, Williams AW, et al. Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol 2011; 6:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGrogan D, Al Shakarchi J, Khawaja A, et al. Arteriovenous fistula outcomes in the elderly. J Vasc Surg 2015; 62:1652–1657. [DOI] [PubMed] [Google Scholar]
- 5. Roy-Chaudhury P, Spergel LM, Besarab A, et al. Biology of arteriovenous fistula failure. J Nephrol 2007; 20:150–163. [PubMed] [Google Scholar]
- 6. Asif A, Gadalean FN, Merrill D, et al. Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int 2005; 67: 1986–1992. [DOI] [PubMed] [Google Scholar]
- 7. Kanterman RY, Vesely TM, Pilgram TK, et al. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology 1995; 195: 135–139. [DOI] [PubMed] [Google Scholar]
- 8. Allon M, Litovsky S, Young CJ, et al. Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 2011; 58: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee T, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 2011; 26: 2264–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tabbara M, Duque JC, Martinez L, et al. Pre-existing and postoperative intimal hyperplasia and arteriovenous fistula outcomes. Am J Kidney Dis 2016; 68: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alpers CE, Imrey PB, Hudkins KL, et al. The Hemodialysis Fistula Maturation Study Group: histopathology of veins obtained at hemodialysis arteriovenous fistula creation surgery. J Am Soc Nephrol 2017; 28: 3076–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 2006; 17: 1112–1127. [DOI] [PubMed] [Google Scholar]
- 13. Brahmbhatt A, Remuzzi, Franzoni M, et al. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int 2016; 89: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baeyens N, Schwartz MA. Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell 2016; 2(7): 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corbett RW, Grechy L, Iori F, et al. Heterogeneity in the nonplanarity and arterial curvature of arteriovenous fistulas in vivo. J Vasc Surg 2018; 68(6S): 152S–163S. [DOI] [PubMed] [Google Scholar]
- 16. Lee T. Novel paradigms for dialysis vascular access: downstream vascular biology–is there a final common pathway? Clin J Am Soc Nephrol 2013; 8: 2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee T. Fistula first initiative: historical impact on vascular access practice patterns and influence on future vascular access care. Cardiovasc Eng Technol 2017; 8: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lok CE. Fistula first initiative: advantages and pitfalls. Clin J Am Soc Nephrol 2007; 2: 1043–1045. [DOI] [PubMed] [Google Scholar]
- 19. Dember GJ, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008; 299: 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quencer KB, Arici M. Arteriovenous fistulas and their characteristic sites of stenosis. AJR Am J Roentgenol 2015; 205: 726–734. [DOI] [PubMed] [Google Scholar]
- 21. Fahrtash F, Kairaitis L, Gruenewald S, et al. Defining a significant stenosis in an autologous radio-cephalic arteriovenous fistula for hemodialysis. Semin Dial 2011; 24: 231–238. [DOI] [PubMed] [Google Scholar]
- 22. Moueta GL, Edwards JM, Chitwood RW, et al. Correlation of North American Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 90% internal carotid artery stenosis with duplex scanning. J Vasc Surg 1993; 17: 152–159. [DOI] [PubMed] [Google Scholar]
- 23. Zierler RE, Bergelin RO, Isaacson JA, et al. Natural history of atherosclerotic renal artery stenosis: a prospective study with duplex ultrasonography. J Vasc Surg 1994; 19: 250–258. [DOI] [PubMed] [Google Scholar]
- 24. Malik J, Slavikova M, Svobodova J, et al. Regular ultrasonographic screening significantly prolongs patency of PTFE grafts. Kidney Int 2005; 67: 1554–1558. [DOI] [PubMed] [Google Scholar]
- 25. Ibeas J, Roca-Tey R, Vallespin J, et al. Spanish clinical guidelines on vascular access for haemodialysis. Nefrologia 2017; 37(Suppl 1): 1–192. [DOI] [PubMed] [Google Scholar]
- 26. Bandyk DF. Interpretation of duplex ultrasound dialysis access testing. Sem Vasc Surg 2014; 6: 120–126. [DOI] [PubMed] [Google Scholar]
- 27. Lomonte C, Meola M, Petrucci I, et al. The key role of color Doppler ultrasound in the work-up of hemodialysis vascular access. Semin Dial 2015; 28: 211–215. [DOI] [PubMed] [Google Scholar]
- 28. Tordoir JH, de Bruin HG, Hoeneveld H, et al. Duplex ultrasound scanning in the assessment of arteriovenous fistulas created for hemodialysis access: comparison with digital subtraction angiography. J Vasc Surg 1989; 10: 122–128. [DOI] [PubMed] [Google Scholar]
- 29. Doelman C, Duijm LEM, Liem YS, et al. Stenosis detection in failing hemodialysis access fistulas and grafts: comparison of color Doppler ultrasonography, contrast-enhanced magnetic resonance angiography, and digital subtraction angiography. Vasc Surg 2005; 2: 739–746. [DOI] [PubMed] [Google Scholar]
- 30. Older RA, Gizienski TA, Wilkowski MJ, et al. Hemodialysis access stenosis: early detection with color Doppler US. Radiology 1998; 207: 161–164. [DOI] [PubMed] [Google Scholar]
- 31. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg 1998; 28: 869–875. [DOI] [PubMed] [Google Scholar]
- 32. Robbin ML, Chamberlain NE, Lockhart ME, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology 2002; 225: 59–64. [DOI] [PubMed] [Google Scholar]
- 33. Lok CE, Huber TS, Lee T, et al. ; KDOQI Vascular Access Guideline Work Group. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75(suppl 2): S1–S164. [DOI] [PubMed] [Google Scholar]
- 34. Robbin ML, Greene T, Allon M, et al. ; for Hemodialysis Fistula Maturation Study Group. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the hemodialysis fistula maturation study. J Am Soc Nephrol 2018; 29: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salmela B, Hartman J, Peltonen S, et al. Thrombophilia and arteriovenous fistula survival in ESRD. Clin J Am Soc Nephrol 2013; 8: 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Padberg FT, Kalligaro KD, Sidawy AN. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg 2008; 48: 5S–55S–80S. [DOI] [PubMed] [Google Scholar]
- 37. Zaheringer M, Strohe D, Stuetzer H, et al. Postcatheterization pseudoaneurysm: result of US-guided percutaneous thrombin injection in 240 patients. Radiology 2005; 236: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 38. Witz M, Werner M, Bernheim J, et al. Ultrasound-guided compression repair of pseudoaneurysm complicating a forearm dialysis arteriovenous fistula. Nephrol Dial Transplant 2000; 15: 1453–1454. [DOI] [PubMed] [Google Scholar]
- 39. Saxena AK, Panhotra BR, Al-Mulhim AS. Vascular access related infections in hemodialysis patients. Saudi J Kidney Dis Transplant 2005; 16: 46–71. [PubMed] [Google Scholar]
- 40. Sivananthan G, Menashe L, Halin NJ. Cephalic arch stenosis in dialysis patients: review of clinical relevance, anatomy, current theories on etiology and management. J Vasc Access 2014; 15: 157–162. [DOI] [PubMed] [Google Scholar]
- 41. D’cruz RT, Leong SW, Syn N, et al. Endovascular treatment of cephalic arch stenosis in brachiocephalic arteriovenous fistulas: a systematic review and meta-analysis. J Vasc Access 2019; 20: 345–355. [DOI] [PubMed] [Google Scholar]