Abstract
Background
Measles, mumps, rubella, and varicella (chickenpox) are serious diseases that can lead to serious complications, disability, and death. However, public debate over the safety of the trivalent MMR vaccine and the resultant drop in vaccination coverage in several countries persists, despite its almost universal use and accepted effectiveness. This is an update of a review published in 2005 and updated in 2012.
Objectives
To assess the effectiveness, safety, and long‐ and short‐term adverse effects associated with the trivalent vaccine, containing measles, rubella, mumps strains (MMR), or concurrent administration of MMR vaccine and varicella vaccine (MMR+V), or tetravalent vaccine containing measles, rubella, mumps, and varicella strains (MMRV), given to children aged up to 15 years.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2019, Issue 5), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to 2 May 2019), Embase (1974 to 2 May 2019), the WHO International Clinical Trials Registry Platform (2 May 2019), and ClinicalTrials.gov (2 May 2019).
Selection criteria
We included randomised controlled trials (RCTs), controlled clinical trials (CCTs), prospective and retrospective cohort studies (PCS/RCS), case‐control studies (CCS), interrupted time‐series (ITS) studies, case cross‐over (CCO) studies, case‐only ecological method (COEM) studies, self‐controlled case series (SCCS) studies, person‐time cohort (PTC) studies, and case‐coverage design/screening methods (CCD/SM) studies, assessing any combined MMR or MMRV / MMR+V vaccine given in any dose, preparation or time schedule compared with no intervention or placebo, on healthy children up to 15 years of age.
Data collection and analysis
Two review authors independently extracted data and assessed the methodological quality of the included studies. We grouped studies for quantitative analysis according to study design, vaccine type (MMR, MMRV, MMR+V), virus strain, and study settings. Outcomes of interest were cases of measles, mumps, rubella, and varicella, and harms. Certainty of evidence of was rated using GRADE.
Main results
We included 138 studies (23,480,668 participants). Fifty‐one studies (10,248,159 children) assessed vaccine effectiveness and 87 studies (13,232,509 children) assessed the association between vaccines and a variety of harms. We included 74 new studies to this 2019 version of the review. Effectiveness Vaccine effectiveness in preventing measles was 95% after one dose (relative risk (RR) 0.05, 95% CI 0.02 to 0.13; 7 cohort studies; 12,039 children; moderate certainty evidence) and 96% after two doses (RR 0.04, 95% CI 0.01 to 0.28; 5 cohort studies; 21,604 children; moderate certainty evidence). The effectiveness in preventing cases among household contacts or preventing transmission to others the children were in contact with after one dose was 81% (RR 0.19, 95% CI 0.04 to 0.89; 3 cohort studies; 151 children; low certainty evidence), after two doses 85% (RR 0.15, 95% CI 0.03 to 0.75; 3 cohort studies; 378 children; low certainty evidence), and after three doses was 96% (RR 0.04, 95% CI 0.01 to 0.23; 2 cohort studies; 151 children; low certainty evidence). The effectiveness (at least one dose) in preventing measles after exposure (post‐exposure prophylaxis) was 74% (RR 0.26, 95% CI 0.14 to 0.50; 2 cohort studies; 283 children; low certainty evidence). The effectiveness of Jeryl Lynn containing MMR vaccine in preventing mumps was 72% after one dose (RR 0.24, 95% CI 0.08 to 0.76; 6 cohort studies; 9915 children; moderate certainty evidence), 86% after two doses (RR 0.12, 95% CI 0.04 to 0.35; 5 cohort studies; 7792 children; moderate certainty evidence). Effectiveness in preventing cases among household contacts was 74% (RR 0.26, 95% CI 0.13 to 0.49; 3 cohort studies; 1036 children; moderate certainty evidence). Vaccine effectiveness against rubella, using a vaccine with the BRD2 strain which is only used in China, is 89% (RR 0.11, 95% CI 0.03 to 0.42; 1 cohort study; 1621 children; moderate certainty evidence). Vaccine effectiveness against varicella (any severity) after two doses in children aged 11 to 22 months is 95% in a 10 years follow‐up (rate ratio (rr) 0.05, 95% CI 0.03 to 0.08; 1 RCT; 2279 children; high certainty evidence). Safety There is evidence supporting an association between aseptic meningitis and MMR vaccines containing Urabe and Leningrad‐Zagreb mumps strains, but no evidence supporting this association for MMR vaccines containing Jeryl Lynn mumps strains (rr 1.30, 95% CI 0.66 to 2.56; low certainty evidence). The analyses provide evidence supporting an association between MMR/MMR+V/MMRV vaccines (Jeryl Lynn strain) and febrile seizures. Febrile seizures normally occur in 2% to 4% of healthy children at least once before the age of 5. The attributable risk febrile seizures vaccine‐induced is estimated to be from 1 per 1700 to 1 per 1150 administered doses. The analyses provide evidence supporting an association between MMR vaccination and idiopathic thrombocytopaenic purpura (ITP). However, the risk of ITP after vaccination is smaller than after natural infection with these viruses. Natural infection of ITP occur in 5 cases per 100,000 (1 case per 20,000) per year. The attributable risk is estimated about 1 case of ITP per 40,000 administered MMR doses. There is no evidence of an association between MMR immunisation and encephalitis or encephalopathy (rate ratio 0.90, 95% CI 0.50 to 1.61; 2 observational studies; 1,071,088 children; low certainty evidence), and autistic spectrum disorders (rate ratio 0.93, 95% CI 0.85 to 1.01; 2 observational studies; 1,194,764 children; moderate certainty). There is insufficient evidence to determine the association between MMR immunisation and inflammatory bowel disease (odds ratio 1.42, 95% CI 0.93 to 2.16; 3 observational studies; 409 cases and 1416 controls; moderate certainty evidence). Additionally, there is no evidence supporting an association between MMR immunisation and cognitive delay, type 1 diabetes, asthma, dermatitis/eczema, hay fever, leukaemia, multiple sclerosis, gait disturbance, and bacterial or viral infections.
Authors' conclusions
Existing evidence on the safety and effectiveness of MMR/MMRV vaccines support their use for mass immunisation. Campaigns aimed at global eradication should assess epidemiological and socioeconomic situations of the countries as well as the capacity to achieve high vaccination coverage. More evidence is needed to assess whether the protective effect of MMR/MMRV could wane with time since immunisation.
Plain language summary
Does the measles, mumps, rubella and varicella (MMRV) vaccine protect children, and does it cause harmful effects?
Background
Measles, mumps, rubella (German measles) and varicella (chickenpox) are infectious diseases caused by viruses. They are most common in children and young adults. They are not always serious, but can cause disability (such as deafness), complications and death. If pregnant women catch rubella, it may cause loss (miscarriage) of, or harm to, their unborn babies. A vaccine is a medicine that prevents infection by a specific disease. The MMR (measles, mumps, rubella) vaccine protects people against all three of these infections (a combined vaccine). Doctors can vaccinate against chickenpox at the same time by mixing the chickenpox (varicella) vaccine with the MMR vaccine (MMRV) or giving it separately at the same time (MMR+V). The MMR vaccine has reduced measles, mumps and rubella infections. However, some people think the MMR vaccine causes unwanted effects such as autism, swelling of the brain (encephalitis), meningitis, learning difficulties, type 1 diabetes, and other conditions. As a result, the number of children being vaccinated has fallen. This is the 2019 update of a review first published in 2005 and previously updated in 2012. Review question
We wanted to find out how effectively MMR, MMR+V and MMRV vaccines stop children (up to 15 years old) from catching measles, mumps, rubella and chickenpox. We also wanted to know if the vaccines cause unwanted effects.
Study characteristics
We looked for studies that assessed MMR, MMRV or MMR+V vaccines, given in any dose or time schedule, compared with not giving the vaccine, or giving a placebo vaccine (a sham treatment), to healthy children up to 15 years old. Studies needed to measure the number of cases of measles, mumps, rubella and chickenpox, and report whether children suffered any unwanted effects attributable to vaccination. We checked each study to make sure it used robust methods so that we could judge how reliable its results were.
Results We found 138 studies with more than 23 million children. Fifty‐one studies (10 million children) assessed how effective the vaccines were at preventing the diseases, and 87 studies (13 million children) assessed unwanted effects. In this 2020 update we have included 74 new studies published since 2012. Measles: results from seven studies (12,000 children) showed that one dose of vaccine was 95% effective in preventing measles. Seven per cent of unvaccinated children would catch measles and this number would fall to less than 0.5% of children who receive one dose of vaccine. Mumps: results from six studies (9915 children) showed that one dose of vaccine was 72% effective in preventing mumps. This rose to 86% after two doses, (3 studies, 7792 children). In unvaccinated children, 7.4% would catch mumps and this would fall to 1% if children were vaccinated with two doses. The results for rubella (1 study, 1621 children) and chickenpox (one study, 2279 children) also showed that vaccines are effective. After one dose, vaccination was 89% effective in preventing rubella, using a vaccine with the BRD2 strain which is only used in China, and after 10 years the MMRV vaccine was 95% effective at preventing chickenpox infection. Unwanted effects Overall, the studies found that MMR, MMRV and MMR+V vaccines did not cause autism (2 studies 1,194,764 children), encephalitis (2 studies 1,071,088 children) or any other suspected unwanted effect. Our analyses showed very small risks of fits due to high temperature or fever (febrile seizures) around two weeks after vaccination, and of a condition where blood does not clot normally (idiopathic thrombocytopenic purpura) in vaccinated children.
Certainty of the evidence
Our certainty (confidence) in the evidence is slightly limited by the design of most of the studies. Nonetheless, we judged the certainty of the evidence for the effectiveness of the MMR vaccine to be moderate, and that for the varicella vaccine to be high. Our certainty in the evidence for autism and febrile seizures was also moderate.
Conclusions
Our review shows that MMR, MMRV and MMR+V vaccines are effective in preventing the infection of children by measles, mumps, rubella and chickenpox, with no evidence of an increased risk of autism or encephalitis and a small risk of febrile seizure.
Search date
This review includes evidence published up to 2 May 2019.
Summary of findings
Background
Description of the condition
Measles, mumps, and rubella (MMR) are serious diseases that can lead to potentially fatal illnesses, disabilities, and death. MMR is particularly prevalent in low‐income countries where vaccination programmes are inconsistent and mortality rates from disease are high. Large‐scale vaccination programmes have reduced MMR incidence, prevalence, and rates of complications in high‐income countries (Hambrosky 2015).
Measles is highly contagious with a case‐fatality rate ranging from 0.01% to 0.1% in high‐income countries to 3% to 30% in low‐income areas (Wolfson 2009). Otitis media (7% to 9%), pneumonia (8%), and diarrhoea (1% to 6%) are the most frequently reported complications of measles. These complications are responsible for the large proportion of measles‐related morbidity and mortality (Perry 2004). Pneumonia is the most common fatal complication of measles, occurring in 56% to 86% of measles‐related deaths (Bester 2016).
Rubella is an acute viral disease mostly affecting school‐aged children and young adults with high incidence and prevalence worldwide in the pre‐vaccine era (Lambert 2015). Women of childbearing age are susceptible to rubella infection before conception or during early pregnancy which can result in miscarriage, fetal death, or congenital rubella syndrome. These conditions are the most serious complications of rubella with incidence varying from fewer than 2 per 100,000 live births in the Americas and Europe to 121 per 100,000 live births in Africa and South East Asia (Vynnycky 2016).
Mumps is a viral infection that mostly affects children. Peak incidence occurs among those aged five to nine years (Hviid 2008). Annual incidence of 100 to 1000 cases/100,000 population was reported in the pre‐vaccine era with greater than 90% reduction after mumps vaccines were introduced (Hambrosky 2015). Orchitis (inflammation of the testicles) is the most common age‐related complication (12% to 66% of cases) (Yung 2011). The most serious complications are aseptic meningitis (1% to 10%) and deafness (4%) (Yung 2011).
Varicella (chickenpox) is a widespread and highly contagious infectious disease with peak incidence in children aged up to 15 years (Gershon 2015). Most epidemiological data are from high‐income countries and account for high pre‐vaccine incidence (from 320 to 1600 cases per 100,000) with case‐fatality rates of approximately 3 per 100,000 cases (Amjadi 2016; Helmuth 2015). Typically, varicella‐zoster virus (VZV) becomes latent in ganglionic neurons after primary infection, and reactivation may occur to cause zoster (shingles); risk increases with age (Gershon 2013).
Description of the intervention
The single‐component live attenuated vaccines of MMR were first licenced in the USA in the early 1960s (Plotkin 2017), and have been shown to be highly effective. Some combination vaccines were available from the early 1970s, including trivalent MMR vaccines; a combination of MMR with varicella (MMRV) was made available from 2005 (Plotkin 2017; WHO Position Paper 2017). At least two MMR vaccines are authorised worldwide and marketed widely:
MMR‐II or MMRVaxPro by Merck/MSD is a live‐virus vaccine. It is a sterile lyophilised preparation of 1000 TCID50 (50% tissue culture infectious doses) Enders' attenuated Edmonston measles strain propagated in chick embryo cell culture; mumps 20000 TCID50 Jeryl Lynn strain propagated in chick embryo cell culture; and rubella 1000 TCID50 Wistar RA 27/3 propagated on human diploid lung fibroblasts. The growth medium is medium 199 (5.7 mg) used with neomycin as stabiliser;
Priorix vaccine, Glaxo SmithKline Beecham (GSK), is a lyophilised mixed preparation of the attenuated Schwarz measles CCID50 (50% cell culture infective dose) strain; RIT 4385 mumps CCID50 (derived from Jeryl Lynn strain); and CCID50 Wistar RA 27/3 rubella strain of viruses. These are obtained separately by propagation either in chick embryo tissue cultures (mumps and measles) or MRC5 human diploid cells (rubella). The vaccine also contains residual amounts of neomycin (25 µg per dose).
A World Health Organization (WHO) pre qualified MMR vaccine has also been licenced by the Serum Institute of India/Masu Co Ltd for Asian markets. It is a sterile lyophilised preparation containing live attenuated Edmonston‐Zagreb measles virus (not less than 1000 CCID50), Leningrad‐Zagreb mumps virus (not less than 5000 CCID50), and Wistar RA 27/3 rubella virus (not less than 1000 CCID50).
Other commercial formulations of MMR vaccines have been used over the past 30 years, and to date are authorised in few countries, or have been withdrawn from marketing for commercial, safety, or both commercial and safety reasons:
Morupar by Chiron contains live attenuated Schwarz measles strain 1000 TCID50, propagated in chick embryo cell culture; Wistar RA 27/3 rubella strain 1000 TCID50, propagated on human diploid lung fibroblasts; and Urabe AM9 mumps 5000 TCID50, propagated in chick embryo cell culture, with neomycin as stabiliser (withdrawn globally because of increased allergic reactions due to the manufacturing process);
Trimovax by Pasteur‐Merieux Serums and Vaccines contains live attenuated Schwarz measles strain, 1000 CCID50; Urabe AM9 mumps strain, 5000 TCID50; and Wistar RA 27/3 rubella strain, 1000 TCID50;
Triviraten Berna contains live attenuated Edmonston‐Zagreb (EZ 19) measles strain, 1000 TCID50; Rubini mumps strain, 5000 TCID50; and Wistar RA 27/3 rubella strain, 1000 TCID50 propagated on human diploid cells. The product contains lactose (14 mg), human albumin (8.8 mg), sodium bicarbonate (0.3 mg), medium 199 (5.7 mg), and distilled water as solvent.
Two main MMRV combined vaccines are authorised for worldwide use and contain live attenuated Oka/Merck strain VZV:
ProQuad by Merck/MSD is a live‐virus vaccine with the same composition as MMR‐II/MMRVaxPro, including live attenuated Oka/Merck VZV strain, 3.99 log10 PFU (plaque forming units) propagated on MRC‐5 human diploid cells; and
Priorix Tetra by GSK is a live‐virus vaccine with the same composition as Priorix, including live attenuated Oka/Merck VZV strain, 103.3 PFU propagated on MRC‐5 human diploid cells.
The components of monovalent and subsequently combined MMR vaccine are described below (Plotkin 2017). Most attenuated measles vaccines currently produced worldwide are derived from the Edmonston strain. Vaccines containing non‐Edmonston‐derived strains are also in use, including Leningrad‐16, Shanghai‐191, CAM‐70, and TD97. In most cases the virus is cultured in chick embryo cells. However, a few vaccines are attenuated in human diploid cells. Most vaccines contain traces of antibiotics (e.g. 25 µg neomycin per dose), but some do not. Sorbitol and gelatine are used as stabilisers (Plotkin 2017; WHO Position Paper 2017).
More than 10 mumps vaccine strains (Jeryl Lynn, Urabe, Hoshino, Rubini, Leningrad‐3, L‐Zagreb, Miyahara, Torii, NK M‐46, S‐12, and RIT 4385) have been used throughout the world, but the Jeryl Lynn strain is the most widely used to date (Plotkin 2017). Although some manufacturers produce live mumps vaccines containing the Urabe AM9 virus strain, some countries have promptly stopped Urabe strain‐containing MMR vaccines because of concerns about vaccine‐associated meningitis. Viruses are often cultured in chick embryo fibroblasts (as with the Jeryl Lynn and Urabe strain‐containing vaccines), but quail and human embryo fibroblasts are also used. Most vaccines also contain neomycin (25 µg per dose) (WHO Position Paper 2017).
Most rubella vaccines used throughout the world contain the RA 27/3 virus strain. Exceptions are vaccines produced in Japan, which use different virus strains: Matsuba, DCRB 19, Takahashi, TO‐336 (cultured in rabbit kidney cells), and Matsuura (produced using quail embryo fibroblasts) (Plotkin 2017). The RA 27/3 strain is used most often because of consistent immunogenicity, induction of resistance to re‐infection, and low rate of adverse effects (WHO Position Paper 2017). The live virus produces viraemia and pharyngeal excretion, but both are of low magnitude and are non‐communicable (Plotkin 2017).
All available monovalent VZV vaccines consist of the Oka virus strain, which was subsequently attenuated by sequential passage in cultures of human embryonic lung cells, embryonic guinea pig cells, and the human diploid cell line WI‐38 or MCR‐5 (Plotkin 2017). The titre of VZV is around 14 times higher in the MMRV vaccines described than in the monovalent VZV vaccine (WHO Position Paper 2014).
How the intervention might work
Combined MMR (trivalent vaccine, containing measles, rubella, mumps strains), MMR+V (concurrent administration of MMR vaccine and varicella (chickenpox) vaccine), and MMRV (tetravalent vaccine containing measles, rubella, mumps, varicella strains) vaccines are widely recommended by health authorities and offer advantages over individual vaccines in the facilitation of current immunisation implementation strategies. Moreover, trivalent vaccines are included in the WHO Expanded Programme on Immunization, and are used in almost all European countries, the USA, Canada, Australia, New Zealand, and 100 other countries around the world (Orenstein 2018; WHO GVAP 2013). Quadrivalent MMRV vaccines are also recommended, but have to date been implemented in a limited number of countries where varicella vaccination is routinely recommended (WHO Immunization Monitoring 2019). According to accepted recommendations, the first dose of both MMR and MMRV should be administered on or after the child's first birthday (from 9 to 15 months of age), and the second dose at least 28 days later, or from 4 to 10 years of age (WHO Immunization Monitoring 2019; WHO Position Paper 2017). Combined vaccines provide a significant improvement in the efficiency of childhood immunisation, and a meaningful reduction in costs through increasing immunisation coverage against specific diseases with a single injection (Vesikari 2007).
Until 2011, single‐component measles vaccine was largely used in nearly all African and several Asian, and Western European WHO member states with different implementation strategies (single‐dose or second‐dose administration) (WHO GVAP 2013). A first dose of measles‐containing vaccine at nine months of age has been recommended in all countries with ongoing transmission and high risk of measles mortality among infants to ensure adequate protection. The introduction of a second measles‐containing vaccine dose at 15 to 18 months of age has been recommended when coverage of at least 80% for the first dose of measles‐containing vaccine has been reached for three consecutive years. By 2011, all 194 WHO member states had introduced or begun the process of introducing a two‐dose measles vaccination strategy through routine immunisation services, supplementary immunisation activity, or both (WHO Strategic Plan 2012). However, this policy was revised in April 2017, and recommended including the second measles vaccine dose in national vaccination schedules regardless of the coverage level (WHO Position Paper 2017). As of December 2010, 131 of the 194 WHO member states included MR or MMR combined vaccines in routine immunisation programmes (WHO Strategic Plan 2012). Relevant progress has been made toward the ambitious goals of the Global Measles and Rubella Strategic Plan 2012 to 2020 (WHO Strategic Plan 2012), with a further 23 of 194 WHO member states introducing a second dose of measles‐containing vaccine, and 17 countries introducing the rubella‐containing vaccine (Orenstein 2018).
Between 2000 and 2017, estimated measles vaccine coverage increased globally from 72% to 85%, with a reported 83% reduction of annual measles incidence and 80% reduction in estimated measles mortality (Dabbagh 2018). Estimated global rubella vaccine coverage increased from 39% to 46%, with high regional variability ranging from 12% in South East Asia to 94% in Europe (Orenstein 2018). According to Regional Verification Commissions in the American, European and Western Pacific Regions, the goal of measles elimination (end of endemic transmission for at least three years) had been reached by the end of 2015 in 61 member states (34/35, 21/53, and 6/27 member states respectively in the Americas, Europe, and western Pacific) and elimination of rubella in 55 member states (35/35 and 20/53 member states in the Americas and Europe, respectively) (Orenstein 2018; Perry 2015). However, measles elimination milestones have not been met in several countries in all WHO regions, and measles resurgence has been reported from 2017 to 2019 because of large outbreaks (Dabbagh 2018; Zimmerman 2019).
A global technical consultation requested by the WHO assessed the feasibility of measles elimination through mass immunisation and convened that eradication is biologically, technically, and operationally feasible (WHO 2011). MMR capability to eliminate the targeted diseases has been demonstrated in a number of countries and different scenarios.
The largest country to have ended endemic measles transmission is the USA, where the elimination of endemic measles had been previously verified in 2000 (CDC 2005; CDC 2012; Orenstein 2004). The interruption of indigenous transmission was first observed in 1993 after refining the elimination strategy to face the large resurgence of measles that occurred from 1989 to 1991 (CDC 1992; Watson 1998). Incidence has remained at less than 1 case per 1 million population continuously since 1997, with most measles cases from 2001 representing importations or import‐associated infections (CDC 2012; Fiebelkorn 2017). The elimination of rubella and congenital rubella syndrome was verified in 2004 by an external expert panel (CDC 2005). The incidence remained below 1 case per 10 million population with an annual median number of 10 cases (range 4 to 18 cases) (CDC 2012; Hinman 2011). Recent studies and reviews of USA measles and rubella outbreaks showed that most imported cases were unvaccinated people in areas with suboptima vaccination coverage and in regions where herd immunity threshold for first or second dose had not been reached, or both (Fiebelkorn 2017; Lee 2019; Papania 2014).
In Europe, measles and rubella outbreaks and endemic transmission persisted at regional levels due to suboptima vaccination coverage (Zimmerman 2019). Despite the substantial reduction of measles and rubella incidence, 21 of 53 countries in the European Union had interrupted the endemic transmission of measles, and 20 member states had interrupted endemic transmission of rubella (Muscat 2014; Orenstein 2018; WHO Regional Office for Europe 2016).
Finland was the first European country to end endemic measles transmission through a national vaccination programme as a two‐dose schedule launched in 1982, with an unremitting 95% coverage for both doses until 2017 (National Institute for Welfare and Health 2017; Peltola 2008). Incidence declined to 1 case per 1 million population for all MMR diseases in 1995, and in 1999 the country was documented as being free of indigenous measles, mumps, and rubella (Davidkin 2010). Since then, a few clusters of MMR imported cases have been observed annually without any outbreaks (WHO 2017).
After the introduction of MMR vaccine in 1988 for children aged 13 to 15 months with a catch‐up campaign for preschool‐aged children, the annual incidence of measles declined sharply in England and Wales, from 160/100,000 in 1989 to 17/100,000 in 1995 (Gay 1997; Ramsay 2003). The interruption of indigenous transmission was first observed in 1996 after a widespread vaccination campaign in 1994 and the introduction of the second MMR dose in 1995 (Vyse 2002). Nevertheless, endemic transmission in the UK re‐established in 2006 because of intense media coverage of the fraudulent Wakefield claim of a suspected link among MMR vaccines and autism (Public Health England 2019a). Moreover, an increased number of mumps‐confirmed cases were reported in England and Wales (Public Health England 2019b). However, after different nationwide vaccination campaigns, the UK had interrupted endemic transmission of measles and rubella by 2014, and elimination was certified in 2017 from the Regional Verification Commission for Measles and Rubella Elimination. Furthermore, a significant reduction of mumps cases in school‐aged children has been observed with persisting outbreaks in young adults (Public Health England 2019c).
Although varicella vaccines are licenced worldwide, a limited number of countries routinely recommend varicella vaccination with a one‐ or two‐dose programme (WHO Immunization Monitoring 2019). The USA was the first country to recommend a routine one‐dose programme in 1996, and an updated routine two‐dose programme in 2006 (Marin 2007). A progressive reduction of overall varicella incidence has been observed in target age groups, with more than 90% decrease in cases when maintaining coverage with two doses over 80%. Moreover, a significant reduction of zoster incidence has been observed in children and adolescents, but it is too early to observe the impact of childhood varicella vaccination in adults and the elderly (Harpaz 2019). Similar data have been reported in some European countries: Italy and Spain reported 75% and 89% reductions, respectively, despite lower rates of immunisation coverage (Bechini 2015; Garcia Cenoz 2013). No evidence suggested a shift of varicella disease burden to older age groups after the introduction of varicella vaccination, but significant reductions in hospitalisations, complications, and deaths have been reported globally (Wutzler 2017).
Why it is important to do this review
Despite its worldwide use, no systematic reviews studying the effectiveness and safety of MMR or MMRV vaccines are available.
Objectives
To assess the effectiveness, safety, and long‐ and short‐term adverse effects associated with the MMR (trivalent vaccine, containing measles, rubella, mumps strains), or MMR+V (concurrent administration of MMR vaccine and varicella vaccine), or MMRV (tetravalent vaccine containing measles, rubella, mumps, varicella strains), given to children aged up to 15 years.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), controlled clinical trials (CCTs), prospective and retrospective cohort studies (PCS/RCS), case‐control studies (CCS), interrupted time‐series (ITS) studies, case cross‐over (CCO) studies, case‐only ecological method (COEM) studies, self‐controlled case series (SCCS) studies, person‐time cohort (PTC) studies, and case‐coverage design/screening methods (CCD/SM) studies. See Appendix 1 for study design definitions (based on Farrington 2004; Harris 2006; Higgins 2011; Jefferson 1999; Last 2001; Maclure 1991; Morgenstern 1995). A study taxonomy is shown in Appendix 2.
Observational study design was crucial in this review because the main concern about MMR/V vaccination is in regard to safety. The cohort, case‐control, and case‐only studies are valid study designs to investigate the possible association between vaccination and rare adverse events (Farrington 2004).
Types of participants
Healthy children aged up to 15 years, or adults who received MMR or MMRV/MMR+V vaccination between 0 and 15 years of age. We included studies (or data sets) where participants received vaccination before 16 years of age. For studies conducted in the general population, only data regarding participants vaccinated under 15 years were included in analyses. Studies where most participants received vaccination when aged 16 years or older were excluded.
Types of interventions
Vaccination with any combined MMR or MMRV/MMR+V vaccine given in any dose, preparation, or time schedule compared with no intervention or placebo.
MMR (trivalent vaccine containing measles, rubella, mumps strains). MMR+V (concurrent administration of MMR vaccine and varicella vaccine). MMRV (tetravalent vaccine containing measles, rubella, mumps, varicella strains).
Types of outcome measures
Primary outcomes
Effectiveness: clinical and/or laboratory‐confirmed cases of measles, mumps, rubella, or varicella.
Safety: encephalitis or encephalopathy, aseptic meningitis, seizure (febrile/afebrile), autism spectrum disorders, inflammatory bowel disease, cognitive delay, developmental delay, idiopathic thrombocytopenic purpura, Henoch‐Schönlein purpura, type 1 diabetes, asthma, dermatitis or eczema, hay fever, rhinoconjunctivitis, hypersensitivity/allergy, acute leukaemia, demyelinating diseases, multiple sclerosis, encephalomyelitis, acute disseminated encephalomyelitis (ADEM), gait disturbances, bacterial or viral infections.
Secondary outcomes
Short‐term side effects: local reactions (e.g. soreness and redness at the site of inoculation) and systemic reactions (e.g. fever, rash, vomiting, and diarrhoea) following MMR or MMRV vaccination.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 2 May 2019:
the Cochrane Central Register of Controlled Trials, which contains the Cochrane Acute Respiratory Infections Group's Specialised Register (CENTRAL; 2019, Issue 5) in the Cochrane Library using the strategy in Appendix 3;
MEDLINE via PubMed (from 1966 to 2 May 2019) using the strategy in Appendix 3; and
Embase via Elsevier (from 1974 to 2 May 2019) using the strategy in Appendix 3.
We searched the following trial registers on 2 May 2019:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov); and
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch).
We used the strategies in Appendix 3 and did not restrict the results by language or publication status (published, unpublished, in press, or in progress).
Searching other resources
For effectiveness trials, we searched bibliographies of all relevant articles obtained and any published reviews for additional studies. We also searched trial registers (WHO ICTRP and ClinicalTrials.gov) for unpublished, prospectively registered trials. For safety trials, we assessed bibliographies of all relevant articles and any published reviews for additional studies. We imposed no language restrictions on all searches.
Data collection and analysis
Selection of studies
Two review authors (CDP, AR) independently applied the inclusion criteria to all identified and retrieved articles. A third review author (VD) arbitrated in case of disagreements about the eligibility of a study.
Data extraction and management
Two review authors (CDP, AR) independently performed data extraction using a data extraction form (Appendix 4). A third review author (VD) checked data extraction and arbitrated in case of disagreement. For each study, relevant information was summarised and reported by main outcomes in Additional tables and Characteristics of included studies.
We used a two‐letter prefix to distinguish types of study designs and whether these related to effectiveness/efficacy or safety (only). The first letter signifies the study design (a = RCT, b = case control, c = cohort, d = self‐controlled case series, e = case cross‐over, f = case‐coverage design, g = case‐only ecological method, h = interrupted time series), and the second letter signifies the endpoint (a = effectiveness/efficacy, b = safety only). See Appendix 2.
We classified the funding sources of included studies as follows.
Government or not‐for‐profit organisation: explicitly stated that funding sources were public institutions, not‐for‐profit organisations, health department, or other government institutions. All authors were affiliated with public institutions, and none were affiliated with the pharmaceutical industry. All critical aspects of the research (participant selection, outcome assessment, statistical analysis, vaccine supplies) were conducted without pharmaceutical industry support.
Pharmaceutical industry: explicitly declared that funding was provided by the pharmaceutical industry. All authors were affiliated with the pharmaceutical industry. All critical aspects of the research (participant selection, outcome assessment, statistical analysis, vaccine supplies) were conducted with pharmaceutical industry support.
Mixed (government and pharmaceutical industry): at least one author was affiliated with the pharmaceutical industry. Statistical analysis was conducted with pharmaceutical industry support. Study vaccines were supplied by the pharmaceutical industry.
Not stated or unclear: funding source was not declared, therefore it was not possible to apply the funding classification criteria.
Assessment of risk of bias in included studies
Two review authors (CDP, AR) independently assessed the methodological quality of the included studies (Appendix 5). We assessed the quality of RCTs and quasi‐RCTs using criteria adapted from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the quality of non‐RCTs in relation to the presence of potential confounders that could make interpretation of the results difficult. We evaluated the quality of case‐control (prospective and retrospective) and cohort studies using the appropriate Newcastle‐Ottawa Scales (Stang 2010; Wells 2000). We applied quality control assessment grids based on those developed by the University of York, NHS Centre for Reviews and Dissemination (Appendix 5) to historical controlled trials (HCTs), interrupted time‐series (Khan 2001).
Experimental and quasi‐experimental studies
See Appendix 5.
Random sequence generation
Low risk of bias: e.g. a table of random numbers or computer‐generated random numbers.
High risk of bias: e.g. alternation, date of birth, day of the week, or case record number.
Unclear risk of bias: if insufficient information was provided.
Allocation concealment
Low risk of bias: e.g. numbered or coded identical containers were administered sequentially; an on‐site computer system that could only be accessed after entering the characteristics of an enrolled participant; or serially numbered, opaque, sealed envelopes, or sealed envelopes that were not sequentially numbered.
High risk of bias: e.g. an open table of random numbers.
Unclear risk of bias: if insufficient information was provided.
Blinding
Low risk of bias: if adequate double‐blinding (e.g. placebo vaccine) or single‐blinding (i.e. blinded outcome assessment) was used.
High risk of bias: if there was no blinding.
Unclear risk of bias: if insufficient information was provided.
Incomplete outcome data
Low risk of bias: no missing data, or the proportion of missing data compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
High risk of bias: when the proportion of missing data compared with observed event risk was large enough to induce clinically relevant bias in the intervention effect estimate.
Unclear risk of bias: if insufficient information was provided.
Non‐experimental studies
See Appendix 5.
We used different methodological quality checklists (unpublished) for the different case‐only design studies for:
self‐controlled case series (SCCS) and person‐time cohort (PTC) checklist based on Farrington 2004 and Petersen 2016;
case cross‐over studies (CCO) checklist was based on Farrington 2004 and Maclure 1991; and
case‐coverage methods/screening method (CCM/SM); and for case‐only ecological method (COEM) studies checklist was based on Farrington 2004.
We assessed evidence quality as a component of interpreting the overall results. We assigned the following 'Risk of bias' categories (Higgins 2011):
low risk of bias: plausible bias unlikely to seriously alter the results;
unclear risk of bias: plausible bias that raises some doubt about the results; and
high risk of bias: plausible bias that seriously weakens confidence in the result.
Measures of treatment effect
We used risk ratio (RR) and its confidence interval (CI) as measures of effect for RCT and cohort studies. We used the odds ratio (OR) and its CI for case‐control studies. The usual effect measure for case‐only studies is the rate ratio (rr). We calculated vaccine efficacy (or effectiveness) as VE = (1 − effect estimate) x 100, expressed as a percentage. For cohort and RCT/CCT studies VE = (1 − RR) x 100. For case‐control studies VE = (1 − OR) x 100. For study designs adopting the rr as effect measure (rate = events/person‐time), the vaccine effectiveness is VE = (1 − rr) x 100.
The inclusion of different studies involved different estimation methods and statistical models, so we are dealing with different measures of effect. Cohort studies may use the RR to compare two groups, or more sophisticated statistical models such as the logistic regression model or the proportional hazard regression model, where the effect measures reported are OR or hazard ratio (HR), respectively. Case‐control studies adopt the logistic regression model, so the effect measure is the OR. Case‐only studies design (SCCS, person‐time cohort, case cross‐over studies) use the Poisson regression model. In this case the effect measure is rr. Consequently, in order to perform meta‐analysis in some cases we had to convert one measure of the effect into another using the formulae described in Higgins 2011.
We converted temperatures to degrees celsius (°C) using the formula °C = (Fahrenheit − 32)/1.8.
Unit of analysis issues
We considered analytical studies that provided data at the person‐level for this review. The only ecological design considered was case‐only ecological study (COES). The differences between ecological study design and case‐only ecological study are described in Appendix 1.
Where several vaccine arms from the same study design were included in the same analysis, we split the placebo group equally between the different arms, so that the total number of participants in a single analysis did not exceed the actual number in the study.
Dealing with missing data
For this update we wrote to study authors to request missing data or for clarification. The response was disappointing, and we desisted from further attempts. Our analysis relies on existing data. Whenever possible we used the intention‐to‐treat (ITT) population. When necessary and possible we used strategies described in Di Pietrantonj 2006 to impute missing outcome data.
Assessment of heterogeneity
We calculated the I² statistic for each pooled estimate to assess the impact of statistical heterogeneity. The I² statistic can be interpreted as the proportion of total variation amongst effect estimates due to heterogeneity rather than sampling error, and is intrinsically independent from the number of studies. When the I² statistic is less than 30%, there is little concern about statistical heterogeneity (Higgins 2011). We used random‐effects models throughout to take account of the between‐study variance in our findings (Higgins 2011). Not all studies reported detail sufficient to enable a full analysis of the sources of heterogeneity.
Assessment of reporting biases
A detailed description of the study quality is provided in the Risk of bias in included studies section. We assessed publication bias by inspecting the funnel plots and heterogeneity (I²) (see Assessment of heterogeneity). Due to the limited number of studies in each comparison, the assessment of publication bias was not applicable. Since the evidence presented in this review originated mainly from published data, we cannot be sure that our results are not affected by publication bias. We were unable to retrieve unpublished papers, thus our results could be affected by publication bias.
Data synthesis
We carried out quantitative and qualitative data syntheses separately for efficacy/effectiveness and safety. We grouped studies for quantitative analysis according to study design (see Types of studies), vaccine type (MMR, MMRV, MMR+V), virus strain, and study settings. We incorporated heterogeneity into the pooled estimates by using the DerSimonian Laird random‐effects model.
Most of the studies included in this review were observational studies, therefore quantitative synthesis is performed on adjusted estimates by multivariate models. The estimates are adjusted for age and gender. The multicentre studies also take into account the geographical area, address, school, paediatric practice, and health organisation/insurance. Some studies adjusted estimates for the health history and health status of the older siblings.
As explained in the Measures of treatment effect section, the different studies involved different statistical models and estimation methods, so we are dealing with different measures of effect. Consequently, in some cases, in order to perform the meta‐analysis, we converted one measure of effect into another using the formulae described in Higgins 2011.
The cohort studies on MMR vaccine effectiveness against measles and mumps present estimates not adjusted by multivariate models but report binary data (fourfold frequency table) stratified by doses. In this case, the quantitative synthesis is performed on binary data. If some studies reported adjusted estimates, we used the method described in Di Pietrantonj 2006 to convert adjusted effect estimates into adjusted binary data.
We used RR for comparisons between vaccine and placebo/control groups for RCTs and cohort studies. We used rr for cohort studies using Poisson regression or the proportional hazard regression model. We OR for case‐control studies and rr for case‐only study designs.
We classified and discussed included studies according to the type of outcomes for which they provided evidence, effectiveness, and possible association with harms or local and systemic adverse effects. We illustrated study characteristics, design, population, and outcomes definitions in Additional tables.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses where data were available, as follows.
-
Age group
aged < 5 years, aged 5 to 10 years;
aged < 6 years, aged 11 to 16 years; and
aged < 1 year, aged 1 to 4 years, aged 5 to 14 years.
-
Number of doses administered
all doses, 1 dose, 2 doses, at least 1 dose (or any dose).
-
Length of follow‐up
< 5 years, 5 to 10 years.
-
Risk period (self‐controlled case series)
0 to 30 days, 31 to 60 days, 61 to 90 days.
-
Disease severity
moderate, severe.
Sensitivity analysis
We had planned to perform a sensitivity analysis on results by applying fixed‐effect and random‐effects models to assess the impact of heterogeneity on our results. We performed a sensitivity analysis by excluding studies at high risk of bias to assess the robustness of our conclusions.
Summary of findings and assessment of the certainty of the evidence
We created 21 'Summary of findings' tables using the outcomes listed in Appendix 6.
Effectiveness against measles
Effectiveness against mumps
Effectiveness against rubella
Effectiveness against varicella
Safety ‐ short‐term side effects
Safety ‐ encephalitis or encephalopathy
Safety ‐ aseptic meningitis
Safety ‐ seizures (febrile/afebrile)
Safety ‐ autism spectrum disorders
Safety ‐ inflammatory bowel disease
Safety ‐ cognitive/developmental delay
Safety ‐ idiopathic thrombocytopenic purpura
Safety ‐ Henoch‐Schönlein purpura
Safety ‐ type 1 diabetes
Safety ‐ asthma
Safety ‐ eczema/dermatitis
Safety ‐ hay fever, rhinoconjunctivitis, hypersensitivity/allergy
Safety ‐ acute leukaemia
Safety ‐ demyelinating diseases ‐ multiple sclerosis ‐ acute disseminated encephalomyelitis (ADEM)
Safety ‐ gait disturbances
Safety ‐ bacterial or viral infections, immune overload
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and made comments to aid readers’ understanding of the review where necessary.
Results
Description of studies
Results of the search
We updated searches on 2 May 2019 and identified 13,196 records for screening. We retrieved 101 papers after reviewing titles and abstracts, 74 of which we considered for this 2019 update. We also evaluated 16 studies identified as awaiting classification in our previous update (Demicheli 2012), of which we considered 12 studies. We included a total of 74 new studies, plus 12 studies from our previous update, for a total of 86 new included studies for this 2019 update. This review includes a total of 138 studies (see Figure 1; Figure 2).
1.
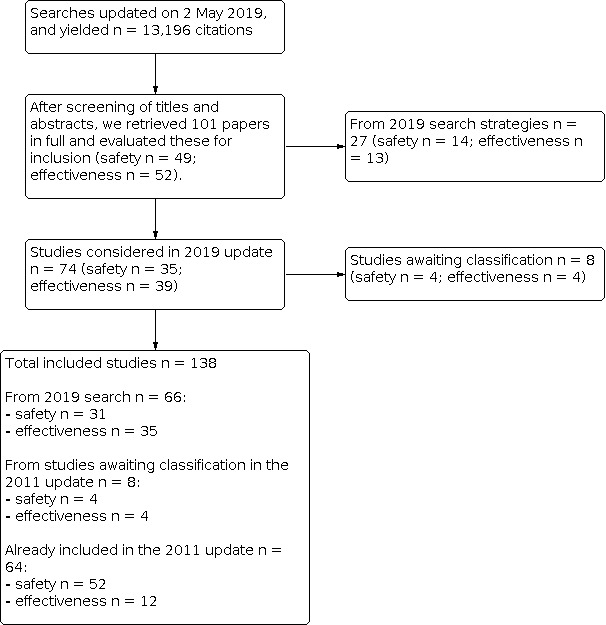
Flow diagram (simplified version).
2.
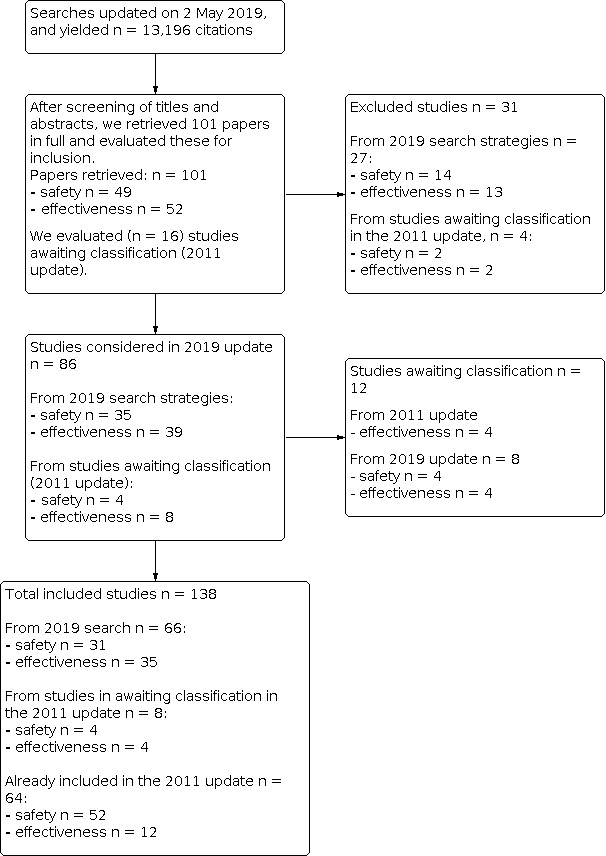
Flow diagram (complete).
Included studies
We included nine randomised controlled trials (RCTs) (aa‐Henry 2018; aa‐Povey 2019; aa‐Prymula 2014; ab‐Bloom 1975; ab‐Edees 1991; ab‐Freeman 1993; ab‐Lerman 1981; ab‐Peltola 1986; ab‐Schwarz 1975); one controlled clinical trial (CCT) (ab‐Ceyhan 2001); 63 cohort studies (PCS/RCS) (ca‐Arciuolo 2017; ca‐Arenz 2005; ca‐Barrabeig 2011a; ca‐Barrabeig 2011b; ca‐Bhuniya 2013; ca‐Chamot 1998; ca‐Chang 2015; ca‐Choe 2017; ca‐Compés‐Dea 2014; ca‐Giaquinto 2018; ca‐Greenland 2012; ca‐Hales 2016; ca‐La Torre 2017; ca‐Livingston 2013; ca‐Lopez Hernandez 2000; ca‐Ma 2018; ca‐Marin 2006; ca‐Marolla 1998; ca‐Musa 2018; ca‐Nelson 2013; ca‐Ogbuanu 2012; ca‐Ong 2005; ca‐Ong 2007; ca‐Rieck 2017; ca‐Schlegel 1999; ca‐Snijders 2012; ca‐Spackova 2010; ca‐Tafuri 2013; ca‐Takla 2014; ca‐Wichmann 2007; ca‐Woudenberg 2017; cb‐Ahlgren 2009; cb‐Barlow 2001; cb‐Beck 1989; cb‐Benjamin 1992; cb‐Benke 2004; cb‐Beyerlein 2017; cb‐DeStefano 2002; cb‐Dunlop 1989; cb‐Gavrielov‐Yusim 2014; cb‐Hviid 2004; cb‐Hviid 2008; cb‐Hviid 2019; cb‐Jacobsen 2009; cb‐Jain 2015; cb‐Klein 2010; cb‐Klein 2012; cb‐Klein 2017; cb‐Madsen 2002; cb‐Makino 1990; cb‐McKeever 2004; cb‐Miller 1989; cb‐Mrozek‐Budzyn 2013; cb‐Robertson 1988; cb‐Rowhani‐Rahbar 2013; cb‐Schink 2014; cb‐Sharma 2010; cb‐Stokes 1971; cb‐Swartz 1974; cb‐Timmermann 2015; cb‐Uchiyama 2007; cb‐Vestergaard 2004; cb‐Weibel 1980); 35 case‐control studies (CCS) (ba‐Andrade 2018; ba‐Castilla 2009; ba‐Cenoz 2013; ba‐Defay 2013; ba‐Fu 2013; ba‐Giovanetti 2002; ba‐Goncalves 1998; ba‐Harling 2005; ba‐Hungerford 2014; ba‐Jick 2010; ba‐Kim 2012; ba‐Liese 2013; ba‐Mackenzie 2006; ba‐Vazquez 2001; bb‐Ahlgren 2009; bb‐Baron 2005; bb‐Bertuola 2010; bb‐Black 1997; bb‐Black 2003; bb‐Bremner 2005; bb‐Bremner 2007; bb‐Chen 2018; bb‐Da Dalt 2016; bb‐Davis 2001; bb‐De Stefano 2004; bb‐Dockerty 1999; bb‐Groves 1999; bb‐Ma 2005; bb‐Mallol‐Mesnard 2007; bb‐Mrozek‐Budzyn 2010; bb‐Ray 2006; bb‐Shaw 2015; bb‐Smeeth 2004; bb‐Uno 2012; bb‐Vcev 2015); 16 self‐controlled case series/person‐time cohort studies (SCCS/PTC) (db‐Andrews 2012; db‐Dourado 2000; db‐Farrington 1995; db‐France 2008; db‐Macartney 2017; db‐MacDonald 2014; db‐Makela 2002; db‐McClure 2019; db‐Miller 2003; db‐Miller 2005; db‐Miller 2007; db‐O'Leary 2012; db‐Perez‐Vilar 2018; db‐Stowe 2009; db‐Taylor 1999; db‐Ward 2007); 3 case cross‐over studies (CCO) (eb‐Ki 2003; eb‐Lafaurie 2018; eb‐Park 2004); and 11 case‐only ecological method studies (COEM) (ga‐Boccalini 2015; ga‐Pozza 2011; ga‐Tafuri 2015; gb‐da Cunha 2002; gb‐da Silveira 2002; gb‐Fombonne 2001; gb‐Fombonne 2006; gb‐Honda 2005; gb‐Jonville‐Bera 1996; gb‐Seagroatt 2005; gb‐Taylor 2002).
We classified studies reported as field trials or controlled trials as cohort studies when the allocation procedure was not mentioned.
Vaccine effectiveness
We included 51 studies on MMR/MMRV effectiveness with the following study designs: 3 RCTs/CCTs, 31 cohorts, 14 case‐control, and 3 COEM. Two studies reported vaccine efficacy data against two diseases (measles and mumps) and were thus included in two different comparisons (ca‐La Torre 2017; ca‐Marolla 1998). We presented studies evaluating effectiveness in four main comparisons, as follows.
Measles: 17 studies included effectiveness data: 14 cohort studies, ca‐Arciuolo 2017; ca‐Arenz 2005; ca‐Barrabeig 2011a; ca‐Barrabeig 2011b; ca‐Bhuniya 2013; ca‐Choe 2017; ca‐Hales 2016; ca‐La Torre 2017; ca‐Marin 2006; ca‐Marolla 1998; ca‐Musa 2018; ca‐Ong 2007; ca‐Wichmann 2007; ca‐Woudenberg 2017, and 3 CCS (ba‐Defay 2013; ba‐Hungerford 2014; ba‐Jick 2010). See also Table 22 and Table 23.
Mumps: 21 studies included effectiveness data: 14 cohort studies, ca‐Chamot 1998; ca‐Compés‐Dea 2014; ca‐Greenland 2012; ca‐La Torre 2017; ca‐Livingston 2013; ca‐Lopez Hernandez 2000; ca‐Ma 2018; ca‐Marolla 1998; ca‐Nelson 2013; ca‐Ogbuanu 2012; ca‐Ong 2005; ca‐Schlegel 1999; ca‐Snijders 2012; ca‐Takla 2014, and 7 CCS (ba‐Castilla 2009; ba‐Fu 2013; ba‐Giovanetti 2002; ba‐Goncalves 1998; ba‐Harling 2005; ba‐Kim 2012; ba‐Mackenzie 2006). See also Table 24 and Table 25.
Rubella: 1 cohort study included effectiveness data (ca‐Chang 2015). See also Table 26.
Varicella: 14 studies included effectiveness data: 3 RCTs (aa‐Henry 2018; aa‐Povey 2019; aa‐Prymula 2014), 4 cohort studies (ca‐Giaquinto 2018; ca‐Rieck 2017; ca‐Spackova 2010; ca‐Tafuri 2013), 4 CCS (ba‐Andrade 2018; ba‐Cenoz 2013; ba‐Liese 2013; ba‐Vazquez 2001), and 3 COEM (ga‐Boccalini 2015; ga‐Pozza 2011; ga‐Tafuri 2015). See also Table 27, Table 28, Table 29, and Table 30.
1. Measles: effectiveness ‐ cohort studies.
| Study | Population characteristics | Case definition | Vaccine/strain | N vaccinated sample size (dose) | N control | N events in exposed/ N total exposed or person‐time versus N events in non‐exposed/ N total non‐exposed or person‐time | Vaccine effectiveness VE% (95% CI) |
| ca‐Barrabeig 2011b | Children attending day‐care and preschool centres (a) ≥ 15 months (all ages) (b) 15 to 23 months (c) 24 to 35 months (d) ≥ 36 months ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (e) Indirect effectiveness (e1) 12 to 23 months (e2) 24 to 35 months (e3) ≥ 36 months |
Confirmed measles was defined as laboratory‐confirmed case or met the WHO clinical case definition and was epidemiologically linked to laboratory‐confirmed case. |
Priorix/Schwarz or
MDS/Enders dose 1 at 9 to 12 months dose 2 at 15 months |
(a) N = 1027 (any dose) (a1) N = 830 (1 dose) (a2) N = 197 (2 doses) (b) N = 269 (any doses) (c) N = 384 (any doses) (d) N = 374 (any doses) |
(a) n = 94 (b) n = 57 (c) n = 20 (d) n = 17 unvaccinated |
(a) 5/1027 versus 12/94 (a1) 5/830 versus 12/94 (a2) 0/197 versus 12/94 (b) 3/296 versus 6/57 (c) 1/384 versus 4/20 (d) 1/374 versus 2/17 |
(a) 96.2% (89.4% to 98.6%) (a1) 95.3% (86.9% to 98.%) (a2) 100% (‐% to ‐%) (b) 89.4% (58.9% to 97.3%) (c) 98.7% (88.9% to 99.8%) (d) 97.7% (76.1% to 99.8%) VE = (1 − RR) x 100 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (e1) 71.1% (63.5% to 78.8%) (e2) 80.0% (56.3% to 94.3%) (e3) 88.2% (63.6% to 98.5%) VE = (ARU − ARV)/ARU x 100 Orenstein 1985 |
| ca‐Bhuniya 2013 | Children aged 9 to 59 months (at 30 June 2011) (a) 9 to 59 months (b) 9 to 12 months (c) > 12 months |
A clinical case of measles is defined as fever with maculopapular rash and either conjunctivitis or cough or coryza (catarrhal inflammation of the mucous membrane in the nose). A confirmed case of measles is defined as a clinical case who is positive for anti‐measles virus nucleoprotein immunoglobulin M antibodies in serological tests but has not been vaccinated against measles during last 1 month. |
MMR vaccine not described | (a) N = 50 (1 dose) | (a) N = 18 | (a) 15/50 versus 16/18 | (a) 66.3% (46.9% to 78.6%) (b) 66.6%(*) (c) 65.4%(*) (*) no statistical evidence VE = (1 − RR) x 100 |
| ca‐Choe 2017 | Outbreak at a university in 2014 Students born between 1984 and 1993. N = 14,465 VE > 10 years after vaccination |
The definition of suspected measles case was individuals with
following features: fever and rash and at least 1 of cough, coryza,
or conjunctivitis. All suspected cases were quarantined and were interviewed using standardised questionnaire, and physical examinations were performed by trained physicians. Presence of symptoms (fever, rash, cough, coryza, or conjunctivitis), travel history, and days of illnesses were assessed. |
MMR/not stated 2 doses |
N = 11448 | N = 3017 | 52/11448 versus 33/3017 | 60% (38.2% to 74.1%) VE = (1 − RR) x 100 |
| ca‐La Torre 2017 | N = 11,004 children born between 2008 and 2010 who underwent vaccination in 2009 to 2011. Follow‐up = 24 months |
Hospitalisation for (a) measles (b) mumps (see also Table 24) (c) measles and mumps (d) all infectious diseases (e) all respiratory diseases The effectiveness of MMR vaccine in reducing hospitalisations for any infection was assessed by analysing 2 distinct databases (vaccination record) and (hospital discharge): Hospital discharge diagnosis which contained the following ICD‐9 codes in primary or secondary diagnosis: 001 to 139 for infectious and parasitic diseases; 460 to 519 for respiratory diseases |
MMR not described the vaccination records of the database of the Roma Local Health Unit from which relevant data were extracted, such as date of birth; MMR vaccination (yes/no); MMR dose (only for vaccinated); personal tax code. The cohort was recomposed through record linkage of the 2 archives, registration and vaccination of hospital discharge records, using personal tax codes as a common identification in both archives. |
(1) 1 dose N = 5392 (2) 2 doses N = 3310 (3) any dose N = 8702 |
Unvaccinated N = 2302 |
(a1) 3/5392 versus 9/2302 (a2) 0/3310 versus 9/2302 (a3) 3/8702 versus 9/2302 (b1) 1/5392 versus 1/2302 (b2) 0/3310 versus 1/2302 (b3) 1/8702 versus 1/2302 (c1) 4/5392 versus 10/2302 (c2) 0/3310 versus 10/2302 (c3) 4/8702 versus 10/2302 (d1) 82/5392 versus 262/2302 (d2) 70/3310 versus 262/2302 (d3) 414/8702 versus 262/2302 (e1) 202/5392 versus 424/2302 (e2) 183/3310 versus 424/2302 (e3) 809/8702 versus 424/2302 |
Unadjusted estimates (a1) 85.8% (47.5% to 96.1%) (a2) 96.3% (37.1% to 99.8%) (a3) 91.2% (67.5% to 97.6%) (b1) 57.3% (−582% to 97.3%)* (b2) 76.8% (−468% to 99.1%)* (b3) 73.5% (−322% to 98.3%)* (c1) 82.9% (45.6% to 94.6%) (c2) 96.7% (43.5% to 99.8%) (c3) 89.4% (66.3% to 96.7%) (d1) 86.6% (83% to 89.5%) (d2) 81.4% (75.9% to 85.6%) (d3) 84.7% (81.4% to 87.4%) (e1) 79.7% (76.1% to 82.7%) (e2) 70% (64.6% to 74.5%) (e3) 76% (72.6% to 78.9%) (*) no statistical evidence VE = (1 − RR) x 100 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Adjusted estimates any doses (a) 91% (68% to 99%) (b) not reported (c) 90% (66% to 97%) (d) 71% (66% to 75%) (e) 82% (52% to 93%) VE = (1 − HR)*100 |
| ca‐Marolla 1998 | Children (19 to 67 months) whose parent required a paediatrician visit during a measles outbreak peak |
Clinical diagnosis patient records and parent interviews | (a) Pluserix
Schwarz
(b) Morupar
Schwarz (c) Triviraten Edmonston‐Zagreb vaccination records |
(a) N = 329 (1 dose) (b) N = 747 (1 dose) (c) N = 1023 (1dose) |
N = 646 unvaccinated |
(a) 0/329 versus 114/646 (b) 2/747 versus 114/646 (c) 8/1023 versus 114/646 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 0/ 19,836 PT (b) 2/ 12,906 PT (c) 8/ 31,329 PT (control) 114/22,188 PT = person‐time in months |
(a) 100% (‐% to ‐%) (b) 97% (88% to 99%) (c) 95% (90% to 98%) VE = (ARU − ARV)/ARU x 100 Orenstein 1985 |
| ca‐Musa 2018 | Children aged up to 14 years. N = 2784 (children aged > 14 years, N = 2300). Data were presented by age group. The study included all students in 40 classes with 1 or more registered measles cases in the period February 2014 to September 2015. VE ≤ 5 years since vaccination 6 to 14 years since vaccination |
Measles diagnosis was confirmed according to WHO guidelines. The clinical criteria
for measles were fever, maculopapular rash (i.e. non‐vesicular
rash), and cough or coryza (i.e. runny nose) or conjunctivitis
(i.e. red eyes). The laboratory criteria for measles surveillance case confirmation were measles IgM antibody detection, or measles virus isolation, or measles viral RNA detection by RT‐PCR, or a significant rise in measles IgG antibody in paired sera. All suspected cases were investigated and classified based on clinical, laboratory, and epidemiological data, based on the WHO case definition. |
MMR/not stated (a) 1 dose (b) 2 doses (c) ≤ 5 years since vaccination (d) 6 to 14 years since vaccination |
(a) N = 100 (b) N = 606 (c) N = 20 (d) N = 76 |
N = 95 | (a) 3/100 versus 35/95 (b) 6/606 versus 35/95 (c) 1/20 versus 35/95 (d) 2/76 versus 35/95 |
(a) 91.9% (74.4% to 97.4%)
(b) 97.3% (93.8% to 98.8%)
(c) 86.4% (6.6% to 98.0%)
(d) 92.9% (71.2% to 98.2%) VE = (1 − RR) x 100 |
| ca‐Ong 2007 | Children from primary school in Singapore (aged 8 to 14 years, > 5 years since vaccination) during a measles outbreak |
Clinical with laboratory confirmation. Active survey and serological confirmation |
MMR vaccine not described Vaccination status was ascertained from health booklet. |
N = 171 (1 dose) | N = 13 unvaccinated |
2/171 versus 7 /13 | 97.8% (90.6% to 99.5%) VE = (1 − RR) x 100 |
| ca‐Wichmann 2007 | School outbreak 2006. Students aged 10 to 15 years (N = 875) 16 to 21 years (N = 139) VE < 10 years after vaccination > 10 years after vaccination |
Clinical or laboratory | MMR/not stated (a) 1 dose (b) 2 doses (c) unknown vaccination status ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
All ages (a) N = 199 (b) N = 561 (c) N = 218 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 10 to 15 years (a) N =196 (b) N = 502 (c) N = 144 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 16 to 21 years (a) N = 3 (b) N = 59 (c) N = 74 |
All ages N = 36 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 10 to 15 years N = 33 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 16 to 21 years N = 3 |
All ages (a) 2/199 versus 19/36 (b) 2/5611 versus 19/36 (c) 30/218 versus 19/36 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 10 to 15 years (a) 2/196 versus 18/33 (b) 2/502 versus 18/33 (c) 25/144 versus 18/33 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 16 to 21 years (a) 0/3 versus 1/3 (b) 0/59 versus 1/3 (c) 5/74 versus 1/3 |
All ages
(a) 98.1% (92.2% to 99.5%)
(b) 99.3% (97.2% to 99.8%) (c) 73.9% (59.0% to 83.4%) VE = (1 − RR) x 100 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 10 to 15 years (a) 98.1% (92.3% to 99.5%) (b) 99.3% (97.0% to 99.8%) (c) 68.2% (48.9% to 80.2%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 16 to 21 years (a) 66.7% (*) (b) 97.8% (53.7% to 99.9%) (c) 79.7% (*) VE = (1 − RR) x 100 (*) no evidence |
| ca‐Woudenberg 2017 | Infants aged 6 to 14 months living in municipalities where coverage with the first dose of MMR vaccine was < 90%. Infants aged 6 to 11 months were offered an extra vaccination (and would thus still be eligible for their second MMR vaccination at the age of 14 months). Infants aged 12 to 14 months were offered an early MMR vaccination as an alternative to the regular time point at 14 months of age. All infants were eligible for another dose of MMR scheduled at 9 years of age. |
Laboratory‐confirmed measles N = 1080 infants eligible for analysis laboratory‐confirmed |
MMR vaccine: (M‐M‐RVAXPRO; Sanofi Pasteur MSD). This vaccine contains measles virus Enders’ Edmonston strain. Vaccination status was checked in the national vaccination register. Parents were asked whether their infant(s) had had measles in the preceding 3 months. |
N = 919 | N = 311 | 3/106,631 (PT‐days) versus 10/23,769 (PT‐days) |
HR (95% CI)(*) 0.29 (0.05 to 1.72) (*) adjusted estimates Cox proportional hazard model VE = 1 − HR |
| ca‐Arenz 2005 |
Household contacts 55 families, 43 children (a) 1 dose (b) 2 doses (c) any dose |
Clinical | MMR/strain not stated | (a) N = 13 (b) N = 4 |
N = 26 | (a) 1/13 versus 19/26 (b) 0/4 versus 19/26 (c) 1/20 versus 19/26 |
(a) 96.9% (71.8% to 99.7%) (b) 95.7% (10.6% to 99.8%) (c) 97.7% (79.3% to 99.7%) VE = (1 − RR) x 100 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 90% (35% to 97%) (b) not reported (c) 92% (48% to 98%) VE = (ARU − ARV)/ARU x 100 Orenstein 1985 |
| ca‐Hales 2016 |
Household contacts adolescents and young adults (10 to 29 years) (a) any dose (b) 1 dose (c) 2 doses (d) 3 doses |
Clinical or laboratory confirmation, or both |
MMR vaccine not described | (a) N = 302 (b) N = 27 (c) N = 205 (d) N = 70 |
(a) N = 16 | Pre‐campaign MMR doses (a) 16/302 versus 2/16 (b) 3/27 versus 2/16 (c) 13/205 versus 2/16 (d) 0/70 versus 2/16 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
Pre‐campaign MMR doses (a) (No data) (b) 23.1% (−425.0% to 87.3%)* (c) 63.4% (−103.0% to 90.6%)* (d) 95.9% (45% to 100%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Campaign MMR doses: 78.7% (10.1% to 97.7%) for pre‐exposure doses ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 50.4% (*) for postexposure doses (*) no statistical evidence ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ VE = (1 − OR) x 100 from logistic regression |
| ca‐Marin 2006 |
Household contacts (6 months to 14 years) of primary measles cases |
Secondary cases Clinical (WHO definition) or IgM positive antibody of secondary cases Standardised questionnaires |
MMR vaccine not described Vaccination records |
(a1) N = 48 (1 dose) (a2) N = 106 (2 doses) (b) N = 44 (> 2 doses) (c) N = 219 any doses contacts |
N = 21 unvaccinated |
(a1) 2/48 versus 11/21 (a2) 3/106 versus 11/21 (b) 1/44 versus 11/21 (c) 17/219 versus 11/21 |
(a1) 92.0% (67.2% to 98.1%) (a2) 94.6% (82.3% to 98.4%) (b) 95.7% (68.6% to 99.4%) (c) 85.2% (72.7% to 92.0%) VE = (1 − RR) x 100 |
| ca‐Arciuolo 2017 |
Postexposure prophylaxis Childrena aged < 19 years N = 208 |
All who subsequently developed measles were considered as contacts. |
MMR not described MMR PEP administered within 72 hours of initial exposure. |
N = 44 | N = 164 | (a) 2/44 versus 45/164 | (a) 83.4% (34.4% to 95.8%) VE = (1 − RR) x 100 |
| ca‐Barrabeig 2011a |
Postexposure prophylaxis N = 166 children with median age of 16.5 months (range 6 to 47 months) Candidates for the intervention were susceptible contacts who had not received either measles‐containing vaccine or had not suffered measles. |
Clinical and laboratory | MMR not stated (a) at least 1 dose (b) vaccinated ≤ 3 days (c) vaccinated 4 to 5 days (d) vaccinated 6 to 7 days (e) vaccinated 8 to 9 days (f) vaccinated 10 to 12 days |
(a) N = 54 (b) N = 17 (c) N = 14 (d) N = 14 (e) N = 8 (f) N = 1 |
N = 21 | (a) 12/54 versus 13/21 (b) 1/17 versus 13/21 (c) 4/14 versus 13/21 (d) 5/14 versus 13/21 (e) 1/8 versus 13/21 (f) 1/1 versus 13/21 |
(a) 64.1% (34.5% to 80.3%) (b) 90.5% (34.5% to 98.6%) (c) 53.8% (0.0% to 81.1%) (d) 42.3% (0.0% to 81.1%) (e) 79.8% (0.0% to 73.5%) (f) not reported VE = (1 − RR) x 100 |
ARU: attack rate amongst unvaccinated ARV: attack rate amongst vaccinated CI: confidence interval HR: hazard ratio ICD: International Statistical Classification of Diseases and Related Health Problems IgG: immunoglobulin G IgM: immunoglobulin M incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine N: number of participants in intervention and control arm OR: odds ratio PEP: postexposure prophylaxis PT: person‐time in months rr: rate ratio (relative incidence, incidence rate ratio, hazard ratio) RR: risk ratio (relative risk) RNA: ribonucleic acid RT‐PCR: reverse‐transcription polymerase chain reaction VE: vaccine effectiveness/efficacy WHO: World Health Organization
2. Measles: effectiveness ‐ case‐control studies.
| Study | Population characteristics | Case definition | Controls/ selection | MMR strain/exposure | N cases vaccinated/N cases versus N controls vaccinated/N controls | OR (95% CI) | VE% (95% CI) |
| ba‐Defay 2013 | Children aged 5 to 17 years (a) outside of outbreak school (b) all participants |
(a) N = 61 (b) N = 102 confirmed by laboratory testing or epidemiologic link is notifiables by both physicians and laboratories in Quebec |
(a) N = 305 (b) N = 510 Controls were matched for date of birth (± 6 months) and school attended in 2010 to 2011. |
MMR‐II (Merck Canada, Montreal, Quebec) Cases and controls received 2 doses of measles‐containing vaccine. |
No data reported amongst unvaccinated. | ‐ | ‐ |
| ba‐Hungerford 2014 | Participants (median age 16 years, upper quartile age 76 years) living in Merseyside (UK) |
N = 42 microbiological confirmation: oral fluid/blood test IgM positive or PCR positive |
N = 42 Control group participants were selected at random, matched 1:1 by general medical practice and aged within 1 year. |
MMR vaccine not described (a) vaccinated appropriately for age (b) under age for vaccination (< 14 months) (c) all ‐ vaccinated Unvaccinated: incompletely or partially vaccinated for age (> 13 months) |
(a) 5/27 versus 23/29 (b) 15/37 versus 12/18 (c) 20/42 versus 35/42 |
Risk factors for measles infection (univariate analysis) age > 13 months and incomplete vaccination 6.3 (1.9 to 33.4) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (Multivariate analysis) under age for routine vaccination 20.4 (2.0 to 300) incomplete/partial vaccination for age > 13 months 22.1 (3.8 to 300) (**) adjusted for confounders |
Risk factors for measles infection (univariate analysis) age > 13 months and incomplete vaccination 84.1% (47.4% to 97.0%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (Multivariate analysis) under age for routine vaccination 95.1% (50.0% to 100%) incomplete/partial vaccination for age > 13 months 95.5% (73.7% to 100%) (**) adjusted for confounders VE = (1 − OR) x 100 |
| ba‐Jick 2010 | Participants aged 1 to 19 years |
N = 1261 clinical definition |
N = 4996 randomly selected, matched for year of birth, gender, general practice attended, index date |
MMR or MR not described (a) 1 dose (b) > 1 dose |
(a) 409/1221 versus 2012/4750 (b) 40/852 versus 246/2984 |
(a) 0.49 (0.41 to 0.58)* (b) 0.39 (0.26 to 0.58)* *adjusted estimates, conditional logistic regression |
(a) 51.0% (42.0% to 59.0%)
(b) 61.0% (42.0% to 74.0%) VE = (1 − OR) x 100 |
**: multivariate analysis CCDC: Consultant in Communicable Disease Control CI: confidence interval IgM: immunoglobulin M MR: measles and rubella vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine N: number of participants OR: odds ratio PCR: polymerase chain reaction VE: vaccine effectiveness/efficacy WHO: World Health Organization
3. Mumps: effectiveness ‐ cohort studies.
| Study | Population characteristics | Case definition | Vaccine/strain | N vaccinated sample size (dose) | N control | N events in exposed/ N total exposed or PT versus N events in non‐exposed/ N total non‐exposed or PT | VE% (95% CI) |
| ca‐Chamot 1998 | Children aged up to 16 years from Geneva were household contacts of primary confirmed mumps cases (clinical or with laboratory confirmation notified by a paediatrician). |
Clinical diagnosis of secondary cases Phone interview |
(a) MMR‐II/Jeryl LynnB (b) Pluserix or Trimovax/Urabe AM9 (c) Triviraten/Rubini (d) any strain Vaccination records Unspecified number of doses |
(a) N = 30 (b) N = 75 (c) N = 83 (d) N = 193 |
N = 72 unvaccinated |
(a) 4/30 versus 25/72 (b) 7/75 versus 25/72 (c) 27/83 versus 25/72 (d) 38/193 versus 25/72 |
(a) 61.6 % (−0.9% to 85.4%) (b) 73.1% (41.8% to 87.6%) (c) 6.3% (−45.9% to 39.8%) (d) 43.0% (12.7% to 62.8%) VE = (1 − RR) x 100 |
| ca‐Compés‐Dea 2014 | 235 students (in Spain) (aged 16 to 17 years) |
Laboratory confirmed | MMR vaccine:
Jeryl Lynn RIT4385 or Rubini (a) 1 dose (b) 2 dose (c) 3 dose (d) any dose |
(a) N = 5 (b) N = 37 (c) N = 2 (d) N = 44 |
N = 2 unvaccinated |
(a) 2/5 versus 1/2 (b) 9/37 versus 1/2 (b) 2/2 versus 1/2 (d) 13/44 versus 1/2 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Incidence (a) 33 versus 50 x 100 person‐day (≥ 2 doses) 16 versus 50 x 100 person‐day |
(a) not reported
(b) not reported
(c) not reported
(d) not reported ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ VE = (1 − rr) x 100 (a) 34% (−44% to 70%)* (≥ 2 doses) 67% (28% to 83%) *no statistical evidence |
| ca‐Greenland 2012 | Students from the 3 university cities N = 989 |
Self‐reported | MMR vaccine: Jeryl Lynn (a) 1 dose (b) 2 doses |
(a) N = 29 (b) N = 706 |
N = 16 unvaccinated |
(a) 2/29 versus 7/16 (b) 92/706 versus 7/16 |
(a) not reported (b) 68% (40.6% to 82.2%) adjusted estimate VE = 1 − RR |
| ca‐La Torre 2017 | N = 11,004 children born between 2008 and 2010, who underwent vaccination in 2009 to 2011. Follow‐up = 24 months |
Hospitalisation for (a) measles (see also Table 22) (b) mumps (c) measles and mumps (d) all infectious diseases (e) all respiratory diseases The effectiveness of MMR vaccine in reducing hospitalisations for any infection was assessed by analysing 2 distinct databases (vaccination record) and (hospital discharge): hospital discharge diagnosis contained the following ICD‐9 codes in primary or secondary diagnosis:
|
MMR not described (we assume Jeryl Lynn) the vaccination records from the Roma Local Health Unit database from which relevant data were extracted, such as date of birth; MMR vaccination (yes/no); MMR dose (only for vaccinated); personal tax code. The cohort was recomposed through record linkage of the 2 archives, registration and vaccination of hospital discharge records, using personal tax codes as a common identification in both archives. |
(1) 1 dose N = 5392 (2) 2 doses N = 3310 (3) any dose N = 8702 |
Unvaccinated N = 2302 |
(a1) 3/5392 versus 9/2302 (a2) 0/3310 versus 9/2302 (a3) 3/8702 versus 9/2302 (b1) 1/5392 versus 1/2302 (b2) 0/3310 versus 1/2302 (b3) 1/8702 versus 1/2302 (c1) 4/5392 versus 10/2302 (c2) 0/3310 versus 10/2302 (c3) 4/8702 versus 10/2302 (d1) 82/5392 versus 262/2302 (d2) 70/3310 versus 262/2302 (d3) 414/8702 versus 262/2302 (e1) 202/5392 versus 424/2302 (e2) 183/3310 versus 424/2302 (e3) 809/8702 versus 424/2302 |
Unadjusted estimates (a1) 85.8% (47.5% to 96.1%) (a2) 96.3% (37.1% to 99.8%) (a3) 91.2% (67.5% to 97.6%) (b1) 57.3% (−582% to 97.3%)* (b2) 76.8% (−468% to 99.1%)* (b3) 73.5% (−322% to 98.3%)* (c1) 82.9% (45.6% to 94.6%) (c2) 96.7% (43.5% to 99.8%) (c3) 89.4% (66.3% to 96.7%) (d1) 86.6% (83% to 89.5%) (d2) 81.4% (75.9% to 85.6%) (d3) 84.7% (81.4% to 87.4%) (e1) 79.7% (76.1% to 82.7%) (e2) 70% (64.6% to 74.5%) (e3) 76% (72.6% to 78.9%) (*) no statistical evidence VE = (1 − RR) x 100 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Adjusted estimates any dose (a) 91% (68% to 99%) (b) not reported (c) 90% (66% to 97%) (d) 71% (66% to 75%) (e) 82% (52% to 93%) VE = 1 − HR |
| ca‐Livingston 2013 | From 2176 household residents from 2009 to 2010 All ages, (age group 1) age ≤ 17 years (age group 2) age ≥ 18 years |
Clinical or laboratory confirmed, or both | MMR vaccine: Jeryl Lynn (a) 1 dose (b) 2 doses (c) unknown (d) any dose |
Age ≤ 17 years (group 1) (1a) 1 dose N = 342 (1b) 2 doses N = 361 (1c) unknown N = 914 (d) any dose ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Age ≥ 18 years (2a) 1 dose N = 9 (2b) 2 doses N = 97 (2c) unknown N = 574 (d) any dose |
Age ≤ 17 years (group 1) N = 126 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Age ≥ 18 years (group 2) N = 6 unvaccinated |
All ages (group 1 + 2) (a) 4/117 versus 4/20 (b) 19/691 versus 4/20 (c) 17/520 versus 4/20 (d) 23/808 versus 4/20 Secondary households contacts age ≥ 5 years N = 1348 |
All ages (a) 82.9% (37.1% to 95.4%) (b) 86.3% (63.3% to 94.9%) (c) 83.7% (55.9% to 93.9%) (d) 85.8% (62.7% to 94.6%) VE = (1 − RR) x 100 assessed amongst 44 secondary cases and 1304 non‐sick household contacts |
| ca‐Lopez Hernandez 2000 | Male children aged between 3 and 15 years attending a scholastic institute in Spain during a mumps outbreak (March to November 1997) |
Clinical diagnosis. Cases notified by the Andalusian survey system. | MMR strain not reported | N = 685 vaccination record |
N = 38 unvaccinated |
73/685 versus 8/38 | 49% (3% to 74%) VE = (1 − RR) x 100 |
| ca‐Ma 2018 | Conducted between 1 December 2014 and 20 September 2015. N = 2303 students aged 6 to 15 years. Of these, 114 were excluded because they had history of mumps illness; 281 students were excluded because of unknown immunisation history. N = 1378 vaccinated and unvaccinated N = 530 children included in the analysis |
A mumps case was defined as a student having unilateral or bilateral parotid or other salivary gland swelling and pain, lasting 2 or more days, with onset between 1 December 2014 and 20 September 2015. All cases were diagnosed by clinical criteria without laboratory confirmation, and no mumps virus genotype information was obtained during this outbreak investigation. |
MMR: S79 strain of mumps vaccine virus, derived through further attenuation of the Jeryl Lynn strain. Students’ vaccination certificates were obtained during the field investigation. (a) 1 dose (≤ 5 years since vaccination) (b) 1 dose (> 5 years since vaccination) (c) any time since vaccination |
(a) N = 363 (b) N = 301 (c) N = 664 |
Unvaccinated N = 530 |
(a) 28/363 versus 93/530 (b) 21/301 versus 93/530 (c) 49/664 versus 93/530 |
(a) 56% (34.4% to 70.6%)
(b) 60.2% (37.5% to 74.7%)
(c) 57.9% (41.7% to 69.7%) VE = (1 − RR) x 100 |
| ca‐Marolla 1998 | Children (19 to 67 months) whose parent required a paediatrician visit during a measles outbreak peak | Clinical diagnosis Patient records and parent interviews |
(a) Pluserix/Urabe (b) Morupar/Urabe (c) Triviraten/Rubini Vaccination records |
(a) N = 329 (1 dose) (b) N = 747 (1 dose) (c) N = 1023 (1 dose) |
N = 646 unvaccinated |
(a) 38 cases/19433 (PT) (b) 28 cases/12785 (PT) (c) 185 cases/29974 (PT) Control = 206 cases/25,816 PT=person‐ time in months |
(a) 75% (65% to 83%) (b) 73% (59% to 82%) (c) 23% (6% to 37%) VE = (ARU − ARV)/ARU x 100 Orenstein 1985 |
| ca‐Nelson 2013 |
During 2009 to 2010 mumps outbreak Children aged 9 to 14 years with a history of 2 MMR vaccine doses, had not previously received a third MMR vaccine dose, and had no history of mumps |
Laboratory confirmed | MMR vaccine not described third dose |
N = 1068 |
Only 2 doses MMR N = 2171 |
1/1068 versus 5/2171 | 59.3% (−247% to 95.2%) VE = (1 − RR) x 100 |
| ca‐Ogbuanu 2012 |
During 2009 to 2010 mumps outbreak Schoolchildren (aged 11 to 17 years) from 3 schools. N = 2665. N = 2178 had validated history of receiving 2 previous doses of MMR. |
Laboratory confirmed | MMR vaccine not described third dose (a) all students with validated 2 doses (b1) postvaccination period 1 to 21 days after third dose (b2) postvaccination period 22 to 41 days after third dose |
Third dose (a) N = 1755 (b1) N = 1751 (b2) N = 1723 |
Only 2 doses MMR (a) N = 432 (b1) N = 420 (b2) N = 413 |
(a) 35/1755 versus 14/432 (b1) 28/1751 versus 7/420 (b2) 1/1723 versus 2/413 |
(a) 39.7% (−11.0% to 67.3%) (b1) 4.1% (−118% to 57.8%) (b2) 88% (−31.9% to 98.9%) VE = (1 − RR) x 100 |
| ca‐Ong 2005 | Children from childcare centres and
primary schools in Singapore, aged 5 to 12 years |
Clinical diagnosis. Standard questionnaire filled by trained public health officer or physician diagnoses. |
(a) Jeryl Lynn (b) Urabe (c) Rubini Health booklet |
(a) N = 711 (b) N = 190 (c) N = 1694 1 or 2 MMR doses |
N = 614 unvaccinated |
(a) 8/711 versus 35/614 (b) 5/190 versus 35/614 (c) 150/1694 versus 35/614 |
(a) 80.3% (57.8% to 90.8%) (b) 53.8%* (c) −55.3% (−121.8% to −8.8%) VE = (1 − RR) x 100 *no statistical evidence |
| ca‐Schlegel 1999 | Children aged 5 to 13 years from a small village in Switzerland | Clinical confirmation after virus isolation or clinical picture observed in sibling of confirmed cases. Parents interview and evaluation by study investigators |
(a) Jeryl Lynn (b) Urabe (c) Rubini Vaccination records |
(a) N = 36 (b) N = 40 (c) N = 79 at least 1 dose |
N = 8 unvaccinated |
(a) 5/36 versus 5/8 (b) 3/40 versus 5/8 (c) 53/79 versus 5/8 |
(a) 78% (64% to 82%) (b) 87% (76% to 94%) (c) −4% VE = (ARU − ARV)/ARU x 100 Orenstein 1985 |
| ca‐Snijders 2012 | Children (aged < 19 years) attending (a) primary schools and (b) their household contacts. (c) index case |
Clinical diagnosis | MMR Jeryl Lynn or RIT 4385 |
(a1) (1 dose) N = 484 (a2) (2 doses) N = 301 (b) (unspecified number of doses) N = 19 (c) (any dose) N = 16 |
(a) N = 351 (b) N = 87 (c) N = 90 unvaccinated |
(a1) 13/484 versus 183/351 (a2) 7/301 versus 183/351 (b) 3/19 versus 44/87 (c) 3/16 versus 44/90 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ adjusted data (a1) 9/484 versus 65/351 (a2) 7/301 versus 86/351 |
(a1) 92% (83% to 96%) (a2) 93% (85% to 97%) (b) 67% (65% to 95%) (c) 11% (−4% to 88%) Adjusted for confounders from Poisson regression VE = 1 − incidence rate In order to include "adjusted data", Di Pietrantonj 2006 method is used to convert adjusted estimates and its 95% CI in "adjusted data". |
| ca‐Takla 2014 | Primary school: 108 students of 5 classes with at least 1 mumps case | Clinical or laboratory confirmed, or both | MMR vaccine: RIT 4385 or Jeryl Lynn strain | (a) (1 dose) N = 4 (b) (2 doses) N = 89 |
N = 6 | (a) 3/4 versus 5/6 (b) 6/89 versus 5/6 |
(a) 10% (−75% to 53%) (b) 91.9% (81.0% to 96.5%) VE = (1 − RR) x 100 |
ARU: attack rate amongst unvaccinated ARV: attack rate amongst vaccinated CI: confidence interval HR: hazard ratio ICD: International Statistical Classification of Diseases and Related Health Problems IgM: immunoglobulin M incidence : cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine N: number of participants OR: odds ratio PT: person‐time in months rr: rate ratio (relative incidence, incidence rate ratio, hazard ratio) RNA: ribonucleic acid RR: risk ratio (relative risk) VE: vaccine effectiveness/efficacy WHO: World Health Organization
4. Mumps: effectiveness ‐ case‐control studies.
| Study | Population characteristics | Case definition | Controls/selection | MMR strain/exposure | N cases vaccinated/ N cases versus N controls vaccinated/ N controls | OR (95% CI) | VE% (95% CI) |
| ba‐Castilla 2009 | Children aged between 15 months and 10 years from Navarre region (Northern Spain) at the time a mumps outbreak occurred (between August 2006 and June 2008) |
(a) N = 181 (b) N = 72 (c) N = 241 Laboratory or epidemiological confirmation of clinical cases: swelling of 1 of more salivary glands for at least 2 days with either laboratory (PCR or IgM positive) or epidemiological confirmation (i.e. epidemiological relation with other laboratory confirmed or clinical mumps cases). Obtained from cases notified to the regional health authority |
(a) N = 875 (b) N = 353 (c) N = 1205 matched for sex, municipality, district of residence, and paediatrician |
(a) 1 dose (b) 2 doses (c) any dose MMR/Jeryl Lynn doses received at least 30 days before symptom disease onset. Blinded review of primary care vaccination registry |
(a) 169/181 versus 852/875 (b) 59/72 versus 330/353 (c) 228/241 versus 1182/1205 |
‐ | (a) 66% (25% to 85%) (b) 83% (54% to 94%) (c) 72% (39% to 87%) adjusted for confounders |
| ba‐Fu 2013 | Children in Guangzhou aged 8 months to 12 years during 2006 to 2012 |
N = 1983 randomly selected clinical definition |
N = 1983 matched 1:1 by birth date, gender, residence not reported breakdown by type of vaccine administrated |
(a) MMR/Jeryl Lynn RIT4385 (b) measles‐mumps (c) missing (vaccine type) (d) any vaccine 1 dose |
(a) 112 versus 145 (b) 242 versus 261 (c) 620 versus 837 (d) 974/1983 versus 1243/1983 |
(a) OR extracted from VE reported 0.49 (0.26 to 0.93) |
(a) 51.3% (7.2% to 95.0%) |
| ba‐Giovanetti 2002 | Children and adolescents aged 14 months to 15 years from urban area of Alba and Bra and 10 rural towns (n = 12,800 residents from 0 to 15 years) during 2000 to 2001 epidemic |
Clinical diagnosis (cases notified by national infectious diseases surveillance system) N = 139 notified mumps cases |
N = 139 randomly selected from immunisation registry, matched for birth year and address. (controls received at least 1 MMR dose) |
MMR vaccine not specified. Vaccination registry and phone interviews, immunisation should have been received at least 30 days before disease onset. |
90/139 versus 111/139 | 0.46 (0.27 to 0.80) | 53.7% (20.4% to 73.0%) |
| ba‐Goncalves 1998 | Children and adolescents (15 months to 16 years) from Oporto (Portugal) |
Clinical diagnosis Cases reported by GPs or hospital doctors, occurred during the 1995 to 1996 mumps outbreak (a) N = 73 (b) N = 133 (c) N = 189 |
2 consecutive
vaccination records
of the same sex, month and birth year as the case
were selected. (a) N = 169 (b) N = 236 (c) N = 378 Controls received at least 1 MMR dose. |
Assuming that before 1 November 1992 MMR mumps Urabe strain was administered, subsequently the Rubini strain (a) Urabe (b) Rubini (c) all at least 1 MMR dose |
(a) 56/73 versus 142/169 (b) 116/133 versus 209/236 (c) 172/189 versus 351/378 |
‐ | (a) 70% (25% to 88%) (b) 1% (−108% to 53%) adjusted for confounders |
| ba‐Harling 2005 | Children and adolescents aged between 1 and 18 years from religious community in Northeast London. Mumps outbreak | Clinical diagnosis N = 156 (GP notification to the local CCDC, mumps diagnoses from electronic practice list, verbal reports by community members) ‐‐‐‐‐‐‐‐‐‐‐‐ Laboratory confirmation of clinical diagnosis N = 43 GP notification to the local CCDC of notified cases, IgM and mumps RNA testing was offered |
N = 175 randomly selected and stratified for age and sex from practice list |
Jeryl Lynn 1 or 2 MMR doses received at least 1 month before index date (a) at least 1 dose (b) 1 dose (c) 2 doses |
79/156 versus 134/175 | (a) 0.31 (0.20 to 0.50) | (a) 69% (50% to 80%) (crude) (a) 69% (41% to 84%) adjusted for age, sex, practice ‐‐‐‐‐‐‐‐‐‐ Laboratory‐confirmed cases (a) 65% (25% to 84%) (b) 64% (40% to 78%) (c) 88% (62% to 96%) All adjusted for age, sex, practice. Proportion of vaccinated in cases and controls not provided. |
| ba‐Kim 2012 | Children (a) prospective case‐control study from March 2010 to October 2011 (b) retrospective case‐control study 2008 to 2009 in western Seoul, Incheon, and Goyang (c) total |
(a) N = 55 (a1) 1 dose (a2) 2 doses (a3) any dose ‐‐‐‐‐‐‐‐‐‐‐‐ (b) N = 122 (b1) 1 dose (b2) 2 doses (b3) any dose ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (c) N = 177 (c1) 1 dose (c2) 2 doses (c3) any dose |
(a) N = 165 (a1) 1 dose (a2) 2 doses (a3) any dose ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (b) N = 449 (b1) 1 dose (b2) 2 doses (b3) any dose ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (c) N = 614 (c1) 1 dose (c2) 2 doses (c3) any dose |
MMR vaccine not described (assumed to be Jeryl Lynn following Park 2015) For (a) and (b): data about demographic characteristics and MMR vaccination status were collected from cases and controls. |
‐‐‐‐‐‐‐‐‐‐(a)‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a1) 0.58 (0.05 to 6.90) (a2) 1.1 (0.09 to 13.3) (a3) 0.67 (0.06 to 7.35) ‐‐‐‐‐‐‐‐‐‐(b)‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (b1) 0.33 (0.02 to 5.33) (b2) 0.11 (0.01 to 2.12) (b3) 0.33 (0.02 to 5.33) ‐‐‐‐‐‐‐‐‐(c)‐‐‐‐‐‐‐‐‐‐‐ (c1) 0.58 (0.10 to 3.56) (c2) 0.42 (0.06 to 2.81) (c3) 0.50 (0.08 to 2.99) |
‐‐‐‐‐‐‐‐(a)‐‐‐‐‐‐‐‐‐ (a1) 42.0%* (a2) −10.0%* (a3) 33.0%* ‐‐‐‐‐‐‐‐‐(b)‐‐‐‐‐‐‐‐‐‐‐ (b1) 67.0%* (b2) 89.0%* (b3) 67.0%* ‐‐‐‐‐‐‐‐‐(c)‐‐‐‐‐‐‐‐‐‐‐ (c1) 42.0%* (c2) 58.0%* (c3) 50.0%* *no statistical evidence |
|
| ba‐Mackenzie 2006 | About 600 pupils attending a boarding school in Scotland during a mumps outbreak that peaked between October and November 2004 |
Virological confirmation
of clinical diagnosis N = 20 (aged 13 to 17 years). Cases notified to consultant in public health medicine. Acute cases with virological positive test |
N = 40 matched for age, sex, residential status, UK or international students |
MMR vaccine not described (a) 1 dose (b) 2 doses (c) any dose Not specified. Pre‐outbreak vaccination status obtained by medical notes held in the school, communication with parents, and from Scottish Immunisation Recall System. |
(a) 9/18 versus 20/34 (b) 2/11 versus 6/20 (c) 11/20 versus 26/40 |
(a) 0.7 (0.22 to 2.21) (b) 0.52 (0.09 to 3.16) (c) 0.66 (0.22 to 1.97) |
(a) 30.0%* (b) 48.1%* (c) 32.4%* *no statistical evidence |
CCDC: Consultant in Communicable Disease Control CI: confidence interval GP: general practitioner ICD: International Statistical Classification of Diseases and Related Health Problems IgM: immunoglobulin M N: number of participants in intervention and control arm MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PCR: polymerase chain reaction PT: person‐time RNA: ribonucleic acid RR: risk ratio (relative risk) VE: vaccine effectiveness/efficacy
5. Rubella: effectiveness ‐ cohort studies.
| Study | Population characteristics | Case definition | Vaccine/strain | N vaccinated sample size (dose) | N control | N events in exposed/ N exposed or person‐months versus N events in non‐exposed/ N non‐exposed or person‐months | VE% (95% CI) |
|
ca‐Chang 2015 Cohort study Secondary attack rate |
Middle school with a total of 1621 students enrolled in the 7th, 8th, and 9th grades, with a total of 37 classes (ages 11 to 13) |
Probable rubella case: defined as a suspected rubella case with fever > 37.5 °C and at least 1 of the following symptoms: arthralgia, arthritis, lymphadenopathy, or conjunctivitis. Laboratory‐confirmed case: required a positive serologic test for rubella IgM antibody. Epidemiologically linked case: confirmed case was defined as a suspected case or a probable case that was not laboratory confirmed, but that was geographically and temporally related to a laboratory‐confirmed case. |
MMR (BRD‐II or RA27/3) A BRD‐II rubella strain vaccine was developed in the 1980s in China, and has been available in the Chinese private market since1993. All monovalent rubella and measles and rubella combined (MR) vaccines in use in China are based on the BRD‐II rubella strain. A domestic measles, mumps, and rubella combined vaccine (MMR) based on BRD‐II strain has been available in China’s private market since 2003. There is also an imported RA27/3 strain‐based vaccine available in China. |
‐ | ‐ | Secondary cases = 2 Exposed person = 47 RR 0.11 (95% CI 0.03 to 0.44) |
89% (56% to 97%) VE = (1 − RR) x 100 |
CI: confidence interval IgM: immunoglobulin M MMR: measles, mumps, rubella vaccine RR: risk ratio (relative risk) VE: vaccine effectiveness/efficacy
6. Varicella: effectiveness ‐ RCTs/CCTs.
| Study ID and design | Population enrolled | Outcome | Vaccine arms n = sample size | Comparator arm n = sample size | Vaccine arm events/n | Comparator arm events/n | VE% (95% CI) |
|
aa‐Prymula 2014 RCT |
This study is the first phase (1 September 2005 to 29 June 2009) of an RCT. The study was done in 111 study centres in Europe: Czech Republic (22), Greece (11), Italy (9), Lithuania (9), Norway (5), Poland (10), Romania (9), Russia (14), Slovakia (17), and Sweden (5). An eligible participant was a healthy child aged 12 to 22 months at the time of the first vaccination; had a negative history of varicella, mumps, measles, and rubella diseases and vaccinations; and was one of the following: (1) at home with at least 1 sibling (with negative history of varicella disease and vaccination), (2) attending a child minder (where at least 1 child was without a known positive history of varicella disease and vaccination), (3) playing for more than 5 min weekly with children without a known positive history of varicella disease and vaccination, (4) registered to attend a day‐care centre from 24 months of age. An eligible participant’s parents or guardians had direct access to a telephone and were deemed by the investigator of being capable of complying with the requirements of the trial protocol. |
The primary efficacy endpoint was the occurrence of confirmed varicella from 42 days after the second vaccine dose to the end of the first phase of the trial. The secondary efficacy endpoint was the occurrence of confirmed varicella graded by severity over the same time period. Varicella cases (a) All (b) Moderate/severe Follow‐up = 3 years |
MMRV group: 2 doses of MMRV (Priorix‐Tetra, GSK) N = 2279 MMR+V group: 1 dose MMR (Priorix, GSK) and monovalent varicella vaccine (Varilrix, GSK) at dose 2 N = 2263 |
MMR group (control): 2 doses of MMR (Priorix, GSK) N = 743 |
MMRV (a) 37/2279 (b) 2/2279 MMR+V (a) 243/2263 (b) 37/2263 |
MMR (a) 201/743 (b) 117/743 |
MMRV (a) 94.9% (92.4% to 96.6%) (b) 99.5% (97.5% to 99.9%) MMR+V (a) 65.4% (57.2% to 72.1%) (b) 90.7% (85.9% to 93.9%) VE = (1 − HR) x 100 |
|
aa‐Henry 2018 RCT linked to aa‐Prymula 2014 |
Healthy children aged 12 to 22 months. n = 5803 children enrolled and vaccinated (TVC) in phase A, n = 4580 in the TVC in phase B, n = 3829 completed the study up to Year 6; n = 5289 ATP cohort for efficacy in phase A + B, n = 3791 in the ATP cohort for efficacy in phase B |
Varicella cases (a) All (b) Moderate/severe (c) Severe Follow‐up = 6 years |
ATP
cohort for efficacy phase A + B MMRV n = 2279 MMR+V n = 2266 Phase B MMRV n = 1802 MMR+V n = 1593 MMRV group 2 doses of MMRV (Priorix‐Tetra, GSK) at Day 0 and Day 42 MMR+V group 1 dose of MMR (Priorix, GSK) at Day 0 and 1 dose of monovalent varicella vaccine (Varilrix, GSK) at Day 42 |
ATP
cohort for efficacy phase A + B MMR n = 744 Phase B MMR n = 396 MMR group 2 doses of the MMR (Priorix, GSK) vaccine at Day 0 and Day 42 |
Phase A + B MMRV (a) 71/2279 (b) 6/2279 (c) 0/2270 MMR+V (a) 419/2266 (b) 58/2266 (c) 1/2266 Phase B MMRV (a) 33/1800 (b) 4/1800 (c) 0/1800 MMR+V (a) 176/1592 (b) 18/1592 (c) 0/1592 |
Phase A + B MMR (a) 325 /744 (b) not reported Phase B (a) 125/396 (b) not reported |
Phase A + B MMRV (a) 95.0% (93.6% to 96.2%) (b) 99.0% (97.7% to 99.6%) (c) undefined MMR+V (a) 67.0% (61.8% to 71.4%) (b) 90.3% (86.9% to 92.8%) (c) 94.6% (55.3% to 99.4%) Phase B MMRV (a) 95.3% (93.1% to 96.8%) (b) 98.4% (95.5% to 99.4%) (c) undefined MMR+V (a) 69.5% (61.5% to 75.8%) (b) 91.8% (85.9% to 95.2%) (c) undefined VE = (1 − HR) x 100 |
|
aa‐Povey 2019 RCT linked to aa‐Prymula 2014 |
Children aged 12 to 22 months were eligible for inclusion if: had not received MMR or varicella vaccines, or both, or had measles‐mumps‐rubella or varicella zoster or herpes zoster diseases, or both, and were at home with at least 1 sibling with negative history of varicella disease and vaccination, at a child‐minders where at least 1 child was without a known positive history of varicella disease and vaccination, playing for more than 5 min/week with children without a known positive history of varicella disease and vaccination, or registered to attend day care from 24 months. |
Varicella cases (a) All (b) Moderate/Severe Follow‐up = 10 years |
Phase A + B MMRV n = 2279 MMR+V n = 2266 Phase B MMRV n = 1800 MMR+V n = 1591 MMRV group 2 doses of MMRV (Priorix‐Tetra, GSK) at Day 0 and Day 42 MMR+V group 1 dose of MMR (Priorix, GSK) at Day 0 and 1 dose of monovalent varicella vaccine (Varilrix, GSK) at Day 42 |
Phase A + B
MMR n = 744 Phase B MMR n = 396 MMR group 2 doses of the MMR (Priorix, GSK) vaccine at Day 0 and Day 42 |
Phase A + B MMRV (a) 71/2279 (b) 6/2279 MMR+V (a) 469/2266 (b) 67/2266 Phase B MMRV (a) 33/1800 (b) 4/1800 MMR+V (a) 176/1592 (b) 18/1592 |
Phase A + B MMR (a) 352/744 (b) 176/744 Phase B (a) 149/396 (b) 59/396 |
Phase A + B MMRV (a) 95.4% (94.0% to 96.4%) (b) 99.1% (97.7% to 99.6%) MMR+V (a) 67.2% (62.3% to 71.5%) (b) 89.5% (86.1% to 92.1%) Phase B MMRV (a) 95.9% (94.1% to 97.1%) (b) 98.7% (96.4% to 99.5%) MMR+V (a) 69.8% (62.8% to 75.5%) (b) 90.0% (84.2% to 93.7%) VE = (1 − HR) x 100 |
ATP: according‐to‐protocol CI: confidence interval MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MMR+V: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time RCT: randomised controlled trial RR: risk ratio (relative risk) TVC: total vaccinated cohort VE: vaccine effectiveness/efficacy
7. Varicella: effectiveness ‐ cohort studies.
| Study | Population characteristics | Case definition | Vaccine/strain | N vaccinated sample size (dose) | N control | N events in exposed/ N exposed or person‐months versus N events in non‐exposed/ N non‐exposed or person‐months | VE% (95% CI) |
| ca‐Giaquinto 2018 | Children aged 0 to 14 registered with 35 Pedianet database physicians across Italy between 1 October 1997 and 30 September 1998 |
Varicella cases recorded in the Pedianet databases are based on physician confirmation only (no laboratory tests were performed). |
MMRV: vaccine ProQuad | n = 2357 | n = 912 unvaccinated | 43/2357 versus 287/912 | unadjusted estimate 94% (92% to 96%) adjusted estimate 94% (91% to 95%) VE = (1 −RR) x 100 |
| ca‐Rieck 2017 | Between January 2006 and October 2013, n = 1,449,411 children |
4‐step algorithm to only select confirmed
and incident varicella cases. Step 1: excluded incompatible or implausible coding combinations for varicella diagnosis reliability; step 2: excluded observations with diagnosis reliability other than confirmed (i.e. suspected, excluded, recovered); step 3: excluded observations with diagnosis type other than incident (i.e. previous state, unknown, not provided); step 4: limited the data selection to the earliest ICD‐10 code per patient whilst also keeping the information about the most severe ICD‐10 code (within up to one‐quarter following the initial diagnosis) using the following ranking (in descending order of severity): varicella with encephalitis, meningitis, pneumonia, other complications, no complications, no further details, with the last equalling ‘no complications’. |
Since 2004, single‐dose varicella vaccination has been recommended for all children aged 11 to 14 months. 2 single‐compound varicella vaccines (VAR; Varivax, Sanofi Pasteur MSD; Varilrix, GSK) were initially available. In 2006, a combined (MMR)‐varicella vaccine (MMRV; Priorix‐Tetra, GlaxoSmithKline) was licenced with a 2‐dose schedule. A 2‐dose schedule has been recommended since 2009 targeting children with the second dose at age 15 to 23 months. Since 2011, the first immunisation has been given preferably as 2 separate injections of VAR and MMR due to higher rates of febrile seizures following immunisation with MMRV. (a) 1 dose MMRV (b) 2 doses MMRV |
‐ | ‐ | ‐ |
VE = (1 − HR) x 100 adjusted estimate (a) 81.7% (81.0% to 82.4%) (b) 94.4% (94.2% to 94.6%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ VE = (1 −RR) x 100 RR obtained from HR and attack rate of varicella in unvaccinated children, Risk in unvaccinated children = 9% (a) 61.8% (60.6% to 63.0%) (b) 86.6% (86.1% to 87.0%) |
| ca‐Spackova 2010 | 1084 children attended day‐care centres in Germany | Varicella was classified clinically as mild (< 50 skin lesions), moderate (≥ 50 skin lesions), severe (any hospitalised case). |
MMRV Priorix‐Tetra (a) All‐brand doses (b1) All‐brand 1 dose (b2) All‐brand 2 dose ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (c) Varivax 1 dose (d) Varilrix 1 dose (e1) Priorix‐Tetra 1 dose (e2) Priorix‐Tetra 2 doses ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (f1) Mild disease (f2) Moderate disease |
(a) n = 244 (b1) n = 167 (b2) n = 77 (c) n = 48 (d) n = 77 (e1) n = 38 (e2) n = 56 (f1) n = 233 (f2) n = 221 |
n = 108 (f1) n = 71 (f2) n = 93 |
(a) 33/244 versus 52/108 (b1) 31/167 versus 52/108 (b2) 2/77 versus 52/108 (c) 4/48 versus 52/108 (d) 19/77 versus 52/108 (e1) 7/38 versus 52/108 (e2) 2/56 versus 52/108 (f1) 22/233 versus 15/71 (f2) 10/221 versus 37/93 |
(a) 71% (57% to 81%) (b1) 62% (43% to 75%) (b2) 94% (75% to 98%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (c) 86% (56% to 96%) (d) 56% (29% to 72%) (e1) 55% (8% to 78%) (e2) 91% (65% to 98%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (f1) 53% (14% to 75%) (f2) 89% (78% to 95%) adjusted for confounders VE = (ARU − ARV)/ARU x 100 Orenstein 1985 VE = (1 − RR) x 100 |
| ca‐Tafuri 2013 | Children at (a) preschool (b) elementary school (c) all ages |
Reported by parents | MMRV (Priorix‐Tetra) Varicella OKA; 1 dose |
(a) n = 170 (b) n = 71 (c) n = 241 |
(a) n = 40 (b) n = 287 (c) n = 327 |
(a) 2/170 versus 14/40 (b) 2/71 versus 223/287 (c) 4/241 versus 237/327 |
(a) Not reported (b) 69.2% (50.5% to 88.1%) (c) 59.9% (48.3% to 69.8%) VE = (ARU − ARV)/ARU x 100 Orenstein 1985 VE = (1 − RR) x 100 |
ARU: attack rate amongst unvaccinated ARV: attack rate amongst vaccinated CI: confidence interval ICD‐10: International Classification of Diseases, Tenth Revision HR: hazards ratio MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time RR: risk ratio (relative risk) VE: vaccine effectiveness/efficacy
8. Varicella: effectiveness ‐ case‐control studies.
| Study | Population characteristics | Case definition | Controls/selection | MMR strain/exposure | N cases vaccinated/N cases versus N controls vaccinated/N controls | OR (95% CI) | VE% (95% CI) |
| ba‐Andrade 2018 | From November 2013 to December 2015, children aged 15 to 32 months | Cases were defined as children aged 15 to 32 months with rash and either suspected as having varicella by an attending physician or being a contact to a confirmed varicella case. Cases were confirmed by either clinical or laboratory criteria. Cases: n = 168 Cases were further classified by severity of disease based on number of skin lesions, being: (1) mild – fewer than 50 lesions; (2) mild/moderate – between 50 and 249 lesions; (3) moderate – between 250 and 499 lesions; or (4) severe – 500 lesions or more, having been hospitalised, or having any complication. |
Controls matched 1:2 by: age (15 to 32 months). Controls were defined as children residing in the neighbourhood of the case, in which no history of varicella or outpatient clinics visits due to skin lesion was reported. To identify controls, houses nearby the cases were visited following a systematic sampling procedure. Controls: n = 301 |
MMRV A combined tetravalent vaccine containing measles, mumps, rubella, and varicella antigens (MMRV), manufactured by GlaxoSmithKline |
(a) Any severity (b) Moderate severe cases > 50 lesions | Adjusted‐estimates (a) 0.14 (0.07 to 0.28) (b) 0.07 (0.03 to 0.18) adjusted for confounders: age in months, day‐care attendance, and pulmonary diseases |
(a) 86% (72% to 92%) (b) 93% (82% to 97%) VE = 1 − OR |
| ba‐Cenoz 2013 | Children between 15 months and 10 years of age | PCR‐confirmed varicella Cases n = 54 |
Matched 1:8 by paediatric practice, district of residence, and date of birth (± 1 year) Controls n = 432 |
MMR+V (Varivax OKA/Merck) not described (a) any doses and age (a1) 1 dose (a2) 2 doses (b) age < 3 years (b1) 1 dose (c) age ≥ 3 years (c1) 1 dose (c2) 2 doses |
(a) 6/54 versus 175/432 (a1) 5/54 versus 112/432 (a2) 1/54 versus 63/432 (b1) 1/6 versus 36/48 (c1) 4/48 versus 76/384 (c2) 1/48 versus 63/384 |
‐ | Adjusted estimates (a) 92% (77% to 97%) (a1) 87% (60% to 97%) (a2) 97% (79.5% to 99.6%) (b1) 84% (−58% to 100%)(*) (c1) 80% (37% to 95%) (c2) 97% (79% to 100%) VE = (1 −OR) x 100 (*) no statistical evidence |
| ba‐Liese 2013 | Children at least 1 year of age, born on or after 1 July 2003, who resided in Germany | PCR‐confirmed varicella n = 432 |
Children matched by age and paediatric practice, fulfilling the same criteria as cases but without history or present clinical diagnosis of varicella n = 432 |
Any varicella vaccine (a1) 1 dose (a2) 2 doses ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ OKA/GSK (b1) 1 dose (b2) 2 doses ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other than OKA/GSK* (c1) 1 dose (c2) 2 doses ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Unknown vaccine (d1) 1 dose (d2) 2 doses ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Any varicella vaccine (after vaccination) (y1) up to 1 year (y2) 1 to 2 year (y3) 4 to 5 year ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (*) includes OKA/Merck and MMR‐OKA/GSK |
(a) 57/432 versus 195/432 (a1) 55/430 versus 153/390 (a2) 2/377 versus 42/279 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (b1) 35/410 versus 63/300 (b2) 0/375 versus 6/243 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (c1) 19/394 versus 87/324 (c2) 2/377 versus 25/262 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (d1) 1/376 versus 3/240 (d2) 0/375 versus 11/248 |
‐ | Adjusted estimates (a1) 86.4% (77.3% to 91.8%) (a2) 94.3% (76.4% to 98.6%) (b1) 71.5% (49.1% to 84.0%) (b2) not reported (c1) not reported (c2) not reported (d1) not reported (d2) not reported (y1) 94.5% (76.9% to 98.7%) (y2) 81.5% (56.8% to 92.1%) (y3) 73.2% (9.1% to 92.1%) VE = (1 − OR) x 100 |
| ba‐Vazquez 2001 | Children between 13 months and 16 years of age. (a) < 5 years old (b) 5 to 10 years old (c) > 10 years old (d) all ages |
PCR‐confirmed varicella n = 202 |
Matched 1:2 according to date of birth (within 1 month) and paediatric practice n = 389 |
MMR+V Vaccine type and number of doses not described |
46/202 versus 238/389 | ‐ | Adjusted estimates (a) 79% (61% to 89%) (b) 89% (80% to 94%) (c) 92% (45% to 99%) (d) 87% (78% to 90%) VE = (1 − OR) x 100 |
CI: confidence interval IgM: immunoglobulin M MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MMR+V: measles, mumps, rubella, and varicella vaccine n: number of participants in intervention and control arm OR: odds ratio PCR: polymerase chain reaction VE: vaccine effectiveness/efficacy WHO: World Health Organization
9. Varicella: effectiveness ‐ case‐only ecological method studies.
| Study | Population characteristics | Case definition | Exposure MMR/MMRV vaccine | Crude data | Estimate (95% CI) | VE% (95% CI) |
|
ga‐Boccalini 2015 Case‐only ecological method |
Hospitalisation between 2004 to 2012 in the Tuscan region. Aged 0 to 14 years (a) age < 1 year (b) age 1 to 4 years (c) age 5 to 14 years |
Hospitalised cases for varicella or its complications, as a primary or secondary discharge diagnosis, with the following ICD‐9‐CM codes (2002 and 2007) were examined: 052.0 (post‐varicella encephalitis), 052.1 (varicella (haemorrhagic) pneumonitis), 052.2 (post‐varicella myelitis), 052.7 (varicella with other specified complications), 052.8 (varicella with unspecified complication), 052.9 (varicella without complication). |
MMRV vaccine: not described and monovalent varicella vaccine Reference period 2004 to 2007 Exposed period 2009 to 2012 Data from 2008, the transition year between the 2 periods, were excluded from our analysis in this study. |
Reference period (a) 73/122,483 (b) 189/478,481 (c) 105/1,141,304 Exposed period (a) 42/128,440 (b) 99/523,810 (c) 55/1,222,222 |
RR (95% CI) (*) (a) 0.55 (0.38 to 0.80) (b) 0.48 (0.38 to 0.61) (c) 0.48 (0.35 to 0.67) (*) Relative risk between exposed and reference period |
VE = 1 − RR (a) 45.1% (19.8% to 62.5%) (b) 52.2% (39% to 62.5%) (c) 51.1% (32.2% to 64.7%) |
| ga‐Pozza 2011 Case‐only ecological method | Hospitalisation between 2000 to 2008 in the Veneto region. Aged 0 to 14 years |
Varicella cases incidence: (a) from surveillance data retrieved from the RDP (b) sentinel surveillance system based on a sample of paediatricians (SPES). Hospitalised cases for varicella hospital discharges that reported in the primary and secondary diagnoses codes 052.X. Admissions with coexistent codes for herpes zoster, i.e. 053.X, were excluded. (c) hospitalisations |
MMRV vaccine: not described and monovalent varicella vaccine Reference period 2000 to 2006 Exposed period 2007 to 2008 |
Cases/person time (RDP) incidence reference period (a) 81,276/438,3097 Exposed period (a) 14,749/1,345,351 (SPES) incidence reference period (b) 13,543/196,949 Exposed period (b) 1344/26,861 Hospitalised reference period (c) 770/4,383,497 Exposed period (c) 126/1,348,474 |
rr (95% CI) (a) 0.59 (0.58 to 0.6) (b) 0.73 (0.69 to 0.77) (c) 0.53 (0.44 to 0.64) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a1) 0.44 (0.43 to 0.45) (b1) 0.58 (0.53 to 0.64) (c1) 0.48 (0.37 to 0.63) Sensitivity analysis Data from 2007, the transition year between the 2 periods, were excluded from analysis. |
VE = (1 − rr) x 100 (a) 40.9% (39.8% to 41.9%) (b) 27.2% (23% to 31.2%) (c) 46.8% (35.8% to 55.9%) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a1) 56.2% (54.9% to 57.3%) (b1) 41.8% (36.2% to 46.8%) (c1) 52.2% (37.4% to 63.5%) |
| ga‐Tafuri 2015 Case‐only ecological method | Hospitalisation between 2003 to 2012 in the Puglia region. Aged 0 to 14 years (a) age < 1 year (b) age 1 to 4 years (c) age 5 to 14 years |
Hospitalised cases for varicella Hospitalisation rates, overall and specific by age, were calculated on data extracted from the regional HDR, selecting all hospital admissions with a main diagnosis of chickenpox or its complications (ICD9‐CM codes: 052.x) in the same period. Incidence rates, overall and specific by age, between 2003 and 2012 were calculated by using data collected in the Apulian computerised surveillance system for communicable diseases. |
MMRV vaccine: not described and monovalent varicella vaccine Reference period 2003 to 2005 Exposed period 2009 to 2012 |
Hospitalised reference period (a) 245/39,618 (b) 2148/163,321 (c) 2201/451,858 Exposed period (a) 39/37,356 (b) 161/152,607 (c) 289/420,058 Incidence reference period (a) 14/39,548 (b) 57/1,623,931 (c) 42/446,809 Exposed period (a) 5/39,063 (b) 9/160,714 (c) 10/434,783 |
rr (95% CI)(*) Hospitalised (a) 0.17 (0.12 to 0.24) (b) 0.08 (0.07 to 0.09) (c) 0.14 (0.12 to 0.16) Incidence (a) 0.36 (0.13 to 1.03) (b) 0.16 (0.08 to 0.33) (c) 0.25 (0.12 to.050) (*) Relative risk between exposed and reference period |
VE = (1 − rr) x 100 Hospitalised (a) 63.8% (−0.4% to 87%) (b) 84% (67.8% to 92.1%) (c) 75.5% (51.2% to 87.7%) Incidence (a) 83.1% (76.3% to 88%) (b) 92% (90.6% to 93.2%) (c) 85.9% (84% to 87.5%) |
CI: confidence interval HDR: hospital discharge registry ICD‐9‐CM MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine n: number of participants in intervention and control arm RDP: Regional Department of Prevention SPES: Sorveglianza PEdiatric Sentinella VE: vaccine effectiveness/efficacy
Vaccine safety‐harms
We included 87 studies on the safety of MMR/MMRV vaccines, with the following study designs: 7 RCTs/CCTs, 21 case control, 32 cohorts, 16 SCCS/PTC, 3 CCO, and 4 COEM. Seven of 87 studies reported data on several adverse effects and were therefore included in each corresponding comparison group (cb‐McKeever 2004; cb‐Timmermann 2015; db‐Farrington 1995; db‐Makela 2002; db‐Miller 2007; db‐Perez‐Vilar 2018; db‐Ward 2007). The studies evaluating adverse events are presented in 18 main groups.
Short‐term side effects: overall 17 studies: 7 RCTs/CCTs, ab‐Bloom 1975; ab‐Ceyhan 2001; ab‐Edees 1991; ab‐Freeman 1993; ab‐Lerman 1981; ab‐Peltola 1986; ab‐Schwarz 1975, and 10 cohort studies (cb‐Beck 1989; cb‐Benjamin 1992; cb‐Dunlop 1989; cb‐Makino 1990; cb‐Miller 1989; cb‐Robertson 1988; cb‐Sharma 2010; cb‐Stokes 1971; cb‐Swartz 1974; cb‐Weibel 1980). See Table 31 and Table 32.
Encephalitis or encephalopathy: overall 3 studies: 1 case control (bb‐Ray 2006), 1 SCCS (db‐Ward 2007), and 1 PTC (db‐Makela 2002). See Table 33.
Aseptic meningitis: overall 10 studies: 1 case control (bb‐Black 1997), 4 SCCS/PTC (db‐Dourado 2000; db‐Farrington 1995; db‐Miller 2007; db‐Perez‐Vilar 2018), 1 PTC (db‐Makela 2002), 2 CCO (eb‐Ki 2003; eb‐Park 2004), and 2 COEM (gb‐da Cunha 2002; gb‐da Silveira 2002). See Table 34.
Seizure ‐ febrile/afebrile: overall 8 studies: 2 cohort (cb‐Barlow 2001; cb‐Vestergaard 2004), 4 SCCS (db‐Farrington 1995; db‐Macartney 2017; db‐Miller 2007; db‐Ward 2007), and 2 PTC (db‐MacDonald 2014; db‐McClure 2019). See Table 35.
MMRV versus MMR/MMR+V ‐ febrile seizures: overall 7 cohort (cb‐Gavrielov‐Yusim 2014; cb‐Jacobsen 2009; cb‐Klein 2010; cb‐Klein 2012; cb‐Klein 2017; cb‐Rowhani‐Rahbar 2013; cb‐Schink 2014). See Table 36.
Autism spectrum disorders: overall 13 studies: 4 cohort (cb‐Hviid 2019; cb‐Jain 2015; cb‐Madsen 2002; cb‐Uchiyama 2007), 4 case control (bb‐De Stefano 2004; bb‐Mrozek‐Budzyn 2010; bb‐Smeeth 2004; bb‐Uno 2012), 1 SCCS (db‐Taylor 1999), 1 PTC (db‐Makela 2002), and 3 COEM (gb‐Fombonne 2001; gb‐Fombonne 2006; gb‐Honda 2005). See Table 37.
Inflammatory bowel disease: overall 6 studies: 4 case control, bb‐Baron 2005; bb‐Davis 2001; bb‐Shaw 2015; bb‐Vcev 2015, and 2 COEM (gb‐Seagroatt 2005; gb‐Taylor 2002). See Table 38.
Cognitive delay, developmental delay: 1 cohort study reported data on cognitive delay (cb‐Mrozek‐Budzyn 2013). See Table 39.
Idiopathic thrombocytopenic purpura: overall 9 studies: 2 case control (bb‐Bertuola 2010; bb‐Black 2003), 5 SCCS (db‐Andrews 2012; db‐Farrington 1995; db‐France 2008; db‐O'Leary 2012; db‐Perez‐Vilar 2018), 1 CCO (eb‐Lafaurie 2018), 1 COEM (gb‐Jonville‐Bera 1996). See Table 40.
Henoch‐Schönlein purpura: 1 case control study (bb‐Da Dalt 2016). See Table 41.
Type 1 diabetes: 2 cohort studies (cb‐Beyerlein 2017; cb‐Hviid 2004). See Table 42.
Asthma: 5 cohort studies (cb‐Benke 2004; cb‐DeStefano 2002; cb‐Hviid 2008; cb‐McKeever 2004; cb‐Timmermann 2015). See Table 43.
Dermatitis or eczema: 2 cohort studies (cb‐McKeever 2004; cb‐Timmermann 2015). See also Table 44.
Hay fever, rhinoconjunctivitis, hypersensitivity/allergy: overall 3 studies: 1 cohort study (cb‐Timmermann 2015), 2 case control (bb‐Bremner 2005; bb‐Bremner 2007). See Table 45.
Acute leukaemia: 4 case control studies (bb‐Dockerty 1999; bb‐Groves 1999; bb‐Ma 2005; bb‐Mallol‐Mesnard 2007). See Table 46.
Demyelinating diseases, multiple sclerosis, encephalomyelitis, acute disseminated encephalomyelitis (ADEM): overall 3 studies reported data on demyelinating diseases, multiple sclerosis, and ADEM: 1 cohort study (cb‐Ahlgren 2009), 2 case control studies (bb‐Ahlgren 2009; bb‐Chen 2018). See Table 47.
Gait disturbances: 1 SCCS (db‐Miller 2005). See Table 48.
Bacterial or viral infections: 2 SCCS reported data on bacterial or viral infections (db‐Miller 2003; db‐Stowe 2009). See Table 49.
10. Safety: short‐term side effects (local or systemic reactions) ‐ RCTs/CCTs.
| Study ID and design | Population enrolled | Vaccine arm n = sample size | Comparator arm n = sample size | Outcome | MMR vaccine arm events/n | Other vaccine arms events/n | Comparator arm events/n |
|
ab‐Bloom 1975; RCT |
Children aged 11 months to 4 years Observation period 21 days |
MMR vaccine Measles Schwarz Mumps Jeryl Lynn Rubella Cendehill n = 183 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature above normal sample size n = 160 Normal temperature rectal 99.6 °F (37.5 °C) (163 children) Oral 98.6 °F (37 °C) (6 children) Axillary 97.6 °F (36.4 °C) (26 children) |
Placebo n = 40 ‐‐‐‐‐‐‐‐‐‐ Temperature sample size n = 35 |
Reactions (a) Rash (b) Lymphadenopathy (c) Coryza (d) Rhinitis (e) Cough (f) Other total ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature above normal (a) 1.5 to 2.4 °F (b) 2.5 to 3.4 °F (c) 3.5 to 4.4 °F (d) 4.5 to 4.9 °F (e) ≥ (normal + 1.5) °F |
MMR vaccine (a) 22/183 (b) 2/183 (c) 4/183 (d) 2/183 (e) 5/183 (f) 35/183 total 70/183 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature above normal (a) 17/160 (b) 1/160 (c) 5/160 (d) 2/160 (e) 25/160 |
‐ |
Placebo arm (a) 2/40 (b) 1/40 (c) 4/40 (d) 4/40 (e) 1/40 (f) 8/40 total 20/40 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature above normal (a) 2/35 (b) 2/35 (c) 0/35 (d) 0/35 (e) 4/35 |
|
ab‐Ceyhan 2001; CCT |
Infants aged 38 to 40 months Observation period 28 days |
Arm A: n = 442 (1) MV/Rouvax Measles Schwarz at 9 months; and (2) MMR/Trimovax Measles Schwarz Mumps Urabe AM9 Rubella Wistar RA 27/3 at 15 months ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Arm B: n = 495 (3) MMR/Trimovax Measles Schwarz Mumps Urabe AM9 Rubella Wistar RA 27/3 at 12 months |
No placebo arm |
Systemic reactions (a) Fever (b) Runny nose (c) Cough (d) Rash (e) Diarrhoea ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Local (f) Redness (g) Swelling ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Total events (x) Fever (y) Systemic (z) Local |
MMR vaccine (2)15 months; (3)12 months (a) 40/442; 55/495 (b) 7/442; 22/495 (c) 36/442; 34/495 (d) 16/442; 19/495 (e) 2/442; 5/495 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Local (2)15 months; (3)12 months (f) 14/442; 19/495 (g) 2/442; 3/495 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Total events (2)15 months; (3)12 months (x) 40/442; 55/495 (y) 61/442; 80/495 (z) 16/442; 22/495 |
MV vaccine (1) 9 months (a) 38/442 (b) 19/442 (c) 28/442 (d) 2/442 (e) 5/442 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Local (f) 7/442 (g) 2/442 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Total events (x) 38/442 (y) 54/442 (z) 9/442 |
|
|
ab‐Edees 1991; RCT |
Children aged 12 to 18 months. Observation period 21 days |
Arm A: n = 196 MV/Rouvax Measles Schwarz ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Arm B: n = 198 MMR/Trimovax Measles Schwarz Mumps Urabe AM9 Rubella Wistar RA 27/3 |
No placebo arm |
Local symptoms
(a) Erythema
(b) Induration
(c) Pain ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Specific systemic (a) Rash (b) Parotitis (c) Conjuntivitis (d) Testicular swelling (e) Arthralgia (f) Arthritis (g) Convulsion ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Non‐specific systemic (a) Fever (b) Adenopathy (c) Nasopharyngeal disorders (d) Gastrointestinal disorders (e) Restlessness Restlessness: used to describe a non‐specifically unwell child; it covers terms such as irritable miserable tearful clingy not sleeping. |
MMR vaccine (Arm B) Local (a) 18/198 (b) 1/198 (c) 9/198 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Specific systemic (a) 87/198 (b) 5/198 (c) 17/198 (d) 0/198 (e) 0/198 (f) 0/198 (g) 0/198 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Non‐specific systemic (a) 76/198 (b) 2/198 (c) 113/198 (d) 83/198 (e) 124/198 |
MV vaccine (Arm A) Local (a) 16/196 (b) 0/196 (c) 14/196 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Specific systemic (a) 100/196 (b) 0/196 (c) 21/196 (d) 0/196 (e) 0/196 (f) 0/196 (g) 0/196 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Non‐specific systemic (a) 74/196 (b) 3/196 (c) 115/196 (d) 74/196 (e) 147/196 |
|
|
ab‐Lerman 1981; RCT |
Children aged 15 months to 5 years Observation period 42 days |
Arm(1): n = 43:
Measles (MSD) Arm(2): n = 41: Mumps (MSD) Jeryl Lynn Arm(3): n = 47: Rubella HPV‐77:CE‐5 Arm(4): n = 142 MMR (MSD) with Rubella HPV‐77:DE‐5 Arm(5): n = 46: Rubella/Wistar RA27/3 Arm(6): n = 141: MMRII (MSD) with Rubella Wistar RA27/3 |
Placebo arm
n = 42 (vaccine diluent) 1 dose subcutaneously |
Reactions (a) Local reaction (b) Fever 101 to 102.9 °F (fever 38.3 to 39.4 °C) (c) Fever 103 to 104.9 °F (fever 39.4 to 40.5 °C) (d) Respiratory symptoms (e) Rash (f) Lymphadenopathy (g) Sore eyes (h) Joint symptoms |
MMR vaccine Arms: (4); (6) (a) 7/142; 11/141 (b) 31/142; 35/141 (c) 11/142; 16/141 (d) 97/142; 102/141 (e) 24/142; 28/141 (f) 6/142; 11/141 (g) 24/142; 23/141 (h) 1/142; 1/141 |
Other vaccine arms: (1); (2); (3); (5) (a) 1/43; 6/41; 3/47; 2/46 (b) 12/43; 6/41; 6/47; 11/46 (c) 2/43; 3/41; 3/47; 2/46 (d) 34/43; 26/41; 31/47; 31/46 (e) 5/43; 1/41; 6/47; 5/46 (f) 1/43; 2/41; 2/47; 2/46 (g) 6/43; 8/41; 8/47; 8/46 (h) 0/43; 0/41; 0/47; 0/46 |
Placebo arm (a) 3/42 (b) 10/42 (c) 0/42 (d) 31/42 (e) 4/42 (f) 0/42 (g) 4/42 (h) 0/42 |
|
ab‐Peltola 1986; RCT |
Pairs of twins aged (a) 14 to 18 months (first dose) (b) 6 years (second dose) Observation period 21 days |
MMR vaccine Vivirac (MSD) 2 doses n = 581 |
Placebo arm n = 581 | No data available for quantitative synthesis | |||
|
ab‐Schwarz 1975; RCT |
Children aged 10 months to 8 years Observation period 21 days |
MMR vaccine Measles Schwarz Mumps Jeryl Lynn Rubella Cendehill n = 403 |
Placebo arm n = 205 |
Temperature
(1) Axillary (2) Rectal ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) < 37.0 °C (b) 37.0 to 37.4 °C (c) < 37.5 °C (d) 37.5 to 37.9 °C (e) 38.0 to 38.4 °C (f) 38.5 to 38.9 °C (g) 39.0 to 39.4 °C (h) 39.5 to 39.9 °C (i) 40.0 to 40.4 C° ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Reactions (s1) Rash (s2) Lymphadenopathy (s3) Conjunctivitis (s4) Otitis media (s5) Coryza (s6) Rhinitis (s7) Pharyngitis (s8) Cough (s9) Headache (s10) Parotitis (s11) Orchitis (s12) Arthralgia (s13) Paraesthesia |
MMR vaccine (1) Temperature axillary (a) 56/244 (b) 154/244 (c) 210/244 (d) 21/244 (e) 6/244 (f) 2/244 (g) 3/244 (h) 2/244 (i) 0/244 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (2) Temperature rectal (a) not reported (b) not reported (c) 48/142 (d) 51/142 (e) 30/142 (f) 8/142 (g) 1/142 (h) 1/142 (i) 3/142 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Reactions (s1) 36/403 (s2) 4/403 (s3) 8/403 (s4) 4/403 (s5) 8/403 (s6) 69/403 (s7) 2/403 (s8) 7/403 (s9) 1/403 (s10) 0/403 (s11) 0/403 (s12) 1/403 (s13) 0/403 |
Placebo arm (1) Axillary temperature (a) 32/176 (b) 132/176 (c) 164/176 (d) 9/176 (e) 2/176 (f) 1/176 (g) 0/176 (h) 0/176 (i) 0/176 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (2) Rectal temperature (a) Not reported (b) Not reported (c) 6/28 (d) 13/28 (e) 6/28 (f) 1/28 (g) 2/28 (h) 0/28 (i) 0/28 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Reactions (s1) 9/205 (s2) 4/205 (s3) 5/205 (s4) 1/205 (s5) 5/205 (s6) 59/205 (s7) 2/205 (s8) 1/205 (s9) 1/205 (s10) 0/205 (s11) 0/205 (s12) 0/205 (s13) 0/205 |
|
|
ab‐Freeman 1993; Cluster‐RCT |
Children aged 13 to 15 months Observation period 30 days |
MMR vaccine MMRII (MSD) n = 253 |
No placebo arm |
Reactions (a) Lymphadenopathy (b) Nasal discharge (c) Rash (d) Otitis media (e) Conjunctival abnormality (f) Abnormal tonsils |
Reactions (a) 57/240 (b) 15/240 (c) 11/240 (d) 8/240 (e) 8/240 (f) 2/240 |
MR: mumps‐rubella vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine RCT: randomised controlled trial
11. Safety: short‐term side effects (local or systemic reactions) ‐ non‐RCT study designs.
| Study ID and design | Population enrolled | Vaccine arm n = sample size | Comparator arm n = sample size | Outcome | MMR vaccine arm events/n | Other vaccine arms events/n | Comparator arm events/n |
|
cb‐Beck 1989 Prospective cohort |
Children aged 12 to 14 months |
MMR vaccine n = 103 containing 4.1 TCID50 mumps strain L‐Zagreb |
Placebo n = 93 |
Reactions (a) Local reactions(*) (b) Fever > 37.5 °C (c) Catarrhal symptoms (d) Swelling of cheeks (*)Local reactions: redness, swelling, tenderness |
MMR vaccine arm (a) 2/103 (b) 2/103 (c) 13/103 (d) 3/103 |
Placebo arm (a) 1/93 (b) 1/93 (c) 9/93 (d) 4/93 |
|
|
cb‐Benjamin 1992 Retrospective cohort |
Children aged 1 to 5 years |
MMR vaccine n = 1588 strain not stated |
Comparator Not immunised n = 1242 |
All episodes
(a) Arthralgia
(b) Possible or probable arthritis
(c) All specific joint syndromes
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
First‐ever episodes
(a1) Arthralgia(*)
(b1) Possible(§)/probable arthritis
(c1) All specific joint syndromes
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(d) Sore eyes (e) Convulsion (f) Coryza (g) Swollen glands (h) Fever (i) Skin rash (j) Hospital admission (k) Doctor consultation (*)Arthralgia was defined as pain experienced in the joint but not accompanied by swelling. (§)Possible arthritis was defined as swelling of joint reported by parent but not corroborated by a doctor. |
MMR vaccine arm All episodes (a) 16/1588 (b) 8/1588 (c) 24/1588 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ First‐ever episodes (a1) 16/1588 (b1) 7/1588 (c1) 23/1588 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (d) 154/1588 (e) 11/1588 (f) 897/1588 (g) 184/1588 (h) 279/1588 (i) 260/1588 (j) 76/1588 (k) 616/1588 |
Placebo arm All episodes (a) 3/1588 (b) 1/1588 (c) 4/1588 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ First‐ever episodes (a1) 3/1588 (b1) 1/1588 (c1) 4/1588 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (d) 150/1588 (e) 5/1588 (f) 797/1588 (g) 135/1588 (h) 262/1588 (i) 216/1588 (j) 78/1588 (k) 554/1588 |
|
|
cb‐Dunlop 1989 Prospective cohort |
Children aged 15 months |
(1) MMR vaccine n = 319 Trimovax Mérieux, measles Schwarz 1000 TCID50, rubella RA 27/3 1000 TCID50, mumps Urabe AM/9 5000 TCID50 (2) MV vaccine n = 16 Mérieux, containing measles Schwarz, 1000 TCID50 |
Local symptoms (a) Injury site bruise ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Systemic symptoms (a) Rash (b) Fever (c) Cough (d) Off‐color (e) Diarrhoea (f) Nappy rash (g) Earache (h) Parotitis (i) Lymphadenopathy (j) Hospital admission ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) Asymptomatic/unrelated |
(1) MMR vaccine Local symptoms (a) 19/319 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Systemic symptoms (a) 93/319 (b) 74/319 (c) 71/319 (d) 55/319 (e) 22/319 (f) 29/319 (g) 16/319 (h) 5/319 (i) 4/319 (j) 1/319 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 138/319 |
(2) MV vaccine Local symptoms (a) 0/16 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Systemic symptoms (a) 4/16 (b) 3/16 (c) 6/16 (d) 8/16 (e) 0/16 (f) 0/16 (g) 0/16 (h) 0/16 (i) 0/16 (j) 0/16 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 9/16 |
||
|
cb‐Makino 1990 Prospective cohort |
Children aged 8 months to 18 years |
(1) MMR vaccine n = 893
Kitasato Institute, Japan
containing
measles AIK‐C
5000 TCID50,
mumps Hoshino
15000 TCID50, rubella Takahashi 32000 TCID50 (2) Measles vaccine n = 147 Kitasato Institute, containing measles AIK‐C 25000 TCID50 (3) Mumps vaccine n = 122 Kitasato Institute, containing mumps Hoshino 10000 TCID50 |
Clinical reactions (a) Fever (≥ 37.5 °C) (b) Fever (≥ 39.0 °C) (c) Rash (d) Rash (mild) (e) Rash (moderate) (f) Rash (severe) (g) Lymphadenopathy (h) Parotitis (i) Cough (j) Vomiting (k) Diarrhoea |
(1) MMR vaccine (a) 139/893 (b) 12/893 (c) 91/893 (d) 81/893 (e) 6/893 (f) 4/893 (g) 12/893 (h) 8/893 (i) 5/893 (j) 2/893 (k) 10/893 |
(2) Measles; (3) Mumps (a) 18/147; 0/122 (b) 1/147; 0/122 (c) 24/147; 0/122 (d) 23/147; 0/122 (e) 1/147; 0/122 (f) 0/147; 0/122 (g) 0/147; 0/122 (h) 0/147; 0/122 (i) 0/147; 0/122 (j) 0/147; 0/122 (k) 0/147; 0/122 |
||
|
cb‐Miller 1989 Prospective Cohort |
Children aged 1 to 2 years |
(1) MMR vaccine n = 6149 Immrawa or Pluserix, both containing measle Schwarz, rubella RA 27/3, mumps Urabe 9) (2) Measles vaccine n = 162 (not described) single dose |
Clinical reactions (a) Symptoms (1 day only) (b) Fever (> 1 day) (c) Rash (> 1 day) (d) Off food (> 1 day) (e) Convulsion (in 1 to 21 days) (f) Convulsion (in 1 to 6 days) observation period 21 days |
(1) MMR vaccine (a) 2319/6149 (b) 976/6149 (c) 1061/6149 (d) 1627/6149 (e) 18/7247 (f) 7/7247 | (2) Measles vaccine (a) 73/162 (b) 23/162 (c) 18/162 (d) 31/162 (e) not reported (f) not reported | ||
|
cb‐Robertson 1988 Prospective cohort |
Children aged 13 months | (1) MMR vaccine n = 236 Mérieux, containing measles Schwarz, mumps Urabe AM/9, rubella Wistar RA 27/3 (2) Measles vaccine n = 52 Schwarz strain |
Clinical reactions (a) Irritability (b) Rash (c) Coryza (d) Fever (e) Cough (f) Lethargy (g) Diarrhoea (h) Vomiting (i) Anorexia (j) Conjunctivitis (k) Lymphadenopathy (l) Parotitis (m) Local reactions (n) No symptoms (o) Given paracetamol (p) Seen by a doctor observation period 21 days |
(1) MMR vaccine
(a) 175/236
(b) 109/236
(c) 104/236
(d) 88/236
(e) 40/236
(f) 65/236 (g) 55/236 (h) 33/236 (i) 48/236 (j) 23/236 (k) 6/236 (l) 3/236 (m) 14/236 (n) 33/236 (o)156/236 (p) 42/236 |
(2) Measles vaccine
(a) 40/52
(b) 23/52
(c) 27/52
(d) 16/52
(e) 12/52
(f) 13/52 (g) 10/52 (h) 7/52 (i) 14/52 (j) 5/52 (k) 0/52 (l) 0/52 (m) 4/52 (n) 4/52 (o) 29/52 (p) 11/52 |
||
|
cb‐Stokes 1971
Costa Rica; prospective cohort |
Costa Rica children aged 7 months to 7 years old |
MMR vaccine
(MSD) containing
measles Moraten
1000 TCID50,
mumps Jeryl Lynn
5000 TCID50,
rubella HPV ‐ 77
1000 TCID50
1 dose subcutaneous n = 457 |
Placebo arm n = 175 |
(a) Conjunctivitis
(b) URTI
(c) Lymphadenopathy
(d) Gastroenteritis
(e) Fever
(f) Irritability
(g) Malaise and anorexia
(h) Measles‐like rash
(i) Arthralgia
(j) Unrelated illness* Observation period 28 days (*)Otitis, allergy, fatigue, headache, viral infection, chickenpox, flush, scarlatina, whooping cough, abdominal pain, herniorrhaphy, heat or diaper rash |
MMR vaccine arm (a) 36/457 (b) 312/457 (c) 31/457 (d) 228/457 (e) 217/457 (f) 175/457 (g) 217/457 (h) 10/457 (i) 0/457 (j) 81/457 | Placebo arm (a) 0/175 (b) 88/175 (c) 9/175 (d) 77/175 (e) 75/175 (f) 49/175 (g) 64/175 (h) 9/175 (i) 2/175 (j) 29/175 | |
|
cb‐Stokes 1971 USA; prospective cohort |
USA children aged 10 months to 6 years old |
MMR vaccine
(MSD) containing
measles Moraten
1000 TCID50,
mumps Jeryl Lynn
5000 TCID50,
rubella HPV ‐ 77
1000 TCID50
1 dose subcutaneous USA n = 228 |
Placebo arm n = 106 | (a) Conjunctivitis
(b) URTI
(c) Lymphadenopathy
(d) Fever > 37.2 °C (orally)
(e) Gastroenteritis
(f) Irritability
(g) Malaise and anorexia
(h) Measles‐like rash
(i) Unrelated illness*
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Temperature (a) < 99 °F, < 37.2 °C (b) 99 to 100.9 °F, 37.2 to 38.3 °C (c) 101 to 102.9 °F, 38.3 to 39.4 °C (d) 103 to 104.9 °F, 39.4 to 40.5 °C (e) Not taken Observation period 28 days (*)Unrelated illness: Otitis, allergy, exanthema, headache, measles, whooping cough, heat rash, boils ‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature 5 to 12 days after vaccination |
MMR vaccine arm
(a) 1/228
(b) 158/228
(c) 3/228
(d) 118/228
(e) 51/228
(f) 43/228
(g) 14/228
(h) 11/228
(i) 89/228 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) 105/228 (b) 86/228 (c) 26/228 (d) 6/228 (e) 5/228 |
Placebo arm (a) 0/106 (b) 48/106 (c) 1/106 (d) 40/106 (e) 6/106 (f) 2/106 (g) 1/106 (h) 0/106 (i) 13/106 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) 57/106 (b) 36/106 (c) 3/106 (d) 1/106 (e) 9/106 | |
|
cb‐Sharma 2010 cohort study |
Prospective cohort | Children aged (1) 16 to 24 months (2) 5 to 7 years |
MMR vaccine
Tresivac, Serum Institute of India measles Edmonston‐Zagreb, 1000 CCID50 mumps Leningrad‐Zagreb, 5000 CCID50, rubella Wistar RA 27/3 1000 CCID50, in each 0.5 mL dose Sample sizes vaccine arms (1) n = 65,423 (2) n = 329,211 |
Placebo arm unvaccinated Sample sizes placebo arms (1) n = 12,253 (2) n = 46,232 observation period 42 days |
Local reactions
(a) Pain
(b) Redness
(c) Swelling Systemic reactions (a) Fever (b) Rash (c) Parotitis (d) Arthralgia (e) Lymphadenopathy |
Vaccine arms (1) age 16 to 24 months Local reactions (a) 1548/65,423 (b) 1157/65,423 (c) 688/65,423 Systemic reactions (a) 1640/65,423 (b) 113/65,423 (c) 25/65,423 (d) 11/65,423 (e) 6/65,423 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (2) age 5 to 7 years Local reactions (a) 4350/329,211 (b) 3728/329,211 (c) 2745/329,211 Systemic reactions (a) 8184/329,211 (b) 391/329,211 (c) 8208/329,211 (d) 200/329,211 (e) 430/329,211 |
Placebo arms (1) age 16 to 24 months Local reactions (a) 10/12,253 (b) 10/12,253 (c) 12/12,253 Systemic reactions (a) 197/12,253 (b) 20/12,253 (c) 21/12,253 (d) 0/12,253 (e) 4/12,253 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (2) age 5 to 7 years Local reactions (a) 0/46,232 (b) 0/46,232 (c) 0/46,232 Systemic reactions (a) 1344/46,232 (b) 11/46,232 (c) 433/46,232 (d) 0/46,232 (e) 2/46,232 |
|
cb‐Swartz 1974 Prospective cohort |
59 children aged 1 to 6 years |
(1) MMR vaccine n = 22
Merck Institute for
Therapeutic Research
(2) Mumps‐rubella vaccine n = 15 Merck Institute for Therapeutic Research (3) Rubella vaccine n = 22 Merck ‐ Meruvax HPV 77‐DE5 Temperature (1) 7 to 11 days (2) 7 to 12 days (3) 7 to 15 days after vaccination |
Reactions (a) Swollen glands (b) Enanthema (c) Conjunctivitis (d) Rash (e) No reactions ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) < 37.2 °C (b) 37.2 to 38.3 °C (c) 38.3 to 39.3 °C (d) ≥ 39.4 °C |
(1) MMR vaccine (a) 12/22 (b) 8/22 (c) 7/22 (d) 1/22 (e) 10/22 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) 15/22 (b) 4/22 (c) 3/22 (d) 0/22 |
(2) MR; (3) Rubella (a) 9/15: 7/22 (b) 8/15; 5/22 (c) 7/15; 7/22 (d) 3/15; 2/22 (e) 6/15; 14/22 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) 9/15; 16/22 (b) 3/15; 3/22 (c) 3/15; 3/22 (d) 0/15; 0/22 |
||
|
cb‐Weibel 1980; Prospective cohort |
(1) MMR vaccine n = 68 (Merck, containing measles Moraten, mumps Jeryl Lynn, rubella RA 27/3) (2) Rubella vaccine n = 67 (strain RA 27/3) 1 dose subcutaneous |
Reactions (a) Rash (b) Lymphadenopathy (c) Arthralgia (d) Myalgia (e) Anorexia ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) < 99 °F < 37.2 °C (b) 99 to 100.9 °F 37.2 to 38.3 °C (c) 101 to 102.9 °F 38.3 to 39.4 °C (d) 103 to 104.9 °F 39.4 to 40.5 °C (e) > 105 °F, ≥ 40.6 °C Temperature 5 to 12 days after vaccination |
(1) MMR vaccine Reactions (a) 16/68 (b) 8/68 (c ) 3/68 (d) 4/68 (e) 60/68 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) 39/68 (b) 14/68 (c ) 9/68 (d) 1/68 (e) 0/68 |
(2) Rubella vaccine Reactions (a) 3/67 (b) 3/67 (c ) 1/67 (d) 3/67 (e) 22/67 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Temperature (a) 37/67 (b) 14/67 (c ) 4/67 (d) 1/67 (e) 0/67 |
CCID50: cell culture infectious dose 50% MR: mumps‐rubella vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine RCT: randomised controlled trial TCID50: Median Tissue Culture Infectious Dose URTI: upper respiratory tract infection
12. Safety: encephalitis or encephalopathy.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Ray 2006 Case‐control |
Cases:
(n = 452) children aged
0 to 6 years
with outcome of interest. Controls: (n = 1280) matching for HMO, location, age within 7 days, sex, and length of enrolment in health plan |
1. Encephalopathy: acute generalised disturbance of brain function requiring hospitalisation and consisting of coma or stupor that cannot be attributed to medication or postictal state. Such cases must have altered consciousness, delirium, obtundation and/or confusion. 2. Reyes syndrome: clinical symptoms of acute encephalopathy with altered level of consciousness as well as:
3. Encephalitis/encephalomyelitis: evidence of acute neurologic disease presenting with non‐specific signs such as fever, seizures, altered consciousness, headache, vomiting, meningismus, or anorexia. Multifocal involvement of the central nervous system and evidence of cerebrospinal fluid inflammation (7 white blood cells/mm3) were required. Diseases with other known aetiologies were excluded. For data analysis, all cases were stratified on the basis of their aetiology: known, unknown, suspected but unconfirmed (this last when a diagnosis was not confirmed by a diagnostic test). Hospitalisation cases for encephalopathy, Reyes syndrome, or encephalitis (primary or secondary diagnosis) in children aged 0 to 6 years, members of the health plan of 4 HMOs in the USA, and occurred between 1 January 1981 and 31 December 1995, were considered as possible cases. Hospital charts were reviewed by abstracter (not blind to vaccination status of the cases) who included in first instance encephalitis diagnoses by a neurologist with clear aetiology and excluded all cases with a condition other than encephalopathy. All other neurologic cases were reviewed by a neurologist (blind to vaccination status of the cases) and included as cases if they met case definition (see column on the right). |
Vaccine exposure
time interval
relative to onset of
encephalopathy (a) 7 to 14 days (b) 0 to 14 days (c) 0 to 30 days (d) 0 to 60 days (e) 0 to 90 days MMR type not reported. Vaccination status of both cases and controls was ascertained from medical records. |
The findings do not support a conclusion that there is an
increased risk of encephalitis or encephalopathy after MMR vaccination. Although this study is large, encephalopathy is rare and thus it is not possible to exclude completely a small increase in the risk of encephalopathy after MMR vaccination. However, if such an increased risk exists, the absolute risk is extremely small and it is much lower after vaccination than after measles. This corresponds roughly to an all‐cause incidence (not an attributable risk) of 1 in 200,000 after MMR, a rate that is not statistically different from background. Consequently, our results support the continued use of DTP and MMR vaccines. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls (a) 1/452 versus 6/1280 (b) 1/452 versus 7/1280 (c) 4/452 versus 13/1280 (d) 8/452 versus 33/1280 (e) 15/452 versus 44/1280 |
OR (95% CI) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 0.40 (0.05 to 3.46) (b) 0.35 (0.04 to 2.95) (c) 0.85 (0.27 to 2.68) (d) 0.64 (0.27 to 1.50) (e) 0.98 (0.47 to 2.01) adjusted estimates |
|
db‐Makela 2002 Person‐time cohort |
Children immunised aged 1 to 7 years old. Between
November 1982 and September 1986 n = 535,544 n = 119 children hospitalised for encephalitis (MMR vaccine was administered before the disease), and only 97 between 0 and 24 months after MMR vaccination. |
Encephalitis: acute or subacute onset of neurologic symptoms. Presence of neurologic symptoms or findings (clinical or laboratory, e.g. microbiological, electroencephalographic, computed tomographic) indicative of involvement of the brain parenchyma, such as coma, seizures, focal neurologic findings, or mental function impairment. Absence of evidence of other diagnoses, including non‐inflammatory conditions, and no microbiological or other laboratory findings suggestive of a non‐viral infection. When pleocytosis in CSF is present, the term encephalitis is used, implying an inflammatory response within the brain. The presence of normal CSF findings does not preclude the diagnosis if the other criteria are satisfied. Encephalopathy: clinically resembles encephalitis but no inflammatory response is evident. Chronic encephalopathy: persistence of acute findings usually over several months. The National Hospital Discharge Register was consulted by using the following ICD‐8 codes: 065.99, 066.01, 066.02, 072.01, 292.20, 292.38, 292.39, 323.00, 323.01, 323.08, 323.09, 781.70, 999, 999.10. Medical records of hospitalised participants were reviewed (in order to evaluate possible other causes of the event) and their correspondence to diagnostic criteria (see column on the right) examined. |
Exposure risk period: (a) 0 to 3 months after vaccination Control period: (b) 4 to 24 months Observation period: (c) 0 to 24 months MMR II vaccine (Merck & Co, West Point, PA) measles: Enders‐Edmonston mumps: Jeryl Lynn rubella: Wistar RA 27/3 Vaccination data were assessed through vaccination register. |
Not significant excess of hospitalisation within 3 months of vaccination (P = 0.28) Incidence of encephalitis of undefined cause amongst 1‐ to 7‐year‐old children decreased from 19.9 per 100,000 in 1983 to 13.0 per 100,000 in 1985. |
(a) 9 cases (3 months) (b) 88 cases (21 months) (c) 97 cases (24 months) |
rr (95% CI)* 0.72 (0.36 to 1.42) (*)rate ratio amongst risk period (b) and control period (a) |
|
db‐Ward 2007 Self‐controlled case series |
Children aged 2 to 35 months (immunised with MMR; NK) with outcome of interest diagnosed between October 1998 and September 2001 (n = 107) | Onset of illness: day of hospital admission Fever: temperature of 37.5 °C; the questionnaire asked whether there was a fever and also for the maximum temperature recorded at any site by any method
Encephalopathy: a depressed or altered level of consciousness
Case definition of serious neurologic disease: any child 2 to 35 months old with a severe illness with fever and convulsions (see Table 35)
and/or encephalitis was included Encephalitis:
Exclude:
Cases of suspected encephalitis and/or severe illness with fever and convulsion occurring in children aged between age 2 and 35 months through Britain and Ireland were identified by consultant paediatricians taking part in a survey (October 1998 to September 2001) and notified to the British Paediatric Surveillance Unit. Details about neurologic illnesses were collected by reporting paediatricians by means of a detailed questionnaire. For diagnostic purposes, saliva, blood, and cerebrospinal samples were also collected. Questionnaires were reviewed by study investigators in order to assess whether reported cases corresponded to an analytical case definition taking into account severe illness with fever and convulsion and encephalitis (see column on the right). |
Exposure risk period:
15 to 35 days after immunisation, because this is the incubation period for postinfectious encephalitis induced by wild‐type measles and for aseptic meningitis induced by the Urabe vaccine strain mumps MMR vaccine type, not reported. Immunisation history of cases was obtained by the Immunisation Department of the Health Protection Agency (other than MMR vaccine, the study also considers DTP, Hib, and MenC vaccines). Only cases with known vaccination history were included in the analysis. |
Regarding MMR vaccine, there was no evidence of a raised relative incidence of serious neurologic disease 15 to 35 days after immunisation. | Within 15 to 35 days with concurrent primary HHV‐6 or HHV‐7 infection (a) all (5 cases) (b) no (4 cases) (c) yes (1 case) |
rr (95% CI) (a) 1.34 (0.52 to 3.47) (b) 1.52 (0.52 to 4.41) (c) 0.86 (0.10 to 7.23) |
incidence: cases/PT CI: confidence interval CSF: cerebrospinal fluid DTP: diphtheria, tetanus, pertussis vaccine Hib: Haemophilus influenzae b vaccine HHV: human herpes virus HMO: health maintenance organisation ICD: International Classification of Diseases MenC: meningococcus C vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MRI: magnetic resonance imaging PT: person‐time OR: odds ratio RR: risk ratio (relative risk) rr = rate ratio (relative incidence; incidence rate ratio)
13. Safety: aseptic meningitis.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Black 1997
Matched case‐control |
Cases n = 59 Controls n = 118 (age 12 to 23 months at the time of discharge diagnosis, between 1984 and 1993). For each ascertained case, 2 controls matched for age, sex, HMO, and HMO membership status were selected. |
Aseptic meningitis Potential cases of aseptic meningitis were identified by computerised hospitalisation at 4 HMOs that participated in the Vaccine Safety Datalink project. They were children aged 12 to 23 months with ICD‐9 discharge diagnoses 045.2, 047.*, 048, 072.1, 321.2 or 322.* between 1984 and 1993. Medical records of potential cases were reviewed and included as cases when corresponding to validation criteria (see column on the right). No evidence of prior underlying meningitis or underlying disease caused by toxoplasmosis, syphilis, cytomegalovirus, neonatal herpes simplex, or HIV. (The same exclusion criteria were used for controls.) In addition, bacterial, mycobacterial, and fungal cultures of the cerebrospinal fluid must have been negative, and the patient must have had a cerebrospinal fluid white blood cell count of >= 10 cells/mm3. |
MMR vaccine: Jeryl Lynn mumps strain. Any vaccines includes: Hib: Haemophilus influenzae type b, DPT: diphtheria‐pertussis‐tetanus toxoids, OPV: oral polio vaccine, HDPT: Haemophilus influenzae type b diphtheria pertussis tetanus toxoid vaccine, HepB: hepatitis B vaccine Vaccine and time window (a) MMR 0 to 14 days (b) MMR 0 to 30 days (c) MMR 8 to 14 days (d) Any vaccine 0 to 14 days (e) Any vaccine 0 to 30 days (f) Any vaccine 8 to 14 days Vaccination status of both cases and controls was derived from medical record review. |
In this analysis of hospitalisation caused by AM, there was no increased risk of AM after MMR vaccine containing Jeryl Lynn strain mumps. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls (a) 1/59 versus 4/118 (b) 3/59 versus 7/118 (c) 1/59 versus 2/118 (d) 2/59 versus 8/118 (e) 7/59 versus 18/118 (f) 2/59 versus 4/118 |
OR (95% CI) (a) 0.50 (0.1 to 4.5) (b) 0.84 (0.2 to 3.5) (c) 1.00 (0.1 to 9.2) (d) 0.44 (0.1 to 2.1) (e) 0.75 (0.3 to 1.9) (f) 1.00 (0.2 to 5.6) |
|
eb‐Park 2004 Case cross‐over |
(1) n = 39. Children with aseptic meningitis aged 13 to 29 months of both sexes, vaccination date confirmed by vaccination record. (2) n = 19. Children with aseptic meningitis aged 12 to 15 months of both sexes, vaccination date confirmed by parents only. |
Aseptic meningitis Generically defined as syndrome characterised by acute onset of meningeal symptoms, fever, and cerebrospinal fluid pleocytosis, with bacteriologically sterile cultures. Cases of aseptic meningitis were identified from insurance claims and hospitalisation data during 1998 in Korea. Authors considered cases corresponding to diagnosis criteria occurred in children aged 8 to 36 months who had received MMR vaccine within 1 year before disease onset and for whom vaccination records were available. |
MMR vaccine:
Strain type not stated (the study was conducted in the same setting of the study eb‐Ki 2003; both studies were performed in Korea, where MMR vaccine containing Urabe or Hoshino mumps strain was routinely administrated in public health, and MMR vaccines containing the Jeryl Lynn or Rubini in the private sector). Risk period (42 days) (a) from disease onset date to 42 days after Control period (323 days) (b) from 42 days up to 365 days after disease onset |
Study results showed that risk increased in the third week after vaccination and was elevated until the sixth week. |
(a) versus(b) (1) 11 versus 28 cases (2) 5 versus 14 cases Sensitivity analysis n = 58, 16 versus 42 cases |
RR (95% CI)(*) (1) 3.02 (1.50 to 6.08) Sensitivity analysis 2.93 (1.65 to 5.22) (*)Mantel‐Haenszel estimator Under the null hypothesis, this estimator is directly analogous to the Mantel‐Haenszel OR for matched‐pair case‐control study. |
|
eb‐Ki 2003 Case cross‐over |
67 children, mean age 19.1 months (standard deviation = 5.4 months) |
Aseptic meningitis Aseptic meningitis is a syndrome characterised by acute onset of meningeal symptoms, fever, and cerebrospinal fluid pleocytosis with bacteriologically sterile cultures. The following criteria were used to define eligible cases of aseptic meningitis for the study: 1) Korean insurance claim cases based on the ICD‐10 (codes A87.9, G03.0, G03.9, and G02.0); and 2) cerebrospinal fluid pleocytosis (leukocytes ≥ 5) with bacteriologically sterile cultures (if measured); or 3) neck stiffness and/or convulsions, or 2 other symptoms (headache or vomiting) in addition to a fever (≥ 38.0 °C, if measured). Patients’ charts were reviewed and their symptoms, laboratory tests, and last diagnoses on the discharge record checked. If patients were diagnosed with aseptic meningitis and were hospitalised in a general hospital, in accordance with these criteria, those who had headache, fever, and vomiting could be included as participants. |
MMR vaccine (1) n = 29 MMR with Urabe or Hoshino mumps strain (2) n = 38 MMR with Jeryl Lynn or Rubini mumps strain Risk period (42 days) (a) from disease onset date to 42 days after Control period (323 days) (b) from 42 days up to 365 days after disease onset |
Study results showed that no significant risk was associated with the Jeryl Lynn or Rubini strain of the vaccine. For the Urabe or Hoshino strain, the risk increased in the third week after vaccination and was elevated until the sixth week. |
(a) versus(b) (1) 13 versus 16 cases (2) 3 versus 35 cases |
RR (95% CI)(*) (1) 5.5 (2.6 to 11.8) (2) 0.6 (0.18 to 1.97) (*)Mantel‐Haenszel estimator Under the null hypothesis, this estimator is directly analogous to the Mantel‐Haenszel OR for matched‐pair case‐control study. |
|
db‐Makela 2002 Person‐time cohort |
Children immunised
aged 1 to 7 years old.
Between November 1982
and September 1986 n = 535,544 n = 120 children hospitalised for encephalitis (MMR vaccine was administered before the disease), and only 64 between 0 and 24 months after MMR vaccination. |
Aseptic meningitis Inflammation of the meninges. Usually a self‐limiting disease of known or suspected viral cause consisting of fever, headache, signs of meningeal irritation, without evidence of brain parenchymal involvement and a lymphocytic and mononuclear pleocytosis of CSF. The term 'meningoencephalitis' does not differentiate cases with prominent involvement of the brain parenchyma from those with meningeal involvement only. Hospitalisation records (ICD‐8 codes: 045.99, 320.88, 320.99) and review of patients' medical records to assess correspondence to case definition. |
Exposure risk period: (a) 0 to 3 months after vaccination Control period: (b) 4 to 24 months after vaccination Observation period: (c) 0 to 24 months after vaccination MMR II vaccine (Merck & Co, West Point, PA) Measles: Enders‐Edmonston Mumps: Jeryl Lynn Rubella: Wistar RA 27/3 Vaccination data were assessed through vaccination register. |
Not significant excess of hospitalisation within 3 months of vaccination (P = 0.57) The incidence of meningitis of undefined causes in 1‐ to 7‐year‐old children decreased from 10.17 per 100,000 in 1983 to 7.71 per 100,000 in 1985. |
(a) 10 cases (3 months) (b) 54 cases (21 months) (c) 64 cases (24 months) |
rr (95% CI)(*) 1.30 (0.66 to 2.55) (*)rate ratio amongst risk (a) and control (b) period |
|
db‐Dourado 2000 Self‐controlled case series ‐‐‐‐‐‐‐‐‐‐‐‐‐ Case‐only ecological method |
Children aged 1 to 11 years (from census) n = 452,344 n = 129 children aged 1 to 11 years old admitted to the referral hospital with a diagnosis of aseptic meningitis between 10th and 43rd epidemiologic surveillance weeks of 1997 (March to October). n = 87 fulfilled inclusion criteria; n = 29 cases of AM occurred prior to the mass immunisation campaign; n = 58 after the immunisation campaign. Of the 58 children, n = 50 were know to have been vaccinated. (The date of vaccination was available for 43 of these children.) |
Aseptic meningitis Data about meningitis were obtained from the state Epidemiology Surveillance System and from the neurologic service of the state referral hospital for infectious disease (Hospital Couto Maia), by reviewing hospital records of children admitted between the 10th and 43rd epidemiological surveillance weeks. Demographic, clinical, and laboratory data were collected on a standardised form. Inclusion/exclusion criteria 1) Residence in the city of Salvador 2) Age 1 to 11 years 3) Cerebrospinal fluid with a cell count of > 10 and < 1200 cells per mL (higher counts could be attributed to unconfirmed bacterial meningitis) 4) Predominance of lymphocytes in the cerebrospinal fluid of > 50% of the total number of cells 5) Exclusion of any bacteriologic or fungal confirmation through the use of Gram stain, latex, immunoelectrophoresis, stain for Cryptococcus neoformans, Ziehl‐Neelsen stain, or culture for bacteria and Mycobacterium tuberculosis 6) Exclusion of all cases with a history of prior meningitis or any neurologic disorder and any cases with sepsis, pneumonia, otitis, or any other disease that might be associated with an increased cell count in the cerebrospinal fluid |
Self‐controlled case series Exposure risk period: (a) 3 to 5 weeks after vaccination (i.e. 15 to 35 days) Control period: (b) 1 to 2 weeks and 6 to 10 weeks after vaccination Observation period: (c) 1 to 10 weeks after vaccination ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Case‐only ecological method (a) Reference period (pre‐vaccination): 10 to 32 epidemiologic surveillance weeks; time interval = 23 weeks (b) Low‐risk period: 34 to 35 epidemiologic surveillance weeks; time interval = 2 weeks (c) High‐risk period: 36 to 39 epidemiologic surveillance weeks (3 to 6 weeks after vaccination day) time interval = 4 weeks (d) Low‐risk period: 40 to 43 epidemiologic surveillance weeks; time interval = 3 weeks MMR vaccine Pluserix vaccine (SmithKline Beecham, UK) containing mumps Urabe strain Vaccination began on 16 August 1997 (National Immunisation Day, surveillance week 33), 45% coverage of the target population was achieved on that day, high coverage (exact data not reported, but very close to 100%) during the 2 following weeks. Vaccination history was obtained by vaccination cards or visits/phone call. |
An elevated risk of aseptic meningitis was observed 3 weeks after Brazil's national vaccination day compared with the risk in the pre‐vaccination period. This result was confirmed by a case series analysis. | (a) 35 cases (b) 3 and 5 cases (c) 43 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Cases/PT (weeks) (a) 29/10,403,912 (b) 3/904,688 (c) 46/1,809,376 (d) 9/1,809,376 |
Self‐controlled case series rr (95% CI)(*) 30.4 (11.5 to 80.8) (*)Poisson regression ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Case‐only ecological method rr (95% CI)(**) (a) reference weeks (b) 1.19 (0.36 to 3.91) (c) 9.12 (5.73 to 14.52) (d) 1.78 (0.84 to 3.77) (**)rate ratio amongst risk periods: (b), (c), (d) and control period (a). |
|
gb‐da Cunha 2002 Case‐only ecological method |
Children aged 1 to 11 years State of Mato Grosso do Sul (MS) n = 580,587 State of Mato Grosso (MT) n = 473,718 |
Aseptic meningitis Data on cases of meningitis were obtained from the routine surveillance system in both states. Notification of meningitis is statutory in Brazil, with a standardised form completed for each case. The attending physician or nurse completes the notification form in the health facility where the diagnosis is made. The notification form includes data on patient’s identification, clinical diagnosis, evolution, treatment, results of vaccination status, and laboratory investigations (the last 2 items not always reported). Reported cases of meningitis were classified into aseptic or not based on information from the notification forms, using 2 different criteria, which are independent but non‐exclusive. In both criteria, AM included only cases with absence of a positive bacteriological isolate in culture or stain of CSF and did not have a positive blood culture or mention of other non‐viral aetiology. Criterion 1: If the diagnosis in the form was of viral aetiology or unknown aetiology, cases were classified as AM. They were classified as not having AM if they had a suspected or confirmed diagnosis of meningitis by a known (non‐viral) agent through any laboratory or clinical finding. Criterion 2 (laboratory): Cases were considered AM if they had a CSF with the following findings: cell count greater than 10 and less than 1500 and presence of lymphocytes greater that 49%. (Applied for the cases in which laboratory data were present in the notification forms. In their absence, cases were excluded.) |
(MS) Unexposed period (a) reference weeks 1 to 31 (MS) Exposed period (b) low‐risk weeks 32 to 34 (c) high‐risk weeks 35 to 37 (d) low‐risk weeks 38 to 42 (e) all weeks 32 to 42 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MT) Unexposed period (a) reference weeks 1 to 37 (MT) Exposed period (b) low‐risk weeks 38 to 40 (c) high‐risk weeks 41 to 43 (d) low‐risk weeks 44 to 48 (e) all weeks 38 to 48 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMR vaccine: Serum Institute of India, Ltd, Pune. Contained Leningrad‐Zagreb mumps strain. 3 different lots were used in each state (MS and MT). Vaccination began in mid‐August 1998 (week 32) in MS and late September in MT (week 38), and lasted for about 1 month, even if the most part of the doses had been administered during the first 2 campaign weeks. Vaccination was reported for 69.4% and 93.5% of the target population in MT and in MS, respectively. |
This study shows an increase in number of notified cases of AM in the 2 states studied, 3 to 4 weeks after the MIC using Leningrad‐Zagreb mumps strain MMR vaccine (3 to 4 weeks after the MIC corresponding to incubation period for wild mumps infection, and the increase was restricted to the age group targeted by the campaign and to the aseptic form of meningitis). The use of the vaccine on a large scale over a short period of time made it possible to identify an increase in risk which may be present, but more difficult to measure when vaccination is spread over longer periods. The risk estimates varied depending on the diagnostic criteria used and the state. There was also an increase in the incidence of notified mumps after the campaign in the state where data were available. |
cases/PT (weeks) (MS) AM criterion 1 (a) 22/14,685,258 (b) 7/1,421,154 (c) 35/1,421,154 (d) 6/2,368,590 (e) 48/5,210,898 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MT) AM criterion 1 (a) 71/21,481,719 (b) 7/1,741,761 (c) 71/1,741,761 (d) 25/2,902,935 (e) 103/6,386,457 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MS) AM criterion 2 (a) 8/14,685,258 (b) 4/1,421,154 (c) 24/1,421,154 (d) 2/2,368,590 (e) 30/ 5,210,898 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MT) AM criterion 2 (a) 36/21,481,719 (b) 3/1,741,761 (c) 54/1,741,761 (d) 15/2,902,935 (e) 72/6,386,457 |
rr (95% CI)* (MS) AM criterion 1 (a) reference weeks (b) 3.3 (1.41 to 7.7) (c) 16.4 (9.65 to 28.0) (d) 1.7 (0.69 to 4.2) (e) 6.2 (3.71 to 10.2) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MT) AM criterion 1 (a) reference weeks (b) 1.2 (0.56 to 2.6) (c) 12.3 (8.88 to 17.1) (d) 2.6 (1.65 to 4.1) (e) 4.9 (3.61 to 6.6) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MS) AM criterion 2 (a) reference weeks (b) 5.2 (1.56 to 17.2) (c) 31.0 (13.93 to 69.0) (d) 1.6 (0.33 to 7.3) (e) 10.6 (4.84 to 23.1) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (MT) AM criterion 2 (a) reference weeks (b) 1.0 (0.32 to 3.3) (c) 18.5 (12.13 to 28.2) (d) 3.1 (1.69 to 5.6) (e) 6.7 (4.51 to 10.0) (*)rate ratio amongst exposed (risk) periods: (b), (c), (d), (e) and unexposed period (a) |
|
gb‐da Silveira 2002 Case‐only ecological method |
Children aged 1 to 11 years target population n = 110,629 (Rio Grande do Sul) dose |
Aseptic meningitis
Any‐cause AM was defined as: occurrence of clinically diagnosed meningitis in a person with a CSF pleocytosis (between 5 and 1500 leucocytes/mL) and a negative Gram stain. Viral isolation is not routinely performed in Rio Grande do Sul. Mumps‐associated AM was defined as: that occurring in conjunction with or following clinically diagnosed mumps. Vaccine‐associated AM was defined as: aseptic meningitis with a pleocytosis of 10 to 1500 leukocytes/mL and occurring within 15 to 35 days after vaccine receipt. |
MMR vaccine: produced by Serum Institute of India, Lot: 180‐X: measles: Edmonston‐Zagreb; mumps: Leningrad‐Zagreb; rubella: Wistar RA 27/3. The campaign was conducted between 8 September and 28 November 1997; weeks 37 to 48. (a) unexposed period in 1995/1996 39 to 47 weeks (b) unexposed period in 1997 1 to 38 weeks (c) exposed period in 1997: High risk: 39 to 47 weeks (d) exposed period in 1997: Low risk: 48 to 53 weeks |
A total of 105,098 doses of Leningrad‐Zagreb were administered to children
aged 1 to 11 years, for an overall coverage of 95%. The risk of vaccine‐associated aseptic meningitis (31 cases) was 2.9 cases per 10,000 doses of Leningrad‐Zagreb administered (equivalent to 1 case per 3390 doses administered). Within the 1‐ to 11‐years age group, the risk did not differ significantly by age group. These findings suggest that Leningrad‐Zagreb is more reactogenic than Urabe and Jeryl‐Lynn strains. |
(a) 2.4 cases per 100,000 person weeks; 4.5 cases in average (b) 10 cases (any cause) (c) 28.7 per 100,000 person weeks 31 cases vaccine associated (55 any cause, 41 vaccinated) (d) 4 cases (any cause) |
rr (95% CI) (c) 12.2 (6.0 to 24.7)(*) (*)rate ratio (c) and (a) |
|
db‐Farrington 1995 Self‐controlled case series |
Children aged 12 to 24 months discharged from hospital in 5 districts in England (Ashford, Leicester, Nottingham, Preston, and Chorley & Ribble) for varying periods between October 1988 and February 1993. Readmissions within 72 h with the same diagnosis were counted as 1 episode. n = 952 children |
Aseptic meningitis Children discharged from hospital with a diagnosis of: meningitis categorised as mumps, aseptic, or viral (ICD 072.1, 047., 321.) Children aged between 366 and 730 days. |
MMR vaccine: Urabe mumps strain Jeryl Lynn mumps strain Rubella strain not specified. Exposure risk period: (a1) 6 to 11 days (1 to 2 weeks after vaccination) (a2) 15 to 35 days (3 to 5 weeks after vaccination) (Urabe strain) Control period: (b) for each vaccine was defined as the time not included in a risk period. The analyses were adjusted for age and were grouped in 6 equal intervals of about 2 months. |
The study shows that there is a true risk of a neurological event attributable to the Urabe strain. |
Urabe strain (a1) 0 cases (a2) 5 cases |
rr (95% CI) (a2) 38.1 (4.3 to 336)(*) (*)Poisson regression |
|
db‐Miller 2007 Self‐controlled case series |
Children aged 12 to 23 months with discharge diagnosis of febrile convulsion or aseptic meningitis |
Aseptic menigitis: Viral meningitis (A87), mumps (B26), meningitis in other infections classified elsewhere (G02), and meningitis due to other and unspecified causes (G03) were identified for the period 1 May 1998 to 30 June 2001, and case notes were reviewed by a paediatrician. In addition, computerised hospital records for children aged 12 to 23 months with an ICD‐9 discharge diagnosis of meningitis categorised as mumps, aseptic, or viral (072.1, 047, 321) were identified for the period 1 January 1991 to 30 September 1992, prior to the withdrawal of Urabe‐containing MMR vaccines, and were linked with MMR vaccination histories. Cases of laboratory‐confirmed mumps meningitis were also ascertained from reports made to the Centre for Infections from laboratories in England and Wales for the period of October 1992 to the end of June 2004. |
MMR vaccine: (1) MMR with Urabe mumps strain up to September 1992 (2) MMRII (Sanofi Pasteur) Edmonston‐Enders measles strain, Jeryl Lynn mumps strain, between September 1992 and May 1998 (3) MMR Priorix (GlaxoSmithKline) Schwarz measles strain RIT4385 (Jeryl Lynn) from May 1998 Exposure risk period: (a) 15 to 35 days after vaccination (from May 1998 to June 2001) (Urabe MMR) (b) 15 to 35 days after vaccination (from January 1991 to September 1992) (Jeryl Lynn MMR) MMR vaccination histories were independently obtained through linkage with computerised immunisation records in the 2 Thames regions, using either the National Health Service number or sex, date of birth, and post code, a highly specific linking algorithm. Information on batch number was sought for any confirmed aseptic meningitis cases with onset 15 to 35 days after MMR vaccination. The formatting of batch numbers differs substantially between manufacturers in length and alphanumeric coding and is a precise means of distinguishing between vaccines from different manufacturers. |
Before after between 2 risk periods, re‐analysis of the data presented in db‐Farrington 1995 This study confirms that the risk of aseptic meningitis with Priorix vaccine, if it exists at all, is significantly lower than with Urabe‐containing mumps vaccine. The study allowed the exclusion of risks as rare as 1 in 437,000 for laboratory‐confirmed mumps meningitis with non‐Urabe‐containing MMR vaccines. |
Comparison between 2 risk periods Aseptic meningitis (a) 4 cases (b) 0 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Laboratory‐confirmed mumps‐positive cerebrospinal fluid (a) 16 cases (b) 0 cases Data from the paper db‐Farrington 1995 |
rr(95%CI) 25.9 (2.8 to 233)(*) (*) rate ratio (a) versus (b) |
|
db‐Perez‐Vilar 2018 Self‐controlled case series |
For this study, WHO selected 26 sentinel sites (49 hospitals) distributed in 16 countries of the 6 WHO regions. The study population included children ages 9 to 23 months admitted to a network‐participating hospital during January 2010 to March 2014, with a discharge diagnosis of either AM or immune thrombocytopenic purpura. |
Aseptic meningitis probable cases ICD‐9 codes in first discharge diagnosis position: 047 (047.0 to 047.9) Meningitis due to enterovirus 049.0 to 049.1 Other non‐arthropod‐borne viral meningitis 072.1 Mumps meningitis 321.2 Meningitis due to viruses not elsewhere classified 322.0, 322.1, 322.9 Meningitis of unspecified cause ICD‐10 codes in first discharge diagnosis position: A87.0 Meningitis due to enterovirus A87.1 Adenoviral meningitis A87.2 Lymphocytic choriomeningitis A87.8 Other viral meningitis A87.9 Viral meningitis, unspecified B26.1 Mumps meningitis G02.0 Meningitis due to viruses not elsewhere classified G03.0, G03.8, G03.9 Meningitis of unspecified cause |
Vaccine(measles strain) (mumps strain) Priorix, GSK (Schwarz) (RIT 4385a) Priorix‐Tetra, GSK (Schwarz) (RIT 4385a) MMR Shanghai Institute (Shanghai‐191) (S79) Measles, Lanzhou Institute (Shanghai‐191) (–) Measles‐Rubella, Beijing Tiantan (Shanghai‐191) (–) M‐M‐R‐II, MSD (Enders’ Edmonston) (Jeryl Lynn (Level B)) MMR, Razi Vaccine and Serum Research (AIK‐C) (Hoshino) M‐M‐RVAXPRO, Sanofi Pasteur‐MSD (Enders’ Edmonston) (Jeryl Lynn (Level B)) Trimovax, Sanofi Pasteur (Schwarz) (Urabe AM9) Measles, Serum Institute of India Pvt. (Edmonston‐Zagreb) (–) Measles‐Rubella, Serum Institute of India Pvt. (Edmonston‐Zagreb) (–) MMR, Serum Institute of India (Edmonston‐Zagreb) (Leningrad‐Zagreb) Tresivac, Serum Institute of India (Edmonston‐Zagreb) (Leningrad‐Zagreb) Rouvax, Sanofi Pasteur (Schwarz) (–) Risk period 8 to 35 days Washout periods 1 to 7 days 36 to 42 days Control period 43 to 84 days |
The elevated risk estimates found for the Leningrad‐Zagreb mumps strain are consistent with previous studies (gb‐da Cunha 2002; gb‐da Silveira 2002). Regarding Jeryl‐Lynn‐derived strain vaccines, although the study did not have enough power to confirm the absence of risk for these strains, the finding of zero cases in the risk window was consistent with the hypothesis of no association (bb‐Black 1997; db‐Makela 2002). | In 16 countries n = 84 confirmed aseptic menigitis cases (Risk versuscontrol) period (a) Overall risk of AM following mumps‐containing vaccines (35 versus 5) (b) Overall risk of AM following mumps‐containing vaccines (excluding cases from Iran) (22 versus 3) (c) Leningrad‐Zagreb strain (7 versus 1) (d) Vaccines products used Hoshino/Leningrad‐Zagreb/Urabe AM9 (27 versus 2) (e) Vaccines products used Hoshino/Leningrad‐Zagreb/Urabe AM9 (excluded cases from Iran) (14 versus 0) |
rr (95% CI) adjusted (a) 10.8 (4.0 to 29.2) (b) 12.4 (3.1 to 49.1) (c) 6.4 (1.3 to 87.4) rr (95% CI) unadjusted (d) 20.3 (48 to 85.2) (e) not estimable |
AM: aseptic meningitis CI: confidence interval CSO: cerebro‐spinal fluid HMO: health maintenance organisation ICD‐10: International Classification of Diseases incidence: cases/PT MIC: mass immunisation campaigns MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine n: number of participants OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) WHO: World Health Organization
14. Safety: seizure (febrile/afebrile).
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Authors' conclusion | Crude data | Estimate (95% CI) |
|
cb‐Vestergaard 2004 Retrospective and prospective cohort |
Children born in Denmark from 1 January 1991 to 31 December 1998 aged 3 months to 5 years n = 537,171 |
Information on febrile seizures and epilepsy was obtained from the National Hospital Register (NHR), which contains information on all patients discharged from Danish hospitals since 1977 (since 1995 information on outpatients (visits to emergency department and hospital clinics)). Diagnostic information was classified according to the Danish version of the ICD as follows: ICD‐8 was used from 1977 to 1993, and ICD‐10 was used from 1994 to the end of 1999. Febrile seizure: (a) within 2 weeks after vaccination (a1) 1 weeks after vaccination (a2) 2 weeks after vaccination ICD‐8 code 780.21 or ICD‐10 code R56.0, were aged between 3 and 60 months at the time of discharge, and had no recorded history of non‐febrile seizures, cerebral palsy, severe head traumas, intracranial tumours, meningitis, or encephalitis. The febrile seizures could not be classified as simple or complex because the NHR contains no information on number of febrile seizures occurring within the febrile episode, duration of the febrile seizures, and type of febrile seizures (generalised or focal onset). (b) Recurrent febrile seizure (b1) within 2 weeks after vaccination (b2) > 2 weeks after vaccination (c) Epilepsy subsequent to a first febrile seizure episode Children were categorised with epilepsy if they had ICD‐8 code 345 or ICD‐10 code G40. (c1) within 2 weeks after vaccination (c2) > 2 weeks after vaccination |
Vaccination status of the children was ascertained by using data of the National Board of Health to which vaccination data were transmitted by general practitioners. MMR vaccine: Moraten measles, Jeryl Lynn mumps, Wistar RA 27/3 rubella The national vaccination program recommended during the entire study period that children should be vaccinated twice, at 15 months and at 12 years. Only the first vaccination is relevant to the endpoint under study. |
MMR vaccination was associated with a transient increased rate of febrile seizures, but the risk difference was small even in high‐risk children. The long‐term rate of epilepsy was not increased in children who had febrile seizures following vaccination compared with children who had febrile seizures of a different aetiology. Febrile seizure: no statistically significant difference in the RR of febrile seizures in the 2 weeks following vaccination between subgroups of children characterised by family history of seizures, sex, birth order, gestational age at birth, birthweight, or socioeconomic factors, compared with non‐vaccinated children within the subgroup under study. The highest rate ratio (2 weeks following vaccination) was found amongst (a1)siblings of children with a history of epilepsy compared with rate of febrile seizures following vaccination in siblings of children with no history of epilepsy. Recurrent febrile seizures and epilepsy The authors found that children who experienced febrile seizures within 2 weeks of MMR vaccination had a 19% increased rate of recurrent febrile seizures but no increased rate of epilepsy during up to 105 months of follow‐up. The reference group consisted of children who had not been vaccinated when having their first febrile seizure. |
Cases/PT (years) vaccinated (a) 7445/1,151,661 versus unvaccinated 10,541/793,568 vaccinated (b1) 236/2212 (b2) 981/12,675 versus unvaccinated 2753/23,560 vaccinated (c1) 9/3825 (c2) 95/21,938 versus unvaccinated 251/41,310 |
rr (95% CI)* (a) 2.75 (2.55 to 2.97) (a1) 2.46 (2.22 to 2.73) (a2) 3.17 (2.89 to 3.49) amongst children with a personal history of febrile seizure (a1) 2.75 (2.32 to 3.26) (b1) 1.19 (1.01 to 1.41) (b2) 1.10 (0.96 to 1.26) (c1) 0.70 (0.33 to 1.50) (c2) 0.92 (0.59 to 1.43) (*) Poisson regression adjusted for age, calendar period, age of first febrile seizure, and current vaccination status |
|
cb‐Barlow 2001 Retrospective cohort study |
Data are collected from 4 HMOs. Children (n = 716) with a confirmed seizure during the study period: from 1 March 1993 to 30 September 1993. n = 679,942 children n = 137,457 vaccinated MMR n = 340,386 vaccinated DTP n = 202,099 (unvaccinated) |
Seizures were identified through the automated data systems of each HMO, on the basis of visits classified according to the ICD‐9‐CM, as code 333.2 (myoclonus), code 345 (epilepsy), code 779.0 (convulsions in a newborn), or code 780.3 (convulsions). Simple febrile seizures were defined as short, generalised seizures, accompanied by documented fever or a parental report of fever. Complex febrile seizures were defined as febrile seizures that occurred more than once in 24 hours and either lasted for at least 12 minutes or were accompanied by focal signs. |
MMR vaccine
strains type not stated Exposure period (after vaccination): (a1) 1 to 7 days (a2) 8 to 14 days (a3) 15 to 30 days Control period (b) The reference group at the time of the seizure was composed of children matched for age, calendar time, and HMO but who had not had a vaccination in the preceding 30 days. |
The study found significantly elevated risks of febrile seizures from 8 to 14 days after the administration of MMR vaccine. The authors did not find a significantly elevated risk of febrile seizures at any other time after vaccination, nor did they find an elevated risk of non‐febrile seizures at any time after vaccination with MMR vaccine. This risk translates into approximately 25 to 34 additional febrile seizures attributable to MMR vaccine for every 100,000. | n = 521 febrile seizures in the absence of vaccination Febrile seizures (a1) 8 cases (a2) 13 cases (a3) 11 cases Non‐febrile seizures (a1) 1 case (a2) 1 case (a3) 1 case |
rr (95% CI)(*) Febrile seizures (a1) 1.73 (0.72 to 4.15) (a2) 2.83 (1.44 to 5.55) (a3) 0.97 (0.49 to 1.95) Non‐febrile seizures (a1) not reported (a2) 1.11 (0.11 to 11.28) (a3) 0.48 (0.05 to 4.64) (*) Cox proportional hazard regression multivariate model estimates adjusted for age, sex, HMO, calendar time, and receipt of DTP vaccine. |
|
db‐Ward 2007 Self‐controlled case series |
Children aged 2 to 35 months (immunised with MMR; NK) with outcome of interest diagnosed between October 1998 and September 2001 (n = 107) | Case definition of serious neurologic disease: any child 2 to 35 months old with a severe illness with fever and convulsions and/or encephalitis (see Table 33) was included. Severe illness with fever and convulsions
Exclude: Viral (aseptic) meningitis without encephalopathy The following confirmed causes were excluded: hypoxic/ischaemic; vascular; toxic; metabolic, neoplastic, traumatic, and pyogenic infections; uncomplicated convulsions; or a series of convulsions lasting 30 min in immunocompromised children. |
Exposure risk period:
6 to 11 days after immunisation MMR vaccine type, not reported Immunisation history of cases was obtained by the Immunisation Department of the Health Protection Agency (other than MMR vaccine the study also considers DTP, Hib, and MenC vaccines). Only cases with known vaccination history were included in the analysis. |
6 to 11 days after measles, mumps, rubella vaccine there is an increased risk of fever and convulsions lasting 30 minutes. All 6 of the episodes temporally related to immunisation met the criteria for complex febrile convulsions. |
Within 6 to 11 days With concurrent primary HHV‐6 or HHV‐7 infection (a) all (6 cases) (b) no (4 cases) (c) yes (2 cases) |
rr (95% CI) (a) 5.68 (2.31 to 13.97) (b) 5.80 (1.98 to 16.99) (c) 5.55 (1.12 to 27.63) |
|
db‐Farrington 1995 Self‐controlled case series |
Children aged 12 to 24 months discharged from hospital in 5 districts in England (Ashford, Leicester, Nottingham, Preston, and Chorley & Ribble) for varying periods between October 1988 and February 1993. Readmissions within 72 h with the same diagnosis were counted as 1 episode. n = 952 children |
Febrile convulsion ICD code 780.3 children aged 29 to 730 days |
MMR vaccine: Urabe mumps strain Jeryl Lynn mumps strain Rubella strain not specified Exposure risk period: (a1) 6 to 11 days (1 to 2 weeks after vaccination) (a2) 15 to 35 days (3 to 5 weeks after vaccination) Control period: (b) for each vaccine was defined as the time not included in a risk period The analyses were adjusted for age and were grouped in 6 equal intervals of about 2 months. |
The study shows that there was an attributable risk of 1 in 2600 doses of a febrile convulsion 15 to 35 days after giving Urabe MMR vaccine. There was no excess of admissions in the same period when Jeryl Lynn vaccine was given. |
Any strain (a1) 49 cases (a2) 85 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Urabe strain (a1) 0 cases (a2) 57 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Jeryl Lynn strain (a1) 0 cases (a2) 9 cases |
rr (95% CI)(*) Any strain (a1) 3.04 (2.27 to 4.07) (a2) 1.51 (1.21 to 1.90) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Urabe strain (a1) 3.77 (1.95 to 7.30) (a2) 1.66 (1.26 to 2.20) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Jeryl Lynn strain (a1) 2.70 (1.81 to 4.01) (a2) 1.04 (0.56 to 1.93) (*) Poisson regression |
|
db‐Miller 2007 Self‐controlled case series |
Children aged 12 to 23 months with discharge diagnosis corresponding to the outcome of interest who received MMR n = 894 |
Febrile convulsion
ICD‐10 code R560 or R568, febrile convulsion or fit, not otherwise specified, who were admitted between 1 January 1998 and 30 June 2002 were identified and linked with computerised immunisation records to obtain dates of MMR vaccination. Episodes within a same individual were considered as separate when they occurred at least 10 days apart. Case review not performed. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Febrile convulsion ICD‐10 codes R560 only |
MMR vaccine: (1) MMRII (Sanofi Pasteur) Edmonston‐Enders measles strain, Jeryl Lynn mumps strain, between September 1992 and May 1998 (2) MMR Priorix (GlaxoSmithKline) Schwarz measles strain RIT4385 (Jeryl Lynn) from May 1998 (3) unknown manufacturer Exposure risk period: (a1) a pre‐vaccination period of 2 weeks (removed from the background risk by treating it as a separate risk period to allow for delayed vaccination due to convulsion) (a2) 6 to 11 days (1 to 2 weeks after vaccination) (a3) 15 to 35 days (3 to 5 weeks after vaccination) . Control period (b) a pre‐vaccination period |
The attributable risk of hospital admission for convulsion following receipt of any MMR vaccine was estimated as 1 in 1150 doses for the 6‐ to 11‐day postvaccination period, based on an estimated relative incidence of 4.09. The excess risk of convulsion in this period was attributable to the measles component of MMR vaccine. The relative incidence of convulsion in the 6‐ to 11‐day period was higher for Priorix than for MMRII, although the difference was not significant. There was no statistically significant evidence that children given MCC vaccine at the same time as MMR vaccine have a somewhat higher risk of convulsion in the 6‐ to 11‐day postvaccination period (rr 7.74, 3.82 to 15.71) than children who receive MMR but not MCC vaccine at the same time (rr 3.81, 2.87 to 5.05). Conclusion: there is no evidence to suggest that the new MMR vaccine used in the UK since mid‐1998 and derived from the Jeryl Lynn‐containing MMR vaccine causes aseptic meningitis attributable to its mumps component. |
Any MMR vaccine (a1) 13 cases (a2) 66 cases (a3) 65 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMRII vaccine Jeryl Lynn (a1) 6 cases (a2) 27 cases (a3) 34 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMR Priorix vaccine RIT4385 (a1) 3 cases (a2) 19 cases (a3) 16 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Unknown manufacturer (a1) 4 cases (a2) 20 cases (a3) 15 cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Febrile convulsion (R560 only) (a1) not reported (a2) 52 cases (a3) 57 cases |
rr (95% CI)(*) Any MMR vaccine (a1) 0.38 (0.22 to 0.64) (a2) 4.09 (3.14 to 5.33) (a3) 1.13 (0.87 to 1.48) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMRII vaccine Jeryl Lynn (a1) 0.39 (0.18 to 0.84) (a2) 3.64 (2.44 to 5.44) (a3) 1.28 (0.89 to 1.84) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMR Priorix vaccine RIT4385 (a1) 0.47 (0.15 to 1.40) (a2) 6.26 (3.85 to 10.18) (a3) 1.48 (0.88 to 2.50) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Unknown manufacturer (a1) 0.32 (0.13 to 0.81) (a2) 3.53 (2.23 to 5.61) (a3) 0.75 (0.44 to 1.26) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Febrile convulsion (R560 only) (a1) not reported (a2) 4.27 (3.17 to 5.76) (a3) 1.33 (1.00 to 1.77) (*) Poisson regression exposure risk period versus control period |
|
db‐McClure 2019 Person‐time cohort |
Children (n = 556,864) were eligible if they had received their first dose of measles‐containing vaccine at age 12 through 23 months from January 2003 through September 2015. Children were excluded if they had a history of seizure or conditions strongly related to seizure prior to 12 months of age. Children born before 37 weeks gestational age were classified as preterm (< 37 weeks) and children born 37 weeks gestational age as full term (≥ 37 weeks). Preterm children were further classified into those born early preterm (< 35 weeks) and late preterm(35 through 36 weeks) gestational age. n = 24,489 were excluded because of documented history of seizures before age 12 months. In analysis n = 532,375 |
Seizure (febrile/afebrile) A seizure was defined as the first emergency department or inpatient hospital encounter with ICD‐9‐CM diagnostic code of 780.3 (convulsions) during the 42 days following vaccination. |
MMR and MMRV vaccines
strains type not stated Risk interval 7 to 10 days after vaccination Control interval 15 to 42 days after vaccination n = number of children (a) Overall (any measles vaccine) (a1) < 37 weeks n = 45,343 (a2) < 35 weeks n = 16,596 (a3) 35 to 36 weeks n = 28,757 (a4) ≥ 37 weeks n = 487,032 (b) MMR (b1) < 37 weeks n = 37,262 (b2) ≥ 37 weeks n = 403,238 (c) MMRV (c1) < 37 weeks n = 8081 (c2) ≥ 37 weeks n = 83,794 Age at vaccination (any measles vaccine) (d) 12 to 15 months (d1) < 37 weeks n = 41,391 (d2) ≥ 37 weeks n = 442,919 (e) 16 to 23 months (e1) < 37 weeks n = 3952 (e2) ≥ 37 weeks n = 4413 |
Conclusion: the results support the current ACIP recommendations to administer the first dose of measles‐containing vaccine at age 12 through 15 months for all children, including those born preterm. Delaying vaccination of measles‐containing vaccines may increase the risk of seizures following vaccination. |
Risk versus control interval
cases/PT‐years (a) Overall (any measles vaccine) (a1) 31/500 versus 56/3500 (a2) 10/182 versus 22/1294 (a3) 21/313 versus 34/2267 (a4) 232/5395 versus 510/36,429 (b) MMR (b1) 22/407 versus 48/2824 (b2) 163/434 versus 425/30,357 (c) MMRV (c1) 9/90 versus 8/615 (c2) 69/908 versus 85/6538 Age at vaccination (any measles vaccine) (d) 12 to 15 months (d1) 27/450 versus 51/3188 (d2) 200/4878 versus 477/34,071 (e) 16 to 23 months (e1) 4/43 versus 5/294 (e2) 32/485 versus 33/3300 |
rr (95% CI)(*) (a) Overall (any measles vaccine) (a1) 3.9 (2.5 to 6.0) (a2) 3.2 (1.5 to 6.7) (a3) 4.3 (2.5 to 7.4) (a4) 3.2 (2.7 to 3.7) (b) MMR (b1) 3.2 (1.9 to 5.3) (b2) 2.7 (2.2 to 3.2) (c) MMRV (c1) 7.9 (3.0 to 20) (c2) 5.7 (4.1 to 7.8) Age at vaccination (any measles vaccine) (d) 12 to 15 months (d1) 3.7 (2.3 to 5.9) (d2) 2.9 (2.5 to 3.5) (e) 16 to 23 months (e1) 5.6 (1.5 to 21) (e2) 6.8 (4.2 to11) (*) Poisson regression risk interval versus control interval |
| db‐Macartney 2017 Self‐controlled case series | Children aged 11 to 23 months. Analysis was further restricted to include only children who had (1) 1 dose of MMR vaccine followed by 1 dose of MMRV vaccine at least 27 days later (consistent with NIP recommendations), (2) 1 dose of MMR vaccine (as some had not yet received MMRV vaccine), or (3) no MMR or MMRV vaccine (unvaccinated children, who contribute to the age‐specific relative incidence). Children who received MMRV vaccine as their first MCV were excluded because this schedule was not consistent with NIP recommendations and occurred rarely. |
Febrile seizures in all children younger than 5 years. Periodic review of all ICD‐10‐Australian Modification coded R56.0 was also conducted to capture additional cases. Clinical and demographic data were collected from the medical records and caregiver interviews, and all FS diagnoses were confirmed. The primary analysis included children who had both first and subsequent FS episodes (considered unique episodes), in which the subsequent FS was separated by at least 7 days from a previous episode. 2 sensitivity analyses were conducted: (1) adjustment for age using finer intervals (1‐month age groups); (2) restriction of the analysis to first FS episodes. |
MMRV Priorix‐Tetra MMR+V Risk period after vaccination (a) 5 to 12 days (b) 13 to 30 days Control period before vaccination excluding interval −13 to −1 days before |
Authors' conclusions: "To our knowledge, this is the first study to provide evidence of the absence of an association between use of MMRV vaccine as the second dose of MCV in toddlers and an increased risk of FSs. Incorporation of MMRV vaccine has facilitated improvements in vaccine coverage that will potentially improve disease control." |
(1) Primary analysis: children who had both first and subsequent episodes (2) Adjustment for age using 1‐month interval (3) Restriction of the first FS episode |
rr (95% CI)(*) (1) MMR (a) 2.71 (1.71 to 4.29) (b) 0.89 (0.54 to 1.48) (1) MMRV (a) 1.08 (0.55 to 2.13) (b) 1.08 (0.67 to 1.74) (2) MMR (a) 2.57 (1.56 to 4.43) (b) 0.83 (0.49 to 1.40) (2) MMRV (a) 1.17 (0.57 to 2.40) (b) 1.10 (0.66 to 1.83) (3) MMR (a) 2.85 (1.78 to 4.56) (b) 0.82 (0.47 to 1.43) (3) MMRV (a) 1.06 (0.49 to 2.27) (b) 1.21 (0.73 to 2.01) (*) Poisson regression |
| db‐MacDonald 2014 Person‐time cohort | Children aged 12 to 23 months who had received either MMRV or MMR+V in Alberta between 2006 and 2012. n = 277,774 |
Seizure events ascertained from 3 administrative databases: 1) the physician claims database; 2) the ambulatory care reporting system, which includes emergency department visits; 3) the hospital discharge abstracts database. From the physician claims database (ICD‐9), codes 780.3* for convulsions and the ambulatory care and hospital discharge databases (ICD, 10th revision, Canadian version, codes R56.0* for febrile convulsions), using coding consistent with other studies of febrile seizures after vaccination. High risk (cohort) Children with a personal history of febrile seizure; seizure disorder; central nervous system injury, infection, or neoplasm; encephalopathy; or a progressive, evolving, or unstable neurologic condition (as identified from physician claims, emergency department visits, or hospital discharges) |
MMRV vaccine (Priorix‐Tetra) administered to children in Alberta, relative to same‐day administration of separate MMR and varicella (MMR+V) vaccines. Risk period (after vaccination) (a) 0 to 42 days (b) 7 to 10 days Control period (before vaccination) 42 days preceding vaccination |
Conclusion:
Combining MMR and varicella into a single vaccine decreases pain for children and distress for parents, thus addressing common barriers to vaccine uptake, and may improve vaccine coverage levels and decrease immunisation delivery costs. These potential benefits must be balanced by the increased risk (albeit small) of febrile seizures with the combination vaccine. Febrile seizures are typically self‐limiting and rarely have long‐term effects, but they can be extremely distressing for parents, may precipitate acute care visits, and may undermine confidence in immunisation programmes. It is a matter for debate whether the choice of separate versus combination vaccine is a policy decision or a choice for parents to make in consultation with their vaccination provider. If MMRV continues to be offered for first‐dose administration, it might be advisable to counsel parents regarding antipyretic use if children experience a fever within the peak risk period. |
Full cohort n = 277,774 MMRV n = 96,686 (a1) 0 to 41 days (b1) 7 to 10 days MMR+V n = 181,088 (a2) 0 to 41 days (b2) 7 to 10 days Low risk n = 266,768 MMRV n = 92,570 (b3) 7 to 10 days MMR+V n = 174,198 (b4) 7 to 10 days High risk n = 11,006 MMRV n = 4116 (b5) 7 to 10 days MMR+V n = 6890 (b5) 7 to 10 days |
rr (95% CI)(*) MMRV (full‐cohort) (a1) 1.80 (1.43 to 2.27) (b1) 6.57 (4.77 to 9.05) MMR+V (full‐cohort) (a2) 1.48 (1.22 to 1.79) (b2) 3.30 (2.40 to 4.52) MMRV (low risk) (b3) 6.69 (4.90 to 9.13) MMR+V (low risk) (b4) 2.94 (2.13 to 4.07) MMRV (high risk) (b5) 4.68 (2.49 to 8.79) MMR+V (high risk) (b6) 3.61 (2.20 to 5.93) (*) Poisson regression |
ACIP: Advisory Committee on Immunization Practice CI: confidence interval CSF: cerebrospinal fluid DTP: diphtheria, tetanus, pertussis vaccine FS: febrile seizures HHV: human herpesvirus Hib: Haemophilus influenzae b vaccine HMO: health maintenance organisation ICD: International Classification of Diseases ICD‐9‐CM: International Classification of Diseases, 9th Revision, Clinical Modification incidence: cases/PT MCV: measles‐containing vaccines MenC: meningococcus C vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MMR+V: measles, mumps, rubella, plus varicella vaccine NIP: National Imminization Program OR: odds ratio PT: person‐time rr: rate ratio (relative incidence; incidence rate ratio) RR: risk ratio (relative risk)
15. Safety: MMRV versus MMR/MMR+V ‐ febrile seizures.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Authors' conclusion | Crude data | Estimate (95% CI) |
|
cb‐Jacobsen 2009 Retrospective cohort study |
Index cohort
(n = 31,298) all children ages 12 to 60 months vaccinated with MMRV at KPSC from February 2006 to June 2007. Children were excluded if they had a history of measles, mumps, rubella, or varicella disease or history of vaccination for any of these diseases. Comparison (matched) cohorts (1) children vaccinated with MMR+V concomitantly before the routine use of MMRV at KPSC (November 2003 to January 2006). Children were optimally matched without replacement to children vaccinated with MMRV, on the basis of age, sex, and vaccination calendar day and month, and had to fulfil the same enrolment criteria. (2) pre‐vaccination self‐comparison period defined by the period from 60 to 30 days prior to vaccination with MMRV. (3) postvaccination self‐comparison period defined by the period from 60 to 90 days following vaccination. |
Febrile convulsion Potential convulsions were identified as occurring on any visit with a diagnosis coded as 779.0 (neonatal seizures), 333.2 (myoclonus), 345 (epilepsy), 780.39 (other convulsion), 780.3 (convulsion), 780.31 (simple febrile convulsion), 780.32 (complex febrile convulsion) regardless of setting (e.g. inpatient, outpatient, emergency department, or outside facility). |
MMRV: ProQuad contains components of 2 Merck vaccines, MMR‐II (MMR) and VARIVAX (V), and was approved in the USA in September 2005. Before MMRV was available, MMR and V were usually given concomitantly as 2 separate injections. Risk interval (a) 0 to 4 days (b) 5 to 12 days (c) 13 to 30 days (d) 0 to 30 days |
Conclusion: "These data suggest that the risk of febrile convulsion is increased in days 5–12 following vaccination with MMRV as compared to MMR+V given separately during the same visit, when post‐vaccination fever and rash are also increased in clinical trials. While there was no evidence of an increase in the overall month following vaccination, the elevated risk during this time period should be communicated and needs to be balanced with the potential benefit of a combined vaccine." |
Cases versus cases MMRV versusMMR+V matched n = 31,298 (a) 9 versus 7 (b) 22 versus 10 (c ) 13 versus 23 (d) 44 versus 40 MMRV versusPre‐Vacc matched n = 31,298 (a) 9 versus 4 (b) 22 versus 3 (c ) 13 versus 9 (d) 44 versus 16 MMRV versusPost‐Vacc matched n = 31,298 (a) 9 versus 5 (b) 22 versus 5 (c ) 13 versus 13 (d) 44 versus 23 |
RR (95% CI)
MMRV versusMMR+V
(a) 1.28 (0.48 to 3.45)
(b) 2.2 (1.04 to 4.65)
(c ) 0.57 (0.29 to 1.12)
(d) 1.1 (0.72 to 1.69) MMRV versusPre‐Vacc (a) 2.25 (0.69 to 7.31) (b) 7.33 (2.2 to 24.5) (c ) 1.44 (0.62 to 3.38) (d) 2.75 (1.55 to 4.87) MMRV versusPost‐Vacc (a) 1.8 (0.6 to 5.37) (b) 4.4 (1.67 to 11.62) (c ) 1 (0.46 to 2.16) (d) 1.91 (1.16 to 3.17) |
|
cb‐Klein 2010 Retrospective cohort study |
Index cohort Children aged 12 to 23 months who were members of the 7 participating versusD sites and had received their first dose of MMRV (n = 83,107) Comparison cohorts (1) children vaccinated with MMR+V between January 2000 and October 2008 (n = 376,354) (2) children vaccinated with MMR vaccine alone (n = 145,302) (2000 to 2008) |
Seizureevent The first instance during the 42 days after MMRV vaccination with ICD‐9 codes 345* (epilepsy) or 780.3* (convulsion) in the emergency department or hospital. Postvaccination outpatient fever visits were examined by using ICD‐9 code 780.6 for fever or febrile illness at all 7 participating versusD sites from January 2000 through October 2008. Similar to seizure cases, fever visits were censored after the first occurrence within the 42 days. |
MMRV (Merck & Co Inc, West Point, PA) Risk interval after vaccination (a) 7 to 10 days (b) 0 to 42 days (c) 0 to 30 days |
Conclusion:
Amongst 12‐ to 23‐month‐olds who had received their first dose of measles‐containing vaccine, fever and seizure were elevated 7 to 10 days after vaccination. Vaccination with MMRV results in 1 additional febrile seizure for every 2300 doses given instead of separate MMR varicella vaccines. Providers who recommend MMRV should communicate to parents that it increases the risk of fever and seizure over that already associated with measles‐containing vaccines. |
Seizures cases from
2000 to 2008 MMRV n = 83,107 (a) 77 cases (b) 189 cases (c) not reported MMR+V n = 376,354 (a) 174 (b) 598 (c) not reported MMR n = 145,302 (a) 42 (b) 151 (c) not reported |
rr (95% CI)(*) MMRV versusMMR+V (a) 1.98 (1.43 to 2.73) (b) 1.42 (1.11 to 1.81) (c) 1.40 (1.06 to 1.85) (*) Poisson regression due to rarity of the event rr (rate ratio) is very close to RR RR (95% CI) MMRV versusMMR (a) 3.21 (2.2 to 4.67) (b) 2.19 (1.77 to 2.71) (c) not reported |
|
cb‐Klein 2012
Retrospective
cohort study linked to cb‐Klein 2010 |
Children
aged 48 to 83 months who were members of the 7 participating versusD sites between January 2000 and October 2008 |
Seizureevent Postvaccination seizure event as the first instance during the 42 days after a measles‐ or varicella‐containing vaccine of the ICD‐9 codes 345* (epilepsy) or 780.3* (convulsion) in the emergency department or hospital. The authors identified postvaccination medically attended outpatient fever events by using ICD‐9 code 780.6 (fever and other physiologic disturbances of temperature regulation). |
1) MMRV (Merck & Co)
2) MMR (Merck & Co Inc, West Point, PA) + varicella (Merck & Co) separately administered on the same day 3) MMR Risk interval after vaccination (a) 7 to 10 days (b) 0 to 42 days |
Conclusions: This study provides reassurance that MMRV and MMR+V were not associated with an increased risk of febrile seizures among 4‐ to 6‐year‐olds. The authors can rule out with 95% confidence a risk greater than 1 febrile seizure per 15,500 MMRV doses and 1 per 18,000 MMR+V doses. |
Cases/PT MMRV n = 86,750 (a) 4/950.1 (b) 19/10,497.2 MMR+V n = 67,438 (a) 0/739 (b) 10/7874 MMR n = 479,311 (a) 9/5252.7 (b) 99/55,618 |
RR (95% CI) MMRV versusMMR+V (a) 7 (0.38 to 130.02) (b) 1.48 (0.69 to 3.18) MMRV versusMMR (a) 2.46 (0.76 to 7.99) (b) 1.06 (0.65 to 1.73) |
|
cb‐Rowhani‐Rahbar 2013 Retrospective cohort study linked to cb‐Klein 2010 |
n = 840,348 children 12 to 23 months of age who had received a measles‐containing vaccine from 2001 through 2011 |
Fever events in the o utpatient setting was defined using ICD‐9 code 780.6*. Seizure events in the postimmunisation medically attended in the emergency department or hospital setting was defined using ICD‐9 code 780.3* (convulsion) or 345* (epilepsy). The authors do not distinguish between febrile and afebrile seizures. |
1) MMRV (Merck & Co)
2) MMR (Merck & Co Inc, West Point, PA) + varicella (Merck & Co) separately administered on the same day 3) MMR Risk interval after vaccination (a) 7 to 10 days (b) 0 to 42 days |
Conclusions: Measles‐containing vaccines are associated with a lower increased risk of seizures when administered at 12 to 15 months of age. Findings of this study that focused on safety outcomes highlight the importance of timely immunisation of children with the first dose of measles‐containing vaccines. |
12 to 15 months
Fever cases (0 to 42 days) (7 to 10 days) MMRV n = 105,578 (2191) (864) MMR+V n = 520,436 (11,300) (3553) MMR n = 102,537 (2558) (760) 16 to 23 months Fever cases (0 to 42 days) (7 to 10 days) MMRV n = 14,799 (300) (116) MMR+V n = 64,551 (1310) (399) MMR n = 32,447 (744) (227) 12 to 15 months Seizures cases (0 to 42 days) (7 to 10 days) MMRV n = 105,578 (255) (99) MMR+V n = 520,436 (997) (244) MMR n = 102,537 (172) (45) 16 to 23 months Fever cases (0 to 42 days) (7 to 10 days) MMRV n = 14,799 (68) (30) MMR+V n = 64,551 (231) (70) MMR n = 32,447 (87) (31) |
MMRV versusMMR+V rr (95% CI)(*) Fever 12 to 15 months (a) 1.4 (1.3 to 1.5) 16 to 23 months (a) 1.4 (1.1 to 1.7) Seizures 12 to 15 months (a) 2.0 (1.4 to 2.8) 16 to 23 months (a) 2.1 (1.3 to 3.3) (*)Poisson regression MMRV versusMMR+V RR (95% CI) (a) 2 (1.63 to 2.45) (b) 1.28 (1.13 to 1.44) MMRV versusMMR RR (95% CI) (a) 1.9 (1.43 to 2.53) (b) 1.4 (1.19 to 1.65) |
|
cb‐Gavrielov‐Yusim 2014
cb‐Gavrielov‐Yusim 2014
Retrospective cohort study |
Index cohort All participants were aged 10 to 24 months. (intervention) n = 8344 MMRV immunised from 1 September 2008 to 31 December 2009 Comparison cohorts n = 90,294 MMR immunised from 1 January 2005 to 31 August 2008 |
Febrile convulsion
Validation FC cases were retrieved using the following coded and free‐text diagnoses: “convulsions in newborn”, “convulsions”, “febrile convulsions”, “complex febrile convulsions”, “other convulsions”. Children diagnosed with FC differential diagnoses during the observational period, i.e. head trauma, epilepsy, or CNS infection, were excluded from the study. The exact coded and free‐text diagnoses used to depict coincidental differential conditions were “concussion”, “cerebral disease”, “acquired hydrocephalus”, “cerebral palsy”, “cerebral cyst”, “epilepsy”, “meningism”, types of “bacterial meningitis”, “encephalitis”, “meningococcal meningitis”, “aseptic viral meningitis”. Children were also excluded from the study if they had a history of mumps, measles, rubella, or varicella prior to vaccination. |
MMRV Priorix‐Tetra MMR (Priorix) GSK Priorix‐Tetra combines the components of 2 of GSK's live attenuated vaccines: MMR (Priorix) and varicella vaccine (Varilrix). Risk intervals Postvaccination (a) 40 days (b) 5 to 12 days (c) 7 to 10 days |
Conclusion:
"The risk of FC is elevated
in children immunized with GSK’s MMRV vaccine. This risk is transient and appears during the second week following immunization. The relative fraction of FC attributable to MMRV vaccine is very low in the target population, and is not detectable in extended follow‐up." |
N cases MMRV/
N MMRV
versus
N cases MMR/
N MMR (a) 19/8344 versus 198/90,294 (b) 8/8344 versus 38/90,294 (c) 7/8344 versus 30/90,294 |
OR (95% CI) unadjusted estimates (a) 1.04 (0.65 to 1.66) (b) 2.28 (1.06 to 4.89) (c) 2.53 (1.11 to 5.76) adjusted estimate(**) (a) 1.00 (0.6 to 1.67) (b) 2.16 (1.01 to 4.64) (c) 2.36 (1.03 to 5.38) (**) 2 different types of multivariate models were used: (a) Cox regression HR (b) logistic‐regression OR (c) logistic‐regression OR Due to rarity of events, HR and OR are very close. |
| cb‐Schink 2014 Matched cohort study | All children born between 1 January 2004 and 31 December 2008 n = 226,267 received an immunisation with 1 of the index vaccines during the study period (2006 to 2008) Index cohort n = 82,656 MMRV Comparison cohorts n = 111,241 MMR n = 32,370 MMR+V |
Febrile convulsions Diagnosis of FC, i.e. an ICD‐10‐GM code R56.0 in any of the hospital diagnoses. 2 outcome definitions, as follows. The primary outcome “FC narrow” was defined as hospitalisation where no alternative plausible cause of FC. This endpoint included: (i) all hospitalisation with FC as main discharge diagnosis; (ii) all hospitalisation with FC as main admission diagnosis and without a main discharge diagnosis of an infectious disease (except measles, mumps, rubella, or chickenpox) or a neurological condition; (iii) all hospitalisation with FC as secondary or ancillary diagnosis and a main discharge diagnosis coded as complication following immunisation (ICD‐10 code T88.0 infection following immunization or T88.1 other complications following immunization, not elsewhere classified). Due to exclusion of alternative causes of FC in this outcome definition, it was assumed that it would have higher specificity, but lower sensitivity. The secondary outcome “FC Jacobsen” was defined as follows: only hospitalisations for FC with a neurological condition coded as main discharge diagnosis were excluded (cb‐Jacobsen 2009). Consequently, “FC Jacobsen” included: (i) all hospitalisation with FC as main discharge diagnosis; (ii) all hospitalisation with FC as main admission diagnosis and without a main discharge diagnosis of a neurological condition; and (iii) all hospitalisation with FC as secondary or ancillary diagnosis and with a main discharge diagnosis coded as complication following immunisation. “FC narrow” cases are a subset of “FC Jacobsen” cases. |
MMRV: Priorix‐Tetra (GSK) compared to MMR and V vaccines (MMR+V). Risk interval postvaccination (a) 0 to 4 days (b) 5 to 12 days (c) 13 to 30 days (d) 0 to 30 days . |
Conclusion: This study suggests a similar risk of FC after a first dose of Priorix‐Tetra as has been observed for a first dose of ProQuad, pointing to a class effect of these quadrivalent vaccines. The elevated risk of FC observed for the quadrivalent vaccines has to be weighed against the advantage of only 1 injection for the child and the potential benefit of an increased varicella immunisation coverage. |
FC narrow MMRV versusMMR matched n = 74,734 case versus cases (a) 4 versus 5 (b) 14 versus 3 (c) 4 versus 9 (d) 22 versus 17 FC narrow MMRV versusMMR+V matched n = 32,180 case versus cases (a) 2 versus 0 (b) 5 versus 1 (c) 4 versus 9 (d) 22 versus 17 FC narrow MMRV versusMMR/MMR+V matched n = 82,561 case versus cases (a) 4 versus 4 (b) 18 versus 4 (c) 4 versus 8 (d) 26 versus 16 FC Jacobsen MMRV versusMMR matched n = 74,734 case versus cases (a) 7 versus 13 (b) 45 versus 19 (c) 35 versus 31 (d) 87 versus 63 FC Jacobsen MMRV versusMMR+V matched n = 32,180 case versus cases (a) 5 versus 4 (b) 21 versus 14 (c) 18 versus 12 (d) 44 versus 30 FC Jacobsen MMRV versusMMR/MMR+V matched n = 82,561 case versus cases (a) 8 versus 15 (b) 51 versus 21 (c) 40 versus 31 (d) 99 versus 67 |
OR (95% CI) FC narrow MMRV versusMMR (a) 0.8 (0.3 to 2.5) (b) 4.1 (1.3 to 12.7) (c ) 0.5 (0.2 to 1.4) (d) 1.3 (0.7 to 2.4) FC narrow MMRV versusMMR+V (a) 5.3 (0.4 to 70) (b) 3.5 (0.76 to 19) (c ) 1.5 (0.3 to 8.7) (d) 3.9 (1 to 14.5) FC narrow MMRV versusMMR/MMR+V (a) 1 (0.3 to 3.3) (b) 4.1 (1.5 to 11.1) (c ) 0.5 (0.2 to 1.6) (d) 1.6 (0.9 to 3) FC Jacobsen MMRV versusMMR (a) 0.5 (0.2 to 1.3) (b) 2.3 (1.4 to 3.9) (c ) 1.1 (0.7 to 1.8) (d) 1.4 (1 to 1.9) FC Jacobsen MMRV versusMMR+V (a) 1.1 (0.3 to 3.5) (b) 1.5 (0.8 to 2.9) (c ) 1.6 (0.8 to 3.2) (d) 1.5 (1 to 2.4) FC Jacobsen MMRV versusMMR/MMR+V (a) 0.5 (0.2 to 1.2) (b) 2.4 (1.5 to 3.9) (c ) 1.3 (0.8 to 2) (d) 1.5 (1.1 to 2) |
| cb‐Klein 2017 Retrospective cohort study linked to cb‐Klein 2012; cb‐Klein 2010 | n = 946,806 children < 36 months of age who had received a first dose of any measles‐containing vaccine from 2000 to 2012 |
Fever visit
Fever visits using ICD‐9 code 780.6. Fever due to an MCV was defined as any clinic or emergency department visit with a fever code 7 to 10 days after a first dose of any MCV (henceforth known as ‘‘MCV‐associated fever”). This study analysed all fevers during postvaccination days 7 to 10 as if they were due to MCV. |
1) MMRV (Merck & Co)
2) MMR (Merck & Co Inc, West Point, PA) + varicella (Merck & Co) separately administered on the same day 3) MMR Risk interval after vaccination (a) 7 to 10 days |
Conclusion: This study identified risk factors associated with developing fever 7 to 10 days after a first dose of measles‐containing vaccines. The study confirmed previous findings that fever was more often associated with receipt of MMRV as compared with MMR vaccine and with older age at time of vaccination during the second year of life, and further found that prior fever and seizure events were associated with fever after measles vaccine and that being fever‐prone in general predicted fever after measles‐containing vaccine. Even after adjusting for general individual and familial susceptibility to fever, fever due to measles vaccine specifically clustered in families. This study suggests an important link between population health (surveillance of a large population for vaccine adverse events) and personalised medicine (possible genetic basis for susceptibility to fever after MCV). Future work is needed to further define this possible relationship of genetics and vaccine‐associated fever. |
MMRV versus MMR (a) MCV‐associated fever (b) MCV‐associated fever (older sibling with MCV‐associated fever) |
OR (95% CI)(*) (a) 1.3 (1.2 to 1.5) (b) 1.5 (1.2 to 1.8) (*)logistic regression |
CI: confidence interval CNS: central nervous system FC: febrile convulsion HR:hazards ratio ICD: International Classification of Diseases ICD‐10‐GM: International Classification of Diseases. Tenth Revision, German Modification incidence: cases/PT MCV: measles‐containing vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MMR+V: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk)
16. Safety: autistic spectrum disorders.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
cb‐Madsen 2002 Retrospective cohort |
Danish children born between January 1991 and December 1998 (n = 537,303) |
(a) Autistic disorders ICD‐10 codes
F84.0 or similar DSM‐IV code 299; (b) Other autistic spectrum disorders ICD‐10 codes F84.1 through F84.9 and DSM‐IV codes 299.1‐ through 299.80. From medical records in Danish Psychiatric Central Register |
MMR vaccine: Moraten (measles), Jeryl Lynn (mumps), Wistar RA 27/3 (rubella) Vaccination data reported in the National Board of Health. Vaccinated n = 440,655 Unvaccinated n = 96,648 |
This study provides 3 strong arguments against a causal relation between MMR vaccination and autism.
Furthermore, the results were derived from a nationwide cohort study with nearly complete follow‐up data. |
(a) Autistic disorders cases unvaccinated n = 53 PT unvaccinated PT(years) = 482,360 versus cases vaccinated n = 263 PT vaccinated PT(years) = 1,647,504 (b) Other autistic spectrum disorters cases unvaccinated n = 77 PT unvaccinated PT(years) = 482,360 versus cases vaccinated n = 345 PT vaccinated PT(years) = 1,647,504 |
rr (95% CI)(*) (a) 0.92 (0.68 to 1.24) (b) 0.83 (0.65 to 1.07) (*) adjusted rr. Log‐linear Poisson regression |
|
cb‐Hviid 2019 Retrospective cohort study |
n = 657,461 children born in Denmark from 1999 through 31 December 2010, with follow‐up from 1 year of age and through 31 August 2013. |
Autism spectrum disorders ICD‐10: F84.0 autistic disorder, F84.1 atypical autism, F84.5 Asperger syndrome, F84.8 (other pervasive developmental disorder), F84.9 (unspecified pervasive developmental disorder). Autism risk score: In a preliminary analysis based on autism risk factors (maternal age, paternal age, smoking during pregnancy, method of delivery, preterm birth, 5‐minute Apgar score, low birthweight, and head circumference) a Risk Score was estimated for each child in the cohort. (b1) very low risk (b2) low risk (b3) moderate risk (b4) high risk Siblings status (at age 1 years): (c1) no siblings with autism (c2) siblings with autism (c3) no siblings |
MMR vaccine
Schwarz (measles, 2000 to 2007) or Enders' Edmonston
(measles, 2008 to 2013),
Jeryl Lynn (mumps), and
Wistar RA 27/3 (rubella) Vaccinated n = 625,842 Unvaccinated n = 31,619 |
The study found: no support for the hypothesis of increased risk for autism after MMR vaccination in a nationwide unselected population of Danish children; no support for the hypothesis of MMR vaccination triggering autism in susceptible subgroups characterised by environmental and familial risk factors; no support for a clustering of autism cases in specific time periods after MMR vaccination. |
Cases vaccinated/vaccinated versus Cases unvaccinated/ unvaccinated All children (a) 5992/625,842 versus 525/31,619 Autism risk score (*) (b1) 1296 versus 91 cases (b2) 1637 versus 133 cases (b3) 2106 versus 206 cases (b4) 953 versus 95 cases Siblings status (*) (c1) 2297 versus 227 (c2) 32 versus 5 (c3) 3594 versus 283 (*) denominator not reported |
HR (95% CI)(*) All children (a) 0.93 (0.85 to 1.02) Autism risk score (b1) 0.93 (0.74 to 1.16 (b2) 0.86 (0.71 to 1.04) (b3) 0.91 (0.78 to 1.06) (b4) 1.06 (0.85 to 1.32) Siblings status (c1) 0.98 (0.84 to 1.13) (c2) 2.96 (0.58 to 12.43) (c3) 0.89 (0.78 to 1.01) (*) adjusted by birth year, sex, other vaccines received, siblings history of autism, and autism risk score). Cox regression |
| cb‐Jain 2015 Retrospective cohort | Children continuously enrolled in the health plan from birth to at least 5 years of age during
2001 to 2012 who also had an older sibling continuously enrolled for at least 6 months between
1997 and 2012. n = 95,727 children in the cohort, (a) n = 93,798 older siblings without ASD (b) n = 1929 older sibling with ASD. |
Autism spectrum disorders Status in index children and older siblings was determined using a claims‐based algorithm that required 2 or more claims on separate dates of service with an ICD‐9‐CM diagnosis code in any position for autistic disorder, other specified pervasive developmental disorder including: Asperger syndrome, or unspecified PDD (299.0x, 299.8x, and 299.9x). Both index child and older sibling ASD status were determined using their entire enrolment time that fell within the study period. Index children had to have at least 1 older sibling with 2 claims with ASD diagnoses or all older siblings with no ASD diagnoses. Children with an older sibling with only 1 claim with an ASD diagnosis were excluded. Index children with only 1 claim with an ASD diagnosis were also excluded. |
MMR vaccine receipt was defined as having a Current Procedural Terminology (CPT) or ICD‐9‐CM procedure code indicating receipt of each component (measles, mumps, and rubella) after 1 year of age. |
The study found: MMR vaccine was not associated with increased risk of ASD, regardless of whether older siblings had ASD. These findings indicate no harmful association between MMR vaccine receipt and ASD even amongst children already at higher risk for ASD. |
Cases vaccinated/vaccinated versus Cases unvaccinated/ unvaccinated age 2 years ‐ 1 dose (a) 53/77,822 versus 13/15,249 (b) 7/1394 versus 6/520 age 3 years ‐ 1 dose (a) 239/79,666 versus 45/12,853 (b) 38/1458 versus 17/438 age 4 years ‐ 1 dose (a) 395/79,691 versus 65/11,957 (b) 64/1491 versus 25/387 age 5 years ‐ 1 dose (a) 339/40,495 versus 56/7735 (b) 51/864 versus 23/269 age 5 years ‐ 2 doses (a) 244/45,568 versus 56/7735 (b) 30/796 versus 23/269 |
HR (95% CI)(*) age 2 years ‐ 1 dose (a) 0.91 (0.68 to 1.20) (b) 0.76 (0.48 to 1.22) age 3 years ‐ 1 dose (a) 0.97 (0.77 to 1.21) (b) 0.81 (0.53 to 1.25) age 4 years ‐ 1 dose (a) 1.03 (0.81 to 1.31) (b) 0.86 (0.56 to 1.34) age 5 years ‐ 1 dose (a) 1.10 (0.79 to 1.53) (b) 0.92 (0.56 to 1.50) age 5 years ‐ 2 doses (a) 1.09 (0.76 to 1.54) (b) 0.56 (0.30 to 1.04) (*) Hazard rate ratio from Cox proportional hazards model adjusting for birth year, sex, region, race/ethnicity, maternal or paternal highest education level, household income, mother’s age at birth of index infant, father’s age at birth of index infant, continuous enrolment with mental health carve‐out benefit, Childhood Chronic Conditions score, seizure, allergies, and preterm birth. Cox regression |
|
cb‐Uchiyama 2007 Retrospective cohort |
Children born between 1976 and 1999 with clinical diagnosis of ASD analysed n = 858 (whole sample n = 904; n = 46 cases were excluded due to insufficient information on ASD regression) |
Regression in autism spectrum disorders ASD regression defined as “a documented deterioration in any aspect of development or reported loss of skills, however transient” Note: over time 2 different diagnostic processes have been adopted at YPCD: until February 2000, the diagnostic process consisted of the assessment of ASD initially conducted by a child psychiatrist using the DSM‐IV (American Psychiatric Association, 1994), after which a clinical psychologist conducted an intelligence test. After admission a psychiatrist followed the patients once or twice a month. All doctors had been trained in using a common concept of diagnosis. From February 2000 onwards, a child psychiatrist with a clinical psychologist conducted the full assessment in 1 day. Diagnosis of ASD was made by 3 experienced child psychiatrists based on clinical observations, intellectual and developmental tests, and interviews with parents and patients. |
MMR vaccine AIK‐C (measles), Urabe AM9 (mumps) To‐336 (rubella) strains. Data concerning MMR vaccination were moreover obtained from records of the Maternal and Child Health Handbook and were referred to the MMR generation group only. Participants were classified according to the chance of having received MMR vaccine (MMR was administered in Japan from April 1989 to April 1993 in children 12 to 36 months of age):
|
The study found: within the MMR era, the rate of regression in those who received MMR was not higher than those who did not. Moreover, there was no indication that the rate of regression in ASD was higher during the era when MMR was used, compared to the ‘‘before’’ period and ‘‘after’’ period, and the ‘‘before" and "after’’ periods combined. |
N cases vaccinated/
N vaccinated
versus
N cases unvaccinated/
N unvaccinated MMR‐generation (a) 15/54 versus 45/132 All generations (*) (b) 15/54 versus 272/715 (*) 98 cases out of 275 (MMR‐generation) were excluded due to unclear vaccination status, analysed n = 186. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMR‐era versusbefore (c) 98/275 versus 34/100 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMR‐era versusafter (d) 98/275 versus 193/483 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ MMR‐era versus(before + after) (e) 98/275 versus 227/583 |
OR (95% CI) (a) 0.744 (0.349 to 1.571) (b) 0.626 (0.323 to 1.200) (c) 1.075 (0.646 to 1.791) (d) 0.832 (0.605 to 1.144) (e) 0.868 (0.638 to 1.182) |
|
bb‐Smeeth 2004 Case‐control |
Children with a first diagnosis of a PDD during the study period registered with a GPRD practice. Cases: n = 1294 Controls: n = 4469 |
Pervasive developmental disorder “Those with autistic disorders and similar presentations were classified as having 'autism' and those with other description (such as Asperger’s syndrome) were classified as having 'other PDD'. Patients who had more than one PDD diagnostic code recorded at different times (for example, autism and then Asperger’s syndrome) were classified as having the most specific diagnosis (in this example Asperger’s syndrome)” From diagnosis contained in UK General Practice Research Database (GPRD electronic records). |
MMR vaccine: No single clinical code was immediately implemented for MMR, then MMR was identified by codes of measles, mumps, and rubella administered on the same day. Information on MMR exposure:
|
The study found: MMR vaccination was not associated with an increased risk of subsequently being diagnosed with a PDD. |
MMR vaccination Before index date (a) at any age (b1) before third birthday (b2) after third birthday (c1) before age 18 months (c2) after age 18 months (d) autism only (e) other PDD only |
OR (95% CI)(*) (a) 0.86 (0.68 to 1.09) (b1) 0.90 (0.70 to 1.15) (b2) 0.77 (0.55 to 1.08) (c1) 0.90 (0.70 to 1.15) (c2) 0.80 (0.61 to 1.05) (d) 0.88 (0.67 to 1.15) (e) 0.75 (0.46 to 1.23) (*)adjusted conditional logistic regression |
|
bb‐De Stefano 2004 Case‐control |
Children with autism aged 3 to 10 years in 1996. All sample Cases: n = 624 Controls: n = 1824 Birth certificate subsample Cases: n = 355 Controls: n = 1020 |
Autism cases were identified through screening and abstraction of source files at schools, hospitals, clinics, and specialty providers. Clinical psychologists with expertise in the diagnosis of autism reviewed the abstracted records according to a standardised coding scheme to determine the presence of behavioural characteristics consistent with the DSM‐IV criteria for ASDs. | MMR vaccine type: not stated MMR vaccination was abstracted from “standardized state immunization forms”. 3 specific years cutoff: (a) 18 months of age, as an indicator of “on‐time” vaccination according to the recommended vaccination schedule for MMR vaccine; (b) 24 months of age, the age by which atypical development has become apparent in most children with autism; (c) 36 months of age, the age by which autistic characteristics must have developed to meet DSM‐IV criteria for autism. |
The study found: no significant associations for vaccinated before 18 months or before 24 months of age, including children with some indication of regression or plateau in development, the group of most concern. Vaccination before 36 months of age was more common amongst case children than control children, although only a small proportion of children in either group received their first MMR vaccination after 36 months of age. Rather than representing causal relationships, associations with the 36‐month cutoff would be more likely than associations with earlier age cutoffs to have been influenced by factors related to the evaluation, management, and treatment of the child, e.g. case children might have been more likely than control children to have been vaccinated as a requirement for enrolment in early intervention or preschool special education programs. This possibility is supported by the finding that the difference between case and control children in the proportion vaccinated before 36 months of age was strongest in the 3‐ to 5‐year‐old age group. A majority of case children who were vaccinated after 36 months of age, however, had indications of developmental problems before 36 months of age. |
All cases (a1) < 18 months (b1) < 24 months (c1) < 36 months Birth certificate (a2) < 18 months (b2) < 24 months (c2) < 36 months |
OR (95% CI) All cases(*) (a1) 1.12 (0.91 to 1.38) (b1) 1.21 (0.93 to 1.57) (c1) 1.49 (1.04 to 2.14) Birth certificate (**) (a2) 0.93 (0.66 to 1.30) (b2) 0.99 (0.63 to 1.55) (c2) 1.23 (0.64 to 2.36) (*)partially adjusted estimates: conditional logistic regression model stratified by the matching variables (age, gender, school). (**)adjusted estimates: conditional logistic regression model stratified by the matching variables (age, gender, school) and adjusted for birthweight, multiple gestation, maternal age, and maternal education. |
|
bb‐Mrozek‐Budzyn 2010 Case‐control |
Children aged 2 to 15 years diagnosed with childhood or atypical autism. Cases: n = 96 Controls: n = 192 children matched for birth year, gender, and practice |
Childhood or atypical autism classified according to ICD‐10 criteria as F84.0 or F84.1, respectively. Every diagnosis of autism was made by child psychiatrist. Dates of these diagnoses were recorded in general practitioner files. Cases with uncertain diagnosis of autism, secondary to disease state or trauma, were excluded. Parents were interviewed. Questions for all children included information about prenatal and postnatal development, mental and physical development, chronic diseases, malformations and injuries, history of bowel disturbances, birth order, family size, and parents’ socioeconomic status. Parents of children with autism were additionally asked about the date of onset of symptom, the period when parents first suspected their child’s symptoms might be related to autism, and their knowledge and beliefs regarding the cause of autism. |
Vaccine type: MMR: not described MV: measles vaccine monovalent: not described Information about vaccination history was extracted from physician records. |
The study found: MMR vaccination was not significantly associated with an increased risk of autism in children. In a separate analysis, a similar result was achieved for the single‐antigen measles vaccine. An unexpected finding was that odds ratios associated with MMR were lower than with the single measles vaccine. The decreased risk of autism amongst vaccinated children may be due to some other confounding factors in their health status. For example, healthcare workers or parents may have noticed signs of developmental delay or disease before the actual autism diagnosis and for this reason have avoided vaccination. |
Any vaccine versusunvaccinated (a1) vaccinated before symptom onset (a2) vaccinated before diagnosis MMR vaccine versusunvaccinated (b1) vaccinated before symptom onset (b2) vaccinated before diagnosis MV vaccine versusunvaccinated (c1) vaccinated before symptom onset (c2) vaccinated before diagnosis |
OR (95% CI)(*) any vaccine versusunvaccinated (a1) 0.65 (0.26 to 1.63) (a2) 0.28 (0.01 to 0.76) MMR versusunvaccinated (b1) 0.42 (0.15 to 1.16) (b2) 0.17 (0.06 to 0.52) MV versusunvaccinated (c1) 0.86 (0.33 to 2.23) (c2) 0.36 (0.13 to 1.00) (*)Adjusted for mother’s age (15 to 35, 36 to 44 years), medication during pregnancy, gestation time (36 to 37, 38 to 43 weeks), perinatal injury, 5‐minute Apgar scale score (3 to 8, 9 to 10). |
|
bb‐Uno 2012 Case‐control |
The study analysed case data from patients of YPDC; the cases consisted of
patients who: (1) were diagnosed with ASD, and (2) had been born
between 1 April 1984 and 30 April 1992, the possible time period
for MMR vaccination. Children aged 6 to 36 months cases: n = 189 control: n = 224 |
Diagnosis of ASD: based on the classifications of pervasive developmental disorders in the DSM‐IV and standardised criteria using the Diagnostic Interview for Social and Communication Disorder (DISCO). | MMR vaccine: not described |
The study found: there was no convincing evidence that MMR vaccination and increasing the number of vaccine injections were associated with an increased risk of ASD in a genetically homogeneous population. Consequently, these findings indicate that there is no basis for avoiding vaccination out of concern for ASD. |
Cases vaccinated/N cases versus Control vaccinated/N controls 47/189 versus 54/224 |
OR (95% CI)(*) 1.04 (0.65 to 1.68) (*) matched odds ratio |
|
gb‐Fombonne 2006 Case‐only ecological method |
Children aged 5 to 11 years (birth cohorts 1987 to 1998 attending a boarding school in Montreal (n = 27,749, out of whom 180 with PDD) |
Pervasive developmental disorders Children with a diagnosis of PDD were identified by school personnel and given a study code to preserve the anonymity of the data. Children’s diagnoses were not verified by direct assessments, but it is worth noting that a majority of these children (N = 155; 86.1%) were diagnosed at the Montreal Children’s Hospital. School personnel further identified the diagnostic subtype using DSM‐IV diagnostic criteria, age, grade, and school the child was attending. When available, place of birth was recorded as well. |
MMR (no description) Identified by vaccination records |
MMR and autism: During the 11‐year interval, rates of PDD significantly increased, whereas MMR vaccine uptake showed a slight opposite trend. The opposite directions of both trends make it even less likely that a true association was not detected in the study data. The study shows a lack of association between MMR uptake and PDD rates applied to the period (1987 to 1995) where a single MMR dose was administered at 12 months of age. Rates of PDD were rapidly increasing well before the introduction of the 2‐dose schedule and, during that first phase, the increase of PDD rate bore no relationship with MMR vaccine uptake. The authors tested whether the introduction of a second MMR dose after 1995 accelerated the increase in PDD rates in the following 3 years. No statistically significant difference could be found between the rate of increase in PDD prevalence between the 1‐dosing and the 2‐dosing periods. In fact, the end point prevalence estimate for 1998 was consistent with the value predicted on the basis of the 1987 to 1995 rate of increase. Consequently, 2‐dosing schedule with MMR before age 2 is not associated with an increased risk of PDD. |
No association. Significant increase in rates of PDDs from 1987 to 1998 (OR 1.10, 95% CI 1.05 to 1.16; P < 0.001) despite decrease in MMR uptake through birth cohorts from 1988 to 1998 (Chi² for trend = 80.7; df = 1; P < 0.001). | No data available for meta‐analysis |
|
gb‐Honda 2005 Case‐only ecological method |
Children born from 1988 to 1996 (n = 31,426) |
Autism spectrum disorders ASD cases defined as all cases of PDD according to ICD guidelines, but an early detection clinical system called DISCOVERY that included items drawn up by the Public Health Bureau of Yokohama called YACHT (Young Autism and other developmental disorders CHeckup Tool) was active in Kohoku Ward. Definite regression Episodes in which caregiver records confirm loss of skills such as aspects of communication skills, including utterances, social behaviours, play activities, adaptive skills, or motor skills that had appeared and become established in the child’s daily life. Probable regression If there was insufficient evidence to confirm that previous skills had become firmly acquired, or that they had not fully disappeared. |
MMR vaccine: no description Exposed period: 1988 to 1992 MMR vaccination rates declined from 69.8% in the 1988 birth cohort to 42.9%, 33.6%, 24.0%, and a mere 1.8% in birth cohorts 1989 to 1992. Reference period: 1993 to 1996 In birth cohorts 1993 to 1996, when not a single child was immunised. |
MMR vaccination is unlikely to be a main cause of ASD, that it cannot explain the rise over time in the incidence of ASD, and that withdrawal of MMR in countries where it is still being used cannot be expected to lead to a reduction in the incidence of ASD. | Risk period (cases/population) versus Reference period (cases/population) (a) Childhood autism 58/17,704 versus 100/13,722 (b) Other ASD 50/17,704 versus 70/13,722 (c) Definite regression 29/17,704 versus 31/13,722 (d) Definite + probable regression 35/17,704 versus 37/13,722 (e) All ASD 108/17,704 versus 170/13,722 |
rr (95% CI) (a) 0.45 (0.33 to 0.62) (b) 0.55 (0.39 to 0.80) (c) 0.73 (0.44 to 1.20) (d) 0.73 (0.46 to 1.16) (e) 0.49 (0.39 to 0.63) |
|
db‐Makela 2002 Person‐time cohort |
Children 1 to 7 years old (n = 535,544) |
Autism Autistic disorder: "Severe qualitative impairment in reciprocal social interaction, in verbal and non verbal communication and in imaginative activity and markedly restricted repertoire of activities and interests" (Steffenburg 1989) Data regarding first hospital visits during the study period identified by ICD‐8/9 codes respectively effective from 1969 to 1986 and from1987 through 1995 (299 ‐ Psychoses ex origine infantia; 2990 ‐ Autismus infantilis; 2998 ‐ Developmental disorder; 2999 ‐ Developmental disorder). |
MMR II ‐ vaccine (Merck & Co, West Point, PA) Measles: Enders‐Edmonston Mumps: Jeryl Lynn Rubella: Wistar RA 27/3 Vaccination data were assessed through vaccination register. For autism the risk period is open‐ended. |
The study found: no distinguishable clustering was detected in the intervals from vaccination to the hospitalisation. The number of hospital admissions remained relatively steady during the first 3 years and then gradually decreased, as was expected because of the increasing age of the vaccinees (Fig 3). 43 children were vaccinated after the first hospitalisation, and 31 were hospitalised but remained unvaccinated between November 1982 and June 1986. Of the children hospitalised for autism, none made hospital visits because of inflammatory bowel diseases in 1982 to 1995. |
ASD cases n = 309 | No data available for meta‐analysis |
|
db‐Taylor 1999 Self‐controlled case series |
Children born since 1979 from 8 health districts (North Thames, UK) |
Autistic disorder “By use of criteria of the International Classification of Diseases, tenth revision (ICD10), the diagnosis of autism was checked against information in the available records on the child’s present condition and his or her condition between the ages of 18 months and 3 years.” ICD‐10 confirmed and non‐confirmed cases from computerised special needs/disability registers at child development centres and from records in special schools. Information on children with such disorders who were younger than 16 years of age was extracted from clinical records by 1 of 3 experienced paediatric registrars. |
MMR vaccination identified by Regional Interactive Child Health Computing System (RICHS) Risk period: (a) Autism diagnosis (a1) < 12 months (a2) < 24 months after vaccination (b) Parental concern (b1) < 6 months (b2) < 12 months after vaccination (c) Regression (c1) < 2 months (c2) < 4 months (c3) < 6 months after vaccination Where vaccination and the event of interest occurred in the same month, the authors assumed that vaccination preceded the event. |
The case‐series analyses showed no evidence of temporal clustering between MMR or other measles‐containing vaccines and diagnosis of autism. Regression occurred in nearly a third of the cases of core autism; regression was not clustered in the months after vaccination. For age at first parental concern, no significant temporal clustering was seen for cases of core autism or atypical autism, with the exception of a single interval within 6 months of MMR vaccine associated with a peak in reported age at first parental concern at 18 months. This peak is likely to reflect the difficulty experienced by parents in defining the precise age at onset of symptoms in their child, particularly those with atypical autism, and consequent approximation with preference for 18 months. Our results do not support the hypothesis that MMR vaccination is causally related to autism, either its initiation or to the onset of regression. |
MMR vaccine (a) Autism diagnosis (n = 357) (b) Parental concern (n = 326) (c) Regression (n = 105) |
rr (95% CI)(*) (a1) 0.94 (0.60 to 1.47) (a2) 1.09 (0.79 to 1.52) (b1) 1.48 (1.04 to 2.12) (b2) 0.90 (0.63 to 1.29) (c1) 0.92 (0.38 to 2.21) (c2) 1.00 (0.52 to 1.95) (c3) 0.85 (0.45 to 1.60) (*) relative incidence, Poisson regression |
|
gb‐Fombonne 2001 Case‐only ecological method |
Pre‐MMR: Maudsley Family Study (MFS) sample: n = 98 probands who had an ICD‐10 diagnosis of autism PDD. Children born between 1954 and 1979. Post‐MMR: Maudsley Hospital Clinical (MHC) sample: n = 68 children born between 1987 and 1996 and had a confirmed diagnosis of PDD. Post‐MMR: Stafford sample: n = 96 children born between 1992 and 1995 selected as part of an epidemiologic survey of PDD conducted in Staffordshire (Midlands, UK) total population n = 15,500. |
Autistic enterocolitis (a) Age (in months) at first parental concern: in the 3 samples, item 2 of the ADI (earlier version of the ADI‐R) was used to assess the first onset of autistic symptoms, or the age of the child at which parents first became concerned about their child’s development. The precise wording of the question is, “How old was your child when you first wondered if there might be something not quite right with his/her development?” (b) Regression: the assessment of regression in the ADI‐R is covered with items 37 to 41 (for language) and items 95 to 103 (for other domains). The regression is assessed for language skills as follows: “Were you ever concerned that your child might have lost language skills during the first years of his/her life? Was there ever a time when he/she stopped speaking for some months after having learned to talk?” Assessment of bowel disorders and symptoms: these data were available only from the epidemiologic sample (Stafford sample). All children were reviewed regularly and are still followed up by the paediatrician, who has records of any additional hospital admissions/medical investigations for bowel disorders in these children. The occurrence of gastrointestinal symptoms was assessed by 2 sources: the parents and the paediatrician. ADI‐R: Autism Diagnostic Interview ‐ Revised was administered with the parents by trained staff. Interrater reliability on the ADI‐R interviews was assessed. |
MMR vaccine type not described MFS sample (pre‐MMR): unvaccinated MHC sample (post‐MMR): likely vaccinated Stafford sample (post ‐MMR): likely vaccinated The MMR immunisation programme was introduced in 1988 in the UK (with first MMR given between 12 and 15 months of age) with coverage rates above 90%. MMR coverage rates in 2‐year‐olds fell from 92% in 1995 to 88% in 2000. |
No evidence was found to support a distinct syndrome of MMR‐induced autism or of “autistic enterocolitis”. No changes in the mean age of parental recognition of first autistic symptoms were found when 2 samples of children, 1 clinical and 1 epidemiologic, all exposed to MMR immunisation, were compared with a pre‐MMR sample. No increase in the rate of regressive autism in recent years. Rates of regression in the development of children with autism were found to be similar in a pre‐ and post‐MMR sample. |
‐‐‐‐MFS sample (n = 98) (a) mean = 19.5 (SD = 13.6) (b) n = 18 ‐‐‐‐MHC sample (n = 68) (a) mean = 19.2 (SD = 8.8) (b) n = 0 ‐‐‐‐Stafford sample (n = 96) (a) mean = 19.3 (SD = 8.7) (b) n = 15 No statistically relevant differences across the 2 samples for the rate of probable or definite regression. |
No data available for meta‐analysis |
ADI‐R: Autism Diagnostic Interview ‐ Revised ASD: autism spectrum disorders CI: confidence interval DSM: Diagnostic and Statistical Manual of Mental Disorders GPRD: General Practice Research Database HMO: health maintenance organisation HR: hazards ratio ICD: International Classification of Diseases ICD‐9‐CM: International Classification of Diseases, Ninth Revision, Clinical Modification incidence: cases/PT KPSC: Kaiser PermanteSsouth California MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PDD: pervasive developmental disorders PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) SD: standard deviation YPDC: Yokohama Psycho‐Developmental Clinic
Definitions:
Childhood autism: children with symptoms before the age of 3 years that meet the necessary criteria under each section of the diagnostic triad for autism: communication difficulties, problems with social interaction, and behaviour problems such as stereotyped repetitions.
Atypical autism cases: with many of the features of childhood autism but not quite meeting the required criteria for that diagnosis, or with atypical features such as onset of symptoms after age 3 years (also known as pervasive developmental disorder not otherwise specified).
Developmental regression: a documented deterioration in any aspect of development or reported loss of skills, however transient (International Classification of Diseases, 10th revision (ICD‐10) and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV)).
17. Safety: inflammatory bowel disease.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Davis 2001 Case‐control |
Vaccine Safety Datalink (versusD) cases were patients born between 1958 and 1989. Case IBD n = 142 (n = 75 Crohn's disease and n = 67 ulcerative colitis) Controls n = 432 matched for sex, HMO, and birth year |
Inflammatory bowel diseases Review of medical records contained in the Vaccine Safety Datalink database of 4 HMOs and identified by using ICD‐9 codes specific for Crohn's disease, ulcerative colitis and idiopathic proctocolitis (555 and 556). Outpatient, emergency department, urgent care clinic visits were available for 3 out of the 4 HMOs and were also taken into account. After abstraction of medical records, IBD cases were classified as: Definite IBD: as individuals diagnosed with IBD by a gastroenterologist at 1 of the HMOs who had at least 1 sign or symptom compatible with IBD (such as bloody stool and/or bloody diarrhoea or severe and/or recurrent abdominal pain) recorded and a diagnostic test result (such as biopsy with pathology specimen, colonoscopy, or sigmoidoscopy) consistent with IBD. Probable IBD: the diagnosis of IBD was made by either an HMO non‐gastroenterologist physician or a gastroenterologist outside the HMO; there was at least 1 sign or symptom compatible with IBD; and there was a diagnostic test result consistent with IBD. IBD cases (suspected or questionable) that did not correspond to these criteria were excluded from analysis. IBD (definite and probable) were further classified as Crohn's disease and ulcerative colitis cases. |
MMR vaccine
not specified MCV vaccine not specified MMR administered at any time before index date |
In this population‐based study of IBD at 4 large HMOs, the authors found no evidence that vaccination with MMR or other MCV, or that the age of vaccination early in life, was associated with an increased risk for development of IBD. In addition, the authors did not find evidence that MMR or other MCV acutely triggers the onset of either ulcerative colitis/proctitis or Crohn's disease. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls Crohn's disease (n = 75) (a) all age and vaccine type (a1) MMR < 12 months (a2) MMR 12 to 18 months (a3) MMR > 18 months Ulcerative colitis (n = 67) (b) all age and vaccine type (b1) MMR < 12 months (b2) MMR 12 to 18 months (b3) MMR > 18 months All IBD (n = 142) (c) all age and vaccine type 132/142 versus 409/432 (c1) MMR < 12 months 6/16 versus 25/48 (c2) MMR 12 to 18 months 84/94 versus 223/246 (c3) MMR > 18 months 4/14 versus 52/75 |
OR (95% CI)(*) Crohn's disease (a) 0.40 (0.08 to 2.00) (a1) 0.38 (0.05 to 2.86) (a2) 0.54 (0.10 to 3.07) (a3) 0.18 (0.03 to 1.21) Ulcerative colitis (b) 0.80 (0.18 to 3.56) (b1) 0.96 (0.12 to 7.57) (b2) 1.14 (0.23 to 5.59) (b3) 0 (0 to 0) All IBD (c) 0.59 (0.21 to 1.69) (c1) 0.61 (0.15 to 2.45) (c2) 0.86 (0.28 to 2.59) (c3) 0.16 (0.04 to 0.68) (*)Conditional logistic regression matched on HMO, sex, birth year adjusted for race. |
|
bb‐Baron 2005 Case‐control |
Cases: patients from the registry of inflammatory bowel diseases January 1988 to December 1997 aged less than 17 years old. Cases n = 222 Crohn's disease Cases n = 60 ulcerative colitis Controls were randomly selected from telephone number lists and matched 1:1 to each case by age (2 years), sex, and living area. |
Crohn's disease; ulcerative colitis Interviewer practitioners collected data on all patients diagnosed between 1 January 1988 and 31 December 1997 from all gastroenterologists (including paediatric gastroenterologists) in the entire area. Only patients who had been residents in the defined study areas at the time of diagnosis of their disease were included. A final diagnosis of CD or UC was made by 2 expert gastroenterologists and recorded as definite, probable, or possible, following criteria previously published. For the purpose of this study, only patients with definite or probable CD or UC were considered. |
MMR vaccine not described | MMR vaccination was negatively associated with a risk of CD. | (a) Crohn's disease (b) ulcerative colitis |
OR (95% CI)(*) (a) 0.5 (0.35 to 0.9) (b) no data available |
|
bb‐Shaw 2015 Case‐control |
Cases n = 117 with IBD diagnosis, born after 1989 and diagnosed before 31 March 2008. Controls n = 834 matched to cases on the basis of age, sex, and region of residence at time of diagnosis. All with an average age of 11 years. |
Inflammatory bowel diseases The administrative data case definition used to identify patients with IBD was validated with the establishment of the population‐based University of Manitoba IBD Epidemiology Database (UMIBDED) in 1995; the UMIBDED contains extracted administrative data of IBD cases and their controls (at a 1:10 ratio) for those individuals with health coverage between 1 April 1984 and 31 March 2008. Residents of Manitoba who resided in the province for at least 2 years were identified as having IBD if they had at least 5 physician visits or hospitalisations with ICD‐9‐CM codes 555.xx (Crohn’s disease) or 556.xx (UC) recorded as a diagnosis at any time. Since 2004, ICD‐10‐CA codes were used for all inpatient contacts and for IBD included K50.xx and K51.xx. |
MMR vaccine not described | No significant association between completed measles‐containing vaccination in the first 2 years of life and paediatric IBD could be demonstrated in this population‐based study. | (a) IBD |
OR (95% CI)(*) (a) 1.54 (0.54 to 4.36) (*)Conditional logistic regression models were fitted to the data, with models adjusted for physician visits in the first 2 years of life and area‐level socioeconomic status at case date. |
|
bb‐Vcev 2015 Case‐control |
Cases inflammatory bowel diseases n = 150 Cases ulcerative colitis n = 119 Cases Crohn's disease n = 31 Controls n = 150 not having a diagnosis of IBD, age and sex matched, were used as the control group. |
Inflammatory bowel diseases Patients diagnosed with IBD (UC or CD), identified according to the hospital’s patient records. Of a total of 150 patients in the sample, 119 patients were diagnosed with UC and 31 were diagnosed with CD. They were identified according to the hospital’s patient records. Documentation of the regional hospitals in Vukovar and Vinkovci was used for this purpose. Hospitals in the near surroundings such as Clinical Hospital Centre Osijek and General Hospital Slavonski Brod were also contacted, as some patients were directly referred to these hospitals by their primary care physicians without prior registration in the resident hospitals. |
MMR vaccine not described | The study found an association between exposure to MMR vaccine in the early childhood and later development of CD |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls (a) IBD 117/150 versus 101/150 (b) UC 89/119 versus 101/150 (c) CD 28/31 versus 101/150 |
OR (95% CI) (a) 1.72 (1.03 to 2.88) (b) 1.44 (0.84 to 2.46) (c) 4.53 (1.31 to 15.63) |
|
gb‐Seagroatt 2005 Case‐only ecological method |
Crohn's Disease emergency admission cases (n = 4463) observed between April 1991 and March 2003 in England population aged below 19 years (about 11.6 million) | Crohn's disease emergency admissions |
MMR vaccine not reported (a) Reference period: 1988 to 1989 (7% children completing a primary course) (b) Risk period: 1990 (68% children completing a primary course) (c) Risk period: 1991 to 2003 (84% children completing a primary course) |
The study found no increase in Crohn’s disease associated with the introduction of the MMR vaccination programme, providing strong evidence against the hypothesis that MMR vaccine increases the risk of Crohn’s disease. | ‐ |
RR (95% CI)(*) 0.95 (0.84 to 1.08) (*) Poisson regression. The estimated rate ratio (populations with a vaccination rate of 84% compared with those with a vaccination rate of 7%). |
|
gb‐Taylor 2002 Case‐only ecological method linked to db‐Taylor 1999 |
Children with childhood (core autism n = 278) and atypical autism (n = 195) born between 1979 and 1998 from computerised health registers of children with disabilities in the community and from special school and child psychiatry records, using the same methods and classifications as in the authors' earlier study. | Recorded bowel problems lasting at least 3 months, age of reported regression of the child's development where it was a feature, and relation of these to MMR vaccination. | MMR vaccine not reported | The study provides no support for an MMR‐associated “new variant” form of autism with developmental regression and bowel problems, and further evidence against involvement of MMR vaccine in the initiation of autism. |
Bowel problem all cases n = 78 unvaccinated cases n = 9 vaccinated before parental concern n = 50 vaccinated after parental concern n = 19 |
OR (95% CI)(*) 0.98 (0.89 to 1.07) (*) logistic regression adjusted for sex, year of birth, district, age at parental concern, and type of autism. |
CD: Crohn's disease CI: confidence interval DSM: Diagnostic and Statistical Manual of Mental Disorders HMO: health maintenance organisation IBD: inflammatory bowel diseases ICD: International Classification of Diseases ICD‐10‐CA: ICD‐9‐CM: incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence; incidence rate ratio) RR: risk ratio (relative risk) UC: ulcerative colitis
Definitions:
Childhood autism: children with symptoms before the age of 3 years that meet the necessary criteria under each section of the diagnostic triad for autism: communication difficulties, problems with social interaction, and behaviour problems such as stereotyped repetitions.
Atypical autism: with many of the features of childhood autism but not quite meeting the required criteria for that diagnosis, or with atypical features such as onset of symptoms after age 3 years (also known as pervasive developmental disorder not otherwise specified).
Developmental regression: a documented deterioration in any aspect of development or reported loss of skills, however transient (International Classification of Diseases, 10th revision (ICD‐10) and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV)).
18. Safety: cognitive delay, developmental delay.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
cb‐Mrozek‐Budzyn 2013 Cohort study |
(Birth‐cohort) The enrolment (3 November 2000 to 22 August 2003) included only non‐smoking women, aged 18 to 35 years, with singleton pregnancy without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension and residing in Krakow for at least 1 year prior to pregnancy. The infants were followed up to 8th year of life. n = 369 children (n = 307 vaccinated MMR; n = 32 vaccinated monovalent; n = 30 unvaccinated) |
Fagan Test of Infant Intelligence (FTII) at 6th month of life. Bayley Scales of Infant Development, second edition (BSID‐II), was administered in the 12th, 24th, and 36th months of life. The Mental Scale of that test includes items that assess memory, habituation, problem solving, early number concepts, generalisation, classification, vocalisation, language, and social skills. Test scores are adjusted to child’s age to obtain the Mental Development Index (MDI). Test results are in 1 of 4 categories (range: from 50 to 150): (1) accelerated performance (score > 115); (2) within normal limits (score 85 to 114); (3) mildly delayed performance (score 70 to 84), and (4) significantly delayed (score < 69). Thetest of Raven’s Colored Progressive Matrices (Raven) was administered twice, in 5th and 8th year of life. The Wechsler Intelligence Scale for Children (WISC‐R) was administered in 6th and 7th year of life, and generated verbal, non‐verbal, and total IQ for evaluated children. Category with IQ < 100 was considered as the poorer outcomes. The outcomes range is from 40 to 160. |
MMR vaccine not described |
MMR and cognitive tests outcomes: No significant differences of cognitive and intelligence tests results were observed between children vaccinated with MMR and unvaccinated in univariable analysis. Their outcomes were on similar level. Conclusion: The results suggest that there is no relationship between MMR exposure and children’s cognitive development. Furthermore, the safety of triple MMR is the same as the single measles vaccine with respect to cognitive development. |
(a1) MDI‐BSID II 24th month (a2) MDI‐BSID II 36th month (b1) Raven (centiles) 5th year (c1) WISC‐R Verbal IQ 6th year |
OR (95% CI)(*) (a1) 1.35 (0.15 to 12.0) (a2) 0.37 (0.03 to 4.02) (b1) 1.22 (0.23 to 6.55) (c1) 1.23 (0.09 to 17.03) (*) adjusted for standardised to child’s gender, maternal education, maternal IQ, maternal economical status, birth order (further child versus first one), and exposure to environmental tobacco smoke during pregnancy (yes versus no). |
CI: confidence interval incidence: cases/PT IQ: intelligence quotient MDI‐BSID II: Mental Development Index of Bayley Scales of Infant Development, second edition MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time
19. Safety: idiopathic thrombocytopenic purpura.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Black 2003 Nested case‐control study |
Cases: n = 23 children with outcome of interest at 12 to 23 months, between 1988 and 1999, GPRD members. Controls: n = 116 participants matching for index date (age), sex, practice. Nested case–control analysis to evaluate whether there was any relationship between recent MMR vaccination and the risk of ITP. Because the data were sparse, the authors grouped case–control sets by 3‐month age bands (13 to 15 months, 16 to 18 months, and so on). In addition, they included boys and girls in sets together because childhood ITP is reported to occur with equal frequency amongst both sexes, and because preliminary analysis of their data showed no evidence for a predominance of cases amongst either sex. The risk ratio of ITP during the specified time periods after MMR vaccination was estimated as the odds ratio using conditional logistic regression. |
Idiopathic thrombocytopenic purpura GPRD electronic records with first‐time diagnosis of thrombocytopenia (ICD‐9 code 287.1) |
MMR vaccine: not reported. Data about MMR vaccination were presumably obtained from GPRD records (type and composition not reported). The authors referred to ITP cases that occurred within 6 weeks after an MMR vaccine as "possible vaccine‐related"; this is a plausible period of risk related to a primary immune response. They also evaluated the risk of ITP during a longer period after MMR vaccination (7 to 26 weeks). Risk time following MMR immunisation (a) 0 to 6 weeks (b) 7 to 26 weeks (c) 0 to 26 weeks Reference time unexposed MMR or > 26 weeks after MMR |
Authors' conclusion: "Although ITP is one of the most frequently diagnosed haematological disorders amongst young children, it is an uncommon condition. The risk of ITP occurring within the 6 weeks after vaccination with MMR is significantly increased. However, the attributable risk of ITP within 6 weeks after MMR vaccination remains low at 1 in 25,000" (95% CI 21,300 to 89,400) "vaccinated children. Complications or long‐term consequences of ITP in this age group are rare. For the majority of children less than 6 years of age, the illness is self‐limiting." |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls Data reported in the study: (a) 8/17 versus 19/84 (b) 6/15 versus 32/97 (c) 14/23 versus 51/116 |
OR (95% CI) unadjusted estimates (a) 3.04 (1.03 to 8.96) (b) 1.35 (0.44 to 4.14) (c) 1.98 (0.79 to 4.95) computed from the data reported in the study. ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ adjusted estimates(*) (a) 6.3 (1.3 to 30.1) (b) 1.5 (0.4 to 4.8) (*) logistic regresson |
|
bb‐Bertuola 2010 Case‐control study |
Cases: n = 387 children aged 1 month to 18 years, hospitalised at emergency department with outcome of interest between November 1999 and September 2007, with outcome of interest. Controls: n = 1924 children of same age interval hospitalised at emergency department for acute neurological disorders or endoscopically confirmed gastroduodenal lesions |
Acute immune thrombocytopenia Platelets count < 100,000/μL at admission. Participants with following conditions were excluded: cancer, immunodeficiency, chronic renal and hepatic failure, so as acute events related to a reactivation of an underlying chronic disease or a congenital anomaly Hospitalisation (emergency department) records review |
Not reported. Exposure to the vaccine (and other drugs) was assessed during hospital admission by means of interview with parents. 0 to 6 weeks following MMR immunisation |
Authors' conclusion: the study confirms an association between MMR vaccination and ITP. As the risk of ITP after vaccination is smaller than after natural infection with these viruses, it is clear that the benefit of vaccination programmes greatly exceed the significance of this possible adverse effect. Although thrombocytopenia is initially severe, the subsequent course is generally benign and short‐lasting. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls 14/387 versus 27/1924 |
OR (95% CI)(*) 2.4 (1.2 to 4.7) (*) adjusted estimates by logistic regression |
|
db‐France 2008 Self‐controlled case series |
Children (n = 63) aged 12 to 23 months with ITP identified from versusD database for the years 1991 to 2000, who had been vaccinated with MMR whilst actively enrolled in their respective MCOs. For each child, follow‐up time was limited to the 365 days before and after MMR vaccination. Vaccinated children with ITP that occurred outside this follow‐up window were excluded. |
Immune thrombocytopenia purpura Participants with 2 platelet counts ≤ 50,000/μL within 6‐week period or with 1 platelets count ≤ 50,000/μL associated with ICD‐9 diagnosis codes 287.0 to 287.9 within 6 weeks, with exclusion of: cases of thrombocytopenia from a known condition (neonatal thrombocytopenia, aplastic anaemia, defibrination syndrome, acquired haemolytic anaemia, chronic liver disease, malignant neoplasm), thrombocytopenia diagnosed within the 30th day of life. By subsequent patient chart reviews, participants who did not have not have ITP, who had drug exposure, with acute illness, or with serendipitous finding during routine care were further excluded. |
MMR vaccine: not reported MMR vaccination date assessed by means of separate audit of patient charts. Exposed period: 42 days after MMR vaccination Unexposed period: defined as the time periods before and after the exposed period. Period of 6 weeks immediately preceding MMR vaccination was excluded from analysis (because this represents a period when a child is most likely to be healthy (the healthy‐vaccinee) and may underestimate the background incidence of ITP) |
Authors' conclusion: since its introduction in the 1960s, the MMR vaccine has reduced the incidence of wild‐type measles by nearly 100% in the USA. Although this vaccine is associated with an increased incidence of ITP, the attributable risk is low (1 case per 40,000 doses of MMR), and the disease associated with MMR vaccination is mild and resolves, on average, within 7 days. Our results, therefore, do not suggest a need to alter current immunisation policies. |
Age groups (a) 12 to 23 months (b) 12 to 15 months |
rr (95% CI)(*) Self‐controlled case series (a) 5.38 (2.72 to 10.62) (b) 7.06 (1.95 to 25.88) (*) conditional Poisson regression controlled by age in three 4‐month age groupings (12 to 15, 16 to 19, 20 to 23 months) and excluding fixed covariate from the model (gender, MCO, MMR dose number) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Person‐time cohort(**) (a) 3.94 (2.01 to 7.69) (b) 7.10 (2.03 to 25.03) (**) Poisson regression model controlled for age, MMR dose number, MCO site, and gender |
|
db‐Farrington 1995 Self‐controlled case series |
Children aged 12 to 24 months discharged from hospital in 5 districts in England (Ashford, Leicester, Nottingham, Preston, and Chorley & Ribble) for varying periods between October 1988, and February 1993. Readmissions within 72 h with the same diagnosis were counted as 1 episode. n = 952 children |
Idiopatric thrombocytopenic purpura (ICD 287.3) children aged between 366 and 730 days |
MMR vaccine: Urabe mumps strain Jeryl Lynn mumps strain Rubella strain not specified Exposure risk period: (a1) 6 to 11 days (1 to 2 weeks after vaccination) (a2) 15 to 35 days (3 to 5 weeks after vaccination) Control period: (b) for each vaccine was defined as the time not included in a risk period. The analyses were adjusted for age and were grouped in 6 equal intervals of about 2 months. |
Authors' conclusion: we demonstrated a causal association between ITP and MMR vaccination, with an absolute risk of 1 in 24,000 doses and an attributable risk of 1 in 29,000 doses. |
Any strain (a1) 0 cases (a2) 4 cases |
rr (95% CI)(*) (a2) 6.44 (1.94 to 21.4) (*) Poisson regression |
|
db‐Andrews 2012 Self‐controlled case series |
Multicountry
collaboration (England and Denmark) study. The chosen study population was children aged 12 to 23 months (365 to 732 days). |
Thrombocytopenic purpura The case definition for TP was based only on the presence of a relevant ICD‐10 code (D69.3) or ICD‐8 code (287.10) in 1 of the diagnostic discharge fields. First episodes were defined as the earliest record found for an individual, further episodes were initially required to be at least 14 days since a previous episode (to prevent double counting of episodes). In England cases (based on ICD‐10) occurring between 1 April 1996 and 31 March 2007 were linked using NHS number or gender/date of birth/postcode to immunisation records. In Denmark the Central Person Registry (CPR) was used to construct a nationwide cohort consisting of all Danish children born in the period 1 January 1990 to 31 December 2007 (∼1.2 million children). |
MMR vaccine: not described Risk periods: (post‐MMR) (a) 0 to 13 days (b) 14 to 27 days (c) 28 to 42 days (d) 0 to 42 days Reference period pre‐vaccination (e) −7 to −1 days (to allow for a vaccination being delayed if the child was ill) |
Authors' conclusion: this study gave consistent estimates of the relative incidence of TP following MMR vaccination in 1‐year‐olds. The 95% CI for the attributable risk of TP can be calculated based on the 95% CI for the relative incidence and gives an interval of 1 in 74,000 to 1 in 40,000 doses. |
(a) 12 cases (b) 26 cases (c) 17 cases (d) 55 cases |
rr (95% CI)(*) (a) 1.30 (0.71 to 2.38) (b) 2.87 (1.85 to 4.46) (c) 1.81 (1.07 to 3.05) (d) 1.98 (1.41 to 2.78) (*) adjusting for age, period, country, and country‐age interaction |
|
db‐O'Leary 2012 Self‐controlled case series |
Children < 18 years old (confirmed ITP cases) who had been vaccinated while actively enrolled in their respective health plans. This investigation was conducted in 5 healthcare systems (Kaiser Permanente: Colorado, Hawaii, Georgia, Northern California, and Harvard Vanguard Medical Associates) by using data from the years 2000 to 2009. |
Thrombocytopenic purpura Case was defined as a child aged 6 weeks to 18 years with a platelet count of ≤ 50,000/μL, with normal red and white blood cell indices, and the presence of clinical signs and symptoms of ITP, such as petechiae, significant bruising, or spontaneous bleeding. |
MMR, MMRV vaccine: not described Follow‐up time: 365 days before and after vaccination. Exposed period: 1 to 42 days after vaccination for all vaccines. Unexposed period: defined as the time before and after the exposed period within 365 days of follow‐up before or after vaccination. Day 0 (the day of vaccination) was excluded, because any cases occurring at this time were most likely coincidental. |
Authors' conclusion: none of the routine childhood vaccines given in the first year of life was significantly associated with an increased risk of ITP. For vaccines routinely administered at 12 to 19 months of age, there was a significant association of ITP with MMR. There was no increased risk of ITP (calculated when not given simultaneously with MMR or MMRV). There were 1.9 cases of ITP per 100,000 doses of MMR. |
Exposed cases versus unexposed cases (a) 12 to 19 months (a1) MMR: 6 versus 5 (a2) MMRV: 4 versus 6 (b) 4 to 6 years (b1) MMR: 2 versus 7 (b2) MMRV: 0 versus 5 (c) 11 to 17 years (c1) MMR: 0 versus 1 |
rr (95% CI) (a1) 5.48 (1.61 to 18.64) (a2) 2.87 (0.78 to 10.56) (b1) 3.06 (0.42 to 22.30) (b2) not estimable (c1) not estimable |
|
db‐Perez‐Vilar 2018 Self‐controlled case series |
For this study, WHO selected 26 sentinel sites (49 hospitals) distributed in 16 countries of the 6 WHO regions. The study population included children aged 9 to 23 months admitted to a network‐participating hospital during January 2010 to March 2014, with a discharge diagnosis of either aseptic menigitis or immune thrombocytopenic purpura. |
Immune thrombocytopenia ICD‐9 codes in first discharge diagnosis position: 287.30 to 287.39 Primary thrombocytopenia 287.41 to 287.49 Secondary thrombocytopenia 287.5 Thrombocytopenia, unspecified ICD‐10 codes in first discharge diagnosis position: D69.3, D69.4 (D69.41 to D69.43) Primary thrombocytopenia D69.5 (D69.51, D69.59) Secondary thrombocytopenia D69.6 Thrombocytopenia, unspecified |
Vaccine (measle strain) (mumps strain) Priorix, GSK (Schwarz) (RIT 4385a) Priorix Tetra, GSK (Schwarz) (RIT 4385a) MMR Shanghai Institute (Shanghai‐191) (S79) Measles, Lanzhou Institute (Shanghai‐191) (–) Measles‐Rubella, Beijing Tiantan (Shanghai‐191) (–) M‐M‐R‐II, MSD (Enders’ Edmonston) (Jeryl Lynn (Level B)) MMR, Razi Vaccine and Serum Research (AIK‐C) (Hoshino) M‐M‐RVAXPRO, Sanofi Pasteur‐MSD (Enders’ Edmonston) (Jeryl Lynn (Level B)) Trimovax, Sanofi Pasteur (Schwarz) (Urabe AM9) Measles, Serum Institute of India Pvt. (Edmonston‐Zagreb) (–) Measles‐Rubella, Serum Institute of India Pvt. (Edmonston‐Zagreb) (–) MMR, Serum Institute of India (Edmonston‐Zagreb) (Leningrad‐Zagreb) Tresivac, Serum Institute of India (Edmonston‐Zagreb) (Leningrad‐Zagreb) Rouvax, Sanofi Pasteur (Schwarz) (–) Risk period 8 to 35 days Washout periods 1 to 7 days 36 to 42 days Control period 43 to 84 days |
The elevated risk of ITP following measles‐containing vaccination is consistent with the literature (db‐O'Leary 2012: db‐France 2008). Our strain‐specific unadjusted analysis showed a significantly elevated ITP risk for measles vaccines containing the Schwarz, Edmonston‐Zagreb, and Enders’ Edmonston strains. No risk of ITP was identified in Iran, which reported the concurrent distribution of 3 vaccine products including the AIK‐C, Edmonston‐Zagreb, and Schwarz strains, without distinguishing between them. | In 16 countries n = 183 ITP cases ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (risk versus control) period (a) overall (36 versus 12) (b) overall (excluding Iran) (36 versus 8) (c) AIK‐C/Edmonston‐Zagreb/Schwarz (2 versus 5) (d) Edmonston‐Zagreb (7 versus 1) (e) Enders' Edmonston (11 versus 3) (f) Schwarz (14 versus 1) (g) Shanghai‐191 (0 versus 1) |
rr (95% CI) adjusted (a) 5.6 (2.7 to 11.9) (b) 9.1 (3.7 to 22.3) (c) 0.54 (0.08 to 3.6) (d) 8.4 (0.7 to 100.3) (e) 28.7 (1.9 to 443.5) rr (95% CI) unadjusted (f) 20.7 (2.7 to 157.6) (g) not estimable |
|
eb‐Lafaurie 2018 Case cross‐over |
Population‐based study in France including all children newly diagnosed for primary ITP between July 2009 and June 2015. n = 2549 |
Immune thrombocytopenia |
MMR vaccine: not described Exposed period 6‐week interval immediately preceding the event (frequency of exposure to vaccines) Control period (1) 6 weeks, 6 months before (2) 6 weeks, 3 months before the case period |
Conclusion: in this nationwide study, no significant risk was observed for vaccines against DTP, pneumococcus, meningococcus, and HBV. The increased risk of MMR‐induced ITP is shown in children (previously demonstrated as lower than after the natural infection with measles). Vaccine‐induced ITP remains an exceptional adverse drug reaction, including for MMR vaccines. The numbers of attributable cases per million MMR doses dispensed were 9.8. |
n = 492 patients included in analysis |
OR (95% CI) 1.62 (1.21 to 2.16) |
|
gb‐Jonville‐Bera 1996 Case‐only ecological study |
Pharmacovigilance reports: case observed after vaccine administration between 1984 and 30 June 1992 (n = 60). Estimated number of administered vaccine doses was 9,205,483. |
Thrombocytopenic purpura Acute haemorrhagic syndrome associated with platelet count of < 100,000/mm³, all cases within 45 days of vaccination, over 8‐year period |
MMR vaccine: (a) ROR, Trimovax (measles Schwarz strain, mumps Urabe AM9 strain, rubella Wistar RA 27/3 M strain) Other measles‐containing vaccines: (b) Rouvax (measles Schwarz strain) (c) Rudi‐Rouvax (measles Schwarz strain, rubella Wistar RA 27/3 M strain) Other vaccine: (d) Rudivax (rubella Wistar RA 27/3 M strain) + DTbis (e) Rudivax (rubella Wistar RA 27/3 M strain, diptheria, tetanus) (e) Imovax Oreillons (mumps Urabe AM9 strain) 2 to 45 days following immunisation |
Authors' conclusion: according to the clinical course and biologic findings, vaccine‐associated TP appears to be similar to that occurring after natural measles or rubella infections and is not distinguishable from acute childhood idiopathic thrombocytopenic purpura not associated with vaccination. Such observation, combined with a clear temporal relationship between MMR vaccination and occurrence of TP, make a causal relationship highly plausible. Nevertheless, the incidence of these events remains relatively low with a favourable immediate outcome. |
Case/doses (a) 42/4,396,645 ‐‐‐ (b) 2/860,938 (c) 12/1,480,058 ‐‐‐ (d) 4/2,295,307 (e) 0/172,535 |
Incidence x 100,000 doses (95% CI)(*) (a) 0.96 (0.71 to 1.29) ‐‐‐ (b) 0.23 (0.06 to 0.85) (c) 0.81 (0.46 to 1.42) ‐‐‐ (d) 0.17 (0.07 to 0.45) (e) 0.00 (0.00 to 2.23) (*) confidence intervals were recomputed by Wilson 1927 method. |
CI: confidence interval DTP: diphtheria, tetanus, and pertussis GPRD: General Practice Research Database HMO: health maintenance organisation HPV: human papillomavirus ICD: International Classification of Diseases ITP: idiopathic thrombocytopenic purpura MCOs: Managed Care Organizations MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) incidence: cases/PT RR: risk ratio (relative risk) TP: thrombocytopenic purpura WHO: World Health Organization
20. Safety: Henoch‐Schönlein purpura.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Da Dalt 2016 Case‐control |
Cases (n = 288) children (aged > 1 month and ≤ 18 years) hospitalised with a diagnosis of Henoch‐Schönlein purpura through the emergency departments (11 Italian paediatric hospitals/wards spread throughout the country (Treviso, Padua, Naples, Genoa, Turin, Florence, Perugia, Palermo, Messina, and Rome, with 2 centres)). Control (n = 617) children hospitalised for gastroduodenal lesions were considered as appropriate controls, since they represent an acute condition admitted through the emergency departments in the same clinical centres in which cases were identified. |
Henoch‐Schönlein purpura All children hospitalised with a diagnosis of HSP at admission were included as cases. Discharge diagnosis was retrieved from clinical records and validated by clinicians, according to EULAR/PRINTO/PRES criteria for classification of HSP. Validation was conducted retrieving data from individual patient clinical records, blinded with respect to drug and vaccine exposure. Only validated cases were analysed. |
Vaccines MMR not described |
Conclusions: the association between MMR vaccination and HSP confirms previous published findings and adds a risk estimate. Further studies are needed to increase our understanding of the role of drugs and vaccines in the aetiology of HSP, a disease with important effects on health of children for its potential, though rare, chronic outcomes. This article confirms that HSP is a rare condition (288 children hospitalised in 14 years). Furthermore, the number of vaccinated cases was only 8, suggesting a very low absolute risk of the condition in children vaccinated with MMR vaccine. The benefit/risk profile of MMR vaccine is thus not affected by our results, being that MMR vaccination is an effective and safe tool against serious diseases in childhood. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls 8/228 versus 6/617 |
OR (95% CI)(*) 3.4 (1.2 to 10.0) (*) Adjusted by age |
CI: confidence interval HSP: Henoch‐Schönlein purpura incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time
21. Safety: type 1 diabetes.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
cb‐Hviid 2004 Cohort study |
A cohort of children born from 1 January 1990 to 31 December 2000 from the Danish Civil Registration System (n = 739,694) |
Type 1 diabetes: information on the diagnosis of type 1 diabetes from 1 January 1990 through 31 December 2000 was obtained from the Danish National Hospital Register. From 1990 through 1993, Denmark used a modified version of the ICD‐8. From 1994 through 2001, the ICD‐10 was used. The authors used codes 249 and E10 (the code 249 does not exist in the standard World Health Organization version of the ICD‐8) to identify all cases of type 1 diabetes. Beginning in 1995, visits to the emergency room and outpatient visits were included in the National Hospital Register. (n = 681 cases of type 1 diabetes) |
MMR vaccine: measles Moraten strain, mumps Jeryl Lynn strain, rubella Wistar RA 27/3 strain. Schedule 15 months and 12 years of age; composition: (a1) 1 dose (a2) unknown (b) unvaccinated |
Authors' conclusion: these results do not support a causal relation between childhood vaccination and type 1 diabetes. |
All children (a1) 499/293,428 (a2) 58/412,830 (b) 124/1,373,401 Children with at least 1 sibling with type 1 diabetes (a1) 20/2795 (a2) 0/361 (b) 6/1053 |
rr (95% CI)(*) All children (a1) 1.14 (0.90 to 1.45) (a2) 1.04 (0.71 to 1.52) Children with at least 1 sibling with type 1 diabetes (a1) 0.86 (0.34 to 2.14) (a2) ‐ (‐ to ‐) (*) Poisson log linear regression |
|
cb‐Beyerlein 2017 Cohort study |
Cohort of children recruited: between 1989 and 2000, a total of 1650 offspring of patients with T1D were recruited for the BABYDIAB study and were followed for 23,856 patient years. Between 2000 and 2006, 791 additional offspring or siblings of patients with T1D were screened in the context of the BABYDIET study and were followed by using the BABYDIAB protocol for 6358 patient years. |
Islet autoimmunity: type 1 diabetes: (T1D) is one of the most common chronic diseases in childhood. The disease is preceded by a preclinical period of islet autoimmunity, which most commonly develops in early infancy. Factors that induce a strong immune response in early life might thus be relevant for the development of T1D‐associated islet autoimmunity. Islet autoantibodies were measured in venous blood samples from scheduled visits. Children in the BABYDIAB study had scheduled visits at birth, at age 9 months, and at 2, 5, 8, 11, 14, 17, and 20 years of age, whereas children in the BABYDIET study had 3‐monthly visits from birth until the age of 3 years, and yearly until the age of 12 years. Measurement of islet autoantibodies in these studies has been described elsewhere. Islet autoimmunity was defined as the development of persistent autoantibodies to 1 or more of the antigens insulin, GAD65, IA‐2 or Zn‐T8, with sample values above the 99th percentile of published population control children classified as positive. In case of single positive antibodies against insulin or GAD65, affinity and epitope reactivity was determined and children with low‐affinity antibodies (< 109 L/mol) were not classified as islet autoantibody positive, as these isolated antibody signals are not T1D specific and are not associated with increased T1D risk. Persistence was defined as positive in at least 2 consecutive samples. Islet autoantibody assays were evaluated according to the Diabetes Autoantibody Standardization Program. |
MMR vaccine not described Age (a) 0 to 24 months |
Conclusions: the authors found no evidence that early vaccinations increase the risk of T1D‐associated islet autoimmunity development. | Total n = 1918 n = 1779 children without confirmed islet autoimmunity n = 139 confirmed islet autoimmunity |
HR (95% CI)(*) (a) 1.08 (0.96 to 1.21) (*) Cox regression |
CI: confidence interval HMO: health maintenance organisation HR:hazards ratio ICD: International Classification of Diseases incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) T1D: type 1 diabetes
22. Safety: asthma.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
cb‐DeStefano 2002 Cohort study |
Children (0 to 6 years) enrolled in VSD project (4 HMOs) between 1991 and 1997 (n = 167,240) |
Asthma: a child had to meet 1 of the following criteria: (1) at least 1 diagnosis of asthma ICD‐9 Code 493 and at least 1 prescription for an asthma medication; the first diagnosis and first prescription had to be within a 2‐year period. Asthma medications included oral or inhaled beta‐agonists, theophyllin, oral or inhaled corticosteroids, cromolyn sodium, adrenergic drugs not elsewhere specified, and unclassified asthma medications; (2) at least 1 prescription for an inhaled beta‐agonist and at least 1 prescription for cromolyn within a 2 year period; (3) at least 5 prescriptions for asthma medications during a 2‐year period. (Total asthma cases n = 18,407) |
MMR vaccine: not reported Exposure to MMR vaccine (and other vaccines). Vaccinations were ascertained through computerised immunisation tracking systems, and onset of asthma was identified through computerised data on medical care encounters and medication dispensing. |
Conclusion: there is no association between MMR vaccine and the risk of asthma. | Not reported |
rr (95% CI)(*) 0.97 (0.91 to 1.04) (*) adjusted rr estimated from a proportional hazard regression model stratified by HMO and month and year of birth, gender, low birthweight status |
|
cb‐McKeever 2004 Cohort study |
Children (n = 16,470) aged from 20 months to 11 years, accounting for 69,602 person‐years n = 29,238 n = 20,845 vaccinated n = 8393 unvaccinated |
Asthma: diagnoses of asthma/wheeze and eczema from the Oxford Medical Information System (which was derived from the ICD‐8) and Read codes (hierarchical codes commonly used in GP practices in England) diagnoses of asthma n = 1753 n = 28 (amongst unvaccinated) |
MMR vaccine: not reported Vaccination status extracted from West Midlands General Practice Research Database. Data are presented stratified by consulting frequency in first 18 months (a1) 0 to 6 (a2) 7 to 10 (a3) 11 to 16 (a4) > 16 |
Conclusion: the study data suggest that currently recommended routine vaccinations are not a risk factor for asthma or eczema. In this observational study analysing computerised primary care records, the authors found an association between MMR and DPT vaccination and the incidence of asthma and eczema, but these associations appeared to be limited to the minority of children who rarely seek care from a GP. This limited association is more likely to be the result of bias than a biological effect. |
Cases vaccinated/PT‐years versus cases unvaccinated/PT‐years ‐‐‐‐‐‐‐‐‐‐‐‐All‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 1725/65,597 versus 28/4006 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ stratified by consulting frequency in first 18 months (a1) 165/12,462 versus 5/2843 (a2) 351/17,522 versus 7/425 (a3) 601/20,693 versus 8/452 (a4) 608 /14,920 versus 8/286 |
rr (95% CI)(*) (a) 2.2 (1.50 to 3.21) (a1) 7.18 (2.95 to 17.49) (a2) 0.95 (0.45 to 2.01) (a3) 1.36 (0.68 to 2.73) (a4) 1.21 (0.60 to 2.43) (*) Adjusted rr estimated from a proportional hazard regression model stratified by consulting frequency, parental smoking, parental allergic disease, maternal age, number of older siblings, use of antibiotics early in life, year of birth, and GP practice. |
|
cb‐Hviid 2008 Cohort study |
Danish birth cohorts 1991 to 2003 followed up between 1 January 1991 and 31 December 2003, or between 1 and 5 years of age |
Asthma hospitalisation: inpatient hospitalisation with asthma diagnosis (occurred between 1 January 1992 and 31 December 2004)
n = 871,234 children (vaccine coverage 85%) PT = 2,926,406 (person‐years) n = 26,880 hospitalisations amongst 17,885 children ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Anti‐asthma medication: prescription of the following cases of anti‐asthma medications have been considered:
n = 600,938 children (vaccine coverage 84%) PT = 1,858,199 (person‐years) n = 833,424 prescriptions anti‐asthma medication amongst 248,907 children |
MMR vaccine: Measles Moraten strain, Mumps Jeryl Lynn strain, Rubella Wistar RA 27/3 strain. Dates of MMR vaccination were obtained from the National Board of Health. |
Conclusion: these results are compatible not with an increased risk of asthma following MMR vaccination, but rather with the hypothesis that MMR vaccination is associated with a reduced risk of asthma‐like disease in young children. | (a) Asthma (b) Status asthmaticus (c) Anti‐asthma medication |
rr (95% CI)(*) (a) 0.75 (0.73 to 0.78) (b) 0.63 (0.49 to 0.82) (c) 0.92 (0.91 to 0.92) (*) Adjusted for age, calendar period, hospitalisations propensity in infancy, birthweight, place of birth, mother’s country of birth, infant vaccine compliance, birth order, maternal age at birth, and child’s sex. Log‐linear Poisson regression. |
|
cb‐Benke 2004 Cohort study |
Participants were aged between 22 and 44 years n = 309 | Participants were surveyed by a validated interviewer‐administered questionnaire covering: history of asthma; details of home and occupation environment; smoking history; medications; dietary information; and respiratory symptoms. The respiratory symptoms included wheezing or whistling in the chest, shortness of breath, chest tightness, and cough and phlegm during the previous 12 months. Atopy was assessed by skin prick testing to common aeroallergens. |
MMR vaccine not described Questionnaire included vaccination history questions, which were not included in the questionnaire used by the other study centres. Vaccination history included measles or MMR vaccinations; hepatitis B; Bacille Calmette‐Guérin (BCG); oral polio vaccine (OPV); and diphtheria, tetanus, and whooping cough (DTP). |
Conclusion: there was no significant association observed for participants diagnosed with asthma who had received measles or MMR vaccinations compared with those who did not receive measles or MMR vaccinations. | (a) Asthma (b) Atopy |
RR (95% CI) (a) 1.33 (0.98 to 1.80) (b) 1.07 (0.88 to 1.30) |
|
cb‐Timmermann 2015 Cohort study |
n = 640 children were followed from birth. Follow‐up examinations at ages 5, 7 and 13 years included a physical examination and a maternal questionnaire about the child’s health. |
Asthma (and dermatitis eczema) At child's age 5, parents were asked whether the child was suspected to suffer from asthma or had been diagnosed with asthma, hypersensitivity, or allergy. At ages 5, 7, and 13 years, the same paediatrician determined the presence of current wheezing by auscultation. At the same ages, the paediatrician also examined all children for dermatitis/eczema. At age 13, the findings from this examination were graded according to a score for atopic dermatitis (SCORAD). At age 7, a blood sample was drawn and total IgE and grass‐specific IgE were quantified. At age 13, parents were asked whether the child had ever suffered from asthma. In accordance with the International Study of Asthma and Allergies in Childhood (ISAAC), they were also asked to indicate whether the child had (i) suffered from wheezing in the past 12 months, (ii) suffered from sneezing, running, or blocked‐up nose except for when the child had a cold or was sick in the past 12 months and, if so, whether it had been accompanied by itching running/tearing eyes (current rhinoconjunctivitis symptoms), and (iii) whether the child had ever suffered from an itching rash that comes and goes for at least 6 months (eczema ever). At age 13, the children underwent a skin prick test with extracts of 5 common allergens (birch/grass pollen, dog/cat dander, and house dust mite (Dermatophagoides pteronyssinus)). |
MMR vaccine: not described The Faroe Islands follow the Danish vaccination schedule, in which MMR vaccination, at the time of this study, was administered at age 15 months and 12 years (Fig. 1). There were no specific contraindications. At the 5‐year examination, the child’s vaccination card was inspected and all vaccination dates were registered. At child's age 13, the mothers were asked whether the child had received the MMR vaccination scheduled at 12 years of age. |
Conclusion: the authors' findings support the notion that MMR vaccination may provide beneficial effects in preventing childhood allergy and asthma. |
Asthma (a) 5 years old (b) 13 years old |
OR (95% CI) (a) 0.33 (0.12 to 0.90)(*) (b) 0.22 (0.08 to 0.56)(*) (a) 0.32 (0.10 to 1.05)(*)(**) (b) 0.16 (0.05 to 0.53)(*)(**) RR (95% CI)(***) (a) 0.44 (0.18 to 0.93)(*) (b) 0.35 (0.14 to 0.71)(*) (*) Adjusted OR (logistic regression model) for birthweight and family history of chronic bronchitis/asthma. The analyses at age 13 years are additionally adjusted for whether the child had received the second MMR vaccine before the 13‐year examination. (**) Additional adjustment for sex, premature birth, maternal smoking during pregnancy, log (cord blood IgE), breastfeeding, number of older siblings, number of younger siblings, parental smoking in the home, day care, family history of eczema in children/allergic eczema/hay fever, family history of allergy, and age at the examination. (***) OR converted in RR (a) CER = 0.36 (b) CER = 0.47 |
ACT: Asthma Control Test CER: control event rate CI: confidence interval DPT: diphtheria, pertussis, and tetanus vaccine GP: general practice HMO: health maintenance organisation ICD: International Classification of Diseases IgE: Immunoglobulin E incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) VSD: Vaccine Safety Datalink
23. Safety: dermatitis or eczema.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
cb‐McKeever 2004 Cohort study |
Children (n = 14,353) aged from 20 months to 11 years, accounting for 59,520 person‐years |
Eczema: diagnoses of asthma/wheeze and eczema from the Oxford Medical Information System (which was derived from the ICD‐8) and Read codes (hierarchical codes commonly used in GP in England) diagnoses of eczema n = 1884 |
MMR vaccine: not reported Vaccination status extracted from West Midlands General Practice Research Database Data are presented stratified by consulting frequency in first 18 months (a1) 0 to 6 (a2) 7 to 10 (a3) 11 to 16 (a4) > 16 |
Conclusion: the study data suggest that currently recommended routine vaccinations are not a risk factor for asthma or eczema. In this observational study analysing computerised primary care records, the authors found an association between MMR and DPPT vaccination and the incidence of asthma and eczema, but these associations appeared to be limited to the minority of children who rarely seek care from a GP. This limited association is more likely to be the result of bias than a biological effect. |
Cases vaccinated/PT‐years versus Cases unvaccinated/PT‐years ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐All‐‐‐‐‐‐‐‐‐‐‐‐‐ (a) 1857/55,651 versus 27/3868 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Stratified by consulting frequency in first 18 months (a1) 244/10,625 versus 6/2768 (a2) 457/14,293 versus 7/402 (a3) 601/17,427 versus 9/400 (a4) 555/13,306 versus 5/297 |
rr (95% CI)(*) (a) 3.50 (2.38 to 5.15) (a1) 10.4 (4.61 to 23.29) (a2) 1.57 (0.75 to 3.32) (a3) 1.36 (0.71 to 2.64) (a4) 2.21 (0.92 to 5.33) (*) Adjusted rr estimated from a proportional hazard regression model stratified by consulting frequency, parental smoking, parental allergic disease, maternal age, number of older siblings, use of antibiotics early in life, year of birth, and GP practice. |
|
cb‐Timmermann 2015 Cohort study |
n = 640 children were followed from birth. Follow‐up examinations at ages 5, 7, and 13 years included a physical examination and a maternal questionnaire about the child’s health. | Asthma and dermatitis eczema At age 5, parents were asked whether the child was suspected to suffer from asthma or had been diagnosed with asthma, hypersensitivity, or allergy. At ages 5, 7, and 13 years, the same paediatrician determined the presence of current wheezing by auscultation. At the same ages, the pediatrician also examined all children for dermatitis/eczema. At age 13, the findings from this examination were graded according to a score for atopic dermatitis (SCORAD). At age 7, a blood sample was drawn and total IgE and grass‐specific IgE were quantified. At child's age 13, parents were asked whether the child had ever suffered from asthma. In accordance with the International Study of Asthma and Allergies in Childhood (ISAAC), they were also asked to indicate whether the child had (i) suffered from wheezing in the past 12 months, (ii) suffered from sneezing, running, or blocked‐up nose except for when the child had a cold or was sick in the past 12 months, and, if so, whether it was accompanied by itching running/tearing eyes (current rhinoconjunctivitis symptoms), and (iii) whether the child had ever suffered from an itching rash that comes and goes for at least 6 months (eczema ever). At age 13, the children underwent a skin prick test with extracts of 5 common allergens (birch/grass pollen, dog/cat dander, and house dust mite (Dermatophagoides pteronyssinus)). |
MMR vaccine: not described The Faroe Islands follow the Danish vaccination schedule, in which MMR vaccination, at the time of this study, was administered at age 15 months and 12 years (Fig. 1). There were no specific contraindications. At the 5‐year examination, the child’s vaccination card was inspected and all vaccination dates were registered. At child's age 13, the mothers were asked whether the child had received the MMR vaccination scheduled at 12 years of age. |
Conclusion: there is no association between MMR vaccine and the risk of eczema. |
Eczema (a) 5 years old (b) 13 years old |
OR (95% CI) (a) no data (*) (b) 0.73 (0.26 to 2.10) (*) (a) no data (*) (**) (b) 0.46 (0.14 to 1.52) (*) (**) RR (95% CI) (***) (a) no data (*) (b) 0.75 (0.28 to 1.87) (*) (*) Adjusted OR (logistic regression model) for birthweight and family history of chronic bronchitis/asthma. The analyses at age 13 years are additionally adjusted for whether the child had received the second MMR vaccine before the 13‐year examination. (**) Additional adjustment for sex, premature birth, maternal smoking during pregnancy, log (cord blood IgE), breastfeeding, number of older siblings, number of younger siblings, parental smoking in the home, day care, family history of eczema in children/allergic eczema/hay fever, family history of allergy, and age at the examination. (***) OR converted in RR (a) no data (b) CER = 0.11 |
CER: control event rate CI: confidence interval HMO: health maintenance organisation ICD: International Classification of Diseases incidence: cases/PT IgE: immunoglobulin E GP: general practice MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) VSD: Vaccine Safety Datalink
24. Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Bremner 2005 Case‐control |
n = 76,310 children from GPRD born between 1989 and 1993 from 464 general practices, and within a DIN cohort of n = 40,183 children born between 1989 and 1997 from 141 general practices. From GPRD cases = 3859 controls = 3859 From DIN cases = 2611 controls = 2611 |
Hay fever Case certain (Definition I): a child with hay fever diagnosis before 24 months of age, and a second diagnosis of hay fever or a relevant therapy in a subsequent years and with a third diagnosis or a relevant therapy in a further year. Case certain (Definition II): a child without first diagnosis before 24 months of age, but with a second diagnosis of hay fever or a relevant therapy in subsequent year. Case less certain (Definition I): a child as a case certain (Definition I) without third diagnosis of hay fever or a relevant therapy in a further year. Case less certain (Definition II): a child with at least a hay fever diagnosis, even if there is not a second diagnosis or a relevant therapy in a subsequent year. The cases and controls were children with at least 5 years of follow‐up from birth and registered “within the practice within 3 months of birth”. Only codes synonymous with "allergic rhinitis” and with seasonal variation in recording were permitted. From GPRD and DIN database. |
MMR vaccine: (first entries) MMR II The time categories for MMR immunisation: (a) 1st to 13th month (b) 14th month (c) 15th month (d) 16th month (e) 17th month (f) 18th to 24th month (g) ≥ 25th month (h) no MMR vaccine |
Conclusions: this study shows that infants vaccinated with MMR are at no greater or lesser risk of developing hay fever than unvaccinated children. This should reassure parents and clinicians, and no opportunity should be missed to immunise. |
n = (cases + controls) From GPRD (a) n = 1688 (b) n = 2311 (c) n = 1638 (d) n = 1183 (e) n = 510 (f) n = 618 (g) n = 234 (h) n = 210 From DIN (a) n = 1128 (b) n = 1769 (c) n = 1192 (d) n = 772 (e) n = 335 (f) n = 379 (g) n = 119 (h) n = 110 |
OR (95% CI) From GPRD(*) (a) 0.97 (0.81 to 1.16) (b) 1.00 (1.00 to 1.00) (c) 0.89 (0.75 to 1.06) (d) 0.93 (0.75 to 1.14) (e) 0.96 (0.73 to 1.25) (f) 0.89 (0.70 to 1.14) (g) 0.83 (0.58 to 1.18) (h) 0.81 (0.53 to 1.24) From DIN(**) (a) 0.90 (0.71 to 1.16) (b) 1.00 (1.00 to 1.00) (c) 1.24 (1.00 to 1.53) (d) 0.96 (0.73 to 1.39) (e) 1.00 (0.69 to 1.45) (f) 1.01 (0.73 to 1.28) (g) 0.54 (0.31 to 0.95) (h) 0.82 (0.45 to 1.50) From GPRD‐DIN Pooled (fixed‐effect) 1.27 (0.93 to 1.72) (*) Adjusted for consultation frequency and restricted to pairs with non‐ghost controls, adjusted for numbers of older and younger siblings and multiple births. (**) Adjusted for consultation frequency and restricted to pairs with non‐ghost controls. |
|
bb‐Bremner 2007 Case‐control |
n = 76,310 children from GPRD born between 1989 and 1993 from 464 practices and within a DIN cohort of n = 40,183 children born between 1989 and 1997 from 141 general practices. case + controls = 13,523 |
Hay fever risk in the first grass pollen season. Case of hay fever were children with diagnostic codes or treatment for hay fever, or both, after 2 years of age. Control was child that matched for general practice, sex, birth month, and follow‐up of control to at least date of diagnosis case. "Cases of hayfever were those who had diagnostic codes and/or treatment for hayfever, after 2 years of age”. From GPRD and DIN database. |
MMR vaccine: MMR II exposure by 24 months in a grass pollen season (May, June, July) versus non‐pollen season exposure |
Conclusion: in 2 population‐based birth cohorts, the authors have not
demonstrated any significant relationship between hay fever
and vaccination with MMR. Having MMR vaccine during grass pollen season by age 24 months (compared with MMR outside grass pollen season only) was not associated with an increased OR. |
Cases + control out season = 9690 in season = 3833 |
OR (95% CI)(*) 1.05 (0.94 to 1.18) (*) Odds ratios were pooled across databases (GPRD and DIN) using a fixed‐effect model. |
|
cb‐Timmermann 2015 Cohort study |
n = 640 children were followed from birth. Follow‐up examinations at ages 5, 7, and 13 years included a physical examination and a maternal questionnaire about the child’s health. | Asthma (and dermatitis eczema) At child's age 5, parents were asked whether the child was suspected to suffer from asthma or had been diagnosed with asthma, hypersensitivity, or allergy. At ages 5, 7, and 13 years, the same paediatrician determined the presence of current wheezing by auscultation. At the same ages, the paediatrician also examined all children for dermatitis/eczema. At age 13, the findings from this examination were graded according to a score for atopic dermatitis (SCORAD). At age 7, a blood sample was drawn and total IgE and grass‐specific IgE were quantified. At age 13, parents were asked whether the child had ever suffered from asthma. In accordance with the International Study of Asthma and Allergies in Childhood (ISAAC), they were also asked to indicate whether the child had (i) suffered from wheezing in the past 12 months, (ii) suffered from sneezing, running, or blocked‐up nose except for when the child had a cold or was sick in the past 12 months, and, if so, whether it had been accompanied by itching running/tearing eyes (current rhinoconjunctivitis symptoms), and (iii) whether the child had ever suffered from an itching rash that comes and goes for at least 6 months (eczema ever). At age 13, the children underwent a skin prick test with extracts of 5 common allergens (birch/grass pollen, dog/cat dander, and house dust mite (Dermatophagoides pteronyssinus)) |
MMR vaccine: not described. The Faroe Islands follow the Danish vaccination schedule, in which MMR vaccination, at the time of this study, was administered at age 15 months and 12 years (Fig. 1). There were no specific contraindications. At the 5‐year examination, the child’s vaccination card was inspected and all vaccination dates were registered. At child's age 13, the mothers were asked whether the child had received the MMR vaccination scheduled at 12 years of age. |
Conclusion: the authors' findings support the notion that MMR vaccination may provide beneficial effects in preventing childhood allergy and asthma. |
Rhinoconjunctivitis (a) 5 years old (b) 13 years old Hypersensitivity/allergy (a) 5 years old (b) 13 years old |
OR (95% CI) Rhinoconjunctivitis (a) no data (*) (b) 0.64 (0.19 to 2.07) (*) (a) no data (*)(**) (b) 0.63 (0.14 to 2.71) (*)(**) Hypersensitivity/allergy (a) 0.32 (0.11 to 0.88) (*) (b) no data (*) (a) 0.36 (0.11 to 1.21) (*)(**) (b) no data (*)(**) (*) Adjusted for birthweight and family history of chronic bronchitis/asthma. The analyses at age 13 years are additionally adjusted for whether the child had received the second MMR vaccine before the 13‐year examination. (**) Additional adjustment for sex, premature birth, maternal smoking during pregnancy, log (cord blood IgE), breastfeeding, number of older siblings, number of younger siblings, parental smoking in the home, day care, family history of eczema in children/allergic eczema/hay fever, family history of allergy, and age at the examination. |
CI: confidence interval DIN: doctors' independent network GPRD: General Practice Research Database HMO: health maintenance organisation incidence: cases/PT IgE: immunoglobulin E MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk)
25. Safety: acute leukaemia.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
bb‐Ma 2005 case control |
Cases: patients with leukaemia or acute lymphoblastic leukaemia, aged 0 to 14 years identified within the NCCLS between 1995 and 2002. Controls: matched to cases for date of birth, gender, Hispanic status (either parent Hispanic), maternal race (white, African‐American, or other), and maternal county of residence, by means of birth certificates. Population coverage initially includes 17 countries in the Greater San Francisco Bay Area, and since 1999 was expanded to a further 18 countries in Northern and Southern California. The present study relies on cases of leukaemia ascertained between 1995 and 2002. |
Leukaemia Acute lymphoblastic leukaemia Within the NCCLS, incident leukaemia cases were ascertained from major paediatric clinical centres within 72 hours after diagnosis. To be eligible, each case or control had to:
|
MMR vaccine: not reported Complete vaccination record was requested to primary caretakers of case or control participants. Other than MMR, vaccinations against diphtheria, pertussis, and tetanus (DPT), DT, Td, poliomyelitis, hepatitis B, or Hib have been considered in the study. (d1) 1 dose (d2) ≥ 2 doses (d0) unvaccinated (a) Leukaemia (a1) born in or before 1995 (a2) born after 1995 (b) Acute lymphoblastic leukaemia (b1) born in or before 1995 (b2) born after 1995 |
Conclusion:
MMR vaccination, measured as the number of doses, was not associated with the risk of overall leukaemia or acute lymphoblastic leukaemia. Each dose of Hib vaccination was associated with a significantly reduced risk of childhood leukaemia, whilst the history of DPT, poliomyelitis, and MMR vaccinations did not differ between cases and controls. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls Leukaemia (0 to 14 years) (d1) 176/323 versus 219/409 (d2) 123/323 versus 162/409 (d0) 24/323 versus 28/409 Leukaemia (> 1 years) (d1) 175/308 versus 219/392 (d2) 123/308 versus 162/392 (d0) 10/308 versus 11/392 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Acute lymphoblastic leukaemia (a) cases = 282; controls = 360 (b1) born in or before 1995 (b2) born after 1995 cases = 270; controls = 346 |
OR (95% CI) leukaemia (*) (a) 1.06 (0.69 to 1.63) (a1) 0.94 (0.75 to 1.53) (a2) 0.79 (0.35 to 1.78) Acute lymphoblastic leukaemia(*) (b) 0.87 (0.55 to 1.37) (b1) 0.95 (0.56 to 1.60) (b2) 0.65 (0.24 to 1.72) (*) Adjusted for maternal education and household income |
|
bb‐Groves 1999 Case‐control |
Cases: patients with acute lymphoblastic leukaemia aged 0 to 14, diagnosed between 1989 and 1993. Participants who resided in Illinois, Indiana, Iowa, Michigan, Minnesota, New Jersey, Ohio, Pennsylvania, or Wisconsin at the time of diagnosis were eligible for the vaccination component of the study. Controls: selected through random‐digit dialling were individually matched to the cases by age (within 25% of the corresponding case's age at diagnosis), the first 8 digits of the telephone number, and race (African‐American/white/other). |
Acute lymphoblastic leukaemia | MMR vaccine: not reported | Conclusion: the MMR vaccine does not alter the risk of subsequent acute lymphoblastic leukaemia. | cases = 395; controls = 394 |
OR (95% CI)(*) 1.19 (0.67 to 2.10) (*)conditional logistic regression adjusted for age at censoring, year of birth, sex, race, family income, parental education, and attendance at day care and/or preschool |
|
bb‐Mallol‐Mesnard 2007 Case‐control |
Each case of acute leukaemia incident in 2003 to 2004 in a child aged < 15 years, residing in France at the time of diagnosis and with no previous history of malignancy, was eligible. Theleukaemia cases(n = 726) were recruited directly by investigators assigned to each French paediatric oncology hospital department, with the support of the French National Registry of Childhood Haematopoietic Malignancies. The controls (n = 1681) were randomly selected from the French population using quotas, a priori determined to make the control group representative of all cancer cases in terms of age and gender. |
(a)Acute leukaemia (b)Acute lymphoblastic leukaemia (c)Acute myeloblastic leukaemia All the childhood leukaemia cases were confirmed by bone marrow analysis. Children whose mother did not speak French or who had been adopted were not eligible. |
MMR vaccine: not reported Note: the study shows measle‐mumps‐rubella vaccination separately, probably because for the study each mother was asked to read out each page of the vaccination record, line by line. |
Conclusion: no association between vaccination and the risk of childhood acute leukaemia: acute lymphoblastic leukaemia or acute myeloblastic leukaemia was observed. No relationship between the risk of leukaemia and the type of vaccine, number of doses of each vaccine, total number of injections, total number of vaccine doses, or number of early vaccinations was evidenced. No confounding factor was observed. The study did not show any evidence of a role of vaccination in the aetiology of childhood leukaemia. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls (a) 541/618 versus 1110/1258 (b) 480/554 versus 1110/1258 (c) 50/62 versus 1110/1258 |
OR (95% CI) (a) 0.94 (0.70 to 1.26) (b) 0.86 (0.64 to 1.17) (c) 0.56 (0.29 to 1.07) |
|
bb‐Dockerty 1999 Case‐control |
The eligible cases were newly diagnosed with childhood leukaemia (aged 0 to 14 years) 1990 to 1993, and born and resident in New Zealand. Controls (matched 1:1 to cases on age and sex) were selected randomly from the New Zealand‐born and resident childhood population, using national birth records. Each control’s birth was registered in the same quarter of the same year as the matched case. Adopted children were not eligible. |
Acute lymphoblastic leukaemia n = 97 matched pairs |
MMR vaccine not described. Vaccination histories were supplemented with information from parent‐held ‘Health and Development’ records. | Conclusion: for MMR, no association was found with leukaemia. |
N cases vaccinated/
N cases
versus
N controls vaccinated/
N controls 6/118 versus 15/272 |
OR (95% CI) (*) 0.8 (0.26 to 2.42) (*)unconditional logistic regression adjusted for age, sex, child’s social class, child’s ethnic group, mother’s marital status, mother’s education, mother’s home ownership, household crowding, delay from reference date to interview, interview year. |
CI: confidence interval DPT: diphtheria, pertussis, tetanus vaccine DT: diphtheria, tetanus vaccine Hib: Haemophilus influenzae b vaccine HMO: health maintenance organisation ICD: International Classification of Diseases incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine NCCLS: Northern California Childhood Leukemia Study OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) Td: tetanus, diphtheria vaccine versusD: Vaccine Safety Datalink
26. Safety: demyelinating diseases, multiple sclerosis, acute disseminated encephalomyelitis.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
cb‐Ahlgren 2009 Cohort study |
Residents in the great Gothenburg area (Sweden) born between 1959 and 1990. The study area was the greater Gothenburg area on the Swedish west coast, with 731,592 residents on 31 December 2000. | Multiple sclerosis (probable or definite) and clinically isolated syndromes. Incidence of multiple sclerosis (4 Poser's criteria) and clinically isolated syndrome with onset between 10 and 39 years of age was assessed in birth cohorts immunised within 4 vaccination programmes. The Gothenburg multiple sclerosis register was established from the 1950s. All records are reviewed with the following MS‐related diagnoses, according to the International Classification of Diseases (ICD) 10, 9, and 8: G359; 340; 340.99 multiple sclerosis; G368; G378; G379; 341W; 341.09 demyelinating disorders in the central nervous system; G360; 341A; 341.01 neuromyelitis optica; G369; 341X acute disseminated encephalomyelitis; G373 acute transverse myelitis: H46; 377D; 367.02 optic neuritis; H48,1; 367.03 retrobulbar neuritis. |
MMR vaccine: not described. Different vaccination programmes carried out from 1971 with different vaccines (single‐component measle, mumps and rubella vaccine so as with MMR vaccine) having as target population children of different ages. 5 population birth cohorts were selected from the total incidence material: (0) born 1959 to 1961: the pre‐vaccine era; (1) born 1962 to 1966: monovalent rubella vaccine; (2) born 1970 to 1973: only received later dose of the MMR vaccine; (3) born 1974 to 1978: monovalent measles; (4) July 1981 to June 1984: combined MMR vaccine. |
Conclusion: there was no significant change in the age‐ and gender‐specific incidence of MS in any of the selected cohorts compared with the incidence in the preceding selected birth cohorts. There was thus no significant change in MS incidence related to the implementation of the rubella vaccination programme in the 12‐year‐old female cohort born in 1962 to 1966 compared with the unvaccinated cohort born in 1959 to 1961. The incidence did not significantly change with all preceding selected cohorts as baseline, neither in the MMR‐vaccinated 12‐year‐old cohort born in 1970 to 1973, nor in the cohort born in 1974 to 1978, half of which were measles vaccinated in the preschool age and the majority MMR vaccinated at 12, nor in the cohort born in July 1981 to June 1984, which were MMR vaccinated at both 18 months and 12 years of age. Restricting the analyses to probable and definite MS cases did not change the results. |
Incidence per 100,000 person‐years (‐) (male female) versus (male female) (*) (1) (14.98; 6.97) versus (17.61; 4.28) (2) (15.28; 6.61) versus (13.17; 5.27) (3) (12.29; 3.85) versus (9.48; 4.62) (4) (4.96; 1.18) versus (3.78; 2.55) (*) including both the unvaccinated cohort 1959 to 1961 and the preceding vaccinated birth cohorts selected for this study, in the corresponding age groups |
No data available for meta‐analysis |
|
bb‐Ahlgren 2009 Case‐control study |
Cases (n = 206): birth years 1959 to 1986, to be resident in the greater Gothenburg area (Sweden), MS onset from age of 10 years onwards, did attend the 6th school grade within study area, availability of CHSH records. Controls (n = 888): matched to cases for year of birth by random selection from the population register. Controls should have attended the 6th school grade within study area, and have available CHSH record. |
Multiple sclerosis (probable or definite) and clinically isolated syndromes |
MMR vaccine: not described MMR vaccination (vaccination with single‐component vaccines has also been considered). The second analysis was therefore restricted to the subgroup of the MMR vaccinations. The first analysis was restricted to the subgroup "MMR vaccination". 4 disjointed vaccination categories were defined: (0) no MMR vaccination; (1) early MMR vaccination only; (3) late MMR vaccination only; (4) both an early and a late MMR vaccination. Comparisons were made within the group of MMR vaccinations. |
Conclusions: no significant association for vaccinated versus unvaccinated. | Cases = 206; controls = 888 |
OR (95% CI) 1.13 (0.62 to 2.05) |
|
bb‐Chen 2018 Case‐control study |
Case (n = 272): acute disseminated encephalomyelitis. Controls (n = 1096): for each ADEM case, 4 control individuals randomly selected from the same hospital with no history of ADEM were matched to the case according to year of birth (within 1 year), gender, and zip code (a surrogate measure for socioeconomic status) during the same period. The control participants were assigned the same index date as their matched case (symptom onset date). Controls were patients referred for headache (except trigeminal neuralgia), migraine, vascular, or other diseases which were thought not to modify the probability of vaccination. Patients with chronic severe neurological diseases or autoimmune diseases were excluded. |
Acute disseminated encephalomyelitis: immune‐mediated central nervous system disorder, characterised by an acute encephalopathy with polyfocal neurological deficits. From the Hospital Information Systems first mention of International Classification of Diseases, Tenth Revision (ICD‐10), diagnostic codes (G04.001, G04.002, G04.051, G04.903, and G04.912) for ADEM from 1 January 2011 to 31 December 2015, for individuals of any age. Diagnoses were confirmed by neurologists from clinical data, such as clinical manifestations, computed tomography, electroencephalograph, cerebrospinal fluid, and magnetic resonance imaging examinations. |
MMR vaccine: not described | Conclusions: findings from the present study do not demonstrate an association of vaccines with an increased risk of ADEM and its recurrence among either paediatric (< 18 years) or adult (≥ 18 years) individuals within the 180 days after vaccinations. | 11/272 versus 36/1096 |
OR (95% CI) adjusted estimate 1.03 (0.68 to 3.75) |
ADEM: acute disseminated encephalomyelitis CI: confidence interval CHSH: child health and school health records CIS: clinically isolated syndromes HMO: health maintenance organisation incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MS: multiple sclerosis OR: odds ratio PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio) RR: risk ratio (relative risk) VSD: Vaccine Safety Datalink
27. Safety: gait disturbances.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
db‐Miller 2005 Self‐controlled case series |
Children hospitalised with gait disturbance between April 1995 and June 2001 (n = 127, age 12 to 24 months). Children with gait disturbance resulting from general practice visit General Practice Research Database. (GPRD archive), born between 1988 and 1997 (n = 1398, age 12 to 24 months) |
(a) Hospitalisation for gait disturbance Review of hospital computerised records (April 1995 to June 2001, children aged 12 to 24 months) with ICD‐10 diagnoses related to acute gait disorder (G111, G112, G25, R26, R27, R29, H55, and F984). Cases were grouped into 5 categories, as follows: (1) presumptive viral/postviral ataxia (clinical history of ataxia and evidence of encephalomyelitis or cerebellitis with lymphocytosis in CSF or encephalographic changes); (2) probable postviral ataxia (history consistent with ataxia but CSF/other investigations inconclusive or not done and no other cause identified); (3) probably not postviral gait disturbance (vague symptoms not suggestive of cerebellar ataxia, e.g. unsteady gait associated with constipation or gastroenteritis); (4) non‐ataxic, non‐viral gait disturbance (including limp after trauma, septic bone or joint disease, unsteadiness following drug ingestion); (5) transient synovitis/‘‘irritable hip’’ (a transient condition described following viral illnesses and with no long‐term sequelae) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (b) GP visits for gait disturbance For the analysis of gait disorders presenting in general practice, information on all children born from 1988 to 1997 with at least 2 years of continuous follow‐up from birth in a GPRD practice deemed as supplying data of research standard was obtained from the Office for National Statistics. Read and OXMIS codes that indicated a consultation for possible gait disturbance in children aged 12 to 24 months were identified by mapping to ICD‐9 codes and by searching on the following keywords: ataxia, gait, co‐ordination, mobility, movement. Read/OXMIS descriptive diagnoses cover a wide range, so were grouped into 6 categories for analysis: (A) ataxia (including cerebellar ataxia and ataxic gait); (B) unsteady/veering/shuffling gait; (C) gait abnormality ‐ unspecified; (D) limp/limping gait; (E) poor mobility; (F) abnormal /involuntary movements. |
MMR vaccine: not reported (a) Risk period: after immunisation (a1) 0 to 30 days (a2) 31 to 60 days (a3) 0 to 60 days (b) Risk period after immunisation (b1) 0 to 5 days (b2) 6 to 30 days (b3) 31 to 60 days (b4) 6 to 60 days |
Conclusion: this study provides no evidence that MMR vaccine causes acute ataxia or other gait disturbance and suggests that the cases observed were chance occurrences, reflecting background incidence. The increased incidence of consultation for any gait disturbance 0 to 5 days after MMR vaccination was attributable to an excess in categories of gait disturbance (B, unsteady; and C, unspecified) that was caused by a clear excess of consultations on the day that MMR was given. It is biologically implausible that any specific MMR effect would be manifest on the day of vaccination since the viraemia induced by the vaccine, which might produce symptoms, does not start until the end of the first week. |
Hospitalisation for gait disturbance any (categories 2, 3, 5) n = 62 (a1) cases = 3 (a2) cases = 1 (a3) cases = 4 GP visits for gait disturbance All cases ((A) to (F)) (b1) cases = 31 (b2) cases = 69 (b3) cases = 102 (b4) cases = 171 |
rr (95% CI) (*) (a1) 0.83 (0.24 to 2.84) (a2) 0.20 (0.03 to 1.47) (a3) 0.46 (0.16 to 1.35) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (b1) 1.88 (1.30 to 2.72) (b2) 0.90 (0.70 to 1.17) (b3) 0.95 (0.77 to 1.19) (b4) 0.93 (0.78 to 1.12) (*) Poisson regression |
CI: confidence interval CSF: cerebrospinal fluid GP: general practitioner GPRD: General Practice Research Database ICD: International Classification of Diseases incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OXMIS: Oxford Medical Information Systems PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio)
28. Safety: bacterial or viral infections.
| Study ID and design | Population | Outcome definition | Exposure MMR/MMRV vaccine | Findings | Crude data | Estimate (95% CI) |
|
db‐Stowe 2009 Self‐controlled case series |
Infants aged 12 to 23 months hospitalised for viral or bacterial infection between April 1995 and May 2005 identified from hospital admission records (n = 2025 accounting for 2077 admissions) |
Lobar pneumonia ICD‐9 codes: 481 ICD‐10 codes: J18.1 Invasive bacterial infections ICD‐9 codes: 036, 038, 320, 711.0, 730.0 ICD‐10 codes: A39, A40, A41, G00, M00, M86, J13X Encephalitis/meningitis ICD‐9 codes: not specified ICD‐10 codes: A85, A86, A87, A88, A89 Herpes ICD‐9 codes: not specified ICD‐10 codes: B00 Pneumonia ICD‐9 codes: not specified ICD‐10 codes: J12 Varicella zoster ICD‐9 codes: not specified ICD‐10 codes: B01, B02 Miscellaneous viral infections ICD‐9 codes: not specified ICD‐10 codes: B08, B09, B15, B17, B25, B27, B34 Review of computerised hospital admission records from North, East, and South London, Essex, East Anglia, Sussex, and Kent using ICD‐9 or ICD‐10 codes |
MMR vaccine: not reported Excluded period from the background from −14 to −1 days before immunisation Risk period after immunisation (a1) 0 to 30 days (a2) 31 to 60 days (a3) 61 to 90 days (a4) 0 to 90 days |
Conclusion: the study confirms that the MMR vaccine does not increase the risk of invasive bacterial or viral infection in the 90 days after the vaccination and does not support the hypothesis that there is an induced immune deficiency due to overload from multi‐antigen vaccines. |
Total cases Lobar pneumonia (a1) cases = 57 (a2) cases = 65 (a3) cases = 69 (a4) cases = 191 Invasive bacterial infections (a1) cases = 30 (a2) cases = 34 (a3) cases = 27 (a4) cases = 91 Encephalitis/meningitis (a1) cases = 1 (a2) cases = 1 (a3) cases = 2 (a4) cases = 4 Herpes (a1) cases = 16 (a2) cases = 25 (a3) cases = 14 (a4) cases = 55 Pneumonia (a1) cases = 0 (a2) cases = 5 (a3) cases = 4 (a4) cases = 9 Varicella zoster (a1) cases = 17 (a2) cases = 32 (a3) cases = 24 (a4) cases = 73 Miscellaneous viral infections (a1) cases = 12 (a2) cases = 12 (a3) cases = 9 (a4) cases = 33 |
rr (95% CI) (*) Lobar pneumonia (a1) 0.65 (0.48 to 0.86) (a2) 0.80 (0.61 to 1.05) (a3) 0.90 (0.69 to 1.18) (a4) 0.77 (0.64 to 0.93) Invasive bacterial infections (a1) 0.75 (0.51 to 1.12) (a2) 1.03 (0.70 to 1.52) (a3) 0.92 (0.61 to 1.41) (a4) 0.89 (0.68 to 1.16) Encephalitis/meningitis (a1) 0.54 (0.06 to 4.83) (a2) 0.74 (0.07 to 7.47) (a3) 1.46 (0.23 to 9.29) (a4) 0.84 (0.20 to 3.49) Herpes (a1) 1.00 (0.57 to 1.74) (a2) 1.69 (1.06 to 2.70) (a3) 0.89 (0.50 to 1.59) (a4) 1.17 (0.56 to 2.47) Pneumonia (a1) 0 (‐ to ‐) (a2) 1.39 (0.49 to 3.90) (a3) 1.27 (0.41 to 3.94) (a4) 0.72 (0.33 to 1.62) Varicella zoster (a1) 0.58 (0.34 to 0.99) (a2) 1.23 (0.81 to 1.87) (a3) 1.05 (0.66 to 1.67) (a4) 0.93 (0.68 to 1.27) Miscellaneous viral infections (a1) 0.71 (0.37 to1.37) (a2) 0.73 (0.37 to 1.14) (a3) 0.61 (0.29 to 1.28) (a4) 0.68 (0.43 to 1.09) (*)Poisson regression |
|
db‐Miller 2003 Self‐controlled case series |
Children aged 12 to 23 months admitted to hospital between April 1991 and March 1995 in selected districts in the Thames region of southern England. Total of 387 admissions with 1 or more of the bacterial infection codes and with a linked MMR vaccination record were identified; occurred in 387 children (169 in 165 females, and 226 in 222 males); 116 had a diagnosis of invasive bacterial infection and 279 had lobar pneumonia. |
Lobar pneumonia Invasive bacterial infections Cases were identified from computerised discharge records using ICD‐9 codes 036 (meningococcal infection), 038 (septicaemia), 320 (bacterial meningitis), 711.0 (pyogenic arthritis), 730.0 (acute osteomyelitis), and 481 (lobar (pneumococcal) pneumonia). Hospital records were linked with computerised district immunisation records by sex, date of birth, and post code. Cases in children with additional diagnostic codes indicating an underlying disorder predisposing to bacterial infection, such as immunosuppression, malignancy, cystic fibrosis, congenital heart defect, or a cerebrospinal fluid shunt, were excluded. |
MMR vaccine: not described Excluded period from the background from −14 to −1 days before immunisation Risk period after immunisation (a1) 0 to 30 days (a2) 31 to 60 days (a3) 61 to 90 days (a4) 0 to 90 days |
Conclusion: combined measles, mumps, and rubella (MMR) vaccine did not increase the risk of hospitalisation with invasive bacterial infection in the 3 months after vaccination; rather there was a protective effect. These results provide no support for the concept of 'immunological overload' induced by multiple‐antigen vaccinations, nor calls for single‐antigen vaccines. |
Total cases Lobar pneumonia (a1) cases = 23 (a2) cases = 24 (a3) cases = 16 (a4) cases = 63 Invasive bacterial infections (a1) cases = 12 (a2) cases = 14 (a3) cases = 7 (a4) cases = 33 Both codes (a1) cases = 35 (a2) cases = 38 (a3) cases = 23 (a4) cases = 96 |
rr (95% CI) (*) Lobar pneumonia (a1) 0.77 (0.48 to 1.23) (a2) 0.80 (0.50 to 1.28) (a3) 0.52 (0.30 to 0.90) (a4) 0.70 (0.50 to 0.97) Invasive bacterial infections (a1) 1.00 (0.52 to 1.94) (a2) 1.17 (0.62 to 2.20) (a3) 0.62 (0.27 to 1.40) (a4) 0.93 (0.58 to 1.49) Both codes (a1) 0.81 (0.56 to 1.19) (a2) 0.90 (0.62 to 1.31) (a3) 0.56 (0.36 to 0.89) (a4) 0.76 (0.58 to 0.99) (*)Poisson regression |
CI: confidence interval CSF: cerebrospinal fluid GP: general practitioner GPRD: General Practice Research Database ICD: International Classification of Diseases incidence: cases/PT MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine PT: person‐time rr: rate ratio (relative incidence, incidence rate ratio)
Excluded studies
We excluded 27 studies of the 101 papers identified and retrieved for this 2019 update. In addition, of 16 studies awaiting classification (see Characteristics of studies awaiting classification) in the previous update (Demicheli 2012), we excluded four studies because they were not comparative; they considered vaccines other than MMR; or they did not present original data (for details see Characteristics of excluded studies). We assessed a further seven studies as awaiting classification and five studies as ongoing because the papers were lacking in some important details (see Characteristics of studies awaiting classification and Characteristics of ongoing studies).
Risk of bias in included studies
Of the 138 included studies, we assessed 53 (38%) as at low risk of bias, 55 (40%) as at unclear risk of bias, and 30 (22%) as at high risk of bias (Figure 3). The quality assessment of each individual study and the description of the quality criteria adopted are shown in Figure 4 and Appendix 5, respectively. The risk of bias by study design and by publication year are shown in Table 50 and Table 51, respectively.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
29. Risk of bias.
| Study design | Low risk of bias | Unclear risk of bias | High risk of bias | |||||
| n | Row % | n | Row % | n | Row % | n total | ||
| Effectiveness studies | RCT/CCT | 3 | 100% | 3 | ||||
| Case‐control | 8 | 57.1% | 4 | 28.6% | 2 | 14.3% | 14 | |
| Prospective/retrospective cohort | 4 | 13.0% | 21 | 67.7% | 6 | 19.4% | 31 | |
| Case‐only ecological method | 2 | 66.7% | 1 | 33.3% | 3 | |||
| Subtotal | 15 | 29.4% | 27 | 53.0% | 9 | 17.6% | 51 | |
| Study design | Low risk of bias | Unclear risk of bias | High risk of bias | |||||
| n | Row % | n | Row % | n | Row % | n total | ||
| Safety studies | RCT/CCT | 2 | 28.6% | 2 | 28.6% | 3 | 42.9% | 7 |
| Case‐control | 8 | 38.1% | 11 | 52.4% | 2 | 9.5% | 21 | |
| Prospective/retrospective cohort | 14 | 43.8% | 4 | 12.5% | 14 | 43.8% | 32 | |
| Self‐controlled case series/person‐time cohort | 11 | 68.8% | 5 | 31.2% | 16 | |||
| Case cross‐over | 1 | 33.3% | 2 | 66.7% | 3 | |||
| Case‐only ecological method | 2 | 25.0% | 4 | 50.0% | 2 | 25.0% | 8 | |
| Subtotal | 38 | 43.7% | 28 | 32.2% | 21 | 24.1% | 87 | |
| Total (all studies) | 53 | 38.4% | 55 | 39.9% | 30 | 21.7% | 138 | |
| Study design | Low risk of bias | Unclear risk of bias | High risk of bias | |||||
| n | Row % | n | Row % | n | Row % | n total | ||
| Safety studies (excluding short‐term side effects studies) | Case‐control | 8 | 38% | 11 | 52% | 2 | 10% | 21 |
| Prospective/retrospective cohort | 14 | 64% | 4 | 18% | 4 | 18% | 22 | |
| Self‐controlled case series/person‐time cohort | 11 | 69% | 5 | 31% | 16 | |||
| Case cross‐over | 1 | 33% | 2 | 67% | 3 | |||
| Case‐only ecological method | 2 | 25% | 4 | 50% | 2 | 25% | 8 | |
| Total | 36 | 51% | 26 | 37% | 8 | 11% | 70 | |
CCT: controlled clinical trial RCT: randomised controlled trial
30. Risk of bias by publication year.
| All studies included | Low risk of bias | Unclear risk of bias | High risk of bias | Total | |||
| Publication year | N | Row % | N | Row % | N | Row % | |
| 1971 to 1980 | 0 | 0% | 1 | 20% | 4 | 80% | 5 |
| 1981 to 1990 | 2 | 29% | 0 | 0% | 5 | 71% | 7 |
| 1991 to 2000 | 3 | 20% | 6 | 40% | 5 | 40% | 15 |
| 2001 to 2010 | 21 | 39% | 23 | 43% | 10 | 18% | 54 |
| 2011 to 2019 | 27 | 47% | 24 | 42% | 6 | 11% | 57 |
| Total | 53 | 36% | 54 | 42% | 30 | 22% | 138 |
| Only safety studies | Low risk of bias | Unclear risk of bias | High risk of bias | Total | |||
| Publication year | N | Row % | N | Row % | N | Row % | |
| 1971 to 1980 | 1 | 20% | 4 | 80% | 5 | ||
| 1981 to 1990 | 2 | 29% | 5 | 71% | 7 | ||
| 1991 to 2000 | 2 | 20% | 4 | 40% | 4 | 40% | 10 |
| 2001 to 2010 | 17 | 40% | 17 | 41% | 8 | 19% | 43 |
| 2011 to 2019 | 17 | 74% | 5 | 22% | 1 | 4% | 22 |
| Total | 38 | 39% | 27 | 37% | 22 | 24% | 87 |
Studies evaluating vaccine effectiveness
Of the 51 studies that assessed the effectiveness of MMR/MMRV vaccines, we assessed 15 (30%) as at low risk of bias, 27 (53%) as at unclear risk of bias, and 9 (17%) as at high risk of bias. These last studies were characterised by poor methodological quality due to poor reporting or missing information about comparability between exposed or non‐exposed groups, and the composition of MMR vaccine is sometimes not reported. See Table 50.
Studies evaluating vaccine safety
Of 87 included studies, we assessed 38 (44%) as at low risk of bias, 28 (32%) as at unclear risk of bias, and 21 (24%) as at high risk of bias. See Table 50.
-
Short‐term side effects: 17 studies (Table 31 and Table 32):
low risk of bias: 2 studies (ab‐Lerman 1981; ab‐Peltola 1986);
unclear risk of bias: 2 studies (ab‐Edees 1991; ab‐Schwarz 1975);
high risk of bias: 13 studies (ab‐Bloom 1975; ab‐Ceyhan 2001; ab‐Freeman 1993; cb‐Beck 1989; cb‐Benjamin 1992; cb‐Dunlop 1989; cb‐Makino 1990; cb‐Miller 1989; cb‐Robertson 1988; cb‐Sharma 2010; cb‐Stokes 1971; cb‐Swartz 1974; cb‐Weibel 1980).
-
Encephalitis or encephalopathy: 3 studies (Table 33):
low risk of bias: 1 study (db‐Ward 2007);
unclear risk of bias: 2 studies (bb‐Ray 2006; db‐Makela 2002).
-
Aseptic meningitis: 10 studies (Table 34):
low risk of bias: 2 studies (db‐Dourado 2000; eb‐Ki 2003);
unclear risk of bias: 8 studies (bb‐Black 1997; db‐Farrington 1995; db‐Makela 2002; db‐Miller 2007; db‐Perez‐Vilar 2018; eb‐Park 2004; gb‐da Cunha 2002; gb‐da Silveira 2002).
-
Seizure ‐ febrile/afebrile: 8 studies (Table 35):
low risk of bias: 5 studies (cb‐Vestergaard 2004; db‐Macartney 2017; db‐MacDonald 2014; db‐McClure 2019; db‐Ward 2007);
unclear risk of bias: 3 studies (cb‐Barlow 2001; db‐Farrington 1995; db‐Miller 2007).
-
MMRV versus MMR/MMR+V ‐ febrile seizures: 7 studies (Table 36):
low risk of bias: 7 studies (cb‐Gavrielov‐Yusim 2014; cb‐Jacobsen 2009; cb‐Klein 2010; cb‐Klein 2012; cb‐Klein 2017; cb‐Rowhani‐Rahbar 2013; cb‐Schink 2014).
-
Autism spectrum disorders: 13 studies (Table 37):
low risk of bias: 8 studies (bb‐De Stefano 2004; bb‐Smeeth 2004; cb‐Hviid 2019; cb‐Jain 2015; cb‐Madsen 2002; db‐Taylor 1999; gb‐Fombonne 2006; gb‐Honda 2005);
unclear risk of bias: 3 studies (bb‐Mrozek‐Budzyn 2010; bb‐Uno 2012; db‐Makela 2002);
high risk of bias: 2 studies (cb‐Uchiyama 2007; gb‐Fombonne 2001).
-
Inflammatory bowel disease: 6 studies (Table 38):
low risk of bias: 1 study (bb‐Shaw 2015);
unclear risk of bias: 4 studies (bb‐Baron 2005; bb‐Davis 2001; gb‐Seagroatt 2005; gb‐Taylor 2002);
high risk of bias: 1 study (bb‐Vcev 2015).
-
Cognitive delay, developmental delay: 1 study (Table 39):
unclear risk of bias (cb‐Mrozek‐Budzyn 2013).
-
Idiopathic thrombocytopenic purpura: 9 studies (Table 40):
low risk of bias: 3 studies (db‐Andrews 2012; db‐France 2008; db‐O'Leary 2012);
unclear risk of bias: 5 studies (bb‐Black 2003; bb‐Bertuola 2010; db‐Farrington 1995; db‐Perez‐Vilar 2018; eb‐Lafaurie 2018);
high risk of bias: 1 study (gb‐Jonville‐Bera 1996).
-
Henoch‐Schönlein purpura: 1 study (Table 41):
unclear risk of bias (bb‐Da Dalt 2016).
-
Type 1 diabetes: 2 studies (Table 42):
low risk of bias (cb‐Beyerlein 2017; cb‐Hviid 2004).
-
Asthma: 5 studies (Table 43):
low risk of bias: 1 study (cb‐Timmermann 2015);
unclear risk of bias: 2 studies (cb‐DeStefano 2002; cb‐Hviid 2008);
high risk of bias: 2 studies (cb‐Benke 2004; cb‐McKeever 2004).
-
Dermatitis or eczema: 2 studies (Table 44):
low risk of bias: 1 study (cb‐Timmermann 2015);
high risk of bias: 1 study (cb‐McKeever 2004).
-
Hay fever, rhinoconjunctivitis, hypersensitivity/allergy: 3 studies (Table 45):
low risk of bias (bb‐Bremner 2005; bb‐Bremner 2007; cb‐Timmermann 2015).
-
Acute leukaemia: 4 studies (Table 46):
low risk of bias: 2 studies (bb‐Ma 2005; bb‐Mallol‐Mesnard 2007);
unclear risk of bias: 2 studies (bb‐Dockerty 1999; bb‐Groves 1999).
-
Demyelinating diseases, multiple sclerosis, ADEM: 3 studies (Table 47):
low risk of bias: 1 study (bb‐Chen 2018);
high risk of bias: 2 studies (bb‐Ahlgren 2009; cb‐Ahlgren 2009).
-
Gait disturbances 1 study (Table 48):
low risk of bias (db‐Miller 2005).
-
Bacterial or viral infections: 2 studies (Table 49):
low risk of bias (db‐Stowe 2009)
unclear risk of bias (db‐Miller 2003).
Allocation
Of 10 RCTs/CCTs, five studies reported adequate concealment (aa‐Henry 2018; aa‐Povey 2019; aa‐Prymula 2014; ab‐Lerman 1981; ab‐Peltola 1986). See Figure 4.
Blinding
Of 10 RCTs/CCTs assessing effectiveness and/or short‐term side effects, six trials were double‐blind (aa‐Henry 2018; aa‐Povey 2019; aa‐Prymula 2014; ab‐Lerman 1981; ab‐Peltola 1986; ab‐Schwarz 1975); one was single‐blind (ab‐Edees 1991); two were not blinded (ab‐Bloom 1975; ab‐Ceyhan 2001); and in one study blinding was not reported (ab‐Freeman 1993).
Incomplete outcome data
In two trials (ab‐Ceyhan 2001; ab‐Lerman 1981), the selection of paediatric practices involved in the recruitment of children was not explained, and the number and assessment of non‐responders were not reported. Similarly in ab‐Edees 1991 there were few details on the refusal and response rate during the recruitment phase, and demographic information from the two UK areas where the trial was conducted was lacking. We considered two trials to be at unclear risk of detection bias affecting the outcomes (ab‐Ceyhan 2001; ab‐Edees 1991).
Selective reporting
In the two trials we assessed as being at high risk of reporting bias, adverse effects were reported for only 60% and 39% of participants, respectively (ab‐Bloom 1975; ab‐Schwarz 1975). We evaluated the only included cluster‐RCT as at high risk of reporting bias (ab‐Freeman 1993). The number of completed weekly diaries varied over the eight‐week study period, with no indication of whether the losses occurred pre‐ or postvaccination. Furthermore, there was an overall attrition rate of 33%.
Other potential sources of bias
Studies evaluating effectiveness
Fifteen (45%) of 33 cohort studies on effectiveness and 8 (57%) of 14 case‐control studies did not report adequate MMR or MMRV vaccine descriptions.
Studies evaluating safety ‐ harms
The association between MMR/MMRV and severe harms (excluding short‐term side effects) was investigated in 70 studies (22 cohort studies, 22 CCS, 13 SCCS, 3 PTC, 3 CCO, 8 COEM). Of 70 studies, we assessed 32 (46%) as at low risk of bias; 28 (40%) as at unclear risk of bias; and 10 (14%) as at high risk of bias. See Table 50.
Several cohort studies used matching procedures to ensure comparability or adopted a multivariate model. When only a few confounders were used to ensure comparability between cohorts, we assigned high risk of bias.
The study by db‐Makela 2002 was weakened by the loss of 14% of the original birth cohorts and the effects of the rather long‐term follow‐up. The impact of either of these factors in terms of confounders is open to debate. It should be taken into account that autism does not often involve hospitalisation, and data about outpatient visits were not available. Limited errors could have been introduced by using population data from a previous census (as estimation of the denominator) in db‐Dourado 2000. Therefore, the number of doses administered (as opposed to supplied) was used to compute the risk of aseptic meningitis in the mass vaccination programme. In eb‐Park 2004, there was an unclear likelihood of selection bias due to missing participants and records (up to 27%). In bb‐Black 1997, there was an unclear likelihood of selection bias due to missing participants and their records (up to 27%) but the study and its methods were well reported. The exclusive use of discharge diagnoses for identification of cases in db‐Miller 2007 could have introduced a noteworthy selection bias. Estimates from cb‐McKeever 2004 (although significant) were strongly affected by ascertainment bias: children who were not taken to the doctor were less likely to be vaccinated and to have fewer opportunities for diagnoses of allergic diseases to be recorded. Lack of clarity over the vaccine exposure status of the controls made the results of the bb‐Black 2003 study difficult to interpret. In bb‐Bertuola 2010, cases and controls were apparently not matched. In bb‐Ma 2005, refusal to participate in the study or inability to locate participants and controls could have introduced an unclear risk of selection bias. Exclusion of participants without completed questionnaires and of those who did not attend the sixth grade at school within the study area could have introduced a relevant selection bias in the bb‐Ahlgren 2009 case‐control study. Assessment of pervasive developmental disorders cases in gb‐Fombonne 2006 was made on the basis of administrative codes only: diagnosis could have been imprecise and did not enable us to consider pervasive developmental disorders subtypes or regression. In gb‐Fombonne 2001, the number and possible impact of bias was so high that interpretation of the results was difficult. The cohort study of cb‐Uchiyama 2007 was potentially affected by a different type of bias, considering that the participants were from a private clinic and that definitions of applied autism spectrum disorders diagnosis and methods used for disorders regression ascertainment were not clearly reported. The long follow‐up for autism could be due to the lack of a properly constructed causal hypothesis. The study of db‐Taylor 1999 demonstrated the difficulties of drawing inferences in the absence of a non‐exposed population or a clearly defined causal hypothesis.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20; Table 21
Summary of findings 1. Effectiveness against measles.
| Effectiveness against measles | |||||
| Patient or population: children 9 months to 15 years old Setting: general population or school or day‐care centre or general practitioner or households Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of measles amongst unvaccinated | Risk of measles amongst vaccinated | ||||
| Cohort studies ‐ 1 dose | Study population | RR 0.05 (0.02 to 0.13) | 12,039 (7 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 66 per 1000 | 3 per 1000 (1 to 9) | ||||
| Cohort studies ‐ 2 doses | Study population | RR 0.04 (0.01 to 0.28) | 21,604 (5 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 19 per 1000 | 1 per 1000 (0 to 5) | ||||
| Cohort studies household contacts ‐ 1 dose | Study population | RR 0.19 (0.04 to 0.89) | 151 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 508 per 1000 | 97 per 1000 (20 to 452) | ||||
| Cohort studies household contacts ‐ 2 doses | Study population | RR 0.15 (0.03 to 0.75) | 378 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 508 per 1000 | 76 per 1000 (15 to 381) | ||||
| Cohort studies household contacts ‐ 3 doses | Study population | RR 0.04 (0.01 to 0.23) | 151 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 351 per 1000 | 14 per 1000 (4 to 81) | ||||
| Cohort studies postexposure prophylaxis | Study population | RR 0.26 (0.14 to 0.50) | 283 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 314 per 1000 | 82 per 1000 (44 to 157) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level for large effect size (non‐critical risk of bias in studies).
Summary of findings 2. Effectiveness against mumps.
| Effectiveness against mumps | |||||
| Patient or population: children 9 months to 15 years old Setting: general population or school or day‐care centre or general practitioner or households Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of mumps amongst unvaccinated | Risk of mumps amongst vaccinated | ||||
| Cohort studies ‐ Jeryl Lynn strain ‐ 1 dose | Study population | RR 0.24 (0.08 to 0.76) | 9915 (6 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 91 per 1000 | 22 per 1000 (7 to 69) | ||||
| Cohort studies ‐ Jeryl Lynn strain ‐ 2 doses | Study population | RR 0.12 (0.04 to 0.35) | 7792 (5 observational studies) | ⊕⊕⊕⊝ MODERATE2 | |
| 74 per 1000 | 9 per 1000 (3 to 26) | ||||
| Cohort studies ‐ Jeryl Lynn strain ‐ unspecified number of doses | Study population | RR 0.23 (0.14 to 0.35) | 2011 (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 97 per 1000 | 22 per 1000 (14 to 34) | ||||
| Cohort studies ‐ Jeryl Lynn strain ‐ household contacts | Study population | RR 0.26 (0.13 to 0.49) | 1036 (3 observational studies) | ⊕⊕⊕⊝ MODERATE2 | |
| 408 per 1000 | 106 per 1000 (53 to 200) | ||||
| Cohort studies ‐ Urabe strain ‐ unspecified numbers or at least 1 dose | Study population | RR 0.23 (0.12 to 0.44) | 2721 (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 202 per 1000 | 47 per 1000 (24 to 89) | ||||
| Cohort studies ‐ Rubini strain ‐ unspecified numbers or at least 1 dose | Study population | RR 0.96 (0.55 to 1.65) | 4219 (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 202 per 1000 | 194 per 1000 (111 to 334) | ||||
| Cohort studies ‐ mumps strain not reported or any strain | Study population | RR 0.52 (0.29 to 0.94) | 769 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 225 per 1000 | 117 per 1000 (65 to 212) | ||||
| Cohort studies ‐ third dose versus 2 doses | Study population | RR 0.59 (0.33 to 1.05) | 5417 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 7 per 1000 | 4 per 1000 (2 to 8) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level for large effect size (non‐critical risk of bias in studies). 2Upgraded one level for large effect size (non‐critical risk of bias in studies).
Summary of findings 3. Effectiveness against rubella.
| Effectiveness against rubella | |||||
| Patient or population: children 9 months to 15 years old Setting: school Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of rubella amongst unvaccinated | Risk of rubella amongst vaccinated | ||||
| Cohort studies secondary cases ‐ any strain | Study population | RR 0.11 (0.03 to 0.42)1 | 1621 (1 observational study) | ⊕⊕⊕⊝ MODERATE2 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Cohort study in China using the BRD2 strain. 2Upgraded one level for large effect size (non‐critical risk of bias in studies).
Summary of findings 4. Effectiveness against varicella.
| Effectiveness against varicella | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMRV or MMR+V vaccine Comparison: MMR vaccine (RCTs), unvaccinated (cohort studies) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of varicella amongst unvaccinated with MMR vaccine | Risk of varicella amongst vaccinated with MMRV vaccine | ||||
| MMRV randomised controlled trial ‐ any severity ‐ 2 doses ‐ follow‐up at 5 years | Study population | Rate ratio 0.05 (0.03 to 0.08) | 3022 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 271 per 1000 | 14 per 1000 (8 to 22) | ||||
| MMRV randomised controlled trial ‐ any severity ‐ 2 doses ‐ follow‐up between 5 and 10 years | Study population | Rate ratio 0.05 (0.04 to 0.06) | 3023 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 437 per 1000 | 22 per 1000 (17 to 26) | ||||
| MMRV randomised controlled trial ‐ any severity ‐ 2 doses ‐ follow‐up at 10 years | Study population | Rate ratio 0.05 (0.04 to 0.06) | 3023 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 473 per 1000 | 24 per 1000 (19 to 28) | ||||
| MMRV randomised controlled trial ‐ moderate/severe cases ‐ 2 doses ‐ follow‐up at 5 years | Study population | Rate ratio 0.00 (0.00 to 0.02) | 3022 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 157 per 1000 | 0 per 1000 (0 to 3) | ||||
| MMRV randomised controlled trial ‐ moderate/severe cases ‐ 2 doses ‐ follow‐up between 5 and 10 years | Study population | Rate ratio 0.01 (0.00 to 0.02) | 3023 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 237 per 1000 | 2 per 1000 (0 to 5) | ||||
| MMRV randomised controlled trial ‐ moderate/severe cases ‐ 2 doses ‐ follow‐up at 10 years | Study population | Rate ratio 0.01 (0.00 to 0.02) | 3023 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 237 per 1000 | 2 per 1000 (0 to 5) | ||||
| MMR+V randomised controlled trial ‐ any severity ‐ 2 doses ‐ follow‐up at 5 years | Study population | Rate ratio 0.35 (0.28 to 0.43) | 3006 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 271 per 1000 | 95 per 1000 (76 to 116) | ||||
| MMR+V randomised controlled trial ‐ any severity ‐ 2 doses ‐ follow‐up between 5 and 10 years | Study population | Rate ratio 0.33 (0.29 to 0.38) | 3010 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 437 per 1000 | 144 per 1000 (127 to 166) | ||||
| MMR+V randomised controlled trial ‐ any severity ‐ 2 doses ‐ follow‐up at 10 years | Study population | Rate ratio 0.33 (0.29 to 0.38) | 3010 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 473 per 1000 | 156 per 1000 (137 to 180) | ||||
| MMR+V randomised controlled trial ‐ moderate/severe cases ‐ 2 doses ‐ follow‐up at 5 years | Study population | Rate ratio 0.09 (0.06 to 0.14) | 3006 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 157 per 1000 | 14 per 1000 (9 to 22) | ||||
| MMR+V randomised controlled trial ‐ moderate/severe cases ‐ 2 doses ‐ follow‐up between 5 and 10 years | Study population | Rate ratio 0.10 (0.07 to 0.13) | 3010 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 237 per 1000 | 24 per 1000 (17 to 31) | ||||
| MMR+V randomised controlled trial ‐ moderate/severe cases ‐ 2 doses ‐ follow‐up at 10 years | Study population | RR 0.10 (0.08 to 0.14) | 3010 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| 237 per 1000 | 24 per 1000 (19 to 33) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; MMRV: measles, mumps, rubella, and varicella vaccine; MMR+V: concurrent administration of MMR vaccine and varicella vaccine; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 5. Safety: short‐term side effects (local or systemic reactions).
| Safety: short‐term side effects (local or systemic reactions) | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Short‐term side effects amongst unvaccinated | Short‐term side effects amongst vaccinated | ||||
| Temperature ‐ RCT/CCT axillary | Study population | RR 2.04 (1.09 to 3.83) | 420 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| 68 per 1000 | 139 per 1000 (74 to 261) | ||||
| Temperature ‐ RCT/CCT rectal | Study population | RR 0.84 (0.67 to 1.06) | 170 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | |
| 786 per 1000 | 660 per 1000 (526 to 833) | ||||
| Temperature ‐ RCT/CCT measurement site not reported | Study population | RR 1.36 (0.83 to 2.23) | 520 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 182 per 1000 | 247 per 1000 (151 to 405) | ||||
| Temperature ‐ cohort studies orally | Study population | RR 1.37 (1.04 to 1.81) | 334 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 377 per 1000 | 517 per 1000 (392 to 683) | ||||
| Temperature ‐ cohort studies measurement site not reported | Study population | RR 1.12 (0.84 to 1.49) | 457,123 (4 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 31 per 1000 | 35 per 1000 (26 to 46) | ||||
| Rash ‐ cohort studies | Study population | RR 1.49 (0.73 to 3.04) | 457,261 (3 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 4 per 1000 | 6 per 1000 (3 to 13) | ||||
| Lymphadenopathy ‐ RCT/CCT | Study population | RR 1.32 (0.52 to 3.33) | 1156 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | |
| 21 per 1000 | 28 per 1000 (11 to 70) | ||||
| Lymphadenopathy ‐ cohort studies | Study population | RR 1.98 (0.19 to 20.97) | 454,085 (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 0 per 1000 | 1 per 1000 (0 to 6) | ||||
| Coryza ‐ RCT/CCT | Study population | RR 0.45 (0.12 to 1.63) | 831 (2 RCTs) | ⊕⊕⊝⊝ MODERATE 1 | |
| 37 per 1000 | 17 per 1000 (4 to 60) | ||||
| Coryza ‐ cohort studies | Study population | RR 1.13 (1.05 to 1.20) | 3176 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 502 per 1000 | 567 per 1000 (527 to 602) | ||||
| URTI (rhinitis pharyngitis) ‐ RCT/CCT | Study population | RR 0.31 (0.06 to 1.56) | 831 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | |
| 265 per 1000 | 82 per 1000 (16 to 414) | ||||
| URTI (rhinitis pharyngitis) ‐ cohort studies | Study population | RR 1.44 (1.26 to 1.64) | 966 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 2 | |
| 484 per 1000 | 697 per 1000 (610 to 794) | ||||
| Cough ‐ RCT/CCT | Study population | RR 1.99 (0.45 to 8.81) | 831 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | |
| 8 per 1000 | 16 per 1000 (4 to 72) | ||||
| Rash ‐ RCT/CCT | Study population | RR 2.05 (1.21 to 3.48) | 1156 (4 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 52 per 1000 | 107 per 1000 (63 to 182) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CCT: controlled clinical trial; MMR: measles, mumps, rubella vaccine; RCT: randomised controlled trial; RR: risk ratio; URTI: upper respiratory tract infection | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded two levels due to selective reporting (reporting bias). 2Downgraded one level due to low comparability amongst groups.
Summary of findings 6. Safety: encephalitis or encephalopathy.
| Safety: encephalitis or encephalopathy | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of encephalitis or encephalopathy amongst unvaccinated | Risk of encephalitis or encephalopathy amongst vaccinated | ||||
| Case‐control: MMR (risk interval from 0 to 90 days) | Study population | OR 0.98 (0.64 to 1.50) | 452 cases, 1280 controls (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 34 per 1000 | 34 per 1000 (22 to 51) | ||||
| Self‐controlled case series/person‐time cohort | Study population | Rate ratio 0.90 (0.50 to 1.61) | 1,071,088 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 22 per 100,000 | 20 per 100,000 (11 to 36) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 7. Safety: aseptic meningitis.
| Safety: aseptic meningitis | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of aseptic meningitis amongst unvaccinated | Risk of aseptic meningitis amongst vaccinated | ||||
| Case‐control ‐ Jeryl Lynn ‐ risk interval 0 to 30 days | Study population | OR 0.85 (0.21 to 3.41) | 59 cases, 118 controls (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 59 per 1000 | 51 per 1000 (13 to 177) | ||||
| Case cross‐over ‐ Urabe or Hoshino | Study population | OR 4.00 (2.23 to 7.20) | (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Case cross‐over ‐ Jeryl Lynn or Rubini | Study population | OR 0.60 (0.18 to 1.99) | (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ any strain | Study population | Rate ratio 12.40 (3.12 to 49.35) | (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ Urabe | Study population | Rate ratio 30.71 (13.45 to 70.10) | 564,635 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 16 per 100,000 | 490 per 100,000 (214 to 1.117) | ||||
| Self controlled case series ‐ Leningrad‐Zagreb | Study population | Rate ratio 6.40 (0.78 to 52.47) | (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Person‐time cohort ‐ Jeryl Lynn | Study population | Rate ratio 1.30 (0.66 to 2.56) | 1,071,088 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 30 per 100,000 | 39 per 100,000 (20 to 77) | ||||
| Case‐only ecological method ‐ Urabe | Study population | Rate ratio 9.12 (5.73 to 14.52) | 1,054,305 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 9 per 100,000 | 80 per 100,000 (51 to 128) | ||||
| Case‐only ecological method ‐ Leningrad‐Zagreb | Study population | Rate ratio 18.56 (12.09 to 28.51) | 1,164,964 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 100,000 | 0 per 100,000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 8. Safety: seizures (febrile/afebrile).
| Safety: seizures (febrile/afebrile) | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of seizures (febrile/afebrile) amongst unvaccinated | Risk of seizures (febrile/afebrile) amongst vaccinated | ||||
| Cohort studies ‐ within 1 week after MMR vaccination | Study population | Rate ratio 2.45 (2.21 to 2.71) | 1,451,990 (2 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 108 per 1000 | 264 per 1000 (238 to 292) | ||||
| Cohort studies ‐ between 1 and 2 weeks after MMR vaccination | Study population | Rate ratio 3.16 (2.89 to 3.46) | 2,147,638 (2 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 13 per 1000 | 42 per 1000 (38 to 46) | ||||
| Cohort studies ‐ > 2 weeks after MMR vaccination | Study population | Rate ratio 0.97 (0.49 to 1.94) | 1,018,998 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 3 per 1000 | 3 per 1000 (1 to 5) | ||||
| Self‐controlled case series/person‐time ‐ between 1 and 2 weeks after MMR vaccination | Study population | Rate ratio 3.36 (2.65 to 4.24) | 505,493 (5 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series/person‐time ‐ > 2 weeks after MMR vaccination | Study population | Rate ratio 1.18 (0.93 to 1.50) | 102,099 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series/person‐time ‐ between 1 and 2 weeks after vaccination; MMRV | Study population | Rate ratio 6.08 (4.95 to 7.47) | 180,480 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series/person‐time ‐ between 1 and 2 weeks after MMR+V vaccination | Study population | Rate ratio 3.13 (2.38 to 4.10) | 181,088 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| MMRV vs MMR+V ‐ by brand ‐ from 0 to 42 days after vaccination (Priorix‐Tetra) | Study population | RR 1.95 (0.85 to 4.48) | 115,022 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 1 per 1000 | 1 per 1000 (0 to 2) | ||||
| MMRV vs MMR+V ‐ by brand ‐ from 7 to 10 days after vaccination (Priorix‐Tetra) | Study population | RR 1.69 (0.93 to 3.07) | 114,922 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 1 per 1000 | 1 per 1000 (0 to 2) | ||||
| MMRV vs MMR+V ‐ by brand ‐ from 0 to 42 days after vaccination (ProQuad) | Study population | RR 1.30 (1.17 to 1.44) | 1,381,609 (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 2 per 1000 | 2 per 1000 (2 to 3) | ||||
| MMRV vs MMR+V ‐ by brand ‐ from 7 to 10 days after vaccination (ProQuad) | Study population | RR 2.01 (1.70 to 2.38) | 1,381,609 (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 2 per 1000 | 4 per 1000 (3 to 4) | ||||
| MMRV vs MMR ‐ by brand ‐ from 0 to 42 days after vaccination (Priorix‐Tetra) | Study population | RR 1.28 (1.00 to 1.64) | 292,535 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 1 per 1000 | 2 per 1000 (1 to 2) | ||||
| MMRV vs MMR ‐ by brand ‐ from 7 to 10 days after vaccination (Priorix‐Tetra) | Study population | RR 2.49 (1.66 to 3.74) | 292,535 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 1 per 1000 | 3 per 1000 (2 to 5) | ||||
| MMRV vs MMR ‐ by brand ‐ from 0 to 42 days after vaccination (ProQuad) | Study population | RR 1.60 (1.42 to 1.82) | 1,049,831 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 43 per 100,000 | 69 per 100,000 (61 to 78) | ||||
| MMRV vs MMR ‐ by brand ‐ from 7 to 10 days after vaccination (ProQuad) | Study population | RR 1.46 (1.32 to 1.61) | 1,989,157 (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 21 per 100,000 | 30 per 100,000 (28 to 34) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; MMRV: measles, mumps, rubella, and varicella vaccine; MMR+V: concurrent administration of MMR vaccine and varicella vaccine; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level due to large effect size
Summary of findings 9. Safety: autistic spectrum disorders.
| Safety: autistic spectrum disorders | ||||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk of ASD amongst unvaccinated | Risk of ASD amongst vaccinated | |||||
| Cohort studies ‐ all children, MMR | Study population | Rate ratio 0.93 (0.85 to 1.01) | 1,194,764 (2 observational studies) | ⊕⊕⊕⊝ MODERATE1 | ||
| 451 per 100,000 | 419 per 100,000 (383 to 455) | |||||
| Cohort studies ‐ autism risk (low), MMR | Study population | Rate ratio 1.00 (0.89 to 1.14) | 93,071 (1 observational study) | ⊕⊕⊕⊝ MODERATE1 | ||
| 85 per 100,000 | 85 per 100,000 (76 to 97) | |||||
| Cohort studies ‐ autism risk (moderate/high), MMR | Study population | Rate ratio 0.80 (0.64 to 0.98) | 1914 (1 observational study) | ⊕⊕⊝⊝ LOW | The apparent protective effect is due to indication bias. | |
| 12 per 1000 | 9 per 1000 (7 to 11) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASD: autism spectrum disorders; CI: confidence interval; MMR: measles, mumps, rubella vaccine | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Upgraded one level due to residual confounding ‐ confounding expected to increase the effect but no effect was observed.
Summary of findings 10. Safety: inflammatory bowel disease.
| Safety: inflammatory bowel disease | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of IBD amongst unvaccinated | Risk of IBD amongst vaccinated | ||||
| Case control ‐ all IBD, MMR | Study population | OR 1.42 (0.93 to 2.16) | 409 cases, 1416 controls (3 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Case control ‐ ulcerative colitis, MMR | Study population | OR 1.35 (0.81 to 2.23) | 292 cases, 582 controls (2 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Case control ‐ Crohn's disease, MMR | Study population | OR 0.64 (0.42 to 0.98) | 514 cases, 804 controls (3 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IBD: inflammatory bowel disease; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level due to residual confounding ‐ confounding expected to increase the effect but no effect was observed.
Summary of findings 11. Safety: cognitive delay ‐ developmental delay.
| Safety: cognitive delay ‐ developmental delay | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of cognitive delay ‐ developmental delay amongst unvaccinated | Risk of cognitive delay ‐ developmental delay amongst vaccinated | ||||
| Cohort study ‐ MDI‐BSID II 24th month, MMR | Study population | OR 1.35 (0.15 to 12.07) | 337 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Cohort study ‐ MDI‐BSID II 36th month, MMR | Study population | OR 0.37 (0.03 to 4.28) | 337 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Cohort study ‐ Raven 5th year, MMR | Study population | OR 1.22 (0.23 to 6.51) | 337 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Cohort study ‐ WISC‐R verbal 6th year, MMR | Study population | OR 1.23 (0.09 to 16.92) | 337 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MDI‐BSID II: Mental Development Index of Bayley Scales of Infant Development, second edition; MMR: measles, mumps, rubella vaccine; OR: odds ratio; WISC‐R: Wechsler Intelligence Scale for Children, Revised Form | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 12. Safety: idiopathic thrombocytopenic purpura.
| Safety: idiopathic thrombocytopenic purpura | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of ITP amongst unvaccinated | Risk of ITP amongst vaccinated | ||||
| Case‐control ‐ case cross‐over ‐ case controls MMR | Study population | OR 2.80 (1.50 to 5.23) | 410 cases, 2040 controls (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ MMR vaccine ‐ age from 9 to 23 months | Study population | Rate ratio 4.21 (2.28 to 7.78) | 3,723,677 (5 observational studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 17 per 100,000 | 72 per 100,000 (39 to 132) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITP: idiopathic thrombocytopenic purpura; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level due to large effect size
Summary of findings 13. Safety: Henoch‐Schönlein purpura.
| Safety: Henoch‐Schönlein purpura | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of HSP amongst unvaccinated | Risk of HSP amongst vaccinated | ||||
| Case‐control ‐ MMR vaccine | Study population | OR 3.40 (1.18 to 9.81) | 288 cases, 617 controls (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HSP: Henoch‐Schönlein purpura; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 14. Safety: type 1 diabetes.
| Safety: type 1 diabetes | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of type 1 diabetes amongst unvaccinated | Risk of type 1 diabetes amongst vaccinated | ||||
| Cohort study MMR ‐ all children | Study population | Rate ratio 1.09 (0.98 to 1.21) | 1,666,829 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Cohort study MMR ‐ children with at least 1 sibling with type 1 diabetes | Study population | Rate ratio 0.86 (0.34 to 2.16) | 3848 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 6 per 1000 | 5 per 1000 (2 to 12) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 15. Safety: asthma.
| Safety: asthma | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of asthma amongst unvaccinated | Risk of asthma amongst vaccinated | ||||
| Cohort study (rate ratio) ‐ all ages | Study population | Rate ratio 1.05 (0.80 to 1.39) | 1,067,712 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 32 per 1000 | 33 per 1000 (25 to 44) | ||||
| Cohort studies (risk ratio) ‐ all ages | Study population | RR 0.63 (0.24 to 1.63) | 886 (3 observational studies) | ⊕⊕⊝⊝ LOW | |
| 414 per 1000 | 261 per 1000 (99 to 674) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level due to non‐critical risk of bias in the study and large number of participants.
Summary of findings 16. Safety: eczema ‐ dermatitis.
| Safety: eczema ‐ dermatitis | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: vaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of eczema ‐ dermatitis amongst unvaccinated | Risk of eczema ‐ dermatitis amongst vaccinated | ||||
| Cohort study (rate ratio) | Study population | Rate ratio 3.50 (2.38 to 5.15) | 14,353 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Cohort study (risk ratio) | Study population | RR 0.75 (0.29 to 1.94) | 555 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level due to ascertainment bias which seriously weakens confidence in the results.
Summary of findings 17. Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy.
| Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of hay fever, rhinoconjunctivitis, hypersensitivity/allergy amongst unvaccinated | Risk of hay fever, rhinoconjunctivitis, hypersensitivity/allergy amongst vaccinated | ||||
| Cohort study ‐ rhinoconjunctivitis | Study population | OR 0.64 (0.19 to 2.11) | 489 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 211 per 1000 | 146 per 1000 (48 to 360) | ||||
| Cohort study ‐ hypersensitivity/allergy | Study population | OR 0.63 (0.14 to 2.77) | 544 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 429 per 1000 | 321 per 1000 (95 to 675) | ||||
| Case control ‐ hay fever | Study population | OR 1.16 (0.92 to 1.45) | 0 cases, 0 controls (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Upgraded one level due to non‐critical risk of bias in the study.
Summary of findings 18. Safety: acute leukaemia.
| Safety: acute leukaemia | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of acute leukaemia amongst unvaccinated | Risk of acute leukaemia amongst vaccinated | ||||
| Case‐control ‐ acute leukaemia | Study population | OR 0.97 (0.76 to 1.24) | 941 cases, 1667 controls (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Case‐control ‐ acute lymphoblastic leukaemia | Study population | OR 0.91 (0.72 to 1.14) | 1375 cases, 2316 controls (4 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Case‐control ‐ acute myeloblastic leukaemia | Study population | OR 0.56 (0.29 to 1.07) | 62 cases, 1258 controls (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 19. Safety: demyelinating diseases ‐ multiple sclerosis ‐ acute disseminated encephalomyelitis.
| Safety: demyelinating diseases ‐ multiple sclerosis ‐ acute disseminated encephalomyelitis | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of demyelinating diseases ‐ multiple sclerosis ‐ ADEM amongst unvaccinated | Risk of demyelinating diseases ‐ multiple sclerosis ‐ ADEM amongst vaccinated | ||||
| Case‐control ‐ multiple sclerosis | Study population | OR 1.13 (0.62 to 2.05) | 206 cases, 888 controls (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Case‐control ‐ ADEM | Study population | OR 1.03 (0.44 to 2.42) | 272 cases, 1096 controls (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADEM: acute disseminated encephalomyelitis; CI: confidence interval; MMR: measles, mumps, rubella vaccine; OR: odds ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 20. Safety: gait disturbances.
| Safety: gait disturbances | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of gait disturbances amongst unvaccinated | Risk of gait disturbances amongst vaccinated | ||||
| Self‐controlled case series (hospitalisations) ‐ hospitalisations ‐ risk period: 0 to 60 days | Study population | Rate ratio 0.46 (0.16 to 1.34) | 127 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series (GP visits) ‐ GP visit ‐ risk period: 0 to 5 days | Study population | Rate ratio 1.88 (1.30 to 2.72) | 1398 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series (GP visits) ‐ GP visit ‐ risk period: 6 to 60 days | Study population | Rate ratio 0.93 (0.78 to 1.11) | 1398 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GP: general practitioner; MMR: measles, mumps, rubella vaccine | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 21. Safety: bacterial or viral infections, immune overload.
| Safety: bacterial or viral infections, immune overload | |||||
| Patient or population: children 9 months to 15 years old Setting: general population Intervention: MMR vaccine Comparison: unvaccinated | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk of bacterial or viral infections, immune overload amongst unvaccinated | Risk of bacterial or viral infections, immune overload amongst vaccinated | ||||
| Self‐controlled case series ‐ lobar pneumonia ‐ lobar pneumonia risk period (0 to 90 days) | Study population | Rate ratio 0.75 (0.64 to 0.89) | 2412 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ invasive bacterial infections ‐ invasive bacterial infections risk period (0 to 90 days) | Study population | Rate ratio 0.90 (0.71 to 1.13) | 2412 (2 observational studies) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ encephalitis meningitis ‐ encephalitis meningitis risk period (0 to 90 days) | Study population | Rate ratio 0.84 (0.20 to 3.51) | 2025 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ herpes ‐ herpes risk period (0 to 90 days) | Study population | Rate ratio 1.17 (0.56 to 2.46) | 2025 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ pneumonia ‐ pneumonia risk period (0 to 90 days) | Study population | Rate ratio 0.72 (0.32 to 1.60) | 2025 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ varicella zoster ‐ varicella zoster risk period (0 to 90 days) | Study population | Rate ratio 0.93 (0.68 to 1.27) | 2025 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Self‐controlled case series ‐ miscellaneous viral infections ‐ miscellaneous viral infections risk period (0 to 90 days) | Study population | Rate ratio 0.68 (0.43 to 1.08) | 2025 (1 observational study) | ⊕⊕⊝⊝ LOW | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MMR: measles, mumps, rubella vaccine | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1. Effectiveness against measles
Seventeen studies included effectiveness data against measles: 14 cohort studies (ca‐Arciuolo 2017; ca‐Arenz 2005; ca‐Barrabeig 2011a; ca‐Barrabeig 2011b; ca‐Bhuniya 2013; ca‐Choe 2017; ca‐Hales 2016; ca‐La Torre 2017; ca‐Marin 2006; ca‐Marolla 1998; ca‐Musa 2018; ca‐Ong 2007; ca‐Wichmann 2007; ca‐Woudenberg 2017), and 3 case‐control studies (ba‐Defay 2013; ba‐Hungerford 2014; ba‐Jick 2010).
The studies are described in Table 22 and Table 23, and the summary of findings is presented in Table 1.
Evidence from cohort studies
Comparison 1.1 (Analysis 1.1) reports on vaccine effectiveness (VE) from eight cohort studies (ca‐Barrabeig 2011b; ca‐Bhuniya 2013; ca‐Choe 2017; ca‐La Torre 2017; ca‐Marolla 1998; ca‐Musa 2018; ca‐Ong 2007; ca‐Wichmann 2007). The VE = (1 − RR) x 100 after one dose is 95% (95% confidence interval (CI) 87% to 98%) and after two doses 96% (95% CI 72% to 99%). Heterogeneity was 88% and 93% for both subgroups, respectively. After exclusion of the two studies at high risk of bias (ca‐Bhuniya 2013ca‐Choe 2017), heterogeneity was reduced to 32% for the first group and 0% for the second. Overall VE for one dose was 96% (95% CI 93% to 98%) and for two doses 98% (95% CI 96% to 99%).
1.1. Analysis.

Comparison 1: Effectiveness against measles, Outcome 1: Cohort studies (vaccinated vs unvaccinated)
One cohort study evaluated the effectiveness of MMR vaccination in preventing clinical cases of measles in children aged from 18 to 90 months from several local health agencies in Rome, Italy (N = 2745) (ca‐Marolla 1998). Vaccination was performed with three different commercial MMR vaccines, two containing both Schwarz strain (Pluserix and Morupar) and one prepared with Edmonston‐Zagreb strain (Triviraten). One other cohort study investigated the effectiveness of MMR immunisation (composition not reported by study authors) in children aged between 8 and 14 years in preventing laboratory‐confirmed measles cases (ca‐Ong 2007). Two laboratory‐confirmed measles cases occurred amongst the vaccinated children (one dose), whereas seven were observed in the unvaccinated group.
Comparison 1.2 (Analysis 1.2) reports on effectiveness of MMR vaccination in preventing secondary measles cases from three cohort studies (ca‐Arenz 2005; ca‐Hales 2016; ca‐Marin 2006). 'Household contacts' was defined as a person residing in the household during the primary case's infection period. A contact was considered vaccinated (one dose or two doses) if there was a documented record of measles vaccination before the rash onset of the primary case. In ca‐Hales 2016 and ca‐Marin 2006, the VE after one dose was 81% (95% CI 11% to 96%), after two doses 85% (95% CI 25% to 97%), and after three doses 96% (95% CI 77% to 99%). Heterogeneity was 61%, 65%, and 0% for each subgroup, respectively. After excluding one study at high risk of bias (ca‐Hales 2016), heterogeneity was reduced to less than 30% for each subgroup, and VE after one dose was 91% (95% CI 73% to 97%), after two doses 94% (95% CI 81% to 98%), and after three doses 96% (95% CI 69% to 99%). Vaccination with one or two doses of MMR vaccine (composition unknown) was highly effective in preventing secondary cases amongst contacts.
1.2. Analysis.

Comparison 1: Effectiveness against measles, Outcome 2: Cohort studies (household contacts: vaccinated vs unvaccinated)
Comparison 1.3 (Analysis 1.3) reports on effectiveness of MMR vaccination for postexposure prophylaxis from two cohort studies (ca‐Arciuolo 2017; ca‐Barrabeig 2011a). Where candidates for the intervention were susceptible contacts who had not received either measles‐containing vaccine or had not suffered measles, the VE was 74% (95% CI 50% to 86%).
1.3. Analysis.

Comparison 1: Effectiveness against measles, Outcome 3: Cohort studies (postexposure prophylaxis: vaccinated vs unvaccinated)
Evidence from case‐control studies
Comparison 1.4 (Analysis 1.4) reports on vaccine effectiveness from two case‐control studies (ba‐Hungerford 2014; ba‐Jick 2010). One study reported insufficient data for quantitative synthesis (ba‐Defay 2013). The VE after one dose was 51% (95% CI 42% to 59%) and after two doses 61% (95% CI 42% to 74%) (ba‐Jick 2010). One case‐control study was conducted during a measles outbreak amongst children and young previously vaccinated children (ba‐Hungerford 2014). The VE amongst "vaccinate appropriately by age" versus "incomplete or partially vaccinated" was 95% (95% CI 60% to 99%).
1.4. Analysis.

Comparison 1: Effectiveness against measles, Outcome 4: Case‐control studies
2. Effectiveness against mumps
Twenty‐one studies reported effectiveness data against mumps: 14 cohort studies, ca‐Chamot 1998; ca‐Compés‐Dea 2014; ca‐Greenland 2012; ca‐La Torre 2017; ca‐Livingston 2013; ca‐Lopez Hernandez 2000; ca‐Ma 2018; ca‐Marolla 1998; ca‐Nelson 2013; ca‐Ogbuanu 2012; ca‐Ong 2005; ca‐Schlegel 1999; ca‐Snijders 2012; ca‐Takla 2014, and 7 case‐control studies (ba‐Castilla 2009; ba‐Fu 2013; ba‐Giovanetti 2002; ba‐Goncalves 1998; ba‐Harling 2005; ba‐Kim 2012; ba‐Mackenzie 2006). The studies are described Table 24 and Table 25, and the summary of findings are presented in Table 2.
All cohort studies present estimates based on binary data as presented in their papers. Only two cohort studies reported binary data and adjusted estimates by multivariate models (ca‐La Torre 2017; ca‐Snijders 2012). The study by ca‐La Torre 2017 reported a combined (measles‐mumps) adjusted (age and gender) estimate, but binary data were reported separately, and we have included these data in a quantitative synthesis. In ca‐Snijders 2012, VE computed from binary data was 95% for one dose and 96% for two doses, when vaccine effectiveness adjusted estimates were 92% (one dose) and 93% (two doses). We used the method described in Di Pietrantonj 2006 to convert the adjusted effect estimates to adjusted binary data.
Evidence from cohort studies
Comparison 2.1 (Analysis 2.1) reports vaccine effectiveness containing Jeryl Lynn strain from nine cohort studies (ca‐Chamot 1998; ca‐Greenland 2012; ca‐La Torre 2017; ca‐Livingston 2013; ca‐Ma 2018; ca‐Ong 2005; ca‐Schlegel 1999; ca‐Snijders 2012; ca‐Takla 2014). Occurrence of clinical mumps cases during outbreaks was retrospectively evaluated by comparing the incidence of disease amongst children who had been immunised with MMR vaccines containing Jeryl Lynn strain. Three cohort studies evaluated the effectiveness of MMR vaccination in household contacts during an outbreak (ca‐Chamot 1998; ca‐Livingston 2013; ca‐Snijders 2012). One cohort study was conducted during a mumps outbreak amongst university students previously vaccinated (once or twice) (ca‐Greenland 2012). Four studies did not specify numbers of doses (ca‐Chamot 1998; ca‐Livingston 2013; ca‐Ong 2005; ca‐Schlegel 1999). The VE after one dose was 72% (95% CI 38% to 87%) and after two doses 86% (95% CI 73% to 93%). The VE from studies that did not specify numbers of doses was 77% (95% CI 65% to 86%). The VE of MMR vaccination in preventing secondary mumps cases (in household contacts) was 74% (95% CI 51% to 87%).
2.1. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 1: Cohort studies ‐ Jeryl Lynn strain
We excluded ca‐Takla 2014 due to its small sample size, which made this study susceptible to bias and low statistical power. We also excluded ca‐Greenland 2012 due to its particular population. The VE after one dose was 79% (95% CI 52% to 81%) and after two doses 83% (95% CI 62% to 93%).
Comparison 2.2 (Analysis 2.2) reports vaccine effectiveness containing Urabe strain from four cohort studies (ca‐Chamot 1998; ca‐Marolla 1998; ca‐Ong 2005; ca‐Schlegel 1999). In ca‐Marolla 1998, two different MMR vaccines containing Urabe strain were evaluated (Pluserix and Morupar). To avoid data duplication, half of the control arm (206/646) were assigned to the Morupar arm (28/747 versus 103/323) and half to the Pluserix arm (38/329 versus 103/323). None of the studies specified numbers of doses administered. The cohort study ca‐Ong 2005 was carried out in childcare centres and primary schools in Singapore (children aged 5 to 12 years), and the cohort study by ca‐Schlegel 1999 was performed amongst children (aged 5 to 13 years) from a small rural village in Switzerland. The VE (at least one dose) was 77% (95% CI 56% to 88%). The high level of heterogeneity seemed to be due to ca‐Marolla 1998, which showed a significant difference in vaccine effectiveness amongst Pluserix and Morupar arms, and partially due to the ca‐Schlegel 1999 cohort study.
2.2. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 2: Cohort studies ‐ Urabe strain
Comparison 2.3 (Analysis 2.3) reports vaccine effectiveness containing Rubini strain from four cohort studies (ca‐Chamot 1998; ca‐Marolla 1998; ca‐Ong 2005; ca‐Schlegel 1999). None of the studies specified numbers of doses administered. Overall, the studies did not show statistical evidence of vaccine (containing Rubini strain) effectiveness. Only ca‐Marolla 1998 showed statistical evidence in favour of vaccine effectiveness 43% (95% CI 33% to 52%). However, ca‐Ong 2005 showed statistical evidence in favour of the control ‐55% (95% CI ‐122% to ‐9%). The other two studies did not show statistical evidence for vaccine effectiveness (ca‐Chamot 1998; ca‐Schlegel 1999).
2.3. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 3: Cohort studies ‐ Rubini strain
Comparison 2.4 (Analysis 2.4) reports vaccine effectiveness from two cohort studies where mumps strain is not reported or any strain (when in the same study population different participants are vaccinated with different MMR vaccines, each containing different mumps strain, but results by mumps strain were not reported) (ca‐Compés‐Dea 2014; ca‐Lopez Hernandez 2000). The cohort study by ca‐Lopez Hernandez 2000 estimated MMR vaccine effectiveness in preventing clinical mumps in male children aged between 3 and 15 years, attending a scholastic institute in Granada, Spain during an outbreak. Occurrence of clinical mumps cases was compared between children who received at least one dose of MMR vaccine (investigators were not able to determine the vaccine composition), and those who did not receive the MMR vaccine. The cohort study by ca‐Compés‐Dea 2014 was performed during an outbreak of mumps that occurred in high school students aged 16 to 17 years in December 2011. The study compared occurrence of clinical mumps between students previously vaccinated with at least one dose of MMR vaccine and those who did not receive the MMR vaccine (vaccine containing different mumps strains were used: Jeryl Lynn RIT‐4385 and Rubini). The overall VE was 48% (95% CI 6% to 71%).
2.4. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 4: Cohort studies ‐ mumps strain not reported or mixed
Comparison 2.5 (Analysis 2.5) includes two cohort studies that assessed the impact of three doses of MMR vaccine against mumps in children aged 9 to 17 years (ca‐Nelson 2013; ca‐Ogbuanu 2012). The overall risk ratio (RR) was 0.59 (95% CI 0.33 to 1.05). There was no evidence of effect of the third MMR dose administered in children aged between 9 to 17 years.
2.5. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 5: Cohort studies ‐ 3 doses vs 2 doses
Evidence from case‐control studies
Comparison 2.6 (Analysis 2.6) reports vaccine effectiveness containing Jeryl Lynn strain from four case‐control studies (ba‐Castilla 2009; ba‐Fu 2013; ba‐Harling 2005; ba‐Kim 2012). The study by ba‐Kim 2012 was available only as a poster presentation and provides very little information. The overall VE after one dose was 57% (95% CI 30% to 73%), after two doses 81% (95% CI 59% to 91%), and the VE irrespective of the number of doses administered was 65% (95% CI 52% to 75%).
2.6. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 6: Case‐control studies ‐ Jeryl Lynn strain
In ba‐Castilla 2009, case definition considers clinical mumps with laboratory or epidemiological confirmation occurring during an outbreak in the Navarre region of northern Spain between August 2006 and June 2008 in children and adolescents (241 cases and 1205 matched controls). The study authors hypothesised a higher risk of having mumps when the first MMR dose was administered after 36 months of age, odds ratio (OR) 3.11 (95% CI 1.15 to 8.43), or when the two MMR doses were administered more than 36 months apart (OR 10.19, 95% CI 1.47 to 70.73).
Comparison 2.7 (Analysis 2.7) reports vaccine effectiveness containing Jeryl Lynn from one case‐control study (ba‐Harling 2005), where cases included in the study were laboratory‐confirmed (by immunoglobulin M radioimmunoassay, detection of mumps ribonucleic acid (RNA) by polymerase chain reaction (PCR), or both). The VE after one, two, and any dose was 64% (95% CI 41% to 78%), 88% (95% CI 63% to 96%), and 65% (95% CI 24% to 84%), respectively.
2.7. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 7: Case‐control studies ‐ Jeryl Lynn strain ‐ lab‐confirmed cases
Comparison 2.8 (Analysis 2.8) reports vaccine effectiveness on vaccines containing Urabe strain, and Comparison 2.9 (Analysis 2.9) reports on vaccines containing Rubini strain. One case‐control study reported evidence from both strains (ba‐Goncalves 1998), assessing the effectiveness of at least one dose of MMR vaccine in preventing clinical mumps cases during an epidemic in a population of children and adolescents. Significant protection was conferred by the Urabe strain‐containing MMR vaccine (VE 70%, 95% CI 25% to 88%), but not by the Rubini strain‐containing MMR (VE 1%, 95% CI −108% to 53%).
2.8. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 8: Case‐control studies ‐ Urabe strain
2.9. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 9: Case‐control studies ‐ Rubini strain
Comparison 2.10 (Analysis 2.10) reports vaccine effectiveness from two case‐control studies where cases and controls were selected from a population where, because of a changing vaccine schedule, different MMR vaccines with different mumps strains were administered (ba‐Giovanetti 2002; ba‐Mackenzie 2006). ba‐Giovanetti 2002 conducted a field study on MMR vaccination effectiveness (at least one dose) in preventing clinical mumps in a population of children and adolescents. ba‐Mackenzie 2006 attempted to estimate the effectiveness of MMR vaccination against virologically confirmed mumps on students aged 13 to 17 years attending a boarding school in Scotland. The study was not large enough to reach statistical evidence of effect. The overall VE (at least one dose) was 50% (95% CI 19% to 69%).
2.10. Analysis.

Comparison 2: Effectiveness against mumps, Outcome 10: Case‐control studies ‐ strain type not reported or any strain
3. Effectiveness against rubella
Comparison 3.1 (Analysis 3.1) reports vaccine effectiveness from one cohort study that attempted to estimate MMR vaccine effectiveness in a population who received two rubella strain‐based MMR vaccines (ca‐Chang 2015): MMR containing the BRD‐II rubella strain, or MMR containing the RA27/3 rubella strain. The VE was 89% (95% CI 56% to 95%). See Table 26 and Table 3.
3.1. Analysis.

Comparison 3: Effectiveness against rubella, Outcome 1: Cohort studies secondary cases
4. Effectiveness against varicella (MMR+V or MMRV)
Fourteen studies reported effectiveness data against varicella: 3 RCTs (aa‐Henry 2018; aa‐Povey 2019; aa‐Prymula 2014), 4 cohort studies (ca‐Giaquinto 2018; ca‐Rieck 2017; ca‐Spackova 2010; ca‐Tafuri 2013), 4 CCS (ba‐Andrade 2018; ba‐Cenoz 2013; ba‐Liese 2013; ba‐Vazquez 2001), and 3 COEM (ga‐Boccalini 2015; ga‐Pozza 2011; ga‐Tafuri 2015). In ga‐Pozza 2011, data from two independent surveillance systems were reported. The studies are described in Table 27, Table 28, Table 29, and Table 30. The summary of findings are presented in Table 4.
Evidence from RCTs/CCTs
Three multicentre RCTs evaluated vaccine effectiveness of 2 doses in children aged 11 to 22 months against varicella (any severity) and against varicella (moderate/severe) during 3 follow‐up time periods: up to 5 years, between 5 and 10 years, and 10 years (aa‐Henry 2018; aa‐Povey 2019; aa‐Prymula 2014). Each of these studies compared three vaccine types: MMRV (Priorix‐Tetra), MMR (Priorix), and MMR+V (Priorix + Varilrix).
Comparison 4.1 and Comparison 4.2. The overall MMRV vaccine effectiveness against varicella (any severity) after 10 years' follow‐up was 95% (95% CI 94% to 96%) (Analysis 4.1). The vaccine effectiveness against varicella (moderate/severe) was 99% (95% CI 98% to 100%) (Analysis 4.2).
4.1. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 1: MMRV randomised clinical trial ‐ any severity
4.2. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 2: MMRV randomised clinical trial ‐ moderate/severe cases
Comparison 4.3, Comparison 4.4, and Comparison 4.5. The overall MMR+V vaccine effectiveness against varicella (any severity) after 10 years' follow‐up was 67% (95% CI 64% to 70%) (Analysis 4.3); against varicella (moderate/severe) 90% (95% CI 88% to 92%) (Analysis 4.4); and against varicella (severe) 95% (95% CI 53% to 99%) (Analysis 4.5).
4.3. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 3: MMR+V randomised clinical trial ‐ any severity
4.4. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 4: MMR+V randomised clinical trial ‐ moderate/severe cases
4.5. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 5: MMR+V randomised clinical trial ‐ severe cases
Evidence from cohort studies
Comparison 4.6 (Analysis 4.6) reports on MMRV vaccine effectiveness from four cohort studies (ca‐Giaquinto 2018; ca‐Rieck 2017; ca‐Spackova 2010; ca‐Tafuri 2013). One study evaluated one dose of the (MMRV ProQuad) vaccine (ca‐Giaquinto 2018), whilst the rest of the cohorts evaluated MMRV (Priorix‐Tetra). The one‐dose MMRV (ProQuad) vaccine effectiveness against varicella was 94% (95% CI 92% to 96%). The overall MMRV (Priorix‐Tetra) vaccine effectiveness against varicella was 62% (95% CI 61% to 63%) after one dose and 87% (95% CI 86% to 87%) after two doses.
4.6. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 6: MMRV cohort study
Evidence from case‐control studies
Comparison 4.7 (Analysis 4.7) includes one case‐control study evaluating the MMRV (GSK) vaccine effectiveness against varicella (any severity) 86% (95% CI 72% to 93%) and against varicella (moderate/severe) 93% (95% CI 83% to 97%) (ba‐Andrade 2018).
4.7. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 7: MMRV case‐control
Comparison 4.8 (Analysis 4.8) includes three studies evaluating MMR+V versus MMR. The overall VE against varicella (any severity) was 86% (95% CI 78% to 92%) after one dose; 95% (95% CI 86% to 99%) after two doses; and 88% (95% CI 82% to 92%) after at least one dose (ba‐Cenoz 2013; ba‐Liese 2013; ba‐Vazquez 2001).
4.8. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 8: MMR+V case control
Evidence from case‐only ecological method studies
Comparison 4.9 (Analysis 4.9) includes three studies evaluating reduction in the number of hospitalisations before and after introduction of MMRV vaccine in children aged 0 to 14 years (ga‐Boccalini 2015; ga‐Pozza 2011; ga‐Tafuri 2015). The overall vaccine effectiveness (VE = (1 − rate ratio) x 100) in reducing hospitalisation in children aged 0 to 14 years was 57% (95% CI 45% to 66%).
4.9. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 9: MMRV case only ecological method ‐ hospitalisation
Comparison 4.10 (Analysis 4.10) includes two studies evaluating incidence reduction before and after introduction of MMRV vaccine in children aged 0 to 14 years (ga‐Pozza 2011; ga‐Tafuri 2015). The overall vaccine effectiveness (VE = (1 − rate ratio) x 100) in reduced incidence was 76% (95% CI 57% to 86%).
4.10. Analysis.

Comparison 4: Effectiveness against varicella, Outcome 10: MMRV case only ecological method ‐ incidence
However, we note that there was a large difference in efficacy amongst subgroups. The highest efficacy was observed in children aged 1 to 4 years, whilst the smallest efficacy was observed in the subgroup of children aged 0 to 14 years (ga‐Pozza 2011). There was no difference between subgroups aged under 1 year and 5 to 14 years. These differences may be due to different methodological quality amongst studies.
5. Safety: short‐term side effects
Seventeen studies reported data on short‐term side effects after MMR vaccination: 7 RCTs/CCTs, ab‐Bloom 1975; ab‐Ceyhan 2001; ab‐Edees 1991; ab‐Freeman 1993; ab‐Lerman 1981; ab‐Peltola 1986; ab‐Schwarz 1975, and 10 cohorts (cb‐Beck 1989; cb‐Benjamin 1992; cb‐Dunlop 1989; cb‐Makino 1990; cb‐Miller 1989; cb‐Robertson 1988; cb‐Sharma 2010; cb‐Stokes 1971; cb‐Swartz 1974; cb‐Weibel 1980). See Table 31, Table 32, and Table 5.
Evidence from RCTs/CCTs and cohort studies
From RCTs: MMR vaccines were compared with monovalent measles vaccine (ab‐Ceyhan 2001; ab‐Edees 1991; ab‐Lerman 1981), two types of monovalent mumps and rubella vaccines (ab‐Lerman 1981), or placebo (ab‐Bloom 1975; ab‐Lerman 1981; ab‐Peltola 1986; ab‐Schwarz 1975). One trial carried out in twins reported a possible protective effect of the MMR vaccine with a lower incidence of respiratory symptoms, nausea and vomiting, and no difference in the incidence of other unintended side effects compared with placebo, with the exception of irritability (ab‐Peltola 1986). Another trial concluded there was no increased clinical reactivity from an MMR vaccine containing two strains of rubella (ab‐Lerman 1981). ab‐Edees 1991 concluded there was no significant difference in numbers of children developing symptoms after MMR or measles vaccination. Two studies concluded that the incidences of raised temperature, rash, lymphadenopathy, coryza, rhinitis, cough, local reactions, or limb and joint symptoms were not significantly different from children who received placebo (ab‐Bloom 1975; ab‐Schwarz 1975). All RCTs and CCTs reported a wide range of outcomes and used different terms, often with no definitions. For example, body temperature higher than 38 °C was measured or reported in 16 ways. When this information was reported, different temperature increments, recording methods, observation periods, and incidence made comparisons amongst trials and pooling of data impossible. In ab‐Freeman 1993, conducted by 22 family physicians, the occurrence of common symptoms following MMR immunisation (type not described) was assessed by means of weekly diaries amongst participants immunised at 13 and 15 months of age, comparing incidence during the four weeks before with four weeks after immunisation. The incidence of rash, lymphadenopathy, and nasal discharge was found to be higher after exposure to MMR immunisation.
From cohort studies: 10 cohort studies assessed the occurrence of short‐term side effects, comparing MMR vaccine with single measles vaccines (cb‐Dunlop 1989; cb‐Makino 1990; cb‐Miller 1989; cb‐Robertson 1988), mumps‐rubella vaccine (cb‐Swartz 1974), single mumps vaccines (cb‐Makino 1990), single rubella vaccines (cb‐Swartz 1974; cb‐Weibel 1980), placebo (cb‐Beck 1989), or no intervention (cb‐Benjamin 1992; cb‐Sharma 2010; cb‐Stokes 1971). cb‐Benjamin 1992 found that the MMR vaccine was associated with an increased risk of episodes of joint and limb symptoms in girls younger than 5 years of age. There was no difference in the incidence of common outcomes such as fever, rash, lymphadenopathy, cough, arthralgia, myalgia, and anorexia between the MMR vaccine and rubella vaccine (cb‐Makino 1990; cb‐Swartz 1974; cb‐Weibel 1980), mumps‐rubella vaccine (cb‐Swartz 1974), single mumps vaccine (cb‐Makino 1990), or measles vaccine (cb‐Dunlop 1989; cb‐Makino 1990). Two studies found that symptoms were similar following MMR and measles vaccination (cb‐Miller 1989; cb‐Robertson 1988), except for a higher incidence of parotitis following MMR vaccination (cb‐Miller 1989). cb‐Makino 1990 reported a higher incidence of diarrhoea in the MMR vaccines arm compared to the single measles or rubella vaccines arms. Two studies reported no difference in the incidence of rash and lymphadenopathy between MMR vaccination and placebo, cb‐Beck 1989, or no treatment (cb‐Stokes 1971). However, cb‐Stokes 1971 reported an increase in the incidence of fever in the period Day 5 to Day 12 postvaccination, but cb‐Beck 1989 reported no difference. Considering the cohort of cb‐Sharma 2010 only within the subgroup of younger children (16 to 24 months of age), fever during the 42 days' postvaccination was reported more frequently amongst children immunised with MMR than in unvaccinated children. This trend appeared to differ when an older population was considered: fever was reported with slightly higher frequency amongst unvaccinated children.
We performed quantitative synthesis for the most common adverse effects: temperature, rash, lymphadenopathy, coryza, upper respiratory tract infections, and cough. The analysis includes only studies comparing MMR versus placebo (or no treatment). The measure of association between MMR vaccination and specific adverse effect is the risk ratio (RR) and its 95% confidence interval (CI). Results from RCTs and cohort studies are presented separately.
Comparison 5.1 (Analysis 5.1). Seven studies assessed the association between MMR vaccination and temperature: 3 RCTs, ab‐Bloom 1975; ab‐Lerman 1981; ab‐Schwarz 1975, and 4 cohort studies (cb‐Beck 1989; cb‐Benjamin 1992; cb‐Sharma 2010; cb‐Stokes 1971). From RCT data the overall RR was 1.29 (95% CI 0.77 to 2.17). A close value is shown from cohort data (RR 1.16, 95% CI 0.90 to 1.51).
5.1. Analysis.

Comparison 5: Safety: short‐term side effects (local or systemic reactions), Outcome 1: Temperature
Comparison 5.2 (Analysis 5.2). Six studies evaluated the association between vaccination and rash: 3 RCTs, ab‐Bloom 1975; ab‐Lerman 1981; ab‐Schwarz 1975, and 3 cohort studies (cb‐Benjamin 1992; cb‐Sharma 2010; cb‐Stokes 1971). From RCT data the overall RR was 2.05 (95% CI 1.21 to 3.48). However, from cohort studies it was RR 1.49 (95% CI 0.73 to 3.04).
5.2. Analysis.

Comparison 5: Safety: short‐term side effects (local or systemic reactions), Outcome 2: Rash
Comparison 5.3 (Analysis 5.3). Five studies evaluated the association between vaccination and lymphadenopathy: 3 RCTs, ab‐Bloom 1975; ab‐Lerman 1981; ab‐Schwarz 1975, and 2 cohort studies (cb‐Sharma 2010; cb‐Stokes 1971). From RCT data the overall association was RR 1.32 (95% CI 0.52 to 3.33); from cohort studies it was RR 1.98 (95% CI 0.19 to 20.97).
5.3. Analysis.

Comparison 5: Safety: short‐term side effects (local or systemic reactions), Outcome 3: Lymphadenopathy
Comparison 5.4 (Analysis 5.4). Three studies assessed the association between vaccination and coryza: 2 RCTs, ab‐Bloom 1975; ab‐Schwarz 1975, and one cohort study (cb‐Benjamin 1992); the association was RR 0.45 (95% CI 0.12 to 1.63) and RR 1.13 (95% CI 1.05 to 1.20), respectively.
5.4. Analysis.

Comparison 5: Safety: short‐term side effects (local or systemic reactions), Outcome 4: Coryza
Comparison 5.5 (Analysis 5.5). Three studies assessed the association between vaccination and coryza: 2 RCTs, ab‐Bloom 1975; ab‐Schwarz 1975, and one cohort study (cb‐Stokes 1971); the association was RR 0.31 (95% CI 0.06 to 1.56) and RR 1.44 (95% CI 1.26 to 1.64), respectively.
5.5. Analysis.

Comparison 5: Safety: short‐term side effects (local or systemic reactions), Outcome 5: URTI (rhinitis, pharyngitis)
Comparison 5.6 (Analysis 5.6). Two RCTs assessed the association between vaccination and coryza: RR 1.99 (95% CI 0.45 to 8.81) (ab‐Bloom 1975; ab‐Schwarz 1975).
5.6. Analysis.

Comparison 5: Safety: short‐term side effects (local or systemic reactions), Outcome 6: Cough
These results must be interpreted cautiously because different MMR vaccines with different strains were used. However, we found a weak association between MMR vaccination and rash (RCT), coryza (cohort), and upper respiratory tract infections (cohort). We found no evidence of association between MMR vaccine and temperature, lymphadenopathy, and cough.
Safety: severe harms
The association between MMR/MMRV and severe harms (excluding short‐term side effects) was investigated in 70 studies (22 cohort studies, 22 CCS, 13 SCCS, 3 PTC, 2 CCO, 8 COEM). The measure of association between MMR vaccination and specific severe harm is the RR for cohort studies, the OR for case‐control studies, and the rate ratio (rr) for cohort studies, self‐controlled case series, and person‐time cohort studies. Each estimate is reported with its 95% CI.
6. Safety: encephalitis or encephalopathy
The potential association between MMR immunisation and the occurrence of encephalopathies was investigated in three studies: one case‐control study, bb‐Ray 2006, and two SCCS (db‐Makela 2002; db‐Ward 2007). See Table 33 and Table 6.
Evidence from case‐control studies
Comparison 6.1 (Analysis 6.1). bb‐Ray 2006 tested if hospitalisations due to encephalopathy, Reye's syndrome, or encephalitis occurring in children aged 0 to 6 years could be linked to MMR vaccine administration (Table 33). Different time intervals between MMR exposure and date of hospitalisation were considered: 7 to 14 days, 0 to 14 days, 0 to 30 days, 0 to 60 days, and 0 to 90 days (Analysis 6.1). A total of 452 cases together with their 1280 matched controls were included in the analysis. Exposure to the MMR vaccine did not differ statistically between cases and controls for any of the time intervals considered.
6.1. Analysis.

Comparison 6: Safety: encephalitis or encephalopathy, Outcome 1: Case‐control: MMR (risk interval from 0 to 90 days)
Evidence from self‐controlled case series studies
Comparison 6.2 (Analysis 6.2). db‐Makela 2002 was based on a surveillance study by the National Public Health Institute that began after the introduction of MMR vaccination in Finland for children aged 14 to 18 months and 6 years (1982). Participants aged 1 to 7 years (N = 535,544) who received the MMR II vaccine between November 1982 and June 1986 were considered in the study (this population corresponds to 86% of all children scheduled for MMR vaccination in Finland). Risk association was evaluated by comparing the number of hospitalisations for encephalitis or encephalopathy (see Table 33 for the outcome definition) within three months after vaccination, with those occurring during the subsequent seven three‐month intervals. Of 199 hospitalisations for encephalitis or encephalopathy, 9 occurred within 3 months after MMR vaccination, 110 occurred more than 3 months after vaccination (88 between 3 and 24 months), whereas 80 occurred before the vaccine was administered. The trial authors stated that no hospitalisation excess for encephalitis or encephalopathy was observed during the three months' postimmunisation. In db‐Ward 2007, to evaluate the association between encephalitis and MMR vaccination (see Table 33 for case definitions), cases (N = 107) diagnosed between the ages of 2 to 35 months were considered (in Britain and Ireland, the MMR vaccine is scheduled at 12 to 15 months of age). The risk period for encephalitis was considered to be the time between 15 and 35 days following MMR immunisation. The incidence of disease within the risk period was compared with the control period. The incidence of encephalitis in the risk period (15 to 35 days) was not statistically different from the control period (rr 1.34, 95% CI 0.52 to 3.47). This estimate did not change in the presence or absence of primary human herpesvirus 6 (HHV‐6) or HHV‐7 infections. The meta‐analysis estimate of the association between MMR immunisation and encephalitis is rr 0.90 (95% CI 0.50 to 1.61; Analysis 6.2).
6.2. Analysis.

Comparison 6: Safety: encephalitis or encephalopathy, Outcome 2: Self‐controlled case series/person‐time cohort
The meta‐analysis did not provide evidence supporting an association between MMR immunisation and encephalitis or encephalopathy.
7. Safety: aseptic meningitis
The association between MMR vaccine and aseptic meningitis was evaluated in the following 10 studies: 1 case‐control (bb‐Black 1997), 2 CCO (eb‐Ki 2003; eb‐Park 2004), 4 SCCS/PTC (db‐Dourado 2000; db‐Farrington 1995; db‐Miller 2007; db‐Perez‐Vilar 2018), 1 PTC (db‐Makela 2002), and 2 COEM (gb‐da Cunha 2002; gb‐da Silveira 2002). The qualitative synthesis is presented in Table 34. The summary of findings are presented in Table 7.
Evidence from case‐control studies ‐ case cross‐over studies
Comparison 7.1 (Analysis 7.1). In bb‐Black 1997, MMR vaccination within defined intervals before the index date (0 to 14 days, 0 to 30 days, 8 to 14 days) was assessed in cases and controls to assess its association with aseptic meningitis (see Table 34 for outcome definitions). Exposure to the MMR vaccine was not statistically different between cases and controls in any of the considered time intervals. The association between MMR vaccination and aseptic meningitis was evaluated in two case cross‐over studies (eb‐Ki 2003; eb‐Park 2004). MMR containing Urabe strain or MMR vaccine containing Hoshino strain was administered to participants of both studies. The overall association between these MMR vaccines and aseptic meningitis is odds ratio (OR) 4.00 (95% CI 2.23 to 7.20; Analysis 7.1). eb‐Ki 2003 presents data from a subgroup for whom only MMR vaccine containing Jeryl Lynn strain was administered. No association between MMR (Jeryl Lynn) vaccine and aseptic meningitis was shown.
7.1. Analysis.

Comparison 7: Safety: aseptic meningitis, Outcome 1: Case‐control ‐ case cross‐over
Evidence from self‐controlled case‐series/person‐time cohort studies
Comparison 7.2 (Analysis 7.2) includes data from five studies. MMR vaccine containing Urabe strain was used in three studies (db‐Dourado 2000; db‐Farrington 1995; db‐Miller 2007). The overall association between MMR (Urabe) and aseptic meningitis is rr 30.71 (95% CI 13.45 to 70.10). In db‐Makela 2002, no association was shown with MMR II vaccine (Enders‐Edmonston, Jeryl Lynn, Wistar RA 27/3). db‐Perez‐Vilar 2018 was conducted on 26 sentinel sites (49 hospitals) distributed in 16 countries of the 6 World Health Organization (WHO) regions, where different MMR vaccines containing different strains were administered. Data showed no association when MMR containing Lenigrad‐Zagreb was administered.
7.2. Analysis.

Comparison 7: Safety: aseptic meningitis, Outcome 2: Self‐controlled case series (SCCS)/person‐time cohort (PT)
Evidence from case‐only ecological method studies
Comparison 7.3 (Analysis 7.3) includes data from three studies (db‐Dourado 2000; gb‐da Cunha 2002; gb‐da Silveira 2002). MMR with Urabe strain was used in db‐Dourado 2000. MMR with Leningrad‐Zagreb was used in gb‐da Cunha 2002 and gb‐da Silveira 2002. The association between MMR and aseptic meningitis was rate ratio (rr) 9.12 (95% CI 5.73 to 14.52) and rr 18.45 (95% CI 13.26 to 25.56), respectively.
7.3. Analysis.

Comparison 7: Safety: aseptic meningitis, Outcome 3: Case only ecological method (COEM)
The association between MMR vaccination and aseptic meningitis was due to the Urabe or Leningrad‐Zagreb strains. The meta‐analysis showed no evidence of an association between MMR containing Jeryl Lynn strain and aseptic meningitis.
8. Safety: seizures (febrile/afebrile)
Fifteen studies evaluated the association between MMR/MMR+V/MMRV immunisation and seizure (febrile/afebrile). Eight studies compared MMR/MMR+V/MMRV versus placebo or no treatment: 2 cohorts (cb‐Barlow 2001; cb‐Vestergaard 2004), 4 SCCS (db‐Farrington 1995; db‐Macartney 2017; db‐Miller 2007; db‐Ward 2007), and 2 PTC (db‐MacDonald 2014; db‐McClure 2019) (see Table 35). Seven cohort studies compared MMRV versus MMR or MMR+V (cb‐Gavrielov‐Yusim 2014; cb‐Jacobsen 2009; cb‐Klein 2010; cb‐Klein 2012; cb‐Klein 2017; cb‐Rowhani‐Rahbar 2013; cb‐Schink 2014). See Table 36 and Table 8.
Evidence from cohort studies
Comparison 8.1 (Analysis 8.1) includes data from two studies (cb‐Barlow 2001; cb‐Vestergaard 2004). cb‐Vestergaard 2004 is a cohort study assessing the risk of febrile seizure after the introduction of routine MMR vaccination in Denmark in 1987 (Table 35). Globally, the risk of febrile seizure was significantly higher amongst vaccinated children (RR 1.10, 95% CI 1.05 to 1.15). When different time frames after vaccination were considered, the RR was at the highest point within two weeks after immunisation (RR 2.75, 95% CI 2.55 to 2.97). The RR did not differ significantly in weeks 3 to 6, and was slightly less than 1 in weeks 7, 8, 9 to 26 and 27 to 52. Amongst children with personal history of febrile seizure, the RR was 2.75 (95% CI 2.32 to 3.26) (adjusted for age, calendar period, age at first febrile seizure) compared with non‐vaccinated children with personal history of febrile seizure. For evaluation of long‐term prognosis, the number of recurrent episodes of febrile seizure and the cases of epilepsy observed in children who received MMR vaccination within 14 days before their first febrile seizure episode, and in those who were vaccinated more than 14 days before their first febrile seizure episode, were compared with those who were not vaccinated at the time of their first febrile seizure episode. A significant risk association was found only for recurrent febrile seizure episodes in children who were immunised with MMR within 14 days before the first episode (RR 1.19, 95% CI 1.10 to 1.41) adjusted for age, calendar period, age at first febrile seizure, and current vaccination status. cb‐Barlow 2001 was a cohort study conducted at four large health maintenance organisations. The study showed statistical evidence of association (within two weeks) between MMR immunisation and febrile seizures. However, there was no evidence of an association with afebrile seizures (RR 1.11, 95% CI 0.11 to 11.28).
8.1. Analysis.

Comparison 8: Safety: seizures (febrile/afebrile), Outcome 1: Cohort studies
The overall RR of having febrile seizures within two weeks after MMR immunisation was 3.16 (95% CI 2.89 to 3.46).
Evidence from self‐controlled case series/person‐time cohort studies
Comparison 8.2 (Analysis 8.2) includes evidence from six studies (db‐Farrington 1995; db‐Macartney 2017; db‐MacDonald 2014; db‐McClure 2019; db‐Miller 2007; db‐Ward 2007). db‐Farrington 1995 shows the rr estimates of febrile seizures amongst people vaccinated with the MMR containing Jeryl Lynn strain and people vaccinated with the MMR containing Urabe strain. The seizure risk associate to MMR (Urabe) was rr 3.77 (95% CI 1.95 to 7.30) within 6 to 11 days, and rr 1.04 (95% CI 0.56 to 1.93) within 15 to 35 days. We only included data from MMR (Jeryl Lynn). db‐Miller 2007 shows the rr estimates of febrile seizures for MMR II vaccine (Jeryl Lynn) and MMR Priorix (RIT 4385). Both estimates were included. In db‐Miller 2007, the risk incidence of febrile convulsion was also analysed considering a more specific definition (Table 37). Considering all MMR vaccine types, the risk incidence remained higher in the 6 to 11 days following vaccination (rr 4.27, 95% CI 3.17 to 5.76), whereas at 15 to 35 days following vaccination it remained at borderline significance (rr 1.33, 95% CI 1.00 to 1.77). db‐McClure 2019 reported data for two vaccines (MMR and MMRV) stratified by gestational age (born before 37 weeks, born ≥ 37 weeks). db‐MacDonald 2014 analysed the risk of febrile seizure amongst people vaccinated with MMRV and people vaccinated with MMR+V; the rr estimates of febrile seizures for each vaccine (MMRV and MMR+V) were presented stratified in two subcohorts (low risk, high risk).
8.2. Analysis.

Comparison 8: Safety: seizures (febrile/afebrile), Outcome 2: Self‐controlled case series/person‐time cohort
The overall rr of having febrile seizures within two weeks after MMR immunisation was 3.36 (95% CI 2.65 to 4.24; Analysis 8.2). No evidence of association was shown beyond two weeks (rr 1.18, 95% CI 0.93 to 1.50). The rr was 6.08 (95% CI 4.95 to 7.47) within two weeks after MMRV immunisation and 3.13 (95% CI 2.38 to 4.10) after MMR+V immunisation.
Evidence from cohort studies ‐ MMRV versus (MMR+V or MMR)
Of seven cohort studies evaluating the risk of having febrile seizures after immunisation with MMRV, four cohort studies evaluated MMRV ProQuad (Merck and Co, USA) (cb‐Jacobsen 2009; cb‐Klein 2010; cb‐Klein 2012; cb‐Rowhani‐Rahbar 2013), and two cohort studies evaluated MMRV Priorix‐Tetra (GSK) (cb‐Gavrielov‐Yusim 2014; cb‐Schink 2014). See Table 36.
Comparison 8.3 (Analysis 8.3). MMRV versus MMR+V includes evidence from five cohort studies (cb‐Jacobsen 2009; cb‐Klein 2010; cb‐Klein 2012; cb‐Rowhani‐Rahbar 2013; cb‐Schink 2014). The studies estimated the risk of febrile seizures after MMRV vaccination compared to MMR+V vaccination. The overall estimate was RR 1.31 (95% CI 1.19 to 1.45) within 42 days after vaccination and RR 1.98 (95% CI 1.69 to 2.33) within 7 to 10 days after vaccination.
8.3. Analysis.

Comparison 8: Safety: seizures (febrile/afebrile), Outcome 3: MMRV versus MMR+V
Comparison8.4 (Analysis 8.4). The RR including only MMRV (Priorix‐Tetra) studies was 1.95 (95% CI 0.85 to 4.48) within 0 to 42 days after vaccination, and RR 1.69 (95% CI 0.93 to 3.07) between 7 and 10 days after vaccination. Including only MMRV (ProQuad) studies, the RR was 1.30 (95% CI 1.17 to 1.44) within 0 to 42 days after vaccination and 2.01 (95% CI 1.70 to 2.38) between 7 and 10 days after vaccination.
8.4. Analysis.

Comparison 8: Safety: seizures (febrile/afebrile), Outcome 4: MMRV versus MMR+V ‐ by brand
Comparison 8.5 (Analysis 8.5). MMRV versus MMR includes evidence from six cohort studies (cb‐Gavrielov‐Yusim 2014; cb‐Klein 2010; cb‐Klein 2012; cb‐Klein 2017; cb‐Rowhani‐Rahbar 2013; cb‐Schink 2014). The studies estimated the risk of febrile seizures after MMRV vaccination compared to MMR vaccination. The overall RR was 1.53 (95% CI 1.37 to 1.71) within 42 days after vaccination and RR 1.50 (95% CI 1.36 to 1.66) within 7 to 10 days after vaccination.
8.5. Analysis.

Comparison 8: Safety: seizures (febrile/afebrile), Outcome 5: MMRV versus MMR
Comparion 8.6 (Analysis 8.6). The RR including only MMRV (Priorix‐Tetra) studies was 1.28 (95% CI 1.00 to 1.64) within 0 to 42 days after vaccination, and 2.49 (95% CI 1.66 to 3.74) between 7 and 10 days after vaccination. However, including only MMRV (ProQuad) studies, the RR was 1.60 (95% CI 1.42 to 1.82) within 0 to 42 days after vaccination, and 1.46 (95% CI 1.32 to 1.61) between 7 and 10 days after vaccination.
8.6. Analysis.

Comparison 8: Safety: seizures (febrile/afebrile), Outcome 6: MMRV versus MMR ‐ by brand
To correctly interpret the associations between MMR/MMRV/MMRV (containing Jeryl Lynn strain) vaccines and febrile seizures, we must consider that vaccine‐induced febrile seizures is an infrequent event, amongst both non‐vaccinated and vaccinated people. cb‐Gavrielov‐Yusim 2014 reported that febrile seizures normally occur in 2% to 4% of healthy children at least once before the age of five years. cb‐Vestergaard 2004 showed a risk difference (RD) of febrile seizures amongst vaccinated and unvaccinated people equal to 0.16% (95% CI 0.14% to 0.17%), and reported a 0.25% absolute cumulative risk of febrile seizures amongst vaccinated people. db‐MacDonald 2014 and db‐McClure 2019 showed a cumulative risk amongst vaccinated people ranging from 0.15% to 0.29%. The attributable risk was estimated to be 1:1700 doses, db‐Farrington 1995, and 1:1150 doses (db‐Miller 2007). db‐McClure 2019 found no difference in RR of febrile seizures by gestational age.
9. Safety: autism spectrum disorders
Thirteen studies investigated the hypothesised link between MMR vaccination and autism spectrum disorders: 4 cohorts (cb‐Hviid 2019; cb‐Jain 2015; cb‐Madsen 2002; cb‐Uchiyama 2007), 4 case‐control (bb‐De Stefano 2004; bb‐Mrozek‐Budzyn 2010; bb‐Smeeth 2004; bb‐Uno 2012), 1 SCCS (db‐Taylor 1999), 1 PTC (db‐Makela 2002), and 3 COEM (gb‐Fombonne 2001; gb‐Fombonne 2006; gb‐Honda 2005). See Table 37 and Table 9.
Evidence from cohort studies
Four retrospective cohort studies investigated the risk of autism and pervasive developmental disorders following MMR immunisation (Table 37) (cb‐Hviid 2019; cb‐Jain 2015; cb‐Madsen 2002; cb‐Uchiyama 2007). Two studies were conducted in Denmark and included all Danish children born between January 1991 and December 1998, and 1999 to December 2010, respectively (cb‐Hviid 2019; cb‐Madsen 2002). The study authors linked vaccination data reported by the National Board of Health with a diagnosis of autism (Table 37) from the Danish Psychiatric Central Register. cb‐Jain 2015 was conducted in the USA and included children born between 2001 to 2012. Data are presented stratified by age (2‐, 3‐, 4‐year‐olds received first dose, 5‐year‐olds received the first and second dose) and subdivided in two subgroups: low risk of autism (older sibling without autism spectrum disorder) and moderate/high risk of autism (older sibling with autism spectrum disorder). The retrospective cohort study cb‐Uchiyama 2007 assessed the association between exposure to MMR vaccination and regression in autistic spectrum disorders. Participants were children with an autism spectrum disorder diagnosis (Table 37) from a private paediatric psychiatric clinic located in Yokohama City, Japan (Yokohama Psycho‐Developmental Clinic, YPCD), which has become recognised as a centre for autism spectrum disorders. Cases of autism spectrum disorders in people born between 1976 and 1999 were considered for study purposes. Regression in autism spectrum could be assessed for 325/904 children who were identified with disorders. Data were analysed in different ways. Within the MMR vaccine generation group, odds ratio (OR) estimates were calculated considering the cases of deterioration observed in children who had received the MMR vaccine from the Mental Child Health Handbook (15/54), and the number of regressions observed amongst participants who did not receive the MMR vaccine (45/132), after exclusion of those with unknown vaccination status (N = 89). Study authors reported a non‐significant OR 0.74 (95% CI 0.35 to 1.52) in people who had received the MMR vaccine versus no MMR vaccination in the MMR period. Furthermore, the OR estimate was calculated considering as the control group (not MMR vaccinated) also both pre‐ and post‐MMR generation groups. Estimates were non‐significant: OR 0.63 (95% CI 0.32 to 1.20). Comparison of regression cases observed within the MMR generation group (independent from documented vaccination status) with that observed in pre‐MMR, post‐MMR, and pre‐ plus post‐MMR groups provided no statistically significant OR estimates. According to the data reported by cb‐Uchiyama 2007, there was no evidence supporting an association between MMR immunisation and autism spectrum disorders (see Table 37). We did not include data in the quantitative synthesis because the study authors did not state which statistical model had been adopted.
Comparison 9.1 (Analysis 9.1) includes evidence from cb‐Hviid 2019, cb‐Jain 2015, and cb‐Madsen 2002.
9.1. Analysis.

Comparison 9: Safety: autism spectrum disorders, Outcome 1: Cohort studies
The meta‐analysis did not provide evidence supporting an association between MMR immunisation and autism spectrum disorder in all children (rr 0.93, 95% CI 0.85 to 1.01). The meta‐analysis did not provide evidence supporting an association between MMR immunisation and autism spectrum disorders amongst low‐risk children (RR 1.00, 95% CI 0.89 to 1.14).
The analysis shows statistical evidence of a protective effect of MMR vaccine amongst high‐risk children (rr 0.80, 95% CI 0.64 to 0.98). This result is clearly due to the effect of indication bias. In previous years, children who had an older sibling with an autism spectrum disorder diagnosis were less likely to be vaccinated. Conversely, children who have an older sibling with an autism spectrum disorder diagnosis have a high risk of autism spectrum disorder diagnosis.
Evidence from case‐control studies
Four case‐control studies investigated the risk of an association between the MMR vaccine and autism (bb‐De Stefano 2004; bb‐Mrozek‐Budzyn 2010; bb‐Smeeth 2004; bb‐Uno 2012) (Table 37). bb‐Smeeth 2004 assessed the association between exposure to the MMR vaccine and the onset of autism and other pervasive developmental disorders (Table 37). The study was based on data from the UK's General Practice Research Database (GPRD), which was established 1 June 1987. bb‐De Stefano 2004 compared the distribution of ages at first MMR vaccination in children with autism (Table 37) cases and controls, divided into three age strata: up to 18, 24, and 36 months. In bb‐Mrozek‐Budzyn 2010, cases of autism in children aged between 2 and 15 years were identified by means of general practitioners' records from Małopolska Province in southern Poland (Table 37). For each case, two controls matching for birth year, gender, and practice were selected. A total of 92 cases with childhood or atypical autism and 192 matched controls were included. Estimate ORs were calculated considering vaccine exposure (MMR or monovalent measles) before autism diagnosis or before onset of symptoms, separately in univariate and multivariate analyses (balanced for mother's age ≥ 35 years, gestation time ≤ 38 weeks, medication during pregnancy, perinatal injuries, and 5‐minute Apgar score). The bb‐Uno 2012 study analysed case data from patients of the Yokohama Psycho‐Developmental Clinic; the cases consisted of children who were diagnosed with autism spectrum disorders born between 1 April 1984 and 30 April 1992, the possible time period for MMR vaccination.
Comparison 9.2 (Analysis 9.2). The meta‐analysis did not provide evidence supporting an association between MMR immunisation and autism spectrum disorders in children vaccinated at any age (18 months to 15 years) (OR 0.62, 95% CI 0.36 to 1.09).
9.2. Analysis.

Comparison 9: Safety: autism spectrum disorders, Outcome 2: Case‐control
The meta‐analysis did not provide evidence supporting an association between MMR immunisation and autism spectrum disorders if the vaccine was administered before 18 months (OR 0.91, 95% CI 0.75 to 1.11) or after 18 months (OR 0.80, 95% CI 0.61 to 1.05).
The meta‐analysis did not provide evidence supporting an association between MMR immunisation and autism spectrum disorders if the vaccine was administered before 36 months (OR 0.94, 95% CI 0.74 to 1.18) or after 36 months (OR 0.77, 95% CI 0.55 to 1.08).
Evidence from self‐controlled case series/person‐time cohort studies
In db‐Makela 2002, described in the section related to neurological diseases, an attempt to evaluate the association between MMR vaccination and hospitalisation for autism was made (Table 37). Unlike for encephalitis and aseptic meningitis, instead of a risk period, changes in the overall number of hospitalisations for autism after MMR vaccination, including only the first hospital visit during the study period, were considered. Times between immunisation and hospitalisation observed amongst the 309 hospitalisations for autism following MMR immunisation were very wide (range 3 days to 12 years and 5 months); their numbers remained relatively steady during the first 3 years and then decreased gradually. No cluster intervals from vaccination could be identified. The study authors concluded that there was no evidence of association, but did not report statistical data supporting this conclusion. Another SCCS assessed clustering of cases of autism by postexposure periods in a cohort of 498 (with 293 confirmed cases) children (db‐Taylor 1999). The study authors reported a significant increase in onset of parental concern at 6 months postvaccination, but no significant clustering of interval to diagnosis or regression was found within any of the considered time periods (2, 4, 6, 12, 24 months).
Comparison 9.3 (Analysis 9.3) includes data from db‐Taylor 1999. The results showed no evidence supporting an association between MMR immunisation and autism spectrum disorder diagnosis or regression (autism spectrum disorder diagnosis < 12 months: rr 0.94, 95% CI 0.60 to 1.47; autism spectrum disorder diagnosis < 24 months: rr 1.09, 95% CI 0.79 to 1.52; regression < 2 months: rr 0.92, 95% CI 0.38 to 2.21; regression < 4 months: rr 1.00, 95% CI 0.52 to 1.95; and regression < 6 months: rr 0.85, 95% CI 0.45 to 1.60).
9.3. Analysis.

Comparison 9: Safety: autism spectrum disorders, Outcome 3: Self‐controlled case series/person‐time cohort
Evidence from case‐only ecological method studies
gb‐Fombonne 2001 tested several causal hypotheses and mechanisms of association between exposure to MMR vaccination and pervasive developmental disorders (Table 37). The population was made up of three cohorts of participants; one was of older children acting as the control (pre‐MMR vaccination introduction). The study authors concluded that there was no evidence that pervasive developmental disorders had become more frequent; the mean age at parental concern had not moved closer to the date of exposure to MMR vaccination. Furthermore, the study authors concluded that there was no evidence that regression with autism had become more common. The parents of children with autism regression did not become concerned about their child in a different time frame than children without regression; children with regressive autism did not have different profiles or severity to those in the control group. There was no evidence that regressive autism was associated with inflammatory bowel disorders. gb‐Fombonne 2006 analysed the trend of pervasive developmental disorder prevalence in cohorts born from 1987 to 1998 attending schools in southern and western Montreal (N = 27,749; 1 October 2003). The relationship between pervasive developmental disorder prevalence trends and MMR vaccination coverage through each birth cohort was assessed. Children with pervasive developmental disorders (N = 180) were identified only if their diagnosis was specifically stated as autism and autism spectrum disorder to allow the schools to receive incremental funding. The study authors reported that whilst a significant trend towards a decrease in MMR uptake through birth cohorts from 1988 to 1998 (Chi² for trend = 80.7; df = 1; P < 0.001) could be assessed, a significant increase in rates of pervasive developmental disorders from 1987 to 1998 was found (OR 1.10, 95% CI 1.05 to 1.16; P < 0.001). By comparing the rate of increase in pervasive developmental disorder prevalence between the one‐ and two‐dose period, no statistically significant differences were detected.
A Japanese study assessed the autism spectrum disorders incidence trend amongst birth cohorts from 1988 to 1996 in Yokohama City in children aged up to 7 years (gb‐Honda 2005). gb‐Honda 2005 assessed the incidence trend in relation to decline of MMR vaccination coverage in the same birth cohorts (before and after termination of MMR vaccination programmes in children in 1993). Examination of risk factor analysis with conditional regression detected a significant increase in cumulative incidence of all autism spectrum disorders amongst birth cohorts from 1988 to 1996 (Chi² = 45.17, df = 8, P < 0.001). This trend was different before and after the 1992 birth cohort: considering the 1996 birth cohort as a reference, incidence of all autism spectrum disorders was significantly lower until 1992 and did not differ after 1993. A significantly increased incidence could be assessed when outcomes definition of childhood autism (Chi² = 31.86, df = 8, P < 0.001) or other autism spectrum disorder (Chi² = 19.25, df = 8, P = 0.01) were considered. The study authors concluded that causal hypothesis involving the MMR vaccine as a risk factor was not supported by the evidence because autism spectrum disorder incidence continued to increase even if the MMR vaccination programme was terminated.
Comparison 9.4 (Analysis 9.4) includes data from gb‐Honda 2005. The analysis showed statistical evidence of a protective effect of MMR vaccine against childhood autism (rr 0.45, 95% CI 0.33 to 0.62); against other autism spectrum disorders (rr 0.55, 95% CI 0.39 to 0.80); and against all autism spectrum disorders (rr 0.49, 95% CI 0.39 to 0.63). These results are surely due to effect of the indication bias.
9.4. Analysis.

Comparison 9: Safety: autism spectrum disorders, Outcome 4: Case only ecological method
The meta‐analysis did not provide evidence supporting an association between MMR immunisation and autism spectrum disorders.
10. Safety: inflammatory bowel disease
Six studies considered the hypothesis of an association between MMR vaccination and inflammatory bowel disease (IBD) or Crohn's disease and ulcerative colitis: 4 case‐control studies, bb‐Baron 2005; bb‐Davis 2001; bb‐Shaw 2015; bb‐Vcev 2015, and 2 COEM (gb‐Seagroatt 2005; gb‐Taylor 2002). See Table 38 and Table 10.
Evidence from case‐control studies
bb‐Baron 2005 was conducted in France between January 1988 and December 1997. Cases were all patients from the EPIMAD (Epidemiology of Inflammatory Bowel Disease) registry who had a diagnosis of either Crohn's disease or ulcerative colitis and were aged under 17 years. bb‐Davis 2001 was conducted in the USA using data from the Vaccine Safety Datalink (versusD). Cases were patients born between 1958 and 1989. bb‐Shaw 2015 was conducted in Canada University of Manitoba IBD Epidemiology Database (UMIBDED) linked to the Manitoba Immunization Monitoring System. All paediatric IBD cases in Manitoba, born after 1989 and diagnosed before 31 March 2008, were included. bb‐Vcev 2015 was conducted in Croatia. IBD patients (> 18 years old) were identified according to the hospital’s patient records. This study has different methodological limitations, a small number of cases, and a weak control for confounders. The region where the study was conducted was affected by the war in Croatia between 1991 and 1997, and experienced large demographic changes during the war and long postwar period.
Comparison 10.1 (Analysis 10.1). The meta‐analysis estimates did not provide evidence supporting an association between MMR immunisation and IBD (OR 1.42, 95% CI 0.93 to 2.16) or an association between MMR and ulcerative colitis (OR 1.35, 95% CI 0.81 to 2.23). Crohn's disease data showed a protective effect (OR 0.64, 95% CI 0.42 to 0.98).
10.1. Analysis.

Comparison 10: Safety: inflammatory bowel disease (IBD), Outcome 1: Case‐control
Evidence from case‐only ecological method studies
gb‐Seagroatt 2005 investigated a possible association between the MMR vaccine and Crohn's disease. Using national data on emergency admissions from England, the authors compared admissions for Crohn's disease in populations with a vaccination coverage of ≥ 84% with populations with MMR vaccination coverage of ≥ 7%. Even if age‐specific rates of emergency admission for Crohn's disease increased during the time considered in the study (April 1991 to March 2003), this trend seems not to have been influenced by the introduction of the MMR vaccine. The introduction of the MMR vaccination programme in England did not increase the risk of Crohn's disease. gb‐Taylor 2002 is linked to db‐Taylor 1999, as the study includes children with childhood and atypical autism born between 1979 and 1998, to investigate whether MMR vaccination is associated with bowel problems and developmental regression in children with autism.
Comparison 10.1 (Analysis 10.2) includes data from gb‐Seagroatt 2005. Results did not show evidence supporting an association between MMR immunisation and Crohn's disease (rr 0.95, 95% CI 0.84 to 1.08).
10.2. Analysis.

Comparison 10: Safety: inflammatory bowel disease (IBD), Outcome 2: Case‐only ecological method (rate ratio)
Comparison 10.2 (Analysis 10.3) includes data from gb‐Taylor 2002. Results did not show evidence supporting an association between MMR immunisation and IBD (in children with autism) (OR 0.98, 95% CI 0.89 to 1.07).
10.3. Analysis.

Comparison 10: Safety: inflammatory bowel disease (IBD), Outcome 3: Case only ecological method (odds ratio)
11. Safety: cognitive delay/developmental delay
The cohort study cb‐Mrozek‐Budzyn 2013 examined the hypothesis that MMR exposure could have a negative influence on cognitive development in children. The Mental Development Index of Bayley Scales of Infant Development, second edition (MDI‐BSID‐II) was administered in the 24th and 36th months of life. The Raven's Colored Scale was administered in the fifth year of life. The Wechsler Intelligence Scale for Children, Revised Form (WISC‐R) was administered in the sixth year of life. See Table 39 and Table 11.
Comparison 11.1 (Analysis 11.1). The estimates did not show evidence supporting an association between MMR vaccine and cognitive development in children.
11.1. Analysis.

Comparison 11: Safety: cognitive delay ‐ developmental delay, Outcome 1: Cohort study
12. Safety: idiopathic thrombocytopenic purpura
Nine studies investigated a suspected association between MMR vaccination and idiopathic thrombocytopenic purpura (ITP): 2 case‐control studies (bb‐Bertuola 2010; bb‐Black 2003), 5 SCCS (db‐Andrews 2012; db‐Farrington 1995; db‐France 2008; db‐O'Leary 2012; db‐Perez‐Vilar 2018), 1 CCO (eb‐Lafaurie 2018), and 1 COEM (gb‐Jonville‐Bera 1996). See Table 40 and Table 12.
Evidence from case‐control and case cross‐over studies
bb‐Black 2003 was a matched case‐control study conducted in children aged 12 to 23 months. The cases were patients with a diagnosis of ITP. The controls were selected within data contained in the General Practice Research Database (GPRD). bb‐Bertuola 2010 tested the association between acute immune thrombocytopenia and MMR vaccination by means of a case‐control design in children and adolescents (aged 1 month to 18 years). eb‐Lafaurie 2018 was a population‐based case cross‐over study. See Table 40.
Comparison 12.1 (Analysis 12.1). The overall meta‐analysis estimate from case‐control studies showed statistical evidence of an association between the MMR vaccination and ITP (OR 2.80, 95% CI 1.50 to 5.23). The estimate from the case cross‐over study showed statistical evidence of an association (OR 1.62, 95% CI 1.21 to 2.16).
12.1. Analysis.

Comparison 12: Safety: idiopathic thrombocytopenic purpura, Outcome 1: Case‐control ‐ case cross‐over
Evidence from self‐controlled case series/person‐time cohort studies
The study by db‐France 2008 was based on data contained in the Vaccines Safety Datalink project from 1991 to 2000, covering eight managed care organisations across the USA. By consulting the database, 63 children aged 12 to 23 months who met the definition (Table 40) could be identified. The incidence rate ratio between the exposed and unexposed time was calculated using two different analytical methods: the self‐controlled case series and the 'risk interval' (i.e. person‐time cohort) method. For the latter method, the estimate rate ratio was rr 3.94 (95% CI 2.01 to 7.69) in children aged 12 to 23 months, and 7.10 (95% CI 2.03 to 25.03) in children aged 12 to 15 months (the age at which about 80% of MMR vaccinations were administered). To avoid data duplication, we included only data from SCCS designs in the meta‐analysis. db‐Andrews 2012 was a multicountry collaboration (England and Denmark) study. db‐O'Leary 2012 involved five healthcare systems. db‐Perez‐Vilar 2018 was conducted on 26 sentinel sites (49 hospitals) in 16 countries of the six WHO regions, that is the Western Pacific region, the South‐East Asia region, the Americas region, the European region, the Eastern Mediterranean region, and the African region.
Comparison 12.2 (Analysis 12.2). The overall meta‐analysis estimate of association between MMR vaccination and ITP in children aged 9 to 23 months was rr 4.21 (95% CI 2.28 to 7.78). There was no statistical evidence in children aged 4 to 6 years (rr 3.06, 95% CI 0.42 to 22.30), and no statistical evidence of association between MMRV vaccination and ITP in children aged 9 to 23 months (rr 2.87, 95% CI 0.78 to 10.56). The latter two results came from one study (db‐O'Leary 2012).
12.2. Analysis.

Comparison 12: Safety: idiopathic thrombocytopenic purpura, Outcome 2: Self‐controlled case series
Evidence from case‐only ecological method studies
The evidence of association between MMR or any of its component vaccines and the onset of thrombocytopenic purpura was also assessed in one ecological study (gb‐Jonville‐Bera 1996). The study concluded that the evidence favoured an association, but in all cases thrombocytopenic purpura appeared to be a benign, self‐limiting condition not distinguishable from its idiopathic counterpart or from thrombocytopenic purpura occurring after natural infection with MMR. The study discussed the weakness of relying on the passive reporting system for the identification of cases and acknowledged a possible under‐reporting of cases of thrombocytopenic purpura.
The results confirm an association between MMR vaccination and ITP. However, the risk of ITP after vaccination is smaller than the one after natural infection with these viruses (bb‐Bertuola 2010; Cecinati 2013). bb‐Bertuola 2010 reported that natural infection of ITP occurs in 5 cases per 100,000 children per year, with a prevalence of 4 to 6 per 100,000. The attributable risk was estimated to be about 1 ITP case per 40,000 administered MMR doses (Cecinati 2013; db‐Andrews 2012; db‐France 2008). bb‐Black 2003 and db‐Farrington 1995 estimate the attributable risk of ITP within six weeks after MMR vaccination about 1 case per 25,000 (95% CI 21,300 to 89,400).
13. Safety: Henoch‐Schönlein purpura
One case control study estimated the association of Henoch‐Schönlein purpura with drug and vaccine (MMR and diphtheria, tetanus, and pertussis (DTaP) vaccine) administration in a paediatric population (bb‐Da Dalt 2016). See Table 41 and Table 13.
Comparison 13.1 (Analysis 13.1). The estimate showed statistical evidence of an association between MMR vaccine and Henoch‐Schönlein purpura (OR 3.40, 95% CI 1.18 to 9.81).
13.1. Analysis.

Comparison 13: Safety: Henoch‐Schönlein purpura, Outcome 1: Case‐control
The result confirmed an association between MMR and Henoch‐Schönlein purpura. However, Henoch‐Schönlein purpura is the most common vasculitis in childhood with an incidence of 10 to 20 cases per 100,000 in children under 17 years, with a peak incidence of 70 cases per 100,000 in the 4‐ to 6‐year age group (bb‐Da Dalt 2016).
14. Safety: type 1 diabetes
Two cohort studies reported on type 1 diabetes (cb‐Beyerlein 2017; cb‐Hviid 2004). See Table 42 and Table 14.
cb‐Beyerlein 2017 analysed data from two German birth cohorts of healthy neonates with a familial increased risk of type 1 diabetes, the BABYDIAB study and the BABYDIET natural follow‐up study, which were combined for association analyses of vaccination patterns and the development of islet autoimmunity. Between 1989 and 2000, a total of 1650 children of people with type 1 diabetes were recruited. Between 2000 and 2006, 791 additional children or siblings of people with type 1 diabetes were screened and followed up. cb‐Hviid 2004 was a retrospective cohort study carried out in Denmark aiming to evaluate if there was an association between childhood vaccinations and the onset of type 1 diabetes. A cohort of children born between 1 January 1990 and 31 December 2000 from the Danish Civil Registration System was recruited.
Comparison 14.1 (Analysis 14.1). The overall meta‐analysis result did not provide evidence supporting an association between MMR vaccination and type 1 diabetes (rr 1.09, 95% CI 0.98 to 1.21). In addition, restricting the analysis to children with at least one sibling with type 1 diabetes did not show evidence of an association (rr 0.86, 95% CI 0.34 to 2.16).
14.1. Analysis.

Comparison 14: Safety: type 1 diabetes, Outcome 1: Cohort study MMR
15. Safety: asthma
Five cohort studies reported on asthma (cb‐Benke 2004; cb‐DeStefano 2002; cb‐Hviid 2008; cb‐McKeever 2004; cb‐Timmermann 2015). See Table 43 and Table 15.
As the studies provided insufficient information to enable us to convert rate ratio (hazard ratio) into RR, we performed two meta‐analyses: Analysis 15.1 includes cb‐DeStefano 2002, cb‐Hviid 2008, and cb‐McKeever 2004, where rate ratio was adopted as the effect measure, and Analysis 15.2 includes cb‐Benke 2004 and cb‐Timmermann 2015, where RR was adopted.
15.1. Analysis.

Comparison 15: Safety: asthma, Outcome 1: Cohort study (rate ratio)
15.2. Analysis.

Comparison 15: Safety: asthma, Outcome 2: Cohort study (risk ratio)
The cohort study cb‐McKeever 2004 used an historical birth cohort of children (from 1988 to 1999) consisting of 29,238 children of both sexes aged between 0 and 11 years and identified through the West Midlands General Practice Research Database (GPRD), to investigate the association between MMR and diphtheria, polio, pertussis, and tetanus (DPPT) vaccination and asthma or eczema (Table 43). Incident diagnoses of asthma/wheeze and eczema (Table 43) were identified using the relevant Oxford Medical Information System (OMIS, derived from the International Classification of Diseases, Revision 8 (ICD‐8)) and Read codes (a hierarchical code used in general practitioner (GP) practices in England). Association with MMR vaccine exposure and risk of asthma was assessed by univariate analyses. Adjusted hazard ratios (HR) were 2.20 (95% CI 1.50 to 3.21) for asthma. Stratifying for GP consultation frequency in the first 18 months, HR estimates remained significant only for the subgroup with lower consulting frequency (0 to 6 times in the first 18 months), and not for the other subgroups (7 to 10 times, 11 to 16 times, and more than 16 times): HR 7.18 (95% CI 2.95 to 17.49) for an association between MMR vaccination and asthma. cb‐Hviid 2008 shows a protective effect of MMR vaccination against asthma hospitalisation and anti‐asthma medications (Table 43). The study was conducted on Danish birth cohorts from 1991 to 2003 using the Danish Civil Registration System. Each participant recorded in the register had an identification number that allowed a link to data contained in other national registers (Danish National Hospital Register, Danish Prescription Drug Database, and National Board of Health). MMR vaccination status was considered as a time‐varying variable, and individuals could contribute to person‐time as both unvaccinated and vaccinated participants. MMR vaccination is protective against all asthma hospitalisations (RR 0.75, 95% CI 0.73 to 0.78); the protective effect of vaccination was greater in younger children (no more significant when the vaccine was administered after 18 months of age), in those with the longest time spent in hospital (18 days to 1 year), in girls, in low‐birthweight children, in children with 1 older sibling, and in those living in rural areas. Vaccination was also protective against hospitalisation for severe asthma (RR 0.63, 95% CI 0.49 to 0.82), even if estimates were not significant within the following stratifications: aged 3 to 4 years; fully immunised children; low hospitalisation propensity; male sex; birthweight below 2499 g or above 4000 g; birth order >/= 3; or born in the capital or in a rural area. Total use of anti‐asthma medications was less frequent amongst participants immunised with MMR (RR 0.92, 95% CI 0.91 to 0.92). No reduction in use of all medications was observed for participants vaccinated between 23 and 26 months old (RR 1.00, 95% CI 0.98 to 1.01) or at 27 months old or later (RR 1.01, 95% CI 0.99 to 1.03). Considering single classes of medication in the unstratified study population, these data were confirmed with the exception for systemic beta2‐agonists, for which reduction in use was not observed (RR 1.02, 95% CI 1.01 to 1.02). Considering only the first use of any anti‐asthma medication in the unstratified population, the RR was 0.93 (95% CI 0.92 to 0.94). Also, cb‐Timmermann 2015 showed a protective effect against asthma. The study was conducted on a birth cohort of consecutive, spontaneous births in the Faroe Islands from 1997 to 2000.
Comparison 15.1 (Analysis 15.1). The overall rr estimate did not provide evidence supporting an association between asthma diagnosis and MMR vaccination (rr 1.05, 95% CI 0.80 to 1.39). Excluding a study at high risk of bias, the new estimate did not show evidence of association (rr 0.85, 95% CI 0.66 to 1.10).
Comparison 15.2 (Analysis 15.2). The overall RR estimate did not provide evidence supporting an association between asthma diagnosis and MMR vaccination (RR 0.63, 95% CI 0.24 to 1.63). Excluding a study at high risk of bias, the new estimate based on cb‐Timmermann 2015 showed evidence of a protective effect of MMR vaccination against asthma (RR 0.39, 95% CI 0.22 to 0.70).
The results did not show evidence supporting an association between MMR vaccination and asthma risk. The association between MMR vaccination and asthma found by cb‐McKeever 2004 appeared to be limited to the minority of children. This limited association is more likely to be the result of bias than a biological effect.
16. Safety: eczema ‐ dermatitis
Two cohort studies reported data on dermatitis/eczema (cb‐McKeever 2004; cb‐Timmermann 2015). See Table 44 and Table 16.
The cb‐McKeever 2004 cohort study used an historical birth cohort of children from 1988 to 1999 consisting of 29,238 children of both sexes aged between 0 and 11 years and identified through the West Midlands General Practice Research Database (GPRD) to investigate the association between MMR and DPPT vaccination and asthma or eczema (Table 44). Incident diagnoses of asthma/wheeze and eczema (Table 44) were identified using the relevant Oxford Medical Information System (OMIS, derived from ICD‐8) and Read codes (a hierarchical code used in GP practices in England). Association with MMR vaccine exposure and the risk of asthma and eczema was assessed by univariate analysis. Correspondent adjusted rate ratio was 3.50 (95% CI 2.38 to 5.15) for eczema (Analysis 16.1). Stratifying for GP consultation frequency in the first 18 months, HR estimates remained significant only for the subgroup with lower consulting frequency (0 to 6 times in the first 18 months) and not for the other subgroups (7 to 10 times, 11 to 16 times, and more than 16 times) for the association between MMR vaccination and asthma (HR 7.18, 95% CI 2.95 to 17.49) and the association between MMR vaccination and eczema (HR 10.4, 95% CI 4.61 to 23.29). Instead, cb‐Timmermann 2015 did not show evidence of an association between MMR vaccination and risk of eczema (RR 0.75, 95% CI 0.29 to 1.94; Analysis 16.2).
16.1. Analysis.

Comparison 16: Safety: eczema ‐ dermatitis, Outcome 1: Cohort study (rate ratio)
16.2. Analysis.

Comparison 16: Safety: eczema ‐ dermatitis, Outcome 2: Cohort study (risk ratio)
Data suggest that currently MMR vaccinations are not a risk factor for eczema. The association found between MMR vaccination and eczema by cb‐McKeever 2004 appeared to be limited to a small subset of children. This limited association is more likely to be the result of bias than a biological effect.
17. Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy
Three studies reported data on hay fever/rhinoconjunctivitis/allergy: 1 cohort study, cb‐Timmermann 2015, and 2 case‐control studies (bb‐Bremner 2005; bb‐Bremner 2007). See Table 45 and Table 17.
Evidence from cohort studies
Comparison 17.1 (Analysis 17.1). The estimate did not provide evidence supporting an association between MMR vaccination and rhinoconjunctivitis (OR 0.64, 95% CI 0.19 to 2.11).
17.1. Analysis.

Comparison 17: Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy, Outcome 1: Cohort study ‐ rhinoconjunctivitis
Comparison 17.2 (Analysis 17.2). The estimate did not provide evidence supporting an association between MMR vaccination and hypersensitivity/allergy (OR 0.63, 95% CI 0.14 to 2.77).
17.2. Analysis.

Comparison 17: Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy, Outcome 2: Cohort study ‐ hypersensitivity/allergy
Evidence from case‐control studies
The two case‐control studies investigated the risk of hay fever in MMR‐vaccinated children in the UK (using the same data source) (bb‐Bremner 2005; bb‐Bremner 2007). The bb‐Bremner 2005 study focused particular attention on the timing of MMR vaccination to identify a critical period for MMR immunisation and hay fever risk (see Table 45 for definitions). The nested case‐control study was conducted within two large databases, the General Practice Research Database (GPRD) and Doctors’ Independent Network (DIN), and involved 7098 hay fever cases and controls. Data were reported by month of life (1st to 13th; 14th, 15th, 16th to 17th, 18th to 24th, and > 25th) by database (GPRD and DIN). bb‐Bremner 2007 specifically investigated if exposure to MMR vaccination during the first grass pollen season of life influences the risk of hay fever more than any other time of the year. The study was conducted within GPRD and DIN databases and involved 7098 hay fever cases matched with controls.
Comparison 17.3 (Analysis 17.3). The overall meta‐analysis estimate did not provide evidence supporting an association between MMR vaccination and hay fever (OR 1.16, 95% CI 0.92 to 1.45). The results showed that infants vaccinated with MMR are not at a greater or lesser risk of developing hay fever or rhinoconjunctivitis than unvaccinated children.
17.3. Analysis.

Comparison 17: Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy, Outcome 3: Case‐control ‐ hay fever
18. Safety: acute leukaemia
Four case‐control studies reported data on acute leukaemia (bb‐Dockerty 1999; bb‐Groves 1999; bb‐Ma 2005; bb‐Mallol‐Mesnard 2007). See Table 46 and Table 18.
Four case‐control studies assessed whether vaccination with MMR (and other vaccines) played a role in the aetiology of leukaemia in children aged between 0 and 14 years (Table 46) (bb‐Dockerty 1999; bb‐Groves 1999; bb‐Ma 2005; bb‐Mallol‐Mesnard 2007).
Comparison 18.1 (Analysis 18.1). The overall meta‐analysis estimate did not provide evidence supporting an association between MMR vaccination and acute leukaemia (OR 0.97, 95% CI 0.76 to 1.24) or acute lymphoblastic leukaemia (OR 0.91, 95% CI 0.72 to 1.14). Moreover, the overall estimate did not provide evidence supporting an association with acute myeloblastic leukaemia (OR 0.56, 95% CI 0.29 to 1.07).
18.1. Analysis.

Comparison 18: Safety: acute leukaemia, Outcome 1: Case‐control
The results showed no evidence of an association between MMR vaccination and the risk of leukaemia.
19. Safety: demyelinating diseases, multiple sclerosis, acute disseminated encephalomyelitis
The possible association between the MMR vaccine and demyelinating diseases was assessed in three studies: 1 cohort study, cb‐Ahlgren 2009, and 2 case‐control studies (bb‐Ahlgren 2009; bb‐Chen 2018). See Table 47 and Table 19.
Two studies used the same population data set (bb‐Ahlgren 2009; cb‐Ahlgren 2009). cb‐Ahlgren 2009 was a cohort study carried out in the Gothenburg area (Swedish west coast, 731,592 residents on 31 December 2000). Cases of multiple sclerosis and clinically isolated syndrome in participants born between 1959 and 1990 with onset between 10 and 39 years of age before July 1984 amongst Gothenburg residents were considered, corresponding to a total of 5.9 million person‐years of observation (Table 47). The incidence of probable or definite multiple sclerosis (Poser criteria) and clinically isolated syndrome (372 and 162 cases, respectively) was analysed in corresponding MMR vaccination programmes, by selecting four birth cohorts corresponding to the first years of a specific vaccination programme.
Birth cohorts 1962 to 1966 (102 multiple sclerosis cases): administration of the monovalent rubella vaccine to 12‐year‐old girls in 1974.
Birth cohorts 1970 to 1973 (62 multiple sclerosis cases): administration of the MMR vaccine at 12 years of age (1982).
Birth cohorts 1974 to 1978 (37 multiple sclerosis cases): administration of monovalent measles vaccine in preschool children. (It was already introduced in 1971, thus adequate coverage was reached only for those born in 1974 and onwards). About 90% of participants from these birth cohorts received the MMR vaccine at 12 years of age.
Born between July 1981 and June 1984 (five multiple sclerosis cases): administration of the MMR vaccine at 18 months and 12 years of age.
The incidence of multiple sclerosis and clinically isolated syndrome within each birth cohort was compared to that calculated for the preceding ones, including that of 1959 to 1961, corresponding to the pre‐vaccine era. No significant changes in age and gender‐specific incidence of multiple sclerosis between selected and preceding selected cohorts was observed. The authors used the same population incidence data in order to assess an association between MMR exposure and multiple sclerosis onset by means of a case‐control design (bb‐Ahlgren 2009). Similar to the cohort study, case definitions included multiple sclerosis or clinically isolated syndrome according to Poser's criteria, residence in Gothenburg, birth date between 1959 and 1986, and disease onset from the age of 10 years onwards. For analysis of vaccine exposure, only cases and controls who attended the sixth grade in school (12 years) within the study area, for whom child health and school health records were available (206 cases and 888 controls), were included.
Evidence from case‐control studies
Comparison 19.1 (Analysis 19.1). The estimate did not show evidence supporting an association between MMR vaccination and multiple sclerosis (OR 1.13, 95% CI 0.62 to 2.05). The estimate did not show evidence supporting an association between MMR vaccination and acute disseminated encephalomyelitis (OR 1.03, 95% CI 0.44 to 2.42).
19.1. Analysis.

Comparison 19: Safety: demyelinating diseases ‐ multiple sclerosis ‐ acute disseminated encephalomyelitis, Outcome 1: Case‐control
The results did not show evidence supporting an association between MMR vaccination and the risk of demyelinating diseases.
20. Safety: gait disturbance
An association between MMR vaccination and gait disturbance was assessed by means of an SCCS, db‐Miller 2005, and considered as cases of hospital admissions (Analysis 20.1) or general practice consultations (Analysis 20.2) in children from the Thames regions of England. Hospital admission cases were obtained from hospital computerised records from April 1995 to June 2001 and considered those relative to children aged 12 to 24 months with ICD‐10 diagnoses related to acute gait disorder (G111, G112, G25, R26, R27, R29, H55, and F984). Cases were validated by reviewing hospital case notes and were grouped into five categories. See Table 48 and Table 20.
20.1. Analysis.

Comparison 20: Safety: gait disturbances, Outcome 1: Self‐controlled case series (hospitalisations)
20.2. Analysis.

Comparison 20: Safety: gait disturbances, Outcome 2: Self‐controlled case series (GP visits)
The vaccination history of cases was obtained from immunisation records. In all, 127 cases with available immunisation status were identified. Of these, 65 belonged to category 4 (i.e. non‐ataxic, non‐viral origin) and were excluded from analysis. No cases corresponding to category 1 definition were found.
Evidence from self‐controlled case series
Comparison 20.1 (Analysis 20.1). The rr within and outside postvaccination time risk (0 to 30 and 31 to 60 days) was calculated after age stratification in one‐month intervals. Rate ratio (rr) estimates for pooled 2, 3, and 5 categories showed no evidence of an association between MMR vaccination and hospitalisations for gait disturbance for 0 to 30 days' risk time (rr 0.83, 95% CI 0.24 to 2.86); 31 to 60 days' risk time (rr 0.20, 95% CI 0.03 to 1.40); and 0 to 60 days' risk time (rr 0.46, 95% CI 0.16 to 1.34).
As gait disturbance does not require hospitalisation, the authors carried out a further analysis based on cases observed in general practices using the General Practice Research Database (GPRD) as the source, and considered children aged 12 to 24 months, born between 1988 and 1997. Read and OXMIS codes indicating a possible consult for gait disturbance were identified in the GPRD by mapping ICD‐9 codes and by searching keywords 'ataxia', 'gait', 'co‐ordination', 'mobility', and 'movement'. Diagnoses were grouped into six categories (Table 48). Vaccination history was obtained from prescription records. In all, 1398 children with diagnoses A to F and known immunisation history were included.
Comparison 20.2 (Analysis 20.2). The relative incidence (RI) within and outside postvaccination time risk (0 to 5, 6 to 30, 31 to 60 days) was calculated. Rate ratio (rr) estimate for 0 to 5 days' risk time shows evidence of association between MMR vaccination and hospitalisations for gait disturbance (rr 1.88, 95% CI 1.30 to 2.72). However, estimates in any other risk period showed no evidence of association for 6 to 30 days' risk time (rr 0.90, 95% CI 0.70 to 1.16); 31 to 60 days' risk time (rr 0.95, 95% CI 0.76 to 1.18); and 6 to 60 days' risk time (rr 0.93, 95% CI 0.78 to 1.11). Early administration of thiomersal‐containing diphtheria, tetanus, and pertussis (DTP)/ diphtheria tetanus (DT) vaccine did not influence this estimate.
The results did not show evidence supporting an association between MMR vaccination and gait disturbance.
In the study authors' opinion, a vaccine‐specific effect would appear one week after immunisation. An excess of B and C diagnoses was observed on vaccination day, caused by an excess of consultations on the day that MMR was given. It is biologically implausible that any specific MMR effect would manifest on the day of vaccination since the viraemia induced by the vaccine, which might produce symptoms, does not start until the end of the first week (db‐Miller 2005).
21. Safety: bacterial or viral infections, immune overload
The incidence of viral and bacterial infection following MMR administration was investigated by means of a SCCS design by db‐Miller 2003 and db‐Stowe 2009. See Table 49 and Table 21.
Episodes of hospitalisation for bacterial or viral infections occurring in children aged between 12 and 23 months were identified by consulting computerised hospital admission records from southern England using ICD‐9 or ICD‐10 codes between April 1991 and March 1995 (db‐Miller 2003); and occurring in children aged between 12 and 23 months were identified by consulting computerised hospital admission records from North, East, and South London, Essex, East Anglia, Sussex, and Kent using ICD‐9 or ICD‐10 codes and covering the time between 1 April 1995 and 1 May 2005 (db‐Stowe 2009). Bacterial infections were characterised as lobar pneumonia or invasive bacterial infection, whereas those of viral aetiology were encephalitis/meningitis, herpes, pneumonia, varicella zoster, or miscellaneous virus (Table 49). Admissions were linked to date of MMR (and meningococcal) immunisation resulting from records held on child health systems. 'At risk' time periods were considered to be the whole risk period (0 to 90) days after immunisation, and subperiods: (0 to 30), (31 to 60), and (61 to 90) days after immunisation.
Comparison 21.1 (Analysis 21.1). The overall meta‐analysis estimate showed that admissions for lobar pneumonia were less frequent in the time between 0 and 90 days after MMR immunisation (protective effect of the MMR vaccine) (rr 0.75, 95% CI 0.64 to 0.89).
21.1. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 1: Self‐controlled case series ‐ lobar pneumonia
Comparison 21.2 (Analysis 21.2). The estimate did not show evidence supporting an association between MMR vaccination and risk of hospitalisations due to invasive bacterial diseases (rr 0.90, 95% CI 0.71 to 1.13) for the whole risk period (0 to 90 days). In addition, no evidence of an association was shown considering the other risk subperiods.
21.2. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 2: Self‐controlled case series ‐ invasive bacterial infections
Comparison 21.3 (Analysis 21.3). The estimate did not show evidence supporting an association between MMR vaccination and encephalitis/meningitis (rr 0.84, 95% CI 0.20 to 3.51) for the whole risk period (0 to 90 days) and other risk subperiods.
21.3. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 3: Self‐controlled case series ‐ encephalitis meningitis
Comparison 21.4 (Analysis 21.4). The risk of hospitalisation due to herpes infection was higher in the risk time interval between 31 and 60 days after MMR vaccine administration (rr 1.69, 95% CI 1.06 to 2.70), but this risk was not statistically significant. Data showed no evidence of association considering the other risk subperiods and the whole risk period (0 to 90 days) (rr 1.17, 95% CI 0.56 to 2.46).
21.4. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 4: Self‐controlled case series ‐ herpes
Comparison 21.5 (Analysis 21.5). The estimate did not show evidence supporting an association between MMR vaccination and hospitalisations due to pneumonia (rr 0.72, 95% CI 0.32 to 1.60) for the whole risk period (0 to 90 days) and the other risk subperiods.
21.5. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 5: Self‐controlled case series ‐ pneumonia
Comparison 21.6 (Analysis 21.6). A significantly lower incidence of varicella zoster was assessed within 30 days after MMR immunisation (protective effect) (rr 0.58, 95% CI 0.34 to 0.99). However, the estimate did not show evidence supporting an association considering the whole risk period (rr 0.93, 95% CI 0.68 to 1.27) and other subperiods.
21.6. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 6: Self‐controlled case series ‐ varicella zoster
Comparison 21.7 (Analysis 21.7). The estimate did not show evidence supporting an association between MMR vaccination and hospitalisations due to other viral infections (rr 0.68, 95% CI 0.43 to 1.08) for the whole risk period (0 to 90 days) and the other risk subperiods. No statistically significant risk of both bacterial and viral infection was detected following concomitant administration of MMR and meningococcal C vaccine.
21.7. Analysis.

Comparison 21: Safety: bacterial or viral infections, immune overload, Outcome 7: Self‐controlled case series ‐ miscellaneous viral infections
The studies confirmed that the MMR vaccine does not increase the risk of invasive bacterial or viral infection in the 90 days after the vaccination and does not support the hypothesis that there is an induced immune deficiency due to overload from multi‐antigen vaccines (db‐Miller 2003; db‐Stowe 2009).
Discussion
Summary of main results
MMR vaccination is ≥ 95% effective in preventing clinically confirmed measles in preschool children. Effectiveness is 95% after one dose (7 cohort studies, n = 12,039) and 96% after two doses (5 cohort studies n = 21,604). The estimates were similar for each of the two measles strains with which participants had been immunised (Schwarz or Edmonston‐Zagreb, 1 cohort study, n = 2745). Effectiveness in preventing secondary measles cases amongst household contacts or preventing transmission of measles to people with which the children were in contact was 81% after one dose (3 cohort studies, 151 participants), 85% after two doses (3 cohort studies, 378 participants), and 96% after three doses (2 cohort studies, 151 participants). The effectiveness of MMR vaccination (at least one dose) in preventing measles after postexposure prophylaxis (at least one dose) was 74% (2 cohort studies, 283 participants). The effectiveness of Jeryl Lynn‐containing MMR vaccine in preventing clinical mumps in children and adolescents was 72% after one dose (6 cohort studies, 9915 participants) and 86% after two doses (5 cohort studies, 7792 participants). The effectiveness of Jeryl Lynn‐containing MMR vaccine in preventing mumps being passed on to contacts was 74% (3 cohort studies, 1036 participants). The Urabe strain was also effective at 77% (4 cohort studies, 2721 participants).
We found no evidence of effect from administering a third MMR dose to prevent mumps among children aged between 9 and 17 years (2 cohort studies, N = 5417). There is an acceptably high effectiveness of the vaccine prepared only with Urabe or Jeryl Lynn strain, but not for vaccines containing the Rubini strain. MMR vaccination effectiveness against rubella is 89%, (1 cohort study, N = 1621). However, this is based on only one cohort study in China using the BRD2 strain (ca‐Chang 2015). This strain is not used anywhere else in the world, and higher vaccine effectiveness has been reported with other strains. MMRV vaccination effectiveness against varicella (any severity) after two doses is 95%; effectiveness against varicella (moderate/severe) is 99%. MMR+V vaccination effectiveness is 67% against any severity of varicella. Effectiveness is 90% against moderate/severe varicella, and 95% against severe varicella (1 RCT, N = 2279).
Association with aseptic meningitis is confirmed for MMR vaccines containing Urabe and Leningrad‐Zagreb mumps strains on the basis of two very large studies at unclear risk of bias, carried out on about 2 million children aged 1 to 11 years and assessing a significant increased risk in the time between 1 and 10 weeks after immunisation, peaking within the third or fifth week. No evidence of association was found for vaccines prepared with mumps Jeryl Lynn strains in results from one case‐control study and one self‐controlled case series study.
We have identified associations between MMR/MMRV/MMRV (containing Jeryl Lynn strain) vaccines and febrile seizures (15 studies, N = 2,166,172). To correctly interpret this association, we must consider that vaccine‐induced febrile seizures is an infrequent event, both amongst non‐vaccinated and vaccinated people. cb‐Gavrielov‐Yusim 2014 reported that febrile seizures normally occur in 2% to 4% of healthy children at least once before the age of 5 years. The risk difference (RD) of febrile seizures amongst vaccinated and unvaccinated was RD 0.16% (95% CI 0.14% to 0.17%). The cumulative risk of having a febrile seizure after vaccination ranges from 0.15% to 0.29%. The attributable risk is estimated to be from 1:1700 to 1:1150 MMR administered doses.
The results confirm an association between MMR vaccination and idiopathic thrombocytopenic purpura (ITP). However, the risk of ITP after vaccination is smaller than the risk after natural infection with these viruses. bb‐Bertuola 2010 reported that natural infection of ITP occurs in 5 cases per 100,000 children per year, with a prevalence of 4 to 6 per 100,000. The attributable risk is estimated to be about 1 ITP case per 40,000 administered MMR doses. The studies estimated the attributable risk of ITP within six weeks after MMR vaccination to be about 1 case/25,000 (95% CI 1/21,300 to 1/89,400) doses. The result confirms an association between MMR and Henoch‐Schönlein purpura. However, Henoch‐Schönlein purpura is the most common vasculitis in childhood with an incidence of 10 to 20 cases per 100,000 in children under 17 years of age, with a peak incidence of 70 cases per 100,000 in the 4‐ to 6‐year age group. Association with acute or idiopathic thrombocytopenic purpura within six weeks of immunisation is assessed in nine studies (n = 6300), but vaccine composition is described in only three studies (db‐Farrington 1995; db‐Perez‐Vilar 2018; gb‐Jonville‐Bera 1996).
Based on the included studies, the meta‐analysis does not provide evidence supporting an association between MMR immunisation and the following conditions: encephalitis or encephalopathy (3 studies, around 500,000 children), autism spectrum disorders (13 studies, around 2 million children), inflammatory bowel disease/Crohn's disease (6 studies, N = 2385 children), cognitive delay (1 study, N = 369 children), type 1 diabetes (2 studies, around 770,000 children), asthma (5 studies, around 1 million children), dermatitis/eczema (2 studies, around 15,000 children), hay fever (3 studies, around 120,000 children), leukaemia (4 studies, N = 4318 children), demyelinating diseases/multiple sclerosis (3 studies, around 730,000 children), gait disturbance (1 study, N = 1525 children), and bacterial or viral infections (2 studies, N = 2412 children).
Overall completeness and applicability of evidence
Internal and external validity of included studies has improved in recent years (Table 51).
Quality of the evidence
Of the 138 included studies, we classified 36% as at low risk of bias with reliable results; 42% as at unclear risk of bias due to a problematic aspect of the study (generally selection bias), but the results remain sufficiently reliable; and 22% as at high risk of bias (Figure 3), for which we found problematic internal validity, and the biases present in the studies (selection, performance, attrition, detection, and reporting) influenced our confidence in their findings. The most common type of bias was selection bias. We analysed reasons presented in the papers to justify missing data. Whilst we accepted as adequate such explanations as 'non‐response to questionnaire' and 'medical records unavailable', not all reports offered adequate explanations for missing data. The overall quality assessment by study design is shown in Table 50 and by publication year in Table 51.
Of the 51 studies on MMR effectiveness, 42 were funded by public or government institutions, and only 5 by the pharmaceutical industry. Of the 87 studies on MMR/MMRV safety, 65 were funded by public or government institutions, 9 by the pharmaceutical industry, and 10 studies were funded in part by industry and in part by government or public institutions.
Potential biases in the review process
There are some weaknesses in our review. The age limit of participants, although substantially justified by public health concerns about the effects of vaccination on the developing child, did lead us to exclude some studies on this basis alone. Additionally, the methodological quality tools used to assess the case‐only designs have not, to our knowledge, been empirically tested. We believe this had a minimal impact on our findings, given the size and nature of the biases present in the design and reporting of the included studies. The range of differing study designs used by authors is partly a reflection of the lack of 'control' children not exposed to MMR, due to the population nature of vaccination programmes. As MMR vaccine is universally recommended, recent studies are constrained by the lack of a non‐exposed control group. This is a methodological difficulty that is likely to be encountered in all comparative studies of established childhood vaccines. We were unable to include some of the retrieved studies because a comparable, clearly defined control group or risk period was not available. This exclusion may be a limitation of our review, or may reflect a more fundamental methodological dilemma: how to carry out meaningful studies in the absence of a representative population not exposed to a vaccine that is universally used in public health programmes? Whichever view one takes, we believe that meaningful inferences from individual studies that lack a non‐exposed control group are difficult to make.
The hypothesis that secondary vaccine failure (waning immunity) could occur and increase over the years after the last immunisation has been considered in some studies (ca‐Greenland 2012; ca‐Nelson 2013; ca‐Ogbuanu 2012), but it needs to be better explained. Two studies, Briss 1994; Hersh 1991, carried out in the USA during mumps epidemics on high school students having high vaccination coverage (over 97% received at least one mumps‐containing vaccine dose before the outbreak), showed that the risk of acquiring mumps was higher in participants who were vaccinated at least three, Briss 1994, or five years, Hersh 1991, before the outbreak, than in those who were more recently vaccinated. This estimate was not statistically relevant. Linear regression analysis demonstrated no significant trend for increasing mumps attack rates by years since last vaccination, after either one or two mumps‐containing vaccine doses (Schaffzin 2007). A Belgian study carried out on pupils from seven kindergartens and primary schools in Bruges (age range 3 to 12 years) during a mumps epidemic in 1995 and 1996 estimated that the odds of developing mumps increased 27% per one‐year increase, from one year after the last MMR immunisation onwards (Vandermeulen 2004). A case‐cohort study carried out at the University in Kansas, USA, during the 2006 outbreak showed that case patients were more likely than their roommates without mumps to have received the second MMR dose more than 10 years before (OR 2.50, 95% CI 1.28 to 5.00) (Cortese 2008). Waning immunity may be secondary to a lack of natural exposure (Cortese 2008; Dayan 2008a). The group with the highest mumps incidence during the 2006 outbreak in the USA were college‐age students (18 to 24 years) born during the 1980s, when the spread of mumps was so low that many of them were never exposed to the disease. They probably received a second dose in the early 1990s, when opportunities for booster shots against exposure to wild viruses became increasingly rare (Dayan 2008a). Moreover, the risk of the contracting mumps virus from abroad should be considered, because in several countries, mumps vaccination was not routinely administered (Cohen 2007; Dayan 2008a). Apart from waning immunity, it must be considered that mumps strains used in vaccine preparation differed phylogenically from those isolated during recent mumps outbreaks (Dayan 2008a; Dayan 2008b). These facts could explain, at least in part, the vaccine failure observed during some mumps outbreaks.
Agreements and disagreements with other studies or reviews
This is currently the only review covering both effectiveness and safety issues of MMR, MMR+V, and MMRV vaccines. In agreement with results from other studies and reviews, we did not find a significant association between autism and MMR exposure. The Wakefield 1998 study which links MMR vaccination with autism has been fully retracted (Editors of the Lancet 2010), as Wakefield was found guilty of ethical, medical, and scientific misconduct in the publication of the paper. Many other authors have shown that the Wakefield data were fraudulent (Flaherty 2011). A formal retraction of the interpretation that there was a causal link between MMR vaccine and autism was issued in 2004 by 10 of the 12 original co‐authors (Murch 2004). In 1998, an excessive and unjustified media coverage of this small study had disastrous consequences (Flaherty 2011; Hilton 2007; Offit 2003; Smith 2008), such as distrust of public health vaccination programmes and suspicion about vaccine safety. The consequence of this was a significant decrease in MMR vaccine coverage and re‐emergence of measles in the UK.
Authors' conclusions
Implications for practice.
Existing evidence on the safety and effectiveness of MMR and MMRV vaccine supports current policies of mass immunisation aimed at global measles eradication in order to reduce morbidity and mortality associated with measles mumps rubella and varicella. Campaigns aimed at global eradication should assess epidemiological and socioeconomic situations of the countries as well as the capacity to achieve high vaccination coverage.
Implications for research.
We have observed an improvement in the quality of the design and reporting of safety outcomes in MMR and MMRV in recent years both pre‐ and post‐marketing. More evidence is needed to assess whether the protective effect of MMR/MMRV could wane with time since immunisation. More evidence is needed to assess efficacy of a third dose against MMRV.
Feedback
Vaccines for MMR in children,
Summary
Based on the title and the introduction, this is a review of the effectiveness and safety of MMR vaccine. However, the authors concluded that they "could find no comparative studies assessing the effectiveness of MMR that fitted [their] inclusion criteria as all had serological outcomes" and then continued to discuss only studies of MMR vaccine safety. The review and discussion of the safety of these vaccines accurately reflects the literature; rather this letter is about the conclusions regarding vaccine effectiveness.
The authors' conclusion that no comparative studies exist about the effectiveness of MMR vaccines do not seem to be borne out by other reviews of the literature. Using the stated inclusion criteria, one can find several studies of the effectiveness of MMR vaccine against individual diseases (measles, mumps or rubella) using cohort and case‐control methods. Numerous retrospective studies have also documented the effectiveness of measles‐containing vaccines (versus. MMR vaccine) for preventing measles. A partial list of articles found in PubMed using the criteria (measles OR mumps OR rubella) AND "vaccine efficacy", screened for articles including calculation of clinical vaccine efficacy, follows this feedback.
The authors also restricted their search to articles appearing in 1966 and later; given that measles vaccines were developed and used in clinical trials in the late 1950s and 1960s, the authors should strongly consider repeating their search for all years? or, at a minimum, from 1954 to the present, given that measles virus was first isolated in 1954.
The authors fail to note that the effectiveness of measles, mumps and rubella vaccines were documented individually before their combination into MMR vaccine, and that the serological correlates of protection are well defined for protection against measles and rubella virus infections. These serological correlates of protection are now used to compare various vaccine virus strains and combinations.
I would strongly suggest that this review be revised so that it includes a discussion of articles that assess the efficacy of MMR vaccines or the individual vaccines included in MMR vaccines against their target diseases using any appropriate methodology. The authors could then compare the efficacy of the individual vaccines with that of the combined vaccine. If they choose not to include any of the articles found that demonstrate clinical vaccine efficacy, it would be helpful if the authors could provide a clear justification for doing so. At the very least, the title and introduction should be changed so that it is clear that the review is of studies of the safety of the vaccines, not their efficacy.
Thank you for your consideration of these comments
Reply
Dear Dr Perry
Many thanks for the attention paid to our MMR vaccines review. We have read with interest you observation, we must though call your attention to the fact that for Cochrane Reviews inclusion criteria are established rigorously from an experienced team of specialists with the aim to made comparisons so homogeneous as possible and to consider preferably those outcomes that have direct implications for decision making in Public Health. For this reason the evaluation of evidences based only on serological parameters is debatable or at least not overall accepted at the rate of their indirect nature.
It shouldn't be forgotten that our review was also performed in order to provide some responses to an important specific question in Public Health regarding the suspected association of MMR vaccine with serious diseases. As reported in the conclusions, vaccine efficacy is in any case out of the question, since we consider as important point of evidence the fact that in many countries eradication of the targeted diseases could be achieved by means of mass immunisation programs.
We agree that studies in which single MMR antigens are tested could contribute some evidence, but in this review the only MMR in comparison with placebo or not intervention was considered. Effectiveness or efficacy of measles vaccine has been already reviewed by other authors (e.g. 1, 2, 3 ; all present in DARE).
Many studies out of those indicated by you in the list, report results of a single component vaccines and are for this reason not includible. In some of them MMR is tested, but all appear results of surveys and consequently their design is markedly affected from different types of biases which would preclude in any case their inclusion in the analysis. To complete background information about efficacy of MMR vaccines (or of different strain combinations), we may comment briefly on the evidence from these and other similar reports in occasion of the next update of the review.
All Authors
1. Aaby P, Samb B, Simondon F, Seck A M, Knudsen K, Whittle H. Non‐specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. 1995; 311:481‐485.
2. Anders J F, Jacobson R M, Poland G A, Jacobsen S J, Wollan P C. Secondary failure rates of measles vaccines: a meta‐analysis of published studies. Pediatric Infectious Disease Journal. 1996; 15(1):62‐66.
3. Cooper W O, Boyce T G, Wright P F, Griffin M R. Do childhood vaccines have non‐specific effects on mortality?. Bulletin of the World Health Organization. 2003; 81(11):821‐826.
Contributors
Robert Perry, MD, MPH Feedback added 09/08/06
Vaccines for measles, mumps and rubella in children, June 2016
Summary
Dear Sir/Madam,
I have a newborn baby and I am reviewing if I should vaccinate her or not. I am an osteopath and I am use to reading research but in this case I'm a little bit confused. And for that I would like some clarification. I would really appreciate some explanations on this as for now I don't feel your review is objective. But I might be mistaken and clarification would be welcome.
Please read my comments ahead on your article http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004407.pub3/full.
The conclusions of your article seem contradictory to your findings. Considering that: Firstly, MMR studies are not well conducted, have low internal and external validity, have medium to high level of biases, don't have control groups, and second, MMR may wain with time (more than natural exposure), is associated with aseptic meningitis, febrile seizures, febrile convulsions, acute or idiopathic thrombocytopaenic purpura, and third, in your conclusion you summarise that MMR vaccine "reduces morbidity and mortality associated with mumps and rubella" contradicting yourself with "we found no studies assessing the effectiveness of MMR vaccine against rubella.
I am seriously wondering and considering if actually MMR vaccine is safe and effective. Therefore I don't understand your conclusions. Thank you very much, Arturo Fernandez
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
Reply
Dear Arturo Fernandez, In this last update the our conclusions do not change, but we have rewritten them. We have understood that in previous version the conclusions were formulated in an unfortunate and apparently contradictory way for most readers. New studies with were added in this update. The quality of the more recent studies is generally better.
In this latest version, we hope to have clarified that: 1) MMR vaccination is highly effective (≥ 95%) 2) aseptic meningitis was associated only to MMR vaccine containing Urabe strain (against mumps), no association was found in MMR vaccine containing Jeryl Lynn strain (against mumps). Currently the MMR and MMRV vaccine formulation use the Jeryl Lynn strain 3) Associations between MMR/MMRV/MMRV (containing Jeryl Lynn strain) vaccines and febrile seizures exist. But we must consider that febrile seizures is a rare event, both amongst the non‐vaccinated and the vaccinated. The attributable risk of febrile seizures vaccine‐induced is estimated to be from 1:1700 to 1:1150 doses. 4) Association between MMR vaccination and idiopathic thrombocytopaenic purpura (ITP). However, the risk of ITP after vaccination is smaller than the one after natural infection with these viruses. The attributable risk of ITP vaccine‐induced is estimated about 1 ITP case per 40,000 administered MMR doses. 5) No evidence of association was found between MMR immunisation and encephalitis or encephalopathy, autistic spectrum disorders, inflammatory bowel disease/Crohn's disease, cognitive delay, type 1 diabetes, asthma, dermatitis/eczema, hay fever, leukaemia, demyelinating diseases/multiple sclerosis, gait disturbance, bacterial or viral Infections. Then we may conclude that: the existing evidence on the safety and effectiveness of MMR and MMRV vaccines support their use for mass immunisation.
Contributors
Arturo Fernandez Feedback added 14/10/2019
Vaccines for measles, mumps, rubella, and varicella in children, June 2020
Summary
The last review: Cochrane Database Systematic Review 2012 (update of 2005) stated: “We did not identify any studies assessing the effectiveness of MMR in preventing rubella.” I was, therefore, interested to see that the new one had discovered a study on Rubella vaccine effectiveness, which is quoted as 89%: “Vaccine effectiveness against rubella is 89% (RR 0.11, 95% CI 0.03 to 0.42; 1 cohort study; 1621 children; moderate certainty evidence.” But on checking the reference: Effectiveness of Rubella vaccine in a rubella outbreak in Guangzhou city, China, 2014. Chang C, Mo X, Hu P, Liang W, Ma H, An Z, Liu J, Zheng H. Vaccine. 2015 Jun 22;33(28):3223‐7 Effectiveness of Rubella Vaccine in a Rubella Outbreak in Guangzhou City, China, 2014 ‐ PubMed https://pubmed.ncbi.nlm.nih.gov/25989448/ I found that it is not the efficacy for the Rubella vaccine that is used worldwide at all ‐ it is one only used in China! “Most licensed rubella vaccines in use globally are based on RA27/3 strains and have estimated vaccine effectiveness (VE) rates of 95‐100%. In contrast, China uses a BRD‐II strain‐based rubella vaccine.” This fact is not even mentioned in the Review. This is a misrepresentation of the facts. People reading the review should be able to rely on the authors being clear and not haveread through the whole 423 pages of the long version themselves in order to 'check up' that what the Review is saying is correct. To have to check every statement for accuracy and veracity negates the whole point of having a Review. Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment?: No
Jayne Donegan Role: GP
Reply
In this review, we believe we have clearly explained the contents of vaccines used in China. Just because a vaccine is only used in one country, we do not believe this constitutes an exclusion criteria. However, we agree with you that it would be clearer to add this information in the summary. Furthermore, this pandemic, and the outbreaks of measles in the United States, has taught us that the level of connection is so great between people in China and the rest of the world, that vaccination coverage for rubella or measles in a single country is in fact a global concern for public health.
Contributors
Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V.
What's new
| Date | Event | Description |
|---|---|---|
| 18 November 2021 | Amended | In the previous publication of this review, Analysis 3.1 and Additional Table 5 reported that vaccine effectiveness from one cohort study that attempted to estimate MMR vaccine effectiveness in a population who received two rubella strain‐based MMR vaccines (ca‐Chang 2015): MMR containing the BRD‐II rubella strain, or MMR containing the RA27/3 rubella strain. The vaccine efficacy was 89% (95% confidence interval 56% to 95%). In response to a feedback comment, we specified in the Abstract, Discussion, and summary of findings table 3, that the vaccine type BRD2 against rubella is only used in China. |
| 18 November 2021 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 8 July 2020 | Amended | The NIHR disclaimer and funding stream detail have been added to the Sources of support section. |
| 2 May 2019 | New search has been performed | A new author joined the team to update this review. We included new vaccines MMRV and MMR+V in an updated search from 4 October 2016 to 2 May 2019. We included 34 studies on safety and 40 studies on effectiveness. We included 4 studies on safety and 8 studies on effectiveness that were previously awaiting classification in our 2012 review update. |
| 2 May 2019 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 4 October 2016 | Feedback has been incorporated | Feedback comment inserted. |
| 12 May 2011 | New search has been performed | We updated the searches and included 33 new trials in the review, including one previously excluded trial (ca‐Marolla 1998). We excluded 50 new trials, and 13 new trials are awaiting classification. The conclusions remain unchanged. |
| 1 February 2011 | New citation required but conclusions have not changed | A new author joined the team to update the review. |
| 6 May 2008 | Amended | Converted to new review format. |
| 8 August 2006 | Feedback has been incorporated | Feedback comment and reply added to review. |
| 18 December 2004 | New search has been performed | Searches conducted. |
Acknowledgements
For this 2019 update, the review authors gratefully acknowledge help received from Liz Dooley, Ann Jones, Janet Wale, David Elliman, Jenny Doust, Kerry Dwan, Tess Moore, Andrew Anglemyer, and Liz Bickerdike. This 2019 update was funded by the UK's National Institute for Health Research (NIHR) Cochrane Incentive Scheme.
The 2012 update was unfunded. The review authors gratefully acknowledge help received from Shelley Deeks, Sulachni Chandwani, Janet Wale, Sree Nair, and Peter Morris for their contribution to the 2012 update. The original review (2004) was funded by the European Union and by the Italian Istituto Superiore di Sanità. We wish to thank the following individuals for commenting on the 2004 review draft: Drs Harald Hejbel, Paddy Farrington, Ms Sally Hopewell, Melanie Rudin, Anne Lusher, Letizia Sampaolo and Valeria Wenzel, Bruce Arroll, Lize van der Merwe, Janet Wale and Leonard Leibovici. The review authors wish to acknowledge Tom Jefferson and Deirdre Price as previous authors.
Appendices
Appendix 1. Study design definitions
Experimental: we defined RCTs (experimental design) as studies in which it appears that the individuals (or other experimental units) followed in the study were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using random allocation.
Randomised controlled trial (RCT): is any study on humans in which the individuals (or other experimental units) followed in the study were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using random allocation.
Quasi‐experimental: the main distinction between randomised and quasi‐experimental studies is the way in which participants are allocated to the intervention and control groups. Quasi‐experimental studies do not use random assignment to create comparison groups. Quasi‐experimental design studies often are conducted where there are practical and ethical barriers to conducting randomised controlled trials. Quasi‐experimental studies are divided into four study design groups: (a) quasi‐experimental designs without control groups; (b) quasi‐experimental designs that use control group but no pre‐intervention measurement; (c) quasi‐experimental designs that use control group and pre‐intervention measurement; (d) interrupted time‐series (Harris 2006).
Quasi‐randomised controlled trial (QRCT): any study on humans in which the individuals (or other experimental units) followed in the study were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using some quasi‐random method of allocation (such as alternation, date of birth, or case record number).
Comparative controlled trial(CCT): a study in which the allocation occurred as the result of some decision or system applied by researcher.
Historical controlled trial(HCT): a study with control participants for whom data were collected at a time preceding that at which the data are gathered on the group being studied.
Interrupted time‐seriesstudy(ITS): a study that uses observations at multiple time points before and after an intervention (the ‘interruption’). The design attempts to detect whether the intervention has had an effect significantly greater than any underlying trend over time (Reeves 2011).
Observational: a study in which natural variation in interventions or exposure amongst participants (i.e. not allocated by an investigator) is investigated to explore the effect of the interventions or exposure on health outcomes.
Prospective cohort study(PCS)/retrospective cohort study (RCS): an epidemiological study where groups of individuals are identified who vary in their exposure to an intervention or hazard and are followed to assess outcomes. Association between exposure and outcome are then estimated. Cohort studies are best performed prospectively (prospective cohort study) but can also be undertaken retrospectively (retrospective cohort study) if suitable data records are available.
Case‐control study(CCS): an epidemiological study usually used to investigate the causes of disease. Study participants who have experienced an adverse outcome or disease are compared with participants who have not. Any differences in the presence or absence of hypothesised risk factors are noted.
Ecologic study (ES): an ecologic study focuses on the comparison of groups, rather than individuals, thus individual‐level data are missing and the occurrence of the exposure and the outcome are measured at the group level. The ES design is classified whether participants are grouped by place (multiple‐group study), by time (time‐trend study), or by place and time (mixed study). Despite several practical advantages of ES, there are many methodologic problems that limit causal inference; ES are subject to the ecological fallacy, which stems from the fact that associations at an individual level are not necessarily replicated at the group level, thus ES may be used to generate hypotheses of an association between exposure and outcome, but these studies cannot confirm causation (Morgenstern 1995).
Case‐only methods: these methods (involving only cases) investigate causality between vaccination and rare adverse events when only data of cases are available. This kind of study must be properly designed; analyses based on haphazardly assembled case reports, sometimes referred to as 'case series' in the medical literature, are unlikely to throw any light on causal mechanisms. Hence the ascertainment of cases must be independent of vaccination status, and it is important to control for confounders, the most important of which is age, since both vaccination and adverse event are often highly age‐dependent. These methods eschew separate controls and denominators, but not control per se. Indeed, case‐only methods using self‐controls provide better control of confounding than standard designs. Nevertheless, appropriate analytic methods are required to avoid bias (Farrington 2004).
Self‐controlled cases seriesstudy(SCCS): uses individuals as their own controls. The ages at vaccination are regarded as fixed, and the age at the time of an adverse event is the random variable of interest within a predetermined observation period (Farrington 2004; Petersen 2016).
Person‐time cohort study(PTC): a study in which outcome rates in lower risk period (or reference period) and higher risk period, for the same individuals, are compared. The time of exposure is regarded as fixed, and person‐time periods for the risk categories are added and the rates are compared. When the risk periods are not summed but are within each individual, the design is that of an SCCS (Farrington 1996; Farrington 2004).
Case cross‐over study(CCO): a study in which the exposure information is obtained from the same case during two different periods of time. In the first period exposure is measured immediately before disease onset. In the second period exposure is measured at an earlier time (background exposure). Exposure amongst cases just prior to disease onset is then compared to exposure amongst the same cases at an earlier time. Each case and its matched control (himself) are therefore automatically matched on many characteristics (age, sex, socioeconomic status, etc.) (Farrington 2004; Maclure 1991).
Case‐coverage design/screening methods (CCD/SM): a study comparing prevalence of exposure in individuals with exposure in the reference population, that is the method makes use of exposure information on cases, supplemented by data on vaccine coverage in the population. No denominator data are required, and the population coverage information is derived from summary statistics. These designs are special cases of case‐base methods using external referents (Farrington 2004).
Case‐only ecological method(COEM): ecologic studies involving only cases. The study is ecological in the sense that it is not based on individual data: cases are not classified as exposed or unexposed. The groups in the analysis are typically defined in place (multiple‐group study) and time (time‐trend study). A strength of this study design is its use of two control mechanisms: a before‐and‐after comparison within the same population, and a comparison between different outcomes within each period. A common feature of such studies is their exploitation of changes in vaccination practice, allowing before‐and‐after comparisons (Farrington 2004).
Appendix 2. Taxonomy: tag ‐ study design ‐ outcome
The only aim of this taxonomy is to permit an ordered list of the studies in the quality assessment figure (Figure 4), grouping them by design and main endpoint. A two‐letter tag is used to distinguish the type of study design and whether it relates to effectiveness/efficacy or safety (only). The first letter (a, b, c, d, e, f, g, h) identifies the study design, the second letter (a, b) identifies the endpoint: (a) effectiveness/efficacy; (b) safety only.
| Study design | Tag ‐ study design ‐ outcomes |
| Randomised controlled trial (RCT); comparative controlled trial (CCT) | aa ‐ RCT/CCT ‐ effectiveness/efficacy ab ‐ RCT/CCT ‐ safety only |
| Case‐control study (CCS) | ba ‐ CCS ‐ effectiveness/efficacy bb ‐ CCS ‐ safety only |
| Prospective cohort study (PCS); retrospective cohort study (RCS) | ca ‐ PCS/RCS ‐ effectiveness/efficacy cb ‐ PCS/RCS ‐ safety only |
| Self‐controlled case series (SCCS); person‐time cohort (PTC) | da ‐ SCCS/PTC‐ effectiveness/efficacy db ‐ SCCS/PTC ‐ safety only |
| Case cross‐over (CCO) | ea ‐ CCO ‐ effectiveness/efficacy eb ‐ CCO ‐ safety only |
| Case coverage method/screening method (CCM/SM) | fa ‐ CCM/SM ‐ effectiveness/efficacy fb ‐ CCM/SM ‐ safety only |
| Case‐only ecological method (COEM) | ga ‐ COEM ‐ effectiveness/efficacy gb ‐ COEM ‐ safety only |
| Interrupted time‐series (ITS) | ha ‐ ITS ‐ effectiveness/efficacy hb ‐ ITS ‐ safety only |
Appendix 3. Search strategies
PubMed
#1 Vaccines[MeSH] OR Vaccines, Combined[MeSH] OR Vaccines, Attenuated[MeSH] #2 Vaccination[MeSH] OR Immunisation[MeSH] #3 vaccin*[tw] or immuni*[tw] or inocula*[tw] #4 #1 OR #2 OR #3 #5 Measles[MeSH] #6 Mumps[MeSH] #7 Rubella[MeSH] #8 Chickenpox[MeSH] #9 measles[tw] AND mumps[tw] AND rubella[tw] #10 #5 OR #6 OR #7 OR #8 OR #9 #11 #4 AND #10 #12 Measles‐Vaccine[MeSH] #13 Mumps‐Vaccine[MeSH] #14 Rubella‐Vaccine[MeSH] #15 Measles‐Mumps‐Rubella‐Vaccine[MeSH] #16 measles, mumps, rubella, varicella vaccine [Supplementary Concept] #17 “measles mumps rubella”[tw] or MMR[tw] #18 "measles mumps rubella varicella"[tw] or "measles mumps rubella chickenpox"[tw] or MMRV[tw] #19 triviraten[tw] or priorix[tw] or trimovax[tw] or virivac[tw] or pluserix[tw] #20 “priorix tetra”[tw] or proquad[tw] #21 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 #22 #11 OR #21
Embase
#1 'vaccine'/exp OR 'immunization'/exp #2 vaccin*:ab,ti OR immuni*:ab,ti OR inoculat*:ab,ti #3 #1 OR #2 #4 'measles'/de AND 'mumps'/de AND 'rubella'/de #5 measles:ab,ti AND mumps:ab,ti AND rubella:ab,ti #6 #4 OR #5 #7 #3 AND #6 #8 'measles mumps rubella vaccine'/de AND 'chickenpox measles mumps rubella vaccine'/de #9 'measles vaccine'/de AND 'mumps vaccine'/de AND 'rubella vaccine'/de #10 mmr:ab,ti OR mmrv:ab,ti OR triviraten:ab,ti OR triorix:ab,ti OR trimovax:ab,ti OR virivac:ab,ti OR pluserix:ab,ti OR 'priorix tetra':ab,ti OR proquad:ab,ti #11 #7 OR #8 OR #9 OR #10 #12 #11 AND [embase]/lim NOT [medline]/lim
CL online
#1 MeSH descriptor: [Vaccines] explode all trees #2 MeSH descriptor: [Vaccines, Attenuated] explode all trees #3 MeSH descriptor: [Vaccination] explode all trees #4 MeSH descriptor: [Immunization] explode all trees #5 vaccin*:ti,ab,kw or immuni*:ti,ab,kw or inocula*:ti,ab,kw #6 MeSH descriptor: [Vaccines, Combined] explode all trees #7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 #8 MeSH descriptor: [Measles] explode all trees #9 MeSH descriptor: [Mumps] explode all trees #10 MeSH descriptor: [Rubella] explode all trees #11 MeSH descriptor: [Chickenpox] explode all trees #12 "measles":ti,ab,kw and "mumps":ti,ab,kw and "rubella":ti,ab,kw #13 #8 OR #9 OR #10 OR #11 OR #12 #14 #7 AND #13 #15 MeSH descriptor: [Measles Vaccine] explode all trees #16 MeSH descriptor: [Mumps Vaccine] explode all trees #17 MeSH descriptor: [Rubella Vaccine] explode all trees #18 MeSH descriptor: [Measles‐Mumps‐Rubella Vaccine] explode all trees #19 "measles mumps rubella":ti,ab,kw #20 "measles mumps rubella varicella":ti,ab,kw #21 "measles mumps rubella chickenpox" #22 "MMR":ti,ab,kw #23 "MMRV":ti,ab,kw #24 "Triviraten":ti,ab,kw or "Priorix":ti,ab,kw or trimovax:ti,ab,kw or "virivac":ti,ab,kw or "pluserix":ti,ab,kw #25 "priorix tetra":ti,ab,kw or proquad:ti,ab,kw #26 #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
WHO ICTRP: Measles Mumps Rubella" OR "measles mumps rubella varicella" OR "triviraten OR priorix OR trimovax OR virivac OR pluserix OR MMR OR MMRV OR MMR V ClinicalTrials.gov: measles AND mumps AND rubella (Diseases) triviraten OR priorix OR trimovax OR virivac OR pluserix OR MMR OR MMR II OR MMRV OR MMR‐V (Treatment)
All searches were performed on 2 May 2019.
Appendix 4. Data extraction form
Description of study
Study_ID | Methods (study design) | Participants | Interventions‐Exposure | Outcomes effectiveness | Outcomes safety | Results | Notes
Description of interventions and outcomes (RCT and CCT only)
Active arms| Vaccines used | Vaccines and composition | Product and manufacturer | Schedule & dosage and status | Route of administration Active Arm 1: Active Arm 2: Active Arm 3:
Placebo or control arm: Rule: index vaccine goes in the Arm 1 line, placebo in the last line Status: primary, secondary or tertiary immunisation.
Details of participants
Active arms Enrolled | Missing | Reasons | Inclusion in analysis | Notes Active arm 1: Active arm 2: Active arm 3: Placebo or Control Arm:
Outcomes list efficacy/effectiveness
Outcome | How defined | Description/Follow‐up/Notes Outcomes 1: Outcomes 2: Outcomes 3:
Outcomes list ‐ safety
Outcome | How defined | Description/Follow‐up/Notes
Outcomes 1: Outcomes 2: Outcomes 3:
Other Information: Investigators to be contacted for more information? Yes/No Contact details (principal investigator, fill in only if further contact is necessary)
Data extraction and manipulation (to be used for dichotomous or continuous outcomes; RCT and CCT only)
Comparison | n/N Index Arm | n/N Comparator Outcomes 1: Outcomes 2: Outcomes 3: Notes (for statistical use only)
Description of interventions and outcomes. Non‐randomised longitudinal studies
Groups | Vaccines and composition | Product and manufacturer | Schedule & dosage and status | Route of administration Group 1: Group 2: Group 3: Comparator
Rule: index vaccine goes in the Group 1 line, placebo in the last line
Vaccine batch numbers
Details of participants
Groups | Enrolled | Missing | Reasons | Inclusion in analysis | Notes Group 1: Group 2: Group 3: Comparator
Outcomes list ‐ effectiveness
Outcome | How defined (including length of follow‐up) | Description/Follow‐up/Notes Outcome 1: Outcome 2: Outcome 3:
Outcomes list ‐ safety
Outcome | How defined (including length of follow‐up) | Description/Follow‐up/Notes Outcome 1: Outcome 2: Outcome 3:
Investigators to be contacted for more information? (a) Yes; (b) No
Contact details (principal investigator, fill in only if further contact is necessary):
Data extraction and manipulation (to be used for dichotomous outcomes). Non‐randomised longitudinal studies only
Comparison|Outcomes | n/N Index Group | n/N Comparator| Notes (for statistical use only) comparison 1: comparison 2: comparison 3:
2.c.Description of studies. Case‐control studies
Event | How defined | Enrolled | Missing | Reasons | Inclusion in analysis | Cases n; Controls n | Exposure | How defined | How ascertained | Notes | Vaccine Exposure 1| Vaccine Exposure 2 Event 1: Event 2: Event 3:
Notes (for statistical use only)
Data extraction and manipulation. Case‐control studies
Status | Numerator | Denominator Cases Control
Notes (for statistical use only)
Appendix 5. Assessing risk of bias ‐ methodological quality assessment
Experimental quasi‐experimental designs: RCT and QRCT/CCT only
-
Random sequence generation:
Type of randomisation: (a) individual participants allocated to vaccine or control group; (b) groups of participants allocated to vaccine or control group
Generation of the allocation sequence: (a) random; (b) quasi‐random; (c) not described.
Allocation concealment: adequate, e.g. numbered or coded identical containers administered sequentially, on‐site computer system that can only be accessed after entering the characteristics of an enrolled participant, or serially numbered, opaque, sealed envelopes; possibly adequate, e.g. sealed envelopes that are not sequentially numbered or opaque; inadequate, e.g. open table of random numbers; not described.
Blinding: (a) double‐blinding; (b) single‐blind; (c) no blinding; (d) unclear.
-
Incomplete outcome data (attrition bias):
Follow‐up: average duration of follow‐up and number of losses to follow‐up.
-
Selective reporting (reporting bias):
Baseline data: (a) reported; (b) not reported.
Participant flow: (a) reported; (b) only described; (c) absent.
Exclusion of participants: (a) mentioned; (b) not mentioned; (c) not applicable.
Quasi‐experimental designs
1. Historical controlled trials (HCT)
Was the assignment to the treatment groups really random? Adequate: random numbers table or computer and central office or coded packages; possibly adequate: sealed envelopes without further description or serially numbered, opaque, sealed envelopes; inadequate: alternation, case record number, birth date, or similar procedures; unknown: just the term ‘randomised’ or ‘randomly allocated’ used.
Was the treatment allocation concealed? Adequate: the person who decides on eligibility cannot distinguish or predict cases from controls centralised or pharmacy‐controlled randomisation, serially numbered, identical vials, unreadable, random sequence, etc.; inadequate: where foreknowledge of allocation to group is possible: use of alternation, case record numbers, birth dates or week days, open random number list; unknown: no details given in text.
Were the groups similar in baseline regarding the prognostic factors? Reported: details reported on which patients were recruited; unknown: no details given.
Were the eligibility criteria specified? Adequate: reported: appropriate criteria listed; inadequate: insufficient, inappropriate criteria given; unknown: no details given.
Were the outcome assessors blinded to the treatment allocation? Adequate: independent person(s) or investigator if secure double‐blind conditions met; inadequate: clinician is assessor on trial were it is possible (from symptoms, lab results, etc.) to distinguish allocation; unknown: no mention in text.
Was the care provider blinded? Adequate: placebo described as indistinguishable; possibly adequate: just ‘double‐blind’ and no further description of procedures or placebo; inadequate: placebo distinguishable from vaccine; unknown: no details in text.
Was the patient blinded? Adequate: placebo described as ‘indistinguishable’ and blinding procedures secure; possibly adequate: the phrase ‘double‐blind’ used in text with no further description; inadequate: no placebo or clearly distinguishable from vaccine; unknown: no details given.
Did the analysis include an intention‐to‐treat analysis? Adequate: details of analysis presented including a) percentage of missing, distribution over groups, and procedure for handling; b) dropout rate less than 20% for each group and reasons given; possibly adequate: incomplete data; inadequate: wrong procedures used; unknown: no mention in text or not deducible from tables.
2. Interrupted time‐series
Were the eligibility criteria specified? Adequate: criteria appropriate to outcomes being measured; inadequate: exclusion criteria impact on outcomes being measured; unknown: no mention in text.
Were objective measurements taken both before and after the intervention? Adequate: relevant data recorded before and after a verifiable intervention; inadequate: non‐verifiable intervention points or incomplete data before/after records.
Was the time frame appropriate? Adequate: the outcomes being measured are detectable within the study time frame; inadequate: brevity of time frame precludes accurate measure, e.g. of long‐term outcomes; unknown: no mention in text.
Was exposure adequate and appropriate? Adequate: sufficient time to allow plausible association was allowed. Exposure was to the vaccine and no obvious confounding interventions were present.
Observational studies
1. Cohort studies ‐ prospective cohort studies (PCS)/retrospective cohort studies (RCS) ‐ Newcastle Ottawa Scale (NOS) (Stang 2010).
PCS/RCS ‐ exposed cohort selection:representation of the exposed cohort: (a) truly representative of the average _______________ (describe) in the community; (b) somewhat representative of the average ______________ in the community; (c) selected group of users, e.g. nurses, volunteers; (d) no description of the derivation of the cohort. Ascertainment of exposure: (a) secure record (e.g. surgical records); (b) structured interview; (c) written self‐report; (d) no description.
PCS/RCS ‐ non‐exposed cohort selection:selection of the non‐exposed cohort: (a) drawn from the same community as the exposed cohort; (b) drawn from a different source; (c) no description of the derivation of the non‐exposed cohort. Demonstration that outcome of interest was not present at start of study: (a) yes; (b) no.
PCS/RCS ‐ comparability:comparability of cohorts on the basis of the design or analysis: (a) study controls for _____________ (select the most important factor); (b) study controls for any additional factor* (this criteria could be modified to indicate specific control for a second important factor).
PCS/RCS ‐ outcome assessment:assessment of outcome: (a) independent blind assessment; (b) record linkage; (c) self‐report; (d) no description. Was follow‐up long enough for outcomes to occur: (a) yes (select an adequate follow‐up period for outcome of interest); (b) no. Losses to follow‐up; adequacy of follow‐up of cohorts: (a) complete follow‐up ‐ all participants accounted for; (b) participants lost to follow‐up unlikely to introduce bias ‐ small number lost ‐ > ____ % (select an adequate %) follow‐up, or description provided of those lost)*; (c) follow‐up rate < ____% (select an adequate %) and no description of those lost; (d) no statement.
2. Case‐control studies (CCS) ‐ Newcastle Ottawa Scale (NOS) (Stang 2010).
CCS ‐ case selection: is the case definition adequate?: (a) yes, with independent validation; (b) yes, e.g. record linkage or based on self‐reports; (c) no description. Representation of the cases: (a) consecutive or obviously representative series of cases (b) potential for selection biases or not stated.
CCS ‐ control selection: control selection: (a) community controls; (b) hospital controls; (c) no description. Definition of controls: (a) no history of disease (endpoint); (b) no description of source.
CCS ‐ comparability: comparability of cases and controls on the basis of the design or analysis: (a) study controls for _______________ (select the most important factor); (b) study controls for any additional factor (this criteria could be modified to indicate specific control for a second important factor).
CCS ‐ exposure: ascertainment of exposure: (a) secure record (e.g. surgical records); (b) structured interview where blind to case/control status; (c) interview not blinded to case/control status; (d) written self‐report or medical record only; (e) no description. Same method of ascertainment for cases and controls: (a) yes; (b) no. Non‐response rate: (a) same rate for both groups; (b) non‐respondents described; (c) rate different and no designation.
Case‐only methods
1. Self‐controlled case series (SCCS) ‐ person‐time cohort design (PTC) (Farrington 2004; Petersen 2016).
SCCS/PTC ‐ case selection: is the case definition adequate? (a) yes, with independent validation; (b) yes, e.g. record linkage or based on self‐reports; (c) no description. (Is the cases ascertainment independent of vaccination status?)
SCCS/PTC ‐ exposure: has exposure been verified? Ascertainment of the exposure: (a) secure record (e.g. surgical records); (b) structured interview; (c) written self‐report; (d) no description. Exposure to multiple vaccines: (a) has been documented in the analysis; (b) has been accounted for in the analysis; (c) unclear.
SCCS/PTC ‐ observation and exposure risk period: are the observation periods well‐defined? Are the full history on the timing of events and exposure available? Risk period: (period when exposure may have had an impact) are the risk periods well‐defined? Has the exposure had an impact within the observation period?
SCCS/PTC ‐ comparability: are the events (cases) well mapped within the different identified periods? Have known confounders been controlled for?
2. Case cross‐overdesign (CCO) (Farrington 2004; Maclure 1991).
CCO ‐ case selection: is the case definition adequate? (a) yes, with independent validation; (b) yes, e.g. record linkage or based on self‐reports; (c) no description. (Is the cases ascertainment independent of vaccination status?)
CCO ‐ exposure: ascertainment of the exposure: (a) secure record (e.g. surgical records); (b) structured interview; (c) written self‐report; (d) no description.
CCO ‐ risk and control period: is the exposure ascertained in a defined time period (immediately) prior to the event (onset)? Are the duration of risk and control periods the same? Are the control and risk periods separated by a 'wash‐out' period in order to avoid mixed‐exposure amongst the control period and the risk period? Is the probability of vaccination the same in all intervals?
CCO ‐ comparability: is the capacity to document exposure identical in the two time periods?
3. Case coverage method/screening method (CCM/SM) (Farrington 2004).
CCM/SM ‐ case selection: are cases drawn from population for which the coverage data exist? (Is the cases ascertainment independent of vaccination status?)
CCM/SM ‐ comparator: are coverage data reliable?
CCM/SM ‐ comparability: do the coverage data permit control of confounding by stratification?
4. Cases‐only ecological method (COEM) (Farrington 2004).
COEM ‐ case selection: is the case definition adequate? (a) yes, with independent validation; (b) yes, e.g. record linkage or based on self‐reports; (c) no description. (Is the case ascertainment independent of vaccination status?)
COEM ‐ exposure: ascertainment of the exposure: (a) secure record (e.g. surgical records); (b) structured interview; (c) written self‐report; (d) no description.
COEM ‐ time trend comparison: unexposed period (or reference period) versus low/high risk (exposed) period: is the full history on the timing of events and exposure available? Has the exposure had an impact within the period?
COEM ‐ comparability: are the events (cases) well mapped within the different identified periods? Have known confounders been controlled for?
Appendix 6. 'Summary of findings' tables
1. Effectiveness against measles (Table 1)
Cohort studies ‐ one dose
Cohort studies ‐ two doses
Cohort studies households contacts ‐ one dose
Cohort studies households contacts ‐ two doses
Cohort studies postexposure prophylaxis
2. Effectiveness against mumps (Table 2)
Cohort studies ‐ Jeryl Lynn strain ‐ one dose
Cohort studies ‐ Jeryl Lynn strain ‐ two doses
Cohort studies ‐ Jeryl Lynn strain ‐ unspecified number of doses
Cohort studies ‐ Jeryl Lynn strain ‐ households contacts
Cohort studies ‐ Urabe strain ‐ unspecified number or at least one dose
Cohort studies ‐ Rubini strain ‐ unspecified number or at least one dose
Cohort studies ‐ mumps strain not reported or any strain
Cohort studies ‐ third dose versus two doses
3. Effectiveness against rubella (Table 3)
Cohort studies secondary cases ‐ any strain
4. Effectiveness against varicella (Table 4)
MMRV randomised clinical trial ‐ any severity ‐ two doses ‐ follow‐up at 5 years
MMRV randomised clinical trial ‐ any severity ‐ two doses ‐ follow‐up between 5 to 10 years
MMRV randomised clinical trial ‐ any severity ‐ two doses ‐ follow‐up at 10 years
MMRV randomised clinical trial ‐ moderate/severe cases ‐ two doses ‐ follow‐up at 5 years
MMRV randomised clinical trial ‐ moderate/severe cases ‐ two doses ‐ follow‐up between 5 to 10 years
MMRV randomised clinical trial ‐ moderate/severe cases ‐ two doses ‐ follow‐up at 10 years
MMR+V randomised clinical trial ‐ any severity ‐ two doses ‐ follow‐up at 5 years
MMR+V randomised clinical trial ‐ any severity ‐ two doses ‐ follow‐up between 5 to 10 years
MMR+V randomised clinical trial ‐ any severity ‐ two doses ‐ follow‐up at 10 years
MMR+V randomised clinical trial ‐ moderate/severe cases ‐ two doses ‐ follow‐up at 5 years
MMR+V randomised clinical trial ‐ moderate/severe cases ‐ two doses ‐ follow‐up between 5 to 10 years
MMR+V randomised clinical trial ‐ moderate/severe cases ‐ two doses ‐ two doses ‐ follow‐up at 10 years
5. Safety ‐ short‐term side effects (Table 5)
Temperature ‐ RCT/CCT axillary
Temperature ‐ RCT/CCT rectal
Temperature ‐ RCT/CCT measurement site not reported
Temperature ‐ cohort studies orally
Temperature ‐ cohort studies measurement site not reported
Rash ‐ cohort studies
Lymphadenopathy ‐ RCT/CCT
Lymphadenopathy ‐ cohort studies
Coryza ‐ RCT/CCT
Coryza ‐ cohort studies
URTI (rhinitis pharyngitis) ‐ RCT/CCT
URTI (rhinitis pharyngitis) ‐ cohort studies
Cough ‐ RCT/CCT
Rash ‐ RCT/CCT
6. Safety ‐ encephalitis or encephalopathy (Table 6)
Case‐control: MMR (risk interval from 0 to 90 days)
Self‐controlled case series/person‐time cohort
7. Safety ‐ aseptic meningitis (Table 7)
Case‐control ‐ case cross‐over ‐ case‐control ‐ Jeryl Lynn ‐ risk interval 0 to 30 days
Case‐control ‐ case cross‐over ‐ case cross‐over ‐ Urabe or Hoshino
Case‐control ‐ case cross‐over ‐ case cross‐over ‐ Jeryl Lynn or Rubini
Self‐controlled case series (SCCS)/person‐time cohort (PT) ‐ SCCS ‐ any strain
Self‐controlled case series (SCCS)/person‐time cohort (PT) ‐ SCCS ‐ Urabe
Self‐controlled case series (SCCS)/person‐time cohort (PT) ‐ SCCS ‐ Leningrad‐Zageb
Self‐controlled case series (SCCS)/person‐time cohort (PT) ‐ PT ‐ Jeryl Lynn
Case‐only ecological method (COEM) ‐ COEM ‐ Urabe
Case‐only ecological method (COEM) ‐ COEM ‐ Leningrad‐Zagreb
8. Safety ‐ seizures (febrile/afebrile) (Table 8)
Cohort studies ‐ within 1 week after vaccination MMR
Cohort studies ‐ between 1 to 2 weeks after vaccination MMR
Cohort studies ‐ > 2 weeks after vaccination MMR
Self‐controlled case series/person‐time ‐ between 1 to 2 weeks after vaccination MMR
Self‐controlled case series/person‐time ‐ > 2 weeks after vaccination MMR
Self‐controlled case series/person‐time ‐ between 1 to 2 weeks after vaccination; MMRV
Self‐controlled case series/person‐time ‐ between 1 to 2 weeks after vaccination MMR+V
MMRV versus MMR+V ‐ by brand ‐ from 0 to 42 days after vaccination (Priorix)
MMRV versus MMR+V ‐ by brand ‐ from 7 to 10 days after vaccination (Priorix)
MMRV versus MMR+V ‐ by brand ‐ from 0 to 42 days after vaccination (ProQuad)
MMRV versus MMR+V ‐ by brand ‐ from 7 to 10 days after vaccination (ProQuad)
MMRV versus MMR ‐ by brand ‐ from 0 to 42 days after vaccination (Priorix)
MMRV versus MMR ‐ by brand ‐ from 7 to 10 days after vaccination (Priorix)
MMRV versus MMR ‐ by brand ‐ from 0 to 42 days after vaccination (ProQuad)
MMRV versus MMR ‐ by brand ‐ from 7 to 10 days after vaccination (ProQuad)
9. Safety ‐ autism spectrum disorders (Table 9)
Cohort studies ‐ all children MMR
Cohort studies ‐ autism risk (low) MMR
Cohort studies ‐ autism risk (moderate/high) MMR
10. Safety ‐ inflammatory bowel disease (IBD) (Table 10)
Case‐control ‐ all IBD. MMR
Case‐control ‐ ulcerative colitis. MMR
Case‐control ‐ Crohn's Disease. MMR
11. Safety ‐ cognitive delay ‐ developmental delay (Table 11)
Cohort study ‐ MDI‐BSID II 24th month. MMR
Cohort study ‐ MDI‐BSID II 36th month. MMR
Cohort study ‐ Raven 5th year. MMR
Cohort study ‐ WISC‐R verbal 6th year. MMR
12. Safety ‐ idiopathic thrombocytopenic purpura (Table 12)
Case‐control ‐ case cross‐over ‐ case‐controls MMR
Self‐controlled case series ‐ MMR vaccine ‐ age from 9 to 23 months
13. Safety ‐ Henoch‐Schönlein purpura (Table 13)
Case‐control ‐ MMR vaccine
14. Safety ‐ type 1 diabetes (Table 14)
Cohort study MMR ‐ all children
Cohort study MMR ‐ children with at least one sibling with type 1 diabetes
15. Safety ‐ asthma (Table 15)
Cohort study (rate ratio) ‐ all ages
Cohort studies (risk ratio) ‐ all ages
16. Safety ‐ eczema ‐ dermatitis (Table 16)
Cohort study (rate ratio)
Cohort study (rate ratio) ‐ all ages
Cohort study (risk ratio)
17. Safety ‐ hay fever, rhinoconjunctivitis, hypersensitivity/allergy (Table 17)
Cohort study ‐ rhinoconjunctivitis
Cohort study ‐ hypersensitivity/allergy
Case‐control ‐ hay fever
18. Safety ‐ acute leukaemia (Table 18)
Case‐control ‐ acute leukaemia
Case‐control ‐ acute lymphoblastic leukaemia
Case‐control ‐ acute myeloblastic leukaemia
19. Safety ‐ demyelinating diseases ‐ multiple sclerosis ‐ acute disseminated encephalomyelitis (Table 19)
Case‐control ‐ multiple sclerosis
Case‐control ‐ acute disseminated encephalomyelitis
20. Safety ‐ gait disturbances (Table 20)
Self‐controlled case series (hospitalisations) ‐ hospitalisations ‐ risk period: (0 to 60 days)
Self‐controlled case series (GP visits) ‐ GP visit ‐ risk period: (0 to 5 days)
Self‐controlled case series (GP visits) ‐ GP visit ‐ risk period: (6 to 60 days)
21. Safety ‐ bacterial or viral infections, immune overload (Table 21)
Self‐controlled case series ‐ lobar pneumonia ‐ lobar pneumonia risk period (0 to 90 days)
Self‐controlled case series ‐ invasive bacterial infections ‐ invasive bacterial infections risk period (0 to 90 days)
Self‐controlled case series ‐ encephalitis meningitis ‐ encephalitis ‐ meningitis risk period (0 to 90 days)
Self‐controlled case series ‐ herpes ‐ herpes risk period (0 to 90 days)
Self‐controlled case series ‐ pneumonia ‐ pneumonia risk period (0 to 90 days)
Self‐controlled case series ‐ varicella zoster ‐ varicella zoster risk period (0 to 90 days)
Self‐controlled case series ‐ miscellaneous viral infections ‐ miscellaneous viral infections risk period (0 to 90 days)
Appendix 7. Previous searches
For effectiveness: for this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, EMBASE (July 2004 to May 2011) and PubMed (July 2004 to May week 2, 2011). We used the following search terms for CENTRAL and PubMed.# 1 explode 'Vaccines‐Combined' / all subheadings # 2 explode 'Vaccines‐Attenuated' / all subheadings # 3 #1 or #2 # 4 trivalen* or combin* or simultan* or tripl* or trebl* # 5 vaccin* or immuni* or inoculat* # 6 # 4 and # 5 # 7 # 3 or # 6 # 8 explode 'Measles‐' / all subheadings # 9 explode 'Mumps‐' / all subheadings # 10 explode 'Rubella‐' / all subheadings # 11 measles and mumps and rubella # 12 #8 or #9 or #10 or #11 # 13 #7 and #12 # 14 explode 'Measles‐Vaccine' # 15 explode 'Mumps‐Vaccine' # 16 explode 'Rubella‐Vaccine' # 17 explode 'Measles‐Mumps‐Rubella‐Vaccine' / all subheadings # 18 measles mumps rubella or MMR # 19 #14 or #15 or #16 or #17 or #18 # 20 #13 or #19We adapted these subject terms for EMBASE (see Appendix 3). We conducted all searches during the second week of May, 2011. We also considered the Cochrane Database of Systematic Reviews (CDSR) and the NHS Database of Abstracts of Reviews of Effects (DARE) for published reviews. For search strategies used in the previous version of the review see Appendix 7.For safetyAgain, for this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, EMBASE (July 2004 to May 2011) and PubMed (July 2004 to May week 2 2011). We used the following search terms for CENTRAL and PubMed.1 Vaccines‐Combined [mesh word (mh)] 2 Vaccines‐Attenuated 3 ((trivalen*[text word (tw)] or combin* (tw) or simultan* (tw) or tripl* (tw) or trebl* (tw) and (vaccin* (tw) or immuni* (tw) or inoculat* (tw))) 4 or/1‐3 5 measles (tw) and mumps (tw) and rubella (tw) 6 4 and 5 7 Measles‐Vaccine(mh) and Mumps‐Vaccine (mh) and Rubella‐Vaccine (mh) 8 MMR [title, abstract (ti,ab)] 9 (measles (tw) and mumps (tw) and rubella (tw) and (vaccin* (tw) or immuni* (tw) or inoculat* (tw)) 10 or/6‐9 11 adverse events [floating sub‐heading (fs)] or chemically induced (fs) or complications (fs) or contraindications (fs) or toxicity (fs) or poisoning (fs) or drug effects (fs) 12 ((adverse (tw) and (effect* (tw) or event* (tw)) or side effect* (tw) or hypersensitiv* (tw) or sensitiv* (tw) or safe* (tw) or pharmacovigil* (tw) 13 explode Product‐Surveillance‐Postmarketing (mh) or Drug‐Monitoring (mh) or Drug‐Evaluation (mh) or explode Risk (mh) or Odds‐Ratio (mh) or explode Causality (mh) 14 relative risk (tw) or risk (tw) or causation (tw) or causal (tw) or odds ratio (tw) or etiol* (tw) or aetiol* (tw) or etiology (fs) or epidemiology (fs) 15 or/11‐14 16 10 and 15
Effectiveness
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2004, Issue 4) which contains the Cochrane Acute Respiratory Infections (ARI) Group's specialised trials register, and MEDLINE (1966 to December 2004) to identify randomised and quasi‐randomised controlled trials identified through electronic databases and handsearches. We used the following search terms.
Embase: effectiveness
#1 'vaccine'/exp OR #2 (trivalen* OR combin* OR simultan* OR tripl* OR trebl*) AND (vaccin* OR immuni* OR inoculat*) #3 ('measles'/exp OR 'mumps'/exp OR 'rubella'/exp) OR (measles:ab,ti AND mumps:ab,ti AND rubella:ab,ti) #4 1# OR #2 #5 #4 AND #3 #6 'measles vaccine'/exp OR 'mumps vaccine'/exp OR 'rubella vaccine'/exp OR 'measles mumps rubella vaccine'/exp #7 'measles mumps rubella':ab,ti OR mmr:ab,ti #8 #5 OR #6 OR #7 #9 #8 AND ([child]/lim OR [adolescent]/lim) #10 #8 AND (child* OR pediatric OR paediatric OR adolescent* OR infant* OR preschool* OR school* OR toddler*) #11 #9 OR #10 #12 #11 AND [embase]/lim AND [01‐06‐2004]/sd
MEDLINE (Webspirs): effectiveness
# 1 explode 'Vaccines‐Combined' / all subheadings # 2 explode 'Vaccines‐Attenuated' / all subheadings # 3 #1 or #2 # 4 trivalen* or combin* or simultan* or tripl* or trebl* # 5 vaccin* or immuni* or inoculat* # 6 # 4 and # 5 # 7 # 3 or # 6 # 8 explode 'Measles‐' / all subheadings # 9 explode 'Mumps‐' / all subheadings # 10 explode 'Rubella‐' / all subheadings # 11 measles and mumps and rubella # 12 #8 or #9 or #10 or #11 # 13 #7 and #12 # 14 explode 'Measles‐Vaccine' # 15 explode 'Mumps‐Vaccine' # 16 explode 'Rubella‐Vaccine' # 17 explode 'Measles‐Mumps‐Rubella‐Vaccine' / all subheadings # 18 measles mumps rubella or MMR # 19 #14 or #15 or #16 or #17 or #18 # 20 #13 or #19
We adapted these subject terms to search the other databases. We searched EMBASE (1980 to the end of 2004) to identify controlled trials in combination with subject terms adapted for EMBASE; Biological Abstracts (1985 to the end of 2004); and Science Citation Index (1980 to present). We also searched the Cochrane Database of Systematic Reviews (CDSR) and NHS Database of Abstracts of Reviews of Effects (DARE) for published reviews. We updated the searches during the third July week of 2010, performing searches on the same databases and using the same search strategy terms.
Safety
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2004, Issue 4) which contains the Cochrane Acute Respiratory Infections (ARI) Group's specialised trials register to identify reports of randomised and quasi‐randomised controlled trials and published reviews. We searched The Cochrane Library to identify reports from the results of handsearching the journal Vaccine (1983 to 2004).We also searched MEDLINE (1966 to December 2004) using the following search terms.
Embase: safety
#1 ('vaccine'/exp) OR ((trivalen* OR combin* OR simultan* OR tripl* OR trebl*) AND (vaccin* OR immuni* OR inoculat*)) #2 measles AND mumps AND rubella #3 #1 AND #2 #4 'measles vaccine'/exp AND 'mumps vaccine'/exp AND 'rubella vaccine'/exp #5 mmr:ti,ab #6 (measles AND mumps AND rubella) AND (vaccin* OR immuni* OR inoculat*) #7 #3 OR #4 OR #5 OR #6 #8 'adverse drug reaction'/exp OR 'chemically induced disorder'/exp OR 'toxicity'/exp #9 ((adverse OR side OR serious OR severe OR threatening OR long AND term OR 'long term') AND (event* OR effect* OR disease* OR condition*)) OR hypersensitiv* OR sensitiv* OR safe* OR pharmacovigil* #10 'postmarketing surveillance'/exp OR 'drug monitoring'/exp OR 'drug screening'/exp OR 'risk'/exp #11 'relative risk' OR risk OR causation OR causal OR 'odds ratio' OR etiol* OR aetiol* #12 #8 OR #9 OR #10 OR #11 #13 #7 AND #12 #14 #7 AND #12 AND ([child]/lim OR [adolescent]/lim) #15 child* OR pediatric OR paediatric OR adolescent* OR infant* OR preschool* OR school* OR toddler* #16 #13 AND #15 #17 #14 OR #16 #18 #14 OR #16 AND [embase]/lim AND [01‐06‐2004]/sd
MEDLINE (OVID): safety
1 Vaccines‐Combined [mesh word (mh)] 2 Vaccines‐Attenuated 3 ((trivalen*[text word (tw)] or combin* (tw) or simultan* (tw) or tripl* (tw) or trebl* (tw) and (vaccin* (tw) or immuni* (tw) or inoculat* (tw))) 4 or/1‐3 5 measles (tw) and mumps (tw) and rubella (tw) 6 4 and 5 7 Measles‐Vaccine(mh) and Mumps‐Vaccine (mh) and Rubella‐Vaccine (mh) 8 MMR [title, abstract (ti,ab)] 9 (measles (tw) and mumps (tw) and rubella (tw) and (vaccin* (tw) or immuni* (tw) or inoculat* (tw)) 10 or/6‐9 11 adverse events [floating sub‐heading (fs)] or chemically induced (fs) or complications (fs) or contraindications (fs) or toxicity (fs) or poisoning (fs) or drug effects (fs) 12 ((adverse (tw) near (effect* (tw) or event* (tw)) or side effect* (tw) or hypersensitiv* (tw) or sensitiv* (tw) or safe* (tw) or pharmacovigil* (tw) 13 explode Product‐Surveillance‐Postmarketing (mh) or Drug‐Monitoring (mh) or Drug‐Evaluation (mh) or explode Risk (mh) or Odds‐Ratio (mh) or explode Causality (mh) 14 relative risk (tw) or risk (tw) or causation (tw) or causal (tw) or odds ratio (tw) or etiol* (tw) or aetiol* (tw) or etiology (fs) or epidemiology (fs) 15 or/11‐14 16 10 and 15
This filter was adapted for searching EMBASE (1980 to the end of 2004), Biological Abstracts (1985 to the end of 2004) and Science Citation Index (1980 to the end of 2004).
Data and analyses
Comparison 1. Effectiveness against measles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cohort studies (vaccinated vs unvaccinated) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 1 dose | 7 | 12039 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.02, 0.13] |
| 1.1.2 2 doses | 5 | 21604 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.28] |
| 1.2 Cohort studies (household contacts: vaccinated vs unvaccinated) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 1 dose | 3 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.89] |
| 1.2.2 2 doses | 3 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.75] |
| 1.2.3 3 doses | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.23] |
| 1.3 Cohort studies (postexposure prophylaxis: vaccinated vs unvaccinated) | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 1.4 Case‐control studies | 2 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.49 [0.41, 0.58] | |
| 1.4.2 2 doses | 1 | Odds Ratio (IV, Random, 95% CI) | 0.39 [0.26, 0.58] | |
| 1.4.3 Unspecified number or at least 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.05 [0.01, 0.40] |
Comparison 2. Effectiveness against mumps.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Cohort studies ‐ Jeryl Lynn strain | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 1 dose | 6 | 9915 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.62] |
| 2.1.2 2 doses | 5 | 7792 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.27] |
| 2.1.3 Unspecified number of doses | 4 | 2011 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.14, 0.35] |
| 2.1.4 Household contacts | 3 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.49] |
| 2.2 Cohort studies ‐ Urabe strain | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Unspecified numbers or at least 1 dose | 4 | 2721 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.12, 0.44] |
| 2.3 Cohort studies ‐ Rubini strain | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Unspecified numbers or at least 1 dose | 4 | 4219 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.55, 1.65] |
| 2.4 Cohort studies ‐ mumps strain not reported or mixed | 2 | 769 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.29, 0.94] |
| 2.5 Cohort studies ‐ 3 doses vs 2 doses | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 3 doses vs 2 doses | 2 | 5417 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 2.6 Case‐control studies ‐ Jeryl Lynn strain | 4 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 1 dose | 3 | Odds Ratio (IV, Random, 95% CI) | 0.43 [0.27, 0.70] | |
| 2.6.2 2 doses | 2 | Odds Ratio (IV, Random, 95% CI) | 0.19 [0.09, 0.41] | |
| 2.6.3 At least 1 dose | 4 | Odds Ratio (IV, Random, 95% CI) | 0.35 [0.25, 0.48] | |
| 2.7 Case‐control studies ‐ Jeryl Lynn strain ‐ lab‐confirmed cases | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.7.1 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.36 [0.22, 0.59] | |
| 2.7.2 2 doses | 1 | Odds Ratio (IV, Random, 95% CI) | 0.12 [0.04, 0.37] | |
| 2.7.3 At least 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.35 [0.16, 0.76] | |
| 2.8 Case‐control studies ‐ Urabe strain | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 At least 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.30 [0.12, 0.75] | |
| 2.9 Case‐control studies ‐ Rubini strain | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.9.1 At least 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.90 [0.43, 1.89] | |
| 2.10 Case‐control studies ‐ strain type not reported or any strain | 2 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.10.1 1 dose | 1 | Odds Ratio (IV, Random, 95% CI) | 0.70 [0.22, 2.21] | |
| 2.10.2 2 doses | 1 | Odds Ratio (IV, Random, 95% CI) | 0.52 [0.09, 3.16] | |
| 2.10.3 At least 1 dose | 2 | Odds Ratio (IV, Random, 95% CI) | 0.50 [0.31, 0.81] |
Comparison 3. Effectiveness against rubella.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Cohort studies secondary cases | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Any strain | 1 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.03, 0.42] |
Comparison 4. Effectiveness against varicella.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 MMRV randomised clinical trial ‐ any severity | 3 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 2 doses ‐ follow up at 5 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.05 [0.03, 0.08] | |
| 4.1.2 2 doses ‐ follow up between 5 to 10 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.05 [0.04, 0.06] | |
| 4.1.3 2 doses ‐ follow up at 10 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.05 [0.04, 0.06] | |
| 4.2 MMRV randomised clinical trial ‐ moderate/severe cases | 3 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 2 doses ‐ Follow up at 5 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.00 [0.00, 0.02] | |
| 4.2.2 2 doses ‐ Follow up between 5 to 10 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.02] | |
| 4.2.3 2 doses ‐ Follow up at 10 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.02] | |
| 4.3 MMR+V randomised clinical trial ‐ any severity | 3 | Rate Ratio (IV, Random, 95% CI) | 0.33 [0.30, 0.36] | |
| 4.3.1 2 doses ‐ follow up at 5 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.35 [0.28, 0.43] | |
| 4.3.2 2 doses ‐ follow up between 5 to 10 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.33 [0.29, 0.38] | |
| 4.3.3 2 doses ‐ follow up at 10 years | 1 | Rate Ratio (IV, Random, 95% CI) | 0.33 [0.29, 0.38] | |
| 4.4 MMR+V randomised clinical trial ‐ moderate/severe cases | 3 | Rate Ratio (IV, Fixed, 95% CI) | 0.10 [0.08, 0.12] | |
| 4.4.1 2 doses ‐ Follow up at 5 years | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.09 [0.06, 0.14] | |
| 4.4.2 2 doses ‐ Follow up between 5 to 10 years | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.10 [0.07, 0.13] | |
| 4.4.3 2 doses ‐ Follow up at 10 years | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.10 [0.08, 0.14] | |
| 4.5 MMR+V randomised clinical trial ‐ severe cases | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.5.1 2 doses ‐ follow up between 5 to 10 years | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.05 [0.01, 0.47] | |
| 4.6 MMRV cohort study | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.6.1 One dose ‐ any severity | 4 | Risk Ratio (IV, Random, 95% CI) | 0.25 [0.11, 0.59] | |
| 4.6.2 Two doses ‐ any severity | 2 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.13, 0.14] | |
| 4.7 MMRV case‐control | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.7.1 Any dose ‐ any severity | 1 | Odds Ratio (IV, Random, 95% CI) | 0.14 [0.07, 0.28] | |
| 4.7.2 Any dose ‐ moderate/severe cases | 1 | Odds Ratio (IV, Random, 95% CI) | 0.07 [0.03, 0.17] | |
| 4.8 MMR+V case control | 3 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.8.1 1 dose ‐ any severity | 2 | Odds Ratio (IV, Random, 95% CI) | 0.14 [0.08, 0.22] | |
| 4.8.2 2 doses ‐ any severity | 2 | Odds Ratio (IV, Random, 95% CI) | 0.05 [0.01, 0.14] | |
| 4.8.3 Any dose ‐ any severity | 2 | Odds Ratio (IV, Random, 95% CI) | 0.12 [0.08, 0.18] | |
| 4.9 MMRV case only ecological method ‐ hospitalisation | 3 | Rate Ratio (IV, Random, 95% CI) | 0.43 [0.34, 0.55] | |
| 4.9.1 Age < 1 year ‐ any dose | 2 | Rate Ratio (IV, Random, 95% CI) | 0.52 [0.37, 0.74] | |
| 4.9.2 Age 1 to 4 years ‐ any dose | 2 | Rate Ratio (IV, Random, 95% CI) | 0.29 [0.10, 0.85] | |
| 4.9.3 Age 5 to 14 years ‐ any dose | 2 | Rate Ratio (IV, Random, 95% CI) | 0.37 [0.19, 0.72] | |
| 4.9.4 Age 0 to 14 years ‐ any doses | 1 | Rate Ratio (IV, Random, 95% CI) | 0.53 [0.44, 0.64] | |
| 4.10 MMRV case only ecological method ‐ incidence | 2 | Rate Ratio (IV, Random, 95% CI) | 0.24 [0.14, 0.43] | |
| 4.10.1 Age < 1 year | 1 | Rate Ratio (IV, Random, 95% CI) | 0.17 [0.12, 0.24] | |
| 4.10.2 Age 1 to 4 years ‐ any dose | 1 | Rate Ratio (IV, Random, 95% CI) | 0.08 [0.07, 0.09] | |
| 4.10.3 Age 5 to 14 years ‐ any dose | 1 | Rate Ratio (IV, Random, 95% CI) | 0.14 [0.12, 0.16] | |
| 4.10.4 Age 0 to 14 years ‐ any doses | 1 | Rate Ratio (IV, Random, 95% CI) | 0.65 [0.53, 0.80] |
Comparison 5. Safety: short‐term side effects (local or systemic reactions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Temperature | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 RCT/CCT axillary | 1 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.09, 3.83] |
| 5.1.2 RCT/CCT rectal | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.06] |
| 5.1.3 RCT/CCT measurement site not reported | 2 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.83, 2.23] |
| 5.1.4 Cohort studies orally | 1 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.04, 1.81] |
| 5.1.5 Cohort studies measurement site not reported | 4 | 457123 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.84, 1.49] |
| 5.2 Rash | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 RCT/CCT | 3 | 1156 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.21, 3.48] |
| 5.2.2 Cohort studies | 3 | 457261 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.73, 3.04] |
| 5.3 Lymphadenopathy | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 RCT/CCT | 3 | 1156 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.52, 3.33] |
| 5.3.2 Cohort studies | 2 | 454085 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.19, 20.97] |
| 5.4 Coryza | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.4.1 RCT/CCT | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.12, 1.63] |
| 5.4.2 Cohort studies | 1 | 3176 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.05, 1.20] |
| 5.5 URTI (rhinitis, pharyngitis) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.5.1 RCT/CCT | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.06, 1.56] |
| 5.5.2 Cohort studies | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.26, 1.64] |
| 5.6 Cough | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.6.1 RCT/CCT | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.81] |
Comparison 6. Safety: encephalitis or encephalopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Case‐control: MMR (risk interval from 0 to 90 days) | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.2 Self‐controlled case series/person‐time cohort | 2 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 Self‐controlled case series: MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 1.34 [0.52, 3.46] | |
| 6.2.2 Person‐time cohort: MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.72 [0.36, 1.43] |
Comparison 7. Safety: aseptic meningitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Case‐control ‐ case cross‐over | 3 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.1.1 Case control ‐ Jeryl Lynn ‐ risk interval 0 to 30 days | 1 | Odds Ratio (IV, Random, 95% CI) | 0.85 [0.21, 3.41] | |
| 7.1.2 Case crossover ‐ Urabe or Hoshino | 2 | Odds Ratio (IV, Random, 95% CI) | 4.00 [2.23, 7.20] | |
| 7.1.3 Case crossover ‐ Jeryl Lynn or Rubini | 1 | Odds Ratio (IV, Random, 95% CI) | 0.60 [0.18, 1.99] | |
| 7.2 Self‐controlled case series (SCCS)/person‐time cohort (PT) | 5 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.2.1 SCCS ‐ any strain | 1 | Rate Ratio (IV, Random, 95% CI) | 12.40 [3.12, 49.35] | |
| 7.2.2 SCCS ‐ Urabe | 3 | Rate Ratio (IV, Random, 95% CI) | 30.71 [13.45, 70.10] | |
| 7.2.3 SCCS ‐ Leningrad‐Zageb | 1 | Rate Ratio (IV, Random, 95% CI) | 6.40 [0.78, 52.47] | |
| 7.2.4 PT ‐ Jeryl Lynn | 1 | Rate Ratio (IV, Random, 95% CI) | 1.30 [0.66, 2.56] | |
| 7.3 Case only ecological method (COEM) | 3 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.3.1 COEM ‐ Urabe | 1 | Rate Ratio (IV, Random, 95% CI) | 9.12 [5.73, 14.52] | |
| 7.3.2 COEM ‐ Leningrad‐Zagreb | 2 | Rate Ratio (IV, Random, 95% CI) | 18.56 [12.09, 28.51] |
Comparison 8. Safety: seizures (febrile/afebrile).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Cohort studies | 2 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 Within 1 week after vaccination MMR | 2 | Rate Ratio (IV, Random, 95% CI) | 2.45 [2.21, 2.71] | |
| 8.1.2 Between 1 to 2 weeks after vaccination MMR | 2 | Rate Ratio (IV, Random, 95% CI) | 3.16 [2.89, 3.46] | |
| 8.1.3 > 2 weeks after vaccination MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.97 [0.49, 1.94] | |
| 8.2 Self‐controlled case series/person‐time cohort | 6 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.2.1 Between 1 to 2 weeks after vaccination MMR | 5 | Rate Ratio (IV, Random, 95% CI) | 3.36 [2.65, 4.24] | |
| 8.2.2 > 2 weeks after vaccination MMR | 3 | Rate Ratio (IV, Random, 95% CI) | 1.18 [0.93, 1.50] | |
| 8.2.3 Between 1 to 2 weeks after vaccination; MMRV | 2 | Rate Ratio (IV, Random, 95% CI) | 6.08 [4.95, 7.47] | |
| 8.2.4 between 1 to 2 weeks after vaccination MMR+V | 1 | Rate Ratio (IV, Random, 95% CI) | 3.13 [2.38, 4.10] | |
| 8.3 MMRV versus MMR+V | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.3.1 from 0 to 42 days after vaccination | 5 | Risk Ratio (IV, Random, 95% CI) | 1.31 [1.19, 1.45] | |
| 8.3.2 from 7 to 10 days after vaccination | 5 | Risk Ratio (IV, Random, 95% CI) | 1.98 [1.69, 2.33] | |
| 8.4 MMRV versus MMR+V ‐ by brand | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.4.1 From 0 to 42 days after vaccination (Priorix) | 1 | Risk Ratio (IV, Random, 95% CI) | 1.95 [0.85, 4.48] | |
| 8.4.2 From 7 to 10 days after vaccination (Priorix) | 1 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.93, 3.07] | |
| 8.4.3 From 0 to 42 days after vaccination (ProQuad) | 4 | Risk Ratio (IV, Random, 95% CI) | 1.30 [1.17, 1.44] | |
| 8.4.4 From 7 to 10 days after vaccination (ProQuad) | 4 | Risk Ratio (IV, Random, 95% CI) | 2.01 [1.70, 2.38] | |
| 8.5 MMRV versus MMR | 6 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 8.5.1 From 0 to 42 days after vaccination | 5 | Risk Ratio (IV, Fixed, 95% CI) | 1.53 [1.37, 1.71] | |
| 8.5.2 From 7 to 10 days after vaccination | 6 | Risk Ratio (IV, Fixed, 95% CI) | 1.50 [1.36, 1.66] | |
| 8.6 MMRV versus MMR ‐ by brand | 6 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 8.6.1 From 0 to 42 days after vaccination (Priorix) | 2 | Risk Ratio (IV, Fixed, 95% CI) | 1.28 [1.00, 1.64] | |
| 8.6.2 From 7 to 10 days after vaccination (Priorix) | 2 | Risk Ratio (IV, Fixed, 95% CI) | 2.49 [1.66, 3.74] | |
| 8.6.3 From 0 to 42 days after vaccination (ProQuad) | 3 | Risk Ratio (IV, Fixed, 95% CI) | 1.60 [1.42, 1.82] | |
| 8.6.4 From 7 to 10 days after vaccination (ProQuad) | 4 | Risk Ratio (IV, Fixed, 95% CI) | 1.46 [1.32, 1.61] |
Comparison 9. Safety: autism spectrum disorders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Cohort studies | 3 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 All children MMR | 2 | Rate Ratio (IV, Random, 95% CI) | 0.93 [0.85, 1.01] | |
| 9.1.2 Autism risk (low) MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 1.00 [0.89, 1.14] | |
| 9.1.3 Autism risk (moderate/high) MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.80 [0.64, 0.98] | |
| 9.2 Case‐control | 4 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.2.1 Any age MMR | 3 | Odds Ratio (IV, Random, 95% CI) | 0.62 [0.36, 1.09] | |
| 9.2.2 Before age 18 months MMR | 2 | Odds Ratio (IV, Random, 95% CI) | 0.91 [0.75, 1.11] | |
| 9.2.3 After age 18 months MMR | 1 | Odds Ratio (IV, Random, 95% CI) | 0.80 [0.61, 1.05] | |
| 9.2.4 Before age 36 months MMR | 2 | Odds Ratio (IV, Random, 95% CI) | 0.94 [0.74, 1.18] | |
| 9.2.5 After age 36 months MMR | 1 | Odds Ratio (IV, Random, 95% CI) | 0.77 [0.55, 1.08] | |
| 9.3 Self‐controlled case series/person‐time cohort | 1 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.3.1 ASD diagnosis < 12 months MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.94 [0.60, 1.47] | |
| 9.3.2 ASD diagnosis < 24 months MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 1.09 [0.79, 1.51] | |
| 9.3.3 Regression < 2 months MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.92 [0.38, 2.22] | |
| 9.3.4 Regression < 4 months MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 1.00 [0.52, 1.94] | |
| 9.3.5 Regression < 6 months MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.85 [0.45, 1.60] | |
| 9.4 Case only ecological method | 1 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.4.1 Childhood autism MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.45 [0.33, 0.62] | |
| 9.4.2 Other ASD. MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.55 [0.39, 0.80] | |
| 9.4.3 Definite regression. MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.73 [0.44, 1.20] | |
| 9.4.4 Definite + probable regression. MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.73 [0.46, 1.16] | |
| 9.4.5 All ASD. MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.49 [0.39, 0.63] |
Comparison 10. Safety: inflammatory bowel disease (IBD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Case‐control | 4 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1.1 All IBD. MMR | 3 | Odds Ratio (IV, Fixed, 95% CI) | 1.42 [0.93, 2.16] | |
| 10.1.2 Ulcerative colitis. MMR | 2 | Odds Ratio (IV, Fixed, 95% CI) | 1.35 [0.81, 2.23] | |
| 10.1.3 Crohn's disease. MMR | 3 | Odds Ratio (IV, Fixed, 95% CI) | 0.64 [0.42, 0.98] | |
| 10.2 Case‐only ecological method (rate ratio) | 1 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 10.2.1 Crohn's disease. MMR | 1 | Rate Ratio (IV, Random, 95% CI) | 0.95 [0.84, 1.08] | |
| 10.3 Case only ecological method (odds ratio) | 1 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 10.3.1 All IBD. MMR | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.98 [0.89, 1.07] |
Comparison 11. Safety: cognitive delay ‐ developmental delay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Cohort study | 1 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 11.1.1 MDI‐BSID II 24th month. MMR | 1 | Odds Ratio (IV, Random, 95% CI) | 1.35 [0.15, 12.07] | |
| 11.1.2 MDI‐BSID II 36th month. MMR | 1 | Odds Ratio (IV, Random, 95% CI) | 0.37 [0.03, 4.28] | |
| 11.1.3 Raven 5th year. MMR | 1 | Odds Ratio (IV, Random, 95% CI) | 1.22 [0.23, 6.51] | |
| 11.1.4 WISC‐R verbal 6th year. MMR | 1 | Odds Ratio (IV, Random, 95% CI) | 1.23 [0.09, 16.92] |
Comparison 12. Safety: idiopathic thrombocytopenic purpura.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Case‐control ‐ case cross‐over | 3 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1.1 Case‐controls MMR | 2 | Odds Ratio (IV, Fixed, 95% CI) | 2.80 [1.50, 5.23] | |
| 12.1.2 Case cross‐over MMR | 1 | Odds Ratio (IV, Fixed, 95% CI) | 1.62 [1.21, 2.16] | |
| 12.2 Self‐controlled case series | 5 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 12.2.1 MMR vaccine ‐ aged from 9 to 23 months | 5 | Rate Ratio (IV, Random, 95% CI) | 4.21 [2.28, 7.78] | |
| 12.2.2 MMR vaccine ‐ aged from 4 to 6 years | 1 | Rate Ratio (IV, Random, 95% CI) | 3.06 [0.42, 22.30] | |
| 12.2.3 MMRV vaccine ‐ aged from 9 to 23 months | 1 | Rate Ratio (IV, Random, 95% CI) | 2.87 [0.78, 10.56] |
Comparison 13. Safety: Henoch‐Schönlein purpura.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Case‐control | 1 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1.1 MMR vaccine | 1 | Odds Ratio (IV, Fixed, 95% CI) | 3.40 [1.18, 9.81] |
Comparison 14. Safety: type 1 diabetes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Cohort study MMR | 2 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1.1 All children | 2 | Rate Ratio (IV, Random, 95% CI) | 1.09 [0.98, 1.21] | |
| 14.1.2 Children with at least 1 sibling with type 1 diabetes | 1 | Rate Ratio (IV, Random, 95% CI) | 0.86 [0.34, 2.16] |
Comparison 15. Safety: asthma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Cohort study (rate ratio) | 3 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 15.1.1 All ages | 3 | Rate Ratio (IV, Random, 95% CI) | 1.05 [0.80, 1.39] | |
| 15.2 Cohort study (risk ratio) | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 15.2.1 All ages | 1 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.98, 1.80] | |
| 15.2.2 Age ≤ 6 years | 1 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.19, 1.00] | |
| 15.2.3 Age between 11 and 16 years | 1 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.16, 0.79] |
Comparison 16. Safety: eczema ‐ dermatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Cohort study (rate ratio) | 1 | Rate Ratio (IV, Random, 95% CI) | Subtotals only | |
| 16.1.1 All ages | 1 | Rate Ratio (IV, Random, 95% CI) | 3.50 [2.38, 5.15] | |
| 16.2 Cohort study (risk ratio) | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 16.2.1 Age between 11 and 16 years | 1 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.29, 1.94] |
Comparison 17. Safety: hay fever, rhinoconjunctivitis, hypersensitivity/allergy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Cohort study ‐ rhinoconjunctivitis | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.64 [0.19, 2.11] | |
| 17.2 Cohort study ‐ hypersensitivity/allergy | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.63 [0.14, 2.77] | |
| 17.3 Case‐control ‐ hay fever | 2 | Odds Ratio (IV, Random, 95% CI) | 1.16 [0.92, 1.45] |
Comparison 18. Safety: acute leukaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Case‐control | 4 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 18.1.1 Acute leukaemia | 2 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.76, 1.24] | |
| 18.1.2 Acute lymphoblastic leukaemia | 4 | Odds Ratio (IV, Random, 95% CI) | 0.91 [0.72, 1.14] | |
| 18.1.3 Acute myeloblastic leukaemia | 1 | Odds Ratio (IV, Random, 95% CI) | 0.56 [0.29, 1.07] |
Comparison 19. Safety: demyelinating diseases ‐ multiple sclerosis ‐ acute disseminated encephalomyelitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Case‐control | 2 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1.1 Multiple sclerosis | 1 | Odds Ratio (IV, Fixed, 95% CI) | 1.13 [0.62, 2.05] | |
| 19.1.2 Acute disseminated encephalomyelitis | 1 | Odds Ratio (IV, Fixed, 95% CI) | 1.03 [0.44, 2.42] |
Comparison 20. Safety: gait disturbances.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Self‐controlled case series (hospitalisations) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1.1 Hospitalisation ‐ risk period: (0 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.83 [0.24, 2.86] | |
| 20.1.2 Hospitalisations ‐ risk period: (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.20 [0.03, 1.40] | |
| 20.1.3 Hospitalisations ‐ risk period: (0 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.46 [0.16, 1.34] | |
| 20.2 Self‐controlled case series (GP visits) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 20.2.1 GP visit ‐ risk period: (0 to 5 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.88 [1.30, 2.72] | |
| 20.2.2 GP visit ‐ risk period: (6 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.90 [0.70, 1.16] | |
| 20.2.3 GP visit ‐ risk period: (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.95 [0.76, 1.18] | |
| 20.2.4 GP visit ‐ risk period: (6 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.93 [0.78, 1.11] |
Comparison 21. Safety: bacterial or viral infections, immune overload.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Self‐controlled case series ‐ lobar pneumonia | 2 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1.1 Lobar pneumonia risk period (0 to 30 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.68 [0.53, 0.87] | |
| 21.1.2 Lobar pneumonia risk period (31 to 60 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.80 [0.63, 1.01] | |
| 21.1.3 Lobar pneumonia risk period (61 to 90 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.81 [0.64, 1.03] | |
| 21.1.4 Lobar pneumonia risk period (0 to 90 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.75 [0.64, 0.89] | |
| 21.2 Self‐controlled case series ‐ invasive bacterial infections | 2 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.2.1 Invasive bacterial infections risk period (0 to 30 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.81 [0.58, 1.13] | |
| 21.2.2 Invasive bacterial infections risk period (31 to 60 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 1.07 [0.77, 1.48] | |
| 21.2.3 Invasive bacterial infections risk period (61 to 90 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.85 [0.58, 1.23] | |
| 21.2.4 Invasive bacterial infections risk period (0 to 90 days) | 2 | Rate Ratio (IV, Fixed, 95% CI) | 0.90 [0.71, 1.13] | |
| 21.3 Self‐controlled case series ‐ encephalitis meningitis | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.3.1 Encephalitis ‐ meningitis risk period (0 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.54 [0.06, 4.84] | |
| 21.3.2 Encephalitis ‐ meningitis risk period (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.74 [0.07, 7.64] | |
| 21.3.3 Encephalitis ‐ meningitis risk period (61 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.46 [0.23, 9.28] | |
| 21.3.4 Encephalitis ‐ meningitis risk period (0 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.84 [0.20, 3.51] | |
| 21.4 Self‐controlled case series ‐ herpes | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.4.1 Herpes risk period (0 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.00 [0.57, 1.75] | |
| 21.4.2 Herpes risk period (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.69 [1.06, 2.70] | |
| 21.4.3 Herpes risk period (61 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.89 [0.50, 1.59] | |
| 21.4.4 Herpes risk period (0 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.17 [0.56, 2.46] | |
| 21.5 Self‐controlled case series ‐ pneumonia | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.5.1 Pneumonia risk period (0 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Not estimable | |
| 21.5.2 Pneumonia risk period (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.39 [0.49, 3.92] | |
| 21.5.3 Pneumonia risk period (61 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.27 [0.41, 3.94] | |
| 21.5.4 Pneumonia risk period (0 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.72 [0.32, 1.60] | |
| 21.6 Self‐controlled case series ‐ varicella zoster | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.6.1 Varicella zoster risk period (0 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.58 [0.34, 0.99] | |
| 21.6.2 Varicella zoster risk period (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.23 [0.81, 1.87] | |
| 21.6.3 Varicella zoster risk period (61 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 1.05 [0.66, 1.67] | |
| 21.6.4 Varicella zoster risk period (0 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.93 [0.68, 1.27] | |
| 21.7 Self‐controlled case series ‐ miscellaneous viral infections | 1 | Rate Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 21.7.1 Miscellaneous viral infections risk period (0 to 30 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.71 [0.37, 1.37] | |
| 21.7.2 Miscellaneous viral infections risk period (31 to 60 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.73 [0.42, 1.28] | |
| 21.7.3 Miscellaneous viral infections risk period (61 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.61 [0.29, 1.28] | |
| 21.7.4 Miscellaneous viral infections risk period (0 to 90 days) | 1 | Rate Ratio (IV, Fixed, 95% CI) | 0.68 [0.43, 1.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
aa‐Henry 2018.
| Study characteristics | ||
| Methods | RCT ‐ Phase A, observer‐blind, controlled study conducted in Czech Republic, Greece, Italy, Lithuania, Norway, Poland, Romania, Russian Federation, Slovakia, and Sweden between 2009 and 2015. Phase B, the study remained observer‐blind for all groups with the exception of the MMR+V group in countries where the national vaccination schedules included a second dose of MMR vaccination at 4 to 8 years of age (Italy, Lithuania, Romania, Russian Federation, Sweden). Phase B follow‐up of an initial multicentre study (NCT00226499) ‐ evaluation of the 10‐year efficacy of 2 doses of the combined MMRV vaccine and 1 dose of the live attenuated varicella vaccine (V) versus an MMR control group for the prevention of clinical varicella disease. This study presents results at 6 years' follow‐up of the study aa‐Prymula 2014. | |
| Participants | Healthy children aged 12 to 22 months. N = 5803 children enrolled and vaccinated. Total vaccinated cohort (TVC), in phase A, N = 4580 were included in the TVC in phase B, N = 3829 completed the study up to Year 6; N = 5289 and N = 3791 were included in the According To Protocol (ATP) cohort for efficacy in phase A + B and phase B, respectively. | |
| Interventions | 3 treatment groups: Phase A
For phase B, the study remained observer‐blind for all groups with the exception of the MMR+V group in countries where the national vaccination schedules included a second dose of MMR vaccination at 4 to 8 years of age (Italy, Lithuania, Romania, Russian Federation, Sweden). Independent data monitoring committee members also remained blinded to the study treatment group when assessing varicella cases. |
|
| Outcomes | Number (percentage) of children with reported contact with varicella or zoster disease, or both | |
| Funding Source | Pharmaceutical Industry | |
| Notes | Conclusion: 2 doses of the MMRV vaccine and 1 dose of the varicella vaccine remain efficacious through 6 years postvaccination | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer‐generated randomisation list ‐ randomised (3:3:1) ‐ block size 7 |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate ‐ participants and their parents or guardians, individuals involved in assessment of any outcome, and sponsor staff involved in review or analysis of data were masked to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate ‐ < 10%. The exclusions are well documented, and it seems unlikely that they could have affected the results. |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ all outcomes are reported |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
aa‐Povey 2019.
| Study characteristics | ||
| Methods | RCT ‐ phase 3b follow‐up of an observer‐blinded, randomised controlled trial. This study presents results at 10 years' follow‐up of the study aa‐Prymula 2014. | |
| Participants | Between 1 September 2005 and 10 May 2006, N = 5803 children aged 12 to 22 months (at first vaccination) from Czech Republic (Czechia), Greece, Italy, Lithuania, Norway, Poland, Romania, Russia, Slovakia, and Sweden | |
| Interventions | 2 doses of MMRV (N = 2279) 1 dose of MMR and 1 dose of varicella vaccine (N = 2266) 2 doses of MMR, 42 days apart (N = 744) |
|
| Outcomes | "All cases of varicella‐like rash identified by the investigator were referred to the independent data monitoring committee for blinded classification using a modified Vázquez scale (mild ≤ 7, moderately severe 8 to 15, severe ≥ 16). The variables for assessing the severity of illness were: rash (number and type of lesions), fever, pain back, or abdomen complications, and investigator’s subjective assessment of the illness. A varicella case was confirmed when it met the clinical case definition and the PCR result was positive for a wild‐type varicella virus, or when it met the clinical definition, was confirmed by the independent data monitoring committee, and was epidemiologically linked to a valid index case". | |
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusion: the 10‐year vaccine efficacy was observed, suggests that a 2‐dose schedule of varicella vaccine provided optimum long‐term protection for the prevention of varicella by offering individual protection against all severities of disease and leading to a potential reduction in transmission, as observed in the USA experience with universal mass vaccination. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer‐generated randomisation list ‐ randomised (3:3:1) ‐ block size 7 |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate ‐ participants and their parents or guardians, individuals involved in assessment of any outcome, and sponsor staff involved in review or analysis of data were masked to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate ‐ < 10% the exclusions are well documented, and seems unlikely that they could have affected the results. |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ all outcomes are reported. |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
aa‐Prymula 2014.
| Study characteristics | ||
| Methods | RCT ‐ the study was conducted in 111 study centres in Europe: Czech Republic (22), Greece (11), Italy (9), Lithuania (9), Norway (5), Poland (10), Romania (9), Russia (14), Slovakia (17), and Sweden (5). | |
| Participants | N = 5285, healthy children aged 12 to 22 months | |
| Interventions | MMRV group: 2 doses of MMRV (Priorix‐Tetra; GSK, Rixensart, Belgium) N = 2279 MMR+V group: MMR (Priorix, GSK) at dose 1 and monovalent varicella vaccine (Varilrix, GSK) at dose 2, N = 2263 MMR group (control): 2 doses of MMR (Priorix, GSK) N = 743. Doses were administered 42 days apart (Day 0 and Day 42). After completion of this first phase of the clinical trial, MMR+V group participants were offered the second dose of MMR in accordance with the immunisation schedule of their respective country. |
|
| Outcomes | The primary efficacy endpoint was occurrence of confirmed varicella (by detection of varicella zoster virus DNA or epidemiological link) from 42 days after the second vaccine dose to the end of the first phase of the trial. Cases were graded for severity. Efficacy analyses were per protocol. | |
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusion: these results support the implementation of 2‐dose varicella vaccination on a short course, to ensure optimum protection from all forms of varicella disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ computer‐generated randomisation list ‐ randomised (3:3:1) ‐ block size 7 |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ centralised randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate ‐ participants and their parents or guardians, individuals involved in assessment of any outcome, and sponsor staff involved in review or analysis of data were masked to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate ‐ < 10% the exclusions are well documented, and seems unlikely that they could have affected the results. |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ all outcomes are reported. |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ab‐Bloom 1975.
| Study characteristics | ||
| Methods | RCT, double‐blind | |
| Participants | 282 children (11 months to 4 years old) absence of any history of natural measles mumps and rubella or immunisation against these diseases. Absence of any usual medical contraindication. | |
| Interventions | 3 lots of MMR vaccine (lot 1, 2, 3 prepared from Schwarz live attenuated measles virus, Jeryl Lynn live attenuated measles virus, and Cenedehill live attenuated measles virus) versus placebo. Vaccines contained at least 1000 TCID50 for measles and rubella and 5000 for mumps. | |
| Outcomes | Observations for intercurrent illness and vaccine reactions made approximately 3 times/child between 7 to 21 days postvaccination:
|
|
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | The study does not say if all children were observed at least once. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unknown, but decoding and tabulation done by computer |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16% of possible total observations missing |
| Selective reporting (reporting bias) | High risk | No explanation for excluding symptom reports |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ab‐Ceyhan 2001.
| Study characteristics | ||
| Methods | Comparative controlled trial | |
| Participants | 1000 infants aged 38 to 40 months from 5 maternity and child health centres in Ankara, Turkey | |
| Interventions | Measles vaccine (Rouvax, Schwarz measles strain, 1000 TCID50) administered at 9 months plus MMR administered at month 15 versus MMR (Trimovax, Schwarz measles strain, 1000 TCID50; AM 9 mumps strain, 5000 TCID50; Wistar RA/27/3 rubella strain, 1000 TCID50) administered at month 12 only | |
| Outcomes | ‐ Fever 39.4 °C
‐ Runny nose
‐ Cough
‐ Rash
‐ Diarrhoea
‐ Redness
‐ Swelling Even if visits by midwife 7, 14, 28 days after vaccination to collect adverse reactions records from parents and every 3 months for 60 months phone call/visit for standard questionnaire were carried out, the time of observation for adverse events is not specified. |
|
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Semi‐randomised |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% (50/500) excluded from arm 2 because immunised with different vaccine batch |
| Selective reporting (reporting bias) | Unclear risk | The time of observations (7, 14 days), if cumulative, number of events or number of children are not specified for adverse reactions. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ab‐Edees 1991.
| Study characteristics | ||
| Methods | RCT, single‐blind | |
| Participants | 420 healthy children aged between 12 and 18 months | |
| Interventions | MMR vaccine Trimovax (Schwarz measles strain, 1000 TCID50; Urabe AM/9 mumps strain, 5000 TCID50; RA/27/3 rubella strain, 1000 TCID50) versus Measles vaccine Rouvax (Schwarz 100 TCID50) Administered in both upper arm or leg | |
| Outcomes | ‐ Local symptoms: erythema, induration, pain ‐ General ‐ specific symptoms: rash, parotitis, conjunctivitis, testicular swelling, arthralgia, arthritis, convulsions ‐ General ‐ non‐specific symptoms: temperature, adenopathy, nasopharyngeal disorders, gastrointestinal disorders, restlessness Diary completed by parents daily for 3 weeks with further 3‐weekly observations. | |
| Funding Source | Pharmaceutical industry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported clearly. |
| Summary Risk of Bias assessment | Unclear risk | The trial is judged to raise some concerns in at least 1 domain, but not to be at high risk of bias for any domain. |
ab‐Freeman 1993.
| Study characteristics | ||
| Methods | Cluster randomised controlled trial Children due to receive MMR (over a 1‐year period) were assigned to receive the vaccine (MMR II) at either 13 or 15 months, depending on the random assignment of their family physician. | |
| Participants | Children receiving MMR | |
| Interventions | MMR ‐ MMRII (Merck Sharp & Dohme) administered at either 13 or 15 months | |
| Outcomes | ‐ Cough ‐ Temperature ‐ Rash ‐ Eyes runny ‐ Nose runny ‐ Lymphadenopathy ‐ Hospital admission Assessed by daily diaries (from 4 weeks before to 4 weeks postvaccination) | |
| Funding Source | Government | |
| Notes | Only ~67% of the participants (253 out of 376) completed the study. It is not explained how delays in vaccination for some participants affected the 8‐week diary. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not reported ‐ there was insufficient information |
| Allocation concealment (selection bias) | High risk | Not reported ‐ there was insufficient information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not reported ‐ there was insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported ‐ there was insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Not reported ‐ there was insufficient information |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ab‐Lerman 1981.
| Study characteristics | ||
| Methods | RCT, double‐blind | |
| Participants | 502 healthy children aged between 15 months and 5 years | |
| Interventions | Arm 1: Rubella virus vaccine (HPV‐77‐DE 5) (Merck Sharp & Dohme) Arm 2: MMR vaccine (MMRII) with Wistar RA 27/3 rubella strain Arm 3: Measles vaccine (Merck Sharp & Dohme) Arm 4: Mumps vaccine (Merck Sharp & Dohme) Arm 5: Rubella vaccine HPV 77: CE ‐ 5 Arm 6: Rubella vaccine Wistar RA 27/3 Placebo (vaccine diluent) 1 dose subcutaneously |
|
| Outcomes | ‐ Local reactions (pain, redness, or swelling at the injection site within 4 days after immunisation) ‐ Temperature > 38 °C at 6 weeks ‐ Respiratory symptoms (6 weeks) ‐ Rash (6 weeks) ‐ Lymphadenopathy (6 weeks) ‐ Sore eyes (6 weeks) ‐ Joint symptoms (6 weeks) | |
| Funding Source | Pharmaceutical Industry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate ‐ randomly selected code |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ centralised |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ all outcomes were reported |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ab‐Peltola 1986.
| Study characteristics | ||
| Methods | RCT, double‐blind ‐ Finland | |
| Participants | 518 pairs of twins aged between 14 months and 6 years | |
| Interventions | MMR vaccine (Vivirac, Merck Sharp & Dohme) versus placebo. One 0.5 mL dose subcutaneously administered. The vaccines were administrated blind, but 1 twin of each pair first received active vaccine. |
|
| Outcomes | ‐ Temperature (< 38.5 °C; 38.6 to 39.5 °C; > 39.5 °C) rectal ‐ Irritability ‐ Drowsiness ‐ Willingness to stay in bed ‐ Rash generalised ‐ Conjunctivitis ‐ Arthropathy ‐ Tremor peripheral ‐ Cough and/or coryza ‐ Nausea or vomiting ‐ Diarrhoea Measured by parental completed questionnaire for 21 days; parents given a thermometer | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ centralised |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequate ‐ no missing |
| Selective reporting (reporting bias) | Low risk | Adequate ‐ all outcomes were reported |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ab‐Schwarz 1975.
| Study characteristics | ||
| Methods | Multicentre RCT, double‐blind | |
| Participants | A total of 1481 healthy children from different countries in North and South America were allocated. | |
| Interventions | 3 lots of MMR vaccine (Liutrin, Do Chemical containing live attenuated measles strain Schwarz, at least 1000 TCID50; mumps live strain Jeryl Lynn, at least 5000 TCID50; live rubella Cenedehill strain, at least 1000 TCID50) versus Placebo 1 dose subcutaneously administered | |
| Outcomes | Axillary and rectal temperature, rash, lymphadenopathy, conjunctivitis, otitis media, coryza, rhinitis, pharyngitis, cough, headache, parotitis, orchitis, arthralgia, paraesthesia, site adverse events, hypersensitivity. Each child was observed for adverse events approximately 3 times between 7 and 21 days. | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | ‐ Age restriction (1 to 4 years) was not enforced. ‐ A large number of participants were missing from all observations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate ‐ not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ba‐Andrade 2018.
| Study characteristics | ||
| Methods | Matched case‐control study (from November 2013 to December 2015) carried out in São Paulo and Goiânia (southeast and Midwest regions, respectively, in Brazil) | |
| Participants | Cases: defined as children aged 15 to 32 months with rash and either suspected as having varicella by an attending physician or being a contact to a confirmed varicella case. Cases were confirmed by either clinical or laboratory criteria. Controls: 2 neighbourhood controls were selected for each case. |
|
| Interventions | MMRV manufactured by GlaxoSmithKline. Evidence of prior vaccination was obtained from vaccination cards. | |
| Outcomes | Cases were further classified by severity of disease based on number of skin lesions, being 1 of:
Having been hospitalised or having any complication |
|
| Funding Source | Government | |
| Notes | Conclusions: effectiveness of single‐dose varicella vaccine in Brazil is comparable to that in other countries where breakthrough varicella cases have also been found to have occurred. The goal of the varicella vaccination programme, along with disease burden and affordability, should be taken into consideration when considering the adoption of a second dose of varicella vaccine into national immunisation programmes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ laboratory‐confirmed |
| CCS ‐ control selection | Low risk | Adequate ‐ community control |
| CCS ‐ comparability | Low risk | Adequate ‐ for each case of varicella, 2 neighbourhood controls were selected, matched by age (15 to 32 months) |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record ‐ vaccination cards |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ba‐Castilla 2009.
| Study characteristics | ||
| Methods | Case‐control study ‐ Navarre, Spain | |
| Participants | The cases were all children residing in Navarre born between 1998 and 2005 who had a diagnosis of mumps confirmed microbiologically or epidemiologically between August 2006 and June 2008. Cases occurring before age 15 months were excluded, as were those whose paediatrician could not be identified. For each case, 5 individually matched controls were selected amongst children with the same sex, municipality, district of residence, and paediatrician. Matching was performed by selecting controls with the closest birth date within the same calendar semester to the corresponding case. We excluded as controls those children who had been diagnosed with mumps before the date the case was diagnosed or who had not fulfilled all the pairing criteria since the beginning of 2006; these children were replaced with the next child who met the inclusion criteria. Cases (N = 241): children aged 1 to 10 years with confirmed (laboratory or epidemiologically) mumps with symptoms of disease between August 2006 and June 2008 Controls (N = 1205): children matched for sex, municipality, district of residence, and paediatrician |
|
| Interventions | MMR vaccine prepared with Jeryl Lynn mumps strain | |
| Outcomes | Exposure to MMR vaccine at least 30 days before mumps onset | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ clinically or laboratory‐confirmed |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ matched by sex, birth date, district of residence, and paediatrician |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record ‐ blinded review |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ba‐Cenoz 2013.
| Study characteristics | ||
| Methods | Case‐control study ‐ Spain | |
| Participants | Case (N = 54): children aged 15 months to 10 years with a diagnosis of varicella confirmed by PCR Control (N = 432): matched (1:8) by paediatric practice, district of residence, and date of birth |
|
| Interventions | Varicella vaccine | |
| Outcomes | Laboratory‐confirmed cases | |
| Funding Source | Government | |
| Notes | The results of this study show that the varicella vaccine is effective in preventing confirmed cases of varicella, although the effect of this vaccine depends on the number of doses and the time since the last dose. Vaccine effectiveness was 87% for 1 dose and 97% for 2 doses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ laboratory‐confirmed |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ matched (1:8) by paediatric practice, district of residence, and date of birth |
| CCS ‐ exposures | Low risk | Adequate ‐ Navarre vaccination registry ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ba‐Defay 2013.
| Study characteristics | ||
| Methods | Matched case–control study ‐ Quebec, Canada | |
| Participants | Cases and controls received 2 doses of measles‐containing vaccine, first dose administered at ≥ 12 months of age, second dose administered ≥ 28 days after dose 1 and ≥ 14 days before rash onset in the matched case, and age between 5 and 17 years. Measles confirmed by laboratory testing or epidemiologic link is notifiable by both physicians and laboratories in Quebec. Laboratory confirmation requires virus detection by culture or PCR or development of measles‐specific immunoglobulin M in absence of recent vaccination. Epidemiologic link requires classic clinical presentation (fever ≥ 38.3 °C (101 °F) and cough or coryza or conjunctivitis and a generalised maculopapular rash for at least 3 days) with epidemiologic link to a laboratory‐confirmed measles case. Cases included only confirmed measles as defined above and reported from across the province to public health between 1 January and 31 December 2011. Controls were matched for the date of birth (more or less 6 months) and school attended in 2010 to 2011. For each case, 5 controls were randomly selected from the provincial measles vaccination registry amongst all students meeting matching criteria. |
|
| Interventions | MMR‐II (Merck Canada, Montreal, Quebec) was the only MMR vaccine administered to the paediatric cohorts included in this study. | |
| Outcomes | The vaccination status and dates of vaccination were ascertained through the provincial vaccination registry and other records. | |
| Funding Source | Government | |
| Notes | Study conclusion: a significantly greater risk of measles amongst 2‐dose recipients whose first dose was given at 12 to 13 months rather than ≥ 15 months of age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ laboratory‐confirmed |
| CCS ‐ control selection | Low risk | Adequate ‐ community controls |
| CCS ‐ comparability | Low risk | Adequate ‐ matching (see above) |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record ‐ vaccination registry |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ba‐Fu 2013.
| Study characteristics | ||
| Methods | Case‐control study. Amongst children in Guangzhou aged 8 months to 12 years during 2006 to 2012 | |
| Participants | Case participants 8 months to 12 years of age were randomly selected from 2 electronic databases in Guangzhou: the Notifiable Disease Reporting System and the Children’s Expanded Programmed Immunization (EPI) Administrative Computerised System. Controls were randomly selected amongst children aged 8 months to 12 years listed in the Children’s EPI Administrative Computerised System, which was designed to manage the immunisation records of children less than 7 years of age in Guangzhou in 1997. Controls were accepted if they did not have prior history of mumps, as confirmed by a phone call by physicians from the Guangzhou Center for Disease Control and Prevention. A list of potential controls with sequence number for each case participant was then created and matched by birth date, gender, and residence (living area, in the same community or village, and residence was categorised into urban, rural, and rural‐urban continuum area). A random number was used to select the potential control. If the potential control declined to participate or had prior history of mumps disease, or both, a control candidate with the next‐closest date of birth to the case participant was enrolled to participate. |
|
| Interventions | The EPI system allows healthcare workers to easily record, retrieve, and analyse all children’s vaccination information; registration of vaccination information in the system is required. Vaccines MMR or measles‐rubella | |
| Outcomes | A mumps case was defined as having acute onset of unilateral or bilateral tender swelling of the parotid of salivary gland lasting 2 or more days without any other apparent cause. Bacterial infection was excluded by the absence of an increase in white blood cell count. | |
| Funding Source | Government | |
| Notes | Only mumps vaccinations received at least 30 days before the onset of mumps disease were considered valid. For controls, we considered only doses administered up to 30 days before the date of symptom onset in the corresponding case participant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ from 2 electronic databases |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Birth date, gender, and residence (living area, in the same community or village, and residence was categorised into urban, rural, and rural‐urban continuum area) |
| CCS ‐ exposures | Unclear risk | The type of vaccine administered is missing in a high percentage of vaccinated. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ba‐Giovanetti 2002.
| Study characteristics | ||
| Methods | Case‐control study | |
| Participants | Children and adolescents aged 14 months to 15 years from an Italian Local Health Agency with 12,880 residents of this age group Cases (N = 139): clinical mumps cases identified by national infectious diseases surveillance system within study area Controls (N = 139): randomly selected from immunisation registry, matched for birth year and address |
|
| Interventions | MMR (Urabe or Rubini or RIT4385‐Jeryl Lynn) vaccine exposure at least 30 days before disease onset (registry and phone interviews) | |
| Outcomes | Association between MMR vaccine exposure and clinical measles within 30 days | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Clinical definition ‐ secure record ‐ representative series of cases |
| CCS ‐ control selection | Unclear risk | Randomly selected ‐ community |
| CCS ‐ comparability | Unclear risk | Possible residual confounding ‐ matched for birth year and address |
| CCS ‐ exposures | Unclear risk | Structured interview ‐ study did not distinguish between mumps strain (Urabe, Jeryl Lynn, and Rubini) |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ba‐Goncalves 1998.
| Study characteristics | ||
| Methods | Case‐control study ‐ Oporto, Portugal | |
| Participants | Only children born after 1979, aged 15 months or more when they developed mumps, were included as cases. This was done to prevent bias against the vaccine because children under 15 months of age and those born before 1980 would not have been vaccinated. Cases that arose in 1995 or 1996 were selected from the notification files of the health authority. Notification forms included the diagnosis, date of onset, and whether the patient was admitted to hospital, but no details of signs and symptoms. Individual vaccination records were traced and reviewed in the health centres where the children were registered. 2 consecutive vaccination records, corresponding to children of the same sex as the case and born in the same month and year, were selected as controls, whether or not they had already had mumps. This sampling scheme for controls was used so that the odds ratio for the exposure would yield an estimate of the relative risk. Before 1 November 1992 (immunisation with Urabe mumps strain): Cases (N = 73): clinical mumps cases reported by GPs or hospital doctors during the 1995 to 1996 mumps outbreak Controls (N = 169): 2 consecutive vaccination records of the same sex, month and birth year as the case, were selected After 1 November 1992 (immunisation with Rubini mumps strain): Cases (N = 133): clinical mumps cases reported by GPs or hospital doctors during the 1995 to 1996 mumps outbreak Controls (N = 236): 2 consecutive vaccination records of the same sex, month and birth year as the case, were selected |
|
| Interventions | MMR vaccination. As strain was not reported in vaccination records, authors assume that until 1 November 1992 Urabe strain has been administered, whereas Rubini strain thereafter. | |
| Outcomes | Association between MMR vaccine exposure and clinical measles | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | High risk | Incompleteness of notification |
| CCS ‐ control selection | High risk | There was insufficient information. |
| CCS ‐ comparability | High risk | There was insufficient information. |
| CCS ‐ exposures | High risk | No vaccination record for all cases |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ba‐Harling 2005.
| Study characteristics | ||
| Methods | Case‐control study carried out on children from a religious community in North East London, as a measles outbreak occurred (June 1998 to May 1999). The community was located in quite a small area, with own schools and amenities, and was served by 2 GPs. MMR vaccination coverage in the community ranged between 67% and 86%. | |
| Participants | Cases (N = 161): clinical or laboratory mumps diagnoses with onset date between 18 June 1998 to 2 May 1999 observed in children aged from 1 to 18 years who belonged to the community, identified through mumps notification from the 2 GPs to the local Consultant Communicable Disease Control, searching of the electronic practice list for diagnoses made using the terms 'mumps' and successive checking, or verbal reports by community members. For notified cases, laboratory testing (oral fluid for IgM antibody and mumps RNA was made available at the enteric, respiratory and neurological virus laboratory (ERNVL)). Altogether, 161 mumps cases with onset during the outbreak were observed (142 notified by GPs, 12 through search in the electronic practice list, and 7 reported by parents). 1 case had no date of onset specified, but illness occurred in the outbreak period. Out of the 142 notified cases, 43 also had laboratory confirmation of infection by IgM radioimmunoassay, PCR detection of mumps RNA, or both. Controls (N = 192) were selected from children in the community registered with the 2 practices. They were chosen by random samples from electronic practices lists in order to match age and sex profile of the cases. Community membership was ascertained by cases. | |
| Interventions | Vaccination status of cases and controls (together with clinical details of cases) was obtained from practice records and cross‐checked with child health immunisation database of the local health authority. Laboratory records were obtained from ERNVL. As vaccination status was available for 156 cases and 175 controls, data analysis was carried out on this population. 79 cases and 134 controls received at least 1 dose of MMR vaccine at least 1 month before disease onset. Even if authors did not report any descriptions of the MMR vaccine used for immunisation, it is assumed that mumps component was Jeryl Lynn strain, as it was in use in the UK at study time. | |
| Outcomes | Association between measles (clinically defined) and receiving of any doses, 1 or 2 doses of MMR vaccine at least 1 month before disease onset Association between laboratory‐confirmed measles cases and receiving of any doses of MMR vaccine at least 1 month before disease onset |
|
| Funding Source | Government | |
| Notes | Composition and description of the administered vaccine was not provided, although it is stated that in UK at study time, MMR vaccine was prepared using the Jeryl Lynn strain. Authors note that the presence of controls who have had mumps infection in the past (i.e. could have developed immunity without vaccination) and the longer exposition to the outbreak for the cases, could have led to underestimation of vaccine effectiveness. Other factors other than sex, age, and practices could moreover have influenced the risk of infection and vaccination status of both cases and controls (e.g. if they were drawn from different residential areas or from groups with different levels of herd immunity and different behaviours). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ representative series of cases |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ match age and sex |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ba‐Hungerford 2014.
| Study characteristics | ||
| Methods | Case‐control study ‐ Liverpool, UK | |
| Participants | Case was defined as a person (median age 16 years old, upper quartile age 76 years old) living in Merseyside with microbiological confirmation of measles (oral fluid/blood test IgM positive or PCR positive) between 1 January and 14 March 2012 with no history of vaccination within 6 weeks of diagnosis. Cases were identified with a computerised case management database, used by Cheshire & Merseyside Health Protection Team. As the assessment focused on possible transmission settings, cases were excluded from the study if they had travelled outside of the UK in the 2 months preceding the onset of illness. In total, there were n = 71 confirmed cases of measles in Merseyside; 1 case was excluded from the study due to travel outside of the UK, leaving n = 70 cases for random allocation in the study. Controls were defined as asymptomatic persons (no history of fever and rash) with no history of travel outside of the UK in the 2 months preceding the onset of illness in the matched case. The controls were selected at random, matched by general medical practice and age (within 1 year). To ensure that all cases were matched to an appropriate number of controls, 5 potential controls were identified for each case to allow for those who refused to participate or were untraceable; if information could not be obtained for the selected control, another control was chosen according to the same principles. |
|
| Interventions | Telephone interviews were undertaken following acquisition of valid consent using an agreed script and a structured questionnaire. Information was collected on demographics and vaccination history. Data were also obtained on community and healthcare settings attended in the 2 weeks preceding the onset of illness in the matched case, therefore any case participants that were hospital inpatients prior to onset were not admitted to hospital due to the measles virus. Information was collected on demographics, vaccination history, community settings visited, and attendance at healthcare settings. The interviews were conducted with a parent or guardian if the case/control was under 16 years of age. | |
| Outcomes | Vaccination status was defined as: (1) vaccinated appropriately for age; (2) incompletely/partially vaccinated for age (> 13 months); (3) under age for vaccination (< 14 months). | |
| Funding Source | Government | |
| Notes | Is not completely clear if vaccination status, collected by interview, was confirmed by the Health Authority. Authors' conclusion: "This matched case‐control study provides further strong evidence that eligible children and young adults who are unimmunized/partially immunized and those who are too young to be vaccinated are at significantly increased risk of measles infection when measles virus is circulating." "This study found that being too young for vaccination increased the risk of measles infection" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ laboratory‐confirmed |
| CCS ‐ control selection | Low risk | Adequate ‐ community control |
| CCS ‐ comparability | Low risk | Adequate ‐ matched for general medical practice and age |
| CCS ‐ exposures | Unclear risk | Adequate ‐ is not completely clear if vaccination status, collected by interview, was confirmed by the Health Authority. |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ba‐Jick 2010.
| Study characteristics | ||
| Methods | Case‐control study carried out in England | |
| Participants | Cases = measles cases diagnosed in 1994, age 1 to 19 years, born from 1982 onwards (n = 1261) Controls = no prior measles, matched to each case on year of birth, gender, general practice attended, index date (n = 4996) Cases who were diagnosed with measles in 1994, age 1 to 19 at the time of the diagnosis, and who were born in or after 1982. The controls were randomly selected up to 4 controls who had no prior diagnosis of measles, matched to each case on year of birth, gender, general practice attended, index date (the date of the case’s measles diagnosis), and the duration of time the patient had been registered in the database. The immunisation history was retrieved for each case and control to determine receipt of a measles vaccine prior to the index date and how many prior measles vaccines had been received. |
|
| Interventions | MMR or MR vaccine A person was considered to have been vaccinated against measles if they had a measles‐containing vaccination recorded in their computerised medical record. |
|
| Outcomes | Case of measles: if they had a clinical diagnosis of measles recorded in their computerised medical record (no laboratory confirmation) | |
| Funding Source | Not stated | |
| Notes | Unclear MMR or MR exposure. Based on the controls, the authors estimate that in 1994, 65% of children age 1 to 2 years had been vaccinated with the MMR vaccine; 87% of children age 3 to 4 years had been vaccinated; 77% of children age 5 to 9 years had been vaccinated; and 28% of those aged 10 to 19 years had been vaccinated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Unclear risk | Possible selection bias ‐ no laboratory confirmation ‐ cases recorded in their computerised medical record |
| CCS ‐ control selection | Unclear risk | Possible selection bias ‐ 4 controls no prior measles |
| CCS ‐ comparability | Low risk | Matching year of birth, gender, general practice attended |
| CCS ‐ exposures | Unclear risk | Unclear MMR or MR exposure ‐ vaccination recorded in their computerised medical record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ba‐Kim 2012.
| Study characteristics | ||
| Methods | Prospective and retrospective case‐control studies in 4 university hospitals in Korea | |
| Participants | Children (a) prospective study: N = 55 cases of mumps were identified and 165 controls were selected from March 2010 to October 2011. Data about their demographic characteristics (b) retrospective study: N = 122 cases of mumps were identified and n = 449 controls were selected. In 2008 to 2009 in western Seoul, Incheon, and Goyang, an outbreak of mumps. |
|
| Interventions | (a) MMR vaccination status were collected in cases and controls. (b) 98% of cases whose vaccination status were available had a history at least 1 MMR vaccination. |
|
| Outcomes | Risk for disease estimated by conditional logistic analysis | |
| Funding Source | Not stated | |
| Notes | Only abstract. Conclusion: mumps vaccine had preventive effect, and 2‐dose vaccination had superior effect than 1 dose, even though there was no statistically significant difference. In addition to the efficacy of the vaccine, other factors that are involved in occurrence of mumps outbreak must be considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Unclear risk | Not stated |
| CCS ‐ control selection | Unclear risk | Not stated |
| CCS ‐ comparability | Unclear risk | Insufficient information |
| CCS ‐ exposures | Unclear risk | Not stated |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ba‐Liese 2013.
| Study characteristics | ||
| Methods | Case‐control study ‐ Munich, Bavaria, Germany | |
| Participants | Children at least 1 year of age, born on or after 1 July 2003, residing in Germany Cases: suspected clinical varicella disease at the time of study entry Control: children matched by age and paediatric practice, fulfilling the same criteria as cases but without history or present clinical diagnosis of varicella |
|
| Interventions | Cases were classified as vaccinated varicella cases if they had received OKA/GSK, OKA/Merck, or the combined MMR‐OKA/GSK vaccine at least 28 days before varicella onset. Controls were classified as vaccinated if they had received OKA/GSK, OKA/Merck, or MMR‐OKA/GSK vaccine at least 28 days before varicella onset in the matched case. |
|
| Outcomes | Laboratory or clinically confirmed | |
| Funding Source | Pharmaceutical industry | |
| Notes | Ascertainment of the vaccination status by practice record and vaccination cards | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ laboratory‐confirmed ‐ representative series of case |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ matched by age and paediatric practice |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record‐ vaccination card |
| Summary Risk of Bias assessment | Low risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ba‐Mackenzie 2006.
| Study characteristics | ||
| Methods | Case‐control study carried out in a private school in Lothian, Scotland to evaluate effectiveness of 1 or 2 doses of MMR vaccine | |
| Participants | October to November 2004 Cases (N = 20): virologically confirmed mumps cases Controls (N = 40): participants matched to cases for age, sex, residential status, and country source (UK or other) |
|
| Interventions | MMR immunisation with 1 or 2 vaccine doses (no description of composition) | |
| Outcomes | Protective effectiveness of MMR immunisation against virologically confirmed mumps | |
| Funding Source | Government | |
| Notes | This study is at high risk of bias due to the following:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | High risk | There was insufficient information. |
| CCS ‐ control selection | High risk | Controls did not have record of previous mumps infections. |
| CCS ‐ comparability | High risk | There was insufficient information. |
| CCS ‐ exposures | High risk | Poor accuracy in reporting vaccination status by parents of some children |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ba‐Vazquez 2001.
| Study characteristics | ||
| Methods | Case–control study | |
| Participants | Healthy children between 13 months and 16 years of age Exclusion criteria: children for whom the vaccine is not routinely recommended. Children who had received the vaccine within the preceding 4 weeks. Cases: identified by means of active surveillance. The parents of eligible children were invited to participate in the study, and written informed consent was obtained. A research assistant (who was unaware of the vaccination status of the child) visited the home of each patient with possible chickenpox (ideally on day 3 of the illness, but as late as day 5 when necessary). In addition, vesicular fluid was collected to test for the presence of varicella–zoster virus by the PCR. Controls: for each child with a potential case of chickenpox, 2 controls, matched according to date of birth (within 1 month) and paediatric practice, were selected. A list of potential controls was generated from the computerised database of the practice, which consisted of all patients in the practice born between 30 days before and 30 days after the birth of the child with the potential case of chickenpox. |
|
| Interventions | MMR vaccine versus MMR+V vaccines The medical records of all the children (from all sources of care) were reviewed to obtain information about all previous immunisations. Children for whom there was written documentation that they had received varicella vaccine 4 weeks or more before the “focal time” ‐ the date of onset of chickenpox or, for the controls, the date of onset in the matched children with chickenpox ‐ were classified as vaccinated. As per current recommendations, children with potential cases of chickenpox and their matched controls who were 13 years of age or older were considered to have been vaccinated if they had received 2 doses of vaccine at least 4 weeks before the focal time. |
|
| Outcomes | Protective effectiveness of MMR+V immunisation against virologically confirmed varicella, all cases and all controls received MMR vaccine | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ laboratory‐confirmed |
| CCS ‐ control selection | Low risk | Adequate ‐ community controls |
| CCS ‐ comparability | Low risk | Adequate ‐ matched according to date of birth (within 1 month) and paediatric practice |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record ‐ medical record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Ahlgren 2009.
| Study characteristics | ||
| Methods | Case‐control study ‐ Sweden | |
| Participants | Cases: participants with MS or clinically isolated syndrome born between 1959 and 1986 and disease onset at age ≥ 10 years, resident in the Gothenburg area. The study area and the greater part of the patient material were the same as in the cohort study cb‐Ahlgren 2009, which was restricted to the age group 10 to 39 years, born between 1959 and 1990. Controls: participants from the same area as the cases (randomly selected from General Population Register) born in the same year as cases. |
|
| Interventions | MMR vaccination (vaccination with single‐component vaccines has also been considered) The second was therefore restricted to the subgroup of the MMR vaccinations. The first analysis was restricted to the subgroup 'MMR vaccination'. 4 disjointed vaccination categories were defined: (0) no MMR vaccination; (1) early MMR vaccination only; (3) late MMR vaccination only; (4) both an early and a late MMR vaccination. Comparisons were made within the group of MMR vaccinations. |
|
| Outcomes | Risk of MS associated with MMR exposure | |
| Funding Source | Government | |
| Notes | Conclusion: there was no overall effect of the MMR vaccinations on MS risk. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Unclear risk | Insufficient information |
| CCS ‐ control selection | Unclear risk | Community control |
| CCS ‐ comparability | Low risk | Matched by age |
| CCS ‐ exposures | High risk | Information bias ‐ by questionnaire not blinded to case or control status |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
bb‐Baron 2005.
| Study characteristics | ||
| Methods | Case control study ‐ to examine environmental risk factors prior to the development of inflammatory bowel disease in a paediatric population‐based case‐control study | |
| Participants | This was a population‐based matched case‐control study. Cases were all patients from the EPIMAD registry (registry of IBD in Northern France since 1988) who had a diagnosis of either CD or UC between January 1988 and December 1997 and were less than 17 years old at the time of IBD diagnosis. Controls were randomly selected from telephone number lists (random‐digit dialling) and matched 1:1 to each case by age (2 years), sex, and living area (region). A total of 222 incident cases of Crohn’s disease and 60 incident cases of ulcerative colitis occurring before 17 years of age between January 1988 and December 1997 were matched with 1 control participant by sex, age, and geographical location. We recorded 140 study variables in a questionnaire that covered familial history of inflammatory bowel disease, events during the perinatal period, infant and child diet, vaccinations and childhood diseases, household amenities, and the family’s socioeconomic status. |
|
| Interventions | MMR vaccination | |
| Outcomes | Crohn’s disease; ulcerative colitis | |
| Funding Source | Government | |
| Notes | Conclusions: whilst family history and appendicectomy are known risk factors, changes in risk based on domestic promiscuity, certain vaccinations, and dietary factors may provide new aetiological clues. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ regional registry |
| CCS ‐ control selection | Unclear risk | Probable selection bias ‐ community ‐ random‐digit dialling |
| CCS ‐ comparability | Low risk | Case by age (2 years), sex, and living area (region) |
| CCS ‐ exposures | Unclear risk | Probable information bias ‐ exposition self‐reported |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Bertuola 2010.
| Study characteristics | ||
| Methods | Case‐control study ‐ from November 1999 to December 2007 | |
| Participants | Cases (N = 387): children aged between 1 month and 18 years of age with acute immune thrombocytopenia (defined as platelets count < 100,000/μL at admission) recorded between November 1999 and September 2007 Controls (N = 1924): children of the same age, hospitalised during the same period as cases with acute neurological disorders and endoscopically confirmed gastroduodenal lesions were considered as controls |
|
| Interventions | MMR vaccine exposure (strain composition not reported) | |
| Outcomes | Risk of acute immune thrombocytopenia during the 6 weeks following MMR immunisation | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ hospital admission |
| CCS ‐ control selection | Low risk | Adequate ‐ hospital control |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ matching by age |
| CCS ‐ exposures | Unclear risk | Probable information bias ‐ structured interview |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Black 1997.
| Study characteristics | ||
| Methods | Multicentre case‐control study, between 1992 and 1993 | |
| Participants | Children 12 to 23 months old from the Vaccine Safety Datalink project. Cases: children 1 to 2 years old with confirmed AM identified by hospital record (discharge diagnosis and cerebrospinal fluid white blood cell count, ICD‐9: 045.2, 047.*; 048.*; 072.1; 321.2 322.*). Cases of AM were reviewed against a predefined case definition of no evidence of prior underlying meninginitis or underlying disease caused by toxoplasmosis, syphilis cytomegalovirus neonatal herpes simplex, or HIV. Bacterial mycobacterial and fungal cultures of cerebrospinal fluid must have been negative. (The same exclusion criteria were used for controls.) N = 59 Controls: children matching cases by age, sex, HMO membership status (N = 188) | |
| Interventions | Vaccination with MMR (Jeryl Lynn strain), data from medical records | |
| Outcomes | Risk of AM within 14 days, 30 days, 8 to 14 days of vaccination | |
| Funding Source | Government | |
| Notes | Authors' conclusion: "no increased risk of aseptic meningitis after MMR vaccine was found" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ hospital record |
| CCS ‐ control selection | Unclear risk | There was insufficient information ‐ probable hospital controls |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ matching cases by age, sex, HMO membership status |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record ‐ medical record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Black 2003.
| Study characteristics | ||
| Methods | Population‐based 1) Case–control study to estimate the relative risk of developing ITP within 6 weeks after MMR vaccination 2) Nested case–control analysis to evaluate whether there was any relationship between recent MMR vaccination and the risk of ITP |
|
| Participants | All children aged less than 6 years old, enrolled in the GPRD within 4 months of birth, and born between 1 January 1988 and 31 December 1999. As an initial broad search, we identified children with a first‐time diagnosis of thrombocytopenia (ICD 287.1) from the base population. Review of the computer records by 2 investigators, blinded to the MMR vaccination status, enabled exclusion of children with illnesses predisposing to thrombocytopenia or purpura (i.e. not ITP). To each case aged 13 to 24 months, up to 6 controls were matched by age at index date (within 1 month), practice, and sex. The index date for each case was assigned as the index date for the matched controls, and the same exclusion criteria were applied. Cases: (N = 23) children enrolled in the GPRD, aged less than 6 years with ITP Controls: (N = 116) matched by age at index date, practice, and sex |
|
| Interventions | MMR vaccine (from GPRD records) | |
| Outcomes | Exposure to MMR within 6 weeks or 7 to 26 weeks | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | Controls are not described very well (e.g. it is unclear from which population they were drawn). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ GPRD |
| CCS ‐ control selection | Unclear risk | Probable selection bias ‐ community ‐ insufficient information |
| CCS ‐ comparability | Low risk | Adequate ‐ matching age at index date, GPRD and sex |
| CCS ‐ exposures | Unclear risk | Probable secure record ‐ insufficient information |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Bremner 2005.
| Study characteristics | ||
| Methods | Nested case‐control studies: carried out in UK (England, Wales, Scotland, Northern Ireland) using 2 large databases of primary care consultation. The GPRD cohort of 76,310 children born between 1989 and 1993 from 464 general practices, and within a DIN cohort of 40,183 children born between 1989 and 1997 from 141 general practices. | |
| Participants | Case Certain (Definition I): a child with hay fever diagnosis before 24 months of age, and a second diagnosis of hay fever or a relevant therapy in a subsequent years and with a 3rd diagnosis or a relevant therapy in a further year Case Certain (Definition II): a child without first diagnosis before 24 months of age, but with a second diagnosis of hay fever or a relevant therapy in subsequent year Case Less Certain (Definition I): a child as a case certain (Definition I) without 3rd diagnosis of hay fever or a relevant therapy in a further year Case Less Certain (Definition II): a child with at least a hay fever diagnosis, even if there is not a second diagnosis or a relevant therapy in a subsequent year For GPRD, 2115 Cases Certain and 2271 Cases Less Certain were selected. After exclusion of cases without a suitable control, left 2025 Cases Certain and 2171 Cases Less Certain. For DIN, 1480 Cases Certain and 1477 Cases Less Certain were selected. After exclusion of cases without a suitable control, left 1459 Cases Certain and 1443 Cases Less Certain. Only codex synonymous with “allergic rhinitis” with seasonal variation in recording were permitted. Description of controls: the controls were children who had no allergic rhinitis or hay fever diagnosis. A suitable control matched a case (1:1) with a practice ID, age, sex, and index date (date of a first diagnosis in a 'Less Certain' case, or date of confirmatory diagnosis or therapy if a certain case). |
|
| Interventions | MMR II (first entries). The time categories for MMR immunisation were: 1st to 13th month, 14th, 15th, 16th, 17th, 18th to 24th, 25th month of life, or later. The study considers also association with DTP and BCG vaccines. | |
| Outcomes | Risk of hay fever at different immunisation ages, using administration at 14 months of age as reference value | |
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusions: immunisation against DTP or MMR does not increase the risk of hay fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ representative series of case ‐ population based |
| CCS ‐ control selection | Low risk | Adequate ‐ community control |
| CCS ‐ comparability | Low risk | Adequate ‐ matching: practice ID, age, sex, and index date |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Bremner 2007.
| Study characteristics | ||
| Methods | Case‐control study | |
| Participants | Case of hay fever were children with diagnostic codes and/or treatment for hay fever (see bb‐Bremner 2005), after 2 years of age. Control was child that matched for general practice, sex, birth month, and follow‐up of control "to at least date of diagnosis case". | |
| Interventions | MMR II | |
| Outcomes | Incidence of hay fever following MMR exposure was compared inside versus outside the grass pollen season. | |
| Funding Source | Pharmaceutical industry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ representative series of case ‐ population based |
| CCS ‐ control selection | Low risk | Adequate ‐ community control |
| CCS ‐ comparability | Low risk | Adequate ‐ matching: practice ID, age, sex, and index date |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Chen 2018.
| Study characteristics | ||
| Methods | Nested case–control study between January 2011 and December 2015 ‐ China | |
| Participants | Case: from the hospital information system's first mention of International Classification of Diseases, 10th Revision (ICD‐10) diagnostic codes (G04.001, G04.002, G04.051, G04.903, G04.912) for ADEM from 1 January 2011 to 31 December 2015, for individuals of any age. Diagnoses were confirmed by neurologists from clinical data, such as clinical manifestations, CT, EEG, CSF, and MRI examinations. N = 272 Controls: for each ADEM case, 4 control individuals randomly selected from the same hospital with no history of ADEM were matched to the case according to year of birth (within 1 year), gender, and zip code (a surrogate measure for socioeconomic status) during the same period. The control participants were assigned the same index date as their matched case (symptom onset date). Controls were patients referred for headache (except trigeminal neuralgia), migraine, vascular, or other diseases that were thought not to modify the probability of vaccination. Patients with chronic severe neurological diseases or autoimmune diseases were excluded. N = 1096 |
|
| Interventions | MMR vaccination | |
| Outcomes | Information on vaccinations was obtained from the Information Management System for Immunization Programming, in which anyone who received vaccinations would have been registered, matched with ID number and verified by paper vaccination records. Any vaccination was considered to be an exposure. The trial authors collected information on all vaccinations received within 180 days. | |
| Funding Source | Government | |
| Notes | Conclusions: findings from the present study do not demonstrate an association of vaccines with an increased risk of ADEM and its recurrence among either paediatric (< 18 years) or adult (≥ 18 years) individuals within the 180 days after vaccinations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ diagnoses were confirmed by neurologists |
| CCS ‐ control selection | Low risk | Adequate ‐ hospital control |
| CCS ‐ comparability | Low risk | Adequate ‐ matching for age, gender, address |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Da Dalt 2016.
| Study characteristics | ||
| Methods | Multicentre case control study ‐ Italy. The aim of this study was to estimate the association of Henoch‐Schönlein purpura with drug and vaccine administration in a paediatric population. | |
| Participants | The study on drug and vaccine safety in children involved 11 Italian paediatric hospitals/wards spread throughout the country (Treviso, Padua, Naples, Genoa, Turin, Florence, Perugia, Palermo, Messina, and Rome, with 2 centres). Enrolled in the study were all children (age > 1 month and ≤ 18 years) hospitalised through the emergency departments for the following acute conditions: thrombocytopenia (platelet count < 100 × 103/L); acute non‐infectious, non‐febrile neurological disorders; endoscopically confirmed gastroduodenal lesions and/or clinically defined haematemesis and melena and non‐infectious muco‐cutaneous diseases and vasculitis. Exclusion criteria were represented by a concomitant diagnosis of cancer or immunodeficiency. All children hospitalised with a diagnosis of Henoch‐Schönlein purpura at admission were included as cases. Discharge diagnosis was retrieved from clinical records and validated by clinicians, according to EULAR/PRINTO/PRES criteria for classification of HSP. Validation was conducted retrieving data from individual patient clinical record, blinded with respect to drug and vaccine exposure. Only validated cases were analysed. Children hospitalised for gastroduodenal lesions were considered as appropriate controls, since they represent an acute condition admitted through the emergency departments in the same clinical centres in which cases were identified. | |
| Interventions | Vaccines MMR and DTaP (diphtheria, tetanus, acellular pertussis) not described. | |
| Outcomes | Diagnosis of Henoch‐Schönlein purpura | |
| Funding Source | Government | |
| Notes | Conclusions: the association between MMR vaccination and HSP confirms previous published findings and adds a risk estimate. Further studies are needed to increase our understanding of the role of drugs and vaccines in the aetiology of HSP, a disease with important effects on the health of children for its potential, though rare, chronic outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ multicentre study |
| CCS ‐ control selection | Low risk | Adequate ‐ hospital control |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ not described |
| CCS ‐ exposures | Unclear risk | Probable information bias ‐ structured interview |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Davis 2001.
| Study characteristics | ||
| Methods | Case‐control study | |
| Participants | Potential cases were selected by ICD‐9 codes specific for Crohn's disease, ulcerative colitis, and idiopathic proctocolitis (ICD‐9 codes 555 and 556) in the computerised databases. Case and control selection was limited to people born after 1979. To be included, cases and controls had to be enrolled from age 6 months up to the index date (the first date of disease diagnosis or symptoms for cases) or reference date for controls. Vaccine Safety Datalink Project (VSDP), children enrolled from the 6th month Cases: cases of definite IBD (VSDP, N = 142) Controls: children matched for sex, HMO, and birth year (N = 432) |
|
| Interventions | Exposure to MMR or other measles‐containing vaccines (MCV) | |
| Outcomes | Exposure to MMR or MCV considering any time, within 2 to 4 months, within 6 months | |
| Funding Source | Government | |
| Notes | There are no details of vaccine type, i.e. manufacturer, strains, dosage, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record |
| CCS ‐ control selection | Unclear risk | Adequate ‐ community |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ matched for sex, HMO, and birth year |
| CCS ‐ exposures | Unclear risk | Probably adequate ‐ secure record, but there are no details of vaccine type, i.e. manufacturer, strains, dosage, etc. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐De Stefano 2004.
| Study characteristics | ||
| Methods | Retrospective case‐control ‐ Atlanta, Georgia, USA | |
| Participants | Children with autism were identified from the Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP), a multiple‐source, population‐based surveillance programme that monitors the occurrence of selected developmental disabilities amongst children in the 5‐county metropolitan Atlanta area. In 1996, the first year in which autism was included, MADDSP identified 987 children 3 to 10 years of age with autism. Autism cases were identified through screening and abstraction of source files at schools, hospitals, clinics, and specialty providers. Clinical psychologists with expertise in the diagnosis of autism reviewed the abstracted records according to a standardised coding scheme to determine the presence of behavioural characteristics consistent with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition 1 criteria for autism spectrum disorders. Cases: case children were derived from MADDSP during the period of 1999 through 2001. N = 624 Controls: control children were selected from regular education programmes and were matched to case children based on age in 1996 (within 1 year), gender, and school of attendance at the time of abstraction. N = 1824 For all case and control children, the authors obtained demographic information, including date of birth, gender, race, and birth state, from the birth certificate or registration form that is kept in each child’s permanent school record. The authors matched 355 (56%) case and 1020 (56%) control children to Georgia state birth certificate records, which allowed them to obtain additional information, such as each child’s birthweight and gestational age and the mother’s parity, age, race, and education. |
|
| Interventions | Exposure to MMR vaccine (not better defined) Trained abstractors collected vaccination histories for both case and control children from the standardised state immunisation forms. Georgia law required at least 1 dose of MMR vaccines, usually administered at 15 months of age as the combined MMR vaccine. Vaccination was also required for enrolment in preschool special education programmes for 3‐ to 5‐year‐old children with disabilities. |
|
| Outcomes | MMR exposure in cases and controls stratified for age groups | |
| Funding Source | Government | |
| Notes | Probable bias in the enrolment in MADDSP, and cases may not be representative of the rest of the autistic population of the city | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ representative series of cases |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ matching for age, gender, and school |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Dockerty 1999.
| Study characteristics | ||
| Methods | A nationwide case‐control study was conducted in New Zealand to test hypotheses about the role of infections in the aetiology of childhood leukaemia. | |
| Participants | The 131 eligible cases were newly diagnosed with childhood leukaemia (ages 0 to 14 years) 1990 to 1993, and born and resident in New Zealand. Controls (matched 1:1 to cases on age and sex) were selected randomly from the New Zealand‐born and resident childhood population, using national birth records. Each control’s birth was registered in the same quarter of the same year as the matched case. Adopted children were not eligible. | |
| Interventions | MMR vaccine not described. Vaccination histories were supplemented with information from parent‐held "Health and Development" records. | |
| Outcomes | Acute lymphoblastic leukaemia | |
| Funding Source | Government | |
| Notes | For MMR, no association was found with leukaemia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ based on population |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ matching for age and sex |
| CCS ‐ exposures | Unclear risk | Probable information bias ‐ vaccine not described ‐ standardised interview |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Groves 1999.
| Study characteristics | ||
| Methods | Case control study ‐ in 9 Midwestern and mid‐Atlantic states (USA) between 1 January 1989 and 30 June 1993 | |
| Participants | Patients with acute lymphoblastic leukaemia aged 0 to 14, diagnosed between 1989 and 1993. Participants who resided in Illinois, Indiana, Iowa, Michigan, Minnesota, New Jersey, Ohio, Pennsylvania, or Wisconsin at the time of diagnosis were eligible for the vaccination component of the study. Controls selected through random‐digit dialling were individually matched to the cases by age (within 25% of the corresponding case's age at diagnosis), the first 8 digits of the telephone number, and race (African‐American/white/other). | |
| Interventions | MMR vaccine ‐ vaccination data were provided by mothers (based on vaccination records from physicians) or obtained directly from the physicians | |
| Outcomes | Acute lymphoblastic leukaemia | |
| Funding Source | Government | |
| Notes | Conclusion: the MMR vaccine does not alter the risk of subsequent acute lymphoblastic leukaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record |
| CCS ‐ control selection | Unclear risk | Probable selection bias ‐ selected through random‐digit dialling |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ matching for age, sex, race, and first 8 digits of the telephone number |
| CCS ‐ exposures | Low risk | Probably adequate ‐ secure record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Ma 2005.
| Study characteristics | ||
| Methods | Case‐control study ‐ Northern California Childhood Leukemia Study (NCCLS). The study area includes 17 counties in the Greater San Francisco Bay Area (1995 to the present), and in 1999 was expanded to a total of 35 counties in Northern and Central California. In the NCCLS, incident cases of newly diagnosed childhood leukaemia (age 0 to 14 years) are rapidly ascertained from major paediatric clinical centres, usually within 72 h after diagnosis. | |
| Participants | Cases (N = 323): newly diagnosed leukaemia in children aged between 0 and 14 years and ascertained from major paediatric clinical centres within 72 h after diagnosis Controls (N = 409): for each case 1/2 controls matched for date of birth, gender, Hispanic status (either parent Hispanic), maternal race (white, African‐American, or other), and maternal county of residence |
|
| Interventions | MMR immunisation (no vaccine description) before index date | |
| Outcomes | Association between MMR exposure and onset of leukaemia or acute lymphoblastic leukaemia | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ population‐based ‐ representative series of cases |
| CCS ‐ control selection | Low risk | Adequate ‐ community controls |
| CCS ‐ comparability | Low risk | Adequate ‐ probable residual confounding ‐ matching for age, gender, race |
| CCS ‐ exposures | Low risk | Adequate ‐ vaccination record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Mallol‐Mesnard 2007.
| Study characteristics | ||
| Methods | Population‐based case‐control study (ESCALE) conducted in France in 2003 and 2004 in order to investigate the role of infectious, environmental, and genetic factors in childhood neoplastic diseases (leukaemia, lymphoma, neuroblastoma, and brain tumour) | |
| Participants | Each case of acute leukaemia incident in 2003 to 2004 in a child aged < 15 years, residing in France at the time of diagnosis and with no previous history of malignancy, was eligible. All the childhood leukaemia cases were confirmed by bone marrow analysis. Children whose mother did not speak French or who had been adopted were not eligible. The leukaemia cases were recruited directly by investigators assigned to each French paediatric oncology hospital department, with the support of the French National Registry of Childhood Haematopoietic Malignancies. Out of the 948 cases of childhood acute leukaemia diagnosed in France from 1 January 2003 to 31 December 2004, 860 cases were eligible. The reasons for exclusion included: absence of a biological mother; non‐French‐speaking mother; serious psychological disorders; physician’s refusal; and death. Finally, 776 case mothers gave consent and were interviewed. The controls were randomly selected from the French population using quotas, a priori determined to make the control group representative of all cancer cases in terms of age and gender. Additional quotas constrained the control group to have the same distribution as the national population in terms of number of children living in the household, conditionally to the age group. Random selection was based on a representative sample of 60,000 addresses from the French national telephone directory plus unlisted numbers, which were randomly retrieved before dialling. Amongst the 2361 eligible control mothers, 679 refused the interview, and 1682 (71.2%) gave their consent and were interviewed. The authors then excluded 1 control that had a prior history of neuroblastoma, to end with a total number of 1681 controls. After exclusion of the cases with conditions that could have resulted in a scheduled vaccination date being modified, 726 cases and 1681 controls were included in analysis. |
|
| Interventions | Each of the case and control biological mothers responded to a personal and standardised telephone interview lasting 40 min. The interview elicited data on demographic and socioeconomic characteristics, parental occupational history, childhood environment, familial and personal medical history, and history of the pregnancy. In France, the vaccination section of a child’s medical record contains a separate page for each vaccine. The healthcare professional reports the proprietary name of the vaccine and the date of vaccination on the appropriate page. For the study, each mother was asked to read out each page of the vaccination record, line by line. | |
| Outcomes | Acute leukaemia, acute lymphoblastic leukaemia, or acute myeloblastic leukaemia | |
| Funding Source | Government | |
| Notes | Conclusion: no association between vaccination and the risk of childhood acute leukaemia, acute lymphoblastic leukaemia, or acute myeloblastic leukaemia was observed. No relationship between the risk of leukaemia and the type of vaccine, number of doses of each vaccine, total number of injections, total number of vaccine doses, or number of early vaccinations was evidenced. No confounding factor was observed. The study did not show any evidence of a role of vaccination in the aetiology of childhood leukaemia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ representative series of cases |
| CCS ‐ control selection | Low risk | Adequate ‐ community control |
| CCS ‐ comparability | Low risk | Adequate ‐ frequency matching for age and gender |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Mrozek‐Budzyn 2010.
| Study characteristics | ||
| Methods | Case‐control study, Poland | |
| Participants | Participants were identified using general practitioner records in the Lesser Poland (Małopolska) Voivodeship in Poland. The sample population of this study included children aged 2 to 15 years diagnosed with childhood or atypical autism, classified according to ICD 10‐criteria as F84.0 or F84.1, respectively. Every diagnosis of autism was made by child psychiatrist. Dates of these diagnoses were recorded in general practitioner files. Cases with uncertain diagnosis of autism, secondary to disease state or trauma, were excluded. 2 controls were selected for each affected child, individually matched by year of birth, gender, and physician’s practice. The first 2 children who visited the physician after the time of the autistic child visit who met entry criteria served as controls. Cases: 96 children with childhood or atypical autism diagnosis aged between 2 and 15 years from Małopolska Province (southern Poland) Controls: 192 children matched for birth year, gender, and practice to the cases |
|
| Interventions | The Polish mandatory vaccinations schedule did not include MMR for all children until 2004. MMR vaccine and monovalent measles |
|
| Outcomes | Parents were interviewed by trained nurses using a standardised questionnaire. Questions for all children included information about prenatal and postnatal development, mental and physical development, chronic diseases, malformations and injuries, history of bowel disturbances, birth order, family size, and parents’ socioeconomic status. Parents of children with autism were additionally asked about the date of onset of symptom, the period when parents first suspected their child’s symptoms might be related to autism, and their knowledge and beliefs regarding the cause of autism. This questionnaire did not contain any questions concerning the child’s vaccination history so as to not bias the parent’s answers (i.e. insinuate a relationship with autism). |
|
| Funding Source | Government | |
| Notes | Conclusion: the study provides evidence against the association of autism with either MMR or a single measles vaccine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ general practitioner records |
| CCS ‐ control selection | Low risk | Adequate ‐ community control |
| CCS ‐ comparability | Unclear risk | Probable residual confounding ‐ matched for age, sex, and general practitioner |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Ray 2006.
| Study characteristics | ||
| Methods | Case‐control study investigating the possible relationship between MMR and DTP immunisation and hospital admission for encephalopathy within 60 days. Data from 4 HMOs (Group Health Cooperative, Washington; Northern and Southern California Kaiser Permanente; Northwest Kaiser Permanente, Oregon and Washington) involving children aged 0 to 6 years who were hospitalised for encephalopathy or related conditions between 1 January 1981 and 31 December 1995 (from 1 August 1998 for Southern California Kaiser Permanente) were reviewed. | |
| Participants | Cases (N = 452): children (aged 0 to 6 years) with encephalopathy, Reye syndrome, or encephalitis defined accordingly to definition (see Table 33) Controls (N = about 1280): for each case up to 3 controls were selected, matching for HMO location, age within 7 days, sex, and length of enrolment in health plan |
|
| Interventions | Vaccination status concerning MMR and DTP vaccine exposure of both cases and controls was assessed by vaccination records. Only the neurologist who made the final case diagnosis was blind to vaccination status, not so the abstracter. Exposure to both vaccines was stratified in the results on the basis of the time elapsed between vaccination and hospital admission (0 to 90 days, 0 to 60 days, 0 to 30 days, 0 to 14 days, 7 to 14 days, 0 to 7 days). | |
| Outcomes | Observed cases (encephalopathy, Reye syndrome, or encephalitis) were further classified considering disease aetiology: known, unknown or suspected but unconfirmed (the latter includes cases in which a diagnosis such as meningitis has not been confirmed by a specific laboratory test). | |
| Funding Source | Government | |
| Notes | Authors did not formally indicate how many controls were included in the analysis. Controls included in each stratification could be calculated from percentages in tables 2, 3, 4. Regarding vaccine exposure, we know only that it has been assessed by means of vaccination record, but any further information (e.g. vaccine type and composition, number of administered doses) is absent in the report. This information would be important, as it would permit the testing of association with diseases and single vaccine strains: cases were enrolled between 1981 and 1995, during which time different vaccine formulations were in use. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ hospital record |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Unclear risk | (See note) ‐ matched for age, sex, HMO location, and length of enrolment in the health plan |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Shaw 2015.
| Study characteristics | ||
| Methods | Case‐control study using the University of Manitoba IBD Epidemiology Database (UMIBDED). The UMIBDED was linked to the Manitoba Immunization Monitoring System (MIMS), a population‐based database of immunisations administered in Manitoba. | |
| Participants | All paediatric IBD cases in Manitoba, born after 1989 and diagnosed before 31 March 2008, were included. Controls were matched to cases on the basis of age, sex, and region of residence at time of diagnosis. Conditional logistic regression models were fitted to the data, with models adjusted for physician visits in the first 2 years of life and area‐level socioeconomic status at case date. A total of 951 individuals (117 cases and 834 controls) met eligibility criteria, with average age of diagnosis amongst cases at 11 years. |
|
| Interventions | Measles‐containing vaccinations (MMR) received in the first 2 years of life were documented, with vaccinations categorised as ‘None’ or ‘Complete’, with completeness defined according to Manitoba’s vaccination schedule. Vaccinations were defined based on the work of Hilderman and colleagues, with the following tariff codes used to define a measles‐containing vaccine: 8621, 8629, 8670, 8673. | |
| Outcomes | The administrative data case definition used to identify patients with IBD was validated with the establishment of the population‐based UMIBDED in 1995; the UMIBDED contains extracted administrative data of IBD cases and their controls (at a 1:10 ratio) for those individuals with health coverage between 1 April 1984 and 31 March 2008. Residents of Manitoba who had resided in the province for at least 2 years were identified as having IBD if they had had at least 5 physician visits or hospitalisations with ICD‐9‐CM codes 555.xx (Crohn’s disease) or 556.xx (ulcerative colitis) recorded as a diagnosis at any time. Since 2004, ICD‐10‐CA codes were used for all inpatient contacts and for IBD included K50.xx and K51.xx. | |
| Funding Source | Government | |
| Notes | Conclusions: no significant association between completed measles‐containing vaccination in the first 2 years of life and paediatric IBD could be demonstrated in this population‐based study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ representative series of cases |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ matched for age, sex, and region of residence at time of diagnosis |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Smeeth 2004.
| Study characteristics | ||
| Methods | Case‐control study using the UK General Practice Research Database (GPRD) | |
| Participants | The study population consisted of all people who were registered in the GPRD at any time between 1 June 1987 (when the database was started) and 31 December 2001, and who were born in 1973 or later, to ensure that virtually all individuals eligible for MMR vaccination were included. Cases: defined as children with a first diagnosis of a PDD during the study period whilst registered with a practice contributing to the GPRD. They were found by searching the electronic records for clinical codes indicating a diagnosis of PDD (codes used are available on request). Those who were first diagnosed outside the study period were excluded from the study and were not eligible to be selected as controls. Those with autistic disorders and similar presentations were classified as having “autism” and those with other descriptions (such as Asperger’s syndrome) were classified as having “other PDD”. Patients who had more than 1 PDD diagnostic code recorded at different times (e.g. autism and then Asperger’s syndrome) were classified as having the most specific diagnosis (in this example Asperger’s syndrome). However, the date of the first diagnosis with a PDD was taken as the date of diagnosis. Controls: 5 controls for every case from amongst individuals in the study population who had no diagnosis of PDD recorded in their general practice record and who were alive and registered with a participating practice on the date of the PDD diagnosis in the case. Controls were individually matched to cases by year of birth (up to 1 year older or younger), sex, and general practice. | |
| Interventions | Exposure to MMR vaccination from birth to index date (date of the first diagnosis with PDD). In 1988, MMR vaccination was introduced in the UK for all children aged 12 to 15 months. During 1988 to 1991, in a catch‐up campaign, MMR vaccine was also offered to all children up until the age of school entry (4 to 5 years). A second dose at school entry was introduced in 1996, with a further catch‐up campaign for children born on or after 1 January 1990, who had not previously received 2 doses of a vaccine containing measles. MMR vaccination is also recommended for non‐immune adults, especially those in residential care or those starting college, and for non‐immune contacts during a measles outbreak. A catch‐up campaign for children aged 5 to 16 years was launched in 1994, but measles‐rubella vaccination was used, not MMR. |
|
| Outcomes | Number of MMR vaccination amongst cases and controls prior to PDD diagnosis and prior to PDD diagnosis and 3rd birthday | |
| Funding Source | Government | |
| Notes | The study method is described in Smeeth 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record ‐ General Practice Research Database |
| CCS ‐ control selection | Low risk | Adequate ‐ community |
| CCS ‐ comparability | Low risk | Adequate ‐ matched for age, sex, general practices |
| CCS ‐ exposures | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
bb‐Uno 2012.
| Study characteristics | ||
| Methods | Case‐control study, Japan | |
| Participants | Data from patients of the Yokohama Psycho‐Developmental Clinic (YPDC), Kanto area, Japan, which accepts only patients with suspected developmental disorders. Of the patients who initially consulted the YPDC from April 1997 (opening of the clinic) until March 2011. Children aged 6 to 36 months Cases: patients (1) were diagnosed with ASD, and (2) had been born between 1 April 1984 and 30 April 1992, the possible time period for MMR vaccination (n = 189). Controls: 1 to 2 controls were selected for each case, matched for sex and year of birth and recruited as volunteers from general schools in the Kanto area, the same area where YPDC patients reside (N = 224). |
|
| Interventions | MMR vaccination was introduced in April 1989, and only 1 vaccination using MMR was included in the immunisation schedule. The monovalent mumps and rubella vaccines remained the choice. After several cases of aseptic meningitis (caused by mumps Urabe strain), the Japanese government ceased extensive inoculation with MMR in April 1993. Consequently, children born from April 1984 to April 1992 could have received the MMR vaccination, and those children were included in the present study. | |
| Outcomes | Diagnosis of ASD. Patients were diagnosed based on the classifications of pervasive developmental disorders in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, and standardised criteria using the Diagnostic Interview for Social and Communication Disorder (DISCO). The DISCO is recognised as one of the best ways to obtain a reliable and valid diagnosis of ASD. | |
| Funding Source | Government | |
| Notes | Same study and data were reported in Uno 2015; this last study reports data by age groups and analyses the possible association between thimerosal and ASD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Adequate ‐ secure record |
| CCS ‐ control selection | Unclear risk | Volunteer from general schools in the same area |
| CCS ‐ comparability | Unclear risk | Matched sex and age (probable residual confounding) |
| CCS ‐ exposures | Low risk | Adequate ‐ data form Maternal and Child Health Handbook |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
bb‐Vcev 2015.
| Study characteristics | ||
| Methods | Case‐control study ‐ part of a wider epidemiologic study aimed at assessing the incidence, prevalence, and clinical expression of IBD in Vukovar‐Srijem County (population in 2001: 204,768), a lesser developed part of continental Croatia that has experienced deep demographic changes in the recent past. | |
| Participants | There were 119 UC patients and 31 CD patients of a total of 150 patients in the cohort. A total of 150 individuals, volunteers, not having a diagnosis of IBD, age and sex matched, were used as the control group. Information on examined risk factors was obtained from all participants in a previously conducted interview. Patients were contacted personally or by phone and interviewed by a gastroenterologist. | |
| Interventions | MMR vaccination | |
| Outcomes | IBD patients were identified according to the hospital’s patient records. | |
| Funding Source | Government | |
| Notes | MMR vaccination rates were higher in CD patients (90.3%) compared to UC patients and the controls (74.8% and 67.3%, respectively) (P = 0.026). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCS ‐ case selection | Low risk | Probably adequate ‐ insufficient information |
| CCS ‐ control selection | High risk | Probable selection bias ‐ insufficient information ‐ recruited on a voluntary basis |
| CCS ‐ comparability | High risk | Not adequate statistical methods |
| CCS ‐ exposures | Unclear risk | Probable information bias ‐ insufficient information |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐Arciuolo 2017.
| Study characteristics | ||
| Methods | Cohort study ‐ postexposure prophylaxis | |
| Participants | Contacts were identified by the New York City Department of Health and Mental Hygiene between 13 March 2013 and 30 June 2013. For the purpose of this analysis, all cases who subsequently developed measles were considered as contacts. All contacts, inclusive of those who developed measles, were then subject to the same exclusion criteria regardless of disease outcome. Contacts who were aged ≥ 19 years at the time of their exposure were excluded from the analysis because adults typically do not have copies of their immunisation records, and reporting of immunisation doses to the CIR is only required for individuals aged < 19 years. | |
| Interventions | MMR PEP | |
| Outcomes | Investigation of suspected cases included patient interviews, medical record reviews, and ascertainment of immunisation records. Testing for measles immunoglobulin G and immunoglobulin M and testing for measles virus RNA by RT‐PCR were performed, and measles genotype was determined. | |
| Funding Source | Government | |
| Notes | Conclusions: contacts who received PEP were less likely to develop disease. Authors' findings support current recommendations for administration of PEP following exposure to measles. These results highlight the importance of a rapid public health outbreak response to limit measles transmission following case identification. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Secure record ‐ immunisation record |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | The cohort was limited to affected classes. |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Arenz 2005.
| Study characteristics | ||
| Methods | Cohort study ‐ Germany | |
| Participants | 55 families and 43 children. Household contacts in families with at least 1 mumps case. 43 exposed children included in the final analysis, of which 25 were female and 18 were male. Median age was 5 years 3 months in measles cases and 6 years 6 months in contacts without measles. None of the included children had a history of measles. |
|
| Interventions | Vaccination with measles‐containing vaccine | |
| Outcomes | Case definition: generalised maculopapular rash with fever 38.4 °C for 3 days and at least 1 of the following signs: cough, coryza, Koplik spots, or conjunctivitis. Primary case: the first household member who acquired measles. Co‐primary cases were defined as measles patients who developed a fever within 4 days after the onset of a rash in the primary case. Secondary cases were confirmed measles patients who developed a fever within 5 to 25 days after the onset of a rash in the primary case. Contacts were all household members who had contact with measles cases in the household during their infectious period. |
|
| Funding Source | Government | |
| Notes | Insufficient information about vaccine composition (if MMR or bivalent) for household contact study. Screening method was used for vaccine effectiveness assessment in Coburg school population aged older than 5 years. Many important details are missing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ comparability | Unclear risk | The cohort was limited to affected classes. |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐Barrabeig 2011a.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ Spain | |
| Participants | A total of 166 children shared a classroom with the index cases, with a median age of 16.5 months (range 6 to 47 months). The median class size was 14.5 children (range 9 to 39). | |
| Interventions | Postexposure prophylaxis with MMR vaccine Candidates for the intervention were susceptible contacts (who had not received either measles‐containing vaccine or had not suffered measles); intervention time was the period between rash onset of the index case and the day of vaccination of the susceptible contact. |
|
| Outcomes | A confirmed case of measles was a laboratory‐confirmed case (positive serology for measles immunoglobulin M antibody by enzyme‐linked immunosorbent assay testing or positive polymerase chain reaction for measles virus in urine sample) or a case that met the WHO clinical case definition and was epidemiologically linked to a laboratory‐confirmed case. An index case was the first case of measles in the classroom; a contact was a child who had shared the same classroom as the index case for at least 1 day during the infectious period of the index case (4 days before rash onset to 4 days after); a secondary case was a contact with rash onset 7 to 18 days after rash onset in the index case. Cases were investigated by public health staff. Susceptible contacts were identified, and PEP immunisation was offered. Active surveillance of centres was performed to detect secondary cases. |
|
| Funding Source | Government | |
| Notes | Insufficient information about study design. Authors' conclusion: "The results of this study show that 1 dose of MMR vaccine reduces the risk of measles when administered in the 3 first days after rash onset in the index case" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ secure record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Barrabeig 2011b.
| Study characteristics | ||
| Methods | Retrospective cohort study carried out between 1 October 2006 and 15 January 2007 in educational centres (day‐care and preschool centres) in Barcelona, Spain. The objective of this study was to evaluate the direct, indirect, and total effectiveness of measles component of the MMR vaccine in the context of a measles outbreak. | |
| Participants | Children attending day‐care and preschool centres. 1) Children were considered as vaccinated against measles if they had received the MMR vaccine on or after the minimum recommended age for vaccination and at least 14 days prior to the onset of disease in the index case for each educational centre. 2) Susceptible children were defined as non‐vaccinated children without measles infection before outbreak. 3) All children and educational staff who could provide evidence of immunity were either vaccinated with the MMR vaccine or excluded and isolated at home until 21 days after the appearance of rash in the last reported case. |
|
| Interventions | MMR vaccine Priorix/Schwarz or MDS/Enders 1 dose at 9 to 12 months. Second dose at 15 months | |
| Outcomes | Confirmed case of measles was defined as laboratory‐confirmed case (positive serology for measles immunoglobulin M antibody by enzyme‐linked immunosorbent assay testing or positive polymerase chain reaction for measles virus in urine sample) or a case that met the WHO clinical case definition and was epidemiologically linked to laboratory‐confirmed case. 1) Direct vaccine effectiveness was estimated from N = 1121 children ≥ 15 months age. 2) Indirect vaccine effectiveness (or herd immunity) was estimated by comparing the risk in non‐vaccinated children from an immunised population and an identical but fully unimmunised population. |
|
| Funding Source | Government | |
| Notes | Study conclusion: over 90% of cases in children aged 12 to 14 months would have been avoided by MMR administration at 12 rather than at 15 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequately defined ‐ vaccination card |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequately defined ‐ vaccination card |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ age‐specific |
| PCS/RCS ‐ assessment of outcome | Low risk | Laboratory‐confirmed or WHO clinical case definition |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ca‐Bhuniya 2013.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ Bengal, India | |
| Participants | Children aged 9 to 59 months (as on 30 June 2011) | |
| Interventions | Vaccine type undeclared ‐ measles vaccination status was determined from immunisation cards. If immunisation card was not available, vaccination status was recorded as unknown. | |
| Outcomes | WHO definitions of clinical and confirmed measles. A clinical case of measles is defined as fever with maculopapular rash and either conjunctivitis or cough or coryza. A confirmed case of measles is defined as a clinical case who is positive for anti‐measles virus nucleoprotein immunoglobulin M antibodies in serological tests but has not been vaccinated against measles during last 1 month. 6 blood samples were collected from selected cases, who were within 5th to 15th day of illness from the onset of rash, for IgM enzyme‐linked immunosorbent assay test. | |
| Funding Source | Government | |
| Notes | Vaccine type undeclared, probably 1 dose was administered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ comparability | High risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Low risk | Clinically confirmed |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐Chamot 1998.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ Switzerland | |
| Participants | Family contacts (N = 265) aged up to 16 years of primary confirmed (N = 223) or probable (N = 60) mumps cases notified at Health Service Cantonal of Geneva from 1 February 1994 to 30 April 1996 | |
| Interventions | Immunisation with MMR containing different mumps strains:
Unvaccinated contact acted as control group. The vaccination status was obtained from vaccination books. |
|
| Outcomes | Clinical mumps cases amongst contacts:
|
|
| Funding Source | Government | |
| Notes | By paediatricians recruiting participants included the serious cases and excluded household with difficult access to Health Service. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Chang 2015.
| Study characteristics | ||
| Methods | Cohort study ‐ China ‐ conducted in 13 classes that had secondary cases of rubella. Using the secondary attack rates, the study authors evaluated VE by the number of RCV doses received and age at vaccination. | |
| Participants | School A is a middle school with a total of 1621 students enrolled in the 7th, 8th, and 9th grades, with a total of 37 classes. All students are day students, and they eat their meals at home. The school canteen only provides meals for some teachers. No school bus is available to students. This school has no full‐time school doctor, only a part‐time health teacher. Students were born between 1998 and 2001. | |
| Interventions | MMR (BRD‐II or RA27/3) A BRD‐II rubella strain vaccine was developed in the 1980s in China, and has been available in the Chinese private market since 1993. All monovalent rubella and measles and rubella combined (MR) vaccines in use in China are based on the BRD‐II rubella strain. A domestic measles, mumps, and rubella combined vaccine (MMR) based on BRD‐II strain has been available in China’s private market since 2003. An imported RA27/3 strain‐based vaccine is also available in China. |
|
| Outcomes | Probable rubella case: defined as a suspected rubella case with fever > 37.5 °C and at least 1 of the following symptoms: arthralgia, arthritis, lymphadenopathy, or conjunctivitis.
A laboratory‐confirmed case: required a positive serologic test for rubella IgM antibody. Epidemiologically linked case: confirmed case was defined as a suspected case or a probable case that was not laboratory confirmed, but that was geographically and temporally related to a laboratory‐confirmed case. |
|
| Funding Source | Government | |
| Notes | Conclusions: the rubella vaccines used in China that are based on the BRD‐II rubella vaccine strain have a VE of 94%, which is similar to the more commonly used RA27/3‐based RCVs. Low vaccination coverage contributed to this outbreak; early reporting of an outbreak is necessary for effective outbreak response immunisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ secure record ‐ vaccination record |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ comparability | Unclear risk | Probably adequate ‐ age 11 to 13 ‐ probable residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Choe 2017.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ during April to June 2014, a measles outbreak occurred at a university in Seoul, Korea. | |
| Participants | N = 14,465 students. A total of 85 cases were confirmed in the university. The median age was 20 years (range 19 to 44 years); cases were born between 1984 to 1993 (the recipients of measles and rubella (MR) vaccine catch‐up campaign in 2001). | |
| Interventions | MR or MMR. Documentation was obtained from measles vaccination records in the National Immunization Registry. | |
| Outcomes | Measles‐specific antibody was tested at Seoul Metropolitan City Research Institute of Health and Environment and Division of Respiratory Viruses of KCDC using a measles enzyme‐linked immunosorbent assay for immunoglobulin M and immunoglobulin G (enzyme immunoassay; Siemens Healthcare Diagnostics Inc, Erlangen, Germany). | |
| Funding Source | Government | |
| Notes | No information on statistical methods used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ immunisation record |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ comparability | High risk | Possible residual confounding ‐ insufficient information |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐Compés‐Dea 2014.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ Zaragoza, Spain | |
| Participants | The reference population were the 235 students (16 to 17 years old) and 27 teachers of the 2011 to 2012 school. | |
| Interventions | Vaccination status ascertainment by vaccination record or by primary care clinical record. Properly vaccinated if 2 doses were registered, the first being after 12 months and the period between doses greater than 4 weeks. | |
| Outcomes | Laboratory‐confirmed case: person in whom mumps virus was isolated in a clinical sample or obtained positive IgM results for serum mumps or obtained positive PCR results in a clinical sample. | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ secure record ‐ vaccination record |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ vaccination record |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Giaquinto 2018.
| Study characteristics | ||
| Methods | Cohort study, Italy; the direct effectiveness of a single dose of ProQuad | |
| Participants | All children born in 2006/2007 N = 2357 children who received ProQuad as a first dose of varicella vaccine (ProQuad‐vaccinated children) N = 912 unvaccinated children Children were followed from age 1 year until the occurrence of varicella, until they received the second dose of varicella vaccine (if vaccinated), their 6th birthday, or exit from the Pedianet database, whichever occurred first. |
|
| Interventions | MMRV ‐ ProQuad | |
| Outcomes | Varicella (chickenpox). Varicella cases recorded in the Pedianet database are based on physician confirmation only; no laboratory tests were performed. | |
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusions: these are the first results on the effectiveness and impact of ProQuad against varicella; data confirmed its high effectiveness, based on immunological correlates for protection. Direct effectiveness is the only ProQuad‐specific measure; all impact measures refer at least partially to the VP and should be interpreted in the context of high vaccine coverage and the use of various varicella vaccines in this region. The Veneto Region offered a unique opportunity for this study due to an individual data linkage between Pedianet and the Regional immunisation database. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ information on the varicella vaccination status of these children and the vaccine brand used was taken from the Regional Immunisation Database |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Probably adequate ‐ vaccination record |
| PCS/RCS ‐ comparability | Unclear risk | Probably adequate ‐ probable residual confounding |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Probably adequate ‐ physician confirmation only |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Greenland 2012.
| Study characteristics | ||
| Methods | Retrospective cohort study amongst students from the 3 university cities most affected by the outbreak: Delft, Utrecht, and Leiden. In May 2010 | |
| Participants | 4988 members of the 4 selected student associations in Delft (N = 356 women; N = 1044 men), Leiden (N = 1400; sex breakdown of members not provided but estimated by society to be an approximately equal sex ratio), and Utrecht (2 societies: N = 1288 women; N = 900 men) were invited to the study by email. The questionnaire asked about demographic characteristics including current living arrangements. N = 989 responded to the questionnaire. | |
| Interventions | The questionnaire asked about MMR vaccination history and history of mumps infection. Informed consent was sought to verify MMR vaccination status using the national vaccination register. | |
| Outcomes | A case was defined as a student with self‐reported mumps (swelling of 1 or both cheeks with symptoms lasting at least 2 days) since 1 September 2009. | |
| Funding Source | Government | |
| Notes | Authors' conclusion: 2 doses of MMR do not confer long‐term protection against mumps. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ secure record ‐ national vaccination register |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ secure record ‐ national vaccination register |
| PCS/RCS ‐ comparability | Unclear risk | Probably adequate ‐ demographic characteristics |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Self‐reported mumps |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Hales 2016.
| Study characteristics | ||
| Methods | Cohort study: secondary attack rate study to evaluate measles vaccine effectiveness in household contacts | |
| Participants | Households were selected for the study by convenience sampling of confirmed measles cases reported to the Pohnpei State Department of Health Services, with laboratory‐confirmed cases prioritised. Was excluded the following from analysis: 1) Co‐primary cases 2) Household contacts aged < 6 months (maternal antibodies may confer protection in these infants) 3) Household contacts aged ≥ 40 years (vaccination records were rarely available for this age group) 4) Individuals with incomplete vaccination records |
|
| Interventions | 1) Vaccinations administered before 1 June 2014 as pre‐campaign doses 2) Vaccinations administered on or after 1 June 2014 as campaign doses 3) Pre‐exposure campaign dose as a dose received ≥ 5 days before rash onset in the primary case 4) Postexposure campaign dose as a dose received between 4 days before to 3 days after rash onset in the primary case Vaccination status of study participants ascertained by vaccination card or vaccine registry. |
|
| Outcomes | A confirmed measles case was defined according to the US Council of State and Territorial Epidemiologists guidelines: a person with acute febrile rash illness with detection of measles‐specific nucleic acid from a clinical specimen using PCR, or a positive serologic test for measles IgM antibody, or direct epidemiologic linkage to another confirmed case. Laboratory testing was performed at the Centers for Disease Control and Prevention. | |
| Funding Source | Government | |
| Notes | Authors' conclusion: "Our results support implementation of a vaccination campaign as soon as possible after introduction of measles into a population with suboptimal levels of measles immunity, as evidenced by the protective effect of both pre‐exposure and postexposure campaign doses." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ comparability | High risk | Only convenience sampling |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐La Torre 2017.
| Study characteristics | ||
| Methods | Retrospective cohort, Italy; the cohort was recomposed through record linkage of 2 archives (vaccination register and hospital discharge records) | |
| Participants | The analysis included 11,004 children. Children born in the period between 2008 and 2010, who subsequently underwent vaccination in 2009 to 2011 and resident in the territories of the ASL Rome. | |
| Interventions | MMR vaccination: 20.9% did not receive the MMR vaccination; 49% and 30.1% received 1 and 2 doses. | |
| Outcomes | Hospitalisation for measles, mumps, or rubella | |
| Funding Source | Government | |
| Notes | Conclusion: MMR vaccination is effective for the primary prevention of target and not‐targeted infectious diseases and may also limit hospitalisations for respiratory diseases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | Retrospective cohort ‐ by vaccination register |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | Retrospective cohort ‐ by vaccination register |
| PCS/RCS ‐ comparability | Unclear risk | Possible residual confounding ‐ no data on family income or at least parents’ educational level that could have an impact on vaccination attitude. No data were available on other vaccinations. |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Livingston 2013.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ vaccine effectiveness in households | |
| Participants | 2176 household residents Between 5 February 2010 and 8 April 2010, 473 index households were contacted for follow‐up. Data were collected using a standard script. An interviewer requested to speak with an adult, who provided information on each household member. A minimum of 3 call attempts were made to each household. During calls, the following information was requested: (1) whether each household contact slept at home on average at least 5 nights per week; (2) total number of bedrooms in the house; and (3) for each household contact: birth date, vaccination status, and whether they had been sick with either cheek swelling that had lasted for at least 2 days or a doctor‐diagnosed case of mumps since September 2009. Households with index cases identified through surveillance from 1 September 2009 to 31 December 2009 were eligible for study inclusion. Case households were excluded if: (1) the index case lived alone; (2) the index case did not live in the house (e.g. lived in a dormitory); (3) the index case did not sleep in the house on average at least 5 nights per week; (4) there was no English‐speaking adult in the household; (5) an adult in the household was not able to be contacted; or (6) an adult in the household refused to provide information on household contacts or provided incomplete information. |
|
| Interventions | Mumps vaccination status was based on documented, valid MMR doses (2 doses). Acceptable documentation included MMR doses recorded in the New York City Citywide Immunization Registry (CIR) or those obtained directly from individual medical provider. | |
| Outcomes | A case of mumps was defined as 1 meeting the Council of State and Territorial Epidemiologist (CSTE) surveillance case definition or a compatible case identified via the phone interview. An index case was defined as the first case in a household to be reported to the DOHMH. Primary cases were those with the earliest onset of mumps in the household. Household members were defined as being exposed 2 days before parotitis onset of the primary case, which is the first day that the primary case was infectious. We defined co‐primary cases as those with onset within 9 days after the primary case’s symptom onset. Secondary cases were defined as those reporting onset of mumps 10 to 25 days after the primary case. Non‐secondary cases were defined as those occurring more than 1 incubation period (> 25 days) after the primary case. The clinical case definition is acute onset of unilateral or bilateral swelling of the parotid or other salivary glands, lasting 2 or more days, and without other apparent cause. Index cases in households were identified through mandated electronic reporting of positive test results by laboratories, or clinical reports of suspect disease by providers. |
|
| Funding Source | Government | |
| Notes | In order to be valid, doses had to be administered in accordance with the recommended vaccination schedule guidelines, meaning the first dose had to be administered no earlier than 4 days before the first birthday and subsequent doses at least 28 days after a previous MMR dose. Individuals lacking MMR documentation from a medical provider and with a record in CIR with at least 1 reported vaccination, but no recorded MMR doses, were considered unvaccinated with MMR. Individuals with a valid provider recorder with no recorded MMR doses were also considered unvaccinated. Individuals lacking MMR documentation from a medical provider and with no recorded vaccinations in CIR were considered to have unknown MMR vaccination status. Vaccination coverage estimates are exclusive to households with known mumps disease, and coverage in the overall Orthodox Jewish community may differ. In addition, the study was conducted during a community‐wide outbreak, so exposure to mumps may have occurred in other settings besides the home. We did not investigate specific exposures during religious holidays and community celebrations when members of the affected community may have had close contact. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ secure record |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ secure record |
| PCS/RCS ‐ comparability | Unclear risk | Amongst secondary cases, 15% were reported by the head of household. These cases were not confirmed by investigation or medical record review and may not have fulfilled the CSTE case definition. The time between the index case onset and the follow‐up interview may have led to cases being missed due to poor recall. |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Lopez Hernandez 2000.
| Study characteristics | ||
| Methods | Retrospective cohort study in Spain assessing the effectiveness of MMR vaccination against clinical mumps on preschool and schoolchildren during an outbreak (March to November 1997) | |
| Participants | Male children aged between 3 and 15 years attending 1 scholastic institute in the district of Cartuja y Almanjàyar (N = 775), which had the highest mumps attack rate in the district | |
| Interventions | MMR immunisation (school, vaccination or register by the local health centre) Composition and strains not reported. | |
| Outcomes | Parotitis. Clinical defined by surveillance (case definition: unilateral or bilateral swelling of parotids or salivary glands, sensible to tasting, lasting more than 2 days, that appears without apparent cause or without contact with affected children) | |
| Funding Source | Government | |
| Notes | It was not possible to assess mumps strain types administered to study population. In Spain, Urabe (AM9 strain) was used until 1993, after which it was replaced by Jeryl Lynn and Rubini. Even if cases are those identified by surveillance, there is no description in the report of how it has been performed (e.g. active or passive surveillance?). In any case, in the paragraph on case definition, the authors declare that included cases are only those identified by surveillance and that real cases are unknown (underestimated). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ register by the local health centre |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ register by the local health centre |
| PCS/RCS ‐ comparability | High risk | No information reported. |
| PCS/RCS ‐ assessment of outcome | High risk | Very unclear reporting |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐Ma 2018.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ China | |
| Participants | Between 1 December 2014 and 20 September 2015 N = 2303 students aged 6 to 15 years were included. 114 were excluded because they had a history of mumps illness, 281 students were excluded because of unknown immunisation history. Included in analysis vaccinated N = 1378 and unvaccinated N = 530 |
|
| Interventions | MMR: S79 strain of mumps vaccine virus, which had been derived through further attenuation of the Jeryl Lynn strain used in the US‐licenced vaccine. Students’ vaccination certificates were obtained during the field investigation. | |
| Outcomes | A mumps case was defined as a student having unilateral or bilateral parotid or other salivary gland swelling and pain, lasting 2 or more days, with onset between 1 December 2014 and 20 September 2015. All cases were diagnosed by clinical criteria without laboratory confirmation, and no mumps virus genotype information was obtained during this outbreak investigation. | |
| Funding Source | Government | |
| Notes | Conclusion: this outbreak was associated with low and declining 1‐dose MuCV effectiveness. China’s immunisation programme should evaluate the potential of a 2‐dose MMR schedule to adequately control mumps. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ vaccination record |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ vaccination record |
| PCS/RCS ‐ comparability | Unclear risk | No adjustment ‐ possible residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ secure record laboratory‐confirmed |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Marin 2006.
| Study characteristics | ||
| Methods | Retrospective cohort study carried out in Republic of the Marshall Islands (South Pacific) after a measles outbreak in 2003 to evaluate MMR vaccine effectiveness in contacts aged 6 months to 14 years with household secondary attack rate (SAR) method | |
| Participants | 72 households (a total of 857 participants) were selected by convenience sampling of measles cases reported in Majuro from 13 July to 7 November 2003. Contacts of these 72 primary cases aged between 6 months and 14 years with available MMR vaccination status were considered for effectiveness analysis (N = 219). | |
| Interventions | MMR vaccine (composition not reported) in 1, 2, 3 or more doses administered. A contact was considered vaccinated if documented record of measles vaccine administration > 4 days before the rash onset of primary case was available. An unvaccinated contact was a person without record of measles vaccination according to criteria in written or electronic records in a centralised electronic database. A person with unknown vaccination status did not have immunisation card and the person's name was not in immunisation record (excluded from analysis). |
|
| Outcomes | Measles case defined as a child who: 1) met the WHO clinical definition for measles (fever, generalised maculopapular rash, and cough, coryza, or conjunctivitis); or 2) had a positive test for measles IgM antibody by any serologic assay with the absence of vaccination 6 to 45 days before testing. Primary case: first case of measles in household Secondary case: a contact (person that resided in household for at least 1 day through the infectious period of primary case ‐ from 4 days before rash to 4 days after) with measles rash onset 7 to 18 days after primary case's rash onset Non‐case: a contact with no clinically apparent disease within 18 days after primary case's rash onset Data were collected by a “standardized questionnaire” and interviews were conducted at home with household member. |
|
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ documented record of measles vaccination ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ no record of measles vaccination meeting the criteria could be found in electronic immunisation record |
| PCS/RCS ‐ comparability | Unclear risk | No adjustment ‐ possible residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ WHO clinical definition for measles or positive test for measles IgM antibody |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Marolla 1998.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | Participants were children born between 1 January 1989 and 31 December 1994, whose parents requested an ambulatory visit by their family paediatrician between 15 May and 30 June 1996. 3050 were enrolled, corresponding to about 40% of the children population in the same age range in care by the 20 paediatricians who participated in the study. | |
| Interventions | During 15 May to 30 June 1996 (period in which the visits were performed), the 20 family paediatricians together with children's parents and by considering the content of medical records filled in a schedule in which the following information was collected: personal data, study titre of both parents, type of trivalent MMR vaccine, date of immunisation, practitioner who administered vaccine, onset of measles or mumps disease, eventual hospital admission, diagnostic criteria used, and the practitioner who diagnosed the disease. For the cases when vaccination status could not be immediately assessed, parents were required to communicate as soon as possible the data contained in vaccination records. During study time, paediatricians received a questionnaire on vaccination modality and how to store and administer it correctly. Out of the 3050 initially enrolled children, 2099 were vaccinated with 1 of 3 MMR commercial preparations, whereas 646 were not vaccinated. A total of 2745 children were included in the effectiveness analysis. The remaining 305 participants were excluded due to receiving monovalent vaccine (167), because schedule was compiled with insufficient detail (124), received vaccine after disease onset (6), or contracted measles or mumps before the 15th month of age. Out of the 2099 vaccinated, 1023 received Pluserix SKB, 747 Morupar Biocine, and 329 Triviraten Berna. | |
| Outcomes | Diseases under investigation were defined as follows:
Even if not described, paediatricians who conducted the study considered as cases those corresponding to these definitions from schedule data. Altogether 124 measles cases (10 amongst vaccinated) and 457 mumps cases (251 amongst vaccinated) were observed. 92 (74.2%) measles and 386 (84.5%) mumps cases occurred in the years 1995 to 1996. |
|
| Funding Source | Not stated | |
| Notes | Diagnosis of measles and mumps disease was made by paediatricians only on clinical parameters and on the basis of data sampled during interviews and of those present in the medical records. Results were managed by the paediatricians themselves, who were not blind to vaccination status of the children. Mean age at enrolment was not statistically different between not‐vaccinated and pooled vaccinated groups (about 52 months), but the authors do not provide these data (or age stratification) within each vaccine arm (considering age interval and visit time, follow‐up time considered could range from 3 to 75 months). Administered vaccine types varied during the time considered for investigation:
Exposition to disease and time since vaccination could be very different amongst children, which was not taken into account by evaluating effectiveness. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Secure record ‐ vaccination card ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Secure record ‐ vaccination card ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ homogeneous age amongst participants |
| PCS/RCS ‐ assessment of outcome | Low risk | Diagnosis of measles and mumps disease was made by paediatricians only on clinical parameters and on the basis of data sampled during interviews and of those present in the medical records. |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ca‐Musa 2018.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ from 1 February 2014 (the first month with confirmed measles cases) to 30 September 2015 | |
| Participants | Data for children aged 0 to 14 years old (N = 2784) (people aged > 14 years (n = 2300)) were presented by age group. The study involved primary school‐aged children in randomly selected schools in 4 cantons where measles cases were registered (Tuzla Canton, Central Bosnia Canton, Zenica‐Doboj Canton, and Herzegovina‐Neretva Canton). 20 primary schools that had registered measles cases were included. The study included all students in 40 classes with 1 or more registered measles cases in the period from February 2014 to September 2015. |
|
| Interventions | Immunisation status, the number of MMR doses, and the date of the last MMR dose were obtained from personal medical records. Since 2001, 2 MMR doses have been scheduled, at 12 to 18 months and 7 years (or at the first grade of primary school). | |
| Outcomes | Measles diagnosis was confirmed according to the WHO guidelines (5). The clinical criteria for measles were fever, maculopapular rash (i.e. non‐vesicular rash), and cough or coryza (i.e. runny nose) or conjunctivitis (i.e. red eyes). The laboratory criteria for measles surveillance case confirmation were measles IgM antibody detection, or measles virus isolation, or measles viral RNA detection by RT‐PCR, or a significant rise in measles IgG antibody in paired sera. | |
| Funding Source | Government | |
| Notes | Conclusions: the results of this study suggest that the resurgence was likely caused by an accumulation of measles‐susceptible children not being vaccinated. This vaccine effectiveness study does not support possible vaccination failure as a contributing factor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ secure record ‐ immunisation status, the number of MMR doses, and the date of the last MMR dose were obtained from personal medical records. |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Nelson 2013.
| Study characteristics | ||
| Methods | Cohort study ‐ during mumps outbreak 2009 to 2010 ‐ USA | |
| Participants | Students in the intervention schools were eligible if they were in the age group with the highest AR (aged 9 to 14 years), had a history of 2 MMR vaccine doses, had not previously received a third MMR vaccine dose, and had no history of mumps. | |
| Interventions | Third‐dose MMR vaccine intervention. Vaccination status of students participating in the study was confirmed either through immunisation card review by parents or immunisation staff, or review of DPHSS and school vaccine registries. For students with unknown or incomplete vaccination status, verification was obtained from healthcare providers. | |
| Outcomes | Mumps laboratory‐confirmed | |
| Funding Source | Government | |
| Notes | Conclusions: after the third‐dose MMR intervention in highly affected schools, 3‐dose recipients had an AR 60% lower than students with ≤ 2 doses, but the difference was not statistically significant, and the intervention occurred after the outbreak had peaked. This outbreak may have persisted due to crowding at home and high student contact rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ secure record ‐ representative cohort |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ secure record ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | Probable residual confounding ‐ there was insufficient information |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Ogbuanu 2012.
| Study characteristics | ||
| Methods | Cohort study ‐ during 2009 to 2010 mumps outbreak, in religious community with a high 2‐dose MMR coverage ‐ northeastern US | |
| Participants | Children who were 6th to 12th grade students (11 to 17 years old) in 3 schools | |
| Interventions | A third dose of MMR vaccine | |
| Outcomes | Mumps clinically and laboratory‐confirmed | |
| Funding Source | Government | |
| Notes | Conclusions: the decline in incidence shortly after the intervention suggests that a third dose of MMR vaccine may help control mumps outbreaks amongst populations with pre‐existing high 2‐dose vaccine coverage. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Secure record ‐ vaccination card ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Secure record ‐ vaccination card ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Ong 2005.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ Singapore | |
| Participants | Children attending childcare centres and primary schools in 1999. Childcare centres (N = 2533) and primary schools (N = 2539) | |
| Interventions | MMR vaccination status of each child (MMR or nothing) was obtained from health booklet (updated in Singapore when a child receives vaccination in accordance with the immunisation schedule). The specific strain type (Rubini, Jeryl Lynn, Urabe, or unknown mumps strain) was identified by matching the batch number of vaccine in health booklet with the record of the vaccine in polyclinic or family doctor's clinic. Even if the number of administered doses was not indicated, it can be supposed that only older children could have received a second MMR dose, as it was routinely introduced in January 1998. | |
| Outcomes | Mumps: clinically defined as fever associated with unilateral or bilateral swelling and tenderness of 1 or more salivary glands, usually the parotid gland. Diagnosed by physician. Serological confirmation was not carried out. | |
| Funding Source | Government | |
| Notes | Authors' conclusions: "Our study confirms the low protection conferred by the Rubini vaccine strain" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | Probably representative of the exposed, but number of administered doses was not indicated |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | Probable residual confounding ‐ the cohort was limited to affected classes |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Only clinical definition |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Ong 2007.
| Study characteristics | ||
| Methods | Retrospective cohort study carried out in Singapore during a measles outbreak in April to May 2004 in primary 3 and 6 school to evaluate MMR vaccine effectiveness | |
| Participants | Participants of the 5 affected classes in primary 3 degree and primary 6 degree (N = 184) (age 8 to 14 years) out of the school enrolment of 1309 students | |
| Interventions | MMR vaccine (no description). Only 1 dose administered. Data about vaccination (date and type of vaccine administered) were noted in health booklet of each child and confirmed with the National Immunisation Registry. |
|
| Outcomes | Measles cases laboratory‐confirmed, defined according to WHO 2001 criteria: "recent absentees who had been clinically diagnosed as measles or who had displayed symptoms and sign characterized by generalized maculopapular rash and fever, with or without cough, coryza or conjunctivitis" | |
| Funding Source | Government | |
| Notes | Very bad reporting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Vaccination status of the cases was obtained from children's booklets and confirmed by National Immunisation Registry. |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | The cohort was limited to affected classes, with a very complex mix of ethnicity. |
| PCS/RCS ‐ assessment of outcome | Low risk | Measles cases laboratory‐confirmed, defined according to WHO 2001 criteria. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Rieck 2017.
| Study characteristics | ||
| Methods | Cohort study ‐ Germany ‐ data from the German Immunisation Information Systems, also called the 'Associations of Statutory Health Insurance Physicians (ASHIPs) vaccination monitoring project'. | |
| Participants | Any individual: (i) born between January 2006 and October 2013; (ii) receiving any vaccination (i.e. not necessarily varicella) soon after birth at 0 to 4 months of age; (iii) in contact with a physician within the second half of 2015; (iv) residing at the time points of (ii) and (iii) in the region of the ASHIP that transferred the data; and (v) born in an ASHIP region where diagnosis information was available and specific vaccination claim codes for varicella vaccines had been introduced since birth. |
|
| Interventions | Since 2004, single‐dose varicella vaccination has been recommended for all children aged 11 to 14 months. 2 single‐compound varicella vaccines (VAR; Varivax, Sanofi Pasteur MSD; Varilrix, GlaxoSmithKline) were initially available. In 2006, a combined MMRV vaccine (Priorix‐Tetra, GlaxoSmithKline) was licenced with a 2‐dose schedule. A universal 2‐dose schedule has been recommended since 2009, targeting children with the second dose at age 15 to 23 months. Since 2011, the first immunisation has preferably been given as 2 separate injections of VAR and MMR due to higher rates of febrile seizures following immunisation with MMRV. Catch‐up vaccinations are recommended until 17 years of age. |
|
| Outcomes | Confirmed and incident varicella diagnoses | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | Data from the German Immunisation Information Systems ‐ approximately 85% of the population in Germany is covered |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | Data from the German Immunisation Information Systems ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | Adjusted for multivariate model ‐ vaccination status, time since vaccination ‐ probable residual confounding |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Schlegel 1999.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ Switzerland | |
| Participants | Participants were children aged 5 to 13 years from a small village in Switzerland (n = 165). Vaccination coverage in this population was high (95%). | |
| Interventions | Immunisation with MMR vaccine prepared with different mumps strain. 79 children were immunised with Rubini‐containing MMR vaccine, 36 with Jeryl Lynn‐containing MMR vaccine, and 40 with Urabe‐containing MMR vaccine. 8 participants were not MMR vaccinated. Vaccine strain was unknown for 2 children without mumps, who were excluded from the study. Vaccination status was ascertained by study investigators from vaccination certificates. All children received immunisation within 2 years of age. | |
| Outcomes | A mumps case was defined by viral isolation of mumps virus in a culture, doctor's confirmation of diagnosis, or if the presence of the typical clinical picture was described in a sibling of a patient with confirmed disease. Investigators who ascertained mumps cases were blind to vaccination status. The absence of IgG antibodies to mumps virus served as confirmation of full susceptibility to mumps in nonvaccinated children without clinical signs of the disease. | |
| Funding Source | Government | |
| Notes | Many study details are insufficiently described in this brief report (e.g. mumps case definition, onset and duration of the outbreak, methods of cases ascertainment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Vaccination status was ascertained by study investigators from vaccination certificates. |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | The absence of IgG antibodies to mumps virus served as confirmation of full susceptibility to mumps in nonvaccinated children without clinical signs of the disease. |
| PCS/RCS ‐ comparability | Unclear risk | No information |
| PCS/RCS ‐ assessment of outcome | Low risk | The person who investigated the cases of mumps was blinded with regard to the vaccination status. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Snijders 2012.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ vaccine effectiveness in primary cases and in households | |
| Participants | Children attending primary schools and their household contacts. Schools were eligible when they had at least 1 laboratory‐confirmed mumps case or more than 1 clinical mumps case. | |
| Interventions | MMR vaccine. Parents of schoolchildren were asked to fill out a questionnaire asking for information on the child's vaccination status (since 2007). To define the vaccination status, the study authors used individual information registered in the national Dutch vaccination register (‘Praeventis’). Information on vaccination status for 69 pupils (6%) could not be obtained from this register (66 no informed consent, 3 unknown vaccination status in register). For these children, authors used the self‐reported vaccination status (vaccinated/not vaccinated), assuming for vaccinated children that 1 dose was received when the child was aged < 8.75 years, and 2 doses when the child was aged ≥ 8.75 years. | |
| Outcomes | Mumps cases were defined by affirmative answer (by parental report) to the question "has your child had mumps after September 2007?". | |
| Funding Source | Government | |
| Notes | The vaccine effectiveness was based on the clinical disease of mumps only. VE is provided adjusted for possible confounders. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | National register or self‐reported |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | National register or self‐reported |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | Unclear risk | By questionnaire |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Spackova 2010.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ local health authorities throughout Germany were encouraged to report varicella outbreaks to the Robert Koch Institute on a voluntary basis. Outbreaks were confirmed by public health professionals. At site visits of day‐care centres (DCC), the authors requested self‐administered questionnaires including varicella history and demographic characteristics from the parents of all children. Furthermore, the authors reviewed children’s vaccination records, which are filled in by the healthcare providers who administer the respective vaccine. Besides information on date of injections and vaccine brands, which the authors collected for all varicella vaccinations, the records also contain the lot numbers of the vaccines. Information regarding general characteristics of the respective DCC (number of children and staff present during the outbreak, number of groups in DCC, joint facilities, etc.) was requested. To protect personal information, study identification numbers were used. A reminding letter was sent to non‐responders to ensure maximum participation. Each outbreak investigation was closed as soon as no further case of varicella had occurred for 42 days (twice the maximal incubation period) after rash onset in the last case. The authors also searched for cases in the 42‐day period before disease onset in the index case to ensure that all outbreak‐related cases were included. | |
| Participants | A case was defined as a child attending 1 of the investigated DCC at the time of the respective outbreak with acute onset of clinical varicella symptoms (maculo‐papulo‐vesicular rash with no other apparent cause) as reported by treating physician or parents. | |
| Interventions | Varilrix 1 dose, Priorix‐Tetra 1 dose and 2 doses, Varivax 1 dose | |
| Outcomes | Varicella was classified clinically as mild (< 50 skin lesions), moderate (≥ 50 skin lesions), or severe (any hospitalised case). Breakthrough varicella (BV) was defined as varicella with rash onset > 42 days after vaccination. | |
| Funding Source | Government | |
| Notes | Potential limitations: case definition, case finding, vaccination status ascertainment, and comparability of vaccinated and unvaccinated regarding exposure to the disease during the study period. The degree of exposure to infection and population susceptibility also influences VE estimates. (1) Exclusion criteria to ensure that only susceptible and vaccinated children were included in VE analyses and that vaccination status did not change during the outbreak. (2) All children under investigation had an equal chance of disease exposure. (3) Vaccination status was verified directly from vaccination records. Information bias might have been present if some parts of the questionnaire were not fully understood or remembered (e.g. duration of skin lesions, previous history of varicella, etc.) by the parents, also if the parent would not recognise mild BV. (1) The authors have considered parental case reporting to be reliable. (2) Additionally, 93% of cases in VE analysis were confirmed by a physician. (3) Each DCC was followed actively until outbreaks, all relevant cases were captured. (4) Both information on disease and vaccination status together was available only in 52% of children, and VE, after exclusions, was calculated only amongst 33% of all children (but amongst all responders who were eligible for VE calculation). (5) Responders (providing either vaccination record or questionnaire) and non‐responders differed significantly by age but not by sex. (6) The failure to demonstrate statistically significant differences regarding brand‐specific VE may be due to sample size. (7) The small number of children with BV and the short time intervals since the last dose of vaccination (up to 4.6 years) limited our ability to explore effects of time since vaccination on BV. (8) Some mild BV cases could have been missed as they might not have been recognised by parents, and thus VE might have been overestimated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ homogeneous age |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ confirmed by physicians |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
ca‐Tafuri 2013.
| Study characteristics | ||
| Methods | Retrospective cohort ‐ this study describes an outbreak of varicella in a small town in the region of Puglia, Southern Italy, in the period between February and March 2011. The investigation subsequent to the outbreak detected at the end of February involved cases that had already been reported and ones that arose subsequently, and were recorded following notification from local doctors. The investigation was conducted by the authors. In the first phase of the investigation, a list of preschools and elementary schools in the town was compiled. Within the town there was 1 state school which was divided into 5 complexes, of which 2 housed elementary schools and 3 preschools. The school principals were contacted, and a list of children enrolled at the schools was requested, as were parents’ telephone numbers. | |
| Participants | The investigation involved 568 children attending school in the town; 358 attended elementary school and 210 attended preschool. | |
| Interventions | Priorix‐Tetra (MMRV; GlaxoSmithKline Biologicals). Varicella vaccination history was verified through the immunisation registry of the Local Health Unit. Parents of the children attending the schools were contacted, and a formal request of informed consent was made for participation in the study, conducted using a standardised questionnaire. | |
| Outcomes | Case definition. A case of natural varicella was defined as an illness involving a pruritic, maculopapulovesicular rash with no other apparent cause, in the period 1 January 2011 through 31 March 2011, in a child attending 1 of the schools in the town, who had not received varicella vaccine or who had been vaccinated less than 14 d before the onset of rash. Breakthrough disease was defined as varicella disease in a child who had been vaccinated 42 d or more before the onset of rash. Illness was classified as mild (fewer than 50 lesions without complications) or moderate‐severe (more than 50 lesions or the occurrence of any serious complications, such as varicella pneumonitis, encephalitis, fever for 5 days, hospitalisations, or death). A child who had attended the schools during this period and did not show signs of the disease was considered as a “non case” patient. | |
| Funding Source | Government | |
| Notes | Children were considered to have asthma, allergies, or eczema if they had a reported history of asthma, allergies, or eczema and were being treated with any medication for these illnesses. Parents were also asked if the child had other chronic illness or had been admitted to hospital in the previous 12 months. The main limitation of the study is the lack of a diagnostic examination of the chickenpox; in fact the study is based on what has been reported by parents, which is due to laboratory‐based confirmation of varicella being very sporadic and to activities supporting molecular diagnostics of epidemiological surveillance not having been initiated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ reported by parent and verified through the immunisation registry of the Local Health Unit |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | High risk | Not reported |
| PCS/RCS ‐ assessment of outcome | High risk | Reported by parents |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
ca‐Takla 2014.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | Primary school: 108 students of 5 classes with ≥ 1 mumps | |
| Interventions | MMR (RIT 4385 or Jeryl Lynn strain) vaccine 2 doses ‐ vaccination status was determined by number who received vaccine up to 18 days prior to disease onset in the index case of the retrospective cohort. | |
| Outcomes | A mumps case was defined as a primary school student who was diagnosed by a physician with acute mumps disease (defined as ≥ 2 d of 1‐ or 2‐sided parotidal swelling without any other cause and/or laboratory detection (IgM detection or significant increase of IgG between 2 specimens) and/or a clinical‐epidemiological link) between 12 March and 9 May 2011. | |
| Funding Source | Government | |
| Notes | The cohort was limited to affected classes because students of same class stay in the same classroom for instruction; mixing with other grades is usually limited. A voluntary parent‐administered questionnaire was handed out to the student collecting information on demography and mumps‐related symptoms and complications. Parents were asked to return the questionnaire with a copy of vaccination card. Very small control sample size. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ representative of the exposed ‐ vaccination card |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | There is insufficient information. |
| PCS/RCS ‐ assessment of outcome | Low risk | Only clinical definition |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Wichmann 2007.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | Students between 10 and 21 years of age (Duisburg, Germany) n = 1098 10 to 12 years old (N = 485); 13 to 15 years old (N = 460); 16 to 21 years old (N = 152) |
|
| Interventions | MMR, but it is unclear if all study population were immunised with only MMR or other single‐component vaccines. Effectiveness of vaccination in preventing measles during an outbreak | |
| Outcomes | Measles cases were identified according to a standard clinical case definition. | |
| Funding Source | Government | |
| Notes | Authors' conclusions: VE was high. Vaccination coverage (92% 1 dose and 70% 2 doses) was insufficient to prevent the outbreak. Immunisation gaps were found, especially in older students. To prevent further outbreaks and to achieve the goal of measles elimination in Germany, vaccination coverage must be increased. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequately defined ‐ by vaccination record ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequately defined ‐ by vaccination record ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | Possible residual confounding ‐ no information about possible confounders |
| PCS/RCS ‐ assessment of outcome | Unclear risk | By questionnaire ‐ in this study 88% of students returned completed questionnaires |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ca‐Woudenberg 2017.
| Study characteristics | ||
| Methods | Prospective observational cohort study during the measles epidemic in the Netherlands in 2013 to 2014 | |
| Participants | Infants between 6 and 14 months of age living in municipalities where coverage with the first dose of MMR vaccine was < 90% Infants 6 to 11 months of age were offered an extra vaccination (and would thus still be eligible for their second MMR vaccination at the age of 14 months); 12‐ to 14‐month‐old infants were offered an early MMR vaccination as an alternative to the regular time point at 14 months of age. All infants are eligible for another dose of MMR scheduled at 9 years of age. |
|
| Interventions | MMR vaccine (M‐M‐RVAXPRO; Sanofi Pasteur MSD). This vaccine contains measles virus Enders’ Edmonston strain. Vaccination status was checked in the national vaccination register. Parents were asked whether their infant(s) had had measles in the preceding 3 months. |
|
| Outcomes | Measles laboratory‐confirmed | |
| Funding Source | Government | |
| Notes | Conclusions: infants vaccinated between 6 and 14 months of age had a lower risk of measles than unvaccinated infants. However, part of the effect was caused by herd immunity, since vaccinated infants were more likely to be surrounded by other vaccinated individuals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ prospective cohort ‐ as part of the vaccination campaign |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ prospective cohort ‐ as part of the vaccination campaign |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ potential confounders: age, breastfeeding, religion, sibling’s vaccination status, day‐care centre attendance, and travel history |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ laboratory‐confirmed |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Ahlgren 2009.
| Study characteristics | ||
| Methods | Cohort study | |
| Participants | 731,592 residents in the great Gothenburg area, Sweden born between 1959 and 1990. The study area was the greater Gothenburg area on the Swedish west coast, on 31 December 2000. | |
| Interventions | Different vaccination programmes carried out from 1971 with different vaccines (single‐component measles, mumps, and rubella vaccine so as with MMR vaccine) having as target population children of different ages. From 534 MS patients, born between 1959 and 1990, the authors selected 1 unvaccinated cohort and 4 cohorts, each corresponding to a vaccination programme: (0) born between 1959 and 1961: the pre‐vaccine era; (1) born between 1962 and 1966: monovalent rubella vaccine; (2) born between 1970 and 1973: only received later dose of the MMR vaccine; (3) born between 1974 and 1978: monovalent measles; and (4) July 1981 to June 1984: combined MMR vaccine. |
|
| Outcomes | Incidence of multiple sclerosis (MS, 4 Poser's criteria) and clinically isolated syndrome with onset between 10 and 39 years of age was assessed in birth cohorts immunised within 4 vaccination programmes. The Gothenburg MS register was established with an intensive case ascertainment from the 1950s and was repeatedly updated. In this study, this register was updated from multiple sources, including the administrative diagnosis registries of the Departments of Neurology, Neuro‐ophthalmology and the Neuropediatric Unit at Sahlgrenska University Hospital, the local MS Society, the National Patient Register of the National Board of Health and Welfare, and by personal visits at the 4 outpatient neurological clinics in the greater Gothenburg area. All records are reviewed with the following MS‐related diagnoses, according to the International Classification of Diseases (ICD) 10, 9, and 8: G359; 340; 340.99 Multiple Sclerosis; G368; G378; G379; 341W; 341.09 Demyelinating disorders of the central nervous system; G360; 341A; 341.01 Neuromyelitis optica; G369; 341X acute disseminated encephalomyelitis; G373 acute transverse myelitis: H46; 377D; 367.02 optic neuritis; H48,1; 367.03 retrobulbar neuritis. 2 of the authors (CA, OA) independently reviewed all medical records retrieved and systematically reassessed the year of onset, the results of diagnostic procedures including CSF analysis and MRI, the course of the disease, and the year of onset of secondary progression. | |
| Funding Source | Government | |
| Notes | Conclusion: there was no significant change in the age‐ and gender‐specific incidence of MS in any of the selected cohorts compared with the incidence in the preceding selected birth cohorts. There was thus no significant change in MS incidence related to the implementation of the rubella vaccination programme in the 12‐year‐old female cohort born 1962 to 1966 compared with the unvaccinated cohort born 1959 to 1961. The incidence did not significantly change with all preceding selected cohorts as baseline, neither in the MMR‐vaccinated 12‐year‐old cohort born 1970 to 1973, nor in the cohort born 1974 to 1978, half of which were measles vaccinated in the preschool age and the majority MMR vaccinated at 12 years, nor in the cohort born July 1981 to June 1984, which was MMR vaccinated at both 18 months and 12 years of age. Restricting the analyses to probable and definite MS cases did not change these results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | Unclear how vaccination status was determined |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | Unclear how vaccination status was determined |
| PCS/RCS ‐ comparability | High risk | Probable residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ clinical definition |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Barlow 2001.
| Study characteristics | ||
| Methods | Cohort study ‐ the design of the Vaccine Safety Datalink ‐ from 1 March 1991 to 30 September 1993 | |
| Participants | Data are collected from 4 HMOs: the Group Health Cooperative in Seattle; Northwest Kaiser Permanente in Portland, Oregon; Kaiser Permanente of Northern California in Oakland; and Southern California Kaiser Permanente in Torrance. Children (N = 137,457). Children entered the cohort at birth, on the date of their enrolment in the HMO, or at the beginning of a study site’s observation period, whichever came last, and remained in the cohort until the age of 7 years, disenrolment from the HMO, or the end of the observation period, whichever occurred first. Using the automated data, the authors identified 2281 possible first seizures. Using the random‐sampling plan previously described, they selected a total of 1094 children for chart review. 716 of these children were confirmed to have had a first seizure during the study period. The reference group at the time of the seizure was composed of children matched for age, calendar time, and HMO but who had not had a vaccination in the preceding 30 days. |
|
| Interventions | Immunisation with MMR vaccine: data on immunisation were derived from automated immunisation tracking systems initially developed to collect information on all routinely administered immunisations. | |
| Outcomes | Risk of febrile seizure within 0 to 7, 8 to 14, 15 to 30 days after immunisation. Potential seizures were identified through the automated data systems of each HMO, on the basis of visits classified according to the ICD‐9‐CM as code 333.2 (myoclonus), code 345 (epilepsy), code 779.0 (convulsions in a newborn), or code 780.3 (convulsions). |
|
| Funding Source | Government | |
| Notes | Conclusions: there are significantly elevated risks of febrile seizures after receipt of DTP vaccine or MMR vaccine, but these risks do not appear to be associated with any long‐term, adverse consequences. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | Based on large HMO ‐ probable selection bias ‐ data on immunisation were derived from automated immunisation tracking system |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | Drawn from the same population ‐ probable selection bias |
| PCS/RCS ‐ comparability | Unclear risk | Adjusted by multivariate model |
| PCS/RCS ‐ assessment of outcome | Unclear risk | Based on hospitalisation record |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
cb‐Beck 1989.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 196 children aged 12 to 14 months | |
| Interventions | MMR containing 4.1 TCID50 of mumps strain L‐Zagreb (information about measles and rubella employed strains not reported, n = 103) versus Placebo (composition unknown, N = 93) No information about doses given and route of immunisation | |
| Outcomes | ‐ Local reactions (redness, swelling, tenderness, 30 days' follow‐up) ‐ Temperature > 37.5 °C ‐ Catarrhal symptoms ‐ Parotid swelling | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | The study is reported with minimal details (no population description, no details given on how the groups are selected, how they are assigned, the total population, how measurements are made). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | No information |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | No information |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding |
| PCS/RCS ‐ assessment of outcome | High risk | No information |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Benjamin 1992.
| Study characteristics | ||
| Methods | Retrospective cohort comparing incidence of joint and limb symptoms in MMR‐vaccinated children versus non‐vaccinated | |
| Participants | 5017 children between 1 and 5 years | |
| Interventions | MMR vaccine (strains and doses not specified, 1588 participants included in analysis) versus no treatment (1242 participants included in analysis) | |
| Outcomes | ‐ Joint complaints, all episodes (arthralgia, possible/probable arthritis) ‐ Joint complaints first‐ever episodes (arthralgia, arthritis possible or probable, joint total first‐ever, limb/joint complaint episodes, hospital admission, GP consultation, sore eyes, convulsion, coryza, parotitis, temperature, rash) Within 6 weeks after immunisation Data based on a 6‐week parental recall questionnaire and clinician home visit. | |
| Funding Source | Government | |
| Notes | Low response rate in non‐immunised group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | Not clearly stated how cohort was selected ‐ high probable selection bias |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | Not clearly stated how cohort was selected ‐ high probable selection bias |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding ‐ high probable selection bias |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Benke 2004.
| Study characteristics | ||
| Methods | Retrospective cohort study in Melbourne, Australia, as part of the European Community Respiratory Health Survey (ECRHS) between 1992 and 1998. To assess possible association between vaccination and asthma | |
| Participants | N = 309 young adults aged between 22 and 44 years and were surveyed by an interviewer‐administered questionnaire | |
| Interventions | Questions were asked about vaccinations to measles, mumps, and rubella (MMR); triple antigen (DTP); hepatitis B; and Sabin polio vaccine (OPV). | |
| Outcomes | Participants were surveyed by a validated interviewer‐administered questionnaire covering: history of asthma; details of home and occupation environment; smoking history; medications; dietary information; and respiratory symptoms. Atopy was assessed by skin prick testing to common aeroallergen. | |
| Funding Source | Government | |
| Notes | Conclusion: there was no significant association observed for participants diagnosed with asthma who had received measles or MMR vaccinations compared with those who did not receive measles or MMR vaccinations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | Randomly selected form electoral rolls ‐ probable selection bias |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | Assessed retrospectively via interview ‐ probable information bias |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding |
| PCS/RCS ‐ assessment of outcome | High risk | Assessed retrospectively via interview ‐ probable information bias |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Beyerlein 2017.
| Study characteristics | ||
| Methods | Cohort study ‐ Germany | |
| Participants | Between 1989 and 2000, a total of 1650 offspring of patients with T1D were recruited for the BABYDIAB study and were followed for 23,856 patient‐years. Between 2000 and 2006, 791 additional offspring or siblings of patients with T1D were screened in the context of the BABYDIET study and were followed by using the BABYDIAB protocol for 6358 patient‐years. |
|
| Interventions | MMR vaccination Vaccines recommended by the German Standing Committee on Vaccination (STIKO), which include diphtheria, hepatitis B, Hib, pertussis, poliomyelitis, tetanus, measles, mumps, rubella, meningococcal, pneumococcal, varicella, TBE, and influenza. Several vaccinations were typically given as a 3‐fold compound (MMR: measles, mumps, rubella) or a 5/6‐fold compound (diphtheria, Hib, pertussis, poliomyelitis, tetanus, and since 2001 additionally hepatitis B). |
|
| Outcomes | Type 1 diabetes (T1D) is one of the most common chronic diseases in childhood, with worldwide increasing incidence. The disease is preceded by a pre‐clinical period of islet autoimmunity, which most commonly develops in early infancy. Factors that induce a strong immune response in early life thus might be relevant for the development of T1D‐associated islet autoimmunity. Islet autoantibodies were measured in venous blood samples from scheduled visits. Children in the BABYDIAB study had scheduled visits at birth and at age 9 months, and at 2, 5, 8, 11, 14, 17, and 20 years of age, whereas children in the BABYDIET study had 3‐monthly visits from birth until the age of 3 years, and yearly until the age of 12 years. Measurement of islet autoantibodies in these studies has been described elsewhere. Islet autoimmunity was defined as the development of persistent autoantibodies to 1 or more of the antigens insulin, GAD65, IA‐2, or Zn‐T8, with sample values above the 99th percentile of published population control children classified as positive. In case of single positive antibodies against insulin or GAD65, affinity and epitope reactivity was determined, and children with low‐affinity antibodies (< 109 L/mol) were not classified as islet autoantibody positive, as these isolated antibody signals are not T1D specific and are not associated with increased T1D risk. Persistence was defined as positive in at least 2 consecutive samples. Islet autoantibody assays were evaluated according to the Diabetes Autoantibody Standardization Program. | |
| Funding Source | Government | |
| Notes | Conclusions: there was no evidence that early vaccinations increase the risk of T1D‐associated islet autoimmunity development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ vaccination record |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ multivariate model |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ Diabetes Autoantibody Standardization Program |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐DeStefano 2002.
| Study characteristics | ||
| Methods | Retrospective cohort study (from the Vaccine Safety Datalink Project) | |
| Participants | N = 167,240 children who were enrolled in 4 large HMOs during 1991 to 1997, with follow‐up from birth until at least 18 months to a maximum of 6 years of age | |
| Interventions | Exposure to MMR vaccine (and other vaccines). Vaccinations were ascertained through computerised immunisation tracking systems, and onset of asthma was identified through computerised data on medical care encounters and medication dispensing. | |
| Outcomes | To be classified as having asthma, a child had to meet 1 of the following criteria: (1) at least 1 diagnosis of asthma ICD‐9 Code 493 and at least 1 prescription for an asthma medication; the first diagnosis and first prescription had to be within a 2‐year period. Asthma medications included oral or inhaled beta‐agonists, theophylline, oral or inhaled corticosteroids, cromolyn sodium, adrenergic drugs not elsewhere specified, and unclassified asthma medications; (2) at least 1 prescription for an inhaled beta‐agonist and at least 1 prescription for cromolyn within a 2‐year period; (3) at least 5 prescriptions for asthma medications during a 2‐year period. |
|
| Funding Source | Government | |
| Notes | Conclusion: there is no association between diphtheria, tetanus, and whole‐cell pertussis vaccine, oral polio vaccine, or measles, mumps, and rubella vaccine and the risk of asthma. The weak associations for Hib and hepatitis B vaccines seem to be at least partially accounted for by healthcare utilisation or information bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | Based on large HMO ‐ probable selection bias ‐ data on immunisation were derived from automated immunisation tracking system |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | Drawn from the same population ‐ probable selection bias |
| PCS/RCS ‐ comparability | Unclear risk | Multivariate model ‐ probable residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ Vaccine Safety Datalink |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
cb‐Dunlop 1989.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 335 healthy children aged about 15 months | |
| Interventions | MMR vaccine Trimovax (Mérieux, containing measles strain Schwarz 1000 TCID50, rubella RA 27/3 1000 TCID50, mumps Urabe AM/9 5000 TCID50)
versus Measles vaccine Rouvax (Mérieux, containing measles strain Schwarz, 1000 TCID50). Single dose IM or sc administered |
|
| Outcomes | Rash, temperature, cough, pallor, diarrhoea, nappy rash, injection site bruise, earache, parotitis, lymphadenopathy, hospitalisation Parental daily diary for 3 weeks and weekly for 3 more weeks | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | Cohort was defined on voluntary basis ‐ probable selection bias |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | Cohort was defined on voluntary basis ‐ probable selection bias |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding |
| PCS/RCS ‐ assessment of outcome | Unclear risk | No information |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Gavrielov‐Yusim 2014.
| Study characteristics | ||
| Methods | A retrospective study design was used to reveal the risk factors associated with febrile convulsion in study participants ‐ Israel | |
| Participants | All participants were aged 10 to 24 months at vaccination, and received the immunisation in community public health well‐child clinics from 1 January 2005 to 31 December 2009. The study group consisted of 8344 MMRV vaccinees immunised from 1 September 2008 (at limitation of national vaccination policy change from MMR to MMRV) until 31 December 2009. The comparison group consisted of 90,294 MMR recipients immunised from 1 January 2005 until 31 August 2008. The observation period captured 40 days following MMR/MMRV administration. Individual data on FC were available for all study participants from birth until 40 days postimmunisation. These data were used to calculate the pre‐vaccination age‐related risk of FC. | |
| Interventions | MMRV and MMR vaccines. Immunisation data were received for the period of 2005 to 2009 from the computerised system of the Israeli Ministry of Health. MMRV cohort N = 32,148 participants; MMR+V cohorts N = 32,145 participants. MMRV Priorix‐Tetra. MMR (Priorix) produced by GSK. Priorix‐Tetra combines the components of 2 of GSK's live attenuated vaccines, MMR (Priorix) and varicella vaccine (Varilrix). | |
| Outcomes | Febrile convulsion: validation FC cases were retrieved using the following coded and free‐text diagnoses: “convulsions in newborn”, “convulsions”, “febrile convulsions”, “complex febrile convulsions”, “other convulsions”. Children diagnosed with FC differential diagnoses during the observational period, i.e. head trauma, epilepsy, or central nervous system infection, were excluded from the study. The exact coded and free‐text diagnoses used to depict coincidental differential conditions were: “concussion”, “cerebral disease”, “acquired hydrocephalus”,“cerebral palsy”, “cerebral cyst”, “epilepsy”, “meningism”, types of “bacterial meningitis”, “encephalitis”, “meningococcal meningitis”, and “aseptic viral meningitis”. Children were also excluded from the study if they had a history of mumps, measles, rubella, or varicella prior to vaccination. | |
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusion: given the low number of MMRV‐specific FC cases, their transient nature, and the benefit of vaccination, the overall benefit‐risk of the vaccine can be considered favourable. Nonetheless, the option of separate immunisation with MMR+V should be offered to parents, in order to maintain sufficient vaccine uptake in the population. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ Clalit Health Services' 53% Israel's population ‐ vaccination status from computerised system of Israeli Ministry of Health |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same population |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ homogeneous age |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ medical record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Hviid 2004.
| Study characteristics | ||
| Methods | Cohort study | |
| Participants | Danish birth cohorts 1990 to 2000. Children in the cohort were followed from birth until 31 December 2001, or until they received a diagnosis of type 1 diabetes, died, were lost to follow‐up or emigrated, or reached 12 years of age, whichever occurred first. A total of 739,694 children were included. | |
| Interventions | MMR (1990 through 2001), Denmark had a nationwide policy of vaccinating children against MMR. The dates of vaccination with the first, second, or third dose of the vaccines were obtained from the National Board of Health. In Denmark, childhood vaccinations are administered solely by general practitioners, who are reimbursed when they report these data to the National Board of Health. The National Board of Health has kept a register of these reports since 1990. Data on the MMR vaccine have been available only since September 1991, thus children born in 1990 were classified as having unknown MMR vaccine status. | |
| Outcomes | Type 1 diabetes:information on the diagnosis of type 1 diabetes from 1 January 1990 through 31 December 2001 was obtained from the Danish National Hospital Register. From 1990 through 1993, Denmark used a modified version of the International Classification of Diseases, 8th Revision (ICD‐8). From 1994 through 2001, the ICD‐10 was used. The authors used codes 249 and E10 (the code 249 does not exist in the standard WHO version of the ICD‐8) to identify all cases of type 1 diabetes. Beginning in 1995, visits to the emergency room and outpatient visits were included in the National Hospital Register (681 cases of type 1 diabetes). | |
| Funding Source | Government | |
| Notes | Conclusions: "These results do not support a causal relation between childhood vaccination and type 1 diabetes" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ National Board of Health |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same population |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ homogeneous age ‐ probable residual confounding |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ National Hospital Register |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Hviid 2008.
| Study characteristics | ||
| Methods | Cohort study ‐ by using data from the Civil Registration System and considering all children born in Denmark between 1 January 1991 and 31 December 2003, the present study investigates the association between MMR immunisation and hospitalisation with asthma diagnosis and use of anti‐asthma medication with a person‐time cohort design. | |
| Participants | For the analysis of association between MMR vaccination and asthma hospitalisation, all those born in Denmark between 1 January 1991 and 31 December 2003, aged between 1 and 5 years, have been considered within the time period from 1 January 1992 and 31 December 2004 (N = 871,234). Children contributed to person‐time follow‐up from 1 year of age until age of 5, or until 31 December 2004, death, or disappearance/emigration. Follow‐up resulted in 2,926,406 person‐years. Due to several reasons, 15,914 children terminated their follow‐up prematurely (5455 because of death, 10,159 emigrated, and 300 disappeared). Follow‐up length for the analysis of use of anti‐asthma medication reached from 1 January 1996 to 31 December 2004, as data about medical prescription were available only from 1996. A total of 600,938 children contributed to follow‐up, corresponding to 1,858,199 person‐years. Follow‐up was prematurely terminated for 12,552 children (4681 due to death, 7710 due to emigration, and 161 disappeared). |
|
| Interventions | Dates of MMR vaccination were obtained from the National Board of Health (in Denmark routine childhood vaccination may be administered by GPs only, who must report them to the National Board of Health). Used preparation contains strain Moraten measles strain, Jeryl Lynn mumps strain, and Wistar RA 27/3 rubella strain. Authors report that 85% of the 871,234 participants in the cohort for asthma hospitalisation and 84% of those considered for anti‐asthma medication (n = 600,938) received MMR before end of follow‐up. MMR vaccination status was considered as time‐varying variable, and individuals could contribute to person‐time as both unvaccinated and vaccinated participants. | |
| Outcomes | Asthma hospitalisation (from the Danish National Hospital Register) Anti‐asthma medication (from the Danish Prescription Drug Database) |
|
| Funding Source | Government | |
| Notes | There is no information about the time considered between vaccination and disease onset or use of medication (i.e. authors do not provide a definition of MMR‐vaccinated and not‐vaccinated status). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ Danish civil registration system ‐ probable selection bias |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same population |
| PCS/RCS ‐ comparability | Low risk | Age and calendar period, sex, child’s place of birth, child’s birthweight, mother’s country of birth, mother’s age at birth of child, birth order, and infant vaccine compliance |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ hospitalisations record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Hviid 2019.
| Study characteristics | ||
| Methods | Nationwide cohort study ‐ Denmark | |
| Participants | 657,461 children born in Denmark from 1999 through 31 December 2010, with follow‐up from 1 year of age and through 31 August 2013. | |
| Interventions | MMR1 and MMR2 vaccinations and other childhood vaccinations administered in the first year of life. There were no thimerosal‐containing vaccines in the Danish programme during the study period. The specific MMR vaccine used in the study period contained the following vaccine strains: Schwarz (measles, 2000 to 2007) or Enders' Edmonston (measles, 2008 to 2013), Jeryl Lynn (mumps), and Wistar RA 27/3 (rubella). | |
| Outcomes | Danish population registries were used to link information on MMR vaccination, autism diagnoses, other childhood vaccines, sibling history of autism, and autism risk factors to children in the cohort. Survival analysis of the time to autism diagnosis with Cox proportional hazards regression was used to estimate hazard ratios of autism according to MMR vaccination status, with adjustment for age, birth year, sex, other childhood vaccines, sibling history of autism, and autism risk factors (based on a disease risk score). | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ Danish population registries ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ Danish population registries ‐ from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ multivariate model ‐ age, sex, other childhood vaccines received, sibling history of autism, and autism risk score |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ Danish Psychiatric Central Register |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Jacobsen 2009.
| Study characteristics | ||
| Methods | Cohort study ‐ USA | |
| Participants | Children aged 12 to 60 months who received a first dose of MMRV in February 2006 to June 2007. Participants were optimally matched on age, sex, and calendar date of vaccination to children who had received MMR+V concomitantly in November 2003 to January 2006, before MMRV licensure. Potential cases of febrile convulsion were identified through administrative data and adjudicated by expert panel, according to prespecified criteria. | |
| Interventions | MMRV: ProQuad, a combined formulation of measles, mumps, rubella, and varicella (MMRV) vaccine that contains components of 2 Merck vaccines, MMR‐II (MMR) and Varivax (V), was approved in the USA in September 2005. Before MMRV was available, MMR and V were usually given concomitantly as 2 separate injections. | |
| Outcomes | Study participants were followed through health encounter and claims records to identify all potential occurrences of convulsion. Potential convulsions were identified as occurring on any visit with a diagnosis coded as 779.0 (neonatal seizures), 333.2 (myoclonus), 345 (epilepsy), 780.39 (other convulsion), 780.3 (convulsion), 780.31 (simple febrile convulsion), or 780.32 (complex febrile convulsion) regardless of setting (e.g. inpatient, outpatient, emergency department, or outside facility). | |
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusion: these data suggest that the risk of febrile convulsion is increased in days 5 to 12 following vaccination with MMRV as compared to MMR+V given separately during the same visit, when postvaccination fever and rash are also increased in clinical trials. Whilst there was no evidence of an increase in the overall month following vaccination, the elevated risk during this time period should be communicated and needs to be balanced with the potential benefit of a combined vaccine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ registry KPSC ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ exposed and non‐exposed were matched for age, sex, vaccination calendar day and month |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ hospital record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Jain 2015.
| Study characteristics | ||
| Methods | Retrospective cohort study using an administrative claims database associated with a large commercial health plan | |
| Participants | Children born between 1 January 2001 and 31 December 2007, continuously enrolled in the health plan from birth to at least 5 years of age during 2001 and 2012 who also had an older sibling continuously enrolled for at least 6 months between 1997 and 2012 | |
| Interventions | MMR vaccine receipt (0, 1, 2 doses) after 1 year of age MMR vaccine receipt was defined as having a Current Procedural Terminology (CPT) or ICD‐9‐CM procedure code indicating receipt of each component (measles, mumps, and rubella) after 1 year of age. |
|
| Outcomes | ASD status in index children and older siblings was determined using a claims‐based algorithm that required 2 or more claims on separate dates of service with an ICD‐9‐CM diagnosis code in any position for autistic disorder, other specified PDD including Asperger syndrome, or unspecified PDD (299.0x, 299.8x, and 299.9x). Both index child and older‐sibling ASD status were determined using their entire enrolment time that fell within the study period. Index children had to have at least 1 older sibling with 2 claims with ASD diagnoses or all older siblings with no ASD diagnoses. Children with an older sibling with only 1 claim with an ASD diagnosis were excluded. Index children with only 1 claim with an ASD diagnosis were also excluded. | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ age at vaccination, ASD status |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ medical record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Klein 2010.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ USA ‐ data from Vaccine Safety Datalink: Group Health Cooperative (Washington state), Kaiser Permanente Colorado, Kaiser Permanente Northwest (Oregon), Harvard Pilgrim Health Care (Massachusetts), HealthPartners (Minnesota), Northern California Kaiser Permanente, and Marshfield Clinic (Wisconsin) | |
| Participants | Children aged 12 to 23 months who were members of the 7 participating VSD sites and received their first dose of MMRV (Merck & Co Inc, West Point, PA) were eligible to be included in study. | |
| Interventions | MMRV (Merck & Co Inc, West Point, PA) | |
| Outcomes | A seizure event was defined as the first instance during the 42 days after MMRV vaccination with ICD‐9 codes 345* (epilepsy) or 780.3* (convulsion) in the emergency department or hospital. | |
| Funding Source | Government | |
| Notes | Conclusion: amongst 12‐ to 23‐month‐olds who received their first dose of measles‐containing vaccine, fever and seizure were elevated 7 to 10 days after vaccination. Vaccination with MMRV results in 1 additional febrile seizure for every 2300 doses given instead of separate MMR varicella vaccines. Providers who recommend MMRV should communicate to parents that it increases the risk of fever and seizure over that already associated with measles‐containing vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ registry Kaiser Permanente ‐ representative of exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ adjusted for age group Vaccine Safety Datalink sites respiratory virus season |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ hospital record with blind assessment |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Klein 2012.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ USA ‐ data from Vaccine Safety Datalink: Group Health Cooperative (Washington state), Kaiser Permanente Colorado, Kaiser Permanente Northwest (Oregon), Harvard Pilgrim Health Care (Massachusetts), HealthPartners (Minnesota), Northern California Kaiser Permanente, and Marshfield Clinic (Wisconsin). Linked to cb‐Klein 2010 | |
| Participants | Children aged 48 to 83 months (2 to 7 years old) who were members of the 7 participating VSD sites between January 2000 through October 2008 and who received MMRV; separately administered, same‐day MMR+V; or MMR or V administered alone were eligible for study inclusion. | |
| Interventions | MMRV (Merck & Co) MMR (Merck & Co Inc, West Point, PA) + V (Merck & Co) | |
| Outcomes | Postvaccination seizure event as the first instance during the 42 days after a measles‐ or varicella‐containing vaccine of ICD‐9 codes 345* (epilepsy) or 780.3* (convulsion) in the emergency department or hospital | |
| Funding Source | Government | |
| Notes | Conclusion: this study provides reassurance that MMRV and MMR+V were not associated with increased risk of febrile seizures among 4‐ to 6‐year‐olds. We can rule out with 95% confidence a risk greater than 1 febrile seizure per 15,500 MMRV doses and 1 per 18,000 MMR+V doses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ registry Kaiser Permanente ‐ representative of exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ adjusted for age group Vaccine Safety Datalink sites respiratory virus season |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ hospital record with blind assessment |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Klein 2017.
| Study characteristics | ||
| Methods | Retrospective cohort study ‐ USA ‐ data from Vaccine Safety Datalink: Group Health Cooperative (Washington state), Kaiser Permanente Colorado, Kaiser Permanente Northwest (Oregon), Harvard Pilgrim Health Care (Massachusetts), HealthPartners (Minnesota), Northern California Kaiser Permanente, and Marshfield Clinic (Wisconsin). Linked to cb‐Klein 2012; cb‐Klein 2010 | |
| Participants | N = 946,806 children who were < 36 months of age who received a first dose of any measles‐containing vaccine from 2000 to 2012 | |
| Interventions | MMRV (Merck & Co) MMR (Merck & Co Inc, West Point, PA) + V (Merck & Co) | |
| Outcomes | Postvaccination seizure event as the first instance during the 42 days after a measles‐ or varicella‐containing vaccine of ICD‐9 codes 345* (epilepsy) or 780.3* (convulsion) in the emergency department or hospital | |
| Funding Source | Government | |
| Notes | Discussion: children who received MMRV vaccine or who had prior medically attended fevers and seizures during the first year of life had increased risk of fever after a first dose of measles vaccine. After adjusting for familial propensity to seek care, MCV‐associated fever still clustered within families, suggesting a possible genetic basis for susceptibility to developing fever due to measles vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ registry Kaiser Permanente ‐ representative of exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ adjusted for age group Vaccine Safety Datalink sites respiratory virus season |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ hospital record with blind assessment |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Madsen 2002.
| Study characteristics | ||
| Methods | Retrospective cohort | |
| Participants | All Danish children born between January 1991 and December 1998: 537,303 | |
| Interventions | MMR vaccine (containing measles strain Moraten, mumps Jeryl Lynn, rubella Wistar RA 27/3) versus pre‐vaccination or non‐vaccinated person‐years | |
| Outcomes |
|
|
| Funding Source | Government | |
| Notes | The follow‐up of diagnostic records ends 1 year (31 December 1999) after the last day of admission to the cohort. Because of the length of time from birth to diagnosis, it becomes increasingly unlikely that those born later in the cohort could have a diagnosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ representative of exposed ‐ National Board of Health |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ adjusted for age, sex, calendar period, other ASD |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ Danish Psychiatric Central Register |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Makino 1990.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 1638 healthy children | |
| Interventions | MMR vaccine MPR (Kitasato Institute, Japan containing measles AIK‐C 5000 TCID50, mumps Hoshino 15000 TCID50, and rubella Takahashi 32000 TCID50) versus Measles vaccine (Kitasato Institute, containing measles AIK‐C 25000 TCID50) versus Mumps vaccine (Kitasato Institute, containing mumps Hoshino 10000 TCID50) | |
| Outcomes | Temperature, axillary (up to 37.5 °C or up to 39.0 °C), rash (mild, moderate, or severe), lymphadenopathy, parotitis, cough, vomiting, diarrhoea within 28 days after vaccination | |
| Funding Source | Not stated | |
| Notes | Inadequate description of the cohorts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | There was insufficient information ‐ probable selection bias. |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | There was insufficient information ‐ probable selection bias. |
| PCS/RCS ‐ comparability | Unclear risk | Homogeneous age ‐ there was insufficient information to assess comparability |
| PCS/RCS ‐ assessment of outcome | High risk | Self‐reported |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐McKeever 2004.
| Study characteristics | ||
| Methods | Cohort study assessing association between MMR and DPPT and asthma or eczema | |
| Participants | Birth cohorts 1988 to 1999 identified through the West Midlands General Practice Research Database (GPRD; N = 16,470, aged from 20 months to 11 years, accounting for 69,602 person‐years) | |
| Interventions | MMR vaccination (data from GPRD; data about other vaccination have also been considered) | |
| Outcomes | Incident diagnoses of asthma/wheeze and eczema were identified using the relevant Oxford Medical Information System (OXMIS, derived from ICD‐8) and Read codes. | |
| Funding Source | Government | |
| Notes | The case definitions used for this study were based on physician‐diagnosed disease and were thus dependent on the child’s being taken to the doctor and receiving a recorded diagnosis. Children who are not taken to the doctor are less likely to be vaccinated and also have less of an opportunity to have a diagnosis of allergic disease recorded. These factors can contribute to show an apparent association between vaccination and allergic reactions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Registry West Midlands General Practice ‐ representative of the exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Registry West Midlands General Practice ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adjusted ‐ parental smoking, parental allergic diseases, maternal age, number of older siblings, use of antibiotics early in life of birth, GP practice |
| PCS/RCS ‐ assessment of outcome | High risk | The case definitions used for this study were based on physician‐diagnosed disease and were thus dependent on the child’s being taken to the doctor and receiving a recorded diagnosis. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Miller 1989.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 12,023 healthy children aged 1 to 2 years | |
| Interventions | MMR vaccine (Immrawa or Pluserix, both containing measles strain Schwarz, rubella RA 27/3, mumps Urabe 9) versus Measles vaccine (not described) Single dose | |
| Outcomes | Temperature (2 or more days over 21 days), rash (2 or more days over 21 days), anorexia (2 or more days over 21 days), number of symptoms for 1 day only (daily diary completed by parents) | |
| Funding Source | Not stated | |
| Notes | The study reports that 84% of diaries/questionnaires completed but only 65% were analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | Probable selection bias ‐ there was insufficient information |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | Probable selection bias ‐ there was insufficient information |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding ‐ there was insufficient information |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Mrozek‐Budzyn 2013.
| Study characteristics | ||
| Methods | Prospective cohort study, Krakow. The aim of the study was to examine the hypothesis that MMR exposure has a negative influence on cognitive development in children. | |
| Participants | The data from an earlier established Krakow birth cohort of children are used (part of collaborative study with Columbia University in New York, on the vulnerability of fetus and child to environmental factors). The enrolment (3 November 2000 to 22 August 2003) included only non‐smoking women, aged 18 to 35 years, with singleton pregnancy without illicit drug use and HIV infection, free from chronic diseases such as diabetes or hypertension and residing in Krakow for at least 1 year prior to pregnancy. The infants were followed up to 8th year of life. Each year mothers were asked to provide information on infants’ health and household characteristics by trained interviewers, who carried out detailed, face‐to‐face standardised interviews. | |
| Interventions | MMR vaccine (and measles vaccine). Data on infants’ vaccination history (date of vaccination and type of vaccine) were extracted from the physician’s records. The vaccination status was based on measles vaccination during the second year of life. | |
| Outcomes | The Fagan Test of Infant Intelligence at 6th month of life. The Bayley Scales of Infants Development, second edition was administered in the 12th, 24th, and 36th months of life. The Mental Scale of that test includes items that assess memory, habituation, problem solving, early number concepts, generalisation, classification, vocalisation, language, and social skills. Test scores are adjusted to child’s age to obtain the Mental Development Index. Test results are in 1 of 4 categories (range 50 to 150): (1) accelerated performance (score > 115); (2) within normal limits (score 85 to 114); (3) mildly delayed performance (score 70 to 84); and (4) significantly delayed (score < 69). The Raven’s Colored Progressive Matrices test was administered twice, in 5th and 8th year of life. The outcomes of the test were measured in terms of centiles. Because the results of this test were generally high, the cut point of poor result category was 74th percentile, which means middle intelligence outcomes. Output scale was presented in centiles standardised to age groups. The Wechsler Intelligence Scale for Children (WISC‐R) was administered in the 6th and 7th years of life, and generated verbal, non‐verbal, and total IQ for evaluated children. Category with IQ < 100 was considered as the poorer outcomes. The outcomes range is from 40 to 160. All neurodevelopment tests were conducted in the Department of Epidemiology and Preventive Medicine by carefully trained examiners who were unaware of the child’s exposure. Bayley Scales as well as Raven test have well‐defined criteria and were considered as fully consent between different examiners. In order to provide fully comparable assessment of WISC‐R test, 1 psychologist rated performed answers for all children. |
|
| Funding Source | Government | |
| Notes | Conclusion: the results suggest that there is no relationship between MMR exposure and children’s cognitive development. Furthermore, the safety of triple MMR is the same as the single measles vaccine with respect to cognitive development. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | From physician record ‐ drawn from the same community |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | Krakow (Poland) birth cohort of children ‐ selected group: women aged 18 to 35 singleton pregnancy |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information ‐ probable residual confounding. |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ standardised method |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
cb‐Robertson 1988.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 319 children aged 13 months | |
| Interventions | MMR vaccine (Mérieux, containing measles strain Schwarz, mumps Urabe AM/9, and rubella Wistar RA 27/3) versus Measles vaccine (Schwarz strain) Allocation by parental choice | |
| Outcomes | Irritability, rash, coryza, temperature (parental touch), cough, lethargy, diarrhoea, vomiting, anorexia, conjunctivitis, lymphadenopathy, parotitis, local reactions, no symptoms, paracetamol use, seen by GP, convulsion Parental‐completed diaries of symptoms. 3‐week follow‐up | |
| Funding Source | Pharmaceutical industry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | Probable selection bias ‐ volunteers |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | Probable selection bias ‐ there was insufficient information |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding |
| PCS/RCS ‐ assessment of outcome | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Rowhani‐Rahbar 2013.
| Study characteristics | ||
| Methods | Retrospective cohort study at 8 Vaccine Safety Datalink sites in the USA. Linked to cb‐Klein 2010 | |
| Participants | N = 840,348 children 12 to 23 months of age who had received a measles‐containing vaccine from 2001 through 2011 | |
| Interventions | MMRV, MMR+V, MMR | |
| Outcomes | Fever events in the outpatient setting were defined using ICD‐9 code 780.6*. Postimmunisation medically attended seizure events in the emergency department or hospital setting were defined using ICD‐9 code 780.3* (convulsion) or 345* (epilepsy). All electronically identified seizure events were included in the analyses; the authors do not distinguish between febrile and afebrile seizures. | |
| Funding Source | Government | |
| Notes | Conclusion: measles‐containing vaccines are associated with a lower increased risk of seizures when administered at 12 to 15 months of age. Findings of this study that focused on safety outcomes highlight the importance of timely immunisation of children with the first dose of measles‐containing vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ 10 managed care organisations |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ 10 managed care organisations |
| PCS/RCS ‐ comparability | Low risk | Adjusting for age group sex respiratory virus season calendar day and VSD site |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ Vaccine Safety Datalink ‐ medical record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Schink 2014.
| Study characteristics | ||
| Methods | Matched cohort study, Germany | |
| Participants | Claims data of more than 17 million insures in the German Pharmacoepidemiological Research Database. All children born between 1 January 2004 and 31 December 2008 who received a 1st dose of MMRV vaccine were matched to children vaccinated with MMR, MMR+V and MMR or MMR+V. | |
| Interventions | MMRV: Priorix‐Tetra (GSK) compared to MMR and V vaccines (MMR+V). Vaccinations were identified by outpatient codes used for reimbursement of administration of vaccines. For MMR and V vaccines, these codes cover all brands available in Germany from different manufacturers. Vaccine dispensations in the pharmacy could not be considered, as physicians generally use vaccines kept in their own medical practices. | |
| Outcomes | Febrile convulsions: diagnosis of FC, i.e. an ICD‐10‐GM code R56.0 in any of the hospital diagnoses 2 outcome definitions: The primary outcome “FC narrow” was defined as hospitalisation where no alternative plausible cause of FC. This endpoint included: (i) all hospitalisations with FC as main discharge diagnosis; (ii) all hospitalisations with FC as main admission diagnosis and without a main discharge diagnosis of an infectious disease (except measles, mumps, rubella, or chickenpox) or a neurological condition; (iii) all hospitalisations with FC as secondary or ancillary diagnosis and a main discharge diagnosis coded as complication following immunisation (ICD‐10‐GM code “T88.0 infection following immunization” or “T88.1 other complications following immunization, not elsewhere classified”). Due to exclusion of alternative causes of FC in this outcome definition, it was assumed that it would have higher specificity, but lower sensitivity. The secondary outcome “FC Jacobsen”: only hospitalisations for FC with a neurological condition coded as main discharge diagnosis were excluded. Consequently, “FC Jacobsen” included: (i) all hospitalisations with FC as main discharge diagnosis; (ii) all hospitalisations with FC as main admission diagnosis and without a main discharge diagnosis of a neurological condition; and (iii) all hospitalisations with FC as secondary or ancillary diagnosis and with a main discharge diagnosis coded as complication following immunisation. “FC narrow” cases are a subset of “FC Jacobsen” cases. |
|
| Funding Source | Pharmaceutical industry | |
| Notes | Conclusions: this study in children younger than 5 years, 90% of them between 11 and 23 months, shows a risk of FC similar in magnitude for Priorix‐Tetra as has previously been reported for ProQuad, suggesting a class effect for these quadrivalent vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ German Pharmacoepidemiological Research Database |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ German Pharmacoepidemiological Research Database |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ matched for age, sex, a prior FC, hospitalisation for an infectious disease 15 days before until 30 days after vaccination, administration of other vaccines 30 days prior to 30 days after immunisation with MMRV, MMR, or MMR+V, and calendar month of vaccination to take into account the seasonality of infectious diseases |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ medical record |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Sharma 2010.
| Study characteristics | ||
| Methods | Cohort study carried out in Egypt, assessing reaction observed after immunisation with MMR in occasion of compulsory vaccinations | |
| Participants | Children aged 16 to 24 months (N = 73,745) from 9 Egyptian governorates and aged 5 to 7 years (N = 371,184) from 8 Egyptian governorates | |
| Interventions | Immunisation with MMR vaccine containing Leningrad‐Zagreb mumps strain (Tresivac, Serum Institute of India) This contains 1000 TCID50 live attenuated measles Edmonston‐Zagreb strains, 5000 TCID50 of mumps strain Leningrad‐Zagreb, 1000 TCID50 of rubella strain Wistar RA 27/3 in each 0.5 mL dose. Partially hydrolysed gelatin (2.5%), sorbitol (5%), neomycin (≤ 15 μg), and water as diluent are also vaccine components. 24 different lots (EU 615V, EU 618V ‐ EU 640V) were used in the study. Younger children were immunised in the thigh; older children were immunised in the deltoid. |
|
| Outcomes | Pain, redness, swelling, fever, rash, parotitis, arthralgia, lymphadenopathy. Data collected by means of a structured questionnaire within 42 days after vaccination. | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | One main purpose of the study was to investigate the association between MMR and aseptic meningitis. No disease cases have been identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Unclear risk | Adequate ‐ representative of exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Unclear risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Unclear risk | There was insufficient information ‐ probable residual confounding. |
| PCS/RCS ‐ assessment of outcome | High risk | Self‐reported ‐ there was insufficient information |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Stokes 1971.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | N = 334 in US children aged 10 months to 6 years old | |
| Interventions | MMR vaccine (Merck Sharp & Dohme containing measles strain Moraten 1000 TCID50, mumps strain Jeryl Lynn 5000 TCID50, rubella strains HPV ‐ 77 1000 TCID50) 1 dose subcutaneous versus No treatment | |
| Outcomes |
|
|
| Funding Source | Government | |
| Notes | Two studies (one in US, one in Costa Rica) were reported in the one study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ comparability | High risk | No adjustment by confounders |
| PCS/RCS ‐ assessment of outcome | High risk | Self‐reported |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Swartz 1974.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 59 children aged 1 to 6 years (mean about 2 years) | |
| Interventions | MMR vaccine (Merck Institute for Therapeutic Research) versus Mumps ‐ rubella vaccine (Merck Institute for Therapeutic Research) versus Rubella vaccine (Merck ‐ Meruvax HPV 77‐DE5) No information about doses and schedule | |
| Outcomes | Temperature (37.2 to 38.2 °C; 38.3 to 39.3 °C; over 39.4 °C), lymphadenopathy, enanthema, conjunctivitis, rash, complaints ‐ any (up to 60 days). Follow‐up 7 to 15 days | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ comparability | High risk | No adjustment for confounding |
| PCS/RCS ‐ assessment of outcome | High risk | There was insufficient information. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Timmermann 2015.
| Study characteristics | ||
| Methods | Cohort study ‐ in the Faroe Islands. A birth cohort was formed from consecutive spontaneous births in the Faroe Islands during 1997 to 2000. | |
| Participants | N = 640 children were followed from birth. Follow‐up examinations at ages 5, 7, and 13 years included a physical examination and a maternal questionnaire about the child’s health. At age 7, total and grass‐specific IgE was quantified in the child’s serum, and at age 13, the children underwent skin prick tests. | |
| Interventions | The Faroe Islands follow the Danish vaccination schedule, in which MMR vaccination, at the time of this study, was administered at age 15 months and 12 years. There were no specific contraindications. At the 5‐year examination, the child’s vaccination card was inspected and all vaccination dates were registered. At age 13, the mothers were asked whether the child had received the MMR vaccination scheduled at 12 years of age. The child’s vaccination card was reviewed at examinations. |
|
| Outcomes | Asthma and dermatitis/eczema At age 5, parents were asked whether the child was suspected as suffering from asthma or had been diagnosed with asthma, hypersensitivity, or allergy. At ages 5, 7, and 13 years, the same paediatrician determined the presence of current wheezing by auscultation. At the same ages, the paediatrician also examined all children for dermatitis/eczema. At age 13, the findings from this examination were graded according to a score for atopic dermatitis (SCORAD). At age 7, a blood sample was drawn and total IgE and grass‐specific IgE were quantified. At age 13, parents were asked whether the child had ever suffered from asthma. In accordance with the International Study of Asthma and Allergies in Childhood (ISAAC), they were also asked to indicate whether the child had (i) suffered from wheezing in the past 12 months; (ii) suffered from sneezing, running, or blocked‐up nose except for when the child had a cold or was sick in the past 12 months and, if so, whether it had been accompanied by itching running/tearing eyes (current rhinoconjunctivitis symptoms); and (iii) whether the child had ever suffered from an itching rash that comes and goes for at least 6 months (eczema ever). At age 13, the children underwent a skin prick test with extracts of 5 common allergens (birch/grass pollen, dog/cat dander, and house dust mite (Dermatophagoides pteronyssinus)). |
|
| Funding Source | Government | |
| Notes | Conclusion: MMR vaccination early in life may have a protective effect against allergy at least up to age 7 and against asthma through age 13 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ representative of exposed |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community |
| PCS/RCS ‐ comparability | Low risk | Adequate ‐ IgE concentration, duration of gestation, birthweight, maternal smoking during pregnancy |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ medical examination |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Uchiyama 2007.
| Study characteristics | ||
| Methods | Retrospective cohort study conducted in Yokohama (Japan) | |
| Participants | Children born between 1976 and 1999 with clinical diagnosis of ASD assessed at the Yokohama Psycho‐Developmental Clinic (N = 904) | |
| Interventions | MMR vaccine containing AIK‐C (measles), Urabe AM9 (mumps), and To‐336 (rubella) strains | |
| Outcomes | ASD regression | |
| Funding Source | Government | |
| Notes | The study analysed data from clients of the Yokohama Psycho‐Developmental Clinic (YPDC). The YPDC, a private child psychiatric clinic specialising in developmental disorder, opened in April 1997. The YPDC has a close relation with the many parental organisations advocating for autism in Japan and has become recognised as a centre for ASD. For this reason, the proportion of clients with ASD is very high. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ Maternal and Child Health Handbook |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ patient of the Yokohama Psycho‐Developmental Clinic ‐ probable selection bias |
| PCS/RCS ‐ comparability | High risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | High risk | The information on regression was totally dependent on parental report. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
cb‐Vestergaard 2004.
| Study characteristics | ||
| Methods | Retrospective and prospective cohort, Denmark | |
| Participants | 537,171 Danish children | |
| Interventions | Exposure to MMR vaccine (containing measles strain Moraten, mumps Jeryl Lynn, and rubella Wistar) | |
| Outcomes | Febrile seizure (ICD definition) in children aged 3 months to 5 years: cases occurred within 2 weeks after vaccination and cases occurred after this time | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | Low risk | Adequate ‐ representative of exposed ‐ Danish civil registration system ‐ National Board of Health |
| PCS/RCS ‐ non‐exposed cohort selection | Low risk | Adequate ‐ drawn from the same community ‐ Danish civil registration system |
| PCS/RCS ‐ comparability | Low risk | Adjusted for age, calendar period, sex, number of siblings with febrile seizures, number of siblings with epilepsy |
| PCS/RCS ‐ assessment of outcome | Low risk | Adequate ‐ national hospital registry |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
cb‐Weibel 1980.
| Study characteristics | ||
| Methods | Prospective cohort | |
| Participants | 135 children | |
| Interventions | MMR vaccine (Merck, containing measles strain Moraten, mumps Jeryl Lynn, rubella RA 27/3) versus Rubella vaccine (strain RA 27/3) 1 dose subcutaneously | |
| Outcomes | Temperature > 38 °C, rash, lymphadenopathy, arthralgia, myalgia, anorexia. Follow‐up 42 days | |
| Funding Source | Government | |
| Notes | No information given on how the children were distributed between the 3 arms. Sparse detail on safety data collection procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS ‐ exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ non‐exposed cohort selection | High risk | There was insufficient information. |
| PCS/RCS ‐ comparability | High risk | There was insufficient information. |
| PCS/RCS ‐ assessment of outcome | High risk | There was insufficient information. |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
db‐Andrews 2012.
| Study characteristics | ||
| Methods | Self‐controlled case series study and cohort study ‐ aimed to estimate the risk of thrombocytopenic purpura following MMR using data on hospital admissions linked to immunisation data from England and Denmark | |
| Participants | In this study the aim was to evaluate the risk of TP following this first MMR dose, therefore a study population of children aged 12 to 23 months (365 to 732 days) was chosen. | |
| Interventions | In England and Denmark, the first MMR dose is scheduled during the second year of life. The risk periods examined were 0 to 13, 14 to 27, 28 to 42, and 0 to 42 days post‐MMR and a pre‐vaccination "low" period: −7 to −1 days, to allow for a vaccination being delayed if the child was ill. |
|
| Outcomes | In England and Denmark, vaccine safety assessment is performed using routinely collected data where health outcomes are linked to immunisation data. For the TP study, both countries used national TP‐coded hospital discharge data linked to immunisation registry data. The case definition for TP was based only on the presence of a relevant ICD‐10 code (D69.3) or ICD‐8 code (287.10) in 1 of the diagnostic discharge fields. First episodes were defined as the earliest record found for an individual; further episodes were initially required to be at least 14 days since a previous episode (to prevent double counting of episodes). In England, cases (based on ICD‐10) occurring between 1 April 1996 and 31 March 2007 were linked using NHS number or gender/date of birth/postcode to immunisation records. In Denmark, the Central Person Registry was used to construct a nationwide cohort consisting of all Danish children born in the period of 1 January 1990 to 31 December 2007 (∼1.2 million children). |
|
| Funding Source | Government | |
| Notes | A cohort analysis is also presented, but only for Denmark data; the results do not differ from those obtained by self‐controlled case series. Consequently, to avoid duplication, we retained only data from self‐controlled case series analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adequate ‐ adjusted by age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Dourado 2000.
| Study characteristics | ||
| Methods | Self‐controlled case series to investigate the association between MMR vaccination and aseptic meningitis in Brazil | |
| Participants | 452,344 children aged 1 to 11 years (from census); 129 children aged 1 to 11 years admitted to the referral hospital with a diagnosis of aseptic meningitis between 10th and 43rd epidemiologic surveillance weeks of 1997 (March to October). N = 87 fulfilled inclusion criteria; n = 29 cases of AM occurred prior to the mass immunisation campaign, N = 58 after the immunisation campaign. Of the 58 children, N = 50 were known to have been vaccinated. (The date of vaccination was available for 43 of these children.) | |
| Interventions | Immunisation with MMR vaccine Pluserix (SmithKline Beecham, containing mumps strain Urabe) Vaccination histories were obtained through home visits or telephone calls. Vaccination cards were required, but if they were not available, information that the child had been vaccinated on the national vaccination day was assumed to be reliable for the MMR vaccine, because it was the only vaccine administered by injection that day. Risk period: 15 to 35 days following MMR vaccination. Observation period: 24 weeks pre‐vaccination and 10 weeks postvaccination were compared. |
|
| Outcomes | The following criteria were used to define eligible cases of aseptic meningitis for the study: (1) residence in the city of Salvador; (2) age 1 to 11 years; (3) cerebrospinal fluid with a cell count of > 10 and < 1200 cells per mL (higher counts could be attributed to unconfirmed bacterial meningitis); (4) predominance of lymphocytes in the cerebrospinal fluid of > 50% of the total number of cells; (5) exclusion of any bacteriologic or fungal confirmation through the use of Gram stain, latex, immunoelectrophoresis, stain for Cryptococcus neoformans, Ziehl‐Neelsen stain, or culture for bacteria and Mycobacterium tuberculosis; and (6) exclusion of all cases with a history of prior meningitis or any neurologic disorder and any cases with sepsis, pneumonia, otitis, or any other disease that might be associated with an increased cell count in the cerebrospinal fluid. |
|
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Unclear risk | Not described |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to seriously alter the results |
db‐Farrington 1995.
| Study characteristics | ||
| Methods | Self‐controlled case series, UK | |
| Participants | Children aged 12 to 24 months in 1 of the 3 diagnostic categories Children discharged from hospital with a diagnosis of:
from computerised hospital records in 5 districts in England (Ashford, Leicester, Nottingham, Preston, and Chorley & Ribble) for varying periods between October 1988 and February 1993. Readmissions within 72 h with the same diagnosis were counted as 1 episode. |
|
| Interventions | MMR vaccines with mumps strain Urabe or Jeryl Lynn | |
| Outcomes | Febrile convulsion, aseptic menigitis, idiopatic thrombocytopenic purpura The risk periods for MMR vaccine (6 to 11 and 15 to 35 days after vaccination) were those in which neurological events attributable to the measles and mumps components might be expected. |
|
| Funding Source | Pharmaceutical industry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Unclear risk | Not described |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ computerised child health and general practice records |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Unclear risk | Not described |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
db‐France 2008.
| Study characteristics | ||
| Methods | Self‐controlled cases series. Study based on Vaccine Safety Datalink investigating association of immune thrombocytopenic purpura and MMR | |
| Participants | Children aged 12 to 23 months with ITP identified from VSD database for the years 1991 to 2000, who had been vaccinated with MMR whilst actively enrolled in their respective MCOs. For each child, follow‐up time was limited to the 365 days before and after MMR vaccination. Vaccinated children with ITP that occurred outside this follow‐up window were excluded. The criteria for cases were defined as children aged < 18 years with a platelet count of 50,000/L with normal red and white blood cell indices, the presence of clinical signs and symptoms of spontaneous bleeding, and the absence of fever. A case was excluded if in the 6 weeks before diagnosis the child had been exposed to platelet‐depleting medication (phenytoin, valproic acid, or sulfonamide antibiotics) or infected with wild‐type varicella or Epstein‐Barr virus. |
|
| Interventions | Exposure to MMR vaccine (composition not provided in the study report) Exposed period: 42 days after MMR vaccination Unexposed period: defined as the time periods before and after the exposed period. Period of 6 weeks immediately preceding MMR vaccination was excluded from analysis because this represents a period when a child is most likely to be healthy (the healthy‐vaccinee) and may underestimate the background incidence of ITP. |
|
| Outcomes | ITP diagnoses within 42 days from immunisation | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record ‐ but probable selection bias |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adequate adjusted for age, sex, MMR doses |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Macartney 2017.
| Study characteristics | ||
| Methods | Self‐controlled case series, Australia. From 2009 to 2012 | |
| Participants | Children aged 11 to 23 months. Analysis was further restricted to include only children who had: (1) 1 dose of MMR vaccine followed by 1 dose of MMRV vaccine at least 27 days later (consistent with NIP recommendations); (2) 1 dose of MMR vaccine (as some had not yet received MMRV vaccine); or (3) no MMR or MMRV vaccine (unvaccinated children, who contribute to the age‐specific relative incidence). Children who received MMRV vaccine as their first MCV were excluded because this schedule was not consistent with NIP recommendations and rarely occurred. Because age is a strong predictor of FS and is time varying, all models were adjusted for the effect of age (using 3 age groups in the base case: 11 to 14, 15 to 18, and 19 to 23 months). We removed the −1‐ to −13‐day period before vaccination from the baseline time because it may be associated with a lower FS risk (an FS occurrence may delay receipt of scheduled vaccines). |
|
| Interventions | MMRV Priorix‐Tetra | |
| Outcomes | Febrile seizures: in all children younger than 5 years. Periodic review of all ICD10‐Australian Modification–coded R56.0 was also conducted to capture additional cases. Clinical and demographic data were collected from the medical records and caregiver interviews, and all FS diagnoses were confirmed. The study outcome was immunisation coverage of consecutive, 3‐month national cohorts of children born between 1 January 2009 and 31 December 2012, who had reached the ages of 24, 36, 48, and 72 months, respectively, for receipt of MMR, varicella, and/or MMRV vaccine by December 2015. |
|
| Funding Source | Government | |
| Notes | Authors' conclusions: "To our knowledge, this is the first study to provide evidence of the absence of an association between use of MMRV vaccine as the second dose of MCV in toddlers and an increased risk of FSs. Incorporation of MMRV vaccine has facilitated improvements in vaccine coverage that will potentially improve disease control." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adjusted by age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐MacDonald 2014.
| Study characteristics | ||
| Methods | Person‐time cohort, Canada. From 2006 to 2012 | |
| Participants | Children 12 to 23 months of age in the province of Alberta For each vaccine administered, the authors compared the incidence of seizures in the 42‐day “observation period” following administration (comparable with clinical trials of Priorix‐Tetra and the postlicensure study of ProQuad) and the 7‐ to 10‐day “peak period” (when previous studies have indicated that febrile seizure risk is expected to be highest) with the incidence in the 42 days preceding vaccination (control period) using a risk interval analysis. |
|
| Interventions | MMRV (Priorix‐Tetra) (administered from mid‐2010 onward) and MMR+V (2006 onward) | |
| Outcomes | Data on seizure events were obtained from the physician claims database ICD‐9 780.3* for convulsions and the ambulatory care and hospital discharge databases (ICD‐10, Canadian version, codes R56.0* for febrile convulsions), using coding consistent with other studies of febrile seizures after vaccination. | |
| Funding Source | Government | |
| Notes | Conclusion: combining MMR and varicella into a single vaccine decreases pain for children and distress for parents, thus addressing common barriers to vaccine uptake, and may improve vaccine coverage levels and decrease immunisation delivery costs. These potential benefits must be balanced by the increased risk (albeit small) of febrile seizures with the combination vaccine. Febrile seizures are typically self‐limiting and rarely have long‐term effects, but they can be extremely distressing for parents, may precipitate acute care visits, and may undermine confidence in immunisation programmes. It is a matter for debate whether the choice of separate versus combination vaccine is a policy decision or a choice for parents to make in consultation with their vaccination provider. If MMRV continues to be offered for first‐dose administration, it might be advisable to counsel parents regarding antipyretic use if children experience a fever within the peak risk period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adjusted for age and calendar year |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Makela 2002.
| Study characteristics | ||
| Methods | Self‐controlled case series study | |
| Participants | 561,089 children aged between 1 and 7 years at the time of vaccination | |
| Interventions | Immunisation with MMR 2 vaccine (Merck, containing measles strain Enders Edmonston, mumps Jeryl Lynn, and rubella Wistar RA 27) during a national immunisation campaign | |
| Outcomes | ‐ Encephalitis ‐ Aseptic meningitis ‐ Autism | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | Incidence of outcomes during the first 3 months after immunisation was compared with that in the following period (from 3 to 24 months after immunisation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ exposure | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ observation and exposure risk period | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ comparability | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
db‐McClure 2019.
| Study characteristics | ||
| Methods | Person‐time cohort (named "risk interval analysis") | |
| Participants | Children were eligible if they had received their first dose of measles‐containing vaccine at age 12 through 23 months from January 2003 through September 2015. Children were excluded if they had a history of seizure or conditions strongly related to seizure prior to 12 months of age. Children born before 37 weeks gestational age were classified as preterm, and children born 37 weeks gestational age as full term. Preterm children were further classified into those born < 35 weeks (early preterm) and 35 through 36 weeks (late preterm) gestational age. The authors conducted a risk‐interval analysis amongst vaccinated children, with each child having 42 days of follow‐up following receipt of a measles‐containing vaccine. Days 7 through 10 following vaccination were defined as the risk interval, and days 15 through 42 following vaccination were defined as the control interval. Days 0 through 6 and 11 through 14 following vaccination were excluded. The first exclusion reduced possible short‐term effects with concomitant vaccines, and the latter exclusion was to avoid residual exposure effects in the control interval. |
|
| Interventions | MMRV vaccination | |
| Outcomes | Seizures were identified by diagnostic codes in the inpatient or emergency department settings. | |
| Funding Source | Government | |
| Notes | Conclusion: vaccination with a measles‐containing vaccine in the second year of life is associated with a similar relative risk of a first seizure in children born preterm as in those who were born full term. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adjusted by age, gestational age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Miller 2003.
| Study characteristics | ||
| Methods | Self‐controlled case series, UK | |
| Participants | Children aged 12 to 23 months admitted to hospital between April 1991 and March 1995 in selected districts in the Thames region of southern England. Total of 387 admissions with 1 or more of the bacterial infection codes and with a linked MMR vaccination record were identified; occurred in 387 children (169 in 165 females, and 226 in 222 males); 116 had a diagnosis of invasive bacterial infection, and 279 had lobar pneumonia. | |
| Interventions | MMR vaccine not reported; risk period 0 to 90 days The incidence of admission for bacterial infection in the 12‐week period after MMR vaccine, and each of the 3 contained 30‐day periods, relative to the background rate was measured using the self‐controlled case series analysis method. Since the incidence of bacterial infection varies with age, the potential confounding effect of age was adjusted for by stratifying age into 26, 2‐week intervals. Seasonal effects were adjusted for by stratifying the analysis by calendar month. A pre‐vaccination low‐risk period of 14 days was defined to allow for a delay to vaccination after hospital admission for an infection. Readmissions within 14 days were considered to be the same episode. Separate analyses were carried out for cases of invasive disease and lobar pneumonia without an invasive code. |
|
| Outcomes | Cases were identified from computerised discharge records using ICD‐9 codes 036 (meningococcal infection), 038 (septicaemia), 320 (bacterial meningitis), 711.0 (pyogenic arthritis), 730.0 (acute osteomyelitis), and 481 (lobar (pneumococcal) pneumonia). Hospital records were linked with computerised district immunisation records by sex, date of birth, and post code. Only MMR vaccine is given in the second year of life. Cases in children with additional diagnostic codes indicating an underlying disorder predisposing to bacterial infection, such as immunosuppression, malignancy, cystic fibrosis, congenital heart defect, or a cerebrospinal fluid shunt, were excluded. | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | Conclusion: combined MMR vaccine did not increase the risk of hospitalisation with invasive bacterial infection in the 3 months after vaccination, rather there was a protective effect. These results provide no support for the concept of 'immunological overload' induced by multiple‐antigen vaccinations, nor calls for single‐antigen vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adjusted by age, calendar month |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
db‐Miller 2005.
| Study characteristics | ||
| Methods | Self‐controlled case series. To determine whether any association between gait disturbance and MMR vaccination exceeds the age‐related background rate of gait disturbance | |
| Participants | Children hospitalised with gait disturbance between April 1995 and June 2001 (N = 127, aged 12 to 24 months). Computerised hospital admission and immunisation records for children in the former North and South Thames regions were obtained for the period April 1995 to June 2001 and linked on National Health Service (NHS) number, or sex, date of birth, and full post code, a highly specific linking algorithm. Admissions in children aged 12 to 24 months with an ICD‐10 diagnosis code indicating a possible acute gait disorder or other condition suggestive of cerebellar dysfunction or disturbed motor control were identified, irrespective of whether a linked MMR record was found. The ICD codes used were G111, G112, G25, R26, R27, R29, H55, and F984. Children with gait disturbance resulting from general practice visit general practice research database (GPRD archive), born between 1988 and 1997 (N = 1398, aged 12 to 24 months). For the analysis of gait disorders presenting in general practice, information on all children born from 1988 to 1997 with at least 2 years of continuous follow‐up from birth in a GPRD practice deemed as supplying data of research standard was obtained from the Office for National Statistics. Read and OXMIS codes that indicated a consultation for possible gait disturbance in children aged 12 to 24 months were identified by mapping to ICD‐9 codes and by searching on the following keywords: ataxia, gait, co‐ordination, mobility, movement. |
|
| Interventions | MMR immunisation | |
| Outcomes | Relative incidence of gait disturbance after MMR immunisation (considered risk periods 0 to 30 and 31 to 60 days | |
| Funding Source | Government | |
| Notes | Conclusion: no evidence of an increased rate of hospital admission or general practice consultations for gait disturbance was found in the putative postvaccination risk periods. This study provides no evidence for a causal association between MMR and gait disturbance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adequate ‐ adjusted for age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Miller 2007.
| Study characteristics | ||
| Methods | Self‐controlled case series | |
| Participants | Children aged 12 to 23 months with discharge diagnosis of febrile convulsion or aseptic meningitis. Febrile convulsion: children aged 12 to 23 months with discharge diagnosis of febrile convulsion (ICD‐10 code R560 or R568, febrile convulsion or fit, not otherwise specified) who were admitted between 1 January 1998 and 30 June 2002 were identified and linked with computerised immunisation records to obtain dates of MMR vaccination. Only those children linked with 1 MMR dose when aged 12 to 23 months were retained for the analysis. Aseptic meningitis: viral meningitis (A87), mumps (B26), meningitis in other infections classified elsewhere (G02), and meningitis due to other and unspecified causes (G03) were identified for the period 1 May 1998 to 30 June 2001, and case notes were reviewed by a paediatrician. In addition, computerised hospital records for children aged 12 to 23 months with an ICD‐9 discharge diagnosis of meningitis categorised as mumps, aseptic or viral (072.1, 047, 321), were identified for the period 1 January 1991 to 30 September 1992 prior to the withdrawal of Urabe‐containing MMR vaccines, and were linked with MMR vaccination histories. |
|
| Interventions | The numbers of doses of Priorix and MMRII given to children aged 1 to 2 years in England and Wales and in the 2 regions during the entire study period (1998 to 2004) were estimated from MMR vaccine coverage rates and the proportions of the total MMR doses distributed nationally and in the 2 regions by manufacturer (UK Department of Health, unpublished data, 2006). MMR vaccination histories were independently obtained through linkage with computerised immunisation records in the 2 Thames regions, using either the National Health Service number or sex, date of birth, and postcode, a highly specific linking algorithm. | |
| Outcomes | Incidence of disease during 2 at‐risk periods (between 6 to 11 and 15 to 35 days after immunisation) | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | For aseptic meningitis, the absolute risk in the 15 to 35 days after MMR vaccination during the period May 1998 to June 2001 was estimated, and this risk was compared with that estimated for the period from January 1991 to the end of September 1992, when Urabe‐containing MMR vaccines were predominantly given. Data presented were obtained from db‐Farrington 1995. 'Risk of bias' table is intended for self‐controlled case series on febrile convulsion. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ computerised hospital record |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ computerised child health |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Unclear risk | Not described |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
db‐O'Leary 2012.
| Study characteristics | ||
| Methods | Self‐controlled case series methods to examine the risk of ITP after childhood vaccines | |
| Participants | Children < 18 years This investigation was conducted in 5 healthcare systems (Kaiser Permanente: Colorado, Hawaii, Georgia, and Northern California, and Harvard Vanguard Medical Associates) using data from the years 2000 to 2009. Included children who had been vaccinated whilst actively enrolled in their respective health plans. |
|
| Interventions | MMR vaccine, MMRV vaccine DTaP (diphtheria‐tetanus‐acellular pertussis vaccine); HBV (hepatitis B virus vaccine); Hep A (hepatitis A vaccine); Hib (Haemophilus influenzae type b vaccine); HPV (human papillomavirus vaccine); IPV (inactivated poliovirus vaccine); MCV (meningococcal conjugate vaccine); PCV (pneumococcal conjugate vaccine); RV (rotavirus vaccine); Tdap (tetanus‐diphtheria‐acellular pertussis vaccine); TIV (trivalent influenza vaccine); VAR (varicella vaccine) |
|
| Outcomes | Identification of possible cases was conducted at the lead site by using electronic databases, with the analyst blinded to vaccination status. The authors reviewed the electronic data to exclude cases of thrombocytopenia from other known conditions by using the ICD‐9 diagnosis codes (such as neonatal thrombocytopenia, aplastic anaemia, disseminated intravascular coagulation, acquired haemolytic anaemia, chronic liver disease, or malignancy). Children < 18 years with either 2 platelet counts of 50,000/mL in a 6‐week period or 1 platelet count of 50,000/mL and an associated ICD‐9 code of 287.0 to 287.9, inclusive, within 6 weeks of the low platelet count were included. A case was excluded if, in the 6 weeks before diagnosis, the child was exposed to a platelet‐depleting medication (such as antiepileptics and sulfonamide antibiotics) or infected with wild‐type varicella or Epstein‐Barr virus. |
|
| Funding Source | Government | |
| Notes | Follow‐up time: 365 days before and after vaccination Exposed period: 1 to 42 days after vaccination for all vaccines Unexposed period was defined as the time before and after the exposed period within 365 days of follow‐up before or after vaccination. Day 0 (the day of vaccination) was excluded, because any cases occurring at this time were most likely coincidental. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ computerised hospital record |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ computerised child health and general practice records |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adequate ‐ stratified for age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Perez‐Vilar 2018.
| Study characteristics | ||
| Methods | International hospital‐based retrospective observational study conducted as proof‐of‐concept for the investigation of rare AEFI using 2 analytical case‐only methods: self‐controlled risk interval (self‐controlled case series) and case cross‐over. For this purpose, WHO selected 26 sentinel sites (49 hospitals) distributed in 16 countries of the 6 WHO regions. | |
| Participants | International hospital‐based retrospective observational study conducted as proof‐of‐concept for the investigation of rare AEFI using 2 analytical case‐only methods: self‐controlled risk interval and case cross‐over. For this purpose, WHO selected 26 sentinel sites (49 hospitals) distributed in 16 countries of the 6 WHO regions. The study population included children ages 9 to 23 months admitted to a network‐participating hospital during January 2010 to March 2014, with a discharge diagnosis of either aseptic menigitis or ITP. |
|
| Interventions | MMR vaccination. Vaccination status was retrieved for confirmed cases only, from vaccine registries, vaccination cards, and medical records. The exposure of interest was first dose of measles/mumps‐containing vaccine. Patients were considered as non‐vaccinated when any other vaccinations, but not measles‐containing vaccines, were registered in the consulted sources. Individuals without any vaccination record were excluded from the study. | |
| Outcomes | Aseptic menigitis and ITP Participating hospitals identified potential cases through hospital discharge databases using prespecified ICD‐9/ICD‐10 codes, whereas hospitals not using a discharge codification system or not having electronic databases used free text. A trained physician or nurse blinded to vaccination status reviewed medical records of potential cases according to established case definitions. Potential cases for which medical records were not available were excluded. Only first episodes of AM or ITP were considered. All cases were classified as either confirmed (Level 1 to 3 of diagnosis certainty) or non‐confirmed. Only confirmed cases entered the analyses. |
|
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ exposure | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ observation and exposure risk period | Unclear risk | There was insufficient information. |
| SCCS/PTC ‐ comparability | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
db‐Stowe 2009.
| Study characteristics | ||
| Methods | Self‐controlled case series, UK | |
| Participants | Children aged 12 to 23 months with hospitalisation for bacterial or viral infections identified from hospital admission records by reviewing ICD‐9 or ICD‐10 codes (n = 2025) for the period 1 April 1995 to 1 May 2005. The present analysis of illnesses in a general population is based on an additional 10 years of data for bacterial infections and also includes admissions with viral infections. |
|
| Interventions | MMR vaccination | |
| Outcomes | Bacterial infections: lobar pneumonia or invasive bacterial infection Viral infections: encephalitis/meningitis, herpes, pneumonia, varicella zoster, or miscellaneous virus Relative incidence of each disease was assessed within specified time risk intervals (0 to 30, 31 to 60, 61 to 90, or 0 to 90 days) after MMR immunisation. |
|
| Funding Source | Government | |
| Notes | Conclusion: the study confirms that the MMR vaccine does not increase the risk of invasive bacterial or viral infection in the 90 days after the vaccination and does not support the hypothesis that there is an induced immune deficiency due to overload from multi‐antigen vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ computerised hospital record |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ computerised child health and general practice records |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adjusted for age and season |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Taylor 1999.
| Study characteristics | ||
| Methods | 3 statistical analyses: 1) Only case ecological method. Trends in the time series of cases were analysed by Poisson regression 2) The age at diagnosis was compared in vaccinated and unvaccinated children with autism diagnosed after the age of 18 months. Children were classified into 3 categories: those who had received MMR vaccine before the age of 18 months; those never vaccinated with MMR; and those who had received MMR vaccine at age 18 months or later. 3) Self‐controlled case series. In each analysis, the reference period for each individual consisted of every month from birth to the end of August 1998 that did not fall during a postvaccination risk period. All analyses were finely stratified for age, particularly in younger age groups, because of the multimodal age distribution of recorded events. |
|
| Participants | Children with autistic disorders born since 1979 were identified in 8 health districts in mid‐1998 from computerised special needs/disability registers at child development centres and from records in special schools. Information on children with such disorders who were younger than 16 years of age was extracted from clinical records by 1 of 3 experienced paediatric registrars. The information extracted included the age at which the autistic disorder was diagnosed, the recorded age at which the parents first became concerned about the child’s developmental state, and the age at which the regression became obvious, if that was a feature. n = 498 children with diagnosis; n = 261 typical autism; N = 166 with atypical autism; N = 71 Asperger's syndrome | |
| Interventions | Immunisation data, which were recorded independently of the clinical record, with exact dates, were obtained from the Regional Interactive Child Health Computing System. | |
| Outcomes | Using ICD‐10 criteria, the diagnosis of autism was checked against information in the available records on the child’s present condition and his or her condition between the ages of 18 months and 3 years. Authors considered periods of within 2 months, 4 months, and 6 months of vaccination. Where vaccination and the event of interest occurred in the same month, it was assumed that vaccination preceded the event. |
|
| Funding Source | Government | |
| Notes | We consider the self‐controlled case series method to be the most reliable analysis; quality assessment is based on this method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record ‐ clinical record ‐ Regional Interactive Child Health Computing System |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation periods are well‐defined, exposure period appears to be well‐documented |
| SCCS/PTC ‐ comparability | Low risk | Adequate ‐ stratified for age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
db‐Ward 2007.
| Study characteristics | ||
| Methods | Self‐controlled case series study carried out to assess whether exposure to MMR and other vaccines (DTP/Hib, MenC) was associated with onset of serious neurological diseases | |
| Participants | 155 children aged between 2 and 35 months from the Republic of Ireland and Britain with serial neurological disease (see outcome definition) and documented vaccination history. Data about cases were collected between October 1998 and September 2001. | |
| Interventions | Immunisation with MMR or DTP vaccine. Data were obtained from child's GP by Immunisation Department and Center for Infection. Vaccination history should cover 1 year after disease onset. Authors consider as at‐risk period the time between 0 and 3 days or 0 and 7 days following DTP, Hib, and MenC vaccinations and the time between 6 and 11 days or 15 and 35 days following MMR vaccination. | |
| Outcomes |
(See Table 33 for detailed definition) Observation period: for 12 to 35 months old: 12 sequential periods of 2 months were used. Exposure risk period: 15 to 35 days. |
|
| Funding Source | Pharmaceutical industry | |
| Notes | Authors' conclusion: "As regards MMR vaccine we no evidence of a raised relative incidence of serious neurologic disease (15 to 35 days) after immunisation" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| SCCS/PTC ‐ case selection | Low risk | Adequate ‐ independent validation |
| SCCS/PTC ‐ exposure | Low risk | Adequate ‐ secure record ‐ immunisation department health protection agency ‐ centre for infections |
| SCCS/PTC ‐ observation and exposure risk period | Low risk | Adequate ‐ observation period and risk period are well‐defined |
| SCCS/PTC ‐ comparability | Low risk | Adequate ‐ adjusted for age |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
eb‐Ki 2003.
| Study characteristics | ||
| Methods | Case cross‐over study to investigate the association between MMR vaccination and aseptic meningitis in Korean children 8 to 36 months old | |
| Participants | 67 children, mean age 19.1 months (standard deviation = 5.4 months) The time period observed was 1 year before the onset of aseptic meningitis. However, of this observed duration, the trial authors excluded the 6 months after birth because of the maternal immunoglobulin effect. A predefined 42‐day hazard period before the onset of meningitis was compared with the previous days of the observed past‐year period. |
|
| Interventions | MMR vaccination: N = 29 MMR with Urabe or Hoshino mumps strain, N = 38 MMR with Jeryl Lynn or Rubini mumps strain | |
| Outcomes | Aseptic meningitis is a syndrome characterised by acute onset of meningeal symptoms, fever, and cerebrospinal fluid pleocytosis with bacteriologically sterile cultures. The following criteria were used to define eligible cases of aseptic meningitis for the study: 1) Korean insurance claim cases based on the ICD‐10 (codes A87.9, G03.0, G03.9, and G02.0); and 2) cerebrospinal fluid pleocytosis (leukocytes ≥ 5) with bacteriologically sterile cultures (if measured); or 3) neck stiffness, and/or convulsions, or 2 other symptoms (headache or vomiting) in addition to a fever (≥ 38.0 °C, if measured). Patients’ charts were reviewed and their symptoms, laboratory tests, and last diagnoses on the discharge record checked. If patients were diagnosed with aseptic meningitis and were hospitalised in a general hospital, in accordance with these criteria, those who had headache, fever, and vomiting could be included as participants. |
|
| Funding Source | Government | |
| Notes | This study uses the same data used by eb‐Park 2004; however, here the authors report separately the data of those who were vaccinated with the Urabe mumps (or Hoshino) strain and the data for those who were vaccinated with the Jeryl Lynn (or Rubini) strain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCO ‐ case selection | Low risk | Adequate ‐ record linkage ‐ independent validation |
| CCO ‐ exposure | Low risk | Adequate ‐ secure record ‐ vaccination record |
| CCO ‐ risk and control periods | Low risk | Adequate ‐ risk and control period are well‐defined |
| CCO ‐ comparability | Low risk | Adequate ‐ adjusted for age, sex, age at vaccination |
| Summary Risk of Bias assessment | Low risk | Plausible bias is unlikely to have seriously altered the results. |
eb‐Lafaurie 2018.
| Study characteristics | ||
| Methods | Case cross‐over, France. To compare the frequency of exposure to vaccines during a 6‐week interval immediately preceding the event (case period) to the frequency of exposure during prior 2 control time intervals (named control periods, 6 and 3 months before the case period, having the same duration as the case period) | |
| Participants | Population‐based study in France including all children newly diagnosed for primary ITP between July 2009 and June 2015 | |
| Interventions | MMR vaccines, combined vaccines containing diphtheria, tetanus, and poliomyelitis (DTP), as well as pneumococcal, meningococcal, and hepatitis B (HBV) vaccines | |
| Outcomes | Immune thrombocytopenia | |
| Funding Source | Not stated | |
| Notes | Conclusion: in this nationwide study, no significant risk was observed for vaccines against DTP, pneumococcus, meningococcus, and HBV. The increased risk of MMR‐induced ITP is shown in children (previously demonstrated as lower than after the natural infection with measles). Vaccine‐induced ITP remains an exceptional adverse drug reaction, including for MMR vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCO ‐ case selection | Unclear risk | There was insufficient information. |
| CCO ‐ exposure | Unclear risk | There was insufficient information. |
| CCO ‐ risk and control periods | Unclear risk | There was insufficient information. |
| CCO ‐ comparability | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
eb‐Park 2004.
| Study characteristics | ||
| Methods | Case cross‐over to investigate the association between MMR vaccination and aseptic meningitis in Korean children | |
| Participants | Children aged 13 to 29 months. The design divides the study period (1 year of 365 days) into a hazard period (42 days after MMR, or before meningitis as defined by the authors) and a control period of 323 days. | |
| Interventions | Immunisation with MMR (vaccine type not stated) | |
| Outcomes | Cases of aseptic meningitis before and after immunisation | |
| Funding Source | Government | |
| Notes | There is a likelihood of selection bias, which the authors dismiss as they say that moving (probable cause of wrong phone numbers) is not associated with MMR exposure. The missing 27% of hospital records is also worrying. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CCO ‐ case selection | Low risk | Adequate ‐ record linkage ‐ independent validation |
| CCO ‐ exposure | Unclear risk | Self‐reported ‐ study does not distinguish between 2 types of MMR vaccine |
| CCO ‐ risk and control periods | Low risk | Adequate ‐ risk and control period are well‐defined |
| CCO ‐ comparability | Unclear risk | Not clearly documented |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ga‐Boccalini 2015.
| Study characteristics | ||
| Methods | Case‐only ecological method study, Italy, to assess the impact of MMRV immunisation programme on varicella‐related hospitalisations | |
| Participants | All hospitalised cases for varicella of all ages | |
| Interventions | MMRV vaccine for children aged 13 to 15 months (first dose) and 5 to 6 years (second dose) or monovalent varicella vaccines for children at 24 months of age. Since July 2008 | |
| Outcomes | From 2004 to 2012, all hospitalised cases for varicella or its complications, as a primary or secondary discharge diagnosis, with the following ICD‐9‐CM codes (2002 and 2007) were examined: 052.0 (post‐varicella encephalitis), 052.1 (varicella (haemorrhagic) pneumonitis), 052.2 (post‐varicella myelitis), 052.7 (varicella with other specified complications), 052.8 (varicella with unspecified complication), and 052.9 (varicella without complication). | |
| Funding Source | Not stated | |
| Notes | Conclusion: the introduction of universal vaccination has already led to a significant decline in hospitalisations due to varicella after just 4 years of implementation. Hospitalisation rates fell noticeably amongst younger individuals involved in the vaccination programme. The decrease in hospitalisation rate in the older age groups suggests a possible indirect protection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ independent validation |
| COEM ‐ exposure | Unclear risk | No description |
| COEM ‐ time trend comparison | Low risk | Adequate ‐ well‐defined periods |
| COEM ‐ comparability | Unclear risk | Stratified by age |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ga‐Pozza 2011.
| Study characteristics | ||
| Methods | Case‐only ecological method | |
| Participants | 66 paediatricians, covering 58,643 children. During the period 2000 to 2008, on average, 44,416 children were followed each month by 51 paediatricians. | |
| Interventions | MMRV: tetravalent combination, which also included MMR vaccines (Priorix, ProQuad) | |
| Outcomes | Varicella cases: the first source consisted of surveillance data retrieved from the Regional Department of Prevention, which is part of the official Italian epidemiological surveillance system of infectious diseases. The second source consisted of a sentinel surveillance system based on a sample of paediatricians, the Sorveglianza Pediatri Sentinella. This is a network of Italian family paediatricians that is co‐ordinated by the Italian Public Health Office (Istituto Superiore di Sanità), the Italian Federation of Family Pediatricians (Federazione Italiana Medici Pediatri), the Italian Society of Pediatrics (Società Italiana di Pediatria), and the Cultural Association of Pediatricians (Associazione Culturale Paediatri). The paediatricians participate in the system on a voluntary basis. | |
| Funding Source | Government | |
| Notes | Conclusion: incidence rates significantly decreased 2.5 years after beginning the universal vaccination, whilst hospitalisation rates showed a significant decrease 1 year earlier. There was a remarkable decline of both varicella incidence and hospitalisations, especially in 1‐ to 4‐year‐old children. This study confirms the positive impact of universal vaccination. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ independent validation |
| COEM ‐ exposure | Unclear risk | No description |
| COEM ‐ time trend comparison | Low risk | Adequate ‐ well‐defined periods |
| COEM ‐ comparability | Unclear risk | Stratified by age and year |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
ga‐Tafuri 2015.
| Study characteristics | ||
| Methods | Case‐only ecological method, Italy. Describes changes in epidemiology and costs of varicella since the introduction of the MMRV vaccination programme. | |
| Participants | All hospitalised cases for varicella of all ages | |
| Interventions | MMRV vaccine | |
| Outcomes | All hospitalised cases for varicella or its complications, as a primary or secondary discharge diagnosis, with the ICD‐9‐CM codes pre‐vaccination era 2003 to 2005, 2‐doses MMRV vaccination era 2009 to 2012 | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Unclear risk | There was insufficient information. |
| COEM ‐ exposure | High risk | There was insufficient information. |
| COEM ‐ time trend comparison | Unclear risk | There was insufficient information. |
| COEM ‐ comparability | Unclear risk | Stratified by age |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
gb‐da Cunha 2002.
| Study characteristics | ||
| Methods | Case‐only ecological method. Study to determine if there is an increased risk of acute aseptic meningitis and mumps in children aged 1 to 11 years in 2 regions of Brazil, Mato Grosso do Sul and Mato Grosso (MS and MT). | |
| Participants | Children aged 1 to 11 years old irrespective of previous vaccination. MS (N = 473,718); MT (N = 580,587). The campaigns started in mid‐August 1998 in MS, and in late September in MT, and lasted for 1 month. The reported numbers of children vaccinated were 442,962 (coverage of 93.5%) and 402,927 (coverage of 69.4%), respectively. Most doses were applied in the first 2 weeks of the campaigns. | |
| Interventions | MMR vaccine containing Leningrad‐Zagreb mumps strain (Serum Institute of India Ltd) | |
| Outcomes | Notification of meningitis is statutory in Brazil, with a standardised form completed for each case. Aseptic meningitis (clinical diagnosis or notification form). 31 (in MT) or 37 (in MS) weeks before and 10 weeks after vaccination campaign |
|
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ medical record |
| COEM ‐ exposure | Unclear risk | There was insufficient information. |
| COEM ‐ time trend comparison | Low risk | Adequate ‐ well‐defined period |
| COEM ‐ comparability | Unclear risk | There was insufficient information. |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
gb‐da Silveira 2002.
| Study characteristics | ||
| Methods | Case‐only ecological method. Surveillance study carried out in Rio Grande do Sul (Brazil) following an immunisation campaign with MMR vaccine containing Leningrad‐Zagreb mumps strain. | |
| Participants | Children between 1 and 11 with aseptic meningitis. | |
| Interventions | Immunisation with Leningrad‐Zagreb MMR vaccine | |
| Outcomes | Risk association with aseptic meningitis | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ independent validation |
| COEM ‐ exposure | Unclear risk | Self‐reported |
| COEM ‐ time trend comparison | Low risk | Adequate ‐ periods are well‐defined |
| COEM ‐ comparability | Unclear risk | Stratified by age |
| Summary Risk of Bias assessment | Unclear risk | We had concerns regarding at least 1 domain such that some doubt is raised about the results. |
gb‐Fombonne 2001.
| Study characteristics | ||
| Methods | Case‐only ecological method Objective to test: if an autistic enterocolitis syndrome occurs in children who have autism and were immunised with MMR, by this set of prediction: 1. childhood disintegrative disorder might have become more frequent; 2. the mean and distribution of age at which parents become concerned has changed and is closer to the mean immunisation age than in children who were not exposed to MMR; 3. regression in the development of children with autism has become more common; 4. the age of onset of symptoms for autistic children with regression clusters around the immunisation date and is different from that of autistic children without regression; 5. children with regressive autism may have distinct symptom and severity profiles; and 6. regressive autism is associated with gastrointestinal symptoms, and children with regressive autism may exhibit increased frequency of inflammatory bowel disorders. |
|
| Participants | 3 samples are used:
Children born between 1992 and 1995 (post‐MMR immunisation programme), selected as part of an epidemiologic survey of PDD conducted in Staffordshire (Midlands, UK) total population N = 15,500. |
|
| Interventions | The MMR immunisation programme was introduced in 1988 in the UK (with first MMR given between 12 and 15 months of age) with coverage rates above 90%; MMR coverage rates in 2‐year‐olds fell from 92% in 1995 to 88% in 2000. | |
| Outcomes | Age at first parental concern: in the 3 samples, item 2 of the Autism Diagnostic Interview (earlier version of the Autism Diagnostic Interview‐Revised) was used to assess the first onset of autistic symptoms, or the age of the child at which parents first became concerned with their child’s development. The precise wording of the question is: “How old was your child when you first wondered if there might be something not quite right with his/her development?” Definition and assessment of regression: the assessment of regression in the ADI‐R is covered with items 37 to 41 (for language) and items 95 to 103 (for other domains). The regression is assessed for language skills as follows: “Were you ever concerned that your child might have lost language skills during the first years of his/her life? Was there ever a time when he/she stopped speaking for some months after having learned to talk?” Assessment of bowel disorders and symptoms: these data were available only from the epidemiologic sample (Stafford sample). All children were reviewed regularly and are still followed up by the paediatrician, who has records of any additional hospital admissions/medical investigations for bowel disorders in these children. The occurrence of gastrointestinal symptoms was assessed by 2 sources: the parents and the paediatrician. ADI‐R was administered with the parents by trained staff. Inter‐rater reliability on the ADI‐R interviews was assessed. |
|
| Funding Source | Government | |
| Notes | The number and possible impact of biases in this study is so high that caution is advised in interpretation of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ epidemiological survey ‐ independent validation |
| COEM ‐ exposure | Low risk | Adequate ‐ secure record |
| COEM ‐ time trend comparison | High risk | Unclear definition ‐ serious risk of confounding |
| COEM ‐ comparability | High risk | Not stated ‐ serious risk of confounding |
| Summary Risk of Bias assessment | High risk | We had concerns regarding multiple domains such that our confidence in the result is substantially lowered. |
gb‐Fombonne 2006.
| Study characteristics | ||
| Methods | Case‐only ecological method | |
| Participants | 1 October 2003 was chosen as the survey date. As of 1 October 2003, a total of 27,749 children were registered within the Lester B. Pearson School Board (LBPSB), the largest school board for Anglophone children in Quebec. The LBPSB has 55 schools (45 elementary and 10 secondary) and provides education from kindergarten through grade 11. Age 5 to 16. | |
| Interventions | MMR doses, at 12 and 18 months of age. Data on MMR uptake for the study period were available through the Direction de Santé Publique de la Capitale Nationale (N Boulianne, BN, MSc, written communication, 2005). These data were routinely collected in the region of Quebec amongst 5‐year‐old children attending kindergarten during the years 1993 to 2004 (i.e. for birth cohorts from 1988 to 1998). Vaccination records from children were used as the main source of information to document MMR vaccination and its date. When this information was not available, vaccination status of the children was obtained through consultation of the regional vaccination registry or else through direct contact with doctors' practices, both from community clinics and private offices. | |
| Outcomes | Children with a diagnosis of PDD were identified by school personnel and given a study code to preserve the anonymity of the data. Children’s diagnoses were not verified by direct assessments, but it is worth noting that a majority of these children (N = 155; 86.1%) were diagnosed at the Montreal Children’s Hospital. School personnel further identified the diagnostic subtype using DSM‐IV diagnostic criteria, age, grade, and school the child was attending. When available, place of birth was also recorded. | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ independent validation |
| COEM ‐ exposure | Low risk | Adequate ‐ secure record ‐ vaccination record |
| COEM ‐ time trend comparison | Low risk | Adequate ‐ well‐defined |
| COEM ‐ comparability | Low risk | Adequate ‐ adjusted by birth cohort, level of ethylmercury |
| Summary Risk of Bias assessment | Low risk | |
gb‐Honda 2005.
| Study characteristics | ||
| Methods | Case‐only ecological method. This study examined cumulative incidence of ASD up to age 7 for children born from 1988 to 1996 in Kohoku Ward (population approximately 300,000), Yokohama, Japan. | |
| Participants | Birth cohorts from 1988 to 1994, and the redistricted Kohoku Ward, for birth cohorts from 1995 to 1996 | |
| Interventions | MMR vaccine exposure | |
| Outcomes | ASD incidence before and after termination of MMR vaccination programme in children (1993) | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | |
| COEM ‐ exposure | Low risk | |
| COEM ‐ time trend comparison | Low risk | |
| COEM ‐ comparability | Low risk | Stratified by birth cohort |
| Summary Risk of Bias assessment | Low risk | |
gb‐Jonville‐Bera 1996.
| Study characteristics | ||
| Methods | Ecological study to assess the association between MMR and the onset of thrombocytopenic purpura | |
| Participants | Data from the French passive survey between 1984 and 30 June 1992. The 60 cases with outcome (TP) were mainly toddlers. | |
| Interventions | Immunisation with MMR (N = 4,396,645), measles (N = 860,938), mumps (N = 172,535), rubella DTP and single rubella (N = 2,295,307), measles/rubella (N = 1,480,058) | |
| Outcomes | Cases of TP diagnosed at 1 of the 30 survey centres after. All cases within 45 days from vaccination. Over 8‐year period of immunisation | |
| Funding Source | Mixed (government and pharmaceutical industry) | |
| Notes | The denominator is determined by the number of doses distributed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Low risk | Adequate ‐ independent validation |
| COEM ‐ exposure | High risk | There was insufficient information. |
| COEM ‐ time trend comparison | Unclear risk | There was insufficient information. |
| COEM ‐ comparability | High risk | There was insufficient information. |
| Summary Risk of Bias assessment | High risk | |
gb‐Seagroatt 2005.
| Study characteristics | ||
| Methods | Case‐only ecological method. Study to determine if the introduction of MMR vaccine in 1988 increased rates in those populations that were offered the vaccine as infants. | |
| Participants | England population aged between 4 and 18 years between April 1991 and March 2003 (about 11.6 million) | |
| Interventions | Introduction of MMR vaccination (1988) | |
| Outcomes | Emergency hospitalisation for Crohn's disease. Age‐specific ranges were calculated such that rates in population with at least 84% coverage and those in population with coverage below 7% were compared. | |
| Funding Source | Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Unclear risk | There was insufficient information. |
| COEM ‐ exposure | Unclear risk | There was insufficient information. |
| COEM ‐ time trend comparison | Unclear risk | There was insufficient information. |
| COEM ‐ comparability | Unclear risk | There was insufficient information. |
gb‐Taylor 2002.
| Study characteristics | ||
| Methods | Case‐only ecological method ‐ linked to db‐Taylor 1999 | |
| Participants | Children with childhood (core autism N = 278) and atypical autism (N = 195) born between 1979 and 1998 from computerised health registers of children with disabilities in the community and from special school and child psychiatry records, using the same methods and classifications as in their earlier study (db‐Taylor 1999) | |
| Interventions | MMR vaccination (not described) | |
| Outcomes | Recorded bowel problems lasting at least 3 months, age of reported regression of the child's development where it was a feature, and relation of these to MMR vaccination | |
| Funding Source | Government | |
| Notes | Conclusions: these findings provide no support for an MMR‐associated “new variant” form of autism with developmental regression and bowel problems, and offer further evidence against involvement of MMR vaccine in the initiation of autism. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| COEM ‐ case selection | Unclear risk | There was insufficient information. |
| COEM ‐ exposure | Unclear risk | There was insufficient information. |
| COEM ‐ time trend comparison | Unclear risk | There was insufficient information. |
| COEM ‐ comparability | Unclear risk | There was insufficient information. |
ADEM: acute disseminated encephalomyelitis ADI‐R: Autism Diagnostic Interview‐Revised AEFI: adverse events following immunisation AIT: acute immune thrombocytopenia AM: aseptic meningitis ASHIPS: Associations of Statutory Health Insurance Physicians ASD: autism spectrum disorders AR: attack rates BCG: Bacillus Calmette‐Guérin CD: Crohn’s disease CI: confidence interval CIR: Citywide Immunization Registry CSF: cerebrospinal fluid CSTE: Council of State and Territorial Epidemiologist CT: computed tomography DIN: Doctors’ Independent Network DOHMH: Department of Health and Mental Hygiene DPHSS: Department of Public Health and Social Services DPPT: diphtheria, polio, pertussis, and tetanus vaccination DSM‐lV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DTP: diphtheria, tetanus, and pertussis EDs: emergency departments EEG: electroencephalograph EPI: Expanded Programmed Immunization FC: febrile convulsion FS: febrile seizures GP: general practice GPRD: General Practice Research Database Hib: Haemophilus influenzae type b HMO: health maintenance organisation HPV: human papillomavirus HSP: Henoch‐Schönlein purpura IBD: inflammatory bowel disease ICD: International Classification of Diseases ICD‐9‐CM: International Classification of Diseases‐Ninth Revision‐Clinical Modification ICD‐10‐CA: International Classification of Diseases, Tenth Revision, Canada ICD‐10‐GM: International Classification of Diseases. Tenth Revision, German Modification IgE: immunoglobulin E IgG: immunoglobulin G IgM: immunoglobulin M IIS: Immunisation Information Systems IM: intramuscular ITP: idiopathic thrombocytopenic purpura KPSC: Kaiser Permanente Southern California MenC: meningitis C MCOs: Managed Care Organizations MuCV: mumps‐containing vaccines MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine MMR+V: measles, mumps, rubella, plus varicella vaccine MR: measles and rubella vaccine MRI: magnetic resonance imaging MS: multiple sclerosis n: number of participants in intervention and control arm or number of cases NIP: National Immunization Program OPV: oral polio vaccine PCR: polymerase chain reaction PDD: pervasive developmental disorder PEP: postexposure prophylaxis RCT: randomised controlled study RCV: rubella‐containing vaccine RNA: ribonucleic acid RT‐PCR: reverse‐transcription polymerase chain reaction SAR: secondary attack rate sc: subcutaneous SCORAD: SCORing Atopic Dermatitis T1D: type 1 diabetes TBE: tick‐borne encephalitis TCID50: Tissue Culture Infectious Dose TP: thrombocytopenic purpura UC: ulcerative colitis V: varicella VE: vaccine effectiveness/efficacy VP: vaccination program VSD: Vaccine Safety Datalink WHO: World Health Organization wks: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akobeng 1999 | Commentary relating to an excluded study (Wakefield 1998) |
| Andre 1984 | No direct data on MMR, only observation that it may interfere with varicella vaccine |
| Anonymous 1982 | Non‐comparative |
| Anonymous 1997 | Review |
| Anonymous 1998 | No safety data |
| Anonymous 1999 | Review |
| Aozasa 1982 | Not MMR vaccine |
| Asaria 2008 | Review |
| Autret 1996 | Epidemiological survey comparing onset of idiopathic thrombocytopenic purpura following vaccination with MMR compared to M, M, and R |
| Bakker 2001 | Authors attribute school mumps outbreak to bad attenuated MMR vaccine lots; uncertain data about relationship between MMR exposure and symptom onset. |
| Balraj 1995 | Review on mumps vaccine |
| Bawankule 2017 | Vaccine type used not described. |
| Beck 1991 | Assessed safety of MMR vaccination in children allergic to eggs |
| Bedford 2010 | Editorial |
| Beeler 1996 | Case series. Reported data were insufficient to assess causal relationship. |
| Benjamin 1991 | Review |
| Berger 1988a | Serological data only |
| Berger 1988b | Serological data only |
| Berlin 1983 | Surveillance data |
| Bernsen 2008 | No review‐relevant outcomes reported. Study assessed association between MMR infection and atopic disorder. |
| Bhargava 1995 | Non‐comparative |
| Bonanni 2005 | Non‐comparative |
| Borchardt 2007 | Non‐comparative |
| Borgono 1973 | Non‐comparative |
| Boxall 2008 | Non‐comparative |
| Brockhoff 2010 | Non‐comparative |
| Brond 2017 | Monovalent varicella vaccine |
| Bruno 1997 | Compared 2 MMR types |
| Bulik 2018 | Review |
| Buntain 1976 | Case report |
| Buynak 1969 | Non‐comparative |
| Byberg 2017 | Monovalent varicella vaccine |
| Cao 2018 | Vaccine type used is unclear; probably monovalent varicella. |
| Cardenosa 2006 | Non‐comparative |
| Cashman 2018 | Letter |
| Chang 1982 | Serological data only |
| Chang 2017 | Serological data only |
| Chen 1991 | Participants aged over 15 years |
| Chen 2000 | Review |
| Cherian 2010 | Environmental factors associated to incidence of type 1 diabetes mellitus only reported. |
| Chiodo 1992 | Non‐comparative |
| Cinquetti 1994 | Compared 2 types of MMR |
| Contardi 1989 | Non‐comparative |
| Contardi 1992 | Non‐comparative |
| Coplan 2000 | Non‐comparative |
| Coronado 2006 | Case‐fatality rate study |
| Cox 2009 | Letter |
| Curtale 2010 | Non‐comparative |
| Czajka 2009 | Non‐comparative |
| D'Souza 2000 | Non‐comparative |
| Dales 2001 | Non‐comparative |
| Dallaire 2009 | Non‐comparative |
| Dankova 1995 | Serological study |
| Dashefsky 1990 | MMR not given independently. |
| Davis 1997 | MMR not given independently. |
| Dayan 2008a | Non‐comparative |
| Deforest 1986 | MMR given with DTP and OPV in different schedules. |
| Deforest 1988 | DTP/OPV +/‐ MMR versus placebo or without MMR |
| De Laval 2010 | Seroprevalence study |
| DeStefano 2000 | Duplicate data of db‐Taylor 1999] |
| Diaz‐Ortega 2010 | Non‐comparative |
| Dobrosavljevic 1999 | Case report |
| Dominguez 2008 | Surveillance study |
| Doshi 2009 | Effectiveness of measles‐containing vaccines was assessed, not MMR specifically. |
| Dos Santos 2002 | Non‐comparative |
| Duderstadt 2012 | Participants' ages (17 to 35 years) did not meet study inclusion criteria (6 months to 15 years). |
| Dyer 2010a | Commentary |
| Dyer 2010b | Commentary |
| Elphinstone 2000 | No data |
| Englund 1989 | MMR not given independently. |
| Fitzpatrick 2007 | Commentary |
| Fletcher 2001 | Commentary |
| Garrido Lestache 1992 | Non‐comparative |
| Geier 2004 | Uncertain MMR focus, mixed with thimerosal |
| Gerber 2009 | Review |
| Goodson 2010 | Monovalent measles vaccine |
| Griffin 1991 | Non‐comparative |
| Grilli 1992 | Comparison of different types of measles in MMR |
| Hasrina 2017 | Poster. No effectiveness or safety data |
| Hilton 2009 | Content analysis |
| Hindiyeh 2009 | No outcomes of interest. The study reported on serological data. |
| Höhle 2011 | Monovalent varicella vaccine only |
| Hooker 2014 | Retracted publication |
| Hornig 2008 | Participants affected by gastrointestinal disturbance. |
| Hu 2007 | Non‐comparative |
| Hua 2009 | Association with Kawasaki disease. Tested for vaccines other than MMR |
| Huang 1990 | Serological data only |
| Huang 2009 | Case‐control study. Study of risk factors for mumps; does not provide effectiveness or safety data for mumps vaccination |
| Ipp 2003 | Head‐to‐head study of 2 MMR types |
| Jiang 2009 | Non‐comparative |
| Jones 1991 | Non‐comparative |
| Just 1985 | Comparison of different types of MMR; CCT with serological outcomes |
| Just 1986 | Compared MMR +/‐ varicella vaccine |
| Just 1987a | Compared MMR +/‐ OPV |
| Just 1987b | Compared MMR +/‐ DTP |
| Kaaber 1990 | Compared MMR with or without other vaccine versus other vaccines (DTP and OPV) |
| Karim 2002 | Case report |
| Kaye 2001 | Non‐comparative |
| Kazarian 1978 | Case report |
| Khalil 2005 | Cross‐sectional study |
| Kiepiela 1991 | RCT investigating 2 types of measles vaccine |
| Kulkarni 2005 | Review |
| Kurtzke 1997 | Case‐control of exposure to anything/measles vaccine and multiple sclerosis |
| Kutty 2014 | Economic evaluation |
| Latasa 2019 | Insufficient information: epidemiological study of mumps incidence |
| Lee 1998 | Commentary |
| Lee 2007 | Non‐comparative |
| Lucena 2002 | No comparator |
| Maekawa 1991 | Non‐comparative |
| Maguire 1991 | Non‐comparative |
| Majwala 2018 | Measles vaccine type not specified |
| Mantadakis 2010 | Review |
| Marshall 2016 | Head‐to‐head study of 2 MMRVs |
| Matter 1995 | Non‐comparative |
| Matter 1997 | Serological data only |
| Meissner 2004 | Review |
| Miller 1983 | Non‐comparative; egg allergy |
| Miller 1993 | Non‐comparative |
| Min 1991 | Compared 2 MMR types |
| Minekawa 1974 | Non‐comparative |
| Mommers 2004 | MMR and all other childhood vaccines, indistinguishable comparison |
| Mupere 2006 | MMR vaccine not included. |
| Nalin 1999 | Serological data only |
| Narwaney 2017 | Non‐comparative |
| Nicoll 1998 | Commentary |
| Ntshoe 2013 | Vaccine type not reported. |
| O'Brien 1998 | Letter |
| O'Connor 2019 | Insufficient information to assess vaccine efficacy; there were no unvaccinated children in the group |
| Ong 2006 | Review |
| Patja 2000 | Non‐comparative |
| Patja 2001 | Non‐comparative |
| Pekmezovic 2004 | MMR not included. |
| Peltola 1998 | Non‐comparative case series |
| Peltola 2007 | Review |
| Petridou 1997 | Case‐control investigation that included all 153 incident cases of leukaemia ascertained throughout the country during 1993 and 1994, and 2 hospital controls for every case matched for gender, age, and place of residence. Data on MMR vaccination are presented as "total viral vaccination shots" (measles, mumps, rubella, hepatitis B vaccines; each antigen counted as a distinct shot). |
| Puvvada 1993 | Non‐comparative case series |
| Rajantie 2007 | Non‐comparative. Unclear study design |
| Roost 2004 | Cross‐sectional study |
| Sabra 1998 | Commentary |
| Saraswathy 2009 | Serological data only |
| Scarpa 1990 | Non‐comparative |
| Schaffzin 2007 | Differences between 2 subpopulations in the study were not taken into account. Partially outside age parameters for this review. Effectiveness was calculated cumulatively for campers (N = 368, age 7 to 15 years, mean 12 years, 366/368 previously immunised with 2 doses of mumps‐containing vaccine, only 2/368 with 1 dose) and staff members (N = 139, age 14 to 65 years, mean 21 years, of whom 74, 44, and 21 received respectively 2, 1, and no doses of a mumps‐containing vaccine). |
| Schettini 1989 | Serological data only |
| Schettini 1990 | Non‐comparative |
| Schmid 2008 | Non‐comparative |
| Schultz 2008 | Assessed a possible relationship between paracetamol and autism. Data were obtained via a parent survey; methods and results are questionable. |
| Schwarz 2010 | No treatment: measles + MMR vaccine |
| Schwarzer 1998 | Compared 2 types of MMR |
| Seagroatt 2003 | Measles vaccine type was unclear. |
| Shah 2017 | Serological data only |
| Shah 2018 | Insufficient information to detect efficacy of the third dose of the MMR vaccine |
| Sharma 2004 | Non‐comparative |
| Shinefield 2002 | MMR not given independently. |
| So 2008 | Korean language, abstract only in English |
| Spitzer 2001 | Commentary |
| Stetler 1985 | DTP vaccine |
| Stokes 1967 | Serological data only |
| Stratton 1994 | Review |
| Sugiura 1982 | Serological data only |
| Svanström 2010 | Non‐comparative |
| Tosun 2017 | Monovalent measles vaccine |
| Ueda 1995 | Compared 2 types of MMR |
| Vesikari 1979 | The study is written in Finnish, and reports on few epidemiological data not suitable for the objective of this review. |
| Vesikari 1984 | Compared 2 types of MMR |
| Wakefield 1998 | Retracted publication |
| Wakefield 1999a | Non‐comparative |
| Wakefield 1999b | No data |
| Wakefield 2000 | Non‐comparative |
| Walker 2011 | Non‐comparative |
| Willocks 2017 | Non‐comparative |
| Wilson 2003 | Systematic review |
| Wilson 2011 | Hospitalisation without specific definition made this endpoint too generic, therefore the study did not provide useful information on vaccine effectiveness or safety. |
| Woyciechowska 1985 | Not MMR |
| Yamashiro 1998 | Paricipants' ages did not meet review inclusion criteria. |
| Yu 2007 | Non‐comparative |
CCT: controlled clinical trial DTP: diphtheria, pertussis, and tetanus vaccine MMR: measles, mumps, rubella vaccine MMRV: measles, mumps, rubella, and varicella vaccine OPV: trivalent oral poliovirus vaccine RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Cardemil 2017.
| Methods | Cohort study ‐ effectiveness of the mumps (MMR) third dose of MMR |
| Participants | Of 20,496 university students who were enrolled during the 2015 to 2016 academic year |
| Interventions | MMR vaccination. 98.1% of the students had received at least 2 doses of MMR vaccine. During the outbreak, 4783 received a third dose. |
| Outcomes | Mumps |
| Notes | Vaccination at 13 years before second doses ‐ age off‐target |
Cohen 2007.
| Methods | Screening method |
| Participants | Children (N = 312) with confirmed mumps in England |
| Interventions | Immunisation with MMR vaccine |
| Outcomes | Effectiveness against mumps diseases |
| Notes | Screening method design (effectiveness is estimated considering the proportion of vaccinated amongst cases and in the general population) |
Deeks 2011.
| Methods | Screening method ‐ to assess vaccine effectiveness of 1 and 2 doses of the MMR vaccine during an outbreak of mumps in Ontario |
| Participants | The outbreak period was defined as 1 September 2009 to 10 June 2010. Vaccination data on cases occurring during this period were provided by all Ontario health units with confirmed cases of mumps. The 6 health units with the highest incidence of mumps supplied data on vaccine coverage by birth cohort from the Ontario Immunization Record Information System database. Coverage was assessed as of 30 April 2009, as this followed a provincial mumps vaccine catch‐up campaign that targeted students at post‐secondary institutions. |
| Interventions | MMR vaccination |
| Outcomes | Effectiveness against mumps |
| Notes | Results: a total of 134 confirmed cases of mumps were identified. Information on receipt of MMR vaccine was available for 114 (85.1%) cases, of whom 63 (55.3%) reported having received only 1 dose of vaccine; 32 (28.1%) reported having received 2 doses. Vaccine effectiveness of 1 dose of the MMR vaccine ranged from 49.2% to 81.6%, whereas vaccine effectiveness of 2 doses ranged from 66.3% to 88.0%. If we assume vaccine effectiveness of 85% for 2 doses of the vaccine, vaccine coverage of 88.2% and 98.0% would be needed to interrupt community transmission of mumps if the corresponding reproductive values were 4 and 6. Interpretation: the trial authors' estimates of vaccine effectiveness of 1 and 2 doses of mumps‐containing vaccine were consistent with the estimates that have been reported in other outbreaks. Outbreaks occurring in Ontario and elsewhere serve as a warning against complacency over vaccination programmes. |
Dominguez 2010.
| Methods | Screening method |
| Participants | Children and adults (N = 381) measles cases |
| Interventions | Immunisation with MMR vaccine |
| Outcomes | Effectiveness against measles diseases |
| Notes | Screening method (effectiveness is estimated considering the proportion of vaccinated amongst cases and in the general population) |
Fantinato 2018.
| Methods | Case‐control ‐ Brazil ‐ anaphylaxis related to MMR vaccine produced by manufacturer A and to assess associated risk factors |
| Participants | From 14 July 2014 to 12 January 2015, in children from 1 year to less than 5 years of age, vaccinated with MMR and reported with anaphylaxis; controls were without anaphylaxis. Cases n = 15, controls n = 60 |
| Interventions | MMR vaccination manufacturer A |
| Outcomes | Anaphylaxis |
| Notes | The bivariate analysis of anaphylaxis and cow’s milk protein allergy showed OR 51.62, with P < 0.001 and 95% CI 5.59 to 476.11. The variables family food allergy, breastfeeding, previous postvaccine adverse event, and simultaneous vaccination were not statistically significant. |
Fiebelkorn 2013.
| Methods | Cohort study ‐ postexposure prophylaxis |
| Participants | 49 households with 239 eligible participants (44 received PEP; 195 did not receive PEP) |
| Interventions | MMR not described |
| Outcomes | Mumps case |
| Notes | Discussion: although the attack rate amongst people who received a third dose of MMR vaccine as PEP was 0%, compared with a 5.2% attack rate for those with 2 doses of MMR who did not receive PEP, the difference was not statistically significant. Nonetheless, MMR vaccine administered as PEP might offer some benefits. Note: quite confused report, main data were not reported in a clear way |
Freitas 2013.
| Methods | Case–control study ‐ Brazil ‐ hypersensitivity‐type adverse events and MMR vaccination |
| Participants | Case‐patients were defined as 1‐ to 4‐year‐old children with suspected HAEs following vaccination with MMR A during the 2004 national campaign and reported to the national AEFI surveillance system by clinicians. Postvaccination HAEs were defined as the acute onset of exanthema, urticaria, or facial or peripheral oedema within 24 h after MMR vaccination during the August 2004 national campaign. For each case, 1 or more asymptomatic children from the same age group vaccinated during the same campaign and residing in nearest‐neighbour households were enrolled as controls.
Parents of both case‐patient children with HAEs and their controls were interviewed, from 2 weeks to 2 months after the HAE, using a standardised questionnaire to collect: basic demographic data, medical history of children (including prior vaccinations; history of known allergy to foods (including gelatin, eggs) and antibiotics); history of recurrent respiratory problems (including asthma), and specifics about symptoms observed after receiving MMR vaccination, as well as the type (if any) of medical care received following vaccination. Case‐patient children n = 49; controls n = 185 |
| Interventions | MMR vaccine (manufacturer A B C) MMR_A contains Dextran 70 (Sigma–Aldrich; St Louis, Missouri, USA) |
| Outcomes | Hypersensitivity‐type adverse events |
| Notes | Discussion: study highlights the importance of a well‐functioning routine AEFI surveillance system linked with mass vaccination campaigns. Such a system in Brazil permitted timely detection of HAEs and validation of a safety signal associated with 1 vaccine manufacturer. Unlike earlier publications, this outbreak linked to a single manufacturer of MMR showed no association with a prior allergic history to eggs or other foods, including gelatin; subsequent studies implicate the dextran stabiliser in MMR from manufacturer A as the likely cause of HAEs. Note: although cases of hypersensitivity after MMR A vaccine occurred in 7 states, the authors only included suspected cases reported in 2 states (Paraná and Santa Catarina) in this case–control study for logistical reasons. Furthermore, the authors investigated only cases reported to the AEFI surveillance system; they did not conduct active surveillance for other cases that might not have been reported. The description of signs and symptoms was based on the recollections of parents or adults who observed children during the episodes, and were not verified by health professionals. Finally, the last interviews were conducted 2 months after the vaccination campaign began. |
Marin 2008.
| Methods | Screening methods |
| Participants | Student population from 2 colleges in Iowa, USA (N = 2363) |
| Interventions | Immunisation with MMR vaccine |
| Outcomes | Mumps cases following an outbreak |
| Notes | Screening method (effectiveness is estimated considering the proportion of vaccinated amongst cases and in the general population) |
Orlikova 2016.
| Methods | Retrospective cohort ‐ case only |
| Participants | All participants analysed in this study had mumps. Data by age groups were provided. 0 to 14 years old |
| Interventions | MMR |
| Outcomes | Clinical complications, and hospital admissions in unvaccinated but also in vaccinated individuals |
| Notes | Conclusions: this study demonstrates a significant preventive effect of 2‐dose vaccination against mumps complications (orchitis, meningitis, or encephalitis) and hospitalisations for mumps. The risk of complications increases with time interval from vaccination. The most affected age groups were teenagers and young adults. |
Prescott 2018.
| Methods | Unclear study design (cohort retrospective) |
| Participants | 1469 patients was extracted from the UK paediatric registry. The vaccination group included those vaccinated in the 6 weeks prior to the onset of immune thrombocytopenia. Their data, including demographics, vaccine type, platelet counts, and treatments, were then analysed using appropriate statistical methods. |
| Interventions | MMR not described |
| Outcomes | Immune thrombocytopenia |
| Notes | Insufficient information |
Sheppeard 2009.
| Methods | Screening method |
| Participants | Notified measles cases in children from New South Wales, Australia during 2006 (N = 56) |
| Interventions | MMR immunisation |
| Outcomes | Effectiveness against measles |
| Notes | Screening method design (effectiveness is estimated considering the proportion of vaccinated amongst cases and in the general population) |
Sorup 2019.
| Methods | Cohort study |
| Participants | 295,559 children born in Denmark from April 2004 to December 2010. The cohort were followed from age 47 months (1 month before turning age 4 years, which is the recommended age of the second MMR (MMR‐2)) until age 60 months. |
| Interventions | MMR vaccination second dose |
| Outcomes | Antibiotic prescriptions and hospital admissions for any off‐targeted infection |
| Notes | Conclusion: in this study, revaccination with MMR appeared safe with regard to off‐target infections and was associated with a lower rate of severe off‐target infections. More studies of the possible association between revaccination with live attenuated vaccines and off‐target infections are needed. |
AEFI: adverse events following immunisation CI: confidence interval HAEs: hypersensitivity‐type adverse events MMR: measles, mumps, rubella vaccine OR: odds ratio PEP: postexposure prophylaxis
Differences between protocol and review
A new vaccine against varicella (MMRV and MMR+V) vaccine has been added for this 2019 update.
Contributions of authors
Carlo Di Pietrantonj (CDP) designed this update. Alessandro Rivetti (AR) performed the searches. CDP, AR, and Maria Grazia Debalini (MGD) applied the inclusion criteria. CDP and AR performed quality assessment of the studies. CDP extracted data and performed quantitative analysis. Pasquale Marchione (PM) wrote the Background section. Vittorio Demicheli (VD) arbitrated on both study inclusion and extraction. All authors contributed to the final draft.
Sources of support
Internal sources
Istituto Superiore di Sanità, Italy
ASL Alessandria, Italy
External sources
European Union Programme for Improved Vaccine Safety Surveillance. EU Contract Number 1999/C64/14, Other
-
NIHR Incentive Scheme 128383, UK
This project is funded by the National Institute for Health Research (NIHR) via the ‘NIHR Cochrane Incentive Award Scheme 2018 ‐ 128383'. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
Carlo Di Pietrantonj: none known Alessandro Rivetti: none known Pasquale Marchione: none known Maria Grazia Debalini: none known Vittorio Demicheli: none known
Edited (no change to conclusions)
References
References to studies included in this review
aa‐Henry 2018 {published data only}
- Henry O, Brzostek J, Czajka H, Leviniene G, Reshetko O, Gasparini R, et al. One or two doses of live varicella virus-containing vaccines: efficacy, persistence of immune responses, and safety six years after administration in healthy children during their second year of life. Vaccine 2018;36(3):381-7. [DOI] [PubMed] [Google Scholar]
aa‐Povey 2019 {published data only}
- Povey M, Henry O, Riise Bergsaker MA, Chlibek R, Esposito S, Flodmark CE, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine or one dose of monovalent varicella vaccine: 10-year follow-up of a phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infectious Diseases 2019;19(3):287-97. [DOI] [PubMed] [Google Scholar]
aa‐Prymula 2014 {published data only}
- Prymula R, Bergsaker MR, Esposito S, Gothefors L, Man S, Snegova N, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind, randomised, controlled trial. Lancet 2014;383(9925):1313-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
ab‐Bloom 1975 {published data only}
- Bloom JL, Schiff GM, Graubarth H, Lipp RW Jr, Jackson JE, Osborn RL, et al. Evaluation of a trivalent measles, mumps, rubella vaccine in children. Journal of Pediatrics 1975;87(1):85-7. [DOI] [PubMed] [Google Scholar]
ab‐Ceyhan 2001 {published data only}
- Ceyhan M, Kanra G, Erdem G, Kanra B. Immunogenicity and efficacy of one dose measles-mumps-rubella (MMR) vaccine at twelve months of age as compared to monovalent measles vaccination at nine months followed by MMR revaccination at fifteen months of age. Vaccine 2001;19(31):4473-8. [DOI] [PubMed] [Google Scholar]
ab‐Edees 1991 {published data only}
- Edees S, Pullan CR, Hull D. A randomised single blind trial of a combined mumps measles rubella vaccine to evaluate serological response and reactions in the UK population. Public Health 1991;105(2):91-7. [DOI] [PubMed] [Google Scholar]
ab‐Freeman 1993 {published data only}
- Freeman TR, Stewart MA, Turner L. Illness after measles-mumps-rubella vaccination. Canadian Medical Association Journal 1993;149(11):1669-74. [PMC free article] [PubMed] [Google Scholar]
ab‐Lerman 1981 {published data only}
- Lerman SJ, Bollinger M, Brunken JM. Clinical and serologic evaluation of measles, mumps, and rubella (HPV-77:DE-5 and RA 27/3) virus vaccines, singly and in combination. Pediatrics 1981;68(1):18-22. [PubMed] [Google Scholar]
ab‐Peltola 1986 {published data only}
- Peltola H, Heinonen OP. Frequency of true adverse reactions to measles-mumps-rubella vaccine. A double-blind placebo-controlled trial in twins. Lancet 1986;1(8487):939-42. [DOI] [PubMed] [Google Scholar]
ab‐Schwarz 1975 {published data only}
- Ehrenkranz NJ, Ventura AK, Medler EM, Jackson JE, Kenny MT. Clinical evaluation of a new measles-mumps-rubella combined live virus vaccine in the Dominican Republic. Bulletin of the World Health Organization 1975;52(1):81-5. [PMC free article] [PubMed] [Google Scholar]
- Ramos-Alvarez M, Bessudo L, Kenny MT, Jackson JE, Schwarz AJ. Simultaneous administration at different dosages of attenuated live virus vaccines against measles, mumps and rubella [Administracion simultanea en diferentes dosificaciones de las vacunas de virus vivos atenuados contra el sarampion, parotiditis y rubeola]. Boletín Médico del Hospital Infantil de México 1976;33(4):875-86. [PubMed] [Google Scholar]
- Schwarz AJ, Jackson JE, Ehrenkranz NJ, Ventura A, Schiff GM, Walters VW. Clinical evaluation of a new measles-mumps-rubella trivalent vaccine. American Journal of Diseases of Children 1975;129(12):1408-12. [DOI] [PubMed] [Google Scholar]
- Walters VW, Miller SA, Jackson JE, Kenny MT. A field with a liver measles-mumps-rubella vaccine. Clinical Pediatrics 1975;14(10):928-33. [DOI] [PubMed] [Google Scholar]
ba‐Andrade 2018 {published data only}
- Andrade AL, da Silva Vieira MA, Minamisava R, Toscano CM, Lima Souza MB, Fiaccadori F, et al. Single-dose varicella vaccine effectiveness in Brazil: a case-control study. Vaccine 2018;36(4):479-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
ba‐Castilla 2009 {published data only}
- Castilla J, Fernandez Alonso M, Garcia Cenoz M, Martinez Artola V, Inigo Pestana M, Rodrigo I, et al. Resurgence of mumps in the vaccine era. Factors involved in an outbreak in Navarre, Spain, 2006-2007 [Rebrote de parotiditis en la era vacunal. Factores implicados en un brote en Navarra, 2006-2007]. Medicina Clínica 2009;133(20):777-82. [DOI] [PubMed] [Google Scholar]
- Castilla J, Garcia Cenoz M, Arriazu M, Fernandez-Alonso M, Martinez-Artola V, Etxeberria J, et al. Effectiveness of Jeryl Lynn-containing vaccine in Spanish children. Vaccine 2009;27(15):2089-93. [DOI] [PubMed] [Google Scholar]
ba‐Cenoz 2013 {published data only}
- Cenoz MG, Martinez-Artola V, Guevara M, Ezpeleta C, Barricarte A, Castilla J. Effectiveness of one and two doses of varicella vaccine in preventing laboratory-confirmed cases in children in Navarre, Spain. Human Vaccines and Immunotherapeutics 2013;9(5):1172-6. [DOI: 10.4161/hv.23451] [DOI] [PMC free article] [PubMed] [Google Scholar]
ba‐Defay 2013 {published data only}
- Defay F, De Serres G, Skowronski DM, Boulianne N, Ouakki M, Landry M, et al. Measles in children vaccinated with 2 doses of MMR. Pediatrics 2013;132(5):e1126-33. [DOI: 10.1542/peds.2012-3975] [DOI] [PubMed] [Google Scholar]
ba‐Fu 2013 {published data only}
- Fu C, Xu J, Cai Y, He Q, Zhang C, Chen J, et al. Effectiveness of one dose of mumps vaccine against clinically diagnoses mumps in Guangzhou, China, 2006-2012. Human Vaccines and Immunotherapeutics 2013;9(12):2524-8. [DOI: 10.4161/hv.26113] [DOI] [PMC free article] [PubMed] [Google Scholar]
ba‐Giovanetti 2002 {published data only}
- Giovanetti F, Laudani E, Marinaro L, Dogliani MG, Giachelli V, Giachino G, et al. Evaluation of the effectiveness of mumps immunization during an outbreak [Valutazione dell'efficacia della vaccinazione contro la parotite durante un'epidemia]. Igiene Moderna 2002;117(3):201-9. [Google Scholar]
ba‐Goncalves 1998 {published data only}
- Goncalves G, De Araujo A, Monteiro Cardoso ML. Outbreak of mumps associated with poor vaccine efficacy - Oporto Portugal 1996. Euro Surveillance 1998;3(12):119-21. [DOI] [PubMed] [Google Scholar]
ba‐Harling 2005 {published data only}
- Harling R, White JM, Ramsay ME, Macsween KF, den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine 2005;23(31):4070-4. [DOI] [PubMed] [Google Scholar]
ba‐Hungerford 2014 {published data only}
- Hungerford D, Clearly P, Ghebrehewet S, Keenan A, Vivancos R. Risk factors for transmission of measles during an outbreak: matched case control study. Journal of Hospital Infections 2014;86(2):138-43. [DOI: 10.1016/j.jhin.2013.11.008] [DOI] [PubMed] [Google Scholar]
ba‐Jick 2010 {published data only}
- Jick H, Hagberg KW. Measles in the United Kingdom 1990-2008 and the effectiveness of measles vaccines. Vaccine 2010;28(29):4588-92. [DOI] [PubMed] [Google Scholar]
ba‐Kim 2012 {published data only}
- Kim HM, Cho HK, Choi KM, Eun BWY, Kim KH. Efficacy of mumps in Korea. International Journal of Infectious Diseases 2012;16s:e306. [DOI: 10.1016/j.ijid.2012.05.997] [DOI] [Google Scholar]
ba‐Liese 2013 {published data only}
- Liese JG, Cohen C, Rack A, Pirzer K, Eber S, Blum M, et al. The effectiveness of varicella vaccination in children in Germany. Pediatric Infectious Disease Journal 2013;32(9):998-1004. [DOI: 10.1097/INF.0b013e31829ac263] [DOI] [PubMed] [Google Scholar]
ba‐Mackenzie 2006 {published data only}
- Mackenzie DG, Craig G, Hallam NF, Moore J, Stevenson J. Mumps in a boarding school: description of an outbreak and control measures. British Journal of General Practice 2006;56(528):526-9. [PMC free article] [PubMed] [Google Scholar]
ba‐Vazquez 2001 {published data only}
- Vazquez M, La Russa PS, Gershon AA, Stainberg SP, Freudigman K, Shapiro E. The effectiveness of the varicella vaccine in clinical practice. New England Journal of Medicine 2001;344(13):955-60. [DOI: 10.1056/NEJM200103293441302] [DOI] [PubMed] [Google Scholar]
bb‐Ahlgren 2009 {published data only}
- Ahlgren C, Toren K, Oden A, Andersen O. A population-based case-control study on viral infections and vaccinations and subsequent multiple sclerosis risk. European Journal of Epidemiology 2009;24(9):541-52. [DOI] [PubMed] [Google Scholar]
bb‐Baron 2005 {published data only}
- Baron S, Turck D, Leplat C, Merle V, Gower-Rousseau C, Marti R, et al. Enviromental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut 2005;54(3):357-63. [DOI: 10.1136/gut.2004.054353] [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Bertuola 2010 {published data only}
- Bertuola F, Morando C, Menniti-Ippolito F, Da Cas R, Capuano A, Perilongo G, et al. Association between drug and vaccine use and acute immune thrombocytopenia in childhood: a case-control study in Italy. Drug Safety 2010;33(1):65-72. [DOI] [PubMed] [Google Scholar]
- Menniti-Ippolito F, Da Cas R, Bolli M, Capuano A, Saglioca L, Traversa G, et al. A multicenter study on drug safety in children [Studo multicentrico sulla sicurezza dei faraci in pediatria]. Quaderni ACP 2007;14(3):98-102. [Google Scholar]
bb‐Black 1997 {published data only}
- Black S, Shinefield H, Ray P, Lewis E, Chen R, Glasser J, et al. Risk of hospitalization because of aseptic meningitis after measles-mumps-rubella vaccination in one- to two-year-old children: an analysis of the Vaccine Safety Datalink (VSD) Project. Pediatric Infectious Disease Journal 1997;16(5):500-3. [DOI] [PubMed] [Google Scholar]
bb‐Black 2003 {published data only}
- Black C, Kaye JA, Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. British Journal of Clinical Pharmacology 2003;55(1):107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Bremner 2005 {published data only}
- Bremner SA, Carey IM, DeWilde S, Richards N, Maier WC, Hilton SR, et al. Timing of routine immunisations and subsequent hay fever risk. Archives of Disease in Childhood 2005;90(6):567-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Bremner 2007 {published data only}
- Bremner SA, Carey IM, De Wilde S, Richards N, Maier WC, Hilton SR, et al. Vaccinations, infections and antibacterials in the first grass pollen season of life and risk of later hayfever. Clinical and Experimental Allergy 2007;37(4):512-7. [DOI] [PubMed] [Google Scholar]
bb‐Chen 2018 {published data only}
- Chen Y, Ma F, Xu Y, Chu X, Zhang J. Vaccines and the risk of acute disseminated encephalomyelitis. Vaccine 2018;36(26):3733-9. [DOI] [PubMed] [Google Scholar]
bb‐Da Dalt 2016 {published data only}
- Da Dalt L, Zerbinati C, Strafella MS, Renna S, Riceputi L, Di Pietro P, et al Italian Multicenter Study Group for Drug and Vaccine Safety in Children. Henoch-Schönlein purpura and drug and vaccine use in childhood: a case-control study. Italian Journal of Pediatrics 2016;42(1):60. [DOI: 10.1186/s13052-016-0267-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Davis 2001 {published data only}
- Davis RL, Kramarz P, Bohlke K, Benson P, Thompson RS, Mullooly J, et al. Measles-mumps-rubella and other measles-containing vaccines do not increase the risk for inflammatory bowel disease: a case-control study from the Vaccine Safety Datalink project. Archives of Pediatric and Adolescent Medicine 2001;155(3):354-9. [DOI] [PubMed] [Google Scholar]
bb‐De Stefano 2004 {published data only}
- De Stefano F, Bhasin TK, Thompson WW, Yeargin-Allsopp M, Boyle C. Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan Atlanta. Pediatrics 2004;113(2):259-66. [DOI] [PubMed] [Google Scholar]
bb‐Dockerty 1999 {published data only}
- Dockerty JD, Skegg DC, Elwood JM, Herbison GP, Becroft DM, Lewis ME. Infections, vaccinations, and the risk of childhood leukaemia. British Journal of Cancer 1999;80(9):1483-9. [DOI: 10.1038/sj.bjc.6690548] [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Groves 1999 {published data only}
- Groves FD, Gridley G, Wacholder S, Shu XO, Robison LL, Neglia JP, et al. Infant vaccinations and risk of childhood acute lymphoblastic leukaemia in the USA. British Journal of Cancer 1999;81(1):175-8. [DOI: 10.1038/sj.bjc.6690668] [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Ma 2005 {published data only}
- Ma X, Does MB, Metayer C, Russo C, Wong A, Buffler PA. Vaccination history and risk of childhood leukaemia. International Journal of Epidemiology 2005;34(5):1100-9. [DOI] [PubMed] [Google Scholar]
bb‐Mallol‐Mesnard 2007 {published data only}
- Mallol-Mesnard N, Menegaux F, Auvrignon A, Auclerc MF, Bertrand Y, Nelken B, et al. Vaccination and the risk of childhood acute leukaemia: the ESCALE study (SFCE). International Journal of Epidemiology 2007;36(1):110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
bb‐Mrozek‐Budzyn 2010 {published data only}
- Mrozek-Budzyn D, Kieltyka A, Majewska R. Lack of association between measles-mumps-rubella vaccination and autism in children: a case-control study. Pediatric Infectious Disease Journal 2010;29(5):397-400. [DOI] [PubMed] [Google Scholar]
bb‐Ray 2006 {published data only}
- Ray P, Hayward J, Michelson D, Lewis E, Schwalbe J, Black S, et al. Encephalopathy after whole-cell pertussis or measles vaccination: lack of evidence for a causal association in a retrospective case-control study. Pediatric Infectious Disease Journal 2006;25(9):768-73. [DOI] [PubMed] [Google Scholar]
bb‐Shaw 2015 {published data only}
- Shaw SY, Blanchard JF, Bernstein CN. Early childhood measles vaccinations are not associated with paediatric IBD: a population based analysis. Journal of Crohn's and Colitis 2015;9(4):334-8. [DOI: 10.1093/ecco-jcc/jjv029] [DOI] [PubMed] [Google Scholar]
bb‐Smeeth 2004 {published data only}
- Smeeth L, Cook C, Fombonne E, Heavey L, Rodrigues LC, Smith PG, et al. MMR vaccination and pervasive developmental disorders: a case-control study. Lancet 2004;364(9438):963-9. [DOI] [PubMed] [Google Scholar]
bb‐Uno 2012 {published data only}
- Uno Y, Uchiyama T, Kurosawa M, Aleksic B, Ozaki N. Early exposure to the combined measles-mumps-rubella vaccine and thimerosal-containing vaccines and risk of autism spectrum disorder. Vaccine 2015;33(21):2511-6. [DOI] [PubMed] [Google Scholar]
- Uno Y, Uchiyama T, Kurosawa M, Aleksic B, Ozaki N. The combined measles, mumps, and rubella vaccines and the total number of vaccines are not associated with development of autism spectrum disorder: the first case-control study in Asia. Vaccine 2012;30(28):4292-8. [DOI: 10.1016/j.vaccine.2012.01.093] [DOI] [PubMed] [Google Scholar]
bb‐Vcev 2015 {published data only}
- Vcev A, Pezerovic D, Jovanovic Z, Nakic D, Vcev I, Majnaric L. A retrospective, case-control study on traditional environmental risk factors in inflammatory bowel disease in Vukovar-Srijem County, north-eastern Croatia, 2010. Wiener Klinische Wochenschrift 2015;127(9-10):345–54. [DOI] [PubMed] [Google Scholar]
ca‐Arciuolo 2017 {published data only}
- Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting - New York City, 2013. Clinical Infectious Diseases 2017;65(11):1843-7. [DOI] [PubMed] [Google Scholar]
ca‐Arenz 2005 {published data only}
- Arenz S, Schmitt HJ, Tischer A, Kries R. Effectiveness of measles vaccination after household exposure during a measles outbreak: a household contact study in Coburg, Bavaria. Pediatric Infectious Disease Journal 2005;24(8):697-9. [DOI] [PubMed] [Google Scholar]
ca‐Barrabeig 2011a {published data only}
- Barrabeig I, Rovira A, Rius C, Munoz P, Soldevila N, Batalla J, et al. Effectiveness of measles vaccination for control of exposed children. Pediatric Infectious Disease Journal 2011;30(1):78-80. [DOI] [PubMed] [Google Scholar]
ca‐Barrabeig 2011b {published data only}
- Barrabeig I, Rovira A, Munoz P, Batalla J, Ruis C, Sanchez JA, et al. MMR vaccine effectiveness in an outbreak that involved day-care and primary schools. Vaccine 2011;29(45):8024-31. [DOI: 10.1016/j.vaccine.2011.08.056] [DOI] [PubMed] [Google Scholar]
ca‐Bhuniya 2013 {published data only}
- Bhuniya S, Maji D, Mandal D, Mondal N. Measles outbreak among Dukpa tribe of Buxa Hills in West Bengal, India: epidemiology and vaccine efficacy. Indian Journal of Public Health 2013;57(4):272-5. [DOI: 10.4103/0019-557X.123273] [DOI] [PubMed] [Google Scholar]
ca‐Chamot 1998 {published data only}
- Chamot E, Toscani L, Egger P, Germann D, Bourquin C. Estimation of the efficacy of three strains of mumps vaccines during an epidemic of mumps in the Geneva canton (Switzerland). Revue d'Epidemiologie et de Sante Publique 1998;46(2):100-7. [PubMed] [Google Scholar]
ca‐Chang 2015 {published data only}
- Chang C, Mo X, Hu P, Liang W, Ma H, An Z, et al. Effectiveness of rubella vaccine in a rubella outbreak in Guangzhou city, China, 2014. Vaccine 2015;33(28):3223-7. [DOI: 10.1016/j.vaccine.2015.04.083] [DOI] [PubMed] [Google Scholar]
ca‐Choe 2017 {published data only}
- Choe YJ, Park YJ, Kim JW, Eom HE, Park O, Oh MD, et al. An outbreak of measles in a university in Korea, 2014. Journal of Korean Medical Science 2017;32(11):1876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Compés‐Dea 2014 {published data only}
- Compés-Dea C, Guimbao-Bescòs J, Gaspar-Escayola JI, Lazaro-Belanche MA, Aznar-Brieba A. An outbreak of mumps in a high school: estimation of vaccine effectiveness. Zaragoza 2011 [Broteescolar de parotiditis: estimacion de la efectividad vacunal Zaragoza 2011]. Enfermedades Infeccionas y Microbiologia Clinical 2015;33(6):385-90. [DOI: 10.1016/j.eimc.2014.09.011] [DOI] [PubMed] [Google Scholar]
ca‐Giaquinto 2018 {published data only}
- Giaquinto C, Gabutti G, Baldo V, Villa M, Tramontan L, Raccanello N, et al. Impact of a vaccination programme in children vaccinated with ProQuad, and ProQuad-specific effectiveness against varicella in the Veneto region of Italy. BMC Infectious Diseases 2018;18(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Greenland 2012 {published data only}
- Greenland K, Whelan J, Fanoy E, Borgert M, Hulshof K, Yap KB, et al. Mumps outbreak among vaccinated university students associated with a large party, the Netherlands 2010. Vaccine 2012;30(31):4676-80. [DOI: 10.1016/j.vaccine.2012.04083] [DOI] [PubMed] [Google Scholar]
ca‐Hales 2016 {published data only}
- Hales CM, Johnson E, Helgenberger L, Papania MJ, Larzelere M, Gopalani SV, et al. Measles outbreak associated with low vaccine effectiveness among adults in Pohnpei State, Federated States of Micronesia 2014. Open Forum Infectious Diseases 2016;3(2):ofw064. [DOI: 10.1093/ofid/ofw064] [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐La Torre 2017 {published data only}
- La Torre G, Saulle R, Unim B, Meggiolaro A, Barbato A, Mannocci A, et al. The effectiveness of measles-mumps-rubella (MMR) vaccination in the prevention of pediatric hospitalizations for targeted and untargeted infections: a retrospective cohort study. Human Vaccination and Immunotherapy 2017;13(8):1879-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Livingston 2013 {published data only}
- Livingston KA, Rosen JB, Zucker JR, Zimmerman CM. Mumps vaccine effectiveness and risk factors for disease in household during an outbreak in New York City. Vaccine 2013;32(3):369-74. [DOI: 10.1016/j.vaccine.2013.11.021] [DOI] [PubMed] [Google Scholar]
ca‐Lopez Hernandez 2000 {published data only}
- Lopez Hernandez B, Martin Velez RM, Roman Garcia C, Penalver Sanchez I, Lopez Rosique JA. An epidemic outbreak of mumps. A study of vaccinal efficacy [Brote epidemico de parotiditis. Estudio de la efectividad vacunal]. Atencion Primaria/Sociedad Española de Medicina de Familia y Comunitaria 2000;25(3):148-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Ma 2018 {published data only}
- Ma C, Liu Y, Tang J, Jia H, Qin W, Su Y, et al. Assessment of mumps-containing vaccine effectiveness during an outbreak: importance to introduce the 2-dose schedule for China. Human Vaccines and Immunotherapy 2018;14(6):1392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Marin 2006 {published data only}
- Marin M, Nguyen HQ, Langidrik JR, Edwards R, Briand K, Papania MJ, et al. Measles transmission and vaccine effectiveness during a large outbreak on a densely populated island: implications for vaccination policy. Clinical infectious Diseases 2006;42(3):315-9. [DOI] [PubMed] [Google Scholar]
ca‐Marolla 1998 {published data only}
- Marolla F, Baviera G, [No Value] Cacciapuoti, Calia V, Cannavavo R, Clemente A, et al. A field study on vaccine efficacy against mumps of three MMR vaccines [Efficacia verso la parotite di tre diversi vaccini a tripla componente: studio sul campo]. Rivista Italiana Di Pediatria 1998;24(3):466-72. [Google Scholar]
ca‐Musa 2018 {published data only}
- Musa S, Topalović B, Ćatić S, Smajlagić Z. Assessment of vaccine effectiveness during measles outbreak in the Federation of Bosnia and Herzegovina, 2014-2015. Central European Journal of Public Health 2018;26(2):79-82. [DOI] [PubMed] [Google Scholar]
ca‐Nelson 2013 {published data only}
- Nelson GE, Aguon A, Valencia E, Oliva R, Guerrero ML, Reyes R, et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles-mumps-rubella vaccine for outbreak control - Guam 2009 to 2010. Pediatric Infectious Disease Journal 2013;32(4):374-80. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Ogbuanu 2012 {published data only}
- Ogbuanu IU, Kutty PK, Hudson JM, Blog D, Abedi GR, Goodell S, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics 2012;130(6):e1567-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
ca‐Ong 2005 {published data only}
- Ong G, Goh KT, Ma S, Chew SK. Comparative efficacy of Rubini, Jeryl-Lynn and Urabe mumps vaccine in an Asian population. Journal of Infections 2005;51(4):294-8. [DOI] [PubMed] [Google Scholar]
ca‐Ong 2007 {published data only}
- Ong G, Rasidah N, Wan S, Cutter J. Outbreak of measles in primary school students with high first dose MMR vaccination coverage. Singapore Medical Journal 2007;48(7):656-61. [PubMed] [Google Scholar]
ca‐Rieck 2017 {published data only}
- Rieck T, Feig M, An der Heiden M, Siedler A, Wichmann O. Assessing varicella vaccine effectiveness and its influencing factors using health insurance claims data, Germany, 2006 to 2015. Euro Surveillance 2017;22(17):30521. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Schlegel 1999 {published data only}
- Schlegel M, Osterwalder JJ, Galeazzi RL, Vernazza PL. Comparative efficacy of three mumps vaccines during disease outbreak in Eastern Switzerland: cohort study. BMJ 1999;319(7206):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Snijders 2012 {published data only}
- Snijders BEP, Lier A, de Kassteele J, Fanoy EB, Ruijs WLM, Hulsof F, et al. Mumps vaccine effectiveness in primary schools and household, the Netherlands, 2008. Vaccine 2012;30(19):2999-3002. [DOI] [PubMed] [Google Scholar]
ca‐Spackova 2010 {published data only}
- Spackova M, Wiese-Posselt M, Dehnert M, Matysiak-Klose D, Heininger U, Siedler A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine 2010;28(3):686-91. [DOI] [PubMed] [Google Scholar]
ca‐Tafuri 2013 {published data only}
- Tafuri S, Martinelli D, Prato R, Germinario C. Vaccine effectiveness evaluation during a varicella outbreak among children of primary schools and day-care centers in region which adopted UMV. Human Vaccines and Immunotherapeutics 2013;9(1):184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Takla 2014 {published data only}
- Takla A, Bohmer MM, Klinc C, Kurz N, Schaffer A, Stich H, et al. Outberak-related mumps vaccine effectiveness among a cohort of children and of young adults in Germany 2011. Human Vaccines and Immunotherapeutics 2014;10(1):140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
ca‐Wichmann 2007 {published data only}
- Wichmann O, Hellenbrand W, Sagebiel D, Santibanez S, Ahlemeyer G, Vogt G, et al. Large measles outbreak at a German public school, 2006. Pediatric Infectious Disease Journal 2007;26(9):782-6. [DOI] [PubMed] [Google Scholar]
ca‐Woudenberg 2017 {published data only}
- Woudenberg T, Maas NAT, Knol MJ, Melker H, Binnendijk RS, Hahne SJM. Effectiveness of early measles, mumps, and rubella vaccination among 6-14-month-old infants during an epidemic in the Netherlands: an observational cohort study. Journal of Infectious Diseases 2017;215(8):1181-7. [DOI] [PubMed] [Google Scholar]
cb‐Ahlgren 2009 {published data only}
- Ahlgren C, Oden A, Toren K, Andersen O. Multiple sclerosis incidence in the era of measles-mumps-rubella mass vaccinations. Acta Neurologica Scandinavica 2009;119(5):313-20. [DOI] [PubMed] [Google Scholar]
cb‐Barlow 2001 {published data only}
- Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. New England Journal of Medicine 2001;345(9):656-61. [DOI] [PubMed] [Google Scholar]
cb‐Beck 1989 {published data only}
- Beck M, Welsz-Malecek R, Mesko-Prejac M, Radman V, Juzbasic M, Rajninger-Miholic M, et al. Mumps vaccine L-Zagreb, prepared in chick fibroblasts. I. Production and field trials. Journal of Biological Standards 1989;17(1):85-90. [DOI] [PubMed] [Google Scholar]
cb‐Benjamin 1992 {published data only}
- Benjamin CM, Chew GC, Silman AJ. Joint and limb symptoms in children after immunisation with measles, mumps, and rubella vaccine. BMJ 1992;304(6834):1075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
cb‐Benke 2004 {published data only}
- Benke G, Abramson M, Raven J, Thien FC, Walters EH. Asthma and vaccination history in a young adult cohort. Australian and New Zealand Journal of Public Health 2004;28(4):336-8. [DOI] [PubMed] [Google Scholar]
cb‐Beyerlein 2017 {published data only}
- Beyerlein A, Strobl AN, Winkler C, Carpus M, Knopff A, Donnachie E, et al. Vaccinations in early life are not associated with development of islet autoimmunity in type 1 diabetes high-risk children: results from prospective cohort data. Vaccine 2017;35(14):1735-41. [DOI] [PubMed] [Google Scholar]
cb‐DeStefano 2002 {published data only}
- DeStefano F, Gu D, Kramarz P, Truman BI, Iademarco MF, Mullooly JP, et al. Childhood vaccinations and risk of asthma. Pediatric Infectious Disease Journal 2002;21(6):498-504. [DOI] [PubMed] [Google Scholar]
cb‐Dunlop 1989 {published data only}
- Dunlop JM, Rai Choudhury K, Roberts JS, Bryett KA. An evaluation of measles, mumps and rubella vaccine in a population of Yorkshire infants. Public Health 1989;103(5):331-5. [DOI] [PubMed] [Google Scholar]
cb‐Gavrielov‐Yusim 2014 {published data only}
- Gavrielov-Yusim N, Hoshen M, Singer SR, Neumann L, Balicer RD. The weight of MMRV-related febrile convulsions among other clinical factors contributing to febrile convulsions in children. Vaccine 2014;32(39):4954-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
cb‐Hviid 2004 {published data only}
- Hiivid AD, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination does not increase the incidence of type 1 diabetes. Evidence-based Healthcare and Public Health 2004;8(5):286-7. [CRSREF: 2955568] [Google Scholar]
- Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and type 1 diabetes. New England Journal of Medicine 2004;350(14):1398-404. [DOI] [PubMed] [Google Scholar]
cb‐Hviid 2008 {published data only}
- Hviid A, Melbye M. Measles-mumps-rubella vaccination and asthma-like disease in early childhood. American Journal of Epidemiology 2008;168(11):1277-83. [DOI] [PubMed] [Google Scholar]
cb‐Hviid 2019 {published data only}
- Hviid A, Hansen JV, Frisch M, Melbye M. Measles, mumps, rubella vaccination and autism: a nationwide cohort study. Annals of Internal Medicine 2019;170(8):513-20. [DOI] [PubMed] [Google Scholar]
cb‐Jacobsen 2009 {published data only}
- Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine 2009;27(34):4656-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
cb‐Jain 2015 {published data only}
- Jain A, Marshall J, Buikema A, Bancroft T, Kelly JP, Newschaffer CJ. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA 2015;313(15):1534-40. [DOI] [PubMed] [Google Scholar]
cb‐Klein 2010 {published data only}
- Klein NP. Measles-mumps-rubella-varicella combination vaccine increases risk of febrile seizure. Pediatrics 2011;126(1):170. [DOI] [PubMed] [Google Scholar]
cb‐Klein 2012 {published data only}
- Klein NP, Lewis E, Baxter R, Weintraub E, Glanz J, Naleway A, et al. Measles-containing vaccines and febrile seizures in children age 4 to 6 years. Pediatrics 2012;129(5):809-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
cb‐Klein 2017 {published data only}
- Klein NP, Lewis E, McDonald J, Fireman B, Naleway A, Glanz J, et al. Risk factors and familial clustering for fever 7-10 days after the first dose of measles vaccines. Vaccine 2017;35(12):1615-21. [DOI] [PubMed] [Google Scholar]
cb‐Madsen 2002 {published data only}
- Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, Thorsen P, et al. A population-based study of measles, mumps, and rubella vaccination and autism. New England Journal of Medicine 2002;347(19):1477-82. [DOI] [PubMed] [Google Scholar]
- Noble KK, Miyasaka K. Measles, mumps, and rubella vaccination and autism. New England Journal of Medicine 2003;348(10):951-4; author reply 951-4. [PubMed] [Google Scholar]
cb‐Makino 1990 {published data only}
- Makino S, Sasaki K, Nakayama T, Oka S, Urano T, Kimura M, et al. A new combined trivalent live measles (AIK-C strain), mumps (Hoshino strain), and rubella (Takahashi strain) vaccine. Findings in clinical and laboratory studies. American Journal of Diseases in Children 1990;144(8):905-10. [DOI] [PubMed] [Google Scholar]
cb‐McKeever 2004 {published data only}
- McKeever TM, Lewis SA, Smith C, Hubbard R. Vaccination and allergic disease: a birth cohort study. American Journal of Public Health 2004;94(6):985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
cb‐Miller 1989 {published data only}
- Miller C, Miller E, Rowe K, Bowie C, Judd M, Walker D. Surveillance of symptoms following MMR vaccine in children. Practitioner 1989;233(1461):69-73. [PubMed] [Google Scholar]
cb‐Mrozek‐Budzyn 2013 {published data only}
- Mrozek-Budzyn D, Kiełtyka A, Majewska R, Augustyniak M. Measles, mumps and rubella (MMR) vaccination has no effect on cognitive development in children - the results of the Polish prospective cohort study. Vaccine 2013;31(22):2551-7. [DOI: 10.1016/j.vaccine.2013.03.057] [DOI] [PMC free article] [PubMed] [Google Scholar]
cb‐Robertson 1988 {published data only}
- Robertson CM, Bennett VJ, Jefferson N, Mayon-White RT. Serological evaluation of a measles, mumps, and rubella vaccine. Archives of Disease in Childhood 1988;63(6):612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
cb‐Rowhani‐Rahbar 2013 {published data only}
- Rowhani-Rahbar A, Fireman B, Lewis E, Nordin J, Naleway A, Jacobsen SJ, et al. Effect of age on the risk of fever and seizures following immunization with measles-containing vaccines in children. JAMA Pediatrics 2013;167(12):1111-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
cb‐Schink 2014 {published data only}
- Gvozdenovic E, Vetter V, Willame C, Rosillon D. Impact of history of febrile convulsions on the risk difference of febrile convulsions with the tetravalent measles-mumps-rubella-varicella vaccine: post-hoc exploratory analysis of results from a matched-cohort study. Vaccine 2018;36(39):5803-6. [DOI] [PubMed] [Google Scholar]
- Schink T, Holstiege J, Kowalzik F, Zepp F, Garbe E. Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination. Vaccine 2014;32(6):645-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
cb‐Sharma 2010 {published data only}
- Sharma HJ, Oun SA, Bakr SS, Kapre SV, Jadhav SS, Dhere RM, et al. No demonstrable association between the Leningrad-Zagreb mumps vaccine strain and aseptic meningitis in a large clinical trial in Egypt. Clinical Microbiology and Infection 2010;16(4):347-52. [DOI] [PubMed] [Google Scholar]
cb‐Stokes 1971 {published data only}
- Stokes JJ, Weibel RE, Villarejos VM, Arguedas JA, Buynak EB, Hilleman MR. Trivalent combined measles-mumps-rubella vaccine. Findings in clinical-laboratory studies. JAMA 1971;218(1):57-61. [PubMed] [Google Scholar]
cb‐Swartz 1974 {published data only}
- Swartz TA, Klingberg W, Klingberg MA. Combined trivalent and bivalent measles, mumps and rubella virus vaccination. A controlled trial. Infection 1974;2(3):115-7. [DOI] [PubMed] [Google Scholar]
cb‐Timmermann 2015 {published data only}
- Timmermann CA, Osuna CE, Steuerwald U, Weihe P, Poulsen LK, Grandjean P. Asthma and allergy in children with and without prior measles, mumps, and rubella vaccination. Pediatric Allergy and Immunology 2015;26(8):742-9. [DOI: 10.1111/pai.12391] [DOI] [PMC free article] [PubMed] [Google Scholar]
cb‐Uchiyama 2007 {published data only}
- Uchiyama T, Kurosawa M, Inaba Y. MMR-vaccine and regression in autism spectrum disorders: negative results presented from Japan. Journal of Autism and Developmental Disorders 2007;37(2):210-7. [DOI] [PubMed] [Google Scholar]
cb‐Vestergaard 2004 {published data only}
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA 2004;292(3):351-7. [DOI] [PubMed] [Google Scholar]
cb‐Weibel 1980 {published data only}
- Weibel RE, Carlson AJ Jr, Villarejos VM, Buynak EB, McLean AA, Hilleman MR. Clinical and laboratory studies of combined live measles, mumps, and rubella vaccines using the RA 27/3 rubella virus. Proceedings of the Society for Experimental Biology and Medicine 1980;165(2):323-6. [DOI] [PubMed] [Google Scholar]
db‐Andrews 2012 {published data only}
- Andrews N, Stoweb J, Miller E, Svanström H, Johansen K, Bonhoeffer J, et al, VAESCO consortium. A collaborative approach to investigating the risk of thrombocytopenic purpura after measles–mumps–rubella vaccination in England and Denmark. Vaccine 2012;30:3042-6. [DOI: 10.1016/j.vaccine.2011.06.009] [DOI] [PubMed] [Google Scholar]
db‐Dourado 2000 {published data only}
- Dourado I, Cunha S, Teixeira MG, Farrington CP, Melo A, Lucena R, et al. Outbreak of aseptic meningitis associated with mass vaccination with a Urabe-containing measles-mumps-rubella vaccine: implications for immunization programs. American Journal of Epidemiology 2000;151(5):524-30. [DOI] [PubMed] [Google Scholar]
db‐Farrington 1995 {published data only}
- Farrington CP, Miller E, Taylor B. MMR and autism: further evidence against a causal association. Vaccine 2001;19(27):3632-5. [DOI] [PubMed] [Google Scholar]
- Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. American Journal of Epidemiology 1996;143(11):1165-73. [DOI] [PubMed] [Google Scholar]
- Farrington P, Pugh S, Colville A, Flower A, Nash J, Morgan-Capner P, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet 1995;345(8949):567-9. [DOI] [PubMed] [Google Scholar]
- Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Archives of Disease in Childhood 2001;84(3):227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. MMR vaccine: review of benefits and risks. Journal of Infection 2002;44(1):1-6. [DOI] [PubMed] [Google Scholar]
db‐France 2008 {published data only}
- France EK, Glanz J, Xu S, Hambidge S, Yamasaki K, Black SB, et al. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics 2008;121(3):e687-92. [DOI] [PubMed] [Google Scholar]
db‐Macartney 2017 {published data only}
- Macartney K, Gidding HF, Trinh L, Wang H, Dey A, Hull B, et al. Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatrics 2017;171(10):992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
db‐MacDonald 2014 {published data only}
- MacDonald SE, Dover DC, Simmonds KA, Svenson LW. Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: a population-based cohort study. CMAJ: Canadian Medical Association Journal 2014;186(11):824-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
db‐Makela 2002 {published data only}
- Makela A, Nuorti JP, Peltola H. Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics 2002;110(5):957-63. [DOI] [PubMed] [Google Scholar]
db‐McClure 2019 {published data only}
- McClure DL, Jacobsen SJ, Klein NP, Naleway AL, Kharbanda EO, Glanz JM, et al. Similar relative risks of seizures following measles containing vaccination in children born preterm compared to full-term without previous seizures or seizure-related disorders. Vaccine 2019;37(1):76-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
db‐Miller 2003 {published data only}
- Miller E, Andrews N, Waight P, Taylor B. Bacterial infections, immune overload, and MMR vaccine. Archives of Disease in Childhood 2003;88:222-3. [DOI: 10.1136/adc.88.3.222] [DOI] [PMC free article] [PubMed] [Google Scholar]
db‐Miller 2005 {published data only}
- Miller E, Andrews N, Grant A, Stowe J, Taylor B. No evidence of an association between MMR vaccine and gait disturbance. Archives of Disease in Childhood 2005;90(3):292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
db‐Miller 2007 {published data only}
- Miller E, Andrews N, Stowe J, Grant A, Waight P, Taylor B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. American Journal of Epidemiology 2007;165(6):704-9. [DOI] [PubMed] [Google Scholar]
db‐O'Leary 2012 {published and unpublished data}
- O’Leary ST, Glanz JM, McClure DL, Akhtar A, Daley MF, Nakasato C, et al. The risk of immune thrombocytopenic purpura after vaccination in children and adolescents. Pediatrics 2012;129:248-55. [DOI: 10.1542/peds.2011-1111] [DOI] [PubMed] [Google Scholar]
db‐Perez‐Vilar 2018 {published data only}
- Perez-Vilar S, Weibel D, Sturkenboom M, Black S, Maure C, Castro JL, et al. Enhancing global vaccine pharmacovigilance: proof-of-concept study on aseptic meningitis and immune thrombocytopenic purpura following measles-mumps containing vaccination. Vaccine 2018;36(3):347-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
db‐Stowe 2009 {published data only}
- Stowe J, Andrews N, Taylor B, Miller E. No evidence of an increase of bacterial and viral infections following measles, mumps and rubella vaccine. Vaccine 2009;27(9):1422-5. [DOI] [PubMed] [Google Scholar]
db‐Taylor 1999 {published data only}
- Taylor B, Miller E, Farrington CP, Petropoulos MC, Favot-Mayaud I, Li J, et al. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet 1999;353(9169):2026-9. [DOI] [PubMed] [Google Scholar]
db‐Ward 2007 {published data only}
- Ward KN, Bryant NJ, Andrews NJ, Bowley JS, Ohrling A, Verity CM, et al. Risk of serious neurologic disease after immunization of young children in Britain and Ireland. Pediatrics 2007;120(2):314-21. [DOI] [PubMed] [Google Scholar]
eb‐Ki 2003 {published data only}
- Ki M, Park T, Yi SG, Oh JK, Choi BY. Risk analysis of aseptic meningitis after measles-mumps-rubella vaccination in Korean children by using a case crossover design. American Journal of Epidemiology 2003;157(2):158-65. [DOI: 10.1093/aje/kwf167] [DOI] [PubMed] [Google Scholar]
eb‐Lafaurie 2018 {published data only}
- Lafaurie M, Baricault B, Lapeyre-Mestre M, Sailler L, Moulis G, Sommet A, et al. Risk of vaccine-induced immune thrombocytopenia in children. Nationwide case cross-over and self controlled case series studies in France. Fundamental & Clinical Pharmacology 2018;32:86. [Google Scholar]
eb‐Park 2004 {published data only}
- Park T, Ki M, Yi SG. Statistical analysis of MMR vaccine adverse events on aseptic meningitis using the case cross-over design. Statistics in Medicine 2004;23(12):1871-83. [DOI] [PubMed] [Google Scholar]
ga‐Boccalini 2015 {published data only}
- Boccalini S, Bonanni P, Bechini A. Preparing to introduce the varicella vaccine into the Italian immunisation programme: varicella-related hospitalisations in Tuscany, 2004–2012. Euro Surveillance 2015;21:1-7. [DOI: 10.2807/1560-7917.ES.2016.21.24.30257] [DOI] [PubMed] [Google Scholar]
ga‐Pozza 2011 {published data only}
- Pozza F, Piovesan C, Russo F, Bella A, Pezzotti P, Emberti Gialloreti L. Impact of universal vaccination on the epidemiology of varicella in Veneto, Italy. Vaccine 2011;29(51):9480-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
ga‐Tafuri 2015 {published data only}
- Tafuri S, Fortunato F, Cappelli MG, Cozza V, Bechini A, Bonanni P, et al. Effectiveness of vaccination against varicella in children under 5 years in Puglia, Italy 2006-2012. Human Vaccines and Immunotherapy 2015;11(1):214-9. [DOI: 10.4161/hv.36153] [DOI] [PMC free article] [PubMed] [Google Scholar]
gb‐da Cunha 2002 {published data only}
- da Cunha SS, Rodrigues LC, Barreto ML, Dourado I. Outbreak of aseptic meningitis and mumps after mass vaccination with MMR vaccine using the Leningrad-Zagreb mumps strain. Vaccine 2002;20(7-8):1106-12. [DOI] [PubMed] [Google Scholar]
gb‐da Silveira 2002 {published data only}
- da Silveira CM, Kmetzsch CI, Mohrdieck R, Sperb AF, Prevots DR. The risk of aseptic meningitis associated with the Leningrad-Zagreb mumps vaccine strain following mass vaccination with measles-mumps-rubella vaccine, Rio Grande do Sul, Brazil, 1997. International Journal of Epidemiology 2002;31(5):978-82. [DOI] [PubMed] [Google Scholar]
gb‐Fombonne 2001 {published data only}
- Fombonne E, Chakrabarti S. No evidence for a new variant of measles-mumps-rubella-induced autism. Pediatrics 2001;108(4):E58. [DOI] [PubMed] [Google Scholar]
gb‐Fombonne 2006 {published data only}
- Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics 2006;118(1):e139-50. [DOI] [PubMed] [Google Scholar]
gb‐Honda 2005 {published data only}
- Honda H, Shimizu Y, Rutter M. No effect of MMR withdrawal on the incidence of autism: a total population study. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2005;46(6):572-9. [DOI] [PubMed] [Google Scholar]
gb‐Jonville‐Bera 1996 {published data only}
- Jonville-Bera AP, Autret E, Galy-Eyraud C, Hessel L. Thrombocytopenic purpura after measles, mumps and rubella vaccination: a retrospective survey by the French Regional Pharmacovigilance Centres and Pasteur-Mérieux Sérums et Vaccins. Pediatric Infectious Disease Journal 1996;15(1):44-8. [DOI] [PubMed] [Google Scholar]
gb‐Seagroatt 2005 {published data only}
- Seagroatt V. MMR vaccine and Crohn's disease: ecological study of hospital admissions in England, 1991 to 2002. BMJ 2005;330(7500):1120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
gb‐Taylor 2002 {published data only}
- Taylor B, Miller E, Lingam R, Andrews N, Simmons A, Stowe J. Measles. mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: population study. BMJ 2002;321(7334):393-6. [DOI: 10.1136/bmj.324.7334.393] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akobeng 1999 {published data only}
- Akobeng AK, Thomas AG. Inflammatory bowel disease, autism, and the measles, mumps, and rubella vaccine. Journal of Pediatric Gastroenterology and Nutrition 1999;28(3):351-2. [DOI] [PubMed] [Google Scholar]
Andre 1984 {published data only}
- Andre FE. Summary of clinical studies with the Oka live varicella vaccine produced by Smith Kline-RIT. Biken Journal 1984;27(2-3):89-98. [PubMed] [Google Scholar]
Anonymous 1982 {published data only}
- Anonymous. Adverse effects of Virivac. Lakartidningen 1982;79(42):3822. [PubMed] [Google Scholar]
Anonymous 1997 {published data only}
- Anonymous. Vaccination: news on precautions, contraindications, and adverse reactions. Consultant 1997;37(3):756-60. [Google Scholar]
Anonymous 1998 {published data only}
- Anonymous. Field evaluation of the clinical effectiveness of vaccines against pertussis, measles, rubella and mumps. The Benevento and Compobasso Pediatrician's Network for the Control of Vaccine-Preventable Diseases. Vaccine 1998;16(8):818-22. [DOI] [PubMed] [Google Scholar]
Anonymous 1999 {published data only}
- Anonymous. Incidence of measles vaccine-associated adverse events is low. Drugs & Therapy Perspectives 1999;14(11):13-6. [Google Scholar]
Aozasa 1982 {published data only}
- Aozasa K, Nara H, Kotoh K, Watanabe Y, Sakai S, Honda M. Malignant histiocytosis with slow clinical course. Pathology, Research and Practice 1982;174(1-2):147-58. [DOI] [PubMed] [Google Scholar]
Asaria 2008 {published data only}
- Asaria P, MacMahon E. Measles in the United Kingdom: can we eradicate it by 2010? BMJ 2006;333(7574):890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Autret 1996 {published data only}
- Autret E, Jonville-Bera AP, Galy-Eyraud C, Hessel L. Thrombocytopenic purpura after isolated or combined vaccination against measles, mumps and rubella [Purpura thrombopenique apres vaccination isolee ou associee contre la rougeole, la rubeole et les oreillons]. Therapie 1996;51(6):677-80. [PubMed] [Google Scholar]
Bakker 2001 {published data only}
- Bakker W, Mathias R. Mumps caused by an inadequately attenuated measles, mumps and rubella vaccine. Canadian Journal of Infectious Diseases 2001;12(3):144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balraj 1995 {published data only}
- Balraj V, Miller E. Complications of mumps vaccines. Reviews in Medical Virology 1995;5(4):219-27. [Google Scholar]
Bawankule 2017 {published data only}
- Bawankule R, Singh A, Kumar K, Shetye S. Does measles vaccination reduce the risk of acute respiratory infection (ARI) and diarrhea in children: a multi-country study? PLoS ONE 2017;12(1):e0169713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1991 {published data only}
- Beck SA, Williams LW, Shirrell MA, Burks AW. Egg hypersensitivity and measles-mumps-rubella vaccine administration. Pediatrics 1991;88(5):913-7. [PubMed] [Google Scholar]
Bedford 2010 {published data only}
- Bedford HE, Elliman DA. MMR vaccine and autism. BMJ 2010;340:c655. [DOI] [PubMed] [Google Scholar]
Beeler 1996 {published data only}
- Beeler J, Varricchio F, Wise R. Thrombocytopenia after immunization with measles vaccines: review of the vaccine adverse events reporting system (1990 to 1994). Pediatric Infectious Disease Journal 1996;15(1):88-90. [DOI] [PubMed] [Google Scholar]
Benjamin 1991 {published data only}
- Benjamin CM, Silman AJ. Adverse reactions and mumps, measles and rubella vaccine. Journal of Public Health Medicine 1991;13(1):32-4. [DOI] [PubMed] [Google Scholar]
Berger 1988a {published data only}
- Berger R, Just M, Gluck R. Interference between strains in live virus vaccines. I: Combined vaccination with measles, mumps and rubella vaccine. Journal of Biological Standardization 1988;16(4):269-73. [DOI] [PubMed] [Google Scholar]
Berger 1988b {published data only}
- Berger R, Just M. Interference between strains in live virus vaccines. II: Combined vaccination with varicella and measles-mumps-rubella vaccine. Journal of Biological Standardization 1988;16(4):275-9. [DOI] [PubMed] [Google Scholar]
Berlin 1983 {published data only}
- Berlin BS. Convulsions after measles immunisation. Lancet 1983;1(8338):1380. [DOI] [PubMed] [Google Scholar]
Bernsen 2008 {published data only}
- Bernsen RM, Wouden JC. Measles, mumps and rubella infections and atopic disorders in MMR-unvaccinated and MMR-vaccinated children. Pediatric Allergy and Immunology 2008;19(6):544-51. [DOI] [PubMed] [Google Scholar]
Bhargava 1995 {published data only}
- Bhargava I, Chhaparwal BC, Phadke MA, Irani SF, Chhaparwal D, Dhorje S, et al. Immunogenicity and reactogenicity of indigenously produced MMR vaccine. Indian Pediatrics 1995;32(9):983-8. [PubMed] [Google Scholar]
Bonanni 2005 {published data only}
- Bonanni P, Bechini A, Pesavento G, Boccalini S, Tiscione E, Graziani G, et al. Implementation of the plan for elimination of measles and congenital rubella infection in Tuscany: evidence of progress towards phase II of measles control. Journal of Preventive Medicine and Hygiene 2005;46(3):111-7. [Google Scholar]
Borchardt 2007 {published data only}
- Borchardt SM, Rao P, Dworkin MS. Is the severity of mumps related to the number of doses of mumps-containing vaccine? Clinical Infectious Diseases 2007;45(7):939-40. [DOI] [PubMed] [Google Scholar]
Borgono 1973 {published data only}
- Borgono JM, Greiber R, Solari G, Concha F, Carrillo B, Hilleman MR. A field trial of combined measles-mumps-rubella vaccine. Satisfactory immunization with 188 children in Chile. Clinical Pediatrics 1973;12(3):170-2. [DOI] [PubMed] [Google Scholar]
Boxall 2008 {published data only}
- Boxall N, Kubinyiova M, Prikazsky V, Benes C, Castkova J. An increase in the number of mumps cases in the Czech Republic, 2005-2006. Euro Surveillance 2008;13(16):18842. [PubMed] [Google Scholar]
Brockhoff 2010 {published data only}
- Brockhoff HJ, Mollema L, Sonder GJ, Postema CA, Binnendijk RS, Kohl RH, et al. Mumps outbreak in a highly vaccinated student population, The Netherlands, 2004. Vaccine 2010;28(17):2932-6. [DOI] [PubMed] [Google Scholar]
Brond 2017 {published data only}
- Brond M, Martins C, Byberg S, Benn C, Whittle H, Garly M, et al. Randomized trial of 2 versus 1 dose of measles vaccine: effect on hospital admission of children after 9 months of age. Journal of the Pediatric Infectious Diseases Society 2017;7(3):226-33. [DOI] [PubMed] [Google Scholar]
Bruno 1997 {published data only}
- Bruno G, Grandolfo M, Lucenti P, Novello F, Ridolfi B, Businco L. Measles vaccine in egg allergic children: poor immunogenicity of the Edmoston-Zagreb strain. Pediatric Allergy and Immunology 1997;8(1):17-20. [DOI] [PubMed] [Google Scholar]
Bulik 2018 {published data only}
- Bulik NB, Bucsa C, Farcas A, Oniga O. Hexavalent vaccine in Europe: safety data from the randomized clinical trials. Farmacia 2018;66(5):747-57. [Google Scholar]
Buntain 1976 {published data only}
- Buntain WL, Missall SR. Letter: Local subcutaneous atrophy following measles, mumps, and rubella vaccination. American Journal of Diseases of Children 1976;130(3):335. [DOI] [PubMed] [Google Scholar]
Buynak 1969 {published data only}
- Buynak EB, Weibel RE, Whitman JE Jr, Stokes J Jr, Hilleman MR. Combined live measles, mumps, and rubella virus vaccines. JAMA 1969;207(12):2259-62. [PubMed] [Google Scholar]
Byberg 2017 {published data only}
- Byberg S, Thysen SM, Rodrigues A, Martins C, Cabral C, Careme M, et al. A general measles vaccination campaign in urban Guinea-Bissau: comparing child mortality among participants and non-participants. Vaccine 2017;35(1):33-9. [DOI] [PubMed] [Google Scholar]
Cao 2018 {published data only}
- Cao Z, Chen D, Yang Y, Zhang D. Effectiveness of post-exposure prophylaxis during varicella outbreaks among primary and middle school students in Shanghai: an analysis of three-year surveillance data. Vaccine 2018;36(38):5754-9. [DOI] [PubMed] [Google Scholar]
Cardenosa 2006 {published data only}
- Cardenosa N, Dominguez A, Camps N, Martinez A, Torner N, Navas E, et al. Non-preventable mumps outbreaks in schoolchildren in Catalonia. Scandinavian Journal of Infectious Diseases 2006;38(8):671-4. [DOI] [PubMed] [Google Scholar]
Cashman 2018 {published data only}
Chang 1982 {published data only}
- Chang HH. Immunisation problems in measles, rubella and mumps. Journal of the Korean Medical Association 1982;25(9):801-6. [Google Scholar]
Chang 2017 {published data only}
- Chang C, Ma H, Liang W, Hu P, Mo X, An Z, et al. Rubella outbreak and outbreak management in a school setting, China, 2014. Human Vaccines and Immunotherapeutics 2017;13(4):772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1991 {published data only}
- Chen RT, Moses JM, Markowitz LE, Orenstein WA. Adverse events following measles-mumps-rubella and measles vaccinations in college students. Vaccine 1991;9(5):297-9. [DOI] [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen RT, Mootrey G, DeStefano F. Safety of routine childhood vaccinations. An epidemiological review. Paediatric Drugs 2000;2(4):273-90. [DOI] [PubMed] [Google Scholar]
Cherian 2010 {published data only}
- Cherian MP, Al-Kanani KA, Al Qahtani SS, Yesurathinam H, Mathew AA, Thomas VS, et al. The rising incidence of type 1 diabetes mellitus and the role of environmental factors - three decade experience in a primary care health center in Saudi Arabia. Journal of Pediatric Endocrinology and Metabolism 2010;23(7):685-95. [DOI] [PubMed] [Google Scholar]
Chiodo 1992 {published data only}
- Chiodo F. Effectiveness and security of the trivalent vaccine against measles, parotitis and rubella (MPR). Igiene Moderna 1992;97(Suppl 1):77-86. [Google Scholar]
Cinquetti 1994 {published data only}
- Cinquetti S, Tonetto L, Portello A, Chermaz E, Sernagiotto F, De Noni R, et al. Adverse reactions following vaccination with two different types of measles mumps-rubella vaccine [Reazioni indesiderate a due diverse preparazioni di vaccine 'triplo' antimorbillo-parotite-rosolia]. Igiene Moderna 1994;101(6):793-800. [Google Scholar]
Contardi 1989 {published data only}
- Contardi I. Clinical and immunologic valuation of a new triple measles, mumps and rubella vaccine. Giornale di Malattie Infettive e Parassitarie 1989;41(11):1106-7. [Google Scholar]
Contardi 1992 {published data only}
- Contardi I, Lusardi C, Cattaneo GG. A comparative study of 3 different types of trivalent measles-mumps-rubella vaccine. Pediatria Medica e Chirurgica 1992;14(4):421-4. [PubMed] [Google Scholar]
Coplan 2000 {published data only}
- Coplan P, Chiacchierini L, Nikas A, Shea J, Baumritter A, Beutner K, et al. Development and evaluation of a standardized questionnaire for identifying adverse events in vaccine clinical trials. Pharmacoepidemiology and Drug Safety 2000;9(6):457-71. [DOI] [PubMed] [Google Scholar]
Coronado 2006 {published data only}
- Coronado F, Musa N, El Tayeb ESA, Haithami S, Dabbagh A, Mahoney F, et al. Retrospective measles outbreak investigation: Sudan, 2004. Journal of Tropical Pediatrics 2006;52(5):329-34. [DOI] [PubMed] [Google Scholar]
Cox 2009 {published data only}
- Cox AR, McDowell S. A response to the article on the association between paracetamol/acetaminophen use and autism by Stephen T. Schultz. Autism: the International Journal of Research and Practice 2009;13(1):123-4; author reply 124-5. [DOI] [PubMed] [Google Scholar]
Curtale 2010 {published data only}
- Curtale F, Perrelli F, Mantovani J, Atti MC, Filia A, Nicoletti L, et al. Description of two measles outbreaks in the Lazio Region, Italy (2006-2007). Importance of pockets of low vaccine coverage in sustaining the infection. BMC Infectious Diseases 2010;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czajka 2009 {published data only}
- Czajka H, Schuster V, Zepp F, Esposito S, Douha M, Willems P. A combined measles, mumps, rubella and varicella vaccine (Priorix-Tetra): immunogenicity and safety profile. Vaccine 2009;27(47):6504-11. [DOI] [PubMed] [Google Scholar]
D'Souza 2000 {published data only}
- D'Souza RM, Campbell-Lloyd S, Isaacs D, Gold M, Burgess M, Turnbull F, et al. Adverse events following immunisation associated with the 1998 Australian Measles Control Campaign. Communicable Diseases Intelligence 2000;24(2):27-33. [DOI] [PubMed] [Google Scholar]
Dales 2001 {published data only}
- Dales L, Hammer SJ, Smith NJ. Time trends in autism and in MMR immunization coverage in California. JAMA 2001;285(9):1183-5. [DOI] [PubMed] [Google Scholar]
Dallaire 2009 {published data only}
- Dallaire F, De Serres G, Tremblay FW, Markowski F, Tipples G. Long-lasting measles outbreak affecting several unrelated networks of unvaccinated persons. Journal of Infectious Diseases 2009;200(10):1602-5. [DOI] [PubMed] [Google Scholar]
Dankova 1995 {published data only}
- Dankova E, Domorazkova E, Skovrankova J, Vodickova M, Honzonova S, Stehlikova J, et al. Immune reactivity and risk of an undesirable response after vaccination. Ceskoslovenská Pediatrie 1995;50(9):515-9. [Google Scholar]
Dashefsky 1990 {published data only}
- Dashefsky B, Wald E, Guerra N, Byers C. Safety, tolerability, and immunogenicity of concurrent administration of Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) with either measles-mumps-rubella vaccine or diphtheria-tetanus-pertussis and oral poliovirus vaccines in 14- to 23-month-old infants. Pediatrics 1990;85(4 Pt 2):682-9. [PubMed] [Google Scholar]
Davis 1997 {published data only}
- Davis RL, Marcuse E, Black S, Shinefield H, Givens B, Schwalbe J, et al. MMR2 immunization at 4 to 5 years and 10 to 12 years of age: a comparison of adverse clinical events after immunization in the Vaccine Safety Datalink project. The Vaccine Safety Datalink Team. Pediatrics 1997;100(5):767-71. [DOI] [PubMed] [Google Scholar]
Dayan 2008a {published data only}
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. New England Journal of Medicine 2008;358(15):1580-9. [DOI] [PubMed] [Google Scholar]
Deforest 1986 {published data only}
- Deforest A, Long SS, Lischner HW. Safety and efficacy of simultaneous administration of measles-mumps-rubella (MMR) with booster doses of diphtheria-tetanus-pertussis (TP) and trivalent oral poliovirus (OPV) vaccines. Developments in Biological Standardization 1986;65:111. [Google Scholar]
Deforest 1988 {published data only}
- Deforest A, Long SS, Lischner HW, Girone JA, Clark JL, Srinivasan R, et al. Simultaneous administration of measles-mumps-rubella vaccine with booster doses of diphtheria-tetanus-pertussis and poliovirus vaccines. Pediatrics 1988;81(2):237-46. [PubMed] [Google Scholar]
De Laval 2010 {published data only}
- De Laval F, Haus R, Spiegel A, Simon F. Lower long-term immunogenicity of mumps component after MMR vaccine. Pediatric Infectious Disease Journal 2010;29(11):1062-3. [DOI] [PubMed] [Google Scholar]
DeStefano 2000 {published data only}
- DeStefano F, Chen RT. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Journal of Pediatrics 2000;136(1):125-6. [PubMed] [Google Scholar]
Diaz‐Ortega 2010 {published data only}
- Diaz-Ortega JL, Bennett JV, Castaneda D, Vieyra JR, Valdespino-Gomez JL, Castro JF. Successful seroresponses to measles and rubella following aerosolized Triviraten vaccine, but poor response to aerosolized mumps (Rubini) component: comparisons with injected MMR. Vaccine 2010;28(3):692-8. [DOI] [PubMed] [Google Scholar]
Dobrosavljevic 1999 {published data only}
- Dobrosavljevic D, Milinkovic MV, Nikolic MM. Toxic epidermal necrolysis following morbilli-parotitis-rubella vaccination. Journal of the European Academy of Dermatology and Venereology 1999;13(1):59-61. [PubMed] [Google Scholar]
Dominguez 2008 {published data only}
- Dominguez A, Torner N, Barrabeig I, Rovira A, Rius C, Cayla J, et al. Large outbreak of measles in a community with high vaccination coverage: implications for the vaccination schedule. Clinical Infectious Diseases 2008;47(9):1143-9. [DOI] [PubMed] [Google Scholar]
Doshi 2009 {published data only}
- Doshi S, Khetsuriani N, Zakhashvili K, Baidoshvili L, Imnadze P, Uzicanin A. Ongoing measles and rubella transmission in Georgia, 2004-05: implications for the national and regional elimination efforts. International Journal of Epidemiology 2009;38(1):182-91. [DOI] [PubMed] [Google Scholar]
Dos Santos 2002 {published data only}
- Dos Santos BA, Ranieri TS, Bercini M, Schermann MT, Famer S, Mohrdieck R, et al. An evaluation of the adverse reaction potential of three measles-mumps-rubella combination vaccines. Revista Panamericana de Salud Pública 2002;12(4):240-6. [DOI] [PubMed] [Google Scholar]
Duderstadt 2012 {published data only}
- Duderstadt SK, Rose CE Jr, Real TM, Sabatier JF, Stewart B, Ma G, et al. Vaccination and risk of type 1 diabetes mellitus in active component U.S. Military, 2002-2008. Vaccine 2012;30(4):813-9. [DOI] [PubMed] [Google Scholar]
Dyer 2010a {published data only}
- Dyer C. Wakefield was dishonest and irresponsible over MMR research, says GMC. BMJ 2010;340:c593. [DOI] [PubMed] [Google Scholar]
Dyer 2010b {published data only}
- Dyer C. Lancet retracts Wakefield's MMR paper. BMJ 2010;340:c696. [DOI] [PubMed] [Google Scholar]
Elphinstone 2000 {published data only}
- Elphinstone P. The MMR question. Lancet 2000;356(9224):161. [DOI] [PubMed] [Google Scholar]
Englund 1989 {published data only}
- Englund JA, Suarez CS, Kelly J, Tate DY, Balfour HH Jr. Placebo-controlled trial of varicella vaccine given with or after measles-mumps-rubella vaccine. Journal of Pediatrics 1989;114(1):37-44. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2007 {published data only}
- Fitzpatrick M. The end of the road for the campaign against MMR. British Journal of General Practice 2007;57(541):679. [PMC free article] [PubMed] [Google Scholar]
Fletcher 2001 {published data only}
- Fletcher AP. MMR safety studies. Adverse Drug Reactions and Toxicological Reviews 2001;20(1):57-60. [PubMed] [Google Scholar]
Garrido Lestache 1992 {published data only}
- Garrido Lestache A, Martin Hernandez D. Triple virus vaccination: study of its efficacy and safety. Pediatrika 1992;12:42-7. [Google Scholar]
Geier 2004 {published data only}
- Geier DA, Geier MR. A comparative evaluation of the effects of MMR immunization and mercury doses from thimerosal-containing childhood vaccines on the population prevalence of autism. Medical Science Monitor 2004;10(3):PI33-9. [PubMed] [Google Scholar]
Gerber 2009 {published data only}
- Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clinical Infectious Diseases 2009;48(4):456-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodson 2010 {published data only}
- Goodson JL, Perry RT, Mach O, Manyanga D, Luman ET, Kitambi M, et al. Measles outbreak in Tanzania, 2006-2007. Vaccine 2010;28(37):5979-85. [DOI] [PubMed] [Google Scholar]
Griffin 1991 {published data only}
- Griffin MR, Ray WA, Mortimer EA, Fenichel GM, Schaffner W. Risk of seizures after measles-mumps-rubella immunization. Pediatrics 1991;88(5):881-5. [PubMed] [Google Scholar]
Grilli 1992 {published data only}
- Grilli G, Cimini D, Vacca F. Vaccination against measles, mumps and rubella: incidence of side effects using different vaccine strains. Giornale di Malattie Infettive e Parassitarie 1992;44(1):38-42. [Google Scholar]
Hasrina 2017 {published data only}
- Hasrina H, Senthilvasan AL, Jayaraman J. A mumps outbreak in a private school in Shah Alam, July 2016. Medical Journal of Malaysia 2017;72:47. [Google Scholar]
Hilton 2009 {published data only}
- Hilton S, Hunt K, Langan M, Hamilton V, Petticrew M. Reporting of MMR evidence in professional publications: 1988-2007. Archives of Disease in Childhood 2009;94(11):831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hindiyeh 2009 {published data only}
- Hindiyeh MY, Aboudy Y, Wohoush M, Shulman LM, Ram D, Levin T, et al. Characterization of large mumps outbreak among vaccinated Palestinian refugees. Journal of Clinical Microbiology 2009;47(3):560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Höhle 2011 {published data only}
- Höhle M, Siedler A, Bader HM, Ludwig M, Heininger U, Von Kries R. Assessment of varicella vaccine effectiveness in Germany: a time series approach. Epidemiology and Infection 2011;139(11):1710-9. [DOI] [PubMed] [Google Scholar]
Hooker 2014 {published data only}
- Hooker BS. Measles-mumps-rubella vaccination timing and autism among young African American boys: a reanalysis of CDC data. Translational Neurodegeneration 2014;3:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Hornig 2008 {published data only}
- Hornig M, Briese T, Buie T, Bauman ML, Lauwers G, Siemetzki U, et al. Lack of association between measles virus vaccine and autism with enteropathy: a case-control study. PLoS ONE 2008;3(9):e3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2007 {published data only}
- Hu JY, Tao LN, Shen J, Wang YC. Study on the epidemiological characteristics of rubella from 1990-2006 in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 2007;28(7):645-8. [PubMed] [Google Scholar]
Hua 2009 {published data only}
- Hua W, Izurieta HS, Slade B, Belay ED, Haber P, Tiernan R, et al. Kawasaki disease after vaccination: reports to the vaccine adverse event reporting system 1990-2007. Pediatric Infectious Disease Journal 2009;28(11):943-7. [DOI] [PubMed] [Google Scholar]
Huang 1990 {published data only}
- Huang LM, Lee CY, Hsu CY, Huang SS, Kao CL, Wu FF. Effect of monovalent measles and trivalent measles-mumps-rubella vaccines at various ages and concurrent administration with hepatitis B vaccine. Pediatric Infectious Disease Journal 1990;9(7):461-5. [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang AS, Cortese MM, Curns AT, Bitsko RH, Jordan HT, Soud F, et al. Risk factors for mumps at a university with a large mumps outbreak. Public Health Reports 2009;124(3):419-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ipp 2003 {published data only}
- Ipp M, Cohen E, Goldbach M, McArthur C. Pain response to measles-mumps-rubella (MMR) vaccination at 12 months of age: a randomised clinical trial. Journal of Paediatrics and Child Health 2003;39(6):A3. [Google Scholar]
Jiang 2009 {published data only}
- Jiang Y, Pang H. Surveillance of adverse events following immunization of MMR in Changning District of Shanghai. Zhongguo Ji Hua Mian Yi 2009;15(6):496-7, 526. [PubMed] [Google Scholar]
Jones 1991 {published data only}
- Jones AG, White JM, Begg NT. The impact of MMR vaccine on mumps infection in England and Wales. CDR (London, England: Review) 1991;1(9):R93-6. [PubMed] [Google Scholar]
Just 1985 {published data only}
- Just M, Berger R, Gluck R, Wegmann A. Field trial with a new human diploid cell vaccine (HDCV) against measles, mumps and rubella [Feldversuch mit einer neuartigen Humandiploidzellvakzine (HDCV) gegen Masern, Mumps und Roteln]. Schweizerische Medizinische Wochenschrift 1985;115(48):1727-30. [PubMed] [Google Scholar]
Just 1986 {published data only}
- Just M, Berger R, Just V. Evaluation of a combined measles-mumps-rubella-chickenpox vaccine. Developments in Biological Standardization 1986;65:85-8. [PubMed] [Google Scholar]
Just 1987a {published data only}
- Just M, Berger R. Immunogenicity of vaccines. A comparative study of a mumps-measles-rubella vaccine given with or without oral polio vaccine [Immunantwort auf Impstoffe. Vergleichende Studie mit Mumps- , Masern-, und Roeteln-Impfstoff allein oder zusammen mit Polio-Impfstoff appliziert]. Münchener Medizinische Wochenschrift 1987;129(11):188-90. [Google Scholar]
Just 1987b {published data only}
- Just M, Berger R. Trivalent vaccines. A comparative study of the immunogenicity of two trivalent mumps-measles-rubella vaccines given with or without diphtheria-tetanus vaccine [Trivalente Impfstoffe. Vergleichende Studie zweier Mumps-Masern-Roeteln-Vakzinen in Kombination mit Diphtherie-Tetanus-Impfstoff]. Münchener Medizinische Wochenschrift 1987;129(23):446-7. [Google Scholar]
Kaaber 1990 {published data only}
- Kaaber K, Samuelsson IS, Larsen SO. Reactions after MMR vaccination [Reaktioner efter MFR-vaccination]. Ugeskrift for Laeger 1990;152(23):1672-6. [PubMed] [Google Scholar]
Karim 2002 {published data only}
- Karim Y, Masood A. Haemolytic uraemic syndrome following mumps, measles, and rubella vaccination. Nephrology, Dialysis, Transplantation 2002;17(5):941-2. [DOI] [PubMed] [Google Scholar]
Kaye 2001 {published data only}
- Kaye JA, Del Mar C, Melero-Montes M, Jick H. Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: a time trend analysis. BMJ 2001;322(7284):460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazarian 1978 {published data only}
- Kazarian EL, Gager WE. Optic neuritis complicating measles, mumps, and rubella vaccination. American Journal of Ophthalmology 1978;86(4):544-7. [DOI] [PubMed] [Google Scholar]
Khalil 2005 {published data only}
- Khalil MK, Al-Mazrou YY, AlHowasi MN, Al-Jeffri M. Measles in Saudi Arabia: from control to elimination. Annals of Saudi Medicine 2005;25(4):324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiepiela 1991 {published data only}
- Kiepiela P, Coovadia HM, Loening WE, Coward P, Botha G, Hugo J, et al. Lack of efficacy of the standard potency Edmonston-Zagreb live, attenuated measles vaccine in African infants. Bulletin of the World Health Organization 1991;69(2):221-7. [PMC free article] [PubMed] [Google Scholar]
Kulkarni 2005 {published data only}
- Kulkarni PS, Phadke MA, Jadhav SS, Kapre SV. No definitive evidence for L-Zagreb mumps strain associated aseptic meningitis: a review with special reference to the da Cunha study. Vaccine 2005;23(46-7):5286-8. [DOI] [PubMed] [Google Scholar]
Kurtzke 1997 {published data only}
- Kurtzke JF, Hyllested K, Arbuckle JD, Brønnum-Hansen H, Wallin MT, Heltberg A, et al. Multiple sclerosis in the Faroe Islands. 7. Results of a case control questionnaire with multiple controls. Acta Neurologica Scandinavica 1997;96(3):149-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kutty 2014 {published data only}
- Kutty PK, Lawler J, Rausch-Phung E, Ortega-Sanchez IR, Goodell S, Schulte C, et al. Epidemiology and the economic assessment of a mumps outbreak in a highly vaccinated population, Orange County, New York, 2009-2010. Human Vaccines and Immunotherapeutics 2014;10(5):1373-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Latasa 2019 {published data only}
- Latasa P, Ordobís M, Garrido-Estepa M, Sanz JC, Gil de Miguel A, Garc¡a-Comas L. Impact of the MMR vaccine on the incidence of mumps in the Community of Madrid and evaluation of the effectiveness of the Jeryl-Lynn strain. Years 1998-2016 [Impacto de la vacuna triple virica sobre la incidencia de la parotiditis en la Comunidad de Madrid y evaluacion de la efectividad de la cepa Jeryl-Lynn entre 1998 y 2016]. Medicina Clinica (Madrid) 2019 Mar 8 [Epub ahead of print]. [DOI: 10.1016/j.medcli.2019.01.012] [DOI] [PubMed]
Lee 1998 {published data only}
- Lee JW, Melgaard B, Clements CJ, Kane M, Mulholland EK, Olive JM. Autism, inflammatory bowel disease, and MMR vaccine. Lancet 1998;351(9106):905; author reply 908-9. [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee KY, Lee HS, Hur JK, Kang JH, Lee BC. The changing epidemiology of hospitalized pediatric patients in three measles outbreaks. Journal of Infection 2007;54(2):167-72. [DOI] [PubMed] [Google Scholar]
Lucena 2002 {published data only}
- Lucena R, Gomes I, Nunes L, Cunha S, Dourado I, Teixeira Mda G, et al. Clinical and laboratory features of aseptic meningitis associated with measles-mumps-rubella vaccine [Caracteristicas clinicas e laboratoriais da meningite asseptica associada a vacina triplice viral]. Revista Panamericana de Salud Pública 2002;12(4):258-61. [DOI] [PubMed] [Google Scholar]
Maekawa 1991 {published data only}
- Maekawa K, Nozaki H, Fukushima K, Sugishita T, Kuriya N. Clinical analysis of measles, mumps and rubella vaccine meningitis - comparative study of mumps, mumps meningitis and MMR meningitis. Jikeikai Medical Journal 1991;38(4):361-8. [Google Scholar]
Maguire 1991 {published data only}
- Maguire HC, Begg NT, Handford SG. Meningoencephalitis associated with MMR vaccine. CDR (London, England: Review) 1991;1(6):R60-1. [PubMed] [Google Scholar]
Majwala 2018 {published data only}
- Majwala RK, Nakiire L, Kadobera D, Ario AR, Kusiima J, Atuhairwe JA, et al. Measles outbreak propagated by children congregating at water collection points in Mayuge District, eastern Uganda, July-October, 2016. BMC Infectious Diseases 2018;18(1):412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantadakis 2010 {published data only}
- Mantadakis E, Farmaki E, Buchanan GR. Thrombocytopenic purpura after measles-mumps-rubella vaccination: a systematic review of the literature and guidance for management. Journal of Pediatrics 2010;156(4):623-8. [DOI] [PubMed] [Google Scholar]
Marshall 2016 {published data only}
- Marshall G, Senders S, Shepard J, Twiggs J, Gardner J, Hille D, et al. A double blind, randomized, active controlled study to assess the safety, tolerability and immunogenicity of measles, mumps rubella, and varicella vaccine (MMRV) manufactured using an alternative process. Human Vaccines and Immunotherapeutics 2016;12(8):2188-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matter 1995 {published data only}
- Matter L, Bally F, Germann D, Schopfer K. The incidence of rubella virus infections in Switzerland after the introduction of the MMR mass vaccination programme. European Journal of Epidemiology 1995;11(3):305-10. [DOI] [PubMed] [Google Scholar]
Matter 1997 {published data only}
- Matter L, Germann D, Bally F, Schopfer K. Age-stratified seroprevalence of measles, mumps and rubella (MMR) virus infections in Switzerland after the introduction of MMR mass vaccination. European Journal of Epidemiology 1997;13(1):61-6. [DOI] [PubMed] [Google Scholar]
Meissner 2004 {published data only}
- Meissner HC, Strebel PM, Orenstein WA. Measles vaccines and the potential for worldwide eradication of measles. Pediatrics 2004;114(4):1065-9. [DOI] [PubMed] [Google Scholar]
Miller 1983 {published data only}
- Miller JR, Orgel HA, Meltzer EO. The safety of egg-containing vaccines for egg-allergic patients. Journal of Clinical Immunology 1983;71(6):568-73. [DOI] [PubMed] [Google Scholar]
Miller 1993 {published data only}
- Miller E, Goldacre M, Pugh S, Colville A, Farrington P, Flower A, et al. Risk of aseptic meningitis after measles, mumps, and rubella vaccine in UK children. Lancet 1993;341(8851):979-82. [DOI] [PubMed] [Google Scholar]
Min 1991 {published data only}
- Min CH, Lee JH, Cho MK. A study of immunogenicity of measles, mumps and rubella vaccine prepared from human diploid cell. Journal of the Korean Society for Microbiology 1991;26(5):487-91. [Google Scholar]
Minekawa 1974 {published data only}
- Minekawa Y, Ueda S, Yamanishi K, Ogino T, Takahashi M. Studies on live rubella vaccine. V. Quantitative aspects of interference between rubella, measles and mumps viruses in their trivalent vaccine. Biken Journal 1974;17(4):161-7. [PubMed] [Google Scholar]
Mommers 2004 {published data only}
- Mommers M, Weishoff-Houben M, Swaen GM, Creemers H, Freund H, Dott W, et al. Infant immunization and the occurrence of atopic disease in Dutch and German children: a nested case-control study. Pediatric Pulmonology 2004;38(4):329-34. [DOI] [PubMed] [Google Scholar]
Mupere 2006 {published data only}
- Mupere E, Karamagi C, Zirembuzi G, Grabowsky M, Swart RL, Nanyunja M, et al. Measles vaccination effectiveness among children under 5 years of age in Kampala, Uganda. Vaccine 2006;24(19):4111-5. [DOI] [PubMed] [Google Scholar]
Nalin 1999 {published data only}
- Nalin D. Comparative study of reactogenicity and immunogenicity of new and established measles, mumps and rubella vaccines in healthy children (Infection 26). Infection 1999;27(2):134-5. [DOI] [PubMed] [Google Scholar]
Narwaney 2017 {published data only}
- Narwaney KJ, Breslin K, Ross CA, Shoup JA, Wain KF, Weintraub ES, et al. Vaccine adverse events in a safety net healthcare system and a managed care organization. Vaccine 2017;35(9):1335-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicoll 1998 {published data only}
- Nicoll A, Elliman D, Ross E. MMR vaccination and autism 1998 [Erratum in: BMJ 1998 Mar 14;316(7134):796]. BMJ 1998;316(7133):715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ntshoe 2013 {published data only}
- Ntshoe GM, McAnerney JM, Archer BN, Smit SB, Harris BN, Tempia S, et al. Measles outbreak in South Africa: epidemiology of laboratory-confirmed measles cases and assessment of intervention, 2009-2011. PLoS ONE 2013;8(2):e55682. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1998 {published data only}
- O'Brien SJ, Jones IG, Christie P. Autism, inflammatory bowel disease, and MMR vaccine. Lancet 1998;351(9106):906-7; author reply 908-9. [DOI] [PubMed] [Google Scholar]
O'Connor 2019 {published data only}
- O'Connor B, Doyle S. Identifying risk factors associated with acquiring measles in an outbreak among age-appropriately vaccinated school children: a cohort analysis. Irish Journal of Medical Science 2019;188(1):193-9. [DOI] [PubMed] [Google Scholar]
Ong 2006 {published data only}
- Ong G, Hoon HB, Ong A, Chua LT, Kai CS, Tai GK. A 24-year review on the epidemiology and control of measles in Singapore, 1981-2004. Southeast Asian Journal of Tropical Medicine and Public Health 2006;37(1):96-101. [PubMed] [Google Scholar]
Patja 2000 {published data only}
- Patja A, Davidkin I, Kurki T, Kallio MJ, Valle M, Peltola H. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. Pediatric Infectious Disease Journal 2000;19(12):1127-34. [DOI] [PubMed] [Google Scholar]
Patja 2001 {published data only}
- Patja A, Paunio M, Kinnunen E, Junttila O, Hovi T, Peltola H. Risk of Guillain-Barre syndrome after measles-mumps-rubella vaccination. Journal of Pediatrics 2001;138(2):250-4. [DOI] [PubMed] [Google Scholar]
Pekmezovic 2004 {published data only}
- Pekmezovic T, Jarebinski M, Drulovic J. Childhood infections as risk factors for multiple sclerosis: Belgrade case-control study. Neuroepidemiology 2004;23(6):285-8. [DOI] [PubMed] [Google Scholar]
Peltola 1998 {published data only}
- Peltola H, Patja A, Leinikki P, Valle M, Davidkin I, Paunio M. No evidence for measles, mumps, and rubella vaccine-associated inflammatory bowel disease or autism in a 14-year prospective study. Lancet 1998;351(9112):1327-8. [DOI] [PubMed] [Google Scholar]
Peltola 2007 {published data only}
- Peltola H, Kulkarni PS, Kapre SV, Paunio M, Jadhav SS, Dhere RM. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clinical Infectious Diseases 2007;45(4):459-66. [DOI] [PubMed] [Google Scholar]
Petridou 1997 {published data only}
- Petridou E, Trichopoulos D, Kalapothaki V, Pourtsidis A, Kogevinas M, Kalmanti M, et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. British Journal of Cancer 1997;76(9):1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puvvada 1993 {published data only}
- Puvvada L, Silverman B, Bassett C, Chiaramonte LT. Systemic reactions to measles-mumps-rubella vaccine skin testing. Pediatrics 1993;91(4):835-6. [PubMed] [Google Scholar]
Rajantie 2007 {published data only}
- Rajantie J, Zeller B, Treutiger I, Rosthoj S. Vaccination associated thrombocytopenic purpura in children. Vaccine 2007;25(10):1838-40. [DOI] [PubMed] [Google Scholar]
Roost 2004 {published data only}
- Roost HP, Gassner M, Grize L, Wuthrich B, Sennhauser FH, Varonier HS, et al. Influence of MMR-vaccinations and diseases on atopic sensitization and allergic symptoms in Swiss schoolchildren. Pediatric Allergy and Immunology 2004;15(5):401-7. [DOI] [PubMed] [Google Scholar]
Sabra 1998 {published data only}
- Sabra A, Bellanti JA, Colon AR. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998;352(9123):234-5. [DOI] [PubMed] [Google Scholar]
Saraswathy 2009 {published data only}
- Saraswathy TS, Zahrin HN, Norhashmimi H, Az-Ulhusna A, Zainah S, Rohani J. Impact of a measles elimination strategy on measles incidence in Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 2009;40(4):742-7. [PubMed] [Google Scholar]
Scarpa 1990 {published data only}
- Scarpa B, Masia G, Contu P, Origa P, Sanna CM, Pintor C, et al. Trivalent vaccine against measles, rubella and parotitis: clinic and serological evaluation [Vaccino trivalente contro morbillo, rosolia e parotite: valutazione clinica e sierologica]. Giornale di Malattie Infettive e Parassitarie 1990;42(6):344-7. [Google Scholar]
Schaffzin 2007 {published data only}
- Schaffzin JK, Pollock L, Schulte C, Henry K, Dayan G, Blog D, et al. Effectiveness of previous mumps vaccination during a summer camp outbreak. Pediatrics 2007;120(4):e862-8. [DOI] [PubMed] [Google Scholar]
Schettini 1989 {published data only}
- Schettini F, Manzionna MM, De Mattia D, Amendola F, Di Bitonto G. Clinico-immunologic evaluation of a trivalent vaccine against measles, rubella and mumps [Valutazione clinico-immunologica di un vaccino trivalente contro morbillo, rosolia e parotite]. Minerva Pediatrica 1989;41(3):117-22. [PubMed] [Google Scholar]
Schettini 1990 {published data only}
- Schettini F, Manzionna MM, De Mattia D, Amendola F, Di Bitonto G. The clinico-immunological evaluation of a bivalent vaccine against measles and rubella [Valutazione clinico-immunologica di un vaccino bivalente contro morbillo e rosolia]. Minerva Pediatrica 1990;42(12):531-6. [PubMed] [Google Scholar]
Schmid 2008 {published data only}
- Schmid D, Holzmann H, Alfery C, Wallenko H, Popow-Kraupp TH, Allerberger F. Mumps outbreak in young adults following a festival in Austria, 2006. Euro Surveillance 2008;13(7):8042. [DOI] [PubMed] [Google Scholar]
Schultz 2008 {published data only}
- Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder: the results of a parent survey. Autism 2008;12(3):293-307. [DOI] [PubMed] [Google Scholar]
Schwarz 2010 {published data only}
- Schwarz NG, Bernard H, Melnic A, Bucov V, Caterinciuc N, an der Heiden M, et al. Mumps outbreak in the Republic of Moldova, 2007-2008. Pediatric Infectious Disease Journal 2010;29(8):703-6. [DOI] [PubMed] [Google Scholar]
Schwarzer 1998 {published data only}
- Schwarzer S, Reibel S, Lang AB, Struck MM, Finkel B, Gerike E, et al. Safety and characterization of the immune response engendered by two combined measles, mumps and rubella vaccines. Vaccine 1998;16(2-3):298-304. [DOI] [PubMed] [Google Scholar]
Seagroatt 2003 {published data only}
- Seagroatt V, Goldacre MJ. Crohn's disease, ulcerative colitis, and measles vaccine in an English population, 1979-1998. Journal of Epidemiology and Community Health 2003;57(11):883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2017 {published data only}
- Shah N, Parikh R, Casabona G, Kolhapure S. A new combined vaccine against measles, mumps, rubella and varicella in India. Indian Pediatrics 2017;54(12):1041-6. [DOI] [PubMed] [Google Scholar]
Shah 2018 {published data only}
- Shah M, Quinlisk P, Weigel A, Riley J, James L, Patterson J, et al. Mumps outbreak in a highly vaccinated university-affiliated setting before and after a measles-mumps-rubella vaccination campaign, Iowa, July 2015 to May 2016. Clinical Infectious Diseases 2018;66(1):81-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2004 {published data only}
- Sharma MK, Bhatia V, Swami HM. Outbreak of measles amongst vaccinated children in a slum of Chandigarh. Indian Journal of Medical Sciences 2004;58(2):47-53. [PubMed] [Google Scholar]
Shinefield 2002 {published data only}
- Shinefield HR, Black SB, Staehle BO, Matthews H, Adelman T, Ensor K, et al. Vaccination with measles, mumps and rubella vaccine and varicella vaccine: safety, tolerability, immunogenicity, persistence of antibody and duration of protection against varicella in healthy children. Pediatric Infectious Disease Journal 2002;21(6):555-61. [DOI] [PubMed] [Google Scholar]
So 2008 {published data only}
- So JS, Go UY, Lee DH, Park KS, Lee JK. Epidemiological investigation of a measles outbreak in a preschool in Incheon, Korea, 2006. Journal of Preventive Medicine and Public Health 2008;41(3):153-8. [DOI] [PubMed] [Google Scholar]
Spitzer 2001 {published data only}
- Spitzer WO. A sixty day war of words: is MMR linked to autism? Adverse Drug Reactions and Toxicological Reviews 2001;20(1):47-55. [PubMed] [Google Scholar]
Stetler 1985 {published data only}
- Stetler HC, Mullen JR, Brennan JP, Orenstein WA, Bart KJ, Hinman AR. Adverse events following immunization with DTP vaccine. Developments in Biological Standardization 1985;61:411-21. [PubMed] [Google Scholar]
Stokes 1967 {published data only}
- Stokes JJ. Studies on active immunization in measles, mumps and rubella. Johns Hopkins Medical Journal 1967;121(5):314-28. [PubMed] [Google Scholar]
Stratton 1994 {published data only}
- Stratton KR, Howe CJ, Johnston RB Jr. Adverse events associated with childhood vaccines other than pertussis and rubella. Summary of a report from the Institute of Medicine. JAMA 1994;271(20):1602-5. [PubMed] [Google Scholar]
Sugiura 1982 {published data only}
- Sugiura A, Ohtawara M, Hayami M, Hisiyama M, Shishido A, Kawana R, et al. Field trial of trivalent measles-rubella-mumps vaccine in Japan. Journal of Infectious Diseases 1982;146(5):709. [DOI] [PubMed] [Google Scholar]
Svanström 2010 {published data only}
- Svanström H, Callréus T, Hviid A. Temporal data mining for adverse events following immunization in nationwide Danish healthcare databases. Drug Safety 2010;33(11):1015-25. [DOI] [PubMed] [Google Scholar]
Tosun 2017 {published data only}
- Tosun S, Olut AI, Tansug N. Adverse effects of single-component measles vaccine in school children. Vaccine 2017;35(52):7309-11. [DOI] [PubMed] [Google Scholar]
Ueda 1995 {published data only}
- Ueda K, Miyazaki C, Hidaka Y, Okada K, Kusuhara K, Kadoya R. Aseptic meningitis caused by measles-mumps-rubella vaccine in Japan. Lancet 1995;346(8976):701-2. [DOI] [PubMed] [Google Scholar]
Vesikari 1979 {published data only}
- Vesikari T, Elo O. Vaccination against measles, mumps and rubella - together or separately? [Tuhkarokon, sikotaudin ja vihurirokon torjunta - yhdessa vai erikseen?]. Duodecim 1979;95(9):527-9. [PubMed] [Google Scholar]
Vesikari 1984 {published data only}
- Vesikari T, Ala-Laurila EL, Heikkinen A, Terho A, D'Hondt E, Andre FE. Clinical trial of a new trivalent measles-mumps-rubella vaccine in young children. American Journal of Diseases in Children 1984;138(9):843-7. [DOI] [PubMed] [Google Scholar]
Wakefield 1998 {published data only}
- Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998;351(9103):637-41. [DOI] [PubMed] [Google Scholar]
Wakefield 1999a {published data only}
- Wakefield AJ, Montgomery SM. Autism, viral infection and measles-mumps-rubella vaccination. Israel Medical Association Journal 1999;1(3):183-7. [PubMed] [Google Scholar]
Wakefield 1999b {published data only}
- Wakefield AJ. MMR vaccination and autism. Lancet 1999;354(9182):949-50. [DOI] [PubMed] [Google Scholar]
Wakefield 2000 {published data only}
- Wakefield AJ, Montgomery SM. Measles, mumps, rubella vaccine: through a glass, darkly. Adverse Drug Reactions and Toxicological Reviews 2000;19(4):265-83; 284-92. [PubMed] [Google Scholar]
Walker 2011 {published data only}
- Walker M, Puenpatom T, Li J, Daniel GW, Brookhart MA, Polakowski L, et al. Search for new safety signals in the patterns of care following MMR vaccine. Pharmacoepidemiology and Drug Safety 2011;20:s347-8. [Google Scholar]
Willocks 2017 {published data only}
- Willocks LJ, Guerendiain D, Austin HI, Morrison KE, Cameron RL, Templeton KE, et al. An outbreak of mumps with genetic strain variation in a highly vaccinated student population in Scotland. Epidemiology and Infection 2017;145(15):3219-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2003 {published data only}
- Wilson K, Mills E, Ross C, McGowan J, Jadad A. Association of autistic spectrum disorder and the measles, mumps, and rubella vaccine: a systematic review of current epidemiological evidence. Archives of Pediatric and Adolescent Medicine 2003;157(7):628-34. [DOI] [PubMed] [Google Scholar]
Wilson 2011 {published data only}
- Wilson K, Hawken S, Kwong JC, Deeks S, Crowcroft NS, Van Walraven C, et al. Adverse events following 12 and 18 month vaccinations: a population-based, self-controlled case series analysis. PLoS ONE 2011;6(12):e27897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woyciechowska 1985 {published data only}
- Woyciechowska JL, Dambrozia J, Leinikki P, Shekarchi C, Wallen W, Sever J, et al. Viral antibodies in twins with multiple sclerosis. Neurology 1985;35(8):1176-80. [DOI] [PubMed] [Google Scholar]
Yamashiro 1998 {published data only}
- Yamashiro Y, Walker-Smith JA, Shimizu T, Oguchi S, Ohtsuka Y. Measles vaccination and inflammatory bowel disease in Japanese children. Journal of Pediatric Gastroenterology and Nutrition 1998;26(2):238. [DOI] [PubMed] [Google Scholar]
Yu 2007 {published data only}
- Yu X, Wang S, Guan J, Mahemuti P, Gou A, Liu Q, et al. Analysis of the cause of increased measles incidence in Xinjiang, China in 2004. Pediatric Infectious Disease Journal 2007;26(6):513-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cardemil 2017 {published data only}
- Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. New England Journal of Medicine 2017;377(10):947-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2007 {published data only}
- Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, et al. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerging Infectious Diseases 2007;13(1):12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011 {published data only}
- Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, et al. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ: Canadian Medical Association Journal 2011;183(9):1014-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dominguez 2010 {published data only}
- Dominguez A, Torner N, Castilla J, Batalla J, Godoy P, Guevara M, et al. Mumps vaccine effectiveness in highly immunized populations. Vaccine 2010;28(20):3567-70. [DOI] [PubMed] [Google Scholar]
Fantinato 2018 {published data only}
- Fantinato FFST, Vargas A, Carvalho SMD, Domingues CMAS, Barreto G, Fialho AS, et al. Anaphylaxis related to measles, mumps, and rubella vaccine in Santa Catarina State, Brazil, 2014 and 2015 [Anafilaxia relacionada à vacina sarampo, caxumba e rubéola, Santa Catarina, Brasil, 2014 e 2015]. Cadernos de Saúde Pública 2018;34(3):e00043617. [DOI] [PubMed] [Google Scholar]
Fiebelkorn 2013 {published data only}
- Fiebelkorn AP, Lawler J, Curns AT, Brandeburg C, Wallace GS. Mumps postexposure prophylaxis with a third dose of measles-mumps-rubella vaccine, Orange County, New York, USA. Emerging Infectious Diseases 2013;19(9):1411-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitas 2013 {published data only}
- Freitas DR, Moura E, Araújo G, Cardoso A, Scheidt P, Ferraz E, et al. Investigation of an outbreak of hypersensitivity-type reactions during the 2004 national measles-mumps-rubella vaccination campaign in Brazil. Vaccine 2013;31(6):950-4. [DOI: 10.1016/j.vaccine.2012.11.095] [DOI] [PubMed] [Google Scholar]
Marin 2008 {published data only}
- Marin M, Quinlisk P, Shimabukuro T, Sawhney C, Brown C, Lebaron CW. Mumps vaccination coverage and vaccine effectiveness in a large outbreak among college students - Iowa, 2006. Vaccine 2008;26(29-30):3601-7. [DOI] [PubMed] [Google Scholar]
Orlikova 2016 {published data only}
- Orlikova H, Maly M, Lexova P, Sebestova H, Limberkova R, Jurzykowska L, et al. Protective effect of vaccination against mumps complications, Czech Republic, 2007-2012. BMC Public Health 2016;16:293. [DOI: 10.1186/s12889-016-2958-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prescott 2018 {published data only}
- Prescott V, Grainger JD. Comparison of vaccination associated and non-vaccination associated paediatric immune thrombocytopenia. British Journal of Haematology 2018;181(Suppl 1, 5–211):114-5. [Google Scholar]
Sheppeard 2009 {published data only}
- Sheppeard V, Forssman B, Ferson MJ, Moreira C, Campbell-Lloyd S, Dwyer DE, et al. Vaccine failures and vaccine effectiveness in children during measles outbreaks in New South Wales, March-May 2006. Communicable Diseases Intelligence 2009;33(1):21-6. [DOI] [PubMed] [Google Scholar]
Sorup 2019 {published data only}
- Sorup S, Jensen AKG, Aaby P, Benn CS. Revaccination with measles-mumps-rubella vaccine and infectious disease morbidity: a Danish register-based cohort study. Clinical Infectious Diseases 2019;68(2):282-90. [DOI] [PubMed] [Google Scholar]
Additional references
Amjadi 2016
- Amjadi O, Rafiei A, Haghshenas M, Navaei RA, Valadan R, Hosseini-Khah Z, et al. A systematic review and meta-analysis of seroprevalence of varicella zoster virus: a nationwide population-based study. Journal of Clinical Virology 2016 Dec 6 [Epub ahead of print];87:49-59. [DOI: 10.1016/j.jcv.2016.12.001] [PMID: ] [DOI] [PubMed]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bechini 2015
- Bechini A, Boccalini S, Baldo V, Cocchio S, Castiglia P, Gallo T, et al. Impact of universal vaccination against varicella in Italy. Human Vaccines and Immunotherapeutics 2015;11(1):63-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bester 2016
- Bester JC. Measles and measles vaccination: a review. JAMA Pediatrics 2016;170(12):1209-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Briss 1994
- Briss PA, Fehrs LJ, Parker RA, Wright PF, Sannella EC, Hutcheson RH, et al. Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. Journal of Infectious Diseases 1994;169(1):77-82. [DOI] [PubMed] [Google Scholar]
CDC 1992
- Centers for Disease Control and Prevention. Public sector vaccination efforts in response to the resurgence of measles among preschool-aged children - United States, 1989–1991. MMWR Weekly 1992;41(29):522-5. [PubMed] [Google Scholar]
CDC 2005
- Centers for Disease Control and Prevention. Achievements in public health: elimination of rubella and congenital rubella syndrome - United States, 1969-2004. MMWR Weekly 2005;54(11):279-82. [PubMed] [Google Scholar]
CDC 2012
- National Center for Immunization and Respiratory Diseases, Division of Viral Diseases, Centers for Disease Control and Prevention. Documentation and verification of measles, rubella and congenital rubella syndrome elimination in the region of the Americas: United States national report, March 28, 2012. www.cdc.gov/measles/downloads/Report-elimination-measles-rubella-crs.pdf [accessed prior to 2 October 2019].
Cecinati 2013
- Cecinati V, Principi N, Brescia L, Giordano P, Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Human Vaccines and Immunotherapeutics 2013;9(5):1158-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cortese 2008
- Cortese MM, Jordan HT, Curns AT, Quinlan PA, Ens KA, Denning PM, et al. Mumps vaccine performance among university students during a mumps outbreak. Clinical Infectious Diseases 2008;46(8):1172-80. [DOI] [PubMed] [Google Scholar]
Dabbagh 2018
- Dabbagh A, Laws RL, Steulet C, Dumolard L, Mulders MN, Kretsinger K, et al. Progress toward regional measles elimination - worldwide, 2000-2017. MMWR. Morbidity and Mortality Weekly Report 2018;67(47):1323-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidkin 2010
- Davidkin I, Kontio M, Paunio M, Peltola H. MMR vaccination and disease elimination: the Finnish experience. Expert Review of Vaccines 2010;9(9):1045-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dayan 2008b
- Dayan GH, Rubin S. Mumps outbreaks in vaccinated populations: are available mumps vaccines effective enough to prevent outbreaks? Clinical Infectious Diseases 2008;47(11):1458-67. [DOI] [PubMed] [Google Scholar]
Di Pietrantonj 2006
- Di Pietrantonj C. Four-fold table cell frequencies imputation in meta analysis. Statistics in Medicine 2006;25(13):2299-322. [PMID: ] [DOI] [PubMed] [Google Scholar]
Editors of the Lancet 2010
Farrington 1996
- Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. American Journal of Epidemiology 1996;143(11):1165-73. [DOI] [PubMed] [Google Scholar]
Farrington 2004
- Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine 2004;22(15-16):2064-70. [DOI] [PubMed] [Google Scholar]
Fiebelkorn 2017
- Fiebelkorn AP, Redd SB, Gastanaduy PA, Clemmons N, Rota PA, Rota JS, et al. A comparison of postelimination measles epidemiology in the United States, 2009-2014 versus 2001-2008. Journal of the Pediatric Infectious Diseases Society 2017;6(1):40-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flaherty 2011
- Flaherty DK. The vaccine-autism connection: a public health crisis caused by unethical medical practices and fraudulent science. Annals of Pharmacotherapy 2011;45(10):1302-4. [DOI] [PubMed] [Google Scholar]
Garcia Cenoz 2013
- Garcia Cenoz M, Castilla J, Chamorro J, Martinez-Baz I, Martinez-Artola V, Irisarri F, et al. Impact of universal two-dose vaccination on varicella epidemiology in Navarre, Spain, 2006 to 2012. Euro Surveillance 2013;18(32):20552. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gay 1997
- Gay N, Ramsay M, Cohen B, Hesketh L, Morgan-Capner P, Brown D, et al. The epidemiology of measles in England and Wales since the 1994 vaccination campaign. Communicable Disease Report. CDR Review 1997;7(2):R17-21. [PMID: ] [PubMed] [Google Scholar]
Gershon 2013
- Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clinical Microbiology Reviews 2013;26(4):728-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gershon 2015
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. Varicella zoster virus infection. Nature Reviews. Disease Primers 2015;1:15016. [DOI: 10.1038/nrdp.2015.16] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 2 October 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hambrosky 2015
- Hambrosky J, Kroger A, Wolfe S. Epidemiology and prevention of vaccine-preventable diseases. In: Hamborsky J, Kroger A, Wolfe S, editors(s). Centers for Disease Control and Prevention. 13th edition. Vol. 1. Washington D.C.: Public Health Foundation, 2015:1. [Google Scholar]
Harpaz 2019
- Harpaz R. Do varicella vaccination programs change the epidemiology of herpes zoster? A comprehensive review, with focus on the United States. Expert Review of Vaccines 2019;18(8):793-811. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2006
- Harris AD, McGregor JC, Perencevich EN, Furuno JP, Zhu J, Peterson DE, et al. The use and interpretation of quasi-experimental studies in medical informatics. Journal of the American Medical Informatics Association 2006;13(1):16-23. [DOI: 10.1197/jamia.M1749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helmuth 2015
- Helmuth IG, Poulsen A, Suppli CH, Molbak K. Varicella in Europe - a review of the epidemiology and experience with vaccination. Vaccine 2015;33(21):2406-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hersh 1991
- Hersh BS, Fine PE, Kent WK, Cochi SL, Kahn LH, Zell ER, et al. Mumps outbreak in a highly vaccinated population. Journal of Pediatrics 1991;119(2):187-93. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hilton 2007
- Hilton S, Hunt K, Petticrew M. MMR: marginalised, misrepresented and rejected? Autism: a focus group study. Archives of Disease in Childhood 2007;92(4):322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinman 2011
- Hinman AR, Hahn C, Maldonado Y, Shult PA, Temte JL. Verification and documentation of elimination of measles and rubella as endemic diseases from the United States: summary and conclusions of an external expert panel. www.cdc.gov/measles/downloads/expert-panel-elimination-measles.pdf (accessed prior to 2 October 2019).
Hviid 2008
- Hviid A, Rubin S, Muhlemann K. Mumps. Lancet 2008;371(9616):932-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jefferson 1999
- Jefferson T, Demicheli V. Relation between experimental and non-experimental study designs. HB vaccines: a case study. Journal of Epidemiology and Community Health 1999;53(1):51-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2001
- Khan SK, ter Riet G, Popay J, Nixon J, Kleijnen J. Stage II conducting the review: phase 5: study quality assessment. In: Khan SK, ter Riet G, Glanville G, Sowden AJ, Kleijnen J, editors(s). Undertaking Systematic Reviews of Research on Effectiveness. CDR's guidance for those carrying out or commissioning reviews. CDR Report No. 4. 2nd edition. York (UK): NHS Centre for Reviews and Dissemination, University of York, 2001. [Google Scholar]
Lambert 2015
- Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet 2015;385(9984):2297-307. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Last 2001
- Last J. A Dictionary of Epidemiology. 4th edition. Oxford (UK): Oxford University Press, 2001. [Google Scholar]
Lee 2019
- Lee AD, Clemmons NS, Patel M, Gastanaduy PA. International importations of measles virus into the United States during the postelimination era, 2001-2016. Journal of Infectious Diseases 2019;219(10):1616-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maclure 1991
- Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. American Journal of Epidemiology 1991;133(2):144-53. [DOI] [PubMed] [Google Scholar]
Marin 2007
- Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Morbidity and Mortality Weekly Report 2007;56:1-40. [PubMed] [Google Scholar]
Morgenstern 1995
- Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Annual Review of Public Health 1995;16:61-81. [DOI] [PubMed] [Google Scholar]
Murch 2004
Muscat 2014
- Muscat M, Shefer A, Ben Mamou M, Spataru R, Jankovic D, Deshevoy S, et al. The state of measles and rubella in the WHO European Region, 2013. Clinical Microbiology and Infection 2014;20(Suppl 5):S12-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
National Institute for Welfare and Health 2017
- National Institute for Welfare and Health (TFL). Vaccination coverage good among young children in Finland, but measles epidemics possible. www.thl.fi/fi/web/thlfi-en/-/while-vaccination-coverage-among-children-in-finland-is-good-measles-epidemics-are-possible (accessed prior to 2 October 2019).
Offit 2003
- Offit PA, Coffin SE. Communicating science to the public: MMR vaccine and autism. Vaccine 2003;22(1):1-6. [DOI] [PubMed] [Google Scholar]
Orenstein 1985
- Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, et al. Field evaluation of vaccine efficacy. Bulletin of the World Health Organization 1985;63(6):1055-68. [PMC free article] [PubMed] [Google Scholar]
Orenstein 2004
- Orenstein WA, Papania MJ, Wharton ME. Measles elimination in the United States. Journal of Infectious Diseases 2004;189(Suppl 1):S1-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Orenstein 2018
- Orenstein WA, Hinman A, Nkowane B, Olive JM, Reingold A. Measles and Rubella Global Strategic Plan 2012-2020 midterm review. Vaccine 2018;36(Suppl 1):A1-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Papania 2014
- Papania MJ, Wallace GS, Rota PA, Icenogle JP, Fiebelkorn AP, Armstrong GL, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatrics 2014;168(2):148-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2015
- Park SH. Resurgence of mumps in Korea. Infection & Chemotherapy 2015;47(1):1-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peltola 2008
- Peltola H, Jokinen S, Paunio M, Hovi T, Davidkin I. Measles, mumps, and rubella in Finland: 25 years of a nationwide elimination programme. Lancet Infectious Diseases 2008;8(12):796-803. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perry 2004
- Perry RT, Halsey NA. The clinical significance of measles: a review. Journal of Infectious Diseases 2004;189(Suppl 1):S4-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Perry 2015
- Perry RT, Murray JS, Gacic-Dobo M, Dabbagh A, Mulders MN, Strebel PM, et al. Progress toward regional measles elimination – worldwide. 2000-2014. MMWR. Morbidity and Mortality Weekly Report 2015;64(44):1246-51. [DOI] [PubMed] [Google Scholar]
Petersen 2016
- Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016;354:1-4. [DOI: 10:1136/bmj.i4515] [DOI] [PubMed] [Google Scholar]
Plotkin 2017
- Plotkin SA, Orestein WA, Offit PA. Plotkin's Vaccines. 7th edition. Vol. 1. Philadelphia: Elsevier Health Sciences Division, 2018. [Google Scholar]
Public Health England 2019a
- Public Health England. UK measles and rubella elimination strategy 2019. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/769970/UK_measles_and_rubella_elimination_strategy.pdf (accessed prior to 2 October 2019).
Public Health England 2019b
- Public Health England. Measles notifications, by age group, region and sex. www.gov.uk/government/publications/measles-notifications-by-age-group-region-and-sex (accessed prior to 2 October 2019).
Public Health England 2019c
- Public Health England. Confirmed cases of measles, mumps and rubella in England and Wales: 1996 to 2018. www.gov.uk/government/publications/measles-confirmed-cases/confirmed-cases-of-measles-mumps-and-rubella-in-england-and-wales-2012-to-2013 (accessed prior to 2 October 2019).
Ramsay 2003
- Ramsay ME, Jin L, White J, Litton P, Cohen B, Brown D. The elimination of indigenous measles transmission in England and Wales. Journal of Infectious Diseases 2003;187(Suppl 1):S198-207. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JP, Wells GA. Chapter 13: Including non-randomised studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Wiley-Blackwell, 2011. [Google Scholar]
Smeeth 2001
- Smeeth L, Hall AJ, Fombonne E, Rodrigues LC, Huang X, Smith PG. A case-control study of autism and mumps-measles-rubella vaccination using the general practice research database: design and methodology. BMC Public Health 2001;1:1-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2008
- Smith MJ, Ellenberg SS, Bell LM, Rubin DM. Media coverage of the measles-mumps-rubella vaccine and autism controversy and its relationship to MMR immunization rates in the United States. Pediatrics 2008;121(4):e836-43. [DOI] [PubMed] [Google Scholar]
Stang 2010
- Stang A. Critical evaluation of Newcastle-Ottawa Scale for the assessment of the quality of non-randomised studies in meta-analyses. European Journal of Epidemiology 2010;25:603-5. [DOI] [PubMed] [Google Scholar]
Steffenburg 1989
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. Journal of Child Psychology and Psychiatry and Allied Disciplines 1989;30(3):405-16. [DOI] [PubMed] [Google Scholar]
Uno 2015
- Uno Y, Uchiyama T, Kurosawa M, Aleksic B, Ozaki N. Early exposure to the combined measles-mumps-rubella vaccine and thimerosal-containing vaccines and risk of autism spectrum disorder. Vaccine 2015;33:2511-6. [DOI: 10.1016/j.vaccine.2014.12.036] [DOI] [PubMed] [Google Scholar]
Vandermeulen 2004
- Vandermeulen C, Roelants M, Vermoere M, Roseeuw K, Goubau P, Hoppenbrouwers K. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine 2004;22(21-22):2713-6. [DOI] [PubMed] [Google Scholar]
Vesikari 2007
- Vesikari T, Sadzot-Delvaux C, Rentier B, Gershon A. Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatric Infectious Disease Journal 2007;26(7):632-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vynnycky 2016
- Vynnycky E, Adams EJ, Cutts FT, Reef SE, Navar AM, Simons E, et al. Using seroprevalence and immunisation coverage data to estimate the global burden of congenital rubella syndrome, 1996-2010: a systematic review. PLoS ONE 2016;11(3):e0149160. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vyse 2002
- Vyse AJ, Gay NJ, White JM, Ramsay ME, Brown DW, Cohen BJ, et al. Evolution of surveillance of measles, mumps, and rubella in England and Wales: providing the platform for evidence-based vaccination policy. Epidemiologic Reviews 2002;24(2):125-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Watson 1998
- Watson JC, Redd SC, Rhodes PH, Hadler SC. The interruption of transmission of indigenous measles in the United States during 1993. Pediatric Infectious Disease Journal 1998;17(5):363-6; discussion 366-7. [DOI] [PubMed] [Google Scholar]
Wells 2000
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. www.Iri.ca/programs/ceu/oxford.htm (accessed 2 October 2019).
WHO 2011
- World Health Organization. Proceedings of the global technical consultation to assess the feasibility of measles eradication, 28-30 July 2010. Journal of Infectious Diseases 2011;204(Suppl 1):S4-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization. Joint external evaluation of IHR core capacities of the Republic of Finland. extranet.who.int/spp/sites/default/files/jeeta/WHO-WHE-CPI-2017.24-Report-eng.pdf (accessed prior to 2 October 2019).
WHO GVAP 2013
- WHO GVAP. Global vaccine action plan 2011-2020. www.who.int/immunization/global_vaccine_action_plan/ GVAP_doc_2011_2020/en/ (accessed prior to 2 October 2019).
WHO Immunization Monitoring 2019
- WHO Immunization Monitoring. WHO. Varicella vaccine. WHO vaccine-preventable diseases: monitoring system. 2019 global summary. apps.who.int/immunization_monitoring/globalsummary/schedules (accessed prior to 2 October 2019).
WHO Position Paper 2014
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. www.who.int/wer/2014/wer8925.pdf (accessed prior to 2 October 2019).
WHO Position Paper 2017
- World Health Organization. WHO Position Paper. Epidemiologic Reviews 2017;17:205-28. [Google Scholar]
WHO Regional Office for Europe 2016
- WHO Regional Office for Europe. 4th meeting of the European regional verification commission for measles and rubella elimination (RVC). www.euro.who.int/__data/assets/pdf_file/0011/304958/4th-RVC-meeting-report.pdf?ua=1 (accessed prior to 2 October 2019).
WHO Strategic Plan 2012
- World Health Organization. Global measles and rubella strategic plan 2012-2020. apps.who.int/iris/bitstream/handle/10665/44855/9789241503396_eng.pdf?sequence=1 (accessed prior to 2 October 2019).
Wilson 1927
- Wilson BE. Probable inference the law of succession and statistical inference. Journal of the American Statistical Association 1927;22:209-12. [Google Scholar]
Wolfson 2009
- Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. International Journal of Epidemiology 2009;38(1):192-205. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wutzler 2017
- Wutzler P, Bonanni P, Burgess M, Gershon A, Safadi MA, Casabona G. Varicella vaccination - the global experience. Expert Review of Vaccines 2017;16(8):833-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yung 2011
- Yung CF, Andrews N, Bukasa A, Brown KE, Ramsay M. Mumps complications and effects of mumps vaccination, England and Wales, 2002-2006. Emerging Infectious Diseases 2011;17(4):661-7; quiz 766. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmerman 2019
- Zimmerman LA, Muscat M, Singh S, Ben Mamou M, Jankovic D, Datta S, et al. Progress toward measles elimination - European region, 2009-2018. MMWR. Morbidity and Mortality Weekly Report 2019;68(17):396-401. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bianco 2003
- Bianco E, Price D, Jefferson T, Demicheli V. Vaccines for measles, mumps and rubella in children. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD004407. [DOI: 10.1002/14651858.CD004407] [DOI] [PubMed] [Google Scholar]
Demicheli 2005
- Demicheli V, Jefferson T, Rivetti A, Price D. Vaccines for measles, mumps and rubella in children. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD004407. [DOI: 10.1002/14651858.CD004407.pub2] [DOI] [PubMed] [Google Scholar]
Demicheli 2012
- Demicheli V, Rivetti A, Debalini MG, Di Pietrantoj C. Vaccines for measles, mumps and rubella in children. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD004407. [DOI: 10.1002/14651858.CD004407.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


