Abstract
Purpose of review
To summarize recent trends in knowledge of HIV status, care and viral suppression, and the status of implementation of relevant contextual requirements for the United States to achieve the 90-90-90 goals. Recently, the US government announced a plan to decrease HIV incidence by over 90% by 2030. Reaching this goal may require higher targets than 90-90-90.
Recent findings
The United States is on course to reach 90-90-90 goals in the near future, with 86% of persons with HIV aware of their infection, 74% of persons with diagnosed infection in care, and 83% of persons in care with viral suppression in 2016. Some high-burden subnational jurisdictions have already achieved these goals.
Summary
The United States is likely to reach 90-90-90 targets in the near future. However, to reduce HIV incidence by at least 90% by 2030, the United States will need to rapidly meet the new 95-95-95 targets and deploy a comprehensive strategy with novel approaches to testing, retaining persons with HIV on treatment, and preventing new infections with preexposure prophylaxis and comprehensive syringe services programs.
Keywords: antiretroviral therapy, diagnosed infections, HIV care, viral suppression
INTRODUCTION
In 2014, UNAIDS set the ambitious 90-90-90 goals that worldwide by 2020, 90% of all people with HIV would have their infection diagnosed, 90% of those with diagnosed infection would be prescribed antiretroviral treatment (ART), and 90% of those treated would by virally suppressed. UNAIDS was not explicit about what this would mean in practical or public health terms, although mathematical modeling suggested that if achieved, these actions would end the HIV epidemic by 2030 [1]. The benchmarks were incorporated in modified form into the 2010 United States National HIV/AIDS Strategy when it was updated to 2020: to increase the percentage of people living with HIV who know their HIV status to at least 90%, increase the percentage of persons with diagnosed infection who are retained in HIV medical care to at least 90%, and increase the percentage of persons with diagnosed infection who are virally suppressed to at least 80% [2]. The second goal focused on retention in care because population-level data on ART prescription were not available for the United States. The US goals if achieved would produce population-based viral suppression rate of 72% comparable with the 73% overall rate achieved with 90-90-90. In 2019, the US government announced a new HIV initiative intended to decrease HIV incidence by 75% by 2025 and over 90% by 2030 [3]. Meeting these goals will require rapidly achieving high levels of diagnosed infections and viral suppression as well as substantially expand use of preexposure prophylaxis (PrEP), syringe services programs, and our capacity to detect and respond to growing clusters of infections.
To achieve these goals require an enabling policy environment (e.g. law, regulations), resources, and the implementation of evidence-based practices in the affected places and populations. Some of these crucial requirements have been in place for a number of years. Since 2006, the Centers for Disease Control and Prevention (CDC) has recommended all Americans aged 13–64 years be tested for HIV once in their lifetime with annual testing for persons at high risk for HIV infection, ideally through routine opt-out HIV screening in healthcare settings [4]. This recommendation was recently given a Grade A endorsement by the US Public Services Task Force [5]. Since 2012, US guidelines have recommended ART for all persons with HIV infection regardless of the stage of disease [6]. Access to ART and comprehensive care has been widely available for decades through public and private insurance programs as well as the Ryan White HIV/AIDS Program (RWHAP), which cares for about half of all people in the United States with diagnosed HIV [7]. Yet similar to other western countries except Sweden, despite these efforts, the United States has not fully realized the UNAIDS 90-90-90 goals [8,9], potentially because these programs and policies have not yet been implemented at full scale. Here, we review recent trends in knowledge of HIV status, care and viral suppression, and the status of implementation of relevant contextual requirements for the United States to achieve the 90-90-90 goals.
PROPORTION OF PERSONS WITH HIV WHOSE INFECTIONS HAVE BEEN DIAGNOSED
In the United States, the percentage of persons with HIV whose infections have been diagnosed increased during 2010 to 2016 from 83% [95% confidence interval (CI) 82–84) to 86% (95% CI 84–87). However, this progress was uneven when considered by race/ethnicity, HIV risk transmission category and age (Table 1). In 2016, CDC estimates that more than 90% of persons who inject drugs and persons aged 45 years or older had their infections diagnosed. Among the 43 jurisdictions with reliable estimates [relative standard error (RSE) of <30%], the percentages of diagnosed HIV infection ranged from 82% in Louisiana to 92% in Pennsylvania. Only one other state, New Jersey, has achieved the 90% diagnosis goal [10■]. However, local successes in cities heavily affected by HIV, such as San Francisco and New York, where 94 and 91% of infections have been diagnosed as of 2016 and 2017, respectively, give hope that the overall target of 90-90-90 may be reached by these other jurisdictions soon [11,12].
Table 1.
HIV prevalencea, careb, and viral suppressionb among persons aged at least 13 years, 2016, by selected characteristics, United States
| Persons living with diagnosed infection | Persons with at least one CD4 or viral load tests Among persons with diagnosed infection |
Viral load less than 200 copies/ml Among persons with at least one CD4 or viral load tests |
||
|---|---|---|---|---|
| (%) | (95% CI) | (%) | (%) | |
| Sex | ||||
| Male | 84.8 | (83.2–86.6) | 74.2 | 83.7 |
| Female | 88.8 | (86.0–91.9) | 74.0 | 80.3 |
| Age at year-end 2015 (year) | ||||
| 13–24 | 56.0 | (54.1–58.0) | 76.3 | 70.5 |
| 25–34 | 70.9 | (69.9–71.8) | 74.1 | 76.4 |
| 35–44 | 84.6 | (83.8–85.5) | 73.2 | 80.7 |
| 45–54 | 91.8 | (91.2–92.5) | 75.3 | 84.7 |
| ≥55 | 94.2 | (93.4–95.0) | 73.2 | 87.7 |
| Race/ethnicity | ||||
| American Indian/Alaska Native | 81.6 | (60.9–100.0) | 73.4 | 81.3 |
| Asian | 80.9 | (70.5–94.8) | 73.5 | 90.6 |
| Black/African American | 85.2 | (82.9–87.6) | 71.7 | 78.3 |
| Hispanic/Latino | 83.3 | (80.2–86.5) | 71.9 | 85.3 |
| Native Hawaiian/other Pacific Islander | 82.4 | (51.6–100.0) | 71.4 | 88.8 |
| White | 88.5 | (85.9–91.3) | 77.8 | 87.2 |
| Multiple races | 86.4 | (80.2–93.5) | 85.9 | 81.3 |
| Transmission category | ||||
| Male-to-male sexual contact | 83.6 | (81.7–85.6) | 75.8 | 85.0 |
| Injection drug use | 94.0 | (89.7–98.8) | ||
| Male | 93.5 | (87.7–100.0) | 63.6 | 80.6 |
| Female | 94.7 | (88.6–100.0) | 72.5 | 78.7 |
| Male-to-male sexual contact and injection drug use | 92.3 | (86.5–99.0) | 78.4 | 79.4 |
| Heterosexual contact | 85.5 | (82.7–88.4) | ||
| Male | 81.6 | (76.4–87.5) | 69.6 | 81.7 |
| Female | 87.3 | (84.2–90.6) | 74.3 | 81.2 |
| Total | 85.8 | (84.3–87.3) | 74.2 | 82.9 |
Although the median time from infection to diagnosis has decreased from 43 months in 2012 to 39 months in 2016 [13], missed opportunities for HIV testing continue, including among persons at high risk for HIV. Among MSM and persons who inject drugs (PWID) who were unaware of their infection and had not tested in the past year but received a diagnosis as part of their participation in National HIV Behavioral Surveillance, 52 and 45%, respectively, had visited a clinician during that year but had not been offered HIV testing [14]. Even focused efforts to increase testing, such as those during the HPTN 065 Test, Link-to-Care Plus Treat (TLC-Plus) study highlight the challenges of screening in emergency departments and hospitals [15]. Progress towards routine screening requires making an HV test standard of care for all adults and instituting structural changes, such as automated and centralized laboratory testing. Other options to increase access to testing include self-testing. MacGowan et al. [16] demonstrated that not only can MSM accurately perform self-testing with kits received in the mail, but that MSM can share kits with other at-risk persons in their social networks and thus support diagnosis of other at-risk persons within their socials networks who might otherwise go undiagnosed [17].
Youth are a particularly high-risk and vulnerable group with a particularly large percentage of undiagnosed infections. Neilan et al. [18■■] assessed the cost-effectiveness and impact on health outcomes of HIV screening among young people. Following CDC guidelines to screen at least once in a lifetime, these investigators determined that for persons aged 15–30 years, a single screening test at age 25 years was the ‘sweet spot’ that maximized diagnoses whereas minimizing the effects of untreated disease to individual health and onward transmission. However, if testing were restricted to this time-frame, many youth with HIV may have a delay in diagnosis. In low-incidence settings, strategies that focus on integrating HIV testing into routine age-related health screenings, such as is currently practiced in the United States to detect breast and colon cancer, merit additional research. For persons at higher risk of HIV infection, such as MSM and people who inject drugs, evidence supports the recommendation for testing at least annually, and there may be benefits of testing more frequently for some MSM [19].
CARE
Retention in care is required to achieve and maintain viral suppression but remains a challenge in the United States, albeit one that is not insurmountable. Among persons with infection diagnosed in 2017, 78% were linked to care within 1 month of diagnosis and 87% within 3 months [20■]. Among persons alive with HIV in 2017, only 74% had accessed care during 2016 when using reports of CD4+ cell counts or viral load tests as a proxy (Table 1). These data from the National HIV Surveillance System rely on complete reporting of laboratory results, deaths, and migration, and thus likely represent lower bound estimates [20■]. CDC’s Medical Monitoring Project (MMP), which provides representative estimates derived from a sample of persons with HIV in selected jurisdictions [21], may provide an upper bound estimate. On the basis of the data for participants with diagnosed HIV interviewed in the 2016 cycle in the selected jurisdictions, MMP has estimated that 97% (95% CI 96–98) received care in the past 12 months. Lastly, a survey of providers at four RWHAP-funded HIV care clinics on standard of care practices reported that 19% of patients were out of care for 6 months or longer [22■■].
Although no direct population level data are available on ART prescription in the United States, several reports offer some indirect insight. Iqbal et al. [23] analyzed insurance claims data and found that 79% of commercially insured persons with HIV had an ARV prescription in 2014, the latest year for which data were available. MMP estimated that 84% (95% CI 81–87) of persons with diagnosed HIV in 2016 had been prescribed ART in the past 12 months [19], an estimate consistent with other data on viral suppression among persons in care presented below.
VIRAL SUPPRESSION
Nance et al. [24■■] reported on the progress in the percentage of adult patients receiving care at eight clinical sites who achieved viral suppression, which increased from 32% in 1997 to 86% in 2015. CDC reported that among persons in care during 2016 (the most recent year for which data are available), viral suppression ranged from 83% for persons with at last one CD4 or viral load test result in 2016 (Table 1) and 86.4% among persons with at least one viral load test result in 2016 [21]. Among clients of the RWHAP with one care visit and at least one viral load test result in 2017, 86% were virally suppressed [7]. However, as with diagnoses in the United States, some sub-national jurisdictions have shown promising progress; specifically as of 2016, Hawaii, Iowa, Maine, Minnesota, New Hampshire, and Rhode Island have all achieved 90% viral suppression among persons in care with an additional 11 states reporting viral suppression of 85–89% (Fig. 1) [21]. Jurisdictions with focused plans to reduce HIV transmission also report high viral suppression rates, such as New York (87% among persons receiving medical care in 2017) [12] and San Francisco [70% viral suppression among all persons with HIV (diagnosed and undiagnosed infections) in 2016, close to the 73% expected for all persons with HIV for the 90-90-90 goals] [11].
FIGURE 1.
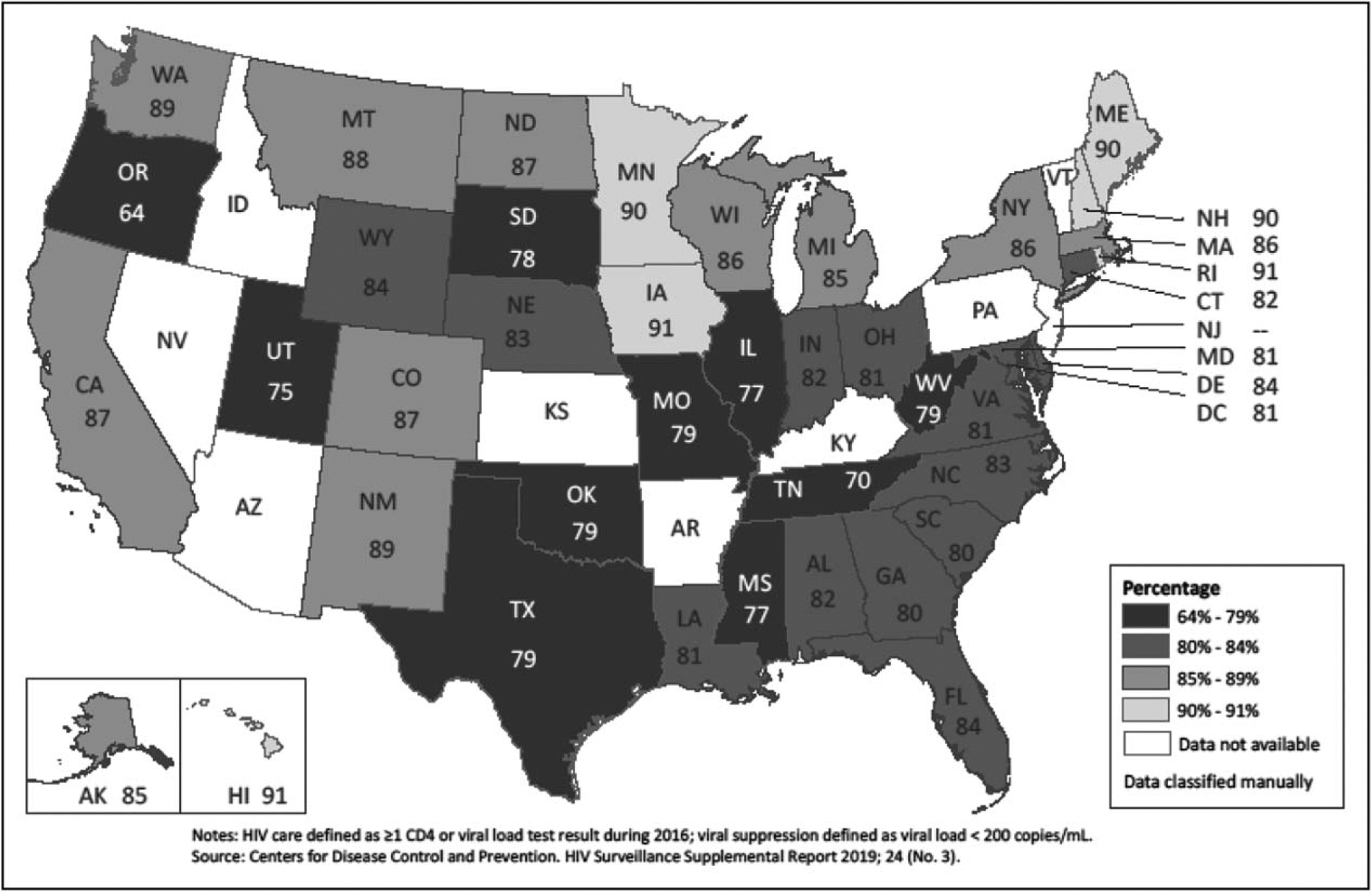
HIV viral suppression during 2016 among persons aged at least 13 years in HIV care, United States.
CDC and HRSA generally use a single assessment, such as the most recent viral load test result, to measure viral suppression in a particular year [7,20■,21]. However, viral suppression estimates based on the last viral load measured do not necessarily represent sustained suppression [25]. Using data from San Francisco, Hughes et al. [26■] attempted to quantify persistence of viral suppression over time. These investigators found that patients in care spent 12% of time during the 2-year study period with unsuppressed viral loads. Assessing persistence in maintaining 90-90-90 and other goals may provide additional, useful insight for HIV programs in the United States and abroad and merit further research.
NEW APPROACHES TO IMPROVING THE CONTINUUM OF CARE
The overall progress in testing, care and treatment outcomes in the United States is encouraging. Yet differences by risk group, race/ethnicity, and geographic region demand a closer look if we want to succeed both in reaching the 90-90-90 goals and ending the HIV epidemic in America. In the realm of testing, in addition to establishing routine opt-out testing as standard of care and expanding self-testing [16,17], other novel approaches, such as testing in community pharmacies [27] and targeted testing among risk networks [28,29] may provide access to testing services for persons not reached by current efforts.
To improve HIV care, Shaw et al. [22■■] recommend systematic use of data to allow providers to regularly review their patients’ engagement in care and adherence to ART, as well as care coordination. Another novel approach is to integrate community-based pharmacists into care services. Byrd et al. have assessed a model of on-going information sharing between pharmacists and HIV medical providers to determine barriers to care and to then develop individual care plans aimed to improve adherence and retention in care. Implementation of this approach increased viral suppression [30■■]. Combining HIV surveillance with other data, health departments can identify persons who may be out of care and work with providers to meet the patients’ service needs and re-engage them. This approach, often called Data-to-Care, has been implemented by health departments across the country as part of their federal program funding. First results reported from the Cooperative Re-Engagement Controlled Trial have shown that the Data-to-Care approach increases both re-engagement and time to re-engagement compared with standard of care [31]. A novel pharmacy-based program that flags persons when they fail to pick up ART prescriptions has helped to identify persons at risk of falling out of care even earlier [32]. Although patient navigation has traditionally been recommended to overcome barriers to linkage to and retention in care as well as viral suppression, a systematic review of the evidence concluded that more evidence is needed to determine best approaches [33■].
Time from diagnosis to viral suppression has decreased from 8 to 5 months among persons with HIV diagnosed in 2012 and 2016, respectively [34]. Viral suppression is expected to further improve as we increasingly start ART as soon possible – ideally the same day – after HIV diagnosis, a practice now recommended by US guidelines [6]. Expanded use of long-acting and more potent antiretroviral formulations is expected to further improve viral suppression by reducing barriers to adherence. Removable ART-delivery systems will likely prove preferable to modalities that cannot be removed in case of toxicity [35] and are the subject of active research in nonhuman primate and murine models at CDC [36] and elsewhere.
CONCLUSION
The United States is on course to reach 90-90-90 goals in the near future, although it is unlikely that reaching these goals will suffice for epidemic control [37,38]. UNAIDS has set new targets of 95-95-95 with ultimately at least 86% of all persons with HIV virally suppressed. UNAIDS has also established measures of epidemic control, including a 90% reduction in incidence compared with 2010 by 2030 [37,39■]. The renewed focus on rapid implementation of efforts to increase testing, treatment, and prevention of HIV infection outlined in the United States’ Ending the HIV Epidemic initiative [3] requires evermore coordinated action of public health, care providers, and affected communities that, when paired with adequate resources, will maximize the prevention and care continua. A recent modeling study predicted that a 67% reduction in HIV incidence can be reached by 2030 if 95-95-95 is achieved by 2025 with 40% PrEP coverage among MSM [38]. These authors note that ‘to substantially reduce HIV prevalence in the next decade [will require] sufficient investments and innovation’. To truly reach the United States goal of reducing HIV incidence by at least 90% by 2030 will require a comprehensive strategy including community-based, targeted, and self-testing, a cohort approach for persons with HIV to meet their service needs, PrEP for all persons at substantial risk for acquiring HIV, expanded comprehensive syringe services programs, housing stability, and detection of and response to growing clusters of infections [3,39■,40■].
KEY POINTS.
The United States is on course to reach 90-90-90 goals in the near future with some high burden subnational jurisdictions having already achieved these goals.
Robust data systems are in place to monitor progress towards achieving success.
Implementation of novel approaches for prevention, HIV testing, tracking and meeting the service needs for care and treatment of persons with HIV, and focus on identifying and interrupting HIV transmission networks will accelerate progress.
To reduce HIV incidence by at least 90% by 2030, the goal of the initiative of ending the HIV epidemic, the United States will need to rapidly meet the new 95-95-95 targets.
Financial support and sponsorship
Source of support:
US Government work.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.UNAIDS. 90-90-90 an ambitious treatment target to help end the AIDS epidemic. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. [Accessed 18 April 2019]
- 2.Office of National AIDS Policy. National HIV/AIDS strategy for the United States: Updated to 2020. Washington, DC: Office of National AIDS Policy; 2015. Available at: https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf. [Accessed 24 April 2019] [Google Scholar]
- 3.Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–845. [DOI] [PubMed] [Google Scholar]
- 4.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55(RR-14):1–17. [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force. Screening for HIV infection, US Preventive Services Task Force Recommendation Statement. JAMA 2019; 321:2326–2336. [DOI] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Accessed 1 July 2019] [Google Scholar]
- 7.Health Resources and Services Administration. Ryan White HIV/AIDS Program Annual Client-Level Data Report 2017. Available at: http://hab.hrsa.gov/data/data-reports. Published December 2018. [Accessed 1 May 2019]
- 8.Granich R, Gupta S, Hall I, et al. Status and methodology of publicly available national HIV care continua and 90-90-90 targets: a systematic review. PLoS Med 2017; 14:e1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisslen M, Svedhem V, Lindborg L, et al. Sweden, the first country to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization (WHO) 90-90-90 continuum of HIV care targets. HIV Med 2017; 18:305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.■.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019; 24 (No. 1). Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. [Accessed 24 April 2019] [Google Scholar]; This report provides the latest information on the first of the 90-90-90 goals, the percentage of persons with HIV whose infection has been diagnosed. Information on this indicator is available starting with 2010 for the United States, by demographic characteristics, risk group, and jurisdiction.
- 11.San Francisco Department of Public Health. HIV Epidemiology Annual Report 2017. September 2018. Available at: https://www.sfdph.org/dph/comupg/oprograms/HIVepiSec/HIVepiSecReports.asp. [Accessed 29 April 2019]
- 12.ETE Dashboard, Ending the AIDS Epidemic. Available at: http://etedashboardny.org/metrics/. [Accessed 26 April 2019]
- 13.Crepaz N, Song R, Hall HI. Duration of infectiousness among persons with HIV diagnosed during 2012–2016. Conference on Retroviruses and Opportunistic Infections (CROI 2019), Seattle, WA, 4–7 March 2019. [Google Scholar]
- 14.Wejnert C, Prejean P, Hoots B, et al. , NHBS Study Group. Prevalence of missed opportunities for HIV testing among persons unaware of their infection. JAMA 2018; 319:2555–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branson BM, Chavez PR, Hanscom B, et al. , HPTN 065 study. Expanding hospital HIV testing in the Bronx, New York and Washington, DC: results from the HPTN 065 study (published online 27 November 2017). Clin Infect Dis 2018; 66:1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacGowan RJ, Chavez PR, Gravens L, et al. , eSTAMP Study Group. Pilot evaluation of the ability of men who have sex with men to self-administer rapid HIV tests, prepare dried blood spot cards, and interpret test results, Atlanta, Georgia, 2013. AIDS Behav 2018; 22:111–126. [DOI] [PubMed] [Google Scholar]
- 17.Wesolowski L, Chavez P, Sullivan P, et al. Distribution of HIV self-tests by HIV-positive men who have sex with men to social and sexual contacts. AIDS Behav 2019; 23:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.■■.Neilan AM, Dunville R, Bańez Ocfemia MC, et al. The optimal age for screening adolescents and young adults without identified risk factors for HIV. J Adolesc Health 2018; 62:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]; This modeling study assessed the cost-effectiveness and impact on health outcomes of screening among young people. Following CDC guidelines of screening at least once in a lifetime, screening at age 25 years is more efficient than screening at earlier ages.
- 19.DiNenno EA, Prejean J, Delaney KP, et al. Evaluating the evidence for more frequent than annual HIV screening of gay, bisexual, and other men who have sex with men in the United States: results from a systematic review and CDC expert consultation [published online November 28, 2017]. Public Health Rep 2018; 133:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.■.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2017. HIV Surveillance Supplemental Report 2019; 24 (No. 3). Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published June 2019. [Accessed 1 July 2019] [Google Scholar]; This report provides the latest information on the third of the 90-90-90 goals, viral suppression, as well as care engagement. Information on these indicators is available for the United States, and by demographic characteristics, risk group, and jurisdiction.
- 21.Centers for Disease Control and Prevention. Behavioral and Clinical Characteristics of Persons with Diagnosed HIV Infection—Medical Monitoring Project, United States, 2016 Cycle (June 2016–May 2017). HIV Surveillance Special Report 21. Available at: https://www.cdc.gov/hiv/library/reports/hivsurveillance.html. Published February 2019. [Accessed 1 May 2019]
- 22.■■.Shaw S, Modi R, Mugavero M, et al. HIV standard of care for ART adherence and retention in care among HIV medical care providers across four CNICS clinics in the US. AIDS Behav 2019; 23:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes results from a survey of providers at four Ryan White funded HIV care clinics on standard-of-care practices. The providers reported that 19% of patients were out of care for 6 months or longer.
- 23.Iqbal K, Huang YA, Peters P, et al. Antiretroviral treatment among commercially insured persons living with HIV in an era of universal treatment in the United States - 2012–2014. AIDS Care 2018; 30:1128–1134. [DOI] [PubMed] [Google Scholar]
- 24.■■.Nance RM, Delaney JAC, Simoni JM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Ann Intern Med 2018; 169:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported on the progress in the percentage of adult patients receiving care at eight clinical sites. The percentage of persons in care who achieved viral suppression increased from 32% in 1997 to 86% in 2015.
- 25.Crepaz N, Dong X, Wang X, et al. Racial and ethnic disparities in sustained viral suppression and transmission risk potential among persons receiving HIV care — United States, 2014. MMWR Morb Mortal Wkly Rep 2018; 67:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.■.Hughes AJ. Rector A, Jimenez V, et al. Cumulative plasma HIV burden disparities among adults in HIV care: implications for HIV transmission in the era of treatment as prevention. AIDS 2018; 32:1881–1889. [DOI] [PubMed] [Google Scholar]; This study assessed the percentage of time persons with HIV in San Francisco had unsuppressed viral loads during a 2-year study period. Overall, patients in care spent 12% of the 2-year study period with unsuppressed viral loads. These findings and the authors assessment of time spent at transmittable viral loads (>1500 copies/ml) have implications for the concept of treatment as prevention.
- 27.Dugdale C, Zaller N, Bratberg J, et al. Missed opportunities for HIV screening in pharmacies and retail clinics. J Manag Care Pharm 2014; 20:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasry A, Medley A, Behel S, et al. Scaling up testing for human immunodeficiency virus infection among contacts of index patients — 20 countries, 2016–2018. MMWR Morb Mortal Wkly Rep 2019; 68:474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oster AM, France AM, Mermin J. Molecular epidemiology and the transformation of HIV prevention. JAMA 2018; 319:1657–1658. [DOI] [PubMed] [Google Scholar]
- 30.■■.Byrd KK, Hou JG, Bush T, et al. , Patient-centered HIV Care Model Team. Adherence and viral suppression among participants of the patient-centered HIV Care Model project—a collaboration between community-based pharmacists and HIV clinical providers. Clin Infect Dis; 10.1093/cid/ciz276. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed a patient-centered HIV care model where information was shared between pharmacists and HIV medical providers to determine barriers to care and develop care plans. Implementation of shared care plans increased viral suppression.
- 31.Fanfair RN, Khalil G, Camp N, et al. Health department randomized trial to re-engage out-of-care HIV infected persons. Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 4–7 March 2019. Available at: http://www.croiconference.org/sites/default/files/posters-2019/1430_Fanfair_1031.pdf. [Accessed 3 June 2019] [Google Scholar]
- 32.Kinsinger L, Savola L, Hale K, et al. ‘Link-up Rx: re-engagement in HIV care using pharmacy refill data’, National HIV Prevention Conference, Atlanta, GA, 18–21 March 2019 (Abstract #5242). [Google Scholar]
- 33.■.Mizuno Y, Higa DH, Leighton CA, et al. Is HIV patient navigation associated with HIV care continuum outcomes? A systematic review. AIDS 2018; 32:2557–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports on the first systematic review of patient navigation to improve outcomes along the HIV continuum of care. The studies reviewed showed improved linkage to care, retention in care, and viral suppression but the weak quality of some of the studies call for additional research to establish best practices.
- 34.Crepaz N, Song R, Hall HI. Duration of infectiousness among persons with HIV diagnosed during 2012–2016. Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 4–7 March 2019. [Google Scholar]
- 35.Pons-Faudoa FP, Ballerini A, Sakamoto J, et al. Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases. Biomed Microdevices 2019; 21:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovarova M, Benhabbour R, Massud I, et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun 2018; 9:4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosh KA, Brooks JT, Hall HI. HIV epidemic control in the United States—assessment of proposed UNAIDS metrics, 2010–2015. Clin Infect Dis ciz 151; 10.1093/cid/ciz151. [DOI] [PubMed] [Google Scholar]
- 38.Bradley H, Rosenberg ES, Holtgrave DR. Data-driven goals for curbing the U.S. HIV epidemic by 2030. AIDS Behav 2019; 23:557–563. [DOI] [PubMed] [Google Scholar]
- 39.■.Granich R, Gupta S, Williams B. 90-90-90, epidemic control and ending AIDS: global situation and recommendations. Int J Virol AIDS 2018; 5:043. [Google Scholar]; This article reviews the concepts of HIV control and the need for a comprehensive approach that includes HIV treatment with case management and outbreak control as well as other prevention interventions, such as preexposure prophylaxis, syringe services, and drug treatment programs.
- 40.■.Clemenzi-Allen A, Geng E, Christopoulos K, et al. Degree of housing in-stability shows independent ‘dose-response’ with virologic suppression rates among people living with human immunodeficiency virus. Open Forum Infect Dis 2018; 5:ofy035. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study assessed the relation between self-reported housing status and viral suppression among patients attending a San Francisco HIV clinic. Persons with unstable housing had substantially lower percentages of viral suppression and higher mean viral load test results compared with persons with stable housing.


