Abstract
The opposing effects on proliferation mediated by G-protein-coupled receptor isoforms differing in their COOH termini could be correlated with the abilities of the receptors to differentially activate p38, implicated in apoptotic events, or phosphatidylinositol 3-kinase (PI 3-K), which provides a source of survival signals. These contrasting growth responses of the somatostatin sst2 receptor isoforms, which couple to identical Gα subunit pools (Gαi3 > Gαi2 >> Gα0), were both inhibited following βγ sequestration. The sst2(a) receptor-mediated ATF-2 activation and inhibition of proliferation induced by basic fibroblast growth factor (bFGF) were dependent on prolonged phosphorylation of p38. In contrast, cell proliferation and the associated transient phosphorylation of Akt and p70rsk induced by sst2(b) receptors were blocked by the PI 3-K inhibitor LY 294002. Stimulation with bFGF alone had no effect on the activity of either p38 or Akt but markedly enhanced p38 phosphorylation mediated by sst2(a) receptors, suggesting that a complex interplay exists between the transduction cascades activated by these distinct receptor types. In addition, although all receptors mediated a sustained activation of extracellular signal-regulated kinases (ERK1 and ERK2), induction of the tumor suppressor p21cip1 was detected only following amplification of ERK and p38 phosphorylation by concomitant bFGF and sst2(a) receptor activation. Expression of constitutively active Akt in the presence of a p38 inhibitor enabled a proliferative response to be detected in sst2(a) receptor-expressing cells. These findings demonstrate that the duration of activation and a critical balance between the mitogen-activated protein kinase and PI 3-K pathways are important for controlling cell proliferation and that the COOH termini of the sst2 receptor isoforms may determine the selection of appropriate βγ-pairings necessary for interaction with distinct kinase cascades.
Mitogen-activated protein (MAP) kinases are proline-directed serine/threonine kinases that play important roles as mediators of cellular responses to a variety of stimuli such as growth factors, cytokines, hormones, and environmental stresses (18, 23). MAP kinases in mammalian cells have been classified into at least four subfamilies: extracellular signal-regulated kinases (ERKs), stress-activated protein kinases/c-Jun NH2-terminal kinase (SAPKs/JNK), p38 kinases, and BMK1/ERK5 (51). ERK is activated by many growth factors and cytokines and is implicated in cell growth as well as differentiation (32). Various stressors such as chemical agents and UV irradiation, tumor necrosis factor, interleukin-1, CD40 ligand, and Fas/CD95 ligand stimulate the activities of SAPKs and p38 (10, 24) which appear to play a decisive role in the control of cell death. Thus, the SAPK pathway is critical during ceramide-induced (49) and stress-induced (56) apoptosis as well as in the Daxx-mediated Fas cascade (55), whereas transfection of a constitutively active mutant of MKK3/6, the physiological activator of p38, is sufficient to induce apoptosis in PC-12 cells (53). In contrast, overexpression of ERK in NIH 3T3 cells impairs a large part of the UV-induced apoptotic response and the inhibition of ERK below a basal threshold level triggers apoptosis (2), suggesting that besides its well-established role in cell cycle progression, ERK controls survival. BMK1 is a redox-regulated kinase and phosphorylates the transcription factor MEF2C, although its physiological role has remained unclear (23).
Recently, considerable attention has also been focused on the role of phosphatidylinositol 3-kinase (PI 3-K) in protecting against apoptosis and promoting cell proliferation (21). Studies indicate that insulin supports the survival of primary cerebellar neurons by activation of the serine/threonine protein kinase Akt (also known as PKB-α) (4, 14). Akt is a widely expressed kinase that is activated by a PI 3-K-dependent mechanism (5), and it has been shown to phosphorylate and inactivate the Bcl-XL/Bcl-2-associated death promoter (11) as well as caspases (48). Another downstream component of the PI 3-K pathway required for G1 cell cycle progression is p70 S6 kinase (p70rsk) (6, 37). This kinase phosphorylates the 40S subunit of ribosomal protein S6 and is involved in the translational control of 5′-oligopyrimidine tract mRNAs. Thus, in addition to the two regulatory pathways mediated by SAPKs and p38 which culminate in apoptotic processes, the ERK and PI 3-K cascades can evoke the induction of cell survival and proliferative events.
In the present study, we have examined the ability of two G-protein-coupled receptors to differentially activate these kinase pathways in order to explain the opposing effects of the receptors on cell proliferation. The somatostatin sst2(a) and sst2(b) receptor splice variants differ only in length and composition of their intracellular COOH termini and inhibit adenylate cyclase activity with similar potency when recombinantly expressed in Chinese hamster ovary (CHO-K1) cells (38). However, initial findings revealed that only the sst2(b) receptor mediated an increase in cell number while having no effect on the proliferation induced by basic fibroblast growth factor (bFGF), which was potently inhibited following activation of sst2(a) receptors.
Whereas the pathway linking cell surface receptors to ERKs has been partially elucidated (51), the mechanism of activation of p38 and SAPKs is poorly understood. This is particularly so for members of the G-protein-coupled receptor family, which have only recently been shown to utilize these alternative MAP kinase cascades for transduction purposes. Activation of p38 (54) and JNK (7) has been demonstrated following stimulation of the Gq/G11-coupled m1 and Gi-coupled m2 muscarinic acetylcholine receptors, and the integration of signals transduced by both these MAP kinase family members appears to be necessary for the m1 muscarinic receptor to activate the c-jun promoter (31). Although the expression of the c-jun proto-oncogene is rapidly induced in response to numerous mitogens and the resulting functional activity of c-Jun proteins appears to be critical for cell proliferation, a role for SAPKs or p38 in controlling cell growth through G-protein-coupled receptors has yet to be demonstrated. Part of this study was to determine if the sst2(a) and sst2(b) receptor isoforms can differentially regulate these alternative MAP kinase cascades and thus subsequently modulate transcription factor activation, cell proliferation, and the expression of the cell cycle inhibitor p21cip1, which has been suggested to play a pivotal role (15) in mediating the well-established antiproliferative effect of somatostatin (3, 35). Changes in proliferative responses with the activation of a particular kinase cascade including that of PI 3-K were also correlated for bFGF. In addition, the time course of the kinase activity was determined. There is much evidence to suggest that the duration of ERK activity is critical for determining the proliferative outcome (32), and in every case examined thus far, only sustained ERK activation induces cytoplasmic-nuclear migration (12, 46). Prolonged stimulation of ERK will therefore have very different consequences for gene expression than will transient activation. Part of this study was thus designed to determine if the duration of the other MAP kinase cascades is similarly important for controlling proliferative events. Our data suggest that prolonged p38 MAP kinase activity plays an essential role in mediating the induction of p21cip1 and the concomitant antiproliferative function of the sst2(a) receptor isoform whereas proliferation induced by the sst2(b) receptor is dependent on Akt and a sustained ERK activity.
MATERIALS AND METHODS
Cell culture and determination of cell number.
The cDNA encoding the rat sst2(a) or sst2(b) receptors was subcloned into the mammalian expression vector pAlphaCA12 harboring a neomycin resistance gene as a selection marker, and stable cell lines expressing the recombinant receptors were prepared as described previously (38). Receptor expression was assessed by binding of 125I-Tyr11-somatostatin. The estimated Bmax values for the two clonal lines were similar, at 2.2 ± 0.6 and 1.9 ± 0.4 pmol/mg of membrane protein for CHOsst2(a) and CHOsst2(b) cells, respectively (n = 3 for both data sets). Recombinant cells were cultured in Dulbecco's modified Eagle's medium-Ham's F12 medium (1:1) containing 10% (vol/vol) fetal calf serum, 0.5 mg of G418 sulfate per ml, and 1 mM Glutamax I. To assess the effect of various treatments on cell number, the clonal lines were grown to confluence in complete medium on Thermanox coverslips. Multiple denuded areas (400 μm wide) were produced by a method described previously (40). The Perspex comb was designed so that 50% of the confluent monolayer was removed by the partial denudation process. Repopulation of the denuded areas was investigated by placing the coverslip into a fresh well containing drug or vehicle in medium without serum. Cells were harvested following incubation for 24 h by washing the coverslip in phosphate-buffered saline and adding 0.05% (wt/vol) trypsin–0.02% (wt/vol) EDTA solution for 2 to 5 min, and the single-cell suspension was counted using a Coulter Counter model Z1. Results are expressed as the mean cell number (± standard error of the mean) harvested from a single coverslip (n = 3, three replicates per test group). Statistical analysis was carried out by Student's t test.
Determination of phosphorylation changes.
To analyze changes in the phosphorylation status of the MAP kinase family members, ATF-2, p70rsk, and Akt at various stages during the repopulation processes following partial denudation, whole-cell protein extract was combined from four coverslips for each treatment group. Termination of the phosphorylation events following the appropriate investigative period was achieved by washing the clonal CHO-K1 cell monolayers in ice-cold phosphate-buffered saline before applying sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer as previously described (40). Total-cell protein for each of the extracts was measured by the microBCA (Pierce) method, and equivalent amounts of protein were electrophoretically resolved on 10% polyacrylamide gels. Following electrophoretic transfer onto nitrocellulose (pore size, 0.22 μm) using a semidry blotter, the membrane was washed briefly in Tris-buffered saline (TBS) and saturated overnight in TBS supplemented with 0.1% (vol/vol) Tween 20 and 5% (wt/vol) dried milk. For detection of the phosphorylated forms of the kinases, the nitrocellulose membrane was incubated with a 1:1,000 dilution of the antiphosphospecific antibodies (New England Biolabs, Inc.). Antibodies recognizing the kinases independent of their phosphorylation state (New England Biolabs, Inc.) were also used at a 1:1,000 dilution, except for those specific to ERK1 and ERK2 (Santa Cruz Biotechnology, Inc.), which were used at a 1:2,000 dilution (1:1 mix of ERK1 and ERK2). Primary incubations were carried out for 1 h at 22°C in TBS containing 0.1% (vol/vol) Tween 20 (TBST), and the membranes were washed five times for 10 min each in TBST. They were then incubated for 1 h at 22°C with a 1:3,000 dilution of the appropriate horseradish peroxidase-conjugated secondary antibody in TBST containing 5% (wt/vol) dried milk. Excess antibody was removed by washing as above, and immunocomplexes were visualized using enhanced chemiluminescence detection as specified by the manufacturer (Amersham Life Science). The Western blots shown are representative of three independent experiments, and each panel is taken from a single immunoblot.
Induction of p21cip1 and transient expression of active Akt and transducin.
Whole-cell protein extracts were prepared 24 h following partial denudation and analyzed by Western blotting using an anti-p21cip1 antibody (Upstate Biotechnology, Inc.) following separation on 15% polyacrylamide gels. The cDNA containing sequences corresponding to amino acids 1 to 11 of avian c-Src at the 5′ end and a Myc-His tag at the 3′ end of the mouse Akt1 open reading frame was inserted into the Klenow-blunted NheI and PmeI sites of pUSEamp (Upstate Biotechnology, Inc.). The eukaryotic expression vector pCDNA3 and that incorporating transducin cDNA were kind gifts of Alan Wise, Receptor Systems, GlaxoWellcome Medicines Research Centre, Stevenage, United Kingdom. Transfections were performed with 2 μg of DNA following complex formation with LipofectAMINE reagent as specified by the manufacturer (Life Technologies). The DNA-containing medium was removed following incubation for 3 h at 37°C, and the cells were incubated for an additional 24 h in complete medium before being transferred onto Thermanox coverslips. Gene expression using immunoblot analysis as described above was determined immediately prior to partial denudation, approximately 48 h posttransfection, using a primary-antibody concentration of 1:1,000. An appropriate antibody for monitoring expression levels of transducin was purchased from NEN Life Science Products. Expression of the myristylated, constitutively active Akt1 was determined using anti-c-Myc Tag antibody.
Identification of Gα subunits involved.
To determine if the contrasting effects on cell growth could be a consequence of the somatostatin receptor isoforms coupling to different G proteins, immunoprecipitation experiments were performed using Gα subunit-selective antibodies following somatostatin-induced labeling with [35S]GTPγS. For experiments involving immunoprecipitation of Gαs, Gα13, and Gαq/11, cell monolayers were pretreated with Bordetella pertussis toxin (100 ng/ml) for 18 h. Membrane fractions from CHOsst2(a) and CHOsst2(b) cells were prepared by Dounce homogenization in ice-cold lysis buffer (50 mM Tris HCl, 5 mM MgCl2, 10 μg of leupeptin per ml, 1 μg of soybean trypsin inhibitor per ml, 100 μg of saponin per ml, 0.2 mg of bacitracin per ml, 1 mM 4-(2-aminoethyl)benzenesulfonylfluoride [pH 7.5]). Membrane protein was adjusted to a concentration of 75 μg/50 μl of assay buffer (50 mM NaCl, 10 mM MgCl2, 10 mM HEPES [pH 7.4]) and incubated for 2 min at 30°C with [35S]GTPγS (2 nM) following a preincubation (2 min at 30°C) with somatostatin (300 nM). The reactions were terminated by the addition of 500 μl of ice-cold assay buffer and subsequent centrifugation at 18,000 × g for 5 min at 4°C. Membrane pellets were vortexed in solubilization buffer (100 mM Tris HCl, 200 mM NaCl, 1 mM EDTA, 1.25% [vol/vol] Nonidet P-40, 0.2% [wt/vol] SDS [pH 7.4]) and precleared for 1 h at 4°C with 20 μl of rabbit serum (1:100 final dilution) and then added as a 20% (vol/vol) protein G-bead suspension in solubilization buffer (without SDS and supplemented with 2% [wt/vol] bovine serum albumin and 0.1% [wt/vol] NaN3). The beads were pelleted, and 100 μl of the supernatant was added to tubes containing protein G suspension (40 μl) and a 1:200 final dilution of the Gα protein antibody, supplied by NEN Life Science Products (polyclonal EC/2 cross-reacting with Gαi3 and Gα0 and polyclonal AS/7 cross-reacting with Gαi1 and Gαi2) or Santa Cruz Biotechnology (for Gαs [K-20], Gα0 [K-20], Gα13 [A-20], and Gαq/11 [C-19]). Samples were gently agitated for 2 to 3 h at 4°C. Washed immunocomplexes were suspended in scintillant and counted. The results are expressed (arithmetic mean ± standard error of the mean) as the percent stimulation over the basal level (n = 3). Statistical analysis was by Student's t test. [35S]GTPγS (specific activity, 1,000 to 1,100 μCi/mmol) and GammaBind G Sepharose beads were from Amersham.
RESULTS
Proliferative properties of G-protein-coupled receptor isoforms.
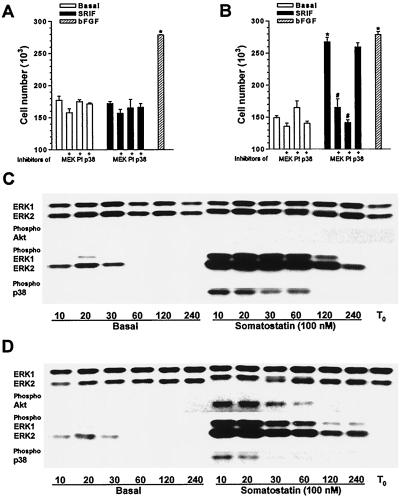
We assessed the ability of various treatments to modulate the proliferative outcome of both recombinant sst2(a) [CHOsst2(a)] and sst2(b) [CHOsst2(b)] receptor-expressing lines by determining the repopulation of denuded areas in confluent monolayers by directly counting viable cells. Parallel denuded areas were created by dragging a Perspex comb across cell monolayers grown on coverslips (40). Application of somatostatin (100 nM) immediately following denudation, in the absence of other exogenously added mitogenic factors, had no significant effect on the number of viable CHOsst2(a) cells counted 24 h later compared to the basal value (Fig. 1A). In contrast, somatostatin (100 nM) caused a significant increase in CHOsst2(b) cell numbers (Fig. 1B), which was comparable to that induced by bFGF (Fig. 1B), using a concentration (10 ng/ml) which produced 80% of its maximal response. The specific inhibitors of MEK1 and p38 kinase, PD 98059 (20 μM) and PD 169316 (10 μM), respectively, had no significant effect on basal proliferation of CHOsst2(a) cells in either the presence or absence of somatostatin (Fig. 1A). However, the increase in proliferation elicited by somatostatin in CHOsst2(b) cells was abolished by PD 98059, while PD 169316 was without effect (Fig. 1B). The PI 3-K inhibitor, LY 294002 (100 μM), also blocked the sst2(b) receptor-mediated proliferative response (Fig. 1B) while having no significant effect on basal proliferation in either the sst2(a) or sst2(b) receptor-expressing lines (Fig. 1A and B).
FIG. 1.
Effect of somatostatin on cell proliferation and the phosphorylation status of MAP kinases and Akt in CHO-K1 cells recombinantly expressing sst2(a) or sst2(b) receptors. (A and B) The number of CHOsst2(a) (A) and CHOsst2(b) (B) cells harvested from a single coverslip following incubation with 100 nM somatostatin (SRIF; solid histograms) in the presence or absence of the MEK1 inhibitor PD 98059 (20 μM) (MEK), the PI 3-K inhibitor LY 294002 (100 μM) (PI), or the p38 inhibitor PD 169316 (10 μM) (p38) 24 h after application to partially denuded cell monolayers is shown. Open histograms indicate basal repopulation; hatched histograms indicate the induced increase in cell number obtained with bFGF (10 ng/ml). Groups labeled with an asterisk are significantly different from basal (P < 0.001), and those labeled with a pound sign are significantly different from that incubated in the presence of somatostatin (P < 0.01). (C and D) Changes induced in the phosphorylation status of ERK1, ERK2, p38, and Akt during initial processes in the repopulation of partially denuded monolayers of CHOsst2(a) (C) or CHOsst2(b) (D) cells, as determined by Western analysis. Whole-cell extracts were prepared from confluent monolayers immediately following denudation (T0) and after incubation with incomplete medium (Basal) or 100 nM somatostatin for the times shown (in minutes). The consistency of protein loading was substantiated by determining the immunoreactivity of samples with phosphorylation state-independent anti-ERK antibodies. Phosphorylation changes were demonstrated by detection with an antibody to ERK1 and ERK2 that recognizes only the doubly phosphorylated (at Thr202 and Tyr204) and hence active forms. Similarly, p38 activation was assessed using an antibody specific for the doubly phosphorylated form at residues Thr180 and Tyr182 within the TGY sequence. The phosphospecific antibody for Akt recognizes this kinase only when phosphorylated at Ser473, which was shown to correlate with Akt activation. Cross-reactivity of the phosphospecific antibodies was not observed in this study.
Kinetics of ERK, p38, and Akt phosphorylation.
To correlate the effects observed on CHOsst2(a) and CHOsst2(b) cell proliferation with the activation of a particular kinase cascade, we analyzed whole-cell protein extract by Western blotting using antibodies specific for the phosphorylated and hence active forms of MAP kinases and Akt. A time course of the immunoreactivity detected with the antiphosphospecific antibodies over the initial 4 h of basal repopulation and that in the presence of somatostatin (100 nM) is shown in Fig. 1. During this period and irrespective of drug treatment, there was no detectable change in the expression of the kinases examined and the immunoreactivity obtained using phosphorylation state-independent pan antibodies to ERK1 and ERK2 was provided for both recombinant lines (Fig. 1C and D). However, electrophoretic mobility shifts for both ERK1 and ERK2 could be observed in the treatment groups where a marked change in the phosphorylation status of these proteins had occurred. Similar observations were apparent using antibodies to p38, SAPKs, and Akt in both cell lines.
Before and immediately following partial denudation, phosphorylated forms of ERK1, ERK2, p38, and Akt were undetectable in either recombinant line (Fig. 1C and D). Under basal repopulation conditions, a small and transient increase in the phosphorylation of ERK1 and ERK2 was observed for both CHOsst2(a) (Fig. 1C) and CHOsst2(b) (Fig. 1D) cells, falling to undetectable levels by 60 min postdenudation. However, no immunoreactivity was detected in either cell line using antibodies to the phosphorylated forms of p38 or Akt, at any time point investigated during the initial basal repopulation processes (Fig. 1C and D). This suggests that the partial denudation process, possibly through disruption of zonular adheren sites, can selectively trigger some signaling pathways which may contribute to basal repopulation. However, the apparent ineffectiveness of the MEK1 inhibitor on this process also suggests that multiple, parallel pathways are involved in basal repopulation and that a blockade of any individual cascade can be effectively circumvented.
Application of somatostatin (100 nM) to either CHOsst2(a) or CHOsst2(b) cells immediately after partial denudation evoked a marked increase in the phosphorylation of ERK1 and ERK2 with a maximal response at 10 min, and although this level subsequently declined, that observed at 4 h postdenudation was increased over basal (Fig. 1C and D). At no time point during the initial 4 h of repopulation processes could phosphorylated Akt be detected in CHOsst2(a) cells treated with somatostatin (Fig. 1C). In CHOsst2(b) cells, however, somatostatin induced a transient phosphorylation of Akt, which peaked 20 min following partial denudation and had declined to basal levels by approximately 2 h (Fig. 1D), suggesting that the onset of this pathway is slower than that for ERK activation. Phosphorylation of p38 by somatostatin was apparent in both recombinant lines, but temporal differences were observed between phosphorylation mediated by the different receptor types. Activation of sst2(a) receptors induced a persistent phosphorylation of p38 (Fig. 1C), whereas activation of sst2(b) receptors caused a transient phosphorylation that had declined to undetectable levels by 30 min postdenudation (Fig. 1D).
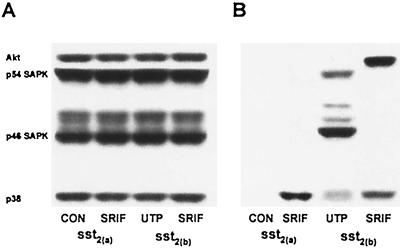
At no time point during the initial 4 h of repopulation processes could phosphorylated forms of the SAPKs be detected in either cell line treated under basal conditions or with somatostatin (100 nM) (determination following incubation for 10 min is shown in Fig. 2). Western analysis, however, revealed CHO-K1 cells to express both the p54 and p46 forms of the SAPKs (Fig. 2A). Phosphorylation of these proteins was detected following application of UTP (100 nM) for 10 min immediately after partial denudation (Fig. 2B), although there was no detectable activation of Akt by UTP at this time point, in contrast to that mediated by sst2(b) receptors, and the UTP-induced phosphorylation of p38 was not as marked as that evoked by sst2(a) receptors (Fig. 2B). It is possible that UTP is acting through endogenous P2Y2 receptors that exhibit a widespread distribution.
FIG. 2.
Changes in the phosphorylation status of SAPKs, p38, and Akt in CHO-K1 cells recombinantly expressing sst2(a) or sst2(b) receptors. Whole-cell extracts were prepared from partially denuded confluent monolayers after incubation for 10 min with incomplete medium (CON), 100 nM somatostatin (SRIF), or 100 nM UTP and analyzed by Western blotting. (A) The consistency of protein loading was substantiated by determining the immunoreactivity of samples with phosphorylation state-independent antibodies to Akt, p38, and the SAPKs. (B) Phosphorylation changes were demonstrated by detection with an antibody to p38 and Akt that recognizes only the phosphorylated and hence active forms. Similarly, SAPK activation was assessed using an antibody specific for the doubly phosphorylated forms of all SAPK isoforms at residues Thr183 and Tyr185 within the TPY sequence.
Antiproliferative function against bFGF.
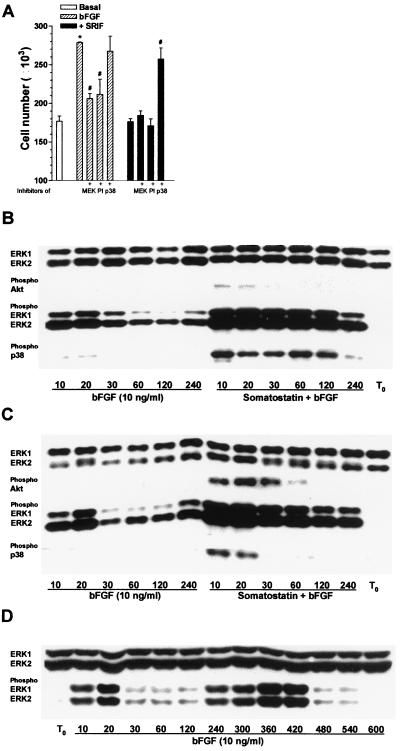
In CHOsst2(a) cells, the proliferative effect induced by bFGF (10 ng/ml) was abolished on coapplication with somatostatin (100 nM) to values not significantly different from basal (Fig. 3A) whereas activated sst2(b) receptors were without effect (data not shown). The increase in cell number induced by bFGF was partially inhibited by PD 98059 or LY 294002 and unaffected by PD 169316 (Fig. 3A). The sst2(a) receptor-mediated antiproliferative effect of somatostatin, however, was blocked by the p38 inhibitor (Fig. 3A).
FIG. 3.
Effect of somatostatin on bFGF-induced cell proliferation in CHOsst2(a) cells and the phosphorylation changes observed in MAP kinases and Akt following concomitant activation of bFGF and either sst2(a) or sst2(b) receptor isoforms. (A) The mean number of CHOsst2(a) cells harvested from a single partially denuded coverslip incubated for 24 h with bFGF (10 ng/ml) (hatched histograms) and the effect of coapplication with 20 μM PD 98059 (MEK), 100 μM LY 294002 (PI), or 10 μM PD 169316 (p38) is shown. Solid histograms show the effect of 100 nM somatostatin (SRIF) and bFGF on CHOsst2(a) cell proliferation with or without the kinase inhibitors present. The group labeled with an asterisk is significantly different (P < 0.001) from basal (open histogram), and those labeled with a pound sign are significantly different from incubation with bFGF (P < 0.01) or with bFGF in the presence of somatostatin (P < 0.001). (B and C) The time dependency of ERK1 and ERK2 phosphorylation induced by bFGF and that evoked by the combined effect of bFGF (10 ng/ml) and somatostatin (100 nM) in both CHOsst2(a) (B) and CHOsst2(b) (C) cells. Whole-cell extracts prepared from confluent monolayers immediately following partial denudation (T0) and after incubation for the times shown (in minutes) were analyzed by Western blotting. Detection of phosphorylated ERK1 and ERK2 as well as Akt and p38 is shown together with the expression levels of ERK1 and ERK2. (D) An extended time course showing the biphasic activation of ERK1 and ERK2 following incubation of partially denuded monolayers of CHO-K1 cells for the times shown (in minutes) with bFGF (10 ng/ml).
In the presence of bFGF (10 ng/ml), both the CHOsst2(a) and CHOsst2(b) cell lines showed a marked increase in the phosphorylation status of ERK1 and ERK2 (Fig. 3B and C). However, in contrast to the activation induced by somatostatin treatment, the time profile of ERK activation by the growth factor appeared biphasic; a transient increase occurred between 10 and 20 min with a second peak following between 4 and 7 h postdenudation (Fig. 3D). Concomitant application of somatostatin and bFGF to partially denuded CHOsst2(a) (Fig. 3B) or CHOsst2(b) (Fig. 3C) cells induced a strong phosphorylation of ERK1 and ERK2 with similar kinetics to that obtained in the presence of somatostatin alone. Application of bFGF to repopulating cell monolayers failed to induce an increase in the phosphorylation of either p38 or Akt at any time point investigated (Fig. 3B and C). The induced phosphorylation of Akt by somatostatin in CHOsst2(b) cells was unaffected by the presence of bFGF in terms of both the duration of the detected immunoreactivity (Fig. 3C) and its intensity (Fig. 4). Application of somatostatin and bFGF to CHOsst2(a) cells could not induce the phosphorylation of Akt (Fig. 3B), although the phosphorylation of p38 by somatostatin alone appeared to be elevated above basal levels for longer in the presence of the growth factor (Fig. 3B).
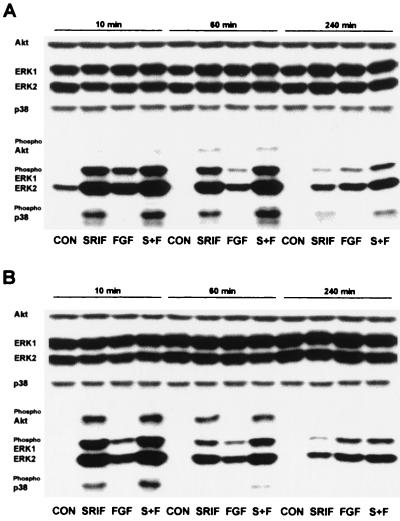
FIG. 4.
Comparison of the changes induced by somatostatin and bFGF in the phosphorylation status of ERK1, ERK2, p38, and Akt during initial processes in the repopulation of partially denuded monolayers of CHOsst2(a) (A) or CHOsst2(b) (B) cells. Analysis at 10, 60, and 240 min following partial denudation of confluent monolayers was determined by Western detection with antibodies specific to Akt, ERK1, ERK2, and p38 or those recognizing the phosphorylated and thus active forms. The immunoreactivity obtained with phosphoindependent antibodies shows that expression of the kinases remained unaffected by the various treatments or between the time points examined. Whole-cell protein extracts were prepared from partially denuded monolayers incubated in the presence of incomplete medium (CON), 100 nM somatostatin (SRIF), 10 ng of bFGF per ml (FGF), or somatostatin in the presence of bFGF (S+F).
This latter observation was confirmed by analyzing samples from the various treatment groups on the same immunoblot (Fig. 4). The detection with phosphorylation state-independent antibodies to Akt, ERK1, ERK2, and p38 shows that the expression of these kinases was unaffected by the various treatments and unchanged over the time course analyzed (Fig. 4). The intensity of the immunoreactivity detected with the anti-phospho-p38 antibody was greater at all time points examined during the initial 4 h of repopulation processes in the presence of somatostatin with bFGF than for CHOsst2(a) cells treated with somatostatin alone (Fig. 4A). This enhancement of the phosphorylation of p38 by the addition of bFGF to somatostatin-treated samples was also evident for CHOsst2(b) cells (Fig. 4B). However, in marked contrast to the prolonged activation by sst2(a) receptors, the phosphorylation of p38 induced by somatostatin in CHOsst2(b) cells, irrespective of the presence of bFGF, was transient (Fig. 4B). Figure 4 also demonstrates that for both cell lines, the level of phosphorylated ERK1 and ERK2 induced by somatostatin in the presence of bFGF was greater than that for either drug alone at all time points examined.
Requirement of prolonged p38 activity for ATF-2 activation.
To correlate the p38 dependency of the sst2(a) receptor-mediated antiproliferative effect with the observed time-related immunoreactivity changes induced by somatostatin in CHOsst2(a) and CHOsst2(b) cells, we examined the effect of PD 169316 on the phosphorylation status of activating transcription factor 2 (ATF-2), a known substrate for p38 kinase. Activation of this transcription factor requires dual phosphorylation at threonine 69 and threonine 71, enabling subsequent binding to both AP-1 and CRE DNA response elements (16). Although both SAPK and p38 MAP kinases phosphorylate ATF-2 at these sites, we can rule out any contribution from SAPKs in this study, since no observable change in the phosphorylation status of SAPKs could be detected under basal conditions or following somatostatin and bFGF treatments at any time point investigated throughout the initial 4 h of repopulation processes for either cell line (Fig. 2).
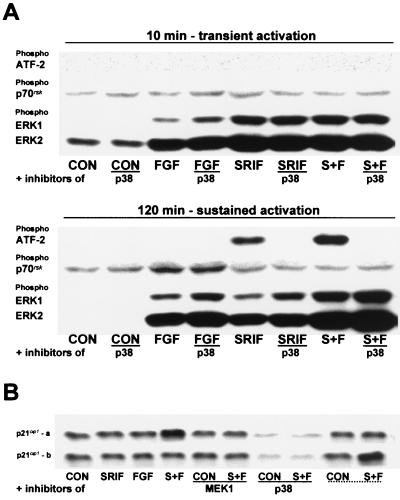
The time points investigated were chosen to represent both the transient (10 min) and sustained (120 min) phases of the kinase activity profiles. The p38 inhibitor had no effect on the phosphorylation of ERK1 or ERK2 (Fig. 5A) or of p38 (data not shown) induced by somatostatin (100 nM) in CHOsst2(a) cells in either the presence or absence of bFGF (10 ng/ml), 10 min following partial denudation. In contrast, ERK phosphorylation induced by somatostatin at 120 min was enhanced by the application of the p38 inhibitor, suggesting that cross talk between the p38 and ERK cascades exists (Fig. 5A). ATF-2 phosphorylation could not be detected under basal conditions or in the presence of bFGF at either time point examined (Fig. 5A). However, ATF-2 phosphorylation was evoked by somatostatin during the sustained phase of p38 activation and was abolished by PD 169316 (Fig. 5A). The phospho-ATF-2 immunoreactivity obtained with somatostatin was also amplified by the presence of bFGF (Fig. 5A), consistent with the enhanced phosphorylation of p38 in the presence of the growth factor. In contrast, ATF-2 phosphorylation could not be detected at either time point by somatostatin treatment in CHOsst2(b) cells (data not shown) and would be in accord with the sst2(b) receptor exhibiting only transient activation of p38.
FIG. 5.
Effect of p38 inhibition on the phosphorylation of ATF-2, p70rsk, ERK1, and ERK2 and the induction of p21cip1 following somatostatin and bFGF application to CHOsst2(a) cells. (A) The effect of 10 μM PD 169316 (p38) on the phosphorylation of ATF-2, p70rsk, ERK1, and ERK2 induced by 10 ng of bFGF per ml (FGF), 100 nM somatostatin (SRIF), or somatostatin in the presence of bFGF (S+F) at both 10 and 120 min following partial denudation of confluent monolayers of CHOsst2(a) cells is shown. Control samples incubated in incomplete medium (CON), with or without the p38 inhibitor at both time points are also shown. Detection was made by Western analysis using phosphospecific antibodies. Transcriptionally active ATF-2 requires phosphorylation of both Thr69 and Thr71, and the antibody used recognizes only this doubly phosphorylated form. The activity of p70rsk is controlled by multiple phosphorylation events. Ser411, Thr421, and Ser424 lie within a Ser-Pro-rich region located in the pseudosubstrate domain, and the antibody used detects the kinase when either Thr421 or Ser424 is phosphorylated. (B) Induction of the cell cycle inhibitor p21cip1 following activation of either sst2(a) (top panel) or sst2(b) (bottom panel) receptors. Immediately postdenudation, cell monolayers were incubated in the presence of incomplete medium (CON), 100 nM somatostatin (SRIF), 10 ng of bFGF per ml (FGF), or somatostatin and bFGF (S+F) with and without 20 μM PD 98059 (MEK1) or 10 μM PD 169316 (p38). Whole-cell protein extracts were prepared 24 h later and analyzed by an anti-p21cip1 antibody following separation on 15% polyacrylamide gels. For comparison, the last two lanes of each panel (underscored with dotted line) show the immunoreactivity obtained from the alternative cell line (i.e., the top panel shows samples from CHOsst2(b) cells and the bottom panel shows samples from CHOsst2(a) cells). Western detection was also performed with an anti-β-actin antibody to demonstrate consistency of protein loading (data not shown).
Requirement of p38 for p21cip1 induction.
There is accumulating evidence (41, 52) suggesting that high-intensity Ras stimulation in a number of cell types can evoke prolonged ERK activation leading to the induction of the cell cycle inhibitor p21cip1. Data provided in this study would also suggest that a strong and sustained activation of ERK1 and ERK2, as observed in both CHOsst2(a) and CHOsst2(b) cells, cannot alone be responsible for the induction of such an effective antiproliferative activity. In addition, ERK phosphorylation was shown to be amplified in the presence of bFGF following activation of either receptor isoform (Fig. 4), and an intense ERK activity in CHOsst2(a) cells resulted following inhibition of p38 (Fig. 5A), despite the ability of this agent to abolish the antiproliferative effect of somatostatin. We thus investigated the involvement of p38 in the induction of p21cip1. In CHOsst2(a) cells, p21cip1 protein expression was elevated over basal levels following treatment for 24 h with somatostatin (100 nM) in the presence of bFGF (10 ng/ml) (Fig. 5B), whereas in CHOsst2(b) cells no change was observed in the immunoreactivity detected with the p21cip1 antibody in the equivalent treatment group (Fig. 5B). The increased expression of p21cip1 was reduced in CHOsst2(a) cells by the MEK1 inhibitor (Fig. 5B). However, the p38 inhibitor not only abolished p21cip1 protein expression induced by somatostatin with bFGF but also reduced basal levels (Fig. 5B). There was no effect on p21cip1 protein expression levels in CHOsst2(a) or CHOsst2(b) cell lines by the application of somatostatin or bFGF alone in either the presence or absence of PD 98059 or PD 169316 (Fig. 5B).
Requirement of PI 3-K for proliferative activity.
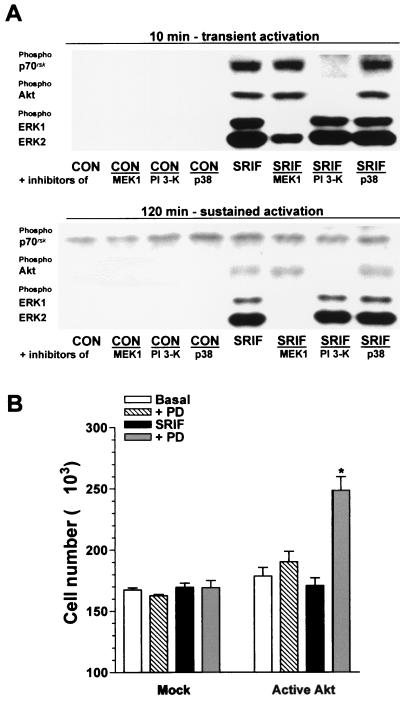
The most obvious differential transduction event between activated sst2(a) and sst2(b) receptors, which might explain their distinct proliferative functions in the absence of exogenously added mitogenic agents, is the phosphorylation of Akt, which, together with a concomitant ERK activity, may promote cell cycle progression in sst2(b) receptor-expressing cells. The somatostatin (100 nM)-induced transient phosphorylation of both Akt and p70rsk was markedly attenuated by the presence of the PI 3-K inhibitor and unaffected by PD 98059 or PD 169316 (Fig. 6A). However, the PI 3-K inhibitor had no effect on the transient or sustained phases of ERK phosphorylation mediated by sst2(b) receptors (Fig. 6A). In contrast, PD 98059 abolished the sustained ERK phosphorylation and partially inhibited the transient phase (Fig. 6A). The sst2(a) receptor had no effect on the phosphorylation of p70rsk (Fig. 5A), in keeping with its lack of effect on Akt.
FIG. 6.
Effect of protein kinase inhibitors on the somatostatin-induced phosphorylation of p70rsk, Akt, ERK1, and ERK2 in CHOsst2(b) cells and the ability of constitutively active Akt to evoke a proliferative activity in CHOsst2(a) cells. (A) The effect of coincubation for 10 and 120 min with 20 μM PD 98059 (MEK1), 100 μM LY 294002 (PI 3-K), or 10 μM PD 169316 (p38) on the phosphorylation of p70rsk, Akt, ERK1, and ERK2 induced by 100 nM somatostatin (SRIF) in partially denuded CHOsst2(b) cell monolayers. The effect of the inhibitors in the presence of incomplete medium (CON) is also shown. Detection was performed by Western analysis using phosphospecific antibodies. (B) Effect of transient expression of tagged Akt1 with c-src-derived residues required for myristylation on the number of cells determined 24 h following application of 100 nM somatostatin (SRIF; solid histograms) in the absence or presence of 10 μM PD 169316 (PD; shaded histograms) to partially denuded CHOsst2(a) monolayers. The cell counts obtained following incubation in the presence of incomplete media (Basal; open histograms) and with PD 169316 present (PD; hatched histograms) are shown, and the effect of transfection with the empty plasmid is represented by the histograms labeled Mock. Values are expressed as the mean cell number harvested from a single coverslip (from separate transfections, four replicates). The group labeled with an asterisk is significantly different from that of mock-transfected cells (P < 0.01).
To demonstrate the importance of Akt as opposed to other substrates of PI 3-K in mediating the proliferative function of the sst2(b) receptor type, we transfected CHOsst2(a) cells with a constitutively active mutant of Akt. There was no significant difference between basal cell counts determined 24 h following partial denudation of confluent CHOsst2(a) cells transfected with either pUSEamp or pUSEamp containing cDNA for tagged Akt1 with c-src-derived residues required for myristylation (Fig. 6B). Application of somatostatin (100 nM) had no significant effect compared to the basal level on the repopulation of mock-transfected cells or those expressing active Akt (Fig. 6B). However, somatostatin in the presence of the p38 inhibitor PD 169316 increased cell counts compared to those obtained with either drug alone for CHOsst2(a) cells expressing active Akt but not for mock-transfected cells (Fig. 6B). PD 169316 on its own was without effect (Fig. 6B).
Identification of the Gα protein mediating the effects of sst2(a) and sst2(b) receptors.
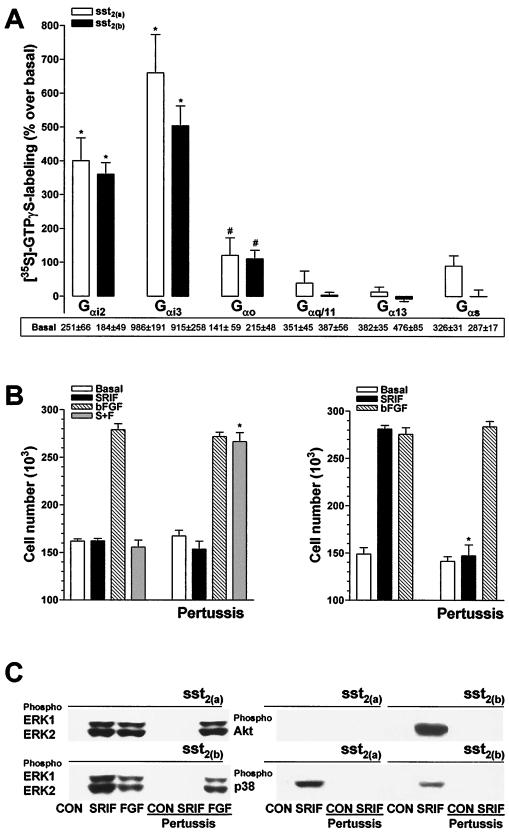
To explain the differential abilities of the somatostatin receptor isoforms to stimulate the PI 3-K and p38 pathways, we examined whether the sst2(a) or sst2(b) receptors exhibited preferential coupling to distinct Gα protein pools. This was attempted using an immunoprecipitation strategy following somatostatin-stimulated labeling of the coupled subunits with [35S]GTPγS. In the presence of 1 μM GDP, somatostatin (300 nM) increased total [35S]GTPγS (0.2 nM) binding to CHOsst2(a) membranes by 644% ± 24% over basal (pEC50 [the negative logarithm of EC50], 8.9 ± 0.1) and increased binding to CHOsst2(b) membranes by 501% ± 17% (pEC50, 8.7 ± 0.1) (n = 5 for both data sets). Optimal agonist-stimulated [35S]GTPγS binding following immunoprecipitation with Gαi1/2, Gαi3, or Gα0 antibodies could be resolved in the presence of 100 μM GDP. Binding and immunoprecipitation with antibodies specific for Gαs, Gαq/11, or Gα13 was performed in the presence of 1 μM GDP using membranes prepared from pertussis toxin-treated cells (100 ng/ml for 18 h).
There was no significant difference in the level of somatostatin (300 nM)-induced labeling between G-protein α subunits immunoprecipitated from either CHOsst2(a) and CHOsst2(b) cells, and the activated receptor isoforms showed the same preference of coupling, Gαi3 > Gαi2 >> Gα0 (Fig. 7A). It should be noted that CHO-K1 cells do not express Gαi1. In addition, both the antiproliferative and proliferative effects of the sst2(a) and sst2(b) receptor types, respectively (24 h following application of 100 nM somatostatin to partially denuded monolayers), were abolished by pertussis toxin pretreatment (100 ng/ml, 18 h), whereas the proliferative activity induced by bFGF (10 ng/ml) in either cell line was unaffected (Fig. 7B). Phosphorylation of ERK1, ERK2, p38, and Akt by somatostatin (100 nM for 10 min) at the appropriate receptor type was also blocked in cells pretreated with pertussis toxin, whereas the level of ERK1 and ERK2 phosphorylation mediated by bFGF was unaffected (Fig. 7C).
FIG. 7.
Involvement of G-protein α subunits in the mediation of the proliferative and phosphorylation effects induced by somatostatin in CHOsst2(a) and CHOsst2(b) cells. (A) Membrane preparations from either sst2(a) (open histograms) or sst2(b) (solid histograms) receptor-expressing cells were preincubated (2 min at 30°C) in the presence or absence of 300 nM somatostatin and then incubated with 2 nM [35S]GTPγS (2 min at 30°C). Subsequent immunoprecipitation of G-protein α subunits was performed using the polyclonal antibodies EC/2 (specific for Gαi3), AS/7 (cross-reacting with Gαi1 and Gαi2), K-20 (for Gαs), K-20 (for Gα0), A-20 (for Gα13), and C-19 (for Gαq/11). Results are expressed as the percent stimulation over basal values, shown as cpm under each histogram. Gαi1 could not be detected by Western analysis in CHO-K1 cells. Groups labeled with an asterisk (P < 0.001) or with a pound sign (P < 0.05) are significantly different from basal values (n = 3 or 4). (B) The effect of pertussis toxin pretreatment (100 ng/ml for 18 h) on cell proliferation induced by 100 nM somatostatin (SRIF; solid histograms), 10 ng of bFGF per ml (hatched histograms), or somatostatin in the presence of bFGF (S+F; shaded histograms), determined 24 h following application to partially denuded monolayers of either CHOsst2(a) (left) or CHOsst2(b) (right) cells. Basal proliferation in the presence of incomplete medium is shown by the open histograms. Values are expressed as the mean cell number harvested from a single coverslip (n = 3, three replicates). Groups labeled with an asterisk are significantly different from the respective treatment group for cells not pretreated with pertussis toxin (P < 0.001). (C) The effect of pertussis toxin pretreatment (100 ng/ml for 18 h) on the phosphorylation of Akt, ERK1, ERK2, and p38 in CHOsst2(a) and CHOsst2(b) cells. Western detection was performed using phosphospecific antibodies of samples prepared from whole-cell extracts of partially denuded monolayers incubated for 10 min in the presence of incomplete medium (CON), 100 nM somatostatin (SRIF), or 10 ng of bFGF per ml (FGF). Samples from cells that had been previously incubated with the toxin are underscored.
Requirement of βγ subunits.
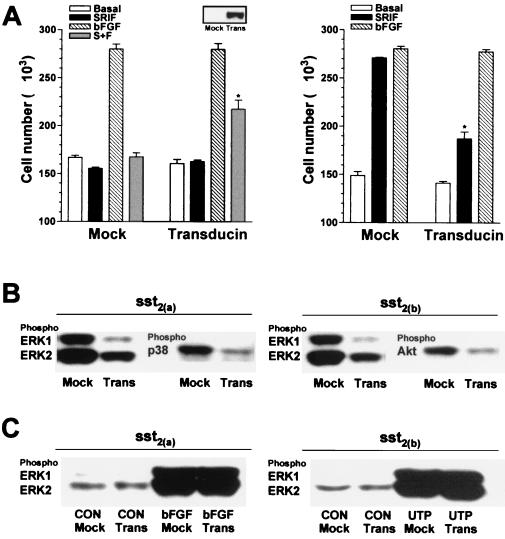
The proliferative effect of the sst2(b) receptor and the antiproliferative effect of the sst2(a) splice variant were both inhibited by overexpression of transducin (Fig. 8A), whereas the proliferative activity induced by bFGF (10 ng/ml) in either cell line was unaffected. Akt-induced phosphorylation in CHOsst2(b) cells and the somatostatin-stimulated (100 nM) phosphorylation of ERK in both recombinant lines was diminished following transducin overexpression (Fig. 8B). The induced phosphorylation of p38 mediated by sst2(a) receptors was also reduced by the expression of the βγ sequestrant (Fig. 8B). However, phosphorylation of ERK1 and ERK2 induced by both bFGF (10 ng/ml) or UPT (100 nM) 10 min following application to partially denuded monolayers was unaffected by transducin overexpression (Fig. 8C).
FIG. 8.
Involvement of βγ subunits in the mediation of the proliferative and phosphorylation effects induced by somatostatin in CHOsst2(a) and CHOsst2(b) cells. (A) The effect of transient expression of the βγ sequestrant transducin on cell proliferation induced by 100 nM somatostatin (SRIF; solid histograms), 10 ng of bFGF per ml (hatched histograms), or somatostatin in the presence of bFGF (S+F; shaded histograms), determined 24 h following application to partially denuded monolayers of either CHOsst2(a) (left graph) or CHOsst2(b) (right graph) cells is shown. The effect of transfection with the empty plasmid, pCDNA3, is represented by the histograms labeled Mock, and open histograms show basal repopulation. Values are expressed as the mean cell number harvested from a single coverslip (from two separate transfections, three replicates). Groups labeled with an asterisk are significantly different from the respective treatment group for cells without transducin expression (P < 0.05). Cell samples extracted immediately prior to partial denudation that had been transfected 48 h previously with either pCDNA3 (Mock) or pCDNA3 incorporating transducin cDNA (Trans) were analyzed by Western detection using an appropriate antibody to confirm expression of transducin (inset). (B) The effect of βγ sequestration on somatostatin-induced phosphorylation of ERK1, ERK2, and p38 in CHOsst2(a) cells or of ERK1, ERK2, and Akt in CHOsst2(b) cells. Western detection was performed using phosphospecific antibodies of samples prepared from whole-cell extracts of partially denuded monolayers incubated in the presence of 100 nM somatostatin for 10 min. Samples from cells transfected with the empty plasmid 48 h prior to partial denudation are labeled Mock, and those from cells expressing transducin are labeled Trans. (C) The effect of βγ sequestration on the phosphorylation of ERK1 and ERK2 stimulated by 10 ng of bFGF per ml in CHOsst2(a) cells or by 100 nM UTP in CHOsst2(b) cells following incubation for 10 min immediately after partial denudation of confluent monolayers. Western detection was performed using phosphospecific anti-ERK antibodies of samples prepared from whole cells that had been either transfected with the empty plasmid 48 h prior to partial denudation (Mock) or transfected to overexpress transducin (Trans).
DISCUSSION
Upon ligand stimulation, G-protein-coupled receptors transduce their effects through both the GTP-bound Gα and the dissociated Gβγ component of the heterotrimeric G protein, directly regulating downstream effectors (33) including adenylate cyclases, phospholipase C isoforms, ion channels, PI 3-K (42), and Tec family tyrosine kinases (25). Several G-protein-coupled receptors, including the somatostatin sst1 (15) and sst4 (39) receptor types, stimulate the ERK pathway through a variety of G-protein subunits (17). For m1 muscarinic acetylcholine and α1-adrenergic receptors, the activation of ERK is mediated by Gαq/11. In contrast, Gi-coupled m2 muscarinic acetylcholine, α2-adrenergic and somatostatin sst4 receptors, and the Gs-coupled β-adrenergic receptor all induce ERK activation through Gβγ. In this report, we demonstrate that both sst2 splice variants similarly stimulate ERK through βγ release from a pertussis toxin-sensitive G protein.
Many studies suggest that a signal transduction pathway from Gβγ to ERK starts at the direct activation of PI 3-Kγ (26), which increases the activities of Src family tyrosine kinases (13, 50), in turn leading to tyrosine phosphorylation of Shc (28). Subsequent recruitment of the Grb2-Sos complex to plasma membranes promotes the exchange of GDP with GTP on Ras and activates a sequential kinase cascade that includes Raf, MAP kinase kinase (MEK), and ERK. Data from this study suggest that the sst2(a) and sst2(b) isoforms have differential abilities to activate PI 3-K, in that Akt and p70rsk phosphorylation was observed following sst2(b) but not sst2(a) receptor stimulation. However, it should be noted that bFGF also failed to phosphorylate Akt, although its proliferative function was partially dependent on a PI 3-K activity. This suggests that the PI 3-K required for bFGF-induced proliferation, in contrast to that for sst2(b) receptors, is not able to stimulate the phosphoinositide-dependent kinase present in CHO-K1 cells and required for Akt activation (1). The growth factor-stimulated PI 3-Kα and the G-protein-coupled receptor-activated PI 3-Kγ forms (42) both have protein kinase activity in addition to their lipid kinase function, and it is possible that distinct signals may be generated through differential activation of their intrinsic kinase domains.
The observed blockade of ERK and PI 3-K by the respective inhibitors in CHOsst2(b) cells is consistent with the dependency of the proliferative function mediated by this receptor type on both these effector activities. However, the lack of effect of LY 294002 on the somatostatin-induced ERK phosphorylation and the ineffectiveness of PD 98059 on Akt and p70rsk activation suggest that these kinase cascades activated by the sst2(b) receptor are parallel but distinct. This is in contrast to the cross talk that has been demonstrated between the ERK and PI 3-K pathways for other G-protein-coupled receptors (39, 43). Although, ERK and PI 3-K activities are critical for somatostatin to induce a proliferative function in CHOsst2(b) cells, it appears that a cooperative effect from both cascades is required since the abolition of either prevents an increase in cell number. The partial dependency on both PI 3-K and ERK activities for the growth factor-induced proliferative response, in contrast to that mediated by sst2(b) receptors, is consistent with the ability of the bFGF receptor to recruit a multitude of secondary effectors and initiate a number of distinct yet parallel signaling pathways with noncooperative functional responses.
The proliferative effect of both the sst2(b) and bFGF receptors was unaffected following inhibition of p38 MAP kinase. In addition, the sst2(b) receptor induced only a transient activation of p38 and the growth factor receptor had no effect on the activity status of either the SAPKs or p38. There are very few reports demonstrating an activation of p38 through bFGF receptors. Its activation has been implicated in bFGF-mediated tube formation by endothelial cells (44) and in bFGF-induced interleukin-6 synthesis in osteoblasts (22) but not in the mechanism controlling neurite outgrowth (36). A transient activation of p38 by bFGF has been shown to occur in PC12 cells, whereas a stronger and more sustained activation has been observed in fibroblasts (30). Here we show that the sst2(a) receptor can induce a marked and sustained phosphorylation of p38, and its antiproliferative function against bFGF-induced growth was critically dependent on this kinase activity. Both the antiproliferative effect and the induced p38 phosphorylation were mediated through Gβγ release, consistent with the demonstration that Gβγ can stimulate p38 activity in HEK293 cells (54) and JNK activity in COS-7 cells (7, 27). The inability of sst2(a) receptors to mediate a proliferative effect in the presence of the p38 inhibitor despite the induced high-intensity ERK stimulation suggests that Akt activation is essential for somatostatin-induced proliferation. This was supported by the demonstration that transient expression of constitutively active Akt in sst2(a) receptor-expressing cells enabled a proliferative function to be detected in response to somatostatin, providing that the p38 cascade was blocked.
An interesting observation from this study was the enhanced phosphorylation of both ERK and p38 MAP kinase by the concomitant effect of somatostatin and the growth factor. Since bFGF and sst2 receptors have the capacity to stimulate ERK1 and ERK2, the amplification of this signal as observed in the presence of both ligands was perhaps expected. However, the mechanism by which bFGF increases the intensity of somatostatin-induced p38 phosphorylation is unclear. It is possible that bFGF may inhibit members of the dual-specificity phosphatase family which reverse MAP kinase activities, enabling high-intensity signals to be observed for both p38 and ERK in the presence of somatostatin. However, despite the amplification of somatostatin-induced p38 by bFGF in CHOsst2(b) cells, the time profile of its activity status remained transient (>30 min), in marked contrast to the sustained p38 activity induced by sst2(a) receptors (<4 h). An enhancement of the prolonged phosphorylation of ERK induced by somatostatin through the sst2(a) receptor was also observed by inhibiting p38, suggesting that cross talk between the p38 and ERK cascades exists. Taken together, these data demonstrate that a complex interplay exists not only between the transduction cascades activated by a single receptor type but also between those activated by distinct receptors types.
Further examples of the influence of stimulating two receptor types on the net activity of a particular signaling pathway were also demonstrated in this study for the induction of the cell cycle inhibitor p21cip1 and the level of activation of the transcription factor ATF-2. The increased expression of p21cip1 required a sustained activation of both p38 and ERK with a critical signal strength that was provided in this system by the cooperative effects of both the growth factor and sst2(a) receptor activities. The importance of a sustained p38 activity in mediating the induction of p21cip1 was further supported by the lack of effect on the expression of this protein by activated sst2(b) receptors in the presence of bFGF. This transduction network combination evoked only transient activation of p38, although a sustained ERK activity was observed, and the inclusion of the PI 3-K inhibitor to prevent any involvement by this kinase also failed to induce p21cip1 (data not shown). This is the first report of p38 MAP kinase being involved in the induction of this cell cycle inhibitor, and it is possible that the antiproliferative function of sst2(a) receptors is mediated through this pathway. However, in addition to the sustained activity of p38, ERK is necessary for the increased expression of p21cip1.
Although this study highlights a correlation between the induction of p21cip1 and the activity status of ATF-2, we have not demonstrated a direct involvement of this transcription factor in the regulation of the cell cycle inhibitor protein. However, it was apparent, as shown for the induction of p21cip1, that a prolonged activation of p38 was also required to phosphorylate ATF-2, since this transcription factor was not stimulated by either sst2(b) or bFGF receptors. An increase in the activity of ATF-2 was observed only during the sustained phase of p38 phosphorylation and was abolished on application of the p38 inhibitor. The activity of ATF-2 was also amplified by the presence of bFGF, consistent with the increased stimulation of p38 by the combined effects of the sst2(a) and growth factor receptors. These data suggest that varying the duration of the p38 stimulus can induce differential transcription factor activation. Phosphorylation of ATF-2 and inhibition of the growth factor-induced proliferative response by somatostatin are both critically dependent on p38 activity, suggesting that the prolonged p38 activity mediated by sst2(a) receptors and not sst2(b) receptors can account for their differential antiproliferative effects.
The contrasting growth responses evoked by the sst2 splice variants can be correlated with their abilities to differentially activate the p38 or Akt pathways, possibly as a consequence of coupling to distinct G-protein pools. The effects on cell growth and the induced changes in the phosphorylation status of ERK1, ERK2, p38, and Akt by the respective somatostatin receptor types were all abolished following pertussis toxin pretreatment, suggesting that the receptor isoforms coupled to Gi proteins. The somatostatin-activated receptor isoforms also exhibited the same preference of coupling to Giα3 over Giα2 subunits, with no significant coupling to Gαs, Gαq/11 or Gα13. It thus seems unlikely that different α subunit coupling can account for the diversity of transductional and functional responses exhibited by these receptor types. However, since all the distinct effects mediated by the splice variants were antagonized by overexpression of transducin, it remains possible that coupling to Gαi3 with different βγ partners may allow the receptor types to selectively activate transduction pathways as well as those that are common to both receptors such as adenylate cyclase inhibition.
Recent reports have suggested a role for chronic ERK activation in mediating the exit from the cell cycle and cellular differentiation (8, 45), whereas in other cell types it is associated with proliferation (9). Such observations indicate the importance of a sustained or transient activation of this particular transduction pathway, as well as cell phenotype, in determining the functional outcome (32). We show in this study that the duration of the p38 MAP kinase cascade, in addition to that of ERK activation, is also critical for dictating functional responses. The p38 and ERK cascades exhibit negative cross talk that may have significant consequences for regulating cellular processes, and the contribution of other input signals, such as that from bFGF receptors, can generate large differences in transcriptional events and subsequent protein expression (Fig. 9). The induction of p21cip1, for example, requires a critical signal strength from the p38 and ERK cascades mediated by the interplay of bFGF and sst2(a) receptor activation, although it has been shown that when Rho is active, induction of p21cip1 by Ras is suppressed (34). The dependency on p38 for p21cip1 expression also suggests that p38 activity may play a dual role not only in mediating apoptotic processes but also as an inhibitor of cell proliferation. This is analogous to that of ERK activation, which can promote mitogenesis as well as providing protection against apoptosis (2). The expression of p21cip1 is transcriptionally regulated by p53 and its function is critical for p53-dependent G1 growth arrest (19). The p53 gene is mutated in approximately half of all human cancers (47), and it is possible that activation of sst2(a) receptors in certain tumors may not result in the induction of this potent antiproliferative activity. This could perhaps explain the poor effects of somatostatin analogues in treating the growth of some cancer cells in the clinical setting (29).
FIG. 9.
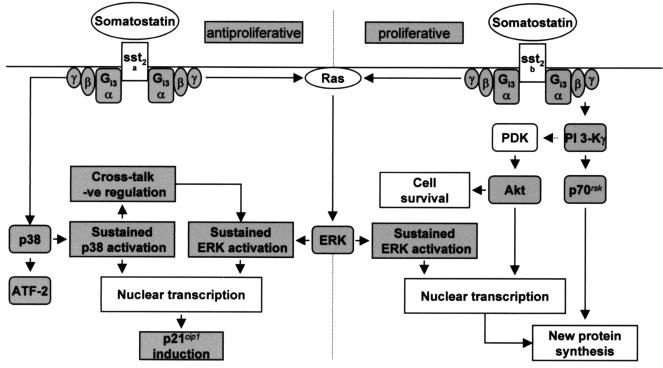
The transduction pathways activated by recombinant somatostatin sst2(a) or sst2(b) receptor isoforms and proposed mechanism which determines the proliferative fate of the host cell. G-protein-coupled receptors and receptor tyrosine kinases stimulate mitogenesis in part via ERKs, which are members of the MAP kinase family. It is becoming evident that many of the same intermediates as those utilized by the receptor tyrosine kinases are involved in the mechanism of ERK activation by G-protein-coupled receptors. For example, ERK1 and ERK2 are regulated by Gi-coupled receptors through a Ras-dependent pathway by stimulating the recruitment of the guanine nucleotide exchange factor, SOS, into a plasma membrane-associated signaling complex, where it activates Ras by catalyzing GTP-for-GDP exchange. This recruitment is the consequence of receptor-mediated stimulation of tyrosine protein kinases, which phosphorylate adapter proteins including Shc, followed by the Grb-2-mediated docking of SOS to the plasma membrane. Considerable evidence supports the role of the Src family kinases in the Gi-mediated stimulation of ERK1 and ERK2 through a mechanism dependent on Gβγ release. Activation of the other MAP kinase pathways, such as p38, also involves a cascade of kinases downstream from Ras family members such as Rac, but little is known about the mechanism by which G-protein-coupled receptors activate these alternative signaling pathways. The proliferative response mediated via the sst2(b) receptor requires a sustained activation of ERK1 and ERK2 that is βγ sensitive. However, the proliferative effect is additionally dependent on a parallel and distinct PI 3-K pathway, which is also mediated by βγ release. Substrates of PI 3-K such as p70rsk, important for protein synthesis and cell cycle progression, and Akt, which affords protection against apoptotic processes, are both phosphorylated by activated sst2(b) receptors. The sustained activation of ERK mediated by the sst2(a) receptor, together with a βγ-dependent prolonged activation of p38, which activates the transcription factor ATF-2, is required to inhibit the cell growth induced by bFGF. Amplification of these MAP kinase cascades by the cooperative effects of the bFGF and sst2(a) receptors enables the induction of p21cip1, which interacts with cyclin-dependent kinases associated with cyclins A, D1, D2, D3, and E to inhibit cyclin-dependent kinase activity and thus block cell cycle progression.
The switch from an antiproliferative to a proliferative activity, as observed for the sst2(b) receptor, appears to be the consequence of poor coupling to the p38 cascade and the selective activation of PI 3-K (Fig. 9). Since the difference between the sst2 receptor isoforms is restricted to their COOH termini, it would imply that this region determines the selection of the appropriate βγ pairings necessary for interaction with the distinct kinase cascades; importantly, these results also demonstrate that even more marked functional outcomes can be derived from the small differences in receptor isoforms than has hitherto been shown (20).
ACKNOWLEDGMENTS
We express our gratitude to John Scott (Vollum Institute) and Peter Parker (ICRF) for helpful comments.
REFERENCES
- 1.Alessi D R, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 2.Berra E, Municio M M, Sanz L, Frutos S, Diaz-Meco M T, Moscat J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol Cell Biol. 1997;17:4346–4354. doi: 10.1128/mcb.17.8.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brevini T A L, Bianchi R, Motta M. Direct inhibitory effect of somatostatin on the growth of the human prostatic cancer cell line LNCaP: possible mechanism of action. J Clin Endocrinol Metab. 1993;77:626–631. doi: 10.1210/jcem.77.3.7690358. [DOI] [PubMed] [Google Scholar]
- 4.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 5.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 6.Chou M M, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 7.Coso O A, Teramoto H, Simonds W F, Gutkind J S. Signaling from G protein-coupled receptors to c-Jun kinase involves βγ subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 8.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 9.Cressman D E, Greenbaum L E, DeAngelis R A, Ciliberto G, Furth E E, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 10.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 11.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 12.Dikic I, Schlessinger J, Lax I. PC12 cells overexpressing the insulin receptor undergo insulin-dependent neuronal differentiation. Curr Biol. 1994;4:702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 13.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 14.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 15.Florio T, Yao H, Carey K D, Dillon T J, Stork P J S. Somatostatin activation of mitogen-activated protein kinase via somatostatin receptor 1 (SSTR1) Mol Endocrinol. 1999;13:24–37. doi: 10.1210/mend.13.1.0224. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 17.Gutkind J S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 18.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 19.Kachnic L A, Wu B, Wunsch H, Mekeel K L, DeFrank J S, Tang W, Powell S N. The ability of p53 to activate downstream genes p21(WAF1/cip1) and MDM2, and cell cycle arrest following DNA damage is delayed and attenuated in scid cells deficient in the DNA-dependent protein kinase. J Biol Chem. 1999;274:13111–13117. doi: 10.1074/jbc.274.19.13111. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick G J, Dautzenberg F M, Martin G R, Eglen R M. 7TM receptors: the splicing on the cake. Trends Pharmacol Sci. 1999;20:294–301. doi: 10.1016/s0165-6147(99)01355-3. [DOI] [PubMed] [Google Scholar]
- 21.Klippel A, Escobedo M A, Wachowicz M S, Apell G, Brown T W, Giedlin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozawa O, Tokuda H, Matsuno H, Uematsu T. Involvement of p38 mitogen-activated protein kinase in basic fibroblast growth factor-induced interleukin-6 synthesis in osteoblasts. J Biol Chem. 1999;74:479–485. [PubMed] [Google Scholar]
- 23.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bio Essays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 24.Kyriakis J M, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 25.Langhans-Rajasekaran S A, Wan Y, Huang X-Y. Activation of Tsk and Btk tyrosine kinases by G protein βγ subunits. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Ilasaca M, Crespo P, Pellici P G, Gutkind J S, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinaseγ. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Ilasaca M, Gutkind J S, Wetzker R. Phosphoinositide 3-kinaseγ is a mediator of the Gβγ-dependent Jun kinase activation. J Biol Chem. 1998;273:2505–2508. doi: 10.1074/jbc.273.5.2505. [DOI] [PubMed] [Google Scholar]
- 28.Luttrell L M, Della Rocca G J, van Biesen T, Luttrell D K, Lefkowitz R J. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 29.Macaulay V M, Smith I E, Everard M J, Teale J D, Reubi J C, Millar J L. Experimental and clinical studies with somatostatin analogue octreotide in small cell lung cancer. Br J Cancer. 1991;64:451–456. doi: 10.1038/bjc.1991.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher P. p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J Biol Chem. 1999;274:17491–17498. doi: 10.1074/jbc.274.25.17491. [DOI] [PubMed] [Google Scholar]
- 31.Marinissen M J, Chiariello M, Pallante M, Gutkind J S. A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol Cell Biol. 1999;19:4289–4301. doi: 10.1128/mcb.19.6.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 33.Neer E J. Heterotrimeric G proteins: organisers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 34.Olson M F, Paterson H F, Marshall C J. Signals from Ras and Rho GTPases interact to regulate expression of p21WAF1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 35.Pagliacci M C, Tognellini R, Grignani F, Nicoletti I. Inhibition of human breast cancer cell (MCF-7) growth in vitro by the somatostatin analog SMS 201-995: effects on cell cycle parameters and apoptotic cell death. Endocrinology. 1991;129:2555–2562. doi: 10.1210/endo-129-5-2555. [DOI] [PubMed] [Google Scholar]
- 36.Perron J C, Bixby J L. Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol Cell Neurosci. 1999;13:362–378. doi: 10.1006/mcne.1999.0753. [DOI] [PubMed] [Google Scholar]
- 37.Price D J, Grove J R, Calvo V, Avruch J, Bierer B E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- 38.Schindler M, Kidd E J, Carruthers A M, Wyatt M A, Jarvie E M, Sellers L A, Feniuk W, Humphrey P P A. Molecular cloning and functional characterisation of a rat somatostatin sst2(b) receptor splice variant. Br J Pharmacol. 1998;125:209–217. doi: 10.1038/sj.bjp.0702064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellers L A. Prolonged activation of extracellular signal-regulated kinase by a protein kinase C-dependent and N17Ras-insensitive mechanism mediates the proliferative response of Gi/0-coupled somatostatin sst4 receptors. J Biol Chem. 1999;274:24280–24288. doi: 10.1074/jbc.274.34.24280. [DOI] [PubMed] [Google Scholar]
- 40.Sellers L A, Feniuk W, Humphrey P P A, Lauder H. Activated G protein-coupled receptor induced tyrosine phosphorylation of STAT3 and agonist-selective serine phosphorylation via sustained stimulation of mitogen-activated protein kinase: resultant effects on cell proliferation. J Biol Chem. 1999;274:16423–16430. doi: 10.1074/jbc.274.23.16423. [DOI] [PubMed] [Google Scholar]
- 41.Sewing A, Wiseman B, Lloyd A C, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21cip1. Mol Cell Biol. 1997;17:5588–5597. doi: 10.1128/mcb.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens L R, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka A S, Thelen M, Cadwallader K, Tempst P, Hawkins P T. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 43.Takeda H, Matozaki T, Takada T, Noguchi T, Yamao T, Tsuda M, Ochi F, Fukunaga K, Inagaki K, Kasuga M. PI 3-kinaseγ and protein kinase Cξ mediate Ras-independent activation of MAP kinase by a Gi protein-coupled receptor. EMBO J. 1999;18:386–395. doi: 10.1093/emboj/18.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka K, Abe M, Sato Y. Roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the signal transduction of basic fibroblast growth factor in endothelial cells during angiogenesis. Jpn J Cancer Res. 1999;90:647–654. doi: 10.1111/j.1349-7006.1999.tb00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tombes R M, Auer K L, Mikkelsen R, Valerie K, Wymanns M P, Marshall C J, McMahon M, Dent P. The mitogen-activated protein (MAP) kinase cascade can either stimulate or inhibit DNA synthesis in primary cultures of rat hepatocytes depending upon whether its activation is acute/phasic or chronic. Biochem J. 1998;330:1451–1460. doi: 10.1042/bj3301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traverse S, Seedorf K, Paterson H, Marshall C J, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 47.Ullrich S J, Sakaguchi K, Lees-Miller S P, Fiscella M, Mercer W E, Anderson C W, Appella E. Phosphorylation at Ser-15 and Ser-392 in mutant p53 molecules from human tumours is altered compared to wild-type p53. Proc Natl Acad Sci USA. 1993;90:5954–5958. doi: 10.1073/pnas.90.13.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaux D L. CED-4: the third horseman of apoptosis. Cell. 1997;90:389–390. doi: 10.1016/s0092-8674(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 49.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 50.Wan Y, Kurosaki T, Huang X Y. Tyrosine kinases in activation of the MAP kinase cascade by G protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- 51.Widmann C, Gibson S, Jarpe M B, Johnson G L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 52.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 54.Yamauchi J, Nagao M, Kaziro Y, Itoh H. Activation of p38 mitogen-activated protein kinase by signaling through G protein-coupled receptors. Involvement of Gβγ and Gαq/11 subunits. J Biol Chem. 1997;272:27771–27777. doi: 10.1074/jbc.272.44.27771. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanke B W, Boudreau K, Reubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]