Abstract
Objective:
Obesity and dysmetabolism are major risk factors for atrial fibrillation (AF). Expansion of fat depots is associated with increased circulating total non-esterified fatty acids (NEFAs), elevated levels of which are associated with incident AF. We undertook comprehensive serum measurement of individual NEFA to identify specific associations with new-onset AF late in life.
Methods:
The present study focused on participants with available serum and free of AF selected from the Cardiovascular Health Study, a community-based longitudinal investigation of older US adults. Thirty-five individual NEFAs were measured by gas chromatography. Cox regression was used to evaluate the association of individual NEFAs with incident AF.
Results:
The study sample included 1,872 participants (age 77.7±4.4). During median follow-up of 11.3 years, 715 cases of incident AF occurred. After concurrent adjustment of all NEFAs and full adjustment for potential confounders, higher serum concentration of nervonic acid (24:1n-9), a long-chain monounsaturated fatty acid, was associated with higher risk of AF (HR per SD: 1.18; 95% CI: 1.08–1.29; p<0.001). Conversely, higher serum concentration of gamma-linolenic acid (18:3n-6), a polyunsaturated n-6 fatty acid, was associated with lower risk of AF (HR per SD: 0.81; 95% CI: 0.71–0.94; p=0.004). None of the remaining NEFAs was significantly associated with AF.
Conclusions:
Among older adults, serum levels of non-esterified nervonic acid were positively associated, while serum levels of non-esterified gamma-linolenic acid were inversely associated, with incident AF. If confirmed, these results could offer new strategies for AF prevention and early intervention in this segment of the population at highest risk.
Keywords: atrial fibrillation, fatty acids, epidemiology
Journal Subject Terms: Atrial Fibrillation, Metabolism, Epidemiology
Introduction
The prevalence of atrial fibrillation (AF) is expected to more than double in the next 3 decades in parallel with aging of the global population.1 The arrhythmia has been categorized as an epidemic, with major implications for the public health in view of its attendant risks of hospitalization, stroke and cognitive impairment, heart failure, and mortality. Soaring rates of obesity are likewise of growing concern worldwide. Among its other adverse effects, obesity is a major modifiable risk factor for AF, accounting for nearly 20% of AF cases.1 There are multiple mechanisms by which obesity can promote AF, such as increased left atrial size and pressure, induction of inflammation, and development of other comorbid conditions that themselves foster AF, such as sleep apnea and hypertension.2 In addition, there are direct effects of visceral and cardiac fat depots which secrete bioactive factors, including non-esterified fatty acids (NEFAs).3
NEFAs play an important role in normal metabolism, serving as the key oxidative fuel for much of the body, and particularly the myocardium. Total circulating NEFA levels have been associated with various outcomes, including incident AF4 and glucose dysregulation.5 The mechanisms by which derangements in NEFA metabolism contribute to these outcomes is a matter of active study. Elucidating the associations of total circulating NEFA with AF depends on studying the contributions of individual NEFAs in order to pinpoint specific determinants and isolate potential therapeutic targets.
Prospective studies of individual fatty acids and cardiovascular outcomes have largely focused on plasma phospholipids, esterified fatty acids that reflect membrane composition rather than the balance between organ-tissue uptake, synthesis and release. Previous measurement of circulating phospholipids, or of both esterified and non-esterified fatty acids, showed individual long-chain n-3 polyunsaturated fatty acids (PUFA) to relate to lower risk of AF in some,6,7 but not all,8,9 studies. Conversely, plasma-phospholipid long-chain saturated fatty acids (SFA) were linked to higher risk of incident AF.10
Available data on individual NEFAs and AF come from a small lipidomic study that compared patients with persistent AF who presented for cardioversion with age-, sex- and body-mass index-matched controls. Circulating levels of 18 individual NEFAs were significantly lower in the AF group compared to the control group, yet within the AF group, NEFA levels did not relate to likelihood of AF recurrence.11 To date, no study has examined the association of individual circulating NEFAs with development of AF in older adults, the highest-risk group. To advance our previous work on total NEFA,4 we undertook comprehensive serum measurement of individual NEFAs in the Cardiovascular Health Study to identify specific associations with new-onset AF late in life.
Methods
Study Population
CHS is a prospective cohort of adults ≥65 years from 4 US locations: Sacramento County, CA; Washington County, MD; Forsyth County, NC; and Allegheny County, PA. Random Medicare eligibility lists stratified by age and sex were used to recruit individuals and age-eligible household members at each site, of whom 57% agreed to participate.12 An initial recruitment wave enrolled 5,201 individuals in 1989–90 (original cohort). Using the same recruitment approach, a second wave enrolled 687 predominantly African-American individuals in 1992–93 from all regions except Washington County (supplemental cohort). Entry criteria stipulated that individuals not be wheelchair dependent or institutionalized, not be currently receiving treatment for cancer, be able to give consent, and be expected to reside in their respective region for the upcoming 3 years.
CHS examinations involved medical history, medication inventory, physical examination, phlebotomy, and non-invasive testing. For the NEFA ancillary study, participants with available serum and eligible for a 2-hour oral glucose tolerance test (not on anti-hyperglycemic therapy) were included.
NEFA Measurement
Fasting serum was collected at the 1996–1997 exam. Blood was maintained at room temperature for 1 hour to allow clotting to occur, prior to centrifugation at 1110 × g for 10 minutes at 4°C, and subsequent storage at −80°C. Samples were stored at the central laboratory at the University of Vermont, Burlington, VT; analyses were performed in the Cardiovascular Nutrition Laboratory at Tufts University, Boston, MA. Serum was quick-thawed at 374°C for analysis, and lipids were extracted after addition of an internal standard (heptadecanoic acid dissolved in hexane) as previously described.13 The NEFA fraction was separated using solid-phase chromatography (aminopropyl columns), then dried under nitrogen, saponified, and methylated to form fatty acid methyl esters as detailed previously.13 An Autosystem XL gas chromatogram (Perkin Elmer, Boston, MA) equipped with a 100 m × 0.25 mm capillary column (HP INNOWax, Agilent Technologies, CA) was used to identify 35 fatty acid peaks, with comparison to authenticated standards (NuCheck Prep, MN). The inter-assay coefficients of variation were 0.5% to 4.3% for fatty acids present at concentrations >5 mol%, 1.8 to 7.1% for fatty acids at concentrations between 1–5 mol%, and 2.8 to 11.1% for fatty acids at concentrations <1 mol%.
AF Ascertainment
The outcome of interest was incident AF or atrial flutter (throughout both are here referred to as AF). Ascertainment was based on annual electrocardiograms from 1989–90 to 1998–99, as well as ICD-9 codes from inpatient or outpatient records throughout follow-up. Episodes associated with coronary bypass or valve replacement surgery during the same hospitalization were excluded. The follow-up period concluded December 2014.
Covariates
Covariates were mostly assessed at the same visit (1996–97) when specimens for NEFA measurement were collected. When not available at the latter visit, measures (height and lipid fractions) were carried over from the 1992–93 visit. Age, sex, race, smoking, and alcohol consumption were based on self-report. Heavy and moderate alcohol consumption were defined as ≥14 drinks/week and 7–13 drinks/week, respectively, for men; and ≥7 drinks/week and 1–6 drinks/week, respectively, for women. Weight, height, and blood pressure were determined using standardized methods. Physical activity level was based on type and intensity of activities reported by participants. Glomerular filtration rate was estimated from serum cystatin C. Other laboratory measures included serum albumin and lipid panels, which were measured at the Central Laboratory soon after collection. Diabetes was defined as fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or use of anti-hyperglycemic medication. Prevalent CHD was determined by participant report of physician diagnosis of angina, myocardial infarction, or coronary revascularization prior to enrollment. This was validated by evidence from the baseline examination and record reviews. At follow-up, incident CHD, similarly defined, was adjudicated by the CHS Events Committee. Prevalent stroke was based on patient report of physician diagnosis, and validated as for CHD. Prevalent HF was assessed using self-report, confirmed by use of HF medications, physician questionnaires, and medical record review. The proportion of missing data for all individual covariates was <5%.
Statistical Analysis
Pairwise correlations for individual NEFAs or sub-classes of NEFAs, expressed as absolute serum concentrations, were assessed with Pearson coefficients. Cox regression was used to estimate the association of individual NEFAs with incident AF after accounting for potential confounders. We first evaluated associations with AF for individual NEFAs separately in adjusted models. We next proceeded to enter all 35 NEFAs in one model in order to derive mutually adjusted risk estimates. This analysis was considered the primary analysis, evaluating the associations of individual NEFAs independent of each other, obviating the need for multiple testing. Restricted cubic splines were assessed for departures from linearity; finding none, we report associations per SD increment in NEFA levels.
We modeled NEFA associations using two levels of adjustment for potential confounders. Such confounders were selected based on prior associations or known biological mechanisms. The initial model (Model 1) adjusted for age, sex, race, and clinic. The fully adjusted model (Model 2) accounted additionally for height, weight, physical activity, systolic blood pressure, antihypertensive medication use, diabetes, smoking status, alcohol intake, serum albumin, LDL cholesterol, HDL cholesterol, triglycerides, prevalent CHD, prevalent stroke, prevalent heart failure, and eGFR. In a sensitivity analysis, we limited follow-up to the first 5 years. We also assessed for effect modification by sex. To assess the impact of missing data, we additionally ran Model 1 only in those with complete Model 2 covariate data.
All analyses were conducted with R (R Development Core Team; http://www.r-project.org). Two-sided P<0.05 was considered statistically significant.
Patient and Public Involvement
Patients were not involved when the Cardiovascular Heart Study was designed in the late 1980s.
Results
Of the 4,413 individuals who took part in the 1996–97 examination, 2,139 had serum NEFA measurements. After exclusion of 267 participants with prevalent AF, 1,872 individuals were eligible for the present analyses (Figure S1). Characteristics of the study sample are presented in Table 1. Participants were on average late into their eighth decade, almost two-thirds were women, and about 1 in 7 was African-American. More than half had hypertension, but, reflecting the study design, a small minority had diabetes. Fewer than 1 in 5 had prevalent CHD, and small fractions had prevalent stroke or heart failure. As compared with excluded participants, the study sample was generally healthier, having a lower burden of cardiovascular risk factors, particularly diabetes, and less prevalent cardiovascular disease (data not shown).
Table 1.
Participant characteristics*
| Characteristics | Study Cohort (n=1872) |
|---|---|
| Age, yrs | 77.7 ± 4.4 |
| Women, n (%) | 1165 (62) |
| Black, n (%) | 266 (14) |
| Clinic, n (%) | |
| Forsyth County, NC | 430 (23) |
| Sacramento County, CA | 540 (29) |
| Washington County, MD | 391 (21) |
| Allegheny County, PA | 511 (27) |
| Height, m | 1.63 ± 0.10 |
| Weight, kg | 71.6 ± 4.4 |
| Systolic blood pressure, mmHg | 137 ± 20 |
| Diastolic blood pressure, mmHg | 70 ± 11 |
| Hypertension, n (%) | 1124 (60) |
| Blood pressure medication use, n (%) | 943 (51) |
| Diabetes, n (%) | 49 (3) |
| Physical activity, n (%) | |
| None/little | 408 (22) |
| Moderate | 932 (50) |
| Strenuous | 513 (28) |
| Smoking, n (%) | |
| Current user | 149 (8) |
| Former user | 782 (43) |
| Never user | 911 (49) |
| Alcohol, n (%) | |
| Heavy use | 153 (8) |
| Moderate use | 677 (36) |
| LDL, mg/dL | 130 ± 33 |
| HDL, mg/dL | 54 ± 14 |
| Triglycerides, mg/dL | 139 ± 77; 121 (89–166) |
| Serum albumin, g/dL | 3.8 ± 0.3 |
| eGFR, ml/min/1.73 m2 | 73 ± 19 |
| Prevalent coronary heart disease, n (%) | 344 (18) |
| Prevalent heart failure, n (%) | 79 (4) |
| Prevalent stroke, n (%) | 86 (5) |
Mean ± standard deviation is presented for continuous variables and number (percent) for categorical variables; median (IQR) additionally provided for triglycerides, which showed substantial positive skew.
N = number, Yrs = years, m = meters, kg = kilograms, mmHg = milometers of mercury, mg/dL = milligrams per deciliter, g/dL = grams per deciliter, ml/min = milliliters per minute, m2 = meters squared.
Mean total saturated fatty acids accounted for 40% of the total serum NEFA concentration, with non-esterified palmitic acid and stearic acid making up most of this group (Figure 1 and Table 2). Monounsaturated fatty acids constituted another 37% of total serum NEFA, for which non-esterified oleic acid was the primary component; non-esterified nervonic acid was present in small amounts. Long-chain n-6 polyunsaturated fatty acids (n-6 PUFA) contributed 18%, most of which was non-esterified linoleic acid; non-esterified gamma-linolenic acid (GLA) was present in small amounts. Small amounts of trans fatty acids (3%) and n-3 PUFA (2%) made up the rest.
Figure 1.
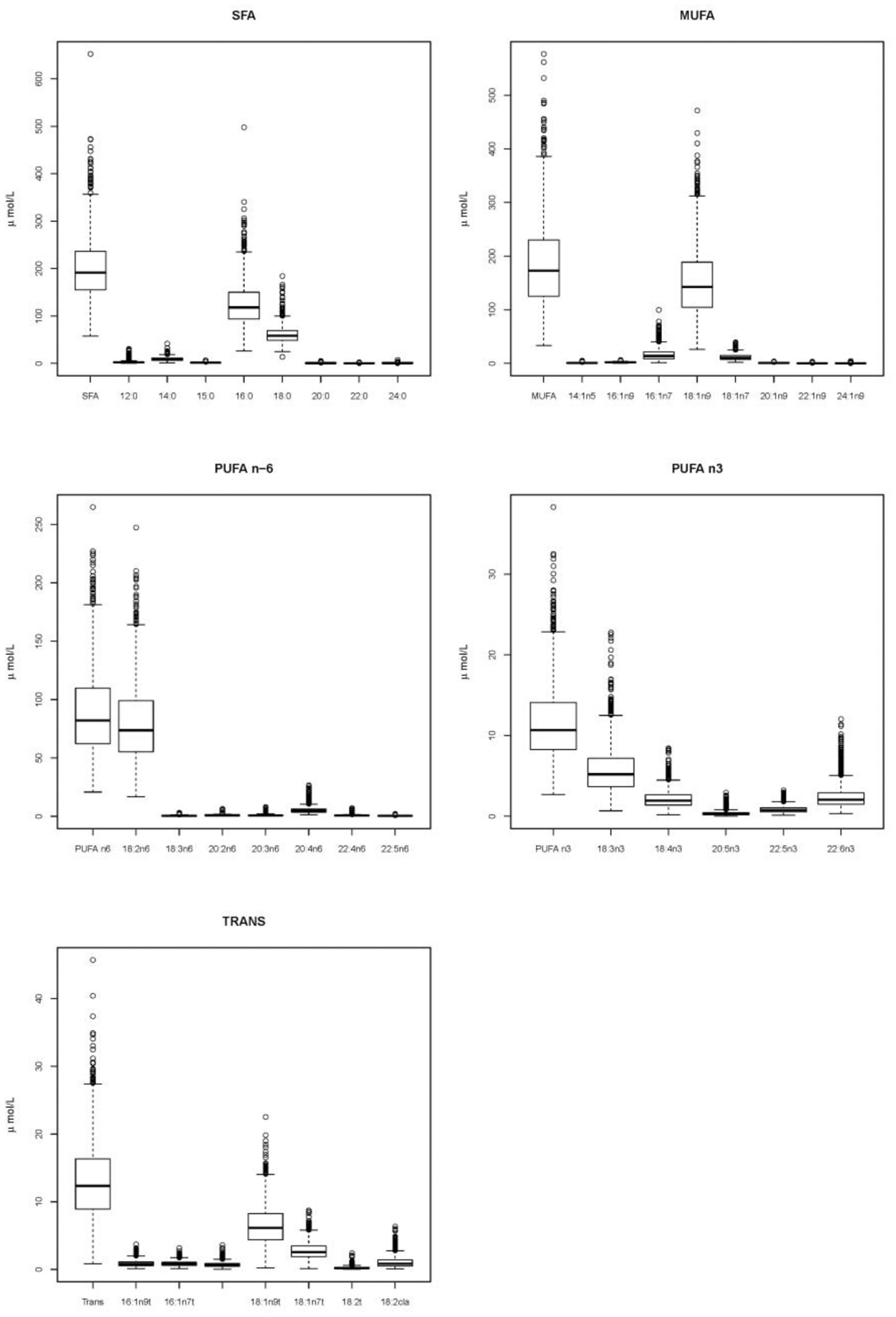
Absolute values of serum non-esterified fatty acids. MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Table 2.
Serum concentrations and associations with incident atrial fibrillation for specific non-esterified fatty acids assessed in separate multivariable models.
| NEFA | Serum Concetration (μmol/L) | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|---|
| Trivial or Systematic Name | Abbrev | Mean ± SD | Median (IQR) | HR per SD (95% CI) | P value | HR per SD (95% CI) | P value |
| SATURATED FATTY ACIDS | |||||||
| Lauric acid | 12:0 | 2.65 ± 2.56 | 2.07 (1.38–3.07) | 0.98 (0.91–1.06) | 0.576 | 1.01 (0.93–1.08) | 0.891 |
| Myristic acid | 14:0 | 9.00 ± 4.06 | 8.15 (6.14–11.19) | 1.06 (0.97–1.15 | 0.198 | 1.10 (1.00–1.20) | 0.044 |
| Pentadecylic acid | 15:0 | 1.61 ± 0.54 | 1.53 (1.24–1.90) | 1.03 (0.95–1.11) | 0.473 | 1.07 (0.98–1.17) | 0.111 |
| Palmitic acid | 16:0 | 124.33 ± 44.50 | 117.96 (93.48–150.00) | 1.06 (0.98–1.15) | 0.180 | 1.09 (0.99–1.19) | 0.075 |
| Stearic acid | 18:0 | 60.31 ± 17.22 | 58.16 (48.58–69.18) | 0.99 (0.92–1.07) | 0.858 | 1.01 (0.93–1.09) | 0.824 |
| Arachidic acid | 20:0 | 0.73 + 0.38 | 0.62 (0.50–0.82) | 0.94 (0.87–1.02) | 0.133 | 0.95 (0.87–1.03) | 0.208 |
| Behenic acid | 22:0 | 0.43 ± 0.18 | 0.39 (0.33–0.48) | 0.95 (0.88 – 1.032 | 0.241 | 0.97 (0.89–1.05) | 0.469 |
| Lignoceric acid | 24:0 | 0.65 ± 0.28 | 0.61 (0.50–0.74) | 0.99 (0.91–1.08) | 0.806 | 1.02 (0.94–1.11) | 0.618 |
| MONOUNSATURATED FATTY ACIDS | |||||||
| Myristoleic acid | 14:1n-5 | 0.87 ± 0.64 | 0.69 (0.43–1.15) | 1.08 (1.00–1.17) | 0.063 | 1.11 (1.01–1.21) | 0.026 |
| cis-7-hexadecenoic acid | 16:1n-9 | 2.00 ± 0.86 | 1.85 (1.37–2.47) | 1.07 (0.99–1.16) | 0.086 | 1.10 (1.01–1.20) | 0.028 |
| Palmitoleic acid | 16:1n-7 | 16.61 ± 11.18 | 13.84 (8.68–21.29) | 1.09 (1.01–1.19) | 0.034 | 1.12 (1.02–1.22) | 0.014 |
| Oleic acid | 18:1n-9 | 150.92 ± 63.19 | 142.91 (104.65–188.86) | 1.04 (0.96–1.13) | 0.324 | 1.07 (0.98–1.17) | 0.133 |
| Cis-Vaccenic acid | 18:1n-7 | 11.47 ± 5.51 | 10.44 (7.45–14.53) | 1.08 (1.00–1.17) | 0.051 | 1.10 (1.01–1.21) | 0.027 |
| Gondoic acid | 20:1n-9 | 1.03 ± 0.47 | 0.94 (0.69–1.28) | 1.00 (0.93–1.09) | 0.941 | 1.02 (0.94–1.11) | 0.634 |
| Erucic acid | 22:1n-9 | 0.38 ± 0.22 | 0.33 (0.24–0.44) | 0.95 (0.88–1.04) | 0.260 | 0.97 (0.89–1.05) | 0.470 |
| Nervonic acid | 24:1n-9 | 0.35 ± 0.18 | 0.32 (0.27–0.38) | 1.17 (1.09–1.25) | <0.001 | 1.13 (1.04–1.22) | 0.004 |
| N-6 POLYUNSATURATED FATTY ACIDS | |||||||
| Linoleic acid | 18:2n-6 | 79.37 ± 32.88 | 73.71 (55.30–98.92) | 1.03 (0.95–1.11) | 0.506 | 1.07 (0.98–1.17) | 0.125 |
| Gamma-Linolenic acid (GLA) | 18:3n-6 | 0.56 ± 0.32 | 0.49 (0.34–0.72) | 0.97 (0.90–1.05) | 0.499 | 0.97 (0.89–1.05) | 0.437 |
| Dihomolinoleic acid | 20:2n-6 | 0.90 ± 0.44 | 0.82 (0.61–1.10) | 1.00 (0.93–1.08) | 0.925 | 1.02 (0.94–1.10) | 0.689 |
| Dihomo-Gamma-Linolenic acid | 20:3n-6 | 0.96 ± 0.69 | 0.79 (0.58–1.13) | 0.99 (0.92–1.07) | 0.878 | 1.00 (0.93–1.09) | 0.934 |
| Arachidonic acid | 20:4n-6 | 5.39 ± 2.96 | 4.74 (3.55–6.34) | 0.96 (0.89–1.03) | 0.242 | 0.97 (0.89–1.05) | 0.423 |
| Adrenic acid | 22:4n-6 | 0.71 ± 0.49 | 0.61 (0.42–0.86) | 1.04 (0.97–1.11) | 0.289 | 1.03 (0.96–1.12) | 0.394 |
| Docosapentaenoic acid | 22:5n-6 | 0.38 ± 0.21 | 0.34 (0.25–0.46) | 1.00 (0.92–1.07) | 0.902 | 1.03 (0.96–1.12) | 0.418 |
| N-3 POLYUNSATURATED FATTY ACIDS | |||||||
| Alpha Linolenic acid (ALA) | 18:3n-3 | 5.79 ± 2.93 | 5.21 (3.68–7.20) | 1.03 (0.95–1.12) | 0.445 | 1.08 (0.9–1.18) | 0.068 |
| Stearidonic acid (SDA) | 18:4n-3 | 2.14 ± 1.05 | 1.93 (1.40–2.65) | 1.03 (0.95–1.11) | 0.516 | 1.04 (0.96–1.13) | 0.311 |
| Eicosapentaenoic acid (EPA) | 20:5n-3 | 0.37 ± 0.29 | 0.29 (0.19–0.45) | 0.97 (0.90–1.15) | 0.441 | 1.01 (0.93–1.09) | 0.884 |
| Docosapentaenoic acid (DPA) | 22:5n-3 | 0.85 ± 0.43 | 0.77 (0.54–1.05) | 1.01 (0.94–1.09) | 0.777 | 1.05 (0.97–1.15) | 0.221 |
| Docosahexaenoic acid (DHA) | 22:6n-3 | 2.43 ± 1.48 | 2.05 (1.49–2.91) | 0.98 (0.91–1.05) | 0.518 | 1.01 (0.93–1.09) | 0.806 |
| TRANS FATTY ACIDS | |||||||
| trans-7-hexadecenoic acid | 16:1n-9 | 0.89 ± 0.48 | 0.81 (0.54–1.12) | 1.07 (0.99–1.16) | 0.096 | 1.09 (1.00–1.20) | 0.051 |
| Palmitelaidic acid | 16:1n-7 | 0.87 ± 0.35 | 0.82 (0.61–1.06) | 1.04 (0.96–1.13) | 0.339 | 1.06 (0.97–1.16) | 0.202 |
| Petroselinic acid | 18:1n-1012* | 0.71 ± 0.37 | 0.65 (0.46–0.88) | 1.07 (0.99–1.15) | 0.108 | 1.08 (0.99–1.17) | 0.080 |
| Elaidic acid | 18:1n-9 | 6.57 ± 2.94 | 6.16 (4.39–8.27) | 1.02 (0.94–1.10) | 0.673 | 1.05 (0.97–1.14) | 0.257 |
| Trans-Vaccenic acid | 18:1n-7 | 2.75 ± 1.21 | 2.58 (1.88–3.45) | 1.00 (0.93–1.08) | 0.965 | 1.03 (0.95–1.12) | 0.484 |
| Linelaidic acid | 18:2 | 0.23 ± 0.19 | 0.18 (0.10–0.30) | 1.00 (0.93–1.07) | 0.976 | 1.01 (0.94–1.09) | 0.802 |
| Conjugated Linoleic acid | 18:2cla | 1.06 ± 0.76 | 0.86 (0.50–1.42) | 1.01 (0.93–1.10) | 0.776 | 1.01 (0.93–1.11) | 0.766 |
Model 1 included age, gender, race, and clinic site. N = 1872
Model 2 included model 1 factors as well as height, weight, physical activity score, systolic blood pressure, antihypertensive medication, diabetes, smoking status, alcohol intake, serum albumin low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, prevalent coronary heart disease, prevalent stroke, prevalent heart failure, and eGFR. N = 1730
sum of 18:1n-10t, 18:1n-11t and 18:1n-12t
NEFA = non-esterified fatty acids, SD = standard deviation, Abbrev = abbreviation, μ mol/L = micromoles per liter, HR = hazard ratio, SD = standard deviation, CI = confidence interval
Figure 2 shows the pairwise correlations for individual NEFAs. These tended to be strong for medium-chain to long-chain saturated fatty acids with one another; and for medium-chain to long-chain monounsaturated fatty acids with one another, or with their saturated or trans fatty acid counterparts, but less so with their n-6 and n-3 polyunsaturated counterparts. Strong correlations were also observed for some n-6 or n-3 PUFAs with one another, as well as with trans fatty acids, which also exhibited strong correlations with one another.
Figure 2.
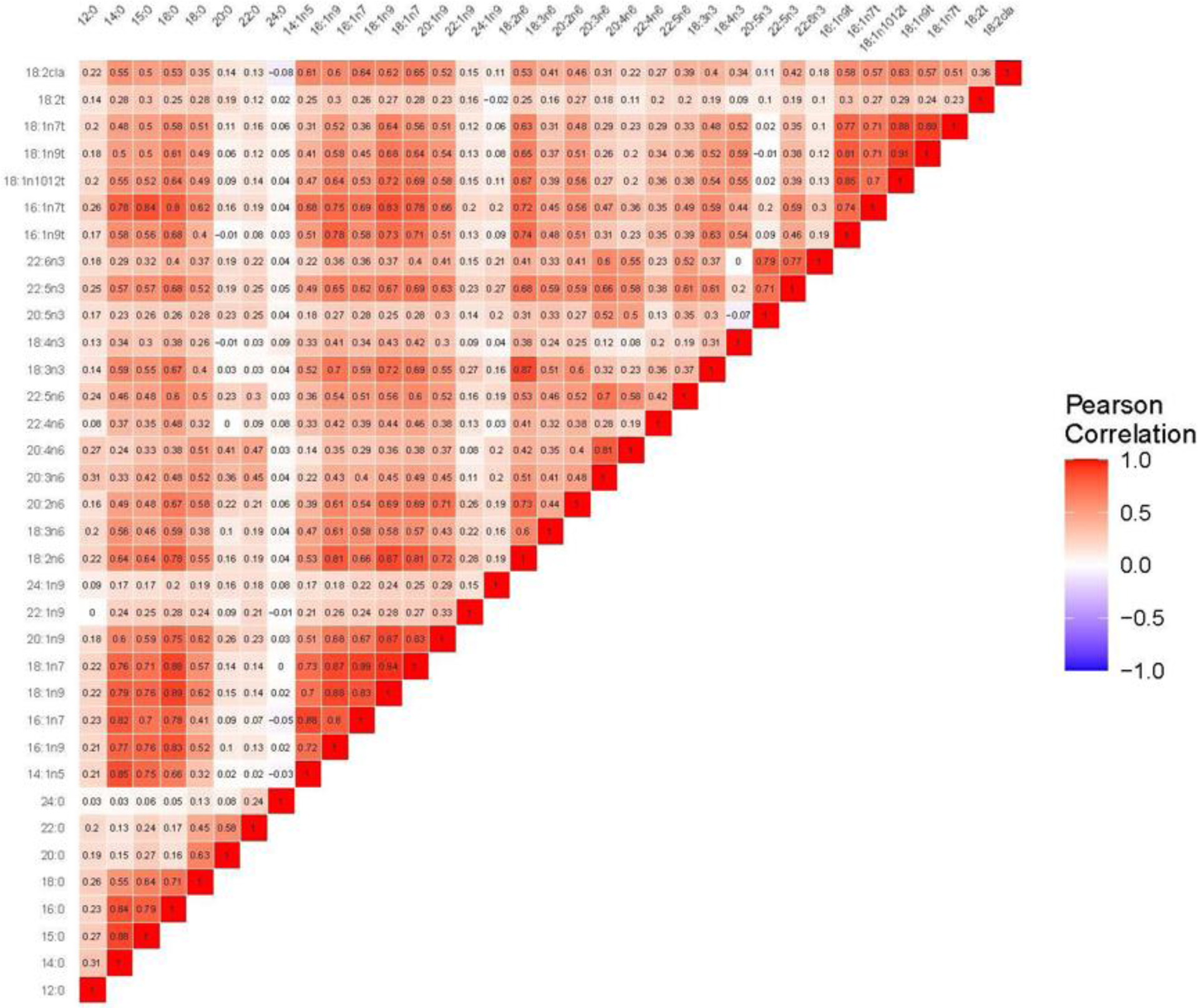
Heat map visually depicting the correlation of the individual serum NEFAs with each other. The redder the box at their intersection, the more the NEFAs cluster together, as quantified by their positive Pearson correlation. Notably, many of the long-chain and medium-chain monounsaturated fatty acids are highly correlated with one another and with the saturated and trans fatty acids, but less so with the PUFAs. Strong correlations are also seen for some PUFAs with one another, and with trans fatty acids, which are also highly correlated with one another. NEFA, non-esterified fatty acid; PUFA, polyunsaturated fatty acid.
During median follow-up of 11.3 years, 715 cases of incident AF occurred, an incidence rate of 4% per year. After full adjustment, several individual NEFAs exhibited significant relationships with incident AF, as shown in Table 2. For each SD increment in individual NEFA concentrations, these ranged from 5% lower relative hazards for arachidic acid, to 12% and 13% higher relative hazards for palmitoleic and nervonic acid.
When all 35 individual NEFAs were entered in the Cox models concurrently, two individual NEFAs showed significant associations with incident AF in both minimally and fully adjusted models, as shown in Table 3. In these analyses, assessment for inflation of the standard errors did not reveal meaningful problems with collinearity. After full adjustment, each SD increment in non-esterified nervonic acid, a long-chain monounsaturated fatty acid, exhibited a significant 18% higher hazard for AF. Conversely, every SD increment of non-esterified GLA, an n-6 fatty acid, was associated with a significant 19% lower hazard for AF. Findings were similar when the follow-up period was restricted to the first 5 years. Stratified analyses did not reveal obvious effect modification by sex. Analysis excluding participants with any missing data (7.6%) did not meaningfully alter results.
Table 3.
Associations with incident atrial fibrillation for specific non-esterified fatty acids after entering all 35 non-esterified fatty acids concurrently in multivariable models.
| NEFA | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| Trivial or Systematic Name | Abbrev | HR per SD (95% CI) | P value | HR per SD (95% CI) | P value |
| SATURATED FATTY ACIDS | |||||
| Lauric acid | 12:0 | 0.97 (0.88–1.06) | 0.492 | 0.99 (0.90–1.08) | 0.806 |
| Myristic acid | 14:0 | 1.10 (0.83–1.47) | 0.517 | 1.12 (0.83–1.50) | 0.475 |
| Pentadecylic acid | 15:0 | 0.96 (0.76–1.21) | 0.725 | 1.04 (0.81–1.32) | 0.783 |
| Palmitic acid | 16:0 | 0.97 (0.74–1.28) | 0.830 | 0.94 (0.70–1.27) | 0.693 |
| Stearic acid | 18:0 | 1.17 (0.95–1.43) | 0.134 | 1.16 (0.93–1.43) | 0.190 |
| Arachidic acid | 20:0 | 0.87 (0.74–1.01) | 0.059 | 0.85 (0.72–1.00) | 0.054 |
| Behenic acid | 22:0 | 0.99 (0.88–1.12) | 0.917 | 1.02 (0.90–1.16) | 0.793 |
| Lignoceric acid | 24:0 | 0.98 (0.89–1.08) | 0.652 | 1.01 (0.92–1.11) | 0.827 |
| MONOUNSATURATED FATTY ACIDS | |||||
| Myristoleic acid | 14:1n-5 | 0.96 (0.76–1.21) | 0.703 | 0.92 (0.73–1.16) | 0.483 |
| cis-7-hexadecenoic acid | 16:1n-9 | 1.11 (0.88–1.41) | 0.379 | 1.10 (0.85–1.41) | 0.479 |
| Palmitoleic acid | 16:1n-7 | 1.06 (0.79–1.42) | 0.700 | 1.15 (0.84–1.57) | 0.396 |
| Oleic acid | 18:1n-9 | 0.69 (0.44–1.08) | 0.104 | 0.76 (0.46–1.26) | 0.290 |
| Cis-Vaccenic acid | 18:1n-7 | 1.35 (0.96–1.90) | 0.081 | 1.25 (0.87–1.80) | 0.236 |
| Gondoic acid | 20:1n-9 | 0.91 (0.72–1.14) | 0.408 | 0.88 (0.69–1.13) | 0.325 |
| Erucic acid | 22:1n-9 | 0.92 (0.84–1.01) | 0.094 | 0.94 (0.85–1.03) | 0.185 |
| Nervonic acid | 24:1n-9 | 1.22 (1.14–1.31) | <0.001 | 1.18 (1.08–1.29) | <0.001 |
| N-6 POLYUNSATURATED FATTY ACIDS | |||||
| Linoleic acid | 18:2n-6 | 1.27 (0.95–1.71) | 0.111 | 1.27 (0.92–1.76) | 0.153 |
| Gamma-Linolenic acid | 18:3n-6 | 0.88 (0.78–0.99) | 0.040 | 0.81 (0.71–0.94) | 0.004 |
| Dihomolinoleic acid | 20:2n-6 | 0.91 (0.78–1.06) | 0.225 | 0.89 (0.75–1.06) | 0.193 |
| Dihomo-Gamma-Linolenic acid | 20:3n-6 | 1.10 (0.93–1.30) | 0.282 | 1.04 (0.87–1.24) | 0.679 |
| Arachidonic acid | 20:4n-6 | 0.91 (0.79–1.05) | 0.196 | 0.91 (0.78–1.06) | 0.231 |
| Adrenic acid | 22:4n-6 | 1.01 (0.93–1.11) | 0.775 | 0.98 (0.89–1.08) | 0.691 |
| Docosapentaenoic acid | 22:5n-6 | 0.95 (0.84–1.08) | 0.424 | 1.02 (0.90–1.16) | 0.712 |
| N-3 POLYUNSATURATED FATTY ACIDS | |||||
| Alpha Linolenic acid (ALA) | 18:3n-3 | 0.92 (0.76–1.11) | 0.368 | 0.97 (0.80–1.19) | 0.784 |
| Stearidonic acid (SDA) | 18:4n-3 | 1.00 (0.91–1.11) | 0.977 | 1.00 (0.90–1.11) | 0.979 |
| Eicosapentaenoic acid (EPA) | 20:5n-3 | 0.98 (0.84–1.13) | 0.734 | 0.99 (0.85–1.15) | 0.850 |
| Docosapentaenoic acid (DPA) | 22:5n-3 | 1.11 (0.90–1.36) | 0.332 | 1.16 (0.94–1.44) | 0.167 |
| Docosahexaenoic acid (DHA) | 22:6n-3 | 0.92 (0.79–1.08) | 0.303 | 0.93 (0.79–1.09) | 0.378 |
| TRANS FATTY ACIDS | |||||
| trans-7-hexadecenoic acid | 16:1n-9 | 0.92 (0.73–1.17) | 0.500 | 0.92 (0.71–1.20) | 0.535 |
| Palmitelaidic acid | 16:1n-7 | 1.03 (0.82–1.29) | 0.801 | 0.92 (0.72–1.18) | 0.523 |
| Petroselinic acid | 18:1n-1012* | 1.44 (1.13–1.82) | 0.003 | 1.28 (0.99–1.66) | 0.058 |
| Elaidic acid | 18:1n-9 | 0.84 (0.67–1.06) | 0.147 | 0.95 (0.75–1.22) | 0.696 |
| Trans-Vaccenic acid | 18:1n-7 | 0.88 (0.71–1.10) | 0.272 | 0.93 (0.73–1.18) | 0.550 |
| Linelaidic acid | 18:2 | 1.00 (0.93–1.09) | 0.929 | 1.01 (0.92–1.10) | 0.907 |
| Conjugated Linoleic acid | 18:2cla | 0.95 (0.84–1.07) | 0.375 | 0.94 (0.83–1.07) | 0.365 |
Model 1 included age, gender, race, and clinic site.
Model 2 included model 1 factors as well as height, weight, smoking status, alcohol usage, physical activity, blood pressure, LDL, HDL, albumin, eGFR, antihypertensive use, prevalent disease (diabetes, hypertension, coronary heart disease, heart failure, and stroke), and other individual NEFAs.
sum of 18:1n-10t, 18:1n-11t and 18:1n-12t
NEFA = non-esterified fatty acids, SD = standard deviation, Abbrev = abbreviation, μ mol/L = micromoles per liter, HR = hazard ratio, SD = standard deviation, CI = confidence interval
Discussion
In this study of older adults with a high incidence of AF, we found two individual NEFAs, nervonic acid (24:1n-9) and GLA (18:3n-6), to be strongly and significantly associated with this outcome after concurrent adjustment of all NEFAs and full adjustment for potential confounders. Higher serum concentration of non-esterified nervonic acid was associated with a higher risk of AF, while higher serum concentration of non-esterified GLA was associated with a lower risk of AF.
Nervonic acid, a long-chain monounsaturated fatty acid, occurs in the seed oils of rapeseed and mustard. It is consumed in low amounts by humans.14 Nervonic acid can be synthesized de novo from stearic acid via desaturation to oleic acid, followed by three consecutive elongation reactions. To date, genome-wide association analyses in human populations have not identified variants associated with circulating nervonic acid levels.15Best known for its role in the nervous system, nervonic acid is an intermediate in the biosynthesis of myelin and is one of the major fatty acids in brain sphingolipids. Nervonic acid has also been associated with increased markers of inflammation and endothelial activation, as well as higher incidence of heart failure and all-cause mortality.16 Nervonic acid has not been examined in previous studies of blood or adipose tissue fatty acids, making this its first reported association with AF to our knowledge.
A dietary study among women showed that low monounsaturated fatty acid intake was associated with higher incidence of persistent, but not paroxysmal, AF.17 Similarly, in the large randomized PREDIMED trial, a Mediterranean diet enriched with extra virgin olive oil, a plant oil rich in the monounsaturated fatty acid oleic acid, was protective against incident AF as compared to a control Mediterranean diet not enriched with extra virgin olive oil or enriched with nuts.18 The nut-rich diet had a lower proportion of monounsaturated fat, indirectly suggesting the potential benefit of monounsaturated fatty acid intake. The basis for the favorable impact on AF of a Mediterranean diet high in monounsaturated fats, specifically as relates to circulating nervonic acid levels, requires further study.
Conversely, oleic acid, a precursor of nervonic acid, has been shown to increase action potential duration and triggered activity in exposed mouse myocytes.19 Calcium transients were increased and sodium handling was likewise dysregulated. These findings support a possible mechanism whereby monounsaturated fatty acids could directly potentiate atrial arrhythmias.
GLA is an n-6 fatty acid present in various botanical seed oils and dietary supplements.20 GLA is the metabolic product of desaturation of linoleic acid by delta-6 desaturase. It is lengthened to dihomo-gamma-linolenic acid (DGLA) by elongase 5 and converted to arachidonic acid (AA) by delta-5 desaturase.20 Both DGLA and AA are substrates for cyclooxygenases and lipoxygenases. For AA, these enzymes lead to generation of pro-inflammatory eicosanoids, namely, 2-series prostaglandins and 4-series leukotrienes.20 In the case of DGLA, however, the resulting species have anti-inflammatory properties.20 These include prostaglandin E1, which affects immune system function and at low levels has been shown to be antiarrhythmic,21 and thromboxane A1, which has vasodilatory and platelet-inhibiting effects.22
To our knowledge, the finding of an inverse association between circulating non-esterified GLA and new-onset AF is new. Studies of n-6 PUFA have been mixed with respect to impact on arrhythmias and other cardiac events. A Danish cohort study that examined dietary intake of PUFAs found no significant association with AF incidence.23 A recent systematic review concluded that the effect of broadly increasing dietary PUFA was unclear with respect to AF, as evidence was of very low quality.24
As relates to PUFA levels in biospecimens, there was a suggestion of less AF following coronary bypass surgery among those with higher plasma AA levels.25 Furthermore, low circulating DGLA levels and a low DGLA/AA ratio were associated with long-term mortality in patients with acute cardiovascular disease and heart failure.26 Among a general cohort undergoing gluteal fat biopsy, wherein treelet analysis was used to derive clusters of adipocyte esterified and non-esterified fatty acids associated with incident AF, the n-6 PUFA cluster was found to be significantly inversely associated with the arrhythmia.27 Notably, DGLA and AA, but not GLA, were part of the n-6 PUFA cluster, suggesting that GLA bears a weaker relationship with others in its sub-class, at least in gluteal fat.
A genome wide association study (GWAS) in the Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) consortium, which includes CHS, found a variant fatty acid desaturase cluster, rs174547,28 which has been shown to have inverse associations with non-arrhythmic cardiovascular outcomes.29 The rs174547 minor allele is associated with decreased delta-5, but increased delta-6, desaturase gene expression.30 Interestingly, in the CHARGE GWAS, the minor allele was associated with higher DGLA and lower AA blood levels, as well as lower blood GLA and linoleic acid levels.28 Thus, the findings with respect to non-arrhythmic cardiovascular disease would appear to run counter to the relationship between higher non-esterified GLA and lower AF risk documented here.
The association in our study only emerged, however, after simultaneous adjustment for circulating linoleic acid, DGLA and AA, as well as the remaining measured NEFAs. This suggests that the association observed with AF could relate to beneficial influences of low GLA levels with respect to this outcome that are otherwise masked by DGLA, AA, and other individual NEFAs. To our knowledge, no direct protective actions of GLA on immune, vascular or platelet function have been described, calling into question attribution of the association to direct GLA effects. But the biology of PUFAs is complex, especially with respect to the implications of circulating levels for membrane function and signaling in different tissue and cell compartments.20 Additional study is necessary to determine the basis for the observed association, and whether it might be responsive to dietary or pharmacologic manipulation.
There are several limitations of our study. As a cohort study, the current analysis may illustrate associations, but cannot demonstrate causality. Individuals originally enrolled in this study represented the subset from random, stratified Medicare eligibility lists who gave consent. Moreover, participants had to survive and attend a follow-up examination up to 8 years later. By design, participants were largely free of diabetes, and overall had a lower burden of cardiovascular disease. Thus, the present results are not necessarily generalizable to the broader population of older adults. AF ascertainment was via annual ECG and inpatient and outpatient ICD-9 codes and therefore likely underrepresented true AF burden. Yet, as this was not expected to be different among the groups, any bias created should be toward the null.
In conclusion, the present study newly identifies positive and inverse associations of non-esterified nervonic acid and GLA with incident AF in older adults, the population at highest risk. These findings require replication in other cohorts. If confirmed, they suggest that enhanced understanding of mechanisms and approaches to modifying levels of these two NEFAs could offer new avenues to reducing the onset of this highly prevalent arrhythmia in our aging population.
Supplementary Material
Key Questions
1. What is already known about this subject?
Prospective studies of individual fatty acids have largely focused on esterified fatty acids that reflect membrane composition rather than the balance between organ-tissue uptake, synthesis, or release. Long-chain n-3 polyunsaturated fatty acids (PUFA) have inconsistently related to lower risk of AF, while long-chain saturated fatty acids (SFA) have been linked to higher risk of incident AF.
2. What does this study add?
This study of older adults is the first to examine the association of individual circulating NEFAs with development of AF, and to document significant independent associations for two specific NEFAs. Nervonic acid was associated with a nearly 20% higher risk of AF per SD increment, and gamma-linolenic acid with a nearly 20% lower risk per SD increment.
3. How might this impact on clinical practice?
Identification of these two individual NEFAs as independently related to incident AF suggests that modification of their levels, or targeting of their associated pathways, could offer potential therapeutic approaches for prevention of AF. These findings provide impetus for replication and further understanding of the nexus between these individual NEFAs and their metabolism and this cardiac dysrhythmia.
Acknowledgements and Sources of Funding
This research was supported by R01 AG053325 from the National Institute on Aging; and by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). JRK was supported by K24 HL135413 from the National Heart, Lung, and Blood Institute. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Kizer discloses stock ownership in Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer.
Footnotes
Ethics Statement
This study complied with the Declaration of Helsinki. All participants provided written informed consent. CHS was approved by the Institutional Review Boards of the Coordinating Center (University of Washington) and field centers (University of Pittsburgh, Johns Hopkins University, Wake Forest University, and UC Davis).
References
- 1.Virani Salim S, Alvaro Alonso, Benjamin Emelia J, Bittencourt Marcio S, Callaway Clifton W, Carson April P, Chamberlain Alanna M, Chang Alexander R, Susan Cheng, Delling Francesca N, Luc Djousse, Elkind Mitchell SV, Ferguson Jane F, Myriam Fornage, Khan Sadiya S, Kissela Brett M, Knutson Kristen L, Kwan Tak W, Lackland Daniel T, Lewis Tené T, Lichtman Judith H, Longenecker Chris T, Loop Matthew Shane, Lutsey Pamela L., Martin Seth S, Matsushita Kunihiro, Moran Andrew E., Mussolino Michael E., Perak Amanda Marma, Rosamond Wayne D., Roth Gregory A., Sampson Uchechukwu K.A., Satou Gary M., Schroeder Emily B., Shah Svati H., Shay Christina M., Spartano Nicole L., Stokes Andrew, Tirschwell David L., VanWagner Lisa B., Tsao Connie W., null null. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J Am Coll Cardiol. 2017;70:2022–2035. [DOI] [PubMed] [Google Scholar]
- 3.Ebbert JO, Jensen MD. Fat depots, free fatty acids, and dyslipidemia. Nutrients. 2013;5:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khawaja O, Bartz TM, Ix JH, Heckbert SR, Kizer JR, Zieman SJ, Mukamal KJ, Tracy RP, Siscovick DS, Djoussé L. Plasma Free Fatty Acids and Risk of Atrial Fibrillation (From the Cardiovascular Health Study). Am J Cardiol. 2012;110:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pankow JS, Duncan BB, Schmidt MI, Ballantyne CM, Couper DJ, Hoogeveen RC, Golden SH, Atherosclerosis Risk in Communities Study. Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. [DOI] [PubMed] [Google Scholar]
- 6.Virtanen Jyrki K, Mursu Jaakko, Voutilainen Sari, Tuomainen Tomi-Pekka. Serum Long-Chain n-3 Polyunsaturated Fatty Acids and Risk of Hospital Diagnosis of Atrial Fibrillation in Men. Circulation. 2009;120:2315–2321. [DOI] [PubMed] [Google Scholar]
- 7.Wu JHY, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, Heckbert SR, Siscovick DS, Mozaffarian D. Association of plasma phospholipid long-chain ω-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronroos NN, Chamberlain AM, Folsom AR, Soliman EZ, Agarwal SK, Nettleton JA, Alonso A. Fish, Fish-Derived n-3 Fatty Acids, and Risk of Incident Atrial Fibrillation in the Atherosclerosis Risk in Communities (ARIC) Study. PLoS ONE [Internet]. 2012. [cited 2020 May 1];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3343018/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JCM. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J. 2006;151:857–862. [DOI] [PubMed] [Google Scholar]
- 10.Deen JF, Adams AK, Fretts A, Jolly S, Navas-Acien A, Devereux RB, Buchwald D, Howard BV. Cardiovascular Disease in American Indian and Alaska Native Youth: Unique Risk Factors and Areas of Scholarly Need. J Am Heart Assoc Cardiovasc Cerebrovasc Dis [Internet]. 2017. [cited 2020 May 1];6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5721901/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung Y, Cho Y, Kim N, Oh I-Y, Kang SW, Choi E-K, Hwang G-S. Lipidomic profiling reveals free fatty acid alterations in plasma from patients with atrial fibrillation. PLOS ONE. 2018;13:e0196709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 13.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res. 2010;51:2826–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y, Meng H-M, Hu G-R, Li F-L. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl Microbiol Biotechnol. 2018;102:3027–3035. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Tanaka T, Zhu J, Guan W, Wu JHY, Psaty BM, McKnight B, King IB, Sun Q, Richard M, Manichaikul A, Frazier-Wood AC, Kabagambe EK, Hopkins PN, Ordovas JM, Ferrucci L, Bandinelli S, Arnett DK, Chen Y-DI, Liang S, Siscovick DS, Tsai MY, Rich SS, Fornage M, Hu FB, Rimm EB, Jensen MK, Lemaitre RN, Mozaffarian D, Steffen LM, Morris AP, Li H, Lin X. Discovery and fine-mapping of loci associated with MUFAs through trans-ethnic meta-analysis in Chinese and European populations. J Lipid Res. 2017;58:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgado GE, Krämer BK, Lorkowski S, März W, Schacky C von, Kleber ME. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J Clin Lipidol. 2017;11:126–135.e5. [DOI] [PubMed] [Google Scholar]
- 17.Chiuve SE, Sandhu RK, Moorthy MV, Glynn RJ, Albert CM. Dietary Fat Intake Is Differentially Associated with Risk of Paroxysmal Compared with Sustained Atrial Fibrillation in Women123. J Nutr. 2015;145:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-González Miguel Á, Toledo Estefanía Arós Fernando, Miquel Fiol, Dolores Corella, Jordi Salas-Salvadó, Emilio Ros, Covas Maria I, Fernández-Crehuet Joaquín, Lapetra José, Muñoz Miguel A., Fitó Monserrat, Serra-Majem Luis, Pintó Xavier, Lamuela-Raventós Rosa M., Sorlí Jose V., Babio Nancy, Buil-Cosiales Pilar, Ruiz-Gutierrez Valentina, Estruch Ramón, Alonso Alvaro. Extravirgin Olive Oil Consumption Reduces Risk of Atrial Fibrillation. Circulation. 2014;130:18–26. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y-K, Chen Y-C, Kao Y-H, Tsai C-F, Yeh Y-H, Huang J-L, Cheng C-C, Chen S-A, Chen Y-J. A monounsaturated fatty acid (oleic acid) modulates electrical activity in atrial myocytes with calcium and sodium dysregulation. Int J Cardiol. 2014;176:191–198. [DOI] [PubMed] [Google Scholar]
- 20.Sergeant S, Rahbar E, Chilton FH. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur J Pharmacol. 2016;785:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Kang JX, Leaf A. Differential effects of various eicosanoids on the production or prevention of arrhythmias in cultured neonatal rat cardiac myocytes. Prostaglandins. 1997;54:511–530. [DOI] [PubMed] [Google Scholar]
- 22.Yeung J, Hawley M, Holinstat M. The expansive role of oxylipins on platelet biology. J Mol Med Berl Ger. 2017;95:575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortensen LM, Lundbye-Christensen S, Schmidt EB, Calder PC, Schierup MH, Tjønneland A, Parner ET, Overvad K. Long-chain n-3 and n-6 polyunsaturated fatty acids and risk of atrial fibrillation: Results from a Danish cohort study. PLOS ONE. 2017;12:e0190262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelhamid AS, Martin N, Bridges C, Brainard JS, Wang X, Brown TJ, Hanson S, Jimoh OF, Ajabnoor SM, Deane KH, Song F, Hooper L. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev [Internet]. 2018. [cited 2020 Apr 10];2018. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6517012/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skuladottir GV, Heidarsdottir R, Arnar DO, Torfason B, Edvardsson V, Gottskalksson G, Palsson R, Indridason OS. Plasma n-3 and n-6 fatty acids and the incidence of atrial fibrillation following coronary artery bypass graft surgery. Eur J Clin Invest. 2011;41:995–1003. [DOI] [PubMed] [Google Scholar]
- 26.Ouchi S, Miyazaki T, Shimada K, Sugita Y, Shimizu M, Murata A, Kato T, Aikawa T, Suda S, Shiozawa T, Hiki M, Takahashi S, Kasai T, Miyauchi K, Daida H. Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis [Internet]. 2017. [cited 2020 Apr 10];16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5556673/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinesen PT, Rix TA, Joensen AM, Dahm CC, Lundbye-Christensen S, Schmidt EB, Overvad K. Patterns of adipose tissue fatty acids and the risk of atrial fibrillation: A case-cohort study. PLoS ONE [Internet]. 2018. [cited 2020 May 3];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6289440/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W, Steffen BT, Lemaitre RN, Wu JHY, Tanaka T, Manichaikul A, Foy M, Rich SS, Wang L, Nettleton JA, Tang W, Gu X, Bandinelli S, King IB, McKnight B, Psaty BM, Siscovick D, Djousse L, Chen Y-DI, Ferrucci L, Fornage M, Mozafarrian D, Tsai MY, Steffen LM. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. 2014;7:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan S, Bäck M, Bruzelius M, Mason AM, Burgess S, Larsson S. Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds LM, Howard TD, Ruczinski I, Kanchan K, Seeds MC, Mathias RA, Chilton FH. Tissue-specific impact of FADS cluster variants on FADS1 and FADS2 gene expression. PloS One. 2018;13:e0194610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


