Abstract
MicroRNAs are major drivers of cell fate specification and differentiation. The post transcriptional regulation of key molecular factors by microRNAs contributes to the progression of embryonic and post-embryonic development in several organisms. Following the discovery of lin-4 and let-7 in C. elegans and bantam microRNAs in D. melanogaster, microRNAs have emerged as orchestrators of cellular differentiation and developmental timing. Spatiotemporal control of microRNAs and associated protein machinery can modulate microRNA activity. Additionally, adaptive modulation of microRNA expression and function in response to changing environmental conditions ensures that robust cell fate specification during development is maintained. Here, we review the role of microRNAs in the regulation of differentiation during development.
Keywords: microRNAs, differentiation, development, cell fate, posttranscriptional gene regulation
Introduction
MicroRNAs are small noncoding RNAs that regulate gene expression at the post-transcriptional level. Following their discovery as regulators of developmental timing in Caenorhabditis elegans (Lee et al., 1993; Wightman et al., 1993; Reinhart et al., 2000), microRNAs have been found to play central roles in determining cell fate and cell identity. Subsequent biochemical isolation of small RNAs launched the search for microRNAs in vertebrates and invertebrates (Lagos-Quintana et al., 2001; Lau et al., 2001; Lee and Ambros, 2001) and the latest version of the public repository miRbase contains annotations for 48,860 mature microRNAs across 271 organisms (Kozomara et al., 2019). MicroRNA-mediated gene regulation is essential in diverse biological processes such as development and differentiation, stress responses, behavior, and longevity (reviewed in Ambros and Ruvkun, 2018). Misregulation of microRNAs and the associated protein machinery have been linked to cancer, cardiovascular, and metabolic disorders (reviewed in Rupaimoole and Slack, 2017).
Many microRNAs, including the let-7 and mir-100 families, are deeply conserved (Grimson et al., 2008; Pasquinelli et al., 2000). By targeting key transcription factors and RNA-binding proteins, for example, microRNAs can have profound effects on the cellular transcriptome in a spatially and temporally regulated manner, especially during development. The mechanisms by which microRNAs promote or inhibit differentiation are diverse (Fig 1). MicroRNAs can 1) prevent premature differentiation by inhibiting the accumulation of differentiation factors; 2) promote cell proliferation by targeting inhibitors of cell cycle arrest; 3) drive differentiation by downregulating stem cell like factors and protein machinery that can elicit broad transcriptomic changes; and 4) help restrict and switch fates of cells derived from the same set of progenitors in a temporally regulated manner. The functions of microRNAs discussed in this review are listed in Table 1.
Fig 1. MicroRNAs regulate different aspects of cell differentiation.
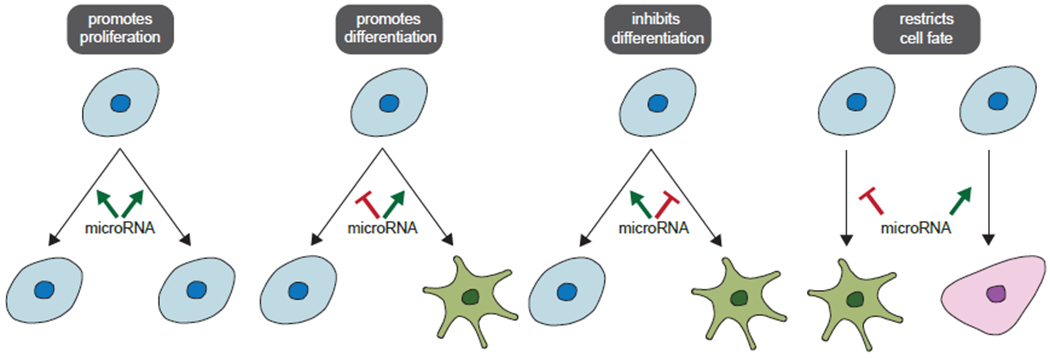
MicroRNAs can promote cell proliferation, differentiation, and regulate cell fates during asymmetric cell division. In some cases, microRNAs act in a spatiotemporal manner to restrict cell fates of daughter cells derived from the same lineage of progenitor cells.
Table 1.
Functions of microRNAs in differentiation
| Conserved family | microRNA | Differentiation function | Process | Organism | Reference |
|---|---|---|---|---|---|
|
| |||||
| mir-125 | miR-125 | promoter of differentiation | gliogenesis | mESCs | Shenoy et al, 2015 |
| lin-4 | promoter of differentiation | hypodermis development | C. elegans | Lee et al, 1993 | |
| lin-4 | inhibitor of differentiation | vulval development | C. elegans | Li and Greenwald, 2010 | |
| lin-4 | promoter of differentiation | HSN neuron development | C. elegans | Olsson-Carter and Slack, 2010 | |
| lin-4 | promoter of differentiation | post dauer development | C. elegans | Karp and Ambros, 2012 | |
| miR-125 | restriction of cell fate | mushroom body development | Drosophila | Wu et al, 2012 | |
| miR-125 | promoter of differentiation | ventral nerve cord development | Drosophila | Wu et al, 2020 | |
|
| |||||
| let-7 | let-7a-f | promoter of differentiation | mESCs | Melton et al, 2010 | |
| let-7b | promoter of differentiation | neurogenesis | Mouse | Shu et al, 2019 | |
| let-7 | promoter of differentiation | gliogenesis | Mouse | Nishino et al, 2013 | |
| let-7 | promoter of differentiation | gliogenesis | Humans | Patterson et al, 2014 | |
| let-7 | promoter of differentiation | gliogenesis | mESCs | Shenoy et al, 2015 | |
| let-7 | promoter of differentiation | hypodermis development | C. elegans | Reinhart et al, 2000 | |
| miR-48, miR-84, miR-241 | promoter of differentiation | hypodermis development | C. elegans | Abbott et al, 2005 | |
| let-7, mir-84 | restriction of cell fate | vulval development | C. elegans | Johnson et al, 2005 | |
| let-7 | promoter of differentiation | male copulatory organ development | C. elegans | Aeschimann et al, 2019 | |
| let-7 | regulation of molting cycle | C. elegans | Hayes et al, 2006; Monsalve and Frand, 2014 | ||
| let-7 | restriction of cell fate | mushroom body development | Drosophila | Wu et al, 2012 | |
| let-7 | promoter of differentiation | mushroom body development | Drosophila | Kucherenko et al, 2012 | |
| let-7 | promoter of differentiation | ventral nerve cord development | Drosophila | Wu et al, 2020 | |
| let-7 | regulation of circadian rhythm | Drosophila | Chen et al, 2014a | ||
|
| |||||
| bantam | miR-58 | anti apoptotic | embryogenesis | C. elegans | Sherrard et al, 2017 |
| miR-58 | dauer entry | C. elegans | Alvarez-Saavedra and Horvitz, 2010; de Lucas et al, 2015; Lozano et al, 2016 | ||
| bantam | anti apoptotic | eye development | Drosophila | Brennecke et al, 2003 | |
| bantam | anti apoptotic | wing development | Drosophila | Gerlach et al , 2019 | |
| bantam | promoter of proliferation | wing development | Drosophila | Herranz et al, 2012a; Gerlach et al, 2019 | |
| bantam | inhibitor of differentiation | wing development | Drosophila | Becam et al, 2011 | |
| bantam | promoter of proliferation | neurogenesis | Drosophila | Weng and Cohen, 2015 | |
| bantam | promoter of proliferation | optic lobe development | Drosophila | Li and Padgett, 2012 | |
| bantam | regulation of circadian rhythm | Drosophila | Kadener et al, 2009 | ||
|
| |||||
| mir-430/mir-294/mir-302 | miR-430 | MZT | Zebrafish | Giraldez et al, 2005 | |
| miR-430 | cell orientation | neurogenesis | Zebrafish | Takacs and Giraldez, 2016 | |
| miR-430 | modulation of cell number | endoderm development | Zebrafish | Choi et al, 2007 | |
| miR-294 | extraembryonic tissue development | Mouse | Paikari et al, 2017; Spruce et al 2010 | ||
| miR-302-367 | inhibitor of differentiation | neurogenesis | Mouse | Parchem et al, 2015 | |
| miR-290-295 | inhibitor of differentiation | mESCs | Wang et al, 2008 | ||
| miR-302-367 | inhibitor of differentiation | mESCs | Wang et al, 2008 | ||
| miR-17-92b | inhibitor of differentiation | mESCs | Wang et al, 2008 | ||
|
| |||||
| mir-100 | miR-51 | embryogenesis | C. elegans | Alvarez-Saavedra and Horvitz, 2010 | |
| miR-51-56 | pharynx development | C. elegans | Shaw et al, 2010 | ||
|
| |||||
| mir-1 | miR-1 | inhibitor of differentiation | cardiac cell fate specification | Mouse | Zhao et al, 2005; Zhao et al, 2007 |
| miR-1 | inhibitor of differentiation | skeletal cell fate specification | Chick | Goljanek-Whysall et al, 2014; Goljanek-Whysall et al, 2011 | |
| miR-1 | promoter of differentiation | skeletal cell fate specification | Zebrafish | Mishima et al, 2009; Stahlhut et al, 2012 | |
| miR-1 | acetyl choline sensitivity | C. elegans | Simon et al, 2008 | ||
|
| |||||
| mir-2 | miR-2 | A/P patterning | early embryogenesis | Drosophila | Rodel et al, 2013 |
|
| |||||
| mir-7 | miR-7 | promoter of differentiation | photoreceptor development | Drosophila | Li and Carthew, 2005 |
| miR-7 | inhibitor of differentiation | spermatogenesis | Drosophila | Pek et al, 2009 | |
| miR-7 | promoter of proliferation | wing development | Drosophila | Aparicio et al, 2014 | |
| miR-7 | promoter of differentiation | optic lobe development | Drosophila | Caygill and Brand, 2017 | |
| miR-7 | promoter of differentiation | sensory organ development | Drosophila | Li et al, 2009 | |
| miR-7 | inhibitor of differentiation | hematopoietic cell development | Drosophila | Tokusumi et al, 2011; Tokusumi et al, 2018 | |
| miR-7 | promoter of proliferation | female germline development | Drosophila | Yu et al, 2009 | |
|
| |||||
| mir-8/mir-200 | miR-200 | promoter of differentiation | olfactory neuron specification | Mouse | Choi et al, 2008 |
| miR-200 | promoter of differentiation | olfactory neuron specification | Zebrafish | Choi et al, 2008 | |
| miR-8 | inhibitor of differentiation | optic lobe development | Drosophila | Morante et al, 2013 | |
| miR-8 | regulation of body size | Drosophila | Hyun et al, 2009 | ||
|
| |||||
| mir-9 | miR-9c | modulation of cell number | PGC | Drosophila | Kugler et al, 2013 |
| miR-9a | modulation of cell number | sensory organ development | Drosophila | Li et al, 2006 | |
| miR-9a | inhibitor of differentiation | sensory organ development | Drosophila | Li et al, 2006 | |
| miR-9a | anti apoptotic | wing development | Drosophila | Bejarano et al, 2010; Biryukova et al, 2009 | |
| miR-9a | regulation of body size | Drosophila | Suh et al, 2015 | ||
| miR-9* | promoter of differentiation | neurogenesis | Mouse | Yoo et al, 2009 | |
|
| |||||
| miR-14 | promoter of proliferation | wing development | Drosophila | Kim et al, 2014 | |
| miR-14 | promoter of cell autophagy | salivary glands development | Drosophila | Nelson et al, 2014 | |
| miR-14 | modulation of starvation sensitivity | Drosophila | Varghese et al, 2010 | ||
|
| |||||
| mir-34 | miR-34 | restriction of cell fate | mESCs | Choi et al, 2017 | |
| miR-34 | pro-apoptotic | Cell lines | Chang et al, 2007a | ||
| miR-34 | robustness of germline development | C. elegans | Burke and Hammell, 2015 | ||
|
| |||||
| miR-35 | inhibitor of differentiation | sex specification | C. elegans | McJunkin and Ambros, 2017; Benner et al, 2019 | |
| miR-35 | anti apoptotic | embryogenesis | C. elegans | Sherrard et al, 2017 | |
|
| |||||
| miR-54 | promoter of differentiation | male sensory ray patterning | C. elegans | Zhang and Emmons, 2009 | |
|
| |||||
| miR-56 | promoter of differentiation | male sensory ray patterning | C. elegans | Zhang and Emmons, 2009 | |
|
| |||||
| miR-61 | promoter of differentiation | vulva development | C. elegans | Yoo and Greenwald, 2005 | |
|
| |||||
| mir-71 | miR-71 | promoter of differentiation | AWC ON/OFF specification | C. elegans | Hsieh et al, 2012 |
| miR-71 | developmental timing | post L1 diapause development | C. elegans | Zhang et al, 2011 | |
|
| |||||
| miR-80/81 | dauer entry | C. elegans | Than et al, 2013 | ||
| mir-29 | miR-83 | robustness of differentiation | germline development | C. elegans | Burke and Hammell, 2015 |
|
| |||||
| mir-92 | miR-92a/b | inhibitor of differentiation | neurogenesis | Drosophila | Yuva-Avdemir et al, 2015 |
| miR-235 | inhibitor of differentiation | L1 diapause | C. elegans | Kasuga et al, 2013 | |
|
| |||||
| mir-124 | miR-124 | promoter of differentiation | neurogenesis | Mouse | Makeyev et al, 2007, Yoo et al, 2009 |
| miR-124 | promoter of proliferation | neurogenesis | Drosophila | Weng and Cohen, 2012 | |
| miR-124 | dauer entry | C. elegans | Than et al, 2013 | ||
|
| |||||
| mir-126 | miR-126 | vascular system development | Zebrafish | Fish et al, 2008 | |
|
| |||||
| mir-128 | miR-128 | promoter of differentiation | neurogenesis | Mouse | Bruno et al, 2011 |
|
| |||||
| mir-133 | miR-133 | inhibitor of differentiation | skeletal cell fate specification | Chick | Goljanek-Whysall et al, 2014 |
| miR-133 | promoter of differentiation | skeletal cell fate specification | Zebrafish | Mishima et al, 2009 | |
|
| |||||
| mir-144 | miR-144 | promoter of differentiation | erythropoesis | Zebrafish | Kretov et al, 2020 |
|
| |||||
| mir-184 | miR-184 | promoter of differentiation | oogenesis | Drosophila | Iovino et al, 2009 |
|
| |||||
| mir-203 | miR-203 | promoter of differentiation | fast-twitch muscle specification | Zebrafish | Lu et al, 2017 |
|
| |||||
| miR-234 | dauer entry | C. elegans | Than et al, 2013 | ||
|
| |||||
| miR-273 | promoter of differentiation | ASE left/right specification | C. elegans | Chang et al, 2004 | |
|
| |||||
| miR-275 | miR-275 | promoter of differentiation | spermatogenesis | Drosophila | Eun et al, 2012 |
| miR-306 | promoter of differentiation | spermatogenesis | Drosophila | Eun et al, 2012 | |
|
| |||||
| miR-278 | promoter of proliferation | oogenesis | Drosophila | Yu et al, 2009 | |
|
| |||||
| mir-279/996 | miR-279 | promoter of differentiation | oogenesis | Drosophila | Yoon et al, 2011 |
| miR-279 | regulation of circadian rhythm | Drosophila | Luo and Sehgal, 2012 | ||
|
| |||||
| mir-309 | miR-309 | MZT | Drosophila | Bushati et al, 2008 | |
|
| |||||
| mir-451 | miR-451 | promoter of differentiation | erythropoesis | Zebrafish | Cheloufi et al, 2010; Cifuentes et al, 2010 |
| miR-451 | promoter of differentiation | erythropoesis | Mouse cell lines | Yang et al, 2010 | |
|
| |||||
| mir-365 | miR-786 | rhythmic defecation cycles | C. elegans | Miska et al, 2007; Kemp et al, 2012 | |
|
| |||||
| miR-791 | promoter of differentiation | CO2 sensing neuron specification | C. elegans | Drexel et al, 2016 | |
|
| |||||
| lsy-6 | promoter of differentiation | ASE left/right specification | C. elegans | Johnston and Hobert, 2003 | |
|
| |||||
| mir-959-964 | regulation of circadian rhythm | Drosophila | Vodala et al, 2012 | ||
|
| |||||
| mir-965 | inhibitor of proliferation | abdominal histoblast development | Drosophila | Verma and Cohen, 2015 | |
|
| |||||
| mir-969 | modulation of cell number | PGC | Drosophila | Kugler et al, 2013 | |
|
| |||||
| mir-iab-4 | promoter of differentiation | ventral nerve cord development | Drosophila | Garaulet et al, 2014 | |
|
| |||||
| mir-iab-8 | promoter of differentiation | ventral nerve cord development | Drosophila | Garaulet et al, 2014 | |
Some recent reviews extensively cover the regulation of microRNAs (Gebert and MacRae, 2019), microRNAs in mammalian stem cells (Shenoy and Blelloch, 2014), and cancer therapeutics (Rupaimoole and Slack, 2017). The overriding theme of this review is the role of microRNAs in maintaining, modifying, and reinforcing cell fate decisions. We will briefly summarize the biogenesis of microRNAs and then elaborate on the role of microRNAs in differentiation during development and stress conditions, with an emphasis on research in model organisms.
MicroRNA biogenesis and action
Canonical microRNAs are transcribed from endogenous genic loci as long primary microRNAs (pri-microRNAs) by RNA polymerase II. MicroRNAs that are transcribed as part of a single polycistronic primary transcript are categorized as belonging to the same microRNA cluster. The transcription of microRNAs can be activated or repressed by cell fate-specific transcription factors. For example, during muscle differentiation, myogenic master transcription factors like myogenin and MYOD1 activate mir-1 (Rao et al., 2006). The oncogenic transcription factor Myc activates the transcription of the pro-tumorigenic mir-17-92 cluster and represses the transcription of let-7 and other tumor suppressive microRNAs (Chang et al., 2007b; He et al., 2005).
The pri-microRNAs are subsequently cleaved by the Microprocessor complex consisting of the Drosha RNase III endonuclease and Pasha/DGCR8, to generate precursor microRNAs (pre-microRNAs) in the nucleus (Lee et al., 2003). Drosha and Pasha are subject to post-translational modifications that affect their localization and stability, thus affecting microRNA processing at a global level (reviewed in Ha and Kim, 2014). Sequence-specific regulation at this level of biogenesis has been reported in the case of pri-let-7, where LIN28 binding to the primary transcript inhibits Drosha-mediated processing in multiple organisms (Viswanathan et al., 2008, Van Wynsberghe et al., 2011, reviewed in Tsialikas and Romer-Seibert, 2015).
Pre-microRNA hairpins are exported into the cytoplasm by Exportin 5, where, after a second round of endonucleolytic cleavage by Dicer, a microRNA duplex is generated (Grishok et al., 2001). In some organisms, Dicer interacts with other RNA-binding proteins such as Loquacious and TRBP to process certain microRNAs (Fukunaga et al., 2012). Post-translational modifications on these RNA-binding proteins can modulate the activity of Dicer and affect biogenesis of specific microRNAs (reviewed in Ha and Kim, 2014). Following unwinding of the duplex and strand selection, the 21–24 nucleotide-long mature microRNA is loaded into the Argonaute protein that forms the core of the microRNA-Induced Silencing Complex (miRISC) (Grishok et al., 2001). The miRISC-associated microRNA binds the 3’UTR of target mRNAs through sequence complementarity and recruits downstream effectors, usually resulting in translational repression and mRNA decay (reviewed in Jonas and Izaurralde, 2015). The 2–8 nucleotides from the 5’ end of the mature microRNA are referred to as the seed sequence. Pairing with a target mRNA requires near-complete complementarity of the seed sequence, with partial complementarity along the rest of the microRNA and the target. MicroRNAs with the same seed sequence belong to the same microRNA family (see Table 1 for details). A bevy of computational and biochemical methods to predict and validate microRNA-mRNA target pairs has been developed (reviewed in Lee and Ule, 2018; Bagnacani et al., 2019; Monga and Kumar, 2019).
Post-translational modifications of Argonaute can regulate steady-state microRNA action (reviewed in Gebert and MacRae, 2019). Mass spectrometry has revealed that conserved phosphorylation sites in the human and C. elegans Argonaute orthologs, AGO2 and ALG-1, respectively, regulate mRNA target binding (Golden et al., 2017; Quévillon Huberdeau et al., 2017). In human cell lines, a phosphorylation cycle mediated by the CSNK1A1 casein kinase and ANKRD52-PPP6A phosphatase has been proposed to maintain the global efficiency of microRNA-target interactions, where target binding triggers AGO2 phosphorylation followed by rapid dephosphorylation (Golden et al., 2017). In a separate report, phosphorylation of the Argonautes AGO1 and AGO2 was found to be required for interaction with LIM domain-containing proteins. These LIMD proteins form a clamp between Argonaute and the adaptor protein GW182 to enhance target silencing by specific microRNAs. In the absence of that phosphorylation event, AGO3, which has a phosphomimetic residue at the site where AGO1 and AGO2 are phosphorylated, is recruited to perform the same silencing function (Bridge et al., 2017). These data suggest that Argonaute function can be context dependent. Global downregulation of microRNA activity in mouse oocytes has been suggested to be a consequence of the expression of a truncated isoform of AGO2, lacking all functional domains (Freimer et al., 2018b). In the Drosophila S2 cell line, absence of mature microRNAs leads to AGO1 poly-ubiquitination by the E3 ubiquitin ligase Iruka at a conserved lysine residue, marking AGO1 for degradation (Kobayashi et al., 2019). Therefore, post-translational modifications of Argonaute play essential roles in modulating miRISC activity.
During early embryogenesis, the transcripts and protein components required for microRNA function are maternally contributed (Denli et al., 2004; Giraldez et al., 2005). As discussed in later sections, maternal and zygotic mutant embryos of DGCR8 and Dicer are inviable. However, these mutants have been instrumental in characterizing non-canonical microRNAs. Pre-microRNA-like RNAs can be derived from direct transcription of small hairpin RNAs, splicing of pre-mRNAs (mirtrons), and processing of tRNAs and snoRNAs. Biogenesis of these microRNAs is independent of the Microprocessor complex, but still requires Dicer (reviewed in Ha and Kim, 2014). m7G-capped pre-microRNAs that are transcribed and processed in a Drosha-independent manner have also been identified in new born mice (Xie et al., 2013). Dicer-independent biogenesis has been reported in the specific case of the conserved microRNA miR-451, which undergoes Drosha-mediated cleavage and then directly loaded into Argonaute for further processing (Cheloufi et al, 2010; Cifuentes et al., 2010; Yang et al., 2010). However, while the existence of non-canonical microRNAs suggests alternate evolutionary pathways for microRNA biogenesis, very few non-canonical microRNAs (e.g., miR-320, miR-451) are conserved.
miRISC composition in context-dependent regulation of target mRNAs
The binding of the microRNA-associated Argonaute to a target mRNA is usually followed by the recruitment of RNA degradation factors. Argonaute interacts with the adaptor protein GW182 to recruit the PAN2-PAN3 and CCR4-NOT deadenylase complexes. Deadenlyation is followed by decapping and mRNA degradation, mediated by the 5’ to 3’ exonuclease XRN1. CCR4-NOT complex has also been hypothesized to interact with the translation initiation factor eIF4A and the DEAD box helicase DDX6 to mediate translational repression (Meijer et al., 2013; Chen et al., 2014b; Mathys et al., 2014). However, the exact mechanism of microRNA-mediated translational repression remains to be fully resolved (reviewed in Jonas and Izaurralde, 2015). Additionally, GW182-independent mechanisms of translational repression have been reported in C. elegans and D. melanogaster (Fukaya and Tomari, 2012; Iwasaki et al., 2009; Jannot et al., 2016). The relative contribution of translational repression and mRNA degradation continues to be a much debated topic in the field. Recent work has revealed that microRNA binding leads to accelerated poly(A) tail shortening and mRNA decay in cell lines (Eisen et al., 2020). However, in the germline and during early embryogenesis, miRISC binding leads to shortening of poly(A) tails and translational repression, with negligible effect on mRNA stability (Bazzini et al., 2012; Fukaya and Tomari, 2011; 2012; Subtelny et al., 2014; Wu et al., 2010; Dallaire et al., 2018). In Drosophila oocytes, increased polyadenylation occurs during late oogenesis and correlates with translational activation (Lim et al., 2016). This suggests that miRISC can act reversibly in a tissue-specific manner to regulate temporal expression of maternally contributed transcripts during development. Similarly, reversible miRISC binding and translational repression has also been reported to maintain synaptic plasticity in neurons (Muddashetty et al., 2011).
The composition of miRISC can also affect mRNA target fate. In vitro experiments with C. elegans lysates have shown that specific microRNAs bind to different protein complexes with varying affinities (Chan et al., 2008). miRISC isolates from C. elegans embryonic extracts reveal interactions with multiple mRNP complexes (Wu et al., 2016). Lysate fractionation experiments across different tissues and cell lines derived from mice have also revealed that functional microRNAs can associate with different protein complexes based on mitogenic signals. Intriguingly, cell lines or tissues associated with enhanced mTOR signaling, such as embryonic cells and activated T cells, show upregulation of the GW182 protein and a shift from a low molecular weight miRISC to a higher molecular weight miRISC (La Rocca et al., 2015). This suggests that regulation of miRISC is more prevalent in cells that make dynamic decisions in response to growth signals or changing environmental conditions. Other RNA-binding proteins such as DEAD-box helicases, Pumilio, and Tudor domain proteins also regulate miRISC activity in a sequence- or tissue-specific manner (e.g., Alessi et al., 2015; Caudy et al., 2003; Hammell et al., 2009b). In C. elegans, different cofactors interact with the Argonaute in the germline and the soma to regulate the fate of target mRNAs. While the GW182 protein AIN-1 is exclusively part of somatic miRISC and mediates target degradation, the DEAD-box helicase GLH-1 specifically associates with germline miRISC and mediates translational repression and target stabilization (Dallaire et al., 2018).
MicroRNA-associated protein machinery has been found in RNP granules in several organisms. In mammalian cells, a “bird’s nest” model involving association of the Microprocessor and pri-microRNAs with paraspeckle components NEAT1, NONO, and PSF has been proposed (Jiang et al., 2017). In D. melanogaster, C. elegans, and human cell lines, miRISC and mRNA degradation machinery are localized to processing bodies (P bodies) (Gallo et al., 2008; reviewed in Parker and Sheth, 2007). Although the factors that drive the possible sequestration and release of these components from P bodies remain unknown, phase separation may represent yet another mechanism by which microRNA action may be regulated.
Embryonic differentiation
Early embryogenesis: MZT and early cell fate specification
During early embryogenesis, as all protein synthesis depends on maternally contributed transcripts, post-transcriptional processing is the predominant gene regulatory mechanism. Maternally contributed and zygotically expressed microRNAs and RNA-binding proteins are essential for degrading maternal transcripts and ensuring faithful maternal-to-zygotic transition (MZT). In addition to MZT, microRNAs also mediate early cell fate and patterning decisions in multiple organisms (Fig 2).
Fig 2. MicroRNAs play important roles throughout embryogenesis.
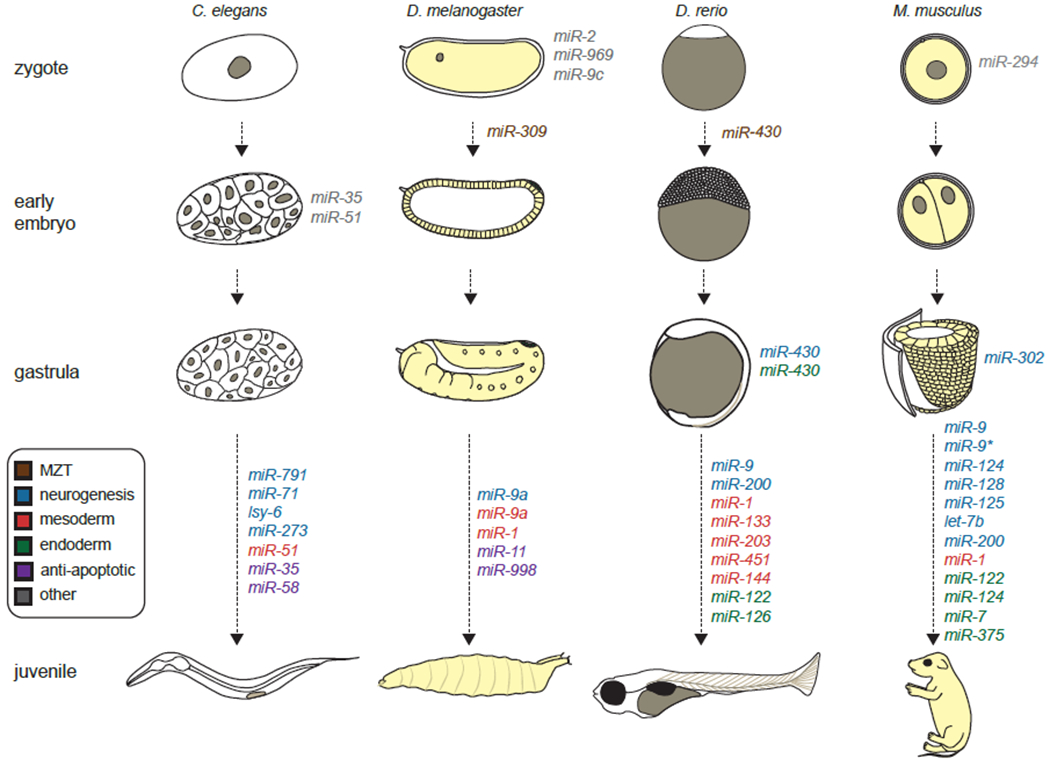
MicroRNAs are mediators of maternal-to-zygotic transition (MZT) and regulators of cell fate specification during early embryogenesis. MicroRNAs continue to modulate proliferation and differentiation in the neurectoderm, mesoderm, and endoderm during and after gastrulation. The developmental time at which the relevant microRNAs function has been indicated.
In C. elegans, the miR-35 family of microRNAs, miR-35–42, is both maternally contributed and zygotically expressed during early embryogenesis (Wu et al., 2010). The miR-35 family is preferentially loaded into the ALG-2 Argonaute (Corrêa et al., 2010) and is required for several aspects of embryonic viability (reviewed in McJunkin, 2018). The maternal or zygotic load of the miR-35 family is sufficient for ensuring viability (Alvarez-Saavedra and Horvitz, 2010; McJunkin and Ambros, 2014). However, both maternal and zygotic populations of the miR-35 family are required for normal sex determination, revealing a novel role for maternally contributed factors in zygotic sex determination. The translational repression of the sup-26 transcript, which encodes an RNA-binding, male-promoting factor, by the miR-35 family is required for normal sex determination. Similarly, miR-35 family-mediated translational repression of the nhl-2 transcript, which encodes an RNA-binding protein, is required for both normal sex determination and embryonic viability (McJunkin and Ambros, 2017). More recently, the mir-35 family has been linked to sex specification and embryonic viability by modulating masculinizing and feminizing pathways to ensure clear sex specification, and thereby embryonic viability (Benner et al., 2019). The levels of miR-35 decrease during mid-embryogenesis (Stoeckius et al., 2009; Wu et al., 2010). Overall, the miR-35 family has been proposed to act as a timer that prevents premature sex specification. The worm homolog of the conserved miR-100 family (the miR-51 family) is also expressed during early embryogenesis and acts redundantly to regulate embryonic viability (Alvarez-Saavedra and Horvitz, 2010).
In D. melanogaster embryos, the miR-2 family is maternally contributed and acts synergistically with the key morphogen and RNA binding protein Bicoid, resulting in translational repression and establishment of the anterior-posterior axis (Rödel et al., 2013). Maternally contributed miR-969 and miR-9c are required to generate primordial germ cells (PGCs) (Kugler et al., 2013). During MZT, the poly(A) polymerase Wispy adds short poly(A) tails to maternal microRNAs, resulting in their degradation and clearance (Lee et al., 2014). The early embryonic transcription factor Zelda induces the expression of several microRNA families, including the miR-309 family (Fu et al., 2014). The miR-309 family binds to maternal mRNA transcripts and degrades them (Bushati et al., 2008). The levels of specific miR-309 family members, miR-3 and miR-309, drop off sharply after early embryogenesis. Overexpression of miR-3 and miR-309 later during embryogenesis leads to the silencing of the target Van Gogh, which mediates the planar cell polarity (PCP) pathway and results in denticle organization defects (Zhou et al., 2018).
During MZT in the zebrafish Danio rerio, the zygotically transcribed miR-430 family initially represses translation of maternally contributed mRNAs and then increases the efficiency of deadenylation and degradation (Giraldez et al., 2006; Subtelny et al., 2014; Yartseva and Giraldez, 2015; Beaudoin et al., 2018). An extensive sequencing and modeling-based approach showed that the temporal and spatial dynamics of miR-430-mediated target mRNA degradation are achieved by a combinatorial effect of stabilizing and destabilizing cis elements in the 3’UTRs of individual transcripts that recruit trans-acting factors (Mishima et al., 2006; Vejnar et al., 2019).
Maternal and zygotic Pasha/DGCR8 null mouse embryos do not exhibit any defects before implantation and differentiate to give rise to the trophectoderm and inner cell mass, suggesting that microRNAs are not necessary for the generation of embryonic pluripotent cells in vivo (Suh et al., 2010). MicroRNAs that are expressed in whole embryos at these stages have been identified. These include the miR-294 family, which are the homologs of the zebrafish mirR-430 family (Yang et al., 2016). However, miR-294 family members do not participate in MZT, and their necessity for establishment of pluripotency in vivo has not been demonstrated (Greve et al., 2013)(reviewed in Greve et al., 2013). DGCR8 null and mir-294 null embryos do not survive, indicating the necessity of microRNAs for post-implantation development (Suh et al., 2010). More recently, the expression of the miR-294 family/miR-290–95 cluster was reported in the yolk sac and placenta. Loss of miR-290 expression resulted in reduced trophoblast progenitor cell proliferation, a reduction of the placental size, and defects in maternal-fetal transport (Paikari et al., 2017; Spruce et al., 2010).
Taken together, microRNAs play essential roles in early cell fate decisions, and mediate degradation of maternal transcripts in some organisms. However, as mentioned in the previous section, translational repression appears to be the initial predominant effect of microRNA binding during early embryogenesis (Bazzini et al., 2012; Fukaya and Tomari, 2011; 2012; Subtelny et al., 2014; Wu et al., 2010). Additionally, in mouse embryonic stem cells (mESCs), the DEAD Box helicase DDX6 has been shown to mediate translational repression with no effect on mRNA stability (Freimer et al., 2018a). These data suggest that microRNAs modulate targets via different mechanisms in a temporally sensitive manner during early embryogenesis.
Embryonic stem cells: anti- and pro-differentiation microRNAs
As DGCR8 embryos are inviable, DGCR8 deficient mouse embryonic stem cells (mESCs) have been used to identify conserved microRNAs that potentially promote or inhibit differentiation in vivo. Each stem cell can undergo symmetric division, giving rise to two equivalent daughter cells, or asymmetric division, giving rise to one stem cell and one differentiated cell. Loss of DGCR8 in mESCs leads to the accumulation of cells in the G1 phase and delayed differentiation (Wang et al., 2007). Clever rescue assays in DGCR8 null cells led to the identification of three microRNA clusters termed the ESC-specific cell cycle regulating microRNAs (ESCC), miR-17-92b, miR-290-295, and miR-302-367. Intriguingly, some members of the ESCC microRNAs share their seed sequence with the zebrafish miR-430 family, suggesting conservation of both sequence and function in regulating early embryonic fate. The ESCC microRNAs downregulate the CDK inhibitors p21, Lats2, and Rbl2 to allow efficient G1/S transitions (Wang et al., 2008).
The biogenesis of the miR-17-92b cluster involves an additional cleavage of the primary transcript by the endonuclease CPSF3 guided by the spliceosome-associated ISY1. This intermediate progenitor microRNA is then processed by the Drosha-DGCR8 complex (Du et al., 2015). Expression of ISY1 is thought to be required for the establishment of the poised pluripotency stage, as the cells transition from the naïve pluripotent stage (capable of giving rise to all embryonic lineages) to the primed pluripotent stage (capable of giving rise to a limited number of embryonic lineages) (Du et al., 2018).
The let-7 family of microRNAs was identified as a promoter of differentiation. Bioinformatic analysis has suggested that FOXO transcription factors may be involved in the transcription of let-7 (Gaeta et al., 2017). The let-7 family microRNAs silence some of the factors that promote stem cell-like potential such as Sall4, Myc, and Lin28 to enhance differentiation. Additionally, the ESCC microRNAs seem to act antagonistically to the let-7 microRNAs by upregulating the same targets indirectly (Melton et al., 2010). The negative regulation of let-7 processing by Lin28 has been hypothesized to be the factor that allows ESCCs to be dominant over the let-7s in ESCs (reviewed in Greve et al., 2013). Single cell sequencing revealed that introducing miR-294 into DGCR8 null ESCs increases the transcriptional homogeneity of the population, making them similar to wild-type ESCs. However, introducing let-7c into the same DGCR8 null ESCs leads to increased transcriptional heterogeneity and enhanced phasing of the cell cycle genes, indicating formation of subpopulations of cells (Gambardella et al., 2017).
mESCs are pluripotent and, upon transplantation, give rise to a chimeric inner cell mass, rarely contributing to the extraembryonic layers. Recently, miR-34a was identified as a regulator that restricts the cell fate potential of mESCs and induced pluripotent stem cells (iPSCs). miR-34a-deficient mESCs have an expanded cell fate potential, forming both embryonic and extra embryonic tissues. miR-34a-deficient mESCs also have a subpopulation of cells expressing high levels of MuERV-L endogenous retroviruses that are usually expressed in the totipotent 2-cell stage embryo. miR-34a was found to directly downregulate the transcription factor Gata2 to repress MuERV-L induction and restrict cell fate potential (Choi et al., 2017).
Therefore, in mESCs, microRNAs inhibit or promote differentiation by targeting cell cycle factors, RNA binding proteins, or key transcription factors. The molecular mechanisms governing the regulation of these microRNAs remain active areas of research.
Neurogenesis: temporally regulated transcriptome reprogramming
The nervous system is derived from the neurectoderm following gastrulation. In vertebrates, the neurectoderm proliferates and differentiates to form neuronal progenitor cells that participate in neural tube formation or neurulation. The dorsal-most neural progenitors form the central nervous system (CNS). MicroRNAs act at critical time points by downregulating factors required for neurulation, as well as differentiation of glia and neurons (Fig 2).
The miR-430 family and the homologous miR-302 family, which have critical roles during embryogenesis, are also involved in early brain morphogenesis in zebrafish and mice (Giraldez et al., 2005; Parchem et al., 2015), albeit by potentially different mechanisms. In zebrafish, the absence of miR-430 does not affect differentiation of neuronal progenitors. However, it does result in misorientation of the mitotic spindle and defective stereotypical cell divisions of the progenitors prior to neural tube formation. The failure of neural progenitors to distribute across the axial midline leads to failure of neuroepithelial integration and defects in neural tube morphogenesis (Takacs and Giraldez, 2016). In mice, the miR-302 family directly downregulates its target Fgf15 to prevent precocious neurogenesis and defective neural tube formation (Parchem et al., 2015).
The microRNAs miR-124, miR-128, miR-9, miR-125, and let-7b have been identified as key regulators of neuronal and glial differentiation in multiple organisms (Cao et al., 2007; Makeyev et al., 2007; Visvanathan et al., 2007; Bruno et al., 2011; Franzoni et al., 2015 Li et al., 2006, Nishino et al., 2013; Shibata et al., 2011). In mouse embryos, miR-124 targets the transcript encoding splicing protein PTBP1 to reprogram the transcriptome and to promote differentiation of neuronal progenitors into all neuronal populations (Makeyev et al., 2007). miR-128 targets the nonsense-mediated decay (NMD) machinery in a neuron-specific manner to promote differentiation and inhibit migration of neurons during the development of the CNS in mice (Bruno et al., 2011; Franzoni et al., 2015). In D. melanogaster embryos, mir-9a prevents the formation of ectopic sensory organ precursors (SOPs), that develop into sensory structures, and the associated Peripheral Nervous System (PNS) (Li et al., 2006). However, miR-9 promotes neuronal migration and neuronal differentiation in mice and zebrafish (Coolen et al., 2012; La Torre et al., 2013; Shibata et al., 2011). miR-125 and let-7b act together late during embryogenesis to promote the switch from neurogenesis to gliogenesis by degrading several targets, including components of the JAK-STAT pathway in mice and the Notch-Delta signaling pathway in humans (Nishino et al., 2013; Patterson et al., 2014; Shenoy et al., 2015).
A recent comprehensive study of microRNAs involved in the development of the mouse neocortex revealed that microRNAs can act in a temporally regulated manner to pattern the different cortical layers. The radial glial cells (RGCs), which are the neocortical progenitors, initially express miR-128 and miR-9. As development proceeds, the levels of miR-128 and miR-9 decrease, and the levels of let-7 increase in the RGCs. The neurons that differentiate from these RGCs maintain the microRNA expression profile that their progenitors expressed. Expression of miR-128, miR-9, and let-7 specify neurons to Layer VI, Layer V, and Layers IV-II, respectively. Although these microRNAs are not required for RGC proliferation or neuron production, altering their levels during development results in a change in the cortical position of the derived neurons. Therefore, opposing gradients of microRNAs specify patterning of the cortex by temporally regulating cell fates (Shu et al., 2019).
MicroRNAs that specify subtypes of sensory neurons have also been identified. In mice and zebrafish, the miR-200 family has been implicated in the terminal differentiation of olfactory neurons (Choi et al., 2008). In C. elegans, miR-791 is expressed in CO2-sensing neurons and represses the broadly expressed genes akap-1 and cah-3 to confer CO2-sensing functionality (Drexel et al., 2016). MicroRNAs are also involved in asymmetric left versus right neuronal specification in C. elegans. The microRNAs lsy-6 and mir-273 repress their respective targets in the ASEL and ASER neurons to ultimately express distinct chemoreceptors that specify asymmetry in this left-right pair of chemosensory neurons (Johnston and Hobert, 2003; Chang et al., 2004; Cochella and Hobert, 2012). miR-71 acts downstream of cell-cell communication events to inhibit its target tir-1 that regulates calcium signaling and specifies stochastic AWC (ON) versus AWC (OFF) neuron differentiation (Hsieh et al., 2012).
The repeated theme of the downregulation of key molecular factors by microRNAs to mediate transitions of cell fates is very evident in the above examples. In particular, the role of miR-9/9* and miR-124 in modulating, as well as reprograming, neural fates has been characterized. In mouse embryos, miR-9* and miR-124 together regulate the composition of the SWI/SNF-like BAF chromatin remodeling complex in post-mitotic neurons to promote dendritic growth and activity (Yoo et al., 2009). Remarkably, in human adult fibroblasts, overexpression of miR-9/9* and miR-124, in combination with neural transcription factors, induces their conversion into neurons (Victor et al., 2014; Yoo et al., 2011). The expression of miR-9/9* and miR-124 triggers reconfiguration of the chromatin state and DNA methylation, allowing transcription factors to access certain genes and induce specific neural cell fates (Abernathy et al., 2017; Lee et al., 2018). Thus, in this particular scenario, microRNA-mediated resetting of the transcriptome occurs in two different contexts, in vivo, during development, and in vitro, during cell reprogramming.
Mesoderm and endoderm: tissue specific microRNAs
In vertebrates, the mesoderm and endoderm are derived from the same set of progenitor cells. The miR-430 family in zebrafish helps maintain the balance between the Nodal agonist squint and antagonist lefty to regulate the number of endodermal cells at the gastrula stage (Choi et al., 2007). Several microRNAs are expressed in an organ- or tissue-specific manner (Wienholds et al., 2005) (Fig 2). For example, miR-122 is expressed specifically in hepatocytes starting in embryogenesis in mice and zebrafish (Chang et al., 2004; Wienholds et al., 2005). miR-126 is expressed specifically in the endothelial cells and is required for the development of an intact vascular system in zebrafish (Fish et al., 2008). Several microRNAs act sequentially during the development of the pancreas. miR-124 regulates the level of the key transcription factor Pdx1 early during pancreatic development, and miR-7 and miR-375 are involved in β-cell proliferation and differentiation (reviewed in Dumortier and Obberghen, 2012).
The microRNA miR-1 functions in muscle development in both invertebrates and vertebrates. Depletion of miR-1 results in poor muscle development and lethality in Drosophila (Kwon et al., 2005; Sokol and Ambros, 2005). miR-1 is expressed in the pharyngeal and body wall muscles in C. elegans but does not appear to regulate their development. Instead, miR-1 functions at the neuromuscular junction to modulate acetyl choline sensitivity (Simon et al., 2008). Studies in mice, frogs, chicks, and zebrafish show that miR-1 is expressed embryonically in mesodermal cells destined to become skeletal muscle cells and cardiac muscle (only in chick and mice) (reviewed in Mok et al., 2017). In mice, miR-1 targets several factors to promote proliferation of myocytes and prevent their premature differentiation into cardiac cells (Zhao et al., 2007; 2005). In chick, miR-1 and miR-133 act to prevent premature differentiation of skeletal muscle cells (Goljanek-Whysall et al., 2014; 2011). In zebrafish, miR-1 and miR-133 function together to promote proper actin organization and sarcomere assembly (Mishima et al., 2009; Stahlhut et al., 2012). A recent report showed that miR-203 specifies fast-twitch muscle cell fate in zebrafish by downregulating the transcription factor Dmrt2a (Lu et al., 2017). miR-51 is required for pharyngeal muscle attachment in C. elegans (Shaw et al., 2010). In Drosophila, mir-9a mutant embryos exhibit cell fate specification defects in tendon-muscle junctions and have 40% lethality at relatively high rearing temperatures of 25–29°C (Yatsenko and Shcherbata, 2014).
The Dicer-independent, but Ago2-dependent, microRNA miR-451 is involved in erythropoiesis in zebrafish and mouse cell lines and functions by directly downregulating the pro-stem cell transcription factor Gata2 (Cheloufi et al., 2010; Cifuentes et al., 2010; Yang et al., 2010). miR-451 is encoded in the same primary transcript as miR-144. Intriguingly, the transcript for Dicer has been reported as a direct target of miR-144 in a recent study. During erythropoiesis, expression of the miR-144/miR-451 primary transcript results in degradation of Dicer mRNA and global downregulation of microRNAs, thus reallocating Ago2 to process and generate mature miR-451, which then promotes the maturation of erythrocytes (Kretov et al., 2020).
Tissue specific microRNAs temporally regulate differentiation of individual tissues and organs. The conserved nature of organ-specific microRNAs hints at coevolution of tissue types, microRNAs and associated machinery.
Apoptosis: anti- and pro-apoptotic microRNAs
Apoptosis or programmed cell death is an integral part of embryogenesis. In C. elegans, the miR-35 family cooperates with the mir-58 family to downregulate the transcript encoding the proapoptotic BH3-only protein EGL-1 to prevent precocious programmed cell death (Sherrard et al., 2017). In Drosophila embryos, the intronic miR-11 and miR-998 clusters inhibit apoptosis by repressing the transcripts encoding the core apoptotic machinery, Hid and Reaper, and the transcript for an inhibitor of the pro-growth EGFR, respectively (Ge et al., 2011; Truscott et al., 2014). In mESCs, several ESCC family microRNAs prevent apoptosis by targeting proapoptotic factors downstream of DNA damage-mediated p53 signaling. In contrast, miR-34 acts downstream of p53 signaling to promote apoptosis (Chang et al., 2007a; reviewed in Greve et al., 2013). MicroRNAs can therefore act as both anti-apoptotic and pro-apoptotic factors.
Post embryonic differentiation
C. elegans
After larvae hatch from embryos, C. elegans undergo a series of four larval molts. Each of the four larval stages, L1 to L4, is characterized by a specific cell division and differentiation pattern. Investigating mutants that exhibited altered cell division patterns led to the identification of the first discovered microRNAs lin-4 (Lee et al., 1993; Wightman et al., 1993) and let-7 (Reinhart et al., 2000). Extensive genetic and biochemical characterization of lin-4 and let-7 has shown that they act across multiple tissue types to regulate the timing of developmental programs. Other microRNAs that are critical for the development of the germline and soma have also been identified (Fig 3).
Fig 3. MicroRNAs are regulators of cell fate during post embryonic development.
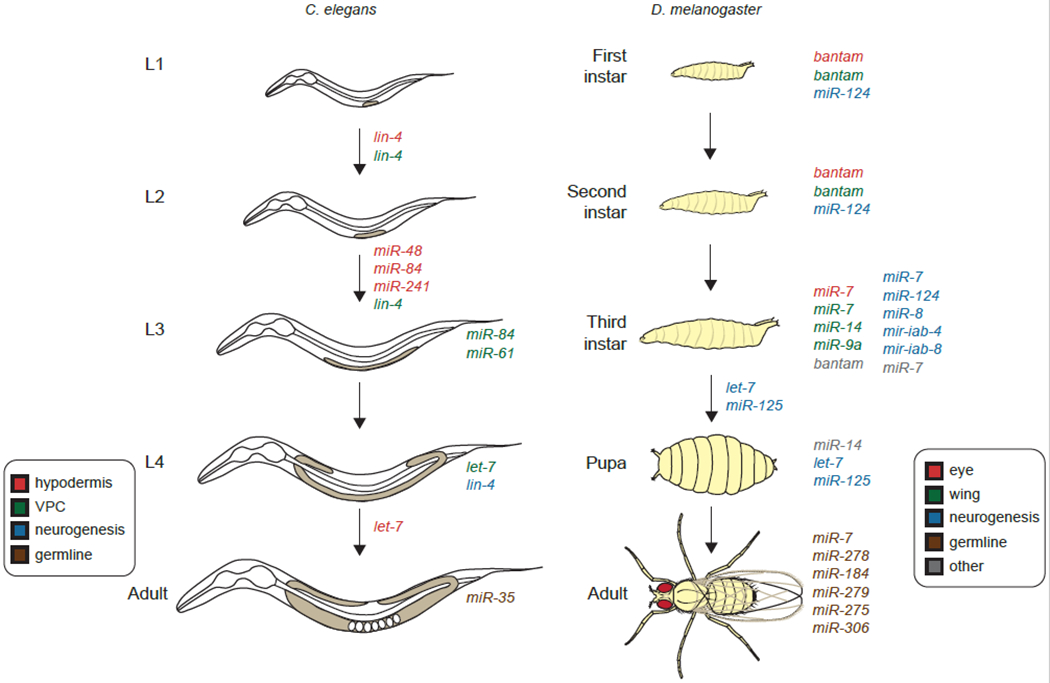
C. elegans L1 larvae undergo four molts before entering adulthood. MicroRNAs regulate differentiation in the hypodermis, vulval precursor cells (VPCs), neurons, and the germline in C. elegans. D. melanogaster first instar larvae undergo two larval molts, pupariation, and metamorphosis to form adults. MicroRNAs modulate cell proliferation and differentiation in the eye imaginal disc, wing imaginal disc, neurons, germline, glia, and salivary glands in D. melanogaster. The microRNAs indicated next to the arrows are key for developmental transitions, whereas, microRNAs indicated next to a developmental stage act during that stage.
Hypodermis: symmetric and asymmetric seam cell divisions
Similar to stem cells, the hypodermal seam cells in C. elegans undergo a series of symmetric and asymmetric cell divisions to self-renew and give rise to differentiated hypodermal cells. The seam cells generated during the larval stages fuse to form cuticular structures called alae at the onset of adulthood. lin-4 and the let-7 family act as heterochronic genes to regulate the timing of these processes. lin-4 is expressed as the animal transitions from L1 to L2. Loss-of-function mutants of lin-4 exhibit inappropriate reiterations of the L1- specific cell division patterns of seam cells, resulting in almost complete lack of adult alae structures and nonfunctional vulva (Chalfie et al., 1981). let-7 regulates the L4 to adult transition. Loss-of-function mutants of let-7 exhibit a supernumerary molt with reiteration of the L4-specific seam cell division pattern and retarded alae formation (Reinhart et al., 2000). However, analysis of the V5 lineage in let-7 loss-of-function males indicates that the action of let-7 begins at the late L3 stage (Vadla et al., 2012). Similarly, the other let-7 family members, miR-48, miR-84 and miR-241, regulate the L2 to L3 transition. (Abbott et al., 2005)
Initial studies identified the direct targets of lin-4 and let-7 responsible for these phenotypes. The targets of lin-4 include the transcription factor lin-14 (Lee et al., 1993; Wightman et al., 1993) and the RNA binding protein lin-28 (Moss et al., 1997). The targets of the let-7 family include the RNA-binding protein lin-41 (Reinhart et al., 2000) and the transcription factor hbl-1 (Abbott et al., 2005). The let-7 family and lin-4 together downregulate the lin-28 transcript (Tsialikas et al., 2017). However, the precise molecular mechanism linking these targets to seam cell differentiation is not fully understood.
Genetic experiments suggest that lin-4 and let-7 regulate the ordering of asymmetric and symmetric divisions of the seam cells by modulating the activity of the Wnt (Wingless) pathway components (Harandi and Ambros, 2015; Mallick et al., 2019). The transcription factor LIN-14 promotes the activity of the polarity-inducing non-canonical Wnt pathway and allows for the first asymmetric division. At the beginning of the L2 stage, lin-4 downregulates lin-14 and promotes a round of symmetric cell division. Toward the end of the L2 stage, the activity of the let-7 family members, miR-48, miR-84, and miR-241 enhances the Wnt pathway through an unknown mechanism, but mediated by the RNA binding protein LIN-28 and the scaffolding protein LIN-46 (Harandi and Ambros, 2015). Additionally, the levels of the let-7 family of microRNAs may be regulated by Wnt signaling, suggesting a layered mechanism involving feedback loops (Mallick et al., 2019). At the end of the L4 stage, let-7-mediated inhibition of lin-41 mRNA leads to the upregulation of the transcription factors LIN-29A and MAB-10 that result in terminal differentiation of the seam cells to form alae (Aeschimann et al., 2017; 2019). Therefore, by targeting key molecular factors, lin-4 and the let-7 family regulate the cell division and differentiation patterns of the stem cell-like seam cells.
Germline and associated soma: specification of cell fates
C. elegans hermaphrodites and males have sexually dimorphic reproductive systems. Both sexes have sex-specific somatic gonads and germlines. Additionally, hermaphrodites have an egg laying apparatus and males have copulatory organs. MicroRNAs are involved in the development of these structures.
In hermaphrodites, microRNAs interact with the epidermal growth factor pathway and the Notch/delta signaling pathway to regulate development of the vulva and the related egg-laying apparatus. During development, the primary vulval precursor cell (VPC) receives high levels of epidermal growth factor (EGF) and has low Notch activation. The activation of Notch/LIN-12 in the adjoining secondary VPCs upregulates the transcription of miR-61. miR-61 directly downregulates the Notch inhibitor vav-1, thereby reinforcing the activated Notch condition in the secondary VPCs (Yoo and Greenwald, 2005). lin-4-mediated modulation of lin-14 levels in the secondary VPCs prevents premature activation of Notch in the secondary VPCs (Li and Greenwald, 2010). The let-7 family members, miR-84 and let-7, also ensure that the EGF signal does not induce primary VPC fate in the secondary VPCs by targeting let-60/RAS (Johnson et al., 2005). Other targets of the let-7 family that contribute to vulval integrity have also been identified (Hunter et al., 2013). However, downregulation of a single target lin-41 by let-7 during L4 is sufficient to maintain vulval integrity (Ecsedi et al., 2015). Similarly, in males, let-7-mediated inhibition of lin-41 during L4 leads to translational activation of the transcription factors mab-3 and dmd-3 that promote the retraction of the tail cells and maturation of the male copulatory organ (Aeschimann et al., 2019).
The hermaphrodite-specific neuron (HSN) that innervates vulval muscles extends its axon during the L4 stage. lin-4-mediated inhibition of lin-14 and lin-28 is critical to promote differentiation of the HSN neuron (Olsson-Carter and Slack, 2010). In males, miR-54 and miR-56 regulate the Hox gene egl-5 to specify the posterior sensory ray patterning (Zhang and Emmons, 2009).
The germline in C. elegans is arranged in a spatio-temporal manner. The distal tip cell (DTC) present at the distal end of the germline promotes mitosis and inhibits meiosis. As germ cells proliferate and move away from the DTC, they transition into meiosis and mature to form oocytes or sperm. In hermaphrodites, spermatogenesis occurs during L4 and oogenesis occurs during adulthood. Males produce sperm throughout their adulthood. Loss-of-function mutations of the microRNA-binding Argonautes alg-1 and alg-2 lead to decreased brood sizes. The defect observed is due to the function of alg-1 and alg-2 in the DTC and the somatic gonad (Bukhari et al., 2012; Rios et al., 2017). Additionally, a distinct germline-enriched microRNA Argonaute alg-5 that regulates the switch between spermatogenesis in L4 and ovulation in adulthood has been identified (Brown et al., 2017). The spatial expression profiles of germline-specific microRNAs have been generated by dissecting gonads and performing small RNA sequencing. Although the miR-35 family and their targets colocalized in the pachytene region of the gonad (Diag et al., 2018; McEwen et al., 2016), their function during ovulation remains unknown. Instead, the miR-35 family has been found to promote spermatogenesis in hermaphrodites and contributes to tail morphogenesis in males (McJunkin and Ambros, 2014). About 80 novel microRNAs have also been identified in the germline, but their functions remain unknown (Diag et al., 2018). Similarly, Drosha-independent microRNAs that regulate oogenesis have also been identified (Minogue et al., 2018). The germline-specific association of the DEAD box helicase GLH-1 with miRISC has been speculated to be indicative of target sequestration into granules (Dallaire et al., 2018). These novel microRNAs expressed in the germline may confer specificity required for the sequestration and modulation of maternal transcript expression during oogenesis.
D. melanogaster
After hatching, first instar Drosophila larvae undergo 2 molts, followed by induction of metamorphosis into the pupal stage. The adult emerges from the pupa. Cells in the juvenile imaginal structures and the larval nervous system undergo temporally appropriate proliferation and differentiation starting at the mid-first instar larval stage. An ecdysone-triggered hormonal cascade initiates major morphogenetic changes during the pupal stage. As outlined below, the interplay between several signaling factors and microRNAs confers robustness to cell fate specification. The first microRNA identified in flies, bantam, was discovered to be an important regulator of cell number and animal size (Brennecke et al., 2003; Hipfner et al., 2002). bantam promotes proliferation and inhibits apoptosis in several tissue types (Brennecke et al., 2003). Since then, several microRNAs that regulate the balance between cell proliferation and cell differentiation have also been discovered (Fig 3).
Eye and wing imaginal discs: Balance between proliferation and differentiation
The eye imaginal disc gives rise to the adult compound eye and antenna. The bantam microRNA represses the transcript encoding apoptotic factor Hid to promote the proliferation in the eye imaginal disc in the first and second instar larva (Brennecke et al., 2003). EGF signaling induces the expression of miR-7 in the third instar larva to promote photoreceptor differentiation by downregulating the transcription factor Yan (Li and Carthew, 2005).
The wing imaginal disc is derived from epidermal cells that divide continuously during the larval stages in Drosophila. The EGF pathway destabilizes the transcriptional repressor Capicua and promotes expression of the pro-proliferation bantam microRNA. bantam also directly downregulates the transcript encoding Capicua, thus forming a positive feedback loop (Herranz et al., 2012b). In addition, bantam promotes cell division by repressing the transcript encoding the anti-proliferation protein Socs36e (Herranz et al., 2012a). Recently, the G2/M inhibitor trbl has also been identified as a target of bantam. Simultaneous depletion of the proapoptotic genes hid and trbl results in the overgrowth phenotype observed in bantam gain-of-function mutants, suggesting that bantam plays both anti-apoptotic and proliferative roles during the development of wing discs (Gerlach et al., 2019).
In the third instar larva, Notch signaling defines dorsal and ventral domains in the wing imaginal disc and represses bantam. The suppression of bantam is critical for wing development in two ways: setting up the zone of non-proliferating cells to maintain the dorsal-ventral border, and the formation of the actomyosin cables via the upregulation of the actin elongation factor (and bantam target) Enabled (Becam et al., 2011). miR-9a downregulates the transcript encoding the transcriptional regulator Lim-only to prevent apoptosis in the dorsal wing primordium (Bejarano et al., 2010; Biryukova et al., 2009). miR-7 also promotes wing growth by enhancing G1/S transitions (Aparicio et al., 2014). miR-14 promotes wing growth by modulating the levels of the Hedgehog signaling pathway components (Kim et al., 2014). Therefore, microRNAs act as regulators of cell differentiation and organ size.
Nervous system: temporal regulation of fates in asymmetrically dividing cells
The Drosophila central nervous system consists of the central brain, the optic lobe, and the ventral nerve cord in the larva. The central brain contains neural stem cells called neuroblasts. Neuroblasts are derived from neurectoderm cells during embryogenesis and become quiescent before hatching. They resume proliferation during the larval stage. Type I neuroblasts undergo asymmetric divisions to self-renew and produce a ganglion mother cell (GMC) that can only undergo another round of division. In contrast to its function in mammals, miR-124 promotes Type I neuroblast proliferation by repressing an inhibitor of proliferation, Anachroism (Weng and Cohen, 2012). Type II neuroblasts undergo asymmetric divisions to self-renew and produce an intermediate neural progenitor (INP) that can undergo several rounds of divisions. The bantam microRNA promotes proliferation of INPs by repressing the transcripts encoding the differentiation factors Brat, Prospero, and Numb (Weng and Cohen, 2015). miR-92a/b prevents the premature differentiation of neuroblast populations by downregulating the transcript encoding the uncharacterized transcription factor Jigr (Yuva-Aydemir et al., 2015).
The mushroom body in the fly brain is associated with olfactory learning and memory. The neuroblasts in the mushroom body divide asymmetrically throughout larval and pupal stages to self-renew, as well as generate the early-born (γ, α’/β’) and late-born (pioneer α/β, α/β) neuronal subtypes. The let-7 and miR-125 microRNAs are essential for specifying fates of the late-born, post-mitotic neurons in the mushroom bodies. Ecdysone signaling induces the expression of let-7 and miR-125 in late-born neurons as the animal transitions from the larval to pupal stage. let-7 and miR-125 downregulate the transcription factor Chinmo that is specific to early-born neurons to allow specification of late-born fates (Wu et al., 2012). let-7 also downregulates the transcription factor Abrupt to promote olfactory functionality in late-born neurons. (Kucherenko et al., 2012).
The optic lobe transduces signals from the photoreceptors in the eye. The neuroepithelial cells in the outer proliferation center of the optic lobe initially undergo symmetric divisions and then transition to form asymmetrically dividing neuroblasts. miR-7 targets Notch effectors to limit Notch signaling at the transition zone and promote formation of neuroblasts (Caygill and Brand, 2017). Neuroblast differentiation is also regulated non-cell autonomously by glia associated with the optic lobe cortex. miR-8 is expressed in these glia and downregulates the secreted EGFR ligand and differentiation factor Spitz. Limiting Spitz in the vicinity of glial cells is speculated to create a niche that promotes neuroepithelial proliferation and inhibits differentiation (Morante et al., 2013). bantam is also expressed in glial cells, regulates the number of glial cells, and is required for the projection of photoreceptor axons (Li and Padgett, 2012).
The ventral nerve cord neuroblasts also arrest short before hatching and divide asymmetrically during the larval stages. Terminal differentiation of the ventral nerve cord neuroblasts occurs at the onset of metamorphosis. Similar to their role in the mushroom body, let-7 and miR-125 repress the transcripts encoding Chinmo and Broad-Z3 to regulate neuronal morphology at the larval-to-pupal transition (Wu et al., 2020). The posterior ventral nerve cord neurons innervate adult ovaries. The Hox cluster microRNAs mir-iab-4 and mir-iab-8 repress transcripts encoding Hox genes Homothorax, and Extradenticle, among others, to specify posterior ventral nerve cord fate and regulate fertility (Garaulet et al., 2014).
The sensory organ precursor (SOP) lineage of the peripheral nervous system (PNS) is also derived from the neurectodermal proneural clusters. miR-9a downregulates the target encoding Senseless specifically in the ectodermal cells of the wing margin to prevent ectopic SOP formation and, thereby, regulates the number of wing sensory bristles (Li et al., 2006). miR-7 promotes SOP formation by repressing the transcript encoding the basic helix-loop-helix transcriptional repressor E(spl) (Li et al., 2009).
Hematopoietic cells: maintenance of stemness
The anterior lymph glands in the third instar Drosophila larva form niches for the production and differentiation of hematopoietic stem cells. miR-7 and the transcription factor bam act together to maintain stemness in the progenitor cells by downregulating the differentiation factor Yan and reinforcing Hedgehog signaling (Tokusumi et al., 2011; 2018). Similarly, microRNA-mediated modulation of transcription factors regulates cell fate decisions during hematopoiesis in mice (reviewed in Kim et al., 2019).
Pupariation: Ecdysone triggered microRNAs
The pupal stage is characterized by extensive tissue morphogenesis. Ecdysone hormone signaling is upregulated during the early stages of pupal formation and coordinates developmental programs. Ecdysone signaling reduces the levels of miR-14 and this, in turn, lifts the direct repression of the Ecdysone Receptor (EcR) (Varghese and Cohen, 2007). A similar positive feedback loop is triggered between miR-965 and EcR in abdominal histoblasts that are destined to form the adult abdominal epithelial cells. Reduction in the levels of miR-965 also releases the histoblasts from G2/M arrest by the resultant increase of cdc25 and wingless (Verma and Cohen, 2015). As described above, ecdysone triggers the expression of let-7 and miR-125 in the neurons of the mushroom body and ventral nerve cord to specify late-stage neurons (Wu et al., 2012; 2020). miR-14 also downregulates its target inositol-1,4,5 triphosphate kinase 1 to promote cell autophagy and shrinkage of the salivary glands (Nelson et al., 2014).
Germline: differentiation of gametes
In male and female Drosophila germlines, the germline stem cells (GSCs) are in contact with a niche that usually secretes a stemness factor. As GSCs divide asymmetrically, the daughter cells in contact with the niche maintain their stem cell identity, and the daughter cells that move away differentiate to form gametes. MicroRNAs help maintain the balance between GSCs and differentiated gametes.
In females, division of GSCs is promoted by miR-7 and miR-278 by inhibiting the CDK inhibitor Decapo (Yu et al., 2009). miR-184 downregulates the receptor for the stemness factor Decapentaplegic (DPP) to promote the differentiation of female GSCs (Iovino et al., 2009). As the differentiated germline cyst cell divides, the 16-cell cluster becomes enveloped by somatic follicle cells. Some of the somatic follicle cells undergo JAK/STAT-dependent differentiation to form migratory border cells and non-migratory squamous follicle cells. The microRNA miR-279 inhibits the STAT transcription factor and helps reinforce squamous follicle cell fates (Yoon et al., 2011).
In males, microRNAs regulate the differentiation factor bag of marbles (bam) during different stages of spermatogenesis. miR-7 promotes stemness in male GSCs by downregulating the transcript encoding Bam. The transcriptional repressor Maelstrom represses miR-7 to allow male GSC differentiation (Pek et al., 2009). Bam is again downregulated when spermatogonia differentiate into spermatocytes by the microRNAs miR-275 and miR-306 (Eun et al., 2012). In aging males, mir-9a downregulates the adhesion molecule N-cadherin in GSCs to promote detachment from the niche and, hence, terminal differentiation (Epstein et al., 2017).
The examples above clearly demonstrate how microRNAs are major drivers of post-embryonic development by temporally regulating the balance between cell proliferation and cell differentiation, as well as symmetric and asymmetric division, to ensure proper cell fate specification.
MicroRNAs as pacemakers
Timekeeping is a repeated theme in microRNA biology. The above examples describe microRNAs as switches, which turn on or off, to specify cell fates at specific times during development. However, varying microRNA activity in a regulated manner can result in complex expression dynamics of the targets. Dynamic microRNA activity, linked to a cyclic signal, can result in repeated repression and derepression of key genes over the course of an organism’s lifetime. Such microRNAs act like pacemakers. For instance, in C. elegans, miR-786 regulates the fatty acid elongase elo-2 in intestinal cells to maintain the rhythmic defecation cycles of about 50 seconds. Disruption of mir-786 alters the initiation of the pace keeping calcium-wave and results in long arrhythmic defection cycles (Kemp et al., 2012; Miska et al., 2007).
MicroRNAs that repress targets as part of the circadian rhythm machinery have been identified in several systems. In Drosophila, bantam represses translation of the core circadian transcription factor Clock (Kadener et al., 2009). let-7 activity in the pacemaker neurons represses the circadian gene clockwork orange to maintain the length of the circadian period (Chen et al., 2014a). mir-279 acts downstream of the pacemaker neurons in the brain by modulating the JAK/STAT pathway (Luo and Sehgal, 2012). The oscillatory miR-959–964 cluster regulates feeding and other circadian behavior in Drosophila (Vodala et al., 2012). Similarly, microRNAs that regulate circadian rhythm in mice, sometimes in an organ specific manner, have also been identified (e.g., Cheng et al., 2007; Smith et al., 2016; Vollmers et al., 2012). However, these studies, largely done in adult animals, do not report defects in differentiation. Here, we discuss a few examples in C. elegans where microRNAs maintain biological rhythm during development.
The transition between larval stages in C. elegans involves a periodic molting cycle, where the worms shed their cuticles as they grow in size. Similar to circadian rhythms in other animals, during every molting cycle, worms exhibit an active period where they forage, feed, and defecate, followed by a period of behavioral quiescence, and then ecdysis. One fifth of the transcripts in C. elegans exhibit oscillatory expression patterns with the same time period as the molting cycle, suggestive of regulated iterations of molecular processes (Hendriks et al., 2014; Kim et al., 2013). LIN-42, the homolog of the core circadian clock protein PERIOD, regulates the length of the molting cycle (Monsalve et al., 2011). Disruption of LIN-42 activity leads to developmental heterochronic defects (Tennessen et al., 2006) and increased biogenesis of several microRNAs, including let-7 and lin-4 (McCulloch and Rougvie, 2014; Perales et al., 2014; Van Wynsberghe et al., 2014). The transcript encoding LIN-42 is also hypothesized to be a target for let-7, setting up a putative mutually repressive dynamic between let-7 and LIN-42 (Reinhart et al., 2000). The let-7 microRNAs have also been proposed to regulate the global molting cycle by modulating levels of key transcription factors nhr-23 and nhr-25, that are homologs of circadian rhythm-associated Retinoic-acid Orphan Receptors (RORs). (Hayes et al., 2006; Monsalve and Frand, 2014). The expression patterns for lin-42, nhr-23 and nhr-25 are all cyclic with one peak per molt. Similarly, other microRNAs, like miR-230 and miR-71, target oscillatory genes (Kim et al., 2013). This suggests that microRNA-mediated post-transcriptional gene regulation may be responsible for the cyclic expression pattern of the targets.
The primary let-7 transcript is expressed in an oscillatory manner during every molt in C. elegans. However, the accumulation of mature let-7 is prevented during L1 and L2 by the action of LIN-28 (Van Wynsberghe et al., 2011). While LIN-28 binds pri-let-7 in C. elegans, the mechanism by which it regulates let-7 levels remains unknown (Stefani et al., 2015). A recent report shows that LIN-28 mediates SL1 trans-splicing of the pri-let-7 transcript downstream of the let-7 stem-loop during L1 and L2. SL1 trans-splicing involves a splicing reaction between a unique snRNP-derived SL1 sequence and the 5’ end of a pre-mRNA containing an SL1-specific trans-splice site. While SL1 trans-splicing upstream of the let-7 stem-loop generates a pri-let-7 transcript that favors processing by the Microprocessor complex (Mondol et al., 2015), SL1 trans-splicing downstream of the let-7 stem-loop inhibits maturation of let-7 in cis. The product generated by the SL1 trans-splicing event downstream of the let-7 stem-loop also contains let-7 complementary elements and acts as a sponge to negatively regulate the let-7 family during the early stages of development (Nelson and Ambros, 2019).
In contrast to the switch like behavior seen in most systems, microRNAs can function with more complex dynamics. Similar to let-7, the expression of mature lin-4 microRNA in C. elegans is pulsatile with one peak per molt. However, lin-14, the target of lin-4, does not exhibit oscillatory expression and has steadily decreasing levels throughout development. Remarkably, the expression pattern of lin-14 becomes oscillatory upon the loss of lin-4. These data suggest that the synchronous pulses of lin-4 dampen the oscillatory expression pattern of lin-14 observed in lin-4 mutants, resulting instead in the steadily decreasing expression pattern of lin-14 in wild type animals during development. Thus, lin-4, in this case, acts more like a rheostat than a switch (Kim et al., 2013).
MicroRNAs can, therefore, orchestrate expression of genes to maintain biological rhythms. However, the mechanisms that coordinate with the external environment to regulate the periodicity, amplitude, and phase of specific microRNAs remain unknown.
Environmental regulation of differentiation
Variable environmental conditions pose challenges to developmental programs. Fluctuations in temperature and availability of nutrients may result in variations in gene expression, biochemical reaction rates, and metabolic rates. Developmental programs need to be robust to adapt to all of these changes and ensure timely cell-fate specifications. Additionally, stress response pathways that are activated during specific conditions also help in coping with harsh environmental conditions. In a special case of adaptation to stress, as elaborated below, C. elegans can enter alternate stress resistant quiescent stages during unfavorable conditions, instead of undergoing continuous development. MicroRNAs form part of the machinery that helps cells maintain robustness of development and respond to changing environments. The phenotypes related to these microRNAs may not be obvious in optimal laboratory conditions. However, in suboptimal, fluctuating conditions in the wild, the function of these microRNAs is likely to maintain the fidelity of gene expression and cell-fate specification.
During Drosophila development, the presence of favorable conditions, such as abundant food and optimal temperature, signal the production of neuropeptides. Neuropeptides act on the insulin-producing cells in the brain to trigger the production of insulin-like peptides (ILPs). miR-9a and miR-14 act through different pathways to modulate the levels of ILPs and regulate body size and starvation sensitivity, respectively (Suh et al., 2015; Varghese et al., 2010). The generated ILPs downregulate the bantam microRNA to trigger ecdysone release from the prothoracic gland in the larval brain (Boulan et al., 2013). The ILPs also act on the fat body to coordinate body growth through miR-8 (Hyun et al., 2009). Similarly, during C. elegans development, the presence of the pro-growth steroid hormone, dafachronic acid, liganded to the nuclear hormone receptor DAF-12, triggers the production of the let-7 family of microRNAs to promote continuous development (Hammell et al., 2009a). Therefore, these microRNAs act as important mediators of favorable environmental conditions and developmental decisions.
In contrast, some microRNA-linked phenotypes only manifest themselves during unfavorable environmental conditions, suggesting that these microRNAs function to ensure fidelity of development. For instance, in Drosophila, mir-9a-linked embryonic lethality and ectopic SOP production phenotypes are enhanced at high-rearing temperatures of 25–29°C (Cassidy et al., 2013; Yatsenko and Shcherbata, 2014). miR-7-mediated regulation of SOP differentiation also becomes more prominent at higher temperatures (Li et al., 2009). Similarly, miR-34 and miR-83 maintain robustness of germline development in C. elegans when there are large fluctuations in temperature (Burke and Hammell, 2015).
C. elegans larvae can enter quiescent stages when exposed to challenging environmental conditions. Larvae that hatch in the absence of food arrest in the L1 diapause stage. The miR-92 homolog, miR-235, is downstream of the insulin/IGF signaling and is required for the inhibition of developmental programs in several tissues during L1 diapause (Kasuga et al., 2013). Upon refeeding, levels of miR-235 decrease and animals exit diapause. miR-71 is required for development after release from L1 diapause and appears to be required in addition to lin-4 and let-7 for regulating heterochronic gene expression post L1 diapause (Zhang et al., 2011).
C. elegans L2 larvae can also enter the stress-resistant dauer stage upon exposure to unfavorable conditions. The dauer stage is a quiescent stage in which larvae can survive for long periods. The bantam/miR-58 family of microRNAs is required for dauer entry (Alvarez-Saavedra and Horvitz, 2010). The miR-58 family regulates components of the TGF-β -signaling cascades to regulate dauer entry (de Lucas et al., 2015; Lozano et al., 2016). miR-124, miR-234, and miR-80/81 are also required to regulate dauer entry (Than et al., 2013). The process of commitment of cells to arrest prior to and during dauer is accompanied by the downregulation of the let-7 family and a rewiring of the genetic program to allow for suppression of the let-7 target hbl-1 (Hammell et al., 2009a; Ilbay and Ambros, 2019; Karp et al., 2011). The nuclear hormone receptor DAF-12, in combination with other stress-induced pathways, downregulates the let-7 family in response to adverse environmental conditions (Bethke et al., 2005; Hammell et al., 2009a; Ilbay and Ambros, 2019). Passage through dauer alters microRNA levels in post-dauer juvenile stages, suggesting that different microRNAs may be required for development post dauer (Karp et al., 2011). Intriguingly, the let-7 family members, miR-48, miR-84 and miR-241 that are essential for the L2 to L3 transition during continuous development, are dispensable if animals undergo dauer-interrupted development. The microRNA lin-4 is able to compensate for the loss of the mir-48, mir-84 and mir-241, and downregulate their target hbl-1 to ensure cell fate specification, only after passage through dauer (Karp and Ambros, 2012).
A recent report showed that lowering metabolism in Drosophila can alleviate nearly all microRNA-related defects, including miR-7-linked photoreceptor differentiation and miR-9-linked SOP differentiation. An information theory-based approach coupled with an analysis of mutants showed that the presence of multiple repressive transcriptional and post-transcriptional mechanisms in a pathway ensures robustness of decision-making. The combined action of multiple repressive pathways becomes especially significant when the pace of the energy-consuming steps is increased by increasing temperature or metabolism (Cassidy et al., 2019). MicroRNAs, therefore, are one of the repressive mechanisms that act at the nexus of environment, metabolism, and developmental decision-making.
MicroRNAs have now been established as key molecular players in cell fate specification events. They function in myriad ways to maintain cell fates and mediate cell differentiation in response to external signals. We now have the tools to manipulate individual microRNAs and mRNA targets in the endogenous context as well as map the global microRNA-mRNA interactome. Future investigations in the field using an integrated approach to understand how microRNA expression and action are regulated in a context-dependent manner during development will likely provide further mechanistic insights.
Acknowledgements:
This work was supported by a grant from the NIH to J.K.K. (NIH R01GM129301). The authors thank Xantha Karp and Amy Pasquinelli for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott AL, Alvarez-Saavedra E, Miska EA, Lau NC, Bartel DP, Horvitz HR, and Ambros V (2005). The let-7 microRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Developmental Cell 9, 403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abernathy DG, Kim WK, McCoy MJ, Lake AM, Ouwenga R, Lee SW, Xing X, Li D, Lee HJ, Heuckeroth RO, et al. (2017). MicroRNAs induce a permissive chromatin environment that enables neuronal subtype-specific reprogramming of adult human fibroblasts. Stem Cell 21, 332–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschimann F, Kumari P, Bartake H, Gaidatzis D, Xu L, Ciosk R, and Großhans H (2017). LIN41 post-transcriptionally silences mRNAs by two distinct and position-dependent mechanisms. Molecular Cell 65, 476–89. [DOI] [PubMed] [Google Scholar]
- Aeschimann F, Neagu A, Rausch M, and Großhans H (2019). let-7 coordinates the transition to adulthood through a single primary and four secondary targets. Life Sci. Alliance 2, e201900335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi AF, Khivansara V, Han T, Freeberg MA, Moresco JJ, Tu PG, Montoye D, Yates JR III, Karp X, and Kim JK (2015). Casein kinase II promotes target silencing by miRISC through direct phosphorylation of the DEAD-box RNA helicase CGH-1. Proc Natl Acad Sci USA 112, E7213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Saavedra E, and Horvitz HR (2010). Many families of Caenorhabditis elegans microRNAs are not essential for development or viability. Current Biology 20, 367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V, and Ruvkun G (2018). Recent molecular genetic explorations of Caenorhabditis elegans microRNAs. Genetics 209, 651–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio R, Simoes Da Silva CJ, and Busturia A (2014). MicroRNA miR-7contributes to the control of Drosophila wing growth. Dev. Dyn 244, 21–30. [DOI] [PubMed] [Google Scholar]
- Bagnacani A, Wolfien M, and Wolkenhauer O (2019). Tools for understanding miRNA–mRNA interactions for reproducible RNA analysis. In: Lai X, Gupta S, Vera J (eds) Computational Biology of Non-Coding RNA. Methods Mol Biol 1912, 199–214. [DOI] [PubMed] [Google Scholar]
- *.Bazzini AA, Lee MT, and Giraldez AJ (2012). Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336, 233–37. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ribosome profiling in early zebrafish embryos revealed that miR-430 binding reduced the ribosome occupancy on its targets, before causing mRNA decay, indicating that microRNA-mediated translational repression could occur prior to microRNA-mediated mRNA decay in vivo.
- Beaudoin J-D, Novoa EM, Vejnar CE, Yartseva V, Takacs CM, Kellis M, and Giraldez AJ (2018). Analyses of mRNA structure dynamics identify embryonic gene regulatory programs. Nat Struct Mol Biol 25, 677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becam I, Rafel N, Hong X, Cohen SM, and Milan M (2011). Notch-mediated repression of bantam miRNA contributes to boundary formation in the Drosophila wing. Development 138, 3781–89. [DOI] [PubMed] [Google Scholar]
- Bejarano F, Smibert P, and Lai EC (2010). miR-9a prevents apoptosis during wing development by repressing Drosophila LIM-only. Developmental Biology 338, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner LK, Prothro KP, and McJunkin K (2019). The mir-35 family links maternal germline sex to embryonic viability in Caenorhabditis elegans. G3: Genes, Genomes, Genetics 9, 901–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke A, Fielenbach N, Wang Z, Mangelsdorf DJ, and Antebi A (2009). Nuclear hormone receptor regulation of microRNAs controls developmental progression. Science 324, 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukova I, Asmar J, Abdesselem H, and Heitzler P (2009). Drosophila mir-9a regulates wing development via fine-tuning expression of the LIM only factor, dLMO. Developmental Biology 327, 487–96. [DOI] [PubMed] [Google Scholar]
- Boulan L, Martín D, and Milán M (2013). bantam miRNA promotes systemic growth by connecting insulin signaling and ecdysone production. Current Biology 23, 473–78. [DOI] [PubMed] [Google Scholar]
- *.Brennecke J, Hipfner DR, Stark A, Russell RB, and Cohen SM (2003). bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene. Cell 113, 25–36. [DOI] [PubMed] [Google Scholar]; The bantam locus in Drosophila, previously identified as a promoter of tissue growth, was cloned and discovered to encode the first Drosophila microRNA. The transcript encoding the pro-apoptotic gene, hid, was identified as a bantam target, suggesting a mechanism for bantam’s pro-proliferation and anti-apoptotic activity.
- Bridge KS, Shah KM, Li Y, Foxler DE, Wong SCK, Miller DC, Davidson KM, Foster JG, Rose R, Hodgkinson MR, et al. (2017). Argonaute utilization for miRNA silencing is determined by phosphorylation-dependent recruitment of LIM-domain-containing proteins. Cell Reports 20, 173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KC, Svendsen JM, Tucci RM, Montgomery BE, and Montgomery TA (2017). ALG-5 is a miRNA-associated Argonaute required for proper developmental timing in the Caenorhabditis elegans germline. Nucleic Acids Research 45, 9093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno IG, Karam R, Huang L, Bhardwaj A, Lou CH, Shum EY, Song H-W, Corbett MA, Gifford WD, Gecz J, et al. (2011). Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Molecular Cell 42, 500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari SIA, Vasquez-Rifo A, Gagné D, Paquet ER, Zetka M, Robert C, Masson J-Y, and Simard MJ (2012). The microRNA pathway controls germ cell proliferation and differentiation in C. elegans. Cell Research 22, 1034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SL, and Hammell M (2015). Robust distal tip cell pathfinding in the face of temperature stress is ensured by two conserved microRNAs in Caenorhabditis elegans. Genetics 200, 1201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Stark A, Brennecke J, and Cohen SM (2008). Temporal reciprocity of miRNAs and their targets during the maternal-to-zygotic transition in Drosophila. Current Biology 18, 501–06. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, and Gage FH (2007). A functional study of miR-124 in the developing neural tube. Genes Dev. 21, 531–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cassidy JJ, Bernasek SM, Bakker R, Giri R, Peláez N, Eder B, Bobrowska A, Bagheri N, Amaral LAN, and Carthew RW (2019). Repressive gene regulation synchronizes development with cellular metabolism. Cell 178, 980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; Genetics and mathematical modeling showed that the presence of multiple repressive mechanisms, including microRNAs, provide robustness to developmental decisions during conditions of high metabolism in Drosophila. The loss of a single repressive mechanism did not affect gene expression dynamics and animal development during conditions of low metabolism.
- Cassidy JJ, Jha AR, Posadas DM, Giri R, Venken KJT, Ji J, Jiang H, Bellen HJ, White KP, and Carthew RW (2013). miR-9a minimizes the phenotypic impact of genomic diversity by buffering a transcription factor. Cell 155, 1556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy A, Ketting R, Hammond S, Denli A, Bathoorn A, Tops B, and Plasterk R (2003). A micrococcal nuclease homologue in RNAi effector complexes. Nature 425, 411–14. [DOI] [PubMed] [Google Scholar]
- Caygill EE, and Brand AH (2017). miR-7 buffers differentiation in the developing Drosophila visual system. Cell Reports 20, 1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Horvitz RH, and Sulston JE (1981). Mutations that lead to reiterations in the cell lineages of C. elegans. Cell 24, 59–69. [DOI] [PubMed] [Google Scholar]
- Chan SP, Ramaswamy G, Choi EY, and Slack FJ (2008). Identification of specific let-7 microRNA binding complexes in Caenorhabditis elegans. RNA 14, 2104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, et al. (2004a). miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biology 1, 106–13. [DOI] [PubMed] [Google Scholar]
- Chang S, Johnston RJ, Frekjaer-Jensen C, Lockery S, and Hobert O (2004b). MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature 430, 785–89. [DOI] [PubMed] [Google Scholar]
- Chang T-C, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, et al. (2007a). Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Molecular Cell 26, 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T-C, Yu D, Lee Y-S, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, and Mendell JT (2007b). Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloufi S, Santos, Dos CO, Chong MMW, and Hannon GJ (2010). A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu Z, Li T, Zhang R, Xue Y, Zhong Y, Bai W, Zhou D, and Zhao Z (2014a). Regulation of Drosophila circadian rhythms by miRNA let-7 is mediated by a regulatory cycle. Nature Communications 5, 5549. [DOI] [PubMed] [Google Scholar]
- Chen Y, Boland A, Kuzuoğlu-Öztürk D, Bawankar P, Loh B, Chang C-T, Weichenrieder O, and Izaurralde E (2014b). A DDX6-CNOT1 complex and W-binding pockets in CNOT9 reveal direct links between miRNA target recognition and silencing. Molecular Cell 54, 737–50. [DOI] [PubMed] [Google Scholar]
- Cheng H-YM, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, et al. (2007). microRNA modulation of circadian-clock period and entrainment. Neuron 54, 813–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi PS, Zakhary L, Choi W-Y, Caron S, Alvarez-Saavedra E, Miska EA, McManus M, Harfe B, Giraldez AJ, Horvitz RH, et al. (2008). Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron 57, 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W-Y, Giraldez AJ, and Schier AF (2007). Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science 318, 271–74 [DOI] [PubMed] [Google Scholar]
- *.Choi YJ, Lin C-P, Risso D, Chen S, Kim TA, Tan MH, Li JB, Wu Y, Chen C, Xuan Z, et al. (2017). Deficiency of microRNA miR-34a expands cell fate potential in pluripotent stem cells. Science 355, eaag1927. [DOI] [PMC free article] [PubMed] [Google Scholar]; Depletion of miR-34a expanded the cell fate potential of mESCs and iPSCs, resulting in the efficient generation of embryonic and extraembryonic lineages. miR-34a restricted cell fate by downregulating the transcription factor Gata2 and thereby, its target MuERV-L.
- Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, et al. (2010). A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328, 1694–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochella L, and Hobert O (2012). Embryonic priming of a miRNA locus predetermines postmitotic neuronal left/right asymmetry in C. elegans. Cell 151, 1229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen M, Thieffry D, Drivenes Ø, Becker TS, and Bally-Cuif L (2012). miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Developmental Cell 22, 1052–64. [DOI] [PubMed] [Google Scholar]
- Corrêa RL, Steiner FA, Berezikov E, and Ketting RF (2010). MicroRNA–directed siRNA biogenesis in Caenorhabditis elegans. PLoS Genet 6, e1000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dallaire A, Frédérick P-M, and Simard MJ (2018). Somatic and germline microRNAs form distinct silencing complexes to regulate their target mRNAs differently. Developmental Cell 47, 239–47. [DOI] [PubMed] [Google Scholar]; Isolation of somatic and germline specific miRISCs showed tissue-specific association of protein co-factors with Argonaute dictated the fate of the target transcript. The DEAD-box helicase, GLH-1, interacted with miRISC specifically in the germline to mediate translational repression and target stabilization, whereas the GW182 protein, AIN-1, interacted with miRISC in the soma to mediate target mRNA degradation.
- de Lucas MP, Sáez AG, and Lozano E (2015). miR-58 family and TGF-β pathways regulate each other in Caenorhabditis elegans. Nucleic Acids Research 126, 9978–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli A, Tops B, Plasterk R, Ketting R, and Hannon GJ (2004). Processing of primary microRNAs by the Microprocessor complex. Nature 432, 231–5. [DOI] [PubMed] [Google Scholar]
- *.Diag A, Schilling M, Klironomos F, Ayoub S, and Rajewsky N (2018). Spatiotemporal m(i)RNA architecture and 3’ UTR regulation in the C. elegans germline. Developmental Cell 47, 785–800. [DOI] [PubMed] [Google Scholar]; Cryo-sectioning followed by RNA seq was performed on the C. elegans gonad to determine the spatial distribution of mRNAs and microRNAs. The data is available on an interactive data visualization SPACEGERM tool.
- Drexel T, Mahofsky K, Latham R, Zimmer M, and Cochella L (2016). Neuron type-specific miRNA represses two broadly expressed genes to modulate an avoidance behavior in C. elegans. Genes Dev. 30, 2042–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Pirouz M, Choi J, Huebner AJ, Clement K, Meissner A, Hochedlinger K, and Gregory RI (2018). An intermediate pluripotent state controlled by microRNAs Is required for the naive-to-primed stem cell transition. Stem Cell 22, 851–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Wang L, Sliz P, and Gregory RI (2015). A biogenesis step upstream of microprocessor controls miR-17~92 expression. Cell 162, 885–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier O, and Obberghen E (2012). MicroRNAs in pancreas development. Diabetes Obes Metab 14, 22–28. [DOI] [PubMed] [Google Scholar]
- Ecsedi M, Rausch M, and Großhans H (2015). The let-7 microRNA directs vulval development through a single target. Developmental Cell 32, 335–44. [DOI] [PubMed] [Google Scholar]
- *.Eisen TJ, Eichhorn SW, Subtelny AO, and Bartel DP (2020). MicroRNAs cause accelerated decay of short-tailed target mRNAs. Molecular Cell 77, 775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]; microRNA binding to target mRNAs recruits deadenylation factors. However, the presence of microRNAs did not alter the distribution of target poly(A) tail lengths at steady state in mouse fibroblasts. Tracking the poly(A) tail lengths of the targets in a time resolved manner revealed that, before achieving steady state, microRNAs accelerated the rate of deadenylation so that there was no accumulation of short tailed isoforms of the target.
- Epstein Y, Perry N, Volin M, Zohar-Fux M, Braun R, Porat-Kuperstein L, and Toledano H (2017). miR-9a modulates maintenance and ageing of Drosophila germline stem cells by limiting N-cadherin expression. Nature Communications 8, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun SH, Stoiber PM, Wright HJ, McMurdie KE, Choi CH, Gan Q, Lim C, and Chen X (2012). MicroRNAs downregulate Bag of marbles to ensure proper terminal differentiation in the Drosophila male germline. Development 140, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DYR, and Srivastava D (2008). miR-126 regulates angiogenic signaling and vascular integrity. Developmental Cell 15, 272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni E, Booker SA, Parthasarathy S, Rehfeld F, Grosser S, Srivatsa S, Fuchs HR, Tarabykin V, Vida I, and Wulczyn FG (2015). miR-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene Phf 6. eLIFE 4, e04263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer JW, Hu TJ, and Blelloch R (2018a). Decoupling the impact of microRNAs on translational repression versus RNA degradation in embryonic stem cells. eLIFE 7, e38014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer JW, Krishnakumar R, Cook MS, and Blelloch R (2018b). Expression of alternative Ago2 isoform associated with loss of microRNA-driven translational repression in mouse oocytes. Current Biology 28, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Nien CY, Liang HL, and Rushlow C (2014). Co-activation of microRNAs by Zelda is essential for early Drosophila development. Development 141, 2108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya T, and Tomari Y (2011). PABP is not essential for microRNA-mediated translational repression and deadenylation in vitro. EMBO J. 30, 4998–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya T, and Tomari Y (2012). MicroRNAs mediate gene silencing via multiple different pathways in Drosophila. Molecular Cell 48, 825–36. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Han BW, Hung J-H, Xu J, Weng Z, and Zamore PD (2012). Dicer partner proteins tune the length of mature miRNAs in flies and mammals. Cell 151, 533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeta X, Le L, Lin Y, Xie Y, and Lowry WE (2017). Defining transcriptional regulatory mechanisms for primary let-7 miRNAs. PLoS One 12, e0169237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo CM, Munro E, Rasoloson D, Merritt C, and Seydoux G (2008). Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Developmental Biology 323, 76–87. [DOI] [PubMed] [Google Scholar]
- Gambardella G, Carissimo A, Chen A, Cutillo L, Nowakowski TJ, di Bernardo D, and Blelloch R (2017). The impact of microRNAs on transcriptional heterogeneity and gene co-expression across single embryonic stem cells. Nature Communications 8, 14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet DL, Castellanos MC, Bejarano F, Sanfilippo P, Tyler DM, Allan DW, Sánchez-Herrero E, and Lai EC (2014). Homeotic function of Drosophila Bithorax-Complex miRNAs mediates fertility by restricting multiple Hox genes and TALE cofactors in the CNS. Developmental Cell 29, 635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, Chen Y-W, Weng R, Lim SF, Buescher M, Zhang R, and Cohen SM (2011). Overlapping functions of microRNAs in control of apoptosis during Drosophila embryogenesis. Cell Death & Differentiation 19, 839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert LFR, and MacRae IJ (2019). Regulation of microRNA function in animals. Nat Rev Mol Cell Biol 20, 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach SU, Sander M, Song S, and Herranz H (2019). The miRNA bantam regulates growth and tumorigenesis by repressing the cell cycle regulator tribbles. Life Sci. Alliance 2, e201900381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, and Schier AF (2006). Zebrafish miR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79. [DOI] [PubMed] [Google Scholar]; miR-430 was found to promote the deadenylation and clearance of maternal mRNAs during early embryogenesis in zebrafish. This was the first mechanistic evidence of in vivo function of microRNAs in vertebrates.
- Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson MJ, Baskerville S, Hammond S, Bartel DP, and Schier AF (2005). MicroRNAs regulate brain morphogenesis in zebrafish. Science 308, 826–33. [DOI] [PubMed] [Google Scholar]
- *.Golden RJ, Chen B, Li T, Braun J, Manjunath H, Chen X, Wu J, Schmid V, Chang T-C, Kopp F, et al. (2017). An Argonaute phosphorylation cycle promotes microRNA-mediated silencing. Nature 542, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]; CRISPR-Cas9 based screens were used to identify CSNK1A1 and ANKRD52-PPP6C as regulators of AGO2 activity in HCT116 cell lines. A phosphorylation cycle triggered by target engagement by AGO2 was found to regulate the efficiency of AGO2-mediated gene silencing.
- Goljanek-Whysall K, Mok GF, Fahad Alrefaei A, Kennerley N, Wheeler GN, and Munsterberg A (2014). myomiR-dependent switching of BAF60 variant incorporation into Brg1 chromatin remodeling complexes during embryo myogenesis. Development 141, 3378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goljanek-Whysall K, Sweetman D, Abu-Elmagd M, Chapnik E, Dalmay T, Hornstein E, and Munsterberg A (2011). MicroRNA regulation of the paired-box transcription factor Pax3 confers robustness to developmental timing of myogenesis. Proc Natl Acad Sci USA 108, 11936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve TS, Judson RL, and Blelloch R (2013). microRNA control of mouse and human pluripotent stem cell behavior. Annu. Rev. Cell Dev. Biol 29, 213–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Grimson A, Srivastava M, Fahey B, Woodcroft BJ, Chiang HR, King N, Degnan BM, Rokhsar DS, and Bartel DP (2008). Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 455, 1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]; MicroRNAs and piRNAs had been identified in bilaterian animals such as humans, flies and worms. This study revealed that microRNAs, piRNAs, and associated protein machinery were present in animal phyla such as Cnidaria and Porifera that diverged before the emergence of Bilateria, suggesting coevolution of small RNAs and multicellular life.
- *.Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, and Mello C (2001). Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34. [DOI] [PubMed] [Google Scholar]; The RNA endonuclease Dicer and the Argonautes ALG-1 and ALG-2 were identified as regulators of microRNA biogenesis in C. elegans. Mutations in dcr-1, alg-1 and alg-2 also resulted in heterochronic defects associated with microRNA mutants.
- Ha M, and Kim VN (2014). Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15, 509–24. [DOI] [PubMed] [Google Scholar]
- Hammell CM, Karp X, and Ambros V (2009a). A feedback circuit involving let-7-family miRNAs and DAF-12 integrates environmental signals and developmental timing in Caenorhabditis elegans. Proc Natl Acad Sci USA 106, 18668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell CM, Lubin I, Boag PR, Blackwell TK, and Ambros V (2009b). nhl-2 modulates microRNA activity in Caenorhabditis elegans. Cell 136, 926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi OF, and Ambros VR (2015). Control of stem cell self-renewal and differentiation by the heterochronic genes and the cellular asymmetry machinery in Caenorhabditis elegans. Proc Natl Acad Sci USA. 112, E287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes GD, Frand AR, and Ruvkun G (2006). The mir-84 and let-7 paralogous microRNA genes of Caenorhabditis elegans direct the cessation of molting via the conserved nuclear hormone receptors NHR-23 and NHR-25. Development 133, 4631–41. [DOI] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. (2005). A microRNA polycistron as a potential human oncogene. Nature 435, 828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks G-J, Gaidatzis D, Aeschimann F, and Großhans H (2014). Extensive oscillatory gene expression during C. elegans larval development. Molecular Cell 53, 380–92. [DOI] [PubMed] [Google Scholar]
- Herranz H, Hong X, Hung NT, Voorhoeve PM, and Cohen SM (2012a). Oncogenic cooperation between SOCS family proteins and EGFR identified using a Drosophila epithelial transformation model. Genes Dev. 26, 1602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz H, Hong X, and Cohen SM (2012b). Mutual repression by bantam miRNA and capicua links the EGFR/MAPK and Hippo pathways in growth control. Current Biology 22, 651–57. [DOI] [PubMed] [Google Scholar]
- Hipfner DR, Weigmann K, and Cohen SM (2002). The bantam gene regulates Drosophila growth. Genetics 161, 1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y-W, Chang C, and Chuang C-F (2012). The MicroRNA mir-71 Inhibits calcium signaling by targeting the TIR-1/Sarm1 adaptor protein to control stochastic L/R neuronal asymmetry in C. elegans. PLoS Genet 8, e1002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SE, Finnegan EF, Zisoulis DG, Lovci MT, Melnik-Martinez KV, Yeo GW, and Pasquinelli AE (2013). Functional genomic analysis of the let-7 regulatory network in Caenorhabditis elegans. PLoS Genet 9, e1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, and Kim VN (2009). Conserved microRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 139, 1096–1108. [DOI] [PubMed] [Google Scholar]
- Ilbay O and Ambros V (2019). Pheromones and nutritional signals regulate the developmental reliance on let-7 family microRNAs in C. elegans. Current Biology 29, 1735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino N, Pane A, and Gaul U (2009). miR-184 has multiple roles in Drosophila female germline development. Developmental Cell 17, 123–33. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kawamata T, and Tomari Y (2009). Drosophila Argonaute1 and Argonaute2 employ distinct mechanisms for translational repression. Molecular Cell 34, 58–67. [DOI] [PubMed] [Google Scholar]
- Jannot G, Michaud P, Quévillon Huberdeau M, Morel-Berryman L, Brackbill JA, Piquet S, McJunkin K, Nakanishi K, and Simard MJ (2016). GW182-free microRNA silencing complex controls post-transcriptional gene expression during Caenorhabditis elegans embryogenesis. PLoS Genet 12, e1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Shao C, Wu Q-J, Chen G, Zhou J, Yang B, Li H, Gou L-T, Zhang Y, Wang Y, et al. (2017). NEAT1 scaffolds RNA-binding proteins and the Microprocessor to globally enhance pri-miRNA processing. Nat Struct Mol Biol 24, 816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Großhans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, and Slack FJ (2005). RAS Is regulated by the let-7 microRNA family. Cell 120, 635–47. [DOI] [PubMed] [Google Scholar]
- *.Johnston RJ, and Hobert O (2003). A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426, 845–49. [DOI] [PubMed] [Google Scholar]; lsy-6 was identified as a regulator of left/right asymmetry in the ASE(L/R) neurons in C. elegans. lsy-6 was the first microRNA identified with a role in neuronal patterning.
- Jonas S, and Izaurralde E (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16, 421–33. [DOI] [PubMed] [Google Scholar]
- Kadener S, Menet JS, Sugino K, Horwich MD, Weissbein U, Nawathean P, Vagin VV, Zamore PD, Nelson SB, and Rosbash M (2009). A role for microRNAs in the Drosophila circadian clock. Genes Dev. 23, 2179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Karp X and Ambros V (2012). Dauer larva quiescence alters the circuitry of microRNA pathways regulating cell fate progression in C. elegans. Development 139, 2177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]; Passage through dauer eliminated the necessity of the let-7 family members, miR-48, miR-84 and miR-241, for post embryonic development in C. elegans. lin-4, could compensate for the loss of miR-48, miR-84 and miR-241, by downregulating their target hbl-1, but only after dauer.
- Karp X, Hammell M, Ow MC, and Ambros V (2011). Effect of life history on microRNA expression during C. elegans development. RNA 17, 639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga H, Fukuyama M, Kitazawa A, Kontani K, and Katada T (2013). The microRNA miR-235 couples blast-cell quiescence to the nutritional state. Nature 497, 503–06. [DOI] [PubMed] [Google Scholar]
- Kemp BJ, Allman E, Immerman L, Mohnen M, Peters MA, Nehrke K, and Abbott AL (2012). miR-786 regulation of a fatty-Acid elongase contributes to rhythmic calcium-wave initiation in C. elegans. Current Biology 22, 2213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kim DH, Grün D, and van Oudenaarden A (2013). Dampening of expression oscillations by synchronous regulation of a microRNA and its target. Nature Genetics 45, 1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; About 2,000 transcripts displayed oscillatory expression across C. elegans development. The pulsatile expression of the microRNA lin-4 dampened the oscillatory expression of its target gene lin-14, which instead showed steadily decreasing levels in wild type animals. lin-4, therefore acts as a rheostat and not as a switch.
- Kim K, Vinayagam A, and Perrimon N (2014). A rapid genome-wide microRNA screen identifies miR-14 as a modulator of hedgehog signaling. Cell Reports 7, 2066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Civin CI, and Kingsbury TJ (2019). MicroRNAs as regulators and effectors of hematopoietic transcription factors. WIREs RNA 15, e1537. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Shoji K, Kiyokawa K, Negishi L, and Tomari Y (2019). Iruka eliminates dysfunctional Argonaute by selective ubiquitination of its empty state. Molecular Cell 73, 119–29. [DOI] [PubMed] [Google Scholar]
- Kozomara A, Birgaoanu M, and Griffiths-Jones S (2019). miRBase: from microRNA sequences to function. Nucleic Acids Research 47, D155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretov DA, Walawalkar IA, Mora-Martin A, Shafik AM, Moxon S, and Cifuentes D (2020). Ago2-dependent processing allows miR-451 to evade the global microRNA turnover elicited during erythropoiesis. Molecular Cell 78, 317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherenko MM, Barth J, Fiala AE, and Shcherbata HR (2012). Steroid-induced microRNA let-7 acts as a spatio-temporal code for neuronal cell fate in the developing Drosophila brain. EMBO J. 31, 4511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler J-M, Chen Y-W, Weng R, and Cohen SM (2013). Maternal loss of miRNAs leads to increased variance in primordial germ cell numbers in Drosophila melanogaster. G3: Genes, Genomes, Genetics 3, 1573–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Han Z, Olson EN, and Srivastava D (2005). MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc Natl Acad Sci USA 102, 18986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rocca G, Olejniczak SH, González AJ, Briskin D, Vidigal JA, Spraggon L, DeMatteo RG, Radler MR, Lindsten T, Ventura A, et al. (2015). In vivo, Argonaute-bound microRNAs exist predominantly in a reservoir of low molecular weight complexes not associated with mRNA. Proc Natl Acad Sci USA 112, 767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Torre A, Georgi S, and Reh TA (2013). Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci USA 110, E2362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lagos-Quintana M, Rauhut R, Lendeckel W, and Tuschl T (2001). Identification of novel genes coding for small expressed RNAs. Science 294, 853–58. [DOI] [PubMed] [Google Scholar]; The discovery of lin-4 and let-7 in C. elegans and the deeply conserved nature of let-7 fueled the attempts to characterize microRNAs in other organisms. This study cloned 16 novel microRNAs from Drosophila melanogaster embryos and 21 novel microRNAs from HeLa cells. Conserved microRNAs were also detected in mouse kidneys, Xenopus laevis ovary and adult zebrafish, providing evidence for the widespread occurrence of microRNAs. Also see Lau et al., 2001 and Lee and Ambros, 2001.
- *.Lau NC, Lim LP, Weinstein EG, and Bartel DP (2001). An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858–62. [DOI] [PubMed] [Google Scholar]; 55 novel microRNAs were cloned from the C. elegans genome. The presence of independent transcriptional units for microRNAs and the possibility of the mature microRNA residing on either strand of the precursor microRNA hairpin were proposed in this study. The presence of a single precursor microRNA for the miR-35-41 cluster was also proved. Also see Lagos-Quintana et al., 2001 and Lee and Ambros, 2001.
- Lee FCY, and Ule J (2018). Advances in CLIP technologies for studies of protein-RNA interactions. Molecular Cell 69, 354–369. [DOI] [PubMed] [Google Scholar]
- Lee M, Choi Y, Kim K, Jin H, Lim J, Nguyen TA, Yang J, Jeong M, Giraldez AJ, Yang H, et al. (2014). Adenylation of maternally inherited microRNAs by Wispy. Molecular Cell 56, 696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lee R and Ambros V (2001). An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–64. [DOI] [PubMed] [Google Scholar]; 15 novel microRNAs were identified in C. elegans. 3 microRNAs were conserved in mammals and Drosophila melanogaster. Some of these microRNAs were expressed in specific tissues at specific developmental time points. Also see Lagos-Quintana et al., 2001 and Lau et al., 2001.
- **.Lee R, Feinbaum R, and Ambros V (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854. [DOI] [PubMed] [Google Scholar]; lin-4 was previously identified as a heterochronic mutant. This study discovered that the lin-4 locus encoded the first microRNA gene. The lin-4 sequence was found to be partially complementary to regions of the lin-14 3’UTR, suggesting that lin-4 mediated the repression of lin-14 through direct RNA-RNA interaction. Also see Wightman et al. 1993.
- Lee SW, Oh YM, Lu Y-L, Kim WK, and Yoo AS (2018). MicroRNAs overcome cell fate barrier by reducing EZH2-controlled REST stability during neuronal conversion of human adult fibroblasts. Developmental Cell 46, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. (2003). The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–19. [DOI] [PubMed] [Google Scholar]; The RNA endonuclease Drosha was identified as the biogenesis factor responsible for cleaving the primary microRNA (pri-microRNA) transcripts to precursor microRNA (pre-microRNA) hairpins. The structural cis elements in the pri-microRNA required for efficient processing by Drosha were also identified.
- Li J, and Greenwald I (2010). LIN-14 Inhibition of LIN-12 contributes to precision and timing of C. elegans vulval fate patterning. Current Biology 20, 1875–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, and Carthew RW (2005). A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 123, 1267–77. [DOI] [PubMed] [Google Scholar]
- *.Li X, Cassidy JJ, Reinke CA, Fischboeck S, and Carthew RW (2009). A microRNA imparts robustness against environmental fluctuation during development. Cell 137, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]; miR-7 was found to be involved in interlocking feedback and feedforward loops during photoreceptor differentiation and sensory organ precursor determination in Drosophila. miR-7 buffered these gene regulatory networks during varying environmental conditions.
- Li Y, Wang F, Lee JA, and Gao FB (2006). MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes Dev. 20, 2793–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, and Padgett RW (2012). bantam is required for optic lobe development and glial cell proliferation. PLoS ONE 7, e32910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lee M, Son A, Chang H, and Kim VN (2016). mTAIL-seq reveals dynamic poly(A) tail regulation in oocyte-to-embryo development. Genes Dev. 30, 1671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano E, de Lucas MP, and Sáez AG (2016). sta-1 is repressed by mir-58 family in Caenorhabditis elegans. Worm 5, e1238560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Wu J, Xiong S, Zhang X, Zhang J, and Mei J (2017). MicroRNA-203a regulates fast muscle differentiation by targeting dmrt2a in zebrafish embryos. Gene 625, 49–54. [DOI] [PubMed] [Google Scholar]
- Luo W, and Sehgal A (2012). Regulation of circadian behavioral output via a microRNA-JAK/STAT circuit. Cell 148, 765–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, and Maniatis T (2007). The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular Cell 27, 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick A, Ranawade A, and Gupta BP (2019). Role of PRY-1/Axin in heterochronic miRNA- mediated seam cell development. BMC Dev Biol 19, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Basquin J, Ozgur S, Czarnocki-Cieciura M, Bonneau F, Aartse A, Dziembowski A, Nowotny M, Conti E, and Filipowicz W (2014). Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Molecular Cell 54, 751–65. [DOI] [PubMed] [Google Scholar]
- McCulloch KA, and Rougvie AE (2014). Caenorhabditis elegans period homolog lin-42 regulates the timing of heterochronic miRNA expression. Proc Natl Acad Sci USA 111, 15450–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen TJ, Yao Q, Yun S, Lee C-Y, and Bennett KL (2016). Small RNA in situ hybridization in Caenorhabditis elegans, combined with RNA-seq, identifies germline-enriched microRNAs. Developmental Biology 418, 248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin K (2018). Maternal effects of microRNAs in early embryogenesis. RNA Biology 15, 165–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin K, and Ambros V (2014). The embryonic mir-35 family of microRNAs promotes multiple aspects of fecundity in Caenorhabditis elegans. G3: Genes, Genomes, Genetics 4, 1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McJunkin K, and Ambros V (2017). A microRNA family exerts maternal control on sex determination in C. elegans. Genes Dev. 31, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer HA, Kong YW, Lu WT, Wilczynska A, Spriggs RV, Robinson SW, Godfrey JD, Willis AE, and Bushell M (2013). Translational repression and elF4A2 activity are critical for microRNA-mediated gene regulation. Science 340, 82–85. [DOI] [PubMed] [Google Scholar]
- *.Melton C, Judson RL, and Blelloch R (2010). Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463, 621–26. [DOI] [PMC free article] [PubMed] [Google Scholar]; The let-7 family of microRNAs inhibited proliferation and promoted differentiation in Dgcr8 knockout mESCs. In wild type conditions, the ESCC and let-7 family microRNAs stabilized self-renewal and differentiation, respectively, by regulating common pathways.
- Minogue AL, Tackett MR, Atabakhsh E, Tejada G, and Arur S (2018). Functional genomic analysis identifies miRNA repertoire regulating C. elegans oocyte development. Nature Communications 9, 5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Abreu-Goodger C, Staton AA, Stahlhut C, Shou C, Cheng C, Gerstein M, Enright AJ, and Giraldez AJ (2009). Zebrafish miR-1 and miR-133 shape muscle gene expression and regulate sarcomeric actin organization. Genes Dev. 23, 619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Giraldez AJ, Takeda Y, Fujiwara T, Sakamoto H, Schier AF, and Inoue K (2006). Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Current Biology 16, 2135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Abbott AL, Lau NC, Hellman AB, McGonagle SM, Bartel DP, Ambros VR, and Horvitz HR (2007). Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet 3, e215–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok GF, Lozano-Velasco E, and MQnsterberg A (2017). microRNAs in skeletal muscle development. Seminars in Cell and Developmental Biology 72, 67–76. [DOI] [PubMed] [Google Scholar]
- Mondol V, Ahn BC, and Pasquinelli AE (2015). Splicing remodels the let-7 primary microRNA to facilitate Drosha processing in Caenorhabditis elegans. RNA 21, 1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga I, Kumar M (2019) Computational resources for prediction and analysis of functional miRNA and their targetome. In: Lai X, Gupta S, Vera J (eds) Computational Biology of Non-Coding RNA. Methods Mol Biol 1912, 215–50. [DOI] [PubMed] [Google Scholar]
- Monsalve GC, and Frand AR (2014). Toward a unified model of developmental timing: A “molting” approach. Worm 1, 221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve GC, Van Buskirk C, and Frand AR (2011). LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Current Biology 21, 2033–45. [DOI] [PubMed] [Google Scholar]
- Morante J, Vallejo DM, Desplan C, and Dominguez M (2013). Conserved miR-8/miR-200 defines a glial niche that controls neuroepithelial expansion and neuroblast transition. Developmental Cell 27, 174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EG, Lee R, and Ambros V (1997). The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88, 637–46. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, and Bassell GJ (2011). Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Molecular Cell 42, 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C, and Ambros V (2019). Trans-splicing of the C. elegans let-7 primary transcript developmentally regulates let-7 microRNA biogenesis and let-7 family microRNA activity. Development 146, dev172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C, Ambros V, and Baehrecke EH (2014). miR-14 regulates autophagy during developmental cell death by targeting ip3-kinase 2. Molecular Cell 56, 376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J, Kim S, Zhu Y, Zhu H, and Morrison SJ (2013). A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. eLIFE 2, 683–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson-Carter K, and Slack FJ (2010). A developmental timing switch promotes axon outgrowth independent of known guidance receptors. PLoS Genet 6, e1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paikari A, Belair CD, Saw D, and Blelloch R (2017). The eutheria-specific miR-290 cluster modulates placental growth and maternal-fetal transport. Development 144, 3731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchem RJ, Moore N, Fish JL, Parchem JG, Braga TT, Shenoy A, Oldham MC, Rubenstein JLR, Schneider RA, and Blelloch R (2015). miR-302 is required for timing of neural differentiation, neural tube closure, and embryonic viability. Cell Reports 12, 760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, and Sheth U (2007). P Bodies and the control of mRNA translation and degradation. Molecular Cell 25, 635–646. [DOI] [PubMed] [Google Scholar]
- **.Pasquinelli AE, Reinhart B, Slack FJ, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, et al. (2000). Conservation of sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86–9. [DOI] [PubMed] [Google Scholar]; The first two microRNAs, lin-4 and let-7, were discovered in C. elegans. This study showed that let-7 was highly conserved across vertebrates, ascidians, hemichordates, mollusks, annelids, and arthropods, revealing that microRNAs were not just a worm phenomenon and launching the search for novel microRNAs and their functions in other organisms.
- Patterson M, Gaeta X, Loo K, Edwards M, Smale S, Cinkornpumin J, Xie Y, Listgarten J, Azghadi S, Douglass SM, et al. (2014). let-7 miRNAs can act through Notch to regulate human gliogenesis. Stem Cell Reports 3, 758–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pek JW, Lim AK, and Kai T (2009). Drosophila maelstrom ensures proper germline stem cell lineage differentiation by repressing microRNA-7. Developmental Cell 17, 417–24. [DOI] [PubMed] [Google Scholar]
- Perales R, King DM, Aguirre-Chen C, and Hammell CM (2014). LIN-42, the Caenorhabditis elegans PERIOD homolog, negatively regulates microRNA transcription. PLoS Genet 10, e1004486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quévillon Huberdeau M, Zeitler DM, Hauptmann J, Bruckmann A, Fressigné L, Danner J, Piquet S, Strieder N, Engelmann JC, Jannot G, et al. (2017). Phosphorylation of Argonaute proteins affects mRNA binding and is essential for microRNA-guided gene silencing in vivo. EMBO J. 36, 2088–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PK, Kumar RM, Farkondeh M, Baskerville S, and Lodish HF (2006). Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA 103, 8721–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Reinhart B, Slack FJ, Basson M, Pasquinelli A, Jill B, Rougvie A, Horvitz HR, and Ruvkun G (2000). The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans, Nature 403, 901–06. [DOI] [PubMed] [Google Scholar]; The let-7 locus was determined to encode the second identified microRNA. let-7 regulated the transition from L4 larvae to adults in C. elegans. The 3’ UTRs of other heterochronic genes had sequences complementary to let-7, including the lin-41 3’ UTR, which was shown to be temporally regulated by let-7.
- Rios C, Warren D, Olson B, and Abbott AL (2017). Functional analysis of microRNA pathway genes in the somatic gonad and germ cells during ovulation in C. elegans. Developmental Biology 426, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel CJ, Gilles AF, and Averof M (2013). MicroRNAs act as cofactors in bicoid-mediated translational repression. Current Biology 23, 1579–84. [DOI] [PubMed] [Google Scholar]
- Rupaimoole R, and Slack FJ (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16, 203–22. [DOI] [PubMed] [Google Scholar]
- Shaw WR, Armisen J, Lehrbach NJ, and Miska EA (2010). The conserved miR-51 microRNA family is redundantly required for embryonic development and pharynx attachment in Caenorhabditis elegans. Genetics 185, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy A, and Blelloch RH (2014). Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol 15, 565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy A, Danial M, and Blelloch RH (2015). let-7 and miR-125 cooperate to prime progenitors for astrogliogenesis. EMBO J. 34, 1143–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard R, Luehr S, Holzkamp H, McJunkin K, Memar N, and Conradt B (2017). miRNAs cooperate in apoptosis regulation during C. elegans development. Genes Dev. 31, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Nakao H, Kiyonari H, Abe T, and Aizawa S (2011). MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. Journal of Neuroscience 31, 3407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu P, Wu C, Ruan X, Liu W, Hou L, Fu H, Wang M, Liu C, Zeng Y, Chen P, et al. (2019). Opposing gradients of microRNA expression temporally pattern layer formation in the developing neocortex. Developmental Cell 49, 764–85. [DOI] [PubMed] [Google Scholar]
- Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, and Kim JK (2008). The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell 133, 903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Dole NS, Franceschetti T, Hrdlicka HC, and Delany AM (2016). MicroRNA-433 dampens glucocorticoid receptor signaling, impacting circadian rhythm and osteoblastic gene expression. J Biol Chem 291, 21717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol NS, and Ambros V (2005). Mesodermally expressed Drosophila microRNA-1 is regulated by Twist and is required in muscles during larval growth. Genes Dev. 19, 2343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce T, Pernaute B, Di-Gregorio A, Cobb BS, Merkenschlager M, Manzanares M, and Rodriguez TA (2010). An early developmental role for miRNAs in the maintenance of extraembryonic stem cells in the mouse embryo. Developmental Cell 19, 207–19. [DOI] [PubMed] [Google Scholar]
- Stahlhut C, Suarez Y, Lu J, Mishima Y, and Giraldez AJ (2012). miR-1 and miR-206 regulate angiogenesis by modulating VegfA expression in zebrafish. Development 139, 4356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Chen X, Zhao H, and Slack FJ (2015). A novel mechanism of LIN-28 regulation of let-7microRNA expression revealed by in vivo HITS-CLIP in C. elegans. RNA 21, 985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckius M, Maaskola J, Colombo T, Rahn H-P, Friedländer MR, Li N, Chen W, Piano F, and Rajewsky N (2009). Large-scale sorting of C. elegans embryos reveals the dynamics of small RNA expression. Nat Meth 6, 745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtelny AO, Eichhorn SW, Chen GR, Sive H, and Bartel DP (2014). Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 508, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N, Baehner L, Moltzahn F, Melton C, Shenoy A, Chen J, and Blelloch R (2010). MicroRNA Function Is Globally Suppressed in Mouse Oocytes and Early Embryos. Current Biology 20, 271–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YS, Bhat S, Hong S-H, Shin M, Bahk S, Cho KS, Kim S-W, Lee K-S, Kim Y-J, Jones WD, et al. (2015). Genome-wide microRNA screening reveals that the evolutionary conserved miR-9a regulates body growth by targeting sNPFR1/NPYR. Nature Communications 6, 7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs CM, and Giraldez AJ (2016). miR-430 regulates oriented cell division during neural tube development in zebrafish. Developmental Biology 409, 442–50. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Gardner HF, Volk ML, and Rougvie AE (2006). Novel heterochronic functions of the Caenorhabditis elegans period-related protein LIN-42. Developmental Biology 289, 30–43. [DOI] [PubMed] [Google Scholar]
- Than MT, Kudlow BA, and Han M (2013). Functional analysis of neuronal microRNAs in Caenorhabditis elegans dauer formation by combinational genetics and neuronal miRISC immunoprecipitation. PLoS Genet 9, e1003592–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T, Tokusumi Y, Hopkins DW, Shoue DA, Corona L, and Schulz RA (2011). Germ line differentiation factor Bag of Marbles is a regulator of hematopoietic progenitor maintenance during Drosophila hematopoiesis. Development 138, 3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T, Tokusumi Y, and Schulz RA (2018). The mir-7 and bag of marbles genes regulate Hedgehog pathway signaling in blood cell progenitors in Drosophila larval lymph glands. Genesis 56, e23210–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott M, Islam ABMMK, Lightfoot J, López-Bigas N, and Frolov MV (2014). An Intronic microRNA Links Rb/E2F and EGFR Signaling. PLoS Genet 10, e1004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsialikas J, and Romer-Seibert J (2015). LIN28: roles and regulation in development and beyond. Development 142, 2397–2404. [DOI] [PubMed] [Google Scholar]
- Tsialikas J, Romens MA, Abbott AL, and Moss EG (2017). Stage-specific timing of the microRNA regulation of lin-28 by the heterochronic gene lin-14 in Caenorhabditis elegans. Genetics 205, 251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadla B, Kemper K, Alaimo J, Heine C, and Moss EG (2012). lin-28 controls the succession of cell fate choices via two distinct activities. PLoS Genet 8, e1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wynsberghe PM, Finnegan EF, Stark T, Angelus EP, Homan KE, Yeo GW, and Pasquinelli AE (2014). The Period protein homolog LIN-42 negatively regulates microRNA biogenesis in C. elegans. Developmental Biology 390, 126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wynsberghe PM, Kai ZS, Massirer KB, Burton VH, Yeo GW, and Pasquinelli AE (2011). LIN-28 co-transcriptionally binds primary let-7 to regulate miRNA maturation in Caenorhabditis elegans. Nat Struct Mol Biol 18, 302–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese J, Lim SF, and Cohen SM (2010). Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev. 24, 2748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese J, and Cohen SM (2007). microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in Drosophila. Genes Dev. 21, 2277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejnar CE, Abdel Messih M, Takacs CM, Yartseva V, Oikonomou P, Christiano R, Stoeckius M, Lau S, Lee MT, Beaudoin J-D, et al. (2019). Genome wide analysis of 3’ UTR sequence elements and proteins regulating mRNA stability during maternal-to-zygotic transition in zebrafish. Genome Research 29, 1100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, and Cohen SM (2015). miR-965 controls cell proliferation and migration during tissue morphogenesis in the Drosophila abdomen. eLIFE 4, e07389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng P-Y, Klyachko VA, Nerbonne JM, and Yoo AS (2014). Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron 84, 311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan J, Lee S, Lee B, Lee JW, and Lee SK (2007). The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 21, 744–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Viswanathan SR, Daley GQ, and Gregory RI (2008). Selective blockade of microRNA processing by Lin28. Science 320, 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]; The RNA binding protein Lin28 selectively inhibited the Microprocessor-mediated processing of pri-let-7 in mESCs. As the expression of Lin28 was developmentally regulated, this suggested a mechanism for the maintenance of low levels of let-7 during self-renewal.
- Vodala S, Pescatore S, Rodriguez J, Buescher M, Chen Y-W, Weng R, Cohen SM, and Rosbash M (2012). The oscillating miRNA 959–964 cluster impacts Drosophila feeding time and other circadian outputs. Cell Metabolism 16, 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Schmitz RJ, Nathanson J, Yeo G, Ecker JR, and Panda S (2012). Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metabolism 16, 833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, and Blelloch R (2008). Embryonic stem cell–specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet 40, 1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elegantly designed rescue assays in Dgcr8 knockout mESCs led to the identification of the ESCC microRNAs. These microRNAs promoted cell proliferation and self-renewal by suppressing inhibitors of the G1-S transition.
- Wang Y, Medvid R, Melton C, Jaenisch R, and Blelloch R (2007). DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet 39, 380–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng R, and Cohen SM (2012). Drosophila miR-124 regulates neuroblast proliferation through its target anachronism. Development 139, 1427–34. [DOI] [PubMed] [Google Scholar]
- Weng R, and Cohen SM (2015). Control of Drosophila Type I and Type II central brain neuroblast proliferation by bantam microRNA. Development 142, 3713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienholds E, Kloosterman WP, Miska EA, Alvarez-Saavedra E, Berezikov E, Bruijn E. de, Horvitz RH, Kauppinen S, Plasterk R, and Plasterk R (2005). MicroRNA expression in zebrafish embryonic development. Science 309, 310–11. [DOI] [PubMed] [Google Scholar]
- **.Wightman B, Ha I, and Ruvkun G (1993). Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–62. [DOI] [PubMed] [Google Scholar]; The 3’UTR of the lin-14 mRNA was shown to be necessary and sufficient for the lin-4 dependent temporal gradient observed for LIN-14 protein. Seven conserved elements that were complementary to the lin-4 sequence were identified in the lin-14 3’UTR, suggesting that base pairing between lin-4 and lin-14 may inhibit translation of lin-14. Also see Lee et al, 1993.
- Wu E, Thivierge C, Flamand M, Mathonnet G, Vashisht AA, Wohlschlegel J, Fabian MR, Sonenberg N, and Duchaine TF (2010). Pervasive and cooperative deadenylation of 3’ UTRs by embryonic microRNA families. Molecular Cell 40, 558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Vashisht AA, Chapat C, Flamand MN, Cohen E, Sarov M, Tabach Y, Sonenberg N, Wohlschlegel J, and Duchaine TF (2016). A continuum of mRNP complexes in embryonic microRNA-mediated silencing. Nucleic Acids Research 45, 2081–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-C, Chawla G, and Sokol N (2020). let-7-complex microRNAs regulate Broad-Z3, which together with Chinmo maintains adult lineage neurons in an immature state. G3: Genes, Genomes, Genetics 10, 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-C, Chen C-H, Mercer A, and Sokol NS (2012). Let-7-complex microRNAs regulate the temporal identity of Drosophila mushroom body neurons via chinmo. Developmental Cell 23, 202–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Li M, Vilborg A, Lee N, Shu M-D, Yartseva V, Šestan N, and Steitz JA (2013). Mammalian 5’-capped microRNA precursors that generate a single microRNA. Cell 155, 1568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-S, Maurin T, Robine N, Rasmussen KD, Jeffrey KL, Chandwani R, Papapetrou EP, Sadelain M, OCarroll D, and Lai EC (2010). Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci USA 107, 15163–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Lin J, Liu M, Li R, Tian B, Zhang X, Xu B, Liu M, Zhang X, Li Y, et al. (2016). Highly sensitive sequencing reveals dynamic modifications and activities of small RNAs in mouse oocytes and early embryos. Sci. Adv 2, e1501482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartseva V, and Giraldez AJ (2015). The maternal-to-zygotic transition during vertebrate development: a model for reprogramming, Curr Top Dev Biol 113, 191–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AS, and Shcherbata HR (2014). Drosophila miR-9a targets the ECM receptor dystroglycan to canalize myotendinous junction formation. Developmental Cell 28, 335–48. [DOI] [PubMed] [Google Scholar]
- Yoo AS, and Greenwald I (2005). LIN-12/Notch activation leads to microRNA-mediated down-regulation of Vav in C. elegans. Science 310, 1330–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Staahl BT, Chen L, and Crabtree GR (2009). MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460, 642–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, and Crabtree GR (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]; The expression of the neuron-specific microRNAs miR-9/9* and miR-124, in combination with neural transcription factors, induced conversion of human fibroblasts into neurons. This suggested a role for microRNAs in cell fate reprogramming.
- Yoon WH, Meinhardt H, and Montell DJ (2011). miRNA-mediated feedback inhibition of JAK/STAT morphogen signalling establishes a cell fate threshold. Nat Cell Biol 13, 1062–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Reynolds SH, Hatfield SD, Shcherbata HR, Fischer KA, Ward EJ, Long D, Ding Y, and Ruohola-Baker H (2009). Dicer-1-dependent Dacapo suppression acts downstream of insulin receptor in regulating cell division of Drosophila germline stem cells. Development 136, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuva-Aydemir Y, Xu X-L, Aydemir O, Gascon E, Sayin S, Zhou W, Hong Y, and Gao F-B (2015). Downregulation of the host gene jigr1 by miR-92 is essential for neuroblast self-renewal in Drosophila. PLoS Genet 11, e1005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, and Emmons SW (2009). Regulation of the Caenorhabditis elegans posterior hox gene egl-5 by microRNA and the polycomb-like gene sop- 2. Dev. Dyn 238, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zabinsky R, Teng Y, Cui M, and Han M (2011). microRNAs play critical roles in the survival and recovery of Caenorhabditis elegans from starvation-induced L1 diapause. Proc Natl Acad Sci USA 108, 17997–18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ransom JF, Li A, Vedantham V, Drehle, von M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, and Srivastava D (2007). Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129, 303–17. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Samal E, and Srivastava D (2005). Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436, 214–20. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lim MYT, Kaur P, Saj A, Bortolamiol-Becet D, Gopal V, Tolwinski N, Tucker-Kellogg G, and Okamura K (2018). Importance of miRNA stability and alternative primary miRNA isoforms in gene regulation during Drosophila development. eLIFE 7, e38389. [DOI] [PMC free article] [PubMed] [Google Scholar]


