Abstract
Messenger RNA vaccines are the main COVID-19 vaccines authorized for use in the United States. Side effects are typically minor and transient. We report a case series of four subjects with an acute myocarditis-like illness following mRNA COVID-19 vaccination who were hospitalized at our hospital in Lubbock, Texas. Three patients were young men who presented with acute chest pain after the second dose of the mRNA-1273 vaccine. Another patient was a 53-year-old white woman who presented with acute left arm pain 3 days after the first dose of the mRNA-1273 vaccine. She was later found to have acute decompensated heart failure, and endomyocardial biopsy revealed eosinophilic injury–mediated myocarditis.
Keywords: Corticosteroids, COVID-19, mRNA vaccine, myocarditis
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a major effect on our lives globally. SARS-CoV-2 vaccines significantly reduce the risk of contracting COVID-19 illness.1,2 The US Food and Drug Administration provided an emergent use authorization for two mRNA COVID-19 vaccines from Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2). Side effects are typically minor and transient.3 Recently, the safety committee of the US Centers for Disease Control and Prevention stated that there was a likely association between SARS-CoV-2 vaccines and myocarditis/pericarditis.4 We report four adult patients who were confirmed to have myocarditis/pericarditis following SARS-CoV-2 vaccination.
CASE DESCRIPTION
A total of four patients were hospitalized at our hospital in Lubbock, Texas, in early 2021 for myocarditis following mRNA-1273 vaccination. The clinical characteristics of the patients are shown in Table 1. Three patients (Cases 2–4) were young white men and presented with hemodynamically stable chest pain. One woman (Case 1) presented with left arm pain that started 3 days after the first dose of vaccine. She was hemodynamically unstable and found to have findings of acute decompensated heart failure on admission.
Table 1.
Characteristics and outcomes of patients with myocarditis following COVID-19 vaccination
| Variable | Case number |
Reference value | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Age (years) | 53 | 22 | 19 | 22 | ||
| Gender | Female | Male | Male | Male | ||
| Race/ethnicity | White | White | White | White | ||
| Comorbidities | Depression, hyperlipidemia | Asthma, kidney stone | None | Asthma, fibrous dysplasia | ||
| Vaccine type | mRNA-1273 | mRNA-1273 | mRNA-1273 | mRNA-1273 | ||
| Vaccine dose | First | Second | Second | Second | ||
| Days: vaccine to hospital | 6 | 3 | 4 | 3 | ||
| Body mass index (kg/m2) | 28.7 | 34.9 | 28 | 24 | ||
| Vital signs on arrival | ||||||
| Temperature (°F) | 96.8 | 99.5 | 99.6 | 98.7 | ||
| Heart rate (beats/min) | 112 | 84 | 76 | 138 | ||
| Blood pressure (mm Hg) | 86/60 | 152/79 | 119/69 | 119/69 | ||
| Respiratory rate (breaths/min) | 17 | 22 | 14 | 20 | ||
| Chest x-ray | Enlarged cardiac silhouette | Enlarged cardiac silhouette | Normal | Normal | ||
| Electrocardiogram | 1st AV block; 3rd AV block later | Normal | Diffuse ST elevations | Diffuse ST elevation | ||
| Echocardiogram | ||||||
| Day from admission | 2 | 2 | 1 | 2 | ||
| LVEF (%) | 60–64 | 55–59 | 65–69 | 60–64 | ≥55 | |
| TAPSE (cm) | 2.1 | 1.9 | 2 | 2 | <1.7 | |
| Right heart catheterization | ||||||
| Day from admission | 4 | 1 | Not done | Not done | ||
| RA pressure (mm Hg) | 26 | 8 | 2.0–6.0 | |||
| RV pressure (mm Hg) | ||||||
| Systolic | 40 | 30 | 15–25 | |||
| Diastolic | 11 | 8 | 0–8 | |||
| PA pressure (mm Hg) | ||||||
| Systolic | 44 | 23 | 15–25 | |||
| Diastolic | 23 | 14 | 8.0–15.0 | |||
| PCWP | 29 | 12 | 6.0–12.0 | |||
| Cardiac output (L/min) | 3.54 | 7 | 4.0–8.0 | |||
| Cardiac index (min × m2) | 1.82 | 2.7 | 2.5–4.0 | |||
| Cardiac MRI | ||||||
| Day after presentation | 8 | 5 | 3 | 25 | ||
| LVEF (%) | 45 | 60 | 53 | 54 | ||
| RVEF (%) | 35 | 44 | 46 | 45 | ||
| LGE abnormality | 0 | + | + | 0 | ||
| Increased T2 signal | 0 | + | 0 | 0 | ||
| Laboratory tests | ||||||
| WBC (K/µL) | 14 | 11.54 | 5.73 | 23.4 | 4.23–9.07 | |
| Hemoglobin (g/dL) | 11.2 | 15 | 15.8 | 15.5 | 13.7–17.5 | |
| Platelet count (K/µL) | 379 | 202 | 230 | 288 | 163–337 | |
| BUN (mg/dL) | 20 | 11 | 11 | 14 | 6.0–20.0 | |
| Creatinine (mg/dL) | 0.8 | 0.8 | 0.9 | 1 | 0.5–1.2 | |
| Creatine kinase (IU/L) | 340 (day 1) | 421 (day 3) | 1747 (day 1) | 111 (day 1) | 26–308 | |
| hs-cTnT (ng/L) | ≤19.0 | |||||
| At presentation | 1582 | 958 | 1509 | 90.3 | ||
| Peak | 1582 (day 1) | 1254 (day 4) | 2472 (day 2) | 90.3 (day 1) | ||
| At discharge | 41.5 (day 12) | 195 (day 8) | 210.6 (day 8) | 25.6 (day 4) | ||
| At follow-up (weeks after discharge) | <6 (2 weeks) | 16.3 (5 weeks) | 13.1 (1 week) | N/A | ||
| Pro-BNP (pg/mL) | 9401 (day 1) | 400 (day 1) | 149 (day 6) | 730 | ≤124 | |
| Peak Pro-BNP (pg/mL) | 14,933 (day 7) | 400 (day 1) | 149 (day 6) | 730 (day 1) | ||
| D-dimer (ng/mL) | 684 | N/A | N/A | N/A | ≤500 | |
| ESR (mm/h) | 0–30 | |||||
| Initial | 12 (day 4) | 14 (day 1) | 15 (day 2) | 29 (day 1) | ||
| Peak | 21 (day 12) | 14 (day 1) | 15 (day 2) | 29 (day 1) | ||
| CRP (mg/dL) | 0.0–0.5 | |||||
| Initial | 15 (day 4) | 5.4 (day 1) | 3.1 (day 2) | 7 (day 1) | ||
| Peak | 15 (day 4) | 5.4 (day 1) | 3.1 (day 2) | 12.4 (day 2) | ||
| Total bilirubin (mg/dL) | 0.4 | 0.3 | 0.5 | 0.7 | 0.0–1.0 | |
| AST (IU/L) | 160 | 72 | 30 | 25 | 5.0–37.0 | |
| ALT (IU/L) | 169 | 41 | 78 | 32 | 5.0–41.0 | |
| ALP (IU/L) | 58 | 56 | 78 | 136 | 35–129 | |
| Ferritin (ng/mL) | 14,345 (day 11) | 234 (day 6) | 92.7 (day 2) | NA | 13–150 | |
| TSH (µIU/mL) | 0.08 | 2.94 | 0.23 | 1.86 | 0.27–4.20 | |
| Free T4 (ng/mL) | 1.47 | 1.23 | 1.15 | 1.24 | 0.93–1.70 | |
| Lactate (mmol/L) | 3.2 | NA | NA | 1.4 | 0.5–2.2 | |
| ANA | Negative | Negative | Negative | Not done | ||
| Ig E (IU/mL) | 104 | Not done | Not done | Not done | 1.53–114 | |
| Clinical course | ||||||
| Hospitalization (days) | 11 | 8 | 7 | 4 | ||
| Methylprednisolone protocol | + | + | + | + | ||
| IV immunoglobulin | + | 0 | + | 0 | ||
ALT indicates alanine transaminase; ANA, antinuclear antibodies; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; hs-cTnT, high-sensitivity cardiac troponin T; IV, intravenous; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; Pro-BNP, pro-brain natriuretic peptide; RA, right atrial; RV, right ventricular; RVEF, right ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; TSH, thyroid-stimulating hormone; WBC, white blood cell.
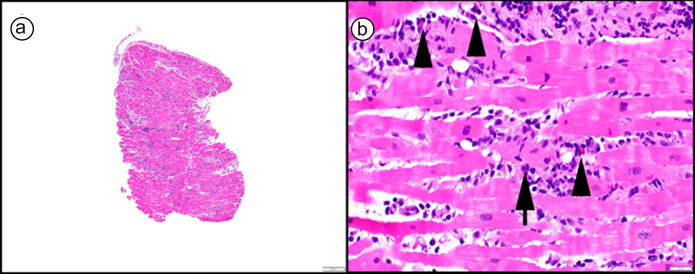
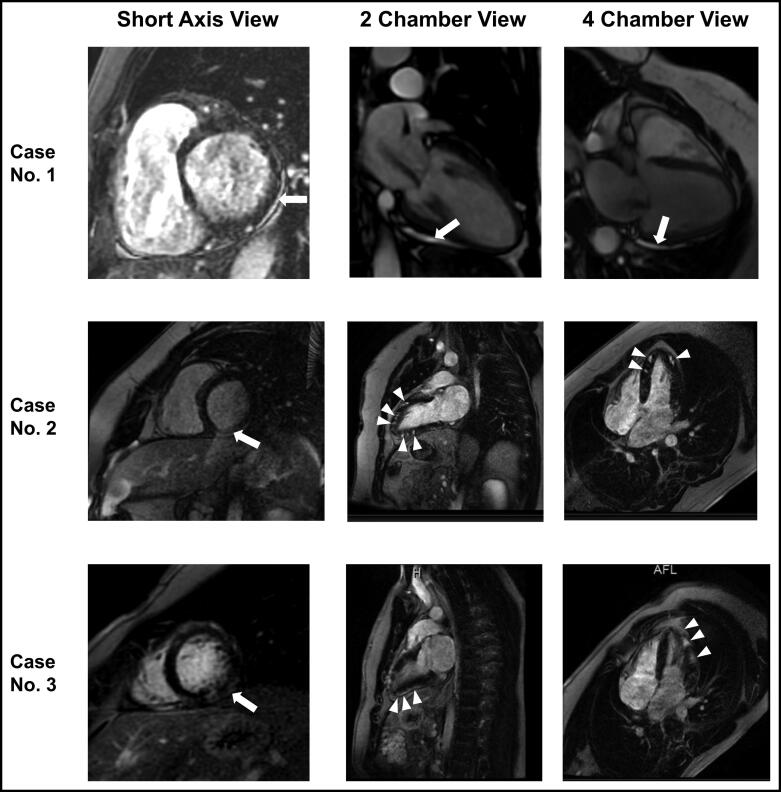
All patients had a negative Sofia 2 SARS Antigen Fluorescent Immunoassay test (Quidel Corporation, San Diego, CA). Electrocardiography upon arrival to the hospital was abnormal in three patients, as shown in Table 1. Serological tests for common viral myocarditis/pericarditis were negative for acute infection. Definitive diagnosis of acute myocarditis was made by endomyocardial biopsy in Case 1 (Figure 1), whereas Cases 2 and 3 had a combination of clinical manifestations and typical cardiac magnetic resonance imaging abnormalities used for diagnosis of myocarditis (Figure 2).5 We used clinical diagnostic criteria from the 2013 European Society of Cardiology for Case 4: new-onset acute chest pain with diffuse ST elevation and high-sensitivity cardiac troponin T.5
Figure 1.
Endomyocardial biopsy showing a polymorphous (mixed) active myocarditis with lymphohistiocytic inflammation and increased eosinophils (b, arrowheads). Myocyte injury was diffuse throughout the sampled tissue (b, arrow). No giant cells were identified throughout extensive sectioning of the biopsy, but vaguely granulomatous inflammation was apparent. (a, 40× original magnification, and b, 400× original magnification; both hematoxylin and eosin stain).
Figure 2.
Cardiac magnetic resonance imaging of Cases 1–3 in short axis (first column), two-chamber (second column), and four-chamber (third column) views. Case 1: Mild late gadolinium enhancement (LGE) is seen in the inferolateral region in the pericardium in all views (arrow). Case 2: LGE is seen in the mid wall region in a multifocal distribution in all views (arrows and arrowheads). Case 3: LGE is seen in the inferolateral and lateral wall in all views (arrows and arrowheads).
Patients were initially treated with oral colchicine and ibuprofen. Because of a lack of clinical improvement, a decision was made to give high-dose intravenous methylprednisolone for 3 to 8 days. Cardiac markers improved significantly after corticosteroid initiation (Supplemental Figures 1 and 2). On clinic follow-up at 4 to 6 weeks, patients’ clinical status was stable, and they had normal high-sensitivity cardiac troponin T levels. Cases were reported to the Vaccine Adverse Event Reporting System.
DISCUSSION
Pericarditis and myocarditis following vaccination are rare, and most case reports have described self-limited and benign conditions.6 The rate of myocarditis/pericarditis following mRNA vaccines is about 8.6 cases per million doses among those aged 16 to 39 years. Myocarditis/pericarditis following the second dose was found more often than after the first dose (2.8 vs. 16.1 cases per million doses). Interestingly, among the two mRNA vaccines available in the US, the BNT162b2 vaccine has a lower rate of myocarditis/pericarditis than the mRNA-1273 vaccine (10.4 vs. 24.7 cases per million of the second doses).7
Two recent published case series reported successful conservative therapies in most patients.8,9 However, our patients symptomatically improved with systemic corticosteroids, which is similar to a case from Verma et al.10 An endomyocardial biopsy performed in Case 1 revealed lymphocytic-eosinophilic injury–mediated myocarditis, which is similar to myocarditis related to smallpox vaccination.11,12 Moreover, Verma et al recently reported pathological findings with fulminant myocarditis after mRNA-1273 vaccinations that showed inflammatory infiltrate admixed with macrophages, T cells, eosinophils, and B cells.10 These findings suggest that the primary mechanism of myocardial injury may be from a maladaptive immune response and could respond to immunosuppressive therapy.11 A recent study by Muthukumar et al reported that patients with myocarditis following COVID-19 mRNA vaccination had an increase in a specific subset of NK cells and several autoantibodies compared with controls.13 However, a cardiac biopsy from one patient reported by Larson et al showed no myocardial infiltration.14
This small case series is not conclusive that COVID-19 vaccination was the cause of myocarditis, as we could not completely exclude spontaneous myocarditis from other causes in these patients. The medical community continues to advocate for universal COVID-19 vaccination in society. Overall, further research and monitoring for adverse events will lead to best treatment practices related to COVID-19 vaccination.
Supplementary Material
References
- 1.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention . Possible side effects after getting a COVID-19 vaccine. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html. Accessed October 1, 2021.
- 4.US Centers for Disease Control and Prevention . COVID-19 VaST Work Group Report. May 17, 2021. https://www.cdc.gov/vaccines/acip/work-groups-vast/report-2021-05-17.html.
- 5.Caforio AL, Pankuweit S, Arbustini E, et al; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 6.Halsell JS, Riddle JR, Atwood JE, et al; Department of Defense Smallpox Vaccination Clinical Evaluation Team. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289(24):3283–3289. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 7.Vaccines and Related Biological Products Advisory Committee. Meeting Presentation , June 10, 2021.
- 8.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6(10):1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shay DK, Shimabukuro TT, DeStefano F.. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021; 6(10):1115–1117. doi: 10.1001/jamacardio.2021.2821. [DOI] [PubMed] [Google Scholar]
- 10.Verma AK, Lavine KJ, Lin C-Y.. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med. 2021;385(14):1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy JG, Wright RS, Bruce GK, et al. Eosinophilic-lymphocytic myocarditis after smallpox vaccination. Lancet. 2003;362(9393):1378–1380. doi: 10.1016/S0140-6736(03)14635-1. [DOI] [PubMed] [Google Scholar]
- 12.Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E.. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70(19):2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Muthukumar A, Narasimhan M, Li QZ, et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144(6):487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson KF, Ammirati E, Adler ED, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144(6):506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.