Abstract
Cardiac disease in pediatric patients due to coronavirus SARS-CoV-2 disease (COVID-19) includes myocarditis and multisystem inflammatory syndrome, both of which can present with a broad range in severity. Here we describe an infant with COVID-19 causing fulminant myocarditis with inotrope-resistant acute heart failure requiring extracorporeal membrane oxygenation. The patient demonstrated an atypical finding of localized septal thickening suggestive of hypertrophic cardiomyopathy, but the diagnosis of myocarditis was confirmed by cardiac MRI. Serial echocardiography illustrated complete resolution of septal hypertrophy and normalized cardiac function. The current report highlights the potential severity of COVID-19 associated myocarditis, the potential for recovery, and the utility of cardiac MRI in confirming the mechanism.
Abbreviations: BNP, brain natriuretic peptide; COVID-19, coronavirus SARS-CoV-2 disease; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MIS-C, multisystem inflammatory syndrome; MRI, magnetic resonance imaging; PCR, polymerase chain reaction
Keywords: COVID-19, Myocarditis, Magnetic resonance imaging, Extracorporeal membrane oxygenation
1. Introduction
Myocarditis can present with a wide range of severity, from a minimally elevated serum troponin level to fulminant presentations with severe myocardial dysfunction. In addition to myocarditis, COVID-19 can also cause myocardial dysfunction as part of a multisystem inflammatory syndrome (MIS-C). Myocarditis is largely diagnosed via magnetic resonance imaging (MRI), using the Lake Louise Criteria. Here we describe an infant presenting with fulminant myocarditis due to COVID-19 and how use of MRI aided in differentiation of severe septal thickening due to edema as opposed to a true cardiomyopathy.
2. Clinical report
A 10-month-old male with a past medical history of Trisomy 18p, monosomy of 8p, and a small conoventricular ventricular septal defect presented with a 4-day history of upper respiratory symptoms and fever that progressed to acute cardiopulmonary failure.
He was directly admitted from the emergency department to the Intensive Care Unit (ICU) on noninvasive positive pressure respiratory support. Limited cardiac evaluation on presentation revealed a low brain natriuretic peptide (BNP, 10 pg/mL). Following his admission to the ICU, he was intubated for worsening hypoxemia. A broad infectious work up was initiated, which revealed a positive coronavirus SARS-CoV-2 disease (COVID-19) PCR from a nasopharyngeal swab. He was treated with dexamethasone (0.1 mg/kg/day), remedesivir, intravenous immunoglobulin and broad-spectrum antibiotics (vancomycin and cefepime). He rapidly deteriorated and due to hemodynamic instability refractory to intravenous epinephrine and norepinephrine, he was cannulated onto veno-arterial extracorporeal membrane oxygenation (ECMO). An echocardiogram demonstrated globally severely diminished left ventricular systolic function, moderate mitral insufficiency, and normal left ventricular wall dimensions (Fig. 1B, Video 1). Subsequent additional workup demonstrated elevated cardiac troponin-I (peaked at 9.04 ng/mL) and repeat BNP of 1278 pg/mL. On the second day of his admission, he was transferred to our institution for further management, at which time he underwent enlargement of the patent foramen ovale by static balloon dilation for left atrial hypertension (38 mmHg).
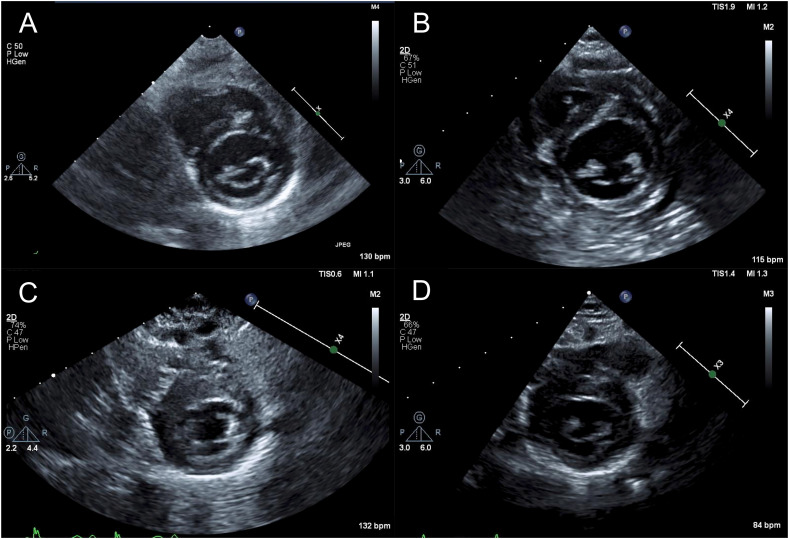
Fig. 1.
(A–D) Serial echocardiography imaging with septal size dimension and z-score in parentheses: A. Baseline echocardiogram performed at age 4 months for follow up of small conoventricular VSD (5.2 mm; z = 0.5). B. Echo performed on illness day 6 (6.5 mm; z = 1.5). C. Echo performed on day 23 of illness (11 mm, z = 7.5). D. Echo performed 75 days after onset of symptoms demonstrating complete resolution of septal thickening (4.7 mm; z = −1.0).
The patient exhibited rapid clinical improvement with recovery of left ventricular systolic function allowing ECMO decannulation to dopamine 3 μg/kg/min and milrinone 1 μg/kg/min on illness day 12 (ECMO day 8), weaning of all inotropic support on illness day 16, and extubation on illness day 19. Echocardiogram at that time revealed normal left ventricular systolic function (shortening fraction 33%) and asymmetric septal hypertrophy.
Due to the asymmetric septal hypertrophy, we considered a pre-existing diagnosis of hypertrophic cardiomyopathy; however, upon review of baseline echocardiograms performed at age 4 months for small conoventricular VSD there was no evidence of septal hypertrophy (Fig. 1A). Instead, we presumed a clinical diagnosis of COVID-19 induced fulminant myocarditis due to the timing of infectious symptoms with onset of myocardial dysfunction and subsequent recovery, the troponin leak, ST abnormalities on EKG, and evidence of inflammation (e.g. pericardial effusion and elevated C-reactive protein mg/dL). To further assess for evidence of myocarditis, a cardiac MRI was performed on day 23 of illness that was consistent with a diagnosis of acute myocarditis and confirmed asymmetric septal wall thickening (interventricular septum 12 mm, z = 6.2 and LV lateral wall 5.5 mm, z = 1.1, Fig. 2). The left ventricular systolic function was hyperdynamic with an ejection fraction measuring 81% (Video 2). T2 weighted imaging demonstrated significantly increased myocardial to skeletal muscle signal intensity (ratio = 2.0) consistent with myocardial edema (Supplemental Fig. 1). T2 mapping measured increased T2 relaxation times most prominently at the ventricular septum (Mid LV septal T2 = 61–63 ms). There was no evidence of discrete myocardial fibrosis with delayed gadolinium enhancement imaging and a mild elevation of the extracellular volume with T1 mapping (34%). Coronary arteries were not dilated. Overall, the findings were consistent with myocarditis. The patient demonstrated continued clinical recovery with resolution of pericardial effusion (day 19), improvement in BNP (187 pg/mL illness day 25), resolution of troponin leak (0.03 ng/mL illness day 45), and reduction in septal hypertrophy by echocardiography (Fig. 1D). He was discharged to home on illness day 47 on no cardiac medications. At follow up, 75 days after onset of illness, there was complete resolution of septal wall thickening with preserved systolic function (Fig. 1D).
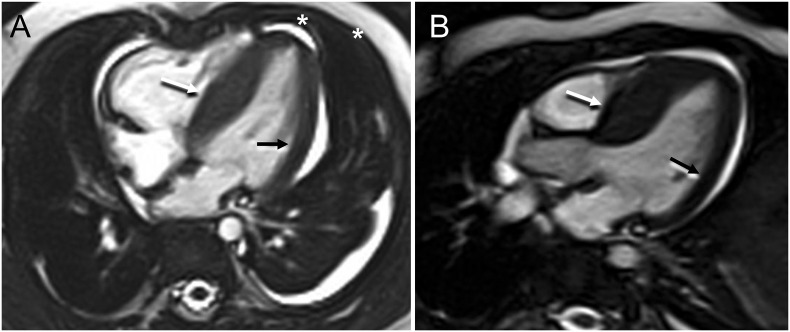
Fig. 2.
(A, B) Cardiac MRI performed after recovery of ventricular function, on day of illness 23, under general anesthesia. Steady state free precession imaging. Four chamber (A) and LVOT (B) views. The wall thickening was most prominent along the ventricular septum (1.2 cm, white arrows) with some extension to part of the superior wall, compared to the lateral wall which measures 0.5 cm (black arrows). On the four chamber view a small pericardial and left pleural effusion are observed (asterisks). LVOT = Left Ventricular Outflow Tract.
Supplemental Fig. 1.
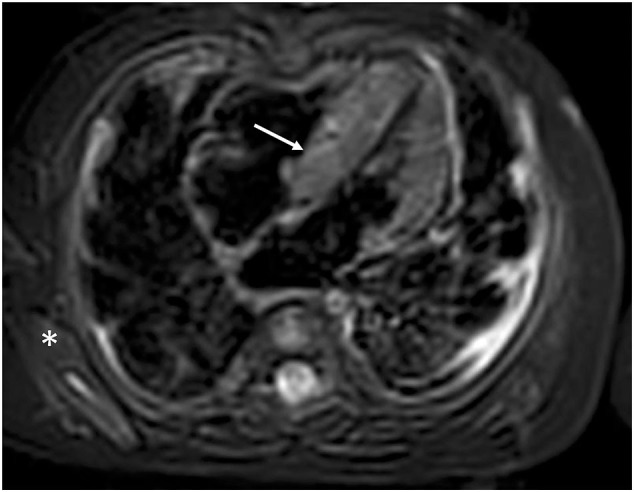
Four chamber view cardiac MRI. T2-weighted dark blood imaging to assess myocardial edema. There is significantly increased myocardial (arrow) to skeletal (*) muscle signal intensity ratio consistent with myocardial edema. T2 relaxation times were increased most prominently in the septum.
3. Discussion
To our knowledge, this is the first report of a patient with severe COVID-19 myocarditis and associated myocardial edema masking as asymmetric septal wall thickening. In addition, this case demonstrates in an infant the severe nature of COVID-19 myocarditis and the utility of cardiac MRI in defining the patient's diagnosis. Cardiac manifestations of COVID-19 virus in pediatric patients include MIS-C and myocarditis. MIS-C presents as a postinfectious immune mediated sequela of COVID-19 infection that can include myocardial dysfunction due to systemic cytokine release (e.g. IL-10 and TNF- α) and hyperinflammatory state [1]. Given the timing of cardiac decompensation in relation to onset of infectious symptoms, evidence of myocardial injury by troponin leak, and rapid decompensation followed by rapid recovery we considered a diagnosis of fulminant myocarditis. Cardiac MRI has been established as the principal noninvasive tool for the diagnosis of myocarditis, with myocardial edema by T2-mapping or T2-weighted imaging forming part of the Modified 2018 Lake Louise main criteria [2]. Our group has previously reported transient left ventricular edema diagnosed by T2-weighted imaging on cardiac MRI in an adolescent patient with influenza A myocarditis that was concerning for hypertrophic cardiomyopathy by echocardiography; however, in contrast to the current report, the pattern of myocardial edema and secondary left ventricular hypertrophy was symmetric in that patient [3]. Although COVID-19 infection rates have been lower for infants and children, preexisting cardiopulmonary comorbidities—similar as in adults—such as hemodynamically significant congenital heart disease, chronic lung disease, prematurity, and obesity are risk factors for requiring ICU admission and mortality in pediatric patients with COVID-19 infection [4]. Here we illustrate the utility of using cardiac MRI to diagnose COVID-19 myocarditis and to differentiate septal wall thickening due to myocardial edema from asymmetric septal hypertrophic cardiomyopathy, which significantly influenced this patient's management and long-term prognosis.
4. Conclusion
We report this case to highlight the potential severity of COVID-19 in an infant, requiring mechanical circulatory support, thus reinforcing the importance of preventative measures for the Pediatric population. Furthermore, this case highlights the utility of cardiac MRI in correctly diagnosing myocarditis associated with pediatric COVID-19 and its implications for treatment and prognosis.
The following are the supplementary data related to this article.
Four chamber view 2-D echocardiogram on illness day 6 demonstrating severely diminished left ventricular systolic function.
Four chamber MRI cine (B) on illness day 23 demonstrating hyperdynamic left ventricular systolic function and marked asymmetric septal wall thickening. The presence of wall thickening secondary to edema lags behind the LV dysfunction.
Ethics approval
This study was approved by the Institutional Review Board of the Children's Hospital of Philadelphia by waiver of consent for a single case report.
Declarations of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Institutes of Health [5T32HL007915, 2021].
References
- 1.Soma V.L., Shust G.F., Ratner A.J. Multisystem inflammatory syndrome in children. Curr Opin Pediatr. 2021;33(1):152–158. doi: 10.1097/MOP.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 3.Wittlieb-Weber C.A., Harris M.A., Rossano J.W. Transient left ventricular wall thickening in a 14-year-old girl with influenza a myocarditis. Cardiol Young. 2015;25:187–190. doi: 10.1017/S1047951114000018. [DOI] [PubMed] [Google Scholar]
- 4.Leeb R.T.P.S., Sliwa S., Kimball A., et al. COVID-19 trends among school-aged children — United States, March 1–September 19. MMWR Morb Mortal Wkly Rep. 2020;2020:1410–1415. doi: 10.15585/mmwr.mm6939e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four chamber view 2-D echocardiogram on illness day 6 demonstrating severely diminished left ventricular systolic function.
Four chamber MRI cine (B) on illness day 23 demonstrating hyperdynamic left ventricular systolic function and marked asymmetric septal wall thickening. The presence of wall thickening secondary to edema lags behind the LV dysfunction.