Abstract
Ca2+ sparks are the elementary Ca2+ release events in cardiomyocytes, altered properties of which lead to impaired Ca2+ handling and finally contribute to cardiac pathology under various diseases. Despite increasing use of machine-learning algorithms in deciphering the content of biological and medical data, Ca2+ spark images and data are yet to be deeply learnt and analyzed. In the present study, we developed a deep residual convolutional neural network method to detect Ca2+ sparks. Compared to traditional detection methods with arbitrarily defined thresholds to distinguish signals from noises, our new method detected more Ca2+ sparks with lower amplitudes but similar spatiotemporal distributions, thereby indicating that our new algorithm detected many very weak events that are usually omitted when using traditional detection methods. Furthermore, we proposed an event-based logistic regression and binary classification model to classify single cardiomyocytes using Ca2+ spark characteristics, which to date have generally been used only for simple statistical analyses and comparison between normal and diseased groups. Using this new detection algorithm and classification model, we succeeded in distinguishing wild type (WT) vs RyR2-R2474S± cardiomyocytes with 100% accuracy, and vehicle vs isoprenaline-insulted WT cardiomyocytes with 95.6% accuracy. The model can be extended to judge whether a small number of cardiomyocytes (and so the whole heart) are under a specific cardiac disease. Thus, this study provides a novel and powerful approach for the research and application of calcium signaling in cardiac diseases.
Keywords: Ca2+ sparks, deep learning, automated detection, classifying single cardiomyocyte, cardiac diseases
Introduction
Since the discovery of Ca2+ sparks in 1993, this elementary sarcoplasmic reticulum (SR) Ca2+ release event in cardiomyocytes has attracted enormous attention (Cheng et al., 1993; Cheng and Lederer, 2008). A typical Ca2+ spark has small spatial size (∼2 μm) and fast kinetics (∼20 ms) (Cheng and Lederer, 2008), and high-resolution line-scan imaging is required to accommodate such spatiotemporally defined fluorescent events as Ca2+ sparks, thereby resulting in noisy images (Cheng and Lederer, 2008). To date, several tools have been developed to detect Ca2+ sparks in line-scan Ca2+ images, most of which introduced an arbitrary threshold comprising the mean intensity μ and background standard deviation σ, usually μ+3.8σ, to distinguish the tiny signals from noises (Cheng et al., 1999; Wegner et al., 2006; Picht et al., 2007; Steele and Steele, 2014). A connected problem was that of “undetected sparks,” i.e., smaller fluorescence signals below the arbitrarily defined threshold (Cheng et al., 1999), which may lead to inaccurate estimates of spark probability, average spark flux, and other parameters, especially under some cardiac diseases for which the average amplitude of Ca2+ sparks in cardiomyocytes could be significantly reduced (Cheng and Lederer, 2008; Brochet et al., 2011; Shan et al., 2012; Santulli et al., 2015).
Excitation-contraction coupling (ECC) converts electrical stimuli to mechanical forces in cardiomyocytes. Impaired ECC are closed associated with cardiac pathology (Kansakar et al., 2021). ECC starts with the minimal influx of Ca2+ via the L-type Ca2+ channels, which triggers SR Ca2+ release by activating type 2 ryanodine receptor Ca2+ release channel (RyR2) (Cheng and Lederer, 2008; Kansakar et al., 2021). A number of reports have revealed altered SR Ca2+ handling in various cardiac diseases such as atrial fibrillation, heart failure (HF), and catecholaminergic polymorphic ventricular tachycardia (CPVT)—as the cause or consequence of cardiac dysfunction (Shan et al., 2012; Santulli et al., 2015; Xie et al., 2015; Zhang et al., 2021). As elementary SR Ca2+ release events in cardiomyocytes, Ca2+ sparks were widely used as readouts to distinguish normal and diseased cardiomyocytes (Shan et al., 2012; Santulli et al., 2015; Xie et al., 2015; Huang et al., 2021; Kansakar et al., 2021; Zhang et al., 2021). While most of those studies simply used the mean values of one or a few characteristics of Ca2+ sparks—including frequency, amplitude, and spatiotemporal parameters—for statistical significance comparison between normal and diseased groups, whereas attempts at deep digging and how to use these learnt data have rarely been reported.
With recent advances in machine learning and deep learning, a collection of algorithms with impressive abilities to decipher the content of images is now available to researchers in medical and biological fields (Esteva et al., 2017; Zhang et al., 2019). Machine learning builds a mathematical model based on structured training data to make predictions or decisions on new data. Deep learning is a subset of machine learning with supervised and unsupervised approaches. It gained attention when a deep-learning-based method won the 2012 ImageNet Large Scale Visual Recognition Challenge. Since then, there has been a major increase in the variety of problems that can be solved with deep learning. In addition, improvements in computer hardware and deep-learning frameworks have placed these tools within reach of the typical software developer, and they are becoming increasingly popular in bio/medical fields. Because deep-learning algorithms can distinguish objects with different characteristics, a deeper processing and analyzing Ca2+ spark images is expected.
In this study, we proposed a deep-learning framework for automated Ca2+ spark detection, analysis, and event-based classification of single cardiomyocytes, including the following. (1) To better characterize Ca2+ sparks in cardiomyocytes under diseased conditions, we developed a deep-learning method for automated spark detection that is free of arbitrarily defined thresholds and can detect a greater number of weak signals. (2) Based on the characteristics of Ca2+ sparks detected above, we proposed an event-based binary classification model to identify single cardiomyocytes into normal or diseased groups with promising accuracy.
Methods
Animal
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Five-month-old male mice harboring CPVT mutation RyR2-R2474S± (RS) (Shan et al., 2012; Xie et al., 2013) or wild-type littermates (WT) were anesthetized with an intraperitoneal injection of sodium pentobarbital (80 mg/kg) and then euthanized by cervical dislocation.
Data Acquisition
Adult murine cardiomyocytes were isolated and subjected to line-scan Ca2+ imaging as described previously (Xie et al., 2015; Huang et al., 2021; Zhang et al., 2021). For Ca2+ spark measurement, cardiomyocytes were preloaded with 5-μmol/L fluo-4 AM (Thermofisher) for 15 min and subjected to line-scan imaging at a speed of 400 lines/s on a Leica TCS SP8 confocal microscope with 40× magnification and a 1.3-NA oil immersion objective. The scan zoom was adjusted to fit the cells, and the scan line was along their long axis. The excitation for Fluo-4 was 488 nm, and emission was collected at 505–530 nm. Ca2+ spark detection and analysis were performed using either the traditional method (Cheng et al., 1999) or the algorithms described in the Results section.
Deep Learning Method Architecture
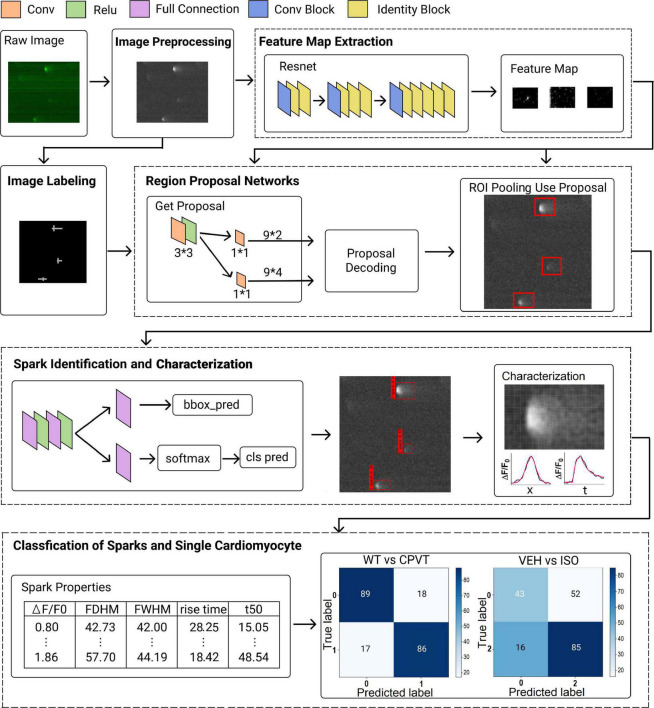
The deep learning method architecture is composed of several major processes, including image pre-processing and labeling, feature map extraction, region proposal network, spark classification and characterization, and classification of sparks and single cardiomyocytes (Figure 1). A detailed description of each process please refers to the Supplementary materials.
FIGURE 1.
Network architecture of proposed framework. The framework comprises several major processes, including image pre-processing and labeling, feature map extraction, region proposal network, spark identification and characterization, and classification of sparks and single cardiomyocytes.
Statistics
Data are reported as mean ± standard error. Student’s t-test (for two groups) or one-way ANOVA with Tukey’s multiple comparison test (for three or more groups) was carried out using GraphPad Prism (version 6.00, GraphPad Software, La Jolla, CA, United States). Differences were considered statistically significant at P < 0.05.
Results
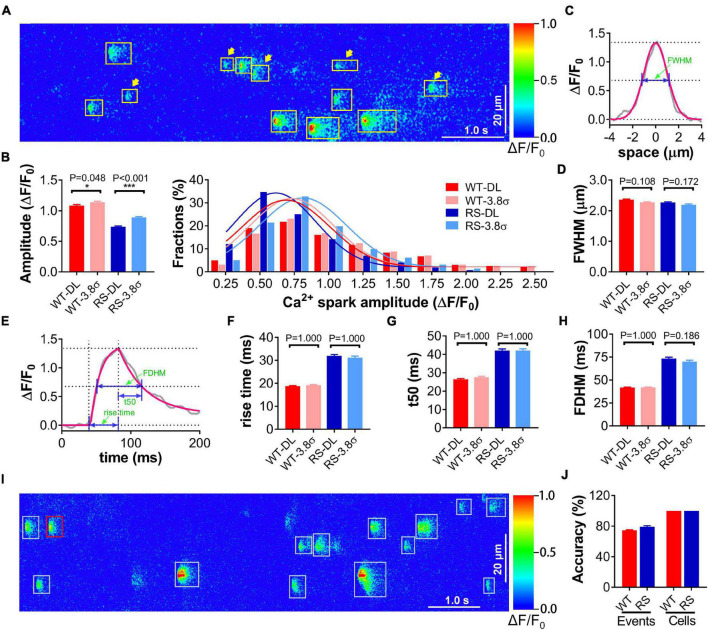
We performed the test for spark detection in cardiomyocytes from RS and WT mice using the proposed deep-learning method with the traditional method (Cheng et al., 1999) as the control. RyR2-R2474S is a typical human CPVT-linked RyR2 mutation. Previous studies have shown that cardiomyocytes from RS mice displayed obviously increased frequency and reduced amplitude of Ca2+ sparks compared to the WT group (Shan et al., 2012; Xie et al., 2013, 2015). The training data set included 3611 Ca2+ sparks from line-scan Ca2+ images of 100 WT and 100 RS cardiomyocytes. The test data set comprised line-scan Ca2+ images from 108 WT and 52 RS cardiomyocytes. Many signals with low peak F/F0 (<3.8σ) that were omitted by the traditional method were detected by the proposed deep-learning method, especially in RS cardiomyocytes (Figure 2A), resulting in a statistically lower amplitude of events (Figure 2B), while the spatiotemporal characteristics remained similar between both methods, indicating that these low-amplitude events were also Ca2+ sparks and not noises (Figures 2C–H).
FIGURE 2.
Application of proposed method for spark detection and event-based classification of single cardiomyocytes using Ca2+ spark characteristics. (A) An example of Ca2+ sparks in a line-scan image of an RS cardiomyocyte detected by deep-learning methods. All detected sparks are labeled with rectangles, and the arrowheads indicate low-amplitude events (peak F/F0 < μ + 3.8σ). (B) Statistics and distributions of Ca2+ spark amplitude in WT and RS cardiomyocytes using conventional (3.8σ) and deep learning (DL) method. (C) Raw (gray)/Gaussian fitting (red) spatial traces of a representative Ca2+ spark. FWHM is calculated from the fitting curve as illustrated. (D) Statistics of FWHM of Ca2+ sparks in each group. (E) Raw (black)/fitting (Lacampagne et al., 1999) (red) time courses of a representative Ca2+ spark. Rise time, t50, and FDHM are calculated from the fitting curve as illustrated. (F–H) Statistics of temporal parameters of Ca2+ sparks in each group. n = 1353 (DL)/1281 (3.8σ) or 1061 (DL)/807 (3.8σ) events in 108 WT or 52 RS cardiomyocytes. (I) An example of spark classification in a line-scan Ca2+ image of an RS cardiomyocyte and (J) statistics of event- and cell-classification accuracy in the independent test dataset comprising 46 WT and 30 RS cardiomyocytes, respectively. Each spark is first classified as being in the “WT” (red) or “RS” (cyan) group, then a cardiomyocyte with more (resp. fewer) “WT” events is classified as a “WT” (resp. “RS”) one.
Furthermore, we attempted to classify single cardiomyocytes as “WT” or “RS” using the logistic regression and classification model trained with the characteristics of Ca2+ sparks detected above. In the independent test dataset including 46 WT and 30 RS cardiomyocytes, 74.71% of the “WT” and 79.19% of the “RS” Ca2+ sparks were classified correctly (Figures 2I,J). The lowest event classification accuracy in single cardiomyocytes was 65.0 or 64.3% for the WT or RS group, respectively. Thus, according to the major event classification in each cardiomyocyte, the accuracy for cell classification into the WT and RS groups is 100% (Figures 2I,J).
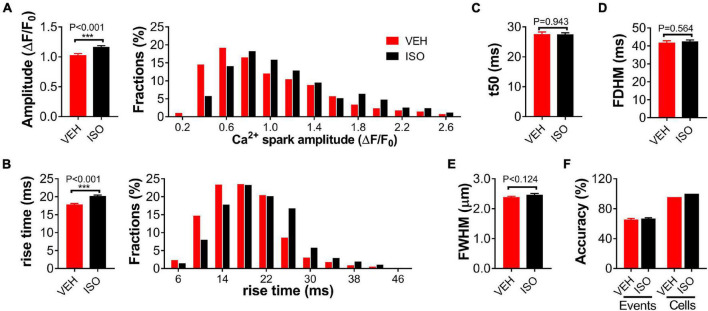
Compared to specific genetic mutation, overactivation of the sympatho-β-adrenergic receptors (β-ARs) system is a common and important mechanism of various cardiac diseases. In vitro application of isoprenaline is a typical model mimicking β-AR stimulation in cardiomyocytes. We also tested the effectiveness of the present method in classifying cardiomyocytes under 0.1-μmol/L isoprenaline (ISO) stimulation or vehicle (VEH) treatment. The amplitude and rise time of the detected Ca2+ sparks differed significantly between the ISO and VEH groups, while the other parameters were similar (Figures 3A–E). Our method achieved 65.51 and 66.81% accuracy for event classification, and 97.8 and 100% accuracy for cardiomyocyte classification, respectively (Figure 3F).
FIGURE 3.
Classification of single WT cardiomyocytes treated with isoprenaline or vehicle using Ca2+ spark characteristics. Statistics and distributions of amplitude (A) and rise time (B) of Ca2+ sparks in WT cardiomyocytes treated with 0.1-μmol/L isoprenaline (ISO) or vehicle (VEH). These parameters show obviously different distributions between the two groups. Statistics of t50 (C), FDHM (D), and FWHM (E) display no difference. n = 557 or 675 events from ≥20 cardiomyocytes per group. (F) Statistics of event and cell classification accuracy in the independent test dataset comprising 46 “VEH” and 20 “ISO” treated WT cardiomyocytes from at least five mice.
Discussion
As elementary SR Ca2+ release events in cardiomyocytes, Ca2+ sparks were widely used as readouts to distinguish normal and diseased cardiomyocytes (Shan et al., 2012; Santulli et al., 2015; Xie et al., 2015; Huang et al., 2021; Kansakar et al., 2021; Zhang et al., 2021). Despite various alterations of Ca2+ spark characteristics in diverse diseases, in most cases only simple statistical analyses and comparison have been performed for using Ca2+ sparks (e.g., as a readout for diastolic SR Ca2+ leakage), whereas attempts at deep digging and how to use these learnt data have rarely been reported. In the present study, we proposed an event-based logistic regression and binary classification model to classify single cardiomyocytes using Ca2+ spark characteristics. With deep learning, our classification model succeeded in distinguishing WT and CPVT-mutated cardiomyocytes with 100% accuracy, and it distinguished isoprenaline-insulted WT cardiomyocytes from vehicle ones with 95.6% accuracy. Chronic overactivation of the β-AR system is a common and important mechanism of various cardiac diseases (e.g., HF). Therefore, the proposed classification model should work well to identify whether a heart is under a specific disease or quantify the disease development using Ca2+ spark images of a small number of cardiomyocytes, through presetting the classification model with Ca2+ spark properties of cardiomyocytes under normal and the specific diseased conditions.
Because Ca2+ sparks are events with small spatial size and fast kinetics, traditional methods have usually introduced an arbitrarily defined threshold (μ+3.8σ) to exclude noises from the true tiny signals in the confocal line-scan Ca2+ images. However, because of the differences in opening times and channels of local Ca2+ release units, the SR Ca2+ content, and the distance between focal plane and release sites, the amplitudes of Ca2+ sparks vary widely. Thus, cutoff with a threshold has resulted in omission of weak but true Ca2+ sparks, while lowering the threshold can lead to false positive detection. The missed and false-positive events can distort the distribution of measured Ca2+ spark properties. Despite continuous progression in confocal microscopy, fluorescent indicators, and denoising algorithms in recent decades that have greatly improved the SNR of line-scan Ca2+ images, the detection problem awaits further resolution, especially under diseased conditions with many Ca2+ sparks in cardiomyocytes. The deep-learning method proposed in this study is free of any hard threshold for better detecting low-amplitude spark events and is shown to be more accurate for characterizing the difference of Ca2+ sparks between normal and diseased cardiomyocytes.
As how to detect the low-amplitude Ca2+ sparks remains to be solved for this fields, the labeling process of Ca2+ sparks in the training set is done manually with the assistance of a spark detection and characterization tool set to exclude false positive events, which could not guarantee that all low-amplitude events in the training images have been included and might lead to false negative detection. However, with these labeled low-amplitude events in the training set, our network can detect many more low-amplitude events in the test set, which is expected to improve further toward the final resolution of detecting low-amplitude Ca2+ sparks by self-adaption training procedure. Our training set included experimental line-scan Ca2+ spark images with a signal-to-noise ratio (SNR) of between ∼1.75 and ∼3.30, thereby allowing our model to detect spark events from images with SNR values in the same range. As our proposed approach is currently much slower than the traditional threshold-based algorithms, a further optimization in our algorithms will be required.
Taken together, the present study provides a novel and powerful approach for the research and application of calcium signaling in diseased hearts.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/BJUT-XJTU-DigitalCell/Software.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Author Contributions
SY and WX designed the research. SY, RL, JC, ZL, and WX performed the research. SY, RL, JC, and WX analyzed the data. SY, ZH, and WX wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by grants from the National Natural Science Foundation of China (11672226 and 31971045) and the Natural Science Basic Research Plan in Shaanxi Province of China (2020JM-001).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.770051/full#supplementary-material
References
- Brochet D. X., Xie W., Yang D., Cheng H., Lederer W. J. (2011). Quarky calcium release in the heart. Circ. Res. 108 210–218. 10.1161/circresaha.110.231258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J. (2008). Calcium sparks. Physiol. Rev. 88 1491–1545. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. (1993). Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262 740–744. 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Cheng H., Song L. S., Shirokova N., Gonzalez A., Lakatta E. G., Rios E., et al. (1999). Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J 76 606–617. 10.1016/S0006-3495(99)77229-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteva A., Kuprel B., Novoa R. A., Ko J., Swetter S. M., Blau H. M., et al. (2017). Dermatologist-level classification of skin cancer with deep neural networks. Nature 542 115–118. 10.1038/nature21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Lei C., Xie W., Yan L., Wang Y., Yuan S., et al. (2021). Oxidation of ryanodine receptors promotes Ca(2+) leakage and contributes to right ventricular dysfunction in pulmonary hypertension. Hypertension 77 59–71. 10.1161/HYPERTENSIONAHA.120.15561 [DOI] [PubMed] [Google Scholar]
- Kansakar U., Varzideh F., Jankauskas S. S., Gambardella J., Trimarco B., Santulli G. (2021). Advances in the understanding of excitation-contraction coupling: the pulsing quest for drugs against heart failure and arrhythmias. Eur. Heart J. Cardiovasc. Pharmacother. [Epub ahead of print]. 10.1093/ehjcvp/pvab069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A., Ward C. W., Klein M. G., Schneider M. F. (1999). Time course of individual Ca2+ sparks in frog skeletal muscle recorded at high time resolution. J. Gen. Physiol. 113 187–198. 10.1085/jgp.113.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht E., Zima A. V., Blatter L. A., Bers D. M. (2007). SparkMaster: automated calcium spark analysis with ImageJ. Am. J. Physiol. Cell Physiol. 293 C1073–C1081. 10.1152/ajpcell.00586.2006 [DOI] [PubMed] [Google Scholar]
- Santulli G., Xie W., Reiken S. R., Marks A. R. (2015). Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. U. S. A. 112 11389–11394. 10.1073/pnas.1513047112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J., Xie W., Betzenhauser M., Reiken S., Chen B. X., Wronska A., et al. (2012). Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 111 708–717. 10.1161/CIRCRESAHA.112.273342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele E. M., Steele D. S. (2014). Automated detection and analysis of Ca(2+) sparks in x-y image stacks using a thresholding algorithm implemented within the open-source image analysis platform ImageJ. Biophys. J. 106 566–576. 10.1016/j.bpj.2013.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner F. V., Both M., Fink R. H. (2006). Automated detection of elementary calcium release events using the a trous wavelet transform. Biophys. J. 90 2151–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Santulli G., Guo X., Gao M., Chen B. X., Marks A. R. (2013). Imaging atrial arrhythmic intracellular calcium in intact heart. J. Mol. Cell. Cardiol. 64 120–123. 10.1016/j.yjmcc.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Santulli G., Reiken S. R., Yuan Q., Osborne B. W., Chen B. X., et al. (2015). Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jiang L., Yang D., Yan J., Lu X. (2019). Urine sediment recognition method based on multi-view deep residual learning in microscopic image. J. Med. Syst. 43:325. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Qi Y., Li J. J., He W. J., Gao X. H., Zhang Y., et al. (2021). Stretch-induced sarcoplasmic reticulum calcium leak is causatively associated with atrial fibrillation in pressure-overloaded hearts. Cardiovasc. Res. 117 1091–1102. 10.1093/cvr/cvaa163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/BJUT-XJTU-DigitalCell/Software.