Abstract
Background
Various studies indicated blunted humoral responses to COVID-19 mRNA and viral vector vaccines among people with multiple sclerosis (pwMS) on sphingosine 1-phosphate receptor (S1PR) modulators and anti-CD20 therapies (aCD20); however, limited evidence was found regarding SARS-CoV-2 serology after inactivated virus vaccination.
Objective
To provide evidence regarding humoral response to COVID-19 inactivated virus vaccination among pwMS on disease-modifying therapies (DMTs).
Methods
A cohort study was carried out in Isfahan, Iran, enrolling DMT-exposed pwMS and unexposed (UX) healthy participants. Post-vaccination anti-SARS-CoV-2 Spike IgG serology testing was carried out among the participants and compared between participants based on their DMT exposure, using proper statistical tests. A multivariable logistic regression model was used to control for confounding. Association between the second vaccine dose-to-phlebotomy (vac2phleb) and the humoral response was investigated in each DMT-exposed cohort, using linear regression. Among the aCD20 cohort, the association of the last aCD20 infusion-to-first vaccine dose period with serostatus was investigated using an unpaired t-test.
Results
After enrolling 358 participants (144 pwMS and 214 healthy), blunted humoral responses were only observed in fingolimod (Log10 mean diff. [SE]: 0.72 [0.18], P = 0.001) and aCD20 (Log10 mean diff. [SE]: 0.75 [0.15], P < 0.001) cohorts compared to the UX cohort. Multivariable analysis confirmed the results. The study did not achieve enough statistical power to detect a significant association between the vac2phleb period and humoral responses. The last aCD20 infusion to first vaccination dose period was longer in the seroconverted pwMS on aCD20 (mean diff. [SE]: 8.43 weeks [2.57], P = 0.005).
Conclusion
The results of this study mirrored the results of previous studies among mRNA- or viral vector-vaccinated pwMS on DMTs. Therefore, it can be concluded that mode of action contributes less than timing, to the efficiency of vaccination strategies among pwMS on DMTs – especially the ones on S1PR modulators and aCD20. Meanwhile, the mentioned pwMS should be advised to receive early boosters and remain vigilant until further data becomes available and more efficient vaccination strategies are crafted.
Keywords: Multiple sclerosis, Disease-modifying therapies, COVID-19 vaccines, SARS-CoV-2, BBIBP-CorV
1. Introduction
People with multiple sclerosis (pwMS) are believed to be more susceptible to worse COVID-19 outcomes due to their disabilities and being on immunomodulatory disease-modifying therapies (DMTs) (Etemadifar et al., 2021). Therefore, prioritization of their COVID-19 vaccination was – and still is – among the adapted strategies in many regions (Zheng et al., 2020). Concerns are, however, raised again among the experts, with various studies reporting blunted humoral responses to mRNA and viral vector vaccines among pwMS treated with anti-CD20 therapies (aCD20) and sphingosine 1-phosphate receptor (S1PR) modulators (Achiron et al., 2021; Chilimuri et al., 2021; Drulovic et al., 2021; Moor et al., 2021; S et al., 2021; Stefanski et al., 2021; Sormani et al., 2021a; Tallantyre et al., 2021a; Apostolidis et al., 2021; Novak et al., 2021; Sabatino et al., 2021). Therefore, adopting strategies for the efficient administration of booster doses has gained more importance among these pwMS. Individualized selection of the booster type may be one of the most important contributors to the efficacy of the adopted strategies, especially among the ones on S1PR modulators who seem to show inconsistent post-infection and post-vaccination humoral responses (Rommer et al., 2021).
The BBIBP-CorV COVID-19 vaccine (Sinopharm, China) uses the whole viral particles of the 19nCoV-CDC-Tan-HB02 strain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in addition to aluminum hydroxide adjuvant, and therefore, presents a wide range of immunogens to the immune system. Therefore, it might be a worthy opponent for mRNA- and viral vector-based vaccines in eliciting humoral responses among pwMS on DMTs, specifically those on aCD20 therapies and S1PR modulators. The present study aimed to provide evidence regarding the responses to COVID-19 inactivated virus vaccination among pwMS on DMTs.
2. Methods
2.1. Design, setting, and participants
This cohort study was designed and conducted in Isfahan, Iran, from August until October 2021. The inclusion criteria for the exposed cohort were predefined as 1) definitive diagnosis of MS based on the revised McDonalds criteria (Thompson et al., 2018) at least one year before the study; and 2) receiving a DMT. The inclusion criteria for the unexposed (UX) cohort were defined as 1) having no history of immunosuppression and 2) absence of any special condition. The common exclusion criteria for all participants were predefined as 1) any history of COVID-19, 2) being under the age of 18 years old, 3) failure of receiving full vaccination based on the regimen proposed by the manufacturer, and 4) receiving any other COVID-19 vaccine than the BBIBP-CorV. Based on the measures calculated by previous studies on mRNA vaccines, aiming for 80% power and a 0.05 alpha error, and considering the six available DMT groups (interferons [IFN], glatiramer acetate [GA], dimethyl fumarate [DMF], teriflunomide [TFN], fingolimod [FNG], and aCD20) we initially aimed to include at least 27 participants per each DMT group; however, no specific limit was set for the sample size. The study was discontinued early after an interim analysis indicating adequate statistical power regarding the primary aim.
2.2. Identification and eligibility
The possibly eligible participants for the exposed cohort were identified from their documentations in three private neurologist offices in Isfahan, Iran. The UX cohort was identified from the referrals to an accredited laboratory (Nobel lab, Isfahan, Iran) for elective post-vaccination screening. After identification, the possibly-eligible pwMS for the exposed cohort were interviewed via telephone calls by an assistant to ensure eligibility, and if considered eligible and provided consent, were considered for phlebotomy and inclusion in the study. After being identified through their documentations in the laboratory, an assistant called the possibly eligible participants for the UX cohort, who underwent phlebotomy for post-vaccination screening within a week, to further confirm their eligibility and obtain their consent for usage of their samples and anonymized data in the study.
2.3. Study procedures
To avoid referring immunocompromised pwMS to the laboratory, an experienced mobile phlebotomist was sent to obtain and bring back samples from the participants of the DMT-exposed cohort at their own houses. The phlebotomist was further instructed to follow strict preventive protocols and to obtain the samples as quickly as possible, to ensure the least direct contact with the immunocompromised pwMS. As mentioned, the participants of the UX cohort underwent phlebotomy at the laboratory before being included in the study. All samples included five milliliters of blood, which were used to prepare sera and stored at −20 °C until further analyses were run.
2.4. Variables and measurements
The study variables and their measurements are interpretable from table 1 . The outcome of the study was defined as the quantitative humoral response to COVID-19 vaccination. Data were either obtained from registered records of participants or via subsequent telephone interviews by an assistant.
Table 1.
Baseline characteristics of participants.
| UX (n = 214) | IFN (n = 28) | GA (n = 15) | DMF (n = 27) | TFN (n = 21) | FNG (n = 22) | aCD20 (n = 29) | P | |
|---|---|---|---|---|---|---|---|---|
| Mean age (SD) [years] | 41.71 (17.41) | 45.00 (11.23) | 37.6 (8.03) | 34.93 (10.91) | 45.29 (9.73) | 35.18 (7.66) | 42.72 (10.17) | 0.044 |
| Sex (female:male) | 67:147 | 24:4 | 13:2 | 22:5 | 17:4 | 16:6 | 21:8 | <0.001 |
| Median MS duration (Range) [years] | NA | 14.5 (26) | 4 (13) | 5 (15) | 7 (24) | 10 (20) | 13 (23) | <0.001 |
| MS type (R:P) | NA | 26:2 | 15:0 | 25:2 | 18:3 | 18:4 | 19:10 | 0.014 |
| Median EDSS (IQR) | NA | 1 (0.5) | 1 (0.5) | 1 (0.5) | 1.5 (1) | 1.5 (1.5) | 2.5 (1.25) | <0.001 |
| Mean duration from second dose to phlebotomy*(SD) [days] | NA | 56.20 (27.76) | 79.15 (39.31) | 58.52 (31.42) | 52.26 (29.29) | 57.28 (33.22) | 61.67 (27.35) | 0.306 |
number of missing values with respect to DMT: IFN, 13; GA, 2; DMF, 6; TFN, 7; FNG, 4; aCD20, 11.
Abbreviations: UX, unexposed; IFN, interferons; GA, glatiramer acetate; DMF, dimethyl fumarate; TFN, teriflunomide; FNG, fingolimod; aCD20, anti-CD20 therapies; SD, standard deviation; MS, multiple sclerosis; NA, not applicable; R, relapsing; P, progressive; EDSS, expanded disability status scale; IQR, interquartile range;.
2.5. Assessment of humoral responses
An anti-Spike IgG enzyme-linked immunosorbent assay (ELISA) kit (Quanti SARS-CoV-2 Anti-Spike IgG, Pishtazteb Diagnostics, Iran) was used to quantify the post-vaccination humoral responses among the participants. The mentioned kit has a reported sensitivity and specificity of 98.16% and 99.01%, respectively, and an approved accuracy (Pishtazteb, 2021). The testing was carried out per manufacturer's instructions (Pishtazteb, 2021) three times for each specimen, and the mean results were reported quantitatively in relative units (RU)/ml with a cut-off index (COI) value equal and above eight considered positive.
2.6. Bias
Three levels of potential bias were addressed in the protocol of this study: 1) information, 2) selection, and 3) confounding. The efforts of minimizing the mentioned biases included double-confirmation of the measurements by an assistant, triple-performing the humoral response testing on each specimen, screening of the whole registries of three different private neurologist offices for eligible participants by two independent assistants, and identifying, controlling, and accounting for the possible confounders in the analysis. All data were anonymized after collection.
2.7. Statistical methods
The distribution of the outcome measures was normalized by Log10-transformation of the results (Pishtazteb, 2021; Resman Rus et al., 2021). Analysis of variance (ANOVA) and Dunnett T3 post-hoc tests were carried out for the inter-group comparisons of outcome measures and for the comparison of each group with the UX cohort, respectively. Further inter-group comparisons were carried out using appropriate parametric and non-parametric tests. Outcome measures were then dichotomized based on the previously-mentioned COI and were used for a multivariable logistic regression model controlling for age, sex, expanded disability status scale (EDSS) score, MS duration, and second dose to phlebotomy days, as possible confounders. A linear regression model was used among each DMT-exposed cohort to investigate the effect of each DMT on the humoral response wear-off pace in subsequent days post-vaccination. Among the participants on aCD20, the number of weeks from the last aCD20 infusion to the first vaccination dose was compared between the participants with and without seroconversion, using an unpaired samples t-test. Missing values were imputed using an automatic multiple imputation model (10 imputations), to maximize the usage from the data. Sensitivity analysis was carried out on the subgroup of participants with no missing data to validate the imputations. Statistical significance was predefined as a two-tailed P value of 0.05 and less. The statistical analyses were carried out using the SPSS 23 (IBM Inc.) and Prism 9 (GraphPad Software LLC.) software for macOS.
2.8. Registration and ethical considerations
The protocol of this study was approved by the Isfahan University of Medical Sciences internal research and ethical boards. Verbal and written consent were obtained from the pwMS included in this study. Only verbal consent was obtainable from the participants of the UX cohort due to the remote nature of the study. No un-anonymized data was stored in any form by the investigators, to ensure privacy protection of the participants.
3. Results
3.1. Baseline characteristics of participants
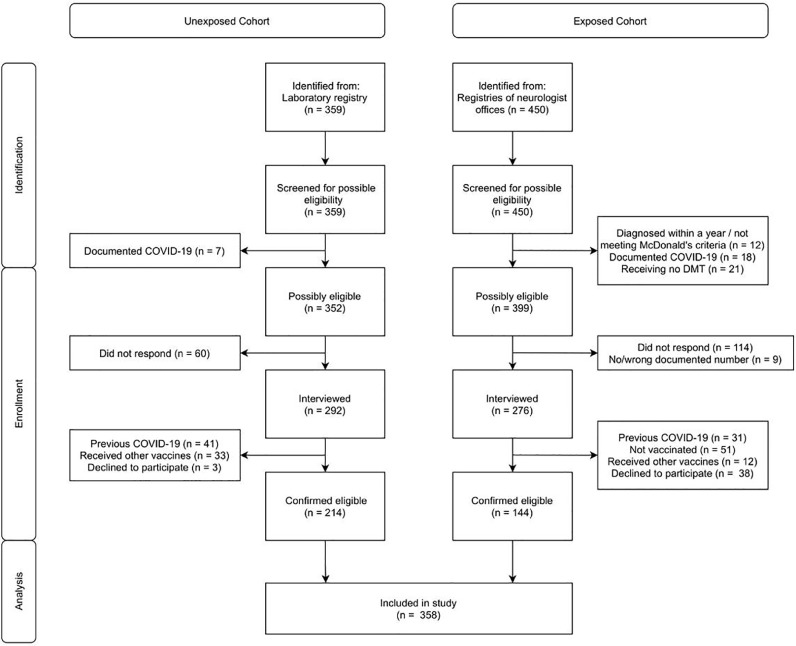
As interpreted from the flow diagram of the study (Fig. 1 ), a total of 358 participants (144 exposed and 214 unexposed) were included in the study. The detailed baseline characteristics of the participants with respect to their DMT exposure can be interpreted from Table 1. The cohorts differed significantly regarding age (F : 2.19, P = 0.04, Table 1), and as expected, the UX cohort consisted of significantly fewer females (, P < 0.001). The aCD20 cohort consisted of 4 participants receiving ocrelizumab and the rest receiving rituximab. The different DMT-exposed cohorts differed significantly regarding MS duration (Kruskal Wallis , P < 0.001), MS type (Pearson , P = 0.01) and EDSS (Kruskal Wallis , P < 0.001) and insignificantly regarding second dose-to-phlebotomy duration (F: 1.22, P = 0.31). Additionally, two pwMS on natalizumab – a male and a female both with positive post-vaccination serostatus – electively underwent phlebotomy but were not included in the analysis.
Fig. 1.
Study flow diagram.
3.2. Results of analyses
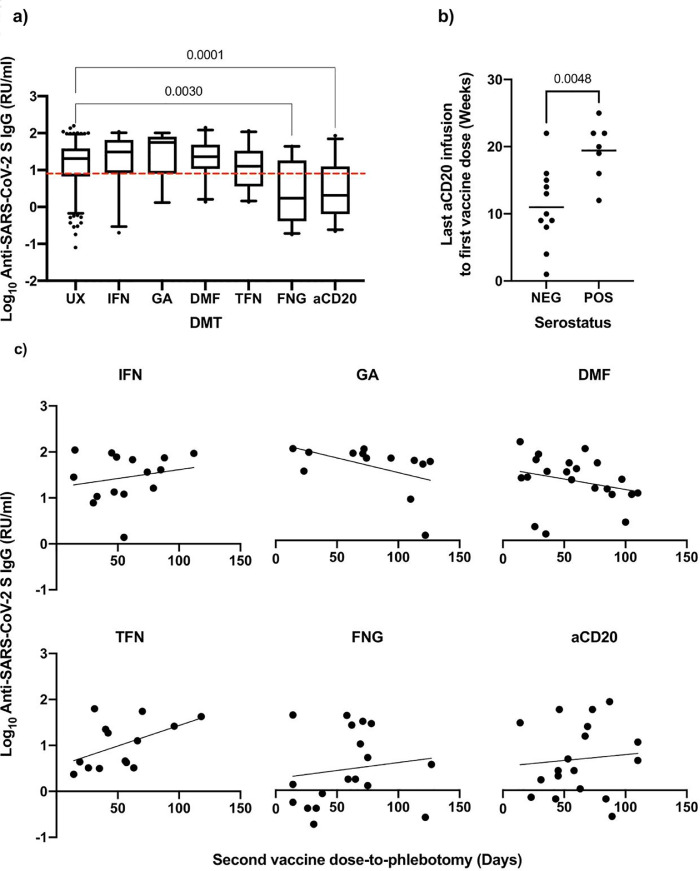
The FNG (Log10 mean diff. [SE]: 0.72 [0.18], P = 0.001) and aCD20 (Log10 mean diff. [SE]: 0.75 [0.15], P < 0.001) cohorts had a significantly lower post-vaccination humoral response than the UX cohort (Fig. 2 a). The IFN cohort showed the least difference with the UX regarding the humoral responses (Log10 mean diff. [SE]: 0.06 [0.14], P = 0.99) (Fig. 2a). The results were maintained after accounting for age, sex, presence of comorbidities, MS duration, MS type, EDSS, and second dose-to-phlebotomy duration in the multivariable logistic regression model (Table 2 ). Linear regression showed no significant association between second dose-to-phlebotomy and Log10 humoral response in any DMT-exposed cohorts (results not shown). Sensitivity analysis by excluding cases with missing values – which only pertained to the second dose-to-phlebotomy variable – did not show any shifts in the results, although positive and negative trends were observed respectively for the TFN and GA cohorts, regarding the association between humoral response and second dose-to-phlebotomy duration (Fig. 2c). These trends should be interpreted with caution as they did not reach statistical significance and may have resulted from variable phlebotomy timings across the cohorts. Among the aCD20 cohort, the last aCD20 infusion-to-first dose period was longer in the seroconverted pwMS (mean diff. [SE]: 8.43 weeks [2.57], P = 0.005) (Fig. 2b).
Fig. 2.
Summary of results. a) Distribution of humoral responses with respect to DMT exposure; The box and whiskers correspond to the 5–95 percentile (line at median) and the dashed line shows the cut-off index for seropositivity; Significant (<0.05) P values pertaining to the pairwise comparisons with the UX cohort are displayed above the plots; b) Distribution of last aCD20 infusion to first vaccine dose duration with respect to the serostatus of pwMS on aCD20; P value of unpaired t-test is displayed above the plots; c) Distribution of humoral responses with respect to second vaccine dose to phlebotomy duration after exclusion of missing values; The regression line is displayed. Abbreviations: RU/ml, relative units per milliliter; UX, unexposed; IFN, interferons; GA. Glatiramer acetate; DMF, dimethyl fumarate; TFN, teriflunomide; FNG, fingolimod; aCD20, anti-CD20 therapies; NEG, negative; POS, positive.
Table 2.
Results of multivariable logistic regression; Significant (<0.05) P values are bolded.
| Predictor | Multivariable logistic regression (n = 142, outcome: seroconversion) | ||
|---|---|---|---|
| B | SE | P | |
| Age (per year) | −0.017 | 0.023 | 0.467 |
| Sex | |||
| - Female | (ref) | ||
| - Male | −0.363 | 0.515 | 0.481 |
| Comorbidities | |||
| - Not present | (ref) | ||
| - Present | 0.475 | 0.506 | 0.348 |
| MS duration (per year) | −0.037 | 0.039 | 0.344 |
| EDSS (per one increase) | −0.048 | 0.329 | 0.885 |
| Second dose-to-phlebotomy duration (per day) | 0.004 | 0.008 | 0.580 |
| MS type | |||
| - relapsing | (ref) | ||
| - progressive | −0.794 | 0.747 | 0.288 |
| DMT | |||
| - IFN | (ref) | ||
| - GA | −0.688 | 0.850 | 0.418 |
| - DMF | 0.614 | 0.824 | 0.456 |
| - TFN | −0.917 | 0.678 | 0.176 |
| - FNG | −1.859 | 0.719 | 0.010 |
| - aCD20 | −1.941 | 0.685 | 0.005 |
Abbreviations: B, unstandardized beta coefficient; SE, standard error; ref, reference; MS, multiple sclerosis; EDSS, expanded disability status scale; DMT, disease-modifying therapy; IFN, interferons; GA, glatiramer acetate; DMF, dimethyl fumarate; TFN, teriflunomide; FNG, fingolimod; aCD20, anti-CD20 therapies.
4. Discussion
In line with the studies regarding the mRNA- and viral vector-based vaccines, the present study showed a blunted humoral response among the pwMS treated with FNG and aCD20 after vaccination with the BBIBP-CorV inactivated virus vaccine. Further measurements e.g., pre-vaccination anti-SARS-CoV-2 N IgG serology, serology after the first dose, cellular responses,%CD19 and%CD20 at the time of vaccination, and anti-SARS-CoV-2 S1 receptor-binding domain (RBD) IgG serology could have contributed to the precision of this study. Other limitations included retrospective enrollment of participants and collection of baseline data, uncontrolled timing of phlebotomy, and the overall remote nature.
The blunted humoral response of pwMS on aCD20 has been well-documented, both after infection with and vaccination against SARS-CoV-2 (Sormani et al., 2021a; Sormani et al., 2021b). Hence, observation of a blunted humoral response was anticipated in these pwMS. Meanwhile, various studies indicated that these pwMS show an adequate cellular response to vaccination (Apostolidis et al., 2021; Madelon et al., 2021; Moor et al., 2021; Sabatino et al., 2021); however, it is unclear whether this could be translated into clinical protectiveness(Evangelou et al., 2021)(Ghadiri et al., 2021). Further studies assessing comparative incidence and severity of COVID-19 among pwMS on aCD20 can provide more clarification in this regard. Furthermore, many studies – including the present study – indicate that delaying aCD20 doses can reverse the humoral blunting in the pwMS receiving them (Tallantyre et al., 2021b; Sormani et al., 2021a; Achiron et al., 2021); however, the exact duration of the required delay is not clear, not to mention that it will cost putting pwMS at risk of possible flares. Temporary or permanent switching to other DMTs and early booster administrations may be reasonable strategies to encounter humoral bluntings, all of which require more data to be evaluated. The present study indicated that the timing outweighs the mode of action, in developing further vaccination strategies for pwMS on aCD20.
As reported by previous studies, the pwMS on S1PR modulators seem to show adequate humoral responses to COVID-19 contraction (Sormani et al., 2021b), which was not the case with mRNA- and viral vector-based vaccines (Sormani et al., 2021a; Tallantyre et al., 2021b) – an interesting dilemma highlighted by Rommer et al. (Rommer et al., 2021). Rommer et al. continued to argue that the blunted immunization observed in people on S1PR modulators, may only be the case for the mRNA-based vaccines (Rommer et al., 2021). Based on their proposed theory, the BBIBP-CorV inactivated virus vaccine, which presents a broader range of antigens to the immune system may better mimic the inflammatory responses to the actual infection, and therefore, should be able to elicit a “complex and diverse” immune response, break through the S1PR modulation, and enhance the seroconversion in pwMS on these therapies. This theory was not supported by the evidence presented in the current study. Hence, the explanation for the mentioned inconsistency remains to be investigated in future studies. Furthermore, unlike the pwMS on aCD20, a recent study showed that cellular responses are also blunted among pwMS on S1PR modulators receiving mRNA vaccines (Sabatino et al., 2021). While future studies provide further data on cellular responses of these pwMS to other vaccine types, and as delaying/switching DMTs can prove more challenging in pwMS receiving S1PR modulators – due to the well-documented probability of rebound disease activity (Barry et al., 2019) – remaining vigilant and receiving early boosters seem to be the most convenient ways of preventing COVID-19 and its unfavorable outcomes in these pwMS.
Some data have highlighted drops in efficacy after approximately six months post-vaccination with COVID-19 vaccines (Chemaitelly et al., 2021; Levin et al., 2021). In pwMS receiving DMTs, this gradual wear-off may theoretically have a higher pace, necessitating earlier booster administration than the general population. The current study lacked enough control and power to accurately estimate of the comparative wear-off pace among these pwMS. Future studies are warranted to provide more clarification in this regard.
5. Conclusion
The current study on humoral responses after inactivated virus vaccination among pwMS on DMTs mirrored the results of previous studies, indicating that the vaccine mode of action does not significantly affect the patterns of humoral bluntings among these pwMS. Therefore, regardless of the type of vaccine they received, people on DMTs, especially on aCD20 and S1PR modulators, should be advised to remain vigilant and receive their booster doses as soon as possible with any available booster type, as the choice between receiving the inactivated virus, viral vector, or mRNA boosters does not seem to bear as much importance as timing. Their neurologists are advised to provide innovative consultations based on the current knowledge (e.g., delaying doses or switching DMTs) to benefit as much as possible from the boosters and prevent the unfavorable outcomes of COVID-19, while minimizing the risk of disease flares.
Declaration of Competing Interest
None.
References
- Achiron A., Mandel M., Dreyer-ALSTER S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021:1–12. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY B., ERWIN A.A., STEVENS J., TORNATORE C. Fingolimod rebound: a review of the clinical experience and management considerations. Neurol. Ther. 2019;8:241–250. doi: 10.1007/s40120-019-00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaitelly H., Tang P., Hasan M.R., Almukdad S., Yassine H.M., Benslimane F.M., Al Khatib H.A., Coyle P., Ayoub H.H., Al Kanaani Z., Al Kuwari E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul Rahim H.F., Nasrallah G.K., Al Kuwari M.G., Al Romaihi H.E., Butt A.A., AL-Thani M.H., Al Khal A., Bertollini R., Abu-Raddad L.J. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. New Eng. J. Med. 2021 doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilimuri S., Mantri N., Gongati S., Zahid M., Sun H. COVID-19 vaccine failure in a patient with multiple sclerosis on ocrelizumab. Vaccines (Basel) 2021:9. doi: 10.3390/vaccines9030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drulovic J., Ivanovic J., Martinovic V., Tamas O., Veselinovic N., Cujic D., Gnjatovic M., Mesaros S., Pekmezovic T. Humoral response to SARS-CoV-2 AND COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M, Nouri H, Maracy MR, Akhavan Sigari A, Salari M, Blanco Y, Sepúlveda M, Zabalza A, Mahdavi S, Baratian M, Sedaghat N. Risk factors of severe COVID-19 in people with multiple sclerosis : A systematic review and meta-analysis. Rev Neurol. 2021 doi: 10.1016/j.neurol.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelou Nikos, Garjani Afagh, Patel Sameer, Bharkhada Dhiren, Rashid Waqar, Coles Alasdair, Law Graham. Impact of mass vaccination on SARS-CoV-2 infections among the total multiple sclerosis population receiving immunomodulatory disease-modifying therapies in England. PREPRINT (Version 1) available at Research Square. 2021 doi: 10.21203/rs.3.rs-1016584/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadiri Fereshteh, Sahraian Mohammad Ali, Azimi Amirreza, Naser Moghadasi Abdorreza. The study of COVID-19 infection following vaccination in patients with multiple sclerosis. Multiple Sclerosis and Related Disorders. 2021 doi: 10.1016/j.msard.2021.103363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C., Freedman L., Kreiss Y., Regev-Yochay G. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. New Eng. J. Med. 2021 doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelon, N., Lauper, K., Breville, G., Royo, I.S., Goldstein, R., Andrey, D.O., Grifoni, A., Sette, A., Siegrist, C.-.A., Finckh, A., Lalive, P.H., Didierlaurent, A.M. & Eberhardt, C.S. 2021. Patients treated with anti-CD20 therapy can mount robust T cell responses to mRNA-based COVID-19 vaccines. medRxiv, 2021.07.21.21260928.
- Moor, M.B., Suter-Riniker, F., Horn, M.P., Aeberli, D., Amsler, J., Möller, B., Njue, L.M., Medri, C., Angelillo-Scherrer, A., Borradori, L., Radonjic-Hoesli, S., Jafari, M.S., Chan, A., Hoepner, R., Bacher, V.U., Mani, L.-.Y., Iype, J.M., Hirzel, C., Maurer, B. & Sidler, D. 2021. Humoral and cellular responses to mRNA vaccines against SARS-CoV2 in patients with a history of CD20-B-cell depleting therapy. medRxiv, 2021.07.04.21259848. [DOI] [PMC free article] [PubMed]
- Novak F., Nilsson A.C., Nielsen C., Holm D.K., Østergaard K., Bystrup A., Byg K.E., Johansen I.S., Mittl K., Rowles W., Mcpolin K., Spencer C., Sagan S., Gerungan C., Wilson M.R., Zamvil S.S., Bove R., Sabatino J.J., Sejbaek T. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2021;56 doi: 10.1016/j.msard.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISHTAZTEB 2021. Quanti SARS-CoV-2 Anti-Spike IgG ELISA Assay Product Catalog; available in Persian from https://pishtazteb.com/wp-content/uploads/2021/04/SARS-Cov-2-Spike-Ab.pdf. Pishtazteb Diagnostics.
- Resman Rus K., Korva M., Knap N., Avšič Županc T., Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021;139 doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommer P.S., Bsteh G., Berger T., Zettl U.K. SARS-CoV-2 antibodies in multiple sclerosis patients depending on the vaccine mode of action? Multiple Sclerosis J. 2021 doi: 10.1177/13524585211039128. 13524585211039128. [DOI] [PubMed] [Google Scholar]
- S G., S L., C Z., A N., M F., L M. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J. Neurol. 2021:1–5. doi: 10.1007/s00415-021-10663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino, J.J., Mittl, K., Rowles, W., Mcpolin, K., Rajan, J.V., Zamecnik, C.R., Dandekar, R., Alvarenga, B.D., Loudermilk, R.P., Gerungan, C., Spencer, C.M., Sagan, S.A., Augusto, D.G., Alexander, J., Hollenbach, J.A., Wilson, M.R., Zamvil, S.S. & Bove, R. 2021. Impact of multiple sclerosis disease-modifying therapies on SARS-CoV-2 vaccine-induced antibody and T cell immunity. medRxiv : the preprint server for health sciences, 2021.09.10.21262933.
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., da Rin G., Serrati C., Gandoglia I., Tassinari T. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Landi D., Carmisciano L., de Rossi N., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: an international cohort study. Multiple Sclerosis J. 2021 doi: 10.1177/13524585211035318. 13524585211035318. [DOI] [PubMed] [Google Scholar]
- Stefanski, A.-.L., Rincon-Arevalo, H., Schrezenmeier, E., Karberg, K., Szelinski, F., Ritter, J., Jahrsdörfer, B., Schrezenmeier, H., Ludwig, C., Sattler, A., Kotsch, K., Chen, Y., Claussnitze, A., Haibel, H., Proft, F., Guerra, G.M., Durek, P., Heinrich, F., Gomes, M.F., Burmester, G.R., Radbruch, A., Mashreghi, M.-.F., Lino, A.C. & Dörner, T. 2021. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. medRxiv, 2021.07.19.21260803.
- Tallantyre, E.C., Vickaryous, N., Anderson, V., Asardag, A.N., BAKER, D., Bestwick, J., Bramhall, K., Chance, R., Evangelou, N. & George, K. 2021a. COVID-19 vaccine response in people with multiple sclerosis. medRxiv. [DOI] [PMC free article] [PubMed]
- Tallantyre, E.C., Vickaryous, N., Anderson, V., Asardag, A.N., Baker, D., Bestwick, J., Bramhall, K., Chance, R., Evangelou, N., George, K., Giovannoni, G., Grant, L., Harding, K.E., Hibbert, A., Ingram, G., Jones, M., Kang, A.S., Loveless, S., Moat, S.J., Robertson, N.P., Schmierer, K., Shah, S.N., Simmons, J., Upcott, M., Willis, M., Jolles, S. & Dobson, R. 2021b. COVID-19 vaccine response in people with multiple sclerosis. medRxiv, 2021.07.31.21261326.
- Thompson A.J., BANWELL B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Zheng C., Kar I., Chen C.K., Sau C., Woodson S., Serra A., Abboud H. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34:879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]