Abstract
Polypyridyl coordinating ligands are common in metal complexes used in medicinal inorganic chemistry. These ligands possess intrinsic cytotoxicity, but detailed data on this phenomenon is sparse, and cytotoxicity values vary widely and are often irreproducible. In order to provide new insights into the biological effects of bipyridyl type ligands and structurally related metal-binding systems, reports of free ligand cytotoxicity were reviewed. The cytotoxicity of 25 derivatives of 2,2′-bipyridine and 1,10-phenanthroline demonstrates that there is no correlation between IC50 values and ligand properties such as pKa, log D, polarizability volume, and electron density, as indicated by NMR shifts. As a result of these observations, as well as the various reported mechanisms of action of polypyridyl ligands, we offer the hypothesis that biological effects are governed by the availability of and affinity for specific metal ions within the experimental model.
Keywords: Ligand; polypyridyl; 1,10-phenanthroline; copper; iron; ruthenium; chemotherapy; natural products
Graphical Abstract

Introduction
Despite the contribution of several drugs which have become essential components of the modern pharmacopeia, and numerous advances in the understanding of inorganic chemical biology, medicinal inorganic chemistry is still considered a nascent discipline. This is due in part to the relatively qualitative nature of the field, which lacks a set of rules (such as Lipinski’s Rules[1]) or structure-activity relationships (SAR) that define the biological activities of inorganic agents. The absence of a common set of features that control the behavior of inorganic systems is a result of the nature of bonding in metal compounds, which exhibit a broad spectrum of interactions. These range from effectively covalent to functionally ionic bonds; strong, weak, and fluctional associations; and bonding interactions through multiple orbitals and angles, resulting in systems with different stoichiometries, geometries, and reactivities. The versatility and plasticity of metal interactions with ligands is showcased by the chemistry and biological activity of cisplatin, which is entirely reliant on ligand exchange for its mechanism of action[2].
Polypyridyl ligands are extensively utilized in medicinal inorganic chemistry, as many physiochemical, photochemical, and biological properties can be altered by modulation of the core structure. However, it is challenging to parse the dividing lines between the biological effects of metals vs. their coordinating groups, and recent publications have renewed attention to this topic[3, 4]. The central question captured our attention: is the cytotoxic activity of certain polypyridyl coordination complexes a result of the intact complex, the metal bound to – and released from – the ligands, or a property of the free ligand?
Our interest was motivated by our use of polypyridyl ligands to construct photocytotoxic ruthenium complexes [5–12]. In an efforts to understand the biological effects of free ligands, we have uncovered information that is important but may have been overlooked. Evidence of the cytotoxicity of specific ligands exists in literature dating back to at least the 1950s, but there has not been a comprehensive attempt to explain the activity of free ligands, to develop SAR, or to account for biological and physicochemical parameters that may influence experiments. We compared the cytotoxicity of 25 derivatives of 2,2′-bypyridine (bpy) and 1,10-phenanthroline (phen) (Figure 1), striving for rigor and reproducibility, yet found that experimental values vary extensively. Our results provide trends in activity and highlight the challenges associated with these biological studies; we also offer plausible explanations for the remarkable variability in experimental results.
Figure 1. A) Timeline of studies on cytotoxic effects of bidentate polypyridyl ligands. B) and C) Bidentate free ligands discussed in this study.A).
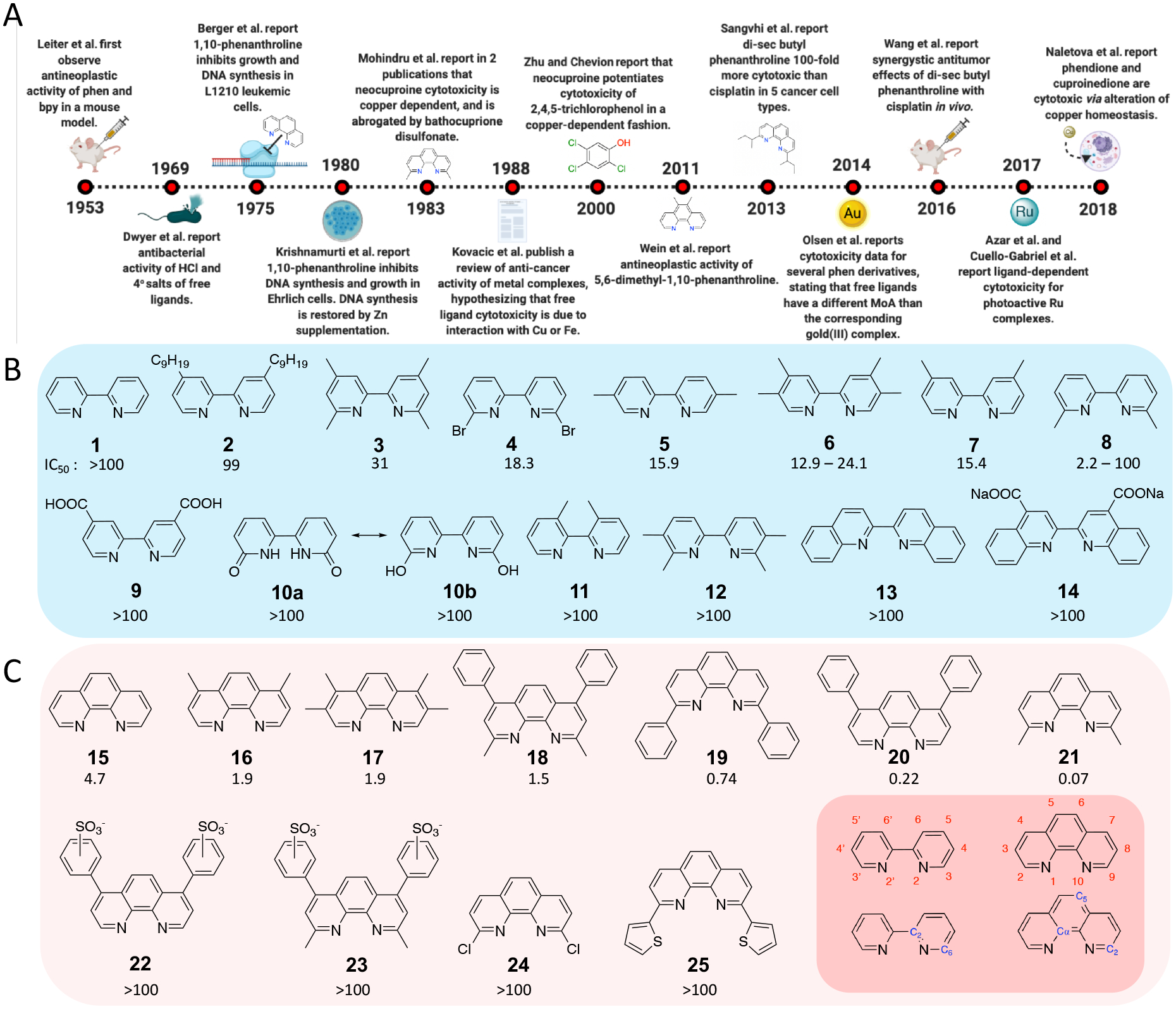
2,2′-bipyridine (1) and derivative compounds 2–14 (blue). C) 1,10-phenanthroline (15) and derivative compounds 16–25 (red). IC50 values (μM) in HL60 cells are included below compound numbers. The numbering schemes for 2,2′-bipyridine and 1,10-phenanthroline (red labels) and 13C-NMR (blue labels) are included (bottom-right inlay).
Biological investigations of polypyridyl ligands
The realization that bpy and phen derivatives (Figure 1) exhibit antineoplastic activity was a coincidental discovery as a side-effect of solving the structure of the gout treatment, colchicine. Prior to Dewar’s structure containing 7-membered rings (26)[13], the accepted model was that of Windaus (27)[14], who contended that colchicine contained a phenanthrene core (Chart 2). Leiter et al. [15] examined the in vivo antineoplastic potential of derivatives of the Windaus structure, the phenanthrenes and phenanthrolines, as well as derivatives of the Dewar structure, the tropolones. Histological examination of murine tumors demonstrated that cytotoxicity was induced by compounds including bpy and phen[16]. Bipyridine and phenanthroline salts where later shown to exhibit bacteriostatic activity[17] by Frances Dwyer. In general, alkyl substituents increased the activity of the ligands, as did metal coordination[18]. The most potent phen complexes were those derived from the labile metal ions (Cd(II), Cu(II), Zn(II) and Mn(II)), and potency correlated with lipophilicity of the ligands[19], suggesting that the activity was a function of the free ligand. Thus, the pre-formed metal complexes might alter cellular uptake, but subsequent ligand exchange and reactions with endogenous intracellular metals likely drove the biological activity.
Mechanistic studies [20, 21] demonstrated that phen inhibited DNA synthesis in cells at concentrations as low as 5 μM; the effect was reversed by addition of Ni(II), Fe(II), Zn(II), Cu(II), or Co(II), following an order that correlated to the stability constant of the 1:1 metal-phen complex[20]. The exception in the trend was Cu(II), and formation of cytotoxic copper species[20] was proposed as the cause of this anomaly. As zinc is critical for DNA synthesis[22], intracellular zinc depletion was presumed to be involved. Cell cycle analysis also revealed that phen prevented entry into S and G2 phases [23], and the effects were reversed by addition of Zn(II), Cu(II), or Fe(II). Surprisingly, cell cycle inhibition occurred in four cell lines, though toxicity was only reported in one, highlighting the variability of cellular sensitivities to the ligand.
Ortho-substituted ligands were first reported by Mohindru et al.[29] who demonstrated that neocuproine (21) was cytotoxic in a manner dependent on Cu(II), but not Zn(II) or Fe(II). This finding contrasted with phen, which became non-cytotoxic upon addition of Cu, Fe, or Zn. Moreover, cellular uptake of neocuproine increased up to 200-fold in the presence of added Cu(II). The ionophoric ability of neocuproine was identified as essential for its activity, contrasting with phen, which was believed to induce cellular metal depletion. As stated succinctly in the conclusion, “the addition of the 2- and 9-methyl groups converts a ligand which inhibits as a chelator to one which inhibits as a copper chelate.”[29]
This finding was complemented by studies on bathocuproine disulfonate (23), which protects the cell from neocuproine by competing for and preventing the uptake of copper[29]. This report stimulated the use of bathocuproine disulfonate as a copper chelator [30], but the activity of neocuproine may have been overlooked, as its experimental continued, with some assuming it was an inert metal binder[31–34]. The implications of unintended ligand-based biological activity were anticipated in an early review that discussed the interactions of the ligands with either copper or iron (rather than zinc)[35], and the ramifications are illustrated by a study showing the copper-dependent antibacterial activity of the pollutant 2,4,5-trichlorophenol was potentiated approximately 100-fold by neocuproine[36]. The large difference in lipophilicity between the neocuproine-Cu(I) and Cu(II) complexes suggested that the ligand acts as an ionophore, facilitating cellular Cu(I) uptake across the cell membrane, resulting in a 5-fold increase in intracellular copper levels.[36]
This uptake process is a result of the fact that Cu(I) is a d10 ion, preferring to adopt the tetrahedral conformation, while Cu(II) is a d9 ion, with an energetic benefit to forming square-planar, trigonal bipyramidal, or distorted octahedral complexes[37]. Intracellular oxidation of Cu(I) neocuproine complexes facilitates elimination of neocuproine and formation of Cu(II) ions, implying that ortho-substituted bidentate ligands act as ionophores, resulting in a catalytic mechanism of metal transport. Several excellent contemporary reviews of Cu(I)/(II) chelators and ionophores as therapeutics were recently published that provide a broad overview of this area[37–43].
The biological activity of a number of polypyridyl ligands were elucidated by Eichler, extending the correlation between ortho-substitution and activity. Free ligands were generally more potent than their Au(III) complexes[44], and in some cases, ligand activity was mechanistically distinct from the corresponding Au(III) complex. The type of substituent was impactful; for example, while 5,6-dimethyl-1,10-phenanthroline was cytotoxic in the low micromolar range [45], 2,9-disecbutyl-1,10-phenanthroline (SBP, 31; also termed dsBPT)[46], was far more potent, with IC50 values in the nanomolar range.
Moreover, in vivo biological data on SBP [47, 48] was promising; treatment reduced murine glioma tumor area by an average of approximately 88%, and increased survival. SBP consistently exhibited IC50 values 20 – 100 fold more potent than cisplatin[46–48], and synergized with cisplatin in vivo and increased apoptosis in cancer lines. Moreover, SBP was nontoxic to noncancerous cell lines and exhibited negligible toxicity to healthy murine ex vivo tissues. Upregulation of apoptosis effectors DR5 and caspase-8, G1-phase cell cycle arrest, and the recruitment of LC3, an autophagy regulatory protein, were observed[48]. P53 upregulation did not occur in all cell lines, indicating differential signaling according to cell type. While SBP is capable of binding Cu, Fe, and Zn, Cu seems the most likely partner.
Ortho substitution alters the stability of Cu(I) complex ions; the aqueous stability constant, log β2 = 15.82 for 1,10-phenanthroline, while log β2 = 19.1 for neocuproine[49]. Higher alkyl substitution at the 2,9 positions likely follows this trend, but may not exhibit a linear correlation with the degree of coordination-induced steric clash. Indeed, mono- vs. disubstituted ligands, and ligands with variable steric bulk near the coordinating nitrogens differ in cytotoxicity by at most 7-fold. Ligand rigidity also matters; 6-sec-butyl-2,2’-bipyridine was less toxic than the analogous 2-substituted 1,10-phenanthroline (> 12 μM vs. 2.92 μM) [4]. Energetic barriers to metal binding may play a role; the copper complex [Cu(8)Cl2] was active in the low micromolar range, while the free ligand 8 had only minor effects on cell growth, and in situ coordination did not result in the same activity as the preformed complex. This is consistent with the frustratingly inconsistent results for 8 in our own studies.
Metal binding in situ is also important for sterically unencumbered ligands such as 35 and APTO-253 (36), which are cytotoxic and downregulate c-Myc[28, 50, 51]. Though iron coordination was not discussed for 35, the structural and mechanistic similarities to APTO-253 suggest the compound forms an active tris-homoleptic iron species. Fe(APTO-253)3 (37) binds to G-quadruplex DNA, causing c-Myc downregulation; it also causes DNA damage[51], likely via the Fenton reaction and production of intracellular hydroxyl radicals[52]. As pre-formed 37 is 1.5-fold less cytotoxic than uncoordinated 36, the complex likely forms intracellularly[51], possibly causing additional effects though Fe depletion. This conjecture is supported by studies on curcumin, which is cytotoxic and known to deplete Fe in a mouse model[53]. Finally, another bipyridyl type ligand, dppz (dipyrido[3,2-a:2′3′-c]phenazine) was recently shown to impair replication fork progression, phosphorylation of Chk1, stalling in G1 phase, and cytotoxicity (IC50 values of 1.9 - > 10 μM, depending on cell line and time point). The effect was distinct from that of its Ru(II) complex, 53, which was generally non-toxic[54]. Again, this supports an important role for binding endogenous metals.
Natural products containing polypyridyl chelates
Several classes of biologically active natural products contain the bipyridyl structure, including the caerulomycins [55, 56], the collismycins [57, 58], and the cyanogrisides [59]. These compounds exhibit antineoplastic activity against several cancer types, and possess other biological effects such as neuroprotection, that may be related to their metal-binding capabilities. Additional naturally occurring chelators have been extensively studied are eilatin [60], streptonigrin [61–65], and lavendamycin[66–68]. As these ligands contain substituents at the ortho-positions, they may exhibit a mechanistic connection to synthetic ligands with similar structures.
Photoactive coordination complexes containing ortho-substituted polypyridyl ligands
In the past decade, we developed a research program utilizing the intrinsic steric clash provided by ortho-substituted bipyridyl ligands to create photocytotoxic ruthenium complexes. The first system reported, [Ru(bpy)2dmbpy]2+ (38), was inert in the dark and highly active upon photochemical release of the ligand (8), with IC50 values of 1.1–1.6 μM[6]. Compound 38 damaged DNA[6] and RNA[7] in vitro, and produced DNA damage responses in cells in a mode similar to cisplatin[69]. Further studies in E. coli depicted a distinctive cytological profile induced by metalating agents. Taken together, this evidence was interpreted to indicate that the metal center generated in the photochemical reaction, nominally [Ru(bpy)2(H2O)2]2+, is the active species.
However, in 2017, the Bonnet group reported that 38 was cytotoxic as a result of the intracellular photorelease of ligand 8, rather than [Ru(bpy)2(H2O)2]2+[70]. In addition, they found that 45, which contained the inactive ligand, 2-methylthiomethylpyridine, and should also produce [Ru(bpy)2(H2O)2]2+, was not cytotoxic. In contrast, 40, which released the same leaving ligand but a different Ru(II) photoproduct, was a light activated cytotoxin. The conclusion was that either the metal photoproduct or the ligand could be cytotoxic, depending on the system. In the same year, the Khnayzer group reported that ligand 21 was more cytotoxic than its corresponding photoactivated Ru(II) complex, 46 [71]. In another study, Ru(II) complex 47 was highly potent upon irradiation[72], but in this case, the system ejected a nontoxic bpy ligand. Thus, the photochemical reaction ostensibly producing an activated [Ru(bpy)(19)(H2O)2]2+ complex that induced cytotoxicity with low micromolar IC50 values.
Following these studies, we attempted to establish a trend for the biological effects of free ligands, but results were deceptive and inconsistent. While 38 possessed constant photocytotoxicity over the years[6, 69, 70], the free ligand 8 was nontoxic at concentrations up to 100 μM in some experiments, while in others it exhibited single micromolar IC50 values. Our review of the literature revealed that the cytotoxicity of the same ligand varies widely between laboratories, cell lines, and experimental replicates. Even 2,2’-bipyridine (1), which is generally inactive, was moderately cytotoxic in the MDA-MB-231 cell line[72], while it was inactive in four other cell lines in the same study.
Several studies suggested the metal center was the active agent. Some Ru(II) complexes with the same “scaffold” (with the same coligands) but different ortho-substituted bpy ligands (3, 8, and 12) possessed the same range of activity upon irradiation[6, 10, 69] while the free ligands exhibited highly variable activity (Figure 1). Alternatively, different scaffolds that contain the same leaving ligand exhibited different potencies; for example, with [Ru(dmphen)2] (39),[73–75] [Ru(bathophen)2] (40–42)[70],[76] or [Ru(dop)2] (43, 44) scaffolds[77, 78] (where bathophen = 4,7-diphenyl-1,10-phenantroline and dop = 2,3-dihydro-[1,4]dioxino[2,3-f][1,10]phenanthroline), which possess higher light mediated potency than analogous complexes with the [Ru(bpy)2] 2+ scaffold. However, the dark cytotoxicity as well as the photocytotoxicity varied drastically for the same Ru(II) scaffolds, depending on the characteristics of both the co-ligands and the photolabile ligand. For example, the [Ru(bathophen)2] scaffold exhibited very different properties for compounds 40, bearing the neutral 2-methylthiomethylpyridine, compared to 41, with a negatively charged ejecting ligand, and 42, with a leaving ligand that could be doubly deprotonated. Compound 40 was toxic in the dark with low micromolar potency, giving a photocytotoxicity index (PI) of 5.5[70], while 41 was 10-fold less toxic under dark conditions and exhibited the same photocytotoxicity[76], and 42 possessed drastically reduced potency in both dark and light (IC50 > 40 μM; IC50 = 17.3 μM)[79]. These complexes release inactive ligands and form the same inorganic product.
Differences in the light mediated potency have also been observed for [Ru(dop)2] complexes 43 and 44[78]. These results highlight the impact of the leaving ligands on the photocytotoxicity of complexes with the same Ru(II) scaffold, but not in a manner that is associated with ligand activity. Rather, in addition to the co-ligands, the leaving ligands designate the charge, hydrophobicity and photoreactivity of the “prodrug” complexes, resulting in different solubility, cellular uptake, subcellular localization, and cytotoxicity before irradiation. Therefore, the disparity in the photocytotoxicity of [Ru(bpy)2]2+ scaffolds can be partly explained by the diversity of the physiochemical features of the prodrug complexes.
Further complicating the matter is the different photophysical and photochemical processes that may occur. The McFarland group extended the principle of controlling prodrug characteristics through ligand design to modulated the photophysics and photochemistry of Ru(II) photoejecting complexes containing ligand 8. In complexes 48–50, 1–3 thiophene units were appended to the third ligand, providing an alternative intraligand (IL) excited state, which could sensitize singlet oxygen (1O2). These “dual action” agents were photocatalysts for 1O2 or could release ligand 8[80]. The switching between excited states also produced environmentally responsive complexes that were active under hypoxia (1% O2)[12]. Photoejection half-life values are not predictive of phototoxicity[12, 77, 78], undercutting the expectation that the toxicity stems from either the free ligand or from the reactive metal center produced. Small structural changes can alter the photochemical efficiency, environmental sensitivity, and photochemical mechanism [81–83]. The behavior of the excited states of Ru(II) compounds are complex and variable, and it appears likely that excited state processes have as large an impact on cellular responses as the ultimate chemical products.
Finally, light-mediated reactions involving Cu and poypyridyl ligands could occur either unintentionally, under room lights, or as a result of photochemical experiments performed in cells or media. Ortho- substituted phen complexes of Cu exhibit radically improved luminescent lifetimes and quantum yields[84]. The Khnayzer group leveraged this feature to develop Cu(I) complexes containing ligands similar to SBP (31) as potential agents for photodynamic therapy[85]. Thus, sterically congested ligands released from light-activated Ru(II) complexes, could plausibly form Cu(I) complexes in situ that act as photosensitizers. A recent report also presented photocleavage of DNA by Cu complexes with uncongested phen ligands containing fluorine[86].
Physicochemical properties of ligands do not correlate to cytotoxicity
Several physicochemical parameters were compared with the IC50 values of the ligands, including pKa, log D (pH 7.4), polarizability volume, and the 13C-NMR chemical shift of bpy carbons C2 and C6, and phen carbons Cα, C2, and C5. No correlations were found with any individual parameter, suggesting the physical organic chemistry of these ligands is not a critical factor. This supports the hypothesis of metal-dependent activity, where the affinity for and interactions with metals within the biological system dictates the cytotoxicity of free ligands. Imbalances in metal homeostasis can lead to cell death[27] [39] through various processes (Figure 2) [3, 27, 51]; this warrants tightly controlled biological studies of ligand systems, and also requires an understanding of mechanistic features of cellular responses to both copper- and iron-binding agents. There are excellent reviews addressing the role of copper [87] [39] and iron [88, 89] in healthy physiology and in disease states, along with possible avenues for treatments. Briefly, Cu and Fe can both impact the proteasome, produce imbalances in redox homeostasis, create ROS, and trigger apoptosis, paraptosis, ferroptosis, and autophagy, as shown in Figure 2.
Figure 2. Proposed model of ligand induced cell death mechanisms.

Ortho-substituted ligands induce cell death. An apoptotic mechanism (top right), driven by ROS production and DNA damage, may compete simultaneously or in a [Cu] dependent manner with a paraptotic mechanism (top left), driven by the unfolded protein response, endoplasmic reticulum stress, and caspase inhibition. A metal-depletion-type mechanism (bottom) is hypothesized for unsubstituted systems.
Possible mechanisms of action of free ligands and explanations for low reproducibility
Free Cu is present at low micromolar concentrations in cellular assay media, and the concentration is highly variable in the formulations of leading suppliers (2.43 μM with 212% coefficient of variation) [91]. Many experiments also rely on supplemental fetal bovine serum (FBS). FBS composition varies[92], but reports show concentrations of 2 μM Cu, and it is another uncontrolled source of trace metals in cell culture experiments. Batch-to-batch discrepancies in media metal concentrations occur within the range of cytotoxicity, and can be significantly higher. Thus, the introduction of a ligand can readily overwhelm metal homeostasis in cells (Figure 3)[91]. Cytotoxicity may occur with the same ligand displaying variable IC50 values (Figure 3A), as levels of Cu in different batches of reagents vary (Figure 3B). Moreover, changes in caspase-3 expression and autophagy regulators can be observed in cells treated with 1 μM copper. Conflicting information exists as to the downstream mechanism of cell death [3], [27], [39], potentially reflecting competition between mechanisms. It is possible that the concentration of intracellular copper dictates how the cell is killed, which frustrates mechanistic studies.
Figure 3. The effect of trace copper contaminants on ligand cytotoxicity.

A. Cu homeostasis is strictly controlled to maintain cell health. Homeostasis is perturbed in the presence of a ligand, and cells experience the cytotoxic effect of Cu overload. B. Cytotoxicity values for 8 from 22 independent experiments collected in our laboratory over two months. Previously published A549 data are included as red triangles.[70, 90] C. In the absence of ligand, Cu levels in different batches of reagents vary but remain below the cytotoxic threshold. In the presence of an ionophoric ligand, different batches of media or FBS elicit cytotoxicity proportional to the metal concentration.
Addition causes for low reproducibility include ligand solubility, or the presence of different crystalline polymorphs. We found the starting stock concentrations, as well as the protocol for generating dilution series, impacted the observed activity of the ligands. While cytotoxicity does not correlate with pKa, the interplay between solubility, pKa, and metal affinity may still be a source of variability. Biological variables further confound understanding the biological activity of ligands, as different cell lines have different sensitivities to metals. This was illustrated by the variable HMOX upregulation observed between cell lines upon treatment with pyrazole-pyridine type Cu binding agents[27]. Alternatively, different uptake and efflux mechanisms may play a role. Despite its likely impact on cytotoxicity, the cellular uptake of free ligands is underexplored. Diffusion seems to be the most widely accepted model of transport in the absence of a metal, but active transport may occur upon complex formation. Finally, a recent analysis of [Cu(phen)2]2+ derivatives over time demonstrated that speciation can be expected to play an important role in biological activity[93].
Conclusions
Having reviewed studies on the cytotoxicity of polypyridyl ligands and the metal complexes that release them, we have come to the happy conclusion that practitioners in the field who have advanced different views on the identity of the active species have all been simultaneously right (or, alternatively, equally wrong). The apparent answer to the question, “is the cytotoxicity of polypyridyl-metal complexes the result of the ligand or the metal?” is that cytotoxicity can be the result of both, though in some cases, it may involve transient, high energy species and mechanisms. Unanticipated chemistry within the biological milieu can easily confound the interpretation of results, as the activity of uncoordinated ligands is highly system dependent, with regards to the metals present and the sensitivity of the biological system to shifts in metal homeostasis. Development of controlled delivery systems, such as liposomes or nanoparticles, will likely benefit the preclinical and clinical development of metal complexes as drugs, as shown in a recent study that demonstrated in vivo efficacy of Cu(phen) complexes encapsulated in nanoliposomes [94]. It will also be critical to develop a physical and systems-biology-based understanding of the dynamics of complex ions within multicomponent systems where many entities compete in binding both the ligand and the metal.
Chart 1. Structures of various compounds investigated with different mechanisms of action.

Colchicine (26) and Windaus’ Colchicine (27); Terpyridine (28), is cytotoxic with an IC50 of 2 μM[24]; Phendione (29) and Cuproinedione (30), which mediate Cu import, and perturb the GSH redox buffer[3]; 2,9-disecbutyl-1,10-phenanthroline (SBP) (31); Cu-pyrrolidine dithiocarbamate (32) and Hinokitiol (33) are ionophores that interfere with the ubiquitin-proteasome system[25, 26]; A Cu complex that activates the paraptotic pathway (34) [27]; c-Myc modulating compounds 35[28], APTO-253 (36), and Fe(APTO-253)3 (37). Ruthenium complexes: [Ru(bpy)2dmbpy]2+ (38); [Ru(dmphen)2]2+ scaffold (39); the third ligand can be any bidentate system; complexes 40–47; [Ru(dmbpy)2]2+ scaffold containing a 1H-imidazo[4,5-f][1,10]phenanthroline ligand appended to various conjugated groups (48–52); [Ru(phen)2dppz]2+ (53). The coordinating atoms of key ligands are highlighted by color.
Acknowledgements:
We gratefully acknowledge the National Institutes of Health (Grant GM107586) for the support of this work.
References:
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Lipinski CA, Lead- and drug-like compounds: the rule-of-five revolution, Drug Discov Today Technol 1 (2004) 337–41. [DOI] [PubMed] [Google Scholar]
- 2.Hoeschele JD, In remembrance of Barnett Rosenberg, Dalton Transactions (2009) 10648–50. [DOI] [PubMed] [Google Scholar]
- **3.Naletova IS C; Curci A; Margiotta N; Natile G; Arena G; La Mendola D; Nicoletti VG; Rizzarelli E, Cytotoxic phenanthroline derivatives alter metallostasis and redox homeostasis in neuroblastoma cells, Oncotarget 9 (2018) 36289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a detailed biological study of the mechanisms of actions of phendione and cuproinedione, and the metal-signaling systems these compounds perturb.
- 4.Angel NR, Khatib RM, Jenkins J, Smith M, Rubalcava JM, Le BK, et al. , Copper (II) complexes possessing alkyl-substituted polypyridyl ligands: Structural characterization and in vitro antitumor activity, J Inorg Biochem 166 (2017) 12–25. [DOI] [PubMed] [Google Scholar]
- 5.Wachter E, Heidary DK, Howerton BS, Parkin S, Glazer EC, Light-activated ruthenium complexes photobind DNA and are cytotoxic in the photodynamic therapy window, ChemComm 48 (2012) 9649–51. [DOI] [PubMed] [Google Scholar]
- **6.Howerton BS, Heidary DK, Glazer EC, Strained ruthenium complexes are potent light-activated anticancer agents, J Am Chem Soc 134 (2012) 8324–7. [DOI] [PubMed] [Google Scholar]; This is the first report using steric effects to activate ligand photoejection for biological applications.
- 7.Heidary DK, Glazer EC, A light-activated metal complex targets both DNA and RNA in a fluorescent in vitro transcription and translation assay, Chembiochem 15 (2014) 507–11. [DOI] [PubMed] [Google Scholar]
- 8.Hidayatullah AN, Wachter E, Heidary DK, Parkin S, Glazer EC, Photoactive Ru(II) complexes with dioxinophenanthroline ligands are potent cytotoxic agents, Inorg Chem 53 (2014) 10030–2. [DOI] [PubMed] [Google Scholar]
- 9.Havrylyuk D, Heidary DK, Nease L, Parkin S, Glazer EC, Photochemical Properties and Structure–Activity Relationships of RuII Complexes with Pyridylbenzazole Ligands as Promising Anticancer Agents, European Journal of Inorganic Chemistry 2017 (2017) 1687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler L, Nease L, Vo P, Garofolo J, Heidary DK, Thummel RP, et al. , Photochemical and Photobiological Activity of Ru(II) Homoleptic and Heteroleptic Complexes Containing Methylated Bipyridyl-type Ligands, Inorg Chem 56 (2017) 12214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havrylyuk D, Heidary DK, Sun Y, Parkin S, Glazer EC, Photochemical and photobiological properties of pyridyl-pyrazol(in)e-based Ruthenium (II) complexes with submicromolar cytotoxicity for phototherapy, ACS Omega ASAP; (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Roque J 3rd, Havrylyuk D, Barrett PC, Sainuddin T, McCain J, Colon K, et al. , Strained, Photoejecting Ru(II) Complexes that are Cytotoxic Under Hypoxic Conditions, Photochem Photobiol 96 (2020) 327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows the design of Ru(II) polypyridyl complexes capable of partitioning between two different photoactivated cytotoxic mechanisms. It also provides a discussion of the multiple features that play a role in the observed biological activity.
- 13.Dewar MJ, Structure of colchicine, Nature 155 (1945) 141–2. [Google Scholar]
- 14.Windaus A, Untersuchungen über die Konstitution des Colchicins, Liebigs Annalen 439 (1924) 59. [Google Scholar]
- 15.Leiter J, Hartwell JL, Kahler JS, Kline I, Shear MJ, Damage induced in sarcoma 37 with chemical agents. VI. Biphenyl, fluorene, phenanthrene, and tropolone derivatives, J Natl Cancer Inst 14 (1953) 365–74. [DOI] [PubMed] [Google Scholar]
- 16.Leiter J, Downing V, Hartwell JL, Shear MJ, Damage Induced in Sarcoma 37 with Chemical Agents, III. Colchicine Derivatives Related to Trimethylcolchicinic Acid and to Colchinol2, JNCI: Journal of the National Cancer Institute 13 (1952) 379–92. [PubMed] [Google Scholar]
- 17.Dwyer FP, Reid IK, Shulman A, Laycock GM, Dixson S, The biological actions of 1,10-phenanthroline and 2,2’-bipyridine hydrochlorides, quaternary salts and metal chelates and related compounds. 1. Bacteriostatic action on selected gram-positive, gram-negative and acid-fast bacteria, Aust J Exp Biol Med Sci 47 (1969) 203–18. [DOI] [PubMed] [Google Scholar]
- 18.Dwyer FP, Gyarfas EC, Rogers WP, Koch JH, Biological activity of complex ions, Nature 170 (1952) 190–1. [DOI] [PubMed] [Google Scholar]
- 19.Shulman A, White DO, Virostatic activity of 1,10-phenanthroline transition metal chelates: A structure-activity analysis, Chemico-Biological Interactions 6 (1973) 407–13. [DOI] [PubMed] [Google Scholar]
- 20.Berger NA, Johnson ES, Skinner AM, Ortho-phenanthroline inhibition of DNA synthesis in mammalian cells, Exp Cell Res 96 (1975) 145–55. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurti C, Saryan LA, Petering DH, Effects of ethylenediaminetetraacetic acid and 1,10-phenanthroline on cell proliferation and DNA synthesis of Ehrlich ascites cells, Cancer Res 40 (1980) 4092–9. [PubMed] [Google Scholar]
- 22.Duncan JR, Dreosti IE, A proposed site of action for zinc in DNA synthesis, J Comp Pathol 86 (1976) 81–5. [DOI] [PubMed] [Google Scholar]
- 23.Falchuk KH, Krishan A, Sullivan J, 1,10-Phenanthroline Inhibition of Lymphoblast Cell Cycle, 37 (1977) 2050–6. [PubMed] [Google Scholar]
- 24.McFadyen WD, Wakelin LP, Roos IA, Leopold VA, Activity of platinum(II) intercalating agents against murine leukemia L1210, J Med Chem 28 (1985) 1113–6. [DOI] [PubMed] [Google Scholar]
- 25.Ding WQ, Lind SE, Metal ionophores - an emerging class of anticancer drugs, IUBMB Life 61 (2009) 1013–8. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Zhang X, Chen J, Yang Q, Yang L, Xu D, et al. , Hinokitiol copper complex inhibits proteasomal deubiquitination and induces paraptosis-like cell death in human cancer cells, Eur J Pharmacol 815 (2017) 147–55. [DOI] [PubMed] [Google Scholar]
- 27.Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, et al. , Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells, J Am Chem Soc 133 (2011) 6235–42. [DOI] [PubMed] [Google Scholar]
- 28.Sun DD, Wang WZ, Mao JW, Mei WJ, Liu J, Imidazo [4,5f][1,10] phenanthroline derivatives as inhibitor of c-myc gene expression in A549 cells via NF-kappaB pathway, Bioorg Med Chem Lett 22 (2012) 102–5. [DOI] [PubMed] [Google Scholar]
- 29.Mohindru A, Fisher JM, Rabinovitz M, 2,9-Dimethyl-1,10-phenanthroline (neocuproine): a potent, copper-dependent cytotoxin with anti-tumor activity, Biochem Pharmacol 32 (1983) 3627–32. [DOI] [PubMed] [Google Scholar]
- 30.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV, Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase, Science 284 (1999) 805–8. [DOI] [PubMed] [Google Scholar]
- 31.Byrnes RW, Antholine WE, Petering DH, Oxidation-reduction reactions in Ehrlich cells treated with copper-neocuproine, Free Radic Biol Med 13 (1992) 469–78. [DOI] [PubMed] [Google Scholar]
- 32.De Man JG, Moreels TG, De Winter BY, Herman AG, Pelckmans PA, Pre- and postjunctional protective effect of neocuproine on the nitrergic neurotransmitter in the mouse gastric fundus, Br J Pharmacol 132 (2001) 277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azmi AS, Bhat SH, Hanif S, Hadi SM, Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties, FEBS Lett 580 (2006) 533–8. [DOI] [PubMed] [Google Scholar]
- 34.Kučírková T, Stiborek M, Dúcka M, Navrátilová J, Bogdanović Pristov J, Popović-Bijelić A, et al. , Anti-cancer effects of wedelolactone: interactions with copper and subcellular localization, Metallomics 10 (2018) 1524–31. [DOI] [PubMed] [Google Scholar]
- 35.Kovacic P, Popp WJ, Ames JR, Ryan MD, Anti-cancer action of metal complexes: electron transfer and oxidative stress?, Anticancer Drug Des 3 (1988) 205–16. [PubMed] [Google Scholar]
- 36.Zhu BZ, Chevion M, Copper-mediated toxicity of 2,4,5-trichlorophenol: biphasic effect of the copper(I)-specific chelator neocuproine, Arch Biochem Biophys 380 (2000) 267–73. [DOI] [PubMed] [Google Scholar]
- **37.Steinbrueck A, Sedgwick AC, Brewster JT 2nd, Yan KC, Shang Y, Knoll DM, et al. , Transition metal chelators, pro-chelators, and ionophores as small molecule cancer chemotherapeutic agents, Chem Soc Rev 49 (2020) 3726–47. [DOI] [PubMed] [Google Scholar]; A contemporary review of the inorganic and biological chemistry of chelators and ionophores as cancer therapeutics.
- **38.Hunsaker EW, Franz KJ, Emerging Opportunities To Manipulate Metal Trafficking for Therapeutic Benefit, Inorg Chem 58 (2019) 13528–45. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work provides a detailed overview of the metallobiology of disease-states and opportunities to leverage this developing concept to therapeutic effect.
- 39.Denoyer D, Masaldan S, La Fontaine S, Cater MA, Targeting copper in cancer therapy: ‘Copper That Cancer’, Metallomics 7 (2015) 1459–76. [DOI] [PubMed] [Google Scholar]
- 40.Denoyer D, Pearson HB, Clatworthy SA, Smith ZM, Francis PS, Llanos RM, et al. , Copper as a target for prostate cancer therapeutics: copper-ionophore pharmacology and altering systemic copper distribution, Oncotarget 7 (2016) 37064–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weekley CM, He C, Developing drugs targeting transition metal homeostasis, Curr Opin Chem Biol 37 (2017) 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutsenko S, Human copper homeostasis: a network of interconnected pathways, Curr Opin Chem Biol 14 (2010) 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tawari PE, Wang Z, Najlah M, Tsang CW, Kannappan V, Liu P, et al. , The cytotoxic mechanisms of disulfiram and copper(ii) in cancer cells, Toxicol Res (Camb) 4 (2015) 1439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen PM, Ruiz C, Lussier D, Le BK, Angel N, Smith M, et al. , Synthesis, characterization, and antitumor activity of unusual pseudo five coordinate gold(III) complexes: distinct cytotoxic mechanism or expensive ligand delivery systems?, J Inorg Biochem 141 (2014) 121–31. [DOI] [PubMed] [Google Scholar]
- 45.Wein AN, Stockhausen AT, Hardcastle KI, Saadein MR, Peng SB, Wang D, et al. , Tumor cytotoxicity of 5,6-dimethyl-1,10-phenanthroline and its corresponding gold(III) complex, J Inorg Biochem 105 (2011) 663–8. [DOI] [PubMed] [Google Scholar]
- 46.Sanghvi CD, Olsen PM, Elix C, Peng SB, Wang D, Chen ZG, et al. , Antitumor properties of five-coordinate gold(III) complexes bearing substituted polypyridyl ligands, J Inorg Biochem 128 (2013) 68–76. [DOI] [PubMed] [Google Scholar]
- 47.David CN, Frias ES, Elix CC, McGovern KE, Walker AM, Eichler JF, et al. , Antitumor activity of a polypyridyl chelating ligand: in vitro and in vivo inhibition of glioma, ASN Neuro 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48.Wang D, Peng S, Amin AR, Rahman MA, Nannapaneni S, Liu Y, et al. , Antitumor Activity of 2,9-Di-Sec-Butyl-1,10-Phenanthroline, PLoS One 11 (2016) e0168450. [DOI] [PMC free article] [PubMed] [Google Scholar]; A report on the biological action and anticancer activity of an unusually cytotoxic free polypyridyl ligand.
- 49.James BRW RJP, 383. The oxidation-reduction potentials of some copper complexes, J Chem Soc (1961) 2007–19. [Google Scholar]
- *50.Local A, Zhang H, Benbatoul KD, Folger P, Sheng X, Tsai CY, et al. , APTO-253 Stabilizes G-quadruplex DNA, Inhibits MYC Expression, and Induces DNA Damage in Acute Myeloid Leukemia Cells, Mol Cancer Ther 17 (2018) 1177–86. [DOI] [PubMed] [Google Scholar]; This reports utilized intracellular pharmacokinetic studies to show that APTO-253 is converted from the free ligand to the ferrous complex [Fe(253)3]. Both the free ligand and [Fe(253)3] stabilize G4 structures, leading to reduced MYC mRNA expression and protein levels.
- *51.Tsai CY, Sun S, Zhang H, Local A, Su Y, Gross LA, et al. , APTO-253 Is a New Addition to the Repertoire of Drugs that Can Exploit DNA BRCA1/2 Deficiency, Mol Cancer Ther 17 (2018) 1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]; APTO-253 was discovered to induce DNA damage, so this report investigated the possible synergistic effect of APTO-253 in cells with loss of either BRCA1 or BRCA2 function, resulting in defects in homologous recombination (HR). Hypersensitivity to APTO-253 was observed similar to the effects of the PARP inhibitor, olaparib, providing an approach to exploit synthetic lethality.
- 52.Winterbourn CC, Toxicity of iron and hydrogen peroxide: the Fenton reaction, Toxicol Lett 82–83 (1995) 969–74. [DOI] [PubMed] [Google Scholar]
- 53.Jiao Y, Wilkinson Jt, Di X, Wang W, Hatcher H, Kock ND, et al. , Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator, Blood 113 (2009) 462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Gill MR, Jarman PJ, Halder S, Walker MG, Saeed HK, Thomas JA, et al. , A three-in-one-bullet for oesophageal cancer: replication fork collapse, spindle attachment failure and enhanced radiosensitivity generated by a ruthenium(II) metallo-intercalator, Chem Sci 9 (2018) 841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrates markedly different biological effects for the free dppz ligand and Ru(II) complexes containing dppz and related ligands.
- 55.Funk A, Divekar PV, Caerulomycin, a new antibiotic from Streptomyces caeruleus Baldacci. I. Production, isolation, assay, and biological properties, Can J Microbiol 5 (1959) 317–21. [DOI] [PubMed] [Google Scholar]
- 56.Fu P, Wang S, Hong K, Li X, Liu P, Wang Y, et al. , Cytotoxic bipyridines from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6, J Nat Prod 74 (2011) 1751–6. [DOI] [PubMed] [Google Scholar]
- 57.Shindo K, Yamagishi Y, Okada Y, Kawai H, Collismycins A and B, novel non-steroidal inhibitors of dexamethasone-glucocorticoid receptor binding, J Antibiot (Tokyo) 47 (1994) 1072–4. [DOI] [PubMed] [Google Scholar]
- 58.Kawatani M, Muroi M, Wada A, Inoue G, Futamura Y, Aono H, et al. , Proteomic profiling reveals that collismycin A is an iron chelator, Sci Rep 6 (2016) 38385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu P, Liu P, Li X, Wang Y, Wang S, Hong K, et al. , Cyclic bipyridine glycosides from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6, Org Lett 13 (2011) 5948–51. [DOI] [PubMed] [Google Scholar]
- 60.Rudi A, Benayahu Y, Goldberg I, Kashman Y, Eilatin, a novel alkaloid from the marine tunicate eudistoma sp, Tetrahedron Letters 29 (1988) 6655–6. [Google Scholar]
- 61.Yeowell HN, White JR, Iron requirement in the bactericidal mechanism of streptonigrin, Antimicrob Agents Chemother 22 (1982) 961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolzan AD, Bianchi MS, Genotoxicity of streptonigrin: a review, Mutat Res 488 (2001) 25–37. [DOI] [PubMed] [Google Scholar]
- 63.Hadju JA EC, Interaction of metal ions with streptonigrin. 1. Formation of copper(II) and zinc(II) complexes of the antitumor antibiotic., J Am Chem Soc 103 (1981) 232–4. [Google Scholar]
- 64.Harris MN, Medrek TJ, Golomb FM, Gumport SL, Postel AH, Wright JC, Chemotherapy with Streptonigrin in Advanced Cancer, Cancer 18 (1965) 49–57. [DOI] [PubMed] [Google Scholar]
- 65.Kaung DT, Wittington RM, Spencer H, Patno ME, Comparison of Chlorambucil and Streptonigrin (NSC-45383) in the Treatment of Malignant Lymphomas, Cancer 23 (1969) 1280–3. [DOI] [PubMed] [Google Scholar]
- 66.Cai W, Hassani M, Karki R, Walter ED, Koelsch KH, Seradj H, et al. , Synthesis, metabolism and in vitro cytotoxicity studies on novel lavendamycin antitumor agents, Bioorg Med Chem 18 (2010) 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassani M, Novel analogues of lavendamycin as antitumor agents: Metabolism by NAD(P)H:quinone oxidoreductase 1 (NQO1) and in vitro cytotoxicity, Cancer Res 64 (2004). [Google Scholar]
- 68.Hassani M, Cai W, Holley DC, Lineswala JP, Maharjan BR, Ebrahimian GR, et al. , Novel lavendamycin analogues as antitumor agents: synthesis, in vitro cytotoxicity, structure-metabolism, and computational molecular modeling studies with NAD(P)H:quinone oxidoreductase 1, J Med Chem 48 (2005) 7733–49. [DOI] [PubMed] [Google Scholar]
- *69.Sun Y, Heidary DK, Zhang Z, Richards CI, Glazer EC, Bacterial Cytological Profiling Reveals the Mechanism of Action of Anticancer Metal Complexes, Mol Pharm 15 (2018) 3404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report details mechanistic studies in mammalian and bacterial cells that support the interpretation that the metal center, not the free ligand, is the active species.
- *70.Cuello-Garibo JA, Meijer MS, Bonnet S, To cage or to be caged? The cytotoxic species in ruthenium-based photoactivated chemotherapy is not always the metal, Chem Commun (Camb) 53 (2017) 6768–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; A report providing evidence that photoejecting Ru(II) polypyridyl complexes can induce cytotoxicity by the action of the free ligand 6,6’-dimethylbipyridine.
- *71.Azar DF, Audi H, Farhat S, El-Sibai M, Abi-Habib RJ, Khnayzer RS, Phototoxicity of strained Ru(II) complexes: is it the metal complex or the dissociating ligand?, Dalton Trans 46 (2017) 11529–32. [DOI] [PubMed] [Google Scholar]; This work shows that photoejecting Ru(II) polypyridyl complexes can induce cytotoxicity by the action of the free ligand neocuprione.
- *72.Mansour N, Mehanna S, Mroueh MA, Audi H, Bodman-Smith K, Daher CF, et al. , Photoactivatable RuII Complex Bearing 2,9-Diphenyl-1,10-phenanthroline: Unusual Photochemistry and Significant Potency on Cisplatin-Resistant Cell Lines, Eur J Inorg Chem 22 (2018) 2524–32. [Google Scholar]; This report demonstrated activity for a Ru(II) center following ligand release. However, due to the inclusion of ligand 19 in the structure, which is potent as a free ligand, it is possible the activity is contributed partly by the ligand. This would be the case if multiple (or sequential) photochmical products are formed.
- 73.Hidayatullah AN, Wachter E, Heidary DK, Parkin S, Glazer EC, Photoactive Ru(II) complexes with dioxinophenanthroline ligands are potent cytotoxic agents, Inorg Chem 53 (2014) 10030–2. [DOI] [PubMed] [Google Scholar]
- 74.Havrylyuk D, Heidary DK, Nease L, Parkin S, Glazer EC, Photochemical Properties and Structure-Activity Relationships of Ru(II) Complexes with Pyridylbenzazole Ligands as Promising Anticancer Agents, Eur J Inorg Chem 2017 (2017) 1687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Havrylyuk D, Heidary DK, Sun Y, Parkin S, Glazer EC, Photochemical and Photobiological Properties of Pyridyl-pyrazol(in)e-Based Ruthenium(II) Complexes with Sub-micromolar Cytotoxicity for Phototherapy, ACS Omega 5 (2020) 18894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian N, Feng Y, Sun W, Lu J, Lu S, Yao Y, et al. , A nuclear permeable Ru(ii)-based photoactivated chemotherapeutic agent towards a series of cancer cells: in vitro and in vivo studies, Dalton Trans 48 (2019) 6492–500. [DOI] [PubMed] [Google Scholar]
- 77.Qu F, Park S, Martinez K, Gray JL, Thowfeik FS, Lundeen JA, et al. , Ruthenium Complexes are pH-Activated Metallo Prodrugs (pHAMPs) with Light-Triggered Selective Toxicity Toward Cancer Cells, Inorg Chem 56 (2017) 7519–32. [DOI] [PubMed] [Google Scholar]
- *78.Qu F, Martinez K, Arcidiacono AM, Park S, Zeller M, Schmehl RH, et al. , Sterically demanding methoxy and methyl groups in ruthenium complexes lead to enhanced quantum yields for blue light triggered photodissociation, Dalton Trans 47 (2018) 15685–93. [DOI] [PubMed] [Google Scholar]; This work revealed the lack of correlation between the quantum yield of photosubstitution and cytotoxicity, highlighting the complexities in the design of light-active anticancer agents.
- 79.Wang Y, Tian N, Sun W, Rena B, Guo X, Feng Y, et al. , A Ru(II)-Based Nanoassembly Exhibiting Theranostic PACT Activity in NIR Region, Particle & Particle Systems Characterization 37 (2020). [Google Scholar]
- *80.Sainuddin T, Pinto M, Yin H, Hetu M, Colpitts J, McFarland SA, Strained ruthenium metal-organic dyads as photocisplatin agents with dual action, J Inorg Biochem 158 (2016) 45–54. [DOI] [PubMed] [Google Scholar]; The mechanistic importance of time-dependent studies for dual action agents is demonstrated, as differences in potency were observed as a function of the compound’s ability to generate 1O2. At 1 hr after irradiation, the three complexes exhibited similar IC50 values, while at 16 hrs significant differences were apparent.
- 81.Wachter E, Howerton BS, Hall EC, Parkin S, Glazer EC, A new type of DNA “light switch”: a dual photochemical sensor and metalating agent for duplex and G-quadruplex DNA, ChemComm 50 (2014) 311–13. [DOI] [PubMed] [Google Scholar]
- 82.Wachter E, Glazer EC, Mechanistic Study on the Photochemical “Light Switch” Behavior of [Ru(bpy)2dmdppz](2+), J Phys Chem A 118 (2014) 10474–86. [DOI] [PubMed] [Google Scholar]
- 83.Wachter E, Moya D, Parkin S, Glazer EC, Ruthenium Complex “Light Switches” that are Selective for Different G-Quadruplex Structures, Chemistry A European Journal 22 (2016) 550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scaltrito DV, Thompson DW, O’Callaghan JA, Meyer GJ, MLCT excited states of cuprous bis-phenanthroline coordination compounds, Coordination Chemistry Reviews 208 (2000) 243–66. [Google Scholar]
- *85.Al Hageh C, Al Assaad M, El Masri Z, Samaan N, El-Sibai M, Khalil C, et al. , A long-lived cuprous bis-phenanthroline complex for the photodynamic therapy of cancer, Dalton Trans 47 (2018) 4959–67. [DOI] [PubMed] [Google Scholar]; This paper showed a range of biological and light-mediated activities for copper complexes containing different ortho-substituted phenantholine ligands.
- *86.Ludtke C, Sobottka S, Heinrich J, Liebing P, Wedepohl S, Sarkar B, et al. , Forty Years after the Discovery of Its Nucleolytic Activity: [Cu(phen)2](2+) Shows Unattended DNA Cleavage Activity upon Fluorination, Chemistry (2021) 10.1002/chem.202004594. [DOI] [PMC free article] [PubMed] [Google Scholar]; A report demonstrating that redox tuning of phen ligands alters the DNA damaging ability of resulting Cu complexes. Both light and DNA were proposed to engage in reductive processes to promote nucleolytic activity.
- 87.Hordyjewska A, Popiolek L, Kocot J, The many “faces” of copper in medicine and treatment, Biometals 27 (2014) 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbaspour N, Hurrell R, Kelishadi R, Review on iron and its importance for human health, J Res Med Sci 19 (2014) 164–74. [PMC free article] [PubMed] [Google Scholar]
- 89.Jung M, Mertens C, Tomat E, Brune B, Iron as a Central Player and Promising Target in Cancer Progression, Int J Mol Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dickerson M, Howerton B, Bae Y, Glazer E, Light-Sensitive Ruthenium Complex-Loaded Cross-linked Polymeric Nanoassemblies for the Treatment of Cancer, J Mater Chem B 4 (2016) 394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **91.Keenan J, Horgan K, Clynes M, Sinkunaite I, Ward P, Murphy R, et al. , Unexpected fluctuations of trace element levels in cell culture medium in vitro: caveat emptor, In Vitro Cell Dev Biol Anim 54 (2018) 555–8. [DOI] [PubMed] [Google Scholar]; This work demonstrates the variability in trace-metal contaminants batch-to-batch in reagents used for common experimental models of cytotoxicity, and that the presence of these metals can be biologically non-innocent.
- 92.Yao T, Asayama Y, Animal-cell culture media: History, characteristics, and current issues, Reprod Med Biol 16 (2017) 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **93.Nunes P, Correia I, Marques F, Matos AP, Dos Santos MMC, Azevedo CG, et al. , Copper Complexes with 1,10-Phenanthroline Derivatives: Underlying Factors Affecting Their Cytotoxicity, Inorg Chem 59 (2020) 9116–34. [DOI] [PubMed] [Google Scholar]; This is an innovative study of metal-ligand chemical dynamics and kinetics within the context of cell-culture models. A speciation study demonstrated that cytotoxicity values for complexes and the corresponding free ligands varied within the first 24 hours of incubation, but by 72 hours the cytotoxicity values were similar. Cu(phen)x rapidly dissociates, thus, the cytotoxicity of Cu(phen)x is largely based on the independent actions of Cu(II) and phen.
- **94.Pinho JO, Amaral JD, Castro RE, Rodrigues CM, Casini A, Soveral G, et al. , Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models, Nanomedicine 14 (2019) 835–50. [DOI] [PubMed] [Google Scholar]; This work demonstrates an effective delivery approach for copper phenanthroline complexes, resulting in in vivo activity and reduced side effects.


