Abstract
Viral infections are considered as etiologic factors of subacute thyroiditis. The true incidence of subacute thyroiditis, caused by SARS‐CoV‐2 infection, is probably considerable since it is often masked by more dramatic affection of the respiratory system. This report presents two female patients who developed de Quervain's thyroiditis after COVID‐19 disease.
Keywords: COVID‐19, de Quervain's thyroiditis, SARS‐CoV‐2, subacute thyroiditis
SARS‐CoV‐2 infection may trigger subacute thyroiditis. Doctors should be aware of the potential development of subacute thyroiditis at SARS‐CoV‐2, which may be masked by dramatic affection of the respiratory system and dexamethasone administration.
1. INTRODUCTION
De Quervain's thyroiditis, also known as subacute granulomatous thyroiditis or giant cell thyroiditis, is an inflammatory disease of the thyroid gland caused by a viral infection. Dissemination of the virus via the blood flow to the thyroid tissue causes inflammation and destruction of the thyroid tissue. It leads to severe pain syndrome and thyrotoxicosis. The disease is characterized by seasonal outbreaks in the autumn‐winter period due to the spread of respiratory viral infections and influenza epidemics and is prone to the recurrent course. 1 , 2 De Quervain's thyroiditis is usually preceded by an upper respiratory tract infection. Any virus can presumably cause inflammation in the thyroid gland. 3 There is evidence on the association of subacute thyroiditis with a variety of viruses, such as coxsackievirus, echovirus, mumps virus, adenovirus, orthomyxovirus, Epstein‐Barr virus, hepatitis E virus, HIV, cytomegalovirus, dengue fever, and SARS‐CoV‐2. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 To the best of our knowledge, there are few reports of subacute thyroiditis as a result of a SARS‐CoV‐2 infection. 12 , 13 , 14 , 15 Here, we present two case reports of de Quervain's subacute thyroiditis after COVID‐19.
2. CASE REPORTS
2.1. Case report 1
A 45‐year‐old woman, referred to our hospital, presented with severe pain in the anterior surface of the neck irradiation to the lower jaw and right ear, pain at swallowing, fever up to 38.0°C, myalgia, palpitation, and sweating. She developed nasal congestion, cough, weakness, muscle pain, and headache. Six days after the first symptoms, the patient was tested positive for SARS‐CoV‐2. Computed tomography (CT) scans revealed signs of viral pneumonia involving 20% of the lungs (Figure 1). Due to the mild course of coronavirus infection, she was not hospitalized. Corticosteroids for coronavirus disease were not administrated, and after 7 days on the symptomatic and antibacterial therapy, respiratory symptoms completely regressed. Two weeks after starting the treatment, repeated polymerase chain reaction (PCR) tests for SARS‐CoV‐2 were negative.
FIGURE 1.
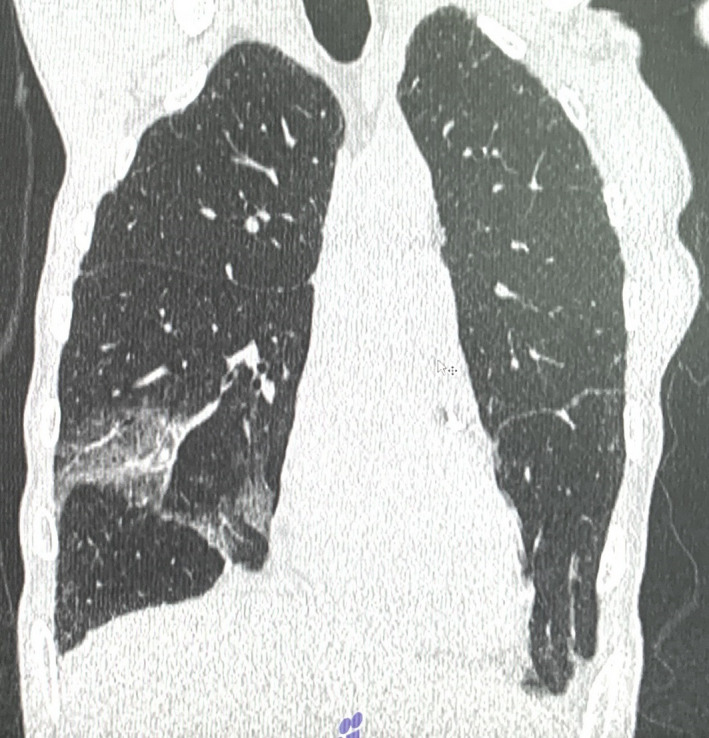
Computed tomography of thorax of the patient N 1 with 20% of viral pneumonia involving 20% of the lungs
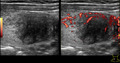
Ten days after the negative test, the patient's condition worsened, characterized by increased general weakness, swelling, and pain in the anterior surface of the neck, and the patient was referred to an endocrinologist. The patient had no previous history of thyroid diseases. On examination, the skin was moist, heart rate was up to 110 per minute, blood pressure 140/80 mm Hg and fever up to 37.8°C. On the anterior surface of the neck, in the projection of the thyroid gland, there was a pronounced swelling without changes in the skin. Palpation of the thyroid gland was sharply painful and with no palpable lymph nodes in the neck. Ultrasound examination revealed diffuse enlargement of the thyroid gland with the right lobe measuring 35 × 39 × 62 mm, the left lobe 16 × 20 × 60 mm, the isthmus 5 mm, and in both lobes were visible multiple hypoechoic areas. In the right lobe, pseudo‐nodule measuring 32 × 36 × 43 mm was visualized (Figure 2; Table 1).
FIGURE 2.
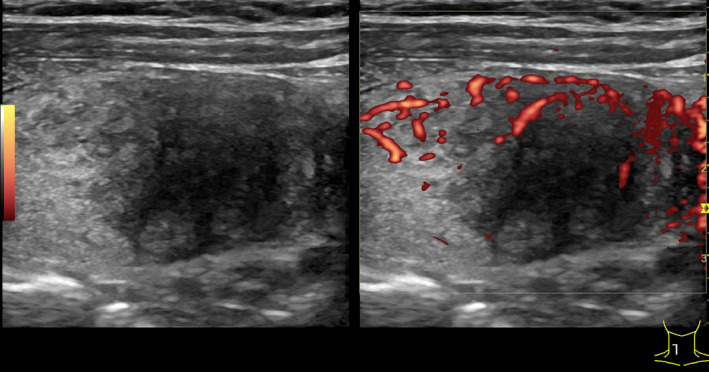
Ultrasonography of the patient N 1. On the left: Longitudinal section of the right thyroid lobe with pseudonodulus in the lower pole. On the right: Energy Doppler shows hypervascularization
TABLE 1.
Demographics, clinical presentation, course, and outcome
| Gender | Age (years) | History of thyroid disease | Presenting complains | COVID−19 status | Time between COVID−19 and SAT symptoms (days) | Hyperthyroid symptoms | Examination | Treatment | Follow‐up | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Female | 45 | No | Neck pain radiating to the lower jaw and right ear, fever, later‐swelling | Ongoing, symptomatic | 0 | Myalgia, palpitation and sweating, tachycardia | Pronounced swelling without changes in the skin, hard thyroid gland and sharply painful at palpation, no palpable lymph nodes. | Prednisone 30 mg per day for 4 weeks, reduction in the dosage by 5 mg weekly | Within a week significant improvement, complete recovery after 30 days | Recovered |
| Patient 2 | Female | 40 | No | Neck pain, fever | Past/resolved | 30 days | Palpitation, tachycardia | Hard thyroid gland, painful at palpation, no palpable lymph nodes | Prednisone 30 mg per day for 4 weeks, reduction in the dosage by 5 mg weekly, symptomatic therapy of thyrotoxicosis | Recovered after 30 days | Recovered |
The typical clinical manifestation and ultrasound examination, the connection with a viral infection, made it possible to diagnose De Quervain's thyroiditis, which was also confirmed by the results of laboratory tests (Table 2):
TABLE 2.
Diagnostics and laboratory investigations
| COVID−19 diagnosis | Chest X‐ray/CT chest | Thyroid ultrasound/imaging | ESR | WBC | Free T4 | TSH | AB‐TPO | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | RT‐PCR, IgM and IgG | Pneumonia involving 20% of the lungs | Diffusely enlarged thyroid gland, visible multiple hypoechoic areas in both lobes, pseudo‐nodule in the right lobe | 32 mm/h | 11.2 × 10⁹/L | 3.2 ng/dl | 0.005 mu/L | 2 IU/ml |
| Patient 2 | RT‐PCR, IgM and IgG | Pneumonia involving 15% of the lungs | Thyroid gland with clear contours, diffuse inhomogeneous echostructure, due to multiple hypoechoic areas in the form of lacunae, but no nodal formations | 47 mm/h | 11.7 × 10⁹/L | 3.4 ng/dl | <0.0083 mu/L | 1 IU/ml |
WBC increased to 11.2 × 10⁹/L without shifting the WBC formula to the left, ESR 32 mm/h, free T4‐3.2 ng/dl (reference values [RF]: 0.8–2.1), TSH–0.005 mu/L (RV: 0.27–4.2), AB‐TPO‐2 IU/ml (RV: 0–34), AB to SARS‐CoV‐2 coronavirus, IgM‐1.2 [RV: ≥1+], IgG‐2.63 [RV: ≥1.4+]).
Thus, the results of laboratory tests indicated an inflammatory process and thyrotoxicosis, which are the main characteristics of the destructive phase of subacute thyroiditis. The results of laboratory tests revealed the development of weak immunity status to coronavirus infection too.
Prednisone 30 mg per day for 4 weeks was prescribed with a reduction in the dosage by 5 mg weekly. Within a week, the patient's condition improved significantly, the pain in the neck area almost completely subsided, and the body temperature normalized. The heart rate was no more than 80 beats per minute. Ten days after starting the treatment, blood tests showed white blood cells–8.9 × 10⁹/L and ESR‐20 mm/h.
After the end of treatment, a follow‐up examination was performed. The patient's condition was satisfactory with normal heart rates and body temperature. The thyroid gland on palpation was soft, mobile, and painless, with no nodular formations. On ultrasound examination, the thyroid gland edges were clear and even, no nodular formation was seen, and a moderate heterogeneity of the echostructure of the tissue remained. Blood tests showed WBC 7.2 × 10⁹/L, ESR 16 mm/h, frT4‐1.6 ng/dl, TSH‐0.1 mu/L, and AB‐TPO‐29 IU/ml (0–34).
2.2. Case report 2
A 40‐year‐old woman, referred to our hospital, presented with 2 days history of pain in the region of the thyroid gland, fever up to 37.5°C, and tachycardia. As the patient noted, the symptoms started 2 days ago. From the anamnesis, it was known that 1 month earlier she had had a mild coronavirus infection with viral pneumonia involving 15% of the lungs. She was treated on an outpatient basis and did not receive corticosteroids. On examination, the condition was of moderate severity. The skin was moist, heart rate up to 100 beats per minute, blood pressure 130/80 mmHg and respiratory rate 20 per minute. The thyroid gland was painful on palpation with no palpable lymph nodes in the neck (Table 1). In ultrasound, the thyroid gland was located in a typical place, the contours were clear, the right lobe was 22 × 24 × 50 mm in size, the left lobe was 20 × 21 × 48 mm, and the isthmus is 4 mm (Figure 3). The echostructure was diffusely inhomogeneous due to multiple hypoechoic areas in the form of lacunae, but no nodal formations. The blood test revealed an increase in WBC to 11.7 × 10⁹/L, ESR–47 mm/h, CRP‐3.32 mg/dl (<0.5), free T4‐43.8 pmol/L (10.3–24.5), TSH < 0.0083 mu/L (0.27–4.2), AB‐TPO‐1 IU/ml (0–34), AB to the TSH receptor‐0.73 IU/L (<1), AB to the coronavirus SARS‐CoV‐2, IgM‐2.98 (≥1+), and IgG‐2.73 (≥1.4+). The obvious clinical signs, the results of ultrasound examination, and laboratory tests made it possible to diagnose subacute thyroiditis manifesting with thyrotoxicosis as a result of coronavirus infection (Table 2).
FIGURE 3.
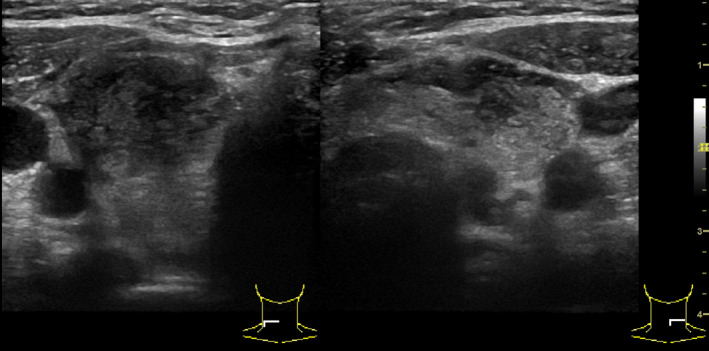
Ultrasonography of the patient N 2. On the left: Cross section of the right thyroid lobe. Hypoechoic tissue with hyperechoic zones. On the right: Cross section of the left thyroid lobe and the isthmus. Heterogeneous echotexture
Prednisone was prescribed according to the scheme with an initial dose of 30 mg per day, followed by a reduction in the dosage by 5 mg weekly and symptomatic therapy of thyrotoxicosis. The patient's condition improved within a week from the start of treatment.
After the completion of prednisone therapy, at the last follow‐up examination, the patient's condition was satisfactory. Heart rate was 80 per minute. The thyroid gland during palpation was soft, painless with no nodular formations. On ultrasound examination, the thyroid gland had clear and even contours, the right lobe measuring 19 × 22 × 49 mm, the left lobe 18 × 20 × 47 mm, the isthmus 4 mm with moderate heterogeneity of the echostructure and no nodular formations. Blood test showed, WBC—8.7 × 10⁹/L, ESR—16 mm/h, CRP—0.61 mg/dl (<0.5), frT4—12.5 pmol/L (10.3–24.5), TSH—0.29 mu/L (0.27–4.2), AB to the coronavirus SARS‐CoV‐2, IgM—0.97 (≥1+), and IgG—4.2 (≥1.4+).
3. DISCUSSION
The nature of the damage to the thyroid tissue by the SARS‐CoV‐2 virus is not fully understood. There may be a uniform mechanism of destruction for all viruses. It has been established that haplotypes of the human leukocyte antigen HLA (HLA‐Bw35, HLA‐B67, HLA‐B15/62, and HLA‐Drw8) predispose to the development of subacute thyroiditis. 7 , 16 , 17 According to the data from pathomorphological studies on subacute thyroiditis, an uneven distribution of loci of noncaseating granulomas, consisting of colloid, small lymphocytes, neutrophils, macrophages, plasma monocytes, and giant cells of foreign bodies, is determined. As a result of cytolytic recognition by T cells of viral and cellular antigens present in thyrocytes, infiltration of follicles occurs, and subsequently rupture of the basement membrane and rupture of follicles. 18 There are data on the thyroid gland damage by the atypical pneumonia virus (SARS‐CoV) in 2002–2003, which had 10% mortality rate. 19 Among those who died of SARS‐CoV, destruction of the follicular epithelium with extensive detachment of apoptotic cells into the lumen of the follicle was observed in the thyroid gland. Inflammatory response and apoptosis are considered as possible mechanisms of thyroid tissue damage in SARS‐CoV. 20 SARS‐CoV is believed to induce a severe inflammatory response and trigger apoptosis through the expression of several viral proteins. 21 , 22 In the report from Wei and colleagues, 11 the data from autopsies of 5 patients with SARS‐CoV revealed damage to the follicular epithelium and cellular apoptosis in the absence of neutrophil or lymphoid infiltration. The authors concluded that the most essential pathogenetic factors were the patient's excessive immune response, emerging immunodeficiency with destruction of lymphocytes and direct destruction of cells. It was also found that apoptosis dominating in the pathogenesis of SARS is triggered by the expression of a number of non‐structural proteins in various cell types, including the thyroid gland. 20 , 22
The direct viral damage to the thyroid gland by the SARS‐CoV‐2 virus is possible. One of the hypotheses explaining the development of subacute thyroiditis in SARS‐CoV‐2 is the interaction with ACE2 receptors. ACE2 is believed to play an essential role in the pathogenesis of coronavirus lung injury. SARS‐CoV and SARS‐CoV‐2 use ACE2 receptors in the kidneys, adrenal glands, adipose tissue, thyroid gland, endothelium, pancreas, testes, ovaries, and human pituitary gland to invade the host cells. 23 ACE2 receptors in the thyroid gland can act as an entry gate for the virus and an important mechanism of damage. SARS‐CoV and SARS‐CoV‐2 use ACE2 receptors on thyroid cell membranes to get inside the cell.
On the ground of the presented case reports, it can be suggested that the novel coronavirus infection COVID‐19 may also cause de Quervain's thyroiditis. Some authors believe that subacute thyroiditis may be one of the variants of COVID‐19 manifestation. 12 , 24 , 25 In a recent report from S. Sohrabpour et al., 15 six cases of COVID‐19–associated subacute thyroiditis were described. In another report from A. Khatri et al., 14 subacute thyroiditis was observed 2–6 weeks after recovery from coronavirus infection, which coincides with the timing of the manifestation of the disease in our patients.
We assume that the true incidence of subacute thyroiditis after a coronavirus infection might be significantly higher than reported in the literature. Not severe symptoms can be conflated with upper respiratory symptoms making it difficult to differentiate underlying subacute thyroiditis; thus, these patients should be assessed carefully through a good history and examination. 1 Chen et al. 26 observed a significant TSH level decrease in 56% of patients with COVID‐19, positively correlating with the severity of the disease, which indirectly indicates destruction of thyroid tissue. An Extensive use of dexamethasone as a first‐line drug in the treatment of COVID‐19 may reduce thyroid damage due to suppression of hypervascular response caused by cytokines making the clinical picture of subacute thyroiditis less vivid. 27 , 28 In our report, thyroid inflammation developed after the manifestation of the COVID‐19 infection in a relatively mild form. Interestingly, both patients were treated on an outpatient basis and did not receive corticosteroids.
The assumed higher true incidence of SARS‐CoV‐2–associated subacute thyroiditis is leveled off by the priorities of the diagnosis of pulmonary complications. Further studies investigating the thyroid status in the long‐term after the coronavirus infection will allow more evident reveal of this relationship. This is of practical importance since, after completion of the destructive phase of subacute thyroiditis, manifested by thyrotoxicosis; thyroid hormone deficiency occurs over time requiring thyroid hormone therapy.
4. CONCLUSION
SARS‐CoV‐2 infection may trigger subacute thyroiditis. Doctors should be aware of the potential development of de Quervain's thyroiditis at SARS‐CoV‐2, which may be masked both by dramatic affection of the respiratory system and by dexamethasone administration in the treatment of COVID‐19 disease.
CONFLICT OF INTEREST
VIS, DLA, AMS, TVK, YKA, GTM, and AMK have no conflict of interest or financial ties to disclose.
AUTHOR CONTRIBUTIONS
VIS gave idea, and involved in material collection, writing, review, revision, and drafting. DLA involved in writing, review, revision, and drafting. AMS and TVK involved in review, revision, and supervision. YKA and GTM involved in review and revision. AMK involved in review, revision, drafting, supervision, and paper submission.
CONSENT
Informed consent was obtained from the patients.
ACKNOWLEDGEMENT
None.
Semikov VI, Aghayan DL, Shulutko AM, et al. Subacute thyroiditis after SARS‐CoV‐2 infection. Clin Case Rep. 2021;9:e5109. doi: 10.1002/ccr3.5109
Funding information
No specific funding. This study was performed as a part of the academic/clinical positions of the authors
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Aemaz Ur Rehman M, Farooq H, Ali MM, Ebaad Ur Rehman M, Dar QA, Hussain A. The association of subacute thyroiditis with COVID‐19: a systematic review. SN Compr Clin Med. 2021;1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hennessey J. Subacute thyroiditis. Endotext. 2018. [Google Scholar]
- 3. Tabassom A, Chippa V, Edens MA. De quervain thyroiditis. In StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 4. Assir MZ, Jawa A, Ahmed HI. Expanded dengue syndrome: subacute thyroiditis and intracerebral hemorrhage. BMC Infect Dis. 2012;12:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute thyroiditis: clinical characteristics and treatment outcome in fifty‐six consecutive patients diagnosed between 1999 and 2005. J Endocrinol Invest. 2007;30(8):631‐635. [DOI] [PubMed] [Google Scholar]
- 6. Bouillet B, Petit JM, Piroth L, Duong M, Bourg JB. A case of subacute thyroiditis associated with primary HIV infection. Am J Med. 2009;122(4):e5‐e6. [DOI] [PubMed] [Google Scholar]
- 7. Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinez‐Artola Y, Poncino D, Garcia ML, Munne MS, Gonzalez J, Garcia DS. Acute hepatitis E virus infection and association with a subacute thyroiditis. Ann Hepatol. 2015;14(1):141‐142. [PubMed] [Google Scholar]
- 9. Martino E, Buratti L, Bartalena L, et al. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest. 1987;10(3):321‐323. [DOI] [PubMed] [Google Scholar]
- 10. Saito S, Sakurada T, Yamamoto M, Yamaguchi T, Yoshida K. Subacute thyroiditis: observations on 98 cases for the last 14 years. Tohoku J Exp Med. 1974;113(2):141‐147. [DOI] [PubMed] [Google Scholar]
- 11. Wei L, Sun S, Xu CH, et al. Pathology of the thyroid in severe acute respiratory syndrome. Hum Pathol. 2007;38(1):95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after Sars‐COV‐2 infection. J Clin Endocrinol Metab. 2020;105(7):2367‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS‐CoV‐2 infection? insights from a case series. J Clin Endocrinol Metab. 2020;105(10):e3742‐e3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khatri A, Charlap E, Kim A. Subacute thyroiditis from COVID‐19 infection: a case report and review of literature. Eur Thyroid J. 2021;9(6):324‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sohrabpour S, Heidari F, Karimi E, Ansari R, Tajdini A, Heidari F. Subacute thyroiditis in COVID‐19 patients. Eur Thyroid J. 2021;9(6):321‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farwell AP, Braverman L, Cooper D. Sporadic painless, painful subacute and acute infectious thyroiditis. Braverman LE (ed.), Werner and Ingbar’s the thyroid: A fundamental and clinical text. 10. Lippincott Williams & Wilkins; 2013;414‐429. [Google Scholar]
- 17. Ohsako N, Tamai H, Sudo T, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80(12):3653‐3656. [DOI] [PubMed] [Google Scholar]
- 18. Kojima M, Nakamura S, Oyama T, Sugihara S, Sakata N, Masawa N. Cellular composition of subacute thyroiditis. An immunohistochemical study of six cases. Pathol Res Pract. 2002;198(12):833‐837. [DOI] [PubMed] [Google Scholar]
- 19. Sørensen MD, Sørensen B, Gonzalez‐Dosal R, et al. Severe acute respiratory syndrome (SARS): development of diagnostics and antivirals. Ann N Y Acad Sci. 2006;1067(1):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Law PT, Wong C‐H, Au TC, et al. The 3a protein of severe acute respiratory syndrome‐associated coronavirus induces apoptosis in vero E6 cells. J Gen Virol. 2005;86(7):1921‐1930. [DOI] [PubMed] [Google Scholar]
- 21. Tan Y‐J, Fielding BC, Goh P‐Y, et al. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase‐dependent pathway. J Virol. 2004;78(24):14043‐14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan X, Shan Y, Zhao Z, Chen J, Cong Y. G0/G1 arrest and apoptosis induced by SARS‐CoV 3b protein in transfected cells. Virol J. 2005;2(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamming I, Timens W, Bulthuis M, Lely A, Gv N, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with covid‐19 infection. J Endocrinol Invest. 2020;43(8):1173‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muller I, Cannavaro D, Dazzi D, et al. SARS‐CoV‐2‐related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID‐19: a retrospective study. Thyroid. 2021;31(1):8‐11. [DOI] [PubMed] [Google Scholar]
- 27. Ippolito S, Dentali F, Tanda ML. SARS‐CoV‐2: a potential trigger for subacute thyroiditis? insights from a case report. J Endocrinol Invest. 2020;43(8):1171‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med. 2020;7:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


