Abstract
COVID-19 survivors are at increased risk of persistent psychopathology after the infection. Despite long-term sequelae are an increasing concern, long-term neuropsychiatric consequences remain largely unclear. This cohort study aimed at investigating the psychopathological impact of COVID-19 in Italy one year after infection, outlining the trajectory of symptomatology at one, six-, and twelve-months follow-up.
We evaluated 402, 216, and 192 COVID-19 survivors respectively at one, six, and 12 months. A subgroup of 95 patients was evaluated longitudinally both at one, six, and 12 months. Validated self-report questionnaires were administered to assess depression, fatigue, anxiety, and post-traumatic distress. Socio-demographics and setting of care information were gathered for each participant.
At six and twelve months, respectively 94 (44%) and 86 (45%) patients self-rated in the clinical range in at least one psychopathological dimension. Pathological fatigue at twelve months was detected in 63 patients (33%). Considering the longitudinal cohort an interaction effect of sex and time was observed for depression (F = 8.63, p < 0.001) and anxiety (F = 5.42, p = 0.005) with males showing a significant increasing trend of symptoms, whereas an opposite course was observed in females.
High prevalence of psychiatric sequelae six and 12 months after COVID-19 was reported for the first time. These findings confirm the need to provide integrated multidisciplinary services to properly address long-lasting mental health sequelae of COVID-19 and to treat them with the aim of reducing the disease burden and related years of life lived with disability.
Keywords: COVID-19, SARS-CoV-2, Depression, Anxiety, Fatigue, Mental health
1. Introduction
Since the coronavirus disease 2019 (COVID-19) pandemic began, it has caused morbidity and mortality at an unprecedented scale globally. The clinical characteristics and complications of patients with COVID-19 during acute infection have been widely explored (Wiersinga et al., 2020). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a broad spectrum of manifestations ranging from asymptomatic infection to life-threatening multi-organ disease. Furthermore, persistent and prolonged symptoms, similarly to previous coronavirus outbreaks (Moldofsky and Patcai, 2011), have been observed in several patients recovered from the acute phase (Carfì et al., 2020). Reports at three, four, and six months following discharge from hospital suggest persisting symptoms such as lung dysfunctions, physical and psychological disturbances, and cognitive impairments (Huang et al., 2021).
Notably, as the pandemic spread, there has been a growing recognition of mental health implications. Delirium and confusion were observed in the context of acute viral infection (Rogers et al., 2020). Moreover, in the months following the infection, COVID-19 survivors were at increased risk of depression, anxiety, insomnia, and post-traumatic stress disorder (PTSD) (Mazza et al., 2020, 2021; Taquet et al., 2021). In this context, females and patients whit a positive previous psychiatric history were found to be at higher risk to present post-COVID psychiatric complaints (Mazza et al., 2020, 2021; Vindegaard and Benros, 2020). Psychiatric long-term complaints affected the quality of life, associated with cognitive impairments, and fatigue syndromes (Manning et al., 2021; Mazza et al., 2021). Considering their prevalence and persistence, neuropsychiatric complaints were recently listed as main symptoms of the “Post-acute COVID-19 syndrome” (Nalbandian et al., 2021). The mechanism underlying the COVID-19 psychiatric consequences seems to be mainly related to the systemic inflammation associated to the viral infection (Troyer et al., 2020) and to the persistent psychological stress before and during infection (Passavanti et al., 2021).
Considering the increasing number of patients recovering from COVID-19, its long-term sequelae are now an increasing concern. Therefore, it is paramount to understand the full spectrum of post-COVID-19 complaints to develop evidence-based knowledge. To date, studies provided information up to six months after discharge, finding that COVID-19 was associated with an increased risk of persistent mental health sequelae (Huang et al., 2021; Taquet et al., 2021), however the long-term psychiatric complaints of the COVID-19 remain largely unclear. Consequently, longitudinal studies with longer follow-up are necessary to better understand the trajectory and full spectrum of mental health consequences from COVID-19.
Considering the high prevalence of psychiatric conditions observed at one-, three-, and six-months follow-up and surmising persistent delayed post-viral psychiatric sequelae, here we aimed at studying the psychopathological impact of COVID-19 in survivors one year after clinical recovery also considering the effect of possible risk factors and the change of psychopathology over time.
2. Materials and methods
2.1. Participants and study design
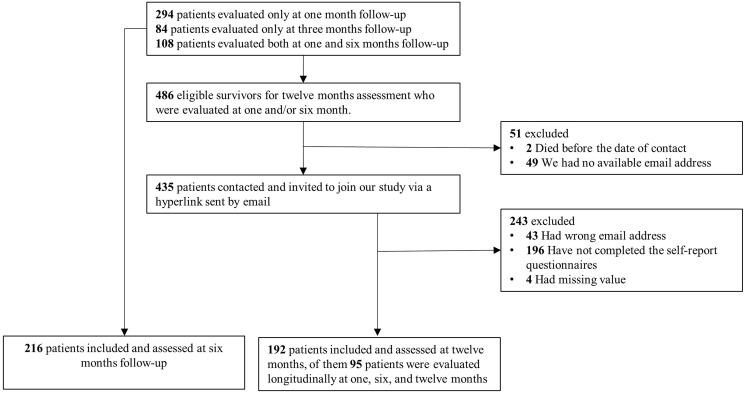
We prospectively evaluated the psychopathological status of COVID-19 survivors, one (31.29 ± 15.7 days), six (190.17 ± 19.78 days), and twelve (387.39 ± 23.67 days) months after hospital discharge during an ongoing longitudinal cohort study at IRCCS San Raffaele Hospital in Milan. From February 25, 2020, since the beginning of COVID-19 outbreak in Italy (WHO), all SARS-CoV-2 infected patients admitted to emergency department were consecutively enrolled in the COVID-BioB study. Then, one month follow-up was performed from April 6 to June 9, 2020; six months follow-up from August 30 to November 25, 2020; twelve months follow-up from April 27, 2021 to May 25, 2021. 402 COVID-19 survivors were evaluated one month after discharge (see Mazza et al., 2020), 216 patients (150 male, mean age 60.13 ± 12.21) were assessed at six months follow-up (108 patients from the initial cohort evaluated at one month follow up), and finally, 192 patients were assessed at one-year follow-up (131 male, mean age 59.16 ± 12.64). In addition, a subgroup of 95 (72 male, mean age 59.92 ± 11.57) patients was evaluated longitudinally at one, six, and 12 months (Fig. 1 ).
Fig. 1.
Flowchart of population selection.
At one- and six-months follow-up, evaluation was performed in an outpatient setting by trained psychiatrists in charge using an unstructured psychiatric interview and validated self-report questionnaires. At one-year follow-up, data were collected using encrypted hyperlinks to an online platform.
Inclusion criteria were SARS-CoV-2 confirmed by positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasopharyngeal and/or throat swab and clinical and radiological findings suggestive of COVID-19 pneumonia at the hospital admission. To keep a naturalistic study design, exclusion criteria were limited to patients under 18 years and difficulty to fully understand self-report questionnaires due to linguistic barrier or intellectual disability.
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (Supplementary Material, eMethods 1). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent to participate in the study was obtained from all participants. All procedures involving human patients were approved by Ethics Committee of San Raffaele Hospital (COVID-BioB protocol NCT04318366).
2.2. Measures
Sociodemographic and clinical data were collected using a data extraction form, including age, sex, psychiatric history, need of hospitalization for COVID-19, duration of hospitalization, need of noninvasive ventilation (NIV), and intensive care unit admission (ICU).
We gathered measures to evaluate the severity of psychopathological sequelae. Specifically, participants were requested to complete the Zung Severity Rating Scale (ZSDS) (Zung, 1965), the Fatigue Severity Scale (FSS) (Krupp, L. B. et al., 1989), the Impact of Event Scale-Revised (IES-R) (Creamer et al., 2003), and the State-Trait Anxiety Inventory form Y (STAI-Y) (Vigneau and Cormier, 2008). ZSDS, IES-R, and STAI-Y were available at one-, six-, and twelve-months follow-up, while FSS was available only at twelve months follow-up. Furthermore, participants were asked about their need for psychopharmacological drugs (antidepressants, anxiolytics, hypnotics, and sedatives) and psychological or psychiatric consultation in the 12 months after COVID-19.
2.2.1. Zung Severity Rating Scale
The ZSDS is a self-reporting instrument which consists of 20-item scale assessing the full spectrum of symptoms related to depressive episodes. Depression is characterized by feelings of sadness and lack of interest or pleasure in previously rewarding or enjoyable activities. The respondent was required to specify on a 4-point Likert scale the frequency with which each symptom occur over the past several days. Commonly accepted cut-off to define depressive's clinical relevance was used (ZSDS index ≥50) (Zung, 1967). The ZSDS scale demonstrated high levels of reliability (Cronbach's alpha = 0.82) (de Jonghe and Baneke, 1989) and it was largely employed in several studies investigating COVID-19 triggered depression (Deng et al., 2020; Mazza et al., 2020, 2021).
2.2.2. Fatigue Severity Scale
FSS questionnaire includes 9 statements aiming at exploring severity of fatigue symptoms by asking subject to choose for each item the number from 1 to 7 which best applied to him/her. The fatigue is defined as a persistent feeling of physical and mental tiredness characterized by lack of energy, weakness, slowed reactions, and drowsiness. The instrument exhibited an excellent internal consistency (Cronbach's alpha = 0.93) and an optimal validity as well (Ozyemisci-Taskiran et al., 2019). Originally conceived to differentiate depression to fatigue, it proves advantageous to assess fatigue in the aftermath of COVID-19 (Ortelli et al., 2021). We employed the standard cut-off (FSS mean ≥ 4) to determine clinically significant levels of fatigue (Krupp, Lauren B et al., 1989).
2.2.3. Impact of Event Scale-Revised
The IES-R consists of 22 item exploring the extent to which one develop trauma-evoking distress. It showed optimal psychometric properties (Cronbach's alpha = 0.96) and resulted effective in evaluating post-traumatic distress triggered by COVID-19 pandemic (Aljaberi et al., 2021). Commonly accepted cut-off (IES-R score ≥33) was implemented to identify participants who fell in the pathological range (Tiemensma et al., 2018).
2.2.4. State-Trait Anxiety Inventory form Y
Finally, we employed STAI-Y inventory to evaluate subject's levels of anxiety on a likert scale ranging from 1 to 4. Anxiety is an emotion characterized by feelings of tension, worried thoughts, and physical changes. The instrument demonstrated optimal internal consistency (0.67–0.91) and was successfully used to define pathological anxiety in several cohorts of COVID-19 survivors in accordance with clinically suggested cut-off score (STAI state ≥40) (Mazza et al., 2020; Prete et al., 2020).
2.3. Statistical analysis
Statistical analyses to compare group means and frequencies (Student's t-test, Pearson χ2 test) exploring effects of sex and previous history of psychiatric illness on symptoms severity were performed.
Listwise deletion method was used in handling missing data, so cases reporting missing data in any single variable were excluded from the analysis. Pearson's correlation analysis was performed to explore the correlation between age, duration of hospitalization, and psychopathology scores at six and twelve months. When appropriate, levels of significance were corrected for multiple comparisons with the adaptive linear step-up procedures that control the FDR and q-values (FDR-adjusted p-value) were considered.
To investigate psychopathology changes over time, repeated measures ANOVAs (according to sex and psychiatric history) were performed, considering ZSDS, IES-R, and STAI-Y at one, six, and twelve months follow-up. Differences in psychopathology scores according to the severity of COVID-19 infection, as proxied by the need of hospitalization, NIV, and ICU admission were assessed through one-way analysis of variance (ANOVA).
To account for the multiple covaring variables, considering the a priori expected collinearity between psychopathological dimensions, we performed three separate multivariate regressions entering as predictors each psychopathological domain at one month (ZSDS, STAI, and IES-R) and as dependent variables twelve months ZSDS, IES-R, and STAI scores while controlling for sex and psychiatric history. We also calculated the statistical significance of the effect of the single independent factors on the dependent variables by parametric estimates of predictor variables (least squares method). Statistical significance was set at p < 0.05.
All the statistical analyses were performed with a commercially available software package (StatSoft Statistica 12, Tulsa, OK, USA) and following standard computational procedures.
3. Results
The sociodemographic and clinical characteristics of the patients are listed in Table 1 . See Mazza et al., 2020 for the clinical and psychopathological description of the sample at one month follow-up.
Table 1.
Psychopathology at six and twelve months in patients surviving COVID-19 infection, divided according to sex and psychiatric history.
| Sex |
Psychiatric History |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Whole sample (n=216) | Male (n=150) | Female (n=66) | t, F or χ2 | q-value | Positive (n=47) | Negative (n=169) | t, F or χ2 | q-value | |
| Six months follow-up | |||||||||
| Male (n-%) | 150–69 | – | – | – | – | 25–53 | 125–74 | 7.48 | 0.007* |
| Age (mean ± SD) | 60.13 ± 12.21 | 60.15 ± 11.44 | 60.09 ± 13.9 | 0.03 | 0.972 | 58.36 ± 11.14 | 60.63 ± 12.48 | −1.13 | 0.262 |
| Depression score (ZSDS index, mean ± SD) | 42.09 ± 10.12 | 40.26 ± 9.6 | 46.27 ± 10.1 | −4.17 | <0.001* | 48.01 ± 9.91 | 40.45 ± 9.58 | 4.75 | <0.001* |
| Presence of depression (ZSDS index>50, Yes-%) | 55–25 | 30–20 | 25–38 | 7.72 | 0.006* | 21–45 | 34–20 | 11.69 | 0.001* |
| PTSD score (IES-R, mean ± SD) | 18.39 ± 18.7 | 15.43 ± 16.88 | 25.14 ± 20.91 | −3.61 | 0.001* | 29.09 ± 21.7 | 15.42 ± 16.66 | 4.64 | <0.001* |
| Presence of PTSD (IES-R>33, Yes-%) | 47-22% | 24–16 | 23–34 | 9.56 | 0.003* | 19–40 | 28–17 | 12.29 | 0.001* |
| Anxiety score (STAI state, mean ± SD) | 36.24 ± 11.13 | 34.56 ± 10.86 | 40.06 ± 10.87 | −3.43 | 0.002* | 43.06 ± 11.68 | 34.34 ± 10.23 | 5.01 | <0.001* |
| Presence of anxiety (STAI state>40, Yes-%) |
71–32 |
39–26 |
32–48 |
10.5 |
0.001* |
27–57 |
44–26 |
16.44 |
<0.001* |
| One year follow-up | |||||||||
|
Whole sample (n=192) |
Male (n=131) |
Female (n=61) |
t, F or χ2 |
q-value |
Positive (n=47) |
Negative (n=145) |
t, F or χ2 |
q-value |
|
| Male (n-%) | 131–68 | – | – | – | – | 22–47 | 110–76 | 13.41 | 0.001* |
| Age (mean ± SD) | 59.16 ± 12.65 | 60.60 ± 11.28 | 56.07 ± 14.82 | 2.34 | 0.023* | 55.00 ± 13.85 | 60.50 ± 11.98 | −2.63 | 0.010* |
| Depression score (ZSDS index, mean ± SD) | 45.79 ± 13.04 | 43.67 ± 12.28 | 50.33 ± 13.55 | −3.38 | 0.004* | 52.13 ± 13.33 | 43.73 ± 12.30 | 3.98 | <0.001* |
| Presence of depression (ZSDS index>50, Yes-%) | 59–31 | 33–25 | 26–43 | 5.94 | 0.019* | 24–51 | 35–24 | 12.09 | 0.001* |
| Fatigue score (FSS corrected, mean ± SD) | 3.40 ± 1.56 | 3.17 ± 1.42 | 3.88 ± 1.73 | −3.02 | 0.004* | 4.05 ± 1.62 | 3.18 ± 1.48 | 3.41 | 0.001* |
| Presence of fatigue (FSS corrected>4 Yes-%) | 63–33 | 37–28 | 26–43 | 3.9 | 0.048* | 23–49 | 40–28 | 7.34 | 0.008* |
| PTSD score (IES-R, mean ± SD) | 22.14 ± 19.89 | 18.28 ± 18.22 | 30.41 ± 20.94 | −4.09 | 0.001* | 30.04 ± 19.71 | 19.57 ± 19.33 | 3.21 | 0.002* |
| Presence of PTSD (IES-R>33, Yes-%) | 53–28 | 27–21 | 26–43 | 10.09 | 0.004* | 19–40 | 34–23 | 5.12 | 0.024* |
| Anxiety score (STAI state, mean ± SD) | 38.79 ± 12.09 | 36.98 ± 11.49 | 42.67 ± 12.52 | −3.11 | 0.004* | 45.53 ± 13.50 | 36.60 ± 10.77 | 4.63 | <0.001* |
| Presence of anxiety (STAI state>40, Yes-%) | 77–40 | 36–27 | 31–51 | 9.98 | 0.004* | 28–60 | 39–27 | 16.68 | <0.001* |
Note. Levels of significance of the observed differences (Student's t-test and Chi-square) is reported as q-value (FDR corrected p-value). Patients self-rated depression on the Zung Self-rating Depression Scale (ZSDS), fatigue on the Fatigue Severity Scale (FSS), Post-Traumatic symptoms on the Impact of Event Scale – Revised (IES-R), anxiety on the State Anxiety Inventory (STAI). *q < 0.05.
At six and twelve months after infection, respectively 44% and 45% of the sample self-rated above the clinical threshold in at least one of the psychopathological dimensions (as rated on ZSDS, IES-R, STAI). Pathological fatigue scores at twelve months were detected in 63 patients (33%). Females and patients with a positive psychiatric history exhibit increased scores in all the psychopathological domains (Table 1). On the contrary, the clinical severity of COVID-19 infection and related setting of care did not affect psychopathology at six and twelve months (Supplementary Material, eTable 1).
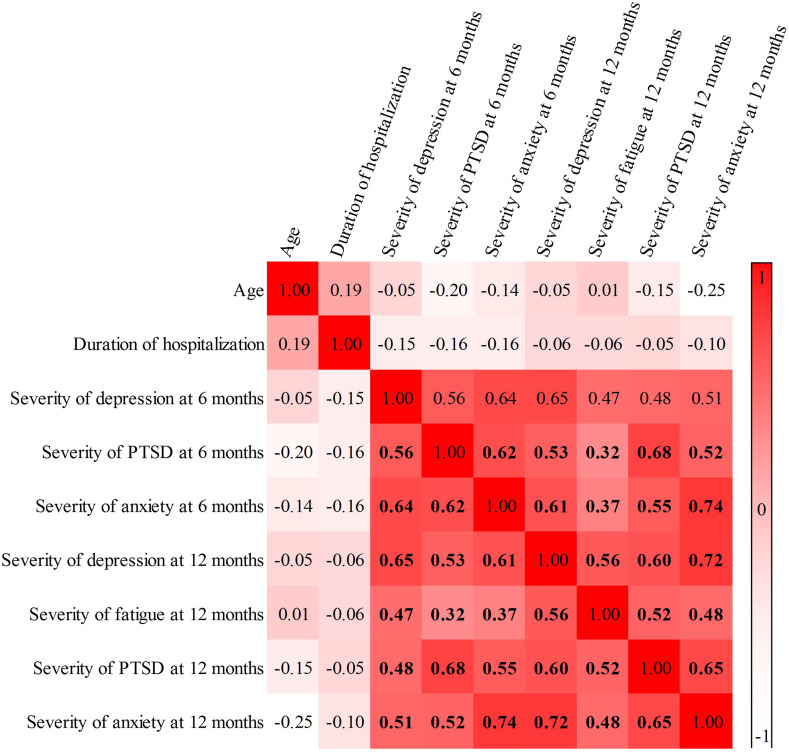
Correlation analysis revealed that self-rated psychopathology indexes were highly correlated over time (Fig. 2 ).
Fig. 2.
Correlation matrix exploring the association of continuous variables. Values in bold are significant (q < 0.05). Patients self-rated depression on the Zung Self-rating Depression Scale, fatigue on the Fatigue Severity Scale Post-Traumatic symptoms on the Impact of Event Scale – Revised, and anxiety on the State Anxiety Inventory.
Considering the need for psychopharmacological treatment in the year following Sars-CoV-2 infection, we found that 53 (27.60%) patients received medications. In detail, 27 (14.06%) started an antidepressant, 22 (11.46%) an anxiolytic, and 28 (14.58%) needed hypnotics. Moreover, 21 (10.94%) of the respondents contacted a mental health care professional during the year after COVID-19: 19 (9.90%) required a psychological intervention and 8 (4.17%) consulted a psychiatrist.
Moreover, the three multivariate GLM models revealed that each psychopathological dimension at one month, irrespectively of sex and previous psychiatric history, significantly predicted current psychopathological status (ZSDS: Wilks = 0.82, F = 10.99, ηp2 = 0.18, p < 0.001; STAI: Wilks = 0.73, F = 16.38, ηp2 = 0.27, p < 0.001; IES-R: Wilks = 0.66, F = 24.56, ηp2 = 0.34, p < 0.001) (Table 2 ). Specifically, in all models, univariate testing confirmed that only psychopathology at one month, and not sex and previous psychiatric history, dictated the entire twelve months psychopathology: i) ZSDS score at one month associated to ZSDS (β = 0.47, F = 31.05, p < 0.001), IES-R (β = 0.42, F = 23.28, p < 0.001), and STAI (β = 0.40, F = 21.15, p < 0.001) at twelve months; ii) STAI score at one month associated to ZSDS (β = 0.47, F = 28.81, p < 0.001), IES-R (β = 0.46, F = 27.56, p < 0.001), and STAI (β = 0.57, F = 49.23, p < 0.001) at twelve months; iii) IES-R score at one month associated to ZSDS (β = 0.48, F = 33.26, p < 0.001), IES-R (β = 0.64, F = 73.94, p < 0.001), and STAI (β = 0.49, F = 36.91, p < 0.001) at twelve months.
Table 2.
Three regression models with depression (ZSDS index), anxiety (STAI-state), and PTSD (IES-R) at one-month respectively as independent variables predicting the entire psychopathology at twelve-month in each model. Multivariate and univariate statistics are reported. *p < 0.05
| Multivariate |
Univariate |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Value | F | p | Beta (ß) | ZSDS index F | ZSDS index p | Beta (ß) | IES-R F | IES-R p | Beta (ß) | STAI F | STAI p | |
| Depression (ZSDS index) | Wilks | 0.82 | 10.99 | <0.001* | 0.47 | 31.05 | <0.001* | 0.42 | 23.28 | <0.001* | 0.40 | 21.15 | <0.001* |
| Sex | Wilks | 0.98 | 1.04 | 0.378 | −0.03 | 0.13 | 0.722 | 0.09 | 1.04 | 0.308 | −0.00 | 0.01 | 0.915 |
| Psychiatric history |
Wilks |
0.97 |
1.50 |
0.216 |
−0.12 |
2.59 |
0.109 |
−0.07 |
0.76 |
0.383 |
−0.16 |
3.92 |
0.050 |
| Anxiety (STAI-state) | Wilks | 0.73 | 16.38 | <0.001* | 0.47 | 28.81 | <0.001* | 0.46 | 27.56 | <0.001* | 0.57 | 49.23 | <0.001* |
| Sex | Wilks | 0.97 | 1.15 | 0.334 | 0.03 | 0.17 | 0.68 | 0.09 | 1.36 | 0.245 | −0.02 | 0.09 | 0.765 |
| Psychiatric history |
Wilks |
0.98 |
1.09 |
0.357 |
−0.12 |
2.08 |
0.15 |
−0.03 |
0.14 |
0.712 |
−0.10 |
1.81 |
0.181 |
| PTSD (IES-R) | Wilks | 0.66 | 24.56 | <0.001* | 0.48 | 33.26 | <0.001* | 0.64 | 73.94 | <0.001* | 0.49 | 36.91 | <0.001* |
| Sex | Wilks | 0.99 | 0.18 | 0.913 | 0.00 | 0.00 | 0.972 | 0.04 | 0.25 | 0.616 | −0.01 | 0.01 | 0.931 |
| Psychiatric history | Wilks | 0.96 | 1.72 | 0.166 | −0.09 | 1.37 | 0.243 | 0.01 | 0.01 | 0.930 | −0.13 | 2.86 | 0.093 |
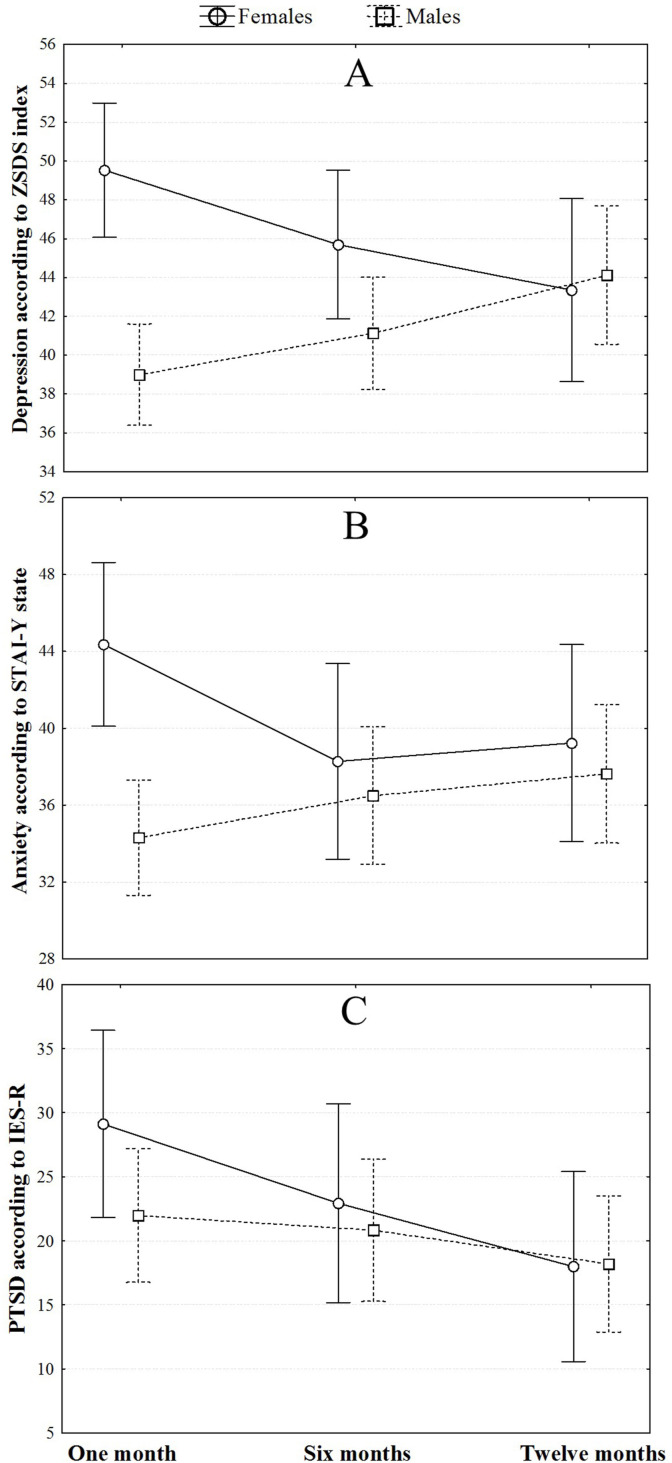
Considering the longitudinal cohort at one, six, and twelve-month follow-up time points, no effect of time was detected in changing depressive (F = 0.20, p = 0.819) and anxiety (F = 0.89, p = 0.414) symptomatology. However, an interaction effect of sex and time was observed both for depression (time*sex interaction F = 8.63, ηp2 = 0.10, p < 0.001) and anxiety (time*sex interaction F = 5.42, ηp2 = 0.08, p = 0.005) with females showing a significant decreasing trend of symptoms, whereas an opposite course was observed in men (Fig. 3 A and B). With regards to post-traumatic symptomatology, repeated measure ANOVA showed a significant reduction of post-traumatic symptoms over time with no effect of sex (F = 5.87, ηp2 = 0.07, p = 0.004) (Fig. 3C). No interaction effect of psychiatric history and time was found for depression, anxiety and PTSD.
Fig. 3.
Repeated measures ANOVAs considering the change over time of ZSDS (A), STAI-Y state (B), and IES-R (C) according to sex.
4. Discussion
This is the first longitudinal cohort study to investigate psychopathological sequelae in COVID-19 survivors during the 12 months after the outbreak. We found that exposure to SARS-CoV-2 has a long-term adverse mental health impact on survivors. We reported a high point prevalence of depression, anxiety, PTSD, and fatigue at both six- and twelve-months follow-up considering that more than 45% of the sample self-rated above the clinical threshold in at least one of the psychopathological dimensions, and 28% needed psychotropic medication. When exploring the longitudinal course of psychopathology during the year after infection, we observed a reduction over time of PTSD irrespectively of sex. Interestingly, we found an increase of depression and anxiety symptomatology in males and an opposite decreasing trend of symptoms in females.
The impact of COVID-19 on mental health can then be long-lasting, prolonging well beyond the acute or subacute stages. Short-term studies investigating one- or three-months follow-up after discharge found significant levels of depression, anxiety, PTSD, and insomnia (Mazza et al., 2020, 2021). To date, the longest follow-up study examining neuropsychiatric sequelae six months after discharge (Taquet et al., 2021). Among 236379 COVID-19 survivors the 24% of them were affected by mood, anxiety, or psychotic disorders. Moreover, more common neuropsychiatric complaints were reported in patients who had COVID-19 than in those who had influenza or other respiratory tract infection. Consistently with our findings, Taquet et al. did not find a relationship between psychiatric disorders and COVID-19 clinical severity but only between neurological outcomes and COVID-19 severity, thus suggesting that psychiatric symptomatology was not a manifestation of physical symptoms.
Despite still largely unknown, the underlying pathophysiological mechanisms of neuropsychiatric post-COVID complications seem to mainly entail immune dysregulation, inflammation, and psychosocial impacts of infection (Muccioli et al., 2020; Troyer et al., 2020). Even if there is no convincing evidence of direct SARS-CoV-2 neuroinvasion, post mortem studies have shown that SARS-CoV-2 related immune dysregulation and systemic inflammation can induce brain damage, disrupting the blood-brain barrier (BBB), thus driving inflammation in the central nervous system (CNS) (Reichard et al., 2020). The link between inflammation and psychiatric pathophysiology is well described and might partially explain the post-COVID sequelae (Najjar et al., 2013). Inflammation is known to induce microglial activation, neurotransmission alteration, indoleamine 2,3-dioxygenase 1 (IDO) activation and subsequent serotonin depletion, and oxidative stress, all of them mechanism involved in psychiatric pathophysiology (Benedetti et al., 2020). Consistently, we have found that anxiety and depression at one month and depression at three months after COVID-19, were predicted by higher baseline systemic immune-inflammation index (SII) (Mazza et al., 2020, 2021) and prevented by cytokine-blocking agents, dampening systemic inflammation. Accordingly, Yuan et al. reported higher depression in convalescent COVID-19 patients with higher NLR. As such, we surmise that COVID-19 could result in prolonged inflammation triggered by infection and by infection-related systemic inflammation, but then persisting on its own causing neuropsychiatric symptomatology.
According to literature and consistent with our findings at one- and three-month's follow-up study, we observed that females and patients with positive psychiatric history showed higher point prevalent psychopathology (Mazza et al., 2020, 2021; Vindegaard and Benros, 2020). However, when entering all clinical predictors in multivariate regression, only psychopathology at one-month follow-up was significantly associated with psychopathology at one year. This finding has a direct clinical relevance in order to prioritize target population for long-term follow-up care basing on acute or sub-acute clinical predictors.
Notably, when exploring the longitudinal course of symptomatology, we observed that only male COVID-19 survivors showed over the year after infection increasing depressive and anxiety psychopathology while an opposite trend was found for females. Males are characterized by a stronger age-dependent activation of the innate pro-inflammatory pathways compared to women (Márquez et al., 2020), leading to male's higher chronic subclinical systemic inflammation (inflammaging) and immune system impairment (immune senescence) (Bonafè et al., 2001). Inflammaging has been associated with both COVID-19 complications in males (Bonafè et al., 2020) and psychopathology (Diniz et al., 2019). Moreover, males are less likely to seek help for mental health difficulties, and they tend to hold more negative attitudes toward the use of mental health services compared to women (Holzinger et al., 2012).
Finally, at one year, we observed a high rate of fatigue symptomatology. Fatigue, is recognized as one of the leading complaints in COVID-19 survivors (Huang et al., 2021). More interesting, we observed that fatigue was not associated to COVID-19 clinical severity, but it highly correlated with psychopathology ratings. This finding suggests that rather independent of pneumonia severity, psychopathology after COVID-19 is associated with persistent fatigue, thus worsening the survivors' global functioning and quality of life.
Our results should be taken in the context of the following limitations. First, the relatively small sample size, also affected by listwise deletion of cases reporting missing data, may have limited the ability to identify risk factors due to low statistical power. Second, the monocentric nature of our study limits the generalizability of the findings raising the possibility of population stratification. Third, only 95 of 486 eligible survivors completed the three-point follow-up over 12 months; thus, the results might not be representative of the entire cohort. Moreover the small sample size of patients assessed longitudinally, and the lack of fatigue assessment at one and six months, limits the interpretation and generalizability of our findings. Fourth, the lack of a comparison group of subjects not affected by COVID-19 but experiencing the same psychological stressful situation (lockdown, fear, doubt, stigma, and social isolation) did not allow to disentangle the effect of COVID-19 infection from the psychological stressors. Finally, limited health care resources forced us to assessed psychopathology at 12 months using only self-rated questionnaires instead of a direct clinical interview. To overcome these limitations, further larger multicentric longitudinal studies also exploring the effect of heterogeneous predictors are needed.
In conclusion, for the first time we report a high rate of persistent mental health complaints at one year follow-up. This finding has significant clinical and healthcare-related implications. Given the size of the pandemic, the highly prevalent long-term post-COVID sequelae, the chronic or recurrent course of psychiatric disorders, and their consequences on the quality of life, substantial impact on health and social care systems are likely to occur in the following years. In this context, according to recent literature (Nalbandian et al., 2021), integrated multidisciplinary services, focused not only on physical sequelae but also on mental health distress, will be required. We suggest to routinely assess the psychopathology of COVID-19 survivors over time in order to promptly diagnose emergent disorders, and to treat them with the aim of reducing the disease burden and related years of life lived with disability.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributors
MGM conceived the study. MGM, MP, RDL, BB, and FB contributed to the inclusion of patients and acquisition of the data. MGM designed the analysis, with input from FB. MGM, MP, and BB carried out the analysis and interpreted the data, with contributions from all the authors. MGM and MP wrote the initial draft of the manuscript. All authors contributed to the final version, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
None.
Acknowledgments
Corporate authors: The COVID-19 BioB Outpatient Clinic Study group includes: Vai Benedetta, Bollettini Irene, Melloni Elisa Maria Teresa, Mazza Elena Beatrice, Aggio Veronica, Calesella Federico, Paolini Marco, Caselani Elisa, Colombo Federica, D’orsi Greta, Di Pasquasio Camilla, Fiore Paola, Calvisi Stefania, Canti Valentina, Castellani Jacopo, Cilla Marta, Cinel Elena, Damanti Sarah, Ferrante Marica, Martinenghi Sabina, Santini Chiara, Vitali Giordano.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2021.11.031.
Contributor Information
The COVID-19 BioB Outpatient Clinic Study group:
Benedetta Vai, Irene Bollettini, Elisa Maria Teresa Melloni, Elena Beatrice Mazza, Veronica Aggio, Federico Calesella, Marco Paolini, Elisa Caselani, Federica Colombo, Greta D’orsi, Camilla Di Pasquasio, Paola Fiore, Stefania Calvisi, Valentina Canti, Jacopo Castellani, Marta Cilla, Elena Cinel, Sarah Damanti, Marica Ferrante, Sabina Martinenghi, Chiara Santini, and Giordano Vitali
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aljaberi M.A., Alareqe N.A., Qasem M.A., Alsalahi A., Noman S., Al-Tammemi A., Mohamed Ibrahim M.I. Rasch modeling and multilevel confirmatory factor analysis for the usability of the impact of event Scale-Revised (IES-R) during the COVID-19 pandemic. 2021. J Available at SSRN 3815681. [DOI] [PMC free article] [PubMed]
- Benedetti F., Aggio V., Pratesi M.L., Greco G., Furlan R. Neuroinflammation in bipolar depression. Front. Psychiatr. 2020;11:71. doi: 10.3389/fpsyt.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafè M., Olivieri F., Cavallone L., Giovagnetti S., Marchegiani F., Cardelli M., Pieri C., Marra M., Antonicelli R., Lisa R. A gender–dependent genetic predisposition to produce high levels of IL‐6 is detrimental for longevity. Eur. J. Immunol. 2001;31(8):2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::AID-IMMU2357>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bonafè M., Prattichizzo F., Giuliani A., Storci G., Sabbatinelli J., Olivieri F. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer M., Bell R., Failla S. Psychometric properties of the impact of event scale—revised. Behav. Res. Ther. 2003;41(12):1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- de Jonghe J.F., Baneke J.J. The Zung self-rating depression scale: a replication study on reliability, validity and prediction. J. Psychol. Rep. 1989;64(3):833–834. doi: 10.2466/pr0.1989.64.3.833. [DOI] [Google Scholar]
- Deng J., Zhou F., Hou W., Silver Z., Wong C.Y., Chang O., Huang E., Zuo Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID‐19 patients: a meta‐analysis. Ann. N. Y. Acad. Sci. 2020 doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz B.S., Reynolds C.F., III, Sibille E., Bot M., Penninx B.W.H. Major depression and enhanced molecular senescence abnormalities in young and middle-aged adults. Transl. Psychiatry. 2019;9(1):1–10. doi: 10.1038/s41398-019-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A., Floris F., Schomerus G., Carta M., Angermeyer M. Gender differences in public beliefs and attitudes about mental disorder in western countries: a systematic review of population studies. Epidemiol. Psychiatr. Sci. 2012;21(1):73–85. doi: 10.1017/S2045796011000552. [DOI] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Manning K., Zvolensky M.J., Garey L., Long L.J., Gallagher M.W. The explanatory role of fatigue severity in the relation between COVID-19 perceived stress and depression, anxiety, and panic severity. Cognit. Behav. Ther. 2021:1–11. doi: 10.1080/16506073.2021.1874503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez E.J., Chung C.-h., Marches R., Rossi R.J., Nehar-Belaid D., Eroglu A., Mellert D.J., Kuchel G.A., Banchereau J., Ucar D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11(1):1–17. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F., group C.-B.O.C.S. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11(1):1–7. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. COVID-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann. Neurol. 2020;88:860–861. doi: 10.1002/ana.25855. [DOI] [PubMed] [Google Scholar]
- Najjar S., Pearlman D.M., Alper K., Najjar A., Devinsky O. Neuroinflammation and psychiatric illness. J. Neuroinflammation. 2013;10(1):1–24. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S. Post-acute COVID-19 syndrome. Nat. Med. 2021:1–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., Engl M., Romanello R., Nardone R., Bonini I., Koch G., Saltuari L., Quartarone A. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J. Neurol. Sci. 2021;420:117271. doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyemisci-Taskiran O., Batur E.B., Yuksel S., Cengiz M., Karatas G.K. Validity and reliability of fatigue severity scale in stroke. Top. Stroke Rehabil. 2019;26(2):122–127. doi: 10.1080/10749357.2018.1550957. [DOI] [PubMed] [Google Scholar]
- Passavanti M., Argentieri A., Barbieri D.M., Lou B., Wijayaratna K., Mirhosseini A.S.F., Wang F., Naseri S., Qamhia I., Tangerås M. The psychological impact of COVID-19 and restrictive measures in the world. J. Affect. Disord. 2021;283:36–51. doi: 10.1016/j.jad.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prete G., Fontanesi L., Porcelli P., Tommasi L. The psychological impact of COVID-19 in Italy: worry leads to protective behavior, but at the cost of anxiety. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.566659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemensma J., Depaoli S., Winter S.D., Felt J.M., Rus H.M., Arroyo A.C. The performance of the IES-R for Latinos and non-Latinos: assessing measurement invariance. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau F., Cormier S. The factor structure of the State-Trait Anxiety Inventory: an alternative view. J. Pers. Assess. 2008;90(3):280–285. doi: 10.1080/00223890701885027. [DOI] [PubMed] [Google Scholar]
- Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav. Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Zung W.W. A self-rating depression scale. Arch. Gen. Psychiatr. 1965;12(1):63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- Zung W.W. Factors influencing the self-rating depression scale. Arch. Gen. Psychiatr. 1967;16(5):543–547. doi: 10.1001/archpsyc.1967.01730230027003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.