Abstract
Background:
Peri-implantitis is an inflammatory response to bacterial biofilm resulting in bone loss and can ultimately lead to implant failure. Because of the lack of predictable treatments available, a thorough understanding of peri-implantitis's pathogenesis is essential. The objective of this study is to evaluate and compare the response of acute induced peri-implantitis and periodontitis lesions after insult removal.
Methods:
Implants were placed in one-month-old C57BL/6J male mice eight weeks post extraction of their left maxillary molars. Once osseointegrated, ligatures were placed around the implants and contralateral second molars of the experimental groups. Controls did not receive ligatures. After one week, half of the ligatures were removed, creating the ligature-retained and ligature-removed groups. Mice were sacrificed at two time points, 5 and 14 days, from ligature removal. The specimens were analyzed via micro-computed tomography and histology.
Results:
By 5 and 14 days after ligature removal, the periodontitis group experienced significant bone gain, whereas the peri-implantitis group did not. Histologically, all implant groups exhibited higher levels of cellular infiltrate than any of the tooth groups. Osteoclast numbers increased in peri-implantitis and periodontitis ligature-retained groups and decreased following insult removal. Collagen was overall more disorganized in peri-implantitis than periodontitis for all groups. Peri-implantitis experimental groups revealed greater matrix metalloproteinase-8 and NF-kB levels than periodontitis.
Conclusions:
Implants respond slower and less favorably to insult removal than teeth. Future research is needed to characterize detailed peri-implantitis disease pathophysiology.
Keywords: dental implant, ligature, mice, peri-implantitis, periodontitis
Titanium dental implants have revolutionized dentistry because of their ability to osseointegrate and have become one of the most desirable options to replace missing teeth in both fully and partially edentulous patients.1 Implant success and survival rates have often been used interchangeably, with a reported number of 94.6%.2 However, it has become clear that a surviving implant is not necessarily a successful implant. It is estimated that 48% of implants will present with some form of complication after 10 to 16 years3; one of these complications is peri-implantitis.
Peri-implantitis is an inflammatory condition characterized by irreversible “changes in the crestal bone in conjunction with bleeding on probing.”4 A 2016 study analyzed data collected from over 9000 patients in a Swedish population, and found that peri-implantitis affects 45% of all patients with implants, with 14.5% presenting with the moderate or severe form of the disease.5 An increasing number of implants are being placed yearly2; given the growing cumulative number of implants in function, the incidence of peri-implantitis cases is expected to rise accordingly.6 Additionally, peri-implantitis does not respond predictably to treatment and no protocol has been shown effective at arresting the condition or reversing it.6,7
There are different hypotheses on the etiology of peri-implantitis, such as a foreign body reaction to the implant, or occlusal overload.8,9 A well-supported hypothesis suggests that the etiology is similar to that of periodontitis; both conditions result as an overwhelming host immune response to bacterial insult.10–14 These two diseases also share the clinical characteristics of soft tissue inflammation and increased pocket depths in association with clinical attachment and bone loss.15–17 Given these similarities, contemporary therapeutic approaches for treating peri-implantitis stem from our understanding of periodontitis treatment.18,19 Both nonsurgical and surgical therapies have proven effective at treating periodontitis.20 However, nonsurgical therapy is ineffective at treating peri-implantitis, and although it has yielded promising outcomes, surgical therapy results in varying and unpredictable degrees of disease resolution.7,13 These diverse responses to treatment are suggestive of fundamental pathophysiological differences between the two diseases. For instance, some studies have found that peri-implantitis lesions had greater inflammatory infiltrates, extended apically to the bone, and did not reside in a well-defined compartment as in periodontitis lesions.17,21
We hypothesize that peri-implantitis is a disease with less potential for disease resolution than periodontitis. To test our hypothesis, we employed a split-mouth ligature murine model to evaluate and compare the response of acute induced (early-stage) peri-implantitis and periodontitis after removal of the noxious stimuli (ligature). The use of ligatures is a well-established model to induce inflammation given its ability to harbor periodontopathogens.22 Therefore, clinically, ligature removal represent the removal of bacteria harboring agents or conditions that lead to inflammation, such as calculus, cement, and overcontoured restorations, to name a few.
1. METHODS
Thirty-one one-month-old C57BL/6J male mice (The Jackson Laboratories, Bar Harbor, ME) were used following the guidelines and protocols of the Chancellor's Animal Research Committee of the University of California, Los Angeles, and that of Animal Research: Reporting In Vivo Experiments (ARRIVE). For the duration of the study, the mice were fed a soft diet ad libitum (Bio Serve; Frenchtown, NJ). Each experiment was performed once.
1.1. Tooth extraction and implant placement
One-month-old mice had their left maxillary molars extracted. After eight weeks of healing, custom-made screw-shaped machined-surface titanium implants (D. P. Machining Inc., La Verne, CA) were placed in the healed extraction sites and allowed to osseointegrate for four weeks (Figure 1A). Osseointegration was assessed through the application of wiggling forces to the implants using two dental explorers and observing or feeling for movement.23 Successfully integrated implants were then included in the study (n ≥ 3 for all groups/time points, Figure 1B).
FIGURE 1.
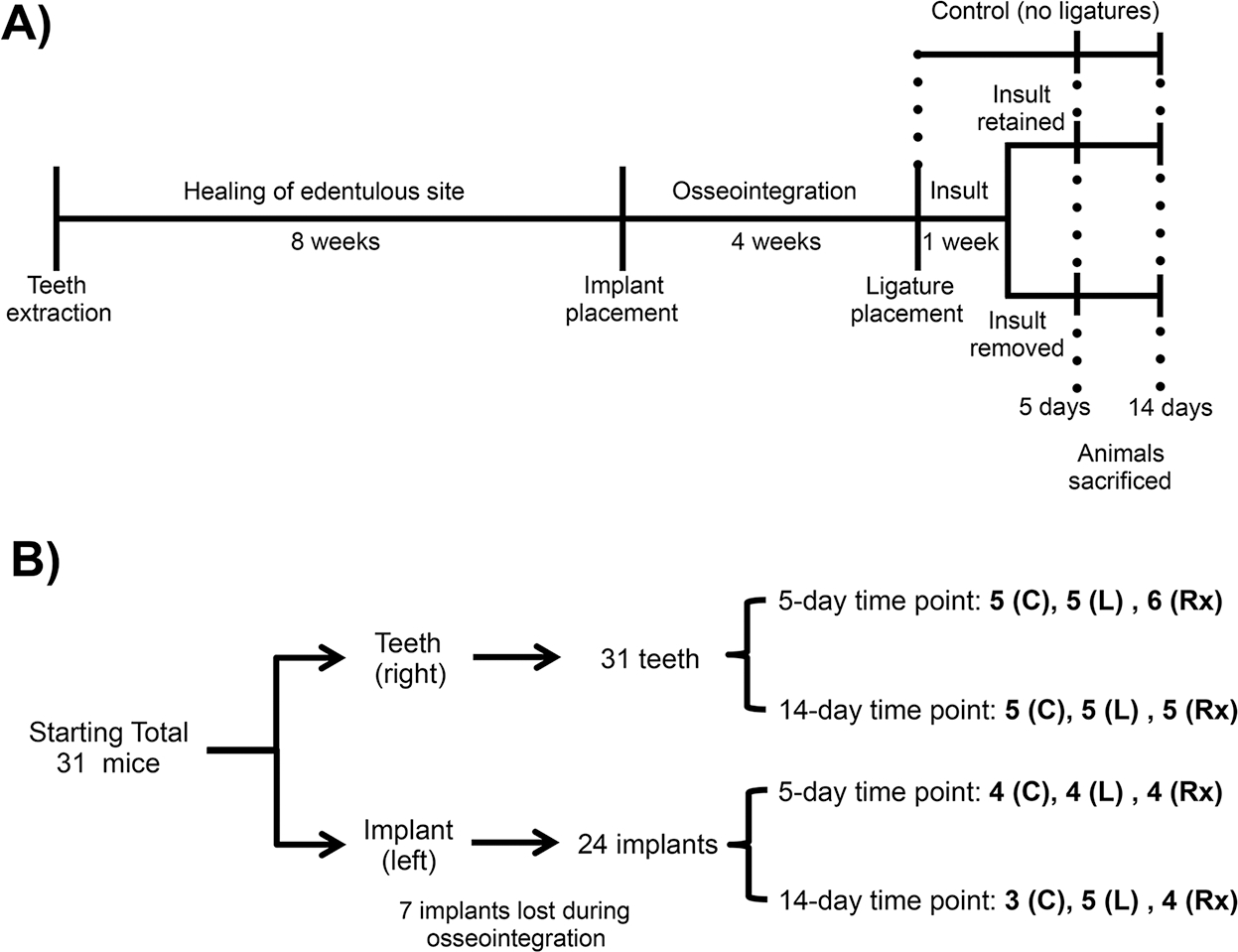
Schematic timetable of experimental design. A) Diagram illustrating sequence of events. B) Diagram showing the sample size for each group, control (C), ligature-retained (L), and ligature-removed (Rx)
1.2. Disease induction and insult removal
Mice were randomly separated into controls (no ligature) and experimental (ligature) groups. Using a split-mouth design, peri-implantitis and periodontitis were induced through the placement of 6–0 silk ligatures (P.B.N. Medicals, Stenløse, Denmark) around the head of the implants and around the contralateral second molars of the experimental groups.24 To assess tissue response to insult removal, ligatures were kept for one week without intervention. Following the initial week of ligature insult, half of the ligatures were removed, creating two subgroups: ligature-retained and ligature-removed. The ligature-retained group kept the ligatures for the duration of the experiment, whereas the ligature-removed group served as the intervention group. This resulted in three final groups: control (no ligature) (10 mice total), ligature-retained (10 mice total), and ligature-removed (11 mice total). At two time points, 5 and 14 days after ligature removal, all mice were sacrificed (Figure 1). After sacrifice, the maxillae were harvested, imaged using a digital optical microscope (VHX-1000; Keyence, Osaka, Japan), fixed in 10% formalin for 24 to 48 hours, and stored in 70% ethanol.
1.3. Micro-computed tomography analysis
Maxillae were scanned using micro-computed tomography (micro-CT) (Model 1172; SkyScan, Kontich, Belgium) at 10 micrometers resolution, and Xray energy of 55 KVp and 181 microampere. Linear (DOLPHIN software, Navantis, Toronto, Canada) bone height and volumetric circumferential (CTAn software, V.1.16 Bruker, Billerica, MA) bone loss analyses were performed. For linear measurements, the implants were oriented so that the body and head of the fixtures were perpendicular to each other in the sagittal and coronal planes; the teeth were oriented so that the cementoenamel junction (CEJ) was parallel in the coronal plane. The distance was measured from the junction of the head and body of the implant, and from the CEJ vertically down to the alveolar bone. A single blinded examiner (RW) collected data from four sites—mesial, distal, buccal, and palatal—and averaged them to a mean linear bone height value for each implant and second molar.24 For the volumetric analysis, the samples were oriented similarly as described in the linear analysis (DataViewer, V.1.5.2 Bruker, Billerica, MA). The range for volumetric measurements in the axial plane was determined by the lowest average of the linear measurement in the control groups and the highest average linear measurement in the ligature-retained groups. For implants, the bone loss around the fixtures was traced and averaged to determine the amount of circumferential volumetric bone loss per group. The criterion used for implants was “tissue volume (mm3).” For teeth, the area of the second molar traced was confined within the distal root of the first molar and the mesial root of the third molar. The criterion used to analyze the level of bone loss on teeth was “tissue volume – bone volume (mm3),” which was averaged per group. A single blinded examiner performed the volumetric analysis (MC).
1.4. Histology
After micro-CT scanning of the maxillae, the samples were decalcified for four weeks in 15% ethylenediaminetetraacetic acid (EDTA). After decalcification, the remaining ligatures were removed, and implants unscrewed by application of a counterclockwise motion. The implant sockets and teeth were sectioned in the sagittal plane. The tissues were then paraffin embedded and 5 μm-thick sections were obtained using a microtome (McBain Instruments, Chatsworth, CA). The tissues were stained with hematoxylin and eosin (H&E), tartrate-resistant acid phosphatase (TRAP) (Sigma Aldrich, MO), and picrosirius red (Polysciences, Inc. Warrington, PA) to assess cellular infiltrates, osteoclasts, and collagen organization. The number of osteoclasts was subsequently quantitated. TRAP+ cells with ≥2 nuclei lining the crestal bone adjacent to implants and teeth were averaged in the different groups and normalized to their respective controls (n = 3/group/time point). Additionally, the 5-day time point was further assessed via immunohistochemistry for matrix metalloproteinase-8 (MMP-8) (Abcam, Cambridge, MA) (1:100-dilution) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Abcam, Cambridge, MA) (1:250-dilution) which recognizes the active form of NFB, and the secondary antibody was anti-rabbit secondary antibody (Santa Cruz, Dallas, TX) (1:200-dilution). Sections were imaged (OLYMPUS U-Pot Lens, Shinjuku Tokyo, Japan) and the picriosirius red slides were captured under polarized light. The remaining stains were digitally imaged (Aperio Image Scope model V11.1.2.752, Vista, CA).
1.5. Statistical analysis
Bone height, volumetric measurements and osteoclast analysis were represented as mean ± standard error of the mean. Significance between groups compared using a two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test with a 95% confidence interval (Prism 5; GraphPad Software, Inc. La Jolla, CA).
2. RESULTS
2.1. Clinical assessment of peri-implantitis and periodontitis after insult removal
Immediately after the maxillae were harvested, images were taken to clinically evaluate soft tissue differences between the groups (Figure 2A). The resulting clinical images revealed soft tissue swelling in both the peri-implantitis and periodontitis ligature-retained groups; this swelling subsided following the removal of the ligatures in both time points. The gingiva of the ligature-retained groups appeared more edematous when compared to controls. In the ligature-removed groups of both peri-implantitis and periodontitis, tissue swelling decreased following the removal of the ligature insult. However, the peri-implantitis ligature-removed group appeared to have more residual soft tissue edema compared to the periodontitis ligature-removed group.
FIGURE 2.
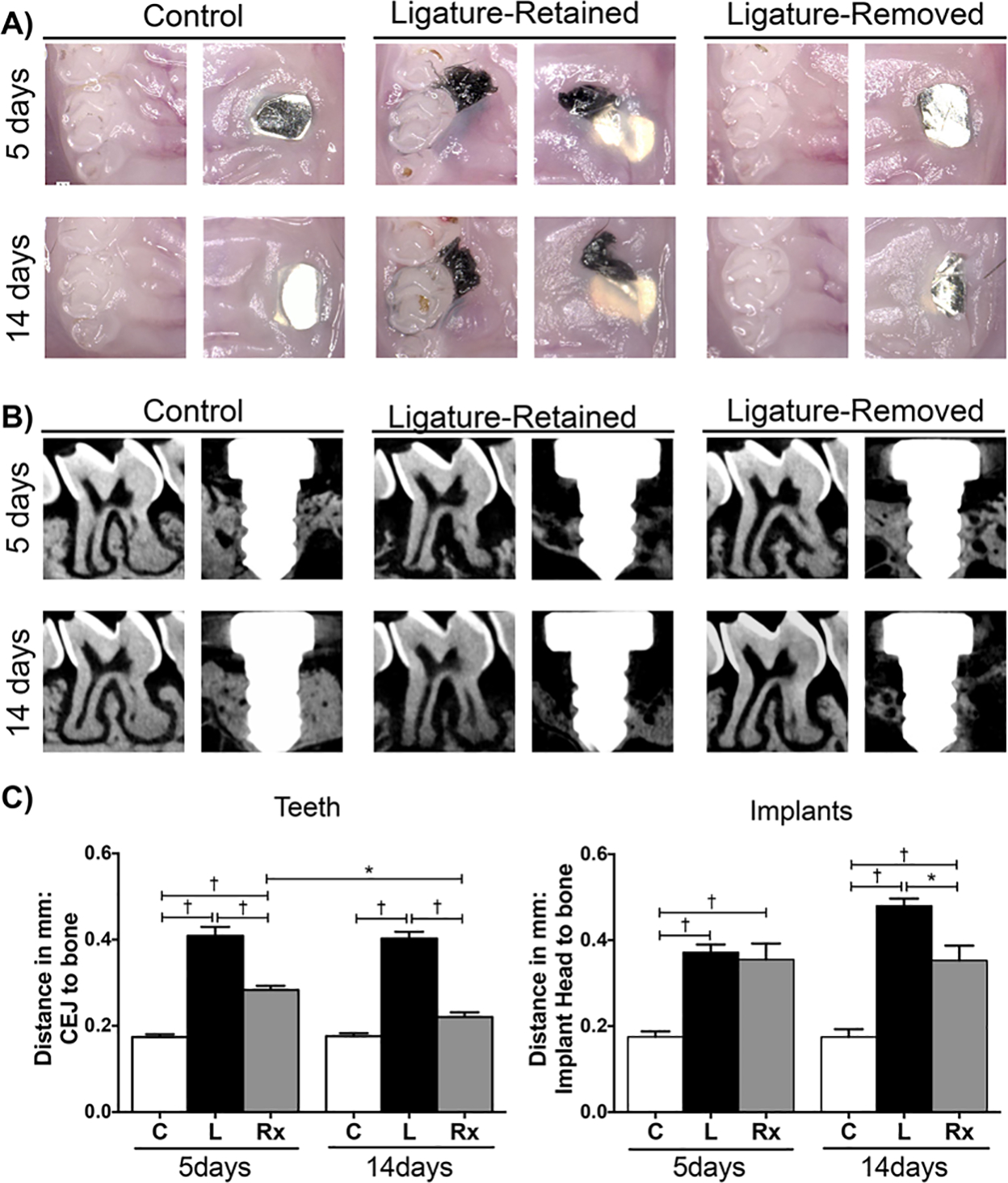
Representative clinical and radiographic images, and linear radiographic analysis of peri-implantitis and periodontitis after insult removal. A) Representative clinical images (50 × magnification) of control (no ligature), ligature-retained, and ligature-removed groups of teeth and implants. Images were taken after 5 (top row) and 14 (bottom row) days of ligature removal immediately after sacrifice. Soft tissue edema/inflammation present in peri-implantitis and periodontitis ligature-retained groups decreased after insult removal for both diseases. B) Representative sagittal micro-CT images of control (C), ligature-retained (L), and ligature-removed (Rx) groups of teeth and implants 5 and 14 days after ligature removal. C) Graphs represent averaged linear bone height measurements. In teeth, distance is measured from CEJ to the alveolar bone crest in teeth (left graph); in implants, from the implant head to the alveolar bone (right graph). Data are mean ± standard error of the mean. *p < 0.01, †p < 0.001, (n ≥ 3 for all groups/time points). Note that when comparing the ligature-removed groups at 5 and 14 days, significant bone formation resulted in teeth, but not in implants
2.2. Radiographic assessment of peri-implantitis and periodontitis after insult removal
To radiographically compare the ability of peri-implantitis and periodontitis to heal after ligature removal, the samples were scanned with micro-CT, and linear bone height and volumetric bone loss measurements were performed (Figure 2B and 2C, Figure 3A and 3B). The micro-CT data for both analyses followed a similar pattern. Utilizing controls as baseline, there was statistically greater bone loss in the peri-implantitis and periodontitis experimental groups at the 5 and 14-day time points. When assessing the intervention response in teeth at both time points following insult removal, the linear bone height was significantly more coronal in the periodontitis ligature-removed groups than the periodontitis ligature-retained groups, and the ligature-removed groups also had greater bone volume. After 14 days, the linear and volumetric values were statistically at the level of the control values, as significant bone gain was observed after ligature removal in teeth. Around implants, however, the linear bone height in the ligature-retained and ligature-removed groups was similar after 5 days. After 14 days, the bone height of the ligature-removed group remained unchanged from the 5-day time point. Volumetrically, the same pattern was observed, as the measured differences between the ligature-removed groups were not significant. Therefore, there was no bone gain nor bone loss progression around implants in the intervention groups, as the bone height and volume did not change between 5 and 14 days after ligature removal. Bone loss progression was observed in the peri-implantitis ligature-retained groups.
FIGURE 3.
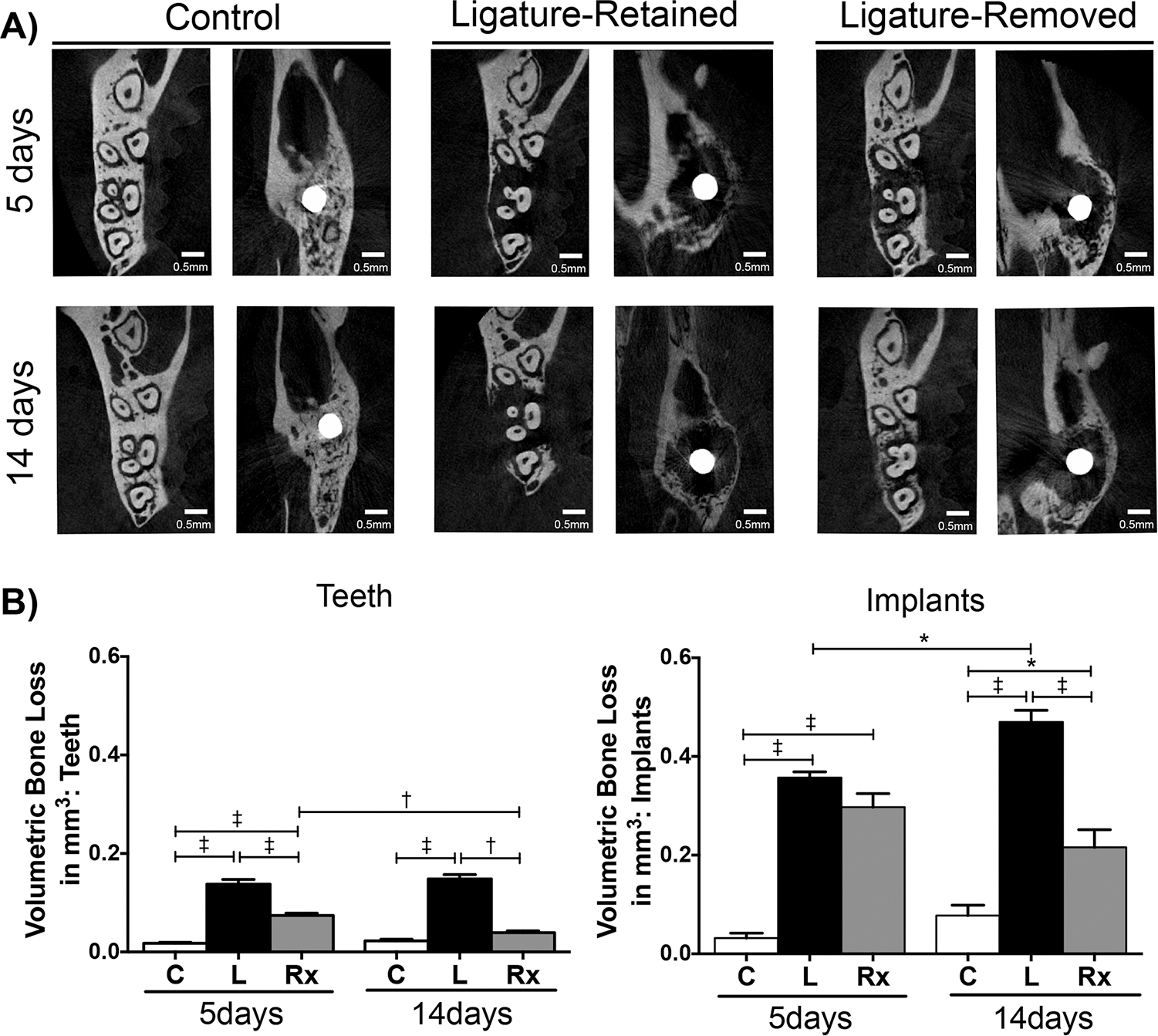
Representative micro-CT images and volumetric bone loss analysis of peri-implantitis and periodontitis after insult removal. A) Representative axial micro-CT images of control (C), ligature-retained (L), and ligature-removed (Rx) groups of teeth and implants 5 and 14 days after ligature removal. B) Graphs represent averaged volumetric bone loss measurements. For teeth (left graph), the criterion used to analyze the level of bone loss was tissue volume – bone volume (mm3). The criterion used for implants was tissue volume (mm3). Data are mean ± standard error of the mean. *p < 0.05, †p < 0.01, ‡p < 0.001, (n ≥ 3 for all groups/time points). Note that when comparing the ligature-removed groups at 5 and 14 days, there was a significant increase in bone volume around teeth, but not around implants
2.3. Histologic assessment of peri-implantitis and periodontitis after insult removal
The results demonstrate a difference in susceptibility between implants and teeth for developing clinically significant peri-implant and periodontal inflammation (Figure 4A and 4B, 5-day time point provided as supplementary Figure 1 in the online Journal of Periodontology). More inflammation was observed in the implants than in any of the groups of teeth, with or without ligature. On ligature placement, peri-implant tissues adjacent to the implants developed more acute inflammation, suggested by greater polymorphonuclear leukocytes infiltration (Figure 4A, green arrow) and vascularity (Figure 4A, yellow arrow), than the tissues adjacent to teeth. Both the peri-implantitis and periodontitis ligature-retained groups appeared to exhibit increased bone porosity after ligature placement. Removal of the ligature allowed the tissues to heal, but the peri-implantitis ligature-removed groups showed signs of slower recovery than the periodontitis ligature-removed groups (Figure 3B). Slight acute inflammation was observed in the peri-implantitis ligature-removed group, whereas only mild chronic inflammation was noted in the periodontitis ligature-removed group. Although lamellar and woven bone, as well as osteoid tissue (Figure 4B, green arrow), were noted in the interproximal bone of the periodontitis ligature-removed group, the alveolar bone surface in the implant ligature-removed group appeared to consist mainly of woven bone.
FIGURE 4.
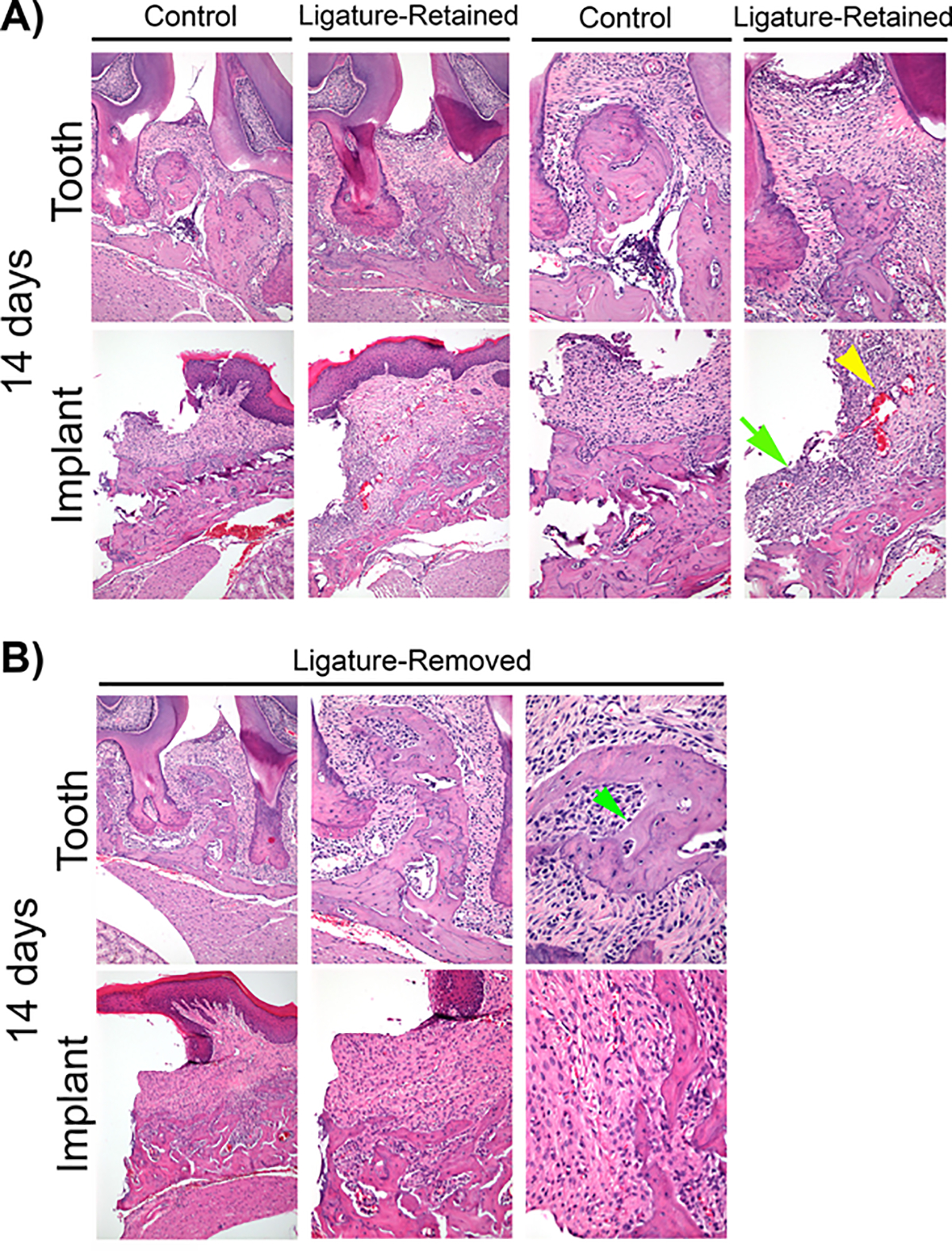
Histological evaluation of peri-implantitis and periodontitis after insult removal. A) Representative H&E images of peri-implantitis and periodontitis control and ligature-retained groups at the 14-day time point at 100 × (left) and 200 × (right) magnification. Note that the peri-implantitis ligature-retained group appeared to exhibit more acute inflammation (green arrow), and vascularity (yellow arrow head) than the periodontitis ligature-retained group. B) Representative H&E images of peri-implantitis and periodontitis ligature-removed groups 14 days after insult removal at 100 ×, 200 ×, and 400 × magnification. Note osteoid (green arrow) on the interproximal bone of the periodontitis ligature-removed group. The peri-implantitis ligature-removed group still exhibited slightly more acute inflammation, while only mild chronic inflammation is noted in the periodontitis ligature-removed group
2.4. Osteoclast assessment of peri-implantitis and periodontitis after insult removal
Osteoclasts play an integral role in bone remodeling and are responsible for the characteristic bone loss seen in peri-implantitis and periodontitis. Through TRAP staining, osteoclasts that had lined the crestal bone adjacent to teeth and implants were counted and normalized to their respective controls (n = 3/group/time point) (Figure 5A and 5B). In the peri-implantitis and periodontitis ligature-retained groups, there was an increase in the fold difference of osteoclasts when compared to their respective controls. After insult removal, there was a significant decrease in osteoclasts for both the peri-implantitis and periodontitis ligature-removed groups.
FIGURE 5.
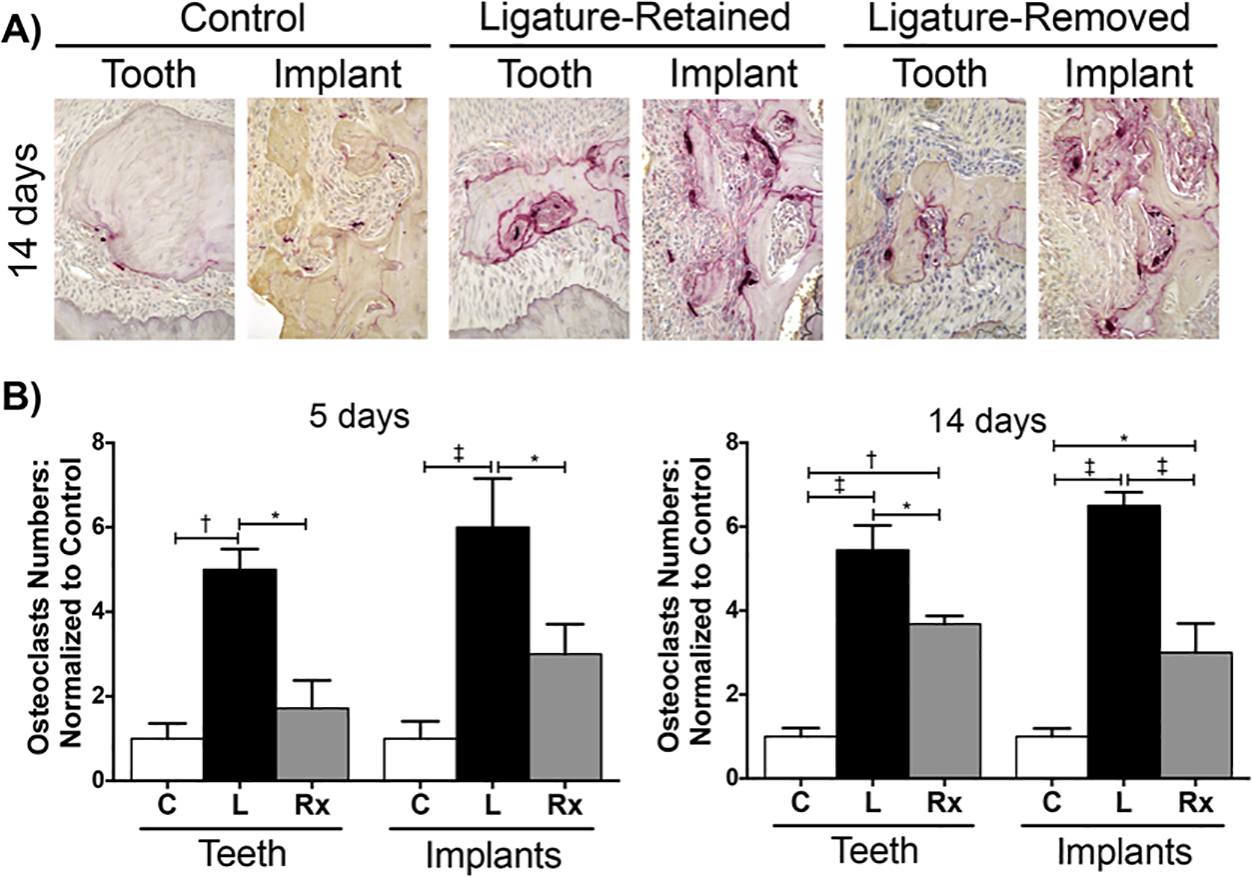
Osteoclast assessment of peri-implantitis and periodontitis after insult removal. A) Representative sagittal of TRAP stained images of peri-implantitis and periodontitis control (C), ligature-retained (L), and ligature-removed (Rx) groups 14 days after insult removal at 400 × magnification. B) Graphs represent the averaged number of osteoclasts at 5 and 14 days after insult removal in peri-implantitis and periodontitis experimental groups normalized to their respective controls (n = 3/group/time point). *p < 0.05, †p < 0.01, ‡p < 0.001 (n ≥ 3 for all groups/time points)
2.5. Collagen assessment of peri-implantitis and periodontitis after insult removal
Picrosirius red staining allows for the visualization of type 1 collagen as yellow birefringence under polarized light (Figure 6A). In control teeth, collagen fibers were present supracrestally and within the periodontal ligament (PDL) space perpendicular to the root surfaces and inserting into the cementum. In control implants, however, the supracrestal soft tissue appeared to contain less collagen and the collagen fibrillar structures were less organized. In the ligature-retained groups of both diseases, a reduction and disorganization of collagen in the supracrestal soft tissue was noted. In peri-implantitis, there was almost a complete obliteration of type 1 collagen, while some was retained in periodontitis. After ligature removal, re-establishment and re-organization of collagen in the soft tissues of both conditions were observed, but these features were more prominent in teeth as new PDL fibers were formed along the root surfaces with the bone gain observed.
FIGURE 6.
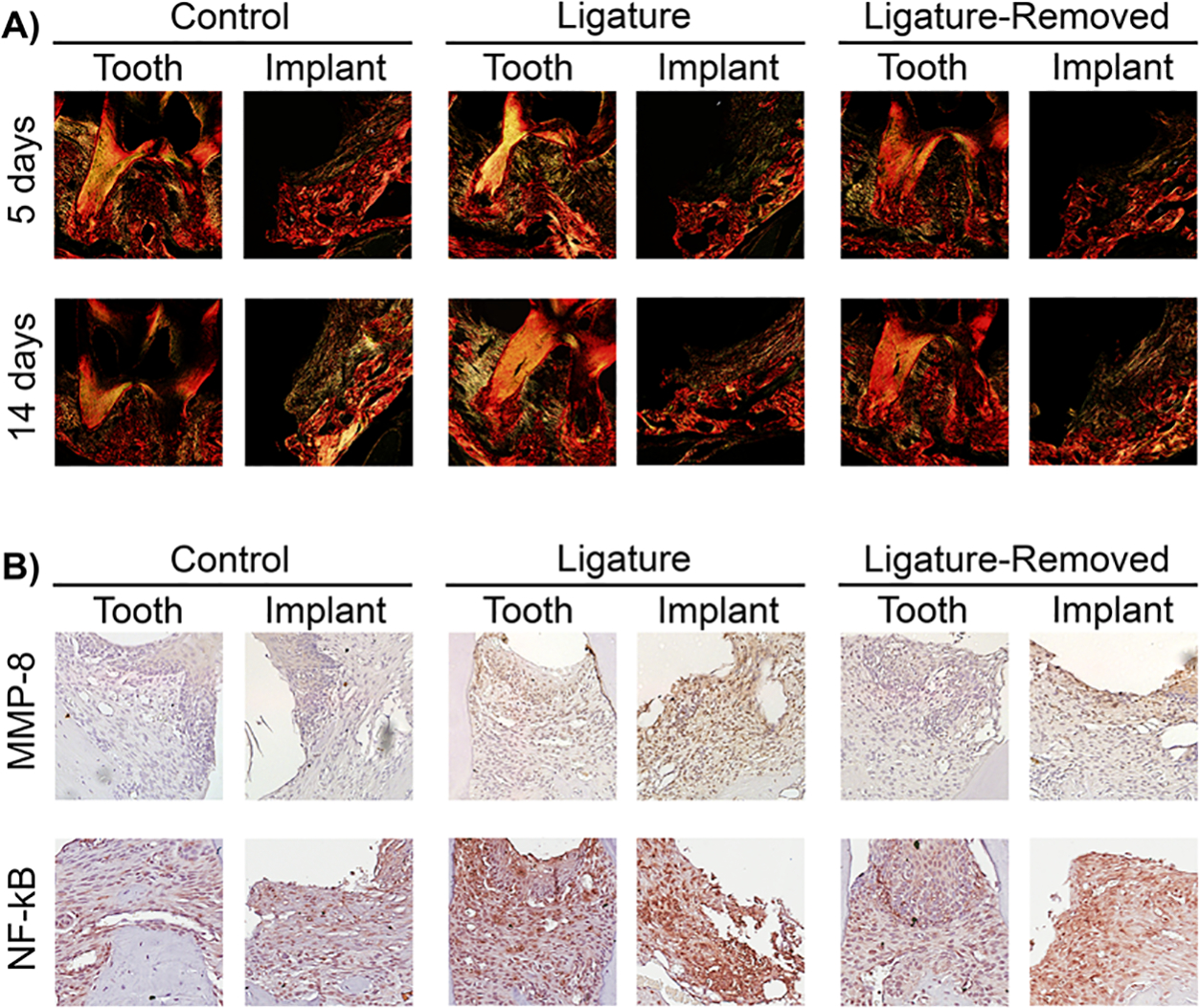
Type 1 collagen, MMP-8, and NF-κB assessment in peri-implantitis and periodontitis after insult removal. A) Representative sagittal picrosirius red stained images of peri-implantitis and periodontitis control, ligature-retained, and ligature-removed groups 5 and 14 days after ligature removal at 10 × magnification under polarized light. Note greater type 1 collagen (yellow birefringence) destruction around implants than around teeth in the ligature-retained groups. New collagen fibers around implants are more apparent after 14 days of insult removal. B) The representative images of MMP-8 staining of peri-implantitis and periodontitis control, ligature-retained, and ligature-removed groups 5 days after ligature removal at 20 × magnification (top row). Note greater MMP-8 in the peri-implantitis ligature-retained and ligature-removed groups than the periodontitis groups. The representative images of NF-κB staining of peri-implantitis and periodontitis control, ligature-retained, and ligature-removed groups 5 days after ligature removal at 20 × magnification (bottom row). Note the greater NF-κB staining in the peri-implantitis ligature-retained and ligature-removed groups than the periodontitis groups
2.6. MMP-8 and NF-κB assessment assessment of peri-implantitis and periodontitis after insult removal
Characterized as inflammatory conditions, peri-implantitis and periodontitis were further assessed via immunohistochemistry (IHC). Using the 5-day time point, MMP-8 (Figure 6B, top row), which is involved in degradation of the extracellular matrix, and NF-κB (Figure 6B, bottom row), which is involved in transcription of pro-inflammatory cytokines, were evaluated. Both IHC staining showed greater MMP-8 and NF-κB in the peri-implantitis and periodontitis ligature-retained groups when compared to their respective controls. Staining was less prominent following ligature removal. When comparing the peri-implantitis and periodontitis experimental groups to each other, peri-implantitis overall showed a greater staining of MMP-8 and NF-κB in both the ligature-retained and the ligature-removed groups.
3. DISCUSSION
In the United States, the market value of dental implants was estimated to be around $1billion in 2013.25 These numbers are expected to increase as more dentists continue to incorporate implant services into their practices. Given the scope of this growing market, dental professionals must be able to place implants, as well as treat complications that may arise. Dental implants can develop peri-implantitis, which is a disease that is not fully understood and current treatment protocols are unpredictable.5,12,26
Categorized as inflammatory conditions, insight of our knowledge of periodontitis has been instrumental in helping define the current understanding and treatment of peri-implantitis.21 However, methods that have been successful at treating periodontitis are not as effective at treating peri-implantitis, as the disease tends to progress despite our best efforts.11,12,27 Moreover, given that surgical therapy provides better results than non-surgical therapy, treatment of peri-implantitis also raises esthetic concerns.28 For the abovementioned reasons, research on peri-implantitis is critical, so as to better understand the disease and to develop predictable treatment protocols.
Researchers have been able to induce experimental periodontal bone loss in animal models through oral gavage of specific pathogens, lipopolysaccharide injections, and through the ligation of foreign materials around teeth.22,29–32 By applying these techniques to implants in animal models, studies have been able to induce some level of experimental peri-implant bone loss.17,23,24,33–35 Our study used the more widely accepted ligature model for several reasons: the bony defect induced by ligatures most closely resembles the configuration in humans, usually a 360 degree circumferential bony defect around the fixtures,36 and to bypass differences in bacterial colonization. In 1995, Marinello, et al, induced peri-implantitis in dogs with ligatures and treated the lesions by removing the ligatures from the implants. The study showed that peri-implantitis invariably progressed and took longer to be arrested.27 Employing this technique in our murine model, we aimed to further characterize how early-stage peri-implantitis and periodontitis respond following ligature removal.
Given that the key feature of peri-implantitis is bone loss, we performed linear bone height and volumetric bone loss measurements radiographically and found noteworthy differences between peri-implantitis and periodontitis in response to early intervention. By comparing the ligature-removed groups to their respective control and ligature-retained groups at the 5- and 14-day time points, significant differences in regenerative potential were observed. Following ligature removal, teeth experienced statistically significant new bone formation, as evidenced by the bone height moving coronally over time and nearing the height of the control group as the bone volume increased. Implants, on the other hand, did not experience radiographic bone formation; at 5 days, the bone height and volume of the ligature-removed group remained relatively unchanged when compared to the results at 14 days. Furthermore, when comparing the peri-implantitis ligature-removed group to the ligature-retained group at 5 days, the bone height was similar. These findings corroborate previous findings that peri-implantitis and periodontitis respond differently to insult removal.17 Moreover, this study supports the proposition that early intervention of peri-implantitis has the potential to halt disease progression as bone loss was discontinued on ligature removal after 14 days.37
Multiple studies have shown histological differences between peri-implantitis and periodontitis, as well as greater soft tissue inflammation around implants than around teeth.4,15,17,21,35 Our study supports these findings, as the implants were more susceptible to developing significant ligature-induced soft tissue inflammation than teeth. Differences between these two conditions were also observed following ligature removal, as the periodontal tissues in the ligature-removed groups showed lower signs of inflammation than the peri-implant soft tissues. Bone formation was observed around teeth, evidenced by the apposition of interproximal bone, which supports our micro-CT findings. Therefore, histologically, our study shows that periodontitis responds more favorably and faster than peri-implantitis after insult removal.
Osteoblasts and osteoclasts comprise the primary bone remodeling cells and intimately interact with each other to produce or resorb bone.38 Having assessed bone loss radiographically, we evaluated the cells responsible for bone resorption, osteoclasts, histologically through TRAP staining. As other studies have shown, ligature-induced lesions have an increase in the number of osteoclasts when compared to controls.24,39 We found the same pattern in our peri-implantitis and periodontitis ligature-retained groups. On insult removal, there was a statistically significant decrease in the osteoclast fold in both peri-implantitis and periodontitis by 5 days. The reduction of osteoclasts around implants supports the radiographic measurements, as bone loss did not progress by the 14-day time point in the peri-implantitis ligature-removed group.
To further explore the healing response of early-stage disease, we assessed the collagen composition and organization of the extracellular matrix in the soft tissues through picrosirius red staining. Under polarized light, collagen types were identified through differences in birefringence. Focusing on the most abundant type of collagen, type 1 collagen (yellow birefringence),40 great differences were found between peri-implantitis and periodontitis. Overall, teeth had more type 1 collagen than implants. In the peri-implantitis ligature-retained groups, almost all supracrestal collagen 1 fibers were lost, as evidenced by an absence of yellow birefringence signals; in the periodontitis ligature-retained groups, however, some supracrestal collagen fibers were preserved. Given that the extracellular matrix plays a major role in the structural integrity of tissues,40 it appears that the soft tissue around implants is highly affected by acute inflammation and its structural integrity is substantially reduced. After intervention, removal of the ligature allowed for the synthesis of new collagen fibers around implants, which became more apparent after 14 days. The new fiber bundles were manifested in a largely disorganized network when compared to controls. A previously published study suggested that peri-implant soft tissues have poorer mechanical resistance than periodontal soft tissues.41 Our study found that peri-implant soft tissue collagen is able to regain some structural integrity after insult removal; however, this newly formed collagen may not be as stable as that found in peri-implant tissues with no history of experimental peri-implantitis.
In order to further evaluate the soft tissue changes before and after intervention, we stained the 5-day time point for MMP-8 and NF-κB. MMP-8 is involved in collagen matrix degradation and has been shown to be elevated in both peri-implantitis and periodontitis.42–44 In our study, the peri-implantitis ligature-retained group stained higher levels of MMP-8 when compared to periodontitis. After ligature removal, the MMP-8 levels remained relatively higher in the peri-implantitis ligature-removed group. These data substantiate our picrosirius red findings, as the elevated MMP-8 levels correlate with the collagen destruction observed. More specifically, because MMP-8 was still mildly expressed after 5 days, it partially explains why new collagen fibers were not readily observed at that specific time point. Moreover, increased MMP-8 levels in humans are detected in disease sites with ongoing bone loss.45 Therefore, the delay in MMP-8 reduction in the peri-implantitis ligature-removed group, relative to the periodontitis group, could help explain why peri-implantitis takes longer to be arrested than periodontitis.
For the transcription factor NF-κB, a similar pattern was observed. NF-κB is involved in many regulatory mechanisms, such as cell survival and cytokine production,46 and its involvement in proinflammatory pathways is well documented.47 In patients with chronic periodontitis, NF- κB is expressed at higher levels than in patients with periodontal health.48 In addition to supporting this finding, our data showed that, relative to the periodontal tissues, the peri-implant tissues expressed greater levels of NF-kB across the ligature-retained and ligature-removed groups. With the assumption that the expressed NF-kB is proinflammatory, it appears that resolution of soft tissue inflammation after insult removal in periodontitis is faster than in peri-implantitis, which correlates with our histologic assessment of the H&E.
4. CONCLUSION
In conclusion, peri-implantitis does not respond as well to insult removal as periodontitis. Lack of bone formation, higher inflammatory levels, disorganized collagen networks, and delayed reduction of MMP-8 and NF-κB after ligature removal suggest that peri-implantitis recovers less favorable and slower than periodontitis. Despite the differences in disease resolution, our study showed that early intervention of peri-implantitis could help arrest the disease, as bone loss did not progress following insult removal. The results of our study demonstrate the use of mice for translational studies as they are in line with related clinic findings, that is, peri-implantitis and periodontitis respond differently to intervention. The data gathered from this experiment come from machined-surface implants and must be regarded as a limitation, because there are various implants on the market with different surface properties. In today's market, there are no machined-surface implants available, as it is believed that rough surface implants have better osseointegration.49 Accordingly, different surface characteristics, such as acid-etched or plasma-sprayed, etc., could potentially lead to different results, which would further elucidate the complex nature of this disease. Nevertheless, this murine model could be useful in further dissecting the pathophysiology of peri-implantitis and assist in the development of better treatment modalities for the disease.
Supplementary Material
ACKNOWLEDGMENTS
The UCLA School of Dentistry Seed Grant supported this work. RW was supported by the UCLA Clinical and Translational Science Institute, NIH 5TL1TR000121-05. SH was supported by NIH/NIDCR T90 DE022734-01. We would like to thank the Translational Pathology Core Laboratory from the UCLA David Geffen School of Medicine for assistance with preparing the decalcified histologic sections. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Narby B, Kronström M, Söderfeldt B, Palmqvist S. Changes in attitudes toward desire for implant treatment: a longitudinal study of a middle-aged and older Swedish population. Int J Prosthodont. 2008;21:481–485. [PubMed] [Google Scholar]
- 2.Moraschini V, Poubel LA da C, Ferreira VF, Barboza E dos SP. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44:377–388. [DOI] [PubMed] [Google Scholar]
- 3.Simonis P, Dufour T, Tenenbaum H. Long-term implant survival and success: a 10–16-year follow-up of non-submerged dental implants. Clin Oral Implants Res. 2010;21:772–777. [DOI] [PubMed] [Google Scholar]
- 4.Lang NP, Berglundh T, Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: where are we now? – Consensus of the seventh European workshop on periodontology. J Clin Periodontol. 2011;38:178–181. [DOI] [PubMed] [Google Scholar]
- 5.Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Efftiveness of implant therapy analyzed in a swedish population. J Dent Res. 2016;95:43–49. [DOI] [PubMed] [Google Scholar]
- 6.Aljateeli M, Fu J-H, Wang H-L. Managing peri-implant bone loss: current understanding. Clin Implant Dent Relat Res. 2012;14: 109–118. [DOI] [PubMed] [Google Scholar]
- 7.Ata-Ali J, Candel-Marti ME, Flichy-Fernández AJ, Peñarrocha-Oltra D, Balaguer-Martinez JF, Peñarrocha Diago M. Peri-implantitis: associated microbiota and treatment. Med Oral Patol Oral Cir Bucal. 2011;16:937–943. [DOI] [PubMed] [Google Scholar]
- 8.Albrektsson T, Canullo L, Cochran D, De Bruyn H. ‘Peri-implantitis’: a complication of a foreign body or a man-made ‘disease’. Facts and fiction. Clin Implant Dent Relat Res. 2016;18: 840–849. [DOI] [PubMed] [Google Scholar]
- 9.Graves CV, Harrel SK, Rossmann JA, et al. The role of occlusion in the dental implant and peri-implant condition: a review. Open Dent J. 2016;10:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Periodontology. Peri-Implant Mucositis and Peri-Implantitis: a Current Understanding of Their Diagnoses and Clinical Implications. J Periodontol. 2013;84:436–443. [DOI] [PubMed] [Google Scholar]
- 11.Khammissa RAG, Feller L, Meyerov R, Lemmer J. Peri-implant mucositis and peri-implantitis: clinical and histopathological characteristics and treatment. SADJ. 2012;67:124–126. [PubMed] [Google Scholar]
- 12.Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. 2008;35: 282–285. [DOI] [PubMed] [Google Scholar]
- 13.Wilson V. An Insight into Peri-Implantitis: a systematic literature review. Prim Dent J. 2013;2:69–73. [DOI] [PubMed] [Google Scholar]
- 14.Heitz-Mayfield LJA, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010;53:167–181. [DOI] [PubMed] [Google Scholar]
- 15.Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions. J Clin Periodontol. 2011;38:188–202. [DOI] [PubMed] [Google Scholar]
- 16.Dhir S, Mahesh L, Kurtzman GM, Vandana KL. Peri-implant and periodontal tissues: a review of differences and similarities. Compend Contin Educ Dent;34:69–75. [PubMed] [Google Scholar]
- 17.Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3: 9–16. [DOI] [PubMed] [Google Scholar]
- 18.Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD. Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol. 1984;11:448–458. [DOI] [PubMed] [Google Scholar]
- 19.Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK. Long-term evaluation of periodontal therapy: i. response to 4 therapeutic modalities. J Periodontol. 1996;67:93–102. [DOI] [PubMed] [Google Scholar]
- 20.Heitz-Mayfield LJA, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs nonsurgical debridement for the treatment of chronic periodontitis. J Clin Periodontol. 2002;29:92–102. [DOI] [PubMed] [Google Scholar]
- 21.Carcuac O, Berglundh T. Composition of human peri-implantitis and periodontitis lesions. J Dent Res. 2014;93:1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Molon RS, Mascarenhas VI, de Avila ED, et al. Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin Oral Investig. 2016;20:1203–1216. [DOI] [PubMed] [Google Scholar]
- 23.Pirih FQ, Hiyari S, Leung H-Y, et al. A murine model of lipopolysaccharide-induced peri-implant mucositis and peri-implantitis. J Oral Implantol. 2015;41:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirih FQ, Hiyari S, Barroso AD V, et al. Ligature-induced peri-implantitis in mice. J Periodontal Res. 2015;50:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Research and Markets: Global Competitor Insights for Dental Implants 2009–2013 | Business Wire. Available at: http://www.businesswire.com/news/home/20100806005319/en/Research-Markets-Global-Competitor-Insights-Dental-Implants Accessed October 24, 2017.
- 26.Valente NA, Andreana S. Peri-implant disease: what we know and what we need to know. J Periodontal Implant Sci. 2016;46:136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marinello CP, Berglundh T, Ericsson I, Klinge B, Glantz PO, Lindhe J. Resolution of ligature-induced peri-implantitis lesions in the dog. J Clin Periodontol. 1995;22:475–479. [DOI] [PubMed] [Google Scholar]
- 28.Romeo E, Ghisolfi M, Murgolo N, Chiapasco M, Lops D, Vogel G. Therapy of peri-implantitis with resective surgery. Clin Oral Implants Res. 2004;16:9–18. [DOI] [PubMed] [Google Scholar]
- 29.Hiyari S, Atti E, Camargo PM, et al. Heritability of periodontal bone loss in mice. J Periodontal Res. 2015;50:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ericsson I, Lindhe J, Rylander H, Okamoto H. Experimental periodontal breakdown in the dog. Scand J Dent Res. 1975;83:189–192. [DOI] [PubMed] [Google Scholar]
- 31.Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. [DOI] [PubMed] [Google Scholar]
- 32.Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35: 89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz F, Sculean A, Engebretson SP, Becker J, Sager M. Animal models for peri-implant mucositis and peri-implantitis. Periodontol 2000. 2015;68:168–181. [DOI] [PubMed] [Google Scholar]
- 34.Tzach-Nahman R, Mizraji G, Shapira L, Nussbaum G, Wilensky A. Oral infection with Porphyromonas gingivalis induces peri-implantitis in a murine model: evaluation of bone loss and the local inflammatory response. J Clin Periodontol. 2017;44:739–748. [DOI] [PubMed] [Google Scholar]
- 35.Hiyari S, Wong R, Yaghsezian A, et al. Ligature-induced peri-implantitis and periodontitis in mice. J Clin Periodontol. 2017. 10.1111/jcpe.12817. Published online ahead of print September 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res. 2007;18:161–170. [DOI] [PubMed] [Google Scholar]
- 37.Chang H-Y, Park S-Y, Kim J-A, Kim Y-K, Lee H-J. Early radiographic diagnosis of peri-implantitis enhances the outcome of peri-implantitis treatment: a 5-year retrospective study after non-surgical treatment. J Periodontal Implant Sci. 2015;45:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast–osteoclast interactions. Connect Tissue Res. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen Vo TN, Hao J, Chou J, et al. Ligature induced peri-implantitis: tissue destruction and inflammatory progression in a murine model. Clin Oral Implants Res. 2017;28:129–136. [DOI] [PubMed] [Google Scholar]
- 40.Deshmukh S, Dive A, Moharil R, Munde P. Enigmatic insight into collagen. J Oral Maxillofac Pathol. 2016;20:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitzmann NU, Berglundh T, Ericsson I, Lindhe J. Spontaneous progression of experimentally induced periimplantitis. J Clin Periodontol. 2004;31:845–849. [DOI] [PubMed] [Google Scholar]
- 42.Teronen O, Konttinen YT, Lindqvist C, et al. Human neutrophil collagenase MMP-8 in peri-implant sulcus fluid and its inhibition by clodronate. J Dent Res. 1997;76:1529–1537. [DOI] [PubMed] [Google Scholar]
- 43.Kivelä-Rajamäki M, Maisi P, Srinivas R, et al. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J Periodontal Res. 2003;38:583–590. [DOI] [PubMed] [Google Scholar]
- 44.Izakovicova Holla L, Hrdlickova B, Vokurka J, Fassmann A. Matrix metalloproteinase 8 (MMP8) gene polymorphisms in chronic periodontitis. Arch Oral Biol. 2012;57:188–196. [DOI] [PubMed] [Google Scholar]
- 45.Arakawa H, Uehara J, Hara ES, et al. Matrix metalloproteinase-8 is the major potential collagenase in active peri-implantitis. J Prosthodont Res. 2012;56:249–255. [DOI] [PubMed] [Google Scholar]
- 46.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence T. The nuclear factor NF- B pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arabaci T, Cicek Y, Canakci V, et al. Immunohistochemical and stereologic analysis of NF-κB activation in chronic periodontitis. Eur J Dent. 2010;4:454–461. [PMC free article] [PubMed] [Google Scholar]
- 49.Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. Br Dent J. 2015;218:1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


