Abstract
Background and Aims:
Inferior vena cava (IVC) diameter and its respiratory variability have been shown to predict post-induction hypotension with high specificity in a mixed population of patients. We assessed whether these parameters could be as reliable in healthy adult patients as in a mixed patient population.
Methods:
In the present prospective observational study, 110 patients of either sex, aged between 18 and 50 years, belonging to American Society of Anesthesiologists class I and II, fasted as per the institutional protocol and scheduled for elective surgery under general anaesthesia were enroled. Prior to induction, ultrasound examination of IVC was done and variation in IVC diameter with respiration was assessed. Maximum and minimum IVC diameters [(dIVCmax) and (dIVCmin), respectively] over a single respiratory cycle were measured and collapsibility index (CI) was calculated. Vitals were recorded just before induction and at every minute after induction for 10 min. Episodes of hypotension (mean arterial pressure [MAP] <65 mmHg or fall in MAP >30% from baseline) during the observation period were recorded. The receiver operating characteristic (ROC) curve was constructed for determining optimum cut-off with sensitivity and specificity of IVC diameters and CI for development of hypotension.
Results:
IVC was not visualised in 22 patients. Out of the remaining 88 patients, 17 (19.3%) patients developed hypotension after induction. The dIVCmax, dIVCmin and CI were comparable between patients who developed and who did not develop hypotension. The area under curve of ROC for CI, dIVCmax and dIVCmin was 0.51, 0.55 and 0.52, respectively, with optimum cut-off value of 0.46, 1.42 and 0.73, respectively.
Conclusion:
Ultrasound-derived IVC parameters demonstrate poor diagnostic accuracy for prediction of hypotension after induction in healthy adult patients.
Keywords: Area under curve, arterial pressure, hypotension, inferior vena cava, Receiver operating characteristic (ROC) curve, ultrasonography
INTRODUCTION
Intravenous (IV) anaesthetic agents induced cardiovascular depression and reduction in peripheral sympathetic tone produces hypotension. This hypotensive response may be accentuated by various underlying conditions like hypovolaemia, pre-existing cardiac dysfunction etc.[1] Hypotension during general anaesthesia (GA) raises the risk of perioperative myocardial infarction, stroke, heart failure, acute renal injury and a longer stay in the hospital. It also increases 1-year mortality rate.[2,3,4]
Hypotension has been shown to be a common side effect of propofol administration, with incidence as high as 49%.[5] American Society of Anesthesiologists (ASA) class III and IV, baseline mean arterial pressure (MAP) less than 70 mmHg, age greater than 50 years and a high fentanyl dose are all predictors of hypotension after induction of anaesthesia with propofol.[4] With a sensitivity of 33% and a specificity of 95%, heart rate (HR) variability could predict post-induction hypotension and bradycardia.[6] Various studies have evaluated the role of the inferior vena cava (IVC) and its respiratory variability, that is collapsibility index (CI), as a predictor of hypotension both during induction of GA and after spinal anaesthesia in a mixed patient population.[7,8]
We aimed to evaluate the diagnostic accuracy of these parameters for prediction of episodes of hypotension after induction of anaesthesia with propofol in healthy adult patients in whom the above-mentioned predictors of hypotension were absent. We anticipated that IVC-derived parameters might accurately predict episodes of post-induction hypotension in healthy adult patients. The primary objective of the study was to find out the diagnostic accuracy in terms of sensitivity and specificity of the IVC-derived parameters for prediction of post-induction hypotension. Secondary objectives were incidence of hypotension and association between IVC-derived parameters and the percentage decrease in MAP from baseline after induction.
METHODS
The present prospective observational study was conducted in the operating room of a tertiary care referral centre during January 2018 to June 2019. Approval from the institute's ethical committee [Certificate Reference Number: AIIMS/IEC/2017/902, dated: 02/11/2017] and written informed consent from patients was obtained. The study was registered with Clinical Trials Registry, India (http://www.ctri.nic.in) (Reg. No.: CTRI/2018/01/011108). A total of 110 patients of either sex, aged between 18 and 50 years, belonging to ASA physical status I and II, fasted as per the institutional protocol (2 h for clear fluids and at least 6 h for solid) and scheduled for elective surgery under GA were enroled. Patients with preoperative MAP <70 mmHg, epidural catheter activated before induction, pregnancy, known or recently diagnosed hypertension, diabetes mellitus on treatment, peripheral vascular disease, anticipated and unanticipated difficult airway, mental incompetence, intra-abdominal hypertension, autonomic nervous system dysfunction and implanted pacemaker/cardioverter were excluded.
On the day of surgery in the preoperative holding area, demographic data were recorded and ultrasound examination of IVC was done. At the time of IVC examination, the patient was lying supine and breathing spontaneously. Ultrasound measurements were performed using a phased array transducer (2.5–5 MHz) of ultrasound machine (“LOGIQ e”, GE Healthcare, Chicago, United States) set to abdominal mode. According to the American Society of Echocardiography's protocol, the IVC was seen with a paramedian long-axis view through a subcostal window.[9] The IVC was seen in two dimensions as it entered the right atrium. The IVC was distinguished from the aorta using pulse wave Doppler. M-mode imaging was used to measure the variation in IVC diameter with breathing at a distance of 2–3 cm from the right atrium. Built-in software was used to measure the maximum and minimum IVC diameters (dIVCmax and dIVCmin, respectively) during a single breathing cycle. The CI was determined using the formula CI = (dIVCmax – dIVCmin)/dIVCmax and expressed as a percentage. Three scans were performed on each patient to guarantee consistent IVC readings. A difference in maximum diameter of IVC values of less than 0.2 cm between any two images was selected as the criterion for data inclusion in the final analysis.
After the IVC examination, patients were shifted to the operating room and standard monitors were attached. IV line was secured with 18 or 20 G cannula and maintenance IV fluid (Ringer's Lactate) was started at the rate of 10 ml/kg/h. The doses of drugs for premedication (midazolam and fentanyl), induction (propofol) and neuromuscular blockade were left to the discretion of the attending anaesthesiologists and were recorded. The endpoint of the propofol induction was loss of response to verbal commands. After achieving adequate relaxation, airway was secured using direct laryngoscopy.
Vitals (HR, MAP and peripheral oxygen saturation [SpO2]) were recorded just before induction and then at every minute after induction for 10 min. Inhalational agent (targeting a minimum alveolar concentration of 0.7–0.8) in air–oxygen mixture (60:40) was started and only minor stimulation (catheterisation and draping) was allowed during the observation period. Hypotension following anaesthesia induction was defined as a drop in MAP below 65 mmHg and/or a drop in MAP of more than 30% from baseline. Significant hypotension (MAP less than 55 mmHg or >40% drop in MAP) or prolonged hypotension (duration greater than or equal to 2 min) was treated with IV ephedrine boluses (3 mg). Significant bradycardia (HR <40 beats/min) was treated with IV atropine (0.3 mg).
Sample size was calculated using software G*Power (version 3.1.9.2, Institute of Experimental Psychology, Heinrich Heine University, Dusseldorf, Germany).[10] Zhang et al.[7] had found good diagnostic accuracy of IVC-CI for predicting hypotension after induction of GA as the area under curve (AUC) was 0.9 (95% confidence interval, 0.82–0.95). We assumed that 20% of patients (allocation ratio 4) in whom the clinical predictors of post-induction hypotension were absent would develop post-induction hypotension, and the AUC of ROC between post-induction hypotension and IVC-CI would be 0.6. Based on this, a sample of 84 patients achieved 80% power using two-tailed z-test at a significance of 0.05.
Data collected during the study were compiled using Microsoft Excel spreadsheet and analysed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 23.0 for Windows). The percentage decrease in MAP from baseline in each patient was calculated using the lowest MAP recorded after induction. The Kolmogorov–Smirnov one-sample test was used to determine whether the data were normal. For continuous variables, data were reported as mean and standard deviation, while for categorical variables, data were presented as absolute numbers or percentages. The development of clinically significant hypotension following induction was investigated using Student's t test, in conjunction with haemodynamic data and IVC measures. The effectiveness of IVC measurements to predict clinically significant post-induction hypotension was determined using a receiver operating characteristic (ROC) curve analysis. The AUC was determined with 95% confidence intervals. The optimal cut-off value with 95% confidence intervals, sensitivity and specificity for each parameter were computed. The association between IVC measurements and the percentage decrease in MAP from baseline following GA induction was tested using the Pearson correlation coefficient (r).
RESULTS
One hundred and forty patients were screened for eligibility and out of them 30 were excluded. Of 110 patients enroled, IVC was not visualised in 22 patients (20%) and data from remaining 88 patients were analysed. The demographic profile (age, gender, height and weight), ASA physical status, preoperative fasting duration, drug doses used for premedication/induction and baseline vitals [HR, systolic blood pressure (SBP), diastolic blood pressure (DBP) and MAP] were comparable between patients who developed and who did not develop hypotension [Table 1].
Table 1.
Comparison of demographics, ASA status, drugs used during induction and baseline vitals between patients who developed and who did not develop hypotension after induction
| Parameter | No hypotension (n=71) | Hypotension (n=17) | P |
|---|---|---|---|
| Age (year) | 30.24±9.44 | 27.82±9.0 | 0.34 |
| Gender (M/F) | 29/42 | 7/10 | 0.98 |
| ASA Class (I/II) | 56/15 | 15/2 | 0.59 |
| Height (cm) | 159.5±6.8 | 160.8±9.1 | 0.51 |
| Weight (kg) | 57.6±12 | 58.6±14.1 | 0.77 |
| Preoperative fasting duration for clear fluids (h) | 2.5±0.35 | 2.6±0.7 | 0.4 |
| Propofol used for induction (mg) | 119.72±25.7 | 124.7±30 | 0.49 |
| Midazolam used during induction (mg) | 1.34±0.42 | 1.24±0.36 | 0.37 |
| Fentanyl used during induction (µg) | 115.35±24 | 119.12±28.1 | 0.58 |
| Baseline heart rate (bpm) | 82.1±14.17 | 90.6±24.35 | 0.06 |
| Baseline systolic blood pressure (mmHg) | 121.08±14.2 | 127±19.2 | 0.15 |
| Baseline diastolic blood pressure (mmHg) | 74.25±12.12 | 80.3±15.33 | 0.08 |
| Baseline mean blood pressure (mmHg) | 92.97±11.92 | 95.24±28.7 | 0.6 |
Values are expressed in mean±SD, or as actual numbers (percentage). Values with *denotes significant P. M: male; F: female; ASA: American Society of Anesthesiologists; cm: centimetres; kg: kilogram; h: hours; mg: milligram; µg: microgram; bpm: beat per minute; mmHg: millimetres of mercury
In the present study, 17 (19.3%) patients developed hypotension and out of them 5 (5.7%) patients developed significant hypotension after induction of anaesthesia. The IVC parameters, i.e. dIVCmax, dIVCmin and CI, were comparable between patients who developed and who did not develop hypotension [Table 2].
Table 2.
Comparison of maximum and minimum diameter of IVC and its collapsibility index between patients who developed and who did not develop hypotension after induction
| Parameter | No hypo tension (n=71) | Hypotension (n=17) | P (95% confidence interval) |
|---|---|---|---|
| IVCmax (cm) | 1.43±0.36 | 1.48±0.29 | 0.57 (−0.24 - 0.13) |
| IVCmin (cm) | 0.77±0.32 | 0.81±0.36 | 0.65 (−0.22 - 0.14) |
| IVC collapsibility Index (proportion) | 0.47±0.14 | 0.47±0.15 | 0.89 (−0.07 - 0.08) |
Values are expressed in mean±SD. Values with *denotes significant P. IVCmax: maximum diameter of inferior vena cava; IVCmin: minimum diameter of inferior vena cava; cm: centimetre; CI: confidence interval
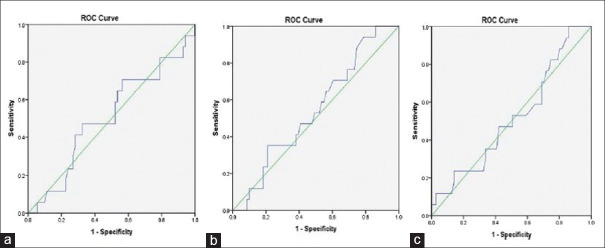
The ROC curve analysis demonstrated that the CI, dIVCmax and dIVCmin had poor diagnostic accuracy as the AUC was 0.51 (95% confidence interval, 0.35–0.67; P value = 0.92), 0.55 (95% confidence interval, 0.41–0.69; P value = 0.56) and 0.52 (95% confidence interval, 0.36–0.67; P value = 0.85), respectively. The optimum cut-off value of IVC-CI was 46% (47–59% sensitivity; 48–50% specificity), of dIVCmax was 1.42 cm (sensitivity 47–53%; specificity 51–53%) and of dIVCmin was 0.73 cm (sensitivity 47–53%; specificity 50%) [Figure 1a-c].
Figure 1.
The Receiver operating characteristic (ROC) curve showing the ability of preoperative inferior vena cava collapsibility index (IVC-CI) (a), IVC maximum diameter (dIVCmax) (b) and IVC minimum diameter (dIVCmin) (c) to predict hypotension after induction of general anaesthesia with propofol
For the prediction of significant hypotension also, the IVC-derived parameters had poor diagnostic accuracy as the AUC of IVC-CI was 0.46 (95% confidence interval, 0.22–0.71; P value = 0.79), of dIVCmax was 0.49 (95% confidence interval, 0.22–0.77; P value = 0.96) and of dIVCmin was 0.50 (95% confidence interval, 0.24–0.77; P value = 0.98) [Figure 2]. There was no correlation between percentage fall in MAP after induction and IVC-derived parameter, that is IVC-CI and dIVCmax [Pearson's correlation (r) = 0.024 and 0.07, respectively] [Figure 3].
Figure 2.
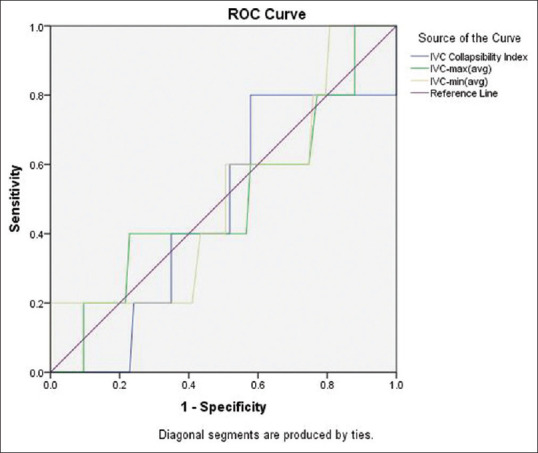
The receiver operating characteristic (ROC) curve showing the ability of preoperative Inferior Vena Cava Collapsibility Index (IVC-CI), IVC maximum diameter dIVCmax and IVC minimum diameter (dIVCmin) to predict significant hypotension after induction of general anaesthesia with propofol
Figure 3.
Scatter plots showing the relationships of preoperative collapsibility index (a) and maximum diameter (b) of inferior vena cava with percentage decrease in mean blood pressure from baseline after induction of general anaesthesia
DISCUSSION
The present study found 19.3% incidence of hypotension after administration of induction dose of propofol in healthy adult patients having no clinical predictors. The ultrasound guided measurement of IVC parameters (CI, dIVCmax and dIVCmin) demonstrated poor diagnostic accuracy for predicting this hypotensive response. Also, there was no correlation between percentage fall in MAP and the ultrasound derived IVC parameters.
Perioperative haemodynamic stability has a favourable impact on the incidence of myocardial injury, acute kidney injury and septic complications.[11] After non-cardiac surgery, it has been shown to affect the 30-day mortality,[2] as well as the 1-year mortality.[3] Safe intraoperative management requires prevention of an undesired hypotensive event. The clinical predictors associated with post-induction hypotension are mostly non-modifiable.[12] The most common modifiable risk factor associated with hypotension after induction is hypovolaemia and fluid therapy to optimise volume status is considered the cornerstone for its management. A method that can accurately detect hypovolaemia may provide the clinician a chance to optimise the volume status before induction and avoid the occurrence of post-induction hypotension.
Accurate assessment of intravascular volume status remains one of the most challenging and important yet unfulfilled tasks.[13] In clinical practice, physical examination, radiography and laboratory parameters are combined to assess the volume status. However, latent hypovolaemia remains undetected as it may not affect the haemodynamic and organ perfusion. Among others, the IVC ultrasound is seen as the 'holy grail' as it is immediately available, non-invasive, easy to learn, quick to perform and applicable in a wide range of patients. The IVC diameter and its respiratory variability is being used to assess the volume status and fluid responsiveness in various settings.[14,15] A greater respiratory variability suggests a low volume status, especially with a small IVC diameter.[16] However, the weight of currently available evidence suggests that these parameters have poor predictive value for fluid responsiveness.[17] These parameters have also been used to predict hypotension after induction of GA in mixed population of patients with variable results.[7,18,19,20,21] We believe that for becoming accurate predictors of post-induction hypotension, these parameters must be applicable over the entire range of surgical patients with volume deficit. Previous studies done on this subject have enroled a mixed population of patients expected to experience higher post-induction hypotension in view of the presence of other predictors of post-induction hypotension. Hence, we enroled healthy adult patients without clinical predictors to evaluate the diagnostic accuracy of the ultrasound derived IVC parameters for predicting the hypotension observed with the induction dose of propofol.
In the present study, none of the patients required regional blocks for intraoperative or postoperative analgesia. We used a standard method for IVC visualisation and measurement of its parameters. The IVC visualisation depends on the ethnicity and the reported non-visualisation varies from 6% to 20%.[12,19] The ethnicity affects the body surface area (racial differences in the body size) which in turn affects the body mass index (BMI) as well the organ size. The IVC cannot be easily visualised by the ultrasound in overweight patients. The IVC was not visualised in 20% of our cases and most of them were overweight with a mean BMI of 25.9 ± 2.5.
Previous studies on role of IVC diameter and CI for prediction of post-induction hypotension have used etomidate[7] or propofol[18,19] and proposed that these parameters have good sensitivity and specificity. In a study by Zhang et al.,[7] the incidence of post-induction hypotension was found to be 47% as most of their patients were older (>50 years) with cardiovascular disease. The CI and maximum diameter of IVC were found to have good diagnostic accuracy with CI having significantly better accuracy compared to maximum diameter of IVC (P value = 0.002). They found almost similar cut-off value for CI (0.43; sensitivity of 78.6% and a specificity of 91.7%) and dIVCmax (1.8 cm; sensitivity of 73.8% and a specificity of 70.8%) compared to our study. Similarly, Au et al.[18] and Szabo et al.[19] conducted their study in emergency department and operation room settings, respectively, to evaluate the role of IVC parameters as predictors of hypotension after induction of GA with propofol. After the IVC scanning they divided their study population into two groups having CI less than and more than 50%. Both studies demonstrated that IVC-CI >50% predicted the development of post-induction hypotension with high specificity [77.3% (95% CI, 64.3–90.3%) and 90.0% (95% CI, 78.2–96.7%), respectively] and moderate-to-low sensitivity [66.7% (95% CI, 52.1–81.3) and 45.5% (95% CI, 28.1–63.7%), respectively]. The subjects enroled in these studies were from mixed population with most of them having clinical predictors for development of post-induction hypotension, so their results cannot be extrapolated to patients without these predictors in whom the incidence of post-induction hypotension might be low.
Our finding suggests that factors apart from the volume status might play a role in the occurrence of post-induction hypotension in healthy adult surgical patients (expected to have adequate volume status). We suggest that well-designed studies with larger sample size are conducted to support or refute our findings and to find the predictors of hypotension after induction in this patient population.
Our study has a few limitations. First, being an observational study the bias inherent to trial design could not be excluded. Second, the calculated sample size might be small as it was based on a similar study[7] done in mixed population of patients expected to experience higher incidence of post-induction hypotension compared to our study population; however, we assumed a lesser incidence (20%) of post-induction hypotension in our study population contrary to what was found by Zhang et al.(47%).[7] Further studies with adequate sample size might produce different results. Third, the BP was measured by non-invasive means at an interval of 1 min for 10 min after intubation, invasive BP measurement might be better as it provides information in real time. Fourth, we could not collect data regarding type of surgery and this heterogeneity might have influenced the power of the study. However, we believe that restriction of type of surgery might not reflect true clinical practice and the results obtained cannot be generalised. Randomised clinical trials comparing the ultrasound-derived IVC parameters and reliable dynamic predictors of volume status are required to confirm our findings.
CONCLUSION
Hence, for healthy adult surgical patients, the ultrasound-derived IVC parameters (CI, dIVCmax and dIVCmin) had poor diagnostic accuracy for prediction of hypotension and significant hypotension after induction of anaesthesia with propofol. Also, these parameters had no correlation with fall in blood pressure after induction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This work was supported by the Department of Anaesthesiology and Critical Care, AIIMS Hospital, Jodhpur, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Assistance with the study: We would like to acknowledge assistance of Dr Naveen KH, Associate Professor, Department of Community Medicine and Family Medicine, AIIMS, Jodhpur, in performing statistical analysis.
REFERENCES
- 1.Lonjaret L, Lairez O, Minville V, Geeraerts T. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49–59. doi: 10.2147/IBPC.S45292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu WJ, Hou BL, Kwong JSW, Tian X, Qian Y, Hao J, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: A meta-analysis of cohort studies. Int J Cardiol. 2018;258:68–73. doi: 10.1016/j.ijcard.2018.01.137. [DOI] [PubMed] [Google Scholar]
- 3.Bijker JB, van Klei WA, Vergouwe Y, Eleveld DJ, van Wolfswinkel L, Moons KG, et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–26. doi: 10.1097/ALN.0b013e3181c14930. [DOI] [PubMed] [Google Scholar]
- 4.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 5.Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: a randomized, controlled trial. J Trauma Acute Care Surg. 2012;73:94–101. doi: 10.1097/TA.0b013e318250cdb8. [DOI] [PubMed] [Google Scholar]
- 6.Hanss R, Renner J, Ilies C, Moikow L, Buell O, Steinfath M, et al. Does heart rate variability predict hypotension and bradycardia after induction of general anaesthesia in high risk cardiovascular patients? Anaesthesia. 2008;63:129–35. doi: 10.1111/j.1365-2044.2007.05321.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Critchley LAH. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124:580–9. doi: 10.1097/ALN.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 8.Ayyanagouda B, Ajay BC, Joshi C, Hulakund SY, Ganeshnavar A, Archana E. Role of ultrasonographic inferior venacaval assessment in averting spinal anaesthesia-induced hypotension for hernia and hydrocele surgeries-A prospective randomised controlled study. Indian J Anaesth. 2020;64:849–54. doi: 10.4103/ija.IJA_244_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 11.Haynes AB, Regenbogen SE, Weiser TG, Lipsitz SR, Dziekan G, Berry WR, et al. Surgical outcome measurement for a global patient population: Validation of the surgical Apgar score in 8 countries. Surgery. 2011;149:519–24. doi: 10.1016/j.surg.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Kouz K, Hoppe P, Briesenick L, Saugel B. Intraoperative hypotension: Pathophysiology, clinical relevance, and therapeutic approaches. Indian J Anaesth. 2020;64:90–6. doi: 10.4103/ija.IJA_939_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett VA, Cecconi M. Perioperative fluid management: From physiology to improving clinical outcomes. Indian J Anaesth. 2017;61:614–21. doi: 10.4103/ija.IJA_456_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das SK, Choupoo NS, Pradhan D, Saikia P, Monnet X. Diagnostic accuracy of inferior vena caval respiratory variation in detecting fluid unresponsiveness: A systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35:831–9. doi: 10.1097/EJA.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 15.Achar SK, Sagar MS, Shetty R, Kini G, Samanth J, Nayak C, et al. Respiratory variation in aortic flow peak velocity and inferior vena cava distensibility as indices of fluid responsiveness in anaesthetised and mechanically ventilated children. Indian J Anaesth. 2016;60:121–6. doi: 10.4103/0019-5049.176285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seif D, Mailhot T, Perera P, Mandavia D. Caval sonography in shock: A noninvasive method for evaluating intravascular volume in critically ill patients. J Ultrasound Med. 2012;31:1885–90. doi: 10.7863/jum.2012.31.12.1885. [DOI] [PubMed] [Google Scholar]
- 17.Millington SJ. Ultrasound assessment of the inferior vena cava for fluid responsiveness: easy, fun, but unlikely to be helpful. Can J Anesth. 2019;66:633–8. doi: 10.1007/s12630-019-01357-0. [DOI] [PubMed] [Google Scholar]
- 18.Au AK, Steinberg D, Thom C, Shirazi M, Papanagnou D, Ku SB, et al. Ultrasound measurement of inferior vena cava collapse predicts propofol induced hypotension. Am J Emerg Med. 2016;34:1125–8. doi: 10.1016/j.ajem.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Szabó M, Bozó A, Darvas K, Horváth A, Iványi ZD. Role of inferior vena cava collapsibility index in the prediction of hypotension associated with general anesthesia: an observational study. BMC Anesthesiol. 2019;19:139. doi: 10.1186/s12871-019-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purushothaman SS, Alex A, Kesavan R, Balakrishnan S, Rajan S, Kumar L. Ultrasound measurement of inferior vena cava collapsibility as a tool to predict propofol-induced hypotension. Anesth Essays Res. 2020;14:199–202. doi: 10.4103/aer.AER_75_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louro J, Rowshanrad A, Epstein RH, Dudaryk R. Preoperative inferior vena cava collapsibility is a poor marker of intraoperative fluid requirements and hypotension: A pilot study. J Anaesthesiol Clin Pharmacol. 2019;35:562–4. doi: 10.4103/joacp.JOACP_136_18. [DOI] [PMC free article] [PubMed] [Google Scholar]