Abstract
Background and Aims:
Appropriate volume assessment and fluid management can prevent maternal deaths in the severely pre-eclamptic (SPE) parturients. We planned a systematic review and meta-analysis (MA) to evaluate the role and ability of point-of-care ultrasound (POCUS) in the assessment of volume status and early detection of lung oedema in an SPE parturient.
Methods:
An e-literature search was done from several databases. Data were extracted under five domains including POCUS-derived parameters like echo comet score (ECS), lung ultrasound (LUS) scores, B-patterns, optic nerve sheath diameter (ONSD), E/e' ratio, presence of pleural effusion, pulmonary interstitial syndrome and pulmonary congestion. The risk of bias was assessed. Extracted data were analysed using MetaXL and Revman 5.3. Heterogeneity in the studies was evaluated using the Cochrane Q test and I2 statistics. Funnel plots were used for the assessment of publication bias.
Results:
Seven prospective studies including 574 parturients (including 396 pre-eclamptics) were selected. POCUS included lung, optic nerve, cardiac and thoracic US. In two studies, the ECS and LUS scores pre-delivery were higher in pre-eclamptics. Two studies found a mean ONSD of 5–5.84 mm before delivery. MA revealed a significantly lower mean ECS score at post-delivery than pre-delivery, and the summary prevalence of B-pattern and pleural effusion among SPE parturients was found to be 0.28 (0.03–0.84) and 0.1 (0–0.2), respectively. A good correlation was observed between B-line patterns and diastolic dysfunction (increased E/e' ratio), LUS score and thoracic fluid content, ONSD and ECS in individual studies.
Conclusion:
POCUS parameters can be useful as early markers of fluid status and serve as useful tools in the precise clinical management of pre-eclampsia.
Keywords: Echocardiography, extravascular lung water, lung, obstetric, point-of-care systems, pre-eclampsia, pregnancy, pulmonary oedema, toxaemia, ultrasonography
INTRODUCTION
The management of severe pre-eclampsia (SPE) has always been a challenging task both for the obstetrician and the anaesthesiologist. Pulmonary oedema is the most frequent and serious complication of SPE and has been observed in 9.5% of women with SPE.[1,2] Poor fluid management leading to maternal deaths have been reported in PE cases.[3,4] The ability to assess fluid status, especially to detect early lung congestion and extravascular lung water (EVLW) before the appearance of clinical signs is considered to be very important in the fluid management of the parturient with SPE.[3,5] Point-of-Care Ultrasound (POCUS) has been defined as a diagnostic or procedural bedside diagnostic tool that can help the clinician in rapid patient evaluation, assessment and treatment.[6] POCUS is nowadays increasingly being applied in pre-eclampsia cases for diagnostic purposes like fluid assessment.[7,8,9] Several researchers have reported lung ultrasound (LUS) as showing excellent properties for the detection of pulmonary oedema.[10] Several others have used optic ultrasound or echocardiographic parameters.[5,7] This area of research was novel with limited possibilities for a randomised controlled trial. Hence, the data were pooled from the available studies to conduct this systematic review (SR) and meta-analysis (MA) to provide quality evidence for the hypothesis that POCUS is a useful adjunct tool to clinical examination in the assessment of fluid status and early detection of pulmonary oedema in a patient with SPE. The primary objective was to evaluate the role and ability of POCUS in assessing volume status in an SPE parturient. The secondary objectives were to find out, whether LUS can detect pulmonary oedema early before the appearance of clinical signs, the correlation between LUS estimates of EVLW with other values like optic nerve sheath diameter (ONSD), thoracic fluid content and echocardiographic ratios and to find the prevalence of POCUS-derived abnormalities.
METHODS
Protocol and registration
This SR and MA were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist and guidelines.[11] The review was registered (CRD 42020192387) in the International Prospective Register of Systematic Reviews [PROSPERO] on 16 July 2020.
Eligibility criteria
The human studies that evaluated the role and use of POCUS in assessing fluid status, lung water/congestion and cerebral oedema in the pre-eclamptic parturients were included in this MA. The inclusion and exclusion criteria of the study were decided by the PICOS strategy [Annexure 1]. Prospective and retrospective observational studies, randomised controlled trials (RCTs) and non-RCTs were included. Study-specific outcomes were not considered as inclusion/exclusion criteria as the review included a significant number of observational studies of cross-sectional nature. Nevertheless, studies reporting at least one outcome were only included. Studies in languages other than English, those without full-text availability, case reports and case series were excluded.
Search strategy and information source
For the present study, we e-searched literature (ranging from January 2000 to June 2020) from databases including PubMed, Google Scholar, Cochrane Library, Web of Science and Science Direct. The cross-references cited in the literature obtained were also searched for additional studies. For the electronic search, search terms like 'Point-of-Care Ultrasound'(POCUS) 'pre-eclampsia', 'fluid management, 'optic ultrasound', 'echocardiography', 'obstetric critical care', 'lung ultrasound', 'pulmonary congestion', 'pulmonary oedema' in all possible combinations were used. Boolean operators and proximity operators were applied as necessary. An example of the search terms used is ('ultrasound'[MeSH] OR 'ultrasonography' [MeSH] OR 'lung ultrasonography'[MeSH]) AND ('pre-eclampsia'[MeSH]) OR ('toxemia'[All Fields] AND 'obstetrics'[All Fields])).
Study selection and data collection
Two primary authors independently conducted the initial e-literature search and checked for study eligibility according to the PICOS framework. Any eligible trials that had got missed in the electronic search were also searched for in the bibliographies of the included trials. There was no discrepancy in the study selection between the authors.
Data items and data extraction
The data were extracted using a tool that included five domains:
Identification of the study: article title, journal title, impact factor of the journal, authors including their names, country and speciality, publication year and host institute of the study (hospital, university research centre, single institute/multicentre study).
Methodological characteristics including study type/design, research question, primary and secondary outcomes and sample characteristics (sample size, presence of healthy control group).
Participant demographics including maternal age, gestational age, pre-eclampsia and severity definition, single/multiple pregnancies, use of diuretic or other anti-hypertensives, body mass index, fluid management protocol, magnesium sulphate prophylaxis, type of delivery (vaginal/caesarean) and presence of clinical signs of pulmonary oedema.
POCUS details including ultrasound probe (transducer shape, frequency), technique, timing in relation to delivery (before/after), POCUS done by whom, POCUS place (emergency room, labour room, radiology suite).
Validated measures and parameters viz:- 1) Point-of-care lung ultrasound (POCLUS)-derived parameters including LUS score, echo comet score (ECS), presence of B-pattern, B-line score, and A-lines; 2) cardiac ultrasound parameters including E, A, E/A, E/E' and optic ultrasound parameters like ONSD; 3) association/correlation between various measures [between ONSD and EVLW/ECS, pulmonary interstitial syndrome (PIS), diastolic dysfunction and increased left ventricular end-diastolic pressure (LVEDP), B-line pattern and increased E/e' ratio, LUS score and thoracic fluid content (TFC)] and 4) EVLW manifestations including pleural effusion, PIS, lung congestion and pulmonary oedema.
The various parameters and measures had precise definitions to their credit in the selected studies [Annexure 2].
Risk of bias assessment (Quality assessment) in individual studies
To assess the risk of bias, the Critical Appraisal Skills Programme (CASP) checklist for the cohort study was applied. It has domains under three sections which are as follows:
Section A – Are the study results valid?
Section B – What are the study results?
Section C – Will the results help locally?
Six items were assessed in Section A, three items in Section B and three in Section C.
Statistical analysis
Extracted data were analysed by using MetaXL and Revman 5.3. MA was done for the variables for which data was available from at least two studies. Before the analysis, the data were standardised into equivalent units. The pooled prevalence for variables like pleural effusion and B-pattern for single-arm studies was calculated using MetaXL software to present pooled prevalence along with its 95% confidence interval (CI) as a forest plot. For comparing continuous variables such as mean ECS between two groups, standardised mean difference and 95% CI were calculated by taking a random-effects model using Revman 5.3. The standard deviation (SD) was calculated by either using the range formula that is, (maximum-minimum)/4 or by using 95% CI [SD = √N (upper limit − lower limit)/3.92. P value less than 0.05 was considered to be significant for MA.
Heterogeneity in the studies was evaluated using the Cochrane Q test and I2 statistics to assess the degree of inter-study variation. I2 values of 0% to 24.9%, 25% to 49.9%, 50% to 74.9% and 75% to 100% were considered as having no, mild, moderate and significant thresholds for statistical heterogeneity, respectively. Funnel plots were used for the assessment of publication bias. The mean difference and overall prevalence are the measures of effect and standard error is used for precision in this paper.
RESULTS
Search results and study selection
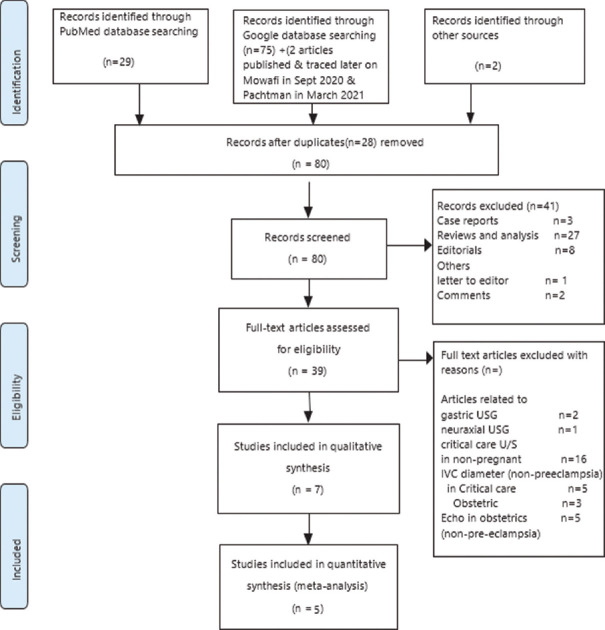
Database search was done in June 2020 and 78 articles were identified. Of these, 27 were review articles and 37 were original articles including studies and surveys. Others were Case reports (3), Letters to the Editor (1), Comments (2) and Editorials/clinical opinions (8) [Figure 1]. Narrative review articles and articles on POCUS related to gastric ultrasonography/neuraxial US were excluded from the study. Seven full-text articles were eventually included as per the selection criteria.[5,8,9,10,12,13,14]
Figure 1.
PRISMA flow diagram of the systematic literature search. USG-Ultrasonography; IVC-Inferior vena cava
Characteristics of included studies
Out of the seven studies, two were prospective observational studies, two were cross-sectional studies and three were prospective cohort studies. A total of 574 parturients were analysed in this SR and MA, out of which 188 were healthy controls, 396 were pre-eclamptics including 220 severely pre-eclamptic cases.[5,8,9,12,13] POCUS for fluid volume status assessment includes LUS, optic nerve ultrasound, cardiac and thoracic ultrasound. In four of the selected studies, lung and cardiac ultrasonography were done.[8,9,10,12] In two studies, optic ultrasound and LUS were done.[5,13] In one study, only LUS was done.[14] For LUS, 28 rib interspace technique was used in two studies,[12,13] eight region technique in two studies,[8,9] four region technique devised from eight region technique in one study[14] and 12 region technique was used in another study.[10] The US imaging and interpretation of images was done by either a certified operator/cardiologist/obstetrician/registered medical sonographer/echocardiography investigator/pulmonary expert. The cases selected in the studies were pre-eclampsia cases and both pre-eclampsia and SPE were diagnosed as per the 2013 American College of Obstetrics and Gynaecology (ACOG) Task Force for hypertension in pregnancy criteria.[15] Healthy parturients were included as controls in three studies.[9,12,14] Late-onset SPE cases (>34 weeks gestation) were included in the study by Ortner et al., whereas thosebeyond 20 weeks of gestation and in first stage of labour were included in the study by Hammad et al. Clinical symptoms of pulmonary oedema were looked for in four studies.[9,10,12,13] The parturient characteristics in the seven individual studies including age, parity, single/multiple pregnancies and blood pressure (BP) were noted [Table 1]. Various parameters related to fluid assessment and EVLW were measured in the selected studies via POCUS [Table 2].
Table 1.
Characteristics of articles and parturients selected for the systematic review
| First author, Country | Journal, year | Title of article | Type of article, study design | Study population | Study groups | Sample size | |||
|---|---|---|---|---|---|---|---|---|---|
| Ambrozic Jana,Slovenia | Ultrasound Obstet Gynecol, 2017 | Lung and cardiac ultrasound for hemodynamic monitoring of patients with severe pre-eclampsia | Prospective observational study | Severe PE | Severe PE group, healthy control group | 33 (21 in severe PE group, 12 in healthy control group) | |||
| Ortner Clemens M, Vienna | Anesth Analg, 2019 | Point-of-Care Ultrasound Abnormalities in Late-Onset Severe Pre-eclampsia: Prevalence and Association With Serum Albumin and Brain Natriuretic Peptide | Prospective cohort study | Acute late-onset severe PE (after 34 weeks gestation) | Study group, No control | 95 enroled | |||
| Zieleskiewicz Laurent, France | Anesthesiology, 2014 | Lung USG Predicts Interstitial Syndrome and Hemodynamic Profile in ParturientsWith Severe PE | Prospective cohort study | Severe PE | Severe PE, healthy parturients | 40(20 in severe PE group. 20 healthy parturients) | |||
| Hammad Y, Egypt | Journal of Clinical Monitoring & Computing, 2019 | Thoracic Fluid Content: A Novel Parameter for Detection of Pulmonary Edema in Parturients With Pre-eclampsia | Prospective observational (cross-sectional) study | Parturient with PE in 1st stage labour | Study group, No control | 62 evaluated for eligibility, 60 analysed | |||
| GabrijelaSimenc, Slovenia | Radiology & Oncology, 2018 English | Optic nerve ultrasound for fluid status assessment in patients with severe pre-eclampsia | Prospective observational (cross-sectional) study | Severe PE | Study group, No control | 30 | |||
| Mowafy SMS, Egypt | Egyptian J Anaesth 2020 | Optic nerve sheath diameter versus extravascular lung water detected by ultrasound in volume status prediction in severe preeclampsia | Prospective cohort study | Severe PE | Study group, No control | 54 | |||
| Sarah LPachtman Shetty, New York | Am J Obstet Gynecol MFM 2021 | Point-of-care lung ultrasound patterns in late third-trimester gravidas with and without pre-eclampsia | Prospective observational study | PE including mild and severe cases | Normotensive gestational age-matched pregnant women were control | 86 PE (48 severe, 38 mild cases), 176 parturients in the control group | |||
|
| |||||||||
| First author, Country | Assessment of POCUS parameters (before/after delivery) | Name of assessed POCUS parameter | Parturient Characteristics | ||||||
|
| |||||||||
| Age (years) | Gravidity | Singleton/multiple pregnancy | Systolic/Diastolic BP (mmHg) (All with PE were hypertensive as per criteria) | GESTATION (weeks) | PARITY | ||||
|
| |||||||||
| Ambrozic Jana,Slovenia | Before and after delivery | ECS | 28 (21-44) Severe pre-eclampsia 33 (24-42) Healthy controls Median (range) | NI | singleton | NI | 33+0 (24+1 to 39+4) severe PE 39+3 (37+0 to 42+1) Healthy controls Median (range) | 76% nulliparous -Severe PE 33% nulliparous -Healthy controls (Frequency %) | |
| Ortner Clemens M, Vienna | Before delivery | ONSD, B-pattern | 27 (6) Mean (SD) | 2(1) Mean (SD) | NI | 165 (18)/105/(12) Mean (SD) | 39 (2.5) Mean (SD) | 1(1) Mean (SD) | |
| Zieleskiewicz Laurent, France | Before delivery After delivery? | ECS, LUS, B-pattern | 31 (26-38) Pre-eclamptics 30 (26-34) (healthy parturents) Median (IQR) | NI | NI | 157 (146-167)/92 (90-101) - PE 110 (100-120)/70 (62-71)-Healthy parturients Median (IQR) | 34 (31-35)-PE 37 (36-39)-Healthy paturients Median (IQR) | 45% in PE 35% in healthy parturients (Frequency) | |
| Hammad Y, Egypt | Before delivery | LUS TFC | 26.8 (4.5) Mean (SD) | NI | NI | 149 (142, 160)/84.6 (8) Median (quartiles)/mean±SD | 35 (32, 37) Median (Quartiles) | 2 (1,3) Median (Quartiles) | |
| GabrijelaSimenc, Slovenia | Before delivery | ECS, ONSD | 31 (21-44) Median (range) | NI | Singleton | NI | 32 5/7 (22 3/7-39 4/7) Median (range) | 77% nulliparous | |
| Mowafy SMS, Egypt | Before and after delivery | ECS, ONSD | 28.31 (5.21) Mean (SD) | NI | Singleton | NI | 34.81 (1.68) Mean (SD) | NI | |
| Sarah LPachtman Shetty, New York | Before delivery | B-pattern | 32.3 (6.2): Mild PE 31.9 (5.4): Severe PE 32.0 (5.1): Healthy gravidas Mean (SD) | NI | Singleton | 158.3(14.3)/93.9(8.0)-Mild PE 172.7 (14.3)/99.5 (10.1) Severe PE 121.1 (15.1)/69.7 (12.4) Healthy gravidas Mean (SD) | 36.6 (2.4): mild PE 36.8 (2.2): severe PE 36.8 (2.8) Healthy gravidas Mean (SD) | Parity 0-27 (71.1) Mild PE 31 (64.6) Severe PE 79 (44.9) healthy gravidas Number (percentages) | |
PE - Pre-eclampsia; ECS - Echo comet score; ONSD - Optic nerve sheath diameter; SD - Standard deviation; LUS - Lung ultrasound score; POCUS - Point-of-care ultrasound; NI - No information; TFC - Thoracic fluid content
Table 2.
Values of parameters assessed and prevalence of POCUS-derived abnormalities in the selected studies
| ECHO COMET SCORES (ECS) | |
|---|---|
|
| |
| STUDY | Value |
| Zeileskiewicz[9] | 31 (0-42) Median (IQR) |
| Ambrozic[12] | P=0.002 (median 22) |
| P=0.02 (median 15) | |
| P<0.001 (median 3) | |
| Gabrizela,[5] | Median 19 |
| Range (0-24) | |
| Mowafy[13] | Mean±SD |
| 20.8±11.32 | |
| 15.09±9.11 | |
|
| |
| OPTIC NERVE SHEATH DIAMETER (mm) | |
|
| |
| Ortner[8] | Mean (SD) |
| 5.4 (0.5) | |
| Mowafy[13] | Mean±SD |
| 5.84±0.82 | |
| 5.24±0.73 | |
| Gabrizela[5] | 5.7 median |
| 3.8-7.5 range | |
| IQR 5.2-6 | |
|
| |
| LUNG ULTRASOUND SCORES (LUS) | |
|
| |
| Hammad[10] | Median 2.6 |
| IQR 2-3.6 | |
| Zeileskiewicz[9] | Median 7 |
| IQR 1-10 | |
|
| |
| Prevalence (%) of POCUS abnormalities B-Pattern/Interstitial oedema | |
|
| |
| Zeileskiewicz[9] | 25 |
| Ortner[8] | 24 |
| Pachtman[14] | 6.9 (by obstetrician) |
| 2.3 (by lung ultrasound expert) | |
|
| |
| Pleural Effusion | |
|
| |
| Zeileskiewicz[9] | 15 |
| Ortner[8] | 4 |
|
| |
| Lung consolidation | |
|
| |
| Zeileskiewicz[9] | 35 |
|
| |
| Increased LVEDP | |
|
| |
| Ortner[8] | 25 |
|
| |
| Increased ONSD | |
|
| |
| Ortner[8] | 28 |
| Gabrizela[5] | 43 |
|
| |
| Increased E/e’ ratio (>3.5 mm) | |
|
| |
| Zieleskiewicz[9] | 20 |
SD: standard deviation; IQR: interquartile range; LVEDP: Left ventricular end-diastolic pressure; ONSD: Optic nerve sheath diameter
Correlation between various POCUS and other parameters was also assessed in the different selected studies [Table 3].
Table 3.
POCUS parameters and correlations that were measured
| Parameters between which correlation measured | Study in which it was measured | Results |
|---|---|---|
| ONSD and EVLW (ECS) | Gabrizela,[5] Mowafy[13] | Correlation will be good if increase in EVLW |
| PIS, diastolic dysfuntion, increased LVEDP | Ortner[8] | PIS associated with diastolic dysfunction & increased LVEDP |
| B-line pattern and increased E/e’ ratio | Zeileskiewicz[9] | B-pattern associated with increased E/e’ ratio; ECS >25 predicts increase in filling pressures (E/e’ ratio >9.5) |
| Lung ultrasound score and thoracic fluid content | Hammad[10] | Excellent correlation in the diagnosis of pulmonary oedema |
| B-lines and oxygen saturation | Pachtman[14] | B-lines seen more frequently in women with decreased oxygen saturation without signs of overt clinical pulmonary oedema |
POCUS: point-of-care ultrasound; ONSD: optic nerve sheath diameter; PIS: pulmonary interstitial syndrome; ECS: echo comet score; EVLW: extravascular lung water
Fluid intake and urine output monitoring were done every hour in three studies.[5,12,13] Euvolaemia was maintained with minimisation of other intravenous (IV)/oral fluid intake (additional IV crystalloid 30 mL/h so that the total IV fluid intake was limited to 80 mL/h) and diuretics. Fluid restriction to <100 mL crystalloid/h in pre-eclamptic women was done in a study by Ortner et al. In one study, diuretics were given if symptomatic.[10] In four studies, magnesium sulphate (MgSO4) prophylaxis with 4 g bolus MgSO4 loading dose was given followed by 1 g/h (50 mL/h) infusion for 24 h.[5,8,13,14] There is no mention of fluid management in three studies.[9,10,14] IV hydralazine, labetalol was given for the acute management of hypertension for a target systolic BP <160 mmHg and diastolic BP <119 mmHg in four studies.[5,8,12,13]) Arterial oxygen saturation was monitored by pulse oximetry in three studies.[9,10,14]
RISK OF BIAS ASSESSMENT [Annexure 3]
Results of individual studies
Primary outcome
Role and ability of POCUS in assessing volume status in a pre-eclamptic parturient
There are several LUS measures of EVLW like B-patterns, LUS score and ECS. Other measures like ONSD also indicate fluid status. In the study by Zieleskiewicz et al., the parturients with SPE had an increased LUS score (7 vs. 1) as compared with healthy parturients.[9] In the study by Hammad et al., the median LUS was 2.6 with an interquartile range (IQR) of 2–3.6; it was high in pre-eclamptics with pulmonary oedema 19 (17–20) (median) (Quartiles) and showed an excellent ability to detect pulmonary oedema with the best cut-off value of 15.7, sensitivity 100%, specificity 90.7%, the positive predictive value of 54% and negative predictive value of 100%. In this study, an LUS score cut-off value of 16.7 was defined to detect pulmonary oedema.[10]
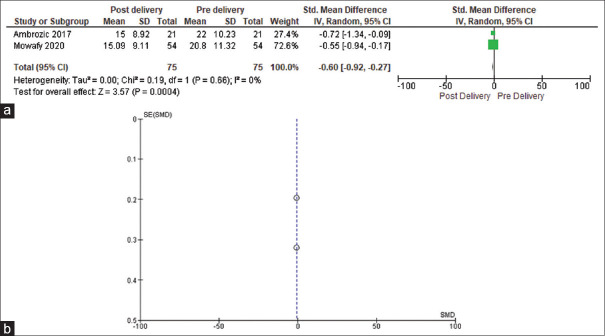
Two studies compared ECS before delivery with after delivery in SPE cases.[12,13] ECS in SPE cases in the study by Mowafy et al. was significantly lower at 24 h after delivery (15.09 ± 9.11) compared with before delivery (20.87 ± 11.32). In the study by Ambrozic et al., ECS was higher in women with pre-eclampsia than in controls, before delivery (P = 0.002) and 1-day post-delivery (P = 0.02).[12] Zieleskiewicz et al. study states that ECS values were measured before and after delivery, in both SPE and healthy controls; however from the values mentioned in the article, it is not clear as to whether they represent pre-/post-delivery values; nonetheless, in the study, the parturients with SPE had increased ECS (31 vs. 3; P = 0.02) and the best cut-off value of ECS was 25. The median (IQR) of ECS was 31 (0–42) in parturients with SPE compared with 3 (0–1.2)[9] in healthy controls. The median ECS value in SPE cases in the study by Gabrizela et al. was 19 with a range of 0–24.[5] We could meta-analyse the results of two studies to compare pre- and post-delivery ECS.[12,13] There was no heterogeneity among the studies (τ2 = 0.00, χ2 = 0.19, df = 1, P = 0.66, I2 = 0%). The mean ECS score was significantly lower in post-delivery phase as compared with pre-delivery scores (mean difference = −0.60, 95% CI = −0.92–0.27, P = 0.0004) [Figure 2a]. The funnel plot for ECS indicates a shortage of literature in this area of the subject [Figure 2b].
Figure 2.
(a) Forest plot comparing pre- and post- delivery ECSs (b) Funnel plot for ECSs
Three studies[5,8,13] found out ONSD values (median/mean) before delivery in SPE cases and one study[13] compared ONSD before delivery with after delivery in SPE cases. The ONSD in the study by Gabrizela et al. had a median value before delivery of 5.7 mm (IQR 5.2–6.0 mm; range: 3.8–7.5 mm), and mean ± SD 5.4 ± 0.5 mm in the study by Ortner et al. In the study by Mowafy et al., ONSD was significantly lower at 24 h after delivery (5.24 ± 0.73) compared with before delivery (5.84 ± 0.82).[13]
The ONSD values before delivery that could categorise pulmonary congestion were 5.04 ± 0.26 mm for mild congestion, 6.11 ± 0.26 for moderate congestion and 6.93 ± 0.22 for severe congestion (P < 0.001). These values decreased after delivery to 4.56 ± 0.42 for mild congestion, 5.61 ± 0.4 for moderate congestion and 6 ± 0.23 for severe congestion. The ECS values before delivery that could categorise pulmonary congestion were mean ± SD value of 10.45 ± 2.41, 23.05 ± 3.96 and 37.23 ± 4.47 for mild, moderate and severe pulmonary congestion, respectively. The ECS values decreased after delivery to 6.75 ± 2.90, 18 ± 4.67 and 26.69 ± 4.9 in mild, moderate and severe pulmonary congestion, respectively.[13] The ONSD cut-off value to diagnose severe pulmonary congestion was >6.4 mm and for mild-to-moderate congestion was 5.6–6.4 mm in the same study.[13] An ECS of '0' corresponded to <5 B-lines and absent EVLW; ECS of '1' to 6–15 B-lines and mild degree EVLW; ECS of '2' to 16–30 B-lines and moderate degree EVLW and ECS 3 to >30 B-lines and severe degree EVLW.[13]
We could subject to MA, the results of three studies regarding the prevalence of B-patterns.[8,9,14] The summary prevalence of B-pattern among women having pre-eclampsia was found to be 0.28 (0.03–0.84). The coefficient of heterogeneity was found to be 97.14, indicating high heterogeneity among the studies [Table 4 and Figure 3a]. The funnel plot clearly indicates inadequate literature on this topic [Figure 3b].
Table 4.
Pooled prevalence of B-pattern with heterogeneity coefficient
| Study | Prevalence | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| Ortner et al.[8] | 0.68 | 0.52 | 0.81 | 33.47 |
| Zeileskiewicz et al.[9] | 0.25 | 0.08 | 0.47 | 32.54 |
| Pachtman et al.[14] | 0.02 | 0.00 | 0.07 | 34.00 |
| Pooled prevalence | 0.28 | 0.03 | 0.84 | 100.00 |
| I 2 | 97.14 | 94.29 | 98.56 | |
| Cochran’s Q | 69.84 | |||
| Chi-square, P | 0.00 | |||
| tau2 | 0.82 |
LCI: Lower confidence interval; HCI: Higher confidence interval
Figure 3.
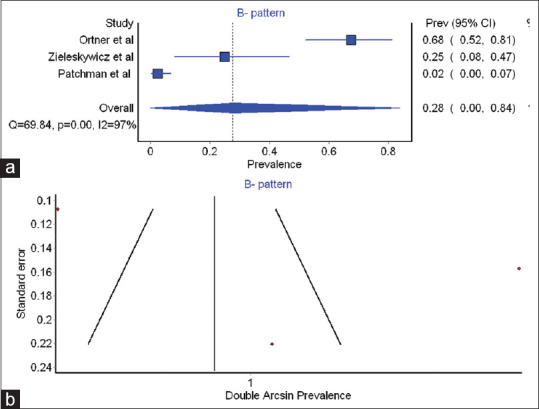
(a) Forest plot for B pattern (b) Funnel plot for-B pattern
Secondary outcomes
Ability of POCUS to find out if LUS can accurately detect lung oedema before the appearance of clinical signs
Four studies worked on this.[8,9,12,14] B-lines are indicative of increased pulmonary congestion and herald interstitial oedema before the appearance of clinical signs of pulmonary oedema. Interstitial oedema is a clinically silent step preceding alveolar oedema.[16] Alveolointerstitial syndrome is characterised by multiple B-lines 7 mm apart caused by thickened interlobular septa.[9] Zieleskiewicz et al. found no B-line pattern in healthy parturients, but found increased B-pattern in parturients with SPE (25 vs. 0%; P = 0.047).[9] Clinically, acute pulmonary congestion was seen in 20% of cases of SPE. A total of 83% of these cases of pulmonary congestion had B-pattern. B-pattern was also seen in asymptomatic SPE cases.[9]
Pachtman et al.[14] found that the LUS patterns in the pre-eclamptics without clinical signs of pulmonary oedema and respiratory symptoms resembled those in healthy gravidas. Also, women with SPE had B-lines more frequently than women without severe features. The authors have said that these B-lines may indicate increased pulmonary congestion and are an early sign of interstitial oedema that precedes clinical signs of pulmonary oedema.
Ortner et al.[8] were able to detect B-patterns on LUS (indicative of PIS) in 24% of women with late-onset SPE.
In the study by Hammad et al., auscultation on hospital admission revealed lung congestion in eleven of the 60 pre-eclamptics. Six of these developed symptoms of pulmonary oedema within 48 h after delivery.[10] The median (IQR) values of LUS scores (cut-off value 16.7 to detect pulmonary oedema) were 19 (17-20) in pre-eclamptic parturients with pulmonary oedema and 3 (2–3) in the pre-eclamptics without pulmonary oedema [Table 2].
Correlation between LUS measures of EVLW with other POCUS values and ratios
Correlations between LUS estimates of EVLW like LUS scores, B-line pattern and other echocardiographic values and ratios were tested in three studies.[8,9,10] Good correlations with clinically significant implications were found between the parameters [Table 3].
Prevalence of POCUS-derived abnormalities in pre-eclampsia [Table 2]
Three studies found the prevalence of POCUS-derived abnormalities including an increase in B-pattern, interstitial oedema and pleural effusion on LUS, increased E/e' ratio and increased LVEDP on echocardiography (cardiac ultrasound) and increased ONSD on optic nerve ultrasound. We could meta-analyse the prevalence of pleural effusion among pre-eclampsia arm patients in two studies.[8,9] The summary prevalence of pleural effusion among women having pre-eclampsia was found to be 0.1% (0–0.2) or 10% (0%–20%). The coefficient of heterogeneity was found to be 63.6, indicating mild heterogeneity among the studies [Table 5 and Figure 4a]. The Funnel plot clearly indicates publication bias [Figure 4b] and hence, a dearth of evidence in this field.
Table 5.
Summary prevalence of pleural effusion
| Study | Prevalence | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| Ortner et al.[8] | 0.0 | 0.0 | 0.1 | 61.8 |
| Zeileskiewicz et al.[9] | 0.2 | 0.0 | 0.3 | 38.2 |
| Pooled prevalence | 0.1 | 0 | 0.2 | 100 |
| Statistics | ||||
| I 2 | 63.6 | 0 | 91.7 |
LCI: Lower confidence interval; HCI: Higher confidence interval
Figure 4.
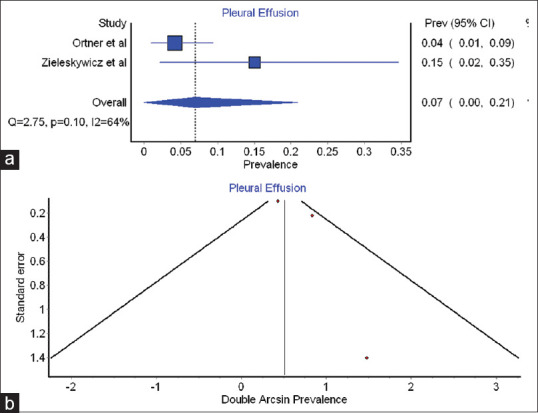
(a) Forest plot indicating summary prevalence of pleural effusion (b) Funnel plot for- pleural effusion
DISCUSSION
Various sonological estimates of EVLW have been performed in the studies and have found their utility in assessing the volume status and grading the level of fluid overload in the patient. LUS has a relatively brief learning curve that is shorter than for some other techniques.[17] The learning curve for the B-line assessment is said to be shorter than 6 weeks.[18] Recommendations for LUS training differ between countries. Most recommendations set a fixed number of examinations that the operator must perform to be competent.[19,20] These recommendations are based on limited evidence. A tool for generic objective structured assessment of LUS skills (LUS-OSAUS) for use in lung ultrasonography competence has also been developed.[21] Studies on both optic and LUS have reported excellent inter and intra-observer reproducibility.[22,23] In the Pachtman study, the LUS expert and obstetric team had different ultrasonographic interpretations of lung findings in all patients. The authors attributed this to the study design wherein the obstetric team was able to analyse the images in real time, unlike the LUS experts who reviewed the images retrospectively on 3 to 6 s video clips, thus impacting the interpretation. Intra and inter-observer agreement of POCUS of lung interpretation were fair in the Pachtman study, excellent in Ambrozic study for ECS score and E/e' ratio, small in Zieleskiewicz study (4%–5% for ECS) and good in Ortner study.
There are several proposed methods to divide each hemithorax during LUS examination, namely, four areas,[24] six areas,[25] three major areas[26] and 20 scanning sites.[27,28] The techniques of LUS and optic ultrasound were variable in the studies selected in our SR. These included the 28 rib interspace technique, eight region/four region/12 region techniques. A variety of transducers can be used for LUS including phased array, linear array, convex and micro-convex.[17] Inappropriate choice of probes can alter the results making it less valid. The convex and microconvex probes allow visualisation of pleural lines and costophrenic angles. The higher frequency probes like linear probes allow a more detailed evaluation of the pleural line and sub-pleural space.[28] Low-frequency probes are the best for B-line detection.[17] Curvilinear probes with a frequency between 3 and 6 MHz, phased array transducers, convex transducers and cardiac transducers were used for lung ultrasonography and linear probe with a frequency between 6.0 and 13. 0 MHz was used for optic ultrasound in the studies selected for this SR. The choice of probes in the studies are thus appropriate and according to the recommendations.[29]
LUS can be performed in any patient position (supine, lateral or prone) since, other than in pleural effusion, lung abnormality distribution on ultrasonography does not change much.[17] In the majority of the studies in the present SR, LUS and optic ultrasound were performed with the patient in the supine position. All the study cases were in the late stages of pregnancy and whether this supine position was modified/unmodified to prevent supine hypotension syndrome is not mentioned in any of the studies; nonetheless, it is important to take precautions to prevent supine hypotension syndrome.
An LUS study can be either A-line dominant pattern or a B-line dominant pattern.[9,14] It has been suggested that the number of B-lines across the imaging fields correlates with the severity of interstitial oedema.[30,31] It is pertinent to note that though B-patterns signal EVLW, they cannot precisely differentiate between a cardiac and non-cardiac source of EVLW.[32] B-lines due to cardiogenic pulmonary oedema are said to be bilateral, start appearing in dependent zones and recover symmetrically.[17]
The ECSs decreased significantly after delivery in Ambrozic et al. and Mowafy et al.[12,13] studies and also as per our metanalysis results. It is well known that there is water and sodium retention with gain in total body water during pregnancy. Around 2 L of this fluid gets lost during the first week of puerperium.[33] This can explain the fall in ECS scores after delivery. However, the IV and oral fluid intake during labour, delivery and in the 24 h following delivery, the urine output, the increase in LVEDP that occurs in normal pregnancy and pre-eclampsia, the increase in cardiac output and stroke volume due to increased fluid administration can all affect the ECS scores. Fluid management can vary as per anaesthesia technique and this can alter the ECS post-operatively.
Factors that can promote pulmonary oedema in pre-eclampsia include decreased plasma oncotic pressure, endothelial dysfunction, acute severe hypertension, elevated pulmonary vascular hydrostatic pressure, abnormal diastolic left ventricular function and iatrogenic causes like fluid overload and medications like MgSO4. Ambrozic et al.[12] study found that women with pre-eclampsia have a higher E/e' ratio both before delivery and on the first day post-delivery. This suggests that higher end-diastolic left ventricular pressure exists in women with SPE compared with controls. A good linear correlation was observed between B-line patterns and diastolic dysfunction and increased LVEDP (E/e' ratio >9.5) in two of our selected studies[8,9] thus showing that increased LVEDP in the SPE parturient can lead to increased EVLW. Ambrozic et al., however, found higher mean E/e' values in women with pre-eclampsia but could not find a correlation between the number of B-lines and raised E/e' ratio.[12] Some studies have shown that pulmonary A-pattern is associated with a low LVEDP.[16] Increased EVLW has several causes in the patient with SPE and considering this, echocardiography parameters like E/e' ratio can be used as surrogate markers for increased EVLW; nevertheless, the lack of supportive findings for this in Ambrozic et al. study goes to say that further research on this topic of echocardiographic surrogate fluid status markers in SPE cases is warranted.
Early detection of pulmonary oedema before the appearance of clinical signs and before progression to severe pulmonary oedema is important in the management of SPE. Alveolar oedema is preceded by interstitial oedema, which is sub-clinical. B-line predominance (B-pattern) is said to indicate PIS, which is related to interstitial oedema.[16] The Pachtman et al., Ortner et al. and Zieleskiewicz et al. studies in this SR showed the ability of POCLUS in detecting B-pattern predominance, PIS and interstitial oedema in women with SPE.[8,9,14] As previously mentioned by some authors, the finding of B-pattern signals the need to restrict fluids as these patients eventually had decreased oxygen saturation and the need for oxygen support.[9,14] Early detection of pulmonary oedema can guide fluid management and allow early appropriate diuretic therapy.[10] In addition, surrogate POCUS markers of EVLW like E/e' ratio, TFC and ONSD bear good correlation with LUS parameters of EVLW as shown in the studies of the present SR[5,9,10,13]; and hence, these too can be used to effectively predict the amount of excess EVLW and degree of pulmonary congestion and thus guide fluid management in the SPE parturient. However, Ortner et al.[8] study of the current SR could not find any association between increased ONSD and any clinical symptom on admission/cardiopulmonary parameter on ultrasound in their study population of 95 late-onset SPE parturients. The normal ONSD is 5.0 mm and ONSD >5 mm is considered abnormal. ONSD is already an established surrogate marker of increased intracranial pressure.[34,35] Researchers have said that the ONSD changes indirectly reflect a state of intracranial oedema that could easily be a part of generalised oedema of the pre-eclamptic state and could signal fluid overload in the pre-eclamptic patient and serve as predictors of volume status in SPE.[36]
Transthoracic ultrasonography is ideal for the detection and quantification of pleural effusions.[37] Pleural effusion was a finding in 15% of the SPE cases in the Zieleskiewicz et al. study and 4% of SPE cases in the Ortner et al. study of this SR.[8,9] Nonetheless, a cross-sectional study in SPE cases revealed a very high frequency (43.48%) of fluid collections including ascites, pleural effusion and ascites with pleural effusion. The factors responsible for this fluid collection include reduced plasma oncotic pressure, increased mean arterial pressure, structural damage of microvasculature, proteinuria and cardiogenic causes.[38] Interstitial lung oedema, by generating positive interstitial pressure, may favour fluid filtration into the pleural cavity; however, the visceral pleura is said to have a low permeability such that the pleural cavity and the lung extravascular space are independent compartments with no fluid exchange in normal conditions.[39] In both Ortner et al. and Zieleskiewicz et al. studies, PIS was prevalent (24% in Ortner's study and 25% in Zileskiewicz's study), but it is not clear from the results of these studies as to whether pleural effusion co-existed in cases with PIS.[8,9]
The technique of POCUS does have some limitations. Both LUS and optic ultrasound require several measurements and may be time-consuming. In most of the studies included in this SR, the POCUS measurements were taken at least three times with a time interval of 5 min in between. Focal B-lines pattern in posterior lung fields at the lung bases can be caused by atelectasis in hospitalised obstetric patients in supine position rather than by pulmonary oedema.[24] Differences in image interpretation can occur depending on the level of training of the expert doing the ultrasonography[14,40] and formal training is required. In the obese parturient, difficulty in access to the lungs may be encountered.
The present study has some limitations. Some studies included in the SR had a small study population mainly because they focused only on SPE cases.[5,9,12] All the studies in the SR are of observational nature. The implementation of RCTs is difficult in relation to this topic because pulmonary oedema in SPE is not a common entity.[9] Most of the studies selected for our SR are single-centre studies except the Pachtman study, which was carried out in two hospitals.[14] Healthy optimal controls were not included in some studies of the present SR.[5,8,10,13] This can lead to over-estimation of prevalence of certain sonological abnormalities common in late pregnancy. Zieleskewicz et al. study had a large number of unadjusted references, which is a weakness of the study.[9] Also, patients with late-onset pre-eclampsia were studied in one study that included 95 pre-eclamptic patients.[8] Assessment in the early stages would have guided fluid management better. The fluid balance during labour and caesarean delivery was not properly accounted for in some studies.[14] This can result in the inclusion of cases of iatrogenic fluid overload in the prevalence. There were some queries and unclear information about certain data in the selected articles. We had tried to contact the authors for the same, but there was no response from their side and hence they were omitted. Substantial heterogeneity was observed in the selected studies. The funnel plots related to the prevalence of pleural effusion, B-patterns and fall in ECS scores after delivery depict shortage of studies in these areas probably due to publication bias or because of novelty of the topic. Though LUS has been found to be an excellent tool to detect EVLW, large outcome studies investigating LUS-guided fluid management are still lacking; nevertheless, prospective observational studies continue to be published on this topic.[41] All the studies selected for this review are observational in nature. Several problems including poor reporting and biased interpretation of results are incorporated in observational studies. Methodological quality assessment of the studies is a very important step of a systematic review. There are severalcritical appraisal tools for assessment of RCTs; however ,there is a paucity of literature on methodological quality assessment of observational studies.[42,43] Certain Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria specified for observational studies could not be applied in the studies selected in the current review due to the cross-sectional nature of the studies. The CASP tool was found suitable for the said study types, and was hence applied. Nevertheless, this indicates that there is a need for the conduct of high quality studies on the topic of POCUS for fluidstatus assessment in the preeclamptic parturient. There has always been a paucity of research in obstetric critical care[44] and studies in this field are the need of the day. Fluid overload and pulmonary oedema are well-known complications in the pre-eclamptic parturient.[45] Even though there is a multitude of factors involving the pathogenesis of pulmonary oedema in these cases, fluid management remains the cornerstone in the treatment and the results of the current review show that POCUS can guide fluid management. Bedside US is now routinely practised in the intensive care unit and operation theatre.[46] It needs to be extended to the labour room and obstetric critical care unit as well. The technology in POCUS can thus improve the quality of care of the obstetric patient including perioperative care which is the need of the day.[47] Further studies are needed to find out faster and more simplified US techniques and to compare various POCUS parameters including their sensitivity and specificity for the early detection of EVLW in the pre-eclamptic parturient. Further studies on the number of B-lines that herald the onset of clinical signs and symptoms of pulmonary oedema are warranted.
CONCLUSION
On the basis of the findings of this review, it is concluded that the POCUS parameters like ECS, LUS score, B-patterns, ONSD, E/e' ratio and TFC can be useful as early markers of fluid status in the parturient with SPE. POCUS can be a useful tool in the clinical management of the parturient with SPE to identify and grade the presence of EVLW before it clinically manifests. Ultrasonography of the lung should accompany that of the abdomen in pre-eclamptic parturients to assess the fluid status and baseline parameters. Scoring and categorising fluid management based on the scores can guide in optimising treatment and decreasing complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Annexure 1
Identification of components of clinical evidence for the systematic review
| Component | Description, Inclusion/Exclusion criteria |
|---|---|
| P-Population | Included: women with pre-eclampsia, healthy parturients in late pregnancy and in labour |
| Excluded: non-hospitalised pre-eclampsia cases, pre-eclampsia cases not in labour | |
| I-Intervention | POCUS in pre-eclamptics |
| POCUS in pre-eclamptics before delivery | |
| C-Comparison | POCUS in healthy controls (non-pre-eclamptics), POCUS in pre-eclamptics after delivery |
| O-Outcome | Detection and estimation of extravascular lung water to guide fluid management in the pre-eclamptic parturient. |
| S-Study design | Observational studies, non-RCTs, RCTs, case series |
| Excluded-Textbook material | |
| Conference abstracts | |
| Editorials, Case reports | |
| Narrative reviews | |
| Letter to the Editor | |
| Comments |
POCUS: point-of-care ultrasound; RCT: randomised controlled trial
ANNEXURE 2: SOME RELEVANT DEFINITIONS AND ELABORATION OF TERMS USED IN THE SELECTED STUDIES
A-lines: They are horizontal, echogenic, parallel lines below the pleura. They are a result of reverberation artefact between the ultrasound beam and the pleural lines and indicate normal lung aeration when lung sliding is present.
B-lines: They are longer, hyperechoic, discrete, vertical 'laser-like' lines of the interlobular septa due to fluid accumulation in the interstitium and alveoli. B-lines extend to the bottom of the screen without fading and move synchronously with the lung.
A-line pattern: Less than two positive regions per side.
-
B-line pattern: Multiple B-lines in a lung field; this is considered a sonographic sign of EVLW and is suggestive of pulmonary oedema/PIS, but does not imply a specific aetiology of pulmonary oedema.
Presence of three or more B-lines in a longitudinal plane between two ribs is defined as a positive region. Two or more positive regions per side are suggested as a B-pattern.
Multiple B-lines 7 mm apart are produced by thickened interlobular septa due to fluid accumulation in the interstitium and alveoli and these lines represent pulmonary interstitial oedema.
Multiple B-lines 3 mm or less apart are caused by 'ground glass' areas representing alveolar oedema.
Pulmonary interstitial oedema: An ultrasound study with 3 or more B-lines in two or more bilateral lung fields. The number of B-lines across imaging fields is semi-quantitative and correlates with the severity of interstitial oedema.
Pulmonary interstitial syndrome (PIS): A bilateral B-line pattern on lung ultrasound and associated with diastolic dysfunction according to an algorithm of the American Society of Echocardiography. PIS as a sonographic entity can be caused by pulmonary oedema (cardiogenic/non-cardiogenic), interstitial pneumonia or lung fibrosis.
Echo comet score (ECS): It is the sum of B-lines found in each of the 28 chest wall areas (12 on left, 16 on right). It is a lung ultrasound parameter. It denotes the amount of extravascular lung water (EVLW) and pulmonary oedema.
-
E wave, E' wave E/e' ratio: In cardiac ultrasound, E wave represents early mitral flow peak velocity and E' wave represents the early diastolic mitral annulus displacement velocity.
E/e' ratio is a marker of diastolic function. It is an echocardiographic parameter for assessing diastolic LV function. Increased E/e' ratio (9–13) signifies increased LVEDP.
-
B-line score – Total number of B-lines counted in all windows. It is calculated for each zone of the six zones of each hemithorax.
0. = no lines
1. = B-7 lines (B lines 7 mm apart)
2. = B-3 lines (B lines 3 mm apart)
3. = Consolidation (sub-pleural, hypoechoic, wedge-shaped, tissue-like structure).
-
Lung Ultrasound score (LUS): It measures lung aeration. The sum of all lung zones (from 0 to 36).
Sum of B-lines found on each scanning site
[0: absence; 1:B7 lines multiple; 2: B3 lines multiple; 3: consolidation].
Annexure 3
Risk of bias assessment (Quality assessment) in individual studies by Critical Appraisal Skills Programme (CASP) checklist
| Article | Ortner[8] | Ambrozic[12] | Zieleskiewicz[9] | Hammad[10] | Simenc[5] | Mowafy[13] | Pachtman[14] |
|---|---|---|---|---|---|---|---|
| Did the study address a clearly focused issue? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the cohort recruited in an acceptable way? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the exposure accurately measured to minimise bias? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the outcome accurately measured to minimise bias? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Have all confounding factors been identified and considered in the design and/or analysis? | Yes | No | No | No | No | Yes | No |
| Was the follow up of subjects long and complete enough | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| How precise are the results? | Moderate | high | high | Moderate | Moderate | Moderate | Low |
| Can the results be applied to the local population? | Yes | Yes | No | No | No | No | Yes |
| Are the results believable | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Do the results of this study fit with other available evidence? | Yes | Yes | Yes | Yes | Yes | Yes | Can’t tell |
| Estimated potential of bias | Low | Moderate | Moderate | High | Low | Low | Low |
REFERENCES
- 1.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: An overview. Circulation. 2014;130:703–14. doi: 10.1161/CIRCULATIONAHA.113.003664. [DOI] [PubMed] [Google Scholar]
- 2.Vaught AJ, Kovell LC, Szymanski LM, Mayer SA, Seifert SM, Vaidya D, et al. Acute cardiac effects of severe pre-eclampsia. J Am Coll Cardiol. 2018;72:1–11. doi: 10.1016/j.jacc.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis AT, Solnordal CB. Acute pulmonary oedema in pregnant women. Anaesthesia. 2012;67:646–59. doi: 10.1111/j.1365-2044.2012.07055.x. [DOI] [PubMed] [Google Scholar]
- 4.Lewis G The confidential enquiry into maternal and child health (CEMACH) The Seventh Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH; 2007. Saving mothers’ Lives: Reviewing maternal deaths to make motherhood safer 2003–2005. [Google Scholar]
- 5.Simenc GB, Ambrozic J, Prokselj K, Tul N, Cvijic M, Mirkovic T, et al. Optic nerve ultrasound for fluid status assessment in patients with severe preeclampsia. Radiol Oncol. 2018;52:377–82. doi: 10.2478/raon-2018-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskins SC, Vaz AM, Garvin S. Perioperative point-of-care ultrasound for the anesthesiologist. J Anesth Perioper Med. 2018;28:92–6. [Google Scholar]
- 7.Dennis AT. Transthoracic echocardiography in obstetric anaesthesia and obstetric critical illness. Int J Obstet Anesth. 2011;20:160–8. doi: 10.1016/j.ijoa.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ortner CM, Krishnamoorthy V, Neethling E, Flint M, Swanevelder JL, Lombard C, et al. Point-of-Care ultrasound abnormalities in late-onset severe preeclampsia: Prevalence and association with serum albumin and brain natriuretic peptide. Anesth Analg. 2019;128:1208–16. doi: 10.1213/ANE.0000000000003759. [DOI] [PubMed] [Google Scholar]
- 9.Zieleskiewicz L, Contargyris C, Brun C, Touret M, Vellin A, Antonini F, et al. Lung ultrasound predicts interstitial syndrome and hemodynamic profile in parturients with severe preeclampsia. Anesthesiology. 2014;120:906–14. doi: 10.1097/ALN.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 10.Hammad Y, Hasanin A, Elsakka A, Refaie A, Abdelfattah D, Rahman SA, et al. Thoracic fluid content: A novel parameter for detection of pulmonary edema in parturients with preeclampsia. J Clin Monit Comput. 2019;33:413–8. doi: 10.1007/s10877-018-0176-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27:889–94. doi: 10.1016/j.arthro.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Ambrozic J, BrzanSimenc G, Prokselj K, Tul N, Cvijic M, Lucovnik M. Lung and cardiac ultrasound for hemodynamic monitoring of patients with severe pre-eclampsia. Ultrasound Obstet Gynecol. 2017;49:104–9. doi: 10.1002/uog.17331. [DOI] [PubMed] [Google Scholar]
- 13.Mowafy SM, Elsayed M. Optic nerve sheath diameter versus extra-vascular lung water detected by ultrasound in volume status prediction in severe preeclampsia. Egypt J Anaesth. 2020;36:184–93. [Google Scholar]
- 14.Pachtman Shetty SL, Koenig S, Tenenbaum S, Meirowitz N. Point-of-care lung ultrasound patterns in late third-trimester gravidas with and without preeclampsia. Am J Obstet Gynecol MFM. 2021;3:100310. doi: 10.1016/j.ajogmf.2021.100310. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists. Task force on Hypertension in Pregnancy. Hypertension in Pregnancy 2013. [Last accessed on 2021 Aug 29]. Available from: https://journals.lww. com/greenjournal/Fulltext/2013/11000/Hypertension_in_ Pregnancy__Executive_Summary. 36.aspx . [DOI] [PubMed]
- 16.Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: Lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136:1014–20. doi: 10.1378/chest.09-0001. [DOI] [PubMed] [Google Scholar]
- 17.Gargani L, Volpicelli G. How I do it: Lung ultrasound. Cardiovasc Ultrasound. 2014;12:25. doi: 10.1186/1476-7120-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble VE, Lamhaut L, Capp R, Bosson N, Liteplo A, Marx JS, et al. Evaluation of a thoracic ultrasound training module for the detection of pneumothorax and pulmonary edema by prehospital physician care providers. BMC Med Educ. 2009;9:3. doi: 10.1186/1472-6920-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzan Simenc GB, Ambrozic J, Prokselj K, Tul N, Cvijic M, Mirkovic T, et al. Ocular ultrasonography for diagnosing increased intracranial pressure in patients with severe preeclampsia. Intl J Obstet Anesth. 2018;36:49–55. doi: 10.1016/j.ijoa.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508–14. doi: 10.1016/j.annemergmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Havelock T, Teoh R, Laws D, Gleeson F. BTS Pleural Disease Guideline Group.Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii61–76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 22.European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Appendix 11. Thoracic ultrasound London: EFSUMB; 2008. Oct, [Last accessed on 2021 Aug 29]. Minimum training requirements for the practice of medical ultrasound in Europe. Available from: https://efsumb.org/wp-content/ uploads/2020/12/2009-04-14apx11.pdf . [Google Scholar]
- 23.Skaarup SH, Laursen CB, Bjerrum AS, Hilberg O. Objective and structured assessment of lung ultrasound competence.A multispecialty Delphi consensus and Construct Validity Study. Ann Am Thorac Soc. 2017;14:555–60. doi: 10.1513/AnnalsATS.201611-894OC. [DOI] [PubMed] [Google Scholar]
- 24.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35(5 Suppl):S250–61. doi: 10.1097/01.CCM.0000260674.60761.85. [DOI] [PubMed] [Google Scholar]
- 26.Cattarossi L. Lung ultrasound: Its role in neonatology and pediatrics. Early Hum Dev. 2013;89(Suppl 1):S17–9. doi: 10.1016/S0378-3782(13)70006-9. [DOI] [PubMed] [Google Scholar]
- 27.Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: A clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356–63. doi: 10.1016/j.echo.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Cantinotti M, Giordano R, Volpicelli G, Kutty S, Murzi B, Assanta N, et al. Lung ultrasound in adult and paediatric cardiac surgery: Is it time for routine use? Interact Cardiovascu Thorac Surg. 2016;22:208–15. doi: 10.1093/icvts/ivv315. [DOI] [PubMed] [Google Scholar]
- 29.Miller A. Practical approach to lung ultrasound. BJA Educ. 2016;16:39–45. [Google Scholar]
- 30.Liteplo AS, Marill KA, Villen T, Miller RM, Murray AF, Croft PE, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): Sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16:201–10. doi: 10.1111/j.1553-2712.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Volpicelli G, Melniker LA, Cardinale L, Lamorte A, Frascisco MF. Lung ultrasound in diagnosing and monitoring pulmonary interstitial fluid. Radiol Med. 2013;118:196–205. doi: 10.1007/s11547-012-0852-4. [DOI] [PubMed] [Google Scholar]
- 32.Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121:320–7. doi: 10.1097/ALN.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 33.Dutta DC, editor. Textbook of Obstetrics. 2nd ed. Kolkata: New Central Book Agency; 1990. Physiological changes during pregnancy; pp. 48–61. [Google Scholar]
- 34.Dubost C, Le Gouez A, Jouffroy V, Roger-Christoph S, Benhamou D, Mercier FJ, et al. Optic nerve sheath diameter used as ultrasonographic assessment of the incidence of raised intracranial pressure in preeclampsia: A pilot study. Anesthesiology. 2012;116:1066–71. doi: 10.1097/ALN.0b013e318246ea1a. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK, Bhatia K. Ultrasonographic optic nerve sheath diameter as a surrogate measure of raised intracranial pressure in severe pregnancy-induced hypertension patients. Anesth Essays Res. 2018;12:42–6. doi: 10.4103/aer.AER_218_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferro F, Rocha E, Nobrega L, Amorim MM, Katz L. Transorbital ultrasonographic measurement of the optic nerve sheath diameter in preeclampsia. Obstet Gynecol. 2016:127–87. [Google Scholar]
- 37.Kocijancic I, Kocijancic K, Cufer T. Imaging of pleural fluid in healthy individuals. Clin Radiol. 2004;59:826–29. doi: 10.1016/j.crad.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez-Rodriguez JG, Veloz-Martinez MG. Pleural effusion and ascites in severe preeclampsia: Frequency and correlation with plasma colloid osmotic pressure and renal filtration function. Cir Cir. 2011;79:299–305. [PubMed] [Google Scholar]
- 39.Miserocchi G. Mechanisms controlling the volume of pleural fluid and extravascular lung water. Eur Respir Rev. 2009;18:244–52. doi: 10.1183/09059180.00002709. [DOI] [PubMed] [Google Scholar]
- 40.Gullet J, Donnelly JP, Sinert R, Hosek B, Fuller D, Hill H, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395–9. doi: 10.1016/j.jcrc.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Mittal AK, Jaipuria J, Patel A, Bhatnagar V, Chawla R, Singh S. Utility of lung ultrasound for extravascular lung water volume estimation during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Indian J Anaesth. 2021;65:458–64. doi: 10.4103/ija.IJA_1513_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manchikanti L, Singh V, Smith HS, Hirsch JA. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 4: observational studies. Pain Physician. 2009;12:73–108. [PubMed] [Google Scholar]
- 43.Lin-Lu M, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment of tools for primary and secondary medical studies: What are they and which is better? Military Medical Research. 2020. [Last accessed on 2021 Oct 13]. pp. 7–7. Available from: https://doi.org/10.1186/s40779-020-00238-8 . [DOI] [PMC free article] [PubMed]
- 44.Bajwa SK, Bajwa SJ. Delivering obstetrical critical care in developing nations. Int J Crit Illn Inj Sci. 2012;2:32–9. doi: 10.4103/2229-5151.94897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Upadya M, Rao ST. Hypertensive disorders in pregnancy. Indian J Anaesth. 2018;62:675–81. doi: 10.4103/ija.IJA_475_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehdiratta L, Bajwa SJS. Technology, engineering and innovations- Power buffers in the COVID driveline…. Indian J Anaesth. 2021;65:351–5. doi: 10.4103/ija.ija_423_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajwa SJS, Mehdiratta L. Adopting newer strategies of perioperative quality improvement: The bandwagon moves on…. Indian J Anaesth. 2021;65:639–43. doi: 10.4103/ija.ija_866_21. [DOI] [PMC free article] [PubMed] [Google Scholar]