Abstract
Cellular DNA-dependent RNA polymerase II (pol II) has been postulated to carry out RNA-dependent RNA replication and transcription of hepatitis delta virus (HDV) RNA, generating a full-length (1.7-kb) RNA genome and a subgenomic-length (0.8-kb) mRNA. However, the supporting evidence for this hypothesis was ambiguous because the previous experiments relied on DNA-templated transcription to initiate HDV RNA synthesis. Furthermore, there is no evidence that the same cellular enzyme is involved in the synthesis of both RNA species. In this study, we used a novel HDV RNA-based transfection approach, devoid of any artificial HDV cDNA intermediates, to determine the enzymatic and metabolic requirements for the synthesis of these two RNA species. We showed that HDV subgenomic mRNA transcription was inhibited by a low concentration of α-amanitin (<3 μg/ml) and could be partially restored by an α-amanitin-resistant mutant pol II; however, surprisingly, the synthesis of the full-length (1.7-kb) antigenomic RNA was not affected by α-amanitin to a concentration higher than 25 μg/ml. By several other criteria, such as the differing requirement for the de novo-synthesized hepatitis delta antigen and temperature dependence, we further showed that the metabolic requirements of subgenomic HDV mRNA synthesis are different from those for the synthesis of genomic-length HDV RNA and cellular pol II transcripts. The synthesis of the two HDV RNA species could also be uncoupled under several different conditions. These findings provide strong evidence that pol II, or proteins derived from pol II transcripts, is involved in mRNA transcription from the HDV RNA template. In contrast, the synthesis of the 1.7-kb HDV antigenomic RNA appears not to be dependent on pol II. These results reveal that there are distinct molecular mechanisms for the synthesis of these two RNA species.
Hepatitis delta virus (HDV) is a subviral particle containing a circular RNA genome of 1.7 kb which resembles plant viroid RNAs and contains ribozyme activities (27). HDV RNA can replicate itself in cultured cells, requiring only a virus-encoded protein, the hepatitis delta antigen (HDAg) (13, 26). HDAg, however, does not possess an RNA polymerase activity. Thus, it has always been assumed that HDV utilizes host cell RNA polymerases to replicate its RNA genome, in a mechanism similar to that of viroid RNA replication (40). However, the dependence of HDV RNA replication on a viral protein (HDAg) distinguishes HDV RNA synthesis from the synthesis of plant viroid RNA, which does not encode any protein. Furthermore, unlike plant cells (41), animal cells are not known to have RNA-dependent RNA polymerases. These issues raised interesting questions concerning which cellular polymerases are responsible for HDV RNA-dependent RNA synthesis and how they are converted from DNA- to RNA-templated polymerases.
The common belief that HDV RNA synthesis is carried out by cellular RNA polymerase II (pol II) came from early in vitro transcription studies using nuclear extracts of HDV-replicating cells, which revealed that HDV RNA synthesis could be inhibited by α-amanitin at concentrations as low as 1 μg/ml (31). However, these studies used nuclear extracts from a cell line (H1δ9) (31) that contains an integrated cDNA trimer of HDV under the control of a foreign promoter. This cDNA first transcribes an HDV RNA, which is then replicated by RNA-dependent RNA synthesis. The presence of HDV cDNA in the assay system rendered it uncertain whether the observed effect of α-amanitin was due to inhibition of pol II-mediated transcription from the HDV cDNA or inhibition of subsequent RNA-dependent RNA synthesis. In fact, when the same experiments were performed using an exogenously added HDV RNA template in an uninfected cell extract, the HDV RNA synthesis was not inhibited by α-amanitin (31). Another piece of evidence in support of the role of pol II in HDV RNA synthesis came from the study using purified pol II and basal transcription factors in a reconstituted in vitro transcription assay, which showed that RNA synthesis from an HDV RNA template was sensitive to α-amanitin (14). This appears to provide the most direct evidence that pol II carries out RNA-dependent synthesis of the HDV RNA species. Unfortunately, this result has so far not been reproduced. Also, in all of these in vitro transcription systems, HDAg was not required and its addition had no effect on HDV RNA synthesis. The absence of a role for HDAg in these assays cast further doubt on the biological relevance of these in vitro studies.
During HDV replication, three HDV RNA species are produced: the 1.7-kb antigenome, the 1.7-kb genome, and the 0.8-kb antigenomic-sense RNA. The former two RNA species form circular RNA and represent the replication products of the HDV RNA genome. The 0.8-kb RNA, however, is polyadenylated and thus resembles cellular pol II transcripts. This RNA is the mRNA for translation of HDAg (29). In the HDV-infected cells, two forms of HDAg are found: a small form (S-HDAg), which is 195 amino acids in length, and a large form (L-HDAg), which is 214 amino acids in length (2, 4, 35, 38). Both forms are translated from the same open reading frame present on the 0.8-kb mRNA; the large form results from an RNA editing event (5, 36, 37), extending the S-HDAg open reading frame by 19 amino acids to encode the L-HDAg. The S-HDAg is required for HDV RNA replication in vivo (26). In contrast, the L-HDAg inhibits HDV RNA replication (7, 17).
Since the 1.7-kb antigenome and the 0.8-kb mRNA are synthesized from the same genomic RNA template, a central question is how the synthesis of these two RNA species is regulated. An earlier hypothesis based on the results from cDNA transfection studies proposed that the primary transcript from the genomic RNA template is the 0.8-kb mRNA (8, 18). Synthesis of the 1.7-kb antigenome occurs only after suppression of the polyadenylation signal by HDAg (19, 20). In this model, the same cellular polymerase is proposed to carry out the synthesis of both the 1.7-kb antigenome and 0.8-kb mRNA. However, using a cDNA-free transfection system (33), we found that the 0.8-kb mRNA continues to be synthesized throughout the replication cycle, and increasing amounts of HDAg do not suppress the synthesis of the 0.8-kb mRNA. These findings suggested that the synthesis of the 1.7-kb antigenome and the synthesis of the 0.8-kb mRNA are under different mechanisms of regulation.
We have investigated the enzymatic requirements of HDV RNA replication (synthesis of the 1.7-kb antigenome) and transcription (synthesis of the 0.8-kb mRNA) from the HDV genomic RNA template in cell culture. Using a cDNA-free HDV RNA transfection system, we established that the synthesis of the 0.8-kb mRNA is sensitive to α-amanitin at a concentration of 3 μg/ml and this inhibition can be relieved by an α-amanitin-resistant pol II mutant. Surprisingly, synthesis of the 1.7-kb antigenome is insensitive to α-amanitin at concentrations as high as 25 μg/ml. Furthermore, the synthesis of the 1.7-kb antigenome, but not the 0.8-kb mRNA, is dependent on the production of newly translated S-HDAg, further discriminating between the mechanisms of synthesis of these two RNA species. Finally, synthesis of the 0.8-kb mRNA from the HDV RNA genome can be distinguished from DNA-templated mRNA synthesis by cellular pol II. Taken together, these results support the hypotheses that pol II, or at least protein products from pol II transcripts, is involved in the synthesis of the 0.8-kb mRNA from the HDV RNA genome and that the mechanisms of synthesis of the 1.7-kb antigenome and the 0.8-kb mRNA are distinct. Furthermore, they raised the interesting possibility that the 1.7-kb HDV antigenomic RNA is synthesized by a cellular polymerase other than pol II.
MATERIALS AND METHODS
Cell culture and transfection.
Huh7 cells (34) were cultured at 37°C in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU of penicillin per ml, 100 mg of streptomycin per ml, 2 mM l-glutamate, and 1% nonessential amino acids (complete DMEM). BC10 M/E cells, a mouse cell line (10), were cultured at 37°C in DMEM supplemented with 10% fetal bovine serum, 100 IU of penicillin per ml, and 100 mg of streptomycin per ml. The BC10ME-derived permanent cell line BCHAWT, which expresses an α-amanitin-resistant mutant pol II, was selected in G418 at 600 μg/ml after transfection with plasmid pHAWT (see below). The resulting clones were further selected in α-amanitin (Sigma) at 10 μg/ml, and the clones resistant to both G418 and α-amanitin were expanded for further analysis. E10 control cells transfected with the empty vector pcDNA3 were selected in G418 only. E10 and BCHAWT cells were cultured at 37°C in DMEM supplemented with 10% fetal bovine serum, 100 IU of penicillin per ml, and 7.5 μg of gentamicin per ml. Tsδ3 cells, which were derived from a temperature-sensitive hamster cell line (42) and stably express S-HDAg from an integrated cDNA copy of the HDAg-encoding mRNA under the control of the cytomegalovirus (CMV) promoter (22), were cultured at 34°C in DMEM supplemented with 10% fetal bovine serum, 100 IU of penicillin per ml, and 7.5 μg of gentamicin per ml. All transfections were performed using the DMRIE-C reagent (Gibco BRL) in accordance with the protocol provided by the manufacturer, with slight modifications. Briefly, 1 day prior to transfection, cells were seeded onto 60-mm-diameter dishes. On the following day, cells were transfected with an appropriate amount of RNA (typically 5 to 10 μg) in 2 ml of transfection mixture in serum-free medium. After 1 to 2 h, 2 ml of culture medium containing 20% fetal bovine serum was added to the cells. Following incubation overnight, the culture medium was replaced with fresh medium and the cells were further incubated for an additional 1 to 5 days. For experiments involving the use of α-amanitin, various amounts of α-amanitin dissolved in sterile water were added directly to the culture medium.
Vectors and plasmid construction.
Construction of plasmid PX9-I/II, which expresses the genotype I/II chimeric S-HDAg, was reported previously (33). Plasmid pKS/HDV1.9, which expresses 1.9-kb genomic-sense HDV RNA (a full-length HDV genome plus 200 additional HDV nucleotides [nt]) under the control of the CMV promoter, and pKS/H2ag, which expresses the antigenomic strand of a mutant trimer HDV RNA under the control of the CMV promoter, were described in a previous study (24). pCMV/neo-Sm (25), which expresses an S-HDAg, was used for HDV cDNA transfection experiments. Plasmid pKS/HDV1.9m, which expresses 1.9-kb genomic-sense HDV RNA with a truncated open reading frame for HDAg, was constructed by digesting pKS/HDV1.9 (24) with AflII (nt 1209), followed by a fill-in reaction with the Klenow fragment to blunt the ends. The blunt-ended product was self-ligated to produce the final plasmid, which contains an insertion of 5 nt to introduce a stop codon. Plasmid pBS/T7GSacII, which expresses nt 25 to 658 of the HDV genomic-sense RNA under the control of the T7 promoter, was constructed by deleting the SacII fragment from plasmid pBS/T7G, which contains the PstI fragment of the American HDV isolate (32) inserted into the PstI site of pBSII/KS+. Plasmid pArg-Maxi, used to measure pol III transcription, is a derivative of a Drosophila tRNAArg gene where 12 nt have been inserted between the internal promoter regions to serve as a marker (15). Plasmid pHAWT, which contains the mouse genomic DNA encoding an α-amanitin-resistant mutant form of the pol II largest subunit, RPII215, with a 793N→D mutation (1), was a gift from J. L. Corden (Johns Hopkins University, Baltimore, Md.). A hemagglutinin tag was placed at the N terminus of the protein.
In vitro transcription.
Genomic HDV RNA (1.9 kb) which contains the entire HDV genome plus approximately 200 additional nt of the HDV sequence was transcribed from pKS/HDV1.9 (24) using the T7 MEGAscript transcription kit (Ambion) after linearization of the plasmid by EcoRV digestion. Antigenomic HDV RNA (1.9 kb) was transcribed from pKS/HDV1.9 using SP6 MEGAscript (Ambion) after linearization of the plasmid by SnaBI digestion. Capped mRNAs used for translation of wild-type and genotype I/II-chimeric S-HDAg were transcribed from plasmids PX9 (33) and PX9-I/II, respectively, using T7 mMESSAGE mMACHINE (Ambion) after linearization of plasmids by HindIII digestion.
Northern blot analysis.
Total RNA was extracted from transfected cells using the guanidinium thiocyanate method (9). The RNA was digested with RQ1 DNase (Promega), treated with formaldehyde, electrophoresed through formaldehyde-containing 1.2% agarose gels, blotted onto a nitrocellulose membrane (Hybond C extra; Amersham), and probed with [32P]UTP-labeled HDV strand-specific riboprobes. Riboprobes were transcribed from plasmid S18 (32) (to detect genomic HDV RNA) after linearization with HindIII digestion or from pBS/T7GSacII (to detect antigenomic HDV RNA in the noncoding region) after linearization of plasmids with HindIII digestion. To detect newly synthesized 0.8-kb mRNA, 32P-end-labeled oligonucleotide 1565A (1565-CCCCGCGGTCTTTCCTTCTTTCGGACC-1581) (33), which is specific for the American isolate of genotype I HDV (32), was used as a probe. The protocol for Northern blots using oligonucleotide probes was adapted from a published protocol (16).
Western blot analysis.
Protein was extracted from transfected cells by the standard method (39) and separated by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred to a nitrocellulose membrane (Hybond C extra; Amersham) and detected by the ECL Western blot detection system (Amersham) using a combination of three monoclonal antibodies against HDAg (23) and visualized by autoradiography.
RNase protection assay.
RNA was analyzed by RNase protection assay using an RPA II RNase Protection Assay Kit (Ambion). Briefly, [32P]UTP-labeled probe (antisense to the expressed Arg maxigene) was transcribed from plasmid pArg-Maxi after linearization with XbaI digestion. Approximately 106 cpm of radiolabeled probe was mixed with 2.5 μg of total RNA from the transfected cells, heat denatured, and incubated at 43°C overnight. After hybridization, the sample was digested by RNase A and T1 and precipitated in accordance with the protocol provided with the kit. The precipitated RNA was resuspended in 8 μl of gel loading buffer (provided with the kit) and subjected to electrophoresis at 20 mA in an 8% acrylamide–8 M urea gel. The protected bands were visualized by autoradiography.
RESULTS
Synthesis of the 0.8-kb mRNA is sensitive to inhibition by a low concentration of α-amanitin in cell culture.
In order to evaluate the possible role of pol II in HDV RNA replication, we examined the sensitivity of HDV RNA synthesis to α-amanitin in cell culture. Previous studies on this issue have used in vitro nuclear lysates that contained an HDV cDNA template and thus were compromised by an artificial requirement for DNA-templated transcription. Furthermore, these assays were not efficient enough for identification of different HDV RNA species (14, 31). To circumvent these problems, we used a recently established HDV RNA transfection approach (33) in which Huh7 cells were transfected with in vitro-transcribed antigenomic-sense 1.9-kb HDV RNA and capped mRNA encoding S-HDAg. As a comparison, we also performed HDV cDNA transfection using a plasmid that would transcribe a 1.9-kb genomic-sense HDV RNA under the control of the CMV promoter. The primary transcripts from both the transfected RNA and DNA are genomic-sense HDV RNAs, and both approaches lead to robust HDV RNA replication. Various concentrations of α-amanitin were added to the culture at the time of transfection, and HDV genomic-sense RNA was examined 3 days posttransfection. We found that Huh7 cells could survive for at least 3 days in the presence of α-amanitin, although the cells showed some cytotoxicity after 2 days of treatment (data not shown). As shown in Fig. 1, HDV RNA (1.7-kb) synthesis in both RNA- and DNA-transfected cells was inhibited by α-amanitin at 3 μg/ml consistent with the previous in vitro data (31) and with the hypothesis that pol II mediates both RNA- and DNA-templated HDV RNA synthesis. Similar results were obtained when the reverse experiment was performed, i.e., the in vitro-transcribed 1.9-kb genomic-sense RNA was used for transfection and antigenomic RNA synthesized in the transfected cells was examined (data not shown).
FIG. 1.
Northern blot demonstrating the effects of α-amanitin on HDV genomic RNA synthesis from either cDNA or RNA templates. Huh7 cells were transfected with either plasmid pKS/1.9 or in vitro-transcribed 1.9-kb antigenomic HDV RNA plus an HDAg-encoding mRNA (33). The indicated amounts of α-amanitin were added to cells at the time of transfection. Cells were harvested 3 days posttransfection, and total RNA was analyzed by Northern blot assay using 32P-labeled antigenomic-sense HDV RNA as a probe. Lanes: 1, total RNA from H1δ9 cells indicating the position of the 1.7-kb genomic RNA; 2 to 5, total RNA from cells transfected with HDV cDNA; 6 to 9, total RNA from cells transfected with HDV RNA.
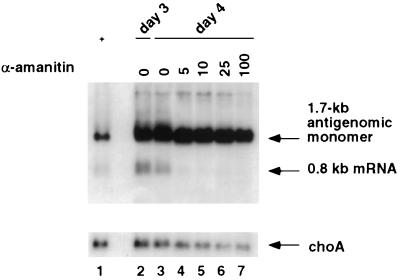
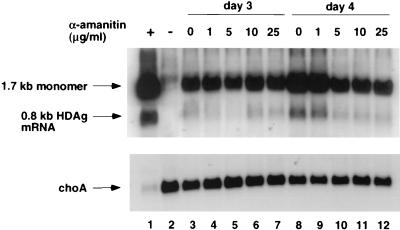
Since α-amanitin was added at the time of transfection, the inhibition of HDV RNA (1.7 kb) replication by α-amanitin observed in this experiment could be an indirect effect of inhibition of 0.8-kb mRNA transcription if a large amount of HDAg needs to be synthesized before HDV RNA replication can occur. Also, the inhibition of HDV RNA synthesis could be a nonspecific cytotoxic effect of prolonged treatment with α-amanitin. To examine these possibilities, we performed another experiment in which α-amanitin was not added until 3 days posttransfection to allow a sufficient amount of HDAg to accumulate prior to α-amanitin treatment. HDV antigenomic-sense RNA was analyzed on day 4 to detect both the 1.7-kb and 0.8-kb RNA species (Fig. 2). The result showed that the synthesis of the 0.8-kb mRNA was completely inhibited by α-amanitin at 5 μg/ml, suggesting that α-amanitin inhibits HDV subgenomic mRNA synthesis per se. Surprisingly, under this condition, the synthesis of the 1.7-kb antigenomic RNA was almost completely resistant to α-amanitin at a concentration as high as 25 μg/ml. α-Amanitin at 100 μg/ml caused a slight decrease in 1.7-kb RNA. The mechanism of resistance of this RNA species to α-amanitin was further investigated, and the findings are presented in later sections.
FIG. 2.
Sensitivity of 0.8-kb mRNA synthesis to α-amanitin. Huh7 cells were cotransfected with in vitro-transcribed 1.9-kb genomic RNA and 0.8-kb HDV mRNA (33). Cells were treated with different concentrations of α-amanitin on day 3 posttransfection, and RNA was harvested on day 4. The RNA blot was probed for HDV antigenomic-sense RNA with 32P-end-labeled oligonucleotide 1565A, which detects the mRNA transcribed from the 1.9-kb genomic RNA but not the transfected mRNA (33). The same membrane was probed for choA mRNA. Lanes: 1, positive control from previously transfected Huh7 cells indicating the positions of the 1.7-kb antigenome and the 0.8-kb HDV mRNA; 2, total RNA from transfected cells on day 3 posttransfection; 3 to 7, total RNA from transfected cells at day 4 posttransfection after treatment with the indicated amounts of α-amanitin on day 3.
As a control, choA RNA, which is an endogenous cellular pol II transcript, was found to be inhibited by a low concentration of α-amanitin, although choA RNA appears to be more resistant than the 0.8-kb HDV RNA, probably because of hte longer half-life of choA mRNA. These results suggest that transcription of the 0.8-kb HDV mRNA is at least as sensitive to α-amanitin as the cellular pol II transcripts.
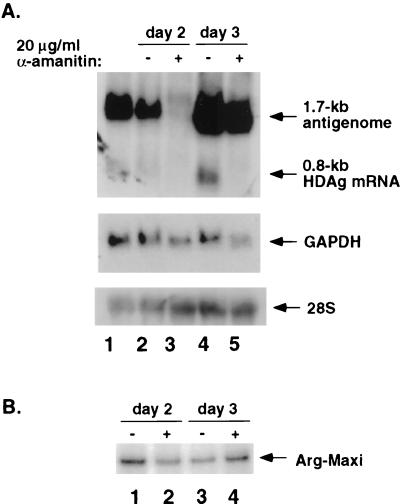
To further rule out the possibility that α-amanitin caused generalized inhibition of transcription, we tested the transcription from an RNA pol III-dependent promoter under the same condition. HDV RNA and a pol III reporter plasmid, pArg-Maxi (15), which expresses tRNAArg containing a 12-nt insertion to allow specific detection of this transcript, were cotransfected into Huh7 cells. We used a higher concentration of α-amanitin for this experiment than necessary for inhibition of the 0.8-kb mRNA. Cells were treated with α-amanitin (20 μg/ml) on days 1 and day 2 posttransfection, and RNA was harvested 1 day later. The results showed that, at both time points, the synthesis of the 0.8-kb HDV mRNA was completely inhibited (Fig. 3A, lanes 2 to 5); in contrast, transcription from the pol III promoter, as detected by RNase protection assay, was not affected by α-amanitin at all (Fig. 3B, lanes 1 to 4). This result supports the conclusion that the inhibition of 0.8-kb HDV mRNA synthesis in α-amanitin-treated cells was due to specific inhibition of pol II transcription but was not a result of the generalized cytotoxicity of α-amanitin. This result also showed that the 1.7-kb antigenomic RNA was resistant to α-amanitin treatment when α-amanitin was added on day 2 and RNA was examined on day 3 (Fig. 3A, lanes 4 and 5). However, when α-amanitin was added on day 1 and RNA was examined 1 day later, the synthesis of the 1.7-kb antigenome was completely inhibited by α-amanitin (Fig. 3A, lanes 2 and 3). These results confirmed those presented in Fig. 1 and 2. As a comparison, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA was inhibited by α-amanitin to the same extent, regardless of when α-amanitin was added. Under the same conditions, the synthesis of 28S rRNA was not inhibited.
FIG. 3.
Comparison of the effects of α-amanitin on the synthesis of various RNA species. Huh7 cells were transfected with in vitro-transcribed 1.9-kb HDV genomic RNA, 0.8-kb HDV mRNA, and pArg-Maxi (under the control of a pol III promoter). Cells were treated with 20 μg of α-amanitin per ml on either day 1 or day 2 posttransfection, and cells were harvested 1 day after α-amanitin treatment. Samples were analyzed by either Northern blot assay (A) to detect antigenomic-sense HDV RNA (upper panel), GAPDH (middle panel), and 28S rRNA (bottom panel) or by RNase protection assay (B) to detect Arg-Maxi tRNA. (A) Antigenomic HDV RNA was detected with 32P-end-labeled oligonucleotide 1565A. The same membrane was probed for GAPDH mRNA and 28S rRNA. Lanes: 1, RNA size markers for the 1.7-kb antigenome and the 0.8-kb mRNA; 2 and 3 and 4 and 5, total RNA from transfected cells on days 2 and 3, respectively, with or without α-amanitin treatment (added 24 h prior to harvest). (B) RNase protection assay of RNA samples in panel A using 32P-labeled antisense Arg-Maxi as a probe. Lanes 1 to 4 correspond to lanes 2 to 5 in panel A.
It should be noted that the amount of the 1.7-kb RNA found on day 3 in the presence of α-amanitin (Fig. 3A, lane 5) was significantly higher than that found on day 2, even in the absence of α-amanitin (lane 2), suggesting that RNA synthesis continued to take place in the presence of α-amanitin. Therefore, the insensitivity of the 1.7-kb RNA to α-amanitin represents the true resistance of its synthesis to α-amanitin treatment and not to stabilization of the RNA. These findings established that the synthesis of the 0.8-kb mRNA is specifically inhibited by the low concentration of α-amanitin, supporting the hypothesis that 0.8-kb mRNA synthesis is pol II dependent. In contrast, the synthesis of the 1.7-kb antigenomic RNA is not sensitive to α-amanitin unless α-amanitin is added early.
Synthesis of the 1.7-kb antigenomic RNA is insensitive to inhibition by α-amanitin after abundant S-HDAg appears.
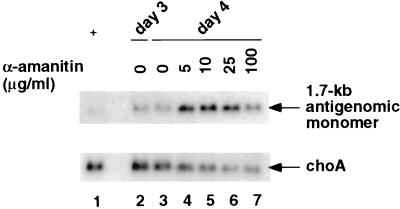
The relative resistance of 1.7-kb antigenomic RNA synthesis to α-amanitin after day 2 or 3 posttransfection was further studied. First, we re-evaluated the data shown in Fig. 2 to focus on the 1.7-kb RNA species. Shorter exposure of the membrane in Fig. 2 demonstrated that the level of the 1.7-kb antigenomic RNA continued to increase from day 3 to day 4, even in the presence of as much as 25 μg of α-amanitin per ml (Fig. 4, compare lanes 4 to 7 with lane 2). This result clearly indicated that α-amanitin does not inhibit the synthesis of 1.7-kb antigenomic RNA. In contrast, the amount of choA RNA decreased in the presence of α-amanitin. The level of the 1.7-kb antigenomic RNA was decreased slightly by α-amanitin at 100 μg/ml. These results suggest that the synthesis of the 1.7-kb antigenomic HDV RNA does not require pol II and that the observed sensitivity of the 1.7-kb RNA to α-amanitin treatment during days 1 and 2 after transfection (Fig. 3A) is likely an indirect effect of the inhibition of 0.8-kb mRNA synthesis by α-amanitin.
FIG. 4.
Northern blot analysis of effects of α-amanitin on antigenomic RNA synthesis on day 3 posttransfection. The blot is the same as that in Fig. 2 but with a shorter exposure time to clearly visualize the 1.7-kb antigenomic RNA. Lanes are identical to those described in the legend to Fig. 2.
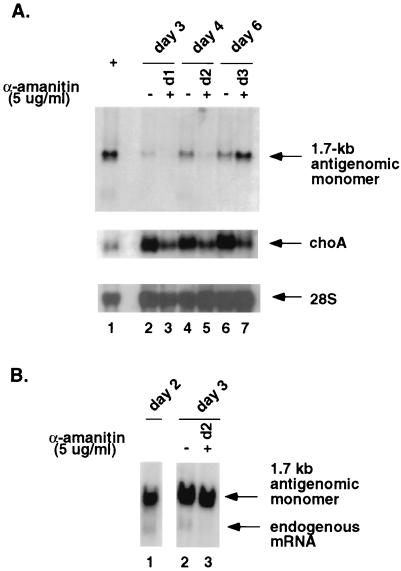
To more carefully establish the time-related effects of α-amanitin on 1.7-kb antigenomic HDV RNA synthesis, α-amanitin was added at several time points after HDV RNA transfection and RNA was examined 2 or 3 days later (Fig. 5A). The results showed that when α-amanitin (5 μg/ml) was added on day 1 and RNA was examined on day 3, the 1.7-kb HDV RNA was not detected (lane 3). When α-amanitin was added on day 2, again virtually no 1.7-kb RNA was detected on day 4 (lane 5). However, when it was added on day 3 and RNA was examined on day 6, the amount of 1.7-kb RNA did not decrease but, in fact, continued to increase (lane 7). The finding that the amount of 1.7-kb RNA actually decreased when α-amanitin was added during the first 2 days indicates that RNA synthesis was indeed inhibited and that the RNA was not unduly stable. Furthermore, the amount of HDV RNA actually increased from day 3 to day 6 (compare lanes 2 and 7), even in the presence of α-amanitin. Therefore, the resistance of 1.7-kb RNA to α-amanitin at later time points represents true resistance of the RNA synthesis and was not due to stabilization of RNA. It should be noted that the timing of the switch of the 1.7-kb RNA from sensitivity to resistance to α-amanitin coincides with the appearance of abundant S-HDAg in HDV RNA-transfected cells on day 3 posttransfection (33) (data not shown), suggesting that 1.7-kb antigenomic RNA synthesis is not inhibited by α-amanitin if S-HDAg is present. choA RNA was inhibited to the same extent by α-amanitin at all three time points, whereas 28S rRNA was not inhibited.
FIG. 5.
(A) Sensitivity of various RNA species to α-amanitin at different time points after HDV RNA transfection. Huh7 cells were transfected with 1.9-kb genomic RNA and 0.8-kb mRNA and treated with α-amanitin (5 μg/ml) at different time points. The RNA blot was probed for the different RNA species. Lanes: 1, RNA size markers; 2, 4, and 6, total RNA harvested at days 3, 4, and 6 from transfected cells without α-amanitin treatment; 3, 5, and 7, total RNA harvested at days 3, 4, and 6 from transfected cells treated with α-amanitin at days 1, 2, and 3, respectively. (B) Northern blot analysis of total RNA harvested from Tsδ3 cells transfected with in vitro-transcribed 1.9-kb HDV genomic HDV RNA only. Cells were treated with 5 μg of α-amanitin per ml on day 2 posttransfection and harvested on day 3. Lanes: 1, RNA from untreated cells harvested on day 2; 2, RNA from untreated cells harvested on day 3; 3, RNA from cells treated with α-amanitin on day 2 and harvested on day 3.
To prove that 1.7-kb RNA synthesis is not inhibited by α-amanitin if abundant S-HDAg is present in the cells, we next examined the α-amanitin sensitivity of HDV RNA synthesis in Tsδ3 cells (22), which constitutively express S-HDAg, after HDV RNA transfection (Fig. 5B). The results showed that the synthesis of the 1.7-kb antigenomic RNA was not inhibited when α-amanitin was added on day 2 posttransfection; in contrast, the endogenous HDAg mRNA (1.1 kb), which was expressed from an integrated cDNA encoding HDAg (22, 33), was inhibited. This result provides further evidence that 1.7-kb antigenomic RNA synthesis is not inhibited by α-amanitin and that its inhibition at early time points is secondary to inhibition of S-HDAg production.
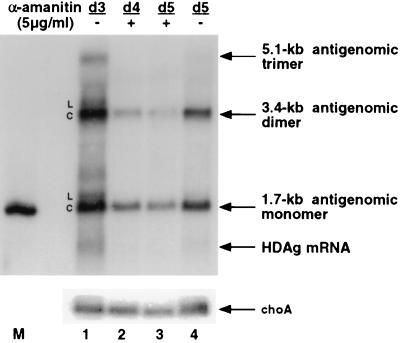
The results above showed that the amount of 1.7-kb HDV RNA continued to increase even in the presence of α-amanitin, indicating that HDV RNA synthesis was not inhibited by α-amanitin. Furthermore, 1.7-kb antigenomic RNA was less stable than the choA mRNA. To further demonstrate that the 1.7-kb HDV RNA was not unusually stable, we examined the stability of the transcript from a plasmid, pKS/H2ag, which transcribes a mutant antigenomic HDV trimer RNA from the CMV promoter (24). The HDV sequence in this plasmid contains a mutation in the ribozyme domain of the genomic strand of HDV RNA, such that the antigenomic RNA transcribed from the plasmid can be processed into 1.7-kb RNA but cannot be replicated (24). Thus, the 1.7-kb antigenomic RNA detected in the transfected cells is derived exclusively from the primary transcript of the HDV cDNA. The synthesis of this transcript from DNA can be inhibited by α-amanitin at any time point, thus allowing measurement of the stability of the 1.7-kb antigenomic RNA. Previous studies have shown that the HDV RNA transcript from this plasmid consists of both linear and circular RNA species and that the RNA transcript derived from the corresponding wild-type plasmid leads to robust RNA replication (24), indicating that the RNA transcript represents the natural HDV RNA species. Huh7 cells were transfected with pKS/H2ag and pCMV/neo-Sm, which expresses a wild-type S-HDAg, mimicking the conditions of the RNA-based transfection studies. Three days after transfection, 5 μg of α-amanitin per ml was added to cells and total RNA was harvested at different time points. RNA was analyzed under conditions in which the linear and circular RNAs were separable (24). The results showed that after 1 day of α-amanitin treatment, the primary transcript (5.1-kb antigenomic trimer) became undetectable. The linear form of 1.7-kb or 3.4-kb RNA was significantly reduced within 1 day of α-amanitin treatment (Fig. 6, compare lanes 1 and 2). The circular form appeared to be more stable than the linear form but is nonetheless not more stable than the choA mRNA (also Fig. 2 and 4). Therefore, the finding that the amount of 1.7-kb antigenomic RNA was not reduced by α-amanitin treatment most likely reflects the true resistance of the synthesis of this RNA to α-amanitin.
FIG. 6.
Comparison of the stability of HDV antigenomic RNA and choA mRNA. Huh7 cells were transfected with plasmids pKS/H2ag and pKS/CMV-sm (see text). Cells were treated with α-amanitin at 5 μg/ml on day 3 posttransfection. Total RNA was harvested at various time points after treatment. RNA was separated by electrophoresis on 1.5% agarose gels as previously described (24, 25). Samples were analyzed by Northern blot, using first a 32P-labeled HDV-specific probe and then a choA probe. Lanes: 1 and 4, total RNA from transfected cells harvested on days 3 and 5, respectively, without α-amanitin treatment; 2 and 3, total RNA from transfected cells to which α-amanitin was added on day 3, harvested on days 4 and 5, respectively. L, linear RNA; C, circular RNA; M, monomer RNA marker.
An α-amanitin-resistant pol II mutant partially restores transcription of the 0.8-kb mRNA.
To further determine if the inhibition of 0.8-kb mRNA synthesis by α-amanitin can be attributed to the inhibition of pol II activity, we examined whether transcription of this mRNA in the presence of α-amanitin could be restored in cells expressing an α-amanitin-resistant pol II mutant. We first established a cell line (BCHWAT) expressing α-amanitin-resistant mutant pol II (1). The expression of this transfected mutant pol II in this cell line was very low but was significantly induced after 2 days of α-amanitin treatment (Fig. 7A). This cell line continued to grow in the presence of α-amanitin at 5 μg/ml but had a slightly slower growth rate than in the absence of α-amanitin, probably due to the slow induction of the mutant pol II. In contrast, the parental cell line (BC10ME) failed to grow in the presence of α-amanitin (Fig. 7B).
FIG. 7.
Characterization of the cell line BCHAWT, which expresses α-amanitin-resistant mutant pol II. (A) Western blot analysis of the mutant pol II in BCHAWT cells. Cells were treated with α-amanitin (5 μg/ml) for 2 days and harvested for Western blotting using an anti-hemagglutinin monoclonal antibody as a probe. Lanes: 1, BC10ME cells; 2, untreated BCHAWT cells; 3, BCHAWT cells treated with α-amanitin for 2 days. (B) Growth kinetics. Culture dishes (60-mm diameter) were seeded with 105 BC10ME or BCHAWT cells and cultured in the presence or absence of α-amanitin at 5 μg/ml. Cells were counted with a hemacytometer on days 1, 2, and 3 after seeding.
This cell line was used to study the effect of α-amanitin on 0.8-kb mRNA synthesis after HDV RNA transfection. In the absence of α-amanitin, HDV RNA replication and mRNA synthesis in this cell line were only slightly less robust than in the control cells that were transfected with the vector alone (data not shown). To fully induce the α-amanitin-resistant pol II, cells were treated with α-amanitin at the time of HDV RNA transfection and newly synthesized HDV antigenomic-sense RNA was examined on days 3 and 4. The results showed that choA RNA transcription in this cell line was not affected by α-amanitin at up to 25 μg/ml, indicating that the mutant pol II conferred resistance to α-amanitin (Fig. 8, bottom). The 0.8-kb mRNA also could be detected even with α-amanitin at 25 μg/ml; however, the overall levels of this RNA were lower than in untreated cells (Fig. 8, top). These results indicate that the synthesis of this mRNA was partially restored by the α-amanitin-resistant mutant pol II. Therefore, we conclude that the 0.8-kb HDV mRNA synthesis is mediated by pol II or requires protein products of pol II transcripts; however, HDV mRNA synthesis appears to require more factors than are involved in the transcription of cellular genes, since the α-amanitin-resistant mutant pol II fully restored choA RNA synthesis but only partially restored 0.8-kb HDV mRNA synthesis. Significantly, the 1.7-kb antigenomic RNA was not inhibited by α-amanitin in this cell line, even when α-amanitin was added at the time of HDV RNA transfection. As shown above, this was likely the result of restoration of 0.8-kb mRNA synthesis.
FIG. 8.
Effects of α-amanitin on HDV RNA synthesis in BCHAWT cells. BCHAWT cells were transfected with in vitro-transcribed 1.9-kb genomic HDV RNA and 0.8-kb mRNA. Various amounts of α-amanitin were added immediately after transfection. Total RNA was harvested on days 3 and 4 posttransfection, and HDV antigenomic RNA and choA mRNA were detected by Northern blot assay as described in the legend to Fig. 2. Lanes: 1, RNA size markers; 2, untransfected BCHAWT cells.
Temperature dependence of 0.8-kb mRNA synthesis from the HDV RNA template.
To further distinguish RNA-templated from DNA-templated pol II transcription, we examined these two types of transcription at different temperatures. We had previously found that HDV RNA replicated much better at 37°C than at 34°C, whereas cellular mRNA synthesis was equivalent at both temperatures (22). Further, we had found that when Tsδ3 cells grown at 34°C were transfected with HDV RNA, only the HDAg mRNA transcribed from the integrated HDAg cDNA, but not the 0.8-kb mRNA synthesized from the transfected HDV RNA, could be detected (33). To establish the differential temperature sensitivity of RNA- versus DNA-templated mRNA transcription, we further studied HDV mRNA synthesis in Tsδ3 cells transfected with HDV RNA at 34°C versus 37°C (Fig. 9A). The results showed that the subgenomic mRNA transcribed from the integrated HDAg cDNA, which is 1.1 kb in length (33), was detectable at both 34 and 37°C; in contrast, the 0.8-kb mRNA transcribed from the transfected RNA was detected only at 37°C. The 1.7-kb HDV RNA was also more abundant at 37°C than at 34°C, which could be the result of increased production of HDAg at 37°C (Fig. 9B). These results further demonstrate that RNA-templated transcription of the HDV mRNA has properties distinct from those of DNA-templated synthesis of a similar transcript.
FIG. 9.
HDV RNA replication in Tsδ3 cells at different temperatures. Tsδ3 cells were transfected with in vitro-transcribed 1.9-kb genomic HDV RNA only and maintained at 34 or 37°C. Total RNA was harvested on days 2 and 3, and protein was harvested on day 3. (A) Northern blot detecting antigenomic HDV RNA. Lane 1, RNA size markers. (B) Western blot analysis of HDAg in HDV RNA-transfected Tsδ3 cells grown at 34 and 37°C.
Transcription of the 0.8-kb HDV mRNA can occur in the absence of HDV RNA replication.
The data shown above indicated that replication of HDV genomic-length RNA and transcription of 0.8-kb mRNA may involve different polymerases and have different metabolic requirements. Furthermore, 1.7-kb HDV RNA synthesis can occur in the absence of 0.8-kb mRNA transcription, once a sufficient amount of HDAg is made. We next examined whether the reverse is also true, i.e., whether 0.8-kb mRNA transcription can occur in the absence of the 1.7-kb RNA synthesis. A previous report showed that an HDV genomic RNA encoding a defective S-HDAg could not replicate even when it was transfected together with a wild-type S-HDAg as a ribonucleoprotein complex (13). We have also found that such a defective HDV RNA could not replicate even when it was transfected together with an in vitro-transcribed mRNA encoding the wild-type S-HDAg (data not shown). We therefore used this approach to determine whether 0.8-kb mRNA transcription could occur in the absence of HDV RNA replication.
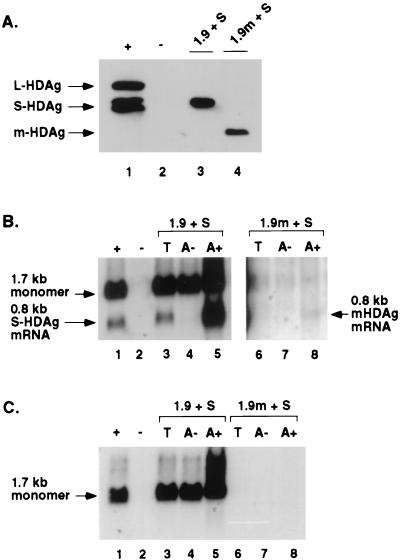
Since the amount of HDV mRNA made, if any, in the absence of HDV RNA replication is expected to be small because the amount of template RNA will be very limited, we designed an experimental approach to increase the sensitivity of detection of HDV mRNA. For this purpose, we used a mutant HDV genomic RNA that encodes a truncated HDAg. Since this protein can be translated only from the 0.8-kb mRNA (29), the accumulation of the truncated HDAg indicates the production of the HDV mRNA. Huh7 cells were transfected with in vitro-transcribed S-HDAg-encoding mRNA and either the wild-type or the mutant 1.9-kb genomic HDV RNA in accordance with the previously reported protocol (33). Western blot analysis of protein from cells harvested on day 2 posttransfection showed that cells transfected with these RNAs produced comparable levels of the wild-type and truncated (m-HDAg) HDAgs, respectively (Fig. 10A). Since m-HDAg can be synthesized only from the subgenomic mRNA (29), this result suggests that the 0.8-kb mRNA was transcribed from the mutant HDV genome.
FIG. 10.
Analysis of 0.8-kb mRNA in the absence of HDV genomic RNA synthesis. Huh7 cells were transfected with in vitro-transcribed 0.8-kb mRNA and either wild-type or mutant 1.9-kb genomic HDV RNA. Cells were harvested 2 days after transfection and analyzed by Western blot assay for HDAg (A) and by Northern blot assay for HDV-specific RNA (B and C). (A) Western blot. Lanes: 1, Huh7 cells transfected with plasmids encoding S- and L-HDAg to serve as protein markers; 2, untransfected cells; 3 and 4, cells transfected with in vitro-transcribed S-HDAg mRNA (S) and either wild-type (lane 3) or mutant (lane 4) 1.9-kb genomic HDV RNA. (B) Northern blot probed with oligonucleotide 1565A. Lanes: 1, RNA size markers; 2, total RNA from untransfected cells; 3 to 5, total (T) poly(A)−, and poly(A)+ RNAs from Huh7 cells transfected with wild-type 1.9-kb genomic HDV RNA and S-HDAg mRNA; 6 to 8, corresponding RNAs from cells transfected with mutant 1.9-kb genomic HDV RNA and S-HDAg mRNA. Lanes 1 to 5 were exposed to autoradiography for 24 h, while lanes 6 to 8 were exposed for 48 h. (C) The same blot as in panel B probed with an HDV RNA detecting antigenomic RNA in the noncoding region of the genome.
This conclusion was confirmed by Northern blot analysis of the poly(A)-enriched RNA from transfected cells (Fig. 10B); a substantial amount of the 0.8-kb mRNA was made from the wild-type genomic HDV RNA, whereas a small amount was also detected in cells transfected with the mutant genomic HDV RNA. This RNA species was not detected in the total RNA fraction of the mutant RNA-transfected cells, indicating that only a small amount of the mRNA was made. Because of cross-hybridization of the oligonucleotide probe to 18S rRNA, the status of the 1.7-kb HDV RNA in cells transfected with the mutant HDV genome was not clear. However, using a different RNA probe detecting a different region of HDV RNA, the 1.7-kb RNA species was clearly not detectable, even after prolonged exposure (Fig. 10C). (This RNA probe does not detect the 0.8-kb mRNA.) We therefore conclude that the 0.8-kb mRNA can be transcribed in the absence of HDV RNA replication.
DISCUSSION
In this paper, we have provided evidence that transcription of the HDV 0.8-kb mRNA and replication of the 1.7-kb antigenomic RNA are differentially regulated and require different cellular polymerases or the products of their transcripts. Synthesis of the 0.8-kb mRNA was sensitive to inhibition by α-amanitin at 3 μg/ml, whereas synthesis of the 1.7-kb antigenomic HDV RNA was resistant to α-amanitin at concentrations as high as 25 μg/ml. Furthermore, HDV mRNA synthesis can occur in the absence of 1.7-kb antigenomic RNA synthesis, and vice-versa, indicating that HDV RNA replication and mRNA transcription are not coupled. Thus, the mechanism of transcription of 0.8-kb mRNA is more similar to that of cellular pol II genes, whereas the synthesis of 1.7-kb RNA is significantly different. However, transcription of the 0.8-kb mRNA from RNA templates shows metabolic requirements distinct from those of DNA-templated transcription in several aspects; for example, an α-amanitin-resistant pol II mutant fails to completely restore the synthesis of 0.8-kb RNA and synthesis of the 0.8-kb HDV mRNA is suppressed at 34°C relative to the transcription of cellular mRNAs. Our results provided the first in vivo evidence for the sensitivity of HDV RNA-templated transcription to a low concentration of α-amanitin. This observed sensitivity clearly was not the result of the general cytotoxicity of α-amanitin, since the synthesis of the 1.7-kb HDV RNA species and cellular pol I- and pol III-mediated transcription were not inhibited under the same conditions.
The above findings lead to several conclusions about the regulation of HDV RNA replication and transcription. First, the cellular machineries that synthesize these two RNA species are different. All of the evidence supports the conclusion that the synthesis of the 0.8-kb mRNA species involves pol II or protein products of pol II-mediated transcripts. In contrast, the resistance of the 1.7-kb antigenomic RNA to α-amanitin at 25 μg/ml suggests that it is synthesized by a polymerase other than pol II. The observed requirements for the synthesis of the 0.8-kb mRNA are consistent with the previous results of nuclear run-on experiments and in vitro reconstitution experiments (14, 31), although the in vitro experiments did not directly examine individual RNA species and their α-amanitin sensitivities. Furthermore, those experiments more likely reflect cDNA-templated transcription than true RNA-templated transcription (14, 31). In addition, the in vitro experiments did not require S-HDAg, which is a necessary factor in HDV RNA synthesis in vivo. Our experiments did not distinguish between the possibilities that pol II is directly involved in HDV RNA-templated transcription and that protein products of pol II transcripts participate in RNA transcription. Nevertheless, our experiments provided unequivocal evidence that pol II is involved directly or indirectly in HDV mRNA synthesis but not in 1.7-kb antigenomic RNA synthesis. The finding that an α-amanitin-resistant mutant pol II failed to completely restore transcription of the HDV mRNA to wild-type levels (Fig. 8) suggests that there are clear differences between transcriptions from DNA and RNA templates. The α-amanitin-resistant mutant pol II is capable of transcribing most cellular mRNAs, as cells that express this mutant pol II had similar growth kinetics in the presence and absence of α-amanitin (after an initial lag). However, another α-amanitin-resistant mutant pol II (11) has been shown to cause selective reduction of transcription of certain cellular genes. Thus, the α-amanitin-resistant mutant pol II used in this study may also have selective defects for transcription from certain genes, including RNA-templated transcription.
The temperature dependence of 0.8-kb mRNA transcription also suggests that RNA-templated transcription has special requirements. One possibility is that temperature affects the conformation of RNA templates; it is possible that more extensive intramolecular base pairing of HDV RNA at the lower temperature prevented adequate unwinding of the template for transcription. Alternatively, temperature may affect the function of the genomic and/or antigenomic ribozymes, which is required for HDV RNA synthesis (24), or may interfere with the formation of an active transcription complex specific for RNA-templated transcription. In any case, the differential temperature sensitivity of DNA- and RNA-templated transcription further suggests that these two types of transcription processes have different requirements.
The failure of α-amanitin to inhibit the 1.7-kb antigenomic RNA synthesis was unexpected and suggests that cellular pol II is not involved in the RNA-templated synthesis of this RNA. Even exposure to α-amanitin for longer than 24 h did not inhibit its synthesis. This result contradicts the previous in vitro transcription data showing the sensitivity of 1.7-kb RNA synthesis to α-amanitin (14, 31). However, the in vitro studies were complicated by the presence of HDV cDNA, whereas our in vivo studies allowed us to focus exclusively on RNA-templated transcription. This conclusion raises an intriguing question regarding the identity of cellular enzymes responsible for HDV antigenomic RNA synthesis. pol I, pol III, or an as yet unidentified cellular enzyme may be responsible. The finding that the synthesis of the 1.7-kb RNA was sensitive to α-amanitin early after HDV RNA transfection but became resistant later suggests that the synthesis of this RNA is dependent on the availability of a large amount of HDAg. This interpretation was supported by the finding that the 1.7-kb RNA synthesis was not inhibited by α-amanitin at any time point after transfection in a cell line (Tsδ3) that constitutively expresses S-HDAg (Fig. 5B). The previous immunolocalization studies have shown that HDAg is not tightly associated with the pol II transcription machinery in the nucleus (12). Some HDAgs have been found to be localized in the nucleolus (3, 6, 43). Also, HDAg has been shown to interact with nucleolin (28), a nucleolar protein. These findings raise a possibility that pol I is responsible for HDV antigenomic RNA synthesis. This possibility is consistent with the resistance of the 1.7-kb RNA to high concentrations of α-amanitin.
The findings that 0.8-kb mRNA transcription can occur in the absence of 1.7-kb antigenome synthesis and vice versa further underscore the conclusion that HDV RNA transcription and replication have different metabolic requirements and are independent of each other. We showed that the 0.8-kb mRNA and HDAg were produced by a mutant HDV RNA which cannot synthesize the 1.7-kb antigenomic RNA. These results suggest that replication of the genome requires a larger quantity of HDAg than does the transcription of the 0.8-kb mRNA. Indeed, we found that when this mutant genome was transfected into Tsδ3 cells, which stably express S-HDAg, HDV RNA replication occurred (L. E. Modahl and M. M. C. Lai, unpublished data). The requirement of newly synthesized S-HDAg for genome replication is also consistent with the finding that the inhibition of 1.7-kb antigenome synthesis by α-amanitin early after transfection is secondary to inhibition of 0.8-kb mRNA synthesis (Fig. 3). However, the question remains as to the mechanism behind the differing requirements of S-HDAg for mRNA synthesis and antigenome synthesis. S-HDAg may be necessary to recruit transcription factors involved in the synthesis of the 1.7-kb antigenome. Indeed, both S- and L-HDAg have been shown to inhibit transcription from a pol II reporter gene (30), suggesting that these proteins sequester factors required for pol II transcription. Alternatively, S-HDAg may be necessary to maintain an HDV RNA structure specific for 1.7-kb antigenome synthesis. HDAg is an RNA-binding protein which enhances ribozyme activity (25) and possesses an RNA chaperone activity (21). These properties may be required for successful replication of the HDV genome. The newly synthesized HDAg may differ from the HDAg provided either by transfected in vitro-transcribed HDAg mRNA or HDAg present in the HDV virion, thus explaining why newly synthesized HDAg is required for 1.7-kb RNA synthesis.
In conclusion, the results presented here suggest that HDV RNA synthesis is different from plant viroid RNA replication in that synthesis of the different HDV RNA species directly or indirectly involves cellular pol II and other as yet unidentified cellular polymerases. Furthermore, HDAg and other cellular factors are also required. The capacity of the mammalian cellular polymerases to carry out RNA-dependent RNA synthesis suggests an exciting new role for the cellular transcription machineries. Identification of the components of the machineries for RNA-templated RNA synthesis will be important for further understanding of these processes.
ACKNOWLEDGMENTS
We thank C.-M. Lee (Chang-Gung Hospital, Kaoshiung, Taiwan, Republic of China), who generously provided the HDV genotype II clones which were used to establish the system for detection of newly synthesized 0.8-kb HDV mRNA. J. L. Corden (Johns Hopkins University, Baltimore, Md.) kindly provided the α-amanitin-resistant pol II clone. We also thank Horng-Dar Wang, who assisted with the RNase protection assay.
L.E.M. is supported by the National Health and Life Insurance Medical Research Fund. M.M.C.L. is an Investigator of Howard Hughes Medical Institute.
REFERENCES
- 1.Bartolomei M S, Corden J L. Localization of an α-amanitin resistance mutation in the gene encoding the largest subunit of mouse RNA polymerase II. Mol Cell Biol. 1987;7:586–594. doi: 10.1128/mcb.7.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann K F, Gerin J L. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986;154:702–706. doi: 10.1093/infdis/154.4.702. [DOI] [PubMed] [Google Scholar]
- 3.Bichko V V, Taylor J M. Redistribution of the delta antigens in cells replicating the genome of hepatitis delta virus. J Virol. 1996;70:8064–8070. doi: 10.1128/jvi.70.11.8064-8070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonino F, Heermann K H, Rizzetto M, Gerlich W H. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986;58:945–950. doi: 10.1128/jvi.58.3.945-950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey J L, Gerin J L. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J Virol. 1995;69:7593–7600. doi: 10.1128/jvi.69.12.7593-7600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M-F, Baker S C, Soe L H, Kamahora T, Keck J G, Makino S, Govindarajan S, Lai M M C. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988;62:2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao M, Hsieh S-Y, Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990;64:5066–5069. doi: 10.1128/jvi.64.10.5066-5069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P J, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J L, Taylor J. Structure and replication of the genome of hepatitis delta virus. Proc Natl Acad Sci USA. 1986;83:8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Collins J L, Patek P Q, Cohn M. Tumorigenicity and lysis by natural killers. J Exp Med. 1981;153:89–106. doi: 10.1084/jem.153.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crerar M M, Leather R, David E, Pearson M L. Myogenic differentiation of L6 rat myoblasts: evidence for pleiotropic effects on myogenesis by RNA polymerase II mutations to α-amanitin resistance. Mol Cell Biol. 1983;3:946–955. doi: 10.1128/mcb.3.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha C, Monjardino J, Chang D, Krause S, Carmo-Fonseca M. Localization of hepatitis delta virus RNA in the nucleus of human cells. RNA. 1998;4:680–693. doi: 10.1017/s135583829898013x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingle K, Bichko V, Zuccola H, Hogle J, Taylor J. Initiation of hepatitis delta virus genome replication. J Virol. 1998;72:4783–4788. doi: 10.1128/jvi.72.6.4783-4788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu T-B, Taylor J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J Virol. 1993;67:6965–6972. doi: 10.1128/jvi.67.12.6965-6972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber M, Panchanathan S, Fan R S, Johnson D L. The phorbol ester, TPA, induces specific transcription by RNA polymerase III in Drosophila Scheider cells. J Biol Chem. 1991;266:20598–20601. [PubMed] [Google Scholar]
- 16.Geliebter J, Zeff R A, Schulze D H, Pease L R, Weiss E H, Mellor A L, Flavell R A, Nathenson S G. Interaction between Kb and Q4 gene sequences generates the Kmb6 mutation. Mol Cell Biol. 1986;6:645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn J S, White J M. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol. 1991;65:2357–2361. doi: 10.1128/jvi.65.5.2357-2361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh S-Y, Chao M, Coates L, Taylor J. Hepatitis delta virus genome replication: a polyadenylated mRNA for delta antigen. J Virol. 1990;64:3192–3198. doi: 10.1128/jvi.64.7.3192-3198.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh S-Y, Taylor J. Regulation of polyadenylation of hepatitis delta virus antigenomic RNA. J Virol. 1991;65:6438–6446. doi: 10.1128/jvi.65.12.6438-6446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh S-Y, Yang P Y, Ou J T, Chu C M, Liaw Y F. Polyadenylation of the mRNA of hepatitis delta virus is dependent upon the structure of the nascent RNA and regulated by the small or large delta antigen. Nucleic Acids Res. 1994;22:391–396. doi: 10.1093/nar/22.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Z S, Wu H N. Identification and characterization of the RNA chaperone activity of hepatitis delta antigen peptides. J Biol Chem. 1998;273:26455–26461. doi: 10.1074/jbc.273.41.26455. [DOI] [PubMed] [Google Scholar]
- 22.Hwang S B, Jeng K S, Lai M M C. Studies of functional roles of hepatitis delta antigen in delta virus RNA replication. In: Dinter-Gottlieb G, editor. The unique hepatitis delta virus. R. G. Austin, Tex: Landes Company; 1995. pp. 95–109. [Google Scholar]
- 23.Hwang S B, Lai M M C. A unique conformation at the carboxyl terminus of the small hepatitis delta antigen revealed by a specific monoclonal antibody. Virology. 1993;193:924–931. doi: 10.1006/viro.1993.1201. [DOI] [PubMed] [Google Scholar]
- 24.Jeng K-S, Daniel A, Lai M M C. A pseudoknot ribozyme structure is active in vivo and required for hepatitis delta virus RNA replication. J Virol. 1996;70:2403–2410. doi: 10.1128/jvi.70.4.2403-2410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeng K-S, Su P-Y, Lai M M C. Hepatitis delta antigens enhance the ribozyme activities of hepatitis delta virus RNA in vivo. J Virol. 1996;70:4205–4209. doi: 10.1128/jvi.70.7.4205-4209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo M Y P, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai M M C. The molecular biology of hepatitis delta virus. Annu Rev Biochem. 1995;64:259–286. doi: 10.1146/annurev.bi.64.070195.001355. [DOI] [PubMed] [Google Scholar]
- 28.Lee C H, Chang S C, Chen C J, Chang M F. The nucleolin binding activity of hepatitis delta antigen is associated with nucleolus targeting. J Biol Chem. 1998;273:7650–7656. doi: 10.1074/jbc.273.13.7650. [DOI] [PubMed] [Google Scholar]
- 29.Lo K, Hwang S B, Duncan R, Trousdale M, Lai M M C. Characterization of mRNA for hepatitis delta antigen: exclusion of the full-length antigenomic RNA as an mRNA. Virology. 1998;250:94–105. doi: 10.1006/viro.1998.9364. [DOI] [PubMed] [Google Scholar]
- 30.Lo K, Sheu G T, Lai M M C. Inhibition of cellular RNA polymerase II transcription by delta antigen of hepatitis delta virus. Virology. 1998;247:178–188. doi: 10.1006/viro.1998.9253. [DOI] [PubMed] [Google Scholar]
- 31.Macnaughton T B, Gowans E J, McNamara S P, Burrell C J. Hepatitis δ antigen is necessary for access of hepatitis δ virus RNA to the cell transcriptional machinery but is not part of the transcriptional complex. Virology. 1991;184:387–390. doi: 10.1016/0042-6822(91)90855-6. [DOI] [PubMed] [Google Scholar]
- 32.Makino S, Chang M F, Shieh C K, Kamahora T, Vannier D M, Govindarajan S, Lai M M C. Molecular cloning and sequencing of a human hepatitis delta virus RNA. Nature. 1987;329:343–346. doi: 10.1038/329343a0. [DOI] [PubMed] [Google Scholar]
- 33.Modahl L E, Lai M M C. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: a new model of HDV RNA transcription and replication. J Virol. 1998;72:5449–5456. doi: 10.1128/jvi.72.7.5449-5456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cell lines with differentiated function in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 35.Pohl C, Baroudy B M, Bergmann K F, Cote P J, Purcell R H, Hoofnagle J, Gerin J L. A human monoclonal antibody that recognizes viral polypeptides and in vitro translation products of the genome of the hepatitis D virus. J Infect Dis. 1987;156:622–629. doi: 10.1093/infdis/156.4.622. [DOI] [PubMed] [Google Scholar]
- 36.Polson A G, Bass B L, Casey J L. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature. 1996;380:454–456. doi: 10.1038/380454a0. [DOI] [PubMed] [Google Scholar]
- 37.Polson A G, Ley III H L, Bass B L, Casey J L. Hepatitis delta virus RNA editing is highly specific for the amber/W site and is suppressed by hepatitis delta antigen. Mol Cell Biol. 1998;18:1919–1926. doi: 10.1128/mcb.18.4.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roggendorf M, Pahlke C, Bohm B, Rasshofer R. Characterization of proteins associated with hepatitis delta virus. J Gen Virol. 1987;68:2953–2959. doi: 10.1099/0022-1317-68-11-2953. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 40.Sanger H L. Viroid function: viroid replication. In: Diener T O, editor. The viroids. New York, N.Y: Plenum Press; 1989. pp. 117–166. [Google Scholar]
- 41.Schiebel W, Haas B, Marinkovic S, Klanner A, Sanger H L. RNA-directed RNA polymerase from tomato leaves. I. Purification and physical properties. J Biol Chem. 1993;268:11851–11857. [PubMed] [Google Scholar]
- 42.Wang E H, Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y-P, Yeh C-T, Ou J-H, Lai M M C. Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex. J Virol. 1992;66:914–921. doi: 10.1128/jvi.66.2.914-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]