Abstract
Objective
To quantitatively evaluate computed tomography (CT) parameters of coronavirus disease 2019 (COVID-19) pneumonia an artificial intelligence (AI)-based software in different clinical severity groups during the disease course.
Methods
From March 11 to April 15, 2020, 51 patients (age, 18–84 years; 28 men) diagnosed and hospitalized with COVID-19 pneumonia with a total of 116 CT scans were enrolled in the study. Patients were divided into mild (n = 12), moderate (n = 31), and severe (n = 8) groups based on clinical severity. An AI-based quantitative CT analysis, including lung volume, opacity score, opacity volume, percentage of opacity, and mean lung density, was performed in initial and follow-up CTs obtained at different time points. Receiver operating characteristic analysis was performed to find the diagnostic ability of quantitative CT parameters for discriminating severe from nonsevere pneumonia.
Results
In baseline assessment, the severe group had significantly higher opacity score, opacity volume, higher percentage of opacity, and higher mean lung density than the moderate group (all P ≤ 0.001). Through consecutive time points, the severe group had a significant decrease in lung volume (P = 0.006), a significant increase in total opacity score (P = 0.003), and percentage of opacity (P = 0.007). A significant increase in total opacity score was also observed for the mild group (P = 0.011). Residual opacities were observed in all groups. The involvement of more than 4 lobes (sensitivity, 100%; specificity, 65.26%), total opacity score greater than 4 (sensitivity, 100%; specificity, 64.21), total opacity volume greater than 337.4 mL (sensitivity, 80.95%; specificity, 84.21%), percentage of opacity greater than 11% (sensitivity, 80.95%; specificity, 88.42%), total high opacity volume greater than 10.5 mL (sensitivity, 95.24%; specificity, 66.32%), percentage of high opacity greater than 0.8% (sensitivity, 85.71%; specificity, 80.00%) and mean lung density HU greater than −705 HU (sensitivity, 57.14%; specificity, 90.53%) were related to severe pneumonia.
Conclusions
An AI-based quantitative CT analysis is an objective tool in demonstrating disease severity and can also assist the clinician in follow-up by providing information about the disease course and prognosis according to different clinical severity groups.
Key Words: CT, COVID-19, quantitative, pneumonia, temporal
Coronavirus disease 2019 (COVID-19) disease was first reported as an unknown pneumonia outbreak in Wuhan, China in December 2019 and has rapidly evolved into a pandemic. The responsible pathogen was a novel coronavirus, which has been called “severe acute respiratory syndrome coronavirus-2”.1,2 The ideal imaging method to be used in the evaluation of COVID-19 pneumonia should be diagnostic. It should also be accurate in terms of quantifying parenchymal findings and objective evaluation of the time course of the disease.3 Chest computed tomography (CT) has been widely used in diagnosis and follow-up with high sensitivity rates.4 Computed tomography findings of COVID-19 pneumonia, correlation of CT findings with clinical severity, and temporal changes of the findings were previously reported in detail in the literature.5–9 Initially, Pan et al6 classified patients according to time from symptom onset and defined 4 consecutive CT stages as follows: early-stage (0–4 days), progressive stage (5–8 days), peak stage (9–13 days), and absorption stage (≥14 days). The peak involvement was observed around day 10, and radiological improvement began after 14 days. In the early stage, the most common CT finding is unilateral or multifocal ground-glass opacities (GGOs); as the disease progresses, the number and extent of GGOs increase, and mixed patterns of GGOs, consolidations, crazy-paving pattern, and linear opacities appear. In the late stage, opacities gradually resolve, while subpleural fibrotic bands and residual GGOs are seen.5,6,10,11
While most patients have mild symptoms, approximately 14% and 5% of the patients progress to severe and critical disease, respectively.12 Different visual and semiquantitative CT scoring systems were used previously to estimate lung involvement, disease severity, and prognosis.13–15 However, adequate assessment in the follow-up period may be challenging. The search for rapid and objective evaluation led to the use of artificial intelligence (AI)-based algorithms. Regarding COVID-19 pneumonia, AI was used to detect and differentiate COVID-19 pneumonia from community-acquired pneumonia, for quantitative evaluation of CT changes and assessment of disease severity.7,9,16–19 Shen et al9 reported a moderate to high correlation between lesion percentage detected by radiologists and the software. Moreover, reduced reader variability was reported in the AI-based approach which is highly critical for the precise evaluation of disease progression.20
In this study, we aimed to quantitatively assess the initial CT findings and temporal CT changes of COVID-19 pneumonia according to different clinical severity groups. Our secondary purpose was to investigate the performance of quantitative CT parameters in differentiating severe from nonsevere pneumonia.
MATERIALS AND METHODS
Study Population
The study was approved by our institution's ethics committee (project number: GO 20/391, approval number: 2020/08-05). Because of the retrospective study design, informed consent was waived. Patients who underwent baseline chest CT for COVID-19 pneumonia between March 11, 2020, and April 15, 2020, were retrospectively analyzed from our institution's database. Patients who had positive real-time reverse transcription-polymerase chain reaction test results for severe acute respiratory syndrome coronavirus 2, older than 18 years, and had at least 1 follow-up CT imaging were included in the study. Exclusion criteria were as follows: (i) negative real-time reverse transcription-polymerase chain reaction result for COVID-19 pneumonia; (ii) CT scans with a poor quality because of artifacts or expiratory phase; (iii) presence of confirmed coinfection; (iv) patients without follow-up CT. Finally, 51 patients with a total of 116 CT scans were included in the study. The enrollment flowchart is shown in Figure 1.
FIGURE 1.
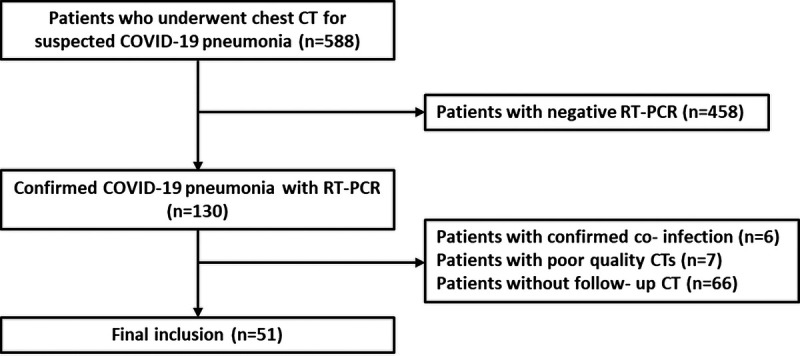
Flowchart showing the inclusion process.
According to disease severity on baseline presentation, patients were divided into 3 groups as mild, moderate, or severe based on the previous guidelines. Patients were classified as “mild” if they had mild symptoms, but no airspace opacities on baseline CT. “Moderate” was defined as having 1 or more symptoms (eg, fever, cough, myalgia) and also imaging findings of pneumonia. Patients were classified as “severe” if they had one of the following conditions: (a) respiratory distress with a respiratory rate of 30 breaths per minute or higher, (b) mean oxygen saturation at rest of 93% or lower, c) partial pressure of oxygen in arterial blood/oxygen concentration of 300 mm Hg or lower.21,22 Also, the time between symptom onset and CT scans was noted for the assessment of temporal changes. Computed tomography scans were classified into 4 groups as follows: stage 1, 0 to 4 days; stage 2, 5 to 8 days; stage 3, 9 to 13 days; and stage 4, 14 days or longer.6
CT Protocol
Computed tomography scans were obtained in supine position, from apices to lung bases, during breath-hold at the end of inspiration. Computed tomography scans were acquired without intravenous contrast administration, on a third-generation dual-source CT scanner (Somatom Force; Siemens Healthineers, Germany). Scanning parameters were as follows: 110 kVp tube voltage, modulated mA using 52 mAs as reference (CareDose 4D; Siemens Healthineers), pitch 3.2, 192 × 0.6 mm detector collimation, 0.25 s rotation time, and slice thickness of 3 mm. Images were reconstructed with a slice thickness of 1 mm. Iterative reconstruction was used (Adaptive Model-based Iterative reconstruction-ADMIRE; Siemens Healthineers).
Quantitative CT Analysis
Lung-lobe segmentation, lesion detection, and quantification were performed using an artificial-intelligence-based algorithm (CT Pneumonia, version 1.0.4, Research Frontier, Syngo.via, VB30; Siemens Healthineers). The parameters quantified for total lung, each lung, and each lobe were as follows: opacity score, volume (mL), the volume of opacity and high opacity, percentage of opacity and high opacity, and mean lung HU. Although the “opacity” term corresponds to areas with density values greater than −700 HU, the “high opacity” term is used for areas with density values higher than −200 HU. The opacity score was calculated automatically based on the percentage of infiltration within a given lobe, and described as: 0, 0%; 1, 1% to 25%; 2, 26% to 50%; 3, 51% to 75%; 4, greater than 75%. So, the maximum CT score is 4 for each lobe and 20 for the whole lung. Two radiologists blinded to patient data, with 8 and 13 years of experience (S.A.D., G.D.), visually checked the precision of segmentation and lesion detection for each slice; incorrectly or incompletely drawn areas were manually corrected.
Statistical Analysis
Kolmogorov-Smirnov test was used to test the normal distribution of continuous variables. Descriptive statistics were presented as mean ± standard deviation or median (interquartile range [IQR]) according to the assumption of normal distribution. Pearson χ2 test was used to compare the difference between groups for categorical variables. When data were not normally distributed, Mann-Whitney U test was used to compare 2 groups. One-way analysis of variance was performed to compare the differences among groups. Kruskal-Wallis was used to compare mean rank of more than 2 groups. After Kruskal-Wallis test, Dunn test (multiple comparisons) was used to determine the differences between the groups. The mixed models analysis of variance test was used to determine whether each of the 2 main effects, and the interaction effect was statistically significant. The receiver operating characteristic (ROC) analysis was performed to evaluate the diagnostic performance of quantitative CT parameters for discriminating severe pneumonia from nonsevere pneumonia. The area under the ROC curve (AUC) and 95% confidence interval (95% CI) were calculated. Cutoff ranges were calculated to maximize sensitivity and specificity to differentiate severe pneumonia by using the Youden index. P values of less than 0.05 were evaluated as statistically significant. All statistical analyses were performed by using IBM SPSS Statistics 21.0 software.
RESULTS
A total of 51 patients (23 women, 28 men) with 116 CT scans were enrolled in the study. The patients were grouped according to clinical severity. There were 12 patients in the mild group (23.5%), 31 patients in the moderate group (60.8%), and 8 patients in the severe group (15.7%). The mean age was 49 ± 15 years (age range, 18–84 years). Age and sex were not significantly different among clinical severity groups (P = 0. 386, and P = 0.344, respectively).
All patients were hospitalized and the median length of hospital stay was 10 days (range, 2–42; IQR, 11). Pairwise comparisons showed that severe patients had significantly longer hospital stay than mild (P = 0.003) or moderate groups (P = 0.022). There was no significant difference between mild and moderate groups in terms of length of hospital stay (P = 0.560) (Fig. 2). Among the groups as disease severity increases, intensive care unit (ICU) admission significantly increased (P = 0.002).
FIGURE 2.
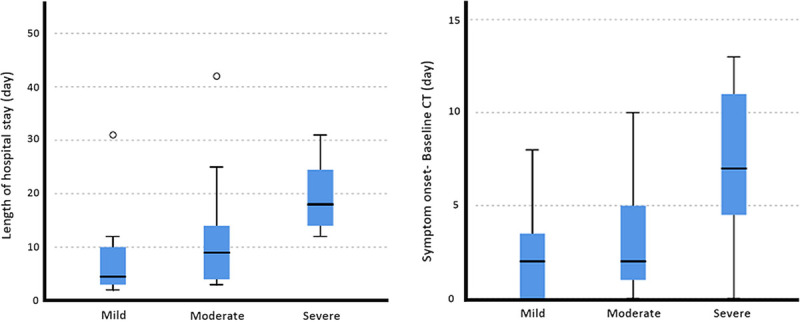
Box plots showing the distribution of the length of hospital stay (A), the time between symptom onset and baseline CT (B) for each clinical severity group. Figure 2 can be viewed online in color at www.jcat.org.
Baseline CT scans were obtained at 3.6 ± 3.27 days (range, 0–13 days) after the symptom onset. The time between the symptom onset and the baseline CT scan in the severe patient group was significantly longer than mild (P = 0.016) and moderate patients (P = 0.046) (Fig. 2).
Baseline CT Analysis
The mild patient group included 12 patients, and none of these patients had abnormal findings on baseline CT. Eight of 31 moderate patients (25.8%), and all of the severe patients (100%) had involvement of the all 5 lobes. All severe patients (8/8, 100%) and 17 of 31 patients (54.8%) in the moderate group had bilateral lung involvement. In the moderate patient group, the right lung (25/31, 80.6%) was affected more than the left lung (23/31, 74.2%). The right lower lobe was the most affected lobe (21/31, 67.7%), and the right middle lobe was the least (10/31, 32.3%) affected lobe.
The opacity score, opacity and high opacity volumes, percentage of opacity and high opacity, the mean lung density (HU), and the number of affected lobes were significantly different among clinical groups (all P ≤ 0.005). Total lung volume did not significantly differ among groups (P = 0.543). The patient characteristics, baseline CT findings, and post hoc comparison results are summarized in Table 1.
TABLE 1.
Patient Characteristics and Baseline Quantitative CT Parameters
| Mild (n = 12) | Moderate (n = 31) | Severe (n = 8) | P | Post Hoc Comparison | |
|---|---|---|---|---|---|
| Sex (F/M) (n) | 7/5 | 14/17 | 2/6 | 0.344 | |
| Age | 45.7 ± 12 | 48.8 ± 16.8 | 55 ± 10 | 0.386 | |
| Symptom onset-baseline CT scan (d) | 2.25 ± 2.4 | 3.16 ± 2.6 | 7.25 ± 4.3 | 0.016 | Mild vs moderate, P = 1.00 Mild vs severe, P = 0.016 Moderate vs severe, P = 0.046 |
| Length of hospital stay (d) | 4.5 (7) [2–31] |
9 (11) [3–42] |
18 (13.75) [12–31] |
0.004 | Mild vs moderate, P = 0.560 Mild vs severe, P = 0.003 Moderate vs severe, P = 0.022 |
| Patients with ICU admission (n,%) | 1 (8.3%) | 5 (16%) | 6 (75%) | 0.002 | |
| The number of affected lobes (n) | 0 | 3 (4) [1–5] |
5 (0) | 0.001 | |
| Total lung volume (mL) | 4332 ± 1463 | 4679 ± 1349 | 4163 ± 1065 | 0.543 | |
| Opacity score | 0 | 3 (4) [1–9] |
6 (1.75) [5–14] |
˂0.001 | Mild vs moderate, P ˂ 0.001 Mild vs severe, P ˂ 0.001, Moderate vs severe, P = 0.014 |
| Opacity volume (mL) | 0 | 8.3 (78) [0.11–564] |
517.5 (642) [27–2731] |
˂0.001 | Mild vs moderate, P ˂ 0.001 Mild vs severe, P ˂ 0.001 Moderate vs severe, P = 0.025 |
| Percentage of opacity (%) | 0 | 0.16 (1.65) [0.0025–23.4] |
13.1 (16.1) [0.46–64.2] |
˂0.001 | Mild vs moderate, P ˂ 0.001 Mild vs severe, P ˂ 0.001 Moderate vs severe, P = 0.029 |
| Volume of high opacity (mL) | 0 | 0.7 (8.18) [0–174] |
86 (152.5) [1.5–546] |
˂0.001 | Mild vs moderate, P ˂ 0.001 Mild vs severe, P ˂ 0.001 Moderate vs severe, P = 0.022 |
| Percentage of high opacity (%) | 0 | 0.02 (0.14) [0–7.2] |
1.73 (4.34) [0.02–12.8] |
˂0.001 | Mild vs moderate, P ˂ 0.001 Mild vs severe, P ˂ 0.001 Moderate vs severe, P = 0.026 |
| Mean lung density (HU) | −806 (61) [−847 to −767] |
−822 (72) [−863 to −620] |
−749 (136) [−818 to −542] |
0.013 | Mild vs moderate, P = 1.00 Mild vs severe, P = 0.026 Moderate vs severe, P = 0.017 |
Categorical variables are expressed as n (%), continuous variables of normal distribution are expressed as mean ± SD, continuous variables of skewed distribution are shown as median (IQR and [range]).
F, female; M, male.
Follow-Up CT Analysis
For the assessment of temporal changes, a total of 116 CT scans in 4 different time stages were analyzed. Most of the follow-up chest CTs were obtained during hospitalization period, between March 25 to June 18, 2020. For mild, moderate, and severe groups, the number of CT examinations obtained after discharge were 2, 7, and 1, respectively. Forty-one patients had 2, 8 patients had 3, 1 patient had 4, and 1 patient had 6 CT scans. The temporal change of CT parameters is shown in Figure 3. Quantitative CT parameters are also shown in detail in Table 2.
FIGURE 3.
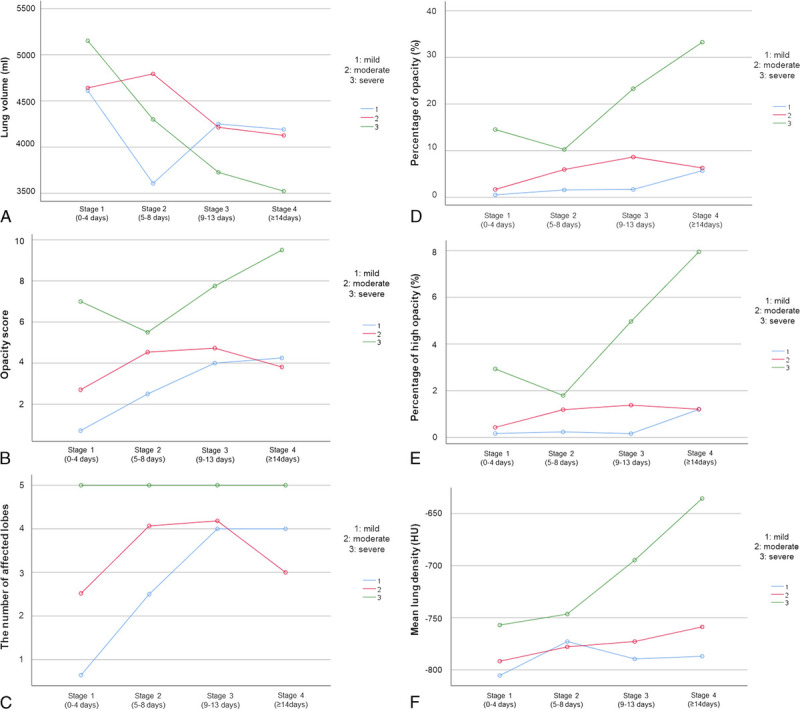
The graphics show the temporal change of (A) lung volume (mL), (B) opacity score, (C) the number of affected lobes, (D) the percentage of opacity (%), (E) the percentage of high opacity (%) and (F) mean lung density (HU) for different clinical severity groups. Figure 3 can be viewed online in color at www.jcat.org.
TABLE 2.
Quantitative CT Parameters on Stage 1 and Stage 4 According to Different Clinical Severity Groups
| Mild (n = 26) | Moderate (n = 69) | Severe (n = 21) | |
|---|---|---|---|
| Lung volume (mL) | |||
| Stage 1 | 4548.86 ± 375 | 4641.1 ± 258.4 | 4848 ± 587.4 |
| Stage 4 | 4161.5 ± 498 | 4674.1 ± 295.8 | 2987.4 ± 445.6 |
| P | 1.000 | 1.000 | 0.008 |
| Opacity score | |||
| Stage 1 | 0.46 ± 0.65 | 2.7 ± 0.47 | 6.9 ± 1.37 |
| Stage 4 | 4.37 ± 1.1 | 3.1 ± 0.6 | 10.2 ± 0.83 |
| P | 0.011 | 1.000 | 0.194 |
| Volume of opacity (mL) | |||
| Stage 1 | 16.36 ± 98.8 | 61.1 ± 70.9 | 657 ± 204.4 |
| Stage 4 | 206.6 ± 175.4 | 171.2 ± 94.3 | 1166.7 ± 124 |
| P | 1.000 | 1.000 | 0.273 |
| Percentage of opacity (%) | |||
| Stage 1 | 0.47 ± 2.72 | 1.93 ± 1.9 | 12.74 ± 6 |
| Stage 4 | 5.7 ± 4.7 | 4.65 ± 2.6 | 36.2 ± 3.44 |
| P | 1.000 | 1.000 | 0.007 |
| Volume of high opacity (mL) | |||
| Stage 1 | 7.4 ± 23.7 | 14.9 ± 19 | 163.1 ± 37 |
| Stage 4 | 35.6 ± 47.6 | 31.15 ± 23.2 | 272.84 ± 30.3 |
| P | 1.000 | 1.000 | 0.374 |
| Percentage of high opacity (%) | |||
| Stage 1 | 0.2 ± 0.75 | 0.5 ± 0.6 | 3.6 ± 1.27 |
| Stage 4 | 1.22 ± 1.48 | 1 ± 0.73 | 8.4 ± 0.97 |
| P | 1.000 | 1.000 | 0.156 |
| Mean lung density (HU) | |||
| Stage 1 | −808 ± 20 | −795 ± 14 | −766 ± 44 |
| Stage 4 | −788 ± 34 | −775 ± 19 | −632 ± 26 |
| P | 1.000 | 1.000 | 0.051 |
Data are expressed as mean ± SD.
Lung Volume
All groups had a decrease in total lung volume at stage 4 compared with baseline volume. However, lung volume loss was statistically significant only in the severe patient group (P = 0.008; mean lung volume at baseline, 4848 ± 587.4 mL and mean lung volume at stage 4, 2987.4 ± 445.6 mL).
Opacity Score
The total opacity score was statistically different between stage 1 and stage 4 in the mild patient group (P = 0.011). In the mild patent group, the total opacity score increased through all stages and peaked at stage 4 (4.37 ± 1.1).
The change of total opacity score was also significant in the severe patient group between stage 2 and stage 4 (P = 0.003). The total opacity score was highest in severe patient groups in all stages reaching 10.2 ± 0.83 in stage 4.
In the moderate patient group, no significance was observed between stages 1 and 4, the total opacity score increased through the first stages, peaked at stage 3 (4.82 ± 0.7), and slightly decreased in stage 4 (3.1 ± 0.6).
Opacity Volume-The Percentage of Opacity
Total opacity volume and total opacity percentage were highest in the severe patient group in all stages. The pairwise comparisons revealed that the total opacity volume was significantly higher in the severe patient group compared with mild and moderate patient groups (both P < 0.001). However, there was no significant difference among clinical groups through different time stages (P = 0.075).
On the other hand, the total percentage of opacity significantly increased between stages 1 and 4 in the severe patient group (P = 0.007). The maximum percentage of opacity in stage 4 reached 36.2 ± 3.44% in severe patients. The temporal change of opacities in a patient with severe pneumonia was demonstrated in Figure 4.
FIGURE 4.
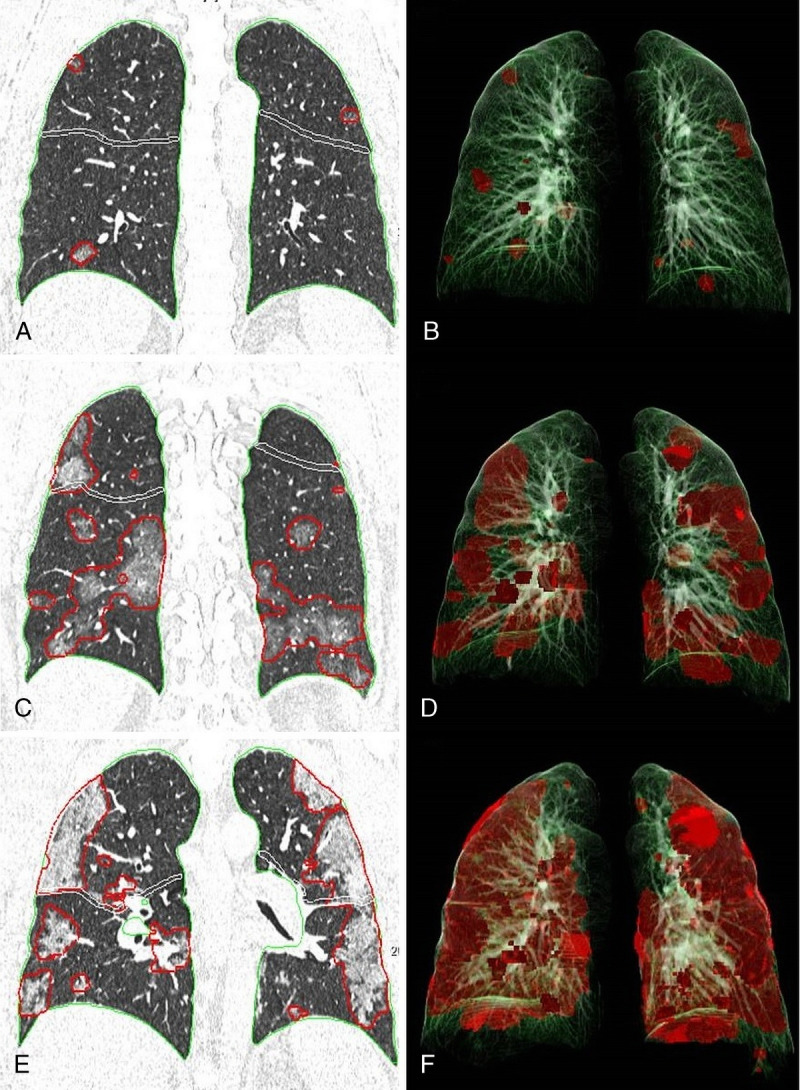
Chest CT images and 3-dimensional (3D) illustrations of lesion segmentation in a severe patient. (A, B) Chest CT obtained on stage 1 (day 4). (A) Coronal CT image shows foci of GGO in both lungs. (B) 3D illustration demonstrating the segmented opacities (percentage of opacity 0.46%). (C, D) Chest CT obtained on stage 2 (day 8). (C) Coronal CT image shows that the GGOs merged, and the size and extent of GGOs increased in both lungs. (D) 3D illustration showing the segmented opacities (percentage of opacity 11,23%). (E, F) Chest CT obtained on stage 4 (day 14). (E) Coronal CT image shows that the density and extent of opacities increased and consolidations developed in the interval period. (F) 3D illustration demonstrating the segmented opacities (percentage of opacity 33.5%). Figure 4 can be viewed online in color at www.jcat.org.
In the mild and moderate patient groups, the temporal change of opacity percentage was not significant. The total percentage of opacity increased through the stages, while peaked at stage 3 (8.9 ± 2.9%), then slightly decreased (4.65 ± 2.6%) in the moderate patient group. The peak was observed in stage 4 in the mild patient group (5.7 ± 4.7%).
High Opacity Volume-The Percentage of High Opacity
High opacity volume and the percentage of high opacity were highest in the severe patient group in all stages. But, the temporal change of high opacity volume and percentage was not significantly different among clinical groups (P = 0.068 and P = 0.064, respectively).
Mean Lung Density
The mean lung density was higher in the severe patient group in all stages. However, the temporal changes in mean lung density were not significantly different among clinical groups (P = 0.319).
Predicting Severity From Quantitative CT Parameters
Patients were classified as severe and nonsevere (mild and moderate) pneumonia for the assessment of diagnostic performance of aforementioned quantitative parameters. For the discrimination of severe from nonsevere pneumonia, the optimal cutoff value was 4 for the number of affected lobes (sensitivity, 100%; specificity, 65.26%; AUC, 0.826; 95% CI, 0.745–0.890, P < 0,0001), 4 for total opacity score (sensitivity, 100%; specificity, 64.21%; AUC, 0.899; 95% CI, 0.830–0.948; P < 0,0001), 337.4 mL for total opacity volume (sensitivity, 80.95%; specificity, 84.21%; AUC, 0.897; 95% CI, 0.827–0.946; P < 0.0001), 11% for total opacity percentage (sensitivity, 80.95%; specificity, 88.42%; AUC, 0.895; 95% CI, 0.825–0.945; P < 0,0001), 10.5 mL for total volume of high opacity (sensitivity, 95.24%; specificity, 66.32%; AUC, 0.882; 95% CI, 0.809–0.935; P < 0,0001), 0.8% for total percentage of high opacity (sensitivity, 85.71%; specificity, 80.00%; AUC, 0.879; 95% CI, 0.805–0.932; P < 0,0001), −705 HU for total mean lung HU (sensitivity, 57.14%; specificity, 90.53%; AUC, 0.806; 95% CI, 0.723–0.874; P < 0.0001).
DISCUSSION
In this study, we evaluated AI-based quantitative CT parameters of the patients diagnosed with COVID-19 pneumonia and the temporal evolution of these parameters in different clinical severity groups. Our major findings are, first, quantitative CT parameters vary according to different clinical severity groups and different time points. Second, between stage 1 and stage 4, although total lung volume decreases, opacity score, opacity and high-opacity volume, percentage of opacity and high-opacity, mean lung density increases in all clinical groups. However, the only parameters that reached statistical significance were total lung volume loss, the increase of opacity score and percentage in the severe patient group, and the increase of opacity score in the mild patient group. Quantitative CT parameters (total opacity score, opacity volume, percentage of opacity, high opacity volume, percentage of high opacity, mean lung density) could differentiate severe from nonsevere pneumonia.
The severe patient group had significantly more extensive involvement with bilateral and multilobar opacities on baseline CT. All 5 lobes were involved in all severe patients. The number of affected lobes, opacity score, opacity volumes, and opacity percentages were significantly higher in the severe group than that of the moderate group as reported previously in the literature.9,15,23 The severe patient group had a significantly longer interval between symptom onset to baseline CT and a longer hospitalization period. These findings indicate that, although the treatment protocols may have slight differences among different institutions and countries, the hospital admission, diagnosis, and therefore, treatment may be delayed in severe patients. In Huang et al.'s study, the hospitalization period was longer, and the CT severity score was higher for patients if the time between symptom onset to diagnosis is more than 3 days.24 Similarly, Liu et al13 reported an association between initial CT severity score and extended hospitalization. Also, individual factors, such as older age, male sex, and comorbidities, were previously associated with disease severity, but age and sex were not significantly different between clinical severity groups in our study.2,25
We observed a decrease in total lung volume during the disease course in all patients, but it reached a statistical significance only in the severe patient group. Significantly lower lung volumes in severe COVID-19 pneumonia were reported in some studies.9,18,26 This might be explained by the histopathological findings of diffuse alveolar damage with alveolar edema, exudation, hyaline membranes, inflammatory cell infiltration, and late phase fibrotic process.27,28 These changes assumably cause a decrease in functional alveolar capacity. In Iwasawa et al.'s29 study using ultra high-resolution CT, they reported lower CT lung volumes in severe cases and smaller secondary lobes in crazy-paving pattern comparing to clear lungs suggesting local volume loss. This was explained by alveolar collapse, which is commonly observed in acute respiratory distress syndrome (ARDS).
It has been previously reported that CT lung volumetry can be used to estimate lung volume and pulmonary function.30 In a recent study, 3 months after discharge, 25.5% of the patients with COVID-19 pneumonia showed lung function abnormalities on spirometry, such as the impaired diffusion capacity for carbon monoxide (DLCO). Also, 71% of these patients exhibited radiological abnormalities.31 Santus et al32 prospectively analyzed respiratory functions and DLCO of COVID-19 patients during hospitalization and 6 weeks after discharge. During the acute phase, a restrictive pattern was observed in approximately 50% of patients with a reduction of vital capacity, forced expiratory volume in 1 second and DLCO impairment. These findings were correlated with disease severity and the presence of consolidations on CT.
In a 6-month follow-up chest CT study, older age, longer hospitalization period, ARDS, noninvasive mechanical ventilation, higher CT opacity score on initial CT were found to be the predictors of fibrotic change development.33 Liu et al34 reported that at 7-month follow-up CT, fibrotic changes were observed in %29 of the patients. Between fibrosis and nonfibrosis groups, no difference was detected in terms of the presence of comorbidities. Patients with older age, severe type disease, and who were mechanically ventilated were more likely to develop fibrosis, and these patients had higher opacity scores, opacity volume, and opacity percentage at discharge CT. Therefore, quantitative CT may give information about the necessity and duration of follow-up imaging. In Yang et al's study, 16% of the patients had lung distortion suggestive of fibrosis on peak CT (median, 20 days), but in 50% of these patients fibrotic-like changes resolved on the last follow-up CTs (median, 56 days).35 It is still early to conclude whether these fibrotic changes are persistent, individual differences and aforementioned risk factors may play a role in the development and persistency of fibrosis. According to these recent studies, early fibrotic changes on CT may not represent actual fibrosis, be related to the recovery process, and may be partly reversible.34,35
The total opacity score, opacity volume, and percentage of opacity were the highest in the severe group in all stages similar to the literature.9,18,26 We found that although the dynamic change of total opacity volume was not significant, the total percentage of opacity and opacity score based on percentages significantly increased in severe patients. As both opacity volume and lung volume vary with the patient's height, weight, disease severity, and time course, the percentage of opacity seems like a more reliable parameter than opacity volume in follow-up period.
Moderate patient group did not have any significant changes in CT parameters over periods. The increasing opacity trend through the first 3 stages was followed by a slight decrease in stage 4. The relatively stable course of nonsevere patients was previously described.15,36 However, this was not the case for the mild group in our study. The increase of opacity score was also statistically significant for the mild patient group from stages 1 to 4. This might be explained by the fact that for the mild group 91.7% of the initial CTs were obtained in stage 1. This rate was 74% for moderate and 25% for severe patients. Patients in the mild group had no airspace opacities on the baseline CT. Therefore, the progression of CT parameters may be because of the expected disease course. On the other hand, follow-up CTs of the mild group might have belonged to the patients with clinical deterioration. Follow-up CT was probably not needed for clinically recovered and discharged mild patients.
The radiological resolution was not complete at the end of our study. The last CTs in each clinical group were obtained on day 29, day 62, and day 49 for mild, moderate, and severe groups, respectively. At stage 4, we observed residual opacities in all groups, especially in the severe patient group reaching approximately 37% of the lung volume. According to Ding et al.'s study, 98.1% of the patients still showed CT abnormalities (mostly GGOs) after 28 days from the symptom onset.10
These residual opacities may be explained with the histopathological and radiological course of the disease. In a recent study, 3 histologic phases of diffuse alveolar damage (exudative, proliferative, and organizing-fibrotic) were correlated with CT findings. Ground-glass opacity was seen in all phases, either isolated in the exudative phase or in a mixed pattern in other phases.37 A major mechanism possibly explaining why the clinical course of the disease is unpredictable might be cytokine storm induced ARDS that aggravates lung injury. In a recent study, a significant correlation between CT severity score and serum IL-2R and IL-6 was reported.38 So, in patients with higher severity scores, there may be intervening or overlapping immunologic mechanisms causing persistent or new opacities.
As for the radiological course, during the absorption stage, whereas consolidative opacities regress, more extensive GGO areas with decreasing density have been described.39,40 Du et al19 reported that while initial GGOs decrease or disappear, fibrotic changes on predischarge CTs evolve into new GGOs. Hu et al8 observed concomitant new lesions with absorbing ones on days 10 to 18. These residual opacities cannot be discriminated by AI-based algorithms, as all GGOs, fibrotic bands, and consolidations account for “opacity.” Thus, although AI-based quantitative CT analysis is more objective on follow-up evaluation, the inability in discriminating between acute versus residual changes still mostly requires the impression of a radiologist. Also, in a study radiologists observed GGOs in 69.7% of the patients, whereas AI could not find any opacities.19 One of the reasons for the higher residual opacity rate in our study might be because of our supervision, as AI results were checked and corrected manually.
In the current study, the involvement of more than 4 lobes (sensitivity, 100%; specificity, 65.26%), mean lung density greater than −705 HU (sensitivity, 57.14%; specificity, 90.53%), total opacity score greater than 4 (sensitivity, 100%; specificity, 64.21), percentage of opacity greater than 11% (sensitivity, 80.95%; specificity, 88.42%), and percentage of high opacity greater than 0.8% (sensitivity, 85.71%; specificity, 80.00%) can discriminate severe from nonsevere pneumonia. Gouda and Yasin41 also analyzed these parameters to differentiate mild to moderate cases from severe-critical cases using the same AI-based software. The cutoff values were −637.7 HU for mean lung density (sensitivity, 81.8%; specificity, 81.9%), 8.5 for total opacity score (sensitivity, 84.1%; specificity, 81.2%), 23.81% for percentage of opacity (sensitivity, 86%; specificity, 81.2%), and 5.61% for percentage of high opacity (sensitivity, 86%; specificity, 81.2%). The reason for our lower cutoff values may be the lack of critical cases and a relatively lower number of severe cases. Also, there was no mortality among our patients. Lower cutoff values in this study may indicate that multilobar (5 lobes) involvement with lower opacity percentages may also show severity.
Our study has several limitations. To begin with, the study design was retrospective and the sample size was small especially in mild and severe groups. The relative scarcity of CT scans obtained on stage 4 in the mild patient group caused inadequate assessment. Residual opacities were present in all groups at the end of the study, thus monitoring the disease resolution completely was not possible.
In conclusion, AI-based quantitative CT analysis is an objective tool in the assessment of baseline and follow-up findings of COVID-19 pneumonia. Computed tomography parameters differ according to clinical severity and stage of the disease. Thus, quantitative CT parameters may help clinicians in predicting disease severity and the disease course. In the follow-up period, quantification of CT findings may give useful information to clinicians in monitoring disease progression and demonstrate the lung changes such as lung volume loss in severe pneumonia. Future prospective studies with larger sample sizes imaged at more time points and with longer follow-up periods may give additional information about the course of the disease and disease resolution.
Footnotes
The authors declare no conflicts of interest.
Author Contributions: S.A.D. wrote the article. All authors made a substantial contribution to the study conception and design. All authors made a substantial contribution to data and image collection. All authors commented on previous versions of the article, and all authors read and approved the final article.
Contributor Information
Gamze Durhan, Email: gamzedurhan@gmail.com.
Figen Basaran Demirkazik, Email: fdemirka@gmail.com.
Ilim Irmak, Email: ilimirmak@hotmail.com.
Jale Karakaya, Email: jalekarakaya@gmail.com.
Erhan Akpinar, Email: erhan.akpinar@gmail.com.
Meltem Gulsun Akpinar, Email: meltemg@hacettepe.edu.tr.
Ahmet Cagkan Inkaya, Email: inkayaac@yahoo.com.
Serpil Ocal, Email: drserpilgocmen@yahoo.com.
Arzu Topeli, Email: atopeli@hacettepe.edu.tr.
Orhan Macit Ariyurek, Email: macit.ariyurek@gmail.com.
REFERENCES
- 1.Zhu N Zhang D Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akpinar E, Erbil B. Is CO-RADS a categorical chest CT assessment scheme or a suspicion scale only? Radiology. 2020. Available at: https://pubs.rsna.org/page/radiology/blog/2020/5/brief_communications_202094.
- 4.Ai T Yang Z Hou H, et al. Correlation of Chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheim A Mei X Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan F Ye T Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F Zhang Q Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10:5613–5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Q Guan H Sun Z, et al. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur J Radiol. 2020;128:109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen C Yu N Cai S, et al. Quantitative computed tomography analysis for stratifying the severity of coronavirus disease 2019. J Pharm Anal. 2020;10:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding X Xu J Zhou J, et al. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol. 2020;127:109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H Han X Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z Jin C Wu CC, et al. Association between initial chest CT or clinical features and clinical course in patients with coronavirus disease 2019 pneumonia. Korean J Radiol. 2020;21:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durhan G Ardalı Düzgün S Başaran Demirkazık F, et al. Visual and software-based quantitative chest CT assessment of COVID-19: correlation with clinical findings. Diagn Interv Radiol. 2020;26:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y Liu Y Gong H, et al. Quantitative lung lesion features and temporal changes on chest CT in patients with common and severe SARS-CoV-2 pneumonia. PLoS One. 2020;15:e0236858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L Qin L Xu Z, et al. Using artificial intelligence to detect COVID-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. 2020;296:E65–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belfiore MP Urraro F Grassi R, et al. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol Med. 2020;125:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YC Luo H Liu S, et al. Dynamic evolution of COVID-19 on chest computed tomography: experience from Jiangsu Province of China. Eur Radiol. 2020;30:6194–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du S Gao S Huang G, et al. Chest lesion CT radiological features and quantitative analysis in RT-PCR turned negative and clinical symptoms resolved COVID-19 patients. Quant Imaging Med Surg. 2020;10:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gieraerts C Dangis A Janssen L, et al. Prognostic value and reproducibility of AI-assisted analysis of lung involvement in COVID-19 on low-dose submillisievert chest CT: sample size implications for clinical trials. Radiol Cardiothorac Imaging. 2020;2:e200441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7).
- 22.Feng Y Ling Y Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K Fang Y Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol. 2020;30:4407–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang G Gong T Wang G, et al. Timely diagnosis and treatment shortens the time to resolution of coronavirus disease (COVID-19) pneumonia and lowers the highest and last CT scores from sequential chest CT. AJR Am J Roentgenol. 2020;215:367–373. [DOI] [PubMed] [Google Scholar]
- 25.Chen N Zhou M Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyu P Liu X Zhang R, et al. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Invest Radiol. 2020;55:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian S Hu W Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao XH Li TY He ZC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. [DOI] [PubMed] [Google Scholar]
- 29.Iwasawa T Sato M Yamaya T, et al. Ultra-high-resolution computed tomography can demonstrate alveolar collapse in novel coronavirus (COVID-19) pneumonia. Jpn J Radiol. 2020;38:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbie H Wells AU Jacob J, et al. Visual and automated CT measurements of lung volume loss in idiopathic pulmonary fibrosis. Am J Roentgenol. 2019;213:318–324. [DOI] [PubMed] [Google Scholar]
- 31.Zhao YM Shang YM Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santus P Flor N Saad M, et al. Trends over time of lung function and radiological abnormalities in COVID-19 pneumonia: a prospective, observational, Cohort Study. J Clin Med. 2021;10:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X Fan Y Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M Lv F Huang Y, et al. Follow-up study of the chest CT characteristics of COVID-19 survivors seven months after recovery. Front Med (Lausanne). 2021;8:636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang ZL Chen C Huang L, et al. Fibrotic changes depicted by thin-section CT in patients with COVID-19 at the early recovery stage: preliminary experience. Front Med (Lausanne). 2020;7:605088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu N He G Yang X, et al. Dynamic changes of Chest CT follow-up in coronavirus disease-19 (COVID-19) pneumonia: relationship to clinical typing. BMC Med Imaging. 2020;20:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barisione E Grillo F Ball L, et al. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. 2021;478:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen LD Zhang ZY Wei XJ, et al. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res. 2020;21:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang Y Xu C Jiang F, et al. Clinical characteristics and changes of chest CT features in 307 patients with common COVID-19 pneumonia infected SARS-CoV-2: a multicenter study in Jiangsu, China. Int J Infect Dis. 2020;96:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D Zhang W Pan F, et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020;21:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouda W, Yasin R. COVID-19 disease: CT pneumonia analysis prototype by using artificial intelligence, predicting the disease severity. Egypt J Radiol Nucl Med. 2020;51:196. [Google Scholar]


