Abstract
Inorganic phosphate (Pi) is essential for signal transduction and cell metabolism, and is also an essential structural component of the extracellular matrix of the skeleton. Pi is sensed in bacteria and yeast at the plasma membrane, which activates intracellular signal transduction to control the expression of Pi transporters and other genes that control intracellular Pi levels. In multicellular organisms, Pi homeostasis must be maintained in the organism and at the cellular level, requiring an endocrine and metabolic Pi-sensing mechanism, about which little is currently known. This Review will discuss the metabolic effects of Pi, which are mediated by Pi transporters, inositol pyrophosphates and SYG1–Pho81–XPR1 (SPX)-domain proteins to maintain cellular phosphate homeostasis in the musculoskeletal system. In addition, we will discuss how Pi is sensed by the human body to regulate the production of fibroblast growth factor 23 (FGF23), parathyroid hormone and calcitriol to maintain serum levels of Pi in a narrow range. New findings on the crosstalk between iron and Pi homeostasis in the regulation of FGF23 expression will also be outlined. Mutations in components of these metabolic and endocrine phosphate sensors result in genetic disorders of phosphate homeostasis, cardiomyopathy and familial basal ganglial calcifications, highlighting the importance of this newly emerging area of research.
Phosphorus found in living organisms is referred to as inorganic phosphate (Pi) when present as phosphoric acid (H2PO4− and HPO42−), in its monovalent or divalent soluble sodium or potassium salts or its less soluble calcium salt (such as hydroxyapatite). Phosphate can also form dimers (such as pyrophosphate) and polymers (polyphosphate) or might be covalently bound in organic molecules (such as inositol pyrophosphates, membrane phospholipids, phosphoproteins and ribonucleic acids)1–3. In mammalian systems, Pi is essential for metabolic functions, such as intracellular signal transduction and energy production in most tissues. In addition, Pi is an important structural component as it is needed to form hydroxyapatite in the extracellular matrix of the skeleton. The intracellular concentration of free (soluble) Pi is approximately equal to the extracellular Pi concentration (3–5 mg/dl in humans), but levels of insoluble salts, multimers or organically bound phosphate are approximately tenfold higher. In total, intracellular phosphate makes up ~14% of total body Pi, whereas 85% of Pi is stored as hydroxyapatite in the extracellular matrix of bone and teeth4. Only 1% of overall phosphate is present in extracellular fluids, where it serves an important additional role as a buffer to maintain total body pH. Dysregulation of the intracellular Pi concentration affects cell metabolism and muscle function, whereas dysregulation of the Pi concentration in the extracellular fluid is implicated in skeletal disorders and in the development of vascular calcification as complications in chronic kidney disease (CKD) and cardiovascular disease (reviewed by us5 and others6–16). Owing to its important function in many cellular processes, bacteria and yeast have developed membrane-anchored and intracellular signalling pathways to sense extracellular P 17. This Pi sensing mechanism is well understood in bacteria and yeast; however, the mechanism of Pi sensing in mammalian systems is still unclear 6,16,18.
This Review will summarize the role of Pi sensing in bacteria, yeast and higher organisms, focusing on the role of Pi sensing in mineral metabolism and the consequences of Pi imbalance in human diseases. We will also discuss new findings about the crosstalk between iron and Pi homeostasis for regulation of fibroblast growth factor 23 (FGF23) expression, human mutations in Pi transporters resulting in genetic disorders of phosphate homeostasis, cardiomyopathy and familial basal ganglial calcifications and functions of inositol pyrophosphates and SYG1–Pho81–XPR1 (SPX)-domain proteins in cellular phosphate homeostasis.
Metabolic Pi sensing and function
Metabolic Pi sensing functions to maintain levels of Pi in the intracellular compartment to support cellular metabolism. Pi uptake in unicellular organisms (such as bacteria and yeast) is regulated at the plasma membrane, where metabolic Pi sensing activates signal transduction pathways that control the expression of phosphatases and the number of Pi transporters. In multicellular organisms, uptake of Pi into the intracellular compartment in muscle, bone and other tissues is regulated by Pi itself like in unicellular organisms19,20, and additionally by hormones such as adrenaline21, platelet-derived growth factor (PDGF)22, insulin, insulin-like growth factor 1 (IGF1)23, FGF2 (REF.24) and transforming growth factor-β (TGFβ)25 (FIG. 1). Some of these genes are highly conserved, as shown in TABLE 1. Therefore, metabolic Pi sensing is distinct from endocrine Pi sensing, which regulates hormones produced by the parathyroid, kidneys and bones (as discussed further in the Review), which in turn regulate Pi in the extracellular compartment.
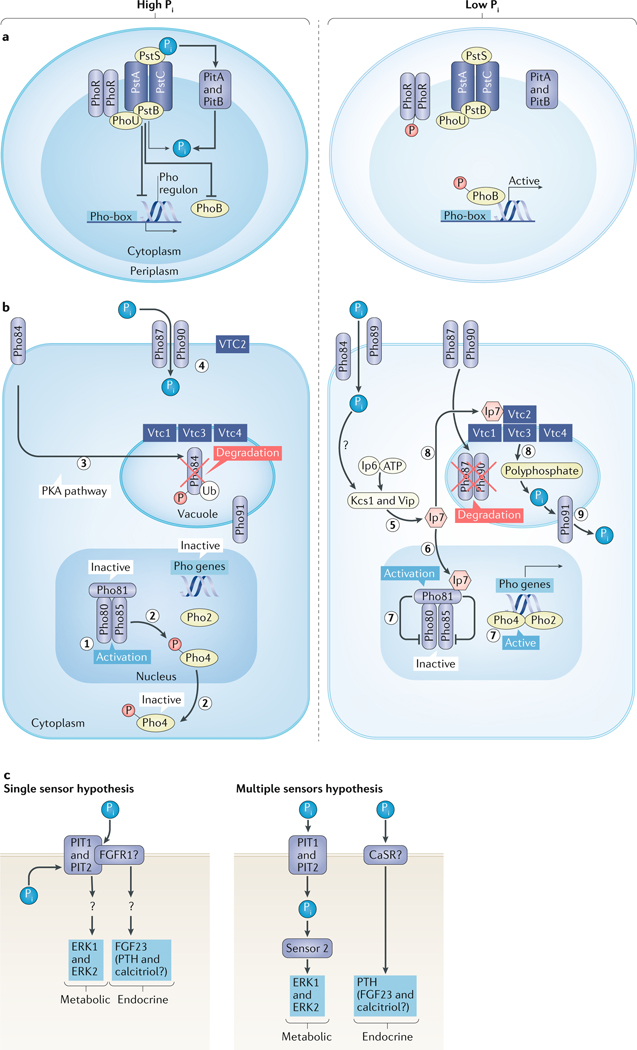
Fig. 1 |. Pi sensing pathways.
a | Pi sensing in bacteria. High Pi levels are sensed by PstS. Then, together with the Pst–ABC complex (PstA, PstB and PstC), it forms a plasma membrane protein complex in the bacterial inner membrane that stimulates the binding of PhoU to PhoB and PhoR. This process inactivates the transcription factor PhoB and the Pho regulon. The low-affinity Pi transporters 1 and 2 (PitA and PitB) facilitate uptake of Pi for cellular metabolism. Under low Pi conditions, PhoR is autophosphorylated and phosphorylates PhoB and activates the Pho box, allowing downstream activation of the Pho regulon. b | In yeast, high Pi levels activate the Pho80 and Pho85 cyclin–cyclin-dependent kinase complex (1), which results in the phosphorylation and export of Pho4 into the cytosol (2) and inactivation of the yeast Pho regulon. The high-affinity Pi transporter and sensor Pho84 is internalized and degraded (3), whereas the low-affinity Pi transporters Pho87 and Pho90 are responsible for Pi uptake in high Pi conditions (4). Low Pi levels stimulate synthesis of Ip7 by the yeast inositol hexakisphosphate (Ip6) kinase 1 (Kcs1) and Vip (5), which activates Pho81 (6). Pho81 inhibits Pho80 and Pho85, preventing phosphorylation of Pho4, resulting in the association of Pho4 with Pho2 (7) in the nucleus to activate the Pho regulon. Ip7 also stimulates Vtc proteins 1–4, which stimulate polyphosphate synthesis from ATP (8) and the conversion of polyphosphate into Pi by endopolyphosphatase (Phm5). Pi is transported by Pho91 from the vacuole to the cytosol (9), thereby indirectly using ATP to supply Pi for metabolic processes. Pho84 and Pho89 are responsible for Pi uptake in low Pi condition, whereas Pho87 and Pho90 are internalized and degraded. c | Metabolic Pi sensing in mammalian cells is mediated by PIT1 and/or PIT2, resulting in activation of the ERK1 and ERK2 pathway, which might also have a role in endocrine Pi sensing but could require co-receptors (single sensor hypothesis), possibly FGFR1, which was shown to be activated by Pi and might regulate FGF23 secretion by osteocytes. Alternatively, endocrine Pi sensing might involve the calcium-sensing receptor (CaSR) or other molecules as a second sensor (multiple sensor hypothesis), which might mediate secondary hyperparathyroidism in the parathyroids. Question marks indicate unknown mechanisms or sensors. P, phosphate; PTH, parathyroid hormone; Ub, ubiquitin.
Table 1 |.
Genes involved in inorganic phosphate (Pi) sensing
| Gene name | Organism | Orthologue | Function | Refs |
|---|---|---|---|---|
| PstS | Bacteria (Escherichia coli) | Human phosphate-binding protein | Phosphate periplasmic-binding component | 46,280 |
| PstA | Bacteria (Escherichia coli) | − | •Phosphate transporter subunit •Membrane component |
281 |
| PstB | Bacteria (Escherichia coli) | − | •Phosphate transporter subunit •ATP-binding component |
282 |
| PstC | Bacteria (Escherichia coli) | − | •Phosphate subunit •Membrane component |
NA |
| PitA | Bacteria (Escherichia coli) | •PIT1 and PIT2 in Homo sapiens •CG7628 in Drosophila melanogaster •Pho89 in yeast |
Low-affinity phosphate transport system | 37 |
| PitB | Bacteria (Escherichia coli) | •PIT1 and PIT2 in Homo sapiens •CG7628 in Drosophila melanogaster •Pho89 in yeast |
Low-affinity phosphate transport system | 37 |
| Pho84 | Yeast | •SLC17A1–9 in Homo sapiens •MFS10 and MFS13 in Drosophila melanogaster |
Major facilitator superfamily (MFS) Pi transporter (H+-coupled) | 32 |
| Pho89 | Yeast | •PIT1 and PIT2 in Homo sapiens •CG7628 in Drosophila melanogaster •PitA and PitB in bacteria |
Pi transporter (Na+-coupled) | 37 |
| Pho87 | Yeast | SLC13A1–4 in Homo sapiens | Putative Pi transporter | 18 |
| Pho90 | Yeast | SLC13A1–4 in Homo sapiens | Putative Pi transporter | 18 |
| Pho91 | Yeast | SLC13A1–4 in Homo sapiens | Putative Pi transporter | 18 |
| SLC17A1 | Mammalian | •MFS10 and MFS13 in Drosophila melanogaster •Pho84 in yeast |
Type 1 sodium-phosphate cotransporters (NPT1) | 32 |
| SLC13A1–4 | Mammalian | Pho87 in yeast | Sodium-dependent sulfate transporters | 18 |
| SLC34A1–A3 | Mammalian | − | Type 2 sodium-phosphate cotransporters NPT2a, NPT2b and NPT2c | 283 |
| SLC20A1 and SLC20A2 | Mammalian | •CG7628 in Drosophila melanogaster •Pho89 in yeast •PitA and PitB in bacteria |
Type 3 sodium-phosphate cotransporters PIT1 and PIT2 | 37 |
| SLC25A3 | Mammalian | MIR1 in yeast | Mitochondrial phosphate transporter PIC | 37,284 |
| SLC53A1 | Mammalian | Pho81 and Syg1 in yeast | Phosphate exporter XPR1 | 285 |
H+, proton; NA, not available.
Bacteria
Unicellular organisms sense Pi through plasma membrane protein complexes to regulate intracellular levels of Pi (REFs26,27) (FIG. 1a). In bacteria, the transporters that make up the membrane protein complex are similar to the ATP-binding cassette (ABC) transporters in higher species and comprise Pi-binding protein PstS (PstS), Pi transport system (Pst) permease protein A (PstA), Pi import ATP-binding protein B (PstB) and Pst permease protein C (PstC). During high Pi conditions (as described in detail in FIG. 1a), Pi binding to the membrane protein complex results in dephosphorylation and deactivation of Pi regulon transcriptional regulatory protein (PhoB)27. PhoB deactivation reduces the expression of high-affinity Pi transporters and phosphatases, which are upregulated in Pi-limiting conditions and permit bacteria to scavenge Pi from the environment to increase intracellular levels of Pi27. Low-affinity Pi transporters 1 and 2 (PitA and PitB) mediate bacterial uptake of Pi during high Pi conditions17. When present in excess, Pi is stored by a specialized polyphosphate kinase (PPK) as a linear polymer (polyphosphate)28,29. This mechanism is conserved from bacterial to human cells; however, the role of polyphosphate as a metabolic regulator is only beginning to be understood in higher species28,29.
Yeast
Whereas Pi is sensed in the inner bacterial membrane, Pi uptake by the Pi transporter Pho84 is required for Pi sensing in yeast30,31 (FIG. 1b). Pho84 is a high-affinity Pi transporter related to the solute carrier family 17 (SLC17) protein family in higher species, and they share 7.6–12.9% amino acid identity32. Pho89, a second high-affinity transporter, has the PIT family signature sequences at the amino terminus and carboxyl terminus, which is shared with bacterial PitA and PitB, plant Pi transporter 2–1 (PHT2–1) and human sodium-dependent Pi transporter 1 and 2 (PIT1 and PIT2; which are encoded by SLC20A1 and SLC20A2, respectively). Both high-affinity Pi transporters Pho84 (587 amino acid residues) and Pho89 (574 amino acid residues) are composed of 12 transmembrane domains. In Pho84, these 12 transmembrane domains are arranged as 2 homologous sequence segments, each containing 6 transmembrane domain regions that are separated by a large cytoplasmic loop33,34, whereas in Pho89, a large intracellular hydrophilic loop is positioned between domains 7 and 8 (REF.35). The amino acid sequence identity in transmembrane domains 4, 8 and 10 for these transporters is 8–11%36. Conversely, the low-affinity transporters Pho87 and Pho90 and the vacuolar transporter Pho91 are related to metazoan sodium–sulfate transporters (solute carrier family 13 member 1–4 (SLC13A1–4))37.
Under high Pi conditions, low-affinity Pi transporters Pho87 and Pho90 are responsible for Pi uptake, whereas under low Pi conditions, Pho84 and Pho89 are required27. Similar to bacteria, high environmental Pi reduces expression of the high-affinity transporters in yeast. This process involves cAMP-dependent protein kinase A (PKA)-dependent internalization and degradation of the Pho84 transporter in the vacuole30. A second mechanism requires the uptake of Pi by Pho84, which activates a signalling cascade (described in detail in FIG. 1b) that results in the repression of the yeast Pho regulon, which encodes yeast Pi starvation-induced genes30,31. Conditions of low Pi induce genes that encode for the high-affinity Pi transporters Pho84 and Pho89, secreted phosphatase Pho5 and putative cyclin-dependent kinase inhibitor SPL2, which in turn suppresses the low-affinity transporter (REF.38).
5-Diphosphoinositol pentakisphosphate (Ip7) is an important intermediary to signal Pi starvation in yeast39. In response to Pi starvation, Ip7 is synthesized from inositol hexakisphosphate (Ip6) by the yeast Ip6 kinase 1 (Kcs1) and diphosphoinositol-pentakisphosphate kinase (Vip1)40. Ip7 binds to the SPX domain, which is named after the plasma membrane protein that suppresses lethality of Gα protein deficiency (Syg1), the cyclin-dependent kinase inhibitor phosphate system positive regulatory protein (Pho81), and the mammalian xenotropic and polytropic retrovirus receptor 1 (XPR1; which is encoded by SLC53A1 and might function as a Pi exporter)41. Ip7 induces the expression of genes with protein products that increase Pi uptake, such as Pho84 and other Pi transporters27,40. In addition, Ip7 can activate another yeast SPX-domain protein, the vacuolar transporter chaperone 4 (Vtc4), which, together with Vtc1, Vtc2 and Vtc3, forms a yeast polyphosphate polymerase that stimulates polyphosphate synthesis from ATP under low Pi conditions in the vacuole42. This polyphosphate is converted to Pi by endopolyphosphatase (Phm5; also known as Ppn1) and transported from the vacuole to the cytosol by the vacuolar Pi transporter (Pho91). Therefore, by synthesizing polyphosphate, ATP can provide Pi for cytosolic processes when the exogenous supply of Pi is low27,43,44. Ip7 finally inhibits transcription of genes important for glycolysis and class 1 histone deacetylase in yeast45, thereby reducing consumption of Pi. In summary, Ip7 might function as a second messenger in yeast to signal reduced cytosolic Pi levels by binding to several SPX-domain proteins, to stimulate Pi uptake from the environment and release of Pi from vacuolar polyphosphate stores and to reduce Pi consumption.
Mammalian
Unlike bacterial and yeast Pi sensing, mammalian Pi sensing is only partially understood. Multicellular organisms require homeostatic regulation in the extracellular and intracellular compartments. Although there are no orthologous proteins of the Pi-sensing histidine kinase Pi regulon sensor protein PhoR in higher species, a human plasma phosphate binding protein has been identified with 25% sequence identity to bacterial Pi binding protein PstS46. Evidence furthermore suggests that the type 3 sodium-dependent Pi transporters PIT1 and PIT2 (encoded by SLC20A1 and SLC20A2, respectively) have an important role in mammalian Pi sensing. Interestingly, PIT1 and PIT2 might sense extracellular Pi without requiring translocation of Pi, which is also referred to as Pi transport-independent sensing47,48; therefore, it is possible that these transporters serve as sensors for extracellular Pi in addition to regulating intracellular levels of Pi. This process might involve a co-receptor (or co-receptors) that switches between extracellular sensing and transport functions (FIG. 1c; one sensor hypothesis). However, it is also possible that there are separate sensors for extracellular and intracellular Pi levels (FIG. 1c; multiple sensor hypothesis). In this hypothesis, extracellular Pi levels are detected by a sensor located only in the cell membrane of endocrine cells, whereas the intracellular Pi level is sensed in most tissues to maintain cell metabolism.
Extracellular Pi sensing.
The mitogen-activated protein kinases (MAPKs) extracellular-signal-regulated kinase 1 (ERK1) and ERK2 (also known as MAPK3 and MAPK1, respectively) are activated by extracellular Pi in most cell types, which is an evolutionarily conserved process between Drosophila melanogaster and humans32. This activation is blocked by pharmacological inhibition or genetic ablation of type 3 sodium-dependent Pi transporters49. Because both PIT1 and PIT2 can fulfil this role, it might be intracellular Pi that is sensed to activate ERK1 and ERK2. However, PIT transporters might also bind and signal to the cell independent of Pi transport, as shown in HeLa cells and vascular smooth muscle cells (VSMCs) expressing the transport-deficient PIT1 mutant Glu70Lys47,48. Activation of PIT1, ERK1 and ERK2 is required for Pi-dependent stimulation of the expression of genes involved in bone mineralization in osteoblasts50 and for the apoptosis of hypertrophic chondrocytes through the mitochondrial caspase 3 pathway51. In addition, Pi regulation of mitochondrial respiration and ATP flux in skeletal muscle requires the activation of ERK1 and ERK2 (REFs52,53). Finally, FGF receptor substrate 2 (FRS2) is phosphorylated following treatment with Pi in human embryonic kidney cells (HEK293)54 and mouse osteoblastic cells (MC3T3)53,55, which is blocked by short interfering RNA silencing of FGF receptor 1 (FGFR1). This result suggests that FGFR1 not only mediates FGF23 signalling but also Pi sensing, as discussed later in the Review.
Intracellular Pi sensing.
Subcellular compartments might further sequester intracellular Pi. The mitochondrial Pi carrier protein (PIC; which is encoded by SLC25A3) is part of the mitochondrial permeability transition pore (mPTP), which is a multiprotein complex. The mPTP regulates mitochondrial membrane potential and mitochondrial apoptosis56 and is important for skeletal and cardiac muscle function57. PIT1 localizes to the endoplasmic reticulum (ER), where it seems to be involved in regulating the ER stress of growth plate chondrocytes58. In addition, large and small conductance chloride channels transport Pi into the sarcoplasmic reticulum of rabbit skeletal muscle59. The advent of novel imaging techniques to visualize subcellular Pi (such as fluorescence-lifetime imaging, fluorescence resonance energy transfer microscopy technologies, synchrotron-based X-ray fluorimetry and nanoscale secondary ion mass spectrometry) might provide insights into this poorly understood area of research in the future60–63.
IP7.
In a genome-wide association study for genetic determinants of serum Pi concentration, two orthologues of yeast Ip6 kinase, inositol hexakisphosphate kinases 2 and 3 (IP6K2 and IP6K3, respectively), were identified as well as PIT1, extracellular calcium-sensing receptor (CaSR) and FGF23 (REF.64). IP6K2 seems to catalyse the synthesis of IP7 in various human cell lines, including HCT116 (human colon colorectal carcinoma) and U2OS (human bone osteosarcoma epithelial) cells65. Similar to yeast, IP7 regulates Pi consumption in mammalian cells. For example, IP7 inhibits insulin signalling by potently inhibiting the phosphorylation of AKT by 3-phosphoinositide-dependent protein kinase 1 (PDPK1), thereby preventing its activation in the human hepatocellular carcinoma cell line HePG2 (REF.66). In addition, IP7 enhances casein kinase 2 (CK2; which is encoded by CSNK2)-mediated phosphorylation of cellular tumour antigen p53 and thereby activates cell death pathways in human U20S bone osteosarcoma cells67. Furthermore, the highly conserved IP7 binding domain, SPX, is present in mammalian XPR1, which may function as a phosphate exporter68. Interestingly, mutations in the SPX domain of XPR1 stimulate the formation of calcium–Pi deposits in the basal ganglia of individuals with primary familial brain calcification, which might be caused by abnormal glial cell Pi export69. XPR1 expression is in turn stimulated by the receptor activator of nuclear factor (NF)-κB (RANK; which is encoded by TNFRSF11A)–RANK ligand (RANKL) pathway in osteoclasts70. Taken together, these findings suggest that IP7 has a role in mammalian metabolic Pi sensing, although the underlying mechanism remains unknown39,69.
Polyphosphate.
Similar to IP7, polyphosphate is found in mammalian cells, although its function is incompletely understood71. Mammalian cells lack orthologues of both yeast polyphosphate kinases (Vtc and Ppk1) (see FIG. 1b), and mammalian polyphosphate synthesis pathways are yet to be discovered. As mammalian polyphosphate synthesis is blocked by oligomycin, a mitochondrial ATP synthase (complex V of the respiratory chain) inhibitor, but does not require ATP as a substrate72, it is suggested that polyphosphate is a by-product of mitochondrial ATP synthesis. Consistent with its role as a Pi store, polyphosphate seems to stimulate Pi consumption by activating the mammalian target of rapamycin (mTOR) pathway73, the mitogenic activities of FGF1 and FGF2 by physically and functionally stabilizing the two, similar to heparin sulfate, or by potentially preventing their degradation in human fibroblasts74. However, polyphosphate also stimulates apoptosis by activating caspase 3 in human plasma cells75 and neurons76. Further research is needed to better understand the role of polyphosphate in intracellular signalling and metabolism of mammalian cells.
Metabolic Pi in musculoskeletal biology
Here, we describe how Pi regulates differentiation and production of cartilage matrix by chondrocytes in joint surfaces and growth plates, how Pi regulates bone remodelling by controlling the differentiation and function of the three types of bone cells (bone-forming osteoblasts, mechano-sensing osteocytes and bone-resorbing osteoclasts) and how Pi regulates vascular, skeletal and cardiac muscle function (FIG. 2). Identification of the phosphaturic hormone FGF23 (REFs77,78), which is secreted by late-stage osteoblasts and osteocytes, also established the skeleton as an important endocrine regulator of Pi homeostasis79, which will be discussed later in the Review.
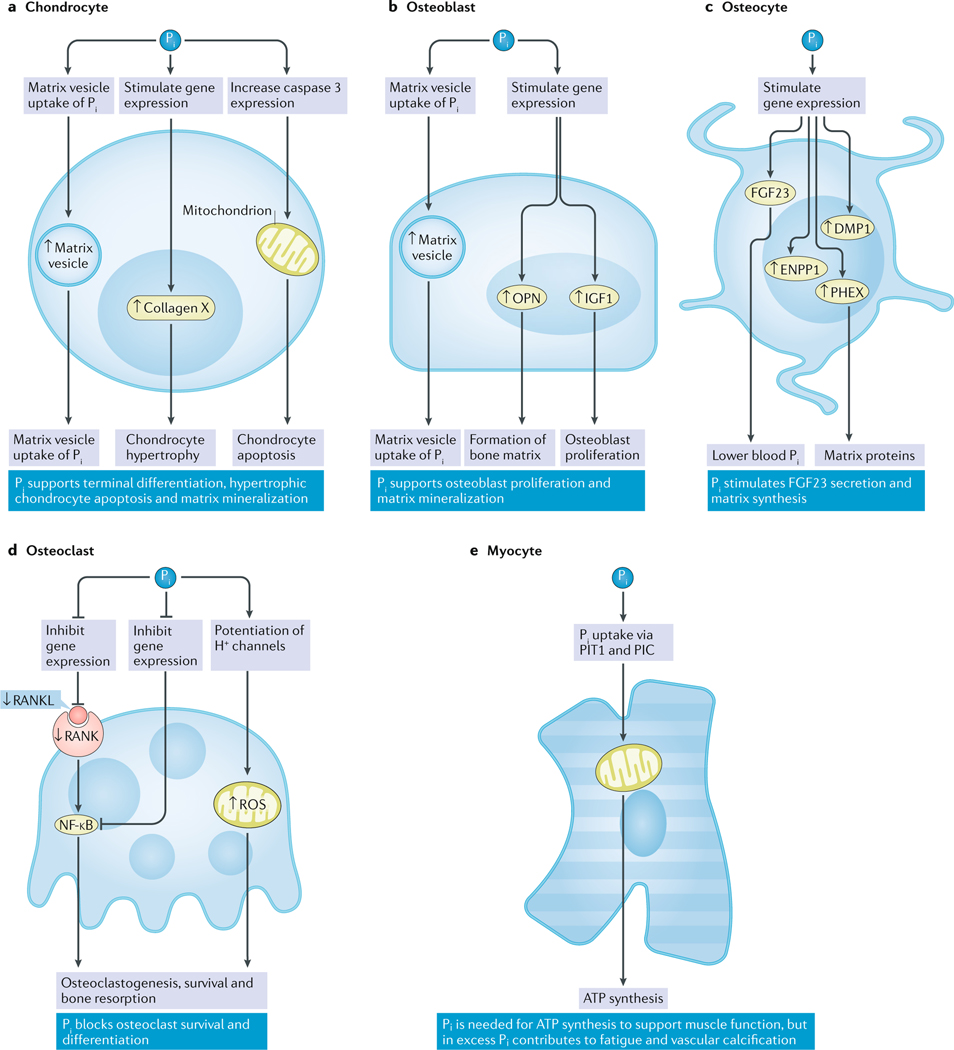
Fig. 2 |. Regulation of bone cell function and matrix mineralization by Pi.
a | In chondrocytes, Pi stimulates the expression of hypertrophic chondrocyte markers (that is, collagen X) and induces apoptosis via the mitochondrial caspase 3 pathway in an ERK1-dependent and ERK2-dependent fashion83. Pi also stimulates PIT1-dependent matrix vesicle mineralization90. b | In osteoblasts, Pi induces the expression of osteopontin (OPN) through an ERK1-dependent and ERK2-dependent mechanism to support the formation of bone matrix98. Pi also stimulates IGF1 secretion, which increases osteoblast proliferation in an autocrine fashion99. c | In osteocytes, DMP1, ENPP1 and PHEX expression are stimulated by Pi (REF.116), which also induces the secretion of bioactive intact FGF23 (REF.103). d | In osteoclasts, Pi reduces gene expression of RANKL and thereby suppresses RANK126, which results in the inhibition of osteoclastogenesis and bone resorption. Pi also induces the production of ROS, possibly through proton (H+) channels, which increases osteoclast function and survival131. e | In myocytes, Pi is important for the function of the mitochondrial respiratory chain and ATP synthesis52. This process is possibly due to the function of the muscle-specific isoform of PIC, which mediates mitochondrial uptake of Pi (REF.57), and PIT1 (REF.277). ↑, upregulation; ↓, downregulation.
Chondrocytes
Chondrocytes produce and maintain the extracellular matrix of joint cartilage and permit longitudinal growth of long bones by a process called endochondral ossification. Abnormal proliferation and differentiation of chondrocytes lead to degenerative diseases such as rickets and osteoarthritis20.
The observation of very low levels of Pi in pre-mineralized cartilage, which increase during mineralization, led to the speculation that Pi might be required for bone growth80,81. A study in which Pi was added to the chondrogenic cell line ATDC5 showed reduced levels of type II collagen and parathyroid hormone receptor–parathyroid hormone-related peptide receptor (PTH–PTHr receptor (PPR1) which is encoded by PTH1R) gene expression and increased type X collagen expression82, suggesting that Pi induces terminal differentiation and apoptosis of chondrocytes. The ability of Pi to rescue delayed differentiation of cultured murine metatarsals, prepared from heterozygous or homozygous knockout of parathyroid hormone-related protein (Pthrp−/+ or Pthrp−/−; which is encoded by Pthlh) in mice, further supported the role Pi has in bone growth83. Additionally, Pi induces hypertrophic differentiation20 and apoptosis84 in the chondrocytic cell line CFK2. Pi also stimulates hypertrophy in primary chondrocytes from human osteoarthritic joints85. PIT1 and PIT2 facilitate Pi uptake into bovine articular chondrocytes86. Ablation or pharmacological inhibition of PIT1 or the inhibition of dual specificity MAPK kinase 1 (MEK1, which is encoded by MAP2K1) blocks the phosphorylation of ERK1 and ERK2 and mitochondrial apoptosis induced by Pi in primary chondrocytes51 and CFK2 cells84. Chondrocytes might also regulate systemic Pi homeostasis by secreting FGF23, independently of ERK1 and ERK2 (REF.85). In addition to the MAPK pathway, Pi increases the nitrate:nitrite (NO3−:NO3−) ratio by stimulating nitric oxide synthase (NOS), which in turn induces chondrocyte apoptosis84.
Studies in genetically modified mice suggest that PIT1 is important for liver development and haematopoesis87,88. Mice carrying two hypomorphic alleles of the Pit1 gene, which results in an 85% reduction of PIT1 expression, show reduced femur length89 but have surprisingly normal bone and mineral metabolism. Therefore, cell-type-specific ablation of PIT1 might be required to fully understand its skeletal functions. For example, mice lacking phosphoethanolamine–phosphocholine phosphatase (PHOSPHO1), an enzyme that hydrolyses phosphoethanolamine to generate Pi for matrix vesicle mineralization, have defective matrix vesicle mineralization in the growth plates90. Mice with double knockout of Pit1 (specifically in the chondrocyte) and Phospho1 (full-body deletion) show worse matrix vesicle mineralization than Phospho1-null mice. Furthermore, acute chondrocyte-specific deletion of Pit1 in mice results in pronounced cell death in the first two postnatal days, possibly owing to Pi transport-independent ER stress58. Finally, universal overexpression of Pit1 in rats caused an incisor enamel defect and decreased bone mineral volume91. Despite normal skeletal development, the mutant animals display biochemical abnormalities, including increased serum levels of Pi, FGF23 and parathyroid hormone (PTH), develop proteinuria and body weight loss and experience premature death.
In summary, Pi stimulates hypertrophic differentiation and apoptosis in chondrocytes via PIT1, ERK1 and ERK2 and possibly via NOS, which is necessary for normal bone growth and possibly articular cartilage function.
Osteoblasts and osteocytes
Osteoblasts and osteocytes synthesize bone matrix92, which is composed of type I collagen, non-collagenous proteins (such as osteocalcin) and small integrin-binding ligand, N-linked glycoprotein (SIBLING) proteins (including dentin matrix acidic phosphoprotein 1 (DMP1), matrix extracellular phosphoglycoprotein (MEPE), osteopontin (OPN; also known as SPP1) and hydroxyapatite)93. When osteoblasts become buried in bone matrix, they undergo terminal differentiation into osteocytes, which serve as mechanosensors and secrete endocrine and paracrine factors to maintain skeletal homeostasis94.
Microarray, next-generation sequencing and proteomics studies show that Pi induces expression of genes important for cell proliferation, energy metabolism and mineralization in osteoblast-like cells53,95,96, suggesting that Pi stimulates osteoblast proliferation and differentiation. Pi, in the form of β-glycerophosphate, is commonly added to cell culture media to stimulate proliferation and mineralization of primary human and mouse mesenchymal stromal cells. This process requires the presence of PIT1 and FGFR1 and activation of the GTPase NRAS53. In MC3T3 cells and primary murine calvaria-derived osteoblasts, Pi induces the expression of Fos-related antigen 1 (Fra1; also known as Fosl1), Opn and matrix Gla protein (Mgp; which are genes required for mineralization), which is dependent on ERK1 and ERK2 (REFs97,98). Pi might also stimulate IGF1 expression in the mouse-derived osteoblast cell line MC3T3-E1, which enhances osteoblast proliferation in an autocrine fashion98,99. The function of polyphosphate is less clear. Similar to pyrophosphate, polyphosphate can inhibit mineralization by preventing apatite crystal growth and by blocking alkaline phosphatase, tissue-nonspecific isozyme (TNSALP; which is encoded by ALPL), which in turn prevents degradation of the mineralization inhibitor pyrophosphate100.
As previously discussed, the Phospho1–Pit1 double-knockout mouse showed reduced matrix vesicle levels of minerals, suggesting that PIT1 is required for Pi uptake into matrix vesicles and initiation of skeletal mineralization through hydroxyapatite formation90. In addition to PIT1, osteoblasts also express PIT2 and the type 2 sodium-dependent phosphate transport protein 2A and 2B (NPT2A and NPT2B; which are encoded by SLC34A1 and SLC34A2, respectively)101–103. In pre-osteoblastic bone marrow stromal cells, nano-hydroxyapatite stimulation of gene expression (which can influence osteoblast lineage commitment and cell function) requires FGFR signalling, phosphate transporters (PIT1 and PIT2) and ERK1 and ERK2 signalling104. Blood levels of calciprotein particles (a complex of calcium, Pi and other proteins that transports hydroxyapatite to bone without crystallization in other tissues) elevate with excess Pi and calcium, contributing to CKD just before the rise of FGF23 (REF.105). Understanding hydroxyapatite formation and signalling might therefore improve clinical outcomes for CKD.
Although ablation of Pit1 does not seem to affect osteoblast or osteocyte functions89, global Pit2-knockout mice show placental calcification, growth retardation, reduced cortical and trabecular BMD106,107, reduced dentin mineralization107, cornea and brain calcification, increased levels of Pi in the cerebrospinal fluid (CSF) and decreased levels of Pi in peripheral blood108. Furthermore, these mice seem to be unable to suppress serum levels of intact FGF23 (iFGF23) in response to a low-Pi diet or to increase levels of iFGF23 in response to high-Pi diet unlike wild-type mice, whereas relative FGF23 gene expression did not show any change109. Global Pit2 haploinsufficiency also causes decreased BMD in response to 5/6 nephrectomy but has no effect on blood levels of calcium and Pi and the mutant mice had normal fractional excretion of Pi (REF.106). Thus, PIT2 is required for normal bone function in mice; however, it remains to be confirmed whether PIT2 is required for the regulation of serum iFGF23.
Unexpectedly, Npt2a−/− mice have increased bone mass, which might be due to a reduction in osteoclast number110. In addition, the difference in osteoclast number is less pronounced at 115 days of age; therefore, there is an increase in mineralizing and osteoblast surfaces later in life in these mice111. Npt2b−/− mice show embryonic lethality at embryonic day 8 (REF.112), and adult Npt2b−/+ mice have pulmonary alveolar microlithiasis but no skeletal abnormalities113. Therefore, the function of NPT2B in bone biology remains poorly understood113.
Osteocytes, similar to osteoblasts, express PIT1 and PIT2 transporters114. Pi stimulates osteocyte maturation and matrix formation in the osteocyte lacuna. Hypophosphataemic Dmp1-null mice have impaired osteocyte maturation and decreased mineralization115. When 10 mM Pi and 10 nM calcitriol (the active form of vitamin D, also known as 1,25-dihydroxyvitamin D3) are added to murine IDG-SW3 osteocyte-like cells, the gene expression of polypeptide N-acetylgalactosaminyltransferase 3 (Galnt3), Dmp1, phosphate regulating endopeptidase homologue, X-linked (Phex), ectonucleotide pyrophosphatase/phosphodiesterase 1 (Enpp1) and Mepe is induced and results in matrix mineralization116. In response to PTH, osteocytes stimulate osteoclast recruitment and differentiation by secreting RANKL and inhibit osteoblast differentiation by secreting sclerostin, which blocks the wingless (WNT)–β-catenin pathway117. Pi and other factors (such as calcitriol and PTH) cause osteocytes to secrete FGF23 to regulate systemic phosphate homeostasis118–120, and in response to IL-6 and myostatin, osteocytes secrete osteocalcin to regulate body energy metabolism121.
In summary, Pi stimulates differentiation of osteoblasts and osteocytes, matrix maturation and bone formation, which involve the function of Pi transporters and ERK1 and ERK2 signalling in vitro. Surprisingly, mild bone and mineral metabolism phenotypes of the global Pit1-null and Pit2-null mice suggest a high degree of redundancy of these generally co-expressed transporters. Bone-specific ablation of Pit1 and Pit2 (individually and in combination) in mice might be required to shed light on their metabolic and endocrine functions.
Osteoclasts
Osteoclasts are large multinucleate cells derived from the monocyte lineage and are responsible for bone resorption122, which is necessary for the remodelling and repair of the skeleton. The formation and function of osteoclasts are stimulated by macrophage colony-stimulating factor (MCSF) and RANKL produced by osteoblasts. In turn, osteoclasts stimulate osteoblastic bone formation through two (and possibly more) signalling pathways (ephrins–ephrin receptors and semaphorins–plexins)123,124.
Osteoclasts express NPT2A, PIT1 and PIT2 (REF.125). High levels of extracellular Pi (4 mM) inhibit osteoclast-like cell formation in mouse bone marrow cells126 and decrease the number and area of resorption pits formed by mature rat osteoclasts on sperm whale dentine slices, which is a common assay for osteoclast function103. This observation presumably reflects a feedback mechanism to limit the degradation of hydroxyapatite and might involve NPT2A-dependent inhibition of RANK–RANKL signalling, inhibition of osteoclast growth by Pi (REF.127) and the suppression of microRNA-223 expression, which was reported in the pre-osteoclast RAW264.7 cell line128. Similar to Pi, polyphosphate might inhibit the maturation of RAW264.7 cells into functional osteoclasts by blocking RANK–RANKL signalling129. However, some Pi is required for normal osteoclast function, as phosphonoformic acid (an inhibitor of sodium-phosphate cotransporters) reduces bone resorption in cultured osteoclasts, possibly by inhibiting ATP production, for which uptake of extracellular Pi is required130. Extracellular Pi also stimulates reactive oxygen species production, which is required for osteoclastogenesis and resorptive function of RAW264.7 cells131. Wild-type mice fed a low-Pi diet and Hyp mice (a mouse model of human X-linked hypophosphataemic rickets) showed similar results. Both wild-type mice fed a low-Pi diet and Hyp mice exhibited decreased osteoclast numbers in osteoclast-like cells derived from bone marrow cells compared with wild-type mice fed normal-Pi diets, and this defect was reversed by a high-Pi diet132. Finally, the high bone mass phenotype observed in 21-day Npt2a-null mice was attributed to impaired osteoclast function133. In summary, osteoclasts express NPT2A, PIT1 and PIT2 transporters. Release of Pi during bone resorption might provide a mechanism of feedback inhibition, limiting survival and differentiation of osteoclasts at high Pi levels. However, some Pi seems to be required for normal osteoclast function.
Myocytes
Myocytes are present in vascular, skeletal and cardiac muscle tissue and contribute to the control of blood pressure, locomotion and cardiac functions.
Hypophosphataemia causes myopathy and heart failure. Although steady-state muscle Pi and ATP concentrations in Hyp mice seem to be preserved134, phosphocreatine recovery time in humans with hypophosphataemia is delayed135, which is similar to findings in individuals with vitamin D deficiency136. After 12 weeks of vitamin D supplementation, there was an increase in serum levels of Pi and restoration of phosphocreatine recovery time in individuals with vitamin D deficiency, suggesting a connection between vitamin D status, serum Pi and mitochondrial muscle ATP synthesis136. By adapting 31P-magnetic resonance spectroscopy techniques137 to noninvasively examine mitochondrial energy production (by measuring basal and insulin-stimulated ATP flux (VATP)), we studied two hypophosphataemic mouse models in vivo (wild-type mice fed a low-Pi diet and Npt2a−/− mice)133. Both mouse models had ~50% reduction in VATP, and this reduction was rapidly restored by intravenous Pi supplementation52. VATP was likewise reduced by ~50% in a patient with untreated hereditary hypophosphataemic rickets with hypercalciuria, which normalized upon treatment with oral Pi supplements52.
At the molecular level, Pi stimulates mitochondrial energy production (measured by VATP) by serving as a substrate for ATP synthesis during oxidative phosphorylation at complex V138. Furthermore, Pi maintains cytochrome b oxidation and cytochrome c reduction139 and stimulates the activity of several Krebs cycle dehydrogenases (2-oxoglutarate dehydrogenase, isocitrate dehydrogenase and malate dehydrogenase)140–143. This action increases the concentrations of mitochondrial electron donors (FADH, NADH and NADPH) to fuel the electron transport chain. In addition, Pi binds to glyceraldehyde 3-phosphate dehydrogenase and is thereby an important cofactor for a rate-limiting glycolytic enzyme that converts triose phosphates (dihydroxyacetone and glyceraldehyde 3-phosphate) to 1,3-bisphosphoglycerate144,145.
By contrast, hyperphosphataemia stimulates the expression of OPN, increases cell proliferation and mineralization and downregulates myocardin and smooth muscle α-actin (SMAαA) in a PIT1 and PIT2 transport-independent manner147–149. This process is dependent on ERK1 and ERK2 and results in osteogenic transdifferentiation147–149, which causes vascular calcification in patients with CKD146. This VSMC transdifferentiation might depend on WNT–β-catenin–runt-related transcription factor 2 (RUNX2) signalling, which is an important anabolic signalling pathway for osteoblast and osteocyte function150–153 and is inhibited by secreted frizzled-related protein 5 (SFRP5)154. Furthermore, research in skinned muscle fibres showed that Pi released during ATP hydrolysis raises cytosolic Pi from 3 mM to 15 mM, which reduces peak force by decreasing force per actin–myosin bridge and/or by increasing the number of low-force bridges in skeletal muscle, by decreasing cytosolic ionized calcium and by causing Ca–Pi precipitations in the sarcoplasmic reticulum. Thus, high intracellular Pi appears to contribute to muscle fatigue155, arguing against the simple depletion of intracellular Pi as a substrate to explain the observed reduction in VATP and hypophosphataemic myopathy.
In summary, Pi is required for maintaining muscle function, but excess Pi leads to calcification, which is best documented in vascular smooth musculature, and fatigue of skeletal muscle. This process might involve the functions of PIT1 and PIT2 transporters and ERK1 and ERK2 signalling.
Phosphate homeostasis and endocrine actions
Different from metabolic Pi sensing, which maintains intracellular Pi levels to support cell metabolism, endocrine Pi sensing maintains extracellular blood levels of Pi. The blood concentration of Pi is regulated within a narrow range (2.5–4.5 mg/dl in humans) through the control of intestinal absorption of Pi from the diet, Pi release from stores by bone modelling and renal excretion (reviewed by REFs5,9,10,156). In turn, Pi feeds back to regulate its intestinal absorption, release from bone mineral and renal excretion by inducing the secretion of PTH and FGF23 and by inhibiting the synthesis of calcitriol.
After its discovery, now almost two decades ago, it rapidly became clear that FGF23 is a key endocrine regulator of renal Pi excretion157–160. Meanwhile, the list of factors regulating FGF23 and renal Pi excretion has grown (FIG. 3; TABLE 2). FGF23 requires the co-receptor Klotho for binding to the receptor FGFR1 to activate ERK1–ERK2 signalling161, whereas non-canonical signalling via FGFR4–nuclear factor of activated T cells (NFAT)–calcineurin seems to be independent of this co-receptor162. Interestingly, there is some evidence that FGFR1 might also contribute to Pi sensing53,55; however, as levels of FGF23 are high, rather than low, in tumoural calcinosis type 3 (REF.163) and Klotho−/− mice164, Klotho is probably not involved in endocrine Pi sensing.
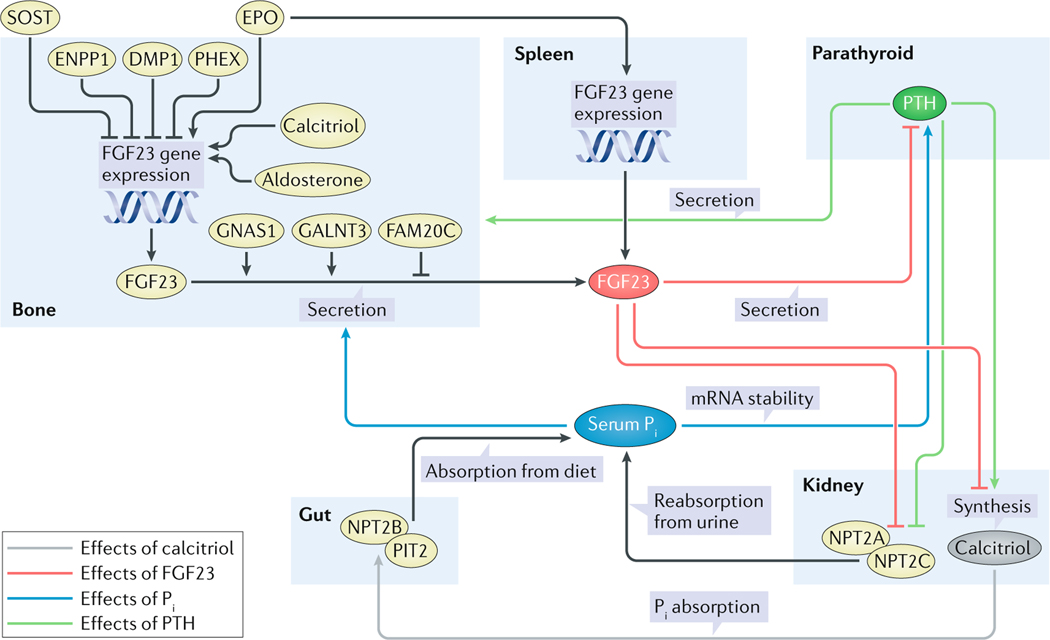
Fig. 3 |. Endocrine regulation of Pi homeostasis.
Serum Pi stimulates secretion of bioactive FGF23 in osteoblasts and osteocytes (blue arrow), which directly or indirectly acts at the proximal tubule of the kidneys to inhibit synthesis of calcitriol and the function of NPT2A and NPT2C (red arrows). Inhibition of calcitriol reduces absorption of Pi from the diet in the gut (grey arrow) and mobilization of Pi from bone mineral. Downregulation of NPT2A and NPT2C reduces renal phosphate reabsorption (black arrows). The net effect of FGF23 action is to lower blood levels of Pi. Similar to FGF23, parathyroid hormone (PTH) downregulates NPT2A and NPT2B and reduces renal phosphate reabsorption (green arrows). However, different from FGF23, PTH induces calcitriol and bone turnover, which increase blood Pi (green arrows). However, the net effect of PTH is to lower blood levels of Pi. Although not completely understood, FAM20C, DMP1, ENPP1 and PHEX reduce FGF23 expression or secretion whereas phosphate, iron deficiency, erythropoietin (EPO), GALNT3 and GNAS1 stimulate it (black arrows)278,279. In addition, sclerostin (SOST) seems to negatively regulate FGF23 (black arrows)117. Furthermore, EPO might directly upregulate FGF23 gene expression in myeloid lineage stem cells of the spleen, providing a link to iron homeostasis (black arrows)195. PTH is suppressed by FGF23 in rodents but not in humans (red arrow).
Table 2 |.
Hormones and growth factors regulated by Pi
| Factors | Regulation by Pi | Target organs | Effect | Blood Pi concentration | Refs |
|---|---|---|---|---|---|
| FGF23 | Up | Kidney | Renal Pi wasting and decreased synthesis of calcitriol | Down | 79 |
| PTH | Up | Kidney and bone | Renal Pi wasting, increased synthesis of calcitriol and release of Pi by bone resorption | Down | 286 |
| Calcitriol | Down | Intestine and bone | Increased intestinal Pi absorption and release of Pi by bone resorption | Up | 287 |
| IGF1 | Up | Bone and kidney | Increased Pi storage in bone mineral and increased renal Pi reabsorption | Up (down) | 99,288 |
| Osteopontin | Up | Bone | Increased Pi storage in bone mineral | (Down) | 98 |
FGF23, fibroblast growth factor 23; IGF1, insulin-like growth factor 1; Pi, inorganic phosphate; PTH, parathyroid hormone.
FGF23
Primarily secreted by osteoblasts, osteocytes and possibly other cell types, FGF23 is a 227-amino-acid intact peptide. Dietary Pi stimulates the increase in serum FGF23 in healthy men, which is inversely related to renal Pi absorption and calcitriol levels165. This inverse relationship is because FGF23 downregulates NPT2A and NPT2C expression in the proximal tubules of the kidneys, which resembles the action of PTH, and results in renal phosphate excretion. Conversely, neutralizing FGF23 antibodies increase serum levels of Pi (REF.166). Different from PTH, FGF23 suppresses 25-hydroxyvitamin D-1 α-hydroxylase (which is encoded by CYP27B1) and stimulates 1,25-hydroxy-vitamin D-24-hydroxylase, mitochondrial (which is encoded by CYP24A1) in the proximal tubules, thereby reducing serum levels of calcitriol167. However, one other study failed to show suppression of CYP27B1 and stimulation of CYP24A1 in response to recombinant human FGF23 in 12-week-old mice, which triggered a phosphaturic effect, possibly owing to suppression of endogenous iFGF23 in these mice168. Both actions of FGF23 at the proximal tubule might be indirect and mediated by WNT–β-catenin signalling as FGFR1 and Klotho are predominantly expressed in the distal tubules156,169. Whole-nephron and global deletion of Klotho cause a similar severe phenotype, characterized by accelerated ageing, disturbed mineral metabolism, growth retardation, organ dysfunction and vascular calcification170,171. Although Klotho ablation in the proximal tubules is not as severe as whole-nephron and global ablation, these mice show impaired Pi excretion and increased NPT2A abundance when fed a high-Pi diet, suggesting that FGF23 also acts directly at the proximal tubules172.
Osteocytes of Dmp1-null mice and Hyp mice that have loss-of-function (LOF) mutations in the Dmp1 and Phex genes, respectively, have elevated FGF23 expression, which at least in part is mediated by the activation of canonical FGF–FGFR signalling173. In addition, mice expressing a transgenic variant of FGF23 that is resistant to proteolytic cleavage within a highly conserved subtilisin-like proprotein convertase site (176RHTR179/S180AE182) overexpress FGF23 when made iron deficient. As a result, intact and carboxy-terminal FGF23 levels are increased, leading to hypophosphataemia. Similarly, iron deficiency stimulates FGF23 expression in wild-type mice; however, only the carboxy-terminal FGF23 levels are increased and hypophosphataemia is absent, suggesting that Pi controls the secretory checkpoint174. Degradation of iFGF23 is enhanced by post-translational phosphorylation of FGF23 by the Golgi-associated secretory pathway kinase FAM20C at Ser180 and reduced by O-glycosylation by polypeptide N-acetylgalactosaminyltransferase 3 (GALNT3) at Thr178 (REF.175).
The effects of iron deficiency on FGF23 gene transcription might be indirect and mediated via erythropoietin. Von Hippel–Lindau disease tumour suppressor (VHL) ablation-mediated overexpression of hypoxia-inducible factor 1α (HIF1α) in the osteoblastic linage induces erythropoietin expression in these cells176. This effect might be relevant in light of emerging data that erythropoietin stimulates FGF23 synthesis and secretion by myeloid lineage LSK cells in the haematopoietic bone marrow177. In turn, FGF23 might feed back to regulate haematopoiesis, as suggested by the low erythrocyte counts found in FGF23-null mice178.
The osteocyte-like mouse cell lines IDG-SW3 (REF.116) and MLO-Y4 (REF.179) reportedly express substantial levels of FGF23 mRNA. Both cell lines differentiate into osteocytes after growing in cell culture on a collagen-coated surface in osteogenic media180. Pi and calcitriol increase FGF23 mRNA expression along with that of other osteocyte markers in IDG-SW3 cells, but these cells do not secrete the FGF23 protein116. In MLO-Y4 cells, in the absence of FGF2, extracellular Pi alone induces DMP1 expression. However, increased extracellular levels of Pi partially inhibited FGF2-induced DMP1, suggesting a coordinated regulation of DMP1 expression by FGF signalling and extracellular Pi (REF.179). Additionally, FGF23 mRNA was detected in UMR-106 rat osteosarcoma cells and MC3T3-E1 osteoblast-like cells181. Pi induces FGF23 mRNA expression in UMR-106 by an ERK-dependent mechanism, which might require the production of NADPH and reactive oxygen species182. In addition, in UMR-106 cells, advanced glycation end products (sugarmodified proteins, nucleic acid and lipids that contribute to FGF23 disorders) induce the transcription of FGF23, partially owing to upregulation of NF-κB183. Pi also induces DMP1 mRNA expression in MC3T3-E1 cells, but not FGF23 mRNA, and this induction is blocked by MEK inhibitor UO126 (REF.55).
In summary, Pi seems to regulate a post-translational checkpoint for secretion of bioactive FGF23 in vivo, and suitable in vitro models are needed to further study this mechanism.
Parathyroid
Intact PTH is a peptide of 84 amino acids that is secreted by the parathyroid glands and signals via its receptor PPR1, which is expressed in osteoblasts, osteocytes, chondrocytes and proximal tubular cells. Its net effect is the reduction of blood levels of Pi. Pi in turn stimulates PTH release from sections of bovine parathyroid glands, an effect not observed in dispersed cells184.
In the parathyroids, FGF23 decreases PTH mRNA levels through the activation of Klotho–FGFR1 and MAPK185. In addition to a Klotho-dependent mechanism, a Klotho-independent calcineurin–NFAT signalling mechanism has been suggested on the basis of the observation that Klotho−/− mice show a preserved PTH response when treated with FGF23, which could be blocked by the calcineurin inhibitor cyclosporine A162. PTH in turn increases serum levels of FGF23 via PPR1 and activation of PKA186, WNT pathways187 and nuclear receptor family 4 group A member 2 (NR4A2)188. Circulating levels of FGF23 are more substantially elevated in patients with humoral hypercalcaemia of malignancy than in patients with primary hyperparathyroidism189,190, suggesting that PTHrP stimulates FGF23 secretion more effectively than PTH (possibly by PPR1-independent pathways)191,192.
Pi stabilizes PTH mRNA levels via A+U-rich element binding factor 1, heterogeneous nuclear ribonucleoprotein K (HNRNPK) and cold shock domain-containing protein E1 (also known as N-ras upstream gene protein (UNR), which is encoded by CSDE1), which might have a role in parathyroid cell proliferation and the pathogenesis of secondary hyperparathyroidism that develops in patients with CKD185. An alternative mechanism for the development of secondary hyperparathyroidism caused by hyperphosphataemia is binding of Pi to the seven-transmembrane-spanning G protein-coupled CaSR193, which might inhibit calcium signalling and thereby stimulate PTH secretion.
Iron deficiency
Iron deficiency might induce FGF23 gene transcription via the transcription factor HIF1α174. HIF1α binding to hypoxia-responsive elements in the FGF23 promoter stimulates FGF23 mRNA expression in bone marrow stromal cells and MC3T3 cultures treated with deferoxamine (an iron-chelating agent)174. However, bone cell-specific ablation of Hif1α does not block the effect of iron deficiency on production of iFGF23; thus, this mechanism might not be relevant in vivo194. Rather, iron deficiency might stimulate erythropoietinexpression in the juxtaglomerular macula densa in the kidneys, which acts on osteoblasts and erythropoietic cells to induce FGF23 expression177,195.
Although co-regulation of Pi and iron homeostasis to support energy metabolism is plausible, it remains unclear how both systems stay sufficiently independent, as hypophosphataemia rarely develops with iron deficiency in otherwise healthy individuals. One possible feedback mechanism is the degradation of iFGF23 during iron deficiency. Hypophosphataemia develops only if FAM20C and GALNT3 are abnormally regulated, which differentially phosphorylate and O-glycosylate FGF23, postmarking the protein for degradation or secretion, respectively, as described previously175. Of interest in this context is that treatment with saccharated ferric oxide196 and iron polymaltose complex197 worsened FGF23-dependent osteomalacia despite correcting the iron deficiency, whereas iron dextran, as expected, corrected it198. The results suggest that saccharated ferric oxide and iron polymaltose complex block the cleavage of iFGF23, resulting in excess FGF23 bioactivity. FGF23 cleavage might also be inhibited by the chronic inflammatory state observed in CKD195,199. This process may be regulated by IL-1β200 and explain why germ-free mice have low FGF23 levels201. In addition, insulin or IGF1 suppresses FGF23 expression in a phosphatidylinositol 3-kinase (PI3K)–AKT–forkhead box protein O1 (FOXO1)-dependent fashion in vitro, in mice and in humans202. Lastly, FGF23 expression is stimulated by the mineralocorticoid hormone aldosterone203. This in vitro observation was corroborated by murine knockout models of the renal thiazide-sensitive NaCl cotransporter; however, renal resistance to FGF23 might be an additional factor as these mice remain normophosphataemic despite increased circulating levels of iFGF23 (REF.204).
Calcitriol
In the proximal tubules of the kidneys, calcitriol is synthesized from its precursor calcifediol (also known as 25-hydroxy vitamin D) by CYP27B1 (REF.205). Pi decreases circulating levels of calcitriol by stimulating gene expression of CYP24A1, which degrades calcitriol, while simultaneously suppressing its synthesis by CYP27B1 (REF.205). This process is predominantly mediated by the actions of FGF23 (REFs206–210).
Cyp24a1-null mice or individuals with LOF mutations in this gene develop hyperphosphataemia, along with severe hypercalcaemia211. Mice lacking Cyp27b1 expression or individuals with LOF mutations in this gene develop hypophosphataemia as well as hypocalcaemia, secondary hyperparathyroidism and rickets212. Calcitriol increases FGF23 levels and FGF23 decreases levels of calcitriol, forming a regulatory feedback loop to regulate plasma Pi (REF.213). Conversely, PTH increases calcitriol by stimulating CYP27B1 and inhibiting CYP24A1 and calcitriol suppresses PTH to regulate plasma calcium levels214. Owing to the opposite actions of both hormones on calcium metabolism, PTH is unable to compensate for the loss or excess of FGF23 in FGF23-dependent disorders of Pi homeostasis.
Pi might exert direct effects as shown in rabbit215 and mouse216 proximal tubule kidney cells in vitro and in vivo. Furthermore, regulation of CYP24A1 by Pi might require expression of NPT2A, as a high-phosphate diet fails to stimulate the gene expression of Cyp24a1 in Npt2a−/− mice217. This observation could explain why severe hypercalcaemia develops in children with NPT2A LOF mutations, which resembles the phenotype of idiopathic infantile hypercalcaemia caused by LOF of CYP24A1 (REF.218).
Calcitriol binds to its nuclear hormone receptor vitamin D3 receptor (VDR) to promote intestinal absorption of Pi and calcium205 by inducing NPT2B expression in the gut219. Elevated circulating levels of calcitriol are an important diagnostic feature in FGF23-independent disorders of renal phosphate wasting. However, this homeostatic response is not sufficiently robust to completely correct the hypophosphataemia owing to the development of hypercalcaemia and because elevated calcitriol also stimulates production of FGF23 in the skeleton, which might further worsen phosphaturia220. Conversely, suppression of calcitriol might contribute to the hypophosphataemia seen in FGF23-dependent disorders of renal phosphate wasting. Because secondary hyperparathyroidism often develops as a consequence of calcitriol deficiency (which should reduce FGF23 levels), PTH is unable to fully suppress and compensate for the phosphaturia caused by FGF23 excess.
Intestinal Pi sensing
Intestinal Pi absorption is stimulated by calcitriol. Both paracellular transport by passive diffusion and active transcellular transport have been described221,222. As suggested by findings in intestine-targeted Npt2b-knockout mice223, active transcellular transport of dietary Pi raises Pi levels in the circulation and results in increased renal phosphate excretion to eliminate excess Pi. Decreased FGF23 levels in these knockout mice suggest that this renal response to Pi is regulated by the stimulation of FGF23. Additionally, it was observed that dietary Pi is a more potent stimulant of renal Pi excretion, when compared with intravenous Pi. Furthermore, homogenate from duodenal mucosa can stimulate renal Pi excretion, suggesting the existence of an intestinal–renal axis (where Pi is sensed in the intestinal mucosa to induce the expression of unknown intestinal phosphatonins that stimulate renal Pi excretion)224. However, an acute intravenous and intestinal Pi load caused nearly identical phosphaturic responses in humans; thus, definitive proof for the presence of these intestinal phosphatonins is still pending225. Paracellular Pi transport has not been well characterized, and it is unknown whether it contributes to the regulation of FGF23 and renal Pi excretion. Targeting dietary Pi absorption and intestinal Pi sensing could reduce the severity of hyperphosphataemia and cardiovascular complications in CKD221.
Disorders of Pi homeostasis
In light of the essential role of Pi in energy metabolism, cell signalling, protein function and bone matrix mineralization, disorders of Pi homeostasis are expected to impair the function of many organ systems. The majority of disorders of Pi homeostasis primarily result in changes of extracellular Pi. However, disorders that primarily change intracellular Pi have been described. Because extracellular and intracellular Pi are intimately connected, symptoms of these disorders might overlap. However, some disorders of excess Pi uptake into cells result in hypophosphataemia and rhabdomyolysis, whereas disorders of reduced Pi uptake into cells result in hyperphosphataemia and matrix calcifications. In addition, some transporters have cell autonomous and systemic functions. For example, LOF mutations in NPT2A reduce intracellular levels of Pi and stimulate the synthesis of calcitriol in the proximal tubules of the kidneys, resulting in hyperabsorption of phosphate from the diet in the gut. However, because of its essential role in reclaiming Pi from the urine, the net effect of NPT2A LOF mutations is reduced extracellular levels of Pi, which results in hypophosphataemic rickets or osteomalacia. Although the mechanisms by which disorders of Pi homeostasis result in extracellular effects, such as loss of bone mineral or vascular calcification, are well defined, how intracellular effects such as myopathy, tumour formation and changes associated with accelerated ageing are mediated is less well understood226.
Extracellular Pi homeostasis
Disorders of extracellular Pi homeostasis can be divided into acquired and familial forms, which might be FGF23-dependent, PTH-dependent or FGF23-independent and PTH-independent. Supplementary Table 1, FIG. 4 and several excellent reviews8,220 provide an in-depth discussion of these disorders. In this section, we focus on the latest discoveries in each category.
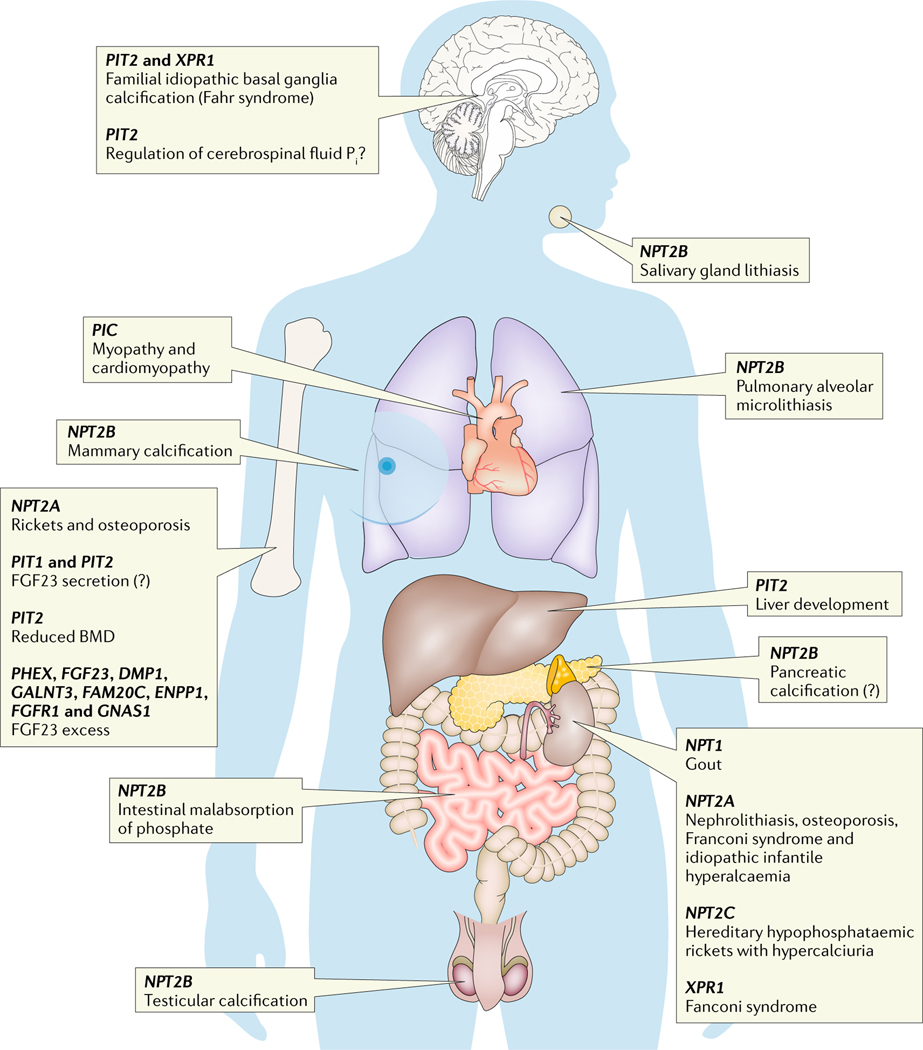
Fig. 4 |. Diseases of phosphate homeostasis organized by organ system.
Linkage analysis in human disorders of inorganic phosphate (Pi) homeostasis, cardiomyopathy and familial basal ganglial calcifications identified several novel genes important for the regulation of Pi homeostasis. DMP1, dentin matrix acidic phosphoprotein 1; ENPP1, ectonucleotide pyrophosphatase-phosphodiesterase family member 1; FAM20C, Golgi-associated secretory pathway kinase; FGF23, fibroblast growth factor 23; FGFR1, FGF receptor 1; GALNT3, polypeptide N-acetylgalactosaminyltransferase 3; GNAS1, guanine nucleotide-binding protein G(s), subunit α; NPT1, sodium-dependent phosphate transport protein 1; NPT2A, sodium-dependent phosphate transport protein 2A; PHEX, phosphate-regulating endopeptidase homologue, X-linked; PIT, sodium-dependent Pi transporter; SLC25A3, solute carrier family 25 member 3; XPR1, xenotropic and polytropic retrovirus receptor 1. Question mark indicates unknown.
Tumour-induced osteomalacia is caused by small benign or low-grade malignant phosphaturic mesenchymal tumours of a mixed connective tissue variant227. This disorder is characterized by the secretion of FGF23, and less commonly by FGF7, MEPE or SFRP4, which cause renal Pi wasting, hypophosphataemia and osteomalacia228. An important discovery is that >60% of phosphaturic mesenchymal tumours of mixed connective tissue variant bear a somatic rearrangement between the fibronectin promoter and FGFR1 (REF.229) or FGF1 genes230. Together with reports that inhibitors of FGFR1 reverse the phenotype of a mouse model of X-linked hypophosphataemia231,232 and reduced FGF23 levels in a patient with malignant tumour-induced osteomalacia after treatment with the FGFR1 inhibitor BGJ398233,234, these findings suggest that FGF1 and/or FGFR1 stimulate tumour growth and FGF23 synthesis and/or secretion. As initial observations with neutralizing FGF23 antibodies did not replicate235 the impressive tumour regression observed with BGJ398 (REFs233,234), excess FGF23 itself does not seem to stimulate tumour-induced osteomalacia tumour growth in an autocrine fashion.
Germline mutations in several genes cause familial forms of FGF23-dependent hypophosphataemia. The most common X-linked hypophosphataemia is caused by mutations in PHEX236, autosomal dominant hypophosphataemic rickets is caused by gain-of-function mutations in FGF23 (REF.237) and autosomal recessive hypophosphataemic rickets is caused by LOF mutations in DMP1. Two additional autosomal recessive forms (autosomal recessive hypophosphataemic rickets types 2 and 3) were described. Autosomal recessive hypophosphataemic rickets type 2 is caused by LOF mutations in ENPP1 (REF.238), and autosomal recessive hypophosphataemic rickets type 3, a variant of Raine syndrome, is caused by LOF mutations in FAM20C239,240.
ENPP1 is a cell membrane protein that is essential for pyrophosphate synthesis. Because LOF mutations in ENPP1 cause hypophosphataemia owing to increased bioactive FGF23, it was speculated that the Pi:pyrophosphate ratio might participate in the post-translational regulation of iFGF23 (REF.241). The mechanism of FAM20C LOF mutations was discussed above and results in underphosphorylation of FGF23 at Ser180 (REF.175), thereby increasing secretion of bioactive iFGF23 in humans239,240 and mice242, whereas LOF mutations in GALNT3 reduce levels of bioactive iFGF23 and lead to hyperphosphataemic tumoural calcinosis243.
Observations made in patients with autosomal dominant hypophosphataemic rickets led to the discovery that the condition often improves after puberty in men, but not women. This discovery raised the possibility that iron deficiency, often a result of menstrual bleeding in women244, might sustain FGF23 excess in women, but not men, which uncovered an important link between iron and phosphate homeostasis244,245. Subsequently, it was found that iron deficiency stimulates FGF23 expression in healthy individuals and those who are affected, but only patients with autosomal dominant hypophosphataemic rickets, who express the autosomal dominant hypophosphataemic rickets variant of FGF23, become hypophosphataemic as discussed previously246.
McCune–Albright syndrome is a condition that causes benign tumours in bones (also known as fibrous dysplasia) and in multiple endocrine glands. Individuals with this condition sometimes develop renal phosphate wasting owing to high circulating levels of FGF23, suggesting that guanine nucleotide-binding protein G(s), subunit α (GNAS1) likewise stimulates secretion of bioactive iFGF23 owing to inhibition of subtilisin or furin-type proteases247. Furthermore, work in mice expressing GNAS-XL, a large splice variant of GNAS1, suggests that GNAS-XL stimulates the secretion of FGF23 by upregulating FGFR1 (REFs248,249).
Current therapies for FGF23-dependent disorders of Pi homeostasis aim to correct blood levels of Pi and calcitriol, which is discussed in several excellent reviews250–252. Additionally, Crysvita (burosumab, an FGF23-inactivating antibody253) was approved by the FDA for FGF23-dependent hypophosphataemic disorders, such as X-linked hypophosphataemia. Also, modified carboxy-terminal FGF23 peptides with increased half-lives are able to improve blood levels of Pi and bone quality in Hyp mice by blocking the action of bioactive FGF23254. As mentioned above, two individuals with tumour-induced osteomalacia showed marked reduction of FGF23 levels upon treatment with the FGFR1 inhibitor BGJ398 (REF.233). Furthermore, FGF23 hormone replacement and blocking excessive degradation of iFGF23 by supplementing GALNT3 enzyme255 were proposed for the treatment of FGF23-deficient hyperphosphataemic disorders.
Different from the already-discussed disorders, hereditary hypophosphataemic rickets with hypercalciuria and autosomal recessive Fanconi syndrome are FGF23-independent and are caused by LOF mutations in NPT2C and NPT2A, respectively218,256,257. Treatment of hereditary hypophosphataemic rickets with hypercalciuria and idiopathic infantile hypercalcaemia 2 is founded on oral Pi supplementation, although there is concern in mouse models lacking the Npt2a gene that Pi supplementation can worsen renal calcifications and Fanconi syndrome, despite normalization of hypercalciuria258–260. NPT2A LOF mutations can also cause idiopathic infantile hypercalcaemia type 2, which clinically resembles idiopathic infantile hypercalcaemia type 1 (caused by LOF mutations in CYP24A1). Both disorders present with hypercalcaemia and elevated calcitriol levels, whereas renal phosphate wasting and hypophosphataemia are mild, which suggests a role of NPT2A in the regulation of CYP24A1, as previously discussed.
Intracellular Pi homeostasis
Mutations in several Pi transporters that cause a new group of disorders of intracellular Pi homeostasis have been reported in the past 5 years. Individuals with hypertrophic cardiomyopathy, muscular dystrophy and lactic acidosis261,262 were found to carry LOF mutations in the mitochondrial phosphate carrier (PIC; which is encoded by SLC25A3), which mediates uptake of Pi by the mitochondria. These findings suggest that Pi is important for the function of the mitochondrial respiratory chain and ATP synthesis, which has been reported by us52 and others134–136,263,264. PIC is also a component of the mPTP, which requires Pi to permit the influx of calcium into the mitochondrial matrix57. Detailed evaluation of hearts in a mouse model with an inducible cardiac-specific deletion of Slc25a3 (REF.265) showed reduced mPTP opening in response to calcium challenge. PIC therefore also has Pi transport-independent roles in mitochondrial function.
LOF mutations in PIT2 (REF.266) and XPR1 (REF.267) were reported in individuals with primary familial brain calcification, or Fahr syndrome. These individuals develop vascular calcifications in the basal ganglia of their brains, leading to seizures and in some cases disturbance of sustained phonation and orofacial apraxia268. Inhibition of Pi uptake into the microglia because of LOF in PIT2 or the inhibition of Pi export from VSMCs due to LOF in XPR1 might stimulate formation of calcium– Pi deposits inside these cells267,269. A similar phenotype was observed in human individuals and mouse models with LOF mutations in PDGFB receptor (PDGFBR) and PDGFB270. Together with reports of a physical interaction between XPR1 and PDGFRB in mice271, it was suggested that PDGFB, PDGFBR and phosphate transporters functionally interact. Similar to humans with PIT2 LOF mutations, Pit2-knockout mice develop brain calcifications272. Interestingly, Pit2-knockout mice have increased CSF levels of Pi and glymphatic pathway-associated arteriolar calcification273, which suggests a role of this transporter in CSF Pi homeostasis274.
Pseudoxanthoma elasticum is caused by LOF mutations in the multidrug resistance-associated protein 6 (encoded by ABCC6 (REF.275)), which, similar to mutations in ENPP1, result in abnormal pyrophosphate metabolism, mineralization and fragmentation of the elastin-containing fibres in connective tissue, which may lead to vascular disease in humans. Mice with LOF mutations of ABCC6 also exhibit renal calcifications that are different from autosomal recessive hypophosphataemic rickets type 2 as hypophosphataemic rickets is absent in pseudoxanthoma elasticum276. As the substrate of ABCC6 is unknown, it remains unclear whether pseudoxanthoma elasticum can be considered a disorder of intracellular Pi homeostasis.
In summary, although the majority of disorders of Pi homeostasis primarily result in changes in extra-cellular Pi, new disorders that primarily change intracellular levels of Pi have been reported. As mammalian Pi sensing is only partially understood, it remains unclear whether these disorders are caused by toxicities due to the lack or excess of Pi in the extracellular or intracellular compartment.
Conclusion
Disorders of Pi homeostasis are important in human health and disease. Major advances in Pi homeostasis in humans have been made with the discovery of FGF23 and its regulation of body Pi levels. However, it is unknown how Pi feeds back to regulate FGF23, PTH and calcitriol. Although the Pi-sensing mechanism has been extensively studied in bacteria and yeast, no Pi sensor has been identified in humans. One possible Pi signalling pathway involves the binding of Pi and/or transport of Pi by the type 3 sodium-phosphate cotransporters PIT1 and PIT2, followed by the activation of ERK1 and ERK2, which modulate gene expression in bone and VSMCs. However, it remains to be shown whether metabolic and endocrine actions of Pi involve the same or different signalling pathways.
Supplementary Material
Key points.
Endocrine regulation of gastrointestinal absorption, storage in the mineral deposits of the skeleton and renal excretion of inorganic phosphate (Pi) maintains the serum concentration of Pi within a narrow range.
Pi activates extracellular-signal-regulated kinases 1 and 2 in mammalian cells, which are required for stimulation of mitochondrial respiration and transcription of bone matrix proteins.
Pi stimulates the synthesis and secretion of parathyroid hormone and fibroblast growth factor 23 and blocks the synthesis of calcitriol; however, the endocrine sensor for Pi remains unknown.
Mutations in the endocrine regulators of Pi lead to genetic disorders characterized by abnormal bone and mineral metabolism and ectopic calcifications.
Identification of loss-of-function mutations in several Pi transporters highlights the importance of intracellular Pi for muscle function and vascular calcifications.
How intracellular Pi causes myopathy, tumour formation and changes associated with acclerated ageing is less well understood.
Acknowledgements
C.B. is supported by the Yale O’Brien Center (P30DK079310), and S.C. is supported by a postdoctoral fellowship from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (T32DK007058-42).
Glossary
- Phosphaturic hormone
Hormone causing excretion of phosphate in the urine
- Hypomorphic alleles
Genes that have a mutation that causes a partial loss of gene function
- Haploinsufficiency
Refers to complete loss of function of one copy of a gene when the remaining functional copy of the gene is not adequate to produce the needed gene product to preserve normal function
- 5/6 nephrectomy
Model of progressive renal failure with reduced nephron number achieved by either infarction or surgical excision of both poles and removal of the contralateral kidney
- Pulmonary alveolar microlithiasis
A rare autosomal recessive disease of widespread intra-alveolar accumulation of minute calcium phosphate calculi called microliths caused by homozygous loss-of-function mutations in SLC34A2 (which encodes NPT2b)
- Tumoural calcinosis
Group of rare autosomal recessive metabolic disorders characterized by the development of severe ectopic calcifications in soft tissues due to homozygous loss-of-function mutations in the GALNT3, FGF23 or KL genes
- Osteomalacia
Osteomalacia is a rare disorder of bone metabolism leading to reduced bone matrix mineralization
- Phosphatonins
Phosphatonins is the collective term used for major regulators of Pi homeostasis, which generally function as phosphaturic hormones and lower blood levels of Pi
Footnotes
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41574–018-0076–3.
Competing interests
The authors declare no competing interests.
References
- 1.Bevington A., Kemp GJ., Graham R. & Russell G. Phosphate-sensitive enzymes: possible molecular basis for cellular disorders of phosphate metabolism. Clin. Chem. Enzym. Comms. 4, 235–257 (1992). [Google Scholar]
- 2.Chakraborty A, Kim S. & Snyder SH Inositol pyrophosphates as mammalian cell signals. Sci. Signal. 4, re1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelova PR, Baev AY, Berezhnov AV & Abramov AY Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death. Biochem. Soc. Trans. 44, 40–45 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Herman H. & Dallemagne MJ The main mineral constituent of bone and teeth. Arch. Oral Biol. 5, 137–144 (1961). [DOI] [PubMed] [Google Scholar]
- 5.Bergwitz C. & Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 61, 91–104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoshniat S. et al. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life Sci. 68, 205–218 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukumoto S. Phosphate metabolism and vitamin D. Bonekey Rep. 3, 497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manghat P, Sodi R. & Swaminathan R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 51, 631–656 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Quarles LD A systems biology preview of the relationships between mineral and metabolic complications in chronic kidney disease. Semin. Nephrol. 33, 130–142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brame LA, White KE & Econs MJ Renal phosphate wasting disorders: clinical features and pathogenesis. Semin. Nephrol. 24, 39–47 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Prie D, Beck L, Silve C. & Friedlander G. Hypophosphatemia and calcium nephrolithiasis. Nephron Exp. Nephrol. 98, e50–54 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Lau WL, Pai A, Moe SM & Giachelli CM Direct effects of phosphate on vascular cell function. Adv. Chron. Kidney Dis. 18, 105–112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scialla JJ & Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat. Rev. Nephrol. 10, 268–278 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Penido MGMG & Alon US Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 27, 2039–2048 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berndt T. & Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiol. (Bethesda) 24, 17–25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabbagh Y. Phosphate as a sensor and signaling molecule. Clin. Nephrol. 79, 57–65 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Hsieh YJ & Wanner BL Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 13, 198–203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergwitz C. & Juppner H. Phosphate sensing. Adv. Chron. Kidney Dis. 18, 132–144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien ML, Foster JL, Douglas JL & Garcia JV The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J. Virol. 71, 4564–4570 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D. et al. Alterations in the sensing and transport of phosphate and calcium by differentiating chondrocytes. J. Biol. Chem. 276, 33995–34005 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Palmer G, Bonjour JP & Caverzasio J. Stimulation of sodium-dependent inorganic phosphate transport by activation of Gi/o-protein-coupled receptors by epinephrine in MC3T3-E1 osteoblast-like cells. Bone 28, 589–594 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Zhen X, B., J. & Caverzasio, J. Platelet-derived growth factor stimulates sodium-dependent Pi transport in osteoblastic cells via phospholipase Cgamma and phosphatidylinositol 3’ -kinase. J. Bone Miner. Res. 12, 36–44 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Polgreen KE, Kemp GJ, Leighton B. & Radda GK Modulation of Pi transport in skeletal muscle by insulin and IGF-1. Biochim. Biophys. Acta 1223, 279–284 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, Palmer G, Bonjour J-P & Caverzasio J. Stimulation of sodium-dependent phosphate transport and signaling mechanisms induced by basic fibroblast growth factor in MC3T3-E1 osteoblast-like cells. J. Bone Miner. Res. 15, 95–102 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Palmer G, Guicheux J. m., Bonjour J-P & Caverzasio J. Transforming growth factor-β stimulates inorganic phosphate transport and expression of the type III phosphate transporter Glvr-1 in chondrogenic ATDC5 cells*. Endocrinology 141, 2236–2243 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Lamarche MG, Wanner BL, Crepin S. & Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32, 461–473 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Qi W, Baldwin SA, Muench SP & Baker A. Pi sensing and signalling: from prokaryotic to eukaryotic cells. Biochem. Soc. Trans. 44, 766–773 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Brown MR & Kornberg A. The long and short of it — polyphosphate, PPK and bacterial survival. Trends Biochem. Sci. 33, 284–290 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Ra NN., Gomez-Garci MR. & Kornber A. Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem. 78, 605–647 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Mouillon JM & Persson BL New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS Yeast Res. 6, 171–176 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Samyn DR & Persson BL Inorganic phosphate and sulfate transport in S. cerevisiae. Adv. Exp. Med. Biol. 892, 253–269 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Bergwitz C. et al. Roles of major facilitator superfamily transporters in phosphate response in Drosophila. PLOS One 7, e31730 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagerstedt JO, Voss JC, Wieslander Å & Persson BL Structural modeling of dual-affinity purified Pho84 phosphate transporter. FEBS Lett. 578, 262–268 (2004). [DOI] [PubMed] [Google Scholar]
- 34.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sengottaiyan P. et al. Characterization of the biochemical and biophysical properties of the Saccharomyces cerevisiae phosphate transporter Pho89. Biochem. Biophys. Res. Commun. 436, 551–556 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Bottger P. & Pedersen L. Mapping of the minimal inorganic phosphate transporting unit of human PiT2 suggests a structure universal to PiT-related proteins from all kingdoms of life. BMC Biochem. 12, 21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner A. & Kinne RKH Evolution of the Na-Pi cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R301–R312 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Secco D, Wang C, Shou H. & Whelan J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 586, 289–295 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Saiardi A. How inositol pyrophosphates control cellular phosphate homeostasis? Adv. Biol. Regul. 52, 351–359 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Lee Y-S, Huang K, Quiocho FA & O’Shea EK Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 4, 25–32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giovannini D, Touhami J, Charnet P, Sitbon M. & Battini JL Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep. 3, 1866–1873 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Gerasimaite R. et al. Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC. ACS Chem. Biol. 12, 648–653 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Auesukaree C, Tochio H, Shirakawa M, Kaneko Y. & Harashima S. Plc1p, Arg82p, and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polyphosphate accumulation in Saccharomyces cerevisiae. J. Biol. Chem. 280, 25127–25133 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Gerasimaitė R, Sharma S, Desfougères Y, Schmidt A. & Mayer A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J. Cell Sci. 127, 5093–5104 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Worley J, Luo X. & Capaldi AP Inositol pyrophosphates regulate cell growth and the environmental stress response by activating the HDAC Rpd3L. Cell Rep. 3, 1476–1482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales R. et al. Serendipitous discovery and X-ray structure of a human phosphate binding apolipoprotein. Structure 14, 601–609 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Chavkin NW, Chia JJ, Crouthamel MH & Giachelli CM. Phosphate uptake-independent signaling functions of the type III sodium-dependent phosphate transporter, PiT-1, in vascular smooth muscle cells. Exp. Cell Res. 333, 39–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beck L. et al. Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity. J. Biol. Chem. 284, e99959 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittrant Y. et al. Inorganic phosphate regulates Glvr-1 and −2 expression: role of calcium and ERK1/2. Biochem. Biophys. Res. Commun. 381, 259–263 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Yoshiko Y, Candeliere GA, Maeda N. & Aubin JE Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol. Cell. Biol. 27, 4465–4474 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papaioannou G. et al. Raf kinases are essential for phosphate induction of ERK1/2 phosphorylation in hypertrophic chondrocytes and normal endochondral bone development. J. Biol. Chem. 292, 3164–3171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pesta DH et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 30, 3378–3387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camalier CE et al. An integrated understanding of the physiological response to elevated extracellular phosphate. J. Cell. Physiol. 228, 1536–1550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamazaki M. et al. Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells. J. Cell. Biochem. 111, 1210–1221 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Nishino J. et al. Extracellular phosphate induces the expression of dentin matrix protein 1 through the FGF receptor in osteoblasts. J. Cell. Biochem. 118, 1151–1163 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Pauleau AL et al. Unexpected role of the phosphate carrier in mitochondrial fragmentation. Cell Death And Differ. 15, 616 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Seifert EL, Ligeti E, Mayr JA, Sondheimer N. & Hajnoczky G. The mitochondrial phosphate carrier: role in oxidative metabolism, calcium handling and mitochondrial disease. Biochem. Biophys. Res. Commun. 464, 369–375 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Couasnay G. et al. Maintenance of chondrocyte survival by PIT1/SLC20A1-mediated regulation of endoplasmic reticulum homeostasis. Osteoarthr. Cartil. 24, S135 (2016). [Google Scholar]
- 59.Laver DR, Lenz GKE & Dulhunty AF Phosphate ion channels in sarcoplasmic reticulum of rabbit skeletal muscle. J. Physiol. 535, 715–728 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paredes JM et al. Real-time phosphate sensing in living cells using fluorescence lifetime imaging microscopy (FLIM). J. Phys. Chem. B 117, 8143–8149 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Banerjee S, Versaw WK & Garcia LR Imaging cellular inorganic phosphate in Caenorhabditis elegans using a genetically encoded FRET-based biosensor. PLOS One 10, e0141128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore KL et al. Combined NanoSIMS and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 201, 104–115 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Braun PD, Schulz-Vogt HN, Vogts A. & Nausch M. Differences in the accumulation of phosphorus between vegetative cells and heterocysts in the cyanobacterium Nodularia spumigena. Sci. Rep. 8, 5651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kestenbaum B. et al. Common genetic variants associate with serum phosphorus concentration. J. Am. Soc. Nephrol. 21, 1223–1232 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koldobskiy MA et al. p53-mediated apoptosis requires inositol hexakisphosphate kinase-2. Proc. Natl Acad. Sci. 107, 20947–20951 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakraborty A. et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143, 897–910 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao F. et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol. Cell 54, 119–132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wild R. et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352, 986–990 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Azevedo C. & Saiardi A. Eukaryotic phosphate homeostasis: the inositol pyrophosphate perspective. Trends Biochem. Sci. 42, 219–231 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Sharma P, Patntirapong S, Hann S. & Hauschka PV RANKL-RANK signaling regulates expression of xenotropic and polytropic virus receptor (XPR1) in osteoclasts. Biochem. Biophys. Res. Commun. 399, 129–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Docampo R, de Souza W, Miranda K, Rohloff P. & Moreno SNJ Acidocalcisomes? conserved from bacteria to man. Nat. Rev. Micro 3, 251–261 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Pavlov E. et al. Inorganic polyphosphate and energy metabolism in mammalian cells. J. Biol. Chem. 285, 9420–9428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmelzle T. & Hall MN TOR, a central controller of cell growth. Cell 103, 253–262 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Shiba T. et al. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J. Biol. Chem. 278, 26788–26792 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Hernandez-Ruiz L, Gonzalez-Garcia I, Castro C, Brieva J. & Ruiz F. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica 91, 1180–1186 (2006). [PubMed] [Google Scholar]
- 76.Holmstrom KM et al. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun. 4, 1362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White KE et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Shimada T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. 98, 6500–6505 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sitara D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bingham PJ & Raisz LG Bone growth in organ culture: effects of phosphate and other nutrients on bone and cartilage. Calcif. Tissue Res. 14, 31–48 (1974). [DOI] [PubMed] [Google Scholar]
- 81.Kakuta S, Golub EE & Shapiro IM Morphochemical analysis of phosphorus pools in calcifying cartilage. Calcif. Tissue Int. 37, 293–299 (1985). [DOI] [PubMed] [Google Scholar]
- 82.Fujita T. et al. Phosphate stimulates differentiation and mineralization of the chondroprogenitor clone ATDC5. Jpn J. Pharmacol. 85, 278–281 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Liu ES et al. Phosphate interacts with PTHrP to regulate endochondral bone formation. Endocrinology 155, 3750–3756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teixeira CC, Mansfield K, Hertkorn C, Ischiropoulos H. & Shapiro IM Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation. Am. J. Physiol. Cell Physiol. 281, C833–C839 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Orfanidou T, Malizos KN, Varitimidis S. & Tsezou A. 1,25-Dihydroxyvitamin D(3) and extracellular inorganic phosphate activate mitogen-activated protein kinase pathway through fibroblast growth factor 23 contributing to hypertrophy and mineralization in osteoarthritic chondrocytes. Exp. Biol. Med. (Maywood) 237, 241–253 (2012). [DOI] [PubMed] [Google Scholar]
- 86.Solomon DH, Wilkins RJ, Meredith D. & Browning JA Characterisation of inorganic phosphate transport in bovine articular chondrocytes. Cell. Physiol. Biochem. 20, 099–108 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Festing MH, Speer MY, Yang HY & Giachelli CM Generation of mouse conditional and null alleles of the type III sodium-dependent phosphate cotransporter PiT-1. Genesis 47, 858–863 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beck L. et al. The phosphate transporter PiT1 (Slc20a1) revealed as a new essential gene for mouse liver development. PLOS One 5, e9148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bourgine A. et al. Mice with hypomorphic expression of the sodium-phosphate cotransporter PiT1/Slc20a1 have an unexpected normal bone mineralization. PLOS One 8, e65979 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yadav MC et al. Skeletal mineralization deficits and impaired biogenesis and function of chondrocyte-derived matrix vesicles in phospho1(−/−) and phospho1/Pi t1 double-knockout mice. J. Bone Miner. Res. 31, 1275–1286 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki A. et al. Effects of transgenic Pit-1 overexpression on calcium phosphate and bone metabolism. J. Bone Miner. Metab. 28, 139–148 (2010). [DOI] [PubMed] [Google Scholar]
- 92.Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch. Biochem. Biophys. 473, 188–192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Staines KA, MacRae VE & Farquharson C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 214, 241–255 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Bellido T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 94, 25–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conrads KA et al. Quantitative proteomic analysis of inorganic phosphate-induced murine MC3T3-E1 osteoblast cells. Electrophoresis 25, 1342–1352 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Conrads KA et al. A combined proteome and microarray investigation of inorganic phosphate-induced pre-osteoblast cells. Mol. Cell Proteom. 4, 1284–1296 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Julien M. et al. Phosphate-dependent regulation of MGP in osteoblasts: role of ERK1/2 and Fra-1. J. Bone Miner. Res. 24, 1856–1868 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Beck GR Jr., Zerler B. & Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl Acad. Sci. USA 97, 8352–8357 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanatani M, Sugimoto T, Kano J. & Chihara K. IGF-I mediates the stimulatory effect of high phosphate concentration on osteoblastic cell proliferation. J. Cell. Physiol. 190, 306–312 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Hoa B., Kiffer-Moreir T., Millá JL. & McKe MD. Polyphosphates inhibit extracellular matrix mineralization in MC3T3-E1 osteoblast cultures. Bone 53, 478–486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caverzasio J, Selz T. & Bonjour JP Characteristics of phosphate transport in osteoblastlike cells. Calcif. Tissue Int. 43, 83–87 (1988). [DOI] [PubMed] [Google Scholar]
- 102.Lundquist P, Murer H. & Biber J. Type II Na+-Pi cotransporters in osteoblast mineral formation: regulation by inorganic phosphate. Cell Physiol. Biochem. 19, 43–56 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Yates AJ, Oreffo RO, Mayor K. & Mundy GR Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts. J. Bone Miner. Res. 6, 473–478 (1991). [DOI] [PubMed] [Google Scholar]
- 104.Ha S-W, Park J, Habib MM & Beck GR Nano-hydroxyapatite stimulation of gene expression requires Fgf receptor, phosphate transporter, and Erk1/2 signaling. ACS Appl. Mater. Interfaces 9, 39185–39196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akiyama K, Kimura T. & Shiizaki K. Biological and clinical effects of calciprotein particles on chronic kidney disease-mineral and bone disorder. Int. J. Endocrinol. 2018, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamada S. et al. in American Society of Nephrology (New Orleans, LA, 2017). [Google Scholar]
- 107.Beck S. et al. PiT2 is essential for normal endochondral and intramembranous ossification, tooth development and the maintenance of adult bone structure and strength [abstract MO0523]. J. Bone Miner. Res. 32, S339 (2017). [Google Scholar]
- 108.Larsen FT, Jensen N, Autzen JK, Kongsfelt IB & Pedersen L. Primary brain calcification causal PiT2 transport-knockout variants can exert dominant negative effects on wild-type PiT2 transport function in mammalian cells. J. Mol. Neurosci. 61, 215–220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nina Bon AB et al. Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J. Biol. Chem. 293, 2102–2114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Segawa H. et al. Type IIc sodium–dependent phosphate transporter regulates calcium metabolism. J. Am. Soc. Nephrol. 20, 104–113 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tenenhouse HS Regulation of phosphorus homeostasis by the type IIA Na/phosphate cotransporter. Annu. Rev. Nutr. 25, 197–214 (2005). [DOI] [PubMed] [Google Scholar]
- 112.Shibasaki Y. et al. Targeted deletion of the tybe IIb Na+-dependent Pi-co-transporter, NaPi-IIb, results in early embryonic lethality. Biochem. Biophys. Res. Commun. 381, 482–486 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Sabbagh Y. et al. Intestinal Npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 20, 2348–2358 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyagawa K. et al. Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic Hyp mice. PLOS ONE 9, e93840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang R. et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 26, 1047–1056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ito N, Findlay DM, Anderson PH, Bonewald LF & Atkins GJ Extracellular phosphate modulates the effect of 1alpha, 25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J. Steroid Biochem. Mol. Biol. 136, 183–186 (2013). [DOI] [PubMed] [Google Scholar]
- 117.Rhee Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Capulli M, Paone R. & Rucci N. Osteoblast and osteocyte: games without frontiers. Arch. Biochem. Biophys. 561, 3–12 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Dallas SL, Prideaux M. & Bonewald LF The osteocyte: an endocrine cell… and more. Endocr. Rev. 34, 658–690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakashima K. & de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 19, 458–466 (2003). [DOI] [PubMed] [Google Scholar]
- 121.Karsenty G. & Olson EN Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication. Cell 164, 1248–1256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Teitelbaum SL Bone resorption by osteoclasts. Science 289, 1504–1508 (2000). [DOI] [PubMed] [Google Scholar]
- 123.Boyce BF Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92, 860–867 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Charles JF & Aliprantis AO Osteoclasts: more than ‘bone eaters’. Trends Mol. Med. 20, 449–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Albano G. et al. Sodium-dependent phosphate transporters in osteoclast differentiation and function. PLOS ONE 10, e0125104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kanatani M, Sugimoto T, Kano J, Kanzawa M. & Chihara K. Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell. Physiol. 196, 180–189 (2003). [DOI] [PubMed] [Google Scholar]
- 127.Mozar A. et al. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J. Cell. Physiol. 215, 47–54 (2008). [DOI] [PubMed] [Google Scholar]
- 128.M’Baya-Moutoula E, Louvet L, Metzinger-Le Meuth V, Massy ZA & Metzinger L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta 1852, 2202–2212 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Wang X. et al. Dual effect of inorganic polymeric phosphate/polyphosphate on osteoblasts and osteoclasts in vitro. J. Tissue Eng. Regen Med. 7, 767–776 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Gupta A, Guo XL, Alvarez UM & Hruska KA Regulation of sodium-dependent phosphate transport in osteoclasts. J. Clin. Invest. 100, 538–549 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li G, Miura K. & Kuno M. Extracellular phosphates enhance activities of voltage-gated proton channels and production of reactive oxygen species in murine osteoclast-like cells. Pflugers Arch. 469, 279–292 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Hayashibara T. et al. Regulation of osteoclast differentiation and function by phosphate: potential role of osteoclasts in the skeletal abnormalities in hypophosphatemic conditions. J. Bone Miner. Res. 22, 1743–1751 (2007). [DOI] [PubMed] [Google Scholar]
- 133.Beck L. et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. 95, 5372–5377 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown CE, Wilkie CA, Meyer MH & Meyer RA Response of tissue phosphate content to acute dietary phosphate deprivation in the X-linked hypophosphatemic mouse. Calcif. Tissue Int. 37, 423–430 (1985). [DOI] [PubMed] [Google Scholar]
- 135.Smith R, Newman RJ, Radda GK, Stokes M. & Young A. Hypophosphataemic osteomalacia and myopathy: studies with nuclear magnetic resonance spectroscopy. Clin. Sci. (Lond.) 67, 505–509 (1984). [DOI] [PubMed] [Google Scholar]
- 136.Sinha A, Hollingsworth KG, Ball S. & Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 98, E509–E513 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Hwang JH & Choi CS Use of in vivo magnetic resonance spectroscopy for studying metabolic diseases. Exp. Mol. Med. 47, e139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Itoh H. et al. Mechanically driven ATP synthesis by F1-ATPase. Nature 427, 465 (2004). [DOI] [PubMed] [Google Scholar]
- 139.Bose S, French S, Evans FJ, Joubert F. & Balaban RS Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J. Biol. Chem. 278, 39155–39165 (2003). [DOI] [PubMed] [Google Scholar]
- 140.Phillips D, Aponte AM, French SA, Chess DJ & Balaban RS Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry 48, 7140–7149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rodriguez-Zavala JS, Pardo JP & Moreno-Sanchez R. Modulation of 2-oxoglutarate dehydrogenase complex by inorganic phosphate, Mg(2+), and other effectors. Arch. Biochem. Biophys. 379, 78–84 (2000). [DOI] [PubMed] [Google Scholar]
- 142.Hansford RG Some properties of pyruvate and 2-oxoglutarate oxidation by blowfly flight-muscle mitochondria. Biochem. J. 127, 271–283 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blonde DJ, Kresack EJ & Kosicki GW The effects of ions and freeze-thawing on supernatant and mitochondrial malate dehydrogenase. Can. J. Biochem. 45, 641–650 (1967). [DOI] [PubMed] [Google Scholar]
- 144.Cook WJ, Senkovich O. & Chattopadhyay D. An unexpected phosphate binding site in glyceraldehyde 3-phosphate dehydrogenase: crystal structures of apo, holo and ternary complex of Cryptosporidium parvum enzyme. BMC Struct. Biol. 9, 9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Travis SF et al. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N. Engl. J. Med. 285, 763–768 (1971). [DOI] [PubMed] [Google Scholar]
- 146.Shanahan CM, Crouthamel MH, Kapustin A. & Giachelli CM Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 109, 697–711 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rangrez AY et al. Inorganic phosphate accelerates the migration of vascular smooth muscle cells: evidence for the involvement of miR-223. PLOS One 7, e47807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giachelli CM Vascular calcification: in vitro evidence for the role of inorganic phosphate. J. Am. Soc. Nephrol. 14, S300–304 (2003). [DOI] [PubMed] [Google Scholar]
- 149.Shobeiri N, Adams MA & Holden RM Phosphate: an old bone molecule but new cardiovascular risk factor. Br. J. Clin. Pharmacol. 77, 39–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duan P. & Bonewald LF The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 77, 23–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gaur T. et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33132–33140 (2005). [DOI] [PubMed] [Google Scholar]
- 152.Komori T. Signaling networks in RUNX2-dependent bone development. J. Cell. Biochem. 112, 750–755 (2011). [DOI] [PubMed] [Google Scholar]
- 153.Cai T. et al. WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 345, 206–217 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Deng D, Diao Z, Han X. & Liu W. Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt/ss-catenin pathway. Calcif. Tissue Int. 99, 66–75 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Allen DG & Trajanovska S. The multiple roles of phosphate in muscle fatigue. Front. Physiol. 3, 463 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lederer E. Regulation of serum phosphate. J. Physiol. 592, 3985–3995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lanske B. & Razzaque MS Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 86, 1072–1074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Imel EA & Econs MJ Fibroblast growth factor 23: roles in health and disease. J. Am. Soc. Nephrol. 16, 2565–2575 (2005). [DOI] [PubMed] [Google Scholar]
- 159.Fukumoto S. & Yamashita T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr. Opin. Nephrol. Hypertens. 11, 385–389 (2002). [DOI] [PubMed] [Google Scholar]
- 160.Liu S, Gupta A. & Quarles LD Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr. Opin. Nephrol. Hypertens. 16, 329–335 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Andrukhova O. et al. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51, 621–628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Olauson H. et al. Parathyroid-specific deletion of Klotho unravels a novel calcineurin-dependent FGF23 signaling pathway that regulates PTH secretion. PLOS Genet. 9, e1003975 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ichikawa S. et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 117, 2684–2691 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K. & Nabeshima Y-I Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 17, 2393–2403 (2003). [DOI] [PubMed] [Google Scholar]
- 165.Ferrari SL, Bonjour JP & Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 90, 1519–1524 (2005). [DOI] [PubMed] [Google Scholar]
- 166.Yamazaki Y. et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 23, 1509–1518 (2008). [DOI] [PubMed] [Google Scholar]
- 167.Perwad F. & Portale AA Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol. Cell. Endocrinol. 347, 17–24 (2011). [DOI] [PubMed] [Google Scholar]
- 168.Kägi L. et al. Regulation of vitamin D metabolizing enzymes in murine renal and extrarenal tissues by dietary phosphate, FGF23, and 1,25(OH)2D3. PLOS One 13, e0195427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feng JQ, Ye L. & Schiavi S. Do osteocytes contribute to phosphate homeostasis? Curr. Opin. Nephrol. Hypertens. 18, 285–291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kuro-o M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45 (1997). [DOI] [PubMed] [Google Scholar]
- 171.Lindberg K. et al. The kidney is the principal organ mediating Klotho effects. J. Am. Soc. Nephrol. 25, 2169–2175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ide N. et al. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney Int. 90, 348–362 (2016). [DOI] [PubMed] [Google Scholar]
- 173.Martin A. et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 25, 2551–2562 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Farrow EG et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl Acad. Sci. 108, E1146–E1155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tagliabracci VS et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl Acad. Sci. 111, 5520–5525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rankin EB et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149, 63–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Clinkenbeard EL et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 102, e427–e430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coe LM et al. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 289, 9795–9810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kyono A, Avishai N, Ouyang Z, Landreth GE & Murakami S. FGF and ERK signaling coordinately regulate mineralization-related genes and play essential roles in osteocyte differentiation. J. Bone Miner. Metab. 30, 19–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Woo SM, Rosser J, Dusevich V, Kalajzic I. & Bonewald LF Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Miner. Res. 26, 2634–2646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee JW, Yamaguchi A. & Iimura T. Functional heterogeneity of osteocytes in FGF23 production: the possible involvement of DMP1 as a direct negative regulator. Bonekey Rep. 3, 543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hori M, Kinoshita Y, Taguchi M. & Fukumoto S. Phosphate enhances Fgf23 expression through reactive oxygen species in UMR-106 cells. J. Bone Miner. Metab. 34, 132–139 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Ludmilla B. et al. Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Mol. Nutr. Food Res. 61, 1601019 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Nielsen PK, Feldt-Rasmussen U. & Olgaard K. A direct effect in vitro of phosphate on PTH release from bovine parathyroid tissue slices but not from dispersed parathyroid cells. Nephrol. Dialysis Transplant. 11, 1762–1768 (1996). [PubMed] [Google Scholar]
- 185.Silver J. & Naveh-Many T. Phosphate and the parathyroid. Kidney Int. 75, 898–905 (2009). [DOI] [PubMed] [Google Scholar]
- 186.Maeda A. et al. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc. Natl Acad. Sci. 110, 5864–5869 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kulkarni NH et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell. Biochem. 95, 1178–1190 (2005). [DOI] [PubMed] [Google Scholar]
- 188.Meir T. et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 86, 1106–1115 (2014). [DOI] [PubMed] [Google Scholar]
- 189.Nilsson IL et al. FGF23, metabolic risk factors, and blood pressure in patients with primary hyperparathyroidism undergoing parathyroid adenomectomy. Surgery 159, 211–217 (2016). [DOI] [PubMed] [Google Scholar]
- 190.Witteveen JE, van Lierop AH, Papapoulos SE & Hamdy NA Increased circulating levels of FGF23: an adaptive response in primary hyperparathyroidism? Eur. J. Endocrinol. 166, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 191.Horwitz MJ et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J. Bone Miner. Res. 20, 1792–1803 (2005). [DOI] [PubMed] [Google Scholar]
- 192.Wysolmerski JJ Parathyroid hormone-related protein: an update. J. Clin. Endocrinol. Metab. 97, 2947–2956 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang C. et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2, e1600241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Clinkenbeard E. et al. Erythropoietin and FGF23 cross-talk during iron-deficiency anemia [abstract 1117]. J. Bone Miner. Res. 30, S39 (2015). [Google Scholar]
- 195.David V, Francis C. & Babitt JL Ironing out the cross talk between FGF23 and inflammation. Am. J. Physiol. Renal Physiol. 312, F1–F8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamamoto S, Okada Y, Mori H, Fukumoto S. & Tanaka Y. Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern. Med. 51, 2375–2378 (2012). [DOI] [PubMed] [Google Scholar]
- 197.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM & Soule SG FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J. Clin. Endocrinol. Metab. 94, 2332–2337 (2009). [DOI] [PubMed] [Google Scholar]
- 198.Hryszko T, Rydzewska-Rosolowska A, Brzosko S, Koc-Zorawska E. & Mysliwiec M. Low molecular weight iron dextran increases fibroblast growth factor-23 concentration, together with parathyroid hormone decrease in hemodialyzed patients. Ther. Apheresis Dialysis 16, 146–151 (2012). [DOI] [PubMed] [Google Scholar]
- 199.Lewerin C. et al. Low serum iron is associated with high serum intact FGF23 in elderly men: the Swedish MrOS study. Bone 98, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Melda O. et al. A novel distal enhancer mediates inflammation-, PTH-, and early onset murine kidney disease-induced expression of the mouse Fgf23 gene. JBMR Plus 2, 31–46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bora SA, Kennett MJ, Smith PB, Patterson AD & Cantorna MT The gut microbiota regulates endocrine vitamin D metabolism through fibroblast growth factor 23. Front. Immunol. 9, 408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bär L. et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc. Natl Acad. Sci. 115, 5804–5809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang B. et al. Up-regulation of FGF23 release by aldosterone. Biochem. Biophys. Res. Commun. 470, 384–390 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Pathare G, Anderegg M, Albano G, Lang F. & Fuster DG Elevated FGF23 levels in mice lacking the thiazide-sensitive NaCl cotransporter (NCC). Sci. Rep. 8, 3590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bikle DD Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 21, 319–329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Norman AW The history of the discovery of vitamin D and its daughter steroid hormone. Ann. Nutr. Metab. 61, 199–206 (2012). [DOI] [PubMed] [Google Scholar]
- 207.Petkovich M. & Jones G. CYP24A1 and kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 337–344 (2011). [DOI] [PubMed] [Google Scholar]
- 208.Jones G, Prosser DE & Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 523, 9–18 (2012). [DOI] [PubMed] [Google Scholar]
- 209.Carpenter TO & Shiratori T. Renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity and mitochondrial phosphate transport in Hyp mice. Am. J. Physiol. 259, E814–821 (1990). [DOI] [PubMed] [Google Scholar]
- 210.Kaufmann M, Lee SM, Pike JW & Jones GA High-calcium and phosphate rescue diet and VDR-expressing transgenes normalize serum vitamin D metabolite profiles and renal Cyp27b1 and Cyp24a1 expression in VDR null mice. Endocrinology 156, 4388–4397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Masuda S. et al. Altered pharmacokinetics of 1alpha, 25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology 146, 825–834 (2005). [DOI] [PubMed] [Google Scholar]
- 212.Vanhooke JL et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3–1alpha-hydroxylase promoter activity in the skin. Proc. Natl Acad. Sci. USA 103, 75–80 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gattineni J. & Friedman PA Regulation of hormone-sensitive renal phosphate transport. Vitam. Horm. 98, 249–306 (2015). [DOI] [PubMed] [Google Scholar]
- 214.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L. & Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin d sufficiency, and calcium intake. JAMA 294, 2336–2341 (2005). [DOI] [PubMed] [Google Scholar]
- 215.Condamine L, Menaa C, Vrtovsnik F, Friedlander G. & Garabédian M. Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25-dihydroxyvitamin D by cultured mammalian kidney cells. J. Clin. Invest. 94, 1673–1679 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang MYH et al. Dietary phosphorus transcriptionally regulates 25-Hydroxyvitamin D-1α-hydroxylase gene expression in the proximal renal tubule. Endocrinology 143, 587–595 (2002). [DOI] [PubMed] [Google Scholar]
- 217.Tenenhouse HS, Martel J, Gauthier C, Zhang MYH & Portale AA Renal expression of the sodium/phosphate cotransporter gene, Npt2, is not required for regulation of renal 1α-hydroxylase by phosphate. Endocrinology 142, 1124–1129 (2001). [DOI] [PubMed] [Google Scholar]
- 218.Schlingmann KP et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 365, 410–421 (2011). [DOI] [PubMed] [Google Scholar]
- 219.Kaneko I. et al. Hypophosphatemia in vitamin D receptor null mice: effect of rescue diet on the developmental changes in renal Na+ -dependent phosphate cotransporters. Pflugers Arch. 461, 77–90 (2011). [DOI] [PubMed] [Google Scholar]
- 220.Christakos S, Ajibade DV, Dhawan P, Fechner AJ & Mady LJ Vitamin D: metabolism. Rheum. Dis. Clin. North Am. 38, 1–11, vii (2012). [DOI] [PubMed] [Google Scholar]
- 221.Lee GJ & Marks J. Intestinal phosphate transport: a therapeutic target in chronic kidney disease and beyond? Pediatr. Nephrol. 30, 363–371 (2015). [DOI] [PubMed] [Google Scholar]
- 222.Sabbagh Y, Giral H, Caldas Y, Levi M. & Schiavi SC Intestinal phosphate transport. Adv. Chron. Kidney Dis. 18, 85–90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Knöpfel T. et al. The intestinal phosphate transporter NaPi-IIb (Slc34a2) is required to protect bone during dietary phosphate restriction. Sci. Rep. 7, 11018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Berndt T. et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc. Natl Acad. Sci. USA 104, 11085–11090 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Scanni R, vonRotz M, Jehle S, Hulter HN & Krapf R. The human response to acute enteral and parenteral phosphate loads. J. Am. Soc. Nephrol. 25, 2730–2739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Razzaque MS Phosphate toxicity: new insights into an old problem. Clin. Sci. 120, 91–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Drezner MK PHEX gene and hypophosphatemia. Kidney Int. 57, 9–18 (2000). [DOI] [PubMed] [Google Scholar]
- 228.Hautmann AH, Hautmann MG, Kolbl O, Herr W. & Fleck M. Tumor-Induced osteomalacia: an up-to-date review. Curr. Rheumatol Rep. 17, 512 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Lee JC et al. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J. Pathol. 235, 539–545 (2015). [DOI] [PubMed] [Google Scholar]
- 230.Lee JC et al. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod. Pathol. 29, 1335–1346 (2016). [DOI] [PubMed] [Google Scholar]
- 231.Wohrle S. et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 28, 899–911 (2013). [DOI] [PubMed] [Google Scholar]
- 232.Xiao Z. et al. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLOS One 9, e104154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Miller CB et al. Response of tumor-induced osteomalacia (TIO) to the FGFR inhibitor BGJ398. J. Clin. Oncol. 34, e22500–e22500 (2016). [Google Scholar]
- 234.Akl MR et al. Molecular and clinical significance of fibroblast growth factor 2 (FGF2 /bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 7, 44735–44762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.De Beur SJ et al. Effects of burosumab (KRN23), a human monoclonal antibody to FGF23, in patients with tumor-induced osteomalacia (TIO) or epidermal nevus syndrome (ENS) [abstract SU0325]. J. Bone Miner. Res. 32, S280 (2017). [Google Scholar]
- 236.Francis F. et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X–linked hypophosphatemic rickets. Nat. Genet. 11, 130 (1995). [DOI] [PubMed] [Google Scholar]
- 237.Bai X-Y, Miao D, Goltzman D. & Karaplis AC The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 278, 9843–9849 (2003). [DOI] [PubMed] [Google Scholar]
- 238.Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G. & Strom TM Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 86, 267–272 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Simpson MA et al. Mutations in FAM20C also identified in non-lethal osteosclerotic bone dysplasia. Clin. Genet. 75, 271–276 (2009). [DOI] [PubMed] [Google Scholar]
- 240.Rafaelsen SH et al. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23–related hypophosphatemia, dental anomalies, and ectopic calcification. J. Bone Miner. Res. 28, 1378–1385 (2013). [DOI] [PubMed] [Google Scholar]
- 241.Nitschke Y. & Rutsch F. Inherited arterial calcification syndromes: etiologies and treatment concepts. Curr. Osteoporosis Rep. 15, 255–270 (2017). [DOI] [PubMed] [Google Scholar]
- 242.Wang X. et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLOS Genet. 8, e1002708 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Topa O. et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 36, 579 (2004). [DOI] [PubMed] [Google Scholar]
- 244.Anand G. & Schmid C. Severe hypophosphataemia after intravenous iron administration. BMJ Case Rep. 2017, bcr2016219160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wolf M, Koch TA & Bregman DB Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 28, 1793–1803 (2013). [DOI] [PubMed] [Google Scholar]
- 246.Wolf M. & White KE Coupling FGF23 production and cleavage: iron deficiency, rickets and kidney disease. Curr. Opin. Nephrol. Hypertension 23, 411–419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dumitrescu CE & Collins MT McCune-Albright syndrome. Orphanet J. Rare Dis. 3, 12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhu Y. et al. Ablation of the stimulatory G protein alpha-subunit in renal proximal tubules leads to parathyroid hormone-resistance with increased renal Cyp24a1 mRNA abundance and reduced serum 1,25-Dihydroxyvitamin D. Endocrinology 157, 497–507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.He Q. et al. The extra-large G protein alpha-subunit (XLαs) mediates FGF23 production by maintaining FGFR1 expression and MAPK signaling in bone [abstract 1138]. J. Bone Miner. Res. 32, S47 (2017). [Google Scholar]
- 250.Carpenter TO The expanding family of hypophosphatemic syndromes. J. Bone Miner. Metab. 30, 1–9 (2012). [DOI] [PubMed] [Google Scholar]
- 251.Sharma A. Physiology of the Developing Kidney: Disorders and Therapy of Calcium and Phosphorous Homeostasis 291–339 (Springer; Berlin Heidelberg, 2016). [Google Scholar]
- 252.White KE, Hum JM & Econs MJ Hypophosphatemic rickets: revealing novel control points for phosphate homeostasis. Curr. Osteoporos. Rep. 12, 252–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Carpenter TO et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Invest. 124, 1587–1597 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Johnson K. et al. Therapeutic Effects of FGF23 c-tail Fc in a murine pre-clinical model of X-linked hypophosphatemia via the selective modulation of phosphate reabsorption. J. Bone Miner. Res. 32, 2062–2073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bhattacharyya N, Chong WH, Gafni RI & Collins MT Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol. Metab. 23, 610–618 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bergwitz C. et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 78, 179–192 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lorenz-Depiereux B. et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am. J. Hum. Genet. 78, 193–201 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Caballero D. et al. Intraperitoneal pyrophosphate treatment reduces renal calcifications in Npt2a null mice. PLOS One 12, e0180098 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li Y. et al. Response of Npt2a knockout mice to dietary calcium and phosphorus. PLOS One 12, e0176232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Caballero D, Li Y, Ponsetto J, Zhu C. & Bergwitz C. Impaired urinary osteopontin excretion in Npt2a−/− mice. Am. J. Physiol. Renal Physiol. 312, F77–F83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bhoj EJ et al. Pathologic variants of the mitochondrial phosphate carrier SLC25A3: two new patients and expansion of the cardiomyopathy/skeletal myopathy phenotype with and without lactic acidosis. JIMD Rep. 19, 59–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mayr JA et al. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 21, 803–808 (2011). [DOI] [PubMed] [Google Scholar]
- 263.Adachi JD et al. Management of corticosteroid-induced osteoporosis. Semin. Arthritis Rheum. 29, 228–251 (2000). [DOI] [PubMed] [Google Scholar]
- 264.Maldonado EN & Lemasters JJ ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19 Pt A, 78–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kwong JQ et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 21, 1209–1217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lemos RR et al. Update and mutational analysis of SLC20A2: a major cause of primary familial brain calcification. Hum. Mutat. 36, 489–495 (2015). [DOI] [PubMed] [Google Scholar]
- 267.Legati A. et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 47, 579–581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nan H. et al. Novel SLC20A2 mutation in primary familial brain calcification with disturbance of sustained phonation and orofacial apraxia. J. Neurol. Sci. 390, 1–3 (2018). [DOI] [PubMed] [Google Scholar]
- 269.Anheim M. et al. XPR1 mutations are a rare cause of primary familial brain calcification. J. Neurol. 263, 1559–1564 (2016). [DOI] [PubMed] [Google Scholar]
- 270.Keller A. et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077–1082 (2013). [DOI] [PubMed] [Google Scholar]
- 271.Yao X-P et al. Analysis of gene expression and functional characterization of XPR1: a pathogenic gene for primary familial brain calcification. Cell Tissue Res. 370, 267–273 (2017). [DOI] [PubMed] [Google Scholar]
- 272.Jensen N. et al. Mice knocked out for the primary brain calcification associated gene Slc20a2 show unimpaired pre-natal survival but retarded growth and nodules in the brain that grow and calcify over time. Am. J. Pathol. 10.1016/j.ajpath.2018.04.010 (2018). [DOI] [PubMed]
- 273.Wallingford MC et al. SLC20A2 deficiency in mice leads to elevated phosphate levels in cerbrospinal fluid and glymphatic pathway-associated arteriolar calcification, and recapitulates human idiopathic basal ganglia calcification. Brain Pathol. 27, 64–76 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jensen N, Autzen JK & Pedersen L. Slc20a2 is critical for maintaining a physiologic inorganic phosphate level in cerebrospinal fluid. Neurogenetics 17, 125–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bergen AAB et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet. 25, 228 (2000). [DOI] [PubMed] [Google Scholar]
- 276.Klement JF et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol. Cell. Biol. 25, 8299–8310 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Li X, Yang HY & Giachelli CM Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 98, 905–912 (2006). [DOI] [PubMed] [Google Scholar]
- 278.Bastepe M. & Juppner H. Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev. Endocr. Metab. Disord. 9, 171–180 (2008). [DOI] [PubMed] [Google Scholar]
- 279.Fukumoto S. FGF23-FGF receptor/Klotho pathway as a new drug target for disorders of bone and mineral metabolism. Calcif. Tissue Int. 98, 334–340 (2016). [DOI] [PubMed] [Google Scholar]
- 280.Moniot S, Elias M, Kim D, Scott K. & Chabriere E. Crystallization, diffraction data collection and preliminary crystallographic analysis of DING protein from Pseudomonas fluorescens. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63, 590–592 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Choi PH, Sureka K, Woodward JJ & Tong L. Molecular basis for the recognition of cyclic-di-AMP by PstA, a P(II)-like signal transduction protein. Microbiologyopen 4, 361–374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hudek L, Premachandra D, Webster WAJ & Bräu L. Role of phosphate transport system component PstB1 in phosphate internalization by Nostoc punctiforme. Appl. Environ. Microbiol. 82, 6344–6356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Murer H, Forster I. & Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Arch. 447, 763–767 (2004). [DOI] [PubMed] [Google Scholar]
- 284.Patrice H. et al. Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol. Microbiol. 51, 307–317 (2004). [DOI] [PubMed] [Google Scholar]
- 285.Secco D. et al. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 193, 842–851 (2012). [DOI] [PubMed] [Google Scholar]
- 286.Yuan Q. et al. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology 152, 4053–4061 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kido S, Kaneko I, Tatsumi S, Segawa H. & Miyamoto K. Vitamin D and type II sodium-dependent phosphate cotransporters. Contrib. Nephrol. 180, 86–97 (2013). [DOI] [PubMed] [Google Scholar]
- 288.Kamenický P, Mazziotti G, Lombès M, Giustina A. & Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: pathophysiological and clinical implications. Endocr. Rev. 35, 234–281 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.