Abstract
Purpose:
We investigated the safety and feasibility of intraoperative portal vein blood (PVB) collection at the time of resection of pancreatic ductal adenocarcinoma (PDAC). Relationships between circulating tumor cells (CTCs) in PVB, peripheral blood (PB), overall survival (OS), and recurrence free survival (RFS) were studied.
Methods:
Patients undergoing PDAC resection were offered enrollment in a prospective liquid biopsy protocol. Patients had PB drawn before incision, PVB drawn before tumor mobilization and again immediately after resection. CTCs were identified using standard CellSearch protocols and compared to OS.
Results:
Of 34 patients enrolled, 23 (68%) underwent pancreaticoduodenectomy, 8 (23%) underwent distal pancreatectomy, and 3 (9%) underwent total pancreatectomy. PB was available for 22 (65%) patients, and PVB was available for 31 (91%). No bleeding or thrombotic complications occurred with PVB draws. CTC counts per 7.5 mL PVB collected before and after resection were highly correlated (R2=0.89). CTCs were present in 11/22 (50%) PB samples and 22/31 (71%) PVB samples. OS rates at 18 months were 92% in patients with <3 CTCs and 71% in patients with ≥3 CTCs per 7.5 mL of PB (p=0.30) and 100% in patients without PVB CTCs and 70% in patients with PVB CTCs (p<0.01).
Conclusions:
PVB collection during PDAC resection is safe. In this pilot study, PVB CTC counts but not PB CTC counts significantly correlated with OS. This opens the door for future studies on selective omission of adjuvant chemotherapy in patients treated preoperatively and tailored surveillance intensity in patients without PVB CTCs at PDAC resection.
Keywords: Pancreatic Cancer, Circulating Tumor Cells, Portal Vein Blood
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy that often presents at a late stage when a patient’s treatment options do not include surgical resection. Patients whose disease is localized and can undergo resection, however, still face a poor prognosis, as recurrence and death from disease are common despite aggressive contemporary medical and surgical options. Within this context, we hope to discover ways to better define PDAC biology to assist patients and clinicians in post-resection prognostication.
While pathologic classification and staging systems are useful, their utility for prognostication is limited.1 Previous work has demonstrated the utility of circulating tumor cells (CTCs) in defining disease biology in a number of solid malignancies.2-8 To date, however, work defining the prognostic utility of CTCs in patients with PDAC and the ideal source of CTC sampling remains limited.6-13
Because the venous drainage of the pancreas runs through the portal circulation and liver, CTCs from PDAC can be detected in both peripheral blood (PB) and portal venous blood (PVB). We hypothesized that PVB could be safely collected intraoperatively for CTC collection. We also hypothesized that because of the venous drainage of the pancreas, CTCs from PVB reflect PDAC biology more accurately than CTCs from PB. The primary aim of this pilot study was to determine the safety and feasibility of drawing PVB through direct needle puncture of the PV to measure CTCs in patients undergoing PDAC resection. Because of previous suggestions that manipulation of the pancreas at the time of resection may alter CTC assessment, we performed PVB sampling both prior to and immediately after resection of malignancy. Secondary aims were to determine the associations between CTC counts obtained from PVB collected before and after resection as well as to determine the associations between CTC counts from PB and PVB. Finally, associations between CTCs in PB or PVB and overall (OS) or recurrence-free survival (RFS) were studied.
PATIENTS AND METHODS
All patients undergoing resection of localized PDAC at our institution between January 2018 and October 2019 were offered enrollment in this institutional review board (IRB)-approved prospective study (PA11-0670). Retrospective review of clinical outcomes was approved by the IRB (PA2020-0309). Patients had their disease staged and discussed in a multidisciplinary format when they presented to our institution. After undergoing neoadjuvant therapy as indicated and tolerated, patients had their disease restaged and proceeded to resection in the absence of clinically evident metastatic disease.
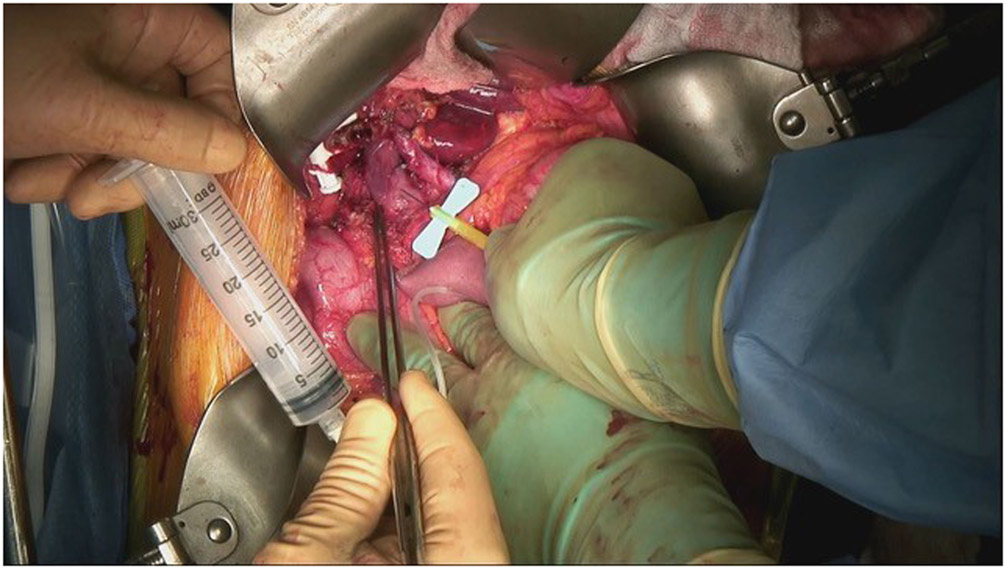
Patients who gave informed consent underwent PB draw in the operating room after general anesthesia induction but before incision. The next blood draw was performed via direct access to the PV after the portal dissection exposed the PV but before significant manipulation of the tumor. After the tumor specimen was mobilized off the portal vein-superior mesenteric vein confluence, the PV was again accessed. PVB samples were obtained by puncture with a 21- or 22-gauge butterfly needle, and the puncture site was covered with a postage-stamp-sized piece of hemostatic agent or dry gauze alone, without need for suture repair (Figure 1). Two separate draws were performed to identify any effect that manipulation of the primary tumor might have on the number of or presence of CTCs. Blood was transferred from the sterile syringes immediately into CellSave tubes (CellSearch, Huntington Valley PA). Resected pathologic specimens were subjected to standard evaluation.
Fig 1.
Placing a 21-gauge butterfly needle into the portal vein (above the splenic vein–superior mesenteric vein confluence) after a pancreatic-head tumor has been resected.
CTC Isolation
CTCs in PVB or PB were measured using the CellSearch system. All specimens were processed within 72 hours of collection. This system processes blood samples by enriching cells that express epithelial cell adhesion molecules. This is done using antibody-coated magnetic beads, labeled with the nuclei of enriched cells with fluorescent dye 4,2-diamidino-2-phenylindole dihydrochloride. Enriched cells are then stained with cytokeratins 8, 18, and 19 and CD45 fluorescent antibodies. A semi-automated fluorescence-based microscope system is used to process blood and identify CTCs, defined as nucleated cells positive for cytokeratin and negative for CD4514. The output of this process was reviewed by a single certified laboratory technician (CH) who was blinded to all patient clinical data.
Statistical Analyses
Demographics for the group and differences based on CTC status were analyzed using t-test, chi-squared, and Wilcoxon rank-sum tests as appropriate. The number of CTCs correlating with OS and RFS was identified using receiver operator characteristics analysis. Survival was measured from the date of tissue diagnosis. RFS was measured to the first clinically confirmed recurrence or censored at the time of last follow-up. OS was measured to the date of death or censored at the time of last know disease status. Survival analysis was performed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. All tests were two-sided, and statistical significance was defined as p<0.05. All analyses were performed with STATA 13 (College Station, TX).15
RESULTS
Patients
A total of 34 patients with PDAC enrolled during the study period and had at least one PB or PVB sample drawn and analyzed for CTCs. The median age at time of diagnosis was 68.0 years (range, 38.3-80.3 years), and 17 (50%) patients were female. Twenty-three patients (68%) underwent a Whipple procedure, 8 (23%) had a distal pancreatectomy, and 3 (9%) had a total pancreatectomy.
Safety of PVB Draws
PB was available for 22 (65%) patients, and PVB was available for 31 (91%) patients. Missing samples were the result of logistical difficulties coordinating blood draws with anesthesia teams, were elected not to be performed by the operating surgeon due to accumulated blood loss at that point in the operation, or were not simply drawn during a technically demanding operation. There were no immediate bleeding or thrombotic events associated with PVB draws.
CTCs in PB and PVB Samples
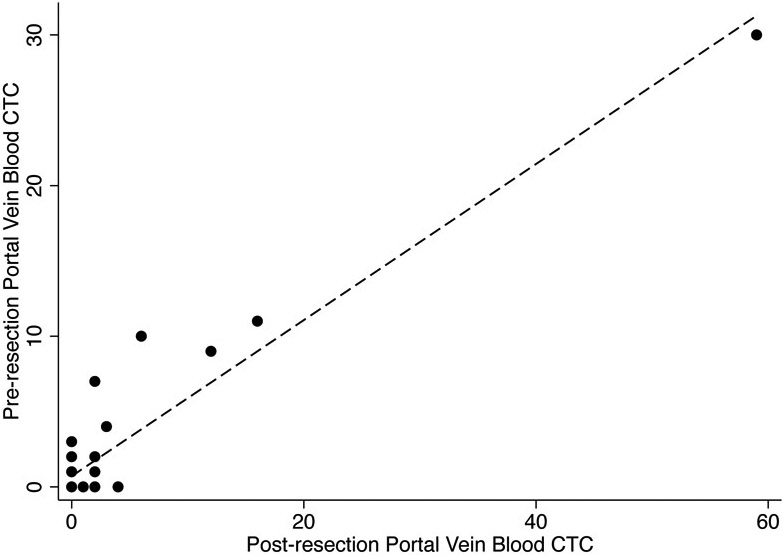
The number of CTCs per 7.5 mL of PVB collected before resection was highly correlated with the number of CTCs per 7.5 mL of PVB collected after resection (R2=0.89, Figure 2). There was no significant association between the number of CTCs per 7.5 mL of blood in PVB samples collected before or after resection and the number of CTCs per 7.5 mL of blood in the PB sample (R2=0.48 and R2=0.46, respectively). A higher proportion of patients had CTCs in pre and/or post resection PVB samples (22 of 31, 71%) than had CTCs in PB samples (11 of 22, 50%), but this difference was not significant (p=0.12).
Fig 2.
Scatter plot of number of circulating tumor cells (CTCs) per 7.5 mL in pre-resection and post-resection portal vein blood samples. Dashed line indicates linear fit. (R2=0.89).
Characteristics of Patients With and Without PVB CTCs
Demographic, clinical, and pathologic characteristics for the patients with and without PVB CTCs are summarized in Table 1. There were no significant differences in demographics, grade, stage, or pathologic parameters between patients with PVB CTCs and those without PVB CTCs. Tumors were slightly larger in the patients without PVB drawn than in the patients with PVB drawn. CTCs were present in 15 of 31 (48%) pre-resection PVB samples and 18 of 31 (58%) post-resection PVB samples.
Table 1.
Demographic, clinical, and pathologic characteristics
| Characteristic | PVB CTC positive (n=22) |
PVB CTC negative(n=9) |
PVB not drawn (n=3) |
p |
|---|---|---|---|---|
| Median age, y (range) | 66 (46-80) | 63 (38-75) | 71 (67-77) | 0.47 |
| Female sex | 12 (55) | 4 (44) | 1 (33) | 0.73 |
| Grade | 0.36 | |||
| IPMN (HGD) | 2 (9) | 0 (0) | 0 (0) | |
| Moderately differentiated | 9 (41) | 5 (56) | 3 (100) | |
| Moderately to poorly differentiated | 7 (32) | 1 (11) | 0 (0) | |
| Poorly differentiated | 4 (18) | 2 (22) | 0 (0) | |
| NA (pathologic CR) | 0 (0) | 1 (11) | 0 (0) | |
| Size at diagnosis, mean ± SD, mm | 26.5 ± 12.5 | 31.9 ± 10.0 | 35.5 ± 2.1 | 0.36 |
| CA 19-9 trend | 0.49 | |||
| Falling | 10 (45) | 8 (89) | 2 (67) | |
| Stable | 1 (5) | 0 (0) | 0 (0) | |
| Rising | 2 (9) | 0 (0) | 0 (0) | |
| Nonproducer | 9 (41) | 1 (11) | 1 (33) | |
| Clinical classification | 0.55 | |||
| Resectable | 11 (50) | 2 (22) | 1 (33) | |
| Borderline resectable | 10 (45) | 7 (78) | 2 (67) | |
| Unresectable | 1 (5) | 0 (0) | 0 (0) | |
| Neoadjuvant chemotherapy | 0.36 | |||
| 5-FU-based | 9 (41) | 7 (78) | 3 (100) | |
| Gemcitabine-based | 9 (41) | 1 (11) | 0 (0) | |
| Immunotherapy | 1 (5) | 0 (0) | 0 (0) | |
| None | 3 (13) | 1 (11) | 0 (0) | |
| Neoadjuvant radiation therapy | 11 (50) | 5 (56) | 3 (100) | 0.31 |
| Resection | 0.42 | |||
| Whipple procedure | 15 (68) | 6 (67) | 2 (67) | |
| Distal pancreatectomy | 5 (23) | 3 (33) | 0 (0) | |
| Total pancreatectomy | 2 (9) | 0 (0) | 1 (33) | |
| Pathologic size, mean ± SD, mm | 29.13 ± 11.5 | 27.3 ± 8.5 | 46.7 ± 5.8 | 0.02 |
| Lymph nodes positive | 9 (41) | 3 (33) | 2 (67) | 0.59 |
5-FU, 5-fluorouracil; CR, complete response; IPMN (HGD), intraductal papillary mucinous neoplasm (high-grade dysplasia); NA, not applicable; SD, standard deviation.
Values in table are number of patients (percentage) unless otherwise indicated.
Relationships between CTCs and Survival
Receiver operator characteristics (ROC) curve analysis of the association between PB CTCs and OS suggested that a cutoff of ≥3 CTCs per 7.5 mL (data not shown) was most closely associated with survival, although this failed to reach the predefined threshold of significance (p=0.13). ROC analysis failed to demonstrate a significant relationship between the number of CTCs in pre- or post-resection PVB samples and OS (p=0.87 and p=0.85 respectively). However, the presence or absence of PVB CTCs in either draw was associated with OS. Therefore, cutoffs of ≥3 PB CTCs and ≥1 PVB CTCs were used in subsequent analyses.
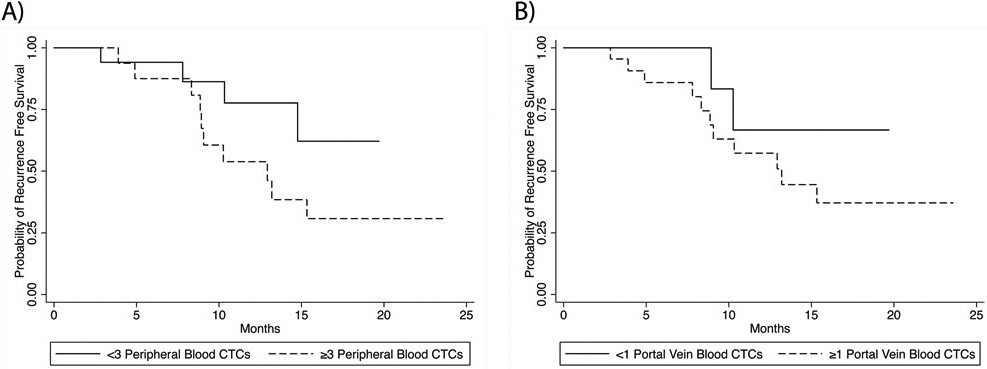
The median RFS for the 34 patients in the analysis was 10.6 months (range, 0.23 to 1.97 months). RFS was not significantly different between the patients with PB CTC levels of < 3 or ≥ 3 per 7.5 mL (hazard ratio [HR] 2.48, 95% CI 0.78-7.94; p=0.13) (Figure 3a) or between the patients with <1 or ≥ 1 PVB CTCs per 7.5 mL (HR 2.26, 95% CI 0.50-10.22; p=0.29) (Figure 3b). Associations between demographic, clinical, or pathologic features and recurrence on univariate analysis are summarized in Table 2.
Fig. 3.
Recurrence free survival (RFS) by circulating tumor cell (CTC) status. (a) RFS in patients with peripheral blood CTC counts of < 3 per 7.5 mL and ≥ 3 per 7.5 mL; 18-month RFS rates, 62% and 31%, respectively (p=0.13). (b) RFS rates in patients with portal vein blood CTC counts of < 1 and ≥ 1 per 7.5 mL; 18-month RFS rates, 67% and 37%, respectively (p=0.29).
Table 2.
Univariate analysis of factors associated with recurrence
| Characteristic | Cox hazard ratio (95% CI) | p |
|---|---|---|
| Age | 0.99 (0.94-1.04) | 0.74 |
| Female sex | 0.99 (0.35-2.84) | 0.99 |
| Grade | ||
| IPMN (HGD) | Colinear | - |
| Moderately differentiated | REF | - |
| Moderately to poorly differentiated | 4.28 (1.3-14.2) | 0.02 |
| Poorly differentiated | 0.62 (0.12-3.11) | 0.56 |
| NA (pathologic CR) | Colinear | - |
| Size at diagnosis, mm | 1.05 (0.99-1.10) | 0.07 |
| CA 19-9 trend | ||
| Falling | REF | - |
| Stable | 0.47 (0.12-1.78) | 0.27 |
| Rising | 13.67 (2.03-91.67) | 0.01 |
| Nonproducer | 6.40 (0.65-63.1) | 0.11 |
| Clinical classification | ||
| Resectable | REF | - |
| Borderline resectable | 1.76 (0.59-5.29) | 0.31 |
| Unresectable | Colinear | - |
| Neoadjuvant chemotherapy | ||
| 5-FU-based | REF | - |
| Gemcitabine-based | 1.50 (0.50-4.50) | 0.46 |
| Immunotherapy | 0.44 (0.05-3.65) | 0.46 |
| None | Colinear | - |
| Neoadjuvant radiation therapy | 1.28 (0.43-3.81) | 0.66 |
| Resection | ||
| Whipple procedure | REF | - |
| Distal pancreatectomy | 1.00 (0.17-6.03) | 1.00 |
| Total pancreatectomy | 0.88 (0.24-3.30) | 0.85 |
| Pathologic size, mm | 1.02 (0.98-1.06) | 0.41 |
| Lymph node positive | 2.64 (0.89-7.77) | 0.08 |
| ≥ 3 PB CTC | 2.48 (0.78-7.94) | 0.13 |
| > 1 PVB CTC | 2.26 (0.50-10.22) | 0.29 |
5-FU, 5-fluorouracil; CR, complete response; IPMN (HGD), intraductal papillary mucinous neoplasm (high-grade dysplasia); NA, not applicable; PB CTC, peripheral blood circulating tumor cell; PVB CTC, portal vein blood circulating tumor cell.
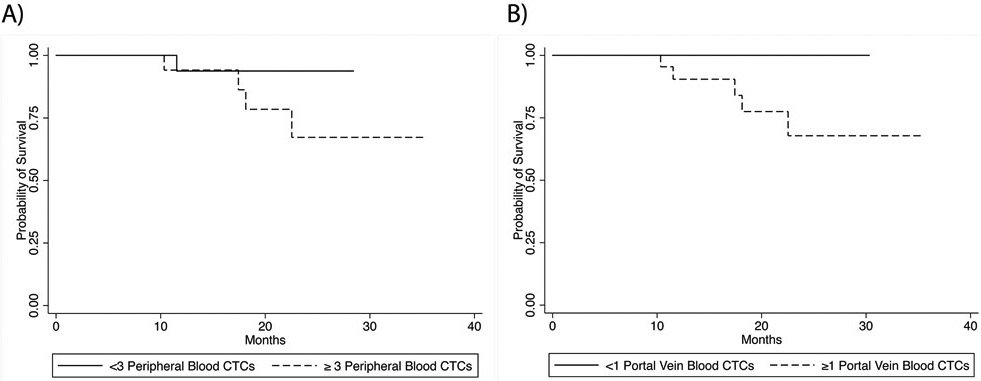
The median follow-up for the 34 patients with any blood sample in the analysis was 14.1 months (range, 0.86 to 1.97 months), and the OS rate at 18 months was 80%. Associations between demographic, clinical, or pathologic features and OS on univariate analysis are summarized in Table 3. OS was not significantly different between the patients with PB CTC levels of < 3 and ≥ 3 per 7.5 mL (HR 3.73, 95% CI 0.42-33.39; p=0.30 Figure 4a). There was a significant colinear association between the absence of PVB CTCs and OS (Figure 4b). The lack of death events within the no–PVB CTCs group did not allow for calculation of an associated HR. However, the patients with no PVB CTCs had an OS rate of 100% at 18 months, while the patients with ≥1 PVB CTCs had an OS rate of 70% at 18 months.
Table 3.
Univariate analysis of factors associated with survival
| Characteristic | Cox hazard ratio (95% CI) | p |
|---|---|---|
| Age | 1.14 (1.00-1.31) | 0.05 |
| Female sex | 0.47 (0.08-2.85) | 0.41 |
| Grade | ||
| IPMN (HGD) | Colinear | - |
| Moderately differentiated | REF | - |
| Moderately to poorly differentiated | 4.17 (0.69-25.1) | 0.12 |
| Poorly differentiated | Colinear | 0 |
| NA (pathologic CR) | Colinear | - |
| Size at diagnosis, mm | 1.05 (0.97-1.14) | 0.22 |
| CA 19-9 trend | ||
| Falling | REF | - |
| Stable | 0.84 (0.08-9.25) | 0.88 |
| Rising | 19.08 (2.27-160.18) | 0.01 |
| Nonproducer | Colinear | - |
| Clinical classification | ||
| Resectable | REF | - |
| Borderline resectable | 2.25 (0.25-20.42) | 0.47 |
| Unresectable | Colinear | - |
| Neoadjuvant chemotherapy | ||
| 5-FU-based | REF | - |
| Gemcitabine-based | 2.15 (0.35-13.02) | 0.41 |
| Immunotherapy | Colinear | - |
| None | Colinear | - |
| Neoadjuvant radiation therapy | 2.18 (0.24-19.8) | 0.49 |
| Resection | ||
| Whipple procedure | REF | - |
| Distal pancreatectomy | Colinear | - |
| Total pancreatectomy | 1.17 (0.13-10.57) | 0.88 |
| Pathologic size, mm | 1.03 (0.96-1.13) | 0.35 |
| Lymph nodes positive | 2.73 (0.45-16.47) | 0.28 |
| ≥ 3 PB CTC | 3.73 (0.42-33.39) | 0.30 |
| > 1 PVB CTC | Colinear | - |
5-FU, 5-fluorouracil; CR, complete response; IPMN (HGD), intraductal papillary mucinous neoplasm (high-grade dysplasia); NA, not applicable; PB CTC, peripheral blood circulating tumor cell; PVB CTC, portal vein blood circulating tumor cell.
Fig 4.
Overall survival (OS) by circulating tumor cell (CTC) status. (a) OS in patients with peripheral blood CTC counts of < 3 and ≥ 3 per 7.5 mL; 18-month OS rates, 92% and 71%, respectively (p=0.30). (b) OS in patients with portal vein blood CTC counts of <1 and ≥ 1 per 7.5 mL; 18-month OS rates, 100% and 70%, respectively (p<0.01).
DISCUSSION
The primary aim of this work was to determine the safety and feasibility of intraoperative sampling of PVB to measure direct shedding of CTCs into the portal and peripheral circulation. In the study period and subsequent follow-up, there were no immediate adverse events directly attributable to PB or PVB blood draws, and the rates of CTC detection ranged from 50% for PB samples to 47% for pre-resection PVB samples to 58% for post-resection PVB samples. While further work will be needed, our finding that intraoperatively sampling the direct venous drainage of the pancreas is safe and feasible for measuring CTCs opens a number of possibilities for further study and identification of markers to direct postoperative therapy and surveillance intensity.
We hypothesized that due to the venous drainage of the pancreas, PVB would represent PDAC disease biology more accurately than PB would. We found no correlation between CTC positivity in PB samples and CTC positivity in PVB samples. This lack of correlation between CTC status in PB and PVB has been noted in other small cohorts as well, suggesting that the liver plays a significant role in trapping or diluting CTCs in the portal circulation.10
There was a high degree of correlation between the number of measured pre- and post-resection PVB CTCs. Despite the fact that CTC counts would be expected to decrease after resection and over time, it has been posited that manipulation of the pancreas during resection may release CTCs, so that the number in circulation increases and remains elevated immediately after resection.16-18 Our findings, however, imply that intraoperative tumor manipulation has little to no effect on the shedding of CTCs, with the caveat that the vast majority of patients (30/34) underwent neoadjuvant therapy.18
As a secondary endpoint, we were interested in any correlation between CTCs and short-term RFS as well as OS. While there was no significant correlation between PB CTC status and OS or RFS, or between PVB CTC status and RFS, our data showed complete collinearity between PVB CTC status and OS. This supports an earlier finding by the group at Johns Hopkins University, who also demonstrated an association between a lack of PVB CTCs and increased OS.8 Taken together, the findings from our study and the Johns Hopkins study suggest the possibility of a CTC shedding phenotype correlating with survival expectations, in a disease associated with high recurrence risk. Further work with larger patient cohorts will be necessary to quantify and delineate this association and to further categorize patients by prognostic groups through liquid biopsy. More promising for patients on the pathway to surgery, circulating biomarkers studies have the potential to be used as a marker for response to neoadjuvant therapy and to augment surgical decision making along with traditional radiographic and tumor marker response. While PB samples are more repeatable than PVB, our PVB data suggest future studies should not be limited solely to peripheral circulation. With select endoscopists’ ability to sample PVB, preoperative plasma biomarker studies of the immediate drainage of pancreatic tumors may be of higher yield in directing neoadjuvant decisions.
CTCs and circulating tumor DNA have been used in work guiding resection of colorectal liver metastases and other solid tumors.2,5,13,19-22 In the case of PDAC, a clinical debate exists regarding the utility of adjuvant chemotherapy in patients who have already received neoadjuvant chemotherapy.23 This debate likely arises from unrecognized heterogeneity within patient populations. If a marker of a disease phenotype corresponding to response to therapy were to be identified, CTCs or otherwise, confirmation of a marker’s utility would require a randomized controlled trial in which patients are randomly assigned to adjuvant therapy or not with stratification based on this marker.
Identification of a low-risk PDAC phenotype could not only guide decision making regarding adjuvant therapy but also help guide the intensity of postoperative surveillance. Currently there is no consensus around the frequency of surveillance, and the National Comprehensive Cancer Network recommendation is simply to perform surveillance studies 3 to 6 months after resection.24,25 The recommended frequency of surveillance is currently determined by the treating physician using clinical factors such as CA19-9 normalization, tumor response to chemotherapy on pathologic evaluation, node positivity, and perineural invasion.26 As demonstrated in this small cohort, these factors do not strongly correlate to either RFS or OS but serve as a suboptimal proxy for a biomarker in making a decision regarding surveillance intensity. Minimizing the need for close surveillance would not only improve survivors’ quality of life but limit the utilization of valuable healthcare resources.
There are significant limitations to this work. Our primary aim was to test the safety and feasibility of PVB sampling in order to establish this platform as safe for CTC detection or other liquid biopsy studies in PDAC. Definitive conclusions are not possible given the small sample size and limited follow-up. Our sample size limits our ability to achieve our secondary endpoint of correlating CTCs with short-term RFS or OS. Selection bias is also significant in this study as patients volunteered for participation and surgeons had to remember to decide to draw PVB at two points during an operation that requires intense focus. Finally, the fact that we obtained PVB at the time of surgical resection greatly limits the applicability of this technique for patients not undergoing resection. This limitation is being addressed at our institution with a separate protocol for endoscopic ultrasound-guided PVB draws at the time of tissue diagnosis.
In conclusion, in this work we were able to demonstrate the safety and feasibility of CTC extraction for PVB. Moreover, our initial findings suggest that PVB could be superior to PB in predicting short-term outcomes in patients. While our findings support work by others suggesting a “CTC shedding” phenotype of PDAC, further work is needed to fully define the biologic implications of CTCs in PDAC.
SYNOPSIS.
Here we demonstrate the safety and feasibility of measuring pancreatic adenocarcinoma circulating tumor cells (CTCs) drawn from the portal vein at the time of resection. CTCs drawn from the portal vein may prove useful as a biomarker for survival.
Acknowledgments
This study was supported by the National Cancer Institute under award number P30CA016672, which supports the MD Anderson Cancer Center Clinical Trials Office.
Dr. White is supported through the National Institute of Health Kirschtein National Research Service (T32) Award. This work was supported using internal research fund through the University of Chicago Department of Surgical Oncology.
The authors thank Stephanie Deming in the Editing Services Division, of the Research Medical Library at the University of Texas, MD Anderson Cancer Center, for her assistance preparing this manuscript.
Footnotes
Author Disclosure Statement: The authors have no conflicts of interest to report.
REFERENCES
- 1.Amin MBES, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. AJCC Cancer Staging Manual (8th edition). 8th ed. Chicago IL: Springer International Publishing; 2017. [Google Scholar]
- 2.Lucci A, Hall CS, Patel SP, et al. Circulating Tumor Cells and Early Relapse in Node-positive Melanoma. Clin Cancer Res. April 15 2020;26(8):1886–1895. [DOI] [PubMed] [Google Scholar]
- 3.Anand K, Roszik J, Gombos D, et al. Pilot Study of Circulating Tumor Cells in Early-Stage and Metastatic Uveal Melanoma. Cancers. June 20 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CS, Ross M, Bowman Bauldry JB, et al. Circulating Tumor Cells in Stage IV Melanoma Patients. J Am Coll Surg. July 2018;227(1):116–124. [DOI] [PubMed] [Google Scholar]
- 5.Hall CS, Karhade MG, Bowman Bauldry JB, et al. Prognostic Value of Circulating Tumor Cells Identified Before Surgical Resection in Nonmetastatic Breast Cancer Patients. J Am Coll Surg. July 2016;223(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Yu X, Hartmann D, Zhou J. Circulating tumor cells in peripheral blood of pancreatic cancer patients and their prognostic role: a systematic review and meta-analysis. HPB (Oxford). November 27 2019. [DOI] [PubMed] [Google Scholar]
- 7.Luchini C, Veronese N, Nottegar A, et al. Liquid Biopsy as Surrogate for Tissue for Molecular Profiling in Pancreatic Cancer: A Meta-Analysis Towards Precision Medicine. Cancers. August 10 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemenetzis G, Groot VP, Yu J, et al. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann Surg. September 2018;268(3):408–420. [DOI] [PubMed] [Google Scholar]
- 9.Hugenschmidt H, Labori KJ, Brunborg C, et al. Circulating Tumor Cells are an Independent Predictor of Shorter Survival in Patients Undergoing Resection for Pancreatic and Periampullary Adenocarcinoma. Ann Surg. March 2020;271(3):549–558. [DOI] [PubMed] [Google Scholar]
- 10.Vicente D, Lee AJ, Hall CS, et al. Circulating Tumor Cells and Transforming Growth Factor Beta in Resected Pancreatic Adenocarcinoma. The Journal of surgical research. November 2019;243:90–99. [DOI] [PubMed] [Google Scholar]
- 11.Kulemann B, Rosch S, Seifert S, et al. Pancreatic cancer: Circulating Tumor Cells and Primary Tumors show Heterogeneous KRAS Mutations. Sci Rep. July 3 2017;7(1):4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissolati M, Sandri MT, Burtulo G, Zorzino L, Balzano G, Braga M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. February 2015;36(2):991–996. [DOI] [PubMed] [Google Scholar]
- 13.Tjensvoll K, Nordgard O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. International journal of cancer. Journal international du cancer. January 1 2014;134(1):1–8. [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. The New England journal of medicine. August 19 2004;351(8):781–791. [DOI] [PubMed] [Google Scholar]
- 15.Stata Statistical Software: Release 13 [computer program]. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 16.Martin OA, Anderson RL, Narayan K, MacManus MP. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol. January 2017;14(1):32–44. [DOI] [PubMed] [Google Scholar]
- 17.Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. December 15 2004;10(24):8152–8162. [DOI] [PubMed] [Google Scholar]
- 18.Gall TM, Jacob J, Frampton AE, et al. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. May 2014;149(5):482–485. [DOI] [PubMed] [Google Scholar]
- 19.Sastre J, Orden V, Martinez A, et al. Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-naive Metastatic Colorectal Cancer Patients Prospectively Collected. Clin Colorectal Cancer. March 6 2020. [DOI] [PubMed] [Google Scholar]
- 20.Osumi H, Shinozaki E, Yamaguchi K, Zembutsu H. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Sci. Apr 2019;110(4):1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang C, Fan C, Wang C, et al. Prognostic value of CD133(+) CD54(+) CD44(+) circulating tumor cells in colorectal cancer with liver metastasis. Cancer Med. December 2017;6(12):2850–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeberg LT, Waage A, Brunborg C, et al. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg. January 2015;261(1):164–171. [DOI] [PubMed] [Google Scholar]
- 23.Perri G, Prakash L, Qiao W, et al. Postoperative Chemotherapy Benefits Patients Who Received Preoperative Therapy and Pancreatectomy for Pancreatic Adenocarcinoma. Ann Surg. December 31 2019. [DOI] [PubMed] [Google Scholar]
- 24.Tzeng CW, Abbott DE, Cantor SB, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol. July 2013;20(7):2197–2203. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng CW, Fleming JB, Lee JE, et al. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB (Oxford). June 2012;14(6):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzeng CW, Balachandran A, Ahmad M, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford). May 2014;16(5):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]