Abstract
Extracellular vesicles (EVs) secreted by living cells are expected to deliver biological cargo molecules, including RNA and proteins, to the cytoplasm of recipient cells. There is an increasing need to understand the mechanism of intercellular cargo delivery by EVs. However, the lack of a feasible bioassay has hampered our understanding of the biological processes of EV uptake, membrane fusion, and cargo delivery to recipient cells. Here, we describe a reporter gene assay that can measure the membrane fusion efficiency of EVs during cargo delivery to recipient cells. When EVs containing tetracycline transactivator (tTA)‐fused tetraspanins are internalized by recipient cells and fuse with cell membranes, the tTA domain is exposed to the cytoplasm and cleaved by tobacco etch virus protease to induce tetracycline responsive element (TRE)‐mediated reporter gene expression in recipient cells. This assay (designated as EV‐mediated tetraspanin‐tTA delivery assay, ETTD assay), enabled us to assess the cytoplasmic cargo delivery efficiency of EVs in recipient cells. With the help of a vesicular stomatitis virus‐derived membrane fusion protein, the ETTD assay could detect significant enhancement of cargo delivery efficiency of EVs. Furthermore, the ETTD assay could evaluate the effect of potential cargo delivery enhancers/inhibitors. Thus, the ETTD assay may contribute to a better understanding of the underlying mechanism of the cytoplasmic cargo delivery by EVs.
Keywords: cargo transfer, extracellular vesicles, membrane fusion, NanoLuc, VSV‐G
1. INTRODUCTION
Extracellular vesicles (EVs) are secreted by living cells and contain biomolecules derived from the donor cells. The physiological role of EVs remains largely unknown and they were formerly known as the “garbage bin” of cells for excretion of the unwanted molecules or organelles. Several studies have shown the cellular disposal role of EVs (Nicolás‐Ávila et al., 2020; Takahashi et al., 2017) although a vast majority of current EV research focuses on the cargo delivery of EVs. Since EVs contain cargo proteins and RNAs, their contents can be transferred from a donor cell to a recipient cell via a paracrine or endocrine mechanism. Recently, EV‐mediated cargo delivery events in pathophysiological settings, such as cancers, have attracted considerable attention. Several studies have reported that EVs are involved in tumour suppression (Putz et al., 2012; Seo et al., 2018) and tumour progression (Bobrie et al., 2012; Ye et al., 2014). Several studies have demonstrated that EVs can deliver small RNAs to recipient cells and elicit phenotypic changes. However, there is limited evidence that demonstrates cargo delivery by EVs into recipient cells (Mateescu et al., 2017). Many confounding factors in the experimental conditions and contaminants in the EV fraction (Whittaker et al., 2020) must be taken into account in the cargo delivery experiments, to prove whether EVs truly deliver their cargo into the recipient cells (we referred to this point as the “EV cargo transfer hypothesis” in the previous review (Somiya, 2020)).
The main challenge in current EV research is the lack of a feasible and reliable assay to evaluate the functional cargo delivery process in the recipient cells (Russell et al., 2019; Somiya, 2020). Several reporter assays that demonstrate the functional delivery of cargo proteins or RNAs have been reported, including miRNA (Albanese et al., 2020; Stevanato et al., 2016; Sutaria et al., 2017), Cre‐LoxP (Heath et al., 2019; Zomer et al., 2016), and CRISPR/Cas9‐gRNA (de Jong et al., 2020), and split luciferase (Somiya & Kuroda, 2021) reporters. However, these assays are influenced by various confounding factors including non‐EV components in the EV fraction. Although the readout of these assays is informative for deciphering the delivery mechanism of EVs in recipient cells, a more precise reporter assay is needed. Mechanistically, cytoplasmic cargo delivery should occur after endocytosis and subsequent membrane fusion, or direct fusion with the plasma membrane (Kalluri & Lebleu, 2020). Upon membrane fusion, the luminal side of EVs is exposed to the cytoplasm of recipient cells and releases their cargo. The functional delivery assay should reflect the biological delivery mechanism, especially the membrane fusion of EVs.
In this study, we developed a reporter assay to quantify the membrane fusion of EVs in recipient cells. In this assay, following the fusion of EVs with the cell membrane of the recipient cells, a transcription factor is released from the EVs and then upregulates the expression of a reporter gene (luciferase or fluorescence protein). This assay provides a biologically orthogonal readout and enables us to accurately interpret the cargo delivery process of EVs.
2. MATERIALS AND METHODS
2.1. Materials
The chemical reagents and antibodies used in this study are listed in Table S1. All NanoLuc substrates were purchased from Promega. The plasmids used in this study are listed in Table S2 and deposited at Addgene. Plasmids were constructed using PCR‐based methods (Gibson Assembly (Gibson et al., 2009)) and confirmed by Sanger sequencing.
2.2. Cell culture and transfection
Human embryonic kidney HEK293T cells (RIKEN Cell Bank) were maintained in 10% (v/v) fetal bovine serum (FBS)‐containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 μg/ml penicillin‐streptomycin solution. Cells were cultured at 37°C under 5% CO2 in humidified conditions.
Transfection of HEK293T cells was performed as follows: cells were plated in a cell culture dish or multi‐well plate and cultured overnight. The next day, the cells were transfected using 25‐kDa branched polyethyleneimine (PEI, Sigma). The ratio of plasmid DNA to PEI was 1: 4 (weight). After 24–96 h, the cells were used in the subsequent experiments. Cell culture supernatant was collected after 2–4 days and centrifuged at 1500 ×g for 5 min to remove cell debris.
2.3. NanoLuc assay
To quantify the expression level of the reporter NanoLuc, the transfected cells were lysed and mixed with NanoLuc substrate (Nano‐Glo Luciferase Assay System; Promega) according to the manufacturer's instructions. Luminescence signal from the cell lysate was measured by using a plate reader, Synergy 2 (BioTek).
2.4. Characterization of tTA‐fused proteins in cell lysate and EVs
Protein expression was assessed by western blotting. Briefly, lysates of the transfected cells (total protein was extracted using radioimmunoprecipitation assay [RIPA] buffer [Nacalai Tesque] containing a protease inhibitor cocktail [Nacalai Tesque]) or the supernatant was mixed with reductant‐free sample buffer and incubated at room temperature for 20 min. Proteins were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane. Proteins on the membrane were detected using antibodies (Table S1) and ImmunoStar LD reagent (FUJIFILM Wako Pure Chemical). As a loading control for cell lysates, the membrane was probed with an anti‐GAPDH antibody.
2.5. Concentration of EVs
EVs were concentrated by PEG precipitation. The 4× PEG solution (40% PEG 6000 [w/v], 1.2 M NaCl, 1 × PBS [pH 7.4]) was added to the supernatant (1:4 dilution), and kept at 4°C overnight. The next day, the supernatant was centrifuged at 1600 ×g for 60 min to pellet the EVs. After decantation, the pellet was resuspended in PBS. Typically, approximately a million donor cells were seeded and cultured for 48–96 h in a 60‐mm dish with 5 ml medium and the supernatant was concentrated to 100–200 μl of PBS solution by PEG precipitation (25 to 50‐fold enrichment).
2.6. Reporter assay
For the membrane fusion reporter assay, recipient HEK293T cells (104 cells/well in 96‐well plate) were transfected with plasmids encoding tobacco etch virus (TEV) protease (TEVp) and TRE3G‐NlucP (PEST motif‐fused NanoLuc [NlucP] (Hall et al., 2012) under tetracycline responsive element [TRE] promoter), and cultured overnight. The next day, the recipient cells were treated with donor culture supernatant or concentrated EVs and further incubated at 37°C for up to 48 h. Unless otherwise stated, 5 or 10 μl of concentrated EVs were applied to recipient cells, which is corresponding to the 250 μl of original conditioned medium, depending on the enrichment factor upon PEG precipitation (25 to 50‐fold enrichment, see above). To assess the effect of various compounds on membrane fusion efficiency, recipient cells were treated with the compound 1 h before the addition of supernatant or EVs. After the incubation, the expression of NanoLuc in the recipient cells was measured as described above.
Reporter expression in recipient cells was also evaluated using an enhanced green fluorescent protein (EGFP) gene. Recipient cells (104 cells/well in 96‐well plate) transfected with pTetOn‐EGFP (EGFP under TRE promoter) and pcDNA3.1‐TEVp were treated with EVs and then observed under a fluorescence microscope IX70 (Olympus) after 24 h. Cre recombinase‐based reporter assay was performed in the same way; recipient HEK293T cells were transfected with a reporter plasmid (encoding LoxP‐flanked mKate and EGFP under the CMV promoter) and a plasmid encoding TEVp, treated with EVs for 24 h the following day, and then observed under a fluorescence microscope.
2.7. Statistical analysis
Data were analysed using Student's t‐test or one‐way ANOVA following either post hoc Tukey's HSD or Dunnett's tests. Statistical analysis was performed using the Real Statistics Resource Pack software created by Charles Zaiontz.
3. RESULTS
3.1. Characterization of tTA‐fused tetraspanins
To establish a reporter assay that can measure the membrane fusion of EVs, we first prepared plasmids encoding human tetraspanins CD9, CD63, or CD81 with C‐terminal fusion of the TEVp cleavage site, followed by tetracycline transactivator (tTA) (Figure 1a). As shown in Figure 1b, tTA‐fused tetraspanin is cleaved in the presence of TEVp and releases the transcription activator tTA. When the EVs containing tTA‐fused tetraspanin are internalized and fused with the endosomal membrane, luminal tTA is exposed to the cytoplasmic side, and TEVp in the recipient cells cleaves the TEVp site, followed by the cytoplasmic release of tTA and induction of the reporter gene expression under the TRE promoter (Figure 1c). We designated this assay the EV‐mediated tetraspanin‐tTA delivery (ETTD) assay.
FIGURE 1.
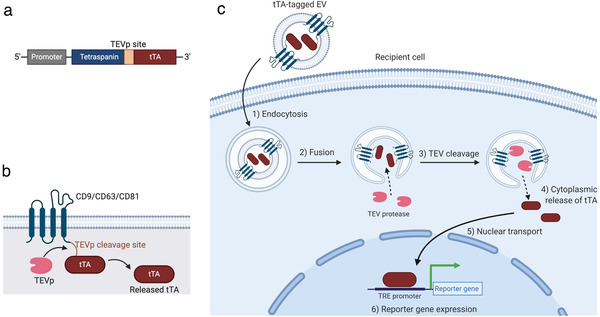
Summary of the ETTD assay. (a) Schematic representation of tTA‐fused tetraspanin. Tetraspanin and tTA flank a TEVp recognition site. (b) Topology of tTA‐fused tetraspanin protein. Upon the cleavage by TEVp, tTA is released from membrane‐anchored tetraspanin. (c) Schematic representation of the ETTD assay. EV containing tTA‐fused tetraspanin is taken up by cells by endocytosis (1), and fuses with the endosomal membrane (2). After cleavage by cytoplasmic TEVp (3), tTAs are released into the cytoplasm (4). Released tTAs are transported to the nucleus (5), and induce expression of a reporter gene under TRE promoter (6)
To demonstrate the feasibility of the above system, HEK293T cells were transfected with plasmids encoding tTA‐fused tetraspanin with or without plasmid encoding TEVp. As shown in Figure 2a, the expression of tTA‐tetraspanins in the cell lysate was confirmed by western blotting. In the presence of TEVp, tTA was cleaved and released from the tTA‐fused protein. While CD9 and CD81 showed obvious tTA bands, CD63 showed only a weak band in the absence of TEVp and no band in the presence of TEVp. This is probably due to the low expression of CD63 in HEK293T cells compared to CD9 and CD81. When HEK293T cells were transfected with both NlucP (under the TRE promoter) and tTA‐fused proteins, co‐expression of TEVp strongly induced Nluc expression (Figure 2b), suggesting that tetraspanin‐anchored tTA was unable to translocate into the nucleus, and therefore could not induce reporter gene expression. In contrast, expression of non‐fused tTA protein continually induced reporter gene expression regardless of the co‐expression of TEVp. These results suggest that tTA‐fused tetraspanins induce reporter gene expression in the recipient cells only when the cells express TEVp.
FIGURE 2.

Characterization of tTA‐fused tetraspanins. (a) Cells were transfected with plasmids encoding tTA‐fused tetraspanins and TEVp. After 48 h, cells were lysed and subjected to western blotting. Upper and lower panels represent immunoblotting using anti‐TetR antibody and anti‐GAPDH antibody, respectively. The expected mass based on the amino acid sequences were as follows: CD63‐tTA, 63.2 kDa; CD81‐tTA, 63.4 kDa; CD9‐tTA, 63.0 kDa; tTA, 36.9 kDa. (b) Expression of NanoLuc under TRE3G promoter in HEK293T cells co‐expressing tTA‐fused tetraspanins and TEVp. As controls, plasmids encoding tTA without tetraspanin fusion and empty expression plasmid were used. Numbers above the bars indicate the fold increase in NanoLuc expression compared to the mock transfection. N = 3, mean ± SD
To characterize the tTA‐fused tetraspanins in EVs, supernatants from transfected HEK293T cells were concentrated by PEG precipitation and analysed by western blotting (Figure 3a to 3c). All tTA‐fused tetraspanins were detected with corresponding antibodies. The tTA‐fused CD81 and CD9 proteins were also detected with an anti‐TetR antibody, indicating that the released EVs contain full‐length tTA‐fused CD81 or CD9, while CD63‐tTA was not clearly detected by the same setting. This might reflect the lower expression of CD63‐tTA in the donor HEK293T cells as demonstrated inFigure 2a. Additional bands seen between 37 and 50 kDa may be degraded proteins or unknown byproducts. Note that substantial contamination of PEG in the EV pellet after the precipitation made blotting deteriorate and observed bands look smear. As a control for the ETTD assay, vesicular stomatitis virus glycoprotein (VSV‐G) was co‐expressed in donor cells, as VSV‐G is known to strongly facilitate membrane fusion and subsequent cargo delivery of EVs (Albanese et al., 2020; Somiya & Kuroda, 2021; Votteler et al., 2016). VSV‐G was detected in the EV fraction, strongly suggesting that released EVs display VSV‐G on their surface along with tTA‐fused tetraspanins. Compared to the supernatant samples, EVs after PEG precipitation showed significantly stronger signals, indicating the successful concentration of EVs from the supernatant. Furthermore, calnexin, a negative marker for EVs (Théry et al., 2018), was only detected in cell lysate, suggesting that EVs are successfully isolated.
FIGURE 3.
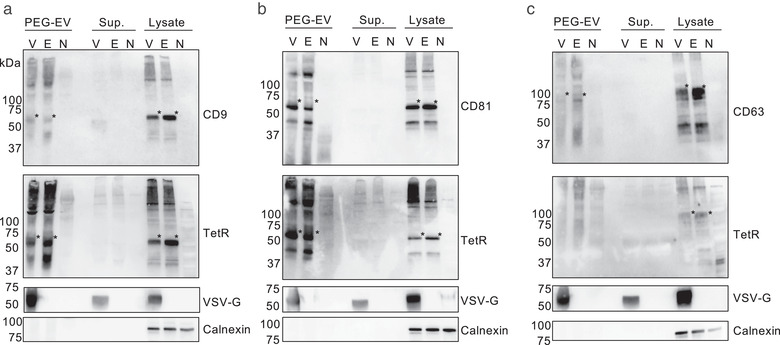
Characterization of HEK293T‐derived EVs containing tTA‐fused tetraspanins. EVs containing (a) CD9‐tTA, (b) CD81‐tTA, and (c) CD63‐tTA were analysed. EVs after the PEG precipitation (PEG‐EV), supernatant before PEG precipitation (Sup.), and total cell lysate (Lysate) were separated by non‐reducing SDS‐PAGE and probed by western blotting. Antibodies used to probe the proteins are indicated on the right. Asterisks indicate tTA‐fused tetraspanins. Other bands in tetraspanin and TetR blots are likely polymerized proteins or degraded proteins. V, tetraspanin‐tTA + VSV‐G; E, tetraspanin‐tTA + EGFP; N, no transfection. The expected mass based on the amino acid sequences were as follows: CD9‐tTA, 63.0 kDa; CD81‐tTA, 63.4 kDa; CD63‐tTA, 63.2 kDa (highly glycosylated); VSV‐G, 57.7 kDa; calnexin, 67.6 kDa (observed mass was approximately 90 kDa probably due to post‐translational modification)
3.2. Validation of ETTD assay for cargo delivery of EVs
We first attempted to assess whether the crude cell culture supernatant from donor cells was capable of inducing reporter gene expression in recipient cells. As shown in Figure 4a, treatment of recipient cells with donor supernatant containing tetraspanin‐tTA fusion protein induced reporter gene expression only when the donor cells were transfected with virus‐derived fusogenic protein VSV‐G. This result suggested that the concentration process is not necessary to evaluate EV membrane fusion in the ETTD assay if the EVs possessed potent fusogenic activity. While the supernatant containing tTA‐fused CD81 and CD9 induced a >10‐fold increase in NanoLuc expression, the supernatant containing tTA‐fused CD63 showed less induction (up to 5‐fold). This may reflect the lower expression level of tTA‐fused CD63 in the donor cells compared to CD9 and CD81 (Figure 2a).
FIGURE 4.
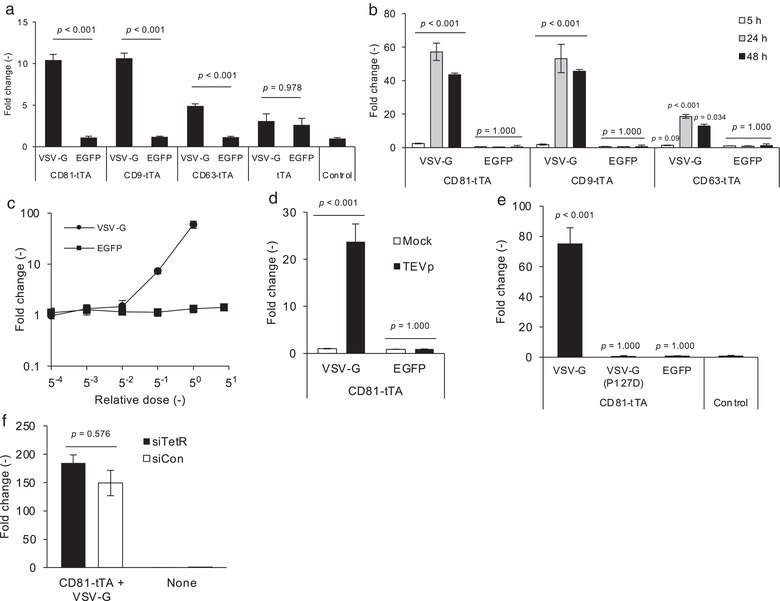
ETTD assay. (a) Donor supernatant (100 μl/well of 2 days‐culture) was applied to recipient HEK293T cells and NanoLuc expression was measured after 24 h. NanoLuc expression was normalized to the control (treatment with supernatant from non‐transfected donor cells). (b) Incubation time‐dependent expression of a reporter gene. Recipient cells were treated with concentrated EVs containing tTA‐fused CD9 or CD81 with or without VSV‐G for 5, 24, and 48 h, followed by luciferase assay. (c) Dose‐dependent reporter expression in recipient cells. Recipient cells were treated with EVs containing tTA‐fused CD81 with or without VSV‐G for 24 h. Concentrated EVs corresponding to the 250 μl of crude supernatant was set as relative dose = 1 (expressed as 50). (d) TEVp‐dependent reporter gene expression. Recipient cells with or without expression of TEVp were treated with EVs containing tTA‐fused CD81 with or without VSV‐G and subjected to the luciferase assay after 24 h. (e) Effect of fusogenicity deficit VSV‐G mutant. Recipient cells were treated with EVs (tTA‐fused CD81) displaying parental VSV‐G, mutant VSV‐G (P127D), or EGFP for 24 h. (f) Recipient cells were pre‐transfected with siRNAs targeting TetR (siTetR) or firefly luciferase (siCon), and further treated with EVs containing tTA‐fused CD81 and VSV‐G for 24 h. In this experiment, concentrated EVs corresponding to the 375 μl of crude supernatant was used. N = 3, mean ± SD. Statistical analysis was performed using one‐way ANOVA followed by post hoc Tukey's HSD (a, d, e, and f) or Dunnett's tests of each treatment group versus non‐treatment control (b)
Next, we used EVs concentrated by PEG precipitation for the ETTD assay. Recipient cells were treated with EVs for 5, 24, and 48 h, and the reporter NanoLuc expression was measured (Figure 4b). In the presence of VSV‐G in EVs, weak but substantial induction of NanoLuc expression was detected as early as 5 h and expression level reached a plateau after 24 h, while EVs without VSV‐G did not induce NanoLuc expression even after 48 h treatment. We chose CD81 for the subsequent analyses.
Induction of NanoLuc expression was dependent on the presence of VSV‐G and the dose of EVs (Figure 4c). Figure 4d indicates that expression of TEVp in the recipient cells was crucial for reporter gene expression, demonstrating that the ETTD assay worked as expected (Figure 1c). Furthermore, the EVs harbouring fusion‐deficient mutants of VSV‐G (P127D) (Fredericksen and Whitt, 1995; Votteler et al., 2016) lost the membrane fusion ability of EVs in the assay compared to the EVs harbouring parent VSV‐G (Figure 4e), strongly supporting that this assay depicted the membrane fusion‐mediated cargo delivery of EVs. Furthermore, the absence of VSV‐G led to no functional delivery (Figure 4a to 4e), indicating the poor cargo delivery efficacy of authentic EVs. In addition to HEK293T, three different cell lines, HeLa, A549, and Huh‐7 were used as alternative recipient cells, and similar results were observed, indicating that the ETTD assay is applicable to other cell lines (Figure S1A). As well as HEK293T‐derived EVs, HeLa‐derived EVs containing CD81‐tTA and VSV‐G induced the reporter gene expression in the recipient HEK293T cells, but greater amount of EVs were necessary to induce the substantial response (Figure S1B). This result demonstrated the versatility of ETTD assay for non‐HEK293T cell lines as donor cells.
There was a possibility the excess of expression plasmid (tTA‐fused tetraspanin) remaining in the supernatant or mRNA of tTA‐fused tetraspanin encapsulated in EVs may induce the reporter gene expression in the recipient cells, which could confound the bona fide reporter expression due to the tTA release of EVs. Therefore, we transfected the reporter cells with siRNA targeting TetR, the TRE‐binding domain of tTA to verify that the reporter gene expression was induced by tTA protein. First, we verified that siRNA targeting TetR (siTetR) efficiently knocked down tTA (Figure S2A). Furthermore, knockdown of tTA by siRNA significantly suppressed TRE‐mediated reporter gene expression (Figure S2B). Based on these results, siRNA targeting tTA should abrogate the confounding factors in the ETTD assay, namely, the excess of expression plasmid remaining in the donor supernatant and mRNA‐mediated expression of tTA. After transfection of siRNA into recipient cells, we applied tTA‐fused EVs to recipient cells. As shown in Figure 4f, transfection of siRNA targeting tTA showed no effect on the reporter gene expression, strongly suggesting that the assay readout of the ETTD assay was solely driven by tTA proteins, neither mRNA nor leftover plasmid DNA.
The PEG precipitation often results in the low purity of isolated EVs compared to conventional methods such as ultracentrifugation or gel filtration (Lobb et al., 2015). To exclude the possibility of the interference on the assay readout from serum‐derived contaminants or other artifacts, we compared the EVs from four different preparations (Figure S3). First, we concentrated the EVs from a serum‐containing conditioned medium by either PEG precipitation or ultracentrifugation and compared the protein contents by western blotting (Figure S3A). PEG‐EV and UC‐EV showed a similar pattern, though the band pattern in PEG‐EV was deteriorated due to the contamination of excess PEG. Compared to the supernatant samples, both PEG‐EV and UC‐EV contained a substantial amount of EV marker proteins (CD81‐tTA and VSV‐G) and no calnexin, suggested the successful concentration of EVs. Next, we examined the membrane fusion activity of four different EV preparations by ETTD assay. In this assay, we prepared EVs from serum‐containing supernatant or serum‐free medium supernatant isolated by either ultracentrifugation or PEG precipitation. Compared to the basic preparation method described so far (S+PEG), all three preparations of EVs (S+UC, SFM+UC, and SFM+PEG) can induce the reporter gene expression in the presence of VSV‐G, despite the different extent of reporter gene expression was observed (Figure S3B). Different induction level was probably due to the EV yield of each preparation method. These results demonstrated that the ETTD assay can assess the membrane fusion activity of EVs regardless of the preparation method or purity.
3.3. Effect of small molecules on the membrane fusion efficiency of EVs
As the novel ETTD assay can evaluate the membrane fusion efficiency of EVs, we next examined the effect of potential delivery enhancers and entry inhibitors in the absence of viral fusogenic protein VSV‐G. According to a previous report, chloroquine enhanced Cre protein delivery of EVs by disrupting endosomes and lysosomes using the Cre‐LoxP reporter assay (Heath et al., 2019). In our reporter assay, chloroquine treatment did not induce any reporter gene expression (Figure 5a), suggesting that chloroquine does not enhance cytoplasmic cargo delivery of EVs without VSV‐G. This is probably because chloroquine treatment induces the destabilization of endosomes/lysosomes and does not enhance membrane fusion.
FIGURE 5.
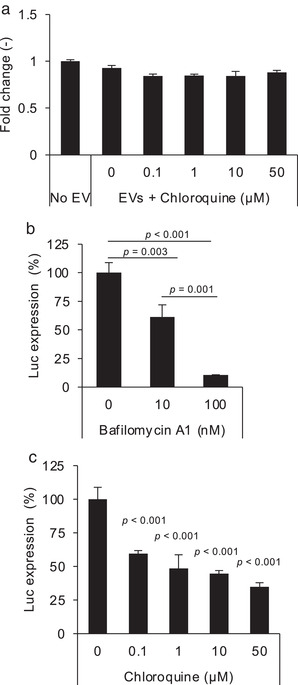
Effect of small molecule compounds on the ETTD assay. (a) EVs (CD81‐tTA) without VSV‐G were applied to recipient HEK293T cells in the presence of indicated concentrations of chloroquine. NanoLuc expression level was normalized to the value of the control (no EV treatment) and is presented as fold‐change. (b) EVs (CD81‐tTA) with VSV‐G were applied to recipient cells in the presence of 10 or 100 nM of bafilomycin A1. (c) EVs (CD81‐tTA) with VSV‐G were applied to recipient cells in the presence of 0.1 to 50 μM of chloroquine. N = 3, mean ± SD. Statistical analysis was performed using one‐way ANOVA followed by a post hoc Tukey's HSD test
In addition to potential delivery enhancers, we assessed the effect of entry inhibitors using the ETTD assay. We used VSV‐G‐modified EVs to assess the effect of compounds that are known to increase the endosomal pH and thereby inhibit the low pH‐dependent fusion activity of VSV‐G (Ci et al., 2018; Kim et al., 2017). Bafilomycin A1 is a selective ATPase inhibitor (Bowman et al., 1988) that prevents the acidification of endosomes/lysosomes and inhibits VSV infection (Yonezawa et al., 2005). When recipient cells were treated with bafilomycin A1, membrane fusion by VSV‐G‐modified EVs was significantly inhibited in a dose‐dependent manner (Figure 5b). In addition, chloroquine, which is known to prevent VSV infection by increasing endosomal/lysosomal pH (Sakata et al., 2017), also blocked the membrane fusion of EVs (Figure 5c). These results strongly support the application of ETTD assay in assessing the pharmacological effects of a potential delivery enhancer/inhibitor of EVs.
3.4. Assessment of membrane fusion efficiency of EVs at the single‐cell level
For the evaluation of EV membrane fusion at the single‐cell level, we changed the reporter gene from NanoLuc to EGFP. As shown in Figure 6a, EVs containing tTA‐fused CD81 and VSV‐G induced EGFP expression in the recipient cells, which was consistent with previous results (Figure 4). This assay enabled us to decipher membrane fusion efficiency at the single‐cell level.
FIGURE 6.
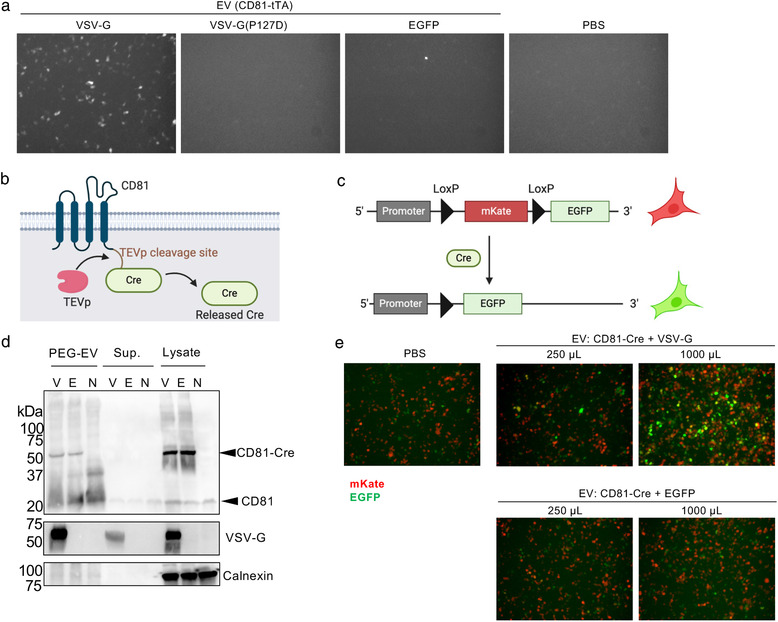
Fluorescence imaging‐based reporter assays. (a) EDDT assay based on EGFP as a reporter readout. Recipient HEK293T cells were transfected with plasmids encoding TRE3G‐EGFP and TEVp, and treated with EVs containing CD81‐tTA with VSV‐G (WT or P127D mutant) or EGFP (negative control). After 24 h, cells were observed under a fluorescence microscope. In this experiment, concentrated EVs corresponding to the 125 μl of crude supernatant was used. (b) Schematic representation of Cre‐fused CD81. (c) Schematic representation of reporter plasmid for Cre‐LoxP reporter assay. After the Cre cleavage, the mKate gene is excised and reporter cells become EGFP positive. (d) Western blotting analysis of donor EVs, supernatant, and cell lysate. Samples were run by non‐reducing SDS‐PAGE, transferred to the membrane, and probed by anti‐CD81 (upper), anti‐VSV‐G (middle), and anti‐calnexin (bottom) antibodies. V, CD81‐Cre + VSV‐G; E, CD81‐Cre + EGFP; N, no transfection. The expected mass based on the amino acid sequences were as follows: CD81‐Cre, 66.1 kDa; VSV‐G, 57.7 kDa; calnexin, 67.6 kDa (observed mass was approximately 90 kDa probably due to post‐translational modification). (e) Fluorescence imaging of Cre‐LoxP reporter assay. Recipient cells were treated with EVs harbouring CD81‐Cre with VSV‐G or EGFP. In this experiment, concentrated EVs equivalent to 250 or 1000 μl of crude supernatant were used
To further validate the general applicability of the ETTD assay, we switched the reporter gene from tTA‐dependent gene expression to the expression of a floxed gene dependent on Cre recombinase. The principle of the Cre‐mediated reporter assay is essentially the same as that of the ETTD assay; however, the readout is driven by Cre‐mediated recombination of the target gene. After the release of Cre from EV by TEVp, Cre recombinases translocate to the nucleus and induce recombination of the target plasmid (Figure 6b & 6c). In this study, we used the mKate/EGFP reporter plasmid. The recipient cells initially expressed the red fluorescence protein mKate, but after Cre‐mediated recombination, cells become EGFP positive (Figure 6c). PEG precipitation successfully enriched the EVs containing both CD81‐Cre and VSV‐G (Figure 6d). As shown in Figure 6e, EVs containing CD81‐Cre with VSV‐G induced EGFP positive cells in a dose‐dependent manner, whereas EVs without VSV‐G showed no induction of EGFP positive cells. This result was consistent with the results of the previous ETTD assay (Figure 4) and again revealed that fusogenic proteins significantly increase the membrane fusion activity of EVs.
4. DISCUSSION
In this study, we developed an ETTD assay that can evaluate the membrane fusion efficiency of EVs in recipient cells. The principle of this assay was inspired by the tango assay (Barnea et al., 2008) that quantitatively assesses receptor activation by the recruitment of genetically engineered TEVp to the receptor, subsequent release of tTA, and expression of TRE‐mediated reporter gene. In the ETTD assay, tetraspanins constrain tTA and are localized at the membrane (Figure 2b). Once the luminal tTA is exposed to the cytoplasm following membrane fusion of the EVs and release by TEVp cleavage, recipient cells express the reporter gene (Figure 4d). This experimental design has rendered the ETTD assay robust and sensitive by avoiding non‐specific background signals.
The ETTD assay enables us to quantitatively assess membrane fusion efficiency and delivery mechanism of EVs. The advantages of this assay are as follows: (1) it is highly sensitive to measure the membrane fusion of EVs with a wide dynamic range owing to the very bright NanoLuc, (2) fewer confounding factors in the ETTD assay compared to conventional assays because expression of the reporter gene under the TRE promoter is highly regulated and specific to the transcription factor tTA, which does not exist in mammalian cells; and (3) it is feasible to assess the membrane fusion efficiency in both the bulk cell population (NanoLuc reporter) and single‐cell level (fluorescence protein reporter). Previously, we reported another assay to study the EV cargo delivery (EV cargo delivery assay or EVCD assay) based on the complementation of split NanoLuc (Somiya & Kuroda, 2021). Compared to the ETTD assay developed in this study, the EVCD assay is capable to measure the real‐time kinetics of the cargo delivery process in the recipient cells. The EVCD assay is also capable to visualize the cytoplasmic cargo delivery process in live cells by using a luminescence microscope, which is currently not feasible in other methods. The EVCD assay measures the amount of EV cargos in the cytoplasm in recipient cells, while the ETTD assay directly measures the exposure of the luminal side of EVs to the cytoplasm of recipient cells, which is a proxy of membrane fusion. In other words, the ETTD assay can assess the upstream membrane fusion event, and the EVCD assay is for the downstream cargo delivery efficiency. It is noteworthy that the dynamic range of the ETTD assay (up to a 100‐fold increase of signal compared to background) is wider than the EVCD assay (typically ∼10‐fold signal increase). Thus, the ETTD assay may be more sensitive to measure a rare EV cargo delivery event. The combination of these different methods is valuable to comprehensively understand the EV cargo delivery process.
The very bright NanoLuc reporter gene enables the ETTD assay to detect rare membrane fusion events in recipient cells. Because the cargo delivery efficiency of EVs is expected to be low (possibly 0.2–10% of recipient cells express reporter gene as a result of the cargo delivery, depending on the reporter assay (de Jong et al., 2020; Zomer et al., 2015)), the assay sensitivity must be high to capture the membrane fusion events in recipient cells. When the EVs harbour the fusogenic protein, VSV‐G, EV‐mediated membrane fusion was sufficient for detection in the ETTD reporter assay, whereas no detectable membrane fusion was observed in the absence of VSV‐G (Figure 4). This result reflected the low efficiency of membrane fusion in the absence of a particular membrane fusion protein. As described in previous studies, the cargo delivery efficiency of EVs is expected to be low (de Jong et al., 2020; Hung & Leonard, 2016; Somiya & Kuroda, 2021; Stremersch et al., 2016; Sutaria et al., 2017; Wang et al., 2018). However, previous reports suggested that EVs can release the cargo into the cytoplasm by yet unknown mechanism (Bonsergent et al., 2021; Joshi et al., 2020). Further study is needed to verify the intrinsic fusion activity of EVs. Our experiments were conducted using a combination of HEK293T or HeLa donor cells and four cell lines as recipient cells (HEK293T, HeLa, A549, and Huh‐7). Other combinations of EV‐donor cells and recipient cells should be examined whether the cargo delivery efficiency is much higher in a future study.
We validated whether the ETTD assay precisely reflects tTA protein‐mediated readout rather than mRNA transfer‐dependent reporter gene expression. EVs can encapsulate overexpressed mRNA in the donor cells in a passive manner and potentially transfer the mRNA to recipient cells (Lai et al., 2015). Since it was postulated that unexpected EV‐mediated transfer of tTA mRNA may lead to a false positive signal in the ETTD assay, recipient cells were pre‐transfected with potent TetR‐targeting siRNA (Figure S2) and blocked the mRNA‐mediated readout. The results demonstrated that siRNA targeting TetR did not affect the assay readout, indicating the absence of mRNA‐dependent tTA expression and subsequent reporter gene expression in the recipient cells (Figure 4f).
Previously, membrane fusion of EVs has been evaluated by fluorescence probes (Parolini et al., 2009) or reporter proteins (Albanese et al., 2020; Votteler et al., 2016). The former approach, especially the membrane‐anchored fluorescence probes, such as R18, are known to often result in false positives due to non‐specific dye transfer between lipid membranes (Wunderli‐Allenspach & Ott, 1990). Joshi et al. developed a sophisticated fluorescence imaging technique to measure membrane fusion and cargo release of EVs in recipient cells (Joshi et al., 2020). Their approach enabled the assessment of membrane fusion at the single‐vesicle level; however, it was still difficult to distinguish the membrane fusion signal from the high background signal of the fluobodies distributed throughout the cytoplasm, and there was a limited capability in terms of throughput. The latter approach, typically using β‐lactamase (BlaM) protein, requires a long incubation time at low temperature for the enzymatic conversion of a fluorescence substrate (7–16 h (Albanese et al., 2020; Cavrois et al., 2002; Votteler et al., 2016)), possibly induce the artifact in recipient cells. The ETTD assay, in contrast, is more feasible, sensitive, and rapid to assess the membrane fusion process of EVs in recipient cells and capable the high‐throughput applications. There are conflicting reports on the effect of chloroquine on EV cargo delivery in a previous study (Heath et al., 2019). In this study, chloroquine was unable to enhance membrane fusion and cargo delivery of EVs (Figure 5a), whereas a previous study showed significant improvement in the Cre delivery of EVs. The inconsistency is probably due to the differences in the experimental settings, sensitivity, and accuracy between assays. The Cre‐LoxP reporter assay is a sensitive and robust method since the assay readout is driven by ideally a single Cre molecule in the recipient cell, and assay readout is exclusively dependent on the Cre‐LoxP excision of target DNA. The different conclusions between these studies should be carefully interpreted and further examined in a future study. Heath et al. demonstrated that small amounts of Cre recombinase (8.9 Cre‐FRB molecules per EV on average) can be passively loaded into EVs and contribute to the recombination in the recipient cells (Heath et al., 2019), whereas our approach involved fusion of the Cre protein to the tetraspanin CD81 and application to the reporter recipient cells (Figure 6b). It appears that our approach may be more convincing because the direct fusion of Cre with the EV marker protein is more reliable and precisely reflects the nature of EV‐mediated cargo transfer. Furthermore, our previous study demonstrated that treatment of recipient cells with chloroquine did not enhance the cytoplasmic cargo delivery of EVs (Somiya & Kuroda, 2021). Taken together, it is unlikely that chloroquine treatment enhances membrane fusion and cytoplasmic cargo delivery.
In addition to the tTA reporter assay, we proved that the Cre‐LoxP reporter system was feasible to assess the membrane fusion of EVs in recipient cells (Figure 6b to 6e). Although it is difficult to directly compare the sensitivity between the ETTD assay and Cre‐LoxP assay, an advantage of the Cre‐LoxP system is the irreversibility. Since the Cre‐mediated excision of a floxed sequence is irreversible, the readout of the Cre‐LoxP reporter system is more stable than the transient induction of TRE promoter‐mediated reporter gene expression. Cre‐LoxP system would be more favourable for in vivo assessment of EV‐mediated membrane fusion and cargo delivery because of the stability of readout and availability of various Cre animals. Together with the ETTD assay, the Cre‐LoxP‐based membrane fusion assay would be a valuable tool to study the membrane fusion efficiency of EVs in vitro and in vivo.
5. CONCLUSIONS
ETTD assay is a novel functional assay to assess the mechanism of EV‐mediated membrane fusion and cargo delivery in a quantitative manner. The lack of reliable functional assays in the EV field has hampered progress in its therapeutic applications (Nguyen et al., 2020) and elucidation of the underlying mechanism of cargo delivery and intercellular communication of EVs (Russell et al., 2019). The ETTD assay is potentially useful for identifying unknown factors that are responsible for the cargo delivery mechanism. Using the ETTD assay, knockout or knockdown of target genes may reveal the unknown cargo delivery pathway as described in a previous study (de Jong et al., 2020), or possibly facilitate the discovery of a methodology that enhances membrane fusion and subsequent cargo delivery of EVs. Together with the previously reported real‐time cargo delivery assay (Somiya & Kuroda, 2021), the ETTD assay may help advance fundamental EV research and its clinical applications.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGEMENTS
We extend our gratitude to Yumi Yukawa for technical assistance in plasmid construction. All illustrations in this work were created using BioRender.com. This work was supported in part by JSPS KAKENHI (Grant‐in‐Aid for Early‐Career Scientists 18K18386 and 20K15790 to MS), Research Grant from JGC‐S Scholarship Foundation (to MS), and the “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” (MEXT).
Somiya, M. , & Kuroda, S. (2021). Reporter gene assay for membrane fusion of extracellular vesicles. Journal of Extracellular Vesicles, 10, e12171. 10.1002/jev2.12171
REFERENCES
- Albanese, M. , et al. (2020). Micro RNAs are minor constituents of extracellular vesicles and are hardly delivered to target cells. bioRxiv 10.1101/2020.05.20.106393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea, G. , Strapps, W. , Herrada, G. , Berman, Y. , Ong, J. , Kloss, B. , Axel, R. , & Lee, K. J. (2008). The genetic design of signaling cascades to record receptor activation. Proceedings of the National Academy of Sciences of United States of America 105, 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie, A. , Krumeich, S. , Reyal, F. , Recchi, C. , Moita, L. F. , Seabra, M. C. , Ostrowski, M. , & Théry, C. (2012). Rab27a supports exosome‐dependent and ‐independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Research. 72, 4920–4930 [DOI] [PubMed] [Google Scholar]
- Bonsergent, E. , Grisard, E. , Buchrieser, J. , Schwartz, O. , Théry, C. , & Lavieu, G. (2021). Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nature Communications 12, 1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, E. J. , Siebers, A. , & Altendorf, K. (1988). Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proceedings of the National Academy of Sciences of the United States of America 85, 7972–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois, M. , De Noronha, C. , & Greene, W. C. (2002). A sensitive and specific enzyme‐based assay detecting HIV‐1 virion fusion in primary T lymphocytes. Nature Biotechnology 20, 1151–1154 [DOI] [PubMed] [Google Scholar]
- Ci, Y. , Yang, Y. , Xu, C. , & Shi, L. (2018). Vesicular stomatitis virus G protein transmembrane region is crucial for the hemi‐fusion to full fusion transition. Scientific Reports 8, 10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, O. G. Murphy, D. E. , Mäger, I. , Willms, E. , Garcia‐Guerra, A. , Gitz‐Francois, J. J. , Lefferts, J. , Gupta, D. , Steenbeek, S. C. , van Rheenen, J. , El Andaloussi, S. , Schiffelers, R. M. , Wood, M. J. A. & Vader, P. (2020) A CRISPR‐Cas9‐based reporter system for single‐cell detection of extracellular vesicle‐mediated functional transfer of RNA. Nature communications 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen, B. L. , & Whitt, M. A. (1995). Vesicular Stomatitis Virus Glycoprotein Mutations That Affect Membrane Fusion Activity and Abolish Virus Infectivity. Journal of Virology 69, 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, D. G. , Young, L. , Chuang, R.‐Y. , Venter, J. C. , Hutchison, C. A. , & Smith, H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods 6, 343–345 [DOI] [PubMed] [Google Scholar]
- Hall, M. P. , Unch, J. , Binkowski, B. F. , Valley, M. P. , Butler, B. L. , Wood, M. G. , Otto, P. , Zimmerman, K. , Vidugiris, G. , Machleidt, T. , Robers, M. B. , Benink, H. A. , Eggers, C. T. , Slater, M. R. , Meisenheimer, P. L. , Klaubert, D. H. , Fan, F. , Encell, L. P. , & Wood, K. V. (2012). Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chemical Biology 10.1021/cb3002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, N. , Osteikoetxea, X. , De Oliveria, T. M. , Lázaro‐Ibáñez, E. , Shatnyeva, O. , Schindler, C. , Tigue, N. , Mayr, L. M. , Dekker, N. , Overman, R. , & Davies, R. (2019). Endosomal escape enhancing compounds facilitate functional delivery of extracellular vesicle cargo. Nanomed. 14, 2799–2814 [DOI] [PubMed] [Google Scholar]
- Hung, M. E. , & Leonard, J. N. (2016). A platform for actively loading cargo RNA to elucidate limiting steps in EV‐mediated delivery. Journal of extracellular vesicles 1, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, B. S. , de Beer, M. A. , Giepmans, B. N. G. , & Zuhorn, I. S. (2020). Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano acsnano.9b10033 14(4), 4444–4455. 10.1021/acsnano.9b10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri, R. , & Lebleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I. S. , Jenni, S. , Stanifer, M. L. , Roth, E. , Whelan, S. P. J. , Van Oijen, A. M. , & Harrison, S. C. (2017). Mechanism of membrane fusion induced by vesicular stomatitis virus G protein. Proceedings of the National Academy of Sciences 114, E28–E36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, C P. , Kim, E Y. , Badr, C E. , Weissleder, R. , Mempel, T R. , Tannous, B A. , & Breakefield, X O. (2015). Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nature communications 6, 7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb, R. J. , Becker, M. , Wen Wen, S. , Wong, C. S. F. , Wiegmans, A. P. , Leimgruber, A. , & Möller, A. (2015). Optimized exosome isolation protocol for cell culture supernatant and human plasma. Journal of extracellular vesicles 4, 27031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateescu, B. , Kowal, E. J. K. , Van Balkom, B. W. M. , Bartel, S. , Bhattacharyya, S. N. , Buzás, E. I. , Buck, A. H. , De Candia, P. , Chow, F. W. N. , Das, S. , Driedonks, T. A. P. , Fernández‐Messina, L. , Haderk, F. , Hill, A. F. , Jones, J. C. , Van Keuren‐Jensen, K. R. , Lai, C. P. , Lässer, C. , Di Liegro, I. … Nolte‐‘T Hoen, E. N. M. (2017). Obstacles and opportunities in the functional analysis of extracellular vesicle RNA ‐ An ISEV position paper. Journal of extracellular vesicles 6, 1286095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, V V.T. , Witwer, K W. , Verhaar, M C. , Strunk, D. , & Balkom, B W.M. (2020). Functional assays to assess the therapeutic potential of extracellular vesicles. Journal of extracellular vesicles 10, e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolás‐Ávila, J. A. , Lechuga‐Vieco, A. V. , Esteban‐Martínez, L. , Sánchez‐Díaz, M. , Díaz‐García, E. , Santiago, D. J. , Rubio‐Ponce, A. , Li, J. L. , Balachander, A. , Quintana, J. A. , Martínez‐De‐Mena, R. , Castejón‐Vega, B. , Pun‐García, A. , Través, P. G. , Bonzón‐Kulichenko, E. , García‐Marqués, F. , Cussó, L. , A‐González, N. , González‐Guerra, A. … Hidalgo, A. (2020). A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e23. [DOI] [PubMed] [Google Scholar]
- Parolini, I. , Federici, C. , Raggi, C. , Lugini, L. , Palleschi, S. , De Milito, A. , Coscia, C. , Iessi, E. , Logozzi, M. , Molinari, A. , Colone, M. , Tatti, M. , Sargiacomo, M. , & Fais, S. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. Journal of Biological Chemistry 284, 34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz, U. , Howitt, J. , Doan, A. , Goh, C. ‐. P. , Low, L. ‐. H. , Silke, J. , & Tan, S. ‐. S. (2012). The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Science signaling 5, ra70–ra70 [DOI] [PubMed] [Google Scholar]
- Russell, A. E. , Sneider, A. , Witwer, K. W. , Bergese, P. , Bhattacharyya, S. N. , Cocks, A. , Cocucci, E. , Erdbrügger, U. , Falcon‐Perez, J. M. , Freeman, D. W. , Gallagher, T. M. , Hu, S. , Huang, Y. , Jay, S. M. , Kano, S. ‐. I. , Lavieu, G. , Leszczynska, A. , Llorente, A. M. , Lu, Q. … Vader, P. (2019). Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: An ISEV position paper arising from the ISEV membranes and EVs workshop. Journal of extracellular vesicles 8, 1684862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata, M. , Tani, H. , Anraku, M. , Kataoka, M. , Nagata, N. , Seki, F. , Tahara, M. , Otsuki, N. , Okamoto, K. , Takeda, M. , Mori, Y. Analysis of VSV pseudotype virus infection mediated by rubella virus envelope proteins. Scientific Reports 7, 11607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, N. , Shirakura, Y. , Tahara, Y. , Momose, F. , Harada, N. , Ikeda, H. , Akiyoshi, K. , & Shiku, H. (2018). Activated CD8+ T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nature Communications 9.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somiya, M. (2020). Where does the cargo go?: Solutions to provide experimental support for the “extracellular vesicle cargo transfer hypothesis”. Journal of Cell Communication and Signaling 14, 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somiya, M. , & Kuroda, S. ’. I. (2021). Real‐time luminescence assay for cytoplasmic cargo delivery of extracellular vesicles. Analytical Chemistry 93, 5612–5620 [DOI] [PubMed] [Google Scholar]
- Stevanato, L. , Thanabalasundaram, L. , Vysokov, N. , & Sinden, J. D. (2016). Investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. Plos One 11, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremersch, S. , Vandenbroucke, R. E. , Van Wonterghem, E. , Hendrix, An , De Smedt, S. C. , & Raemdonck, K. (2016). Comparing exosome‐like vesicles with liposomes for the functional cellular delivery of small RNAs. Journal of Controlled Release 232, 51–61 [DOI] [PubMed] [Google Scholar]
- Sutaria, D. S. , Jiang, J. , Elgamal, O. A. , Pomeroy, S. M. , Badawi, M. , Zhu, X. , Pavlovicz, R. , Azevedo‐Pouly, A. C. P. , Chalmers, J. , Li, C. , Phelps, M. A. , & Schmittgen, T. D. (2017). Low active loading of cargo into engineered extracellular vesicles results in inefficient miRNA mimic delivery. Journal of Extracellular Vesicles 6, 1333882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A. , Okada, R. , Nagao, K. , Kawamata, Y. , Hanyu, A. , Yoshimoto, S. , Takasugi, M. , Watanabe, S. , Kanemaki, M. T. , Obuse, C. , & Hara, E. (2017). Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Communications 8, 15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , Atkin‐Smith, G. K. , Ayre, D. C. , Bach, J. ‐. M. , Bachurski, D. , Baharvand, H. , Balaj, L. , Baldacchino, S. , Bauer, N. N. , Baxter, A. A. , Bebawy, M. … Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 8, 1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votteler, J. , Ogohara, C. , Yi, S. , Hsia, Y. , Nattermann, U. , Belnap, D. M. , King, N. P. , & Sundquist, W. I. (2016). Designed proteins induce the formation of nanocage‐containing extracellular vesicles. Nature 540, 292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, M. P. , Unch, J. , Binkowski, B. F. , Valley, M. P. , Butler, B. L. , Wood, M. G. , Otto, P. , Zimmerman, K. , Vidugiris, G. , Machleidt, T. , Robers, M. B. , Benink, H. A. , Eggers, C. T. , Slater, M. R. , Meisenheimer, P. L. , Klaubert, D. H. , Fan, F. , Encell, L. P. , & Wood, K. V. (2018)ARMMs as a versatile platform for intracellular delivery of macromolecules. Nature communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker, T. E. , Nagelkerke, A. , Nele, V. , Kauscher, U. , & Stevens, M. M. (2020). Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles. Journal of Extracellular Vesicles 9, 1807674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderli‐Allenspach, H. , & Ott, S. (1990). Kinetics of fusion and lipid transfer between virus receptor‐containing liposomes and influenza viruses as measured with the octadecylrhodamine B chloride assay. Biochemistry 29, 1990–1997 [DOI] [PubMed] [Google Scholar]
- Ye, S.‐B. , Li, Ze‐L. , Luo, D.‐H. , Huang, Bi‐J. , Chen, Yu‐S. , Zhang, X.‐S. , Cui, J. , Zeng, Yi‐X. , & Li, J. (2014). Tumor‐derived exosomes promote tumor progression and T‐cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 5, 5439–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa, A. , Cavrois, M. , & Greene, W. C. (2005). Studies of ebola virus glycoprotein‐mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: Involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. Journal of Virology 79, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer, A. , Maynard, C. , Verweij, F. . J. , Kamermans, A. , Schäfer, R. , Beerling, E. , Schiffelers, R. . M. , De Wit, E. , Berenguer, J. , Ellenbroek, S. . I. . J. , Wurdinger, T. , Pegtel, D. . M. , & Van Rheenen, J. (2015). In vivo imaging reveals extracellular vesicle‐mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer, A. , Steenbeek, S. C. , Maynard, C. , Van Rheenen, J. Studying extracellular vesicle transfer by a Cre‐loxP method. Nature Protocols 11, 87–101 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


