Abstract
Interleukin-6 (IL-6) is a major cytokine released by skeletal muscle. Although IL-6 plays complex but well-known roles in host defense, the specific contribution of skeletal muscle IL-6 to innate immunity remains unknown. We tested its functional relevance by exposing inducible skeletal muscle IL-6 knockdown (skmIL-6KD) mice to a cecal slurry model of polymicrobial peritonitis and compared responses to strain-matched controls and skeletal muscle Cre-matched controls at 3, 6, and 12 h postinfection. In both sexes, skmIL-6KD mice at 6 h of infection exhibited marked changes to leukocyte trafficking in the peritoneum, characterized by ~1.75-fold elevation in %neutrophils, a ~3-fold reduction in %lymphocytes and a ~2 to 3-fold reduction in %basophils. A similar pattern was seen at 12 h. No changes were observed in plasma leukocyte counts. Circulating cytokines in female skmIL-6KD mice at 6 h consistently showed modest reductions in IL-6, but marked reductions in a broad range of both pro- and anti-inflammatory cytokines, e.g., TNFα and IL-10. In both sexes at 12 h, a generalized suppression of plasma cytokines was also seen after the effects of Cre-induction with raloxifene were addressed. There were no significant effects of skmIL-6KD on mortality in either sex. Collectively, our results are consistent with skmIL-6 playing an important and previously unrecognized role in immune cell trafficking and cytokine regulation during septic shock.
Keywords: Cecal slurry, diaphragm, myokine, raloxifene, septic shock, sexual dimorphism
INTRODUCTION
Interleukin-6 (IL-6) is one of the most prevalent cytokines expressed in response to skeletal muscle contraction (1). Experiments sampling blood across the vasculature or in the interstitial space of the human leg during exercise reveal net secretion of IL-6 (2, 3). This discovery shifted our paradigm regarding the function of skeletal muscles from solely movement-related activities to include possible endocrine and immune functions (4). Because of the linkage of skeletal muscle IL-6 secretion to exercise, most research on its isolated function, to date, has focused on the role skeletal muscle IL-6 plays in regulating metabolism (5, 6); whereas, much less is known about other functions.
One potential role of skeletal muscle IL-6 is in host defense. It is well accepted that IL-6 plays important, yet complex, roles in immunity. These roles include the transition from innate to adaptive immunity, modulation of inflammatory cell trafficking at the site of infection (7), and initiation and regulation of the acute phase responses (8). IL-6 is also known to be produced by skeletal myofibers in response to exposure to pathogen-associated-molecular-patterns like lipopolysaccharides (LPS) (9) and to other stress stimuli that are common in conditions of severe illness, such as heat shock (10, 11), catecholamines (12, 13), and endoplasmic reticular stress (14). Since skeletal muscle comprises ~40% of lean body mass in both man and mouse (15), the potential contributions of skeletal muscle IL-6 knockdown (skmIL-6) to host defense are substantial.
In this study, we tested the hypothesis that skeletal muscle IL-6 production contributes to net circulating IL-6 during exposure to septic peritoneal infection. Furthermore, we hypothesized that the IL-6 arising from skeletal muscle affects immune cell trafficking, expression of other circulating cytokines and the ability to fight the underlying infection. To test these hypotheses, we utilized an inducible skeletal muscle-specific IL-6 knockdown mouse, exposed to severe intraperitoneal polymicrobial sepsis. Our results demonstrate profound effects on immune cell trafficking at the site of infection and broad influences on circulating cytokines, particularly in females at 6 h.
MATERIALS AND METHODS
Animal care and handling
All protocols were approved by the University of Florida Institutional Animal Care and Use Committee. Animals were bred, in house, or purchased from The Jackson Laboratory (naive mice used in mortality experiments and for cecal slurry production) and singly housed at the University of Florida Animal Care Facilities. The temperature and relative humidity of the vivarium ranged from 20°C to 25°C and 31% to 67%, respectively. Mice were under a 12:12-h light–dark cycle and had access to standard chow (2,918, Envigo) and automatic tap water ad libitum. All reported information about the procedures conformed to the Animal Research Reporting of in vivo Experiments— ARRIVE guidelines (16).
Muscle-specific conditional IL-6 knockdown
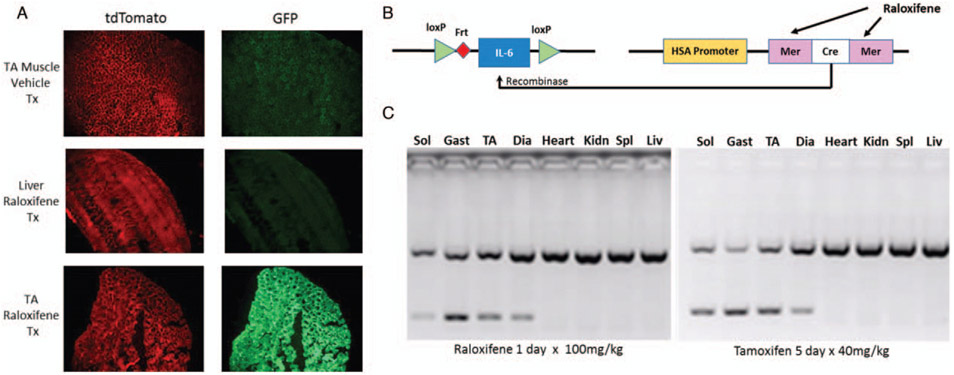
We obtained embryos of floxed IL-6 mice (IL-6lox/lox) which were developed on a C57BL/6 background (17). The mice were bred with a muscle-specific inducible Cre mouse developed by McCarthy et al. (18), and provided by Dr Karyn Esser. The Cre construct employs a human skeletal muscle α-actin promoter (HSA) and a mutated estrogen receptor ligand binding domain to activate the gene (HSA-CreERT2/−), also on a C57BL/6 background (Fig. 1B). Cre expression with this construct is specific to skeletal muscles following exposure of the animal to an exogenous selective estrogen receptor-activating drug like tamoxifen or raloxifene. Mice were bred in colonies to produce male and female experimental mice, i.e., IL-6lox/loxCreERT2/−, which we will refer to as “skmIL-6” and separate sets of CreERT2/−, which we will refer to as “Cre” mice. For experiments, both strains were treated with either tamoxifen, raloxifene, or a matched vehicle-injected intraperitoneally (IP) to activate the recombinase, as described below and illustrated in Figure 1.
Fig. 1. Study design.
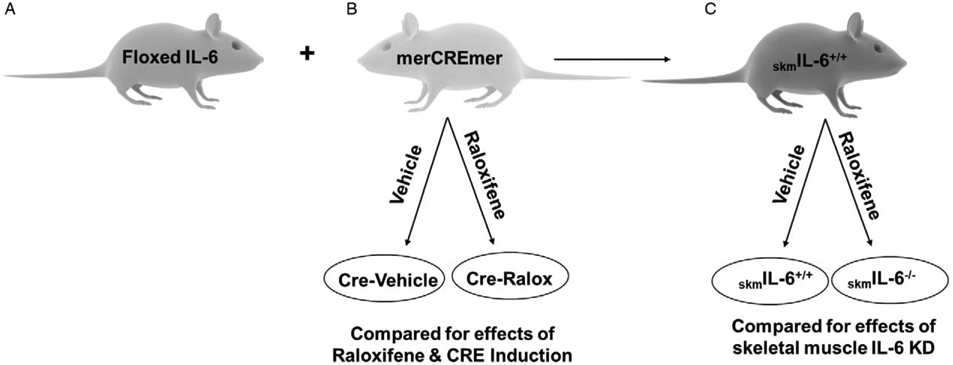
(A) Floxed IL-6 mice (IL-6lox/lox) were bred with (B) alpha actin merCREmer mice (HSA-CreERT2/−) to form (C) homozygote floxed SkmIL-6+/+ mice with a heterozygote HSA-CreErt/+/−. Groups B and C were then randomly divided into raloxifene treatment (to induce Cre) or vehicle treatment to produce a total of four experimental groups. Comparisons within group B were used to test for effects of raloxifene treatment and CRE induction. Comparisons in Group C were used to test for effects of SkmIL-6 knockdown. SkmIL-6 indicates skeletal muscle interleukin-6, IL; HSA, human skeletal muscle α-actin promoter.
In initial experiments, we utilized two common tamoxifen doses to induce Cre recombination, 80 mg/kg IP (19), or 40 mg/kg IP (20) dissolved in sterile sunflower oil, daily for 5 consecutive days. However, concerns regarding drug effects of tamoxifen emerged including gut and heart injury (21-23) effects on immunity (24-26), and survival during infection (27). Therefore, we developed a protocol for raloxifene administration to induce the CreERT2/− which has previously been reported to reduce the toxic effects observed with tamoxifen (21, 22). All mice reported in this study were treated with either a single injection of raloxifene, 110 mg/kg, prepared in polyethylene glycol 400 (PEG400) at 55 mg/mL and combined in a suspension with equal parts of deionized water (DIH2O) or PEG400 and DIH2O as vehicle. We observed no apparent toxicity or ill effects to this treatment. We allowed mice to recover from raloxifene injections for at least 4 weeks prior to sepsis experiments. To test for the effectiveness of raloxifene in inducing the Cre in muscle, we injected Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter mice (28) with raloxifene, as described above and evaluated the distributions of green fluorescent protein (GFP) and tdTomato using fluorescent microscopy.
Cecal slurry protocol
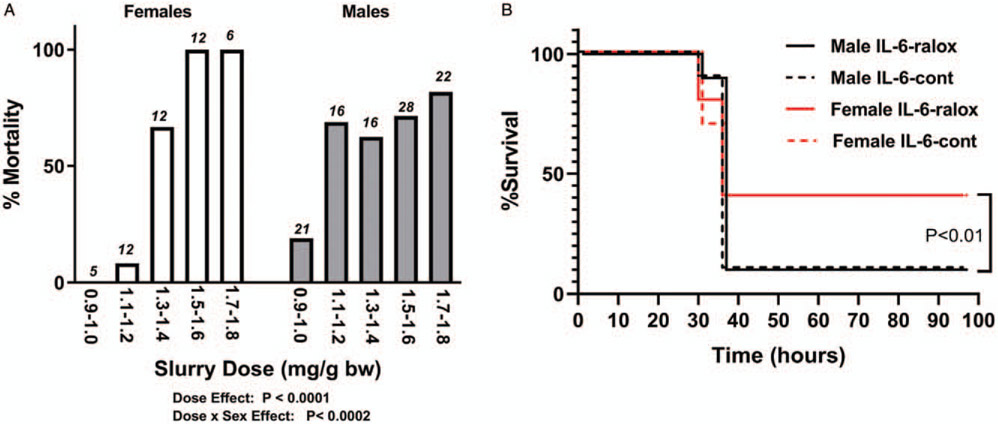
To induce sepsis we utilized a cecal slurry model previously described (29). In short, large numbers of donor mice were used to generate a pool of diluted cecal contents. These were mixed in a 15% glycerol/85%PBS to a concentration of 100mg slurry/mL. The slurry was aliquoted into ~2 mL cryovials and frozen at −80° C within Mr Frosty freezing containers (Thermo Fisher Scientific, Waltham, MA) for later use. Vials were allowed to thaw at room temperature and were vortexed immediately before an intraperitoneal injection was performed. Inoculation was performed on the lower right quadrant of the abdominal cavity. All mice were singly housed. To reduce animal suffering when large groups of mice were given sepsis, e.g., to determine rates of mortality, we developed a sensitive and specific method of predicting death based on reductions in body surface temperature, measured at the xiphoid process (30). Mortality rates were then determined for male and female mice at slurry doses ranging from 1.0 mg/g to 1.8 mg/g of mouse. For all the remaining studies, an equivalent dosage was used that we predicted would result in 40% to 60% mortality over 5 days in each sex, i.e., 1.6 mg/g in females and 1.2 mg/g in males. In all experiments, the xiphoid temperature was monitored every 6 to 12 h prior to death.
Polymicrobial sepsis and sample collection
For all experiments, mice were injected with cecal slurry between 2:00 am and 5:00 am and then specimens were collected at 3, 6, or 12 h post. Sixty minutes prior to sample collection, mice were injected with 0.5 cc of subcutaneous sterile warm saline, which resulted in more reliable collection of blood samples. At the targeted collection time, mice were anesthetized under isoflurane and 2 mL of sterile PBS was injected into the peritoneum for collection of a peritoneal lavage sample. Mice were then placed in a supine position and a sample of arterialized blood collected in EDTA from the heart using a caudal transcardiac stick, just below the xiphoid process. In most cases > 0.6 mL of blood was collected. Whole blood and peritoneal lavage samples were immediately run in a hematology analyzer (Heska Element HT5) for differential cell counts using laser impedance and colorimetric technologies. Blood samples were then centrifuged at 2,000 g for 10 min at 4°C and the plasma was immediately snap frozen and stored at −80°C for later analyses of cytokines and chemokines.
Plasma cytokine and chemokine concentrations were measured using Luminex Magpix multiplex analyzer (25 Plex MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel, Millipore). We followed manufacturer’s instructions for sample preparation. In short, plasma samples were diluted six times in serum matrix prior to analysis. Following 18-h incubation at 4°C with magnetic beads coated with primary antibodies, we washed the microplate twice with wash buffer. Thereafter, detection antibodies were added to the wells and incubated for 1 h at room temperature. Finally, streptavidin was added to each well for 30-min incubation. Magnetic beads were then resuspended in assay buffer and run on the MagPix system.
Experimental design and statistical analysis
For 3, 6-, and 12-h time points, experiments were run as four matched groups, parallel in time, i.e. skmIL-6/raloxifene, skmIL-6/vehicle, Cre/raloxifene, Cre/vehicle, 10 animals per group, male and female (Fig. 1). Initial analyses of variance demonstrated strong differences between the responses of the two strains of mice, Cre and skmIL-6, independent of raloxifene treatment. Since this comprised a large source of variance in these populations, which interfered with detection of differences within groups, and because we were not interested in strain differences, we treated the Cre mice (with and without raloxifene) and the skmIL-6 mice (with and without raloxifene) as completely separate groups. We used the Cre mice to determine any effects of raloxifene or recombinase induction on the immune response and the skmIL-6 mice to determine the effects of skeletal muscle fiber IL-6 knockdown.
All statistics were performed with SAS JMP Pro 14.1. For each variable we identified outliers from sample groups where all of the cytokine measurements were below or above detection limits suggesting a blood sample error or by Dixon Q test for parametric and nonparametric outlier detection (31), set at the two sided, P = 0.01 level. This method detected only very extreme outliers and no more than one sample per group was eliminated. Less than 0.5% of the total data was removed by outlier detection and the same approach was used on all variables. Each population was then tested for normality using the Shapiro–Wilk test. When one or more populations were non-parametric, we utilized the Wilcoxon signed-ranks test. Otherwise, we used the two-sample t test or Welch’s two-sample t test, for populations of equal or unequal variances, respectively. Thus, the data were handled as preplanned comparisons between matched groups. To avoid errors due to multiple comparisons, the P values were tested for significance after Benjamini-Hochberg analyses, using a false discovery rate threshold level of <15%. When appropriate, we evaluated mortality using parameterized Kaplan–Meier survival analyses factors, with sex, treatment, and crossed effects being included in parameterization analyses. For comparisons of dose effects on mortality rates in males and females we utilized a nominal logistic fit with effects of sex, dose, and crossed effects evaluated. Any other statistical approaches are outlined in the figure legends.
RESULTS
Raloxifene treatment induces Cre recombination in skmIL-6KD
Skeletal muscles from Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice treated with vehicle (PEG400) did not display Cre recombination (Fig. 2A). Raloxifene treatment was effective in inducing skeletal muscle-specific Cre recombination in skeletal muscle of Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice. Sections of Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J liver did not reveal GFP, which is consistent with lack of Cre recombination. These experiments confirmed that our raloxifene treatment protocol was effective to induce Cre recombination, specifically in skeletal muscle fibers. Polymerase chain reaction analyses confirmed IL-6 knockdown induced by raloxifene, in soleus, gastrocnemius, tibialis anterior, and diaphragm. Importantly, the extent of knockdown was similar to that observed with tamoxifen treatment at doses traditionally employed (Fig. 2C). The IL-6 gene was preserved in other tissues such as heart, kidneys, spleen, and liver.
Fig. 2. Raloxifene induction of Cre transgene.
A, Effects of vehicle versus raloxifene treatment on GFP expression using ROSA mice (B6.129(Cg)-Gt(Rosa)26Sortm4(ACTB-tdTomato,-EGFP)/Lou/J) in the tibialis muscle and liver. B, Schematic of the conditional muscle-specific IL-6 knockdown used in the experiments. C, PCR genotyping of conditional knockdown in representative skeletal muscles and liver in raloxifene treated and tamoxifen treated mice. Identical contrast was applied to the fluorescent images across all tissues, using Power Point software. IL indicates interleukin.
Effects of skmIL-6KD on leukocyte trafficking at the site of infection
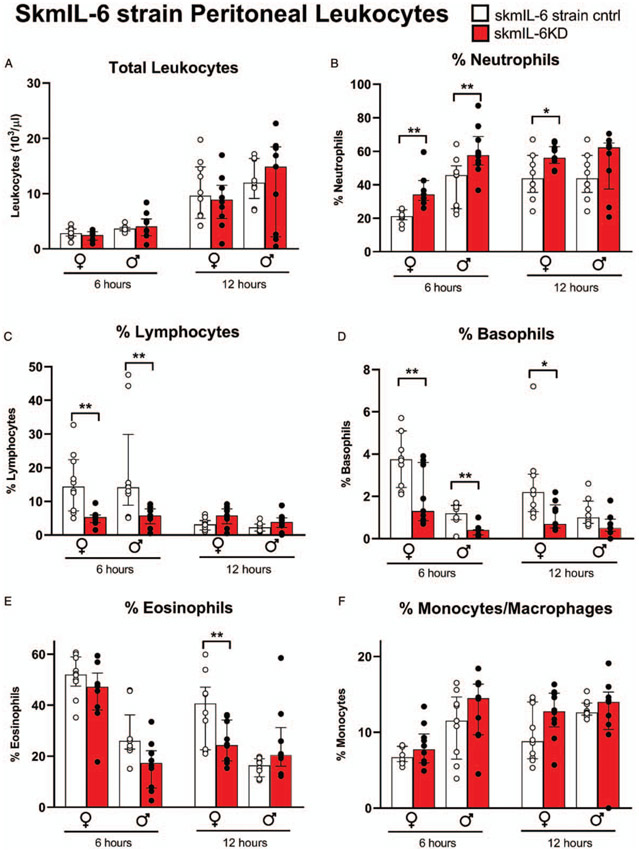
Marked changes in the leukocyte differential counts were observed within the peritoneal lavage (i.e., the site of infection). Total white blood cell counts in the peritoneum were not significantly affected by skmIL-6KD (Fig. 3A). However, at 6 h post-cecal slurry injection, we observed marked increases in peritoneal %neutrophils (Fig. 3B), reductions in %lymphocytes (Fig. 3C), and reductions in %basophils (Fig. 3D) in both male and female mice. In female mice there was a similar response of peritoneal neutrophils and basophils at 12 h; whereas, in males, any apparent changes did not reach statistical significance at this time point. These observations were mirrored in the absolute differential counts in females at 6 h, where lymphocytes averaged only ~0.25 of controls (P < 0.003) basophils averaged ~0.35 of controls (P < 0.0003), and neutrophils averaged ~1.36 of controls (P = 0.1), data not shown. Absolute cell counts in the lavage in males followed a similar pattern but did not reach statistical significance. In females, a reduction in %eosinophils was also observed at 12 h in skmIL-6KD mice (Fig. 3E). In the blood samples, there were no significant effects of skmIL-6KD on blood differential counts at any time point in either males or females (Supplement Fig. S1, http://links.lww.com/SHK/B110).
Fig. 3. Effects of skmIL-6 knockdown on immune cell recruitment to the peritoneum.
SkmIL-6 mice treated with vehicle; gray bars: skmIL-6KD mice 3 to 4 weeks following raloxifene treatment. Time points are 6 and 12 h post-cecal slurry IP injection. Following ANOVA, groups were tested by orthogonal comparisons within sex and strain matched controls. * = P < 0.05, ** = P < 0.01. n = 9–10 in each group. Data expressed as medians ± 25% to 75% quartiles. SkmIL-6KD indicates skeletal muscle interleukin-6 knockdown; IP intraperitoneally.
Because of concerns regarding the impact of estrogen receptor activity on the immunological responses to sepsis (25, 26), we also measured responses in matched pairs of mice sharing only the Cre-gene, with and without raloxifene treatment. There were no significant effects of raloxifene/Cre induction in the peritoneal samples on total leukocyte counts or on any specific leukocyte subtypes, as shown in the Supplement Figure S2, http://links.lww.com/SHK/B110. There were also no significant effects of Cre induction on blood leukocyte counts (data not shown).
To further resolve the time course of the response of the transgenic mice to cecal slurry injection, we repeated the experiments in all four groups of mice at the 3 h time point. The data can be found in the supplementary Tables S1, S2, and S3, http://links.lww.com/SHK/B110. There were no significant effects of skmIL-6 KD at this time point on peritoneal lavage cell populations (Table S1). There were almost no effects of skmIL-6 KD on plasma cytokines in either males or females (Tables S2, S3). In males IL-15 and in females IL-13 reached statistical significance, which we did not attribute any biological significance.
Effects SkmIL-6KD on circulatory cytokines and chemokines
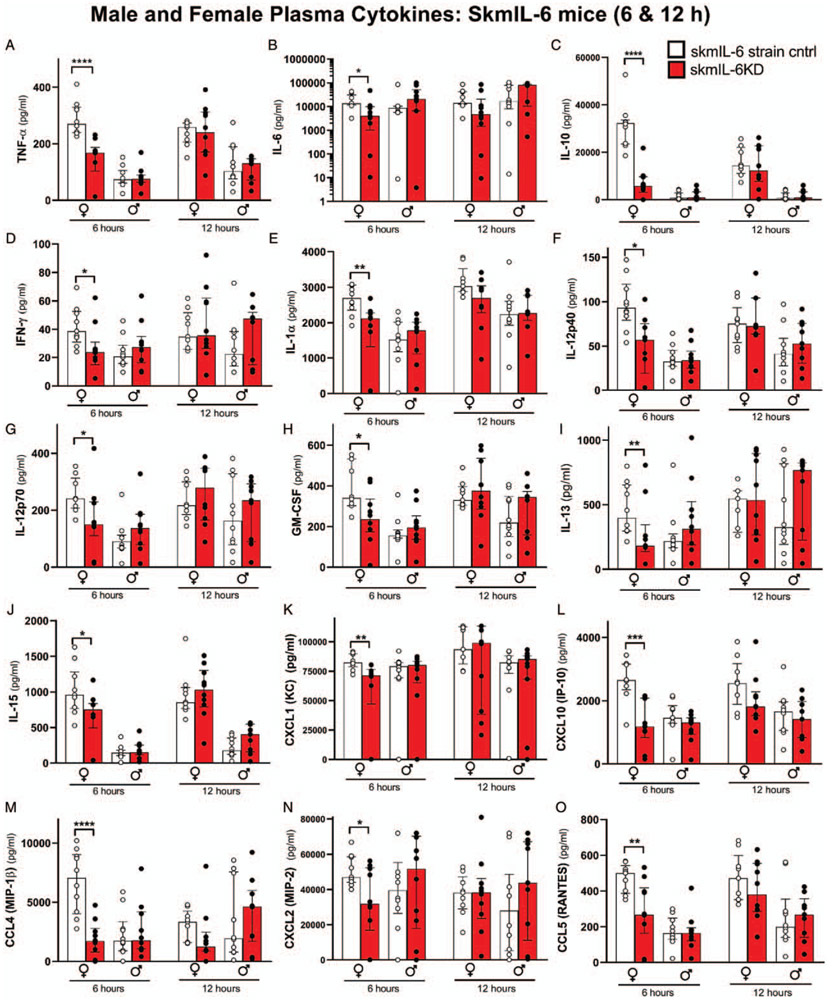
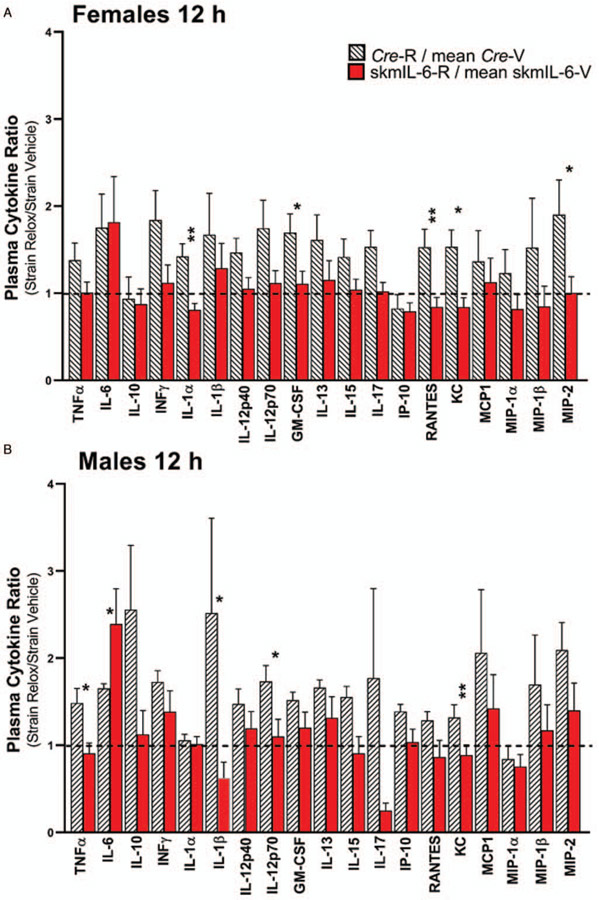
Female skmIL-6KD mice showed consistent reductions in circulating cytokines involved in the early (6 h) innate immune response (Fig. 4). This generalized response carried over to nearly every measured cytokine and chemokine. Interestingly, skmIL-6KD resulted in only a modest reduction in circulating IL-6 (Fig. 4B). Male mice did not show significant alterations in plasma cytokines at either the 6 or 12 h time point. The circulating cytokine responses in the Cre strain showed no significant effects of raloxifene treatment at 6 h (Supplement Fig S3, http://links.lww.com/SHK/B110). However, at 12 h, raloxifene-treated mice tended to have elevations in circulating cytokines compared with vehicle-treated mice, e.g. (IFNγ, IL-1β, IL-5). Because of these background raloxifene-Cre induction influences, we utilized a correction at the 12 h timepoint for this effect by calculating the ratios of the individual responses of the skmIL-6 KD as a fraction of the average strain control and comparing these to the ratio of the responses in the raloxifene treated Cre mice as a fraction of their vehicle control. The results for both males and females are illustrated in Figure 5. As shown, most of the skmIL-6KD mice had lower cytokine ratios than their Cre counterparts with a number of the comparisons reaching statistical significance.
Fig. 4. Effects of skmIL-6KD on representative circulating cytokines/chemokines.
Data shown females and males at 6 and 12 h after cecal slurry injection. White bars = SkmIL-6 strain control, red bars = SkmIL-6KD. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** P < 0.0001. n = 9–10 in each group. Data expressed as medians ±25% to 75% quartiles. SkmIL-6KD indicates skeletal muscle interleukin-6 knockdown.
Fig. 5. Results of circulating cytokines at 12 h post cecal slurry.
(A) Females and (B) males. To account for effects of raloxifene and Cre induction on the response this data is represented as fractions of the effects of raloxifene-Cre induction in Cre mice (hatched bars) versus effects of raloxifene-Cre induction in skmIL-6strain (i.e., skmIL-6KD). The raw data can be observed in both males and females in Supplemental Figs. S3 and S4, http://links.lww.com/SHK/B110. *= P < 0.05, ** = P < 0.01. n = 8–10 in each group. SkmIL-6KD indicates skeletal muscle interleukin-6 knockdown.
Effects of skmIL-6KD on Mortality
Male and female mice, in general, had different relationships between cecal slurry dose/body weight and mortality as shown in Figure 6A. Female mice appeared to be less sensitive to low doses of slurry, but once a threshold was reached, nearly all died. In contrast, the males were more sensitive to lower doses and once a threshold was reached, a death rate of ~70% to 80% was sustained, regardless of dose. This outcome in males resembles the results described in the original model by Starr et al. (29). Using a refined approach to reduce suffering during mortality evaluations (30), we identified no significant effects of skmIL-6KD on mortality rates in either males or females (Fig. 6B). However, female mice of the skmIL-6 strain, at the doses used here, had a lower mortality rate than males (P < 0.01). These sex differences in mortality were not seen in identical studies done in male and female Cre mice, where there were no effects of either sex or Cre induction on mortality (data not shown).
Fig. 6. Mortality responses.
A, Effects of sex on the dose response to cecal slurry in wild-type C57/Bl6 mice. Above each bar are the number of animals tested at each dose. P values obtained from multivariate nominal logistic analysis. B, Effects of skmIL-6 knockdown on survival to cecal slurry. Females received higher doses. No significant effects of skmIL-6KD were observed in either sex but striking differences in mortality were observed between sex. n = 10 animals in each group (total n = 40). SkmIL-6KD indicates skeletal muscle interleukin-6 knockdown.
DISCUSSION
The results demonstrate that suppression of skeletal muscle IL-6 during the early responses to severe polymicrobial sepsis results in an altered pattern of leukocyte trafficking at the site of infection and a generalized suppression of circulatory cytokines. The results do not support a major role of skeletal muscle myofibers in providing a substantial and sustained quantity of circulating IL-6 during septic shock. However, the data do support a role for skeletal muscle IL-6 in orchestrating the timing of immune cell trafficking in the peritoneum and modulating overall circulating cytokine responsiveness.
Leukocyte trafficking
The pattern of leukocyte recruitment and retention observed in the peritoneal lavage of skmIL-6KD mice is entirely aligned with what is currently known about the role of IL-6 in directing immune cell trafficking at the site of infection (32). A key inflammatory response to bacterial infection is the early recruitment of neutrophils, which is classically considered pivotal for the first line of defense against invading pathogens (33). Inadequate neutrophilic response results in difficulty eliminating the infection, and eventually, impaired wound healing (34). Conversely, excessive neutrophil migration or reduced clearance may lead to exaggerated and possibly damaging inflammatory responses, i.e., systemic inflammatory response syndrome (SIRS) (34). We observed that skmIL-6KD mice exhibited a marked increase in the percentage of neutrophils in the peritoneal cavity, suggesting that when skeletal muscle IL-6 functions normally, it somehow attenuates the flux of neutrophils into the site of infection, or contributes to an accelerated rate of neutrophil clearance. Both responses are well-known effects of the IL-6/JAK/STAT3 signaling pathway (7, 32, 35).
In contrast to the response of neutrophils, peritoneal lymphocyte numbers were consistently reduced in the skmIL-6KD mice, 6 h after sepsis. This result ascribes a reverse role of skeletal muscle IL-6 in facilitating lymphocyte movement into the infection site and/or promoting lymphocyte survival. Again, this response is consistent with well-known IL-6 effects in promoting adhesion of lymphocytes on venules (36-38) and increasing migration into the site of infection (35). Overall, these actions of IL-6 would ultimately facilitate the transition from innate to acquired host defense.
The other major impact seen in the skmIL-6KD mice was a marked reduction in basophils in the peritoneum, suggesting that endogenous skmIL-6 in sepsis is somehow directly or indirectly influencing basophil migration. Basophils can improve survival in murine peritoneal sepsis via enhancement of bacterial clearance (39). Though they comprise a very small proportion of the total number of leukocytes in the peritoneum (1%–6%, Fig. 3D), recent studies have revealed that they have an unexpectantly potent effect in resolving infection and elevating intraperitoneal and circulating levels of TNFα (39). For example, knockout of basophil TNFα expression during peritonitis drops peritoneal TNFα to ~1/5 and plasma TNFα to ~1/3 of control levels (39).
One of the difficulties in understanding these results is how the secretory behavior of a remote organ (i.e., skeletal muscle) could influence specifically the population of immune cells trafficking into the peritoneum. It is particularly curious how this effect could happen in male mice in which demonstrable changes in circulating IL-6 were not seen at any time point studied. We have considered several hypotheses. One was that skeletal muscle IL-6 secretion could be a very early response that was missed at the 6 or 12 h collection time points. We tested this hypothesis in groups of 10 male and female mice at 3 h, post-cecal slurry injection as shown in Tables S1, S2, and S3. However, there were no significant differences in circulating IL-6 and no notable differences in other circulating cytokines or inflammatory cell populations in the peritoneum at this time.
A second hypothesis involves the unique potential of the diaphragm to contribute to inflammatory cell trafficking into the peritoneum. Intraperitoneal fluid primarily exits the peritoneal cavity via stomata on the peritoneal surface of the diaphragm and then into diaphragmatic lymphatic lacunae (40), eventually flowing through a variety of thoracic and abdominal lymphatic nodes and ducts into the circulation. Consider that in response to cecal slurry injection into the peritoneum, the diaphragm would be exposed to nearly all the septic fluid being cleared from the peritoneal space. Thus, the potential for toll-like receptor stimulation in the diaphragm during peritonitis is great. The primary pathway for neutrophils and lymphocytes to enter the peritoneum is believed to be through “high endothelial venules” which are vascular structures within the lymph nodes and vessels (41, 42). The extensive network of lymphatic vessels in the diaphragm, its direct communication with the interstitial compartment around the muscle fibers, the probable exposure of the fibers to high concentrations of pathogens in this area, and the network of abdominal and thoracic lymphatics that lead from the diaphragm make this muscle uniquely positioned to influence leukocyte trafficking into the peritoneum. Finally, the diaphragm itself is the site of intensive localization of inflammatory cells, particularly monocytes, on the peritoneal surface during peritonitis induced by LPS (43). A potential immunological role for the diaphragm muscle fibers via cytokine and chemokine secretion in severe peritonitis warrants further investigation.
The cytokine/chemokine response
Another surprising outcome of these experiments was the marked suppression of essentially all circulating cytokines and chemokines in skmIL-6KD mice, particularly in females at the 6 h time point but with some indication of a similar response at 12 h in both sexes. We predicted that if a substantial quantity of circulating IL-6 was reduced in the skmIL-6KD mice, we would observe an elevation in pro-inflammatory cytokines like TNFα (44) and IL-1β (45), a reduction in anti-inflammatory cytokines like IL-10 (46) and mixed effects on chemokines (47, 48). Instead, we observed a global suppression of circulating cytokines and chemokines (both inflammatory and anti-inflammatory).
We have considered several alternative hypotheses for this unusual observation as well. One possibility is the influence of skeletal muscle IL-6 on acute phase protein (APP) secretion in the liver and in skeletal muscle (49, 50). IL-6, through JAK/STAT3 signaling, is a primary stimulus for APP transcription (51). An early surge in IL-6 coming from skeletal muscle could elevate serum amyloid A protein (SAA) secretion (49), which behaves as a damage-associated molecular pattern, interacting with toll-like receptors, increasing generalized cytokine responses (52). In this secondary way, IL-6 could stimulate a wide range of circulating cytokines during sepsis, from a wide variety of organs and cells. However, we tested for effects of skmIL-6 KD in both males and females on liver and muscle SAA1 and found no significant effects over time (data not shown). Another hypothesis centers on the greatly reduced basophil concentrations in the peritoneum in skmIL-6KD mice (Fig. 3D). As previously mentioned, recent studies have emphasized the critical importance of these cells in promoting inflammation and resolving infection during peritoneal sepsis (39). Their presence contributes to the largest fraction of total TNFα found in both the peritoneal space and in the circulation during murine septic shock (39). A marked reduction in basophils (as observed in Fig. 3D for both sexes) could therefore reduce circulating TNFα. As a consequence, this would reduce any TNFα-stimulated cytokines. We cannot rule out that there may have been baseline differences (e.g., before sepsis induction) in peritoneal cell populations and plasma cytokine measures in the skmIL-6 KD versus their strain controls. The data collected at 3 h (Tables S1, S2, and S3) suggest that at least early in the development of sepsis there were few differences between skmIL-6 KD and their strain controls, or with the Cre strain and its raloxifene-treated strain control. Therefore, if there were significant baseline differences it would seem unlikely that they could account for the large changes in these variables seen at 6 h into sepsis.
Finally, the differences in cytokine responses in male and female mice are difficult to explain. One possibility is the differences in regulation of the IL-6 gene through the effects of estrogen. Several investigators have demonstrated that estrogen receptor activation interferes with NFκB transignaling on the IL-6 gene (53, 54). It is possible that this background inhibition lowers the IL-6 signal sufficiently in all tissues that the effects of knockdown within muscle become more evident. Nevertheless, the values for circulating IL-6 in males and females are not strikingly different in this model (Fig. 4B). Another possibility is the larger basophil populations in the peritoneum of all female versus male mice during sepsis. At the 6 h timepoint the percentage of basophils is nearly four times higher in females than males (Fig. 3D). If cytokines in plasma are reflecting inflammatory events in the peritoneum as shown by Piliponsky et al. (39), then perhaps in males, peritoneal basophils are not having proportionately as high of an effect on plasma IL-6 or other cytokines compared with females.
Lack of effects on mortality
Global IL-6 KO or anti-IL-6 treatment in mice can either increase mortality (55, 56), decrease mortality (57, 58), or have no effect (59, 60). Thus, considering the wide range of reported outcomes with global knockout, it is not surprising that we saw no significant effect of eliminating a single tissue source of IL-6 (e.g., skeletal muscle) on mortality. The mortality outcome may reflect in part the severity of the model. The cecal slurry model used here develops sepsis very rapidly, over the course of several hours. Animals generally die or resolve the infection within 24 to 36 h (30) and our mortality rates at these doses were high. There are, no doubt, numerous comorbidities that may be only indirectly related to resolution of the bacterial infection, such as when SIRS reaches a point of no return or when dehydration and hypothermia contribute to the deterioration of the animals’ health. These comorbidities may not be affected by subtle changes in cytokine signaling, yet they may have ultimately determined the fate of the animals. We utilized the model as it was originally described (29), prior to more recent recommendations that bring this and other models more closely aligned with conditions that resemble the human patient (61, 62). It is possible in a more physiological or prolonged model that the skmIL-6 response could have a greater influence on the ability of the mice to resolve massive infection and survive.
CONCLUSIONS AND IMPLICATIONS
Our data lend support to a contributing role of skmIL-6 in host defense, but probably not by greatly affecting the overall levels of circulating IL-6 that we expected, but rather by orchestrating the behavior of immune cell trafficking in the peritoneum. What is the significance of muscle? Epidemiological studies have observed that sedentary versus minimally active individuals are approximately ~2.4 times more likely to die from sepsis (63), but the mechanistic links between exercise conditioning, muscle health, and a healthy immune system are not yet understood. This study suggests that, when considering the many functional roles of skeletal muscle, it is reasonable to consider its role in contributing to effective innate immunity. How aging, exercise, and various disease states influence this new element of immune regulation, which could be called the “myoimmune axis” is a new vector of research.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr Shannon M. Wallet for her technical contributions.
This work was supported by NIH R01GM118895-0 (TLC) and the BK and Betty Stevens Endowment (TLC). JH was supported in part by Ministerio de Economía y Competitividad y Fondo Europeo de Desarrollo Regional SAF2014-56546-R and RTI2018-101105-B-I00. The MerCreMer mouse line was provided by a kind donation of Dr Karyn Esser, University of Florida.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.shockjournal.com).
REFERENCES
- 1.Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van der Ven-Jongekrijg J, Van der Meer JW: Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. J Appl Physiol 79(5):1497–1503, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK: Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537(2):633–639, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosendahl L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J: Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol 98:477–491, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Welc SS, Clanton TL: The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp Physiol 98(2):359–371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knudsen JG, Gudiksen A, Bertholdt L, Overby P, Villesen I, Schwartz CL, Pilegaard H: Skeletal muscle IL-6 regulates muscle substrate utilization and adipose tissue metabolism during recovery from an acute bout of exercise. PLoS One 12(12):e0189301, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molinero A, Fernandez-Perez A, Mogas A, Giralt M, Comes G, Fernandez-Gayol O, Vallejo M, Hidalgo J: Role of muscle IL-6 in gender-specific metabolism in mice. PLoS One 12(3):e0173675, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C: IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 24(1):25–29, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Arras D, Rose-John S: IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol 64(6):1403–1415, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Frost RA, Nystrom GJ, Lang CH: Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol 285(5):R1153–R1164, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Welc SS, Clanton TL, Dineen SM, Leon LR: Heat stroke activates a stress-induced cytokine response in skeletal muscle. J Appl Physiol 115(8):1126–1137, 2013. [DOI] [PubMed] [Google Scholar]
- 11.King MA, Leon LR, Morse DA, Clanton TL: Unique cytokine and chemokine responses to exertional heat stroke in mice. J Appl Physiol 122(2):296–306, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Welc SS, Morse DA, Mattingly AJ, Laitano O, King MA, Clanton TL: The impact of hyperthermia on receptor-mediated interleukin-6 regulation in mouse skeletal muscle. PLoS One 11(2):e0148927, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattingly AJ, Laitano O, Clanton TL: Epinephrine stimulates CXCL1 IL-1α, IL-6 secretion in isolated mouse limb muscle. Physiol Rep 5(23):e13519, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welc SS, Judge AR, Clanton TL: Skeletal muscle interleukin-6 regulation in hyperthermia. Am J Physiol Cell Physiol 305(4):C406–C413, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Griffin GE, Goldspink G: The increase in skeletal muscle mass in male and female mice. Anat Rec 177(3):465–469, 1973. [DOI] [PubMed] [Google Scholar]
- 16.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG: Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintana A, Erta M, Ferrer B, Comes G, Giralt M, Hidalgo J: Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav Immun 27(1):162–173, 2013. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA: Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet Muscle 2(1):8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kivelä R, Salmela I, Nguyen YH, Petrova TV, Koistinen HA, Wiener Z, Alitalo K: The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat Commun 7:13124, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feil S, Valtcheva N, Feil R: Inducible Cre mice. Methods Mol Biol 530:343–363, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA: Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105(1):12–15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC: Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142(1):21–24. e7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Cheng Y, Luan Y, Zhong W, Lai H, Wang H, Yu H, Yang Y, Feng N, Yuan F, et al. : Short-term tamoxifen treatment has long-term effects on metabolism in high-fat diet-fed mice with involvement of Nmnat2 in POMC neurons. FEBS Lett 592(19):3305–3316, 2018. [DOI] [PubMed] [Google Scholar]
- 24.Faulkner L, Altmann DM, Ellmerich S, Huhtaniemi I, Stamp G, Sriskandan S: Sexual dimorphism in superantigen shock involves elevated TNF-alpha and TNF-alpha induced hepatic apoptosis. Am J Respir Crit Care Med 176(5):473–482, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Behjati S, Frank MH: The effects of tamoxifen on immunity. Curr Med Chem 16(24):3076–3080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corriden R, Hollands A, Olson J, Derieux J, Lopez J, Chang JT, Gonzalez DJ, Nizet V: Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun 6:8369, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuebler JF, Jarrar D, Toth B, Bland KI, Rue L, Wang P, Chaudry IH: Estradiol administration improves splanchnic perfusion following trauma-hemorrhage and sepsis. Arch Surg 137(1):74–79, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45(9):593–605, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H: A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One 9(12):e115705, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laitano O, Van Steenbergen D, Mattingly AJ, Garcia CK, Robinson GP, Murray KO, Clanton TL, Nunamaker EA: Xiphoid surface temperature predicts mortality in a murine model of septic shock. Shock 50(2):226–232, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dean RB, Dixon WJ: Simplified statistics for small numbers of observations. Anal Chem 23(4):636–638, 1951. [Google Scholar]
- 32.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ: IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 181(3):2189–2195, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Ma AC, Kubes P: Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost 6(3):415–420, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ: The role of neutrophils in severe sepsis. Shock 30(7):3–9, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Jones SA: Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 175(6):3463–3468, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Wang WC, Bruce R, Li H, Schleider DM, Mulbury MJ, Bain MD, Wallace PK, Baumann H, Evans SS: Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity 20(1):59–70, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Vardam TD, Zhou L, Appenheimer MM, Chen Q, Wang WC, Baumann H, Evans SS: Regulation of a lymphocyte-endothelial-IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. Cytokine 39(1):84–96, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC, Brown KA: IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol 105(1):112–119, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piliponsky AM, Shubin NJ, Lahiri AK, Truong P, Clauson M, Niino K, Tsuha AL, Nedospasov SA, Karasuyama H, Reber LL, et al. : Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol 20(2):129–140, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Hijleh MF, Habbal OA, Moqattash ST: The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat 186(pt 3):453–467, 1995. [PMC free article] [PubMed] [Google Scholar]
- 41.Buscher K, Wang H, Zhang X, Striewski P, Wirth B, Saggu G, Lütke-Enking S, Mayadas TN, Ley K, Sorokin L, et al. : Protection from septic peritonitis by rapid neutrophil recruitment through omental high endothelial venules. Nat Commun 7:10828, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ager A: High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front Immunol 8:45, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim KE, Koh Y-J, Jeon B-H, Jang C, Han J, Kataru RP, Schwendener RA, Kim J-M, Koh GY: Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol 175(4):1733–1745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK: Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 17(8):884–886, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK: IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 101(2):311–320, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK: IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 285:E433–E437, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, et al. : Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6(3):315–325, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S: The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813(5):878–888, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Langhans C, Weber-Carstens S, Schmidt F, Hamati J, Kny M, Zhu X, Woller-sheim T, Koch S, Krebs M, Schulz H, et al. : Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PLoS One 9(3):e92048, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwaniec J, Robinson GP, Garcia CK, Murray KO, de Carvalho L, Clanton TL, Laitano O: Acute phase response to exertional heat stroke in mice. Exp Physiol; 2020. doi: 10.1113/EP088501. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW: Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut 34(1):41–45, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song C, Hsu K, Yamen E, Yan W, Fock J, Witting PK, Geczy CL, Freedman SB: Serum amyloid A induction of cytokines in monocytes/macrophages and lymphocytes. Atherosclerosis 207(2):374–383, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Liu K, Bodenner DL: Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor kappaB transactivation. Cytokine 31(4):251–257, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Stein B, Yang MX: Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol 15(9): 4971–4979, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deutschman CS, Cereda M, Ochroch EA, Raj NR: Sepsis-induced cholestasis, steatosis, hepatocellular injury, and impaired hepatocellular regeneration are enhanced in interleukin-6 −/− mice. Crit Care Med 34(10):2613–2620, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Leon LR, White AA, Kluger MJ: Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol 275(1 pt 2):R269–R277, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Fang CH, Hasselgren PO: Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regulatory Integrative Comp Physiol 281:R1013–R1023, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Barton BE, Jackson JV: Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun 61(4):1496–1499, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM: Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol 289(4):R1048–R1053, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remick DG, Bolgos G, Copeland S, Siddiqui J: Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun 73(5): 2751–2757, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stortz JA, Raymond SL, Mira JC, Moldawer LL, Mohr AM, Efron PA: Murine models of sepsis and trauma: can we bridge the gap? ILAR J 58(1):90–105, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steele AM, Starr ME, Saito H: Late therapeutic intervention with antibiotics and fluid resuscitation allows for a prolonged disease course with high survival in a severe murine model of sepsis. Shock 47(6):726–734, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams PT: Inadequate exercise as a risk factor for sepsis mortality. PLoS One 8(12):e79344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.