Abstract
Objective:
To investigate the association of maternal body mass index (BMI) and recurrent pregnancy loss
Design:
Systematic review and meta-analysis
Setting:
Not applicable
Patients:
3833 women with recurrent pregnancy loss and 4083 controls
Intervention:
Studies were identified through a PubMed, Embase, Scopus and Cochrane search.
Main outcome measure:
The primary outcome of interest was maternal BMI. The results of the meta-analysis were reported as the mean difference with a 95% confidence interval (CI)
Results:
892 studies were reviewed. Pooled data from 25 studies suggest that the maternal BMI of women with a history of recurrent pregnancy loss is significantly higher than the BMI of controls, mean difference 0.7 kg/m2 [95% CI 0.2- 1.3].
Conclusion(s):
These findings support an association between maternal BMI and recurrent pregnancy loss. Large prospective studies are needed to evaluate the influence of maternal BMI on pregnancy outcomes in women with RPL.
Keywords: Recurrent Pregnancy Loss, Recurrent Miscarriage, Obesity, Body Mass Index
Capsule:
Maternal body mass index is significantly higher in women with recurrent pregnancy loss compared to controls.
INTRODUCTION
Recurrent pregnancy loss (RPL), defined as the spontaneous loss of two or more clinical pregnancies is a devastating disease and is estimated to affect 5% of couples hoping to grow their family (1-7). Despite having a wide prevalence, the mechanisms underlying RPL remain incompletely understood, with more than 50% of RPL cases unexplained (8).
There is an established link between risk of RPL and maternal underweight and obese state (9-12). Based on data from 2011-2012 in the United States, one in three women of reproductive age was obese (13) and the obesity pandemic is on the rise, worldwide. There are also significant racial and socio-economic disparities associated with obesity (14).
There are several areas of research that suggest mechanisms by which changes in BMI may influence pregnancy loss. Increased adiposity has been shown to disrupt the hypothalamic-pituitary-ovarian axis and steroidogenic activity in the ovary through decreased insulin sensitivity and increased inflammation (12, 15). Further, animal studies suggest inappropriate meiotic progression and meiotic spindle defects in oocytes (16). Together, these data suggest that obesity may affect reproductive outcomes by interfering with normal oocyte development, embryo development (17), or by a disrupted endometrium (18, 19). The suboptimal reproductive outcomes associated with BMI has been studies in donor oocyte IVF treatment (20,21)
The available studies evaluating the association between BMI and RPL present conflicting results due to differences in study design, varying definitions of RPL and BMI ranges and the final reproductive outcomes of interest.
The association of RPL and subtle changes in maternal BMI are not well studied. Given the large proportion of women affected, gaining a more comprehensive understanding of the influence of BMI on reproduction is pivotal. This may help to further explain the mechanisms driving idiopathic RPL. Establishing whether difference in BMI is associated with RPL may allow for the development and implementation of new interventions to prevent and treat RPL.
We, therefore, aim to perform a systematic review and meta-analysis to evaluate the association between mean differences in maternal BMI and RPL.
MATERIALS AND METHODS
The conduct and reporting of this systematic review closely adhered to guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (22).
Search strategy
A systematic search strategy was created for the concepts of recurrent pregnancy loss and body mass index. The search strategies were launched in PubMed (MEDLINE) 1946-, Embase (Elsevier) 1947-, Scopus (Elsevier) 1823-, and the Cochrane Library (Wiley). The search strategies for the Embase, Cochrane, and Scopus, databases were adapted from the MEDLINE search strategy. All databases were searched back to their inception and no language or date limits were applied. Searches were completed June 2019. The full strategies are available in Supplemental figure 1. All results were exported to Rayyan. The automatic duplicate finder was applied, and duplicates were removed, resulting in a total of 892 unique citations. No additional studies were identified by reviewing the references of included studies.
Study selection criteria
Studies that compared a cohort of women with a history of RPL to controls and reported body mass index in both groups were included. There were no language restrictions applied in the study identification phase; however, only articles with a full English translation were included in the final analysis. Data in the abstract form only were excluded. Randomized controlled trials were excluded.
Data Extraction and Risk of Bias:
The results of the systematic search were thoroughly reviewed independently by three authors (EH, AE, DM). Data from included studies were then extracted for study design, study location, and year of publication. The definition of recurrent pregnancy loss was noted. Patient characteristics including age and body mass index were also extracted. The primary outcome was the mean difference in BMI between women with RPL and controls. A subgroup analysis was performed for two or more versus three or more miscarriages. A second subgroup analysis was performed to compare the mean age in the RPL and control groups.
Risk of bias assessment was performed by two authors separately (DM, AE) and described in Supplemental figure 1. The Newcastle-Ottawa quality assessment scale for case control studies was used to evaluate the study quality. A total of nine points can be awarded to any study where a maximum of one star for each category within the selection and exposure categories, and a maximum of two stars can be given for comparability.
Ethical approval
Institutional review board approval was not required due to study design and lack of identifiable data.
Statistical methods
Using the meta and metafor packages in R, we produced forest, funnel, and meta-regression plots comparing the mean differences of age and BMI between RPL and controls for each analysis set of studies. The forest plots summarize RPL and control groups with counts, means, and standard deviations. The between-group mean difference is displayed visually and numerically with the mean difference and confidence interval and used random effect weights in the calculation of the composite statistics. A random effects model was used to meta-analyze the data due to the variability within the studies and between the studies.
RESULTS:
A flow diagram of the systematic review (PRISMA template) is shown in Figure 1. Of 892 articles identified in the initial searches, 860 underwent full-text assessment. Of these, 28 trials were included in the qualitative analysis. The study characteristics are detailed in Table 1. Three studies appeared to be conducted at the same site using the same group of participants (cases and controls), but with different study designs and date, so we included the most recent study with the largest number of participants. Another study was also excluded from metanalysis as the standard deviation of mean BMI was not mentioned. A retrospective study of 306 participants provided separate data based on two different ethnicities within Chinese women, therefore, we included this as two separate studies in the meta-analysis (23).
Figure 1.
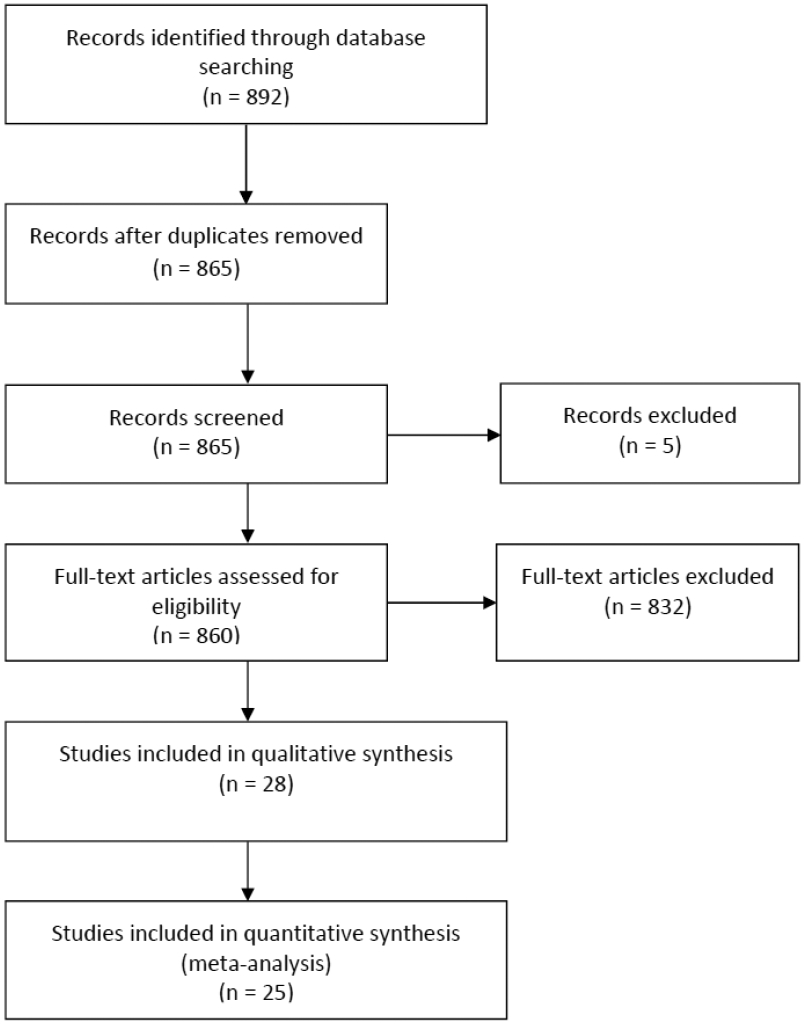
PRISMA flow diagram of studies identified in the systematic review. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
Table 1.
Description of included studies
| Author, Country, Year |
Study Setting |
Study recruitment duration, Number of study sites |
RPL definition | RPL (n) |
Control (n) |
Primary Outcome |
Description of Participants | Description of Comparator |
|---|---|---|---|---|---|---|---|---|
| Ahmed, S, Bahrain (2015) | Outpatient OB/GYN clinics | Jan 12-Apr 13, Single site | 3 or more consecutive early pregnancy losses | 275 | 290 | Association of RPL with serum CRP and genetic variation in CRP gene | Non-pregnant women mean age 31.6 [5.4], with u- RPL. Additional exclusion criteria included women over 40 years at first pregnancy, Rh incompatibility, preeclampsia and biochemical pregnancy. | Women, mean age 31.6 [4.9], with at least two live births, no personal or family history of miscarriage, preeclampsia, ectopic pregnancy or preterm delivery. Controls were matched to cases according to age and self-identified ethnic origin. |
| Almawi, W, Bahrain (2013) | OBGYN clinics | Jan 11 – Apr 12, Single site | 3 or more consecutive miscarriages before 24 weeks | 296 | 305 | VEGF polymorphism in RPL | Non-pregnant women mean age 31.6 [5.4], with u- RPL. | Age matched, multiparous women, mean age 31.6 [4.9] with no previous miscarriages and at least two LB. |
| Al-Shaikh, F, Bahrain, (2013) | OBGYN clinics | NM, Single site | 3 or more consecutive miscarriages before 24 weeks | 287 | 308 | Protien Z variants in I-RPL | Non-pregnant women mean age 31.6 [5.4], with u- RPL. | Age matched, multiparous women, mean age 31.7 [3.9] with no previous miscarriages and at least two LB. |
| Al-Khateeb, G, Bahrain (2011) | Outpatient OB/GYN clinics | NM, Two sites | 3 or more consecutive early pregnancy losses | 282 | 289 | Association of RPL with IL-18 genotyping | Non-pregnant women mean age 31.6 [5.4], with u- RPL. Additional exclusion criteria included women over 40 years at first pregnancy, Rh incompatibility, preeclampsia and biochemical pregnancy. | Women, mean age 31.7 [4.5], with at least two live births, no personal or family history of miscarriage, preeclampsia, ectopic pregnancy or preterm delivery. Controls were matched to cases according to age and self-identified ethnic origin. |
| Bagheri, A, India (2017) | ART clinic | NM, Single site | At least 2 pregnancy losses before 20 weeks | 90 | 70 | Investigate the relationship between serum level of VEGF and URM | Non-pregnant women mean age 30.6 [6.3], with u- RPL. | Age matched women, mean age 28.9 [5.8], without history of recurrent abortion with at least one LB. |
| Bahia, W, 2017, Tunisia | OB/GYN clinics | Jan 2014-Apr 2016, two sites | 3 or more miscarriages | 396 | 361 | Genetic variation in progesterone receptor gene in RPL | Non-pregnant women mean age 32.4 [6.2], with u- RPL. | Hospital employees or volunteer women, mean age 36.1 [7.9] with 2 or more natural pregnancies |
| Bennett, S, UK, 2014 | RM Clinic | Mar 11 – Oct 12, Single site | 3 or more miscarriages ≤14 weeks or 1 or more miscarriages >14 weeks | 50 | 41 | Pro-coagulation potential in RPL | Non-pregnant women mean age 36.4 [5.3], with u- RPL. | Parous women, mean age 34.9 [5.6], with no miscarriages. |
| Bussen, S, Germany, 1999 | RM Clinic | NM, Single site | 3 or more miscarriages | 42 | 42 | Endocrine abnormalities in RPL | Non-pregnant women, mean age 33.2 [4.2] with u- RSA | Nulligravid women attending ART clinic, mean age 33.3 (4.7] with no previous miscarriage, and no clinical evidence of endocrine abnormality. |
| Cao, Y, China, 2013 | Maternal and Child Health Center | NM, Two sites | At least two consecutive pregnancy losses before 20 weeks | 94 | 169 | Hemostasis-related gene polymorphism in RPL | Women, mean age 28.4 [3.7] with RPL | Ethnically matched healthy women, mean age 28.1 [3.6] with regular menstrual cycles, at least one naturally conceived pregnancy and no history of pregnancy loss or other pregnancy complication |
| Chin, J, USA, 2013 | OB/GYN tissue bank | NM, Two sites | At least two consecutive pregnancy losses before 20 weeks | 99 | 108 | Leptin receptor polymorphism in RPL | Non-pregnant women mean age 30.6 [5.1], with u- RPL. | Women, mean age 30.5 [4.8] with a history of at least two live births and no pregnancy losses |
| Comba, C, Turkey, 2015 | Gyn and Infertility clinics | NM, Single site | 2 or more consecutive failed clinical pregnancies | 21 | 20 | Inflammatory mediators in RPL | Non-pregnant women mean age 36.4 [5.3], with u- RPL. | Fertile women, mean age 28 [2.6], who had regular menstrual cycles with a history of at least one live birth, no history of abortion or infertility, and who were admitted for annual gynecologic examination. |
| Dundar, O, Turkey, 2015 | OBGYN clinic | Jan 01 – Jan 14, Single site | 3 or more consecutive first trimester miscarriages, two or more second third trimester fetal loss combined with at least one first-trimester miscarriage | 60 | 60 | RBC and Platelet distribution width in RPL | Women, mean age 27 [5.2], with history of RPL. | Healthy parous women, mean age 27.6 [5.3], with no history of previous miscarriage. |
| Eser, A, Turkey, 2016 | OBGYN clinic | NM, Single site | 2 or more miscarriages prior to 12 weeks | 42 | 36 | Carboxypeptidase B2 in RPL | Caucasian women mean age 33.3 [7.9], with RPL and normal thrombophilia panel test. | Healthy Caucasian women mean age 32.7[4], who had no history of miscarriage or obstetric morbidity. |
| Granfors, M, Sweden, 2012 | OBGYN clinics | Apr 09 – Jun 10, Four sites | 3 or more verified consecutive miscarriages in the first or second trimester of pregnancy (5–21 completed weeks of gestation). | 188 | 391 | Phosphodiesterase 8B gene polymorphism in RPL | Women, mean age 30.1[5.8], with RPL | Age matched women, mean age 30.1 [5.8], with no previous history of miscarriage and 74.9% had at least two spontaneous pregnancies, including the ongoing pregnancy, resulting in LB. |
| Ispasoiu, CA, Romania, 2013 | Obstetrics and Fertility clinics | Jan 11 – Dec 12, Single site | 2 or more pregnancy losses | 65 | 53 | High Fasting Insulin Levels and Insulin Resistance | Women, mean age 30.1 [4.9], with RPL. | Women, mean age 29.3 [5.2], with no pregnancy loss, with at least one live birth. |
| Jiao, Y 1, China, 2016 | OBGYN clinics | 2012-2014 | 3 or more consecutive pregnancies prior to 20 weeks | 154 | 155 | Toll-like receptor 4 gene in Uygur women with RPL | Women, mean age 35.2 [3.7], with u- RPL. | Age-matched healthy women, mean age 35.1 [4.5] with no history of abortions or fertility treatments. |
| Jiao, Y 2, China, 2016 | OBGYN clinics | 2012-2014 | 3 or more consecutive pregnancies prior to 20 weeks | 152 | 151 | Toll-like receptor 4 gene in Han women with RPL | Women, mean age 35.6 [4.1], with u- RPL. | Age-matched healthy women, mean age 35.7 [3.8] with no history of abortions or fertility treatments. |
| Krause, M, Germany & Switzerland, 2005 | Obstetric clinics | Jan 98-Dec 03, Four sites | 3 or more abortions < 23 gestational weeks with the same partner | 133 | 133 | Lipoprotein (a) and other prothrombotic risk factors in RPL | Caucasian women, median age 29 [range=17-40], with u- RM | Age-matched healthy women, median age 28.5 [range=18-40], who had delivered at least one child without complications and who had no history of spontaneous abortion. |
| Li, L, China, 2018 | OBGYN in-patients and outpatient clinics | Jan 14 – May 16 | 2 or more spontaneous abortion; the couple without abnormal karyotype or thrombotic diseases. | 129 | 116 | Polymorphism in promoter region of MMP2 and MMP9 in RPL. | Women, mean age 28.1 [4.5], with u- RPL. | Women, mean age [27.4 [4.2], with history of normal pregnancy without complications in the age of 17 to 43 years. |
| Li, S, China, 2017 | Maternal and Child Care Service Centre | Jan 15 – Dec 15 | 2 or more miscarriages less than 12 weeks | 80 | 100 | TNF-α in decidual tissue and peripheral blood in RPL | Women, mean age 29.03 [4.4], with u-RSA. | Women, mean age 28.5 [5.2], with a normal early pregnancy but who voluntarily decided to terminate the pregnancy |
| Park, H, Korea, 2019 | Obstetrics and Fertility clinics | Mar 99-Feb 10, Two sites | At least 2 consecutive pregnancy losses | 375 | 276 | MicroRNA polymorphism in miR-150 and miR-1179 in RPL | Women, mean age 33.02 [4.2], with idiopathic RPL. | Pregnant women, mean age 33.01 [5.3], previous regular menstrual cycles, history of LB, no history of pregnancy loss, and karyotype 46, XX. |
| Pekcan, M, Turkey, 2017 | Infertility OP clinic | Feb 15 – Jan 16 | 2 or more clinically diagnosed unexplained pregnancy loss before 20 weeks | 45 | 41 | ADAMTS-3, −13, −16, and −19 levels in RPL | Women, median age [range=20-45], with u- RPL. | Women, median age 31 [range=21-41], with at least two healthy children, regular menstrual cycles, requesting contraception, no history of recurrent miscarriage, no acute or chronic illness, and no drug use. |
| Romero, S, USA, 2016 | OBGYN and Internal Medicine clinics | NM, Two sites | 2 or more clinically diagnosed unexplained pregnancy loss before 20 weeks | 117 | 117 | Serum fructosamine and RPL | Women, mean age 30.1 [4.5], with idiopathic RPL. | Women, mean age 30.1 [4.6], with at least one LB and no miscarriage or major medical obstetric history. |
| Sater, M, Bahrain, 2011 | OBGYN clinics | Oct 07 – May 09, Single site | 3 or more miscarriages before 12 weeks | 265 | 283 | Anti-PZ IgM and IgG level in RPL | Non-pregnant women mean age 31.6 [5.4], with u- RPL. | Age matched, multiparous women, mean age 31.7 [4.5], with no previous miscarriages. |
| Sater, M, Bahrain, 2012 | OBGYN clinics | Feb 10 – Oct 10, Single site | 3 or more miscarriages with the same partner | 277 | 288 | Anti-β2 GP1 antibodies in RPL | Non-pregnant women mean age 31.6 [5.4], with u- RPL. | Age matched, multiparous women, mean age 31.7 [4.5], with no previous miscarriages. |
| Sharshiner, R, USA, 2013 | OBGYN and Internal Medicine clinics | NM, Two sites | 2 or more clinically diagnosed unexplained pregnancy loss before 20 weeks | 116 | 116 | Celiac disease serum markers and RPL | Women, mean age 30.1 [4.4], with idiopathic RPL. | Women, mean age 30.1 [4.5], with at least one LB and no miscarriage or major medical obstetric history. |
| Trifonova, E.A, Russia, 2019 | Genetic clinics | 2010-2014, Single site | At least 2 or more miscarriages before 20 weeks | 253 | 339 | Angiogenesis and endothelial dysfunction related gene variants in RPL | Women, mean age 29.5 [4.5], with idiopathic RPL | Women, mean age 27.3 [4.6], with at least 2 live births and no history of miscarriage. |
| Xu, Z, China, 2017 | Gyn clinic | Aug 16-Sep 16, Single site | 3 or more consecutive miscarriages before 24 weeks | 30 | 30 | Expression of LRH-1 in RPL | Women, mean age 27.6 [2.9], in early pregnancy after a diagnosis of u-RPL | Women, mean age 27.2 [1.5], in early pregnancy with no previous history of miscarriages. |
| Zahraei, M, Iran, 2014 | Infertility clinic | NM, Single site | 2 or more miscarriages with no previous LB | 100 | 100 | Sulf1 gene polymorphism in RPL | Women, mean age 30.9 [5.1], with u- RM. | Age-matched healthy women, mean age 29 [4.4] with two LB and no history of abortions or fertility treatments. |
A total of 10 studies presented results from gene polymorphism studies, nine from angiogenesis and hematological factors, three from autoantibody assessment and four from assessment of endocrine factors. Overall, three studies were from North America, six from Europe, 12 from the Middle East, and seven from Asia. All studies had pre-specified inclusion and exclusion criteria.
RPL was defined as a history of two or more pregnancy losses in 14 trials and as a history of three or more pregnancy losses in 11 trials.
Synthesis of Results
A total of 7916 women were included in the final meta-analysis, 3833 (48%) women with RPL and 4083 (52%) controls. The mean BMI in the RPL group ranged from 20.3 to 29.3 kg/m2. The mean BMI in the control group ranged from 20.1 to 26.9 kg/m2. Women with recurrent pregnancy loss had a significantly higher BMI compared to fertile controls, mean difference 0.7 kg/m2 [95% CI 0.2; 1.3] (Figure 2). Statistical heterogeneity was 90% (p<0.01) within the included studies.
Figure 2.
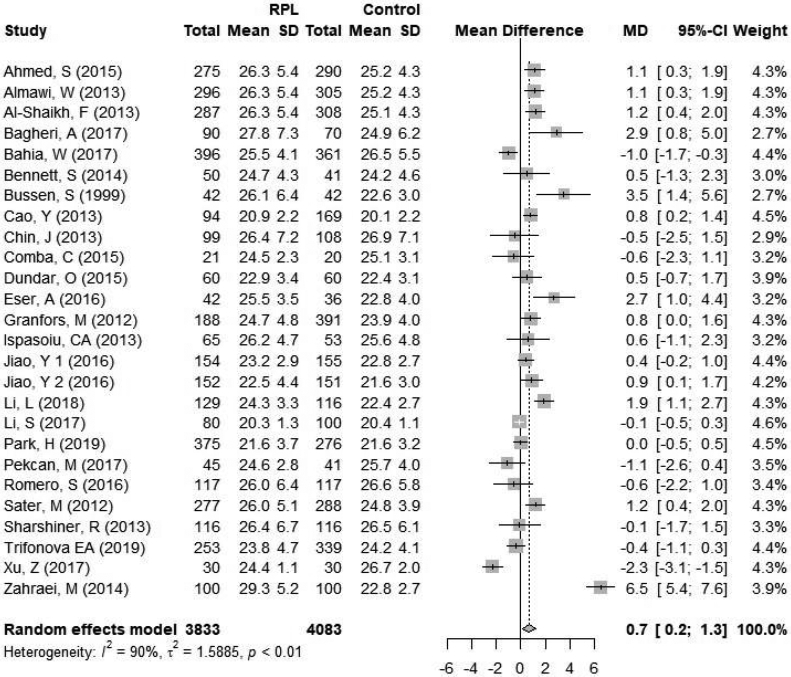
Forest plot of primary outcome in the overall analysis. CI = confidence interval; RPL = recurrent pregnancy loss.
A subgroup analysis was performed when RPL was defined as two versus three or more pregnancy losses. A total of 14 studies defined RPL as two or more pregnancy losses and included 626 women with RPL and 1661 controls (Figure 3). The mean BMI in the RPL group ranged from 20.3 to 29.3 kg/m2 and the mean BMI in the control group ranged from 20.1 to 26.9 kg/m2. When RPL was defined as two or more pregnancy losses, there was a significantly higher BMI in the RPL group, with a mean difference of 0.9 kg/m2 [95% CI 0.0; 1.7] between women with RPL and controls (Figure 3a). Statistical heterogeneity was 92% (p<0.01) within the studies included.
Figure 3.
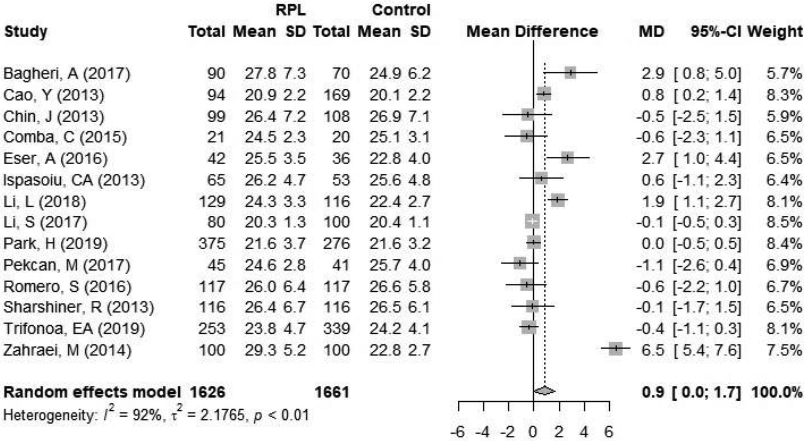
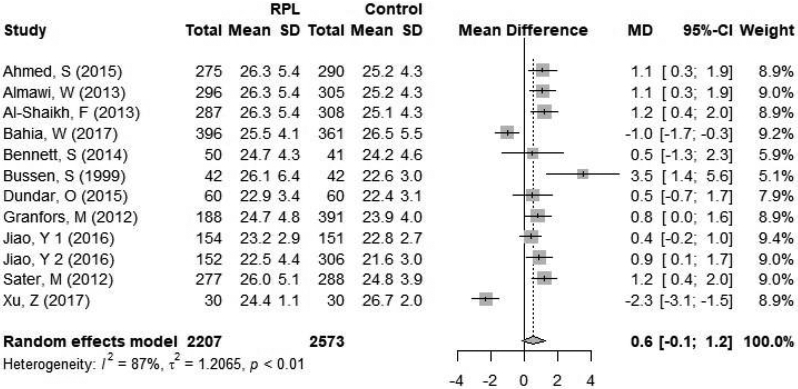
Forest plot of subgroup analysis by definition of RPL A, RPL ≥2; B, RPL ≥3. CI = confidence interval; RPL = recurrent pregnancy loss.
A total of 11 studies defined RPL as three or more pregnancy losses and included 2207 women with RPL and 2573 controls. The mean BMI in the RPL group ranged from 22.5 to 26.3 kg/m2 and the mean BMI in the control group ranged from 21.6 to 26.7 kg/m2. When RPL was defined as three or more pregnancy losses, the difference in BMI between women with RPL and controls was non-significant, mean difference 0.43 kg/m2 [95% CI −0.5; 1.3] (Figure 3b). Statistical heterogeneity was 91% (p<0.01) within the included studies.
To evaluate maternal age as a potential confounder, the mean age in the RPL and control groups were compared (Figure 4). The mean age in the RPL group ranged from 27 to 35.6 years and the mean age in the control group ranged from 27.2 to 35.9 years. One study (24) did not provide standard deviation of age and was therefore excluded from analysis. There was no significant difference in the mean age between women with RPL and controls, mean difference of 0.2 years [−0.1; 0.6]. Statistical heterogeneity was 58% (p<0.01) within the studies included.
Figure 4.
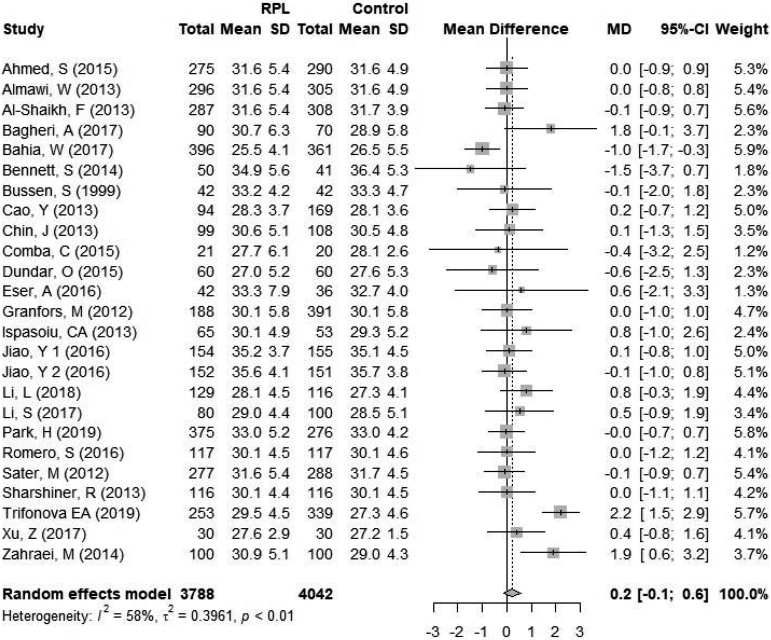
Forest plot of mean maternal age. CI = confidence interval; RPL = recurrent pregnancy loss.
Discussion
We report evidence that women with RPL have a significantly higher BMI compared to controls. This is the largest systematic review and meta-analysis comparing the difference in maternal body mass index in RPL and control cohorts. Our analysis confirms that maternal obesity is a risk factor for recurrent pregnancy loss.
We were unable to identify any studies evaluating the association of mean differences in maternal BMI and the risk of RPL. Our study shows a higher mean maternal BMI in the RPL group, but this does not imply that all women in the RPL group were overweight or obese. Previously, many studies have only evaluated the risk of RPL to either, maternal obesity (26) or an underweight state (27). Furthermore, an association of an increased frequency of euploid miscarriage among obese women with RPL was shown in 482 patients with a history of two or more consecutive miscarriages (28).
The exact mechanism of sub-optimal reproductive outcomes associated with changes in maternal BMI and RPL is unknown.
It is well known that elevated BMI may result in increased oxidative stress (29) and systemic inflammation (30). Furthermore, changes in body mass index is associated with reduced uterine receptivity (21), impairment of oocyte metabolism and maturation (31), increased risk of endocrine abnormalities (32) leading to metabolic syndrome, and shorter telomere length (33) which in turn is associated with poor reproductive outcomes.
Despite the difference in mean BMI between women with RPL and controls being small, the association may be clinically significant and increase a patient’s risk of miscarriage. It is important to note, however, that this correlation does not determine causation. While there was no difference in maternal age between groups, we were unable to control for other possible confounding variables that may be associated with both changes in BMI and RPL, such as increased parity (34, 35) or increased rates of depression (36, 37).
This study also has the inherent limitations associated with a meta-analysis of observational studies. Although all studies specify a case and a control group, there are variations in case definitions, primary outcomes, participant numbers, study design and data collection. As a result, there is substantial heterogeneity between studies pooled in the meta-analyses. In addition, we were not able to include randomized control studies in the analysis as BMI is typically matched between cases and controls. Finally, we would have liked to perform a meta-analysis on underweight, normal weight, overweight and obese women with RPL, however, the data were insufficiently reported in studies to allow for such an analysis.
Nevertheless, this comprehensive review with a large number of women and narrow confidence intervals supports the validity of our conclusions. The study is further strengthened through a subgroup analysis based on two or three previous miscarriages, and meta-regression to assess publication bias.
In this systematic review and meta-analysis, we report that women with RPL have a significantly higher mean BMI compared to controls. Healthcare professionals should include a discussion of BMI as part of pre-conception and miscarriage counseling. BMI is not only a measure of weight and height; BMI can also be a sign or symptom for other conditions, such thyroid dysfunction, insulin resistance/diabetes, depression/anxiety, disordered eating habits, poor nutrition and physical activity, all of which are modifiable risks that when addressed could potentially improve the success of their next pregnancy and the health of their children. Further research to include conducting large, well-designed cohort studies to analyze relation of changes in maternal BMI and reproductive outcomes in RPL would be valuable.
Supplementary Material
Acknowledgements:
This study was supported in part by the University of Iowa Institute for Clinical and Translational Science, which is granted with Clinical and Translational Science Award funds from the National Institutes of Health (UL1TR002537).
REFERENCES:
- 1.Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertility and sterility. 2012. ;98(5):1103–11. [DOI] [PubMed] [Google Scholar]
- 2.Stirrat GM. Recurrent miscarriage. Lancet (London, England). 1990. 15;336(8716):673–5. [DOI] [PubMed] [Google Scholar]
- 3.Jauniaux E, Farquharson RG, Christiansen OB, Exalto N. Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Human reproduction (Oxford, England). 2006. ;21(9):2216–22. [DOI] [PubMed] [Google Scholar]
- 4.Killick S Recurrent pregnancy loss: Causes, controversies and treatment. Taylor & Francis; 2008. [Google Scholar]
- 5.Alberman E, Beard R, Sharp F. Early pregnancy loss: mechanisms and treatment. New York: Springer-Verlag; 1988. [Google Scholar]
- 6.Stray-Pedersen B, Lorentzen-Styr A-M. The prevalence of toxoplasma antibodies among 11 736 pregnant women in Norway. Scandinavian journal of infectious diseases. 1979;11(2):159–65. [DOI] [PubMed] [Google Scholar]
- 7.Cohn D, Goddijn M, Middeldorp S, Korevaar J, Dawood F, Farquharson R. Recurrent miscarriage and antiphospholipid antibodies: prognosis of subsequent pregnancy. Journal of Thrombosis and Haemostasis. 2010;8(10):2208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertility and sterility. 1996. ;66(1):24–9. [PubMed] [Google Scholar]
- 9.Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reproductive biomedicine online. 2007. ;15(5):532–8. [DOI] [PubMed] [Google Scholar]
- 10.Boots CE, Bernardi LA, Stephenson MD. Frequency of euploid miscarriage is increased in obese women with recurrent early pregnancy loss. Fertility and sterility. 2014. ;102(2):455–9. [DOI] [PubMed] [Google Scholar]
- 11.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Human reproduction (Oxford, England). 2004. ;19(7):1644–6. [DOI] [PubMed] [Google Scholar]
- 12.Dağ Z, Dilbaz B. Impact of obesity on infertility in women. Journal of the Turkish German Gynecological Association. 2015;16(2):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014. ;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (London, England). 2016. ;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nteeba J, Ganesan S, Keating AF. Progressive Obesity Alters Ovarian Folliculogenesis with Impacts on Pro-Inflammatory and Steroidogenic Signaling in Female Mice1. Biology of Reproduction. 2014;91(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015. ;27(4):716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrell DT, Jones KP, Peterson CM, Aoki V, Emery BR, Campbell BR. Body mass index is inversely related to intrafollicular HCG concentrations, embryo quality and IVF outcome. Reproductive biomedicine online. 2001;3(2):109–11. [DOI] [PubMed] [Google Scholar]
- 18.Rhee JS, Saben JL, Mayer AL, Schulte MB, Asghar Z, Stephens C, et al. Diet-induced obesity impairs endometrial stromal cell decidualization: a potential role for impaired autophagy. Human Reproduction. 2016;31(6):1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellver J, Melo MAB, Bosch E, Serra V, Remohí J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertility and sterility. 2007;88(2):446–51. [DOI] [PubMed] [Google Scholar]
- 20.Cardozo ER, Karmon AE, Gold J, Petrozza JC, Styer AK. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Human Reproduction. 2015;31(2):385–92. [DOI] [PubMed] [Google Scholar]
- 21.Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertility and sterility. 2013. ;100(4):1050–8. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009. ;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 23.Jiao Y, Zhao J, Hu B, La X, Gong X, Huang Y, et al. Toll-like receptor 4 gene is associated with recurrent spontaneous miscarriage in Uygur and Han women in Xinjiang. Exp Ther Med. 2016. ;12(5):3268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pekcan MK, Sarikaya E, Tokmak A, Alisik M, Alkan A, Ozaksit G, et al. ADAMTS-3, −13, −16, and −19 levels in patients with habitual abortion. Kaohsiung J Med Sci. 2017. ;33(1):30–5. [DOI] [PubMed] [Google Scholar]
- 25.Cavalcante MB, Sarno M, Peixoto AB, Araujo Junior E, Barini R. Obesity and recurrent miscarriage: A systematic review and meta-analysis. The journal of obstetrics and gynaecology research. 2019. ;45(1):30–8. [DOI] [PubMed] [Google Scholar]
- 26.Sugiura-Ogasawara M Recurrent pregnancy loss and obesity. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):489–97. [DOI] [PubMed] [Google Scholar]
- 27.Metwally M, Saravelos SH, Ledger WL, Li TC. Body mass index and risk of miscarriage in women with recurrent miscarriage. Fertility and sterility. 2010;94(1):290–5. [DOI] [PubMed] [Google Scholar]
- 28.Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Human reproduction (Oxford, England). 2012;27(8):2297–303. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004. ;114(12):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das UN. Is obesity an inflammatory condition? Nutrition. 2001. ;17(11-12):953–66. [DOI] [PubMed] [Google Scholar]
- 31.Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011. ;28(6):517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robker RL, Akison LK, Russell DL. Control of oocyte release by progesterone receptor-regulated gene expression. Nucl Recept Signal. 2009. 31;7:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009. March;18(3):816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrams B, Heggeseth B, Rehkopf D, Davis E. Parity and body mass index in US women: a prospective 25-year study. Obesity (Silver Spring). 2013. ;21(8):1514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohain JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth. 2017. ; 22;17(1):437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolte AM, Olsen LR, Mikkelsen EM, Christiansen OB, Nielsen HS. Depression and emotional stress is highly prevalent among women with recurrent pregnancy loss. Human reproduction (Oxford, England). 2015; 30(4):777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010. ;67(3):220–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


