Abstract
Purpose
Primary progressive aphasia (PPA) and the amnestic variant of Alzheimer's disease (AD) are neurodegenerative conditions characterized by a profound loss of functional communication abilities. Communicative impairment in AD and PPA is especially apparent in the domain of naming common objects and familiar faces. We evaluated the effectiveness of a language intervention targeting maintenance of an individualized core vocabulary in a longitudinal cohort of older adults experiencing either PPA or AD.
Method
PPA (n = 9) and AD (n = 1) patients were administered a semantically based language treatment for up to 2 years. Patients repeatedly named and generated semantic features for a personalized lexicon consisting of 100 words. We evaluated naming accuracy and off-line neuropsychological measures at four successive timepoints. Naming accuracy was assessed in patients (n = 7) who completed at least three recurrent evaluations. Off-line neuropsychological performance was assessed across timepoints in all patients.
Results
Patients demonstrated relative preservation of naming trained words relative to a steep decline for untrained (control) words. The greatest decrements were observed for naming people relative to objects.
Conclusion
These results suggest that consistent training of a finite set of words can protect a core lexicon composed of crucial target concepts (e.g., a spouse's name). We discuss potential benefits and clinical implications of maintenance-based approaches to promoting language functioning in the context of neurodegeneration.
One of the most common and functionally debilitating symptoms of Alzheimer's disease (AD) and primary progressive aphasia (PPA) is anomia, a disorder that manifests as an ever-worsening impairment in the ability to name common people and objects (Leyton et al., 2017; Reilly et al., 2011; Thompson et al., 2012; Wilson et al., 2017). The past decade has seen an increase in research targeting progressive anomia using both behavioral and noninvasive neurostimulation paradigms (Heredia et al., 2009; Hung et al., 2017; Jokel & Anderson, 2012; Meyer et al., 2016; Reilly, 2016; Savage et al., 2013; Tsapkini et al., 2015). One of the most significant challenges in the management of progressive anomia is the dynamic nature of the disorder. Whereas the pattern of language in poststroke aphasia is typically either static or improving, treatment gains in dementia are more vulnerable to erosion from neurodegeneration.
Traditional speech-language interventions involve either restorative or compensatory approaches. The primary aim of a restorative treatment is to regain lost function (e.g., retraining a forgotten face–name pairing). In contrast, compensatory approaches promote more indirect functional gains by optimizing alternative cognitive and communicative supports. Compensatory techniques for progressive anomia vary in technological sophistication from immersive virtual reality and smartphone applications to memory books/banks and structured communication guidelines for caregivers (Conway & Chenery, 2016; Lanzi et al., 2017; Lavoie et al., 2019).
Maintenance has recently evolved as another viable approach for the management of progressive language impairment. The rationale for maintenance-based treatment is that patients with progressive anomia often struggle to relearn forgotten words. For example, paired associate learning techniques such as repeated naming of probe–object pairings can produce ephemeral and rigid gains (Graham et al., 1999). An alternative strategy involves training more intact lexical–semantic knowledge with the goal of protecting against loss of known concepts. A growing body of research has demonstrated the benefits of maintenance-based treatment approaches that target retention of a constrained or restricted target lexicon (Jokel et al., 2014; Meyer et al., 2018; Reilly, 2016). A compelling advantage of this approach is that repeated training on a relatively small number of target items capitalizes on repetition and reduces cognitive demand, in turn producing sustained treatment effects as disease severity worsens. In an effort to develop the most effective, patient-specific interventions for progressive anomia, it is imperative to explore the longitudinal efficacy of such a treatment.
Semantic and Lexical Foundations of Progressive Anomia
Treatment specificity is a paradox within clinical aphasiology. Phonological treatments often improve language functioning in patients who experience predominant semantic impairments (Beeson et al., 2011). Conversely, people with phonological impairments often benefit from semantic treatments (Meyer et al., 2018). A naïve interpretation of this phenomenon is that treatment specificity is unnecessary in aphasia treatment. However, this does not appear to be the case. A common denominator across such studies is the strengthening of lexical representations and the reestablishment of durable links between word forms and their corresponding concepts.
One of the primary assumptions of classical aphasiology is that naming impairments are predominantly rooted in impaired lexical access to core semantic knowledge (Dell et al., 1997; Nickels, 2002; van Ewijk & Avrutin, 2016). A person with poststroke aphasia who experiences lexical retrieval impairment might have difficulty naming an apple, but it would be unusual for the same patient to confuse the apple for a ball. In contrast, anomia in dementia is thought to reflect loss of the conceptual substrate for word meaning. “Apple” is no longer in their semantic store (Hodges et al., 1996; McCarthy & Warrington, 2016; Warrington & Shallice, 1979). The hallmark of a semantic storage deficit is homogeneity of impairment across representational modalities. A patient with degraded knowledge of apples might struggle to name an apple from a photograph. However, a pure storage deficit would manifest as a struggle to describe apples, categorize them (e.g., fruit vs. toy), and acknowledge that apples are edible. Such uniformity of impairment has been reported in semantic dementia (or semantic variant PPA [svPPA]) and AD, where anomia tends to be accompanied by a wide range of nonverbal semantic deficits for the same items (Hodges et al., 1996; Perri et al., 2012; Reilly et al., 2011; Ungrady et al., 2019).
Patients may in theory exemplify a pure dissociation between storage versus access impairment. However, this distinction is perhaps more accurately characterized along a continuum. Many patients with PPA and AD experience symptoms that are not entirely in accord with their canonical diagnostic criteria, especially during advanced disease severity (Irish et al., 2018; Rogalski, Cobia, Harrison, Wieneke, Weintraub, & Mesulam, 2011). For example, patients with svPPA and AD have both been reported to show priming and cueing effects (Flanagan et al., 2016; Noonan et al., 2012). In addition, it has been argued that naming impairments in both PPA and AD are moderated by pre- and postsemantic processing deficits impacting visual object recognition, phonological encoding, executive functioning, lexical retrieval, verbal working memory, and output form encoding (Leyton et al., 2011; Lukic et al., 2019; Patterson et al., 2006; Rogalski, Cobia, Harrison, Wieneke, Thompson, et al., 2011; Rohrer et al., 2010; Wilson et al., 2017). Postsemantic deficits have been demonstrated when patients cannot name items but can provide some lexical knowledge related to the target. Wilson et al. (2017) reported a subset of French-speaking patients with svPPA who could successfully identify grammatical gender markers (a feature of French nouns) or initial letters of items they could not name. Though these items were unnamed, the successful production of lexical markers suggests intact coordination between semantic and lexical–phonological forms.
Despite a potential multifactorial locus of anomia, patients with svPPA typically experience more impaired conceptual knowledge for items they cannot name (Bozeat et al., 2003; Gorno-Tempini et al., 2011; Hodges et al., 1992; van Scherpenberg et al., 2019). Patients with svPPA experience impoverished abilities to describe, identify, or even recognize salient features of objects they cannot name (Bozeat et al., 2003; Hodges et al., 1992). Ungrady et al. (2019) recently presented further confirmatory evidence of this inability to target diagnostic features using eyetracking during confrontation naming. Patients showed aberrant eye gaze patterns for items they could not name. These unusual gaze patterns were characterized by delays or failures to successfully fixate on diagnostic semantic features of items required for successful identification. The authors interpreted this finding as evidence for a progressive reduction in top-down support for visual search.
Our premise is that progressive anomia in AD and PPA often results from a dual impairment compromising both access to word forms and underlying semantic knowledge (Reilly et al., 2011). In order to facilitate lasting treatment gains, an anomia intervention should aim to preserve both lexical and semantic processes. A maintenance-based intervention that targets a personalized lexicon may offer a compelling means of achieving this goal (Jokel et al., 2006; Reilly, 2016).
Approaches for Treating Progressive Anomia
Treatments utilizing semantic featural support have shown promise for patients with progressive anomia (Henry et al., 2008; Hung et al., 2017; Jokel & Anderson, 2012). Semantic feature analysis (SFA) provides a structured framework for activating semantic networks that support lexical retrieval in poststroke aphasia (Boyle, 2010; Boyle & Coelho, 1995). A common implementation of SFA includes generating semantic features of a target item, which actuates featural and associated properties of the item (Massaro & Tompkins, 1994). Specifically, when a patient demonstrates difficulty in labeling an item or picture (i.e., hammer), they will either be prompted or explicitly cued with “semantic features” (i.e., superordinate GROUP [is a tool], FUNCTION [used for building], ACTION [hits nails], PROPERTIES [has a handle/metal head], LOCATION [is found in a toolshed], ASSOCIATION [reminds me of carpentry]). Successful naming induced through SFA is thought to result from strengthened connections between an object's semantic features and its corresponding lexical form (Boyle, 2010).
Previous studies have demonstrated positive effects of semantic treatment for PPA patients who present with impaired semantic (Henry et al., 2008; Jokel & Anderson, 2012) and phonological performance (Beeson et al., 2011). However, relatively little is known regarding the benefits of such approaches over an extended duration. Moreover, previous studies typically focus on retraining lost words as they are identified over time. This item selection approach is reactive in that therapy targets are identified after they are forgotten.
Studies of learning in PPA tend to show some commonalities. Naming often improves with training, but these improvements are typically rigid, often failing to generalize to untrained items (Graham et al., 1999; Savage et al., 2015). Furthermore, treatment gains tend to be relatively short-lived with the cessation of treatment (Graham et al., 1999). One explanation for this phenomenon is that training techniques such as repetition or repeated naming employ shallow processing mechanisms that engage paired associate learning. This learning strategy would be analogous to a neurotypical adult practicing the pairing of nonwords with novel objects. The absence of a semantic or deep processing component makes any such associations more susceptible to forgetting. People with dementia are losing many of the cognitive mechanisms that support such deep learning in addition to the compromise of supportive functions (e.g., episodic memory encoding; Dignam et al., 2017; Yeung & Law, 2010). In this study, we evaluated the longitudinal effectiveness of a maintenance-based treatment approach using both deep (semantic) and more relatively shallow (lexical) training mechanisms.
Effects of a Personalized Lexicon in Treating Progressive Anomia
Many previous studies have emphasized the importance of treating words that have high functional utility and personal relevance. Reilly (2016) proposed a formal system and set of criteria for crafting a target lexicon, including the recommendation that stimuli should be highly imageable, functional, and frequent. Another potential consideration is that items should represent a range of semantic categories and thematic roles (e.g., agents, recipients of actions). Reilly (2016) argued that careful selection of a constrained lexicon using these principles could maximize scarce, diminishing resources and provide a proactive approach to training known words rather than attempting to teach forgotten words on an ad hoc basis.
Jokel et al. have conducted perhaps some of the earliest longitudinal studies of language maintenance in PPA. In one such study, Jokel et al. (2006) characterized language functioning over time in a patient (A. K.) with PPA. A. K. was assigned to review and practice naming a set of 30 known items for 30 min per day over the span of 3 weeks. At posttest, A. K. retained all target items (N = 30). However, 6 months later, A. K. successfully named 24 of 30 items (a loss of 20%) relative to a steeper decline for untrained words (18 of 30 or a 40% loss). The authors argued that the maintenance treatment was a potential contributor to lexical retention for A. K. (see also Jokel et al., 2010).
Recent work has affirmed the capacity for patients to maintain successful lexical retrieval of a personalized lexicon for up to 12 months. In a study reported by Henry et al. (2019), 18 PPA patients were enrolled in a lexical retrieval therapy which utilized both semantic and phonological self-cueing strategies for the treatment of 20–40 personally relevant target items. Treatment consisted of 1- to 2-hr sessions per week for 5–6 weeks with a clinician. Treatment also included an additional 15 min of daily homework. Patients made significant improvements in naming accuracy of trained items. At the conclusion of treatment, patients were encouraged to maintain practice with treated items, and treatment gains were maintained for trained targets over a 1-year follow-up.
To our knowledge, the lengthiest longitudinal treatment study for progressive anomia was conducted by Meyer et al. (2019). The authors implemented a phonological and orthographic treatment involving repeated naming of items that were either unknown or unstable (remediation items) relative to known (prophylaxis items) at baseline. Patients completed naming treatment over the span of 6 months. In the first month, sessions occurred twice per week for 45 min. For the remaining 5-month treatment period, sessions occurred in home practice for 10- to 15-min sessions 3 times per week. In the orthographic condition, patients observed word–picture cards. Patients were instructed to observe the card, read the label, and write the name of the item on a response sheet. In the phonological condition, patients also observed the items on a picture card, but caregivers provided the verbal label for patients to repeat. Results indicated that both remediation and prophylaxis items responded to both treatments at 1-month posttreatment testing. However, by 15 months posttreatment, only prophylaxis items demonstrated sustained performance in naming accuracy.
Language maintenance of a personalized lexicon has garnered recent support in progressive anomia. However, the role of caregiver implementation is not well understood in this context. Such approaches have proven successful with other populations, like stroke aphasia, (Arroyo et al., 2012; Simmons-Mackie et al., 2005) though progressive neurodegeneration in the current population negates the ability to adopt these findings. Support for caregiver implementation in progressive anomia can be gleaned from Grasso et al. (2019) where two patients demonstrated medium to large effect sizes for both clinician and caregiver implementations of a lexical retrieval treatment. Expanded investigations could be a valuable avenue for exploration provided limitations in treatment accessibility (e.g., cost, proximity to services) for some patients. Nevertheless, limited support has been demonstrated for a larger cohort of patients with progressive anomia.
A growing body of research suggests that it is possible to treat, slow, and even reverse some degree of language loss in progressive aphasia. However, the data suggest that these treatment effects are relatively item-specific and moderated by disease severity. In the study to follow, we evaluate the effectiveness of a largely maintenance-based, caregiver-implemented treatment over an unprecedented time span (average time in treatment 19.7 months) in a cohort of patients with progressive anomia. As a secondary aim, we evaluated whether patients were more impaired for particular semantic categories targeted for training (e.g., people vs. common objects).
Semantic Category Effects in Neurodegeneration
Many studies have investigated category-specific effects in neurological disorders (Cotelli et al., 2006; Humphreys & Forde, 2001; Lambon Ralph et al., 2006; Martin et al., 1996; Warrington & Shallice, 1984). The most common findings are that proper nouns are more susceptible to loss than common nouns (Barbarotto et al., 1995; Gainotti, 2003; Gentileschi et al., 1999) and that biological natural kinds are more vulnerable than manufactured artifacts (Farah & McClelland, 1991; Warrington & Shallice, 1984). Some have described deficits in more specific domains, such as naming persons and faces of people (Mendez et al., 2015) and the selective loss of fruits and vegetables (Samson & Pillon, 2003) or musical instruments (Barbarotto et al., 2001).
The etiology of such category-specific impairments remains widely debated in cognitive neuropsychology (Capitani et al., 2003; Mahon & Caramazza, 2009). Here, we remain agnostic to the root causes of such impairments. That is, our focus in the study to follow is not on the cause of category-specific advantages but rather whether certain types of words are more susceptible to language loss in progressive anomia. This information may prove useful in calibrating item selection or calling attention to particular classes of items (e.g., face naming) that might have a reduced naming accuracy.
Study Aims
We hypothesize that an optimal approach to treating progressive anomia will target both the lexical and semantic substrates that support naming. It is reasonably well-established that it is more difficult for patients to relearn forgotten concepts than maintain known concepts. Moreover, repeated training on a closed/finite set of items offers the significant advantage of repeated exposure. These aspects of learning and memory support the rationale for a maintenance-based treatment. Furthermore, by incorporating both shallow (i.e., repeated naming) and deep (e.g., semantic feature generation) semantic tasks in a regular treatment regimen, patients may benefit from distributed and consistent practice. Finally, the use of personally relevant stimuli (e.g., spouse, family dog) may additionally serve as a motivating factor to continue with treatment even as other language functions decline. Our goal is to preserve a small lexicon of personally valuable target items for as long as possible, and these principles can provide a useful framework for implementing a naming treatment focused on preservation. It is important to note that we prioritize personally salient targets for patients. As a result, some items can be included for a patient even though they may not be able to consistently provide the name at the onset of the study (e.g., name of spouse/loved one). Therefore, not all treatment targets are required to be consistently “maintained” at the onset of treatment. In the current study, we evaluated the effects of a largely maintenance-based treatment for up to 2 years, with a secondary aim exploring the potential for any differences in category-specific effects (e.g., “people” > “artifacts”). Finally, we explore the associations between naming accuracies with off-line neuropsychological measures to investigate the various underpinnings that typically contribute to progressive anomia (i.e., lexical access deficit, semantic storage deficit).
Method
Overview
We evaluated the efficacy of a caregiver implemented naming treatment for language retention in a cohort of 10 patients with varied dementia types: either PPA (n = 9) or AD (n = 1) over the span of 2 years. Patients selected 100 items to be trained 3 times a week via confrontation naming where errored words were provided semantic feature description. We compared performance of each patient's personalized trained items to 100 untrained control items of matched categorical distribution. Additionally, at each time point every 4–7, patient performance was assessed with an off-line neuropsychological battery.
Participants
Participants included older adults experiencing progressive anomia secondary to diagnoses of either AD (n = 1) or PPA (n = 9). The PPA subgroup included seven patients with svPPA (n = 7) and two patients with logopenic variant PPA (n = 2). Diagnoses were established by an experienced behavioral neurologist in accord with published criteria (Gorno-Tempini et al., 2011; McKhann et al., 2011). Once enrolled, we obtained baseline neuropsychological and naming performances for each patient. This testing battery was completed every 4–7 months. Due to life circumstances (e.g., advanced disease progression, illness, relocation), not all patients completed the 2-year testing interval, but all patients participated in at least three timepoints. Some participants (e.g., P10) did not complete full sessions at each time point (e.g., due to fatigue, frustration) and were not responsive for reschedules in a timely manner. Other participants (e.g., P02, P06) requested multiple changes to training stimuli, precluding repeated assessments of their ability to maintain a closed set of target items. Table 1 reflects demographic and study participation details. Patients averaged 3.8 sessions spaced over 19.7 months. Table 2 illustrates neuropsychological performance at baseline and the conclusion of the study for each patient.
Table 1.
Demographics and participation details.
| ID | Age | Dx | Years postonset | Edu | Duration in study (months) | N testing sessions |
|---|---|---|---|---|---|---|
| P01 | 65 | svPPA | 3 | 15 | 19 | 4 |
| P02 | 69 | lvPPA | 2 | 13 | 10 | 3 |
| P03 | 65 | lvPPA | 3 | 14 | 19 | 4 |
| P04 | 79 | AD | 4 | 16 | 17 | 4 |
| P05 | 64 | svPPA | 3 | 18 | 18 | 4 |
| P06 | 65 | svPPA | 6 | 19 | 31 | 4 |
| P07 | 65 | svPPA | 2 | 12 | 21 | 4 |
| P08 | 66 | svPPA | 1 | 16 | 23 | 4 |
| P09 | 59 | svPPA | 5 | 12 | 20 | 4 |
| P10 | 60 | svPPA | 7 | 16 | 19 | 3 |
Note. Age = age at baseline; Dx = diagnosis; Years postonset = approximate interval in years between self-reported symptom onset and initiation of treatment study; Edu = education; Duration in study = months; N testing sessions = total no. of times tested in the study; svPPA = semantic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; AD = Alzheimer's disease.
Table 2.
Neuropsychological performance at baseline and endpoint.
| ID | Dx | BNT |
MoCA |
PPT-W |
PPT-P |
Trail-A |
Trial-B |
Digits-F |
Digits-B |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | TF | T1 | TF | T1 | TF | T1 | TF | T1 | TF | T1 | TF | T1 | TF | T1 | TF | ||
| P01 | svPPA | 2 | 1 | 14 | 5 | 14 | 10 | 26 | 13 | 70 | 101 | 245 | 300 | 7 | 6 | 5 | 5 |
| P02 | lvPPA | 11 | 8 | 15 | 16 | 26 | 25 | 26 | 25 | 67 | 49 | 118 | 155 | 5 | 7 | 6 | 4 |
| P03 | lvPPA | 12 | 12 | 15 | 11 | 24 | 24 | 25 | 21 | 95 | 58 | 300 | 300 | 4 | 3 | 2 | 2 |
| P04 | AD | 4 | 4 | 15 | 10 | 20 | 17 | 20 | 15 | 25 | 40 | 60 | 93 | 9 | 6 | 7 | 7 |
| P05 | svPPA | 3 | 3 | 17 | 18 | 25 | 22 | 23 | 19 | 41 | 25 | 96 | 85 | 7 | 6 | 7 | 6 |
| P06 | svPPA | 1 | 2 | 17 | 16 | 13 | 14 | 12 | 15 | 42 | 55 | 101 | 90 | 12 | 11 | 8 | 10 |
| P07 | svPPA | 4 | 4 | 16 | 17 | 18 | 18 | 22 | 25 | 16 | 24 | 45 | 44 | 6 | 3 | 5 | 7 |
| P08 | svPPA | 4 | 4 | 22 | 18 | 19 | 17 | 23 | 19 | 27 | 45 | 44 | 60 | 6 | 7 | 7 | 10 |
| P09 | svPPA | 2 | 1 | 21 | 18 | NA | 14 | 24 | 22 | NA | 34 | NA | 74 | NA | 9 | NA | 7 |
| P10 | svPPA | 4 | 3 | 16 | 19 | 19 | 22 | 22 | 21 | 40.29 | 40 | 117 | 109 | 5 | 7 | 5 | 6 |
Note. T1 = baseline; TF = final time point; BNT = Boston Naming Test short form of 15 items (Mack et al., 1992; Version 4) where a mean of 13.2 correct is normative (Mack et al., 1992); MoCA = Montreal Cognitive Assessment (Nasreddine et al., 2005) score out of 30 where < 26 is considered impaired global cognition; PPT Word = Pyramids and Palm Trees Test–Words; PPT Pic = Pyramids and Palm Trees Test–Pictures, short form versions both have a maximum score of 26 (Howard & Patterson, 1992); Trail Making Test–Form A (maximum time is 150 s); Trails-B = Trail Making Test–Form B (maximum time is 300 s), scores rounded to the nearest second (Tombaugh, 2004; Weintraub et al., 2009); Digit F/B = Digit Span Forward & Backward (total number correct), maximum score is 14 for both, normative data suggest 8.6 items correct for digits forward and 6.9 items correct for digits backward (Morris et al., 2006); svPPA = semantic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; AD = Alzheimer's disease; NA = not applicable.
Structure of the Naming Treatment
Patients and their primary caregivers were informed that they would be taking part in a language treatment study targeting a set of important words. They were free to continue the treatment for as long as they felt it was beneficial but were told that we planned for 2 years of data collection. Once participants were recruited into the study, we obtained a baseline neuropsychological profile (see Table 2) and developed a set of treatment targets (N = 100) in tandem with caregivers.
Item selection procedures are described in detail in Reilly (2016). In brief, participants with caregiver support were instructed to select 100 treatment items of personal salience (i.e., familiar to them, frequently used, and high utility) from fixed lists of 220 possible items representing a range of six semantic categories, which included activities (e.g., cooking, reading), clothes (e.g., hat, jacket), hygiene (e.g., toothbrush, shaving cream), household items (e.g., book, couch), places (e.g., living room, supermarket), and food (e.g., banana, coffee). A seventh category, “people” (e.g., spouse, child) was also included for selection, but was not included on the fixed lists. These targets were independently generated by participants and caregivers. Unselected items served as controls and followed a roughly matched distribution of items per category. For example, 15 “activity” target items would result in semirandom selection of 15 control, “activity” items. Since every patient had a personally selected target lexicon from a fixed list, it was not possible to explicitly match target and control items based on psycholinguistic measures (i.e., frequency, length). However, as stated, the greater item selection pool followed familiarity, frequency, and utility constraints. Stimulus lists are available for examination and use at https://osf.io/uvh6y/. Once a suitable target lexicon was established, we visited patients in their homes and took photographs of the target items that had “personalized” forms. Specifically, photographs were taken for targets such as “phone” or “keys,” which referred to the patient's personal belonging, while other target items, like “banana” or “swimming,” did not warrant personalized forms, so canonical views of common objects or actions (for “activities”) were used. Canonical views were also used for target items if the patient or caregiver did not feel a personalized version was necessary. Additionally, all pictures of the control items were canonical views of common objects or actions. These canonical forms were downloaded from Google Images and cropped to 500 × 500 pixels. The control items for familiar people included famous faces. All photographs were printed four to a page and arrayed in a binder distributed to each patient for home use. Each binder contained a copy of the pictures and the answer key with blanks for the caregivers to track the patient's accuracy, along with instructions for how to fill out the answer key (described below).
Treatment Procedures
The clinician trained caregivers on how to correctly administer the treatment at home by modeling the treatment to follow. Every 3 weeks, the clinical technician administered the treatment (in person or by phone) to support caregiver adherence and continuity and to answer any problems or concerns raised by caregivers. For example, we made efforts to keep the treatment items updated in order to reflect real-time alterations of the items (i.e., updating pictures of grandchildren, updating items to reflect a patient's move to a new home). The treatment protocol required the patient to review their personal lexicon with a caregiver (e.g., family member, friend) 3 times per week.
During treatment sessions, the patient was instructed to progress through the binder and name each item in the picture, while their caregiver simultaneously followed along with the answer key. When the patients named the item correctly, caregivers marked the answer key with a check mark. If the patient named an item incorrectly, caregivers were instructed to provide cues; first, a semantic cue (e.g., for shampoo: “you use it to wash your hair”) was followed by a phonological cue if still unsuccessful (e.g., “it starts with the sound ‘sh-’”). If the patient still was unable to successfully name the item, caregivers gave him/her the correct answer and then marked the answer key with an “x.” In an attempt to mitigate order effects, caregivers were instructed to mix up the pages.
When the clinician visited the family to administer this treatment, they collected the answer key from the caregiver to monitor weekly progress. The clinician then began the same treatment protocol as described above, however, following a more detailed corrective instruction approach, by imputing incorrect responses with semantic featural support. That is, on failed naming trials, patients were provided a semantic and phonological cues, followed by the correct name (“This is a toothbrush.”) and then a semantic feature profile of the item composed of use (“It is used to brush, or clean, your teeth.”), action (“You put it in your mouth and scrub your teeth.”), properties (“It has a long handle for you to hold and bristles on the top.”), location (“You can find this in your bathroom.”), and association (“This is something you do when you wake up in the morning and right before you go to sleep.”). The clinician then enforced these semantic feature characteristics by asking the patients to repeat them (e.g., “Why do you use this toothbrush?” “When do you use this toothbrush?” “Where can you find this toothbrush?”).
Patients completed a baseline neuropsychological battery at the onset of the study. Every 4–7 months, patients completed the identical battery to gauge disease progression. In addition to neuropsychological testing, participants were also evaluated on their naming accuracy for their training and control items. Here, patients were scored on their spontaneous responses, or self-corrections. That is, patients were provided with cues if they were experiencing distress while naming; however, only their spontaneous answers were recorded and scored. Each item was probed once per testing period. Each individual response was scored as incorrect if the patient failed to respond (e.g., “I don't know”) or produced an inaccurate response (e.g., “ball” or “banana” for apple). During treatment, patients repeatedly named printed images. During testing, however, we probed naming accuracy for the same items as digital images in completely randomized order using a laptop computer running a stimulus delivery program (Experiment Center).
Data Analysis
At each discrete time point, we derived two accuracy scores for each patient, including percent accuracy for naming treated and control stimuli. This aggregation procedure resulted in a maximum possible total of eight observations per patient reflecting naming accuracy at four timepoints for both treated and control items over the span of 2 years. Several patients lacked full 2-year data sets. We evaluated the longitudinal data using a linear mixed-effects model with the “lme4” package in R (Bates et al., 2015). This statistical model included “Condition,” “Time point,” and the “Condition × Time point” interaction as fixed effects. “Patient” was entered as a random effect with random intercept.
In a separate analysis of category-specific effects for “objects” versus “people,” we aggregated all naming attempts for “people” and “objects” across all time points within participants. This involved isolated naming attempts exclusively for the following categories: “people,” “clothes,” “food,” “hygiene products,” and “household items.” We analyzed all naming attempts for this subset of the original item pool by calculating means per participant in each category for comparison.
Results
Overall Impact of Treatment Across Time
Table 3 reflects naming accuracy for each patient expressed as proportion correct at each time point; Table 4 reflects aggregate means across patients. The linear mixed-effects model revealed main effects of condition, F(1, 45.6.9) = 225.8, p < .001, time, F(3, 44.5) = 3.98, p = .01, and a significant Time × Condition interaction, F(3, 44.5) = 3.03, p = . 04, using Satterthwaite's approximation. This interaction was such that naming accuracy of trained items (1.5% decrease) was more preserved than control items (20% decrease) from baseline to the conclusion of treatment. To explicitly investigate the impact of treatment onset, a paired t test was conducted contrasting baseline performance to Time point 2 in the treatment condition. Results indicated significant differences, t(7) = −3, p < .03, d = −.52, where Time point 2 (92%) showed an increase in naming accuracy relative to baseline (83.1%). Figure 1 illustrates these relationships across (a) and between (b) subjects.
Table 3.
Naming accuracy for control versus trained items across time.
| Patient | Time 1 |
Time 2 |
Time 3 |
Time 4 |
||||
|---|---|---|---|---|---|---|---|---|
| Control | Train | Control | Train | Control | Train | Control | Train | |
| P01 | 0.45 | 0.58 | 0.40 | 0.87 | 0.34 | 0.84 | 0.20 | 0.62 |
| P02 | 0.69 | NA | 0.73 | NA | 0.61 | NA | NA | NA |
| P03 | 0.70 | 0.88 | 0.67 | 0.95 | 0.71 | 0.92 | 0.68 | 0.89 |
| P04 | 0.57 | 0.69 | 0.63 | 0.81 | 0.51 | 0.77 | 0.42 | 0.58 |
| P05 | 0.57 | 0.88 | 0.57 | 1.00 | 0.38 | 0.93 | 0.46 | 0.98 |
| P06 | 0.24 | NA | 0.26 | NA | NA | NA | 0.14 | NA |
| P07 | 0.54 | 0.89 | 0.45 | 0.92 | 0.50 | 0.98 | 0.33 | 0.98 |
| P08 | 0.56 | 1.00 | 0.42 | 0.96 | 0.34 | 0.98 | 0.22 | 0.92 |
| P09 | 0.51 | 0.90 | 0.32 | 0.93 | 0.23 | 0.85 | 0.17 | 0.75 |
| P10 | NA | 0.82 | NA | 0.95 | NA | 0.95 | NA | NA |
Note. Values represent mean performance of naming accuracy (proportion correct). NA indicates inability to determine accuracy (e.g., participant dropout, fatigue during testing session).
Table 4.
Differences in mean accuracy in trained and control words.
| Variable | Time Point 1 | Time Point 2 | Time Point 3 | Time Point 4 |
|---|---|---|---|---|
| Train | 83.1 (4.6) | 92.0 (6.2) | 89.5 (7.8) | 81.6 (16.6) |
| Control | 55.7 (7.7) | 49.3 (13.8) | 43.1 (15.6) | 35.5 (18.2) |
| Difference score | 27.2** | 43.0*** | 47.0*** | 46.1*** |
Note. Values are mean (SD) accuracy scores (%) across patients. Difference scores are “Train” minus “Control” means at each time point.
indicates p < .005,
indicates p < .001.
Figure 1.
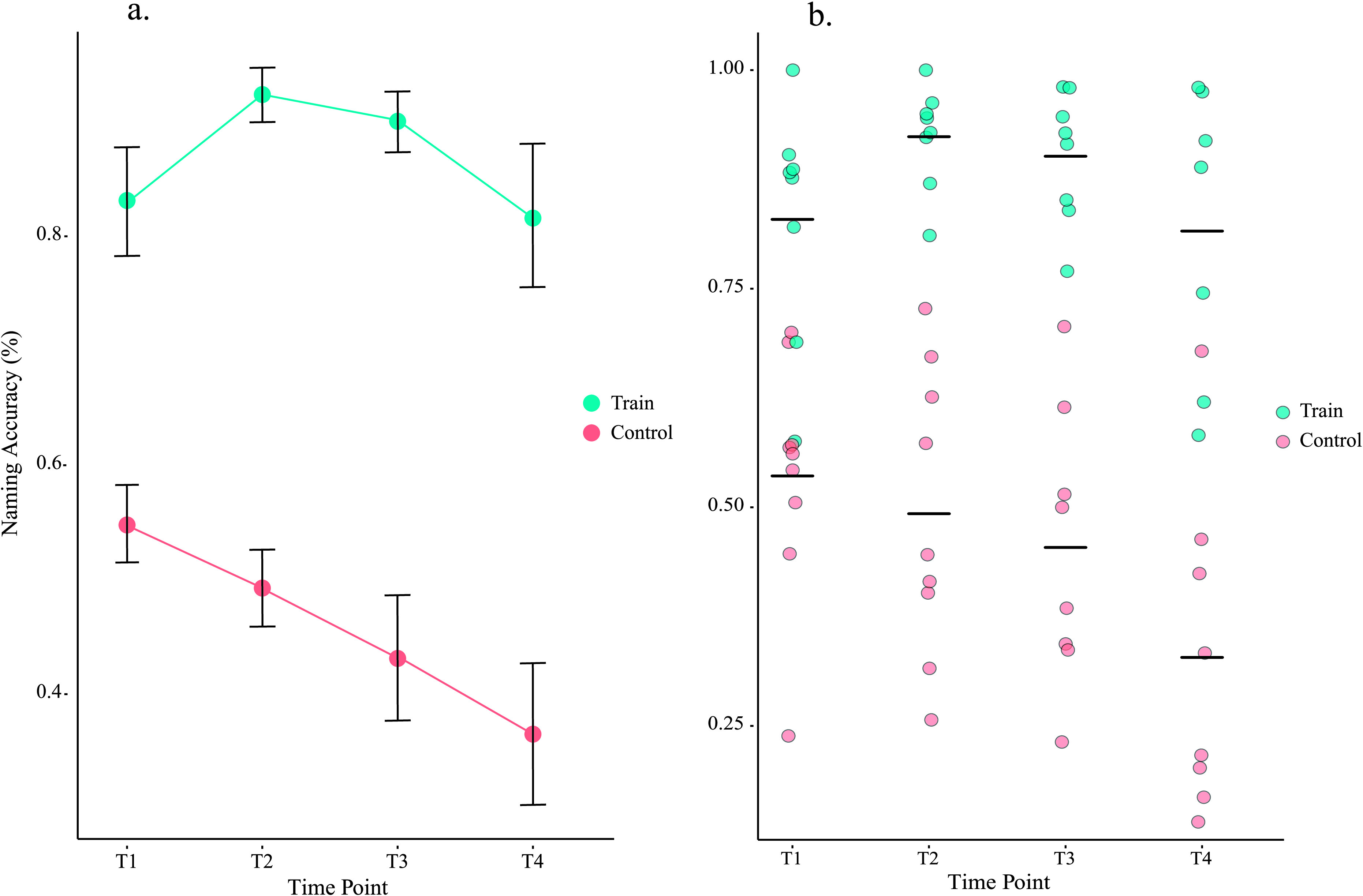
Naming accuracy by condition and time. (a) Illustrates mean naming accuracy of all participants at each time point for trained and untrained/control words. Errors bars in Figure 1a. reflect standard error of the mean. Trained words did not drop below 80% accuracy and initially improved to 92% accuracy with the onset of treatment. Overall, trained words remained within 1.5% accuracy of baseline performance. Control words steadily declined, dropping from a 56% accuracy average to 36% by T4. (b) Here, we see mean naming accuracy at each time point for each patient.
Figure 2 illustrates the magnitude of the overall treatment effect size reflected as a difference score from baseline to the study's conclusion (i.e., conclusion minus baseline) independent of variability between the intervening timepoints. Over the 2-year treatment interval, control items declined in accuracy by a margin of 20.2% from a baseline of 55.7%. In contrast, treated items declined by a margin of 1.5% from a baseline of 83.1%. This differential rate of decline between treatment and control items was statistically significant (Paired t test, t(6) = −4, p < .005, d = −.77).
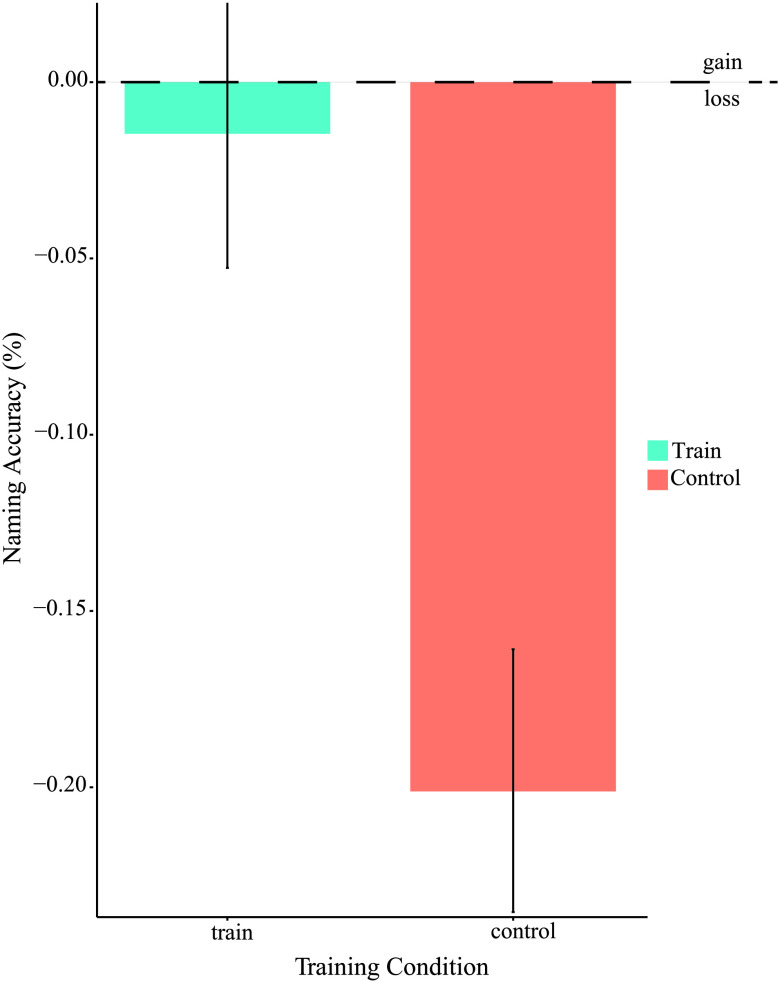
Figure 2.
Naming accuracy difference scores. Shows differences scores of T4 (final time point) minus T1 (baseline) in each condition. Trained words achieved a difference of less than 2%, while control words declined over 20%. Errors bars represent standard error of the mean.
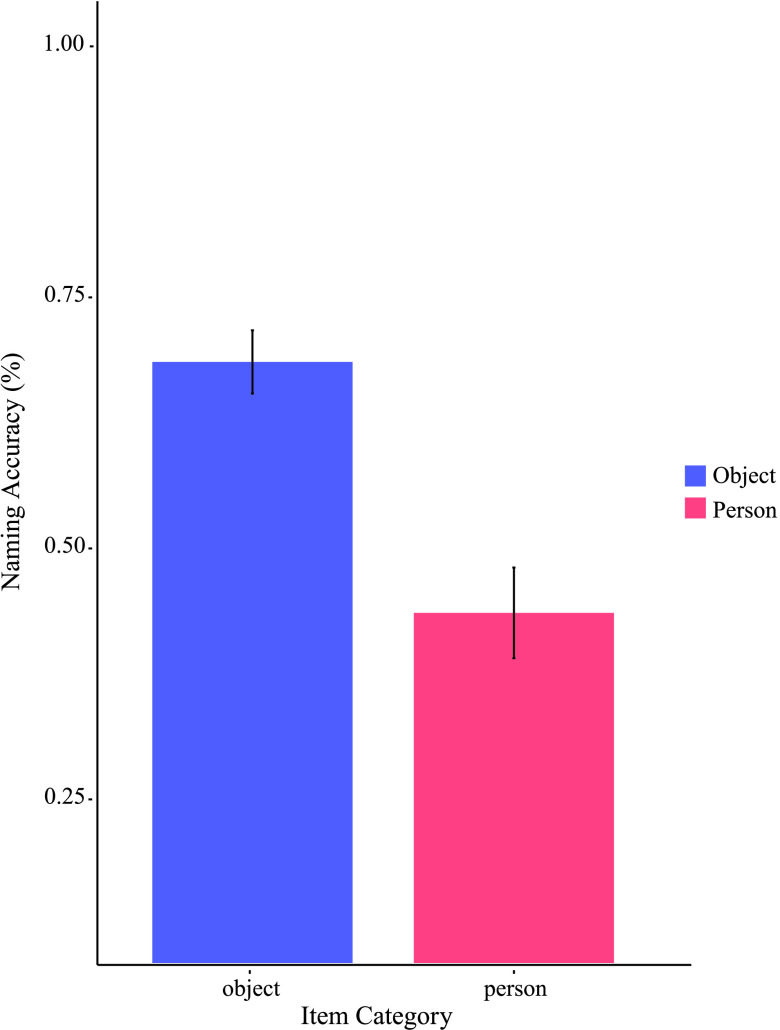
Figure 3 represents naming accuracy differences for “people” versus “objects” collapsed across patients and condition. Patients more accurately named “objects” (M = .69, SD = .19) than “people” (M = .44, SD = .27; paired t test indicated significant differences, t(9) = 3, p < .01, d = .86).
Figure 3.
Categorical discrepancy in naming ability. Demonstrates mean naming accuracy in “object” and “people” item categories. “Objects” achieved about a 69% accuracy while “person” (i.e., “people”) averaged 44% accuracy. Errors bars represent standard error of the mean.
Neuropsychological Correlates of Naming Accuracy
Figure 4 represents correlations between off-line neuropsychological measures of semantic (Pyramids and Palm Trees Test–Words [PPTwords], Pyramids and Palm Trees Test–Pictures [PPTpics]; Howard & Patterson, 1992) and lexical performance (Boston Naming Test [BNT]; Mack et al., 1992) and naming accuracy. Observations for analysis included mean neuropsychological performance and naming accuracy (collapsed across conditions) for each patient collapsed across timepoints. Pearson correlations are of complete observations.
Figure 4.
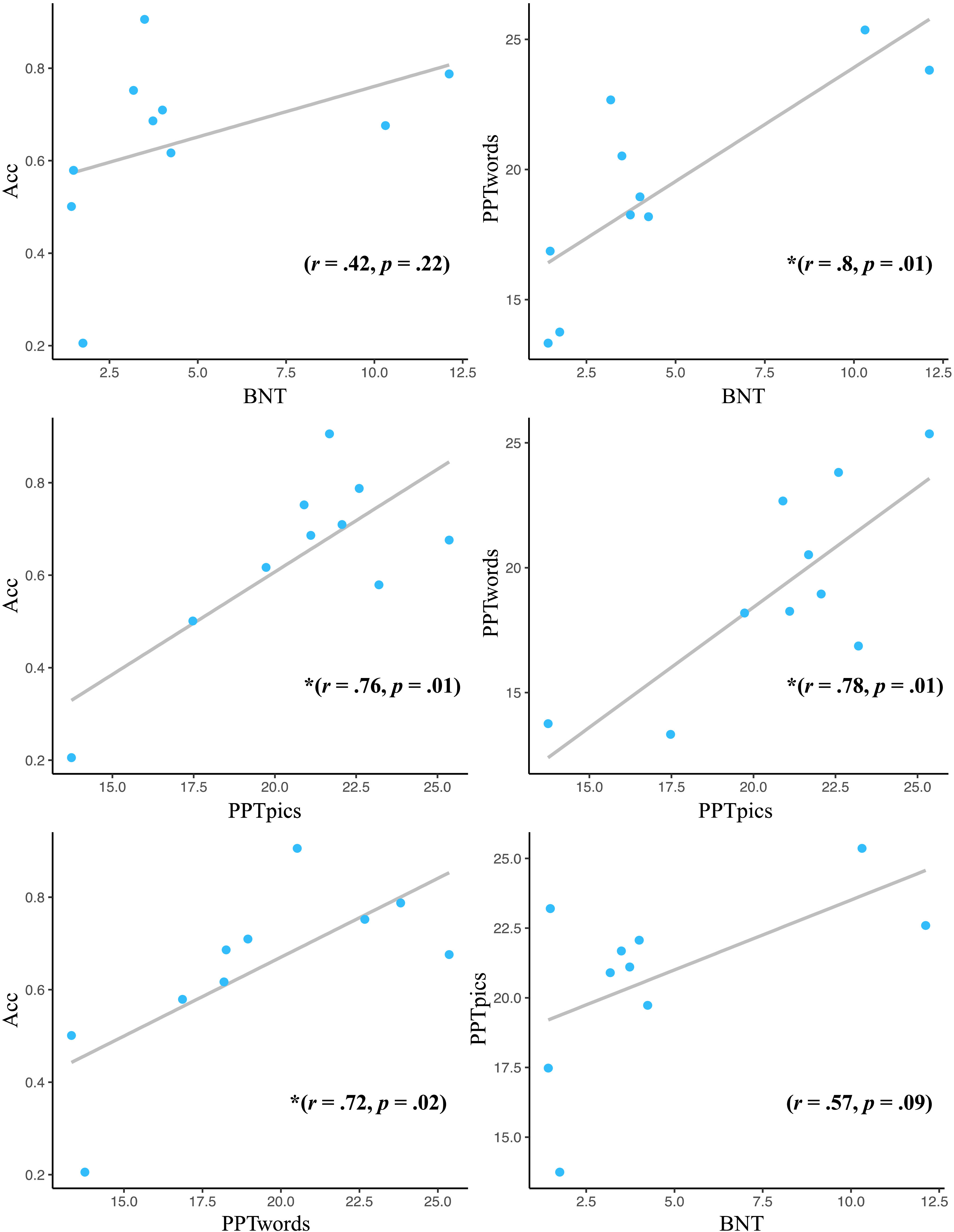
Neuropsychological performance and naming correlations. Values are Pearson correlation coefficients. PPTpics and PPTwords achieved significant, positive correlations with naming accuracy. Additionally, positive correlations were identified between PPTwords and BNT, and PPTwords and PPTpics. Acc = Naming Accuracy (proportion correct); BNT = Boston Naming Test; PPTwords = Pyramids and Palm Trees Test–Words; PPTpics = Pyramids and Palm Trees Test–Pictures.
General Discussion
The incidence of dementia and associated language disorders is exponentially increasing across much of the industrialized world (World Health Organization, 2019). Effective dementia management involves promoting aging in place such that patients maintain functional independence for as long as possible. A key element of this strategy involves maintaining functional communication skills. Yet, our understanding of how best to intervene in progressive language disorders remains limited. Few proven treatment options exist for people experiencing neurodegenerative language loss. One intuitive solution is to extrapolate treatment approaches to dementia from other neurogenic disorders, such as stroke aphasia. We have discussed several potential limitations of such an extension, including differences in etiology of impairments (i.e., linguistic access vs. storage) and differing trajectories of loss or recovery (i.e., neurodegeneration in dementia vs. functional reorganization in stroke aphasia). These fundamental differences across clinical populations necessitate etiology-specific interventions.
Interventions for progressive language impairments have tended to fall within two overarching philosophical approaches: restoration and compensation. A third area recently gaining traction is maintenance. The rationale for a maintenance-based approach is that preservation of a targeted set of known words offers significant advantages over training words that are actively being lost. The two major advantages of maintenance include item-level constraint (i.e., a focused set of treatment targets) and anchoring treatment targets to preserved semantic knowledge (i.e., targets are supported by intact conceptual knowledge). That is, finite training on a finite set of items allows patients to capitalize on frequency and personal familiarity via repeated training. A focus on known words may allow patients to retain highly functional and salient concepts. This is not to say that item-level constraint cannot be implemented in a restorative treatment. In fact, restorative treatment on a closed set of items has been successfully employed (Meyer et al., 2019). Restoring words on a purely ad hoc basis is likely not as successful. Lost words in progressive neurodegeneration is an ever-growing selection pool of potential items, so treating words as they drop out of the lexicon is a laborious and possibly ineffective approach without a priori selection of a constrained lexicon. Here, we demonstrated the effects of a largely maintenance-based approach delivered over four successive timepoints (4–7 months between sessions, 19.7 month average time in study). Patients completed a combination of semantic feature–based training and repeated naming of a set of approximately 100 personalized photographs. We examined off-line neuropsychological performance to assess potential degradation in related cognitive capacities in conjunction with naming ability.
Maintenance and Improvement of Trained Items
Patients showed an advantage in naming trained relative to control items. At baseline, patients named treatment target photographs with 83.1% accuracy in comparison to naming a set of control photographs at 55.7%. With the onset of treatment, patients showed significant improvement in naming accuracy for target items (92%), and at the conclusion of the study period, patients showed evidence of treatment-induced retention with 81.6% naming accuracy of trained items relative to 35.5% accuracy for control items. Though both categories declined in accuracy, the slope of decline was significantly steeper for untrained items. Patient's naming accuracy of trained items remained within 1.5% of their baseline performance while control items dropped in accuracy by more than 20%.
In a secondary analysis, we contrasted naming accuracy for “objects” versus “people” with the aim of examining differences in complexity, which may underlie responsivity to treatment or spontaneous loss. We hypothesized that patients would experience a disproportionate impairment for naming people relative to objects, and indeed, this prediction was borne out (people = 44% accuracy, objects = 69% accuracy). This discrepancy is consistent with previous observations of category specificity in both PPA (Barbarotto et al., 1995; Gentileschi et al., 1999; Yi et al., 2007) and AD (Grossman et al., 2003; Laws & Sartori, 2005; Whatmough et al., 2003).
Neuropsychological Correlates of Naming Performance
Our final analysis sought to investigate factors that contribute to naming performance in progressive anomia by examining bivariate correlations between naming capabilities and off-line measures of cognitive and linguistic performance. Noteworthy correlations were identified between naming accuracy and semantic knowledge. Specifically, semantic memory was positively correlated with naming accuracy (PPTwords and naming accuracy [r = .72, p = .02], and PPTpics and naming accuracy [r = .76, p = .01]), indicating that the integrity of semantic memory was a strong predictor of naming ability. This association was further demonstrated with a strong correlation between semantic memory and naming performance on the BNT (PPTwords and BNT [r = .80, p = .01]), a standardized measure of naming ability (Kaplan et al., 1983). Significant correlations were also observed between semantic memory measures (PPTwords and PPTpics [r = .78, p = .01]), suggesting performance on semantic measures was largely based on semantic knowledge, as opposed to a potential deficit in visual processing of words or images. Naming accuracy for tested items did not show a significant correlation with BNT performance. This is likely due to a floor effect in BNT performance, where most participants received low scores (only two participants scored above 5 at any time point) with little variation across time.
Limitations
The gold standard of clinical research involves randomized control trials (RCTs). That is, a patient is assigned to either the experiment treatment or an equivalent alternative treatment matched in dose and numerous other variables (e.g., intensity). Other recommended procedures involve double-blinding and placebo control. These procedures are implemented to reduce bias and expectancy effects known to have a profound influence on outcome measurement. Neurorehabilitation is moving toward achieving this standard. However, true random assignment (e.g., items, equivalent treatments) can conflict with the unique constraints of administering personalized treatment for patients in cognitive decline. For example, it would have been possible to expand the item selection pool to 500 or more items to allow matched treatment and control items (i.e., word length, frequency); however, this would be at the sacrifice of a constrained pool that is limited to highly imageable, highly frequent, and high utility words. Furthermore, assigning the word “mug” to a person who does not drink coffee would be futile. Conversely, it would be counterproductive to leave selection entirely up to the patients and caregivers. Previous research following such an approach resulted in patients selecting highly specialized, low frequency targets (e.g., a wildlife biologist selecting multiple bird species) that diminish at an accelerated rate (see Reilly, 2016, for an expanded discussion) Therefore, we implemented a quasirandom assignment where the item selection pool was balanced across semantic categories and patients were given the opportunity to exclude items with low personal utility. These procedures increased the ecological validity of the treatment, with the use of caregiver-implemented therapy and personalized treatment targets. However, these procedures resulted in a list of training items for each person that were biased by personal salience. As a result, the training items tended to be named more accurately than control items at baseline—a trend that runs counter to the practice of baseline equivalence. It is possible differences in baseline accuracy reflect a confound of complexity where trained items were inherently easier to name than control items. Prioritizing selection of targets based on personal salience also resulted in a subset of items that were not truly “maintained” at the onset of treatment, as patients could not consistently name them. Most items were preserved at baseline (83.1% of training items), but a true assessment of longitudinal maintenance would require 100% accuracy at baseline. Nevertheless, treatment items were marked by significant improvement with the onset of treatment and were largely maintained over the course of study, warranting continued investigation.
Conclusions and Future Directions
Patients with progressive language loss retained a set of words with strategic importance for their daily lives as a result of regular training. These benefits of a maintenance-focused treatment were evident exclusively for the treated stimuli, whereas untrained words showed precipitous declines in accuracy over the span of 2 years. In all, patients were able to retain the names of their loved ones and other highly functional words even as other aspects of language and cognition declined. These results yield a much-needed empirical rationale for administering maintenance-based treatment to people with progressive language disorders. Still, differential performance between item categories (“objects” > “people”) highlights the need to examine whether parameters of the treatment (e.g., dose, feature) should be optimized by category of the target item. That is, SFA may prove more effective for training objects, whereas an alternative approach rooted more in socioemotional and/or autobiographical knowledge may prove more effective for retaining the names of loved ones. Furthermore, these contrasts warrant the need for a longitudinal investigation of trajectories over time where certain categories might decline at different rates.
A more comprehensive demonstration of treatment efficacy will involve evaluating this maintenance-based approach in the context of a true RCT. This would include a more tightly controlled range for repeated assessment (e.g., evaluating performance every 6 months exactly), an expanded timeline (e.g., average participation > 18 months across all participants), and a focal assessment of maintenance (e.g., 100% accuracy in naming targets at baseline). A more comprehensive RCT might also include an expanded exploration of contributing cognitive–linguistic factors by specifically analyzing the longitudinal relationship between neuropsychological performance and treatment/naming performance. In the current study, the integrity of semantic memory as indexed by the PPT (Howard & Patterson, 1992) was strongly predictive of naming accuracy, suggestive of a primary semantic locus for naming ability in this patient cohort. Nevertheless, semantic memory is just one component of an extensive supporting cast of cognitive (e.g., executive functioning) and perceptual abilities that are likely to moderate treatment gains. Our understanding of the complex interaction between these variables in the context of environmental and psychosocial supports will be essential for optimizing this and other caregiver-administered language maintenance treatments.
An exponential increase in the incidence of dementia and associated disorders represents a pressing public health crisis (Hebert et al., 2013). A cornerstone of our national management strategy involves cultivating treatments that allow patients to age in place by prolonging functional independence for as long as possible. Toward this end, it is essential to develop interventions that have widespread accessibility in terms of cost and technological demands. Furthermore, feelings of stress and powerlessness seen in caregivers can be reduced by allowing them to take a direct role in a treatment (Jokel et al., 2017). Caregiver-administered treatment supplemented with intermittent calibration by a skilled clinician may offer a cost-effective approach to promoting functional communication. The data reported here show promise for the effectiveness of such a hybrid model of service delivery. Future investigations will expand its scope, generalizability, and accessibility.
Acknowledgments
This work was funded by U.S. Public Health Service Grant R01 DC013063, awarded to James Joseph Reilly.
Funding Statement
This work was funded by U.S. Public Health Service Grant R01 DC013063, awarded to James Joseph Reilly.
References
- Arroyo, C. , Goldfarb, R. , & Sands, E. (2012). Caregiver training in an AAC intervention for severe aphasia. The Journal of Speech-Language Pathology and Applied Behavior Analysis, 5(3–4). [Google Scholar]
- Barbarotto, R. , Capitani, E. , & Laiacona, M. (2001). Living musical instruments and inanimate body parts? Neuropsychologia, 39(4), 406–414. https://doi.org/10.1016/S0028-3932(00)00128-7 [DOI] [PubMed] [Google Scholar]
- Barbarotto, R. , Capitani, E. , Spinnler, H. , & Trivelli, C. (1995). Slowly progressive semantic impairment with category specificity. Neurocase, 1(2), 107–119. https://doi.org/10.1080/13554799508402355 [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01 [Google Scholar]
- Beeson, P. , King, R. , Bonakdarpour, B. , Henry, M. , Cho, H. , & Rapcsak, S. (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45(3), 724–736. https://doi.org/10.1007/s12031-011-9579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, M. (2010). Semantic feature analysis treatment for aphasic word retrieval impairments: What's in a name? Topics in Stroke Rehabilitation, 17(6), 411–422. https://doi.org/10.1310/tsr1706-411 [DOI] [PubMed] [Google Scholar]
- Boyle, M. , & Coelho, C. (1995). Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech-Language Pathology, 4(4), 94–98. https://doi.org/10.1044/1058-0360.0404.94 [Google Scholar]
- Bozeat, S. , Lambon Ralph, M. , Graham, K. , Patterson, K. , Wilkin, H. , Rowland, J. , Rogers, T. , & Hodges, J. (2003). A duck with four legs: Investigating the structure of conceptual knowledge using picture drawing in semantic dementia. Cognitive Neuropsychology, 20(1), 27–47. https://doi.org/10.1080/02643290244000176 [DOI] [PubMed] [Google Scholar]
- Capitani, E. , Laiacona, M. , Mahon, B. Z. , & Caramazza, A. (2003). What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cognitive Neuropsychology, 20(3–6), 213–261. https://doi.org/10.1080/02643290244000266 [DOI] [PubMed] [Google Scholar]
- Conway, E. , & Chenery, H. (2016). Evaluating the MESSAGE Communication Strategies in Dementia training for use with community-based aged care staff working with people with dementia: A controlled pretest–post-test study. Journal of Clinical Nursing, 25(7–8), 1145–1155. https://doi.org/10.1111/jocn.13134 [DOI] [PubMed] [Google Scholar]
- Cotelli, M. , Borroni, B. , Manenti, R. , Alberici, A. , Calabria, M. , Agosti, C. , Arévalo, A. , Ginex, V. , Ortelli, P. , Binetti, G. , Zanetti, O. , Padovani, A. , & Cappa, S. (2006). Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology, 20(5), 558–565. https://doi.org/10.1037/0894-4105.20.5.558 [DOI] [PubMed] [Google Scholar]
- Dell, G. , Schwartz, M. , Martin, N. , Saffran, E. , & Gagnon, D. (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104(4), 801–838. https://doi.org/10.1037/0033-295X.104.4.801 [DOI] [PubMed] [Google Scholar]
- Dignam, J. , Copland, D. , O'Brien, K. , Burfein, P. , Khan, A. , & Rodriguez, A. (2017). Influence of cognitive ability on therapy outcomes for anomia in adults with chronic poststroke aphasia. Journal of Speech, Language, and Hearing Research, 60(2), 406–421. https://doi.org/10.1044/2016_JSLHR-L-15-0384 [DOI] [PubMed] [Google Scholar]
- Farah, M. J. , & McClelland, J. L. (1991). A computational model of semantic memory impairment: Modality specificity and emergent category specificity. Journal of Experimental Psychology: General, 120(4), 339–357. https://doi.org/10.1037/0096-3445.120.4.339 [PubMed] [Google Scholar]
- Flanagan, K. , Copland, D. , Hees, S. , Byrne, G. , & Angwin, A. (2016). Semantic feature training for the treatment of anomia in Alzheimer disease: A preliminary investigation. Cognitive and Behavioral Neurology, 29(1), 32–43. https://doi.org/10.1097/WNN.0000000000000088 [DOI] [PubMed] [Google Scholar]
- Gainotti, G. (2003). Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain, 126(4), 792–803. https://doi.org/10.1093/brain/awg092 [DOI] [PubMed] [Google Scholar]
- Gentileschi, V. , Sperber, S. , & Spinnler, H. (1999). Progressive defective recognition of familiar people. Neurocase, 5(5), 407–424. https://doi.org/10.1080/13554799908402735 [Google Scholar]
- Gorno-Tempini, M. , Hillis, A. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. , Ogar, J. , Rohrer, J. , Black, S. , Boeve, B. , Manes, F. , Dronkers, N. , Vandenberghe, R. , Rascovsky, K. , Patterson, K. , Miller, B. , Knopman, D. , Hodges, J. , Mesulam, M. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, K. , Patterson, K. , Pratt, K. , & Hodges, J. (1999). Relearning and subsequent forgetting of semantic category exemplars in a case of semantic dementia. Neuropsychology, 13(3), 359–380. https://doi.org/10.1037/0894-4105.13.3.359 [DOI] [PubMed] [Google Scholar]
- Grasso, S. , Shuster, K. , & Henry, M. (2019). Comparing the effects of clinician and caregiver-administered lexical retrieval training for progressive anomia. Neuropsychological Rehabilitation, 29(6), 866–895. https://doi.org/10.1080/09602011.2017.1339358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, M. , Smith, E. E. , Koenig, P. , Glosser, G. , Rhee, J. , & Dennis, K. (2003). Categorization of object descriptions in Alzheimer's disease and frontotemporal dementia: Limitation in rule-based processing. Cognitive, Affective, & Behavioral Neuroscience, 3(2), 120–132. https://doi.org/10.3758/CABN.3.2.120 [DOI] [PubMed] [Google Scholar]
- Hebert, L. , Weuve, J. , Scherr, P. , & Evans, D. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. https://doi.org/10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. , Beeson, P. , & Rapcsak, S. (2008). Treatment for lexical retrieval in progressive aphasia. Aphasiology, 22(7–8), 826–838. https://doi.org/10.1080/02687030701820055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, M. , Hubbard, H. , Grasso, S. , Dial, H. , Beeson, P. , Miller, B. , & Gorno-Tempini, M. (2019). Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: Immediate and long-term outcomes. Journal of Speech, Language, and Hearing Research, 62(8), 2723–2749. https://doi.org/10.1044/2018_JSLHR-L-18-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia, C. , Sage, K. , Lambon Ralph, M. , & Berthier, M. (2009). Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology, 23(2), 192–209. https://doi.org/10.1080/02687030801942999 [Google Scholar]
- Hodges, J. , Patterson, K. , Graham, N. , & Dawson, K. (1996). Naming and knowing in dementia of Alzheimer's type. Brain and Language, 54(2), 302–325. https://doi.org/10.1006/brln.1996.0077 [DOI] [PubMed] [Google Scholar]
- Hodges, J. , Patterson, K. , & Oxbury, S. (1992). Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain, 115(Pt. 6), 1783–1806. https://doi.org/10.1093/brain/115.6.1783 [DOI] [PubMed] [Google Scholar]
- Howard, D. , & Patterson, K. (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Humphreys, G. , & Forde, E. (2001). Hierarchies, similarity, and interactivity in object recognition: “Category-specific” neuropsychological deficits. Behavioral and Brain Sciences, 24(3), 453–476. https://doi.org/10.1017/S0140525X01004150 [PubMed] [Google Scholar]
- Hung, J. , Bauer, A. , Grossman, M. , Hamilton, R. , Coslett, H. , & Reilly, J. (2017). Semantic feature training in combination with transcranial direct current stimulation (tDCS) for progressive anomia. Frontiers in Human Neuroscience. Advance online publication. https://doi.org/10.3389/fnhum.2017.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish, M. , Landin-Romero, R. , Mothakunnel, A. , Ramanan, S. , Hsieh, S. , Hodges, J. , & Piguet, O. (2018). Evolution of autobiographical memory impairments in Alzheimer's disease and frontotemporal dementia—A longitudinal neuroimaging study. Neuropsychologia, 110, 14–25. https://doi.org/10.1016/j.neuropsychologia.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Jokel, R. , & Anderson, N. (2012). Quest for the best: Effects of errorless and active encoding on word re-learning in semantic dementia. Neuropsychological Rehabilitation, 22(2), 187–214. https://doi.org/10.1080/09602011.2011.639626 [DOI] [PubMed] [Google Scholar]
- Jokel, R. , Graham, N. , Rochon, E. , & Leonard, C. (2014). Word retrieval therapies in primary progressive aphasia. Aphasiology, 28(8–9), 1038–1068. https://doi.org/10.1080/02687038.2014.899306 [Google Scholar]
- Jokel, R. , Meltzer, J. , D. R., J. , D. M., L. , J. C., J. , A. N., E. , & D. T., C. (2017). Group intervention for individuals with primary progressive aphasia and their spouses: Who comes first? Journal of Communication Disorders, 66, 51–64. https://doi.org/10.1016/j.jcomdis.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Jokel, R. , Rochon, E. , & Anderson, N. D. (2010). Errorless learning of computer-generated words in a patient with semantic dementia. Neuropsychological Rehabilitation, 20(1), 16–41. https://doi.org/10.1080/09602010902879859 [DOI] [PubMed] [Google Scholar]
- Jokel, R. , Rochon, E. , & Leonard, C. (2006). Treating anomia in semantic dementia: Improvement, maintenance, or both? Neuropsychological Rehabilitation, 16(3), 241–256. https://doi.org/10.1080/09602010500176757 [DOI] [PubMed] [Google Scholar]
- Kaplan, E. , Goodglass, H. , & Weintraub, S. (1983). The Boston Naming Test. Lea and Febiger. [Google Scholar]
- Lambon Ralph, M. , Lowe, C. , & Rogers, T. (2006). Neural basis of category-specific semantic deficits for living things: Evidence from semantic dementia, HSVE and a neural network model. Brain, 130(4), 1127–1137. https://doi.org/10.1093/brain/awm025 [DOI] [PubMed] [Google Scholar]
- Lanzi, A. , Burshnic, V. , & Bourgeois, M. (2017). Person-centered memory and communication strategies for adults with dementia. Topics in Language Disorders, 37(4), 361–374. https://doi.org/10.1097/TLD.0000000000000136 [Google Scholar]
- Lavoie, M. , Bier, N. , Laforce, R. , & Macoir, J. (2019). Improvement in functional vocabulary and generalization to conversation following a self-administered treatment using a smart tablet in primary progressive aphasia. Neuropsychological Rehabilitation, 30(7), 1224–1254. https://doi.org/10.1080/09602011.2019.1570943 [DOI] [PubMed] [Google Scholar]
- Laws, K. R. , & Sartori, G. (2005). Category deficits and paradoxical dissociations in alzheimer's disease and herpes simplex encephalitis. Journal of Cognitive Neuroscience, 17(9), 1453–1459. https://doi.org/10.1162/0898929054985428 [DOI] [PubMed] [Google Scholar]
- Leyton, C. E. , Hodges, J. R. , Piguet, O. , & Ballard, K. J. (2017). Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer's disease. Cortex, 86, 45–54. https://doi.org/10.1016/j.cortex.2016.10.019 [DOI] [PubMed] [Google Scholar]
- Leyton, C. E. , Villemagne, V. L. , Savage, S. , Pike, K. E. , Ballard, K. J. , Piguet, O. , Burrell, J. R. , Rowe, C. C. , & Hodges, J. R. (2011). Subtypes of progressive aphasia: Application of the international consensus criteria and validation using β-amyloid imaging. Brain, 134(10), 3030–3043. https://doi.org/10.1093/brain/awr216 [DOI] [PubMed] [Google Scholar]
- Lukic, S. , Mandelli, M. , Welch, A. , Jordan, K. , Shwe, W. , Neuhaus, J. , Miller, Z. , Hubbard, H. , Henry, M. , Miller, B. , Dronkers, N. , & Gorno-Tempini, M. (2019). Neurocognitive basis of repetition deficits in primary progressive aphasia. Brain and Language, 194, 35–45. https://doi.org/10.1016/j.bandl.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, W. J. , Freed, D. M. , Williams, B. W. , & Henderson, V. W. (1992). Boston Naming Test: Shortened versions for use in Alzheimer's disease. Journal of Gerontology, 47(3), P154–P158. https://doi.org/10.1093/geronj/47.3.P154 [DOI] [PubMed] [Google Scholar]
- Mahon, B. Z. , & Caramazza, A. (2009). Concepts and categories: A cognitive neuropsychological perspective. Annual Review of Psychology, 60, 27–51. https://doi.org/10.1146/annurev.psych.60.110707.163532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A. , Wiggs, C. , Ungerleider, L. , & Haxby, J. (1996). Neural correlates of category-specific knowledge. Nature, 379(6566), 649–652. https://doi.org/10.1038/379649a0 [DOI] [PubMed] [Google Scholar]
- Massaro, M. , & Tompkins, C. (1994). Feature analysis for treatment of communication disorders in traumatically brain-injured patients: An efficacy study. Clinical Aphasiology, 22, 245–256. [Google Scholar]
- McCarthy, R. , & Warrington, E. (2016). Past, present, and prospects: Reflections 40 years on from the selective impairment of semantic memory (Warrington, 1975). Quarterly Journal of Experimental Psychology, 69(10), 1941–1968. https://doi.org/10.1080/17470218.2014.980280 [DOI] [PubMed] [Google Scholar]
- McKhann, G. M. , Knopman, D. S. , Chertkow, H. , Hyman, B. T. , Jack , C. R., Jr. , Kawas, C. H. , Klunk, W. E. , Koroshetz, W. J. , Manly, J. J. , Mayeux, R. , Mohs, R. C. , Morris, J. C. , Rossor, M. N. , Scheltens, P. , Carrillo, M. C. , Thies, B. , Weintraub, S. , & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging—Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia, 7(3), 263–269. https://doi.org/10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez, M. , Ringman, J. , & Shapira, J. (2015). Impairments in the face-processing network in developmental prosopagnosia and semantic dementia. Cognitive and Behavioral Neurology, 28(4), 188–197. https://doi.org/10.1097/WNN.0000000000000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Getz, H. , Brennan, D. , Hu, T. , & Friedman, R. (2016). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology, 30(4), 483–507. https://doi.org/10.1080/02687038.2015.1081142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Tippett, D. , & Friedman, R. (2018). Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychological Rehabilitation, 28(3), 352–368. https://doi.org/10.1080/09602011.2016.1148619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. , Tippett, D. , Turner, R. , & Friedman, R. (2019). Long-term maintenance of anomia treatment effects in primary progressive aphasia. Neuropsychological Rehabilitation, 29(9), 1439–1463. https://doi.org/10.1080/09602011.2018.1425146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. C. , Weintraub, S. , Chui, H. C. , Cummings, J. , DeCarli, C. , Ferris, S. , Foster, N. L. , Galasko, D. , Graff-Radford, N. , & Peskind, E. R. (2006). The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Disease & Associated Disorders, 20(4), 210–216. https://doi.org/10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Badirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. L. , & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nickels, L. (2002). Therapy for naming disorders: Revisiting, revising, and reviewing. Aphasiology, 16(10–11), 935–979. https://doi.org/10.1080/02687030244000563 [Google Scholar]
- Noonan, K. , Pryer, L. , Jones, R. , Burns, A. , & Lambon Ralph, M. (2012). A direct comparison of errorless and errorful therapy for object name relearning in Alzheimer's disease. Neuropsychological Rehabilitation, 22(2), 215–234. https://doi.org/10.1080/09602011.2012.655002 [DOI] [PubMed] [Google Scholar]
- Patterson, K. , Lambon Ralph, M. , Jefferies, E. , Woollams, A. , Jones, R. , Hodges, J. , & Rogers, T. (2006). “Presemantic” cognition in semantic dementia: Six deficits in search of an explanation. Journal of Cognitive Neuroscience, 18(2), 169–183. https://doi.org/10.1162/jocn.2006.18.2.169 [DOI] [PubMed] [Google Scholar]
- Perri, R. , Zannino, G. , Caltagirone, C. , & Carlesimo, G. (2012). Alzheimer's disease and semantic deficits: A feature-listing study. Neuropsychology, 26(5), 652–663. https://doi.org/10.1037/a0029302 [DOI] [PubMed] [Google Scholar]
- Reilly, J. (2016). How to constrain and maintain a lexicon for the treatment of progressive semantic naming deficits: Principles of item selection for formal semantic therapy. Neuropsychological Rehabilitation, 26(1), 126–156. https://doi.org/10.1080/09602011.2014.1003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, J. , Peelle, J. , Antonucci, S. , & Grossman, M. (2011). Anomia as a marker of distinct semantic memory impairments in Alzheimer's disease and semantic dementia. Neuropsychology, 25(4), 413–426. https://doi.org/10.1037/a0022738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, E. , Cobia, D. , Harrison, T. , Wieneke, C. , Thompson, C. , Weintraub, S. , & Mesulam, M. (2011). Anatomy of language impairments in primary progressive aphasia. Journal of Neuroscience, 31(9), 3344–3350. https://doi.org/10.1523/JNEUROSCI.5544-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, E. , Cobia, D. , Harrison, T. , Wieneke, C. , Weintraub, S. , & Mesulam, M. (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. https://doi.org/10.1212/WNL.0b013e31821ccd3c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer, J. , Ridgway, G. , Crutch, S. , Hailstone, J. , Goll, J. , Clarkson, M. , Mead, S. , Beck, J. , Mummery, C. , Ourselin, S. , Warrington, E. , Rossor, M. , & Warren, J. (2010). Progressive logopenic/phonological aphasia: Erosion of the language network. NeuroImage, 49(1), 984–993. https://doi.org/10.1016/j.neuroimage.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson, D. , & Pillon, A. (2003). A case of impaired knowledge for fruit and vegetables. Cognitive Neuropsychology, 20(3–6), 373–400. https://doi.org/10.1080/02643290244000329 [DOI] [PubMed] [Google Scholar]
- Savage, S. , Ballard, K. , Piguet, O. , & Hodges, J. (2013). Bringing words back to mind-improving word production in semantic dementia. Cortex, 49(7), 1823–1832. https://doi.org/10.1016/j.cortex.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Savage, S. , Piguet, O. , & Hodges, J. (2015). Cognitive intervention in semantic dementia: Maintaining words over time. Alzheimer Disease & Associated Disorders, 29(1), 55–62. https://doi.org/10.1097/WAD.0000000000000053 [DOI] [PubMed] [Google Scholar]
- Simmons-Mackie, N. , Kearns, K. , & Potechin, G. (2005). Treatment of aphasia through family member training. Aphasiology, 19(6), 583–593. https://doi.org/10.1080/02687030444000408 [Google Scholar]
- Thompson, C. , Cho, S. , Price, C. , Wieneke, C. , Bonakdarpour, B. , Rogalski, E. , Weintraub, S. , & Mesulam, M. (2012). Semantic interference during object naming in agrammatic and logopenic primary progressive aphasia (PPA). Brain and Language, 120(3), 237–250. https://doi.org/10.1016/j.bandl.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh, T. (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19(2), 203–214. https://doi.org/10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- Tsapkini, K. , Vargas, Y. , & Hillis, A. (2015). Effects of different language and tDCS interventions in PPA and their neural correlates. Frontiers in Psychology, 6. https://doi.org/10.3389/conf.fpsyg.2015.65.00034 [Google Scholar]
- Ungrady, M. , Flurie, M. , Zuckerman, B. , Mirman, D. , & Reilly, J. (2019). Naming and knowing revisited: Eyetracking correlates of anomia in progressive aphasia. Frontiers in Human Neuroscience, 13, 354. https://doi.org/10.3389/fnhum.2019.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk, L. , & Avrutin, S. (2016). Lexical access in non-fluent aphasia: A bit more on reduced processing. Aphasiology, 30(11), 1264–1282. https://doi.org/10.1080/02687038.2015.1135867 [Google Scholar]
- van Scherpenberg, C. , Fieder, N. , Savage, S. , & Nickels, L. (2019). The relationship between response consistency in picture naming and storage impairment in people with semantic variant primary progressive aphasia. Neuropsychology, 33(1), 13–34. https://doi.org/10.1037/neu0000485 [DOI] [PubMed] [Google Scholar]
- Warrington, E. K. , & Shallice, T. (1979). Semantic access dyslexia. Brain, 102(1), 43–63. https://doi.org/10.1093/brain/102.1.43 [DOI] [PubMed] [Google Scholar]
- Warrington, E. K. , & Shallice, T. (1984). Category specific semantic impairments. Brain, 107(3), 829–853. https://doi.org/10.1093/brain/107.3.829 [DOI] [PubMed] [Google Scholar]
- Weintraub, S. , Salmon, D. , Mercaldo, N. , Ferris, S. , Graff-Radford, N. R. , Chui, H. , Cummings, J. , DeCarli, C. , Foster, N. L. , Galasko, D. , Ferris, S. , & Peskind, E. (2009). The Alzheimer's disease centers' uniform data set (UDS): The neuropsychological test battery. Alzheimer Disease and Associated Disorders, 23(2), 91–101. https://doi.org/10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmough, C. , Chertkow, H. , Murtha, S. , Templeman, D. , Babins, L. , & Kelner, N. (2003). The semantic category effect increases with worsening anomia in Alzheimer's type dementia. Brain and Language, 84(1), 134–147. https://doi.org/10.1016/S0093-934X(02)00524-2 [DOI] [PubMed] [Google Scholar]
- Wilson, S. , Dehollain, C. , Ferrieux, S. , Christensen, L. , & Teichmann, M. (2017). Lexical access in semantic variant PPA: Evidence for a post-semantic contribution to naming deficits. Neuropsychologia, 106, 90–99. https://doi.org/10.1016/j.neuropsychologia.2017.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2019, September 19). Dementia [Who.int] . https://www.who.int/news-room/fact-sheets/detail/dementia
- Yeung, O. , & Law, S. (2010). Executive functions and aphasia treatment outcomes: Data from an ortho-phonological cueing therapy for anomia in Chinese. International Journal of Speech-Language Pathology, 12(6), 529–544. https://doi.org/10.3109/17549507.2011.516840 [DOI] [PubMed] [Google Scholar]
- Yi, H. , Moore, P. , & Grossman, M. (2007). Reversal of the concreteness effect for verbs in patients with semantic dementia. Neuropsychology, 21(1), 9–19. https://doi.org/10.1037/0894-4105.21.1.9 [DOI] [PubMed] [Google Scholar]