Abstract
Introduction:
The dementia experience is not a monolithic phenomenon—and while core elements of dementia are considered universal—people living with dementia experience the disorder differently. Understanding the patterning of ADRD in the population with regards to incidence, risk factors, impacts on dementia care, and economic costs associated with ADRD can provide clues to target risk and protective factors for all populations as well as addressing health disparities.
Methods:
We discuss information presented at the 2020 National Research Summit on Care, Services, and Supports for Persons with Dementia and Their Caregivers, Theme 1: Impact of Dementia. In this paper, we describe select population trends, care interventions, and economic impacts, health disparities and implications for future research from the perspective of our diverse panel comprised of academic stakeholders, and persons living with dementia, and care partners.
Results:
Dementia incidence is decreasing yet the advances in population health are uneven. Studies examining the educational, geographic and race/ethnic distribution of ADRD have identified clear disparities. Disparities in health and healthcare may be amplified by significant gaps in the evidence base for pharmacological and non-pharmacological interventions. The economic costs for persons living with dementia and the value of family care partners’ time are high, and may persist into future generations.
Conclusions.
Significant research gaps remain. Ensuring that ADRD healthcare services and long term care services and supports are accessible, affordable and effective for all segments of our population is essential for health equity. Policy-level interventions are in short supply to redress broad unmet needs and systemic sources of disparities. Whole of society challenges demand research producing whole of society solutions. The urgency, complexity and scale merit a “whole of government” approach involving collaboration across numerous federal agencies.
Keywords: dementia, disparities, care interventions, economic costs, race/ethnicity
INTRODUCTION
Dementia is a major health problem that exerts considerable health, social, and economic costs on individuals, families, and societies, and presents enormous challenges to systems providing healthcare and long term services and support1. Considered a heterogeneous disorder, dementia is characterized by clinical variability as evidenced by differences in etiology, risk, clinical presentation, pathologic patterns, progression, and prognosis. Variability extends to epidemiologic trends in rates, population-based risk factors, social determinants of health, economic impacts, and access to healthcare and long-term services and supports across population subgroups. It is often said that, “When you meet a person living with dementia, you have only met one person living with dementia.” This statement highlights that the dementia experience is not a monolithic phenomenon, and while some elements of dementia may be considered universal, people living with dementia (PLWD) experience the disorder differently depending on their disease risk, life and social circumstances, environment, preferences, and resources/supports. These inherent sources of heterogeneity present substantial challenges to research given the complex interplay of diverse presenting conditions, study participants, care settings, interventions, uptake, and variability in treatment efficacy, and organizational policies. Such variability can obscure the mechanisms of action of treatment efficacy, implementation, and large-scale dissemination of dementia care and services.
This paper is based on discussions during the Theme 1: Impact of Dementia panel of the 2020 National Research Summit on Care, Services, and Supports for Persons with Dementia and Their Caregivers. Given that the theme encompasses a wide range of impacts that go beyond the scope of this paper (see Supplementary File S1), we focus on describing select population trends, care interventions, and economic impacts, with particular attention to health disparities. We define health disparity as any health difference closely aligned with social, economic and environmental disadvantages in a society2---which if taken broadly can include the impact of disadvantages on diverse populations identified by sex and gender, race and ethnicity, language, education, socioeconomic status, geography, living arrangements, including people living alone or without caregivers, among others.
METHODS
In summer 2020, the National Institute on Aging (NIA), in conjunction with the Department of Health and Human Services as part of the National Alzheimer’s Project Act, hosted the second National Research Summit on Care, Services, and Supports for Persons with Dementia and Their Caregivers. The goal was to bring together individuals with a variety of backgrounds to identify evidence-based programs, strategies, approaches, and research that can be used to improve the care, services, and supports of persons with dementia and their care partners. The panels were conducted virtually due to the COVID-19 pandemic, and all materials are available online at https://www.nia.nih.gov/2020-dementia-care-summit. This paper discusses information from the Theme 1: Impact of Dementia panel comprised of co-chairs (MPA, INK), speakers (RW, LH, JZ), and discussants (CHH, LT, CF) with input from PLWD, other stakeholder groups convened by summit leaders, and public comments received by NIA.
RESULTS
Heterogeneity and ADRD Population Trends
Although the incidence of dementia is declining in the US, the number of persons and families living with dementia is increasing with population aging as older age is a dementia risk factor.3 As the US becomes more diverse, describing population risk patterns for Alzheimer’s disease and related dementias (ADRD) will help clinical, public health and long-term services and support providers prepare for the needs of their patients, consumers, and communities.
Understanding the epidemiology of ADRD can also provide insights about risk and protective factors that may be pertinent to risk reduction and the attenuation of health disparities. Importantly, health disparities by definition are socially derived and distinct from biological differences.4 If the observed racial/ethnic patterning in ADRD and cognitive aging is driven by health disparities rather than biological differences – and indeed there are clear indications this is the case5 – the disproportionate burden of ADRD could be mitigated through intervening on identified social, economic and behavioral determinants.
Based on national and regional data, significant racial/ethnic as well as gender disparities in ADRD prevalence exist.1, 4, 6 Although more Whites live with ADRD than any other US racial/ethnic group, older Latinos and Blacks are disproportionately more likely to have ADRD. Latest population projection data by age, sex, and race/ethnicity (2015–2060) indicate that over 43% of Blacks and 40% of Latinos have the greatest estimated burden of disease due to ADRD (Figure 1). Regardless of subgroup, ADRD prevalence was higher for women than men. Latinos are expected to have the largest increase in dementia cases over the projection period.
Figure 1.
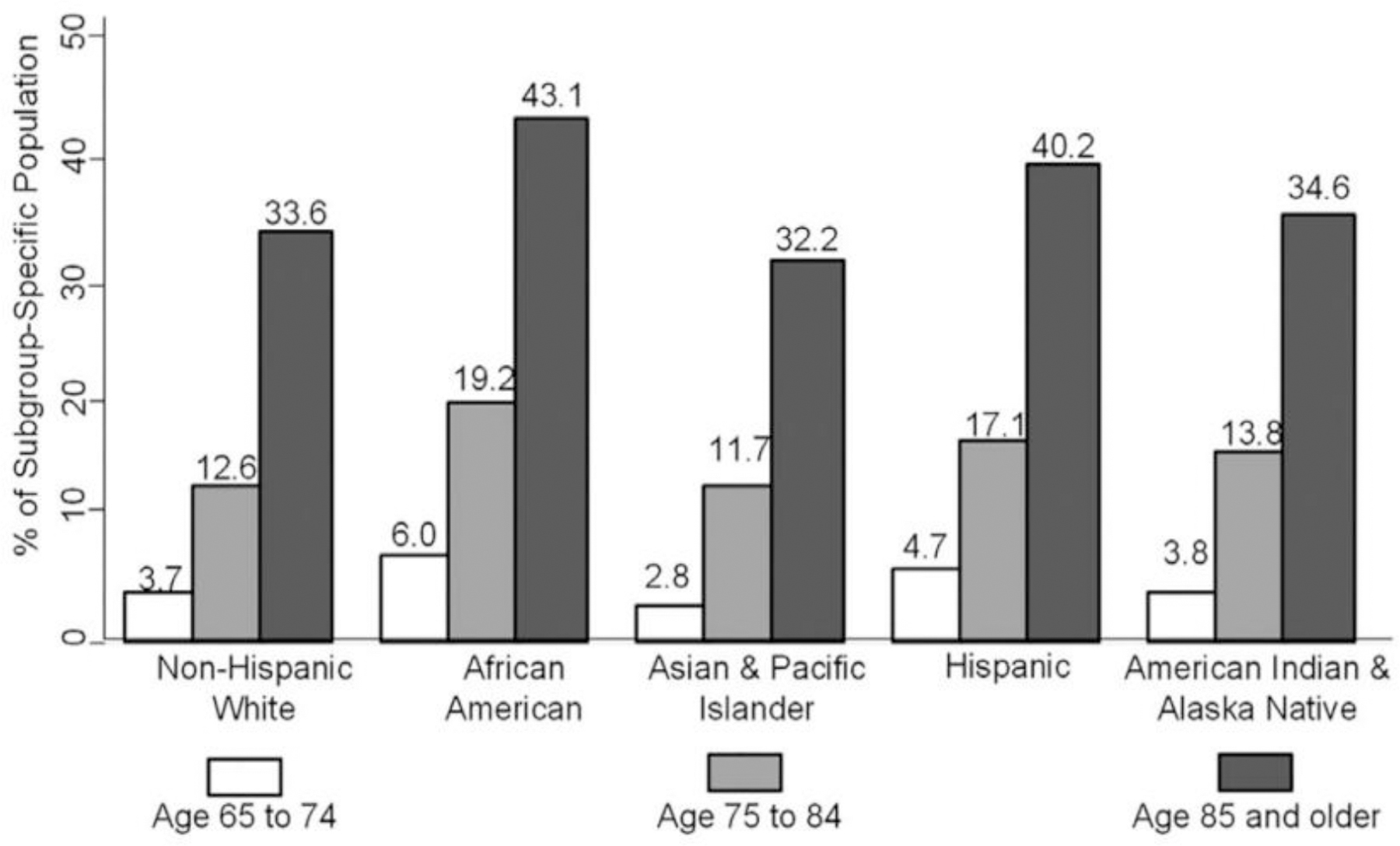
Estimated prevalence of Alzheimer’s disease and related dementias in the US Population aged ≥65 years, by sex and race and ethnicity; United States, 2014.
The mechanisms by which racial and ethnic disparities exist remain unclear. In a regional study of members with Type II diabetes across six racially- and ethnically-diverse groups in a large managed healthcare system,7 Blacks, Native Americans and Latinos had higher dementia incidence and Asians lower incidence, a trend that persisted among individuals in their 90s.7 The rates followed the same racial/ethnic pattern observed in non-diabetic populations suggesting that neither access to healthcare nor the disproportionate burden of Type II diabetes explain the observed racial disparities in ADRD.8 Thus, other mechanisms exist that may account for the disparity over and beyond comorbidity.
Individuals are embedded within community contexts that serve as environmental determinants of health or disease risk. Recent work underscores complex overlapping and interacting factors that shape ADRD risk over time from birth to adulthood such as place of birth and education. For individuals born in US states with high stroke mortality rates, the risk of ADRD increases regardless of race. This may help to partly explain the disparate burden of ADRD among Blacks, who are 9.6 times more likely to be born in a high stroke mortality state.9 These findings suggest the need for clinical, public health, and policy interventions that target cardiovascular risk and life course factors that are place-based (health behaviors, educational quality, discrimination) especially in high-risk groups as a pathway to reduce ADRD incidence.
High-income countries, such as the US, have reported declining age-specific incidence of dementia, yet the putative factors that contribute to the decline have not been adequately identified possibly due to the many possible mechanisms for health disparities. Although incident dementia is decreasing, the benefits are concentrated among people with higher education.10, 11 Education is a key non-biological correlate of cognitive ability that is determined early in life, a source of cognitive disparities,12 and implicated in cognitive reserve, improved health, health behaviors, and healthcare access.1 Other putative mechanisms for ADRD health disparities include variations in medical conditions, health behaviors, environmental exposures, discrimination and structural racism, and epigenetic factors, among others. 1, 13, 14
Emerging Evidence for Dementia Care Disparities
PLWD receive care in healthcare systems and often rely heavily on families and close others for support. We use the term dementia care disparities to encompass equity concerns in healthcare and long-term services and supports. Ensuring that services and supports are accessible and effective for all segments of our population is essential for health equity.15 Because the impacts of dementia and disparities in health and health outcomes for PLWD and families often are substantial, equity demands that these disproportionate impacts be addressed. For example, racial and ethnic minorities, persons with lower socioeconomic status, sexual and gender minorities, and rural populations experience greater challenges accessing and receiving quality services – disparities that are growing more extreme and entrenched as our older adult population becomes increasingly diverse and stratified.1
Higher dementia prevalence and incidence in certain racial and ethnic populations (i.e., African Americans, Native Americans, and Latinos) translates to a higher likelihood that families from these populations will be caring for a relative with dementia.3, 7 African American and Latino families provide higher intensity caregiving,16, 17 report more unmet needs,18 and provide care to PLWD with higher levels of dementia-related behavioral symptoms. 19, 20 Studies have found variability in caregiver psychological well-being among race and ethnic subgroups.21, 22 Moreover, evidence indicates racial and ethnic minority care partners may be in poorer health when entering a caregiving role.23 Racial and ethnic minorities and care partners with lower socioeconomic status are also more likely to experience adverse social determinants of health and discrimination which increase stress and create barriers to healthcare access.1, 24
There is growing evidence, most of it based on Black and Latino PLWD, of healthcare-related disparities (Figure 2). At early stages of help-seeking, there are too often delayed diagnosis25, 26 and misdiagnosis,4, 27 and challenges in accessing services and referrals to dementia specialist care especially for Latinos and Asians.28 Evidence suggests different pharmacological treatment for racial and ethnic minority groups. Post-diagnosis, Black and Latino PLWD are less likely to be prescribed and more likely to discontinue anti-dementia medications.29, 30 Prior work suggests a potentially problematic increase in antipsychotic utilization among Latinos even after controlling for dementia severity.31 and higher hospital mortality rates among African Americans and Latinos.32 Black and Latino PLWD are more likely to reside in under-resourced nursing homes33 where higher rates COVID-related deaths have occurred.34 There is less likelihood for advanced care planning among Blacks and Latinos compared with white non-Hispanics35 which could account for more aggressive, higher-intensity, costly care at end of life,36, 37 with the latter findings potentially explained by care preferences.38
Figure 2.
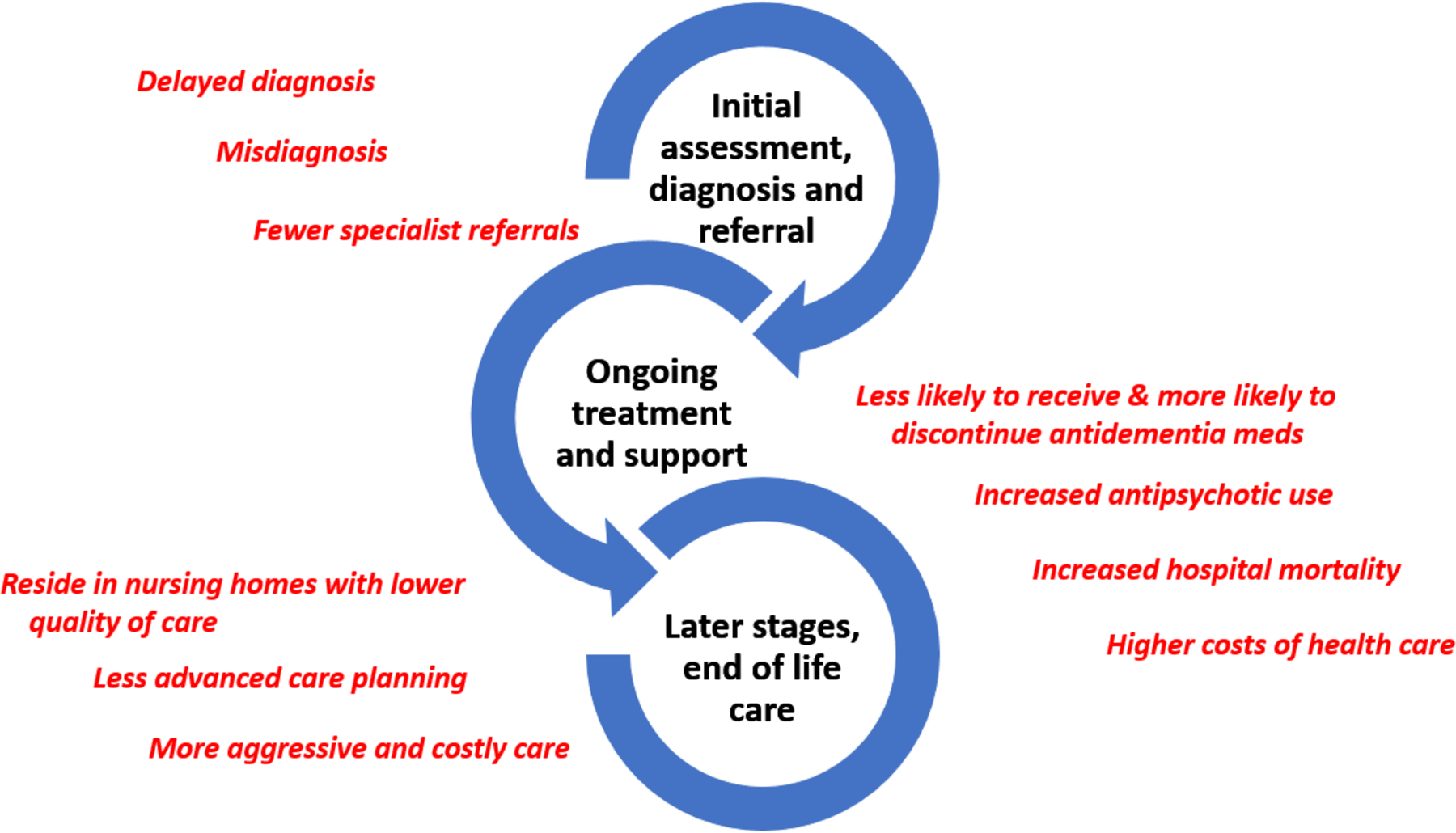
Emerging Evidence of Care Disparities for African-American and Latinx Persons Living with Dementia.
Healthcare system disparities are amplified by significant gaps in the evidence base for pharmacological and non-pharmacological interventions,39, 40 including care partner support interventions and recruitment strategies. 41 There is well-documented and striking under-representation of disparities populations in ADRD clinical trials and caregiving intervention research. For example, findings from a review of 48 studies,42 found that 67% of the studies did not report results by racial/ethnic group, or gender. Among studies that did, nearly 80% reported statistically significant differences in treatment outcomes by racial/ethnic group membership. Using the NIH Stage Model for Behavioral Intervention Development as a guide, we find many interventions that reach Stage IV or Stage V trials have been tested inadequately in diverse populations, with minimal or no evidence of efficacy and risk, or alignment with study participant preferences. Thus, addressing these evidence gaps will require that interventions are sufficiently examined in diverse populations prior to being evaluated at the pragmatic trials stage.43
Economic Costs of Dementia
Growth in the ADRD population raises questions about the full extent of the economic impact, who bears this burden, and how interventions could improve quality of life while reducing unequal burden and access. Evidence regarding disparities in economic costs is has received limited attention, yet some subgroup disparities are emerging. As new care models and innovations in diagnostics and treatments rise, assessments of their costs and effectiveness will aid in equitable distribution of resources.
The combined medical and caregiving costs associated with the care of all persons in the US with Alzheimer’s dementia were estimated to exceed $500 billion in 2020 and are projected to rise to $1.6 trillion (inflation-adjusted) by 2050.44, 45 Of the costs of paid services and supports, about 25% are paid out-of-pocket and 75% are paid for by Medicare or Medicaid. For approximately 200,000 Americans who have younger-onset of Alzheimer’s,45 the proportion of costs paid out-of-pocket may be significantly higher. The per capital annual medical costs of persons living with Alzheimer’s age 70 and older, inclusive of family care partners’ time, is over $81,000 and will reach $92,060 by 2030 (Figure 3). For persons with no Alzheimer’s diagnosis the costs are almost two-thirds less through 2050.44 The value of time spent caregiving, including all hours across multiple care partners, comprises 30% to 50% of the total costs of dementia care, depending on the method used for valuing the time of care partners.44, 46 Out-of-pocket and caregiving costs are particularly high in the last year of life among community dwelling PLWD, and account for just over 50% of total expenditures.47
Figure 3.
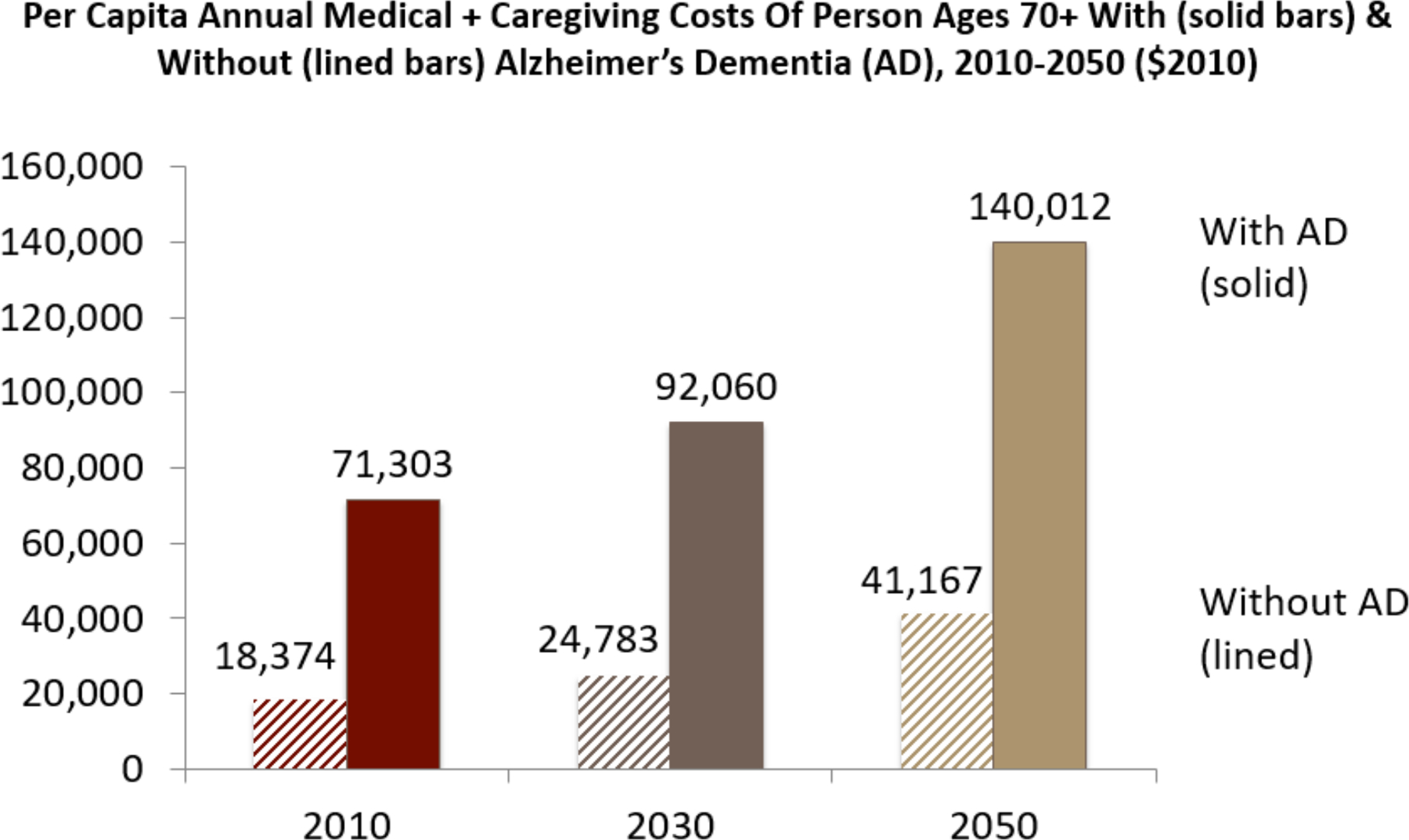
Average Annual Costs Are Increasing and Higher for Persons Living with Alzheimer’s Disease
There is a paucity of studies that examine heterogeneity of costs by demographic characteristics of PLWD such as race, ethnicity, and living arrangement.48 Studies find both higher and lower medical expenditures among non-whites compared to whites and variation across types of medical care expenditures likely reflecting differential access to care and preference for types of care.49 Typically, PLWD have multiple co-morbid conditions that contribute to healthcare use and costs.50 Persons with Alzheimer’s dementia are more likely to be hospitalized and have longer stays and use more post-acute skilled nursing care and home health care than otherwise similar adults without dementia.51–53 Lifetime out-of-pocket medical spending at age 65 is estimated to be more than $38,000 higher for PLWD compared to those without dementia.54 Similarly, average lifetime medical care, long term care costs and unpaid care costs after age 70, for someone who acquired Alzheimer’s dementia, has been estimated to be just over $700,000 compared to $250,000 for persons who never acquired Alzheimer’s dementia death.44
Economic costs are devastating for individuals and families and likely unsustainable for healthcare systems if numbers of PLWD rise as projected. These estimates are likely an underestimate as they often exclude the effects for care partners. Care partners experience wage and productivity loss, and increased healthcare utilization. 23, 55 Other financial costs to PLWD and their families include changes to living environments to accommodate health issues, other paid services, loss of wealth due to financial decision making errors or susceptibility to scams,56 and early withdrawals from retirement funds along with lost Social Security earnings. Economic impacts may reverberate across generations as adult children may bear income and wealth costs now and years later (selling the family home to cover long-term care costs, and being at higher risk for acquiring dementia in later life).57 ADRD family care partners often incur devastating out-of-pocket costs, and uncompensated labor.46 Black, Latino and less than high-school educated care partners experience greater out of pocket costs.58, 59 Latino and African American women devoted significant portions of their annual income to caregiving, 47% and 40%, respectively,58 which may account for increased economic impacts for them as they enter later adulthood.
Blacks pay higher healthcare costs compared with white non-Hispanics.60, 61 The economic costs of dementia for African Americans is high as evidenced by the fact that although they comprise 13.6% of the US population, they represent one-third of the costs of ADRD. Families of African-American women with AD bear the lion’s share: 60% of the costs of dementia care are borne by families of African-American women with AD.62 For US Latinos 65 years and older living with dementia, it is projected that the direct costs (medical plus long-term care) will be $169.1 billion after adjusting for inflation, or 24 times greater than costs in 2012 ($6.9 billion) due to the accelerated growth in the older Latino population. Lost earnings for Latinos due to AD are projected to increase over 10 times from $272 million in 2012 to $2.7 billion in 2060 (in 2012 dollars).63
Costs vary across the continuum of disease from preclinical to prodromal to moderate and severe stages.64 Studies report higher cost of mild Alzheimer’s disease dementia compared to mild cognitive impairment65 and from mild to severe dementia66 but for whom and why is not fully understood. Costs vary across the etiological dementia types,67 although population level information on dementia subtype is limited and 30% of dementia diagnoses reported in health care claims are for unspecified dementia.28
Misdiagnosis of Alzheimer’s disease is common; about 20% of persons initially diagnosed with Alzheimer’s had subsequent diagnoses of non-Alzheimer’s dementia.28 Evidence suggests Alzheimer’s misdiagnosis (first diagnosed as Alzheimer’s and subsequently diagnosed as non-Alzheimer’s dementia) is associated with higher costs due to more inpatient days, emergency department visits and out-patient visits. Persons with diabetes-related complications and dementia had higher costs than those without dementia68 although less is known about the full range of co-occurring diseases for PLWD and their impacts on inpatient and outpatient healthcare utilization.
Policies, services, and care delivery environments also impact costs as well as who bears the costs. While Medicare pays for home health care, for persons requiring nursing home care, Medicare only pays for the first 100 days while Medicaid will cover longer stays and memory care units in nursing homes. Newer Medicare benefits such as the Annual Wellness Visit, with required cognitive screening, may increase early detection and reduce costs, but whether this occurs remains unknown.69 Utilization rates for the Annual Wellness Visit cognitive assessment benefit remain stubbornly low nearly a decade after implementation. 70 Other changes such as reimbursement incentives to reduce post-acute institutional care, have decreased Medicare costs but may be shifting costs to families.71 Most studies examine costs among beneficiaries in traditional Medicare. Yet over one-third of beneficiaries are in Medicare Advantage plans with incentives for care coordination not found in traditional Medicare. Medicare Advantage plans have lower hospitalizations and readmissions than traditional Medicare72 but whether these differences persist for PLWD is unknown. Dementia cost estimates in Medicare Advantage plans vary widely in the few studies reporting them.52
Conclusion
Dementia imposes enormous and unsustainable burdens to individuals, families, communities, systems of care, government and society at large. These are borne disproportionately and increasingly, by certain populations including older people, underrepresented racial and ethnic groups, women, people living or having lived in high stroke mortality states, and people with low education and socioeconomic status, among others.
Disparities research begins with recognition of disparities, then focuses on understanding the mechanisms of those disparities, and ultimately should lead to disparities reduction.73 In much of the existing work on dementia healthcare disparities, we have identified differences in services and treatment, but it remains unclear to what extent these reflect personal preferences, clinically appropriate decisions, or true disparities. Relatively little work has moved to understanding mechanisms to reduce disparities although certain areas appear ready for this stage of research such as misdiagnosis, anti-dementia medications. Care partner support is a critical aspect of routine dementia care, yet we lack sufficient data to examine potential disparities in receipt of support.22, 41, 74 There is a paucity of research for many disparity populations and glaring gaps in the evidence base for intervention efficacy and effectiveness. More research is needed to understand to what extent disparities are driven by unequal access, lower quality of care, exposure to discrimination versus patient/family preferences and clinical appropriateness.
To advance knowledge we need to prioritize research that examines individuals and groups that are disproportionately affected by ADRD and disease burden yet have limited resources to prevent, treat, or manage cognitive decline. Research opportunities and care strategies will need to identify risk factors and social determinants of health and promote multilevel strategies that incorporate multiple action points similar to the breadth of determinants presented in the NIH Health Disparities Frameworks. 14
Towards this end, future research will need to integrate multiple sources of population-level and clinical data and to employ rigorous methods for quantifying heterogeneity in genetic, health, functional, social, behavioral, financial, and neighborhood impacts for PLWD and care partners across diverse groups. Employing rigorous methods for identifying the drivers of heterogeneity would facilitate opportunities to reduce the impact of dementia and to derive greater value from funds expended by individuals, families, public and private payers, and taxpayers.
Whole of society challenges demand research producing whole of society solutions.
As noted by a recent NASEM report,74 most dementia care research has focused on interventions at the individual level, and underrepresented groups have not received adequate attention. Thus, although significant evidence gaps exist, opportunities to study interventions at the community, policy and society levels can pave the way for innovation. Given the enormous number and heterogeneity of PLWD and informal care partners along with insidious health disparities, individual, family, and policy level interventions are of heightened urgency to redress broad unmet need and institutionalized disparities.
The urgency, complexity and scale merit a “whole of government” approach involving collaboration across numerous federal agencies. The National Institutes of Health (NIH) is the most well-resourced and visible federal agency sponsoring and conducting dementia care research. Important complementary activities are led by agencies including the Center for Medicare and Medicaid Innovation (CMMI), the Administration for Community Living, the Centers for Disease Control and Prevention, the Health Resources and Services Administration, the Veterans Administration, the Agency for Health Research and Quality, among others. To the extent practicable, the engagement of the Departments of Labor, Commerce, Justice and Education would strengthen these efforts by addressing challenges in the dementia care workforce, economic burdens of dementia, and redressing abuse, and exploitation. Thanks to congressional action, NIH funding for ADRD research has grown from approximately $500 million in fiscal year 2012 to $3.1 billion in fiscal year 2021.75 While the bulk of funding remains centered on biomedical research, NIH expanded its dementia care research portfolio; in fiscal year 2020, NIH supported 67 dementia care and caregiver intervention research projects.75 However, other agencies lag behind. For example, only four CMMI Health Care Innovation Awards focused on dementia. Despite the National Alzheimer’s Project Act’s decade-old mandates for an annual national plan and creation of a federal advisory council with representatives from many of these agencies, no formal mechanism exists to facilitate coordination of dementia research agendas across agencies. Additional resources would allow agencies to accelerate ADRD disparities research and then scaling, disseminating and improving effective dementia care interventions in ways that optimize quality of life, improve value, and ensure health equity.
Supplementary Material
Supplementary File S1. Description of Summit Theme 1. Impact of Dementia
Key Points.
Dementia incidence is decreasing yet the advances in population health are uneven.
Significant gaps exist in the evidence base for pharmacological and non-pharmacological interventions, and readiness to disseminate in real-world settings for underrepresented groups is lagging.
The combined medical and caregiving costs associated with the care of all persons in the US with dementia is high and unsustainable based on projection estimates.
Why Does this Paper Matter?
Ensuring ADRD healthcare services and long term care services and supports are accessible, affordable and effective for all segments of our population is essential for health equity. Research addressing risk factors and social determinants of health is needed to reduce individual, family, healthcare and societal disease burden of ADRD across all groups, and groups that disproportionately bear the burden.
ACKNOWLEDGEMENTS
We thank all our summit participants but most importantly persons living with dementia and their care partners who were instrumental in planning, executing, and documenting the summit proceedings. Our appreciation goes to summit Co-Chairs, Jennifer Wolff and David Reuben, for their leadership and reviews of prior versions of the manuscript.
FINANCIAL DISCLOSURE
María P. Aranda, PhD, and Ladson Hinton, MD were supported by the National Institute on Aging (NIA) of the National Institutes of Health under Award Number U54AG063546, which funds NIA Imbedded Pragmatic Alzheimer’s Disease and AD-Related Dementias Clinical Trials Collaboratory (NIA IMPACT Collaboratory). María P. Aranda, PhD was supported by the National Institute on Aging (NIA) P50AG05142, and R13AG063477. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Julie Zissimopoulos, PhD, was supported by the National Institute on Aging (NIA) of the National Institutes of Health under Award Numbers R01AG055401, P30AG066589 and P30AG043073 which fund social science research on Alzheimer’s disease and other dementias. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Rachel A. Whitmer PhD was supported by National Institutes on Aging (NIA) of the National Institutes of Health under award numbers RF1AG056519, RF1AG050782, and RF1AG052132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Chanee Fabius, PhD was supported by the National Institute on Aging (NIA) of the National Institutes of Health under Award Numbers P30AG066587 and P30AG059298.
The content of this paper is solely the responsibility of the authors as noted above and does not necessarily represent the official views of the funder, the National Institutes of Health.
SPONSOR SUPPORT
The summit was sponsored by the National Institute on Aging, Division of Behavioral and Social Research (NIA/BSR). The views expressed in this document reflect both individual and collective opinions of the summit meeting participants and authors of this paper, and not necessarily those of NIA/BSR.
Footnotes
This paper was presented at the 2020 National Research Summit on Care, Services, and Supports for Persons with Dementia and Their Caregivers in Bethesda, MD
CONFLICT OF INTEREST
No conflicts reported.
REFERENCES
- 1.Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures Special Report Race, Ethnicity and Alzheimer’s in America 2021. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf
- 2.Braveman P. What are health disparities and health equity? We need to be clear. Public health reports 2014;129(1_suppl2):5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia 2017;13(1):72–83. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2019;5:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology review 2008;18(3):223–254. [DOI] [PubMed] [Google Scholar]
- 6.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer’s & Dementia 2019;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia 2016;12(3):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes Care 2014;37(4):1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Association between birth in a high stroke mortality state, race, and risk of dementia. JAMA neurology 2017;74(9):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoeni RF, Freedman VA, Langa KM. Introduction to a supplement on population level trends in dementia: causes, disparities, and projections. The Journals of Gerontology: Series B 2018;73(suppl_1):S1–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. New England Journal of Medicine 2016;374(6):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez FS, Aranda MP, Lloyd DA, Vega WA. Racial and Ethnic Disparities in Dementia Risk Among Individuals With Low Education. The American Journal of Geriatric Psychiatry 2018/09/01/ 2018;26(9):966–976. [DOI] [PubMed] [Google Scholar]
- 13.Aranda MP, Vega WA, Richardson JR, Resendez J. Priorities for Optimizing Brain Health Interventions Across the Life Course in Socially Disadvantaged Groups 2019. https://www.usagainstalzheimers.org/sites/default/files/2019-10/fiu_paper_10.18.19%20%281%29.pdf [Google Scholar]
- 14.Hill CV, Perez-Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis August 7 2015;25(3):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans MK. Health Equity—Are We Finally on the Edge of a New Frontier? New England Journal of Medicine 2020;383(11):997–999. [DOI] [PubMed] [Google Scholar]
- 16.Friedman EM, Shih RA, Langa KM, Hurd MD. US prevalence and predictors of informal caregiving for dementia. Health Affairs 2015;34(10):1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabius CD, Wolff JL, Kasper JD. Race differences in characteristics and experiences of black and white caregivers of older Americans. The Gerontologist 2020;60(7):1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison KL, Ritchie CS, Patel K, et al. Care settings and clinical characteristics of older adults with moderately severe dementia. Journal of the American Geriatrics Society 2019;67(9):1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sink KM, Covinsky KE, Newcomer R, Yaffe K. Ethnic differences in the prevalence and pattern of dementia‐related behaviors. Journal of the American Geriatrics Society 2004;52(8):1277–1283. [DOI] [PubMed] [Google Scholar]
- 20.Salazar R, Dwivedi AK, Royall DR. Cross-ethnic differences in the severity of neuropsychiatric symptoms in persons with mild cognitive impairment and Alzheimer’s disease. The Journal of neuropsychiatry and clinical neurosciences 2017;29(1):13–21. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Badana AN, Burgdorf J, Fabius CD, Roth DL, Haley WE. Systematic review and meta-analysis of racial and ethnic differences in dementia caregivers’ well-being. The Gerontologist 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eden J, Schulz R, National Academies of Sciences EaM, Division HaM, Services BoHC, Adults CoFCfO. Families Caring for an Aging America 2016:1–345. 2016. [PubMed] [Google Scholar]
- 23.Chen C, Thunell J, Zissimopoulos J. Changes in physical and mental health of Black, Hispanic, and White caregivers and non‐caregivers associated with onset of spousal dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2020;6(1):e12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young HM, Bell JF, Whitney RL, Ridberg RA, Reed SC, Vitaliano PP. Social determinants of health: Underreported heterogeneity in systematic reviews of caregiver interventions. The Gerontologist 2020;60(Supplement_1):S14–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper C, Tandy AR, Balamurali TBS, Livingston G. A Systematic Review and Meta-Analysis of Ethnic Differences in Use of Dementia Treatment, Care, and Research. The American Journal of Geriatric Psychiatry 2010;18(3):193–203. [DOI] [PubMed] [Google Scholar]
- 26.Livney MG, Clark CM, Karlawish JH, et al. Ethnoracial differences in the clinical characteristics of Alzheimer’s disease at initial presentation at an urban Alzheimer’s disease center. The American Journal of Geriatric Psychiatry 2011;19(5):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2019;5:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drabo EF, Barthold D, Joyce G, Ferido P, Chui HC, Zissimopoulos J. Longitudinal analysis of dementia diagnosis and specialty care among racially diverse Medicare beneficiaries. Alzheimer’s & Dementia 2019;15(11):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuckerman IH, Ryder PT, Simoni-Wastila L, et al. Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 2008;63(5):S328–S333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorpe CT, Fowler NR, Harrigan K, et al. Racial and Ethnic Differences in Initiation and Discontinuation of Antidementia Drugs by Medicare Beneficiaries. J Am Geriatr Soc September 2016;64(9):1806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filshtein T, Beckett LA, Godwind H, Hinton L, Xiong GL. Incident Antipsychotic Use in a Diverse Population with Dementia. Journal of the American Geriatrics Society 2016;64(9):e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherzai D, Sherzai A, Sahak M, Ani C. Age and Race Specific Trends and Mortality for Dementia Hospitalization in the US. Journal of Neurology & Neurophysiology 2016;7(1):1–6. [Google Scholar]
- 33.Rivera-Hernandez M, Kumar A, Epstein-Lubow G, Thomas KS. Disparities in nursing home use and quality among African American, Hispanic, and white Medicare residents with Alzheimer’s disease and related dementias. Journal of aging and health 2019;31(7):1259–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Cen X, Cai X, Temkin‐Greener H. Racial and ethnic disparities in COVID‐19 infections and deaths across US nursing homes. Journal of the American Geriatrics Society 2020;68(11):2454–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison K, Adrion E, Ritchie C. Low Completion and Disparities in Advance Care Planning Activities Among Older Medicare. JAMA 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byhoff E, Harris JA, Langa KM, Iwashyna TJ. Racial and ethnic differences in end‐of‐life medicare expenditures. Journal of the American Geriatrics Society 2016;64(9):1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ornstein KA, Zhu CW, Bollens-Lund E, et al. Medicare Expenditures and Healthcare Utilization in a Multi-ethnic Community-based Population with Dementia from Incidence to Death. Alzheimer disease and associated disorders 2018;32(4):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. Journal of general internal medicine 2009;24(6):695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vyas MV, Raval PK, Watt JA, Tang-Wai DF. Representation of ethnic groups in dementia trials: systematic review and meta-analysis. Journal of the neurological sciences 2018;394:107–111. [DOI] [PubMed] [Google Scholar]
- 40.Canevelli M, Bruno G, Grande G, et al. Race reporting and disparities in clinical trials on Alzheimer’s disease: a systematic review. Neuroscience & Biobehavioral Reviews 2019;101:122–128. [DOI] [PubMed] [Google Scholar]
- 41.Dilworth-Anderson P, Moon H, Aranda MP. Dementia caregiving research: Expanding and reframing the lens of diversity, inclusivity, and intersectionality. The Gerontologist 2020;60(5):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmore-Bykovskyi A, Johnson R, Walljasper L, Block L, Werner N. Underreporting of gender and race/ethnicity differences in NIH-funded dementia caregiver support interventions. American Journal of Alzheimer’s Disease & Other Dementias® 2018;33(3):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quiñones AR, Mitchell SL, Jackson JD, et al. Achieving health equity in embedded pragmatic trials for people living with dementia and their family caregivers. Journal of the American Geriatrics Society 2020;68:S8–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zissimopoulos J, Crimmins E, Clair PS. The value of delaying Alzheimer’s disease onset De Gruyter; 2015:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia 2019;15(3):321–387. [DOI] [PubMed] [Google Scholar]
- 46.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. New England Journal of Medicine 2013;368(14):1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelley AS, McGarry K, Bollens‐Lund E, et al. Residential setting and the cumulative financial burden of dementia in the 7 years before death. Journal of the American Geriatrics Society 2020;68(6):1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantarero-Prieto D, Leon PL, Blazquez-Fernandez C, Juan PS, Cobo CS. The economic cost of dementia: A systematic review. Dementia 2019:1471301219837776. [DOI] [PubMed] [Google Scholar]
- 49.Park S, Chen J. Racial and ethnic patterns and differences in health care expenditures among Medicare beneficiaries with and without cognitive deficits or Alzheimer’s disease and related dementias. BMC geriatrics 2020;20(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacNeil‐Vroomen JL, Thompson M, Leo‐Summers L, Marottoli RA, Tai‐Seale M, Allore HG. Health‐care use and cost for multimorbid persons with dementia in the National Health and Aging Trends Study. Alzheimer’s & Dementia 2020;16(9):1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White L, Fishman P, Basu A, Crane PK, Larson EB, Coe NB. Medicare expenditures attributable to dementia. Health services research 2019;54(4):773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fishman P, Coe NB, White L, et al. Cost of dementia in Medicare managed care: a systematic literature review. The American journal of managed care 2019;25(8):e247. [PMC free article] [PubMed] [Google Scholar]
- 53.Lin PJ, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare expenditures of individuals with Alzheimer’s disease and related dementias or mild cognitive impairment before and after diagnosis. Journal of the American Geriatrics Society 2016;64(8):1549–1557. [DOI] [PubMed] [Google Scholar]
- 54.Hudomiet P, Hurd MD, Rohwedder S. The relationship between lifetime out-of-pocket medical expenditures, dementia, and socioeconomic status in the US. The Journal of the Economics of Ageing 2019;14:100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goren A, Montgomery W, Kahle-Wrobleski K, Nakamura T, Ueda K. Impact of caring for persons with Alzheimer’s disease or dementia on caregivers’ health outcomes: findings from a community based survey in Japan. BMC geriatrics 2016;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholas LH, Langa KM, Bynum JP, Hsu JW. Financial presentation of Alzheimer disease and related dementias. JAMA internal medicine 2021;181(2):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coe NB, Skira MM, Larson EB. A comprehensive measure of the costs of caring for a parent: differences according to functional status. Journal of the American Geriatrics Society 2018;66(10):2003–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rainville C, Skufca L, Mehegan L. Family Caregiving and Out-of-Pocket Costs: 2016 Report 2016. https://www.aarp.org/content/dam/aarp/research/surveys_statistics/ltc/2016/family-caregiving-costs. [Google Scholar]
- 59.Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Annals of internal medicine 2015;163(10):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilligan AM, Malone DC, Warholak TL, Armstrong EP. Health disparities in cost of care in patients with Alzheimer’s disease: an analysis across 4 state Medicaid populations. American Journal of Alzheimer’s Disease & Other Dementias® 2013;28(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatr Serv January 2003;54(1):92–6. [DOI] [PubMed] [Google Scholar]
- 62.Gaskin DJ, LaVeist TA, Richard P. African American Network Against Alzheimer’s: The Costs of Alzheimer’s and Other Dementia for African Americans 2013. https://www.usagainstalzheimers.org/sites/default/files/USA2_AAN_CostsReport.pdf [Google Scholar]
- 63.Wu S, Vega WA, Resendez J, Jin H. Latinos and Alzheimer’s Disease: New Numbers Behind the Crisis Aging UERRIo; 2016. https://roybal.usc.edu/wp-content/uploads/2016/10/Latinos-and-AD_USC_UsA2-Impact-Report.pdf [Google Scholar]
- 64.Jonsson L, Lin PJ, Khachaturian A. Editorial. Alzheimer’s and Dementia; 2017. p. 201–204. [DOI] [PubMed] [Google Scholar]
- 65.Robinson RL, Rentz DM, Andrews JS, et al. Costs of Early Stage Alzheimer’s Disease in the United States: Cross-Sectional Analysis of a Prospective Cohort Study (GERAS-US). Journal of Alzheimer’s Disease 2020;(Preprint):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky‐Rabas PL. The main cost drivers in dementia: a systematic review. International journal of geriatric psychiatry 2015;30(2):111–129. [DOI] [PubMed] [Google Scholar]
- 67.Chen MS. Rectifying Disparities in Funding of Asian American, Native Hawaiian, and Pacific Islander Research by the US National Institutes of Health. JAMA network open 2019;2(7):e197561–e197561. [DOI] [PubMed] [Google Scholar]
- 68.Lin PJ, Fillit HM, Cohen JT, Neumann PJ. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related disorders. Alzheimer’s & Dementia 2013;9(1):30–38. [DOI] [PubMed] [Google Scholar]
- 69.Institute R. Impact of Medicare annual wellness visit on detection of cognitive impairment is minimal 2018. https://medicalxpress.com/news/2018-04-impact-medicare-annual-wellness-cognitive.html
- 70.Jacobson M, Thunell J, Zissimopoulos J. Cognitive Assessment At Medicare’s Annual Wellness Visit In Fee-For-Service And Medicare Advantage Plans: Study examines the use of Medicare’s annual wellness visit and receipt of cognitive assessment among Medicare beneficiaries enrolled in fee-for-service Medicare or Medicare Advantage. Health Affairs 2020;39(11):1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chatterjee P, Hoffman A, Werner R. Shifting the burden? Consequences of postacute care payment reform on informal caregivers. Health Affairs Blog 2019; [Google Scholar]
- 72.Cohen R, Lemieux J, Schoenborn J, Mulligan T. Medicare Advantage Chronic Special Needs Plan boosted primary care, reduced hospital use among diabetes patients. Health Affairs 2012;31(1):110–119. [DOI] [PubMed] [Google Scholar]
- 73.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. American journal of public health 2006;96(12):2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Academies of Science EaM. Meeting the Challenge of Caring for Persons Living with Dementia and Their Care Partners and Caregivers: A Way Forward 2021. [PubMed] [Google Scholar]
- 75.RePORT NIH. Estimates of Funding for Various Researchers, Condition, and Disease Categories (RCDC) 2020. https://report.nih.gov/funding/categorical-spending#/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File S1. Description of Summit Theme 1. Impact of Dementia


