Abstract
Purpose
The purpose of this study was to analyze the impact of cumulative hearing hour percentage (HHP) on pediatric cochlear implant users' speech and language development at age 3 years and to determine an evidence-based wear time recommendation that yields typical spoken language standard scores.
Method
A retrospective chart review of 40 pediatric cochlear implant recipients was completed. Children met the following criteria: prelingually deafened, implanted at age 2 years or younger, utilized a speech processor with datalogging capabilities, a minimum of 1 year of cochlear implant use, and language testing completed at approximately age 3 years. Exclusion criteria included significant inner ear malformation (i.e., common cavity) or developmental delay that would preclude spoken language development.
Results
Multiple regression analysis revealed that age and implantation and HHP were predictive of spoken language skills at age 3 years. Further analysis yielded wear time recommendations associated with age-appropriate spoken language based on the age at implantation.
Conclusions
When the goal is age-appropriate spoken language, wear time recommendations should reflect a child's current age, age at implantation, and the comparative daily sound access of age-matched normal-hearing peers. The HHP measurement can help provide that information. The minimum wear time recommendation should be set to 80% HHP with the ultimate goal of 100% HHP to give pediatric cochlear implant recipients enough access to sound and language to achieve their spoken language goals.
For children diagnosed with congenital severe-to-profound hearing loss, cochlear implantation has become a standard of care, with overwhelming literature to support younger age at implantation for improved spoken language outcomes (Ching et al., 2013; Colletti et al., 2011; Dettman et al., 2016; Houston & Miyamoto, 2010; Leigh et al., 2016; Nicholas & Geers, 2018; Niparko et al., 2010). Implantation before a child's first birthday has become typical practice in many European countries and Australia (Karltorp et al., 2020). Retention at this young age can be challenging with coil-offs and coil-off time found to be higher the younger the child's age (Easwar et al. 2016). Variability in moods, time spent in car seats, strollers or highchairs, and the general instability of infants and toddlers are retention obstacles unique to this age group (Easwar et al., 2016; Walker et al., 2013). To overcome these challenges, clinicians and parents navigate the vast array of hearing technology retention options such as headbands, shirt clips, pilot caps, toupee tape, and manufacturer specific accessories. At device activation, clinicians counsel parents about the importance of wear time with clinical jargon such as full-time use, consistent use, all waking hours, eyes open ears on, and all the time. Yet, these phrases do not give a parent a specific and tangible goal for their child's cochlear implant device use.
The datalogging feature in cochlear implant speech processors allows clinicians to give objective feedback to recipients and parents regarding cochlear implant wear time. A variety of information from time the device is maintaining lock, the number of coil-offs, coil-off time, streaming, and program usage are just a few of the measures clinicians may view in the programming software. Clinicians have access to a plethora of datalogging metrics; however, there is limited research assessing what wear time a child should be striving to meet. Current research does not have a universal approach to quantifying full-time use, nor are full-time use metrics based on spoken language outcomes for cochlear implant recipients.
There is ample research assessing pediatric cochlear implant device use; however, there is not cohesion regarding how to quantify or label full-time use. Some studies have focused on subjective descriptions or reports. Contrera et al. (2014) used e-mail or postal surveys to assess device wear time and set regular cochlear implant device use at 8 hr per day in a study of long-term rates of cochlear implant use. Similarly, 8 hr was the definition for consistent device used by Spencer et al. (2004) when obtaining wear time via patient questionnaire in a long-term outcomes study, though this was below the average reported length of device use of 10.56 hr. A study by Archbold et al. (2013) utilized parent judgment to rank their child's device use into the following categories: all of the time, most of the time, some of the time, or none of the time. These categories were not given any further definitions or hour ranges. Marnane and Ching (2015) utilized the Parents' Evaluation of Aural/Oral Performance of Children (PEACH) questionnaire (Ching & Hill, 2007) in their usage pattern study. Response options on the PEACH are never (0%), seldom (1%–25%), sometimes (26%–50%), often (51%–75%), and always (75%–100%). Fitzgerald et al. (2013) studied acceptance of sequential cochlear implants by wear time between the devices, consistent bilateral use was denoted as bilateral use all waking hours (parent report), and inconsistent bilateral use was defined as wearing one device all waking hours while sometimes turning off the second device. These studies all address subjective device use but are not universal or objective in how they define or quantify cochlear implant use.
Further studies have analyzed objective datalogging evidence and reveal average wear time found for the study population. Easwar et al. (2016) found that children use their cochlear implant for an average of 9.86 hr. In a subsequent study of children capable of completing speech perception testing, Easwar et al. (2018) found that increased device use and increased duration of cochlear implant use were associated with increased speech perception outcomes. Busch et al. (2017) reviewed 1,501 logs from various clinics across the world, with recipients ranging in age from 0 to 96 years with the aim of analyzing the users' auditory environment. This study found that average wear time increased with age, with an average for early childhood use at 8.5 hr. A recent article from Busch et al. (2020) reveals a positive association receptive vocabulary scores on the Peabody Picture Vocabulary Test (PPVT) and daily cochlear implant use in congenitally deafened children. Daily cochlear implant use in this study was calculated based on average wear time in the year prior to the PPVT assessment. The mean wear time was 10.07 hours per day. These studies provide insight into objective cochlear implant wear time and the range of average wear time based on age and how use impacts speech perception and vocabulary development, yet, there are no current evidenced-based wear time recommendations based on spoken language outcomes. Wiseman and Warner-Czyz (2018) state:
The current study defined ‘full-time' use as 8 hours of daily device use to roughly match the earlier research and clinical recommendations. However, it is unknown the amount of device use a child with a CI needs to yield optimal outcomes. (p. 135)
This illustrates the current void in datalogging research and the need to quantify the requisite cochlear implant wear time necessary to achieve the desired spoken language outcome. As standard of care is pivoting toward younger age at implantation, the need for a wear time recommendation based on language outcomes is magnified. Two recent studies have introduced the hearing hour percentage (HHP) as a wear time metric to account for both a child's age and wake time (Gagnon et al., 2020; Park et al., 2019). The HHP uses the objective wear time datalog as a percentage of the mean wake time for children based on their age, or the estimated percentage of the day that the child has access to sound. This uses typical hearing peers as the baseline metric for hearing, as a typical hearing child hears 100% of their waking hours. Gagnon et al. (2020) found that HHP was a significant predictor of receptive language scores in congenitally deafened cochlear implant recipients after 1 year of device use. Park et al. (2019) found that the age at which a child established full-time use was a better predictor of spoken language development at age 3 years than age at implantation. Full-time use was defined as 80% HHP and was compared to a traditional metric of 8 hr. HHP accounted for more variability in spoken language outcomes making HHP a stronger metric. Park et al. (2019) relied on 80% HHP as full-time use because “it was the highest percentage that greater than 50% of the recipients achieved prior to the language test date” (p. 988). These studies are unique, as they demonstrate the relationship between early cochlear implant use via HHP and language outcomes instead of speech perception outcomes, and focus on young children who are acquiring spoken language. However, they do not reveal what HHP is necessary to obtain typical spoken language development.
The premise made by Park et al. (2019) that age at which a child establishes full-time use predicts outcomes could be reflective of a child's cumulative device use. A child who establishes full-time use early would theoretically have more access to sound for the first 3 years of use. Language abilities at age 3 years were chosen because it is the age when a child makes the transition from an Individualized Family Service Plan, with home-based parent-centered early intervention services (Part C), to an Individualized Education Program with school-based services (Part B). While this is a necessary transition, it is a critical time to have a language foundation and a benchmark age for language assessments, as results from these language assessments will impact the child's educational and classroom placement. Other studies have only utilized the most recent datalog period (Easwar et al. 2016, 2018), which is not reflective of the entirety of the subject's device use. The purpose of this study is twofold: as an extension of Gagnon et al. (2020) to analyze cumulative HHP and spoken language outcomes by the age of 3 years and to produce an evidence-based definition of full-time use that yields typical spoken language.
Method
Participants
This study was approved by the institutional review board at the University of North Carolina at Chapel Hill (UNC). A retrospective chart review was completed from 2014 to 2019, and 40 children met the following criteria: prelingually deafened, completed a speech and language evaluation at age 3 years, a minimum of 1 year of device use by the date of the speech and language evaluation, and utilized a speech processor capable of datalogging for the entirety of device use. There was one exception to these criteria: A participant was included who was deafened due to meningitis at 14 months of age. The following were exclusion criteria: a major anatomical malformation (i.e., common cavity) and no significant developmental delay that would preclude spoken language development. The mean age at implantation was 1.2 years (SD = .47), and the mean duration of cochlear implant use at the time of language evaluation was 1.8 years (SD = .43; see Table 1). Of the 40 subjects, 30 were bilateral cochlear implant recipients, while 10 were unilateral.
Table 1.
This table displays the etiology of hearing loss for the study sample.
| Etiology | Total |
|---|---|
| Connexin 26 | 7 |
| EVA/Pendred syndrome | 5 |
| Infection (CMV, meningitis) | 5 |
| Malformation | 2 |
| Other | 5 |
| Unknown | 13 |
| Waardenburg syndrome | 3 |
| Total | 40 |
Note. EVA = enlarged vestibular aqueduct; CMV = cytomegalovirus.
Data Collection and HHP Calculation
For subjects utilizing Nucleus 6, Nucleus 7 or Kanso processors, datalog files were extracted from the Custom Sound software using the Clinical Datalog Extractor software provided by Cochlear Ltd. Extracted datalogs from the entirety of device use, from activation to speech and language evaluation at age 3 years, were obtained. For recipients utilizing the Med-El Sonnet processor, datalogging information from every clinical visit was input in the UNC Hearing and Language database in the FileMaker platform. The time from one visit date to the next was defined as the datalog period.
The equation for HHP uses the inverse of the median sleep recommendations from the American Academy of Sleep Medicine, which aligns with a sleep meta-analysis to find the child's wake time based on age (Gagnon et al., 2020; Park et al., 2019; Paruthi et al., 2016). A polynomial equation was used to calculate average wake time by age using the data provided in Paruthi et al. (2016).
| (1) |
This calculation results in values of 10.8 hours for 6-month-olds, 10.9 hours for 9-month-olds, 11.1 hours for 12-month-olds, 11.7 hours for 2-year-olds, and 12.3 hours for 3-year-olds. The standard HHP equation, (wear time/mean awake time) × 100, was adjusted to calculate cumulative HHP. Average daily wear time for each log was multiplied by the cumulative number of days for each datalog period and totaled from activation through the date of the language evaluation. For bilateral users, the ear with the greatest total hours was utilized. Average awake hours based on the subject's age during the datalog period were multiplied by the number of days in each datalog period and were totaled as well. The cumulative hours of device use was the numerator with cumulative awake hours at the denominator multiplied by 100 to calculate cumulative HHP. See Table 2 for mean wake time by age.
Table 2.
This table displays the inverse of the average sleep time by age based on pediatric sleep meta-analysis from Paruthi et al. (2016).
| Age | Average wake time (hours) |
|---|---|
| Under 3 Months | 9.4 |
| 3 Months | 10.4 |
| 6 Months | 11.1 |
| 9 Months | 11.4 |
| 12 Months | 11.1 |
| 2 Years | 12 |
| 3 Years | 12.25 |
Note. Average wake time displays the potential time for auditory input based on age to calculate the hearing hour percentage.
Language Testing
Testing was completed using either the Preschool Language Scale–Fifth Edition or Oral and Written Language Scales–Second Edition (Carrow-Woolfolk, 2011; Zimmerman et al., 2011). Both tests yield standard scores with the same mean of 100 and a standard deviation of 15, meaning that scores within the range of typical language development would be between 85 and 115.
Analysis
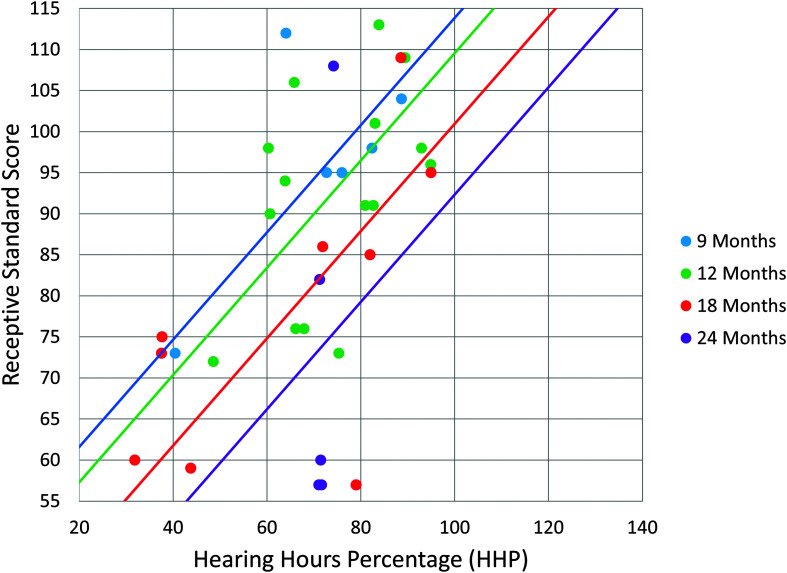
Two multiple regression analyses were run, the first with receptive language scores at age 3 years as the dependent variable and the second with expressive language as the dependent variable. Independent variables for both analyses were cumulative HHP and age at implantation (see Figure 1).
Figure 1.
This graph displays the predicted receptive language standard score based on cumulative HHP and age at implantation. Normal range for standard scores is 85–115. The four regression lines account for the differences in age at implantation. These have the same slope but a different intercept, meaning that increased cumulative HHP is required with increased age at implantation.
Results
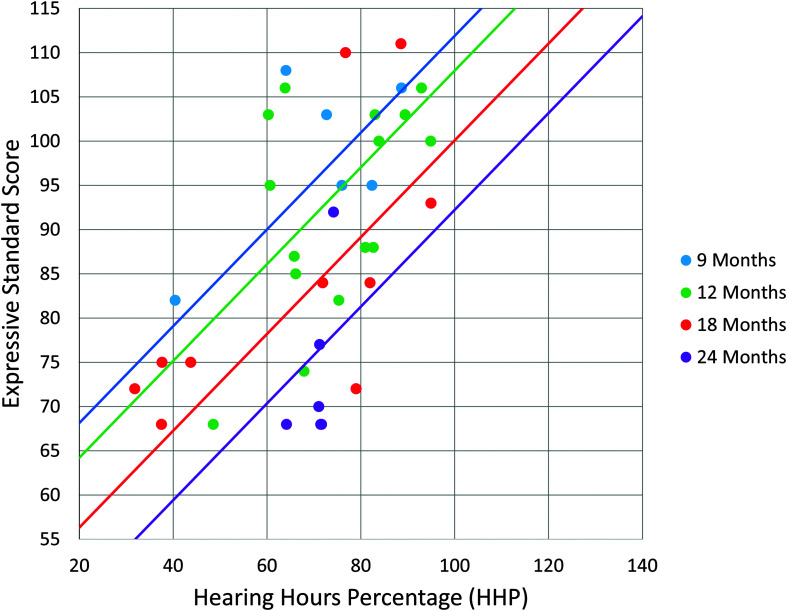
The multiple regression analyses were significant, for both receptive, F(2, 37) = 22.23, p < .001, R 2 = .55, and expressive language, F(2, 37) = 32.48, p < .001, R 2 = .64. This indicated that there was a collective significant effect between cumulative HHP and age at cochlear implantation on language outcomes at age 3 years. When investigating individual predictors, both age at implantation and cumulative HHP were significant predictors for receptive language (p = .001 and p < .001, respectively; see Table 3) and expressive language (p < .001 for both predictors; see Table 4). Based on these regression models, the initial intention was to create a single wear time recommendation from the regression line. However, because age was a significant factor for both models, equations were derived from each model to account for age at implantation. The equation for receptive language was [Y = 61.476–1.435 (age in months) + .653 (HHP)] and the equation for expressive language was [Y = 68.994–1.311(age in months) + .547 (HHP)]. Utilizing these equations, the predicted cumulative HHP required for mean age-appropriate spoken language (standard score of 100) was computed for the following ages of implantation: 9, 12, 18, and 24 months. While the slope of the regression line was constant for each age group, the intercept varied based on age at cochlear implantation (see Figures 1 and 2). The results revealed a requisite cumulative HHP for a 9-month-old to obtain a receptive language standard score of 100 was 79%. For a 12-month-old, this increased to 85% cumulative HHP; for a 18-month-old, this increased to a 99% cumulative HHP; and this increased to a 112% cumulative HHP for a child implanted at 24 months old. For expressive language, to obtain a 100 standard score, a 78% cumulative HHP was required at 9 months, an 85% cumulative HHP was needed at 12 months, a 100% cumulative HHP at 18 months old, and finally increasing to 114% for a child implanted at 24 months old (see Table 5).
Table 3.
This table displays the summary of the multiple regression analysis for receptive language at age 3 years.
| Variable | B | SE B | ϐ | p |
|---|---|---|---|---|
| Intercept | 61.476 | 9.088 | ||
| Age at CI | −1.435 | 0.4 | −0.4 | .001 |
| Cumulative HHP | 0.653 | 0.109 | 0.667 | < .001 |
Note. Both age at implantation and cumulative HHP were found to be significant predictors of receptive language scores. B = unstandardized regression coefficient; SE B = Standard error of the coefficient; ϐ = standardized coefficient; CI = cochlear implantation; HHP = hearing hour percentage.
Table 4.
This table displays the summary of the multiple regression analysis for expressive language at age 3 years.
| Variable | B | SE B | ϐ | p |
|---|---|---|---|---|
| Intercept | 68.994 | 6.442 | ||
| Age at CI | −1.311 | 0.283 | −0.461 | < .001 |
| Cumulative HHP | 0.547 | 0.077 | 0.705 | < .001 |
Note. Both age at implantation and cumulative HHP were found to be significant predictors of receptive language scores. B = unstandardized regression coefficient; SE B = standard error of the coefficient; ϐ = standardized coefficient; CI = cochlear implantation; HHP = hearing hour percentage.
Figure 2.
This graph displays the predicted expressive language standard score based on cumulative HHP and age at implantation. Normal range for standard scores is 85–115. The four regression lines account for the differences in age at implantation. These have the same slope but a different intercept, meaning that increased cumulative HHP is required with increased age at implantation.
Table 5.
This table displays the requisite HHP to obtain a standard score of 100 for both receptive and expressive spoken language measures accounting for age at implantation.
| HHP | 9 Months | 12 Months | 18 Months | 24 Months |
|---|---|---|---|---|
| Receptive language | 79% | 85% | 99% | 112% |
| Expressive language | 78% | 85% | 100% | 114% |
Note. HHP = hearing hour percentage.
Discussion
Gagnon et al. (2020) hypothesized that increased HHP would be predictive of both receptive and expressive standard language scores, but found that HHP after 1 year of device use was predictive of only receptive speech and language standard scores. After longer than 1 year of device use, results of this study confirm the prior hypothesis. Cumulative HHP and age of cochlear implantation were found to be significant predictors of both receptive and expressive spoken language outcomes at age 3 years.
Cumulative HHP takes the entirety of device use into account. With known delays in establishing full-time use (Park et al., 2019), this metric reflects the aggregate of the child's device use. The requisite HHP for receptive language standard scores of 100 is 79% for a child implanted at 9 months old; this is an attainable wear time goal based on findings from Park et al. (2019). However, the requisite HHP increases to 114% for expressive language for children implanted at 24 months old. Based on the findings of Park et al. (2019), this is not a practical wear time goal and likely not feasible based on pediatric sleep meta-analysis by Paruthi et al. (2016). This difference between wear time needs could be due to a reduced hearing and language gap, due to shorter duration of deafness (Leigh et al., 2016). To help obtain practical and feasible wear time recommendations, this study further validates younger age at implantation but emphasizes that, for optimal spoken language outcomes to be achieved, device wear time is a significant variable.
The results of this study suggest the following HHP goals: 80% for those implanted at 9 months, 85% for those implanted at 12 months, 100% for children implanted at 18 months, and 100% with the likely addition of aggressive listening and spoken language therapy to help close the language gap for children implanted at 24 months. Pediatric recipients should be striving to meet their language potential, which could be much higher than a standard score of 100. This necessitates increased device use and therapy. An 80% HHP in a 12-month-old child translates into 8.88 hr of wear time, supporting the notion that the traditional 8-hr definition of full-time use is too conservative to achieve optimal spoken language outcomes. In addition, an 80% HHP has been found to be achievable by the majority of pediatric listeners (Park et al. 2019), but higher levels may be more difficult to achieve. Further work should be completed to find effective counseling strategies, device retention, and speech processor wearing options to optimize use. While this study provides information on cumulative device use and language development, there were limitations. This was a retrospective study; one of the clinical assessments utilized in young children was the PLS-5. Studies have raised concerns that this measure may inflate scores (Smith, 2014). In addition, datalogs from multiple manufacturers were used (Cochlear and Med-El). Datalogs from Cochlear indicated the length of time the internal and external devices were locked, while datalog information from Med-El provided the length of time the device was powered on, not necessarily transmitting to the internal device. Quality and quantity of therapy could have an impact on language outcomes, but these could not be studied in this retrospective work. In North Carolina, all children birth to 3 years receive Early Intervention services with a teacher of the deaf, generally once a week; however, some opt for additional private therapy services. The focus of therapies at this age is to teach the parent how to integrate language learning into daily life, thus making the quality of language intervention in the home an unknown variable. The current study did not investigate the impact of types of input as measured by the datalog. Busch et al. (2020) found a connection between speech variables from datalog metrics and PPTV scores and negative association between music and PPTV scores. They hypothesize that television use could have been classified as music. Further research is needed to better understand the relationship between cochlear implant wear time and listening environments on early language development. Furthermore, the study sample included a heterogeneous group of both unilateral and bilateral cochlear implant recipients. A variety of studies have revealed the positive impact bilateral cochlear implantation conveys on language as opposed to unilateral cochlear implantation (Boons et al., 2012; De Raeve et al., 2015; Sarant et al., 2014). This variable was not controlled for in the current study but would be an interesting addition to further research regarding device wear time.
This study fills a void in the literature by providing cochlear implant wear time recommendations based on age at implantation that predict age-appropriate language outcomes. These recommendations can support counseling tools for parents as they work through establishing a cochlear implant wear time routine and navigate the various pediatric retention options. As standard of care pivots toward younger age at implantation, a tangible wear time goal can be utilized to help achieve typical spoken language outcomes.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, through Grant Award UL1TR002489. The authors would like to thank the team at the Children's Cochlear Implant Center at The University of North Carolina at Chapel Hill for their hard work and tedious entries into the The University of North Carolina at Chapel Hill Hearing and Language Database. We would also like to thank Cochlear Ltd. for use of the Clinical Datalog Extractor software. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
The project described was supported by the National Center for Advancing Translational Sciences, through Grant Award UL1TR002489.
References
- Archbold, S. M. , Nikolopoulos, T. P. , & Lloyd-Richmond, H. (2013). Long-term use of cochlear implant systems in paediatric recipients and factors contributing to non-use. Cochlear Implants International, 10(1), 25–40. https://doi.org/10.1179/cim.2009.10.1.25 [DOI] [PubMed] [Google Scholar]
- Boons, T. , Brokx, J. P. L. , Frijns, J. H. M. , Peeraer, L. , Philips, B. , Vermeulen, A. , Wouters, J. , & van Wieringen, A. (2012). Effect of pediatric bilateral cochlear implantation on language development. Archives of Pediatrics & Adolescent Medicine, 166(1), 28–34. https://doi.org/10.1001/archpediatrics.2011.748 [DOI] [PubMed] [Google Scholar]
- Busch, T. , Vanpoucke, F. , & Van Wieringen, A. (2017). Auditory environment across the life span of cochlear implant users: Insights from datalogging. Journal of Speech, Language, and Hearing Research, 60(5), 1362–1377. https://doi.org/10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- Busch, T. , Vermeulen, A. , Langereis, M. , Vanpoucke, F. , & van Wieringen, A. (2020). Cochlear implant data logs predict children’s receptive vocabulary. Ear and Hearing, 41(4), 733–746. https://doi.org/10.1097/AUD.0000000000000818 [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk, E. (2011). Oral and Written Language Scales–Second Edition. Western Psychological Services. [Google Scholar]
- Ching, T. Y. , Dillon, H. , Marnane, V. , Hou, S. , Day, J. , Seeto, M. , Crowe, K. , Street, L. , Thomson, J. , Van Buynder, P. , Zhang, V. , Wong, A. , Burns, L. , Flynn, C. , Cupples, L. , Cowan, R. S. C. , Leigh, G. , Sjahalam-King, J. , & Yeh, A. (2013). Outcomes of early- and late-identified children at 3 years of age: findings from a prospective population-based study. Ear and Hearing, 34(5), 535–552. https://doi.org/10.1097/AUD.0b013e3182857718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, T. Y. C. , & Hill, M. (2007). The Parents' Evaluation of Aural Oral Performance of Children (PEACH) scale normative data. Journal of the American Academy of Audiology, 18(3), 221–237. https://doi.org/10.3766/jaaa.18.3.4 [DOI] [PubMed] [Google Scholar]
- Colletti, L. , Mandala, M. , Zoccante, L. , Shannon, R. V. , & Colletti, V. (2011). Years, infants versus older children fitting with cochlear implants: Performance over 10. International Journal of Pediatric Otorhinolaryngology, 75, 504–509. https://doi.org/10.1016/j.ijporl.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Contrera, K. J. , Choi, J. S. , Blake, C. R. , Betz, J. F. , Niparko, J. K. , & Lim, F. R. (2014). Rates of long-term cochlear implant use in children. Otology and Neurotology, 35(30), 426–420. https://doi.org/10.1097/MAO.0000000000000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raeve, L. , Vermeulen, A. , & Snik, A. (2015). Verbal cognition in deaf children using cochlear implants: Effect of unilateral and bilateral stimulation. Audiology and Neurotology, 20(4), 261–266. https://doi.org/10.1159/000381003 [DOI] [PubMed] [Google Scholar]
- Dettman, S. J. , Dowell, R. C. , Choo, D. , Arnott, W. , Abrahams, Y. , Davis, A. , Dornan, D. , Leigh, J. , Constantinescu, G. , Cowan, R. , & Briggs, R. J. (2016). Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otology & Neurotology, 37(2), e82–e95. https://doi.org/10.1097/mao.0000000000000915 [DOI] [PubMed] [Google Scholar]
- Easwar, V. , Sanfilippo, J. , Papsin, B. , & Gordon, K. (2016). Factors affecting daily cochlear implant use in children: Datalogging evidence. Journal of the American Academy of Audiology, 27(10), 824–838. https://doi.org/10.3766/jaaa.15138 [DOI] [PubMed] [Google Scholar]
- Easwar, V. , Sanfilippo, J. , Papsin, B. , & Gordon, K. (2018). Impact of consistency in daily device use on speech perception abilities in children with cochlear implants: Datalogging evidence. Journal of the American Academy of Audiology, 29(9), 835–846. https://doi.org/10.3766/jaaa.17051 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M. B. , Green, J. E. , Fang, Y. , & Waltzman, S. B. (2013). Factors influencing consistent device use in pediatric recipients of bilateral cochlear implants. Cochlear Implants International, 14(5), 257–265. https://doi.org/10.1179/1754762812Y.0000000026 [DOI] [PubMed] [Google Scholar]
- Gagnon, E. B. , Eskridge, H. , & Brown, K. D. (2020). Pediatric cochlear implant wear time and early language development. Cochlear Implants International, 21(2), 92–97. https://doi.org/10.1080/14670100.2019.1670487 [DOI] [PubMed] [Google Scholar]
- Houston, D. M. , & Miyamoto, R. T. (2010). Effects of Early Auditory experience on word learning and speech perception in deaf children with cochlear implants: Implications for sensitive periods of language development. Otology and Neurotology, 31(8), 1248–1253. https://doi.org/10.1097/MAO.0b013e3181f1cc6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karltorp, E. , Eklöf, M. , Östlund, E. , Asp, F. , Tideholm, B. , & Löfkvist, U. (2020). Cochlear implants before 9 months of age led to more natural spoken language development without increased surgical risks. Acta Paediatrica, 109(2), 332–341. https://doi.org/10.1111/apa.14954 [DOI] [PubMed] [Google Scholar]
- Leigh, J. R. , Dettman, S. J. , & Dowell, R. C. (2016). Evidence-based guidelines for recommending cochlear implantation for younger children: Audiological criteria and optimizing age a implantation. International Journal of Audiology, 55(Suppl. 2), S9–S18. https://doi.org/10.3109/14992027.2016.1157268 , https://doi.org/10.3109/14992027.2016.1146415 [DOI] [PubMed] [Google Scholar]
- Marnane, V. , & Ching, T. Y. (2015). Hearing aid and cochlear implant use in children with hearing loss at three years of age: Predictors of use and predictors of changes in use. International Journal of Audiology, 54(8), 544–551. https://doi.org/10.3109/14992027.2015.1017660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas, J. G. , & Geers, A. E. (2018). Sensitivity of expressive linguistic domains to surgery age and audibility of speech in preschoolers with cochlear implants. Cochlear Implants International, 19(1), 26–37. https://doi.org/10.1080/14670100.2017.1380114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko, J. K. , Tobey, E. A. , Thal, D. J. , Eisenberg, L. S. , Wang, N.-Y. , Quittner, A. L. , & Fink, N. E. (2010). Spoken language development in children following cochlear implantation. Journal of the American Medical Association, 303(15), 1498–1506. https://doi.org/10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, L. R. , Gagnon, E. B. , Thompson, E. , & Brown, K. D. (2019, December). Age at full-time use predicts language outcomes better than age of surgery in children who use cochlear implants. American Journal of Audiology, 28(4), 986–992. https://doi.org/10.1044/2019_AJA-19-0073 [DOI] [PubMed] [Google Scholar]
- Paruthi, S. , Brooks, L. J. , D'Ambrosio, C. , Hall, W. A. , Kotagal, S. , Lloyd, R. M. , Malow, B. A. , Maski, K. , Nichols, C. , Quan, S. F. , Rosen, C. L. , Troester, M. M. , & Wise, M. S. (2016). A consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine, 12(6), 785–786. https://doi.org/10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarant, J. , Harris, D. , Bennet, L. , & Bant, S. (2014). Bilateral versus unilateral cochlear implant in children: A study of spoken language outcomes. Ear and Hearing, 35(4), 396–409. https://doi.org/10.1097/AUD.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. (2014, October 28). PLS-5 validity study—A comparison oftest scores. ASHA Community. https://community.asha.org/blogs/kristin-smith/2014/10/28/pls-5 [Google Scholar]
- Spencer, L. J. , Gantz, B. J. , & Knutson, J. F. (2004). Outcomes and achievement of students who grew up with access to cochlear implants. The Laryngoscope, 114(9), 1576–1581. https://doi.org/10.1097/00005537-200409000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, E. A. , Spratford, M. , Moeller, M. , Oleson, J. , Ou, H. , & Jacobs, S. (2013). Predictors of hearing aid use time in children with mild-to-severe hearing loss. Language, Speech, and Hearing Services in Schools, 44(1), 73–88. https://doi.org/10.1044/0161-1461(2012/12-0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman, K. B. , & Warner-Czyz, A. D. (2018). Inconsistent device use in pediatric cochlear implant users: Prevalence and risk factors. Cochlear Implants International, 19(3), 131–141. https://doi.org/10.1080/14670100.2017.1418161 [DOI] [PubMed] [Google Scholar]
- Zimmerman, I. L. , Steiner, V. G. , & Pond, R. E. (2011). Preschool Language Scales–Fifth Edition. Pearson. https://doi.org/10.1037/t15141-000. [Google Scholar]