Structured Abstract:
Background:
Acute kidney injury (AKI) after cardiac surgery remains a common complication that has been associated with increased morbidity and mortality. This study implemented Kidney Disease Improving Global Outcomes criteria to evaluate renal outcomes after concomitant surgical ablation for atrial fibrillation.
Methods:
Patients with a history of atrial fibrillation who underwent elective cardiac surgery at our institution from 2008–2018 were retrospectively reviewed. Those with preoperative renal dysfunction were excluded. Patients were classified as those who underwent concomitant Cox-Maze IV (CMP-IV) (n=376) or no surgical ablation (n=498). Nearest neighbor 1:1 propensity matching was conducted on fourteen covariates. AKI was evaluated by mixed effects logistic regression analysis. Long-term survival was evaluated by proportional hazards regression.
Results:
Propensity matching yielded 308 patients in each group (n=616). All preoperative variables were similar between groups. The concomitant CMP-IV group had a higher incidence of AKI: 32% (n=99) vs 16% (n=49), P<0.001. After accounting for bypass time and non-ablation operations on mixed effects analysis, concomitant CMP-IV was associated with increased risk of AKI (Odds Ratio 1.89 (1.12–3.18); P=0.017). While AKI was associated with decreased late survival (P<0.001), patients who received a concomitant CMP-IV maintained superior seven-year survival to patients who received no ablation (P<0.001). No patients required permanent dialysis.
Conclusions:
Concomitant CMP-IV was independently associated with increased risk of AKI in the acute postoperative period. However, the long-term risks of AKI were offset by the significant survival benefit of CMP-IV. Concerns regarding new onset renal dysfunction should not prohibit recommendation of this procedure in appropriate patients.
Keywords: Surgical Ablation, Cox Maze Procedure, Acute Kidney Injury, Renal Failure, Dialysis, Propensity Score Match
Introduction
Acute kidney injury (AKI) is one of the most common complications after cardiac surgery, though the true incidence has been obscured by a lack of standardized reporting[1]. Renal dysfunction at mild thresholds has been independently associated with higher rates of morbidity and mortality, with a stepwise progression in risk by level of postoperative serum creatinine[2]. Among many factors related to cardiopulmonary bypass (CPB) associated AKI, the heightened systemic inflammatory response, ischemia-reperfusion injury, and pigment nephropathy from rhabdomyolysis and hemolysis have been proposed as possible mechanisms[3].
The relationship between surgical ablation and postoperative AKI has not been well-characterized and has remained controversial[4–8]. The Cox-Maze IV procedure (CMP-IV) has demonstrated superior results to catheter ablation and remains the single most effective treatment for atrial fibrillation (AF)[9,10]. Prior generations of the procedure were associated with postoperative fluid imbalances that resulted in significant revisions to the original cut-and-sew operation[11,12]. The combination of radiofrequency and cryoablation in the CMP-IV has replaced most of the operative incisions of the Cox-Maze III and has resulted in substantial decreases in perioperative morbidity and mortality[13]. The late survival benefit of CMP-IV has also been well-established[14].
While current guidelines indicate that surgical ablation does not increase the risk of renal failure or dialysis[4,5], recent large-scale studies have produced conflicting results and the added risk of AKI has been overlooked[6–8]. National database studies have reported increases in both renal failure and dialysis after concomitant CMP-IV, relative to cardiac surgery without ablation[6–8]. However, the added CPB time required to perform CMP-IV was not accounted for and prior renal dysfunction did not preclude study evaluation. While CPB duration has been identified as an important predictor of renal failure in patients with preexisting renal dysfunction, it has been shown to be exponentially less impactful in patients with glomerular filtration rate (GFR)≥60 mL/min/1.73m3[15].
Existing literature has focused on renal failure or dialysis as major complications following concomitant CMP-IV, but the scope of analysis on postoperative AKI has been limited[4–9,14,21,22]. In this report, we examined CMP-IV as an independent risk factor for new onset renal dysfunction in a large cohort of patients who underwent cardiac surgery at a single high-volume center (Video 1).
Video 1:
Ralph J. Damiano, Jr., MD summarizes the findings of this study and implications for clinical practice.
Methods
Data Sources
This study was approved by the Washington University School of Medicine Institutional Review Board with a waiver for patient consent. Preoperative demographic data, operative details, and perioperative results were obtained from our institutional Society of Thoracic Surgeons (STS) database and our custom AF ablation database. Missing data were obtained through chart review.
Patient Population
Between January 2008 and August 2018, 10,928 patients underwent cardiac surgery at our institution. Patients without preexisting AF, and those who underwent urgent, emergent, or salvage procedures, and any surgical ablations other than a biatrial or a complete left-sided lesion set CMP-IV were excluded. A left atrial CMP-IV was performed in carefully selected patients with small right atria, normal pulmonary pressures, and either short duration or paroxysmal AF. Patients who underwent stand-alone CMP-IV, had missing CPB times or underwent off-pump procedures, and those with preexisting renal dysfunction or chronic kidney disease (preoperative GFR<60 mL/min/1.73m3 and/or receiving hemodialysis) also were excluded. The remaining 874 patients were stratified into two groups: patients with a history of AF who underwent concomitant CMP-IV (n=376) and patients with a history of AF that did not undergo concomitant ablation (n=498)(Figure E1). Preoperative and perioperative characteristics were retrospectively evaluated and compared between concomitant CMP-IV and no AF surgery groups.
Left atrial appendage (LAA) excision or exclusion has remained an integral part of the CMP-IV. While the method of LAA management varied by individual patient, closure was generally achieved by amputation with epicardial oversewing at the base in a median sternotomy approach. If the LAA was excluded in this approach, an AtriClip (AtriCure Inc., West Chester, OH) device was placed epicardially at the base of the LAA. In a right mini-thoracotomy approach, the LAA was oversewn endocardially in two layers.
Evaluation of Renal Dysfunction
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines were established in 2012 to standardize the continuum of acute renal dysfunction[18]. In this study, preoperative serum creatinine was measured immediately prior to surgery. Postoperative serum creatinine was measured as the highest creatinine level in the acute postoperative period. By KDIGO serum creatinine guidelines, AKI was defined as ≥1.5-fold increase in serum creatinine from baseline within seven days or an acute rise of ≥0.3 mg/dL within 48 hours[16]. Renal failure was defined as ≥3.0-fold increase from baseline, acute rise of ≥4.0 mg/dL, or initiation of dialysis. KDIGO urine output criteria were not used due to poor hourly documentation in the inpatient hospital setting[1] with lack of standardization over the study period. GFR was calculated by Modified Diet in Renal Disease formulation as 175×(serum creatinine)−1.154×(age)−0.203×0.742 (if female)×1.210 (if African American). Renal failure and both temporary and permanent dialysis requirements were evaluated as major complications.
Statistical Analysis
Continuous variables were reported as mean ± standard deviation (SD) or median with interquartile range [IQR], as appropriate. Comparisons were made using either the Wilcoxon signed rank test for paired data or Student’s t-test for unpaired comparisons. Categorical variables were expressed as frequencies and percentages. Comparisons were made using either the paired McNemar’s test or unpaired χ2 analysis. All data analysis was performed using SPSS 25.0 (IBM Corp, Armonk, NY) and R version 3.3.3 with the MatchIt package (The R Foundation for Statistical Computing, Vienna, Austria).
Propensity score matching was conducted to improve covariate balance and account for both baseline and demographic differences between CMP-IV and no AF surgery groups. Fourteen preoperative variables were selected based on clinical relevance in association with AKI. The variables used in the propensity score match were male gender, age, body mass index (BMI), race, left ventricular ejection fraction, New York Heart Association class III/IV, previous myocardial infarction, smoking history, diabetes, hypertension, hypercholesterolemia, preoperative GFR, peripheral vascular disease, and recent era (2013–2018). Propensity scores were estimated using a logistic model with the nearest neighbor algorithm and a 1:1 match ratio without replacement. A 0.1 caliper width was imposed. Covariate balance after matching was assessed using the distribution of propensity scores (Figure E2) and standardized mean differences (SMDs). An |SMD|≤0.10 indicates negligible difference between groups.
Postoperative AKI was evaluated by mixed effects logistic regression with surgeon as a random effect. Fixed effects included baseline demographics, perioperative variables, and year of surgery to account for time bias. Operations were categorized as lone coronary artery bypass graft (CABG), aortic valve surgery with or without concomitant procedures (excluding mitral valve surgery), mitral valve repair or replacement with or without concomitant procedures, and other elective surgeries. Significant covariates with P<0.1 were entered into a multivariable mixed effects logistic regression model.
Survival analysis was conducted with the propensity matched cohort using Cox proportional hazards regression with robust variance estimators to account for clustering. Baseline demographics, concomitant surgical ablation, and postsurgical AKI were evaluated as potential predictors of late mortality. Subgroup analysis was conducted for CMP-IV patients.
Results
Baseline Characteristics
Prior to the propensity score match, patients in the concomitant CMP-IV cohort (n=376) had fewer comorbidities than those in the no AF surgery cohort (n=498). Propensity score matching yielded 616 total patients, with 308 patients in the concomitant CMP-IV and no AF surgery groups. The matched groups were similar in all preoperative characteristics (Table 1).
Table 1.
Preoperative Characteristics Before and After Propensity Score Match
| Variable | No AF Surgery n=498 |
Concomitant CMP-IV n=376 |
SMD | No AF Surgery n=308 |
Concomitant CMP-IV n=308 |
SMD |
|---|---|---|---|---|---|---|
| Male Gender | 325 (79%) | 225 (60%) | −0.112 | 188 (61%) | 188 (61%) | <0.001 |
| Age (years) | 67 ± 13 | 65 ± 12 | −0.206 | 66 ± 14 | 66 ± 11 | −0.006 |
| BMI (kg/m2) | 29.6 ± 7.3 | 29.3 ± 7.1 | −0.048 | 29.4 ± 7.5 | 29.5 ± 7.0 | 0.009 |
| African American Race | 36 (7%) | 14 (4%) | −0.154 | 16 (5%) | 14 (5%) | −0.030 |
| LVEF | 52 ± 15 | 57 ± 12 | 0.400 | 56 ± 13 | 56 ± 12 | 0.037 |
| NYHA Class III/IV | 308 (62%) | 204 (54%) | −0.154 | 178 (58%) | 175 (57%) | −0.020 |
| Prior Myocardial Infarction | 133 (27%) | 35 (9%) | −0.464 | 29 (9%) | 34 (11%) | 0.054 |
| Smoking History | 260 (52%) | 153 (41%) | −0.232 | 132 (43%) | 142 (46%) | 0.065 |
| Diabetes | 169 (34%) | 68 (18%) | −0.367 | 66 (21%) | 66 (21%) | <0.001 |
| Hypertension | 400 (80%) | 273 (73%) | −0.182 | 231 (75%) | 235 (76%) | 0.030 |
| Hyperlipidemia | 370 (74%) | 224 (60%) | −0.316 | 205 (67%) | 200 (65%) | −0.034 |
| GFR (mL/min/1.73m3) | 83 ± 20 | 83 ± 0.16 | −0.024 | 83 ± 20 | 83 ± 16 | 0.013 |
| Peripheral Vascular Disease | 123 (25%) | 44 (12%) | −0.341 | 47 (15%) | 44 (14%) | −0.027 |
| Recent Era (2013–2018) | 251 (50%) | 208 (55%) | 0.099 | 162 (53%) | 162 (53%) | <0.001 |
| Arrhythmia AF Type | ||||||
| Paroxysmal AF | - | 163 (43%) | - | - | 127 (41%) | - |
| Persistent AF | - | 56 (15%) | - | - | 49 (16%) | - |
| Longstanding Persistent AF | - | 157 (42%) | - | - | 132 (43%) | - |
| Propensity Score | 0.781 | 0.006 |
Values are n (%) or mean ± standard deviation (SD) unless otherwise indicated. AF, atrial fibrillation; BMI, body mass index; CMP-IV, Cox-Maze IV procedure; CPB, cardiopulmonary bypass; LVEF, left ventricular ejection fraction; GFR, glomerular filtration rate; NYHA, New York Heart Association; SMD, standardized mean difference. |SMD|≤0.10 indicates no significant difference.
Perioperative Results
The majority of patients who underwent concomitant CMP-IV received a biatrial lesion set (90%; n=277). Subgroup analysis by Cox-Maze lesion set can be found in Table E1. Relative to patients who did not receive surgical ablation, the concomitant CMP-IV group had longer CPB (181 mins [154–211] vs 113 mins [90–147]; P<0.001) and aortic cross-clamp times (94 mins [75–112] vs 74 mins [56–94]; P<0.001). Concomitant CMP-IV patients had longer hospital stays (9 days [7–13] vs 8 days [6–12]; P<0.001), but there was no difference in thirty-day mortality between groups (3% (n=10) vs 2% (n=7); P=0.467). Operative variables and complications are reported in Table 2. The most common primary operation was valve surgery in both groups; mitral valve repair/replacement in the concomitant CMP-IV group (59%; n=182) and aortic valve replacement (43%; n=132) in the no AF surgery group (Table E2).
Table 2.
Perioperative Outcomes and Complications
| Variable | No AF Surgery n=308 | Concomitant CMP-IV n=308 |
P-value |
|---|---|---|---|
| Perioperative Outcomes | |||
| CMP-IV Lesion Set | |||
| Biatrial | - | 277 (90%) | - |
| Left Sided | - | 31 (10%) | - |
| Sternotomy | 287 (93%) | 253 (82%) | <0.001 |
| CPB time (mins) | 113 [90–147] | 181 [154–211] | <0.001 |
| Aortic Cross-Clamp Time (mins) | 74 [56–94] | 94 [75–112] | <0.001 |
| Intraoperative Blood Products | 247 (80%) | 225 (73%) | 0.039 |
| Length of Stay Post-Surgery (days) | 8 [6–12] | 9 [7–13] | 0.013 |
| ICU Length of Stay (hours) | 52 [29–99] | 70 [40–120] | 0.064 |
| 30-Day Mortality | 7 (2%) | 10 (3%) | 0.467 |
| Complications | |||
| Intra-aortic Balloon Pump | 18 (6%) | 13 (4%) | 0.369 |
| Myocardial Infarction | 1 (<1%) | 0 (0%) | 0.317 |
| Mediastinitis | 3 (1%) | 1 (<1%) | 0.317 |
| Reoperation for Bleeding | 13 (4%) | 8 (3%) | 0.275 |
| Stroke | 8 (3%) | 7 (2%) | 0.821 |
| Sepsis | 7 (2%) | 7 (2%) | 1.000 |
| Atrial Tachyarrhythmias | 76 (25%) | 142 (49%) | <0.001 |
| Pacemaker | 25 (8%) | 43 (14%) | 0.020 |
| Pneumonia | 24 (8%) | 22 (7%) | 0.768 |
| Pulmonary Embolism | 0 (0%) | 0 (0%) | 1.000 |
| Pleural Effusion | 8 (3%) | 19 (6%) | 0.022 |
| Renal Outcomes | |||
| AKI | 49 (16%) | 99 (32%) | <0.001 |
| Renal Failure | 12 (4%) | 19 (6%) | 0.209 |
| Dialysis | 8 (3%) | 14 (5%) | 0.201 |
| Postoperative Dialysis | |||
| Sent Home on Dialysis | 0 (0%) | 3 (1%) | 0.083 |
| Sent Home Without Dialysis | 4 (1%) | 6 (2%) | 0.527 |
| Deceased Before Discharge | 4 (1%) | 5 (2%) | 0.739 |
| Relative increase in serum creatinine from baseline | 1.15 [1.03–1.33] | 1.27 [1.11–1.57] | <0.001 |
| Postoperative GFR (mL/min/1.73m3) | 67 [57–82] | 61 [45–73] | <0.001 |
Values are n (%) or median with interquartile range [IQR], unless otherwise indicated. AF, atrial fibrillation; AKI, acute kidney injury; BMI, body mass index; CABG, coronary artery bypass graft; CMP-IV, Cox-Maze IV procedure; CPB, cardiopulmonary bypass; ICU, Intensive care unit; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Evaluation of Postoperative Renal Dysfunction
Concomitant CMP-IV patients experienced significantly higher rates of postoperative AKI, relative to the group not receiving surgical ablation (32% (n=99) vs 16% (n=49); P<0.001)(Figure 1A). The incidences of renal failure (6% (n=19) vs 4% (n=12); ablation vs no ablation respectively, P=0.209) and dialysis (5% (n=14) vs 3% (n=8); P=0.201) did not reach the level of statistical significance. Patients with a new requirement for postoperative dialysis were either weaned off dialysis before discharge, discharged on dialysis, or deceased before discharge. The proportion of patients sent home on dialysis was not significantly higher in the concomitant CMP-IV group (1% (n=3) vs 0% (n=0); P=0.083). Among patients discharged on dialysis, all required dialysis past one month. Two patients were weaned off dialysis at two months and the third was weaned off dialysis at four months. No patient required permanent dialysis.
Figure 1A:
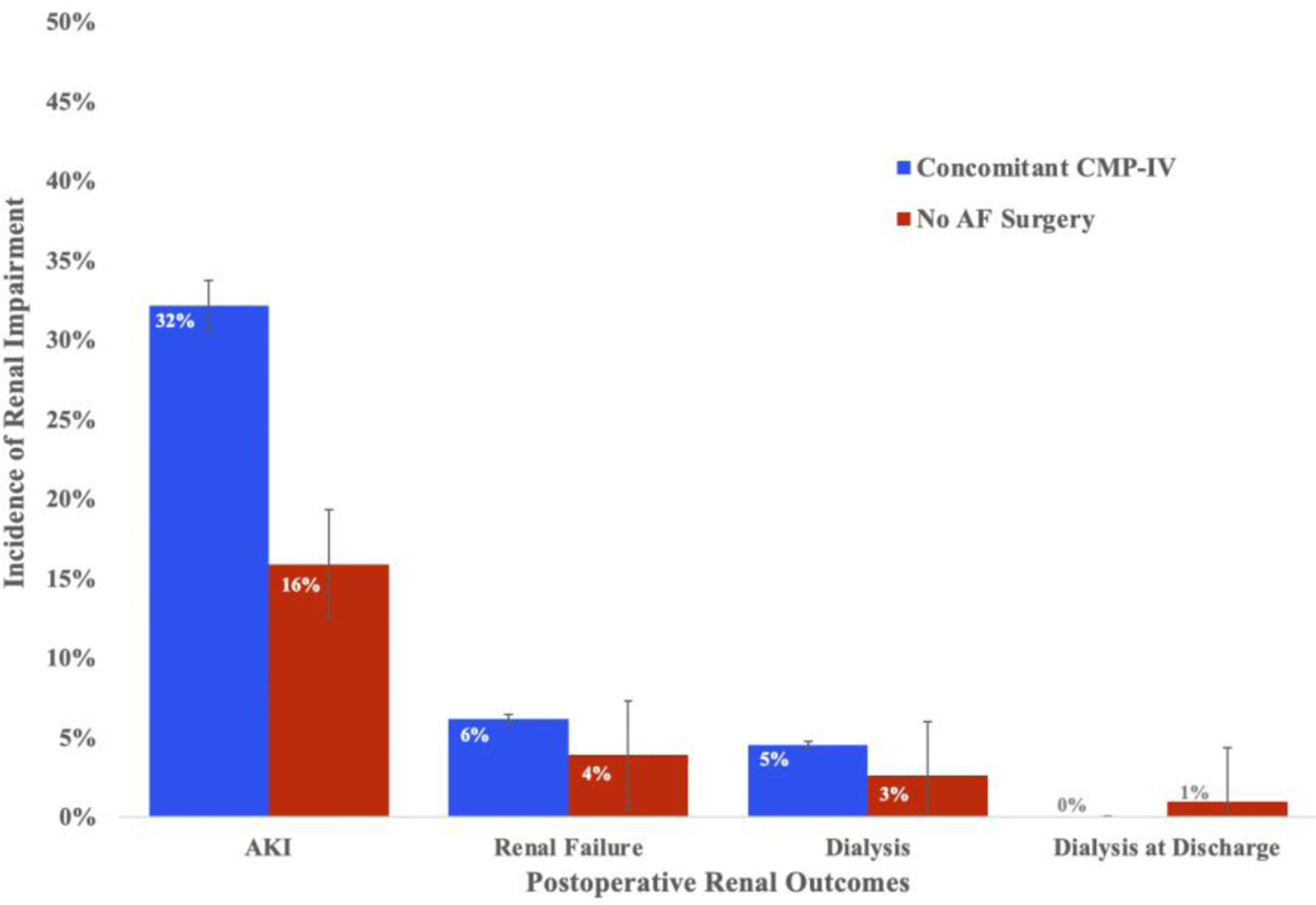
Distribution of the incidence of renal impairment after surgery between concomitant CMP-IV (n=308) and no AF surgery (n=308) groups after propensity score matching. Renal impairment was evaluated by clinical severity: acute kidney injury, renal failure, dialysis, and dialysis at discharge. Absolute numbers can be found in Table 2.
AF, atrial fibrillation; AKI, acute kidney injury; CMP-IV, Cox-Maze IV.
Patients who received concomitant CMP-IV experienced a median 1.27-fold increase in serum creatinine from baseline (1.27 [1.11–1.57]), relative to the 1.15-fold increase seen by the no surgical ablation group (1.15 [1.03–1.33]; P<0.001). Postoperative GFR was lower in the concomitant CMP-IV group (61 mL/min/1.73m3 [45–73] vs 67 mL/min/1.73m3 [57–82]; P<0.001).
The incidence of AKI by type of operation is detailed in Figure 1B. When grouped by primary operation, there was no significant difference in main procedure among patients who underwent no AF ablation (χ2=0.228; P=0.973) or concomitant CMP-IV (χ2=4.405; P=0.256).
Figure 1B.
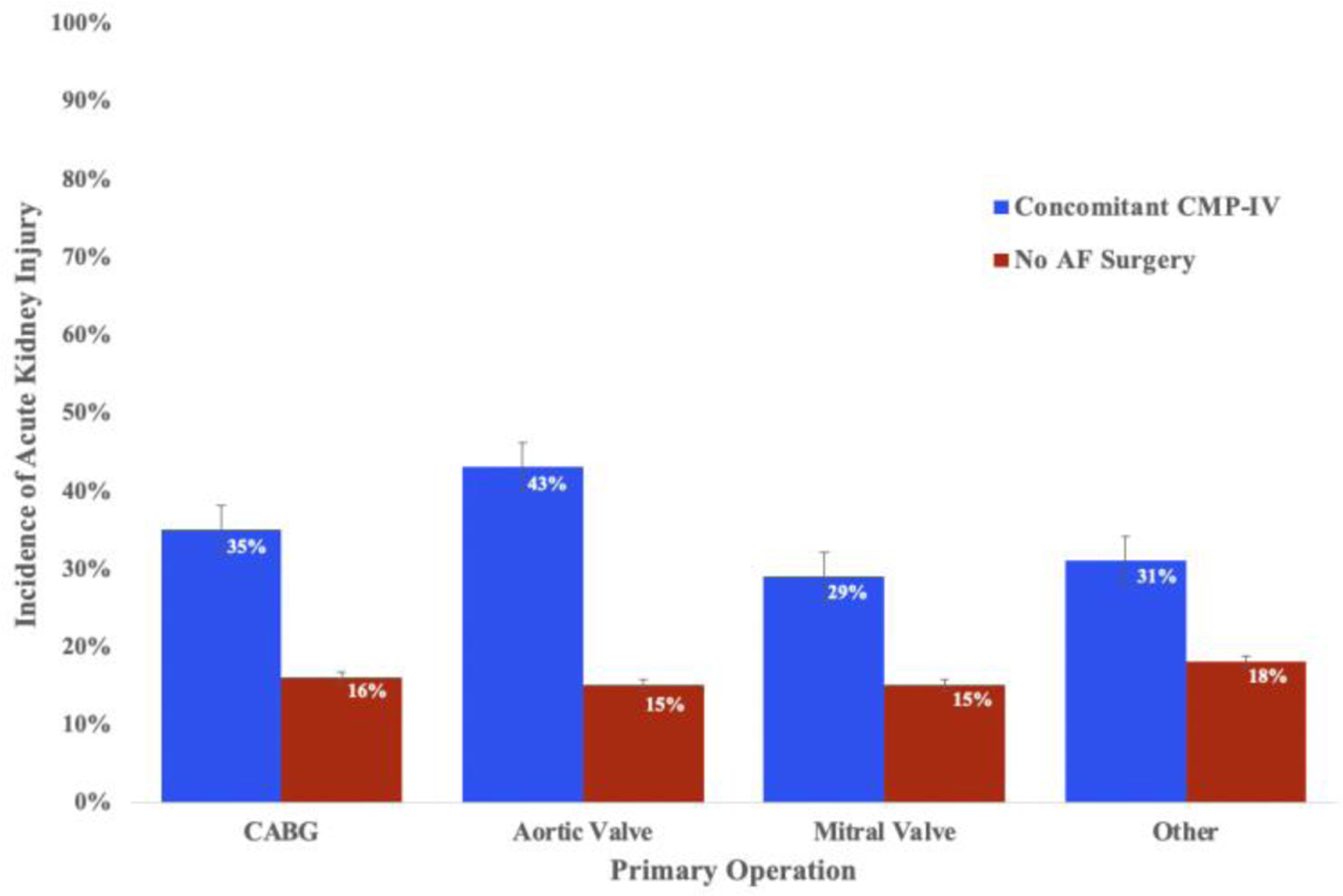
Distribution of the incidence of AKI after surgery between concomitant CMP-IV and no AF surgery groups, separated by primary operation. Absolute procedure numbers can be found in Table E2.
AF, atrial fibrillation; AKI, acute kidney injury; AV, aortic valve; MV, mitral valve; CMP-IV, Cox-Maze IV; CABG, coronary artery bypass graft.
Predictors of Postoperative Acute Kidney Injury
The following were identified as predictors of AKI on multivariable mixed effects logistic regression: concomitant CMP-IV (Odds Ratio (OR) 1.88, 95% confidence interval (CI) (1.12–3.18); P=0.017), CPB time (OR 1.59 (1.22–2.07); P=0.001), length of stay (OR 1.11 (1.07–1.16); P<0.001), age (OR 1.03 (1.01–1.05); P=0.011), BMI (OR 1.05 (1.02–1.08; P=0.003), and preoperative GFR (OR 1.02 (1.01–1.04); P<0.001)(Table 3). The final multivariable model fit well by the Hosmer-Lemeshow goodness of fit test (P=0.985) with a C-statistic of 0.824.
Table 3.
Univariable and Multivariable Mixed Effects Logistic Regression for AKI
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Concomitant CMP-IV | 2.55 (1.70–3.80) | <0.001 | 1.89 (1.12–3.18) | 0.017 |
| CPB Time (hours) | 1.91 (1.55–2.37) | <0.001 | 1.59 (1.22–2.07) | 0.001 |
| Type of Operation | ||||
| Reference: MV ± AVR | ||||
| AVR ± CABG | 1.10 (0.70–1.72) | 0.685 | ||
| CABG | 1.14 (0.62–2.12) | 0.671 | ||
| Other | 1.02 (0.57–1.85) | 0.940 | ||
| Sternotomy | 2.20 (1.14– 4.26) | 0.019 | 0.120 | |
| Intraoperative Transfusion | 1.34 (0.84–2.13) | 0.213 | ||
| Length of Stay (days) | 1.13 (1.09–1.17) | <0.001 | 1.11 (1.07–1.16) | <0.001 |
| Year of Surgery | 1.06 (0.99–1.13) | 0.072 | 0.580 | |
| Male Gender | 1.13 (0.77–1.66) | 0.544 | ||
| Age (years) | 1.02 (1.00–1.04) | 0.018 | 1.03 (1.01–1.05) | 0.012 |
| BMI (kg/m2) | 1.04 (1.01–1.06) | 0.005 | 1.05 (1.02–1.09) | 0.003 |
| African American Race | 1.02 (0.42–2.49) | 0.957 | ||
| LVEF | 0.99 (0.98–1.01) | 0.514 | ||
| NYHA Class III/IV | 1.26 (0.86–1.85) | 0.234 | ||
| Prior Myocardial Infarction | 1.08 (0.59–1.99) | 0.798 | ||
| Smoking History | 0.75 (0.51–1.10) | 0.141 | ||
| Diabetes | 1.57 (1.02–2.43) | 0.042 | 0.456 | |
| Hypertension | 2.11 (1.28–3.48) | 0.003 | 0.200 | |
| Hyperlipidemia | 1.30 (0.86–1.94) | 0.210 | ||
| GFR (mL/min/1.73m3) | 1.02 (1.01–1.03) | 0.001 | 1.02 (1.01–1.04) | <0.001 |
| Peripheral Vascular Disease | 1.39 (0.84–2.32) | 0.203 | ||
AKI, acute kidney injury; AVR, aortic valve replacement; BMI, body mass index; CABG, coronary artery bypass graft; CMP-IV, Cox-Maze IV procedure; CI, confidence interval; CPB, cardiopulmonary bypass; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MV, mitral valve; NYHA, New York Heart Association; OR, odds ratio. CPB time was scaled to hours. Fixed effects are listed above. Surgeon (n=15) was included as a random effect.
Predictors of Late Survival
Cox proportional hazards regression with robust variance estimators was used to identify potential predictors of late survival (Table 4). On multivariable analysis, concomitant CMP-IV was associated with prolonged late survival (Hazard Ratio (HR) 0.53 (0.40–0.70); P<0.001), while AKI in the acute postoperative period was associated with decreased late survival (HR 1.85 (1.33–2.58); P<0.001). The Kaplan-Meier depiction of seven-year survival stratified by postoperative AKI is shown in Figure 2 for patients who underwent concomitant CMP-IV and in Figure E3 for patients who underwent no AF ablation. Late survival status stratified by concomitant CMP-IV is shown in Figure 3. Despite the decremental effect of increased risk of AKI, CMP-IV patients experienced prolonged late survival.
Table 4.
Univariable and Multivariable Cox Proportional Marginal Hazards Analysis
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Post-Surgical AKI | 1.98 (1.44–2.72) | <0.001 | 1.85 (1.33–2.58) | <0.001 |
| Concomitant CMP-IV | 0.57 (0.43–0.76) | <0.001 | 0.53 (0.40–0.70) | <0.001 |
| Recent Era (2013–2018) | 0.87 (0.62–1.23) | 0.427 | ||
| Male Gender | 0.96 (0.71–1.29) | 0.771 | ||
| Age (years) | 1.05 (1.04–1.06) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
| BMI (kg/m2) | 0.99 (0.97–1.01) | 0.375 | ||
| African American Race | 0.64 (0.28–1.47) | 0.294 | ||
| LVEF | 1.00 (0.99–1.01) | 0.648 | ||
| NYHA Class III/IV | 1.49 (1.10–2.02) | 0.010 | 1.53 (1.13–2.07) | 0.006 |
| Prior Myocardial Infarction | 1.50 (0.98–2.31) | 0.061 | 1.57 (1.02–2.45) | 0.044 |
| Smoking History | 1.03 (0.77–1.37) | 0.869 | ||
| Diabetes | 1.21 (0.87–1.66) | 0.253 | ||
| Hypertension | 1.76 (1.20–2.57) | 0.004 | 0.098 | |
| Hyperlipidemia | 1.32 (0.97–1.80) | 0.074 | 0.475 | |
| GFR (mL/min/1.73m3) | 1.00 (0.99–1.01) | 0.774 | ||
| Peripheral Vascular Disease | 1.12 (0.76–1.66) | 0.554 | ||
AKI, acute kidney injury; CMP-IV, Cox-Maze IV procedure; CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Figure 2.
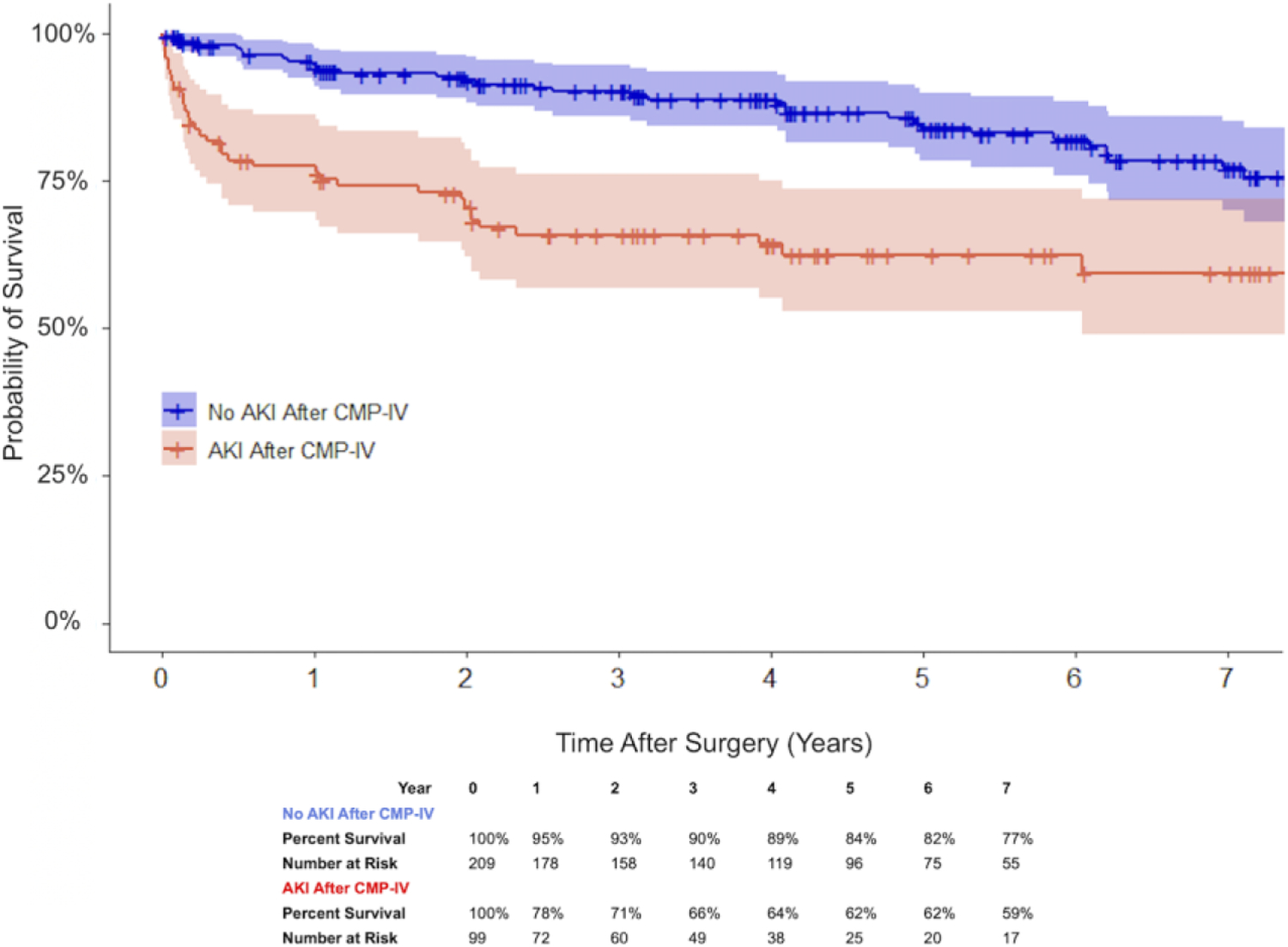
Kaplan-Meier depiction of late survival for patients who underwent concomitant CMP-IV, stratified by new onset postoperative AKI with 95% confidence intervals: no AKI after concomitant CMP-IV (blue) and AKI after concomitant CMP-IV (red) (P<0.001).
AKI, acute kidney injury; CMP-IV, Cox-Maze IV.
Figure 3.
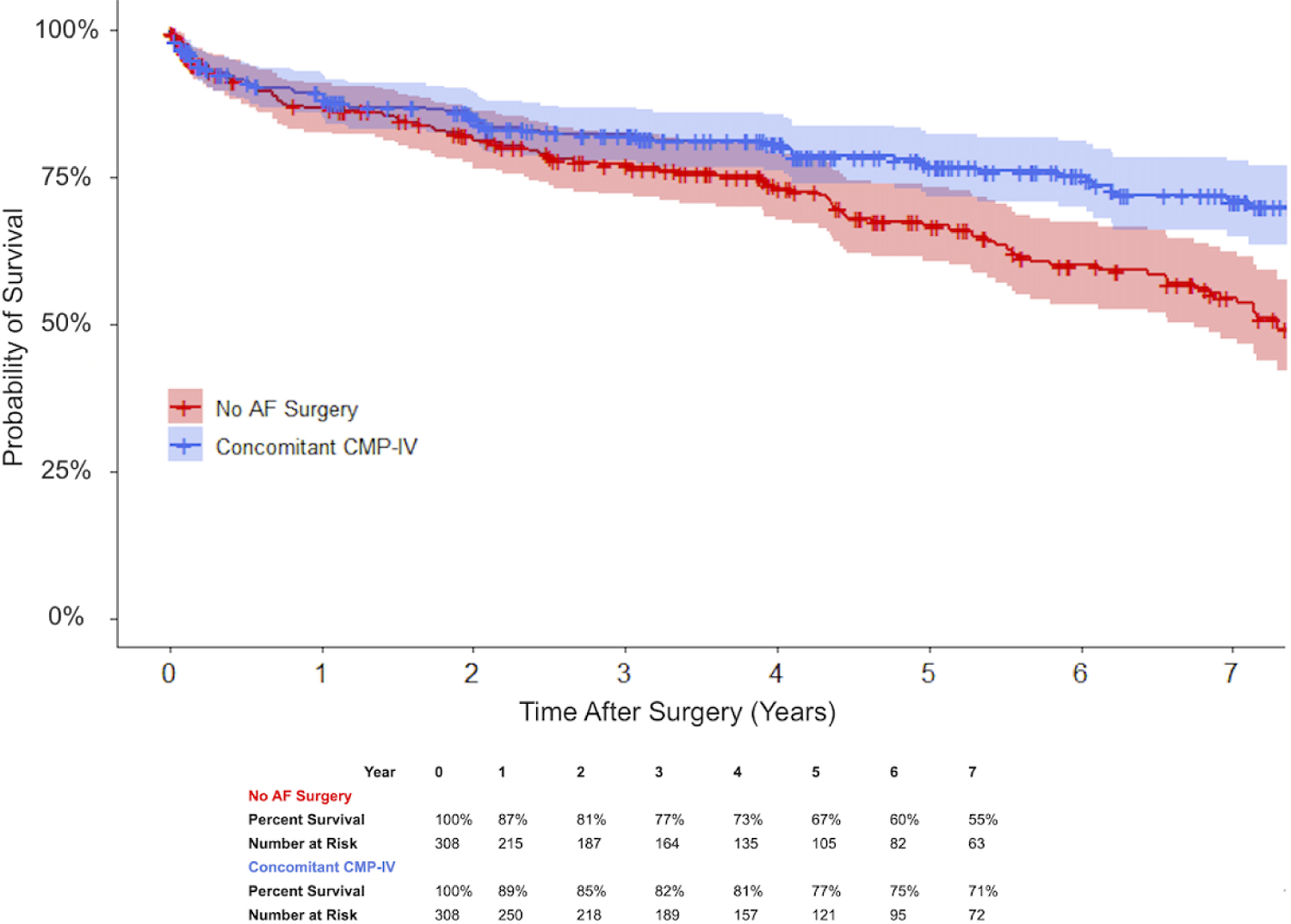
Kaplan-Meier depiction of late survival for patients with AF after cardiac surgery, stratified by receipt of concomitant surgical ablation with 95% confidence intervals: concomitant CMP-IV (blue) and no AF surgery (red) (P<0.001).
AF, atrial fibrillation; CMP-IV, Cox-Maze IV.
Discussion
This study provided a unique and comprehensive analysis of new onset renal dysfunction following surgical ablation of AF (Figure 4). Concomitant CMP-IV was identified as an independent risk factor for AKI after accounting for comorbidities and operative variables. While AKI in the acute postoperative period was associated with increased late mortality, this was overcome by the substantial survival benefit of concomitant CMP-IV. For patients who required temporary dialysis after concomitant CMP-IV, the most probable outcome was renal recovery prior to discharge. All patients who were discharged with a new dialysis requirement experienced renal recovery within four months.
Figure 4.
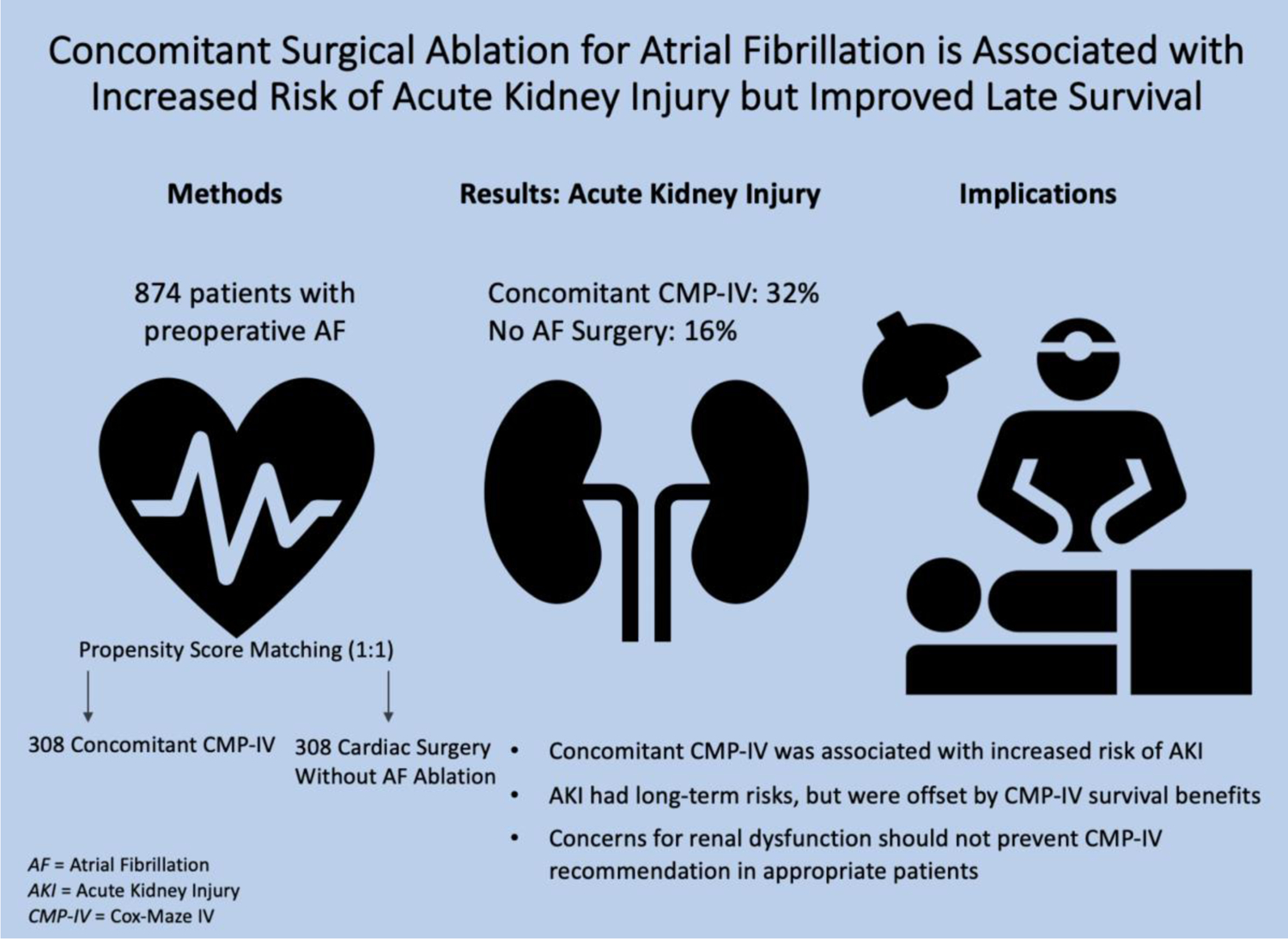
Depiction of the propensity score matched analysis of acute kidney injury. Patients with atrial fibrillation were stratified based on ablation decision at the time of surgery (concomitant Cox-Maze IV vs no AF ablation). Concomitant CMP-IV was associated with increased risk of AKI in the acute postoperative period (P<0.001), which was counterbalanced by prolonged late survival due to restoration of sinus rhythm.
AF, atrial fibrillation; AKI, acute kidney injury; CMP-IV, Cox-Maze IV.
Previously reported complications of concomitant CMP-IV have limited the evaluation of postoperative renal dysfunction to renal failure with or without dialysis. Renal failure and/or dialysis are easily queried outcomes from the STS database that are often reported as secondary endpoints[4–9,14]. According to the 2017 American Association for Thoracic Surgery guidelines, concomitant surgical ablation does not increase the incidence of postoperative dialysis (Class IIa, Level A evidence) or renal failure (Class IIa, Level B-NR evidence)[5]. This statement unfortunately did not address the impact of surgical ablation on AKI, since few publications have reported the incidence of AKI with standardized criteria. In this study, we defined AKI by serum creatinine criteria according to well-accepted KDIGO guidelines.
Identification of patients at increased risk for AKI is crucial to allow for prompt intervention and improved patient management. AKI after cardiac surgery has been independently associated with several complications, resulting in increased ICU and hospital stays[17]. Following renal recovery, AKI after cardiac surgery has also associated with increased risks of both short- and long- term mortality, up to ten years after surgery[18]. The late mortality risks noted in other studies are consistent with our findings.
The trend analysis of the STS national database conducted by Badhwar et al. reported an increased relative risk of perioperative renal failure (Relative Risk 1.12, (1.03, 1.22), P=0.011) with concomitant surgical ablation, which was deemed likely due to the increased operative times associated with CMP-IV[6]. The aforementioned study did not evaluate CPB time, but recent literature has demonstrated that the risk of renal failure with prolonged CPB time was exponentially higher in patients with preoperative renal dysfunction. For patients with preoperative GFR >60 mL/min/1.73m3, predicted probabilities of renal failure at 1 and 5 hours of CPB time were 0.2% and 3%, respectively; these rates increased to 6% and 25% for patients with GFR <30 mL/min/1.73m3[15]. In order to minimize the impact of CPB time, we excluded patients with GFR ≤60 mL/min/1.73m3. We found that concomitant CMP-IV was significantly associated with AKI after accounting for the marginal effect of CPB time and other operative variables. Our novel reporting of clinically significant renal outcomes after CMP-IV helps to clarify its impact on AKI and address conflicting guidelines and prior publications.
In our study, the addition of concomitant CMP-IV added an average of 68 minutes to CPB time. While it has been historically stated that concomitant surgical ablation should add approximately 20–30 minutes of CPB time[19,20], this has not been the case in recent studies[6–9,14,21,22]. Ad and colleagues showed in a propensity matched cohort of patients receiving mitral and tricuspid valve surgery (n=416) that patients receiving a concomitant CM procedure experienced increases in CPB time (174 vs 129 mins) of more than double the anecdotal amount[21]. This was also shown in a study from our group that evaluated CABG and valve procedures; relative to patients with AF who underwent cardiac surgery, patients who underwent concomitant CMP-IV also experienced prolonged CPB time (193 vs 132 mins)[14]. In our current study, the increases in CPB time of just over an hour(181 vs 113 mins) for patients who received concomitant CMP-IV are consistent with previous works at our institution and others[9,24,25]. We feel this is a realistic picture of the extra time required to perform a concomitant CMP-IV at an experienced, high-volume, academic center, representing a spectrum of surgeons on our faculty. Moreover, the confounding influence of CPB was adjusted for in our analysis.
The reason that surgical ablation was independently associated with AKI is not obvious from our data. Fluid retention after AF surgery was a documented complication with previous generations of the Cox-Maze procedure. It was suggested that decreased atrial natriuretic peptide (ANP) from multiple atriotomy incisions and ligation of both atrial appendages may play a role in its pathogenesis[11]. It has also been proposed that temporary dysfunction of atrial stretch receptors after surgical ablation may lead to increased antidiuretic hormone and aldosterone, resulting in postoperative fluid retention[11]. Excision of the right atrial appendage was removed from the earlier versions of the Cox-Maze procedure to reduce the risk of fluid overload.
The role of surgical LAA closure in relation to AKI has been evaluated to a limited extent in recent studies[23,24]. One study noted an increase in the incidence in AKI after concomitant LAA exclusion, but did not account for increased CPB time[23]. After accounting for prolonged CPB time, another study found no difference in the incidence of AKI after concomitant LAA excision[24]. The relationship between CMP-IV, LAA closure, and neurohormonal biomarkers should be elucidated in future studies, perhaps by evaluation of surgical LAA closure techniques or a prospective study design.
Another mechanism for AKI may be related to the extensive ablation-induced cardiac necrosis and release of inflammatory mediators or cytokines. Evaluation of cardiac biomarkers after ablation has been limited to catheter ablation. Radiofrequency (RF) ablation has been shown to increase N-terminal pro-ANP levels in the immediate post-procedure period[24] However, the paucity of research on cardiac necrosis or inflammatory biomarkers after CMP-IV limits inference. Previous studies on RF catheter ablation have shown that the number of applied RF lesions is correlated with the degree of myocardial injury, as assessed by cardiac troponin I levels[26]. The amount of RF energy delivered has also been associated with increased levels of both inflammatory and oxidative markers[27]. The radiofrequency and cryothermal ablation technology that are used for CMP-IV, and the pro-inflammatory state induced after ablation may play a role in the higher incidence of AKI seen in these patients. This remains an important area in need of further clinical research and is presently undergoing active investigation at our institution.
While concomitant CMP-IV was associated with increased risk of postoperative AKI in this study, this needs to be balanced against the marked benefits of this operation. AKI in the acute postoperative period was associated with increased late mortality. This has been shown for other surgical procedures[21], but is a novel finding after CMP-IV. Importantly, however, the substantial survival benefit of surgical ablation greatly offset the long-term risks of AKI. Restoration of sinus rhythm and LAA exclusion have been shown to significantly improve hemodynamics and reduce the incidence of stroke in these patients[28]. Our group and others have shown improved late survival in AF patients who underwent concomitant CMP-IV. In a prior study, we showed markedly improved ten-year survival at 62% vs 42% in patients with AF who underwent surgical ablation, relative to those not receiving a concomitant CMP-IV[14]. Thus, the increased risk of AKI acutely needs to be balanced against improved survival and hemodynamics due to the restoration of sinus rhythm, along with decreased risk of stroke. Additionally, it is important to remember that the CMP-IV did not significantly increase the need for dialysis following surgical ablation. For patients who undergo CMP-IV, postoperative renal function should be monitored closely for impending signs of AKI. Further investigation is warranted into the development of renal protection strategies in the perioperative period to ameliorate renal insult.
Limitations
Although this study provided a comprehensive evaluation of renal dysfunction after concomitant surgical ablation, it is not without limitations. Operations were performed at a single institution, which may prevent generalizability to other centers. Most of the CMP-IV operations were performed by a single, highly experienced surgeon (76%; n=233). Inclusion of surgeon as a random effect addressed this selection bias. Time bias was addressed by evaluation in both the propensity score match and regression analysis. The retrospective nature of this study precluded randomization, but propensity matching allowed for equalization of baseline covariates. Type of operation was not included in the propensity match, as many patients received multiple concomitant procedures and the relatively small number of participants for each combination of procedures precluded evaluation by this methodology. A limitation of propensity matching also extends to baseline covariates present at the time of treatment allocation (CMP-IV or no AF surgery) and exclusion of procedure in the propensity score match was statistically appropriate. Numbers were too few to conduct multiple propensity matched subgroup analyses, but there was no significant difference in the incidence of AKI among major subgroups by primary procedure. It is important to emphasize that procedure was included in the mixed effects regression and was not a significant effect modifier, and all cases were elective. The evaluation of CPB time by regression analysis with only elective cases served as an additional proxy for surgical complexity in relation to AKI. Statistical evaluation precluded propensity score matching on CPB time, as there is no way to perform CMP-IV without adding time on bypass. Therefore, both CMP-IV and CPB time were included in the regression separately and evaluated in terms of predictive value and contribution to model fit. LAA closure technique was not evaluated in this study because of the relationship between technique and operative approach at our center (i.e., sternotomy was usually associated with excision, thoracotomy with oversewing. However, sternotomy was not predictive for AKI in the final multivariable model. Despite these limitations, this study offers a uniquely in-depth analysis of renal impairment following concomitant CMP-IV.
Conclusion
Concomitant CMP-IV was independently associated with increased risk for postoperative AKI. After accounting for increased CPB time, CMP-IV was an independent predictor of postoperative AKI but did not increase the incidence of renal failure, dialysis, or thirty-day mortality. Patients should be monitored closely in the acute perioperative period for signs of impending renal dysfunction. Further investigation into the mechanism of renal injury and development of renal protection strategies is warranted in this cohort. While there are many benefits to performing surgical ablation, including improved late survival, lower stroke rates, and restoration of sinus rhythm, the independent association between AKI and concomitant CMP-IV should be considered in the decision-making when counseling and consenting patients prior to surgical ablation.
Supplementary Material
Acknowledgements
The authors thank Marci Damiano for assistance with study preparation.
Funding: This work was supported by the National Institutes of Health R01-HL032257 to R.J.D., and R.B.S., T32-HL007776 to R.J.D., A.J.K, R.M.M., and M.O.K., the AATS Summer Intern Scholarship to N.B., and grants from the Barnes-Jewish foundation.
Abbreviations and Acronyms:
- AF
Atrial fibrillation
- AKI
Acute kidney injury
- ANP
Atrial natriuretic peptide
- BMI
Body mass index
- CABG
Coronary artery bypass graft
- CI
Confidence interval
- CMP
Cox-Maze procedure
- CPB
Cardiopulmonary bypass
- GFR
Glomerular filtration rate
- HR
Hazard ratio
- ICU
Intensive care unit
- IQR
Interquartile range
- KDIGO
Kidney Disease Improving Global Outcomes
- LAA
Left atrial appendage
- OR
Odds ratio
- RF
Radiofrequency
- SD
Standard deviation
- SMD
Standardized mean difference
- STS
Society of Thoracic Surgeons
Footnotes
Conflict of Interest Disclosure:
R.J.D. - AtriCure, Inc: Speaker and receives research funding; LivaNova, Inc.: Speaker. Medtronic: Consultant; Edwards Lifesciences: Speaker.
WUSM IRB: IRB ID# 202010026, current approval date: 10/6/2020
This study was approved by the Washington University School of Medicine Institutional Review Board with a waiver for patient consent.
Meeting Presentation: Abstract presented at the STS Annual Meeting by N.H.B., Jan 25–28, 2020, New Orleans, LA
References
- 1.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1(1):19–32. [DOI] [PubMed] [Google Scholar]
- 2.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004;15(6):1597–605. [DOI] [PubMed] [Google Scholar]
- 3.Laffey JG, Boylan JF, Cheng DCH. The systemic inflammatory response to cardiac surgery. Anesthesiol 2002;97:215–52. [DOI] [PubMed] [Google Scholar]
- 4.Badhwar V, Rankin JS, Damiano RJ, et al. The Society of Thoracic Surgeons 2017 Clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2017;103(1):329–341. [DOI] [PubMed] [Google Scholar]
- 5.Ad N, Damiano RJ, Badhwar V, et al. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2017;153(6):1330–1354.e1. [DOI] [PubMed] [Google Scholar]
- 6.Badhwar V, Rankin JS, Ad N, et al. Surgical ablation of atrial fibrillation in the United States: Trends and propensity matched outcomes. Ann Thorac Surg 2017;104(2):493–500. [DOI] [PubMed] [Google Scholar]
- 7.Malaisrie SC, McCarthy PM, Kruse J, et al. Ablation of atrial fibrillation during coronary artery bypass grafting: Late outcomes in a Medicare population. J Thorac Cardiovasc Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churyla A, Andrei A-C, Kruse J, et al. Safety of atrial fibrillation ablation with isolated surgical aortic valve replacement. Ann Thorac Surg 2020. [DOI] [PubMed] [Google Scholar]
- 9.Henn MC, Lancaster TS, Miller JR, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2015;150(5):1168–76, 1178.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up?. J Am Coll Cardiol 2011;57(2):160–6. [DOI] [PubMed] [Google Scholar]
- 11.Yoshihara F, Nishikimi T, Kosakai Y, et al. Atrial natriuretic peptide secretion and body fluid balance after bilateral atrial appendectomy by the maze procedure. The Journal of Thoracic and Cardiovascular Surgery 1998;116(2):213–219. [DOI] [PubMed] [Google Scholar]
- 12.Ad N, Tian YY, Verbalis J, Imahara SD, Cox JL. The effect of the maze procedure on the secretion of arginine-vasopressin and aldosterone. The Journal of Thoracic and Cardiovascular Surgery 2003;126(4):1095–1100. [DOI] [PubMed] [Google Scholar]
- 13.Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg 2007;133(2):389–96. [DOI] [PubMed] [Google Scholar]
- 14.Musharbash FN, Schill MR, Sinn LA, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg 2018;155(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Axtell AL, Fiedler AG, Melnitchouk S, et al. Correlation of cardiopulmonary bypass duration with acute renal failure after cardiac surgery 2020;159(1):170–178.e2. [DOI] [PubMed] [Google Scholar]
- 16.Birnie K, Verheyden V, Pagano D, et al. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care 2014;18(6):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmistekawy E, Mcdonald B, Hudson C, et al. Clinical impact of mild acute kidney injury after cardiac surgery. Ann Thorac Surg 2014;98(3):815–22. [DOI] [PubMed] [Google Scholar]
- 18.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009;119(18):2444–53. [DOI] [PubMed] [Google Scholar]
- 19.Ghavidel AA, Javadpour H, Shafiee M, Tabatabaie MB, Raiesi K, Hosseini S. Cryoablation for surgical treatment of chronic atrial fibrillation combined with mitral valve surgery: a clinical observation. Eur J Cardiothorac Surg 2008;33(6):1043–8. [DOI] [PubMed] [Google Scholar]
- 20.Gillinov M, Soltesz E. Surgical treatment of atrial fibrillation: today’s questions and answers. Semin Thorac Cardiovasc Surg 2013;25(3):197–205. [DOI] [PubMed] [Google Scholar]
- 21.Ad N, Holmes SD, Massimiano PS, Pritchard G, Stone LE, Henry L. The effect of the Cox-maze procedure for atrial fibrillation concomitant to mitral and tricuspid valve surgery. J Thorac Cardiovasc Surg 2013;146(6):1426–34. [DOI] [PubMed] [Google Scholar]
- 22.Saint LL, Damiano RJ, Cuculich PS, et al. Incremental risk of the Cox-maze IV procedure for patients with atrial fibrillation undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2013;146(5):1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmood E, Matyal R, Mahmood F, et al. Impact of left atrial appendage exclusion on short-term outcomes in isolated coronary artery bypass graft surgery. Circulation 2020;142(1):20–28. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Rao CF, Chen SP, He L, Hou JF, Zheng Z. Surgical left atrial appendage occlusion in patients with atrial fibrillation undergoing mechanical heart valve replacement. Chin Med J (Engl) 2020;133(16):1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charitakis E, Walfridsson H, Alehagen U. Short-term influence of radiofrequency ablation on NT-proBNP, MR-proANP, Copeptin, and MR-proADM in patients with atrial fibrillation: Data from the observational SMURF study. J Am Heart Assoc 2016;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolis AS, Vassilikos V, Maounis T, et al. Detection of myocardial injury during radiofrequency catheter ablation by measuring serum cardiac troponin I levels: Procedural correlates. J Am Coll Cardiol 1999;34:1099–105. [DOI] [PubMed] [Google Scholar]
- 27.Richter B, Gwechenberger M, Socas A, et al. Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol 2012;101(3):217–25. [DOI] [PubMed] [Google Scholar]
- 28.Pet M, Robertson JO, Bailey M, et al. The impact of CHADS2 score on late stroke after the Cox maze procedure. J Thorac Cardiovasc Surg 2013;146(1):85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


