Introduction
Gastrointestinal (GI) disorders are amongst the most common medical conditions that are comorbid with Autism spectrum disorders (ASD) 1,2,3. Despite their prevalence, GI disorders are often overlooked 3. Untreated GI distress in children with ASD has been linked to many issues in this population, including sleep, behavioral and psychiatric disorders 4,5. It is thus essential to understand the presentations of GI problems in children with ASD. In this chapter we will discuss the GI disorders commonly associated with ASD, how they present, and studied risk factors.
Prevalence and Types of Gastrointestinal Disorders in Children with ASD
GI disorders were first associated with ASD through the presentation of feeding disorders in affected children. In Dr. Leo Kanner’s seminal report describing ASD, ‘eating problems’ were identified in the majority of children presented 6. Since that time, it has been found that children with ASD are up to five times more likely to develop feeding problems, such as food selectivity, food refusal, and poor oral intake, than neurodevelopmentally normal children. Food intake is also often predicated on food category and/or texture aversion 7,8. Food selectivity in this population is often manifested as a preference for carbohydrates and processed foods 8,9. This behavior tends to be more severe than in age-matched children and lasts past childhood 10,11. As with other GI problems, food selectivity may be more common in ASD than in children with other causes of developmental delay 12. GI symptoms are also more common in toddlers with ASD than children with typical development or other developmental delays 13, implying that there may be something unique in gut development and/or function that occurs in ASD relative to not only neurotypical children but also other special needs populations.
It has been increasingly recognized that GI problems may underlie some of the feeding disorders seen in this population. In fact, the prevalence of GI symptoms in children with ASD varies from 9-91% 14. The most comprehensive meta-analysis to date revealed that children with ASD were more than four-fold more likely to develop GI problems than those without ASD and, further, that constipation, diarrhea and abdominal pain are reported most commonly 3. Other studies have reported constipation as the primary GI comorbidity with ASD, where the odds of constipation increase with greater social impairment and less verbal ability 15. GI disorders are also associated with increased ASD severity 16.
Alternating constipation and diarrhea has also been reported in this population 17. Whether this is a true alternating picture or, rather, constipation accompanied by periods of encopresis, has not been examined in prospective studies 18. In 2010, a multi-expert panel published a consensus report on GI disorders in ASD that outlined the best practices for evaluating and treating GI disorders in children with ASD. These guidelines focused on abdominal pain, constipation, chronic diarrhea and gastroesophageal reflux disease (GERD), which were again noted as the most common causes of GI problems in ASD 14. The commonality of these conditions has been published in multiple studies 18,3.
Pica, the ingestion of non-nutritive items, is also reported as a problem in children with development delays, including ASD. Pica has been associated with GI problems, such as irritable bowel syndrome and constipation, though it is not known whether the GI issues are the cause of the pica or whether the pica causes GI problems 19. In one study, 60% of ASD patients displayed pica at some stage in their lives 20,21. In some cases pica is associated with dangerous outcomes, including elevated blood lead levels, bezoars, obstructions, perforation and poisoning, necessitating close monitoring of these patients 22,21,23,24.
Clinical Comorbidities associated with Gastrointestinal Disorders in ASD
Of the medical comorbidities associated with ASD, seizures, sleep disorders and psychiatric problems tend to be the conditions most commonly associated with GI dysfunction (Figure 1) 5,25.
Figure 1:
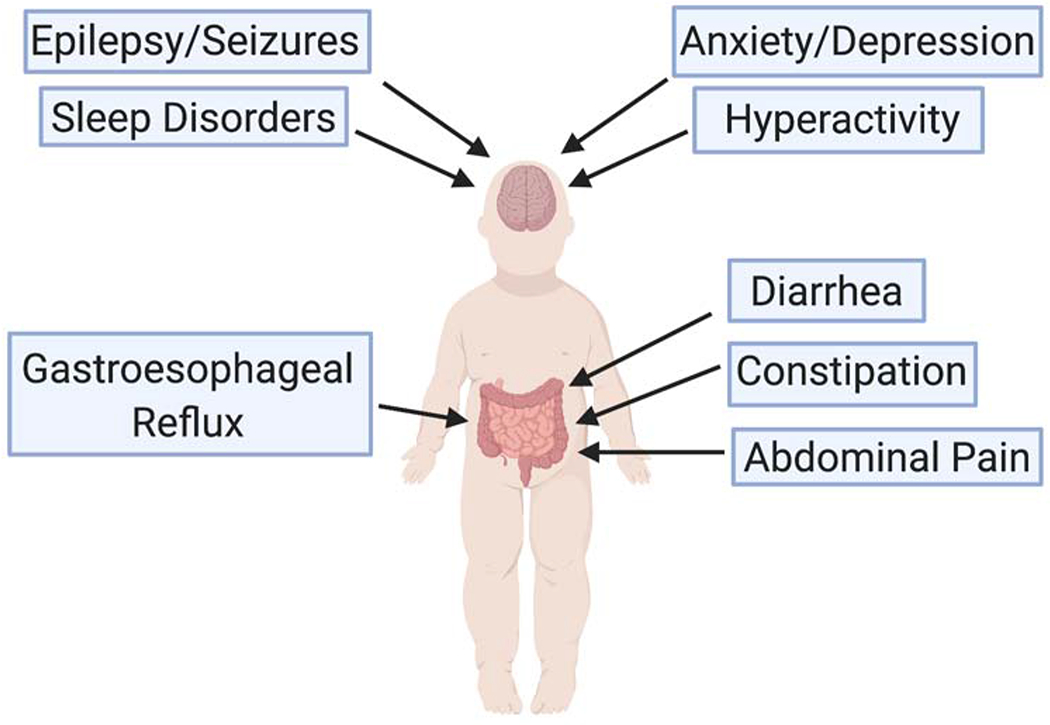
The major brain and intestinal comorbidities associated with ASD
Sleep abnormalities affect 80% of children with ASD and range from reduced sleep duration to parasomnias 26,27. Sleep disorders have been described to be associated with other psychiatric and/or other clinical co-morbidities or as an autonomous issue 28. Both upper and lower GI tract problems have been associated with ASD 29. The predominant GI conditions seen in ASD, including constipation and abdominal pain, cause abdominal discomfort which could be an impediment to good sleep hygiene 14. ASD children with gastroesophageal reflux, which is also associated with GI discomfort, have higher comorbidity with sleep disorders 30. This heightened sleep disturbance seen with GI problems in ASD children may play an important role in the quality of life for these children.
Psychiatric disorders occur in up to 70% of patients with ASD. The most common psychiatric disorder associated with ASD is anxiety 31, although others that often present include attention deficit/hyper-reactivity disorder (ADHD) and oppositional defiant disorder 31. Anxiety has been highly associated with chronic GI problems in children with ASD 32. The common manifestations of anxiety in these children include simple phobias, generalized anxiety, separation anxiety, obsessive-compulsive disorder and social phobias. Anxiety disorders are commonly found across all levels of cognitive functioning seen in ASD 33. These comorbidities do not differ between males and females and often persist from childhood into adolescence 34,35.
Children with ASD and anxiety have been shown to be at greater risk for lower GI problems, which may in part be mediated through an enhanced stress response. ASD patients with GI problems have greater stress reactivity than non-ASD controls. Children with ASD were also shown to have greater GI symptoms correlated to higher post-stress cortisol levels 36.
It has also been suggested that GI issues may be related to a subset of patients with autonomic nervous system dysfunction. For example, lower heart rate variability (a measure of parasympathetic activity on the heart) was associated with greater GI problems, especially in ASD patients with regression 29 The relationship between anxiety and GI issues in ASD is an active area of investigation 29,36.
In addition to psychiatric comorbidities, maladaptive disorders, such as irritability and social withdrawal, have also been associated with GI dysfunction in ASD 37. Children with ASD who experience abdominal pain, gas, diarrhea and constipation have more irritability, social withdrawal and hyperactivity compared to those without the GI issues 37. Argumentative, oppositional, defiant and destructive behaviors are also more often seen in ASD children with accompanying GI problems 5. GI distress experienced by children with ASD may thus play an important role in the behavioral problems demonstrated in this population.
Difficult and unexplained behaviors may be due in part to the inability of some children with ASD to verbalize their discomfort in response to GI distress 14. Consequently, GI distress may manifest in seemingly unassociated ways. For example, unexplained irritable behavior was found in 43% of ASD children with esophagitis 38 and functional constipation has been associated with rigid-compulsive behavior in children with ASD 39. Non-verbal patients may also demonstrate GI distress as constant eating or drinking, chewing on non-edible objects and abdominal pressure 40.
Risk Factors for Gastrointestinal Disorders in ASD
Though studies have identified potential genetic risk factors for ASD and GI dysfunction, most alone have been insufficient in explaining its cause. For example, a polymorphism of the MET tyrosine receptor kinase is associated with both ASD and GI dysfunction in family samples, elucidating a potential genetic connection of the two pathologies 41. Not all individuals with this genetic mutation, however, exhibit symptoms of GI dysfunction. Gene x environment interactions are likely to play a role in most etiologies 42. Since genetic susceptibility is often elicited by environmental exposure, understanding the role(s) that environmental risk factors play in ASD symptomatology and/or pathogenesis is critical and an active area of investigation 43.
Diet is an environmental factor that may affect ASD symptomatology. The most common restrictive diet utilized in the ASD population is one that lacks gluten and/or casein 44. Though anecdotal reports suggest an improvement in GI and/or behavioral symptoms in individuals on gluten- and/or casein-free diets, these findings have not been confirmed in double-blind placebo-controlled trials 45. It has also not been shown that children with ASD suffer from an increased incidence of celiac disease, wheat allergy or milk allergy 46. It is thus not currently recommended that children with ASD be placed on these diets indiscriminately. It is possible, however, that some children may respond positively to being on exclusionary diets, but it is not known how to identify these children or why they may benefit. There are also disadvantages to these diets; they can be highly restrictive and can thus further compounds the highly selective diets children with ASD often already have 47. A restrictive diet and picky eating can both lead to nutrient deficiencies. If families are interested in trying an exclusionary diet, they should do so in close collaboration with a nutritionist.
An important way in which the diet may impact behavior and/or GI function is based on its ability to alter the intestinal microbiome. Diet rapidly alters gut microbiota composition and specific microbiota environments have been associated with changes in behavior, mood, cognition and GI problems, in both preclinical and clinical studies (as reviewed in 48,49,50) (Figure 2). Although children with ASD have been shown to have different intestinal microbiota populations than neurotypical children, the studies have been small and the results have been extremely variable 51.
Figure 2:
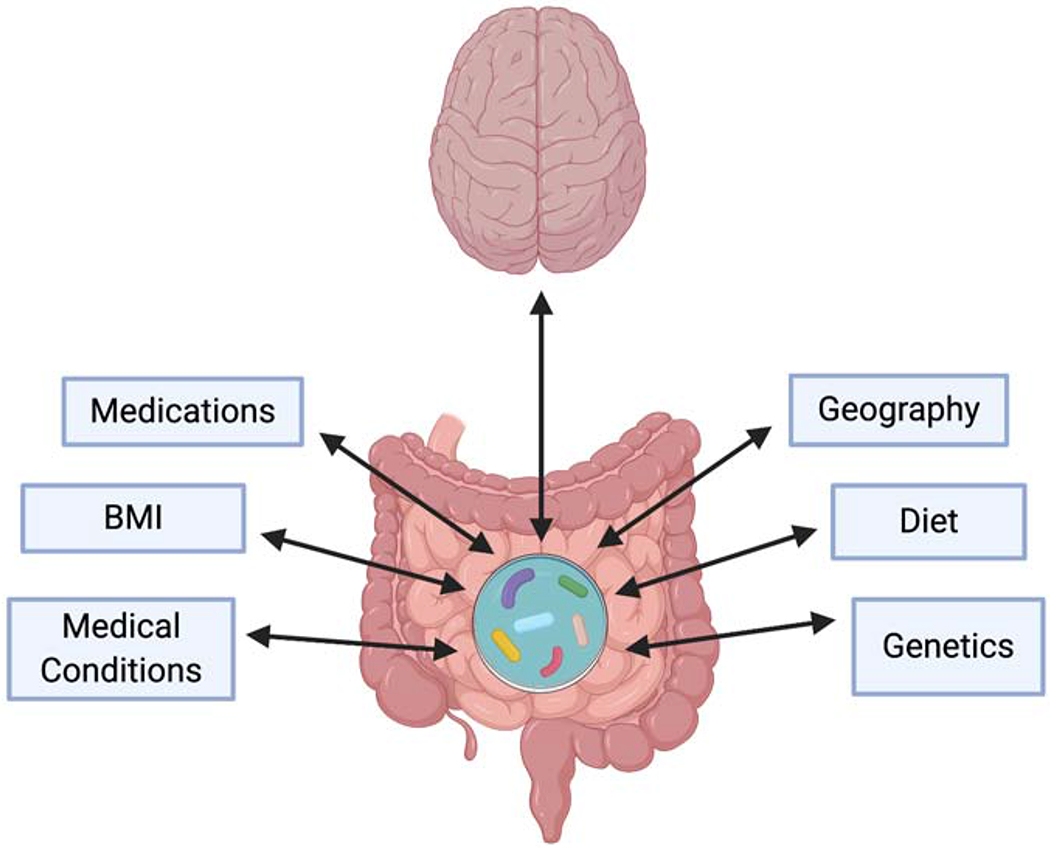
The microbiome can have a significant effect on gut function as well as on mood and behavior. The studies that have sought to evaluate the gut microbiome in ASD have demonstrated variable results. This is likely because there are many factors that impact the microbiome that may differ, on an individual basis, in patients with ASD. These factors include diet, geography, genetics, body mass index (BMI), medications and other medical conditions.
Interestingly, some of the microbial differences seen in children with ASD have been associated with impaired transcription of genes involved in carbohydrate metabolism 52. This could thus be a reason why some kids with ASD respond poorly to gluten ingestion. There is also one report showing that children with ASD have increased immune reactivity to gluten and this immune response is enhanced when GI symptoms are present, but this study, like the aforementioned one, has not been replicated 53. In addition to the lack of confirmatory data in this area, there are also contradictory findings; a recent study showed that dietary patterns were not associated with the GI symptoms seen in children with ASD 54. These data are suggestive of the notion that diet is not the exclusive cause of underlying GI dysfunction in these children. Alternatively, it is also possible that subsets of children with ASD harbor abnormalities in carbohydrate digestion and/or immunity. At this point, however, it is not known whether these physiological differences actually exist and, if they do, how physicians would be able to identify these specific patients in a reliable fashion.
Maternal factors, through prenatal exposure, have been associated with ASD occurrence. In particular, maternal obesity and gestational diabetes mellitus (GDM) are two factors that have been more extensively studied and were found to be associated with a 1.5 times greater odds of ASD in exposed offspring 55,56,57,58. In line with these clinical studies, animal research has shown that a maternal high fat diet (HFD) is associated with gut microbiota dysbiosis, that results in changes in central neurobiology and abnormal social behaviors, linking the maternal diet and dysbiosis to neurodevelopmental disorders 59. Interestingly, studies have also shown that changing the microbiome may correct the brain and behavioral defects induced by a dysbiosis, suggesting that the intestinal microbiota may be an underlying cause of the CNS dysfunction in ASD and also that its manipulation could be a novel therapeutic option 59,60. In one of these studies, administration of a HFD during gestation in mice resulted, in the pups, in a gut microbiota dysbiosis, reduced sociability, fewer oxytocin neurons in the hypothalamus and reduced synaptic plasticity in the ventral tegmental area (VTA) of offspring. A lack of Lactobacillus reuteri was implicated as one cause of the dysbiosis seen in this diet-induced phenotype and treatment with Lactobacillus reuteri resulted in improvements in the CNS and social deficits 59. Another study showed similar results; HFD-exposed offspring expressed less vocalizations during maternal separation experiments, a measure of early life stress in the offspring, than control diet offspring. In these experiments, members of the Firmicutes phyla were implicated in the gut microbiome dysbiosis and behavioral correlates 61. Recently, L. reuteri restored social impairments in multiple ASD mouse models and novel insights into this mechanism have revealed that this process is mediated directly through the nervous system and not via changes in the gut microbiome 60. These basic experiments have demonstrated a possible role for the gut microbiome in the generation of ASD-like phenotypes and, excitingly, possible novel treatment modalities.
Both human and animal studies have indicated an association between maternal inflammation and ASD risk 62. The inflammation resulting from human maternal infection and/or maternal autoimmune disease has been associated with an increased prevalence of ASD in progeny 63. Similarly, mice and/or non-human primates that are exposed to maternal immune activation (MIA) through maternal infection with the viral mimetic, polyinosinic: polycitidylic acid (poly (I:C)), or maternal influenza infection, have offspring that develop abnormal social behaviors 64,65,66, deficits in their immune profiles and anxiety-like phenotypes 67. Interestingly, MIA has also been shown to cause increased gut permeability and gut dysbiosis 68,69. Treatment of MIA-exposed mice with Bacteriodes fragilis has been shown to normalize intestinal permeability, prevents migration of neurotransmitters through the gut wall and improves multiple hallmarks of ASD-like behaviors 68,70. These experiments demonstrate how maternal infection may result in concomitant ASD and GI dysfunction. It is not known whether MIA-exposed pups also manifest other GI abnormalities like motility dysfunction or abdominal pain.
Mouse studies have shown evidence that the link between maternal inflammation and ASD development may involve placental inflammation. Placental interleukin-6 (IL-6), a pro-inflammatory cytokine, was shown to mediate the maternal infection-induced ASD risk to the developing fetus. Whereas progeny of MIA mice develop phenotypes consistent with ASD, those mice with selective deletion of IL-6 in placental trophoblasts had offspring that did not display these neurobiological or behavioral abnormalities 71. This alludes to a potential mechanism through which MIA can interfere with gestation, opening the door to future research in this area.
Diagnosis and Treatment of GI problems in ASD
Although a reliable diagnosis of GI problems in ASD in important, they can be extremely difficult to recognize because their presentations often lack the classic signs of GI distress such as verbal complaints of abdominal pain or other signs of localization of discomfort (i.e., holding of the stomach). Two of the major reasons that this occurs is because children with ASD often have limited verbal ability and, even if their verbal ability is intact, sensory perception is often abnormal, making it difficult for these individuals to localize and/or describe sources of their discomfort 72. Consequently, children with ASD and GI pain may present with non-specific signs or symptoms to express their discomfort, including aggression, self-injury, irritability, abnormal vocalizations (i.e. frequent swallowing, moaning), motor signs (i.e. grimacing, tapping), hyperactivity, changes in sleep patterns and/or anxiety 73,25,4,14.
The gold standard screening tool used to diagnose some of the most common pediatric GI conditions is the Rome criteria. A major problem with the Rome criteria for evaluating individuals with ASD is that its accuracy is dependent on the ability of the patient to speak and also to localize pain. In order to facilitate more accurate diagnoses in the ASD population, the Autism Treatment Network created a GI symptom inventory questionnaire to diagnose the most common GI conditions found in ASD. In contrast to the Rome criteria, GI problems were identified in this questionnaire by utilizing observable signs for caretakers, as opposed to verbal complaints, and thus included physical behaviors associated with GI distress (i.e. applying abdominal pressure). Recently, a concise version of this screening tool was created and validated. This 17-question screen, which relies on caretaker observation, was administered prospectively to caretakers and then to pediatric gastroenterologists who had evaluated the patients. Using this screen, GI problems were effectively diagnosed with a sensitivity of 86%, a specificity of 43% and a positive predictive value of 67%. Importantly, the screen detected new diagnoses of GI problems in over 20% of the patients 18.
Although there are anecdotal reports of behavioral improvements following effective treatment for GI problems, prospective studies are still required 74,75. These anecdotal reports, however, suggest that effective treatment of GI disorders is important not only for the attenuation of the GI problems, but potential improvements in associated behavioral correlates as well 74,76,77.
Treatment of GI problems in children with ASD should be addressed in the same ways as for those without ASD. Those with ASD, however, may benefit from having an interdisciplinary medical team that can diagnose and treat the different complex, and often interrelated medical conditions (noted above) that affect this population 14. Depending on the issues involved, a sleep specialist, psychiatrist and/or neurologist may be most commonly useful. Nutritionists also often also need to be involved proactively in the long-term nutritional treatment strategies for these individuals.
Some of the major novel therapeutic approaches that have been evaluated in recent years in the ASD population involve manipulation of the gut microbiota. The theory that gut dysbiosis underlies some of the behavioral manifestations in ASD originated from a study in children who developed regressive ASD and diarrhea after a course of antibiotics. The study showed that short-term administration of oral vancomycin treatment resulted in improvements in behavioral correlates. Although the initial findings were promising, the behavioral improvements ceased almost immediately after the antibiotic was stopped 78. Though not effective as a treatment, this was an important step in linking the microbiome with ASD.
Although multiple studies have shown that children with ASD have different intestinal microbiota than neurotypical children, the studies have been small with variable results 51. The variability may be linked to the many confounding factors that can alter the microbiome (Figure 2). There are a number of studies, however, that demonstrate a difference in Clostridia levels in children with ASD that, interestingly, is a target for vancomycin 79–82.
Another way that the gut microbiome may be manipulated is through delivery of a fecal microbiota transplant (FMT). There one study that evaluated the efficacy of FMT in individuals with ASD showed that the patients given a FMT exhibited significantly improved GI and behavioral outcomes following fecal transfer that persisted eight weeks post-treatment 76. Moreover, in a recent two-year follow-up of this same patient cohort, the GI and behavioral improvements persisted, demonstrating long-term effects of this therapy 77. Though the results of this study are exciting, the patient sample was small (18 patients) and the trial was open-label, leaving outcomes open to the effects of placebo. A fecal microbiota transplant study that is conducted in a larger cohort and in a double-blind, placebo-controlled manner is thus warranted.
As discussed briefly above, administration of some probiotics have resulted in improvements in ASD-like phenotypes in animal models 68. Recently, a small-randomized pilot trial of probiotics in children with ASD and GI problems was conducted. A probiotic mixture of eight Lactobacillus and Bifidobacterium species was given for the 19-week trial period. Although parents reported a significant improvement in GI symptoms, the overall resulting quality of life measures were not significant 74. In another randomized pilot study, children with ASD and GI problems were given both pre- and pro-biotic treatments intermittently for 12 weeks. Although some of these patients showed improvements in GI problems and aberrant behaviors, the small sample size and lack of a true control group makes this data harder to generalize 75. These early studies on probiotic treatment in children with ASD and GI problems thus show promise, but larger trials are necessary to better implicate the role of probiotics in ASD treatment 14.
Summary
It has become increasingly clear that GI problems are common in the ASD population and also morbidity causing. It is thus critical that clinicians understand the different presentations of GI distress in this population so efficient treatment and/or referral to a gastroenterologist can be implemented. Once GI problems are diagnosed other clinical comorbidities commonly co-occurring with GI dysfunction in ASD should also be promptly considered.
Although the treatment for GI conditions in ASD is similar in many ways to those of neurotypical individuals, additional considerations must be made. Comprehensive nutritional evaluations should be considered because of the high incidence of food aversions as well as the common use of exclusionary diets. Although not a currently recommended treatment, gut microbiota modulation (i.e. FMT, probiotics, etc.) may be a distinct novel therapeutic option for this population in the future, as a treatment for not only GI problems but behavioral issues as well.
KEY POINTS.
GI disorders are highly prevalent in ASD
GI disorders are highly associated with other clinical comorbidities in ASD, and must be screened for accordingly
Diagnosis and treatment of GI issues in ASD can be challenging and often benefits from a multidisciplinary approach
Large, double-blind, placebo-controlled trials are required to confirm the effectiveness of dietary and microbiome-focused therapies
SYNOPSIS.
Gastrointestinal (GI) disorders are one of the most common medical conditions that are comorbid with Autism spectrum disorders (ASD). These comorbidities can cause greater severity in ASD symptoms, other associated clinical manifestations and lower quality of life if left untreated. Clinicians need to understand how these GI issues present and apply effective therapies. Effective treatment of GI problems in ASD may result in marked improvements in ASD behavioral outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The Authors have nothing to disclose
Reference:
- 1.Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133(1):e54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldinger KA, Lane CJ, Veenstra-VanderWeele J, Levitt P. Patterns of Risk for Multiple Co-Occurring Medical Conditions Replicate Across Distinct Cohorts of Children with Autism Spectrum Disorder. Autism Res. 2015;8(6):771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133(5):872–883. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson BJ, Dovgan K, Takahashi N, Beversdorf DQ. The Relationship Among Gastrointestinal Symptoms, Problem Behaviors, and Internalizing Symptoms in Children and Adolescents With Autism Spectrum Disorder. Front Psychiatry. 2019;10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maenner MJ, Arneson CL, Levy SE, Kirby RS, Nicholas JS, Durkin MS. Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J Autism Dev Disord. 2012;42(7):1520–1525. [DOI] [PubMed] [Google Scholar]
- 6.Kanner L Austic disturbances of affective contact. Nervous Child. 1943;2:217–250. [Google Scholar]
- 7.Sharp WG, Berry RC, McCracken C, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. 2013;43(9):2159–2173. [DOI] [PubMed] [Google Scholar]
- 8.Ahearn WH, Castine T, Nault K, Green G. An assessment of food acceptance in children with autism or pervasive developmental disorder-not otherwise specified. J Autism Dev Disord. 2001;31(5):505–511. [DOI] [PubMed] [Google Scholar]
- 9.Bandini LG, Curtin C, Phillips S, Anderson SE, Maslin M, Must A. Changes in Food Selectivity in Children with Autism Spectrum Disorder. J Autism Dev Disord. 2017;47(2):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J Am Diet Assoc. 2010;110(2):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding disorders in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008;39(2):261–272. [DOI] [PubMed] [Google Scholar]
- 12.Beighley JS, Matson JL, Rieske RD, Adams HL. Food selectivity in children with and without an autism spectrum disorder: investigation of diagnosis and age. Res Dev Disabil. 2013;34(10):3497–3503. [DOI] [PubMed] [Google Scholar]
- 13.Bresnahan M, Hornig M, Schultz AF, et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015;72(5):466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buie T, Campbell DB, Fuchs GJ 3rd, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125 Suppl 1:S1–18. [DOI] [PubMed] [Google Scholar]
- 15.Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. 2012;5(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr. 2011;32(5):351–360. [DOI] [PubMed] [Google Scholar]
- 17.Molloy CA, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7(2):165–171. [DOI] [PubMed] [Google Scholar]
- 18.Margolis KG, Buie TM, Turner JB, et al. Development of a Brief Parent-Report Screen for Common Gastrointestinal Disorders in Autism Spectrum Disorder. J Autism Dev Disord. 2019;49(1):349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Call NA, Simmons CA, Mevers JE, Alvarez JP. Clinical Outcomes of Behavioral Treatments for Pica in Children with Developmental Disabilities. J Autism Dev Disord. 2015;45(7):2105–2114. [DOI] [PubMed] [Google Scholar]
- 20.Kinnell HG. Pica as a feature of autism. Br J Psychiatry. 1985;147:80–82. [DOI] [PubMed] [Google Scholar]
- 21.Cohen DJ, Johnson WT, Caparulo BK. Pica and elevated blood lead level in autistic and atypical children. Am J Dis Child. 1976;130(1):47–48. [DOI] [PubMed] [Google Scholar]
- 22.Serour F, Witzling M, Frenkel-Laufer D, Gorenstein A. Intestinal obstruction in an autistic adolescent. Pediatr Emerg Care. 2008;24(10):688–690. [DOI] [PubMed] [Google Scholar]
- 23.George M, Heeney MM, Woolf AD. Encephalopathy from lead poisoning masquerading as a flu-like syndrome in an autistic child. Pediatr Emerg Care. 2010;26(5):370–373. [DOI] [PubMed] [Google Scholar]
- 24.Oestreich AE. Multiple magnet ingestion alert. Radiology. 2004;233(2):615. [DOI] [PubMed] [Google Scholar]
- 25.Kang V, Wagner GC, Ming X. Gastrointestinal dysfunction in children with autism spectrum disorders. Autism Res. 2014;7(4):501–506. [DOI] [PubMed] [Google Scholar]
- 26.Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53(9):783–792. [DOI] [PubMed] [Google Scholar]
- 27.Mannion ALG, Healy O. An investigation of comorbid pyschological disorders, sleep problems, gastrointestical symptoms and epilepsy in children and adolescents with Autism Spectrum Disoder. Research in Autism Spectrum Disorders. 2012;7(1):35–42. [Google Scholar]
- 28.Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev. 2009;13(6):403–411. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson BJ, Marler S, Altstein LL, et al. Psychophysiological Associations with Gastrointestinal Symptomatology in Autism Spectrum Disorder. Autism Res. 2017;10(2):276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trickett J, Heald M, Oliver C, Richards C. A cross-syndrome cohort comparison of sleep disturbance in children with Smith-Magenis syndrome, Angelman syndrome, autism spectrum disorder and tuberous sclerosis complex. J Neurodev Disord. 2018;10(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. [DOI] [PubMed] [Google Scholar]
- 32.Mazurek MO, Vasa RA, Kalb LG, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J Abnorm Child Psychol. 2013;41(1):165–176. [DOI] [PubMed] [Google Scholar]
- 33.White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 2009;29(3):216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofvander B, Delorme R, Chaste P, et al. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonoff E, Jones CR, Baird G, Pickles A, Happe F, Charman T. The persistence and stability of psychiatric problems in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2013;54(2):186–194. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson BJ, Marler S, Altstein LL, et al. Associations between cytokines, endocrine stress response, and gastrointestinal symptoms in autism spectrum disorder. Brain Behav Immun. 2016;58:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath K, Perman JA. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002;14(5):583–587. [DOI] [PubMed] [Google Scholar]
- 39.Marler S, Ferguson BJ, Lee EB, et al. Association of Rigid-Compulsive Behavior with Functional Constipation in Autism Spectrum Disorder. J Autism Dev Disord. 2017;47(6):1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7(3):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell DB, Buie TM, Winter H, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123(3):1018–1024. [DOI] [PubMed] [Google Scholar]
- 42.Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beversdorf DQ, Stevens HE, Jones KL. Prenatal Stress, Maternal Immune Dysregulation, and Their Association With Autism Spectrum Disorders. Curr Psychiatry Rep. 2018;20(9):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrin JM, Coury DL, Hyman SL, Cole L, Reynolds AM, Clemons T. Complementary and alternative medicine use in a large pediatric autism sample. Pediatrics. 2012;130 Suppl 2:S77–82. [DOI] [PubMed] [Google Scholar]
- 45.Hyman SL, Stewart PA, Foley J, et al. The Gluten-Free/Casein-Free Diet: A Double-Blind Challenge Trial in Children with Autism. J Autism Dev Disord. 2016;46(1):205–220. [DOI] [PubMed] [Google Scholar]
- 46.Buie T The relationship of autism and gluten. Clin Ther. 2013;35(5):578–583. [DOI] [PubMed] [Google Scholar]
- 47.Bandini LG, Anderson SE, Curtin C, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157(2):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murtaza N, P OC, Morrison M. Diet and the Microbiome. Gastroenterol Clin North Am. 2017;46(1):49–60. [DOI] [PubMed] [Google Scholar]
- 49.Vuong HE, Yano JM, Fung TC, Hsiao EY. The Microbiome and Host Behavior. Annu Rev Neurosci. 2017;40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parekh PJ, Balart LA, Johnson DA. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin Transl Gastroenterol. 2015;6:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vuong HE, Hsiao EY. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol Psychiatry. 2017;81(5):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6(9):e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lau NM, Green PH, Taylor AK, et al. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS One. 2013;8(6):e66155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson BJ, Dovgan K, Severns D, et al. Lack of Associations Between Dietary Intake and Gastrointestinal Symptoms in Autism Spectrum Disorder. Front Psychiatry. 2019;10:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connolly N, Anixt J, Manning P, Ping ILD, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder-An analysis of electronic medical records and linked birth data. Autism Res. 2016;9(8):829–837. [DOI] [PubMed] [Google Scholar]
- 56.Li M, Fallin MD, Riley A, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics. 2016;137(2):e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425–1434. [DOI] [PubMed] [Google Scholar]
- 58.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 2016;165(7):1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019;101(2):246–259 e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruce-Keller AJ, Fernandez-Kim SO, Townsend RL, et al. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS One. 2017;12(4):e0175577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frye RE, Rossignol DA. Identification and Treatment of Pathophysiological Comorbidities of Autism Spectrum Disorder to Achieve Optimal Outcomes. Clin Med Insights Pediatr. 2016;10:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang HY, Xu LL, Shao L, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav Immun. 2016;58:165–172. [DOI] [PubMed] [Google Scholar]
- 64.Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23(1):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75(4):332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109(31):12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. [DOI] [PubMed] [Google Scholar]
- 71.Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav Immun. 2017;62:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitney DG, Shapiro DN. National Prevalence of Pain Among Children and Adolescents With Autism Spectrum Disorders. JAMA Pediatr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang XL, Liang S, Zou MY, et al. Are gastrointestinal and sleep problems associated with behavioral symptoms of autism spectrum disorder? Psychiatry Res. 2018;259:229–235. [DOI] [PubMed] [Google Scholar]
- 74.Arnold LE, Luna RA, Williams K, et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J Child Adolesc Psychopharmacol. 2019;29(9):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanctuary MR, Kain JN, Chen SY, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. 2019;14(1):e0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang DW, Adams JB, Gregory AC, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang DW, Adams JB, Coleman DM, et al. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9(1):5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandler RH, Finegold SM, Bolte ER, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15(7):429–435. [DOI] [PubMed] [Google Scholar]
- 79.Bolte ER. Autism and Clostridium tetani. Med Hypotheses. 1998;51(2):133–144. [DOI] [PubMed] [Google Scholar]
- 80.Rodakis J An n=1 case report of a child with autism improving on antibiotics and a father’s quest to understand what it may mean. Microb Ecol Health Dis. 2015;26:26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finegold SM, Summanen PH, Downes J, Corbett K, Komoriya T. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe. 2017;45:133–137. [DOI] [PubMed] [Google Scholar]
- 82.Luna RA, Oezguen N, Balderas M, et al. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell Mol Gastroenterol Hepatol. 2017;3(2):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]


