PURPOSE
Breast cancer risk prediction models are used to identify high-risk women for early detection, targeted interventions, and enrollment into prevention trials. We sought to develop and evaluate a risk prediction model for breast cancer in US Black women, suitable for use in primary care settings.
METHODS
Breast cancer relative risks and attributable risks were estimated using data from Black women in three US population-based case-control studies (3,468 breast cancer cases; 3,578 controls age 30-69 years) and combined with SEER age- and race-specific incidence rates, with incorporation of competing mortality, to develop an absolute risk model. The model was validated in prospective data among 51,798 participants of the Black Women's Health Study, including 1,515 who developed invasive breast cancer. A second risk prediction model was developed on the basis of estrogen receptor (ER)–specific relative risks and attributable risks. Model performance was assessed by calibration (expected/observed cases) and discriminatory accuracy (C-statistic).
RESULTS
The expected/observed ratio was 1.01 (95% CI, 0.95 to 1.07). Age-adjusted C-statistics were 0.58 (95% CI, 0.56 to 0.59) overall and 0.63 (95% CI, 0.58 to 0.68) among women younger than 40 years. These measures were almost identical in the model based on estrogen receptor–specific relative risks and attributable risks.
CONCLUSION
Discriminatory accuracy of the new model was similar to that of the most frequently used questionnaire-based breast cancer risk prediction models in White women, suggesting that effective risk stratification for Black women is now possible. This model may be especially valuable for risk stratification of young Black women, who are below the ages at which breast cancer screening is typically begun.
INTRODUCTION
Risk prediction modeling is used to identify women at high risk of breast cancer for early detection, targeted interventions, and enrollment into prevention trials.1 However, prediction models have not performed as well in Black women.2-6 Lack of a breast cancer risk prediction model tailored to Black women represents a critical gap, given that US Black women have, on average, earlier ages at diagnosis than US White women and are more likely to be diagnosed with poor-prognosis breast cancers.7-15 Many young Black women are diagnosed with and die from breast cancer before they even reach the ages at which mammographic screening is typically recommended.8,16,17 Until now, the relatively small number of Black women enrolled in epidemiologic studies of breast cancer has hampered efforts to derive and test models for use in Black women.
CONTEXT
Key Objective
To date, breast cancer risk prediction models have underperformed in US Black women, among whom there is a disproportionately high breast cancer mortality and a younger age at diagnosis. There is a critical need for a prediction model developed and validated in data from large studies of Black women.
Knowledge Generated
A new model, developed in data from more than 3,000 Black women with breast cancer and 3,000 controls, had excellent calibration in a prospective cohort of Black women. Discriminatory accuracy was better than for currently available models and was best for women younger than 40 years. All variables included in the model can be obtained from the women themselves.
Relevance
This new tool for personalized prediction of breast cancer risk in Black women can be easily used by primary health care providers to guide screening recommendations and/or referrals for genetic testing, particularly for young Black women, thus leading to earlier diagnosis and reduced mortality.
Established breast cancer risk prediction models have considered all invasive breast cancers together, using risk parameters that are primarily associated with estrogen receptor–positive (ER+) breast cancer.18-21 Recent research has indicated that some risk factors differ for ER+ and estrogen receptor–negative (ER–) breast cancer.22-26 Although a single-disease approach may work well for White women, among whom approximately 85% of cancers are ER+, consideration of differential associations with ER– breast cancer may be more important for Black women, among whom a smaller proportion of breast cancers are ER+.12,15
We used data from the largest case-control27-29 and cohort30 studies of breast cancer in US Black women to develop and test a risk prediction tool to be used in the primary care setting to identify Black women at elevated breast cancer risk. In addition to modeling all breast cancers as one entity, we derived a model that incorporated ER-specific relative risks and attributable risks.
METHODS
Model Development
Study population.
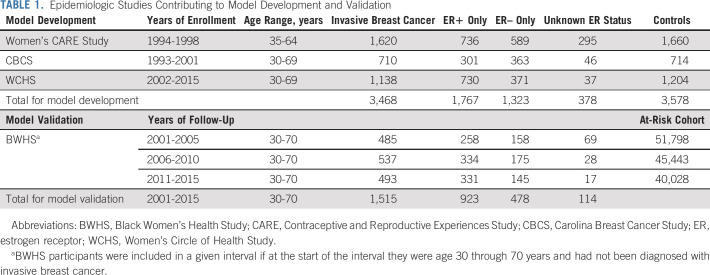
Questionnaire-based data on self-identified Black women from three population-based case-control studies were used to derive relative risk models (training data set; Table 1).
TABLE 1.
Epidemiologic Studies Contributing to Model Development and Validation
The National Institute of Child Health and Human Development Women's Contraceptive and Reproductive Experiences Study (CARE)27 is a breast cancer case-control study conducted in five US locations in 1994-1998. Cases were identified through SEER Rapid Case Ascertainment (RCA) for four sites and through staff review of pathology reports from hospitals, clinics, and pathology laboratories for the fifth site. Controls were identified by random digit dialing (RDD). In-person interviews were conducted to obtain a detailed history of reproductive events and other potential risk factors.
The Carolina Breast Cancer Study (CBCS) is a case-control study conducted in 44 counties in North Carolina in 1993-2001.28 Cases were identified from the North Carolina Central Cancer Registry using RCA, and controls were from Division of Motor Vehicles and Health Care Financing Administration lists. In-home interviews were conducted to obtain information on breast cancer risk factors.
The Women's Circle of Health Study is a case-control study conducted in New York and New Jersey.29 Recruitment in New York took place in 2002-2008, with hospital-based ascertainment of cases and controls identified through RDD. Recruitment in New Jersey was conducted in 2006-2015, with cases identified by the New Jersey State Cancer Registry using RCA in 10 counties. Controls were initially recruited through RDD (2006-2010) and later through community-based efforts (2009-2015).31 In-person interviews used questionnaires closely modeled on the CBCS questionnaire.
Across the three studies, participants ranged in age from 30 to 69 years; 1,767 had ER+ and 1,323 had ER– cancer (Table 1).
Statistical methods for model development.
Two separate breast cancer absolute risk prediction models were developed.32,33 Model A treated invasive breast cancer as a single disease, with predictors retained on the basis of associations with overall breast cancer risk. Model B was based on ER+ and ER– specific relative risks and attributable risks. Potential predictors evaluated were factors previously associated with breast cancer risk in the literature: recent body mass index (BMI); BMI at age 18 years; adult height; alcohol use; number of births; breastfeeding; age at first birth; oral contraceptive use duration; duration of use of estrogen plus progestin hormone supplements; first-degree family history of breast cancer; first-degree family history of each of ovarian, colorectal, and prostate cancer; age at menarche; history of past breast biopsy or diagnosis of benign breast disease; type 2 diabetes; menopausal status; and history of bilateral oophorectomy. We examined age interactions with each predictor and interactions of BMI with menopausal status. We used multiple imputations with chained equations to handle missing values (Data Supplement, online only; IVEware 0.3). We generated 50 imputed data sets and used Rubin's rules to combine coefficients and their SEs across the imputed data sets.34
We selected predictors separately for all relative risk models through backward elimination on the basis of the Akaike's Information Criterion. We assessed the importance of each variable on the basis of statistics calculated from the imputed data, removed the predictor that showed the smallest contribution to the model, and repeated the process until no further predictor could be removed (Data Supplement).34,35
To calculate 5-year predicted absolute risks, we combined relative risks and attributable risks with age-specific breast cancer incidence rates for 2000-2016 for non-Hispanic Black women from the National Cancer Institute (NCI) SEER program and age-specific mortality rates from the CDC Wide-ranging ONline Data for Epidemiologic Research.36 We describe detailed methods and formulas in the Data Supplement. Because CBCS and CARE oversampled younger women with breast cancer, we used reweighting to ensure that the attributable risk corresponded to the SEER population.33
Model Validation
Study population.
The Black Women's Health Study (BWHS) is an ongoing cohort study of 59,000 self-identified US Black women.30,37 Participants, age 21-69 years, enrolled in 1995 by completing a detailed 14-page health questionnaire. Biennial follow-up questionnaires update exposure variables and ascertain new diagnoses, including cancer. Follow-up has been successful for > 85% of potential person-years through 10 follow-up cycles. Incident breast cancers are ascertained by self-report, by linkage with state cancer registries of 24 states in which 95% of participants reside, and by linkage with the National Death Index. Pathology reports or state cancer registry data have been obtained for more than 90% of breast cancer cases to date and diagnosis confirmed for 99% of the cases for whom records were obtained.
Statistical methods for external validation.
Models were validated using BWHS data from 2001 through 2015. We constructed three 5-year follow-up intervals: 2001-2005, 2006-2010, and 2011-2015. Women were included in an interval if at the start they had not been diagnosed with invasive breast cancer and were age 30 through 70 years. We updated age and the time-dependent predictors (BMI, menopausal status, parity, breastfeeding, oral contraceptive use, and bilateral oophorectomy at the start of subsequent intervals). We computed separate 5-year predictions for each interval to which a woman contributed.38 Risks were predicted over 5 years for each woman. Across the 15 years of follow-up, there were 923 ER+, 478 ER–, and 114 unknown ER status incident invasive cases of breast cancer (Table 1).
We assessed calibration for a 5-year projection period by the ratio of the expected number, E (sum of the absolute predicted risk across BWHS participants), to the observed number, O, of invasive breast cancers. We used multiple imputation (IVEware 0.3) to address missing data (< 2% missing for each predictor; 5% missing ER status; Data Supplement). We calculated the log expected/observed (E/O) ratio and its SE for each of 10 imputed data sets and combined the values according to Rubin's rules.35 Calibration estimates were obtained by summing the observed and expected 5-year risks over the three time periods and then computing the ratio. We estimated E/O ratio overall and within strata of age and risk factors. To evaluate models across levels of risk, we stratified the data by 5-year age group and created age-specific quintile groups of 5-year predicted risk.
To assess discriminatory accuracy, we used the concordance statistic (C-statistic),39,40 which corresponds to the probability that a randomly selected woman with breast cancer has a higher predicted risk than a randomly selected unaffected woman. We estimated age-specific C-statistics and then calculated an age-adjusted C-statistic as the inverse-variance weighted average of the age-specific estimates.
For comparison, we also computed calibration and discrimination measures for the current NCI Breast Cancer Risk Assessment Tool (BCRAT) model41 in BWHS follow-up data.
RESULTS
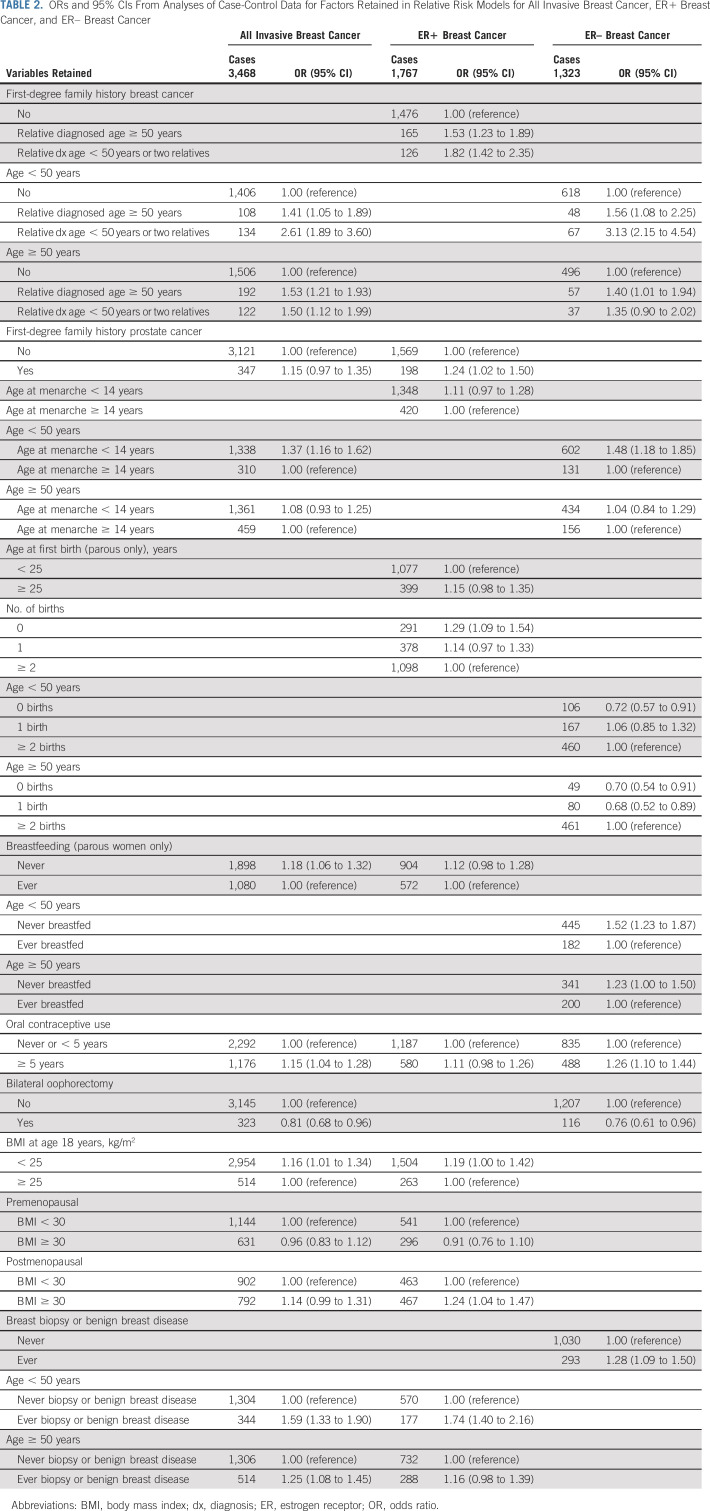
Variables included in each relative risk model (all breast cancer, ER+, and ER–) are shown in Table 2. First-degree family history of breast cancer, breast biopsy, 5 or more years of oral contraceptive use, earlier age at menarche, and lack of breastfeeding were associated with increased risk of both subtypes and bilateral oophorectomy was associated with reduced risk. Family history of prostate cancer, lower BMI at age 18 years, BMI > 30 kg/m2 during the postmenopausal period, later age at first birth, and nulliparity were associated with increased risk of ER+ but not ER– breast cancer. Higher parity was associated with increased risk of ER– breast cancer. Age interaction terms were retained for family history of breast cancer, age at menarche, and breast biopsy in analyses of all breast cancer.
TABLE 2.
ORs and 95% CIs From Analyses of Case-Control Data for Factors Retained in Relative Risk Models for All Invasive Breast Cancer, ER+ Breast Cancer, and ER– Breast Cancer
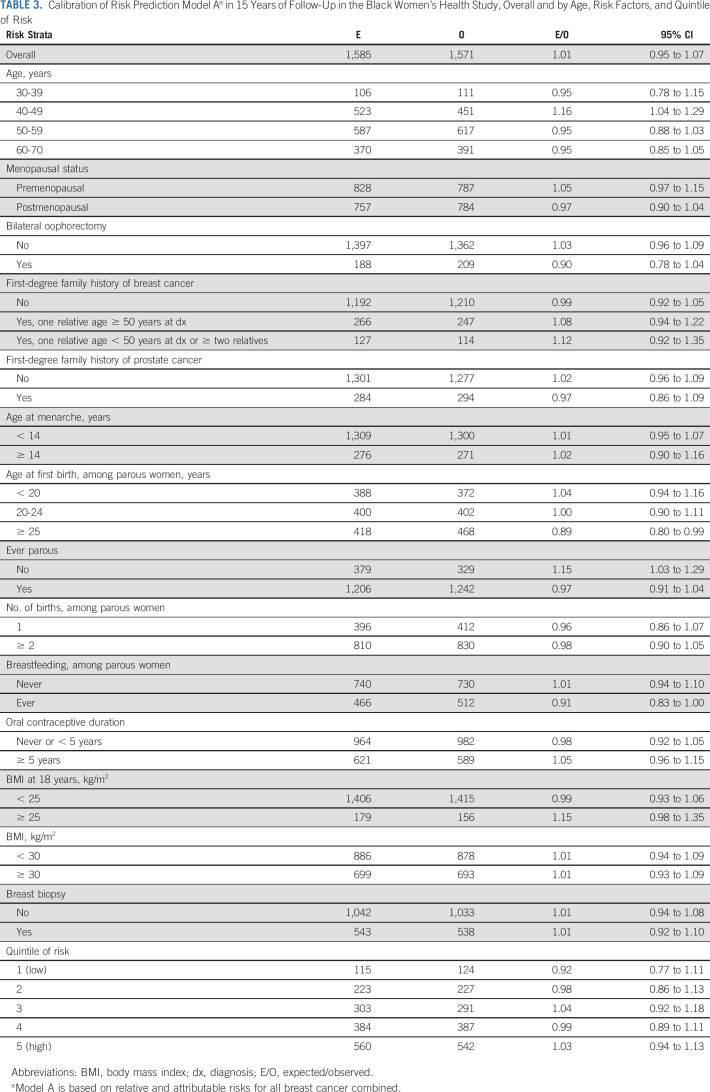
Both models were well-calibrated overall (E/O 1.01, 95% CI, 0.95 to 1.07 for model A; 1.06, 95% CI, 1.00 to 1.13 for model B) and within strata of age, individual risk factors, and risk quintiles (Table 3 and Data Supplement).
TABLE 3.
Calibration of Risk Prediction Model Aa in 15 Years of Follow-Up in the Black Women's Health Study, Overall and by Age, Risk Factors, and Quintile of Risk
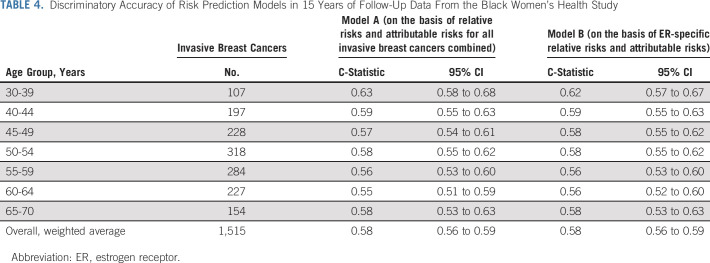
Discriminatory accuracy was similar for models A and B, with an age-adjusted C-statistic of 0.58 (95% CI, 0.56 to 0.59) for each model (Table 4); discrimination was somewhat better for ER+ than ER– disease (C-statistic 0.59 v 0.56; Data Supplement). Discrimination was best among women younger than 40 years, with a C-statistic of 0.63 (95% CI, 0.58 to 0.68). When we used 5-year predicted absolute risk divided into quintiles in a Cox regression model fit to breast cancer as the outcome, women younger than 40 years also had the largest estimated relative risk (highest v lowest risk quintile, 4.56, 95% CI, 2.02 to 10.3; Data Supplement).
TABLE 4.
Discriminatory Accuracy of Risk Prediction Models in 15 Years of Follow-Up Data From the Black Women's Health Study
The overall C-statistic without age adjustment was 0.64 (95% CI, 0.62 to 0.65) in both models. Although this measure can be misleading because the age distribution in a specific study influences discriminatory accuracy, it is reported here for comparison with previous studies.21,42
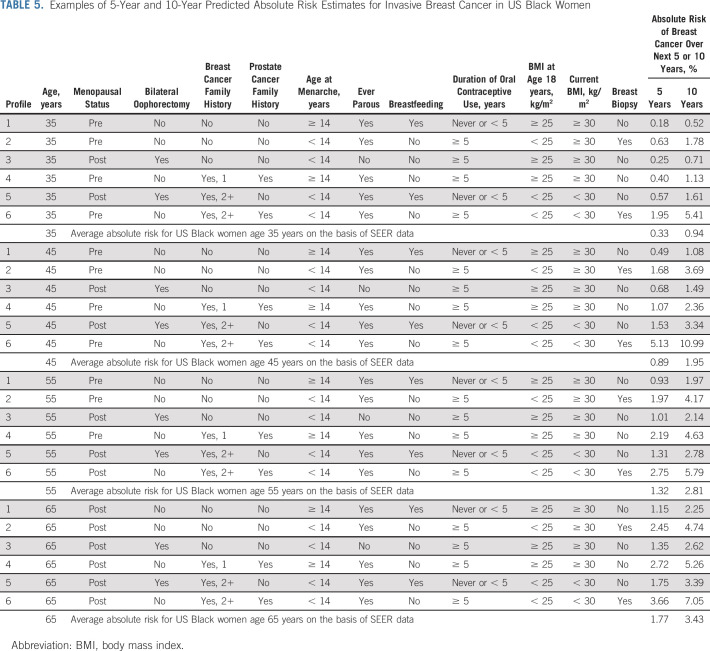
Table 5 shows estimated 5- and 10-year absolute risks for women age 35, 45, 55, and 65 years with differing risk profiles. Although family history of breast cancer is clearly an important factor, predicted risk differed noticeably across profiles even for women with the same family history (eg, profiles 5 and 6).
TABLE 5.
Examples of 5-Year and 10-Year Predicted Absolute Risk Estimates for Invasive Breast Cancer in US Black Women
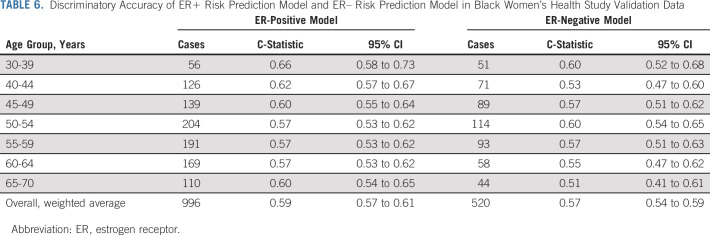
Table 6 presents discriminatory accuracy for separate ER+ and ER– absolute risk models; age-adjusted C-statistics were 0.59 for the ER+ prediction model and 0.57 for the ER– model.
TABLE 6.
Discriminatory Accuracy of ER+ Risk Prediction Model and ER– Risk Prediction Model in Black Women's Health Study Validation Data
Finally, for the NCI BCRAT model, we obtained an E/O of 0.97 (95% CI, 0.92 to 1.03) and age-adjusted C-statistic of 0.56 (95% CI, 0.55 to 0.59; Data Supplement).
DISCUSSION
We developed and externally validated two absolute risk prediction models for breast cancer in Black women, one that considered all breast cancers together and another that incorporated heterogeneity by ER status. The overall model, derived from 3,468 cases and 3,578 controls in three studies and validated with prospective follow-up data from the BWHS, had excellent calibration overall, by age, and in both low- and high-risk women. Similar to other questionnaire-based breast cancer risk models,2,18,20,43-45 our model had only moderate predictive ability. Importantly, however, discriminatory accuracy was the highest (C-statistic 0.63) among women younger than 40 years, arguably those most in need of a tool to guide personalized prevention and screening. Furthermore, we demonstrated that a risk prediction model on the basis of separate relative risks and attributable risks for ER+ and ER– breast cancers does not improve performance relative to a single-disease model.
Numerous risk prediction models for breast cancer have been developed and validated, most often in data from White women.2,18,20,21,43,44 The most widely used and validated questionnaire-based models intended for use in the general US population are the BCRAT2 and International Breast Intervention Study (IBIS) (Tyrer-Cuzick)20 models. In our BWHS data set, the BCRAT model yielded a C-statistic of 0.56 and E/O of 0.97. In Nurses' Health Study (NHS) data, age-adjusted C-statistics were 0.57 for the BCRAT model and 0.60 for the IBIS model.42 In a third model, derived from data on White women in two prospective cohorts, the corresponding C-statistic in NHS data was 0.58 (0.57 to 0.59).44 Similarly, for a breast cancer risk prediction model derived from European Prospective Investigation of Cancer study data and tested in Women's Health Initiative data, the age-adjusted C-statistic was 0.57 (0.56 to 59).46 These results based in White women are consistent with the discriminatory performance of our model. C-statistics reported for the Rosner-Colditz model in the NHS have been higher, as expected when the same study is used for both development and calibration assessment.19,42,47 Models that incorporate mammographic density are not considered here because they are useful only for women who have already had a first screening mammogram.
In a recent validation of the IBIS model version 7.0 (no mammographic or genetic variables) in Women's Health Initiative data, discriminatory accuracy was lower for non-Hispanic Black women than for any other group, albeit not statistically significant (P-interaction of discrimination by race .24).5 Because discriminatory accuracy was quantified using hazard ratios rather than C-statistics, we could not compare performance with our model.
We had hypothesized that prediction of breast cancer risk in Black women would be improved by a model that considered specific ER subtypes. Most established risk factors for breast cancer are more strongly associated with ER+ cancer. Because recent research has demonstrated differential associations by ER status for reproductive factors,23-25 we evaluated separate relative risk models for ER+ and ER– breast cancer. Glynn et al previously reported no benefit to using ER-specific relative risks in the NHS,47 but this might be expected given that 81% of the breast cancers were ER+. However, even in BWHS data, with a more balanced distribution of subtypes (66% ER+, 34% ER–), C-statistics were virtually identical for models based on ER-specific relative risks and overall breast cancer relative risks, suggesting that a model based on all breast cancer is sufficient.
The performance of our new model for prediction of breast cancer in Black women was best for women younger than 40 years, with an E/O of 0.99 and a C-statistic of 0.63. Given their higher breast cancer mortality rates, Black women younger than 40 years are most in need of a personalized tool to guide decision making about risk-reduction strategies, including when to pursue genetic testing for a high-risk gene mutation and when to initiate mammographic screening. As shown in Table 5, women with two or more first-degree relatives generally had a high 5-year predicted breast cancer risk and may meet guidelines for genetic testing. The prevalence of breast cancer genetic testing is lower among Black than White women48 despite similar prevalence of mutations and magnitudes of associations.49,50 As the key predictors of our new model include the same risk factors for carrying high-penetrance gene mutations such as BRCA1, BRCA2, and PALB2, quantifying risk from this new model may improve referral of Black women for genetic testing, which would have important implications for risk reduction of both breast and ovarian cancer.
Current guidelines variably recommend starting mammographic screening at age 40, 45, and 50 years, with an emphasis on shared decision making (SDM) between patients and clinicians. Uncertainty about personal risk is a well-documented barrier to SDM.51-54 With this new tool, young Black women, along with their primary health care providers, can estimate their risk of developing breast cancer over the next 5 years. National Comprehensive Cancer Network guidelines also include specific screening recommendations for women younger than 40 years, including women age ≥ 35 years with a 5-year risk of invasive breast cancer ≥ 1.7% and women age ≥ 30 years with a lifetime risk of breast cancer > 20% on the basis of the age of their affected first-degree relative.55 In either case, the new tool will support SDM between young Black women and their primary care clinicians.
Our ER+ risk prediction model could be useful for identifying Black women eligible for trials of chemopreventive agents for ER+ breast cancer; Black women have been underrepresented in such trials, in part because of their lower predicted absolute risk based on the most widely used models.56,57 In accord with models from other populations, our models did not perform well for ER– breast cancer.46 Further improvements may require identification of additional risk factors for ER– cancer.
Addition of a polygenic risk score (PRS) to breast cancer risk prediction models typically improves discriminatory accuracy.58,59 However, existing PRSs have been shown to stratify risk poorly in Black women, probably because of greater genetic variation and different patterns of linkage disequilibrium in women of African ancestry.60,61 In the future, addition of a race-specific PRS developed from a large ongoing consortial genome-wide association study of breast cancer in Black women could be a useful extension of our model.
Similarly, adding mammographic density to the model would likely improve discriminatory accuracy. We could not evaluate this factor because data on mammographic density were not available. As one goal of the new tool is identification of women who should begin screening earlier than usual recommendations, this is only a partial limitation.
The present study has notable strengths. Sample sizes for model development and validation were the largest to date for breast cancer prediction in Black women, and a substantial number of cases were diagnosed at young ages. The large number of ER– cases provided an opportunity to compare the performance of an ER-specific model with an overall breast cancer risk model. Repeated measures on time-varying potential predictors such as BMI, parity, and breastfeeding were available, permitting updating of predictors at the start of each 5-year period of risk.
In conclusion, this work has produced a breast cancer risk prediction tool that can be used by US Black women age 30 to 70 years and their primary care providers. The new model was well-calibrated in prospective cohort data and the discriminatory accuracy was on par with the models most widely used in US White women. It is based on variables that can be easily obtained from women themselves and entered into an online risk calculator, available at BWHS Breast Cancer Risk Calculator.62 Validation data indicated better performance among women younger than 40 years, for whom personalized referral for breast cancer screening may be most important. The ER+ model has the potential to increase representation of Black women in breast cancer chemoprevention trials. Both risk-based screening recommendations and increased representation in chemoprevention trials may help to reduce racial disparities in breast cancer mortality.
ACKNOWLEDGMENT
Breast cancer pathology data were obtained from several state cancer registries (AZ, CA, CO, CT, DE, DC, FL, GA, IL, IN, KY, LA, MD, MA, MI, NJ, NY, NC, OK, PA, SC, TN, TX, and VA) and the BWHS study protocol was approved by the institutional review boards of those cancer registries.
Tracy Battaglia
Research Funding: American Cancer Society, Patient Centered Outcomes Research Institute, NIH NCATs (Inst)
Elisa V. Bandera
Consulting or Advisory Role: Pfizer
No other potential conflicts of interest were reported.
DISCLAIMER
This article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the state cancer registries.
SUPPORT
Supported in part by National Institutes of Health grants R01CA228357 to J.R.P., U01CA164974 to L.R. and J.R.P., R01CA058420 to L.R., R01CA100598 to C.B.A. and E.V.B., P50CA58223 to M.A.T., the Susan G Komen Foundation SAC180086 to J.R.P., and the Karin Grunebaum Foundation.
AUTHOR CONTRIBUTIONS
Conception and design: Julie R. Palmer, Gary Zirpoli, Ruth M. Pfeiffer, Ludovic Trinquart
Financial support: Julie R. Palmer
Provision of study materials or patients: Leslie Bernstein, Christine B. Ambrosone, Elisa V. Bandera, Melissa A. Troester, Lynn Rosenberg
Collection and assembly of data: Julie R. Palmer, Gary Zirpoli, Leslie Bernstein, Christine B. Ambrosone, Elisa V. Bandera, Melissa A. Troester, Lynn Rosenberg
Data analysis and interpretation: Julie R. Palmer, Gary Zirpoli, Kimberly A. Bertrand, Tracy Battaglia, Melissa A. Troester, Ruth M. Pfeiffer, Ludovic Trinquart
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
A Validated Risk Prediction Model for Breast Cancer in US Black Women
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tracy Battaglia
Research Funding: American Cancer Society, Patient Centered Outcomes Research Institute, NIH NCATs (Inst)
Elisa V. Bandera
Consulting or Advisory Role: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gail MH, Pfeiffer RM: Breast cancer risk model requirements for counseling, prevention, and screening. J Natl Cancer Inst 110:994-1002, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gail MH, Costantino JP, Pee D, et al. : Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst 99:1782-1792, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Boggs DA, Rosenberg L, Pencina MJ, et al. : Validation of a breast cancer risk prediction model developed for Black women. J Natl Cancer Inst 105:361-367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams-Campbell LL, Makambi KH, Frederick WA, et al. : Breast cancer risk assessments comparing Gail and CARE models in African-American women. Breast J 15:S72-S75, 2009. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurian AW, Hughes E, Simmons T, et al. : Performance of the IBIS/Tyrer-Cuzick model of breast cancer risk by race and ethnicity in the Women's Health Initiative. Cancer 127:3742-3750, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Tice JA, Bissell MCS, Miglioretti DL, et al. : Validation of the breast cancer surveillance consortium model of breast cancer risk. Breast Cancer Res Treat 175:519-523, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen VW, Correa P, Kurman RJ, et al. : Histological characteristics of breast carcinoma in blacks and whites. Cancer Epidemiol Biomarkers Prev 3:127-135, 1994 [PubMed] [Google Scholar]
- 8.Dignam JJ: Differences in breast cancer prognosis among African-American and Caucasian women. CA Cancer J Clin 50:50-64, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Rose DP, Royak-Schaler R: Tumor biology and prognosis in black breast cancer patients: A review. Cancer Detect Prev 25:16-31, 2001 [PubMed] [Google Scholar]
- 10.American Cancer Society : Cancer Facts and Figures for African Americans 2013-2014. Atlanta, GA, American Cancer Society, 2013 [Google Scholar]
- 11.Silber JH, Rosenbaum PR, Clark AS, et al. : Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 310:389-397, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Clarke CA, Keegan TH, Yang J, et al. : Age-specific incidence of breast cancer subtypes: Understanding the black-white crossover. J Natl Cancer Inst 104:1094-1101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson WF, Rosenberg PS, Menashe I, et al. : Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst 100:1804-1814, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu KC, Anderson WF: Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat 74:199-211, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Howlader N, Altekruse SF, Li CI, et al. : US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106:dju055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Cancer Society : Cancer Facts & Figures 2020. Atlanta, GA, American Cancer Society, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy AM, Yang J, Armstrong K: Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. Am J Public Health 105:S446-S448, 2015. (suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry MB, Liao Y, Whittemore AS, et al. : 10-Year performance of four models of breast cancer risk: A validation study. Lancet Oncol 20:504-517, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Rice MS, Tworoger SS, Hankinson SE, et al. : Breast cancer risk prediction: An update to the Rosner-Colditz breast cancer incidence model. Breast Cancer Res Treat 166:227-240, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyrer J, Duffy SW, Cuzick J: A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111-1130, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Tice JA, Cummings SR, Smith-Bindman R, et al. : Using clinical factors and mammographic breast density to estimate breast cancer risk: Development and validation of a new predictive model. Ann Intern Med 148:337-347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandera EV, Chandran U, Hong CC, et al. : Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat 150:655-666, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortner RT, Sisti J, Chai B, et al. : Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: Results from the Nurses' Health Studies. Breast Cancer Res 21:40, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer JR, Viscidi E, Troester MA, et al. : Parity, lactation, and breast cancer subtypes in African American women: Results from the AMBER consortium. J Natl Cancer Inst 106:dju237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Work ME, John EM, Andrulis IL, et al. : Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer 110:1367-1377, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg L, Bethea TN, Viscidi E, et al. : Postmenopausal female hormone use and risk of estrogen receptor positive and negative breast cancer in African American women. J Natl Cancer Inst 108:djv361, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchbanks PA, McDonald JA, Wilson HG, et al. : The NICHD Women's Contraceptive and Reproductive Experiences Study: Methods and operational results. Ann Epidemiol 12:213-221, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Newman B, Moorman PG, Millikan R, et al. : The Carolina Breast Cancer Study: Integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat 35:51-60, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Ambrosone CB, Ciupak GL, Bandera EV, et al. : Conducting molecular epidemiological research in the age of HIPAA: A multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol 2009:871250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg L, Adams-Campbell L, Palmer JR: The Black Women's Health Study: A follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972) 50:56-58, 1995 [PubMed] [Google Scholar]
- 31.Bandera EV, Chandran U, Zirpoli G, et al. : Rethinking sources of representative controls for the conduct of case-control studies in minority populations. BMC Med Res Methodol 13:71, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfeiffer RM, Gail MH: Absolute Risk: Methods and Applications in Clinical Management and Public Health. Boca Raton, FL, CRC Press, 2017 [Google Scholar]
- 33.Bruzzi P, Green SB, Byar DP, et al. : Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 122:904-914, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Rubin DB, Schenker N: Multiple imputation in health-care databases: An overview and some applications. Stat Med 10:585-598, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Wood AM, White IR, Royston P: How should variable selection be performed with multiply imputed data?. Stat Med 27:3227-3246, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention, National Center for Health Statistics : Underlying Cause of Death 1999-2018 on CDC WONDER Online Database, Released in 2020. Data Are From the Multiple Cause of Death Files, 1999-2018, as Compiled from Data Provided by the 57 Vital Statistics Jurisdictions Through the Vital Statistics Cooperative Program. http://wonder.cdc.gov/ucd-icd10.html
- 37.Palmer JR, Wise LA, Horton NJ, et al. : Dual effect of parity on breast cancer risk in African-American women. J Natl Cancer Inst 95:478-483, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Cupples LA, D'Agostino RB, Anderson K, et al. : Comparison of baseline and repeated measure covariate techniques in the Framingham Heart Study. Stat Med 7:205-222, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Harrell FE: Regression Modeling Strategies (ed 1). New York, NY, Springer-Verlag, 2001 [Google Scholar]
- 40.Hanley JA, McNeil BJ: The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29-36, 1982 [DOI] [PubMed] [Google Scholar]
- 41.Breast Cancer Risk Assessment Tool SAS Macro. https://dceg.cancer.gov/tools/risk-assessment/bcrasasmacro [Google Scholar]
- 42.Glynn RJ, Colditz GA, Tamimi RM, et al. : Comparison of questionnaire-based breast cancer prediction models in the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev 28:1187-1194, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosner B, Colditz GA, Iglehart JD, et al. : Risk prediction models with incomplete data with application to prediction of estrogen receptor-positive breast cancer: Prospective data from the Nurses' Health Study. Breast Cancer Res 10:R55, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeiffer RM, Park Y, Kreimer AR, et al. : Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: Derivation and validation from population-based cohort studies. PLoS Med 10:e1001492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, Ogundiran T, Ademola A, et al. : Development of a breast cancer risk prediction model for women in Nigeria. Cancer Epidemiol Biomarkers Prev 27:636-643, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li K, Anderson G, Viallon V, et al. : Risk prediction for estrogen receptor-specific breast cancers in two large prospective cohorts. Breast Cancer Res 20:147, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glynn RJ, Colditz GA, Tamimi RM, et al. : Extensions of the Rosner-Colditz breast cancer prediction model to include older women and type-specific predicted risk. Breast Cancer Res Treat 165:215-223, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy AM, Bristol M, Domchek SM, et al. : Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol 34:2610-2618, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer JR, Polley EC, Hu C, et al. : Contribution of germline predisposition gene mutations to breast cancer risk in African American women. J Natl Cancer Inst 112:1213-1221, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domchek SM, Yao S, Chen F, et al. : Comparison of the prevalence of pathogenic variants in cancer susceptibility genes in Black women and non-Hispanic White women with breast cancer in the United States. JAMA Oncol 7:1045-1050, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis S, Stewart S, Bloom J: Increasing the accuracy of perceived breast cancer risk: Results from a randomized trial with Cancer Information Service callers. Prev Med 39:64-73, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Edwards A, Unigwe S, Elwyn G, et al. : Effects of communicating individual risks in screening programmes: Cochrane systematic review. BMJ 327:703-709, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillespie C: The experience of risk as 'measured vulnerability': Health screening and lay uses of numerical risk. Sociol Health Illn 34:194-207, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Gunn CM, Bokhour B, Parker VA, et al. : Exploring explanatory models of risk in breast cancer risk counseling discussions: NSABP/NRG Oncology Decision-Making Project 1. Cancer Nurs 42:3-11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bevers TB, Helvie M, Bonaccio E, et al. : Breast Cancer Screening and Diagnosis, Version 1.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf [DOI] [PubMed] [Google Scholar]
- 56.Ford JG, Howerton MW, Lai GY, et al. : Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer 112:228-242, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Kwiatkowski K, Coe K, Bailar JC, et al. : Inclusion of minorities and women in cancer clinical trials, a decade later: Have we improved? Cancer 119:2956-2963, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Mealiffe ME, Stokowski RP, Rhees BK, et al. : Assessment of clinical validity of a breast cancer risk model combining genetic and clinical information. J Natl Cancer Inst 102:1618-1627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dite GS, MacInnis RJ, Bickerstaffe A, et al. : Breast cancer risk prediction using clinical models and 77 independent risk-associated SNPs for women aged under 50 Years: Australian Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev 25:359-365, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palmer JR: Polygenic risk scores for breast cancer risk prediction: Lessons learned and future opportunities. J Natl Cancer Inst 112:555-556, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du Z, Gao G, Adedokun B, et al. : Evaluating polygenic risk scores for breast cancer in women of African ancestry. J Natl Cancer Inst 113:1168-1176, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.BWHS Breast Cancer Risk Calculator. https://www.bu.edu/slone/bwhs-brcarisk-calculator/ [Google Scholar]