PURPOSE
The Pediatric Oncology COVID-19 Case Report registry supplies pediatric oncologists with data surrounding the clinical course and outcomes in children with cancer and SARS-CoV-2.
METHODS
This observational study captured clinical and sociodemographic characteristics for children (≤ 21 years) receiving cancer therapy and infected with SARS-CoV-2 from the pandemic onset through February 19, 2021. The demographic and clinical characteristics of the cohort were compared with population-level pediatric oncology data (SEER). Multivariable binomial regression models evaluated patient characteristics associated with hospitalization, intensive care unit (ICU) admission, and changes in cancer therapy.
RESULTS
Ninety-four institutions contributed details on 917 children with cancer and SARS-CoV-2. Median age at SARS-CoV-2 infection was 11 years (range, 0-21 years). Compared with SEER, there was an over-representation of Hispanics (43.6% v 29.7%, P < .01), publicly insured (59.3% v 33.5%, P < .01), and patients with hematologic malignancies (65.8% v 38.3%, P < .01) in our cohort. The majority (64.1%) were symptomatic; 31.2% were hospitalized, 10.9% required respiratory support, 9.2% were admitted to the ICU, and 1.6% died because of SARS-CoV-2. Cancer therapy was modified in 44.9%. Hispanic ethnicity was associated with changes in cancer-directed therapy (adjusted risk ratio [aRR] = 1.3; 95% CI, 1.1 to 1.6]). Presence of comorbidities was associated with hospitalization (aRR = 1.3; 95% CI, 1.1 to 1.6) and ICU admission (aRR = 2.3; 95% CI, 1.5 to 3.6). Hematologic malignancies were associated with hospitalization (aRR = 1.6; 95% CI, 1.3 to 2.1).
CONCLUSION
These findings provide critical information for decision making among pediatric oncologists, including inpatient versus outpatient management, cancer therapy modifications, consideration of monoclonal antibody therapy, and counseling families on infection risks in the setting of the SARS-CoV-2 pandemic. The over-representation of Hispanic and publicly insured patients in this national cohort suggests disparities that require attention.
INTRODUCTION
After its emergence in December 2019, the novel virus SARS-CoV-2 rapidly led to a global pandemic. As of this writing, more than 33 million cases and 590,000 deaths have occurred in the United States.1 Compared with adults, children have a lower risk of becoming infected with SARS-CoV-2.2,3 Children are also more likely to have mild disease,4 but severe infection, multisystem inflammatory syndrome, and death do occur.4-6 Risk factors associated with serious illness in children include comorbidities such as complex congenital conditions, obesity, diabetes, and cancer.4,7,8 Reports of SARS-CoV-2 in children with cancer have been small and/or regional (United Kingdom [UK]: N = 54, New York and New Jersey [NY and NJ]: N = 98) and have not reported any deaths solely attributable to SARS-CoV-2.9,10 Despite lower rates of SARS-CoV-2 testing,4 Black and Hispanic adults have higher infection rates and worse outcomes, including 3.3-fold higher mortality than non-Hispanic Whites.11-13 Neither the UK nor the NY and NJ studies evaluated whether non-White children with cancer had an increased risk of SARS-CoV-2 nor whether they had more severe infection.
CONTEXT
Key Objective
The Pediatric Oncology COVID-19 Case Report was established to provide pediatric oncologists real-time information about the clinical course of COVID-19 in children with cancer during the ongoing pandemic, including identifying which children with cancer are most likely to get COVID, determining the clinical course of COVID-19 is in children with cancer, and identifying factors associated with a severe course of COVID-19.
Knowledge Generated
Children with cancer and COVID-19 are at risk of having severe infection and having their cancer therapy modified because of COVID-19. Children age ≥ 11 years, with comorbidities, neutropenia, and/or hematologic malignancies, are more likely to get sick than their peers. Hispanic children with cancer are more likely to get SARS-CoV-2 and have their cancer therapy modified because of infection despite not having a more severe SARS-CoV-2 course.
Relevance
These findings provide critical information for decision making among pediatric oncologists, including inpatient versus outpatient management of COVID-19, cancer therapy modifications, consideration of monoclonal antibody therapy, and counseling families on infection risks in the setting of the SARS-CoV-2 pandemic.
Although these early reports indicate that children with cancer may develop severe SARS-CoV-2, they provide little understanding of the role of comorbidities and race or ethnicity in SARS-CoV-2 risk and severity in children with cancer and SARS-CoV-2. The paucity of data surrounding SARS-CoV-2 infection in children with cancer makes it challenging for pediatric oncologists to provide optimal care for their patients during the ongoing pandemic. We sought to address this gap by creating a national US registry to collect sociodemographic data and the clinical course of children with cancer who acquired SARS-CoV-2.
METHODS
Study Design
This observational study aimed to provide pediatric oncologists real-time information about the clinical course of SARS-CoV-2 in children with cancer. To facilitate rapid regulatory approval, no personal health information (PHI) was collected. Outreach occurred via e-mail solicitation to colleagues at the US pediatric oncology programs and via pediatric oncology–specific social media outlets. Institutions entered deidentified clinical and sociodemographic data online (REDCap; Data Supplement, online only) in the Pediatric Oncology COVID-19 Case Report (POCC Report) registry for each consecutive pediatric cancer patient with SARS-CoV-2 infection. Sites entered data retrospectively and prospectively, providing baseline data regarding SARS-CoV-2 infection, with updates at 4 and 12 weeks after initial infection. Data briefs were e-mailed biweekly to all US pediatric oncology sites (Data Supplement). The institutional review board at the University of Alabama at Birmingham approved the study with a waiver of consent; all participating institutions adhered to local institutional review board policies and procedures.
Study Population
Registry eligibility included age 0-39 years at the time of SARS-CoV-2 infection and receipt of cancer-directed therapy within 1 year of infection. Cancer-directed therapy was defined as chemotherapy, radiation, immunotherapy, blood or marrow transplantation (BMT) for malignancy, or graft-versus-host-disease treatment following a malignancy-related BMT. Analyses presented here are limited to the pediatric population (≤ 21 years at SARS-CoV-2 infection) registered with POCC between April 17, 2020, and May 10, 2021, to evaluate the pediatric experience; this age group is more commonly treated with pediatric protocols at pediatric centers.
General Pediatric Oncology Comparison
We compared the study cohort with the general pediatric oncology population using SEER18.14,15 Sociodemographic (age, sex, insurance, and race or ethnicity) and diagnosis data for children diagnosed with any malignancy between 2011 and 2016 in SEER18 at age ≤ 21 years14,15 were used for comparison.
Independent Variables
Sites provided clinical details regarding cancer diagnosis, recent cancer therapy, complete blood count and differential at the time of infection, history, and type of BMT, history of relapse or progressive disease, and presence of comorbidities. Cancer diagnoses were categorized as hematologic malignancies or solid tumors; absolute neutrophil count (ANC), and absolute lymphocyte count were dichotomized into clinically relevant categories (ANC: ≥ 500 cells/µL, < 500; absolute lymphocyte count ≥ 1,000, < 1,000).16-18 Sites also provided sociodemographics, including age at SARS-CoV-2 infection (dichotomized using median age), sex, race or ethnicity (non-Hispanic White, Black, Hispanic or Latino, Asian, and unknown or other), insurance (private, public, none, and unknown), and state where SARS-CoV-2 infection was identified.
Dependent Variables
Sites reported the following for each patient: symptoms likely attributable to SARS-CoV-2 including symptom duration, multisystem inflammatory syndrome in children, and death; level of support required for SARS-CoV-2; changes in cancer-directed therapy because of SARS-CoV-2 (related or unrelated to neutropenia or thrombocytopenia); and SARS-CoV-2–directed treatment. The dependent variables included: (1) hospitalization, (2) intensive care unit (ICU) admission, (3) duration of SARS-CoV-2 symptoms, and (4) changes in cancer-directed therapy (overall; related or unrelated to neutropenia or thrombocytopenia).
Statistical Analysis
Standard parametric and nonparametric tests were used to compare characteristics of the cohort with SEER patients as well as to compare patients with hematologic malignancy and solid tumor with respect to symptoms, level of support, and changes in cancer therapy. We constructed multivariable binomial regression models to examine factors associated with hospitalization, ICU admission, and changes in cancer-directed therapy; the latter only included patients who had cancer therapy within 90 days of SARS-CoV-2 infection. However, because of the sparseness of data, we removed BMT and those with unknown ANC from the ICU model. We constructed multivariable negative binomial regression models to determine factors associated with duration of COVID-19 symptoms (univariate results in the Data Supplement). Patients without complete data for the outcome and independent variables included in a given model were excluded from regression analyses (complete case analysis) using listwise deletion. We performed the following sensitivity analyses (1) To better compare our cohort and SEER: we matched the geographic distribution SEER to the POCC cohort and repeated the parametric and nonparametric tests mentioned above (Data Supplement), (2) included only patients with 12-week follow-up data in our regression models, (3) conducted multiple imputation to account for missing data in our regression models (Data Supplement), and (4) conducted regression analysis to evaluate for risk of hospitalization and ICU admission only among those who were symptomatic to determine whether they were distinct from the general cohort (Data Supplement). SAS 9.4 (Cary, NC) was used for all analyses.
RESULTS
Sites
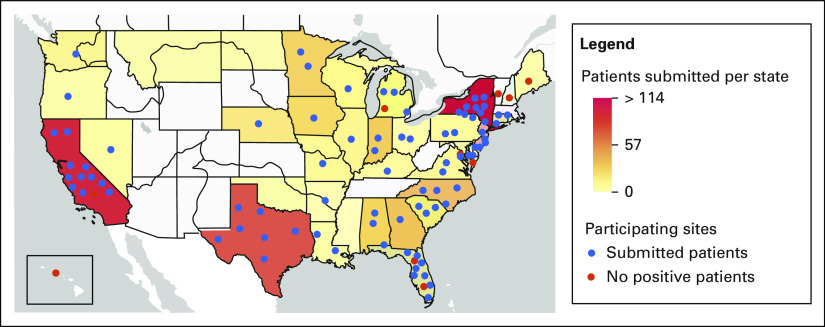
Ninety-four pediatric oncology institutions (from 36 US states) reported data on 917 children with cancer and SARS-CoV-2 (Fig 1). Sites ranged in patient volume, reporting 10-500 new pediatric cancer patients per year (median = 80).
FIG 1.
Distribution of study patients and sites. The state color represents the number of patients submitted to the registry who were diagnosed in that state. The dots represent participating pediatric oncology sites in the state: blue, those who have submitted patients (86), and red, those who are participating but have no children with cancer and SARS-CoV-2 (four). The dots are arranged randomly within the state—they do not represent the sites' actual location. Of note, there are states (eg, Hawaii) that have participating centers but no positive patients, and states (eg, North Dakota) that have positive patients but no participating sites.
Patients
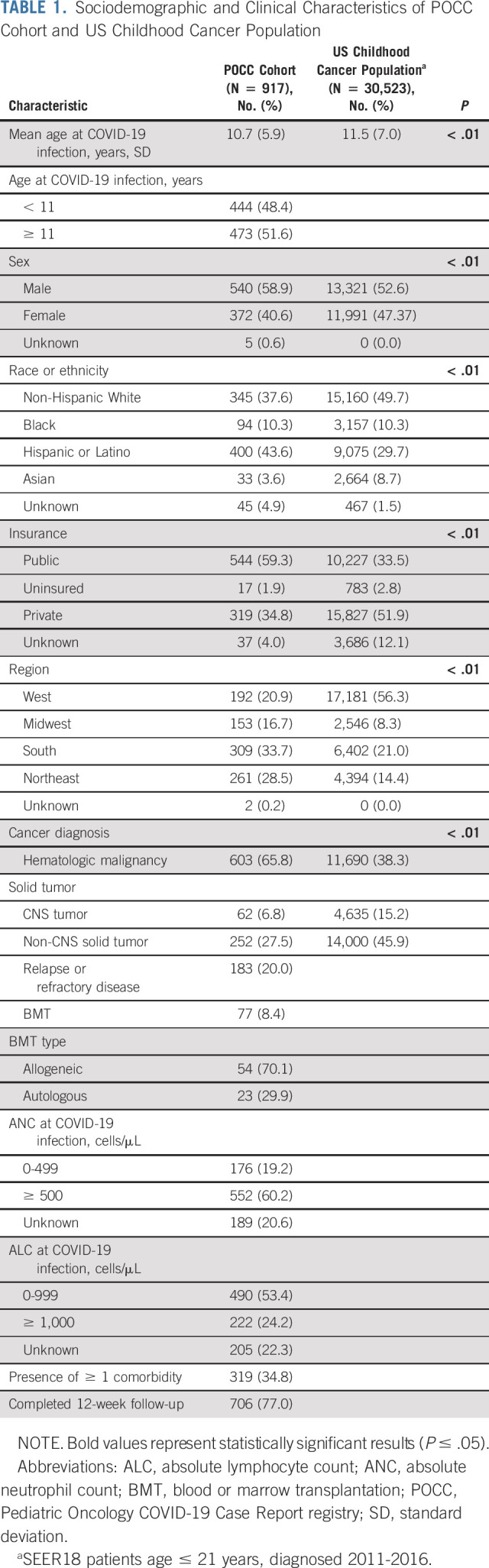
Mean age at SARS-CoV-2 infection was 10.8 years (range, 0-21 years). Nearly half of the cohort were Hispanic or Latino (43.6%), 37.6% were non-Hispanic White, and 10.3% Black (Table 1). The majority were publicly insured (59.3%). More children had hematologic malignancies (65.8%) than solid tumors (non-CNS: 28.9%; CNS: 3.7%). Only 18.5% had relapsed/refractory disease and 9% carried a history of BMT or were receiving graft-versus-host-disease prophylaxis. At the time of SARS-CoV-2 infection, 19.2% had an ANC < 500. Ninety-two percent (844) of children received cancer treatment within 90 days of their SARS-CoV-2 diagnosis. Sixty-five percent of the cohort had at least one noncancer comorbidity. Obesity (10.3%), asthma (4.8%), and hypertension (4.1%) were the most common specific comorbidities (Data Supplement). Follow-up data at 12 weeks were available for the majority of the cohort (77.0%).
TABLE 1.
Sociodemographic and Clinical Characteristics of POCC Cohort and US Childhood Cancer Population
Comparison Between Study Cohort and General Pediatric Oncology Population
The study cohort had an over-representation of patients who were Hispanic or Latino (POCC: 43.6% v SEER: 29.7%; P ≤ .01), publicly insured (POCC: 59.3% v SEER: 33.5%; P ≤ .01), and had hematologic malignancies (POCC: 65.8% v SEER: n = 38.3%; P ≤ .01) compared with the general pediatric oncology population (Table 1).
Symptoms
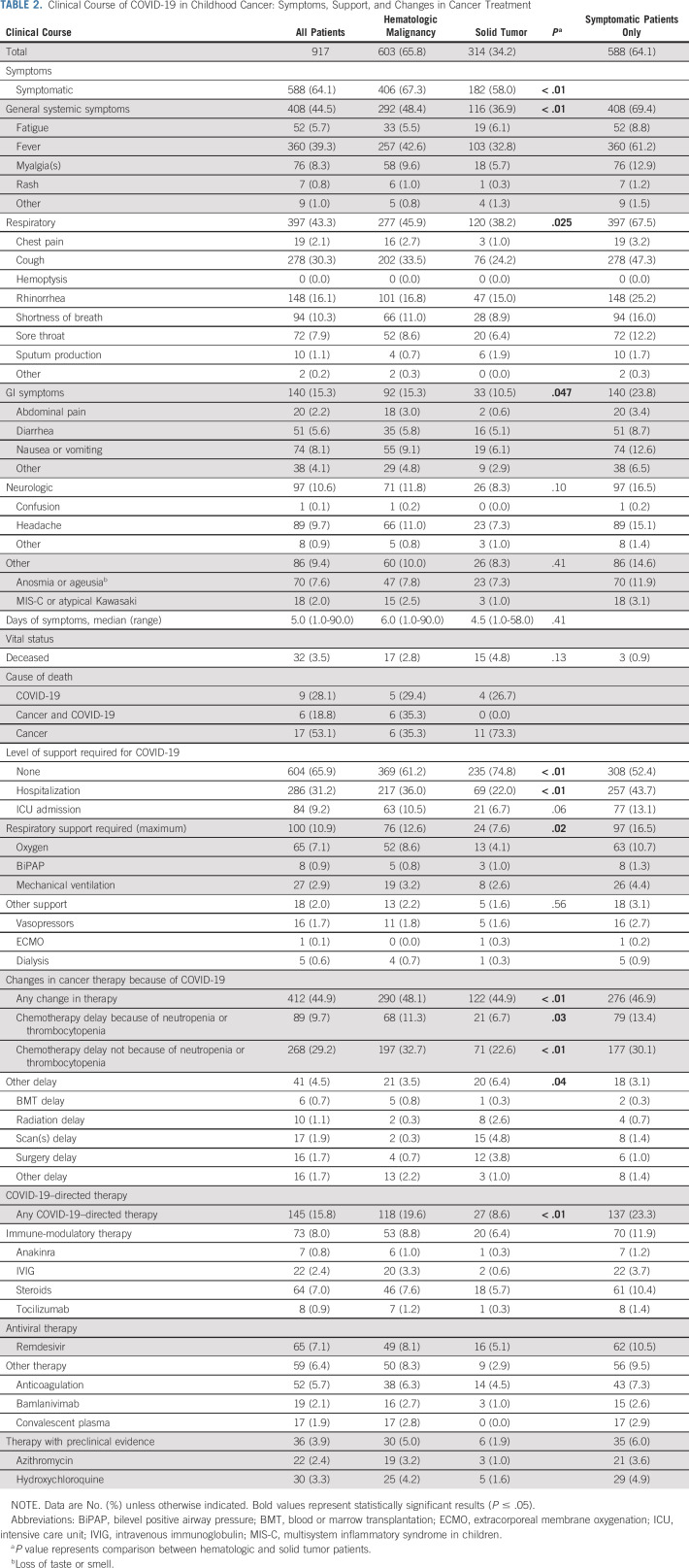
Sixty-four percent of children had SARS-CoV-2–related symptoms. Patients with hematologic malignancies were more likely to be symptomatic (67.3%) than those with solid tumors (58.0%, P < .01; Table 2). Among those with symptoms, the median symptom duration was 5 days (range, 1-90 days) and did not vary by diagnosis type (hematologic malignancies: 6 days [range, 1-90 days] v solid tumors: 4.5 days [range, 1-58 days], P = .41). Nearly half of the cohort (44.5%) had systemic symptoms (eg, fever, fatigue, etc); fever was the most prevalent systemic symptom (39.3%). Systemic symptoms were more common in patients with hematologic malignancies (48.4%) than in those with solid tumors (36.9%, P < .01). Respiratory symptoms were the second most common symptom type (43.3%) and were more common in children with hematologic malignancies (45.9%) versus solid tumors (38.2%), P = .03. Among the 32 (3.5%) children who died during the study period, the cause of death was SARS-CoV-2 in 15 (1.6% of the study population; Table 2, Data Supplement). Of these 15 patients, 60% died from SARS-CoV-2 alone, whereas 40% died from a combination of cancer and SARS-CoV-2.
TABLE 2.
Clinical Course of COVID-19 in Childhood Cancer: Symptoms, Support, and Changes in Cancer Treatment
Risk of Prolonged Symptoms
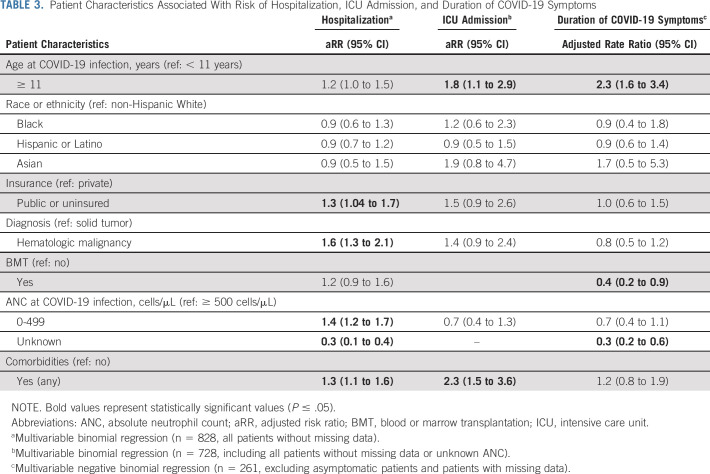
Longer duration of SARS-CoV-2 symptoms (days of symptoms as a continuous variable) was associated with age ≥ 11 years and BMT (adjusted rate ratios: 2.3, 95% CI = 1.6 to 3.4 and 0.4, 0.2-0.9, respectively; Table 3).
TABLE 3.
Patient Characteristics Associated With Risk of Hospitalization, ICU Admission, and Duration of COVID-19 Symptoms
Level of Support
One third of the cohort was hospitalized (31.2%) and one tenth (9.2%) was admitted to the ICU (Table 2). Although a higher proportion of patients with hematologic malignancies were hospitalized than solid tumors (36.0% v 22.0%, P < .01), ICU admissions did not vary with diagnosis (hematologic malignancies: 10.5% v solid tumors: 6.7%, P = .06). One hundred children (10.9%) required respiratory support, with maximal support of oxygen (7.1%), bilevel positive airway pressure (0.6%), and mechanical ventilation (2.9%), which varied by diagnosis (hematologic malignancies: 12.6% v solid tumors: 7.6%, P = .02).
Hospitalization and ICU Admission
Hospitalization and ICU admission were associated with age, comorbidities, and diagnosis. Patients with comorbidities were at increased risk of hospitalization (adjusted risk ratio [aRR] = 1.3; 95% CI, 1.1 to 1.6) and ICU admission (aRR = 2.3; 95% CI, 1.5 to 3.6; Table 3). Additionally, patients with public insurance (aRR = 1.3; 95% CI, 1.04 to 1.7), hematologic malignancies (aRR = 1.6; 95% CI, 1.6; 95% CI, 1.3 to 2.1), and ANC 0-499 (aRR = 1.4; 95% CI, 1.2 to 1.7) were at increased risk of hospitalization.
Changes to Cancer Therapy
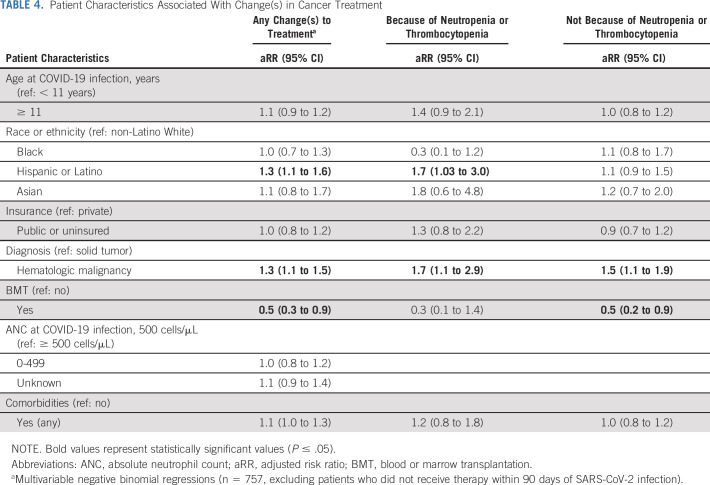
Cancer therapy was changed because of SARS-CoV-2 in 44.9% of children (Table 2). Cancer therapy changes were more common among patients with hematologic malignancies (48.1%) than solid tumors (44.9%, P < .01). Delays in therapy not related to cytopenias (29.2%) were more common than delays related to cytopenias (9.7%). Treatment delays unrelated to cytopenia were more common in patients with hematologic malignancies (32.7%) than solid tumors (22.6%, P < .01); cytopenia-related delays were also more common among patients with hematologic malignancies (11.3% v 6.7%; P = .03).
Hispanic patients faced a higher risk of therapy changes than non-Hispanic Whites (aRR = 1.3; 95% CI, 1.1 to 1.6), driven by an increased risk of changes in cancer-directed therapy because of neutropenia or thrombocytopenia (aRR = 1.7; 95% CI, 1.03 to 3.0). Patients with BMT had a lower risk of change to therapy (aRR = 0.5; 95% CI, 0.3 to 0.9; Table 4). Patients with hematologic malignancies had a higher risk of any change in therapy (aRR = 1.3; 95% CI, 1.1 to 1.5), changes because of neutropenia (aRR = 1.7; 95% CI, 1.1 to 2.9), and changes in chemotherapy not related to neutropenia (aRR = 1.5; 95% CI, 1.1 to 1.9).
TABLE 4.
Patient Characteristics Associated With Change(s) in Cancer Treatment
SARS-CoV-2 Treatment
Only 15.8% of the patients received SARS-CoV-2–directed therapy. Immune-modulatory therapy (8.0%) and antiviral therapy (7.1%) were the most frequently used. A larger proportion of patients with hematologic malignancies (19.6%) than solid tumors (8.6%, P < .01) received SARS-CoV-2–directed therapy (Table 2).
DISCUSSION
We report the largest study of children with cancer and SARS-CoV-2, providing critical data regarding the clinical course in this population. Many children in our cohort had severe SARS-CoV-2 courses, with 31% admitted to the hospital, 9% admitted to the ICU, and 4% dying because of SARS-CoV-2. This suggests a more severe clinical course of SARS-CoV-2 in children with cancer than what has been reported in children without cancer.4 The over-representation of patients who were Hispanic, uninsured, and with hematologic malignancies suggests that SARS-CoV-2 infection is more likely in these patients. A more severe clinical course was observed among children ≥ 11 years, with hematologic malignancies, neutropenia, and comorbidities. SARS-CoV-2 infection led to cancer treatment modifications for nearly half of the children. During the ongoing pandemic, these findings can guide clinical decision making among pediatric oncologists based on evidence surrounding who is at risk for severe infection; these include decisions about when to admit for observation, holding cancer therapy, or whether to treat with monoclonal antibodies. It will also allow clinicians to provide families with information about the course of SARS-CoV-2 in children with cancer.
Many children with cancer had a severe course of SARS-CoV-2. Early in the pandemic, some hypothesized that immunosuppressed patients would have a milder clinical course.19 It also became apparent that children generally experienced less severe disease than adults. Early experiences suggested that children with cancer would exhibit clinical courses similar to children without cancer.20 However, 31.2% of our pediatric cancer cohort was hospitalized (v 6.7% in general pediatrics), 9.2% had an ICU admission (v 1.8%), and 3.5% died because of COVID (v 0.2%).4 The higher hospitalization rate seen here is similar to the NY and NJ pediatric oncology SARS-CoV-2 experience (29%)10; the UK cohort did not report hospitalization rates.9 Our rate of ICU admissions falls between the other cohorts (17% and 5.5%, respectively). However, mortality rates in our cohort differed from the others, with no deaths attributable to COVID-19 reported in the NY and NJ and UK cohorts. It is plausible that the larger patient population represented in our study was necessary to identify the differences in mortality between general pediatric and pediatric cancer patients. With this in mind, clinicians can appropriately counsel childhood patients with cancer about the importance of continuing prevention measures for SARS-CoV-2 and have a low threshold to consider monoclonal antibody administration for relevant age groups and inpatient management when children with cancer become symptomatic from SARS-CoV-2. When the SARS-CoV-2 vaccine becomes available for children, these data may facilitate discussions surrounding risks, benefits, and prioritization of the vaccine among children with cancer. In the meantime, pediatric oncologists can better advise families as they decide whether or not to vaccinate their child with cancer and other family members.
Hispanic and publicly insured children are over-represented in our cohort. A hallmark of the pandemic in the United States has been increased rates of SARS-CoV-2 in under-represented minorities.21,22 Emerging SARS-CoV-2 data in the general pediatric population also show higher rates in Black, Hispanic, and Asian children.4 Individuals from non-White racial or ethnic groups and lower socioeconomic strata may be more likely to live in multifamily dwellings and dense urban neighborhoods, work in essential industries such as food services, and take public transportation—all increasing the risk of SARS-CoV-2 infection.23 Despite the geographic and racial or ethnic diversity of our cohort, the proportion of Black patients did not differ from the general pediatric oncology population. Many programs have standardized approaches to SARS-CoV-2 testing and screening and discussion of risk-reduction strategies. Teams may consider whether these data warrant a lower threshold to test and/or counsel on risk-reduction strategies in Hispanic and publicly insured children to optimize prevention and treatment.
Despite comparable rates of hospitalization or ICU admission to their peers, Hispanic children were more likely to have changes in cancer-directed therapy because of SARS-CoV-2. Among children with cancer, Hispanic children have worse overall survival than their non-Hispanic White peers.21,22 Socioeconomic status,24 adherence to oral chemotherapy,25 and genetics26 contribute to this survival disparity. In pediatric oncology, increased intensity of cancer-directed therapy has contributed to dramatic improvements in survival.27 Thus, if SARS-CoV-2 is more likely to lead to changes in cancer-directed therapy in Hispanic children, the pandemic has the potential to worsen the survival gap for Hispanic children with cancer. Futher work is needed to determine whether Hispanic children with SARS-CoV-2 are more likely to be neutropenic than their peers, whether clinicians are more likely to check blood counts in Hispanic children with cancer and SARS-CoV-2 because of a history of Hispanic children experiencing worse chemotherapy-related side effects,28,29 or other reasons.
Older children, those with comorbidities, neutropenia, and/or hematologic malignancies, were more likely to have severe SARS-CoV-2. In the general pediatrics population, children with comorbidities are more likely to have severe COVID-19.4,26,30 Even with the heterogeneous comorbidities in our study population, we saw an increased risk of severe SARS-CoV-2 infection with a comorbidity. It will be essential to determine the specific comorbidities most associated with severe SARS-CoV-2. The emergency use authorizations for monoclonal antibodies have a lower age threshold of 12 years despite a lack of pediatric data; thus, these findings provide additional data, which have the potential to guide decisions regarding which patients may be considered for these therapies.
By creating a national registry of SARS-CoV-2 in children with cancer early in the pandemic, we were able to provide our colleagues regular snapshots of the emerging clinical course of this disease. Pediatric oncology has been lauded for its collaborative nature which led to improvements in survival31; the rapidity with which we engaged approximately half of the US pediatric oncology sites speaks to this collaborative nature. Like many initial studies, we have answered a number of questions and are now faced with many additional questions. As we anticipate the pandemic continuing to affect patient care for the foreseeable future, we must better understand the roles played by biology, immunosuppression, and sociodemographic factors in SARS-CoV-2 vulnerability and clinical course, and how to respond. For instance, examining length of polymerase chain reaction positivity and viral shedding in children with cancer can help pediatric oncology programs make informed policies about retesting and isolation practices after SARS-CoV-2 infection. We must also examine how SARS-CoV-2 treatment and changes in cancer-directed therapy affect the course of SARS-CoV-2 in children with cancer, particularly given the risk of decreased cancer survival with delays in cancer-directed therapy. Finally, we continue to extend data collection to respond to the evolving pandemic, including the emergence of the variants and vaccine uptake.
Despite the essential information this study provides for pediatric oncologists, it faces limitations. Excluding PHI facilitated rapid regulatory approvals, allowing for 94 sites (approximately half of the US pediatric oncology sites) to open the study over 10 months. However, the lack of PHI prevents comparison of our cohort with children with cancer but without COVID-19 at participating sites, which would have allowed for a more nuanced comparison than the SEER comparison allows. However, we have approximately half of the US pediatric oncology centers participating with broad geographic representation, we have done sensitivity analyses to address this limitation to the best of our abilities, and our findings that Hispanic and publically insured children with cancer are more likely to become infected with SARS-CoV-2 than their peers mirror the risk factors for SARS-CoV-2 in the general population.4,21-23 To avoid PHI, we also did not collect information on the date of diagnosis of SARS-CoV-2 infection, which limits our understanding of how treatment and/or clinical course changed over the course of the pandemic. As the study reports 10 months of data and optimal treatment for SARS-CoV-2 remains on the table, we do not expect the findings to have varied much over the study period. Although we have follow-up data for the majority of patients (4-week: 86%, 12-week: 61%), these data do not encompass the entire course of symptoms, additional support, SARS-CoV-2 treatment, or chemotherapy changes. Thus, we may under-report these outcomes in our data, implying that the course of SARS-CoV-2 in children with cancer is worse than presented. However, sensitivity analysis with those who completed 12-week follow-up data yielded similar results (data not shown). Determining whether symptoms, support, and treatment changes are because of SARS-CoV-2 or cancer is difficult; we have been transparent in our reporting process and encourage clinicians to understand these limitations as they consider the generalizability of these findings to other clinical situations.
Children with cancer and SARS-CoV-2 are at risk of having severe infection, especially those ≥ 11 years, with comorbidities, neutropenia, and/or hematologic malignancies. Hispanic children with cancer appear more likely to get SARS-CoV-2 and have their cancer therapy modified because of infection despite not having a more severe SARS-CoV-2 course. These findings provide critical data for pediatric oncologists when they are considering inpatient versus outpatient management, cancer treatment modifications, and the potential role of monoclonal antibody therapy. Additionally, these findings provide data that clinicians can use to guide families (regarding infection risks in the setting of SARS-CoV-2) and systems (regarding vaccine prioritization among pediatric patient groups). The over-representation of Hispanic and publicly insured patients in this national cohort and the higher likelihood of cancer treatment modifications in Hispanic children are concerning and require attention.
ACKNOWLEDGMENT
This work would not have been possible without the contributions of each member of the Pediatric Oncology COVID-19 Case Consortium. The full list of consortium members is in the Appendix. The study team would also like to acknowledge the work of Brook Araya in creating the study maps.
APPENDIX
Julienne Brackett
Research Funding: Bristol Myers Squibb
Smita Bhatia
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Jennifer M. Levine
Stock and Other Ownership Interests: UMotif
No other potential conflicts of interest were reported.
DISCLAIMER
There are other registries of SARS-COVID 19 in children with cancer—both single-institution and multi-institution registries. Institutions could submit data to multiple registries. Therefore, patients in this registry may be represented in publications of other registries. The POCC registry has been sending reports to the pediatric oncology community at least monthly; the report has been reported by media outlets as well.
J.A.W. and J.M.L. are co-senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Emily E. Johnston, Isaac Martinez, Julienne Brackett, David S. Dickens, Alissa Kahn, Carla Schwalm, Archana Sharma, Jennifer M. Levine, Julie A. Wolfson
Financial support: Emily E. Johnston, Julie A. Wolfson
Administrative support: Emily E. Johnston, Isaac Martinez, Julie A. Wolfson
Provision of study materials or patients: Julienne Brackett, Carla Schwalm, Julie A. Wolfson
Collection and assembly of data: Emily E. Johnston, Isaac Martinez, Elizabeth S. Davis, Caroline Caudill, Julienne Brackett, David S. Dickens, Alissa Kahn, Pratik A. Patel, Julie A. Wolfson
Data analysis and interpretation: Emily E. Johnston, Elizabeth S. Davis, Joshua Richman, Julienne Brackett, David S. Dickens, Alissa Kahn, Smita Bhatia, Jennifer M. Levine, Julie A. Wolfson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
SARS-CoV-2 in Childhood Cancer in 2020: A Disease of Disparities
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Julienne Brackett
Research Funding: Bristol Myers Squibb
Smita Bhatia
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Jennifer M. Levine
Stock and Other Ownership Interests: UMotif
No other potential conflicts of interest were reported.
REFERENCES
- 1.COVID-19 United States Cases by County. Johns Hopkins Coronavirus Resource Center, 2021. https://coronavirus.jhu.edu/us-map [Google Scholar]
- 2.Chin-Hong P, Alexander KM, Haynes N, et al. : Pulling at the heart: COVID-19, race/ethnicity and ongoing disparities. Nat Rev Cardiol 17:533-535, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Reports Highest One-Week Increase in Child Cases of COVID-19 Since Onset of Pandemic 2020. https://services.aap.org/en/news-room/news-releases/aap/2020/american-academy-of-pediatrics-reports-highest-one-week-increase-in-child-cases-of-covid-19-since-onset-of-pandemic/ [Google Scholar]
- 4.Bailey LC, Razzaghi H, Burrows EK, et al. : Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr 175:176-184, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang L, Tang K, Levin M, et al. : COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 20:e276-e288, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuhara J, Kuno T, Takagi H, et al. : Clinical characteristics of COVID‐19 in children: A systematic review. Pediatr Pulmonology 55:2565-2575, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Lingappan K, Karmouty-Quintana H, Davies J, et al. : Understanding the age divide in COVID-19: Why are children overwhelmingly spared? Am J Physiol Lung Cell Mol Physiol 319:L39-L44, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. : Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 174:868-873, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millen GC, Arnold R, Cazier J-B, et al. : Severity of COVID-19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br J Cancer 124:754-759, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhusoodhan PP, Pierro J, Musante J, et al. : Characterization of COVID-19 disease in pediatric oncology patients: The New York-New Jersey regional experience. Pediatr Blood Cancer 68:e28843, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogedegbe G, Ravenell J, Adhikari S, et al. : Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open 3:e2026881, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabarriti R, Brodin NP, Maron MI, et al. : Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw Open 3:e2019795, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVIDView : A Weekly Surveillance Summary of U.S. COVID-19 Activity 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results Program 2020. https://seer.cancer.gov/index.html [Google Scholar]
- 15.Registry Groupings in SEER Data and Statistics—SEER Registries 2020. https://seer.cancer.gov/registries/terms.html [Google Scholar]
- 16.Yan W, Chen D, Bigambo FM, et al. : Differences of blood cells, lymphocyte subsets and cytokines in COVID-19 patients with different clinical stages: A network meta-analysis. BMC Infect Dis 21:156, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Liu C, Mao Z, et al. : Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit Care 24:647, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah V, Ko Ko T, Zuckerman M, et al. : Poor outcome and prolonged persistence of SARS‐CoV‐2 RNA in COVID‐19 patients with haematological malignancies; King's College Hospital experience. Br J Haematol 190:e279, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung M, Babik J: COVID-19 in immunocompromised hosts: What we know so far. Clin Infect Dis 72:340-350, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulad F, Kamboj M, Bouvier N, et al. : COVID-19 in children with cancer in New York City. JAMA Oncol 6:1459-1460, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vahidy FS, Nicolas JC, Meeks JR, et al. : Racial and ethnic disparities in SARS-CoV-2 pandemic: Analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 10:e039849, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackey K, Ayers C, Kondo K, et al. : Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths: A systematic review. Ann Intern Med 174:362, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC : COVID-19: Health Equity Considerations and Racial and Ethnic Minority Groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html [Google Scholar]
- 24.Kehm R, Spector L, Poynter J, et al. : Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer 124:4090-4097, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia S, Landier W, Shangguan M, et al. : Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic White children with acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol 30:2094–2101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim JY, Bhatia S, Robison LL, et al. : Genomics of racial and ethnic disparities in childhood acute lymphoblastic leukemia. Cancer 120:955-962, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson MM, Neglia JP, Woods WG, et al. : Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer 58:334-343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JJ, Landier W, Yang W, et al. : Inherited NUDT15 variant is a Genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 33:1235-1242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor OA, Brown AL, Brackett J, et al. : Disparities in neurotoxicity risk and outcomes among pediatric acute lymphoblastic leukemia patients. Clin Cancer Res 24:5012-5017, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graff K, Smith C, Silveira L, et al. : Risk factors for severe COVID-19 in children. Pediatr Infect Dis J 140:e137-e145, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Hudson M, Meyer WH, Pui C-H: Progress born from a legacy of collaboration. J Clin Oncol 33:2935-2937, 2015 [DOI] [PubMed] [Google Scholar]