PURPOSE
Liquid biopsies can be used to investigate tumor-derived DNA, circulating in the cell-free DNA (cfDNA) pool in blood. We aimed to develop a droplet digital polymerase chain reaction (ddPCR) assay detecting hypermethylation of tumor suppressor gene RASSF1A as a simple standard test to detect various pediatric tumor types in small volume blood samples and to evaluate this test for monitoring treatment response of patients with high-risk neuroblastoma.
METHODS
We developed a ddPCR assay to sensitively detect tumor-derived hypermethylated RASSF1A DNA in liquid biopsies. We tested this assay in plasma of 96 patients with neuroblastoma, renal tumors, rhabdomyosarcoma, or Hodgkin lymphoma at diagnosis and in cerebrospinal fluid of four patients with brain tumors. We evaluated the presence of hypermethylated RASSF1A in plasma samples during treatment and follow-up in 47 patients with neuroblastoma treated according to high-risk protocol and correlated results with blood mRNA–based and bone marrow mRNA–based minimal residual disease detection and clinical outcomes.
RESULTS
The total cfDNA level was significantly higher in patients with metastatic neuroblastoma and nephroblastoma compared with healthy adult and pediatric controls. Hypermethylated RASSF1A was present in 41 of 42 patients with metastatic neuroblastoma and in all patients with nephroblastoma, with the median percentage of 69% and 21% of total RASSF1A, respectively. Hypermethylated RASSF1A levels decreased during therapy and recurred at relapse.
CONCLUSION
Our findings demonstrate the value of ddPCR-based detection of hypermethylated RASSF1A as a circulating molecular tumor marker in neuroblastoma. Our preliminary investigation of RASSF1A hypermethylation detection in circulating cfDNA of other pediatric tumor entities demonstrates potential as a pan-tumor marker, but requires investigation in larger cohorts to evaluate its use and limitations.
INTRODUCTION
Cancer remains one of the most common causes of childhood death in high-income countries.1 Although the combination of intensive chemotherapy, surgery, radiation therapy, and immunotherapy has improved outcomes in children with solid tumors, disease still recurs in 50% of patients with neuroblastomas;2,3 46% of patients with Ewing sarcomas;4 and approximately 30% of patients with localized rhabdomyosarcomas,5 osteosarcomas,6 and renal tumors.7 Response to treatment is primarily based on imaging. In patients with neuroblastoma, bone marrow (BM) histology or (immuno)cytology assesses the extent of disease.8 In neuroblastoma and rhabdomyosarcoma, reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR) for the detection of minimal residual disease (MRD) in peripheral blood or BM is shown to be more sensitive9-13 and predictive of outcomes, but even patients with low or negative MRD results can suffer from recurrent disease,9,14 or mRNA markers can be downregulated upon epithelial-to-mesenchymal transition.15
CONTEXT
Key Objective
Molecular testing of circulating tumor DNA (ctDNA) has the potential to improve pediatric solid tumor diagnosis and discrimination of subtypes and monitoring of treatment response. Our aim was to develop a RASSF1A hypermethylation droplet digital polymerase chain reaction as a standard test to detect ctDNA in several pediatric tumor types using small blood volumes and as a test to monitor treatment response of patients with neuroblastoma.
Knowledge Generated
We developed a sensitive and quantitative droplet digital polymerase chain reaction–based assay for hypermethylated RASSF1A detection. Our findings demonstrate the value of hypermethylated RASSF1A as a molecular circulating tumor marker in neuroblastoma. RASSF1A was frequently hypermethylated in plasma samples from patients with nephroblastoma, rhabdomyosarcoma, and Hodgkin lymphoma.
Relevance
Our study supports the use of ctDNA in assisting the monitoring of therapy response in patients with neuroblastoma and shows the potential of ctDNA in assisting the diagnosis of other pediatric solid tumor entities.
Liquid biopsies, for example, peripheral blood, can also be a source for tumor-derived cell-free DNA (cfDNA). As the genomic view is not limited to the boundaries of a tissue biopsy, liquid biopsies better represent spatial and intratumor heterogeneity. Liquid biopsies have shown promise in assisting diagnosis and monitoring therapy response in adult oncology.16-18 Pediatric tumors have lower mutational burdens with few recurrent mutations19 but a variety of copy number alterations20 and epigenetic changes.21 The tumor suppressor gene RASSF1A is silenced in nearly all adult cancers and associated with poor prognosis and high-risk disease.22-24 Promotor hypermethylation23,25,26 or, less frequently, a combination of hypermethylation and 3p21.3 allelic loss22,23,27 causes inactivation. RASSF1A is hypermethylated in neuroblastoma,22,28-35 hepatoblastoma,29,36 nephroblastoma,29,37,38 medulloblastoma and primitive neuroectodermal tumors,29,39 and osteosarcoma and Ewing sarcoma.29,40-42 These accumulating data suggest RASSF1A hypermethylation to be as common in pediatric tumor entities as in adult tumor entities. RASSF1A hypermethylation is rare in normal tissues,23 but present in placenta, and therefore is also suited for fetal DNA detection in maternal plasma.43,44 We previously investigated hypermethylated RASSF1A in cfDNA from patients with neuroblastoma by performing qPCR.33 We demonstrated the promise of this marker, but observed loss of cfDNA because of bisulfite conversion, and were unable to quantify the low amounts of circulating tumor DNA (ctDNA).33 In this study, we harnessed the sensitivity and accuracy of droplet digital PCR (ddPCR) and developed a ddPCR method with methylation-sensitive restriction enzymes (MSREs) to overcome these limitations. We furthermore investigated the feasibility of our hypermethylated RASSF1A ddPCR assay in detecting different pediatric tumor types in small volume patient plasma samples.
METHODS
Methods on patient inclusion, sample collection, cfDNA isolation, and RT-qPCR for mRNA markers45 and single nucleotide polymorphism array can be found in the Data Supplement.
Hypermethylated RASSF1A ddPCR
To discriminate between methylated and unmethylated RASSF1A, every sample was subjected to two different ddPCR reactions (Fig 1): one with MSRE and the other without; all remaining conditions were identical. ACTB-1 primer-probe set was added to control for cfDNA input, and this amplicon is unaffected by the MSRE. ACTB-2 primer-probe set was added to control for MSRE performance since this amplicon is digested by the enzymes. RASSF1A, ACTB-1, and ACTB-2 primer and probe sets are listed in the Data Supplement. Primer and probe sequences for RASSF1A and ACTB-2 have been described before by O'Brien et al.44 A detailed protocol can be found in the Data Supplement. To avoid false positivity, a threshold was based on healthy donors for both the single- and double-digest reactions (see the Results) and a minimum of four positive droplets per duplicate. If a sample was scored positive, the percentage of hypermethylated RASSF1A was calculated as (RASSF1A/ACTBwith MSRE)/(RASSF1A/ACTBwithout MSRE) × 100%. RASSF1A ddPCR performance was compared with that of RASSF1A qPCR by testing 16 rhabdomyosarcoma and renal tumor cfDNA samples. RASSF1A qPCR was performed as described previously.33
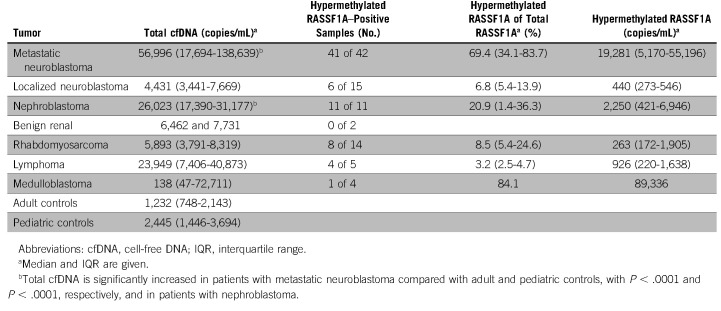
FIG 1.
Concept of quantifying methylated RASSF1A using MSRE and ddPCR. (A) An MSRE incubation of a cfDNA sample results in the digestion of unmethylated RASSF1A, whereas methylated RASSF1A remains intact. Two amplicons of ACTB are added, and ACTB-1 is unaffected by the MSRE, whereas ACTB-2 is digested by the MSRE, as a control for MSRE performance. Every sample is subjected to two different ddPCR reactions, (B) one without the MSRE and (C) the other with the MSRE. ACTB-2 primers and probe are added in a lower concentration, resulting in a lower amplitude to discriminate between the ACTB-1 and ACTB-2 clusters. (C) Only in cfDNA from patients with circulating tumor DNA present, RASSF1A will be detected after digestion with the MSRE, as the absence of RASSF1A methylation will result in RASSF1A digestion, preventing the detection of this unmethylated RASSF1A allele by ddPCR. cfDNA, cell-free DNA; ddPCR, droplet digital polymerase chain reaction; MSRE, methylation-sensitive restriction enzymes.
Statistical Analysis
As cfDNA and ctDNA levels were not normally distributed, they are presented as median (interquartile range) and statistical significance was determined by the Kruskal–Wallis test. Fisher's exact test was used to analyze the correlation between ctDNA and/or mRNA positivity and outcomes. Correlation analysis between cfDNA, ctDNA, and mRNA levels was performed using Spearman's test. Events were defined as relapse, progressive,8 or refractory disease, when the progression was not according to the International Neuroblastoma Response Criteria but resulted in change of treatment protocol. Receiver operating characteristic analysis was used to identify a cutoff for hypermethylated RASSF1A copies/mL. This cutoff was used to identify two subgroups for the comparison of event-free survival using Kaplan-Meier method. All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA) software. Results were considered significant if P ≤ .05.
RESULTS
Limit of Detection and Limit of Blank: Single and Double MSRE Digest
The dilution series of neuroblastoma cell line IMR32 DNA (100% hypermethylated RASSF1A) in DNA from blood from a healthy male and in H2O showed a good linearity (a detailed description is given in the Data Supplement). The limit of detection, however, is defined by the level of positivity in the control samples, also called the limit of blank. For the limit of blank, we evaluated RASSF1A positivity in 22 samples stored at room temperature from adult male controls from which plasma was separated after 24, 48, 72, or 144 hours and 18 pediatric control samples (plasma separation within 24 hours). To test the efficacy of single-digest MSRE (BstUI-only), both hypermethylated RASSF1A and ACTB were measured in these control samples after digestion. We observed a correlation between the number of hypermethylated RASSF1A copies and ACTB copies in the adult controls (Spearman rs = 0.91, P < .0001) and to a lesser extent in the pediatric controls (Spearman rs = 0.69, P = .002), with a maximum of 0.039 RASSF1A copies per ACTB copies/mL plasma (Data Supplement). Although we cannot formally exclude that hypermethylated RASSF1A is derived from necrotic cells during storage of the samples, these data suggest that, although the ACTB-2 cluster was not clearly present, BstUI-only was not able to digest all cfDNA in our samples. A threshold on the basis of this ratio would greatly reduce the sensitivity of the assay and result in many inconclusive samples, and therefore, we investigated the use of two MSREs in a double-digest reaction. Double digestion by MSREs HhaI and Bsh1236I instead of BstUI in 43 adult and 18 pediatric control samples resulted in a more efficient digestion of RASSF1A. The number of hypermethylated RASSF1A copies was no longer dependent on the cfDNA concentration (Data Supplement). A prolonged time to plasma separation did not result in a significant increase in RASSF1A copies/mL, neither for the single-digest nor double-digest method (Data Supplement). On the basis of mean + 3 × standard deviation in hypermethylated RASSF1A copies/mL plasma of these controls, we set the threshold on 14 copies/mL plasma. As a large number of patient samples were already tested using the single-digest method, all patient samples with ≥ 4 positive droplets and a ratio ≤ 0.039 RASSF1A/ACTB copies/µL were also tested using the double-digest method and scored according to the new double-digest threshold. To compare RASSF1A ddPCR performance with that of RASSF1A qPCR,33 we tested 16 diagnostic rhabdomyosarcoma and renal tumor plasma samples using both techniques. All 11 samples that were positive by qPCR, of which three were positive-not-quantifiable, tested positive by ddPCR, and 1 in 5 qPCR-negative samples were tested positive by ddPCR.
Total cfDNA Is Increased in Patients With Neuroblastoma and Nephroblastoma
We investigated plasma samples from patients with high-risk neuroblastoma (47) at diagnosis and during therapy and diagnostic plasma samples from pediatric patients with non–high-risk neuroblastoma (17), rhabdomyosarcoma (14), renal tumor (13), Hodgkin lymphoma (five), and cerebrospinal fluid (CSF) from CNS tumors (four). For clinical details, see the Data Supplement. We isolated cfDNA from 200 to 1,000 µL plasma or CSF and compared diagnostic plasma cfDNA levels (ACTB) with 24 healthy adult and 18 healthy pediatric plasma control samples, processed within 24 hours (Fig 2A, Table 1). Total cfDNA levels were significantly higher in patients with metastatic neuroblastoma and nephroblastoma compared with adult and pediatric controls (P < .0001, P < .0001, P < .0001, and P = .0117, respectively). Patients with localized neuroblastoma had significantly lower cfDNA levels compared with metastatic neuroblastoma (P = .0004) and were not significantly different from the adult and pediatric controls (P = .4 and P > .99, respectively). There was a trend to higher cfDNA levels in patients with rhabdomyosarcoma and Hodgkin lymphoma, which was only significant compared with adult controls (P = .015 and P = .013, respectively; Table 1).
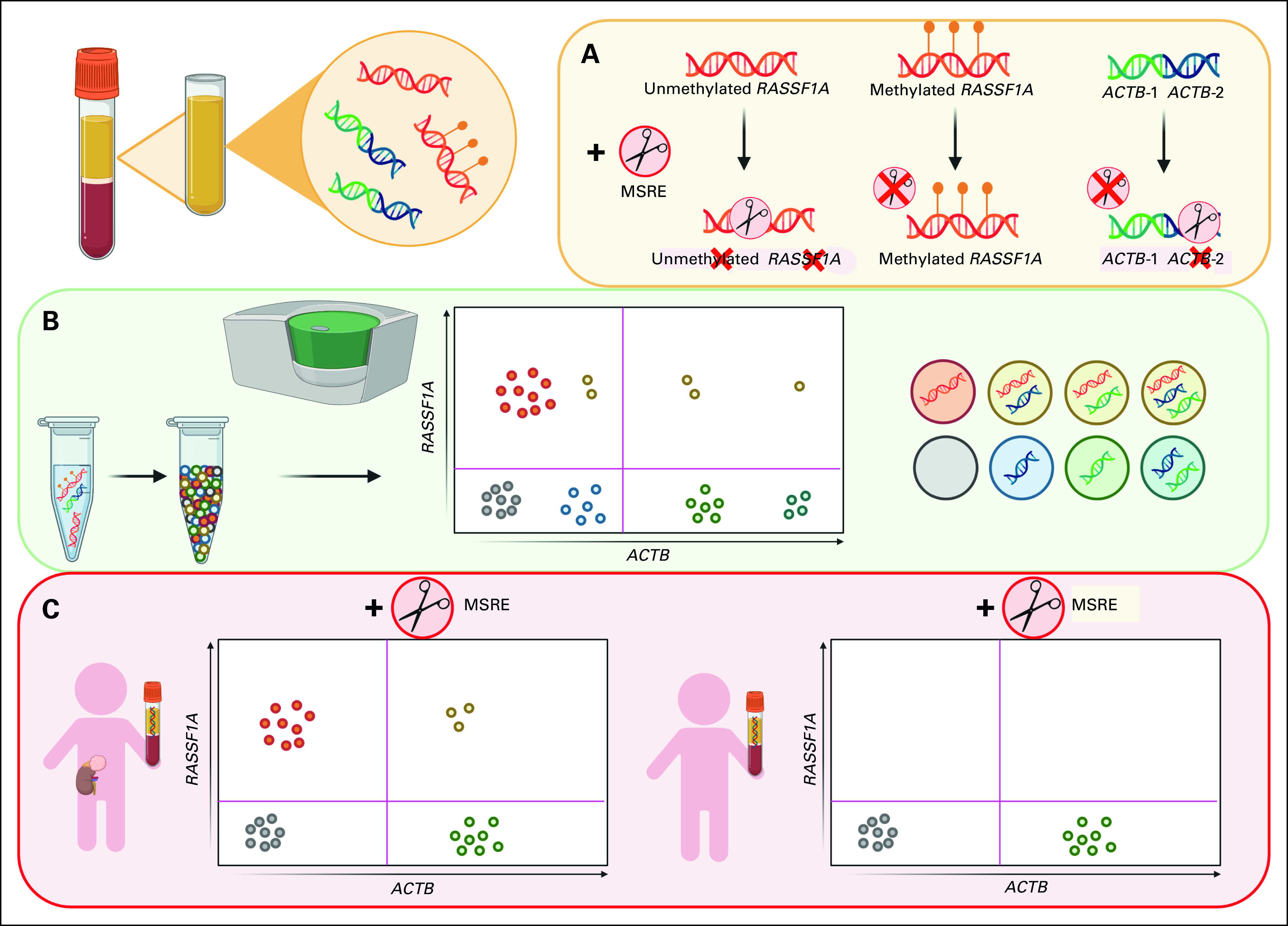
FIG 2.
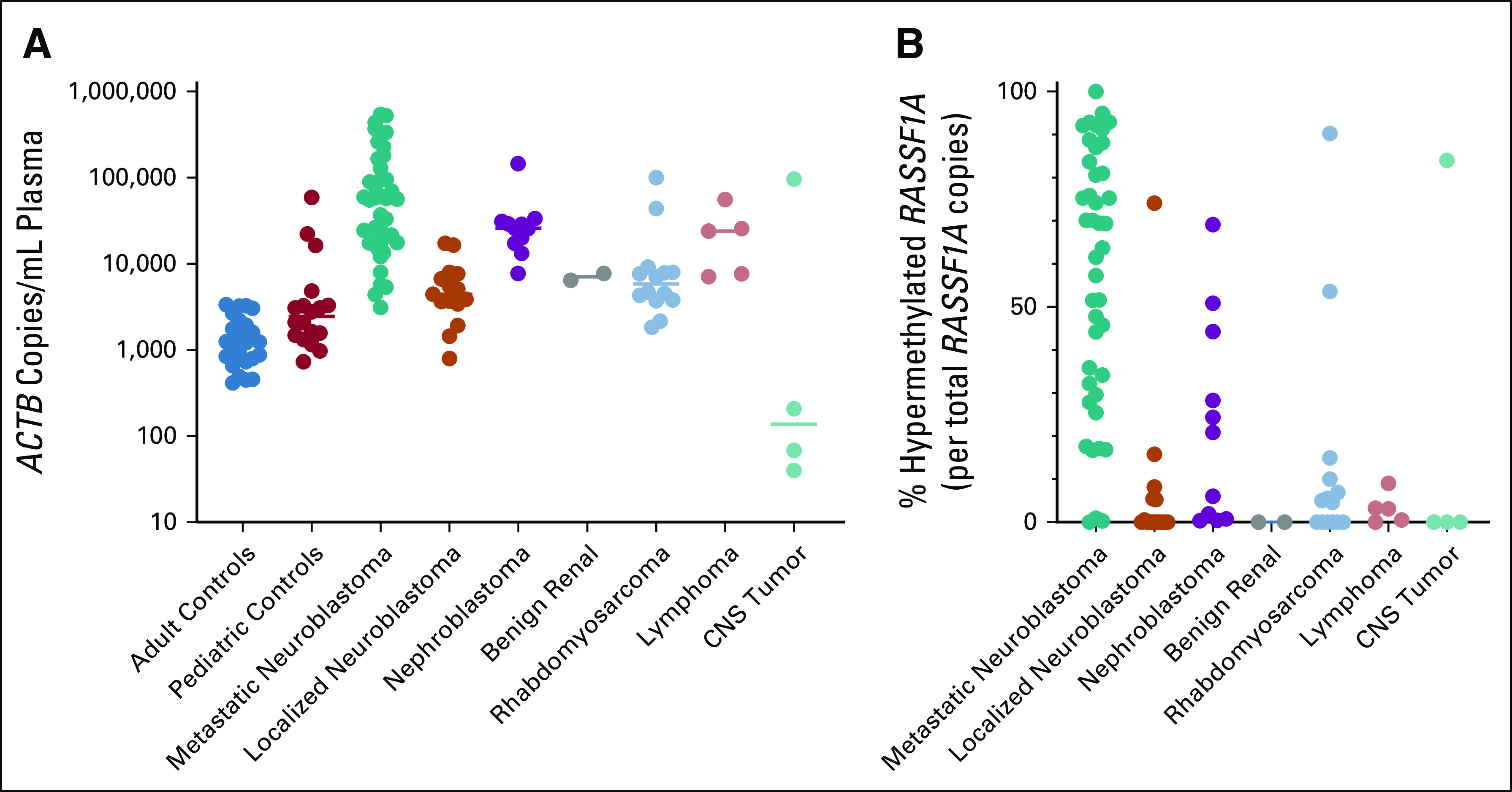
Amount of cfDNA and circulating hypermethylated RASSF1A. (A) Level of cfDNA at diagnosis in patients with various pediatric solid tumor entities, compared with healthy adult and pediatric controls. cfDNA was quantified by β-actin (ACTB), in copies/mL plasma or CSF (cerebrospinal fluid). Lines indicate the median. (B) The percentage of hypermethylated RASSF1A of total RASSF1A copies at diagnosis in patients with metastatic neuroblastoma (n = 42), localized neuroblastoma (n = 15), nephroblastoma (n = 11), rhabdomyosarcoma (n = 14), lymphoma (n = 5), and CNS tumors (n = 4). Adult and pediatric controls were used to establish a threshold for positivity. In 41 of 42 patients with metastatic neuroblastoma and 6 of 15 patients with localized neuroblastoma, hypermethylated RASSF1A was detected. In all 11 patients with nephroblastoma, 8 of 14 patients with rhabdomyosarcoma, 4 of 5 patients with lymphoma, and 1 of 4 patients with CNS tumor, hypermethylated RASSF1A was detected. Two plasma samples of patients with benign renal tumors (a Cystic Partially Differentiated Nephroblastoma and a bilateral differentiated nephroblastomatosis) were negative for hypermethylated RASSF1A. cfDNA, cell-free DNA; CFS, cerebrospinal fluid.
TABLE 1.
Levels of cfDNA and Circulating Hypermethylated RASSF1A in Various Pediatric Solid Tumor Entities and Adult and Pediatric Controls
Hypermethylated RASSF1A Is Detected in Diagnostic Plasma of Patients With Different Tumor Entities
At diagnosis, RASSF1A hypermethylation was detected in 41 of 42 patients with metastatic neuroblastoma (Fig 2B and Table 1). The one negative patient was stage MS and upstaged to stage M because of two new bone lesions. Hypermethylated RASSF1A was detected in all diagnostic plasma samples from patients with nephroblastoma and absent in plasma from two patients with Cystic Partially Differentiated Nephroblastoma and bilateral differentiated nephroblastomatosis, providing the possibility that only malignant tumors are detected by this marker. Eight of 14 plasma samples from patients with rhabdomyosarcoma were positive, as were 4 of 5 Hodgkin lymphoma plasma samples. Only one CSF sample from a patient with medulloblastoma was positive, and this was the sample with the highest cfDNA concentration.
Cell-Free Detection of Hypermethylated RASSF1A at Diagnosis and During Therapy
Plasma was available from 47 patients with high-risk neuroblastoma during the course of treatment. Clinical details, time of sampling, and ctDNA and mRNA results per sample can be found in the Data Supplement. Single nucleotide polymorphism array data confirmed 3p loss in 9 of 32 tumor samples, and in all nine patients, hypermethylated RASSF1A was detected in plasma, indicating that RASSF1A hypermethylation can still be identified in neuroblastoma with only one RASSF1A allele. At diagnosis, the absolute and relative levels of hypermethylated RASSF1A were significantly higher in the group of patients who will experience an event, although with a substantial overlap (median 37,243 copies/mL [interquartile range: 6,749-174,727] v 8,221 copies/mL [3,951-18,339], P = .012, 70.2% [45.0-91.7] v 56.5% [17.1-74.5], P = .030, respectively; Figs 3A and 3B). Receiver operating characteristic analysis revealed a cutoff of 27,681 hypermethylated RASSF1A copies/mL with a sensitivity of 64% and a specificity of 89% (Data Supplement) that identifies a group that has a significantly poorer event-free survival (Data Supplement, log-rank P = .0007). As the majority of the total cfDNA was tumor-derived, this led to a significant increase in cfDNA at diagnosis for patients who will experience an event (59,714 copies/mL [27,547-246,149] v 21,450 copies/mL [16,107-63,446], P = .023; Fig 3C). For other time points, there was no significant difference in total cfDNA levels between the patients with and without an event. At relapse, ctDNA levels were comparable with levels at diagnosis. Hypermethylated RASSF1A positivity did not correlate with an event for any of the time points (Fig 3D).
FIG 3.
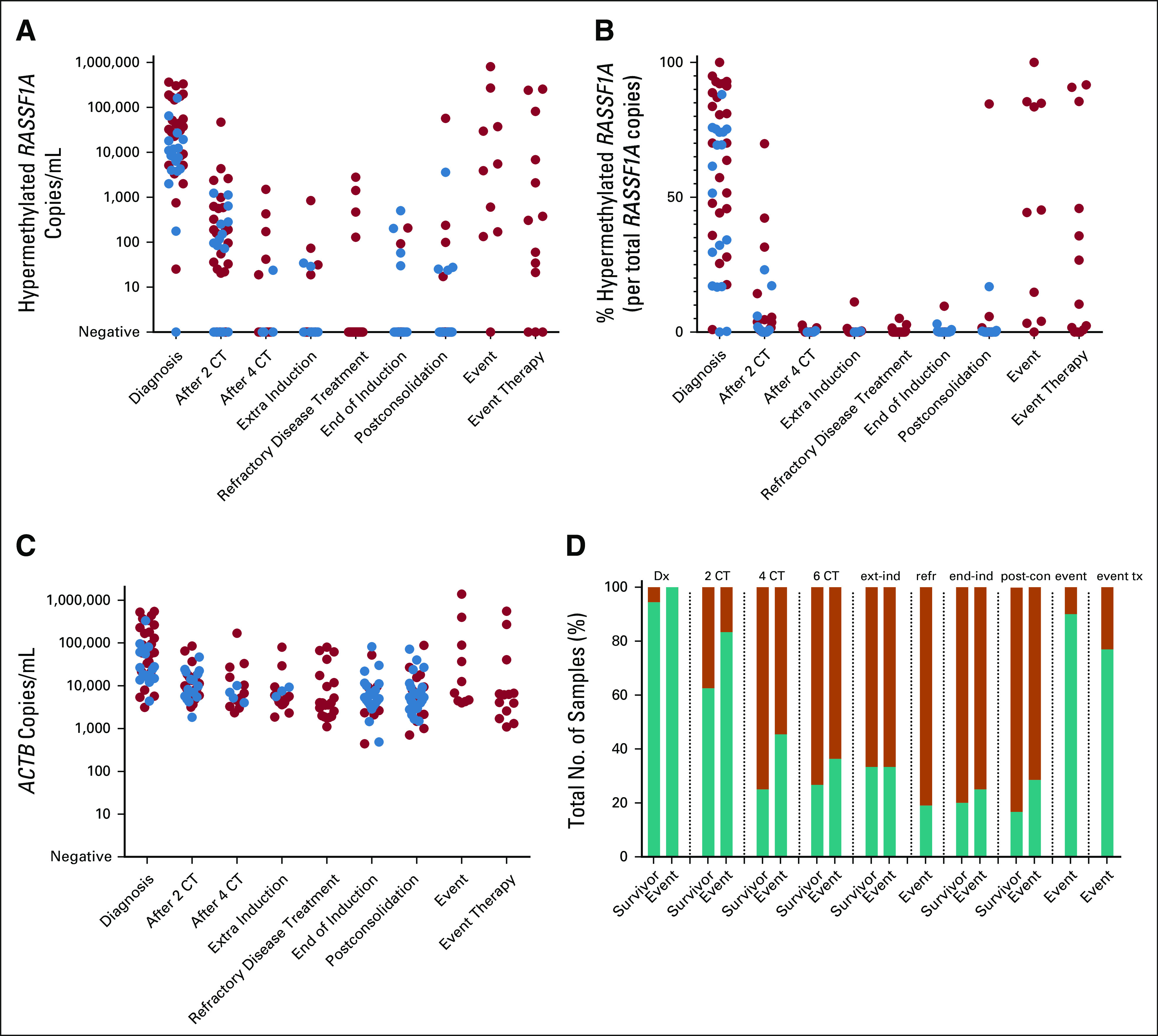
Positivity and levels of circulating hypermethylated RASSF1A and total cfDNA during therapy in patients with high-risk neuroblastoma. Red circles indicate samples from a patient who will suffer from an event, and blue circles indicate samples from patients who remain in complete remission (survivor). (A) Amount of hypermethylated RASSF1A in copies/mL plasma during therapy. (B) Relative levels of hypermethylated RASSF1A per total RASSF1A copies during therapy. (C) Levels of cfDNA, measured by β-actin (ACTB), in copies/mL plasma during therapy. (D) Fraction of total number of samples tested that were positive for circulating hypermethylated RASSF1A. Green bar represents positive samples, and orange bar represents negative samples. cfDNA, cell-free DNA; CT, cycles of chemotherapy; Dx, diagnosis; end-ind, end of induction; event tx, event therapy; ext-ind, extra induction therapy (not for refractory disease); post-con, postconsolidation; refr, refractory disease treatment.
Comparison of ctDNA With the Detection of mRNA in BM and Blood
We previously showed that qPCR-based RASSF1A hypermethylation correlated with mRNA marker panel positivity or negativity in BM cells in patients when tumor burden was high or no tumor was detected.33 Marker discrepancies indicated either low-level BM infiltration (ctDNA–&mRNA panel+) or primary tumor or soft tissue lesions without BM involvement (ctDNA+&mRNA panel–). To confirm these results in the current cohort, we tested cell fractions of corresponding blood (227) and BM (224) samples for mRNA markers45 and compared them with hypermethylated RASSF1A in plasma by ddPCR. We again observe a strong correlation when the tumor load is to be expected high (at time of diagnosis or event) or absent (Fig 4), but see both ctDNA−&mRNA+ and vice versa when the tumor load is expected to be lower, for example, during therapy. In 227 matched blood samples, ctDNA was concordant with blood mRNA in 73% (75 ctDNA+&mRNA+ and 91 ctDNA–&mRNA–), 47 samples were ctDNA-positive only, and 14 samples mRNA-positive only. Spearman correlation of those 75 ctDNA+&mRNA+ indicated an association between ctDNA and mRNA results (rs = 0.65, P > .001). In 224 matched BM mRNA and ctDNA blood samples, paired positive or negative results were found in 65% (103 and 43 samples, respectively). In contrast to the blood samples, BM mRNA–only identified more positive samples (62) compared with ctDNA-only (16). Twenty-seven of those 62 samples were taken during induction chemotherapy. In 103 ctDNA+&mRNA+ samples, Spearman correlation indicated a moderate association between ctDNA and mRNA results (rs = 0.49, P > .001).
FIG 4.
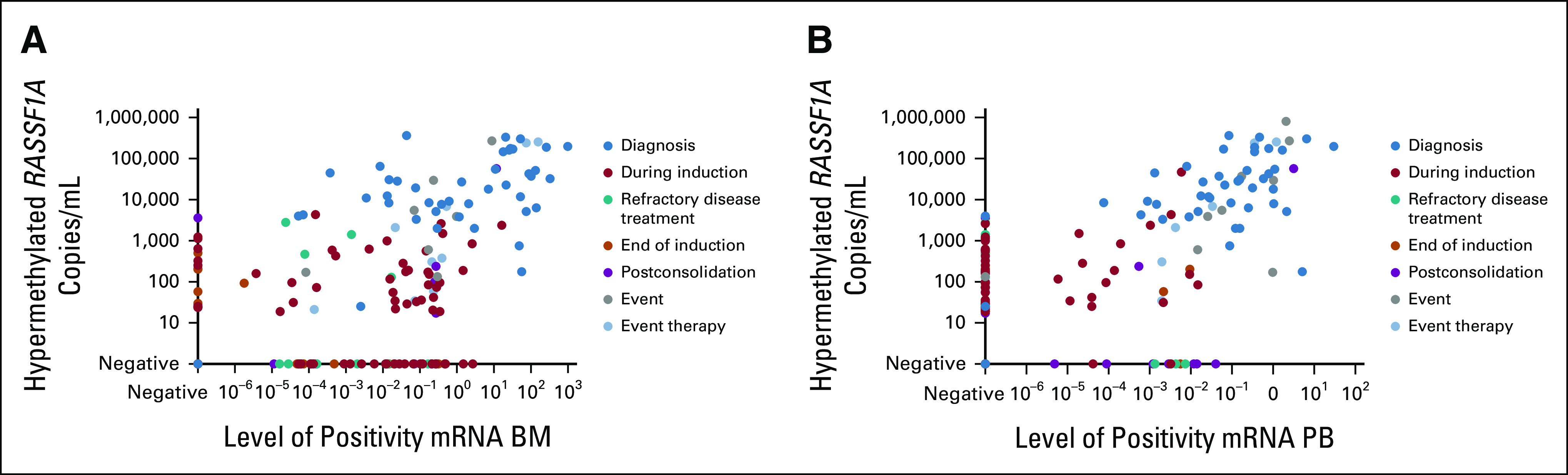
(A) Association between mRNA in BM samples and circulating hypermethylated RASSF1A and (B) association between mRNA in blood samples and circulating hypermethylated RASSF1A. BM, bone marrow; PB, peripheral blood.
Combined ctDNA and mRNA Detection Correlates With Outcomes
We next studied the kinetics of circulating hypermethylated RASSF1A and the mRNA markers from the corresponding BM and blood samples. Representative examples from five patients are depicted in Figures 5A-5E, and the combined outcome of circulating hypermethylated RASSF1A and BM mRNA for different time points is shown in Figure 5F. We showed that during therapy, the presence of hypermethylated RASSF1A in plasma was not associated with poorer prognosis at any of the time points in this patient cohort (Fig 3D). However, when circulating hypermethylated RASSF1A results were combined with BM mRNA, positivity with both techniques after two cycles of chemotherapy was associated with unfavorable clinical outcomes of these patients (P = .046; Fig 5F), with the sensitivity and specificity of the ctDNA+&mRNA+ profile being 74% and 63%, respectively. BM mRNA positivity alone at this time point was not predictive of the outcome in this cohort (P = .12). The trend that ctDNA+&mRNA+ positivity at other time points also correlates with an event was not significant in this small cohort. Remarkably, BM mRNA positivity alone during postconsolidation was associated with unfavorable outcomes (P = .077). In summary, the level of hypermethylated RASSF1A at diagnosis was correlated with unfavorable outcomes. Moreover, the combination of ctDNA with BM mRNA improved the predictive value after two cycles of chemotherapy in this cohort.
FIG 5.
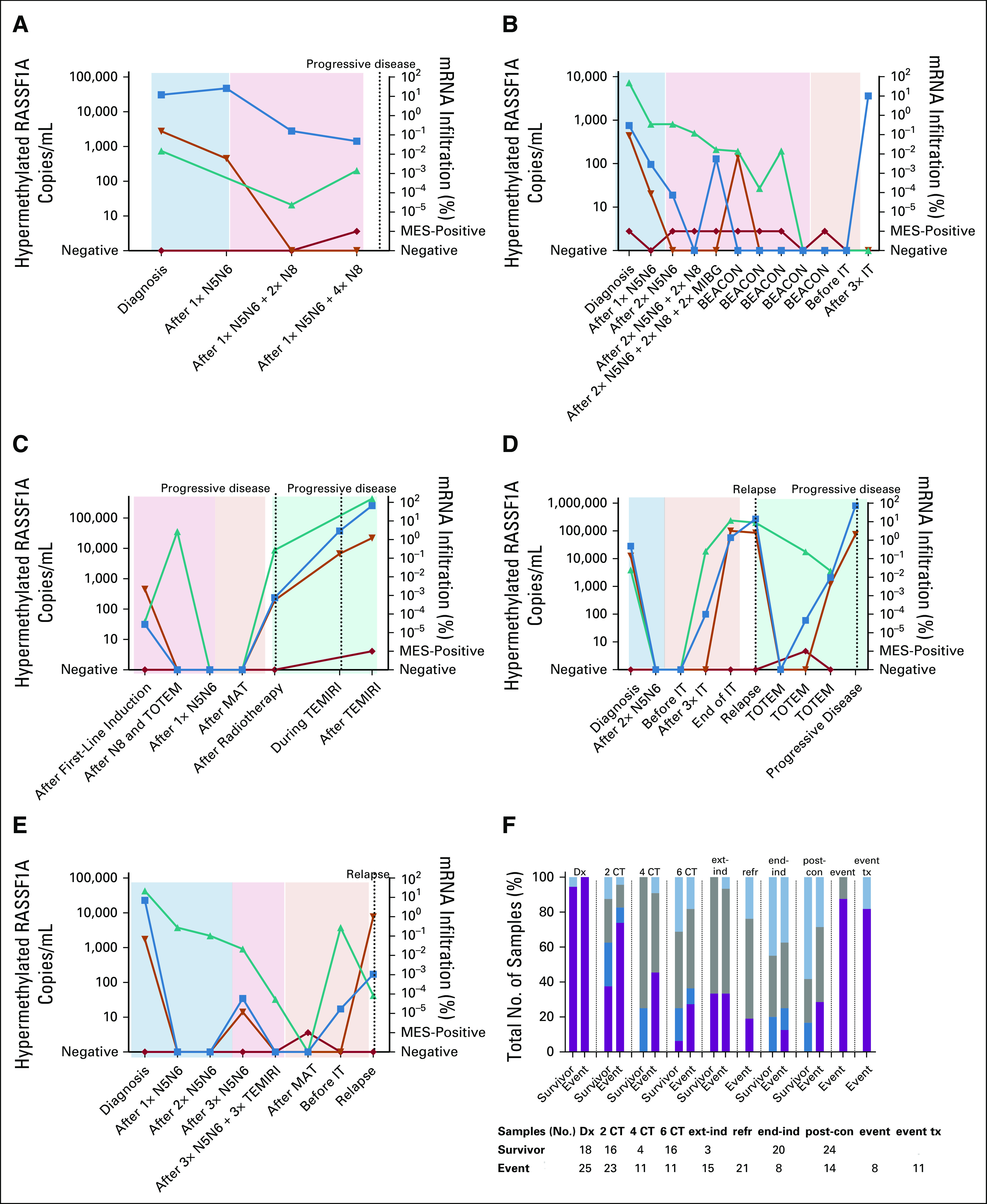
(A-E) For patients with refractory, relapse, or progressive disease, all sequential samples, if available, were analyzed for hypermethylated RASSF1A (blue squares; N2063, N2071, N2099, N2101, and N2123, respectively). Corresponding blood (orange triangles) and BM (green triangles for adrenergic markers and red diamonds for MES markers) samples were tested for mRNA. Colored blocks indicate the treatment: light blue, induction therapy; light red, extra induction therapy; light orange, postconsolidation therapy; light green, relapse or progressive disease treatment. (F) Fraction of total number of tested samples, which were positive for circulating hypermethylated RASSF1A and/or BM mRNA, of patients who will suffer an event compared with those who remain in complete remission (survivor). Purple bar represents hypermethylated RASSF1A+ and mRNA panel+ samples, dark blue bar represents hypermethylated RASSF1A ctDNA+ and mRNA panel– samples, gray bar represents hypermethylated RASSF1A– and mRNA panel+ samples, and light blue bar represents hypermethylated RASSF1A ctDNA–/mRNA panel– samples. BEACON, TEMIRI, and TOTEM are treatment for refractory or relapsed disease. BEACON, BEACON-Neuroblastoma Trial: bevacizumab, temozolomide ± irinotecan; BM, bone marrow; CT, cycles of chemotherapy; ctDNA; circulating tumor DNA; Dx, diagnosis; end-ind, end of induction; event tx, event therapy; ext-ind, extra induction therapy (not for refractory disease); IT, immunotherapy; MAT, myeloablative therapy; MES, mesenchymal; MIBG, iodine-131-meta-iodobenzylguanidine; N5, N6, and N8; courses of induction chemotherapy; post-con, postconsolidation; refr, refractory disease treatment; TEMIRI, temozolomide and irinotecan; TOTEM, temozolomide and topotecan.
DISCUSSION
Molecular testing of cfDNA has the potential to improve pediatric solid tumor diagnosis, discrimination of subtypes, and MRD monitoring. Our aim was to complete a first step in this evolution of diagnostic modalities by evaluating our RASSF1A hypermethylation ddPCR as a standard test to detect ctDNA in several pediatric tumor types using small blood volumes and as a test to monitor treatment response of patients with neuroblastoma.
We previously described qPCR-based detection of circulating hypermethylated RASSF1A in patients with neuroblastoma.33 In our previous study, the majority of positive samples could not be quantified reliably by qPCR, whereas ddPCR technology is adept for precise quantification of low abundant targets.46 Furthermore, like in many other widely used methods to analyze DNA methylation, cfDNA samples in the qPCR study were bisulfite converted, which is known to degrade the majority of DNA.47 As cfDNA is often present in low quantities, we investigated the use of an MSRE, previously described by Chan et al and O'Brien et al, as an alternative to bisulfite conversion.43,44 We noticed higher hypermethylated RASSF1A levels in control samples with high total cfDNA levels, also reported by O'Brien et al.44 We successfully introduced a combination of two MSREs, which resulted in better digestion of unmethylated RASSF1A. cfDNA may not always be present as double-stranded DNA, but can also appear as (partially) single-stranded DNA fragments.48,49 Although the enzyme BstUI performed well in genomic DNA experiments, it is reported to be less active on single-stranded DNA.50 The addition of HhaI overcomes this, as this enzyme is capable of digesting single-stranded DNA. The use of two different MSRE, and thus an increase in digestion sites, may result in digestion of DNA that is only partially methylated,51 potentially underestimating present hypermethylated RASSF1A. However, as BstUI-only was clearly unable to digest all unmethylated RASSF1A, we proceeded with the use of two MSREs. The frequency of low-level positive results detected in healthy adult and pediatric controls defined the limit of detection. Since lack of remnants precluded the retesting of our qPCR study samples,33 we showed in 16 rhabdomyosarcoma and renal tumor samples the slight superiority of the ddPCR method. In summary, the ddPCR is our preferred method to use for hypermethylated RASSF1A detection in plasma samples because the MSRE-ddPCR can reliably quantify ctDNA and saves time and sample.
We corroborate the potential of hypermethylated RASSF1A as a ctDNA marker for neuroblastoma, for monitoring treatment response and early relapse detection. This study confirms that cell-free hypermethylated RASSF1A correlates with mRNA marker panel positivity in BM and blood in patients at the opposite ends of the disease spectrum, when tumor burden was high or no tumor was detected.32,33 The difference in kinetics of ctDNA and BM mRNA is illustrated by the prolonged presence of BM mRNA during induction therapy, whereas ctDNA rapidly declines during therapy, but is present again at relapse. The results of this study further support the finding, in an independent cohort, that both ctDNA and mRNA complement each other for the detection of MRD, with the combination showing a correlation with the outcome after two cycles of chemotherapy. Although the detection of ctDNA was shown to be very promising for future MRD studies in neuroblastoma, no definitive conclusions can be made as samples for this study were not prospectively collected, resulting in missing samples. Future research should be undertaken to investigate whether hypermethylated RASSF1A can be used as a marker during follow-up for early relapse detection and whether a cutoff can be used to predict event-free survival. As inactivation of RASSF1A, for example, by hypermethylation, is advantageous for many tumor entities, in melanoma, demethylation agents lead to apoptosis and cell death52; we think that this marker is not lost in time. We will test this hypothesis in prospective collaborative studies on the use of ctDNA in the new SIOPEN HR-2 (NCT04221035) patient cohort, which are being initiated within the SIOPEN liquid biopsy group.
Comparison of the total cfDNA levels in pediatric solid tumors with those of other studies confirms higher levels in patients with neuroblastoma and nephroblastoma tumors.53-57 Consistent with literature, a high tumor-derived fraction of total cfDNA was found in patients with neuroblastoma and nephroblastoma, demonstrating the potential of liquid biopsies in these tumor entities.54,56,58 Plasma samples from patients with other tumor entities in this study were less conclusive, which may indicate differences in the extent that different tumor types shed tumor DNA into circulation, a lower frequency of RASSF1A hypermethylation in other tumor entities,29 or may just be artifacts of low sample numbers in the preliminary sample collection evaluated.
In this study, we developed a sensitive and quantitative ddPCR-based assay for hypermethylated RASSF1A detection and determined threshold values for positive results. Our findings demonstrate the value of hypermethylated RASSF1A as a molecular circulating tumor marker in neuroblastoma. Furthermore, our preliminary investigation of RASSF1A hypermethylation detection in circulating cfDNA demonstrates potential as a pan-tumor marker, but requires further investigation to evaluate its use and limitations.
Johannes H. M. Merks
Consulting or Advisory Role: Bayer, GlaxoSmithKline
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the AACR Advances in Liquid Biopsies Congress, Miami, FL, January 13-16, 2020; Advances in Neuroblastoma Research Congress, Amsterdam, the Netherlands, January 25-27, 2021; and 52nd Congress of the International Society of Paediatric Oncology, Ottowa, Canada, October 14-17, 2020.
SUPPORT
Supported by Liquidhope, a TranScan-2 project by Koningin Wilhelmina Fund, KWF Kankerbestrijding TRANSCAN 8352/TRS-2018-00000715 (L.M.J.v.Z. and N.U.G.), Foundation AMeesing Mees, and Foundation Koppie Au.
AUTHOR CONTRIBUTIONS
Conception and design: Lieke M. J. van Zogchel, Mohammed Gussmalla Nuru, Johannes H. M. Merks, Antoinette Y. N. Schouten-van Meeteren, C. Ellen van der Schoot, Godelieve A. M. Tytgat
Financial support: Godelieve A. M. Tytgat
Administrative support: Lieke M. J. van Zogchel, Nathalie S. M. Lak, Mohammed Gussmalla Nuru, Ahmad Javadi, Johannes H. M. Merks, Janine Stutterheim, Godelieve A. M. Tytgat
Provision of study materials or patients: Lily Zappeij-Kannengieter, Eline A. M. Zijtregtop, Johannes H. M. Merks, Antoinette Y. N. Schouten-van Meeteren, Godelieve A. M. Tytgat
Collection and assembly of data: Lieke M. J. van Zogchel, Onno J. H. M. Verhagen, Ahmed Tissoudali, Mohammed Gussmalla Nuru, Nina U. Gelineau, Lily Zappeij-Kannengieter, Eline A. M. Zijtregtop, Johannes H. M. Merks, Antoinette Y. N. Schouten-van Meeteren
Data analysis and interpretation: Lieke M. J. van Zogchel, Nathalie S. M. Lak, Onno J. H. M. Verhagen, Mohammed Gussmalla Nuru, Ahmad Javadi, Johannes H. M. Merks, Marry van den Heuvel-Eibrink, Antoinette Y. N. Schouten-van Meeteren, Janine Stutterheim
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Johannes H. M. Merks
Consulting or Advisory Role: Bayer, GlaxoSmithKline
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7-34, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Park JR, Kreissman SG, London WB, et al. : Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma. JAMA 322:746, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Wezel EM, Zwijnenburg D, Zappeij-Kannegieter L, et al. : Whole-genome sequencing identifies patient-specific DNA minimal residual disease markers in neuroblastoma. J Mol Diagn 17:43-52, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Stahl M, Ranft A, Paulussen M, et al. : Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 57:549-553, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Malempati S, Hawkins DS: Rhabdomyosarcoma: review of the Children's Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer 59:5-10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelan JS, Davis LE: Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol 36:188-193, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Brok J, Lopez-Yurda M, Tinteren HV, et al. : Relapse of Wilms' tumour and detection methods: A retrospective analysis of the 2001 Renal Tumour Study Group–International Society of Paediatric Oncology Wilms' tumour protocol database. Lancet Oncol 19:1072-1081, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Park JR, Bagatell R, Cohn SL, et al. : Revisions to the International Neuroblastoma Response Criteria: A consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol 35:2580-2587, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viprey VF, Gregory WM, Corrias MV, et al. : Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: A European HR-NBL1/SIOPEN study. J Clin Oncol 32:1074-1083, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Cheung NK, Ostrovnaya I, Kuk D, et al. : Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti-GD2 immunotherapy. J Clin Oncol 33:755-763, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreissman SG, Seeger RC, Matthay KK, et al. : Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol 14:999-1008, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchill SA, Beiske K, Shimada H, et al. : Recommendations for the standardization of bone marrow disease assessment and reporting in children with neuroblastoma on behalf of the International Neuroblastoma Response Criteria Bone Marrow Working Group. Cancer 123:1095-1105, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Lak NS, Voormanns TL, Zappeij-Kannegieter L, et al. : Improving risk stratification for pediatric patients with rhabdomyosarcoma by molecular detection of disseminated disease. Clin Cancer Res 10.1158/1078-0432.CCR-21-1083 [epub ahead of print on July 20, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stutterheim J, Zappeij-Kannegieter L, Versteeg R, et al. : The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. Eur J Cancer 47:1193-1202, 2011 [DOI] [PubMed] [Google Scholar]
- 15.van Wezel EM, van Zogchel LM, van Wijk J, et al. : Mesenchymal neuroblastoma cells are undetected by current mRNA marker panels: The development of a specific neuroblastoma mesenchymal minimal residual disease panel. JCO Precis Oncol 3:1-11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan JCM, Massie C, Garcia-Corbacho J, et al. : Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat Rev Cancer 17:223-238, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Siravegna G, Marsoni S, Siena S, et al. : Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 14:531-548, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Rolfo C, Cardona AF, Cristofanilli M, et al. : Challenges and opportunities of cfDNA analysis implementation in clinical practice: Perspective of the International Society of Liquid Biopsy (ISLB). Crit Rev Oncol Hematol 151:102978, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Lawrence MS, Stojanov P, Polak P, et al. : Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499:214-218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klega K, Imamovic-Tuco A, Ha G, et al. : Detection of somatic structural variants enables quantification and characterization of circulating tumor DNA in children with solid tumors. JCO Precis Oncol 10.1200/PO.17.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor ER, Thiele CJ: Epigenetic changes in pediatric solid tumors: Promising new targets. Clin Cancer Res 18:2768-2779, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hesson LB, Cooper WN, Latif F: The role of RASSF1A methylation in cancer. Dis Markers 23:73-87, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donninger H, Vos MD, Clark GJ: The RASSF1A tumor suppressor. J Cell Sci 120:3163-3172, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Grawenda AM, O'Neill E: Clinical utility of RASSF1A methylation in human malignancies. Br J Cancer 113:372-381, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois F, Bergot E, Zalcman G, et al. : RASSF1A, puppeteer of cellular homeostasis, fights tumorigenesis, and metastasis—An updated review. Cell Death Dis 10:928, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malpeli G, Innamorati G, Decimo I, et al. : Methylation dynamics of RASSF1A and its impact on cancer. Cancers (Basel) 11:959, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg RP, Honorio S, Martinez A, et al. : Frequent 3p allele loss and epigenetic inactivation of the RASSF1A tumour suppressor gene from region 3p21.3 in head and neck squamous cell carcinoma. Eur J Cancer 38:1585-1592, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Abe M, Ohira M, Kaneda A, et al. : CpG island methylator phenotype is a strong determinant of poor prognosis in neuroblastomas. Cancer Res 65:828-834, 2005 [PubMed] [Google Scholar]
- 29.Harada K, Toyooka S, Maitra A, et al. : Aberrant promoter methylation and silencing of the RASSF1A gene in pediatric tumors and cell lines. Oncogene 21:4345-4349, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hoebeeck J, Michels E, Pattyn F, et al. : Aberrant methylation of candidate tumor suppressor genes in neuroblastoma. Cancer Lett 273:336-346, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Misawa a, Tanaka S, Yagyu S, et al. : RASSF1A hypermethylation in pretreatment serum DNA of neuroblastoma patients: A prognostic marker. Br J Cancer 100:399-404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stutterheim J, Ichou FA, Den Ouden E, et al. : Methylated RASSF1a is the first specific DNA marker for minimal residual disease testing in neuroblastoma. Clin Cancer Res 18:808-814, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Van Zogchel LMJ, Van Wezel EM, Van Wijk J, et al. : Hypermethylated RASSF1A as circulating tumor DNA marker for disease monitoring in neuroblastoma. JCO Precis Oncol 4:291-306, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong IHN, Chan J, Wong J, et al. : Ubiquitous aberrant RASSF1A promoter methylation in childhood neoplasia. Clin Cancer Res 10:994-1002, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Kiss NB, Kogner P, Johnsen JI, et al. : Quantitative global and gene-specific promoter methylation in relation to biological properties of neuroblastomas. BMC Med Genet 13:1-12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda S, Miyagi H, Suzuki H, et al. : RASSF1A methylation indicates a poor prognosis in hepatoblastoma patients. Pediatr Surg Int 29:1147-1152, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Wagner KJ, Cooper WN, Grundy RG, et al. : Frequent RASSF1A tumour suppressor gene promoter methylation in Wilms' tumour and colorectal cancer. Oncogene 21:7277-7282, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich M, Jiang G, Fiala E, et al. : Hypomethylation and hypermethylation of DNA in Wilms tumors. Oncogene 21:6694-6702, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Mühlisch J, Schwering A, Grotzer M, et al. : Epigenetic repression of RASSF1A but not CASP8 in supratentorial PNET (sPNET) and atypical teratoid/rhabdoid tumors (AT/RT) of childhood. Oncogene 25:1111-1117, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lim S, Yang MH, Park JH, et al. : Inactivation of the RASSF1A in osteosarcoma. Oncol Rep 10:897-901, 2003 [PubMed] [Google Scholar]
- 41.Wang WG, Chen SJ, He JS, et al. : The tumor suppressive role of RASSF1A in osteosarcoma through the Wnt signaling pathway. Tumour Biol 37:8869-8877, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Avigad S, Shukla S, Naumov I, et al. : Aberrant methylation and reduced expression of RASSF1A in Ewing sarcoma. Pediatr Blood Cancer 53:1023-1028, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Chan KC, Ding C, Gerovassili A, et al. : Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem 52:2211-2218, 2006 [DOI] [PubMed] [Google Scholar]
- 44.O’Brien H, Hyland C, Schoeman E, et al. : Non‐invasive prenatal testing (NIPT) for fetal Kell, Duffy and Rh blood group antigen prediction in alloimmunised pregnant women: Power of droplet digital PCR. Br J Haematol 189:e90-e94, 2020 [DOI] [PubMed] [Google Scholar]
- 45.van Zogchel LM, Zappeij-Kannegieter L, Javadi A, et al. : Specific and sensitive detection of neuroblastoma mRNA markers by multiplex RT-qPCR. Cancers (Basel) 13:150, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor SC, Laperriere G, Germain H: Droplet digital PCR versus qPCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Scientific Rep 7:1-8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grunau C, Clark S, Rosenthal A: Bisulfite genomic sequencing: Systematic investigation of critical experimental parameters. Nucleic Acids Res 29:e65, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez C, Snyder MW, Tanos R, et al. : New insights into structural features and optimal detection of circulating tumor DNA determined by single-strand DNA analysis. NPJ Genom Med 3:31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burnham P, Kim MS, Agbor-Enoh S, et al. : Single-stranded DNA library preparation uncovers the origin and diversity of ultrashort cell-free DNA in plasma. Scientific Rep 6:1-9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Restriction Enzyme Database (REBASE). http://rebase.neb.com/rebase/rebase.html
- 51.Mikeska T, Candiloro IL, Dobrovic A: The implications of heterogeneous DNA methylation for the accurate quantification of methylation. Epigenomics 2:561-573, 2010 [DOI] [PubMed] [Google Scholar]
- 52.McKenna S, García-Gutiérrez L: Resistance to targeted therapy and RASSF1A loss in melanoma: What are we missing? Int J Mol Sci 22:5115, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bettegowda C, Sausen M, Leary RJ, et al. : Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6:224ra24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chicard M, Boyault S, Daage LC, et al. : Genomic copy number profiling using circulating free tumor DNA highlights heterogeneity in neuroblastoma. Clin Cancer Res 22:5564-5573, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Chicard M, Colmet-Daage L, Clement N, et al. : Whole-exome sequencing of cell-free DNA reveals temporo-spatial heterogeneity and identifies treatment-resistant clones in neuroblastoma. Clin Cancer Res 24:939-949, 2018 [DOI] [PubMed] [Google Scholar]
- 56.Jiménez I, Chicard M, Colmet-Daage L, et al. : Circulating tumor DNA analysis enables molecular characterization of pediatric renal tumors at diagnosis. Int J Cancer 144:68-79, 2019 [DOI] [PubMed] [Google Scholar]
- 57.Su Y, Wang L, Jiang C, et al. : Increased plasma concentration of cell-free DNA precedes disease recurrence in children with high-risk neuroblastoma. BMC Cancer 20:102, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paemel RV, Koker AD, Vandeputte C, et al. : Minimally invasive classification of paediatric solid tumours using reduced representation bisulphite sequencing of cell-free DNA: A proof-of-principle study. Epigenetics 16:196-208, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]