Abstract
The ability to exploit the immune system as a weapon against cancer has revolutionised the treatment of cancer patients, especially through immune checkpoint inhibitors (ICIs). However, ICIs demonstrated a modest benefit in treating breast cancer (BC), with the exception of certain subsets of triple-negative BCs. An immune-suppressive tumour microenvironment (TME), typically present in BC, is an important factor in the poor response to immunotherapy.
After almost two decades of poor clinical trial results, cancer vaccines (CVs), an active immunotherapy, have come back in the spotlight because of some technological advancements, ultimately boosted by coronavirus disease 2019 pandemic. In particular, neoantigens are emerging as the preferred targets for CVs, with gene-based and viral vector–based platforms in development. Moreover, lipid nanoparticles proved to be immunogenic and efficient delivery vehicles.
Past clinical trials investigating CVs focused especially on the metastatic disease, where the TME is more likely compromised by inhibitory mechanisms. In this sense, favouring the use of CVs as monotherapy in premalignant or in the adjuvant setting and establishing combination treatments (i.e. CV plus ICI) in late-stage disease are promising strategies. This review provides a full overview of the past and current breast cancer vaccine landscape.
Keywords: Cancer, Vaccines, Immunotherapies, Covid-19, Breast cancer, Pandemic, Immunogenicity, Neoantigens
1. Introduction
Breast cancer (BC) is the second most frequently diagnosed malignancy worldwide, accounting for over two million cases each year and the leading cause of cancer death in women [1]. For years BC has been considered immunologically quiescent compared with other tumour types [2]. This is partially because of its lower somatic mutational burden and consequent lower neoantigen load [2,3]. The hormonal asset plays an important role, as well [4]. In fact, human epidermal growth factor receptor 2 (HER2)–positive and triple-negative breast cancer (TNBC) show a higher mutational burden than hormone receptor (HR)–positive BC [5,6]. Consistently, tumour-infiltrating lymphocyte (TIL) rates are higher in HER2-positive BC and TNBC, in comparison with HR-positive BCs [[7], [8], [9], [10], [11], [12]]. Interestingly, higher TIL levels have been associated with improved prognosis in HER2-positive BC and TNBC, with a 10% increase in TILs associated with a 15–25% decrease in risk of relapse and death [2,13]. The lymphocytic infiltrate is also predictive of pathologic complete response (pCR) to neoadjuvant chemotherapy (NACT) [[14], [15], [16], [17], [18]]. Of note, the association between TILs and a better outcome after anthracycline-based chemotherapy supports the immunogenic role of certain chemotherapy regimens, which may awaken a pre-existing host immune response against cancer cells [19].
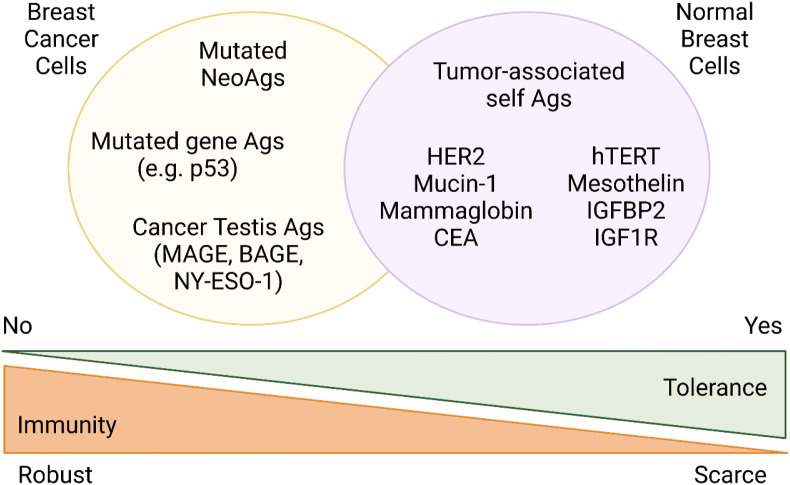
Other strategies to stimulate host immune responses lie in immune checkpoint inhibitors (ICI) and active immunotherapies, such as therapeutic cancer vaccines (CVs). The latter aim at stimulating type I (Th1) CD4+ and CD8+ T-cell immune responses against tumour-associated antigens (TAAs) or tumour specific antigens (TSAs). Cancer vaccines rely on the ability of the immune system to differentiate between self-antigens, normally expressed on the surface of healthy cells, and those abnormally expressed on cancer cells [20,21]. The main advantages of CVs are minimal toxicity, the generation of highly specific adaptive immune responses and the establishment of immunologic memory, with the potential to control or eliminate residual disease by swiftly responding to TAA/TSA exposure over time [22].
In this regard, BC represents the third most studied tumour for cancer vaccination, following melanoma and cervical cancer [23]. Historically, the most common TAAs targeted in BC are HER2, Mucin 1, cell surface associated (MUC-1), carcinoembryonic antigen (CEA) and human telomerase reverse transcriptase (hTERT) [23,24].
However, results from therapeutic BC vaccine clinical trials have been disappointing, so far [24]. Two phase-III BC vaccine clinical trials have failed to demonstrate any benefit in the metastatic and adjuvant setting, respectively [25,26]. The former investigated a STn keyhole limpet haemocyanin (KLH) vaccine targeting a tumour associated carbohydrate (TACA) epitope found on cancer-associated mucins, in the metastatic setting (Theratope) [26]. The latter assessed the risk of disease relapse after administering a HER2-based peptide vaccine Nelipepimut-S (NeuVax) to high-risk BC patients in the adjuvant setting (PRESENT trial) [25].
Overall, during a 20-year period (2000–2019) of BC vaccine clinical trials, the estimated objective response rate (ORR) was around 9%, without a clear survival benefit [24]. Such results did not seem to be statistically different when comparing different traditional vaccine platforms or different TAAs [24]. Similarly, the use of vaccines alone or in combination with other treatments did not result in a significant change in ORR outcomes [24].
Negative results from BC vaccine trials may be associated to different factors: first, the potentially immunologically inadequate clinical settings (metastatic disease among often heavily pretreated BC patients, mainly characterised by a large tumour burden) [27]; second, the predominance of TAAs as targets; third, the vaccine delivery platform used [28,29]; fourth, the presence and type of concurrent therapies administered in combination with the CV [30,31]; fifth, the presence of many non-controlled mechanisms of immune escape, including alterations in antigens (Ags) processing machinery, the loss of Human Leukocyte Ag class I (HLA-I) expression, the down-regulation of tumour Ags expression and, in general, factors favouring a ‘cold’ tumour microenvironment (TME) [6,[32], [33], [34], [35], [36]]. In fact, a major hurdle undermining therapeutic CVs efficacy is the presence of immune-evasive mechanisms, particularly in solid tumours [37,38]. Major recognised immune-suppressive mechanisms are T-cell exhaustion in inflamed tumours, lack of T-cell infiltration in immune excluded tumours and defective antigen presentation processes in immune desert tumours [37].
Based on this experience, current CVs research has been mostly directed towards novel technologies for vaccine development, combination immunotherapies – especially with ICIs – and new clinical settings, as later discussed [29]. Incidentally, the struggle to face the coronavirus disease 2019 (COVID-19) pandemic, not only provides large-scale clinical evidence of the safety and efficacy of new vaccine platforms, but also offers a technological boost for effective BC vaccines in the next future [29,39].
This review focuses on the current landscape of therapeutic vaccines for BC, with insights on their potential use in the clinical practice and the main challenges to overcome in the next future.
2. Current landscape of therapeutic vaccines for breast cancer
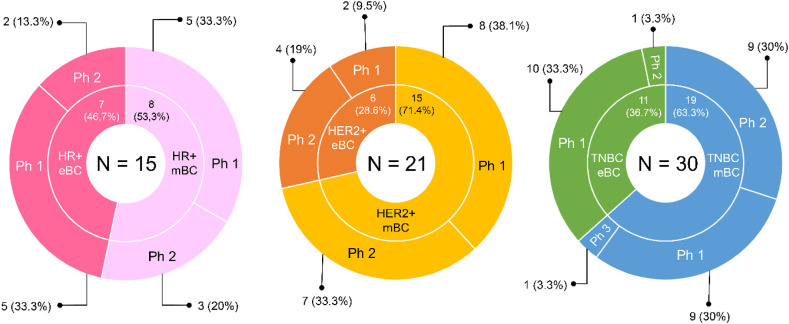
Briefly, a total of 44 ongoing (i.e. active, not recruiting; recruiting; not yet recruiting) clinical trials are currently investigating therapeutic CVs in BC, as of 23rd June 2021 (Fig. 1 ). Overall, 30 (68.2%) clinical trials are enrolling patients with TNBC, 21 (47.7%) clinical trials are open to patients with HER2-positive BC and only 15 (34.1%) trials have HR-positive BC as inclusion criteria. Of note, seven (15.9%) trials are open to BC patients, irrespective of the biological subtype. As for the clinical setting, 21 (47.7%) clinical trials are including BC patients in the early setting, while 23 (52.3%) clinical trials are investigating the role of CVs in the advanced setting. No study is testing any vaccine in a more preventive setting. Interestingly, only one (2.3%) clinical trial has a phase-III experimental design, whereas the other 43 (97.7%) are early-phase studies.
Fig. 1.
Summary of the ongoing trials investigating therapeutic vaccines in BC, as of 23rd June 2021. Abbreviations: HR, hormone receptor; mBC, metastatic breast cancer; eBC, early breast cancer; TNBC, triple-negative breast cancer; Ph, phase; HER2, human epidermal growth factor receptor 2. Source: clinicaltrials.gov.
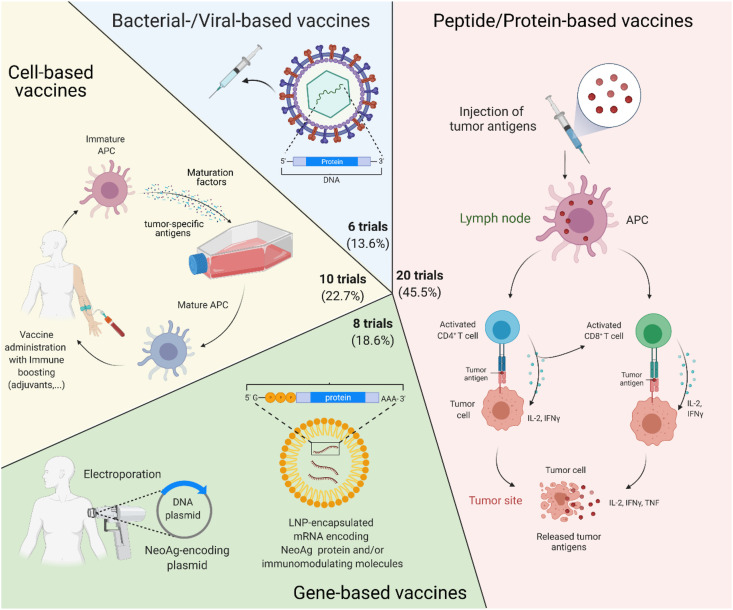
As for the vaccine platform, the majority of BC vaccines are peptide-based (N = 20, 45.5%), while almost 10 (22.7%) clinical trials are testing cell-based BC vaccines; new technologies such as viral-vectored vaccines account for six (13.6%) clinical trials, whereas gene-based BC vaccines are currently investigated in 8 (18.2%) clinical trials, as depicted in Fig. 2 .
Fig. 2.
Summary of the main platforms used in current clinical trials investigating therapeutic vaccines in BC, as of 23rd June 2021. Abbreviations: Ph, phase; HER2, human epidermal growth factor receptor; NeoAg, neoantigens; mRNA, messenger ribonucleic acid; LNP, lipid nanoparticle; APC, antigen presenting cell; IL-2, interleukin 2; INFγ, interferon gamma; TNF, tumour necrosis factor. Created with biorender.com.
3. Vaccines targeting HER2 in breast cancer
Human epidermal growth factor receptor is a transmembrane growth factor receptor, member of the HER protein family, along with HER1 (EGFR), HER3 and HER4. Numerous epidermal growth factor (EGF) ligands have been defined, although none specific to HER2 [40,41]. When a ligand binds to HER1, HER3 or HER4, receptor dimerisation occurs, with HER2 as the preferred dimer partner. Then, the activation of the tyrosine-kinase intracellular domain triggers different downstream pathways [40]. The oncoprotein HER2 is overexpressed with complete membrane staining that is intense and >10% of tumour cells (i.e. immunohistochemistry, IHC, 3+) in 10–15% of BC cases [42]. The recently introduced HER2-low status is defined as an IHC assay score of 1+ or 2+ without amplification at in situ hybridisation (ISH) assay.
When HER2 plays its role as an oncogene, it is by means of gene overexpression and consequent HER2 easier heterodimerization [41]. Next, both cell transformation and tumorigenic growth are promoted [[43], [44], [45]]. Of note, HER2 somatic mutations have been described as well [46,47]. Typically, BC harbouring high expression of HER2 is associated with an aggressive clinical behaviour, both in terms of invasiveness and risk of recurrence, in absence of appropriate treatment [48].
A strong correlation between T cell–based immunity and reduction of cancer recurrence rates has been observed in HER2-positive BCs [49]. In particular, a 3% relative reduction risk of recurrence for each percentage unit increase in TILs has been reported in a study of 387 cases of HER2-positive BC patients [50]. In addition, the specificities of the interaction between HER2-positive tumour cells with potential ligands in the TME are thought to promote immune responses against HER2 protein [51]. Precisely, immune responses against HER2-positive BC are induced by both cytotoxic T lymphocytes (CD8+) and CD4+ T-cells [52]. Cytotoxic T lymphocytes recognise HER2 peptides presented by major histocompatibility complex (MHC) class I molecules and induce cell cycle arrest, apoptosis and an interferon gamma (IFN-γ)–mediated killing of tumour cells [53]. Finally, CD4+ T cells and anti-HER2 antibodies are associated with stronger anti-HER2 immunity response [54,55].
For these reasons, stimulation of anti-HER2 immune responses has been exploited to eliminate cancerous cells in HER2-positive BC [56,57]. An overview of the clinical trials currently investigating therapeutic vaccines targeting HER2-positive BC is provided in Table 1 .
Table 1.
Summary of ongoing trials on cancer vaccines enrolling HER2-positive breast cancer patients, as of 23rd June 2021.
| ID | Ph | Platform | Setting | # pts | Intervention | Patient cohort | Target/Moiety | Outcome |
|---|---|---|---|---|---|---|---|---|
| NCT02061423a | 1 | Cell-based | eBC | 7 | 6 HER2 pulsed dendritic cell vaccines injections followed by 3 booster vaccines | Stage I–III HER2-positive BC with residual disease post-neoadjuvant CT | HER2 | Safety (compliance, AEs) |
| NCT00880464a | 1 | Cell-based | eBC | 8 | Irradiated, autologous BC cells engineered by adenoviral mediated gene transfer to secrete GM-CSF | Stage II–III (any subtype) BC who have at least 2 cm of disease after neoadjuvant CT or 4 cm without neoadjuvant CT | GM-CSF release | Safety (DLT) |
| NCT03384914c | 2 | Cell-based and gene-based | eBC | 110 | 9 dendritic cells vaccine injections or 6 WOKVAC vaccine injections | Stage I–III HER2-positive BC treated with neoadjuvant CT without pCR | HER2 | Immune response |
| NCT02297698a | 2 | Peptide-based | eBC | 100 | 6 Nelipepimut-S vaccine injections followed by 4 booster vaccine injections + trastuzumab for one year | HLA-A2+/A3+/A24+/A26+ HER2+ BC at high risk of relapse (no pCR, >3 LNs, 1–3 LNs with ER-/PR-) | HER2 E75 (369–377) | 5-year iDFS |
| NCT04329065c | 2 | Gene-based | eBC | 16 | 3 polyepitope plasmid–based WOKVAC vaccine injections and 3 infusions of paclitaxel + trastuzumab and pertuzumab | Stage I–III ER-/HER2-positive BC before surgery | pUMVC3-IGFBP2-HER2-IGF1R | Immune response |
| NCT04197687c | 2 | Peptide-based | eBC | 480 | 6 TPIV100 vaccine injections + T-DM1 as maintenance therapy, followed by 2 booster vaccine injections | Stage II–III HER2-positive BC who did not achieve pCR after NACT | HER2 | 5-year iDFS |
| NCT00317603a | 1 | Cell-based | mBC | 20 | Irradiated, autologous BC cells engineered by adenoviral mediated gene transfer to secrete GM-CSF | Stage IV BC (any subtype) in II line of treatment | GM-CSF release | Safety (DLT) |
| NCT00393783a | 1 | Gene-based (xenogenic) | mBC | 12 | 5 HER2 ECD DNA vaccine injections ± ET ± trastuzumab (as per local practice) | Stage III–IV HER2-positive BC | HER2 (ECD) | Safety (DLT) |
| (BrEAsT) NCT04296942c |
1 | Viral-based | mBC | 65 | MVA-BN-Brachyury vaccine injections followed by boosters with FPV-BN-Brachyury until disease progression + M7824 + TDM-1 (for HER2+ BC) | Stage IV TNBC and ER-/HER2-positive | Brachyury | ORR |
| NCT00436254a | 1 | Gene-based | mBC | 66 | 3 pNGVL3-hICD vaccine injections | Stage III–IV HER2-positive BC | HER2 | Safety (AEs) Immune response |
| NCT04521764c | 1 | Viral-based | mBC | 33 | 4 MV-s-NAP administered intratumorally | Stage IV BC (any subtype) | Pylori neutrophil activating protein (NAP) | Safety (DLT) |
| NCT01376505c | 1 | Peptide-based | mBC | 100 | 3 MVF-HER-2 (597–626 and 266–296) vaccine injections followed by 6 booster shots | Stage IV HER2-positive BC | HER2 (597–626, 266–296) | Immune response Clinical benefit (tumour markers, RECIST criteria) |
| NCT03689192c | 1 | Peptide-based | mBC | 10 | ARG1 vaccine injections every third week for 45 weeks | Stage IV BC (any subtype) | Arginase-1 | Safety (AEs) |
| NCT04418219b | 1/2 | Cell-based | mBC | 42 | SV-BR-1-GM + cyclophosphamide, pembrolizumab, interferon-alpha-2b for 2 years | Stage IV BC (any subtype)d | GM-CSF release | Safety (AEs) ORR (RECIST 1.1) |
| NCT03632941c | 2 | Viral-based | mBC | 39 | 3 VRP-HER2 vaccine injections + pembrolizumab | ER-/PR-/HER2-positive mBC | HER2 | Immune response (TILs and anti-HER2 Abs) |
| NCT04348747b | 2 | Cell-based | mBC | 23 | 3 anti-HER2/HER3 DCs vaccine injections + celecoxib, recombinant INFalfa-2b, rintatolimod followed by pembrolizumab | TNBC or HER2-positive BC with brain metastasis | HER2/HER3 | CNS ORR |
| NCT03328026c | 2 | Cell-based + TME modulator | mBC | 60 | 4 SV-BR-1-GM + 24 INCMGA00012 vaccine injections ± epacadostat (twice daily) | Stage IV BC (any subtype) | GM-CSF release PD-L1 IDO |
Safety (AEs) |
| NCT00194714b | 2 | Peptide-based | mBC | 26 | 6 HER2 peptide vaccine + trastuzumab | HLA-A2+, HER2-positive stage IV BC | HER2 | Immune response Safety (AEs) |
| NCT02491697a | 2 | Cell-based | mBC | 400 | 4 cycles of DC-CIK treatment (every 1 year) + capecitabine (2500 mg/m2 twice daily for 2 weeks followed by a 1-week rest period q21) | Stage IV BC (any subtype) | CIK cells agonist | 1-year OS |
| NCT04246671c | 2 | Viral-based | mBC | 45 | 3 TAEK-VAC-HerBy vaccine injections + trastuzumab/pertuzumab/T-DM1/anti-PD-L1 (not disclosed) | Stage IV HER2-positive BC (ER+/−) | HER2/neu | Safety (DLT) |
| NCT00722228c | 2 | Cell-based | mBC | 50 | 5 modified autologous or allogenic tumour cells vaccine injections | Stage IV BC (any subtype) | NA | NA |
Abbreviations: ID, identifier; #, number; pts, patients; BC, breast cancer; eBC, early breast cancer; mBC, metastatic breast cancer; DLT, dose-limiting toxicity; HER2, human epidermal growth factor receptor 2; CT, chemotherapy; NACT, neoadjuvant chemotherapy; ET, endocrine therapy; HR, hormone receptor; TPC, treatment of physician's choice; DCIS, ductal carcinoma in situ; TNBC, triple-negative breast cancer; AEs, adverse events; SoC, standard of care; ORR, objective response rate; CNS, central nervous system; PFS, progression-free survival; DFS, disease-free survival; pCR, pathologic complete response; IV, intravenous; LNs, lymph nodes; DCs, dendritic cells; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA, human leukocyte antigen; iDFS, invasive disease-free survival; MVA-BN, Modified Vaccinia Ankara-Bavarian Nordic; T-DM1, trastuzumab emtasine; MVF, measles virus fusion; ARG1, Arginase 1; VRP, Virus-like replicon particles; CIK, cytokine-induced killer cells; PD-L1, programmed-death ligand 1; IDO, indoleamine-pyrrole 2,3-dioxygenase; TME, tumour microenvironment; INF, interferon alpha; ECD, extracellular domain; IGF1R, insulin-like growth factor 1 receptor; IGFBP2, insulin-like growth factor binding protein 2. Source: clinicaltrials.gov.
Active, not recruiting.
Not yet recruiting.
Recruiting.
This trial has been described in the section dedicated to clinical trials open to BC patients, irrespective of the biological subtype.
3.1. Peptide-based vaccines targeting HER2 in breast cancer
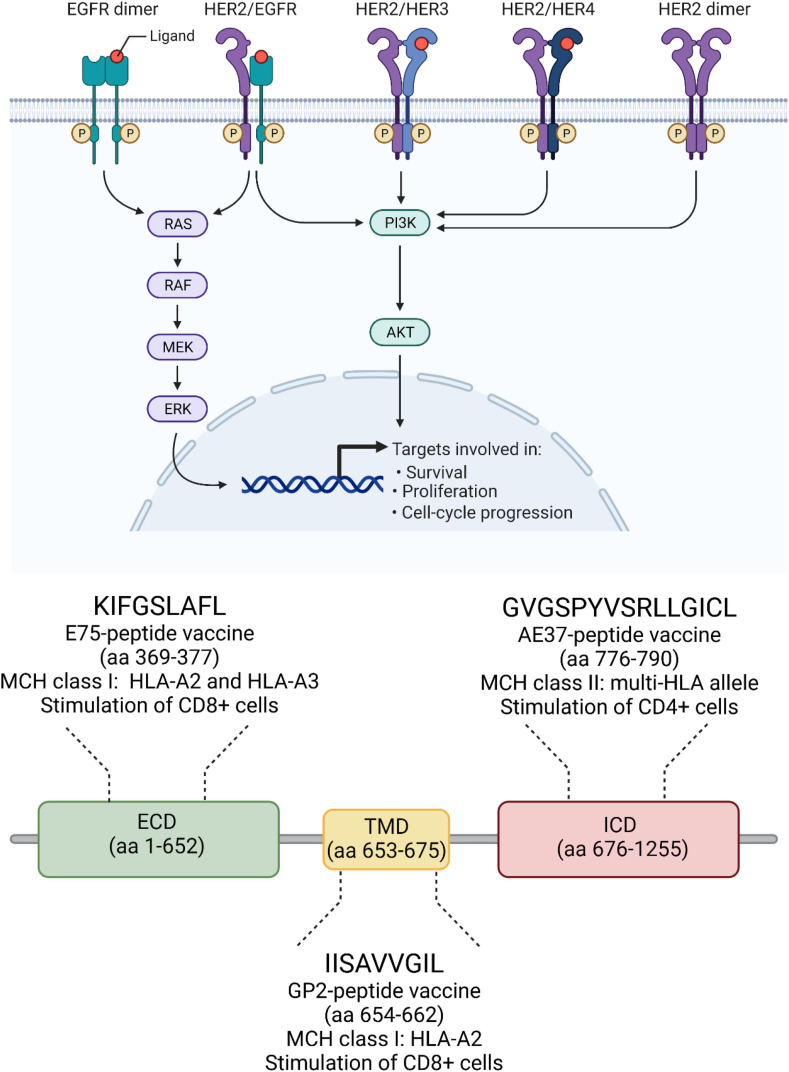
Immunogenic HER2-derived peptides include those derived from different parts of HER2 protein, specifically E75, from the extracellular domain (ECD); GP2, from the transmembrane domain (TMD); AE37, from the intracellular domain (ICD), as depicted in Fig. 3 [54].
Fig. 3.
Most used HER2-derived peptides for the development of therapeutic peptide-based cancer vaccines. Abbreviations: EGFR; epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; HER3, human epidermal growth factor receptor 3; HER4, human epidermal growth factor receptor 4; P, phosphate; RAF, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein kinase; ERK, extracellular signal–regulated kinases; PI3K, phosphoinositide 3 kinase; MHC, major histocompatibility complex; HLA, human leukocyte antigen; aa, amino acids; ECD, extracellular domain; TMD, transmembrane domain; ICD, intracellular domain. Created with biorender.com.
3.1.1. E75 (HER2/neu 369–377, KIFGSLAFL), Nelipepimut-S/NeuVax
The E75-based vaccination is the most widely investigated strategy for the treatment of BC patients [58]. E75 is a 9-amino-acid-long peptide from the ECD of the HER2 receptor and works as an immunodominant CTL epitope with high affinity for human leukocyte antigen HLA-A2 and HLA-A3 molecules (HLA-restricted) (Fig. 3) [21]. E75 is expressed in almost 60–75% of the population [58].
Although a meta-analysis of delayed-type hypersensitivity (DTH) reactions and CD8+ T cell immune responses confirmed significant differences between the vaccinated groups compared with their non-vaccinated counterparts in several phase I–II clinical trials, a phase III randomised controlled trial (PRESENT) in the adjuvant setting failed to demonstrate a clinical benefit [25]. Specifically, over 750 HLA-A2+ BC patients were randomised after standard treatment to receive NeuVax with granulocyte-macrophage colony-stimulating factor (GM-CSF) or GM-CSF alone, as the control arm. The PRESENT trial was terminated due to futility after an interim analysis conducted after 70 disease-free survival (DFS, primary end-point) events in the overall population; however, the study was also hampered by an important imbalance in the practice of image detection of metastatic relapse [25]. Interestingly, patients harbouring HER2-low BC had more robust immune responses after vaccination, in comparison with their HER2-high (i.e. HER2 3+ by IHC, or HER2 gene amplification assessed by fluorescence in situ hybridisation, FISH, test) counterparts [25]. These findings could reflect the presence of immunologic tolerance in HER2 over-expressing patients and suggest further clinical investigations tailored on the HER2-low subgroup [21].
Moreover, a potential synergistic effect between trastuzumab and E75 vaccine has been observed [59]. Consequently, a phase IIb, multicenter, randomised, single-blinded, controlled trial–enrolled disease-free BC patients after standard therapy completion for high-risk HER2-low BC [59]. Briefly, patients received trastuzumab for 1 year and were randomised to placebo GM-CSF as control or Nelipepimut-S with GM-CSF [59]. While in HER2 low-expressing BCs, no significant difference in DFS was seen in the intention-to-treat (ITT) analysis, significant clinical benefit was seen in patients expressing HLA-A24 (hazard ratio, HR = 0.41; p = 0.05) and in the TNBC subgroup (i.e. HR, DFS, 0.25; 95% CI: 0.08–0.81; p = 0.01) [59,60]. Based on the present data, Nelipepimut-S will be further evaluated in a phase III trial in combination with trastuzumab for the treatment of patients with TNBC in the adjuvant setting, after standard treatment [60,61].
3.1.2. GP2 (654–662, IISAVVGIL)
GP2 is a subdominant epitope with poor binding affinity to the HLA-A2 molecule (Fig. 3) [62]. A GP2-based CV has been investigated in a prospective, randomised, placebo-controlled, single-blinded, multicenter phase IIb basket trial. GP2+GM-CSF was administered in the adjuvant setting to node-positive and high-risk node-negative BC patients with tumours expressing any degree of HER2 [63]. Patients were randomised to receive six doses of vaccine (500 mcg GP2:125 mcg GM-CSF) or placebo (125 mcg GM-CSF alone), intradermally, every 3–4 weeks, as part of the primary immunisation series (PIS) for the first 6 months, then four GP2 + GM-CSF booster versus placebo intradermal injections every 6 months, thereafter [63]. Overall, 168 patients were included. Specifically, 96 HER2-positive patients received a standard course of trastuzumab after surgery and subsequently completed the full PIS or placebo; 72 HER2-low patients (IHC 1–2+ or absent HER2 gene amplification assessed by the FISH test) did not receive trastuzumab after surgery and subsequently completed the full PIS or placebo [63]. Of note, since GP2 is synergistic with trastuzumab, and the HER2-low patients did not receive trastuzumab, a comparison between recurrence rates in intention-to-treat (ITT) population (n = 180) versus per protocol analysis population was pre-specified. After a 5-year follow up, the DFS rate in 46 HER2 IHC 3+ BC fully vaccinated patients was 100% versus 89.4% in the 50 HER2 IHC 3+ patients who received placebo (95% CI: 76.2–95.5; p = 0.0338). In the 35 HER2 IHC 1–2+ BC fully vaccinated patients assessed, the 5-year DFS rate was 77.1% (95% CI: 59.5–87.9%) versus 77.6% in the 37 patients who received a placebo (95% CI: 60.1–88.2; p = 0.9142). As for the toxicity profile, the most common toxicities were mild local reactions (i.e. erythema, pruritis) and fatigue. Grade ≥3 accounted for only five events: induration and maculopapular rash/pruritis in two GP2+GM-CSF subjects; chest pain and hypersensitivity reaction in two GM-CSF-only patients [63]. In conclusion, the clinical trial demonstrated that completion of the GP2+GM-CSF PIS safely elicited an immune response and reduced recurrence rates to 0% in HER2 3+ patients, who received a standard course of trastuzumab after surgery [63]. Consequently, a pivotal phase III trial has been initiated to treat HER2 IHC 3+ patients in the (neo)adjuvant setting [63].
3.1.3. AE37 (HER2/Neu 776–790, GVGSPYVSRLLGICL)
AE37 is a HER2-directed vaccine designed on the AE36 hybrid peptide (aa 776–790), which is derived from the intracellular portion of the HER2 protein, and the core portion of the MHC class II invariant chain (the Ii-Key peptide, Fig. 3). This hybrid peptide and the GM-CSF immunoadjuvant constitute the AE37 vaccine and is able to induce both CD8+ and CD4+ cells both in vitro and in vivo [64]. A phase II trials testing AE37-based vaccine enrolled BC patients with node-positive or high-risk node-negative disease and tumours that presented with HER2 1–3+ on IHC, after completion of standard therapies [65]. In the final survival analysis, with a median follow-up of 59.8 months, no statistically significant difference in 5-year DFS, the primary end-point, was observed. Interestingly, in patients with advanced stage BC and HER2 low-expression, the 5-year estimated DFS in subjects treated with AE37 was 83% compared with 62.5% in the GM-CSF-only arm (HR = 0.375; CI: 0.142–0.988; p = 0.039). Similarly, in the subgroup with advanced stage TNBC, a trend toward improved DFS with vaccination was found (AE37 85.7% versus control 36.4%, HR = 0.184; CI: 0.022–1.510; p = 0.078) [65].
The growing body of evidence coupled with subset analyses of this phase II trial led to another phase II clinical trial investigating AE37 in combination with pembrolizumab (NSABP FB-14) in TNBC patients with metastatic disease (NCT04024800, Table 2 ).
Table 2.
Summary of ongoing trials on cancer vaccines enrolling TNBC patients, as of 23rd June 2021.
| ID | Ph | Platform | Setting | # of pts | Intervention | Patient cohort | Target/Moiety | Outcome |
|---|---|---|---|---|---|---|---|---|
| NCT02427581c | 1 | Peptide-based | eBC | 15 | single synthetic long peptide vaccine injections | TNBC at least cT2 with no pCR post-neoadjuvant CT | Neoantigens | Safety (AEs) |
| NCT00880464a | 1 | Cell-based | eBC | 8 | Irradiated, autologous BC cells engineered by adenoviral-mediated gene transfer to secrete GM-CSF | Stage II–III BC (any subtype) who have at least 2 cm of disease after neoadjuvant CT or 4 cm without neoadjuvant CTd | GM-CSF release | Safety (DLT) |
| NCT03199040c | 1 | Gene-based | eBC | 24 | 6 neoantigen DNA vaccine injections (+/− durvalumab) | cT1c-T4 TNBC with residual invasive BC after neoadjuvant CT | Polyepitope neoantigens | Safety (AEs) |
| NCT02780401a | 1 | Gene-based | eBC | 24 | 3 polyepitope plasmid–based WOKVAC vaccine injections | Stage I–III HER2-negative and node-positive BC after standard treatment | pUMVC3-IGFBP2-HER2-IGF1R | Safety (AEs) |
| NCT04105582a | 1 | Cell-based | eBC | 5 | NA | Non-metastatic TNBC following standard treatment | Tailored neoantigen synthetic peptide | Safety (AEs) |
| NCT02364492a | 1 | Peptide-based | eBC | 20 | 6 MAG-Tn3 vaccine injections | TNBC (any T/N) or ER+/HER2− (with at least one lymph node) pts following standard treatment | Tn carbohydrate antigen | Safety (DLT) |
| TNBC-MERIT NCT02316457a |
1 | Gene-based | eBC | 42 | 4 RNAs vaccine injections | Stage II–III TNBC following standard treatment | Neo-antigens | Safety (AEs) |
| NCT02826434a | 1b | Peptide-based | eBC | 22 | 6 PVX-410 vaccine injections and 2 infusions of durvalumab | Stage II–III HLA-A2+ TNBC following standard treatment | XBP1 CD138 |
Safety (DLT) |
| NCT02938442c | 1/2 | Peptide-based | eBC | 102 | 3 P10s-PADRE vaccine injections + SoC CT (AC + T) | Stage I–III TNBC who will undergo SoC neoadjuvant treatment | Carbohydrate mimetic peptide P10s | Safety Clinical response (pCR) |
| NCT02593227a | 2 | Peptide-based | eBC | 80 | +/− priming with cyclophosphamide IV, then 6 TPIV200 monthly vaccine injections followed by 6 booster injections | Stage I–III TNBC following standard treatment | Folate receptor alpha + GM-CSF adjuvant | Immune response |
| NCT00317603a | 1 | Cell-based | mBC | 20 | Irradiated, autologous BC cells engineered by adenoviral mediated gene transfer to secrete GM-CSF | Stage IV BC (any subtype) in II line of treatmentd | GM-CSF release | Safety (DLT) |
| NCT03674827c | 1 | Viral-based and gene-based | mBC | 36 | 4 PF-06936308 vaccine injections per cycle + tremelimumab, sasanlimab | Stage IV TNBC | Multi TAAs (not yet disclosed) | ORR (RECIST 1.1) |
| NCT04296942c | 1 | Viral-based | mBC | 65 | MVA-BN-Brachyury vaccine injections followed by boosters with FPV-BN-Brachyury until disease progression + M7824 + TDM-1 (for HER2+ BC) | Stage IV TNBC and ER-/HER2-positived | Brachyury | ORR |
| NCT04521764c | 1 | Viral-based | mBC | 33 | 4 MV-s-NAP administered intratumorally | Stage IV BC (any subtype)e | Pylori neutrophil–activating protein (NAP) | Safety (DLT) |
| NCT02432963a | 1 | Viral-based | mBC | 19 | 3 modified vaccinia virus Ankara vaccine expressing p53 injections and 7 infusions of Pembrolizumab | Stage IV TNBC | p53 | Safety (AEs) |
| NCT03689192c | 1 | Peptide-based | mBC | 10 | ARG1 vaccine injections every third week for 45 weeks | Stage IV BC (any subtype) | Arginase-1 | Safety (AEs) |
| NCT02157051c | 1 | Gene-based | mBC | 40 | 3/6/9 polyepitope plasmid DNA vaccine injections followed by 1 or 2 booster vaccine injections | Stage III–IV HER2-negative BC | CD105/Yb-1/SOX2/CDH3/MDM2 | Safety (AEs) Immune efficacy |
| NCT03362060a | 1b | Peptide-based | mBC | 20 | 8 PVX-410 vaccine injections followed by 2 booster doses + Pembrolizumab | HLA-A2+ mTNBC | XBP1 SLAMF7 CD138 |
Immune response (T-cell activation) |
| NCT04418219b | 1/2 | Cell-based | mBC | 42 | SV-BR-1-GM + cyclophosphamide, pembrolizumab, interferon-alpha-2b for 2 years | Stage IV BC (any subtype)e | GM-CSF release | Safety (AEs) ORR (RECIST 1.1) |
| NSABP FB-14 NCT04024800a |
2 | Peptide-based | mBC | 29 | 5 AE37 peptide vaccine injections + pembrolizumab | Stage IV TNBC with no more than one line of CT for mBCd | HER2 (776–790) | Safety (AEs) ORR (RECIST 1.1) |
| NCT04348747b | 2 | Cell-based | mBC | 23 | 3 anti-HER2/HER3 dendritic cell vaccine injections + celecoxib, recombinant interferon alfa-2b, rintatolimod followed by pembrolizumab | TNBC or HER2+ pts with brain metastasisd | HER2/HER3 | CNS ORR |
| NCT03606967c | 2 | Peptide-based | mBC | 70 | personalised synthetic long peptide vaccine + durvalumab + 4 infusions of tremelimumab + CT | Stage IV TNBC | Personalised synthetic long peptide vaccine | 12-month PFS |
| NCT03012100c | 2 | Peptide-based | eBC | 280 | Cyclophosphamide orally + 6 multiepitope folate receptor alpha vaccine (TPIV200) injections + booster injections | cT1-4 or N+ TNBC following standard treatment | Folate receptor alpha | 5-year DFS |
| NCT03328026c | 2 | Cell-based + TME modulator | mBC | 60 | 4 SV-BR-1-GM + 24 INCMGA00012 vaccine injections ± epacadostat (twice daily) | Stage IV BC (any subtype)e | GM-CSF release PD-L1 IDO |
Safety (AEs) |
| NCT04634747b | 2 | Peptide-based | mBC | 53 | PVX-410 vaccine injections + pembrolizumab + CT | HLA-A2+, PD-L1+ stage IV TNBC | XBP1 SLAMF7 CD138 |
PFS |
| NCT02491697a | 2 | Cell-based | mBC | 400 | 4 cycles of DC-CIK treatment (every 1 year) + capecitabine (2500 mg/m2 twice daily for 2 weeks followed by a 1-week rest period q21) | Stage IV BC (any subtype)e | Cytokine-induced killer cells agonist | 1-year OS |
| NCT03761914c | 2 | Peptide-based | mBC | 90 (15 TNBC) | 16 galinpepimut-S vaccine injections + Pembrolizumab | Stage IV TNBC after one line of therapy in metastatic setting | Wilms’ tumour (WT1) | Safety (AEs) ORR (RECIST 1.1) |
| NCT00722228c | 2 | Cell-based | mBC | 50 | 5 modified autologous or allogenic tumour cells vaccine injections | Stage IV BC (any subtype) | N/A | N/A |
| NCT03562637c | 3 | Peptide-based | eBC | 668 | 21 Adagloxad simolenin vaccine injections | Stage IIB–IIIC TNBC Globo-H–positive | Globo-H | 5-year iDFS |
Abbreviations: ID, identifier; ph, phase; #, number; mBC, metastatic breast cancer; eBC, early breast cancer; pCR, pathologic complete response; TNBC, triple-negative breast cancer; CT, chemotherapy, AE, adverse events; DLT, dose-limiting toxicity; GM-CSF, granulocyte-macrophage colony-stimulating factor; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; SoC, standard of care; IV, intravenous; AC, adriamycin and cyclophosphamide; T, paclitaxel; TAAs, tumour-associated antigens; ORR, objective response rate; CNS, central nervous system; PFS, progression-free survival; (i) DFS, (invasive) disease-free survival; XBP1, X-box–binding protein 1; SLAMF7, SLAM family member 7; SOX2, (sex-determining region Y)-box 2; CDH3, Cadherin-3; PD-L1, programmed-death ligand 1; IDO, indoleamine-pyrrole 2,3-dioxygenase; NSABP, National Surgical Adjuvant Breast and Bowel Project; WT1, Wilms' tumour protein; DC, dendritic cell; IGF1R, insulin-like growth factor 1 receptor; IGFBP2, insulin-like growth factor–binding protein 2. Source: ClinicalTrials.gov.
Active, not recruiting.
Not yet recruiting.
Recruiting.
This trial has already been described in the section dedicated to vaccines targeting HER2 in BC.
This trial has been described in the section dedicated to clinical trials open to BC patients, irrespective of the biological subtype.
3.1.4. TPIV100 and sargramostim
A phase II clinical trial is currently enrolling HER2-positive BC patients (stages II–III) with residual disease after neoadjuvant treatment (NCT04197687, Table 1). TPIV is a multi-epitope–based vaccine targeting HER2. Sargramostim (a recombinant GM-CSF) serves as adjuvant. Patients are randomised to receive standard-of-care (SoC) maintenance therapy with trastuzumab emtansine (T-DM1) and receive TPIV100/placebo and sargramostim every 21 days, for up to 6 cycles, in the absence of disease progression or unacceptable toxicity. After completion of T-DM1 maintenance therapy, patients receive two additional booster injections of TPIV100 and sargramostim at 3 and 12 months. The primary end-point of the study is invasive disease-free survival (iDFS), with an estimated enrolment of 480 patients and an estimated completion date in January 2025.
3.1.5. MVF-HER-2 (597-626) and MVF-HER-2 (266-296)
A phase I clinical trial is investigating safety and the optimal biological dose (OBD) for a combination of two chimeric trastuzumab-like (MVF-HER-2, aa 597–626) and pertuzumab-like (MVF-HER-2, aa 266–296) HER2 B-cell peptide-based vaccine emulsified in Montanide (ISA-720) and muramyldipeptide derivative (nor-MDP) as adjuvants in patients with metastatic or unresectable breast, ovarian and gastrointestinal cancer, progressive after one line of standard therapy. The estimated enrolment is of 100 patients and the estimated completion date is in December 2021 (NCT01376505, Table 1).
3.2. Whole protein-based vaccines targeting HER2 in breast cancer
Whole protein vaccines are not HLA restricted and include both HLA class I and II epitopes [57]. A vaccine made with the HER2 ICD protein in combination with GM-CSF was investigated in 25 patients with HER2-positive BC in different stages (II, III, IV) [57]. With a good safety profile, 89% of patients developed HER-2/neu ICD-specific T-cell immunity, and 82% of patients also developed HER-2/neu-specific immunoglobulin G antibody immunity [57]. Of note, although higher doses of vaccine were not associated with increased T-cell response, time to development of detectable HER2/neu-specific immunity was earlier for the high- versus low-dose vaccine group (p = 0.003) [57]. The vaccine elicited and promoted HER2-specific cellular immunity for 9–12 months [57].
Another whole protein-based vaccine candidate combines a truncated (aa 1–146) HER2 protein with cholesterylpullulan (CHP) [66]. In a clinical trial involving nine metastatic patients, four of which with BC, CHP-HER2 vaccine was administered three times, on a 2-week schedule, followed by a booster injection [66]. Specific anti-HER2 CD8+ and/or CD4+ responses were developed, with a good safety and tolerability profile [66]. However, seven patients experienced disease progression within a median of 5 months, and 5 patients died. The remaining two patients showed no disease progression over a period of 19 and 25 months, respectively [66]. No tumour regression was observed [66]. In the second part of the trial, nine patients were immunised with the vaccine alone followed by administration of the adjuvant GM-CSF or OK-432, a lyophilised mixture of group A Streptococcus pyogenes with antineoplastic activity, whereas six patients received CHP-HER2 plus GM-CSF from cycle one [67]. One hundred and forty-six HER2-specific durable IgG responses were detected in 14 patients, who were negative at baseline. Of note, the antibodies induced by the vaccine were not reactive with the HER2 antigen expressed on the cell surface in any of the patients.
The third whole protein-based HER2-targeting vaccine exploited a fusion protein, namely dHER2, consisting of the ECD of HER2 and a portion of the ICD, plus the immune stimulant AS15 [51]. Forty HER2-positive metastatic BC patients were vaccinated with dHER2 [51]. As for the safety profile, only grade I–II adverse events (AEs) were reported, such as myalgia, back pain and diarrhoea [51]. Twenty-five percent of patients produced anti-HER2 long-term immunity [68].
3.3. Cell-based vaccines targeting HER2 in breast cancer
Cell-based CVs are patient-specific and a safe approach for personalised vaccine development [21]. Autologous vaccines consist of tumour cell lysates obtained from a patient's own tumour antigens, subsequently exploited to develop an effective immune response [21]. Conversely, in allogeneic cell–based vaccines, an additional antigen, immune modulator or cytokine is combined with autologous tumour cells [21,69]. Overall, the major disadvantages of cell-based vaccines lie in the inherent poor immunogenicity of tumour cells and in the variability of production methods [21]. In addition, the presence of endogenous cellular antigens could elicit autoimmune reactions [70].
3.3.1. Lapuleucel-T (APC8024)
In a phase I study, 18 patients with metastatic HER2 overexpressing BC have been evaluated for the toxicity and the immune response induced by Lapuleucel-T (APC8024), an autologous active cellular immunotherapy (IO) [71]. This cell-based therapeutic vaccine consists of peripheral blood mononuclear cells (PBMCs), previously activated with a recombinant fusion protein containing the ICD and ECD of HER2, plus GM-CSF. The vaccine was well-tolerated and provided specific anti-HER2 cellular immune responses [71]. Of note, one patient experienced a partial response (PR) lasting 6 months, and three additional patients had stable disease (SD), lasting more than 1 year [71].
Currently, in two phase I clinical trials, other autologous BC cells secreting GM-CSF are being evaluated for activation of immune responses. In one study (NCT00317603, Table 1) the safety and biological activity of the vaccine is being evaluated also in HER2-positive metastatic BC patients previously treated with trastuzumab. In a second study (NCT00880464, Table 1), the same vaccine is being investigated in women with operable, stage II and III BC.
3.3.2. Allogeneic cell–based cancer vaccines
A 3 × 3 factorial dose-ranging phase I study investigated the use of cyclophosphamide, doxorubicin and an HER2-positive, allogeneic, GM-CSF-secreting tumour vaccine in 28 patients with metastatic BC [72]. This vaccine was made of two cell lines (T47D parent, HER2-low and SKBR3, HER2-high) which were genetically modified to secrete GM-CSF. Patients received three monthly immunisations, with a boost at 6–8 months from study entry. Primary end-points were safety and determination of the chemotherapy doses that maximise HER2-specific immunity. The vaccine was safe, and no dose limiting toxicity was observed. HER2-specific Th-dependent immunity was induced with vaccine alone or with low doses of chemotherapy agents [72]. Of note, the immunomodulatory activity of low-dose cyclophosphamide showed a narrow therapeutic window, with an optimal dose not exceeding 200 mg/m2 [72]. Such results suggested that low dose chemotherapy could break the immune tolerance and paved the way for further clinical trials (e.g. PERICLES, NCT03971045) [72]. A similar phase II trial (NCT00847171) assessed a GM-CSF secreting cell–based vaccine in combination with trastuzumab and cyclophosphamide in patients with high-risk or metastatic BC. No serious adverse effects were reported.
3.3.3. Dendritic cells (DCs)–based cancer vaccines
Dendritic cells regulate immune tolerance and are involved in initiation of anti-tumour effects, by presenting TAAs to T lymphocytes through the MHC pathway [73]. Moreover, DCs have crucial roles in controlling antibody-based responses as they can interact with CD4+ T cells and B cells, ultimately inducing specific humoral immunity [21,73]. Preclinical and clinical evidence support the ability of DCs to induce strong anti-tumour responses against BC cells [74]. For example, DCs-based CVs combined with chemotherapy are being investigated in a phase I study aiming at preventing disease recurrence in BC patients with residual disease, in the post-neoadjuvant setting (NCT02061423, Table 1). The estimated enrolment is of seven patients, and the estimated completion date is in December 2021.
Similarly, an upcoming phase II clinical trial will investigate the role of a DCs-based vaccine targeting HER2/HER3 in combination with pembrolizumab and a cytokine modulation regimen (rintatolimod, interferon alpha-2b and celecoxib) to treat brain metastases from HER2-positive and TNBC. The combined strategy may enhance the effectiveness of the ICI (NCT04348747, Table 1, Table 2). The primary end-point of the study is the best overall central nervous system response as per Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM). The estimated enrolment is of 23 patients, and the estimated completion date is in June 2024.
To date, although multiple approaches, such as selection of suitable DC subsets, adjuvants and different DC engineering methods, are being developed to improve the efficiency of DC-based vaccines, their clinical benefit is still limited [[75], [76], [77]].
3.4. Viral vector–based vaccines targeting HER2 in breast cancer
Viruses are naturally immunogenic, and their genetic material can be engineered to carry any transgenes to be expressed in the host cells. Several recombinant viruses are capable of infecting and expressing the transgene in immune cells like antigen-presenting cells [78].
For example, a multifunctional vaccine made from a modified lentivirus, loaded with two BC antigens, including alpha lactalbumin, and HER2, could directly target the resident DCs in preclinical models [79]. Single injections of the DC-targeted lentiviral vectors resulted in tumour self-antigen–specific cellular immunity and decreasing tumour growth in mice [79]. Moreover, certain viruses have naturally oncolytic properties or can be engineered to target tumour cells (oncolytic virotherapy). Oncolytic virotherapy has the additional advantage to elicit immune T-cell responses against viral antigens, as well as to tumour cell antigens, which seems to be long lasting [80].
3.4.1. VRP-HER2
A phase II clinical trial aims at elucidating the immunogenicity (number of TILs and anti-HER2 antibodies) of alphavirus-like replicon particles (VRPs)–containing self-amplifying replicon RNA-encoding HER2 (NCT03632941, Table 1). In the initial safety arm, the subjects receive the VRP-HER2 vaccinations plus pembrolizumab. If no dose limiting toxicity is observed, then subjects are randomised into three arms (VRP-HER2 alone versus pembrolizumab alone versus VRP-HER2 plus pembrolizumab). The combination treatment is scheduled as three-dose vaccine every 2 weeks with five administrations of pembrolizumab flat dose (200 mg every 3 weeks). The estimated enrolment is of 39 patients with an expected completion date in October 2022.
3.4.2. MVA-BN-brachyury/FPV-BN-brachyury (BrEAsT)
This phase 1b clinical trial is assessing safety and activity of two different viral vector–based vaccines in patients with HER2-positive and metastatic TNBC (NCT04296942, Table 1). The priming vaccine is called modified vaccine Ankara–Bavarian Nordic brachyury, whereas the boosting vaccine is called fowlpox virus brachyury. The participants are assigned to three different arms. Arm I of the trial receives M7824, an investigational bifunctional antibody that targets transforming growth factor beta (TGF-β) and programmed–death ligand 1 (PD-L1), and the viral vector–based vaccines alone; arm II receives M7824, T-DM1 and the viral vector–based vaccines; arm III of the trial is treated with M7824, T-DM1, Entinostat and the viral vector–based vaccines. Treatment is continued until disease progression or unacceptable toxicity. The estimated enrolment is of 65 patients with an expected completion date in January 2024.
3.5. Gene-based vaccines targeting HER2 in breast cancer
Typically, in gene-based vaccines, the deoxyribonucleic acid (DNA) encoding the tumour antigen is carried by a plasmid which is injected into the host. This kind of vaccine can be used to stimulate both an antigen-specific adoptive and a non-specific innate immunity [81]. This strategy is considered as one of the most practical ways for cancer IO because of its simplicity, safety and cost effectiveness [82]. Many vaccine studies have already provided evidence for the efficacy of HER2 DNA-based vaccines in the prevention of tumour development in HER2 transgenic mice and transplantable tumour models [82,83].
The first gene-based CV investigated in a clinical trial was constituted of a DNA plasmid encoding the total length of HER2 protein [84]. Eight patients with advanced/metastatic HER2-positive BC on treatment with trastuzumab received the gene-based vaccine with low doses of GM-CSF and interleukin-2. Six patients completed the three vaccination cycles, while the other two patients only received one dose of the vaccine due to clinical complications or disease progression. The vaccine was safe and well-tolerated. Specific anti-HER2 T cells as well as antibodies significantly increased after vaccination; long-lasting cellular and humoral immunity were observed in some patients [84]. The median survival was 76 months [21,84]. In another phase I clinical study, the safety and immunogenicity of a gene-based vaccine with a dual component, namely V930 and V932, was investigated (NCT00250419 and NCT00647114) [85]. Precisely, V930 vaccine consisted of two plasmids expressing the ECD and TMD of HER2 and CEA fused to the B-subunit of Escherichia coli heat labile toxin (LTB) [85]. V932 exploited an adenoviral vector encoding the CEA-B-subunit fusion and the truncated HER2. The study enrolled patients with different solid tumours, including BC (stages II, III or IV), with safety and tolerability as primary end-points [85]. V930 vaccination with electroporation alone or in combination with V932 was well-tolerated without any serious AEs. No measurable cell-mediated immune response to CEA or HER2 was detected in patients by enzyme-linked immunospot (ELISPOT). However, a significant increase of both cell-mediated immunity and antibody titre against the bacterial LTB were observed [85].
3.5.1. Xenogeneic HER2/Neu DNA-based vaccine
A phase I clinical trial is investigating a gene-based CV which contains a plasmid carrying a rat-derived HER2-encoding gene. The trial enrolled patients with stage III–IV HER2-positive BC. The gene-based vaccine is administered intramuscularly at four different dose levels (0.5 mg, 1 mg, 3 mg, or 6 mg) during weeks 1, 4, 7, 10 and 13, for five injections. The primary end-point is dose-limiting toxicity (DLT). The estimated enrolment is of 12 patients, and the projected completion date was in May 2021 (NCT00393783, Table 1).
3.5.2. pNGVL3-hICD, plasmid-based vaccine
Another phase I clinical trial is assessing the safety, tolerability and immunogenicity of a pNGVL3-hICD plasmid-based vaccine, containing genetic information about the ICD of HER2 (NCT00436254, Table 1). The trial was open to 66 patients either with stage III–IV HER2-positive BC or with stage III–IV ovarian cancer, with metastases in remission. The enrolled subjects received the pNGVL3-hICD vaccine combined with GM-CSF intradermally once a month for 3 months, in the absence of disease progression or unacceptable toxicity. The vaccine was administered at the three different doses of 10 μg, 100 μg and 500 μg. At a median follow-up of 68 months after vaccination, patients who received the intermediate dosing schedule (100 μg) demonstrated the best overall survival (OS, p = 0.02), as compared with the other two dosing schedules, with a good safety profile [86].
3.5.3. WOKVAC (pUMVC3-IGFBP2-HER2-IGF1R), plasmid-based vaccine
After a promising phase I dose escalation study, a multicenter, two-arms, phase II clinical trial is currently investigating the safety of DC1 and WOKVAC vaccines, to prevent disease recurrence in clinical stage II–III HER2-positive BC treated with neoadjuvant chemotherapy and HER2-targeted therapies and who did not achieve pCR (NCT03384914, Table 1) [87]. DC1 is a DC-based vaccine, whereas WOKVAC (also pUMVC3-IGFBP2-HER2-IGF1R) is a plasmid-based DNA vaccine which contains expression vector pUMVC3 encoding the three epitopes insulin-like growth factor–binding protein 2 (IGFBP2), HER2 and insulin-like growth factor receptor-1 (IGF1R) that are overexpressed in BC as TAAs. In accordance with the vaccination schedule, patients will receive the first of 3 booster vaccines, approximately 6 months after the first vaccine. The primary end-point of the study is immunogenicity assessed by ELISPOT assay for six distinct peptides. Secondary end-point of the study is DFS. The estimated enrolment is of 110 patients, and the projected completion date is in March 2023.
WOKVAC is being investigated also in a phase II clinical trial that combines the BC vaccine with paclitaxel, trastuzumab, and pertuzumab in the neoadjuvant setting (NCT04329065, Table 1). WOKVAC is administered intradermally on day 13 and treatment repeats for up to 3 cycles, in the absence of disease progression or unacceptable toxicity. The primary end-point is immunogenicity, that is the number of T-bet+, CD4+, and CD8+ T cells in TILs after combination immune-chemotherapy. The likely enrolment is of 16 patients, and the estimated completion date is in June 2026.
4. Vaccines targeting triple-negative breast cancer
With almost 200.000 cases each year, TNBC accounts for approximately 15% of BCs diagnosed worldwide [1,88]. Besides the typical aggressiveness of TNBC, its poor prognosis lies also in the scarcity of predictive biomarkers of treatment response [41]. However, the recent advances in IO for TNBC allowed for a rapid surge of different strategies to enhance its efficacy [29]. In particular, besides ICIs and combination treatment with chemotherapy, several CVs are currently investigated to heat-up the TME against cancer cells, as shown in Table 2 [24].
4.1. Peptide-based vaccines for triple-negative breast cancer
4.1.1. PVX-410 (PVX, OncoPep)
Two phase Ib and a phase II clinical trials are investigating the role of this vaccine in TNBC in the early (stage II–III) and metastatic setting. PVX-410 is a novel tetra-peptide HLA-A2-restricted vaccine constituted by three of the four antigens most commonly overexpressed in TNBC, namely X-box–binding protein 1, with two splice variants, syndecan-1 (CD138) and cell surface glycoprotein SLAM family member 7 (SLAMF7 or CD319). X-box–binding protein 1 has been found to drive relapse and progression in TNBC by controlling the hypoxia-inducing factor 1α [[88], [89], [90]]. Syndecan-1 promotes cell growth and is associated with epithelial–mesenchymal transition (EMT) [91]. SLAMF7 is a natural killer (NK) cell receptor, which results upregulated in primary TNBCs, although its messenger ribonucleid acid (mRNA) expression has been found decreased in metastatic lesions [92].
In the early setting, the phase Ib multi-centre, single-arm clinical trial included HLA-A2–positive patients with TNBC at least 1 cm in size or with positive loco-regional lymph nodes [93]. Of note, patients with local-regional recurrence without evidence of distant metastases were also eligible as long as they had not received treatment for the metastatic setting [93]. Patients received subcutaneous injections of PVX-410, emulsified in Montanide and co-administered with polyinosinic-polycytidylic acid (Poly-ICLC, Hiltonol), an intramuscular immunostimulant. The vaccination was planned on a 2-week schedule, and two doses of durvalumab 1500 mg were concurrently administered during the 4th and 6th vaccine treatments, respectively. After a run-in phase with six patients enrolled to assess for DLT, 14 further patients constituted the expansion phase. The primary objectives were safety and tolerability. A key secondary objective was PVX-410 specific immune response, defined at week 14 as a two-fold or greater change over baseline in the proportion of CD3+ CD8+ T cells expressing IFNγ and the proportion of CD3+ CD8+ T cells positive for PVX tetramers, after an in vitro stimulation of PBMC with PVX peptides and a flow cytometric determination. Among a total of 22 patients enrolled, the median age was aged 48 years, and the majority of the study population had a stage II disease (N = 14 patients, 64%). No DLTs were observed in the run-in phase. The most common any-grade AEs were injection site reaction (96%), flu-like symptoms (41%), fatigue (41%) and arthralgias (36%). Twenty patients were evaluable for immune response at week 14. At the time of the analysis, 12 patients had a complete immune-response assessment. Ten of these 12 patients showed a PVX-410–specific immune response, that persisted in all patients tested at 6 months. With a median follow-up of 15.4 months at the time of data cut-off, four of 22 (18%) patients had a local [1] or metastatic [3] recurrence and two patients (9%) have died. Updated results are expected in December 2021 (NCT02826434, Table 2).
In the metastatic setting, PVX-410 was first investigated in a phase Ib clinical trial in combination with pembrolizumab. The clinical trial started in 2017 with a planned enrolment of 22 patients with HLA-A2–positive TNBC, in the second-line treatment for metastatic disease or beyond. The primary end-point is immune response as assessed by the fold activation of T cells from patient blood at week 10, compared to baseline. The estimated completion date is 31st December 2022 (NCT03362060, Table 2). A second clinical trial, starting in 2021, will assess PVX-410 in HLA-A2–positive metastatic TNBC (NCT04634747, Table 2). The vaccine will be administered in combination with pembrolizumab and chemotherapy, with progression-free survival (PFS) as primary end-point. The estimated enrolment is of 53 patients and the projected completion date is in April 2025.
4.1.2. P10s-PADRE
P10s-PADRE is a peptide-based vaccine, containing a carbohydrate mimetic peptide P10s, fused to the pan-HLA-DR–binding epitope (PADRE) peptide, with immunoadjuvant activity and potential antineoplastic activity [94]. It is usually administered with the Montanide ISA 51 VG adjuvant. The P10s peptide mimics gangliosides and other TACAs. Upon injection, it both stimulates a CTL response towards TAAs-expressing cells and induces the production of antibodies that are reactive with a broad set of TACAs. In addition, the anti-TACA antibodies may interfere with cellular pathways involved in tumour cell survival and may induce antibody-dependent cellular cytotoxicity [94]. This vaccine has been tested in a randomised two-arm, open-label, multi-center phase II clinical trial in the early setting for TNBC (NCT02938442, Table 2). Patients were randomised to receive either only standard dose-dense neoadjuvant chemotherapy (doxorubicin, cyclophosphamide and paclitaxel) or neoadjuvant chemotherapy plus the vaccine. The vaccine is injected on weeks 1, 2 and 3, prior to chemotherapy. Primary end-points are safety, tolerability and clinical response as assessed by pCR. The estimated enrolment is of 102 participants, and the projected completion date is in November 2024.
4.1.3. TPIV200
Metastatic lesions of TNBC are characterised by an overexpression (up to 86% of patients) of the TAA folate–receptor alpha (FRα) [95]. Because FRα is poorly expressed in healthy tissues, the target has been selected for the development of a peptide-based CV. TPIV200 is a penta-epitope vaccine, carrying five fragments of the FRα, able to elicit both a CD4+ and a CD8+ immune response. A phase I trial first investigated TPIV200 in BC (stage II–III), ovarian (stage II–IV) and other gynaecological cancers (NCT01606241). Twenty-two were treated with cyclophosphamide (days 1–7 and 15–22 of 28, for one cycle). Subsequently, the TPIV200 was administered intradermally at three sites with a mixture of the five FRα peptides on day 1 of a 28-day cycle for a maximum of six vaccination cycles. FRα-specific T cell responses were observed in 20 of 21 patients with immune response data. The vaccine showed a good safety profile with lymphopenia, neutropenia and injection site reactions as the most severe AEs (grade 2).
A randomised four-arm phase II clinical trial investigated TPIV200 in patients with early TNBC. Patients were randomised to receive either 165 μg or 500 μg of TPIV200 with or without cyclophosphamide [96]. Eighty patients with stage I–III TNBC were planned for enrolment (NCT02593227, Table 2). The vaccine was administered on a monthly basis for six months, then a booster was dispensed every six months, for a total of three years of treatment or until disease recurrence. Although results of the study are expected in the second half of 2021, a double-blind efficacy study has already started (NCT03012100, Table 2), with an enrolment plan of 280 patients and an estimated completion date is in July 2026.
4.1.4. Tumour-specific mutant antigens (TSMA)–based synthetic long peptide vaccine
A phase II clinical trial is investigating the role of a peptide-based vaccine targeting TNBC neoantigens in the metastatic setting (NCT03606967, Table 2). In one arm, the vaccine is administered in combination with chemotherapy, durvalumab and tremelimumab while in the other arm the patients receive the triplet therapy with a placebo instead of the vaccine. The latter is administered in combination with a poly-ICLC on days 1, 4, 8, 15, 22, 50, and 78 until disease progression on unacceptable toxicity. The primary end-point is PFS, with an estimated enrolment of 70 participants and an estimated study completion date in December 2021.
4.1.5. Galinpepimut-S
After initial assessments in haematological malignancies, Galinpepimut-S is being investigated in TNBC in an open-label, non-comparative, multicenter, multi-arm phase I/II clinical trial in combination with pembrolizumab (NCT03761914, Table 2) [97]. Galinpepimut-S is an investigational peptide-based vaccine constituted by the four peptide chains of Wilms' tumour gene 1 (WT-1) protein. Specifically, the vaccine is comprised of one WT1-derived peptide (WT-A1), possibly stimulating CD8+ T cells; two WT1 peptides (WT1-427 long, WT1-331 long), that may stimulate CD4+ T-cell responses, and one modified peptide (WT1-122A1 long) that may stimulate both CD4+ and CD8+ T cells and a targeted innate immune response against WT1-expressing tumour cells [98]. WT1, a zinc-finger DNA-binding protein and transcription factor, is overexpressed in leukaemic cells and in many non-haematological solid tumours [98].
In the clinical trial, Galinpepimut-S is administered as second-line treatment every three weeks, co-administered with pembrolizumab every three weeks for four times. Subsequently, pembrolizumab is administered either alone or in combination with the vaccine in accordance with the study schedule. After 84 weeks, non-progressors will continue in the study on pembrolizumab alone. The primary end-points are safety and ORR. The estimated enrolment is of 90 participants, and the projected study completion date in April 2024.
4.1.6. Adagloxad simolenin (GLORIA study)
Globohexaosylceramide (Globo-H) is overexpressed in a variety of epithelial cancer cell types including human pancreatic, gastric, lung, colorectal, oesophageal, and BC [99]. Adagloxad simolenin (AS) is constituted by the TAA Globo-H, linked to the carrier protein keyhole limpet haemocyanin (KLH). The KLH provides antigenic immune recognition and T-cell responses. AS is co-administered with a saponin-based adjuvant OBI-821 to induce a humoral response [100]. After a phase II trial showed a trend for superior PFS for Adagloxad simolenin (AS)/OBI-821 compared to placebo in patients with Globo-H-high BC, the GLORIA study was initiated [101]. It is a phase III, randomised, open-label study investigating the peptide-based vaccine AS/OBI-821 in the adjuvant treatment of patients with high-risk, early-stage Globo-H–positive TNBC (NCT03562637, Table 2). In particular, eligible patients must have either ≥1 cm residual invasive local disease or at least one positive lymph node after neoadjuvant chemotherapy or ≥4 axillary lymph nodes after adjuvant chemotherapy [100]. Patients will receive either subcutaneous injection of AS (30 μg) with OBI-821 (100 μg) or a volume-matched placebo. Up to 21 doses of vaccine or placebo will be administered over 100 weeks with a schedule as per study protocol, provided that no disease recurrence or unacceptable toxicity occur [100]. The primary end-point is iDFS, with quality of life and OS as secondary outcome measures [100]. The planned enrolment is of 668 participants, with a study completion date estimated in December 2027.
4.2. Viral vector–based vaccines for triple-negative breast cancer
4.2.1. p53MVA
Mutations in TP53 gene are present in a majority of solid tumours, resulting in the accumulation of oncogenic and potentially immunogenic p53 protein product within tumour cells [102]. A MVA virus has been engineered to express wild-type TP53 transgene (p53MVA) as an immunotherapeutic strategy. Robust p53-specific CD8+ T-cell responses were observed, and they were further enhanced by anti–PD-1 treatment, in preclinical models [103]. Consequently, a phase I clinical trial was designed to evaluate the safety and tolerability profiles of the p53MVA vaccine in combination with pembrolizumab. Patients with different solid tumours, including TNBC, failing standard treatment were eligible, provided that p53 involvement was confirmed by IHC or by mutational analysis [103]. A 3-at-risk rolling design was used as patients received 5.6 × 108 plaque-forming unit (PFU) p53MVA for three doses in combination with 200 mg pembrolizumab for seven doses every 3 weeks [103]. The preliminary results showed clinical benefit associated with durable p53-specific CD8+ T-cell responses, especially in two cases of TNBC and head and neck squamous cell carcinoma [103]. In particular, a TNBC patient had complete regression of cutaneous metastases as well as stable disease for >6 months. However, four patients (pancreatic ductal adenocarcinoma, hepatocellular carcinoma and two TNBC) had rapidly progressive disease. Preliminarily, the viral vaccine p53MVA in combination with the PD-1 blockade represents a novel immunotherapeutic approach capable of stimulating systemic immune responses and associated clinical benefit. The study completion date is estimated in December 2021 (NCT02432963, Table 2).
4.3. Gene-based vaccines for triple-negative breast cancer
4.3.1. Neoantigen vaccine with durvalumab
A single institution, open-label randomised phase I trial is testing a neoantigen gene-based vaccine in stage II–III TNBC patients. After SoC therapy, patients are randomised to receive either the personalised neoantigen vaccine alone, or the vaccine plus durvalumab, in accordance with a defined schedule. In patients randomised to the neoantigen vaccine plus durvalumab arm, the neoantigen-specific T-cell response will be assessed prior to Day 85. If a neoantigen-specific T cell response is present, durvalumab 1500 mg will be started on Day 85, and will be administered every four weeks. The primary outcome of the study is the safety of the vaccine given alone or in combination with durvalumab (number of AEs). Immunogenicity will be assessed via different assays as a secondary outcome measure. The estimated enrolment is of 24 participants, with a study completion date foreseen in December 2023 (NCT03199040, Table 2).
4.3.2. IVAC_W_bre1_uID and IVAC_M_uID vaccines
The Mutanome Engineered RNA Immuno-Therapy (TNBC-MERIT) is a three-arm phase I clinical trial investigating a personalised RNA-based vaccine in TNBC patients. Two main strategies have been developed for this trial. First, a ready-to-use, off-the-shelf RNA-based vaccine targeting TAAs (IVAC_W_bre1_uID); second, a personalised vaccine constituted by on-demand RNA manufacturing to target up to 20 individual tumour neo-antigens derived from mutated epitopes (IVAC_M_uID). Tumour-specific mutations are identified by next-generation sequencing (NGS). Besides this novel platform, liposomes will be used as vaccine vehicles.
Patients in arm 1 and 3 receive treatment with four TAAs-targeting RNAs, plus a p53 RNA. A selection process of RNAs from different TAAs is based on reverse transcriptase-polymerase chain reaction (RT-PCR) profiling of RNA extracted from patient's tumour sample specimens. Predefined cut-offs and algorithms have been implemented to select the three most relevant RNAs to each patient. The immunostimulant RBLTet.1 is added in arm 3. Finally, patients enrolled in arm 2 optionally receive both the TAAs-targeting RNA-based vaccine (IVAC_W_bre1_uID) and a personalised TSA-targeting RNA-based vaccine (IVAC_M_uID). The mutation selection is carried out through identification of tumour-specific somatic mutation by NGS, mutation confirmation, prioritisation, and on-demand manufacturing. The primary outcomes of the study are safety and tolerability of the vaccine. Changes of induced T-cell responses will be assessed as secondary outcomes. The planned enrolment is of 42 participants, with a study completion date estimated in December 2023 (NCT02316457, Table 2).
4.3.3. STEMVAC
A phase I dose-escalation clinical trial is investigating the role of DNA plasmid-based vaccine in stage III–IV HER2-negative BC, irrespective of HR status (both TNBC and HR-positive HER2-negative subtypes). This vaccine platform is a CD105/Y-box–binding protein 1(Yb-1)/sex-determining region Y-box 2 (SOX2)/cadherin 3 (CDH3)/mouse double minute 2 homolog (MDM2)-polyepitope plasmid that targets immunogenic TAAs associated to breast cancer stem cells (BCSC). Preliminary data demonstrated an increased humoral immune response against eight BCSC proteins in advanced BC and suggested an association between autoantibodies and a more aggressive disease [104].
The vaccine is administered with recombinant GM-CSF (rhuGM-CSF) every 28 days for 3 months. Patients may also receive two additional boosters of STEMVAC vaccines at 3 and 9 months after the third vaccine, in the absence of unacceptable toxicity or disease progression. After completion of study treatment, patients are followed up twice yearly for up to 5 years. The primary outcomes of the study are safety and immunologic efficacy, defined as achievement of a statistically significant increase in Th1 cell immunity for at least 50% of the immunising antigens, as compared to baseline. The estimated enrolment is of 40 participants, with a study completion date likely in February 2027 (NCT02157051, Table 2).
5. Vaccines targeting HR-positive breast cancer
HR-positive BC is a well-known less immunogenic subtype in comparison with TNBC and HER2-positive BC [88]. Generally, HR-positive BC establishes a TME devoid of TILs, have low HLA class I expression, and recruit immune cells, other than T cells, which impact response to therapy [4]. This phenomenon could be partially due a crosstalk between the hormone receptor–related pathways and the immune checkpoint pathways [105]. In this context, CVs could represent either a means to convert a cold TME into a hot one or a new strategy to enhance immunosurveillance against possible micrometastases in the early setting [24]. A summary of the ongoing clinical trials investigating CVs, specifically in HR-positive BC is provided in Table 3 .
Table 3.
Summary of ongoing trials on cancer vaccines enrolling patients with HR-positive BC, as of 23rd June 2021.
| ID | Ph | Platform | Setting | # of pts | Intervention | Patient cohort | Target/Moiety | Outcome |
|---|---|---|---|---|---|---|---|---|
| NCT04270149c | 1 | Peptide-based | eBC | 18 | 6 ESR1 peptides vaccine injections | HLA-A0201+ with at least pT3 (any N) ER+ (any HER2) BC following standard treatment | ESR1 | Safety (AEs) |
| NCT00880464a | 1 | Cell-based | eBC | 8 | Irradiated, autologous BC cells engineered by adenoviral mediated gene transfer to secrete GM-CSF | Stage II–III (any subtype) who have at least 2 cm of disease after neoadjuvant CT or 4 cm without neoadjuvant CTd | GM-CSF release | Safety (DLT) |
| NCT02204098c | 1 | Gene-based | eBC | 56 | 3 Mammaglobin-A DNA vaccine injections + CT/ET | cT2-T4 (any N) ER+/HER2-negative BC undergoing neoadjuvant therapy | Mammaglobin-A | Safety (AEs) |
| NCT02780401a | 1 | Gene-based | eBC | 24 | 3 polyepitope plasmid–based WOKVAC vaccine injections | Stage I–III HER2-negative and node-positive BC after standard treatment | pUMVC3-IGFBP2-HER2-IGF1R | Safety (AEs) |
| NCT02364492a | 1 | Peptide-based | eBC | 20 | 6 MAG-Tn3 vaccine injections | TNBC (any T/N) or ER+/HER2- (with at least one lymph node), following standard treatment | Tn carbohydrate antigen | Safety (DLT) |
| NCT02229084c | 2 | Peptide-based | eBC | 61 | 3 P10s-PADRE injections + neoadjuvant CT with different schedules | Stage I–III ER+/HER2-negative BC who undergo neoadjuvant CT | Carbohydrate mimetic peptide P10s | Safety Immune response Clinical response (pCR) |
| NCT03804944c | 2 | Peptide-based | eBC | 100 | CDX-301 + pembrolizumab + radiotherapy | Post-menopausal stage II–III ER+/HER2− BC before surgery | Ftl-3 (binds to CD135) | Safety (AEs) Clinical response rate pCR |
| NCT02157051c | 1 | Gene-based | mBC | 40 | 3/6/9 polyepitope plasmid DNA vaccine injections followed by 1 or 2 booster vaccine injections | Stage III–IV HER2-negative BC | CD105/Yb-1/SOX2/CDH3/MDM2 | Safety (AEs) Immune efficacy |
| NCT00317603a | 1 | Cell-based | mBC | 20 | Irradiated, autologous BC cells engineered by adenoviral mediated gene transfer to secrete GM-CSF | Stage IV BC (any subtype) in II line of treatmentd | GM-CSF | Safety (DLT) |
| NCT04521764c | 1 | Viral-based | mBC | 33 | 4 MV-s-NAP administered intratumorally | Stage IV BC (any subtype)e | Pylori NAP | Safety (DLT) |
| NCT03689192c | 1 | Peptide-based | mBC | 10 | ARG1 vaccine injections every third week for 45 weeks | Stage IV BC (any subtype) | Arginase-1 | Safety (AEs) |
| NCT04418219b | 1/2 | Cell-based | mBC | 42 | SV-BR-1-GM + cyclophosphamide, pembrolizumab, interferon-alpha-2b for 2 years | Stage IV BC (any subtype)e | GM-CSF release | Safety (AEs) ORR (RECIST 1.1) |
| NCT03328026c | 2 | Cell-based + TME modulator | mBC | 60 | 4 SV-BR-1-GM + 24 INCMGA00012 vaccine injections ± epacadostat (twice daily) | Stage IV BC (any subtype)e | GM-CSF release PD-L1 IDO |
Safety (AEs) |
| NCT02491697a | 2 | Cell-based | mBC | 400 | 4 cycles of DC-CIK treatment (every 1 year) + capecitabine (2500 mg/m2 twice daily for 2 weeks followed by a 1-week rest period q21) | Stage IV BC (any subtype)e | Cytokine-induced killer cells agonist | 1-year OS |
| NCT00722228c | 2 | Cell-based | mBC | 50 | 5 modified autologous or allogenic tumour cells vaccine injections | Stage IV BC (any subtype) | N/A | N/A |
Abbreviations: ID, identifier; ph, phase; #, number; mBC, metastatic breast cancer; eBC, early breast cancer; pCR, pathologic complete response; OS, overall survival; TNBC, triple-negative breast cancer; AE, adverse events; DLT, dose-limiting toxicity; GM-CSF, granulocyte-macrophage colony-stimulating factor; DNA, deoxyribonucleic acid; CT, chemotherapy; ET, endocrine therapy; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; SoC, standard of care; IGF1R, insulin-like growth factor receptor 1; IV, intravenous; ORR, objective response rate; XBP1, X-box–binding protein 1; SLAMF7, SLAM family member 7; SOX2, (sex determining region Y)-box 2; CDH3, cadherin-3; PD-L1, programmed-death ligand 1; IDO, indoleamine-pyrrole 2,3-dioxygenase; WT1, Wilms' tumour protein; DC, dendritic cell; CIK, cytokine-induced killer; NAP, neutrophil-activating protein; IGF1R, insulin-like growth factor 1 receptor; IGFBP2, insulin-like growth factor–binding protein 2. Source: ClinicalTrials.gov.
Active, not recruiting.
Not yet recruiting.
Recruiting.
This trial has already been described in the section dedicated to vaccines targeting HER2 in breast cancer.
This trial has been described in the section dedicated to clinical trials open to BC patients, irrespective of the biological subtype.
5.1. Peptide-based vaccines for HR-positive breast cancer
5.1.1. ESR1 peptide vaccine
Mutations in the oestrogen receptor gene (ESR1) are frequently acquired in metastatic HR-positive BC [106]. A phase I clinical trial is investigating a peptide-based vaccine aiming at inducing ESR mutant–specific memory T cells in early BC. In particular, the oestrogen receptor–derived peptides used in this vaccine are combined with the adjuvant GM-CSF to enhance immune response. The primary outcome of the study is the safety profile of the IO. The proposed enrolment is of 18 participants, with a study completion date estimated in August 2024 (NCT04270149, Table 3).
5.1.2. Magtrivacsein (MAG-Tn3)
Tumorigenesis is often associated with aberrant glycosylation processes that lead to the expression of new carbohydrate antigens at the surface of tumour cells. In particular, Tn is a mucin carbohydrate antigen, highly expressed in many carcinomas, especially in BC. MAG-Tn3 is a CV based on three consecutive Tn moieties that are linked to a CD4+ T-cell epitope, to induce anti-Tn antibodies. In animal models, the MAG-Tn3 vaccine was able to induce anti-Tn antibody responses, which targeted Tn-expressing tumour cells and mediated tumour cell death both in vitro and in vivo [107].
A three dose-level first-in-human, open-label, non-randomised, dose-escalation phase I study assessed the vaccine candidate MAG-Tn3+AS15 administered to patients with high-risklocalised HR-positive HER2-negative BC (HR status <10% is included, as well). Each patient received one of the three escalating doses ofMAG-Tn3 in combination with a fixed dose of AS15 adjuvant. Six vaccine injections are scheduled and administered with a 3-weeksinterval between each dose. The primary outcome of the study is the safety profile of the IO. The planned enrolment is of 20 participants, with a study completion date estimated in December 2021 (NCT02364492, Table 3). Preliminary findings demonstrated the induction of tumour-specific cytotoxic antibodies [108].
5.2. Gene-based vaccines for HR-positive breast cancer
5.2.1. Mammaglobin-A (MAM-A) vaccine
MAM-A is a protein that is highly expressed by BC cells (TAA). A phase Ib study is investigating a gene-based CV targeting mammaglobin-A in HR-positive early BC. The DNA is purified from bacteria and carries the gene encoding for the target protein. Patients are divided into four cohorts, in accordance with their planned treatment for early disease. In arm 1 and 2 patients receive adjuvant endocrine therapy alone versus endocrine therapy plus the vaccine; in arm 3 and 4 patients receive (neo)adjuvant chemotherapy alone versus endocrine therapy plus the vaccine. The gene-based CV is administered at the dose of 4 mg via electroporation at three time points (days 28, 56, and 84). The primary outcome of the study is the safety profile of the IO. The estimated enrolment is of 56 participants, with a study completion date projected in January 2027 (NCT02204098, Table 3).
6. Vaccines targeting breast cancer irrespective of the biological subtype
6.1. SV-BR-1-GM (allogeneic cell-based vaccine)
Compared to normal human breast cells, SV-BR-1-GM, a GM-CSF-secreting BC cell line overexpresses genes encoding TAAs, such as Preferentially Expressed Antigen in Melanoma (PRAME), a cancer/testis antigen, and HLA class II genes (HLA-DRA, HLA-DRB3, HLA-DMA, HLA-DMB), in addition to several other factors known to play immunostimulatory roles [109]. In a phase I pilot study (NCT00095862), a subject with stage IV BC treated with SV-BR-1-GM in combination with low-dose interferon alfa and low-dose cyclophosphamide experienced substantial regression of breast, lung and brain metastatic lesions [109]. On these promising results, a phase I/II study (NCT03328026, Table 1, Table 2, Table 3) is enrolling patients with metastatic or locally recurrent BC, irrespective of the biological subtype, to assess the activity of SV-BR-1-GM, in combination with the PD-1 inhibitor INCMGA00012 and the indoleamine-pyrrole 2,3-dioxygenase inhibitor epacadostat, a TME modulator, on a 3-week schedule. The primary end-point is the safety profile, with an estimated enrolment of 60 participant and an expected completion date in December 2022. Conversely, the phase I/II clinical trial investigating SV-BR-1-GM in combination with pembrolizumab in HLA-matched recurrent or metastatic BC has been withdrawn for funding discontinuation on 7th June 2021 (NCT04418219, Table 1, Table 2, Table 3).
6.2. DC-CIK (DCs-based cancer vaccine)
In vitro generated DCs co-cultured with cytokine-induced killer cells (CIK) IO proved to prolong survival in several cancers, but its role in advanced BC is still unclear [110]. Thus, a phase II clinical trial is assessing the efficacy and safety of DC-CIK IO combined with capecitabine versus capecitabine monotherapy as second or third-line treatment for advanced BC, irrespective of the biological subtype (NCT02491697, Table 1, Table 2, Table 3). The primary end-point is OS, with an estimated enrolment of 400 participants and an expected completion date in August 2033.
6.3. Modified vaccination measles virus (MV-s-NAP)
A phase I trial is currently investigating the safety profile and optimal dose of the oncolytic virus MV-s-NAP in metastatic BC patients, irrespective of the biological subtype (NCT04521764, Table 1, Table 2, Table 3). MV-s-NAP has been engineered to have an extra gene that allows the virus to produce the Helicobacter pylori neutrophil-activating protein (NAP), which is normally expressed in inflammatory reactions. The expected enrolment of 33 participants, and the estimated completion date in August 2022.
7. Other targets for breast cancer vaccine development
Research on TAA peptides has identified a large collection of peptide epitopes that have been and are being used for the vaccination of cancer patients [89]. Among all recognised TAAs, interest has been focused on cancer testis and differentiation antigens in the past few years. These antigens are frequently downregulated in somatic adult tissues, while becoming aberrantly re-expressed in various malignancies [89]. As a result, several clinical trials investigated vaccines containing TAAs, such as New York oesophageal squamous cell carcinoma-1 (NY-ESO-1), Wilms tumour antigen (WT-1), melanoma-associated antigen 3 (MAGE-A3) and PRAME in different solid tumours, including BC. In this regard, WT1 and NYESO-1 show overexpression in a group of patients with TNBC for whom therapeutic options are limited [89]. However, none of these CVs showed striking efficacy in both the early and the advanced setting for BC [24].
A summary of the most frequently studied antigens for the treatment of BC is provided in Fig. 4 .
Fig. 4.
Summary of the most frequently studied antigens for the treatment of BC with information about their immunogenicity. Abbreviations: NeoAgs, neoantigens; MAGE, melanoma-associated antigen; BAGE, B melanoma antigen; NY-ESO-1, New York oesophageal squamous cell carcinoma 1; HER2, human epidermal growth factor receptor 2; CEA, carcinoembryonic antigen; hTERT, human telomerase reverse transcriptase; IGFBP2, insulin-like growth factor–binding protein 2; IGF1R, insulin-like growth factor receptor-1. Created with biorender.com.
8. Conclusions and future perspectives
The ability to exploit the immune system as a weapon against cancer has revolutionised the treatment of cancer patients. So far, the main clinical benefit has been achieved with the administration of ICIs [88]. However, ICIs have demonstrated only a modest benefit in treating advanced BC, as an immune suppressive TME typically characterises this disease [88]. Of note, HER2-positive and, above all, TNBC are associated with TILs and tend to better respond to ICIs. In contrast, HR-positive BC displays low TIL infiltration and minimal response to ICIs [4].
In this context, active IO, such as CVs, represent for BC additional means to convert a cold TME into a hot one. Moreover, CVs harbour well-known advantages over passive IO or chemotherapy, as they are tumour-specific, usually well-tolerated, can be used as a combination with other treatment modalities and are expected to induce long-lasting immune responses [24]. After almost two decades of poor clinical trial results, CVs have come back in the spotlight because of the technological advancements occurred in the recent years and ultimately boosted by the COVID-19 vaccination campaign [24,111]. In particular, because of poor immunogenicity of single-peptide vaccines, polypeptide vaccines in addition to the inclusion of different adjuvants and delivery vectors have been implemented [29]. As well, new platforms are approaching the field of CVs for BC, such as gene-based and viral vector–based vaccines. In addition, delivery vehicles are undergoing an evolution towards lipid nanoparticles, virus-like particles, polymeric and non-degradable nanoparticles [29]. Specifically, liposomes not only help the peptide-based or nucleic acid–based antigens to be delivered to the lymph nodes, but they also increase the cellular uptake by DCs, resulting in enhanced vaccine immunogenicity [112]. Moreover, liposomes themselves act as immune adjuvant to increase and prolong the immune responses [113].
Unlike the past two decades, neoantigens are emerging as the preferred targets for CVs [29]. In particular, the recent development of methods for high-throughput detection of mutation-associated epitopes, as well as of bioinformatic tools for neoepitope prediction have been developed, with promising results [24,29].
Regarding the treatment setting, past clinical trials investigating CVs focused especially on the metastatic setting, where the TME is more likely compromised by inhibitory mechanisms [24]. In this sense, new strategies are focussing on favouring the use of CVs as monotherapy in premalignant or in the (neo)adjuvant setting and on establishing combination treatments in late-stage disease [24]. For example, combining BC vaccines with ICIs, gut microbiome modulators or novel immune targeting agents have been proposed to promote the transition from a ‘cold’ to a ‘hot’ TME [24]. In fact, the synergistic effect between CVs and ICIs has already been established in many tumour types [29]. This aspect could be of particular interest, especially given the recent approvals for the first-line treatment setting of pembrolizumab in combination with chemotherapy in TNBC patients with PD-L1 combined positive score ≥10 and of atezolizumab in combination with nab-paclitaxel in TNBC patients with PD-L1 ≥ 1% as determined by VENTANA SP142 assay [114,115]. More importantly, on 26th July 2021, the Food and Drug Administration approved pembrolizumab in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery for high-risk, early-stage, TNBC. Therefore, in an attempt to impact mechanisms of primary and secondary resistance to the anti-tumour immune response, novel combination strategies should be further investigated in BC [[116], [117], [118]]. In this sense, both technological advancements and a better understanding of the tumour–host immune interactions are needed.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
CC and PPMBG have no potential conflicts of interest to disclose. AMME served as consultant or advisor for Ellipses Pharma, GlaxoSmithKline, ISA Pharmaceuticals, MSD, Novartis, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BioInvent, IO Biotech, CatalYm, and Nektar; served on the speaker's bureau for MSD and BIOCAD; received travel funding from Bristol Myers Squibb; received honoraria from Ellipses Pharma, GlaxoSmithKline, ISA Pharmaceuticals, MSD, Novartis, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BIOCAD, CatalYm, BioInvent, IO Biotech, and Nektar; provided expert testimony for Novartis; and has stock and other ownership interest with RiverD, Skyline Diagnostics, Theranovir, all outside the submitted work. SD served as consultant or advisor for AstraZeneca; received research funding from AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma (Inst), Eli Lilly (Inst), Novartis (Inst), Sanofi (Inst); and received travel funding from Pfizer, AstraZeneca and Roche. GC served as consultant or advisor for Roche, Lilly, and Bristol-Myers Squibb; served on the speaker's bureau for Roche, Pfizer, and Lilly; received travel funding from Pfizer and Roche; and received honoraria from Roche, Pfizer, Lilly, Novartis, and SEAGEN, all outside the submitted work.
Acknowledgements
CC contributed to the literature search, conception, design of the article and drafted the first version of the manuscript. PPMBG contributed to the literature search, conception and design of the article. AMME and SD provided critical revisions of the manuscript. GC contributed to conception and design of the article, provided critical revisions of the manuscript and supervision. Fig. 2, Fig. 3, Fig. 4 were created with biorender.com. All the authors provided final approval to the submitted work.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Adams S., Gatti-Mays M.E., Kalinsky K., et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019 Aug 1;5(8):1205–1214. doi: 10.1001/jamaoncol.2018.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence M.S., Stojanov P., Polak P., et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg J., Pastorello R.G., Vallius T., et al. The immunology of hormone receptor positive breast cancer. Front Immunol. 2021;12:674192. doi: 10.3389/fimmu.2021.674192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budczies J., Bockmayr M., Denkert C., et al. Classical pathology and mutational load of breast cancer - integration of two worlds. J Pathol Clin Res. 2015;1(4):225–238. doi: 10.1002/cjp2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luen S., Virassamy B., Savas P., et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–250. doi: 10.1016/j.breast.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Criscitiello C., Vingiani A., Maisonneuve P., et al. Tumor-infiltrating lymphocytes (TILs) in ER+/HER2- breast cancer. Breast Cancer Res Treat. 2020;183(2):347–354. doi: 10.1007/s10549-020-05771-7. [DOI] [PubMed] [Google Scholar]
- 8.Stanton S.E., Adams S., Disis M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 9.Loi S., Sirtaine N., Piette F., et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 10.Dieci M.V., Mathieu M.C., Guarneri V., et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26(8):1698–1704. doi: 10.1093/annonc/mdv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loi S., Michiels S., Salgado R., et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 12.Denkert C., von Minckwitz G., Darb-Esfahani S., et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim E.M., Al-Foheidi M.E., Al-Mansour M.M., Kazkaz G.A. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148(3):467–476. doi: 10.1007/s10549-014-3185-2. [DOI] [PubMed] [Google Scholar]
- 14.Denkert C., Loibl S., Noske A., et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 15.Denkert C., von Minckwitz G., Brase J.C., et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 16.Issa-Nummer Y., Darb-Esfahani S., Loibl S., et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer - a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali H.R., Chlon L., Pharoah P.D., et al. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med. 2016;13(12) doi: 10.1371/journal.pmed.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono M., Tsuda H., Shimizu C., et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132(3):793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L., Buqué A., Kepp O., et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 20.Williams A.D., Payne K.K., Posey A.D., et al. Immunotherapy for breast cancer: current and future strategies. Curr Surg Rep. 2017;5 doi: 10.1007/s40137-017-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arab A., Yazdian-Robati R., Behravan J. HER2-Positive breast cancer immunotherapy: a focus on vaccine development. Arch Immunol Ther Exp. 2020;68(1):2. doi: 10.1007/s00005-019-00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solinas C., Aiello M., Migliori E., et al. Breast cancer vaccines: heeding the lessons of the past to guide a path forward. Cancer Treat Rev. 2020;84:101947. doi: 10.1016/j.ctrv.2019.101947. [DOI] [PubMed] [Google Scholar]
- 23.Migali C., Milano M., Trapani D., et al. Strategies to modulate the immune system in breast cancer: checkpoint inhibitors and beyond. Ther Adv Med Oncol. 2016;8(5):360–374. doi: 10.1177/1758834016658423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dafni U., Martín-Lluesma S., Balint K., et al. Efficacy of cancer vaccines in selected gynaecological breast and ovarian cancers: a 20-year systematic review and meta-analysis. Eur J Cancer. 2021;142:63–82. doi: 10.1016/j.ejca.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorf E.A., Lu B., Melisko M., et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25(14):4248–4254. doi: 10.1158/1078-0432.CCR-18-2867. [DOI] [PubMed] [Google Scholar]
- 26.Miles D., Roché H., Martin M., et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16(8):1092–1100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curigliano G., Spitaleri G., Pietri E., et al. Breast cancer vaccines: a clinical reality or fairy tale? Ann Oncol. 2006;17(5):750–762. doi: 10.1093/annonc/mdj083. [DOI] [PubMed] [Google Scholar]
- 28.Rosalia R.A., Quakkelaar E.D., Redeker A., et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. 2013;43(10):2554–2565. doi: 10.1002/eji.201343324. [DOI] [PubMed] [Google Scholar]
- 29.Antonarelli G., Corti C., Tarantino P., et al. Therapeutic cancer vaccines revamping: technology advancements and pitfalls. Ann Oncol. 2021 Sep 6 doi: 10.1016/j.annonc.2021.08.2153. S0923-7534(21)04457-4. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton G., Silcocks P., Cox T., et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15(8):829–840. doi: 10.1016/S1470-2045(14)70236-0. [DOI] [PubMed] [Google Scholar]
- 31.Rini B.I., Stenzl A., Zdrojowy R., et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(11):1599–1611. doi: 10.1016/S1470-2045(16)30408-9. [DOI] [PubMed] [Google Scholar]
- 32.Blum J.S., Wearsch P.A., Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leone P., Shin E.C., Perosa F., et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105(16):1172–1187. doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera T., Lara E., Romero J.M., et al. HLA class I expression in metastatic melanoma correlates with tumor development during autologous vaccination. Cancer Immunol Immunother. 2007;56(5):709–717. doi: 10.1007/s00262-006-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westcott P., Sacks N., Schenkel J., et al. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat Cancer. 2021 Oct;2(10):1071–1085. doi: 10.1038/s43018-021-00247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells D.K., van Buuren M.M., Dang K.K., et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell. 2020;183(3):818–834.e13. doi: 10.1016/j.cell.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtkamp S., Kreiter S., Selmi A., et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108(13):4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 38.Luo N., Nixon M.J., Gonzalez-Ericsson P.I., et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun. 2018;9(1):248. doi: 10.1038/s41467-017-02630-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corti C., Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol. 2021 Apr;32(4):569–571. doi: 10.1016/j.annonc.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel C.L., Cobleigh M.A., Tripathy D., et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 41.Corti C., Giugliano F., Nicolò E., et al. Antibody–drug conjugates for the treatment of breast cancer. Cancers. 2021;13(12):2898. doi: 10.3390/cancers13122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarantino P., Prat A., Cortes J., et al. Third-line treatment of HER2-positive advanced breast cancer: from no standard to a Pandora's box. Biochim Biophys Acta Rev Cancer. 2020;1875(1):188487. doi: 10.1016/j.bbcan.2020.188487. [DOI] [PubMed] [Google Scholar]
- 43.Moasser M.M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mimura K., Kono K., Hanawa M., et al. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2005;92(7):1253–1260. doi: 10.1038/sj.bjc.6602499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valtorta E., Martino C., Sartore-Bianchi A., et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28(11):1481–1491. doi: 10.1038/modpathol.2015.98. [DOI] [PubMed] [Google Scholar]
- 46.Cocco E., Lopez S., Santin A.D., Scaltriti M. Prevalence and role of HER2 mutations in cancer. Pharmacol Ther. 2019;199:188–196. doi: 10.1016/j.pharmthera.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meric-Bernstam F., Johnson A.M., Dumbrava E.E.I., et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019;25(7):2033–2041. doi: 10.1158/1078-0432.CCR-18-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corti C., Criscitiello C. Tucatinib approval by EMA expands options for HER2-positive locally advanced or metastatic breast cancer. ESMO Open. 2021;6(2) doi: 10.1016/j.esmoop.2021.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexe G., Dalgin G.S., Scanfeld D., et al. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;67(22):10669–10676. doi: 10.1158/0008-5472.CAN-07-0539. [DOI] [PubMed] [Google Scholar]
- 50.Salgado R., Denkert C., Campbell C., et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curigliano G., Romieu G., Campone M., et al. A phase I/II trial of the safety and clinical activity of a HER2-protein based immunotherapeutic for treating women with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2016;156(2):301–310. doi: 10.1007/s10549-016-3750-y. [DOI] [PubMed] [Google Scholar]
- 52.Costa R.L.B., Czerniecki B.J. Clinical development of immunotherapies for HER2. NPJ Breast Cancer. 2020;6:10. doi: 10.1038/s41523-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costa R.L.B., Soliman H., Czerniecki B.J. The clinical development of vaccines for HER2. Cancer Treat Rev. 2017;61:107–115. doi: 10.1016/j.ctrv.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Datta J., Fracol M., McMillan M.T., et al. Association of depressed anti-HER2 T-helper type 1 response with recurrence in patients with completely treated HER2-positive breast cancer: role for immune monitoring. JAMA Oncol. 2016;2(2):242–246. doi: 10.1001/jamaoncol.2015.5482. [DOI] [PubMed] [Google Scholar]
- 55.Ohlschlegel C., Zahel K., Kradolfer D., et al. HER2 genetic heterogeneity in breast carcinoma. J Clin Pathol. 2011;64(12):1112–1116. doi: 10.1136/jclinpath-2011-200265. [DOI] [PubMed] [Google Scholar]
- 56.De La Cruz L.M., Nocera N.F., Czerniecki B.J. Restoring anti-oncodriver Th1 responses with dendritic cell vaccines in HER2/neu-positive breast cancer: progress and potential. Immunotherapy. 2016;8(10):1219–1232. doi: 10.2217/imt-2016-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Disis M.L., Schiffman K., Guthrie K., et al. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein--based vaccine. J Clin Oncol. 2004;22(10):1916–1925. doi: 10.1200/JCO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Clifton G.T., Peoples G.E., Mittendorf E.A. The development and use of the E75 (HER2 369-377) peptide vaccine. Future Oncol. 2016;12(11):1321–1329. doi: 10.2217/fon-2015-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clifton G.T., Hale D., Vreeland T.J., et al. Results of a randomized phase IIb trial of nelipepimut-S + trastuzumab versus trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer. Clin Cancer Res. 2020;26(11):2515–2523. doi: 10.1158/1078-0432.CCR-19-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chick R.C., Clifton G.T., Hale D.F., et al. Subgroup analysis of nelipepimut-S plus GM-CSF combined with trastuzumab versus trastuzumab alone to prevent recurrences in patients with high-risk, HER2 low-expressing breast cancer. Clin Immunol. 2021;225:108679. doi: 10.1016/j.clim.2021.108679. [DOI] [PubMed] [Google Scholar]
- 61.SELLAS Life Sciences Provides Regulatory Update for Nelipepimut-S (NPS) for Triple Negative Breast Cancer (TNBC) Following FDA Feedback. 14 February 2020. [press release]. New York. [Google Scholar]
- 62.Ayoub N.M., Al-Shami K.M., Yaghan R.J. Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer. 2019;11:53–69. doi: 10.2147/BCTT.S175360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel S., McWilliams D., Fischette C.T., et al. Final five-year median follow-up safety data from a prospective, randomized, placebo-controlled, single-blinded, multicenter, phase IIb study evaluating the use of HER2/neu peptide GP2 + GM-CSF vs. GM-CSF alone after adjuvant trastuzumab in HER2-positive women with operable breast cancer. J Clin Oncol. 2021;39(15_suppl):542. [Google Scholar]
- 64.McCarthy P.M., Clifton G.T., Vreeland T.J., et al. AE37: a HER2-targeted vaccine for the prevention of breast cancer recurrence. Expert Opin Investig Drugs. 2021;30(1):5–11. doi: 10.1080/13543784.2021.1849140. [DOI] [PubMed] [Google Scholar]
- 65.Brown T.A., Mittendorf E.A., Hale D.F., et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res Treat. 2020;181(2):391–401. doi: 10.1007/s10549-020-05638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitano S., Kageyama S., Nagata Y., et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006;12(24):7397–7405. doi: 10.1158/1078-0432.CCR-06-1546. [DOI] [PubMed] [Google Scholar]
- 67.Kageyama S., Kitano S., Hirayama M., et al. Humoral immune responses in patients vaccinated with 1-146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008;99(3):601–607. doi: 10.1111/j.1349-7006.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kroemer G., Senovilla L., Galluzzi L., et al. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21(10):1128–1138. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 69.Kurtz S.L., Ravindranathan S., Zaharoff D.A. Current status of autologous breast tumor cell-based vaccines. Expert Rev Vaccines. 2014;13(12):1439–1445. doi: 10.1586/14760584.2014.969714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Awadhi A., Lee Murray J., Ibrahim N.K. Developing anti-HER2 vaccines: breast cancer experience. Int J Cancer. 2018;143(9):2126–2132. doi: 10.1002/ijc.31551. [DOI] [PubMed] [Google Scholar]
- 71.Park J.W., Melisko M.E., Esserman L.J., et al. Treatment with autologous antigen-presenting cells activated with the HER-2 based antigen lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. J Clin Oncol. 2007;25(24):3680–3687. doi: 10.1200/JCO.2006.10.5718. [DOI] [PubMed] [Google Scholar]
- 72.Emens L.A., Asquith J.M., Leatherman J.M., et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27(35):5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gelao L., Criscitiello C., Esposito A., et al. Dendritic cell-based vaccines: clinical applications in breast cancer. Immunotherapy. 2014;6(3):349–360. doi: 10.2217/imt.13.169. [DOI] [PubMed] [Google Scholar]
- 75.Czerniecki B.J., Koski G.K., Koldovsky U., et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 76.Viehl C.T., Becker-Hapak M., Lewis J.S., et al. A tat fusion protein-based tumor vaccine for breast cancer. Ann Surg Oncol. 2005;12(7):517–525. doi: 10.1245/ASO.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 77.Xie Y., Chen Y., Ahmed K.A., et al. Potent CD4+ T-cell epitope P30 enhances HER2/neu-engineered dendritic cell-induced immunity against Tg1-1 breast cancer in transgenic FVBneuN mice by enhanced CD4+ T-cell-stimulated CTL responses. Cancer Gene Ther. 2013;20(10):590–598. doi: 10.1038/cgt.2013.60. [DOI] [PubMed] [Google Scholar]
- 78.Larocca C., Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17(5):359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bryson P.D., Han X., Truong N., Wang P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine. 2017;35(43):5842–5849. doi: 10.1016/j.vaccine.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shanmugaraj B., Priya L.B., Mahalakshmi B., et al. Bacterial and viral vectors as vaccine delivery vehicles for breast cancer therapy. Life Sci. 2020;250:117550. doi: 10.1016/j.lfs.2020.117550. [DOI] [PubMed] [Google Scholar]
- 81.Li L., Saade F., Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162(2–3):171–182. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang B., Jeang J., Yang A., et al. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother. 2014;10(11):3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchini C., Kalogris C., Garulli C., et al. Tailoring DNA vaccines: designing strategies against HER2-positive cancers. Front Oncol. 2013;3:122. doi: 10.3389/fonc.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norell H., Poschke I., Charo J., et al. Vaccination with a plasmid DNA encoding HER-2/neu together with low doses of GM-CSF and IL-2 in patients with metastatic breast carcinoma: a pilot clinical trial. J Transl Med. 2010;8:53. doi: 10.1186/1479-5876-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diaz C.M., Chiappori A., Aurisicchio L., et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J Transl Med. 2013;11:62. doi: 10.1186/1479-5876-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Disis M.L., Coveler A.L., Higgins D., et al. A phase I trial of the safety and immunogenicity of a DNA-based vaccine encoding the HER2/neu (HER2) intracellular domain in subjects with HER2+ breast cancer. J Clin Oncol. May 20, 2014;32(15_suppl):616. [Google Scholar]
- 87.Childs J., Higgins D., DeShong K., et al. San Antonio Breast Cancer Symposium. February 2017. Abstract OT3-01-03: A phase I trial of the safety and immunogenicity of a DNA plasmid based vaccine (WOKVAC) encoding epitopes derived from three breast cancer antigens (IGFBP2, HER2, and IGF1R) in patients with breast cancer. San Antonio, Texas. [Google Scholar]
- 88.Criscitiello C., Corti C., Pravettoni G., Curigliano G. Managing side effects of immune checkpoint inhibitors in breast cancer. Crit Rev Oncol Hematol. 2021;162:103354. doi: 10.1016/j.critrevonc.2021.103354. [DOI] [PubMed] [Google Scholar]
- 89.Curigliano G., Bagnardi V., Ghioni M., et al. Expression of tumor-associated antigens in breast cancer subtypes. Breast. 2020;49:202–209. doi: 10.1016/j.breast.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belli C., Trapani D., Viale G., et al. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Couchman J.R. Syndecan-1 (CD138), carcinomas and EMT. Int J Mol Sci. 2021;22(8) doi: 10.3390/ijms22084227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buller C.W., Mathew P.A., Mathew S.O. Roles of NK cell receptors 2B4 (CD244), CS1 (CD319), and LLT1 (CLEC2D) in cancer. Cancers (Basel) 2020;12(7) doi: 10.3390/cancers12071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Isakoff J.S., Adams S., Soliman H.H., et al. San Antonio Breast Cancer Symposium; December 10-14, 2019; San Antonio, Texas. 2020. Abstract P3-09-15: a phase 1b study of PVX-410 (PVX) vaccine plus durvalumab (DUR) as adjuvant therapy in HLA-A2+ early stage triple negative breast cancer (eTNBC) to assess safety and immune response. [Google Scholar]
- 94.Hutchins L.F., Makhoul I., Emanuel P.D., et al. Targeting tumor-associated carbohydrate antigens: a phase I study of a carbohydrate mimetic-peptide vaccine in stage IV breast cancer subjects. Oncotarget. 2017;8(58):99161–99178. doi: 10.18632/oncotarget.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norton N., Youssef B., Hillman D.W., et al. Folate receptor alpha expression associates with improved disease-free survival in triple negative breast cancer patients. NPJ Breast Cancer. 2020;6:4. doi: 10.1038/s41523-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murtaza Kasi P., Kalli K., Block M.S., et al. A phase I trial of the safety and immunogenicity of a multi-epitope folate receptor alpha peptide vaccine used in combination with cyclophosphamide in subjects previously treated for breast or ovarian cancer. J Clin Oncol. May 20, 2015;33(15_suppl) e14028-e14028. [Google Scholar]
- 97.Jain A.G., Talati C., Pinilla-Ibarz J. Galinpepimut-S (GPS): an investigational agent for the treatment of acute myeloid leukemia. Expert Opin Investig Drugs. 2021:1–7. doi: 10.1080/13543784.2021.1928635. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y., Yan W.T., Yang Z.Y., et al. The role of WT1 in breast cancer: clinical implications, biological effects and molecular mechanism. Int J Biol Sci. 2020;16(8):1474–1480. doi: 10.7150/ijbs.39958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chuang P.K., Hsiao M., Hsu T.L., et al. Signaling pathway of globo-series glycosphingolipids and β1,3-galactosyltransferase V (β3GalT5) in breast cancer. Proc Natl Acad Sci U S A. 2019;116(9):3518–3523. doi: 10.1073/pnas.1816946116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rugo H.S., Chow L.W.C., Cortes J., et al. Phase III, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of adagloxad simolenin (OBI-822) and OBI-821 treatment in patients with early-stage triple-negative breast cancer (TNBC) at high risk for recurrence. J Clin Oncol. May 25, 2020;38(15_suppl) [Google Scholar]
- 101.Huang C.S., Yu A.L., Tseng L.M., et al. Globo H-KLH vaccine adagloxad simolenin (OBI-822)/OBI-821 in patients with metastatic breast cancer: phase II randomized, placebo-controlled study. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2019-000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bykov V.J.N., Eriksson S.E., Bianchi J., Wiman K.G. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 103.Chung V.M., Kos F., Hardwick N., et al. A phase 1 study of p53MVA vaccine in combination with pembrolizumab. J Clin Oncol. February 26, 2018;36(5_suppl):206. [Google Scholar]
- 104.Stanton S.E., Cecil D.L., O'Meara M.M., et al. Breast cancer stem cell autoantibodies to identify women with advanced breast cancer. J Clin Oncol. June 01, 2018;36:1079. [Google Scholar]
- 105.Conforti F., Pala L., Bagnardi V., et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(8):772–781. doi: 10.1093/jnci/djz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turner N.C., Swift C., Kilburn L., et al. Mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res. 2020;26(19):5172–5177. doi: 10.1158/1078-0432.CCR-20-0224. [DOI] [PubMed] [Google Scholar]
- 107.Laubreton D., Bay S., Sedlik C., et al. The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity. Cancer Immunol Immunother. 2016;65(3):315–325. doi: 10.1007/s00262-016-1802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosenbaum P., Artaud C., Bay S., et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol Immunother. 2020;69(5):703–716. doi: 10.1007/s00262-020-02503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lacher M.D., Bauer G., Fury B., et al. SV-BR-1-GM, a clinically effective GM-CSF-secreting breast cancer cell line, expresses an immune signature and directly activates CD4. Front Immunol. 2018;9:776. doi: 10.3389/fimmu.2018.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang S., Wang X., Zhou X., et al. DC-CIK as a widely applicable cancer immunotherapy. Expert Opin Biol Ther. 2020;20(6):601–607. doi: 10.1080/14712598.2020.1728250. [DOI] [PubMed] [Google Scholar]
- 111.Corti C., Crimini E., Tarantino P., et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer. 2021;148:316–327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watson D.S., Endsley A.N., Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nordly P., Madsen H.B., Nielsen H.M., Foged C. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin Drug Deliv. 2009;6(7):657–672. doi: 10.1517/17425240903018863. [DOI] [PubMed] [Google Scholar]
- 114.FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast cancer. March 8, 2019. Available from: https://bit.ly/39Slkl5 [DOI] [PubMed]
- 115.FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Available from: https://bit.ly/3bWSsdW
- 116.Goldberg J., Qiao N., Gross B., et al. ESR1 mutations provide novel targets for breast cancer immunotherapy. J Clin Oncol. May 25, 2020;38:3135. [Google Scholar]
- 117.Harbeck N., Rastogi P., Martin M., et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021 Sep 29 doi: 10.1016/j.annonc.2021.09.015. S0923-7534(21)04494-X. [DOI] [PubMed] [Google Scholar]
- 118.Griffiths J., Chen J., Cosgrove P., et al. Serial single-cell genomics reveals convergent subclonal evolution of resistance as patients with early-stage breast cancer progress on endocrine plus CDK4/6 therapy. Nat Cancer. 2021;2:658–671. doi: 10.1038/s43018-021-00215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]