Abstract
High Intensity Focused Ultrasound (HIFU) is an emerging and increasingly useful modality in the treatment of cancer and other diseases. Although traditional use of ultrasound at lower frequencies has primarily been for diagnostic imaging purposes, the development of HIFU has allowed this particular modality to expand into therapeutic use. This non-invasive and acoustic method involves the use of a piezoelectric transducer to deliver high-energy pulses in a spatially coordinated manner, while minimizing damage to tissue outside the target area. This review describes the history of the development of diagnostic and therapeutic ultrasound and explores the biomedical applications utilizing HIFU technology including thermally ablative treatment, therapeutic delivery mechanisms, and neuromodulatory phenomena. The application of HIFU across various tumor types in multiple organ systems is explored in depth, with particular attention to successful models of HIFU in the treatment of various medical conditions. Basic mechanisms, preclinical models, previous clinical use, and ongoing clinical trials are comparatively discussed. Recent advances in HIFU across multiple medical fields reveal the growing importance of this biomedical technology for the care of patients and for the development of possible pathways for the future use of HIFU as a commonplace treatment modality.
Keywords: Ablation, Drug Delivery, Neuromodulation, Sonoporation, Focused Ultrasound
Introduction
Ultrasound technology was first discovered in 1880 by Pierre and Jacques Curie when examining the effects of mechanical vibration on quartz crystals 86. While early applications included underwater visualization during World War I and metal impurity testing for industrial uses, ultrasound was eventually introduced to the medical setting 87. Since then, it has broadly grown into a fundamental clinical modality (Table 1). From fetal imaging and bone sonometry to echocardiograms and biopsy guidance, ultrasound technology has become a diagnostic mainstay in many medical fields 88. Diagnostic ultrasound is usually delivered at 0.1 W/cm2 with higher energy dose administration categorized as either high intensity (1,000 W/cm2 - 10,000 W/cm2) 108, medium intensity, or low intensity (< 3 W/cm2) 79. This review focuses on the applications of high intensity focused ultrasound (HIFU) in which acoustic waves are administered at the highest energy level and converge at a focal point.
Table 1:
Milestone table of notable advancements in ultrasound technology.
| 1880 | Jacques and Pierre Curie discover piezoelectricity; ultrasound technology discovered 86 |
| 1927 | Effects of ultrasound on biological tissue investigated 107 |
| 1932 | Ultrasound first suggested in a therapeutic context 68 |
| 1942 | HIFU effects in animals (Lynn et al.) – first focused ultrasound device and first tissue lesion 40 |
| 1944 | First preclinical ultrasound study (Lynn and Putcham) 89 |
| 1950-1969 | Molecular study on HIFU effects (Fry brother) 30 |
| 1951-1960 | Radiofrequency generator and electrode developments 48 |
| 1955 | Fry brothers use focused ultrasound to perform partial ablation of basal ganglia after craniotomy 30 |
| 1962 | Focused ultrasound is explored as a treatment for multiple brain pathologies, including Parkinson’s Disease 51 |
| 1964 | First cancer application of FU (M. Oka reported treatment of thyroid and breast cancer) 90 |
| 1968 | First brain cancer treated using FU (Dr. Robert Heimberger) 30 |
| 1980s-present | MRI technology |
| 1988 | First FDA approval for Sonocare CST-100 Therapeutic Ultrasound System (to treat glaucoma) 50 |
| 1994 | First commercial HIFU machine receives FDA approval, approved for benign prostatic hyperplasia (BPH) (Sonablate 200) 52 |
| 1996 | First blood-brain barrier (BBB) opening moderate-intensity FU application, monitored by MRI 28 |
| 2004 | The FDA approved INSIGHTEC's Exablate 2000 to treat uterine fibroids; first FDA approval of integrated MRgFUS machine 91 |
| 2009 | First ultrasound to treat neuropathic pain 92 |
| 2014 | Focused ultrasound for bone cancer pain 64 |
| 2016 | Ultrasound approved to treat essential tremor (ET) 109 |
| 2018 | First clinical trial conveying local drug delivery published in Lancet Oncology 41. BBB clinical trials begin at University of Maryland in brain tumor patients 93 |
HIFU was first therapeutically suggested in 1932 when H. Freundlich, K. Collner, and F. Rogowski discovered the medium’s propensity to heat tissue 40. In 1942, Lynn et al. explored the localized impact of targeted beams on tissue blocks and live animal organs 40. The researchers specifically noted the method’s ability to cause intense change at the energy’s focal point while leaving tissue in the path of the beams unharmed 40. In the 1960s, interest in HIFU greatly increased due to contributions by the Fry brothers, who created cortical lesions in patients with Parkinson’s and other hyperkinetic disorders 18 in an effort to slow disease progression. HIFU further gained momentum as a viable treatment option in the fields of ophthalmology and neurosurgery through the later 1900s, but research was stalled due to limited imaging modalities and the temperature-monitoring software required for precision during treatment 80.
The advent of Magnetic Resonance Imaging (MRI) technology in the 1980s led to a renewed interest in high-intensity focused ultrasound due to the potential for precise spatial guidance via imaging and the development of MR-thermometry, allowing for accurate temperature tracking 30. The first coupled MR-guided focused ultrasound machine (MRgFUS) in 2003 80, set the stage for HIFU to become a useful treatment option with broader applications.
While HIFU is increasingly being used across disparate areas of medicine, one field of particular interest is oncology. Current strategies for treating malignant neoplasms include a combination of surgery, radiation, chemotherapy, and immunotherapy. Chemotherapy is the most widely employed systemic treatment, yet, even the most promising chemotherapies have been unable to demonstrate desired efficacy due in large part to barriers in delivery, tumor heterogeneity, and cancer resistance. In the pursuit to optimize cancer treatment, HIFU is emerging as a promising and versatile technology that presents itself as both a novel standalone treatment and also one that can enhance the effectiveness of currently available agents. Modulating the intensity of ultrasound treatment allows for its usage as either a drug delivery mechanism or ablative modality, both of which show promise as treatments for neoplasms.
HIFU Mechanism
High intensity focused ultrasound is traditionally delivered by a piezoelectric transducer with a fixed aperture and focal length. The transducer generates an ultrasound field with frequencies ranging from 1 to 7 MHz 32. These sound waves are then converted to thermal energy and travel through the body, converging at a focal point and capable of causing coagulative necrosis. Similar to general ultrasound, there are two categories of treatment effects on tissue: thermal and mechanical.
Thermal effects include the physical heating of targeted tissue due to absorption of ultrasound waves. At lower deposited energy doses (< 55 °C), the induced hyperthermia can lead to increased cellular permeability, better facilitating the delivery of nanoparticles 47. This can be advantageous in tandem with thermally modulated carrier molecules. At higher deposited energy doses (> 55 °C), a state of cell death is induced by coagulative necrosis 22. This level is characteristic of tumor ablative therapies where the lesioned area is mapped using diagnostic ultrasound (USgFUS) or preferably MR imaging (MRgFUS). The precision of HIFU delivery allows the distance between ablated and normal tissue to be minimal. Yu-Feng reported an almost imperceptible margin between affected and unaffected myocytes, even providing images depicting histological differences across a single cell soon after ablation; the half that was within lesion boundaries demonstrated dramatic subcellular fragmentation while the other half of the cell outside the margins remained intact 85. ter Haar et al reported this distance to be approximately 10 cells (250-300 microns) when ablating hepatocytes 23. Even accounting for tissue variability, HIFU ablation results in a very thin boundary between affected and unaffected regions 85.
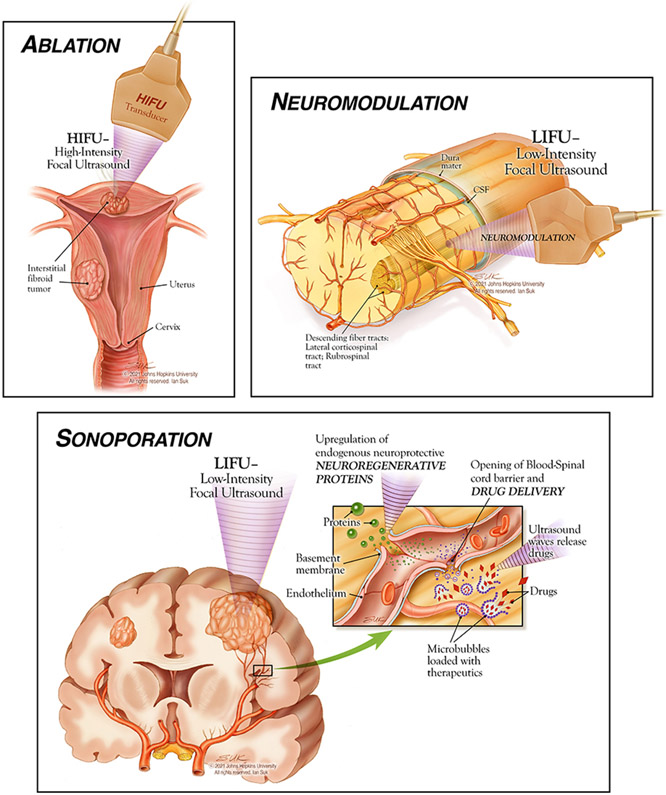
The mechanical effects of HIFU include radiation force, increased pressure, and most importantly, acoustic cavitation. Acoustic cavitation describes the process by which pressure field differences in the targeted tissue lead to the formation, oscillation, and collapse of microbubbles. While ultrasound administered at a low intensity causes sheer stress on nearby structures, ultrasound administered at high intensity leads to the formation of jet streams and shock waves. This increased frequency fosters the creation of transient pores in the plasma membrane, increasing cellular permeability - a process known as sonoporation 47. Sonoporation (Figure 1) is useful from a drug delivery standpoint, as the pores allow for increased particle uptake in target tissues and the crossing of intercellular and intracellular barriers.
Figure 1:
Illustrative depictions of HIFU applications, © 2021 Johns Hopkins University All rights reserved. Ian Suk.
Applications
Focused Ultrasound and Ablation
Due to its heating effects, the most commonly explored utilization of HIFU is thermal ablation (Figure 1) 94. The idea was first introduced by Lynn et al. in the 1940s when exploring inducible hyperthermia108, and further expanded upon in the 1980s when Wang et al. correlated the scope of ablative injury with wave intensity and irradiation time in porcine liver tissue 74. Since its first application, ablation has become a popular therapeutic option for treatment in the bone, liver, pancreas, breast, and kidney 15.
To adequately ablate an area with high-intensity focused ultrasound, certain parameters are determined including the treatment zone, safety margins, radiation dose, and duration of ablation. The treatment zone is the most variable and includes both the target tumor volume and a surrounding perimeter of normal tissue as a safety margin, which is similar to the surgical excising approach 13. Lesion depth is taken into account as well; deeper structures (>10 cm) result in more attenuation of the acoustic waves as they pass through the body and are less effective at depositing the set energy dose 13. Reflective interfaces between tissues and dense structure obstruction may lead to under-treatment of the target region. In addition, the delivery path should avoid gas-filled organs due to their muffling of HIFU effects through focal point displacement and sound wave modulation 27.
Monitoring lesion formation during ablative pulses is of the utmost importance from an efficacy and safety standpoint. Transducer dose deposition is actively adjusted to control temperature fluctuations, allowing those delivering care to optimize ablative impact within safety limits. Because of its ability to monitor lesion formation and tissue temperature in real time, MRI and thermometry is preferred over diagnostic ultrasound. For accurate delivery, anxiolytic, analgesic, and antispasmodic medications are administered to decrease movement during the procedure and temporarily block digestive peristaltic motion 83.
Focused Ultrasound and Drug Delivery
Though traditionally used in an ablative setting, HIFU has more recently been explored as an adjunct to drug delivery due to its effect on membrane permeability. Specifically, there are two leading justifications for nanoparticles to be utilized in conjunction with ultrasound. First, nanoparticles can serve as nucleation sites, lowering the cavitation threshold during the formation of microbubbles; this potentiates the mechanical effects of HIFU and results in more efficient treatment applications. A 2019 study by Khirallah et al. demonstrated the increased capacity of perfluorohexane nanoparticles in reducing the cavitation threshold during ablation of tissue phantoms contained red blood cells 33. Second, carrier particles themselves can be loaded with drug molecules and ablated at the appropriate delivery site by selectively applying HIFU to the region. Thus, externally triggered drug release can be accomplished with spatiotemporal control, with HIFU “activating” select particles via thermal and/or mechanical effects 1. A description of select nanoparticles used with HIFU for cancer therapy shown in Table 2.
Table 2.
Select nanoparticles used with HIFU for cancer therapy
| HIFU Effect |
Particle Type |
Particle | In Vitro | In Vivo | Model | Ref. |
|---|---|---|---|---|---|---|
| Thermal | Conjugated Polymer | HMME+PFP/PLGA-Ab (liquid fluorocarbon) | X | X | Breast cancer | 84 |
| Liposome | Low temperature-sensitive liposomes | X | X | Mammary adenocarcinoma | 12 | |
| High temperature-sensitive liposomal cerasomes | X | Breast adenocarcinoma | 38 | |||
| Mechanical | Metallic | Gold | X | Colon cancer | 63 | |
| Porous Silicon | X | X | Laryngeal cancer | 53 | ||
| Titanium Dioxide | X | Oral squamous cell carcinoma | 49 | |||
| Magnetite (Fe3O2) | X | Breast cancer | 5 | |||
| Conjugated Polymer | Fe3O4-PFH/PLGA | X | X | Hepatocellular carcinoma | 82 |
To rely on the thermal effects of HIFU, particles must be temperature-sensitive such that above or below a certain heat or energy threshold, drug is released. Dromi et al. explored the use of thermo-sensitive liposomes 12; in vitro and in vivo mouse models demonstrated a more rapid and concentrated release of doxorubicin following administration of HIFU pulses and injection of low temperature-sensitive liposomes. In contrast, Liang et al. demonstrated that high temperature-sensitive cerasomes underwent a burst-release of drug molecules over a 5 °C temperature increase in their target region when administered to treat adenocarcinoma of the breast in mice 38.
The study of inducible characteristics relying on the mechanical effects of HIFU has centered on nanoparticles; this is due to their capacity to present as additional nucleation sites and also act as carrier molecules, allowing for increased drug unloading via sonoporation at the site of HIFU application. These nanoparticles can be organic, such as lipid- or polymer-based, or they can be inorganic, such as metallic, or they can be a hybrid combination. You et al. explored the use of a metal oxide conjugated polymeric nanoparticle to unload perfluorohexane and treat hepatocellular carcinoma in a xenograft rabbit model. In addition to demonstrating in vitro efficacy, the nanoparticle + HIFU experimental group demonstrated a significantly (p < 0.05) lower tumor proliferative index than the HIFU alone control group 82. Along with its efficacy in rabbit liver tumor xenograft tissue, nanoparticles in combination with HIFU has also been successfully utilized in mouse models. For example, researchers have demonstrated that pulsed HIFU (administered across burst intervals) effectively synergized with glycol chitosan nanoparticles in murine models 84. Furthermore, in a study by You et al, specific HIFU pulsed dosing of 10, 20, and 50 W resulted in leaky murine femoral vasculature, demonstrated by increased fluorescence signals as compared to untreated tissue 81. HIFU treatment has increasingly been shown to increased extravasation of drug-loaded carrier nanoparticles, overcoming tissue penetration, one of the critical obstacles to nanoparticle use.
Limitations of HIFU in facilitating drug delivery include its short duration of effect and variable drug uptake. In clinical practice, solid tumors benefit from sustained release of chemotherapeutic agents to most fully penetrate the mass. Because the delivery mechanism of HIFU has inherent limitations in the number of pulses per session due to safety, large tumors may require longer and more complicated treatment protocols 55. Additionally, the delivery of nanoparticles depends on transport through extracellular barriers to reach the target area. With variance within heterogenous tumors as well as from patient to patient, there can be dramatic differences in drug penetration and uptake based on tumor type, treatment area, and other biological characteristics.
Focused Ultrasound and Neuromodulation
In addition to ablative and drug delivery applications, focused ultrasound techniques have the potential to be used in neuromodulation therapies especially when administered at a lower intensity. Neuromodulation (Figure 1, Figure 2) refers to the alteration of neuronal activity by a therapeutic agent, including electrical stimulation and pharmacologic chemicals 95. With the FDA having only recently approved therapeutic ultrasound, neuromodulatory treatments are a newly emerging target of investigation with limited current literature. In contrast with the ablation caused by high intensity focused ultrasound (HIFU), low intensity focused ultrasound (LIFU) has been theorized to play a useful role in neuromodulation 14. Mechanistically, LIFU creates a nonthermal mechanical disturbance in voltage-gated ion channels, affecting electrical signaling across membranes and therefore impacting neuronal activity 14. In order for neuromodulation to occur as opposed to thermal ablation, ultrasound must be delivered at lower energy (< 3 W/cm2) and provide marginally enough stimulation to modify channels short of causing mechanical damage 79. The reversibility of this mechanism, as first demonstrated by the Fry brothers 17, provided the basis for the investigation of ultrasound for neuromodulation. Furthermore, the resulting changes in neuronal activity are not limited to the duration of the LIFU therapy and can last for hours to days 14,25. HIFU, conversely, is believed to not function through this neuromodulation mechanism, given its overt thermal destruction of tissue at higher frequencies 3,14.
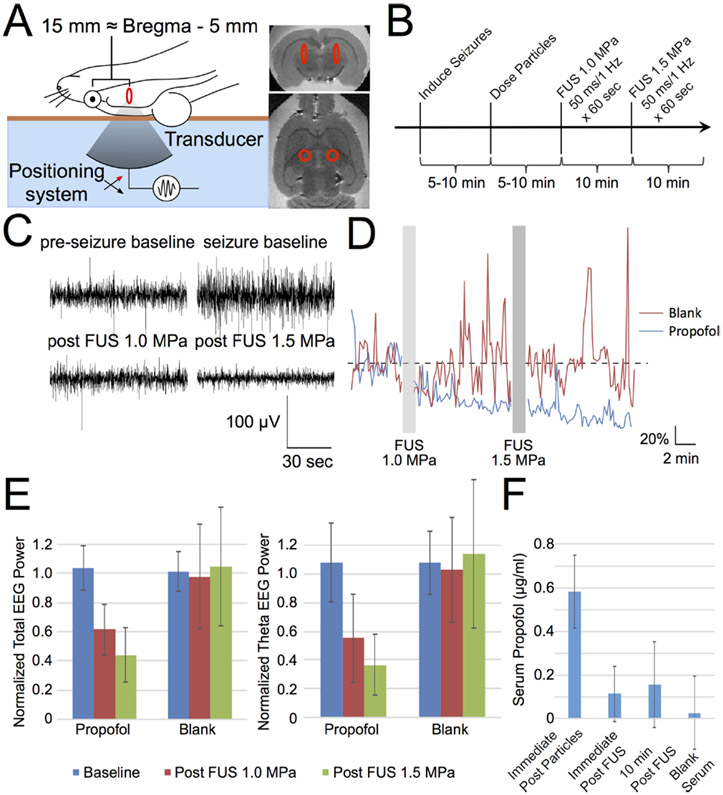
Figure 2.
Focused ultrasound-gated propofol release is potent enough to silence seizure activity. (A) Schematic of rat positioning for this demonstration of in vivo efficacy. Rats were placed supine on the bed of a focused ultrasound transducer and underwent seizure induction, coupled to the transducer via degassed water (light blue), a Kapton membrane filled with degassed water (orange-brown), and ultrasound gel (not pictured). Expected location of the two sonication foci are overlaid onto ex vivo MRI images with the red ellipse indicating the fwhm of the sonication focus, located ~5 mm caudal to bregma. (B) Schematic of experiment timing for seizure induction, particle administration, and FUS application. (C) Sample traces of EEG voltage from one rat receiving propofol-loaded particles before and after seizure-induction and focused ultrasound application at the indicated pressures. (D) Total EEG power normalized by baseline averaged across rats receiving particles loaded with either propofol (blue) or no drug (blank, red) across experiment time (N = 7 propofol, 5 blank). Gray bars indicate time of FUS application. (E) Mean ± SD of normalized total (left) and theta band (right) EEG power in the indicated time period across rats receiving propofol-loaded particles or blank particles (N = 7 propofol, 5 blank). (F) Mean ± SD of the HPLC-quantified serum propofol concentration of samples from N = 4 rats taken immediately after propofol-loaded particle administration, immediately after sonication, and 10 min post sonication, compared to a blank serum sample. There was no appreciable serum propofol peak for the post sonication samples.
Figure reproduced with permission from Nano Lett. 2017, 17, 2, 652–659, Publication Date: January 17, 2017, https://doi.org/10.1021/acs.nanolett.6b03517, Copyright © 2017 American Chemical Society1.
Preclinical animal studies have repeatedly suggested the relative safety and efficacy of LIFU for neuromodulation. A study by Deffieux and colleagues in 2017 investigated LIFU as a tool to modulate prefrontal cortex activity, specifically visuomotor actions, in awake macaque rhesus monkeys 11. By training the monkeys to initiate specific saccade movements based on a stimulus, they found that this behavior could be modified through the application of LIFU, suggesting potential for similar behavior-modifying capacity in humans 11. A study by Dallapiazza et al. in 2018 explored the use of LIFU to modulate the swine sensory thalamus as a means of noninvasively mapping the brain 9. The authors found success in inhibiting specific thalamic nuclei without affecting neighboring nuclei, creating any tissue damage, or inducing any thermal effects, affirming the safety and specificity of delivered ultrasound signals and opening the possibility of developing neuromodulation as a brain-mapping tool pending future investigation in patients 9. A setup and analysis of FU-guided neuromodulation is exemplified in Figure 2, in which Airan et al. in 2017 demonstrated that sonication can safely deliver seizure-silencing nanoparticles without brain parenchymal damage in a rat model1.
Several studies have investigated the use of ultrasound for neuromodulation in patients. A recent study by Sanguinetti and colleagues demonstrated the application of transcranial focused ultrasound to the right prefrontal cortex in healthy patients through a randomized, placebo-controlled, double-blind study; the authors found that this ultrasound use improved mood and affected the connectivity of neural networks related to emotional regulation 61. These results provide a positive projection for future studies that may investigate the potential use of LIFU as a psychiatric neuromodulation treatment in patients suffering from mood disorders. An active clinical trial (NCT04197921), is examining LIFU as an adjunctive treatment in opioid use disorder. As researchers further develop an understanding of the mechanisms of LIFU as a neuromodulator, these studies will shed light on both the utilities of neuromodulation with LIFU and the impact this therapeutic modality could have on the treatment of various neurologic disorders.
HIFU Clinical Trials
Uterine Leiomyomas/Myomas and Adenomyosis
One of the most established applications of HIFU is in the female reproductive system. HIFU is FDA-approved for treatment of uterine leiomyomas and currently in the clinical trial stage for treatment of uterine adenomyosis 96. Adenomyosis occurs when the inner endometrial lining of the uterus grows into the muscular wall and leads to a thickening of the organ. Though the cause has not been fully elucidated, it results in painful menstrual cramps and abnormal bleeding in many affected women. There are several current treatment protocols for adenomyosis include hysteroscopic resection, focal excision, uterine artery ligation, and myometrial electrocoagulation 70. Yet, many of these procedures have not gained widespread acceptance due to negative side effects and serious contraindications in certain populations.
Studies on the treatment of adenomyosis with HIFU have yielded positive results but still indicated the need to standardize protocols and optimize parameters. In a 2016 study, Gong et al. investigated factors affecting HIFU ablative efficiency in 245 patients diagnosed with adenomyosis. Increased abdominal wall thickness, distance from skin to lesion, richer blood supply, and high T2 signals on MRI scans were all found to be predictive factors for lower HIFU ablative efficiency 20. Marques et al. released a meta-analysis of all English language studies examining HIFU-treatment of adenomyotic lesions between 2010 and 2020 45. Results indicated that uterine volume and dysmenorrhea significantly decreased with a standard mean difference of 0.85 and 2.37 respectively at the 12 month-interval. Patients further reported a significant improvement in quality of life at both the 3 month and 12 month mark 45. Still, comparative studies have not been conducted to evaluate HIFU treatment against more traditional standards of care 45, indicating a need to assess direct impacts of treatment protocols prior to adjusting clinical decisions.
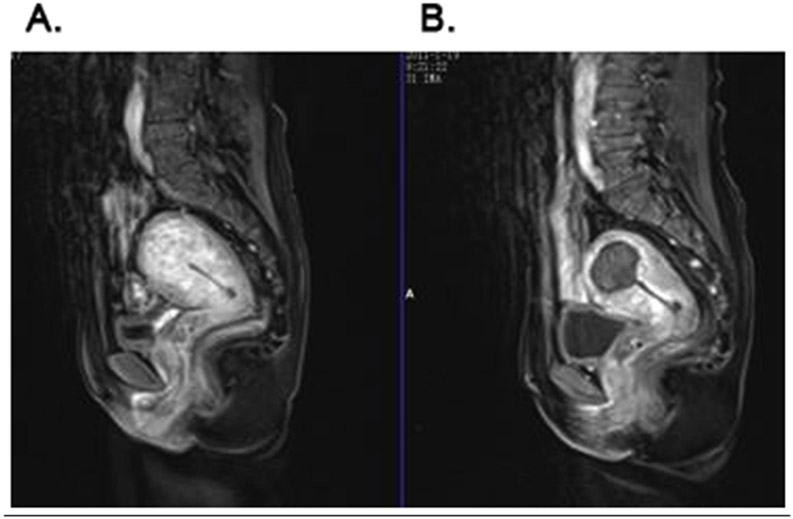
In 2004, HIFU was approved by the Food and Drug Administration (FDA) for the treatment of leiomyomas, otherwise known as uterine fibroids 106. Uterine leiomyomas are benign monoclonal tumors arising from smooth muscle cells of the myometrium. They are the most prevalent pelvic tumors in premenopausal females, currently affecting 11 million females in the United States 46. Treatments for uterine fibroids include hysterectomy, myomectomy, endometrial ablation, myolysis, and MRgHIFU 59. Compared to more traditional treatments, MRgHIFU has been theorized to be more safe and effective due to its noninvasiveness, rapid recovery time, and ability to spare the uterus 97. In 2015 Shui et al. evaluated the long-term improvement of clinical symptoms of adenomyosis after USgHIFU. 224 patients were followed for two years (Figure 3)65. All patients completed HIFU ablation without severe postoperative complications. Dysmenorrhea significantly decreased after treatment (P< 0.001) and the relief rate was 84.7%, 84.7%, and 82.3%, respectively at 3 months, 1 year, and 2 years after treatment. The menstrual volume in 109 patients with menorrhagia was also significantly improved after treatment (P< 0.001) with a relief rate of 79.8%, 80.7%, and 78.9%, respectively at 3 months, 1 year, and 2 years after HIFU treatment. This clinical follow up study determined that HIFU was a safe and effective treatment for adenomyosis.
Figure 3.
Preoperative and postoperative enhanced MRI. (A) Enhanced MRI of adenomyosis before treatment which shows the thickening of the myometrium in fundus of uterus and rich blood supply. (B) Enhanced MRI from a patient with adenomyosis 1 day after HIFU treatment, which shows non-perfused area in the lesion. Reprinted from Ultrasonics Sonochemistry, Vol. 27, Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: Two-year follow-up results, Pages 677-681 (2015) with permission from Elsevier65.
A retrospective observational trial published by Li et al. in 2020 analyzed long-term reintervention rates among their cohort of patients with uterine fibroids who were treated with ultrasound-guided HIFU 37. With an overall reintervention rate of 20.7% and 86.4% of patients reporting relief from distressing symptoms, HIFU was concluded to be an effective treatment for leiomyomas 37. Similarly, Wang et al. collected data on 245 women who were treated with ultrasound-guided HIFU for their uterine fibroids and 129 women who underwent uterus-sparing surgery for symptomatic fibroids 73. The treatment resulted in reduced procedural complications and significantly higher symptom relief (p < 0.05). Furthermore, long-term clinical outcomes were reported to be better in the group that was administered HIFU as compared to the uterus-sparing surgical group 73. While these studies are promising, larger-scale clinical trials should also be conducted to further validate these findings.
There are two completed or currently active clinical trials examining the treatment effects of HIFU 98 on adenomyosis and 13 on uterine leiomyomas 99. The vast majority of these trials are focused on HIFU as an ablative treatment in comparison with previously mentioned protocols such as uterine artery ligation, myomectomy, etc. The results of these ongoing trials will help to further elucidate the long-term effects of HIFU in the context of uterine leiomyomas/adenomyosis and aide in protocol optimization.
HIFU and Prostate Cancer
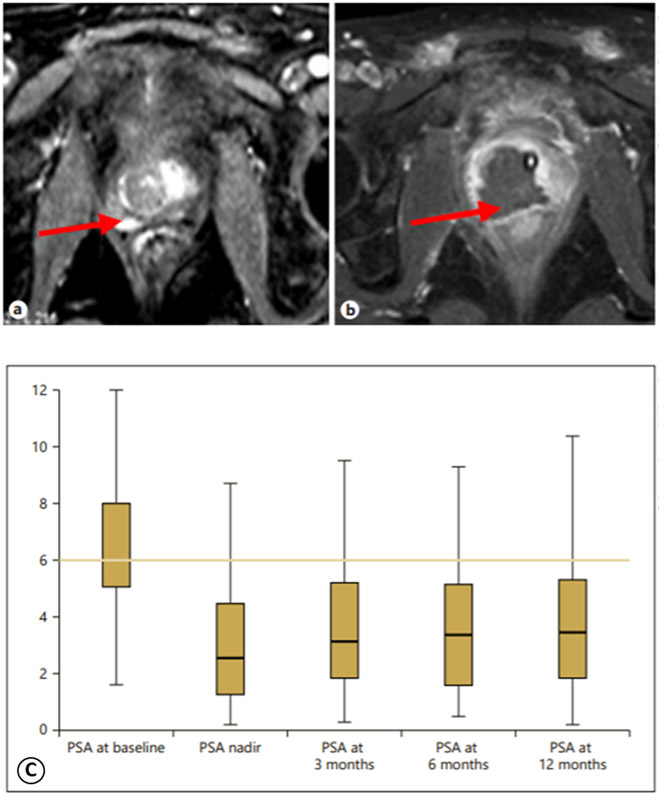
Prostate cancer is the second most common cancer in men and a major cause of mortality due to its high recurrence rates, necessitating extensive research into various potential tumor treatments to render affected patients disease-free 57. HIFU has been examined and employed for several years as a treatment method for ablation of prostate cancer 69. A multi-center study published in 2018 by Guillaumier and colleagues investigated 625 patients with nonmetastatic prostate cancer treated with HIFU between 2006 and 2015 21. They found that at the five-year mark, metastasis-free survival was 98%, cancer-specific survival levels were 100%, and morbidities were low as compared to whole-gland radical prostatectomy and radical radiotherapy, which are interventions that though extremely successful are commonly known to have urinary, sexual function, and bowel side effects. The authors concluded that though long-term data is unavailable, HIFU is an advantageous therapy for prostate cancer care, lacking the morbidities of more aggressive and invasive therapies, and can be offered as a treatment to certain patients with nonmetastatic disease 21. Glybochko et al. found similarly low morbidity rates in their 35-case retrospective study on patients who received HIFU hemiablation of the prostate cancer and too noted that HIFU shows promise as a low-risk and feasible procedure 19 (Figure 4).
Figure 4.
Preoperative ultrasound shear wave elastography and prostate histoscanning and dynamics of prostate-specific antigen (PSA) before and after hemiablation. Representative MRI control findings of the pathological focus (arrows) before HIFU hemiablation (a) and its disappearance 3 months after the procedure (b). (C) demonstrates a box plot showing changes in PSA level before and after HIFU hemiablation (n=35), The line indicated the mean, the box indicated the interquartile range, and whiskers indicate the maximum and minimum values. The final, published version of this article is available at https://www.karger.com/Article/Fulltext/499739 19.
Due to the high likelihood of recurrence of prostate cancer, the use of HIFU in prostate cancer has also been proposed and investigated as a salvage therapy after traditional therapy fails to prevent local recurrences. A study in 2017 by Crouzet and colleagues examined 418 patients with locally recurrent prostate cancer across several institutions treated with external beam radiotherapy followed by HIFU. The authors found that 7-year survival rates increased with the utilization of salvage HIFU 8. Furthermore, a study the next year by von Hardenburg et al. examined 24 patients who underwent MRI and transrectal ultrasound (TRUS) guided HIFU (nineteen patients with focal HIFU and five with zonal HIFU) as an ablative therapy for prostate cancer. The study found that though HIFU was capable of achieving successful local tumor ablation, 40% of patients actually had a positive biopsy at short-term follow-up 26, indicating a current need for a more robust treatment regimen as well as further investigation and development in the use of this technology before it can become a standalone therapy.
A search of clinicaltrials.gov provides a robust list of over 30 completed or active clinical trials investigating the use of HIFU in the treatment of prostate cancer, particularly regarding different guidance techniques and the utility of PET scans for prostate cancer identification and HIFU ablation. A few such trials include NCT03350529, investigating MRI guided transurethral ultrasound in prostate cancer and benign prostatic hyperplasia, and NCT03927521 and NCT04461509, both investigating using PET-MRI as a selection tool for HIFU treatment. No current trials exist regarding enhancement of drug delivery with the use of HIFU in prostate cancer, but this is would be an interesting are to explore in future studies in the prostatic cancer care field.
HIFU and Breast Cancer
HIFU has undergone extensive examination in multiple cancer types as a technique for cancer ablation and drug delivery, and results from these studies provide encouragement for further exploration. One such cancer investigated is breast cancer, which is the most common cancer in women (276,480 new cases/yr and 42,170 deaths/yr) 100; Although it has a high survival rate (5-year survival of 89% between 2005 and 2011) 58, there remains a significant population who suffer from more aggressive disease refractory to standard surgical intervention, radiation, and chemotherapy protocols, demanding the exploration of advanced techniques, including HIFU.
Several studies have examined the use of HIFU as a method of ablation for breast cancer treatment. One of the earliest published results came from Wu et al. in 2003, who examined a cohort of 48 women with biopsy-proven breast cancer staged at T1-2, N0-2, M0 77. The patients were randomized to either the control group, who received modified radical mastectomy, or the treatment group, who received ultrasound-guided HIFU and modified radical mastectomy within 1-2 weeks of ablation. The authors found that HIFU left no severe short-term side effects and that cells treated with HIFU underwent severe damage, achieving complete coagulative necrosis and losing the ability to proliferate and metastasize, indicating its worth as a potential noninvasive treatment of breast cancer 77. Wu and colleagues further explored this technique in 2007, affirming its ability to achieve wide local ablation in localized breast cancer 76,78.
Other studies since have reinforced the value of HIFU as an ablative technique for breast cancer treatment 56. In 2016, Knuttel et al. examined the histopathological changes of MR-guided HIFU versus that of traditional radiofrequency ablation (RFA) 34. The authors found that there were several distinctions between histopathologic changes in HIFU and RFA. For HIFU, there were more necrotic-type changes in vivo that were more subtle ex vivo, whereas for RFA, in vivo and ex vivo histopathologic changes were similar in character, with hyper-eosinophilic stroma and elongated nuclei. Further, RFA created large transition zones, while HIFU created smaller ones, suggesting a more defined area of effect with HIFU 34. Several ongoing clinical trials (NCT02407613, NCT03560102, NCT03342625, and NCT00008437) are continuing to examine both short- and long-term effects of HIFU as a method of noninvasive tumor ablation and will continue to guide clinical practice as their results are determined in the coming years.
HIFU has also been examined as a method to enhance drug delivery in breast cancer. Based on prior research indicating that pulsed HIFU could enhance systemic delivery of various drugs, Frenkel and colleagues in 2006 performed one of the earliest experiments by examining the delivery of liposome-encapsulated doxorubicin in a murine breast cancer model using pulsed HIFU. These authors injected a cell suspension of either a mouse mammary adenocarcinoma or squamous cell carcinoma into the bilateral flanks of their mice, and unilaterally treated with pulsed HIFU and/or doxorubicin as compared to a saline control on day 21 of tumor growth via tail-vein injection. Their aim was to use HIFU to enhance uptake, but not specifically through hyperthermia. The results from this study indicated that HIFU did not sensitize tissue to doxorubicin delivery 16. Although such results were not immediately promising, other tumor types investigated have shown HIFU to be a viable method of enhancing doxorubicin delivery via induction of hyperthermia in rabbits with a unilateral Vx2 tumor in the thigh. The rabbits were treated at 11-13 days after inoculation with the tumor, with thermosensitive liposomal doxorubicin via ear-vein injection. For rabbits receiving the hyperthermia variable, this was injected once the HIFU created a mild hyperthermia state of 40-43°C, with a target mean of 42°C. The rabbits who underwent hyperthermia-focused HIFU treatment had better uptake of doxorubicin into their tumors and longer survival times. 2,67 An ongoing clinical trial (NCT03749850) is taking place to further examine how MR-guided HIFU can enhance doxorubicin delivery in breast cancer. Another mechanism by which HIFU may enhance drug delivery is through the disruption of microbubbles; a study by Lee and colleagues found that HIFU burst chemically-generated microbubbles containing the chemotherapeutic drug methotrexate, allowing for highly targeted local delivery 35. A final mechanism by which HIFU may augment drug delivery is through enhancing antigen presentation. Specifically, HIFU can cause in situ tumor emulsification, facilitating an increased breakdown of antigenic proteins and stimulating an inflammatory response. This response upregulates chemoattractants and allows for local delivery of exogenous drug-carrier molecules 44. A current trial (NCT03237572) is examining the mechanisms behind this therapeutic regimen, specific to pembrolizumab therapy in patients with metastatic breast cancer. With careful titration and examination, HIFU may become a useful tactic to enhance drug delivery to treat multiple types of solid tumors.
HIFU and CNS Diseases/Tumors
With neurological disorders a major cause of death and disability globally, dysfunction in the central nervous system (CNS) is associated with serious consequences 71. Comprising the brain and spinal cord, the CNS is responsible for sensory integration, response coordination, and motor output. High intensity focused ultrasound was first applied to the human CNS when the Fry brothers discovered its ability to treat neurological disorders in the early 1950s 30. Since then, it has been utilized to treat essential tremor (ET), neuropathic pain, and CNS tumors. HIFU has also been used as a technique to transiently make the brain more accessible for the delivery of systemically administered agents.
Essential Tremor
In 2016, focused ultrasound was approved in the United States as a treatment for essential tremor (ET) by the FDA109. In this treatment, the ventral intermediate nucleus of the thalamus is ablated, selectively creating brain lesions at the focal point of soundwave convergence. With structures in the wave path largely unaffected, HIFU is relatively safe for non-targeted regions and results in lower risk of thrombosis and lower risk of infection as compared to traditional ET treatments such as deep brain stimulation and radiofrequency ablation 24,36,60. A study by Ito et al. explored long-term clinical outcomes of MRgFUS in patients with medication-refractory ET 29. Focal left-sided thalamotomy resulted in a 60% reduction of clinical rating scale for tremor scores (CRST) in the right hand. Though patients reported significant symptoms including headache during treatment and sensory disturbances post-treatment, the study validated MRgFUS unilateral thalamotomy as a viable choice for refractory ET 29. Furthermore, Park et al. demonstrated sustained clinical mitigation of refractory ET by unilateral MRgFUS thalamotomy 4 years after initial treatment 54. Current clinical trials include NCT04112381 in which scientists are examining the effect of bilateral thalamotomies on left and right-sided tremors 101. NCT03465761 is another similar prospective single-arm trial, studying outcomes of bilateral focused ultrasound ablative treatment to treat drug-refractory ET 102. These larger-scale studies will set the stage for further treatment optimization of HIFU technology.
Neuropathic Pain
Neuropathic pain is generally caused by abnormalities in the somatosensory system and impacts 7-10% of the population 7. Dealing with a chronic issue, those affected report a lower quality of life due to persistent symptoms and limited treatment options. Primary central neuropathic pain is frequently a result of injury to the spinal cord whereas peripheral neuralgias can be due to an array of disease states including diabetes, inherited disorders, autoimmune diseases, etc 103. Conventional methods to treat neuropathic pain include medications, nerve blocks, and invasive surgery, yet many patients are unable to find long-term relief.
Focused ultrasound was first theorized to have an effect on neuropathic pain in 1996 10. A prospective clinical trial released in 2012 followed 12 patients who suffered from treatment-resistant chronic neuropathic pain. MRgFUS was utilized to perform central lateral thalamotomies, with patients reporting a mean pain relief score of 57% at the 1-year follow-up interval 31. In a 2020 study, Ma et al. further concluded the high potential of HIFU to become a non-invasive treatment modality for primary trigeminal neuralgia 42. There are currently 8 clinical trials underway to examine the short- and long-term effects of focused ultrasound on neuropathic pain, including trigeminal neuralgia (NCT04579692), phantom limb pain (NCT03111277), and craniofacial neuralgias (NCT04202783). Though preliminary studies have speculated the ability of HIFU to relieve neuropathic pain, the results of these trials will dictate its potential to become a mainstay treatment in the field.
CNS Tumors and the Blood-Brain Barrier
In 2020, approximately 23,890 malignant tumors of the brain and spinal cord will be diagnosed 66. Current strategies for treating brain tumors include a combination of surgery, radiation, and chemotherapy. Yet, the most promising chemotherapies have been unable to demonstrate as much success in the central nervous system as they have in other systemic locations 75. This is due to the blood-brain barrier (BBB), which is a tight layer of cells lining vessels in the brain that serve to protect the brain from potentially harmful substances in the circulation. In addition to functioning in an ablative capacity, HIFU has been shown to open this endothelial barrier in a safe, minimally invasive, and regional manner by means of cavitation and other physical mechanisms 104. The ability to non-destructively penetrate the blood-brain barrier in a targeted manner and deliver appropriate concentrations of select drugs has the potential to revolutionize the way we treat some of our most vulnerable patients.
The utilization of focused ultrasound to transiently and reversibly open the blood-brain barrier poses several advantages over traditional delivery methods. Transcranial application eradicates the need for invasive procedures, reducing mechanical shift of brain tissue and infection risk. Tandem MRI use would allow for focal application of FUS, increasing the precision of treatment. And furthermore, current research has indicated no long-term deficits in blood-brain barrier function 4.
Proof of concept has been shown in multiple in vivo trials, demonstrating targeted delivery of nanoparticles to selective brain regions in animal models 6,39,72. Recently, human trials are emerging evaluating the feasibility of focused ultrasound applications in this regard. A clinical trial published by Mainprize et al. reported a 15-50% transient contrast enhancement in five patients who were administrated chemotherapy after MRgFUS pulses for high-grade glioma 43. Barrier function reportedly returned to normal within 20 hours, affirming the transient and reversible nature of the treatment modality. Sattiraju et al. further posited on the compounded treatment effects of focused ultrasound to bypass the blood-brain barrier and surpass the efficacy of therapeutic drugs 62. Chan et al. delivered varying sizes of Cy5-DNA-Au nanoparticles across the BBB using focused ultrasound and showed smaller NPs were delivered 6 times more efficiently than the largest ones tested, even though the difference in diameter between the particles was < 5nm), suggesting that optimizing NPs for intracranial delivery needs to be precisely fine-tuned (Figure 5)6. To establish translational data and substantiate theorized efficacy in humans, there are currently four clinical trials examining the effect of focused ultrasound on blood-brain barrier disruptions. One trial (NCT02343991) is examining the potential of MRgFUS to facilitate doxorubicin accumulation in the brain tumors of ten patients. Another prospective, single arm, non-randomized trial (NCT03714243) will evaluate focused ultrasound as a tool to intentionally disrupt the blood-brain barrier in a transient and targeted manner in patients with breast cancer and brain metastases. The larger goal of both trials is to examine and determine safety factors in order to provide quantitative parameters when assessing treatment efficacy in the future.
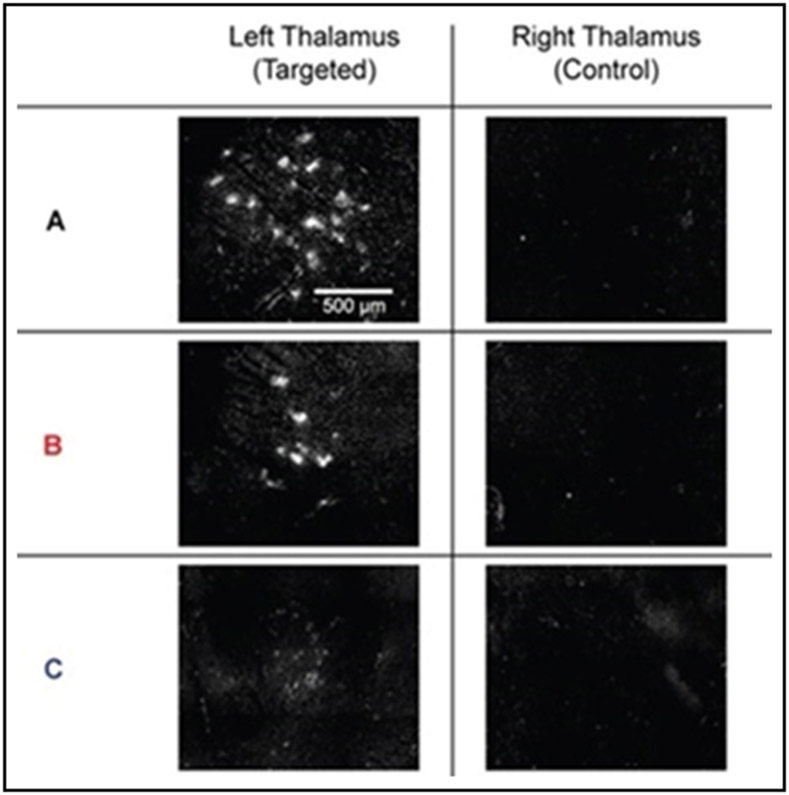
Figure 5.
Fluorescence images from the delivery of Cy5-DNA-Au NPs across the BBB using focused ultrasound. There is a size-dependency associated with the delivery of Cy5-DNA-Au NPs across the BBB using focused ultrasound, with the smallest NPs tested in this study (A) delivered across the BBB six times more efficiently than the larger/largest NPs tested (B/C). A spot-like distribution of fluorescence was observed in the left thalamus, while no fluorescence signal is detected in the right thalamus. Reprinted with permission from John Wiley and Sons 6.
As evidenced throughout this discussion, the application of HIFU in a therapeutic rather than diagnostic method has invigorated the medical community in recent years. Though published literature on HIFU as a treatment modality on humans is limited, several clinical trials are underway in the hopes to introduce HIFU into common practice (Table 3). Promising preliminary results from a number of these trials indicate that HIFU will become a valuable therapeutic tool in the years to come.
Table 3.
Current active ongoing HIFU clinical trials described at clinicaltrials.gov (last updated on 04/11/2021) 105
| Disease | N | Primary Outcome |
ClinicalTrials.gov Identifier |
|---|---|---|---|
| Uterine Adenomyosis | 10 | Perceived symptom change after HIFU treatment based on menstrual pain score | NCT02954757 |
| Uterine Leiomyoma | 40 | Post-treatment myoma stiffness and ablathermy efficiency | NCT04345003 |
| 50 | Temperature elevation, non-perfused volume (NPV) of fibroid, adverse events related to potential damage to tissue outside the treatment zone, and over treatment volume | NCT03323905 | |
| Uterine Adenomyosis + Leiomyoma | 500 | Technical efficacy of HIFU for treatment of uterine fibroids as assessed by a change in the symptom severity | NCT02914704 |
| Prostate Cancer | 40 | Feasibility to use the PET-MRI imaging for focal-HIFU guidance | NCT03927521 |
| 170 | Patient proportion with controlled disease, treatment efficacy (percentage of positive biopsies in the treated lobe at 12 months after inclusion) | NCT03568188 | |
| 250 | Number of patients without clinically significant prostate cancer, functional results, patients who need repeated focal treatment, disease-free survival, treatment-free survival, overall survival, metastasis-free survival, patients who need radical (surgery or radiation), or palliative treatment (hormone therapy) | NCT04549688 | |
| 146 | Patient proportion who needed to seek further radical treatment | NCT03531099 | |
| 10 | Positron emission tomography (PET) based of Assessment of Local Therapeutic Response | NCT03949517 | |
| 117 | Absence of biochemical failure (defined as achieving a PSA nadir of ≤ 0.5 ng/mL within 12 months of treatment) | NCT00772317 | |
| 4022 | Recurrence-free survival | NCT04307056 | |
| 130 | Recurrence free survival after focal therapy, pathological persistence after prostate cancer focal therapy | NCT03255135 | |
| 20 | Absence of prostate cancer on Biopsy | NCT03927924 | |
| 200 | Treatment failure | NCT03668652 | |
| 354 | Conversion to radical therapy and/or requiring systemic therapy and/or developing metastases and/or dying of prostate cancer | NCT01194648 | |
| 70 | Targeting accuracy of HIFU ablation and volume of HIFU ablation separately in each study arm/group, radiologically and histopathologically determined treatment accuracy, safety of MRI-guided transurethral HIFU ablation in various prostate diseases | NCT03350529 | |
| 200 | Prostate biopsy Gleason grade | NCT03492424 | |
| 2450 | Progression-free survival (PFS) rates of focal therapy alone compared to radical therapy, Failure-Free-Survival (FFS) rates of focal therapy alone compared to focal therapy combined with other therapies | NCT04049747 | |
| 10 | Micro-wave ablation area | NCT04831905 | |
| 918 | Post-standard of care prostate biopsy, safety, progression-free survival | NCT03763253 | |
| Breast Cancer | 10 | Amount of ablated tissue at histopathological examination, presence of non-perfused volumes on DCE-MRI | NCT02407613 |
| 15 | Change in tumor infiltrating lymphocytes | NCT03237572 | |
| 10 | Accuracy of MRI as method for assessment of quantitative/qualitative treatment success (correlation with results of the histopathological analysis performed as reference method | NCT03560102 | |
| 15 | Efficacy of HIFU for the treatment of breast tumors based on histological criteria | NCT03342625 | |
| 12 | Safety and tolerability of the study treatment, logistical MR thermometry and administration | NCT03749850 | |
| 32 | Incidence and severity of adverse events and incidence of dose-limiting toxicities, proportion of patients with increased CD8+ T cell infiltration of spot-treated metastasis. | NCT04116320 | |
| Essential Tremor | 50 | Change in QUEST Score, patient-based Assessment of Utility | NCT04501484 |
| CNS Tumor | 10 | Incidence of Treatment-Emergent Adverse Events Safety and Tolerability, Measurement of Tumor Volume | NCT03028246 |
| CNS Blood-Brain Barrier Disruption | 10 | To evaluate the incidence and severity of adverse events associated with the ExAblate transcranial treatment | NCT02343991 |
| 10 | Rate of adverse events following each treatment through end of study | NCT03714243 |
High-intensity focused ultrasound is an emerging treatment modality with ground-breaking potential. Since its integration with imaging software in the early 2000s, HIFU’s applicability has increased as both an ablative treatment and technique to improve drug delivery of multiple agents. Significant applications of HIFU have been demonstrated clinically for CNS disorders, pain, and cancer. For oncological diseases in particular, including uterine leiomyomas, adenomyosis, breast, prostate, and CNS cancers, the field has significantly advanced over the last decade and has great promise. Despite these gains, larger clinical trials are still needed to further substantiate results and increase the therapeutic options to best utilize this innovative modality.
Acknowledgements
The authors thank the NIH for support (R01CA228133).
References
- 1.Airan RD, Meyer RA, Ellens NPK, Rhodes KR, Farahani K, Pomper MG, Kadam SD, and Green JJ. Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions. Nano Letters 17:652–659, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bing C, Patel P, Staruch RM, Shaikh S, Nofiele J, Wodzak Staruch M, Szczepanski D, Williams NS, Laetsch T, and Chopra R. Longer heating duration increases localized doxorubicin deposition and therapeutic index in Vx2 tumors using MR-HIFU mild hyperthermia and thermosensitive liposomal doxorubicin. International Journal of Hyperthermia 36:196–203, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowary P, and Greenberg BD. Noninvasive Focused Ultrasound for Neuromodulation: A Review. , 2018. [DOI] [PubMed] [Google Scholar]
- 4.Burgess A, Shah K, Hough O, and Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. , 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canavese G, Ancona A, Racca L, Canta M, Dumontel B, Barbaresco F, Limongi T, and Cauda V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chemical Engineering Journal 340:155–172, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan TG, Morse S. v., Copping MJ, Choi JJ, and Vilar R. Targeted Delivery of DNA-Au Nanoparticles across the Blood–Brain Barrier Using Focused Ultrasound. ChemMedChem 13:1311–1314, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, and Raja SN. Neuropathic pain. Nature Reviews Disease Primers 3:17002, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crouzet S, Blana A, Murat FJ, Pasticier G, Brown SCW, Conti GN, Ganzer R, Chapet O, Gelet A, Chaussy CG, Robertson CN, Thuroff S, and Ward JF. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU International 119:896–904, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Dallapiazza RF, Timbie KF, Holmberg S, Gatesman J, Lopes MB, Price RJ, Miller GW, and Elias WJ. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. Journal of Neurosurgery 128:875–884, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies IAI, Gavrilov LR, and Tsirulnikov EM. Application of focused ultrasound for research on pain. , 1996. [DOI] [PubMed] [Google Scholar]
- 11.Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, and Aubry JF. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Current Biology 23:2430–2433, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KCP, and Wood BJ. Pulsed-high intensity focused ultrasound and low temperature - Sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clinical Cancer Research 13:2722–2727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endo M, and Lin PP. Surgical margins in the management of extremity soft tissue sarcoma. Chinese Clinical Oncology 7:4, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Fishman PS, and Frenkel V. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapeutics 14:393–404, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley JL, Vaezy S, and Crum LA. Applications of high-intensity focused ultrasound in medicine: Spotlight on neurological applications. Applied Acoustics 68:245–259, 2007. [Google Scholar]
- 16.Frenkel V, Etherington A, Greene M, Quijano J, Xie J, Hunter F, Dromi S, and Li KCP. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: Investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Academic Radiology 13:469–479, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Fry FJ, Ades HW, and Fry WJ. Production of reversible changes in the central nervous system by ultrasound. Science 127:83–84, 1958. [DOI] [PubMed] [Google Scholar]
- 18.Fry WJ, and Fry FJ. Fundamental Neurological Research and Human Neurosurgery Using Intense Ultrasound. IRE Transactions on Medical Electronics ME-7:166–181, 1960. [DOI] [PubMed] [Google Scholar]
- 19.Glybochko P. v., Amosov A. v., Krupinov GE, Petrovskii N. v., andLumpov IS. Hemiablation of Localized Prostate Cancer by High-Intensity Focused Ultrasound: A Series of 35 Cases. Oncology (Switzerland) 97:44–48, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong C, Yang B, Shi Y, Liu Z, Wan L, Zhang H, Jiang D, and Zhang L. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: A retrospective study. International Journal of Hyperthermia 32:496–503, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, Hosking-Jervis F, Hindley RG, Lewi H, McCartan N, Moore CM, Nigam R, Ogden C, Persad R, Shah K, van der Meulen J, Virdi J, Winkler M, Emberton M, and Ahmed HU. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. European Urology 74:422–429, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ter Haar Gail, and Coussios C. High intensity focused ultrasound: Physical principles and devices. International Journal of Hyperthermia 23:89–104, 2007. [DOI] [PubMed] [Google Scholar]
- 23.ter Haar GR, and Robertson D. Tissue Destruction with Focused Ultrasound in vivo. European Urology 23:8–11, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Harary M, Segar DJ, Hayes MT, and Cosgrove GR. Unilateral Thalamic Deep Brain Stimulation Versus Focused Ultrasound Thalamotomy for Essential Tremor. World Neurosurgery 126:e144–e152, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Harary M, Segar DJ, Huang KT, Tafel IJ, Valdes PA, and Cosgrove GR. Focused ultrasound in neurosurgery: A historical perspective. Neurosurgical Focus 44:E2, 2018. [DOI] [PubMed] [Google Scholar]
- 26.von Hardenberg J, Westhoff N, Baumunk D, Hausmann D, Martini T, Marx A, Porubsky S, Schostak M, Michel MS, and Ritter M. Prostate cancer treatment by the latest focal HIFU device with MRI/TRUS-fusion control biopsies: A prospective evaluation. Urologic Oncology: Seminars and Original Investigations 36:401.e1–401.e9, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Hosseini SHR, Zheng X, and Vaezy S. Effects of gas pockets on high-intensity focused ultrasound field. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 58:1203–1210, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Hynynen K, McDannold N, Vykhodtseva N, and Jolesz FA. Non-invasive opening of BBB by focused ultrasound. Acta Neurochirurgica, Supplementum 86:555–558, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Yamamoto K, Fukutake S, Odo T, and Kamei T. Two-year Follow-up Results of Magnetic Resonance Imaging-guided Focused Ultrasound Unilateral Thalamotomy for Medication-refractory Essential Tremor. Internal Medicine 59:, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagannathan J, Sanghvi NK, Crum LA, Yen C-P, Medel R, Dumont AS, Sheehan JP, Steiner L, Jolesz F, and Kassell NF. High intensity focused ultrasound surgery (HIFU) of the brain: A historical perspective, with modern applications. 64:201–211, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, and Martin E. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurgical focus 32:, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Jenne JW, Preusser T, and Günther M. High-intensity focused ultrasound: Principles, therapy guidance, simulations and applications. Zeitschrift fur Medizinische Physik 22:311–322, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Khirallah J, Schmieley R, Demirel E, Rehman TU, Howell J, Durmaz YY, and Vlaisavljevich E. Nanoparticle-mediated histotripsy (NMH) using perfluorohexane “nanocones.” Physics in Medicine and Biology 64:, 2019. [DOI] [PubMed] [Google Scholar]
- 34.Knuttel FM, Waaijer L, Merckel LG, van den Bosch MAAJ, Witkamp AJ, Deckers R, and van Diest PJ. Histopathology of breast cancer after magnetic resonance-guided high-intensity focused ultrasound and radiofrequency ablation. Histopathology 69:250–259, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Al-Kaabi L, Mawart A, Khandoker A, Alsafar H, Jelinek HF, Khalaf K, Park JH, and Kim YC. Ultrasound-mediated drug delivery by gas bubbles generated from a chemical reaction. Journal of Drug Targeting 26:172–181, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Levi V, Eleopra R, Franzini A, and Romito L. Is Deep Brain Stimulation still an option for tremor recurrence after Focused Ultrasound thalamotomy? A case report. Journal of Clinical Neuroscience 68:344–346, 2019. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Jiang Z, Deng X, and Xu D. Long-term follow-up outcome and reintervention analysis of ultrasound-guided high intensity focused ultrasound treatment for uterine fibroids. International Journal of Hyperthermia 37:1046–1051, 2020. [DOI] [PubMed] [Google Scholar]
- 38.Liang X, Gao J, Jiang L, Luo J, Jing L, Li X, Jin Y, and Dai Z. Nanohybrid liposomal cerasomes with good physiological stability and rapid temperature responsiveness for high intensity focused ultrasound triggered local chemotherapy of cancer. ACS Nano 9:1280–1293, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Luo Z, Jin K, Pang Q, Shen S, Yan Z, Jiang T, Zhu X, Yu L, Pang Z, and Jiang X. On-Demand Drug Release from Dual-Targeting Small Nanoparticles Triggered by High-Intensity Focused Ultrasound Enhanced Glioblastoma-Targeting Therapy. ACS Applied Materials and Interfaces 9:31612–31625, 2017. [DOI] [PubMed] [Google Scholar]
- 40.Lynn JG, Zwemer RL, Chick AJ, and Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. Journal of General Physiology 26:179–193, 1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon PC, Gray MD, Mannaris C, Folkes LK, Stratford M, Campo L, Chung DYF, Scott S, Anderson M, Goldin R, Carlisle R, Wu F, Middleton MR, Gleeson FV, and Coussios CC. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. The Lancet Oncology 19:1027–1039, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Hsu G, and Zhang F. The applicability and efficacy of magnetic resonance-guided high intensity focused ultrasound system in the treatment of primary trigeminal neuralgia. Medical Hypotheses 139:109688, 2020. [DOI] [PubMed] [Google Scholar]
- 43.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Perry J, and Hynynen K. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Scientific Reports 9:, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maloney E, Khokhlova T, Pillarisetty VG, Schade GR, Repasky EA, Wang YN, Giuliani L, Primavera M, and Hwang JH. Focused ultrasound for immuno-adjuvant treatment of pancreatic cancer: An emerging clinical paradigm in the era of personalized oncotherapy. , 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marques ALS, Andres MP, Kho RM, and Abrão MS. Is High-intensity Focused Ultrasound Effective for the Treatment of Adenomyosis? A Systematic Review and Meta-analysis. , 2020. [DOI] [PubMed] [Google Scholar]
- 46.Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, and Kerolous M. Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. Journal of Women’s Health 27:1359–1367, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClure A Using High-Intensity Focused Ultrasound as a Means to Provide Targeted Drug Delivery. Journal of Diagnostic Medical Sonography 32:343–350, 2016. [Google Scholar]
- 48.McRury ID, Panescu D, Mitchell MA, and Haines DE. Nonuniform heating during radiofrequency catheter ablation with long electrodes: Monitoring the edge effect. Circulation 96:4057–4064, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Moosavi Nejad S, Takahashi H, Hosseini H, Watanabe A, Endo H, Narihira K, Kikuta T, and Tachibana K. Acute effects of sono-activated photocatalytic titanium dioxide nanoparticles on oral squamous cell carcinoma. Ultrasonics Sonochemistry 32:95–101, 2016. [DOI] [PubMed] [Google Scholar]
- 50.Muratore R A history of the sonocare CST-100: The first FDA-approved HIFU device. , 2006. [Google Scholar]
- 51.Mylonas N Development of positioning devices for MRI-guided high intensity focused ultrasound (HIFU) for abdominal, thyroid and brain, tumours. 2012. [Google Scholar]
- 52.Nakamura K, Baba S, Saito S, Tachibana M, and Murai M. High-intensity focused ultrasound energy for benign prostatic hyperplasia: Clinical response at 6 months to treatment using Sonablate 200®. Journal of Endourology 11:197–201, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Osminkina LA, Nikolaev AL, Sviridov AP, Andronova NV, Tamarov KP, Gongalsky MB, Kudryavtsev AA, Treshalina HM, and Timoshenko VY. Porous silicon nanoparticles as efficient sensitizers for sonodynamic therapy of cancer. Microporous and Mesoporous Materials 210:169–175, 2015. [Google Scholar]
- 54.Park YS, Jung NY, Na YC, and Chang JW. Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Movement Disorders 34:727–734, 2019. [DOI] [PubMed] [Google Scholar]
- 55.Payne A, Vyas U, Blankespoor A, Christensen D, and Roemer R. Minimisation of HIFU pulse heating and interpulse cooling times. International Journal of Hyperthermia 26:198–208, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Peek MCL, and Douek M. Ablative techniques for the treatment of benign and malignant breast tumours. , 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rawla P Epidemiology of Prostate Cancer. World Journal of Oncology 10:63–89, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rojas K, and Stuckey A. Breast Cancer Epidemiology and Risk Factors. Clinical Obstetrics and Gynecology 59:651–672, 2016. [DOI] [PubMed] [Google Scholar]
- 59.Sabry M, and Al-Hendy A. Medical treatment of uterine leiomyoma. , 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saluja S, Barbosa DAN, Parker JJ, Huang Y, Jensen MR, Ngo V, Santini VE, Pauly KB, Ghanouni P, McNab JA, and Halpern CH. Case Report on Deep Brain Stimulation Rescue After Suboptimal MR-Guided Focused Ultrasound Thalamotomy for Essential Tremor: A Tractography-Based Investigation. Frontiers in Human Neuroscience 14:, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanguinetti JL, Hameroff S, Smith EE, Sato T, Daft CMW, Tyler WJ, and Allen JJB. Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Frontiers in Human Neuroscience 14:52, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.SATTIRAJU A, SUN Y, SOLINGAPURAM SAI KK, LI KCP, and MINTZ A. Maximizing Local Access to Therapeutic Deliveries in Glioblastoma. Part IV: Image-Guided, Remote-Controlled Opening of the Blood–Brain Barrier for Systemic Brain Tumor Therapy. In: Glioblastoma. Codon Publications, 2017, pp. 395–404.doi: 10.15586/codon.glioblastoma.2017.ch20 [DOI] [PubMed] [Google Scholar]
- 63.Sazgarnia A, Shanei A, Meibodi NT, Eshghi H, and Nassirli H. A novel nanosonosensitizer for sonodynamic therapy: In vivo study on a colon tumor model. Journal of Ultrasound in Medicine 30:1321–1329, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Scipione R, Anzidei M, Bazzocchi A, Gagliardo C, Catalano C, and Napoli A. HIFU for Bone Metastases and other Musculoskeletal Applications. Seminars in Interventional Radiology 35:261–267, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shui L, Mao S, Wu Q, Huang G, Wang J, Zhang R, Li K, He J, and Zhang L. High-intensity focused ultrasound (HIFU) for adenomyosis: Two-year follow-up results. Ultrasonics Sonochemistry 27:677–681, 2015. [DOI] [PubMed] [Google Scholar]
- 66.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians 70:7–30, 2020. [DOI] [PubMed] [Google Scholar]
- 67.Staruch RM, Hynynen K, and Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: Therapeutic effect in rabbit Vx2 tumours. International Journal of Hyperthermia 31:118–133, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Stewart HF, Repacholi MH, and Benwell DA. Ultrasound Therapy. In: Essentials of Medical Ultrasound. Totowa, NJ: Humana Press, 1982, pp. 181–213.doi: 10.1007/978-1-4612-5805-6_6 [DOI] [Google Scholar]
- 69.Sundaram KM, Chang SS, Penson DF, and Arora S. Therapeutic Ultrasound and Prostate Cancer. Seminars in Interventional Radiology 34:187–200, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taran FA, Stewart EA, and Brucker S. Adenomyosis: Epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe und Frauenheilkunde 73:924–931, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thakur KT, Albanese E, Giannakopoulos P, Jette N, Linde M, Prince MJ, Steiner TJ, and Dua T. Neurological Disorders. In: Disease Control Priorities, Third Edition (Volume 4): Mental, Neurological, and Substance Use Disorders. The World Bank, 2016, pp. 87–107.doi: 10.1596/978-1-4648-0426-7_ch5 [DOI] [Google Scholar]
- 72.Treat LH, McDannold N, Zhang Y, Vykhodtseva N, and Hynynen K. Improved Anti-Tumor Effect of Liposomal Doxorubicin After Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound in Medicine and Biology 38:1716–1725, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Liu X, Wang W, Tang J, and Song L. Long-term Clinical Outcomes of US-Guided High-Intensity Focused Ultrasound Ablation for Symptomatic Submucosal Fibroids: A Retrospective Comparison with Uterus–Sparing Surgery. Academic Radiology, 2020.doi: 10.1016/j.acra.2020.05.010 [DOI] [PubMed] [Google Scholar]
- 74.Wang ZB, Wu F, Wang ZL, Zhang Z, Zou JZ, Liu C, Liu YG, Cheng X, Du YH, He ZC, Gu ML, Wang ZG, and Feng R. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou Province miniswine. Ultrasonics Sonochemistry 4:181–182, 1997. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Sun H, and Yakisich J. Overcoming the Blood-Brain Barrier for Chemotherapy: Limitations, Challenges and Rising Problems. Anti-Cancer Agents in Medicinal Chemistry 14:1085–1093, 2014. [DOI] [PubMed] [Google Scholar]
- 76.Wu F, ter Haar G, and Chen WR. High-intensity focused ultrasound ablation of breast cancer. Expert Review of Anticancer Therapy 7:823–831, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Wu F, Wang ZB, De Cao Y, Chen WZ, Bai J, Zou JZ, and Zhu H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. British Journal of Cancer 89:2227–2233, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu F, Wang Z-B, Cao Y-D, Zhu X-Q, Zhu H, Chen W-Z, and Zou J-Z. “‘Wide Local Ablation’” of Localized Breast Cancer Using High Intensity Focused Ultrasound. Journal of Surgical Oncology 96:130–136, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Xin Z, Lin G, Lei H, Lue TF, and Guo Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. , 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yiannakou M, Menikou G, Yiallouras C, and Damianou C. MRI-guided coupling for a focused ultrasound system using a top-to-bottom propagation. Journal of Therapeutic Ultrasound 5:, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.You DG, Yoon HY, Jeon S, Um W, Son S, Park JH, Kwon IC, and Kim K. Deep tissue penetration of nanoparticles using pulsed-high intensity focused ultrasound. Nano Convergence 4:30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.You Y, Wang Z, Ran H, Zheng Y, Wang D, Xu J, Wang Z, Chen Y, and Li P. Nanoparticle-enhanced synergistic HIFU ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale 8:4324–4339, 2016. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, and Wang ZB. High-intensity focused ultrasound tumor ablation: Review of ten years of clinical experience. , 2010. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Yong L, Luo Y, Ding X, Xu D, Gao X, Yan S, Wang Q, Luo J, Pu D, and Zou J. Enhancement of HIFU ablation by sonosensitizer-loading liquid fluorocarbon nanoparticles with pre-targeting in a mouse model. Scientific Reports 9:, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Y-F High intensity focused ultrasound in clinical tumor ablation. World Journal of Clinical Oncology 2:8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.This Month In Physics Historyat <https://www.aps.org/publications/apsnews/201403/physicshistory.cfm>
- 87.History of ultrasound in medicine ∣ Radiology Reference Article ∣ Radiopaedia.orgat <https://radiopaedia.org/articles/history-of-ultrasound-in-medicine?lang=us>
- 88.Different Uses for Ultrasound - Medical Associates of Northwest Arkansasat <https://www.mana.md/different-uses-for-ultrasound/>
- 89.First Preclinical Study - Focused Ultrasound Foundationat <https://www.fusfoundation.org/timeline-of-focused-ultrasound/first-preclinical-study>
- 90.First Cancer Application - Focused Ultrasound Foundationat <https://www.fusfoundation.org/the-technology/timeline-of-focused-ultrasound?view=article&id=2274:first-cancer-application&catid=135:timeline>
- 91.FDA Approves Use of InSightec’s ExAblate(R) 2000 With GE Healthcare’s 3 Tesla Magnetic Resonance Imaging System ∣ BioSpaceat <https://www.biospace.com/article/releases/fda-approves-use-of-insightec-s-exablate-r-2000-with-ge-healthcare-s-3-tesla-magnetic-resonance-imaging-system-/>
- 92.Neuropathic Pain - Focused Ultrasound Foundationat <https://www.fusfoundation.org/diseases-and-conditions/neurological/neuropathic-pain>
- 93.Clinical Trial to Disrupt the Blood-brain Barrier for Brain Tumor Treatment Launched at University of Maryland - Focused Ultrasound Foundationat <https://www.fusfoundation.org/news/clinical-trial-to-disrupt-the-blood-brain-barrier-for-brain-tumor-treatment-launched-at-university-of-maryland>
- 94.High-Intensity Focused Ultrasound (HIFU) ∣ UCSF Radiologyat <https://radiology.ucsf.edu/patient-care/services/high-intensity-focused-ultrasound-hifu>
- 95.About Neuromodulationat <https://www.neuromodulation.com/about-neuromodulation>
- 96.Search of: focused ultrasound ∣ Adenomyosis - List Results - ClinicalTrials.govat <https://clinicaltrials.gov/ct2/results?cond=Adenomyosis&term=focused+ultrasound&cntry=&state=&city=&dist=>
- 97.Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MRgHIFU) Treatment of Symptomatic Uterine Fibroids: An Evidence-Based Analysis - PubMedat <https://pubmed.ncbi.nlm.nih.gov/26357530/> [PMC free article] [PubMed]
- 98.Search of: HIFU ∣ Recruiting, Active, not recruiting, Completed Studies ∣ Uterine Adenomyosis - List Results - ClinicalTrials.govat <https://www.clinicaltrials.gov/ct2/results?term=HIFU&cond=Uterine+Adenomyosis&Search=Apply&recrs=a&recrs=d&recrs=e&age_v=&gndr=&type=&rslt=>
- 99.Search of: HIFU ∣ Recruiting, Active, not recruiting, Completed Studies ∣ Uterine Leiomyoma - List Results - ClinicalTrials.govat <https://www.clinicaltrials.gov/ct2/results?term=HIFU&cond=Uterine+Leiomyoma&Search=Apply&recrs=a&recrs=d&recrs=e&age_v=&gndr=&type=&rslt=>
- 100.Common Cancer Types - National Cancer Instituteat <https://www.cancer.gov/types/common-cancers>
- 101.Bilateral Treatment of Medication Refractory Essential Tremor - Full Text View - ClinicalTrials.govat <https://clinicaltrials.gov/ct2/show/NCT04112381?term=04112381&draw=2&rank=1>
- 102.Staged Bilateral Exablate Treatment of Medication Refractory Essential Tremor - Full Text View - ClinicalTrials.govat <https://clinicaltrials.gov/ct2/show/NCT03465761?term=03465761&draw=2&rank=1>
- 103.Peripheral neuropathy - Symptoms and causes - Mayo Clinicat <https://www.mayoclinic.org/diseases-conditions/peripheral-neuropathy/symptoms-causes/syc-20352061>
- 104.Blood-Brain Barrier Opening - Focused Ultrasound Foundationat <https://www.fusfoundation.org/mechanisms-of-action/blood-brain-barrier-opening>
- 105.Home - ClinicalTrials.govat <https://www.clinicaltrials.gov/>
- 106.Premarket Approval (PMA). In: Wiley Encyclopedia of Clinical Trials, edited by D’Agostino RB, Sullivan L, and Massaro J. 2008.doi: 10.1002/9780471462422.eoct545 [DOI] [Google Scholar]
- 107.Effect of Ultrasound on Tissues. Acta Ophthalmologica; 41:17–20, 2009. [Google Scholar]
- 108.High-intensity focused ultrasound use widens in research, practiceat <https://www.diagnosticimaging.com/view/high-intensity-focused-ultrasound-use-widens-research-practice>
- 109.FDA approves first MRI-guided focused ultrasound device to treat essential tremor ∣ FDAat <https://www.fda.gov/news-events/press-announcements/fda-approves-first-mri-guided-focused-ultrasound-device-treat-essential-tremor>