Abstract
The tumor necrosis factor alpha (TNF-α) gene is rapidly activated by lipopolysaccharide (LPS). Here, we show that extracellular signal-regulated kinase (ERK) kinase activity but not calcineurin phosphatase activity is required for LPS-stimulated TNF-α gene expression. In LPS-stimulated macrophages, the ERK substrates Ets and Elk-1 bind to the TNF-α promoter in vivo. Strikingly, Ets and Elk-1 bind to two TNF-α nuclear factor of activated T cells (NFAT)-binding sites, which are required for calcineurin and NFAT-dependent TNF-α gene expression in lymphocytes. The transcription factors ATF-2, c-jun, Egr-1, and Sp1 are also inducibly recruited to the TNF-α promoter in vivo, and the binding sites for each of these activators are required for LPS-stimulated TNF-α gene expression. Furthermore, assembly of the LPS-stimulated TNF-α enhancer complex is dependent upon the coactivator proteins CREB binding protein and p300. The finding that a distinct set of transcription factors associates with a fixed set of binding sites on the TNF-α promoter in response to LPS stimulation lends new insights into the mechanisms by which complex patterns of gene regulation are achieved.
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that activates multiple-signal transduction pathways and influences a broad range of immunological processes. Multiple extracellular stimuli induce the synthesis of TNF-α in a wide variety of cell types, including T and B cells, monocytes and macrophages, mast cells, and fibroblasts (reviewed in reference 1). We have shown that induction of TNF-α gene transcription by T or B cell receptor engagement, virus infection, and treatment with a calcium ionophore depends upon the activity of the phosphatase calcineurin (15, 18, 20). Calcineurin targets the nuclear factor of activated T cells (NFAT) family of proteins (reviewed in references 11 and 38), which are critical for TNF-α gene expression by calcineurin-dependent signal transduction pathways (15, 48, 49).
Production of TNF-α in response to lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria, is of particular clinical importance because TNF-α is a mediator of septic shock (reviewed in reference 1). Exposure of monocytes and macrophages to LPS results in activation of the mitogen-activated protein kinase (MAPK) pathway, including the extracellular signal-related kinase (ERK), c-jun NH2-terminal kinase (JNK), and p38 cascades (reviewed in reference 12).
Here, we show that ERK, but not calcineurin or p38, is required for full transcriptional induction of TNF-α gene expression by LPS. We identify TNF-α promoter elements critical for LPS induction of the gene and demonstrate that two Sp1 binding sites and three Ets binding sites, in addition to a cyclic AMP response element (CRE)-like site and an Egr site, are critical for LPS induction of the TNF-α gene. Consistent with this functional analysis of the TNF-α promoter, using chromatin immunoprecipitation and formaldehyde crosslinking (ChIP) assays, we directly detect LPS-inducible binding of the transcription factors ATF-2, c-jun, Ets-1 and -2, Elk-1, Egr-1, and Sp1 to the endogenous TNF-α promoter. Furthermore, we show that LPS-mediated TNF-α transcription is dependent upon CREB binding protein (CBP) and p300 coactivator proteins and, moreover, that the intrinsic transcriptional activity of CBP and p300 is potentiated by LPS.
Thus, a unique TNF-α enhancer complex, including Ets, Elk-1, Sp1, ATF-2–Jun, and the coactivator proteins CBP and p300, is assembled on the TNF-α promoter in LPS-stimulated monocytes. Remarkably, a set of TNF-α promoter elements, which bind NFAT upon induction of the gene by calcineurin-dependent stimuli, also bind the ERK-targeted Ets and Elk proteins and are required in LPS-stimulated TNF-α gene expression. Thus, these studies reveal that a distinct group of activators is recruited to a fixed set of TNF-α promoter binding sites, depending on the stimulus. This work therefore provides direct evidence for a general mechanism by which a single gene may be regulated in an inducer-specific manner.
MATERIALS AND METHODS
Cell culture and transfection.
J774 (49), P388D1 (17), Mono Mac-6 (60), and ANA-1 cells (10) were maintained as previously described. Transfections in J774, ANA-1, and Mono Mac-6 cells were performed using FuGene6 (Boehringer-Mannheim) according to the manufacturer's protocol. Transfections in RAW264.7 cells were performed using Super-Fect (Qiagen) as described previously (35). Thirty-six hours after transfection, cells were treated with LPS (Sigma; Escherichia coli O111:B4) at a concentration of 1 μg/ml and harvested approximately 16 h later. Chloramphenicol acetyltransferase (CAT) assays were performed as previously described (18). As a transfection control, the pCMVβ plasmid (Clontech) was cotransfected and extracts were normalized to β-galactosidase (β-Gal) activity prior to performance of CAT assays. Luciferase assays were performed according to the manufacturer's instructions (Dual Luciferase Reporter Assay System; Promega) using a Dynex luminometer, with Renilla luciferase (pRL-TK) as a control.
RNA analysis.
RNA was prepared from J774, P388D1, and Mono Mac-6 cells, or splenocytes from ATF-2 mutant mice or NFATp-deficient mice, and 32P-labeled RNA probes were prepared from SP6 γ-actin and murine TNF-α probes. RNase protection assays were performed as previously described (18) and quantified with a phosphorimager (Molecular Dynamics). The ATF-2 mutant mice (40) contain low levels of a mutant ATF-2 protein; ATF-2-deficient mice die immediately after birth (33) and are thus not suitable for in vivo LPS experiments. ATF-2 mutant mice and wild-type littermates were injected with 50 μg of LPS, and RNA was prepared from whole spleens as previously described (40). Spleens were removed from mice deficient in NFATp (23) and stimulated in vitro with LPS (1 μg/ml) for 1 h as described (17). Where indicated, cells were pretreated for 10 min with cyclosporin A (CsA) (Sandoz), SB203580 (a gift from Genetics Institute, synthesized based on a published procedure) (5), or PD98059 (BioMol Research Labs) at the concentrations indicated in the figure legends.
Plasmids.
The −200 TNF-α CAT, −1045 TNF-α CAT, −200 TNF-α Luc, −39 TNF-α CAT, (CRE/κ3)1 −39 TNF-α CAT, (CRE/κ3)2 −39 TNF-α CAT, and (CRE/κ3)2 −39 TNF-α Luc constructs have been described previously (6, 17, 21, 48). The −982 TNF-α Luc reporter was created by subcloning the SmaI-HincII fragment of −982 TNF-α CAT (50) into the SmaI site of pGL3-Basic (Promega, Madison, Wis.). The (C1M)2 −39 TNF-α Luc and (3′M)2 −39 TNF-α Luc reporters were created by subcloning the SmaI-HincII fragment of the (C1M)2 −39 TNF-α CAT and (3′M)2 −39 TNF-α CAT reporters (48) into the SmaI site of pGL3-Basic. The −117M, −113M, C1M, κ3 5′ M, κ3 3′M, −76M, AP1M, and SP1M luciferase reporters were created by subcloning the BamHI-XbaI fragment of the corresponding CAT constructs (18, 48, 49) into pBluescript (Stratagene) and subcloning the KpnI-XbaI fragment of the resulting vector into the KpnI-NheI sites of pGL3-Basic. The −84M CAT reporter from which the −84M luciferase reporter was created was constructed by M13 in vitro mutagenesis as described (49). The Up-Sp1M, EgrM, and Egr/Up-Sp1M luciferase reporters were prepared by standard PCR mutagenesis. All mutations were confirmed by sequencing. The G5E1b-Luc reporter and the Gal4-p300 vector have been described elsewhere (14, 59). The Gal4-CBP expression vector was a generous gift of D. Thanos (Columbia University).
DNase I footprinting.
DNase I footprinting of the human TNF-α promoter was performed using recombinant NFATp, Ets-1, Elk-1, and PU.1 proteins (generous gifts of D. Thanos, B. Nikolajczyk, A. Sharrocks, and H. Singh, respectively) at concentrations indicated in the figure legends, as described previously (49). The −200 to +87 fragment of the wild-type TNF-α promoter or isogenic constructs bearing the −76M, −84M, κ3 3′M, −113M, and −117M mutations as shown in Fig. 7 were used as templates.
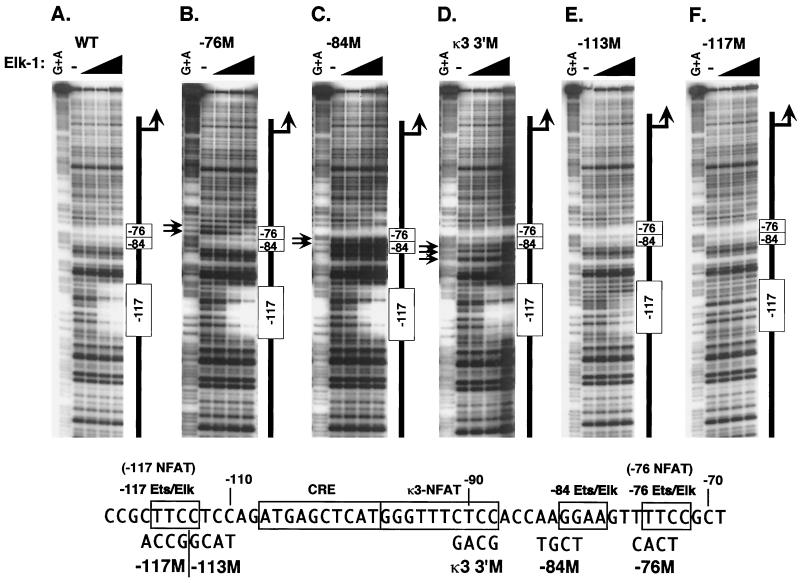
FIG. 7.
Mutation of the −76-NFAT, κ3-NFAT, −117-NFAT, or −84 sites in the TNF-α promoter inhibit Elk-1 binding. Quantitative DNase I footprinting using the wild-type human TNF-α promoter (nt −200 to +87 relative to the transcription start site) (A) or isogenic probes bearing mutations in the −76-NFAT site (−76M) (B), the −84 Elk-Ets site (−84M) (C), or the κ3 site (3′M) (D), as well as two mutations in the −117-NFAT site, −117M (E) and −113M (F), is shown. The sequences of the mutant sites are shown at the bottom of the figure. Probes were incubated with increasing concentrations of recombinant Elk-1 (50 ng, 200 ng, or 1 μg). Alterations in the cleavage pattern observed with the −76-NFAT and κ3-NFAT mutant templates in the vicinity of the −84 Ets-Elk site are indicated with arrows (B to D).
Western blot analysis.
For Western blot analysis, after nuclear extract preparation (18), equal amounts of protein (30 μg/sample) were analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis, transferred on nitrocellulose membranes and immunoblotted with anti-ERK1 and anti-ERK2 (anti-ERK1/2) antibody (Upstate Biotechnology, Lake Placid, N.Y.) or anti-phospho-ERK1/2 (anti-phospho-p44 or -p42 MAPK [Thr202-Tyr204]) antibody (New England Biolabs). Immunodetection was performed by incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (Promega) and developed by chemiluminescence (New England Nuclear, Boston, Mass.).
Formaldehyde crosslinking and chromatin immunoprecipitation.
J774 cells (∼2 × 108 cells) and control samples were treated with LPS for 3 h as indicated and then were treated with formaldehyde (1% final concentration) for 30 min at 37°C. Cells were harvested, and fixed chromatin was sonicated, extracted, and purified, followed by immunoprecipitation with anti-c-jun, anti-ATF-2, anti-Sp1, anti-Egr-1, anti-Ets-1 and -2, anti-Elk-1, or anti-C/EBPβ (Santa Cruz Biotechnology). Immunoprecipitated DNA was then amplified by PCR with primers specific to the TNF-α promoter as previously described (15). Titrations of PCR cycles were performed to ensure that experiments were performed in the linear range of amplification.
RESULTS
The region from nucleotide (nt) −200 to the TNF-α transcription start site is sufficient for maximal LPS inducibility of TNF-α.
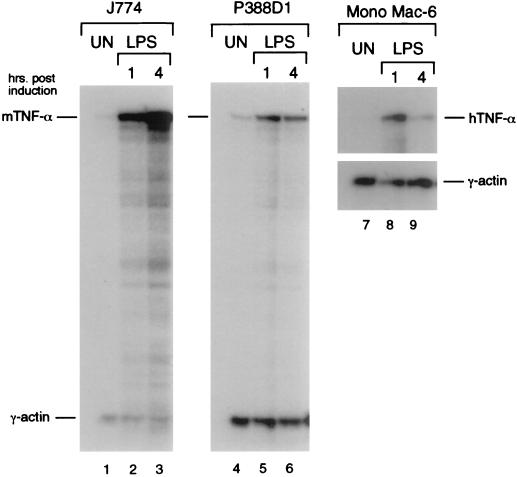
We first compared LPS induction of the endogenous TNF-α gene in murine (J774, P388D1) and human (Mono Mac-6) monocytic cell lines stimulated with LPS to determine an appropriate system in which to study LPS-stimulated TNF-α gene expression. As shown in Fig. 1, TNF-α mRNA was highly inducible by LPS in all three cell lines; however, relatively higher levels of inducible TNF-α mRNA were achieved in the J774 cells than in the P388D1 and Mono Mac-6 cells (Fig. 1).
FIG. 1.
Induction of TNF-α mRNA by LPS in monocytic cell lines. Autoradiograms are shown of RNase protection assays mapping TNF-α and γ-actin mRNAs from untreated or LPS-stimulated J774 (lanes 1 to 3), P388D1 (lanes 4 to 6), and Mono Mac-6 (lanes 7 to 9). RNA was analyzed at 1 or 4 h poststimulation as indicated.
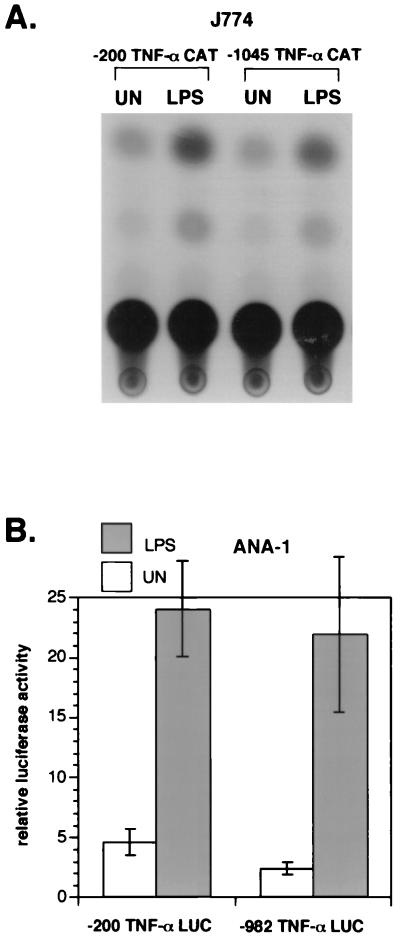
The region from nt −200 to the human TNF-α mRNA cap site is sufficient for maximal induction of the TNF-α gene by LPS in murine P388D1 cells (17), THP-1 human monocytic cells (47, 58), and murine RAW264.7 monocytic cells (22). As shown in Fig. 2A, deletion of the sequences between nt −1045 and −200 had no effect on LPS induction of the TNF-α CAT reporter gene, and thus this region is also sufficient for maximal induction of the TNF-α gene by LPS in J774 cells.
FIG. 2.
The region from nt −200 upstream of the TNF-α mRNA cap site suffices for LPS induction. J774 (A) and ANA-1 (B) cells were transfected with 2 μg of CAT (A) or luciferase (B) reporters linked to human TNF-α promoters containing −200 and −1045 (CAT) or −200 and −982 (luciferase) nucleotides upstream of the mRNA cap site and treated with LPS as shown. (A) A representative CAT assay of three independent transfections is shown. Transfections included 2 μg of pCMVβ as a control, and CAT activity was normalized to β-Gal activity. (B) Histograms of luciferase activity from five independent experiments are shown; error bars indicate standard errors of the means. All transfections included a control Renilla luciferase plasmid (2 μg), and reporter luciferase activity was normalized to Renilla luciferase activity.
A recent report claimed that sequences around nt −600 relative to the human TNF-α mRNA cap site that contain a strong NF-κB binding motif, κ1 (17, 47), were required for maximal expression of the TNF-α gene in murine ANA-1 and human Mono Mac-6 cells (27). Thus, to rule out a cell-type-specific difference in TNF-α gene regulation by LPS, we also transfected the ANA-1, Mono Mac-6 cell lines and RAW264.7 cells with TNF-α luciferase reporter genes containing nt −982 or −200 upstream of the TNF-α transcription start site. We found that there were only minimal differences in induction between the nt −200 and −982 luciferase reporter constructs in ANA-1 (Fig. 2B), Mono Mac-6 cells, and RAW264.7 (data not shown), in agreement with results obtained with J774, THP-1, and P388D1 cells. Thus, consistent with experiments performed with multiple cell types, including monocytes, T, B, and fibroblast cells stimulated with a variety of inducers (6, 17–19, 22, 47, 48, 58), the region from nt −200 upstream of the start site of transcription contained the critical sequences required for inducibility of the TNF-α gene. We are unable to explain the discrepancy between our findings and those previously reported for ANA-1 and Mono Mac-6 cells (27).
The CRE, Sp1, Ets-Elk, and Egr sites are required for LPS induction of TNF-α gene expression.
To identify the promoter elements required for LPS induction of the TNF-α gene, we transfected J774 cells with human TNF-α luciferase reporter constructs bearing mutations in different regulatory elements. Mutation of the −117-NFAT (−117M), CRE (C1M), κ3-NFAT (5′M and 3′M), −76-NFAT (−76M), and Sp1 (SP1M) sites, as well as sequences that match an Ets–Elk-1 motif located between nt −84 and −80 (−84M), significantly reduced LPS induction of the gene (Fig. 3A). It should be noted that both the C1M and κ3 5′M mutants inhibit binding of ATF-2/c-jun to the TNF-α promoter (48, 49). By contrast, mutation of a putative AP-1 site (AP1M) had no effect on LPS induction of the gene expression. Mutation of this site, which bears limited sequence similarity to a consensus AP-1 site, also had no effect on the regulation of the gene by LPS in THP-1 cells (58) and by a variety of inducers in multiple cell types (6, 15, 21, 48).
FIG. 3.
Identification of activator binding sites required for LPS induction of TNF-α. (A) The CRE, Sp1, and Ets-NFAT sites are required for LPS induction of TNF-α. J774 cells were transfected with 2 μg of the wild-type −200 TNF-α luciferase reporter or with isogenic reporters containing mutations in the −117 NFAT (−117M), CRE (C1M), κ3-NFAT (5′M and 3′M), Ets-Elk (−84M), −76-NFAT (−76M), Sp1 (SP1M), or AP-1 (AP1M) sites and treated with LPS as shown. (B) The upstream Sp1 and Egr-1 binding sites are required for LPS induction of TNF-α. J774 cells were transfected with 2 μg of the wild-type −200 TNF-α luciferase reporter or with isogenic reporters containing mutations in the Egr-1 and/or upstream Sp1 sites and treated with LPS as shown. Renilla luciferase (2 μg) was used to normalize transfection efficiency as shown in Fig. 2. Histograms show average results of three independent experiments; error bars represent the standard errors of the means.
We note that in contrast to induction of TNF-α gene expression by ionophore (18, 49), induction of TNF-α gene expression by LPS requires an intact Sp1 site, which is also required for virus induction of the gene (15). Furthermore, there is a second Sp1 site in the −200 TNF-α promoter region, which is located between nt −172 and −163 relative to the TNF-α transcription start site. Mutation of this upstream Sp1 site also greatly reduced LPS induction, while mutation of the Egr site reduced induction of the gene by approximately 50% (Fig. 3B), consistent with a previous study (58). Thus, the upstream Sp1 binding site, like the downstream Sp1 site, is critical for induction of the TNF-α gene by LPS.
The composite CRE/κ3/−84Ets element is sufficient for LPS induction of a truncated TNF-α promoter.
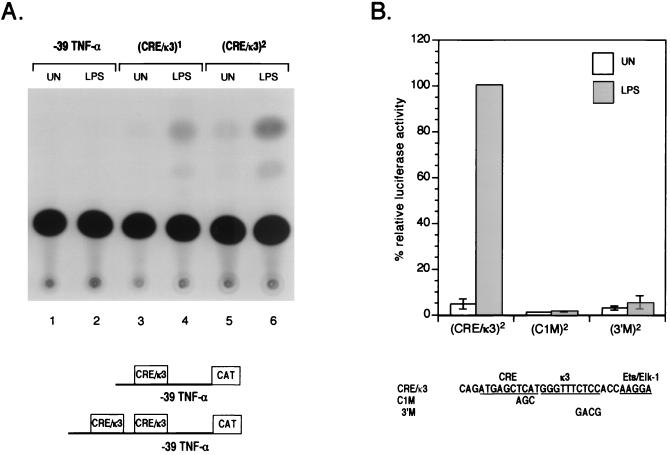
Previous studies have shown that multiple copies of the κ3 site, which bears some resemblance to an NF-κB binding site, do not confer LPS inducibility upon a heterologous promoter or a truncated TNF-α promoter, consistent with the lack of binding of NF-κB p50 or p65 to this element in DNase I footprinting assays (49). Given the importance of the CRE and the κ3 site in induction of TNF-α gene expression by LPS, we next tested whether a synthetic construct containing the CRE site in addition to the κ3 site and the −84 Ets site would be capable of conferring LPS inducibility upon a truncated TNF-α promoter. Only one copy of this composite element is sufficient to confer LPS induction upon the truncated −39 TNF-α promoter (Fig. 4A), whereas up to six copies of the κ3 site alone were not capable of conferring LPS inducibility (17, 58), underscoring the importance of the CRE in LPS induction of TNF-α gene expression. Furthermore, a mutation of the CRE, which abolishes binding of ATF-2/c-jun to the site (48), abrogated LPS induction of this synthetic promoter construct. Intriguingly, a mutation of the 3′ aspect of the κ3 site also abrogates LPS inducibility. These results are consistent with a previous study in THP-1 monocytic cells, which showed that two or three copies of the composite CRE/κ3 element conferred LPS inducibility to a minimal simian virus 40 promoter and that this induction depended on the integrity of the CRE and κ3 sites (58). Moreover, the data presented here and the study by Yao et al. demonstrate that the κ3 site alone does not function as an LPS-inducible NF-κB site.
FIG. 4.
Activation of the CRE/κ3 region of the TNF-α promoter in response to LPS. (A) A single copy of the CRE/κ3 region confers LPS inducibility to a minimal TNF-α promoter. J774 cells were transfected with 2 μg of a minimal TNF-α promoter CAT reporter (−39 TNF-α CAT) CAT or reporters with one [(CRE/κ3)2 −39 TNF-α CAT] or two [(CRE/κ3)1 −39 TNF-α CAT] copies of the CRE/κ3 region. A representative CAT assay of three independent transfections is shown, illustrating CAT activity from uninduced cells and cells treated with LPS. Transfections included 2 μg of pCMVβ as a control, and CAT activity was normalized to β-Gal activity. (B) The CRE and κ3 sequences are critical for LPS inducibility of the CRE/κ3 region. J774 cells were transfected with luciferase reporters (2 μg) consisting of a minimal TNF-α promoter fused to two copies of the wild-type CRE/κ3 sequence [(CRE/κ3)2 −39 TNF-α Luc] or two copies of the CRE/κ3 sequence with mutations in the CRE [(C1M)2 −39 TNF-α Luc] or κ3 sequences [(3′M)2 −39 TNF-α Luc]. The mutations are shown at the bottom of the figure. Histograms of luciferase activity from five independent experiments are shown; error bars indicate the standard errors of the means. All transfections included a control Renilla luciferase plasmid (2 μg), and reporter luciferase activity was normalized to Renilla luciferase activity. UN, uninduced.
ERK and ATF-2 are required for LPS induction of TNF-α.
Stimulation of the monocyte lineage by LPS triggers the activation of the MAPKs ERK, JNK, and p38 (reviewed in reference 12). Phosphorylation of Ets proteins is generally dependent upon MAPKs (reviewed in reference 53). For example, upon LPS stimulation of macrophages, the Ets protein Elk-1 is phosphorylated via the ERK pathway (39). LPS activation of the Ets protein PU.1 is dependent upon a distinct pathway involving protein kinase CK2 (30), which is in turn ERK dependent (35). The transcriptional activity of c-jun is dependent upon phosphorylation by JNK, while that of ATF-2 is dependent upon JNK or p38 (reviewed in reference 56). By contrast, NFAT proteins are targeted by the calcium-dependent phosphatase calcineurin, which is selectively inhibited by CsA (reviewed in references 11 and 38).
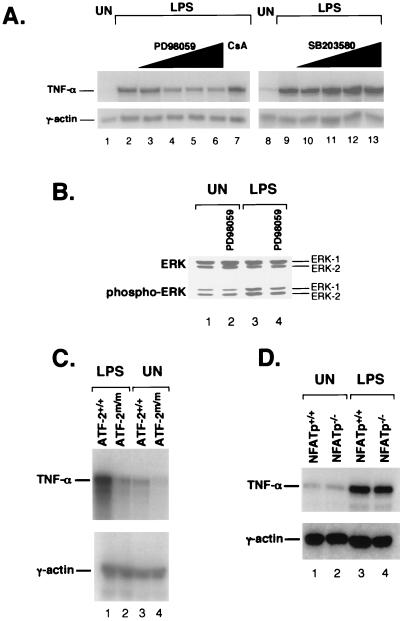
In order to investigate the role of these distinct signal transduction pathways in LPS induction of TNF-α gene expression, we performed quantitative RNase protection assays in J774 cells using inhibitors of p38 (SB203580), ERK (PD98059), and calcineurin (CsA). As shown in Fig. 5A, LPS induction of TNF-α mRNA levels was selectively inhibited (approximately 50%) by the ERK inhibitor PD98059 at a concentration of 10 μM (compare lanes 2 and 4) and was not affected by CsA (lane 7) or the p38 inhibitor SB203580 even up to a concentration of 20 μM (lanes 10 to 13). Thus, ERK but not p38 or calcineurin activity is required for full induction of TNF-α transcription by LPS in J774 cells.
FIG. 5.
LPS-stimulated TNF-α induction is dependent upon ERK and ATF-2 but not calcineurin, p38, or NFATp. (A) ERK-dependent TNF-α mRNA induction by LPS. Autoradiograms of RNase protection assays mapping TNF-α and γ-actin mRNAs from untreated (UN) or LPS-stimulated J774 cells in the absence or presence of the ERK inhibitor PD98059, the calcineurin inhibitor CsA, or the p38 inhibitor SB203580 are shown. RNA was analyzed 1 h poststimulation, and inhibitors were added 10 min prior to LPS stimulation. Concentrations were as follows: PD98059, 2, 10, 20, and 30 μM (lanes 3 to 6); CsA, 1 μM (lane 7); and SB203580, 1, 5, 10, and 20 μM (lanes 10 to 13). We note that TNF-α-stimulated TNF-α gene transcription is inhibited by SB203580 at a concentration of 10 μM (6). (B) Activation of ERK by LPS. A Western blot of total ERK1/2 and phosphorylated ERK1/2 from untreated or LPS-stimulated J774 cells in the presence or absence of PD98059 is shown. Nuclear extracts were prepared from J774 cells stimulated with LPS for 15 min in the presence or absence of pretreatment (10 min) with 10 μM PD98059. Extracts were analyzed by sodium dodecyl sulfate–6% polyacrylamide gel electrophoresis, and Western analysis was performed using antibodies to phosphorylated ERK1/2 (bottom), followed by reprobing with an antibody against ERK1/2 (top) to ensure equal protein loading in all samples. The result displayed is representative of three independent experiments. (C) Induction of TNF-α by LPS is impaired in ATF-2 mutant mice. Results of an RNase protection assay of spleen cells from wild-type (ATF-2+/+) and ATF-2 mutant (ATF-2m/m) mice using TNF-α and γ-actin probes as described for panel A are shown. Mice were injected with LPS intraperitoneally, and spleens were collected 2 h later. (D) Induction of TNF-α by LPS is not impaired in NFATp-deficient mice. Results of an RNase protection assay of spleen cells from wild-type (NFATp+/+) and NFATp-deficient (NFATp−/−) mice using TNF-α and γ-actin probes as described for panel A are shown. Spleens were isolated from the mice and stimulated in vitro with LPS for 1 h.
To confirm that ERK was activated by LPS, we next performed a Western blot analysis using nuclear extracts from J774 cells and a specific antibody to the phosphorylated forms of ERK1 and ERK2. As shown in Fig. 5B, the phosphorylated ERK levels were stimulated by LPS, while total ERK levels were unaffected (compare lanes 1 and 3). The LPS-induced levels of phosphorylated ERK were in turn inhibited by PD98059 (lane 4). Thus, in J774 cells, LPS stimulation leads to activation of ERK1/2 through phosphorylation of specific tyrosine residues, and PD98059 functions as an inhibitor of LPS-induced ERK phosphorylation.
Parallel RNase protection assays to assess the role of JNK in LPS-mediated TNF-α transcription were not possible due to the lack of JNK-specific inhibitory compounds, so instead we focused on the role of a downstream target of JNK and p38, ATF-2, which binds the TNF-α CRE site (48). Using RNase protection assays, we examined LPS-stimulated TNF-α gene regulation in mice homozygous for a mutant form of the ATF-2 gene (40). As shown in Fig. 5C, constitutive and LPS-stimulated TNF-α mRNA levels were reduced approximately 50% in spleens from ATF-2 mutant mice compared to levels in wild-type littermates (compare lanes 1 and 3 to lanes 2 and 4). For comparison, we also examined LPS-stimulated TNF-α mRNA levels in mice deficient in NFATp (23). Consistent with our finding that LPS induction of TNF-α was not sensitive to CsA, LPS-stimulated TNF-α mRNA levels from NFATp-deficient and wild-type mice were equivalent (Fig. 5D, compare lanes 1 and 3 to 2 and 4). Taken together, these results establish that ERK and ATF-2, but not p38, calcineurin, or NFATp, play a critical role in induction of TNF-α transcription by LPS. These findings are consistent with results of multiple studies that support a role for ERK in LPS-mediated TNF-α transcription and protein synthesis in macrophages (3, 16, 31, 41, 51, 52).
Ets proteins bind to three sites in the TNF-α promoter.
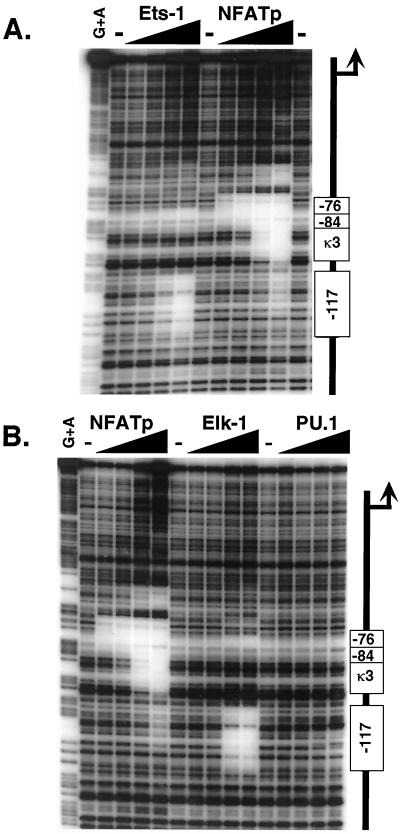
Although our results indicated that induction of the TNF-α gene by LPS did not involve calcineurin or NFATp, induction of the TNF-α gene by LPS was strongly inhibited by mutation of the −117-NFAT, −76-NFAT, and κ3-NFAT sites (Fig. 3A). Since the binding sites for NFAT and Ets proteins both contain a 5′-GGAA-3′ core element, we next examined the binding of the ERK-dependent Ets proteins Elk-1, Ets-1, and PU.1 to the TNF-α promoter by quantitative DNase I footprinting, using NFATp for comparison. We note that proteins that recognize Ets-like binding motifs, such as Ets-1 (26) and C/EBPβ (37), have previously been implicated in TNF-α gene regulation in T cells and myelomonocytic cells, respectively.
As shown in Fig. 6, two regions of the TNF-α promoter are protected by Ets-1 (Fig. 6A) and Elk-1 (Fig. 6B). Notably, these regions overlap the −117-NFAT and −76-NFAT sites (49). Moreover, the latter protected region overlaps an Elk-1 consensus site (43) located between nt −84 and −80. We also note that the region containing the −117-NFAT site was previously shown to bind Ets in a gel shift assay (26). By contrast, however, binding of PU.1 at the same concentrations used for Ets-1 and Elk-1 was not discernible (Fig. 6B). Since the −76, −84, and κ3-3′ mutations compromise induction of TNF-α by LPS (Fig. 3A), we next examined the effects of these mutations upon Ets protein binding.
FIG. 6.
Ets-1 and Elk-1 bind to three sites in the TNF-α promoter. (A) Ets-1 binds to the −84 Ets and the −76- and −117-NFAT sites. Quantitative DNase I footprinting using the wild-type human TNF-α promoter (nt −200 to +87 relative to the transcription start site) and increasing concentrations of recombinant NFATp or Ets-1 (20 ng, 100 ng, 400 ng, and 2 μg) is shown. The positions of the κ3, −76, −84, and −117 binding sites are shown. NFATp binds with high affinity to κ3 and −76 and with lower affinity to −117 and a novel site centered around −55 (Tsytsykova and Goldfeld, unpublished data). (B) Elk-1 binds to the −84 Ets and the −76- and −117-NFAT sites. Quantitative DNase I footprinting using the wild-type human TNF-α promoter (nt −200 to +87 relative to the transcription start site) and increasing concentrations of recombinant NFATp or Elk-1 (20 ng, 100 ng, 400 ng, and 2 μg) is shown. Two independent preparations of PU.1 (gifts of H. Singh and B. Nikolajczyk) were tested, and no binding of PU.1 with significant affinity was observed.
The region protected by Elk-1 near the −76-NFAT site overlaps two 5′-GGAA-3′ Ets-binding motifs at positions −76 and −84 (Fig. 7). Mutation of the −76-NFAT site, however, did not abolish binding of Elk-1 to the −84 site (Fig. 7B); conversely, mutation of the −84 site did not abolish binding of Elk-1 to the −76-NFAT site (Fig. 7C). We note that using the −84 mutant template, at the highest concentrations of Elk-1, some additional binding to a downstream GGA sequence overlapping a novel NFAT site at −55 (A. V. Tsytsykova and A. E. Goldfeld, unpublished data) not normally protected is observed (Fig. 7C). Thus, two distinct Ets sites are discernible by DNase I footprinting using mutant TNF-α promoter templates. In results consistent with those of previous studies, mutation of the Ets-Elk motif that overlaps the −117 NFAT site abrogated binding of Elk-1 to the site (Fig. 7E and F). We note that for the −113 M template, in which only one base pair of the Ets-Elk site is altered, some binding of Elk-1 was discernible, but only at the highest protein concentrations (Fig. 7E).
Mutation of the 3′ aspect of the κ3-NFAT site (κ3 3′M), like mutation of the −76-NFAT and −84 sites, changed the cleavage pattern by DNase I on the naked DNA template. We note that the cleavage pattern of the naked −76 mutant template significantly varied from that of the wild-type template in this region (compare Fig. 7A and D). At the highest concentrations of Elk-1, partial protection of this altered cleavage pattern on the mutant template was observed; however, even at the highest concentrations of Elk-1 there was not full protection of this site, consistent with its deleterious effect upon LPS-stimulated TNF-α gene induction. Strikingly, the κ3 3′M mutation not only caused a change in the cleavage pattern in the vicinity of the nearby −84 site but also inhibited binding of Elk-1 to the site (Fig. 7D). Thus, the inhibition of LPS-stimulated TNF-α gene expression by the −76, −84, and κ3 3′ mutations is consistent with their interference with Ets and Elk binding to the −76 and −84-Ets-Elk sites.
Elk-1, Ets, ATF-2–Jun, Egr1, and Sp1 proteins interact with the endogenous TNF-α promoter upon LPS stimulation.
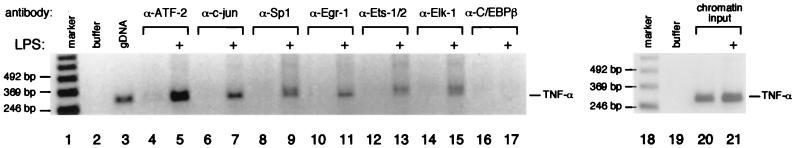
To establish which of the transcriptional activator proteins bind to the TNF-α promoter in J774 cells in vivo, we next performed ChIP assays using specific antibodies against the different activators. This technique has been used to detect binding of transcription factors to the beta interferon promoter following virus infection (55) and to the TNF-α promoter following virus infection and ionophore stimulation (15). TNF-α promoter DNA was amplified by PCR of formaldehyde-fixed chromatin immunoprecipitated by the antibodies shown in Fig. 8, which provided an indication of the amount of transcription factor binding to the promoter following stimulation by LPS in vivo. Based on our site-directed mutagenesis studies of the TNF-α promoter function, characterization of the upstream signaling pathways involved in LPS induction of the gene, and quantitative DNase I footprinting, we used antibodies directed against proteins that recognize Ets binding sites (Ets-1 and -2, Elk-1, C/EBPβ), the CRE (ATF-2 and c-jun), and the Sp1 and Egr-1 sites. As shown in Fig. 8, LPS stimulation of J774 cells resulted in the inducible binding of Ets-1 and -2, Elk-1, ATF-2, c-jun, Egr-1, and Sp1 to the TNF-α promoter in vivo. By contrast, binding of C/EBPβ to the TNF-α promoter was not induced by LPS, consistent with the observation that macrophages from mice lacking C/EBPβ produce wild-type levels of TNF-α mRNA following LPS stimulation (45).
FIG. 8.
LPS-induced binding of transcription factors to the endogenous TNF-α promoter. Formaldehyde cross-linking and chromatin immunoprecipitation of unstimulated and LPS-stimulated J774 cells are shown. Following induction, cells were treated with formaldehyde to cross-link endogenous protein and DNA. Samples of sonicated and purified chromatin were immunoprecipitated with the indicated antibodies, and DNA isolated from immunoprecipitated material was amplified by PCR with primers specific for the TNF-α gene. An increase in the relative amount of the amplified TNF-α promoter-specific PCR product indicates binding of the protein to the endogenous TNF-α promoter. Control amplifications with buffer, genomic DNA (gDNA), or the chromatin used as input for the immunoprecipitations are shown, along with a 123-bp marker (Life Technologies). We used the human immunodeficiency virus type 1 long terminal repeat as a template in the ChIP analysis as a positive control for the C/EBPβ antibody (B. M. N. Brinkman and A. E. Goldfeld, unpublished data).
Due to the variable sizes of promoter DNA fragments that are generated when DNA is sheared in the ChIP assay (36), binding of factors to nonfunctional flanking sequences can also be detected in this sensitive assay. Thus, correlation of findings obtained with ChIP with functional data, including the roles of specific promoter binding sites, is necessary. We note that LPS causes some calcium influx in J774 cells (54), which would be expected to activate calcineurin and cause the nuclear translocation of NFAT proteins, and that LPS also causes the phosphorylation of IκB, resulting in the nuclear translocation of NF-κB (reviewed in reference 46). Consistent with these observations, we detected binding of NFAT and p50-p65 proteins to the TNF-α promoter upon LPS treatment of J774 cells (data not shown). However, induction of TNF-α gene transcription by LPS is insensitive to CsA and is not compromised in NFATp-deficient mice. Furthermore, the only NF-κB-like site in the −200 TNF-α promoter that is required for maximal induction by LPS, κ3, does not bind high concentrations of recombinant p50-p65 in DNase I footprinting assays (49), nor does it confer LPS inducibility upon a heterologous promoter as would a functional NF-κB site (17, 58). We thus conclude that NFAT binds to the subset of TNF-α NFAT sites and/or NFAT sites in flanking sequences of no functional relevance in LPS stimulation, and that p50-p65 proteins bind to nonfunctional NF-κB motifs, which are in flanking sequences not involved in LPS-stimulated TNF-α gene expression.
Taken together, the LPS-inducible recruitment of ATF-2, c-jun, Ets, Elk-1, and Sp1 to the TNF-α promoter observed in the ChIP analysis strongly correlates with the critical functional roles that the Sp1, CRE, Ets, and Egr-1 TNF-α promoter sites play in the activation of the gene by LPS.
CBP and p300 proteins are required for LPS stimulation of TNF-α and are transcriptionally activated by LPS.
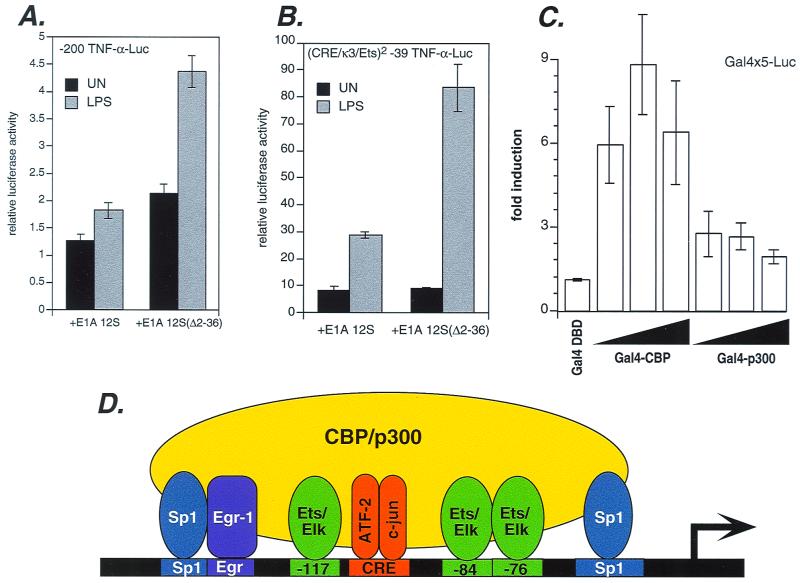
CBP and p300 proteins play a critical role in the induction of TNF-α transcription by virus and T cell receptor ligands (14). CBP and p300 proteins function as coactivators for multiple transcription factors (reviewed in reference 42). We thus next examined the potential role of these proteins in TNF-α gene expression in response to LPS. Using the adenovirus E1A 12S protein, which inhibits CBP and p300 function (13), we performed cotransfection studies with the TNF-α luciferase reporter gene in J774 cells. As a control, we used a mutant form of the E1A 12S protein (E1A 12S Δ2–36), which lacks the CBP and p300 interaction domain and fails to inhibit CBP and p300 activity (28). As shown in Fig. 9A, activation of the TNF-α reporter upon stimulation of J774 cells with LPS was inhibited by E1A 12S but not by E1A 12S Δ2–36, indicating a specific role for CBP and p300 coactivators in LPS-mediated TNF-α transcription.
FIG. 9.
CBP and p300 proteins mediate LPS induction of TNF-α. (A) Inhibition of CBP and p300 impairs TNF-α transcription induced by LPS. J774 cells were cotransfected with 2 μg of −200 TNF-α luciferase reporter and 2 μg of the vectors expressing wild-type or mutant (Δ2–36) forms of E1A 12S. Wild-type E1A represses CBP and p300 activity, while the mutant form does not. Histograms of uninduced (UN) or LPS-induced cells are shown, representing at least three independent experiments. Cotransfection of an empty vector with luciferase reporter yielded results essentially identical to those obtained with E1A(Δ2–36) (data not shown). Transfection efficiency was normalized as described in the legend to Fig. 2, and error bars represent the standard errors of the means. (B) The CRE/κ3/Ets sequence functions as a CBP- and p300-dependent element. J774 cells were cotransfected with (CRE/κ3)2 −39 TNF-α luciferase reporter and vectors expressing wild-type or mutant E1A 12S and analyzed as described above. Histograms of uninduced and LPS-induced cells are shown, representing at least three independent experiments; error bars represent the standard errors of the means. Cotransfection of empty vector with luciferase reporter yielded results essentially identical to those obtained with E1A(Δ2–36) (data not shown). (C) LPS potentiates transcriptional activity of CBP and p300. J774 cells were cotransfected with a Gal4-dependent luciferase reporter (2 μg) and vectors expressing full-length CBP or p300 fused to the Gal4 DNA-binding domain (0.2, 0.7, or 2 μg) or the Gal4 DNA-binding domain alone (2 μg). The fold induction of LPS-induced activity relative to uninduced activity is shown. The total amount of DNA was kept constant with empty vector. Assays were quantified as described above. (D) Model of an LPS-specific TNF-α enhancer complex. A diagram of TNF-α promoter elements and the cognate transcription factors recruited upon LPS stimulation is shown. These factors are known to interact, constitutively or inducibly, with CBP and p300 proteins, which are required for LPS induction of TNF-α gene expression.
We next tested the effect of inhibition of CBP and p300 upon the synthetic promoter construct containing two copies of the −117 to −80 sequence fused to a truncated −39 TNF-α promoter. We have shown that this sequence, which includes the composite CRE/κ3 element and the flanking Ets-Elk sites at nt −117 and −84, is highly inducible by LPS (Fig. 4A). Induction of the (CRE/κ3/Ets)2 reporter construct was also blocked by E1A 12S but not by E1A 12S Δ2–36 (Fig. 9B). Thus, the CRE/κ3/Ets element is sufficient for functional interaction with the coactivator proteins CBP and p300 in LPS-mediated TNF-α gene expression.
CBP and p300 contain transcriptional activation domains (9, 32). Thus, our results raised the possibility that the transactivation of CBP and p300 proteins might be potentiated by LPS. To examine this, we used CBP or p300 proteins fused to the DNA binding domain of Gal4 to determine the effect of LPS stimulation upon Gal4 binding site-dependent transcription. Strikingly, both Gal4-CBP and Gal4-p300 were activated in response to LPS stimulation (Fig. 9C). We note that the levels of transcriptional activity of CBP induced by LPS stimulation were consistently higher than those of p300 (Fig. 9C). Taken together, these data provide the first demonstration of a role for the CBP and p300 proteins in LPS-mediated gene expression and furthermore demonstrate that LPS-stimulated assembly of the TNF-α enhancer complex is CBP and p300 coactivator dependent.
DISCUSSION
We have shown that LPS-induced activation of TNF-α gene transcription in macrophages leads to the formation of a distinct enhancer complex that includes transcription factors that bind the CRE, Egr, Ets, and Sp1 sites in the promoter. Strikingly, a set of core promoter elements, which comprise functional NFAT binding sites required for induction of the gene by calcineurin-dependent stimuli, are also functional binding sites for the ERK-targeted Ets and Elk proteins in LPS-stimulated TNF-α gene expression.
We previously demonstrated that the TNF-α gene is regulated in a cell-type-specific manner in T and B cells activated through their antigen receptors through the differential binding of NFAT to the same composite promoter element, the CRE/κ3 site (49). In more recent studies, we have shown that within a specific cell type, two different stimuli result in the formation of a distinct set of protein complexes at the TNF-α promoter. Specifically, in T and B cells, ionophore stimulation leads to the formation of a nucleoprotein complex containing ATF-2–c-jun and NFATp, while virus infection leads to the formation of a nucleoprotein complex containing ATF-2–c-jun and NFATp and Sp1 (15).
Here, we have characterized the TNF-α enhancer complex that forms upon LPS stimulation of macrophages and find that there is no role for NFAT proteins. Rather, the Ets proteins Ets-1 and Elk-1, in combination with ATF-2–c-jun, Sp1, and Egr-1, are involved in LPS-mediated TNF-α gene transcription. Furthermore, a given set of promoter elements that match Ets-Elk and NFAT motifs in the TNF-α promoter are functional sites for distinct proteins, depending on the stimulus. In the case of T cell receptor engagement and ionophore stimulation, these sites are functional NFAT motifs (15, 18, 49). By contrast, in the case of LPS stimulation, these sites are no longer functional NFAT motifs but rather can function as binding sites for the Ets proteins Elk-1 and Ets-1 in vitro and in vivo. Thus, this study demonstrates that a gene can respond to different signaling pathways through the recruitment of different proteins to the same enhancer element.
Similar to virus infection, LPS-induced TNF-α gene transcription also involves the inducible binding of Sp1, generally considered to be a constitutive transcription factor. Notably, quantitative DNase I footprinting reveals that both of the TNF-α promoter Sp1 sites are in fact low-affinity Sp1 sites (Tsytsykova and Goldfeld, unpublished observations). Thus, inducible rather than constitutive Sp1 binding correlates with the relatively lower affinity of Sp1 for its binding sites in the TNF-α promoter and with its inducer-specific requirement in TNF-α gene regulation. There is no evidence of cooperative binding between Sp1 and the other activators involved in TNF-α gene regulation by LPS, since binding of Sp1 with Elk-1, ATF-2/c-jun, or NFATp is not cooperative in quantitative DNase I footprinting analyses (Tsytsykova and Goldfeld, unpublished data). Inducer-specific recruitment of Sp1 might thus be achieved by enhancing the affinity of Sp1 for these binding sites in the TNF-α promoter via posttranslational modification or by inducing a change in the accessibility of the sites in the context of chromatin.
Our findings also demonstrate a role for the CRE site in LPS-induced TNF-α gene transcription and have shown the inducible recruitment of ATF-2 and c-jun to the TNF-α promoter by LPS in vivo. Our results with ATF-2 mutant mice further demonstrate a role for ATF-2 in LPS activation of TNF-α gene transcription. The CRE site and binding of ATF-2–Jun to this site are required for induction of the TNF-α gene by calcium ionophore or antigen receptor engagement of T and B cells (48, 49), by FcɛRI engagement in mast cells (21), by TNF-α treatment in fibroblasts (6), and by virus infection of T and B cells and fibroblasts (15). Thus, binding of ATF-2–Jun to the TNF-α CRE is the endpoint of distinct signal transduction pathways that are set into motion by multiple extracellular stimuli that induce TNF-α gene expression, and this site serves to integrate these various signals at the level of transcription.
The CBP and p300 transactivation domains were initially characterized as targets of protein kinase A (9, 32), and it has recently been shown that transactivation mediated by CBP is potentiated by calcium influx and by nerve growth factor in neuronal cells (8, 29). Here, we have demonstrated that the transcriptional activity of CBP and p300 is also potentiated by LPS. Moreover, in our studies of TNF-α, we have established a role for the CBP and p300 proteins in LPS-mediated expression of a specific gene. The CBP and p300 proteins function as transcriptional integrators, interacting with multiple transcription factors and the basal transcription machinery (reviewed in reference 42). Our demonstration that the CRE/κ3 element is sufficient for functional interaction with the coactivator proteins CBP and p300 is therefore consistent with the role of this composite element in integrating diverse signal transduction pathways at the TNF-α promoter.
Notably, all of the transcription factors we have detected binding to the TNF-α promoter in vivo upon LPS stimulation (ATF-2, c-jun, Ets, Elk-1, Egr-1, and Sp1) have been shown to interact with CBP and p300 in an inducible or constitutive manner (2, 4, 24, 25, 44, 57). We thus model the LPS-induced enhancer complex at the TNF-α promoter as a complex involving multiple interactions between the DNA-bound transcription factors and the CBP and p300 coactivators (Fig. 9D).
Assembly of transcription factors into higher-order nucleoprotein complexes, or enhanceosomes, typically ensures that a gene is transcribed in response to a given stimulus (reviewed in references 7 and 34). In the case of the TNF-α promoter, distinct sets of transcription factors are recruited to a fixed set of binding sites depending upon the stimulus. This finding thus illustrates a mechanism by which a single gene may respond to distinct induction signals through the same regulatory region and may indeed be a general means by which temporal and stimulus-specific transcription is achieved.
ACKNOWLEDGMENTS
We thank Tom Maniatis for comments on the manuscript and for research support to J.V.F. Critical support was also provided by Fred Rosen and The Center for Blood Research. We gratefully acknowledge the generosity of the following individuals who provided essential reagents for this study: Dimitris Thanos (NFATp protein and Gal4-CBP), Harinder Singh (PU.1 protein), Andrew Sharrocks (Elk-1 protein), and Barbara Nikolajczyk (Ets-1 and PU.1 proteins). We also thank the following individuals for their generous gifts: L.-L. Lin and Genetics Institute for SB203580, G. Cox for the ANA-1 cells, H. W. L. Ziegler-Heitbrock for the Mono Mac-6 cells, A. Giordano and Y. Shi for Gal 4-p300, T. Collins for the E1A expression vectors, T. Kawakami for the −200 TNF-α luciferase reporter, and Jessica Leung and Patricia Pesavento for assistance with transfections.
This work was supported by grants from the NIH to A.E.G. (GM-56492) and L.H.G. (AI-32412), a gift from the G. Harold and Leila Y. Mathers Charitable Foundation to L.H.G., a grant from the Arthritis Foundation to A.M.R., and an Established Investigator Award from the American Heart Association to A.E.G.
E.Y.T., J.V.F., and A.V.T. made equal contributions to this work.
REFERENCES
- 1.Aggarwal B B, Puri R K. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Science; 1995. [Google Scholar]
- 2.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 3.Baldassare J J, Bi Y, Bellone C J. The role of p38 mitogen-activated protein kinase in IL-1β transcription. J Immunol. 1999;162:5367–5373. [PubMed] [Google Scholar]
- 4.Billon N, Carlisi D, Datto M B, van Grunsven L A, Watt A, Wang X-F, Rudkin B B. Cooperation of Sp1 and p300 in the induction of the CDK inhibitor p21WAF1/CIP1 during NGF-mediated neuronal differentiation. Oncogene. 1999;18:2872–2882. doi: 10.1038/sj.onc.1202712. [DOI] [PubMed] [Google Scholar]
- 5.Boehm J C, Smietana J M, Sorenson M E, Garigipati R S, Gallagher T F, Sheldrake P L, Bradbeer J, Badger A M, Laydon J T, Lee J C, Hillegass L M, Griswold D E, Breton J J, Chabot-Fletcher M C, Adams J L. 1-substituted 4-aryl-5-pyridinylimidazoles: a new class of cytokine suppressive drugs with low 5-lipoxygenase and cyclooxygenase inhibitory potency. J Med Chem. 1996;39:3929–3937. doi: 10.1021/jm960415o. [DOI] [PubMed] [Google Scholar]
- 6.Brinkman B M N, Telliez J-B, Schievella A R, Lin L-L, Goldfeld A E. Engagement of TNF receptor 1 leads to ATF-2 and p38 MAP kinase-dependent TNF-α gene expression. J Biol Chem. 1999;274:30882–30886. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- 7.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 8.Chawla S, Hardingham G E, Quinn D R, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 9.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 10.Cox G W, Mathieson B J, Gandino L, Blasi E, Radzioch D, Varesio L. Heterogeneity of hematopoietic cells immortalized by v-myc/v-raf recombinant retrovirus infection of bone marrow or fetal liver. J Natl Cancer Inst. 1989;81:1492–1496. doi: 10.1093/jnci/81.19.1492. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree G R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 12.DeFranco A L, Crowley M T, Finn A, Hambleton J, Weinstein S L. The role of tyrosine kinases and map kinases in LPS-induced signaling. Prog Clin Biol Res. 1998;397:119–136. [PubMed] [Google Scholar]
- 13.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 14.Falvo J V, Brinkman B M N, Tsytsykova A V, Tsai E Y, Yao T-P, Kung A L, Goldfeld A E. A stimulus-specific role for CREB-binding protein (CBP) in T cell receptor-activated tumor necrosis factor α gene expression. Proc Natl Acad Sci USA. 2000;97:3925–3929. doi: 10.1073/pnas.97.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falvo J V, Uglialoro A M, Brinkman B N M, Merika M, Parekh B S, King H C, Tsai E Y, Morielli A D, Peralta E G, Maniatis T, Thanos D, Goldfeld A E. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter. Mol Cell Biol. 2000;20:2239–2247. doi: 10.1128/mcb.20.6.2239-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geppert T D, Whitehurst C E, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93–103. [PMC free article] [PubMed] [Google Scholar]
- 17.Goldfeld A E, Doyle C, Maniatis T. Human tumor necrosis factor α gene regulation by virus and lipopolysaccharide. Proc Natl Acad Sci USA. 1990;87:9769–9773. doi: 10.1073/pnas.87.24.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldfeld A E, McCaffrey P G, Strominger J L, Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J Exp Med. 1993;178:1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldfeld A E, Strominger J L, Doyle C. Human tumor necrosis factor α gene regulation in phorbol ester stimulated T and B cell lines. J Exp Med. 1991;174:73–81. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfeld A E, Tsai E, Kincaid R, Belshaw P J, Schrieber S L, Strominger J L, Rao A. Calcineurin mediates human tumor necrosis factor α gene induction in stimulated T and B cells. J Exp Med. 1994;180:763–768. doi: 10.1084/jem.180.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hata D, Kawakami Y, Inagaki N, Lantz C S, Kitamura T, Khan W N, Maeda-Yamamoto M, Miura T, Han W, Hartman S E, Yao L, Nagai H, Goldfeld A E, Alt F W, Galli S J, Witte O N, Kawakami T. Involvement of Bruton's tyrosine kinase in FcɛRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haudek S B, Natmebnig B E, Redl H, Schlag G, Giroir B P. Genetic sequences and transcriptional regulation of the TNFA promoter: comparison of human and baboon. Immunogenetics. 1998;48:202–207. doi: 10.1007/s002510050424. [DOI] [PubMed] [Google Scholar]
- 23.Hodge M R, Ranger A M, Charles de la Brousse F, Hoey T, Grusby M J, Glimcher L H. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 24.Janknecht R, Nordheim A. MAP kinase-dependent transcriptional coactivation by Elk-1 and its cofactor CBP. Biochem Biophys Res Commun. 1996;228:831–837. doi: 10.1006/bbrc.1996.1740. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki H, Song J, Eckner R, Ugai H, Chiu R, Taira K, Shi Y, Jones N, Yokoyama K K. p300 and ATF-2 are components of the DRF complex, which regulates retinoic acid- and E1A-mediated transcription of the c-jun gene in F9 cells. Genes Dev. 1998;12:233–245. doi: 10.1101/gad.12.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer B, Wiegmann K, Kronke M. Regulation of the human TNF promoter by the transcription factor Ets. J Biol Chem. 1995;270:6577–6583. doi: 10.1074/jbc.270.12.6577. [DOI] [PubMed] [Google Scholar]
- 27.Kuprash D V, Udalova I A, Turetskaya R L, Kwiatkowski D, Rice N R, Nedospasov S A. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–4052. [PubMed] [Google Scholar]
- 28.Lee J S, Galvin K M, See R H, Eckner R, Livingston D, Moran E, Shi Y. Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev. 1995;10:1188–1198. doi: 10.1101/gad.9.10.1188. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y Z, Chrivia J C, Latchman D S. Nerve growth factor up-regulates the transcriptional activity of CBP through activation of the p42/p44(MAPK) cascade. J Biol Chem. 1998;273:32400–32407. doi: 10.1074/jbc.273.49.32400. [DOI] [PubMed] [Google Scholar]
- 30.Lodie T A, Savedra R, Jr, Golenbock D T, Van Beveren C P, Maki R A, Fenton M J. Stimulation of macrophages by lipopolysaccharide alters the phosphorylation state, conformation, and function of PU.1 via activation of casein kinase II. J Immunol. 1997;158:1848–1856. [PubMed] [Google Scholar]
- 31.Lu H T, Yang D D, Wysk M, Gatti E, Mellman I, Davis R J, Flavell R A. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundblad J R, Kwok R P, Laurance M E, Harter M L, Goodman R H. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 33.Maekawa T, Bernier F, Sato M, Nomura S, Singh M, Inoue Y, Tokunaga T, Imai H, Yokoyama M, Reimold A, Glimcher L H, Ishii S. Mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome. J Biol Chem. 1999;274:17813–17819. doi: 10.1074/jbc.274.25.17813. [DOI] [PubMed] [Google Scholar]
- 34.Maniatis T, Falvo J V, Kim T H, Lin C H, Parekh B S, Wathelet M G. Structure and function of the interferon-β enhanceosome. Cold Spring Harbor Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 35.Means T K, Lodie T A, Fenton M J. Activation of protein kinase CK2 by LPS is mediated by the MAP kinase pathway. J Endotoxin Res. 1999;5:37–40. [Google Scholar]
- 36.Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- 37.Pope R M, Leutz A, Ness S A. C/EBPβ regulation of the tumor necrosis factor α gene. J Clin Investig. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 39.Reimann T, Buscher D, Hipskind R A, Krautwald S, Lohmann-Matthes M L, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1β and the TNF-α genes. J Immunol. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 40.Reimold A M, Grusby M J, Kosaras B, Fries J W, Mori R, Maniwa S, Clauss I M, Collins T, Sidman R L, Glimcher M J, Glimcher L H. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature. 1996;379:262–265. doi: 10.1038/379262a0. [DOI] [PubMed] [Google Scholar]
- 41.Scherle P A, Jones E A, Favata M F, Daulerio A J, Covington M B, Nurnberg S A, Magolda R L, Trzaskos J M. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161:5681–5686. [PubMed] [Google Scholar]
- 42.Shikama N, Lyon J, La Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 43.Shore P, Sharrocks A D. The ETS-domain transcription factors Elk-1 and SAP-1 exhibit differential DNA binding specificities. Nucleic Acids Res. 1995;23:4698–4706. doi: 10.1093/nar/23.22.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman E S, Du J, Williams A J, Wadgaonkar R, Drazen J M, Collins T. cAMP-response-element-binding-protein-binding protein (CBP) and p300 are transcriptional co-activators of early growth response factor-1 (Egr-1) Biochem J. 1998;336:183–189. doi: 10.1042/bj3360183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 46.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 47.Trede N S, Tsytsykova A V, Chatila T, Goldfeld A E, Geha R S. Transcriptional activation of the human TNF-α promoter by superantigen in human monocytic cells: role of NF-κB. J Immunol. 1995;155:902–908. [PubMed] [Google Scholar]
- 48.Tsai E Y, Jain J, Pesavento P A, Rao A, Goldfeld A E. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai E Y, Yie J, Thanos D, Goldfeld A E. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol. 1996;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uglialoro A M, Turbay D, Pesavento P A, Delgado J C, McKenzie F E, Gribben J G, Hartl D, Yunis E J, Goldfeld A E. Identification of three new single nucleotide polymorphisms in the human tumor necrosis factor-α gene promoter. Tissue Antigens. 1998;52:359–367. doi: 10.1111/j.1399-0039.1998.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Bruggen T, Nijenhuis S, van Raaij E, Verhoef J, van Asbeck B S. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun. 1999;67:3824–3829. doi: 10.1128/iai.67.8.3824-3829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vittimberga F J, Jr, McDade T P, Perugini R A, Callery M P. Sodium salicylate inhibits macrophage TNF-α production and alters MAPK activation. J Surg Res. 1999;84:143–149. doi: 10.1006/jsre.1999.5630. [DOI] [PubMed] [Google Scholar]
- 53.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe N, Suzuki J, Kobayashi Y. Role of calcium in tumor necrosis factor-α production by activated macrophages. J Biochem (Tokyo) 1996;120:1190–1195. doi: 10.1093/oxfordjournals.jbchem.a021540. [DOI] [PubMed] [Google Scholar]
- 55.Wathelet M G, Lin C H, Parekh B S, Ronco L V, Howley P M, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 56.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 57.Yang C, Shapiro L H, Rivera M, Kumar A, Brindle P K. A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol Cell Biol. 1998;18:2218–2229. doi: 10.1128/mcb.18.4.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao J, Mackman N, Edgington T S, Fan S T. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-κB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 59.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–9013. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler-Heitbrock H W, Sternsdorf T, Liese J, Belohradsky B, Weber C, Wedel A, Schreck R, Bauerle P, Strobel M. Pyrrolidine dithiocarbamate inhibits NF-κB mobilization and TNF production in human monocytes. J Immunol. 1993;151:6986–6993. [PubMed] [Google Scholar]