Biological functions, from signal transduction to cell fate commitment, are mediated by complex molecular interactions and conformational changes.1,2 Indeed, protein–protein interactions are pervasive in shaping biological phenotype, yet they are challenging to characterize.1,2 The weaker interactions are especially challenging and are rarely detected by widely used methods, such as affinity purification mass spectrometry. Fortunately, methods for analyzing protein localization at the subcellular level are able to identify even weakly interacting proteins, although these methods do not directly identify protein–protein interactions.3 Qiu et al.4 used one such method, proximity-dependent biotin labeling (BioID),5 to comprehensively map the set of proteins around key regulators of vesicle trafficking.
Vesicle trafficking is regulated in part by phosphatidylinositols, which are hydrophobic, membrane-associated signaling molecules.6 Phosphatidylinositols regulate many other cellular functions, including cell growth, proliferation, differentiation, motility, survival, and intracellular trafficking.2 Their phosphorylation is tightly controlled as it carries regulatory biological signals. In the case of endosomal trafficking, the phosphorylation of phosphatidylinositols is regulated by the PIKfyve–Vac14–Fig4 complex, but the direct mechanisms, effector functions, and protein–protein interactions remain to be fully elucidated.
IDENTIFYING A PROXIMITY INTERACTOME
Toward this goal, Qiu et al.4 set out to identify proteins surrounding the PIKfyve–Vac14–Fig4 complex, including those that interact only weakly with the complex. To achieve this goal, they used BioID,5 which involves the fusion of a biotin ligase to proteins of interest, Vac14 and Fig4 in this case. Thus BioID constitutes a proximity-based labeling method used to map proteins proximal to Vac14 and Fig4. The expression of Vac14 and Fig4 fused to biotin ligase resulted in the biotinylation of proximal proteins. Subsequent isolation and mass spectrometry analysis of biotinylated proteins revealed novel proteins associated with Vac14–Fig4 with functions in the cell division cycle and mitochondrial regulation. Identifying the sets of proteins proximal to Vac14 and Fig4 allowed the authors to intersect these sets and derive a higher confidence set of proteins in the vicinity of both Vac14 and Fig4. The high overlap between the proteins identified as proximal to Vac14 and Fig4 supports the internal consistency of the results. The authors further validated these BioID results in situ using a proximity ligation assay and complementary inhibition of both complexes. These assays suggest that Vac14–COPI interactions result in preferential recruitment of the COPI complex to the late endosomal compartment. This recruitment does not depend on PIKfyve kinase activity and is insensitive to the GEF inhibitors tested.
INTEGRATIVE INFERENCE OF REGULATORY PROTEIN NETWORKS
Qiu et al.4 used cross-validation both within BioID assays and with other assays to identify a comprehensive set of proteins in the proximity of Vac14–Fig4 and to further evaluate some of the suggested interactions. Whereas these results do not identify the protein interaction interfaces, that is, the full set of protein regulatory interactions of the network, they provide a foundation toward building such a network.
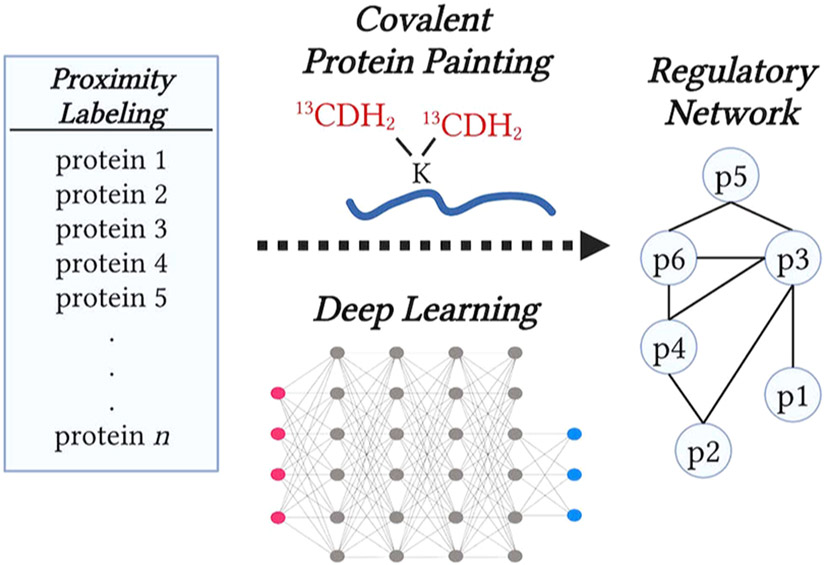
To build upon this foundation, future research can combine orthogonal sources of information, such as (i) experimental measurements of the changes in exposed protein surfaces, as measured by covalent protein painting (CPP),7,8 and (ii) computational predictions of protein interactions based on deep learning, as shown in Figure 1.9,10 The empirical data will provide essential constraints, including a small set of proteins identified by proximity labeling and their conformational changes measured by CPP. Applying these empirical constraints on protein interactions predicted by deep learning may prioritize the set of protein interfaces most likely to interact and directly mediate biological regulation, Figure 1. The regulatory networks formed by these interactions can be further evaluated and refined by complementary methods, such as cross-linking and native mass spectrometry. This integrative approach, leveraging the strengths of proximity labeling, covalent protein painting, and deep learning, may bring us closer to understanding the molecular interactions in protein regulatory networks and how these interactions give rise to biological functions.
Figure 1.
Integration of (i) protein sets identified by proximity labeling with (ii) CPP data for exposed protein surfaces and (iii) the likely protein–protein interactions predicted by deep learning may enable the inference of regulatory protein networks.
REFERENCES
- (1).Bludau I; Aebersold R Proteomic and Interactomic Insights into the Molecular Basis of Cell Functional Diversity. Nat. Rev. Mol. Cell Biol 2020, 21 (6), 327–340. [DOI] [PubMed] [Google Scholar]
- (2).Balla T Phosphoinositides: Tiny Lipids with Giant Impact on Cell Regulation. Physiol. Rev 2013, 93 (3), 1019–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Christopher JA; Stadler C; Martin CE; Morgenstern M; Pan Y; Betsinger CN; Rattray DG; Mahdessian D; Gingras A-C; Warscheid B; Lehtiö J; Cristea IM; Foster LJ; Emili A; Lilley KS Subcellular Proteomics. Nat. Rev. Methods Primers 2021, 1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Qiu S; Lavallée-Adam M; Côté M Proximity Interactome Map of the Vac14–Fig4 Complex Using BioID. J. Proteome Res 2021, DOI: 10.1021/acs.jproteome.1c00408. [DOI] [PubMed] [Google Scholar]
- (5).Roux KJ; Kim DI; Raida M; Burke B A Promiscuous Biotin Ligase Fusion Protein Identifies Proximal and Interacting Proteins in Mammalian Cells. J. Cell Biol 2012, 196 (6), 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Berridge MJ; Irvine RF Inositol Trisphosphate, a Novel Second Messenger in Cellular Signal Transduction. Nature 1984, 312 (5992), 315–321. [DOI] [PubMed] [Google Scholar]
- (7).Bamberger C; Pankow S; Martínez-Bartolomé S; Ma M; Diedrich J; Rissman RA; Yates JR Protein Footprinting via Covalent Protein Painting Reveals Structural Changes of the Proteome in Alzheimer’s Disease. Journal of Proteome Research. 2021, 20, 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Slavov N Measuring Protein Shapes in Living Cells. J. Proteome Res 2021, 20 (6), 3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Jumper J; Evans R; Pritzel A; Green T; Figurnov M; Ronneberger O; Tunyasuvunakool K; Bates R; Žídek A; Potapenko A; Bridgland A; Meyer C; Kohl SAA; Ballard AJ; Cowie A; Romera-Paredes B; Nikolov S; Jain R; Adler J; Back T; Petersen S; Reiman D; Clancy E; Zielinski M; Steinegger M; Pacholska M; Berghammer T; Bodenstein S; Silver D; Vinyals O; Senior AW; Kavukcuoglu K; Kohli P; Hassabis D Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596 (7873), 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Humphreys IR; Pei J; Baek M; Krishnakumar A; Anishchenko I; Ovchinnikov SR; Zheng J; Ness T; Banjade S; Bagde SR; Stancheva V; Li X; Liu K; Zheng Z; Barerro D; Roy U; Fernandez IS; Szakal B; Branzei D; Greene EC; Biggins S; Keeney S; Miller EA; Christopher Fromme J; Hendrickson T; Cong Q; Baker D Structures of Core Eukaryotic Protein Complexes. bioRxiv 2021, No. 2021.09.30.462231. [DOI] [PMC free article] [PubMed] [Google Scholar]