Abstract
Background
The COVID-19 pandemic disrupted routine screening for and treatment of gastrointestinal (GI) cancers. We analyzed changes in GI cancer pathology specimens resulting from diagnostic and therapeutic procedures at a single academic center in an epicenter of the COVID-19 pandemic. Our aim was to determine which cancer types, procedures, and patients were impacted by the pandemic.
Methods
This was a retrospective, cohort study of patients identified based on carcinoma containing pathologic specimens reviewed in our institution resulting from diagnostic or resection procedures. Pathology and medical records of patients with GI and liver carcinoma and high-grade dysplasia were reviewed from February 1 to April 30 in 2018, 2019 and 2020. We used March 16, 2020 to delineate the pre-COVID-19 and COVID-19 period in 2020. Chi-squared or t-tests, as appropriate, were used to compare these time periods in each year. Mann Kendall test was used to test for trend in volume. ANCOVA was used to compare differences across years.
Results
A total of 1028 pathology samples from 949 unique patients were identified during the study period. There was a 57% drop in samples within 2020 (p = 0.01) that was not present in either 2018 or 2019 (p<0.01). In 2020, there were significantly fewer resections compared to biopsies overall in the COVID-19 period (p = 0.01). There were fewer colorectal cancer specimens (p = 0.04) which were procured from older patients (p<0.01) in the 2020 COVID-19 period compared to pre-COVID-19.
Conclusions
In our institution, there was a significant drop in diagnostic and resection specimens of GI cancers during the COVID-19 pandemic, disproportionately affecting older colorectal cancer patients.
Keywords: Colon cancer, COVID, Hepatocellular carcinoma, Endoscopy
Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic has had a rapid global spread. After the first confirmed case on March 1, 2020, New York City (NYC) quickly became an epicenter for the disease. In April and early May, NYC accounted for up to one quarter of global cases [1]. In order to “flatten the curve” a stay-at-home order was mandated by the Governor of the State of New York, which additionally mandated the cancelation of elective surgeries and procedures in mid-March. While containment has been critical to limit the spread of COVID-19, this strategy comes at a detrimental cost in terms of delaying the diagnosis and treatment of important diseases, especially cancer.
The COVID-19 pandemic has had a major impact on public health beyond the toll of the virus itself. There are increasing reports of postponed medical care in the United States attributed to COVID-19, for example due to cancelation of office visits and procedures and from patient fears of contracting the virus while seeking care for other health issues [2]. According to the CDC, emergency department visits dropped 42% in April 2020 compared to the same time period in 2019 [3]. Similarly, there were decreases in number of ST-segment elevation cardiac catheterization activations [4] and delayed diagnosis and treatment of acute hematological malignancies [5]. The New York City Department of Health and Mental Hygiene (DOHMH) identified 5293 (22%) excess deaths beyond those officially attributed to COVID-19, which may be due in part to patients avoiding needed care [6].
Of particular concern are patients with symptoms concerning for malignancy needing an initial diagnostic procedure and those with cancer diagnoses awaiting surgical intervention or adjuvant oncologic therapy. Additionally, with the postponement of preventative health appointments, diagnoses of new cancers through screening and surveillance may be delayed or even entirely missed during this time [7]. In the Netherlands, this is already evident given a significant drop in cancer diagnoses since their first case of COVID-19 in late February [8]. Delays in cancer diagnosis and treatment might be expected to result in more advanced stage disease at diagnosis, requirement for additional or more complicated treatment interventions, worse prognosis, and possibly decreased overall survival. Indeed, the National Institute of Health predicts an estimated 10,000 excess deaths from delayed diagnosis and staging of colorectal and breast cancer over the next ten years [9].
In this study, we aimed to quantify changes in the number of gastrointestinal (GI) cancer specimens that resulted from endoscopies, Interventional Radiology (IR)-guided biopsies and surgeries, both diagnostic and therapeutic, performed at a single academic medical center in New York City during the time that it was an epicenter of the COVID-19 pandemic. Our aim was to determine the demographic, histopathologic and clinical characteristics of cancer patients most impacted by COVID-19.
Methods
The study was approved by the Mount Sinai Institutional Review Board (IRB). This was a retrospective, cohort study of patients identified based on carcinoma containing pathologic specimens reviewed by Mount Sinai Pathology Department resulting from diagnostic or resective procedures. Surgical pathology records of patients with a diagnosis of GI and liver carcinoma or high-grade dysplasia were identified from February 1 to April 30, 2020 as well as during the same period in 2018 and 2019. March 16, 2020 was chosen as the date to delineate the pre- and COVID-19 period, since this was the date that the New York State Executive Order banning large gatherings and closing schools and non-essential businesses went into effect. This was the same date that all elective procedures were canceled at the Mount Sinai Health system. All specimens (which included both diagnostic biopsies and surgical and endoscopic resections) from the tubular GI tract and associated solid organs (esophagus, stomach, small intestine, appendix, colon, rectum, anus, liver, pancreas, and biliary tract, including intrahepatic, extrahepatic and gallbladder) were included. Consultation cases submitted to the pathology department from referring physicians were included only when sufficient information was contained within the pathology report. Metastatic carcinomas to the GI tract were included provided other parameters fulfilled study criteria. Cases with non-carcinoma diagnoses (sarcomas, mixed tumors, hematologic malignancies), diagnoses of indefinite or low-grade dysplasia, patients scoped or operated on for liver transplant (regardless of underlying etiology), and cytology specimens were excluded. The type of procedure performed was recorded as surgical, endoscopic or percutaneous (most commonly performed by interventional radiologists).
Data on demographic and clinicopathological features including patient age, sex, and race, type of procedure, anatomical and primary site (if applicable), histological type and grade, and overall tumor stage were obtained from electronic medical records, including pathology reports. Specimens were considered upper GI cancers if they originated in the esophagus, gastroesophageal junction (GEJ), stomach or proximal small bowel (duodenum). Extraluminal GI cancers were those that originated in the liver, biliary tract or pancreas. Lower GI cancers had origin in the distal small bowel (jejunum and ileum), colon (including appendix), rectum or anus. Specimens were classified as either resection (if the tumor was fully removed either surgically or endoscopically) or biopsy if it was only sampled (endoscopically, percutaneously, or through a surgical biopsy). Advanced pathologic stage was defined as the presence of any of the following: pT3, pT4 or metastatic disease to any lymph node or distant organ.
Patient and clinical characteristics from the 6 weeks prior to COVID-19 (February 1-March 15, 2020) were compared to the COVID-19 time period (March 16-April 30, 2020), as well as to the same time periods (February-April) of 2018 and 2019 using chi-squared t-test, as appropriate. Mann Kendall test was used to analyze trends in volume of cases over bi-weekly periods from February to April 30 in each year. Analysis of Covariance (ANCOVA) was used to compare the slope of the regression line across years. Time to treatment (surgery and/or chemoradiation) for CRC and all other GI cancers was compared using the Mann Whitney U test.
All data was analyzed using SAS version 9.4 (Cary, NC) with p < 0.05 considered statistically significant throughout.
Results
Overall study cohort
A total of 949 unique patients were identified in 2018, 2019 and 2020 (Table 1 ). The mean age of the patients in each time period ranged from 62.7 ± 14.9 to 65.3 ± 11.2 years. There were more male than female patients overall in both 2019 and 2020, in contrast with 2018 when there were more female patients. Across all time periods, most patients were white (33.3%−39.5%). Black and Asian patients comprised less than 20% of the population during each six-week period.
Table 1.
Clinicopathologic Characteristics of Gastrointestinal Neoplastic Specimens in 2018, 2019 and 2020.
| 2018 |
2019 |
2020 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | February 1-March 15 | March 16-April 30 | February 1-March 15 | March 16-April 30 | February 1-March 15 | March 16-April 30 | |||
| N = 949 | N = 148 (47.0%) | N = 167 (53.0%) | N = 154 (43.2%) | N = 202 (56.7%) | N = 194 (69.8%) | N = 84 (30.2%) | p value (2020^) | ||
| Age (mean [standard deviation]) | 63.9 (13.2) | 63.6 (13.1) | 63.7 (12.6) | 62.7 (14.9) | 64.6 (13.4) | 64.1 (13.0) | 65.3 (11.2) | <0.01 | |
| Sex | Male | 55.3% | 45.9% | 41.9% | 54.5% | 61.9% | 61.9% | 69.0% | 0.25 |
| Female | 44.7% | 54.1% | 58.1% | 45.5% | 38.1% | 38.1% | 31.0% | ||
| Race | White | 37.2% | 37.8% | 39.5% | 33.8% | 37.6% | 38.7% | 33.3% | 0.46 |
| Black | 14.3% | 14.2% | 14.4% | 11.7% | 13.9% | 16.0% | 16.7% | ||
| Asian | 14.1% | 14.2% | 19.8% | 17.5% | 10.9% | 12.4% | 8.3% | ||
| Other/unknown | 34.4% | 33.8% | 26.3% | 37.0% | 37.6% | 33.0% | 41.7% | ||
| Consults | 18.2% | 13.5% | 13.2% | 22.1% | 25.2% | 14.4% | 21.4% | 0.15 | |
| Procedure | Surgery | 49.1% | 57.9% | 47.9% | 50.0% | 47.0% | 50.5% | 36.1% | 0.07 |
| *(n = 939) | Endoscopy | 28.2% | 25.5% | 28.2% | 24.0% | 24.8% | 32.8% | 38.6% | |
| Percutaneous | 22.7% | 16.6% | 26.9% | 26.0% | 28.2% | 16.7% | 25.3% | ||
| Specimen | Resection | 50.2% | 57.4% | 45.5% | 51.3% | 48.0% | 50.5% | 34.5% | 0.01 |
| ⁎⁎(n = 940) | Biopsy | 48.9% | 40.5% | 52.1% | 48.7% | 52.0% | 48.5% | 65.5% | |
| Anatomic Site | Colorectal⁎⁎⁎ | 45.6% | 50.7% | 45.5% | 37.7% | 47.0% | 51.5% | 34.5% | 0.04 |
| Hepatobiliary | 26.4% | 21.6% | 26.9% | 32.5% | 25.7% | 24.2% | 29.8% | ||
| GEJ | 4.6% | 3.4% | 2.4% | 7.8% | 4.5% | 3.1% | 9.5% | ||
| Stomach | 9.4% | 8.1% | 12.0% | 7.8% | 11.4% | 8.8% | 6.0% | ||
| Pancreas/duodenum | 8.3% | 8.8% | 7.2% | 7.8% | 7.4% | 8.8% | 11.9% | ||
| Anus Other |
2.3% 3.3% |
2.0% 5.4% |
1.8% 4.2% |
3.9% 2.6% |
2.5% 1.5% |
1.5% 2.1% |
2.4% 6.0% |
||
| Histologic Type | Adenocarcinoma | 74.0% | 77.0% | 78.4% | 64.9% | 77.2% | 76.8% | 61.9% | 0.04 |
| HCC | 10.4% | 5.4% | 9.0% | 17.5% | 9.9% | (7.7% | 16.7% | ||
| Cholangiocarcinoma | 5.4% | 4.7% | 5.4% | 6.5% | 4.0% | (5.2% | 8.3% | ||
| SCC | 5.0% | 3.4% | 4.8% | 8.4% | 4.0% | 3.1% | 8.3% | ||
| AMN | 0.5% | 1.4% | 0.0% | 0.0% | 1.0% | 0.5% | 0.0% | ||
| Neuroendocrine | 2.5% | 2.0% | 1.2% | 1.9% | 2.5% | 5.2% | 1.2% | ||
| PCN | 0.8% | 3.4% | 0.0% | 0.0% | 0.5% | 0.5% | 1.2% | ||
| Other | 1.4% | 2.7% | 1.2% | 0.6% | 1.0% | 1.0% | 2.4% | ||
| Grade⁎⁎⁎⁎ | Well to Moderately Differentiated | 67.8% | 63.1% | 70.4% | 73.2% | 70.2% | 67.3% | 60.6% | 0.32 |
| (n = 805) | Poorly Differentiated | 32.2% | 36.9% | 31.6% | 26.8% | 29.8% | 32.7% | 39.4% | |
| Metastases to GI Tract | 15.7% | 18.9% | 22.2% | 11.0% | 12.4% | 15.5% | 14.3% | 0.15 | |
| Stage⁎⁎⁎⁎⁎ | Localized | 31.7% | 22.8% | 20.6% | 46.3% | 34.7% | 22.2% | 35.1% | 0.92 |
| (n = 515) | Advanced | 68.3% | 77.2% | 79.4% | 53.8% | 65.3% | 42.7% | 64.9% | |
GEJ: Gastroesophageal Junction.
HCC: Hepatocellular Carcinoma.
SCC: Squamous Cell Carcinoma.
AMN: Appendiceal Mucinous Neoplasm.
PCN: Pancreatic Cystic Neoplasm.
p value refers to chi-squared test for categorical variables or t-test for continuous variables for February 1-March 15 vs. March 16-April 30, 2020 only.
Procedure refers to whether the procedure whereby the tissue was obtained was surgical, endoscopic or percutaneous (performed by interventional radiology). Included from n = 939 where data was known.
Specimen refers to whether the pathology tissue was a diagnostic biopsy or a full resection of tumor. Included from n = 940 where data was known.
Colorectal includes appendiceal.
Included from n = 805 where data was known.
Localized stage defined as pT0, pT1 or pT2 without any distant disease; advanced stage defined as pT3 or pT4, or the presence of metastatic disease in lymph nodes or distant organs. Included from n = 515 where data was known.
The procedures were most commonly surgeries (461/939 or 49.1%) and the remainder were endoscopies (265/939 or 28.2%) or IR-guided biopsies (213/939 or 22.7%). Of these, 476/940 (50.2%) specimens were fully resected, either endoscopically or surgically. The largest number (433/949 or 45.6%) came from the colon and rectum. An additional 251 (26.4%) came from hepatobiliary tumors. Correspondingly the majority (702/949 or 74.0%) of cancers were adenocarcinoma; the remainder were cholangiocarcinoma, hepatocellular carcinoma, squamous cell carcinoma, neuroendocrine tumor, pancreatic cystic neoplasms and metastatic appendiceal mucinous neoplasms. Amongst those with grade, 32.2% of tumors were poorly differentiated. 15.7% of tumors resected or biopsied were from metastatic cancers.
Trends across years
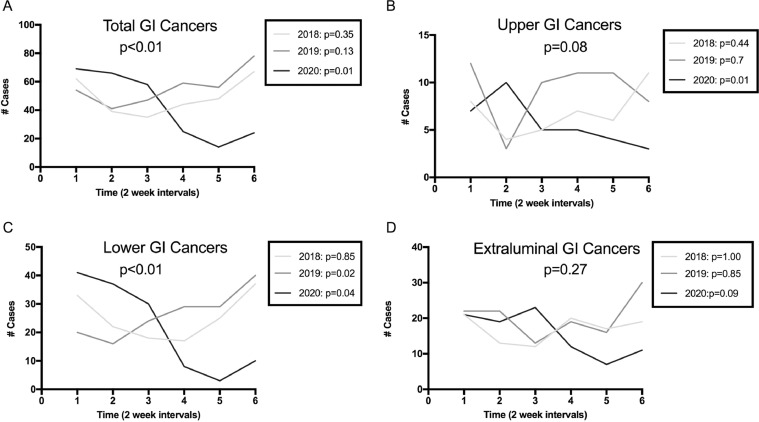
There was a consistent decrease in biweekly volume of pathology specimens during 2020 that was not present in either 2018 or 2019 (p = 0.01 for 2020, Fig. 1 a). A similar decrease was also seen for upper GI cancers (p = 0.01 for 2020, Fig. 1b). Lower GI cancers increased during the 2019 study period (p = 0.02, Fig. 1c) but fell significantly during 2020 (p = 0.04). No differences were noted for extraluminal cancers (p = 0.09 for 2020, Fig. 1d).
Fig. 1.
Numbers of gastrointestinal cancer specimens over time. Overall p value in each graph reflects comparison of slopes between years, while p values for each individual years' trend are indicated in the inset.
When comparing the slopes of the regression lines by year across all three years, there was a significant difference in the total number of pathology specimens sampled (p < 0.01). The same was true for lower GI cancers specifically (p < 0.01). There was no significant difference for upper GI or extraluminal cancers.
Differences within 2020
A total of 278 unique specimens were analyzed during 2020, 84 of which were in the COVID-19 period, representing a 57% drop in volume. Patients in the COVID-19 cohort were older than pre-COVID-19 (65.3 vs 64.1 years, p < 0.01). Sex and race were not significantly different between the two groups.
During the COVID-19 period, the proportions of the procedures by which specimens were obtained changed as well. There was a 69% decline in the volume of surgical specimens, 49% decline in endoscopy specimens and 34% decrease in percutaneous biopsy specimens. These changes resulted in overall lower proportion of surgical samples (50.5% vs 36.1%, p = 0.07). Similarly, there was a 70% decline in resections and 41% decline in biopsy samples resulting in a lower proportion of cancers that were fully resected (50.5% vs 34.5%, p = 0.01). While the absolute volume of most GI cancer specimens decreased during COVID-19, there was a notable decrease in the proportion of colorectal cancers (51.5% vs 34.5%, p = 0.04) and a relative increase in overall proportion of extraluminal GI cancers (Table 1). The overall number of GEJ tumors sampled was similar (6 vs 8) but increased as a share of total cancers (3.1% vs 9.5%). Corresponding to the significant drop in colorectal cancers, there were fewer adenocarcinomas (76.8% vs 61.9%, p = 0.04). There was no significant difference in histologic grade or pathologic staging in the COVID-19 time period.
Colorectal cancer
Since our results show a more dramatic decrease in colorectal cancer specimens, we examined the colorectal cancer staging in more detail. Although the volume fell from 95 patients in the pre-COVID-19 period to 28 in the COVID-19 period, resulting in 71% overall drop in volume of cases, there was no significant difference in the distribution by patient sex or race (Table 2 ). Those who underwent colorectal cancer-related procedures in the COVID-19 time period were older than those in the pre-COVID-19 time period (65.5 ± 13.1 years vs 68.4 ± 11.6 years, p < 0.01). There was no significant difference in pathologic stage (p = 0.62). Additionally, there were no differences noted in the proportion of surgical resections and biopsies.
Table 2.
Clinicopathologic Characteristics of Colorectal Carcinomas in 2020.
| Total | February 1-March 15 | March 16-April 30 | |||
|---|---|---|---|---|---|
| N = 123(100%) | N = 95 (77.2%) | N = 28 (22.8%) | p value | ||
| Consult | 17(13.8) | 11(11.6) | 6(21.4) | 0.18 | |
| Age | Mean (Standard Deviation) | 66.0(12.6) | 65.5(13.1) | 68.4(11.0) | <0.01 |
| Sex | Male | 57.7% | 56.8% | 60.7% | |
| Female | 42.3% | 43.2% | 39.3% | 0.72 | |
| Race | White | 42.3% | 46.3% | 28.6% | 0.23 |
| Black | 11.4% | 11.6% | 10.7% | ||
| Asian | 12.2% | 12.6% | 10.7% | ||
| Other/unknown | 34.1% | 29.5% | 50.0% | ||
| Procedure | Surgery | 60.6% | 62.8% | 53.5% | 0.15 |
| *(n = 122) | Endoscopy | 38.5% | 37.2% | 42.9% | |
| Percutaneous | 0.8% | 0.0% | 3.6% | ||
| Specimen | Resection | 64.8% | 66.0% | 60.7% | 0.61 |
| ⁎⁎(n = 122) | Biopsy | 35.2% | 34.0% | 39.3% | |
| Grade⁎⁎⁎ | Well to Moderately Differentiated | 82.2% | 82.7% | 80.8% | 0.82 |
| (n = 107) | Poorly Differentiated | 17.8% | 17.3% | 19.2% | |
| Stage⁎⁎⁎⁎ | Localized | 41.8% | 40.3% | 47.1% | 0.62 |
| (n = 79) | Advanced | 58.2% | 59.7% | 52.9% |
Table excludes appendiceal cancers.
Procedure refers to whether the procedure whereby the tissue was obtained was surgical, endoscopic or percutaneous (performed by interventional radiology). Included from n = 122 where data was known.
Specimen refers to whether the pathology tissue was a diagnostic biopsy or a full resection of tumor. Included from n = 122 where data was known.
Included from n = 107 where data was known.
Localized stage defined as pT0, pT1 or pT2 without any distant disease; advanced stage defined as pT3 or pT4, or the presence of metastatic disease in lymph nodes or distant organs. Included from n = 79 where data was known.
We further examined treatment outcomes among 87 patients with available follow-up who had been diagnosed with cancer based on a biopsy during the 2020 study period. There was a median time to surgery of 82 days (interquartile range [IQR] 51.25–111) for CRC and 74 days (IQR 36–153.5) for all other cancer types, difference not statistically significant). There was a median time to chemoradiation of 52.5 days (IQR 29.25–140.5) for CRC and 35.50 (IQR 27.5–54.75) for all other cancer types (difference not statistically significant). Time to any treatment (chemoradiation or surgery) was a median 77.0 days (IQR. 35.75–127) for CRC and 40 days (IQR 30.5–77) for all other GI cancers (difference not statistically significant).
Discussion
Since the onset of the COVID-19 pandemic, the consequence of delayed cancer care has been of great concern world-wide [7,8,[10], [11], [12], [13], [14], [15]]. A recent modeling study suggests 10,000 excess deaths due to colon and breast cancer over the next 10 years, but the assumptions of this model are based on hypothetical estimates [9]. To our knowledge, this is the first study to measure the impact on volume of diagnostic and surgical procedures for all types of GI cancers in a U.S. epicenter of the COVID-19 pandemic. Not surprisingly, we found that there was a significant decline in overall number of pathology-yielding procedures for GI cancers during the COVID-19 pandemic, with a 57% reduction in total GI pathology specimens submitted. Patients who underwent these procedures during the COVID-19 period were older, but did not differ by sex or race. We also found that the number of resection specimens fell even more than biopsies. Finally, we found that colorectal cancers were disproportionately impacted by the pandemic when compared to upper and extraluminal GI cancers.
The reasons for this reduction in diagnoses and surgeries for cancer during the pandemic are multifaceted. From the healthcare delivery perspective, elective surgeries and procedures were suspended to conserve ventilators and personal protective equipment as well as to maximize medical capacity in the hospital. In fact, many of the endoscopy suites and post-anesthesia care units were converted to COVID-19 units during the pandemic. Additionally, gastroenterologists, surgeons, radiologists and anesthesiologists were redeployed to COVID-19 wards. An international survey found an 83% reduction in endoscopy procedure volumes worldwide [16]. From the patient perspective, social distancing practices along with fear and anxiety about COVID-19 have led to delays in seeking medical care [2]. Moving forward, with resumption of elective procedures, delays in diagnosis may persist for weeks or months due to ongoing transmission concerns, unemployment and subsequent loss of health insurance, and the large number of patients whose missed procedures need to be triaged for urgency [17].
There are multiple possible explanations for the discrepancy between primary cancer types. Cancers of the upper GI and hepatobiliary tracts often present with significant symptoms such as obstruction leading to jaundice or dysphagia, or are evaluated in the inpatient setting where procedures were still being performed at our hospital. In contrast, screening and diagnostic procedures for less urgent diagnoses such as iron deficiency anemia, which may be the initial presentation of colorectal cancer, were deferred. Furthermore, pancreatic, hepatobiliary or esophageal cancers continued to be evaluated by surgeons or therapeutic endoscopists who maintained hospital- and endoscopy-based practices throughout the study period, whereas many general gastroenterologists were not performing outpatient endoscopies as per the hospital protocol.
A strength of our study is that it includes procedures from multiple different disciplines including IR, GI and surgery. Additionally, this study describes that patients with colorectal cancer were disproportionately affected, which may help our colleagues to triage and prepare for future waves of the pandemic where there may be a significant restriction in health care resources. In particular, for providers whose protocols limit use of endoscopy, they should consider how best to identify patients at high risk of colorectal cancer and prioritize these patients’ care.
Limitations to this study are that we did not assess delays to imaging or oncology follow up. Additionally, staging was only reported on resection specimens based on the tissue that was submitted to pathology department and may have been limited by incomplete medical records. We did not find significant differences in colorectal cancer stage between the pre-COVID-19 and COVID-19 cohorts; however, this study is limited to six weeks after the stay-at-home order was issued and is likely too short a time period to find such an effect. Finally, this study was limited by the fact that it was carried out at a single institution.
Given the significant decrease in cancers identified and treated, it will be imperative to recapture these patients to mitigate delays in diagnosis and treatment. Herein we have described the changes in diagnoses that were seen during the initial surge to help providers be aware of patients who may have been at risk of delayed cancer diagnosis. Future studies that evaluate patient and provider factors leading to delays in surgical and endoscopic procedures due to COVID, both during the pandemic and in the months that follow, will be important to determine barriers to care. Identifying and evaluating practices for triaging surgical and endoscopic procedures is key [11,13,18]. Finally, to understand the impact of COVID-19 on cancer care as a whole, from diagnosis to resection to oncological treatment, further studies are needed to evaluate the long-term impact of the pandemic on cancer staging, mortality and health care costs as patients with delayed care may present with more advanced disease in the coming months and years. Moving forward, as we face additional waves of the pandemic where health care resources may be severely restricted, we must ensure that we are capturing patients whose clinical course will be impacted by delays in care and not allow their cancer diagnosis to be missed or their treatment to be postponed.
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Declaration of funding
Aimee L. Lucas has a grant from the American Cancer Society: 129,387-MRSG-16-015-01-CPHPS.
CRediT authorship contribution statement
Lauren Tal Grinspan: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Sheila D. Rustgi: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. Steven H. Itzkowitz: Writing – review & editing. Alexandros D. Polydorides: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. Aimee L. Lucas: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.clinre.2021.101839.
Appendix. Supplementary materials
References
- 1.COVID-19 dashboard by the center for systems science and engineering (CSSE) at johns hopkins university (JHU) 2020. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- 2.Physicians ACoE. COVID-19 2020. Available from: https://www.emergencyphysicians.org/globalassets/emphysicians/all-pdfs/acep-mc-COVID19-april-poll-analysis.pdf.
- 3.Kathleen P., Hartnett A.K.P., DeVies Jourdan, Coletta Michael A., Boehmer Tegan K., Jennifer Adjemian A.V.G. Impact of the COVID-19 pandemic on emergency department visits —United States, January 1, 2019–May 30, 2020. Cent Dis Control Prev Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6923e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia S., Albaghdadi M.S., Meraj P.M., Schmidt C., Garberich R., Jaffer F.A., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molica M., Mazzone C., Cordone I., Pasquale A., Niscola P., de Fabritiis P. SARS-CoV-2 infection anxieties and general population restrictions delay diagnosis and treatment of acute haematological malignancies. Br J Haematol. 2020;190(1):e5–e8. doi: 10.1111/bjh.16785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.New York City Department of Health and Mental Hygiene (DOHMH) COVID-19 Response Team Preliminary estimate of excess mortality during the COVID-19 outbreak — New York City, March 11–May 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:603–605. doi: 10.15585/mmwr.mm6919e5. [DOI] [PubMed] [Google Scholar]
- 7.The Lancet O Safeguarding cancer care in a post-COVID-19 world. Lancet Oncol. 2020;21(5):603. doi: 10.1016/S1470-2045(20)30243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpless N.E. COVID-19 and cancer. Science. 2020;368(6497):1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 10.Pellino G., Spinelli A. How coronavirus disease 2019 outbreak is impacting colorectal cancer patients in italy: a long shadow beyond infection. Dis Colon Rectum. 2020;63(6):720–722. doi: 10.1097/DCR.0000000000001685. [DOI] [PubMed] [Google Scholar]
- 11.Romesser P.B., Wu A.J., Cercek A., Smith J.J., Weiser M., Saltz L., et al. Management of locally advanced rectal cancer during the COVID-19 pandemic: a necessary paradigm change at memorial Sloan kettering cancer center. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saini K.S., de Las Heras B., de Castro J., Venkitaraman R., Poelman M., Srinivasan G., et al. Effect of the COVID-19 pandemic on cancer treatment and research. Lancet Haematol. 2020;7(6):e432–e435. doi: 10.1016/S2352-3026(20)30123-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett D.L., Howe J.R., Chang G., Crago A., Hogg M., Karakousis G., et al. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27(6):1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrag D., Hershman D.L., Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323(20):2005–2006. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 15.Lui T.K., Leung K., Guo C.G., Tsui V.W., Wu J.T., Leung W.K. Impacts of COVID-19 pandemic on gastrointestinal endoscopy volume and diagnosis of gastric and colorectal cancers: a population-based study. Gastroenterology. 2020;159(3):1164–1166. doi: 10.1053/j.gastro.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parasa S., Reddy N., Faigel D.O., Repici A., Emura F., Sharma P. Global Impact of the COVID-19 pandemic on endoscopy: an international survey of 252 centers from 55 countries. Gastroenterology. 2020;159(4):1579–1581. doi: 10.1053/j.gastro.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soreide K., Hallet J., Matthews J.B., Schnitzbauer A.A., Line P.D., Lai P.B.S., et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg. 2020;107(10):1250–1261. doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaukat A., Church T. Colorectal cancer screening in the USA in the wake of COVID-19. Lancet Gastroenterol Hepatol. 2020;5(8):726–727. doi: 10.1016/S2468-1253(20)30191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.