Abstract
Background
In an effort to improve outcomes of in vitro fertilisation (IVF) cycles, the use of growth hormone (GH) has been considered as adjuvant treatment in ovarian stimulation. Improving the outcomes of IVF is especially important for women with infertility who are considered 'poor responders'. We have compared the outcomes of IVF with adjuvant GH versus no adjuvant treatment in routine use, and specifically in poor responders.
Objectives
To assess the effectiveness and safety of growth hormone as an adjunct to IVF compared to standard IVF for women with infertility
Search methods
We searched the following databases (to November 2020): Cochrane Gynaecology and Fertility (CGF) Group specialised register, CENTRAL, MEDLINE, Embase, CINAHL, Epistemonikos database and trial registers together with reference checking and contact with study authors and experts in the field to identify additional trials.
Selection criteria
We included all randomised controlled trials (RCTs) of adjuvant GH treatment in IVF compared with no adjuvant treatment for women with infertility. We excluded trials where additional adjuvant treatments were used with GH. We also excluded trials comparing different IVF protocols.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. Two review authors independently performed assessment of trial risk of bias and extraction of relevant data. The primary review outcome was live birth rate. The secondary outcomes were clinical pregnancy rate, oocytes retrieved, embryo transfer, units of gonadotropin used and adverse events, i.e. ectopic pregnancy, multiple pregnancy, ovarian hyperstimulation syndrome (OHSS), congenital anomalies, oedema.
Main results
We included 16 RCTs (1352 women). Two RCTs (80 women) studied GH in routine use, and 14 RCTs (1272 women) studied GH in poor responders. The evidence was low to very low certainty, the main limitations being risk of bias, imprecision and heterogeneity.
Adjuvant growth hormone compared to no adjuvant: routine use for in vitro fertilisation (IVF)
The evidence is very uncertain about the effect of GH on live birth rate per woman randomised for routine use in IVF (odds ratio (OR) 1.32, 95% confidence interval (CI) 0.40 to 4.43; I2 = 0%; 2 trials, 80 participants; very low‐certainty evidence). If the chance of live birth without adjuvant GH is assumed to be 15%, the chance of live birth with GH would be between 6% and 43%.
There was insufficient evidence to reach a conclusion regarding clinical pregnancy rates per woman randomised, number of women with at least one oocyte retrieved per woman randomised and embryo transfer achieved per woman randomised; reported data were unsuitable for analysis.
The evidence is very uncertain about the effect of GH on mean number of oocytes retrieved in normal responders (mean difference (MD) ‐0.02, 95% CI ‐0.79 to 0.74; I2 = 0%; 2 trials, 80 participants; very low‐certainty evidence).
The evidence is very uncertain about the effect of GH on mean units of gonadotropin used in normal responders (MD 13.57, 95% CI ‐112.88 to 140.01; I2 = 0%; 2 trials, 80 participants; very low‐certainty evidence).
We are uncertain of the effect of GH on adverse events in normal responders.
Adjuvant growth hormone compared to no adjuvant: use in poor responders for in vitro fertilisation (IVF)
The evidence is very uncertain about the effect of GH on live birth rate per woman randomised for poor responders (OR 1.77, 95% CI 1.17 to 2.70; I2 = 0%; 8 trials, 737 participants; very low‐certainty evidence). If the chance of live birth without adjuvant GH is assumed to be 11%, the chance of live birth with GH would be between 13% and 25%. Adjuvant GH results in a slight increase in pregnancy rates in poor responders (OR 1.85, 95% CI 1.35 to 2.53; I2 = 15%; 11 trials, 1033 participants; low‐certainty evidence). The results suggest, if the pregnancy rate without adjuvant GH is assumed to be 15%, with GH the pregnancy rate in poor responders would be between 19% and 31%. The evidence suggests that GH results in little to no difference in number of women with at least one oocyte retrieved (OR 5.67, 95% CI 1.54 to 20.83; I2 = 0%; 2 trials, 148 participants; low‐certainty evidence). If the chance of retrieving at least one oocyte in poor responders was 81%, with GH the chance is between 87% and 99%. There is a slight increase in mean number of oocytes retrieved with the use of GH for poor responders (MD 1.40, 95% CI 1.16 to 1.64; I2 = 87%; 12 trials, 1153 participants; low‐certainty evidence). The evidence is very uncertain about the effect of GH on embryo transfer achieved (OR 2.32, 95% CI 1.08 to 4.96; I2 = 25%; 4 trials, 214 participants; very low‐certainty evidence). If the chance of achieving embryo transfer is assumed to be 77%, the chance with GH will be 78% to 94%. Use of GH results in reduction of mean units of gonadotropins used for stimulation in poor responders (MD ‐1088.19, 95% CI ‐1203.20 to ‐973.18; I2 = 91%; 8 trials, 685 participants; low‐certainty evidence).
High heterogeneity in the analyses for mean number of oocytes retrieved and units of GH used suggests quite different effects according to differences including in trial protocols (populations, GH dose and schedule), so these results should be interpreted with caution.
We are uncertain of the effect of GH on adverse events in poor responders as six of the 14 included trials failed to report this outcome.
Authors' conclusions
The use of adjuvant GH in IVF treatment protocols has uncertain effect on live birth rates and mean number of oocytes retrieved in normal responders. However, it slightly increases the number of oocytes retrieved and pregnancy rates in poor responders, while there is an uncertain effect on live birth rates in this group. The results however, need to be interpreted with caution, as the included trials were small and few in number, with significant bias and imprecision. Also, the dose and regimen of GH used in trials was variable. Therefore, further research is necessary to fully define the role of GH as adjuvant therapy in IVF.
Plain language summary
Growth hormone for in vitro fertilisation (IVF)
Review question
Cochrane researchers reviewed the evidence about giving growth hormone as an additional treatment to women undergoing IVF compared to not giving this treatment to such women.
Background
During an IVF cycle, women need to be given gonadotrophin therapy to stimulate ovaries to produce eggs. Theoretically, the use of growth hormone as an added treatment may enhance the response of gonadotrophin therapy. We assessed the benefits and risks of using growth hormone compared with no growth hormone treatment in women undergoing IVF. 'Poor responders' in IVF treatment are usually older women with low ovarian reserve or women who had previous IVF treatment with less than five eggs collected despite a maximum dose of stimulation medication. Younger women with good ovarian reserve and good ovarian response (> 5 eggs collected) after ovarian stimulation are considered normal responders.
Study characteristics
We found 16 randomised controlled trials with 1352 women. This type of trial randomly assigns people into two groups. In this case, one group received IVF plus growth hormone and the other group received IVF only. The evidence is current to 11 November 2020.
Key results
In normal responders, with adjuvant GH use, the effect on live birth rate is very uncertain; if the chance of live birth without growth hormone is assumed to be 15%, the chance of live birth with growth hormone would be between 6% and 43%. There was not enough evidence to reach a conclusion regarding clinical pregnancy rates, number of women with at least one egg retrieved, embryo transfer achieved, and number of eggs retrieved in normal responders. The evidence is also very uncertain about the effect of growth hormone on mean units of gonadotropin used in normal responders.
The evidence is very uncertain about the effect of growth hormone on live birth rate for poor responders, based on eight trials. If the chance of live birth without growth hormone is assumed to be 11%, the chance of live birth with growth hormone would be between 13% and 25%. Growth hormone results in a slight increase in pregnancy rates in poor responders, based on 11 trials with low‐certainty evidence. The results suggest, if the pregnancy rate without growth hormone is assumed to be 15%, with growth hormone use, the pregnancy rate in poor responders would be between 19% and 31%. The evidence suggests that growth hormone results in little to no difference in the number of women with at least one oocyte retrieved, based on two trials with low‐certainty evidence. If the chance of retrieving at least 1 egg in poor responders was 81%, with growth hormone the chance is between 87% and 99%. There is a slight increase in the mean number of oocytes retrieved with the use of growth hormone for poor responders, based on 12 trials with low‐certainty evidence. The evidence is very uncertain about the effect of growth hormone on embryo transfer achieved, based on four trials. If the chance of achieving embryo transfer is assumed to be 77%, the chance with use of growth hormone will be between 78% and 94%. Use of growth hormone results in reduction of mean units of gonadotropins used for stimulation in poor responders, based on eight trials with low‐certainty evidence.
High heterogeneity in the analyses for the mean number of oocytes retrieved and the mean units of GH used suggests quite different effects according to differences including in trial protocols (populations, GH dose and schedule), so these results should be interpreted with caution.
We are uncertain of the effect of growth hormone on adverse events in normal or poor responders as 6 of the 16 included trials failed to report this outcome.
Quality of the evidence
The evidence was of low to very low certainty, with the main limitations being poor reporting of study methods, imprecise data and variability among the trials.
Summary of findings
Summary of findings 1. Adjuvant growth hormone compared to no adjuvant: routine use for in vitro fertilisation (IVF).
| Adjuvant growth hormone compared to no adjuvant: routine use for in vitro fertilisation (IVF) | ||||||
| Patient or population: women with infertility Setting: IVF Intervention: growth hormone Comparison: no adjuvant | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants(trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no adjuvant: routine use | Risk with growth hormone | |||||
| Live birth rate per woman randomised | Study population | OR 1.32 (0.40 to 4.43) | 80 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of growth hormone on live birth rate per woman randomised in normal responders | |
| 15 per 100 | 18 per 100 (6 to 43) | |||||
| Clinical pregnancy rate per woman randomised | Study population | OR 1.78 (0.49 to 6.50) | 42 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c,d | Only one study reported this outcome, hence conclusions cannot be drawn. | |
| 27 per 100 | 40 per 100 (16 to 71) | |||||
| Number of women with at least one oocyte retrieved per woman randomised | Study population | OR 2.86 (0.11 to 74.31) | 42 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c,d | Only one study reported this outcome, hence conclusions cannot be drawn. | |
| 95 per 100 | 98 per 100 (70 to 100) | |||||
| Mean number of oocytes retrieved | The mean number of oocytes retrieved ranged from 6 to 13 | MD 0.02 lower (0.79 lower to 0.74 higher) | ‐ | 80 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | The evidence is very uncertain about the effect of growth hormone on mean number of oocytes retrieved in normal responders. |
| Embryo transfer achieved per woman randomised | Study population | OR 7.36 (0.36 to 151.91) | 42 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c,d | Only one study reported this outcome, hence conclusions cannot be drawn. | |
| 86 per 100 | 98 per 100 (70 to 100) | |||||
| Mean units of gonadotrophin used | The mean units of gonadotrophin used ranged from 1327 to 2820 units | MD 13.57 units higher (112.88 lower to 140.01 higher) | ‐ | 80 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of growth hormone on mean units of gonadotrophin used in normal responders. |
| Adverse events |
Younis 1992 reported: ‐ ectopic pregnancy 0/20 in GH group and 2/22 in control group ‐ multiple pregnancy 5/20 in GH group and 2/22 in control group ‐ OHSS 0/20 in GH group and 0/22 in control group Tapanainen 1992 reported: ‐ multiple pregnancy 0/19 in GH group and 1/19 in control group. Other adverse events were not reported. |
‐ | 80 (2 RCTs) | ⊕⊕⊝⊝ Lowe,f | The evidence suggests that growth hormone does not increase or reduce adverse events in normal responders. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GH: growth hormone; IVF: in vitro fertilisation; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to randomisation bias and selective reporting. There are only 2 trials in this analysis. bDowngraded 2 levels due to imprecision, small study numbers and very wide confidence intervals. cDowngraded one level due to randomisation bias and selective reporting. dOnly one study reported this outcome, hence downgraded. eNot all outcomes reported uniformly. fSmall study numbers.
Summary of findings 2. Adjuvant growth hormone compared to no adjuvant: poor responders for in vitro fertilisation (IVF).
| Growth hormone compared to no adjuvant: poor responders for in vitro fertilisation (IVF) | ||||||
| Patient or population: women with infertility subclassified as poor responders Setting: IVF Intervention: growth hormone Comparison: no adjuvant | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no adjuvant: poor responders | Risk with growth hormone | |||||
| Live birth rate per woman randomised | Study population | OR 1.77 (1.17 to 2.70) | 737 (8 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The evidence is very uncertain about the effect of growth hormone on live birth rate per woman randomised in poor responders. | |
| 11 per 100 | 18 per 100 (13 to 25) | |||||
| Clinical pregnancy rate per woman randomised | Study population | OR 1.85 (1.35 to 2.53) | 1033 (11 RCTs) | ⊕⊕⊝⊝ Lowc,d,e | Growth hormone may result in a slight increase in clinical pregnancy rate per woman randomised in poor responders. | |
| 15 per 100 | 25 per 100 (19 to 31) | |||||
| Number of women with at least one oocyte retrieved per woman randomised | Study population | OR 5.67 (1.54 to 20.83) | 148 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d | The evidence suggests that growth hormone results in little to no difference in number of women with at least one oocyte retrieved per woman randomised. | |
| 81 per 100 | 96 per 100 (87 to 99) | |||||
| Mean number of oocytes retrieved | The mean number of oocytes retrieved ranged from 2 to 6 | MD 1.40 higher (1.16 higher to 1.64 higher) | ‐ | 1153 (12 RCTs) | ⊕⊕⊝⊝ Lowc,d,f | Growth hormone may result in a slight increase in mean number of oocytes retrieved in poor responders. |
| Embryo transfer achieved per woman randomised | Study population | OR 2.32 (1.08 to 4.96) | 214 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,c,f | The evidence is very uncertain about the effect of growth hormone on embryo transfer achieved per woman randomised in poor responders. | |
| 77 per 100 | 89 per 100 (78 to 94) | |||||
| Mean units gonadotropin used | The mean units gonadotropin used ranged from 2548 to 5590 units | MD 1088.19 units lower (1203.2 lower to 973.18 lower) | ‐ | 685 (8 RCTs) | ⊕⊕⊝⊝ Lowc,d | The evidence suggests growth hormone results in a slight reduction in mean units of gonadotropin used in poor responders. |
| Adverse events | Six trials did not report adverse events (Choe 2017; Dakhly 2018; Dor 1995; Hazout 2003; Tesarik 2005; Zhuang 1994). The other 8 trials reported adverse events. Owen 1991 reported ectopic pregnancies: 0/13 in GH group and 1/12 in control; the other trials in poor responder group reported no cases of ectopic pregnancy in either group. Multiple pregnancy was reported in Owen 1991: 2/13 in GH group and 0/12 in control group; Mohammad 2019 reported 1/78 in GH group and 1/78 in control group; Suikkari 1996 reported 1/10 in 4 IU GH group and 0/6 in control group; and other trials reported no cases of multiple pregnancies in either group. Norman 2019 reported congenital anomalies: 1/65 in GH group and 1/65 in control group; other trials reported no cases of congenital anomalies in either group. Bergh 1994 reported that 2/29 cases in GH group had oedema, but this outcome was not reported by any other study. Kucuk 2008, Safdarian 2019 and Lee 2019 reported that no adverse events were seen in either group. This information has been presented in tabular form (Table 3). | ‐ | 512 (7 RCTs) | ⊕⊝⊝⊝ Very lowe,g | Results reported varied across the studies, from an increase to a decrease of AEs with use of GH, but the evidence is very uncertain. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IVF: in vitro fertilisation; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to high risk of attrition bias. bDowngraded one level due to imprecision, small numbers and wide confidence intervals. cDowngraded one level due to publication bias as per funnel plot. dDowngraded one level due to randomisation bias and selective reporting. eThe dose, form, timing of administration was inconsistent across various trials. fDowngraded one level due to allocation bias and selective reporting. gAdverse events have not been reported by all trials, and the ones reporting have not reported adverse events long term. In particular effects on developing foetus have only been reported by one study.
1. Adverse events.
| Trial | Response type | AE reported | Ectopic | Multiple pregnancy | Congenital anomalies | OHSS | Oedema | |||||
| GH | Control | GH | Control | GH | Control | GH | Control | GH | Control | |||
| Bergh 1994 | Poor | Yes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2/29 | 0 |
| Choe 2017 | Poor | No | ||||||||||
| Dakhly 2018 | Poor | No | ||||||||||
| Dor 1995 | Poor | No | ||||||||||
| Hazout 2003 | Poor | No | ||||||||||
| Kucuk 2008 | Poor | Yes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lee 2019 | Poor | Yes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mohammad 2019 | Poor | Yes | 0 | 0 | 1/78 | 1/78 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norman 2019 | Poor | Yes | 0 | 0 | 0 | 0 | 1/65 | 1/65 | 0 | 0 | 0 | 0 |
| Owen 1991 | Poor | Yes | 0/13 | 1/12 | 2/13 | 0/12 | 0 | 0 | 0 | |||
| Safdarian 2019 | Poor | Yes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Suikkari 1996 | Poor | Yes | 0 | 0 | 1/10 (4IU group) | 0/6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tapanainen 1992 | Normal | Yes | 0 | 0/19 | 1/19 | 0 | 0 | 0 | ||||
| Tesarik 2005 | Poor | No | ||||||||||
| Younis 1992 | Normal | Yes | 0/20 | 2/22 | 5/20 | 2/22 | 0 | 0 | 0/20 | 0/22 | 0 | 0 |
| Zhuang 1994 | Poor | No | ||||||||||
- IU: international units
- AE: adverse effects
- OHSS: ovarian hyperstimulation syndrome
Background
Description of the condition
Infertility, usually defined as absence of conception after one year of regular intercourse, is a common problem affecting as many as one in six couples(NICE CG156). The main causes include sperm dysfunction, ovulation disorder and fallopian tube damage (Cahill 2002). Even after undergoing diagnostic tests, approximately 30% cases of infertility remain unexplained. One method of treating infertile couples is assisted conception via in vitro fertilisation (IVF). IVF involves using hormones to stimulate ovaries in order to increase follicular growth and thus develop more than one oocyte. Ovulation is then triggered with human chorionic gonadotropin and the oocytes are retrieved and fertilised with sperm in the laboratory setting outside the body (in vitro) (Bhandari 2018). The fertilised oocytes (embryos) are then transferred into the uterus, two to five days after egg retrieval. IVF protocols are constantly under review in an attempt to decrease hormone (gonadotrophin) requirement, improve follicular recruitment, and ultimately increase live birth rates (Bhandari 2018). A challenge for IVF practitioners is to optimise the outcome in 'poor responders'. As per the consensus from the European Society of Human Reproduction and Embryology (ESHRE), poor ovarian response has been defined if at least two of the following three features are present: i) increased maternal age (> 40 years); ii) any other risk factor for poor ovarian response (3 or fewer oocytes with ovulation induction); and iii) low scores on tests of ovarian reserve (i.e. antral follicle count < 5 to 7 follicles or anti‐Müllerian hormone < 0.5 ng/mL to 1.1 ng/mL (Ferraratti 2011). A newer classification system has been proposed by the POSEIDON group (Patient‐Oriented Strategies Encompassing IndividualizeD Oocyte Number; POSEIDON Group 2016). In this classification, four subgroups have been suggested based on quantitative and qualitative parameters, namely, age and the expected aneuploidy rate; ovarian biomarkers (i.e. antral follicle count and anti‐Müllerian hormone); and ovarian response ‐ provided a previous stimulation cycle was performed (POSEIDON criteria 2016). The POSEIDON group also introduced a new measure for successful assisted reproductive technology treatment, namely, the ability to retrieve the number of oocytes needed for the patient to obtain at least one euploid embryo for transfer. This feature represents a pragmatic endpoint for clinicians and enables the development of prediction models aiming to reduce the time‐to‐pregnancy. This however, can only be applied to prospective RCTs, and has not been used by any of the trials included in this meta‐analysis.
Description of the intervention
Over the last 25 years growth hormone (GH) has been used in IVF treatment (Jacobs 1995; Landolfi 1994). GH is a biological peptide hormone, synthesised, stored and secreted by somatotroph cells located in the anterior pituitary gland (Regan 2018). GH can be synthetically produced using recombinant DNA technology and is licensed to be used in the human population. There is currently no consensus as to the route, dose or timing of GH administration in IVF protocols (Ahmad 2009)
How the intervention might work
The administration of GH may potentiate the effect of exogenous gonadotrophins (Homburg 1988; Zhou 2013). GH is reported to modulate the action of follicular stimulating hormones on granulosa cells by up‐regulating the local synthesis of insulin‐like growth factor‐1 (IGF‐1) (Regan 2018). This interest has been stimulated by animal trials which suggest that GH may increase the intraovarian production of IGF‐1 (Hsu 1987; Yoshimura 1996). IGF‐1 displays GH dependence both in vivo and in vitro (Blumenfeld 1996). The interaction between GH and IGF‐1 is of significance since IGF‐1 has been shown to play an important part in ovarian function in both animal and human models (Adashi 1985; Erickson 1989; Zhou 2013). The addition of IGF‐1 to gonadotrophins in granulosa cell cultures increased gonadotrophin action on the ovary by several mechanisms including augmentation of aromatase activity, 17 beta‐oestradiol and progesterone production and luteinising hormone receptor formation (Erickson 1989; Mason 1990). In human ovarian cells, IGF‐1, in synergy with FSH (follicle stimulating hormone), stimulates protein synthesis and steroidogenesis. Following the presence of luteinising hormone receptors, IGF‐1 enhances luteinising hormone‐induced progesterone synthesis and stimulates proliferation of granulosa‐luteal cells. IGF‐1, in synergy with FSH, is very influential in stimulating aromatase activity in preovulatory follicles. Thus, IGF‐1 can be involved in both estradiol and progesterone synthesis (Zhou 2013). In other words, the existence of GH is essential in follicle development and ovarian steroidogenesis. IGF‐1 has also been found to stimulate follicular development, oestrogen production and oocyte maturation (Regan 2018; Yoshimura 1996). Trials have shown that maturation and evolution of oocytes have been impaired or severely reduced by inhibiting the GH receptor in animal models (Lucy 2011). GH is an essential requirement in treatment of infertility for women with GH deficiency, with many such women presenting with ovulation disorder (Park 2007).
Why it is important to do this review
The aim of this review is to establish the role of GH in IVF. Improving the outcomes of IVF with the use of GH adjuvant therapy is important particularly in those women who are considered poor responders. Since GH treatment is expensive, it is important to examine the available evidence as to the effectiveness and safety of its use as an adjunct to IVF, as its routine use could render IVF treatment unaffordable for many more patients, than is the case now (Kucuk 2008).
Objectives
To assess the effectiveness and safety of growth hormone as an adjunct to IVF compared to standard IVF for women with infertility
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) were eligible for inclusion.
Types of participants
Women with infertility undergoing ovarian stimulation for IVF
Types of interventions
We included all RCTs comparing the use of adjuvant GH in IVF cycles with standard IVF cycles, with or without placebo control.
Types of outcome measures
Primary outcomes
Live birth rate per woman randomised: number of women achieving a live birth divided by the number of women randomised
Secondary outcomes
Cinical pregnancy rate per woman randomised: number of women achieving a clinical pregnancy (established with confirmation of ongoing intrauterine pregnancy at 6 weeks on ultrasound), divided by the number of women randomised
Oocyte retrieval per woman randomised: number of women with at least one oocyte retrieved divided by the number of women randomised
Mean number of oocytes retrieved
Embryo transfer per woman randomised: number of women with at least one embryo transferred divided by the number of women randomised
Mean units of gonadotrophin used
Adverse events (ectopic pregnancy, multiple pregnancy, ovarian hyperstimulation syndrome (OHSS), congenital anomalies, oedema).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for relevant trials to 11 November 2020:
The Cochrane Gynaecology and Fertility (CGF) Group Specialised Register of Controlled Trials, ProCite platform (searched 11 November 2020) (Appendix 1);
CENTRAL, via the Cochrane Register of Studies Online (CRSO); Web platform (searched 11 November 2020) (Appendix 2);
MEDLINE, Ovid platform (searched from 1946 to 11 November 2020) (Appendix 3);
Embase, Ovid platform (searched from 1980 to 11 November 2020) (Appendix 4);
PsycINFO, Ovid platform (searched from 1806 to 11 November 2020) (Appendix 5);
CINAHL Plus, Ebsco platform (searched from 1961 to 20 January 2020 and any later CINAHL search output from the 11 November 2020 search is contained in the CENTRAL output) (Appendix 6).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0 chapter 6, 6.4.11)(Higgins 2021). We combined the Embase searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/what-we-do/methodology/search-filters/)
Other electronic sources of trials include:
LILACS (Latin American and Caribbean Health Science Information database (searched from 1982 to 11 November 2020), found in the Virtual Health Library Regional Portal (VHL) (pesquisa.bvsalud.org/portal);
Google Scholar (for recent trials not yet indexed in the major databases).
Searching other resources
We handsearched the reference lists of articles retrieved by the search and made personal contact with experts in the field and with the manufacturers of GH to obtain any additional relevant trials. In liaison with the Information Specialist, we handsearched any relevant journals and conference abstracts that were not covered in the CGF register.
We also rescreened the trials included in the previous version of this review.
Data collection and analysis
Selection of studies
Two review authors (AS and GM) scanned retrieved searches for relevant titles and abstracts and retrieved the full text of all potentially eligible trials. The same review authors independently examined the full text articles for compliance with the inclusion criteria and elected trials eligible for inclusion in the review. We corresponded with study investigators to clarify study eligibility (for example, with respect to participant eligibility criteria and allocation method). Disagreements as to study eligibility were resolved by discussion with a third review author (LM). We used a PRISMA flow chart to explain this process (PRISMA 2021)
Data extraction and management
Two review authors (GM and AS) independently extracted data from eligible trials using a data extraction form designed and pilot tested by review authors. Any disagreements were resolved by a third review author (LM). Where trials have multiple publications, we used the main trial report as the reference and supplemented this with additional details from secondary papers. Review authors corresponded with study investigators in order to resolve data queries.
Assessment of risk of bias in included studies
Two review authors (GM and AS) assessed the included trials for risk of bias using the Cochrane RoB 1 tool (Higgins 2011); any disagreements were resolved by discussion with a third review author (LM). We assessed: sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias (Higgins 2011). The conclusions are presented in the risk of bias table and incorporated in the interpretation of review findings by means of sensitivity analyses (see below). Where identified trials failed to report the primary outcomes of live birth, but did report secondary outcomes such as clinical pregnancy, we undertook informal assessment as to whether those reporting the primary outcomes have typical values of the secondary outcomes.
Measures of treatment effect
For dichotomous data (e.g. live birth rates), we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs) or (where events are very rare) Peto ORs*. For continuous data (e.g. weight gain), if all trials report exactly the same outcomes, we calculated the mean difference (MD) between treatment groups. If similar outcomes are reported on different scales (e.g. change in weight) we calculated the standardised mean difference (SMD). We reversed the direction of effect of individual trials, if required, to ensure consistency across trials. We treated ordinal data (e.g. quality of life scores) as continuous data. We presented 95% confidence intervals (CIs) for all outcomes. Where data to calculate ORs or MDs were not available, we utilised the most detailed numerical data available that facilitated similar analyses of included trials (e.g. test statistics, P values). We assessed whether the estimates calculated in the review for individual trials are compatible in each case with the estimates reported in the study publications. Three trails reported data as median and range (Bergh 1994; Dor 1995; Owen 1991). We converted the data to mean and standard deviation (SD) using Hozo's method (Hozo 2005).
Unit of analysis issues
The primary analysis was per woman randomised. Multiple live births (e.g. twins or triplets) were to be counted as one live birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and made attempts to obtain missing data from the original investigators. If trials reported sufficient detail to calculate MDs but no information on associated SD, we planned to assume that the outcome had a SD equal to the highest SD from other trials within the same analysis. We assumed live births and pregnancies not to have occurred in participants with unreported outcomes. Where these were unobtainable, imputation of individual values was undertaken for the primary outcomes only. For other outcomes, we only analysed the available data. Any imputation undertaken was subjected to sensitivity analysis (see below).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included trials were sufficiently similar for meta‐analysis to provide a meaningful summary. We assessed statistical heterogeneity using the I2 statistic. An I2 measurement greater than 50% indicated substantial heterogeneity (Higgins 2011), and if present, we addressed this through sensitivity analysis, subgroup analysis, or both.
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible trials, by being alert for duplication of data, and by constructing a funnel plot if there were sufficient trials (10 or more) in one analysis.
Data synthesis
We combined the data from primary trials using fixed‐effect models in the following comparisons.
GH versus no adjuvant treatment: routine use of adjuvant GH in IVF protocols
GH versus no adjuvant treatment: use of GH in poor responders
GH versus no adjuvant treatment: subgroup analysis based on age
Subgroup analysis and investigation of heterogeneity
We subgrouped the poor responders as follows.
Women identified as poor responders by definition or based on test results showing low ovarian reserve who did not have IVF cycle before
Women identified as poor responders based on previous poor response in IVF stimulation cycle
If we identified substantial heterogeneity, we planned to explore methodological and clinical differences between the trials
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions are robust to arbitrary decisions made regarding eligibility and analysis. These analyses considered whether conclusions would have differed under the following circumstances.
If eligibility was restricted to trials without high risk of bias. Risk of bias assessment conducted as per Cochrane RoB 1 tool (Higgins 2011). Serial exclusion of each study from the meta‐analysis did not produce significant changes in any outcome.
If trials with outlying results had been excluded. Serial exclusion of each study from the meta‐analysis did not produce significant changes in any outcome.
If a random‐effects model had been adopted. Changing from fixed‐effect to random‐effects model did not change the conclusions, but the results showed wider CIs.
If a sensitivity analysis was performed to detect whether the inclusion of RCTs with high numbers of participants affected the results. Serial exclusion of each study from the meta‐analysis did not produce significant changes in any outcome.
Summary of findings and assessment of the certainty of the evidence
We prepared a summary of findings table using GRADEpro GDT and Cochrane methods (GRADEpro GDT 2015; Higgins 2021; Schünemann 2013). We prepared two summary of findings tables for GH compared to no adjuvant in: i) normal responders; and ii) poor responders. Each table evaluated the overall certainty of the body of evidence for live birth rate, clinical pregnancy rate, number of women with at least one oocyte retrieved, mean number of oocytes retrieved, embryo transfer rate, mean units of gonadotrophin used, and adverse events for adjuvant GH treatment versus no adjuvant in normal responders. We assessed the certainty of the evidence using GRADE criteria (Schünemann 2013): risk of bias, consistency of effect, imprecision, indirectness and publication bias). Two review authors (AS and LM) working independently made judgements about evidence quality (high, moderate, low or very low), with disagreements resolved by discussion. We justified, documented, and incorporated judgements into reporting of results for each outcome.
We extracted the study data, formatting our comparisons in data tables and prepared the summary of findings tables before writing the results and conclusions of our review.
Results
Description of studies
We only included randomised controlled trials (RCTs) with growth hormone (GH) used as an adjuvant treatment for ovarian stimulation with the control group using placebo or no placebo.
Results of the search
We included 16 RCTs in the meta‐analysis. The search retrieved 436 articles. After removing duplicates, 304 abstracts were screened. Fifty five studies were potentially eligible and were retrieved in full text. Sixteen studies met our inclusion criteria. We excluded 35 studies, 4 are awaiting classification and 7 are ongoing. See study tables: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies; Table 4. For details of the screening and selection process see Figure 1.
2. Trial characteristics.
| Trial | Age | Poor response type | GH | Dose | Schedule | Placebo | ET day | GH | Total |
| BMI | Protocol | Control | |||||||
| Bergh 1994 | < 38 | Response x 2 | HGH Agonist |
0.1 IU/kg | Pre‐ treatment for 7 days followed by with/without stimulation | Saline | Day 2/3 | 10 x 4 groups | 40 |
| Choe 2017 | ≥ 40 | Bologna | Sustained‐release GH Antagonist |
20 mg | 3 doses ‐ previous cycle mid luteal, late luteal and D2 | No | Not stated | 62 | 127 |
| < 30 | 65 | ||||||||
| Dakhly 2018 | ≥ 40 | Bologna | HGH Agonist |
7.5 IU | Daily from D21 of previous cycle | No | Day 3 up to 3 | 120 | 240 |
| 120 | |||||||||
| Dor 1995 | Response | HGH Flare |
18 IU | Days 2, 4, 6, 8 | Mannitol | Day 2 | 7 | 14 | |
| 7 | |||||||||
| Hazout 2003 | < 39 | Oocyte dysmorphia > 50% | HGH Agonist |
3 groups 4 IU, 8 IU, placebo | Daily from day 1 of stimulation until trigger | Yes ‐ not stated what | Day 3 | 4 IU: 12 8 IU: 11 |
35 |
| 12 | |||||||||
| Kucuk 2008 | Response | HGH Agonist |
12 IU | Daily from D21 of previous cycle | No | Day 3 | 31 | 61 | |
| 30 | |||||||||
| Lee 2019 | ≥ 40 | Bologna | HGH Agonist ultra‐long protocol |
4 IU, 4 IU, 2 IU ‐ total 10 IU | 3 consecutive days with stimulation | No | Day 3 | 94 | 184 |
| 90 | |||||||||
| Mohammad 2019 | 25‐38 | Response | HGH Antagonist |
4 IU | D2 until 1 day before egg collection | Saline | Day 2/3 | 78 | 156 |
| 78 | |||||||||
| Norman 2019 | < 41 | Response | HGH Antagonist |
12 IU | D1 until 1 day before egg collection | Metacresol in water | Not stated | 65 | 130 |
| < 33 | 65 | ||||||||
| Owen 1991 | < 38 | Response | HGH Agonist |
24 IU | Alternate day from D1 stimulation (maximum 2 weeks) | Yes ‐ not stated what | Day 2 (1‐4) | 13 | 25 |
| 12 | |||||||||
| Safdarian 2019 | Bologna | HGH Antagonist |
3 groups: 7.5 IU from day 8 0.3 IU from day 3 previous cycle saline from day 8 | 3 groups:
7.5 IU from day 8 X 5 d 0.3 IU from day 3 previous cycle x 20 d saline from day 8 X 5 d |
Saline | Day 5 up to 3 | Group 1: 34 Group 2: 32 |
105 | |
| Group 3: 26 | |||||||||
| Suikkari 1996 | < 40 | Response x 2 | HGH Flare |
3 groups 4 IU, 12 IU, placebo | Daily from day 3 | Saline | Day 2 | 4 IU: 10 12 IU: 6 |
22 |
| < 27 | 6 | ||||||||
| Tapanainen 1992 | 27‐37 | N | HGH Flare |
24 IU | Alternate day from D4 stimulation until last HMG | Saline | Not stated | 19 | 38 |
| 19 | |||||||||
| Tesarik 2005 | > 40 | As per study | HGH Agonist |
8 IU | Daily from D7 of stimulation until 1 day before egg collection | Yes ‐ not stated what | Day 3 (1‐5) | 50 | 100 |
| 50 | |||||||||
| Younis 1992 | < 38 | N | HGH Agonist |
12 IU | Days 1, 3, 5, 7 | Mannitol | Not stated | 20 | 42 |
| 22 | |||||||||
| Zhuang 1994 | Response | HGH Agonist |
12 IU | Alternate day | No | Not stated | 12 | 27 | |
| 15 |
- HMG: human menopausal gonadotropin
- IU: international units
- HGH: human recombinant growth hormone
- GH: growth hormone
1.
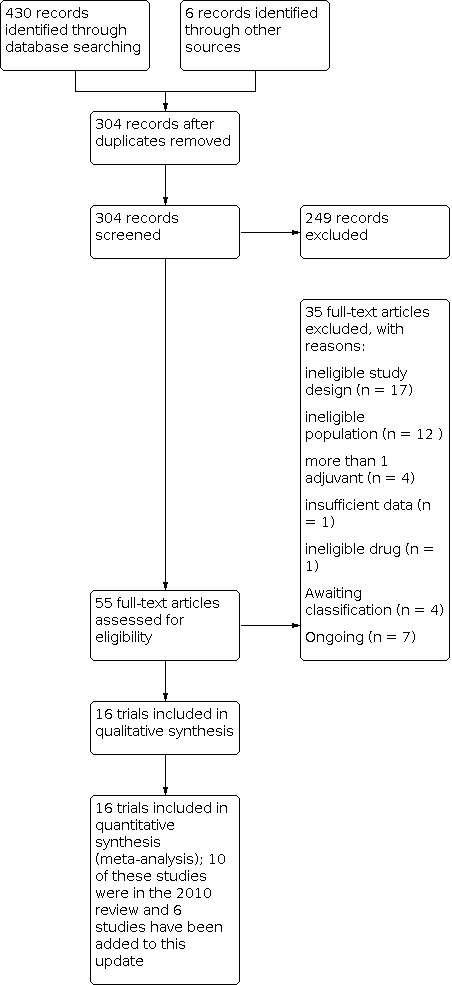
Included studies
Design
We included 16 parallel‐group RCTs in this review (Bergh 1994; Choe 2017; Dakhly 2018; Dor 1995; Hazout 2003; Kucuk 2008; Lee 2019; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tapanainen 1992; Tesarik 2005; Younis 1992; Zhuang 1994). Further descriptive details about the included trials are provided in Characteristics of included studies. All included trials were published reports either as full papers or as conference abstracts (Hazout 2003).
There are 4 studies on which we are awaiting further information (Bassiouny 2016; Bayoumi 2015; Eftekhar 2012; Gong 2020). Further details for these are available under Characteristics of studies awaiting classification.
In addition, there are 7 ongoing trials for which the results are awaited: (ChiCTR1800016106; CTRI/2019/03/018047; NCT01715324; NCT02179255; NCT03027843; NCT03373149; NCT03759301). The details for these trials are provided in Characteristics of ongoing studies.
Participants
We included 16 trials with a total of 1352 subfertile couples in the review. The number of couples included in each trial ranged from 14 in Dor 1995 to 240 in Dakhly 2018.
Two trials included women who were not identified as poor responders (Tapanainen 1992; Younis 1992). The other 14 trials were conducted in poor responders. The subgroups were as follows.
-
Poor responder by definition/test results showing low ovarian reserve
Over 40 years old (Tesarik 2005)
-
ESHRE criteria
age > 40 years
previous treatment that resulted with < 3 oocytes
anti‐Müllerian hormone level < 0.5 ng/mL to 1.1 ng/mL
antral follicle count < 5 to 7 follicles (Lee 2019)
-
Bologna criteria
age ≥ 40 years or other factor for poor ovarian response
previous poor ovarian response (≤ 3 oocytes on ovulation induction)
low ovarian reserve test (anti‐Müllerian hormone level < 0.5 ng/mL to 1.1 ng/mL
antral follicle count < 5 to 7 follicles (Choe 2017; Dakhly 2018; Safdarian 2019)
-
Poor responder based on previous low response to ovarian stimulation
< 3 oocytes retrieved in previous cycle or at least 48 ampoules of human menopausal gonadotrophin (hMG) used (Suikkari 1996)
< 5 oocytes retrieved and > 250 IU follicle stimulating hormone (FSH) in previous cycle (Norman 2019)
≥ 2 previous cycles with < 5 oocytes retrieved (Bergh 1994)
< 6 oocytes retrieved and < 3 embryos developed in previous cycle (Owen 1991)
Previous poor response (not further defined) (Zhuang 1994)
History of oocyte dysmorphia (Hazout 2003)
Previous low response to high‐dose gonadotrophin treatment (Kucuk 2008)
Oestradiol < 500 pg/mL, < 3 oocytes retrieved in two previous IVF cycles (Dor 1995)
IVF in previous poor responders with ≥ 2 failed cycles with < 5 oocytes (Mohammad 2019)
Exclusion criteria were not stated in Hazout 2003, Lee 2019, Owen 1991, Safdarian 2019, Suikkari 1996 and Tapanainen 1992. The remaining trials based their exclusion criteria on serum FSH concentrations (Kucuk 2008; Tesarik 2005), obesity (Bergh 1994), ovarian pathology (Bergh 1994), endometriosis (Bergh 1994), severe intercurrent illness (Bergh 1994), and unsatisfactory sperm quality (Tesarik 2005). Women with high FSH levels (> 20 IU/L), a history of infertility due to other causes such as azoospermia and diabetes (type 1 or 2) were excluded in Safdarian 2019.
Interventions
There was no consistency as to the dose or timing of GH administration (see Characteristics of included studies tables). The dose of GH ranged from 4 IU in Mohammad 2019 and Suikkari 1996 to 24 IU in Owen 1991 and Tapanainen 1992. Both Hazout 2003 and Suikkari 1996 conducted a multiple‐arm study comparing two different doses of GH to a placebo arm, and their data have been reported separately in the analyses, with explanatory footnotes. The timing of GH administration varied between trials from daily administration prestimulation to alternate doses after the start of stimulation. Sustained‐release GH preparation was used by Choe 2017; all other trials used recombinant GH.
Placebo was not used in five trials (Choe 2017; Dakhly 2018; Kucuk 2008; Lee 2019; Zhuang 1994). The remaining 11 trials used placebo in the control group: five used saline (Bergh 1994; Mohammad 2019; Safdarian 2019; Suikkari 1996; Tapanainen 1992), two used mannitol (Dor 1995; Younis 1992), one used metacresol in water (Norman 2019), and in three trials the nature of placebo used was not stated (Hazout 2003; Owen 1991; Tesarik 2005).
Outcomes
Primary outcome measure
Live birth rates were reported in 10 of the included trials (Dakhly 2018; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tapanainen 1992; Tesarik 2005; Younis 1992; Zhuang 1994).
Secondary outcome measures
Pregnancy rates were reported in 13 of the included trials (Bergh 1994; Choe 2017; Dakhly 2018; Hazout 2003; Kucuk 2008; Lee 2019; Mohammad 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tesarik 2005; Younis 1992; Zhuang 1994). The number of oocytes retrieved per women was reported in 15 trials, except Hazout 2003, where SD was not mentioned, hence data could not be used. Adverse events were reported in 10 trials (Bergh 1994; Kucuk 2008; Lee 2019; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tapanainen 1992; Younis 1992).
Excluded studies
Thirty five studies were excluded from the review, for the following reasons:
17/35 were not RCTs
12/35 were not done in women undergoing IVF (ineligible population)
4/35 had used more than 1 adjuvant treatment
1/35 had insufficient data
1/35 had used ineligible drug
Risk of bias in included studies
Please refer to Characteristics of included studies table, Figure 2 and Figure 3.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Random sequence generation
The method of randomisation was clearly stated in seven trials and we assessed them at low risk of selection bias (Bergh 1994; Dakhly 2018; Kucuk 2008; Norman 2019; Tesarik 2005; Mohammad 2019; Safdarian 2019). The method of randomisation was unclear in the remaining trials.
Allocation concealment
Five studies were rated as at low risk of selection bias related to allocation concealment as they used sequentially labelled, sealed, opaque envelopes (Mohammad 2019; Tesarik 2005; Younis 1992; Norman 2019; Dakhly 2018). No allocation concealment was described in 3 studies, which we rated as high risk of bias for this domain (Dor 1995; Lee 2019; Safdarian 2019). The other eight studies failed to describe methods of allocation concealment or opaque envelopes were not used and we rated these as at unclear risk of bias for this domain.
Blinding
We did not consider that blinding of participants and personnel was likely to influence findings for the primary review outcome (live birth rate). Eleven trials were rated as low risk for selection bias related to blinding; among which two trials reported single‐blinding (Safdarian 2019; Zhuang 1994), seven trials were double‐blinded (Bergh 1994; Hazout 2003; Norman 2019; Owen 1991; Suikkari 1996; Tapanainen 1992; Tesarik 2005), and two trials reported triple‐blinding (Kucuk 2008; Younis 1992). The remaining 5 trials were not blinded.
Incomplete outcome data
Two women were lost to follow‐up in the Bergh 1994 study and four women were lost to follow‐up in the Suikkari 1996 study, and both these were rated as high risk of attrition bias. Also, 3 studies with > 10% cycle cancellation rate were rated as high risk of attrition bias (Dakhly 2018; Lee 2019; Safdarian 2019). The remaining trials reported no losses and had a cycle cancellation rate < 10%.
Selective reporting
Six trials were rated as high risk of selective reporting as these did not report adverse events (Choe 2017; Dakhly 2018; Dor 1995; Hazout 2003; Tesarik 2005, Zhuang 1994). A registered protocol was available for four trials (Dakhly 2018; Norman 2019, Safdarian 2019, Mohammad 2019), 3 of these were rated as low risk of selection bias; adverse effects were not reported by Dakhly 2018. We rated the remaining 7 studies as at unclear risk of bias although they reported our review’s primary outcomes; we could not obtain a study protocol and the study was not prospectively registered so there was no information we could use to verify study details.
Other potential sources of bias
Five trials have been rated as high risk: three trials received a free supply of GH from the manufacture (Owen 1991; Tapanainen 1992; Younis 1992), one trial received a grant (Choe 2017) and one trial had very low numbers (Dor 1995). Five trials have been rated as unclear risk of this bias: placebo was not used in four (Dakhly 2018; Kucuk 2008; Lee 2019; Zhuang 1994) and one was a conference abstract (Hazout 2003). Placebo was not used in Choe 2017 as well but this has been rated as high risk. No other bias was noted in the remaining six trials.
Effects of interventions
1 Adjuvant growth hormone compared to no adjuvant or placebo: routine use for IVF
Primary outcomes
1.1 Live birth rate per woman randomised
Only two of the RCTs were conducted in women who were not identified as poor responders (Tapanainen 1992; Younis 1992). The evidence is very uncertain about the effect of GH on live birth rate per woman randomised for routine use in IVF (odds ratio (OR) 1.32, 95% confidence interval (CI) 0.40 to 4.43; I2 = 0%; 2 trials, 80 participants; very low‐certainty evidence) Analysis 1.1, Figure 4). If the chance of live birth without use of GH as adjuvant is assumed to be 15%, the chance of live birth with use of GH would be between 6% and 43%.
1.1. Analysis.

Comparison 1: Adjuvant GH compared to no adjuvant: routine use for IVF, Outcome 1: Live birth rate per woman randomised
4.

Forest plot of comparison: 1 Adjuvant GH compared to no adjuvant: routine use for IVF, outcome: 1.1 Live birth rate per woman randomised.
Secondary outcomes
1.2 Clinical pregnancy rate per woman randomised
Only one RCT was conducted in women who were not identified as poor responders (Younis 1992), hence we could not perform meta‐analysis (Analysis 1.2).
1.2. Analysis.

Comparison 1: Adjuvant GH compared to no adjuvant: routine use for IVF, Outcome 2: Clinical pregnancy rate per woman randomised
1.3 Number of women with at least one oocyte retrieved per woman randomised
One trial reported number of women with at least one oocyte retrieved (Younis 1992), hence we could not perform meta‐analysis (Analysis 1.3).
1.3. Analysis.

Comparison 1: Adjuvant GH compared to no adjuvant: routine use for IVF, Outcome 3: Number of women with at least one oocyte retrieved per woman randomised
1.4 Mean number of oocytes retrieved
Two trials reported the mean number of oocytes retrieved per woman randomised (Tapanainen 1992; Younis 1992). The evidence is very uncertain about the effect of GH on mean number of oocytes retrieved in normal responders (mean difference (MD) ‐0.02, 95% CI ‐0.79 to 0.74; I2 = 0%; 2 trials, 80 participants; very low‐certainty evidence) ( Analysis 1.4). The mean number of oocytes retrieved were 6 to 13.
1.4. Analysis.

Comparison 1: Adjuvant GH compared to no adjuvant: routine use for IVF, Outcome 4: Mean number of oocytes retrieved
1.5 Embryo transfer per woman randomised
One trial reported the number of embryos transferred per woman randomised (Younis 1992), hence we could not perform meta‐analysis (Analysis 1.5).
1.5. Analysis.

Comparison 1: Adjuvant GH compared to no adjuvant: routine use for IVF, Outcome 5: Embryo transfer achieved per woman randomised
1.6 Mean units of gonadotrophin used
Two trials reported the mean number of ampoules of gonadotrophin used per woman randomised (Tapanainen 1992; Younis 1992). We converted the ampoules into units for standardisation throughout the review. We are uncertain if the mean number of ampoules of gonadotropin used changed with GH in IVF protocols when compared to standard IVF protocols (MD 13.57, 95% CI ‐112.88 to 140.01; I2 = 0%; 2 trials, 80 participants; very low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Adjuvant GH compared to no adjuvant: routine use for IVF, Outcome 6: Mean units of gonadotrophin used
1.7 Adverse events
Low‐certainty evidence suggests that GH does not increase or reduce adverse events in normal responders. Adverse events were reported by Younis 1992 and Tapanainen 1992. Younis 1992 reported ectopic pregnancy 0/20 in GH group and 2/22 in control group, multiple pregnancy 5/20 in GH group and 2/22 in control group, ovarian hyperstimulation syndrome (OHSS) 0/20 in GH group and 0/22 in control group. Tapanainen 1992 reported multiple pregnancy 0/19 in GH group and 1/19 in control group. Other adverse events were not reported (Table 3).
2 Adjuvant GH compared to no adjuvant or placebo: poor responders for IVF
Primary outcome
2.1 Live birth rate per woman randomised
Eight trials reported the live birth rate per woman randomised (Dakhly 2018; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tesarik 2005; Zhuang 1994). The evidence is very uncertain about the effect of GH on live birth rate per woman randomised for poor responders (OR 1.77, 95% CI 1.17 to 2.70; I2 = 0%; 8 trials, 737 participants; very low‐certainty evidence; Analysis 2.1, Figure 5). If the chance of live birth without use of GH as adjuvant is assumed to be 11%, the chance of live birth with use of GH would be between 13% and 25%.
2.1. Analysis.

Comparison 2: Adjuvant GH compared to no adjuvant: poor responders for IVF, Outcome 1: Live birth rate per woman randomised
5.

Forest plot of comparison: 2 Growth hormone versus no adjuvant: poor responders, outcome: 2.1 Live birth rate per woman randomised.
Subgroup analysis
Live birth rates in the poor responder by definition subgroup (Dakhly 2018; Safdarian 2019; Tesarik 2005), evidence is uncertain if adjuvant GH increases live birth rates (OR 1.85, 95% CI 1.02 to 3.38; I2 = 46%; 3 trials, 400 participants); and in the poor responder based on previous response subgroup (Mohammad 2019; Norman 2019; Owen 1991; Suikkari 1996; Zhuang 1994), evidence is uncertain if adjuvant GH increases live birth rates (OR 1.70, 95% CI 0.95 to 3.06; I2 = 0%; 5 trials, 337 participants).
We did not find evidence that the treatment effect differed between the groups of studies (test for subgroup differences: Chi² = 0.04, df = 1 (P = 0.84), I² = 0%).
Secondary outcomes
2.2 Clinical pregnancy rate per woman randomised
Eleven trials reported the pregnancy birth rate per woman randomised (Bergh 1994; Choe 2017; Dakhly 2018; Hazout 2003; Kucuk 2008; Lee 2019; Mohammad 2019; Owen 1991; Safdarian 2019; Tesarik 2005; Zhuang 1994). GH used as adjuvant results in a slight increase in pregnancy rates per woman randomised in poor responders (OR 1.85, 95% CI 1.35 to 2.53; I2 = 15%; 11 trials, 1033 participants; low‐certainty evidence; Analysis 2.2; Figure 6). The results suggest, if the pregnancy rate without adjuvant GH is assumed to be 15%, with GH use, the pregnancy rate in poor responders would be between 19% and 31%.
2.2. Analysis.

Comparison 2: Adjuvant GH compared to no adjuvant: poor responders for IVF, Outcome 2: Clinical pregnancy rate per woman randomised
6.

Forest plot of comparison: 2 Growth hormone versus no adjuvant: poor responders, outcome: 2.2 Pregnancy rate per woman randomised.
Subgroup analysis
In both subgroups, pregnancy rates probably increased in women who received adjuvant GH. Poor responder by definition group: OR 1.70, 95% CI 1.16 to 2.50; I2 = 59%; 5 trials, 711 participants; and the poor responder based on previous response group: OR 2.19, 95% CI 1.26 to 3.81; I2 = 0%; 6 trials, 322 participants; Analysis 2.2).
We did not find evidence that the treatment effect differed between the groups of studies (test for subgroup differences: Chi² = 0.53, df = 1 (P = 0.46), I² = 0%).
2.3 Number of women with at least one oocyte retrieved per woman randomised
Two trials reported number of women with at least 1 oocyte retrieved per woman randomised (Bergh 1994; Norman 2019). The evidence suggests that GH results in little to no difference in the number of women with at least one oocyte retrieved per woman randomised (OR 5.67, 95% CI 1.54 to 20.83; I2 = 0; 2 trials, 148 participants; low‐certainty evidence; Analysis 2.3). If the chance of retrieving at least one oocyte in poor responders was 81%, with GH the chance is between 87% and 99%.
2.3. Analysis.

Comparison 2: Adjuvant GH compared to no adjuvant: poor responders for IVF, Outcome 3: Number of women with at least one oocyte retrieved per woman randomised
2.4 Mean number of oocytes retrieved
Twelve trials reported number of oocytes retrieved (Bergh 1994; Choe 2017; Dakhly 2018; Dor 1995; Kucuk 2008; Lee 2019; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tesarik 2005). Hazout 2003 reported the mean numbers but standard deviation (SD) was not mentioned, hence data could not be used in the analysis. There is a slight increase in the mean number of oocytes retrieved with the use of GH for poor responders (MD 1.40, 95% CI 1.16 to 1.64; I2 = 87%; 12 trials, 1153 participants; low‐certainty evidence; Analysis 2.4). The mean number of oocytes retrieved were 2 to 6. However heterogeneity of over 85% suggests quite different effects according to differences including in trial protocols (populations, GH dose and schedule), so the result should be interpreted with caution.
2.4. Analysis.

Comparison 2: Adjuvant GH compared to no adjuvant: poor responders for IVF, Outcome 4: Mean number of oocytes retrieved
We did not find evidence that the treatment effect differed between the groups of studies (test for subgroup differences: Chi² = 0.08, df = 1 (P = 0.77), I² = 0%).
2.5 Embryo transfer achieved per woman randomised
Four trials reported embryo transfer per woman randomised (Bergh 1994; Kucuk 2008; Norman 2019; Suikkari 1996). The evidence is very uncertain about the effect of GH on embryo transfer achieved per woman randomised (OR 2.32, 95% CI 1.08 to 4.96; I2 = 25%; 4 trials, 214 participants; very low‐certainty evidence; Analysis 2.5). If the chance of achieving embryo transfer per woman randomised is assumed to be 77%, the chance with use of GH will be 78% to 94%.
2.5. Analysis.

Comparison 2: Adjuvant GH compared to no adjuvant: poor responders for IVF, Outcome 5: Embryo transfer acheived per woman randomised
2.6 Mean units of gonadotrophin used
Eight trials reported mean units of gonadotropin used (Bergh 1994; Choe 2017; Dakhly 2018; Dor 1995; Kucuk 2008; Norman 2019; Owen 1991; Safdarian 2019). Use of GH results in reduction of mean units of gonadotropins used for stimulation in poor responders (MD ‐1088.19, 95% CI ‐1203.20 to ‐973.18; I2 = 91%; 8 trials, 685 participants; low‐certainty evidence; Analysis 2.6). However amongst studies including poor responders based on a definition there was considerable heterogeneity, such that the pooled estimate may not represent a useful summary.
2.6. Analysis.

Comparison 2: Adjuvant GH compared to no adjuvant: poor responders for IVF, Outcome 6: Mean units gonadotropin used
The test for subgroup differences showed that treatment effect differed between the groups of trials (Chi² = 14.41, df = 1 (P = 0.0001), I² = 93.1%).
2.7 Adverse events
Six trials did not report adverse events (Choe 2017; Dakhly 2018; Dor 1995; Hazout 2003; Tesarik 2005, Zhuang 1994). Owen 1991 reported ectopic pregnancies: 0/13 in GH group and 1/12 in control; the other trials in poor responder group reported no cases of ectopic pregnancy in either group. Multiple pregnancy was reported in Owen 1991: 2/13 in GH group and 0/12 in control group; Mohammad 2019 reported 1/78 in GH group and 1/78 in control group; Suikkari 1996 reported 1/10 in 4 IU GH group and 0/6 in control group; and other trials reported no cases of multiple pregnancies in either group. Norman 2019 reported congenital anomalies: 1/65 in GH group and 1/65 in control group; other trials reported no cases of congenital anomalies in either group. Bergh 1994 reported that 2/29 cases in GH group had oedema, but this outcome was not reported by any other study. Kucuk 2008, Safdarian 2019 and Lee 2019 reported that no adverse events were seen in either group. This information has been presented in tabular form in Table 3.
2.8 Other analyses
We conducted sensitivity analyses for the primary outcome, live birth rate, to determine whether the conclusions are robust to arbitrary decisions made regarding eligibility and analysis. These analyses considered whether conclusions would have differed under the following circumstances.
If eligibility was restricted to trials without high risk of bias. Risk of bias assessment conducted as per Cochrane RoB 1 tool (Higgins 2011). Serial exclusion of each study from the meta‐analysis did not produce significant changes in this outcome.
If trials with outlying results had been excluded. Serial exclusion of each study from the meta‐analysis did not produce significant changes in this outcome.
If a random‐effects model had been adopted. Changing from fixed‐effect to random‐effects model did not change the conclusions, but the results showed wider CIs.
If a sensitivity analysis was performed to detect whether the inclusion of RCTs with high numbers of participants affected the results. Serial exclusion of each study from the meta‐analysis did not produce significant changes in this outcome.
3 Adjuvant GH compared to no adjuvant or placebo: subgroup analysis based on age
3.1 Live birth rate per woman randomised
Ten trials reported the live birth rate per woman randomised (Dakhly 2018; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tesarik 2005; Zhuang 1994; Tapanainen 1992; Younis 1992).
Subgroup analysis based on age showed that it is uncertain if adjuvant GH increases live birth rates both in < 40 years group (OR 1.45, 95% CI 0.82 to 2.56; I2 = 0%; 6 trials, 390 participants) and > 40 years group (OR 1.69, 95% CI 0.90 to 3.20; I2 = 72%; 2 trials, 340 participants; Analysis 3.1, Figure 7). High heterogeneity was noted in the subgroup > 40 years, suggesting different effects according to differences including in trial protocols (populations, GH dose and schedule).
3.1. Analysis.

Comparison 3: Adjuvant GH compared to no adjuvant: subgroup analysis based on age, Outcome 1: Live birth rate per woman randomised
7.

We did not find evidence that the treatment effect differed between the groups of studies (test for subgroup differences: Chi² = 1.71, df = 2 (P = 0.43), I² = 0%).
Secondary outcomes
3.2 Clinical pregnancy rate per woman randomised
Twelve trials reported the pregnancy birth rate per woman randomised (Bergh 1994; Choe 2017; Dakhly 2018; Hazout 2003; Kucuk 2008; Lee 2019; Mohammad 2019; Owen 1991; Safdarian 2019; Tesarik 2005; Zhuang 1994; Younis 1992).
On conducting further subgroup analysis based on age, pregnancy rates were slightly improved in < 40 years of age (OR 1.98, 95% CI 1.12 to 3.50; I2 = 0%; 5 trials, 288 participants) be it in poor responder or normal responder, but this was not seen in the age group > 40 years (OR 1.64, 95% CI 1.11 to 2.42; I2 = 67%; 4 trials, 651 participants; Analysis 3.2, Figure 8). There is high heterogeneity noted in clinical pregnancy rates in the > 40 years subgroup, suggesting different effects according to differences including in trial protocols, study population and also difference in GH dose and schedule.
3.2. Analysis.

Comparison 3: Adjuvant GH compared to no adjuvant: subgroup analysis based on age, Outcome 2: Clinical pregnancy rate per woman randomised
8.

We did not find evidence that the treatment effect differed between the groups of studies (test for subgroup differences: Chi² = 0.69, df = 2 (P = 0.71), I² = 0%).
3.3 Number of women with at least one oocyte retrieved per woman randomised
Three trials reported number of women with at least 1 oocyte retrieved per woman randomised (Bergh 1994; Norman 2019; Younis 1992).
On conducting further subgroup analysis based on age, the number of women with at least one oocyte retrieved was higher in those < 40 years of age (OR 5.19, 95% CI 1.56 to 17.32; I2 = 0; 3 trials, 190 participants; (Analysis 3.3)
3.3. Analysis.

Comparison 3: Adjuvant GH compared to no adjuvant: subgroup analysis based on age, Outcome 3: No of women with at least one oocyte retrieved per woman randomised
3.4 Mean number of oocytes retrieved
Fifteen trials reported number of oocytes retrieved (Bergh 1994; Choe 2017; Dakhly 2018; Dor 1995; Kucuk 2008; Lee 2019; Mohammad 2019; Norman 2019; Owen 1991; Safdarian 2019; Suikkari 1996; Tesarik 2005; Tapanainen 1992; Younis 1992). Hazout 2003 reported the mean numbers but standard deviation (SD) was not mentioned, hence data could not be used in the analysis.
On further subgroup analysis based on age, there was uncertain effect on mean number of oocytes retrieved in women < 40 years (MD 0.73, 95% CI 0.34 to 1.13; I2 = 67%; 7 trials, 437 participants; Analysis 3.4), whereas trials with participants > 40 years showed a slight increase in oocytes retrieved (MD 1.35, 95% CI 1.03 to 1.68; I2 = 94%; 4 trials, 651 participants; Analysis 3.4), and similarly trials in which age criteria were not defined showed a slight increase in oocytes retrieved (MD 2.12, 95% CI 1.55 to 2.69; I2 = 83%; 4 trials, 172 participants) and the pooled result for all trials showed a slight increase in oocytes retrieved (MD 1.27, 95% CI 1.04 to 1.49; I2 = 86%; 15 trials, 1260 participants; Analysis 3.4, Figure 9). There is high heterogeneity noted in the number of oocytes retrieved for all subgroups, suggesting different effects according to differences including in trial protocols, study population and also difference in GH dose and schedule.
3.4. Analysis.

Comparison 3: Adjuvant GH compared to no adjuvant: subgroup analysis based on age, Outcome 4: Mean number of oocytes retrieved
9.

The test for subgroup differences showed that the treatment effect differed between the groups of trials (Chi² = 16.04, df = 2 (P = 0.0003), I² = 87.5%).
3.5 Embryo transfer achieved per woman randomised
Five trials reported embryo transfer per woman randomised (Bergh 1994; Kucuk 2008; Norman 2019; Suikkari 1996; Younis 1992). On sub‐group analysis, the evidence is very uncertain about the effect of GH on embryo transfer achieved per woman randomised < 40 years (OR 1.73, 95% CI 0.80 to 3.74; participants = 201; studies = 5; I2 = 0%); Analysis 3.5.
3.5. Analysis.

Comparison 3: Adjuvant GH compared to no adjuvant: subgroup analysis based on age, Outcome 5: Embryo transfer achieved per woman randomised
We found evidence that the treatment effect differed between the groups of trials (test for subgroup differences: Chi² = 2.92, df = 2 (P < 0.09), I² = 65.8%).
3.6 Mean units of gonadotrophin used
Ten trials reported mean units of gonadotropin used (Bergh 1994; Choe 2017; Dakhly 2018; Dor 1995; Kucuk 2008; Norman 2019; Owen 1991; Safdarian 2019; Younis 1992; Tapanainen 1992).
On further subgroup analysis based on age, there was uncertain effect in < 40 years (MD ‐24.30, 95% CI ‐145.14 to 96.55; participants = 253; studies = 5; I2 = 34%; Analysis 3.6), whereas trials with participants > 40 years showed a slight reduction in mean units of gonadotropin used (MD ‐782.66, 95% CI ‐1004.35 to ‐560.97; participants = 367; studies = 2; I2 = 97%; Analysis 3.6), and similarly trials in which age criteria were not defined showed there was a reduction in mean units of gonadotropins used (MD ‐1294.15, 95% CI ‐1436.54 to ‐1151.77; participants = 145; studies = 3; I2 = 91%; Analysis 3.6) and the pooled result for all trials showed a slight reduction in mean units of gonadotropin used (MD ‐589.38, 95% CI ‐674.47 to ‐504.30; participants = 765; studies = 10; I2 = 96%);Analysis 3.6. There is high heterogeneity noted in the number of oocytes retrieved for the subgroups of women > 40 years and where the age criteria were not defined, such that the pooled estimates may not represent useful summaries.
3.6. Analysis.

Comparison 3: Adjuvant GH compared to no adjuvant: subgroup analysis based on age, Outcome 6: Mean units of gonadotropin used
The test for subgroup differences showed that the treatment effect differed between the groups of trials (Chi² = 181.03, df = 2 (P < 0.00001), I² = 98.9%).
Discussion
Summary of main results
This review was undertaken to establish the role of adjuvant GH therapy for IVF in improving IVF outcomes, particularly in those women who are considered poor responders. We included 16 RCTs (1352 women analysed). Two RCTs (80 women analysed) studied GH in routine use, and 14 RCTs (1272 women analysed) studied GH in poor responders. The evidence was low to very low certainty, with the main limitations being risk of bias, imprecision and heterogeneity.
Adjuvant growth hormone compared to no adjuvant: routine use for in vitro fertilisation (IVF)
The evidence is very uncertain about the effect of GH on live birth rate per woman randomised for routine use in IVF (low‐certainty evidence). There was insufficient evidence to reach a conclusion regarding clinical pregnancy rates per woman randomised, number of women with at least one oocyte retrieved per woman randomised and embryo transfer achieved per woman randomised; reported data were unsuitable for analysis. The evidence is very uncertain about the effect of GH on mean number of oocytes retrieved in normal responders (very low‐certainty evidence). The evidence suggests that GH does not increase or reduce adverse events in normal responders. This information has been presented in tabular form in Table 3.
Adjuvant growth hormone compared to no adjuvant: use in poor responders for in vitro fertilisation (IVF)
The evidence is very uncertain about the effect of GH on live birth rate per woman randomised for poor responders (very low‐certainty evidence). GH used as adjuvant results in a slight increase in pregnancy rates per woman randomised in poor responders compared to no use of GH (low‐certainty evidence). The evidence suggests that GH results in little to no difference in the number of women with at least one oocyte retrieved per woman randomised (low‐certainty evidence). There is a slight increase in the mean number of oocytes retrieved with the use of GH for poor responders (low‐certainty evidence). The evidence is very uncertain about the effect of GH on embryo transfers achieved per woman randomised (very low‐certainty evidence). Use of GH results in reduction of mean units of gonadotropins used for stimulation in poor responders (low‐certainty evidence). Eight of 14 included trials reported adverse events. These included ectopic pregnancy, multiple pregnancy and congenital abnormality. This information has been presented in tabular form in Table 3.
High heterogeneity in the analyses for mean number of oocytes retrieved and units of GH used suggests quite different effects according to differences including in trial protocols (populations, GH dose and schedule), so these results should be interpreted with caution.
Overall completeness and applicability of evidence
The included trials did not answer the review question satisfactorily. Mainly, the primary outcome, live birth rate, was not reported in all trials. Also, since the dose, preparation and timing of administration of GH varied across the trials, robust conclusions cannot be derived.
The causative factors for poor response to controlled ovarian stimulation are not well described in the literature. Consequently, the definitions of a 'poor responder' are varied, ranging from age to poor responders to gonadotrophin stimulation on previous IVF cycles. Therefore the inclusion criteria of the included trials varied greatly. The evidence is low to very low certainty.
There was no uniformity of dose, preparation or timing of the intervention, and this can introduce bias (see Table 4). Also, the funnel plot could indicate publication bias (Figure 10), and this can skew the results of the review. Furthermore, some of the included trials had relatively small sample sizes, and this may have influenced the validity and reliability of the conclusions. Finally, not all the included trials had strict methods of randomisation, blinding and allocation concealment, which may affect conclusions.
10.

Funnel plot ‐ Preganacy rate per woman randomised in poor responder group.
Quality of the evidence
Of the 16 RCTs included in the review, there were significant differences in the number of participants, variations in the cause of subfertility and variations in the IVF treatment protocol. The dose of GH also varied in the trials and the outcomes measured all varied considerably between the trials. As such, the certainity of evidence is very low due to imprecision, small sample size and heterogeneity. Also, there was asymmetry in the funnel plot (Figure 10), which could indicate publication bias, or small study effects or use of an inappropriate effect measure.
Potential biases in the review process
The methods established to conduct the current review were agreed by all review authors and any potential bias that could have been introduced was bypassed through independent screening, assessment, selection and data extraction with discrepancies resolved through team consensus. The search was supported by the CGFG Information Specialist.
We made every effort to identify all potentially eligible trials, and sought additional data from study authors as necessary. However, it is possible that there are unpublished trials that were not retrieved.
Also, there was asymmetry in the funnel plot (Figure 10), which could indicate publication bias, or small study effects or use of an inappropriate effect measure.
Agreements and disagreements with other studies or reviews
Currently, no national or international guidelines recommend the routine use of GH augmentation in IVF protocols. Unfortunately, due to the problems inherent with recruiting women who have undergone unsuccessful IVF treatment cycles and their inevitable low live birth rate per initiated cycle, many trials performed to date have been underpowered. However, a previous systematic review and meta‐analysis concerning the evaluation of strategies to improve pregnancy rates in poor responders undergoing IVF concluded there was some evidence to suggest the addition of GH could improve live birth rates, but further research was required (Kyrou 2009). Another meta‐analysis demonstrated a benefit for the use of adjunct GH, with a reduction in the duration of ovarian stimulation required for oocyte retrieval, the collection of a greater number of oocytes than placebo, and an improvement in many of the early clinical parameters; however, there was no evidence of an increased chance of a live birth with the use of GH (Hart 2017). Similar conclusions suggesting an increase in clinical pregnancy rate but no increase in live birth rate was also seen in a meta‐analysis in Cozzolino 2020. However, another recent meta‐analysis concluded that GH supplementation might improve live birth rates, clinical pregnancy rates and oocytes retrieved (Yang 2020). The difference in results could possibly be explained by the further information we are awaiting from trials included in this review.
A retrospective analysis based on real‐world data suggests a role for GH in POSEIDON (Patient‐Oriented Strategies Encompassing IndividualizeDOocyte Number) group 4 patients (Cai 2019). This is the first publication to detect specific subgroups of poor ovarian responders that would benefit from GH supplementation. The authors explored the effects of GH in the POSEIDON groups 3 and 4, but they were capable of detecting an improvement in live birth rates together with a decrease in miscarriage rates only in POSEIDON group 4. A future prospective trial based on POSEIDON groups with a standardised protocol of GH supplementation may provide further answers.
New avenues are being explored for the use of GH in IVF. In a recent study of recurrent implantation failure patients undergoing IVF. The clinical pregnancy and live birth rates in the treatment group were significantly higher than those in the control group (Chen 2018). The mechanism was postulated to be increased GH receptors in granulosa cells. Another study reported improved implantation, pregnancy, and live birth rates among infertile patients with recurrent implantation failure; treatment with GH suggested that GH improves uterine receptivity (Altmae 2018). We await further investigation and clarification from a recently conducted meta‐analysis that questioned whether the role of GH resides in the treatment of poor oocyte quality, the treatment of the 'suboptimal' responder, the treatment of the 'thin endometrium' or 'recurrent implantation failure' (Cui 2019; Hart 2019).
Authors' conclusions
Implications for practice.
Use of adjuvant GH in IVF treatment protocols slightly increases the number of oocytes retrieved and pregnancy rates in 'poor responders' but has uncertain effects on live birth rates. The results however, need to be interpreted with caution, as the included trials were small and few in number, with significant bias and imprecision. Also, the dose and regimen of GH used in trials was variable. Therefore, further research is necessary to fully define the role of GH as adjuvant therapy in IVF. Furthermore, the cost of the intervention has been reported by only one study (Kucuk 2008), which was nearly double the cost without adjuvant GH use. This could affect the applicability of GH in practice, as the cost of IVF treatment with GH will be significantly higher than without, making treatment with GH unaffordable for some women.
Implications for research.
With regards to women who are known poor responders to IVF, a multiple‐centre randomised double‐blinded trial is warranted to investigate the effect of GH augmentation. Key elements of design should include a power calculation to ensure the minimum number of participants needed for a significant result are included, the standardisation of controlled ovarian hyperstimulation protocols, and dose of GH and subgroups based on POSEIDON criteria (POSEIDON criteria 2016). The primary outcome of live birth rate should be measured. Only by considering such outcomes can this therapy be truly tested. Also, adverse events should be routinely reported. Given the high cost of GH treatment, one component of new trials should also be an economic evaluation. Newer avenues of research include the potential role of GH in improving uterine receptivity and improved outcomes in women with recurrent implantation failure.
What's new
| Date | Event | Description |
|---|---|---|
| 28 September 2021 | New citation required but conclusions have not changed | The addition of 6 new trials has not led to any changes in the conclusions of this review. Also, we added a separate subgroup analysis based on age at the request of a referee. Methods updated to current Cochrane standards, including provision for summary of findings tables, sensitivity analyses and a funnel plot. |
| 28 September 2021 | New search has been performed | We updated our contact details. We added 6 trials to the review (Choe 2017; Dakhly 2018; Lee 2019; Mohammad 2019; Norman 2019; Safdarian 2019). We made a list of ongoing research and contacted all authors. |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 24 August 2009 | New citation required but conclusions have not changed | Authors changed |
| 11 August 2009 | New citation required but conclusions have not changed | New authors added |
| 14 June 2009 | New search has been performed | Since the last published review (1995 & 2003), the authorship of the review has changed. New authors involved in updating the review in 2009 included G Ahmad, J Brown, JMN Duffy, L Nardo, I Salim and AJ Watson. New randomised controlled trials were included in the review, resulting from repeating the search strategy In June 2009. Subgroup analysis of poor responders was performed in the 2009 update, the first subgroup defined as poor responders as demonstrated by sub‐optimal response following controlled ovarian stimulation and the second subgroup defined as poor ovarian performance as demonstrated by abnormal ovarian reserve tests. |
| 28 April 2008 | Amended | Converted to new review format. |
| 28 May 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the members of the Cochrane Gynaecology and Fertility Group (CGFG) based in Auckland, New Zealand, who assisted with this review. The authors of the 2021 update thank Dr Emily Liu, Professor Rob Norman, Dr Jack Wilkinson and Ms Rana Zarean who provided referee comments on the draft.
We thank several study authors who replied to requests for additional information including Lee 2019; Mohammad 2019, Hazout 2003, Bergh 1994, Tapanainen 1992, Younis 1992 and Zhuang 1994.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
ProCite platform
Searched 11 November 2020
Keywords CONTAINS "growth hormone" or "growth hormone derivative" or "human growth hormone" or "growth hormone releasing factor" or "grf" or r‐hGH or rhGH or Title CONTAINS "growth hormone" or "growth hormone derivative" or "human growth hormone" or "growth hormone releasing factor" or "grf" or r‐hGH or rhGH
(102 records)
Appendix 2. CENTRAL via the Cochrane Register of Studies Online (CRSO) search strategy
Web platform
Searched 11 November 2020
#1 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 2062
#2 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1097
#3 (embryo transfer*):TI,AB,KY 3809
#4 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 538
#5 (vitro fertili?ation ):TI,AB,KY 3460
#6 (intracytoplasmic sperm injection* ):TI,AB,KY 1933
#7 ((Ivf or icsi)):TI,AB,KY 6604
#8 (blastocyst* adj2 transfer*):TI,AB,KY 417
#9 MESH DESCRIPTOR Ovulation Induction EXPLODE ALL TREES 1349
#10 (ovulat* adj3 stimulat*):TI,AB,KY 82
#11 (ovar* adj3 stimulat*):TI,AB,KY 2364
#12 (ovar* adj3 induc*):TI,AB,KY 661
#13 (ovulat* adj3 induc*):TI,AB,KY 2666
#14 (infertil* or subfertil*):TI,AB,KY 9043
#15 (assisted reproduct*):TI,AB,KY 1430
#16 (poor adj3 respon*):TI,AB,KY 2423
#17 (sub‐optimal respon*):TI,AB,KY 37
#18 (ovar* adj3 function*):TI,AB,KY OR (ovar* adj3 reserv*):TI,AB,KY 1385
#19 (ovar* adj2 hyperstimulat*):TI,AB,KY 1646
#20 (implantation failure*):TI,AB,KY 452
#21 superovulat*:TI,AB,KY 218
#22 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 17409
#23 MESH DESCRIPTOR Human Growth Hormone EXPLODE ALL TREES 1541
#24 MESH DESCRIPTOR Growth Hormone EXPLODE ALL TREES 3135
#25 somatotrop*:TI,AB,KY 289
#26 somatrop*:TI,AB,KY 195
#27 (growth hormone ):TI,AB,KY 5917
#28 grf:TI,AB,KY 159
#29 rHGH:TI,AB,KY 520
#30 HGH:TI,AB,KY 403
#31 sermorelin:TI,AB,KY 32
#32 norditropin:TI,AB,KY 102
#33 #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 6167
#34 #22 AND #33 163
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 11 November 2020 1 exp Reproductive Techniques, Assisted/ (70255) 2 embryo transfer$.tw. (12474) 3 in vitro fertili?ation.tw. (23676) 4 ivf.tw. (24222) 5 icsi.tw. (8622) 6 intracytoplasmic sperm injection$.tw. (7378) 7 (blastocyst adj2 transfer$).tw. (1140) 8 exp Ovulation Induction/ (13241) 9 ((ovar$ or ovulat$) adj5 (induct$ or stimulat$)).tw. (16271) 10 (infertil$ or subfertil$).tw. (65487) 11 assisted reproduct$.tw. (15458) 12 (poor adj2 respon$).tw. (17407) 13 (ovar$ adj2 respon$).tw. (4652) 14 (ovar$ adj2 reserv*).tw. (3386) 15 sub‐optimal respon$.tw. (102) 16 ovar$ function$.tw. (7465) 17 (ovar$ adj2 hyperstimulat$).tw. (5223) 18 poor prognosis.tw. (83842) 19 implantation failure$.tw. (1705) 20 or/1‐19 (248311) 21 growth hormone/ or human growth hormone/ (55298) 22 exp growth hormone‐releasing hormone/ or sermorelin/ (5017) 23 somatotrop$.tw. (7495) 24 (somatrop$ or norditropin).tw. (363) 25 (growth adj3 hormone$).tw. (63161) 26 grf.tw. (2951) 27 rHGH.tw. (1852) 28 GHRF.tw. (102) 29 HGH.tw. (4380) 30 sermorelin.tw. (10) 31 Humatrope.tw. (26) 32 or/21‐31 (84325) 33 20 and 32 (1346) 34 randomized controlled trial.pt. (516636) 35 controlled clinical trial.pt. (93916) 36 randomized.ab. (498254) 37 randomised.ab. (99429) 38 placebo.tw. (218158) 39 clinical trials as topic.sh. (193603) 40 randomly.ab. (344514) 41 trial.ti. (228357) 42 (crossover or cross‐over or cross over).tw. (86662) 43 or/34‐42 (1392770) 44 exp animals/ not humans.sh. (4754125) 45 43 not 44 (1282851) 46 33 and 45 (145)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 11 November 2020 1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (72124) 2 embryo$ transfer$.tw. (22137) 3 in vitro fertili?ation.tw. (31361) 4 icsi.tw. (16653) 5 intracytoplasmic sperm injection$.tw. (9979) 6 (blastocyst adj2 transfer$).tw. (2561) 7 ivf.tw. (41854) 8 assisted reproduct$.tw. (23687) 9 ovulation induc$.tw. (5714) 10 (ovari$ adj2 stimulat$).tw. (11462) 11 superovulat$.tw. (3952) 12 ovarian hyperstimulation.tw. (7612) 13 COH.tw. (2567) 14 infertil$.tw. (87096) 15 subfertil$.tw. (7145) 16 (ovari$ adj2 induction).tw. (332) 17 exp ovulation induction/ (14827) 18 assisted reproduct$.tw. (23687) 19 (poor adj2 respon$).tw. (28231) 20 (ovar$ adj2 respon$).tw. (6617) 21 (ovar$ adj2 reserv*).tw. (6549) 22 sub‐optimal respon$.tw. (304) 23 ovar$ function$.tw. (8958) 24 ovar$ reserv$.tw. (6362) 25 (ovar$ adj2 hyperstimulat$).tw. (7737) 26 implantation failure$.tw. (3394) 27 poor prognosis.tw. (128750) 28 or/1‐27 (342471) 29 exp growth hormone/ (55943) 30 exp human growth hormone/ (11631) 31 exp growth hormone releasing factor/ (7244) 32 exp sermorelin/ (317) 33 somatotrop$.tw. (7094) 34 (somatrop$ or norditropin).tw. (1212) 35 (growth adj3 hormone$).tw. (67557) 36 grf.tw. (3322) 37 rHGH.tw. (2631) 38 GHRF.tw. (100) 39 HGH.tw. (4691) 40 sermorelin.tw. (23) 41 Humatrope.tw. (442) 42 or/29‐41 (95989) 43 28 and 42 (1852) 44 Clinical Trial/ (982892) 45 Randomized Controlled Trial/ (627750) 46 exp randomization/ (89123) 47 Single Blind Procedure/ (40905) 48 Double Blind Procedure/ (175571) 49 Crossover Procedure/ (65151) 50 Placebo/ (345175) 51 Randomi?ed controlled trial$.tw. (242701) 52 Rct.tw. (39309) 53 random allocation.tw. (2090) 54 randomly allocated.tw. (36699) 55 allocated randomly.tw. (2593) 56 (allocated adj2 random).tw. (833) 57 Single blind$.tw. (25671) 58 Double blind$.tw. (207879) 59 ((treble or triple) adj blind$).tw. (1229) 60 placebo$.tw. (311063) 61 prospective study/ (640581) 62 or/44‐61 (2266870) 63 case study/ (73510) 64 case report.tw. (418898) 65 abstract report/ or letter/ (1129538) 66 or/63‐65 (1610773) 67 62 not 66 (2211744) 68 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6108712) 69 67 not 68 (2131981) 70 43 and 69 (233)
Appendix 5. PsycINFO search strategy
Ovid platform
Searched from 1806 to 11 November 2020
1 exp Somatotropin/ (1302) 2 somatotrop$.tw. (259) 3 (somatrop$ or norditropin).tw. (9) 4 (growth adj5 hormone$).tw. (2667) 5 1 or 2 or 3 or 4 (2869) 6 exp reproductive technology/ (1881) 7 in vitro fertili?ation.tw. (772) 8 ivf‐et.tw. (20) 9 (ivf or et).tw. (143737) 10 icsi.tw. (75) 11 intracytoplasmic sperm injection$.tw. (57) 12 (blastocyst adj2 transfer$).tw. (4) 13 assisted reproduct$.tw. (1014) 14 artificial insemination.tw. (262) 15 iui.tw. (44) 16 intrauterine insemination$.tw. (34) 17 ovulation induc$.tw. (33) 18 (ovari$ adj2 stimulat$).tw. (60) 19 ovarian hyperstimulation.tw. (13) 20 COH.tw. (135) 21 superovulat$.tw. (8) 22 infertil$.tw. (3633) 23 subfertil$.tw. (97) 24 (ovari$ adj2 induction).tw. (8) 25 poor responder$.tw. (131) 26 or/6‐25 (148811) 27 5 and 26 (112) 28 random.tw. (59858) 29 control.tw. (453410) 30 double‐blind.tw. (23231) 31 clinical trials/ (11806) 32 placebo/ (5779) 33 exp Treatment/ (1068486) 34 or/28‐33 (1472642) 35 27 and 34 (37)
Appendix 6. CINAHL search strategy
Ebsco platform
Searched from 1961 until 20 January 2020. Search output from the 11 November 2020 search is included in the CENTRAL output
| # | Query | Results |
| S50 | S26 AND S49 | 19 |
| S49 | S48 NOT S47 | 622,364 |
| S48 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 | 650,729 |
| S47 | S45 NOT S46 | 166,691 |
| S46 | MH (human) | 2,022,943 |
| S45 | S42 OR S43 OR S44 | 189,382 |
| S44 | TI (animal model*) | 2,879 |
| S43 | MH (animal studies) | 110,140 |
| S42 | MH animals+ | 87,026 |
| S41 | AB (cluster W3 RCT) | 317 |
| S40 | MH (crossover design) OR MH (comparative studies) | 251,708 |
| S39 | AB (control W5 group) | 98,479 |
| S38 | PT (randomized controlled trial) | 86,212 |
| S37 | MH (placebos) | 11,573 |
| S36 | MH (sample size) AND AB (assigned OR allocated OR control) | 3,753 |
| S35 | TI (trial) | 98,599 |
| S34 | AB (random*) | 279,402 |
| S33 | TI (randomised OR randomized) | 96,562 |
| S32 | MH cluster sample | 3,996 |
| S31 | MH pretest‐posttest design | 39,260 |
| S30 | MH random assignment | 56,981 |
| S29 | MH single‐blind studies | 13,027 |
| S28 | MH double‐blind studies | 43,273 |
| S27 | MH randomized controlled trials | 89,512 |
| S26 | S16 AND S25 | 57 |
| S25 | S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 | 5,505 |
| S24 | TX HGH | 157 |
| S23 | TX HGH | 157 |
| S22 | TX rHGH | 155 |
| S21 | TX grf | 574 |
| S20 | TX (somatrop* or norditropin) | 64 |
| S19 | TX somatotrop* | 152 |
| S18 | TX growth N3 hormone* | 4,803 |
| S17 | (MM "Human Growth Hormone") | 1,552 |
| S16 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 | 15,741 |
| S15 | TX (ovari* N2 induction) | 34 |
| S14 | TX COH | 244 |
| S13 | TX ovarian hyperstimulation | 835 |
| S12 | TX superovulat* | 86 |
| S11 | TX ovulation induc* | 1,756 |
| S10 | TX assisted reproduct* | 3,853 |
| S9 | (MM "Reproduction Techniques+") | 8,993 |
| S8 | TX intracytoplasmic sperm injection* | 902 |
| S7 | TX embryo* N3 transfer* | 3,102 |
| S6 | TX ovar* N3 hyperstimulat* | 840 |
| S5 | TX ovari* N3 stimulat* | 1,007 |
| S4 | TX IVF or TX ICSI | 5,030 |
| S3 | (MM "Fertilization in Vitro") | 3,439 |
| S2 | TX vitro fertilization | 7,002 |
| S1 | TX vitro fertilisation | 7,002 |
Data and analyses
Comparison 1. Adjuvant GH compared to no adjuvant: routine use for IVF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth rate per woman randomised | 2 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.40, 4.43] |
| 1.2 Clinical pregnancy rate per woman randomised | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.49, 6.50] |
| 1.3 Number of women with at least one oocyte retrieved per woman randomised | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.11, 74.31] |
| 1.4 Mean number of oocytes retrieved | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.79, 0.74] |
| 1.5 Embryo transfer achieved per woman randomised | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.36 [0.36, 151.91] |
| 1.6 Mean units of gonadotrophin used | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 13.57 [‐112.88, 140.01] |
Comparison 2. Adjuvant GH compared to no adjuvant: poor responders for IVF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth rate per woman randomised | 8 | 737 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.17, 2.70] |
| 2.1.1 Poor responder by definition | 3 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.02, 3.38] |
| 2.1.2 Poor responder based on previous response | 5 | 337 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.95, 3.06] |
| 2.2 Clinical pregnancy rate per woman randomised | 11 | 1033 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.35, 2.53] |
| 2.2.1 Poor responder by definition | 5 | 711 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.16, 2.50] |
| 2.2.2 Poor responder based on previous response | 6 | 322 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.26, 3.81] |
| 2.3 Number of women with at least one oocyte retrieved per woman randomised | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.67 [1.54, 20.83] |
| 2.3.1 Poor responder based on previous response | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.67 [1.54, 20.83] |
| 2.4 Mean number of oocytes retrieved | 12 | 1153 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [1.16, 1.64] |
| 2.4.1 Poor responder based on definition | 5 | 721 | Mean Difference (IV, Fixed, 95% CI) | 1.42 [1.12, 1.73] |
| 2.4.2 Poor responder based on previous response | 7 | 432 | Mean Difference (IV, Fixed, 95% CI) | 1.35 [0.96, 1.74] |
| 2.5 Embryo transfer acheived per woman randomised | 4 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.08, 4.96] |
| 2.5.1 Poor responder based on previous response | 4 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.08, 4.96] |
| 2.6 Mean units gonadotropin used | 8 | 685 | Mean Difference (IV, Fixed, 95% CI) | ‐1088.19 [‐1203.20, ‐973.18] |
| 2.6.1 Poor responder based on definition | 3 | 437 | Mean Difference (IV, Fixed, 95% CI) | ‐1246.99 [‐1388.24, ‐1105.74] |
| 2.6.2 Poor responder based on previous response | 5 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐775.79 [‐973.90, ‐577.67] |
Comparison 3. Adjuvant GH compared to no adjuvant: subgroup analysis based on age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Live birth rate per woman randomised | 10 | 817 | Odds Ratio (IV, Fixed, 95% CI) | 1.62 [1.07, 2.44] |
| 3.1.1 Age < 40 years | 6 | 390 | Odds Ratio (IV, Fixed, 95% CI) | 1.45 [0.82, 2.56] |
| 3.1.2 Age > 40 years | 2 | 340 | Odds Ratio (IV, Fixed, 95% CI) | 1.69 [0.90, 3.20] |
| 3.1.3 Age criteria not defined | 2 | 87 | Odds Ratio (IV, Fixed, 95% CI) | 3.00 [0.58, 15.56] |
| 3.2 Clinical pregnancy rate per woman randomised | 12 | 1087 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.38, 2.52] |
| 3.2.1 Age < 40 years | 5 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.12, 3.50] |
| 3.2.2 Age > 40 years | 4 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.11, 2.42] |
| 3.2.3 Age criteria not defined | 3 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.15 [1.25, 7.94] |
| 3.3 No of women with at least one oocyte retrieved per woman randomised | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.19 [1.56, 17.32] |
| 3.3.1 Age < 40 years | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.19 [1.56, 17.32] |
| 3.4 Mean number of oocytes retrieved | 15 | 1260 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [1.04, 1.49] |
| 3.4.1 Age < 40 years | 7 | 437 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.34, 1.13] |
| 3.4.2 Age > 40 years | 4 | 651 | Mean Difference (IV, Fixed, 95% CI) | 1.35 [1.03, 1.68] |
| 3.4.3 Age criteria not defined | 4 | 172 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [1.55, 2.69] |
| 3.5 Embryo transfer achieved per woman randomised | 5 | 262 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.24, 5.03] |
| 3.5.1 Age < 40 years | 4 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.80, 3.74] |
| 3.5.2 Age criteria not defined | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 23.80 [1.31, 433.85] |
| 3.6 Mean units of gonadotropin used | 10 | 765 | Mean Difference (IV, Fixed, 95% CI) | ‐589.38 [‐674.47, ‐504.30] |
| 3.6.1 Age < 40 years | 5 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐24.30 [‐145.14, 96.55] |
| 3.6.2 Age > 40 years | 2 | 367 | Mean Difference (IV, Fixed, 95% CI) | ‐782.66 [‐1004.35, ‐560.97] |
| 3.6.3 Age criteria not defined | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐1294.15 [‐1436.54, ‐1151.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergh 1994.
| Study characteristics | ||
| Methods | Randomisation: using a computerised list women were randomised to one of four arms Allocation concealment: unclear Blinding: double‐blind Trial design: parallel Analysis: power calculation was performed, no intention‐to‐treat analysis performed Study setting: multicentre study ‐ three IVF programmes in Sweden Withdrawals: two women (< 10%) Cancelled cycles: one woman in placebo group (< 10%) |
|
| Participants |
|
|
| Interventions | Intervention
Treatment protocol
|
|
| Outcomes |
|
|
| Notes | This trial involved four treatment arms (and 40 women) but only data comparing GH use in conjunction with GnRHa/hMG versus standard treatment (groups I, II) were included. Groups III and IV involved GH pretreatment and were excluded. The placebo used was NaCl. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation: using a computerised list women were randomised to one of four arms |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 women lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol found |
| Other bias | Low risk | No other source of bias identified |
Choe 2017.
| Study characteristics | ||
| Methods | Trial design: open‐label parallel randomised control trial Analysis: power calculation performed Study setting: single study centre (Seoul, South Korea) Cancelled cycles: 30 (12.5%) failed egg collection = 18, failed fertilisation = 12 |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | Initially 164 patients screened for the study. 28 were excluded due to loss to follow‐up, 8 were excluded due to abnormal findings at screening. No patients discontinued due to adverse events. Improved number of mature oocytes noted in GH group. Authors contacted regarding more information on adverse effects and allocation concealment ‐ no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all 127 women randomised to GH versus standard treatment |
| Selective reporting (reporting bias) | High risk | The study did not report adverse events |
| Other bias | High risk | This study was supported by a research grant from LG Life Sciences, Seoul, Korea (manufacturer of sustained release GH preparation used) |
Dakhly 2018.
| Study characteristics | ||
| Methods | Randomisation: using specific computer programmes Allocation concealment: results placed in opaque sealed envelopes with patients' number written outside (and after opening the envelope, it would reveal which group patient belonged to) Blinding: non‐blinded Trial design: open‐label randomised control trial Analysis: power calculation performed Study setting: single study centre (Egypt) Withdrawals: none Cancelled cycles: 30 (12.5%) failed egg collection = 18, failed fertilisation = 12 |
|
| Participants |
|
|
| Interventions | Both groups
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Registered protocol NCT02338206 Improved number of MII oocytes noted Authors contacted regarding further information on adverse events data ‐ no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes reported for per cycle started, 12.5% cancellation |
| Selective reporting (reporting bias) | High risk | Registered protocol NCT02338206 The study did not report adverse events |
| Other bias | Unclear risk | No placebo was used in control group |
Dor 1995.
| Study characteristics | ||
| Methods | Randomisation: method not described Allocation concealment: none Blinding: double‐blind Trial design: prospective, randomised, placebo‐controlled, double‐blind study Analysis: power calculation not performed Study setting: Department of Obstetrics and Gynecblogy, The Chaim Sheba Medical Center, Tel Hashomer and Sackler School of Medicine, Tel Aviv University, Israel Withdrawals: none |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported per cycle started |
| Selective reporting (reporting bias) | High risk | The study did not report adverse events |
| Other bias | High risk | Very low number of participants ‐ no power calculation |
Hazout 2003.
| Study characteristics | ||
| Methods | Randomisation: stated as randomised Allocation concealment: unclear Blinding: double‐blind Intention‐to‐treat analysis: not performed Power calculation: not performed Study setting: single centre (Paris, France) Withdrawals: none Cancelled cycles: < 10% |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention
Induction protocol
|
|
| Outcomes |
|
|
| Notes | Thirty‐five women in total were included in Hazout 2003 and they were divided into three groups: placebo, GH 4 IU and GH 8 IU. Since only two groups could be compared for the table of comparisons the two GH groups were separated and compared with half the placebo data for the meta‐analysis but throughout the text the trial is referred to singly as Hazout 2003. Received a response from author regarding clarification of queries in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised; no other details |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: none. Cancelled cycles < 10% |
| Selective reporting (reporting bias) | High risk | The study did not report adverse events |
| Other bias | Unclear risk | Conference abstract only available as data not published as article, author contacted for additional information ‐ response received by email |
Kucuk 2008.
| Study characteristics | ||
| Methods | Randomisation: computer generated randomisation Allocation concealment: sealed envelopes Blinding: triple Intention‐to‐treat analysis: not performed Power calculation: not performed Study setting: single centre ‐ Bursa, Turkey Withdrawals: none Cancelled cycles: < 10% |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; no details as to whether opaque |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: none. Cancelled cycles < 10% |
| Selective reporting (reporting bias) | Unclear risk | There is no indication the study has reported outcomes selectively |
| Other bias | Unclear risk | No placebo used in control group |
Lee 2019.
| Study characteristics | ||
| Methods | Randomisation: simple randomisation using a coin toss method Allocation concealment: none Blinding: not blinded Trial design: parallel Analysis: no power calculation or intention‐to‐treat analysis performed Study setting: single centre, location Taipei Medical University Hospital from January 2010 to October 2012 Withdrawals: none Cancelled cycles: 40% |
|
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The second part of the study was a retrospective comparison of poor responders treated with adjuvant GH with normal responders ‐ these data were not included in the review. The data presented for number of oocytes and number of embryos transferred was an average and number of women who had the procedure not mentioned. Authors contacted regarding further data on adverse events if available ‐ reply received to confirm that no adverse events were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Tossing a coin used for randomisation |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of cycle cancellation or withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol |
| Other bias | Unclear risk | Live birth rates were not reported. Second half of the study was retrospective in nature, hence only data from the prospective part was analysed No placebo used in control group |
Mohammad 2019.
| Study characteristics | ||
| Methods | Randomisation: using computer generated tables Allocation concealment: closed envelope technique Blinding: double‐blind Trial design: parallel randomised controlled trial Analysis: power calculation performed Study setting: single study centre (Egypt) Withdrawals: 8 (< 10%) Cancelled cycles: 16 |
|
| Participants |
Inclusion criteria: age 25 to 38 years, IVF previous poor responders with at least two failed cycles with < 5 oocytes, abnormal ovarian reserve tests e.g. AMH < 1 ng/mL, patients with unexplained infertility, normal hormonal profile (FSH, LH, PRL), normal ovarian ultrasound, normal pelvic ultrasound, women that were willing to do ICSE‐ET. Poor responders were identified according to the Bologna Criteria but without advanced maternal age. Exclusion criteria: women with known medical disease (e.g. severe hypertension or hepatic disease), history of altered karyotype in one or both partners, history of chronic, autoimmune or metabolic diseases, presence of endocrinopathies, male factor infertility, participation in any other clinical trial during enrolment, women who in the investigator's judgment cannot be expected to comply with the protocol or study procedures, and refusal to participate in the study. |
|
| Interventions | Stimulation protocol
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT03759301 Authors contacted regarding further data on adverse effects if available‐ reply received to confirm that only multiple pregnancy was noted. No other adverse events noted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated tables used |
| Allocation concealment (selection bias) | Low risk | Closed envelope technique |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported per cycle started |
| Selective reporting (reporting bias) | Low risk | There is no indication that the data were reported selectively |
| Other bias | Low risk | No other bias noted |
Norman 2019.
| Study characteristics | ||
| Methods | Trial design ‐ multicentre, double‐blind, placebo‐controlled trial performed in 10 participating centres throughout Australia and New Zealand. Analysis ‐ Intention to treat |
|
| Participants | Total participants randomised ‐ 130, 65 to GH and 65 to placebo
|
|
| Interventions | Study drug‐ GH (Recombinant GH‐ Saizen 8 mg, Merck, Australia), in a syringe of 24 IU with a daily administered dose of 12 IU. Placebo control‐ identical syringe provided by Merck but containing 0.3% metacresol in water. Gonadotrophin‐releasing hormone (GnRH) antagonist cycle with the study drug being started at the same time as recombinant FSH on Day 2 or 3 of the cycle. GnRH antagonist was started on Day 5 or 6. When at least two follicles were available at 17 mm or more, injection of 250 μg recombinant HCG was given to trigger ovulation and an oocyte recovery organised for 36 h later. |
|
| Outcomes | Primary outcome ‐
Secondary outcomes‐
|
|
| Notes | Australian New Zealand Clinical Trials Registry ACTRN12609001060235. Based on sample size calculation before the start of the study, sample size ‐195. These numbers were not reached and the study was ended early as provided drug had expired. A post hoc sub group analysis of poor ovarian response according to Bologna criteria was performed ‐ no statistically significant differences were observed between the 2 groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (1:1) was with a computer‐generated block randomisation |
| Allocation concealment (selection bias) | Low risk | A prenumbered drug kit which was allocated on day 1 of the FSH stimulation. Sites telephoned a central office to obtain the randomisation numbers. Drugs were stored onsite and the code determined which injections were given to the participant |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, study investigators, care providers and the trial statistician were all blinded to treatment allocation until the statistical analysis was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Low risk | There is no indication the study has reported outcomes selectively |
| Other bias | Low risk | No other bias noted |
Owen 1991.
| Study characteristics | ||
| Methods | Randomisation: two randomisation lists were made with 20 women on each list and block randomised into blocks of four. Allocation concealment: method unclear Blinding: double‐blind Trial design: parallel. Analysis: no power calculation or intention to treat analysis performed. Study setting: single centre, location London. Withdrawals: none (< 10%). Cancelled Cycles: < 10%. |
|
| Participants | Number of women:n = 25 (13 GH, 12 placebo).
|
|
| Interventions | Intervention: GH 24 IU intramuscular (IM), days 1, 3, 5, 7, 9, and 11 of hMG treatment, during long GnRHa protocol, vs placebo given IM on same cycle days as active treatment groups. Recombinant GH used. Dose of human chorionic gonadotropin: 5000 IU | |
| Outcomes |
|
|
| Notes | Nature of placebo not described. Follicular fluid IG1 increased by 27% with GH treatment. The data from Jacobs 1995 are also presented in Owen 1991. Authors contacted regarding further information on allocation concealment ‐ no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Two randomisation lists were made with 20 women on each list and block randomised into blocks of four |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals: none (< 10%). Cancelled Cycles < 10% |
| Selective reporting (reporting bias) | Unclear risk | There is no indication the study has reported outcomes selectively |
| Other bias | High risk | Nature of placebo not described. Follicular fluid IG‐1 increased by 27% with GH treatment Free supply of growth hormone received |
Safdarian 2019.
| Study characteristics | ||
| Methods | Randomisation: computerized random sampling table Allocation concealment: none Blinding: single blinded. Analysis: no power calculation or intention to treat analysis performed. Study setting: single centre, location Shariati Hospital of Tehran University of Medical Sciences. Withdrawals: none. Cancelled cycles: 14.2% ( Failed fertilisation ‐7, no oocytes retrieved ‐3) |
|
| Participants |
|
|
| Interventions | The patients in all groups received gonadotropin (Gonal‐f 300 to 450 IU/day, subcutaneously, based on age, AFC, and the level of AMH) plus GnRH antagonist (Cetrotide, 0.25mg/day, subcutaneously, after production of 14mm follicles until HCG injection) from the third day of their cycle. In addition to common regimens, group A received recombinant GH (Somatropin, 2.5mg/day, subcutaneously from the eighth day of the cycle until the injection of HCG) and group C received placebo (normal saline, 0.1mg/day, subcutaneously) from the eighth day of the cycle until the injection of HCG). | |
| Outcomes |
|
|
| Notes | Study registered in Iranian Registry of Clinical Trials (IRCT20140818842N14). Third group ‐ group B received GH (Somatropin, 0.1mg/day, subcutaneously from the third day of the previous cycle)‐ this group was excluded from analysis as GH was started in the previous cycle. Number of collected oocytes and embryo transfer given as average, hence could not be included in the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random sampling table |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'Single blinded' but mentioned patients' blinding was also considered. In one of the groups, intervention started in previous menstrual cycle, hence blinding not possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals: none. Cancelled cycles 14.2% |
| Selective reporting (reporting bias) | Low risk | There is no indication the study has reported outcomes selectively. Registered protocol |
| Other bias | Low risk | No other bias |
Suikkari 1996.
| Study characteristics | ||
| Methods | Randomisation: stated as randomised. Allocation concealment: unclear. Blinding: double blind. Trial design: parallel. Analysis: no power calculation and no intention to treat analysis performed Study setting: two centres. Analysis: no power calculation or intention‐to‐treat analysis performed. Withdrawals: < 10%. Cancelled Cycles: > 10% (therefore include in meta‐analysis but perform sensitivity analysis). |
|
| Participants |
|
|
| Interventions | Intervention: six women received 12 IU GH and 10 women received 4 IU GH daily SC from day three of spontaneous menstrual cycle. Recombinant GH used. Study Protocol: A boost "flare‐up" protocol was used for ovarian stimulation. On day two of spontaneous menstrual cycle leuprolide acetate was administered SC 0.75mg in the morning. On day three gonadotrophin Metrodin was started at 300IU SC for four days then adjusted according to serum E2 and follicular growth. Dose of human chorionic gonadotropin 5000 IU IM given when the largest follicle(s) reached a diameter of 18 to 20mm. | |
| Outcomes |
|
|
| Notes | Twenty two women in total were included in Suikkari 1996 and they were divided into three groups, placebo, GH four IU and GH 12IU. Since only two groups could be compared for the table of comparisons the two GH groups were separated and compared with half the placebo data for the meta‐analysis but throughout the text the trial is referred to singly as Suikkari 1996. Authors contacted for further information on allocation concealment ‐ no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals < 10%. Cancelled Cycles > 10% |
| Selective reporting (reporting bias) | Unclear risk | There is no indication the study has reported outcomes selectively |
| Other bias | Low risk | No other bias |
Tapanainen 1992.
| Study characteristics | ||
| Methods | Randomisation: Stated as randomised, method unclear. Allocation concealment: trial codes kept in sealed envelopes until the study was completed. Blinding: double‐blind Trial design: parallel. Analysis: power calculation not done, no intention to treat analysis but no withdrawals. Study setting: single centre. Finland Withdrawals: none (<10%). Cancelled cycles: <10%. |
|
| Participants |
|
|
| Interventions | Intervention: Recombinant GH 24 IU IM beginning on cycle day four, then every 2 days until human chorionic gonadotropin, vs sterile saline IM on same cycle days. Treatment Protocol: Short GnRHa protocol used for ovulation induction, 300 µg BA 3 times daily on cycle days 1‐4. Three ampoules of hMG given IM on day 4 and then 150‐223 IU daily until human chorionic gonadotropin injection. 5000 IU human chorionic gonadotropin given. Clinical Pregnancy Diagnosis: USS at six weeks gestation | |
| Outcomes |
|
|
| Notes | There were two parts to this trial, A and B. Only data from part A was included as part B studied the effect of GH on gene expression of steroidogenic enzymes in granulosa cells and the women were not followed up for live birth or pregnancy data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but method unclear |
| Allocation concealment (selection bias) | Unclear risk | Trial codes kept in sealed envelopes until the end of the study, no details as to whether centralised or envelopes opaque |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals none, cancelled cycles < 10% |
| Selective reporting (reporting bias) | Unclear risk | There is no indication the study has reported outcomes selectively |
| Other bias | High risk | Free supply of growth hormone received |
Tesarik 2005.
| Study characteristics | ||
| Methods | Randomisation: truly randomised, computer generated random number tables. Allocation concealment: clear, opaque envelopes. Blinding: double‐blinded. Analysis: Power calculation performed and intention to treat analysis not performed. Study setting: multi‐centre, Spain and France. Withdrawals: none. Cancelled cycles: <10%. |
|
| Participants |
|
|
| Interventions | Intevention: Recombinant GH 8IU Subcut. Treatment Protocol: Long. Dose of human chorionic gonadotropin: 25mg when at least 1 follicle measured > 18mm in diameter. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals none, cancelled cycles < 10% |
| Selective reporting (reporting bias) | High risk | The study did not report adverse events |
| Other bias | Low risk | No other bias noted |
Younis 1992.
| Study characteristics | ||
| Methods | Randomisation: prospectively randomised, method unclear. Allocation concealment: allocation not revealed until all outcome measures were calculated and comparison between the two groups had been performed. Blinding: double‐blind. Study design: placebo controlled trial. Sensitivity analysis: no power calculation or intention to treat analysis performed. Study setting: single centre, location Israel. Withdrawals: none (< 10%). Cancelled Cycles: < 10%. |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Mannitol chosen as placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but method unclear |
| Allocation concealment (selection bias) | Low risk | Allocation not revealed until all outcomes calculated and comparisons between groups performed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals none, cancelled cycles < 10% |
| Selective reporting (reporting bias) | Unclear risk | There is no indication the study has reported outcomes selectively |
| Other bias | High risk | Free supply of growth hormone received |
Zhuang 1994.
| Study characteristics | ||
| Methods | Randomisation: stated as randomised, method unclear. Allocation concealment: unclear of method Blinding: outcome assessors were blind to treatment allocation. Study design: parallel. Study setting: unclear. Analysis: power calculation done. Withdrawals: none. Cancelled cycles: none. |
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Some information will have been stated in the trial but was not translated. The sections that were translated were kindly done so by Teresa Gu. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but method unclear |
| Allocation concealment (selection bias) | Unclear risk | Not stated within the text |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No cancelled cycles, no withdrawals |
| Selective reporting (reporting bias) | High risk | The study did not report adverse events |
| Other bias | Unclear risk | Translator used as publication was not in English |
Only outcomes relevant to the review were stated in the table of included studies.
- hMG: human menopausal gonadotropin
- AFC: antral follicle count
- AMH: anti‐Müllerian hormone
- FSH: follicle stimulating hormone
- GnRHa‐ gonadotropin‐releasing hormone agonist
- GH: growth hormone
- IU: international units
- LH: luteinising hormone
- NaCl: sodium chloride
- COS: controlled ovarian stimulation
- Mii oocytes: metaphase 2 oocytes
- WHO: World Health Organisation
- IM: intramuscular
- SC: sub‐cutaneous
- POR: poor ovarian response
- PRL: prolactin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balasubramanyam 2017 | Case series. Testosterone gel and GH used together |
| Bhattacharya 2014 | Not a RCT; non‐randomised, case‐control study |
| Blumenfeld 1994 | Additional data were sought to clarify which women included in the trial received which method of assisted conception and the definition of "poor responder" used, but a response was not received |
| Busacca 1996 | Method of assisted conception used was not IVF, but artificial insemination by husband or GIFT |
| Cui 2018 | Role of GH in frozen embryo replacement cycle in women with thin endometrium |
| Dakhly 2016 | All four groups received GH as adjuvant with different protocols to compare outcomes |
| Demoulin 1992 | Not randomised, published abstract with no data available |
| European and Australian Multicentre study 1995 | All hypogonadotropic hypogonadism participants included in the study for comparison of various doses of GH with hMG versus placebo with hMG to evaluate the dose‐response relationship |
| Fernandez 2015 | Pre‐implantation genetic testing done on all embryos, therefore prone to bias |
| Guan 2007 | Co‐administration of GH and aspirin |
| Hassan 1998 | The study involved identifying poor responders on day 10 and then alteration of the protocol by stopping GnRH agonist and adding GH/no GH at that stage. Abstract published |
| Hassan 2001 | Study on effect of GH on in vitro maturation |
| Hazout 2009 | Not randomised and non‐comparative |
| Homburg 1990a | Not stated as randomised, no useful outcomes reported |
| Homburg 1990b | Women did not undergo IVF |
| Homburg 1995 | Women did not undergo IVF |
| Howles 1999 | Intervention is GH‐releasing factor, not GH |
| Hughes 1994 | Stimulation cycles with less that 3 follicles > 20 mm after ovarian stimulation were cancelled. These women were not included in the analysis. This unpublished information could not be obtained from the author. |
| Jacobs 1995 | Only concerns ovulation induction, not IVF |
| Landolfi 1994 | Only concerns ovulation induction, not IVF |
| Latte 2013 | Study not randomised |
| Li 2020 | The participants included had a history of poor embryonic development; not based on ovarian response |
| Matsumoto 2020 | Retrospective study |
| Merdassi 2010 | Non‐randomised retrospective study |
| Nayar 2018 | Comparison of two adjuvants: GH versus rLH instead of placebo, hence not included |
| Ob'edkova 2017 | Prospective observational study |
| Owen 1991b | There are two publications for this trial. The analysis used women randomised to receive GH in the trial and retrospective cases of women who had also received GH in the past. |
| Regan 2018 | Laboratory study; pregnancy rates mentioned but included the fresh as well as frozen cycles |
| Rinehart 1999 | Allocation was stated as "alternating randomisation", suggesting allocation to groups by alternation, not randomisation. |
| Sakr 2012 | Comparative study of the effect of GH versus corticosteroids to ICSI in low responding patient (instead of placebo) |
| Schoolcraft 1997 | Both treatment groups received the same dose of GH, the intervention was oral contraceptive |
| Tulandi 1993 | Method of assisted conception was intrauterine insemination, not IVF |
| Viardot‐Foucault 2016 | Retrospective study, 3 different regimens compared: FSH, FSH + LH, FSH + GH |
| Xue‐Mei 2016 | Effect of GH on clinical outcomes in frozen embryo replacement cycles |
| Yovich 2010 | Study was non‐randomised sequential cross‐over study |
- FSH: follicle stimulating hormone
- GH: growth hormone
- GnRH: gonadotropin‐releasing hormone
- IVF: in vitro fertilisation
- LH: luteinising hormone
- GIFT: Gamete intrafallopian transfer
- hMG: human menopausal gonadotropin
- ICSI: Intracytoplasmic sperm injection
Characteristics of studies awaiting classification [ordered by study ID]
Bassiouny 2016.
| Methods | Trial design: parallel Analysis: no power calculation or intention‐to‐treat analysis performed Study setting: single study centre (Egypt) Withdrawals: none Cancelled cycles: 25 (17.73%), failed egg collection: 9, failed fertilisation: 16 |
| Participants | Number of women: 141 (68 GH, 73 gonadotropin only). IVF previous poor responders as defined by study design (ESHRE consensus):
|
| Interventions | GnRH antagonist protocol
|
| Outcomes |
|
| Notes | Registered protocol NCT02195947 |
Bayoumi 2015.
| Methods | Randomisation: using a computerised list women were randomised to one of four arms Allocation concealment: unclear Blinding: non‐blind Trial design: parallel Analysis: no power calculation or intention‐to‐treat analysis performed Study setting: single study centre (Egypt) Withdrawals: none Cancelled cycles: 27 patients (15.6%) |
| Participants | Number of women: 172 (84 GH, 88 placebo). IVF previous poor responders as defined by study design (ESHRE consensus):
|
| Interventions | Intervention: GH 2.5 mg subcutaneously daily until ovulation induction with HCG trigger Treatment protocol: microflare stimulation protocol ‐ combined OCP (drosperinone plus ethinyl estradiol) for 21 days before ovarian stimulation. Short GnRHa (0.05 mg triptorelin/hMG 300 IU to 450 IU intramuscularly daily from day 3. Dose of HCG: 10000 IU when at least two follicles were > 17 mm in diameter |
| Outcomes |
|
| Notes | Registered protocol NCT02185326 Method: inserted 3 embryos per cycle. Primary outcome of clinical pregnancy was changed after 3 months into the trial. No data for live birth rate obtained |
Eftekhar 2012.
| Methods | Randomisation: stated as randomised Allocation concealment: sealed opaque identical envelopes Blinding: not blinded Trial design: parallel Analysis: no power calculation or intention‐to‐treat analysis performed Study setting: single centre, location Iran Withdrawals: none. Cancelled cycles: 40% |
| Participants | Number of women: 82 (40 GH, 42 not treated) Inclusion criteria: women who had one or more previous failed IVF‐ET cycles with three or fewer retrieved oocytes and with subsequent three or less obtained embryos using GnRH agonist long protocol, and/or E2 levels B 500 pg/mL on the day of HCG injection Age: unlimited |
| Interventions | Intervention
Treatment protocol
|
| Outcomes |
|
| Notes | Waiting for additional information from trial authors |
Gong 2020.
| Methods | Prospective randomised open‐label study |
| Participants | Total participants in POR group: 105, GH (52), no adjuvant POR control (53) Inclusion criteria
Exclusion criteria
Women with tubal factor infertility (aged 20 to 35 years) with a normal ovarian reserve and regular menstrual cycles who underwent IVF‐ET were recruited as non‐POR controls during the same period. The exclusion criteria for the non‐POR group were the same as those for the POR group. |
| Interventions |
|
| Outcomes |
|
| Notes | Chinese Clinical Trial Registry Centre Registration No. ChiCTR1900021269 The sample size calculation was based on assumption that the clinical pregnancy rate would increase 3‐fold after GH pretreatment. Rationale for the proposed GH treatment: low physiological dose and longer treatment (from the antral follicle stage) might be more beneficial to follicular growth and development. Oxidative stress makers studied: follicular fluid malondialdehyde, superoxide dismutase, total oxidant status, oxidative stress index and total antioxidant capacity were significantly lower in the POR‐C group (P < 0.05). |
- AFC: antral follicle count
- AMH: anti‐mullerian hormone
- ESHRE: European Society of Human Reproduction and Embryology
- ET: embryo transfer
- GH: growth hormone
- GnRH: gonadotropin releasing hormone
- HCG: human chorionic gonadotropin
- hMG:human menopausal gonadotropin
- ICSI: intracytoplasmic sperm injection
- IVF: in vitro fertilisation
- OCP: oral contraceptive pills
- POR: poor ovarian response
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800016106.
| Study name | Application of growth hormone in patients with poor ovarian response and study of mechanism |
| Methods | |
| Participants | Patients with poor ovarian response |
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcome
|
| Starting date | 2018 |
| Contact information | Not available |
| Notes |
CTRI/2019/03/018047.
| Study name | Study to compare the effect of giving growth hormone in poor responders during IVF |
| Methods | |
| Participants |
|
| Interventions | Intervention 1
Control
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 2019 |
| Contact information | Not available |
| Notes |
NCT01715324.
| Study name | Adjuvant growth therapy in vitro fertilization |
| Methods | Randomised, parallel, open‐label interventional trial |
| Participants | 528 participants
|
| Interventions | The treatment group will receive 2.5 mg of saizen daily via subcutaneous injections, from the beginning of the ovarian reserve stimulation until the day of the ovulation triggering |
| Outcomes | Aim: to determine if the clinical pregnancy rate during the course of one treatment cycle in women receiving GH daily in addition to gonadotropin‐releasing hormone antagonist protocol is significantly higher than those receiving only gonadotropin‐releasing hormone antagonist protocol (control group) Primary outcomes
Aim: to evaluate the effectiveness of GH adjuvant therapy in gonadotropin‐releasing hormone antagonist protocol when compared to the control group Secondary outcomes
|
| Starting date | October 2012 |
| Contact information | Jacques Kadoch, MD. Clinique Ovo, Montreal, Quebec, Canada |
| Notes |
NCT02179255.
| Study name | Human growth hormone pre‐treatment for 6 weeks prior to ovulation induction for IVF |
| Methods | Randomised, parallel, open‐label interventional trial |
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | July 2017 |
| Contact information | David H Barad, MD, Center for Human Reproduction New York, New York, USA |
| Notes | This study is for pretreatment unlike other trials where GH has been used during stimulation |
NCT03027843.
| Study name | The effect of growth hormone in assisted reproductive technology clinical outcome of poor responder |
| Methods | Randomised, parallel, open‐label, interventional trial |
| Participants | 80 participants
|
| Interventions | GH group patients have weekly injections of GH dose 14 IU, until the day of hCG |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | January 2017 |
| Contact information | Xing Yang, MD, PhD. The Sixth Affiliated Hospital, Sun Yat‐Sen University |
| Notes |
NCT03373149.
| Study name | Growth hormone co‐treatment within a GnRH antagonist protocol in patients with poor ovarian response |
| Methods | Randomised, parallel, single‐blind interventional trial |
| Participants | 228 participants
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | December 2017 |
| Contact information | Usama M Fouda, Prof Riyadh Fertility and Reproductive Health Center, Giza, Egypt |
| Notes |
NCT03759301.
| Study name | Efficacy of growth hormone supplementation with gonadotrophins in IVF/ICSI for poor responders |
| Methods | Randomised, parallel, triple‐blind, interventional trial |
| Participants | 156 participants.
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | November 2018 |
| Contact information | Mohamed M Shafeek, MSc Al Azhar University Hospitals (Kasr Al‐Aini), Cairo, Egypt |
| Notes |
- AMH: anti‐Müllerian hormone
- COS: controlled ovarian stimulation
- FSH: follicle stimulating hormone
- GH: growth hormone
- GnRH:gonadotropin releasing hormone
- HCG: human chorionic gonadotropin
- HPuFSH: highly purified urinary FSH
- ICSI:intracytoplasmic sperm injection
- IVF: in vitro fertilization
- LH: luteinising hormone
- mRNA: messenger ribonucleic acid
- ORT: ovarian reserve test
- PCOS: polycystic ovarian syndrome
- rHGH: recombinant human growth hormone
- E: estradiol
Differences between protocol and review
New authors of the protocol were added for the first full review; additional authors were included in the 2009 update: G Ahmad, J Brown, JMN Duffy, L Nardo, L Mohiyiddeen and AJ Watson. Further additional authors for the 2021 review were A Sood and G Mohiyiddeen.
We added a separate analysis for subgroups, based on age, at the request of a referee in the 2021 update.
The protocol stated that Peto ORs would be used. We used Maentel‐Haenszel ORs for the 2021 update, as the Cochrane Handbook for Systematic Reviews of Interventions recommends this as the default method for meta‐analysis (Higgins 2021).
At the 2021 update we updated the methods to current Cochrane standards, including provision for summary of findings tables, sensitivity analyses and a funnel plot.
Contributions of authors
D Kotarba, J Kotarba, and E Hughes prepared the original version of this review published in 1995. The 2003 update of the review was prepared by K Harper and M Proctor. The 2009 update of the review was prepared by G Ahmad, J Brown, JMN Duffy and L Nardo, L Mohiyiddeen and AJ Watson.
The 2021 update was prepared by A Sood, G Mohiyiddeen and L Mohiyiddeen. AS and GM retrieved the searches, selected studies, extracted and analysed the data with guidance from LM. CF, GA and AW made contributions to concept and design, reading and approving the draft.
Sources of support
Internal sources
-
Dept of Obstetrics and Gynaecology, University of Auckland, New Zealand
Editorial support
External sources
-
Department of Health, UK
₤5000 initiative fund
Declarations of interest
AS has no conflicts of interest to declare.
GM has no conflicts of interest to declare.
GA has no conflicts of interest to declare.
CF has no conflicts of interest to declare.
AW has no conflicts of interest to declare.
LM has no conflicts of interest to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bergh 1994 {published data only}
- Bergh C, Carlstrom K, Selleskog U, Hillensjo T. Effect of growth hormone on follicular fluid androgen levels in patients treated with gonadotropins before in vitro fertilization. European Journal of Endocrinology 1996;134:190-6. [DOI] [PubMed] [Google Scholar]
- Bergh C, Nilsson L, Hillensjo T, Borg G, Wikland M, Hamgerger L. Adjuvant growth hormone treatment during in vitro fertilization: a randomized, placebo-controlled study. Fertility and Sterility 1994;62:113-20. [PubMed] [Google Scholar]
Choe 2017 {published data only}
- Choe S, Kim MJ, Lee HJ, Lee WS, Yoon TK, Kim YS, et al. Increased proportion of mature oocytes with sustained release growth hormone treatment in poor responders: a prospective randomised controlled study. Archives of Gynecology and Obstetrics 2017;297(3):791-6. [DOI] [PubMed] [Google Scholar]
- Kim YS, Yoon TK, Kim JW, Kim MJ, Kim J, Choe SA, et al. Increased proportion of mature oocytes with sustained releasing growth hormone treatment in poor responders: a randomized controlled study. Human reproduction (Oxford, England) abstract from 33rd Annual Meeting of the European Society of Human Reproduction and Embryology 2017;32(0):i464-5. [Google Scholar]
Dakhly 2018 {published data only}
- Dakhly D, Bassiouny YA, Bayoumi YA, Hassan MA, Gouda HM, Hassan AA. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: Randomised control trial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2018;228:161-5. [DOI] [PubMed] [Google Scholar]
Dor 1995 {published data only}
- Dor J, Seidman DS, Amudai E, Bider D, Levran D, Mashiach S. Adjuvant growth hormone therapy in poor responders to in-vitro fertilization: a prospective randomized placebo-controlled double blind study. Human Reproduction 1995;10(1):40-3. [DOI] [PubMed] [Google Scholar]
Hazout 2003 {published data only (unpublished sought but not used)}
- Hazout A, Junca AM. A pilot, prospective, randomised, double blind, placebo-controlled study to compare protocols for ovarian stimulation with r-hRSH (Gonal-F) combined with two different doses of r-hGH or placebo in patients with oocyte dysmorphy undergoing ICSI. Human Reproduction 2003;18(Suppl 1):299-312. [Google Scholar]
Kucuk 2008 {published data only}
- Kucuk T, Kozinoglu H, Kaba A. Growth hormone co-treatment within a GnRH agonist long protocol in patients with poor ovarian response:a prospective, randomized, clinical trial. Journal of Assisted Reproduction and Genetics 2008;25:123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2019 {published data only}
- Lee YX, Shen MS, Tzeng CR. Low dose growth hormone adjuvant treatment with ultra-long ovarian stimulation protocol in poor responders showed non-inferior pregnancy outcome compared with normal responders. Frontiers in Endocrinology 2019;10:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammad 2019 {published data only}
- Mohammad EH, Abou El Serour AG, Mohamed EA, Abbasy AH, Zaatar M, Rageh KA, et al. Efficacy of growth hormone supplementation with gonadotropins in IVF/ICSI for poor responders: randomised controlled trial. Current Science International 2019;8(3):585-94. [Google Scholar]
Norman 2019 {published data only}
- Norman RJ, Alvino H, Hart R, Rombauts L, LIGHT Investigators. A randomised double blind placebo controlled trial of recombinant human growth hormone (r-HGH) on live birth rates in women who are poor responders. Human Reproduction (Oxford, England) 2016;31(Suppl 1):i37. [Google Scholar]
- Norman RJ, Alvino H, Hull LM, Mol BW, Hart RJ, Kelly T-L, et al. Human growth hormone for poor responders: a randomised placebo-controlled trial provides no evidence for improved live birth rate. Reproductive BioMedicine Online 2019;00:1-8. [DOI] [PubMed] [Google Scholar]
Owen 1991 {published data only}
- Jacobs HS. Growth hormone and ovulation: Is there an indication for treatment of infertile women with growth hormone? Hormone Research 1992;38:14-21. [DOI] [PubMed] [Google Scholar]
- Owen EJ, Ostergaard H, Shoham Z, Jacobs HS, Mason BA. Cotreatment with growth hormone, after pituitary suppression, for ovarian stimulation in vitro fertilization: a randomized, double-blind, placebo-control trial. Fertility and Sterility 1991;56:1104-10. [DOI] [PubMed] [Google Scholar]
Safdarian 2019 {published data only}
- Safdarian L, Aghahosseini M, Alyasin A, Samaei-Nouroozi A, Rashidi S, Shabani-Nashtaei M, et al. Growth hormone (GH) improvement of ovarian responses and pregnancy outcome in poor ovarian responders: a randomized study. Asian Pacific Journal for Cancer Prevention 2019;20(7):2033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suikkari 1996 {published data only}
- Suikkari AM, MacLachlan V, Koistinen R, Seppälä M, Healy DL. Double-blind placebo controlled study: human biosynthetic growth hormone for assisted reproductive technology. Fertility and Sterility 1996;65(4):800-5. [DOI] [PubMed] [Google Scholar]
Tapanainen 1992 {published data only}
- Tapanainen J, Orava M, Martikainen H, Ruokonen A, Voutilainen R, Ronnberg L. Effect of growth hormone administration on human ovarian function and steroidogenic gene expression in granulosa-luteal cells. Fertility and Sterility 1992;58:726-32. [DOI] [PubMed] [Google Scholar]
Tesarik 2005 {published data only}
- Tesarik J, Hazout A, Mendoza C. Improvement of delivery and live birth rates after ICSI in women aged > 40 years by ovarian co-stimulation with growth hormone. Human Reproduction 2005;20(9):2536-41. [DOI] [PubMed] [Google Scholar]
Younis 1992 {published data only}
- Younis JS, Dorembus D, Simon A, Schenker JG, Koren R, Laufer N. The effect of growth hormone supplementation on in vitro fertilization outcome: a prospective randomized placebo-controlled double-blind study. Fertility and Sterility 1992;58:575-80. [DOI] [PubMed] [Google Scholar]
- Younis JS, Ezra Y, Brzezinnski, Fibich T, Schenker JG, Laufer N. The effect of growth hormone on granulosa cell function during in-vitro fertilization. Human Reproduction 1993;8(10):1588-92. [DOI] [PubMed] [Google Scholar]
Zhuang 1994 {published data only}
- Zhuang GL, Wong SX, Zhou CQ. The effect of co-administration of low dosage growth hormone and gonadotropin for ovarian hyperstimulation in vitro fertilization and embryo transfer. Chung-Hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics and Gynaecology) 1994;29(8):471-4. [PubMed] [Google Scholar]
References to studies excluded from this review
Balasubramanyam 2017 {published data only}
- Balasubramanyam S. Sequestial use of testosterone gel and growth hormone in expected poor responders and those with previous poor assisted reproductive outcomes: a pilot study. International Journal of Infertility and Fetal Medicine 2017;8(1):1-4. [Google Scholar]
Bhattacharya 2014 {unpublished data only}
- Bhattacharya V, Verma S, Maity M, Bhattacharya N, Sinha SS. Effect of addition of growth hormone to gonadotropins in ovarian stimulation of poor responders in IVF. Abstracts of the 30th Annual Meeting of ESHRE, Munich, Germany July 2014:343.
Blumenfeld 1994 {published data only}
- Blumenfeld Z, Amit T. The role of growth hormone (GH), GH-receptor and GH-binding protein in reproduction and ovulation induction. Journal of Pediatric Endocrinology & Metabolism 1996;9:145-62. [PubMed] [Google Scholar]
- Blumenfeld Z, Amit T. The role of growth hormone in ovulation induction. Annals of Medicine 1994;26:249-54. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Dirnfeld M, Gonen Y, Abramovici H. Growth hormone co-treatment for ovulation induction may enhance conception in the co-treatment and succeeding cycles, in clonidine negative but not clonidine positive patients. Human Reproduction 1994;9:209-13. [DOI] [PubMed] [Google Scholar]
Busacca 1996 {published data only}
- Busacca M, Fusi MF, Brigante C, Bonzi V, Gonfiantini C, Vignali M, et al. Use of growth hormone-releasing factor in ovulation induction in poor responders. Journal of Reproductive Medicine 1996;41(9):699-703. [PubMed] [Google Scholar]
Cui 2018 {published data only}
- Cui N, Li A M, Luo Z Y, Zhao Z M, Xu Y M, Zhang J et al. Effects of growth hormone on pregnancy rates of patients with thin endometrium. Journal of Endocrinological Investigation 2018;0(0):1-9. [DOI] [PubMed] [Google Scholar]
Dakhly 2016 {published data only}
- Dakhly DMR, Bayoumi YA, Gad Allah SH. Which is the best IVF/ICSI protocol to be used in poor responders receiving growth hormone as an adjuvant treatment? A prospective randomized trial. Gynaecological Endocrinology 2016;32(2):116-9. [DOI] [PubMed] [Google Scholar]
Demoulin 1992 {published data only}
- Demoulin A, Pignon M, Dubois M. Co-treatment with growth hormone and gonadotropins after pituitary desensitization in unselected patients does not improve success rates in in vitro fertilisation: a double blind- placebo controlled trial. Fertility and Sterility 1992;58:S129-S130.
European and Australian Multicentre study 1995 {published data only}
- European and Australian Multicentre Study. Cotreatment with growth hormone and gonadotropin for ovulation induction in hypogonadotropic patients: a prospective, randomized, placebo-controlled, dose-response study.. Fertility and Sterility 1995;64(5):917-23. [PubMed] [Google Scholar]
Fernandez 2015 {published data only}
- Fernandez M, Rocafort E, Guijarro M, Ramos B, Medrano L, Rogel S, et al. The use of human growth hormone (HGH) in poor prognosis patients improves euploidy and implantation rates. a patient-controlled trial. Fertility and Sterility 2015;104:51. [Google Scholar]
Guan 2007 {published data only}
- Guan Q, Ma HG, Wang YY, Zhang F. Effects of co-administration of growth hormone(GH) and aspirin to women during in vitro fertilization and embryo transfer (IVF-ET) cycles. Zhonghua Nan Ke Xue 2007;13(9):798-800. [PubMed] [Google Scholar]
Hassan 1998 {published data only}
- Hassan HA, Saleh HA, El Gezeiry D, Baghdady I, Abdel Rahman AH. Agonist stop and adjuvant growth hormone (GH): saving the intra-cytoplasmic sperm injection (ICSI) cycle in low responders with good ovarian reserve. Fertility and Sterility 1998;70(3):S58-9.
Hassan 2001 {published data only}
- Hassan HA, Azab H, Rahman AA, Nafee TM. Effects of growth hormone on in vitro maturation of germinal vesicle of human oocytes retrieved from small antral follicles. Journal of Assisted Reproduction and Genetics 2001;18(8):417-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazout 2009 {published data only}
- Hazout A, Junca AM, Ménézo Y, De Mouzon J, Cohen-Bacrie P. Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reproductive BioMedicine Online 2009;18:664-70. [DOI] [PubMed] [Google Scholar]
Homburg 1990a {published data only}
- Homburg R, West C, Torresani T, Jacobs HS. A comparative study of single-dose growth hormone as an adjuvant to gonadotrophin treatment for ovulation induction. Clinical Endocrinology 1990;32:781-5. [DOI] [PubMed] [Google Scholar]
Homburg 1990b {published data only}
- Homburg R, West C, Torresani T, Jacobs HS. Cotreatment with human growth hormone and gonadotropins for induction of ovulation: a controlled clinical trial. Fertility and Sterility 1990;53(2):254-60. [DOI] [PubMed] [Google Scholar]
Homburg 1995 {published data only}
- Homburg R, Levy T, Ben-Rafael Z. Adjuvant growth hormone for induction of ovulation with gonadotrophin-releasing hormone agonist and gonadotrophins in polycystic ovary syndrome: a randomized, double-blind, placebo controlled trial. Human Reproduction 1995;10:2550-3. [DOI] [PubMed] [Google Scholar]
Howles 1999 {published data only}
- Howles CM, Loumaye E, Germond M, Yates R, Brinsden P, Healy D, et al. Does growth hormone-releasing factor assist follicular development in poor responder patients undergoing ovarian stimulation for in-vitro fertilisation? Human Reproduction 1999;14(8):1939-43. [DOI] [PubMed] [Google Scholar]
Hughes 1994 {published data only}
- Huang ZH, Baxter RC, Hughes SM, Matson PL, Lieberman BA, Morris ID. Supplementary growth hormone treatment of women with poor ovarian response to exogenous gonadotrophins: changes in serum and follicular fluid insulin-like growth factor-1 (IGF-1) and IGF binding protein-3 (IGFBP-3). Human Reproduction 1993;8:850-7. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Huang ZH, Morris ID, Matson PL, Buck P, Lieberman BA. A double blind cross over controlled study to evaluate the effect of human biosynthetic growth hormone on ovarian stimulation in previous IVF poor responders. Journal of Reproductive Fertility 1992;96:21. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Huang ZH, Morris ID, Matson PL, Buck P, Lieberman BA. A double-blind cross-over controlled study to evaluate the effect of human biosynthetic growth hormone on ovarian stimulation in previous poor responders to in-vitro fertilization. Human Reproduction 1994;9:13-8. [DOI] [PubMed] [Google Scholar]
Jacobs 1995 {published data only}
- Jacobs HS, Shoham Z, Schachter M, Braat DD, Franks S, Hamilton-Fairley D. Cotreatment with growth hormone and gonadotropin for ovulation induction in hypogonadotropic patients: A prospective, randomized, placebo-controlled, dose-response study. Fertility and Sterility 1995;64(5):917-23. [PubMed] [Google Scholar]
Landolfi 1994 {published data only}
- Landolfi L, Marra V, Gallina N. Ovulation induction with growth hormone and GnRH in polycystic ovarian disease. Rassegna Internazionale di Clinica e Terapia 1994;74(12):529-32. [Google Scholar]
Latte 2013 {unpublished data only}
- Latte K, Prats L, Urrest J, Checa MA. Abstract -Use of low-dose growth hormone co-treatment in poor-responders. Fertility and Sterility 2013;100(3):267. [Google Scholar]
Li 2020 {published data only}
- Li J, Chen Q, Wang J, Huang G, Ye H. Does growth hormone supplementation improve oocyte competence and IVF outcomes in patients with poor embryonic development? A randomised controlled trial. BMC Pregnancy and Childbirth 2020;20(310):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsumoto 2020 {published data only}
- Matsumoto L, Pires EK, Nagai M, Granjo FI, Costa CR, Lo Turco EG et al. Effect of Growth Hormone on blastocyst formation and implantation rates in women undergoing in vitro fertilization. Fertility and Sterility 2020;114(3):e447. [Google Scholar]
Merdassi 2010 {published data only}
- Merdassi G, Chaker A, Kacem K, Benmeftah M, Fourati S, Wahabi D, et al. Increased clinical pregnancy rates with GH addition in patients undergoing ovarian stimulation with dysmorphic oocytes. ESHRE conference 2010;0:316-17. [Google Scholar]
Nayar 2018 {published data only}
- Nayar KD, Gupta S, Singh M, Gupta M, Kant G, Sharma N et al. Adjuvant recombinant Lh (rLH) or growth hormone (GH) to the antagonist protocol in poor responders undergoing IVF. Fertility and Sterility. Conference: 74th annual congress of the American society for reproductive medicine, ASRM 2018. Denver Colorado, USA 2018;110(4):e101-e102. [Google Scholar]
Ob'edkova 2017 {published data only}
- Ob'edkova K, Kogan I, Krikheli I, Dzhemlikhanova L, Muller V, Mekina I et al. Growth hormone co-treatment in IVF/ICSI cycles in poor responders. Gynecological Endocrinology 2017;33:15-17. [DOI] [PubMed] [Google Scholar]
Owen 1991b {published data only}
- Owen EJ, Torresani T, West C, Mason BA, Jacobs HS. Serum and follicular fluid insulin like growth factors I and II during growth hormone co-treatment for in-vitro fertilization and embryo transfer. Clinical Endocrinology 1991;35:3217-34. [DOI] [PubMed] [Google Scholar]
- Owen EJ, West C, Mason BA, Jacobs HS. Cotreatment with growth hormone of sub-optimal responders in IVF-ET. Human Reproduction 1991;6:524-8. [DOI] [PubMed] [Google Scholar]
Regan 2018 {published data only}
- Regan SLP, Knight PG, Yovich JL, Arfuso F, Dharmarajan A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertility and Sterility Dec 2018;110(7):1298-1309. [DOI] [PubMed] [Google Scholar]
Rinehart 1999 {published data only}
- Rinehart JS. Randomized, prospective trial comparing the addition of growth hormone for the ovulation induction of "poor responders" in IVF. Fertility and Sterility 1999;72(3):S91. [Google Scholar]
Sakr 2012 {published data only}
- Sakr SH. Comparing the effects of Growth Hormone versus Corticosteroids on ICSI outcome in potentially Low responder patients. ASRM abstracts 2012;98:280. [Google Scholar]
Schoolcraft 1997 {published data only}
- Schoolcraft W, Schlenker T, Gee M, Stevens J, Wagley L. Improved controlled ovarian hyperstimulation in poor responder in vitro fertilization patients with a microdose follicle-stimulating hormone flare, growth hormone protocol. Fertility and Sterility 1997;67(1):93-7. [DOI] [PubMed] [Google Scholar]
Tulandi 1993 {published data only}
- Tulandi T, Falcone T, Guyda H, Hemmings R, Billiar R, Morris D. Effects of synthetic growth hormone-releasing factor in women treated with gonadotrophin. Human Reproduction 1993;8(4):525-7. [DOI] [PubMed] [Google Scholar]
Viardot‐Foucault 2016 {published data only}
- Viardot-Foucault V, Lye WK, Edrus E, Tan H, Nadarajah S. Poor ovarian responders' best therapeutic options: a centre's experience. Journal of Assisted Reproduction and Genetics. Conference: Translational Rproduction Biology and Clinical Reproductive Endocrinology Conference 2016. USA 2016;33(12):1694-5.
Xue‐Mei 2016 {published data only}
- Xue-Mei W, Hong J, Wen-Xiang Z, Yang L. The effects of growth hormone on clinical outcomes after frozen-thawed embryo transfer. International Journal of Gynaecology and Obstetrics 2016;133(3):347-50. [DOI] [PubMed] [Google Scholar]
Yovich 2010 {published data only}
- Yovich JL, Stanger JD. Growth hormone supplementation improves implantation and pregnancy productivity rates for poor-prognosis patients undertaking IVF. Reproductive BioMedicine Online 2010;21:37-49. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bassiouny 2016 {published data only}
- Bassiouny YA, Dakhly DM, Bayoumi YA, Hashish NM. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertility and Sterility 2016;105(3):697-702. [DOI] [PubMed] [Google Scholar]
Bayoumi 2015 {published data only}
- Bayoumi Y, Dakhly DMR, Bassiouny Y, Hashish N. Addition of growth hormone to the micro flare stimulation protocol among women with poor ovarian response. International Journal of Gynaecology and Obstetrics 2015;131:305-8. [DOI] [PubMed] [Google Scholar]
Eftekhar 2012 {published data only}
- Eftekhar M, Aflatoonian A, Mohammadian F, Eftekhar T. Adjuvant growth hormone therapy in antagonist protocol in poor responders undergoing assisted reproductive technology. Archives of Gynecology and Obstetrics 2013;287:1017-21. [DOI] [PubMed] [Google Scholar]
Gong 2020 {published data only}
- Gong Y, Zhang K, Xiong D, Wei J, Tan H, Qin S. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: a randomised controlled trial. Reproductive Biology and Endocrinology 2020;18(91):1-10. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800016106 {published data only}
- ChiCTR1800016106. Application of Growth Hormone in Patients with Poor Ovarian Response and Study of Mechanism [Application of Growth Hormone in Patients with Poor Ovarian Response and Study of Mechanism]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800016106 (first received 11 May 2018).
CTRI/2019/03/018047 {published data only}
- CTRI/2019/03/018047. Study to compare the effect of giving growth hormone in poor responders during IVF [Assisted reproductive technique outcomes after growth hormone supplementation in poor responders undergoing in-vitro fertilisation- a randomized controlled trial]. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/03/018047 (First received 12 March 2019).
NCT01715324 {unpublished data only}
- NCT01715324. Adjuvant Growth Therapy in in Vitro Fertilization [Adjuvant Growth Therapy in in Vitro Fertilization: A Randomized Control Trial]. https://clinicaltrials.gov/ct2/show/study/NCT01715324?term=NCT01715324&draw=2&rank=1 (First received 24 Oct 2012). [NCT01715324]
NCT02179255 {unpublished data only}
- NCT02179255. Human Growth Hormone Pre-treatment for 6 Weeks Prior to Ovulation Induction for IVF [An Open-Label Randomized Controlled Trial (RCT) of 6 Weeks of Human Growth Hormone (HGH) Prior to Ovulation Induction for In Vitro Fertilization (IVF)]. https://clinicaltrials.gov/ct2/show/study/NCT02179255?term=NCT02179255&draw=2&rank=1 (First received 15 Jun 2014).
NCT03027843 {unpublished data only}
- NCT03027843. The Effect of Growth Hormone in Assisted Reproductive Technology Clinical Outcome of Poor Responder [A Pilot Study of the Effect of Growth Hormone in Assisted Reproductive Technology Clinical Outcome of Poor Responder]. https://clinicaltrials.gov/ct2/show/NCT03027843?term=NCT03027843&draw=2&rank=1 (First received 13 Jan 2017).
NCT03373149 {unpublished data only}
- NCT03373149. Growth Hormone Co-treatment Within a GnRH Antagonist Protocol in Patients With Poor Ovarian Response [Growth Hormone Co-treatment Within a GnRH Antagonist Protocol in Patients With Poor Ovarian Response]. https://clinicaltrials.gov/ct2/show/NCT03373149?term=NCT03373149&draw=2&rank=1 (First received 7 Dec 2017).
NCT03759301 {unpublished data only}
- NCT03759301. Efficacy of Growth Hormone Supplementation With Gonadotrophins in IVF/ICSI for Poor Responders [Efficacy of Growth Hormone Supplementation With Gonadotrophins in IVF/ICSI for Poor Responders; a Randomized Controlled Trial]. https://clinicaltrials.gov/ct2/show/NCT03759301?term=NCT03759301&draw=2&rank=1 (First received 22 Nov 2018).
Additional references
Adashi 1985
- Adashi EY, Resnick CE, D'Ercole AJ, Svoboda ME, Van Wyke J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocrinology Review 1985;6:400-20. [DOI] [PubMed] [Google Scholar]
Altmae 2018
- Altmae S, Mendoza-Tesarik R, Mendoza C, Mendoza N, Cucinelli F, Tesarik J. Effect of growth hormone on uterine receptivity in women with repeated implantation failure in an oocyte donation program: a randomized controlled trial. Journal of the Endocrine Society 2018;2(1):96-105. [DOI: 10.1210/js.2017-00359] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhandari 2018
- Bhandari, HM, Choudhary, MK, Stewart, JA. An overview of assisted reproductive technology procedures.. The Obstetrician &Gynaecologist. 2018;20:167–176. 2018;20:167-176. [DOI: ] [Google Scholar]
Blumenfeld 1996
- Blumenfeld Z, Amit T. The role of growth hormone (GH), GH-receptor and GH-binding protein in reproduction and ovulation induction. Journal of Pediatric Endocrinology & Metabolism 1996;9:145-62. [PubMed] [Google Scholar]
Cahill 2002
- Cahill D, Wardle PG. Management of Infertility. BMJ 2002;325(7354):28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2019
- Cai MH, Gao LZ, Liang XY, Fang C, Wu YQ, Yang X. The effect of growth hormone on the clinical outcomes of poor ovarian reserve patients undergo- ing in vitro fertilization/intracytoplasmic sperm injection treatment: a retrospective study based on POSEIDON criteria. Frontiers in Endocrinology (Lausanne) 2019;10:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2018
- Chen Y, Liu F, Nong Y, Ruan J, Guo Q, Luo M, et al. Clinical efficacy and mechanism of growth hormone action in patients experiencing repeat implantation failure. Canadian Journal of Physiology and Pharmacology 2018;96:929-32. [DOI] [PubMed] [Google Scholar]
Cozzolino 2020
- Cozzolino M, Cecchino GN, Troiano G, Romanelli C. Growth hormone co-treatment for poor responders undergoing in vitro fertilization cycles: a systematic review and meta-analysis. Fertility and Sterility 2020;114(1):97-109. [DOI: doi: 10.1016/j.fertnstert.2020.03.007.] [DOI] [PubMed] [Google Scholar]
Cui 2019
- Cui N, Li AM, Luo ZY, Zhao ZM, Xu YM, Zhang J, et al. Effects of growth hormone on pregnancy rates of patients with thin endometrium. Journal of Endocrinological Investigation 2019;42:27-35. [DOI] [PubMed] [Google Scholar]
Erickson 1989
- Erickson GF, Gabriel VG, Magoffin DA. Insulin-like factor-I regulates aromatase activity in human granulosa and granulosa luteal cells. Journal of Clinical Endocrinological Metabolism 1989;69:716-24. [DOI] [PubMed] [Google Scholar]
Ferraratti 2011
- Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Human Reproduction 2011;26(7):1616-24. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT. Version accessed 10 April 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org. [.Available at gradepro.org.]
Hart 2017
- Hart RJ, Rombauts L, Norman RJ. Growth hormone in IVF cycles: any hope? Current Opinion in Obstetrics and Gynecology 2017;29(3):119-25. [DOI] [PubMed] [Google Scholar]
Hart 2019
- Hart RJ. Use of growth hormone in the IVF treatment of women with poor ovarian reserve. Frontiers in Endocrinology 2019;10:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Homburg 1988
- Homburg R, Eshel A, Abdulla HI, Jacobs HS. Growth hormone facilitates ovulation induction by gonadotrophins. Clinical Endocrinology 1988;29:113-8. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 1987
- Hsu DJ, Hammond JM. Concomitant effects of growth hormone on secretion of insulin-like growth factor I and progesterone by cultured porcine granulosa cells. Endocrinology 1987;121(4):1343-8. [DOI] [PubMed] [Google Scholar]
Kyrou 2009
- Kyrou D, Kolibianakis EM, Venetis CA, Papanikolaou EG, Bontis J, Tarlatzis BC. How to improve the probability of pregnancy in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Fertility and Sterility 2009;91(3):749-66. [DOI] [PubMed] [Google Scholar]
Lucy 2011
- Lucy MC. Growth hormone regulation of follicular growth. Reproduction, Fertility and Development 2011;24:19-28. [DOI] [PubMed] [Google Scholar]
Mason 1990
- Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cell in vitro. Journal of Endocrinology 1990;126:R1-R2. [DOI] [PubMed] [Google Scholar]
NICE CG156
- Fertility: Assessment and Treatment for People with Fertility Problems. National Collaborating Centre for Women’s and Children’s Health (UK). Feb 2013. [PMID: 25340218]25340218
Park 2007
- Park JK, Murphy AA, Bordeaux BL, Dominguez CE, Session DR. Ovulation induction in a poor responder with panhypopituitarism: a case report and review of the literature. Gynecological Endocrinology 2007;23:82-6. [DOI] [PubMed] [Google Scholar]
POSEIDON criteria 2016
- Humaidan P, Alviggi C, Fischer R, Esteves SC. The novel POSEIDON stratification of 'Low prognosis patients in Assisted Reproductive Technology' and its proposed marker of successful outcome. F1000 Research 2016;5:2911. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
POSEIDON Group 2016
- Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Ferility and Sterility 2016;105(6):1452-3. [DOI: doi: 10.1016/j.fertnstert.2016.02.005.] [PMID: Epub 2016 Feb 26. PMID: 26921622.] [DOI] [PubMed] [Google Scholar]
PRISMA 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.. Systematic Reviews 2021;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Yang 2020
- Yang P, Wu R, Zhang H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: a meta-analysis of randomized controlled trials. Reproductive Biology and Endocrinology 2020;18(1):76. [DOI: doi: 10.1186/s12958-020-00632-w.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshimura 1996
- Yoshimura Y, Ando M, Nagamatsu S, Iwashita M, Adachi T, Sueoka K. Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biology of Reproduction 1996;55(1):152-60. [DOI] [PubMed] [Google Scholar]
Zhou 2013
- Zhou P, Baumgarten SC, Wu Y. IGF-I signalling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells.. Molecular Endocrinology 2013;27:511-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ahmad 2009
- Ahmad G, Brown J, Duffy JM, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD000099. [DOI: 10.1002/14651858.CD000099.pub2] [DOI] [PubMed] [Google Scholar]
Harper 2003
- Harper K, Proctor M, Hughes E, Duffy JMN. Growth hormone for in vitro fertilization. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD000099. [DOI: 10.1002/14651858.CD000099] [DOI] [PubMed] [Google Scholar]
Kotarba 1996
- Kotarba D, Kotarba J, Hughes E. Growth hormone in in vitro fertilization. Cochrane Database of Systematic Reviews 1996, Issue 1. Art. No: CD000099. [DOI: 10.1002/14651858.CD000099] [DOI] [PubMed] [Google Scholar]


