Abstract
Introduction
The need for intubation and mechanical ventilation among COVID-19 patients is associated with high mortality rates and places a substantial burden on the healthcare system. There is a strong pathophysiological rationale suggesting that hyperbaric oxygen treatment (HBOT), a low-risk and non-invasive treatment, may be beneficial for COVID-19 patients. This systematic review aimed to explore the potential effectiveness and safety of HBOT for treating patients with COVID-19.
Methods
Medline, Embase, Scopus, and Google Scholar were searched from December 2019 to February 2021, without language restrictions. The grey literature was searched via an internet search engine and targeted website and database searches. Reference lists of included studies were searched. Independent reviewers assessed studies for eligibility and extracted data, with disagreements resolved by consensus or a third reviewer. Risk of bias was assessed using the Newcastle Ottawa Scale. Data were summarised descriptively.
Results
Six publications (one cohort study, five case reports/series) met the inclusion criteria with a total of 37 hypoxaemic COVID-19 patients treated with HBOT. Of these 37 patients, the need for intubation and mechanical ventilation and in-hospital survival were assessed for 26 patients across three studies. Of these 26 patients, intubation and mechanical ventilation were not required for 24, and 23 patients survived. No serious adverse events of HBOT in COVID-19 patients were reported. No randomised trials have been published.
Conclusions
Limited and weak evidence from non-randomised studies including one propensity-matched cohort study suggests HBOT is safe and may be a promising intervention to optimise treatment and outcomes in hypoxaemic COVID-19 patients. Randomised controlled studies are urgently needed.
Keywords: Hyperbaric medicine, Hypoxia, Infection
Introduction
The current SARS-CoV-2 (COVID-19) viral pandemic has infected over 143 million individuals, with over 3.1 million deaths worldwide as of April 21, 2021.[ 1] Approximately 15 to 20% of patients present with hypoxaemic respiratory failure requiring oxygen supplementation.[ 2] Although outcomes may vary depending on factors such as age, comorbidities and initial oxygen requirements,[ 3 , 4] overall one in five of these patients die in hospital.[ 5 - 7] Among hospitalised hypoxaemic COVID-19 patients, one in four require intensive care (ICU) admission and among these, 60% require intubation and 30% die in-hospital.[ 3 , 5 , 8 , 9] Mechanical ventilation and ICU admission are limited resources, placing a substantial burden on the healthcare system.
As the COVID-19 pandemic continues to evolve, there remains a need for a low risk and non-invasive intervention that can both prevent the adverse progression of moderate cases and improve survival in severe cases. Hyperbaric oxygen treatment (HBOT) is one potential solution. HBOT is defined as breathing 100% oxygen at a pressure > 142 kPa (1.4 atmospheres absolute [atm abs]).[ 10] HBOT is a well-established and safe[ 11] method to increase tissue oxygen delivery up to 10-20 fold at 203 to 304 kPa (2-3 atm abs) pressure.[ 12] HBOT is currently approved by the US Food and Drug Administration and Health Canada for 14 indications for both elective (e.g., late radiation tissue injury, non-healing chronic wounds) and urgent conditions (e.g., carbon monoxide poisoning, decompression sickness, gas embolism).[ 10]
The clinical use of HBOT for patients with severe COVID-19 is supported by physiological and preclinical rationales.[ 13] First, hyper-oxygenation of arterial blood with oxygen dissolved in plasma corrects tissue oxygen debt. Second, HBOT has a strong anti-inflammatory effect.[ 14 , 15] Indeed, preclinical and clinical studies show that HBOT has a strong immunomodulatory effect regulating the inflammatory response through several pathways.[ 14 , 15] HBOT stimulates both the humoral and cellular immune response, resulting in decreasing pro-inflammatory cytokines while increasing anti-inflammatory cytokines.[ 15] Intervening early to limit the increase of plasma IL-6 may be beneficial since elevated levels of IL-6 are independently associated with mortality for COVID-19 patients.[ 16] Furthermore, intermittent hyperoxia (i.e., HBOT) promotes stem cell mobilization and cytokine expression.[ 17] Stem cells represent another pathway through which HBOT may have positive effects on COVID-19 patients. Mesenchymal stem cells (MSCs) are known to have strong anti-inflammatory and immunomodulatory properties.[ 18] Therefore, MSCs may contribute to preventing overreaction of the immune system, referred to as the cytokine storm, by limiting pro-inflammatory cytokines and increasing anti-inflammatory cytokines.[ 19] Finally, HBOT may have a direct viricidal effect on SARS-CoV-2 similar to the direct viricidal action that has been demonstrated in preclinical research in other enveloped viruses.[ 20]
Despite the potential for HBOT to reduce the rate of invasive mechanical ventilation and possibly mortality for COVID-19 patients, its effectiveness and safety has yet to be quantified. As the number of new COVID-19 cases continues to rise,[ 1] a systematic review of the efficacy and safety of HBOT is urgently needed. Results will inform COVID-19 research and practice in order to optimise recovery for patients and reduce the burden of the pandemic on the healthcare system as quickly as possible.
We aimed to systematically summarise the existing literature on the clinical effect of HBOT for COVID-19 patients to inform future clinical trials and practice decisions.
Methods
PROTOCOL
This review was planned and conducted according to the Cochrane Handbook for Systematic Reviews of Interventions and reported in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.[ 21] The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD 42020209933).
ELIGIBILITY CRITERIA
Eligibility criteria were pre-specified as described in Box 1.
Box 1.
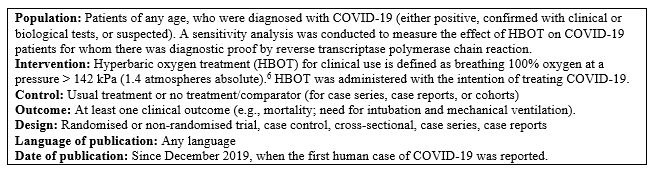
Study eligibility criteria
Articles were included if they involved patients of any age undergoing at least one HBOT session with the intention of treating patients with confirmed positive or suspected COVID-19. Studies involving patients who received HBOT for other purposes while also having confirmed or suspected COVID-19 were not included. To be eligible for inclusion, studies had to assess at least one clinical outcome measured at any time point after HBOT was initiated. Of primary interest were the clinical outcomes of mortality and the need for intubation and mechanical ventilation. In addition to clinical outcomes, studies could also assess biological outcomes (e.g., inflammation markers), imaging outcomes (e.g., chest computed tomography [CT]), cost outcomes (e.g., length of stay), and safety outcomes (any adverse events related to HBOT).
In the context of a new disease leading to a pandemic with no definitive cure, we elected to include all types of study designs, such as randomised and non-randomised trials, case-control studies, cross-sectional studies, case series and case reports. Both peer-reviewed studies and pre-prints were eligible for inclusion. We did not impose language restrictions. We excluded editorials and conference abstracts.
SEARCH STRATEGY AND INFORMATION SOURCES
The search strategy was developed by an experienced information specialist (LS) in close collaboration with the research team (Appendix 1* (72.5KB, pdf) ). It was then reviewed by a second information specialist, following the Peer Review of Electronic Search Strategies (PRESS) guidelines.[ 22] The databases MEDLINE via Ovid, EMBASE via Ovid, Scopus, and Google Scholar were searched without language restrictions from 01 December 2019 to 04 February 2021. References not published in English or French were translated using DeepL Translator (DeepL GmbH, Cologne, Germany). The reference lists of included studies were also searched in addition to related reviews. Our systematic grey literature (i.e., difficult to locate/unpublished) search strategy was developed by the co-author team and consisted of three parts: internet search engine (using anonymous browser to avoid geographical bias); targeted website searching of hyperbaric medicine organisations (e.g., Undersea and Hyberbaric Medical Society, International Association of Francophone Hyperbaric Centres); and targeted grey literature database searching (e.g., World Health Organization, Centres for Disease Control and Prevention, clinicaltrials.gov, medRxiv, bioRxiv, LitCovid, COVID-END). We also consulted content experts and the authors' personal files to ensure literature saturation.
STUDY SELECTION AND DATA EXTRACTION
Following removal of duplicates and a pilot test of a screening form, identified studies were screened using DistillerSR (Evidence Partners, Ottawa, Canada), a systematic review software. Two independent reviewers (RK, GD) assessed titles and abstracts for eligibility. The full-text of articles of included studies and those deemed 'unclear' were subsequently screened. Screening for inclusion at each level was always conducted in duplicate, with disagreements resolved by consensus or involvement of a third reviewer as needed (SB).
A data extraction form was developed and used by the two independent reviewers (RK, GD) to extract relevant information with Microsoft Excel. Extracted data included publication details (e.g., first author name, year of publication, country of data collection, funding, trial registration), study characteristics (e.g., study design, sample size, inclusion/exclusion criteria), patient demographics, intervention and comparator details, the type of hyperbaric chamber, and the effect of intervention on reported clinical outcomes. Discrepancies in extraction were resolved by a third reviewer (SB).
RISK OF BIAS
An estimate of the risk of bias was not performed for case series data given the inherent limitations and high possibility of bias for these types of studies. For included comparative cohort studies, two independent reviewers (RK, GD) assessed risk of bias using the Newcastle Ottawa Scale.[ 23] The Newcastle Ottawa Scale can be used to assess case and cohort studies across three domains: selection, comparability, and outcome/exposure. Each domain is comprised of a series of questions and stars are given according to each item. A maximum of four stars can be given in the Selection and Outcome/exposure domains and a maximum of two stars can be given in the Comparability domain. The star system can then be converted to the Agency for Health Research and Quality standards of good, fair, and poor quality. Good quality is assigned to studies with at least three stars in the Selection domain, one star in the Comparability domain and two stars in the Outcome/exposure domain. Fair quality is assigned to studies with two stars in the Selection domain, at least one star in the Comparability domain and at least two stars in the Outcome/exposure domain. Poor quality is assigned to studies with zero or one stars across the domains. A pilot test was conducted with one article prior to risk of bias assessment.
ASSESSMENT OF THE CERTAINTY OF EVIDENCE
The certainty of the evidence for included comparative studies was assessed using the GRADE approach, which considers five domains: risk of bias, indirectness, inconsistency, imprecision and publication bias.[ 24] Based on the grading of these domains, the certainty of the evidence was rated as high, moderate, low or very low.
DATA SYNTHESIS
A meta-analysis was planned for our pre-specified primary and secondary outcomes but was not conducted based on the heterogeneity of the included studies. Therefore we provide a descriptive synthesis of the current literature.
Results
The literature search yielded 165 studies. After removal of duplicates, 111 studies were assessed for eligibility (Figure 1). The majority of these studies either did not involve the use of HBOT to treat COVID-19 patients, did not assess at least one clinical patient outcome, or were not an original study or case report (e.g., commentaries, position statements). Subsequently, six studies met the inclusion criteria and were included in this systematic review.
Figure 1.
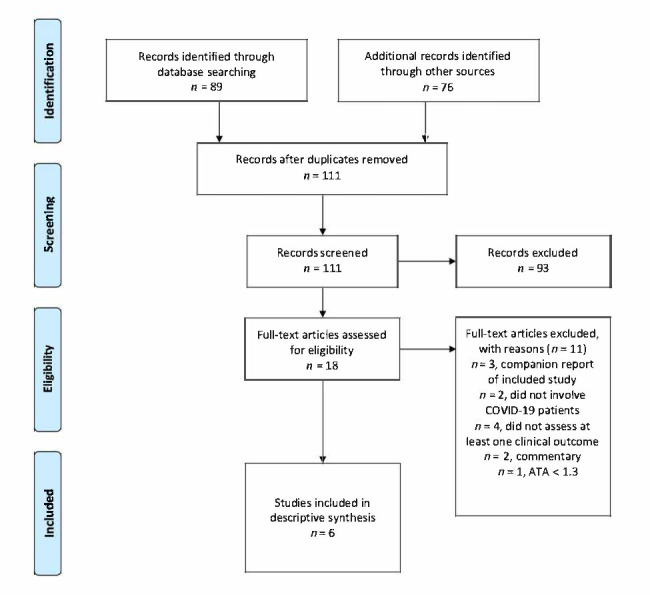
PRISMA flow diagram
STUDY AND PATIENT CHARACTERISTICS
Details of included study and patient characteristics are provided in Table 1. Three studies were case series[ 25 - 27] and two studies involved case reports of one[ 28] or two[ 29] patients. One study was a cohort design with propensity-matched controls, where 20 COVID-19 patients were treated with HBOT and compared to 60 similar patients (matched based on age, sex, body mass index, coronary artery disease, troponin, D-dimer, hospital day, and oxygen requirement) from the same hospital who received usual care (no HBOT).[ 30]
Table 1. Study characteristics; all studies were single centre. ARDS – acute respiratory distress syndrome; NR − not reported; P/F ratio − PaO2/FiO2, where PaO2 is the arterial oxygen partial pressure and FiO2 is the fraction of inhaled oxygen); SpO2 – peripheral oxygen saturation .
| Study | Study design | Inclusion criteria | Exclusions | Intervention |
| Chen[25] China | Case series, n = 5 | Progressive hypoxaemia, moderate-severe ARDS, laboratory confirmed COVID-19 | NR | Multiplace 203 kPa for one patient, 162 kPa for four patients, 60−90 min. Mean five HBOT sessions (range 3−8) |
| Gorenstein[30] USA | Cohort study with controls chosen from patients treated in the same hospital and time period using propensity score matching, n = 80 (20 intervention; 60 control) | Age ≥ 18, laboratory confirmed COVID-19, SaO2 < 93% on air | Pregnancy, pneumothorax, positive troponin | Monoplace 203 kPa, 90 min per day, once daily for five days |
| Guo[29] China | Case reports, n = 2 | Confirmed COVID-19, and one of: shortness of breath; respiratory rate ≥ 30 breaths·min-1; SpO2 ≤ 93% at rest; and P/F ratio ≤ 300 mmHg | Pneumothorax, pulmonary bullae | Monoplace, 152 kPa, 60 min, once daily for seven days |
| Qian[26] China | Prospective case series, n=4 | Progressive dyspnoea, lung CT lesion area > 30%, SpO2 < 90% on air, clear consciousness, able to communicate in words, minimal education to junior high | Pneumothorax, pulmonary bullae | Portable monoplace chamber; 152 kPa, 90 min, once daily for seven days |
| Thibodeaux[27] USA | Retrospective case series, n=5 | Impending respiratory failure, imminent intubation | NR | Type of chamber not reported. 203 kPa, 90 min. Mean five HBOT sessions (range 1−6) |
| Zhong [28] China | Case report, n = 1 | Critically ill with pneumonia and tracheal intubation, confirmed COVID-19 from tracheal aspirate | NR | Multiplace chamber, 162−182 kPa for 70–100 min. Four HBOT sessions |
Across the six included studies, 37 participants were treated with HBOT for COVID-19. All the patients treated with HBOT were hypoxaemic in room air and required (normobaric) oxygen supplementation. The COVID-19 cases treated by HBOT were from the United States of America (two publications, 25 patients) and China (four publications, 12 patients).
All patients treated with HBOT were adults (ranging from 24 to 87 years old) and 12 patients were female (32%) (Table 2). Only one study included a control group with no HBOT, and was a propensity matched design.[ 30] Only one case was intubated and mechanically ventilated while being treated with HBOT.[ 28]
Table 2. Patient demographic characteristics; #60 controls and 20 patients treated with HBOT; COPD – chronic obstructive pulmonary disease; HBOT – hyperbaric oxygen treatment; NR − not reported .
| Study | n | Female n | Age (years) | Ethnicity | Comorbidities |
| Chen[25] | 5 | 1 | Mean 47 Range 24−69 | Chinese: 5 | Hypertension: 1 Cardiovascular disease: 1 |
| Gorenstein[30] | 80# | 7 | HBOT: Median 58 Range 30−79 Control: Median 62 Range 24−80 | White: 23 Black: 13 Asian: 7 Other: 37 | Hypertension: 40 (50%) Diabetes: 24 (30%) Cardiovascular disease: 8 (10%) COPD: 4 (5%) |
| Guo[29] | 2 | 0 | 57 and 64 | Chinese: 2 | Hypertension: 1 Diabetes: 1 Cardiovascular disease: 1 |
| Qian[26] | 4 | 0 | Range 56−67 | Chinese: 4 | NR |
| Thibodeaux[27] | 5 | 4 | Median 48 Range 39−63 | White: 2 Black: 3 | Obese: 4 Hypertension: 4 Diabetes: 3 |
| Zhong [28] | 1 | 0 | 87 | Chinese | Cardiovascular disease COPD |
HBOT ranged from one to seven sessions, each session lasting between 60 minutes29 and 100 minutes[ 28] at a pressure between 152[ 26 , 29] to 203 kPa[ 25 , 27 , 30] (1.5-2.0 atm abs).
EFFECTIVENESS OF HBOT FOR COVID-19
The seven publications included clinical, biological and imaging outcomes. Cost outcomes were not reported by any of the studies. Clinical outcomes were the most frequently reported (six studies, 37 patients), followed by biological (5 studies, 17 patients), and imaging outcomes (4 studies, 11 patients). Detailed outcomes of included studies are provided in Table 3 and Table 4. Improvements in clinical (e.g., survival), biological (e.g., lymphocyte count, renal function) and imaging (e.g., chest CT) outcomes were observed for the majority of the 37 hypoxaemic COVID-19 patients across all studies (Table 3).
Table 3. Effect of HBOT on COVID-19 patient outcomes; *results that were reported as significant at P < 0.05; #60 controls and 20 patients treated with HBOT; bpm – breaths per minute; HCO3- − bicarbonate; HBOT – hyperbaric oxygen treatment; NR − not reported; P/F ratio − PaO2/FiO2, where PaO2 is the arterial oxygen partial pressure and FiO2 is the fraction of inhaled oxygen); SaO2 – arterial oxygen saturation; SpO2 – peripheral oxygen saturation .
| Study | n | Timing of outcome measurement | Clinical outcomes Data are mean (SD) | Biological outcomes Data are mean (SD) | Imaging outcomes | Safety outcomes |
| Chen[25] | 5 | Assessed before and after course of HBOT (average of five sessions per patient) | SpO2 improved: 73 (6) to 94 (2)%* | PaO2 and SaO2 increased* Lymphocyte count increased: 0.61(0.35) to 1.09 (0.24) x109·L-1* C-reactive protein levels decreased: data NR D-dimer decreased: data NR* Fibrinogen decreased: data NR* | CT improved (qualitatively) | NR |
| Gorenstein[30] | 80# | Assessed at end of study (patients received up to five daily treatments as long as supplemental oxygen still required) | Inpatient mortality: HBOT: two (10%) died, none remained hospitalised at end study. Controls: 13 (22%) died, three (5%) remained hospitalised at end study. Mechanical ventilation: HBOT: 2 (10%) Controls: 18 (30%) Adjusted hazard ratios: Inpatient mortality = 0.37 (P = 0.14); Mechanical ventilation = 0.26* | N/A | N/A | Claustrophobia, ear pain (n = NR) Hypoxic arrest in unclear circumstances after transferring to the floor (n = 1) |
| Guo[29] | 2 | Assessed over 7-day course of HBOT | Dyspnoea eliminated immediately after the first HBOT session. Respiratory rate decreased daily; no need for mechanical ventilation SpO2 > 93% after the first session and continued to improve | D-dimers reduced. Lymphocyte counts improved. PaO2, P/F ratio, HCO3-, lactate improved Liver function (cholinesterase) improved. Data NR for any outcomes | CT pulmonary inflammation gradually improved | No adverse effects |
| Thibodeaux[27] | 5 | Assessed before and after course of HBOT (average of 5 treatments per patient [range: 1−6]) | SpO2 improved: 96 (3) to 96 (1)%. All patients recovered without need for mechanical ventilation. Respiratory rate decreased: 35.4 (8.5) to 28 (7.6) bpm | Inflammatory markers decreased (reported for 1 patient, not reported for 4 patients) | N/A | No adverse effects |
| Qian[26] | 4 | Assessed before and one day after 7-day course of HBOT | SpO2 improved: 86 (5) to 92 (4)%*. Six-minute walk distance improved: 272 (62) to 346 (43) m*. Dyspnoea improved | Blood gas analysis indexes improved | CT resolution of inflammation to different degrees | NR |
| Zhong [28] | 1 | Assessed before and after course of HBOT (4 sessions) | Oxygenation improved. Patient was eventually extubated | CO2 reduced. Coagulation normalised. Kidney function improved | N/A | NR |
Table 4. Clinical outcome summary of 37 COVID-19 patients treated with hyperbaric oxygen .
| Outcome | Patients assessed (n) | Patients improved (n) |
| Avoided mechanical ventilation | 26 | 24 |
| In-hospital survival | 26 | 23 |
| Oxygen saturation | 17 | 17 |
| Respiratory rate | 7 | 7 |
| Walking distance | 4 | 4 |
| Shortness of breath | 6 | 6 |
Of the 37 included hypoxaemic COVID-19 patients treated with HBOT, the need for mechanical ventilation and in-hospital survival was reported for 26 patients across three studies (Table 4). Of these 26 patients, 24 did not require mechanical ventilation and 23 survived. Improvements in oxygen saturation (17 patients, 5 studies), respiratory rate (7 patients, 2 studies), walking distance (4 patients, 1 study), and shortness of breath (6 patients, 2 studies) were also observed (Table 4).
RISK OF BIAS AND GRADE ASSESSMENT
There was only one included comparative study for which risk of bias assessment and GRADE assessment could be conducted.[ 30] This study was deemed to be of good quality with moderate certainty in the evidence (Table 5).
Table 5. Risk of bias assessment using the Newcastle-Ottawa Scale. A maximum of 4 stars can be given in the Selection and Outcome/exposure domains and a maximum 2 stars can be given in the Comparability domain .
| Newcastle Ottawa scale for risk of bias assessment | GRADE assessment | ||||
| Selection | Comparability | Outcome/exposure | Overall quality assessment | Certainty of evidence | |
| Gorenstein[30] | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | Good | Moderate |
Discussion
This systematic review found six studies reporting on 37 hypoxaemic COVID-19 patients who were treated with HBOT. Available data from the included cohort and case studies suggest that the use of HBOT to treat COVID-19 patients may be promising and urgently requires randomised controlled trials (RCTs). These early data show that HBOT may be useful for preventing deterioration requiring intubation and mechanical ventilation in COVID-19 patients. However, since many admitted hypoxaemic COVID-19 patients will not require intubation and will survive, studies with comparative data are paramount. In addition, no serious adverse events were reported by any of the included studies. However, all studies were of poor quality and the certainty of the evidence was low or very low, with the exception of the one comparative study.[ 30] While a few RCTs were registered, none were published.
Although conclusions are limited by study quality and risk of bias, the positive clinical outcomes observed by the included studies are supported by concordant biological and radiological data. Findings of effectiveness across multiple outcome categories are also supported by a compelling physiological rationale. While HBOT was first considered for treating hypoxaemic COVID-19 patients to quickly correct their tissue hypoxia, more evidence suggests that the immunomodulatory effect of HBOT may also be relevant.[ 14 , 15] COVID-19 is increasingly considered as an endothelial disease,[ 31 , 32] which provides a unifying pathophysiological picture. This endothelial dysfunction explains both the range of symptoms experienced by COVID-19 patients (e.g., thrombotic events, neurologic manifestations), and why patients with pre-existing impaired endothelial function (e.g., age, diabetes, cardiovascular disease) are also more at risk for severe COVID-19. Evidence suggests that endothelial cells contribute to the initiation and propagation of pneumonia by altering vessel barrier integrity, promoting a pro-coagulative state, and inducing vascular inflammation.[ 33] The anti-inflammatory effect of HBOT may counteract the inflammation of the endothelium caused by COVID-19 and prevent a cytokine storm leading to multi-organ dysfunction and death. Accordingly, positive findings for both safety and effectiveness across each of the included studies conducted in diverse locations, combined with the pathophysiological mechanisms, suggest an urgent need for rigorous RCTs to fully assess the effectiveness of HBOT for hypoxaemic patients.
There are currently eight registered RCTs that aim to test the effectiveness of HBOT as a treatment for COVID-19. Of these, two are completed (results not yet available),[ 34 , 35] two are recruiting,[ 36 , 37] and four are not yet recruiting.[ 38 - 41] This suggests the need for a living systematic review to continue to inform practice throughout the pandemic. Our present review represents an initial step toward this end. One of the strengths of our systematic review is its early summary of the current evidence for a safe non-invasive therapy that could potentially improve mortality and morbidity in severe COVID-19 patients. Given the pandemic context, patients and clinicians need to access current evidence with no delay. Our study may be helpful to clinicians, decision makers and patients to make an evidence-based decision when contemplating use of HBOT for hypoxaemic COVID-19 patients. Although the studies included in our review were mostly case series, this does not negate the potential of these studies to promote a broader understanding of COVID-19 treatments and outcomes. It may also assist clinicians in making a decision regarding the compassionate use of HBOT in the context of COVID-19 until results from RCTs are available.
It should be acknowledged that there may be more evidence that was not captured in this review. This may be due to the fast-moving nature of the pandemic, the delay of the peer-review system, indexation of publications in databases, and the possible publication of cases in non-peer-reviewed media. Only one study was of good quality with a moderate level of certainty in the evidence, and that study reported mortality and mechanical ventilation outcomes.
Finally, no cost data were reported in any of the included studies. However, if HBOT can prevent intubation, mechanical ventilation, and intensive care admission for hypoxaemic COVID-19 patients, then it is likely to be cost-effective. If the positive outcomes identified by our systematic review are confirmed by RCTs, the prompt use of mobile or portable chambers may be part of the solution to fight the current pandemic and avoid overwhelming the limited critical care resource at hospitals.
Conclusions
Limited and weak evidence suggests that HBOT may be a safe and promising intervention to improve COVID-19, including prevention of intubation and death in hypoxaemic COVID-19 patients. Studies were mostly of poor quality and the certainty of the evidence was low or very low. This systematic review supports the urgent need for a large-scale clinical trial to provide a rigorous level of evidence that could guide practice during the COVID-19 pandemic.
Supplementary Material
Footnotes
Conflict of interest and funding
No conflicts of interest were declared. Dr Tricco was funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. Dr Boet was supported by The Ottawa Hospital Anesthesia Alternate Funds Association and the Faculty of Medicine, University of Ottawa with a Tier 2 Clinical Research Chair.
Contributor Information
Sylvain Boet, Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Canada; Francophone Affairs, Faculty of Medicine, University of Ottawa, Ottawa, Canada.
Cole Etherington, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Canada.
George Djaiani, Department of Anesthesia and Pain Management, University Health Network, Toronto, Canada.
Andrea C Tricco, Knowledge Translation Program, Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Unity Health Toronto, Toronto, Canada; Epidemiology Department and Institute for Health Policy, Management and Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Lindsey Sikora, Health Sciences Library, University of Ottawa, Ottawa, Canada.
Rita Katznelson, Department of Anesthesia and Pain Management, University Health Network, Toronto, Canada.
References
- World Health Organization . WHO Coronavirus Disease (COVID-19) Dashboard; c2021. [cited 2021 May 19]. Available from: https://covid19.who.int/.
- Chinese Center for Disease Control and Prevention . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020; 41: 145- 51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Guan W-J, Ni Z-Y, Hu Y, Liang Y, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institute for Health Information . COVID-19 hospitalization and emergency department statistics; c2021. [cited 2021 May 19]. Available from: https://www.cihi.ca/en/covid-19-hospitalization-and-emergency-department-statistics.
- Dequin PF, Heming N, Meziani F, Plantefève G, Voirot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324(13):1298–306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santus P, Radovanovic D, Saderi L, Marino P, Cogliati C, De Filippis G, et al. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: A prospective observational multicentre study. BMJ Open. 2010;10(10):e043651. doi: 10.1136/bmjopen-2020-043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, So C, Shum HP, Chan PKS, Lai CKC, Kandamby DH, et al. Critically ill patients with COVID-19 in Hong Kong: A multicentre retrospective observational cohort study. Crit Care Resusc. 2020;22:119–25. doi: 10.51893/2020.2.oa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayya D, Neill OJO, Feiertag TD, Tuazon-Boer R, Sullivan B, Perez L, et al. The use of oxygen hoods in patients failing on conventional high-flow oxygen delivery systems, the effects of oxygenation, mechanical ventilation and mortality rates in hypoxic patients with COVID-19. A prospective controlled cohort study. Respir Med. 2021;179:106312. doi: 10.1016/j.rmed.2021.106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L, editor. Hyperbaric oxygen therapy indications. 14th ed. Palm Beach (FL): Undersea and Hyperbaric Medical Society; 2019. [Google Scholar]
- Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: Retrospective analysis of 62,614 treatment sessions. Undersea Hyperb Med. 2016;43:21–8. [PubMed] [Google Scholar]
- Blatteau J, Coulange M, Parmentier-Decrucq E, Poussard J, Louge P, de Mastre S, et al. Oxygénothérapie hyperbare, principes et indications. EMC-Anesthésie-Réanimation. 2019;45(4):1–18. doi: 10.1016/S0246-0289(19)83082-7. [DOI] [Google Scholar]
- Feldmeier JJ, Kirby JP, Buckey JC, Denham DW, Evangelista JS, Gelly HB, et al. Physiologic and biochemical rationale for treating COVID-19 patients with hyperbaric oxygen. Undersea Hyperb Med. 2021;48:1–12. [PubMed] [Google Scholar]
- Boet S, Martin L, Cheng-boivin O, Etherington N, Louge P, Pignel R, et al. Can preventive hyperbaric oxygen therapy optimise surgical outcome?: A systematic review of randomised controlled trials. Eur J Anaesthesiol. 2020;37:636–48. doi: 10.1097/EJA.0000000000001219. [DOI] [PubMed] [Google Scholar]
- Shinomiya N, Asai Y. Hyperbaric oxygenation therapy: Molecular mechanisms and clinical applications. Singapore; Springer Nature: 2019. [Google Scholar]
- Cummings M, Baldwin M, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–70. doi: 10.1101/2020.04.15.20067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin KJ, Barton GP, Braun RK, Eldridge MW. Effect of intermittent hyperoxia on stem cell mobilization and cytokine expression. Med Gas Res. 2019;9(3):139–44. doi: 10.4103/2045-9912.266989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41:653–64. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh MA. HIV: reactive oxygen species, enveloped viruses and hyperbaric oxygen. Med Hypotheses. 2000;55:232–8. doi: 10.1054/mehy.2000.1048. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffman TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; c2021. [cited 2021 May 19]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Guyatt G, Oxman AD, Akl EA, Akl R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhong X, Tang Y, Liang Y, Li B, Tao X. The Outcomes of hyperbaric oxygen therapy to severe and critically ill patients with COVID-19 pneumonia; c2020. [cited 2021 May 19]. Available from: https://oxycamaras.com.br/wp-content/uploads/2020/04/Outcome-of-HBOT-to-COVID19.pdf.
- Qian Z, Hai-Xia W, Li-Ying Y, Ying X, Yi C. Infection control of coronavirus disease 2019 patients receiving hyperbaric oxygen therapy in mobile single air compression chamber. Acad J Second Mil Med Univ. 2020;41:628–32. World Health Organization ID: covidwho-743074. [Google Scholar]
- Thibodeaux K, Speyrer M, Raza A, Yaakov R, Serena TE. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: A retrospective case series. J Wound Care. 2020;29:S4–S8. doi: 10.12968/jowc.2020.29.Sup5a.S4. [DOI] [PubMed] [Google Scholar]
- Zhong X, Chen R, Niu X, Tao X, Liang Y, Tang Y. Hyperbaric oxygen therapy in an elderly critical coronavirus disease 2019 patient with endotracheal intubation: clinical effect analysis. Acad J Second Mil Med Univ. 2020;41:621–7. World Health Organization ID: covidwho-727547. [Google Scholar]
- Guo D, Pan S, Wang MM, Guo Y. Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID-2019 pneumonia: two case reports. Undersea Hyperb Med. 2020;47:181–7. [PubMed] [Google Scholar]
- Gorenstein SA, Castellano ML, Slone ES, Gillette B, Liu H, Alsomarraie C, et al. Hyperbaric oxygen therapy for covid-19 patients with respiratory distress: Treated cases versus propensity-matched controls. Undersea Hyperb Med. 2020;47:405–13. [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Habrecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–44. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: The vasculature unleashed. Nat Rev Immunol. 2020;20:389–91. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadanny A. Hyperbaric oxygen therapy effect in COVID-19 RCT; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04358926.
- Duarte M Jorda-Vargas L Verdini F Hyperbaric Oxygen as an adjuvant treatment for patients with COVID-19 severe hypoxemia; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04477954.
- Kjellberg A Lindholm P Rodriguez-Wallberg K Safety and efficacy of hyperbaric oxygen for ARDS in patients with COVID-19; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04327505.
- Blatteau JE. Management by hyperbaric oxygen therapy of patients with hypoxaemic pneumonia with SARS-CoV-2 (COVID-19); c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04344431.
- Engle J. Hyperbaric oxygen therapy (HBOT) as a treatment for COVID-19 infection; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04343183.
- Boet S. Hyperbaric versus normobaric oxygen therapy for COVID-19 patients; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04500626. [Google Scholar]
- Huang E, Lee D. Hyperbaric oxygen for COVID-19 patients with moderate to severe respiratory distress; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04619719.
- Wiesel O. Hyperbaric oxygen therapy in non-ventilated COVID-19 patient; c2021. [cited 2021 May 19]. Available from: https://clinicaltrials.gov/ct2/show/NCT04409886. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


