Abstract
Coronary artery fistula (CAF) in adults is a rare but significant coronary artery anomaly. Main data on that rare disease were mostly obtained from case reports and small studies. In presented study, we share our two-decade experience on the clinical and angiographic characteristics of CAF.
The data were collected retrospectively by analyzing the angiographic data between January 1, 2000 and December 31, 2019. Demographic data, clinical data, laboratory, and cardiac catheterization reports were reviewed.
CAFs were found in 40 patients (0.06%). There were 22 male (55%) patients. The mean age was 61.2 years. Twenty-nine patients (72.5%) had small, 4 patients (10%) had medium, and 7 patients (17.5%) had large CAFs. The majority of study population had solitary CAF ( n = 31, 77.5%). The pulmonary artery is the major side of fistula drainage ( n = 20, 50%). The study population was divided into two groups as follow: group 1—small CAFs 29 (72.5%), group 2—medium and large CAF (MLCAF) 11 (27.5%). Patients with MLCAFs had more atrial fibrillation, abnormal coronary morphology, and multiple fistulae. In patients with hemodynamically significant CAFs, 7 (17.5%) patients had surgical ligation and 3 (7.5%) patients had transcutaneous closure. Three patients died during mean follow-up period of 5 years.
The incidence and the pattern of CAFs in our study were similar to previous studies. Clinical course of small fistulae was benign. Symptomatic MLCAFs need to be treated by transcatheter or surgical way and should be individualized per patient.
Keywords: cardiac catheterization, coronary artery, fistula, coronary intervention, cardiac surgery
Coronary artery fistula (CAF) is rare but important coronary anomaly. It was first described by Krause in 1865. 1 These involve abnormal communication between one or more coronary arteries with either one of the cardiac chambers (CAF) or the great vessels adjacent to the heart (coronary artery or arteriovenous fistulae) without an interposed capillary bed. 2 3 4 5 While CAFs are present in only 0.002% of the general population, they are detected in 0.3 to 0.8% of the patients undergoing diagnostic cardiac catheterization. 5 6 7 8 9 Interestingly, CAFs account for 48.7% of all congenital coronary anomalies. 10 Due to their rarity, the natural history of CAFs remains unclear. Acquired forms due to trauma and iatrogenic causes have been reported. 5 11 12 Many CAFs are incidental findings on conventional coronary angiography. Advanced cardiac imaging modalities including cardiac magnetic resonance (MR) and cardiac computed tomography (CT) provide additional anatomic and morphologic information of CAF. 2 5 6 10 13 14 Due to the rarity of CAFs, their natural history remains unclear. The hemodynamic consequences of the fistula vary, depending on shunt size, location, and presence of other underlying cardiac disorders. 4 5 15 16 Management of CAFs is controversial, and recommendations are based on case reports and small retrospective series. 4 5 16 17 Unfortunately, data regarding angiographic characteristics of these patients is limited.
In this study, we report our institution's 20-year experience of with CAFs, and detail their prevalence, clinical course, angiographic feature, and prognosis.
Materials and Methods
We retrospectively reviewed 65,686 consecutive coronary angiograms performed between January 1, 2000 and December 31, 2019 at two MercyOne-Iowa Heart hospitals: MercyOne in Des Moines, Iowa and MercyOne in West Des Moines, Iowa. A total of 40 cases of CAF were identified on angiography by board certified interventional cardiologists. The following definitions were applied: CAFs were described according to their origin and drainage sites. “Solitary fistula” describes cases in which the CAF originates from a single vessel and has a single termination. “Complex fistulae” are characterized by plexiform variants and multiple fistulae. “Coronary aneurysm” is defined as localized dilation of a portion or diffuse segment of the coronary artery, and has a diameter of more than 1.5 times the adjacent normal coronary artery. “Coronary dilatation” refers to an increase in coronary artery diameter insufficient to meet criterion for aneurysm. A fistula size is defined in reference to the size of the distal reference vessel, being “large” if its diameter is more than twice its size, medium if one to two times its diameter, and small when its diameter is narrower than the distal reference vessel. 5 Patient's fistula size was measured by the interventional cardiologist the time of the patient's study. We extracted demographic, clinical, and laboratory data from medical records. Cardiac catheterization, electrocardiogram, and echocardiogram imaging and reports were reviewed in detail.
The study population was divided into two groups based on the size of CAF. Group 1 had small CAFs (SCAF), and group 2 had medium or large CAFs (MLCAFs). Statistical analysis was performed utilizing SPSS (IBM, Version 25). We calculated mean ± standard deviation for continuous baseline demographic, clinical, and laboratory variables. For categorical data, relative frequencies were determined. Student's t -test was utilized when comparing groups of demographic and clinical variables. Similarly, proportions were compared by means of a Fisher's exact test.
The study received approval from the Institutional Review Board, and was conducted in accordance with institutional guidelines.
Results
Video 1 ( A ) Right anterior oblique (RAO) cranial view of the left coronary artery showed distal left anterior descending to left ventricle small coronary cameral fistula. ( B ) RAO cranial view of right coronary artery (RCA) showed RCA AM distal small coronary cameral fistula.
Video 2 ( A ) RAO caudal view of left coronary artery showed aneurysmal dilatation of D2 along with small D2 to pulmonary artery fistula. ( B ) Left anterior oblique view of right coronary artery (RCA) showed mid-size proximal RCA conus branch artery to pulmonary artery fistula.
Video 3 ( A ) Left anterior oblique (LAO) view of right coronary artery (RCA) showed large coronary artery fistula between RCA proximal to bilateral pulmonary artery. ( B ) LAO view of RCA showed complete closure of coronary artery fistula after three Terumo Auzor CX18 detachable coils placement.
Video 4 ( A ) Right anterior oblique (RAO) caudal view showed large aneurysmal dilatation of left circumflex artery (LCX) and large LCX to coronary sinus fistula. ( B ) RAO caudal view showed delivered Amplatzer duct occluder to large LCX to coronary sinus fistula. ( C ) RAO caudal view showed closed large LCX to coronary sinus fistula by Amplatzer duct occluder with small residual flow.
Video 5 ( A ) Right anterior oblique (RAO) caudal view showed large ectatic left circumflex artery (LCX), and large LCX to coronary sinus fistula. ( B ) RAO caudal view showed cardiac catheter injection from coronary sinus which showed large fistula and severely tortuous LCX.
Among 65,686 consecutive cardiac catheterizations, 40 (0.06%) unique cases of CAFs were identified in 22 males and 18 females. The mean age was 61.2 ± 14.5 years (range: 20–88 years). Thirty-nine patients had congenital CAF, and one patient had postcoronary artery bypass graft surgery (CABG) CAF. Three patients had associated congenital heart disease, including bicuspid aortic valve, myocardial bridging, interatrial septal aneurysm, and persistent left superior vena cava. Common noncardiac comorbidities among CAF patients were hypertension (60%), diabetes mellitus (15%), and chronic kidney disease (15%). Prior or current history of cigarette smoking was reported in 50% of patients ( Table 1 ).
Table 1. Baseline characteristics and clinical data.
| Variables | Entire cohort, N = 40, n (%) |
|---|---|
| Age mean (min-max) | 61.2 ± 14.5 (20–88) |
| Gender (male) | 22 (55%) |
| HTN | 24 (60%) |
| DM | 6 (15%) |
| CKD | 6 (15%) |
| CHF | 4 (10%) |
| HLD | 32 (80%) |
| Smoker | 20 (50%) |
| Clinical presentation | |
| SAP | 12 (30%) |
| ACS | 23 (57.5%) |
| Heart failure | 3 (7.5%) |
| Other | 2 (5%) |
| Cardiac stress testing | |
| Myocardial perfusion SPECT | 12 (30%) |
| Abnormal | 7 (17.5) |
| Normal | 5 (20%) |
| Stress echocardiography | 2 (5%) |
| Abnormal | 2 (5%) |
| Treadmill stress test | 1 (2.5%) |
| Abnormal | 1 (2.5%) |
| Etiology of CAF | |
| Congenital | 39 (97.5%) |
| Acquired | 1 (2.5%) |
| Accompanied CHD | |
| None | 37 (92.5%) |
| BAV, myocardial bridge | 1 (2.5%) |
| Interatrial septal aneurysm | 1 (2.5%) |
| Persistent left superior vena cava | 1 (2.5%) |
| Transthoracic Echocardiogram, LVEF (%) | 57.7 ± 10 |
| ECG | |
| Sinus rhythm | 35 (87.5%) |
| Atrial fibrillation | 5 (12.5%) |
Abbreviations: ACS, acute coronary syndrome; BAV, bicuspid aortic valve; CAF, coronary artery fistula; CHD, congenital heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus, ECG, electrocardiography; HLD, hyperlipidemia, HTN, hypertension; LVEF, left ventricular ejection fraction; SAP, stable angina pectoris, SPECT, single photon emission computed tomography.
The main presenting symptom was chest pain. Twenty-three patients (57.5%) had undergone angiography for acute coronary syndrome, and the balance had undergone angiography for stable angina pectoris or heart failure ( Table 1 ). Electrocardiogram analysis showed no abnormal waveform to suggest congenital conduction abnormality. Rhythm analysis detected five patients with atrial fibrillation, with the remaining in sinus rhythm. The mean left ventricular ejection fraction was 57% on two-dimensional echocardiogram ( Table1 ).
Twelve patients had myocardial perfusion single-photon emission computed tomography (SPECT) study before coronary angiogram, of which seven patients had normal and five had abnormal myocardial perfusion ( Table 1 ). Three patients underwent myocardial perfusion SPECT after being incidental diagnose of CAF, and the myocardial perfusion SPECT was unremarkable in all three of these patients. Two patients had abnormal stress echocardiography before coronary angiogram, and three patients had normal stress echocardiography after incidental diagnosis of CAF ( Table 1 ). There was one patient with an abnormal treadmill stress test before coronary angiography.
Angiographic data showed 29 patients (72.5%) had small, 4 (10%) had medium, and 7 (17.5%) had large hemodynamically significant CAFs ( Figs 1 2 3 4 5 ) ( Videos 1 2 3 4 5 ).
Fig. 1.
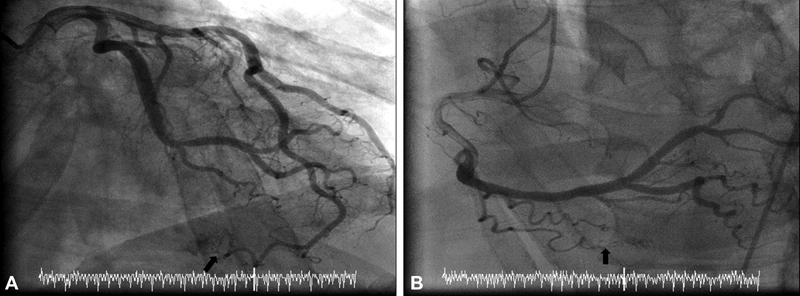
A 72-year-old male with chest pain and unequivocal stress test. Coronary angiogram showed multiple small coronary artery fistula. He was managed medically and did well during follow-up. ( A ) Right anterior oblique (RAO) cranial view of the left coronary artery showed distal left anterior descending to left ventricle small coronary cameral fistula (black arrow) ( B ) RAO cranial view of right coronary artery (RCA) showed RCA AM distal small coronary cameral fistula (black arrow).
Fig. 2.
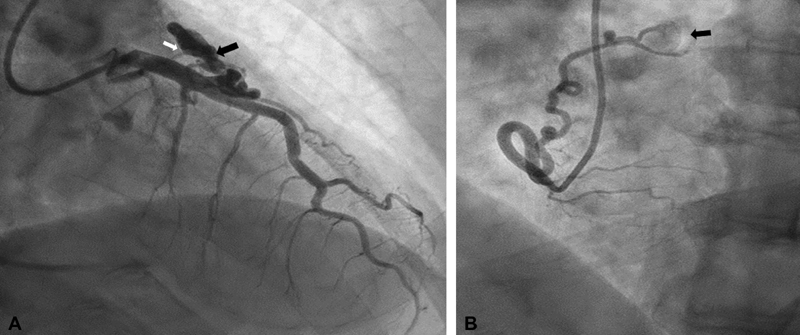
A 68-year-old male presented with inferior ST elevation myocardial infarction. He had coronary artery aneurysm with significant thrombus in right coronary artery (RCA) proximal. He had cover stent placed to proximal RCA. He was found to have two coronary artery fistulae. His stress test was negative. Cardiac computed tomography confirms the size and location of fistula. He was managed medically and did well in follow-up. ( A ) Right anterior oblique caudal view of left coronary artery showed aneurysmal dilatation of D2 (black arrow) along with small D2 to pulmonary artery fistula (white arrow). ( B ) Left anterior oblique view of RCA showed mid-size proximal RCA conus branch artery to pulmonary artery fistula (black arrow).
Fig. 3.
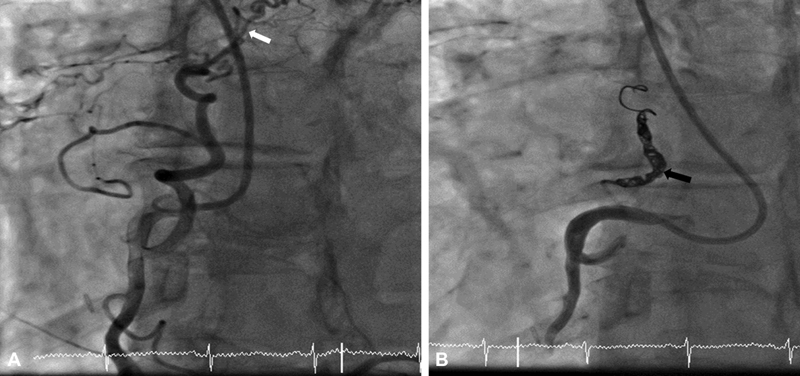
A 70-year-old female presented with atrial fibrillation and symptomatic mid-range heart failure. During coronary angiogram, she was found to have coronary artery fistulae. The coronary artery fistula was closed with transcatheter technique by using Terumo Auzor detachable coils. Patient clinically improved. ( A ) Left anterior oblique (LAO) view of right coronary artery (RCA) showed large coronary artery fistula between RCA proximal to bilateral pulmonary artery (white arrow). ( B ) LAO view of right coronary artery (RCA) showed complete closure of coronary artery fistula after three Terumo Auzor CX18 detachable coils placement (black arrow).
Fig. 4.
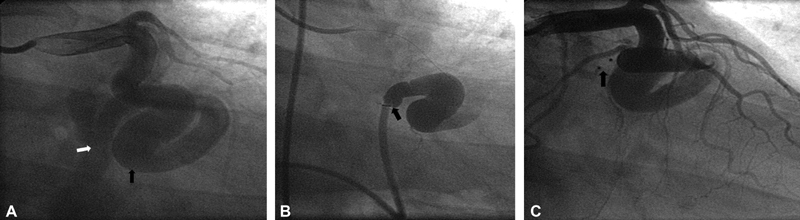
A 58-year-old man presented for exertional chest pain and dyspnea. Echocardiogram showed dilated tortuous left subclavian vein, persistent left superior vena cava, and a possible coronary arterial fistula. Coronary angiogram showed large aneurysmal left circumflex artery (LCX) with large LCX to coronary sinus fistula. Coronary artery fistula was closed with transcatheter techniques by using Amplatzer duct occluder. There was small and clinically insignificant flow observe after the procedure. ( A ) Right anterior oblique (RAO) caudal view showed large aneurysmal dilatation of LCX (black arrow) and large LCX to coronary sinus fistula (white arrow). ( B ): RAO caudal view showed cardiac catheter at ostium of coronary artery fistula between LCX and coronary sinus (black arrow). ( C ) RAO caudal view showed closed large LCX to coronary sinus fistula by Amplatzer duct occluder.
Fig. 5.
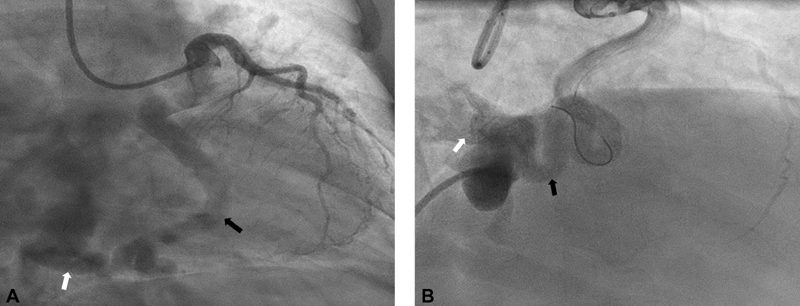
A 60-year-old female presented with chest pain. Cardiac computed tomography showed large left circumflex artery (LCX) to coronary sinus fistula. Coronary angiogram confirmed and gave more detail information about fistula. Due to severe LCX tortuosity patient had surgical correction. She recovered well and continue to improve clinically. ( A ) Right anterior oblique (RAO) caudal view showed large ectatic LCX (black arrow) and large LCX to coronary sinus fistula (white arrow). ( B ) RAO caudal view showed cardiac catheter at ostium of coronary artery fistula between coronary sinus to LCX (white arrow). There was severe tortuosity in LCX (white arrow).
The vast majority were solitary fistulae ( n = 31, 77.5%), and originated from the left anterior descending artery (LAD) 11 (32.5%), right coronary artery (RCA 11) (27.5%), and left circumflex artery 7 (17.5%). Of the nine patients (22.5%) had multiple CAFs ( Table 2 ). The majority of patients with multiple CAFs had origins in the LAD and RCA and drained into the pulmonary artery (PA) ( Figs. 2 and 3 ) ( Video 2A, B , 3A, B ). The PA was the major site of fistula drainage among all patients ( n = 20, 50%), followed by the left ventricle, coronary sinus, pulmonary vein, and right heart ( Table 2 ).
Table 2. Angiographic data.
| Variables | Entire cohort N = 40, n (%) |
|---|---|
| Size of CAF | |
| Small | 29 (72.5%) |
| Medium | 4 (10%) |
| Large | 7 (17.5%) |
| CAF origin | |
| Solitary | 31 (77.5%) |
| LAD | 13 (32.5%) |
| RCA | 11 (27.5%) |
| LCX | 7 (17.5%) |
| Multiple/complex | 9 (22.5%) |
| CAF termination | |
| PA | 20 (50%) |
| LV | 9 (22.5%) |
| Coronary sinus | 5 (12.5%) |
| Pulmonary vein, LA | 2 (5%) |
| RA, RV | 1 (2.5%) |
| Other | 3 (7.5%) |
| Angiography result | |
| Normal coronary | 8 (20%) |
| Nonobstructive | 18 (45%) |
| Obstructive | 14 (35%) |
| RCA dominant | 36 (90%) |
| LCX dominant | 2 (5%) |
| Codominant | 2 (5%) |
| Coronary artery morphology | |
| Normal | 24 (60%) |
| Dilatation | 8 (20%) |
| Aneurysm | 8 (20%) |
| CAF therapy | |
| Medical | 30 (75%) |
| Transcutaneous | 3 (7.5%) |
| Surgical | 7 (17.5%) |
| CAF prognosis | |
| Mean follow-up (year) | 5.8 ± 4.1 |
| Mortality | |
| Cardiac | 1 (2.5%) |
| Noncardiac | 2 (5%) |
| Alive | 35 (87.5%) |
| Lost follow-up | 2 (5%) |
Abbreviations: CAF, coronary artery fistula; LAD, left anterior descending artery; LCX, left circumflex artery; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RCA, right coronary artery; RV, right ventricle.
Normal coronary morphology was observed in 24 (60%) patients, while eight patients (20%) were found to have a dilated coronary artery, and another eight (20%) had coronary artery aneurysm ( Table 2 ). Fourteen patients (35%) had obstructive coronary artery disease (CAD), ten of which required revascularization (percutaneous coronary intervention n = 8, CABG n = 2).
There were 29 patients (72.5%) with SCAFs, whereas 11 (27.5%) had MLCAF, group 2. There were no significant difference in demographic variables between these two groups, including age, gender, hypertension, diabetes mellitus, hyperlipidemia, and smoking. However, presence of abnormal coronary morphology was statistically significantly greater in patients with MLCAF ( p = 0.04). There was a trend toward increased incidence of atrial fibrillation and multiple fistulae in patients with MLCAF; however, these were not statistically significant, so possibly because of the small study size. MLCAF were prone to drain the coronary sinus ( Table 3 ). Conservative medical management was applied in 30 patients ( Figs 1A,B , 2A,B ) ( Videos 1A,B , 2A,B ). In patients with hemodynamically significant CAFs, 7 (17.5%) had surgical ligation and 3 (7.5%) had transcutaneous closure (TCC). TCC of CAFs was performed utilizing a variety of techniques and devices, including Terumo auzor, Amplatzer patent ductus arteriosus (PDA) closure, and tornado coil ( Figs. 3A,B , 4A,B,C ) ( Videos 3A,B , 4A,B,C ). One patient was planned to do TCC but due to severe tortuosity of fistula plan had to change to surgical correction ( Figs. 5A,B ) ( Video 5A,B ).
Table 3. Comparison of small- (SCAF) versus medium-to-large CAF (MLCAF) groups clinical characteristic and angiographic results.
| Variable | SCAF N = 29, n (%) |
MLCAF N = 11, n (%) | p -Value |
|---|---|---|---|
| Gender, male | 14 (48%) | 8 (72%) | NS |
| Age | 62 ± 13 | 59 ± 16 | NS |
| HTN | 19 (70%) | 6 (54%) | NS |
| Low LVEF <50% | 3 (11.5%) | 1(9.1%) | NS |
| DM | 5 (17.9%) | 1 (9.1%) | NS |
| HLD | 23 (79%) | 9 (90%) | NS |
| Smoking | 14 (48.3%) | 6 (54.5%) | NS |
| ECG, atrial fibrillation | 2 (6.9%) | 3 (27.3%) | 0.08 |
| Echocardiography, LVEF % | 58 ± 10 | 54 ± 12 | NS |
| Coronary angiogram | NS | ||
| Nonobstructive CAD | 20 (69%) | 6 (55%) | |
| Obstructive CAD | 9 (31%) | 5(45%) | |
| Coronary morphology | 0.04 | ||
| Normal | 20 (69%) | 4 (36.4%) | |
| Dilatation | 6 (20%) | 2 (18.2%) | |
| Aneurysmal | 3 (10%) | 5 (45.5%) | |
| Number of fistulas | NS | ||
| Solitary | 24 (89%) | 7 (64%) | |
| Multiple | 5 (17%) | 4 (36%) | |
| Fistula originate | NS | ||
| LAD | 12(41%) | 1 (11%) | |
| LCX | 4 (14%) | 3 (27%) | |
| RCA | 8 (27%) | 3 (27%) | |
| Others | 5 (18%) | 4 (35%) | |
| Fistula termination | 0.05 | ||
| PA | 15 (52%) | 5 (37.5%) | |
| LA/pulmonary vein | 1 (3.4%) | 2 (18.2%) | |
| LV | 9 (31%) | 0 | |
| RV | 3 (10%) | 0 | |
| Coronary sinus | 1 (3.4%) | 4 (36.4%) | |
| Mortality | 2 (7.1%) | 1 (10%) | NS |
Abbreviations: CAF, coronary artery fistula; DM, diabetes mellitus; ECG, electrocardiography; HLD, hyperlipidemia; HTN, hypertension; LAD, left anterior descending artery; LCX, left circumflex artery; LV, left ventricle; LVEF, left ventricular ejection fraction; NS, not significant; PA, pulmonary artery; RA, right atrium; RCA, right coronary artery; RV, right ventricle; SAP, stable angina pectoris.
No complications of CAF spontaneous rupture, endocarditis, pulmonary embolus, or tamponade were reported, nor was there any mortality difference between the groups over the 5-year follow-up period ( Table 3 ). Two patients in the SCAF group died, one in hospital following surgery for mitral and tricuspid valve along with ligation of a SCAF. The other patient's demise was related to lung cancer, which was found 7 years after diagnosis of SCAF. One patient with large CAF died in hospital after CABG and fistula ligation.
Cardiac CT was done for five patients to better define the anatomy and morphology of the CAF and comorbid structural disease.
While two patients were lost to contact, the rest of the patients had average of 5.8 years of follow-up, with event-free survival rate of 87.5%.
Discussion
The prevalence of CAF in our study population was 0.06%, which was slightly lower than reported in the other study series. 7 8 14 15 18 19 The vast majority of CAFs in this study were congenital. 5 9 12 Previous CAF studies, including pediatric cases, found ∼20% of patients had coexisting congenital heart disease. Our data presented here was limited to adult patients that may account for the fact that only two patients (5%) were found to have congenital heart disease ( Table 1 ). 8 9 20
There was no gender predominance in our study population. 8 9 21 Around 40% of our study population had abnormal coronary morphology. Not surprisingly, the MLCAF group had a relatively high prevalence (63%) of abnormal coronary morphology. It is believed that morphologic abnormality of the coronary vessels was probably the result of congenital malformation in addition to a high prevalence of cardiovascular risk factors ( Tables 1 , 2 ).
Consistent with previous studies on CAFs, the majority of the CAFs in our patients originated from the LAD. 7 15 19 20 21 In present study, CAFs most frequently drained into the PA and left ventricle. While former studies found right heart drainage of fistulas to be more common, several recent studies have shown drainage into the PA and left ventricle to be more common, as supported by our data ( Table 3 ). 8 20
CAFs usually remain asymptomatic. 6 13 15 19 Fistulae resulting in left to right shunts can lead to pulmonary hypertension and/or biventricular volume overload. Large fistulae connecting a coronary artery to the left heart can result in left ventricular volume overload and heart failure. 5 9 21 22 CAF can also cause disturbances in coronary hemodynamics. The low pressure sink of the fistula results in preferential flow through it, and can produce a coronary steal phenomenon. 5 22
The natural course of CAF can be complicated by infection, thrombosis, embolism, and dissection. 5 9 21 While we did not see any of these rare complications, ischemia and heart failure were two common clinical presentations in our study. 5 8 13 16 Arrhythmias can occur in CAF patients due to cardiac chamber enlargement and altered hemodynamics. 5 13 16 The most commonly seen arrhythmia is atrial fibrillation like seen in our study.
History and physical, electrocardiography, chest radiograph, and echocardiography need to be done in patients with suspicion of CAF. Cardiac stress tests are useful in patients with chest pain, and also very useful to evaluate the hemodynamic significance of incidentally noted CAFs. Conventional coronary angiogram has been the gold standard diagnostic tool. 2 5 7 8 15 19 21 Cardiac MR and CT coronary angiography are newer, advanced, and noninvasive imaging techniques for the detection of major coronary artery anomalies including CAFs. 5 9 13 16
There is no medical therapy proven to slow the progression of CAF. SCAFs are not clearly associated with significant long-term complications. In our study, two patients died in SCAF group from non-CAF-related causes. SCAFs can be ligated if patient gets cardiac surgery for other coexisting cardiac pathology because of the previously mentioned complication risks. Guideline recommendations for asymptomatic, small or small-to-medium CAFs were to conservative management and follow-up with echocardiography every 3 to 5 years. 4 9 16 23
Medium and large fistulas have the potential to cause significant long-term complications, including angina, arrhythmia, endocarditis, and heart failure. The 2008 American College of Cardiology/American Heart Association (ACC/AHA) adult congenital heart disease management guidelines give a Class I recommendation for closure of large CAFs, regardless of symptom status, by transcatheter or surgical route. 4 Additional Class I recommendation of closure of mild-to-moderate CAF is given in the settings of myocardial ischemia, arrhythmia, heart failure, or endarteritis. 4 Two of our patients with incidentally diagnosed medium CAFs were asymptomatic and had negative cardiac stress tests. They were managed conservatively, and did well over the 3-year follow-up.
Transcatheter approaches are often feasible, especially if the fistulous communication arises from the proximal aspect of a coronary artery. 8 9 23 24 Three out of four study patients had successful TCC, while one patient was deferred to surgery due to severe tortuosity. The patient that underwent Amplatzer PDA closure device was left with a small residual flow, but patient was asymptomatic and doing well in his 9-year follow-up ( Figs. 4A,B ) ( Video 4A,B ) ). It is critical to understand the anatomy of the fistula, especially if intervention is planned via TCC. 5 16 25
Surgical closure of CAFs can be performed with a variety of techniques. 20 26 Complex CAFs with aneurysmatic change and tortuous vessels require surgical treatment ( Figs. 5A,B ) ( Video 5A,B ). 9 20 26 Here, four patients underwent surgical CAF closure due to complex and large size of CAFs. Unfortunately, two patients died soon after surgery. One from cardiac arrest and the other from respiratory and renal failure. While surgical closure remains the standard therapy for symptomatic or large CAF, it is important to consider the patients surgical risk as well as risk for complications during patient selection. 9 20 26 Patients with symptomatic large coronary fistulae and high surgical mortality risk should be reconsidered for TCC therapy.
Limitations of the Study
Despite presenting one of the largest series of CAF cases, the number of study patients remains relatively small because adult coronary arteriovenous fistulae are rare. Due to retrospective nature of study, we could not obtain complete data from all study subjects. Unfortunately, the follow-up period was relatively short and two patients were lost to follow-up.
Conclusions
CAF is a rare congenital coronary abnormality, and conventional coronary angiogram is the gold standard for diagnosis and treatment. In our study, the left coronary system was the most common origin of fistulae, with a significant portion of those fistulae draining into the PA or left ventricle. In light of the good prognosis and risk of complications associated with interventional techniques, patients with small and small-to-medium size fistula can be managed conservatively. Large and symptomatic fistulae require intervention, either by surgical repair or via transcatheter techniques. The type of intervention should be individualized based on patient factors, surgical risk, and the anatomy of the CAF.
Funding Statement
Funding This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interest The all authors have no conflict of interest.
References
- 1.W. K. Uber den ursprung einer akzessorischen A. coronaria aus der a. pulmonalis. Z Ratl Med. 1865;24(24):225–227. [Google Scholar]
- 2.Ata Y, Turk T, Bicer M, Yalcin M, Ata F, Yavuz S. Coronary arteriovenous fistulas in the adults: natural history and management strategies. J Cardiothorac Surg. 2009;4:62. doi: 10.1186/1749-8090-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Challoumas D, Pericleous A, Dimitrakaki I A, Danelatos C, Dimitrakakis G. Coronary arteriovenous fistulae: a review. The Int J Angiol. 2014;23(01):1–10. doi: 10.1055/s-0033-1349162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnes C A, Williams R G, Bashore T M. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(23):e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Reddy G, Davies J E, Holmes D R, Schaff H V, Singh S P, Alli O O. Coronary artery fistulae. Circ Cardiovasc Interv. 2015;8(11):e003062. doi: 10.1161/CIRCINTERVENTIONS.115.003062. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka O, Hobbs R E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21(01):28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 7.Said S A, van der Werf T. Dutch survey of coronary artery fistulas in adults: congenital solitary fistulas. Int J Cardiol. 2006;106(03):323–332. doi: 10.1016/j.ijcard.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Valente A M, Lock J E, Gauvreau K. Predictors of long-term adverse outcomes in patients with congenital coronary artery fistulae. Circ Cardiovasc Interv. 2010;3(02):134–139. doi: 10.1161/CIRCINTERVENTIONS.109.883884. [DOI] [PubMed] [Google Scholar]
- 9.Buccheri D, Chirco P R, Geraci S, Caramanno G, Cortese B. Coronary artery fistulae: anatomy, diagnosis and management strategies. Heart Lung Circ. 2018;27(08):940–951. doi: 10.1016/j.hlc.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes E D, Kadivar H, Hallman G L, Reul G J, Ott D A, Cooley D A. Congenital malformations of the coronary arteries: the Texas Heart Institute experience. Ann Thorac Surg. 1992;54(04):732–740. doi: 10.1016/0003-4975(92)91019-6. [DOI] [PubMed] [Google Scholar]
- 11.Calkins J B, Jr, Talley J D, Kim N H. Iatrogenic aorto-coronary venous fistula as a complication of coronary artery bypass surgery: patient report and review of the literature. Cathet Cardiovasc Diagn. 1996;37(01):55–59. doi: 10.1002/(SICI)1097-0304(199601)37:1<55::AID-CCD14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Mangukia C V. Coronary artery fistula. Ann Thorac Surg. 2012;93(06):2084–2092. doi: 10.1016/j.athoracsur.2012.01.114. [DOI] [PubMed] [Google Scholar]
- 13.Pan Y Y, Chen G, Chen B. Prevalence of coronary artery fistula in a single center of China. Chin Med J (Engl) 2018;131(12):1492–1495. doi: 10.4103/0366-6999.233955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim J J, Jung J I, Lee B Y, Lee H G. Prevalence and types of coronary artery fistulas detected with coronary CT angiography. AJR Am J Roentgenol. 2014;203(03):W237-43. doi: 10.2214/AJR.13.11613. [DOI] [PubMed] [Google Scholar]
- 15.Canga Y, Ozcan K S, Emre A. Coronary artery fistula: review of 54 cases from single center experience. Cardiol J. 2012;19(03):278–286. doi: 10.5603/cj.2012.0050. [DOI] [PubMed] [Google Scholar]
- 16.Ali M, Kassem K M, Osei K, Effat M. Coronary artery fistulae. J Thromb Thrombolysis. 2019;48(02):345–351. doi: 10.1007/s11239-019-01897-8. [DOI] [PubMed] [Google Scholar]
- 17.Gowda R M, Vasavada B C, Khan I A. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006;107(01):7–10. doi: 10.1016/j.ijcard.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 18.Luo L, Kebede S, Wu S, Stouffer G A. Coronary artery fistulae. Am J Med Sci. 2006;332(02):79–84. doi: 10.1097/00000441-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Tuncer C, Eryonucu B, Batyraliev T. Angiographic characteristics of coronary artery fistulas. Turk Kardiyol Dern Ars. 2014;42(05):456–460. doi: 10.5543/tkda.2014.66281. [DOI] [PubMed] [Google Scholar]
- 20.Albeyoglu S, Aldag M, Ciloglu U. Coronary arteriovenous fistulas in adult patients: surgical management and outcomes. Rev Bras Cir Cardiovasc. 2017;32(01):15–21. doi: 10.21470/1678-9741-2017-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Said S A. Current characteristics of congenital coronary artery fistulas in adults: a decade of global experience. World J Cardiol. 2011;3(08):267–277. doi: 10.4330/wjc.v3.i8.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunkara A, Chebrolu L H, Chang S M, Barker C. Coronary artery fistula. Methodist DeBakey Cardiovasc J. 2017;13(02):78–80. doi: 10.14797/mdcj-13-2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochar A, Kiefer T. Coronary artery anomalies: when you need to worry. Curr Cardiol Rep. 2017;19(05):39. doi: 10.1007/s11886-017-0854-x. [DOI] [PubMed] [Google Scholar]
- 24.Kiefer T L, Crowley A L, Jaggers J, Harrison J K. Coronary arteriovenous fistulae: the complexity of coronary artery-to-coronary sinus connections. Tex Heart Inst J. 2012;39(02):218–222. [PMC free article] [PubMed] [Google Scholar]
- 25.Bruckheimer E, Harris M, Kornowski R, Dagan T, Birk E. Transcatheter closure of large congenital coronary-cameral fistulae with Amplatzer devices. Catheter Cardiovasc Interv. 2010;75(06):850–854. doi: 10.1002/ccd.22365. [DOI] [PubMed] [Google Scholar]
- 26.Said S M, Burkhart H M, Schaff H V. Late outcome of repair of congenital coronary artery fistulas--a word of caution. J Thorac Cardiovasc Surg. 2013;145(02):455–460. doi: 10.1016/j.jtcvs.2012.11.028. [DOI] [PubMed] [Google Scholar]


