Abstract
In this article, we reported a patient with Crigler–Najjar syndrome type II with high-unconjugated bilirubin levels that decreased after phenobarbital treatment. The patient had two novel missense mutations in the UGT1A1 gene and a promoter variant in one allele. One mutation was c.1001T > C, that predicted leucine to proline substitution at position 334 (p.Leu334Pro). The other, c.1139A > G, predicted glutamic acid to glycine replacement at position 380 (p.Glu380Gly). In silico analysis indicated that both mutations are likely pathogenic.
Keywords: Crigler–Najjar syndrome, hyperbilirubinemia, hereditary, genetic variation
Introduction
Crigler–Najjar syndrome (CNS) is a rare inherited liver disorder caused by pathogenic variants in the UGT1A1 gene, resulting in severe nonhemolytic unconjugated hyperbilirubinemia. In its most severe form, CNS type I (OMIN: 218800), bilirubin glucuronidation is completely absent, while in CNS type II (OMIN: 606785), there is some residual activity and the disorder is responsive to phenobarbital treatment. 1
We reported a patient who had unconjugated hyperbilirubinemia that was responsive to phenobarbital and carried two novel mutations in the UGT1A1 gene.
Case Report
A 6-day-old male infant presented with jaundice and a total serum bilirubin (TSB) level of 463 µmol/L (27.1 mg/dL). Direct bilirubin level was at 10.4 µmol/L (0.6 mg/dL). He was born after 40 weeks of an uncomplicated pregnancy by vacuum-assisted vaginal delivery. There were no signs of hemolysis. After receiving one dose of human serum albumin and intensive phototherapy for 5 days, TSB levels decreased to 203 µmol/L (11.9 mg/dL). During follow-up, an increase in jaundice was observed and, at 30 days of age, the boy was admitted again to the hospital with TSB levels of 374 µmol/L (21.9 mg/dL). Besides phototherapy (just 2 days), phenobarbital treatment was started at a dose of 4 mg/kg/day. TSB levels dropped to 46 µmol/L (2.7 mg/dL) in 5 weeks. Then treatment was stopped for 2 weeks, but TSB levels increased to 128 µmol/L (7.5 mg/dL). Phenobarbital was resumed and TSB levels dropped again to 50 µmol/L (2.9 mg/dL). In the next years, TSB levels ranged from 60 to 200 µmol/L with 1 to 2 mg/kg/day of phenobarbital.
All UGT1A1 gene exons and the promoter region were sequenced after amplification by PCR. The patient was found to be heterozygous for the A(TA) 7 TAA allele. The analysis also revealed two missense mutations in the UGT1A1 gene that have not been previously described in literature or databases, including populational exon/genome databases. One mutation was c.1001T > C that would result in a leucine to proline substitution at position 334 (p.Leu334Pro). The other mutation was c.1139A > G, that would result in glutamic acid to glycine replacement at position 380 (p.Glu380Gly). In silico analysis with 11 prediction software tools (DANN, DEOGEN2, EIGEN, FATHMM-MKL, M-CAP, MVP, MutationAssessor, MutationTaster, Primate AI, REVEL, and SIFT) indicated that both mutations are likely deleterious/pathogenic.
Segregation analysis in his parents ( Fig. 1 ) showed that the c.1139A > G mutation and A(TA) 7 TAA allele were in the same UGT1A1 allele and inherited from his father, and the c.1001T > C was on the other UGT1A1 allele and inherited from his mother.
Fig. 1.
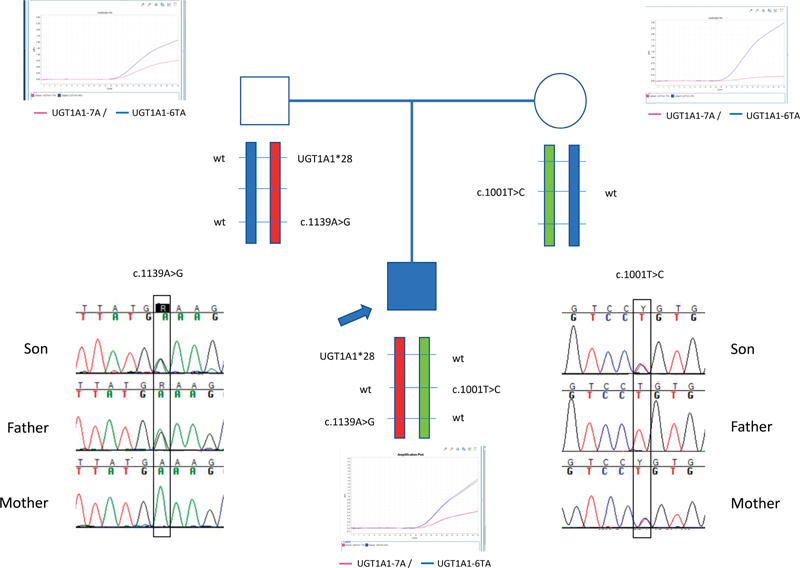
Family tree and segregation analysis of the UGT1A1 gene mutations.
During follow-up, a BiliChek transcutaneous bilirubinometer (Respironics Inc.; Monroeville, Pennsylvania, United States) was used to control bilirubin levels. In eleven checkups, transcutaneous bilirubin and TSB were simultaneously determined. Pearson's correlation coefficient was 0.928 ( p = 0.0001, 95% confidence interval: 0.853–0.986). At the age of 7 years, the patient has had normal psychomotor development and audiological tests, including auditory evoked potentials.
Discussion
Here, we presented a patient who had unconjugated serum bilirubin level consistent with patients with CNS-II that was responsive to phenobarbital treatment. A single normal UGT1A1 allele is sufficient to maintain a normal plasma bilirubin, and almost all cases of UGT1A1 deficiencies are transmitted in an autosomal recessive manner that requires homozygous or compound heterozygous alleles. 2 3 Compound heterozygotes with a promoter variant, such as A(TA) 7 TAA (as found in Gilbert's syndrome), on one allele and a coding region variant on the other allele have been found to have a CNS-II phenotype. 4 5
Our patient was found to be heterozygous for the A(TA) 7 TAA allele and also to have two novel heterozygous mutations in the UGT1A1 gene. Both the father and mother had one normal allele. That explains why they had never presented with jaundice and both had normal TSB levels. In cases where one allele produces nonfunctional UGT1A1 and the expression of the only structurally normal allele is reduced because of the presence of the variant promoter, the residual UGT1A1 activity can be only 10 to 15% of normal, 4 that may result in CNS-II. So, it seems highly plausible that c.1001T > C, the mutation carried by the mother, is a structural pathogenic mutation. The paternally inherited allele had both the A(TA) 7 TAA promoter variant and c.1139A > G mutation. This last mutation might also be pathogenic, further aggravated by the promoter variant, but it is difficult to know the exact effects of each in this allele.
The use of transcutaneous bilirubinometry to measure TSB has been widely validated in newborns, 6 but there is little evidence as to its usefulness at other stages of life. One study found a good correlation of BiliChek transcutaneous bilirubinometer and TSB in hospitalized adults, but it became less accurate at highest TSB levels. 7 In our patient, we found a very strong correlation between transcutaneous bilirubin and TSB, and the maximum difference between two simultaneous measurements during the newborn period was 51 µmol/L (3 mg/dL). This allowed us to heavily reduce the number of venipunctures needed during the patient's follow-up.
Conclusion
In conclusion, we presented a case of CNS-II with compound heterozygosity for two missense mutations in the UGT1A1 gene and a promoter variant in one of the alleles. Transcutaneous bilirubinometry can be a useful tool for the follow-up of such patients.
Funding Statement
Funding None.
Conflict of Interest None declared.
Note
Written informed parental consent was obtained for both print and online publication of this case.
References
- 1.Arias I M, Gartner L M, Cohen M, Ezzer J B, Levi A J. Chronic nonhemolytic unconjugated hyperbilirubinemia with glucuronyl transferase deficiency. Clinical, biochemical, pharmacologic and genetic evidence for heterogeneity. Am J Med. 1969;47(03):395–409. doi: 10.1016/0002-9343(69)90224-1. [DOI] [PubMed] [Google Scholar]
- 2.Erlinger S, Arias I M, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology. 2014;146(07):1625–1638. doi: 10.1053/j.gastro.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Abuduxikuer K, Fang L-J, Li L-T, Gong J-Y, Wang J-S. UGT1A1 genotypes and unconjugated hyperbilirubinemia phenotypes in post-neonatal Chinese children: a retrospective analysis and quantitative correlation. Medicine (Baltimore) 2018;97(49):e13576. doi: 10.1097/MD.0000000000013576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadakol A, Sappal B S, Ghosh S S. Interaction of coding region mutations and the Gilbert-type promoter abnormality of the UGT1A1 gene causes moderate degrees of unconjugated hyperbilirubinaemia and may lead to neonatal kernicterus. J Med Genet. 2001;38(04):244–249. doi: 10.1136/jmg.38.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servedio V, d'Apolito M, Maiorano N. Spectrum of UGT1A1 mutations in Crigler-Najjar (CN) syndrome patients: identification of twelve novel alleles and genotype-phenotype correlation. Hum Mutat. 2005;25(03):325. doi: 10.1002/humu.9322. [DOI] [PubMed] [Google Scholar]
- 6.Romagnoli C, Tiberi E, Barone G. Validation of transcutaneous bilirubin nomogram in identifying neonates not at risk of hyperbilirubinaemia: a prospective, observational, multicenter study. Early Hum Dev. 2012;88(01):51–55. doi: 10.1016/j.earlhumdev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Harbrecht B G, Rosengart M R, Bukauskas K, Zenati M S, Marsh J W, Jr, Geller D A. Assessment of transcutaneous bilirubinometry in hospitalized adults. J Am Coll Surg. 2008;206(06):1129–1136. doi: 10.1016/j.jamcollsurg.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


