Abstract
Introduction
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) imposes a large burden on economy and society worldwide. In addition to western medicine, multiple kinds of qi-tonifying Chinese medicine injections have been widely used in China as adjunctive treatments. Previous small-sample clinical trials have proven their efficacy in the treatment of AECOPD. However, data on comparative effectiveness and safety of qi-tonifying injections are limited. We conducted this network meta-analysis to compare the efficacy and safety of 7 commonly used qi-tonifying injections in patients with AECOPD.
Methods
Literature search was conducted through electronic databases, including PubMed, the Cochrane Library, EMBASE, CINAHL, AMED, CBM, CNKI, Wanfang database, and VIP database. Randomized clinical trials (RCTs) exploring the efficacy of any of these 7 qi-tonifying injections were included. The primary outcome was lung function (FEV1 and FVC). R 4.0.0 and STATA 12.0 were adopted to perform the network meta-analysis using Bayesian statistics.
Results
A total of 36 RCTs involving 2657 participants were included. The results of network meta-analyses indicated that Chuankezhi injection (CKZ) combined with routine treatment (RT) was superior to other qi-tonifying injections combined with RT in terms of FEV1 improvement (MD = 0.63, 95% CI: 0.22, 1.04). For improving FVC, Shengmai injection (SGM) combined with RT showed the greatest therapeutic effect (MD = 0.38, 95% CI: 0.13, 0.61). Moreover, SGM combined with RT revealed the best estimates for response rate (MD = 4.00, 95% CI: 1.34, 13.63). The main adverse events in this study were gastrointestinal reactions and injection site reactions. No serious adverse events were reported.
Conclusion
In this network meta-analysis, SGM and CKZ were potential best adjunctive therapies in the treatment of AECOPD.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is defined by persistent respiratory symptoms and airflow limitation which is due to airway and/or alveolar abnormalities [1]. Over the past few decades, COPD has become a serious public health concern worldwide. COPD caused around 3,000,000 deaths each year globally, making it the 3rd leading cause of deaths [2, 3]. Moreover, due to the increasing environmental exposures (cigarette smoking, ambient particulate matter, etc.) and the aging population [4–7], COPD-related mortality was projected to increase progressively [8]. In China, it was reported that the overall prevalence of COPD was 8.6%, accounting for 99.9 million people [9]; and the death rate was estimated to range from 50 to 100 per 100,000 people [10].
Among them, acute exacerbation of COPD (AECOPD), defined as acute worsening of respiratory symptoms which needs additional therapy [1], is a major factor for the high mortality of COPD. It is established that AECOPD contributes to worse health status, higher rates of readmission, and worse disease progression [11]. Apart from this, the prolonged stay, oxygen therapy, and other medications caused by AECOPD needs made up more than 50% of the total COPD burden on economy and society [12, 13]. Therefore, the treatment of AECOPD is critical for reducing burden of COPD.
The treatment strategies of AECOPD are to minimize the negative impact of the current acute exacerbation and to prevent subsequent events. Currently, routine western medicine for AECOPD mainly includes bronchodilators, corticosteroids, antibiotics, and supplemental oxygen for emergency [1]. Owing to their clinical benefits in relieving symptoms, these therapies are widely used. However, their definite effect in exacerbations remains controversial [14], and their side effects received a growing concern. For instance, corticosteroids are widely applied for the treatment to prevent complications of exacerbation among AECOPD patients. A Danish observational cohort study showed that long course of oral corticosteroids treatment was associated with pneumonia hospitalization or all-cause mortality. Another retrospective cohort study demonstrated that short-term use of oral corticosteroids increased risk of sepsis, venous thromboembolism, and fracture [15, 16]. For the past two decades, multiple studies demonstrated that qi-tonifying Chinese medicine injections, an example of the popular traditional Chinese medicines (TCM) for AECOPD, can overcome acute exacerbation and improve lung function. According to TCM theory, the pathological basis of AECOPD is “exterior excess and interior deficiency.” Qi deficiency runs through the process of AECOPD development, which means qi-tonifying strategy is one of the most important treatment options for AECOPD [17]. Coupled with the high bioavailability of injections [18, 19], multiple qi-tonifying injections were widely used. However, some existing evidence demonstrated that different types of qi-tonifying injections varied in their mechanism of action and clinical efficacy [20–22]. If the best choice of qi-tonifying injections becomes available, clinicians can make better therapeutic choices. However, to date, it is unknown which qi-tonifying injections are more effective; and there were no head-to head studies to compare all these qi-tonifying injections. The Bayesian network meta-analysis can synthesize evidence from direct and indirect comparisons to estimate comparative efficacy [23]. Here, in order to provide the best available treatment, a network meta-analysis was conducted to compare the efficacy and safety of 7 commonly used qi-tonifying injections in patients with AECOPD.
2. Methods
The prospective protocol was created and registered in the International Prospective Register of Systematic Reviews (PROSPERO CRD42020200297). The PRISMA checklist for network meta-analysis is presented in Supplementary Materials (Table S1).
2.1. Data Sources and Searches
We searched PubMed, the Cochrane Central Register of Controlled Trials, EMBASE, CINAHL Nursing Journal Databases (CINAHL), Allied and Complementary Medicine Database (AMED), Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang database, and VIP database to find relevant studies from inception to September 20, 2019. All randomized controlled trials (RCTs) in Chinese and English were included without any other restrictions. The search terms and their combinations were “Pulmonary Disease, Chronic Obstructive,” “Randomized controlled trial,” and “Systematic review” combined with seven included injections. These seven included injections were recommended in Chinese medicine monograph and were commonly used in clinical practice [24, 25]. They were as follows: Shenmai injection (SM), Huangqi injection (HQ), Chuankezhi injection (CKZ), Shenqi Fuzheng injection (SQFZ), Shenfu injection (SF), Kangai injection (KA), and Shengmai injection (SGM). Furthermore, the reference lists of the publications were searched for additional articles. The detailed search strategy was described in Supplementary Table S2.
2.2. Inclusion Criteria and Exclusion Criteria
We included published RCTs that met the following criteria: (1) trials that enrolled patients with definite diagnostic criteria of AECOPD; (2) trials that explored the efficacy of any of these 7 qi-tonifying injections; (3) qi-tonifying injections were given as intravenous except CKZ, whose conventional usage is intramuscular injection; and (4) trials that reported at least one of the following outcomes: the primary outcome was lung function (including FEV1、FVC); secondary outcomes were FEV1%, arterial blood gas analysis (including PaO2 and PaCO2), response rate, the six-minute walking distance (6MWD), the length of hospitalization, and modified British medical research council (mMRC). It is noteworthy that response rate was defined according to efficacy criteria [26, 27]. Clinical recovery, markedly effective, effective were classified into response, and noneffective was classified into nonresponse.
Exclusion criteria were as follows: (1) trials that combined other types of Chinese medicine product, such as Chinese medicine decoction, Chinese patent medicine, and acupuncture; (2) duplicate studies; (3) literature review; (4) studies with only abstracts.
2.3. Study Selection
All titles and abstracts were screened by two reviewers (Xueyi Deng and Jiaqi Lai), and the full texts of eligible articles were obtained for final inclusion.
2.4. Data Extraction and Quality Assessment
Two reviewers (Fuqin Kang and Xuanchen Guan) used a designed form independently to extract and summarize the following data: first author, year of publication, study ID, Journal, study design, sample size, treatment regimens, follow-up time, and adverse event. Two researchers (Xueyi Deng and Jiaqi Lai) independently assessed risk of bias of each study using the Cochrane Risk of Bias Tool [28]. Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases were assessed. If any discrepancies were raised, they were resolved by discussion to achieve consensus and arbitration.
2.5. Statistical Analysis
For all outcomes, we conducted pairwise meta-analyses in random-effects model using Cochrane collaboration software RevMan (5.3). Odds ratios (ORs) were reported for dichotomous outcomes and mean differences for continuous outcomes. P value < 0.05 was considered to be statistically significant. Random-effects network meta-analyses were conducted with STATA (12.0) software and R software (4.0.0) using gemtc package, if there were enough available RCTs for each outcome and intervention. We generated network plots for several outcomes to clarify the direct comparisons or indirect comparisons. The rank probability was generated to show which treatment is the best. Funnel plots and Egger's test were conducted to assess the publication bias.
3. Results
3.1. Literature Retrieval and Study Characteristics
A total of 1226 articles were identified, and 81 articles were assessed for full-text screening. Finally, 36 eligible RCTs involving 2657 participants were included. The details of the literature screening are presented in Figure 1. All 36 studies were published between 2004 and 2019, and the sample size ranged from 36 to 128. There were 31 RCTs reporting the age, and the average age of participants in these studies was 66 years. The characteristics of included studies are reported in Table 1. Overall, baseline characteristics of participants were comparable among different studies. In addition to targeted interventions (7 qi-tonifying injections), all participants received RT, with treatment duration about 2 weeks. The primary outcome lung function (FEV1, FVC) was reported in more than 10 studies.
Figure 1.

Study flow diagram.
Table 1.
Characteristics of included studies.
| Study ID | Sample size assessed (I/C) | Mean age (I/C) | Severity | Intervention arm | Control arm | Treatment duration | Reported outcomes | Adverse events (I/C) |
|---|---|---|---|---|---|---|---|---|
| Cai et al. [29] | 60/60 | 59.89/61.21 | NR | CKZ + RT | RT | 2 w | FEV1; PaCO2 | |
| Chen et al. [30] | 41/43 | 67.1/65.7 | NR | CKZ + RT | RT | 14 d | FEV1% | I: tolerable injection site pain (4 cases), injection site induration (1 case) |
| Chen et al. [31] | 55/53 | NR | NR | HQ + RT | RT | 10~14 d | FVC; response rate | |
| Chi et al. [32] | 48/48 | 76.45/77.68 | NR | SF + RT | RT | 14 d | PaO2; PaCO2; response rate | I: injection site pruritus (1 case) |
| Deng et al. [33] | 30/30 | 67.5/65.5 | NR | SM + RT | RT | 10 d | PaO2; PaCO2; response rate | |
| Guo et al. [34] | 35/35 | 67/66 | NR | SM + RT | RT | 15 d | Response rate | |
| Han et al. [35] | 36/36 | NR | NR | SF + RT | RT | 2 w | FEV1%; PaO2; PaCO2; response rate | |
| Hu et al. [36] | 43/43 | 64.39/65.18 | NR | CKZ + RT | RT | 2 w | FEV1%; 6MWD; response rate | |
| Zhang et al. [37] | 26/25 | 61/66 | NR | HQ + RT | RT | 10 d | Response rate | |
| Jiang et al. [38] | 18/18 | 65.8/66.1 | NR | SQFZ + RT | RT | 10 d | Response rate | |
| Jin et al. [39] | 34/36 | 66.44/66.56 | NR | SF + RT | RT | 2 w | FEV1%; response rate | |
| Li et al. [40] | 36/36 | NR | NR | KA + RT | RT | 7 d | mMRC; response rate | |
| Li et al. [41] | 42/42 | 60.3/60.3 | 1–4 | SQFZ + RT | RT | 7 d | FEV1; FVC; mMRC | I: oral fungal infection (2 cases), lethargy (1 case), low fever (1 case) |
| C: oral fungal infection (1 case), lethargy (1 case) | ||||||||
| Li et al. [42] | 40/40 | 60.13/58.81 | NR | CKZ + RT | RT | 7 d | FEV1; FVC; FEV1%; response rate | I: dizziness, nausea (1 case); |
| C: dizziness, nausea (1 case) | ||||||||
| Liang et al. [43] | 25/25 | 66.27/65.34 | NR | HQ + RT | RT | 10 d | FEV1; FEV1%; PaO2; PaCO2; response rate | |
| Liao et al. [44] | 30/28 | 68.3/65.2 | 1–3 | SF + RT | RT | 14 d | FVC; FEV1%; response rate | |
| Liu et al. [45] | 60/60 | 65.2/65.0 | NR | SQFZ + RT | RT | 10 d | FEV1; FVC; FEV1%; PaO2; PaCO2 | |
| Liu et al. [46] | 25/25 | 68.72/69.56 | NR | CKZ + RT | RT | 7 d | PaO2; PaCO2; mMRC | |
| Lv et al. [47] | 36/36 | NR | NR | SGM + RT | RT | 7 d | PaO2; PaCO2; response rate | I: gastrointestinal reactions (4 cases); |
| C: gastrointestinal reactions (3 cases) | ||||||||
| Qin et al. [48] | 35/35 | 60.5/61.3 | NR | SF + RT | RT | 7 d | FEV1%; PaO2; PaCO2; response rate | |
| Ren et al. [49] | 35/35 | 62.5/62.8 | NR | SF + RT | RT | 2 w | Response rate | |
| Ruan et al. [50] | 64/64 | 63.4/62.8 | 2-3 | SM + RT | RT | 2 w | FEV1; FVC; response rate | |
| Tang et al. [51] | 44/42 | 72.89/71.23 | 2–4 | SF + RT | RT | 7 d | PaO2; PaCO2 | |
| Wang et al. [52] | 30/30 | 62.8/64.1 | NR | SF + RT | RT | NR | PaO2; PaCO2; the length of hospitalization; response rate | |
| Wang et al. [53] | 32/28 | 69.5/69.3 | 1–4 | SGM + RT | RT | 2 w | PaO2; PaCO2; the length of hospitalization; response rate | |
| Wu [54] | 25/25 | 75.35/74 | NR | CKZ + RT | RT | 7 d | PaO2; PaCO2; response rate | |
| Xiao et al. [55] | 32/32 | 63.7/62.6 | NR | SM + RT | RT | 14 d | FEV1; FEV1% | |
| Xiong et al. [56] | 56/56 | 66.7/66.5 | NR | HQ + RT | RT | 14 d | FEV1%; PaO2; PaCO2; response rate | |
| Yin et al. [57] | 30/30 | 49.38/47.62 | NR | SGM + RT | RT | 14 d | FEV1; FVC; FEV1%; PaO2; PaCO2 | |
| Yuan et al. [58] | 39/39 | 74.4/74.6 | 1–3 | CKZ + RT | RT | 21 d | FEV1; FVC; PaO2; PaCO2; response rate | |
| Yue et al. [59] | 35/35 | 62.1/61.8 | 2–3 | SGM + RT | RT | 2 w | FEV1; FVC; PaO2 | |
| Zhang et al. [60] | 39/39 | 64.3/65.1 | NR | SF + RT | RT | 2 w | FEV1%; PaO2; PaCO2; response rate | |
| Zheng et al. [61] | 30/28 | 67.3/67.5 | NR | SQFZ + RT | RT | 10 d | FVC; PaO2; PaCO2 | |
| Zhou et al. [62] | 31/31 | 64.63/63.57 | NR | SGM + RT | RT | 2 w | Response rate | |
| Zhou et al. [63] | 30/30 | NR | NR | HQ + RT | RT | 14 d | FEV1; FVC; FEV1% | |
| Zhu et al. [64] | 26/26 | 72.04/71.69 | NR | HQ + RT | RT | 2 w | FEV1%; PaO2; PaCO2 |
I: intervention; C: control; NR: not reported; CKZ: Chuankezhi injection; HQ: Huangqi injection; SF: Shenfu injection; SM: Shenmai injection; SQFZ: Shenqi Fuzheng injection; SGM: Shengmai injection; KA: Kangai injection; RT: routine treatment.
3.2. Risk of Bias Assessment
For random sequence generation, 13 RCTs [32, 39, 41, 43, 45, 46, 50, 52, 57, 58, 61] performed randomization using the random digital table method or random draws, so they were evaluated as low risk; and the remaining RCTs were assessed as unclear because they only mentioned “random” without providing description of randomization in detail. For allocation concealment, all RCTs were estimated as unclear. 35 studies [29, 37, 39–64] were assessed as high risk in terms of blinding of participants and personnel due to no information on blinding. No studies mentioned blinding of outcome assessors. All included studies were deemed to be low risk on incomplete outcome data. As for selective reporting bias, 2 RCTs [29, 41] were identified as high risk since not all prespecified outcome were reported. For other biases, all RCTs were unclear due to inadequate information. The detailed risk of bias assessments summary is reported in Figure 2.
Figure 2.
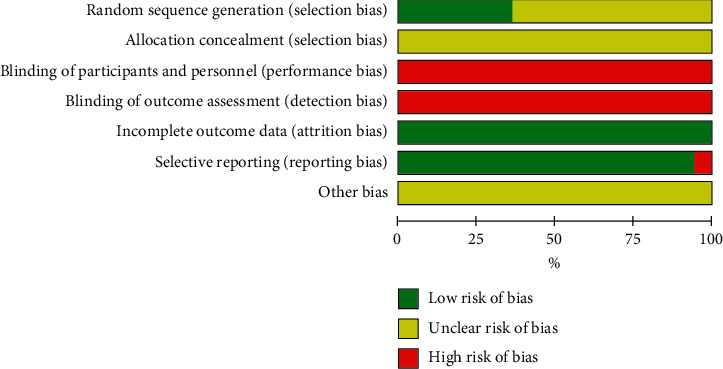
The detailed risk of bias assessments.
3.3. Results of the Pairwise Meta-Analyses
All included studies were two-arm RCTs. In comparison between 7 qi-tonifying injections combined with RT, respectively, SM (MD = 0.34, 95% CI: 0.22, 0.46), HQ (MD = 0.25, 95% CI: 0.23, 0.27), SQFZ (MD = 0.26, 95% CI: 0.09, 0.42), and SGM (MD = 0.39, 95% CI: 0.29, 0.49) combined with RT showed significant effect in FEV1. In addition, CKZ + RT (MD = 0.62, 95% CI: 0.53, 0.72), SQFZ + RT (MD = 0.20, 95% CI: 0.03, 0.37), and SGM + RT (MD = 0.39, 95% CI: 0.26, 0.51) achieved better FVC compared with RT. Most of the qi-tonifying injections plus RT were superior than RT in arterial blood gases and response rate. Detailed results of pairwise comparisons are summarized in Table S3.
3.4. Results of the Network Meta-Analyses
For qi-tonifying injections, network meta-analysis included 5 treatments for FEV1 and FVC, 5 treatments for FEV1%, 6 treatments for PaO2, and 7 treatments for PaCO2 and response rate. The networks of eligible comparisons for lung function, arterial blood gases, and response rate are presented in Figure 3. The pooled estimates of the network meta-analysis are shown in Tables 2–4. The rank probability SUCRA was generated for included interventions and is presented in Figure 4.
Figure 3.
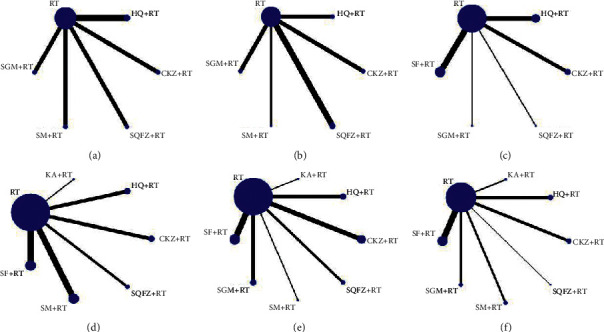
Network meta-analyses of eligible comparisons for lung function (FEV, FVC, and FEV1%), arterial blood gases (PaO2 and PaCO2), and response rate. (a) FEV1. (b) FVC. (c) FEV1%. (d) PaO2. (e) PaCO2. (f) Response rate.
Table 2.
Pooled estimates of the network meta-analysis on response rate and FVC.
| Response rate | ||||||||
|---|---|---|---|---|---|---|---|---|
| FVC | RT | 3.36 (1.56, 7.90) | 3.72 (1.82, 7.87) | 2.85 (0.60, 17.55) | 2.79 (1.60, 5.05) | 4.00 (1.34, 13.63) | 3.98 (1.57, 11.14) | 2.62 (0.18, 88.25) |
| −0.63 (−1.04, −0.22) | CKZ + RT | 1.10 (0.36, 3.26) | 0.86 (0.15, 5.94) | 0.83 (0.30, 2.20) | 1.20 (0.30, 4.87) | 1.19 (0.34, 4.33) | 0.78 (0.05, 27.79) | |
| 0.07 (−0.31, 0.51) | 0.70 (0.15, 1.31) | HQ + RT | 0.76 (0.14, 5.41) | 0.75 (0.30, 1.90) | 1.07 (0.29, 4.41) | 1.07 (0.32, 3.73) | 0.70 (0.04, 25.22) | |
| — | — | — | KA + RT | 0.98 (0.15, 5.26) | 1.40 (0.17, 10.23) | 1.39 (0.18, 9.25) | 0.90 (0.04, 38.29) | |
| — | — | — | — | SF + RT | 1.43 (0.41, 5.53) | 1.43 (0.47, 4.62) | 0.94 (0.06, 32.96) | |
| −0.36 (−0.78, 0.06) | 0.27 (−0.31, 0.86) | −0.43 (−1.04, 0.13) | — | — | SGM + RT | 0.99 (0.21, 4.57) | 0.65 (0.03, 25.09) | |
| 0.75 (0.17, 1.33) | 1.38 (0.67, 2.10) | 0.68 (−0.06, 1.36) | — | — | 1.11 (0.39, 1.83) | SM + RT | 0.65 (0.04, 24.60) | |
| −0.21 (−0.55, 0.11) | 0.42 (−0.11, 0.95) | −0.28 (−0.84, 0.21) | — | — | 0.15 (−0.39, 0.68) | −0.96 (−1.64, −0.29) | SQFZ + RT | |
Values in bold indicate statistical difference.
Table 3.
Pooled estimates of the network meta-analysis on FEV1 and FEV1%.
| FEV1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| FEV1% | RT | 0.18 (−0.01, 0.45) | 0.25 (0.04, 0.46) | — | — | 0.38 (0.13, 0.61) | 0.35 (0.14, 0.58) | 0.26 (0.02, 0.49) |
| −5.57 (−8.62, −3.14) | CKZ + RT | 0.07 (−0.28, 0.35) | — | — | 0.20 (−0.18, 0.49) | 0.17 (−0.17, 0.46) | 0.07 (−0.29, 0.37) | |
| −6.50 (−8.85, −4.23) | — | HQ + RT | — | — | 0.13 (−0.20, 0.44) | 0.09 (−0.20, 0.41) | 0.00 (−0.32, 0.32) | |
| — | — | — | KA + RT | — | — | — | — | |
| −4.37 (−6.51, −2.55) | 1.21 (−1.98, 4.70) | 2.13 (−1.02, 5.05) | − | SF + RT | — | — | — | |
| −3.65 (−11.45, 4.02) | 1.98 (−6.18, 10.21) | 2.86 (−5.25, 10.81) | − | 0.74 (−7.21, 8.72) | SGM + RT | −0.03 (−0.34, 0.31) | −0.12 (−0.45, 0.22) | |
| — | — | — | — | — | — | SM + RT | −0.09 (−0.43, 0.22) | |
| −3.67 (−9.67, 2.26) | 1.94 (−4.47, 8.63) | 2.83 (−3.56, 9.19) | — | 0.71 (−5.50, 7.03) | −0.04 (−9.85, 9.84) | — | SQFZ + RT | |
Values in bold indicate statistical difference.
Table 4.
Pooled estimates of the network meta-analysis on PaO2 and PaCO2.
| PaO2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| PaCO2 | RT | 10.05 (6.02, 15.21) | 7.83 (3.57, 11.75) | — | 5.68 (2.50, 9.10) | 6.80 (4.10, 9.98) | 4.65 (−1.77, 11.10) | 5.89 (1.76, 9.83) |
| −1.76 (−5.80, 2.55) | CKZ + RT | −2.23 (−9.11, 3.21) | — | −4.40 (−10.38, 0.80) | −3.21 (−9.05, 1.77) | −5.41 (−13.82, 1.92) | −4.15 (−10.98, 1.24) | |
| 4.74 (−0.02, 9.68) | 6.51 (0.05, 12.80) | HQ + RT | — | −2.15 (−7.13, 3.38) | −1.00 (−5.67, 4.41) | −3.17 (−10.62, 4.63) | −1.97 (−7.59, 3.84) | |
| 11.71 (3.77, 19.63) | 13.48 (4.34, 22.32) | 6.97 (−2.42, 16.21) | KA + RT | — | — | — | — | |
| 4.22 (0.71, 7.75) | 5.98 (0.39, 11.31) | −0.54 (−6.57, 5.39) | −7.52 (−16.19,1.23) | SF + RT | 1.17 (−3.20, 5.61) | −1.01 (−8.40, 6.04) | 0.21 (−5.21, 5.15) | |
| 10.84 (6.05, 15.56) | 12.61 (6.10, 18.76) | 6.10 (−0.81, 12.75) | −0.88 (−10.11, 8.38) | 6.62 (0.66, 12.52) | SGM + RT | −2.20 (−9.41, 4.70) | −0.90 (−6.24, 3.74) | |
| 3.76 (−4.37, 11.98) | 5.52 (−3.73, 14.59) | −1.00 (−10.57, 8.51) | −7.95 (−19.34, 3.43) | −0.45 (−9.37, 8.46) | −7.09 (−16.50, 2.41) | SM + RT | 1.22 (−6.42, 8.68) | |
| 8.70 (2.79, 14.68) | 10.46 (3.07, 17.61) | 3.96 (−3.76 11.61) | −3.01 (−12.97, 6.98) | 4.49 (−2.44, 11.43) | −2.14 (−9.71, 5.54) | 4.94 (−5.17, 15.08) | SQFZ + RT | |
Values in bold indicate statistical difference.
Figure 4.
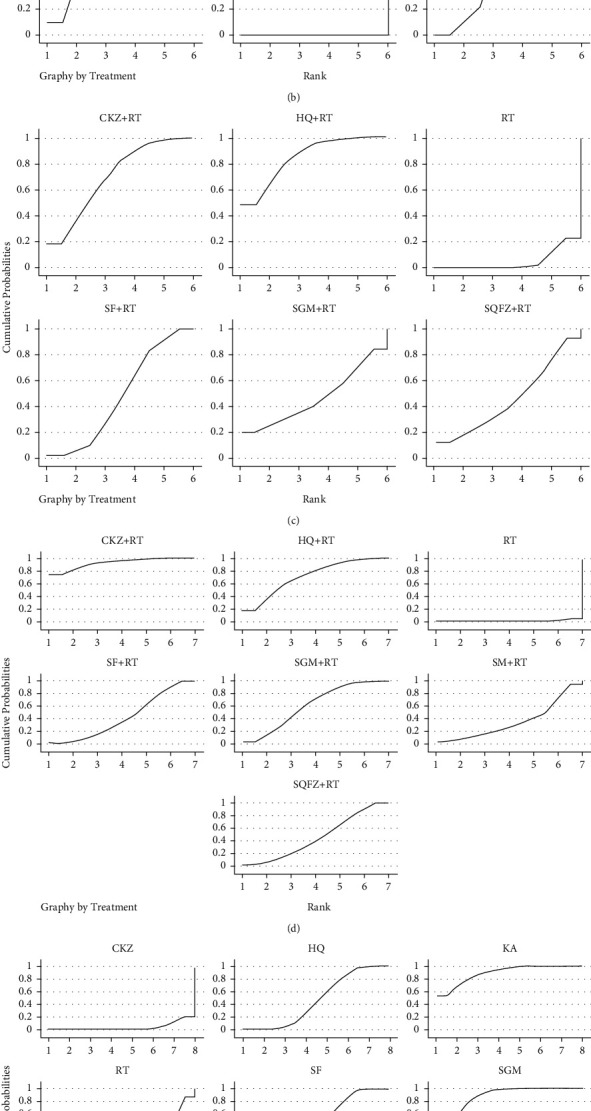
The rank probability of lung function, arterial blood gases (PaO2 and PaCO2), and response rate for included interventions. (a) FEV1. (b) FVC. (c) FEV1%. (d) PaO2. (e) PaCO2. (f) Response rate.
3.4.1. Comparison of the Lung Function (FEV1, FVC, and FEV1%)
Of these RCTs, a total of 11 studies [29, 41, 43, 45, 50, 55, 57, 59, 63] reported the outcome of FEV1, involving 5 different qi-tonifying injections. All 5 qi-tonifying injections combined with RT were more beneficial than RT, with MD of 0.38 (95% CI: 0.13, 0.61) for SGM + RT, MD of 0.35 (95% CI: 0.14, 0.58) for SM + RT, MD of 0.25 (95% CI: 0.04, 0.46) for HQ + RT, and MD of 0.26 (95% CI: 0.02, 0.49) for SQFZ + RT. In addition, SGM yielded the best result among these five injections.
In terms of FVC improvement, the random-effects network meta-analyses summarized the MDs for 5 qi-tonifying injections. The results revealed that CKZ combined with RT was associated with the best FVC (MD = 0.63, 95% CI: 0.22, 1.04). Moreover, CKZ + RT was the only treatment that was significantly better than RT, followed by SGM + RT (MD = 0.36, 95% CI: −0.06, 0.78) and SQFZ + RT (MD = 0.21, 95% CI: −0.11, 0.55). Notably, there were no statistically significant differences between SGM + RT, SQFZ + RT, and RT. Therefore, CKZ may be the optimal treatment for improving FVC.
In the analysis of FEV1%, the result of network meta-analyses revealed that HQ + RT may yield the best FEV1% (MD = 6.51, 95% CI: 4.23, 8.85), followed by CKZ (MD = 5.57, 95% CI: 3.14, 8.62) and SF (MD = 4.37, 95% CI: 2.55, 6.51). Both of them combined with RT were approved to be with higher FEV1% when compared with RT alone. There was no significant difference in the association when comparing SGM + RT (MD = 3.65, 95% CI: −4.02,11.45) and SQFZ + RT (MD = 3.70, 95% CI: −2.26, 9.67) with RT alone in network meta-analyses.
3.4.2. Comparison of the Arterial Blood Gases (PaO2 and PaCO2)
For arterial blood gases, PaO2 and PaCO2 were reported in 18 RCTs, respectively, in which 6 qi-tonifying injections were evaluated in these trials. The network meta-analyses for PaO2 indicated that CKZ (MD = 10.05, 95% CI: 6.02, 15.21) had the highest probability of increasing PaO2. Also, HQ + RT (MD = 7.83, 95% CI: 3.57, 11.75), SGM + RT (MD = 6.80, 95% CI: 4.10, 9.98), SQFZ + RT (MD = 5.89, 95% CI: 1.76, 9.83), and SF + RT (MD = 5.68, 95% CI: 2.50, 9.10) were more effective than RT alone.
In terms of PaCO2, KA plus RT (MD = −11.71, 95% CI: −19.63, −3.77) was likely to be the best choice. SGM (MD = −10.84, 95% CI: −15.56, −6.05), SQFZ (MD = −8.70, 95% CI: −14.68, −2.79), and SF (MD = −4.22, 95%CI: −7.75 to −0.71) combined with RT resulted in a significantly better outcome than RT. The result of network meta-analysis was consistent with pairwise comparisons. Overall, the effect estimates of SGM were high in both PaO2 and PaCO2 outcome measurements.
3.4.3. Comparison of the Response Rate
In total, 24 of 36 RCTs [31–40, 42–44, 47–50, 52–54, 56, 58, 60, 62] tested the response rate. For data that were available on all 7 qi-tonifying injections of interest, network meta-analyses were conducted addressing these 7 interventions. SGM + RT (OR = 4.00, 95% CI: 1.34, 13.63) was considered as the best response rate of 7 qi-tonifying injections, although there was no significant difference observed among SGM and the other 6 qi-tonifying injections. Besides, the random-effects network meta-analyses demonstrated that SM + RT (OR = 3.98, 95% CI: 1.57, 11.14), HQ + RT (OR = 3.72, 95% CI: 1.82, 7.87), CKZ + RT (OR = 3.36, 95% CI: 1.56, 7.90), and SF + RT (OR = 2.79, 95% CI: 1.60, 5.05) performed significantly better than RT. However, all of them had similar effects with respect to response rate.
3.5. Adverse Events
Ten of the 36 RCTs [30, 32, 35, 40, 42, 47, 53, 54, 60] reported outcomes of adverse events. Among them, 5 RCTs [39, 44, 57, 58, 64] reported no intervention related adverse events, and the other 5 RCTs [30, 32, 41, 42, 47] reported at least one adverse event. A trial evaluated SF reported injection site pruritus (1 case) in the intervention group, and symptom disappeared after withdrawal of infusion [32]. Gastrointestinal reactions were observed in another trial that evaluated SGM, including 4 cases in the treatment group and 3 cases in the control group [47]. Injection-related adverse events (5 cases) were observed after administration of CKZ, including tolerable injection site pain (4 cases) and injection site induration (1 case), which resolved within days after treatment [30]. One patient in CKZ group developed AE symptoms like dizziness and nausea. The symptoms completely disappeared after rest [42]. Serious adverse events were not reported. Further details of side effects are presented in Table 1. Basically, the main adverse events were gastrointestinal reactions and injection site reactions, which would spontaneously relieve without any specific treatment. However, the safety of these seven qi-tonifying injections was still unclear due to limited information.
3.6. Publication Bias
Publication bias was evaluated by funnel plot (Figure 5) and Egger's test. There was not any evidence of publication bias for FEV1(t = 0.41, P=0.691).
Figure 5.
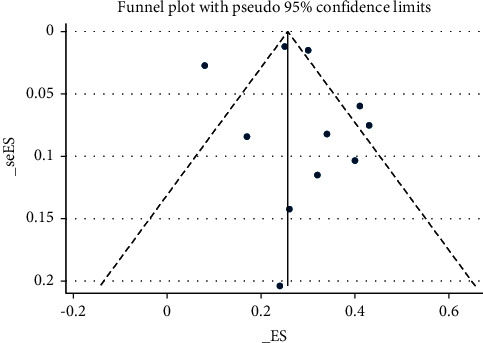
Funnel plot for the publication bias.
4. Discussion
In this review, we comprehensively summarized the efficacy and safety of 7 commonly utilized qi-tonifying injections for patients with AECOPD. Our analyses showed that SGM + RT, CKZ + RT, and HQ + RT revealed a highest probability to be the best choice to improve the lung function. In addition, CKZ and KA combined with RT had a similar first ranking in the analysis about arterial blood gases. In terms of response rate, SGM + RT showed the best improvement in network meta-analysis, although no significant difference was observed among these 7 qi-tonifying injections. The wide confidence intervals on ORs may be due to the small sample size. Thus, the results should be treated with caution. In conclusion, our assessment overall found that SGM and CKZ may be the most effective qi-tonifying injections.
Possible explanation about high effect of SGM is that it contains ginsenoside, organic acid, schizandra, and multiple microelements, which may help to decrease pulmonary artery pressure and improve gas exchange function. On the other hand, they can improve hypoxia tolerance by inhibiting Na+/K+ ATPase to improve myocardial contractility and microcirculation [65, 66]. In addition to the efficacy, the safety of SGM should be considered. However, due to the limited information reported, we cannot draw a specific conclusion. Only 1 RCT reported the side effect related to gastrointestinal reactions [47]. This may be attributed to the excessive secretion of gastric acid and bile promoted by schizandra [67]. Given that, the patient should be evaluated for drug tolerance when SGM was used in excess. Additionally, in 2017, China National Medical Products Administration had informed that SGM-induced allergic shock should be paid more attention in clinical practice [68].
Numerous studies have helped to verify the mechanism of action of epimedins A, B, and C and icariin, which are major constituents of CKZ. It is reported that icariin can not only increase expression of the anti-inflammatory factor interleukin-10 but also decrease expression of various proinflammatory factors IL-8 and tumor necrosis factor-α. Besides this, icariin can regulate the expression of Glucocorticoids (GC) resistance-related factors, which was beneficial for reversing GC resistance in COPD [69, 70]. Regarding the administration ways, intravenous mode was the preferred mode of administration (6 studies), followed by intermuscular administration (1 study). Different ways of administration may lead to difference in their bioavailability [19]. In order to get better effect, physicians should adjust the administration strategies timely according to individualized treatment.
4.1. Strengths and Limitations
The main strength of this study is that we creatively applied a network meta-analysis to comprehensively compare the efficacy and safety of commonly used qi-tonifying injections. Furthermore, it was not feasible to include all kinds of qi-tonifying injections of interest due to the limited clinical application of some kinds of qi-tonifying injection. Therefore, we focused on seven injections recommended by clinical practice guidelines.
This study had several limitations. First of all, network meta-analyses based on the assumption that comparators among different trials are compared are similar [23, 71]. In addition to characteristics of participants, routine care strategies should be adjusted for any discordance in comparators among trials. However, 25 of 36 trials did not describe routine treatment measures in detail. This may cause inconsistency and heterogeneity. Thus, the limitations need to be considered when interpreting the result. We recommend that the detailed information of RT should be reported in future research. Secondly, response rate was the only outcome being evaluated by all qi-tonifying injections. The bias induced by its subjectivity and uncertainty needs to be noted.
5. Conclusions
In conclusion, our results suggested that SGM and CKZ were optimal injections when they combined with RT for the treatment of AECOPD. The safety of these seven qi-tonifying injections was still uncertain due to the limited information. Further studies with direct comparisons of these injections are warranted to confirm our results. Moreover, the safety also needs to be monitored rigorously in the clinical practice.
Acknowledgments
The authors would like to thank Professor Lei Wu for her clinical advice on this manuscript. The project was supported by a grant from the National Key R&D Program of China (no. 2019YFC1709804) and the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (Grant nos. YN2019QL16 and YN2016QL09).
Contributor Information
Xinfeng Guo, Email: guoxinfeng@gzucm.edu.cn.
Shaonan Liu, Email: shaonanliu819@gzucm.edu.cn.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Supplementary Materials
S1: PRISMA NMA Checklist of Items to Include When Reporting A Systematic Review Involving a Network Meta-Analysis. S2: search strategy of EMBASE. S3: pairwise random-effects meta-analyses of lung function, arterial blood gases, and response rate.
References
- 1. https://www.who.int/respiratory/copd/burden/en/, accessed 29 Jan 2020.
- 2.World Health Organization. The top 10 causes of death. 2018. https://www.who.int/data/gho/data/themes/topics/causes-of-death .
- 3.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet . 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9.10100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisner M. D., Anthonisen N., Coultas D., et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine . 2010;182(5):693–718. doi: 10.1164/rccm.200811-1757st. [DOI] [PubMed] [Google Scholar]
- 5.Anne G. Wheaton, division of population health, national center for chronic disease prevention and health promotion, CDC; laura kurth, respiratory health division, national institute for occupational safety and health, CDC. Morbidity and Mortality Weekly Report . 2019;68(13):303–307. [Google Scholar]
- 6.Yin P., Jiang C., Cheng K., et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. The Lancet . 2007;370(9589):751–757. doi: 10.1016/s0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 7.Mercado N., Ito K., Barnes P. J. Accelerated ageing of the lung in COPD: new concepts. Thorax . 2015;70(5):482–489. doi: 10.1136/thoraxjnl-2014-206084. [DOI] [PubMed] [Google Scholar]
- 8.Mathers C. D, Loncar D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine . 2006;3(11) doi: 10.1371/journal.pmed.0030442.e442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C., Xu J., Yang L., et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet . 2018;391(10131):1706–1717. doi: 10.1016/s0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M., Wang H., Zhu J., et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. The Lancet . 2016;387(10015):251–272. doi: 10.1016/s0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 11.Hillas G., Perlikos F., Tzanakis N. Acute exacerbation of COPD: is it the stroke of the lungs? International Journal of Chronic Obstructive Pulmonary Disease . 2016;11:1579–1586. doi: 10.2147/COPD.S106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi H., Sharafkhaneh A., Hanania N. A. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Therapeutic Advances in Chronic Disease . 2014;5(5):212–227. doi: 10.1177/2040622314532862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad Hassali A. U., Muhammad S. A., Shah S., Abbas S., Hyder Ali I. A. B., Salman A. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Review of Pharmacoeconomics & Outcomes Research . 2020;20(6):661–672. doi: 10.1080/14737167.2020.1678385. [DOI] [PubMed] [Google Scholar]
- 14.Vollenweider D. J., Frei A., Steurer-Stey C. A., Garcia-Aymerich J., Puhan M. A. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews . 2018;10(10) doi: 10.1002/14651858.CD010257.pub2.CD010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivapalan P., Ingebrigtsen T. S., Rasmussen D. B., et al. COPD exacerbations: the impact of long versus short courses of oral corticosteroids on mortality and pneumonia: nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respiratory Research . 2019;6(1) doi: 10.1136/bmjresp-2019-000407.e000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waljee A. K., Rogers M. A., Lin P., et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ . 2017;357 doi: 10.1136/bmj.j1415.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Key Laboratory of COPD Lung Qi Deficiency Syndrome. Expert consensus on the evolution of TCM syndromes of chronic obstructive pulmonary disease and its combined syndrome based on the theory of lung qi deficiency. Chinese Journal of Integrated Traditional Chinese and Western Medicine Emergency Medicine . 2015;2:113–114. [Google Scholar]
- 18.Li H., Wan H., Xia T., et al. Therapeutic angiogenesis in ischemic muscles after local injection of fragmented fibers with loaded traditional Chinese medicine. Nanoscale . 2015;7(30):13075–13087. doi: 10.1039/c5nr02005k. [DOI] [PubMed] [Google Scholar]
- 19.Xu S., Yu J., Yang L., Zhu Y., Sun S., Xu Z. Comparative pharmacokinetics and bioavailability of epimedin C in rat after intramuscular administration of epimedin C, a combination of four flavonoid glycosides and purified herba epimedii extract. Journal of Analytical Methods in Chemistry . 2016;2016:9. doi: 10.1155/2016/5093537.5093537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W., Li C., Huang J., et al. Application of pathways activity profiling to urine metabolomics for screening Qi-tonifying biomarkers and metabolic pathways of honey-processed Astragalus. Journal of Separation Science . 2018;41(12):2661–2671. doi: 10.1002/jssc.201701371. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y. X., Chen Y., Yang Y., Chen X. X., Zhang D. D. Screening five qi-tonifying herbs on M2 phenotype macrophages. Evidence-Based Complementary and Alternative Medicine: ECAM . 2019;2019:8. doi: 10.1155/2019/9549315.9549315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding H., Wang C., Li Z. Clinical study on the treatment of chronic obstructive pulmonary disease by invigorating the lung. Clinical Journal of Chinese Medicine . 2018;30(2):191–194. [Google Scholar]
- 23.Bucher H. C., Guyatt G. H., Griffith L. E., Walter S. D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology . 1997;50(6):683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 24.Chinese Academy of Chinese Medical Sciences. Evidence-Based Guidelines of Clinical Practice in Chinese Medicine . Beijing, China: Chinese Academy of Chinese Medical Sciences Publishing House; 2011. [Google Scholar]
- 25.Xue C. C., Lu C. Evidence-based Clinical Chinese Medicine . Singapore: World Scientific; 2016. Chronic Obstructive Pulmonary Disease. [Google Scholar]
- 26.Zheng X. Implementation of the Guiding Principles for Clinical Research of New Chinese Medicines . Beijing, China: China Medical Science and Technology Press; 2002. pp. 54–58. [Google Scholar]
- 27.State Administration of Traditional Chinese Medicine. TCM Disease Diagnosis and Therapeutic Effect Standard . Nanjing, China: Nanjing University Press; 1994. [Google Scholar]
- 28.Higgins J. P. T., Altman D. G., Gøtzsche P. C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. The BMJ . 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai H., Li Z., Li P. Clinical study on the treatment of chronic obstructive pulmonary disease with Chuankezhi injection combined with tiotropium bromide inhalation. Journal of Hunan University of Traditional Chinese Medicine . 2018;A01:62–63. [Google Scholar]
- 30.Chen G. Effects of chuankezhi injection on blood gas analysis and pulmonary function of patients with moderate-severe COPD. Journal of Yunyang Medical College . 2008;27(5):429–431. [Google Scholar]
- 31.Chen S. Curative effects of milkvetch root injections in acute chronic obstructive pulmonary disease and immunically functional effects of lymphocyte. Modern Chinese Doctor . 2008;46(32):40–41.45 [Google Scholar]
- 32.Chi Y. Efficacy observation of Shenfu injection in adjuvant treatment of chronic obstructive pulmonary disease in acute exacerbation period. China Emergency in Traditional Chinese Medicine . 2015;24(3):553–554. [Google Scholar]
- 33.Deng Y. The effect of Shenmai injection in adjuvant treatment of acute exacerbation of chronic obstructive pulmonary disease. China Health Care and Nutrition . 2019;17(17)31 [Google Scholar]
- 34.Guo X. Observation on the clinical efficacy of integrated traditional Chinese and western medicine in the treatment of acute exacerbation of chronic obstructive pulmonary disease. China Modern Medicine Application . 2009;3(15):153–154. [Google Scholar]
- 35.Han B. Clinical study on guiding fire to origin method in acute aggravating period of chronic obstructive pulmonary disease. Modern Journal of Integrated Traditional Chinese and Western Medicine . 2013;22(17):1825–1827.1832 [Google Scholar]
- 36.Hu K. The curative effect of Chuankezhi injection combined with western medicine in the treatment of AECOPD with deficiency of lung and kidney qi and its effect on the immune function and blood NF-κB. Journal of Medical Theory and Practice . 2018;31(18):2735–2737. [Google Scholar]
- 37.Zhang T. Effects of astragalus injection on acute exacerbation chronic obstructive pulmonary disease. Medical Journal of West . 2012;24(1):33–35.37 [Google Scholar]
- 38.Jiang Z. Effects of shenqi fuzheng liquid on the quality of life and T lymphocyte subgroups in patients with AECOPD. Medical Information . 2015;29:72–73. [Google Scholar]
- 39.Jin W. A clinical trial on the effect of shenfu injection on cellular immune function in patients with AECOPD. Journal of Emergency in Traditional Chinese Medicine . 2018;27(7):1182–1185. [Google Scholar]
- 40.Li H. Clinical observation on Kang-ai-zhu-she-ye combined with antibiotics in the treatment of acute exacerbations of chronic obstructive pulmonary disease in elderly patients. International Journal of Respiration . 2012;32(5):344–346. [Google Scholar]
- 41.Li L. The effect of Shenqi Fuzheng injection combined with budesonide nebulization inhalation in the treatment of acute exacerbation of chronic obstructive pulmonary disease. China Rural Medicine . 2019;26(4):37–38. [Google Scholar]
- 42.Li X. Effect of chuankezhi injection combined with doxofylline in the treatment of acute exacerbation of chronic obstructive pulmonary disease and its influence on immune function and lung function. Modern Hospital . 2019;19(3):434–436. [Google Scholar]
- 43.Liang F. The curative effect and X-ray changes of Huangqi injection in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Medical Aesthetics and Cosmetology (Mid-Term Journal) . 2014;10:152–153.154 [Google Scholar]
- 44.Liao W. Influence of Shenfu injection on tumor necrosis factor-α,interleukin-2 and lung function in patients with chronic obstructive pulmonary disease at acute exacerbation stage. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care . 2008;15(3):149–151. [Google Scholar]
- 45.Liu K., Liu P., Chen L., Sun J., Tan Y. Effects of shenqi fuzheng liquid on pulmonary function and blood gas analysis in patients with acute exacerbation of chronic obstructive pulmonary disease. Journal of New Chinese Medicine . 2011;43(11):24–25. [Google Scholar]
- 46.Liu W. The Clinical Research on the Therapy of AECOPD by Traditional Chinese Medicine CKZ Injection . Guangzhou, China: Guangzhou University of Chinese Medicine; 2015. [Google Scholar]
- 47.Lv R., Tuo Z., Cheng Y., Cai Z., Ding H. The effect of Shengmai injection combined with piperacillin sodium/tazobactam sodium in the treatment of elderly AECOPD. Journal of Bethune Medical Science . 2015;3:316–317. [Google Scholar]
- 48.Qin H. Clinical observation of Shenfu injection in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Chinese Journal of Clinical Medicine . 2010;17(5):659–660. [Google Scholar]
- 49.Ren Y. Clinical observation of Shenfu Injection in the treatment of acute exacerbation chronic obstructive pulmonary disease. Nei Mongol Journal of Traditional Chinese Medicine . 2013;32(7):58–59. [Google Scholar]
- 50.Ruan Z. Clinical study of shenmai injection combined with salmeterol/fluticasone in treating AECOPD. Journal of New Chinese Medicine . 2017;49(1):40–43. [Google Scholar]
- 51.Tang Q. Clinical effect of Shenfu injection on the treatment of acute exacerbation of chronic obstructive pulmonary disease: an analysis of 86 cases. Journal of Southwest Medical University . 2018;41(4):372–376. [Google Scholar]
- 52.Wang X. Shenfu injection combined with mechanical ventilation for 30 cases of acute exacerbation of chronic obstructive pulmonary disease. Journal of Traditional Chinese Medicine . 2013;54(16):1386–1389. [Google Scholar]
- 53.Wang Y., Shi C., Liu Y. Clinical observation of Shengmai injection in acute exacerbations of chronic obstructive pulmonary disease(COPD) Chinese Journal of New Drugs . 2007;16(16):1298–1300. [Google Scholar]
- 54.Wu S. The Clinical Research on the Treatment of AECOPD by Traditional Chinese Medicine CKZ . Guangzhou, China: Guangzhou University of Chinese Medicine; 2010. [Google Scholar]
- 55.Xiao B., Liu H., Chen K., Ling C. The intervention of acute aggregation stage of chronic obstructive pulmonary disease by shenmai injection. Journal of New Chinese Medicine . 2007;39(6):11–12. [Google Scholar]
- 56.Xiong S. Influence of astragalus injection on serum cytokines and lung function in acute exacerbation of chronic obstructive pulmonary disease. China Modern Doctor . 2013;51(9):43–45. [Google Scholar]
- 57.Yin S. Effect of shengmai injection on pulmonary function and blood gas analysis during COPD attack. Clinical Journal of Traditional Chinese Medicine . 2006;18(6):557–558. [Google Scholar]
- 58.Yuan G. Clinical observation of chuankezhi injection on acute exacerbation of COPD. Guiding Journal of Traditional Chinese Medicine and Pharmacy . 2014;20(12):65–68. [Google Scholar]
- 59.Yue Y. Effect of Shengmai injection on serum BNP, pulmonary function and oxygen metabolism in patients with chronic obstructive pulmonary disease at attack stage. Hainan Medical Journal . 2013;24(2):225–227. [Google Scholar]
- 60.Zhang Y. Effects of shenfu injection on the patients with acute ecacerbation of chronic obstruction pulmonary disease. International Journal of Geriatrics . 2017;38(2):63–66. [Google Scholar]
- 61.Zheng G. An observation on clinical efficacy of Shenqi Fuzheng injection for treatment of patients with acute exacerbation of chronic obstructive pulmonary disease. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care . 2015;4:357–360. [Google Scholar]
- 62.Zhou Q. Effects of the Shengmai injection on clinical symptoms and inflammatory markers of chronic obstructive pulmonary disease during the acute attack stage. Clinical Journal of Chinese Medicine . 2015;11:12–13. [Google Scholar]
- 63.Zhou Y. Huangqi injection on cytokines and pulmonary function in patients with AECOPD. Journal of Changchun University of Chinese Medicine . 2016;32(2):337–338. [Google Scholar]
- 64.Zhu Y., Ying K., Cai W., Jiang X., Wang X. The effect on oxidauts/antioxidants imblance of huangqi injection to patients with acute exacerbations of COPD. Journal of Emergency in Traditional Chinese Medicine . 2004;13(9):597–598. [Google Scholar]
- 65.Huang X., Duan X., Wang K., Wu J., Zhang X. Shengmai injection as an adjunctive therapy for the treatment of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complementary Therapies in Medicine . 2019;43:140–147. doi: 10.1016/j.ctim.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 66.Wang H. Clinical manifestations and preventive measures of adverse reactions of Shengmai injection. China Pharmacy . 2010;21(16):1505–1507. [Google Scholar]
- 67. Adverse drug reaction information bulletin (issue 44) beware of severe allergic reactions to shengmai injection, 2012, https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/yjjsh/ypblfytb/20120110120001828.html.
- 68. Announcement of the general administration on revising the instructions for shengmai injection (no. 142 of 2017), 2017, https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/ypshmshxdgg/20171128100001181.html.
- 69.Hu L., Liu F., Li L., et al. Effects of icariin on cell injury and glucocorticoid resistance in BEAS‑2B cells exposed to cigarette smoke extract. Experimental and therapeutic medicine . 2020;20(1):283–292. doi: 10.3892/etm.2020.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen S., Yang X.-Y., Tang X.-Y., et al. Systematic review of Chuankezhi injection for treating acute exacerbation of chronic obstructive pulmonary disease. China Journal of Chinese Materia Medica . 2017;42(14):2789–2795. doi: 10.19540/j.cnki.cjcmm.20170523.009. [DOI] [PubMed] [Google Scholar]
- 71.Kim H., Gurrin L., Ademi Z., Liew D. Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. British Journal of Clinical Pharmacology . 2014;77(1):116–121. doi: 10.1111/bcp.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: PRISMA NMA Checklist of Items to Include When Reporting A Systematic Review Involving a Network Meta-Analysis. S2: search strategy of EMBASE. S3: pairwise random-effects meta-analyses of lung function, arterial blood gases, and response rate.


