Abstract
The aryl hydrocarbon receptor (AHR) contains signals for both nuclear import and nuclear export (NES). The purpose of the studies in this report was to determine the relationship between the nuclear export of the AHR and AHR-mediated gene regulation. Blockage of nuclear export in HepG2 cells with leptomycin B (LMB) resulted in increased levels of AHR-AHR nuclear translocator (ARNT) complex in the nucleus and correlative reductions in agonist-stimulated AHR degradation. However, LMB exposure inhibited agonist-mediated induction of numerous AHR-responsive reporter genes by 75 to 89% and also inhibited induction of endogenous CYP1A1. LMB did not transform the AHR to a ligand binding species or affect activation by TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin). Mutagenesis of leucines 66 and 71 of the putative AHR NES resulted in a protein with reduced function in dimerization to ARNT and binding to DNA, while alanine substitution at leucine 69 (AHRA69) resulted in an AHR that bound with ARNT and associated with DNA. AHRA69 protein injected directly into the nuclei of E36 cells remained nuclear following 6 h of agonist stimulation. In transient-transfection assays, AHRA69 accumulated within the nucleus was not degraded efficiently following agonist exposure. Finally, AHRA69 supported induction of AHR-responsive reporter genes in an agonist-dependent manner. These findings show that it is possible to generate an AHR protein defective in nuclear export that is functional in agonist-mediated gene induction. This implies that the negative effect of LMB on agonist-mediated gene induction is independent of the nuclear export of the AHR.
Nuclear export is an ATP-dependent process that is essential for transport of RNA and protein from the nucleus. The best-understood model of nuclear export proposes that a protein containing a nuclear export signal (NES) associates with an export receptor (the exportin or CRM-1 protein) and the complex then exits the nucleus through the nuclear pore (6, 7, 10, 18, 19, 42, 47). The putative NES appears to be a sequence of 10 to 12 amino acids rich in leucine (XLXXLXXLXLX), and this sequence has been shown to directly associate with CRM-1. The function of nuclear export appears to be dependent on the function of the protein containing the NES sequence, but a theme is developing in which nuclear export functions to deliver active nuclear transcription factors to the cytoplasm for degradation. Examples of this type of mechanism include the p53 protein where function is controlled in part through nuclear export and subsequent degradation by cytoplasmic proteases (8, 44, 45), and the cyclin-dependent kinase p27Kip1 (40, 43, 46). The common theme in both pathways is that the export of the effector protein from the nucleus results in reduced levels of gene regulation since the protein is being removed from its site of action.
Recently, it has been clearly established that the aryl hydrocarbon receptor (AHR) protein is rapidly degraded both in vivo and in vitro subsequent to agonist binding (11, 12, 28, 29). It has also been shown elsewhere that the AHR contains a putative NES sequence (36, 41). Thus, studies have been performed to establish the relationship between the nuclear export of the AHR and protein degradation. The studies have shown that blockage of AHR degradation through inhibition of the 26S proteasome results in (i) increased levels of AHR in the nucleus, (ii) increased levels of AHR-AHR nuclear translocator (ARNT) complexes associated with DNA, and (iii) increased levels of gene induction following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (4). In addition, studies have shown that when nuclear export of the AHR is blocked by the fungal antibiotic leptomycin B (LMB), the AHR is accumulated within the nucleus following ligand binding and is not degraded efficiently (4). Taken together, these findings support the hypothesis that nuclear export is required for the ligand-bound AHR to be degraded and that this type of biological mechanism is consistent with the mechanism involved in the degradation of p53 and p27Kip1.
The purpose of the studies detailed in this report was to further evaluate the function of nuclear export of the AHR with focus on the relationship between nuclear export and AHR-mediated gene regulation. The studies show that it is possible to generate an AHR protein with mutations in the NES that dimerizes with ARNT, binds DNA, and supports ligand-mediated gene induction. However, the studies also demonstrate that treatment of cells with LMB results in inhibition of AHR-mediated gene regulation even though high levels of AHR-ARNT complex are bound to DNA. Thus, the results indicate that there is a clear relationship between nuclear export and AHR-mediated gene regulation but that the relationship is complex and involves mechanisms that are directly and indirectly related to the AHR protein.
MATERIALS AND METHODS
Chemicals.
TCDD (98% stated chemical purity) was obtained from Radian Corp. (Austin, Tex.) and was solubilized in dimethyl sulfoxide (Me2SO). LMB was a generous gift from M. Yoshida (Osaka University Medical School, Osaka, Japan) and was stored as a concentrated stock in ethanol under N2. Dextran conjugated to Texas red was purchased from Molecular Probes.
Buffers.
Phosphate-buffered saline (PBS) is 0.8% NaCl, 0.02% KCl, 0.14% Na2HPO4, and 0.02% K2HPO4, pH 7.4. Gel sample buffer (2×) is 125 mM Tris (pH 6.8), 4% sodium dodecyl sulfate (SDS), 25% glycerol, 4 mM EDTA, 20 mM dithiothreitol, and 0.005% bromophenol blue. Tris-buffered saline is 50 mM Tris and 150 mM NaCl, pH 7.5. TTBS is 50 mM Tris, 0.2% Tween 20, and 150 mM NaCl, pH 7.5. TTBS+ is 50 mM Tris, 0.5% Tween 20, and 300 mM NaCl, pH 7.5. BLOTTO is 5% dry milk in TTBS. Lysis buffer (2×) is 50 mM HEPES (pH 7.4), 40 mM sodium molybdate, 10 mM EGTA, 6 mM MgCl2, and 20% glycerol. Gel shift buffer (5×) is 50 mM HEPES (pH 7.5), 15 mM MgCl2, and 50% glycerol. Tris-borate-EDTA (0.5×) is 45 mM Tris-borate and 1 mM EDTA.
Antibodies.
Specific antibodies against either the AHR (A-1 and A-1A) or ARNT (R-1) protein are identical to those described previously (16, 30). All antibodies are affinity-purified immunoglobulin G (IgG) fractions. For Western blot analysis, goat anti-rabbit antibodies conjugated to horseradish peroxidase (GAR-HRP) were utilized. For immunohistochemical studies, goat anti-rabbit IgG conjugated to Texas red or fluorescein isothiocyanate (FITC) was used. Both of these reagents were purchased from Jackson Immunoresearch (West Grove, Pa.). Polyclonal rabbit β-actin antibodies were purchased from Sigma (St. Louis, Mo.).
Cell culture lines and growth conditions.
Wild-type Hepa-1c1c7 (Hepa-1) and type I Hepa-1 variants were a generous gift from James Whitlock, Jr. (Department of Pharmacology, Stanford University). These cells were propagated in Dulbecco's minimum essential medium supplemented with 5% fetal bovine serum (FBS). E36 cells are a Chinese hamster ovary cell line obtained from Alan Schwartz (Washington University, St. Louis, Mo.) that has minimal levels of AHR protein expression (4). Cells were propagated at 30°C in α minimum essential medium supplemented with 10% FBS and 4.5 g of glucose per liter. All other cells were obtained from the American Type Culture Collection (Manassas, Va.). HepG2 cells were propagated in Dulbecco's minimum essential medium supplemented with 10% FBS. All cells were passaged at 1-week intervals and used in experiments over a 2-month period. For treatment regimens, TCDD (2 nM) or LMB (5 nM) was administered directly into growth medium for the indicated incubation times. The vehicle used for TCDD was Me2SO at a final concentration of 0.02%. The vehicle used for LMB was ethanol at a final concentration of 0.1%.
Preparation of total cell lysates, cytosol, nuclear lysates, and nuclear extracts.
Following treatment, cell monolayers were washed twice with PBS and detached from plates by trypsinization (0.05% trypsin–0.5 mM EDTA). Cell pellets were processed for total cell lysates, cytosol, or total nuclear lysates essentially as detailed previously (4, 16, 28). Nuclear extracts were prepared by incubating isolated nuclei in MENG buffer supplemented with 400 mM KCl as detailed previously (4, 31, 32). Protein concentrations were determined by the Coomassie Blue Plus assay (Pierce, Rockford, Ill.) with bovine serum albumin as the standard.
Quantitative Western blot analysis of AHR.
The linearity of the AHR, ARNT, and β-actin antibodies for detection of target proteins and the quantitative Western blotting procedure has been detailed previously (16, 28, 29, 32, 41). Briefly, enhanced chemiluminescence (ECL) exposures were scanned into a Power Macintosh computer utilizing an HP Sanjet II cx/T with Adobe Photoshop 5.0 software. Images were quantified utilizing NIH Image 1.55 software. The raw level of AHR protein was then divided by the level of β-actin protein to generate normalized values for the concentration of the AHR in each sample. In all studies, the trend of the data was never affected by the normalization procedure.
Immunofluorescence staining and microscopy.
All immunocytochemical procedures (cell plating, fixation, staining, and photography) were carried out as previously described (4, 28, 30, 32). Cells were observed on a Zeiss Axiophot microscope using the 568-nm filter. On average, 15 to 20 fields (5 to 20 cells each) were evaluated on each coverslip and three to four fields were photographed to generate the raw data. Experiments were repeated at least two times.
Electrophoretic mobility shift assay (EMSA).
A double-stranded xenobiotic response element (XRE) fragment corresponding to the consensus XRE-1 of the CYP1A1 promoter (39) was labeled with [32P]dCTP by Klenow fill-in (37). Five micrograms of nuclear extract was then incubated at 22°C for 15 min in 1× gel shift buffer supplemented with KCl (80 mM) and poly(dI-dC) (0.1 mg/ml). In some samples, 0.25 μg of affinity-purified IgG specific to AHR (1-A1) or ARNT (R-1) or preimmune IgG was also added to samples. Approximately 4 ng of 32P-labeled XRE was then added to each sample, and the incubation was continued for an additional 15 min at 22°C. The samples were resolved on 5% acrylamide–0.5% Tris-borate-EDTA gels, dried, and exposed to film.
Construction of AHR NES plasmids and eukaryotic transfections.
All NES mutations were generated with the Quick Change in vitro mutagenesis kit as detailed by the manufacturer (Stratagene, Palo Alto, Calif.). The NES is located between amino acids 63 and 71 in the mouse AHR. Previous studies of this NES have used the human sequence which is located between amino acids 64 and 71 (4, 17). The base plasmid utilized was pSportM′AHR, which contains the full-length mouse AHR cDNA. The NES mutations are indicated in the text. All constructs were sequenced to confirm the presence of the mutation. For transfection studies, cells were plated into 35- or 60-mm-diameter culture dishes and incubated at 30°C for 16 to 24 h. DNA was introduced into the cell with Lipofectamine reagent as detailed by the manufacturer (Gibco). Cells were treated with TCDD (2 nM) or Me2SO 8 to 24 h after transfection. Cells were harvested from plates by trypsinization, and total cell lysates were prepared as detailed above. For studies of protein localization, 5 × 105 E36 cells were plated into 60-mm-diameter culture dishes containing poly-l-lysine-coated glass coverslips. Cells were then transfected, treated with TCDD or vehicle, and stained for AHR as detailed previously (4, 28, 30, 32).
In vitro protein expression, activation, and immunoprecipitation.
Protein was produced in vitro with the TNT coupled reticulolysate system (Promega, Madison, Wis.). In vitro activation of the AHR was carried out for 2 h at 30°C with 10 nM TCDD as previously detailed (4, 26, 32). Immunoprecipitation of 35S-AHR–ARNT complexes was carried out with the anti-AHR (A-1A) antibody following in vitro activation as previously detailed (16, 32).
Luciferase reporter gene assay.
The reporter gene constructs used in these studies were pGudLuc 1.1, which contains a 484-bp fragment from the murine CYP1A1 promoter (9); p-1897Om1A3luc, which contains an 1,897-bp fragment isolated from the rainbow trout CYP1A3 promoter (2); pWFG101, which contains a 2,321-bp fragment (−2296 to +25) isolated from the mouse CYP1B1 promoter; and pMinLuc, which was produced by ligating an oligonucleotide corresponding to the consensus XRE-1 of the CYP1A1 promoter (39) into pGL2 (Promega). Cells were transfected with the indicated plasmids and a constitutive β-galactosidase expression vector (pSV-β-galactosidase) and treated with TCDD or vehicle for the times indicated in the text. Cells were then scraped from plates in 1× reporter gene buffer (Promega), and luciferase and β-galactosidase activities were quantified as detailed by the manufacturer. The raw luciferase activity was then divided by the β-galactosidase activity to control for transfection efficiency. In all experiments, the overall trend of the data was never changed by the normalization procedure.
Microinjection of AHR.
AHR protein was produced by the in vitro TNT system, and expression was quantified by Western blotting. The volume of the TNT sample was then adjusted with PBS so that the level of expressed AHR protein was equal, and the sample was then supplemented with 70-kDa dextran-conjugated Texas red (final concentration of 0.25%). Equal amounts of the AHR-dextran sample were then injected directly into the nuclei of 15 to 20 E36 cells growing on a glass coverslip. Following the injection, the cells were immediately treated with TCDD or Me2SO for 4 to 8 h and then fixed and stained as detailed previously (4, 28, 30, 32).
RESULTS AND DISCUSSION
Treatment of HepG2 cells with LMB results in accumulation of AHR-ARNT complexes in the nucleus but reduced levels of TCDD-induced gene induction.
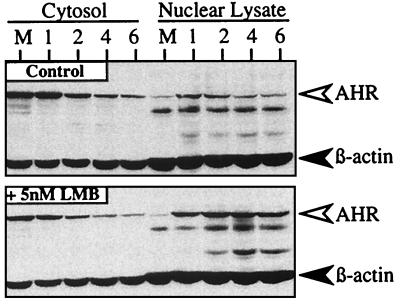
Previous studies have established that one function of the AHR NES is to facilitate export of the liganded AHR from the nucleus so that the protein can be degraded by the cytoplasmic 26S proteasome (4). Since this process removes the liganded AHR from its site of action, it has been hypothesized that nuclear export may play an important role in the activity of AHR-responsive genes by controlling the level of AHR protein (4). To begin analysis of this hypothesis, studies were initiated to evaluate whether transcriptionally active AHR protein would be increased in the nucleus when nuclear export was blocked by LMB. HepG2 cells were preincubated with LMB or vehicle (ethanol) for 3 h and exposed to TCDD for an additional 1 to 8 h, and the levels of AHR and β-actin protein were evaluated in nuclear lysate and cytosolic fractions by Western blotting. In the absence of LMB, TCDD treatment resulted in detectable AHR protein in the nuclear lysate fraction between the 1- and 2-h points that declined to basal levels by 8 h (Fig. 1). This transient pattern is consistent with the rapid degradation of the AHR observed in other cell lines (12, 24, 28) and was directly confirmed by indirect immunofluorescence microscopy (unpublished results). In contrast, high levels of AHR protein were detected in the nuclear lysate fraction at all time points when cells were pretreated with LMB for 3 h prior to TCDD exposure (Fig. 1). Importantly, the majority of AHR protein remained nuclear and was not redistributed to the cytoplasmic fraction at later time points. The high level of nuclear AHR was also confirmed by indirect immunofluorescence microscopy (unpublished results). Since Western blot analysis does not provide information on the functionality of the nuclear AHR, studies were performed to directly test whether the nuclear AHR represented AHR-ARNT complexes bound to DNA.
FIG. 1.
Accumulation of AHR in the nucleus of HepG2 cells exposed to LMB and TCDD. Duplicate plates of HepG2 cells were exposed to ethanol (0.1%) or LMB (5 nM) for 3 h followed by TCDD (2 nM) for an additional 1 to 6 h. Cell pellets were then solubilized in lysis buffer, and cytosol (10 μg) and nuclear lysates (18 μg) were resolved by SDS-PAGE. Gels were then blotted and stained with A-1A IgG (1.0 μg/ml) and β-actin IgG (1:1,000) and visualized by ECL with GAR-HRP IgG (1:10,000). 1, 2, 4, and 6 represent hours of TCDD exposure. M = cells exposed to Me2SO for 6 h.
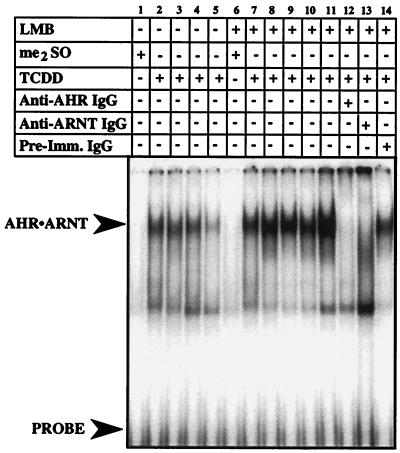
HepG2 cells were preincubated with LMB or ethanol for 3 h and exposed to TCDD for an additional 1 to 8 h, and nuclear extracts were evaluated by EMSA. Figure 2 shows a representative EMSA. In control cells, AHR-ARNT complexes were detected following 1 to 4 h of TCDD exposure but were present at greatly reduced levels by the 8-h point (Fig. 2, lanes 1 to 5). In contrast, high levels of AHR-ARNT complexes were detected at all time points when cells were pretreated with LMB for 3 h prior to TCDD exposure (Fig. 2, lanes 6 to 11). To confirm that the shifted XRE detected in the LMB-treated cells was caused by binding of AHR-ARNT complexes, nuclear extracts were incubated with antibodies specific to AHR or ARNT prior to addition of the XRE oligonucleotide. The addition of the specific antibodies but not preimmune IgG dramatically reduced the observed shift (Fig. 2, lanes 11 to 14). Thus, these results are consistent with the Western blot data (Fig. 1) and demonstrate that inhibition of nuclear export by LMB results in increased levels of AHR-ARNT complex tightly associated with DNA following ligand exposure. Importantly, these results are identical to those obtained when AHR degradation was blocked at the level of the 26S proteasome (4).
FIG. 2.
Potentiation of XRE binding activity in nuclear extracts isolated from HepG2 cells treated with LMB and TCDD. Duplicate plates of HepG2 cells were exposed to ethanol (0.1%) or LMB (5 nM) for 3 h followed by TCDD (2 nM) for an additional 1 to 8 h. Nuclear extracts were prepared, and 5 μg was evaluated by EMSA. Lanes 1 and 6, 8-h exposure to Me2SO. Lanes 2, 7, and 12 to 14, 1-h TCDD exposure. Lanes 3 and 8, 2-h TCDD exposure. Lanes 4 and 9, 4-h TCDD exposure. Lane 10, 6-h TCDD exposure. Lanes 5 and 11, 8-h TCDD exposure.
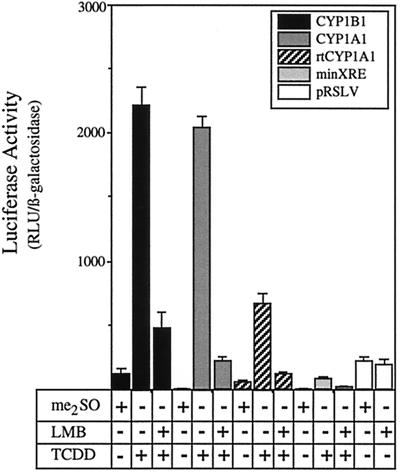
Having demonstrated that inhibition of nuclear export resulted in increased levels of AHR-ARNT complexes in the nucleus, it was pertinent to investigate whether the increase in these complexes would affect AHR-mediated gene regulation. To address this issue, HepG2 cells were transfected with luciferase reporter constructs containing promoter domains isolated from the murine CYP1A1 gene (pGudLuc 1.1 [9]), the murine CYP1B1 gene (pWFG101), or the rainbow trout CYP1A3 gene (p-1897Om1A3luc [2]). In addition, gene induction was evaluated with a luciferase reporter driven by the minimal simian virus 40 promoter and a single XRE oligonucleotide (pMinLuc). Following transfection, cells were treated with LMB or ethanol for 3 h, exposed to TCDD for an additional 10 h, and then assayed for luciferase activity. Surprisingly, pretreatment of cells with LMB markedly decreased the TCDD-mediated luciferase activity of all the reporter constructs by 75 to 89% (Fig. 3). For example, the pGudLuc 1.1 reporter, consisting of a 484-bp fragment from the murine CYP1A1 gene with four XREs (9), was induced 165-fold under control conditions but only 19-fold when exposed to TCDD in the presence of LMB (89% reduction in activity). Similar levels of inhibition were observed for the murine CYP1B1 promoter (81% reduction in activity), the rainbow trout CYP1A3 promoter (79% reduction in activity), and the minimal promoter construct as well (75% reduction in activity). Importantly, LMB treatment had no effect on the luciferase activity of a constitutive luciferase reporter (Fig. 3), suggesting that the reduced levels of reporter gene activity were not due to direct inhibition of luciferase activity or nuclear export of luciferase mRNA.
FIG. 3.
Inhibition of TCDD-induced reporter gene activity in HepG2 cells exposed to LMB. HepG2 cells were cotransfected with reporter constructs containing the indicated promoter sequences and pSV-β-galactosidase. Twenty-four hours after transfection, triplicate plates were treated with ethanol (0.1%) or LMB (5 nM) for 3 h followed by exposure to TCDD (2 nM) or Me2SO (0.02%) for an additional 10 h. A set of cells were also transfected with a constitutive luciferase reporter (pRSLV) under the control of the Rous sarcoma virus promoter (white bars). Cells were then harvested into reporter lysis buffer and assayed for luciferase and β-galactosidase activity. Data are expressed as the means ± standard deviations for three samples of the normalized luciferase activity which represents the relative luciferase units (RLU) divided by the β-galactosidase activity. Note that LMB did not affect the activity associated with the constitutive luciferase reporter.
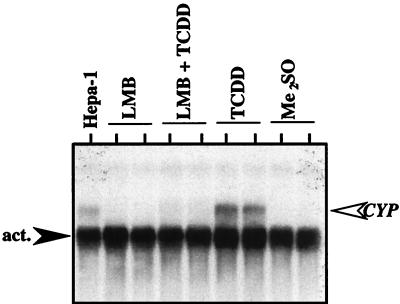
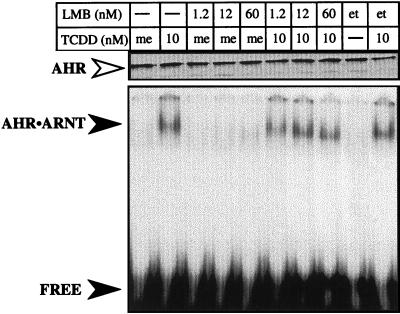
Since the promoter region of a reporter construct does not represent the true context of the endogenous gene, studies were initiated to determine whether LMB would affect TCDD-mediated induction of endogenous CYP1A1. HepG2 cells were treated with LMB or ethanol for 3 h and exposed to TCDD for an additional 10 h, and total RNA was isolated. The level of CYP1A1 mRNA was then determined by Northern blotting. As expected, CYP1A1 mRNA was induced in HepG2 cells following exposure to TCDD (Fig. 4). However, induction of CYP1A1 by TCDD was strongly reduced following preincubation of cells with LMB (Fig. 4). These results are consistent with the data from the reporter gene studies and suggest that some aspect of nuclear export is required for efficient transcription from several AHR-regulated promoters and at the endogenous CYP1A1 gene. However, it is also possible that LMB could be acting directly at the AHR to affect TCDD binding or the ability of TCDD to transform the AHR into a functional AHR-ARNT complex. To address this issue in a functional manner, AHR protein was produced in vitro, mixed with ARNT, and activated with TCDD or LMB. The formation of an AHR-ARNT complex was then evaluated by EMSA as detailed above. The results show that LMB alone does not affect the formation of AHR-ARNT complexes and that a fivefold excess of LMB (60 nM) does not compete with the ability of TCDD to transform the AHR to a DNA binding protein (Fig. 5). Importantly, the highest concentration of LMB used (60 nM) was 12-fold higher than the concentration used in cell culture experiments (Fig. 1 to 4). Thus, taken together with the results of previous studies (4), these data add further support to the hypothesis that the inhibitory effect of LMB on TCDD-mediated gene induction is the result of a block in nuclear export and not the interaction of LMB directly at the AHR protein. However, since LMB is a global inhibitor of CRM-1-mediated export and has numerous effects on cell function independent of the AHR (17, 18), it is not possible to directly determine the relationship between nuclear export of the AHR and AHR-mediated gene regulation using LMB. Thus, experiments shifted to the generation of a functional AHR protein that was defective in nuclear export.
FIG. 4.
Inhibition of CYP1A1 mRNA induction in HepG2 cells exposed to LMB. LMB, HepG2 cells treated with LMB (5 nM) for 13 h. LMB + TCDD, HepG2 cells treated with LMB (5 nM) for 3 h followed by exposure to TCDD (2 nM) for an additional 10 h. TCDD, HepG2 cells treated with ethanol (0.1%) for 3 h followed by exposure to TCDD (2 nM) for an additional 10 h. Me2SO, HepG2 cells treated with ethanol (0.1%) for 3 h followed by Me2SO (0.02%) for an additional 10 h. After each incubation, total RNA was extracted from cells and evaluated for CYP1A1 and β-actin expression as described in Materials and Methods. Each lane represents an independent plate of cells. Hepa-1 = 10 μg of total RNA from murine Hepa-1 cells treated with TCDD for 24 h.
FIG. 5.
Analysis of LMB with AHR protein. wtAHR protein (approximately 15 ng) was expressed in vitro and mixed with an equal amount of ARNT protein. The samples were then incubated in the presence of TCDD (10 nM) or Me2SO (0.1%) in the presence or absence of varying concentrations of LMB (1.2, 12, or 60 nM) or ethanol (0.1%) for 2 h at 30°C and analyzed immediately. Equal amounts of each sample were analyzed by EMSA with 32P-labeled XRE as detailed in Materials and Methods. The specifically shifted AHR-ARNT complex and free probe are indicated by the closed arrowheads. The Western blot at the top of the EMSA represents an aliquot of the exact sample used for the EMSA that was stained for AHR (open arrowhead). Note that the concentration of AHR in each sample is similar. Numbers indicate nanomolar concentrations. me, Me2SO; et, ethanol.
Amino acid substitutions within the NES negatively affect AHR function independent of nuclear export.
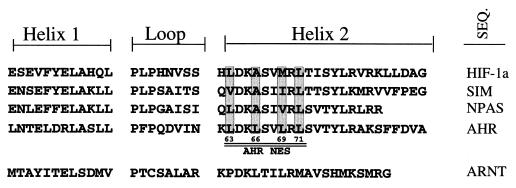
The putative NES of the AHR is a leucine-rich sequence (LDKLSVLRL) located between amino acids 63 and 71 in the proximal region of the helix 2 domain of the murine AHR. Since the helix-loop-helix is directly involved in dimerization of basic helix-loop-helix–periodicity-ARNT-single-minded protein (bHLH-PAS) proteins (1, 5, 21, 34), it was first necessary to compare several ARNT binding bHLH-PAS proteins to determine overlap between dimerization and nuclear export. Interestingly, sequence alignment showed that five of the nine amino acids of the NES were conserved among hypoxia factor 1α (HIF-1α), the single-minded protein, and the nuclear PAS protein, even though nuclear export has not been described for these proteins (Fig. 6). Importantly, only the leucine residues that bracketed the AHR NES (positions 63 and 71) were conserved among all the proteins, while leucine 66 was an alanine in the other sequences and the residues that aligned with leucine 69 were valine, isoleucine, or methionine. Therefore, leucines 66 and 69 were selected for change, and four AHR NES mutants were produced by in vitro mutagenesis. AHRA66 contains a leucine-to-alanine change at amino acid 66. AHRA69 contains a leucine-to-alanine change at amino acid 69. AHRAM contains a leucine-to-alanine change at amino acid 66 and a leucine-to-methionine change at amino acid 69. This amino acid change was made so that AHRAM showed 100% identity to the HIF-1α protein from amino acids 64 to 71 (Fig. 6). Finally, AHRΔNES contains leucine-to-alanine changes at amino acids 69 and 71.
FIG. 6.
Alignment of HLH regions of bHLH-PAS proteins. The HLH regions for the mouse HIF-1α, single-minded protein, nuclear PAS, AHR, and ARNT proteins were analyzed by Lasergene software (DNASTAR Inc., Madison, Wis.). The helix 1, loop, and helix 2 regions are shown. The putative NES within helix 2 of AHR is underlined. Conserved leucine residues with the AHR NES are indicated. SEQ., sequence.
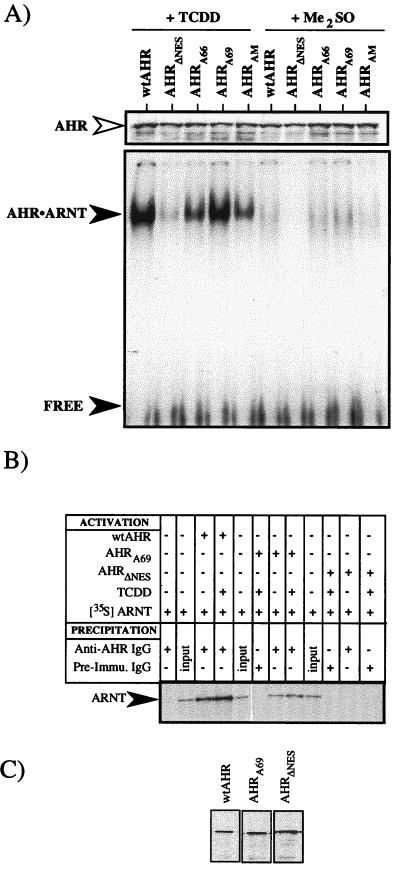
To assess the function of the various NES mutants, wild-type AHR (wtAHR), AHRA66, AHRA69, AHRAM, and AHRΔNES proteins were expressed in vitro and quantified by Western blotting. Equal amounts of each AHR were then mixed with an equal amount of ARNT protein, and the sample was incubated in the presence of TCDD or Me2SO for 2 h at 30°C. The formation of AHR-ARNT complexes was then evaluated by EMSA. Figure 7A shows that all the AHR NES mutants exhibited some level of TCDD-dependent binding to the XRE compared to the wtAHR protein. However, AHRΔNES bound at only 18% of the level of wtAHR, while AHRA66 and AHRAM bound at approximately 50% of the level of wtAHR. In contrast, the AHRA69 protein functioned at approximately 92% of the level of the wtAHR in the context of the in vitro EMSA. To further assess the binding of AHRA69, EMSA studies were repeated with varying levels of in vitro-activated wtAHR and AHRA69 proteins. Analysis of the shifted XRE showed that both wtAHR and AHRA69 produced similar levels of shifted XRE at all concentrations, and the compiled data showed an identical slope over the concentration range used (R. S. Pollenz, unpublished data). Thus, the A69 mutation does not appear to affect the activation or DNA binding of the AHR and the protein functions at the same level as the wtAHR.
FIG. 7.
Functional analysis of mutations within the NES of the AHR. The indicated AHR protein was expressed in vitro and mixed with an equal amount of cold or 35S-labeled ARNT protein (approximately 15 ng). The samples were then incubated in the presence of TCDD (10 nM) or Me2SO (0.1%) for 2 h at 30°C and analyzed immediately. (A) Equal amounts of each sample were analyzed by EMSA with 32P-labeled XRE as detailed in Materials and Methods. The specifically shifted AHR-ARNT complex and free probe are indicated by the closed arrowheads. The Western blot at the top of the EMSA represents an aliquot of the exact sample used for the EMSA that was stained for AHR (open arrowhead). Note that the concentration of AHR in each sample is similar. (B) Equal amounts of each sample were precipitated with anti-AHR IgG (A-1A) or preimmune IgG and resolved by SDS-PAGE. Specificity is demonstrated by the lack of 35S-labeled ARNT in samples precipitated with preimmune IgG. (C) The Western blot shown represents an aliquot of the exact AHR sample used for the immunoprecipitation that was stained for AHR (open arrowhead). Note that the concentration of AHR in each sample is similar.
To better understand why the AHRΔNES exhibited reduced activity in the in vitro activation assay, immunoprecipitation studies were carried out. Equal amounts of wtAHR, AHRΔNES, and AHRA69 were mixed with 35S-labeled ARNT and activated with TCDD or Me2SO for 2 h at 30°C. The samples were then immunoprecipitated with IgG against AHR (A-1A) and protein A-Sepharose, and the pelleted material was resolved by SDS-polyacrylamide gel electrophoresis (PAGE). The results show that wtAHR and AHRA69 associate with 35S-labeled ARNT in a TCDD-dependent manner, while samples containing AHRΔNES do not appear to associate with ARNT to a significant degree (Fig. 7B). Thus, the reduced level of DNA binding observed in the gel shift assay (Fig. 7A) is explained by the reduced level of dimerization of AHRΔNES and is consistent with a mutation of the conserved leucine at position 71. Collectively, these results show that the AHRA69 protein exhibits TCDD-dependent function at the level of dimerization and DNA binding in vitro. However, substitutions at residue 66 or 71 appear to negatively affect AHR function at the level of dimerization and DNA binding that is independent of any nuclear export function.
AHRA69 is defective in nuclear export and is not degraded efficiently.
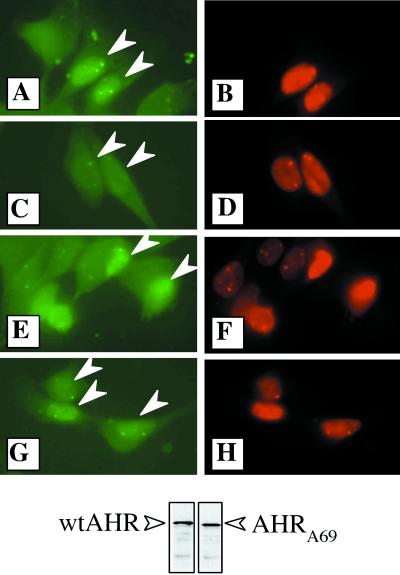
It was next necessary to determine whether the AHRA69 protein was defective in nuclear export. In the first set of studies, a microinjection strategy was utilized. First, wtAHR and AHRA69 proteins were produced in vitro and evaluated by Western blotting to ensure that equal levels were expressed (Fig. 8, bottom panel). The AHR was then mixed with 70-kDa dextran-conjugated Texas red (0.5% final concentration) and microinjected directly into the nuclei of E36 cells. Following microinjection, cells were treated with either TCDD or Me2SO for 6 h and then fixed and stained for AHR which was visualized with goat anti-rabbit–FITC. The dextran-Texas red conjugate serves as a control to identify those cells that were injected and as a marker of nuclear integrity since 70-kDa dextran is too large to freely diffuse through the nuclear pore complex. The results show that specific staining for the AHR is observed only in the nuclei of cells that exhibit concomitant Texas red fluorescence (Fig. 8A, B, and E to H, white arrowheads). However, when cells containing wtAHR were exposed to TCDD for 6 h, there was a dramatic reduction in AHR immunoreactivity compared to that for Me2SO-treated cells (Fig. 8, compare panels A and C). Importantly, while wtAHR immunofluorescence was reduced following TCDD treatment, Texas red fluorescence remained essentially unchanged, illustrating that reductions were not related to a loss of nuclear integrity. In contrast, high levels of AHRA69 were observed in the nuclei of TCDD-treated cells and the staining intensity remained similar to that observed for cells treated with Me2SO (Fig. 8, compare panel G to panels A and E). Collectively, these results suggest that the AHRA69 is not efficiently exported from the nucleus and thus is not readily degraded following TCDD exposure. These findings are in agreement with previous studies that have established that the liganded AHR is rapidly degraded in the cytoplasm following nuclear export (4, 28) and support a hypothesis that AHRA69 is defective in nuclear export.
FIG. 8.
Distribution of wtAHR and AHRA69 protein injected into E36 cells. wtAHR and AHRA69 proteins were expressed in vitro and mixed with 70-kDa dextran-conjugated Texas red. The samples were then injected into the nuclei of E36 cells and exposed to TCDD (2 nM) or Me2SO (0.02%) for 6 h at 30°C. Following the incubation, cells were fixed and stained with 1 μg of A-1 IgG per ml followed by goat anti-rabbit–FITC (1:500). Identical fields showing FITC fluorescence (A, C, E, and G) and Texas red fluorescence (B, D, F, and H) are shown. (A and B) Cells injected with wtAHR and exposed to Me2SO. (C and D) Cells injected with wtAHR and exposed to TCDD. (E and F) Cells injected with AHRA69 and exposed to Me2SO. (G and H) Cells injected with AHRA69 and exposed to TCDD. Arrowheads indicate the nuclei injected with AHR protein. The Western blot shown at the bottom represents an aliquot of the exact wtAHR and AHRA69 proteins used for the microinjection that were stained for AHR (open arrows).
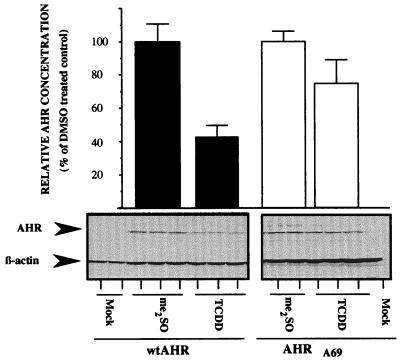
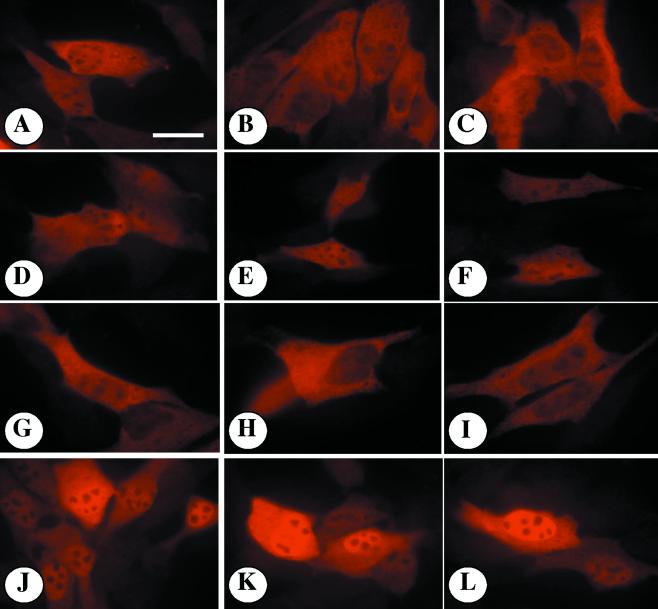
To directly quantify the TCDD-induced degradation of AHRA69, expression vectors containing wtAHR or AHRA69 were transfected into E36 cells. Ten hours after transfection, cells were exposed to TCDD or Me2SO for 16 h and either lysed to produce total cell lysates or fixed and stained for AHR to evaluate subcellular distribution. Figure 9 shows a representative Western blot stained for AHR and β-actin. In mock-transfected cells, there is little detectable AHR, while cells expressing wtAHR and AHRA69 express a protein that migrates at approximately 95 kDa. When the transfected cells were treated with TCDD for 16 h, wtAHR was reduced by 55% compared to the level for Me2SO-treated cells. In contrast, AHRA69 protein was depleted by only 22% compared to the level in Me2SO-treated cells following 16 h of exposure (Fig. 9). These findings support the results of the microinjection studies and are consistent with the observation that the AHRA69 is not degraded efficiently. To correlate these results with the subcellular location of wtAHR or AHRA69, transfected cells were fixed and stained. Representative fields of cells are presented in Fig. 10. The wtAHR or AHRA69 protein was highly expressed in transfected cells and exhibited a predominately cytoplasmic pattern of fluorescence when treated with Me2SO (Fig. 10A to C and G to I). The specificity of the A-1 antibody is demonstrated by the lack of staining in nontransfected cells. When cells expressing wtAHR were treated with TCDD for 24 h, there was a general reduction in the fluorescence intensity, but the overall staining pattern remained predominately cytoplasmic with modest accumulation of AHR in the nuclear compartment (Fig. 10D to F). In contrast, cells expressing the AHRA69 protein showed markedly increased levels of AHR staining in the nucleus following 24 h of TCDD exposure and exhibited low to moderate levels of cytoplasmic fluorescence (Fig. 9J to L). Taken together with the results of the microinjection studies (Fig. 8), these data provide further evidence that the putative NES present in the AHR has reduced function when leucine 69 is changed to an alanine and that blockage of nuclear export at the level of the AHR inhibits ligand-mediated degradation of the AHR protein.
FIG. 9.
Western blot analysis of recombinant AHR protein expression in E36 cells exposed to TCDD. E36 cells were transfected with expression vectors for wtAHR or AHRA69. Triplicate plates were exposed to TCDD (2 nM) or Me2SO (0.02%) for 16 h, and 18 μg of total cell lysate was resolved by SDS-PAGE. Blots were stained with A-1A IgG (1.0 μg/ml) and β-actin IgG (1:1,000) and visualized by ECL with GAR-HRP IgG (1:10,000). Bands were quantified and normalized as detailed previously (14, 17, 19, 23, 25). Data are expressed as the percentages of wtAHR or AHRA69 protein compared to that for Me2SO (DMSO)-treated controls. Bars represent the averages ± standard errors of three independent samples.
FIG. 10.
Subcellular localization of recombinant AHR protein expression in E36 cells exposed to TCDD. All slips were incubated with A-1 IgG (1.0 μg/ml) and visualized with goat anti-rabbit–Texas red IgG (1:750). (A to C) E36 cells expressing wtAHR and stained for AHR following a 24-h exposure to Me2SO (0.02%). (D to F) E36 cells expressing wtAHR and stained for AHR following a 24-h exposure to TCDD (2 nM). (G to I) E36 cells expressing AHRA69 and stained for AHR following a 24-h exposure to Me2SO (0.02%). (J to L) E36 cells expressing AHRA69 and stained for AHR following a 24-h exposure to TCDD (2 nM). All panels were photographed and printed for identical times. Note the greatly reduced AHR reactivity in untransfected cells. Bar = 10 μm.
TCDD-mediated gene induction is not inhibited in cells expressing an AHR defective in nuclear export.
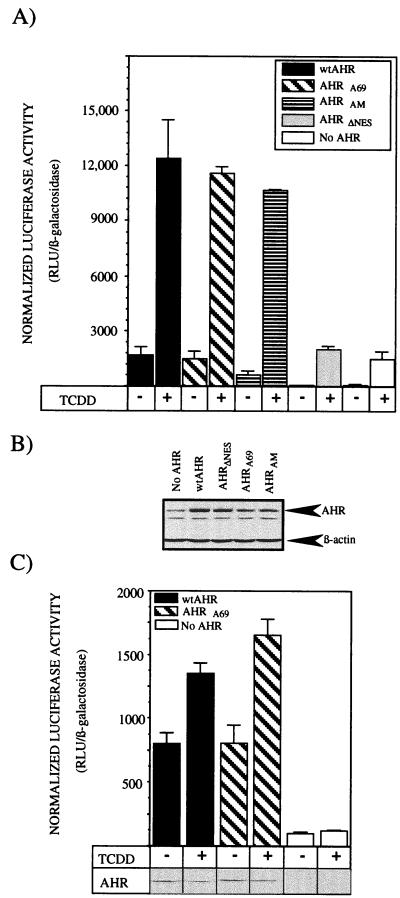
The previous studies have demonstrated that the AHRA69 (i) accumulates in the nucleus in a ligand-dependent manner, (ii) is capable of forming a functional dimer with ARNT protein, (iii) associates with putative XRE oligonucleotides in vitro, and (iv) is not efficiently exported from the nucleus following ligand exposure. Thus, the final set of studies focused on whether AHRA69 could support TCDD-mediated induction of CYP1A1. To address this issue, expression vectors containing wtAHR, AHRA69, AHRAM, or AHRΔNES were cotransfected into the type I Hepa-1 or E36 cell line along with the TCDD-responsive pGudLuc 1.1 luciferase reporter and a constitutive β-galactosidase construct. Similar to the E36 cell line, type I cells have reduced levels of AHR protein and show minimal levels of TCDD-inducible gene induction (24, 48). Ten hours after transfection, cells were exposed to TCDD or Me2SO for 16 h and either processed for total cell lysates or analyzed for the level of luciferase and β-galactosidase activity. A representative experiment for the type I cells is shown in Fig. 11A. Type I cells transfected with only pGudLuc 1.1 exhibited low levels of constitutive reporter gene activity that were induced approximately 21-fold following exposure to TCDD. When wtAHR was expressed in the type I cells, however, the basal luciferase activity increased 25-fold above background and was induced an additional 7-fold following TCDD exposure. The increased level of basal luciferase activity in the absence of exogenous ligand was consistent with numerous other reports that have utilized reporter genes to assess AHR function and likely reflects the high level of AHR or ARNT protein that is expressed in the transfected cells (14, 20, 22, 23). Importantly, AHRA69 and AHRAM also showed increased levels of basal luciferase activity in the absence of exogenous ligand but were induced an additional 7- to 13-fold following addition of TCDD. In contrast, the cells expressing the AHRΔNES did not show increased levels of luciferase activity above background and also did not support induction of luciferase above the level induced by the endogenous AHR (Fig. 11A). These results are consistent with the reduced ability of AHRΔNES to dimerize with ARNT and bind DNA. To confirm that exogenous AHR protein was expressed in the transfected cells, Western blot analysis was performed on plates of cells that were transfected with the identical DNA solutions used to generate the data in Fig. 11A. The results show that the level of AHR in each population is increased above the endogenous level found in cells transfected with parental vector alone. Importantly, the high level of expression of AHRΔNES indicates that the reduced functionality of this protein is not due to reduced expression. The lower level of AHRA69 and AHRAM protein than of wtAHR was consistent with a reduced level of transfection efficiency as determined by analysis of β-galactosidase activity.
FIG. 11.
Induction of TCDD-induced reporter gene activity in cells expressing AHRA69. Type I Hepa-1 E36 CHO cells were cotransfected with pGudLuc 1.1, pSV-β-galactosidase, and pSport expression vectors containing wtAHR, AHRA69, AHRAM, or AHRΔNES. Control plates of cells were transfected with pGudLuc 1.1, pSV-β-galactosidase, and pSport (no AHR). Eight hours after transfection, triplicate plates of cells were treated with TCDD (2 nM) or Me2SO (0.02%) for 16 h. (A) Type I cells were harvested into reporter lysis buffer and assayed for luciferase and β-galactosidase activity. Data are expressed as the means ± standard deviations for three samples of normalized luciferase activity which represents the relative luciferase units (RLU) divided by the β-galactosidase activity. (B) Western blot analysis of total cell lysates from plates of type I cells transfected with the identical DNA solutions used for panel A. Blots were stained with A-1A IgG and β-actin IgG as detailed in the text. (C) E36 cells were harvested into reporter lysis buffer and assayed for luciferase and β-galactosidase activity. Data are expressed as the means ± standard deviations for three samples of normalized luciferase activity which represents the relative luciferase units (RLU) divided by the β-galactosidase activity. Shown are results of Western blot analysis of total cell lysates from representative plates of E36 cells transfected with the identical DNA solutions used to generate the luciferase data. Blots were stained with A-1A IgG as detailed in the text.
In the E36 cells, the expression of both wtAHR and AHRA69 increased luciferase activity above basal levels as observed in the type I studies and also induced increased levels of luciferase in the presence of TCDD (Fig. 11C). Interestingly, the overall level of induction above basal activity was not as high as that observed in the type I cells (1.5- to 2.5-fold), and the AHRA69 was able to induce activity to a slightly higher level than was wtAHR. These findings may be the result of a higher level of AHR expression in the transfected E36 cells or reduced levels of endogenous AHR protein. To confirm the expression of the AHR, Western blot analysis was carried out on representative cell lysates produced from cells transfected with the identical DNA samples used to produce the luciferase data. In all samples, AHR was expressed with the wtAHR showing a 50% reduction in concentration following TCDD exposure while the AHRA69 remained at a level similar to that in Me2SO-treated cells (as shown in Fig. 9). Collectively, the results of these experiments indicate that the AHRA69 protein functions in AHR-mediated gene induction. Thus, the inhibition of nuclear export directly at the level of the AHR is not a component of the inhibition of gene induction observed when cells are exposed to LMB.
Conclusions and implications.
Currently, there are several signaling cascades that define a novel biological theme in which the interplay between nuclear export and protein degradation plays a major role in determining the magnitude and duration of gene regulation. For example, the level of p53 protein is controlled in part by nuclear export and subsequent degradation by cytoplasmic proteases (8, 14, 44, 45). If nuclear export of p53 is inhibited, there is a general upregulation of p53-dependent genes (8, 37). Another pathway involves p27Kip1, p27Kip1 is a cyclin-dependent kinase that arrests cells in G1 (40) and appears to be reduced in certain types of cancer (43). Importantly, the level of p27Kip1 is controlled in part through nuclear export following association with p38 (JAB1), a protein that contains an NES and allows export of p27Kip1 to the cytoplasm for degradation (27, 46). The common theme in both pathways is that the export of the effector protein from the nucleus results in reduced levels of gene regulation since the protein is being removed from its site of action. The studies presented in this report show that nuclear export and protein degradation are essential components of the AHR-mediated signal transduction pathway as well.
Interestingly, the relationship between nuclear export and AHR-mediated gene regulation appears to involve the AHR directly and indirectly. In the first instance, the presence of the NES within the AHR sequence appears to be directly related to the transport of the AHR from the nucleus so that the protein is degraded by the cytoplasmic proteasome (4). Since the result of nuclear export is an actual reduction in the level of transcriptionally active AHR protein, proper functioning of nuclear export and the proteolytic machinery likely serves to control the magnitude and duration of gene induction or repression by the AHR-ARNT complex. This view is supported by the observation that ligand exposure results in rapid reductions in the level of AHR protein and AHR-ARNT complexes in vivo and in vitro (28, 29, 31, 33, 36, 41) and the finding that mutations within the AHR NES or direct inhibition of the cytoplasmic proteasome results in reduced degradation of the AHR, high levels of nuclear AHR, and high levels of AHR-mediated gene induction (4). Thus, the direct role of nuclear export would be similar to that observed for the p53 and p27Kip1 pathways (8, 40, 43–46) and would involve attenuation of AHR-mediated gene induction when the pathway is functioning properly. Alternatively, a recent report suggests that the AHR protein is rapidly degraded once it enters the nuclear compartment regardless of ligand exposure, dimerization with ARNT, or the presence of hsp90 (35). While compelling, this hypothesis is not supported by any of the previous (4, 15) or current studies from this lab where high levels of the endogenous AHR protein have been shown to accumulate in the nucleus and exhibit reduced levels of degradation. It must be noted, though, that comparisons of the results of Robert and Whitelaw (35) to studies that have evaluated endogenous AHR protein are difficult because Robert and Whitelaw (35) analyzed a stable cell line expressing a modified form of the AHR (DRNLS) that is likely functioning in an aberrant manner compared to wtAHR protein (which was not tested in the studies). For example, it is intriguing that the kinetics of DRNLS degradation were not affected by the presence of AHR ligands, hsp90, or ARNT (35). Such findings call into question the physiological significance of the DRNLS model for studying AHR proteolysis, since it is clear that the endogenous AHR can be translocated to the nucleus in the presence of hsp90 and is protected from degradation (4, 15). Thus, while it cannot be ruled out that some level of ligand-bound AHR might be degraded by a nuclear protease in certain cells or tissues, the current data overwhelmingly support a model in which AHR is degraded in the cytoplasm by the 26S proteasome following ligand binding, nuclear import, and nuclear export (references 4 and 15 and present studies).
While the function of the AHR NES correlates well with ligand-mediated degradation of the AHR (4), an explanation for the inhibition of AHR-mediated gene induction following exposure of cells to LMB is less clear. The data suggest that a nuclear export-dependent process is also important in AHR-mediated signaling but that it is independent of the nuclear export of the AHR. This view is supported by the following observations. First, the AHR is accumulated in the nucleus following LMB exposure and appears to be associated with ARNT in a complex bound to DNA. Second, inhibition of AHR degradation by proteasome inhibitors results in high levels of accumulation of AHR-ARNT in the nucleus that have a positive impact on AHR-mediated gene regulation (4). Finally, direct mutation of the AHR NES does not have a negative impact on AHR-mediated gene regulation even though the AHR appears to accumulate in the nucleus just as if export is blocked by the use of LMB. Thus, these results are consistent with the presence of an additional negatively acting factor that may or may not associate with AHR and/or ARNT.
There are currently a number of reports that have characterized proteins that act negatively on AHR-mediated signaling. These include a dominant-negative ARNT isoform identified in rainbow trout (26, 32), a factor that associates with ARNT in human fibroblasts (13), and an AHR repressor protein termed AHRR that has the ability to bind ARNT and DNA without inducing genes (25). Thus, there is growing evidence that negative regulators are involved in AHR-mediated signaling. Interestingly, the AHRR appears to be the product of an AHR-inducible gene, and the protein itself shares homology to the AHR within the bHLH region (25). Indeed, amino acid sequence analysis of the AHRR indicates that the protein contains a putative NES within the helix 2 domain that is identical to that of the AHR (KLDKLSVLRLS). While the presence of the NES does not prove that it is functional in the AHRR, it is compelling to hypothesize that the AHRR or a similar factor containing an NES may be responsible for the inhibitory results obtained with LMB. Studies are currently under way to explore these possibilities and further assess the consequences for AHR-mediated gene regulation when nuclear export is inhibited.
ACKNOWLEDGMENTS
Several individuals deserve special thanks for their input in the project. We acknowledge the contribution of Steve Frawley and Bill Faught, who carried out the microinjections; William Greenlee, who provided the CYP1B1 reporter construct and shared unpublished data with the laboratory; Michael Carvan, who provided the rtCYP1A3 reporter construct; Michael Denison, who provided the minimal XRE construct; Chris Bradfield, for the original pSportM′AHR vector; Minoru Yoshida, who generously donated LMB; Brian Necela, who carried out the immunoprecipitation studies; and Alan Schwartz, for providing the E36 cell line and also offering his insights into protein degradation. Finally, we thank Nikos Davarinos for his participation in aspects of this project and Michael Kern for his support and helpful discussions of the work. The reviewers of this work are also acknowledged for their critical insights with regard to the work.
This work was supported in part by National Institutes of Health grants ES-08980 and ES-10401 to R.S.P., and by Institutional Research Funds to the Medical University of South Carolina for 1999-2000.
REFERENCES
- 1.Basci S G, Hankinson O. Functional characterization of DNA binding domains of the heterodimeric AHR complex imputing canonical bHLH protein-DNA interactions. J Biol Chem. 1996;271:8843–8850. doi: 10.1074/jbc.271.15.8843. [DOI] [PubMed] [Google Scholar]
- 2.Carvan M J, Ponomareva L V, Solis W A, Matlib R S, Puga A, Nebert D W. Trout CYP1A3 gene: recognition of fish DNA motifs by mouse regulatory proteins. Mar Biotechnol. 1999;1:155–166. doi: 10.1007/pl00011763. [DOI] [PubMed] [Google Scholar]
- 3.Chang C-Y, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol. 1998;18:525–535. doi: 10.1128/mcb.18.1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davarinos N A, Pollenz R S. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytoplasmic proteasome following nuclear export. J Biol Chem. 1999;274:28707–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 5.Dolwick K M, Swanson H I, Bradfield C A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci USA. 1993;90:8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti K G, Fransen J, Grosveld G. The human homologue of yeast CRM1 is a dynamic subcomplex with Can/nup214 and a novel nuclear pore component Nup 88. EMBO J. 1997;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornerod M, Ohno M, Yoshida M, Mattaj I A. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 8.Freedman D H, Levine A J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrison P M, Tullis K, Aarts J M, Brouwer A, Giesy J P, Denison M S. Species specific recombinant cell lines as bioassay systems for the detection of TCDD-like chemicals. Fundam Appl Toxicol. 1996;30:194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 10.Gerace L. Nuclear export signals and the fast track to the cytoplasm. Cell. 1995;82:341–344. doi: 10.1016/0092-8674(95)90420-4. [DOI] [PubMed] [Google Scholar]
- 11.Giannone J, Li W, Probst M, Okey A. Prolonged depletion of AH receptor without alteration of receptor mRNA levels after treatment of cells in culture with 2,3,7,8-tetrachloro-dibenzo-p-dioxin. Biochem Pharmacol. 1998;55:489–497. doi: 10.1016/s0006-2952(97)00493-0. [DOI] [PubMed] [Google Scholar]
- 12.Giannone J V, Okey A B, Harper P A. Characterization of polyclonal antibodies to the aromatic hydrocarbon receptor. Can J Physiol Pharmacol. 1995;73:7–17. doi: 10.1139/y95-002. [DOI] [PubMed] [Google Scholar]
- 13.Gradin K, Toftgard R, Poellinger L, Berghard A. Repression of dioxin signal transduction in fibroblasts. J Biol Chem. 1999;274:13511–13518. doi: 10.1074/jbc.274.19.13511. [DOI] [PubMed] [Google Scholar]
- 14.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 15.Heid S E, Pollenz R S, Swanson H I. Role of heat shock protein 90 in mediating agonist-induced activation of the aryl hydrocarbon receptor. Mol Pharmacol. 2000;57:82–92. [PubMed] [Google Scholar]
- 16.Holmes J L, Pollenz R S. Determination of ARNT protein concentration and subcellular localization in hepatic and non-hepatic cell culture line. Mol Pharmacol. 1997;52:202–211. doi: 10.1124/mol.52.2.202. [DOI] [PubMed] [Google Scholar]
- 17.Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. 1998;273:2895–2904. doi: 10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- 18.Kudo N, Wolff B, Sekimoto T, Schreiner E, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 19.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle-dependent expression of mammalian CRM-1, a protein involved in nuclear export of proteins. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Dong L, Whitlock J P. Transcriptional activation function of the ARNT protein. J Biol Chem. 1994;269:28098–28105. [PubMed] [Google Scholar]
- 21.Lindebro M C, Poellinger L, Whitelaw M L. Protein-protein interactions via PAS domains: role of the PAS domain in positive and negative regulation of the bHLH/PAS dioxin receptor-ARNT transcription factor complex. EMBO J. 1995;14:3528–3539. doi: 10.1002/j.1460-2075.1995.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason G G F, Witte A-M, Whitelaw M L, Antonsson C, McGuire J, Wilhelmsson A, Poellinger L, Gustafsson J-K. Purification of the DNA binding form of dioxin receptor. Role of the Arnt cofactor in regulation of dioxin receptor function. J Biol Chem. 1994;269:4438–4449. [PubMed] [Google Scholar]
- 23.Matsushita N, Sogawa K, Ema M, Yoshida A, Fujii-Kuriyama Y. A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt. J Biol Chem. 1993;268:21002–21006. [PubMed] [Google Scholar]
- 24.Miller A G, Isreal D, Whitlock J P. Biochemical and genetic analysis of variant mouse hepatoma cells defective in the induction of benzo(a)pyrene-metabolizing enzyme activity. J Biol Chem. 1983;258:3523–3527. [PubMed] [Google Scholar]
- 25.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Necela B, Pollenz R S. Functional analysis of activation and repression domains with the rainbow trout ARNT protein. Biochem Pharmacol. 1999;57:1177–1190. doi: 10.1016/s0006-2952(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 27.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 28.Pollenz R S. The Ah-receptor but not the Arnt protein is rapidly depleted in hepatic and non-hepatic culture cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1996;49:391–398. [PubMed] [Google Scholar]
- 29.Pollenz R S, Santostefano M J, Klett E, Richardson V M, Necela B, Birnbaum L S. A single oral dose of TCDD results in sustained depletion of AHR protein in female Sprague-Dawley rats. Toxicol Sci. 1998;42:117–128. doi: 10.1006/toxs.1998.2439. [DOI] [PubMed] [Google Scholar]
- 30.Pollenz R S, Sattler C A, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994;45:428–438. [PubMed] [Google Scholar]
- 31.Pollenz R S, Necela B. Characterization of two continuous cell lines from Oncorhynchus mykiss for models of AHR-mediated signal-transduction. Aquat Toxicol. 1998;41:31–49. [Google Scholar]
- 32.Pollenz R S, Sullivan H R, Holmes J, Necela B, Peterson R E. Isolation and expression of cDNAs from rainbow trout that encode two novel bHLH/PAS proteins with distinct functions in the presence of the aryl hydrocarbon receptor. J Biol Chem. 1996;271:30886–30896. doi: 10.1074/jbc.271.48.30886. [DOI] [PubMed] [Google Scholar]
- 33.Reick M, Robertson R W, Pasco D S, Fagan J B. Down-regulation of nuclear aryl hydrocarbon receptor DNA-binding and transactivation functions. Mol Cell Biol. 1994;14:5653–5660. doi: 10.1128/mcb.14.9.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reisz-Porszasz S, Probst M R, Fukunaga B N, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor nuclear translocator protein (ARNT) Mol Cell Biol. 1994;14:6075–6086. doi: 10.1128/mcb.14.9.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts B J, Whitelaw M L. Degradation of the basic-helix-loop-helix/Per-ARNT-Sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. J Biol Chem. 1999;274:36351–36356. doi: 10.1074/jbc.274.51.36351. [DOI] [PubMed] [Google Scholar]
- 36.Roman B L, Pollenz R S, Peterson R E. AHR and ARNT expression and CYP1A1 induction in the adult male rat reproductive tract. Toxicol Appl Pharmacol. 1998;150:228–239. doi: 10.1006/taap.1998.8388. [DOI] [PubMed] [Google Scholar]
- 37.Roth J, Dobbelstein M, Freedman D A, Shenk T, Levine A J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Shen E S, Whitlock J P., Jr Protein-DNA interactions at the dioxin responsive enhancer. J Biol Chem. 1992;267:6815–6819. [PubMed] [Google Scholar]
- 40.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 41.Sommer R J, Sojka K, Pollenz R S, Cooke P, Peterson R E. AHR and ARNT protein and mRNA concentrations in rat prostate: effects of stage of development and TCDD. Toxicol Appl Pharmacol. 1999;155:177–189. doi: 10.1006/taap.1998.8597. [DOI] [PubMed] [Google Scholar]
- 42.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (CRM1) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 43.Steeg P S, Abrams J S. Cancer prognostics: past, present and p27. Nat Med. 1997;3:152–154. doi: 10.1038/nm0297-152. [DOI] [PubMed] [Google Scholar]
- 44.Tao W, Levine A J. P19ARF stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao W, Levine A J. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci USA. 1999;96:3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomoda K, Kubota Y, Kato J-Y. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–165. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 47.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 48.Whitlock J P, Galeazzi D R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin receptors in wild type and variant mouse hepatoma cells. Nuclear location and strength of nuclear binding. J Biol Chem. 1984;259:980–985. [PubMed] [Google Scholar]