Abstract
BACKGROUND:
Heart failure is a prominent complication of type 2 diabetes mellitus (T2D). The goal of this study was to provide longitudinal data on cardiac structure and function (and cross-sectional comparison to normal-weight and obese controls without T2D) in individuals followed from adolescence with youth-onset T2D.
METHODS:
In the TODAY study (Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth), echocardiograms were performed at study years 4 to 5 and 9 to 10. Echocardiograms were also obtained at years 8 to 9 in a control population of age, race/ethnicity, and sex-matched normal-weight and obese individuals without diabetes mellitus. Study outcomes were measures of left ventricular structure and function. The cohort included 411 participants with T2D, 194 obese controls, and 51 normal-weight controls.
RESULTS:
At follow-up, mean participant age was 23 years, 65% women, 20% non-Hispanic white, 35% non-Hispanic black, and 39% Hispanic. Ejection fraction was <52% in 11.7% of male participants with T2D. Diastolic function declined during follow-up in participants with T2D (mitral valve lateral E/Em increased 0.72±0.12 in women and 0.50±0.17 in men; P<0.01) and was significantly higher than obese controls (women, 6.65±1.89 versus 5.66±1.37; men, 6.15±1.90 versus 5.26±1.31; P<0.0001). Predictors of adverse changes included hypertension, obesity, female sex, Hispanic and non-Hispanic black ethnicity, worse glycemic control, and elevated heart rate. Cardiac structural abnormalities, left ventricular hypertrophy, or concentric geometry, were highest in those with T2D (15.8% versus 5.7% obese versus 0% normal weight).
CONCLUSIONS:
Adverse changes in cardiac structure and function changed significantly from adolescence to early adulthood in participants with youth-onset T2D.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00081328.
Keywords: cardiovascular diseases, epidemiology, heart rate, risk factors
Heart failure with both reduced and preserved ejection fraction are long-term complications of type 2 diabetes mellitus (T2D). Increases in early-onset T2D and1 obesity prevalence over the last 20 years parallel the lack of decline in cardiovascular morbidity and mortality in younger adults.2,3 Accordingly, efforts to understand the early stages of T2D-related cardiovascular dysfunction are of paramount importance, to characterize an at-risk population at a time when intervention may be more effective. While subclinical cardiac dysfunction (eg, alterations in ventricular structure, strain, and diastolic function) has been noted in4 cross-sectional studies in middle aged adults, there remains limited information at the adolescent-adulthood transition, where many of these changes may be5 reversible. Prior work from our group in the TODAY study (Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth) has demonstrated the3 antecedents of future cardiovascular dysfunction in adolescents with T2D, with higher left ventricular (LV) mass, lower diastolic function, and higher left atrial size relative to normative values in normal-weight and obese control adolescent cohorts. However, whether these are an epiphenomenon of T2D and obesity or represent6 the origin of a progressive decline in cardiac structure and function remains unclear. The most comparable T2D cohort in age to this study and with longitudinal data is the population-based CARDIA study (Coronary Artery Risk Development in Young Adults), where in middle age, T2D was a significant risk factor for changes in cardiac systolic and diastolic function.7,8 No CARDIA participants had T2D at baseline, when the cohort was aged 18 to 30 years.
In this study, we directly address this knowledge gap by examining 5-year longitudinal changes in echocardiographic markers of systolic and diastolic function at the adolescent-adulthood transition. We hypothesized that participants with youth-onset T2D would have measurable progression in parameters linked to cardiac dysfunction later in life. We studied young adults with youth-onset T2D, now in the second and third decade of life, who were enrolled in the TODAY randomized trial and were followed in the observational phase of this study. We used standard regression-based techniques to describe the secular patterns and metabolic correlates of 5-year change in cardiac structure and function in the TODAY participants who had mean durations of T2D of 5 years at the end of the TODAY study and of 10 years during observational follow-up. We further compared these echocardiographic characteristics in individuals with T2D to normal-weight and obese controls without T2D, to highlight differences in LV structure and function compared with those without T2D.
METHODS
Data Sharing
Anonymized data and materials from the TODAY study have been made publicly available at the National Institute of Diabetes and Digestive and Kidney Disease repository (https://repository.niddk.nih.gov/home/). Data from the observational follow-up phase will be available, through the repository, at the end of the funding period.
Study Participants
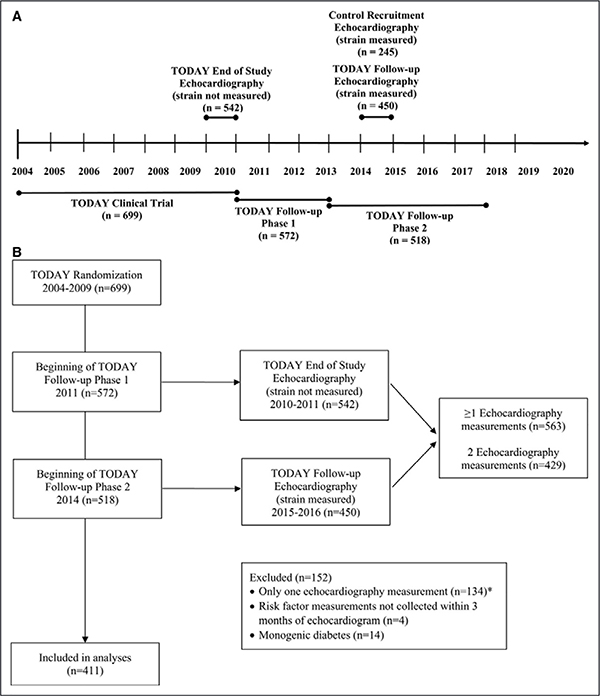
The study flow is shown in Figure 1. The TODAY, TODAY control, and TODAY follow-up studies were approved by an institutional review board, and all participants provided written informed consent. Both informed parental consent and minor child assent were obtained where needed (based on age). Participants included in this analysis were initially enrolled in the TODAY study (2004–2011)—a randomized controlled trial of 3 treatments for T2D. In 2011, 572 TODAY participants enrolled in9 the TODAY follow-up study, which was conducted in 2 phases. Between 2011 and 2014, participants continued to receive diabetes mellitus–related care from the TODAY study team and were treated with metformin or insulin as needed to maintain glycemic control. From 2014 to 2019, 518 participants transitioned to community diabetes mellitus care but continued to be followed-up during annual observational study visits. Participants included in this analysis were similar to nonparticipants in terms of mean age, duration, body mass index (BMI), Hemoglobin A1c (HbA1c), and blood pressure at the beginning of TODAY follow-up, as well as with respect to the distribution of sex, race/ethnicity, and smoking (Table I in the Data Supplement).
Figure 1. TODAY (Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth) study flow.
The analyses were performed on the cohort of control participants and on 411 participants with echocardiographic measurements both at the end of the TODAY trial and 5 y later during observational follow-up. Excluded were 14 participants with monogenic diabetes mellitus and 4 participants without risk factor measurements within 3 mo of the echocardiogram. A, Timeline. B, Consolidated Standards of Reporting Trials diagram. *n=113 participants only had a TODAY end of study visit; n=21 participants only had a TODAY follow-up study visit.
Control individuals without diabetes mellitus who were normal weight and obese were recruited for the TODAY study by the echocardiography reading center from Baltimore, MD and Philadelphia, PA for a 1-time echocardiogram visit. Frequency matching was used to ensure that the controls were similar to the TODAY study participants with respect to the distribution of age, sex, and race/ethnicity. The obese controls were also recruited to approximate the average BMI of the TODAY study participants. Sample size for the normal-weight (n=51) and obese (n=194) control cohorts was determined from power calculations based on differences in tissue Doppler imaging measures from the CARDIA study among normal-weight, T2D, and nondiabetic obese participants. Data collected from control participants included age, sex, race/ethnicity, blood pressure, and HbA1c using methods identical to that of the TODAY study.
Study Design
The TODAY clinical trial (2004–2011) was designed to evaluate the effects of 3 treatment arms (metformin alone, metformin+rosiglitazone, and metformin+lifestyle) on time to failure to maintain glycemic control (HbA1c ≥8% for 6 months or inability to wean from temporary insulin started for acute metabolic decompensation). Detailed methods have been published.9,10 Briefly, 699 participants with recent onset T2D, ages 10 to 17 years, were enrolled among 15 participating diabetes mellitus centers. Eligibility criteria included negative diabetes mellitus autoantibodies (glutamic acid decarboxylase-65 and tyrosine phosphatase), measurable C-peptide, BMI ≥85th percentile, and <2 years’ duration of T2D. Participants were followed for an average of 3.86 years. Treatment with metformin+rosiglitazone was superior to metformin in preventing loss of glycemic control in youth with T2D. The TODAY follow-up study (2011–2019) was designed to provide longitudinal follow-up data on the original9 TODAY cohort. T2D treatment during the TODAY follow-up study was not randomized and guided by existing best practice recommendations.
Evaluations
All study visits included a detailed medical history, self-reported medication usage, and a physical examination with measurements of height, weight, waist circumference, and blood pressure taken using a CAS 740 monitor with standardized oscillometric cuff sizes. At every visit, use of blood pressure medications was recorded. Participants self-reported cigarette use, categorized as either used within the past month or never used/not used within the past month.
Fasting laboratory studies and a 2-hour oral glucose tolerance test were also obtained, and measurements of lipids, glucose, insulin, C-peptide, and HbA1c were performed centrally at the TODAY Central Biochemistry Laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle WA).
Echocardiography
A central Echocardiography Reading Center was used (Johns Hopkins University, A.I. duPont Hospital for Children). Transthoracic echocardiography was performed at the end of the TODAY randomized trial and at follow-up examinations conducted 6 ≈5 years later. The protocol for measurement of LV structure and function was identical at each examination with the exception that LV global strain was also measured during the second examination. In brief, 2-dimensional transthoracic echocardiograms were performed with the participant lying in a left lateral decubitus position to maximize image quality. Parasternal short-axis, long-axis, and apical views were obtained. This allowed measurement of LV size and structure, tissue Doppler imaging of right and LV inflow tracts, and for 2-dimensional images to allow later retrieval to obtain measurements of LV strain. Left atrial diameter, rather than left atrial area, was reported, as previous quality control studies have demonstrated poor reproducibility of left atrial area in obese cohorts. Heart rate was measured as part of the echocardiogram.6 Heart rate was measured as part of the echocardiogram.
For each TODAY site, a single Echocardiography Reading Center conducted web-based centralized training, and 3 practice case submissions, to certify each field center regarding conduct of the TODAY protocol. Echocardiograms for the TODAY control study were performed by technicians from the Echocardiography Reading Center using a Toshiba Artida cardiac ultrasound system (Canon Medical Systems, Otawara, Japan) and followed the TODAY echocardiogram protocol. All TODAY and control echocardiograms were read by a single technician with random rereads for quality control using commercially available software (Digisonics, Houston, TX). All speckle tracking and strain measurements were analyzed by a single technician with TomTec at a frame rate per second of 50 (Unterschleissheim, Germany) for global and regional myocardial deformation and strain, volumes, mass, and ejection fraction. For strain measurements, the intraobserver variability was <10%. Measurements were made and abnormal thresholds were chosen according to the American Society of Echocardiography standards.11 The analyses were performed on the cohort of control participants and on the 411 participants with echocardiographic measurements available both at the end of the TODAY trial and 5 years later during observational follow-up. Excluded were 14 participants with monogenic diabetes mellitus and 4 participants without risk factor measurements within 3 months of the echocardiogram.
Statistical Analyses
Descriptive statistics presented are mean (SD) or percentage. Slopes were estimated from unadjusted repeated measures linear regression models and represent the change over time between the 2 echocardiogram assessments. Separately, multivariable linear regression models were used to assess relationships among echocardiography outcomes and independent predictors at TODAY baseline (age, sex, race/ethnicity, treatment group assignment at randomization), TODAY 5-year follow-up (cigarette use, hypertension medication use, urinary albumin), and change from TODAY baseline to TODAY follow-up (BMI, systolic blood pressure, diastolic blood pressure, heart rate, HbA1c). For each echocardiography outcome, the TODAY follow-up measurement was included as the dependent variable and the TODAY end of study measurement as an independent predictor along with all of the fixed covariates (baseline, follow-up, change) listed above. Normal-weight and obese participants were compared with the TODAY cohort at the 5-year follow-up using separate ANCOVA models, after adjustment for BMI, systolic blood pressure, smoking, and heart rate. We report the results by sex because of the known differences by sex in heart size and because of the substantial differences between men and women in the prevalence of low systolic function. All analyses are considered exploratory; therefore, no adjustments were made for multiple testing. Statistical significance was defined as P<0.05.
RESULTS
Cohort Description
Table 1 presents participant characteristics, by sex, at TODAY randomization, the end of study visit, and 5-year follow-up visit. A total of 411 (79%) TODAY participants had both end of study and follow-up echocardiograms available. The cohort had a mean age of 23 to 24 years and a 10-year duration of T2D at the time of the second (follow-up) echocardiogram, and 65% were women, 20% non-Hispanic white, 35% non-Hispanic black, and 39% Hispanic. During the 5-year period between echocardiograms, blood pressure increased from a mean 116.3±11.7 to 120.3±13.0 mm Hg, and the number of participants prescribed blood pressure medications increased from 27% to 32%; men had higher blood pressure than women at both time points. BMI remained stable (36.9±8.5 to 36.2±8.4 kg/m ) and prevalent2 smoking increased from 16% to 24%. The level of glycemic control worsened over time, with an HbA1c of 8.0±2.7% at the end of TODAY to 9.6±3.1% at TODAY follow-up (46% receiving insulin at the end of TODAY, 68% receiving insulin by TODAY 5-year follow-up). Characteristics of the control participants are in Table 2.
Table 1.
Sex-Specific Characteristics of Participants (n=411) at Randomization, End of Study, and 5-y Follow-Up (Table view)
| TODAY Randomization (2004–2009) | TODAY End of Study (2010–2011) | TODAY 5-y Follow-Up (2015–2016) | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| 266 | 145 | 266 | 145 | 266 | 145 | |
| Race/ethnicity, % | ||||||
| Non-Hispanic white | 18.4 | 21.4 | 18.4 | 21.4 | 18.4 | 21.4 |
| Non-Hispanic black | 38.0 | 29.0 | 38.0 | 29.0 | 38.0 | 29.0 |
| Hispanic | 36.1 | 44.8 | 36.1 | 44.8 | 36.1 | 44.8 |
| Other | 7.5 | 4.8 | 7.5 | 4.8 | 7.5 | 4.8 |
| Education, % | ||||||
| Less than high school | ... | ... | ... | ... | 10.9 | 10.3 |
| Completed high school or GED | 75.9 | 75.9 | ||||
| Attended college | 13.2 | 13.8 | ||||
| Total annual income | ||||||
| <$25 000 | ... | ... | ... | ... | 84.4 | 74.4 |
| $25 000–$49 999 | 15.2 | 19.2 | ||||
| ≥$50 000 | 0.5 | 6.4 | ||||
| Age, y | 13.5 (2.0) | 14.4 (1.9) | 18.1 (2.5) | 19.1 (2.3) | 23.0 (2.5) | 24.0 (2.3) |
| Diabetes mellitus duration, y | 0.7 (0.5) | 0.6 (0.5) | 4.8 (1.5) | 4.9 (1.5) | 9.6 (1.6) | 9.8 (1.5) |
| Body mass index, kg/m2 | 34.6 (7.6) | 35.5 (8.3) | 37.0 (8.3) | 36.8 (9.0) | 36.5 (8.3) | 35.7 (8.7) |
| HbA1c, % | 6.0 (0.8) | 5.9 (0.7) | 7.8 (2.5) | 8.3 (3.0) | 9.4 (3.1) | 9.9 (3.2) |
| Blood pressure | ||||||
| Systolic, mm Hg | 109.9 (10.0) | 116.6 (10.8) | 113.6 (11.1) | 121.0 (11.3) | 117.6 (11.9) | 125.3 (13.5) |
| Diastolic, mm Hg | 65.4 (8.3) | 67.4 (7.7) | 69.1 (9.1) | 71.6 (9.5) | 74.0 (9.7) | 77.3 (11.5) |
| Medication use, % | 4.5 | 6.9 | 22.9 | 33.8 | 27.1 | 42.1 |
| Smoking, % | 3.4 | 2.8 | 12.7 | 22.6 | 24.1 | 24.1 |
| Failed to maintain glycemic control, % | ... | ... | 42.9 | 53.1 | 66.5 | 69.7 |
| Heart rate, bpm | ... | ... | 76.5 (12.5) | 75.4 (12.8) | 75.7 (12.5) | 75.4 (14.2) |
Data are mean (SD) or percentage. Information on education attainment and income was collected from the participants during TODAY follow-up. GED indicates general education diploma; HbA1c, Hemoglobin A1c; and TODAY, Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth.
Table 2.
Sex-Specific Characteristics at 5-y Follow-Up of Participants and Normal-Weight and Obese Controls (Table view)
| TODAY 5-y Follow-Up Participants With T2D | Normal-Weight Controls | Obese Controls | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| n | 266 | 145 | 29 | 22 | 147 | 47 |
| Race/ethnicity, % | ||||||
| Non-Hispanic white | 18.4 | 21.4 | 55.2 | 45.5 | 21.1 | 23.4 |
| Non-Hispanic black | 38.0 | 29.0 | 31.0 | 40.9 | 68.7 | 59.6 |
| Hispanic | 36.1 | 44.8 | 13.8 | 4.6 | 7.5 | 14.9 |
| Other | 7.5 | 4.8 | 0.0 | 9.1 | 2.7 | 2.1 |
| Age, y | 23.0 (2.5) | 24.0 (2.3) | 23.3 (3.0) | 22.2 (2.9) | 24.4 (3.6) | 24.9 (3.8) |
| Body mass index, kg/m2 | 36.5 (8.3) | 35.7 (8.7) | 21.5 (1.6) | 22.8 (1.7) | 38.1 (7.0) | 38.5 (7.1) |
| HbA1c, % | 9.4 (3.1) | 9.9 (3.2) | 5.1 (0.3) | 4.8 (0.4) | 5.1 (0.5) | 5.2 (0.5) |
| Blood pressure, mm Hg | ||||||
| Systolic | 117.6 (11.9) | 125.3 (13.5) | 106.3 (6.9) | 113.6 (6.4) | 111.2 (9.0) | 118.4 (8.1) |
| Diastolic | 74.0 (9.7) | 77.3 (11.5) | 68.5 (5.8) | 68.8 (6.7) | 67.9 (6.2) | 69.3 (7.5) |
| Smoking, % | 24.1 | 24.1 | 24.1 | 54.6 | 44.2 | 57.5 |
| Heart rate, bpm | 75.7 (12.5) | 75.4 (14.2) | 62.8 (10.0) | 55.3 (6.4) | 66.8 (10.0) | 61.4 (9.8) |
Data are mean (SD) or percentage. Smoking is ever for controls and in the past month for TODAY participants. HbA1c indicates Hemoglobin A1c; T2D, type 2 diabetes mellitus; and TODAY, Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth.
Longitudinal Changes in Echocardiographic Characteristics Across 5 Years and Their Correlates
Table 3 presents the echocardiographic measurements at the TODAY end of study visit and at the TODAY 5-year follow-up visit, as well as the change between the 2 assessments. For both sexes, there was a significant decrease in mitral valve lateral Em leading to a rise in E/Em (0.72±0.12 in women and 0.50±0.17 men; P<0.01 for both). There was a significant decline in LV ejection fraction in both sexes (−0.98±0.39 in women, P<0.05, and −2.28±0.59 in men, P<0.01). At the 5-year follow-up echocardiogram, 11.7% of male participants had an abnormal resting LV ejection fraction <52% versus 1.5% of female participants with <54%.11 Men also had a slight but significant increase in tricuspid annular plane systolic excursion (0.14±0.03; P<0.01), with no significant change in women. Values for LV strain were within the normal range for both sexes. Each model was further evaluated with an interaction term for sex by time, to evaluate whether or not the changes over time differed by sex. The interaction term was not significant in any of the 7 models presented in Table 3, indicating that although there are known differences by sex in heart size, the change (or worsening) over time is similar in both sexes.
Table 3.
Echocardiography Outcomes of Participants at the End of Study and 5-y Follow-Up (Table view)
| TODAY End of Study | TODAY 5-y Follow-Up | Change* | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| n | 266 | 145 | 266 | 145 | 266 | 145 |
| LV mass/height2.7, g/m2.7 | 36.93 (8.89) | 40.27 (9.71) | 37.63 (8.98) | 40.43 (10.31) | 0.79 (−0.16 to 1.74), P=0.1009 | 0.14 (−1.29 to 1.56), P=0.8493 |
| LV relative wall thickness | 0.33 (0.06) | 0.35 (0.06) | 0.34 (0.05) | 0.35 (0.05) | 0.003 (−0.005 to 0.012), P=0.4305 | −0.002 (−0.014 to 0.010), P=0.7370 |
| LV ejection fraction, % | 67.94 (6.10) | 66.97 (6.55) | 66.99 (5.80) | 64.57 (6.64) | −0.98 (−1.75 to −0.20), P=0.0134 | −2.28 (−3.44 to −1.11), P=0.0002 |
| LA internal dimension, cm | 3.58 (0.45) | 3.71 (0.46) | 3.63 (0.45) | 3.77 (0.45) | 0.04 (−0.01 to 0.09), P=0.0791 | 0.05 (−0.01 to 0.12), P=0.1228 |
| TAPSE, cm | 2.18 (0.37) | 2.13 (0.35) | 2.23 (0.33) | 2.27 (0.34) | 0.05 (−0.01 to 0.10), P=0.0756 | 0.14 (0.07 to 0.21), P<0.0001 |
| Doppler diastology, cm/s | ||||||
| Mitral valve lateral Em | 17.12 (4.32) | 16.72 (4.71) | 14.44 (3.04) | 14.14 (3.03) | −2.66 (−3.17 to −2.15), P<0.0001 | −2.57 (−3.31 to −1.83), P<0.0001 |
| Mitral valve lateral E/Em | 5.92 (1.81) | 5.65 (1.79) | 6.65 (1.89) | 6.15 (1.90) | 0.72 (0.49 to 0.96), P<0.0001 | 0.50 (0.16 to 0.83), P=0.0040 |
| Strain | ||||||
| n | 262 | 140 | ||||
| Longitudinal 4-chamber strain | −20.27 (3.34) | −18.12 (3.11) | ||||
| Longitudinal 2-chamber strain | −21.08 (3.80) | −19.16 (3.73) | ||||
| Circumferential strain | −22.65 (4.40) | −21.31 (4.36) | ||||
| Radial strain | 36.47 (21.57) | 31.21 (20.79) | ||||
Data are mean (SD). LA indicates left atrium; LV, left ventricle; TAPSE, tricuspid annular plane systolic excursion; and TODAY, Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth.
β-Estimates (95% CIs) and P value from separate repeated measures linear regression models. β-Estimates are equal to the slope (change) between the 2 assessments.
Associations of risk factors with changes in echocardiographic structure and function parameters during TODAY follow-up are shown in Table 4 and Table II in the Data Supplement. LV mass increase was associated with Hispanic versus non-Hispanic white ethnicity, non-Hispanic black versus non-Hispanic white ethnicity, antihypertensive medication use, higher systolic blood pressure, and lower HbA1c. Overall, LV mass was the greatest in non-Hispanic blacks. LV relative wall thickness increase was associated with antihypertensive medication use. LV ejection fraction increase was associated with female sex. Left atrial internal dimension increase was associated with antihypertensive medication use, higher BMI, and lower heart rate. Tricuspid annular plane systolic excursion increase was associated with male sex, non-Hispanic black versus non-Hispanic white ethnicity, metformin treatment during TODAY versus metformin+rosiglitazone, and lower heart rate. There were no significant treatment group differences for any of the other echocardiography outcomes. Mitral valve lateral E/Em increase was associated with female sex, higher urinary albumin, and higher BMI.
Table 4.
Association of Risk Factors With Changes in Echocardiography Outcomes During 5-y Follow-Up Among Participants (Table view)
| LV Mass/Height2.7, g/m2.7 | LV Relative Wall Thickness | LV Ejection Fraction, % | LA Internal Dimension, cm | TAPSE, cm | Mitral Valve Lateral Em | Mitral Valve Lateral E/Em | |
|---|---|---|---|---|---|---|---|
| Risk factors at baseline | |||||||
| Age (per y) | 0.8407 | 0.3658 | 0.0788 | 0.7504 | 0.0901 | 0.0004 | 0.3632 |
| Sex (female vs male) | 0.3566 | 0.9885 | 0.0156 | 0.3227 | 0.0076 | 0.7273 | 0.0163 |
| Race/ethnicity | |||||||
| Hispanic vs non-Hispanic white | 0.0017 | 0.4088 | 0.8286 | 0.1935 | 0.0720 | 0.0669 | 0.6190 |
| Non-Hispanic black vs non-Hispanic white | 0.0028 | 0.4038 | 0.8602 | 0.6118 | 0.0232 | 0.2337 | 0.2113 |
| Other vs non-Hispanic white | 0.8500 | 0.4296 | 0.3097 | 0.1562 | 0.3228 | 0.2485 | 0.0682 |
| Treatment group | |||||||
| Metformin+rosiglitazone vs metformin | 0.5974 | 0.1541 | 0.6007 | 0.0696 | 0.0003 | 0.3349 | 0.7253 |
| Lifestyle vs metformin | 0.4061 | 0.9863 | 0.4912 | 0.1475 | 0.3808 | 0.3116 | 0.9318 |
| Risk factors at TODAY 5-y follow-up | |||||||
| Cigarette use (yes vs no) | 0.2544 | 0.7773 | 0.5607 | 0.0881 | 0.8064 | 0.7224 | 0.2607 |
| Hypertension medication use (yes vs no) | 0.0066 | 0.0234 | 0.6397 | 0.0030 | 0.8797 | 0.0411 | 0.1449 |
| Urinary albumin (per 10 mg/dL) | 0.9641 | 0.1041 | 0.9841 | 0.6387 | 0.8055 | 0.0004 | <0.0001 |
| Risk factor changes* from TODAY baseline to TODAY 5-y follow-up | |||||||
| Body mass index (per kg/m2) | 0.0730 | 0.2305 | 0.5368 | 0.0325 | 0.1857 | 0.2076 | 0.0195 |
| Systolic BP (per 10 mm Hg) | 0.0142 | 0.2012 | 0.7667 | 0.9292 | 0.8024 | 0.5050 | 0.1228 |
| Diastolic BP (per 10 mm Hg) | 0.7873 | 0.4414 | 0.1477 | 0.6583 | 0.9830 | 0.0192 | 0.5638 |
| Heart rate (per bpm) | 0.4146 | 0.0959 | 0.3528 | 0.0005 | 0.3258 | 0.0011 | 0.8678 |
| HbA1c (per %) | 0.0007 | 0.9404 | 0.2208 | 0.3974 | 0.0055 | 0.6641 | 0.6538 |
Data are P value. Each column represents a separate multivariable linear regression model with all predictors included. Significant P value for exploratory risk factors are presented. β-Coefficients (SEs) are presented in Table I in the Data Supplement. BP indicates blood pressure; HbA1c, Hemoglobin A1c; LA, left atrium; LV, left ventricular; TAPSE, tricuspid annular plane systolic excursion; and TODAY, Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth.
Change was determined as the difference of the TODAY follow-up value minus the TODAY baseline value.
Cross-Sectional Comparisons of Echocardiographic Characteristics Among T2D, Obese, and Normal-Weight Participants
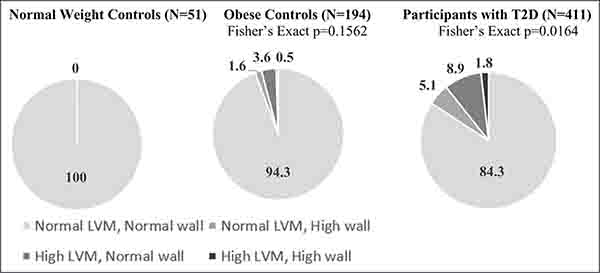
Table 5 compares echocardiographic structure and function parameters among normal-weight controls, obese controls, and TODAY follow-up participants, after adjusting for relevant covariates. Parameters that were significantly lower in T2D participants to normal-weight and obese controls were tricuspid annular plane systolic excursion and mitral valve lateral Em. Participants with T2D had significantly higher ejection fraction, left atrial internal dimension, and longitudinal 2-chamber strain compared with normal-weight controls. Compared with obese controls, TODAY follow-up participants had significantly higher LV relative wall thickness and E/Em. Prevalence of cardiac structural abnormalities, LV hypertrophy, or concentric geometry, was highest in the participants with T2D (15.8% versus 5.7% obese versus 0% normal weight; Figure 2).
Table 5.
Echocardiography Outcomes at 5-y Follow-Up by Sex for Participants and Normal-Weight and Obese Controls (Table view)
| Participants With T2D | Normal-Weight Controls | Obese Controls | Normal-Weight Controls vs T2D* | Obese Controls vs T2D* | ||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |||
| n | 266 | 145 | 29 | 22 | 147 | 47 | ||
| LV mass/height2.7, g/m2.7 | 37.63 (8.98) | 40.43 (10.31) | 27.43 (5.41) | 33.26 (5.58) | 36.70 (6.46) | 41.36 (7.59) | 0.2917 | 0.0462 |
| LV relative wall thickness | 0.34 (0.05) | 0.35 (0.05) | 0.30 (0.04) | 0.29 (0.04) | 0.30 (0.05) | 0.32 (0.06) | 0.0423 | <0.0001 |
| LV ejection fraction, % | 66.99 (5.80) | 64.57 (6.64) | 64.19 (4.06) | 62.68 (5.87) | 65.26 (5.15) | 65.10 (6.48) | 0.0072 | 0.0930 |
| LA internal dimension, cm | 3.63 (0.45) | 3.77 (0.45) | 2.95 (0.38) | 3.48 (0.35) | 3.66 (0.37) | 3.96 (0.35) | 0.0016 | 0.4268 |
| TAPSE, cm | 2.23 (0.33) | 2.27 (0.34) | 2.42 (0.30) | 2.47 (0.26) | 2.44 (0.31) | 2.46 (0.35) | <0.0001 | <0.0001 |
| Doppler diastology, cm/s | ||||||||
| Mitral valve lateral Em | 14.44 (3.04) | 14.14 (3.03) | 17.88 (1.89) | 18.55 (3.94) | 16.45 (3.19) | 15.74 (2.27) | 0.0009 | <0.0001 |
| Mitral valve lateral E/Em | 6.65 (1.89) | 6.15 (1.90) | 5.28 (0.71) | 4.53 (1.22) | 5.66 (1.37) | 5.26 (1.31) | 0.2651 | <0.0001 |
| Strain | ||||||||
| Longitudinal 4-chamber strain | −20.27 (3.34) | −18.12 (3.11) | −22.06 (2.66) | −20.33 (2.98) | −20.92 (3.10) | −18.86 (3.07) | 0.7001 | 0.9003 |
| Longitudinal 2-chamber strain | −21.08 (3.80) | −19.16 (3.73) | −24.89 (4.10) | −22.48 (3.55) | −22.32 (3.87) | −20.57 (2.92) | 0.0031 | 0.2753 |
| Circumferential strain | −22.65 (4.40) | −21.31 (4.36) | −24.41 (2.63) | −22.90 (3.94) | −23.71 (4.36) | −22.47 (3.80) | 0.2968 | 0.1359 |
| Radial strain | 36.47 (21.57) | 31.21 (20.79) | 36.76 (23.70) | 30.36 (16.83) | 36.92 (18.36) | 26.20 (22.74) | 0.0349 | 0.1743 |
Data are mean (SD). LA indicates left atrial; LV, left ventricular; T2D, type 2 diabetes mellitus; and TAPSE, tricuspid annular plane systolic excursion.
P values are from separate ANCOVA models adjusted for body mass index, systolic blood pressure, smoking, and heart rate.
Figure 2. Distribution of left ventricular (LV) geometry at 5-y follow-up in participants and normal-weight and obese controls.
Each cohort was stratified into 4 groups according to LV mass (cutoff at 51 g/m2.7) and relative wall thickness (cutoff at 0.42): normal, LV hypertrophy (increased LV mass only), concentric geometry (increased relative wall thickness only), and LV hypertrophy with concentric geometry. Data are percentage. LVM indicates left ventricular mass; and T2D, type 2 diabetes mellitus.
DISCUSSION
The overall hypothesis of this study was that T2D early in life would be associated with a cardiac phenotype commonly aligned with progression to heart failure with both reduced and preserved ejection fraction. Our principal findings were that (1) LV diastolic function (as measured by transmitral flow) worsened from 5- to 10-year duration T2D in adolescents and young adults with youth-onset T2D in the TODAY study and (2) the measures obtained at the follow-up were significantly worse relative to age- and sex-similar normal-weight and obese control participants without T2D. Cardiometabolic risk in the early adulthood period, most notably glycemic control, BMI, hypertension, and presence of microalbuminuria, appeared to be associated with adverse cardiac remodeling. Importantly, a significant fraction of youth with T2D manifest clinically abnormal echocardiographic parameters, supporting our hypothesis suggesting an early genesis of diastolic dysfunction at this critical period in development. In addition, a significant proportion of men had reduced LV systolic function, and tricuspid annular plane systolic excursion was lower in those with T2D, consistent with either elevated pulmonary vascular resistance or increased LV diastolic pressure.11 Collectively, these data show adverse trends in diastolic function in both sexes and for men, systolic cardiac function over a relatively short follow-up interval and duration of T2D (5–10 years). Studies in adults with T2D in the third and fourth decades of life are needed to determine whether these changes predict the premature development of cardiac dysfunction phenotypes now thought to occur much later in life.
While there is an extensive literature on diabetes mellitus and heart function, the majority of studies have been conducted in much older cohorts.1,2,4 Longitudinal data on adults with T2D exist in epidemiological studies such as CARDIA and the Framingham Heart Study, but other reports of echocardiographic findings are reported in cross-sectional studies.1,2,7,8,12 Therefore, it is difficult to provide an exact quantification of the clinical significance of these findings. Given the age group under study, late adolescence to young adulthood, no significant changes in LV structural or functional parameters would be expected in a healthy cohort.13,14 Though tissue Doppler parameters change with age, such changes typically occur much later in life.15 We believe our results are concerning given that 12% of men had decreased systolic function, lateral E/Em ratio was 20% to 30% higher in participants with T2D compared with controls without T2D, and lateral E/Em ratio increased in participants with T2D by 10% in just 5 years of follow-up.
These data extend the extensive literature on the evolution of subclinical cardiac dysfunction in T2D to the youngest population studied to date. Prior findings from1 the TODAY study based on the initial echocardiographic examination have shown the high prevalence of LV hypertrophy, higher-than-normal LV relative wall thickness, and increased left atrial dimension.16 Many meet criteria for American Heart Association/American College of Cardiology stage B heart failure.17 Studies comparing adolescents with T2D to obese and normal-weight controls in several additional cohorts have reported similar findings; those with diabetes mellitus have worse measures of diastolic function, that is, subclinical diastolic dysfunction,18 and also both LV hypertrophy and reduced cardiac strain.19,20
These results are consistent with longitudinal studies conducted in adults occurring over a longer time course. In the CARDIA study, long-standing adult-onset T2D was associated with longitudinal increases in LV mass, left atrial size, and adverse cardiac geometry from young adulthood to middle age. With regard to systolic7 function, increased LV mass in young adulthood predicted decline in systolic function over the following 20 years.21 These findings translated into adverse subclinical changes in both systolic and diastolic functional parameters in CARDIA participants with T2D. In the Framingham Heart Study, the presence of diabetes mellitus8 adversely impacted LV structure (increased LV mass and relative wall thickness) in a pattern predicted to worsen diastolic function without changes in systolic function over 16 years of follow-up.12 These findings are consistent with cross-sectional observations in adults with T2D.22,23
The results of our study should be viewed in the context of its design. Strengths of our study include the careful longitudinal follow-up, detailed phenotyping, use of a single, central reading center, a large sample size in individuals of this age and disease type (youth-onset T2D), and examination of outcomes by sex and race/ethnicity. Another strength is the prospectively chosen control group that included both normal-weight and obese participants with similar demographic characteristics. Limitations of echocardiography in obese individuals are important to note, leading to high inter- and intraobserver variability. In addition, the parent population was in a clinical trial setting (eg, TODAY), potentially leading to improved care and follow-up relative to a real-world setting, but this would tend to underestimate the problem. Nevertheless, we observed significant advance of cardiometabolic risk and worsening of glycemic control over time, which characterizes youth with T2D in this age range clinically.
In summary, we have shown deterioration in subclinical measures of cardiac function, particularly diastolic function, over 5 years, in adolescents and young adults with T2D, despite follow-up in a clinical trial setting and standardized protocols to manage hypertension and control dysglycemia. Significant progressive myocardial remodeling and dysfunction related to adolescent-onset T2D is apparent within a decade of disease onset. These results demand increased attention to this vulnerable T2D population to prevent development of heart failure.
Supplementary Material
WHAT IS NEW?
Adolescents and young adults with type 2 diabetes mellitus have a high prevalence of cardiac structural changes often associated with future cardiovascular morbidity.
There are progressive changes in systolic (men) and diastolic (both sexes) subclinical cardiac function over 5 years in youth with type 2 diabetes mellitus.
WHAT ARE THE CLINICAL IMPLICATIONS?
Early-onset type 2 diabetes mellitus presents risks for future early-onset heart failure.
Adolescents and young adults with type 2 diabetes mellitus should have close monitoring and aggressive treatment of cardiovascular risk factors.
Acknowledgments
Industry contributions: the TODAY (Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth) Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc; Pfizer; and Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Sources of Funding
This work was completed with funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institutes of Health Office of the Director through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NIDDK project office was involved in all aspects of the study, including design and conduct; collection, management, analysis, and interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication.
For Sources of Funding and Disclosures, see page 43.
Nonstandard Abbreviations and Acronyms
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- LV
left ventricular
- T2D
type 2 diabetes mellitus
- TODAY
Treatment Options for Type 2 Diabetes Mellitus in Adolescents and Youth
Footnotes
Appendix
Writing Committee: Samuel S. Gidding, MD, Barbara H. Braffett, PhD, Rachana D. Shah, MD, Joao Lima, MD, Henrique Doria de Vasconcellos, MD, Ravi Shah, MD, Kristen J. Nadeau, MD, Jeanie B. Tryggestad, MD, Kara S. Hughan, MD, Ruban Dhaliwal, MD, MPH, Lorraine E. Levitt Katz, MD.
Disclosures
Dr Shah discloses his relationship with Mycardia, Amgen, and Best Doctors. The other authors report no conflicts.
A list of all writing committee members in the TODAY Study Group is given in the Appendix.
A complete list of individuals in the TODAY Study Group is presented in the Data Supplement.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.119.006685.
REFERENCES
- 1.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovasculardisease in diabetesmellitus: atheroscleroticcardiovasculardisease and heartfailure in type 2 diabetesmellitus - mechanisms, management, and clinicalconsiderations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, Yancy CW, Green JB, Altman N, Hernandez AF. Heart failurewithpreservedejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:602–611. doi: 10.1016/j.jacc.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 3.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V Coronary heartdiseasemortalitydeclines in the United States From 1979 Through 2011: evidence for stagnation in youngadults, especiallywomen. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosmala W, Marwick TH. Asymptomatic leftventriculardiastolic dysfunction: predictingprogression to symptomaticheartfailure. JACC Cardiovasc Imaging. 2020;13(1 pt 2):215–227. doi: 10.1016/j.jcmg.2018.10.039 [DOI] [PubMed] [Google Scholar]
- 5.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitt Katz L, Gidding SS, Bacha F, Hirst K, McKay S, Pyle L, Lima J; TODAY Study Group. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes. 2015;16:39–47. doi: 10.1111/pedi.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidding SS, Liu K, Colangelo LA, Cook NL, Goff DC, Glasser SP, Gardin JM, Lima JA. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Circ Cardiovasc Imaging. 2013;6:769–775. doi: 10.1161/CIRCIMAGING.112.000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, Isogawa A, Lewis CE, Wu C, Jacobs DR Jr, et al. Association of insulinresistance and glycemicmetabolicabnormalitieswith LV structure and function in middleage: the CARDIA Study. JACC Cardiovasc Imaging. 2017;10:105–114. doi: 10.1016/j.jcmg.2016.02.033 [DOI] [PubMed] [Google Scholar]
- 9.Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, et al. ; TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D; TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91:2400–2406. doi: 10.1161/01.cir.91.9.2400 [DOI] [PubMed] [Google Scholar]
- 14.Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41:955–960. doi: 10.1016/s0735-1097(03)00052-4 [DOI] [PubMed] [Google Scholar]
- 15.Keller KM, Howlett SE. Sex Differences in the Biology and Pathology of the Aging Heart. Can J Cardiol. 2016;32:1065–1073. doi: 10.1016/j.cjca.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 16.Bacha F, Gidding SS, Pyle L, Levitt Katz L, Kriska A, Nadeau KJ, Lima JA.; Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group. Relationship of cardiac structure and function to cardiorespiratory fitness and lean body mass in adolescents and young adults with type 2 diabetes. J Pediatr. 2016;177:159–166.e1. doi: 10.1016/j.jpeds.2016.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 18.Shah AS, Khoury PR, Dolan LM, Ippisch HM, Urbina EM, Daniels SR, Kimball TR. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011;54:722–730. doi: 10.1007/s00125-010-1974-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjornstad P, Truong U, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Pyle L, Regensteiner JG, Reusch JE, Nadeau KJ. Cardiopulmonary dysfunction and adiponectin in adolescentswithtype 2 diabetes. J Am Heart Assoc. 2016;5:e002804. doi: 10.1161/JAHA.115.002804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishi S, Armstrong AC, Gidding SS, Jacobs DR Jr, Sidney S, Lewis CE, Schreiner PJ, Liu K, Lima JA. Relation of left ventricular mass at age 23 to 35 years to global left ventricular systolic function 20 years later (from the Coronary Artery Risk Development in Young Adults study). Am J Cardiol. 2014;113:377–383. doi: 10.1016/j.amjcard.2013.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102–107. doi: 10.1161/01.cir.103.1.102 [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.