Abstract
Background:
People who inject drugs (PWID) lag behind other key populations in HIV care continuum outcomes. The impacts of criminal justice reform and increasing drug treatment access on HIV have been underexplored.
Methods:
We developed agent-based models (ABM) of sexual partnerships among PWID and non-PWID, and injection equipment-sharing partnerships among PWID in five US cities (Baltimore, Boston, Miami, New York City, San Francisco) over 3 years. The first set of ABM projected changes in partnership discordance among PWID as a function of decreasing ZIP code-level incarceration rates. The second set projected discordance as a function of increasing ZIP code-level drug treatment access. ABM were parameterized and validated overall, and by city and PWID race/ethnicity (Black, Latino, White) using National HIV Behavioral Surveillance data, administrative ZIP code-level data, surveillance reports and prior literature. Informed by research on prisoner release and community-level HIV prevalence, reductions in incarceration rates were fixed at 5% and 30% and respectively projected to increase ZIP code-level HIV prevalence by 2% and 12%. Increases in drug treatment access were fixed at 30% and 58%.
Results:
In each city, a 30% reduction in ZIP code-level incarceration rates and 12% increase in ZIP code-level HIV prevalence significantly increased sero-discordance among at least one racial/ethnic group of PWID by 1–3 percentage points. A 5% reduction in incarceration rates, and 30% and 58% increases in drug treatment access, led to isolated significant changes in sero-discordance among Black and White PWID that were less than 1 percentage point.
Conclusion:
Reductions in incarceration rates may lead to short-term increases in sero-discordant partnerships among some PWID by increasing community-level HIV prevalence. Efforts to increase HIV testing, engagement in care and community reintegration post release, should be strengthened in the wake of incarceration reform. Additional research should confirm these findings and explore the lack of widespread impacts of drug treatment in this study.
Keywords: HIV, people who inject drugs, incarceration, drug treatment, agent-based models
Background
People who inject drugs (PWID) remain at risk of HIV acquisition (Burnett, Broz, Spiller, Wejnert, & Paz-Bailey, 2018). In the era of treatment as prevention, gaps in viral suppression among PWID and non-PWID in some cities is narrowing (Don C. Des Jarlais, Kerr, Carrieri, Feelemyer, & Arasteh, 2016; Lesko, Tong, Moore, & Lau, 2017), but national estimates point to disproportionately low viral suppression among PWID (Karch, Gray, Shi, & Hall, 2016), and racial disparities still exist, with Black and Latino/Hispanic PWID bearing most of the burden of HIV diagnoses and sub-optimal HIV treatment outcomes (Burnett, et al., 2018; Gant, Dailey, Hu, & Johnson, 2017; Karch, et al., 2016). Collectively, these circumstances suggest structural interventions should be coupled with biomedical approaches to reach national goals of ending the HIV epidemic in the United States (Fauci, Redfield, Sigounas, Weahkee, & Giroir, 2019). Criminal justice reform and increasing drug treatment access under the Affordable Care Act are among the structural approaches that may combat HIV transmission among PWID.
Drug control policy, incarceration and HIV transmission
The US has the highest incarceration rate in the world (“World Prison Brief,” 2020). Drug-related offenses account for the largest share of incarcerations (“Bureau of Prisons Statistics,” 2020), but are documented to neither reduce drug use nor drug market activity (Melo, et al., 2018; “More imprisonment does not reduce state drug problems,” 2018; Werb, et al., 2008). Instead drug control policies have led to stark racial inequities in incarceration (“The Color of Justice: Racial and Ethnic Disparity in State Prisons,” 2016) (Alexander, 2010; Bronson & Carson, 2019; Musto, 1987).
Incarceration is also a determinant of HIV acquisition. HIV infection in US prisons (1.5%) is five times greater than the general population (Maruschak, 2015), and the mechanisms linking incarceration to HIV risk are behavioral, social and structural. Threat of arrest increases syringe and other equipment sharing and underutilization of syringe service programs among PWID (Beletsky, et al., 2015; Burris, et al., 2004; H. Cooper, Moore, Gruskin, & Krieger, 2005; DeBeck, et al., 2017; Park, Linton, Sherman, & German, 2019; Small, Rhodes, Wood, & Kerr, 2007; Werb, et al., 2008). Incarceration encourages sexual network dynamics that accelerate HIV transmission (Farel, et al., 2013; James & Glaze, 2006; McClelland, Teplin, Abram, & Jacobs, 2002; Mumola & Karberg, 2007; Rosen, et al., 2009; Seal, Eldridge, Zack, & Sosman, 2010) ((Adimora & Schoenbach, 2005; H. L. F. Cooper, et al., 2015; Khan, et al., 2011; Nunn, et al., 2012; Nunn, et al., 2011; Thomas & Torrone, 2008) (Lucas, et al., 2016)). Seroconversion can occur in correctional settings (Jafa, et al., 2009; Macher, Kibble, & Wheeler, 2006). Lastly, incarceration creates vulnerability to HIV post-release, by disrupting drug treatment, HIV care and treatment, and disqualifying men and women from employment and social benefits (Alexander, 2010; Curtis, Garlington, & Schottenfeld, 2013; Musto, 1987; Silva, 2015).
Role of criminal justice reform on HIV transmission
In response to US drug control policies’ inability to reduce substance use and instead increase health and social inequities, several US states have legalized cannabis for medicinal and recreational use and possession, and/or reclassified possession of small amounts of cannabis to be a civil offense. Under the Trump administration, the US government enacted the First Step Act, which included efforts to retroactively eliminate sentencing disparities between crack and cocaine and reduce mandatory minimums for men and women enrolled in eligible rehabilitation programs. The Biden-Harris administration has promised to take further steps toward justice reform, by completely eliminating the federal crack and cocaine disparity, decriminalizing cannabis use, and investing in mental health and substance use services and treatment.
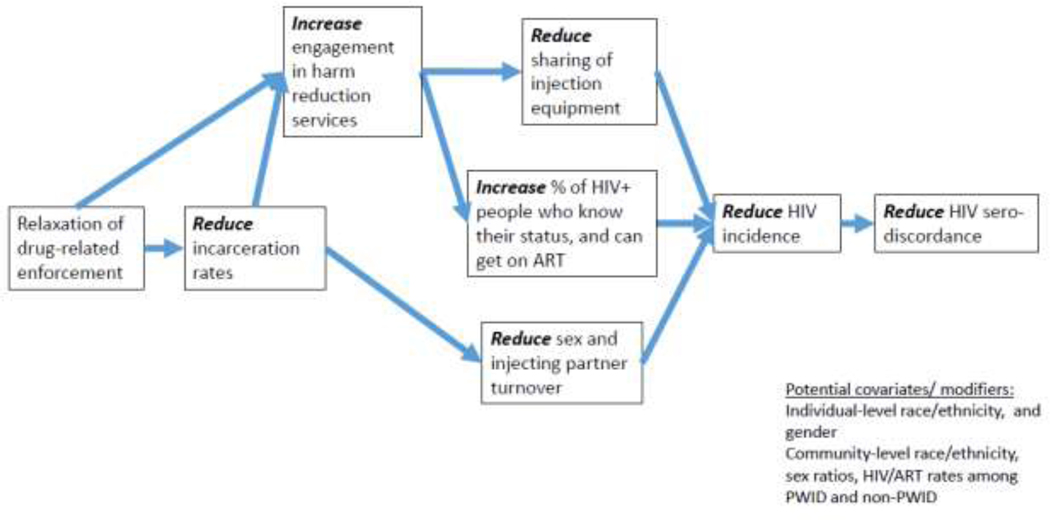
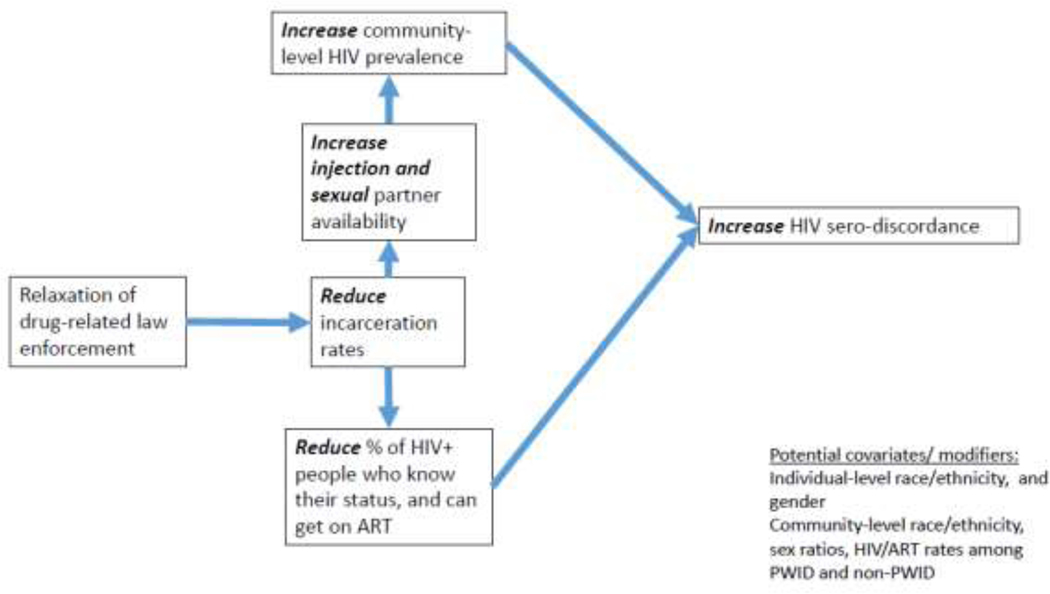
These criminal justice reform policies have the potential to influence HIV transmission through behavioral, network, and structural mechanisms, but the paths linking criminal justice reform to HIV transmission may be countervailing (figures 1–2). Reductions in incarceration rates as part of criminal justice reform efforts may reduce HIV risk behaviors and transmission within correctional settings, stabilize sexual networks and increase access to drug treatment, HIV care and treatment, and social benefits. However, because of the disproportionate rate of HIV among incarcerated adults, community-level HIV prevalence may initially increase when persons living with HIV or who are at risk of acquiring HIV remain in the community, or are released to the community (Ojikutu, Srinivasan, Bogart, Subramanian, & Mayer, 2018).
Figure 1. Pathways linking reductions in incarceration rates to reductions in HIV sero-discordance among PWID.
Figure 2. Pathways linking reductions in incarceration rates to increases in HIV sero-discordance among PWID.
The impacts of criminal justice reform on HIV may also vary according to where people live, their racial/ethnic background, and their social networks. Residential segregation disproportionately sorts Black and Latino people into neighborhoods with higher levels of intensified policing and HIV determinants (e.g., poverty) than White people (Alexander, 2010; Pietila, 2012; Reardon, Fox, & Townsend, 2015; Wilson, 1997). Further, racial differences in partner homophily (Aral, et al., 1999; Laumann & Youm, 1999; Momplaisir, et al., 2017), and prevalence of HIV in sexual and drug-using networks (Burnett, et al., 2018; Momplaisir, et al., 2017; Williams, et al., 2013), may modify the impacts of criminal justice reform on HIV prevalence.
Medicaid expansion and access to drug treatment
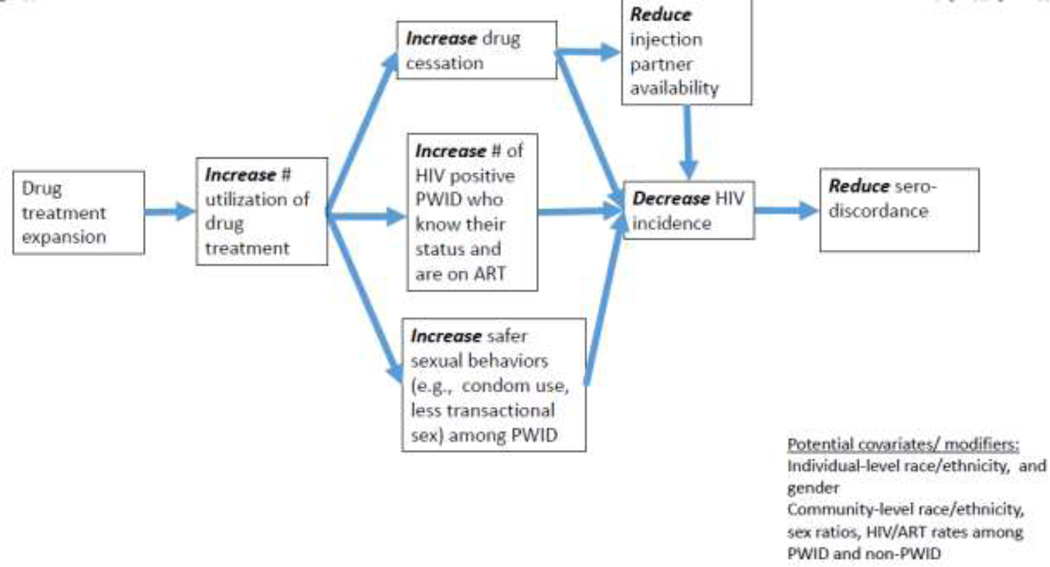
Similar to criminal justice reform, increases in drug treatment access, particularly medication-assisted treatment (MAT), may impact HIV transmission via behavioral, network and structural mechanisms. However, evidence suggests that these impacts are largely ameliorative, as MAT has been associated with protective sexual behaviors and engagement in HIV care and treatment (Brown, Eriksen, Gause, Brody, & Sales, 2018; Culbert, et al., 2018; D. C. Des Jarlais, et al., 2013; Farrell, Marsden, Ling, Ali, & Gowing, 2005; Larney, 2010; Mathers, et al., 2010; Millson, et al., 2007; Shoptaw, et al., 2005; Sorensen & Copeland, 2000; Volkow, Jones, Einstein, & Wargo, 2018).
Role of drug treatment expansion on HIV risk
The expansion of Medicaid to include lower-income people (e.g., annual incomes below 138 % of the federal poverty level) as part of the 2014 Affordable Care Act has increased insurance coverage among low-income people. Thus, a prominent hypothesis is that by doing so, drug treatment access and utilization will increase among this population. To date, research investigating the impacts of Medicaid expansion on drug treatment utilization have been mixed, (Baicker, Allen, Wright, & Finkelstein, 2017; Feder, et al., 2017; McKenna, 2017; Meinhofer & Witman, 2018; Saloner, Landis, Stein, & Barry, 2019; Saloner, Levin, Chang, Jones, & Alexander, 2018; Wen, Hockenberry, Borders, & Druss, 2017) (Feder, et al., 2017; Olfson, Wall, Barry, Mauro, & Mojtabai, 2018)). However, in cases when Medicaid expansion increases drug treatment access and utilization, it may protect against HIV acquisition and transmission by encouraging safer drug use and sexual behaviors, and decreasing partnerships with other people who use drugs (Bohnert, Bradshaw, & Latkin, 2009; Brown, et al., 2018; Culbert, et al., 2018; Davey-Rothwell, Frydl, & Latkin, 2009; D. C. Des Jarlais, et al., 2013; Farrell, et al., 2005; Larney, 2010; Mathers, et al., 2010; Millson, et al., 2007; Shoptaw, et al., 2005; Sorensen & Copeland, 2000; Volkow, et al., 2018). Like incarceration reform efforts, the potential for increases in drug treatment access to impact HIV transmission may vary according to place-based residential characteristics, local policies, personal networks and racial/ethnic background. For example, our prior research suggests predominantly-Black and Latino neighborhoods have less spatial access to drug treatment programs (H. L. F. Cooper, et al., 2016).
Modeling the impact of reducing incarceration rates and increasing access to drug treatment on sero-discordant partnerships
Informed by prior literature, figures 1–3 illustrate the multiple pathways linking reductions in incarceration rates and increases in drug treatment access to sero-discordant sexual and injection networks. Analysis of the multiple mediating and feedback pathways displayed in these conceptual frameworks require a complex systems approach. Agent-based modeling (ABM) has increasingly been used as a tool to evaluate HIV epidemiology and impacts of prevention (Marshall, et al., 2012; Escudero, et al., 2017;(Adams, et al., 2018, 2019).
Figure 3. Pathways linking increases in drug treatment expansion to reductions in HIV sero-discordance among PWID.
In an era when federal and state-level efforts to reform drug policy by reducing incarceration rates and increasing access to drug treatment coexist with consistent debates over rejecting or imposing restrictions on Medicaid expansion within local jurisdictions (e.g., issuing work requirements, disqualifying people living below the poverty line) (Luhby, 2019; “Status of State Action on the Medicaid Expansion Decision,”), application of ABM to examine whether the impacts of these policies can be leveraged to prevent and control HIV transmission is timely. Although progress along the HIV care continuum is documented among PWID in some cities, like Miami, New York City and Baltimore (Lesko, et al., 2017), national surveillance data and local data from some cities suggest PWID lag behind other key populations in engagement in HIV care, achievement of viral suppression (Karch, et al., 2016), and utilization of PREP (Biello, et al., 2018; McFarland, et al., 2019; Roth, et al., 2019). Furthermore, racial disparities in HIV diagnoses, engagement in HIV care and viral suppression persist (Burnett, et al., 2018; Gant, et al., 2017; Karch, et al., 2016; Klevens, et al., 2018; McFarland, et al., 2019). Collectively, this literature strengthens rationale for investigating the role of policies and structural approaches, including incarceration reform and increasing access to drug treatment on HIV transmission among this population.
This study expands application of ABM in HIV epidemiological research to project whether reductions in incarceration rates and increases in drug treatment access impact sero-discordant sexual and injection equipment-sharing partnerships among Black, Latino and White PWID living in 5 large US cities between 2009 and 2012. Sexual and injection equipment-sharing partnerships were selected as the measurable outcome in this study due to low incidence rates of HIV among the sample of PWID used to validate the ABM.
Methods
Overview and Data Sources
We created a series of agent-based models (ABM) of sexual and injection equipment-sharing relationships among PWID, and sexual relationships of people who do not inject drugs over time in order to explore the potential impacts of reducing incarceration rates and increasing access to drug treatment on sero-discordant sexual and injection equipment-sharing partnerships among PWID exclusively. Sero-discordance was defined as one outcome which jointly combined sexual and injection equipment-sharing partnerships. The ABM was parameterized using individual-level demographic, health behavior, network, and HIV-related outcome information from multiple sources, including data extracted from prior literature and surveillance reports and observed individual-level data from PWID enrolled in the 2009 and 2012 cycles of the Centers for Disease Control and Prevention’s National HIV Behavioral Surveillance (NHBS) in Baltimore, Boston, New York City, San Francisco and Miami. Data on ZIP code and MSA characteristics were based on individual-level data from NHBS participants aggregated to the ZIP codes where NHBS participants lived, and data extracted from publicly available datasets, including the US Census Bureau’s American Community Survey, and from published literature and CDC surveillance reports. NHBS individual-level data aggregated to ZIP codes were not considered for parameterization of ZIP code-level features in the model when there were fewer than 5 NHBS participants in a given ZIP code.
Briefly, we provide background on the NHBS study from which most individual-level data used to parameterize the model were drawn. Data from the five sites, Baltimore, Boston, New York City, San Francisco and Miami, were selected from a larger sampling frame of 21 MSAs included in NHBS because of data availability for model inputs, and their representation of different US regions and policy environments. Participants enrolled in NHBS were recruited using respondent-driven sampling and met eligibility criteria if they were ≥18 years of age; reported injection drug use in the past year; demonstrated evidence of injection (e.g., track marks); residence in an NHBS-eligible MSA; and provided oral consent (Gallagher, Sullivan, Lansky, & Onorato, 2007). The analytic sample used for model parameterization was further restricted to eligible PWID who completed the NHBS questionnaire; reported valid survey and ZIP-code level information (note: because different people were sampled between these years, the sample of residential ZIP codes varied between cycles); were male or female (transgender PWID were excluded because of small sample size (n=14 in 2009; n=12 in 2012); and were Black, White or Hispanic/Latino. PWID who did not identify with these racial/ethnic groups were excluded because of small sample size (n=48 in 2009; n=52 in 2012).
Descriptive Analysis
The distribution of key characteristics of NHBS PWID participants, the ZIP codes where NHBS PWID lived were described, as were values of parameters extracted from literature, surveillance reports, and the US Census Bureau.
Agent based model
Model Output
The model output sexual or injection equipment-sharing HIV discordance, whereby an HIV infected person who injects drugs and not on ART was in a sexual or injection equipment-sharing partnership with an HIV-negative partner. Aggregate metrics of discordance (i.e., the fraction of agents in a discordant sexual or injection equipment-sharing relationships) were estimated in the agent-based model. The first set of computational ABM projected sero-discordance overall and for each racial/ethnic group of PWID in each ZIP code and MSA. A second and third series of ABM respectively simulated how reductions in incarceration rates and increases in drug treatment access impacted mean sero-discordance for PWID overall and within each racial/ethnic group of PWID in each MSA.
Calibration
Agents in the ABM represented both PWID and people who do not inject drugs that interacted with each other. Model parameters included static properties and behaviors that were estimated from observed data from PWID enrolled in NHBS and data extracted from prior literature and surveillance reports.
Agent properties
Individual-level data from participants enrolled in NHBS, prior literature, and surveillance reports were used to initialize the following set of static agent properties: age, race/ethnicity, gender, number of sexual partners, number of injection equipment-sharing partners (among PWID), HIV status, and anti-retroviral therapy (ART) status. ZIP code-level data on HIV prevalence, ART rates, racial/ethnic composition and male: female sex ratios from the US Census Bureau’s American Community Survey and from published literature and CDC surveillance reports were used to initialize the population of agents within a given ZIP code, such that the distribution of these characteristics across the population of agents within the given ZIP code matched the empirical distributions in each zip code estimated from the sources used for parameterization.
Agent Behaviors
We ran our model separately for each ZIP code, with every model run simulating 36 months, which corresponded with the 36-month period between NHBS data collection cycles (2009 and 2012). During each simulated month, agents regardless of injection status could: (1) create or (2) break sexual partnerships; (3) become incarcerated or (4) return from jail/prison. Among agents who injected drugs, during each simulated month, in addition to the 4 behaviors mentioned, they could: (1) create or (2) break injection equipment-sharing partnerships, or (3) enter drug treatment or (4) return from drug treatment. Agent’s behaviors were determined by their static properties and the static properties and behaviors of other agents in the model and the distribution of ZIP code-level characteristics (i.e., male-female sex ratios, racial/ethnic composition, proportion of non-PWID and PWID with HIV, proportion of non-PWID and PWID with HIV who use antiretroviral therapy).
Preset rules based on data from participants enrolled in NHBS across the 5 sites of interest also determined sexual and injection behaviors and incarceration and drug treatment status. Specifically, agents searched for new sexual partners (regardless of status of injecting drugs) when agents had less sexual partners than their target network degree (See Appendix A). Propensity to establish partnerships with same race/ethnicity members as documented in prior research (Aral, et al., 1999; Laumann & Youm, 1999; Momplaisir, et al., 2017), was modeled according to NHBS participants’ predicted probability of selecting a sexual partner of the same race/ethnicity (calculation presented in Appendix A).
New injection equipment-sharing partnerships were likewise established when PWID agents had fewer injection equipment-sharing partners than their target degree (see Appendix A). Propensity to establish same-gender injection equipment-sharing partnerships (Mayock, Cronly, & Clatts, 2015; Powis, Griffiths, Gossop, & Strang, 1996) was modeled according to NHBS participant’s predicated probability of selected an injection equipment-sharing partner of the same gender (see Appendix A).
NHBS participant data on race/ethnicity was used to determine drug treatment and incarceration rates among PWID and people not injecting drugs (see Appendix A).
Injection equipment-sharing and sexual partnerships were simulated to become dormant during periods of incarceration, and injection equipment-sharing partnerships were simulated to become dormant during periods of drug treatment (see Appendix A). To maintain parsimony, duration of incarceration and drug treatment did not vary. When partnerships were dormant during periods of incarceration or drug treatment, sexual partnerships and injection equipment-sharing partnerships were inactive and were not considered in the estimations of discordance. However, they were modeled to similarly dissolve at the same rate as they would have otherwise dissolved when active (i.e., when not incarcerated or in drug treatment). Furthermore, agents were simulated to not become incarcerated when in drug treatment. Sexual and injection equipment-sharing partnerships were reestablished after periods of incarceration or drug treatment according to partnership rules described above.
Validation
To ensure that the model was producing discordance estimates that were within empirical bounds, we compared the model estimates produced at baseline when policy impacts were not tested to the observed proportion of sero-discordant sexual and injection equipment-sharing partnerships in the past 12 months among PWID enrolled in NHBS (n=2592 in 2009; n=2580 in 2012). The proportion of sero-discordant partnerships among NHBS participants was defined using data on participants’ self-reported HIV status and the status of their last sexual and injection equipment-sharing partner. HIV status of the last sexual partner was asked of PWID who reported vaginal or anal sex in the past year. Same-sex and heterosexual partnerships among men were considered in the determination of sero-discordant partnerships. If both were reported by a male participant, the most recent partnership was considered in analysis. Heterosexual partnerships were only considered among women because detailed characteristics of same-sex partners among women were not captured in the NHBS questionnaire. Small sample size precluded stratification by main and casual partnerships. HIV status of the last injection equipment-sharing partner was asked of PWID who reported sharing injection equipment with others in the past year. To accommodate the structure of questions used to determine HIV status of participants’ partners, we established two definitions of sero-discordance for validation: whereby “strict” and “loose”. “Strict” denoted partnerships in which: (1) the NHBS participant self-reported HIV infected status of herself/himself and uninfected HIV status of her/his sexual or injection equipment-sharing partner, or (2) the NHBS participant self-reported uninfected HIV status of herself/himself and HIV infected status of their sexual or injection equipment-sharing partner. “Loose” denote partnerships in which: (1) the self-reported status of the participant differs from that of the partner, or (2) a participant who self-reports HIV infection or uninfected HIV status of herself/himself and reports not knowing the HIV status of their partner. The model output fell in between the strict and loose empirical estimates.
“Policy impacts”: reducing incarceration rates and increasing access to drug treatment Reducing incarceration rates
The policy impacts simulated in the ABM were reductions in incarceration rates and increases in drug treatment access. Research by Ojikutu and colleagues (Ojikutu, et al., 2018) informed the projected impacts of reducing incarceration rates on ZIP code-level HIV prevalence. Specifically, we projected the impacts of small and large reductions in incarceration rates, whereby a 5% reduction in ZIP code-level incarceration was simulated to result in a 2% increase in ZIP code-level HIV prevalence and a 30% reduction in ZIP code-level incarceration was projected to result in a 12% increase in ZIP code-level HIV prevalence.
Drug treatment expansion
We modeled drug treatment expansion as directly increasing individual-level drug treatment utilization at low and high magnitudes. Informed by prior research (McKenna, 2017), we defined a low magnitude increase as 30%, and defined a high magnitude increase as 58%.
Sensitivity analysis
To ensure robustness of the model results to changes in key parameters, we investigated whether varying dissolution rates and estimates of HIV prevalence and ART rates changed the results.
Results
Description of PWID enrolled in NHBS and data extracted from other sources
Overall, across MSAs, the mean age of PWID recruited to NHBS in 2009 and 2012 was 43.6 and 45 years, respectively. Most PWID were male (71% in 2009; 72% in 2012). Racial/ethnic composition of PWID varied across site MSAs for both cycles (Table 1). The Baltimore site had a predominantly Black PWID population (83–91%) followed by White (8–17%) and Latino PWID (<1%); Boston PWID were predominantly White (59–72%); New York PWID were predominantly Latino (56–64%); San Francisco had an almost equal mix of Black (37–46%) and White (43–48%) PWID, and Miami was the most diverse with 38–44% Latino, 27–42% Black and 20–29% White PWID. Overall, 8–9% of PWID self-reported HIV positive status, and half of them reported utilizing antiretroviral therapy (55–65%). Overall, Black PWID reported past-year incarceration (27%−28%) less frequently than Latino (37–43%) and White PWID (30–39%). Black PWID also reported past-year drug treatment (31–36%) less frequently than Latino (43%) and White PWID (44–46%). Distributions of incarceration and drug treatment by race/ethnicity and MSA, and HIV status are shown in Table 1.
Table 1.
Distribution and sources of individual-level, network and ZIP-code level input variables
| Variables | Proportion (n) or mean (SD) | Source | |||||
|---|---|---|---|---|---|---|---|
| Overall | Baltimore | Boston | Miami | New York | San Francisco | ||
| Individual-level characteristics1 | |||||||
| Age | |||||||
| 43.6 (10.8) | 46.7 (9.3) | 38.1 (11.1) | 46.1 (10.2) | 40.0 (9.4) | 47.3 (10.0) | NHBS 2009 | |
| 45.0 (11.1) | 50.4 (8.1) | 37.6 (10.6) | 42.7 (11.9) | 43.8 (9.9) | 48.9 (10.1) | NHBS 2012 | |
| Gender (Male) | |||||||
| 72.0% (1865) | 73.0% (371) | 67.5% (394) | 76.2% (432) | 75.8% (342) | 67.6% (326) | NHBS 2009 | |
| 70.9% (1830) | 66.5% (417) | 69.8% (324) | 73.3% (368) | 74.7% (380) | 71.3% (341) | NHBS 2012 | |
| Race/Ethnicity | |||||||
| Latino | 25.7% (665) | 0.4% (2) | 21.1% (123) | 38.3% (217) | 55.9% (252) | 14.7% (71) | NHBS 2009 |
| 27.0% (697) | 0.6% (4) | 19.6% (91) | 44.4% (223) | 63.7% (324) | 11.5% (55) | NHBS 2012 | |
| Non-Hispanic/ Latino Black | 38.4% (995) | 82.9% (421) | 19.9% (116) | 41.8% (237) | 9.5% (43) | 36.9% (178) | NHBS 2009 |
| 41.6% (1072) | 91.2% (572) | 8.0% (37) | 26.5% (133) | 21.6% (110) | 46.0% (220) | NHBS 2012 | |
| Non-Hispanic/ Latino White | 36.0% (932) | 16.7% (85) | 59.1% (345) | 19.9% (113) | 34.6% (156) | 48.3% (233) | NHBS 2009 |
| 31.4% (811) | 8.13% (51) | 72.4% (336) | 29.1% (146) | 14.7% (75) | 42.5% (203) | NHBS 2012 | |
| Self-reported HIV infection2 | |||||||
| 8.18% (212) | NA | NA | NA | NA | NA | NHBS 2009 | |
| 9.4% (242) | NA | NA | NA | NA | NA | NHBS 2012 | |
| Self-reported ART use among HIV infected | |||||||
| 55.2% (117) | NA | NA | NA | NA | NA | NHBS 2009 | |
| 65.3% (158) | NA | NA | NA | NA | NA | NHBS 2012 | |
| Proportion incarcerated in the last 12 months by race/ethnicity3 | |||||||
| Latino | 37.0% (Suppressed) | Suppressed (Suppressed) | 26.0% (32) | 52.1% (113) | 30.3% (76) | 33.8% (24) | NHBS 2009 |
| 42.6% (Suppressed) | Suppressed (Suppressed) | 40.7% (37) | 55.2% (123) | 34.0% (110) | 47.3% (26) | NHBS 2012 | |
| Non-Hispanic/ Latino Black | 28.1% (Suppressed) | 24.8% (Suppressed) | 25.0% (29) | 34.6% (82) | 39.5% (17) | 26.4% (47) | NHBS 2009 |
| 27.4% (Suppressed) | 22.2% (Suppressed) | 35.1% (13) | 40.6% (54) | 39.1% (43) | 25.9% (57) | NHBS 2012 | |
| Non- Hispanic Latino White | 30.0% (Suppressed) | 25.9% (Suppressed) | 28.8% (99) | 38.9% (44) | 31.4% (49) | 27.9% (65) | NHBS 2009 |
| 38.8% (Suppressed) | 35.3% (Suppressed) | 42.0% (141) | 51.4% (75) | 38.7% (29) | 25.6% (52) | NHBS 2012 | |
| Proportion incarcerated in the last 12 months by self-reported HIV infection2 | |||||||
| Self-reported HIV infection | 28.0% (59) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | NHBS 2009 |
| 29.8% (72) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | NHBS 2012 | |
| Self-reported HIV uninfected | 31.3% (634) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | Suppressed (Suppressed) | NHBS 2009 |
| 36.0% (747) | NHBS 2012 | ||||||
| Relative proportion of incarcerated HIV infected to proportion of incarcerated HIV uninfected | |||||||
| 1.0 | 0.90 | 1.0 | 1.0 | 0.4 | NHBS 2012 | ||
| Incarceration rate by race/ethnicity4 | |||||||
| Latino | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | NYC NHBS 2012 |
| Non-Hispanic/ Latino Black | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | NYC NHBS 2012 |
| Non-Hispanic/ Latino White | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | 0.4×10−1 | NYC NHBS 2012 |
| Incarceration duration among those previously arrested/detained in past 12 months | |||||||
| 1 | 1 | 1 | 1 | 1 | 1 | NHBS 2009 | |
| Proportion participating in alcohol or drug treatment in the past 12 months by race/ethnicity5 |
|||||||
| Latino | Suppressed | Suppressed | 52.0% (64) | 30.4% (66) | 53.6% (135) | 29.6% (21) | NHBS 2009 |
| Suppressed (Suppressed) | Suppressed (Suppressed) | 57.1% (52) | 23.3% (52) | 55.6% (180) | 30.9% (17) | NHBS 2012 | |
| Non-Hispanic/ Latino Black | 31.0% (Suppressed) | 39.2% (Suppressed) | 42.2% (49) | 13.5% (32) | 48.8% (21) | 23.0% (41) | NHBS 2009 |
| 36.3% (Suppressed | 39.9(Suppressed) | 67.6% (25) | 18.8% (25) | 50.0% (55) | 25.5% (56) | NHBS 2012 | |
| Non-Hispanic/ Latino White | 46.0% (Suppressed) | 51.8% (Suppressed) | 67.2% (231) | 23.0% (26) | 46.8% (73) | 23.2% (54) | NHBS 2009 |
| 43.8% (Suppressed) | 41.2% (Suppressed) | 62.1% (208) | 27.4% (40) | 49.3% (37) | 24.1% (49) | NHBS 2012 | |
| Proportion participating in alcohol or drug | |||||||
| treatment in the past 12 months by self-reported HIV infection4 | |||||||
| Self-reported HIV infection | 38.7% (82) | NHBS 2009 | |||||
| 47.5% (115) | NHBS 2012 | ||||||
| Self-reported HIV uninfected | 39.8% (806) | NHBS 2009 | |||||
| 40.5% (840) | NHBS 2012 | ||||||
| Relative proportion of drug treatment among HIV infected6 to proportion of drug treatment among HIV uninfected | |||||||
| 1.5 | 1.0 | 1.4 | 1.0 | 1.2 | NHBS 2012 | ||
| Drug treatment rate7 | |||||||
| Latino | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | NYC NHBS 2012 |
| Non- Hispanic/ Latino Black | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | NYC NHBS 2012 |
| Non- Hispanic/Latino White | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | NYC NHBS 2012 |
| Drug treatment duration | |||||||
| 3 | 3 | 3 | 3 | 3 | 3 | (Cunningham, et al.,2011) | |
| Network-level characteristics | |||||||
| Monthly sexual link dissolution rate8 | |||||||
| 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | (Kapadia, et al., 2007) | |
| Monthly injection link dissolution rate | |||||||
| 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | 0.3×10−1 | (Kapadia, et al., 2007) | |
| Predicted probability of having a same-sex injection equipment sharing-partner among men | |||||||
| 0.7 | 0.6 | 0.7 | 0.7 | 0.5 | NHBS 2012 | ||
| Predicted probability of having a same-sex injection equipment-sharing partner among women | |||||||
| 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | NHBS 2012 | ||
| ZIP code-level characteristics | |||||||
| Percent poverty | |||||||
| 26.0 (9.4) | 27.4 (8.9) | 24.9 (7.8) | 30.1 (10.4) | 26.3 (10.6) | 20.7 (5.6) | ACS920072011 NHBS 2009 | |
| 27.0 (10.4) | 28.1 (8.3) | 18.4 (7.1) | 34.1 (12.4) | 30.3 (9.5) | 23.2 (6.4) | ACS 2009-2013 NHBS 2012 | |
| Sex ratio10 | |||||||
| 1.0 (0.3) | 0.9 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.9 (0.1) | 1.4 (0.3) | 2010 | |
| Census NHBS 2009 | |||||||
| 1.0 (0.3) | 0.9 (0.1) | 1.0 (0.1) | 1.0 (0.1) | 0.9 (0.1) | 1.5 (0.3) | 2010Census NHBS 2012 | |
| Racial/ethnic composition | |||||||
| Percent of Non-Hispanic/Latino White residents | |||||||
| 27.4 (22.3) | 23.8 (19.0) | 39.5 (23.3) | 10.5 (12.1) | 23.5 (25.6) | 39.8 (12.5) | ACS 20072011 | |
| 27.3 (22.7) | 22.5 (16.7) | 53.9 (19.9) | 11.3 (12.3) | 14.0 (20.2) | 38.9 (11.2) | ACS 20092013 | |
| Percent Non-Hispanic/Latino Black residents | |||||||
| 34.6 (27.5) | 68.4 (22.6) | 26.5 (20.4) | 39.2 (23.2) | 25.3 (21.6) | 12.1 (7.5) | ACS 20072011 | |
| 36.2 (29.0) | 69.1 (20.4) | 15.9 (15.6) | 37.3 (22.1) | 37.3 (26.4) | 10.6 (5.7) | ACS 20092013 | |
| Percent Hispanic/Latino residents | |||||||
| 25.4 (21.5) | 2.88 (3.5) | 18.7 (9.7) | 48.1 (18.8) | 38.2 (23.1) | 18.4 (6.6) | ACS 20072011 | |
| 24.4 (22.6) | 3.2 (3.2) | 15.2 (12.6) | 49.4 (18.9) | 39.7 (22.9) | 18.8 (6.2) | ACS 20092013 | |
| Proportion of HIV infected people who | |||||||
| inject drugs11 | |||||||
| Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | NHBS 2009 | |
| Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | NHBS 2012 | |
| Proportion of HIV infected people who inject drugs on ART | |||||||
| Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | NHBS 2009 | |
| Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | Suppressed | NHBS 2012 | |
| HIV rates among people who do not inject drugs 12 | |||||||
| 15.0 | 36.5 | 15.7 (or 22.0 for Boston Division) | 44.3 (or 50.9 for Miami Division) | 24.8 (or 27.1 for New YorkDivision) | 18.2 (or 29.3 for SF Division) | CDC HIVSurveillance Report(2013) | |
| Percent of HIV infected people who do not inject drugs on ART | |||||||
| 37% | 37% | 37% | 37% | 37% | 37% | Bradley et al. (2014) | |
With the exceptions of rate and duration of incarceration, rate and duration of drug treatment, relative proportion of drug treatment by HIV status, and relative proportion of drug treatment by HIV status, individual-level characteristics are presented in two rows, with the first row reflecting 2009 and the second row reflecting 2012.
To protect privacy and sensitivity of data, and account for non-representative sampling procedures of NHBS, MSA-level data on HIV and ART status among NHBS participants was suppressed
Definition of incarceration differed between 2009 (i.e., arrested by police and booked in the past 12 months) and 2012 (i.e., held in detention center, jail or prison for more than 24 hours in the past 12 months). Data for Latino persons in Baltimore were suppressed because of a count less than 5, rendering the percentages less reliable
Predicted monthly hazard derived from New York City NHBS 2012 data. Monthly Hazard = ( ), where is the proportion incarcerated over a 12-month period.
Total counts for PWID incarcerated and in drug treatment in the last 12 months and counts and frequencies of Latino PWID incarcerated and in drug treatment in the last 12 months were suppressed because less than 5 Latino PWID were sampled in Baltimore, rendering percentages less reliable.
HIV status based on self-report
Predicted monthly hazard derived from New York City NHBS 2012 data. Monthly Hazard = ( ), where is the proportion in drug treatment over a 12-month period
Monthly hazard rate that generates a median duration of 20 months; estimate from: Kapadia (2006).
US Census Bureau’s American Community Survey (ACS) estimates were computed across ZIP codes where NHBS participants lived.
M-F Sex Ratio = (Males 18–64, excluding institutionalized populations 18–64)/(Females 18–64, excluding institutionalized populations 18–64)
To protect privacy and sensitivity of data, and account for non-representative sampling procedures of NHBS, ZIP code-specific data on HIV and ART status of PWID was suppressed
Values reflect MSA (Metro Division) rates that were applied to each ZIP code within a given MSA in the ABM
On average, the ZIP codes where this sample of PWID reported residing were home to approximately 18–34% of residents living below the poverty line. With the exception of San Francisco, which was projected to have a higher proportion of males to females in ZIP codes within that MSA, the ZIP codes where most PWID lived had similar proportions of males to females. Across ZIP codes in Baltimore, the highest mean percentage of residents were Black (68–69%) followed by White (23–24%) and Latino residents (3%). In Boston and San Francisco, the mean percentage of residents across ZIP codes was highest among White residents (39–54%) as compared to Black (11–27%) and Latino residents (15–19%). Latino residents (38–49%) accounted for the highest mean percentage across ZIP codes in Miami and New York, as compared to Black (25–39%) and White residents (11–26%) in these cities. (Table 1).
The values and distribution of parameters extracted from literature and surveillance reports, which were used to parameterize agent, network, and ZIP code level characteristics in the model are described in Table 1.
Agent based model
MSA-specific distributions of proportions of HIV sero-discordance at baseline
In the absence of reducing incarceration rates and increasing access to drug treatment, the “baseline” mean proportions of HIV sero-discordance among sexually active and injection equipment-sharing PWID varied across MSAs, with San Francisco having the lowest overall mean proportion of sero-discordance (mean=0.1179, standard error=0.003) and New York having the highest (mean=0.1853; standard error=0.0003). The magnitude of mean proportions also varied by racial/ethnic groups of PWID within and across MSAs. (Table 2; Figures 4–8).
Table 2.
Projected distribution of sero-discordance across MSAs, by race/ethnicity and magnitude of drug treatment expansion and incarceration reform by agent-based modeling
| MSA | Race/ Ethnicit y | Baseline | Drug Treatment Expansion (Low Magnitude) | Drug Treatment Expansion (High Magnitude) | Incarceration Reform (Low magnitude) | Incarceration Reform (High magnitude) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Error | Mean | Standard Error | Pvalue | Mean | Standard Error | Pvalue | Mean | Standard Error | Pvalue | Mean | Standard Error | P-value | ||
| Baltimore | Total | 0.1474 | 0.0003 | 0.1471 | 0.0002 | 0.8948 | 0.142 2 | 0.0002 | 0.029 1 | 0.1432 | 0.0002 | 0.0736 | 0.158 9 | 0.0002 | <0.000 1 |
| Baltimore | Black | 0.1336 | 0.0003 | 0.1340 | 0.0002 | 0.8659 | 0.127 1 | 0.0002 | 0.015 2 | 0.1304 | 0.0002 | 0.2100 | 0.144 6 | 0.0003 | 0.0001 |
| Baltimore | White | 0.1782 | 0.0004 | 0.1741 | 0.0005 | 0.3382 | 0.1756 | 0.0004 | 0.4854 | 0.170 9 | 0.0004 | 0.049 8 | 0.190 7 | 0.0004 | 0.0008 |
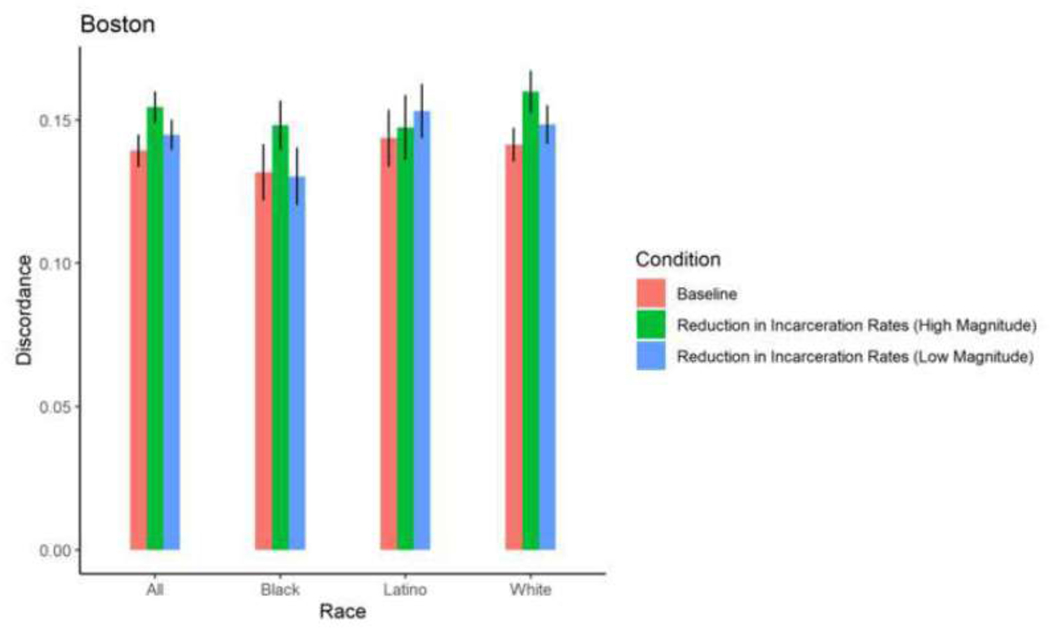
| Boston | Total | 0.139 | 0.0004 | 0.144 | 0.0003 | 0.158 | 0.140 | 0.0004 | 0.751 | 0.144 | 0.0004 | 0.168 | 0.154 | 0.0004 | 0.0004 |
| 3 | 2 | 0 | 5 | 9 | 8 | 0 | 5 | ||||||||
| Boston | Black | 0.1317 | 0.0008 | 0.1320 | 0.0007 | 0.9699 | 0.1291 | 0.0008 | 0.7302 | 0.1303 | 0.0008 | 0.8434 | 0.148 2 | 0.0007 | 0.0166 |
| Boston | Latino | 0.1437 | 0.0008 | 0.1536 | 0.0008 | 0.1712 | 0.1406 | 0.0007 | 0.6428 | 0.1531 | 0.0007 | 0.1849 | 0.1473 | 0.0009 | 0.6405 |
| Boston | White | 0.1413 | 0.0005 | 0.1465 | 0.0004 | 0.1821 | 0.1453 | 0.0005 | 0.3546 | 0.1484 | 0.0005 | 0.1271 | 0.159 8 | 0.0006 | 0.0004 |
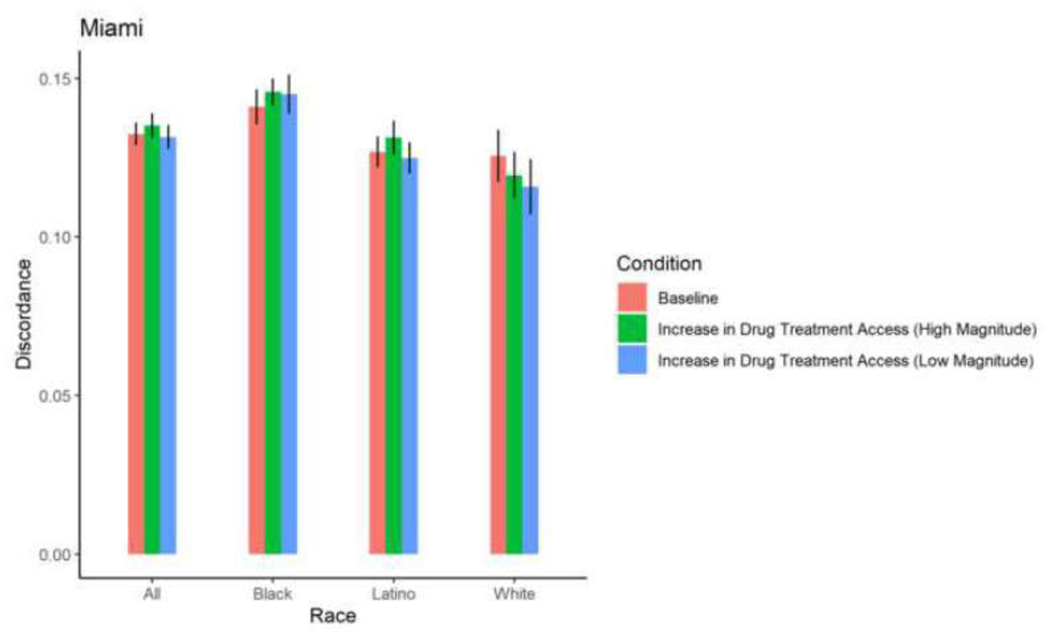
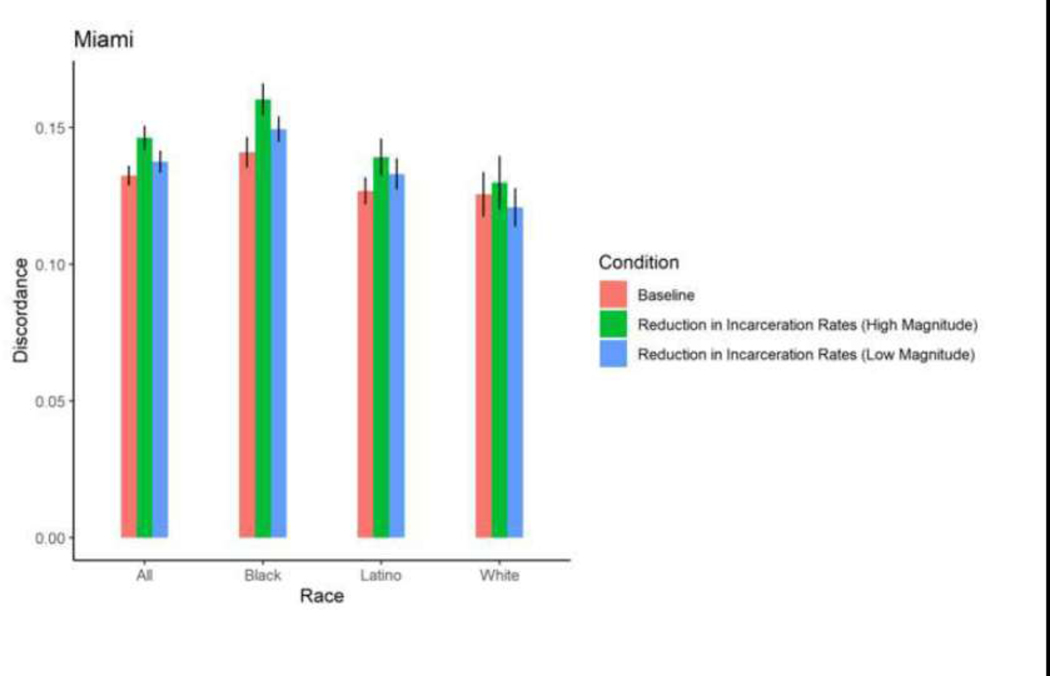
| Miami | Total | 0.1324 | 0.0003 | 0.1315 | 0.0003 | 0.7350 | 0.1351 | 0.0003 | 0.3197 | 0.1375 | 0.0003 | 0.0724 | 0.146 2 | 0.0003 | <0.000 1 |
| Miami | Black | 0.1410 | 0.0004 | 0.1450 | 0.0005 | 0.3528 | 0.1457 | 0.0003 | 0.1920 | 0.149 4 | 0.0004 | 0.027 5 | 0.160 3 | 0.0005 | <0.000 1 |
| Miami | Latino | 0.1268 | 0.0004 | 0.1249 | 0.0004 | 0.5982 | 0.1313 | 0.0004 | 0.2360 | 0.1330 | 0.0004 | 0.1161 | 0.139 2 | 0.0005 | 0.0055 |
| Miami | White | 0.1256 | 0.0006 | 0.1158 | 0.0007 | 0.1170 | 0.1194 | 0.0006 | 0.2777 | 0.1208 | 0.0006 | 0.3951 | 0.1299 | 0.0008 | 0.5121 |
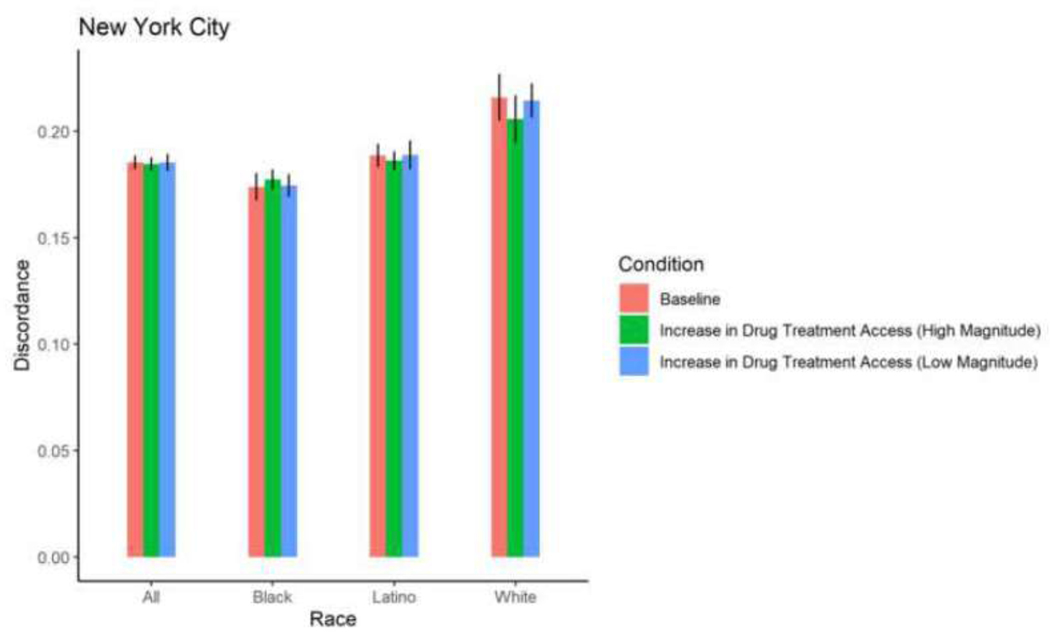
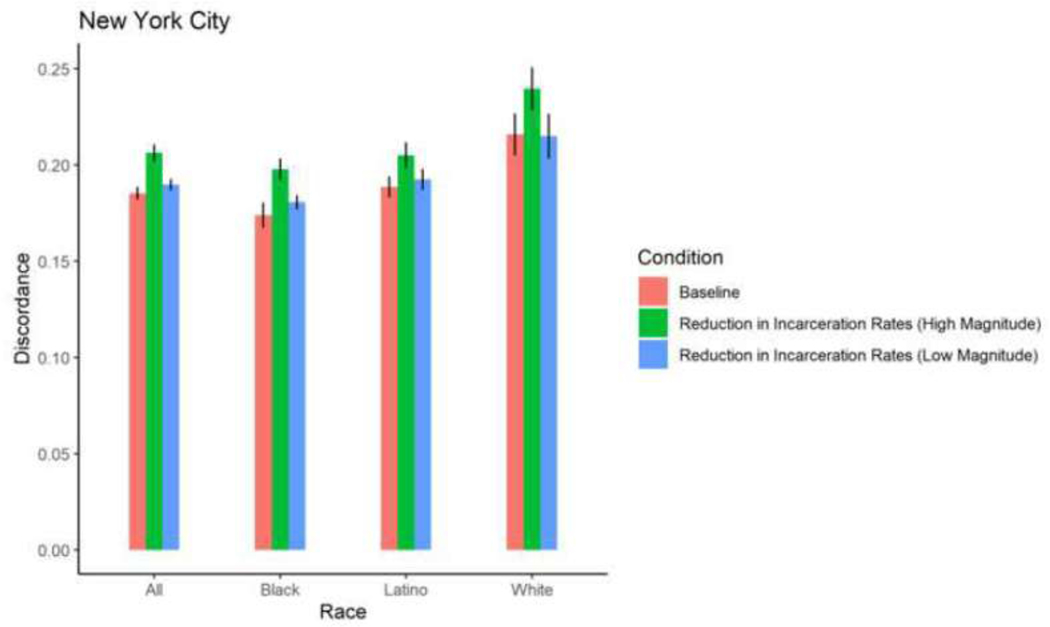
| New York City | Total | 0.1853 | 0.0003 | 0.1852 | 0.0003 | 0.9716 | 0.1844 | 0.0003 | 0.7054 | 0.189 7 | 0.0002 | 0.049 6 | 0.206 3 | 0.0003 | <0.000 1 |
| New York City | Black | 0.1738 | 0.0005 | 0.1744 | 0.0004 | 0.9024 | 0.1772 | 0.0004 | 0.4127 | 0.1806 | 0.0003 | 0.0858 | 0.197 8 | 0.0004 | <0.000 1 |
| New York City | Latino | 0.1886 | 0.0004 | 0.1888 | 0.0005 | 0.9524 | 0.1861 | 0.0003 | 0.4905 | 0.1924 | 0.0004 | 0.3251 | 0.205 0 | 0.0005 | 0.0004 |
| New York City | White | 0.2158 | 0.0009 | 0.2142 | 0.0006 | 0.8180 | 0.2057 | 0.0009 | 0.2094 | 0.2149 | 0.0009 | 0.9131 | 0.239 5 | 0.0009 | 0.0044 |
| San Francisco | Total | 0.1179 | 0.0003 | 0.1222 | 0.0004 | 0.1596 | 0.1143 | 0.0004 | 0.2526 | 0.1206 | 0.0003 | 0.3185 | 0.131 5 | 0.0003 | <0.000 1 |
| San Francisco | Black | 0.1362 | 0.0008 | 0.1317 | 0.0008 | 0.5448 | 0.1355 | 0.0009 | 0.9333 | 0.1408 | 0.0008 | 0.5349 | 0.1492 | 0.0009 | 0.0967 |
| San Francisco | Latino | 0.0961 | 0.0006 | 0.0939 | 0.0007 | 0.7007 | 0.0900 | 0.0005 | 0.2303 | 0.0924 | 0.0004 | 0.4335 | 0.0993 | 0.0005 | 0.5454 |
| San Francisco | White | 0.1231 | 0.0004 | 0.131 5 | 0.0004 | 0.021 2 | 0.1199 | 0.0004 | 0.3891 | 0.1278 | 0.0004 | 0.1614 | 0.140 7 | 0.0004 | <0.000 1 |
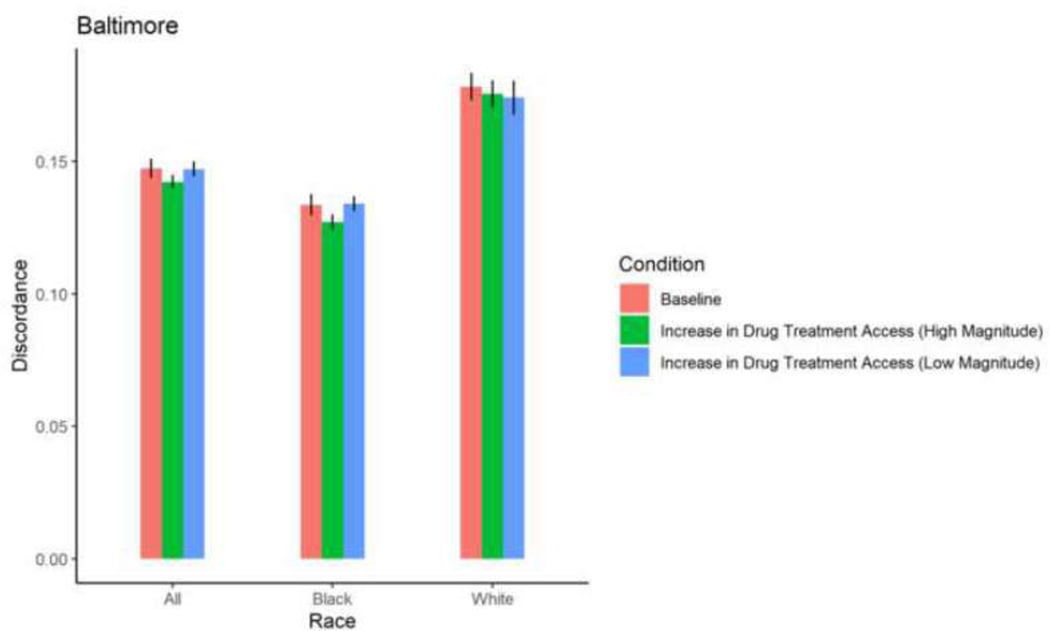
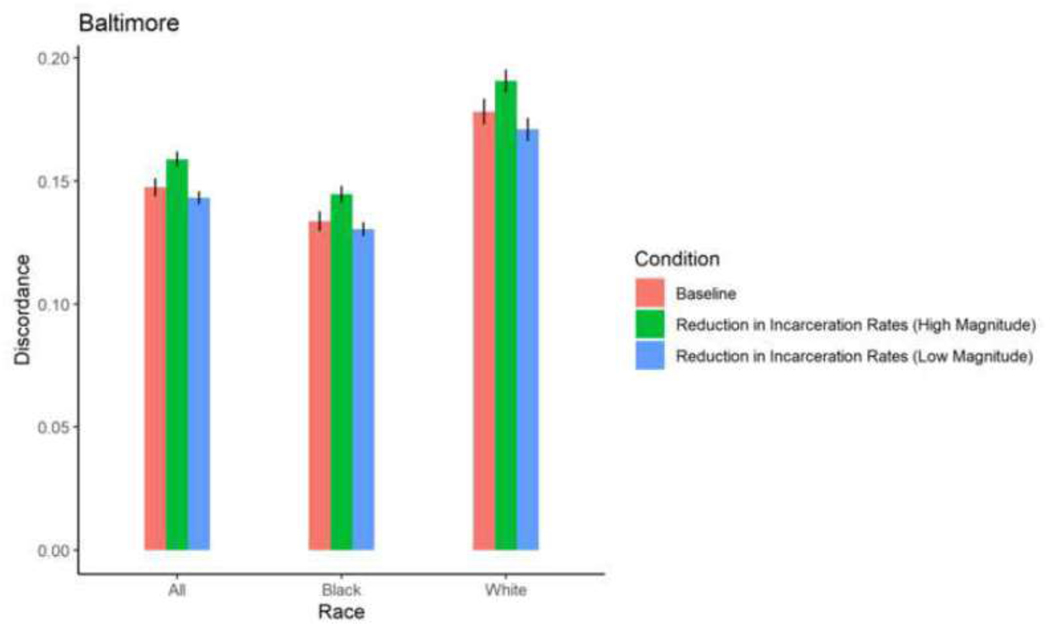
Figure 4. Proportion HIV sero-discordance in Baltimore, by race/ethnicity and magnitude of increases in drug treatment access (error bars represent 95% confidence intervals).
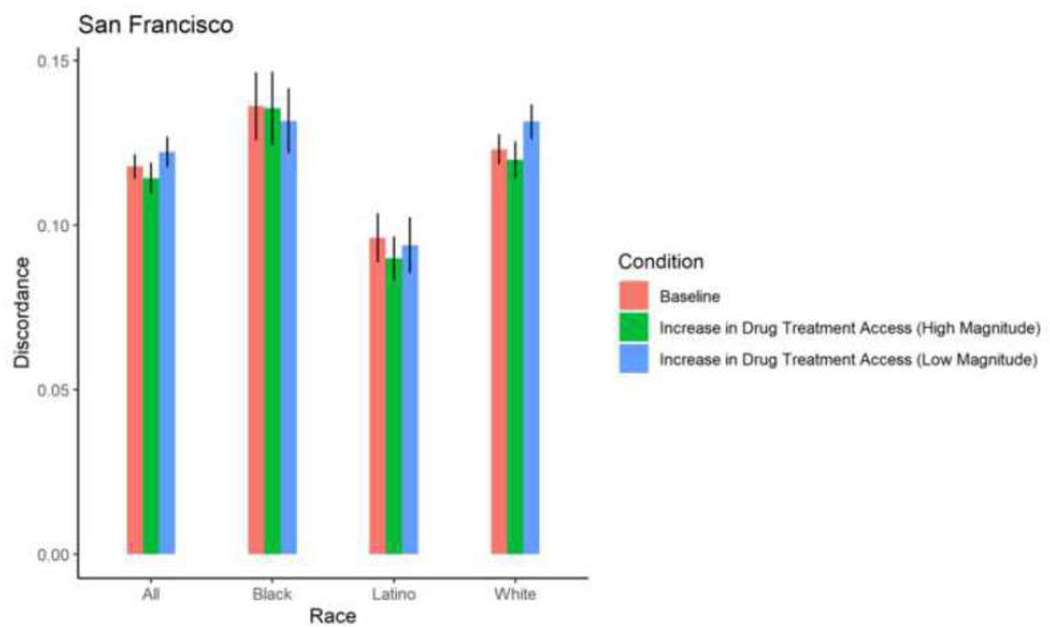
Figure 8. Proportion HIV sero-discordance in San Francisco, by race/ethnicity and magnitude of increases in drug treatment access (error bars represent 95% confidence intervals).
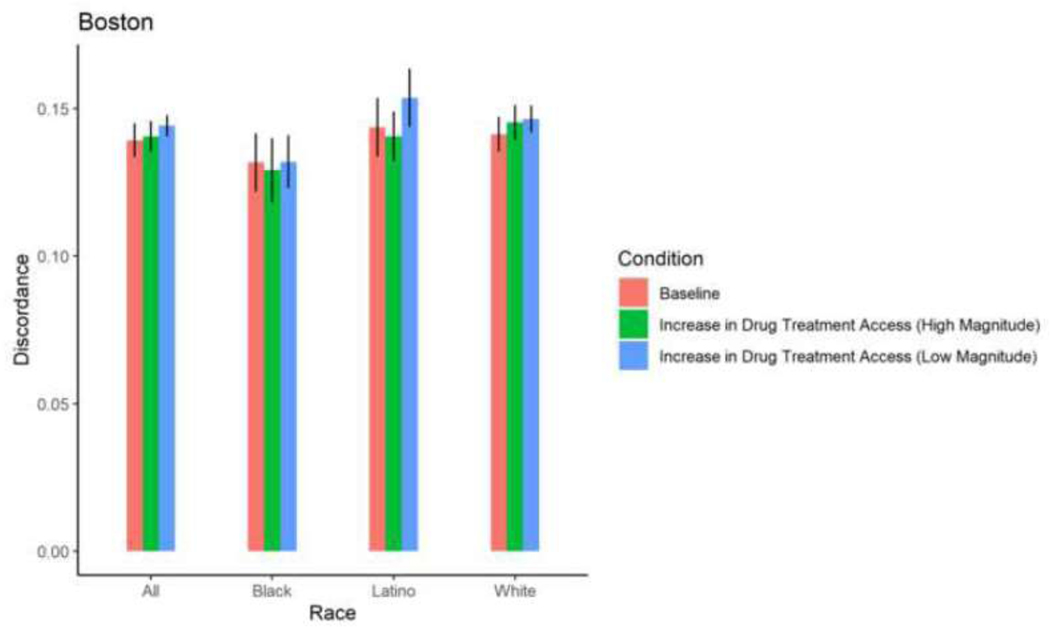
Simulated change in mean HIV sero-discordance as a result of increasing drug treatment access
With the exception of White PWID in San Francisco, among whom a 30% increase in drug treatment access resulted in a 0.84 percentage point-increase in sero-discordance, a 30% increase in drug treatment access did not result in significant changes in sero-discordance among PWID. With the exception of Black PWID in Baltimore, among whom a 58% increase in drug treatment access resulted in a decrease in sero-discordance by 0.65 percentage points, a 58% increase in drug treatment did not result in a significant change in sero-discordance among PWID. (Table 2; Figures 4–9).
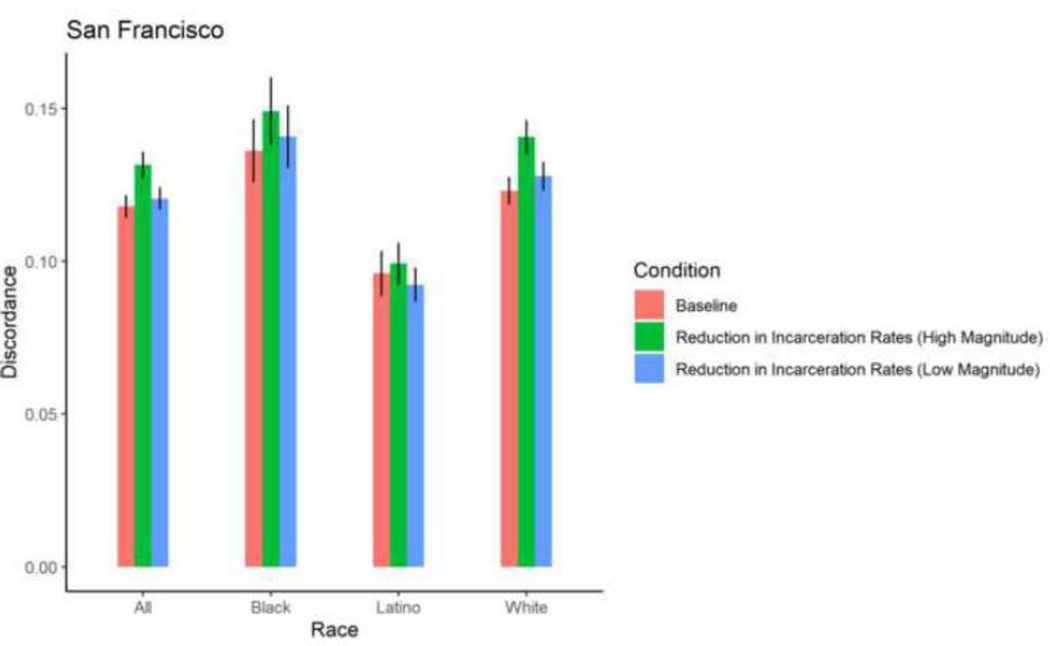
Figure 9. Proportion HIV sero-discordance in Baltimore, by race/ethnicity and magnitude of reductions in incarceration rates (error bars represent 95% confidence intervals).
Simulated change in mean HIV sero-discordance as a result of reducing incarceration rates
Among Black, Latino, and White PWID in most cities, exposure to a 5% reduction in incarceration rates and 2% increase in ZIP code-level HIV prevalence did not result in significant changes in mean proportions of HIV sero-discordance. Isolated changes were observed among White PWID in Baltimore, who experienced a marginally, significant 0.73 percentage point-reduction in mean proportion of sero-discordance as a result of a 5% decrease in incarceration rates, and Black PWID in Miami, who experienced a 0.84 percentage point-increase in mean proportion of sero-discordance following a 5% decrease in incarceration rates.
A 30% reduction in incarceration rates led to significant, 1–3 percentage point- increases in sero-discordance among at least one racial/ethnic group of PWID in all 5 cities. Specifically, in Baltimore, significantly higher mean proportions of sero-discordance were observed among Black and White PWID exposed to a 30% reduction in incarceration rates. Similar trends were observed among White PWID in San Francisco, Black and White PWID in Baltimore and Boston; Black and Latino PWID in Miami; and Black, White and Latino PWID in New York (Table 2, Figures 9–13).
Figure 13. Proportion HIV sero-discordance in San Francisco, by race/ethnicity and magnitude of reductions in incarceration rates (error bars represent 95% confidence intervals).
Sensitivity analyses demonstrated that the results were robust to changes in key parameters (see Appendix B)
Discussion
This study is the first to apply agent-based modeling to project short-term (i.e., 36 months) impacts of decreasing incarceration rates and increasing drug treatment access on sero-discordant partnerships among recently sexually active and injection equipment-sharing PWID. In each city of interest, large reductions in incarceration rates were projected to increase sero-discordant partnerships among at least one of the sampled racial/ethnic groups of PWID. Specifically, increases in sero-discordance in the wake of reductions in incarceration rates, were observed among Black and White PWID in Baltimore and Boston, Black and Latino PWID in Miami, Black, Latino and White PWID in New York, and White PWID in San Francisco. These increases in mean sero-discordance among racial/ethnic groups ranged from 1–3 percentage points. In contrast, significant impacts of reducing incarceration rates by 5% and increasing access to drug treatment programs on sero-discordance were isolated to Black and White PWID in a handful of cities. The significant changes in mean discordance that occurred among these PWID were below 1 percentage point, and in some cases, only marginally significant.
The potential for reductions in incarceration rates to increase sero-discordance over a short period time for some PWID, is supported by research of Ojikutu and colleagues. This work projected that over a five-year period, a 10-person increase in prison release would lead to a 4% increase in HIV diagnoses in 9 southern cities (Ojikutu, et al., 2018), including Miami — an MSA sampled in this study. Findings also mirror those observed by Adams and colleagues, who projected an increase in HIV acquisition among Black women following the release of Black men from prison and jail (Adams, et al., 2018, 2019).
In the context of a growing body of literature linking incarceration to sub-optimal HIV-related outcomes, the increase in sero-discordance that resulted from reductions in incarceration rates is counterintuitive. However, because the ABM created here reflected sexual and injection partnership dynamics over a short time frame of 3 years, it is possible that hypothesized positive consequences of incarceration reform, including decreases in HIV risk behaviors, stabilization of sexual networks, and increased access to drug treatment, and HIV care and treatment may not occur quickly enough to initially offset a potential uptick in community-level HIV prevalence that results after men and women are released from correctional settings, or men and women at risk for incarceration and HIV remain in their communities(Binswanger, et al., 2012; Merrall, et al., 2010; Vlahov & Putnam, 2006). Additionally, men and women who might otherwise receive HIV testing and treatment services while incarcerated, may not be reached by community-based programs (Bartlett, et al., 2008; Branson, et al., 2006; Seal, et al., 2010)
Although the increases in sero-discordance due to reductions in incarceration rates were slight, an examination of the ABM results for New York City can be used to further illustrate how reductions in incarceration rates might meaningfully increase HIV transmission within a population. Specifically, our ABM projected an increase in prevalence of discordant relationships from 19% to 21% as a result of a 58% decrease in incarceration rates from 2009 to 2012 in New York City. Projected HIV incidence among PWID in New York City during that time frame was approximately 100 incident cases per year among the 100,000 PWID in the city (Don C. Des Jarlais, Arasteh, et al., 2016). Thus, assuming a perfect correlation between prevalence of discordant relationships and HIV incidence among PWID, the numbers of incident cases among PWID would have increased to approximately 110/year under high implementation of criminal justice reform.
Criminal justice reform initiatives are rooted in efforts to advance social justice and racial/ethnic equity, and most evidence suggests that they can have ameliorative impacts on the public’s health in the long-term. Prior research suggests that ensuring HIV prevention and treatment among justice-involved populations and the persons with whom they interact in the community requires a multipronged approach that accomplishes the following: (1) bridges gaps between HIV and behavioral health services in correctional settings and those in the community, (2) engages formerly incarcerated men and women into HIV testing and treatment, and behavioral health programs in the community, (3) builds trust in the medical system and autonomy among formerly incarcerated men and women, (4) facilitates community reintegration by reducing barriers to employment, housing, and social benefits, leveraging social capital, and (5) integrates case management and patient navigation in post-release and reentry programs (Binswanger et al., 2012; Kim et al., 2019; Kutnick, Leonard, & Gwadz, 2019; McLean, Robarge, & Sherman, 2006; van Olphen, Freudenberg, Fortin, & Galea, 2006). Many of these approaches are documented to have strengthened reintegration and to have increased HIV care engagement and retention (Myers, et al., 2018; Rich, et al., 2001; Westergaard, et al., 2019), and thus offer evidence on what steps are critical to creating infrastructures that buffer unintended, negative consequences of incarceration reform.
The finding that the impact of reductions in incarceration rates on mean sero-discordance may vary across geographic settings for specific racial/ethnic groups suggests that underlying differences in contextual and HIV endemicities should be considered when tailoring strategies to specific racial/ethnic groups. These differential findings by MSA and race/ethnicity may reflect the racial/ethnic distribution of the PWID in the five cities of interest. Based on our prior research we also hypothesize exposure to place-based factors may partly influence these findings (H. L. F. Cooper, et al., 2016). However, we were unable to model such nuances in the ABM.
Given the important role that substance use treatment plays in HIV prevention, the lack of impact of increasing drug treatment access on reducing sero-discordance among PWID in most cities in the ABM is surprising, as is the counter-intuitive relationship of increased access to drug treatment and increases in sero-discordance among White PWID in San Francisco. The low frequency of discordant relationships in injection equipment-sharing partnerships and our inability to model some mediating pathways that link drug treatment access to sero-discordant partnerships may explain the null findings observed among PWID in most cities. Across the MSAs in the ABM, approximately 10% of PWID who knew their status and that of their partners reported sero-discordant injection equipment-sharing partnerships. Thus, expansion might only impact a small number of PWID in discordant injection equipment-sharing partnerships. Additionally, because the ABM is operationalized such that PWID are subtracted from the injection-equipment sharing partner pool and not from the sexual partner pool when treatment access increases, the absolute and relative number of discordant sexual partnerships would not be affected by changes in treatment utilization. Lastly, the ABM did not account for institutional barriers (e.g., provider stigma) to accessing drug treatment, and did not simultaneously evaluate the potential combined impact of reducing incarceration rates and increasing drug treatment access on sero-discordance to maintain parsimony. Future studies should consider the intersection of drug treatment and incarceration policies, given high rates of recidivism among people who use drugs (Belenko & Peugh, 2005; Chandler, Fletcher, & Volkow, 2009; Polcin, 2018; Zarkin, et al., 2012), and greater likelihood of drug cessation and HIV care retention among people who have received drug treatment in correctional settings and in community-based after-care programs. (Swan, 2015; Zarkin, et al., 2012). Further, the counterintuitive results among White PWID in San Francisco warrants further investigation.
The interpretation of findings from this study are subject to additional limitations. This analysis was restricted to 3 years, and we lacked data necessary to simulate subsequent increases in the stability of injecting and sexual partnerships among PWID that may offset the initial increase in HIV prevalence. We parameterized the ABM using data from PWID who were recruited using RDS but did not account for this sampling procedure in analysis to maintain parsimony of the model. Prior research documents inconsistencies in the extent to which RDS sampling methods result in similar or different distribution of demographic characteristics, including age, racial/ethnic background, HIV status and residential location as compared to other recruitment methods (Abdul-Quader, et al., 2006; Burt, Hagan, Sabin, & Thiede, 2010; Burt & Thiede, 2014; Collier, Garfein, Cuevas-Mota, & Teshale, 2017; Robinson, et al., 2006; Rudolph, Young, & Lewis, 2015). Because the sample of PWID used for parameterizing the model exclusively lived in urban centers across the United States, this study’s findings lacked generalizability to PWID living in rural and suburban settings. Further, while we attempted to sample cities from multiple regions of the United States, the small sample of cities included did not reflect a full snapshot of regional diversity. Additionally, NHBS participant data used to validate model output, were drawn from participants’ perceptions of their partner’s HIV status, therefore the reported partnerships may have been misclassified, but it is assumed that potential misclassification was non-differential. With the exception of Boston, the period of data collection for NHBS occurred prior to when Medicaid expansion occurred in the cities of interest. Most criminal justice reform policies were also implemented after the data collection period for most cities included in this analysis. It took several years to build these models and secure approval from sites, and we were restricted to using NHBS 2009 and 2012 data for model parameterization and validation.
ABM include simplifications of “reality,” and do not include all the potential causal pathways that affect the outcomes in question. This is true for this analysis, where several hypothesized pathways linking reductions in incarceration rates and increases in drug treatment access to potential mediators could not be projected (e.g., changes in sexual and injection drug use-related access and utilization of community- and corrections-based HIV prevention and treatment services). Some covariates (e.g., homelessness) were also excluded to maintain parsimony of the models. Further, the model is only reflective of the data that is used to parameterized it, and different values for some metrics could have resulted in different estimations of sero-discordance. For example, increasing the duration of drug treatment and incarceration, would have possibly caused the model to underestimate sero-discordance because links between agents and their partners would be deemed inactive for longer periods of time in the model. However, at the same time, we believe that changing the values of some parameters in the model might not have changed the model’s estimation of sero-discordance. For example, changing the sexual network and injection equipment-sharing network dissolution rates would have only impacted the extent to which agents and their partners cycle through partnerships, not the extent to which discordant dyads are formed. Our sensitivity analyses supports this assumption, by demonstrating that the impacts of increasing drug treatment access and reducing incarceration rates on seroconversion were generally consistent when we varied the injection equipment-sharing and sexual network dissolution rates (see Appendix B).
Lastly, this study did not directly measure HIV transmission, which limits our abilities to make strong inferences regarding the direct impacts of reducing incarceration rates and increasing access to drug treatment on HIV transmission. Our interpretation of sero-discordant partnerships as proxies for HIV transmission is also limited by our inability to account for the frequency of condom-less sexual intercourse and to distinguish between main and casual partnerships, which have different potentialities for behaviors that influence vulnerability to HIV acquisition. For example, studies document higher condom use among adults who have histories of using drugs with casual partners than with main partners, and that condom use decreases as the duration of an intimate partnership increases (Bernstein, et al., 2013; Sherman & Latkin, 2001). Among PWID enrolled in NHBS, whose data was used to estimate the model, partnership type (main vs. casual) and length of partnerships varies, with our prior research documenting that more than half of PWID in 2012 reported that their last sexual partner was a main partner; the median duration of sexual partnerships was 2 years, and nearly 75% reported condomless sex (Linton, et al., 2020).
Other model specifications need to be considered when interpreting the implications of the ABM to understanding HIV transmission. To maintain parsimony, injection equipment-sharing and sexual partnerships were modeled independently and not jointly in the model. Therefore, we were unable to explore the potential impacts of multiplex relationships, wherein partners serve as both injection equipment-sharing partners and sexual partners, a circumstance that is not uncommon among people who use drugs and increases their vulnerability to HIV acquisition (Rudolph, Crawford, Latkin, & Lewis, 2017). Additionally, in our model, pre-existing relationships and new relationships had similar probability of formation, such that agents were not restricted from reestablishing a partnership with a prior partner. Reconnections among pre-existing partners would therefore cause sero-discordance among these partnerships to overestimate the effect on HIV transmission. However, because the agent population was large relative to the number of network links per agent, the probability of reconnecting with a previous partner in the model was low. We were also unable to model ART treatment as prevention (TasP), as there was relatively little ART TasP in the data we used, and data on viral suppression among PWID in this sample was not available. We also did not consider PrEP use in the model, given low prevalence of uptake (0.13%) of PrEP among PWID who self-reported HIV negative status and lived in the five cities sampled in this study. Varying the HIV prevalence and ART rate in sensitivity analyses (Appendix B), however, did not change the interpretation of results.
Conclusion
Despite these limitations, this study provides an example of how ABM can be applied to generate early insight into whether changes in public policies may impact vulnerability to HIV transmission in a vulnerable population. Although increasing access to drug treatment demonstrated little impact on sero-discordance, reductions in incarceration rates, which were simulated to lead to a small-order increase in community-level HIV transmission, increased sero-discordance. If criminal justice reform has the potential to increase sero-discordance by increasing community-level HIV prevalence over the short-term, local agencies and community organizations must make a concerted effort to build social service and public health program infrastructure in ways that prevent these unintended negative consequences. Further the observation of this finding among different racial/ethnic groups in different MSAs, suggests the response must be tailored appropriately according to geography and race/ethnicity of the client population. Future longitudinal studies should extend the modeling applied here to both quantify and elucidate the temporal and longer-term impacts of criminal justice reform and Medicaid expansion on HIV-related outcomes, especially in cities where these policies have been implemented over a longer period of observation, an on overdose. Additionally, because actual local-level equity in enforcement of criminal justice reform, including decriminalization of cannabis and the First Step Act has been questioned, future studies should investigate this inequity and its implications to racial/ethnic health disparities. This line of research will augment other analyses uncovering the impact of Medicaid expansion and criminal justice reform on the health of people who use drugs.
Supplementary Material
Figure 5. Proportion HIV sero-discordance in Boston, by race/ethnicity and magnitude of increases in drug treatment access (error bars represent 95% confidence intervals).
Figure 6. Proportion HIV sero-discordance in Miami, by race/ethnicity and magnitude of increases in drug treatment access (error bars represent 95% confidence intervals).
Figure 7. Proportion HIV sero-discordance in New York, by race/ethnicity and magnitude of increases in drug treatment access (error bars represent 95% confidence intervals).
Figure 10. Proportion HIV sero-discordance in Boston, by race/ethnicity and magnitude of reductions in incarceration rates (error bars represent 95% confidence intervals).
Figure 11. Proportion HIV sero-discordance in Miami, by race/ethnicity and magnitude of reductions in incarceration rates (error bars represent 95% confidence intervals).
Figure 12. Proportion HIV sero-discordance in New York, by race/ethnicity and magnitude of reductions in incarceration rates (error bars represent 95% confidence intervals).
Acknowledgements
This research was supported by three grants from the National Institutes of Health: (R01DA035101; R01DA046197 Cooper, PI, and P30 AI050409; Curran, PI). We thank the Centers for Disease Control and Prevention and extend our gratitude to Sara Braunstein for sharing data collected by the New York City NHBS site, all staff at the Baltimore, Boston, Miami, New York City, and San Francisco NHBS sites, and men and women who volunteered their time and participated in NHBS. We also acknowledge Elizabeth Horinek for assisting the literature review, Stephanie Beane for reviewing the paper, and the CDC clearance review team for providing final feedback on the paper.
The Institutional Review Boards (IRBs) of each NHBS site and the CDC approved study protocols.
Footnotes
Conflict of interest
None declared
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Quader AS, Heckathorn DD, McKnight C, Bramson H, Nemeth C, Sabin K, Gallagher K, & Des Jarlais DC (2006). Effectiveness of respondent-driven sampling for recruiting drug users in New York City: findings from a pilot study. Journal of urban health : bulletin of the New York Academy of Medicine, 83, 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JW, Lurie MN, King MRF, Brady KA, Galea S, Friedman SR, Khan MR, & Marshall BDL (2018). Potential drivers of HIV acquisition in African-American women related to mass incarceration: an agent-based modelling study. BMC public health, 18, 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JW, Lurie MN, King MRF, Brady KA, Galea S, Friedman SR, Khan MR, & Marshall BDL (2019). Decreasing HIV transmissions to African American women through interventions for men living with HIV post-incarceration: An agent-based modeling study. PloS one, 14, e0219361–e0219361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adimora AA, & Schoenbach VJ (2005). Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis, 191 Suppl 1, S115–122. [DOI] [PubMed] [Google Scholar]
- Alexander M. (2010). The new Jim Crow : mass incarceration in the age of colorblindness: New York : New Press; [Jackson Tenn.] : Distributed by Perseus Distribution, 2010. [Google Scholar]
- Aral SO, Hughes JP, Stoner B, Whittington W, Handsfield HH, Anderson RM, & Holmes KK (1999). Sexual mixing patterns in the spread of gonococcal and chlamydial infections. American journal of public health, 89, 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicker K, Allen HL, Wright BJ, & Finkelstein AN (2017). The Effect Of Medicaid On Medication Use Among Poor Adults: Evidence From Oregon. Health Aff (Millwood), 36, 2110–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, & Mayer KH (2008). Opt-Out Testing for Human Immunodeficiency Virus in the United States: Progress and Challenges. JAMA, 300, 945–951. [DOI] [PubMed] [Google Scholar]
- Belenko S, & Peugh J. (2005). Estimating drug treatment needs among state prison inmates. Drug Alcohol Depend, 77, 269–281. [DOI] [PubMed] [Google Scholar]
- Beletsky L, Cochrane J, Sawyer AL, Serio-Chapman C, Smelyanskaya M, Han J, Robinowitz N, & Sherman SG (2015). Police Encounters Among Needle Exchange Clients in Baltimore: Drug Law Enforcement as a Structural Determinant of Health. American Journal of Public Health, 105, 1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Ng V, McCloskey L, Vazquez K, Ashong D, Stapleton S, Cromwell J, & Bernstein J. (2013). Qualitative analysis of cocaine and heroin users’ main partner sex-risk behavior: is safety in love safety in health? Addiction science & clinical practice, 8, 10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello KB, Bazzi AR, Mimiaga MJ, Biancarelli DL, Edeza A, Salhaney P, Childs E, & Drainoni ML (2018). Perspectives on HIV pre-exposure prophylaxis (PrEP) utilization and related intervention needs among people who inject drugs. Harm reduction journal, 15, 55–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Nowels C, Corsi KF, Glanz J, Long J, Booth RE, & Steiner JF (2012). Return to drug use and overdose after release from prison: a qualitative study of risk and protective factors. Addiction science & clinical practice, 7, 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert ASB, Bradshaw CP, & Latkin CA (2009). A social network perspective on heroin and cocaine use among adults: evidence of bidirectional influences. Addiction, 104, 1210–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, & Clark JE (2006). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in healthcare settings. Morbidity and Mortality Weekly Report, 55, 1–14. Morbidity and Mortality Weekly Report, 55, 1–14. [PubMed] [Google Scholar]
- Bronson J, & Carson AE (2019). Prisoners in 2017. In Bulletin. Washington, D.C.: U.S. Department of Justice, Office of Justice Programs, Bureau of Justice Statistic; s. [Google Scholar]
- Brown JL, Eriksen MD, Gause NK, Brody GH, & Sales JM (2018). Impact of Behavioral Drug Abuse Treatment on Sexual Risk Behaviors: An Integrative Data Analysis of Eight Trials Conducted Within the National Drug Abuse Treatment Clinical Trials Network. Prev Sci, 19, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Prisons Statistics. Retrieved January 3 2020 from https://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. [Google Scholar]
- Burnett JC, Broz D, Spiller MW, Wejnert C, & Paz-Bailey G. (2018). HIV Infection and HIV-Associated Behaviors Among Persons Who Inject Drugs - 20 Cities, United States, 2015. MMWR Morb Mortal Wkly Rep, 67, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris S, Blankenship KM, Donoghoe M, Sherman S, Vernick JS, Case P, Lazzarini Z, & Koester S. (2004). Addressing the “risk environment” for injection drug users: the mysterious case of the missing cop. The Milbank quarterly, 82, 125–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RD, Hagan H, Sabin K, & Thiede H. (2010). Evaluating respondent-driven sampling in a major metropolitan area: Comparing injection drug users in the 2005 Seattle area national HIV behavioral surveillance system survey with participants in the RAVEN and Kiwi studies. Annals of epidemiology, 20, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RD, & Thiede H. (2014). Assessing differences in groups randomized by recruitment chain in a respondent-driven sample of Seattle-area injection drug users. Annals of epidemiology, 24, 861–867.e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RK, Fletcher BW, & Volkow ND (2009). Treating drug abuse and addiction in the criminal justice system: improving public health and safety. JAMA, 301, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier MG, Garfein RS, Cuevas-Mota J, & Teshale EH (2017). Comparison of Three Popular Methods for Recruiting Young Persons Who Inject Drugs for Interventional Studies. Journal of urban health : bulletin of the New York Academy of Medicine, 94, 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Color of Justice: Racial and Ethnic Disparity in State Prisons. In. (2016). Washington, DC: The Sentencing Project. [Google Scholar]
- Cooper H, Moore L, Gruskin S, & Krieger N. (2005). The impact of a police drug crackdown on drug injectors’ ability to practice harm reduction: A qualitative study. Social Science & Medicine, 61, 673–684. [DOI] [PubMed] [Google Scholar]
- Cooper HLF, Caruso B, Barham T, Embry V, Dauria E, Clark CD, & Comfort ML (2015). Partner Incarceration and African-American Women’s Sexual Relationships and Risk: A Longitudinal Qualitative Study. Journal of urban health : bulletin of the New York Academy of Medicine, 92, 527–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HLF, Linton S, Kelley ME, Ross Z, Wolfe ME, Chen Y-T, Zlotorzynska M, Hunter-Jones J, Friedman SR, Des Jarlais D, Semaan S, Tempalski B, DiNenno E, Broz D, Wejnert C, & Paz-Bailey G. (2016). Racialized risk environments in a large sample of people who inject drugs in the United States. International Journal of Drug Policy, 27, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert GJ, Waluyo A, Wang M, Putri TA, Bazazi AR, & Altice FL (2018). Adherence to Antiretroviral Therapy Among Incarcerated Persons with HIV: Associations with Methadone and Perceived Safety. AIDS and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Garlington S, & Schottenfeld LS (2013). Alcohol, Drug, and Criminal History Restrictions in Public Housing. Cityscape: A Journal of Policy Development and Research, 15. [Google Scholar]
- Davey-Rothwell M, Frydl A, & Latkin C. (2009). Does taking steps to control one’s drug use predict entry into treatment? The American journal of drug and alcohol abuse, 35, 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBeck K, Cheng T, Montaner JS, Beyrer C, Elliott R, Sherman S, Wood E, & Baral S. (2017). HIV and the criminalisation of drug use among people who inject drugs: a systematic review. The lancet. HIV, 4, e357–e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Arasteh K, McKnight C, Feelemyer J, Campbell ANC, Tross S, Smith L, Cooper HLF, Hagan H, & Perlman D. (2016). Consistent Estimates of Very Low HIV Incidence Among People Who Inject Drugs: New York City, 2005–2014. American journal of public health, 106, 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, & Arasteh K. (2016). HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. AIDS (London, England), 30, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Pinkerton S, Hagan H, Guardino V, Feelemyer J, Cooper H, Hatzatkis A, & Uuskula A. (2013). 30 Years on Selected Issues in the Prevention of HIV among Persons Who Inject Drugs. Advances in preventive medicine, 2013, 346372–346372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farel CE, Parker SD, Muessig KE, Grodensky CA, Jones C, Golin CE, Fogel CI, & Wohl DA (2013). Sexuality, sexual practices, and HIV risk among incarcerated African-American women in North Carolina. Women’s health issues : official publication of the Jacobs Institute of Women’s Health, 23, e357–e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M, Marsden J, Ling W, Ali R, & Gowing L. (2005). Effectiveness of drug dependence treatment in preventing HIV among injecting drug users. In. [Google Scholar]
- Fauci AS, Redfield RR, Sigounas G, Weahkee MD, & Giroir BP (2019). Ending the HIV Epidemic: A Plan for the United StatesEnding the HIV EpidemicEditorial. JAMA, 321, 844–845. [DOI] [PubMed] [Google Scholar]
- Feder KA, Mojtabai R, Krawczyk N, Young AS, Kealhofer M, Tormohlen KN, & Crum RM (2017). Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug Alcohol Depend, 179, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KM, Sullivan PS, Lansky A, & Onorato IM (2007). Behavioral surveillance among people at risk for HIV infection in the U.S.: the National HIV Behavioral Surveillance System. Public health reports (Washington, D.C. : 1974), 122 Suppl 1, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant Z, Dailey A, Hu X, & Johnson AS (2017). HIV Care Outcomes Among Hispanics or Latinos with Diagnosed HIV Infection - United States, 2015. MMWR Morb Mortal Wkly Rep, 66, 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafa K, McElroy P, Fitzpatrick L, Borkowf CB, MacGowan R, Margolis A, Robbins K, Youngpairoj AS, Stratford D, Greenberg A, Taussig J, Shouse RL, LaMarre M, McLellan-Lemal E, Heneine W, & Sullivan PS (2009). HIV Transmission in a State Prison System, 1988–2005. PloS one, 4, e5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DJ, & Glaze LE (2006). Mental Health Problems of Prison and Jail Inmates. In. Washington, DC: Bureau of Justice Statistics, U.S. Department of Justice. [Google Scholar]
- Karch DL, Gray KM, Shi J, & Hall HI (2016). HIV Infection Care and Viral Suppression Among People Who Inject Drugs, 28 U.S. Jurisdictions, 2012–2013. Open AIDS J, 10, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MR, Behrend L, Adimora AA, Weir SS, Tisdale C, & Wohl DA (2011). Dissolution of primary intimate relationships during incarceration and associations with post-release STI/HIV risk behavior in a Southeastern city. Sexually transmitted diseases, 38, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Martin BM, Doherty R, Fukuda HD, Cranston K, & DeMaria A. (2018). Factors Associated with Pre-exposure Prophylaxis in a Highly Insured Population of Urban Men Who Have Sex with Men, 2014. AIDS and Behavior, 22, 1201–1208. [DOI] [PubMed] [Google Scholar]
- Larney S. (2010). Does opioid substitution treatment in prisons reduce injecting-related HIV risk behaviours? A systematic review. Addiction, 105, 216–223. [DOI] [PubMed] [Google Scholar]
- Laumann EO, & Youm Y. (1999). Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis, 26, 250–261. [DOI] [PubMed] [Google Scholar]
- Lesko CR, Tong W, Moore RD, & Lau B. (2017). Retention, Antiretroviral Therapy Use and Viral Suppression by History of Injection Drug Use Among HIV-Infected Patients in an Urban HIV Clinical Cohort. AIDS and Behavior, 21, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton SL, Cooper HLF, Chen YT, Khan MA, Wolfe ME, Ross Z, Des Jarlais DC, Friedman SR, Tempalski B, Broz D, Semaan S, Wejnert C, & Paz-Bailey G. (2020). Mortgage Discrimination and Racial/Ethnic Concentration Are Associated with Same-Race/Ethnicity Partnering among People Who Inject Drugs in 19 US Cities. J Urban Health, 97, 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas KD, Eckert V, Behrends CN, Wheeler C, MacGowan RJ, & Mohle-Boetani JC (2016). Evaluation of Routine HIV Opt-Out Screening and Continuum of Care Services Following Entry into Eight Prison Reception Centers--California, 2012. MMWR Morb Mortal Wkly Rep, 65, 178–181. [DOI] [PubMed] [Google Scholar]
- Luhby T. (2019). GOP lawmakers set up roadblocks to voter-approved Medicaid expansion. In CNN Politics. [Google Scholar]
- Macher A, Kibble D, & Wheeler D. (2006). HIV transmission in correctional facility. Emerg Infect Dis, 12, 669–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruschak LM (2015). HIV in Prisons, 2001–2010. In. Washington, DC: Bureau of Justice Statistics, U.S. Department of Justice. [Google Scholar]
- Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, Myers B, Ambekar A, & Strathdee SA (2010). HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. The Lancet, 375, 1014–1028. [DOI] [PubMed] [Google Scholar]
- Mayock P, Cronly J, & Clatts MC (2015). The Risk Environment of Heroin Use Initiation: Young Women, Intimate Partners, and “Drug Relationships”. Substance use & misuse, 50, 771–782. [DOI] [PubMed] [Google Scholar]
- McClelland GM, Teplin LA, Abram KM, & Jacobs N. (2002). HIV and AIDS risk behaviors among female jail detainees: implications for public health policy. American journal of public health, 92, 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland W, Lin J, Santos G-M, Arayasirikul S, Raymond HF, & Wilson E. (2019). Low PrEP Awareness and Use Among People Who Inject Drugs, San Francisco, 2018. AIDS and Behavior. [DOI] [PubMed] [Google Scholar]
- McKenna RM (2017). Treatment use, sources of payment, and financial barriers to treatment among individuals with opioid use disorder following the national implementation of the ACA. Drug and Alcohol Dependence, 179, 87–92. [DOI] [PubMed] [Google Scholar]
- Meinhofer A, & Witman AE (2018). The role of health insurance on treatment for opioid use disorders: Evidence from the Affordable Care Act Medicaid expansion. Journal of Health Economics, 60, 177–197. [DOI] [PubMed] [Google Scholar]
- Melo JS, Garfein RS, Hayashi K, Milloy MJ, DeBeck K, Sun S, Jain S, Strathdee SA, & Werb D. (2018). Do law enforcement interactions reduce the initiation of injection drug use? An investigation in three North American settings. Drug and Alcohol Dependence, 182, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, & Bird SM (2010). Meta-analysis of drug-related deaths soon after release from prison. Addiction, 105, 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, Shore R, & Hopkins S. (2007). Reduction in injection-related HIV risk after 6 months in a low-threshold methadone treatment program. AIDS Educ Prev, 19, 124–136. [DOI] [PubMed] [Google Scholar]
- Momplaisir F, Hussein M, Tobin-Fiore D, Smith L, Bennett D, Latkin C, & Metzger DS (2017). Racial inequities in HIV prevalence and composition of risk networks among people who inject drugs in HIV Prevention Trial Network 037. Journal of Acquired Immuno Deficiency Syndromes, 76, 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More imprisonment does not reduce state drug problems. In. (2018): The Pew Charitable Trusts. [Google Scholar]
- Mumola CJ, & Karberg JC (2007). Drug Use and Dependence, State and Federal Prisoners, 2004. In. Washington, DC: Bureau of Justice Statistics, U.S. Department of Justice. [Google Scholar]
- Musto DF (1987). The American Disease: Origins of Narcotic Control: Oxford University Press. [Google Scholar]
- Myers JJ, Dufour M-SK, Koester KA, Morewitz M, Packard R, Klein KM, Estes M, Williams B, Riker A, & Tulsky J. (2018). The Effect of Patient Navigation on the Likelihood of Engagement in Clinical Care for HIV-Infected Individuals Leaving Jail. American journal of public health, 108, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn A, Dickman S, Cornwall A, Kwakwa H, Mayer KH, Rana A, & Rosengard C. (2012). Concurrent sexual partnerships among African American women in Philadelphia: results from a qualitative study. Sexual health, 9, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn A, Dickman S, Cornwall A, Rosengard C, Kwakwa H, Kim D, James G, & Mayer KH (2011). Social, structural and behavioral drivers of concurrent partnerships among African American men in Philadelphia. AIDS care, 23, 1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojikutu BO, Srinivasan S, Bogart LM, Subramanian SV, & Mayer KH (2018). Mass incarceration and the impact of prison release on HIV diagnoses in the US South. PloS one, 13, e0198258–e0198258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Wall M, Barry CL, Mauro C, & Mojtabai R. (2018). Impact Of Medicaid Expansion On Coverage And Treatment Of Low-Income Adults With Substance Use Disorders. Health Aff (Millwood), 37, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JN, Linton SL, Sherman SG, & German D. (2019). Police violence among people who inject drugs in Baltimore, Maryland. International Journal of Drug Policy, 64, 5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietila A. (2012). Not in My Neighborhood: How Bigotry Shaped a Great American City: Ivan R Dee, Incorporated. [Google Scholar]
- Polcin DL (2018). Role of recovery residences in criminal justice reform. The International journal on drug policy, 53, 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis B, Griffiths P, Gossop M, & Strang J. (1996). The Differences between Male and Female Drug Users: Community Samples of Heroin and Cocaine Users Compared. Substance use & misuse, 31, 529–543. [DOI] [PubMed] [Google Scholar]
- Reardon SF, Fox L, & Townsend J. (2015). Neighborhood income composition by household age and income, 1990–2009. The Annals of the American Academy of Political and Social Science, 660, 78–97. [Google Scholar]
- Rich JD, Holmes L, Salas C, Macalino G, Davis D, Ryczek J, & Flanigan T. (2001). Successful linkage of medical care and community services for HIV-positive offenders being released from prison. Journal of urban health : bulletin of the New York Academy of Medicine, 78, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WT, Risser JMH, McGoy S, Becker AB, Rehman H, Jefferson M, Griffin V, Wolverton M, & Tortu S. (2006). Recruiting injection drug users: a three-site comparison of results and experiences with respondent-driven and targeted sampling procedures. Journal of urban health : bulletin of the New York Academy of Medicine, 83, i29–i38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DL, Schoenbach VJ, Wohl DA, White BL, Stewart PW, & Golin CE (2009). Characteristics and behaviors associated with HIV infection among inmates in the North Carolina prison system. American journal of public health, 99, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Tran N, Piecara B, Welles S, Shinefeld J, & Brady K. (2019). Factors Associated with Awareness of Pre-exposure Prophylaxis for HIV Among Persons Who Inject Drugs in Philadelphia: National HIV Behavioral Surveillance, 2015. AIDS Behav, 23, 18331840. [DOI] [PubMed] [Google Scholar]
- Rudolph AE, Crawford ND, Latkin C, & Lewis CF (2017). Multiplex Relationships and HIV: Implications for Network-Based Interventions. AIDS and Behavior, 21, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph AE, Young AM, & Lewis CF (2015). Assessing the geographic coverage and spatial clustering of illicit drug users recruited through respondent-driven sampling in [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York City. Journal of urban health : bulletin of the New York Academy of Medicine, 92, 352–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Landis R, Stein BD, & Barry CL (2019). The Affordable Care Act In The Heart Of The Opioid Crisis: Evidence From West Virginia. Health Aff (Millwood), 38, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Levin J, Chang HY, Jones C, & Alexander GC (2018). Changes in Buprenorphine-Naloxone and Opioid Pain Reliever Prescriptions After the Affordable Care Act Medicaid Expansion. JAMA Netw Open, 1, e181588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal DW, Eldridge GD, Zack B, & Sosman J. (2010). HIV testing and treatment with correctional populations: people, not prisoners. Journal of health care for the poor and underserved, 21, 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, & Latkin CA (2001). Intimate relationship characteristics associated with condom use among drug users and their sex partners: a multilevel analysis. Drug Alcohol Depend, 64, 97–104. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, & Hucks-Ortiz C. (2005). Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence, 78, 125–134. [DOI] [PubMed] [Google Scholar]
- Silva L. (2015). Collateral Damage: A Public Housing Consequences of the ‘War on Drugs’. [Google Scholar]
- Small W, Rhodes T, Wood E, & Kerr T. (2007). Public injection settings in Vancouver: Physical environment, social context and risk. International Journal of Drug Policy, 18, 27–36. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, & Copeland AL (2000). Drug abuse treatment as an HIV prevention strategy: a review. Drug and Alcohol Dependence, 59, 17–31. [DOI] [PubMed] [Google Scholar]
- Status of State Action on the Medicaid Expansion Decision. Retrieved April 14, 2019 from https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. [Google Scholar]
- Swan H. (2015). Different Patterns of Drug Use and Barriers to Continuous HIV Care Post-Incarceration. Journal of drug issues, 45, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JC, & Torrone E. (2008). Incarceration as forced migration: effects on selected community health outcomes. American journal of public health, 98, S181–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov D, & Putnam S. (2006). From corrections to communities as an HIV priority. Journal of urban health : bulletin of the New York Academy of Medicine, 83, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Jones EB, Einstein EB, & Wargo EM (2018). Prevention and treatment of opioid misuse and addiction: A review. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- Wen H, Hockenberry JM, Borders TF, & Druss BG (2017). Impact of Medicaid Expansion on Medicaid-covered Utilization of Buprenorphine for Opioid Use Disorder Treatment. Med Care, 55, 336–341. [DOI] [PubMed] [Google Scholar]
- Werb D, Wood E, Small W, Strathdee S, Li K, Montaner J, & Kerr T. (2008). Effects of police confiscation of illicit drugs and syringes among injection drug users in Vancouver. The International journal on drug policy, 19, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard RP, Hochstatter KR, Andrews PN, Kahn D, Schumann CL, Winzenried AE, Sethi AK, Gangnon RE, & Sosman JM (2019). Effect of Patient Navigation on Transitions of HIV Care After Release from Prison: A Retrospective Cohort Study. AIDS and Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Eisenberg M, Becher J, Davis-Vogel A, Fiore D, & Metzger D. (2013). Racial disparities in HIV prevalence and risk behaviors among injection drug users and members of their risk networks. Journal of Acquired Immune Deficiency Syndromes, 63 Suppl 1, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WJ (1997). When work disappears : the world of the new urban poor. New York: Knopf: : Distributed by Random House. [Google Scholar]
- World Prison Brief. In. (2020): Institute for Crime and Justice Policy Research. [Google Scholar]
- Zarkin GA, Cowell AJ, Hicks KA, Mills MJ, Belenko S, Dunlap LJ, Houser KA, & Keyes V. (2012). Benefits and costs of substance abuse treatment programs for state prison inmates: results from a lifetime simulation model. Health economics, 21, 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.