Abstract
Background:
Bile acid (BA) and short chain fatty acid (SCFA) production is impacted by diet and microbial metabolism. These metabolites may play important roles in human carcinogenesis.
Methods:
We used a fully quantitative targeted liquid chromatography-mass spectrometry (LC-MS/MS) system to measure serum and fecal BA and SCFA concentrations in 136 Costa Rican adults at study baseline and 6-months. We randomly selected 50 participants and measured their baseline samples in duplicate. Our objective was to evaluate: technical reproducibility; 6-month temporal variability; and concordance between sample type collected from the same individual at approximately the same time.
Results:
Technical reproducibility was excellent, with intraclass correlation coefficients (ICCs) ≥0.83 for all BAs except serum tauroursodeoxycholic acid (ICC=0.72) and fecal glycolithocholic acid (ICC=0.66) and ICCs ≥0.81 for all SCFAs except serum 2-methylbutyric acid (ICC=0.56) and serum isobutyric acid (ICC=0.64). Temporal variability ICCs were generally low, but several BAs (i.e., deoxycholic, glycoursodeoxycholic, lithocholic, taurocholic, and tauroursodeoxycholic acid) and SCFAs (i.e., 2-methylbutyric, butyric, propionic, and valeric acid) had 6-month ICCs ≥0.44. The highest degree of concordance was observed for secondary and tertiary BAs.
Conclusions:
Serum and fecal BAs and SCFAs were reproducibly measured. However, 6-month ICCs were generally low, indicating that serial biospecimen collections would increase statistical power in etiologic studies. The low concordance for most serum and fecal metabolites suggests that consideration should be paid to treating these as proxies.
Impact:
Our findings will inform the design and interpretation of future human studies on associations of BAs, SCFAs, and potentially other microbial metabolites, with disease risk.
Keywords: bile acids, short chain fatty acids, microbial metabolites, reproducibility, temporal variability
Introduction
Bile acids (BAs) and short chain fatty acids (SCFAs) are two groups of metabolites that have gained considerable scientific attention, owing to their connections to diet, the gut microbiome, and cancer. Epidemiological studies of BAs and SCFAs in relation to colonic inflammation and cancer have generally measured either circulating metabolites in blood or excreted metabolites in feces. However, it remains unclear how concentrations of these metabolites correlate between biospecimen types and ultimately whether blood concentrations can be used as surrogate measures for fecal concentrations in nested studies within existing cohorts that have biobanked blood samples but not fecal samples.
BAs are critical to the digestion and absorption of fat and also help regulate intestinal epithelial homeostasis in the gastrointestinal tract [1, 2]. BAs are mostly reabsorbed in the distal ileum, but those that are not reabsorbed may be transformed into secondary BAs by gut bacteria. Secondary BAs, namely deoxycholic (DCA) and lithocholic acid (LCA), are known to promote colon carcinogenesis in animals [3–5]. However, epidemiological studies in humans of fecal BA composition and colon cancer risk have yielded inconsistent results. A meta-analysis of 20 studies, including 18 case control studies, found that fecal chenodeoxycholic acid (CDCA), but not fecal DCA and LCA, was associated with colon cancer. In contrast, case control studies of serum BAs have found positive associations of DCA with colorectal adenomas [6, 7], and one prospective study of BAs and colon cancer found that several conjugated, primary and secondary BAs were associated with increased risk of colon cancer [8]. In addition to colon cancer, BAs have been associated with increased risk of cancer of the laryngopharyngeal tract, esophagus, stomach, pancreas, small intestine [9] and liver [10] as well as inflammatory bowel diseases [11].
SCFAs, including acetate, propionate, and butyrate, are derived from microbial fermentation of non-digestible carbohydrates, mainly prebiotic dietary fibers, in the gut. Experimental studies have demonstrated that SCFAs suppress colonic inflammation and may prevent colorectal cancer via induction of apoptosis as well as inhibition of proliferation, histone acetylation, and cell differentiation [12, 13]. Epidemiological studies have suggested that diets rich in fiber, particularly fiber from whole grains, are associated decreased risk of colon cancer [14]. There are few human studies of SCFAs and colorectal cancer risk; however, research on SCFA production and absorption has shown inverse associations between colonic fluid SCFA and fecal SCFA concentrations, suggesting that fecal measures may better reflect absorption than production [15, 16]. More recently, epidemiologic studies of plasma and fecal SCFAs found that plasma levels may be more relevant to cardiovascular disease (CVD) pathogenesis. Interestingly, the associations with CVD were not in line with a hypothesized protective effect [17, 18], raising the possibility that circulating SCFA concentrations, like fecal concentrations, are not a reflection of colonic production but rather inversely related to colonic absorption.
To date, much of the knowledge on how BAs and SCFAs impact carcinogenesis derives from laboratory studies, but, as this research moves more into large epidemiological studies, it is important to understand sources of variability in BA and SCFA measures including technical variability, which impacts reproducibility, and within-person or biological variability. Within-person variability impacts the temporal variability of a biomarker over time and provides critical information on whether a single biospecimen can be used to measure an individual’s long-term or usual level [19–21]. In addition, few epidemiological studies have prospectively collected fecal samples [15]. Thus, studies of gut microbiome-related metabolites, like BAs and SCFAs, and cancer risk generally rely on blood levels as a proxy of fecal levels without directly examining the concordance between blood and fecal metabolites. Poor reproducibility and temporal variability reduce statistical power to detect associations, and a lack of knowledge regarding the correlation between measures in relevant biospecimen types may result in a misleading or incorrect interpretation of findings. Therefore, we evaluated the reproducibility, temporal variability, and concordance of serum and fecal BAs and SCFAs, using a population-based study with blood and fecal biospecimens collected at study baseline and again at 6 months.
Materials and Methods
Study participants
This study is nested within a study conducted in Costa Rica designed to evaluate the temporal variability of the skin, colonic, vaginal, penile, and oral microbiome. Eligible participants were 18 years or older and planning to reside in the study area for the 6-month study period. The baseline sample collection for participants who reported recent antibiotic use was deferred until at least 6 weeks post-antibiotic use. At baseline 151 participants were enrolled and 145 participants completed follow up at 6 months. Participants completed questionnaires on medical history, education, and employment at baseline. Participants also completed limited questionnaires on lifestyle factors, including cigarette smoking, at baseline and 6 months. Of the 145 participants with questionnaire data, we included all 136 participants with baseline biospecimen collections. We also randomly selected a subset of 50 participants for whom we included duplicate baseline serum and fecal samples as well as 6-month serum and fecal samples.
All participants provided written informed consent. The study was approved by the Special Studies Institutional Review Board (IRB) at the National Institute of Health (NIH) and the Costa Rican IRB CEC-UNA (Comité Ético Científico de la Universidad Nacional).
Biospecimen collection
Non-fasting blood samples were collected at the baseline and 6-month clinic visits using serum separation tubes without clot activator and kept at 2ºC – 10ºC until serum separation. Serum was separated by centrifugation, aliquoted, and frozen on the day of blood collection. Stool samples were collected at home within 24 hours of each clinic visit. Participants were provided with a self-collection kit, including 4 stool sample collection containers and a Stainless-steel insulated food flask with dry ice for immediate freezing of samples. The collection containers used for the current analysis of BAs and SCFAs contained no preservatives. Participants were asked to collect a scoop from their first bowel movement of the day, and samples were retrieved by study staff or returned to the center by participants within 24 hours of collection. At the center, fecal samples were frozen in liquid nitrogen.
Metabolomics analysis
Human serum (275 ul) and feces (200 mg without fixative) were analyzed by Metabolon, using high throughput targeted assays that provide quantification of bile acids (BAs) and short chain fatty acids (SCFAs) and are increasingly being used in human research [8, 22]. In brief, serum and fecal samples were spiked with a solution of corresponding labeled internal standards for each of the measured metabolites (note BA and SCFA panels are run separately) and were subjected to protein precipitation with acidified methanol for the BA panel and with an organic solvent for the SCFA panel. For the BA panel, samples were centrifuged, and a portion of the clear supernatant was evaporated to dryness in a gentle stream of nitrogen at 40ºC. The dried extract was reconstituted, and an aliquot was injected onto an Agilent 1290/Sciex QTrap 6500 mass spectrometer LC-MS/MS system equipped with a C18 reverse phase HPLC column with acquisition in negative ion mode. For the SCFA panel, samples were centrifuged, and an aliquot of the supernatant was derivatized forming the corresponding SCFA aryl hydrazides. The reaction mixture was diluted, and an aliquot was injected onto an Agilent 1290/AB Sciex QTrap 5500 LC MS/MS system equipped with a C18 reversed phase UHPLC column. The mass spectrometer was operated in negative mode using electrospray ionization (ESI). For both panels, the peak area of each parent or product ion was measured against the peak area of the respective internal standard parent or product ion. Quantitation was performed using a weighted and linear least squares regression analysis generated from fortified calibration standards prepared immediately prior to each run. Analyte concentrations that were below or above the limit of quantitation were extrapolated using calibration curves based on internal standards. Analytes that were below the limit of quantitation that could not be extrapolated were considered not quantifiable, and fecal samples weighing less than a 5 mg were not analyzed owing to insufficient material.
Serial samples from the same participant were placed next to each other, but in random order, and duplicate samples were distributed within and across batches to estimate technical variability. Additionally, for each sample type, BA and SCFA analyses were run using a 96-well plate format containing two calibration curves as well as 6 and 8 quality control (QC) samples for serum and fecal analyses, respectively, to monitor assay performance. QC replicates at three levels (low, medium, and high) were prepared by Metabolon and met the acceptance criteria (i.e., ≥50% of QC samples at each concentration level per analyte were within ±20.0% of the corresponding historical mean) and at least 75% of all QC samples per analyte were within ±20.0% of the corresponding historical mean for all analytes.
Statistical analysis
For the analyses of technical reproducibility, temporal variability, and concordance between serum and fecal measures, metabolites with concentrations that exceeded the lower limit of detection (LLOD) in at least 50% of the samples were treated as continuous variables. Metabolite concentrations that fell below the LLOD were assigned to be half of the minimum observed concentration for a given metabolite. Metabolite values that could not be measured owing to insufficient sample volume were treated as missing. Finally, continuous metabolite concentrations were natural log-transformed to improve normality.
For two fecal metabolites with concentrations below the LLOD in more than 50% of samples, we categorized concentrations as quantifiable (i.e., 1) or not quantifiable (i.e., 0) and estimated classification agreement via Cohen’s kappa statistics. First, we estimated the coefficient of variation (CV) for duplicate samples per individual (i.e., ) per analyte and averaged them across participants; a lower CV indicates better technical reproducibility but does not factor in the differences between individuals.
To account for variability within and between individuals, we considered the total variance, , of each metabolite, which is comprised of three components, the between-individual variance, , which represents the variance of the “usual” level in a population; the within-individual variance, , which represents variability over time around the “usual” level within an individual, and the technical variance, , which represents variance introduced by measurement error in the laboratory procedures (19,21). Using these variance components, we defined the following quantities:
For each metabolite, , , were estimated using a linear mixed effects regression model with hierarchal random effects for subject and time of sample collection. The three variance components were estimated using linear mixed models with the normalized log-transformed metabolite level for subject i at time j, Yij, as the outcome and random effects for subject, Si, and time period, Tij (nested within subject) [19, 21, 23].
For ICC values, we calculated 95% confidence intervals (CIs) using the bootstrap procedure. An ICC less than 0.4 indicates poor reproducibility or low temporal variability, an ICC of 0.4 up to 0.75 indicates fair to good reproducibility or moderate temporal variability, and an ICC exceeding 0.75 indicates excellent reproducibility or high temporal variability [24].
Concordance between baseline serum and fecal metabolite measures was estimated using the Spearman correlation coefficient (rs). For the concordance analysis, three participants were excluded owing to insufficient sample volume.
We also estimated the statistical power to detect an association between a metabolite, given its observed between-individual, within-individual, and technical variance, and case status in a hypothetical 1:1 matched case-control study, varying the number of cases (i.e., 250, 500, or 1000), the number of serial samples pooled together (1, 2, or 3), and the effect size. As examples, we present the power estimates for one BA (i.e., deoxycholic acid) and one SCFA (i.e., butyric acid) that are each microbial metabolites and thought to play a role in human disease. The effect size was defined as the relative risk of disease for those in the top quartile of the metabolite concentration versus those in the bottom quartile. We assumed metabolites values were normally distributed and associations were tested by a t-test at an alpha level of =0.05.
Analyses were conducted with R version 4.0.2 (R Core Team, Vienna, Austria. URL https://www.R-project.org/) and with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Of the 136 participants who contributed serum and fecal samples at baseline, age ranged from 21 to 83 years old, 61% were female, 65% married, and 66% had completed primary school. Overall, 85% of participants reported ever drinking alcohol, and 23% reported ever smoking cigarettes (Supplementary Table S1). Median baseline concentrations and interquartile ranges (IQR) of BAs and SCFAs for serum and fecal samples are presented in Supplementary Table S2.
We evaluated technical reproducibility of targeted BA and SCFA measurements in both serum and fecal samples using CVs and ICCs. For serum, CVs were generally less than 10%, (median=7%; IQR:5%−13%) (Table 1), and technical ICCs were excellent, with ICCs ≥0.88 for all BAs except tauroursodeoxycholic acid (ICC=0.72, 95% CI, 0.50 to 0.89) and ICCs ≥0.81 for all SCFAs except 2-methylbutyric acid (ICC=0.56, 95% CI, 0.41 to 0.79) and isobutyric acid (ICC=0.64, 95% CI, 0.37 to 0.77) (Table 1). For feces, CVs were generally less than 10% for SCFAs (median=9%; IQR:9%−10%) but were higher for BAs (median=9%; IQR:9%−10%). Nevertheless, technical ICCs were excellent, with ICCs ≥0.83 for all BAs except glycolithocholic acid (ICC=0.66 95% CI, 0.26 to 0.92) and ICCs ≥0.89 for all SCFAs (Table 2).
Table 1.
Technical reproducibility and temporal variability of serum bile acids and short chain fatty acids.
| SERUM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline visit | Duplicate | Reproducibility | 6-month visit | Temporal variability | |||||||
| Bile acid | Na | NNQb | Na | NNQb | CVc | ICC | 95 % CI | Na | NNQb | ICC | 95 % CI |
| Chenodeoxycholic | 136 | 0 | 50 | 0 | 2.9% | >0.99 | (>0.99, >0.99) | 50 | 0 | 0.31 | (0.10, 0.54) |
| Cholic | 136 | 0 | 50 | 0 | 4.1% | >0.99 | (>0.99, >0.99) | 50 | 0 | 0.28 | (0.06, 0.57) |
| Deoxycholic | 136 | 2 | 50 | 0 | 2.4% | >0.99 | (>0.99, >0.99) | 50 | 1 | 0.51 | (0.30, 0.75) |
| Glycochenodeoxycholic | 136 | 0 | 50 | 0 | 4.7% | >0.99 | (>0.99, >0.99) | 50 | 0 | 0 | (0.00, 0.32) |
| Glycocholic | 136 | 0 | 50 | 0 | 6.5% | >0.99 | (0.99, >0.99) | 50 | 0 | 0.22 | (0.00, 0.64) |
| Glycodeoxycholic | 136 | 1 | 50 | 0 | 3.3% | >0.99 | (>0.99, >0.99) | 50 | 1 | 0.29 | (0.03, 0.68) |
| Glycolithocholic | 136 | 1 | 50 | 0 | 9.1% | 0.99 | (0.96, >0.99) | 50 | 1 | 0.37 | (0.12, 0.62) |
| Glycoursodeoxycholic | 136 | 0 | 50 | 0 | 5.5% | >0.99 | (>0.99, >0.99) | 50 | 0 | 0.50 | (0.30, 0.67) |
| Lithocholic | 136 | 4 | 50 | 2 | 8.1% | 0.99 | (0.98, 0.99) | 50 | 3 | 0.57 | (0.42, 0.79) |
| Taurochenodeoxycholic | 136 | 1 | 50 | 0 | 5.4% | >0.99 | (>0.99, >0.99) | 50 | 0 | 0.28 | (0.06, 0.54) |
| Taurocholic | 136 | 2 | 50 | 0 | 5.2% | >0.99 | (>0.99, >0.99) | 50 | 1 | 0.44 | (0.16, 0.66) |
| Taurodeoxycholic | 136 | 7 | 50 | 2 | 7.9% | 0.99 | (0.97, >0.99) | 50 | 1 | 0.38 | (0.15, 0.59) |
| Ursodeoxycholic | 136 | 33 | 50 | 9 | 20.8% | 0.89 | (0.79, 0.98) | 50 | 4 | 0.34 | (0.06, 0.57) |
| Taurolithocholic | 136 | 14 | 50 | 5 | 13.4% | 0.88 | (0.73, >0.99) | 50 | 11 | 0.16 | (0.00, 0.43) |
| Tauroursodeoxycholic | 136 | 51 | 50 | 10 | 40.6% | 0.72 | (0.50, 0.89) | 50 | 16 | 0.38 | (0.17, 0.54) |
| Short chain fatty acid | |||||||||||
| 2-Methylbutyric | 136 | 0 | 50 | 0 | 18.8% | 0.56 | (0.41, 0.79) | 50 | 0 | 1.00d | -- |
| Acetic | 136 | 0 | 50 | 0 | 7.2% | 0.97 | (0.95, 0.99) | 50 | 0 | 0.06 | (0.00, 0.24) |
| Butyric | 136 | 0 | 50 | 0 | 7.1% | 0.97 | (0.95, 0.98) | 50 | 0 | 0.39 | (0.17, 0.56) |
| Hexanoic | 136 | 0 | 50 | 0 | 11.2% | 0.81 | (0.69, 0.88) | 50 | 0 | 0.23 | (0.00, 0.47) |
| Isobutyric | 136 | 0 | 50 | 0 | 13.2% | 0.64 | (0.37, 0.77) | 50 | 0 | 0.53 | (0.23, 0.66) |
| Isovaleric | 136 | 0 | 50 | 0 | 10.8% | 0.91 | (0.87, 0.94) | 50 | 0 | 0.25 | (0.00, 0.54) |
| Propionic | 136 | 0 | 50 | 0 | 20.7% | 0.81 | (0.74, 0.88) | 50 | 0 | 0.43 | (0.20, 0.61) |
| Valeric | 136 | 0 | 50 | 0 | 10.7% | 0.93 | (0.89, 0.96) | 50 | 0 | 0.58 | (0.37, 0.72) |
Abbreviations: NQ, not quantifiable; CV, coefficient of variation; ICC, intraclass correlation coefficient; CI, confidence interval.
N denotes the total number of samples used in the analysis
NNQ denotes samples for which concentrations were not quantifiable and were replaced by ½ lowest observation value for a given analyte and sample type except for fecal taurolithocholic and tauroursodeoxycholic
CV is estimated as the mean value of CVs from sample duplicates
ICC reliability over time is estimated as 1. This may be because the ICC reproducibility is low, so it can inflate the ICC variability over time.
Table 2.
Technical reproducibility and temporal variability of fecal bile acids and short chain fatty acids.
| FECESc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline visit | Duplicate | Reproducibility | 6-month visit | Temporal variability | |||||||
| Bile acid | Na | NNQb | Na | NNQb | CVd | ICC | 95 % CI | Na | NNQb | ICC | 95 % CI |
| Chenodeoxycholic | 135 | 0 | 49 | 0 | 26% | 0.97 | (0.95, 0.98) | 50 | 0 | 0.15 | (0.00, 0.37) |
| Cholic | 135 | 0 | 49 | 0 | 30% | 0.97 | (0.96, 0.98) | 50 | 1 | 0.25 | (0.02, 0.46) |
| Deoxycholic | 135 | 0 | 49 | 0 | 11% | 0.99 | (0.98, >0.99) | 50 | 0 | 0.25 | (0.00, 0.62) |
| Glycochenodeoxycholic | 135 | 0 | 49 | 0 | 33% | 0.88 | (0.78, 0.95) | 50 | 0 | 0.04 | (0.00, 0.22) |
| Glycocholic | 135 | 4 | 49 | 0 | 38% | 0.92 | (0.88, 0.95) | 50 | 3 | 0.06 | (0.00, 0.27) |
| Glycodeoxycholic | 135 | 2 | 49 | 0 | 30% | 0.88 | (0.77, 0.94) | 50 | 0 | 0.02 | (0.00, 0.27) |
| Glycolithocholic | 135 | 39 | 49 | 13 | 20% | 0.66 | (0.26, 0.92) | 50 | 21 | 0.20 | (0.00, 0.45) |
| Glycoursodeoxycholic | 135 | 55 | 49 | 18 | 47% | 0.89 | (0.83, 0.93) | 50 | 20 | 0.21 | (0.00, 0.44) |
| Lithocholic | 135 | 0 | 49 | 0 | 12% | 0.96 | (0.93, 0.98) | 50 | 0 | 0.40 | (0.12, 0.70) |
| Taurochenodeoxycholic | 135 | 10 | 49 | 4 | 36% | 0.91 | (0.84, 0.96) | 50 | 9 | 0.12 | (0.00, 0.34) |
| Taurocholic | 135 | 23 | 49 | 4 | 31% | 0.93 | (0.88, 0.96) | 50 | 13 | 0.11 | (0.00, 0.29) |
| Taurodeoxycholic | 135 | 44 | 49 | 11 | 42% | 0.89 | (0.79, 0.93) | 50 | 21 | 0.17 | (0.00, 0.36) |
| Ursodeoxycholic | 135 | 41 | 49 | 6 | 22% | 0.97 | (0.95, 0.98) | 50 | 9 | 0.34 | (0.03, 0.58) |
| Taurolithocholice | 50 | 29 | 49 | 29 | 26% | 0.83 | (0.67, 0.99) | 50 | 36 | 0.18 | (−0.08, 0.44) |
| Tauroursodeoxycholice | 50 | 35 | 49 | 35 | 42% | 0.90 | (0.76, 1.00) | 50 | 34 | 0 | (−0.34, 0.19) |
| Short chain fatty acid | |||||||||||
| 2-Methylbutyric | 134 | 0 | 46 | 0 | 10% | 0.97 | (0.94, 0.99) | 47 | 0 | 0.22 | (0.00, 0.57) |
| Acetic | 134 | 0 | 46 | 0 | 8% | 0.89 | (0.79, 0.96) | 47 | 0 | 0.13 | (0.00, 0.42) |
| Butyric | 134 | 0 | 46 | 0 | 10% | 0.94 | (0.90, 0.97) | 47 | 0 | 0.43 | (0.16, 0.69) |
| Hexanoic | 134 | 0 | 46 | 0 | 11% | 0.99 | (0.99, >0.99) | 47 | 0 | 0.53 | (0.26, 0.76) |
| Isobutyric | 134 | 0 | 46 | 0 | 8% | 0.96 | (0.91, 0.99) | 47 | 0 | 0.21 | (0.00, 0.57) |
| Isovaleric | 134 | 0 | 46 | 0 | 9% | 0.96 | (0.93, 0.98) | 47 | 0 | 0.22 | (0.00, 0.56) |
| Propionic | 134 | 0 | 46 | 0 | 9% | 0.94 | (0.88, 0.97) | 47 | 0 | 0.43 | (0.18, 0.64) |
| Valeric | 134 | 0 | 46 | 0 | 9% | 0.98 | (0.97, 0.99) | 47 | 0 | 0.27 | (0.04, 0.80) |
Abbreviations: NQ, not quantifiable; CV, coefficient of variation; ICC, intraclass correlation coefficient; CI, confidence interval.
N denotes the total number of samples used in the analysis
NNQ denotes samples for which concentrations were not quantifiable (NQ) and were replaced by ½ lowest observation value for a given analyte and sample type except for fecal taurolithocholic and tauroursodeoxycholic
There was insufficient sample volume for 1 baseline and 1 duplicate fecal samples for BAs and for 2 baseline, 4 duplicate and, 3 follow-up fecal samples for SCFAs
CV is estimated as the mean value of CVs from sample duplicates
Taurolithocholic and Tauroursodeoxycholic <50% detectability assessed with Cohen’s kappa statistics as a measure of reproducibility
We assessed temporal variability over 6 months for serum and fecal BAs and SCFAs using samples collected at baseline and 6 months. For serum, ICCs for BA and SCFA temporal variability were generally low; however, a number of BAs (i.e., deoxycholic, glycoursodeoxycholic, lithocholic, taurocholic, and tauroursodeoxycholic acid) as well as a few SCFAs (i.e., 2-methylbutyric, butyric, propionic, and valeric acid) had 6-month ICCs, with estimates ranging from 0.44 to 0.58 (Table 1). For feces, BA and SCFA ICCs for temporal variability were also low, falling below 0.40 for all fecal BAs except lithocholic acid (ICC=0.40, 95% CI, 0.12 to 0.70) and for all SCFAs except butyric, hexanoic, and propionic acid (ICC= 0.43, 0.53, 0.43, respectively) (Table 2).
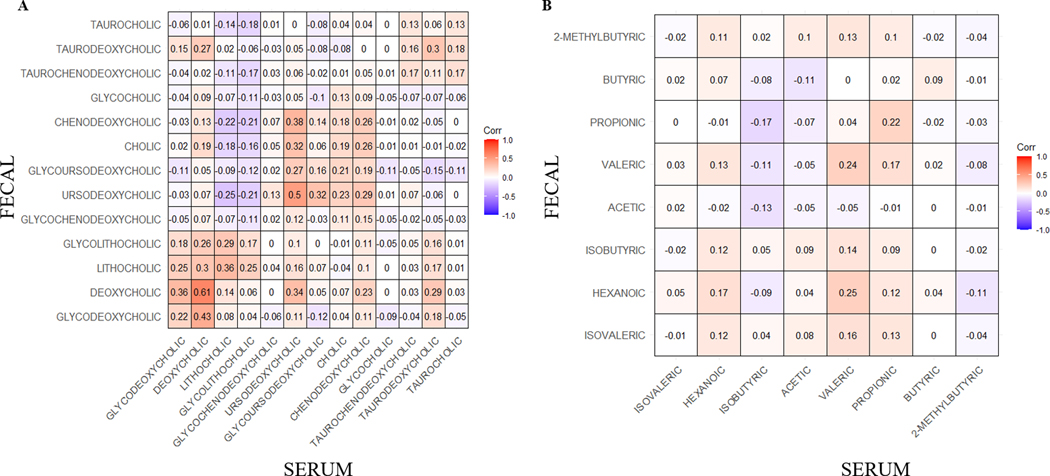
As a measure of concordance between specimen types, we evaluated the correlation between serum and fecal BAs as well as serum and fecal SCFAs (Fig. 1). In general, modest to low levels of concordance were observed between serum and fecal BAs and SCFAs. The highest degree of concordance was observed between secondary and tertiary BAs, with the strongest correlation between serum and fecal deoxycholic acid (r=0.61, p<.0001), followed by ursodeoxycholic acid (r=0.50, p<.0001) and then lithocholic acid (r=0.36, p<.0001). Serum and fecal SCFAs generally showed a low degree of concordance, with correlations ranging from r=0.24 for valeric acid to r=−0.05 for acetic acid (Fig. 1b). The results were similar between serum and fecal BAs as well as serum and fecal SCFAs at 6 months (Supplementary Table S4). Furthermore, metabolites were highly intercorrelated (Supplementary Fig. S1) and within a given sample type, many BAs and SCFAs were highly correlated with each other (Supplementary Fig. S2).
Figure 1.
Spearman correlations of A) bile acids and B) short chain fatty acid serum and fecal samples (n=133 complete serum and fecal pairs were used for this analysis).
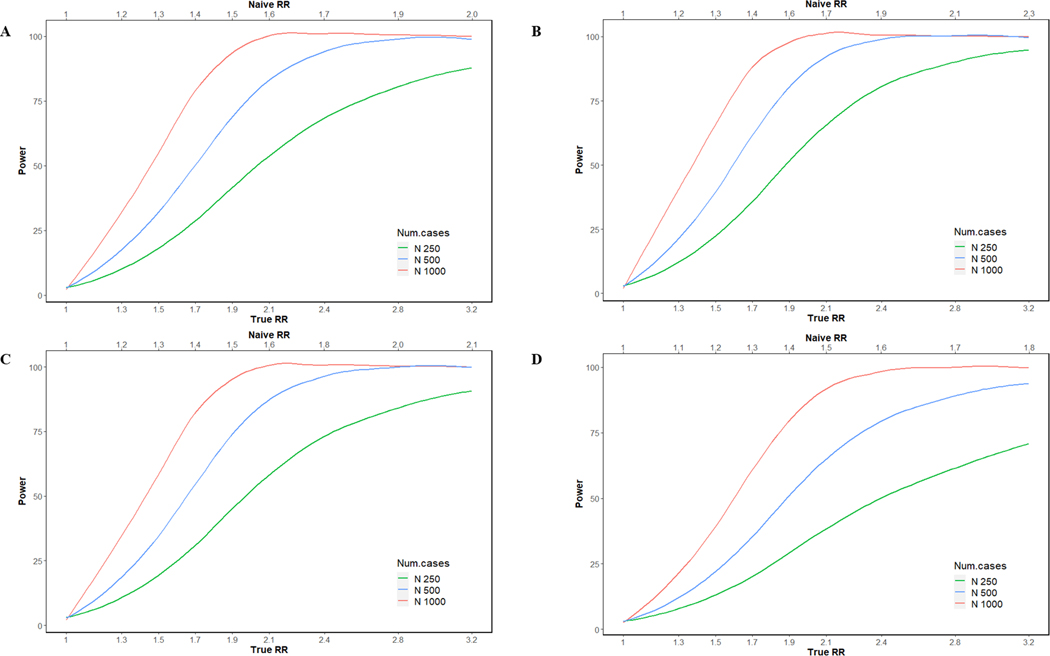
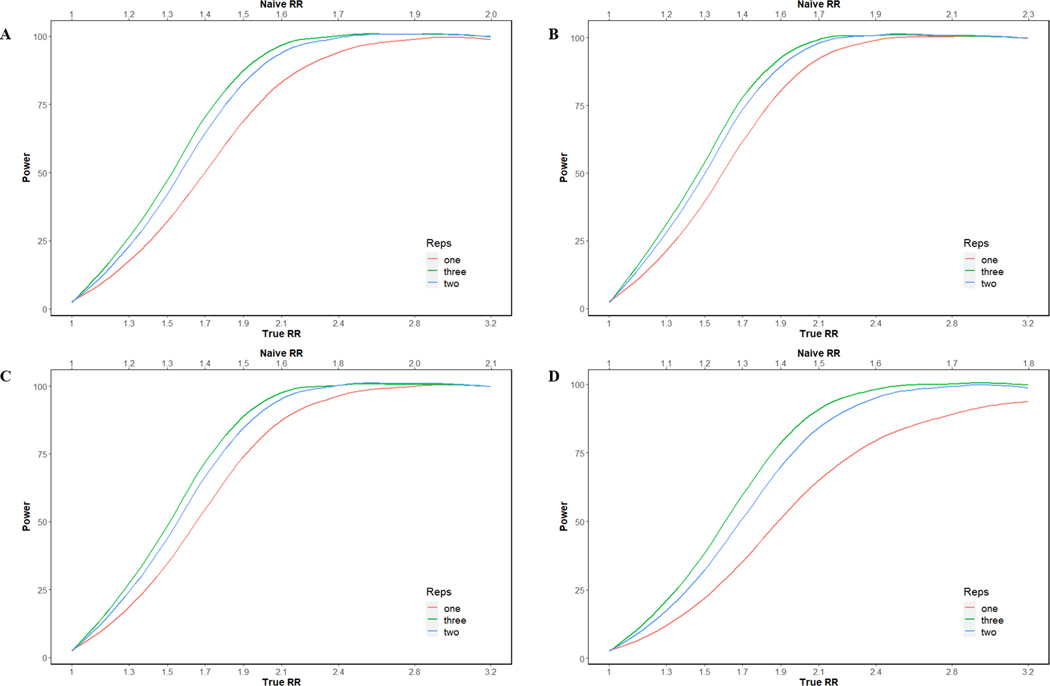
Finally, we estimated the statistical power to detect various effect sizes, assuming an α of 0.05, using a 1:1 case-control design for two illustrative metabolites, deoxycholic and butyric acid, representing BAs and SCFAs, respectively. For serum deoxycholic acid, which had near perfect reproducibility and a moderate 6-month ICC (), the statistical power to detect true RRs of 1.3, 1.7, 2.1, and 2.8 was 20%, 62%, 92%, and 99%, respectively, in a study with a single serum sample for each of 500 cases and 500 controls. For fecal samples, given the same parameters but a lower 6-month ICC () statistical power was lower. For serum butyric acid, which also had near perfect reproducibility but a lower 6-month ICC (), the statistical power to detect true RRs of 1.3, 1.7, 2.1, and 2.8 was 17%, 50%, 84%, and 98%, respectively, and for fecal samples, given the same parameters as well as similar reproducibility and a slightly higher 6-month ICC () statistical power was marginally higher (Fig. 2). Statistical power improved with the addition of a second biospecimen collection as this reduced within-individual and technical variability. For example, collecting two serial samples per person over 6 months and using the average to define an individual’s usual concentration in a case-control study of 500 cases and 500 controls, we expect that the statistical power to detect an association between serum deoxycholic acid and case status would increase from 62% to 74% for a true RR of 1.7. Similarly, the statistical power to detect an association between serum butyric acid and case status would increase from 69% to 83% for a true RR of 1.9 (Fig. 3).
Figure 2. The curves represent the expected power in a case–control study for butyric acid ((A) serum and (B) fecal) and deoxycholic acid ((C) serum and (D) fecal) as a function of the observed RR and changes in sample size (250, 500, and 1000).
Effect size is defined by the relative risk (RR, x-axis) of disease comparing individuals within the highest quartile of the “usual” metabolite level to the lowest quartile. The top axis indicates the “naïve” relative risk that would be observed in the specified case–control study when not adjusting for measurement error.
Figure 3. The curves represent the expected power in a case–control study for butyric acid ((A) serum and (B) fecal) and deoxycholic acid ((C) serum and (D) fecal) as a function of the observed RR and changes in number of repeated samples/individual (one, two, and three).
Effect size is defined by the relative risk (RR, x-axis) of disease comparing individuals within the highest quartile of the “usual” metabolite level to the lowest quartile. The top axis indicates the “naïve” relative risk that would be observed in the specified case–control study when not adjusting for measurement error.
Discussion
In our novel methods study examining reproducibility, temporal variability, and concordance of serum and fecal BAs and SCFAs, we found that technical reproducibility was generally high (ICCs>0.80) for serum and fecal BAs and SCFAs indicating that these compounds were well-measured using Metabolon’s targeted LC-MS/MS platform. However, temporal variability, based on biospecimens collected 6 months apart, was also high (i.e., 6-month ICCs were low), indicating that studies of these, and likely other diet and microbial-related, metabolites would benefit from serial biospecimen collections. Interestingly, unconjugated secondary and tertiary BA exhibited the highest degree of concordance between serum and fecal samples, with a particularly strong positive correlation between serum and fecal DCA, which have both been linked to increased risk of colorectal adenoma and cancer in human [6, 25, 26] and animal studies [27–29]. On the other hand, we found that SCFA serum and fecal measures were not well correlated, suggesting that they may not be good proxy measures of each other.
High technical reproducibility and low temporal variability reduce unwanted variation in measurements and improve statistical power to detect an association between a biomarker and an outcome [19]. Previous studies using an LC-MS/MS platform for global or untargeted metabolite profiling have also reported good technical ICCs for many serum metabolites, including some of the BAs considered herein, such as glycochenodeoxycholic acid, but have also generally reported low 6-month or 1-year ICCs, indicating considerable temporal variability of for many metabolites [19]. Relying on a single baseline measurement, assumes that the within-person variance over time is small compared to the between-person variance, and violating this assumption may bias measures of association towards the null [30]. Nevertheless, few epidemiological studies include serial biospecimen measurements to account for the variability around the “usual” level of a biomarker within an individual. Based on the current study, we estimated that a single measurement of a serum metabolite with excellent technical reproducibility (ICC>0.95) but good (e.g., DCA with =0.51) or fair 6-month reproducibility over time (e.g., butyric acid with =0.39), would have 62% and 50% power to detect an expected RR of 1.7 (observed RR: 1.4) comparing the top to the bottom quartile, in a nested case-control study with 500 cases and 500 controls. However, given the same parameters but adding a second measurement during follow-up would increase power to 74% and 65%, respectively, making it possible to detect more modest associations without requiring the addition of cases.
We found that correlations of BAs and SCFAs between serum and feces were generally low. However, concentrations of unconjugated secondary and tertiary BAs were moderately correlated in serum and feces, particularly the secondary BA, DCA. Secondary BAs are formed via the action of the bacterial enzyme 7 alpha-dehydroylase on a small fraction of primary BAs that escape enterohepatic circulation and enter the colon. Experimental evidence indicates that secondary BAs are carcinogenic to the colon [31, 32] and DCA has been shown both to cause DNA damage and to promote colon [33] as well as liver tumor growth [34]. Few studies have considered the relationship between BA concentrations in the gut versus those circulation or in feces, but some studies suggest that colonic BAs are present in the blood due to spillover from the enterohepatic system into system circulation [35]. In a rodent study that measured BAs in both feces and blood following treatment with antibiotics, investigators found that taurocholic acid was upregulated in feces and plasma, glycochenodeoxycholic acid was downregulated in both biospecimens, and cholic acid was upregulated in feces but downregulated in plasma [36]. In another animal study, investigators showed that plasma and fecal BA profiles differed significantly but that plasma and fecal DCA were strongly and positively correlated [37], which is in line with our main finding.
Currently, epidemiological evidence linking BAs to colon cancer has primarily derived from case-control studies with cross-sectional assessments of fecal BA levels. Some studies suggest that fecal BAs levels, particularly DCA and LCA, are higher in patients with colon cancer than control subjects [38–40]; however, others have found no association [41, 42]. Heterogeneity in results have been attributed to differences in study design, timing of sample collection (morning or afternoon), excretion of bile acids in feces, selection of subjects, sample preparation methods, and the method used to quantify feces (e.g. LC-MS/MS vs. NMR). Our findings on high within-person variability for many serum and fecal BAs coupled with varying degrees of concordance between serum and fecal BA concentrations sheds further light on possible sources of heterogeneity, suggests that potential associations are much larger than previously thought, and should inform the design of future studies, including consideration of measurement error, and the subsequent interpretation of the results. For example, a prospective study, nested in the European Prospective Investigation into Cancer and Nutrition (EPIC), found that pre-diagnostic serum levels of conjugated primary and secondary BAs were positively associated with risk of colon cancer [8]. Our finding that serum and fecal BA concentrations are highly correlated suggests that for some microbial metabolites, like BAs, assessment in both blood and feces is unnecessary. This may be particularly relevant to existing that only collected blood samples or future studies that plan to collect one biospecimen type but not the other.
Epidemiological evidence linking SCFAs to colorectal cancer primarily comes from prospective studies of fiber intake [14, 43]. Herein, we found that serum and fecal SCFAs were not well correlated, suggesting that studies of these analytes may not be prudent without a better understanding of how circulating and fecal SCFA concentrations compare to gut concentrations. About 95% of SCFAs are absorbed from the gut while only a minor fraction (< 5%) are excreted in feces [44]. Butyrate and propionate are largely used up as energy sources by colonocytes and hepatocytes, respectively [45, 46]; whereas, acetate is more likely to make it into peripheral circulation [47]. Consequently, SCFA concentrations in human feces as well as blood may not reflect those in the colon. This idea was supported by a recent randomized trial that found that administration of SCFAs from pH-dependent colon-delivery capsules did not modulate fecal SCFA concentrations since the SCFAs were likely absorbed in the colon rather than excreted [48]. Nevertheless, studies have explored the associations of human fecal SCFA concentrations, presumably as a surrogate for gut concentrations, with diet [49] and disease risk with inconsistent results [50]. While our study cannot address whether serum or fecal SCFA concentrations reflect local concentrations in the colon, we did find that serum and fecal measures were not well correlated, suggesting that these are not good proxy measures of each other.
Our study is not without limitations. First, for practical reasons, blood was drawn at the clinic and fecal samples were collected within 24-hours at home; thus, diurnal variation in BA and SCFA concentrations could have contributed to low concordance of metabolites between sample types [51]. Additionally, samples were collected at baseline and at 6 months, so we were unable to evaluate shorter (i.e., 24-hour) or longer (i.e., 1-year) term temporal variability, and 6-month temporal variability estimates were based on a subset of participants (n=50) potentially leading to less robust estimates. As previously mentioned, we could not directly compare the concentrations of these metabolites in the colon with those in blood and feces. However, understanding the relationship between metabolite concentrations in blood and stool is particularly relevant to human studies given that these are the types of biospecimens most often used to measure metabolites and the gut microbiome, and our findings caution against broadly treating circulating metabolites as proxies of those in feces. Finally, baseline and 6 month data on dietary intake and physical activity were not available so we were unable to explore whether changes in these modifiable lifestyle factors impacted temporal variability in BA and SCFA concentrations. Research evaluating sources of variability, including modifiable factors (e.g., diet), demographics (e.g., race/ethnicity), and aspects of study design (e.g., fasting versus non-fasting collections), are needed to further inform the interpretation of etiologic studies on microbial metabolites and health. Strengths of our study include our use of targeted panels to measure absolute concentrations, which permit comparisons across studies and populations, as well as duplicate and serial samples to estimate both technical reproducibility and temporal variability of a given metabolite in serum and feces and to translate these estimates into statistical power calculations that should inform future study designs.
In conclusion, we found that serum and fecal BA and SCFA concentrations could be reproducibly measured using a targeted LC-MS/MS approach. However, within-person variability over 6 months was generally low, indicating that etiologic studies of these, and likely other diet and microbiome-related metabolites, would benefit from serial biospecimen collections and analysis, particularly for rarer disease outcomes for which case number are likely to be limited. Finally, we showed that serum and fecal secondary and tertiary BAs were strongly correlated, indicating that circulating concentrations may better reflect those in feces than those of primary BAs.
Supplementary Material
Acknowledgements
We would like to acknowledge the leadership and contribution to this work of Dr Paula Gonzalez, who served as one of the original principal investigators of this study. Paula passed away in March 2020.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health (to Z. Farhat, E. Loftfield, J.N. Sampson, A. Hildesheim, E. Vogtmann, and R. Sinha).
Footnotes
Conflict of Interest
The authors declare no potential conflicts of interest.
References
- 1.Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci 2019;20(5):1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014;28(4):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomchai C, Bhadrachari N, Nigro ND. The effect of bile on the induction of experimental intestinal tumors in rats. Dis. Colon Rectum 1974;17(3):310–2. [DOI] [PubMed] [Google Scholar]
- 4.Rafter J, Eng V, Furrer R, Medline A, Bruce W. Effects of calcium and pH on the mucosal damage produced by deoxycholic acid in the rat colon. Gut 1986;27(11):1320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting Effect of Bile Acids on Colon Carcinogenesis After Intrarectal Instillation of N-Methyl-N′ nitro-N-nitrosoguanidine in Rats. J. Natl. Cancer Inst 1974;53(4):1093–7. [DOI] [PubMed] [Google Scholar]
- 6.Bayerdörffer E, Mannes GA, Richter WO, Ochsenkühn T, Wiebecke B, Köpcke W, et al. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology 1993;104(1):145–51. [DOI] [PubMed] [Google Scholar]
- 7.Bayerdörffer E, Mannes G, Ochsenkühn T, Dirschedl P, Wiebecke B, Paumgartner G. Unconjugated secondary bile acids in the serum of patients with colorectal adenomas. Gut 1995;36(2):268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kühn T, Stepien M, López-Nogueroles M, Damms-Machado A, Sookthai D, Johnson T, et al. Prediagnostic Plasma Bile Acid Levels and Colon Cancer Risk: A Prospective Study. J. Natl. Cancer Inst 2020;112(5):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 2005;589(1):47–65. [DOI] [PubMed] [Google Scholar]
- 10.Loftfield E, Rothwell JA, Sinha R, Keski-Rahkonen P, Robinot N, Albanes D, et al. Prospective investigation of serum metabolites, coffee drinking, liver cancer incidence, and liver disease mortality. J. Natl. Cancer Inst 2020;112(3):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duboc H, Rajca S, Rainteau D, Benarous D, Maubert M-A, Quervain E, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013;62(4):531–9. [DOI] [PubMed] [Google Scholar]
- 12.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002;132(5):1012–7. [DOI] [PubMed] [Google Scholar]
- 13.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost F, Brummer RJ. The role of butyrate on colonic function. Aliment. Pharmacol. Ther 2008;27(2):104–19. [DOI] [PubMed] [Google Scholar]
- 14.Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J. Natl. Cancer Inst 1990;82(8):650–61. [DOI] [PubMed] [Google Scholar]
- 15.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PloS one 2013;8(8):e70803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt JA, Wolever TM. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J. Nutr. 2003;133(10):3145–8. [DOI] [PubMed] [Google Scholar]
- 17.la Cuesta-Zuluaga D, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2019;11(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurilshikov A, van den Munckhof IC, Chen L, Bonder MJ, Schraa K, Rutten JH, et al. Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk: a cross-sectional study. Circ. Res. 2019;124(12):1808–20. [DOI] [PubMed] [Google Scholar]
- 19.Sampson JN, Boca SM, Shu XO, Stolzenberg-Solomon RZ, Matthews CE, Hsing AW, et al. Metabolomics in epidemiology: sources of variability in metabolite measurements and implications. Cancer Epidemiol. Biomark. Prev 2013;22(4):631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin. Chem. 2013;59(11):1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Q, Moore SC, Boca SM, Matthews CE, Rothman N, Stolzenberg-Solomon RZ, et al. Sources of variability in metabolite measurements from urinary samples. PLoS One 2014;9(5):e95749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Z, Wang X, Yin P, Wu R, Zhou L, Xu G, et al. Serum metabolome and targeted bile acid profiling reveals potential novel biomarkers for drug-induced liver injury. Medicine 2019;98(31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird NM and Ware JH, Random-effects models for longitudinal data. Biometrics, 1982: p. 963–974. [PubMed] [Google Scholar]
- 24.Rosner B. Multisample Inference. Fundamentals of biostatistics. Boston, MA: Nelson Education; 2015. p. 610. [Google Scholar]
- 25.Ochsenkühn T, Bayerdörffer E, Meining A, Schinkel M, Thiede C, Nüssler V, et al. Colonic mucosal proliferation is related to serum deoxycholic acid levels. Cancer 1999;85(8):1664–9. [PubMed] [Google Scholar]
- 26.Ou J, DeLany JP, Zhang M, Sharma S, O’Keefe SJ. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr Cancer 2012;64(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen BI, Raicht RF, Deschner EE, Takahashi M, Sarwal AN, Fazzini E. Effect of cholic acid feeding on N-methyl-N-nitrosourea-induced colon tumors and cell kinetics in rats. J. Natl. Cancer Inst 1980;64(3):573–8. [PubMed] [Google Scholar]
- 28.Reddy BS, Narasawa T, Weisburger J, Wynder E. Promoting effect of sodium deoxycholate on colon adenocarcinomas in germfree rats. J. Natl. Cancer Inst 1976;56(2):441–2. [DOI] [PubMed] [Google Scholar]
- 29.Hori T, Matsumoto K, Sakaitani Y, Sato M, Morotomi M. Effect of dietary deoxycholic acid and cholesterol on fecal steroid concentration and its impact on the colonic crypt cell proliferation in azoxymethane-treated rats. Cancer Lett 1998;124(1):79–84. [DOI] [PubMed] [Google Scholar]
- 30.Fleiss JL. Repeated Measurements Studies. Design and analysis of clinical experiments. New York: John Wiley & Sons; 2011, p. 220–241. [Google Scholar]
- 31.O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 2016;13(12):691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol 2014;12(10):661–72. [DOI] [PubMed] [Google Scholar]
- 33.Farhana L, Nangia-Makker P, Arbit E, Shango K, Sarkar S, Mahmud H, et al. Bile acid: a potential inducer of colon cancer stem cells. Stem Cell Res Ther 2016;7(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013;499(7456):97–101. [DOI] [PubMed] [Google Scholar]
- 35.Stellaard F, Sackmann M, Sauerbruch T, Paumgartner G. Simultaneous determination of cholic acid and chenodeoxycholic acid pool sizes and fractional turnover rates in human serum using 13C-labeled bile acids. J Lipid Res 1984;25(12):1313–9. [PubMed] [Google Scholar]
- 36.Behr C, Ramirez-Hincapie S, Cameron HJ, Strauss V, Walk T, Herold M, et al. Impact of lincosamides antibiotics on the composition of the rat gut microbiota and the metabolite profile of plasma and feces. Toxicol Lett 2018;296:139–51. [DOI] [PubMed] [Google Scholar]
- 37.Kasbo J, Saleem M, Perwaiz S, Mignault D, Lamireau T, Tuchweber B, et al. Biliary, fecal and plasma deoxycholic acid in rabbit, hamster, guinea pig, and rat: comparative study and implication in colon cancer. Biol Pharm Bull 2002;25(10):1381–4. [DOI] [PubMed] [Google Scholar]
- 38.Hill M, Drasar B, Williams R, Meade T, Cox A, Simpson J, et al. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet 1975;305(7906):535–9. [DOI] [PubMed] [Google Scholar]
- 39.Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer: fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer 1977;39(6):2533–9. [DOI] [PubMed] [Google Scholar]
- 40.Stadler J, Yeung KS, Furrer R, Marcon N, Himal HS, Bruce WR. Proliferative activity of rectal mucosa and soluble fecal bile acids in patients with normal colons and in patients with colonic polyps or cancer. Cancer Lett 1988;38(3):315–20. [DOI] [PubMed] [Google Scholar]
- 41.Mudd D, McKelvey S, Norwood W, Elmore D. Carcinoma of the large bowel and faecal bile acids. 1979. Br J Surg 1979;66:355. [Google Scholar]
- 42.Murray W, Backwood A, Trotter J, Calman K, MacKay C. Faecal bile acids and clostridia in the aetiology of colorectal cancer. Br. J. Cancer 1980;41(6):923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr 2020;112(3):603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28(1):42–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jørgensen J, Clausen M, Mortensen P. Oxidation of short and medium chain C2-C8 fatty acids in Sprague-Dawley rat colonocytes. Gut 1997;40(3):400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brass EP, Beyerinck RA. Effects of propionate and carnitine on the hepatic oxidation of short-and medium-chain-length fatty acids. Biochem. J 1988;250(3):819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy 2007;108(8):354–8. [PubMed] [Google Scholar]
- 48.Dalile B, Vervliet B, Bergonzelli G, Verbeke K, Van Oudenhove L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacology 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, González S. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr Res 2013;33(10):811–6. [DOI] [PubMed] [Google Scholar]
- 50.Sze MA, Topçuoğlu BD, Lesniak NA, Ruffin MT, Schloss PD. Fecal short-chain fatty acids are not predictive of colonic tumor status and cannot be predicted based on bacterial community structure. MBio 2019;10(4):e01454–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.