Abstract
Farnesyltransferase inhibitors (FTIs) are in clinical trials, but how they selectively inhibit malignant cell growth remains uncertain. One important player in this process appears to be RhoB, an endosomal Rho protein that regulates receptor trafficking. FTI treatment elicits a gain of the geranylgeranylated RhoB isoform (RhoB-GG) that occurs due to modification of RhoB by geranylgeranyltransferase I in drug-treated cells. Notably, this event is sufficient to mediate antineoplastic effects in murine models and human carcinoma cells. To further assess this gain-of-function mechanism and determine whether RhoB-GG has a necessary role in drug action, we examined the FTI response of murine fibroblasts that cannot express RhoB-GG due to homozygous deletion of the rhoB gene. Nullizygous (−/−) cells were susceptible to cotransformation by adenovirus E1A plus activated H-Ras but defective in their FTI response, despite complete inhibition of H-Ras prenylation. Actin cytoskeletal and phenotypic events were disrupted in −/− cells, implicating RhoB-GG in these effects. Interestingly, −/− cells were resistant to FTI-induced growth inhibition under anchorage-dependent but not anchorage-independent conditions, indicating that, while RhoB-GG is sufficient, it is not necessary for growth inhibition under all conditions. In contrast, −/− cells were resistant to FTI-induced apoptosis in vitro and in vivo. Significantly, the apoptotic defect of −/− cells compromised the antitumor efficacy of FTI in xenograft assays. This study offers genetic proof of the hypothesis that RhoB-GG is a crucial mediator of the antineoplastic effects of FTIs.
Rho proteins are Ras superfamily GTPases that regulate the actin cytoskeleton, cell adhesion, motility, proliferation, and apoptosis (1, 45). RhoB is a member of the Rho family that is closely related to RhoA, its better-studied relative, which is a key regulator of actin stress fiber formation and integrin signaling. However, RhoB differs from RhoA in its localization and regulation and has a unique function in cells. First, RhoB is located in early-endosome and nuclear membranes and has a specialized function in intracellular trafficking of cytokine receptors such as the epidermal growth factor (EGF) receptor (16). Second, RhoB is short lived and is part of the genetic response to Src activation or EGF stimulation leading to cell cycle progression (19). RhoB also has cell cycle inhibitory roles and is upregulated during stress responses (14, 15) and by the inhibitory growth factor transforming growth factor β (12). Last, RhoB proteins are posttranslationally modified by geranylgeranylation or farnesylation, and the different isoforms may have distinct functions in cell growth control (11, 24).
Rho functions are crucial for malignant transformation, and there is evidence that RhoB alteration is part of the mechanism through which farnesyltransferase inhibitors (FTIs) inhibit malignant cell growth (26, 35). Like Ras proteins, Rho proteins are posttranslationally modified by farnesyl (C15) or geranylgeranyl (C20) isoprenoids at their C-terminal CAAX box sequences, where C is cysteine, A is generally an aliphatic amino acid, and X is usually methionine, serine, glutamine, or leucine (6). Where it occurs, protein isoprenylation is crucial for appropriate membrane localization, protein-protein interactions, and physiological functions. There are three protein-isoprenyltransferases in cells, namely, farnesyltransferase (FT), geranylgeranyltransferase I (GGT-I), and GGT-II. Most Rho proteins in cells are geranylgeranylated by GGT-I, but some, including RhoB, are farnesylated by FT (24). Despite some initial confusion about how RhoB becomes isoprenylated in cells, it is now clear that FT is solely responsible for farnesylation and that GGT-I is solely responsible for geranylgeranylation and that other reactions are in vitro artifacts (i.e., nonphysiological reactions). RhoB is unusual among Ras superfamily proteins in its ability to be isoprenylated by either FT or GGT-I, and the mechanistic impact of its differential prenylation remains to be fully understood.
FTIs were developed as anticancer therapeutics to exploit the farnesylation requirement of Ras for its oncogenic activity (17). In support of this potential, FTIs revert Ras-transformed cells to a normal phenotype and cause tumor growth inhibition, stasis, or regression in various animal models without discernible toxicity to normal cells (reviewed in reference 42). However, it has become apparent that inhibition of Ras prenylation is not crucial for FTIs to exert their antineoplastic effects (8, 26). Instead, an alternate model, in which alteration of RhoB prenylation and function is crucial, has been corroborated (26, 35, 37). Specifically, elevation of the geranylgeranylated isoform (RhoB-GG), rather than loss of the farnesylated isoform, appears to be the key step in mediating the biological response to FTI treatment. Thus, the FTI-Rho hypothesis for the mechanism suggests a different target for drug action but also proposes a gain-of-function mechanism involving increased production of RhoB-GG, an event that occurs due to the unencumbered activity of GGT-I in FTI-treated cells (35). The shift in RhoB prenylation pattern is correlated with an apparent depletion from its normal endosomal localization and a loss of growth-potentiating function in fibroblast models (24, 25). Strikingly, elevation of RhoB-GG is sufficient to mediate the effects of FTI treatment in transformed murine fibroblasts or in frank human carcinoma cells (9, 11). One caveat to these studies was that the engineered RhoB-GG isoform used was not exactly identical to cellular RhoB-GG in structure. Moreover, although RhoB-GG was sufficient, it remained to be shown whether it was necessary or whether alteration of other farnesylated proteins by FTIs was also sufficient. To address these questions, we examined the FTI response in cells where the rhoB gene was deleted by homologous recombination, eliminating the ability of FTIs to elevate a bona fide RhoB-GG isoform.
MATERIALS AND METHODS
Plasmid constructions.
Hemagglutinin (HA) epitope-tagged RhoB-WT (wild-type) and RhoB-GG expression vectors have been described previously (9, 24). A HindIII-BstXI restriction fragment excised from pCMV-HA-RhoB-WT was blunt-ended by Klenow treatment and subcloned into the HpaI site of the puromycin resistance gene-tagged retroviral vector pacMSCV (18) to generate pacMSCV-HA-RhoB-WT. A HindIII/XbaI restriction fragment excised from pCMV-HA-RhoB-GG was subcloned similarly to generate MSCV-HA-RhoB-GG.
Tissue culture.
The generation and characterization of rhoB nullizygous (−/−) mice, which lack apparent developmental abnormalities and which are fertile, will be described elsewhere. The heads, limbs, and internal organs were removed from a litter of E16.5 embryos and a litter of E14.5 embryos from pregnant mice. The carcasses were minced in Dulbecco modified Eagle medium (DMEM; Life Technologies) and trypsinized for 20 min at 37°C individually. Fetal bovine serum (FBS) was added to stop the trypsin, and the cell suspension was seeded into 25-cm2 flasks containing DMEM and 10% FBS. Cells were maintained in the same media containing 10 U of penicillin-streptomycin (Cellgro)/ml. Focus formation assays were performed with murine embryo fibroblasts (MEFs) using a modified calcium phosphate protocol for DNA-mediated transfection (7). Briefly, 5 × 105 cells were transfected with 10 μg of the human oncogenic H-Ras vector pT22 and the adenovirus E1A vector p1A/neo (39). Cells were passaged at a 1:5 split ratio the day after transfection, and transformed cell foci were scored 10 to 14 days later. Single foci were cloned and expanded into mass culture for analysis. Derivatives of E1A- plus Ras-transformed MEFs were generated by infection with filtered supernatants harvested 48 h after transfection of the ecotropic packaging cell line Bosc23, essentially as described previously (34), with 20 μg of recombinant pacMSCV retrovirus vector DNAs. Cells were selected in 2 μg of puromycin (Sigma), and transgene expression was confirmed by Western analysis with anti-HA antibody 12CA5 as described previously (9).
Cell morphology, viability, and proliferation assays.
To document cell morphology changes, 104 cells were seeded in a 10-cm-diameter dish and treated with 10 μM FTI L-744832 or dimethyl sulfoxide (DMSO) carrier (22). After 48 h cells were photographed on an Olympus microscope with a 35-mm camera attachment. Cell viability was monitored by trypan blue exclusion. Cells (5 × 105) were seeded overnight onto 60-mm-diameter dishes. The next day 10 μM FTI was added in DMEM containing 0.1% FBS. Viable cells excluding trypan blue were counted at 12-h intervals. Anchorage-dependent growth curves and anchorage-independent proliferation in soft-agar culture were determined as described previously (9).
Apoptosis assays.
FTIs have previously been shown to induce apoptosis of Ras-transformed cells under low-serum conditions (10, 44). For in vitro assays, 5 × 105 cells were seeded onto 60-mm-diameter dishes and treated 16 h later with FTI L-744832 or methanol carrier in DMEM containing 0.1% FBS. After 16 to 24 h cells were harvested by trypsinization, washed once with phosphate-buffered saline (PBS), fixed in 70% ethanol, and stained in PBS containing 5 μg of propidium iodide and 10 μg of RNase A/ml and 0.1% glucose. Flow cytometry was performed using an EPIC/XL cell analyzer (Coulter). The proportion of cells in the sub-G1 phase DNA fraction, indicating DNA degradation, was taken as the apoptotic population. The following protocol was used for in vivo determinations of apoptosis. Severe combined immunodeficient (SCID) mice were injected in each leg with 106 heterozygous (+/−) or −/− transformed MEFs and 3 weeks later were dosed once daily for 2 days by intraperitoneal injection with FTI L-744832 (40 mg/kg of body weight/day) or 30% DMSO carrier in a 0.2-ml total volume. Twenty-four hours after the second dose, the mice were sacrificed and tumor samples from each leg were processed for apoptotic determination using a commercial fluorescence in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit, following the instructions of the vendor (Roche Molecular Biochemicals). Positive cells on tumor sections were assessed by fluorescence microscopy.
Tumorigenicity assay.
E1A- plus Ras-transformed cell clones were tested for tumorigenic potential in 4- to 6-week-old SCID mice (Fox Chase CB-17 SCID mouse strain established at The Wistar Institute by D. Bosma). Mice were injected subcutaneously in the upper thighs of different legs of the same animal with 106 +/− or −/− cells suspended in 200 ml of DMEM. Palpable tumors appeared at the injection site within 1 week, and visible nodules of >0.5 cm in diameter were apparent within 2 weeks. For FTI experiments, when the tumor reached ∼50 to 100 mm3, mice were dosed once daily essentially as described previously (22) by intraperitoneal injection with FTI L-744832 (40 mg/kg/day) or 30% DMSO carrier in a 0.2-ml total volume. Tumor volumes were calculated using the formula width squared × length × 0.52.
Akt kinase assay.
Cells were lysed in extraction buffer containing 20 mM Tris-HCl (pH 7.5), 137 mM NaCl, 15% glycerol, 1% NP-40, 2 mM phenylmethylsulfonyl fluoride, 2 μg of aprotinin and leupeptin/ml, 2 mM benzamidine, 20 mM sodium fluoride, 10 mM sodium pyrophosphate, and 1 mM sodium vanadate. Lysates were aliquoted for protein expression and enzyme activity. For in vitro kinase assays, lysates were first subjected to immunoprecipitation with anti-Akt-1 antibody (Santa Cruz Biotechnology; catalog no. sc-1618) in the presence of 30 μl of protein A-Sepharose beads for 4 h at 4°C and the precipitate was incubated in 10 μCi of [γ-32P]ATP (NEN)–4 μM cold ATP in a 25-μl reaction buffer that contained 20 mM HEPES (pH 7.5), 10 mM MgCl2, 10 mM MnCl2, 1 mM dithiothreitol, and 1 μg of histone H2B as the substrate. The reaction mixture was incubated for 30 min at room temperature and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Relative incorporation of radioactivity was determined by gel autoradiography.
Northern analysis.
Endogenous RhoB RNA levels in wild-type (+/+), +/−, and −/− cells were examined by fractionation of 10 μg of total cytoplasmic RNA on 1% formaldehyde gels, Northern blotting, and hybridization with a 32P-labeled rat RhoB cDNA probe, using standard methods.
Western analysis.
Cells were washed in cold PBS and lysed in 1% NP-40 lysis buffer. Cellular protein was quantitated by a Bradford assay, and 50 μg of cellular protein was fractionated by SDS-PAGE. Gels were analyzed by standard Western blotting methods using 1 μg of anti-HA antibody 12CA5 (Boehringer Mannheim Biochemicals)/ml or anti-Akt-1 antibody (Santa Cruz Biotechnology; catalog no. sc-1618). Detection of the primary antibody was carried out using a chemiluminescence system for detection of murine antibodies (Amersham).
Actin immunofluorescence.
Cells were seeded onto coverslips in 24-well dishes and treated beginning the next day for 48 h with 10 μM L-744832 or carrier. Cells were fixed and stained with fluorescein-phalloidin (Molecular Probes) as described previously (36) and analyzed using a confocal scanning microscope (Leica).
RESULTS
Similar susceptibilities of embryo fibroblasts lacking RhoB to E1A and Ras transformation or to FT inhibition.
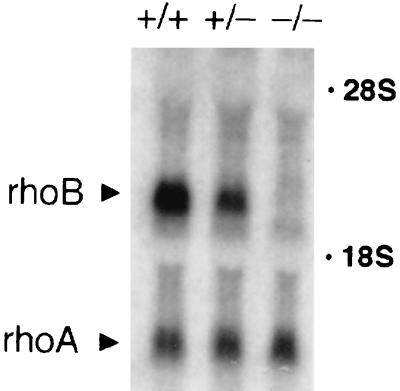
Elevated levels of RhoB-GG are sufficient to elicit the effects of FTI treatment in transformed Rat1 fibroblasts as well as in human carcinoma cell lines with or without activated K-Ras genes (9, 11). We reasoned that, if RhoB-GG was also necessary for these effects, then malignantly transformed cells lacking RhoB should be refractory to FTI treatment, because RhoB-GG could not be elevated in these cells in response to the drug. To test this hypothesis, we employed a model system using embryo fibroblasts isolated from mice in which the single exon carrying the rhoB gene was targeted for deletion by standard homologous recombination methodology (the exon was replaced with a neomycin resistance gene cassette). Generation and characterization of these mice, which develop apparently normally and are fertile, will be described elsewhere. MEFs were isolated from E14.5 or E16.5 embryos that were +/+, +/−, or −/− for the rhoB gene. Northern analysis confirmed loss of the rhoB message in −/− MEFs relative to +/− or +/+ cells (Fig. 1). Where examined +/+ and +/− cells exhibited similar responses, but −/− cells differed in most cases; this report presents observed differences between +/− and −/− cells.
FIG. 1.
Northern analysis of MEF RNA. Total cytoplasmic RNA isolated from +/+, +/−, and −/− primary MEFs was fractionated by Northern analysis and hybridized to radiolabeled rhoB and rhoA cDNA probes.
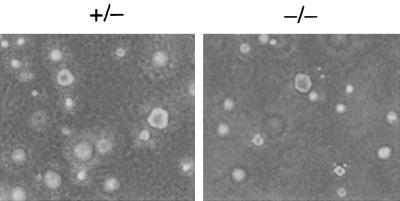
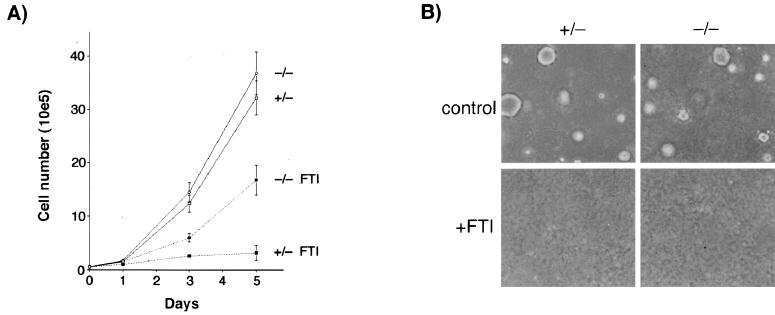
Primary MEFs of each genotype derived from different embryos from different mice were similarly susceptible to transformation by adenovirus E1A plus the activated human T22 allele of H-Ras, as determined by focus formation efficiency and soft-agar cloning efficiency (data not shown). Transformed cell foci were cloned and expanded into cell lines for analysis; cells used in this study were derived from different embryos and were all transformed by E1A plus Ras, so for simplicity they are referred to below only by genotype. Both −/− and +/− cell lines formed colonies under anchorage-independent conditions in soft-agar culture (Fig. 2) and formed histologically similar tumors in immunocompromised SCID mice with the same efficiency (data not shown). We concluded that MEFs lacking RhoB were susceptible to malignant transformation by E1A and Ras.
FIG. 2.
RhoB deletion does not compromise E1A plus Ras cotransformation. Primary MEFs transformed by adenovirus E1A plus mutant H-Ras in a focus formation assay were cloned and seeded into soft-agar culture to confirm their ability to proliferate under anchorage-independent conditions.
To confirm that rhoB deletion did not affect the susceptibility of endogenous cellular FT to suppression by FTIs, we determined whether H-Ras isoprenylation could be blocked with similar efficiencies in +/− and −/− cells by the FT-specific peptidomimetic inhibitor L-744832 (22). Following 24 h of treatment with FTI or vehicle only, cells were lysed and processed for Western analysis with anti-Ras antibody Y13-259. Similar shifts in Ras mobility, which are diagnostic for inhibition of isoprenylation, were observed in both cell types (Fig. 3). This result was confirmed in additional clones of each genotype (data not shown). We concluded that loss of RhoB did not affect the ability of FTI to suppress cellular FT activity. Furthermore, since Ras isoprenylation was blocked with identical efficiency in cells nullizygous for RhoB, we inferred that any differences in biological response reflected Ras-independent effects.
FIG. 3.
RhoB deletion does not affect the ability of FTI L-744832 to inhibit farnesylation of H-Ras. Processing of H-Ras in cells treated with vehicle (control) or FTI L-744832 was monitored as described previously (29) except that anti-Ras monoclonal antibody Y13-259 was used for hybridization to the Western blot. The decrease in apparent mobility is diagnostic in the assay for loss of farnesylation.
Loss of RhoB abolishes the actin cytoskeletal changes and alters the phenotypic shift elicited in response to FTI treatment.
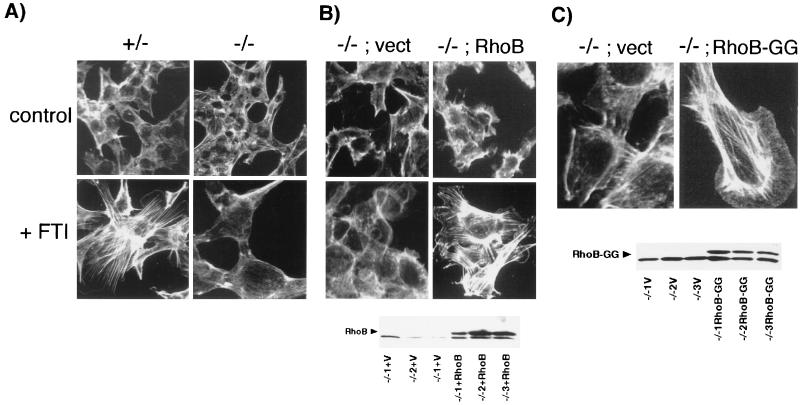
When treated with FTI, Ras-transformed fibroblasts undergo a rapid and pronounced phenotypic shift that is associated with the appearance of a flat morphology and an extensive network of actin stress fibers (36). The stress fiber response was monitored by immunofluorescence microscopy following cell staining with fluorescein-conjugated phalloidin, which binds specifically to filamentous actin. The results of the experiments are shown in Fig. 4. Stress fibers were induced within 24 h of starting treatment with FTI in +/− cells but not in −/− cells (Fig. 4A). Longer drug treatment times did not elicit any change in actin structure. In addition, Western analysis confirmed that the lack of stress fiber response in −/− cells was not caused by reduced expression of β actin in either untreated or drug-treated cells (data not shown). To confirm that the effect was due to loss of RhoB rather than some nonspecific effect, we examined the response of −/− cells that were infected with retroviral vectors expressing no insert or an HA epitope-tagged wild-type RhoB cDNA (24). Western analysis confirmed the expression of RhoB in −/− cells infected with the appropriate vector (Fig. 4B and C, bottom). In three different clones tested, expression of RhoB complemented the defective actin response whereas the empty vector had no effect (Fig. 4B). Moreover, results for cells stably expressing an engineered RhoB-GG isoform (9) from the same retroviral vector indicated that RhoB-GG was sufficient to induce stress fibers (Fig. 4C). This observation supported the notion that the defective actin response was due to the inability of FTI to elevate RhoB-GG levels. Whether this feature is specific to RhoB-GG was unclear because of the inability to engineer a RhoB isoform that is strictly farnesylated (i.e., RhoB-F). However, this issue is not germane to the FTI mechanism since FTIs abolish rather than elevate RhoB-F levels. We concluded that RhoB alteration was crucial for the actin cytoskeletal response to FTI treatment.
FIG. 4.
Defective actin stress fiber response to FTI treatment. (A) RhoB deletion abolishes FTI-induced stress fiber formation. Cells were treated with 10 μM FTI or vehicle for 30 h and F-actin was visualized in paraformaldehyde-fixed cells by phalloidin staining and confocal microscopy. (B) Ectopic RhoB rescues the defective actin response. Top, representative results for −/− cells that ectopically express vector sequences or HA epitope-tagged wild-type RhoB (24); bottom, Western analysis of HA-RhoB in retrovirally transduced cell clones. (C) Ectopic RhoB-GG is sufficient to mediate stress fiber formation. Top, representative results for −/− cells that ectopically express vector sequences or HA epitope-tagged RhoB-GG (9); bottom, Western analysis of HA–RhoB-GG in retrovirally transduced cell clones. V, vector only.
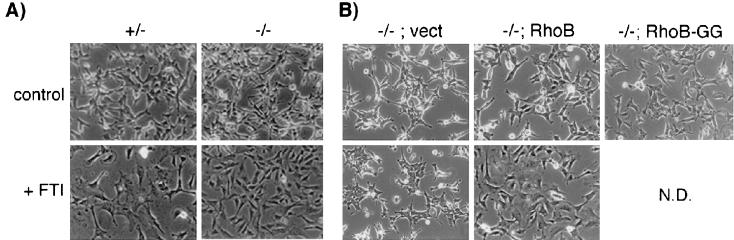
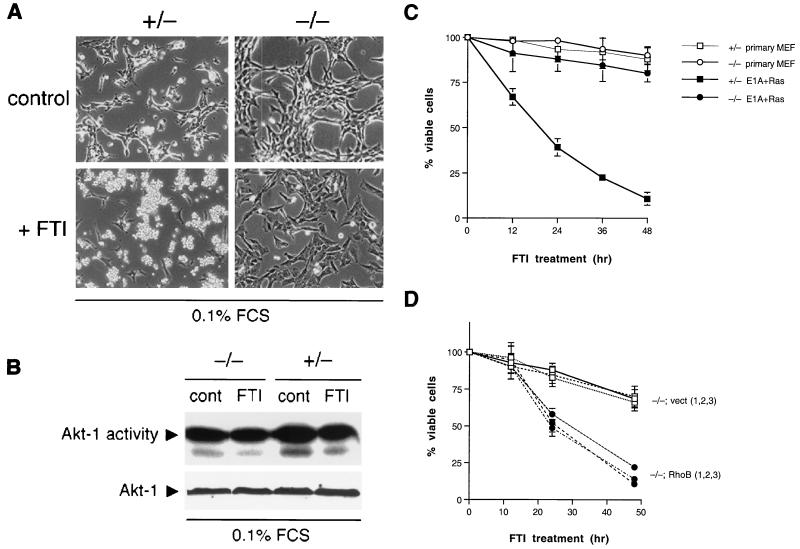
In the absence of RhoB, the morphological response to FTI was also blunted but not so dramatically as the actin response. Within 24 h of FTI treatment, +/− cells underwent a dramatic phenotypic shift to an enlarged flat morphology (Fig. 5A), similar to that seen in other transformed-fibroblast models (20, 30, 36). In contrast, −/− cells exhibited only a partial shift, suggesting a role for altered isoprenylation of proteins other than H-Ras and RhoB. To confirm that the partial effect seen was due to loss of RhoB, we examined the response of the cells infected with the retroviral vectors described above. Expression of RhoB complemented the morphology defect, whereas the empty vector had no effect, and RhoB-GG mimicked the limited effect of FTI (Fig. 5B). We concluded that RhoB alteration played a partial role in phenotypic reversion by FTI but that alteration of other non-Ras proteins was also involved.
FIG. 5.
Defective morphological response to FTI treatment. (A) RhoB deletion partially compromises FTI-induced phenotypic reversion. Cells were photographed 30 h after treatment with 10 μM FTI or vehicle. (B) Ectopic rescue by RhoB and effect of RhoB-GG. Cells were infected with recombinant retroviruses as indicated, and the phenotypic response was monitored as for panel A. N.D., not done.
Loss of RhoB compromises anchorage-dependent but not anchorage-independent growth inhibition by FTI.
To analyze the consequences for FTI-induced growth suppression, we treated +/− and −/− cells grown in serum-containing media on plastic dishes or in soft-agar culture (to monitor anchorage-dependent or anchorage-independent proliferation, respectively). When serum growth factors are present, transformed rodent fibroblasts exhibit cytostatic but not cytotoxic responses to FTI treatment. Interestingly, −/− cells were resistant to growth inhibition when they were cultured on plastic dishes but sensitive to growth inhibition when they were cultured in soft agar (Fig. 6). These results were not due to clonal variation, because three different −/− cell lines tested exhibited the same effect (data not shown). In contrast, +/− cells were sensitive to growth inhibition under both conditions. Flow-cytometric analyses indicated that growth inhibition was not associated with a block to cell cycle transit in a particular phase of the cell cycle (data not shown). Stable expression of RhoB-GG achieved by retroviral transfer showed that RhoB-GG was sufficient to inhibit the growth of −/− cells approximately twofold under anchorage-dependent conditions (data not shown), consistent with previous observations of H-Ras-transformed Rat1 fibroblasts (9). Thus, when taken together, the results indicated that RhoB alteration was only one of at least two events elicited by FTI that were sufficient to inhibit anchorage-independent growth. In addition, the results implied that RhoB-GG was sufficient to mediate growth inhibition but that it only contributed a partial necessary role if cells had substratum attachment.
FIG. 6.
Defect in FTI-induced growth inhibition under anchorage-dependent but not anchorage-independent conditions. (A) Anchorage-dependent growth curve in plastic dishes. The results represent the averages ± and standard errors of the means for three cell lines of each genotype tested. Vehicle or FTI was added at days 0, 2, and 4. (B) Anchorage-independent growth in soft-agar culture. Cells (104) for three cell lines of each genotype were seeded into soft-agar culture as described previously (9) and were photographed 14 days later. A representative field is shown.
Loss of RhoB abolishes the apoptotic response to FTI.
To assay the effects of rhoB deletion on FTI-induced apoptosis, we treated cells cultured in suboptimal concentrations of serum. Under these conditions, the response of transformed fibroblasts shifts from a cytostatic response to a cytotoxic response (10, 44). Strikingly, whereas +/− cells underwent massive apoptosis when treated with FTI, −/− cells remained fully viable (Fig. 7A). Similar effects were observed if cells were deprived of substratum attachment, as seen previously in H-Ras-transformed Rat1 cells (27). A partial flattening seen in −/− cells under low-serum conditions was consistent with observations made in growth media, confirming that additional FTI targets contribute to full phenotypic reversion. In previous work we established that Akt kinase is not targeted for inhibition by FTIs in H-Ras-transformed cells (in fact, activated Akt opposes FTI action [10]). This was confirmed for apoptosis-sensitive +/− MEFs by the finding that endogenous Akt-1 activity was not altered during FTI-induced cell death (Fig. 7B). Neither rhoB deletion nor FTI treatment affected basal levels of Bcl-2 or Bax expressed in cells, as shown by Western blotting (data not shown). Flow cytometry performed to quantitate the extent of DNA degradation showed that cell death occurred rapidly in +/− cells and that −/− cells were as resistant to FTI-induced apoptosis as primary untransformed +/− or −/− MEFs (Fig. 7C). To confirm that the resistance of −/− cells was due to lack of RhoB rather than to some nonspecific cause, we compared the response of −/− cells that were retrovirally transduced with wild-type RhoB with that of those transduced with engineered RhoB-GG. In three separate wild-type RhoB-expressing clones tested we observed complementation of the defect by RhoB, whereas the vector had no effect (Fig. 7D). Ectopic RhoB-expressing clones exhibited a slight delay in response relative to parental cells. In cells that stably expressed RhoB-GG, we did not observe apoptosis upon serum deprivation. However, transient expression experiments using green fluorescent protein to mark transfected cells revealed a major population of rounded, apoptotic cells (data not shown). Thus a negative selection against overexpressed RhoB-GG appears to occur, even in the presence of serum, possibly reflecting differences of bona fide and engineered RhoB-GG or the increased dosage of the retrovirally delivered gene. Nevertheless, the data supported the interpretation that RhoB-GG was proapoptotic and that FTIs must elicit RhoB-GG to drive apoptosis in transformed cells. We concluded that RhoB alteration was a required part of the mechanism through which FTIs exert their cytotoxic effects.
FIG. 7.
Defect in FTI-induced apoptosis. (A) Morphology. Cells were seeded into 35-mm-diameter dishes and photographed after treatment for 24 h in DMEM containing 0.1% FBS plus either 10 μM FTI or an equivalent volume of vehicle. Flow cytometry and viable cell determination by trypan blue exclusion confirmed the appearance of degraded DNA and loss of viability. (B) FTI L-744832 does not inhibit endogenous Akt-1. Cells were treated as for panel A and then processed for Akt kinase activity using histone H2B as the substrate as described in Materials and Methods. Bottom, Western analysis of Akt-1 showing equivalent protein in the assay. FCS, fetal calf serum. (C) RhoB deletion renders E1A-plus Ras-transformed cells resistant to FTI-induced apoptosis. Cells (5 × 105) were seeded into 60-mm-diameter dishes, treated as for panel A, and processed for flow cytometry. The proportion of cells in the sub-G1 phase DNA fraction (degraded DNA) relative to control cells is presented as a measurement of loss of viability. The means and standard errors computed from three trials are shown. (D) Complementation by ectopic RhoB. −/− cells transformed by E1A plus Ras were infected with recombinant retroviruses expressing vector sequences only (vect) or HA-RhoB (RhoB), treated as for panel A, and analyzed for apoptosis by flow cytometry. Three trials using three different clones are presented to address caveats of clonal variation.
Loss of RhoB blunts the antitumor efficacy of FTIs due to reduced apoptotic response.
RhoB-GG was dispensable for anchorage-independent growth inhibition by FTIs, so one might predict that −/− cells grown as tumor xenografts would remain sensitive to FTI treatment. However, RhoB-GG had a crucial role in the apoptotic response to FTIs, so −/− cells grown as tumor xenografts might exhibit significant resistance to FTIs. To distinguish these possibilities, we compared the FTI responses of +/− and −/− cells grown in SCID mice. Cells (107) of different genotypes were injected into the opposite thighs of the same animal to control for nonspecific environmental effects. A total of 16 mice in each group were treated in this manner. Palpable +/− and −/− tumors in each animal formed within 7 days of injection. One week after the xenograft was initiated mice were assigned randomly to control or drug treatment groups. The drug treatment group was dosed once daily for 16 days by intraperitoneal injection with 40 mg of L-744832/kg as described previously (22). Control mice were given vehicle carrier only. Tumor volumes were calculated every 4 days from caliper measurements taken at various times during the trial. We noted no adverse side effects of L-744832 administration consistent with previous observations (3, 22, 29). At the end of the trial, mice were euthanized and their tumors were surgically excised for weighing and examination.
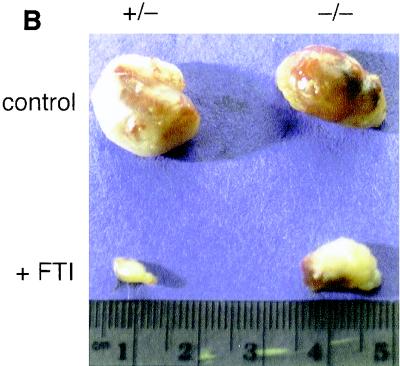
Significantly, while +/− and −/− xenografts grew at similar rates in control mice they exhibited dramatic differences in their susceptibilities to suppression by FTI treatment (Fig. 8A). Tumors of both genotypes grew at similar rapid rates in control animals during the trial. +/− xenografts were strongly inhibited by the drug protocol and grew little relative to controls. By the end of the experiment, +/− tumors in the drug-treated group were quite small and 2 of 8 were only apparent by surgical examination. In contrast, the −/− tumors continued to grow under FTI treatment and by the end of the experiment were grossly apparent on all mice and had achieved ∼50% of the size of control tumors (Fig. 8B). The approximately twofold suppression in the growth of −/− tumors was consistent with the susceptibility of −/− cells to anchorage-independent cell growth inhibition by FTIs, because while RhoB-GG was sufficient it was not necessary for this effect. However, the fact that −/− tumors continued to grow during the trial implied that the blunted apoptotic capacity of −/− cells played a predominant role in the in vivo response to FTIs.
FIG. 8.
RhoB deletion compromises the antitumor efficacy of FTIs. (A) Xenograft assay. +/− and −/− E1A- plus Ras-transformed cells were injected subcutaneously into opposite thighs of each of 16 SCID mice. Mice were assigned randomly to drug treatment or control groups and 1 week later were dosed daily for 16 days by intraperitoneal injection with FTI L-744832 (40 mg/kg) or vehicle carrier as described in the text. Tumor size was measured by caliper, and tumor volume was computed as described in Materials and Methods. The data represent the average tumor volumes ± standard errors of the means computed at the times indicated. (B) Representative tumors from control or drug treatment animals. Tumors were excised at the end of the experiment from euthanized animals and fixed in formalin before being photographed.
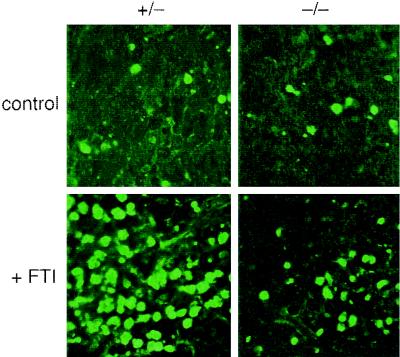
To explicitly determine whether −/− tumors were less susceptible to FTI-induced apoptosis, we performed a second trial in which four mice were challenged with 106 +/− or −/− cells per thigh and tumors were allowed to form for 3 weeks before drug treatment began. As before, mice were then dosed daily except that 24 h after the second dose mice were euthanized and their tumors were harvested and processed for in situ TUNEL analysis using a fluorescence detection assay. Analysis of the sections by immunofluorescence microscopy revealed a significant difference in the extent of TUNEL-positive cells in −/− and +/− tumors (Fig. 9). +/− tumors exhibited extensive apoptosis relative to −/− tumors, consistent with the in vitro resistance of −/− cells to FTI treatment. Thus, the reduced apoptotic susceptibility of −/− cells was well correlated with the blunted antitumor response. We concluded that RhoB alteration was required for efficient suppression of malignant tumor growth by FTIs.
FIG. 9.
RhoB nullizygous tumors are resistant to FTI-induced apoptosis. Four animals were injected in each thigh with 106 cells of +/− or −/− genotype, and tumors were allowed to grow untreated for 3 weeks. Two mice each were dosed with FTI L-744832 (40 mg/kg) or vehicle as described for Fig. 8 except that 24 h after the second dose mice were euthanized and tumors were excised and processed for in situ TUNEL analysis using a fluorescence-based method. Representative fluorescence microscope fields of processed tumor sections are shown.
DISCUSSION
This report offers genetic proof of the hypothesis that RhoB alteration is central to the antineoplastic mechanism engaged by FTI treatment. Previous work implicated the increased expression of RhoB-GG elicited by FTIs as a sufficient cause for growth inhibition in transformed rodent cells and human carcinoma cell lines (9, 11). In this study, we showed that RhoB-GG is necessary for FTI-induced apoptosis and crucial for antitumor efficacy in a xenograft model. The results indicated that RhoB-GG also has a necessary role for efficient growth inhibition by FTI under anchorage-dependent conditions. However, RhoB-GG was dispensable for inhibition of anchorage-independent growth, implying that if RhoB is absent the alteration of another protein(s) (possibly H-Ras in this model) could also provide a sufficient event for this process. One question addressed here was whether the engineered RhoB-GG construction previously used to establish RhoB-GG as a sufficient condition for growth inhibition and apoptosis by FTI accurately mimicked a bona fide cellular RhoB-GG (the construction had included a small number of RhoA-derived residues). The finding that loss of cellular RhoB-GG significantly blunts the FTI response argues against the concern that the engineered construct acted through a nonspecific rather than specific gain-of-function mechanism. Some differences in the apoptotic potency of exogenous engineered RhoB-GG versus that of the endogenous isoform were noted. Nevertheless the data supported the conclusion that RhoB-GG has proapoptotic activity in transformed cells.
Despite the close structural similarity between RhoB-GG and RhoA, it was clear that RhoA could not complement losses in RhoB-GG, because RhoA was expressed similarly regardless of rhoB genotype. As mentioned above, the exact basis for the growth-inhibitory and apoptotic properties of RhoB-GG is unclear, but we favor the interpretation that mislocalization of this isoform in FTI-treated cells (25) is key to understanding its action. We previously suggested that loss of the farnesylated RhoB isoform (RhoB-F) may have some role in the FTI response but this study argues strongly against this possibility. In summary, our observations provide direct evidence that RhoB-GG elevation by FTIs is not only sufficient but also necessary for apoptosis and a robust antineoplastic response.
A significant finding of this study was that the apoptotic defect of −/− cells treated with FTIs was correlated with a loss of antitumor efficacy in vivo. This observation implied that RhoB-GG was needed to mediate efficient tumor suppression because it was needed to drive apoptosis. One question concerns the strength of the apoptotic response of E1A- plus Ras-transformed MEFs in vivo, which differed from the more-limited apoptosis that occurs in other xenograft models that have been published. To our knowledge, this is the first study in which oncogene-transformed primary cells were tested. Primary cells have greater sensitivity to apoptosis, especially anoikis (which may be crucial in in vivo settings), than established lines which have been examined previously in xenograft assays. Moreover, this greater sensitivity is retained in oncogene-transformed primary cells (31). In addition, since E1A “epithelializes” cells (13), the more pronounced apoptotic response to FTI that was seen in the MEF model might reflect a sensitization of the model to anoikis, which is characteristic of epithelial cells but not fibroblasts. In any case, while accentuated, apoptosis of murine tumors in response to FTI is not unique, insofar as it has been seen in a variety of transgenic models (3, 22, 28, 29, 33).
Dominant inhibitory genetic methods have argued that Rho functions are required for efficient transformation of Rat1 cells by activated H-Ras (38, 40), but we found that rhoB null MEFs were fully susceptible to cotransformation by adenovirus E1A plus activated H-Ras. This susceptibility could mean either that RhoB is dispensable in this context or that E1A complements a RhoB requirement. An additional possibility is that RhoB may have a Janus or modifier role, perhaps determined by positive- or negative-acting cell surface receptors relevant to different cellular contexts. In any case, it is clear that RhoB has nonredundant functions, insofar as other ubiquitous and even highly related Rho proteins such as RhoA cannot complement the effects of RhoB deficiency. To further investigate the function of RhoB in cell transformation and growth regulation, we are generating 3T3 cells for analysis as well as comparing the susceptibilities of rhoB null mice to 7,12-dimethyl-benz[a]anthracene-induced skin carcinogenesis, which involves Ras activation as the initiating step (41).
Concerning the relevance of genetic context, it is worth noting that mutant K-Ras and mutant H-Ras have similar abilities to sensitize fibroblasts to apoptosis by FTIs (10, 44). Therefore, we believe that the proapoptotic action of FTI and RhoB-GG is unlikely to be a specific feature of H-Ras in the model. Nevertheless, it will be interesting to learn whether loss of RhoB compromises the FTI response in K-Ras-transformed cells and cells with other genetic backgrounds. K-Ras- plus E1A-transformed MEFs might be expected to differ in growth suppression but to remain similarly susceptible to apoptosis, given the observations of Suzuki and colleagues with K-Ras-transformed NRK cells (44). The effects of rhoB deletion on apoptosis in different genetic backgrounds will be important since levels of reliance on Rho signaling pathways may differ in such backgrounds. Crossing rhoB nullizygous animals with animals carrying various oncogenes is one way in which FTI susceptibilities to apoptosis, growth suppression, and tumorigenesis could be addressed in future work.
We observed that RhoB-GG promoted apoptosis in a manner that was not correlated with inhibition of endogenous Akt activity in E1A-plus Ras-transformed MEFs. These observations were consistent with previous evidence that FTI-induced apoptosis is not associated with Akt inhibition (10, 23). However, this issue deserves careful examination in a variety of models because of evidence that FTIs may influence Akt activity in certain settings. The RhoB effector kinase Prk has been found to associate with and regulate the activity of Pdk1, the kinase responsible for activating Akt following its membrane recruitment (2). For reasons that remain unclear, in FTI-treated cells RhoB-GG is mislocalized away from the endosomal site where RhoB is normally found (25). Therefore, RhoB-GG may sequester RhoB effector signaling molecules such as Prk away from the endosome. If so, this sequestration could impact the activity of Pdk1 and Akt, due to the ability of Prk to regulate Pdk1. While this biochemical linkage must be considered tentative at this time, it is interesting that one recent study has suggested that FTI may promote apoptosis in certain human epithelial tumor cell lines by inhibiting the Akt-2 isoform (21). In transient expression assays of COS epithelial cells, we have observed that both FTIs and RhoB-GG can inhibit Akt-1 activation by mutant Ras or epidermal growth factor (A.-X. Liu and G. C. Prendergast, submitted for publication). These observations are consistent with a potential Prk-Pdk1 connection and raise the possibility that cell type or genetic background may dictate the mechanism through which RhoB-GG acts. However, the physiological relevance of these observations remains to be established, since the weight of the evidence to date suggests that FTIs do not influence endogenous Akt-1 activity in transformed Rat1 or MEF cells under conditions where the drugs can induce apoptosis. Recognizing the above caveats, we interpret our findings to mean that RhoB-GG can mediate the proapoptotic effects of FTIs through an Akt-1-independent mechanism.
Apoptosis would be expected to be an important component of any successful clinical application of FTIs. In this regard, we note that the results of preclinical experiments and human clinical trials to date suggest strongly that FTIs will need to be applied combinatorially with other agents to achieve efficacy (i.e., cell death). We have reported that inhibitors of cell adhesion or phosphatidylinositol 3′-kinase cooperate with FTI to efficiently kill Ras-transformed cells (10, 27). In addition, others have shown that FTIs synergize with classical cytotoxic cancer therapeutics and irradiation (4, 32, 43). Based on this and other studies, we propose that RhoB-GG provides a crucial signal(s) needed to mediate the synergistic effect of FTI when combined with other modalities. In support of this proposal, we have observed that E1A- plus Ras-transformed −/− cells are resistant to apoptosis induced by irradiation or doxorubicin treatment and that they lack susceptibility to FTI-induced sensitization to these treatments (A.-x. Liu and G. C. Prendergast, unpublished observations).
In closing, we remain intrigued by the connections between Rho and apoptosis (5) and how the organization of integrins and the actin cytoskeleton regulated by Rho impact the antineoplastic response to FTIs. We have suggested that the means by which FTIs cause cell death ultimately involve partial or abortive responses of the transformed cell to induction of some integrin-dependent process (27). Normal cells would be immune to this process since they already exist in a state where such processes are fully operative. Since RhoB-GG induces actin stress fiber formation, it is tempting to speculate that it acts by facilitating the organization of focal adhesions and integrins in malignant cells, leading to a conflict with oncogene-mediated signals which suppress this organization. As mentioned, since focal adhesions and integrins are already in a highly ordered state in normal cells, this model offers an appealing explanation for why FTIs do not affect normal cells (35).
ACKNOWLEDGMENTS
We thank Allen Oliff for supplying FTI L-744832. Contributions from the Microscopy and Flow Cytometry Core Facilities at The Wistar Institute are gratefully acknowledged.
This research was supported by NIH grants CA65892 and CA82222. W.D. was supported in part by a grant from Merck and Co., Inc. G.C.P. is a Pew Scholar in the Biomedical Sciences.
REFERENCES
- 1.Aspenstrom P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 2.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes C P, Alessi D R. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 3.Barrington R E, Subler M A, Rands E, Omer C A, Miller P J, Hundley J E, Koester S K, Troyer D A, Bearss D J, Conner M W, Gibbs J B, Hamilton K, Koblan K S, Mosser S D, O'Neill T J, Schaber M D, Senderak E T, Windle J J, Oliff A, Kohl N E. A farnesyltransferase inhibitor induces tumor regression in transgenic mice harboring multiple oncogenic mutations by mediating alterations in both cell cycle control and apoptosis. Mol Cell Biol. 1998;18:85–92. doi: 10.1128/mcb.18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhard E J, McKenna W G, Hamilton A D, Sebti S M, Qian Y, Wu J M, Muschel R J. Inhibiting Ras prenylation increases the radiosensitivity of human tumor cell lines with activating mutations of ras oncogenes. Cancer Res. 1998;58:1754–1761. [PubMed] [Google Scholar]
- 5.Bobak D, Moorman J, Guanzon A, Gilmer L, Hahn C. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene. 1997;15:2179–2189. doi: 10.1038/sj.onc.1201396. [DOI] [PubMed] [Google Scholar]
- 6.Casey P J, Seabra M C. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox A D, Der C J. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta. 1997;1333:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 9.Du W, Lebowitz P, Prendergast G C. Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol Cell Biol. 1999;19:1831–1840. doi: 10.1128/mcb.19.3.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du W, Liu A, Prendergast G C. Activation of the PI3′K-AKT pathway masks the proapoptotic effect of farnesyltransferase inhibitors. Cancer Res. 1999;59:4808–4812. [PubMed] [Google Scholar]
- 11.Du W, Prendergast G C. Geranylgeranylated RhoB mediates inhibition of human tumor cell growth by farnesyltransferase inhibitors. Cancer Res. 1999;59:5492–5496. [PubMed] [Google Scholar]
- 12.Engel M E, Datta P K, Moses H L. RhoB is stabilized by transforming growth factor β and antagonizes transcriptional activation. J Biol Chem. 1998;273:9921–9926. doi: 10.1074/jbc.273.16.9921. [DOI] [PubMed] [Google Scholar]
- 13.Frisch S M. The epithelial cell default-phenotype hypothesis and its implications for cancer. Bioessays. 1997;19:705–709. doi: 10.1002/bies.950190811. [DOI] [PubMed] [Google Scholar]
- 14.Fritz G, Kaina B. rhoB encoding a UV-inducible ras-related small GTP-binding protein is regulated by GTPases of the rho family and independent of JNK, ERK, and p38 MAP kinase. J Biol Chem. 1997;272:30637–30644. doi: 10.1074/jbc.272.49.30637. [DOI] [PubMed] [Google Scholar]
- 15.Fritz G, Kaina B, Aktories K. The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J Biol Chem. 1995;270:25172–25177. doi: 10.1074/jbc.270.42.25172. [DOI] [PubMed] [Google Scholar]
- 16.Gampel A, Parker P J, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase RhoB. Curr Biol. 1999;9:955–958. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs J B, Oliff A. The potential of farnesyltransferase inhibitors as cancer chemotherapeutics. Annu Rev Pharmacol Toxicol. 1997;37:143–166. doi: 10.1146/annurev.pharmtox.37.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Hawley R G, Lieu F H, Fong A Z, Hawley T S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 19.Jahner D, Hunter T. The ras-related gene rhoB is an immediate-early gene inducible by v-Fps, epidermal growth factor, and platelet-derived growth factor in rat fibroblasts. Mol Cell Biol. 1991;11:3682–3690. doi: 10.1128/mcb.11.7.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James G L, Brown M S, Cobb M H, Goldstein J L. Benzodiazepine peptidomimetic BZA-5B interrupts the MAP kinase activation pathway in H-Ras-transformed Rat-1 cells, but not in untransformed cells. J Biol Chem. 1994;269:27705–27714. [PubMed] [Google Scholar]
- 21.Jiang K, Coppola D, Crespo N C, Nicosia S V, Hamilton A D, Sebti S M, Cheng J Q. The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol. 2000;20:139–148. doi: 10.1128/mcb.20.1.139-148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohl N E, Omer C A, Conner M W, Anthony N J, Davide J P, deSolms S J, Giuliani E A, Gomez R P, Graham S L, Hamilton K, Handt L K, Hartman G D, Koblan K S, Kral A M, Miller P J, Mosser S D, O'Neill T J, Rands E, Schaber M D, Gibbs J B, Oliff A. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995;1:792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- 23.Law B K, Norgaard P, Gnudi L, Kahn B B, Poulson H S, Moses H L. Inhibition of DNA synthesis by a farnesyltransferase inhibitor involves inhibition of the p70s6k pathway. J Biol Chem. 1999;274:4743–4748. doi: 10.1074/jbc.274.8.4743. [DOI] [PubMed] [Google Scholar]
- 24.Lebowitz P, Casey P J, Prendergast G C, Thissen J. Farnesyltransferase inhibitors alter the prenylation and growth-stimulating function of RhoB. J Biol Chem. 1997;272:15591–15594. doi: 10.1074/jbc.272.25.15591. [DOI] [PubMed] [Google Scholar]
- 25.Lebowitz P F, Davide J P, Prendergast G C. Evidence that farnesyl transferase inhibitors suppress Ras transformation by interfering with Rho activity. Mol Cell Biol. 1995;15:6613–6622. doi: 10.1128/mcb.15.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebowitz P F, Prendergast G C. Non-Ras targets for farnesyltransferase inhibitors: focus on Rho. Oncogene. 1998;17:1439–1447. doi: 10.1038/sj.onc.1202175. [DOI] [PubMed] [Google Scholar]
- 27.Lebowitz P F, Sakamuro D, Prendergast G C. Farnesyltransferase inhibitors induce apoptosis in Ras-transformed cells denied substratum attachment. Cancer Res. 1997;57:708–713. [PubMed] [Google Scholar]
- 28.Liu M, Bryant M S, Chen J, Lee S, Yaremko B, Lipari P, Malkowski M, Ferrari E, Nielsen L, Prioli N, Dell J, Sinha D, Syed J, Dorfmacher W A, Nomeir A A, Lin C-C, Wang L, Taveras A G, Doll R J, Njoroge F G, Mallams A K, Remiszewski S, Catino J J, Girijavallabhan V M, Kirschmeier P, Bishop W R. Antitumor activity of SCH 6636, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer Res. 1998;58:4947–4956. [PubMed] [Google Scholar]
- 29.Mangues R, Corral T, Kohl N E, Symmans W F, Lu S, Malumbres M, Gibbs J B, Oliff A, Pellicer A. Antitumor effect of a farnesyl protein transferase inhibitor in mammary and lymphoid tumors overexpressing N-ras in transgenic mice. Cancer Res. 1998;58:1253–1259. [PubMed] [Google Scholar]
- 30.Manne V, Yan N, Carboni J M, Tuomari A V, Ricca C S, Brown J G, Andahazy M L, Schmidt R J, Patel D, Zahler R, Weinmann R, Der C J, Cox A D, Hunt J T, Gordon E M, Barbacid M, Seizinger B R. Bisubstrate inhibitors of farnesyltransferase: a novel class of specific inhibitors of ras transformed cells. Oncogene. 1995;10:1763–1779. [PubMed] [Google Scholar]
- 31.McGill G, Shimamura A, Bates R C, Savage R E, Fisher D E. Loss of matrix adhesion triggers rapid transformation-selective apoptosis in fibroblasts. J Cell Biol. 1997;138:901–911. doi: 10.1083/jcb.138.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moasser M M, Sepp-Lorenzino L, Kohl N E, Oliff A, Balog A, Su D S, Danishefsky S J, Rosen N. Farnesyl transferase inhibitors cause enhanced mitotic sensitivity to taxol and epothilones. Proc Natl Acad Sci USA. 1998;95:1369–1374. doi: 10.1073/pnas.95.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norgaard P, Law B, Joseph H, Page D L, Shry Y, Mays D, Peitenpol J A, Kohl N E, Oliff A, Coffey R J, Poulsen H S, Moses H L. Treatment with farnesyl-protein transferase inhibitor induces regression of mammary tumors in transforming growth factor (TGF) alpha and TGF alpha/neu transgenic mice by inhibition of mitogenic activity and induction of apoptosis. Clin Cancer Res. 1999;5:35–42. [PubMed] [Google Scholar]
- 34.Pear W, Nolan G, Scott M, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prendergast G C. Farnesyltransferase inhibitors: antineoplastic mechanism and clinical prospects. Curr Opin Cell Biol. 2000;12:166–174. doi: 10.1016/s0955-0674(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 36.Prendergast G C, Davide J P, deSolms S J, Giuliani E, Graham S, Gibbs J B, Oliff A, Kohl N E. Farnesyltransferase inhibition causes morphological reversion of ras-transformed cells by a complex mechanism that involves regulation of the actin cytoskeleton. Mol Cell Biol. 1994;14:4193–4202. doi: 10.1128/mcb.14.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prendergast G C, Du W. Targeting farnesyltransferase: is Ras relevant? Drug Resist Updates. 1999;2:81–84. doi: 10.1054/drup.1999.0070. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast G C, Khosravi-Far R, Solski P, Kurzawa H, Lebowitz P, Der C J. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- 39.Prendergast G C, Lawe D, Ziff E B. Association of Myn, the murine homolog of Max, with c-Myc stimulates methylation-sensitive DNA binding and Ras cotransformation. Cell. 1991;65:395–407. doi: 10.1016/0092-8674(91)90457-a. [DOI] [PubMed] [Google Scholar]
- 40.Qiu R G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 42.Rowinsky E K, Windle J J, von Hoff D D. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, Blaskovich M A, Knowles D, Qian Y, Ohkanda J, Bailey R D, Hamilton A D, Sebti S M. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 1999;59:4919–4926. [PubMed] [Google Scholar]
- 44.Suzuki N, Urano J, Tamanoi F. Farnesyltransferase inhibitors induce cytochrome c release and caspase 3 activation preferentially in transformed cells. Proc Natl Acad Sci USA. 1998;95:15356–15361. doi: 10.1073/pnas.95.26.15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]