ABSTRACT
Background and objectives
Drug–drug interactions (DDIs) can create a burden on prescribers to preserve patient safety. This study aimed to identify common DDIs in critically ill patients with chronic kidney disease (CKD) and to evaluate clinical pharmacist's interventions in managing DDIs among these patients.
Methods
A prospective observational study was conducted from October 2018 to March 2019. The clinical pharmacist performed a medication chart review; DDIs were identified by using Lexicomp® drug interaction. Based on the occurrence of DDIs, patients were divided into group A: patients with DDI (n = 76) and group B: patients without DDI (n = 15). Clinical pharmacist's interventions were classified according to Pharmaceutical Care Network Europe. The National Coordinating Council for Medication Error Reporting and Prevention was used to categorize the severity outcomes of DDIs and the degree of patient harm.
Results
A total of 273 DDIs were identified. The majority of DDIs (63.7%) required close monitoring of the therapeutic outcome to ensure maintaining patient safety. DDIs that needed to be managed by considering therapy modification and avoiding drug combination were accounted for 17.2 and 12.8% of the most common detected interactions, respectively. Seventy-eight percent of DDIs induced no harm to patient. Clinical pharmacist provided different types of recommendations to manage detected interactions, which ranged from therapy outcome monitoring to stop DDIs. A great proportion of pharmacist's interventions (92%) were accepted by prescribers. Compared to patients with stage 3 and 4 CKD, patients with stage 5 had a significantly higher number of DDIs (stage 3 vs 5: p = 0.0019, stage 4 vs 5: p = 0.0456). The number of comorbidities (p = 0.0003) and (p <0.0001) medications were found to be significantly greater in group A.
Conclusion
Clinical pharmacist performed important interventions in timely identifying, managing DDIs, and prevention of associated patient harms.
How to cite this article
Aghili M, Kasturirangan MN. Management of Drug–Drug Interactions among Critically Ill Patients with Chronic Kidney Disease: Impact of Clinical Pharmacist's Interventions. Indian J Crit Care Med 2021;25(11):1226–1231.
Keywords: Chronic kidney disease, Clinical pharmacist's intervention, Critically ill patients, Drug–drug interaction
INTRODUCTION
Chronic kidney disease (CKD) is a progressive loss of renal function that occurs over a period of months or years and can affect people at any ages of any races. CKD is a global health concern. Approximately 1 out of 10 people in the world's population have some degree of CKD. However, the risk of CKD is higher among African Americans, Hispanics, American Indians, and people of South Asian origin, which can be due to a higher rate of diabetes and hypertension among these populations. CKD is associated with a high rate of morbidity, healthcare expenditures, and mortality.1,2 Patients with CKD are prescribed multiple medications (polypharmacy) due to either slowing deterioration of kidney function or managing comorbidities, such as hypertension, diabetes, cardiovascular diseases, and anemia.3 The presence of comorbidities and associated polypharmacy have major implications on patients’ ability to cope with treatment.4 The need for complex drug regimens in patients with CKD potentiates the risk of occurrence of medication-related problems (MRPs), such as drug–drug interactions (DDIs),5 and the risk increases as CKD progresses.6 In addition, the influence of the disease on the pharmacokinetic and pharmacodynamic mechanism of medications increases the risk of the occurrence of DDIs-related adverse outcome in this cohort.7,8 Therefore, the significant consideration about CKD lies not only in the burden associated with the progressive nature of the disease itself but also in the difficulties related to the acquisition of medications to manage this disease in patients. Clinical pharmacy is defined as an area of pharmacy concerned with the science and practice of rational medication use, and clinical pharmacists are practitioners who are trained in pharmacotherapeutics and are involved in the implementation and monitoring of therapeutic regimens.9 Clinical pharmacists have been demonstrated to alleviate MRPs, including DDIs,10 and contribute to improving medication usage6 and management of comorbidity in patients with CKD.11 Moreover, a clinical pharmacist has a beneficial role in adjusting medication dose regimens of patients with CKD admitted in a critical care unit,12 where patients are more vulnerable to medication-related complications.13 Pharmacy practice services were introduced in the medical intensive care unit (MICU) of our hospital since 2016. Thus, the documentation and evaluation of clinical pharmacy services in the study setting are still under development. Given the complexity of medication regimens for patients with CKD, existing comorbidities, alteration of pharmacokinetic, and pharmacodynamic of medications prescribed for these patients,3,7,8 there is the requirement to evaluate clinical pharmacist's interventions in critically ill patients with CKD to establish enhanced clinical pharmacy services. In addition, the integration of clinical pharmacist into the MICU healthcare team for identifying and managing DDIs, and improving therapeutic outcomes in patients with CKD is crucial for continued drug safety monitoring.14 Therefore, this study aimed to identify common DDIs in patients with CKD admitted in MICU and to evaluate clinical pharmacist's interventions in managing DDIs among these patients.
MATERIALS AND METHODS
Study Design, Setting, and Participants
A prospective observational study was conducted in the MICU (10 beds) of a tertiary care academic hospital located in Bengaluru, India. The study was performed over a period of 6 months (October 2018 to March 2019). Patients with CKD admitted to the MICU of this hospital are treated by a multidisciplinary team mainly composed of physicians, nephrologists, senior and junior residents, and nurses. In addition, clinical pharmacy services have been introduced to the MICU team since 2016. The clinical pharmacist is involved in the monitoring of pharmacotherapeutic regimens of patients, attending medical rounds, and answering drug queries. Patients (age ≥18 years) admitted to MICU and diagnosed with CKD were included in this study, and patients with CKD who stayed less than 24 hours in MICU and discharged against medical advice were excluded.
Informed consent was obtained from patients or the patient's caregiver whenever the patient was not able to communicate. The study received approval from the Institutional Human Ethics Committee of Visveswarapura Institute of Pharmaceutical Sciences, Bengaluru, India (Reference number: VIPS/IEC/2016-08).
Data Collection and Identifying DDIs
The clinical pharmacist reviewed patients’ medication charts and documented prescribed medications during working hours (Monday to Saturday, 09:00–17:00). The process of medication chart review was performed twice daily (after morning and afternoon medical rounds) to avoid miss out of newly added medication, STAT, and Si Opus Sit (S.O.S, if necessary) medication orders. In addition, demographic information of patients and relevant medical history including main complaints, history of present illness, and past medical/medication history were collected by the clinical pharmacist. The glomerular filtration rate was estimated by using the modification of diet in the renal disease equation.15 CKD definition and categorization of CKD stages were according to the Kidney Disease Outcomes Quality Initiative.16 DDIs were identified and categorized by using Lexicomp® drug interaction. According to Lexicomp® drug interaction, DDIs with a risk-rating category of “C” (drug interaction required monitoring to detect potential adverse outcome), “D” (drug interaction has a high risk of the occurrence of adverse outcome, and safer alternative treatment should be considered), and “X” (drug combination is contraindicated and must be avoided) are clinically significant. Lexicomp® DDIs with the risk-rating categories of A and B (no action is required to manage drug interaction) do not imply the clinically significant impact on the patient's outcome of therapy. Therefore, we only considered DDIs with the risk-rating categories of C, D, and X (clinically significant DDIs), which require particular intervention and management.
Clinical Pharmacist's Interventions and Outcome Measures
The clinical pharmacist participated in daily multidisciplinary MICU rounds, delivered proposed recommendations to prescribers, and intervened in managing identified DDIs. Clinical pharmacist's interventions were classified according to the Pharmaceutical Care Network Europe version 8.02.17 The study outcomes were numbers and types of clinical pharmacist's interventions in managing encountered DDIs. The type of these interventions was based on the risk-rating categories of identified DDIs and recommendations provided by Lexicomp® drug interaction. For the risk-rating category of C (monitor therapy), the clinical pharmacist recommended monitoring patients’ clinical outcomes, such as blood pressure, heart rate, blood glucose, serum electrolytes, and serum creatinine more frequently or more closely. For example, the interaction between furosemide and insulin may diminish the therapeutic effect of insulin. Hence, for managing this interaction, the patient's blood glucose required more frequent monitoring to ensure appropriate glycemic control. On the contrary, for DDIs with the risk-rating categories of D and X, the clinical pharmacist recommended considering therapy modification and avoiding the combination, respectively. For example, to avoid the occurrence of severe hypotension, the clinical pharmacist recommended stopping the combination of nitroglycerine and sildenafil.
The clinical pharmacist closely monitored the outcome of identified DDIs. The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) Index was used to categorize the severity outcomes of DDIs and the degree of patient harm.18 The clinical pharmacist's recommendations for the management of DDIs and the severity outcomes of DDIs were validated by a panel of an academic clinical pharmacist and two senior MICU physicians.
Statistical Analysis
Collected data of study patients were entered into a Microsoft Excel spreadsheet during the study period. Descriptive statistics were calculated for variables, such as demographic characteristics of patients, prescribed medications, CKD stages, clinical pharmacist's interventions, severity, and risk-rating categories of identified DDIs. The clinical pharmacist reviewed patients’ medication charts and depending on the occurrence of DDIs, study patients were classified into two groups. Group A was a group of patients who had DDIs, whereas group B was a group of patients with no detected DDI. The number of comorbidities (>5) and medications prescribed (>7) were compared between group A and B patients. Statistically significant differences between the numbers of DDIs identified in patients with different stages of CKD were examined. A Chi-square test was applied for comparison between clinical variables obtained from study groups. The p value <0.05 was considered statistically significant. The Statistical Package for Social Sciences for Windows, version 22.0 was applied to study data analysis.
RESULTS
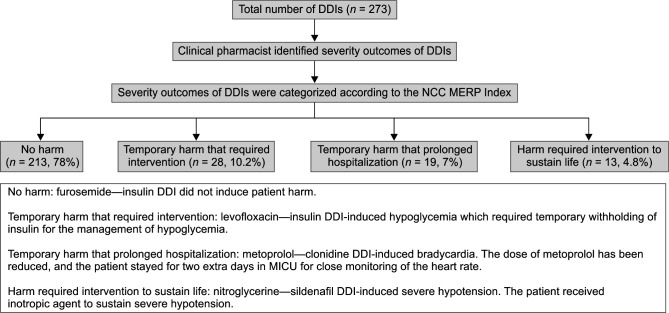
A total of 91 patients met the study criteria and were included during the study period. The clinical pharmacist reviewed these patients’ medication charts to identify DDIs (risk-rating categories of C, D, and X) and provide interventions accordingly. The mean age of 60.2 ± 12.1 years was calculated for study patients of whom 59 (64.8%) patients were male. The mean length (days) of MICU stay and the mean number of prescribed medications were 13.8 ± 6.7 and 19.1 ± 6.5, respectively. Approximately half of the patients (43, 47.2%) were in stage 5 of CKD (Table 1). Diabetes mellitus (62, 68.1%), hypertension (58, 63.7%), electrolyte imbalance (49, 53.8%), and anemia (37, 40.6%) were found to be the most frequent comorbidities among patients (Table 2). Seventy-six (83.5%) patients had at least one DDI in the risk-rating categories of C, D, or X (clinically significant DDIs), which required clinical pharmacist's intervention to manage encountered interaction. These patients were categorized in group A (N = 76). The remaining 15 (16.5%) patients did not have such clinically significant DDIs and were classified in group B. Overall, the clinical pharmacist identified a total of 273 DDIs among 76 patients (group A), giving an average ± standard deviation of 3.6 ± 1.8 DDIs per patient. The majority of these DDIs (174, 63.7%) belonged to the risk-rating category of C, which indicates these interactions required close monitoring of patients’ therapy for the detection of any potential adverse outcome. Forty-seven (17.2%) and 35 (12.8%) of the most common detected DDIs were classified in the risk-rating categories of D and X, which specifies that prescribers needed to consider therapy modification and stop drug combinations, respectively, to prevent the occurrence of adverse outcome (Table 3). The severity outcomes of DDIs (degree of patient harm) were categorized according to the NCC MERP Index. The majority of DDIs induced no harm to patients (n = 213, 78%). Only 22% (n = 60) of DDIs led to patient harm, which included 10.2% temporary harm that required intervention, 7% temporary harm that prolonged hospitalization (longer MICU stay), and 4.8% harm required intervention to sustain patient's life (Flowchart 1). The clinical pharmacist informed prescribers regarding detected DDIs and provided appropriate solutions to minimize the occurrence of DDIs-related adverse outcomes. Most of the clinical pharmacist's interventions at the prescriber level (181, 66.3%) were performed through discussion with the concerned prescriber. Analysis of clinical pharmacist's interventions at the drug level showed that the outcome monitored (112, 41%), dosage changed (53, 19.4%), and drug stopped (47, 17.2%) were the most frequent types of provided interventions for managing DDIs. A great proportion of these interventions (251, 92%) was accepted and fully implemented by the prescribers (Table 4). A comparison between the numbers of DDIs identified in patients with different stages of CKD showed that patients with the last stage of CKD (stage 5) had a significantly higher number of DDIs (stage 3 vs 5: p = 0.0019, stage 4 vs 5: p = 0.0456).
Table 1.
Demographic and clinical characteristics of study participants (N = 91)
| Variables | |
|---|---|
| Age (years) | |
| Mean ± SD | 60.2 ± 12.1 |
| Sex, n (%) | |
| Male | 59 (64.8) |
| Female | 32 (35.2) |
| Length of MICU stay (days) | |
| Mean ± SD | 13.8 ± 6.7 |
| Number of prescribed medications | |
| Mean ± SD | 19.1 ± 6.5 |
| Number of comorbidities | |
| Mean ± SD | 3.5 ± 1.9 |
| Alcohol intake, n (%) | |
| Yes | 39 (42.8) |
| Smoking, n (%) | |
| Yes | 44 (48.3) |
| CKD stage, n (%) | |
| 3 | 12 (13.2) |
| 4 | 36 (39.6) |
| 5 | 43 (47.2) |
SD, standard deviation; MICU, medical intensive care unit; CKD, chronic kidney disease
Table 2.
Frequency of comorbidities in study participants (N = 91)
| Comorbidities | n (%) |
|---|---|
| Diabetes mellitus | 62 (68.1) |
| Hypertension | 58 (63.7) |
| Electrolyte imbalance | 49 (53.8) |
| Anemia | 37 (40.6) |
| Urinary tract infection | 31 (34.1) |
| Ischemic heart disease | 19 (20.9) |
| Cardiac failure | 13 (14.3) |
| Sepsis | 13 (14.3) |
| COPD | 13 (14.3) |
| Bone mineral disease | 11 (12.1) |
| CVA | 7 (7.7) |
| Pulmonary thromboembolism | 6 (6.6) |
| Pneumonia | 6 (6.6) |
COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident
Table 3.
Most common identified drug–drug interactions
| Drug interaction pair | N (%)* | Potential outcome | Risk rating | Severity rating |
|---|---|---|---|---|
| Furosemide–Insulin | 39 (14.3) | Diminished therapeutic effect of insulin. | C, Monitor therapy | Moderate |
| Amlodipine–Calcium carbonate/vitamin D3 | 32 (11.7) | Diminished therapeutic effect of amlodipine. | C, Monitor therapy | Moderate |
| Linezolid–Insulin | 26 (9.5) | Enhanced hypoglycemic effect of insulin. | C, Monitor therapy | Moderate |
| Amlodipine–Calcium gluconate | 21 (7.7) | Diminished therapeutic effect of amlodipine. | C, Monitor therapy | Moderate |
| Nitroglycerine–Sildenafil | 19 (6.9) | Enhanced vasodilatory effect of nitroglycerine. | X, Avoid combination | Major |
| Metoprolol–Clonidine | 19 (6.9) | Enhanced AV-blocking effect of metoprolol. Enhanced rebound hypertensive effect of clonidine. | D, Consider therapy modification | Moderate |
| Atenolol–Clonidine | 18 (6.6) | Enhanced AV-blocking effect of atenolol. Enhanced rebound hypertensive effect of clonidine. | D, Consider therapy modification | Moderate |
| Salbutamol–Metoprolol | 17 (6.2) | Diminished bronchodilatory effect of salbutamol. | C, Monitor therapy | Moderate |
| Tolvaptan–Sodium chloride (3%) | 16 (5.9) | Enhanced adverse/toxic effect of tolvaptan. | X, Avoid combination | Major |
| Furosemide–Salbutamol | 16 (5.9) | Enhanced hypokalemic effect of furosemide. | C, Monitor therapy | Moderate |
| Levofloxacin–Insulin | 15 (5.5) | Enhanced hypoglycemic effect of insulin or can diminish therapeutic effect of insulin. | C, Monitor therapy | Moderate |
| Digoxin–Amiodarone | 10 (3.7) | Increased serum concentration of digoxin. | D, Consider therapy modification | Major |
| Furosemide–Amikacin | 8 (2.9) | Enhanced adverse/toxic effect of amikacin. | C, Monitor therapy | Moderate |
AV, atrioventricular;
The total number of drug interactions (N = 273) was considered for calculating percentages
Flowchart 1.
Flowchart of severity outcomes of identified drug–drug interactions. DDIs, drug–drug interactions; NCC MERP, National Coordinating Council for Medication Error Reporting and Prevention
Table 4.
Clinical pharmacist's interventions in managing identified drug–drug interactions (N = 273)
| Clinical pharmacist's intervention | n (%) |
|---|---|
| At prescriber level | |
| Prescriber informed only | 12308 (2.9) |
| Prescriber asked for information | 23 (8.4) |
| Intervention proposed to prescriber | 61 (22.4) |
| Intervention discussed with prescriber | 181 (66.3) |
| At drug level | |
| Drug changed | 23 (8.4) |
| Dosage changed | 53 (19.4) |
| Formulation changed | 11 (4) |
| Instructions for use changed | 27 (10) |
| Drug stopped | 47 (17.2) |
| Other intervention (outcome monitored) | |
| Blood glucose monitored more frequently | 33 (12.1) |
| Blood pressure monitored more closely | 29 (10.6) |
| Serum electrolytes monitored more frequently | 23 (8.4) |
| Heart rate monitored more closely | 19 (7) |
| Serum creatinine monitored more frequently | 8 (2.9) |
| Acceptance of the intervention by prescriber | |
| Intervention accepted and fully implemented | 251 (92) |
| Intervention accepted, partially implemented | 17 (6.2) |
| Intervention accepted but not implemented | 5 (1.8) |
A comparison of clinical variables between study groups revealed that group A patients had significantly higher numbers of comorbidities (>5) (p = 0.0003) and medications (>7) (p <0.0001) than the study group with no detected DDI (group B) (Table 5).
Table 5.
Clinical variables comparison of study participants
| Clinical variables | Comparison of clinical variables N (%) | p value |
|---|---|---|
| CKD stage 3 vs 4 19 (6.9)a vs 96 (35.2)a | 0.0617 | |
| DDIs identified in patients with different stages of CKD | CKD stage 3 vs 5 19 (6.9)a vs 158 (57.9)a | 0.0019* |
| CKD stage 4 vs 5 96 (35.2)a vs 158 (57.9)a | 0.0456* | |
| Patients with >5 numbers of comorbidities | A vs B 49 (64.4)b vs 2 (13.3)b | 0.0003* |
| Patients who received >7 numbers of medications | A vs B 74 (97.3)b vs 6 (40)b | <0.0001* |
DDIs, drug–drug interactions; CKD, chronic kidney disease;
The total number of drug interactions (N = 273) was considered for calculating percentages;
Patient number in each study group was considered as the total number (group A: N = 76, group B: N = 15) for calculating percentages;
Significant at p <0.05
DISCUSSION
The current study aimed to evaluate clinical pharmacist's interventions in managing DDIs in patients with CKD admitted to the MICU. Our study showed that 83.5% of study patients had DDIs with an average of 3.6 per patient, which required clinical pharmacist's intervention for managing these interactions. To minimize the occurrence of DDIs-related adverse outcomes, the clinical pharmacist performed different types of interventions that ranged from therapy outcome monitoring to stop the drug combination. Our finding indicates that DDIs occur commonly among critically ill patients with CKD. This finding is in line with several pieces of literature that demonstrated the common occurrence of DDIs among these patients.14,19–21
The common occurrence of DDIs in critically ill patients with CKD can be due to several potential reasons. Our analysis showed that patients with DDIs (group A) had significantly higher numbers of comorbidities (p = 0.0003) and prescribed medications (p <0.0001) than patients with no DDI identified (group B). The presence of comorbidities and multiple medications (polypharmacy) are found to be the predictors for the occurrence of MRPs, such as DDIs in patients with CKD.5,20,22 These patients are prescribed more complex medication regimens not only to slow the progression of disease but for the management of associated comorbidities. As the number and the severity of these comorbidities advance, the number of prescribed medications increases, in turn, the risk of DDIs is higher.3
Our most common identified pair of DDIs were furosemide–insulin and amlodipine–calcium carbonate/vitamin D3. These DDIs are the paradigm of interactions that occurred between medications prescribed for the management of CKD-related symptoms, such as volume overload, and for the management of comorbidities, such as diabetes mellitus, which is known as the most common CKD-related comorbidity.1 Nineteen (6.9%) contraindicated type of interaction occurred due to the prescription of nitroglycerine and sildenafil for the management of cardiovascular comorbidities among study patients. It is, therefore, plausible to consider medication prescribed for the management of comorbidity as a contributing factor in the occurrence of DDIs among critically ill patients with CKD. Additionally, our findings showed that patients with a more advanced stage of CKD had a significantly higher number of DDIs than patients in other stages of the disease. The stage of CKD may affect the likelihood of DDIs occurrence. While renal function declines and CKD stage progresses, the number of prescribed medication increases, thereby the risk of DDIs will increase consequently.19,21 Therefore, while managing patients with CKD, especially with the advanced stage of the disease, it is important to consider the stage of CKD as another contributing factor for the occurrence of MRP, including DDIs.5
Overall, 83.5% of study patients had DDIs. DDIs with severe risk-rating categories (category D: therapy modification, n = 47 and category X: avoid the combination, n = 35), which required intensive interventions, were accounted for 30% of the most common identified DDIs. The lower rate of severe DDIs was reported among CKD patients admitted to the hospital ward (normal nephrology ward) other than critical care settings.5,14,19 Our higher rate of severe DDIs (30%) partially can be explained by the complexity of care provided in the study setting (MICU), which is known to be a high-risk setting where several factors bond together and potentiate the occurrence of medication-related complications in critically ill patients.13
The clinical pharmacist intervened with prescribers and provided evidence-based DDIs solutions. The majority of these interventions were performed during medical rounds through direct discussion with prescribers, which indicates the close collaboration of the clinical pharmacist in the delivery of drug therapy management. This activity of clinical pharmacists can improve their recognition among healthcare providers and gain a higher acceptance rate by prescribers.23 Moreover, the delivery of interventions during medical rounds can increase the visibility of clinical pharmacists in the healthcare team, which is an important factor for the integration of clinical pharmacists, especially in the newly established clinical pharmacy practice setting.24 Therefore, our high acceptance rate can reflect the recognition of the clinical pharmacist as a source of drug knowledge among our healthcare providers and clinically significant recommendations delivered by the clinical pharmacist.
The nature of clinical pharmacist's interventions depended on the risk-rating categories of identified DDIs. Monitoring the patient's therapy outcome (n = 112, 41%) was the most frequent type of intervention provided to manage the most common category of DDIs (category C, monitor therapy). The change of dosage, formulation, and instructions for drug use were interventions delivered for the management of DDIs with the risk-rating category of D. The order for immediate discontinuation of drugs was the type of intervention mostly provided for the management of contraindicated DDIs (risk-rating category of X). The provision of these interventions at the prescriber level highlights the need for a clear and thorough collaboration of the clinical pharmacist in evaluating, monitoring, and managing pharmacotherapeutic regimens of critically ill patients with CKD. A systematic review reported a wide range of interventions performed by clinical pharmacists in the care of patients with CKD.25 These interventions include modifying drug doses, recommending new pharmacotherapy, interacting with the multidisciplinary team, requesting and monitoring laboratory parameters, and assessing the appropriateness of prescribed medications for identifying MRPs, such as DDIs. Our types of interventions are largely in line with the report of this review study.
The majority of detected DDIs did not induce patient harm, which can be related to the participation of the clinical pharmacist in timely identifying and managing DDIs among study patients. Similarly, the effective participation of clinical pharmacists in reducing the medication-related adverse outcomes has been addressed in critically ill patients receiving continuous renal replacement therapy.12,26 However, 60 (22%) DDIs induced patient harm when the clinical pharmacist's interventions were not implemented by prescribers. One such interaction was between nitroglycerine and sildenafil that induced severe hypotension (patient harm), which required the administration of an inotropic agent to sustain the patient's life. The clinical pharmacist informed the prescriber about this contraindicated DDI and the related harmful consequence. The intervention was initially not implemented by the prescriber. In the next 24 hours, patient harm (severe hypotension) occurred, subsequently, sildenafil was withdrawn, and an inotropic agent was administered to manage severe hypotension. Further, with the improvement in blood pressure, the inotropic agent was also stopped. Clinical pharmacists as an invaluable source of drug knowledge27 can have a medication-related solution role in managing DDIs and prevention of associated patient harm in critically ill patients with CKD.
Our study has several limitations. Due to the unavailability of documented data before this study, we could not evaluate the impact of the clinical pharmacists’ interventions on reducing clinical outcomes, such as LOS, the number of hospital readmission, or financial saving. This study was conducted in one MICU of the tertiary care hospital, and our findings may not be generalized. We did not evaluate the long-term impact of clinical pharmacist's interventions on patient outcomes. The long-term impact of clinical pharmacist's interventions on the improvement of clinical outcomes of patients with CKD admitted in the critical care unit can be an area for future research.
CONCLUSION
This study suggests a common occurrence of clinically significant DDIs in critically ill patients with CKD. The majority of identified DDIs did not induce patient harm as clinical pharmacist's interventions played an essential role in timely identifying and managing DDIs, preventing and alleviating their further adverse outcomes. The provided interventions were well-accepted by prescribers, and the continuation of clinical pharmacy services in the study setting may further improve the appropriate selection of medications and patient safety.
Footnotes
Source of support: Nil
Conflict of interest: None
ORCID
Mina Aghili https://orcid.org/0000-0002-4960-242X
REFERENCES
- 1.Schoolwerth AC, Engelgau MM, Rufo KH, Vinicor F, Hostetter TH, Chianchiano D, et al. Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis. 2006;3:A57. doi: 10.1053/j.ackd.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Kidney Day. Chronic kidney disease 2015. Available from: https://www.worldkidneyday.org/faqs/chronic-kidney-disease/
- 3.Fraser SDS, Roderick PJ, May CR, McIntyre N, McIntyre C, Fluck RJ, et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2015;16:193. doi: 10.1186/s12882-015-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2015;65(10):1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Njeri LW, Ogallo WO, Nyamu DG, Opanga SA, Birichi AR. Medication-related problems among adult chronic kidney disease patients in a sub-Saharan tertiary hospital. Int J Clin Pharm. 2018;40(5):1217–1224. doi: 10.1007/s11096-018-0651-7. [DOI] [PubMed] [Google Scholar]
- 6.Patel HR, Pruchnicki MC, Hall LE. Assessment for chronic kidney disease service in high-risk patients at community health clinics. Ann Pharmacother. 2005;39(1):22–27. doi: 10.1345/aph.1E269. [DOI] [PubMed] [Google Scholar]
- 7.Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM. Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol. 2018;13(7):1085–1095. doi: 10.2215/CJN.00340118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller F, Hann A. Clinical pharmacodynamics: principles of drug response and alterations in kidney disease. Clin J Am Soc Nephrol. 2018;13(9):1413–1420. doi: 10.2215/CJN.10960917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Clinical Pharmacy. The definition of clinical pharmacy. Pharmacotherapy. 2008;28(6):816–817. doi: 10.1592/phco.28.6.816. [DOI] [PubMed] [Google Scholar]
- 10.Grabe DW, Low CL, Bailie GR, Eisele G. Evaluation of drug-related problems in an outpatient hemodialysis unit and the impact of a clinical pharmacist. Clin Nephrol. 1997;47(2):117–121. [PubMed] [Google Scholar]
- 11.Allenet B, Chen C, Romanet T, Vialtel P, Calop J. Assessing a pharmacist-run anaemia educational programme for patients with chronic renal insufficiency. Pharm World Sci. 2007;29(1):7–11. doi: 10.1007/s11096-005-4800-4. [DOI] [PubMed] [Google Scholar]
- 12.Jiang SP, Zhu ZY, Wu XL, Lu XY, Zhang XG, Wu BH. Effectiveness of pharmacist dosing adjustment for critically ill patients receiving continuous renal replacement therapy: a comparative study. Ther Clin Risk Manag. 2014;10:405–412. doi: 10.2147/TCRM.S59187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyen E, Camiré E, Stelfox HT. Clinical review: medication errors in critical care. Crit Care. 2008;12(2):208. doi: 10.1186/cc6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rama M, Viswanathan G, Acharya LD, Attur RP, Reddy PN, Raghavan SV. Assessment of drug-drug interactions among renal failure patients of nephrology ward in a south Indian tertiary care hospital. Indian J Pharm Sci. 2012;74(1):63–68. doi: 10.4103/0250-474X.102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. HERO ID: 658418. [Google Scholar]
- 16.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–S266. [PubMed] [Google Scholar]
- 17.Pharmaceutical Care Network Europe. Classification for drug related problems version 8.02, 2017. Available from: https://www.pcne.org/upload/files/230_PCNE_classification_V8-02.pdf.
- 18.The National Coordinating Council for Medication Error Reporting and Prevention. NCC MERP index for categorizing medication errors. Available from: https://www.nccmerp.org/types-medication-errors. [DOI] [PubMed]
- 19.Marquito AB, Fernandes NM, Colugnati FA, de Paula RB. Identifying potential drug interactions in chronic kidney disease patients. J Bras Nefrol. 2014;36(1):26–34. doi: 10.5935/0101-2800.20140006. [DOI] [PubMed] [Google Scholar]
- 20.Saleem A, Masood I, Khan TM. Clinical relevancy and determinants of potential drug-drug interactions in chronic kidney disease patients: results from a retrospective analysis. Integr Pharm Res Pract. 2017;6:71–77. doi: 10.2147/IPRP.S128816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasipe OJ, Akhideno PE, Nwaiwu O, Adelosoye AA. Assessment of prescribed medications and pattern of distribution for potential drug-drug interactions among chronic kidney disease patients attending the Nephrology Clinic of Lagos University Teaching Hospital in Sub-Saharan West Africa. Clin Pharmacol. 2017;9:125–132. doi: 10.2147/CPAA.S147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason NA, Bakus JL. Strategies for reducing polypharmacy and other medication-related problems in chronic kidney disease. Semin Dial. 2010;23(1):55–61. doi: 10.1111/j.1525-139X.2009.00629.x. [DOI] [PubMed] [Google Scholar]
- 23.McBane SE, Dopp AL, Abe A, Benavides S, Chester EA, Dixon DL, et al. Collaborative drug therapy management and comprehensive medication management-2015. Pharmacotherapy. 2015;35(4):e39–e50. doi: 10.1002/phar.1563. [DOI] [PubMed] [Google Scholar]
- 24.Jorgenson D, Dalton D, Farrell B, Tsuyuki RT, Dolovich L. Guidelines for pharmacists integrating into primary care teams. Can Pharm J(Ott) 2013;146(6):342–352. doi: 10.1177/1715163513504528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Raiisi F, Stewart D, Fernandez-Llimos F, Salgado TM, Mohamed MF, Cunningham S. Clinical pharmacy practice in the care of Chronic Kidney Disease patients: a systematic review. Int J Clin Pharm. 2019;41(3):630–666. doi: 10.1007/s11096-019-00816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang SP, Xu YY, Ping-Yang, Wu WF, Zhang XG, Lu XY, et al. Improving antimicrobial dosing in critically ill patients receiving continuous venovenous hemofiltration and the effect of pharmacist dosing adjustment. Eur J Intern Med. 2014;25:930–935. doi: 10.1016/j.ejim.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Ghaibi S, Ipema H, Gabay M. ASHP guidelines on the pharmacist's role in providing drug information. Am J Health Syst Pharm. 2015;72(7):573–577. doi: 10.2146/sp150002. [DOI] [PubMed] [Google Scholar]