Abstract
Objective
The objective of this study was to measure humoral responses after SARS-CoV-2 vaccination in MS patients treated with ocrelizumab (OCR) compared to MS patients without disease modifying therapies (DMTs) in relation to timing of vaccination and B-cell count.
Methods
OCR treated patients were divided into an early and a late group (cut-off time 12 weeks between infusion and first vaccination). Patients were vaccinated with mRNA-1273 (Moderna). B-cells were measured at baseline (time of first vaccination) and SARS-CoV-2 antibodies were measured at baseline, day 28, 42, 52 and 70.
Results
87 patients were included (62 OCR patients, 29 patients without DMTs). At day 70, seroconversion occurred in 39.3% of OCR patients compared to 100% of MS patients without DMTs. In OCR patients, seroconversion varied between 26% (early group) to 50% (late group) and between 27% (low B-cells) to 56% (at least 1 detectable B-cell/µL).
Conclusions
Low B-cell counts prior to vaccination and shorter time between OCR infusion and vaccination may negatively influence humoral response but does not preclude seroconversion. We advise OCR treated patients to get their first vaccination as soon as possible. In case of an additional booster vaccination, timing of vaccination based on B-cell count and time after last infusion may be considered.
Keywords: MS, multiple sclerosis; OCR, ocrelizumab; DMT, disease modifying therapy
Keywords: Multiple sclerosis, SARS-CoV-2, COVID-19, Ocrelizumab, Antibodies
1. Introduction
In patients with multiple sclerosis (MS), ocrelizumab (OCR) is a risk factor for a severe course of COVID-19 (Simpson-Yap et al., 2021). Therefore, inducing protective immunity in this patient group is of great importance. Unfortunately, OCR negatively influences the humoral response after vaccination, as previously shown for pneumococcal and tetanus vaccination (Bar-Or et al., 2020). It has been suggested that the humoral response after SARS-CoV-2 vaccination can improve by adjusting timing of vaccination to OCR infusion or repopulation of B-cells (Otero-Romero et al., 2021). In the updated advice (October 13th 2021) of the MS International Federation, it is advised to schedule SARS-CoV-2 vaccination minimally 12 weeks after the last OCR infusion to optimize vaccination response. However, data supporting this advice are currently lacking.
The objective of this study was to longitudinally investigate the humoral response after SARS-CoV-2 vaccination in OCR treated MS patients and MS patients without disease modifying therapies (DMTs). We aimed to assess the influence of timing of vaccination after OCR infusion and B-cell count on the humoral immune response.
2. Methods
This was a substudy of a prospective multicenter multi-arm cohort study on SARS-CoV-2 vaccination in patients with various immune mediated inflammatory diseases (T2B!; Trial NL8900; Dutch Trial register). All MS participants in this study were included at the MS Center Amsterdam. The medical ethical committee of the Amsterdam UMC, location AMC (2020.194) approved the study and participants provided written informed consent. This study was supported by ZonMw (The Netherlands Organization for Health Research and Development). The sponsor had no role in the design, analyses or reporting of the study.
For this substudy, participants with a current diagnosis of MS using OCR and patients without DMTs were included. Patients previously infected with SARS-CoV-2 or SARS-CoV-2 antibodies at baseline (day of first vaccination, just prior to vaccination) were excluded. OCR treatment was given at 6-month intervals as part of routine medical care. OCR patients were selected based on the interval to their last infusion and formed an early group (vaccinated < 12 weeks after OCR infusion) and a late group (vaccinated ≥12 weeks after the OCR infusion). All participants were vaccinated by the study team with mRNA-1273 (Moderna) with the second vaccination administered after 42 days following national protocols.
Clinical data were retrieved from the medical files. To calculate the number of OCR infusions, the first two 300 mg infusions were counted as one. The SARS-CoV-2 antibody response was evaluated at baseline, day 28 after first vaccination, day 42 (just prior to second vaccination), day 52 (10 days after second vaccination) and day 70 (day 28 after second vaccination). SARS-CoV-2 antibodies against RBD were measured at Sanquin using an IgG specific ELISA (Steenhuis et al., 2021). The cut-off for a positive SARS-CoV-2 IgG anti-RDB response was 4.0 arbitrary units per ml (AU/ml, Sanquin units), lower limit of quantification was 0.1 AU/ml. At baseline, a qualitative anti-RBD bridging assay was also used as this assay has better sensitivity to detect low levels of antibodies (Vogelzang et al., 2020). Fresh whole blood was drawn at baseline (prior to vaccination) for measuring CD19+ B-cells with a highly sensitive assay (Koutsakos et al., 2021). We used a cut-off of 1 B-cell/µL to define low/absent B-cell counts as this is the lower limit of quantification in most routine assays used in clinical care.
Proportions are compared between groups using the chi-square test and are presented with 95% confidence intervals. Differences in antibody titers are compared using the Mann-Whitney U test. The correlation between the B-cell count, time since last OCR infusion, cumulative number of OCR infusions prior to vaccination and SARS-CoV-2 titers at day 70 was calculated using Spearman's rho.
3. Results
A total of 89 participants were screened, of whom two patients in the no DMT group were excluded because of SARS-CoV-2 antibodies at baseline. Sixty-two patients were treated with ocrelizumab and 25 patients were not treated with DMTs. Patient characteristics are described in Table 1 .
Table 1.
Baseline characteristics.
| No DMT (n = 25) | Ocrelizumab (n = 62) | Overall (n = 87) | |
|---|---|---|---|
| Age, years | 55.3 (10.3) | 43.8 (10.0) | 47.0 (11.4) |
| Sex, female | 18 (72.0) | 44 (71.0) | 61 (70.9) |
| MS type | |||
| PPMS | 4 (16.0) | 9 (14.5) | 13 (14.0) |
| RRMS | 15 (60.0) | 53 (85.5) | 68 (79.1) |
| SPMS | 6 (24.0) | 0 (0) | 6 (6.98) |
| CD19+ B- cells/µL at baseline | 133 (50–912) | 1 (0–25) | 2 (0–912) |
Baseline characteristics. Values are presented as mean age (±standard deviation), numbers (percentages) and median for B-cell count (ranges). There was one B cell outlier with a newly diagnosed clonal B cell lymphocytosis (912 B cells/µL). DMT: disease modifying therapy, MS: multiple sclerosis, PPMS: primary progressive multiple sclerosis, RRMS: relapsing remitting multiple sclerosis, SPMS: secondary progressive multiple sclerosis.
3.1. Seroconversion after vaccination
At day 70 after first vaccination, 39.3% (95% CI: 26.5–52.1) of OCR treated patients seroconverted versus 100% of the patients without DMT (p < 0.01). Serological responses in OCR treated patients were incremental until day 70. OCR treated patients in the late group (N = 33) seroconverted in 50% (95 CI: 32.1–67.9) versus 26% (95 CI: 9.9–44.0) of patients in the early group (N = 29; p:0.13). OCR treated patients with at least 1 detectable B-cell/µL at the day of first vaccination (N = 29) seroconverted in 56% (95 CI: 36.5–75.5) versus 27% (95 CI: 10.8–42.5) of patients absent/low B-cells (N = 33; p:0.05). Seroconversion in relation to number of B-cells and timing of vaccination is presented in Table 2 .
Table 2.
SARS-CoV-2 seroconversion.
| Ocrelizumab (n = 62) | All OCR (n = 62) | No DMT (n = 25) | ||||
|---|---|---|---|---|---|---|
| CD19+ B-cells <1/µL (n = 33) | CD19+ B-cells ≥1/µL (n = 29) | |||||
| Early group (<12 weeks) (n = 20) | Late group (>12 weeks) (n = 13) | Early group (<12 weeks) (n = 9) | Late group (>12 weeks) (n = 20) | |||
| Baseline | ||||||
| negative | 20 (100) | 13 (100) | 9 (100) | 20 (100) | 62 (100) | 25 (100) |
| positive | 0 | 0 | 0 | 0 | 0 | 0 |
| missing | 0 | 0 | 0 | 0 | 0 | 0 |
| Day 28 | ||||||
| negative | 15 (93.8) | 9 (100) | 7 (100) | 11 (68.8) | 42 (87.5) | 1 (4.2) |
| positive | 1 (6.2) | 0 | 0 | 5 (31.3) | 6 (12.5) | 23 (95.8) |
| missing | 4 | 4 | 2 | 4 | 14 | 1 |
| Day 42 | ||||||
| negative | 18 (90.0) | 13 (100) | 9 (100) | 13 (68.4) | 53 (86.9) | 1 (4.2) |
| positive | 2 (10.0) | 0 | 0 | 6 (31.6) | 8 (13.1) | 23 (95.8) |
| missing | 0 | 0 | 0 | 1 | 1 | 1 |
| Day 52 | ||||||
| negative | 17 (89.5) | 13 (100) | 8 (88.9) | 11 (55.0) | 49 (80.3) | 1 (4.3) |
| positive | 2 (10.5) | 0 | 1 (11.1) | 9 (45.0) | 12 (19.7) | 22 (95.7) |
| missing | 1 | 0 | 0 | 0 | 1 | 2 |
| Day 70 | ||||||
| negative | 15 (78.9) | 8 (66.7) | 4 (57.1) | 7 (38.9) | 34 (60.7) | 0 |
| positive | 4 (21.1) | 4 (33.3) | 3 (42.9) | 11 (61.1) | 22 (39.3) | 23 (100) |
| missing | 1 | 1 | 2 | 2 | 6 | 2 |
SARS-CoV-2 antibody response at baseline and 4 timepoints after vaccination. Days are indicated as days after the first vaccination. Day 42 was time of the second vaccination, day 70 is 28 days after the second vaccination. The cut-off for CD19+ B-cells was set at 1 cell/µL. Early and late groups were defined as less or more than 12 weeks between the last OCR infusion and the first vaccination, respectively. Values are presented as numbers (percentages). OCR: ocrelizumab, DMT: disease modifying therapy.
3.2. Antibody titers after vaccination
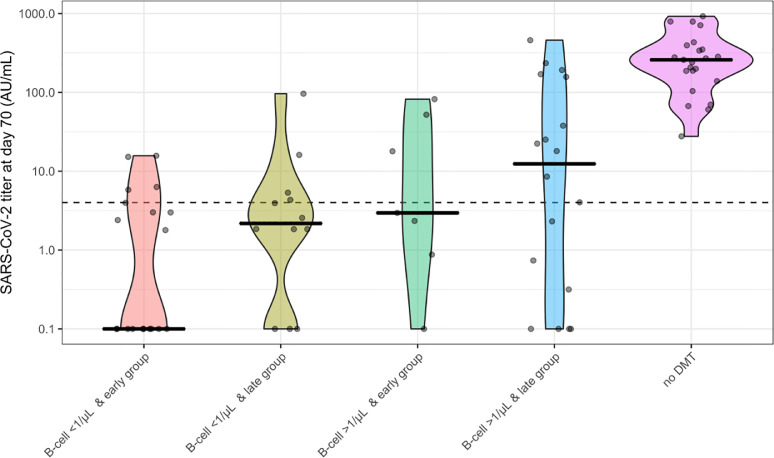
SARS-CoV-2 antibody titers were lower in OCR patients who seroconverted than in controls without DMTs (median 20.2 AU/mL versus 259 AU/mL; p < 0.01, Fig. 1 ).
Fig. 1.
SARS-CoV-2 antibody titers in relation to B cells and timing of vaccination.
SARS-CoV-2 antibody titers 70 days after first vaccination (28 days after second vaccination) in OCR treated patients and patients without DMTs. Dots indicate antibody titers for individual participants. The black line indicates the median per group. The dotted line indicates the cut-off for seroconversion (> 4 AU/mL). OCR treated patients are divided into four groups regarding the presence or absence of CD19+ B-cells and timing of vaccination (early or late). B-cell cut-off is set at 1 cell/µL. Early and late groups were defined as less or more than 12 weeks between the last OCR infusion and the first vaccination, respectively. DMT: disease modifying therapy.
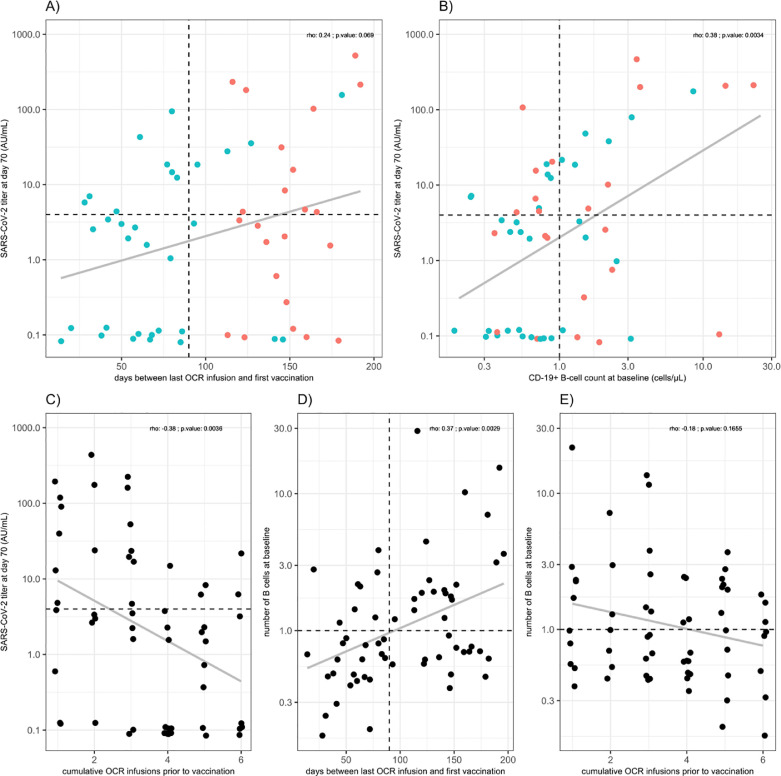
The number of B-cells and the number of cumulative OCR infusions prior to first vaccination weakly correlated with SARS-CoV-2 antibody titers at day 70 (rho=0.38; p < 0.01 and rho=−0.38; p < 0.01, respectively; Fig. 2 ; panel B&C). We observed a trend between the timing of infusion (days between last infusion and first vaccination) and SARS-CoV-2 antibody titer at day 70 (rho=0.24; p:0.07; Fig. 2; panel A). The number of B-cells were correlated with the timing of infusion (rho: 0.37; p < 0.01) but not with the number or OCR infusions (rho=−0.18; p:0.17; Fig. 2; panel D&E).
Fig. 2.
SARS-CoV-2 antibody titers in OCR patients.
Days between last OCR infusion and vaccination (A), correlation plots of SARS-CoV-2 titers in relation to B-cell count (B), cumulative number of prior OCR infusions (C) and B-cell count in relation to interval between last OCR infusion and vaccination (D) and cumulative number of prior OCR infusions (E). Red dots in panels (A) and (B) indicate participants who received their next OCR infusion after first vaccination prior to day 70; participants with blue dots received their next OCR infusion after day 70. The dotted horizontal line indicates the cut-off for seroconversion (> 4 AU/mL). The dotted vertical line in panel B indicates the cut-off for B-cells (1 cell/µL). The dotted vertical line in panel A indicates the cut-off for patients vaccinated early or late after their last OCR infusion. In each panel the correlation coefficient is shown (presented as Spearman's rho with associated p-value) and the gray line which represent a Pearson trend line for visualization. OCR: ocrelizumab.
4. Discussion
In this study, we found a clear difference in the humoral response after SARS-CoV-2 vaccination between OCR treated MS patients and MS patients without DMTs, which is in agreement with other reports (Achiron et al., 2021; Bigaut et al., 2021; Louapre et al., 2021). Although T cell immunity may largely be intact in OCR treated patients after SARS-CoV-2 vaccination (Apostolidis et al., 2021), anti-CD20 therapies are a risk factor for severe COVID-19 in fully vaccinated patients (Brosh-Nissimov et al., 2021), underlining the importance of a humoral response to efficiently counter severe COVID-19.
Our study shows that seroconversion at day 70 in OCR treated patients varied between 21% in the early group with B-cells <1 cell/µL to 61% in patients in the late group with ≥1 B-cell/µL. The significant correlation between the B-cell counts with antibody titer and the trend between time from OCR infusion to vaccination further support the finding that B-cell count and timing influences the humoral response. However, we consider them both as suboptimal predictors, as they neither preclude nor guarantee a humoral response.
In a recent study, OCR treated MS patients showed robust T cell responses after SARS-CoV-2 vaccination (Apostolidis et al., 2021). Given the modest influence of timing of vaccination on the humoral response in our study, we would advise OCR treated patients to get vaccinated as early as possible to induce protective immunity (including T cell mediated immunity) as soon as possible. In regards of a potential additional booster vaccination, which would mainly be directed to increase humoral responses, B-cell count and possibly the timing in relation to the last OCR infusion could be taken into account.
Strengths of this study are the prospective design and longitudinal assessment of humoral responses. Limitations of our study were the sample size that led to broad confidence intervals for some estimates. Finally, we did not address T-cell immunity in this analysis.
5. Conclusions
Our data show that B-cell count and timing of SARS-CoV-2 vaccination after OCR infusion weakly influences the humoral response after vaccination. We advise a first vaccination to be offered as soon as possible, rather than waiting until 12 weeks after infusion. A possible additional booster vaccination could be guided by B-cell count and timing versus last OCR infusion to potentially increase the humoral response.
Funding
This study was funded by ZonMw (The Netherlands Organization for Health Research and Development) with grant number 10,430,072,010,007. The sponsor had no role in the design, analyses or reporting of the study.
Declaration of Competing Interest
Prof. dr. J. Killestein reported speaking and consulting relationships with Biogen, Genzyme, Merck, Novartis, Roche, Sanofi and TEVA. Amsterdam UMC, location VUmc, MS Center Amsterdam has received financial support for research activities from Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi and TEVA.
Dr. M. Löwenberg has served as speaker and/or principal investigator for Abbvie, Alimentiv, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, Tramedico
Dr. F. Eftimov, dr. G.J Wolbink and Prof. dr. T.W. Kuijpers reported a grant from ZonMW for COVID research in patients with auto-immune diseases.
No other disclosures were reported.
Acknowledgments
None.
References
- Achiron A., Mandel M., Dreyer-Alster S., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med.. 2021:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fabacher T., et al. Impact of disease-modifying treatments of multiple sclerosis on anti-SARS-CoV-2 antibodies: an observational study. Neurol. Neuroimmunol. Neuroinflamm. 2021;8 doi: 10.1212/NXI.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh-Nissimov T., Orenbuch-Harroch E., Chowers M., et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin. Microbiol. Infect. 2021:1652–1657. doi: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsakos M., Rowntree L.C., Hensen L., et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Ibrahim M., Maillart E., et al. Anti-CD20 therapies decrease humoral immune response to SARS-CoV-2 in patients with multiple sclerosis or neuromyelitis optica spectrum disorders. J. Neurol. Neurosurg. Psychiatry. 2021 doi: 10.1136/jnnp-2021-326904. [DOI] [PubMed] [Google Scholar]
- Otero-Romero S., Ascherio A., Lebrun-Frenay C. Vaccinations in multiple sclerosis patients receiving disease-modifying drugs. Curr. Opin. Neurol. 2021;34:322–328. doi: 10.1097/WCO.0000000000000929. [DOI] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021 doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhuis M., van Mierlo G., Derksen N.I., et al. Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors. Clin. Transl. Immunol. 2021;10:e1285. doi: 10.1002/cti2.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang E.H., Loeff F.C., Derksen N.I.L., et al. Development of a SARS-cov-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J. Immunol. 2020:3491–3499. doi: 10.4049/jimmunol.2000767. [DOI] [PubMed] [Google Scholar]