Abstract
Rationale & Objective:
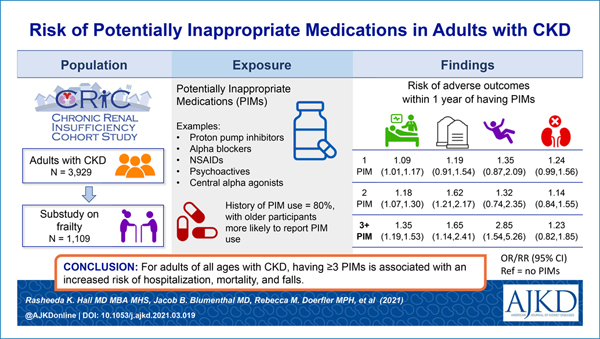
Adults with chronic kidney disease (CKD) may be at increased risk of adverse effects from use of potentially inappropriate medications (PIMs). Our objective was to assess whether PIM exposure has an independent association with CKD progression, hospitalizations, mortality, or falls.
Study Design:
Retrospective observational study.
Setting & Participants:
Chronic Renal Insufficiency Cohort (CRIC) study; 3,929 adults with CKD enrolled 2003–2008 and followed prospectively until December 2011.
Exposure:
PIM exposure was defined as prescriptions for any medications to be avoided in older adults as defined by the 2015 American Geriatrics Society Beers Criteria.
Outcome:
Hospitalization count, death, a composite kidney disease end point of CKD progression or initiation of kidney replacement therapy (KRT), KRT, and fall events assessed 1 year after PIM exposure.
Analytical Approach:
Logistic regression and Poisson regression to estimate the associations of PIM exposure with each outcome.
Results:
The most commonly prescribed PIMs were proton pump inhibitors and α-blockers. In unadjusted models, any PIM exposure (compared to none) was associated with hospitalizations, death, and fall events. After adjustment, exposure to 1, 2, or ≥3 PIMs had a graded association with a higher hospitalization rate (rate ratios of 1.09 [95% CI, 1.01–1.17], 1.18 [95% CI, 1.07–1.30], and 1.35 [95% CI, 1.19–1.53], respectively) and higher odds of mortality (odds ratios of 1.19 [95% CI, 0.91–1.54], 1.62 [95% CI, 1.21–2.17], and 1.65 [95% CI, 1.14–2.41], respectively). In a cohort subset reporting falls (n = 1,109), prescriptions for ≥3 PIMs were associated with an increased risk of falls (adjusted OR, 2.85 [95% CI, 1.54–5.26]). PIMs were not associated with CKD progression or KRT. Age did not modify the association between PIM count and outcomes.
Limitations:
Measurement bias; confounding by indication.
Conclusions:
Adults of any age with CKD who are prescribed PIMs have an increased risk of hospitalization, mortality, and falls with the greatest risk occurring after more than 1 PIM prescription.
Graphical Abstract
Adults with chronic kidney disease (CKD) of all ages have a greater risk of geriatric complications compared with an age-matched general population.1,2 Geriatric conditions known to coexist with CKD include frailty, cognitive dysfunction, and impaired physical function,3–5 and these geriatric conditions likely contribute to the increased risk of both mortality and hospitalizations in adults with CKD.6,7 Notably, risk of mortality and hospitalizations are both increased among older adults taking American Geriatrics Society (AGS) Beers Criteria potentially inappropriate medications (PIMs), which are medications that carry more risk of harm than benefit in older adults.8–10 Although the AGS Beers Criteria have offered guidance for prescribing for older adults, the frequency of use and association of these medications with adverse outcomes in the adult CKD population has not been well studied.
The altered pharmacokinetics of many medications are likely to be compounded in older patients in CKD.11,12 Adults with CKD also tend to have polypharmacy, and the increased prevalence of conditions such as cardiovascular disease (CVD) and hypertension results in even higher rates of PIM use.13 Although some studies have shown PIMs are common in older adults with CKD,14,15 it is paramount to understand whether PIMs independently contribute to adverse outcomes in the CKD population. With that information, providers will have additional guidance for PIM prescribing decisions. We examined data from participants in the Chronic Renal Insufficiency Cohort (CRIC) study to determine the prevalence of PIMs and the extent of an independent association between PIMs and adverse outcomes, including death, hospitalization, falls, and progression of kidney disease in participants of any age.
Methods
Study Design and Population
The CRIC study commenced in 2003 with phase I enrollment completed in 2008, and continued follow-up observation still ongoing. The cohort study design was described previously elsewhere.16,17 CRIC participant eligibility included age 21 to 74 years old with age-specific estimated glomerular filtration rate (eGFR) eligibility criteria of 20–70 mL/min/1.73 m2, from 7 US centers with 13 clinical sites, and with institutional review board approval at each site. All participants provided written informed consent, and the research was conducted in accordance with the principles of the Declaration of Helsinki.
Briefly, CRIC participants underwent annual in-center visits during which they provided demographic information, medical history and status update, vital signs, blood and urine samples, and other survey-based information. Using this cohort, we identified participants with medication records as of December 1, 2011 (n = 3,930). Among those, we identified participants who had annual study visit data to include in this longitudinal study to estimate risks of hospitalization, death, and kidney disease outcomes associated with exposure to PIMs (n = 3,929). In a subcohort with available data (n = 1,109) derived from an ancillary study of vitamin D and frailty with identical inclusion and exclusion criteria,4 we also estimated risk of falls and its association with exposure to PIMs.
Medication Ascertainment
Since the study’s inception, the coordinators have recorded the CRIC participants’ prescription and over-the-counter medications, herbal and dietary supplements, and vitamins from 30 days preceding each study visit. To reduce recall bias, participants were asked to maintain a medication list or bring the medications with them to the visits. The drug name, frequency, total daily dosage, dosage units, and administration route were documented. Individual medications were identified using the First Databank dictionary for common medications and supplements available on the market.
Identification of Potentially Inappropriate Medications
We identified PIMs designated in the 2015 AGS Beers Criteria as medications to avoid for many or most older adults (Table S1).18 These medications were selected because the majority are not primarily renally cleared, and there is limited evidence on adverse outcomes of these medications in general adults with CKD. These PIMs include the following therapeutic classes: anticholinergics, antithrombotics, anti-infectives, cardiovascular medications, central nervous system medications, endocrine, gastrointestinal, genitourinary, and pain medications. PIM exposure was updated at each annual visit. As a result, a participant with PIM exposure at one visit was not assumed to have PIM exposure at subsequent annual visits.
Outcomes
We examined 5 separate outcomes within a year after PIM exposure: (1) number of all-cause hospitalizations; (2) death, comprising all deaths, irrespective of initiation of kidney replacement therapy (KRT); (3) KRT; (4) a composite kidney disease outcome of KRT and halving of eGFR from baseline ascertained from laboratory results obtained at the next annual visit; and (5) occurrence of a fall event (in a subcohort). Hospitalizations and falls (only in the subcohort) from the prior year were ascertained during interviews at annual study visits.
In the subcohort, annual visits included a separate assessment for falls during which a fall was defined as falling on the ground or at some other level, excluding sports-related falls in the prior 3 months. Death was ascertained through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Mortality Master File.19 GFR was estimated annually using the CRIC GFR estimating equation.20 KRT was defined as initiation of dialysis or kidney transplantation as ascertained by CRIC study personnel and cross-reference to the US Renal Data System.21 Participants were followed until study withdrawal, loss to follow-up, or the end of the follow-up period (December 2011), whichever occurred first.
Covariates
We identified the following covariates ascertained at baseline and updated at annual study visits: age, sex, race/ethnicity, participant’s clinical center (geographic site of enrollment), eGFR, body mass index (BMI), comorbidities—diabetes (defined as self-reported or use of diabetes medications), cardiovascular disease (defined as self-reported coronary disease, prior revascularization, heart failure, stroke, or peripheral vascular disease), hypertension (defined as blood pressure [BP] of >140/90 or self-report of antihypertensive use), arthritis (defined as self-reported rheumatoid arthritis)—history of nephrology care, and medication count (prescription and nonprescription medications within the prior 30 days).
Statistical Methods
Baseline demographics and clinical characteristics were reported as proportions for categorical variables and as mean with standard deviation (SD) for continuous variables, stratified by PIM exposure during the entire follow-up period. We counted the number of PIMs reported at each clinic visit. We also estimated the prevalence of each PIM category as the number of study visits with PIM medicines reported from each PIM category divided by the total number of visits and compared between 3 age groups: <65, 65–70, and >70 years, based on the age at the time of annual visit. The >70 age group was delineated from ages 65–70 to examine the characteristics and PIM use of the cohort’s oldest older adults.
Logistic regression was used to estimate the association of number of PIMs (1, 2, and 3 or more vs no PIM exposure) with each of the following outcomes that had occurred within 1 year of an annual study visit at which the PIM exposure was reported or measured: death, fall event, KRT, and composite of KRT and halving of the baseline eGFR. Robust variance estimation was used to account for repeated measures per individual.22 Similarly, Poisson regression with robust variance estimation was used to estimate the association of PIM exposure with number of hospitalizations within 1 year of PIM exposure.
To control for measured confounding, all models were sequentially adjusted: model 1 (demographics, participant site), model 2 (model 1 covariates plus eGFR, BMI, diabetes, CVD, hypertension, arthritis, and prior nephrology care), and model 3 (model 2 covariates plus medication count). All covariates in the model were updated over time.
To examine whether the association of PIM exposure with outcomes may be modified by age or medication count, we performed separate tests for interaction between PIM exposure and age and PIM exposure and medication count (dichotomized at the median medication count) using variables from each study visit. Additional analyses included: (1) separated models in which the cohort was stratified by age group (<65 and ≥65) and each model was repeated to examine the risk associated with any PIM exposure (compared with none); (2) sensitivity analyses to examine if modeling results were sustained when the number of hospitalizations in the prior year was added as a covariate to model 3 or in a subgroup of visits at which eGFR < 45 mL/min/1.73 m2; and (3) repeated models of mortality and hospitalizations to assess the risk associated with selected individual PIM classes (compared with not having the specific PIM) that were both frequently used and highly prevalent among participants who died.
In addition, we reported the most prevalent PIM classes, stratified by age categories among those who died during the follow-up period. Participants with missing covariates were excluded from multivariable analyses. Among covariates, there were low rates of missingness, with the highest detected for BMI (3.6%), arthritis (2.6%), and hypertension (0.2%) across all visits. All statistical analyses were performed with SAS, version 9.4.
Results
Cohort Characteristics
Of 3,930 participants, 3,929 met criteria for inclusion in our analytic cohort (Fig S1). The mean age in our analytic cohort was 58 years, and the mean age for the subcohort (for falls outcome) was 63 years.4 In the larger analytic cohort, 2,789 (71.0%), 741 (18.9%), and 399 (10.2%) were aged <65, 65–70, and >70 years, respectively.
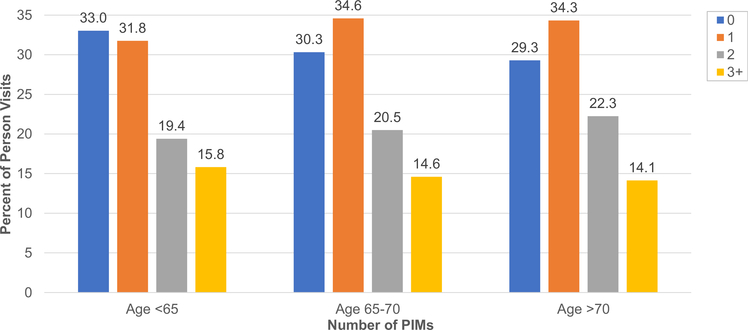
The majority of the cohort, 80% (n = 3,151), had a history of PIM use (mean PIM count was 1.2 ± 1.1 [SD]). Those with history of PIM use (vs without) were older, were more likely to be female, Black race, have a BMI >30 kg/m2, have cardiovascular disease, have arthritis, and take more medications, and they had lower urinary protein-creatinine ratios versus participants without (Table S2). The proportion with a history of PIM use increased by age group: for those aged <65, 65–70, and >70 years they were 78.9% (n = 2,200), 82.1% (n = 608), and 85.9% (n = 343), respectively (Fig 1). With each older age group, those with (vs without) PIM exposure had similarly greater comorbidity burden, eGFR reduction, and receipt of nephrology care (Table 1).
Figure 1.
Proportion of potentially inappropriate medications (PIM) by age group. The percent represents the number of clinic visits during which PIMs were reported divided by total number of clinic visits.
Table 1.
Baseline Cohort Characteristics Based on Ever Taking a PIM During Observation Period Overall and by Age Group
| Characteristic | Overall (N = 3,929) |
Age < 65 y |
Age 65–70 y |
Age > 70 y |
|||
|---|---|---|---|---|---|---|---|
| Ever (n = 2,200) |
Never (n = 589) |
Ever (n = 608) |
Never (n = 133) |
Ever (n = 343) |
Never (n = 56) |
||
|
| |||||||
| Demographics | |||||||
|
| |||||||
| Age (y) | 57.7 ± 10.9 | 53.8 ± 8.9 | 50.3 ± 10.8 | 67.5 ± 1.7 | 67.4 ± 1.8 | 72.5 ± 1.1 | 72.5 ± 1.2 |
|
| |||||||
| Female sex | 1,775 (45.2%) | 1,041 (47.3%) | 220 (37.4%) | 285 (46.9%) | 61 (45.9%) | 1 40 (40.8%) | 28 (50.0%) |
|
| |||||||
| Race/ethnicity | |||||||
|
| |||||||
| Non-Hispanic Black | 1,646 (41.9%) | 973 (44.2%) | 208 (35.3%) | 264 (43.4%) | 58 (43.6%) | 119 (34.7%) | 24 (42.9%) |
|
| |||||||
| Non-Hispanic White | 1,635 (41.6%) | 889 (40.4%) | 234 (39.7%) | 263 (43.3%) | 42 (31.6%) | 181 (52.8%) | 26 (46.4%) |
|
| |||||||
| Hispanic | 495 (12.6%) | 253 (11.5%) | 116 (19.7%) | 60 (9.9%) | 26 (19.5%) | 36 (10.5%) | 4 (7.1%) |
|
| |||||||
| Other | 153 (3.9%) | 85 (3.9%) | 31 (5.3%) | 21 (3.5%) | 7 (5.3%) | 7 (2.0%) | 2 (3.6%) |
|
| |||||||
| Clinical Characteristics | |||||||
|
| |||||||
| eGFR, mL/min/1.73 m2 | 44.8 ± 16.8 | 474 ± 1 7.9 | 45.87 ± 1 7.5 | 39.9 ± 12.7 | 38.8 ± 11.3 | 38.5 ± 11.1 | 38.2 ± 11.8 |
|
| |||||||
| CKD at baseline | |||||||
|
| |||||||
| Stage 1–2 | 694 (177%) | 522 (23.7%) | 123 (20.9%) | 35 (5.8%) | 2 (1.5%) | 1 2 (3.5%) | 0 (0.0) |
|
| |||||||
| Stage 3a | 1,090 (27.7%) | 614 (27.9%) | 162 (27.5%) | 1 75 (28.8%) | 36 (27.1%) | 87 (25.4%) | 1 6 (28.6%) |
|
| |||||||
| Stage 3b | 1,338 (34.1%) | 653 (29.7%) | 182 (30.9%) | 252 (41.4%) | 66 (49.6%) | 161 (46.9%) | 24 (42.9%) |
|
| |||||||
| Stage 4–5 | 807 (20.5%) | 411 (18.7%) | 122 (20.7%) | 146 (24%) | 29 (21.8%) | 83 (24.2%) | 1 6 (28.6%) |
|
| |||||||
| UPCR, (mg/dL)/(mg/dL) | 1.0 ± 2.4 | 1.1 ± 2.6 | 1.30 ± 2.5 | 0.6 ± 1.5 | 0.9 ± 2.2 | 0.6 ± 1.6 | 0.5 ± 1.0 |
|
| |||||||
| BMI category | |||||||
|
| |||||||
| >30 kg/m2 | 2,174 (55.5%) | 1,305 (59.5%) | 268 (45.5%) | 351 (57.8%) | 62 (46.6%) | 1 68 (49.4%) | 20 (35.7%) |
|
| |||||||
| 25–30 kg/m2 | 1,121 (28.6%) | 553 (25.2%) | 194 (32.9%) | 174 (28.7%) | 50 (37.6%) | 126 (37.1 %) | 24 (42.9%) |
|
| |||||||
| <25 kg/m2 | 623 (15.9%) | 335 (15.3%) | 127 (21.6%) | 82 (13.5%) | 21 (15.8%) | 46 (13.5%) | 12 (21.4%) |
|
| |||||||
| Diabetes | 1,908 (48.6%) | 1,061 (48.2%) | 254 (43.1%) | 325 (53.5%) | 70 (52.6%) | 1 70 (49.6%) | 28 (50%) |
|
| |||||||
| CVD | 1,315 (33.5%) | 678 (30.8%) | 133 (22.6%) | 273 (44.9%) | 44 (33.1%) | 165 (48.1 %) | 22 (39.3%) |
|
| |||||||
| Hypertension | 3,390 (86.3%) | 1,861 (84.6%) | 477 (81 %) | 561 (92.3%) | 120 (90.2%) | 319 (93.0%) | 52 (92.9%) |
|
| |||||||
| Arthritis | 492 (13.2%) | 264 (12.6%) | 39 (6.8%) | 105 (18.5%) | 13 (10.4%) | 63 (19.6%) | 8 (16.3%) |
|
| |||||||
| No. of medications | 8.9 ± 4.5 | 9.2 ± 4.7 | 5.9 ± 3.2 | 10.2 ± 4.4 | 7.5 ± 3.1 | 10.3 ± 3.9 | 7.9 ± 3.7 |
|
| |||||||
| No. of PI Ms | 0.9 ± 1.1 | 1.1 ± 1.1 | 0.0 ± 0.0 | 1.1 ± 1.0 | 0.0 ± 0.0 | 1.3 ± 1.0 | 0.0 ± 0.0 |
|
| |||||||
| Nephrology care | 2,596 (66.1%) | 1,423 (64.7%) | 411 (69.8%) | 417 (68.6%) | 77 (57.9%) | 231 (67.3%) | 37 (66.1%) |
Data for continuous variables given as mean ± SD and for categorical variables as count (%). Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease, eGFR, estimated glomerular filtration rate; PIMs, potentially inappropriate medications; UPCR, urinary protein-creatinine ratio.
PIM Prevalence
In descending order, the 3 most common PIMs in the cohort were proton pump inhibitors (PPIs), α-blockers, and nonsteroidal anti-inflammatory drugs (NSAIDs) (Table 2). The participants older than 70 years had the highest rates of PPIs and α-blockers, digoxin, and non-benzodiazepine benzodiazepine receptor agonist hypnotics (or Z-drugs). Meanwhile those in younger age groups had higher rates of NSAID, anticholinergic, antidepressant, muscle relaxant, and estrogen use.
Table 2.
Number and Rate of PIM Classes in Total Cohort and by Age Group
| Age |
|||||
|---|---|---|---|---|---|
| Medication | Total Cohort | <65 ya | 65–70 y | >70 y | P |
|
| |||||
| Proton pump inhibitor | 5,147 (21.2) | 2,750 (1 7.9) | 1,271 (23.2) | 1,126 (24.8) | <0.001 |
|
| |||||
| α-Blocker | 2,471 (10.2) | 937 (6.1) | 715 (13.0) | 819 (18.0) | <0.001 |
|
| |||||
| NSAIDs | 2,386 (9.8) | 1,540 (10.0) | 524 (9.5) | 322 (7.1) | <0.001 |
|
| |||||
| Anticholinergic | 2,048 (8.4) | 1,296 (8.4) | 424 (7.7) | 328 (7.2) | 0.03 |
|
| |||||
| Central α agonist | 1,844 (7.6) | 1,152 (7.5) | 390 (7.1) | 302 (6.7) | 0.2 |
|
| |||||
| Benzodiazepine | 1,473 (6.1) | 883 (5.7) | 307 (5.6) | 283 (6.2) | 0.4 |
|
| |||||
| Antidepressant | 1,351 (5.6) | 863 (5.6) | 271 (4.9) | 217 (4.8) | 0.04 |
|
| |||||
| Muscle relaxant | 770 (3.2) | 531 (3.4) | 160 (2.9) | 79 (1.7) | <0.001 |
|
| |||||
| Estrogen | 722 (3.0) | 498 (3.2) | 136 (2.5) | 88 (1.9) | <0.001 |
|
| |||||
| Digoxin | 622 (2.6) | 286 (1.9) | 180 (3.3) | 156 (3.4) | <0.001 |
|
| |||||
| Nonbenzodiazepine | 553 (2.3) | 322 (2.1) | 83 (1.5) | 148 (3.3) | <0.001 |
Data reported as number of visits (rate). Rate defined as number of PIMs per 100 person-years. Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; PIM, potentially inappropriate medication.
Age group is based on age at reported medication use.
PIMs and Adverse Outcomes
In unadjusted models, compared with those with no PIMs, exposure to 1, 2, and ≥3 PIMs had a graded association with hospitalizations, death, and fall events (Table 3). In fully adjusted models, this graded association of PIM exposure with hospitalization and death within a year persisted (Table 3). In the vitamin D subcohort, the increased odds of a fall event were sustained after covariate adjustment in participants with 3+ PIMs but not so in those with fewer than 3 PIMs (OR, 2.85 [95% CI, 1.54–5.26]). In both unadjusted and adjusted models, there was no association between PIM exposure and kidney disease outcomes. Age and total medications did not modify the association between number of PIMs and each outcome.
Table 3.
Sequentially Adjusted Association Between PIM Exposure and Adverse Outcomes
| Outcome/Exposure | No. of Events (Event Rate) | OR (95% CI) or RR (95% CI)a | |||
|---|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2c | Model 3d | ||
| Hospitalizations | |||||
| No PIM | 4,724 (57.9) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 PIM | 4,711 (72.7) | 1.16 (1.08–1.24) | 1.16 (1.08–1.24) | 1.13 (1.05–1.21) | 1.09 (1.01–1.17) |
| 2 PIMs | 2,818 (88.0) | 1.32 (1.20–1.44) | 1.33 (1.21–1.46) | 1.26 (1.15–1.39) | 1.18 (1.07–1.30) |
| ≥3 PIMs | 2,045 (104.7) | 1.52 (1.35–1.71) | 1.55 (1.38–1.74) | 1.53 (1.36–1.72) | 1.35 (1.19–1.53) |
| Death | |||||
| No PIM | 135 (1.3) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 PIM | 152 (1.8) | 1.42 (1.12–1.80) | 1.34 (1.06–1.69) | 1.25 (0.97–1.62) | 1.19 (0.91–1.54) |
| 2 PIMs | 113 (2.6) | 2.14 (1.66–2.76) | 1.99 (1.54–2.58) | 1.79 (1.36–2.37) | 1.62 (1.21–2.17) |
| ≥3 PIMs | 75 (3.1) | 2.32 (1.74–3.09) | 2.28 (1.70–3.05) | 1.97 (1.42–2.75) | 1.65 (1.14–2.41) |
| KRT | |||||
| No PIM | 285 (2.8) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 PIM | 236 (3.1) | 1.04 (0.88–1.24) | 1.15 (0.96–1.37) | 1.29 (1.04–1.61) | 1.24 (0.99–1.56) |
| 2 PIMs | 108 (2.8) | 0.95 (0.76–1.19) | 1.12 (0.89–1.41) | 1.23 (0.93–1.64) | 1.14 (0.84–1.55) |
| ≥3 PIMs | 55 (2.5) | 0.77 (0.58–1.04) | 1.12 (0.89–1.41) | 1.38 (0.95–2.01) | 1.23 (0.82–1.85) |
| Kidney disease composite outcomee | |||||
| No PIM | 333 (3.4) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 PIM | 279 (3.6) | 1.05 (0.89–1.24) | 1.14 (0.97–1.35) | 1.18 (0.98–1.43) | 1.15 (0.95–1.39) |
| 2 PIMs | 126 (3.3) | 0.95 (0.77–1.17) | 1.11 (0.89–1.37) | 1.15 (0.90–1.48) | 1.09 (0.84–1.41) |
| ≥3 PIMs | 71 (3.3) | 0.86 (0.66–1.12) | 1.01 (0.77–1.32) | 1.33 (0.96–1.83) | 1.21 (0.85–1.72) |
| Falls | |||||
| No PIM | 48 (2.6) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 PIM | 58 (4.0) | 1.66 (1.10–2.50) | 1.59 (1.05–2.41) | 1.44 (0.94–2.22) | 1.35 (0.87–2.09) |
| 2 PIMs | 26 (4.5) | 1.84 (1.10–3.09) | 1.75 (1.04–2.94) | 1.46 (0.84–2.56) | 1.32 (0.74–2.35) |
| ≥3 PIMs | 30 (8.4) | 4.06 (2.40–6.85) | 3.81 (2.22–6.55) | 3.50 (2.00–6.11) | 2.85 (1.54–5.26) |
Abbreviations: KRT, initiation of kidney replacement therapy; OR, odds ratio; PIM, potentially inappropriate medication; RR, rate ratio.
Rate ratio for hospitalization model.
Model 1 adjusted for age, other demographics, and participant site.
Model 2 is adjusted for model 1 covariates and eGFR, body mass index, diabetes, any cardiovascular disease, hypertension, arthritis, and prior nephrology care.
Model 3 is adjusted for model 2 covariates and number of medications.
Defined as occurrence of KRT or halving of baseline estimated glomerular filtration rate.
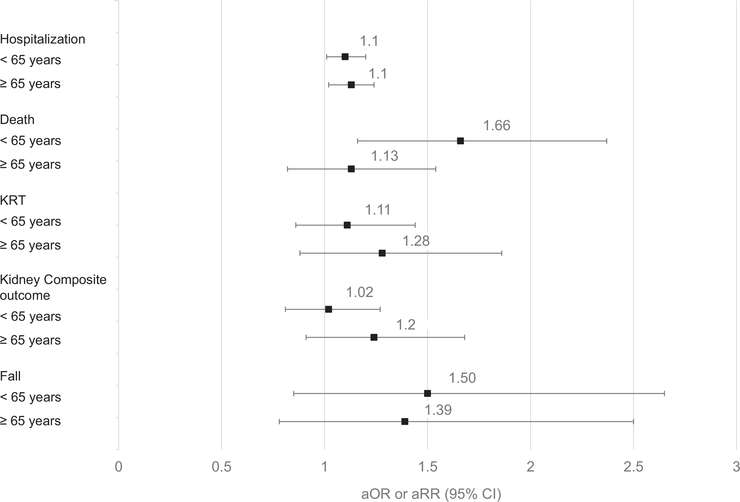
In post hoc analysis, adjusted models stratified by age demonstrate that participants aged <65 years with any PIM exposure had an increased risk of death (compared with those in the same age group with no PIMs) (Fig 2). However, the participants aged ≥65 years with any PIM exposure did not have increased risk of death (compared with those in the same age group with no PIMs). Our 2 sensitivity analyses (adding number of hospitalizations in the prior year as a covariate and restriction to visits with eGFR <45 mL/min/1.73 m2) show the associations of PIM exposure with each outcome were mostly sustained (Tables S3 and S4). The PIMs with the highest prevalence among those who experienced death were PPIs, α-blockers, central α-agonists, antidepressants, and anticholinergics (Table S5). In separate models, we found the participants with PPIs or α-blockers (compared with those without either PIM) did not have an increased risk of death within the next year, but there was an association of having PPIs with experiencing hospitalization within a year in the fully adjusted model (rate ratio, 1.13 [95% CI, 1.04–1.23]) (Table S6).
Figure 2.
Association of any potentially inappropriate medication (PIM) exposure and adverse outcomes stratified by age group. Models repeated separately for age <65 and age ≥65 and adjusted for demographics, study site, estimated glomerular filtration rate, body mass index, diabetes, cardiovascular disease, hypertension, arthritis, prior nephrology care, medication count, all were updated over time. Fall defined as fall within 3 months of medication exposure. Renal composite outcome is defined as initiation of KRT or halving of baseline estimated glomerular filtration rate. Reference group for each model was participants with no PIMs within the same age group. Abbreviations: KRT, kidney replacement therapy; aOR, adjusted odds ratio; aRR, adjusted rate ratio.
Discussion
In this retrospective study of CRIC participants, we describe prevalence and risk of harm associated with PIMs in the adult CKD population. PIMs were used by 80% of the cohort, were increasingly prevalent at older ages, and represented medication classes for common CKD comorbid conditions (eg, hypertension, depression, gastroesophageal reflux disease). Reported PIM use had a graded association with hospitalizations, death, and to a lesser extent falls, but not with progression of CKD. Older age did not increase the strength of association of PIMs with adverse outcomes; however, adults aged <65 with at least 1 PIM (compared with similarly aged cohort members without PIM exposure) had increased risk of death within a year. These findings suggest additional evidence is needed to guide prescribing of PIMs in the general adult CKD population regardless of age to both minimize adverse outcomes and optimally manage comorbid conditions.
Our study importantly adds to the literature because our analyses were not limited to older adults and uniquely identified that PIMs can contribute to adverse outcomes in community-dwelling adults with CKD with no variation in risk across age groups. Similar to our findings, several studies that focused on the general older adult population have shown an association between PIMs and hospitalizations, falls, and mortality.23–25 However, one recent study of community-dwelling older adults, including some with CKD, did not find an association between PIMs and hospitalization or death.14 Compared with our study, that study’s cohort had a smaller proportion (29%) with CKD and fewer adverse events. Unlike prior studies, our study examined kidney disease outcomes, revealing that PIMs are not associated with an increased risk of CKD progression.
Although individual medication classes have been associated with adverse outcomes in adults with CKD (eg, PPIs and NSAIDs),26,27 the PIMs evaluated in our study represent several therapeutic categories. This heterogeneity supports the idea that using a PIM could be a surrogate for other unmeasured factors related to comorbidity burden, such as poor health status, that contribute to morbidity and mortality.28 PIMs have been associated with incident frailty, which could itself drive adverse outcomes.29 In part, this unmeasured factor could explain why we found an increased risk of mortality among adults aged <65 with at least 1 PIM compared with those in the same age group with no PIMs. The heterogeneity of the PIMs represented in our exposure variable could also point to a commonly observed series of events that can possibly occur if, as the result of patient complications from PIM use, adverse drug events led to a prescribing cascade and subsequent acute hospitalizations.30 Additionally, seeing multiple providers through many health care visits can increase the risk of a patient using PIMs and their association with future adverse events.31,32
Because post hoc analyses show differential association with death and hospitalization outcomes for individual medication classes (α-blockers and PPIs), we acknowledge that all PIMs may not carry the same risk of harm. These findings highlight the need for additional studies to understand the risks attributable to individual medication classes in the AGS Beers Criteria and the mechanisms by which these medications classes may heighten the risk of harm in the adult CKD population.
Although our findings suggest age and eGFR had minimal impact on our risk estimates, there remains a need to consider the possibility that CKD itself may contribute to the association between PIM use and adverse outcomes. Because our cohort is limited to adults with CKD, our findings may imply that CKD itself may increase susceptibility to PIM complications. Further research is warranted, but mechanistic studies suggest that CKD-associated inflammation and mineral metabolism derangements yield premature aging.33 As a result, this premature aging could manifest as lower physiologic reserve when adverse drug events occur.34 However, not all CRIC participants with PIMs experienced morbidity and mortality; therefore, additional research is needed to understand whether there is a causal pathway and to identify the patient characteristics, instead of age and eGFR, that may modify risk.
This study has implications for clinical practice. This study revealed that 80% of adults of all ages with CKD reported taking PIMs, suggesting that the AGS Beers Criteria holds value as guidance for prescribing in both younger and older adults with CKD. The absence of an association of PIM exposure with kidney disease progression in our study, even among a subgroup of visits among participants with an annual eGFR of <45 mL/min/1.73 m2, may provide reassurance on the independence of these drugs’ safety profile with regard to kidney function. However, PIMs are not limited to medications that require adjustment for kidney function, so providers should be alert to medications—PIM or non-PIM—that have reduced clearance in CKD.
The most prevalent PIMs in this cohort are medications prescribed for significant comorbidities, such as hypertension, benign prostatic hypertrophy, diabetes, and depression, so it might be challenging to deprescribe all PIMs. Instead, providers are encouraged to promote non-pharmacologic interventions, repeatedly inquire about the continual need for specific PIMs, taper PIM doses when possible, and discuss potential risks with patients about PIMs use. Overall, providers should continue to employ an individualized approach to clinical decision making. Meanwhile, there is a need for future studies that elucidate which patients are at increased risk of adverse events from these medications to facilitate prescribing decisions.
This study’s strength lies in the robust and unique data collection inherent to the CRIC study; however, there are limitations. First, measurement bias related to ascertainment of PIMs from self-report may not account for medication adherence, underreporting of PIM combination pill use, and unknown medication dose and duration of use. Second, confounding by indication could not be captured in our analyses and may explain treatment choices for some medications, especially among participants who may have received palliative or hospice care during the observation period.
Our study design captures a heterogeneous group of PIMs in our exposure variable, so we are unable to report whether all PIMs are truly unsafe for this population. Analyses of each PIM class that minimize confounding by indication through new-user design and/or high-dimensional propensity score matching would be ideal for ascertaining risk.35 Second, our PIM list was not derived from the more recent 2019 AGS Beers Criteria.9 However, the similarities between the lists suggest our findings would be unchanged with the updated list. Finally, only ~20% of our cohort had eGFR < 30 mL/min/1.73 m2, so our findings may not be specifically generalizable to this subpopulation who are highly likely to experience adverse drug reactions.36 Still, our study captured a broad spectrum of the CKD population.
In summary, these analyses of PIM use and risk of harm in the CRIC cohort demonstrate that PIM use is very common and is associated, in a graded manner, with hospitalizations and death in an adult CKD population irrespective of age. Because many of these PIMs are indicated for common comorbidities, additional studies are needed to understand and minimize these risks.
Supplementary Material
Figure S1: Study flowchart.
Table S1: PIMs included in exposure variable.
Table S2: Baseline cohort characteristics based on ever taking a PIM during study observation period.
Table S3: Sensitivity analysis of fully adjusted model with additional adjustment for number of hospitalizations reported from the prior year.
Table S4: Sensitivity analysis of fully adjusted model in a subgroup of visits from the cohort with eGFR < 45.
Table S5: Number of all visits when PIMs reported used by cohort members who died in total and by age group.
Table S6: Adjusted association between common PIMs in the cohort and adverse outcomes of hospitalization and death.
PLAIN-LANGUAGE SUMMARY.
Potentially inappropriate medications (PIMs) bring more risk of harm than benefit in older adults. The frequency of use of these medications and their association with adverse outcomes in the adult population with CKD has not been well studied. To address this knowledge gap, we examined data from participants in the Chronic Renal Insufficiency Cohort (CRIC) study to determine the prevalence of PIM prescriptions and the association between PIMs and adverse outcomes, including death, hospitalization, falls, and progression of kidney disease. The most commonly used PIMS were proton pump inhibitors, α-blockers, and nonsteroidal anti-inflammatory drugs (NSAIDs). The first 2 were used more frequently in older study participants, and NSAIDs were more commonly used by the younger participants. Study participants, regardless of age, were more likely to have an increased risk of hospitalization, mortality, and falls after prescriptions for PIMs, with the greatest risk occurring with prescriptions for more than 1 type of PIM.
Acknowledgements:
The authors would like to thank Donna Crabtree at Duke University School of Medicine for her assistance with building supplemental table.
Support: Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (NIH) under Award Number K76AG059930 (to RH) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under Award Number R01DK090008 (to JCF). This study was also supported by the ASN Foundation for Kidney Research (to RH) and the Doris Duke Charitable Foundation Fund to Retain Clinical Scientist Award (to RH). Funding for the CRIC Study was obtained under a cooperative agreement from NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, and Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The NIH (and other sponsors) did not have a role in study design, data collection, analysis, or interpretation, or manuscript preparation.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CRIC Study Investigators: In addition to author Chen, the CRIC Study Investigators are Lawrence J. Appel, MD, MPH, Harold I. Feldman MD, MSCE, Alan S. Go, MD, Mahboob Rahman, MD, Panduranga S. Rao, MD, Vallabh O. Shah, PhD, MS, Raymond R. Townsend, MD, and Mark L. Unruh, MD, MS.
Contributor Information
Rasheeda K. Hall, Renal Section, Department of Medicine, School of Medicine, Duke University, and Durham Veterans Affairs Healthcare System, Durham, North Carolina
Jacob B. Blumenthal, Division of Gerontology & Geriatric Medicine, School of Medicine, University of Maryland, Baltimore, Maryland Baltimore Geriatrics Research, Department of Medicine, Education and Clinical Center (GRECC), Baltimore Veterans Affairs and Medical Center, Baltimore, Maryland.
Rebecca M. Doerfler, Department of Medicine, School of Medicine, University of Maryland, Baltimore, Maryland
Jing Chen, Department of Medicine, School of Medicine, Tulane University, New Orleans, Louisiana.
Clarissa J. Diamantidis, Renal Section, Department of Medicine, School of Medicine, Duke University, and Durham Veterans Affairs Healthcare System, Durham, North Carolina
Bernard G. Jaar, Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland
John W. Kusek, Center for Clinical Epidemiology and Biostatistics, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania
Krishna Kallem, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Mary B. Leonard, Department of Pediatrics, Stanford University School of Medicine, Palo Alto, California
Sankar D. Navaneethan, Section of Nephrology, Department of Medicine, Baylor College of Medicine, Houston, Texas
Daohang Sha, Center for Clinical Epidemiology and Biostatistics, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
James H. Sondheimer, Department of Medicine, School of Medicine, Wayne State University, Detroit, Michigan
Lee-Ann Wagner, Department of Medicine, School of Medicine, University of Maryland, Baltimore, Maryland.
Wei Yang, Center for Clinical Epidemiology and Biostatistics, School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Min Zhan, Department of Epidemiology and Public Health, School of Medicine, University of Maryland, Baltimore, Maryland.
Jeffrey C. Fink, Department of Medicine, School of Medicine, University of Maryland, Baltimore, Maryland
References
- 1.Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69(3):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowling CB, Booth JN 3rd, Gutierrez OM, et al. Nondisease-specific problems and all-cause mortality among older adults with CKD: the REGARDS Study. Clin J Am Soc Nephrol. 2014;9(10):1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11): 1863–1869. [DOI] [PubMed] [Google Scholar]
- 4.Reese PP, Cappola AR, Shults J, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38(4):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart A, Paudel ML, Taylor BC, et al. Cystatin C and frailty in older men. J Am Geriatr Soc. 2013;61(9):1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. [DOI] [PubMed] [Google Scholar]
- 7.Wong E, Ballew SH, Daya N, et al. Hospitalization risk among older adults with chronic kidney disease. Am J Nephrol. 2019;50(3):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fick DM, Mion LC, Beers MH, Waller JL. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 10.Dedhiya SD, Hancock E, Craig BA, Doebbeling CC, Thomas J 3rd. Incident use and outcomes associated with potentially inappropriate medication use in older adults. Am J Geriatr Pharmacother. 2010;8(6):562–570. [DOI] [PubMed] [Google Scholar]
- 11.Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Momper JD, Venkataramanan R, Nolin TD. Nonrenal drug clearance in CKD: searching for the path less traveled. Adv Chronic Kidney Dis. 2010;17(5):384–391. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt IM, Hubner S, Nadal J, et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J. 2019;12(5):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Secora A, Alexander GC, Ballew SH, Coresh J, Grams ME. Kidney function, polypharmacy, and potentially inappropriate medication use in a community-based cohort of older adults. Drugs Aging. 2018;35(8):735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tesfaye WH, Wimmer BC, Peterson GM, et al. The effect of hospitalization on potentially inappropriate medication use in older adults with chronic kidney disease. Curr Med Res Opin. 2019;35(6):1119–1126. [DOI] [PubMed] [Google Scholar]
- 16.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(suppl 2):S148–S153. 7. [DOI] [PubMed] [Google Scholar]
- 17.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8): 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
- 19.Deo R, Shou H, Soliman EZ, et al. Electrocardiographic measures and prediction of cardiovascular and noncardiovascular death in CKD. J Am Soc Nephrol. 2016;27(2):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Xie D, Anderson AH, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63(2):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed. Wiley; 2011. [Google Scholar]
- 23.Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schottker B. The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc. 2017;18(3):211–220. [DOI] [PubMed] [Google Scholar]
- 24.Hyttinen V, Jyrkka J, Valtonen H. A systematic review of the impact of potentially inappropriate medication on health care utilization and costs among older adults. Med Care. 2016;54(10):950–964. [DOI] [PubMed] [Google Scholar]
- 25.Berdot S, Bertrand M, Dartigues JF, et al. Inappropriate medication use and risk of falls—a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009; 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan M, Doerfler RM, Xie D, et al. Association of opioids and nonsteroidal anti-inflammatory drugs with outcomes in CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2020;76(2):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackwell SA, Montgomery MA, Baugh DK, Ciborowski GM, Riley GF. Applying the 2003 Beers update to elderly Medicare enrollees in the Part D program. Medicare Medicaid Res Rev. 2012;2(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlack DC, Hoppe LK, Saum KU, Haefeli WE, Brenner H, Schottker B. Investigation of a possible association of potentially inappropriate medication for older adults and frailty in a prospective cohort study from Germany. Age Ageing. 2019;49(1):20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeRhodes KH. The dangers of ignoring the Beers Criteria—the prescribing cascade. JAMA Intern Med. 2019;179(7):863–864. [DOI] [PubMed] [Google Scholar]
- 31.Jiron M, Pate V, Hanson LC, Lund JL, Jonsson Funk M, Sturmer T. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc. 2016;64(4):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nothelle SK, Sharma R, Oakes A, Jackson M, Segal JB. Factors associated with potentially inappropriate medication use in community-dwelling older adults in the United States: a systematic review. Int J Pharm Pract. 2019;27(5):408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nat Rev Nephrol. 2014;10(12):732–742. [DOI] [PubMed] [Google Scholar]
- 34.Hanlon JT, Fillenbaum GG, Kuchibhatla M, et al. Impact of inappropriate drug use on mortality and functional status in representative community dwelling elders. Med Care. 2002;40(2):166–176. [DOI] [PubMed] [Google Scholar]
- 35.Hundemer GL, Knoll GA, Petrcich W, et al. Kidney, cardiac, and safety outcomes associated with alpha-blockers in patients with CKD: a population-based cohort study. Am J Kidney Dis. 2021;77(2):178–189 e171. [DOI] [PubMed] [Google Scholar]
- 36.Laville SM, Gras-Champel V, Moragny J, et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol. 2020;15(8):1090–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Study flowchart.
Table S1: PIMs included in exposure variable.
Table S2: Baseline cohort characteristics based on ever taking a PIM during study observation period.
Table S3: Sensitivity analysis of fully adjusted model with additional adjustment for number of hospitalizations reported from the prior year.
Table S4: Sensitivity analysis of fully adjusted model in a subgroup of visits from the cohort with eGFR < 45.
Table S5: Number of all visits when PIMs reported used by cohort members who died in total and by age group.
Table S6: Adjusted association between common PIMs in the cohort and adverse outcomes of hospitalization and death.